Patents
Literature
36 results about "Edoxaban" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
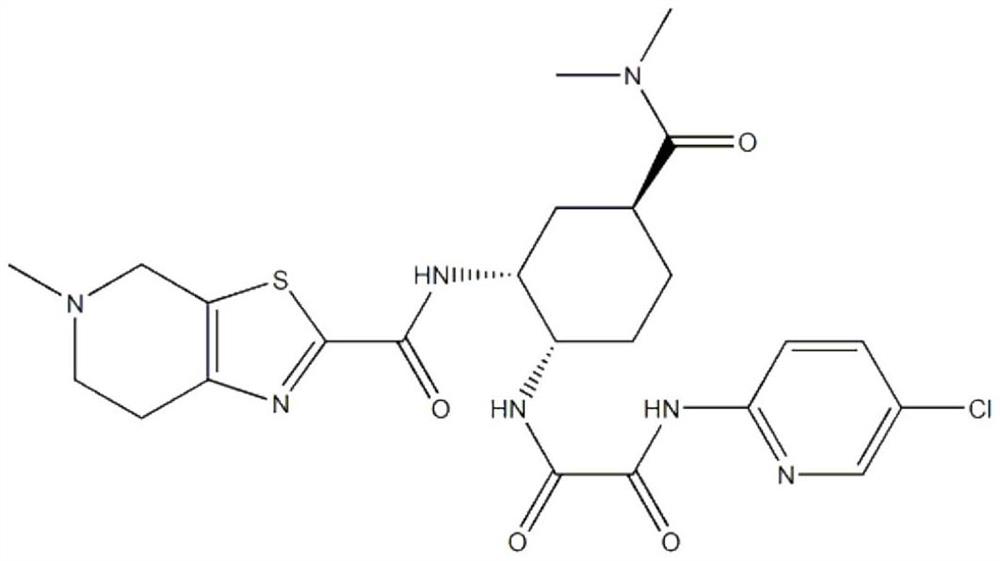
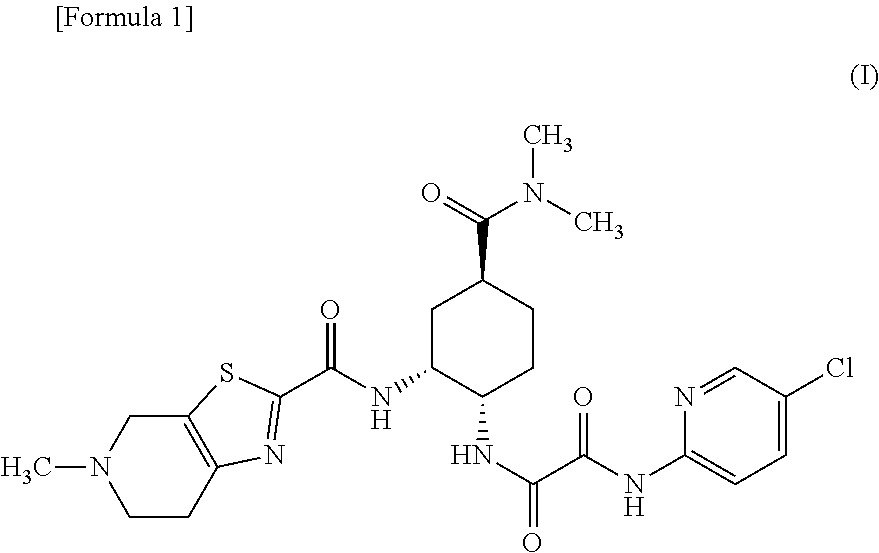
Edoxaban is used to prevent serious blood clots from forming due to a certain irregular heartbeat (atrial fibrillation). It is also used to treat certain blood clots (such as in deep vein thrombosis-DVT or pulmonary embolus-PE).
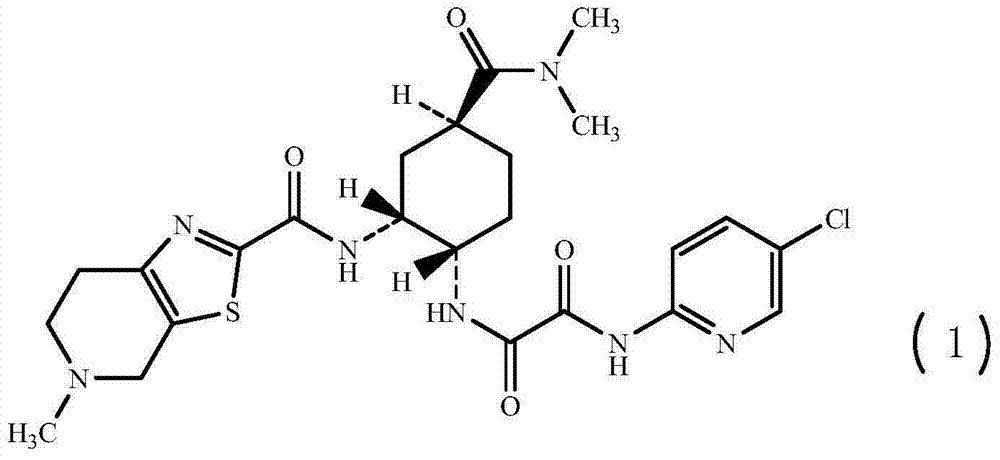
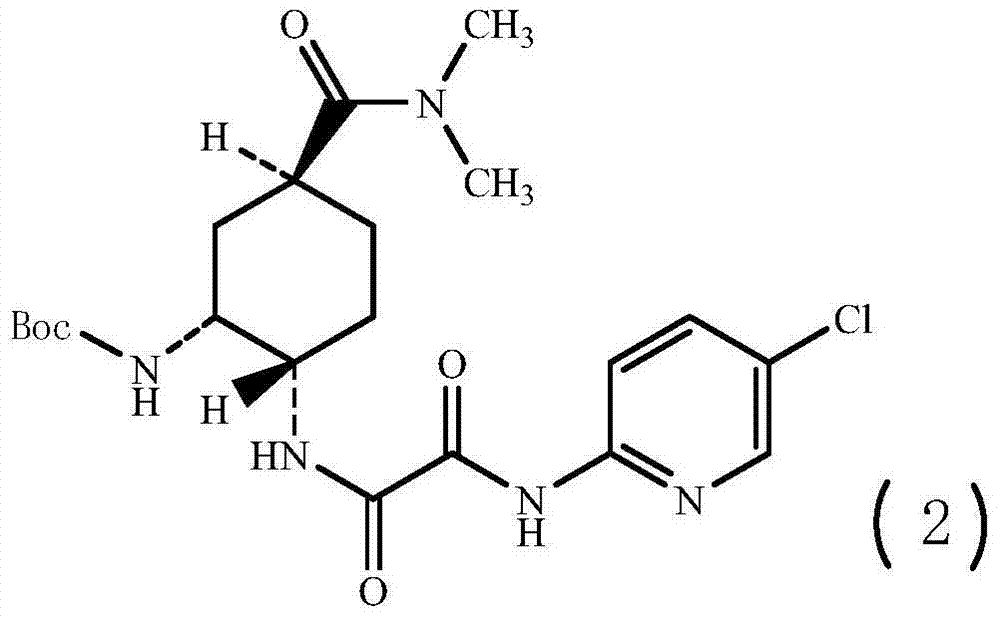
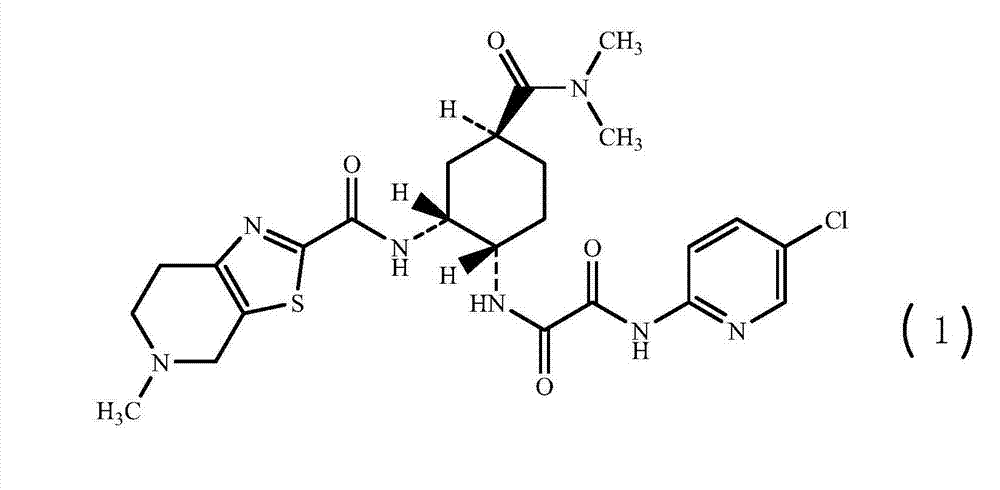
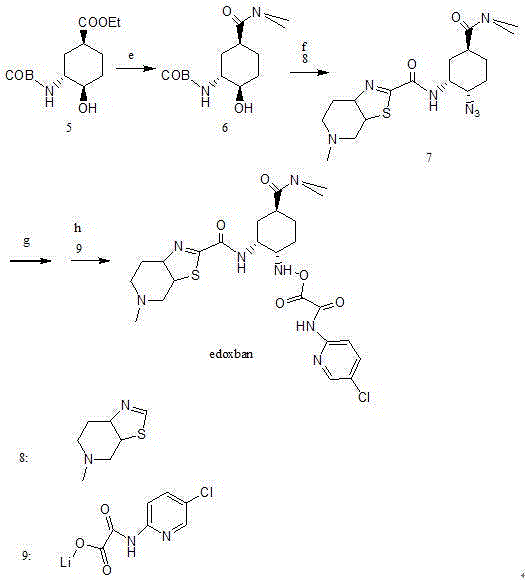
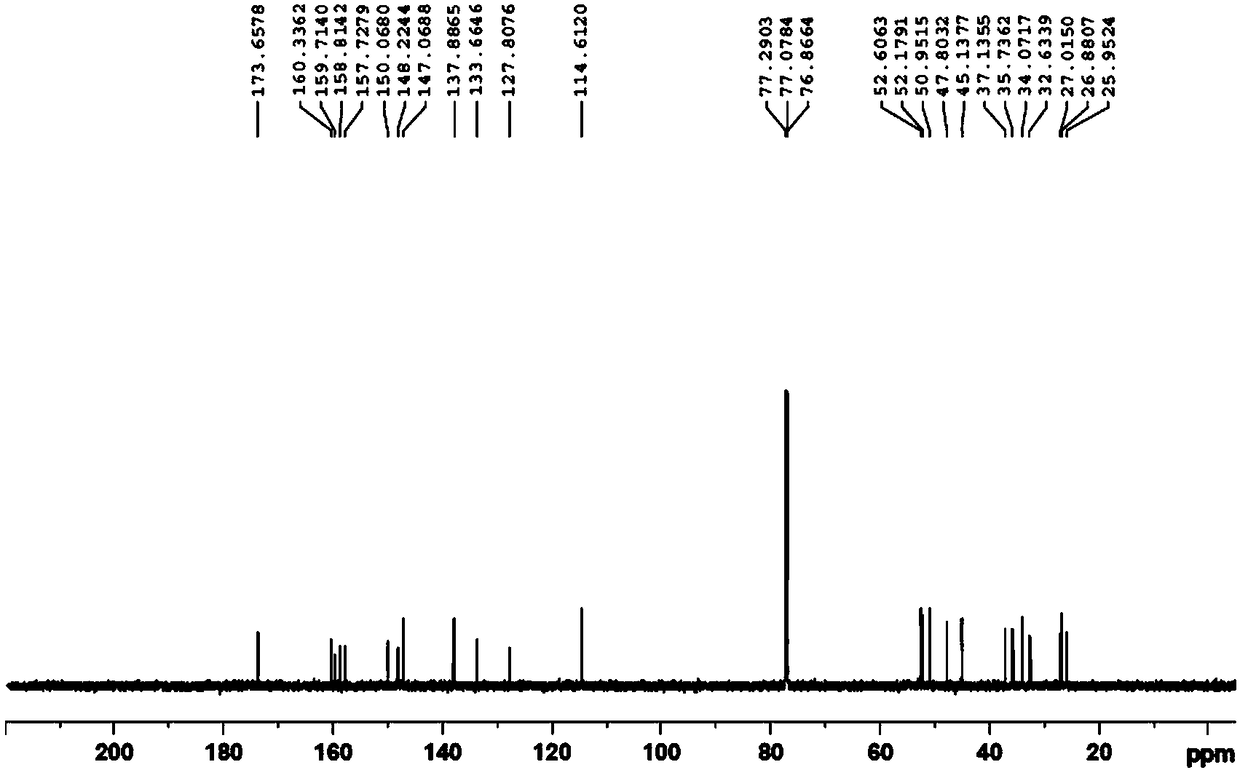
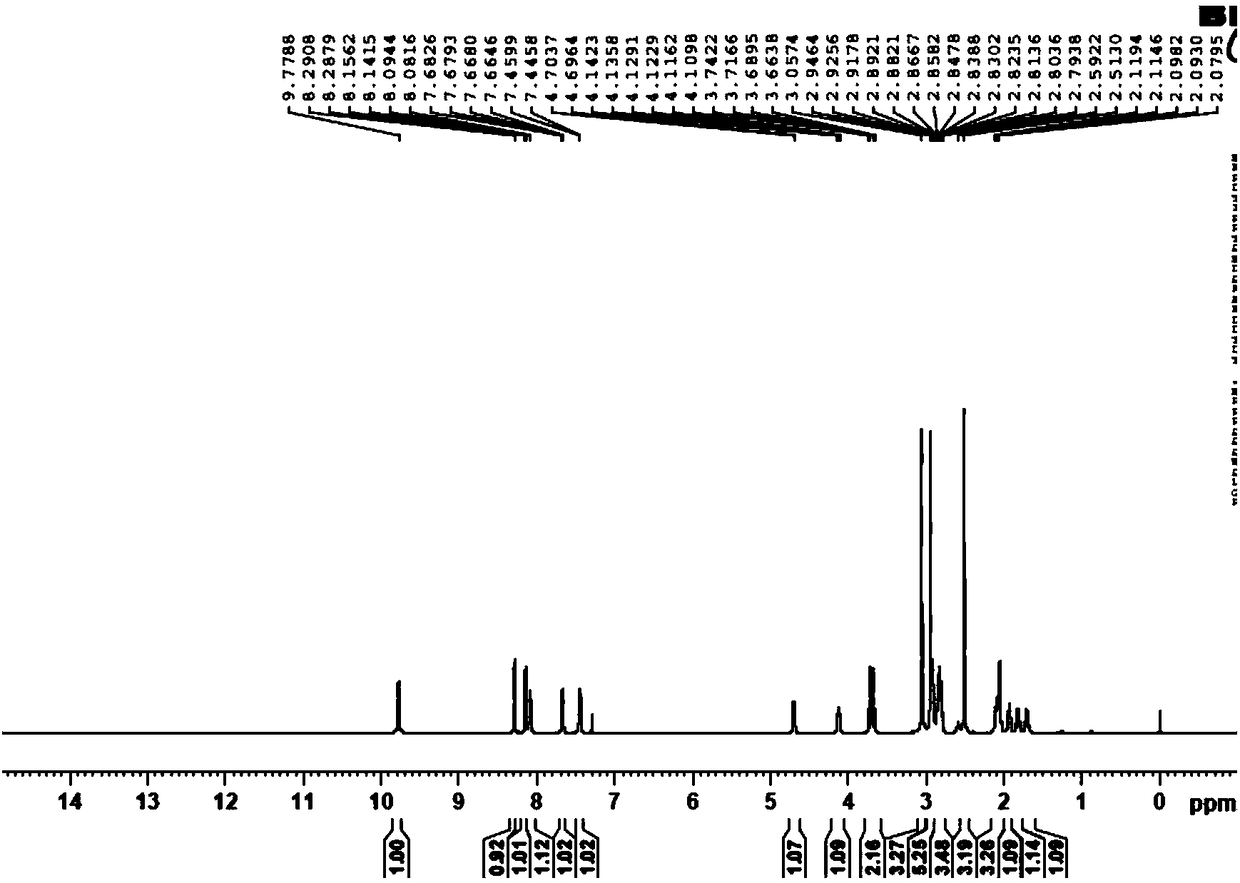
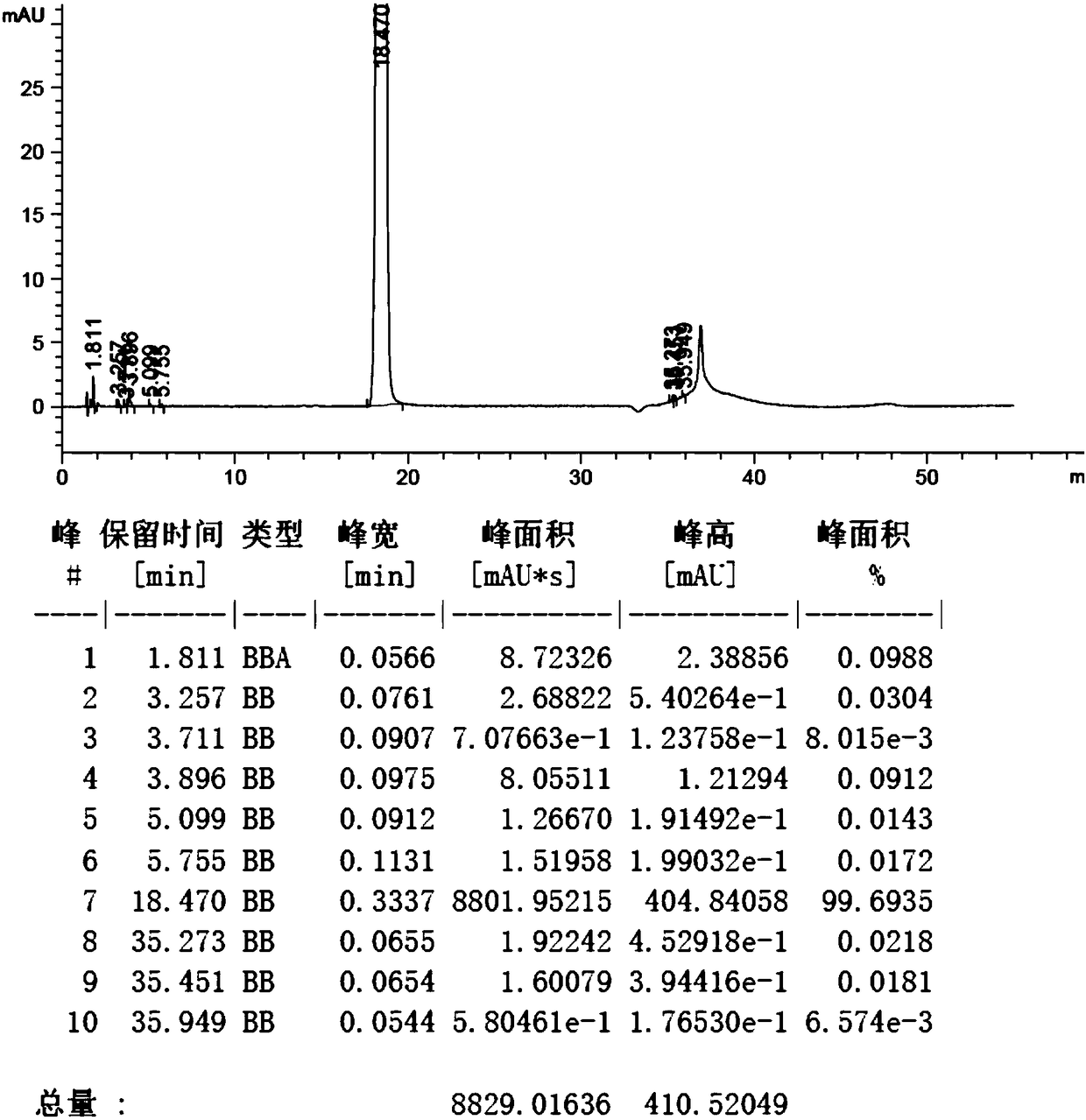
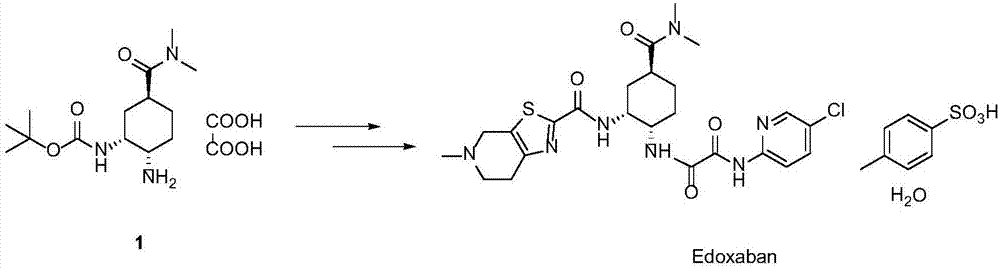
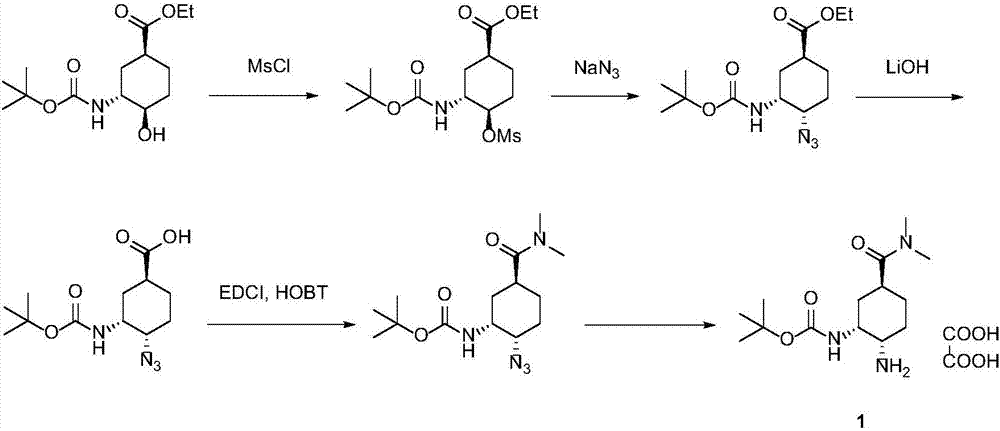
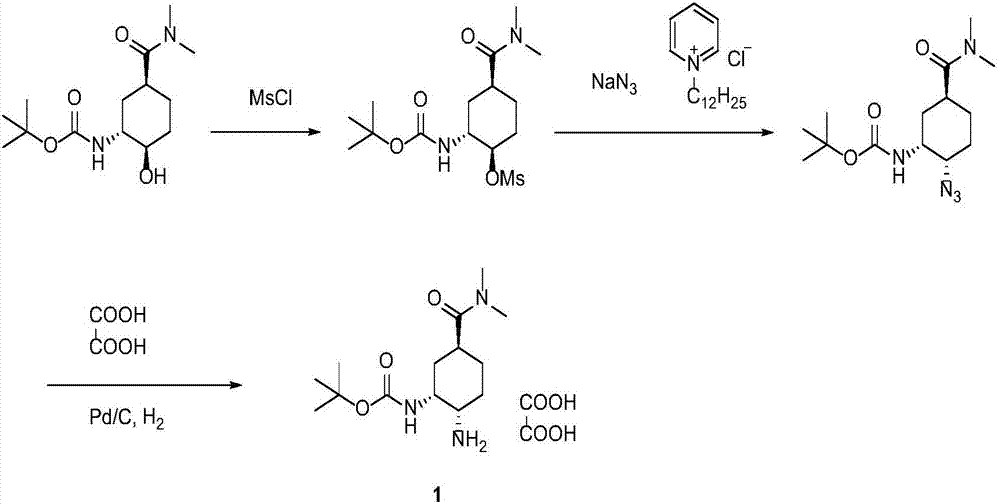
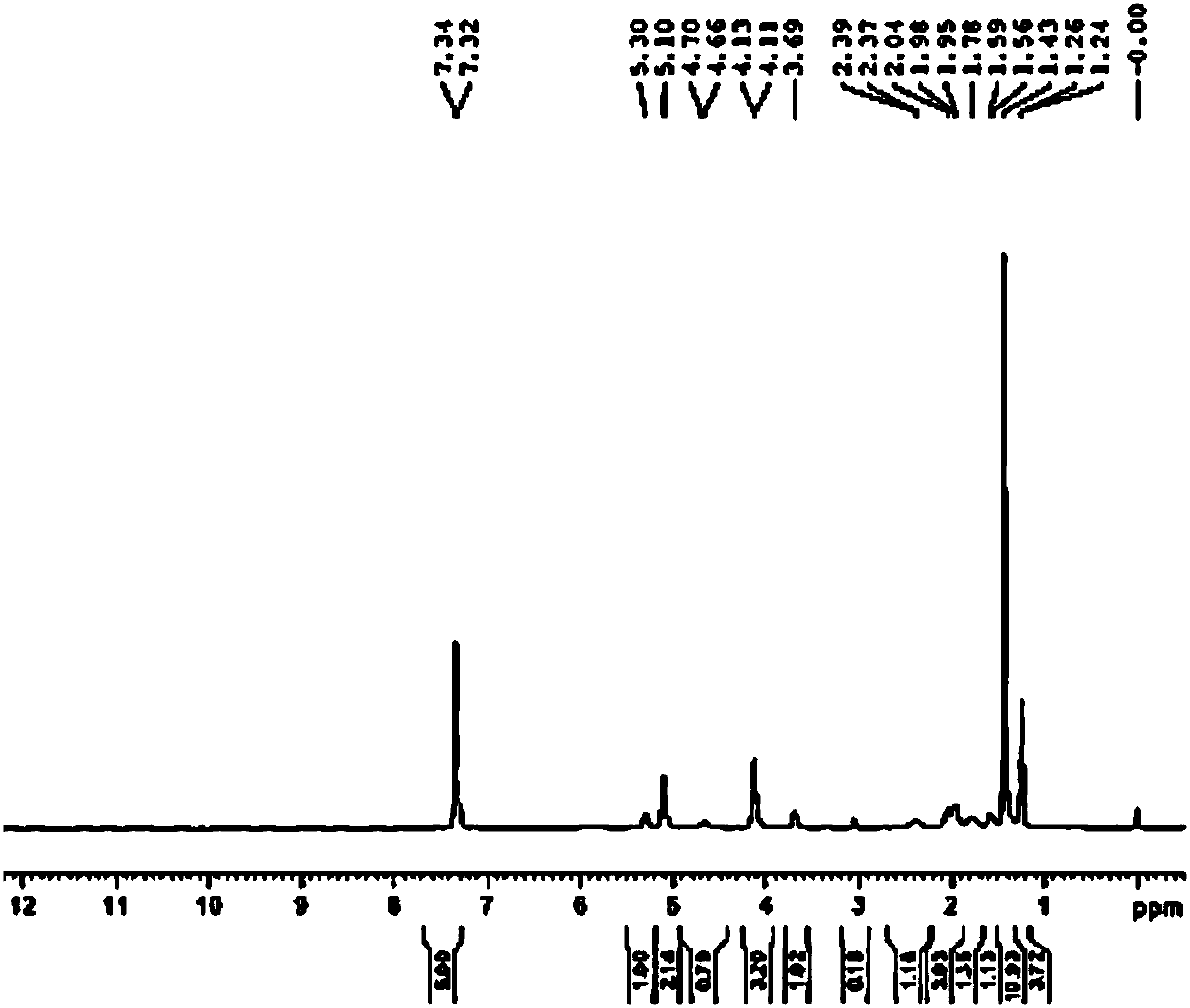
Synthesis method of edoxaban
ActiveCN104761571AHigh purityHigh yieldOrganic chemistryBulk chemical productionSynthesis methodsEdoxaban
The invention provides a synthesis method of edoxaban, which includes: [step 1]: adding a compound (2) to a solvent of a nitrile being 2-4 in carbon atom number to remove an N-Boc protective group under an acidic condition to obtain a compound (2-a); and [step 2]: treating a reaction liquid in the (step 1) with an tertiary amine and adding a compound (3) to carry out a reaction, and finally treating the reaction liquid after the reaction finished with an alkali liquid. Compared with a method of synthesizing the edoxaban in the prior art in references, the method is mild in the conditions of the reactions and is high in yield. The raw materials are easy to obtain. The method is simple in operations and is stable in processes, is free of column chromatography for purifying the product and can enable the product to achieve a medicinal requirement just by one time of purification, so that the method is more suitable for industrialized production.
Owner:SHENZHEN KEXING PHARM CO LTD +1
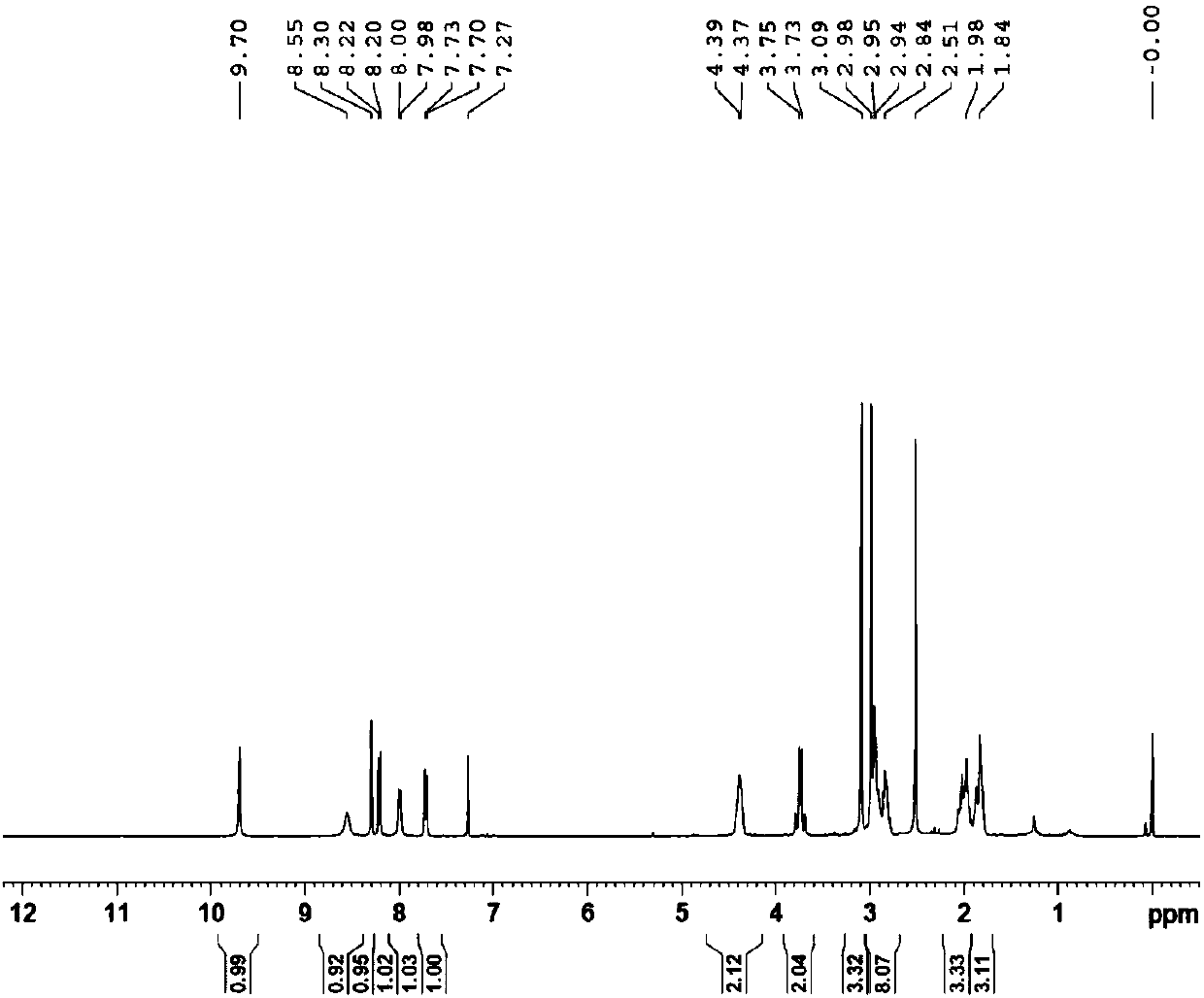
Preparation method for free-state edoxaban
The invention discloses a preparation method for free-state edoxaban.The synthetic route is shown in the description.According to the preparation method, the charging sequence is changed, and a curing problem caused by a traditional method is reduced; according to an existing method, a starting material A and triethylamine are added at first, a starting material B is added, a large amount of solid will appear in the temperature increasing process, and the stirring uniformity is greatly affected, so that the yield is greatly lowered, and the method is adopted for greatly solving the problem and improving the yield; when the compound free-state edoxaban is synthetized, a first amount of a saturated sodium bicarbonate water solution is added at first, the phenomenon that a product is rapidly separated out and impurities are included due to the fact that the added saturated sodium bicarbonate water solution is excessive is avoided, and meanwhile introduction of genotoxicity impurity methanesulfonate is effectively avoided; after the impurities are completely hydrolyzed, a second amount of the saturated sodium bicarbonate water solution is added so that the product can be separated out thoroughly; the method improves the crystallization way, the yield is improved, and generation of impurities is reduced.
Owner:LEPU PHARMACEUTICAL CO LTD
Edoxaban intermediate and preparation method thereof
InactiveCN105198776AStable and reliable quantityHigh yieldCarbamic acid derivatives preparationOrganic compound preparationPtru catalystFormic acid ethyl ester
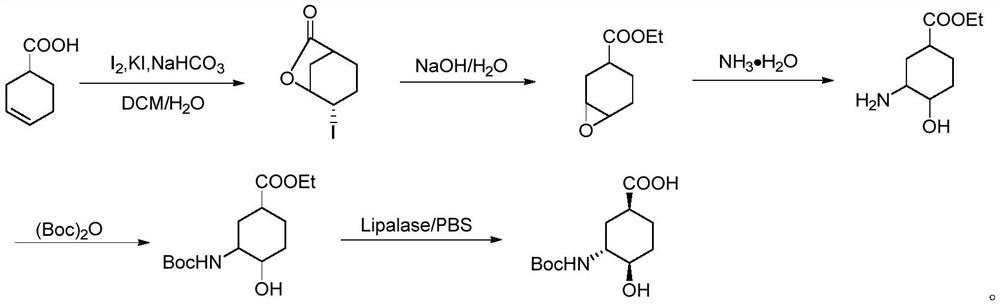
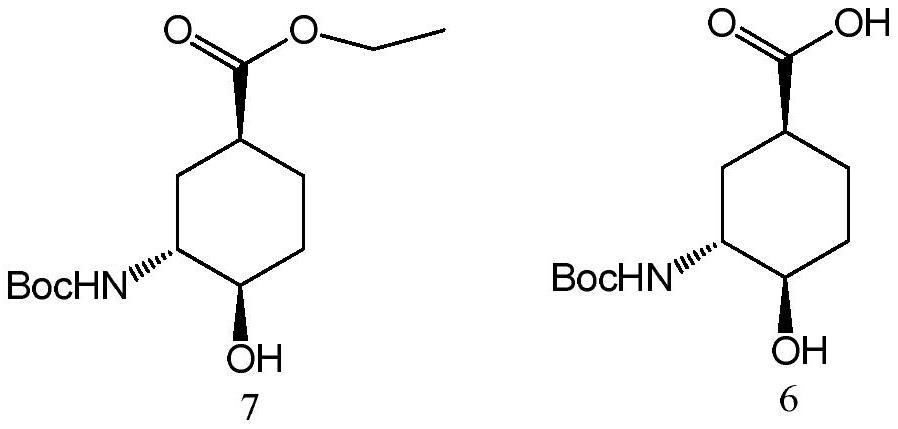
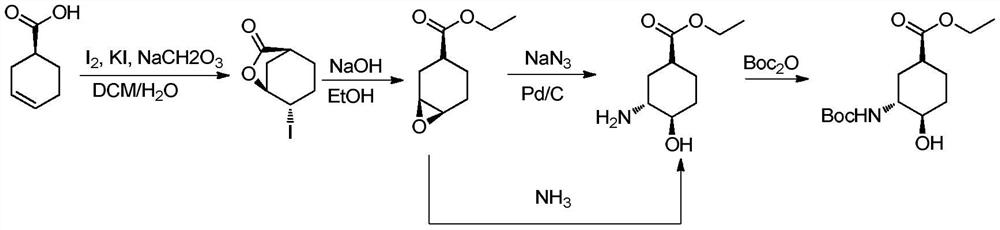
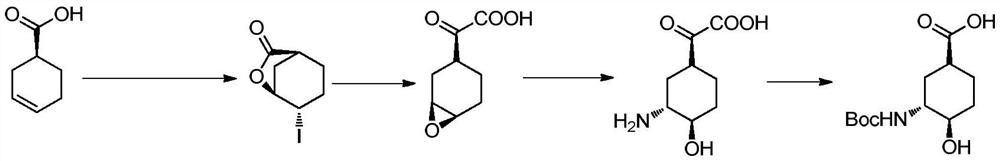
The invention relates to an edoxaban intermediate and a preparation method thereof. The preparation method of the edoxaban intermediate comprises steps as follows: (a), (1s)-3- cyclohexene-1-formic acid is taken as a raw material, and a compound 2 is generated in the presence of a catalyst, a halogen and a weak base; (b), the compound 2 generates a compound 3 under the action of strong base in an absolute ethanol solution; (c), the compound 3 generates a compound 4 in an ammonia and ethanol solution; (d), the compound 4 reacts with a protecting group of amino under the action of a catalyst, and a compound 5, (1S,3R,4R)-3-[(t-butyloxycarboryl)amino]-4-hydroxycyclohexyl ethyl formate, is generated. Raw materials and reagents required in the synthesis method of the edoxaban intermediate are easy to obtain, the yield is high, the cost is low, reaction conditions are mild, three wastes are relatively fewer, and the quality of the product is stable and reliable.
Owner:天津药物研究院药业有限责任公司
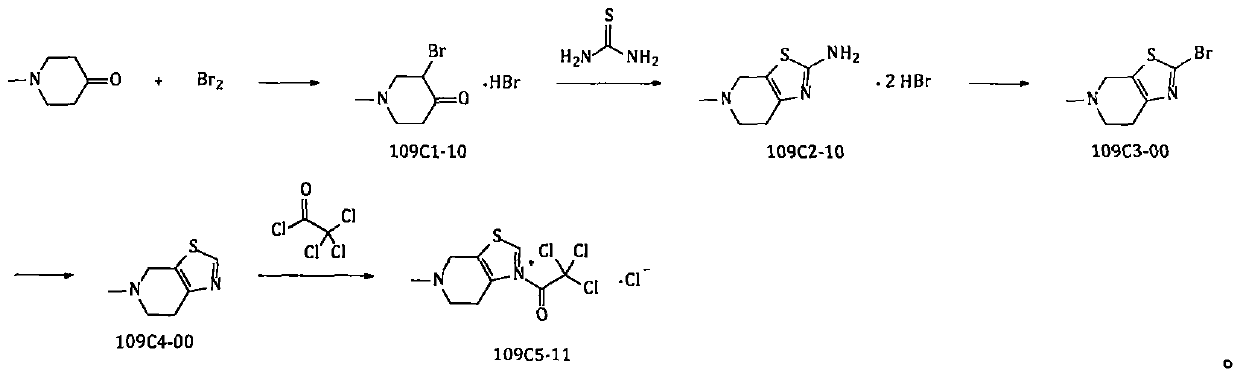
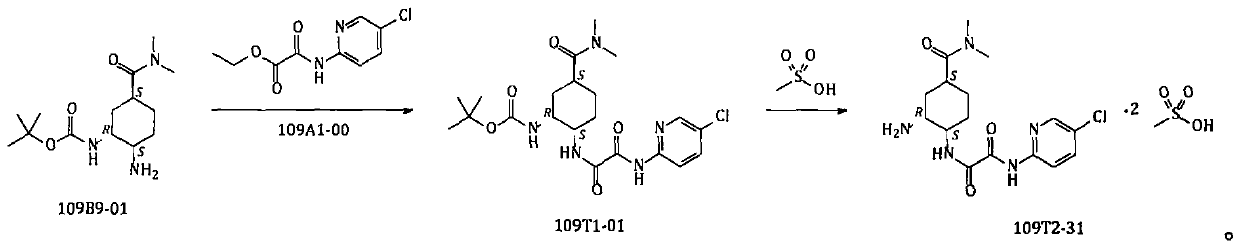
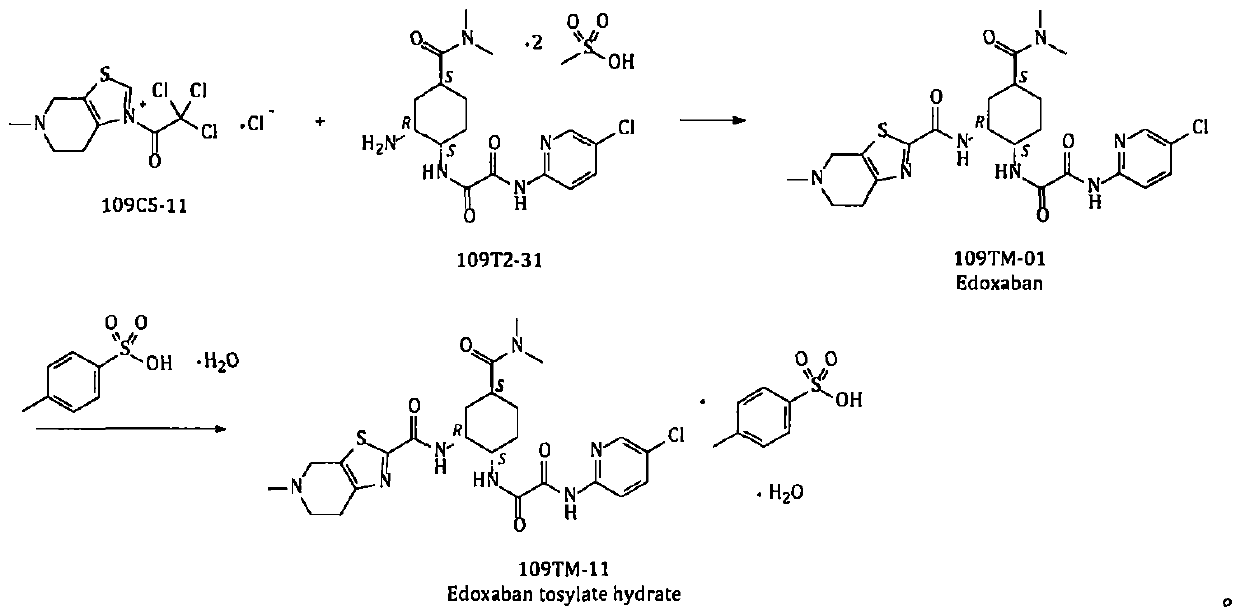
Method for preparing edoxaban from trichloroacetophenone onium salt derivatives
ActiveCN111393456AReduce dosagePost-processing is simpleSulfonic acids salts preparationBulk chemical productionPyridiniumCyclohexyl chloride
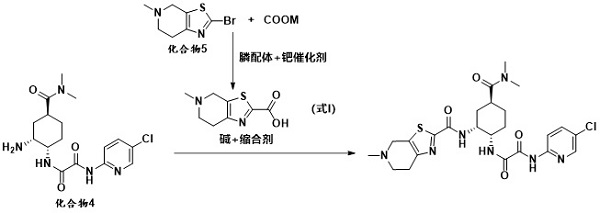
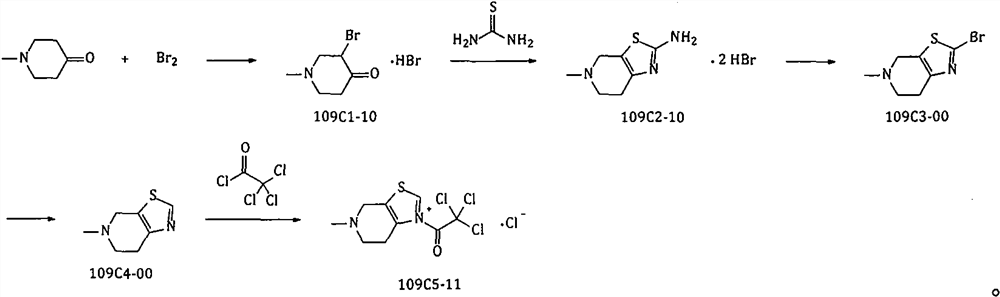
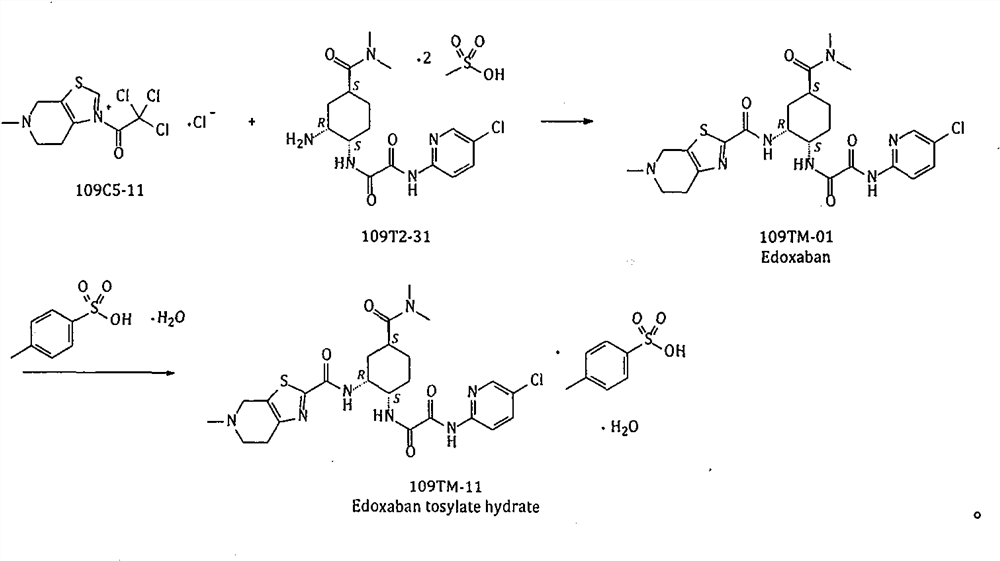
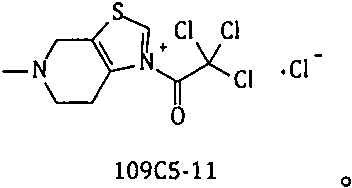
The invention provides a method for preparing edoxaban by using 2, 2, 2-trichloro-1-(4, 5, 6, 7-tetrahydro-5-methylthiazolo[5, 4-c]pyridinium-1-yl) ethanone chloride. The preparation method comprisesthe following steps: preparing 2, 2, 2-trichloro-1-(4, 5, 6, 7-tetrahydro-5-methylthiazolo[5, 4-c]pyridinium-1-yl) ethanone chloride, namely 109C5-11; the invention discloses a preparation method of N1[(1S, 2R, 4S)-2-amino-4-[(dimethylamino) carbonyl]cyclohexyl]-N2(5-chloro-2-pyridyl) oxalamide dimesylate, namely 109T2-31. The 109C5-11 is used as an acylation reagent to prepare the edoxaban with 109T2-31. The preparation method comprises the following steps: preparing the edoxaban by using the 109C5-11 as the acylation reagent; the novel method overcomes the defect that expensive condensing agents EDCI.HCl and activating agents HOBt need to be used in the prior art. The new method provided by the invention is beneficial to more economically and more efficiently realizing industrial scale production of the Edoxaban p-toluenesulfonate hydrate.
Owner:内蒙古京东药业有限公司
Preparation method of edoxaban p-toluenesulfonate monohydrate
ActiveCN108484641ARapid responseHigh yieldOrganic chemistry methodsSulfonic acids salts preparationProduction ratePyridine
The invention provides a preparation method of edoxaban p-toluenesulfonate monohydrate. According to the preparation method, an appropriate amount of a specific ionic liquid is added, a specific ratioof a combination of triethylamine and pyridine is selected as a base, the rapid progress of a reaction is facilitated, and improvements on the production rate and the product purity are facilitated.
Owner:北京阳光诺和药物研究股份有限公司
Synthesis method of edoxaban intermediate and intermediate product
ActiveCN106866452APromote conversionReduce industrial production risksOrganic compound preparationCarboxylic acid amides preparationLower yieldSodium azide
Relating to the field of pharmaceutical chemistry, the invention a synthesis method of an edoxaban intermediate (compound 1) and an intermediate product (compound 4) to solve the disadvantages of tedious conversion steps, high risk of production explosiveness, low yield and the like in the prior art. The reaction steps are shown as the specification. The synthesis process of the edoxaban intermediate provided by the invention has the characteirsitcs of easy acquisition of ammonia source and high reaction conversion rate, effectively reduces the cost of industrial production, avoids the use of dangerous reagent sodium azide, and improves the safety of the synthesis process.
Owner:ZHEJIANG TIANYU PHARMA
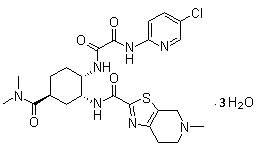
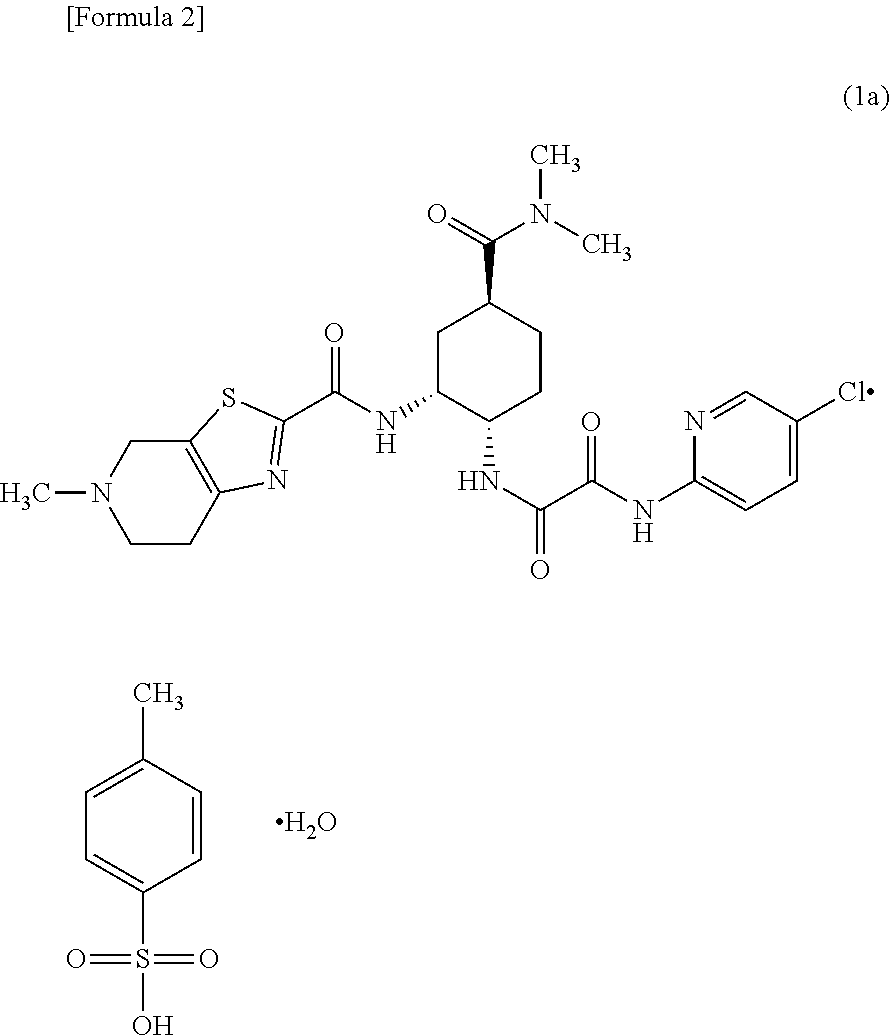
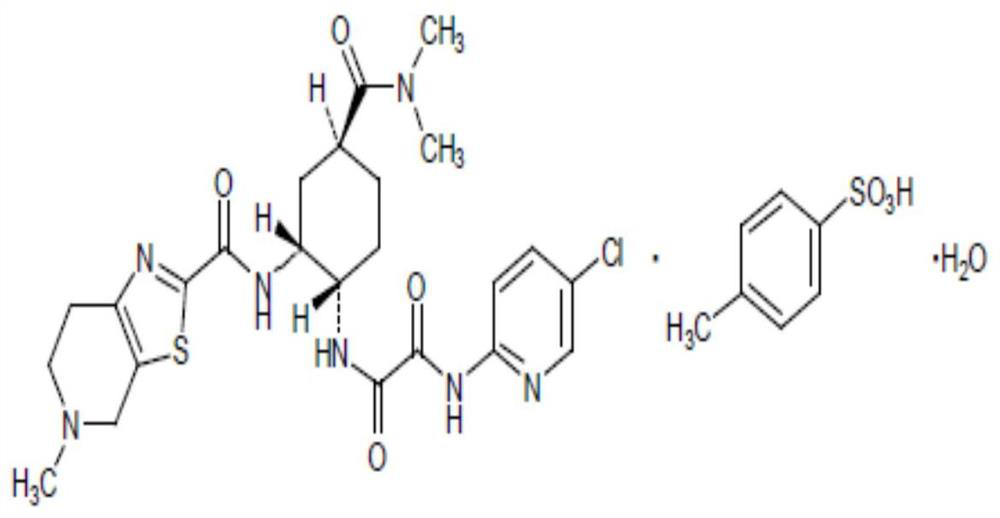
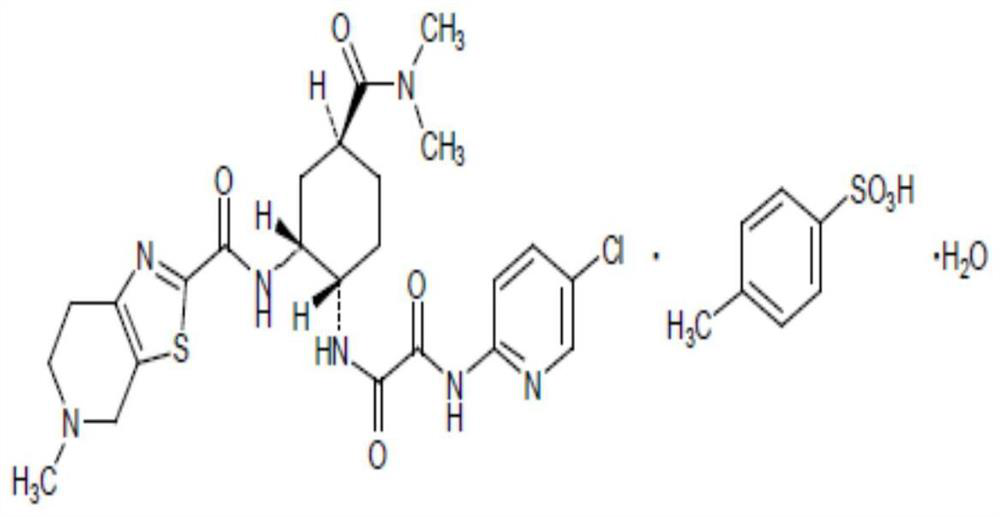
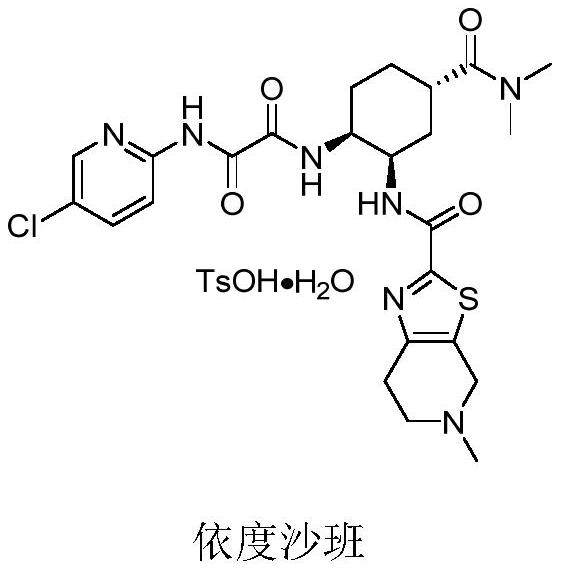
Edoxaban tosilate hydrate
ActiveCN105777779ALow impurity contentPrinciples of safe and controllable drug useOrganic active ingredientsOrganic chemistryBlood Coagulation Factor XEdoxaban Tosilate
The invention provides edoxaban tosilate hydrate. Edoxaban is a micromolecule oral anticoagulant drug, and is a blood coagulation factor X(FXa) retarding agent. In the process of blood coagulation, the activated coagulation factor X(FXa) activates prothrombin (FII) into thrombin (FIIa), formation of fibrin is promoted, and thrombus is formed, so that FXa is developed as a main target of a new generation anticoagulant drug. Edoxaban is the oral anticoagulant drug by selectively, reversibly and directly inhibiting FXa in order to inhibit formation of thrombus.
Owner:海思科制药(眉山)有限公司
Use of a Factor Xa Inhibitor for Treating and Preventing Bleeding Events And Related Disorders in Patients Having Sensitivity to Vitamin K Antagonists Used As Anticoagulants
InactiveUS20150272935A1Reduce riskIncreased riskBiocideMicrobiological testing/measurementVKORC1Thrombus
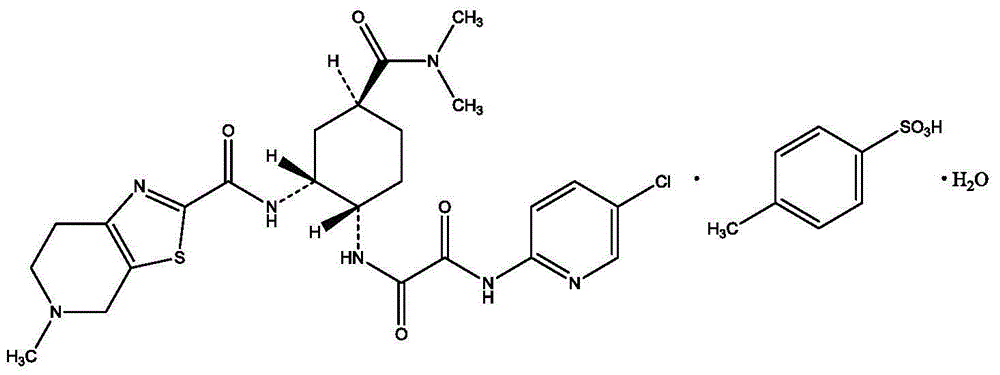
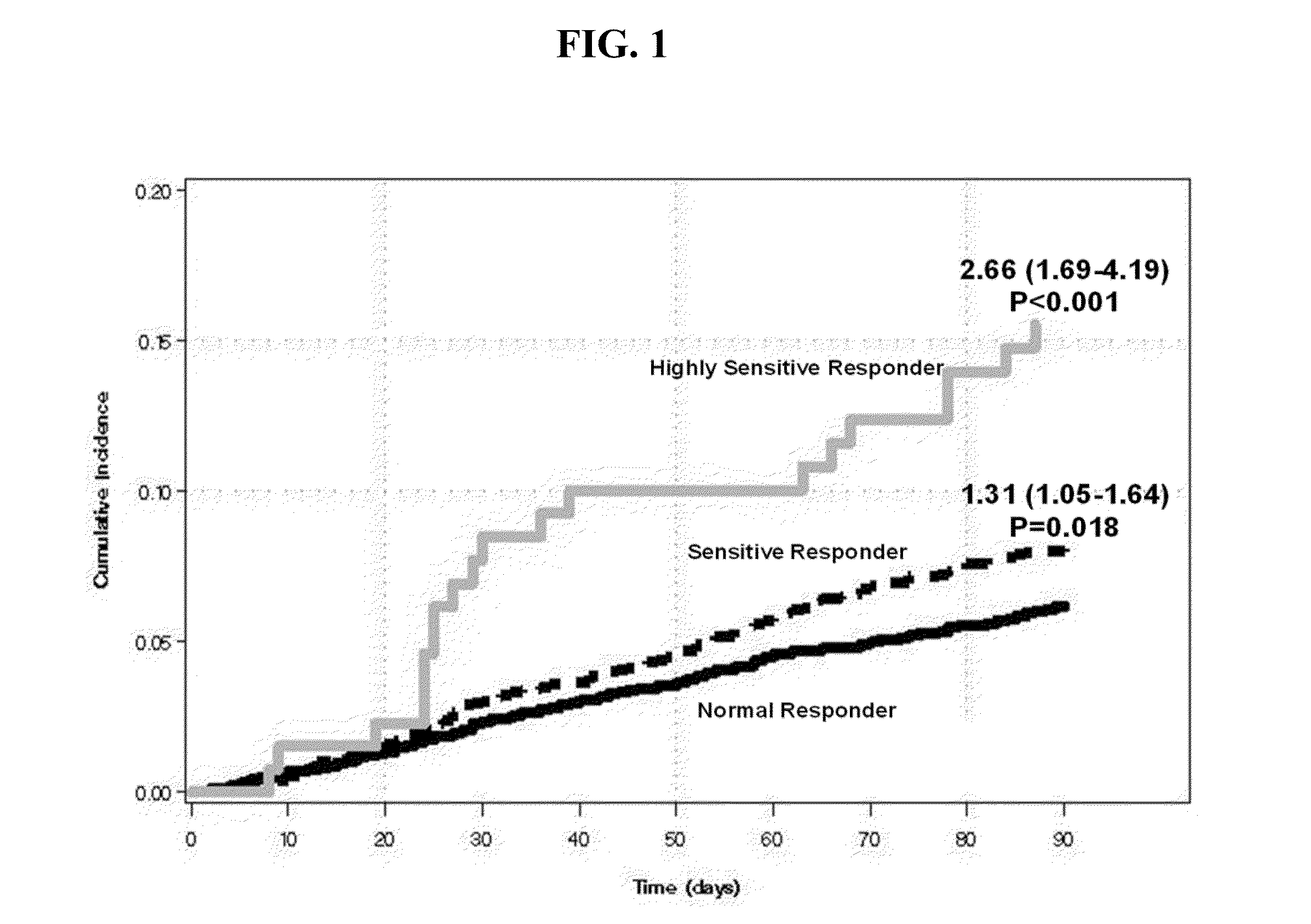
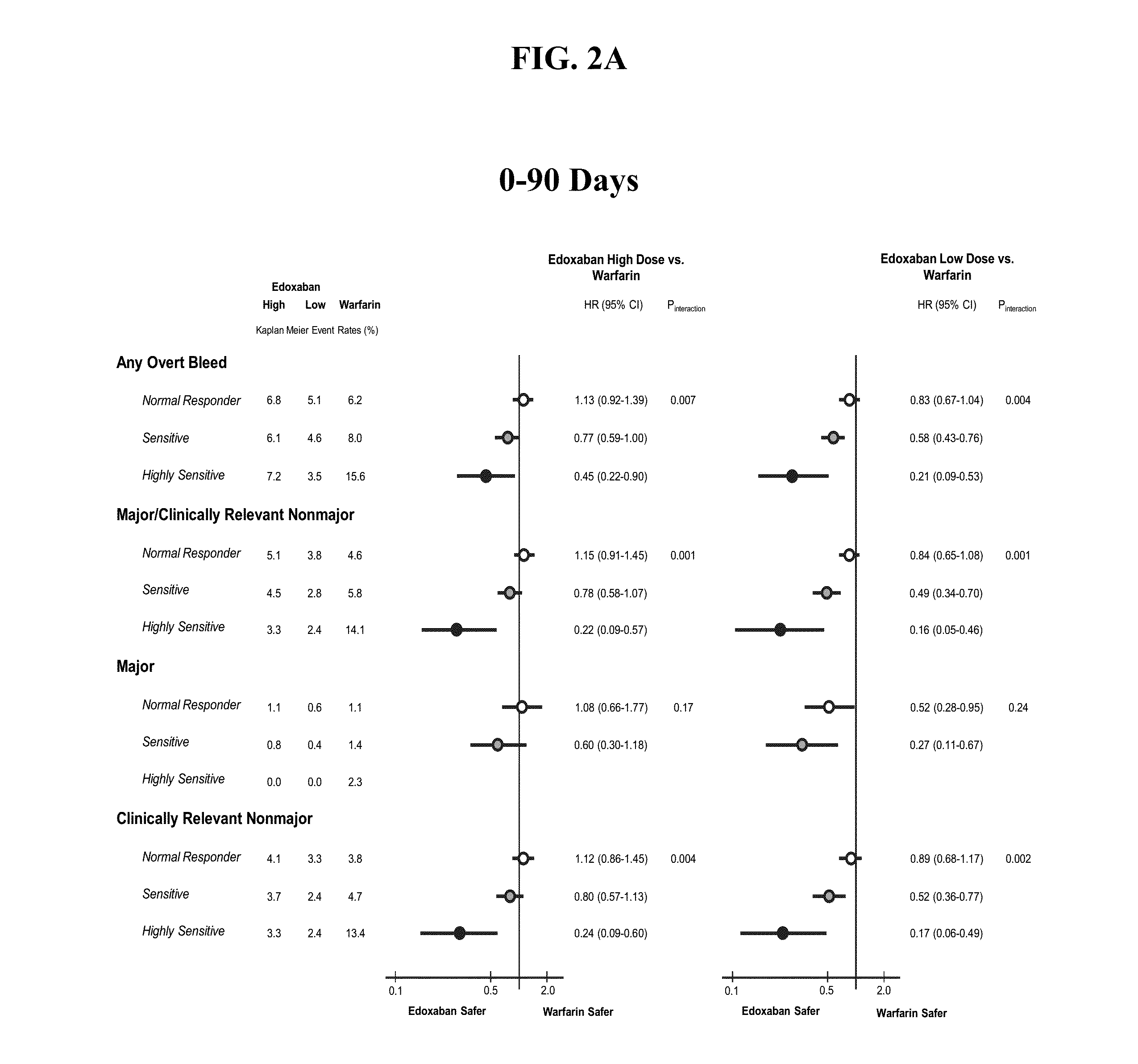
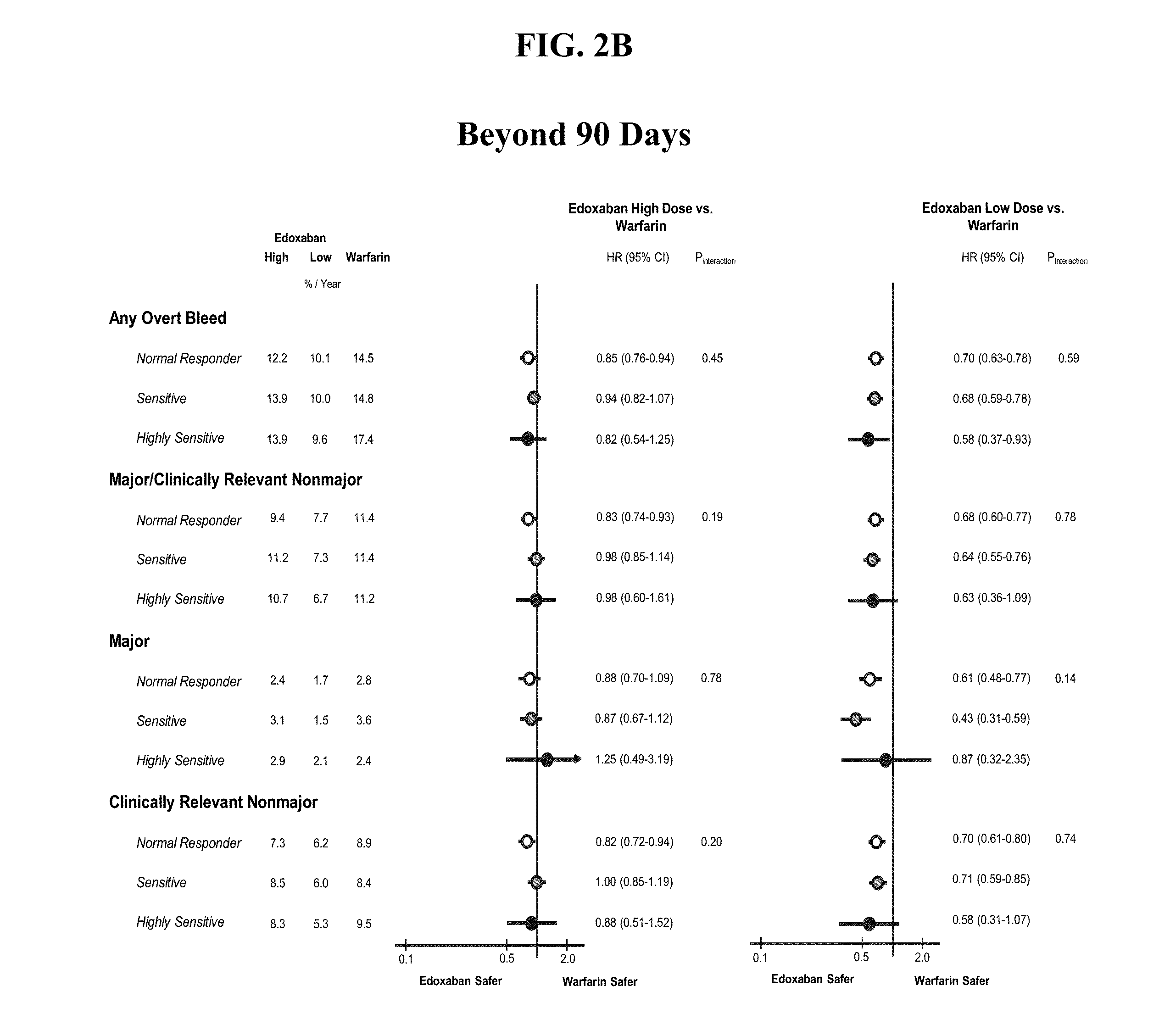
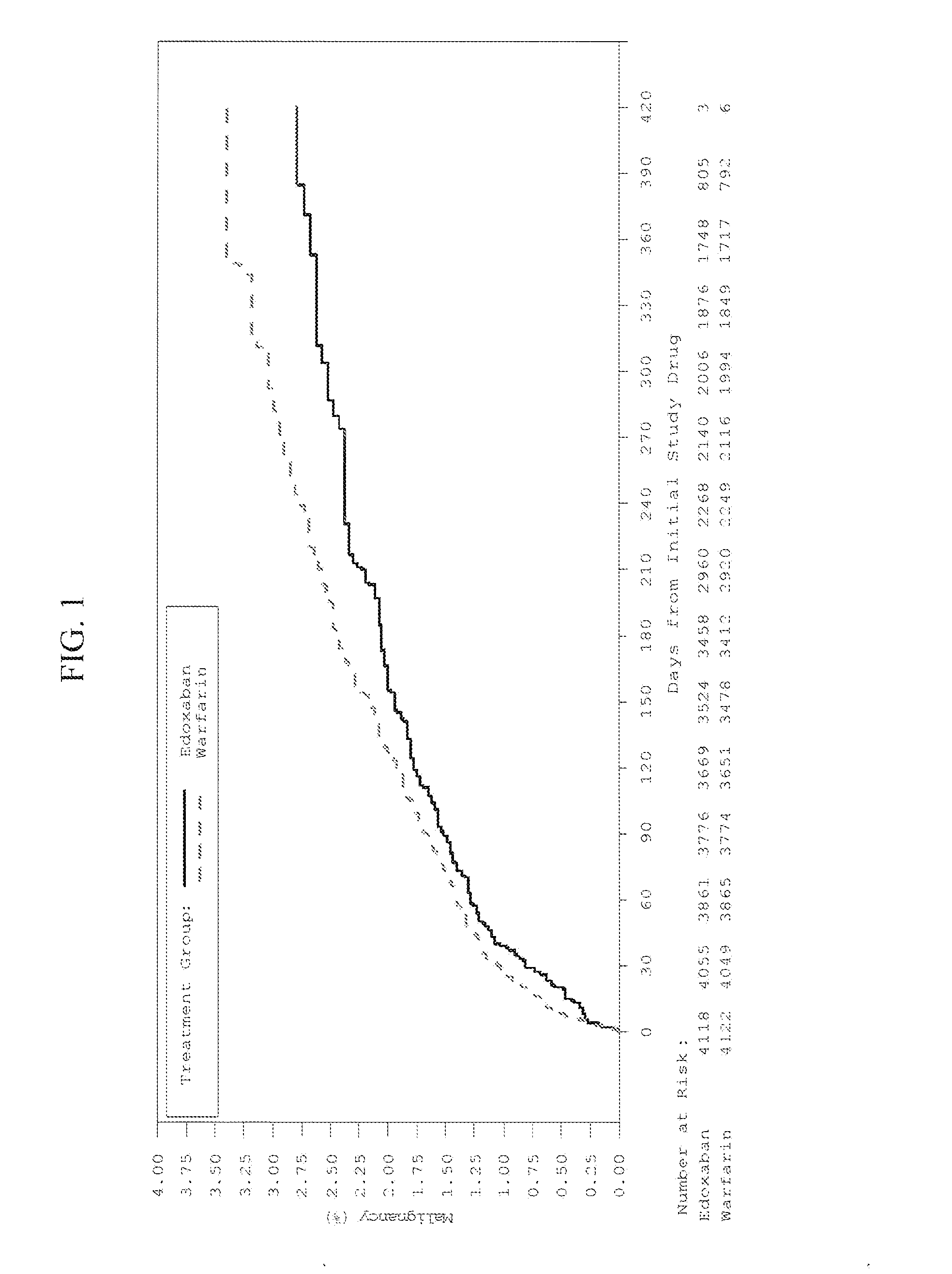
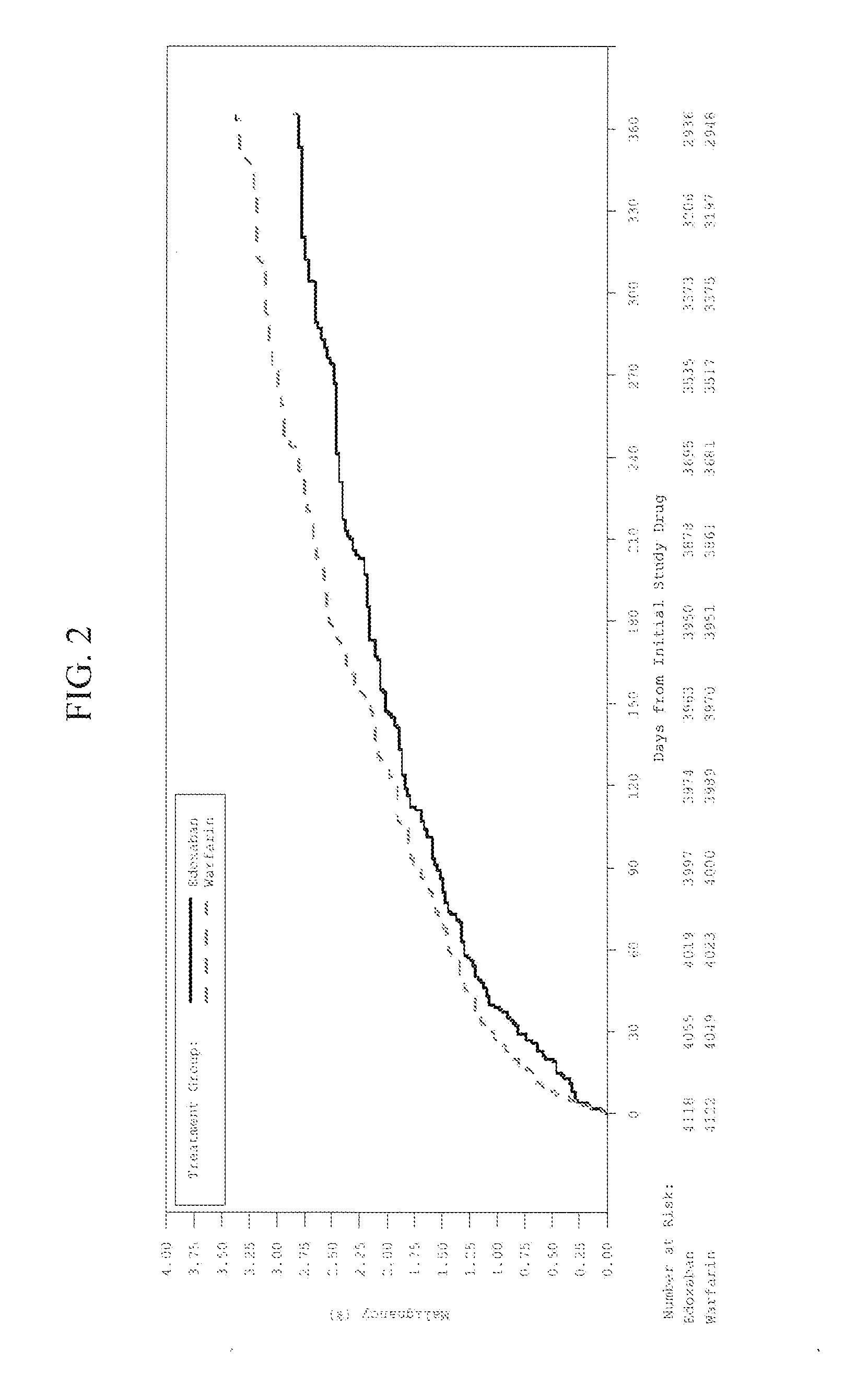
The invention provides methods of treating or preventing bleeding events or over-anticoagulation in a subject in need thereof who is identified as having sensitivity to a vitamin K antagonist such as warfarin by administering to the subject a therapeutically effective amount of an FXa inhibitor, which can be a direct or indirect FXa inhibitor, or a warfarin or VKA alternative drug or compound. The direct FXa inhibitor can be the small molecule edoxaban p-toluenesulfonate monohydrate, edoxaban, or a pharmaceutically acceptable salt and / or hydrate thereof. In aspects, the subject is identified as having one or more genetic polymorphisms in genes CYP2C9 and / or VKORC1 resulting in loss of function, reduction in function, or aberrant function of these genes and / or their protein products, and sensitivity to warfarin. The invention provides methods of administering an FXa inhibitor or warfarin alternative to safely and effectively reduce, prevent, reduce the risk of, prevent the recurrence of, or prevent the risk of recurrence of, conditions such as embolism, thrombosis, thromboembolism, etc. in a subject who is in need of anticoagulant therapy and who is identified as having one or more genetic polymorphisms resulting in warfarin sensitivity.
Owner:DAIICHI SANKYO CO LTD
Method for refining edoxaban
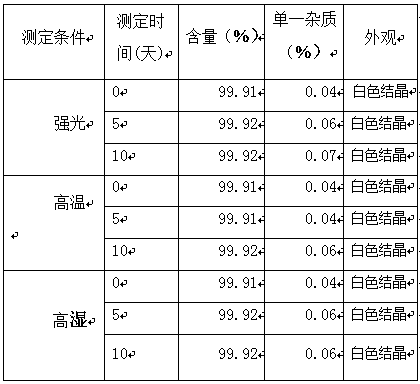
The invention belongs to the field of medicinal chemistry, and relates to a refined method for edoxaban. The crude edoxaban is first stirred and dissolved in an ethanol pure aqueous solution, then a mixed solution of ethers and ketones is added, and the mixture is stirred and crystallized; Washing, vacuum drying to obtain pure edoxaban crystals, the yield is over 90%, the purity (HPLC) reaches 99.4%, and the impurities are reduced to about 0.1%.
Owner:TIANJIN HANKANG PHARMA BIOTECH
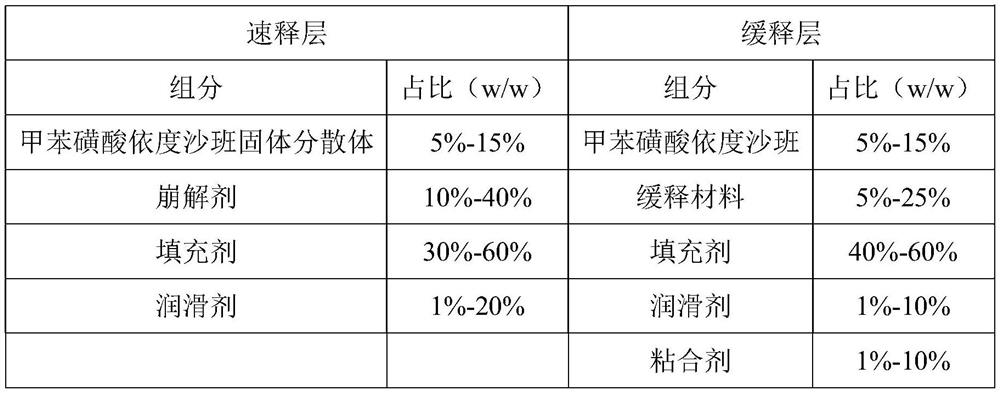
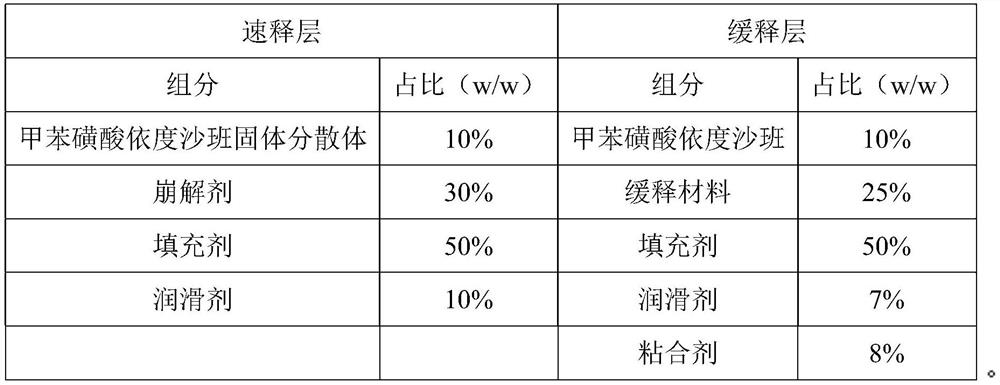
Edoxaban-containing sustained release preparation and preparation method thereof
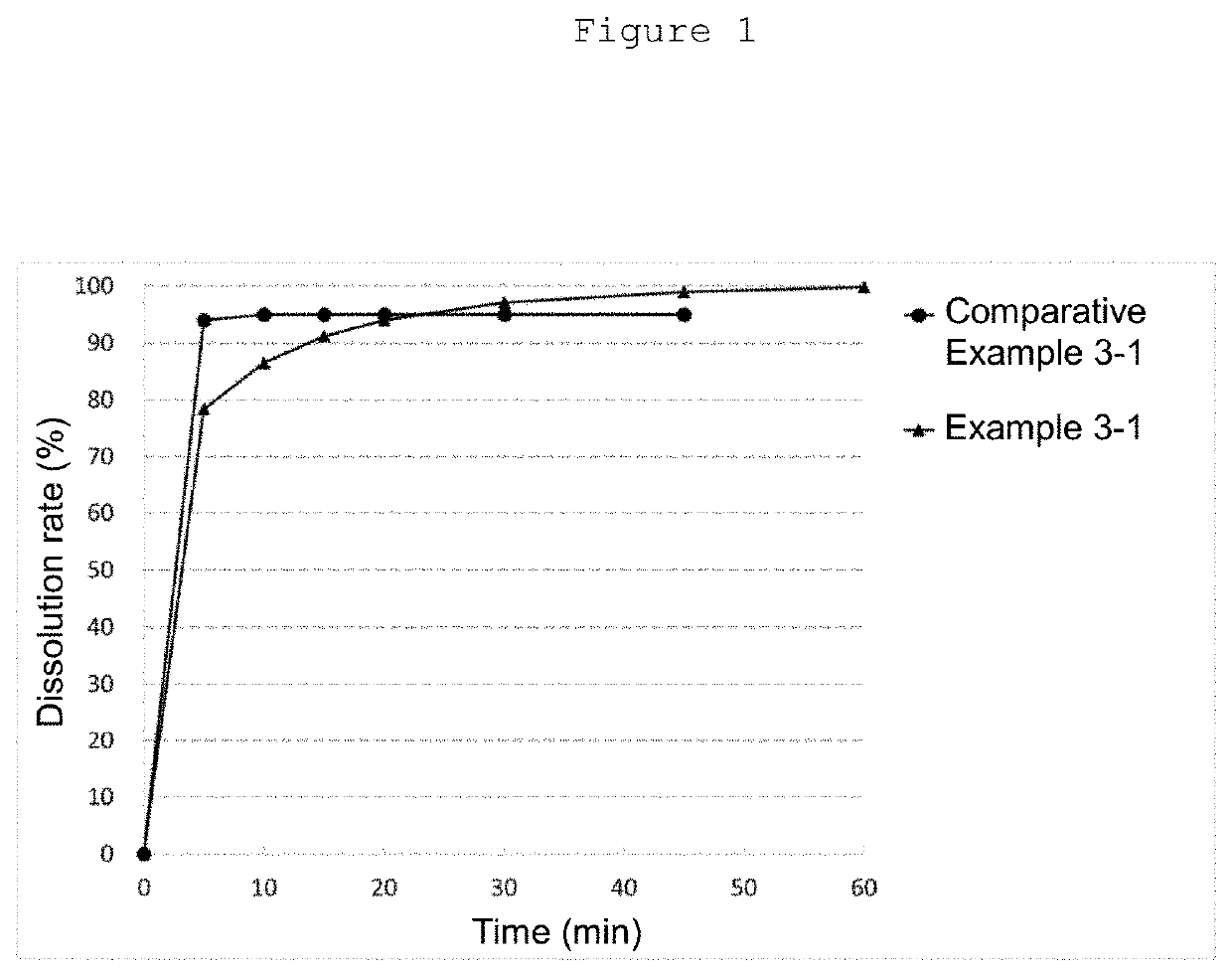
ActiveCN112791057AHigh dissolution rateQuality improvementOrganic active ingredientsInorganic non-active ingredientsControl releaseEdoxaban
The invention belongs to the technical field of drug sustained release preparations, and particularly relates to an edoxaban-containing sustained release preparation and a preparation method thereof. The sustained release preparation consists of a quick release layer and a sustained release layer, wherein the quick release layer mainly consists of an edoxaban solid dispersion, a disintegrating agent, a filling agent and a lubricating agent; the sustained release layer is mainly composed of an edoxaban bulk drug, a sustained-release material, a filler, a lubricant and an adhesive. The sustained release preparation containing edoxaban, which is excellent in controlled release effect and high in dissolution stability, is provided by optimizing the types, the proportion and the dosage of the filling agent and the sustained release material and optimizing the preparation method of the edoxaban solid dispersion in the process, and the problems that a sustained release preparation containing edoxaban is poor in release effect, low in dissolution rate and the like are solved.
Owner:齐飞
Method for Separating Edoxaban and Its Isomers
The invention provides a method for separating and purifying edoxaban by chromatography. The separation method is chromatography, and the chromatographic conditions are as follows: the filler is an amylose-tri(3,5-xylylcarbamate) bonded silica gel, and the mobile phase is selected from a mixed solution comprising a non-polar solvent and a polar solvent. By selecting the chromatographic conditions, the edoxaban and seven enantiomers and nonenantiomers thereof can be simultaneously separated, and thus, the method has the characteristics of high speed, high simpleness, high efficiency and the like.
Owner:北京康派森医药科技有限公司
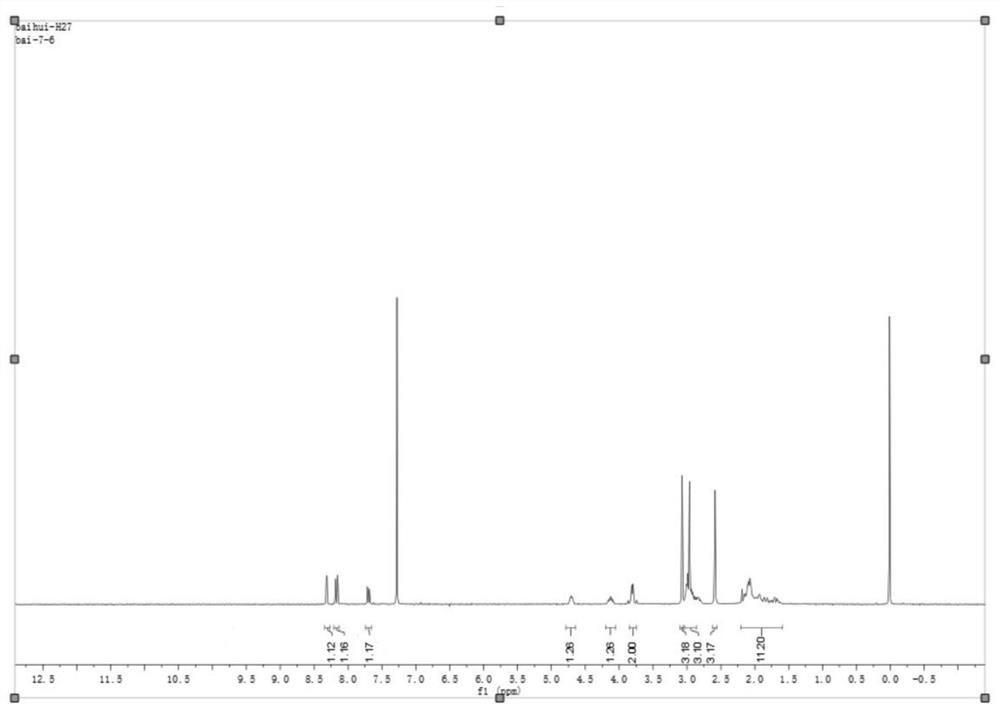
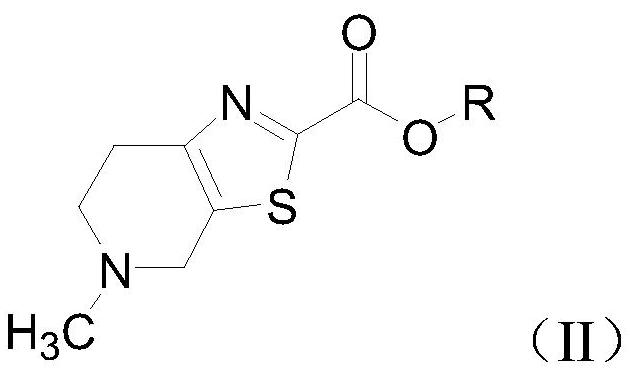
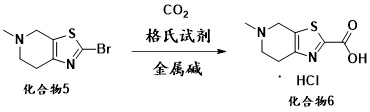
Intermediate for preparing edoxaban free alkali, and preparation method and application of intermediate
ActiveCN111763222ASynthetic safetyHigh drug safetyOrganic chemistry methodsThiazoleChemical compound
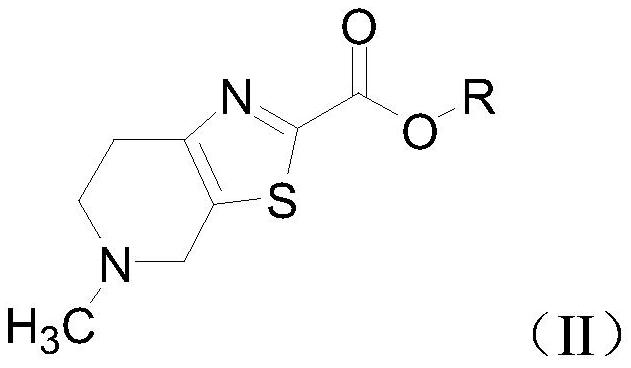
The invention relates to an intermediate for preparing edoxaban free alkali, and a preparation method and application of the intermediate. The preparation method of the intermediate comprises the following step: reacting 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5, 4-c]pyridine-2-carboxylic acid or a salt thereof with acyl chloride in an organic solvent under the action of alkali to obtain the intermediate. By utilizing the intermediate, the edoxaban free alkali can be synthesized in a low-cost, green, environment-friendly, simple, efficient and safe manner, and the drug safety of an obtained edoxaban product can be improved. A preparation method of the edoxaban free alkali comprises the following steps: reacting a compound (II) with a compound (IV) in an organic solvent to obtain the edoxaban free alkali, or reacting the salt of the compound (II) with the salt of the compound (IV) in an organic solvent under the action of alkali. The structures of the compound (II), the edoxaban free alkali and the compound (IV) are respectively shown in the specification.
Owner:ZHUHAI HAIRUIDE NEW MATERIAL TECH CO LTD
Preparation method of key intermediate of edoxaban
The invention relates to a preparation method of a key intermediate of edoxaban which is a blood coagulation factor X (FXa) blocker drug, in particular to a preparation method of a compound I that is tert-butyl [(1R,2S,5S)-2-[[2-[(5-chloropyridin-2-yl) amino]-2-oxoacetyl] amino]-5-(dimethylaminocarbonyl) cyclohexyl] carbamate. According to the method, the compound I is prepared from a compound A and a compound B in a free state in a reaction system with N,N-dimethylformamide as a solvent in the presence of sodium acetate. The raw materials adopted by the method are safe and stable, the reaction yield is high, and the method is suitable for industrial mass production.
Owner:JIANGSU VCARE PHARMATECH
Method for simultaneously determining concentrations of anticoagulant drugs and active metabolite in plasma
ActiveCN113030312AHigh sensitivityMeet analysis requirementsComponent separationPharmaceutical drugEdoxaban
The invention discloses a method for simultaneously determining the concentrations of anticoagulant drugs and an active metabolite in plasma. The anticoagulant drugs are dabigatran etexilate, rivaroxaban, edoxaban and apixaban respectively, and the active metabolite is dabigatran. The specificity, precision, accuracy, linearity, stability and the like of the method all meet the analysis requirements of biological samples, the sensitivity is high, and the method can be used for clinical monitoring of therapeutic drugs of dabigatran etexilate, dabigatran, rivaroxaban, edoxaban and apixaban.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Edoxaban trihydrate as well as preparation method and application thereof
InactiveCN107663213AOrganic active ingredientsOrganic chemistry methodsMedicineAntithrombotic treatment
The invention belongs to the technical field of medicine, and in particular relates to a crystal form of Edoxaban trihydrate and a preparation method thereof. The invention also relates to a pharmaceutical composition containing the above-mentioned crystal form of Edoxaban trihydrate and its preparation method for treating antithrombotic drugs. in the application.
Owner:TIANJIN HANRUI PHARMA
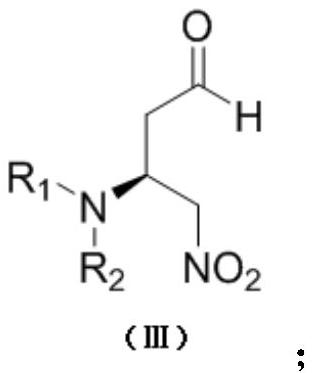
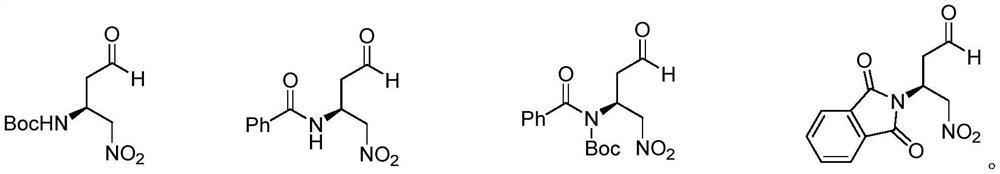
Chiral amino compound, preparation method and application thereof, and preparation method for preparing edoxaban intermediate from chiral amino compound
ActiveCN111763157AEasy to makeEfficient preparationCarbamic acid derivatives preparationOrganic compound preparationCyclohexeneSodium azide
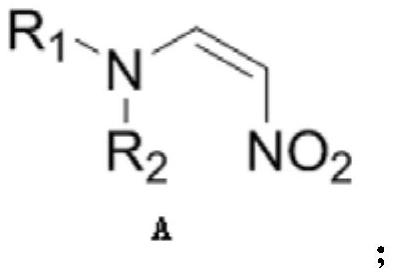
The invention provides a chiral amino compound, a preparation method and application thereof, and a preparation method for preparing an edoxaban intermediate from the chiral amino compound. The structure of the chiral amino compound is shown as a formula (III); the structure of the edoxaban intermediate prepared by the formula (III) is as shown in the formula (I). The R1 and R2 are independently aprotecting group of nitrogen or hydrogen; wherein R is a C1-6 alkoxy group, an N, N-dimethylamino group, an N-methylamino group or a benzylamino group. The compound shown in the formula (III) can beprepared on a large scale with high optical purity only through one-step reaction without a chiral resolution process, and the preparation process is simple, efficient and low in cost; according to the present invention, the edoxaban intermediate represented by the formula (I) can be prepared by using the compound represented by the formula (III) as the raw material through the one-step reaction,wherein the expensive (S)-3-cyclohexene-1-formic acid does not need to be used as the starting material, and the chiral resolution is not required, the route is short, and the use of sodium azide canbe effectively avoided; therefore, the reaction condition is mild, the yield is high, and the method is suitable for large-scale industrial production.
Owner:SUN YAT SEN UNIV
Preventive and/or therapeutic agent for thromboembolism in thromboembolism patient with severe renal impairment
InactiveUS20140371262A1Avoid bleeding riskAvoid security issuesBiocideAnimal repellantsMedicineThrombus
It is intended to provide a highly safe, orally administrable preventive and / or therapeutic agent for thrombosis and / or embolism that can be applied to a thrombosis and / or embolism patient with severe renal impairment. The present inventors have found that even for a thrombosis and / or embolism patient with severe renal impairment, use of edoxaban at a dose of 15 mg once a day can effectively prevent thrombosis and / or embolism while avoiding the risk of bleeding. The present inventors have also found that even for a thrombosis and / or embolism patient with severe renal impairment, edoxaban at a dose of 15 mg once a day can effectively prevent thrombosis and / or embolism with safety over a long period.
Owner:DAIICHI SANKYO CO LTD
Compound sustained-release preparation containing apixaban and preparation method thereof
ActiveCN112494489ADisintegrates quicklyReduce the number of dosesPowder deliveryOrganic active ingredientsImmediate releaseSpray coating
The invention discloses a compound sustained-release preparation containing apixaban and a preparation method thereof. The preparation is composed of apixaban particles, edoxaban sustained-release pellets and other pharmaceutically acceptable auxiliary materials, wherein the edoxaban sustained-release pellets are composed of blank pellet cores, isolation coating layers and drug-containing layers from inside to outside in order; the drug-containing layers are composed of edoxaban, hydroxypropyl cellulose and a filler; a drug-containing dispersion solution is sprayed onto the coated pellet coresby a bottom spraying coating method to obtain drug-loaded quick-release pellets, and then the drug-loaded quick-release pellets are coated with sustained-release coating layers to obtain the drug-containing layers; and the apixaban particles are obtained by granulation of apixaban, microcrystalline cellulose, hydroxypropyl cellulose and cross-linked sodium carboxymethyl cellulose. The compound sustained-release preparation provided by the invention can take effect quickly, can release drugs stably for a long time, reduces the number of drug taking times of patients, improves the compliance, and improves the safety of drug use of patients, thereby meeting the needs of treatment and meeting the requirements of clinical medication.
Owner:浙江诺得药业有限公司 +1
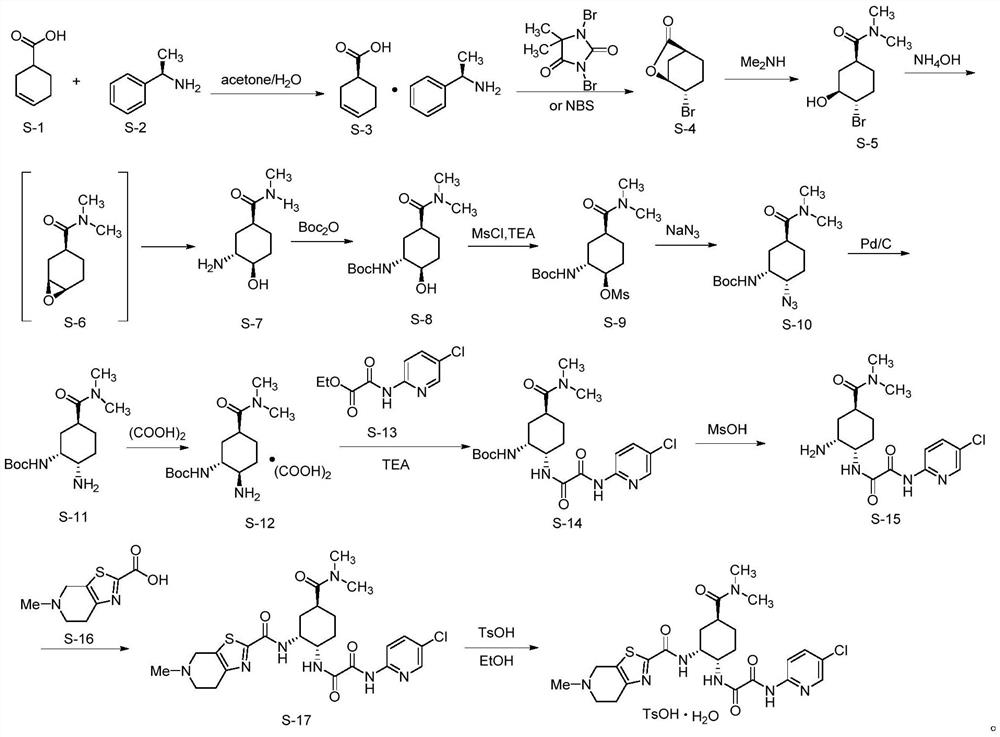
A kind of preparation method of edoxaban and its intermediate
Owner:SHANGHAI HANSOH BIOMEDICAL +1
A kind of method for preparing edoxaban from trichloroethyl ketonium salt derivative
ActiveCN111393456BReduce dosagePost-processing is simpleSulfonic acids salts preparationBulk chemical productionPyridiniumCyclohexyl chloride
The present invention provides 2,2,2-trichloro-1-(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridinium-1-yl)ethanone Method for preparing edoxaban from chloride. Among them: 2,2,2-trichloro-1-(4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridinium-1-yl)ethanone chloride , namely the preparation method of 109C5-11; N 1 -[(1S, 2R, 4S)-2-amino-4-[(dimethylamino)carbonyl]cyclohexyl]-N 2 -(5-Chloro-2-pyridyl)oxalamide bis-methanesulfonate, namely the preparation method of 109T2-31; edoxaban was prepared by using 109C5-11 as an acylating reagent and 109T2-31. The new method avoids the disadvantages of using expensive condensing agent EDCI·HCl and activating agent HOBt in the existing process. The novel method of the invention is beneficial to realize the industrialized scale production of edoxaban p-toluenesulfonate hydrate more economically and efficiently.
Owner:内蒙古京东药业有限公司
A compound slow-release preparation containing apixaban and its preparation method
ActiveCN112494489BDisintegrates quicklyReduce the number of dosesPowder deliveryOrganic active ingredientsImmediate releaseSpray coating
Owner:浙江诺得药业有限公司 +1
Preparation method of edoxaban intermediate
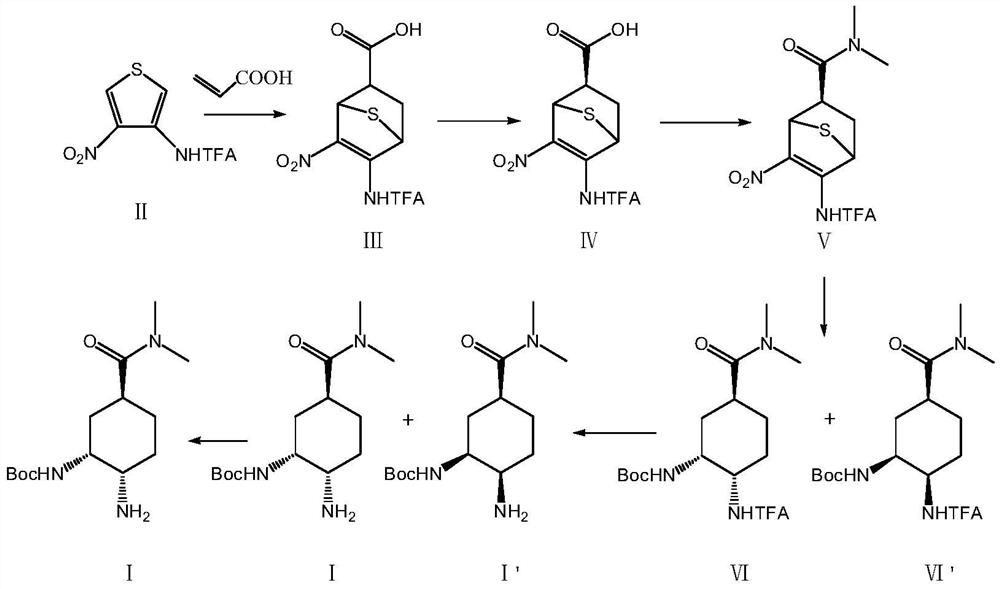
ActiveCN111606826AHigh selectivityReduce usageCarbamic acid derivatives preparationOrganic compound preparationHydrogen atmosphereThiophene derivatives
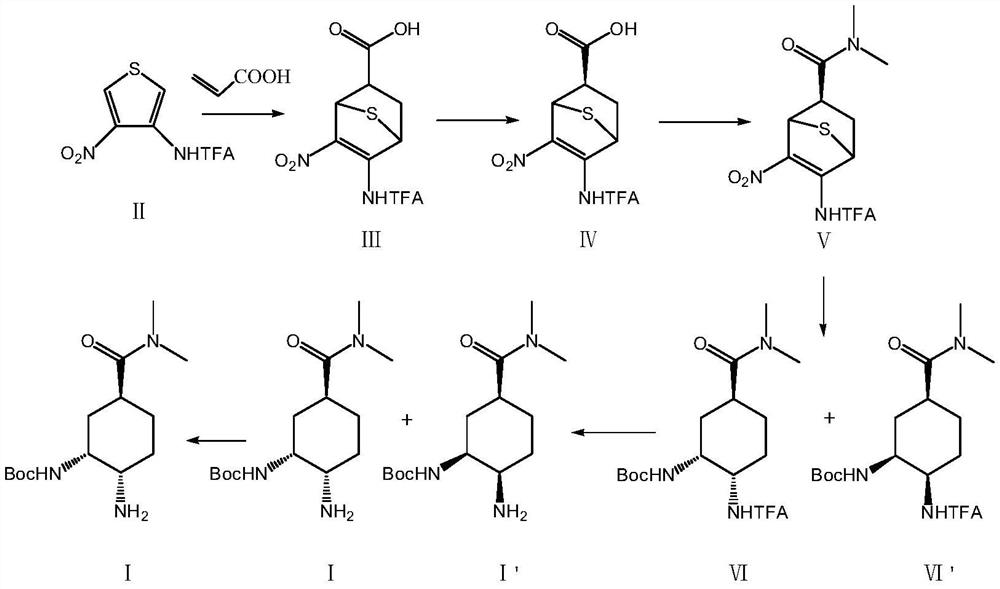
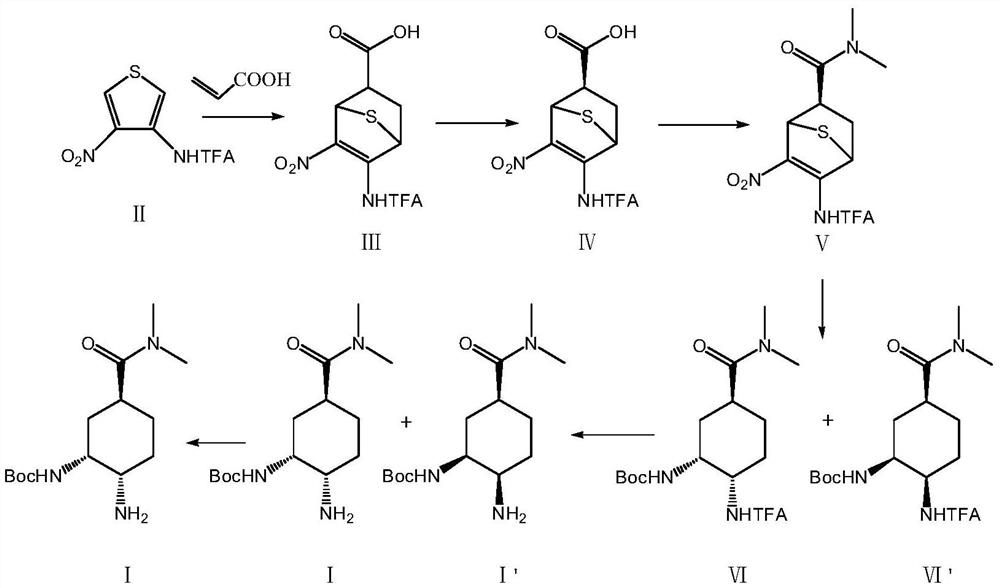
The invention relates to the technical field of medicines, and particularly discloses a preparation method of an edoxaban intermediate. The preparation method comprises the following steps: carrying out diene synthesis on a thiophene derivative of a compound II and acrylic acid, and carrying out chiral resolution to obtain a compound IV; carrying out amidation reaction with dimethylamine hydrochloride to obtain a compound V; reacting with di-tert-butyl dicarbonate in a hydrogen atmosphere to obtain a compound VI and a compound VI'; and carrying out amino deprotection and chiral resolution to obtain a compound I, namely the edoxaban intermediate. The preparation method provided by the invention has the advantages of concise operation steps, high diastereomer selectivity, facilitation of improvement of the product yield and reduction of the production cost, no use of a dangerous reagent sodium azide, no involvement of a low-temperature reaction, reduction of the production risk, and guarantee of the safety and the operability of the reaction.
Owner:CANGZHOU SENARY CHEM SCI TEC
Agent for treatment and prevention of cancer
InactiveUS20150065456A1Reduce and prevent metastasisIncreased risk of bleedingBiocideAnimal repellantsNeoplasmDirect factor Xa inhibitors
The invention provides methods of treating or preventing in a subject a cancer, tumor, or neoplasm, including malignancies or metastases thereof, using a direct Factor Xa inhibitor. The methods particularly involve the treatment of human patients afflicted with a malignant cancer, tumor, or neoplasm with an effective amount of the Factor Xa inhibitor. In an embodiment, the Factor Xa inhibitor is the small molecule edoxaban p-toluenesulfonate monohydrate, also termed “DU-176b”. The invention provides methods of administering a Factor Xa inhibitor to effectively reduce or suppress, or otherwise abrogate, the growth of cancers, tumors, or neoplasms in a subject who has, or is at risk for, cancer and, optionally, who also has, or is at risk for, thrombotic disease or embolism. The methods of the invention can also reduce the incidence of migration or invasion of metastatic or malignant cancers in a human subject by administration of a Factor Xa inhibitor.
Owner:DAIICHI SANKYO CO LTD
Intermediate for the preparation of edoxaban free base and its preparation method and application
The present invention relates to an intermediate for preparing edoxaban free base and its preparation method and application. The preparation method of the intermediate comprises the following steps: in an organic solvent, under the action of a base, 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c] Pyridine-2-carboxylic acid or its salt is reacted with acid chloride to obtain. Using the intermediate, the free base of edoxaban can be synthesized in a low-cost, green, environmentally friendly, simple, efficient and safe manner, and the drug safety of the obtained edoxaban product can be improved. The steps for preparing edoxaban free base are as follows: in an organic solvent, compound (II) and compound (IV) react to obtain; or, in an organic solvent, under the action of a base, compound (II) and compound ( IV) salt reaction, that is, obtained. The structures of compound (II), edoxaban free base, and compound (IV) are respectively as follows.
Owner:ZHUHAI HAIRUIDE NEW MATERIAL TECH CO LTD
Process for preparation of edoxaban
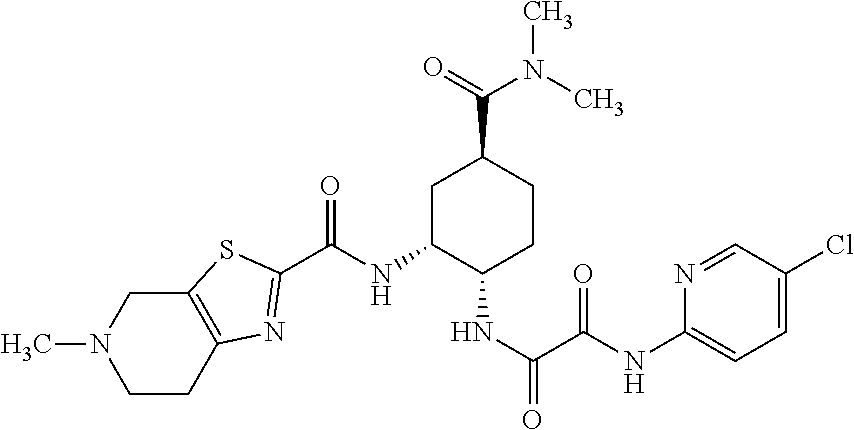
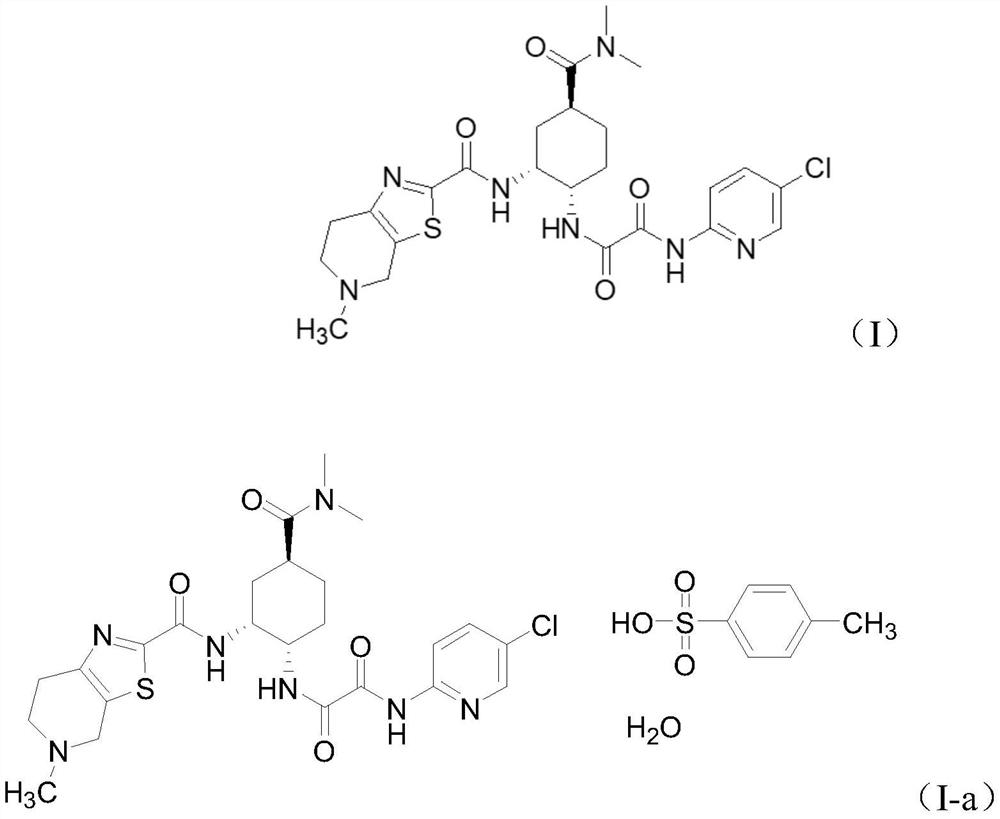
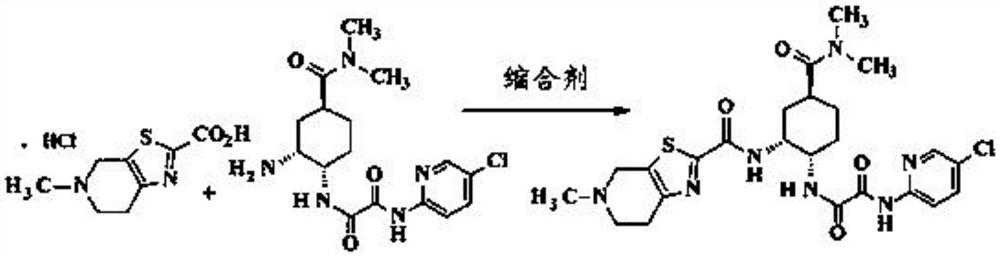
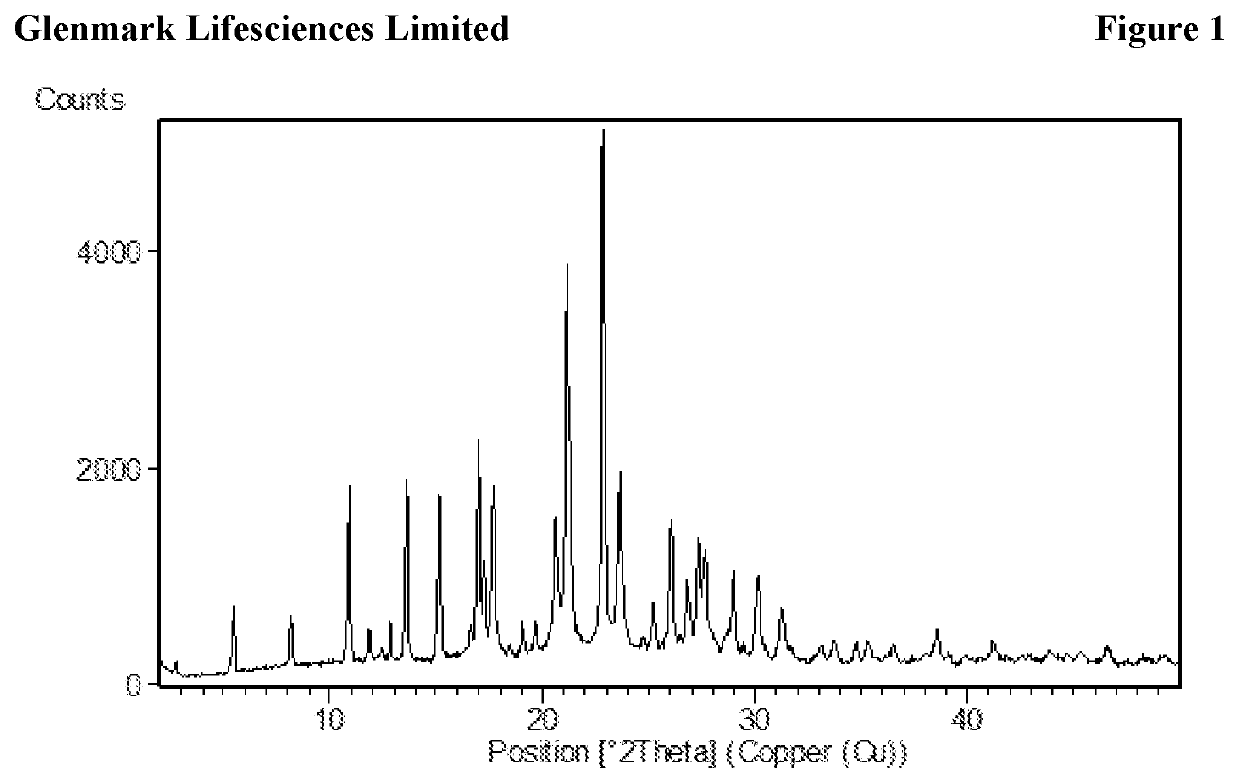
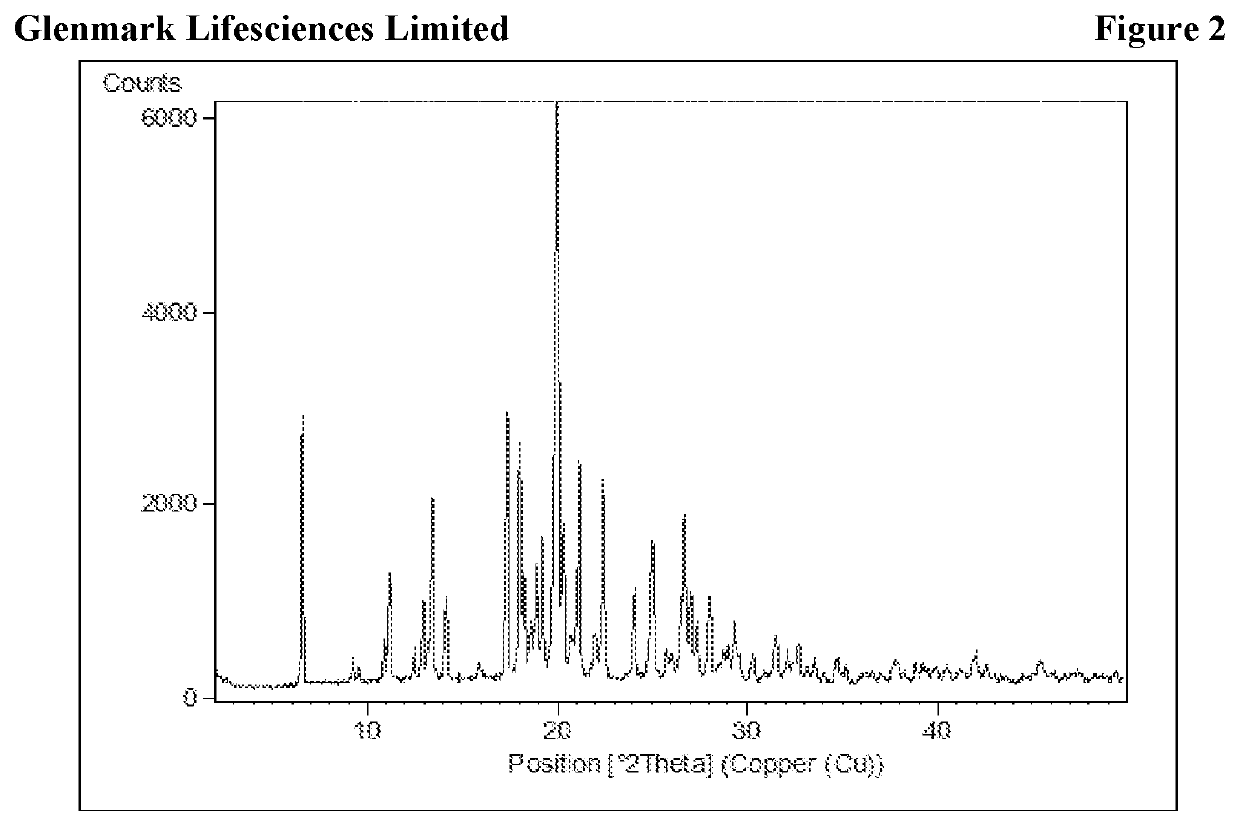
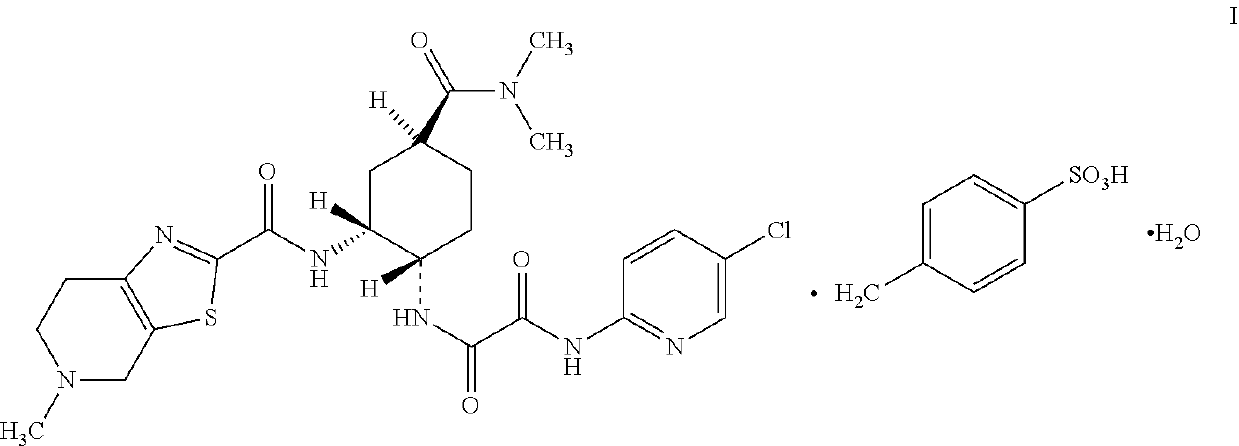
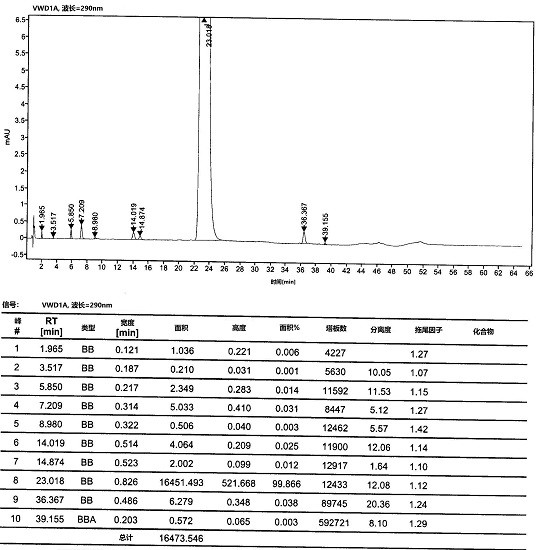
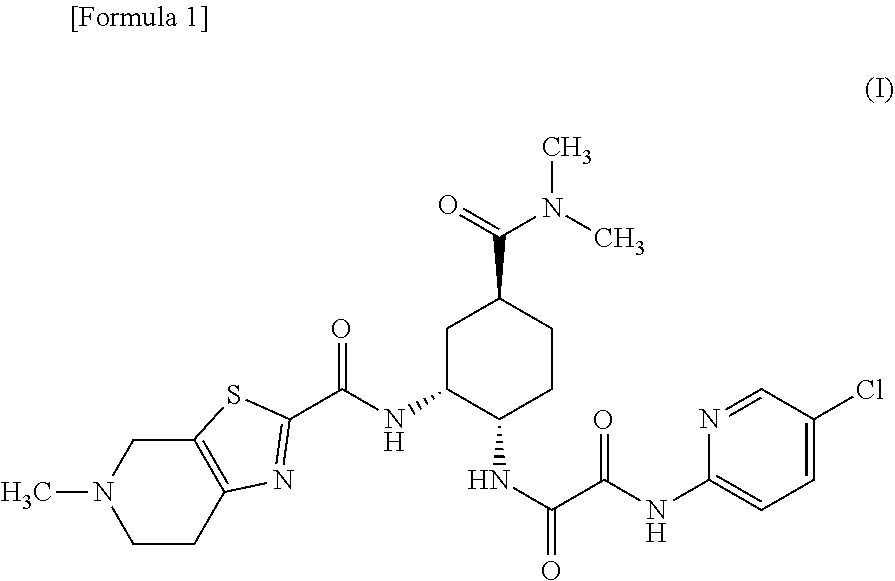
The present invention relates to process for preparation of N1-(5-Chloropyridin-2-yl)-N2-[(1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydrothiazolo [5,4-c]pyridin-2-ylcarboxamido)cyclohexyl]oxamide p-toluene sulfonate monohydrate [edoxaban tosylate monohydrate], the compound of formula I, comprising reacting compound of formula VI with compound of formula V to obtain the compound of formula IV and further converting it to edoxaban tosylate monohydrate in an industrially feasible process.
Owner:GLENMARK LIFE SCI LTD
Preparation method of edoxaban and intermediate thereof
ActiveCN112940012AOperational securityEasy to operateOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention relates to a preparation method of edoxaban and an intermediate thereof. A compound 1, a compound 2 and a compound 5 which are taken as initial raw materials are subjected to an ammonolysis reaction, a deprotection reaction, a carboxylation reaction and a condensation reaction to generate edoxaban. Compared with the prior art, the preparation method is higher in operability and safety during amplified production, and the obtained product is high in yield and purity.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
A chiral amino compound, its preparation method and application, and its preparation method for preparing an edoxaban intermediate
ActiveCN111763157BHigh optical purityEasy to makeCarbamic acid derivatives preparationOrganic compound preparationSodium azideCombinatorial chemistry
The invention provides a chiral amino compound, a preparation method and application thereof, and a preparation method for preparing an edoxaban intermediate from the chiral amino compound. The structure of the chiral amino compound is as shown in formula (III); the structure of the edoxaban intermediate prepared by formula (III) is as described in formula (I); wherein, R 1 and R 2 is independently a nitrogen protecting group or hydrogen; R is C 1~6 Alkoxy, N,N-dimethylamino, N-methylamino or benzylamino. The compound of formula (Ⅲ) can be prepared in large quantities with high optical purity in only one step reaction, without the need for chiral resolution process, the preparation process is simple, efficient and low in cost; The reaction can prepare the edoxaban intermediate of formula (I), without using expensive (S)-3-cyclohexene-1-carboxylic acid as a starting material, and without chiral resolution, the route is short, and The use of sodium azide can be effectively avoided, the reaction conditions are mild, the yield is high, and the method is suitable for large-scale industrial production.
Owner:SUN YAT SEN UNIV
Granules containing diamine derivative
ActiveUS20210267954A1Improve solubilityReduce bitternessOrganic active ingredientsDispersion deliveryCarmellose SodiumCellulose
Provision of a granular preparation that contains edoxaban or a pharmacologically acceptable salt thereof, and has the property of being rapidly dissolved or suspended by the addition of water. A granular preparation comprising first granules containing (A) edoxaban or a pharmacologically acceptable salt thereof, (B) a sugar alcohol, and (C) a water-swelling additive, and second granules containing (D) 0.5 to 10% by weight of carmellose sodium with respect to the total weight of the preparation, and (E) 70 to 90% by weight of xylitol or sorbitol with respect to the total weight of the preparation.
Owner:DAIICHI SANKYO CO LTD
A kind of preparation method of edoxaban intermediate
ActiveCN107721866BEfficient manufacturingReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationTert-Butyloxycarbonyl protecting groupLithium hydroxide
The invention discloses a preparation method of edoxaban intermediate (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxy-cyclohexanecarboxylic acid. According to the preparation method, 3-amino-4-hydroxy-cyclohexane carboxylate represented by formula IV is taken as a raw material, protective reaction with amino protection groups is carried out so as to obtain 3-tert-butoxycarbonylamino-4-hydroxy-cyclohexane carboxylate represented by formula V, enzyme catalyzed esterification resolution reaction is carried out so as to obtain optically pure (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxy-cyclohexane carboxylate represented by formula VII, and at last lithium hydroxide hydrolysis is adopted so as to obtain the (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxy-cyclohexanecarboxylic acid represented by formula VI. The preparation method possesses following advantages: operation is simple, the preparation method is green, is friendly to the environment, and is high in selectivity and lowin cost, large scale industrialized production can be realized, and it is convenient for industrial popularization.
Owner:ASTATECH CHENGDU BIOPHARM CORP
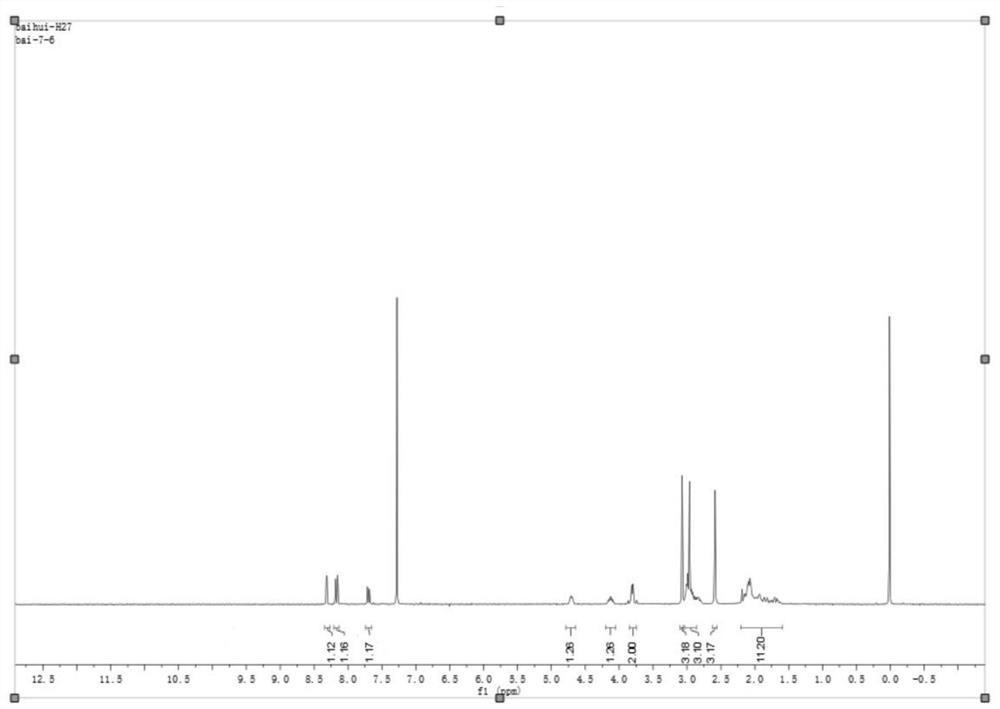
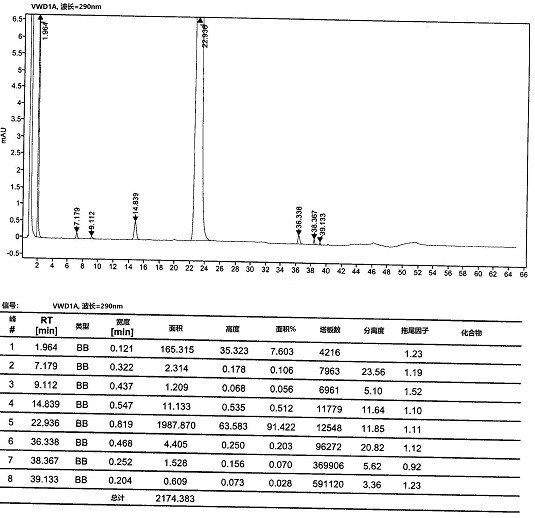
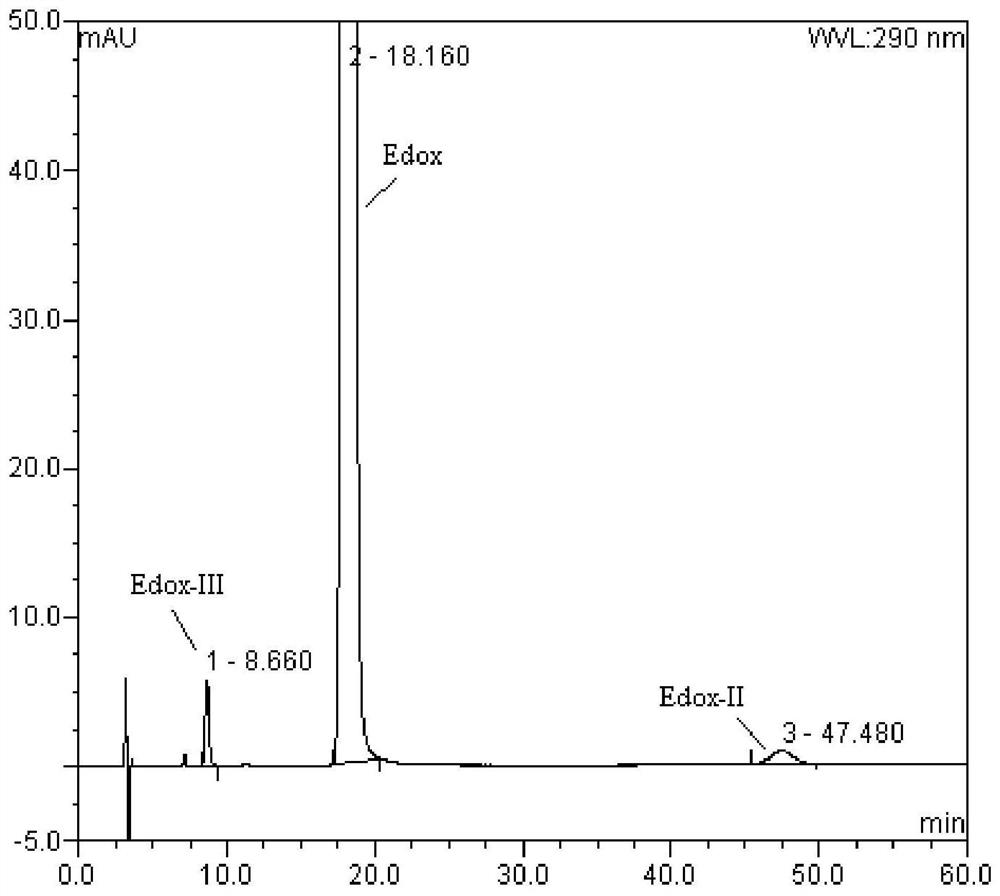
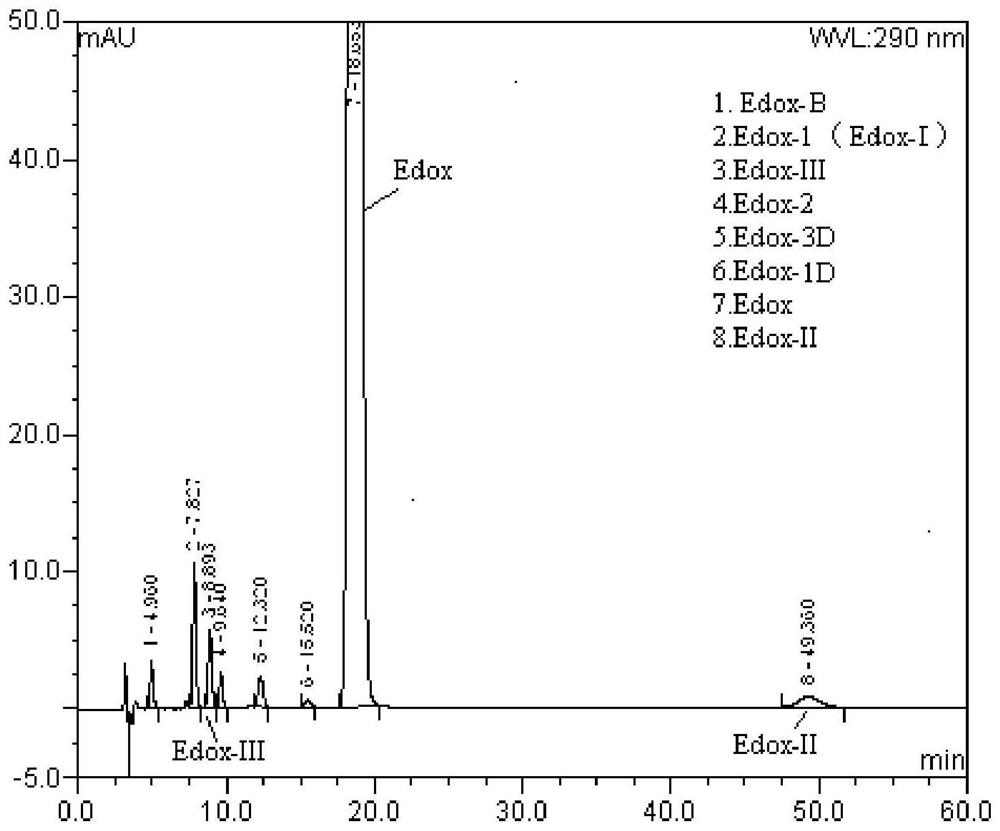
Method for separation and determination of edoxaban tosylate hydrate and its isomer impurities by chiral high performance liquid chromatography
ActiveCN107543872BEnsure quality controllabilityAchieve separationComponent separationCelluloseCarbamate
The invention discloses a method for separating and measuring edoxaban tosylate hydrate and its isomer impurities by chiral high-performance liquid chromatography. The silica gel of methyl phenyl carbamate) is the chiral chromatographic column of filler, and mobile phase is the mixed solution of the methanol-ethanol that adds basic additive. By adopting the method of the present invention, complete separation of Edoxaban from the isomers Edox-II and Edox-III can be realized. The method is convenient to operate, has been verified by the method, has good method specificity, high sensitivity, and precision and accuracy. The method can accurately carry out the quantitative analysis of the isomers Edox-II and Edox-III of the edoxaban toluenesulfonate hydrate raw material drug and its preparations, thereby ensuring that the edoxaban toluenesulfonate hydrate and its preparations quality controllability.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com