Patents
Literature
282 results about "Cyanoacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyanoacetic acid is an organic compound. It is a blue, hygroscopic solid. The compound contains two functional groups, a nitrile NC and a carboxylic acid. It is a precursor to cyanoacrylates, components of adhesives.
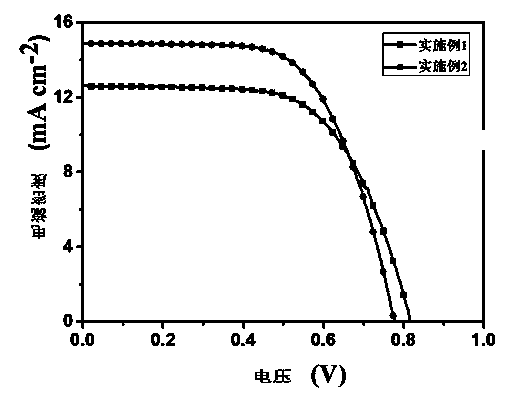
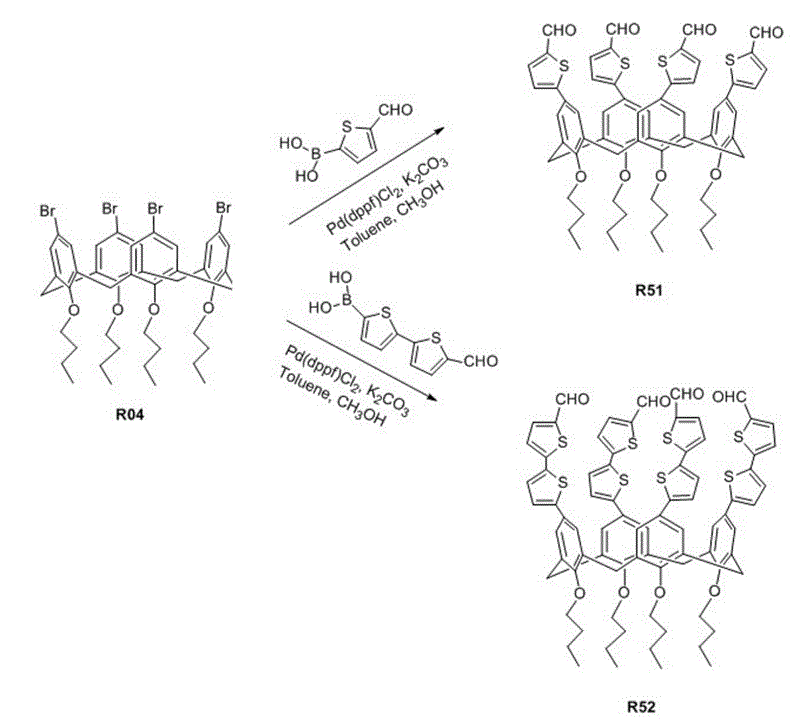
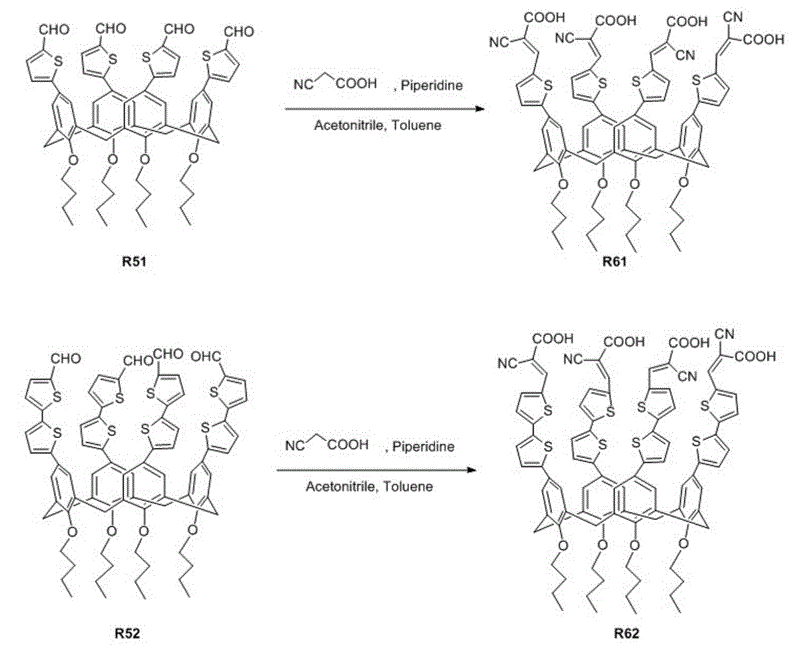
Organic dye with five-element heterocycle and derivative thereof as conjugate unit, and dye sensitization solar cell prepared thereby
ActiveCN101407639APromote absorptionSimple structure and easy accessLight-sensitive devicesMethine/polymethine dyesOrganic dyeCyanoacetic acid
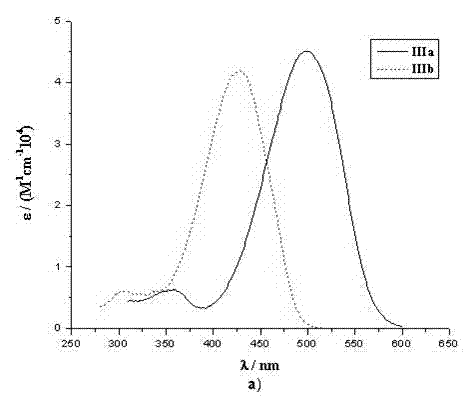
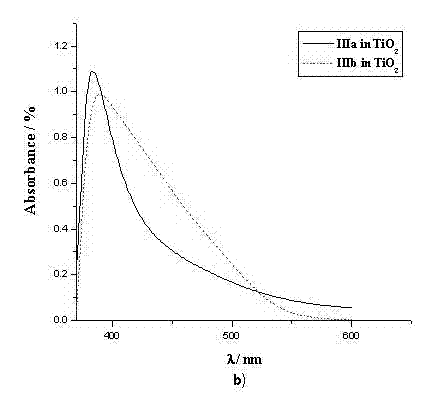
The invention provides an organic dye which uses five-membered heterocyclic ring, derivatives thereof and a dye-sensitized solar cell prepared by the organic dye. The dye uses the five-membered heterocyclic ring or the derivatives thereof as a Pi unit, uses substituted triarylamine as an electron donor, uses cyanoacetic acid structure as an electron acceptor, and belongs to molecule of D-Pi-A structure. The dye molecule belongs to pure organic compounds, the structure of the materials is simple and is easy to be obtained; expensive materials of metal of Ru and purifying agent of polydextrose do not need to use, the compound yield is higher, so the total yield is between 40 percent and 70 percent; besides, the performance of spectral absorption and molar extinction coefficient and the like of the dye are excellent; and the peak value of the spectral absorption is over 550nm at the most, the molar extinction coefficient is over 48000M<-1>cm<-1>, the peak value and the coefficient are more than the Ru dye, therefore, the dye has wider absorption range to the sunlight. The dye-sensitized solar cell prepared by the organic dye has the maximum of quantum conversion efficiency of 97 percent and the highest photoelectric conversion efficiency of more than 8.0 percent.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Method for synthesizing citric acid tofacitinib
ActiveCN106146517AEliminate potential safety hazardsShort reaction timeCarboxylic acid salt preparation4-methylpiperidineTofacitinib
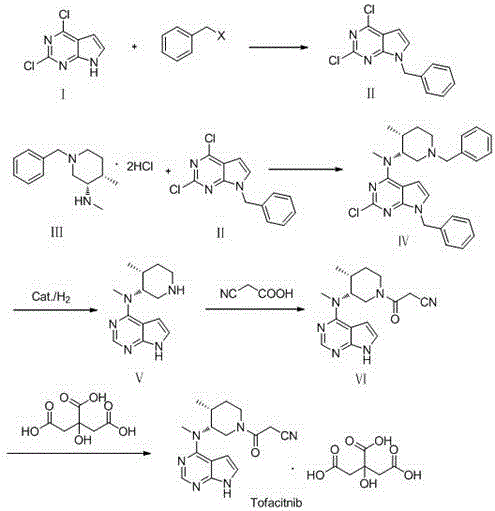
The invention discloses an efficient and safe method for synthesizing citric acid tofacitinib. N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-base]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine is used as the raw material, Pd / C and HCOOH reduction debenzylation is conducted, condensation is conducted under the catalysis of an EDCI or EDCI, HOBT and triethylamine compound system with cyanoacetic acid, and salifying is conducted with citric acid in acetone to obtain citric acid tofacitinib. By the adoption of the synthesis method, potential safety hazards caused by hydrogen and ammonium formate are avoided, debenzylation reaction and amidation are thorough, no side reaction is caused basically, reaction time is shortened greatly, yield is high, aftertreatment is easy, and citric acid tofacitinib can be prepared efficiently and safely.
Owner:ZHEJIANG LEPU PHARMA CO LTD
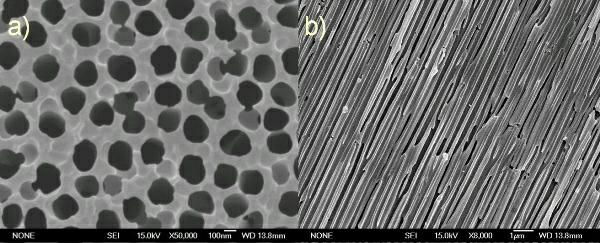
Preparation method of self-supporting molecularly imprinted polymer film
ActiveCN102432778ASolve the larger particlesSolve for uniformityOther chemical processesAlkali metal oxides/hydroxidesPolymer scienceCyanoacetic acid
The invention relates to a preparation method of a self-supporting molecularly imprinted polymer film. The preparation method comprises the following steps of: firstly, reacting divalent metal ions with a porous alumina film to obtain a porous alumina-based layered double hydroxide; secondly, carrying out further reaction to obtain an SBC (Sulpho-Benzoyl Cyanoacetic Acid) intercalated porous alumina-based layered double hydroxide; and finally, mixing the SBC intercalated porous alumina-based layered double hydroxide with a polymerizable monomer, a polymerized cross-linking agent, a molecularly imprinted template agent and an organic solvent, dispersing by using ultrasound wave, adding a free base polymerization initiator for polymerizing, washing a product obtained by reacting and then drying to obtain the self-supporting molecularly imprinted polymer film. According to the self-supporting molecularly imprinted polymer film disclosed by the invention, the advantages of high strength, corrosion resistance, high temperature resistance and great specific area of an inorganic material as well as high load information quantity and adjustable performance of an organic polymer material can be fully exerted; the active free base polymerization is assisted in controlling; and the controllable synthesis of an ultrathin nano self-supporting structure of a molecularly imprinted material can be realized by using the controllability of the growth process of an active chain.
Owner:SHANGHAI UNIV
Method for preparing high purity solid cyanoacetic acid
InactiveCN101270063AHigh purityImprove efficiencyPreparation by cyanide reactionAcetic acidSodium chloroacetate
The present invention discloses a preparation method of high-purity solid cyano acetic acid. The preparation method comprises the following steps: (1) chloroactic acid and sodium hydroxide have the reaction of neutralization and cyanidation to prepare sodium chloroacetate aqueous solution; hydrochloric acid is added for neutralization; then the mixed solution of cyano acetic acid and sodium chloride can be prepared; (2) at the temperature of 20 to 90 DEG C, ester solvents are used for extracting the mixed solution prepared in the first step; the volume ratio of the mixed solution and the ester solvents is 1 : 1 to 5; the time of extraction is 4 to 10; the time of each extraction step is 5 to 15 minutes; (3) the extract is condensed through vacuum evaporation to prepare the high-purity solid cyano acetic acid. The preparation method has the advantages of simple process and low investment. The extractant is stable, cheap and easily available; and the extractant can be recycled and reused. More importantly, the preparation method can be used for preparing the high-purity solid cyano acetic acid and can satisfy the requirements for refining the high-purity solid cyano acetic acid in the industry.
Owner:TIANJIN UNIV
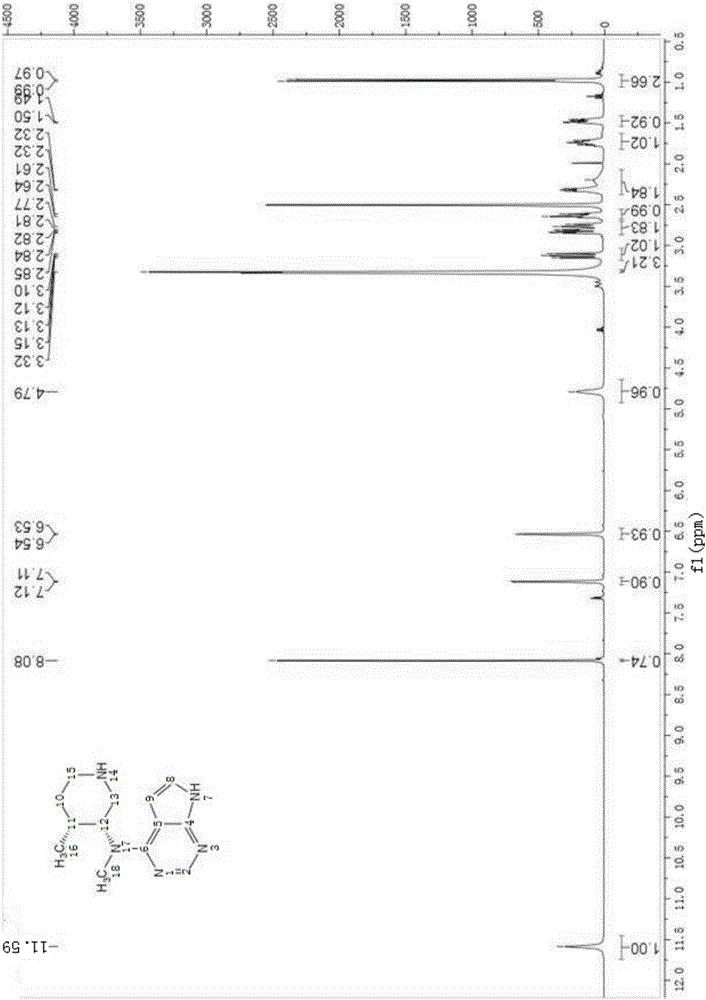
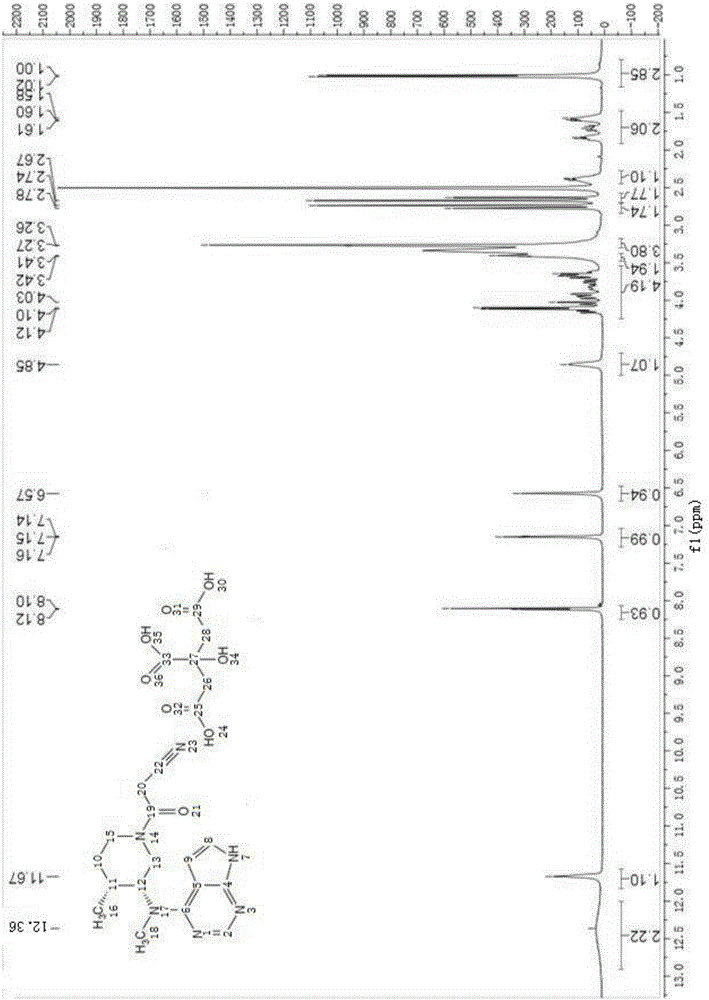
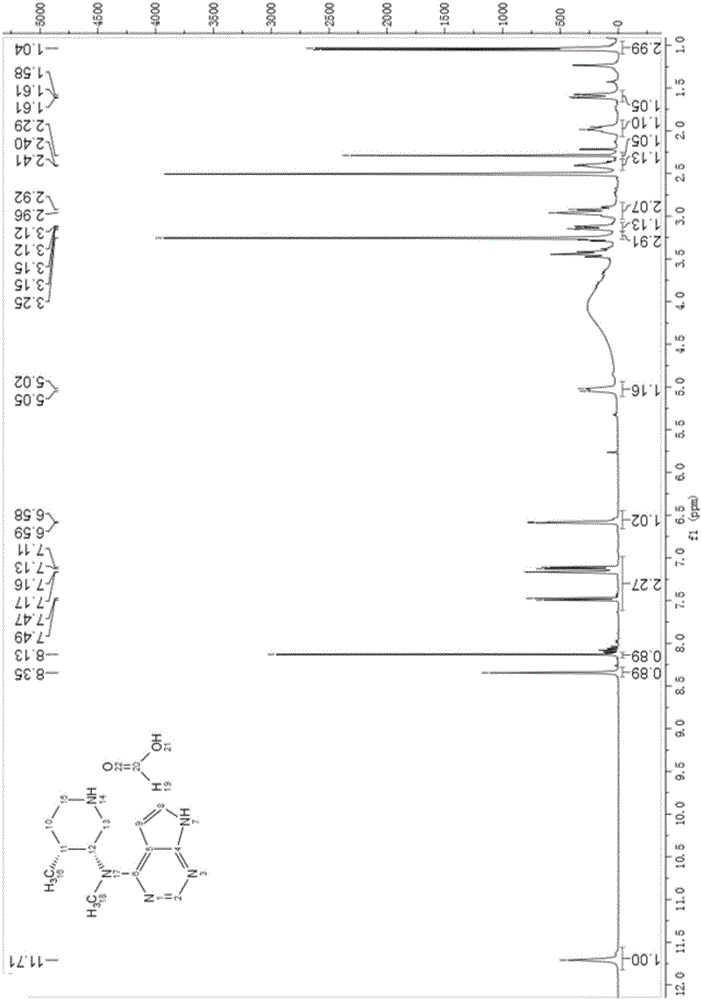
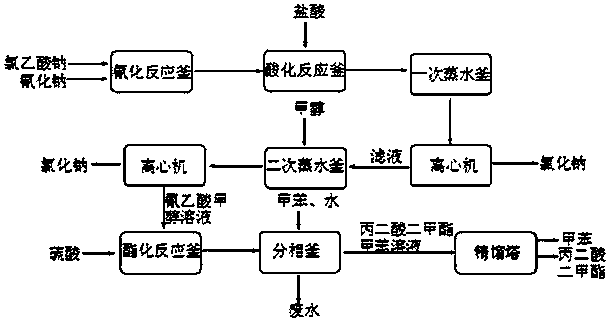
Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine
InactiveCN105622616AIncrease productivityImprove product qualityOrganic chemistryCyanoacetic acidFormamidine acetate
The invention relates to a preparation method of 4-chloropyrrolo[2,3-d]pyrimidine.The method includes the following steps of obtaining 2-cyano-3-(1,3-dioxolan)ethyl propionate after 2-bromomethyl-1,3-dioxolane and ethyl cyanoacetate which are used as the raw materials react with alkaline matter as the catalyst; conducting cyclization on obtained 2-cyano-3-(1,3-dioxolan)ethyl propionate and formamidine acetate with alkaline matter as the catalyst, and adding hydrochloric acid for hydrolysis cyclization to obtain pyrrolo[2,3-d]pyrimidin-4-ol; making obtained pyrrolo[2,3-d]pyrimidin-4-ol react with phosphorus oxychloride to obtain 4-chloropyrrolo[2,3-d]pyrimidine.The method for preparing 4-chloropyrrolo[2,3-d]pyrimidine is simple in technological process, the requirement for production conditions is low, the product is easy to purify and high in yield, and the production efficiency and product quality of 4-chloropyrrolo[2,3-d]pyrimidine are remarkably improved.
Owner:ABA CHEM SHANGHAI
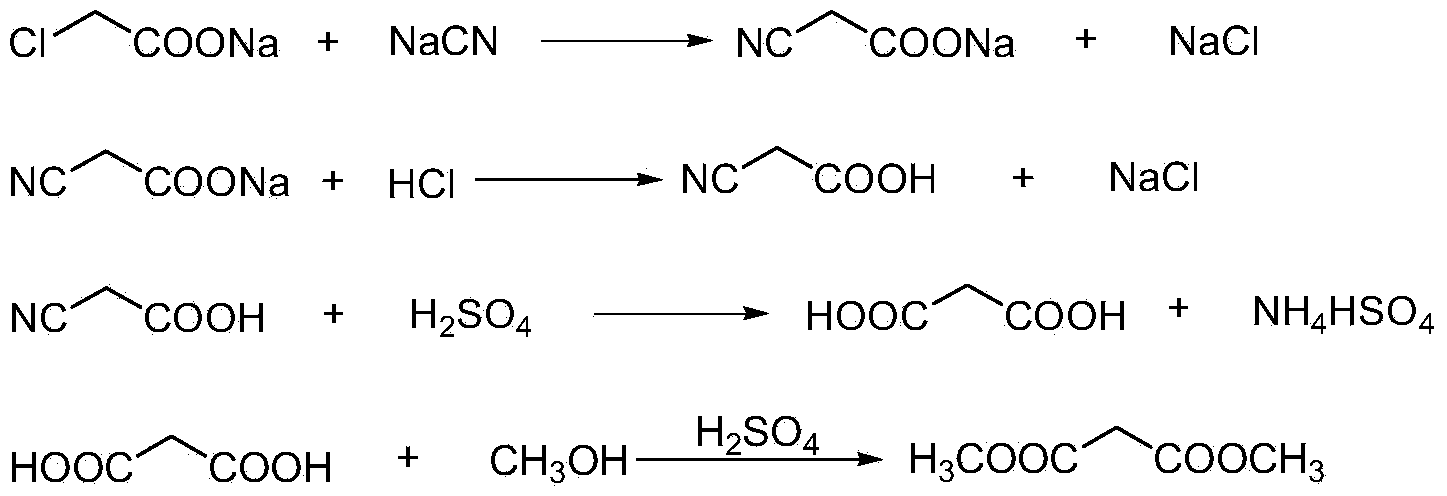
Process of preparing malonic ester
InactiveCN1834081AReduce consumptionCause a lot of decompositionOrganic compound preparationCarboxylic acid esters preparationAcetic acidMalonic acid
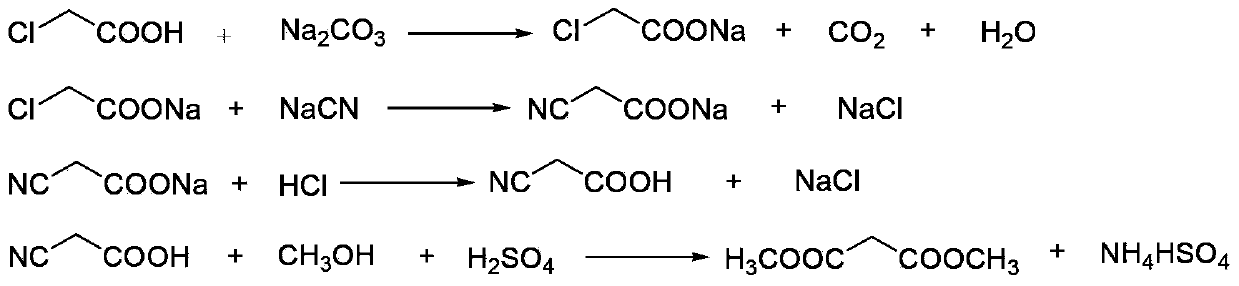
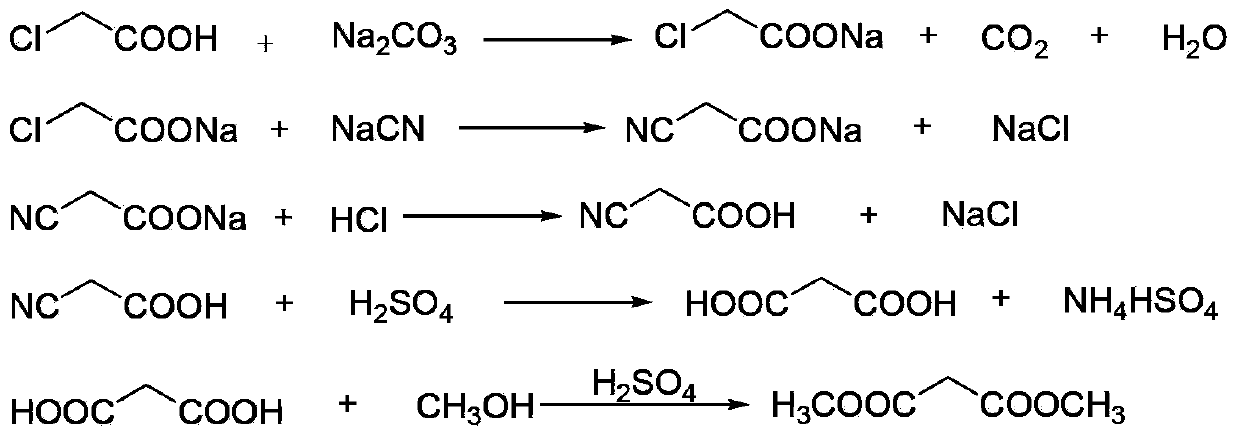
This invention relates to a method to synthesize malonate from chloroacetic acid, which includes neutralization, cyanidation, acidification, dehydration and esterification. It has the characteristics that dilute sulphuric acid is added after dehydration so that cyanoacetic acid is hydrolyzed into malonic acid which is then esterified to obtain malonate. This invention has the advantages of low cost, easily-controlled technique and high yield.
Owner:CHONGQING UNISPLENDOUR CHEM
Synthetic method of pregabalin
InactiveCN104496832AImprove conversion rateShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationPregabalinHydrolysis
The invention discloses a synthetic method of pregabalin. According to the synthetic method, isovaleraldehyde and cyanoacetic alkyl ester are used as starting materials; condensation reaction, Michael addition reaction, hydrolysis reaction, amidation reaction and the like are carried out successively to obtain 3-(carbamoyl methyl)-5-methyl-hexanoic acid; and resolution and Hofmann elimination are carried out to obtain pregabalin. The greatest improvement of the synthetic method is to carry out the hydrolysis reaction and the amidation reaction under the condition of near-critical water. Thus, addition of a catalyst is avoided, and yield of the reaction is raised. In addition, a flow reactor can be applied to the reaction so as to obtain a better reaction effect.
Owner:ZHEJIANG MENOVO PHARMA
Carbazole-thiophene compound for dye-sensitized solar cell material, and preparation method thereof
InactiveCN104211691AHelps fixIncreased conjugate bridge lengthMethine/polymethine dyesLight-sensitive devicesAcetic acidTerthiophene
The invention discloses a carbazole-thiophene compound for a dye-sensitized solar cell material, and a preparation method thereof. The compound is a carbazolyl-thienyl group-containing dye-sensitized solar cell material obtained through a Knoevenagel condensation reaction of carbazole-thiophene-based aldehyde and cyanoacetic acid, and the molecule of the compound is characterized in that the structure of the compound contains an N-substituted carbazolyl group, a thienyl group, a cyan group and a carboxyl group; and the compound has a long conjugate D-pi-A structure, and contains the cyan group and the carboxyl group, an electron-withdrawing group is cyanoacetic acid, the introduction of the thienyl group increases the conjugate bridge length of the dye molecule, so the compound is an ideal solar cell sensitizer. The carbazole-thiophene compound has the advantages of simple synthesis route, mild reaction conditions, simple and convenient post-treatment, high yield, and convenience for application.
Owner:ANHUI UNIVERSITY
Method for recovering and refining sodium bromide from dipropyl cyanoacetate mixture
The invention provides a method for recovering refining sodium bromide from dipropyl cyanoacetate mixture. The method comprises the following steps: filtering a dipropyl cyanoacetate mixture containing sodium bromide, filtering and colleting sodium bromide, dissolving sodium bromide, adjusting the pH value to 6-6.5 by hydrobromic acid, adding an extraction agent of an ether and alkane mixture, stirring for mixing, standing and layering; separating water liquid, boiling, cooling to 80 DEG C, adding an active carbon and diatomite mixed decolorizing agent, decolorizing at 50-80 DEG C, filtering, and conducting spray drying on the filtrate to obtain a white powdered anhydrous sodium bromide with high purity and high recycling rate.
Owner:湖南省湘中制药有限公司
Environment-friendly clean method for preparing dimethyl malonate
InactiveCN103420834AAvoid decompositionAvoid reactionOrganic compound preparationCarboxylic acid esters preparationInorganic saltsWater desalination
The invention discloses an environment-friendly clean method for preparing dimethyl malonate. The method comprises steps of neutral reaction, cyaniding reaction, acidizing reaction, steaming water desalination, esterification reaction and the like, wherein inorganic salt in a cyanoacetic acid aqueous solution is removed in the step of steaming water desalination, and the preferable technical scheme of steaming water desalination is carried out twice. The inorganic salt-sodium chloride mixed in cyanoacetic acid is removed through steaming water desalination before the esterification reaction, the reaction of sulfuric acid and sodium chloride are avoided in the esterification process, and the consumption of sulfuric acid and the waste water generating capacity are reduced, so that the method for preparing the dimethyl malonate is environment-friendly and clean. The steaming water desalination is carried out twice, so that the phenomenon that cyanoacetic acid is decomposed due to local overtemperature caused by large salt content in the later stage of one-time steaming concentration is avoided.
Owner:CHONGQING UNISPLENDOUR CHEM
Preparation method of JAKs inhibitor drug tofacitinib
InactiveCN105732641ALess side effectsReduce generationOrganic chemistryAntipyreticAcetic acidTofacitinib
The invention discloses a preparation method of JAKs inhibitor drug tofacitinib, which is characterized in that it comprises the following steps: (1) using 2,4-dichloro-7H pyrrole [2,3-D] pyrimidine as a raw material, Under the action of base, react with halobenzyl to prepare compound II; (2) (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride React with 7-benzyl-2,4-dichloro-7H pyrrole [2,3-D] pyrimidine under the action of a base to prepare compound IV; (3) the compound IV obtained in step (2) is catalyzed under the action of a metal catalyst Hydrogenation reaction to obtain compound V; (4) Compound V obtained in step (3) is coupled and docked with cyanoacetic acid under the action of a condensing agent to obtain compound VI; (5) Compound VI obtained in step (4) is prepared by salting with citric acid Get tofacitinib. The synthesis route of the method is short, the reaction process of each step is easy to operate, the solvent can be recycled and used mechanically, the pollution is small, and it is suitable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Organic dye containing calixarene derivative and preparation method and application thereof
InactiveCN102618066AInhibit molecular aggregationProlonged absorption spectrum red shiftLight-sensitive devicesSolid-state devicesCyanoacetic acidElectron donor
The invention discloses organic dye containing calixarene derivative and a preparation method and application thereof. The organic dye has the characteristics of a classical D-pi-A structure in the organic dye, a calixarene framework and a derivative unit of the calixarene framework are introduced to serve as electron donors, and cyanoacetic acid connected with a conjugate bridge group serves as an electron acceptor and is used to prepare dye sensitized solar cells. Dye molecules are introduced to the calixarene derivative unit, thereby prolonging a conjugated chain to absorb absorption spectrum and effectively restraining molecular aggregation caused by the dye absorbed onto TiO2. In addition, the dye molecules contain a plurality of light absorption units so that the light trapping capability can be improved greatly. The organic dye serving as photosensitive dye is used for preparing the dye sensitized solar cells. The organic dye enables the service life of the cells to be prolonged through photo-thermal stability of the calixarene framework.
Owner:SUN YAT SEN UNIV
Synthesis and application of coumarin type dye sensitizer
InactiveCN103087051AImprove electrochemical performanceOrganic chemistryLight-sensitive devicesCyanoacetic acidElectron donor
The invention relates to a coumarin functional dye containing a thiophene bridge chain in the field of fine chemical industry and organic photoelectric material applications. The structure of the coumarin functional dye takes coumarin and a derivative thereof as an electron donor, contains a thiophene structure unit capable of adjusting an absorption spectrum and a fluorescence emission spectrum as the bridge chain and is further connected with a cyanoacetic acid electron withdrawing group. Coumarin-thiophene, POCl3 / DMF (dimethyl fumarate) are added into a reaction container by adopting general reaction for reaction so as to get a 5-(7-substiutted-2-carbonyl-2H-benzopyran-3-yl) thiophene-2-formaldehyde intermediate (II) with an aldehyde group; and the intermediate II with the aldehyde group further reacts with cyanoacetic acid to get the coumarin dye connected by the thiophene. As the coumarin is taken as a chromophore, the electron donating capability is good; the thiophene has high electron cloud density and special optical properties and electron transmission capability; and the electron withdrawing group of the cyanoacetic acid is further connected for enabling the dye to have good light, thermal and chemical stability and photoelectric properties. Therefore, the dye can be used as a photosensitive dye for dye-sensitized solar cells.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of 6-amino-1,3-dimethyluracil
InactiveCN107540618AImprove long-term stabilityImprove stabilityOrganic chemistryAcetic anhydrideCyanide
The invention discloses a preparation method of 6-amino-1,3-dimethyluracil. According to the preparation method, cyanoacetic acid and 1,3-dimethylurea undergo condensation under the action of acetic anhydride so as to obtain 2-cyano-N-methyl-N-[(methylamino)carbonyl]acetamide; and then 2-cyano-N-methyl-N-[(methylamino)carbonyl]acetamide undergoes a cyclization reaction under an alkaline conditionso as to obtain 6-amino-1,3-dimethyluracil. The 6-amino-1,3-dimethyluracil has a good system stabilizing effect when used as a main component of a PVC stabilizer along with zinc stearate, and has a better synergistic effect than a commonly-used counterpart in the industry at present. The 6-amino-1,3-dimethyluracil can absorb HCl and also can replace unstable chlorine atoms. On the other hand, the6-amino-1,3-dimethyluracil also can react with ZnCl2 to generate a metal complex and inactivate ZnCl2. Thus, long-term stability of a PVC sample is greatly enhanced.
Owner:XINHUA PHARM (SHOUGUANG) CO LTD
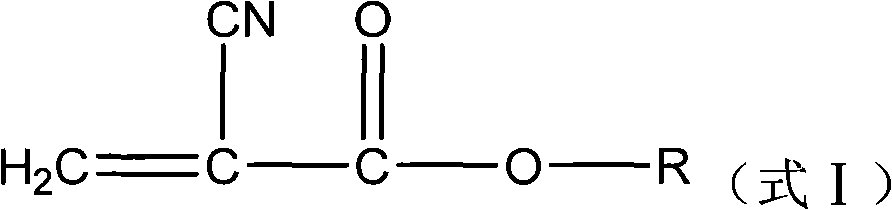
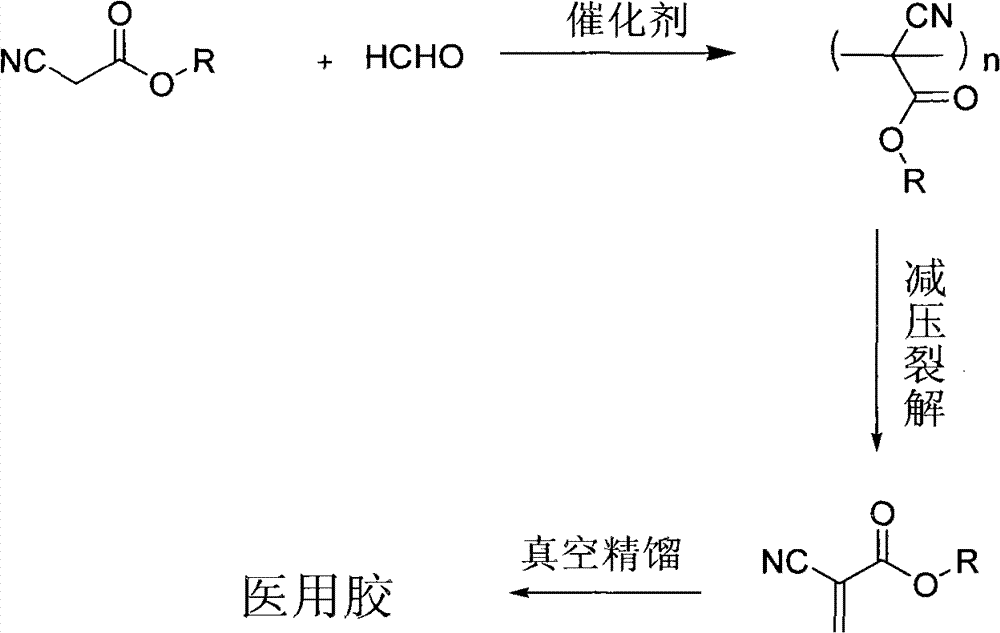
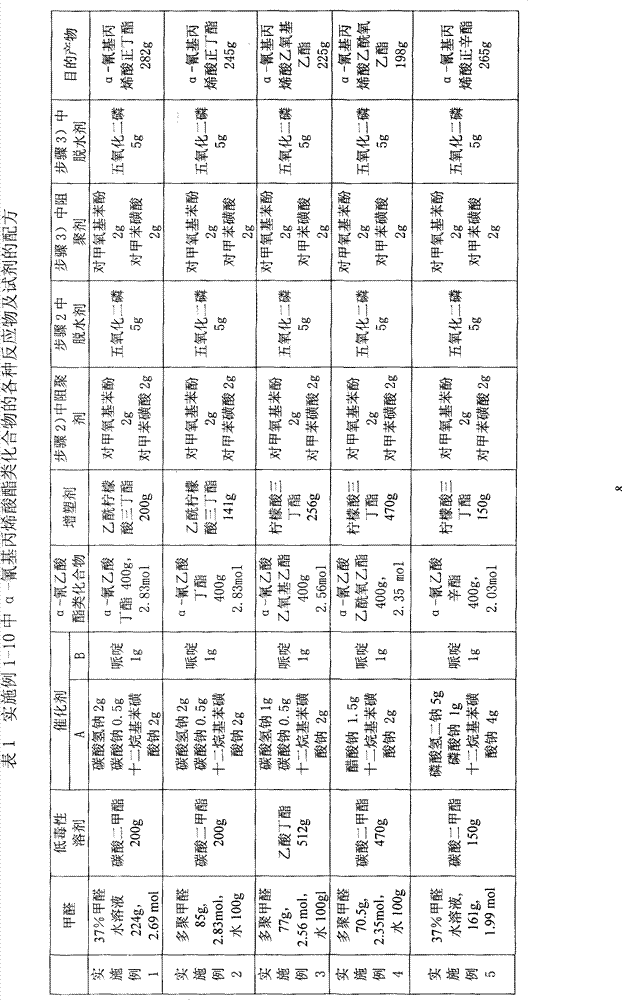
Synthesis technique of alpha-cyanoacrylate monomer
ActiveCN102603564AHigh yieldSave energyCarboxylic acid nitrile preparationOrganic compound preparationHexahydropyridineCyanoacetic acid
The invention belongs to the field of adhesives, and particularly relates to a synthesis technique of an alpha-cyanoacrylate monomer, which comprises the following steps: (1) directly reacting cyanoacetate and polyformaldehyde, which are used as raw materials, under solventless conditions by using a basic catalyst; (2) naturally cooling, keeping the temperature, and dehydrating to obtain the crude product; and (3) in the presence of a polymerization inhibitor, preparing the alpha-cyanoacrylate monomer, wherein the basic catalyst in the step (1) is one or mixture of hexahydropyridine, pyridine and KOH, the cyanoacetate is one or mixture of methyl cyanoacetate, ethyl cyanoacetate and n-butyl cyanoacetate, the polymerization inhibitor in the step (3) is hydroquinone and sulfur dioxide, a dehydrating agent is used for dehydration in the step (2); and the dehydrating agent is one or mixture of benzene, methylbenzene and methanol. The reaction process has the advantages of no need of organic solvent, low energy consumption, obvious environmental protection effect and high target product yield.
Owner:GLEIHOW NEW MATERIALS CO LTD
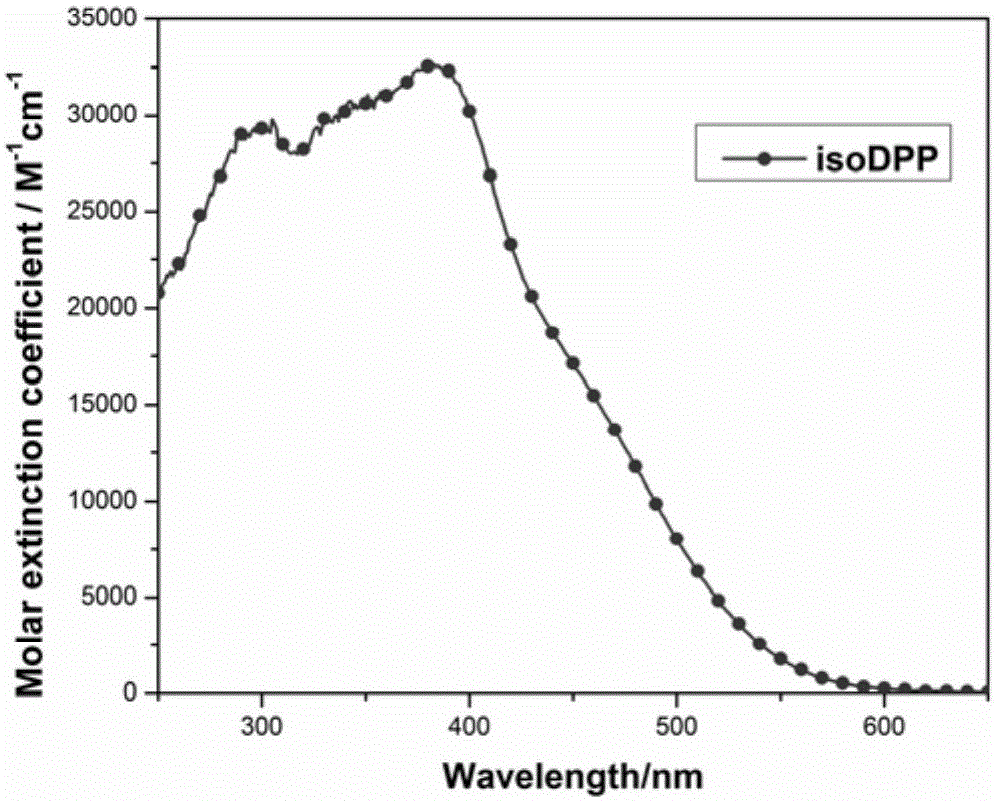
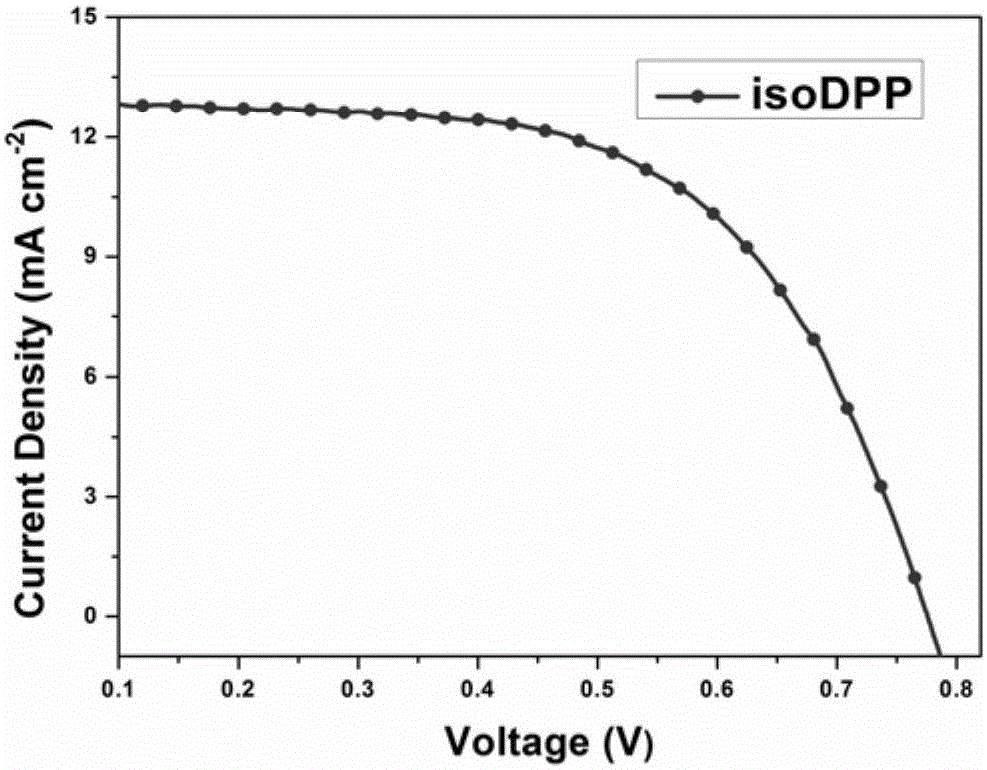
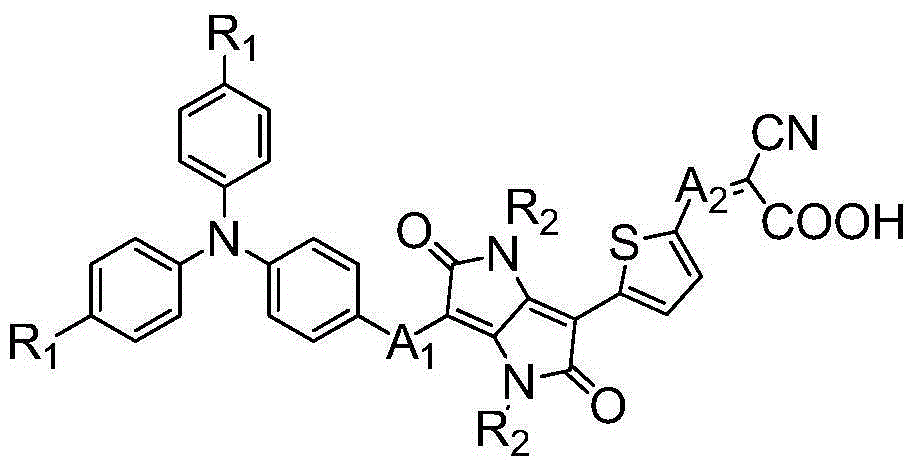
Isodiketopyrrolopyrrole dyes and application thereof
ActiveCN105131640AThe synthesis method is simpleRaw materials are cheap and easy to getMethine/polymethine dyesLight-sensitive devicesElectron donorOrganic dye
The invention discloses isodiketopyrrolopyrrole dyes and application thereof. According to the invention, 4,4'-dihexyloxytriphenylamine is used as an electron donor, isodiketopyrrolopyrrole is used as a Pi bridge, cyanoacetic acid is used as an electron acceptor and an anchoring group, and an alkyl chain is introduced on an isodiketopyrrolopyrrole group, so a series of purely organic dyes based on isodiketopyrrolopyrrole are synthesized. The dyes have good light-harvesting performance and large steric hindrance and hardly aggregate when adsorbing on a semiconductor membrane. The purely organic dyes with isodiketopyrrolopyrrole as the Pi bridge of electrons are applied in dye-sensitized solar cells and have good capability in inhibiting electron recombination; and the dye-sensitized solar cells have high photoelectric conversion efficiency.
Owner:SOUTH CHINA UNIV OF TECH
Dimethyl malonate preparation method
InactiveCN103724191AReduce productionReduce dosageOrganic compound preparationCarboxylic acid esters preparationCyanoacetic acidDecomposition
The invention discloses a dimethyl malonate preparation method. The shortcomings of mass decomposition of cyanoacetic acid and low yield caused by the traditional distillation concentration desalination are avoided by introducing hydrogen chloride into cyanoacetic acid reaction liquid to separate out sodium chloride; meanwhile, malonic acid and ammonium chloride are obtained by hydrolysis in a closed system, and hydrogen chloride gas is introduced to separate out ammonium chloride; after desalination, an esterification reaction can reduce the catalyst dosage and wastewater generation while a byproduct ammonium chloride is obtained. The method disclosed by the invention is simple to operate, low in production cost and high in product yield and generates a small quantity of three wastes, thereby being an environment-friendly and clean production method.
Owner:CHONGQING UNISPLENDOUR CHEM
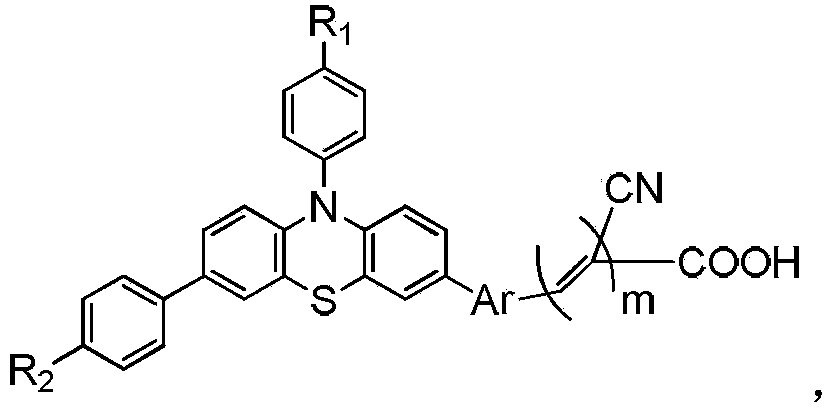
Double-strand phenothiazine dye with benzotriazole led into Pi bridge and application thereof in preparation of dye-sensitized solar cells
ActiveCN103788679AThe synthesis method is simpleRaw materials are cheap and easy to getLight-sensitive devicesThiazine dyesElectron donorCyanoacetic acid
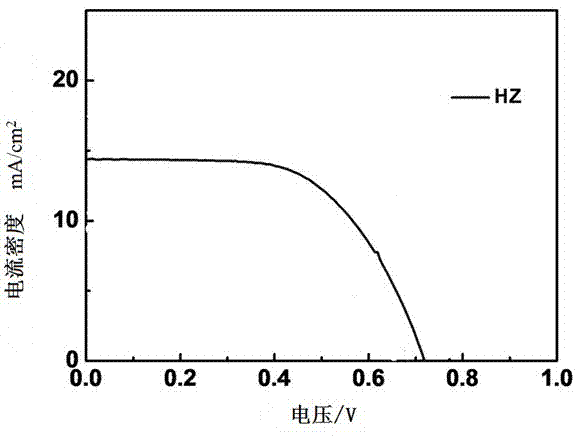
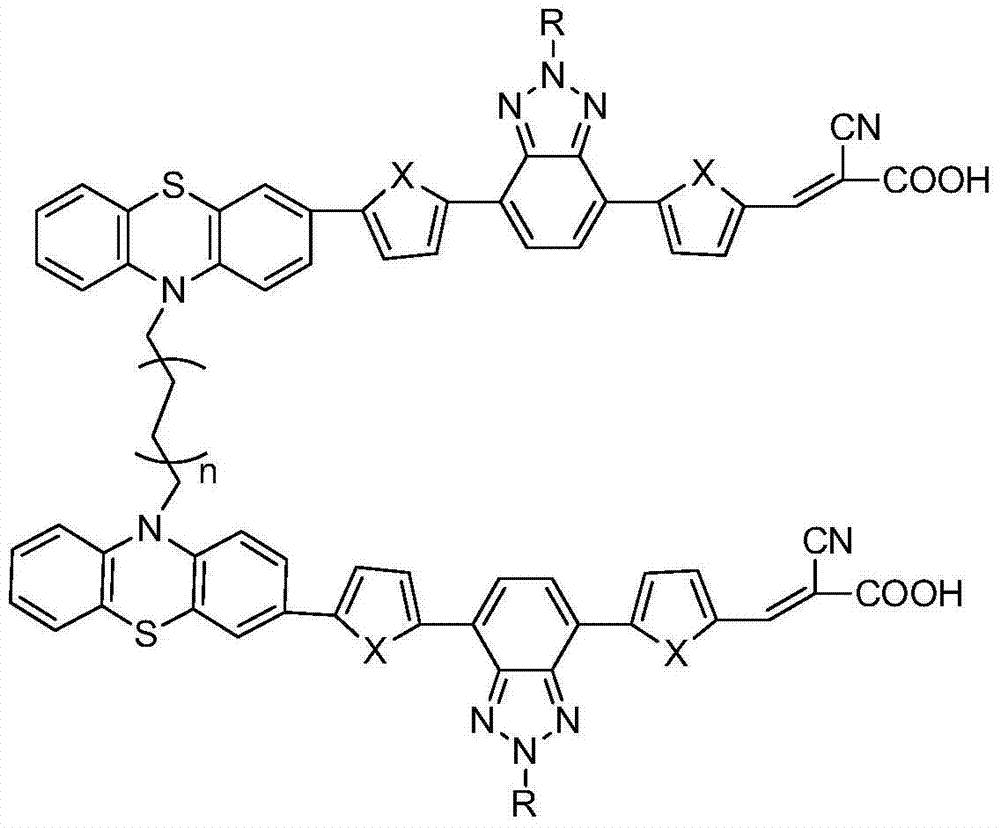
The invention discloses a double-strand phenothiazine dye with benzotriazole led into a Pi bridge and an application thereof in the preparation of dye-sensitized solar cells, and belongs to the field of photoelectric converting material application in fine chemical engineering. A preparation method of the double-strand phenothiazine dye with the benzotriazole led into the Pi bridge comprises the following steps: leading benzotriazole which is an electron-deficient group into the Pi bridge by using phenothiazine as an electron donor, and synthesizing a series of double-strand phenothiazine dyes by using cyanoacetic acid as an electron acceptor and anchoring group. The double-strand phenothiazine dye is capable of increasing adsorbing capacity and inhibiting the aggregation of the dye. In addition, benzotriazole which is an electron-deficient additional acceptor is led into the Pi bridge and plays a role in widening an absorption spectrum. In consideration of the two effects, the double-strand phenothiazine dye has more excellent performances than common phenothiazine dye under the same conditions and can be used for effectively improving the photoelectric conversion efficiency of the dye-sensitized solar cells.
Owner:SOUTH CHINA UNIV OF TECH
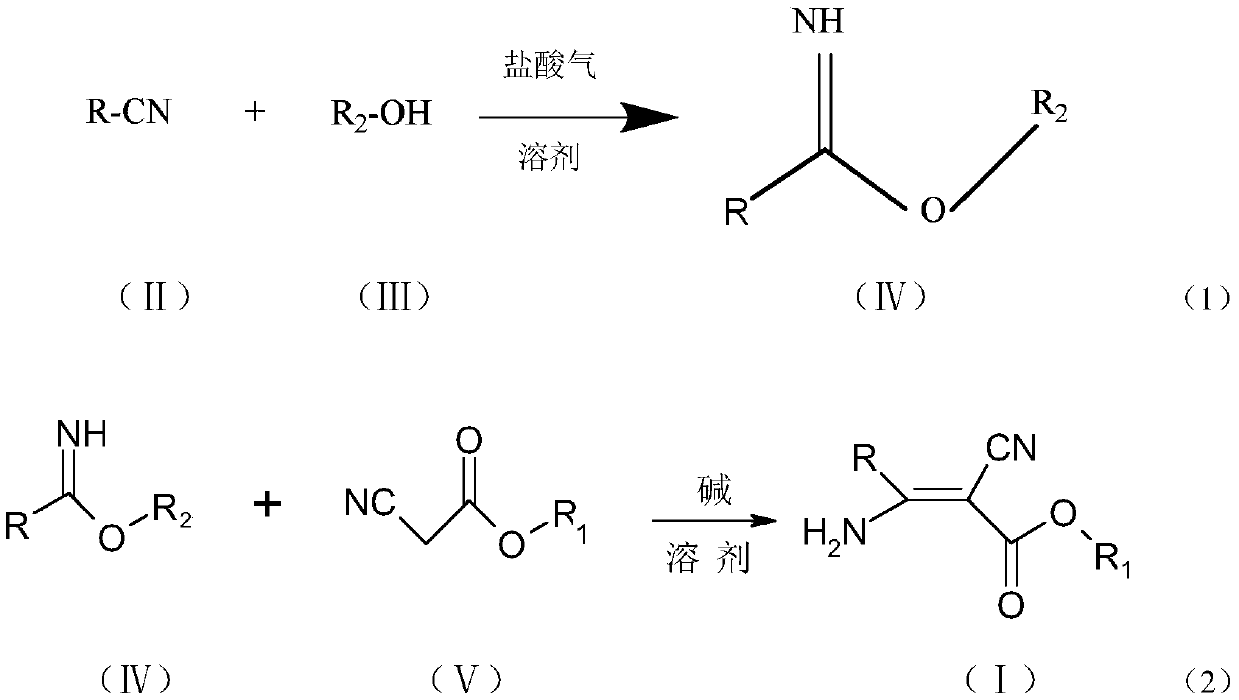
3-pyridyl-3-amino-2-cyanoacrylate compound, and preparation method and application thereof
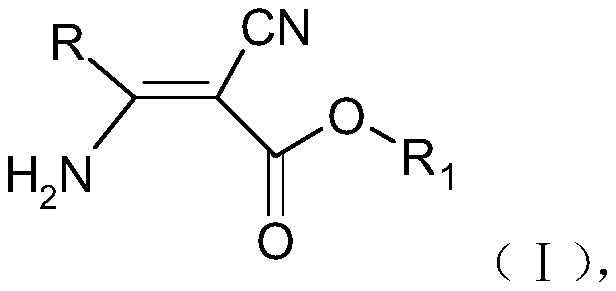
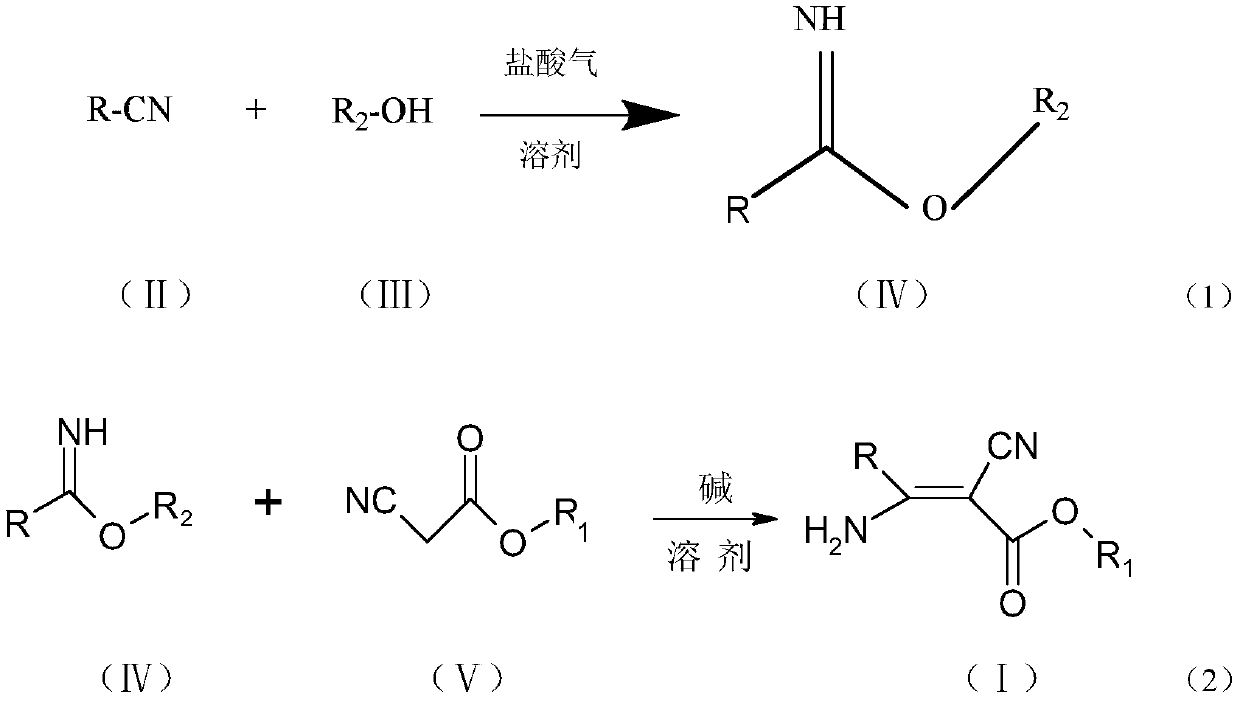
The invention discloses a 3-pyridyl-3-amino-2-cyanoacrylate compound, and a preparation method and application thereof. The structure of the compound is shown in a general formula (1). The preparationmethod of the compound comprises the following steps: taking a compound shown in a formula (II) as a raw material to carry out a reaction with a fatty alcohol in an organic solvent in the presence ofhydrogen chloride to obtain pyridyl imidate, and carrying out a reaction on the pyridyl imidate with cyanoacetate in an organic solvent under catalysis of a catalyst for condensation to prepare the 3-pyridyl-3-amino-2-cyanoacrylate compound. The compound has bactericidal activity and can be used for preventing and treating diseases.
Owner:JIANGSU PESTICIDE RES INST
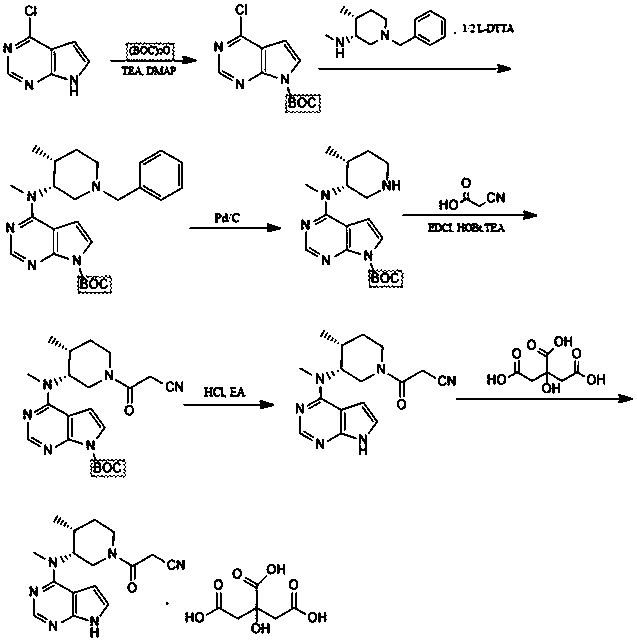
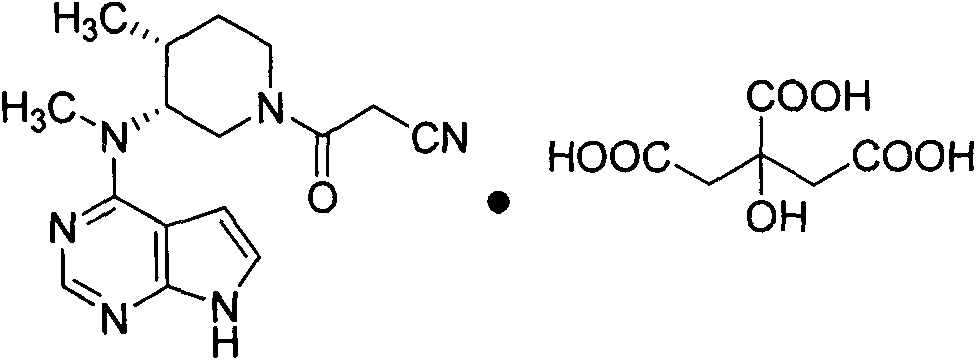
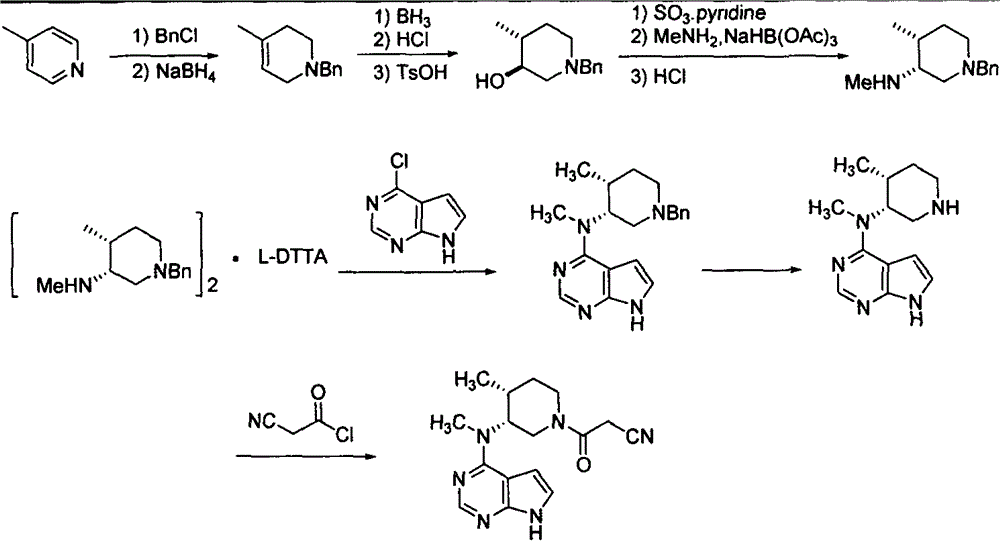
Preparation method of tofacitinib citrate
The invention relates to a preparation method of tofacitinib citrate, in particular to high-yield synthesis of tofacitinib citrate. The tofacitinib citrate is synthesized from raw materials including4-chloro-7-pyrrolo[2,3-d]pyrimidine, (BOC)2O, (3R,4R)-(1-benzyl-4-methylpiperidine-3-yl)methylamine-L-di-p-toluoyl tartrate, Pd / C, cyanoacetic acid and citric acid through six steps including an aminoprotection reaction, an amination reaction, a debenzylation reaction, a condensation reaction, a deprotection reaction and a salt forming reaction. The synthesis route provides the preparation methodof tofacitinib citrate, and the preparation method is high in yield, low in cost, easy to operate and suitable for industrialization.
Owner:南京法恩化学有限公司
Dimethyl malonate preparation method
InactiveCN103724196AReduce dosageReduce productionOrganic compound preparationCarboxylic acid esters preparationDecompositionCyanoacetic acid
The invention discloses an environment-friendly and clean dimethyl malonate preparation method. Hydrogen chloride gas is introduced into the mixed liquid of cyanoacetic acid and sodium chloride, and then methanol and sulfuric acid are added for an esterification reaction to obtain dimethyl malonate. The shortcomings of mass decomposition of cyanoacetic acid and low yield caused by the traditional distillation concentration desalination are avoided; meanwhile, after desalination, the esterification reaction can reduce the dosage of sulfuric acid and wastewater generation. The method is simple to operate, low in production cost, high in product yield and suitable for large-scale industrial production.
Owner:CHONGQING UNISPLENDOUR CHEM
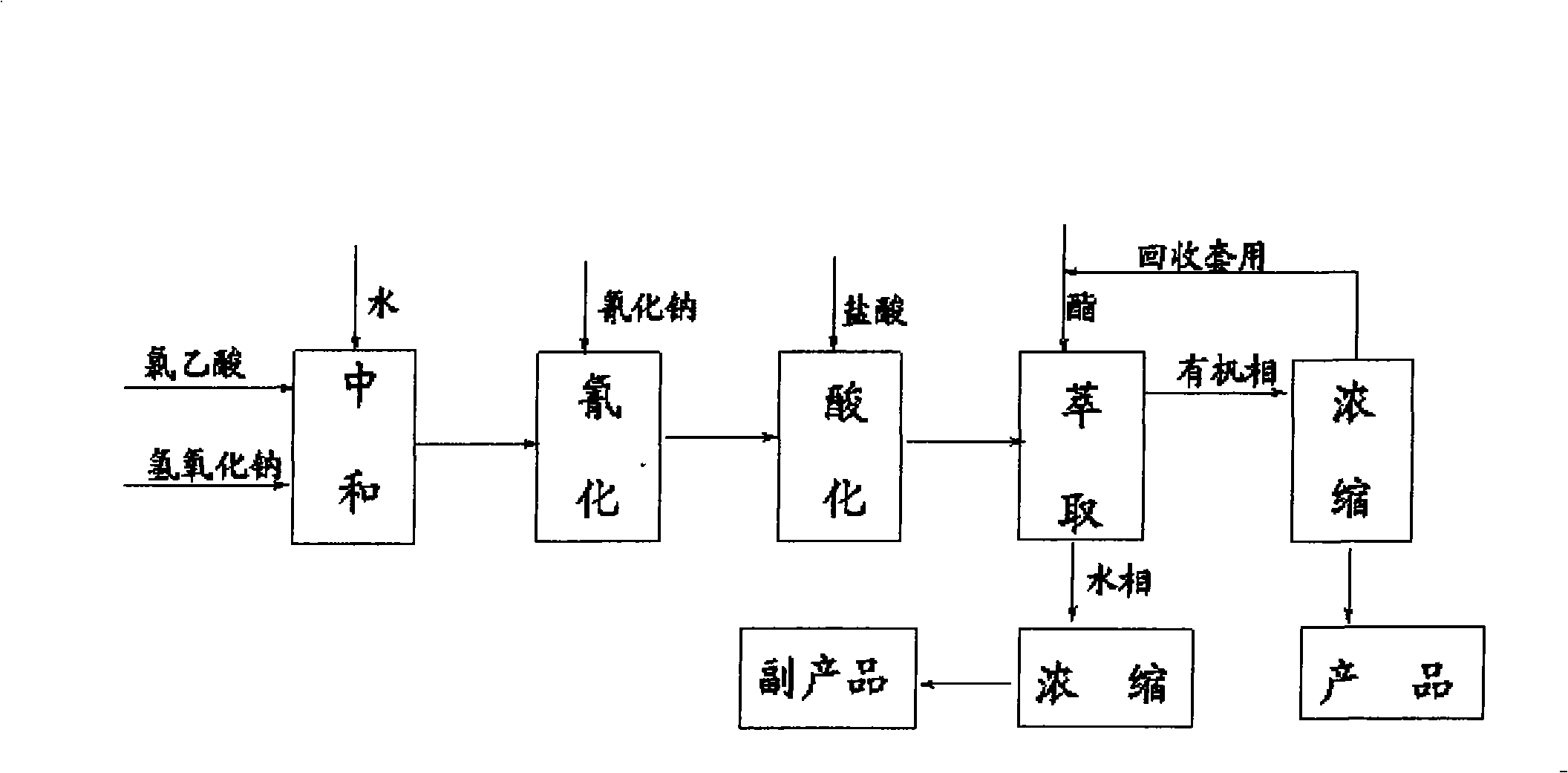
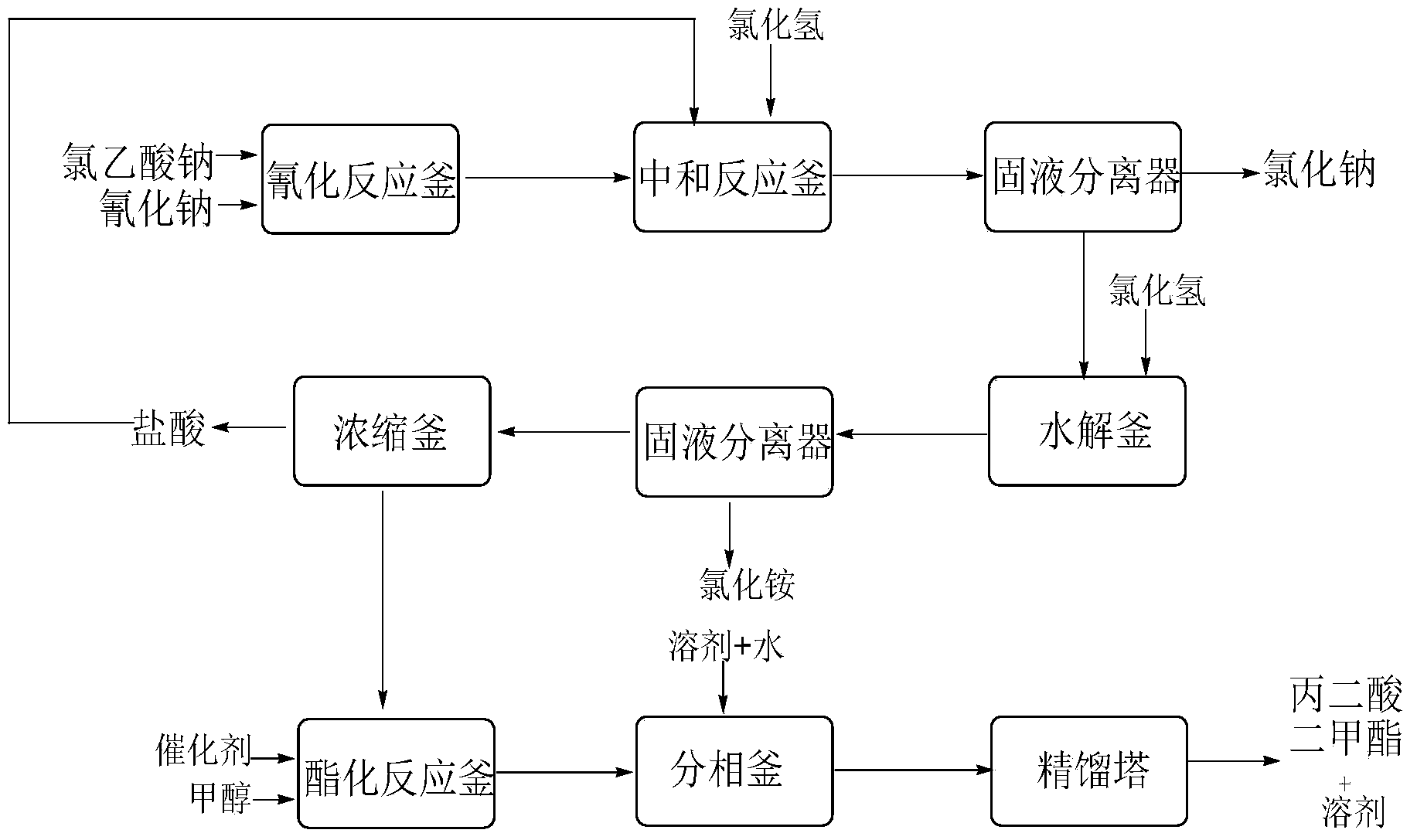
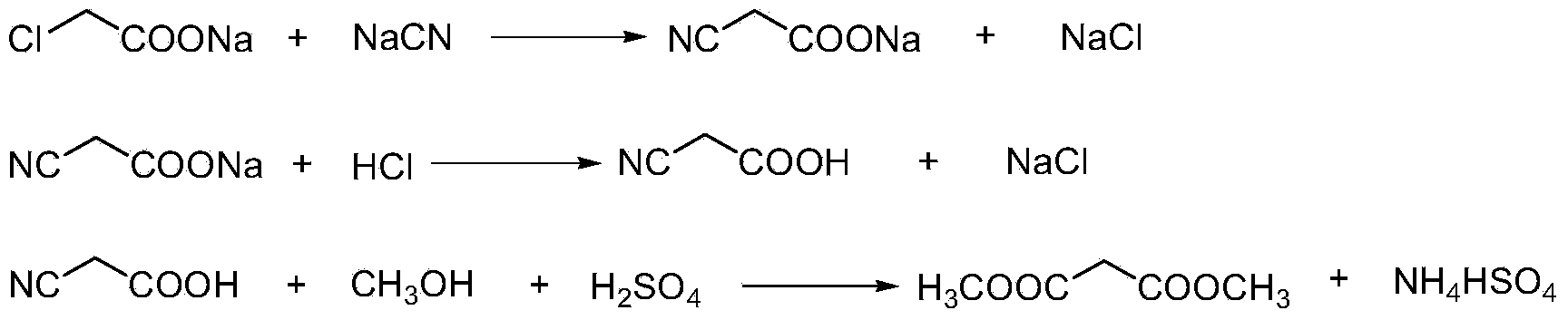
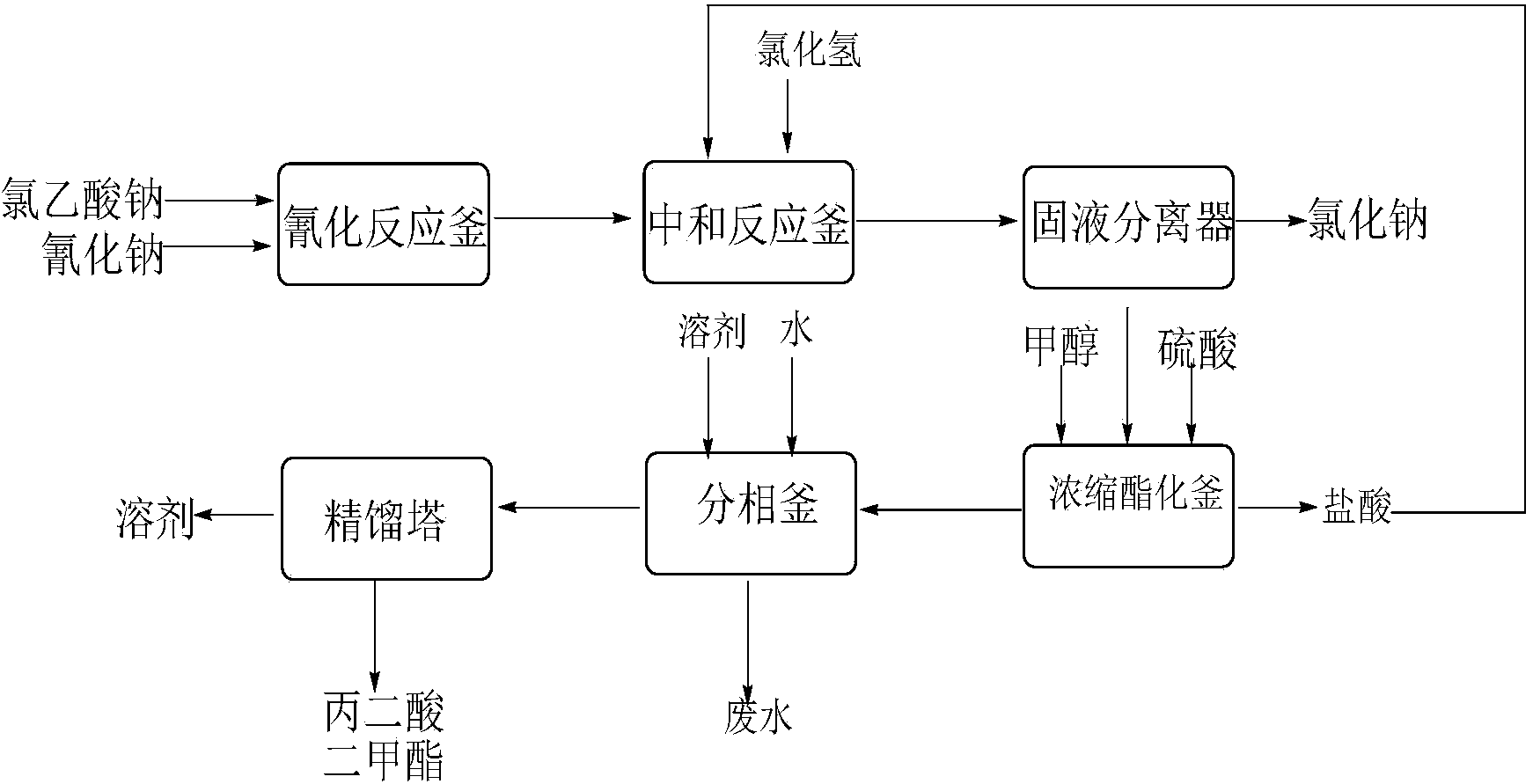
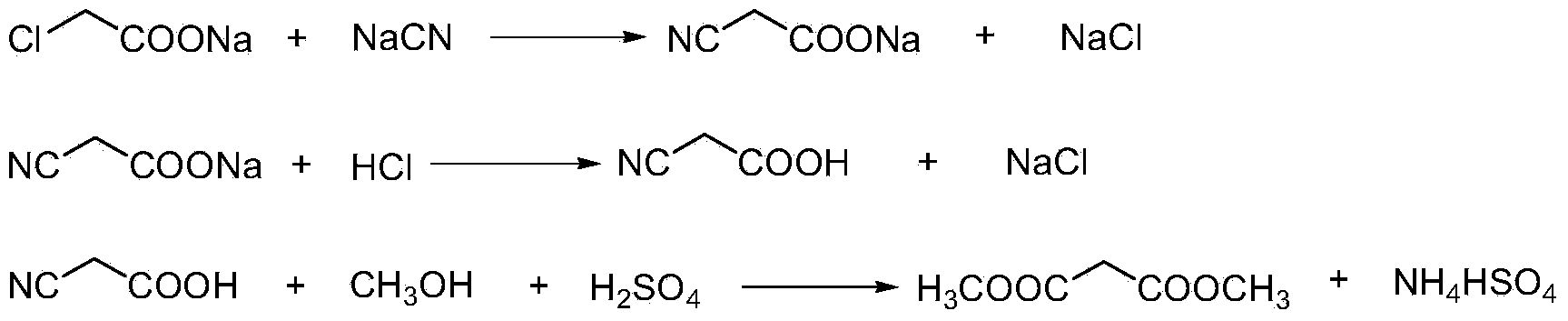
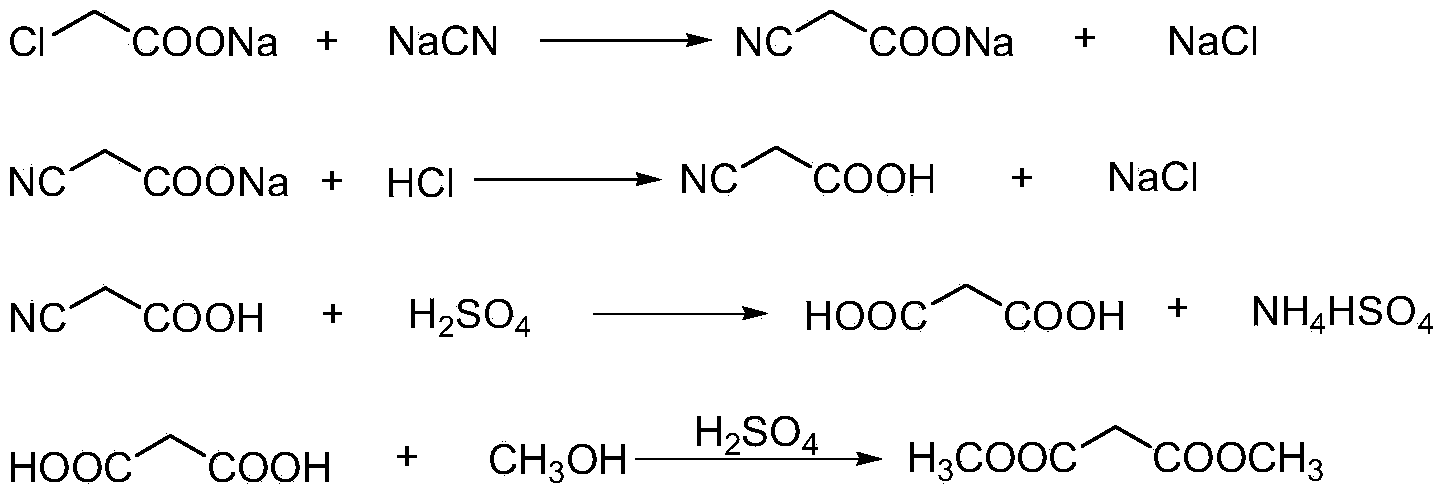
Preparation method of cyanoacetic acid
InactiveCN102336684AReduce energy consumptionReduce moisturePreparation by cyanide reactionSodium acetateAcetic acid
The invention discloses a preparation method of cyanoacetic acid, which comprises the following steps that: chloroacetic acid and alkali take neutralization reaction to generate sodium chloroacetate, in addition, the sodium chloroacetate and sodium cyanide carry out cyaniding reaction to obtain sodium cyanoacetate water solution, hydrogen chloride gas is absorbed by the sodium cyanoacetate water solution, and cyanoacetic acid water solution is obtained through reaction. The sodium cyanoacetate water solution is used for directly absorbing the hydrogen chloride gas. Compared with the prior art, the preparation method has the advantages that the production process is simplified, the equipment investment is also not increased, and in addition, the production consumption is greatly reduced, so the production cost is reduced.
Owner:WEIFANG BAILI CHEM
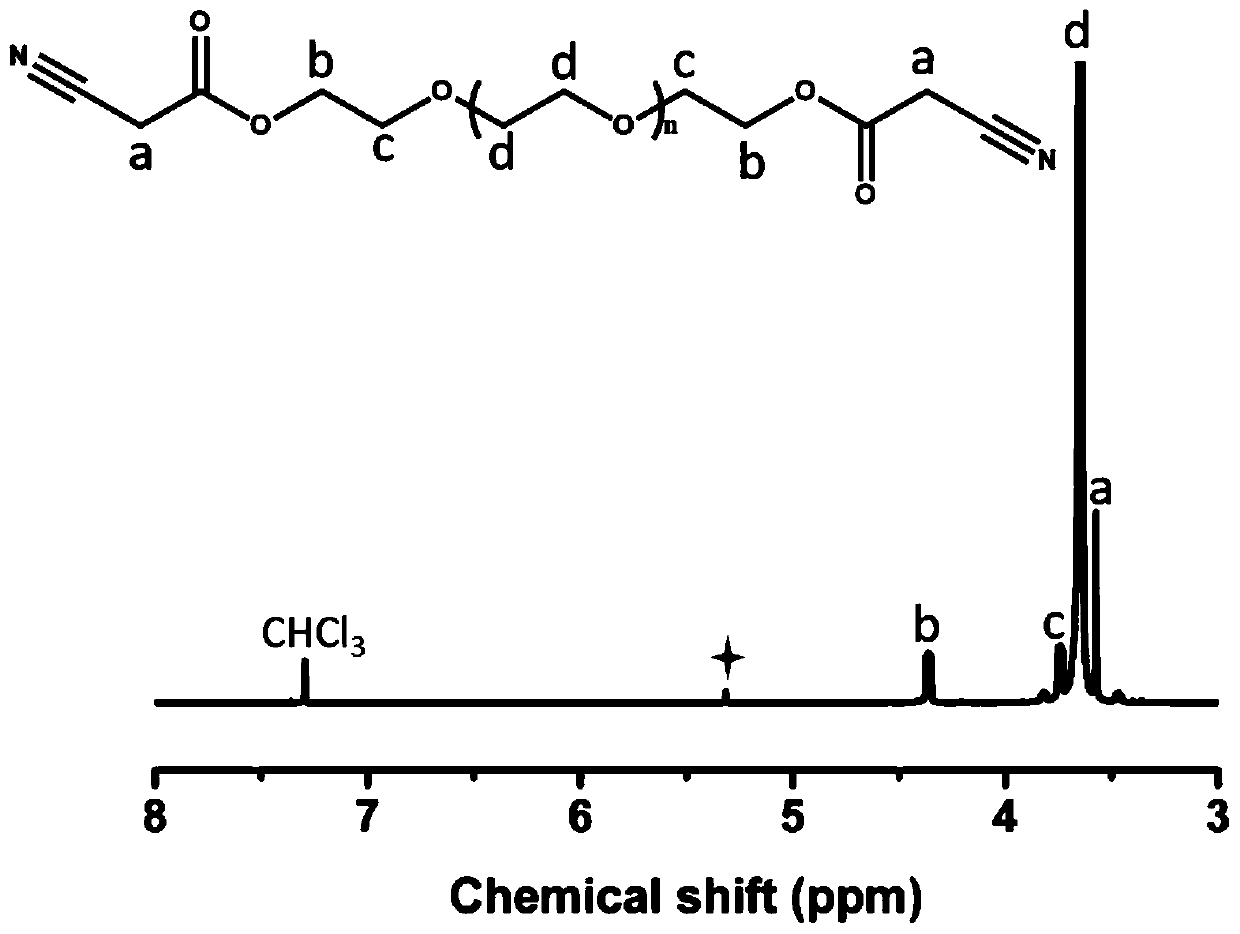
Preparation method of injectable hydrogel precursor solution, injectable hydrogel and application of injectable hydrogel
ActiveCN111171339AMix evenlyImprove delivery efficiencyPharmaceutical delivery mechanismProsthesis3D cell cultureCyanoacetic acid
The invention provides a preparation method of an injectable hydrogel precursor solution, an injectable hydrogel prepared by the preparation method and a use method of the injectable hydrogel. According to the preparation method, PEG2000 cyanoacetate and water-soluble polyaldehyde are used as raw materials, active methylene groups provided by the PEG2000 cyanoacetate and formyl groups provided bythe water-soluble polyaldehyde form the injectable hydrogel in situ through a Knoevenagel reaction under the catalysis of PBS. The injectable hydrogel has excellent biocompatibility and biodegradability and can be used as a carrier material for 3D cell culture.
Owner:QINGDAO UNIV
Preparation method of (3R,5R)-3,5-dihydroxy-6-methyl cyan-caproate
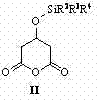
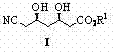
InactiveCN102827030AHigh yieldHigh stereoselectivityCarboxylic acid nitrile preparationOrganic compound preparationCyanide compoundPtru catalyst
The invention relates to a preparation method of (3R, 5R)-3,5-dihydroxy-6-methyl cyan-caproate (I) and belongs to the technical field of pharmaceutical chemistry. The preparation method concretely comprises the following steps of: firstly, carrying out asymmetric catalysis alcoholysis on 3-siloxy cyclopentane anhydride (II) to prepare (R)-3-siloxy-5-alkoxy-5-oxo-pentanoate (III); secondly, condensing the (R)-3-siloxy-5-alkoxy-5-oxo-pentanoate (III) and methyl cyanoacetate to prepare (R)-2-cyano-3-oxo-5-siloxy diethyl pimelate (IV); thirdly, carrying out decarboxylation on the (R)-2-cyano-3-oxo-5-siloxy diethyl pimelate (IV) to prepare (R)-3-hydroxy-5-oxo-6-benzyl cyanohexanoate (V) by using desilicication protective groups; and fourthly, carrying out asymmetric reduction on the (R)-3-hydroxy-5-oxo-6-benzyl cyanohexanoate to prepare a product of (3R,5R)-3,5-dihydroxy-6-methyl cyan-caproate (I). The preparation method is mild in reaction conditions, simple and convenient to operate, high in stereoselectivity, environment-friendly, and suitable for industrial production; and products have high yield, the used chiral catalyst is small in dosage and can be recovered with fix quantify, raw materials which are easily obtained are low in cost, and particularly hypertoxic cyanides are avoided.
Owner:FUDAN UNIV
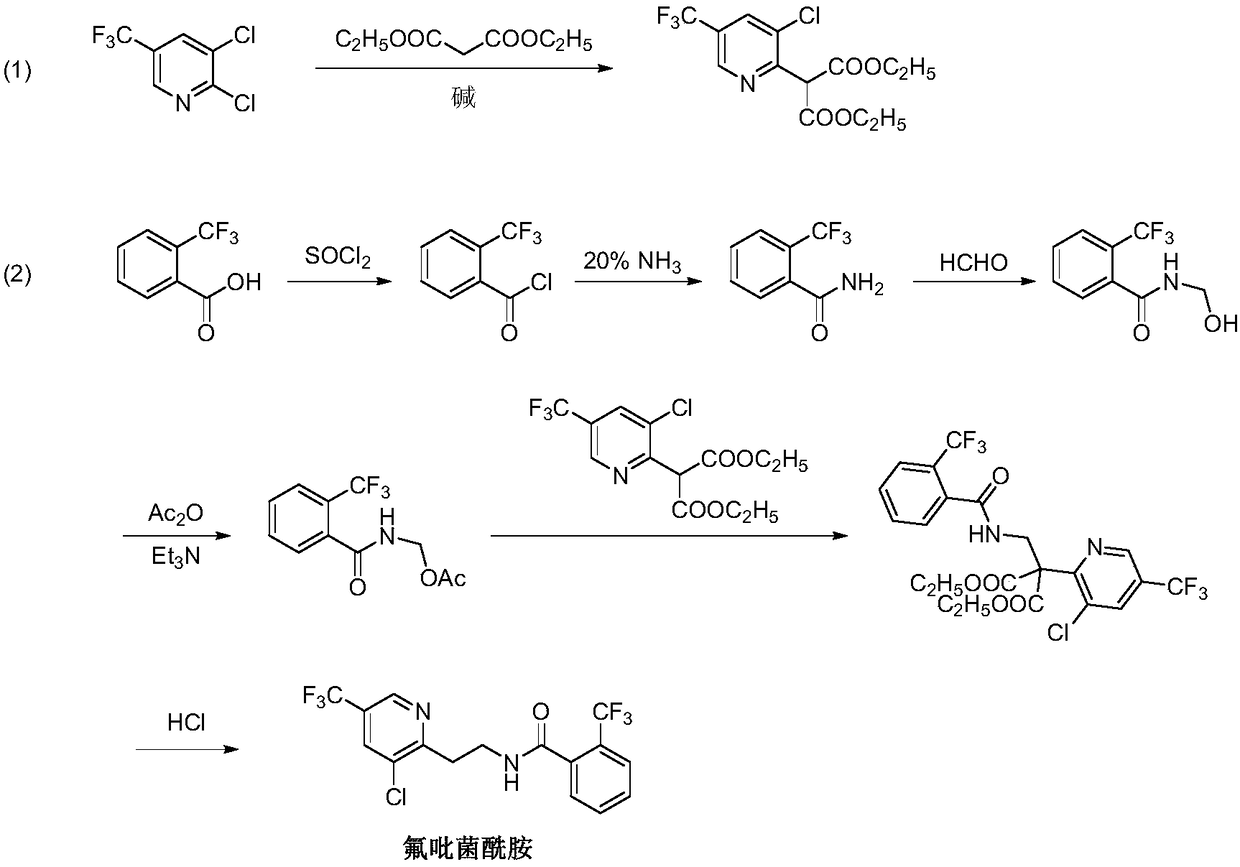
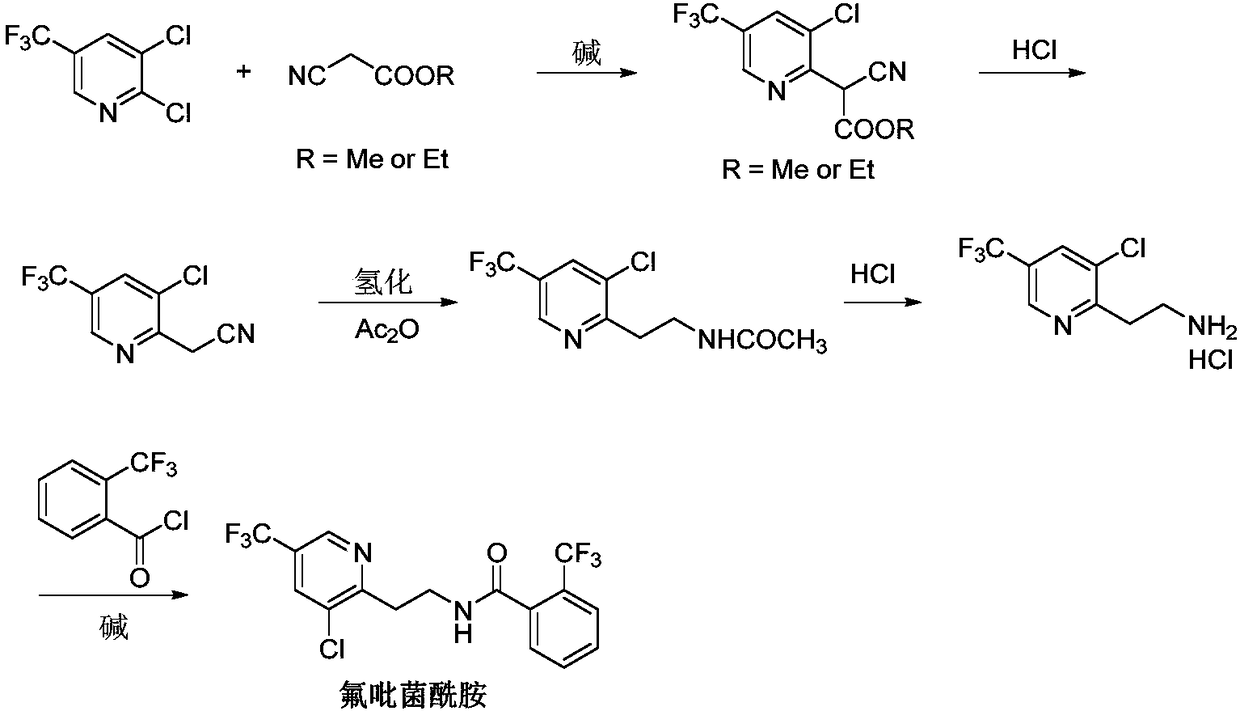
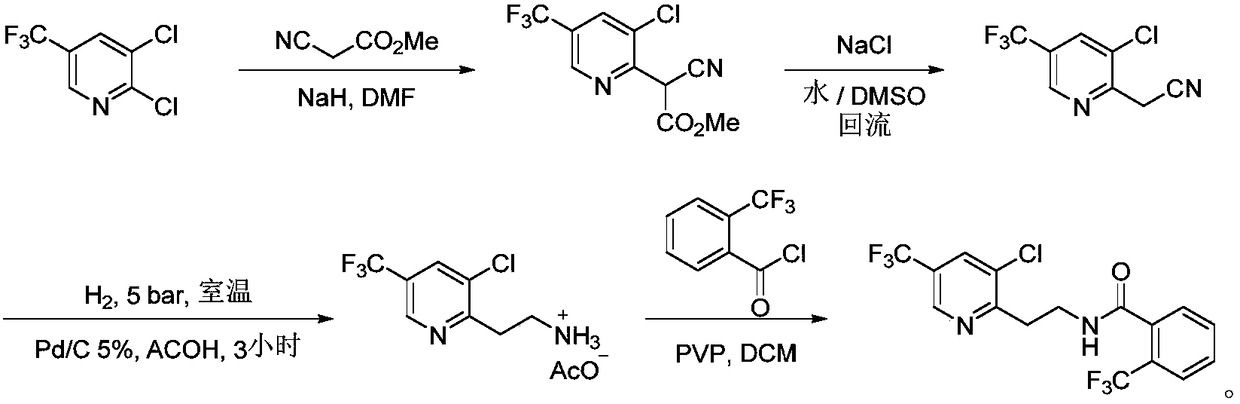
Fluopyram preparation method
ActiveCN109293565AShort stepsAvoid protection‐deprotection stepsOrganic chemistryHydrogenCyanoacetic acid
The invention relates to a fluopyram preparation method, which sequentially comprises: (1) carrying out a substitution reaction on 2,3-dichloro-5-trifluoromethylpyridine and ethyl cyanoacetate or methyl cyanoacetate at a temperature of 30-160 DEG C in the presence of an alkali and a solvent, adjusting the reaction liquid to an acidic pH value after completing the reaction, and carrying out a decarboxylation reaction at a temperature of 80-160 DEG C to obtain 3-chloro-5-(trifluoromethyl)-2-acetonitrilepyridine; and (2) carrying out tandem hydrogenation and condensation reaction on the 3-chloro-5-(trifluoromethyl)-2-acetonitrilepyridine prepared in the step (1) and o-trifluoromethylbenzoyl chloride at a temperature of 20-100 DEG C in the presence of a catalyst, hydrogen, an alkali and a solvent to obtain fluopyram. According to the present invention, the preparation method has the short steps, avoids the unnecessary protection-deprotection step, is economical and environmentally friendly, and is suitable for industrial production.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
Synthesis method for tofacitinib
The invention relates to a synthesis method for tofacitinib serving as a JAK inhibitor. According to the method, the tofacitinib is prepared by taking (4-picoline-3-yl)methyl carbamate as a raw material, and performing catalytic hydrogenation, benzyl protection, reduction, salification, separation, deprotection and amidation salification. The method specifically comprises the following steps: (1) performing catalytic hydrogenation and reduction on (4-picoline-3-yl)methyl carbamate in sulfuric acid and Pd / C; (2) reacting cis-(4-picoline-3-yl)methyl carbamate and benzyl chloride to obtain cis-(1-benzyl-4-methylpiperidine-3-yl)methyl carbamate; (3) performing HOBT catalytic condensation on N-[(3R,4R)-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine and cyanoacetic acid to obtain tofacitinib free alkali. The preparation method provided by the invention has easily obtained raw materials, mild reaction conditions, easiness and convenience in operation and high yield, and is suitable for industrial production.
Owner:济南扬诺生物科技有限公司
Environment-friendly preparation method of alpha-cyanoacrylate compound
ActiveCN102775328AHigh boiling pointReduce pollutionOrganic compound preparationCarboxylic acid nitrile purification/separationDepolymerizationCyanoacetic acid
The invention discloses a preparation method of the alpha-cyanoacrylate compound, comprising the following steps: (1) subjecting the mixture of the alpha-cyanoacrylate compound and a catalyst B to reaction with formaldehyde in a low-toxicity solvent under the existence of a catalyst A to generate the mixture of a prepolymer and an oligomer; (2) removing the catalyst in the mixture of the prepolymer and the oligomer, and adding a plasticizer and a polymerization inhibitor to subject the prepared mixture of reaction to depolymerization reaction to generate a crude product; and (3) rectifying the crude product to obtain the alpha-cyanoacrylate compound with high purity. The reaction is carried out in the solvent with low toxicity under the existence of the special catalyst, and the plasticizer and the polymerization inhibitor with high boiling point and less toxicity are adopted, thus the final product has the advantages of less toxicity and low biohazard, and the biological safety of the medical adhesive product of the alpha-cyanoacrylate compound is improved greatly when being used clinically.
Owner:BEIJING COMPONT MEDICAL DEVICES
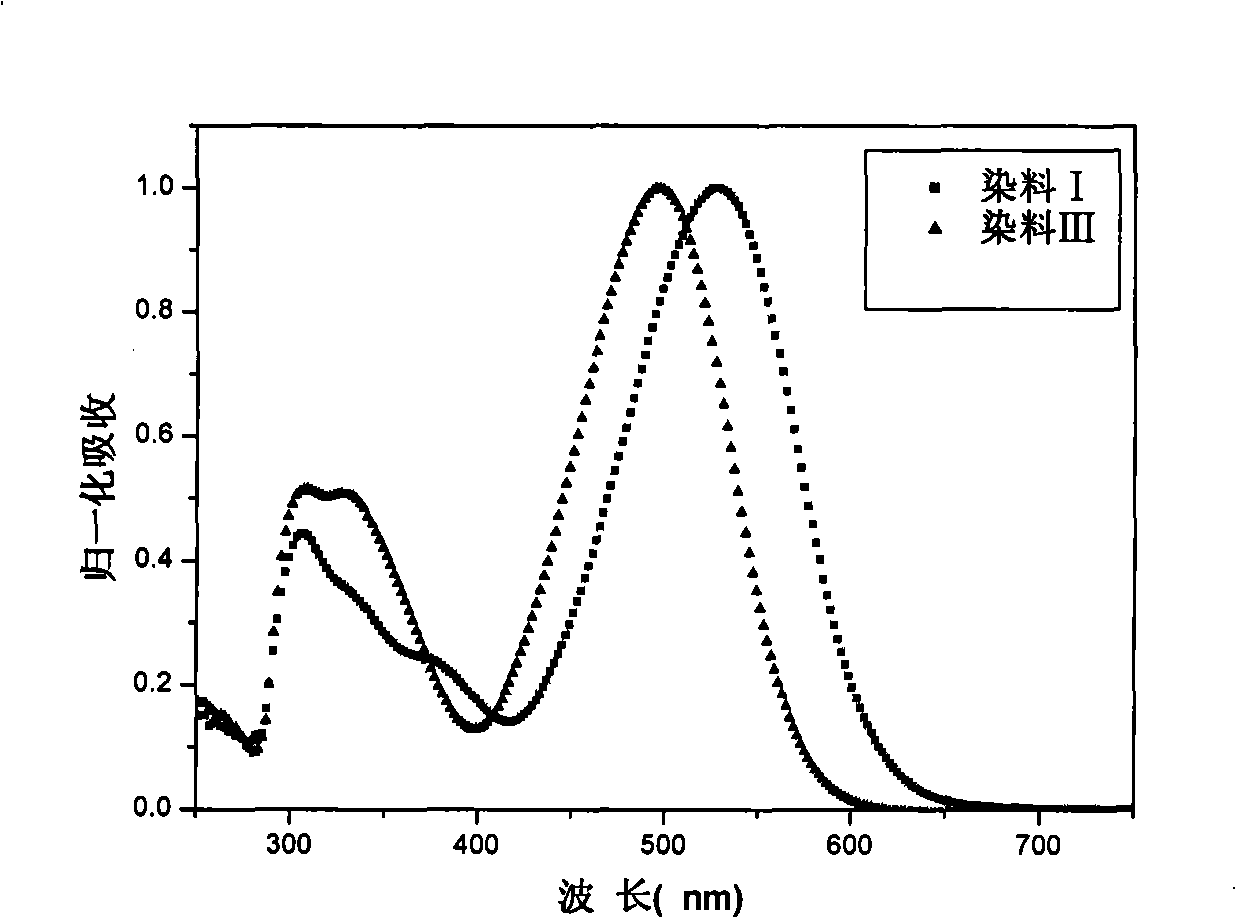
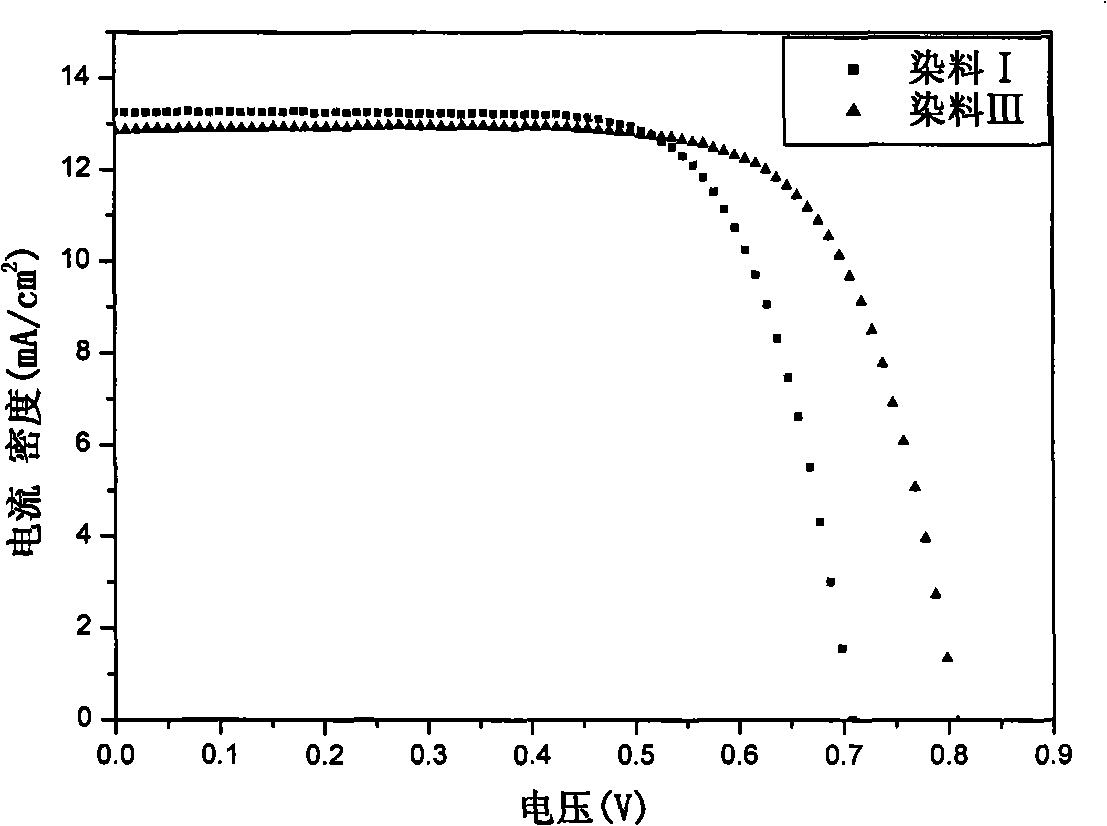
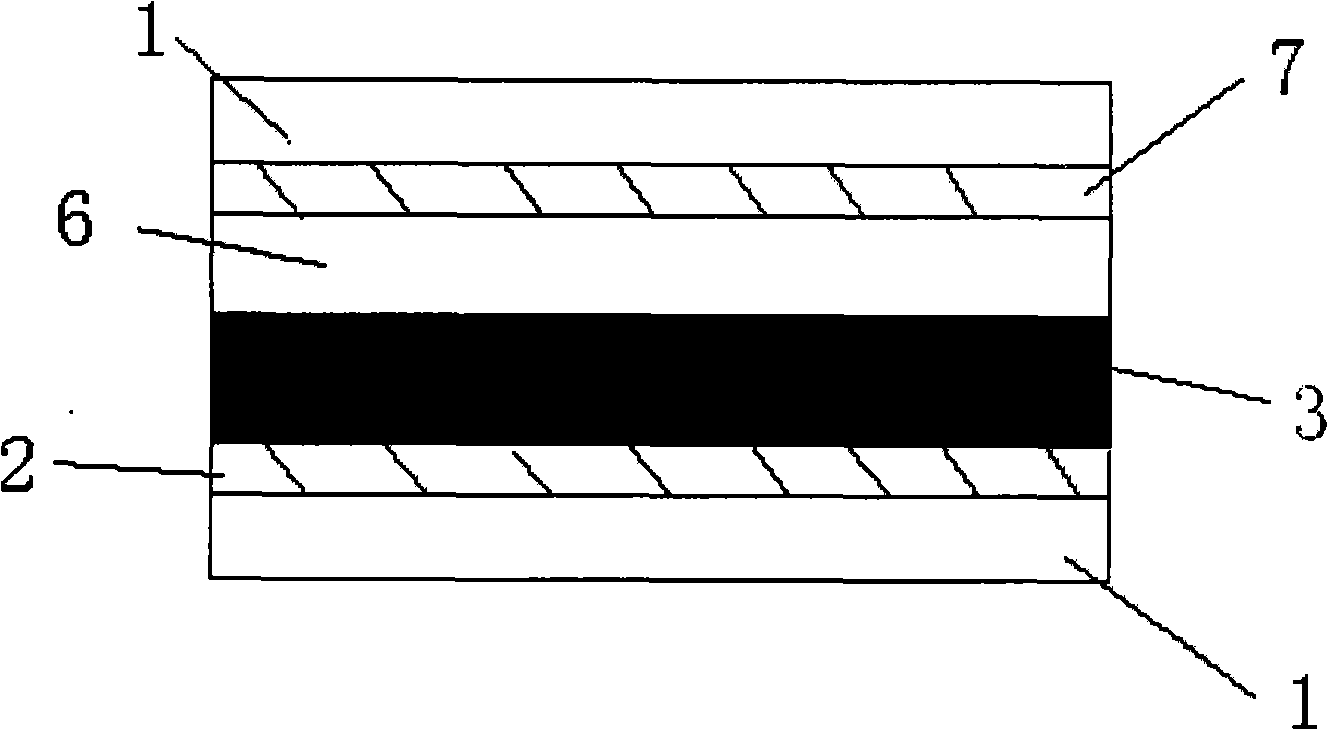
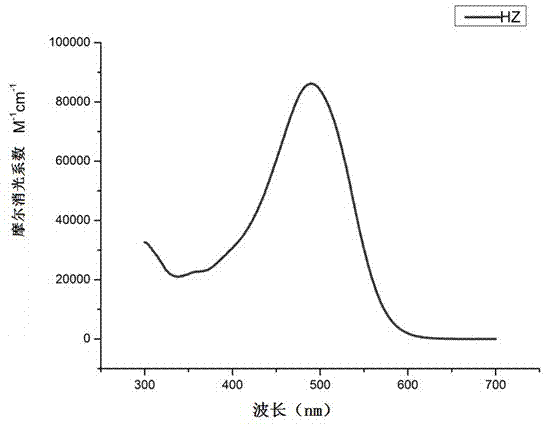
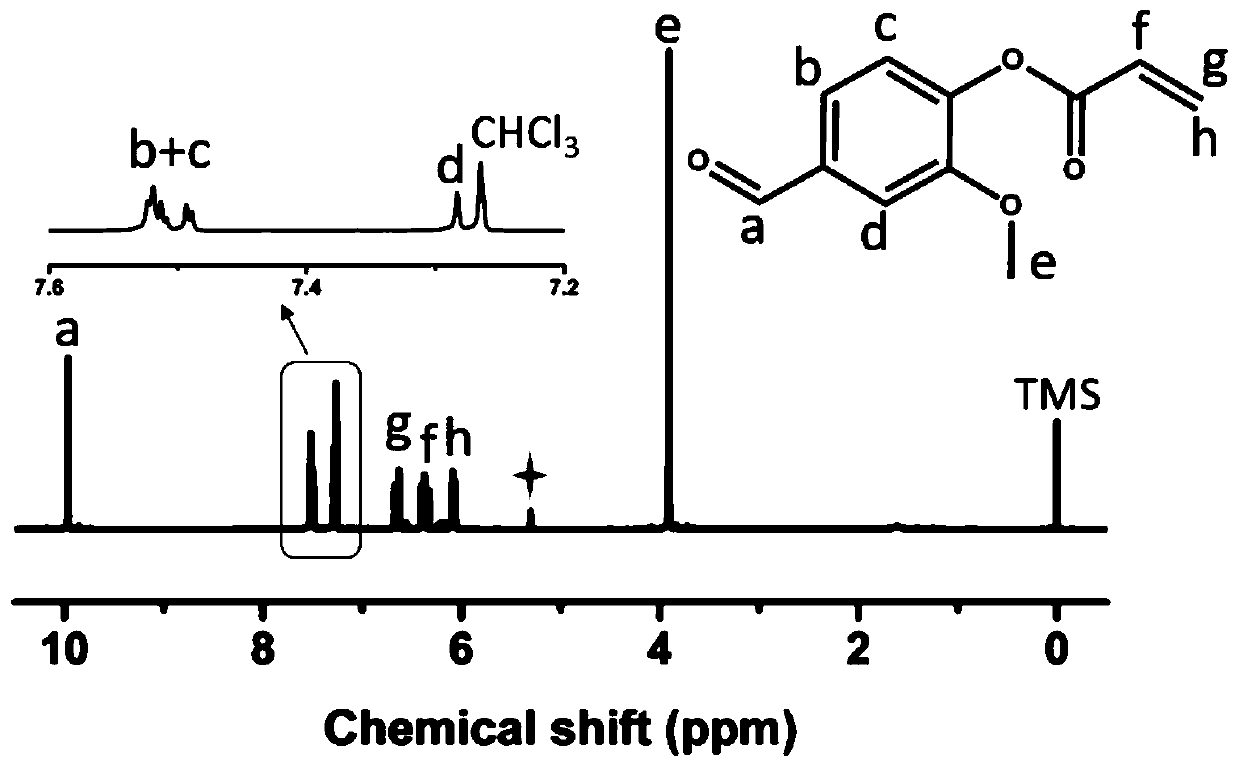
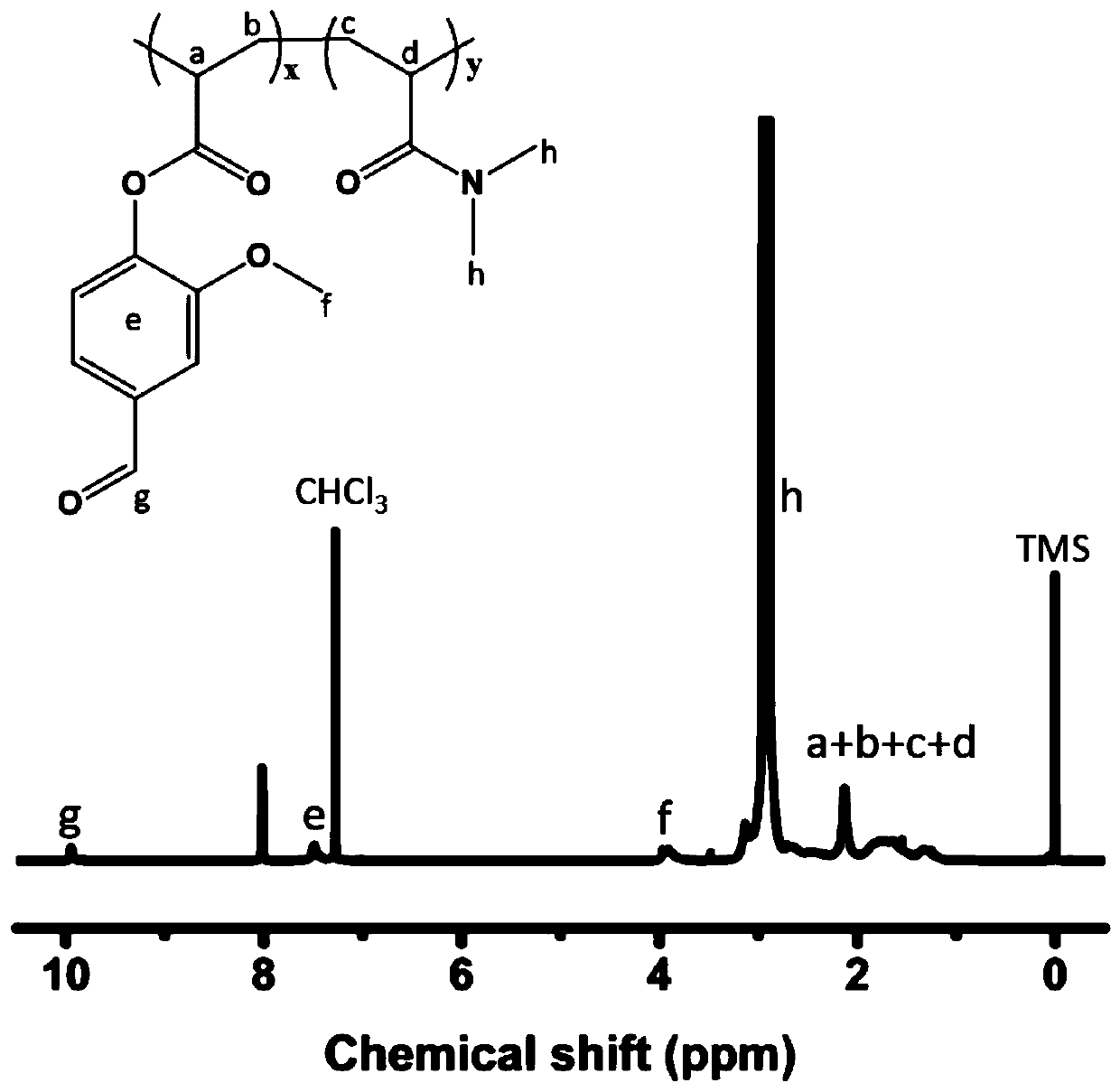
Indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of dye to dye-sensitized solar cell
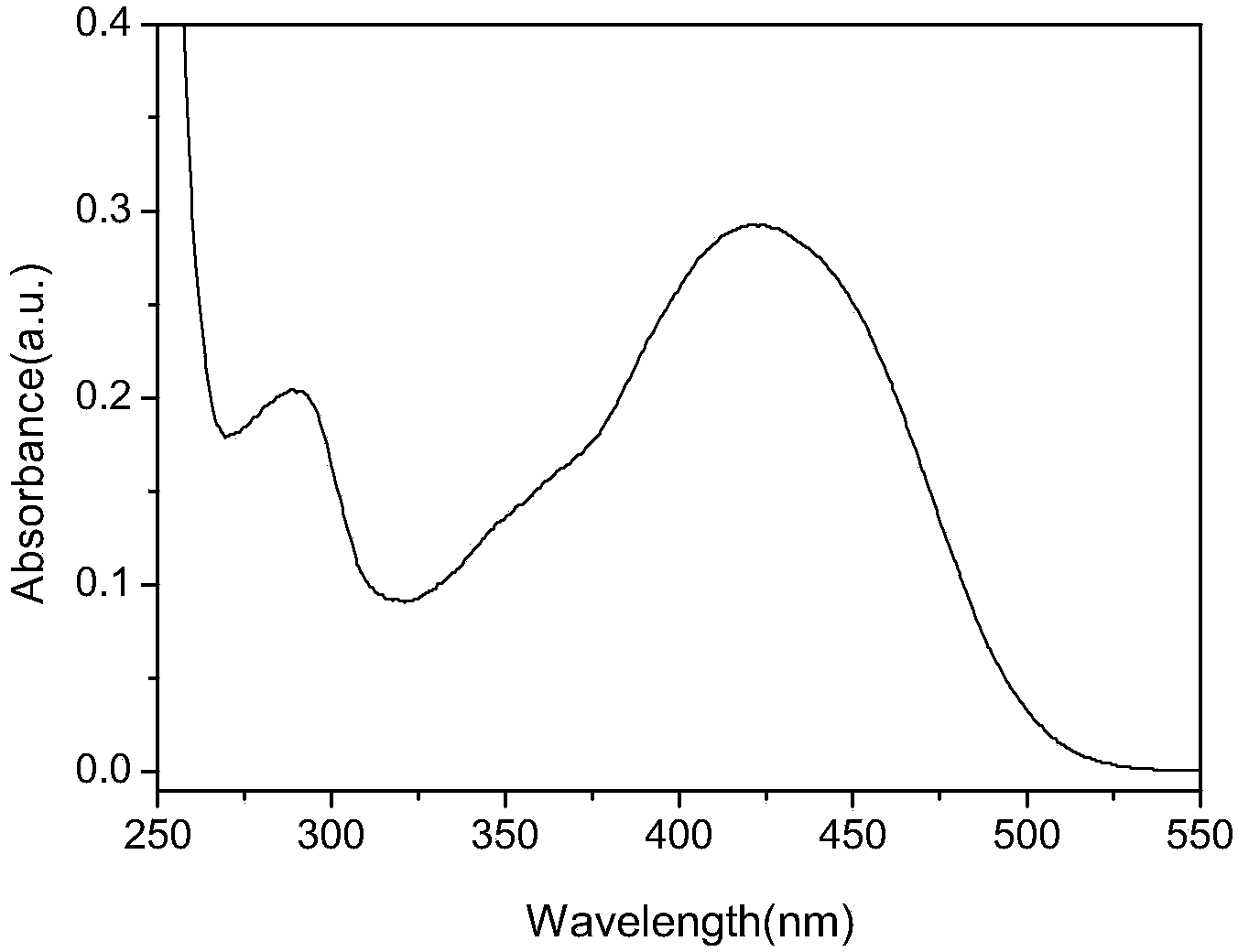
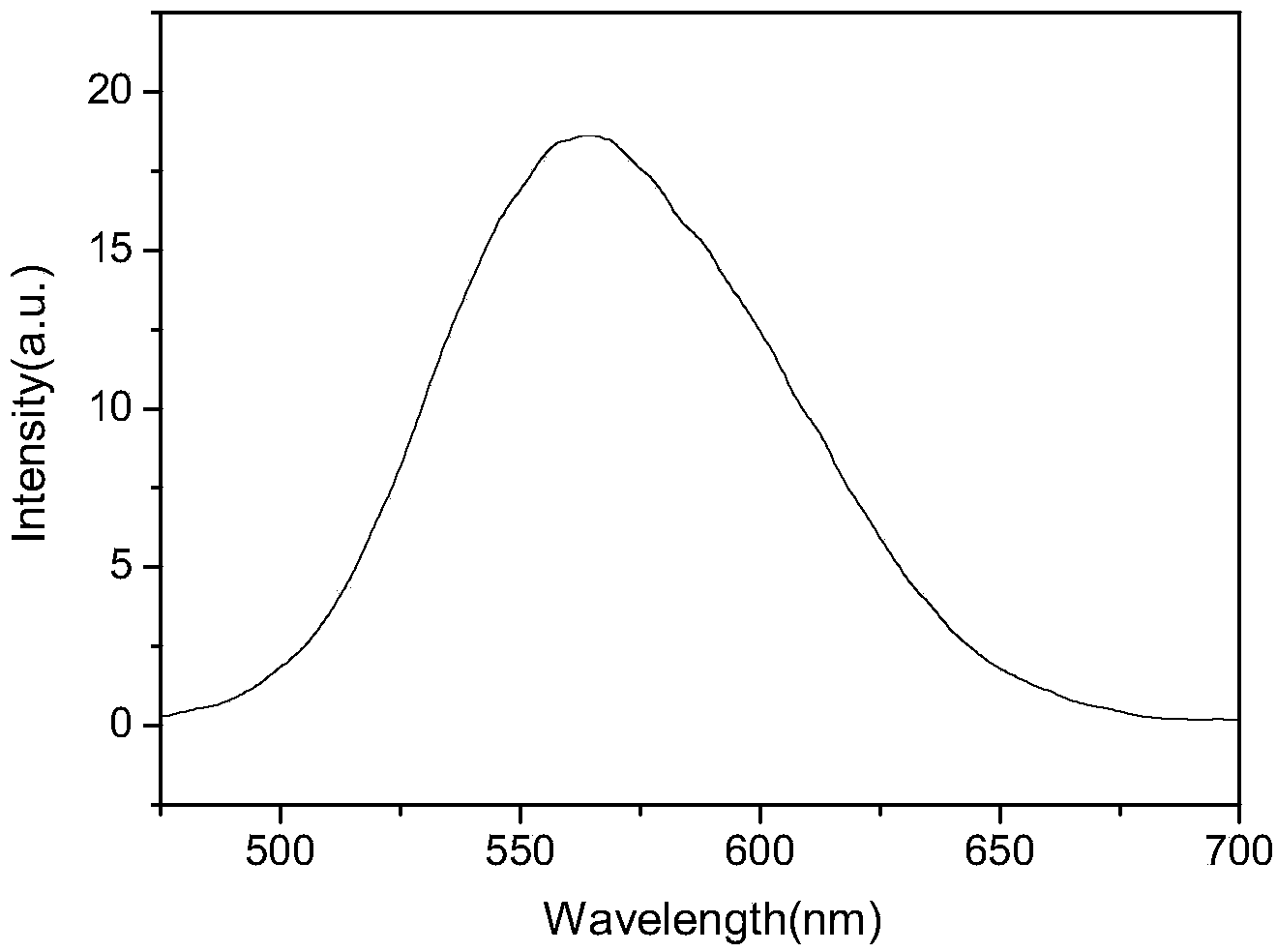
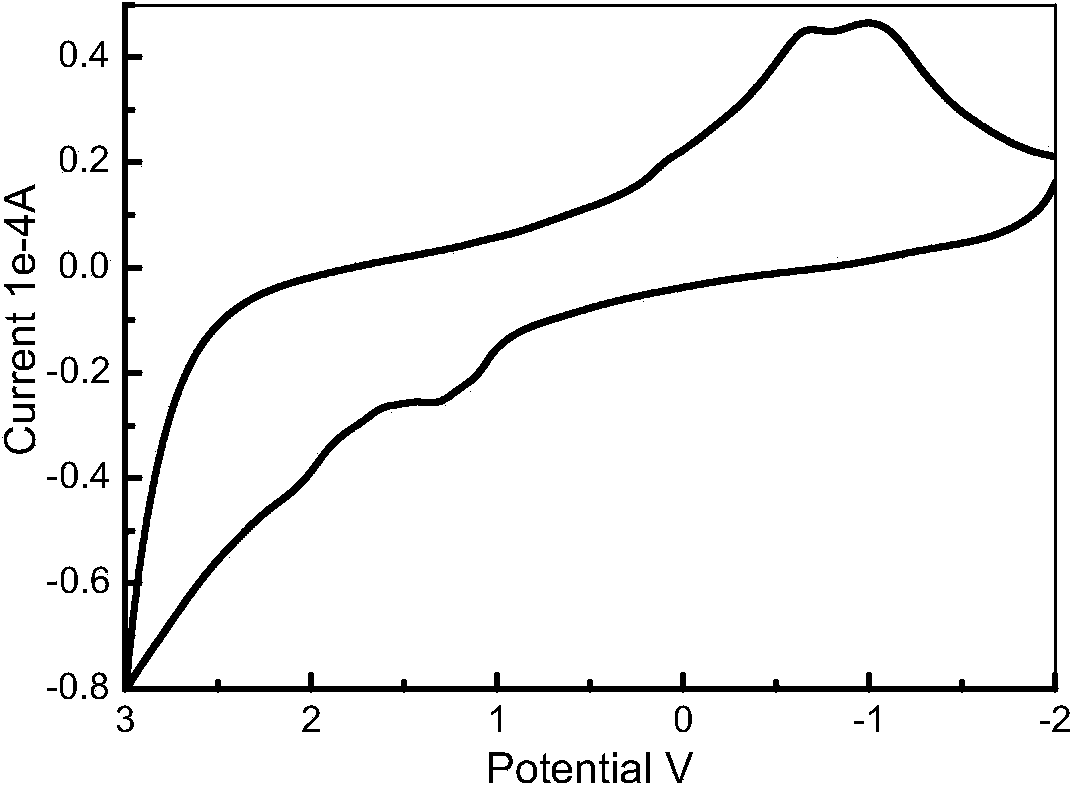
ActiveCN108164546AExtended conjugation propertiesExtend your lifeOrganic chemistryLight-sensitive devicesCyanoacetic acidOrganic dye
The invention discloses indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of the dye to a dye-sensitized solar cell. The dye is a D-A-pi-A pure organic dye, wherein an indoline group is taken as an electron donor; a dithienoquinoxaline group and a thiophene group are taken as pi bridges; a cyanoacetic acid group is taken as an electron donor as well as an anchoring group; meanwhile a dibenzo[a,c]phenazine group is taken as an electronic assisted donor. The dibenzo[a,c]phenazine assisted electronic donor is introduced into dye molecules, so that the conjugacy of the dye molecules is enhanced, and the molecular orbital energy level is regulated; intramolecular electron transfer is promoted through a rigid plane of conjugated big pi-bridge dioctyl dithienoquinoxaline, andthe accumulation of the dye is restrained through an alkyl chain, so that the spectrum response range is expanded effectively, and the electronic life is prolonged; the dye has good light absorptioncapability; the dye-sensitized solar cell based on the dye can obtain big photocurrent, so that relatively high photoelectric conversion efficiency is achieved.
Owner:SOUTH CHINA UNIV OF TECH
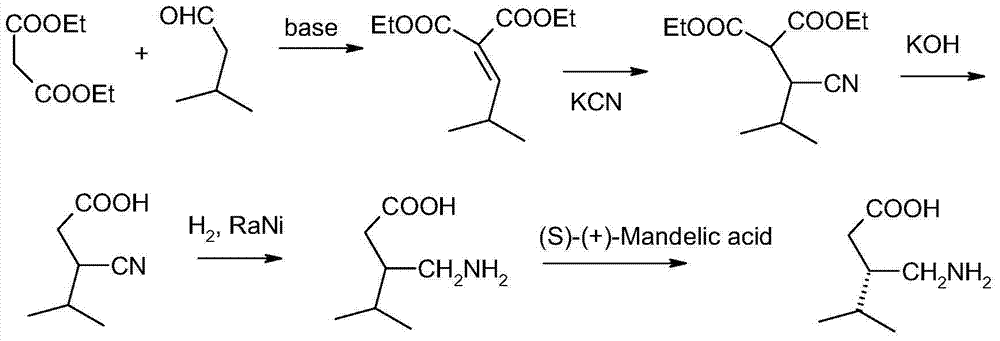
Preparation method of antiepileptic drug intermediate
InactiveCN102863326APromote precipitationEasy to operatePreparation from carboxylic acid esters/lactonesGlutaric acidCyanoacetic acid
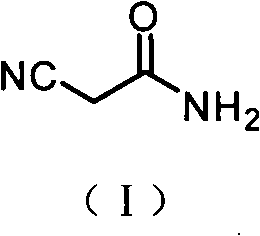
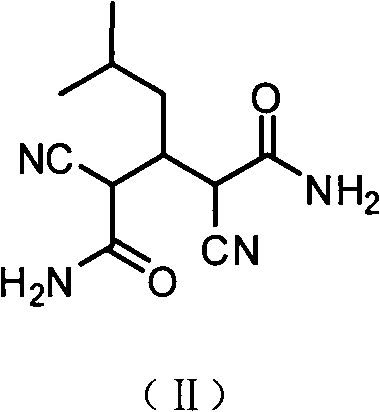
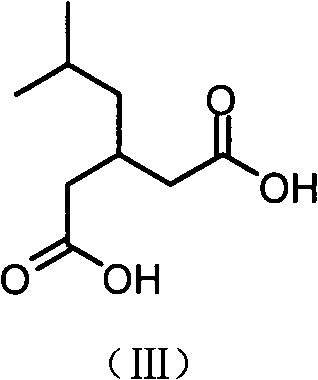
The invention relates to a preparation method of an antiepileptic drug intermediate 3-isobutyl glutaric acid. The preparation method includes that cyanoacetic acid ester ammonia is decomposed to obtain cyanoacetamide, condensation is performed on the cyanoacetamide and isovaleraldehyde to generate 2, 4-dicyan-3-isobutyl glutaric acid amide, the 2, 4-dicyan-3-isobutyl glutaric acid amide is hydrolysed and decarboxylated to obtain the 3-isobutyl glutaric acid, and total yield in the three steps can reach 77%. The preparation method of the antiepileptic drug intermediate is simple to operate, intermediate products and end products can be separated out easily from reaction, and the preparation method is low in cost and suitable for industrial production.
Owner:CHANGZHOU PHARMA FACTORY
Three-dimensionally prolonged conjugated chain phenothiazine dyes and application to dye-sensitized solar cells thereof
ActiveCN103497532AThe synthesis method is simpleRaw materials are cheap and easy to getLight-sensitive devicesThiazine dyesAcetic acidCyanoacetic acid
The invention discloses three-dimensionally prolonged conjugated chain phenothiazine dyes and an application to dye-sensitized solar cells thereof, and belongs to the field of photoelectric conversion material application in fine chemical engineering. According to the invention, an aromatic nucleus is introduced at 3 position of phenothiazine; phenyl rings are introduced at 7 and 9 positions; the conjugated chain is prolonged at the three directions simultaneously; thus the three-dimensionally prolonged conjugated chain phenothiazine is used as an electron donor; cyanoacetic acid is used as an electron receptor; and a series of three-dimensionally prolonged conjugated chain phenothiazine dyes are synthesized. The three-dimensionally prolonged conjugated chain phenothiazine dyes have stronger light-capturing capability in visible region, thus have superior performance to common phenothiazine dyes under the same conditions, and can effectively improve the photoelectric conversion efficiency of dye-sensitized solar cells.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000011.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000012.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka.patsnap.com/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000021.PNG)

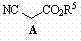

![Indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of dye to dye-sensitized solar cell Indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of dye to dye-sensitized solar cell](https://images-eureka.patsnap.com/patent_img/19494de4-6e77-4d05-a754-d069d63c9895/HDA0001583963530000011.png)
![Indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of dye to dye-sensitized solar cell Indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of dye to dye-sensitized solar cell](https://images-eureka.patsnap.com/patent_img/19494de4-6e77-4d05-a754-d069d63c9895/HDA0001583963530000021.png)
![Indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of dye to dye-sensitized solar cell Indoline-dithienoquinoxaline-dibenzo[a,c]phenazine dye and application of dye to dye-sensitized solar cell](https://images-eureka.patsnap.com/patent_img/19494de4-6e77-4d05-a754-d069d63c9895/BDA0001583963520000021.png)