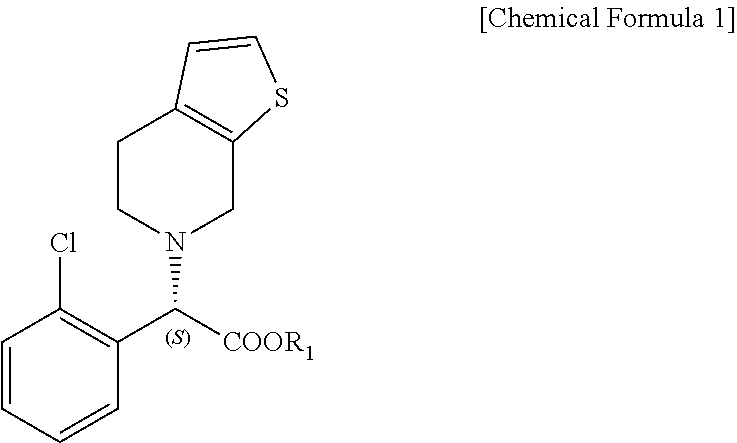
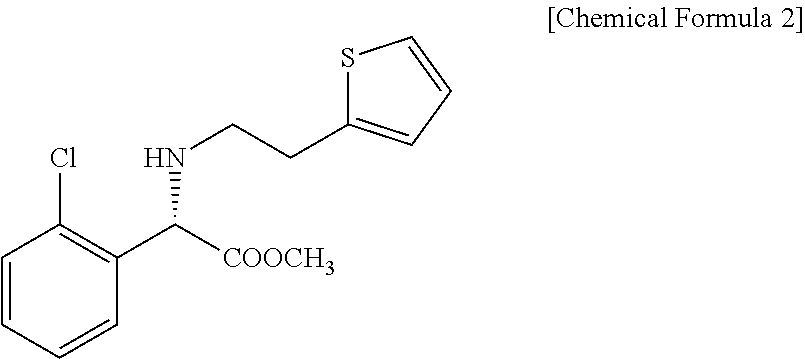
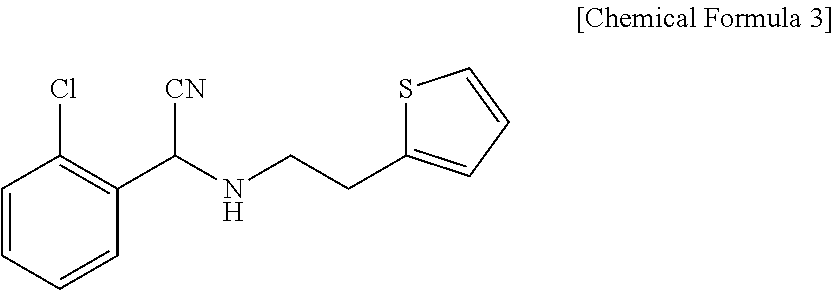
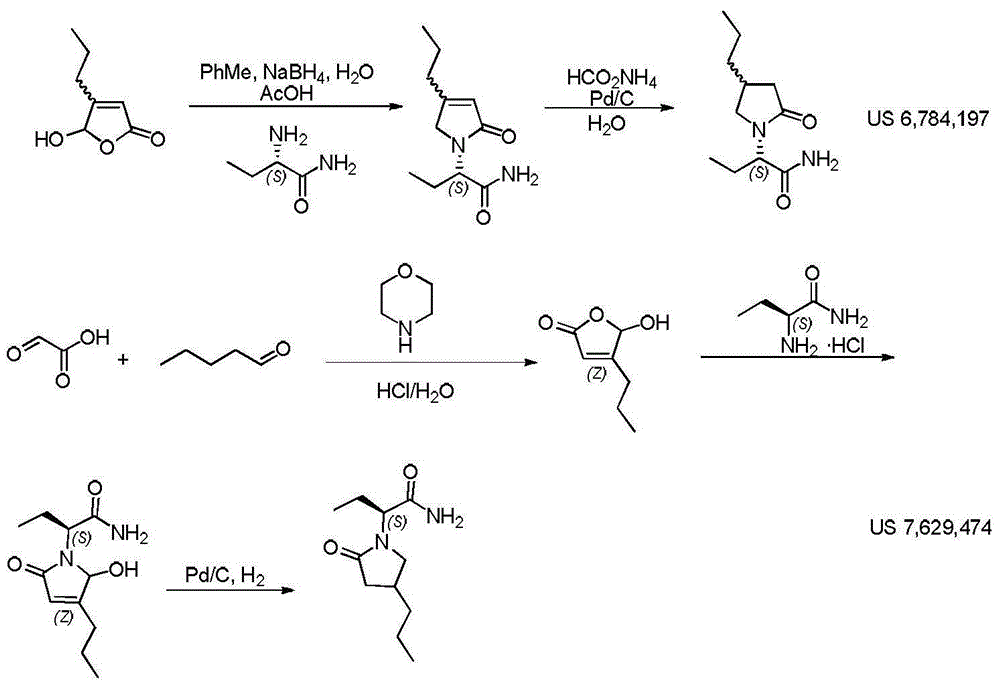
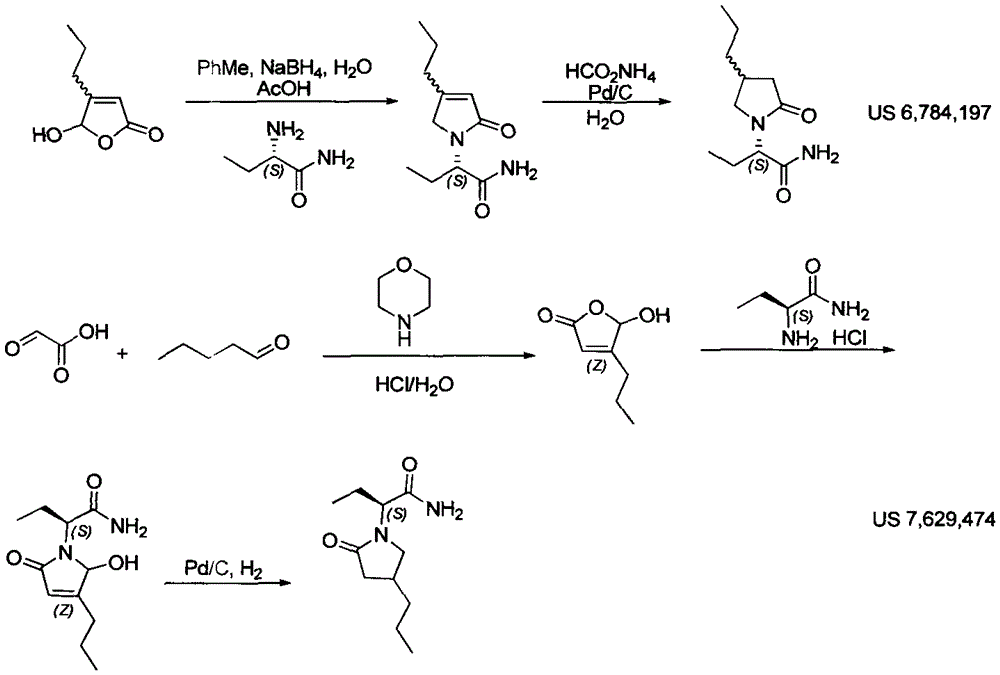
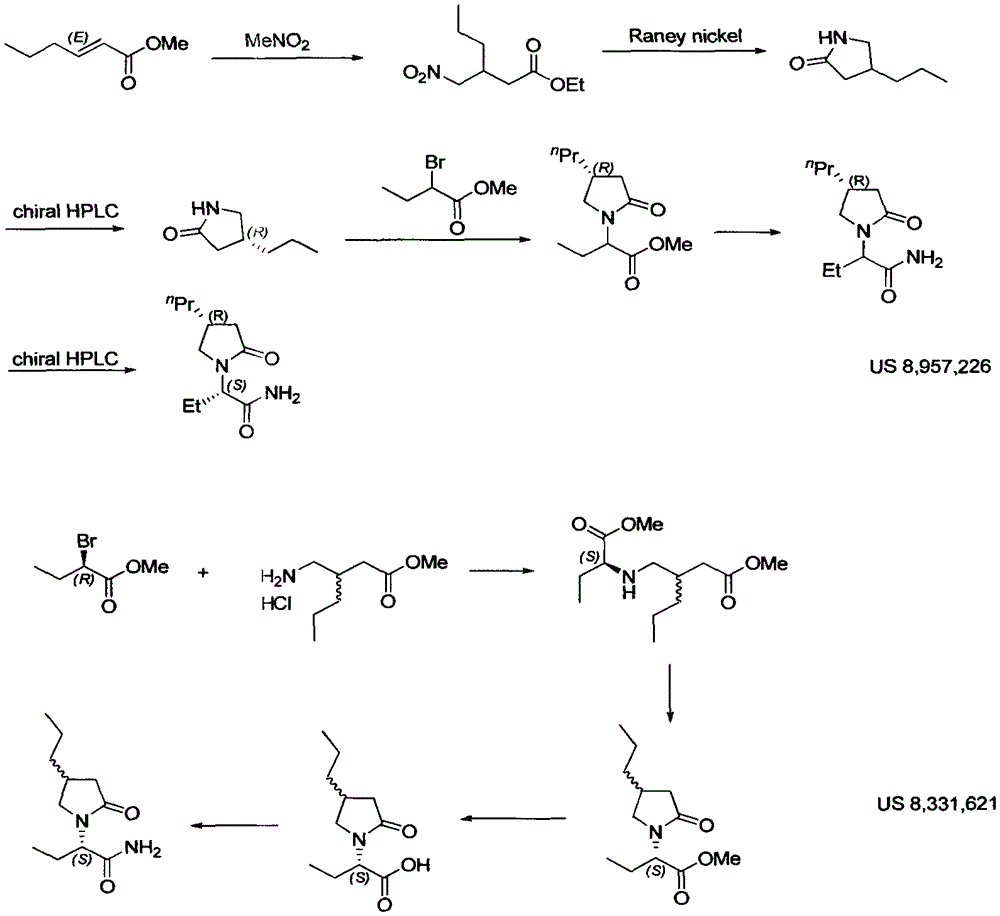
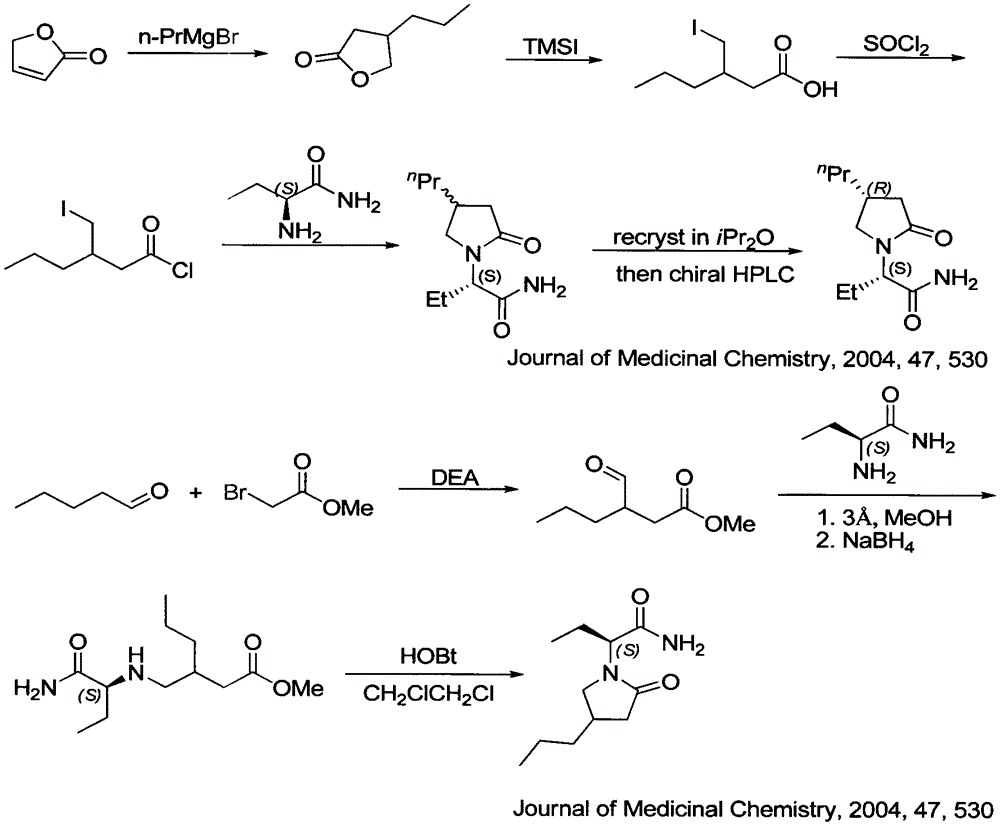
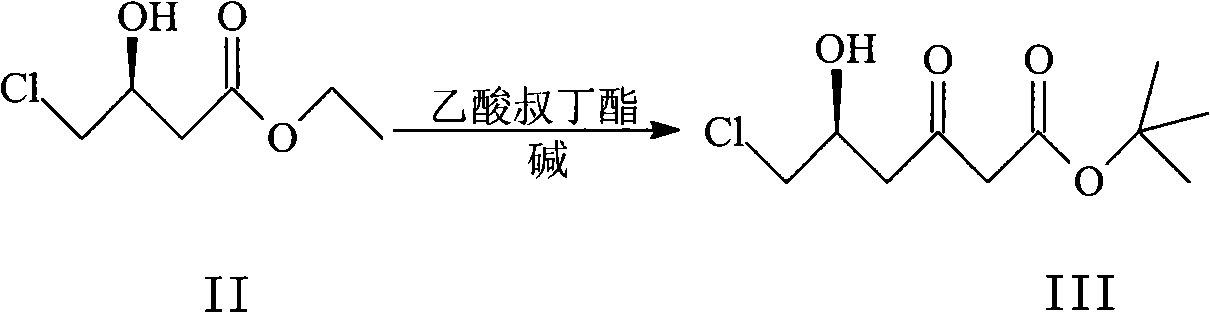
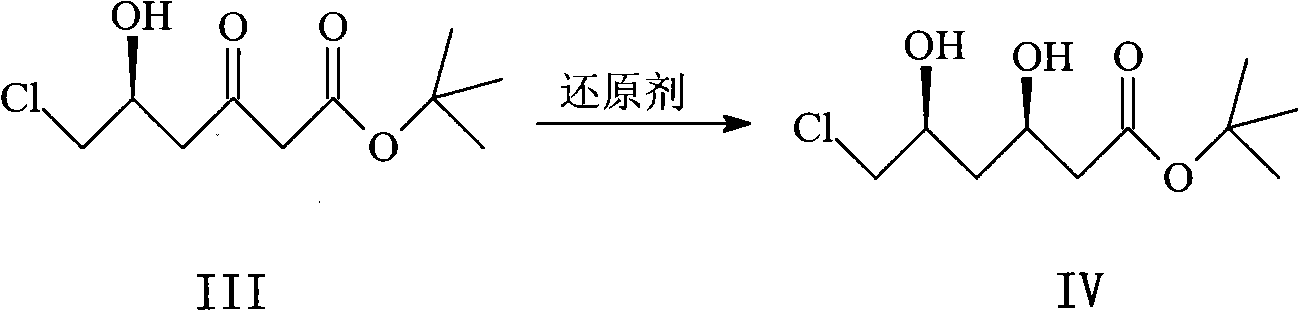
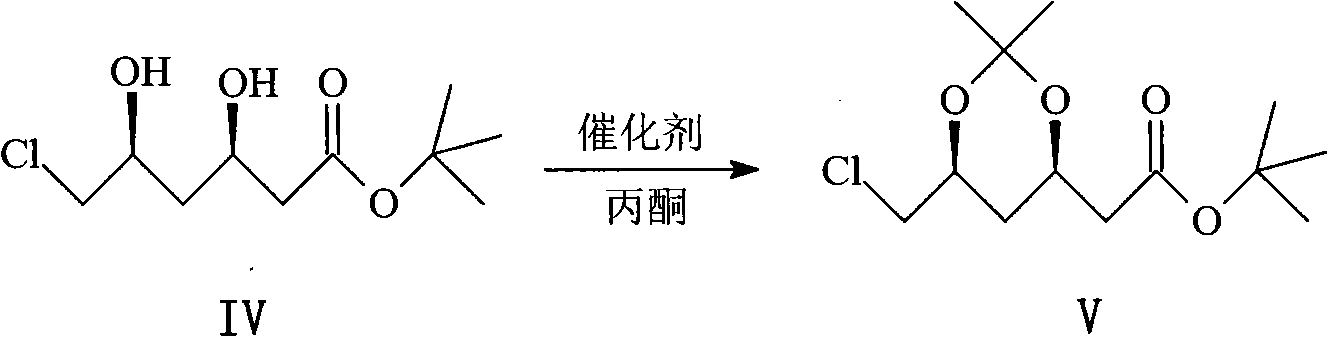
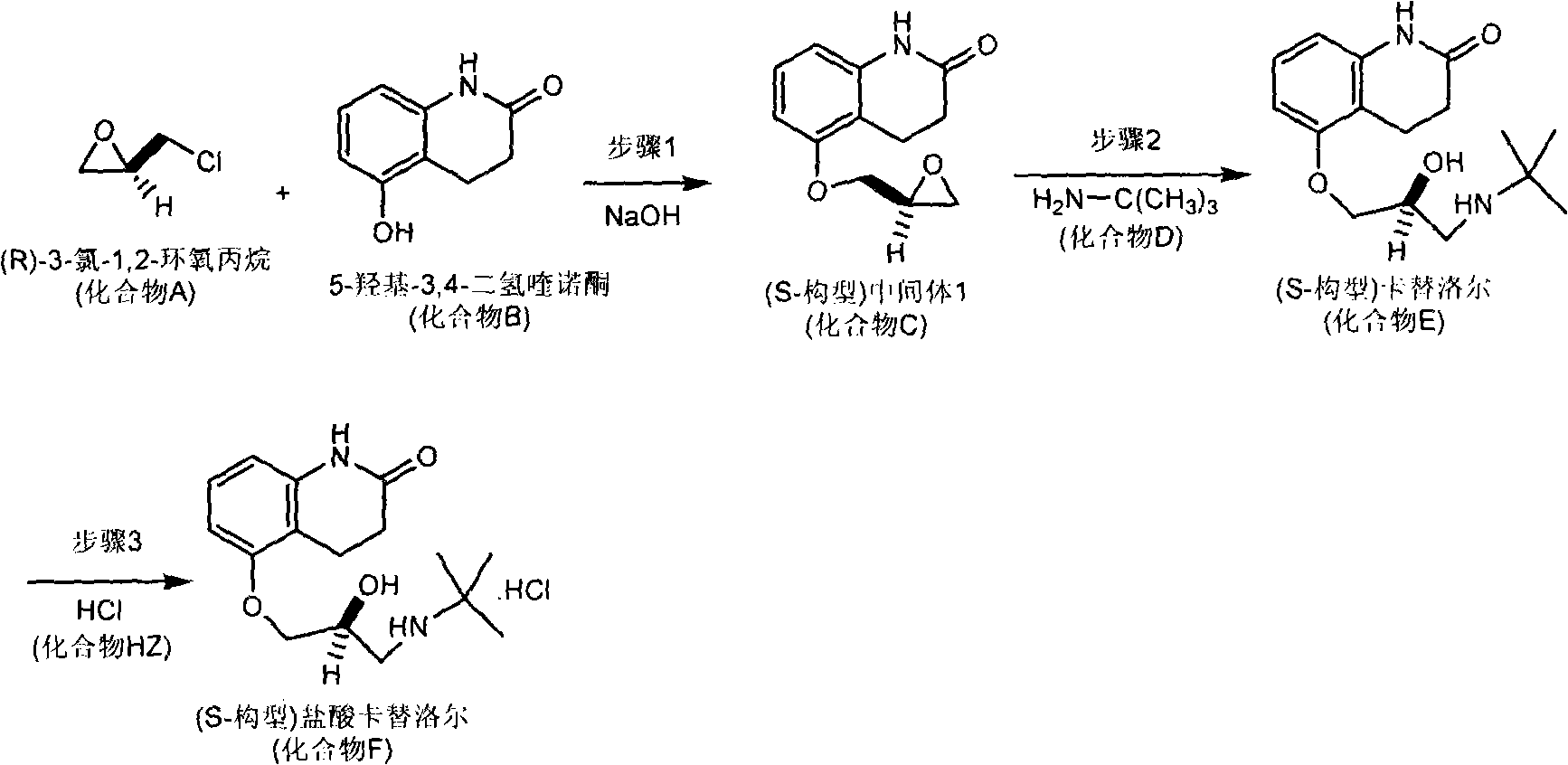
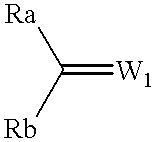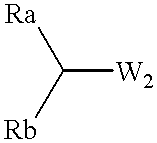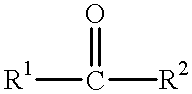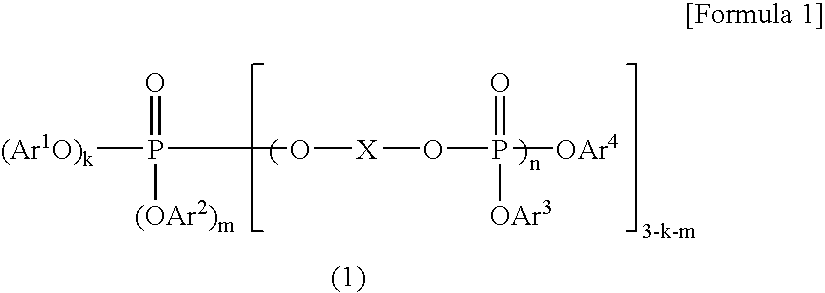Patents
Literature
1644results about How to "High optical purity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
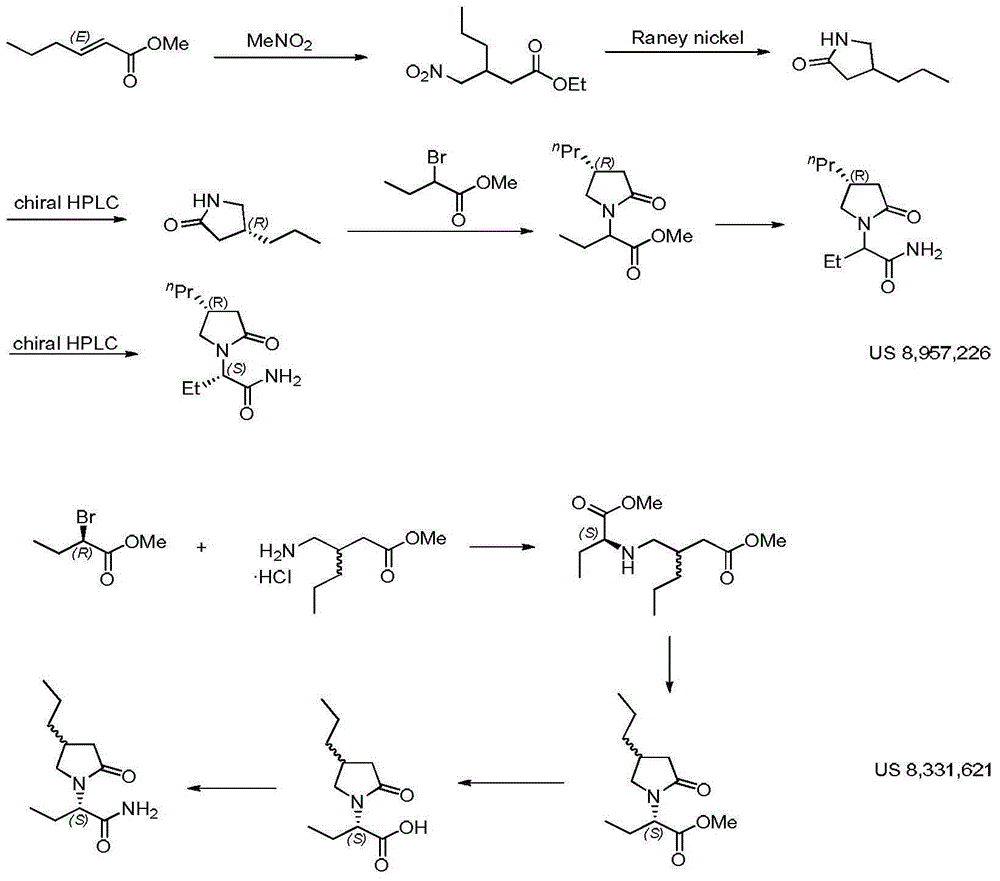
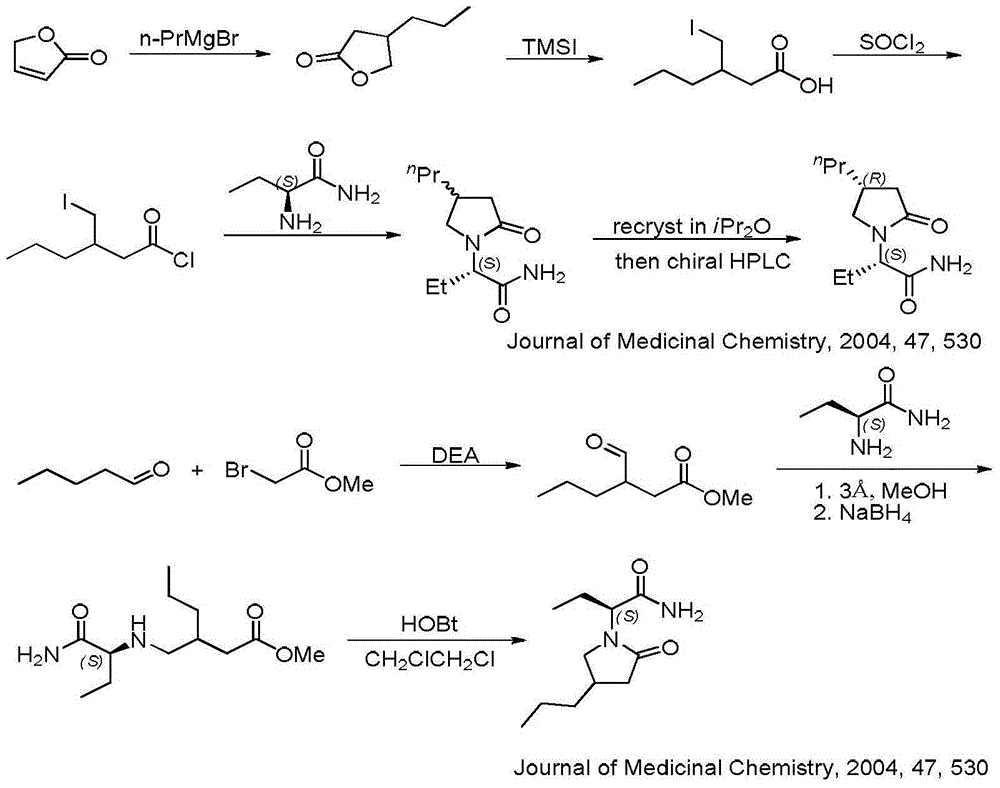
Process for preparing optically active compounds
InactiveUS6184381B1High yieldReadily produceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOptical resolutionLiquid crystal
This document describes a novel and practically excellent process for the preparation of optically active compounds, such as optically active alcohols or amines which are useful for various applications, for example, as synthetic intermediates of pharmaceuticals, liquid crystal materials, and reagents for optical resolution, wherein a hydrogen transfer type asymmetric reduction is carried out in the presence of both a transition metal complex and an optically active nitrogen compound or a transition metal complex having an optically active nitrogen compounds as an asymmetric ligand, and a hydrogen-donating organic or inorganic compound.
Owner:ASAHI KASEI PHARMA +4
Carbonyl reductases, polynucleotides comprising DNA encoding the same, methods for producing the same, and methods for producing optically active alcohol utilizing the same
Owner:DAICEL CHEM IND LTD
Resin Composition and Molded Article Comprising the Same
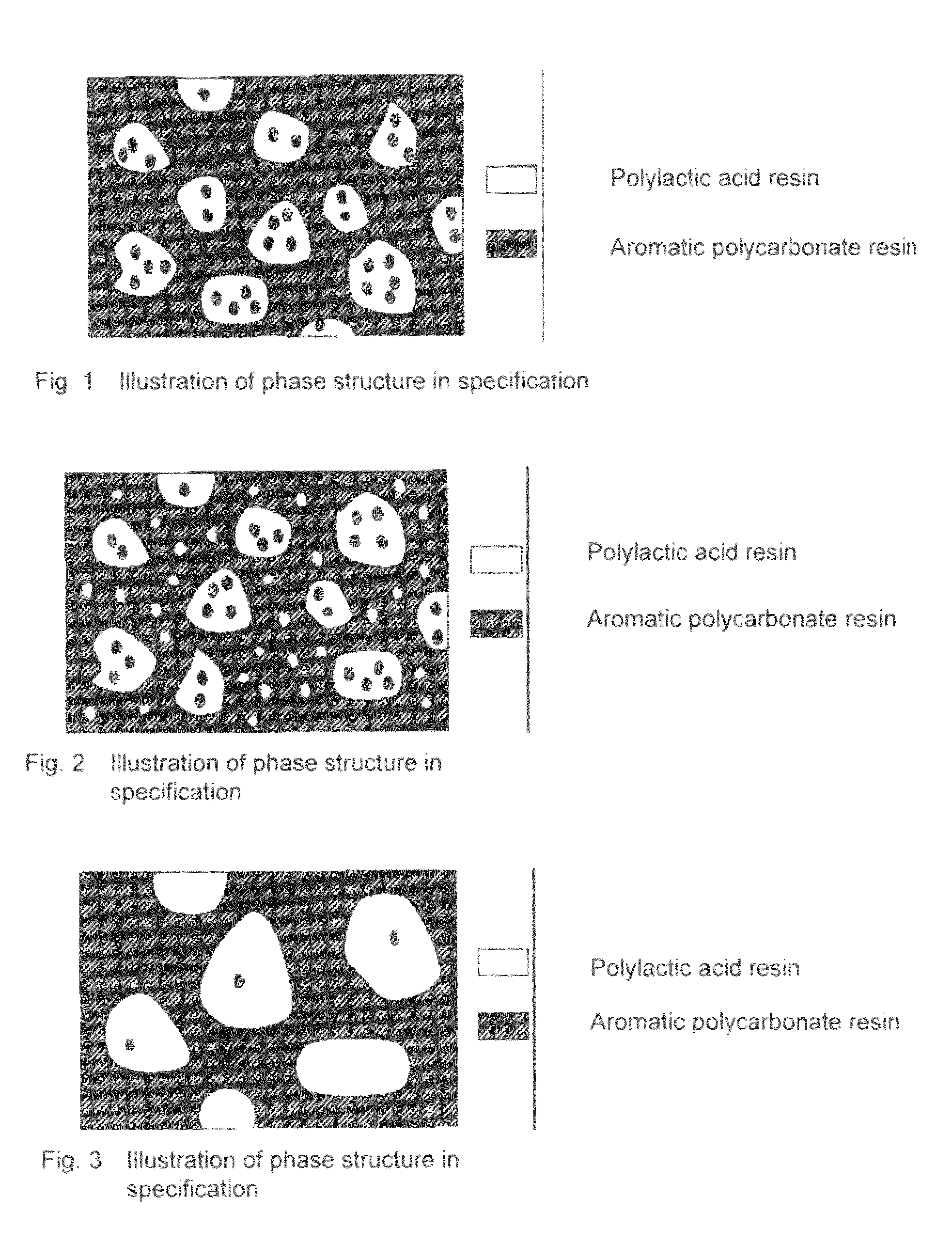
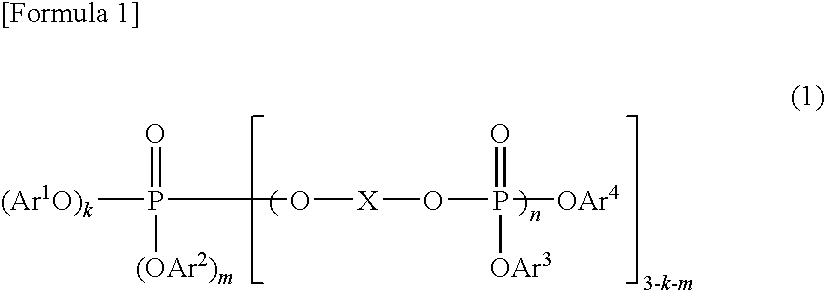
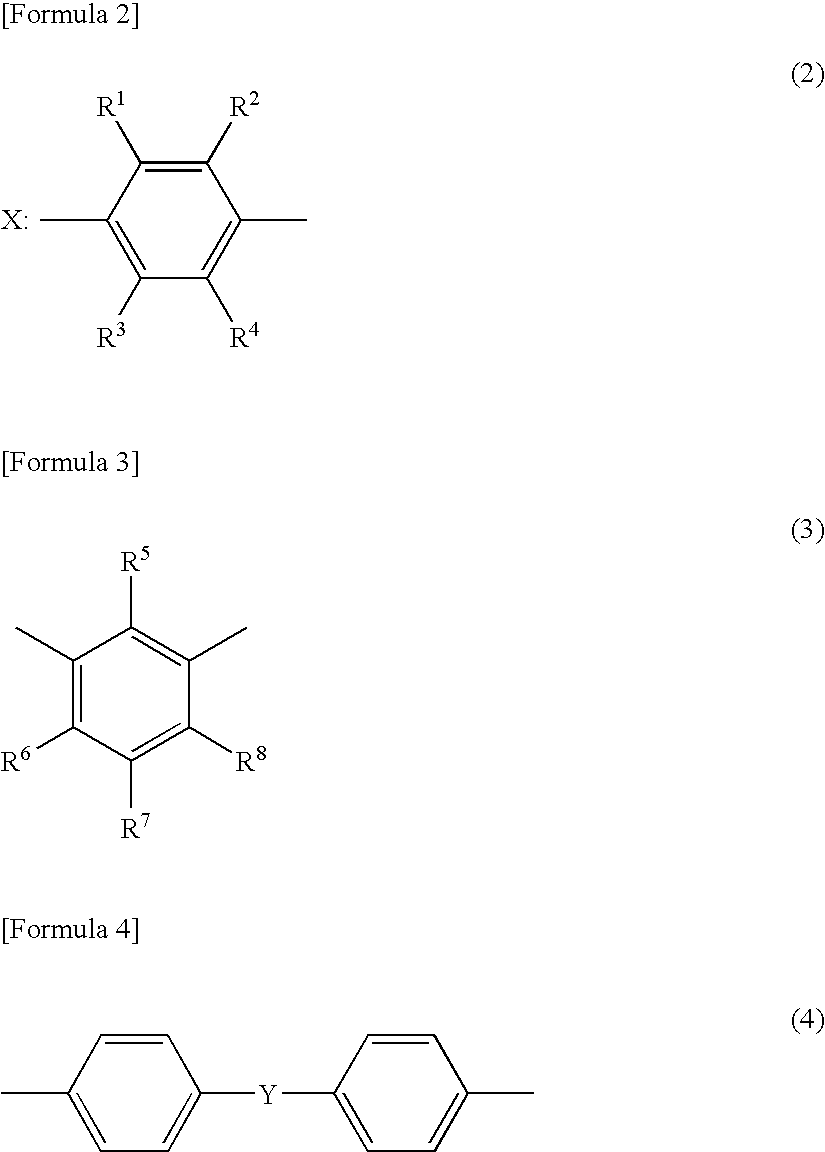
A resin composition in which a polylactic acid resin (A) 95-5 wt %, an aromatic polycarbonate resin (B) 5-95 wt %, and, with respect to 100 wt parts of the total of the (A) and the (B), at least one compatibilizer selected from a polymer compound containing an acrylic resin or styrene resin unit as a graft (C), a polymer compound to which a glycidyl compound or an acid anhydride is grafted or copolymerized (D) and an oxazoline compound, an oxazine compound and a carbodiimide compound (E) are compounded.
Owner:TORAY IND INC
Resin composition and molded article comprising the same
Owner:TORAY IND INC
Method for preparing clopidogrel and its derivatives
The present invention relates to a method for preparing Clopidogrel and its derivatives. More particularly, the present invention is a method for preparation of (S)-2-Clopidogrel and its derivatives, which are active inhibitors of platelet aggregation, from an optically active (S)-2-chlorophenyl glycine alkyl ester through hydrolysis of racemic 2-chlorophenylglycine alkyl esters using an enzyme. The present invention employs a simple procedure to prepare Clopidogrel and its derivatives. Because no chiral resolving agents are used except for a small amount of enzyme, the cost of preparation can be reduced. In addition, the present invention is suitable for synthesizing highly optical-active Clopidogrel and its derivatives on a large scale by using optically active (S)-2-chlorophenylglycine alkyl ester obtained in high yield as an intermediate, and is also environmentally friendly since no highly toxic reagents are employed.
Owner:ENZYTECH LTD
Compound and its preparation method and use in brivaracetam synthesis
ActiveCN106279074ARaw materials are easy to getLow priceOrganic chemistrySynthesis methodsCombinatorial chemistry
The invention provides a compound shown in the formula I and a preparation method thereof. The invention also provides a use of the compound shown in the formula I in brivaracetam synthesis and a synthesis method of brivaracetam. The synthesis method utilizes cheap and easily available raw materials and can produce high optical purity brivaracetam.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
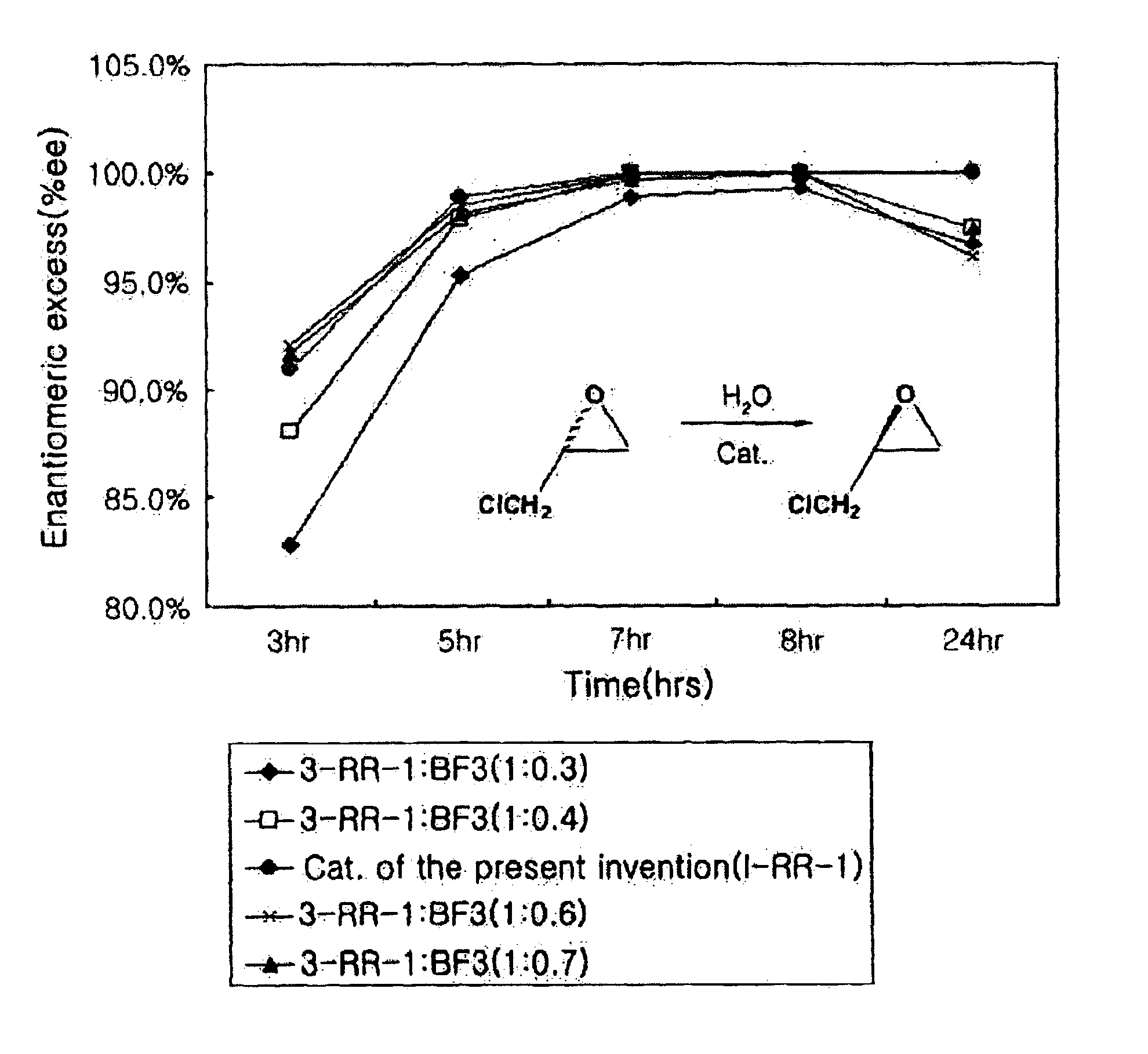
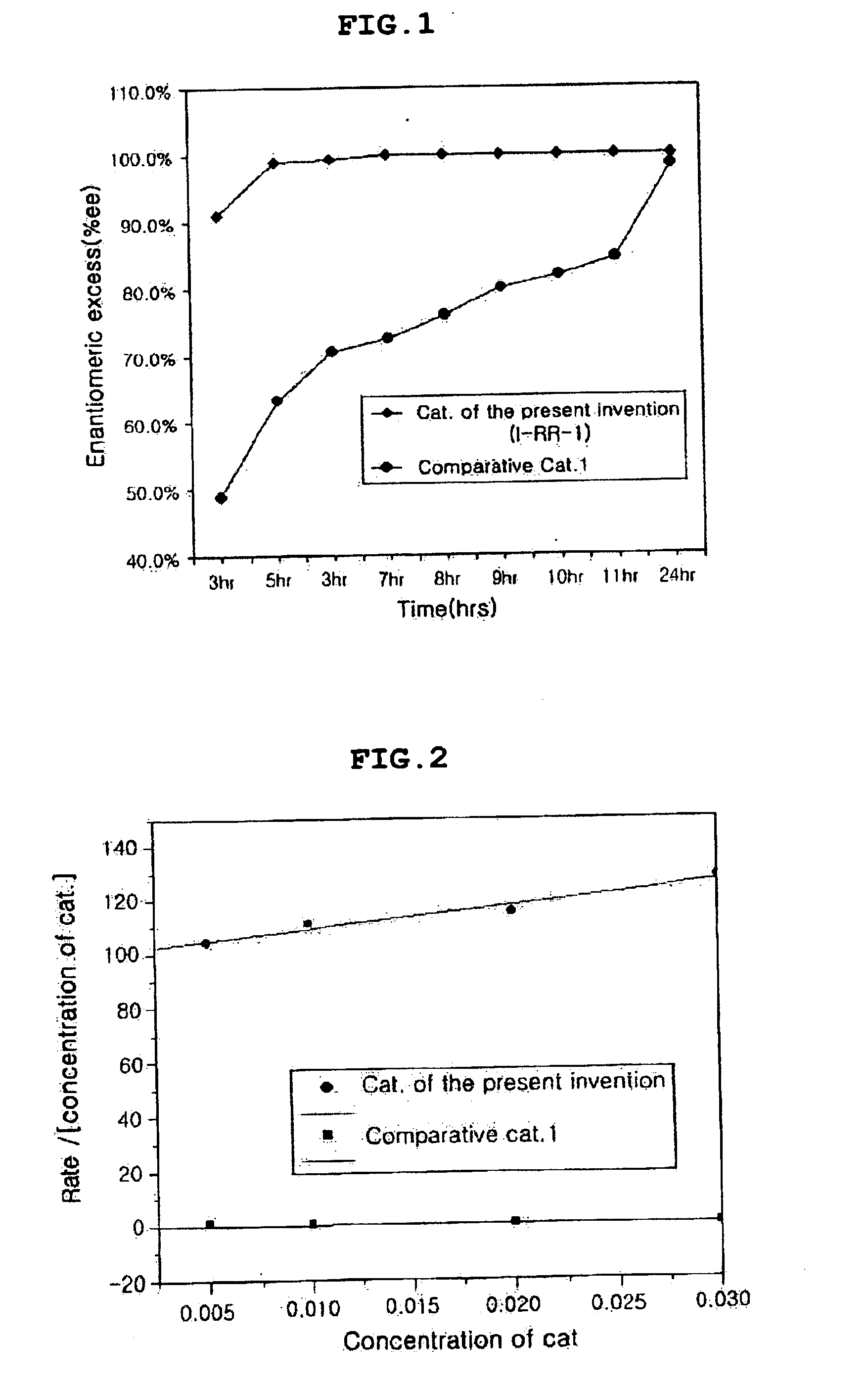
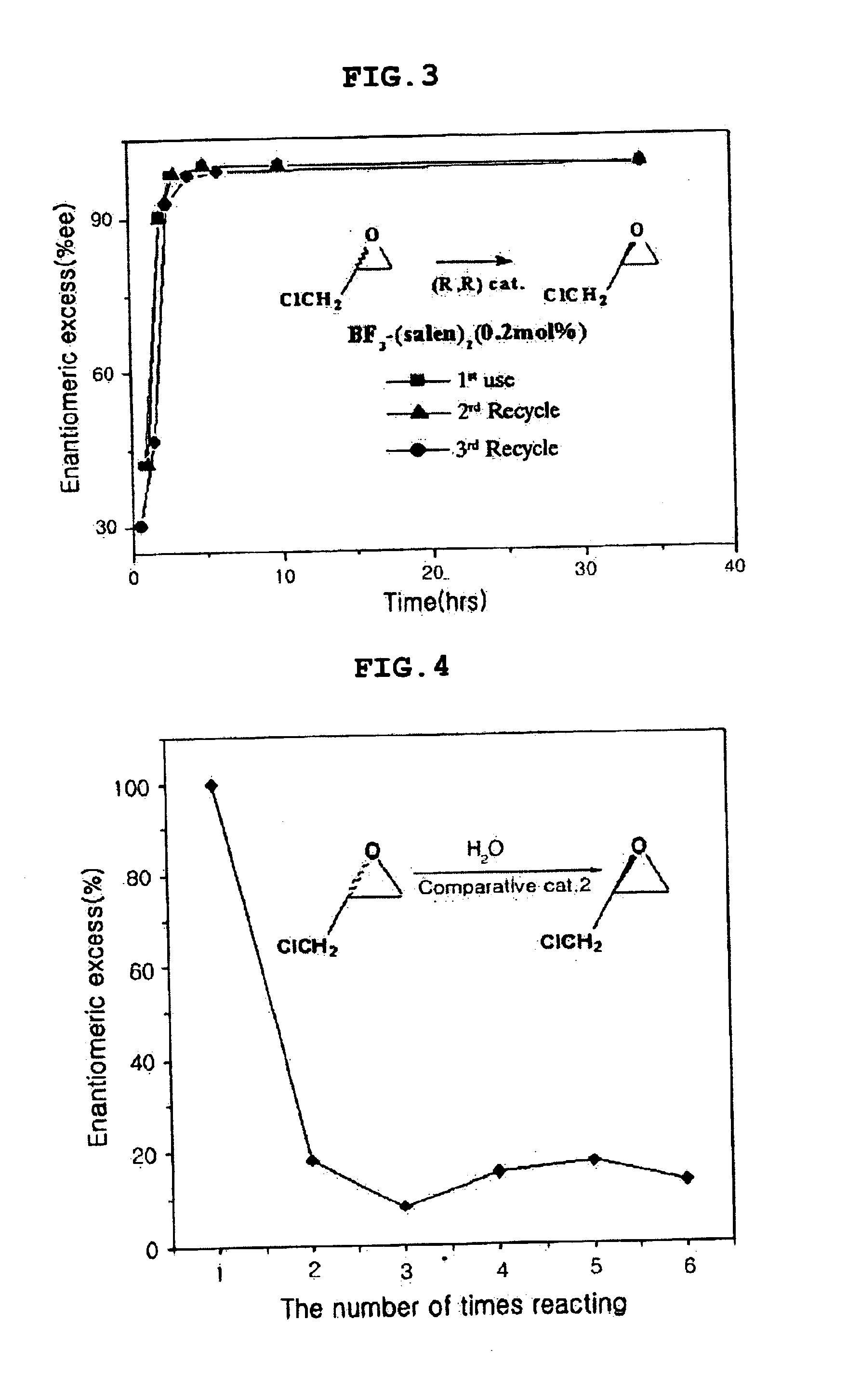
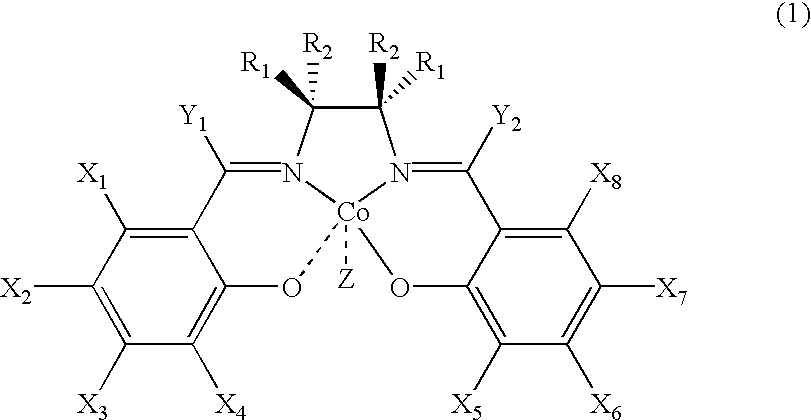
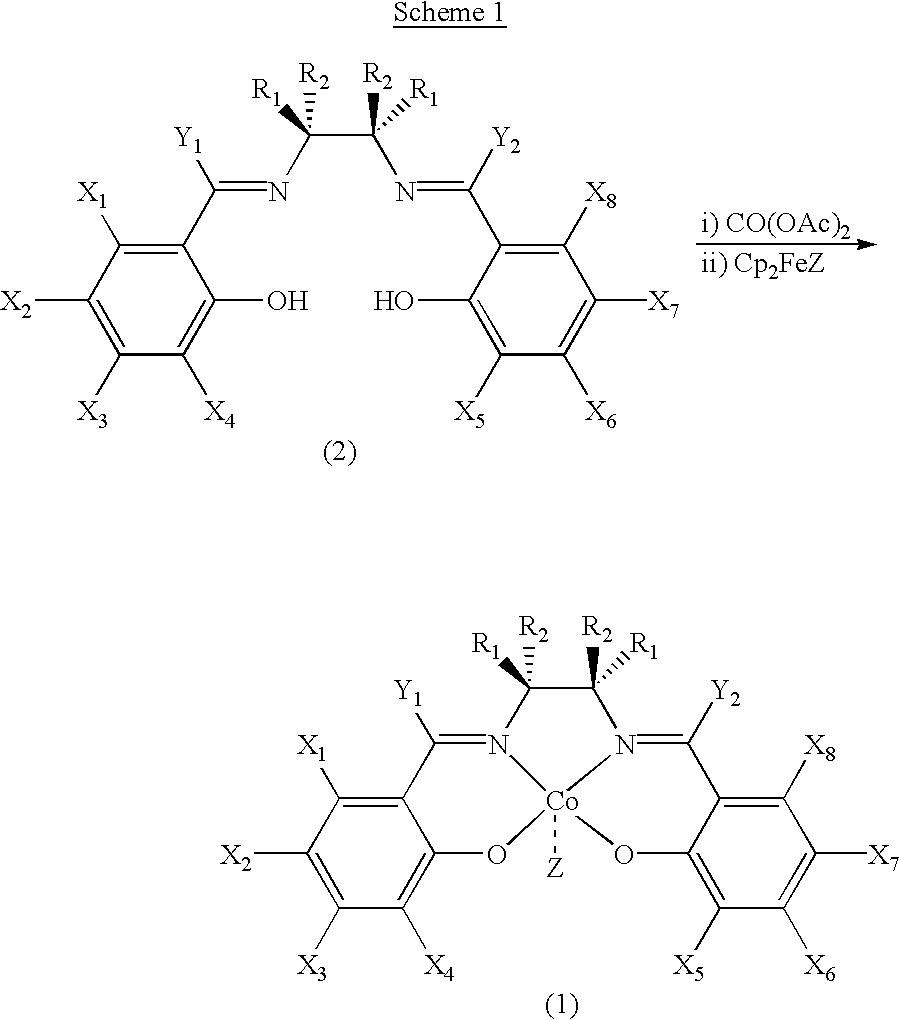
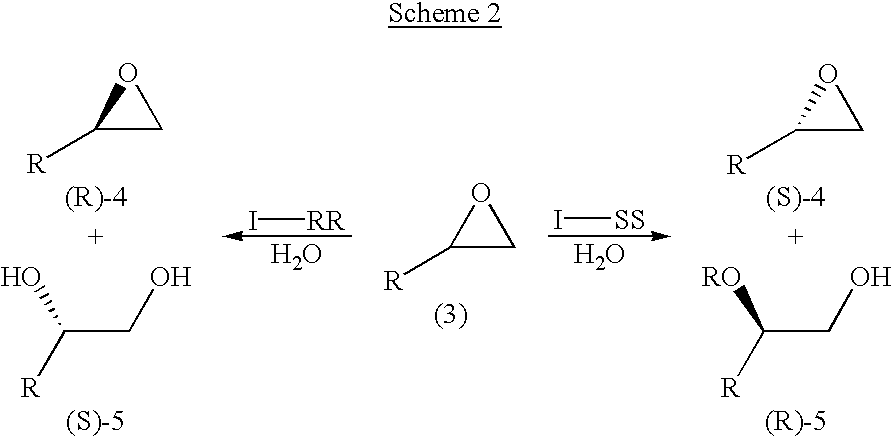
Chiral salen catalyst and methods for the preparation of chiral compounds from racemic epoxides by using new catalyst
InactiveUS6884750B2High optical purityHigh activityOrganic-compounds/hydrides/coordination-complexes catalystsCobalt organic compoundsFood additiveEpoxide
The present invention relates to new chiral salen catalysts and methods for the preparation of chiral compounds from racemic epoxides by using new catalyst. More particularly, the present invention is to provide novel chiral salen catalysts and their uses for producing chiral compounds having high optical purity to be used as raw materials for preparing chiral medicines or food additives in a large scale economically, wherein the chirl salen catalyst having a particular molecules structure can be reused continuously without any activating process of used catalysts and cause no or little racemization after the reaction is completed because it maintains its catalytic activity after the reaction process.
Owner:RS TECH CORP
Resin Composition and Molded Article Composed of the Same
ActiveUS20090030132A1Excellent in flowabilityImprove heat resistanceSpecial tyresOrganic dyesShell moldingGlass transition
A resin composition comprising a polylactic acid-based resin (A) and methacrylic resins (B), wherein the methacrylic resins having at least (a) a difference of 10° C. or more in glass transition temperature or (b) a difference of 3% or more in syndiotacticity; it is preferred that at least one of the methacrylic resins (B) is a methacrylic resin having a weight average molecular weight of 50,000 to 450,000, a glass transition temperature of 110° C. or higher and a syndiotacticity of 40% or more, and that the resin composition further contains a multilayer structure polymer formed as particles each consisting of a core layer and one or more shell layers covering it (C) A molded article made of said resin composition.
Owner:TORAY IND INC
Carbonyl reductase, gene and mutant and application thereof to asymmetrical reduced carbonyl compound
ActiveCN102618513AHigh optical purityMild reaction conditionsBacteriaMicroorganism based processesHigh concentrationMethyl o-chloromandelate
The invention discloses a novel carbonyl reductase, a gene, a mutant thereof, a recombinant expression vector containing the gene and the mutant, a recombinant expression transformant, a recombinase preparation method, and applications of the carbonyl reductase and recombinase to preparation of active chiral alcohols with a chiral carbonyl compound before asymmetrical reduction. The carbonyl reductase is derived from candida glabrata, is applied to preparation of a plurality of optically-active chiral alcohols such as (R)-chloromandelic acid methyl ester, (R)-2-hydroxy-4-phenyl ethyl butyrate, (R)-4-chlorin-3-phenyl ethyl butyrate and the like. Compared with other preparation methods, a product prepared through the method has high concentration, does not require additionally or slightly adding any expensive coenzyme, has high optical purity, and has the advantages of mild reaction conditions, easiness and convenience for operating, easiness for amplifying and the like, and has a good industrial application prospect in the production of clopidogrel, L-carnitine and perindopril antihypertensive medicinal intermediates.
Owner:EAST CHINA UNIV OF SCI & TECH
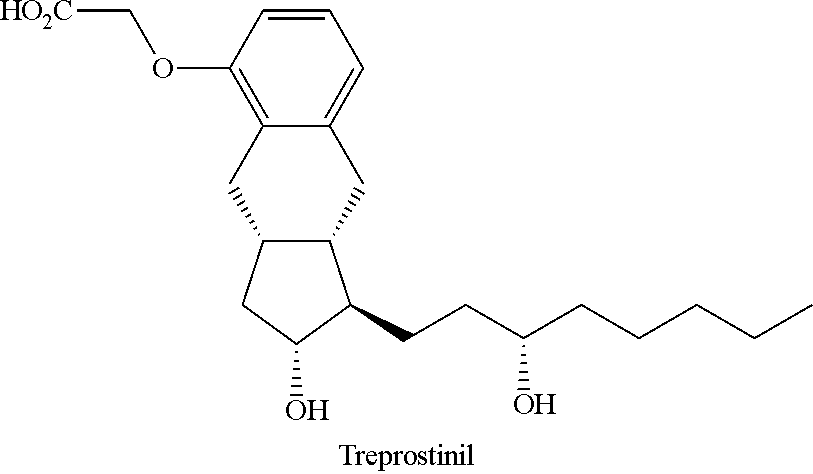
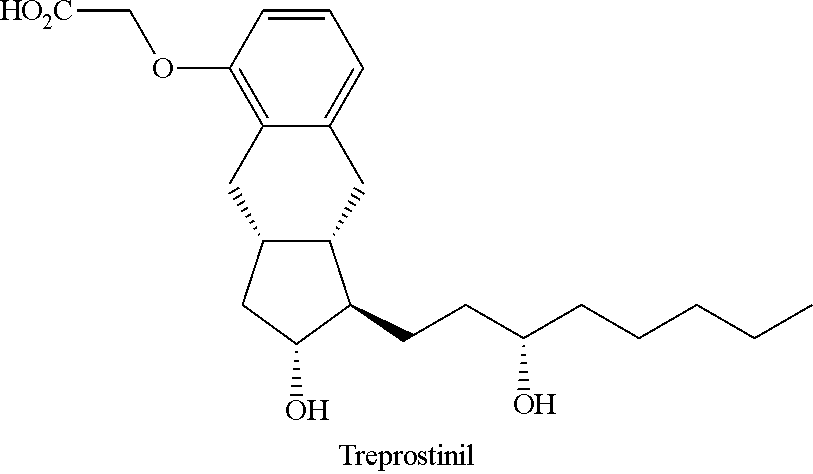
Novel intermediate for synthesizing treprostinil diethanolamine and method for preparing the same
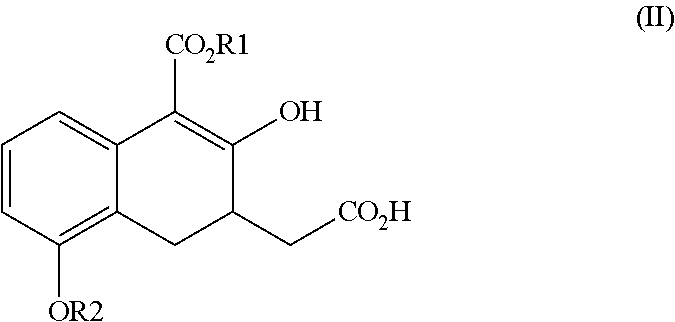
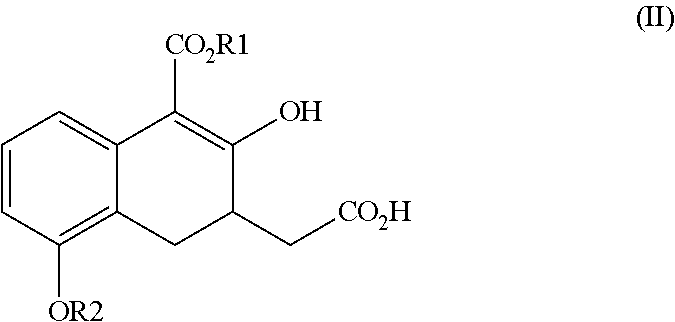
ActiveUS20160152548A1High optical purityHigh purityOrganic compound preparationPreparation by ester-hydroxy reactionCombinatorial chemistryEthanolamine synthesis
The present invention relates to a method for treprostinil diethanolamine synthesis. The present invention also relates to a novel intermediate used in the method for treprostinil diethanolamine synthesis. The novel intermediate is shown in the following formula (II):wherein R1 and R2 are described in the description.
Owner:EVERLIGHT CHEMICAL INDUSTRIAL CORPORATION
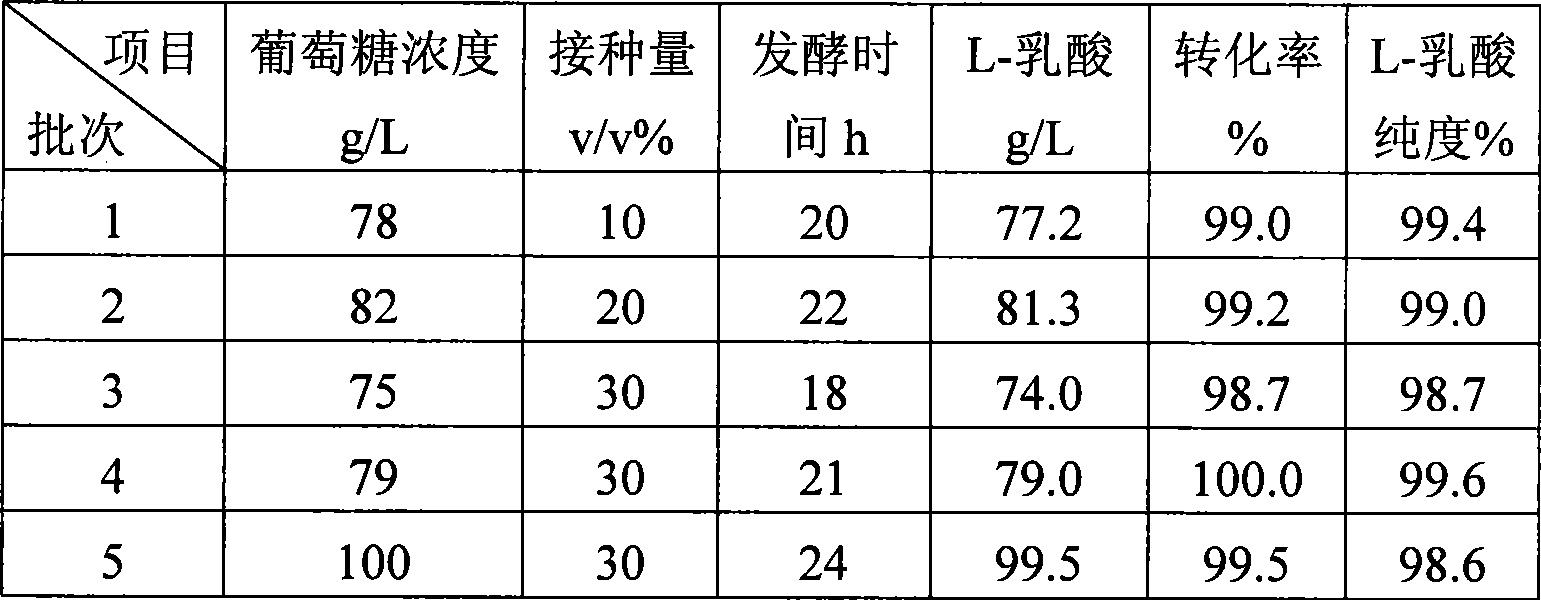
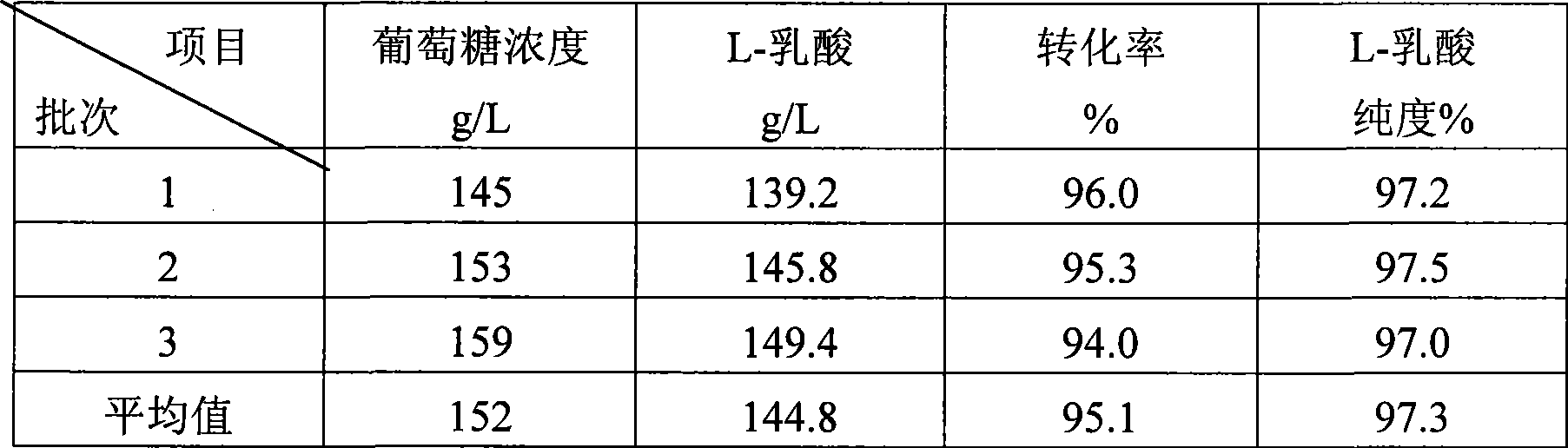
Method for producing L-lactic acid by Bacillus coagulans CGMCC No.2602
ActiveCN101544993AReduce fermentation costsSimple nutritional requirementsMicroorganism based processesFermentationMicroorganismGlucose polymers
A method for producing L-lactic acid by Bacillus coagulans CGMCC No.2602 belongs to the technical field of microorganisms. In the invention, Bacillus coagulans CGMCC No.2602 is adopted, and under the condition of no oxygen supply, starchiness hydrolyzed sugar or dextrose is fermented by a semicontinuous intermittent fermentation way or an inter-sugar-compensating fermentation way to generate L-lactic acid with high optical purity. The invention has the advantages that the gemma property of the Bacillus coagulans is stable, and the L-lactic acid obtained by inoculating and fermenting the starchiness hydrolyzed sugar has high optical purity and rate of conversion of sugar and acid and short fermentation period; in addition, the semicontinuous intermittent fermentation way saves the time for preparing seeds by a continuous reladling and subculturing method, shortens the fermentation period, enhances the fermentation strength and obtains the L-lactic acid with both relatively high rate of conversion of sugar and acid and purity.
Owner:江苏省苏微微生物研究有限公司
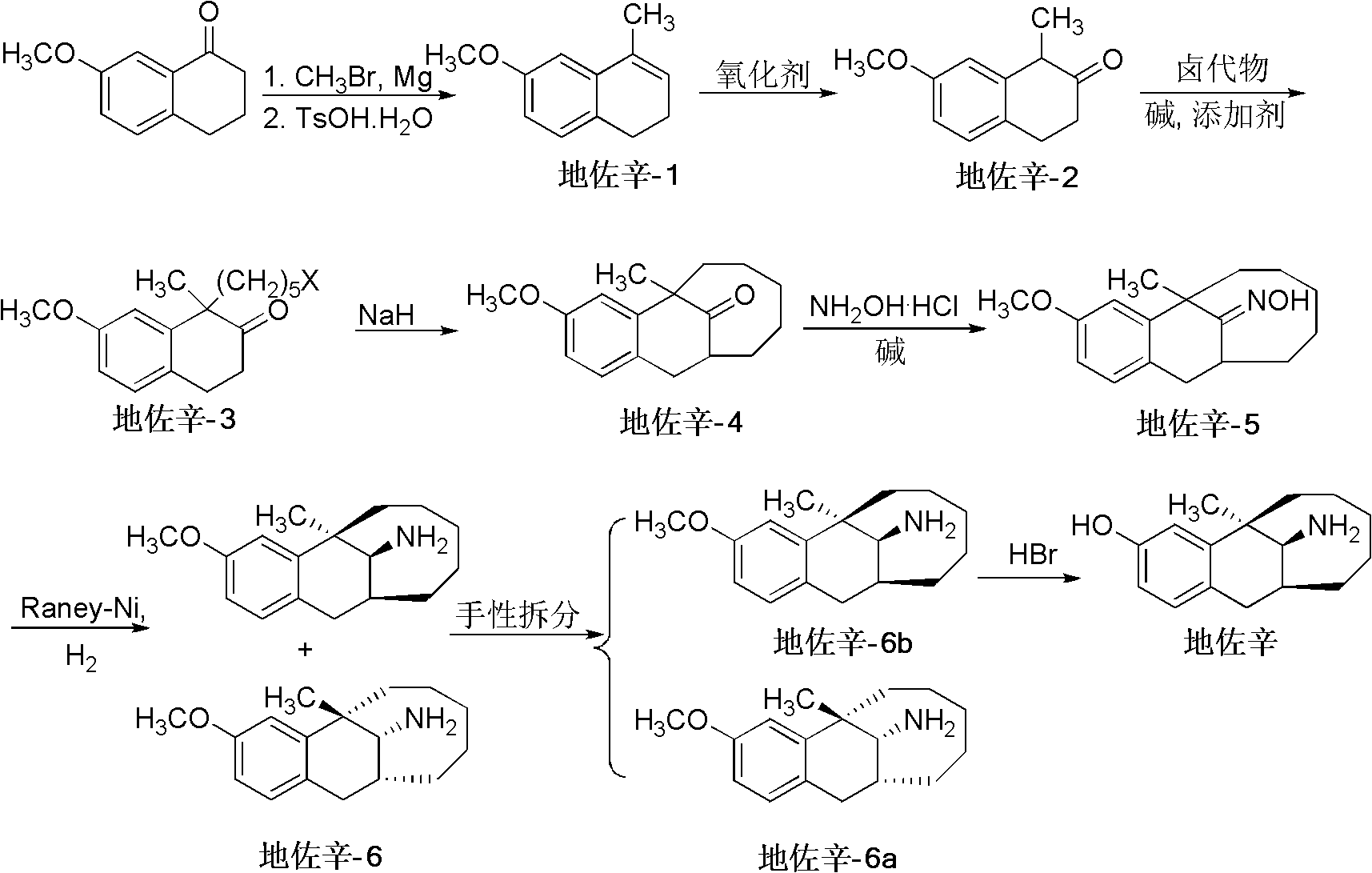
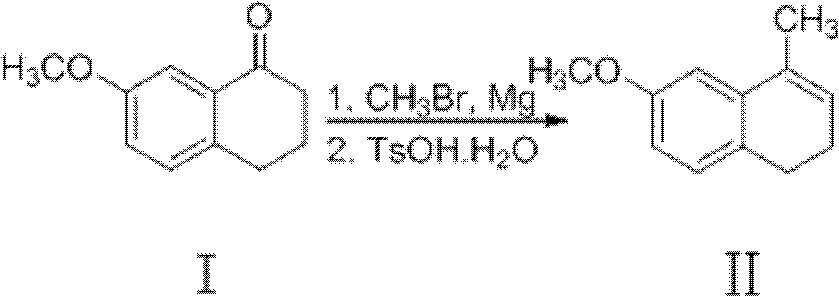
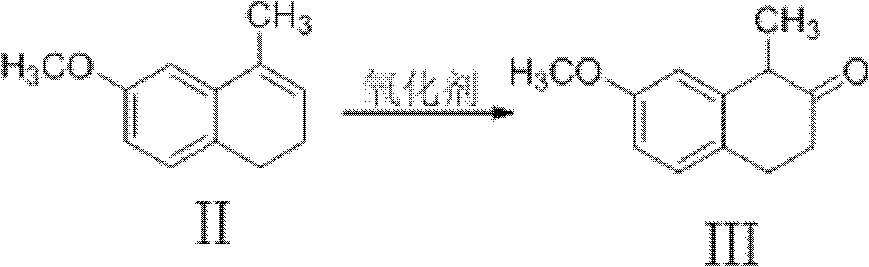
Preparation method of dezocine
ActiveCN102503840ASimple post-processingHigh optical purityOrganic compound preparationAmino-hyroxy compound preparationOrganic acidAlcohol
The invention provides a preparation method of dezocine, which has the following advantages: (1) when an intermediate which is dezocine-1 is used as a raw material to prepare another intermediate which is dezocine-2, the obtained oxidants are acid peroxide, alcohol peroxide or hydrogen peroxide which have low cost and are easy to obtain and post-process; (2) when the intermediate which is dezocine-2 is used as a raw material to prepare an intermediate which is dezocine-3, the obtained bases are bases which have low cost and are easy to obtain; (3) when an intermediate which is dezocine-6 is used as a raw material to prepare an intermediate which is dezocine-6b, an optically pure chiral organic acid and an racemic intermediate which is the dezocine-6 react for generate a pair of diastereomeric salts, and then the pair of diastereomeric salts are separated through recrystallization to obtain the intermediate which is the dezocine-6 with high optical purity; and (4), the operation is simple and efficient, the reaction condition is mild, and the used reagents are cheap, readily available, highly safe and suitable for industrial mass production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP +1
Method for preparing (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol by utilizing microbial catalysis
ActiveCN102559520AHigh excessLess side effectsFungiMicroorganism based processesPhosphateReaction temperature
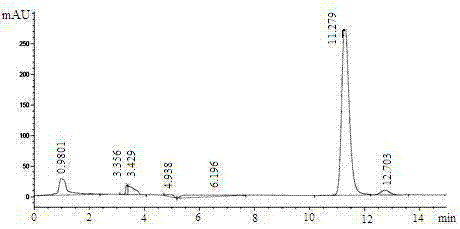
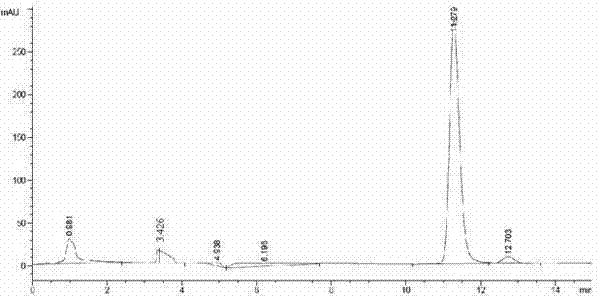
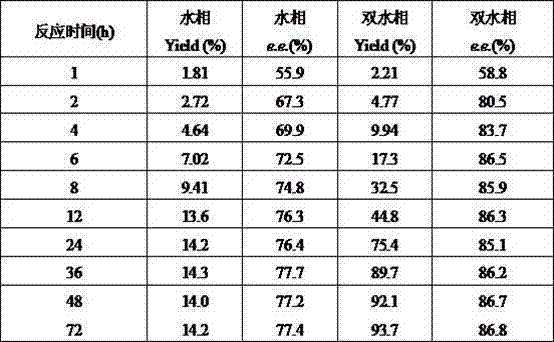
The invention relates to a method for preparing (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol by utilizing microbial catalysis, and belongs to the technical field of biological catalysis. According to the method, 4-chlorphenyl-(pyridine-2-yl)-ketone is subjected to asymmetrical reduction by utilizing kluyveromycessp (CCTCCM 2011385) whole cells to synthesize the (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol. The method comprises the following steps of: sieving a microbe which has high-stereoselectivity carbonyl reductase activity on a prochiral ketone substrate, determining a series of conditions of asymmetrical reduction reaction, such as reaction temperature, pH, the concentration of cells, the concentration of the substrate and reaction time and additives (including various secondary solvents, polyethyleneglycol (PEG), phosphates and the like), wherein the enantiomer excess value and yield of the (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol serving as a product can reach 86.7 percent e.e and 92.1. The product is separated and extracted initially by silicagel column chromatography, so that the purity of the separated product is 99.2 percent, and the extraction yield is 56.7 percent.
Owner:JIANGNAN UNIV
Asymmetric synthesis method of (+)-tanshinol
InactiveCN102924265ARaw materials are easy to getSimple and fast operationOrganic compound preparationCarboxylic compound preparationPropanoic acidCombinatorial chemistry
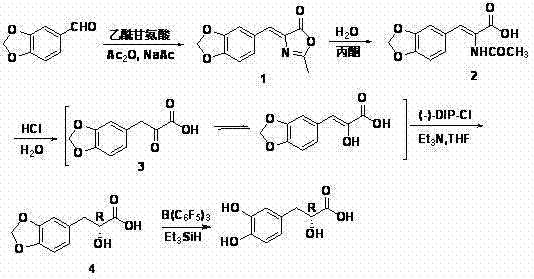
The invention relates to an asymmetric synthesis method of (+)-tanshinol, which comprises the following steps: carrying out Knoevenagel condensation on the raw material heliotropin, hydrolyzing for ring opening to obtain beta-(3,4-3,4-methylenedioxyphenyl)pyruvic acid, carrying out key asymmetric reduction reaction to obtain R-beta-(3,4-dibenzyloxyphenyl)-alpha-hydracrylic acid, and finally, deprotecting to obtain the (+)-tanshinol. The method has the advantages of accessible raw material and high optical purity of the product, is simple to operate, and can implement large-scale preparation.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method of brivaracetam
ActiveCN106432030ARaw materials are easy to getLow priceOrganic chemistryBrivaracetamColumn chromatography
The invention provides a method for synthesizing brivaracetam. Raw materials used in the method are available and inexpensive, a preparation process avoids appearance of chiral isomers difficult to separate, purification means such as column chromatography purification and the like adverse to industrial amplification production is avoided, a synthesis process is not involved with expensive and toxic heavy metal and chiral ligands, and a product brivaracetam with high quality and high optical purity can be obtained.
Owner:SUZHOU PENGXU PHARM TECH CO LTD +1
Preparation method for optical activity active 3-amino butanol and optical activity 3-amino butyric acid
ActiveCN104370755ANon-hazardousImprove conversion rateOrganic compound preparationAmino-carboxyl compound preparationSolventHydrolysis
The present invention discloses a preparation method for optical activity active 3-amino butanol and optical activity 3-amino butyric acid. The optical activity active 3-amino butanol preparation method comprises: in a solvent, under effects of a hydroboration reduction agent and a Lewis acid, carrying out a reduction reaction on a compound represented by a formlu 65 to produce a compound represented by a formlu 14. The optical activity active 3-amino butyric acid preparation method comprises: carrying out a hydrolysis reaction on a compound represented by a formlu 64 to produce a compound represented by a formlu 65. According to the present invention, the preparation method has characteristics of cheap and easily-available raw materials, simple operation, short process route, no hazard of raw materials, high yield, little waste production, environment protection, high raw material conversion rate, high product chemical purity and high product optical purity, and the industrialization is easily achieved. The formulas 64, 65 and 14 are defined in the instruction.
Owner:JIANGXI LONGLIFE BIO PHARM CO LTD +1
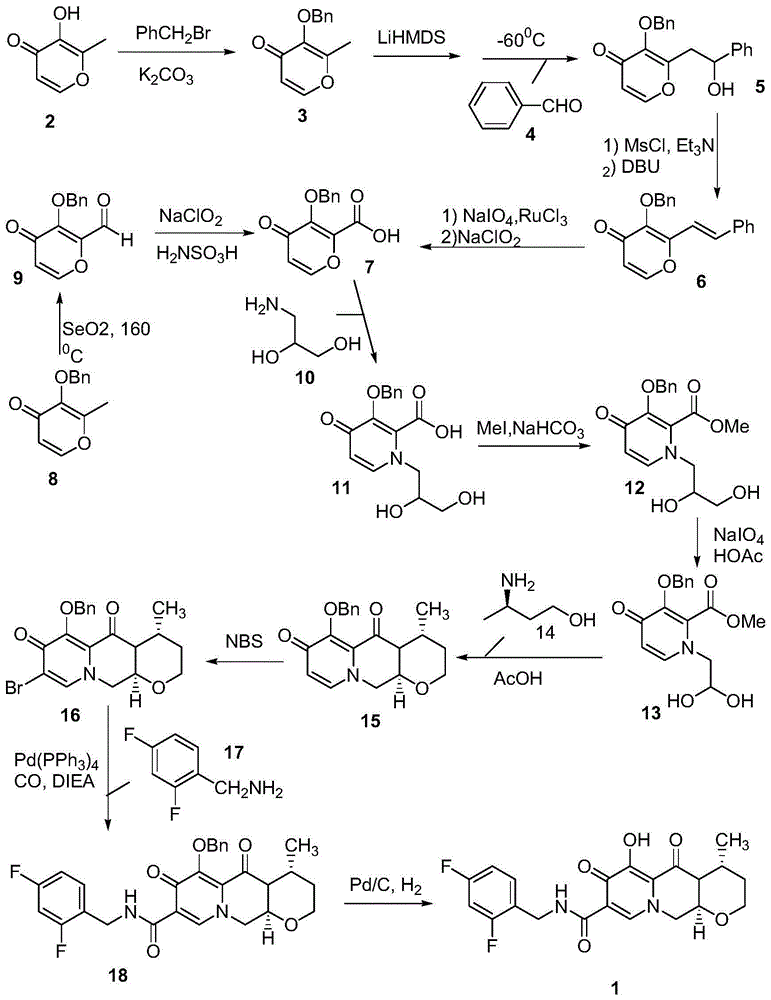
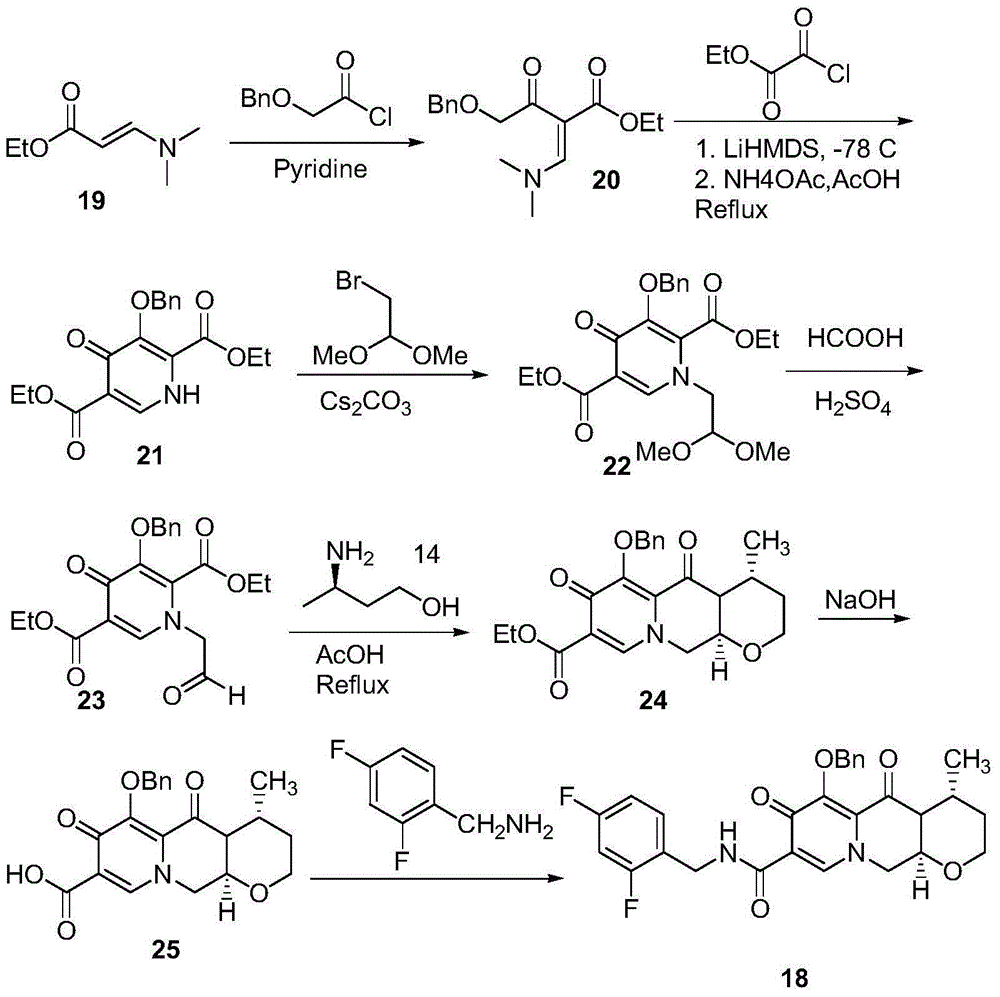
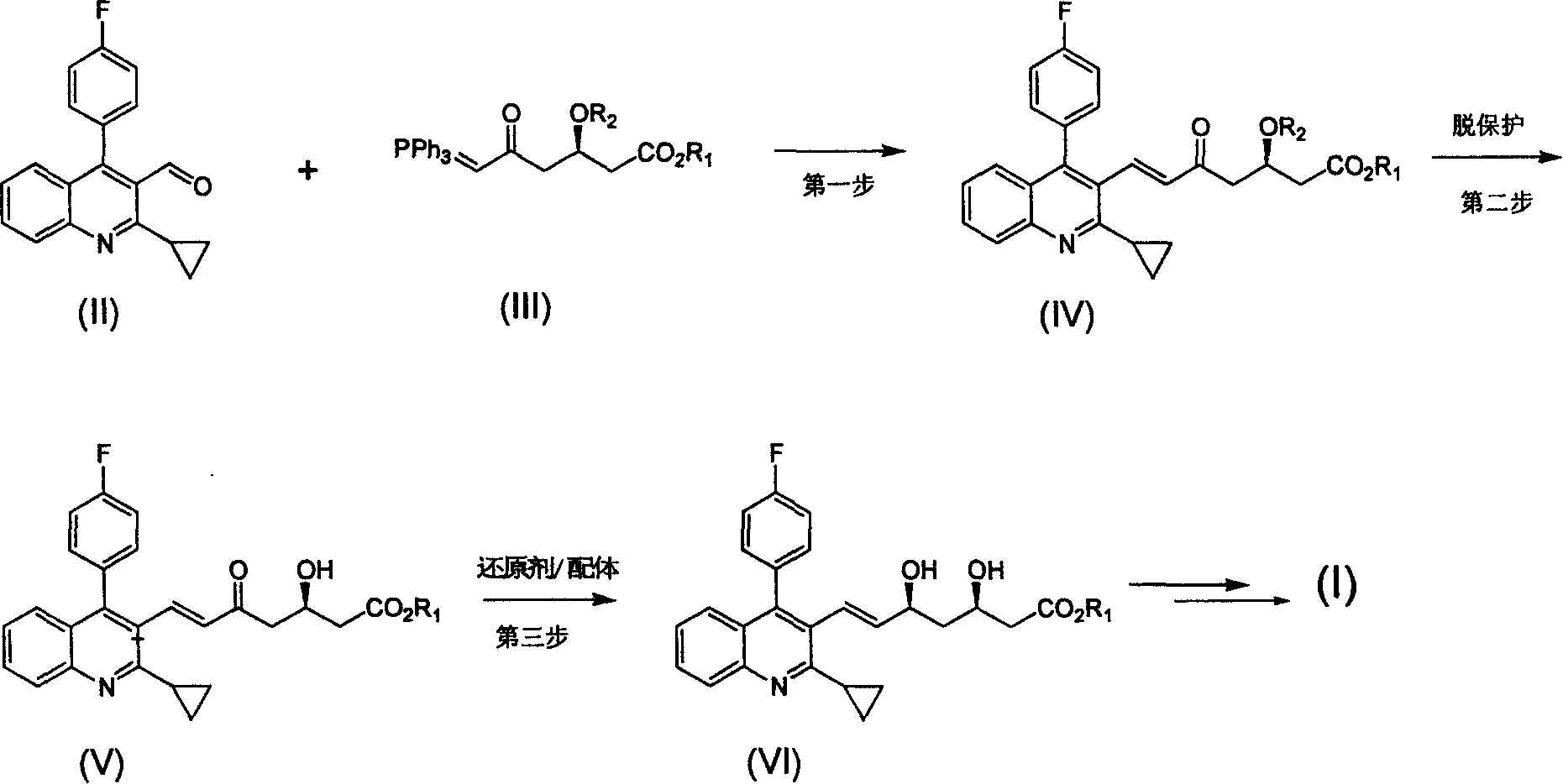
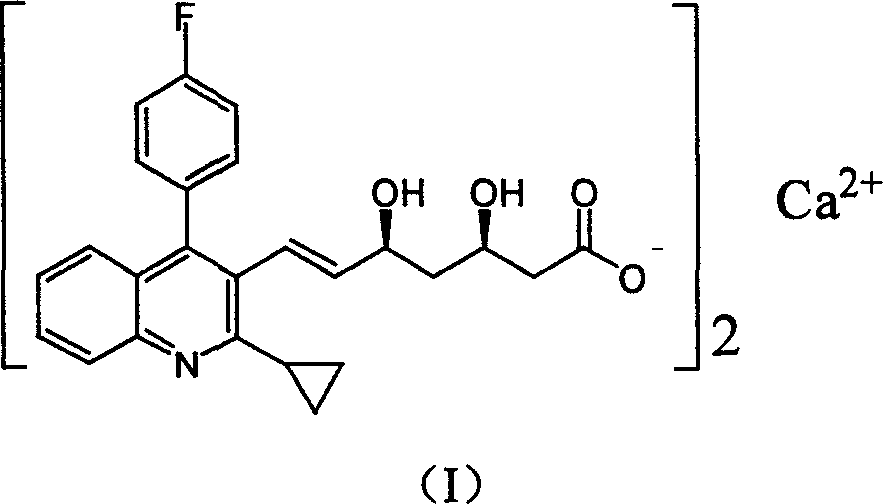
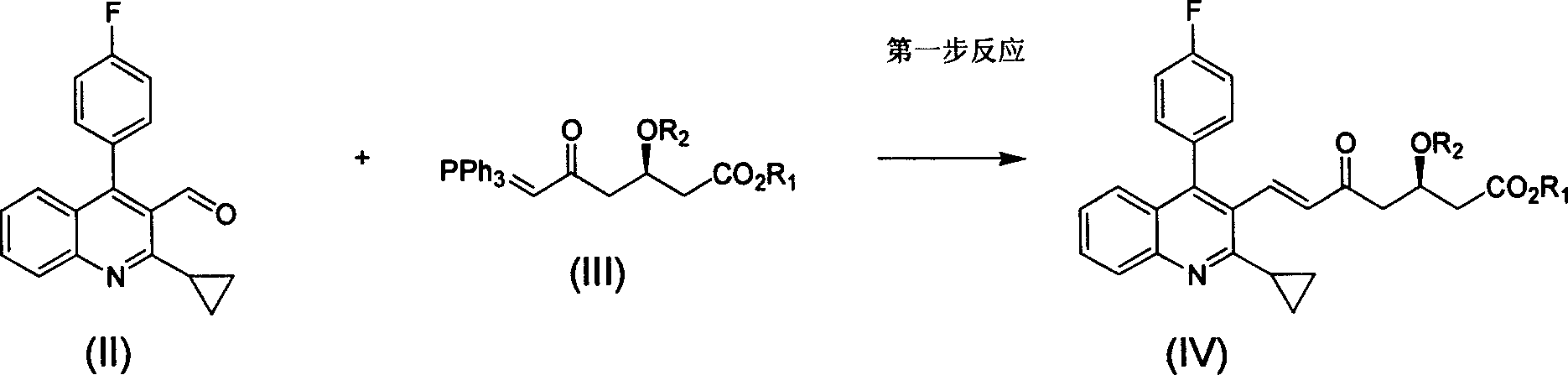
Method for preparing high optical purity pitavastatin calcium raw material drug
The invention relates the method of preparing the raw material of high optical purity pravastatin calcium. The method comprises the following steps: adding the 2- cyclopropyl-4-(4- fluorophenyl)-3-quinoline aldehyde II and (3R)-3- alkoxy silane-5- carbonyl-6- triphenyl phosphor heptene acid ester III in dissolvent, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)-3- alkoxy silane-6- triphenyl phosphor heptene acid ester IV, removing the protection of IV, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)- hydroxyl -6- triphenyl phosphor heptene acid ester V, deacidizing it in the mixture dissolvent of alcohol and ether with NaBH4 or KBH4 at -100-0Deg.C, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-(3R, 5S)- dihydroxy -6- triphenyl phosphor heptene acid ester VI, hydrolyzing it with alkali, and getting pravastatin calcium. The material is used to prepare HMG-CoA reductase inhibiting agent.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Method for catalyzing dynamic kinetic resolution of arylamine via racemization catalyst
InactiveCN102533922AGood stability for repeated useMild reaction conditionsOrganic chemistry methodsChemical recyclingChlorobenzenePtru catalyst
The invention discloses a method for catalyzing dynamic kinetic resolution of arylamine via a racemization catalyst, comprising the following steps of: 1) adding p-chlorophenol, n-pentanoic acid, dicyclohexylcarbodiimide and 4-dimethylamino-pyridine, and carrying out mixing, filtration, drying, concentration and column chromatography to obtain a pentanoic acid p-chlorophenyl ester acyl donor; 2) carrying out coprecipitation on magnesium chloride solution and aluminum chloride solution and carrying out water-heat treatment to obtain chloridion intercalated hydrotalcite, adding the chloridion intercalated hydrotalcite in lauryl sodium sulfate aqueous solution, and carrying out backflow, cooling, centrifugation, water washing, acetone washing and drying to obtain a carrier; 3) adding palladium salt and the carrier, and carrying out heating, ascorbic acid addition, centrifugation, water washing, acetone washing and freeze-drying to obtain the racemization catalyst; and 4) adding arylamine, the acyl donor, lipase and the racemization catalyst in toluene and placing in a stainless steel reactor to add hydrogen so as to obtain amide. The method provided by the invention is used for catalyzing the dynamic kinetic resolution of arylamine, has rapid reaction rate, low temperature, high conversion rate and high product optical purity, and has great application value.
Owner:ZHEJIANG UNIV
Method for producing substituted polycyclic pyridone derivative and crystal of same
ActiveCN109311911AHigh optical purityEfficient manufacturingOrganic active ingredientsOrganic chemistry methodsPerylene derivativesCombinatorial chemistry
Provided is a method for producing a substituted polycyclic pyridone derivative. A method for producing a compound represented by formula (II), which is characterized in that a compound represented byformula (I) is reacted with a compound represented by formula R2-OH (wherein R2 represents an unsubstituted alkyl group) in the presence of a sodium salt and / or a magnesium salt. (In formula (I), R1represents a hydrogen atom or a protecting group other than an unsubstituted alkyl group.) (In formula (II), R2 is as defined above.)
Owner:SHIONOGI & CO LTD
Carbonyl reductase, gene and applications of carbonyl reductase in asymmetric reduction of prochiral carbonyl compound
ActiveCN102876734AIncrease concentrationHigh optical purityMicroorganism based processesOxidoreductasesCarbonyl groupMutant
The invention discloses applications of carbonyl reductase being as a catalyst in the preparation of chiral alcohol by asymmetric reduction of a prochiral carbonyl compound, and a gene, protein and a mutant of the carbonyl reductase. The invention also provides a recombinant expression vector and a recombinant expression transformant containing the gene of the carbonyl reductase. When the carbonyl reductase disclosed by the invention is utilized for catalyzing the asymmetric reduction reaction of the prochiral carbonyl compound, the reaction conditions of the asymmetric reduction are moderate, the operation process is simple and convenient, and the amplification is easy; and the chiral alcohol product prepared by the asymmetric reduction reaction is high in concentration and optical purity. Therefore, the carbonyl reductase has a good industrial application and development prospect in the preparation of the optically pure chiral alcohol.
Owner:EAST CHINA UNIV OF SCI & TECH
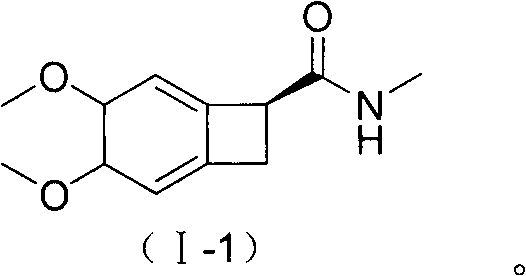
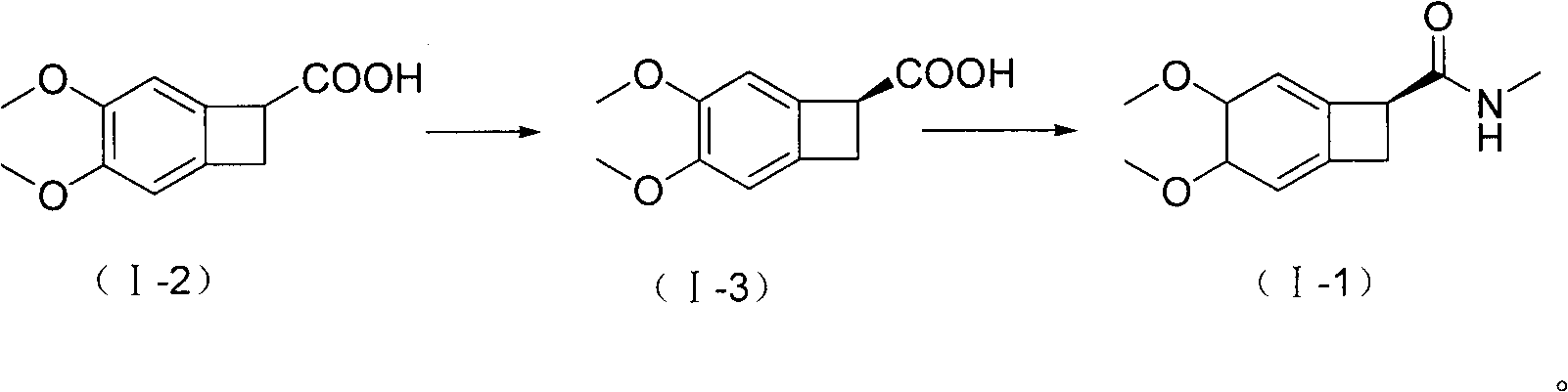
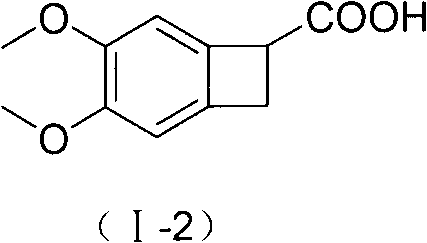
New benzocyclobutane, preparation method thereof and application thereof
InactiveCN101671265AEasy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationIVABRADINE HYDROCHLORIDECombinatorial chemistry
The invention relates to new benzocyclobutane, a preparation method thereof and application thereof. The invention provides a new preparation method for (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane serving as a key intermediate of ivabradine hydrochloride and addition salt thereof, and meanwhile provides a new benzocyclobutane compound which is an intermediate for preparing the (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane. In addition, the invention also provides a method for preparing the new benzocyclobutane compound, and meanwhile provides a method for splitting an intermediate product during preparing the new benzocyclobutane compound.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Preparation method of key intermediate of rosuvastatin calcium side chain
ActiveCN101624390AEase of industrial productionSimple and fast operationOrganic compound preparationCarboxylic acid esters preparationSide chainRosuvastatin Calcium
The invention provides a preparation method of a key intermediate of a rosuvastatin calcium side chain, comprising the following steps: using (S)-4-chlorine-3-hydroxybutanoate as an initial raw material; and preparing the key intermediate through four-step reactions of condensation, reduction, hydroxy group protection and condensation. The reaction process is simple to operate, the products in each step are easy to separate and purify, the purification and separation step is carried out without a silicagel column, and the yield is more than 80 percent, therefore, the intermediate with higher chemical purity and optical purity can be obtained. The GC determination shows that the chemical purity is more than or equal to 99.5 percent and the optical purity is more than or equal to 99.2 percent ee.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthetic methods of chiral aryloxy propanol amine compounds and salts thereof
InactiveCN101323580ASimple post-processingHigh yieldOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsBeta adrenoceptors
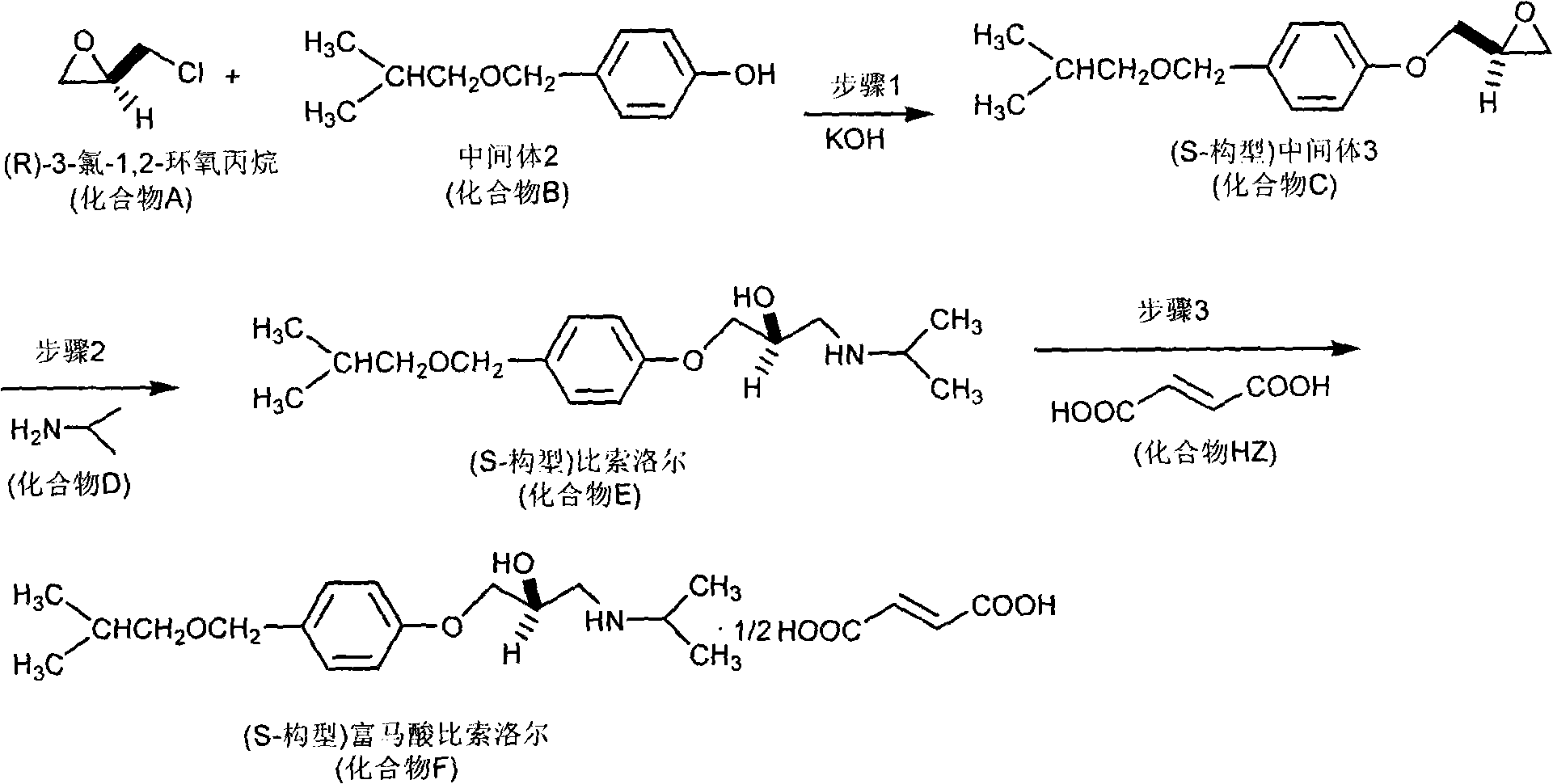
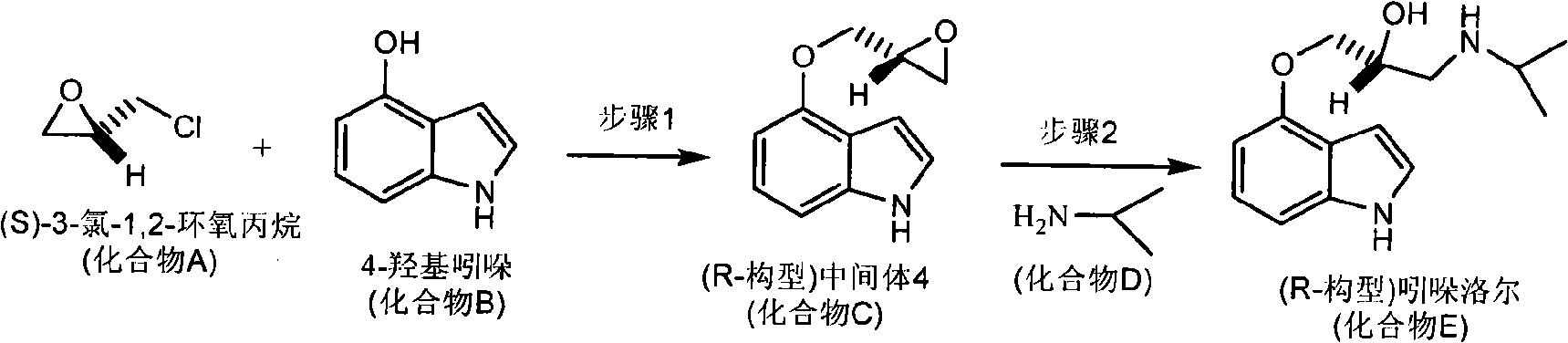
The invention relates to a synthesis method of chiral aryloxy propanol amine compound and salts thereof, which pertains to chemical pharmacy field. The method of the invention takes chiral source (R or S) halogenated propylene oxide and phenol compound as initial raw materials, (R or S) aryloxy propanol amine compound of high optical purity is obtained through etherification reaction and amination reaction, and the method of the invention can be applied to the preparation of various salts or compounds through salt forming reaction. The products of the invention usually has the characteristics of retarder of beta adrenoceptor and can be used as medicaments for curing diseases such as high blood pressure, angina pectoris, cardiac neurosis, arrhythmia and glaucoma, etc.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Intermediate for synthesizing treprostinil diethanolamine and method for preparing the same
ActiveUS9505704B2Improve the tedious and lengthy synthetic processHigh optical purityOrganic compound preparationCarboxylic acid esters preparationCombinatorial chemistryEthanolamine synthesis
The present invention relates to a method for treprostinil diethanolamine synthesis. The present invention also relates to a novel intermediate used in the method for treprostinil diethanolamine synthesis. The novel intermediate is shown in the following formula (II):wherein R1 and R2 are described in the description.
Owner:EVERLIGHT CHEMICAL INDUSTRIAL CORPORATION
Non-enantioselective prepn process of emtricitabine
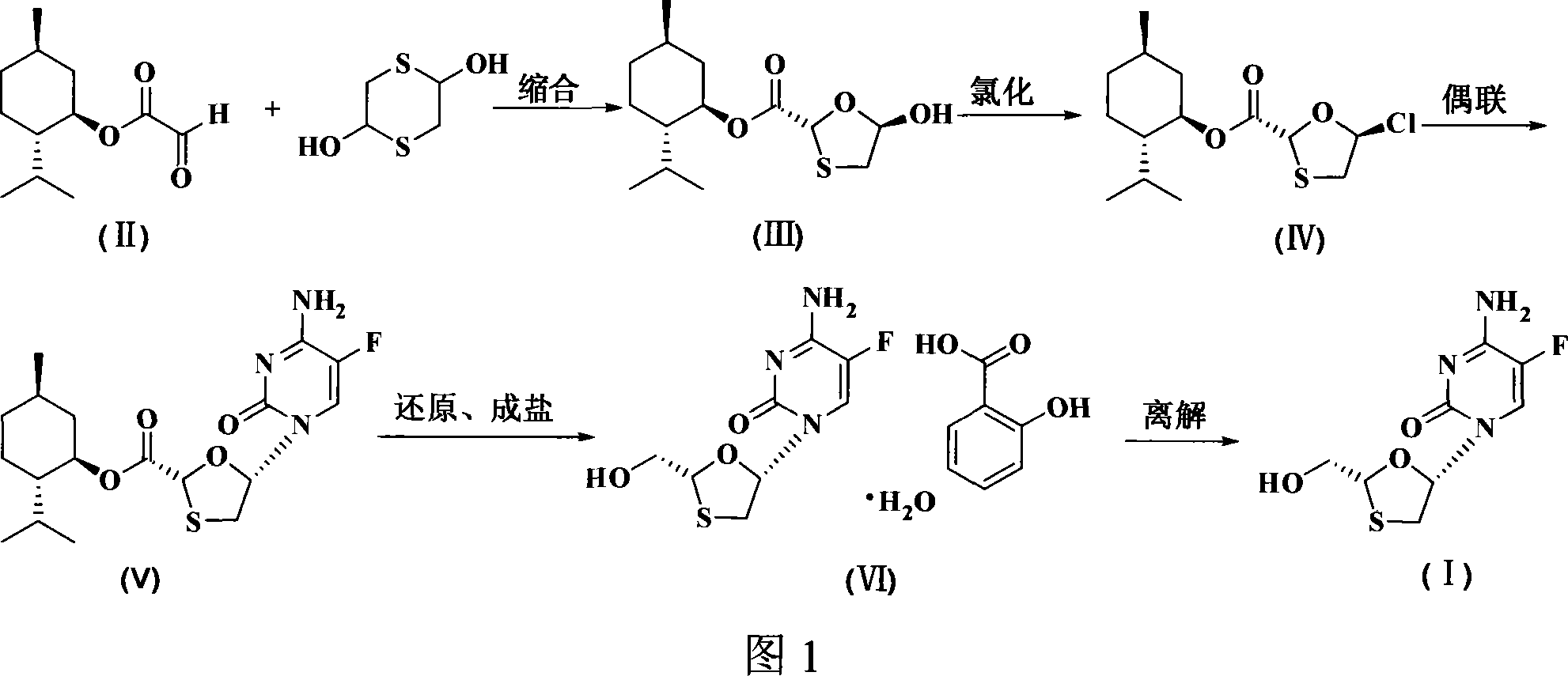
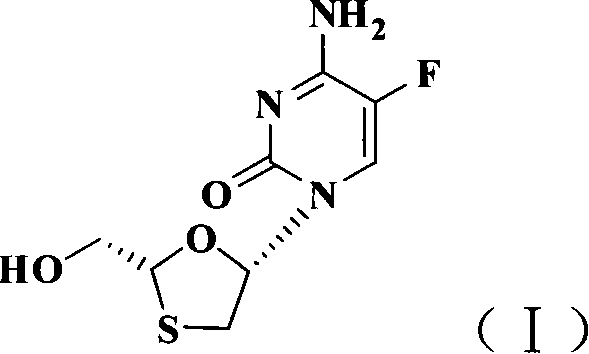
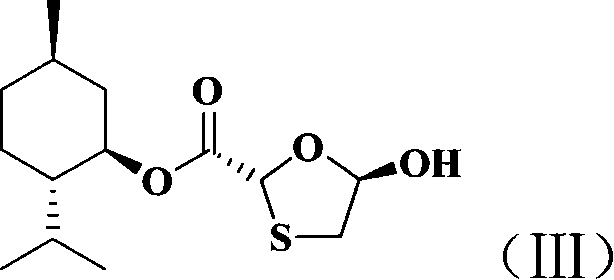
InactiveCN101066971AMild reaction conditionsSimple and fast operationOrganic chemistryDithianeAlcohol
The present invention discloses non-enantioselective preparation process of emtricitabine. The preparation process includes condensation of glyoxalic acid as the initial material and 2, 5-dihydroxy-1, 4-dithiane under the asymmetric induction of optically active alcohol ester to obtain trans-5-hydroxy-1, 3-oxythiacyclopentane-2-carboxylate; halogenating and coupling with silylated 5-flucytosine, and reducing to obtain initial product; reaction with salicylic acid to form salt, purification and separation to obtain optically pure emtricitabine. The present invention has mild reaction condition, simple operation, low cost, less environmental pollution and high product purity reaching medicinal standard, and is suitable for industrial production. The product is used in treating hepatitis B and AIDS.
Owner:葛建利
High optical purity copolymer film
ActiveUS20070178325A1High optical purityLiquid surface applicatorsFilm/foil adhesivesOptical propertyPolymer science
The invention relates to a high optical purity toughened copolymer film or coating. The copolymer is a graft or block copolymer, preferably acrylic, preferably produced by a controlled radical polymerization having an extremely low degree of particulate contamination and excellent optical properties. The film or coating is preferably formed by solvent-casting on a temporary substrate or solvent-coating on a permanent substrate.
Owner:TRINSEO EURO GMBH
Compounds and preparation methods thereof, and uses of compounds in synthesis of brivaracetam
ActiveCN106365986AAvoid wastingHigh optical purityOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsBrivaracetam
The present invention provides compounds represented by formulas II and IV. The invention further provides uses of the compound represented by the formula II in synthesis of brivaracetam, and a synthesis method. According to the present invention, the used raw materials are easy to obtain and have low price, and the high optical-purity brivaracetam can be prepared. The formulas II and IV are defined in the specification.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
Process for producing optically active 2-substituted carboxylic acid
InactiveUS20050245764A1High optical purityHigh chemical purityOrganic compound preparationOrganic chemistry methodsMedicinal chemistrySubstituted carboxylic acid
The present invention relates to a process for efficiently producing an optically active 2-bromocarboylic acid and an optically active 2-sulfonyloxycarboxylic acid, which are important in the production of medicinal compounds and so forth. An optically active 2-sulfonyloxycarboxylic acid ester is subjected to deprotection under acid conditions to obtain an optically active 2-sulfonyloxycarboxylic acid. A metal bromide is caused to act on the acid to brominate it with configuration inversion at position 2 to thereby produce an optically active 2-bromocarboxylic acid. The resultant optically active 2-bromocarboxylic acid is isolated / purified by subjecting it to a step in which the acid is crystallized and separated as a salt with a base. Thus, an optically active 2-bromocarboxylic acid having a high chemical purity and high optical purity can be produced.
Owner:KANEKA CORP
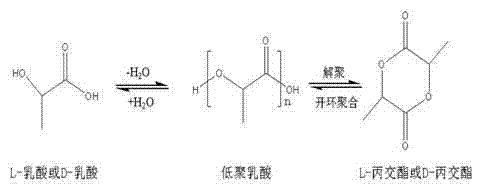
High-purity lactide and preparation method thereof
The invention discloses high-purity lactide and a preparation method thereof. The preparation method comprises the steps of raw material addition, free water removal, polycondensation, depolymerization, distillation and secondary distillation. The raw material addition step refers to that a catalyst is added into L-lactic acid or D-lactic acid, wherein the catalyst is one or more of zinc oxide, stannous oxide and stannous octoate. In the preparation process, the use of an organic solvent, N2 and inert gas and environmental pollution are avoided, the vacuum degree is moderate, purification loss is reduced, the production cost is reduced, and the method is suitable for industrial production. The L-lactide or D-lactide product prepared by the method is high in yield, optical purity and quality, the yield is more than or equal to 97 percent, and the optical purity of the purified lactide is 99.45 percent or above.
Owner:SHANDONG SHOUGUANG JUNENG GOLDEN CORN CO LTD
Process for preparing chiral compounds from recemic epoxides by using chiral salen catalysts
InactiveUS6720434B2Quick responseHigh optical purityOxygen-containing compound preparationOrganic compound preparationSalen ligandContinuous use
The present invention relates to chiral salen catalysts and a process for preparing chiral compounds from racemic epoxides by using them. More particularly, the present invention is to provide chiral salen catalysts and its use for producing chiral compounds such as chiral epoxides and chiral 1,2-diols economically in high yield and high optical purity by performing stereoselective hydrolysis of racemic epoxides, wherein the chiral salen catalyst comprises a cationic cobalt as a center metal of chiral salen ligand and counterions having weak nucleophilic property to resolve disadvantages associated with conventional chiral salen catalysts, and can be used continuously without any activating process of used catalysts because it does not loose a catalytic activity during the reaction process.
Owner:RSTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com