Carbazole-thiophene compound for dye-sensitized solar cell material, and preparation method thereof
A technology for solar cells and dye sensitization, which is applied in the field of carbazole-thiophene compounds and their preparation, can solve the problems of toxic and expensive, and achieve the effects of simple and convenient post-processing, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of dyestuff 3-(4-cyanoacrylate thiophene)-N-n-hexylcarbazole
[0037] (1) Synthesis of N-hexylcarbazole
[0038] Weigh 1.65 g (10 mmol) of carbazole into a round bottom flask equipped with a stirring bar, add 10 mL of anhydrous DMF to dissolve completely at room temperature, then add 0.4 g NaH to the flask, seal with oil, stir at room temperature for 20 min, and use a constant pressure funnel to Add 2 mL of n-C solution dropwise 6 h13 Br in DMF solution 3mL, 80 ℃ oil bath reflux 3h. After cooling to room temperature, pour it into water and stir thoroughly, a beige solid is formed, stand until the solution becomes clear, filter with suction, recrystallize with ethanol to obtain white needle crystals, filter with suction, and air-dry to obtain 1.48g of needle crystal products, Yield 58.85%. 1H-NMR (CDCl 3 ,400Hz):δ(ppm):8.09(d,J=7.6Hz,2H),7.38~7.48(m,4H),7.21(d,J=8.0Hz,2H),4.28(t,2H),1.86 (m,2H), 1.33(m,6H), 0.86(t,3H). FT-IR (KBr, cm -1...
Embodiment 2
[0045] Embodiment 2: The ultraviolet absorption spectrum of 3-(4-cyanoacrylic acid thiophene)-N-n-hexylcarbazole and the measurement of fluorescence spectrum
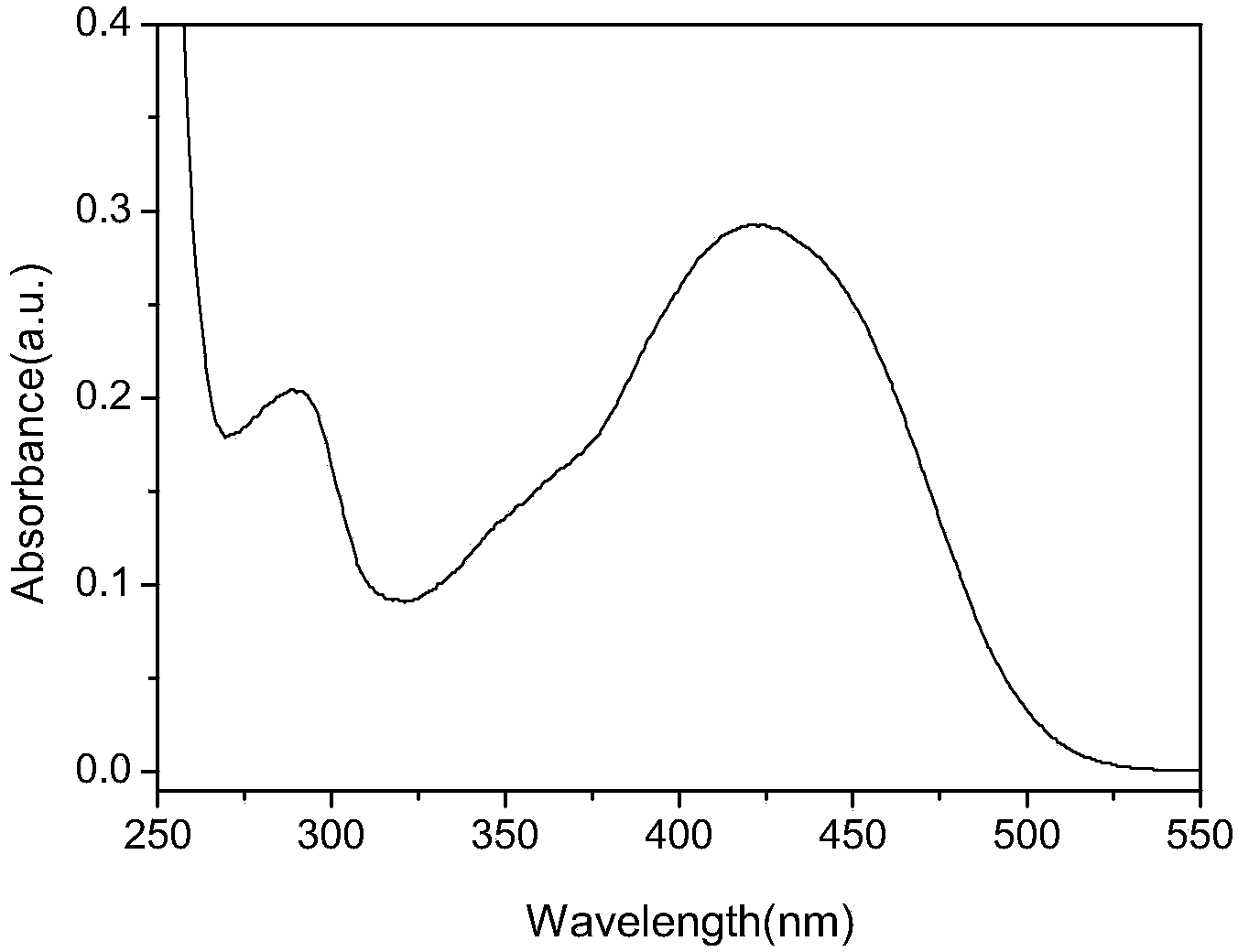
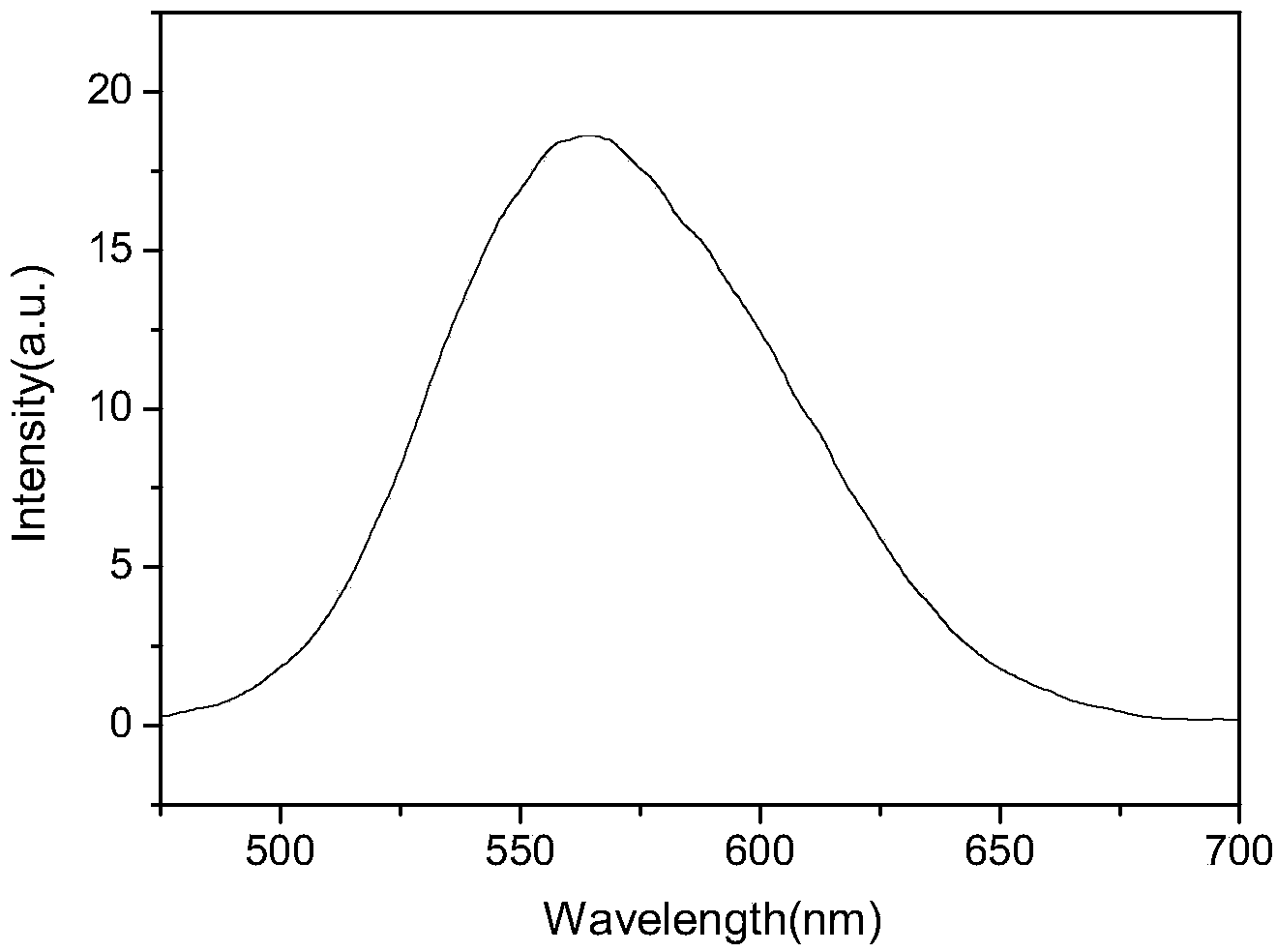
[0046] The ultraviolet-visible absorption spectrum was measured on a Shimadzu UV-3600 ultraviolet-visible spectrophotometer, and the measurement range was between 200-1000nm. The concentration of the sample to be tested is 1.0×10 -5 mol / L test solution. The ultraviolet-visible spectrum and fluorescence spectrum were measured respectively. From figure 1 Visible dyes have two absorption peaks: the absorption peak at 291nm in the high energy region is the carbazole functional group π-π * The characteristic absorption peak, the position of the absorption peak in the low energy region is located at 426nm, which is attributed to the intramolecular charge transfer (ICT) transition of the whole molecule. From the analysis of the ultraviolet-visible absorption spectrum, the electron-withdrawing group of the dye is cyanoaceti...
Embodiment 3
[0048] Embodiment 3: the mensuration of the electrochemical voltammetry characteristic of 3-(4-cyanoacrylic acid thiophene)-N-n-hexylcarbazole
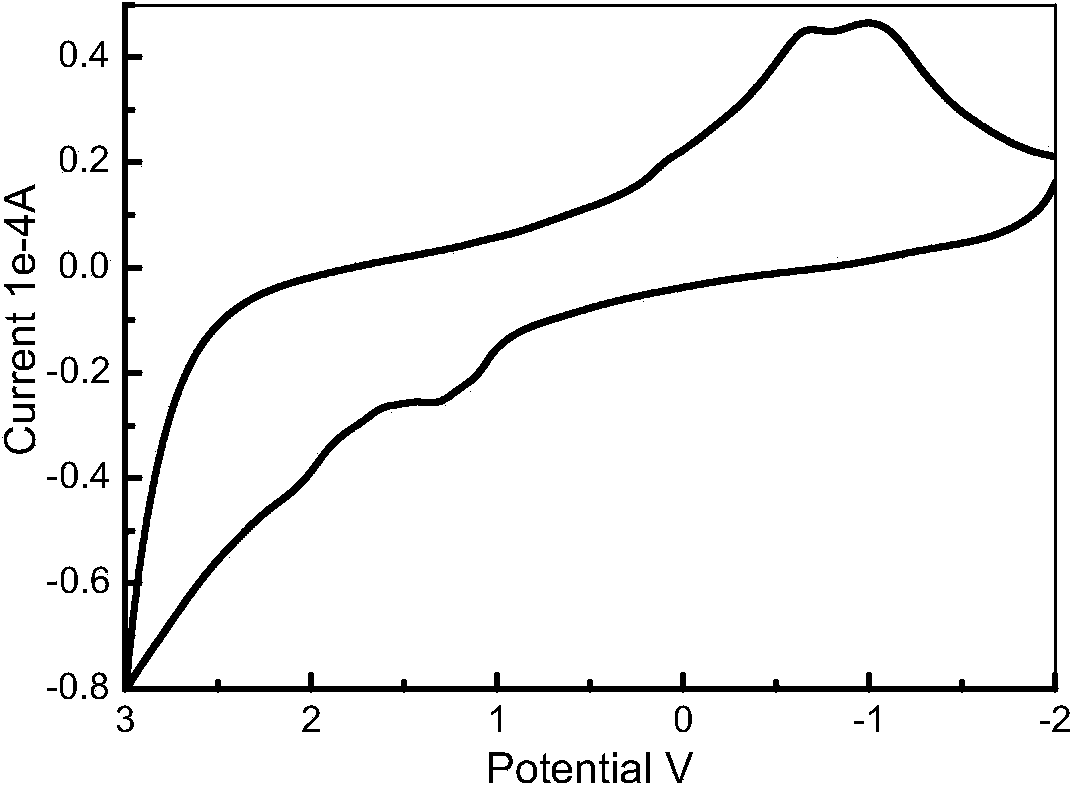
[0049] The redox energy level of the dye in the solution was determined by cyclic voltammetry on Chenhua CHI660 instrument electrochemical workstation. The test uses a three-electrode working system: the working electrode is a glassy carbon electrode, the counter electrode is a Pt wire electrode, and the reference electrode is a saturated calomel electrode. The solvent used in the test was chromatographically pure acetonitrile. The electrolyte was 0.05M tetrabutylammonium perchlorate. In order to reduce the error, each sample was scanned 3 times.
[0050] The reference electrode used in the experiment is a saturated calomel electrode, and its potential needs to be converted into a standard hydrogen electrode potential, which is 0.24 eV relative to the NHE potential, HOMO=eE ox +0.24. image 3 is the cyclic voltammetry characterist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com