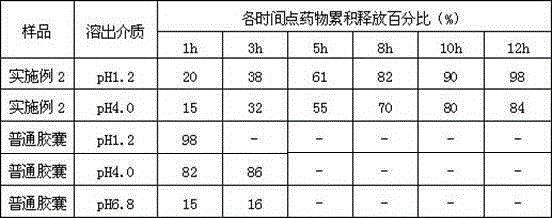
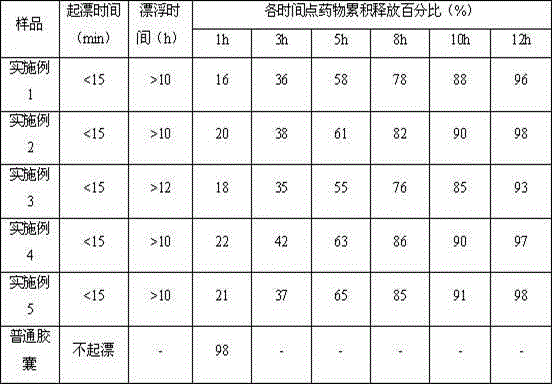
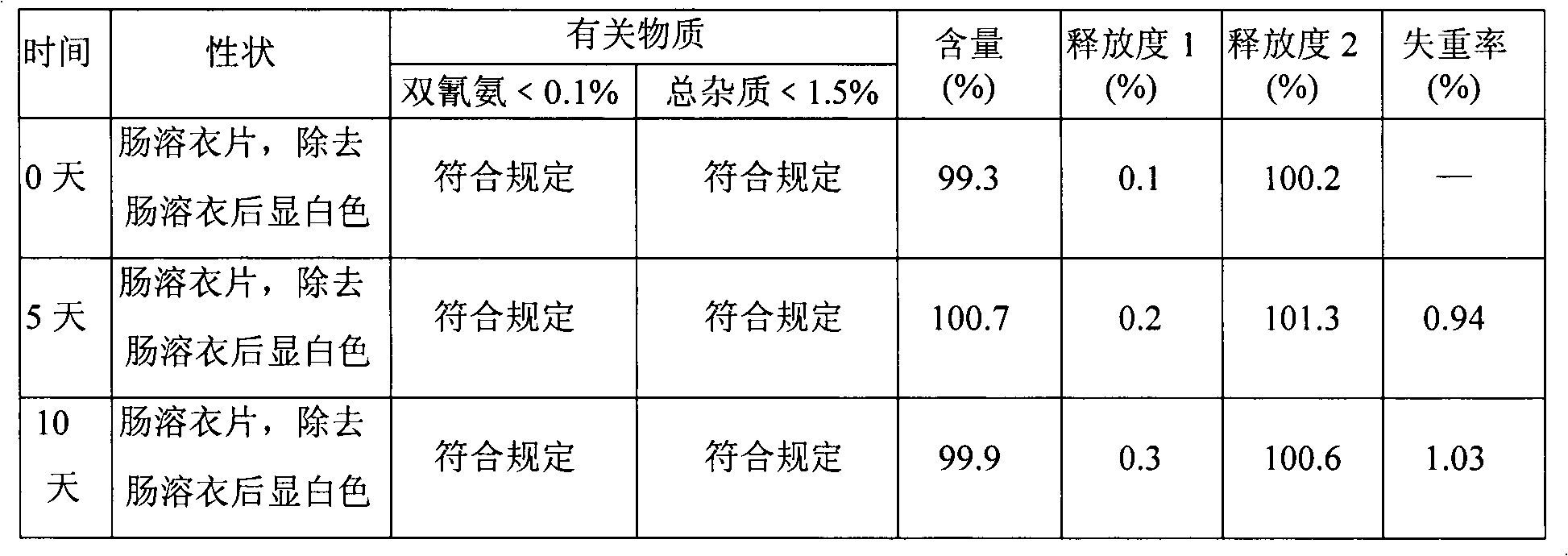
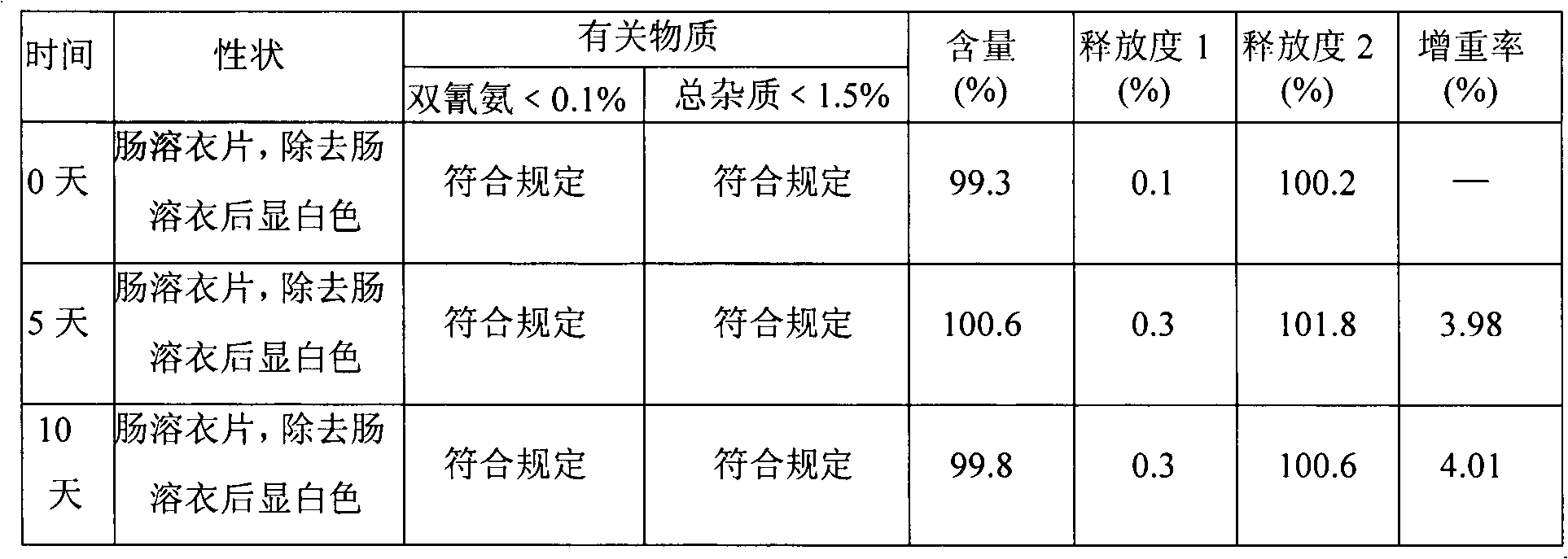
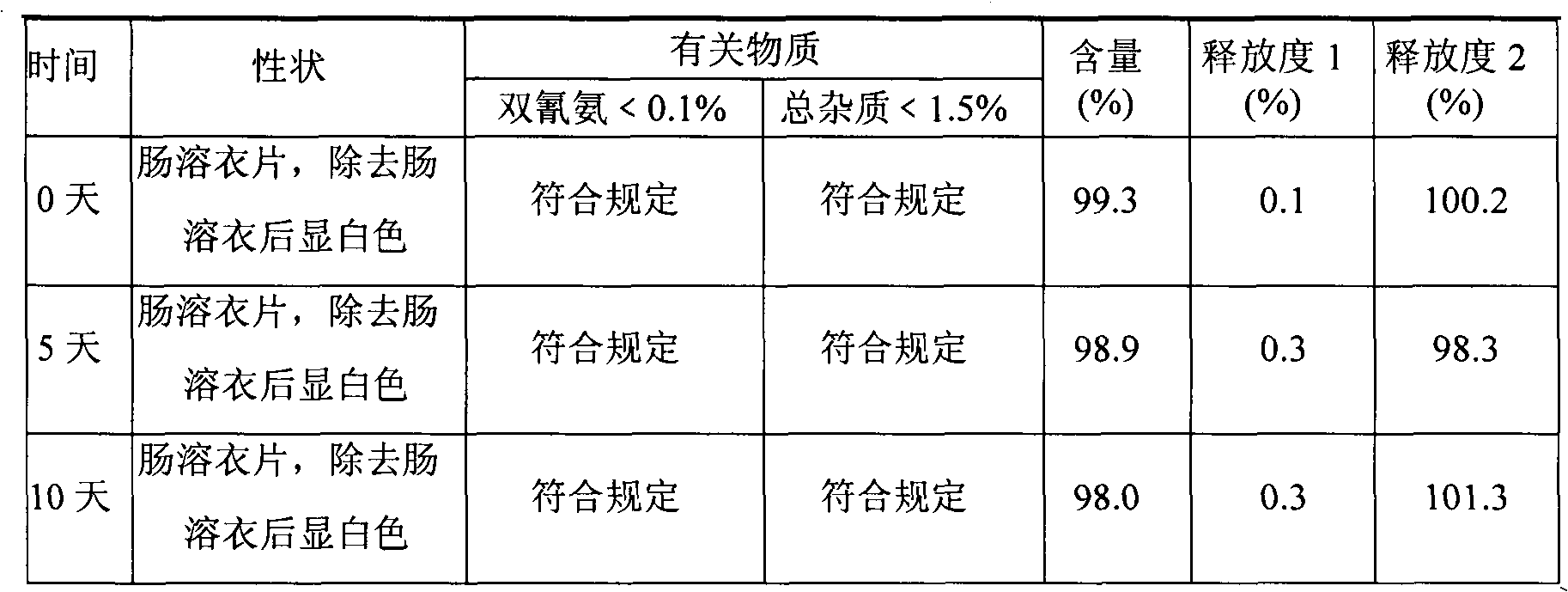
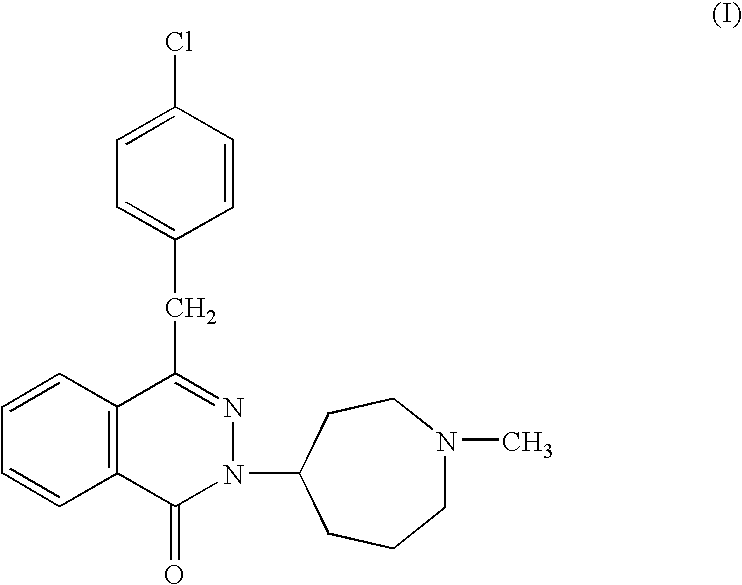
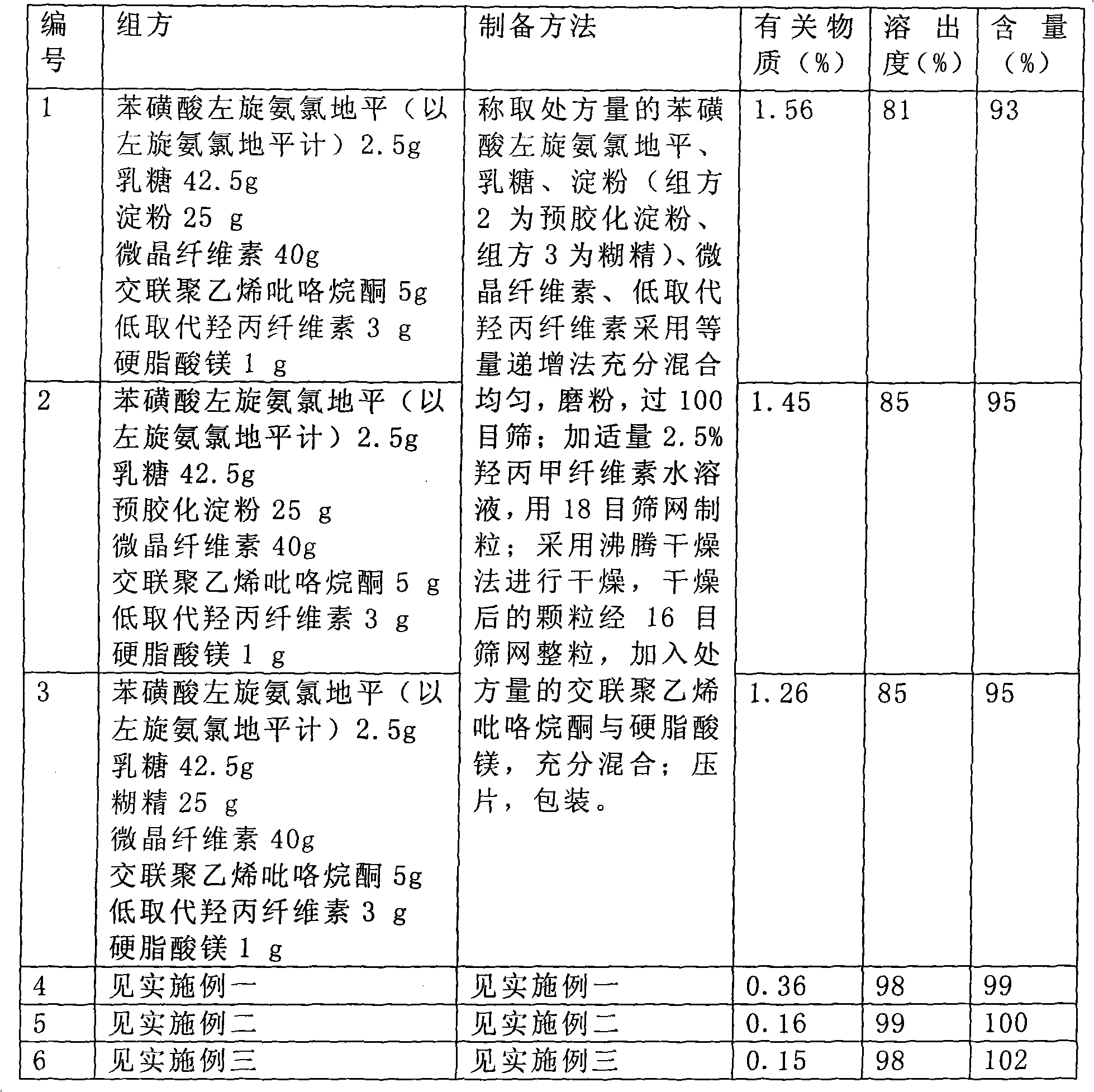
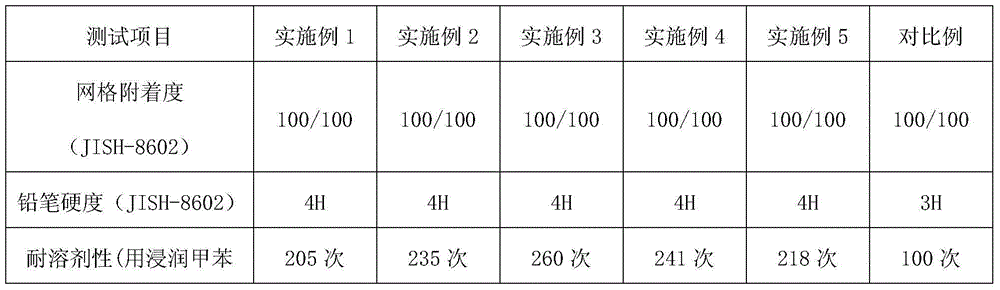
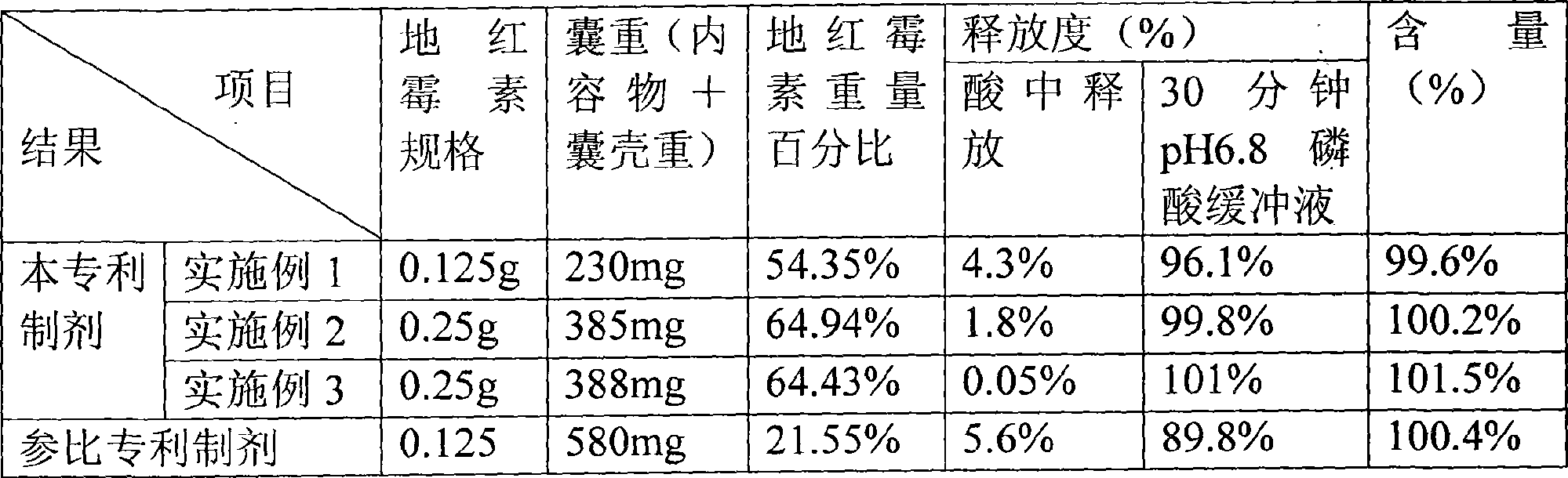
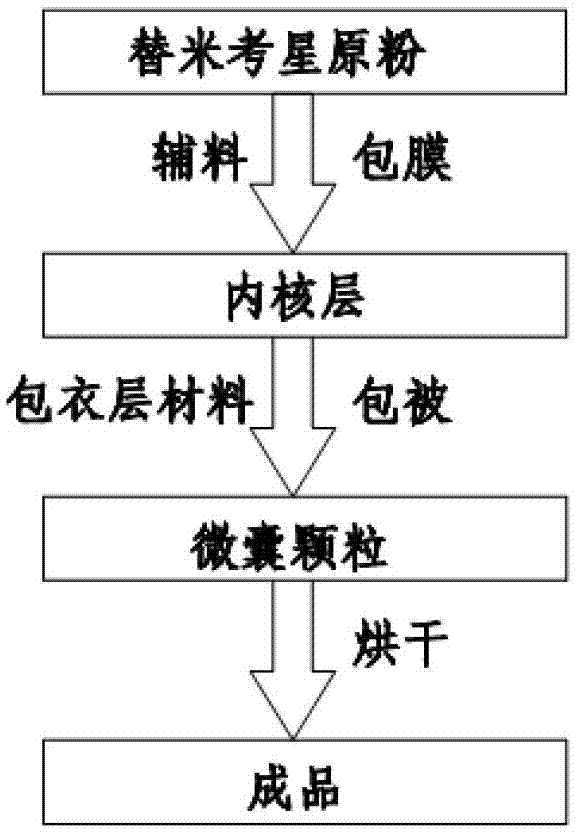
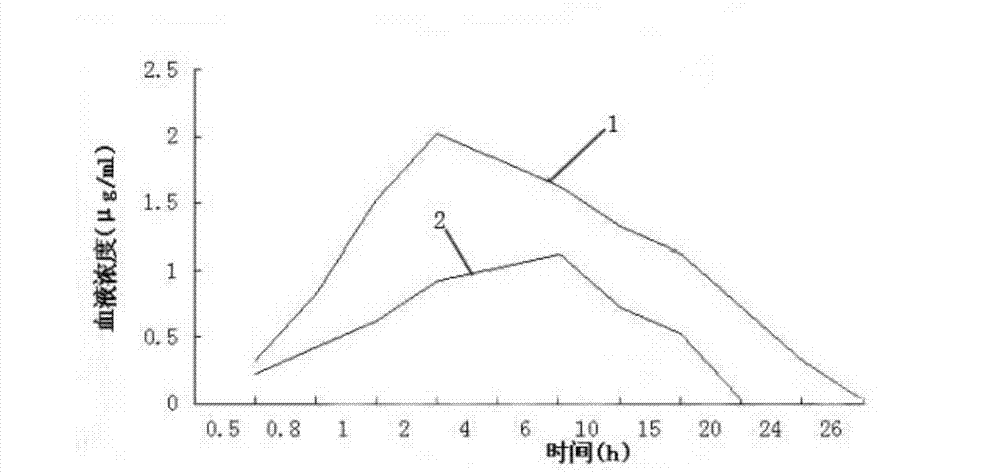
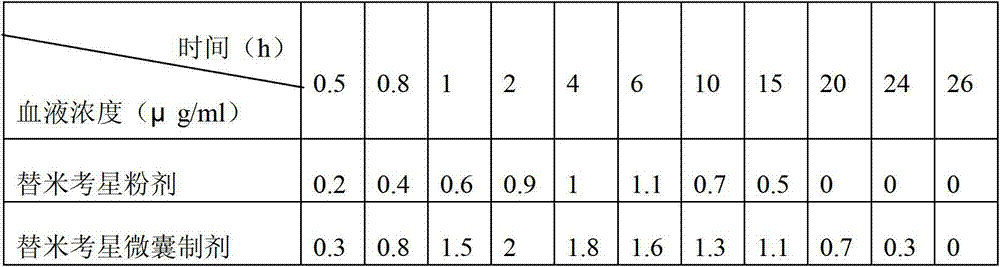
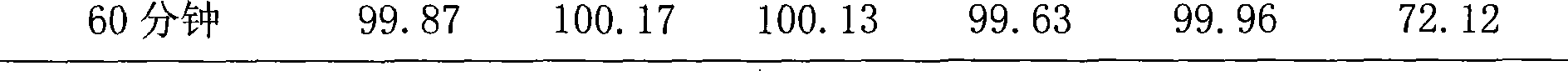Patents
Literature
1106 results about "Hypromellose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hypromellose (INN), short for hydroxypropyl methylcellulose (HPMC), is a semisynthetic, inert, viscoelastic polymer used as eye drops, as well as an excipient and controlled-delivery component in oral medicaments, found in a variety of commercial products.
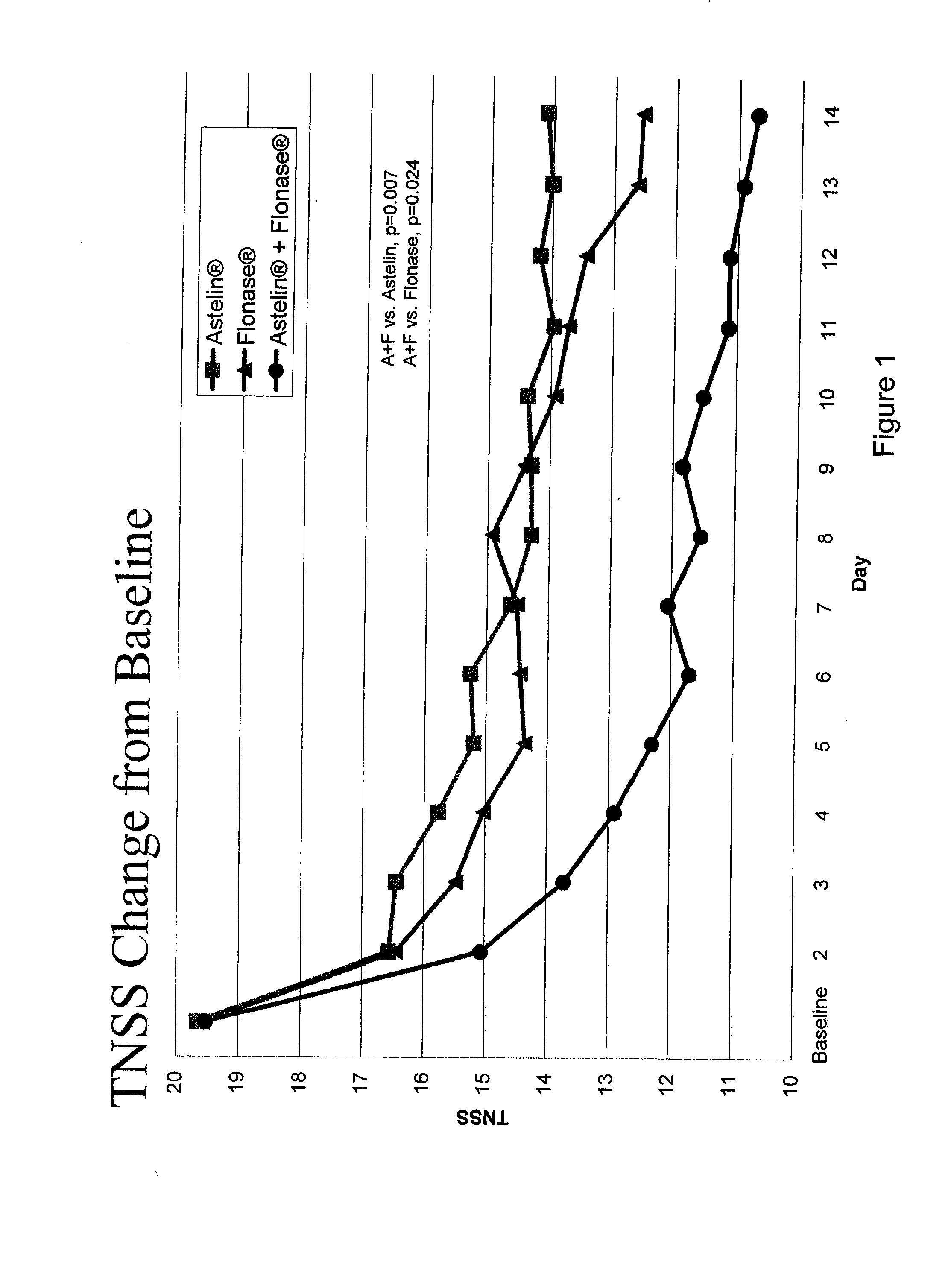
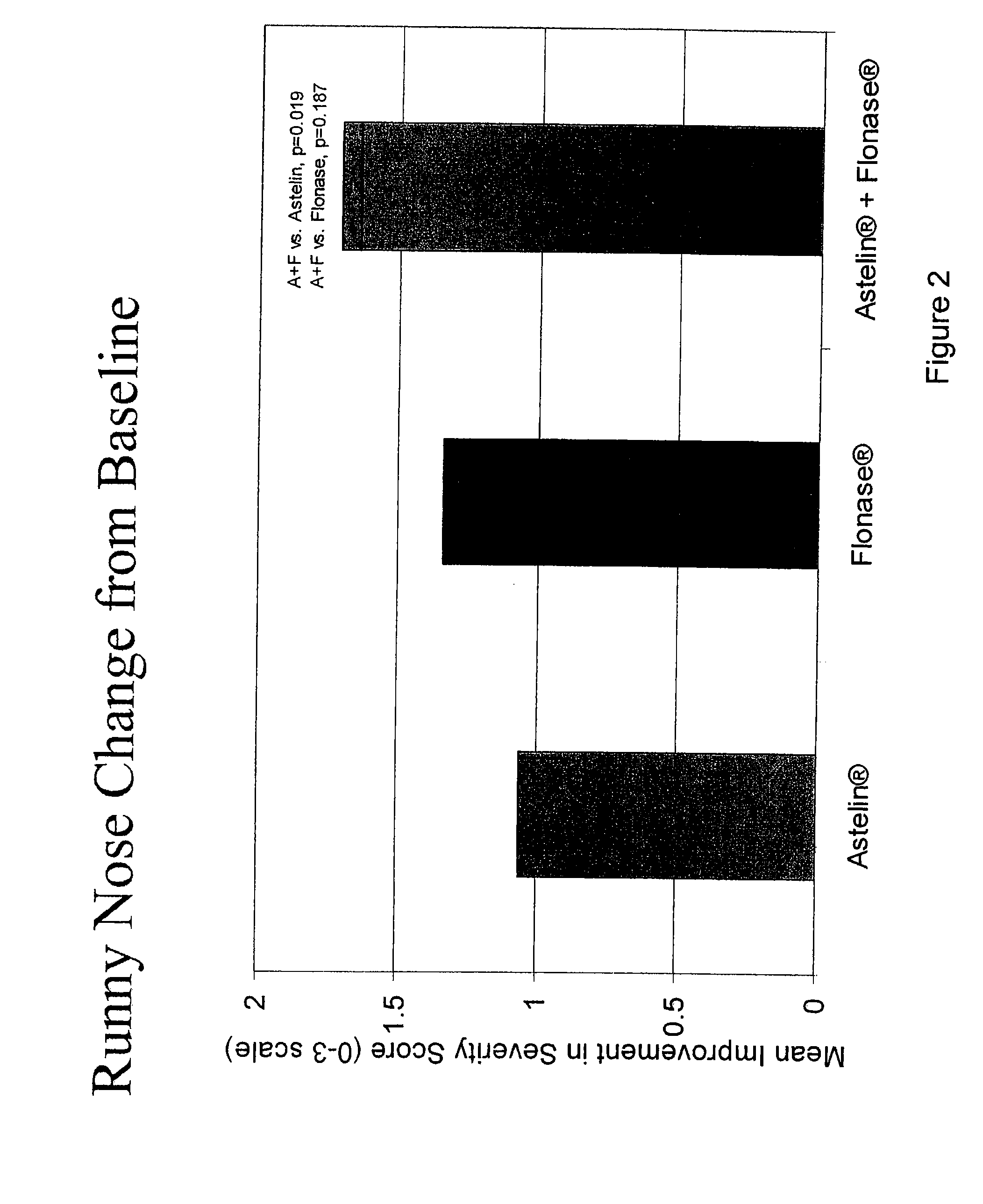
Compositions comprising azelastine and methods of use thereof
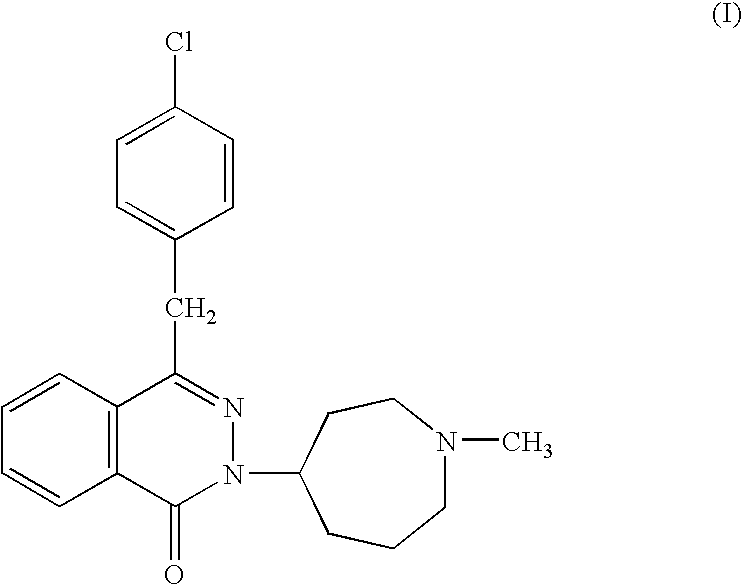
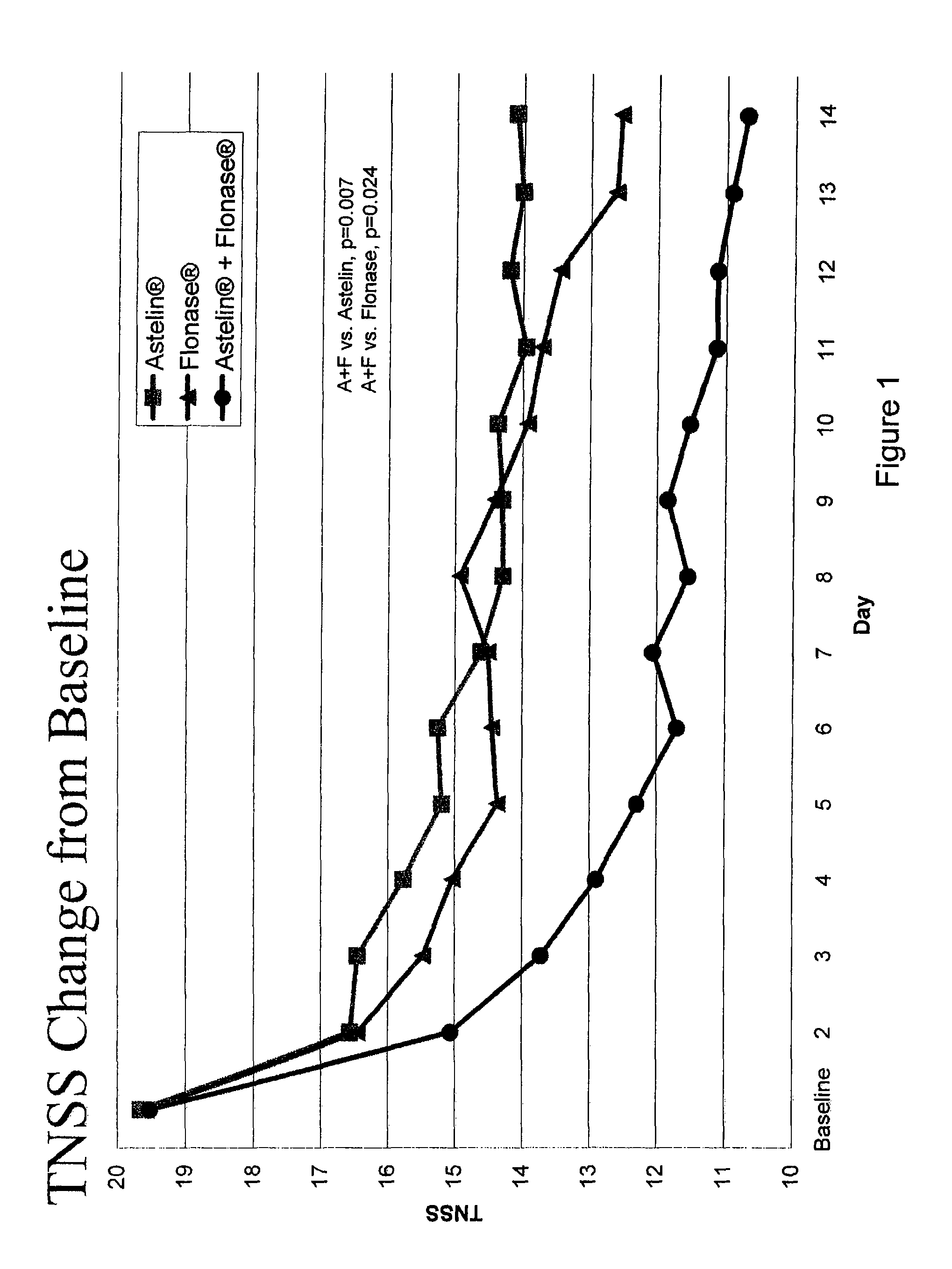
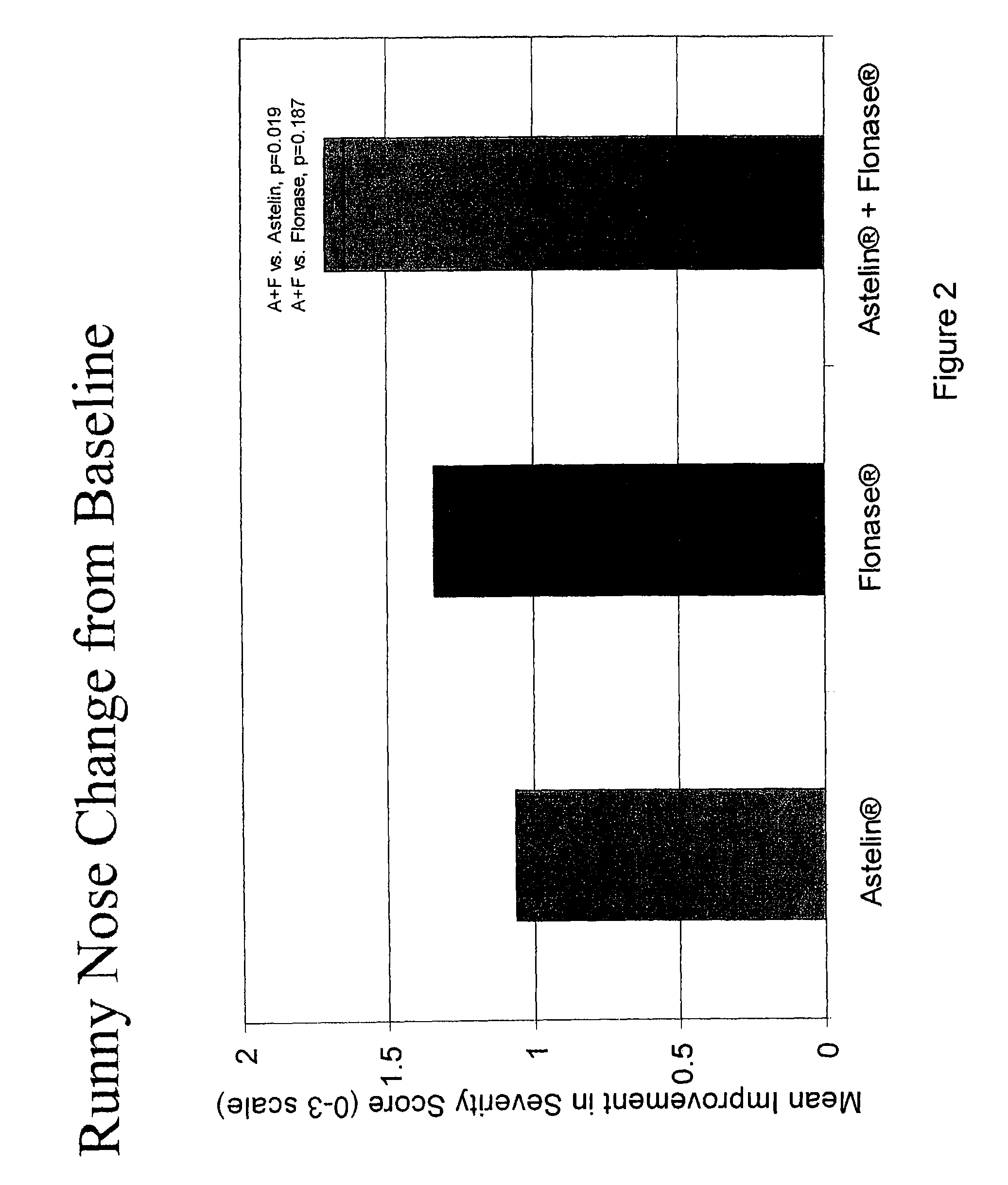
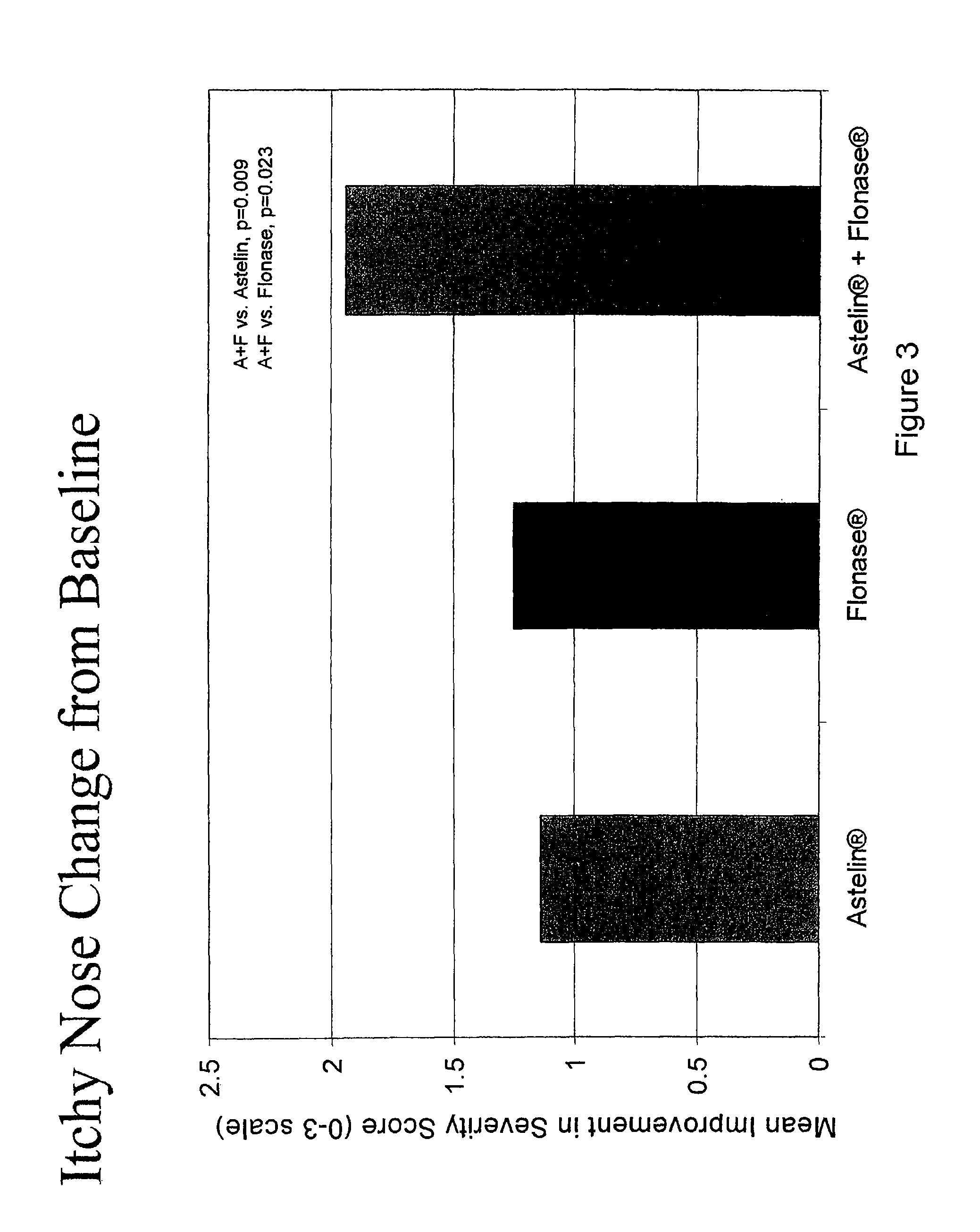
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Pantoprazole multiparticulate formulations
ActiveUS20050129761A1Low variabilityProlong the action timeBiocideDispersion deliveryMedicineEnantiomer
Pantoprazole sodium multiparticulates are described which avoid sticking to nasogastric and gastronomy tubes. The pantoprazole multiparticulates have a spheroid core of pantoprazole or an enantiomer thereof, or a salt thereof, a surfactant, and a distintegrant; a sub coat which is comprised of hydroxypropyl methylcellulose (hypromellose) and water, an enteric coat on the sub-coat, and a final seal coat over the enteric coat, which is composed of hydroxypropyl methylcellulose (hypromellose) and water.
Owner:WYETH LLC
Thermal insulation building mortar and preparation process thereof
InactiveCN101759416AWith thermal insulationWith phase change energy storageCement mixing apparatusSodium BentoniteThermal insulation
The invention discloses a thermal insulation building mortar. The thermal insulation building mortar is prepared by the mixing portland cement, pulverized fuel ash, calcium bentonite, re-scattering glue powder, hypromellose, polyacrylamide powder, polypropylene fibre, wood fiber, permeating crystal waterproof agent, air entraining agent, vitrified micro-bead, sizing phase-change material and hollow glass bead. A preparation process thereof comprises the steps of: a) preparing the sizing phase-change material, b) preparing the permeating crystal waterproof agent and c) preparing the thermal insulation building mortar. The mortar of the invention is characterized by the functions of thermal insulation, phase-change energy storage, temperature control, water resistance and energy saving, the wide application scope and low cost. The mortar is widely applied in thermal insulation work inside or outside the buildings and has a broad prospect.
Owner:唐山市思远涂料有限公司
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Soluble microneedle patch used for skin whitening and preparation method thereof
ActiveCN107375008AHigh mechanical strengthSolve the lack of hardnessCosmetic preparationsToilet preparationsSolubilitySodium hyaluronate
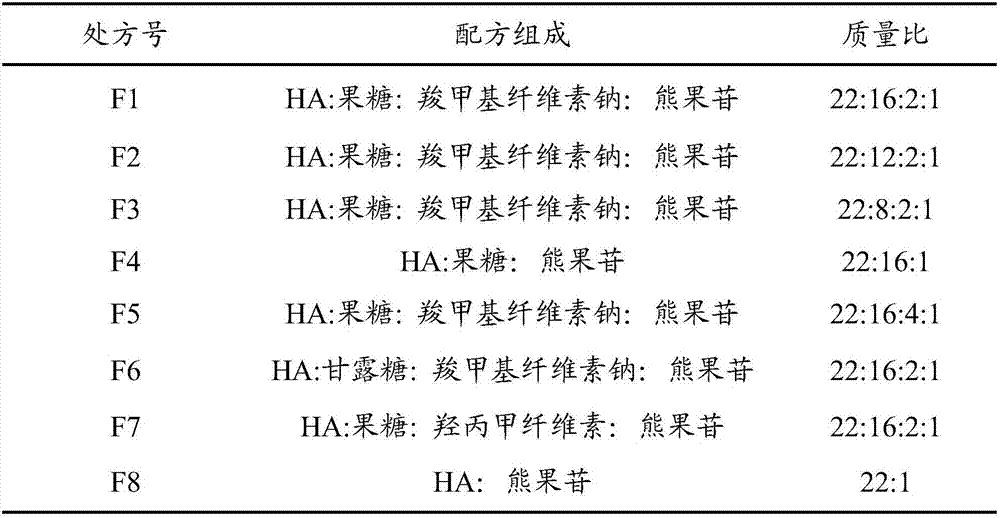
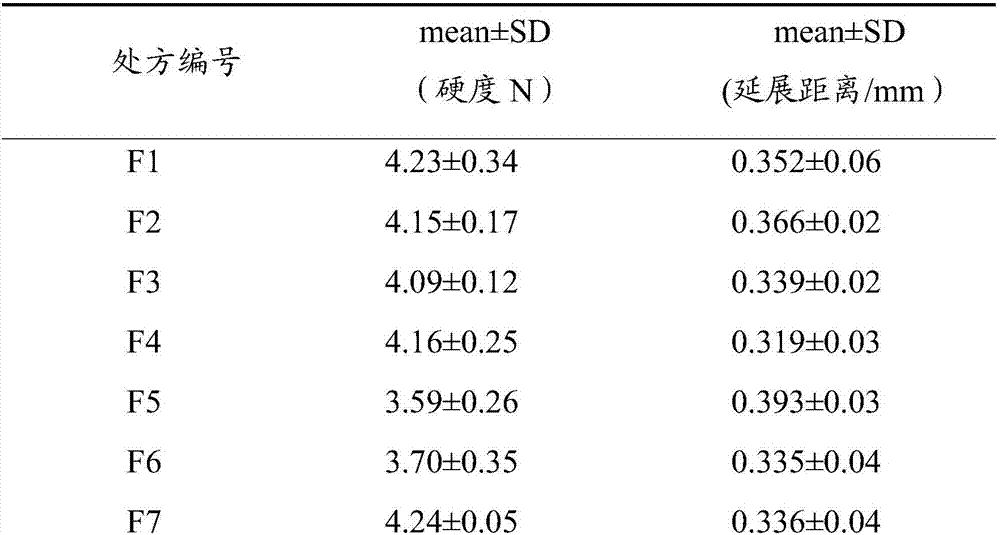
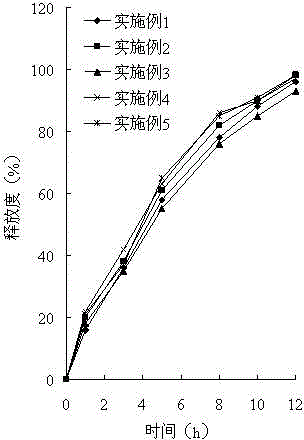
The invention relates to a soluble microneedle patch used for skin whitening and a preparation method thereof. The soluble microneedle patch comprises a needle point and a substrate, the needle point comprises the following raw materials in parts by weight: 1 part of arbutin, 15-30 parts of hyaluronic acid or its salt, and 5-25 parts of a forming material; wherein the hyaluronic acid or its salt is hyaluronic acid or sodium hyaluronate; and the forming material is selected from at least one of fructose, mannose, carboxymethylcellulose sodium and hydroxypropyl methylcellulose. The soluble microneedle patch has good mechanical strength, hardness and dissolvability, avoids the disadvantage that a traditional skin-caring mode cannot perform arbutin advantage, and has obvious effects for lightning color spots and whitening skin.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD +1
Palbociclib gastric-floating tablet and preparation method thereof
ActiveCN104887641ALow drift timeReduce dosageOrganic active ingredientsPill deliveryUse medicationPharmaceutical drug
The invention belongs to the technical field of medicine, and relates to a palbociclib gastric-floating tablet and a preparation method thereof. The palbociclib gastric-floating tablet comprises, by mass, 10%-30% of palbociclib, 20%-50% of hydroxypropyl methylcellulose, 20%-40% of bleaching auxiliaries, 2%-10% of foaming agents, 0%-25% of microcrystalline cellulose and 0.5%-3% of magnesium stearate. A dry granulating technology or a wet granulating technology can be used as the preparation technology. The palbociclib gastric-floating tablet is high in bioavailability, has a slow release tendency, and effectively lowers the total dosage. The palbociclib gastric-floating tablet and the preparation method thereof have the unique advantages that two different mechanisms are used for preparing the gastric-floating tablet, and accordingly the prepared tablet can keep floating in gastric juice by more than 10 hours and continuously release drugs in the hydrochloric acid solution with the pH being 1.2; the problem that the bioavailability is low due to the fact that drugs are extremely difficult to dissolve after the pH is higher than four is effectively solved; the medicine taking frequency is reduced; toxic and side effects are lightened; and the complaisance of a patient is effectively improved.
Owner:上海润泰医药科技有限公司
Vaginal pharmaceutical compositions and methods for preparing them
Vaginal pharmaceutical compositions are described. These compositions contain (i) an active pharmaceutical ingredient selected from the group consisting of antimicrobial imidazoles and mixtures thereof, and (ii) a polysaccharide, wherein the pH of the composition is greater than 4.25 and less than about 8. In particularly preferred compositions, the active pharmaceutical ingredient includes metronidazole and the polysaccharide includes hypromellose. These compositions can be applied to vaginal tissue for treatment of various diseases, such as bacterial vaginosis, or for prophylaxis.
Owner:TEVA PHARM USA INC
Metformin hydrochloride enteric-coated tablets and preparation method thereof
The invention belongs to the technical field of medicinal preparations, in particular relates to metformin hydrochloride enteric-coated tablets and a preparation method thereof, and provides stable metformin hydrochloride enteric-coated tablets. Each metformin hydrochloride enteric-coated tablet comprises a tablet core, an insulation coating and an enteric coating, wherein the tablet core is prepared from metformin hydrochloride, dextrin, hyprolose, magnesium stearate and talcpowder by adopting the ethanol aqueous solution of hypromellose as an adhesive; the insulation coating is prepared from a gastric soluble film coating premixed suspension agent and purified water; and the enteric coating is prepared from an enteric film coating premixed suspension agent and the purified water.
Owner:BEIJING JINGFENG PHARMA GRP
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Levamlodipine besylate tablet, preparation process thereof and control method for relevant materials
ActiveCN102028662AGood treatment effectStable buckOrganic active ingredientsPill deliveryCross-linkSolubility
The invention relates to a levamlodipine besylate tablet, a preparation process thereof and a control method for relevant materials. Each 1000 levamlodipine besylate tablets provided by the invention comprise the following compositions: 2.5g of levamlodipine besylate (measured in besylate), 30 to 50g of lactose (as a filler), 20 to 40g of beta-cyclodextrin (as an inclusion agent), 25 to 45g of microcrystalline cellulose (as a disintegrating agent), 5 to 15g of cross-linked polyvinylpyrrolidone (as a disintegrating agent), 1 to 2g of magnesium stearate (as lubricant), and 50 to 80g of 2.5% HPMC (hydroxypropyl methylcellulose) and 50% ethanol (as an adhesive). The levamlodipine besylate tablet provided by the invention has the advantages of making multi-item improvements on the properties of the levamlodipine besylate, increasing the solubility and apparent dissolution rate of the tablet, improving the stability of the tablet, reducing the excitability of the tablet, significantly reducing the limit of the relevant material, and having better clinical treatment effect, so that the blood pressure lowering of the patient with hypertension is more stable.
Owner:JIANGXI SHIMEI PHARM CO LTD
Vaginal pharmaceutical compositions and methods for preparing them
Vaginal pharmaceutical compositions are described. These compositions contain (i) an active pharmaceutical ingredient selected from the group consisting of antimicrobial imidazoles and mixtures thereof, and (ii) a polysaccharide, wherein the pH of the composition is greater than 4.25 and less than about 8. In particularly preferred compositions, the active pharmaceutical ingredient includes metronidazole and the polysaccharide includes hypromellose. These compositions can be applied to vaginal tissue for treatment of various diseases, such as bacterial vaginosis, or for prophylaxis.
Owner:TEVA PHARM USA INC
Metallic cathode electrophoretic paint and preparation method thereof
ActiveCN104312246AImprove stabilityImprove acid resistancePaints for electrolytic applicationsEpoxySilanes
The invention discloses metallic cathode electrophoretic paint and a preparation method thereof. The metallic cathode electrophoretic paint comprises the following components: modified epoxy resin, methyl trimethoxy silane, hydroxy propyl cellulose acetic ester, 2-cyan ethyl acrylate, acrylamide, 2-acrylamide-2-methyl propanesulfonic acid, propanesulfonic acid and zinc stearate. The preparation method comprises the following steps: adding modified epoxy resin, hydroxy propyl cellulose acetic ester and 2-cyan ethyl acrylate into a reaction kettle, heating till the above components are molten, adding hydroxypropyl methylcellulose and methyl trimethoxy silane, heating, stirring to react, further adding deionized water, further reacting at certain temperature and vacuum degree, finally adding acrylamide, heating, stirring, subsequently cooling, adding 2-acrylamide-2-methyl propanesulfonic acid and zinc stearate, stirring, reacting, and finally cooling to room temperature, thereby obtaining the metallic cathode electrophoretic paint. By adopting the metallic cathode electrophoretic paint disclosed by the invention, the properties of a coated paint membrane are greatly improved, and the use range of the cathode electrophoretic paint is further widened and expanded.
Owner:江苏悠谷未来科技有限公司
Butylphthalide dripping pill and preparing method
ActiveCN1943571AFast oral onsetPromote absorptionOrganic active ingredientsDispersion deliveryButylphthalideHypromellose
The invention relates to a drip pills of butylbenzene phthalein and its preparation method and it is characterized by comprising butylbenzene phthalein, base material, dispersing agent and coating materials. PEG4000, PEG6000, PEG20000 and poloxamer are selected as base materials. Dispersing agent is from lightweight aerosol and crospovidone. And coating materials are from hypromellose, hydroxypropyl cellulose, ethyl cellulose and Eudragit E30D.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Secnidazole tablet and its prepn process
InactiveCN1973838AHigh dose of main drugPiece weight smallAntibacterial agentsOrganic active ingredientsCarboxymethyl celluloseMagnesium stearate
The secnidazole tablet contains secnidazole 50-90 wt%, microcrystalline cellulose, starch, pre-gelatinized starch and / or lactose 0.5-40 wt%, cross-linked sodium carboxymethyl cellulose and / or cross-linked polyvinyl pyrrolidone 0.5-9 wt%, polyvinyl pyrrolidone or hydroxypropylmethyl cellulose 0.1-2.5 wt%, magnesium stearate 0.5-1 wt%, and talcum powder and / or fine silica gel powder 0.5-1 wt%. The preparation process of the secnidazole tablet includes the steps of mixing the said materials, palletizing and tabletting. The secnidazole tablet has high effective component content, easy swallowing, no bitter taste and fast leaching.
Owner:湖北科益药业股份有限公司
Nicardipine hydrochloride dispersion piece and method for making same
InactiveCN101156851AShort disintegration timeGood dispersionOrganic active ingredientsPill deliveryCross-linked polyethyleneStevioside
The invention discloses a nicardipine hydrochloride dispersible tablet which is characterized in that the nicardipine hydrochloride dispersible tablet is composed of components with the following weight percentage: 8 to 12 percent of nicardipine hydrochloride, 35 to 55 percent of starch, one or more than one of microcrystalline cellulose and lactose, 35 to 50 percent of sodium carboxymethyl starch, one or more than one of hydroxypropyl cellulose, hydroxypropyl methylcellulose, crosslinked polyvinyl pyrrolidone, and crosslinked sodium carboxymethyl cellulose, 5 to 2.5 percent of saccharin sodium or stevioside, and 0.25 to 1 percent of magnesium stearate. The disintegration time of the nicardipine hydrochloride dispersible tablet is short, the dispersed state is good, the drug is dissolved rapidly, the administration is convenient and flexible, the tablet can not only be sucked and swallowed, but also can be taken after being dispersed by adding water. The invention also discloses a preparation method of the nicardipine hydrochloride dispersible tablet.
Owner:刘全胜
Edoxaban sustained release tablet and preparation method thereof
InactiveCN103919746AAnticoagulant therapy is stableReduce adverse reactionsPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSulfonateCellulose
The invention provides a preparation method of an edoxaban sustained release matrix tablet. The specific prescription of the edoxaban sustained release tablet consists of the following components in percentage by weight: 14-21 percent of edoxaban p-toluene sulfonate hydrate, 0-36 percent of hydroxypropyl methylcellulose, 0-28 percent of carbomer, 10-28 percent of lactose, 29-38 percent of diluent, 0-3 percent of povidone and 0.6-4 percent of lubricant.
Owner:SHANDONG INST OF PHARMA IND
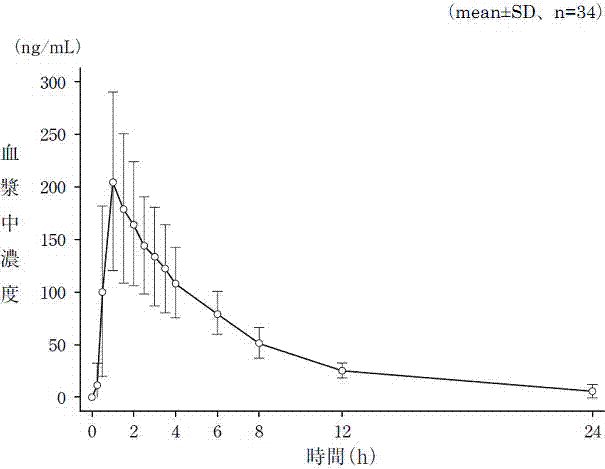
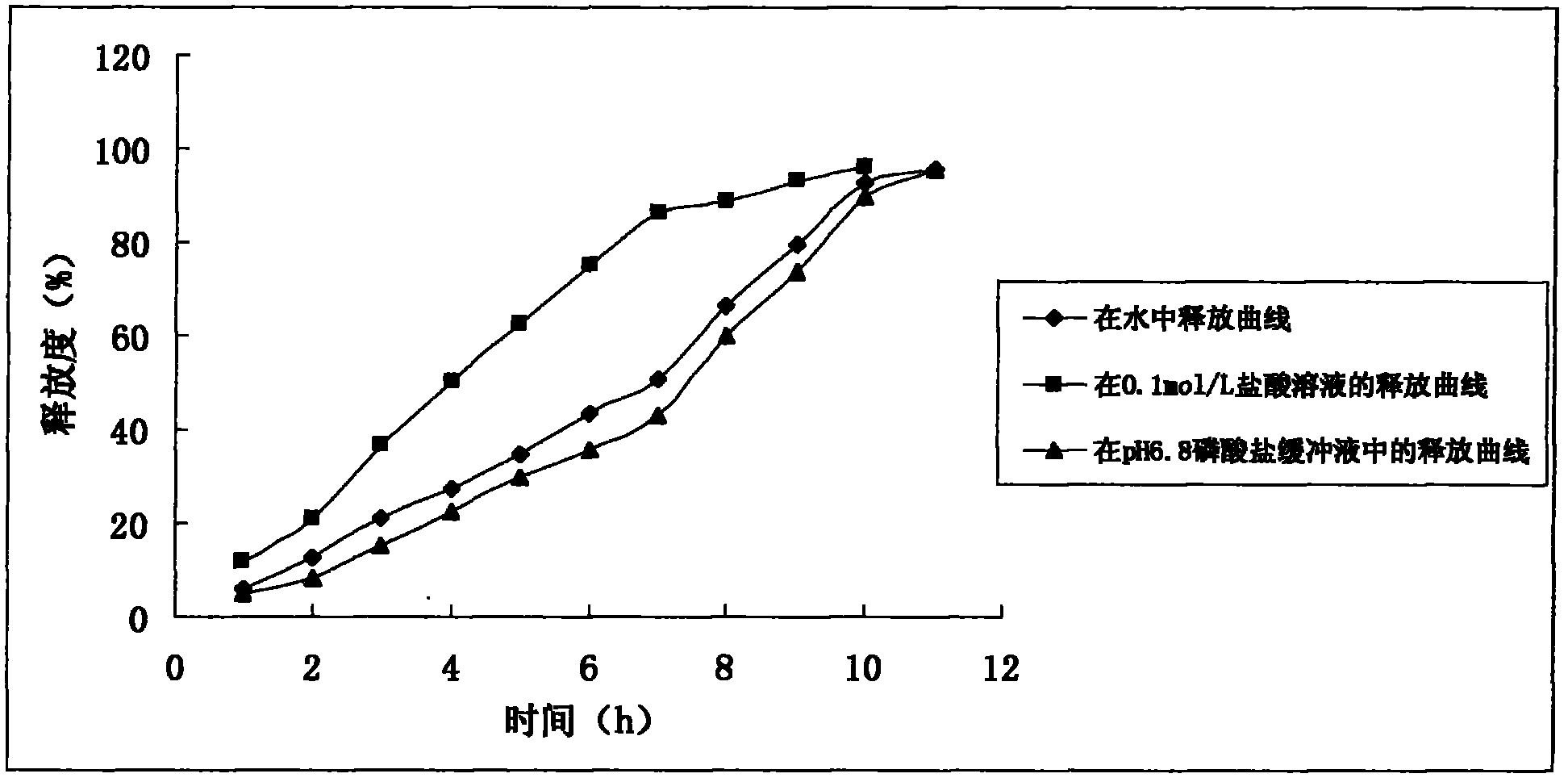
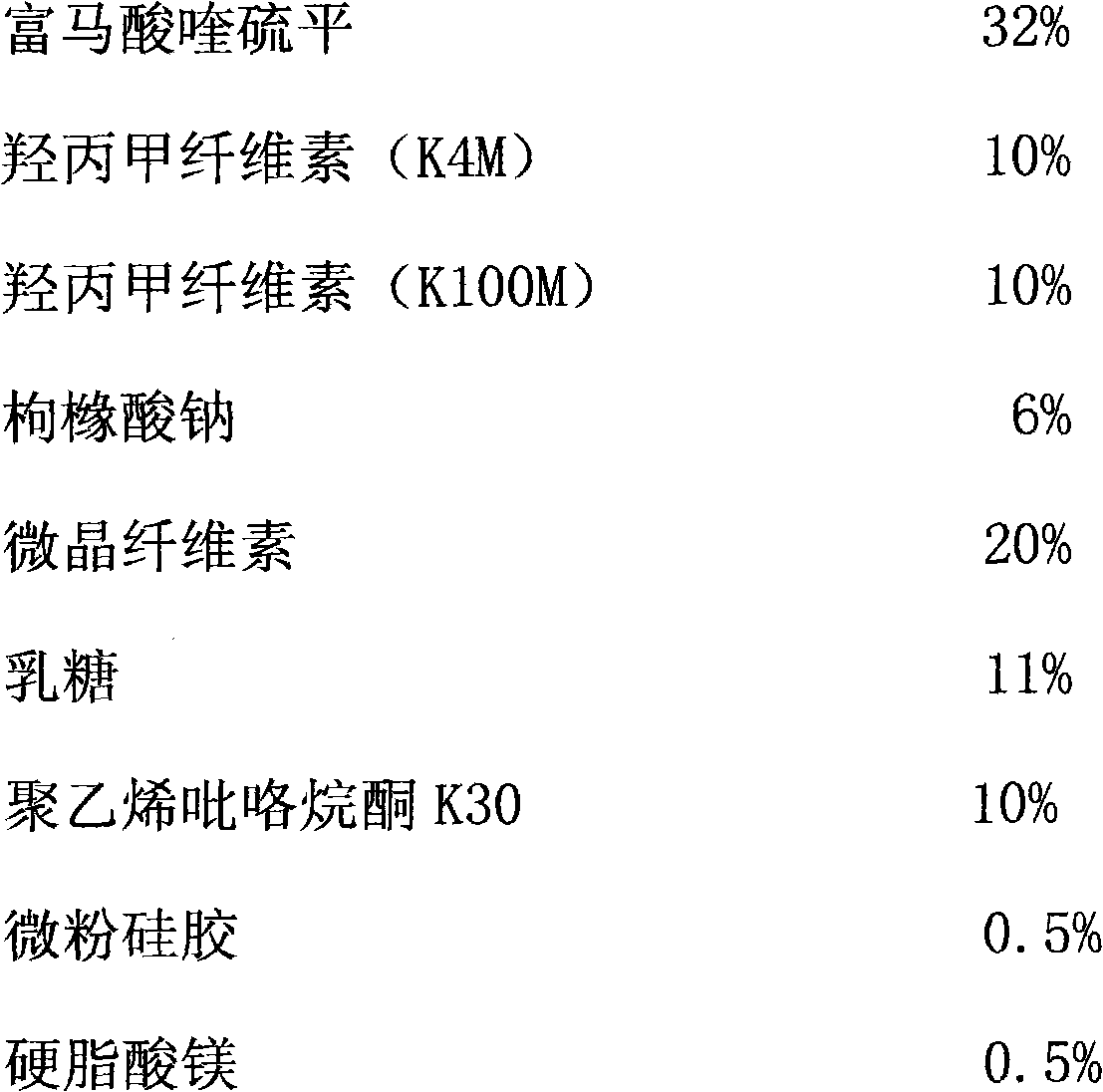
Sustained release tablet of quetiapine fumarate composition and preparation method of sustained release tablet
InactiveCN102218042AReduce weightIncrease toleranceOrganic active ingredientsNervous disorderSustained Release TabletOrganic acid
The invention discloses a sustained release tablet of a quetiapine fumarate composition, comprising the following components in percentage by weight: 25 to 40% of quetiapine fumarate, 2 to 8% of organic acid salt, 5 to 30% of a sustained release material and the balance of other pharmaceutical adjuvants, wherein the sustained release material is K type hydroxypropyl methylcellulose. The sustained release material is the K type hydroxypropyl methylcellulose and the organic acid salt is added, therefore, a stable sustained release skeleton can be formed by few sustained release materials, the raw material can be saved, the weight of unit preparation can be reduced, and the problem of difficulty in swallowing caused by large size of the preparation can be released and / or solved; the hydroxypropyl methylcellulose with different viscosities are used as the material of the skeleton so that the preparation can reach formulated sustained release effects and has the characteristics of strong controllability and stability in storage. By use of the preparation method capable of granulating in one step, the operation is simplified and the production efficiency can be improved.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Process for producing diabecron sustained release tablet
InactiveCN101428007AImprove bioavailabilityOrganic active ingredientsMetabolism disorderCelluloseMetformin Hydrochloride
The invention relates to a preparation method for metformin hydrochloride sustained-release tablets. The preparation method comprises the following steps: firstly, dissolving ethyl cellulose into ethanol solution, blending the ethyl cellulose with metformin hydrochloride to produce granules, and drying and sieving the granules at 45 to 55 DEG C; secondly, dissolving hydroxypropyl methylcellulose into ethanol solution to produce bonding agent, blending the granules prepared in step one, hydroxypropyl methylcellulose, ethyl cellulose and pore forming agent, adding the bonding agent, performing sieving and granulation, adding hydroxypropyl methylcellulose and lubricant, evenly blending, detecting the content, determining the tablet weight, and pressing into plain tablets; thirdly, dissolving film forming agent, plasticizer, masking agent and glidant for hydroxypropyl methylcellulose and ethyl cellulose into ethanol solution, completely stirring for more than 1 hour to produce sustained-release preparation coating solution, and finally obtaining the metformin hydrochloride sustained-release tablets by performing film coating to the plain tablets. The sustained-release preparation not only embodies the main characteristics of the sustained-release preparation for continuous sustained release of drug, but also presents high bioavailability.
Owner:上海天赐福生物工程有限公司
Dirithromycin enteric-coated formulation
The invention relates to a dirithromycin (DRM) enteric preparation with hydroxypropyl methylcellulose phthalate (HPMCP) being the framework material of drug-containing core and the coating material of an enteric coating layer. The enteric preparation has the advantages that the drug content is higher, the drug dissolution rate is less affected by the coating material, the preparation cost is low and the preparation process is simple.
Owner:SHANDONG INST OF PHARMA IND
Enteric-coated tilmicosin slow-release micro-capsule preparation and preparation method thereof
InactiveCN103083281AProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsMonoglycerideAcrylic resin
The invention relates to an enteric-coated tilmicosin slow-release micro-capsule preparation and a preparation method thereof and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin slow-release micro-capsule preparation provided by the invention comprises an inner core layer and a coating layer, wherein the inner core layer comprises tilmicosin raw powder and an auxiliary material; the auxiliary material comprises one or more than one of stearic acid, glycerin monostearate, stearyl alcohol, saturated triglyceride, monoglyceride and paraffin; and the coating layer is made from one or more than one of cellulose acetate phthalate, hydroxypropyl methyl cellulose phthalate, acrylic resin, polyvinyl acetate phthalate and acetic hydroxypropyl methylcellulose succinate. The preparation method comprises the following steps of: carrying out primary coating on the tilmicosin raw powder and the auxiliary material, carrying out secondary coating by using the materials of the coating layer, and drying to obtain the finished product. According to the invention, the tilmicosin is coated by using high polymer materials and the coated tilmicosin micro-capsule is undissolved in acid environment and slowly dissolved in alkaline environment of enteric canal, so that the purpose of slow release is achieved and the action time of the tilmicosin is prolonged.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Pantoprazole sodium enteric-coated tablet and preparation method thereof
InactiveCN102626398AImprove acid resistanceFacilitated releaseOrganic active ingredientsDigestive systemAcrylic resinDissolution
The invention provides a pantoprazole sodium enteric-coated tablet, which consists of the following components in parts by weight: 1 part of a pantoprazole sodium plain film, 0.1-0.5 part of an isolating layer and 0.5-1 part of an enteric-coated layer, wherein the isolating layer consists of hydroxypropyl methylcellulose and an alkali in the weight part ratio of 1:5-5:1; and the enteric-coated layer is prepared from 0.5-1 part of acrylic resin. The invention provides a preparation method of the pantoprazole sodium enteric-coated tablet. Parameters such as spray speed, spray pressure, the rotating speed of a coating pan and the like are improved through process parameters of the isolating layer, an enteric liquid and a coating, so that the prepared pantoprazole sodium enteric-coated tablet has high acid tolerance and dissolution rate, the acid tolerance of a finished product is over 90 percent, the dissolution rate is over 75 percent, and the pantoprazole sodium enteric-coated tablet is accordant with and superior to the requirements of the Chinese Pharmacopoeia on the enteric tablet. The product has the advantages of stable quality, convenience for storing and transporting and contribution to clinical application. The method is simple, is suitable for industrial production, and has high application value.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
Carragheenan and potassium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule
ActiveCN103394093AReduced risk of hygroscopicityReduce the impactCosmetic preparationsToilet preparationsPutrefactionVegetable fibers
The invention relates to a carragheenan and potassium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule, and belongs to the technical field of the production of hollow capsules. The hollow capsule is characterized in that the hollow capsule is prepared through mixing a main raw material hydroxypropyl methylcellulose with carragheenan and potassium chloride under a special condition, the hollow capsule has the characteristics of timely disintegration, low water content, suitableness for medicines having strong hygroscopic property and being sensitive to water, small influences of a dissolve-out medium to the shell of the capsule, high drug compliance, long storage duration data, no deterioration within 3-4 years, low condition requirements on the storage and the transportation, difficult crushing in the low humidity environment, difficult deformation, putrefaction and deterioration in the high temperature environment, according with the requirements of populations having all culture backgrounds because of pure vegetable fibers, almost no pollution in the production process, secondary re-dissolving utilization of tailings, omission of the commonly-used surfactants and plasticizers, acceleration of the gelling speed by reducing the gelling temperature only through utilizing potassium chloride to assist the carragheenan, additive reduction, production cost reduction, and suitableness for the mass popularization.
Owner:SICHUAN TIANSHENG PHARMA
Edible cat toy
InactiveUS20150164047A1Easy to manageHealth and safetyMilk preparationMeat/fish preservationFlavorWater soluble polysaccharides
An edible toy for cats is made from a base material made of edible material; and an edible coating for the base material. The base material can be any of hypromellose; an edible, water-soluble polysaccharide; fermented tapioca; fish gelatin, beef gelatin, catnip, valerian root or a combination thereof. The base material is combined with water, an inorganic pH buffer and a metal-ion bonding agent. A coating has flavors such as chicken, turkey, beef, pork, lamb, duck, seafood, freshwater fish, grass, catnip, valerian root, lemon grass, sweet grass or a combination thereof. Also disclosed is a method of making edible and medicated toys.
Owner:URBAN PET HAUS
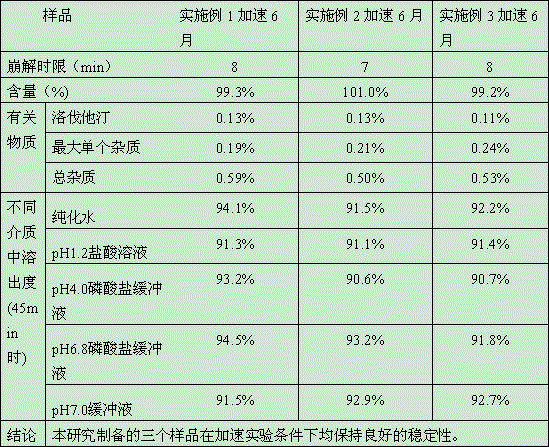
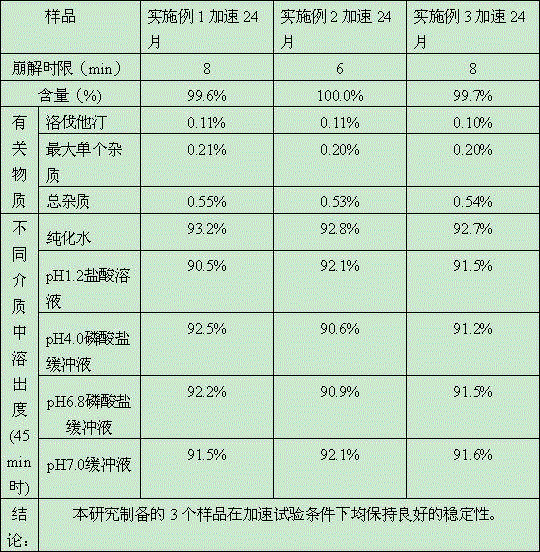
Simvastatin tablet and preparation method thereof
The invention discloses a simvastatin tablet and a preparation method thereof. The simvastatin tablet comprises an active ingredient simvastatin and pharmaceutical excipients, wherein the pharmaceutical excipients are spherical lactose, cross-linked sodium carboxymethyl cellulose, butylhydroxyanisole, hydroxypropyl methyl cellulose, silicon dioxide, magnesium stearate and film coating premix, which are added according to a specific mass ratio and process. The simvastatin and the pharmaceutical excipients are incompatible, and are prone to hydrolysis and oxidation, lactone bonds break to open loop to generate an active metabolite simvastatin hydroxy acid under the high-humidity condition, the intramolecular diene bond is subjected to a slow oxidative copolymerization reaction to generate a dimer or polymer under the high-temperature condition, and the preparation of a stable preparation is greatly difficult. In recent years, with the continuous disclosure of information, the difference between the quality standards of simvastatin tablets produced by different manufacturers is great, wherein the dissolution behavior difference is more significant, so that the situation that the simvastatin tablets have the same name but have different quality is very obvious. The prescription process determined by the study can continuously produce the simvastatin tablet at large scale, and the prepared simvastatin tablet has a good dissolution performance in various PH-value dissolution media, and keeps good stability in the long-term storage process.
Owner:DIAO GRP CHENGDU PHARMA
Enteric omeprazole micropill and its preparing method
ActiveCN1985822ALow priceGood for healthOrganic active ingredientsDigestive systemClinical efficacyOmeprazole
The present invention discloses a kind of enteric omeprazole micropill preparation and its preparation process. The enteric omeprazole micropill preparation has core of omeprazole or its single antimer subsalt as the active component and excipient and middle isolating coating and enteric protecting layer to coat the core. The enteric omeprazole micropill preparation is prepared directly with the main medicine component and supplementary materials including magnesia as the stabilizer, hydroxypropylmethyl cellulose and talcum powder.
Owner:KAMP PHARMA
Starch-based hemostasis sponge and preparation method of hemostasis sponge
ActiveCN104623720AImprove water absorptionGood formabilityAbsorbent padsBandagesCross-linkFreeze-drying
The invention relates to a medical hemostasis instrument and in particular relates to a starch-based hemostasis sponge and a preparation method of the hemostasis sponge. The starch-based hemostasis sponge is prepared from the following raw materials in parts by weight: 80-95 parts of starch, 2-10 parts of hydroxypropyl methylcellulose calcium, 2-5 parts of a cross-linking agent and 1-5 parts of a plasticizer. The preparation method of the hemostasis sponge comprises the following steps: step one, crosslinking starch with hydroxypropyl methylcellulose calcium by virtue of the cross-linking agent; step two, purifying; and step three, freeze-drying and sterilizing to prepare the finished starch-based hemostasis sponge. According to the starch-based hemostasis sponge and the preparation method of the hemostasis sponge, the hemostasis sponge is prepared by crosslinking starch with hydroxypropyl methylcellulose calcium; the hydroxypropyl methylcellulose calcium is high in water absorption rate, excellent in formability, high in strength and not liable to break, and has a plurality of functions of stopping bleeding, packing and repairing.
Owner:北京爱特康医疗科技有限公司
Heat-sensitive gel containing matrine alkaloid and its preparing method
InactiveCN1850076AGood thermal propertiesEasy and effective applicationOrganic active ingredientsPharmaceutical delivery mechanismGel preparationPhosphate
The present invention relates to a heat-sensitive gel preparation containing matrine alkaloid and its preparation method. Said preparation is composed of matrine alkaloid, hydroxypropyl methylcellulose, poloxamer 407, phosphate buffer solution whose pH value is 5.0-7.0, glycerin and preservative. It is flowable fluid at normal temperature, and when the temperature is 28-33 deg.C, it can produce phase change, and can be formed into gel, so that it can be conveniently applied to vagina, mucous membrane of external genitalia, skin and eye.
Owner:SHANGHAI HUATUO MEDICAL SCI CO LTD
Rumen-protected chlorine chloride microcapsule additive and preparation method thereof
The invention discloses chlorine rumen-protected microcapsule additive and a preparation method thereof. The additive comprises a kernel and a surface layer which wraps the kernel; and the kernel comprises the ingredients of chlorine-based mixture, wherein the chlorine-based mixture comprises the following components by weight percent of 83 to 98.9 percent of choline chloride adopting silicon dioxide as a carrier, 1 to 10 percent of No.4 polyacrylic resin and 0.1 to 10 percent of hypromellose. Due to the adoption of the chlorine rumen-protected microcapsule additive and the preparation methodthereof, deliquesce of the choline chloride in the air can be reduced, damage of the choline chloride on other ingredients in the feed can be reduced, and the storage and application of the choline chloride can be facilitated. When the additive is added into feed for dairy cow, the productivity and health of the dairy cow can be improved, the utilization efficiency of the feed can be improved, environmental pollution caused by excretion of the dairy cow during the breeding process can be reduced, the rearing cost of the dairy cow can be reduced, and an important significance on promoting the sustainable development of the agricultural economy in China and the society progress can be realized.
Owner:HEBEI AGRICULTURAL UNIV.
Preparation method of bitter gourd peptide arabinose composite tablet
InactiveCN103861086AGood for blood sugar controlBeneficial to blood lipid metabolismOrganic active ingredientsPeptide/protein ingredientsLow density lipoprotein cholesterolLiver and kidney
The invention relates to a preparation method of a bitter gourd peptide arabinose composite tablet. The bitter gourd peptide arabinose composite tablet consists of the following components in parts by weight: 8-15 parts of bitter gourd polypeptides, 15-25 parts of L-arabinose, 0.1-0.3 part of chromium-enriched yeast, 25-35 parts of inulin, 5-15 parts of phytosterol, 10-15 parts of D- mannitol, 10-20 parts of microcrystalline cellulose, 0.5-1 part of magnesium stearate, and 2-5 parts of hydroxypropyl methylcellulose. The preparation method has beneficial effects that the bitter gourd peptide arabinose composite tablet is prepared by adopting a plurality of components beneficial to blood sugar control and blood lipid metabolism; compared with a way of independently adopting the bitter gourd polypeptides, the inulin or the phytosterol, and the like, a way of combining the bitter gourd polypeptides, the inulin or the phytosterol, and the like is better in effect, beneficial to blood sugar control and blood lipid metabolism of a patient with diabetes mellitus II, and free of influences on functions of livers and kidneys. Besides, the preparation method can improve sensibility of insulin better and can remarkably lower total cholesterol and low-density lipoprotein cholesterol of the patient with diabetes mellitus II.
Owner:DONGYING BEIKANG BIOLOGICAL SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com