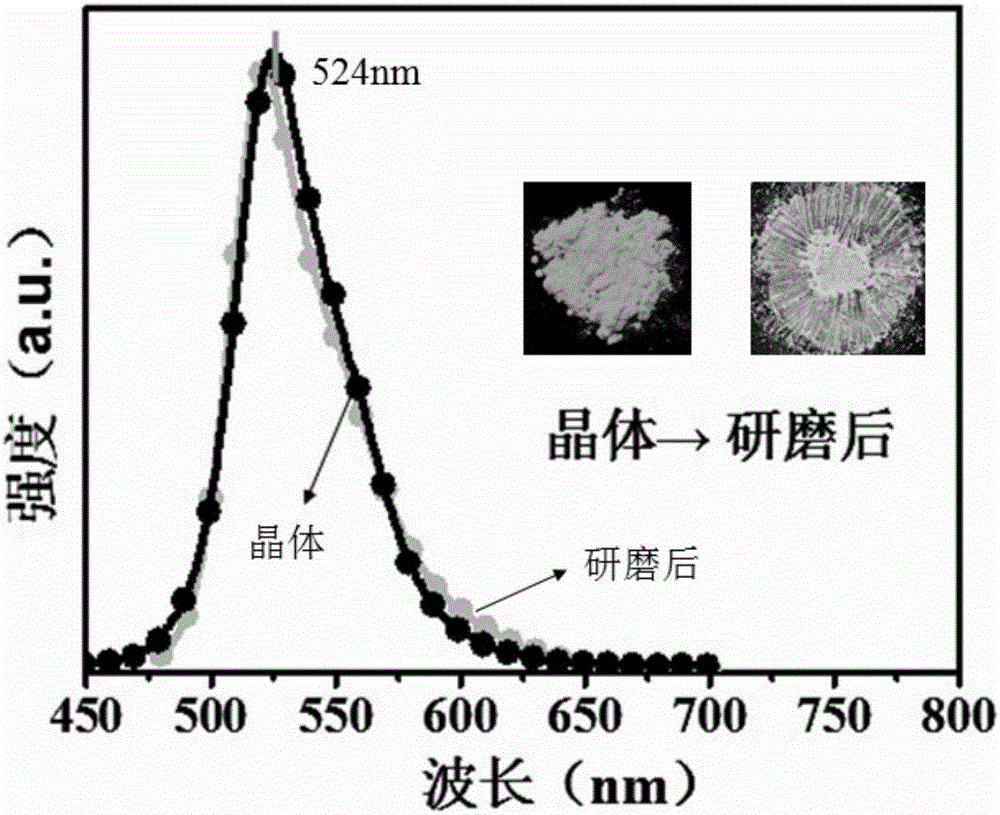
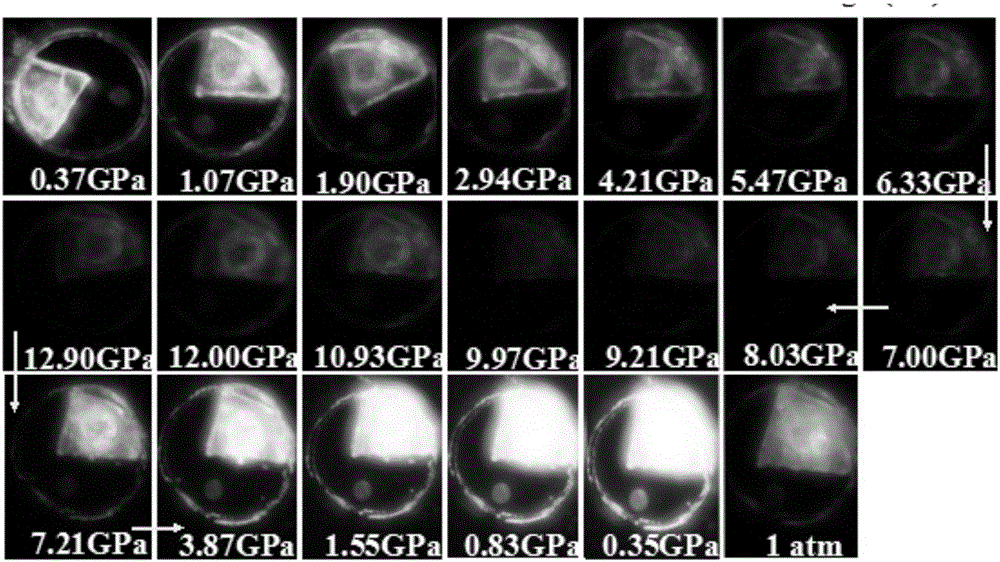
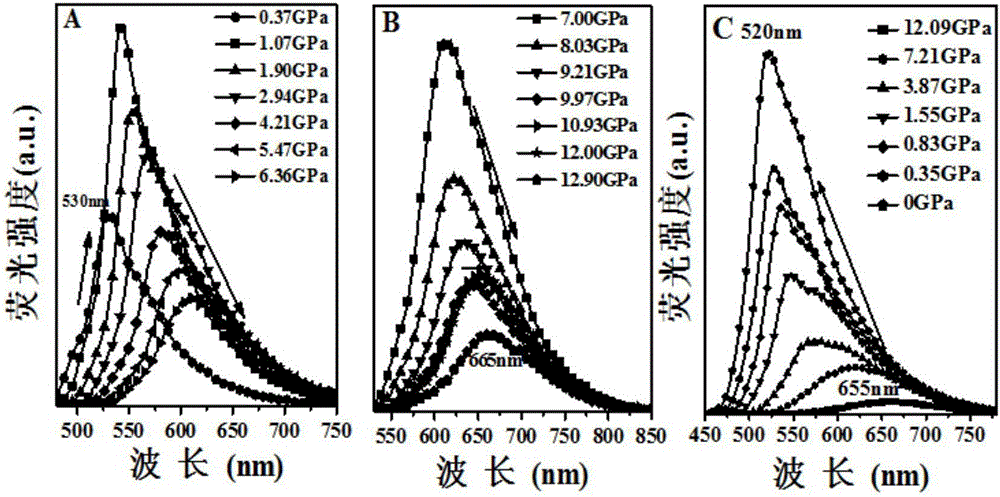
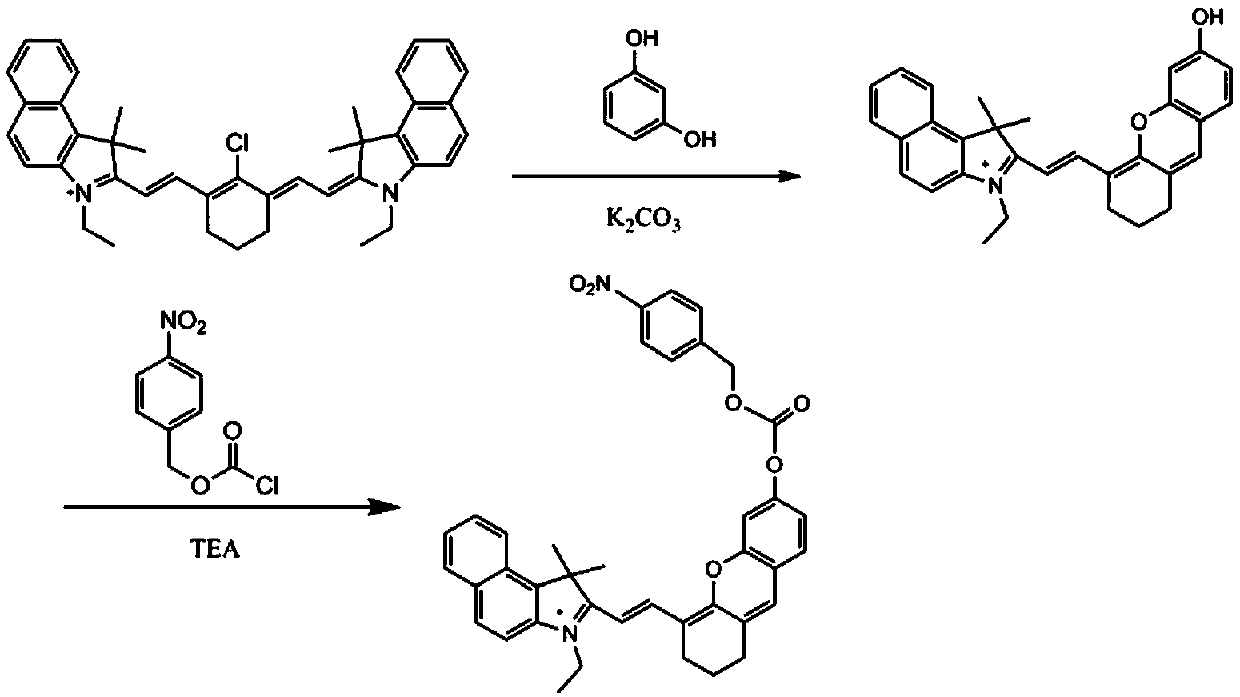
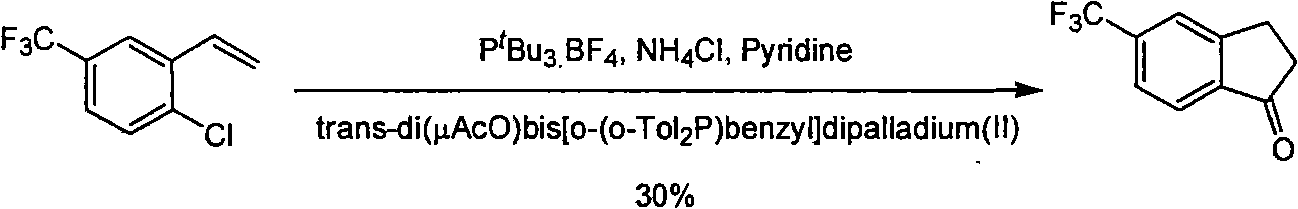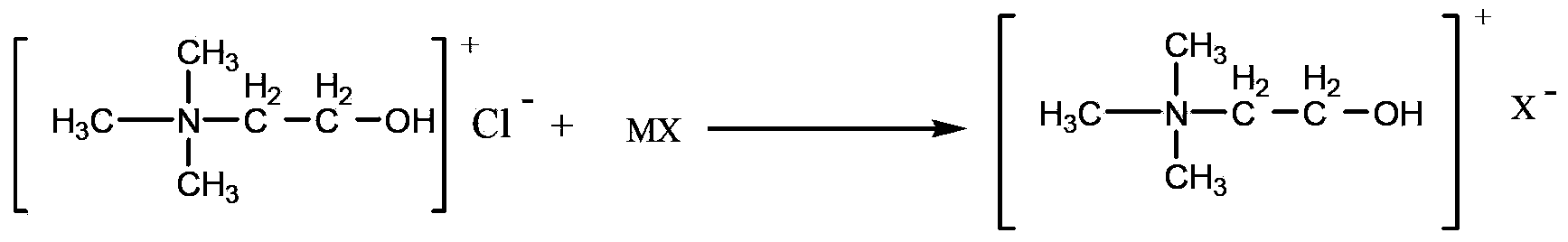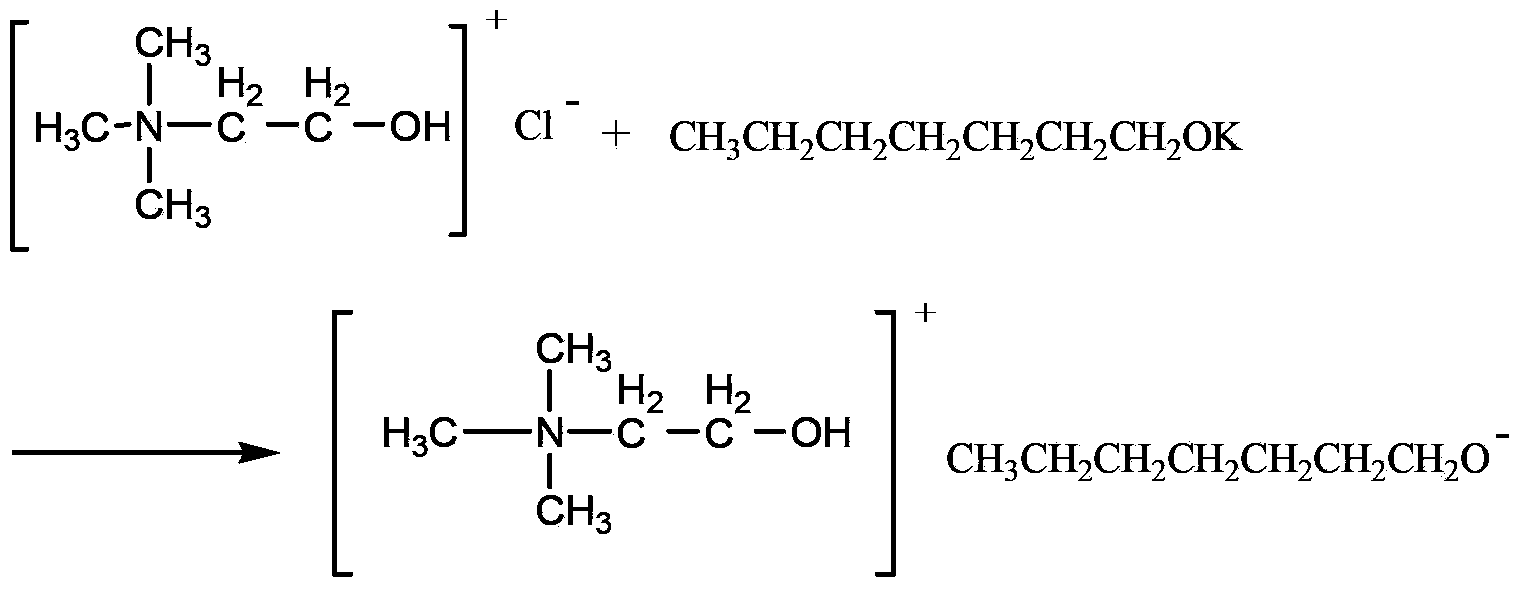Patents
Literature
237 results about "Knoevenagel condensation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Knoevenagel condensation ([ˈknøːvənaːɡl̩]) reaction is an organic reaction named after Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence condensation). The product is often an α,β-unsaturated ketone (a conjugated enone).
Near infrared BODIPY fluorescence dye and preparation method thereof
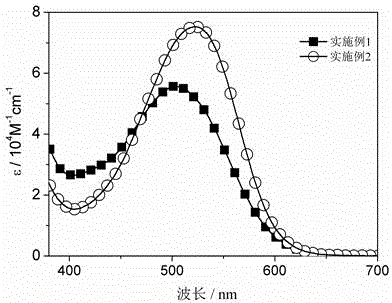
ActiveCN105462576AHigh molar absorptivityHigh fluorescence quantum efficiencyMethine/polymethine dyesGroup 3/13 element organic compoundsInfraredSolubility
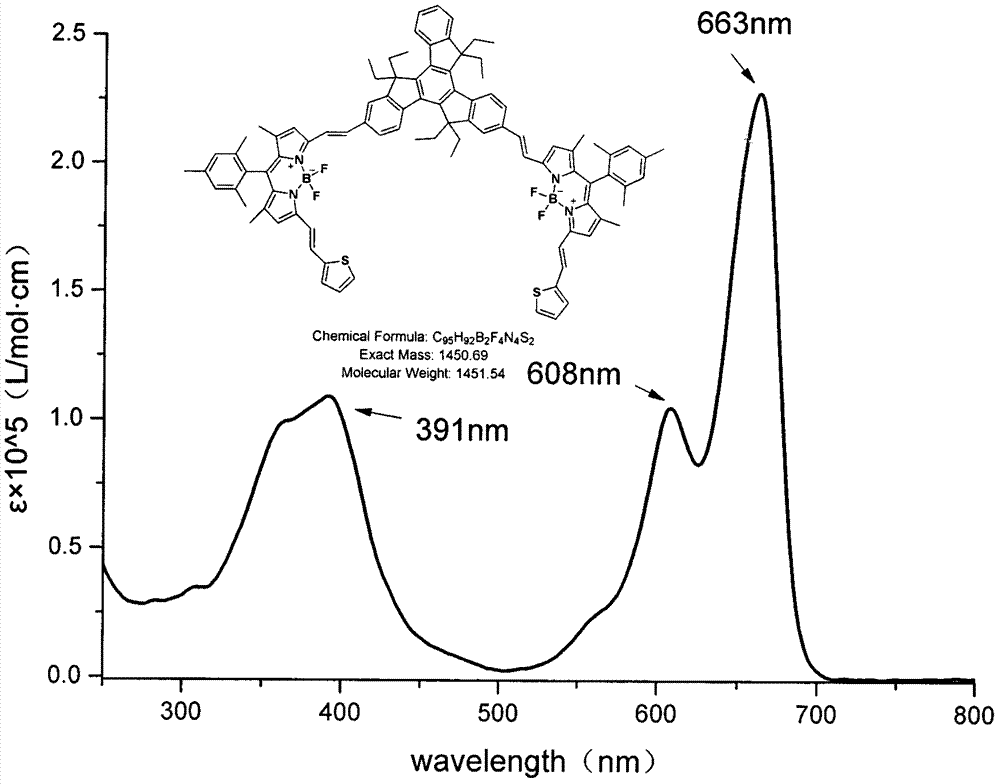
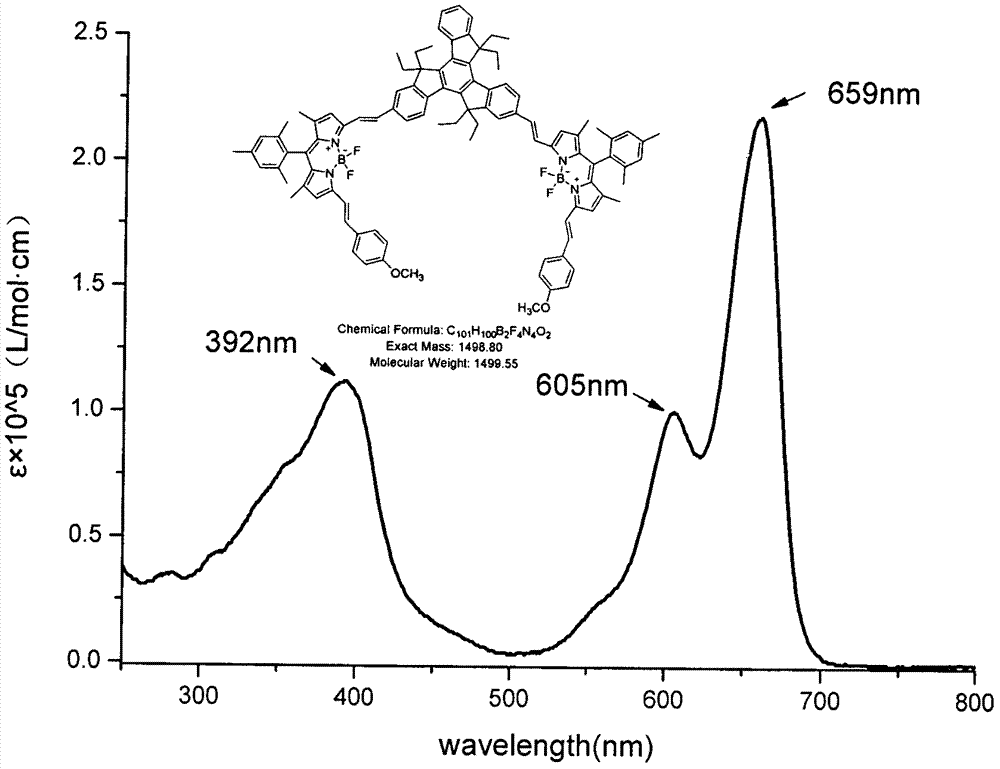
The invention relates to a near infrared BODIPY fluorescence dye and a preparation method thereof. A BODIPY derivative and 2-aldotruxene undergo a Knoevenagel condensation reaction under the catalysis action of p-toluenesulfonic acid and piperidine to synthesize the dye, and the maximal absorption wavelength and the maximal emission wavelength of the dye in an organic solvent are 650nm or above respectively. The preparation method has the advantages of simple reaction steps, mild reaction conditions and good selectivity. Like fluorescence dyes have the advantages of high molar extinction coefficient, good solubility and light stability, excellent photophysical performances, and good application prospect in cell imaging and biological marking.
Owner:南京颐维环保科技有限公司
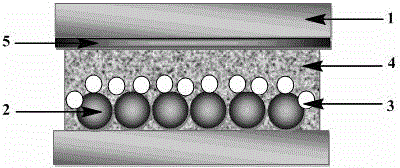
Metal-organic framework nanocrystal loaded polyurethane foam as well as preparation and application thereof
ActiveCN106366636AGood dispersionOvercoming the defects of difficult processingCarboxylic acid nitrile preparationOrganic compound preparationChemical industryMetal-organic framework
The invention discloses a metal-organic framework nanocrystal loaded polyurethane foam as well as a preparation and an application thereof. Polyurethane prepolymer and active amino on MOFs (Metal-Organic Frameworks) are taken to perform chemical doping, the great challenge that MOFs materials are difficult to process is overcome, MOFs nanoparticles are uniformly loaded in the polyurethane foam, a foam reactor is obtained by means of in-situ forming, a new process of building a complex mesoporous-microporous assembly based on microporous metal-organic skeleton nanoparticles as structural units is provided to observe catalysis of the MOFs nanoparticles on knoevenagel condensation; as water serves as a foaming agent and a green chemical method is adopted to prepare an integral flow-by foam reactor to catalyze an organic reaction, aftertreatment of a catalyst is simplified, loss of the catalyst is avoided, continuous flow-by cyclic catalyzation can also be performed, and a new method is provided for the treatment of environmental pollution and chemical industry.
Owner:SHANDONG NORMAL UNIV
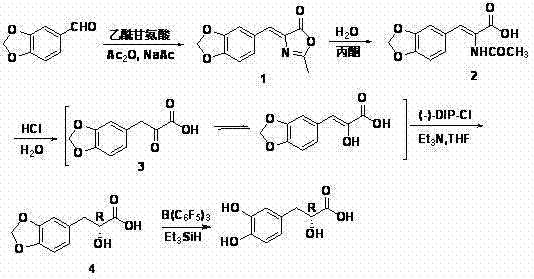
Asymmetric synthesis method of (+)-tanshinol
InactiveCN102924265ARaw materials are easy to getSimple and fast operationOrganic compound preparationCarboxylic compound preparationPropanoic acidCombinatorial chemistry
The invention relates to an asymmetric synthesis method of (+)-tanshinol, which comprises the following steps: carrying out Knoevenagel condensation on the raw material heliotropin, hydrolyzing for ring opening to obtain beta-(3,4-3,4-methylenedioxyphenyl)pyruvic acid, carrying out key asymmetric reduction reaction to obtain R-beta-(3,4-dibenzyloxyphenyl)-alpha-hydracrylic acid, and finally, deprotecting to obtain the (+)-tanshinol. The method has the advantages of accessible raw material and high optical purity of the product, is simple to operate, and can implement large-scale preparation.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Catalyst of basic ionic liquid immobilized on metal-organic frameworks and preparation method thereof
InactiveCN103920534AEasy to separateEasy to prepareCarboxylic acid nitrile preparationOrganic compound preparationMetal-organic frameworkReusability
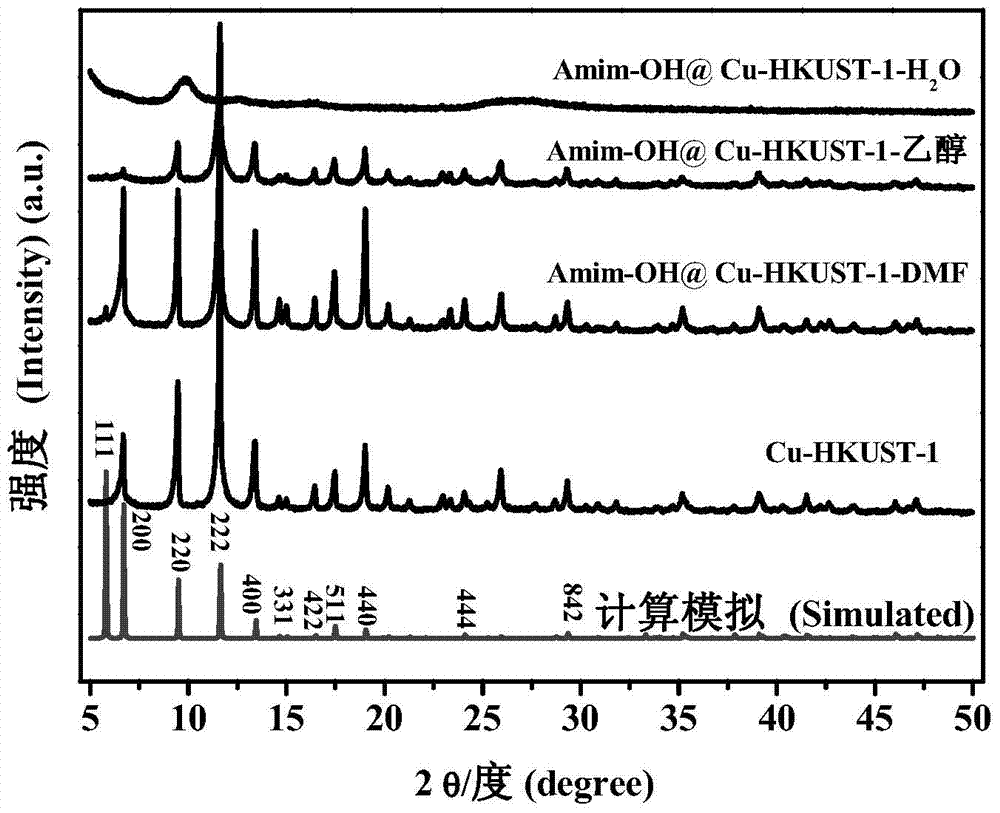
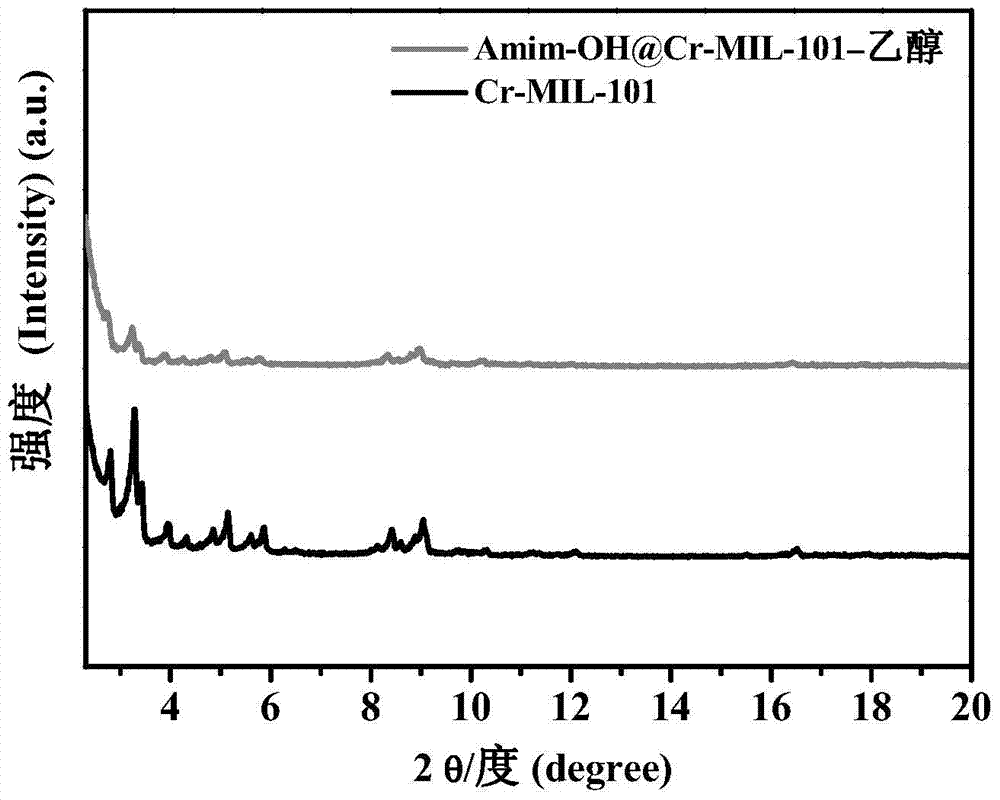
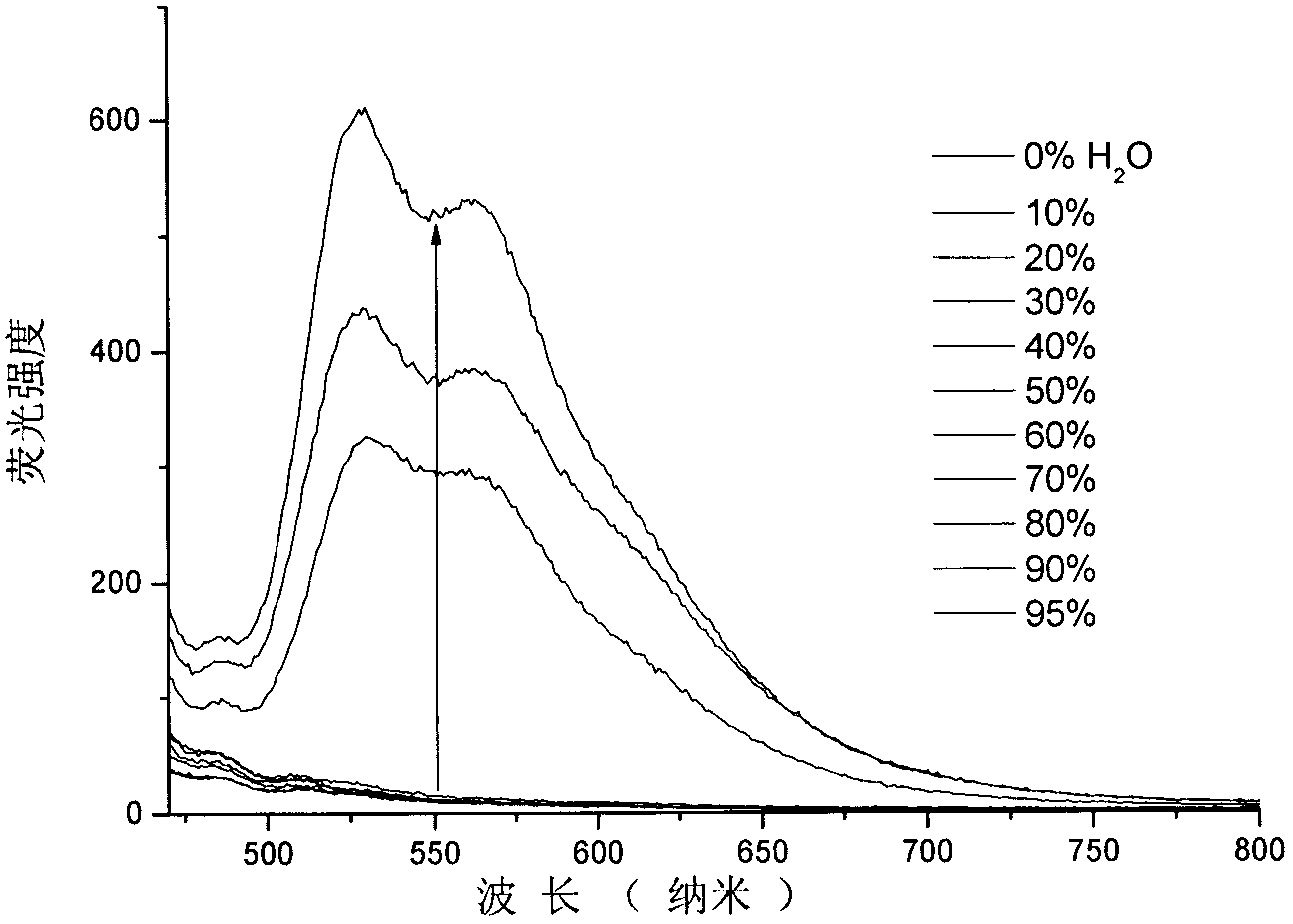
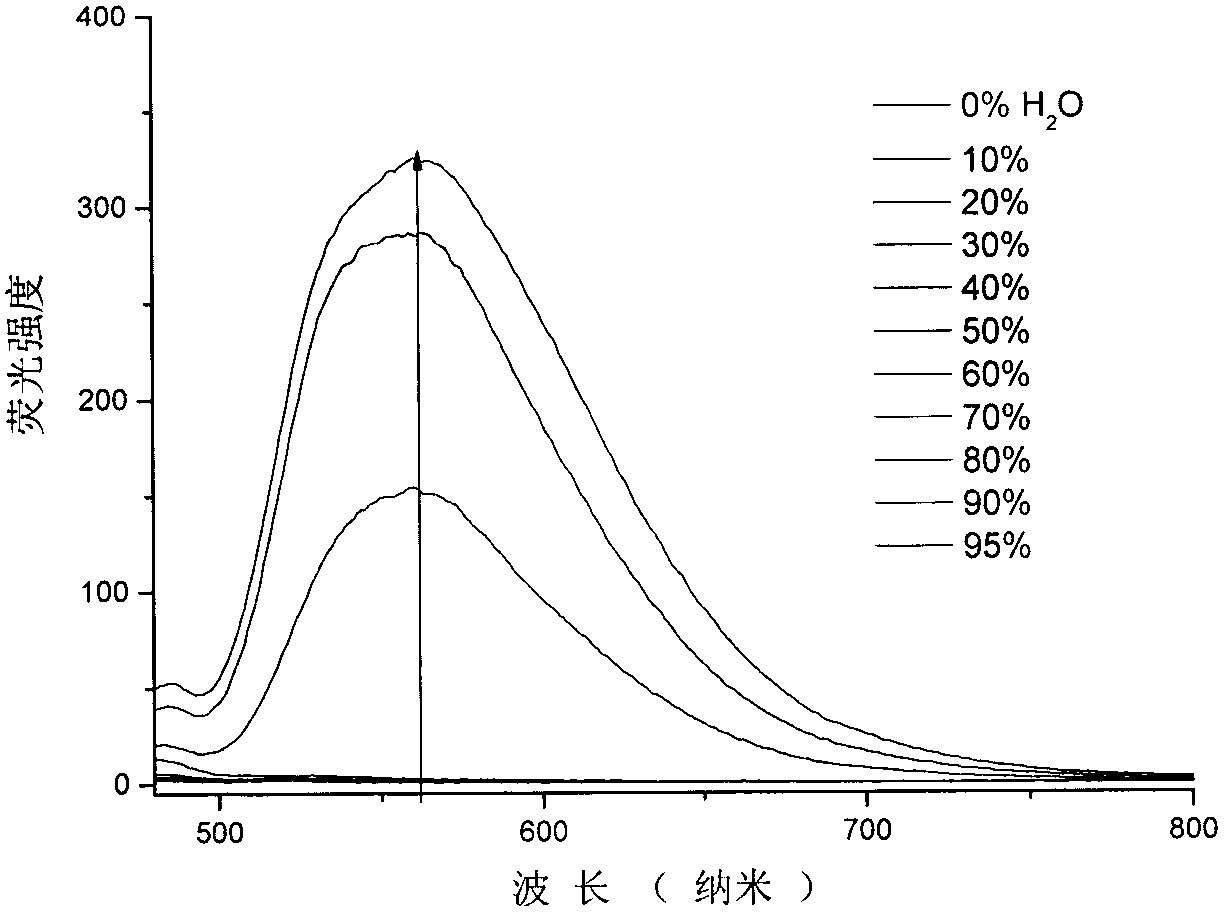
The invention relates to a catalyst of a basic ionic liquid immobilized on metal-organic frameworks and a preparation method thereof and belongs to the field of catalyst preparation technology and application. Through chemical anchoring post-synthetic modification, an amino-functionalized imidazolyl basic ionic liquid Amim-OH is introduced into nanocavities of MOFs; by chemical bonds between amino group and metal nonsaturated vacancy, an Amim-OH ionic liquid catalyst is immobilized so as to prepare a novel basic ionic liquid immobilized catalyst. The basic heterogeneous catalyst shows excellent catalytic performance and reusability in a Knoevenagel condensation reaction.
Owner:DALIAN UNIV OF TECH
Quinoline nitrile derivative with aggregation-induced emission performance
ActiveCN102702096AStrong fluorescenceSignificant aggregation-induced luminescence characteristicsOrganic chemistryLuminescent compositionsQuinolineOrganism
The invention relates to a quinoline nitrile derivative. A method comprises the following steps of: reacting 2-methylquinoline serving as an initial raw material with the corresponding alkyl halide to obtain quaternary ammonium salt, performing Michael addition-elimination reaction on the quaternary ammonium salt and malononitrile, and performing Knoevenagel condensation reaction on the obtained compound and the corresponding aromatic aldehyde to obtain a target product. The aggregate or solid substance of the derivative has strong fluorescence and large wavelength, is a good aggregation-induced emission material, and has considerable application prospects in the fields of electroluminescent devices, fluorescent probes, intelligent materials, organism imaging and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
Gold-MOFs-polymer composite membrane, and production method and application thereof
ActiveCN106397797AEfficient use ofSolving the Recycling ConundrumSemi-permeable membranesMembranesRoom temperatureContinuous flow
The invention discloses a gold-MOFs-polymer composite membrane, and a production method and an application thereof, and belongs to the technical field of polymeric functional membranes. An MOFs-polymer composite membrane obtained through self assembling of a covalent bond driven NMOFs material and mercapto group-containing functional polysiloxane is adopted to load gold nano-particles as a matrix in order to obtain the gold-MOFs-polymer composite membrane material. The gold-MOFs-polymer composite membrane has a high catalysis effect on Knoevenagel condensation of 4-nitrobenzaldehyde at room temperature and reduction of 4-nitrophenol as a continuous flow-through membrane reactor. The composite membrane has great application prospect in the field of membrane catalysis.
Owner:SHANDONG NORMAL UNIV
Near-infrared truxene-based conjugate dual-BODIPY fluorescent dye and preparation method thereof
ActiveCN106928262ANarrow absorbencyNarrow emission peakAzo dyesGroup 3/13 element organic compoundsSolubilityFluorescence
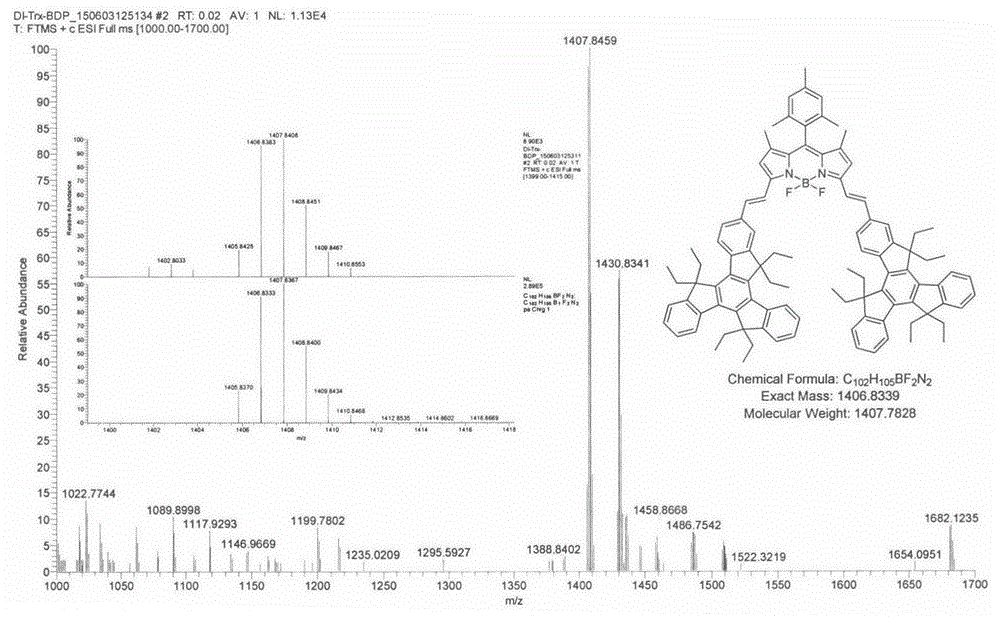
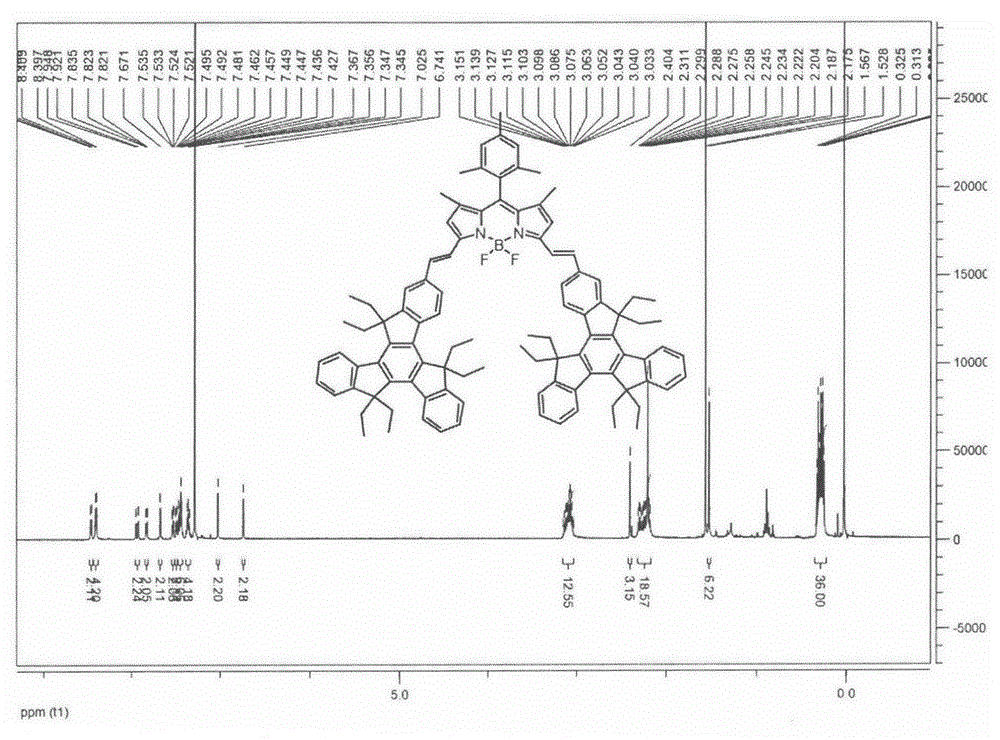
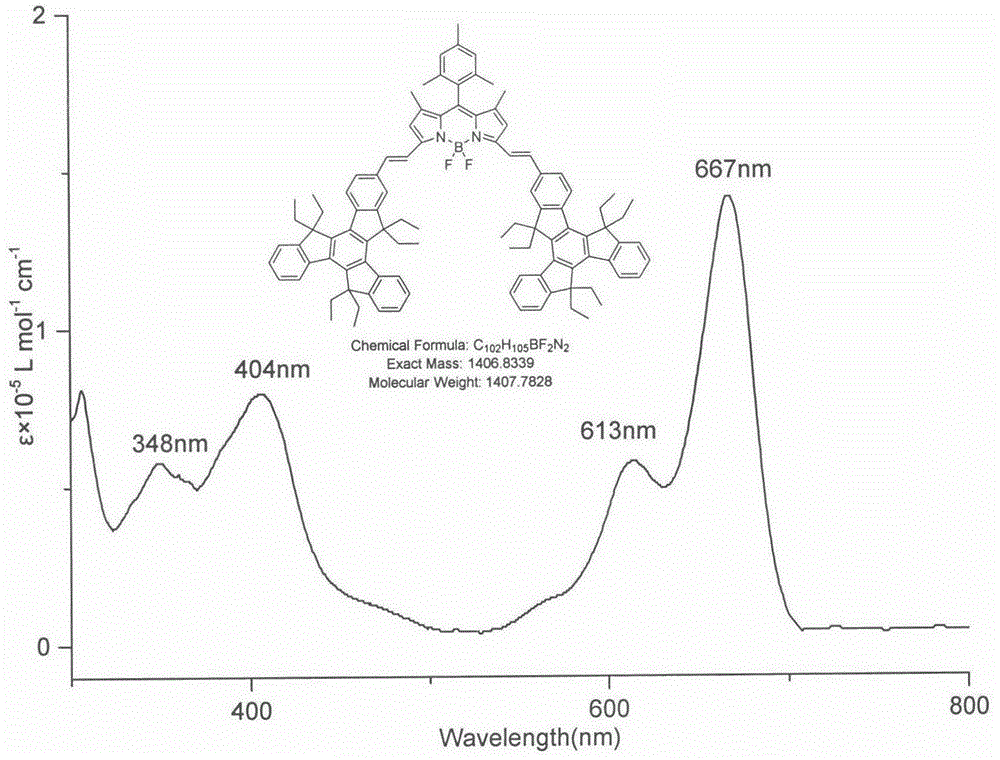
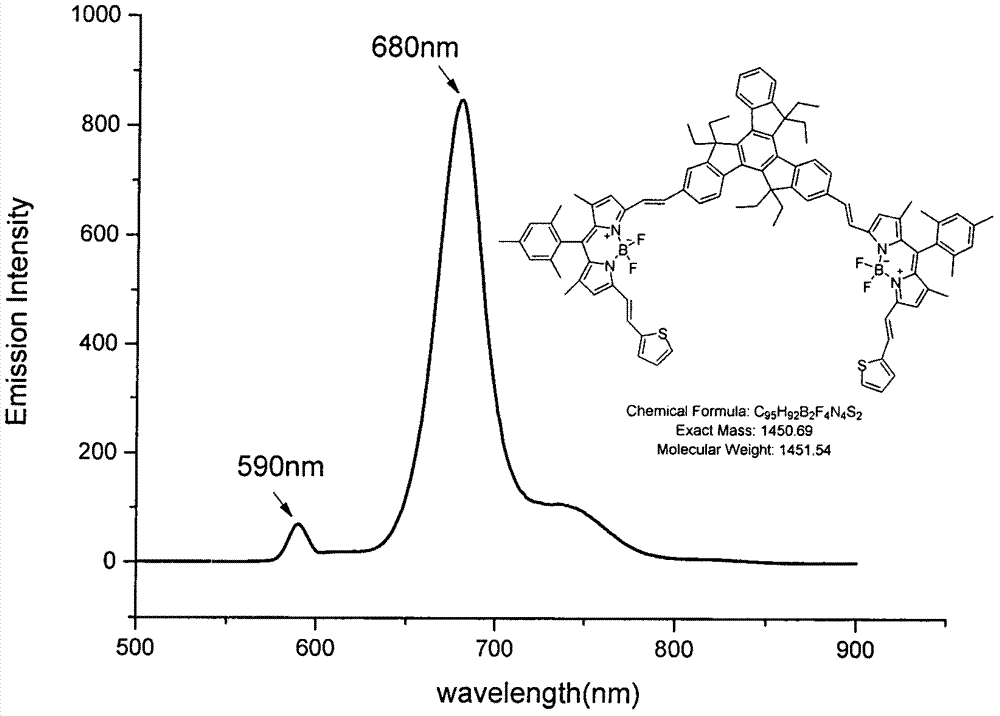
The invention relates to a near-infrared truxene-based conjugate dual-BODIPY fluorescent dye and a preparation method thereof. The fluorescent dye is synthesized by virtue of Knoevenagel condensation reaction of a BODIPY derivative and dialdehyde-containing truxene under the catalysis of p-toluenesulfonic acid and piperidine. The preparation method has the beneficial effects of simple reaction steps, mild reaction conditions and relatively good selectivity. The fluorescent dye has excellent physical properties of relatively high molar extinction coefficients, good solubility and light stability and the like, the highest electronic absorption spectrum red shift reaches above 650nm, the fluorescence emission wavelength reaches 680nm, and the fluorescent dye has very high application prospects. The fluorescent dye has good potential application prospects in the fields of cell imaging, bio-labeling or photoelectric materials and the like.
Owner:南京颐维环保科技有限公司
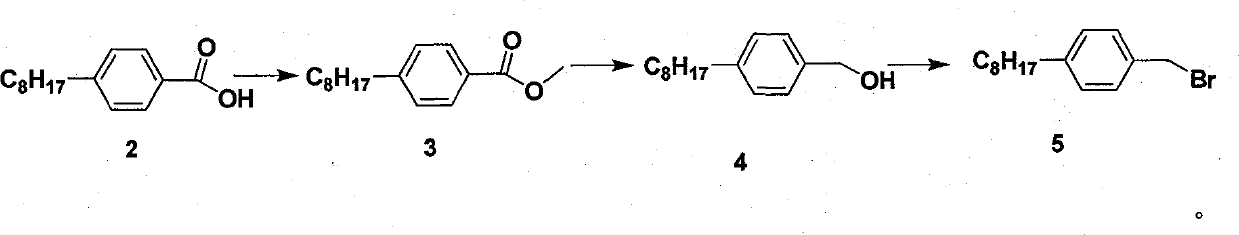
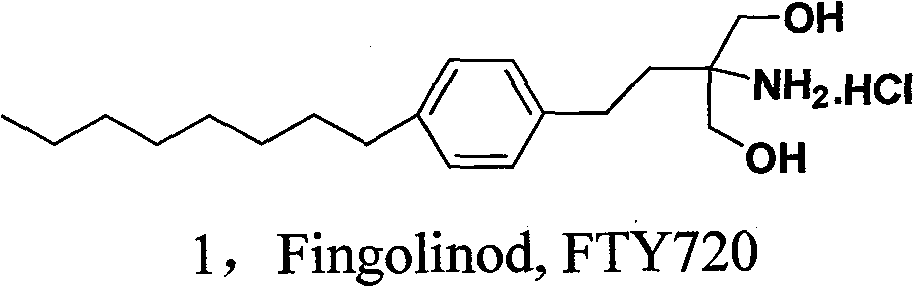
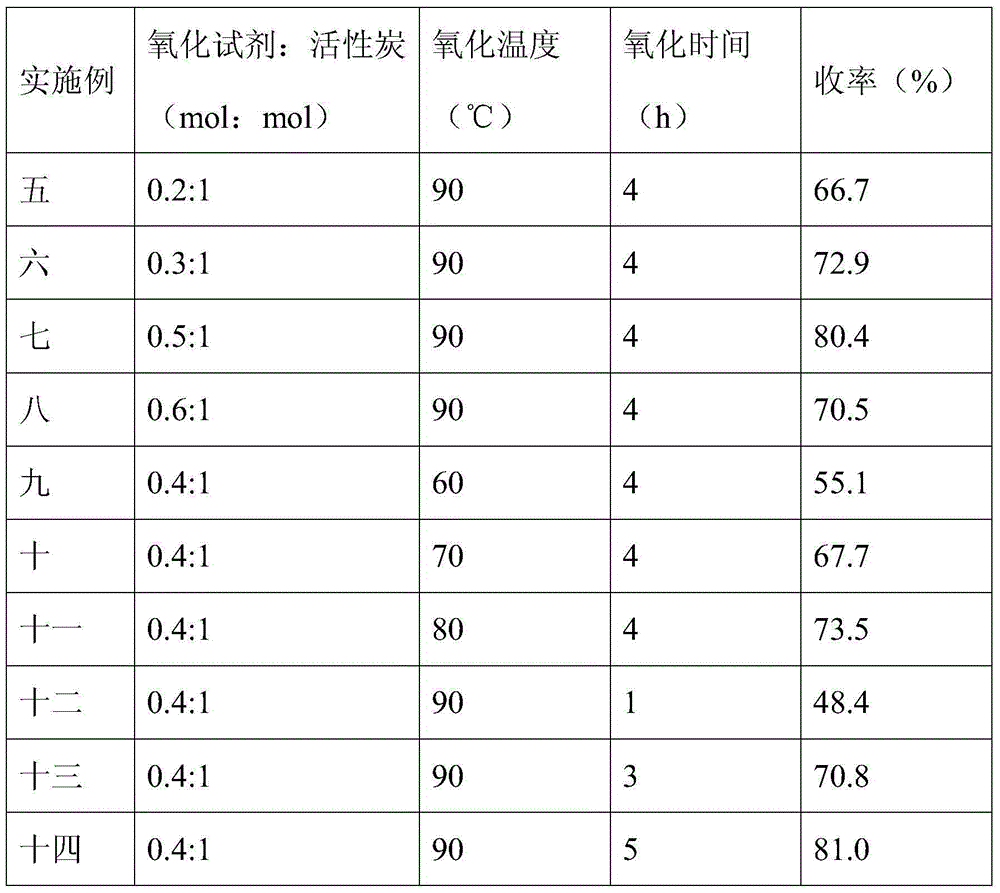
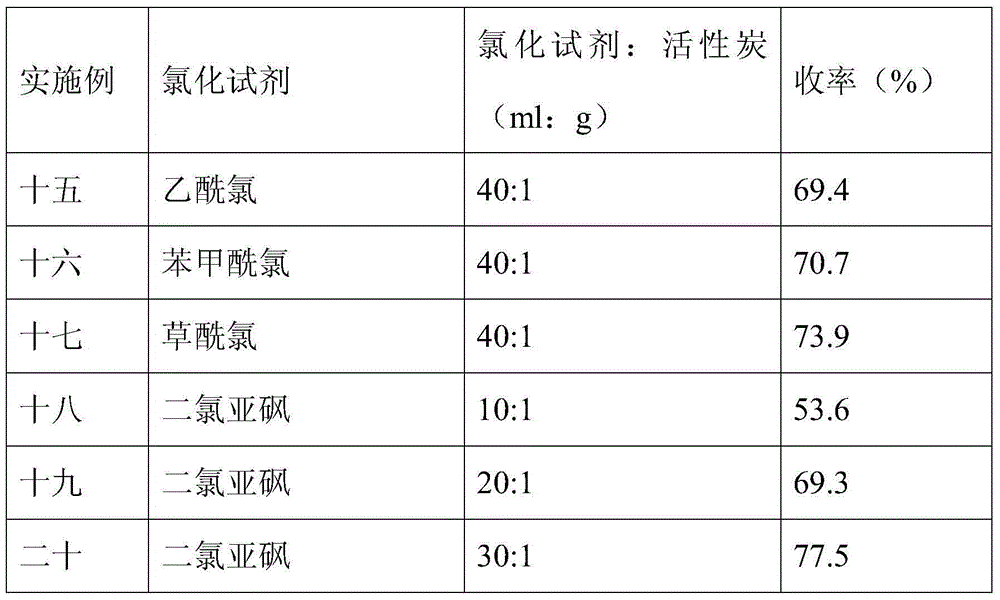
Novel synthesis method of fingolimod hydrochloride
ActiveCN102120720AReduce usageReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsFingolimod Hydrochloride
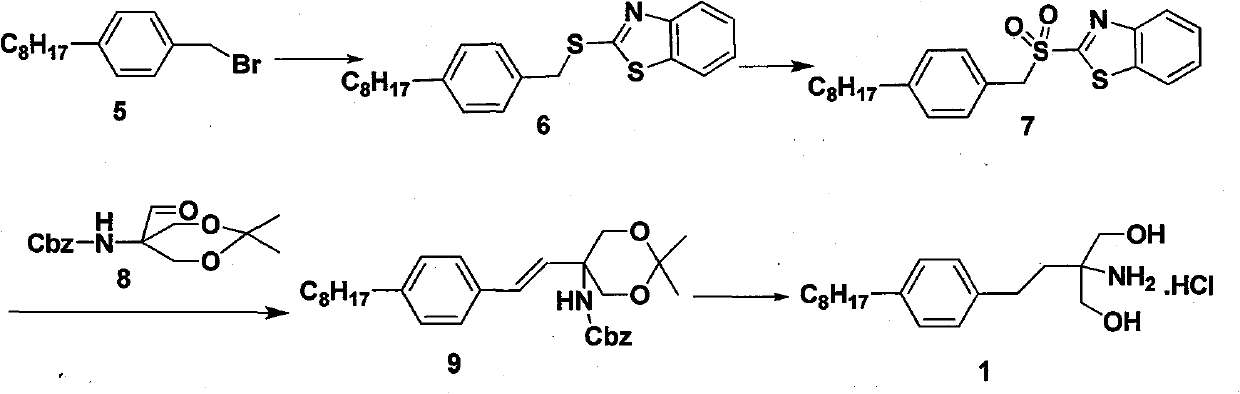
The invention discloses a novel synthesis method of fingolimod hydrochloride. The method comprises the following steps of: 1, reacting a compound (5) with a sulfhydryl compound under alkaline conditions to generate a key intermediate product (6); 2, performing oxidation reaction on the product (6) to generate a sulfonyl compound (7); 3, performing Knoevenagel condensation reaction on the sulfonylcompound (7) and a raw material aldehyde group compound (8) under the alkaline conditions to obtain a key intermediate (9); and 4, performing hydrogenation reduction reaction on the intermediate (9) and performing amino group deprotection simultaneously to prepare 5-amino-5-[2-(4-n-octylphenyl)ethyl]-2,2-dimethyl-1,3-dioxane; and performing acetonylidene deprotection in diluted hydrochloric acid and salifying simultaneously to obtain a target compound fingolimod hydrochloride (1). In the method, raw materials are readily available, reaction conditions are mild, aftertreatment is convenient, and the method is high in yield, low in cost, environmentally-friendly, and high in utilization value in industrial production.
Owner:安庆润科生物医药科技有限公司
Activated carbon immobilized ionic liquid catalyst and application thereof
InactiveCN104549495AEasy to separateCatalytic activity did not decreaseOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingActivated carbonAfter treatment
The invention discloses an activated carbon immobilized ionic liquid catalyst and application thereof. The activated carbon immobilized ionic liquid catalyst is prepared from the following steps: performing oxidation treatment and chlorination on activated carbon sequentially, so as to obtain oxidized and chlorinated activated carbon; performing refluxing on the oxidized and chlorinated activated carbon, bromized 1-(2-amino ethyl hydrobromic acid)-3 methylimidazole onium salt ionic liquid and organic amine in tetrahydrofuran for 20-30 h, filtering, and washing and drying filter cakes B, so as to obtain solid products; adding the solid products into a potassium hydroxide solution, stirring for 20-30 h at the temperature of 0-5 DEG C, filtering, washing filter cakes C and drying, so as to obtain the activated carbon immobilized ionic liquid catalyst. The activated carbon immobilized ionic liquid catalyst provided by the invention can be used for catalyzing of knoevenagel condensation, during after-treatment, the activated carbon immobilized ionic liquid catalyst can be separated from reaction mixed liquor through simple centrifuging or filtering only, and the activated carbon immobilized ionic liquid catalyst can be used repeatedly.
Owner:ZHEJIANG UNIV OF TECH
Fluorescent probe for detecting cyanide ion and synthesis and application method thereof
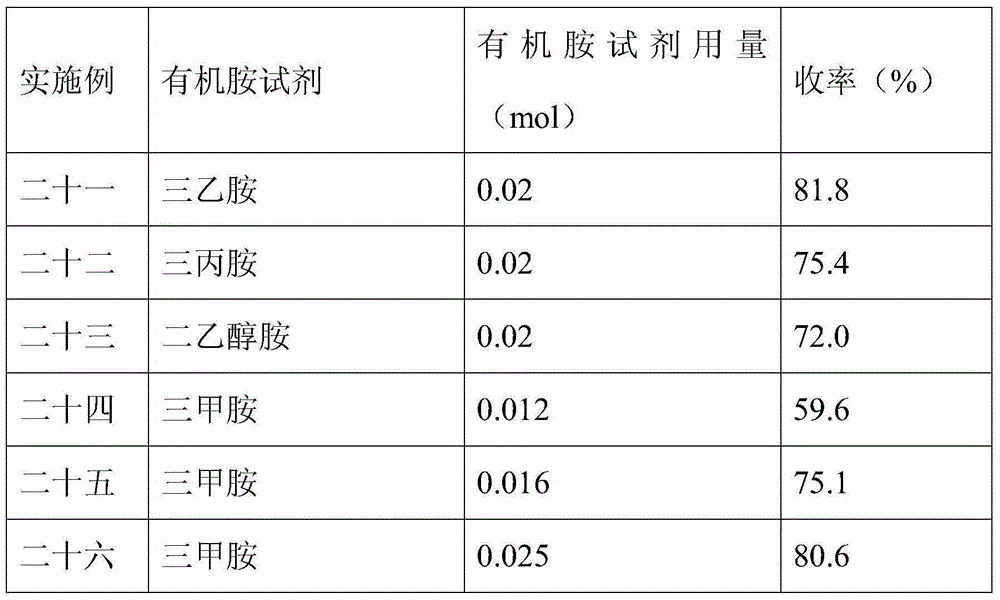
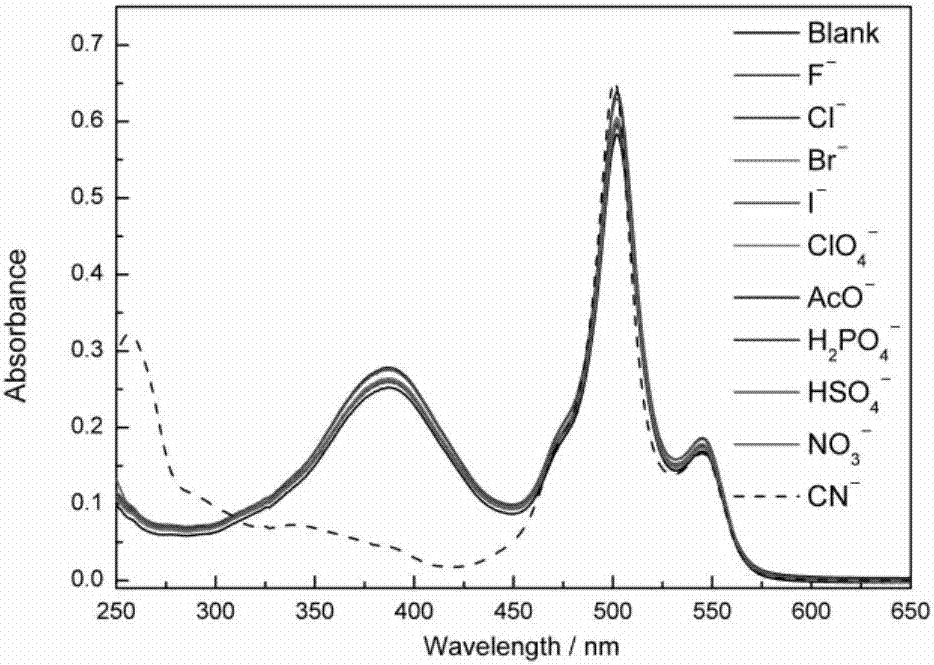
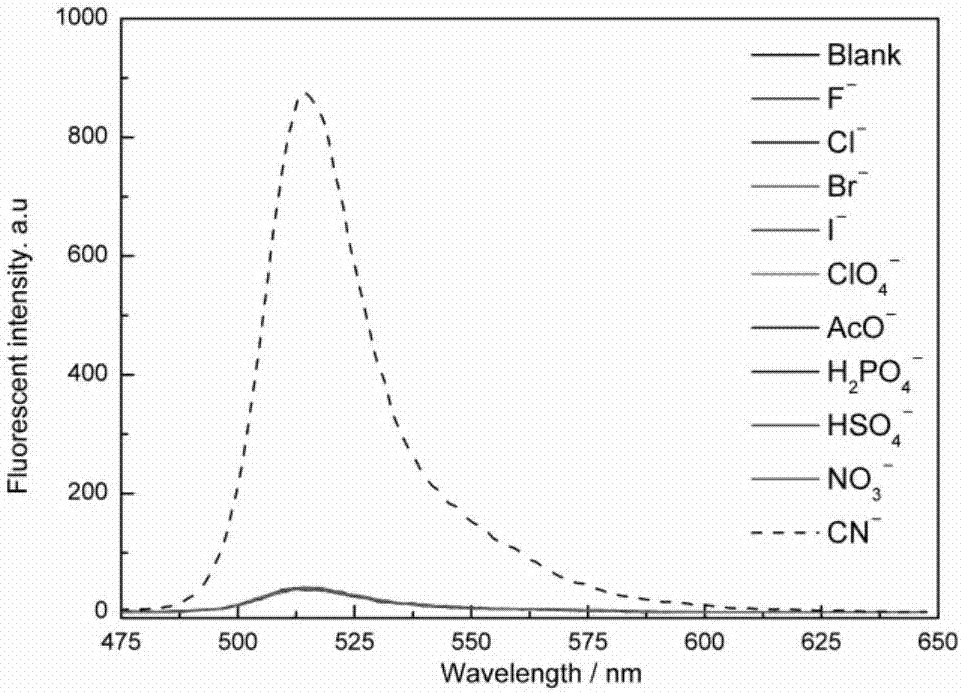
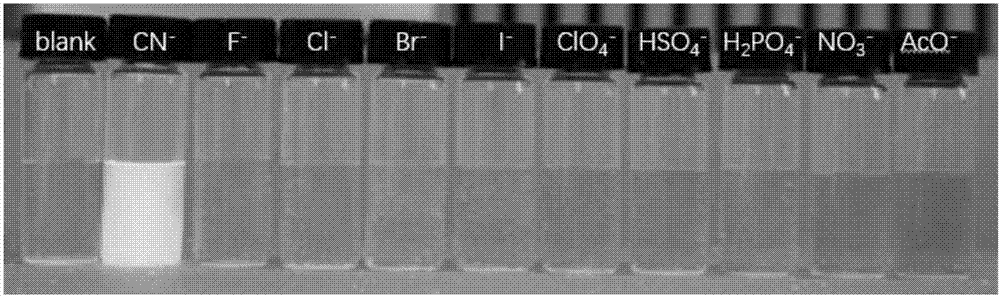
ActiveCN107082785AThe synthesis method is simpleLow costGroup 3/13 element organic compoundsFluorescence/phosphorescenceIodideRelative standard deviation
The invention discloses a fluorescent probe for detecting a cyanide ion and a synthesis and application method thereof. A cyanide ion fluorescent probe takes boron fluoride dipyrrole (BODIPY) as a fluorescent signal group and hemicyanine as a recognition site, and the probe is obtained by carrying out Knoevenagel condensation reaction on 1,3,5,6-tetramethyl 8-(4(formylphenyl)) boron fluoride dipyrrole and 1,2,3,3-tetramethyl-3H-indolium iodide. Ultraviolet absorption and fluorescence emission spectroscopy research finds that the probe can solely identify the cyanide ion, the lowest detection limit can reach 59 nM, the identification process is not interfered by other negative ions, and response speed is high. The fluorescent probe disclosed by the invention can be used for detection of the actual water sample, and relative standard deviation is less than 5%. Besides, the fluorescent probe is simple in synthesis method and relatively low in cost and has a good application prospect in cyanide ion detection.
Owner:JIANGHAN UNIVERSITY
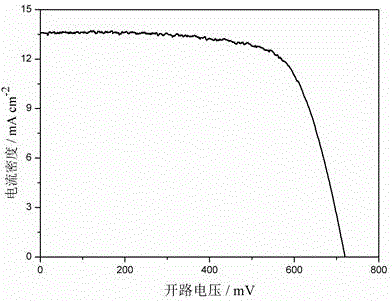
Triphenylamine-thiophene organic dyestuff as well as preparation method and application thereof
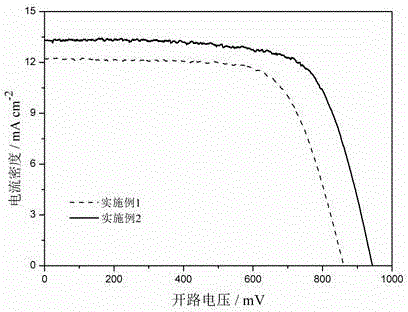
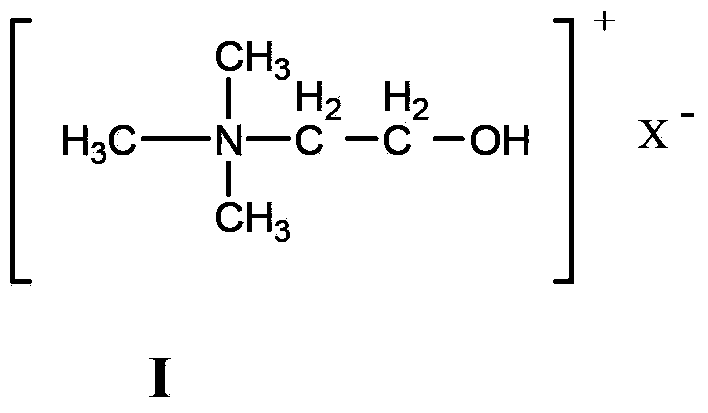
InactiveCN103554957AInhibitory complexIncrease the open circuit voltageLight-sensitive devicesOrganic chemistryOrganic dyeThiophene derivatives
The invention provides a triphenylamine-thiophene organic dyestuff. The structural general formula of the triphenylamine-thiophene organic dyestuff is as shown in a formula (I), wherein in the formula (I), R1 is C1-C6 alkoxy, the structural formula of Ar is as shown in a formula (II) or a formula (III), and R2 in the formulas (II) and (III) is C1-C6 alkyls. A preparation method of the triphenylamine-thiophene organic dyestuff comprises the following steps: sequentially carrying out Suzuki coupled reaction and Knoevenagel condensation reaction by using aryl bromal, tetrakis(triphenylphosphine)palladium(0), potassium carbonate and alkoxy substituted triphenylamine boron ester as raw materials, thus obtaining a target product. The triphenylamine-thiophene organic dyestuff can serve as a photosensitizer of a dye-sensitized solar cell. The triphenylamine-thiophene organic dyestuff has the advantages that the distortion spatial structure of alkyl triphenylamine is capable of effectively suppressing the electron recombination and increasing the open-circuit voltage; a thiophene derivative serving as a dyestuff molecule conjugated bridge is capable of ensuring the high molar absorption coefficient and has relatively strong power supply performance, so that the ideal photoelectric conversion efficiency is ensured.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
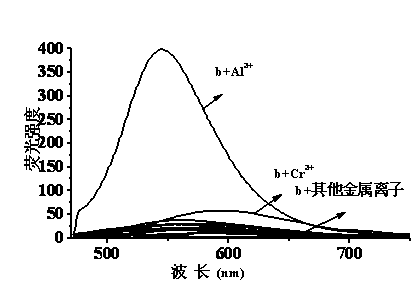
Quinaldine derivative b fluorescent and colorimetric reagent as well as preparation method and application thereof
InactiveCN103641779AOrganic chemistryColor/spectral properties measurementsOrganic synthesisBenzaldehyde
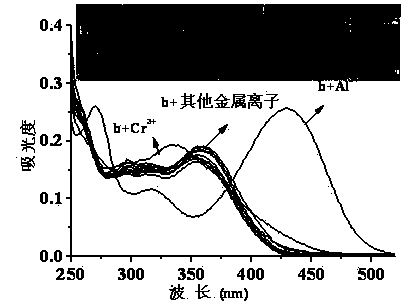
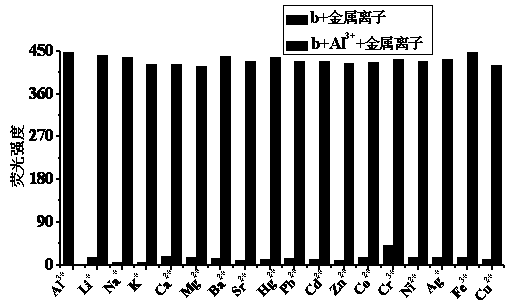
The invention discloses a quinaldine derivative b fluorescent and colorimetric reagent as well as a preparation method and an application thereof and belongs to the field of organic synthesis and analytical chemistry. 8-hydroxyquinaldine serving as a raw material has a Knoevenagel condensation reaction with 2,4-dihydroxy benzaldehyde to obtain an intermediate a, wherein the chemical name of the intermediate a is (E)-2-(2,4-didiacetoxylphenyl)ethenyl-8-acetoxyquinoline; the intermediate a is hydrolyzed in a mixed medium of pyridine and water to obtain a derivative b, wherein the chemical name of the derivative b is (E)-2-(2,4-dihydroxylphenyl)ethenyl-8-hydroxyquinoline. The reaction materials are easily available; the synthesis method is simple; a target product can be obtained by two-step reaction. In a special solvent medium, the derivative b can act as fluorescent and colorimetric reagent for detecting Al<3+> and F-. The structural formula of the derivative is shown in the specification.
Owner:GUIZHOU UNIV
Triphenylamine derivatives, and preparation method and application thereof
ActiveCN102911082ASelf-recoverableIncrease contrastCarboxylic acid nitrile preparationOrganic compound preparationPhenylboronic acidFluorescence
The invention discloses triphenylamine derivatives, and a preparation method and application thereof. The structure of the triphenylamine derivatives is disclosed as Formula (I). The preparation method comprises the following steps: (1) carrying out Knoevenagel condensation reaction on anisaldehyde and p-bromobenzyl cyanide to generate a bromated distyrene nitrile intermediate; and (2) carrying out Suzuki reaction on the bromated distyrene nitrile intermediate and 4-(diphenylamino)phenylboronic acid to generate the triphenylamine derivatives disclosed as Formula (I). The triphenylamine derivatives (I) disclosed by the invention have reversible-force-stimulated fluorescence switching performance, thus, can be used as a reversible-force-stimulated fluorescence switching material, and can be used in the fields of fluorescence switching, sensors, memories, display and the like.
Owner:ZHEJIANG UNIV OF TECH
Hydrosulfate radical ion nanometer sensing material with up-conversion luminescence property and preparation method thereof
InactiveCN103756667ASmall particle sizeUniform particle sizeMaterial nanotechnologyFluorescence/phosphorescenceSolubilityBenzaldehyde
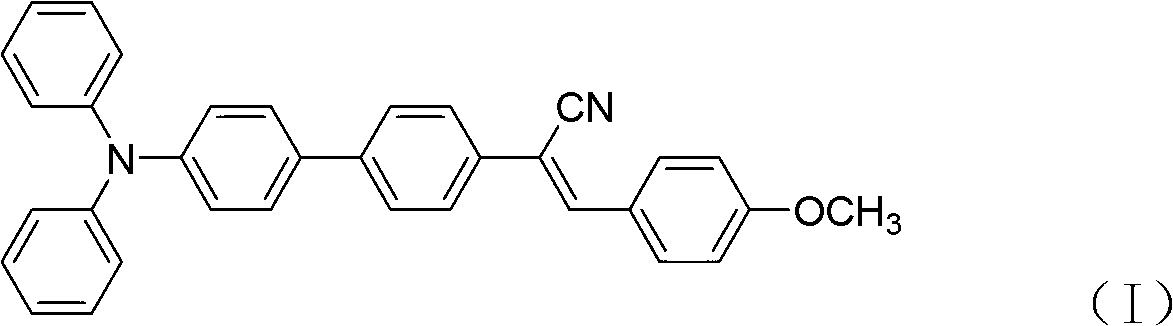
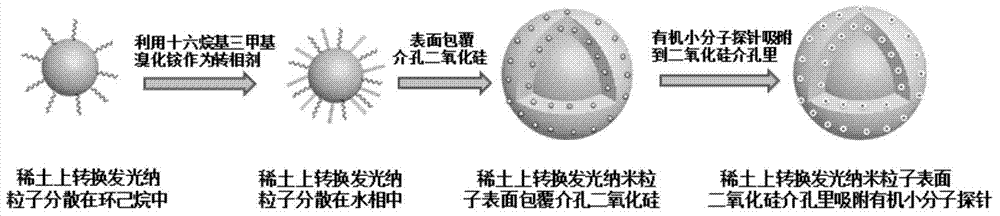
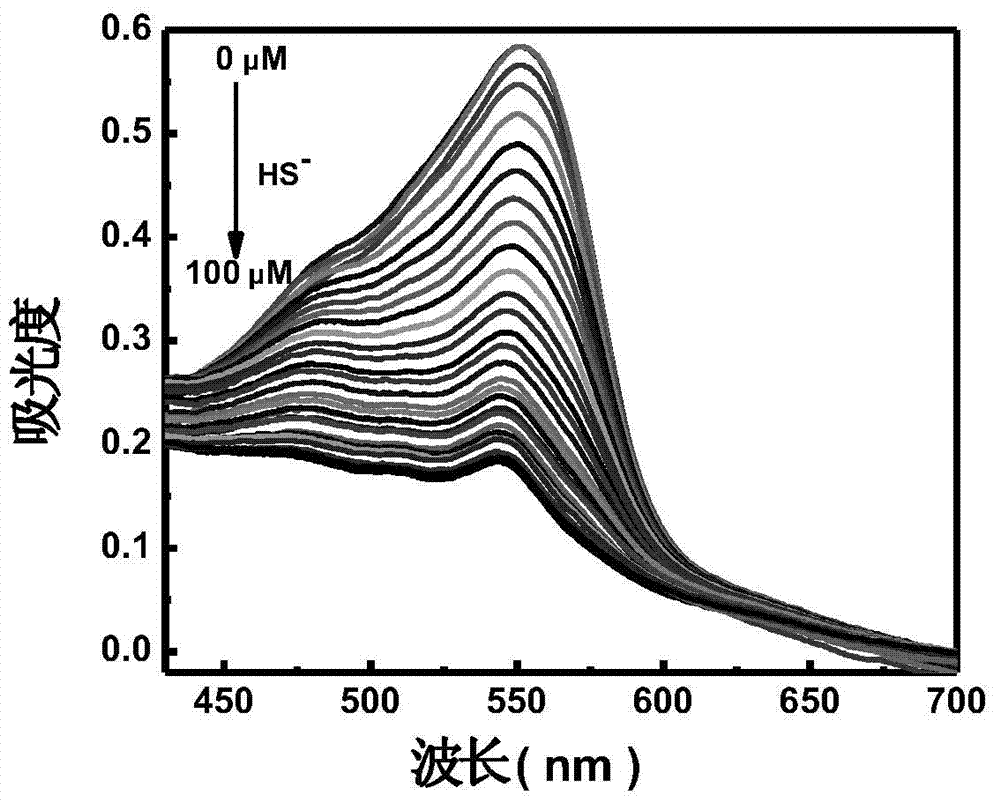
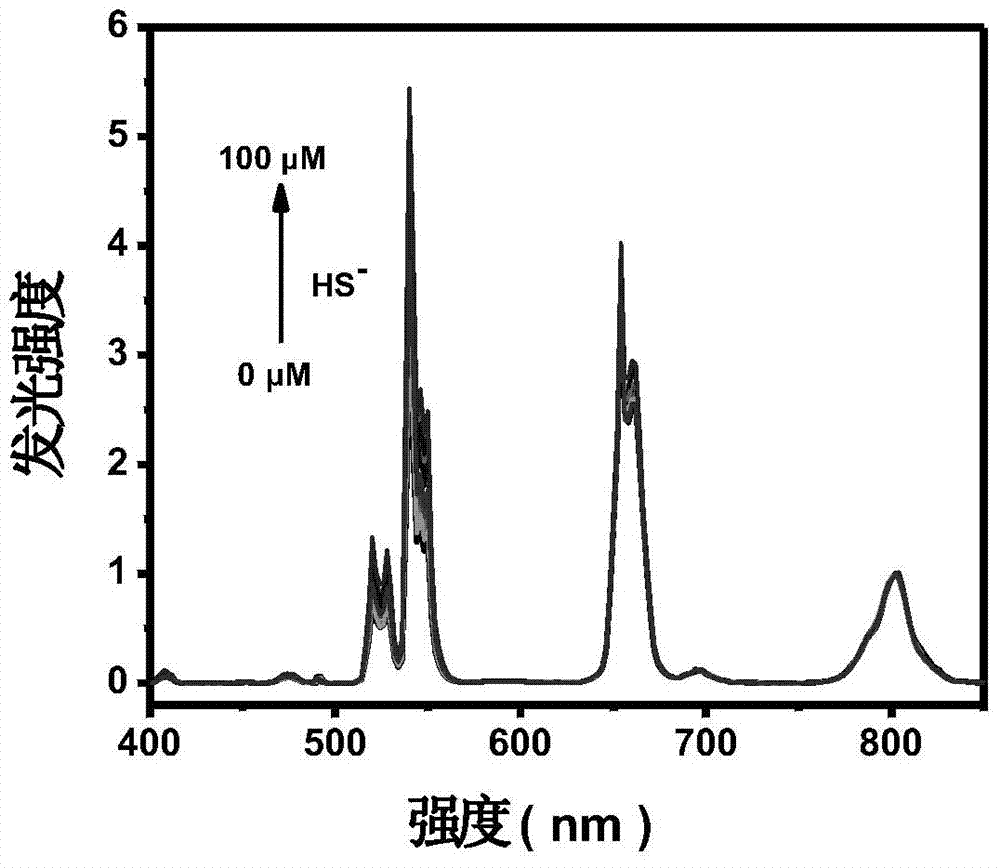
The invention relates to a hydrosulfate radical ion nanometer sensing material with the up-conversion luminescence property, and a preparation method thereof. According to the invention,?a fluoride is adopted as a matrix and doped with trivalent rare earth ions to prepare a rare earth up-conversion luminescence nanometer crystal by using a solvothermal process; the rare earth up-conversion luminescence nanometer crystal is endowed with excellent water-solubility and biocompatibility through surface modification, the surface of the rare earth up-conversion luminescence nanometer crystal is modified by a mesoporous silicon dioxide layer, and an organic micromolecular probe responsive to hydrosulfate radical ions is prepared from a quaternary ammonium salt of an indole derivative and 4-(dimethylamino)benzaldehyde through Knoevenagel condensation; and the organic micromolecular probe is absorbed into the meso pore of silica on the surface of the rare earth up-conversion luminescence nanometer material through physical absorption. The organic-inorganic-hybrid rare earth up-conversion luminescence nanometer material has a controllable size, a uniform particle size and good biocompatibility; the material has a good mesoporous structure and good biocompatibility and can realize high-capacity loading of guest molecules; and the material can be used for detection of hydrosulfate radical ions in a solution and living cells.
Owner:NANJING UNIV OF POSTS & TELECOMM
Synthetic method of effective component-royaljelly acid of royal jelly
InactiveCN101747181AAvoid purification difficultiesAvoid Yield ProblemsOrganic compound preparationCarboxylic compound preparation2-PyrrolidoneCopper
The invention provides a synthetic method of an effective component-royaljelly acid of royal jelly, comprising the following steps: allowing (1,1-bialkoxy-6-hexyl magnesiumhalide) to react with bromoethanol alkyl acid ester in the solvent in the presence of an auxiliary agent N-methyl-2-pyrrolidone under catalysis of catalytic amount of a copper catalyst; removing acetal protection under acid conditions to obtain 8-alkyl acyloxy caprylicaldehyde; and then adopting Knoevenagel condensation method to obtain the royaljelly acid in formula (1). The method adopted by the invention is characterized by mild reaction conditions, simple operation ('one pot method'), high yield and the like, and avoids the problems of difficult purification, low yield and the like encountered by the traditional synthetic technology of the compound, thus greatly reducing production cost. The reagent used in the whole reaction is available, thus being convenient for industrial implementation.
Owner:邵阳市科瑞化学品有限公司
Carbazole-thiophene compound for dye-sensitized solar cell material, and preparation method thereof
InactiveCN104211691AHelps fixIncreased conjugate bridge lengthMethine/polymethine dyesLight-sensitive devicesAcetic acidTerthiophene
The invention discloses a carbazole-thiophene compound for a dye-sensitized solar cell material, and a preparation method thereof. The compound is a carbazolyl-thienyl group-containing dye-sensitized solar cell material obtained through a Knoevenagel condensation reaction of carbazole-thiophene-based aldehyde and cyanoacetic acid, and the molecule of the compound is characterized in that the structure of the compound contains an N-substituted carbazolyl group, a thienyl group, a cyan group and a carboxyl group; and the compound has a long conjugate D-pi-A structure, and contains the cyan group and the carboxyl group, an electron-withdrawing group is cyanoacetic acid, the introduction of the thienyl group increases the conjugate bridge length of the dye molecule, so the compound is an ideal solar cell sensitizer. The carbazole-thiophene compound has the advantages of simple synthesis route, mild reaction conditions, simple and convenient post-treatment, high yield, and convenience for application.
Owner:ANHUI UNIVERSITY
Method for preparing solid base catalyst with high specific surface by hybrid composite precursors
InactiveCN101554596APore Structure RegulationControl areaCarboxylic acid nitrile preparationOrganic compound preparationAcetic acidBenzaldehyde
Owner:BEIJING UNIV OF CHEM TECH
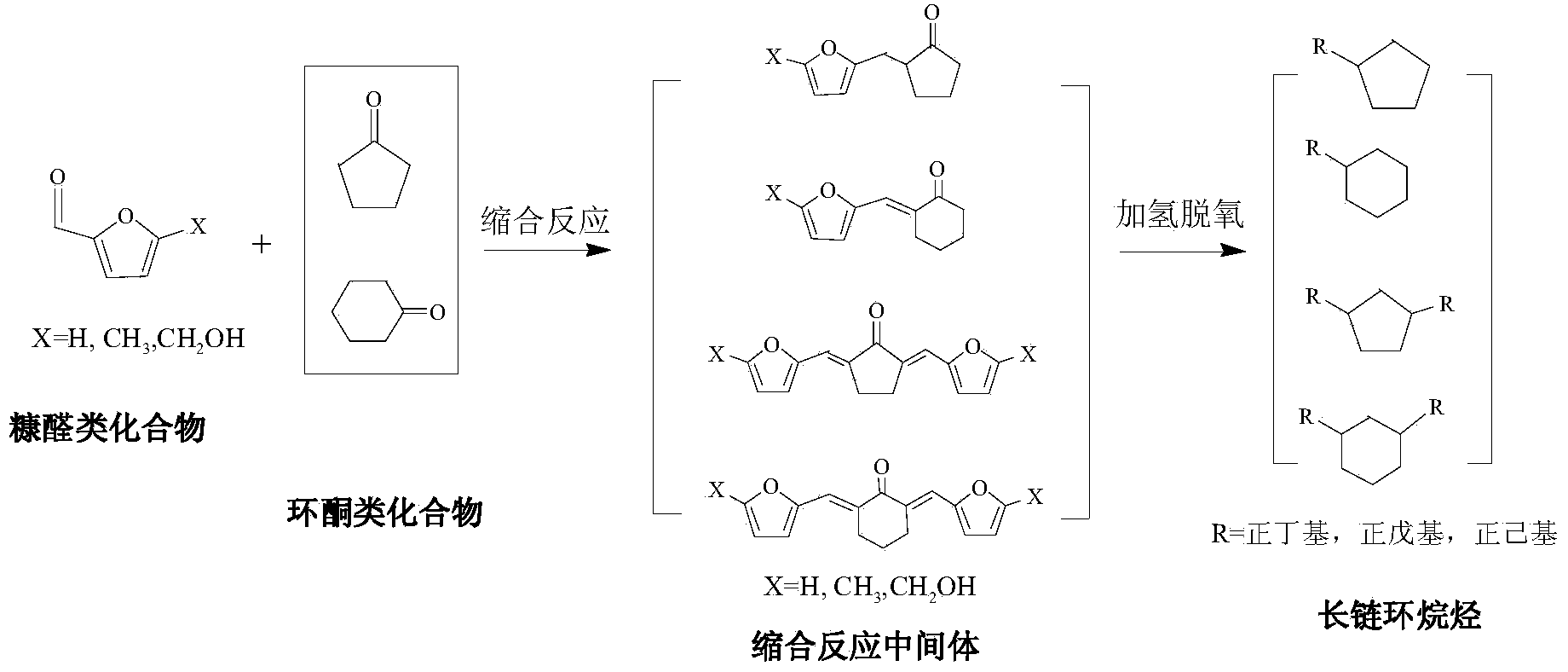
Method for preparing C10-C18 long chain naphthenic hydrocarbon by utilizing furfural compound
ActiveCN104045503AIncrease volumetric energy densitySimple stepsLiquid hydrocarbon mixture productionHydrocarbonsAlkaneKerosene
The invention provides a method for preparing C10-C18 long chain naphthenic hydrocarbon by utilizing furfural compounds obtained from agricultural and forestry wastes. The method mainly uses furfural compounds and cyclic ketone to generate C10-C18 containing oxygen and ring organic compounds through the Knoevenagel condensation reaction, and then the hydrodeoxygenation is conducted to obtain C10 C18 long chain naphthenic hydrocarbon. The method has the advantages of high selectivity, simple steps, simple operation, easily available raw materials, mild reaction conditions, low cost, low equipment requirement and high product yield. The product is long chain naphthenic hydrocarbon with high volume energy density, and is a potential aviation kerosene and a high quality diesel component.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
Preparation and application of solid super alkali catalyst
InactiveCN103212398AEasy to prepareHigh catalytic activityCarboxylic acid nitrile preparationOrganic compound preparationTube furnaceNitrogen atmosphere
The invention provides a preparation method of a composite metal-oxide-based solid super alkali catalyst. According to the method, magnesium nitrate and aluminum nitrate with a molar ratio of [1:1]-[7:1] are prepared into a solution; 1mol / L of NaOH or KOH is used for regulating the pH to 10-12; refluxing is carried out for 12-72h; a precipitate is washed by using water with different amounts; the precipitate is dried, and is heated to 400-700 DEG C in a tube furnace under a nitrogen atmosphere; roasting is carried out for 1-5h, such that the solid super alkali catalyst with K or Na mass content of 1-20% is obtained. According to the solid super alkali catalyst preparation method, synthesizing and modification are carried out simultaneously, wherein modification object K or Na and carrier magnesium-aluminum oxide are simultaneously produced during the roasting process, such that time and energy consumption are saved. When the catalyst is applied in a Knoevenagel condensation reaction, catalyst dose is low, reaction conditions are mild, and catalytic activity is high. The catalyst can be repeatedly used through centrifugal separation.
Owner:HUNAN UNIV
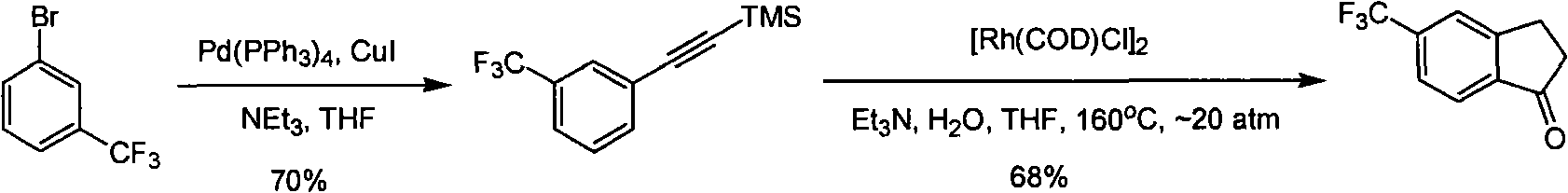
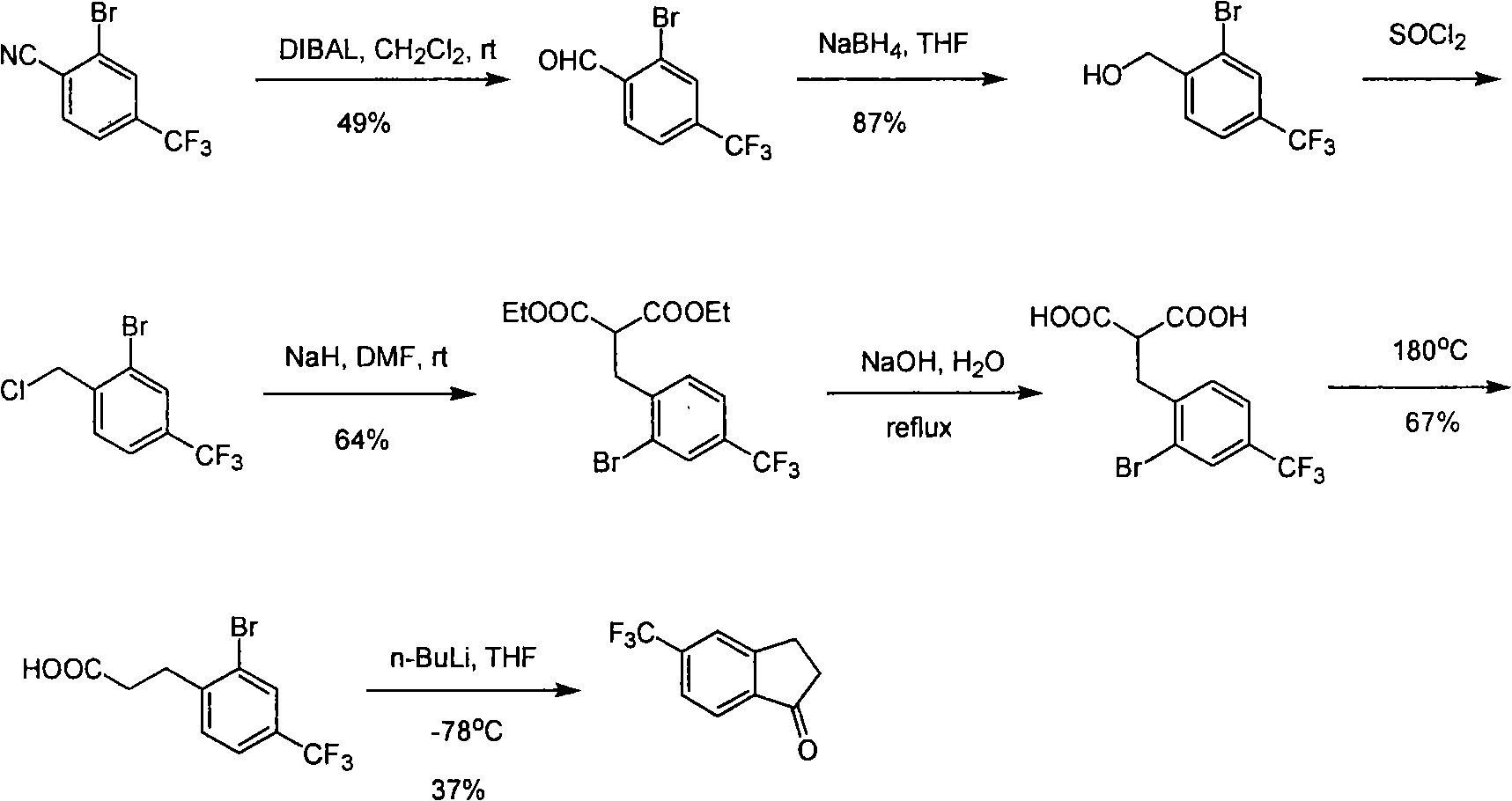
Process for synthesizing 5-trifluoromethyl-1-indene ketone
ActiveCN101293820AEasy to solveRealize large-scale productionCarbonyl compound preparation by condensationBenzaldehydeReaction temperature
The invention relates to a novel method for synthesizing 5-trifluoromethyl-1-indanone. The method comprises the following steps: adopting industrial m-trifluoromethyl benzaldehyde as raw material and preparing m-trifluoromethyl cinnamic acid by Knoevenagel condensation; hydrogenating to obtain m-trifluoromethyl phenylpropionic acid; and preparing 5-trifluoromethyl-1-indanone by intramolecular Friedel-Crafts acylation. The first step is carried out in the presence of malonic acid as organic reagent and pyridine or piperidine as catalyst under reflux at 100 DEG C. The second step is carried out in the presence of palladium / carbon or palladium hydroxide / carbon as catalyst and reaction solvent such as methanol, ethanol, ethyl acetate or tetrahydrofuran under a pressure of 40psi at room temperature. The third step is carried out with trifluoromethanesulfonic acid for closing rings at -20 DEG C-90 DEG C. The method provides a synthesis process for 5-trifluoromethyl-1-indanone which is simple and easy to be scaled, and solves the technical problems in prior art on relative long synthetic route, expensive catalyst or raw material, and severe reaction conditions, high cost, etc.
Owner:WUXI APPTEC (TIANJIN) CO LTD +1
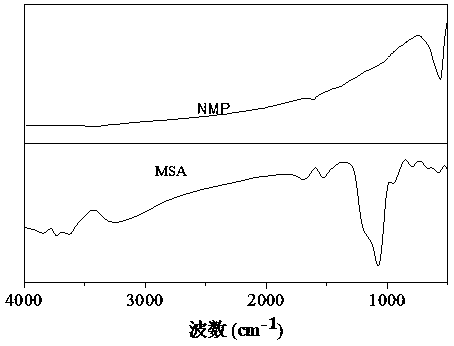
Method for preparing composite magnetic catalyst of magnetic solid sulfonic acid loaded amine catalyst and application thereof
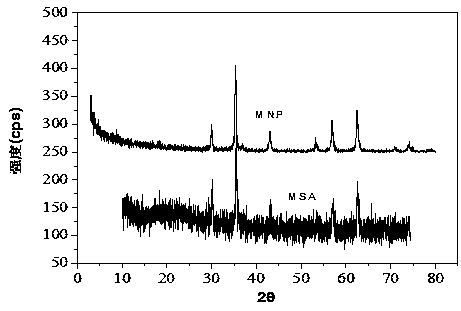
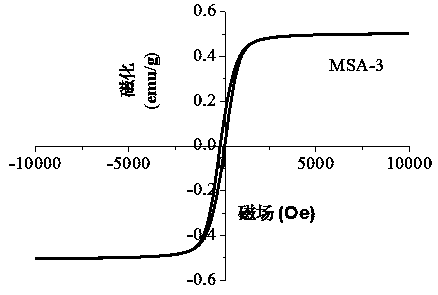
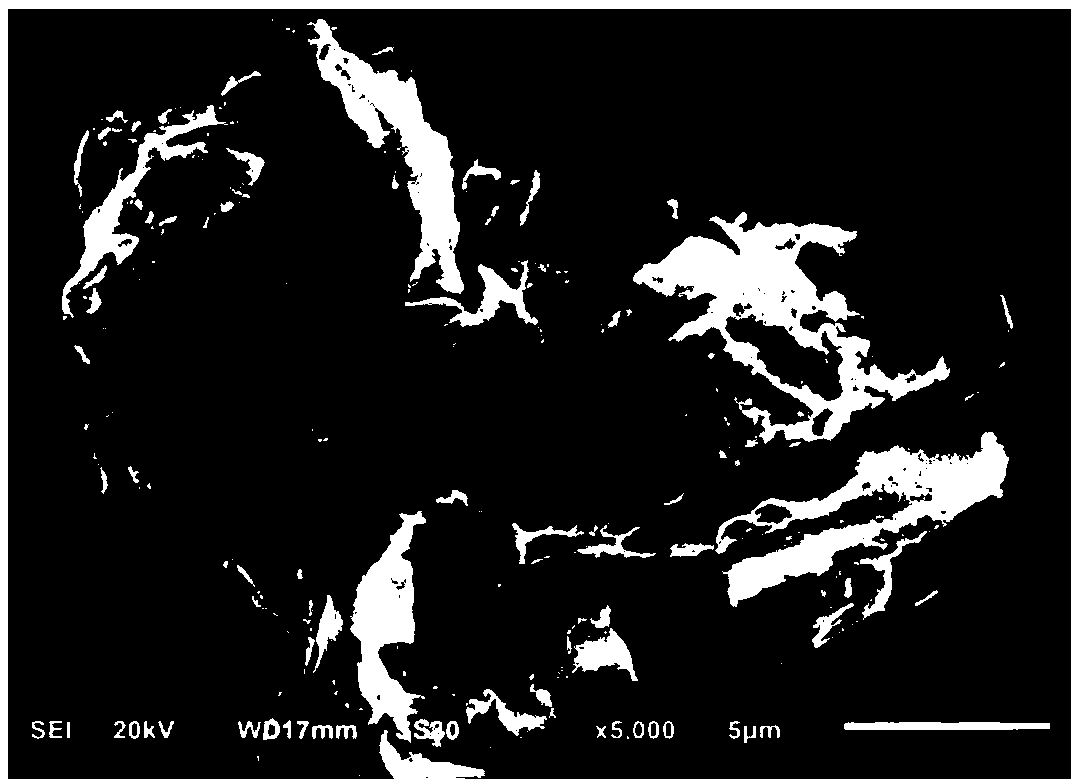
ActiveCN108435248AHigh activityCarboxylic acid nitrile preparationOrganic compound preparationDiamineKnoevenagel condensation
The invention discloses a method for preparing a composite magnetic catalyst of a magnetic solid sulfonic acid loaded amine catalyst. A one-kettle method is used for preparing mercapto coated ball ferriferrous oxide Fe3O4-coated SiO2-SH, hydrogen peroxide is employed for oxidizing a mercapto group (-SH) in Fe3O4-coated SiO2-SH to obtain magnetic sulfonic acid MSA, then magnetic solid sulfonic acidis taken as a carrier, an organic micromolecule amine catalyst is subjected to non covalent loading through acid-base effect, and a magnetic solid sulfonic acid-loaded diamine composite catalyst is prepared. The magnetic solid sulfonic acid of the composite catalyst can be used as the carrier, and a catalytic process can be directly participated and influenced through soda acid effect, so that activity of the composite catalyst for catalysis of a Knoevenagel condensation reaction is effectively increased; through an external magnet, direct attraction and separating can be realized, multitimecycle utilization is realized, and the problems of immobilization, separating recovery and reuse of the catalyst can be effectively solved.
Owner:NORTHWEST NORMAL UNIVERSITY
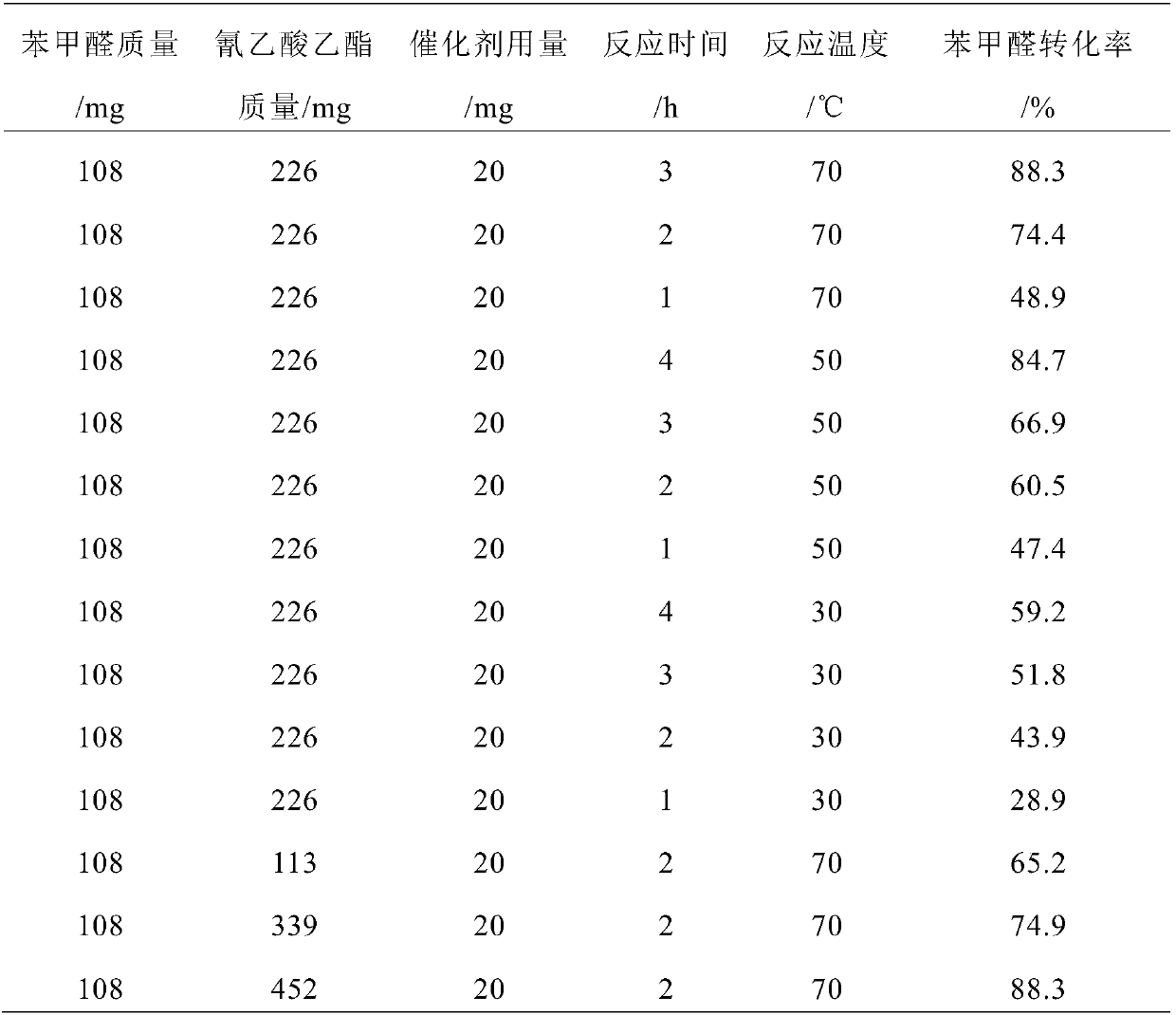
Preparation method of carbon nitride nanosheet catalyst and Knoevenagel condensation reaction method based on catalyst
InactiveCN107824211ARaw materials are easy to getLow reaction temperatureCarboxylic acid nitrile preparationOrganic compound preparationBenzaldehydeSolvent
The invention discloses a preparation method of a carbon nitride nanosheet catalyst. The preparation method comprises mixing a chloride salt, a carbon nitrogen source and a solvent in a mass ratio of(0.1-28):(2-6):(100-500), and performing uniform stirring to form a mixture; removing the solvent in the mixture, performing drying and roasting, performing repeated water washing on the roasted solid, performing separation and drying to obtain the carbon nitride nanosheet catalyst. The invention also discloses a condensation reaction method by using the carbon nitride nanosheet catalyst. According to the invention, when the carbon nitride nanosheet is prepared, the reactants are thoroughly mixed in an aqueous solution, and after one-step pyrolysis, the metal cations and chloride ions in the chloride salts destroy the acting force between layers of the laminar carbon nitride to achieve thermal stripping and further to form laminar carbon nitride nanosheets, thereby improving the catalyticperformance. When the catalyst is applied to a condensation reaction of benzaldehyde and ethyl cyanoacetate, the temperature can be as low as 30 DEG C, and the required longest reaction time is 4 h.
Owner:HUBEI UNIV
Novel 2,6-site-substituted BODIPY organic dye sensitizer and preparation method therefor
ActiveCN105238092ACommonly available raw materialsReduce manufacturing costMethine/polymethine dyesLight-sensitive devicesSynthesis methodsElectron donor
The present invention relates to a novel 2,6-site-substituted BODIPY organic dye sensitizer and a preparation method therefor. The organic dye sensitizer is organic photovoltaic materials with a D-pi-A structure. By taking a meso-site-substituted BODIPY core as a pi bridge framework, the sites 2 and 6 of the BODIPY core is respectively substituted by an electron donor and an electron acceptor. The present invention further discloses a preparation method for the above the dye sensitizer. The preparation method obtains a dye molecule having a general structural formula I by taking 2,4-dimethylpyrrole as an initial reaction raw material, performing a serials of simple synthesis reactions, and finally performing classical Suzuki coupling and Knoevenagel condensation reactions. The dye sensitizer synthesis method is simple, easy to control and high in yield, and has general applicability. When the method is applied to dye sensitized solar cell preparation, a dye sensitized solar cell material with a high fill factor and an ideal photoelectric conversion efficiency can be obtained. The formula I is shown in the description.
Owner:邳州市润宏实业有限公司
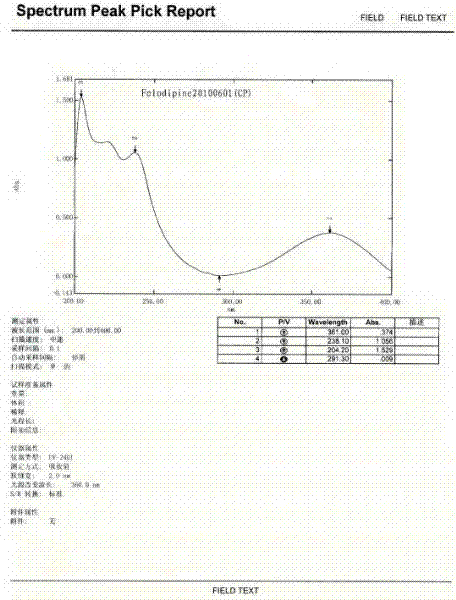
The preparation method of felodipine
InactiveCN102285911AEasy to operatePreparative route steps are short and effectiveOrganic chemistryAcetic acidEthyl ester
The invention discloses a method for preparing felodipine, which belongs to the technical field of felodipine synthesis. The method is characterized by comprising the following steps of: performing a Knoevenagel condensation reaction of 2,3-dichlorobenzaldehyde and methyl acetoacetate which are used as initial raw materials to obtain 2,3-dichlorobenzylidene acetoacetate; and performing a Michael cyclization reaction of 2,3-dichlorobenzylidene acetoacetate and 3-amino ethyl crotonate to obtain the felodipine to obtain the felodipine. The method has the advantages that the preparation route is simple, short and reliable; the preparation period is short; the method is simple and convenient to operate and has high safety and low environmental pollution; residual toxicity of a medicament is low; and the post treatment process has high efficiency.
Owner:SHAOXING UNIVERSITY
Dithiophene pyrrole bridge-indoline organic dyes as well as preparation method and application thereof
InactiveCN103554958AEffective printingHigh molar absorptivityLight-sensitive devicesOrganic chemistryOrganic dyePyrrole
The invention discloses dithiophene pyrrole bridge-indoline organic dyes with the following structural formula which is shown in the specification, wherein R1 is C1-C6 alkoxy, a structural formula of Ar is (II) or (III); in the formula (II) and (III) which are shown in the specification, R2 is C1-C6 alkyls. A preparation method of the dithiophene pyrrole bridge-indoline organic dyes comprises the following steps of: using indoline boron ester, tetra triphenylphosphine, potassium carbonate and dithiophene pyrrole bromal substituted by alkoxy triphenylamine as materials; and obtaining a target through Suzuki coupling reaction and Knoevenagel condensation reaction in sequence, wherein the target can be used as a photosensitizer of a dye-sensitization solar cell. The preparation method disclosed by the invention has the advantage that a molar absorption coefficient and absorbing ability to light of dye molecules can be effectively improved by introducing the dithiophene pyrrole bridge into the indoline dyes; printed electrons can be effectively compounded and open-circuit voltage can be effectively improved by introducing large steric-hindrance groups into the indoline and the dithiophene pyrrole bridge, so that photoelectric conversion efficiency of the solar cell is improved.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Method for catalyzing Knoevenagel condensation reaction by using function ion liquid
InactiveCN103351270ASpecial featuresSpecial thermodynamic stabilityCarboxylic acid nitrile preparationOrganic compound preparationRoom temperatureIonic liquid
The present invention discloses a method for catalyzing a Knoevenagel condensation reaction by using a function ion liquid. The method comprises that: a choline chloride function ion liquid is adopted as a catalyst, and aromatic aldehyde and an active methylene compound are subjected to a catalysis condensation reaction at a room temperature under a normal pressure to obtain a condensation product. The method has the following characteristics that: operations are simple, a yield is high, the catalysis reaction system has good reusability, reaction conditions are mild, and good industrialization prospects are provided.
Owner:TAIZHOU UNIV
Cyanostilbene derivative, preparation method and application thereof
ActiveCN105111102AReversible Force Stimulation of Fluorescent Switching PropertiesIncrease contrastCarboxylic acid nitrile preparationOrganic compound preparationPhenylboronic acidFluorescence
The invention provides a cyanostilbene derivative shown as formula (I). The preparation method includes: reacting 4-bromobenzaldehyde shown as formula (II) with 4-(diphenylamino)phenylboronic acid shown as formula (III) to generate a triphenylamine intermediate shown as formula (IV); and then subjecting the triphenylamine intermediate shown as formula (IV) and 4-methoxybenzonitrile shown as formula (V) to Knoevenagel condensation reaction so as to generate the cyanostilbene derivative. The force stimulation responsive fluorescence switch performance of the cyanostilbene derivative has the characteristics of high contrast, self restorability and good cyclicity, the synthesis method is simple, and the cyanostilbene derivative can be applied as a reversible force stimulation fluorescence switch material in fluorescent switch, sensor, storage, display and other fields. (formula (I), formula (II), formula (III), formula (IV) and formula (V)).
Owner:ZHEJIANG UNIV OF TECH
Opto-acoustic probe used for detecting nitroreductase in living bodies and preparation method and application thereof
InactiveCN109627236AOvercoming scatteringOvercome absorbencyOrganic chemistryMaterial analysis by optical meansKnoevenagel condensationTumor marker
The invention discloses an opto-acoustic probe used for detecting nitroreductase in living bodies and a preparation method and application thereof, and belongs to the field of detection and imaging oftumor markers. The structure formula of the opto-acoustic probe used for detecting nitroreductase in living bodies is shown in the formula I. The probe mainly takes benzo Cy7-C1 as a raw material, and through nucleophilic substitution, the crewengel condensation reaction and the chloroformate nucleophilic substitution reaction, the probe is obtained. The problems of scattering of a fluorescence signal and tissue absorption in the process of detection for the living bodies by adopting a fluorescence probe are solved, the prepared probe has the advantages that the penetration depth of an acoustical signal is large, imaging of deep tissue is achieved, response is quick, the specificity is good, and ratio detection is more accurate. The probe can be used for detecting the nitroreductase in the living bodies, thereby monitoring anaerobic conditions of tumors; the probe can also be applied to detection of the nitroreductase in cells and biological samples.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Preparation method of micro-molecular receptor for polymeric solar batteries
The invention relates to a preparation method of a micro-molecular receptor for polymeric solar batteries. The preparation method comprises the following steps: 1, carrying out a Suzuki coupling reaction on dithienoindacene trimethyltin and 4-bromo-7-aldehyde-2,1,3-benzothiadiazole to prepare an aldehyde-terminated intermediate; and 2, carrying out a Knoevenagel condensation reaction on the terminated intermediate through using 3-ethylrhodanine to obtain the micro-molecular receptor product. The micro-molecular receptor prepared in the invention can be used for the polymeric solar batteries, and has the characteristics of low price, simple preparation and excellent photovoltaic conversion efficiency.
Owner:NANCHANG HANGKONG UNIVERSITY
Preparation method of piperazine functionalized ordered mesoporous phenolic resin solid base catalyst
InactiveCN101811068AHigh mechanical strengthOrdered mesoporous structureCarboxylic acid nitrile preparationOrganic compound preparationSolvent evaporationSolvent
The invention discloses a preparation method of a piperazine functionalized ordered mesoporous phenolic resin solid base catalyst. The method comprises the specific steps: under the alkali condition, phenol, formaldehyde and piperazine functionalized formaldehyde are copolymerized; the solvent evaporation induced self-assembly (EISA) method is adopted to be in hot polymerization to prepare piperazine a functionalized ordered mesoporous phenolic resin heterogeneous catalyst (Piperazidine-MPs). The invention also relates to application thereof in Knoevenagel condensation reaction in aqueous medium. In the Knoevenagel condensation reaction in the aqueous medium, the Piperazidine-MPs prepared by the EISA method has similar catalytic activity as the heterogeneous catalyst.
Owner:SHANGHAI NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com