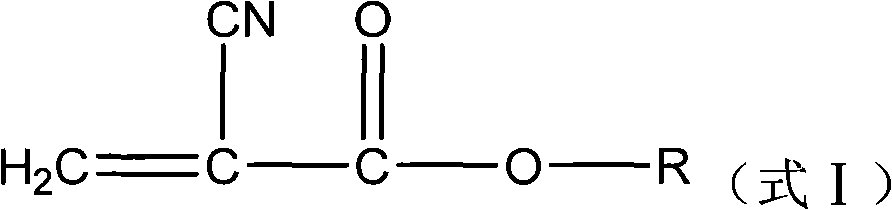
Environment-friendly preparation method of alpha-cyanoacrylate compound
A technology of cyanoacrylate and cyanoacrylic acid, which is applied in the field of environmentally friendly preparation of α-cyanoacrylate compounds, can solve the problems of easily affecting the health of production personnel, reducing biological safety, and contacting personnel injuries, etc., to achieve reduction Contamination and operator injury, improvement of biological safety, and reduction of difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
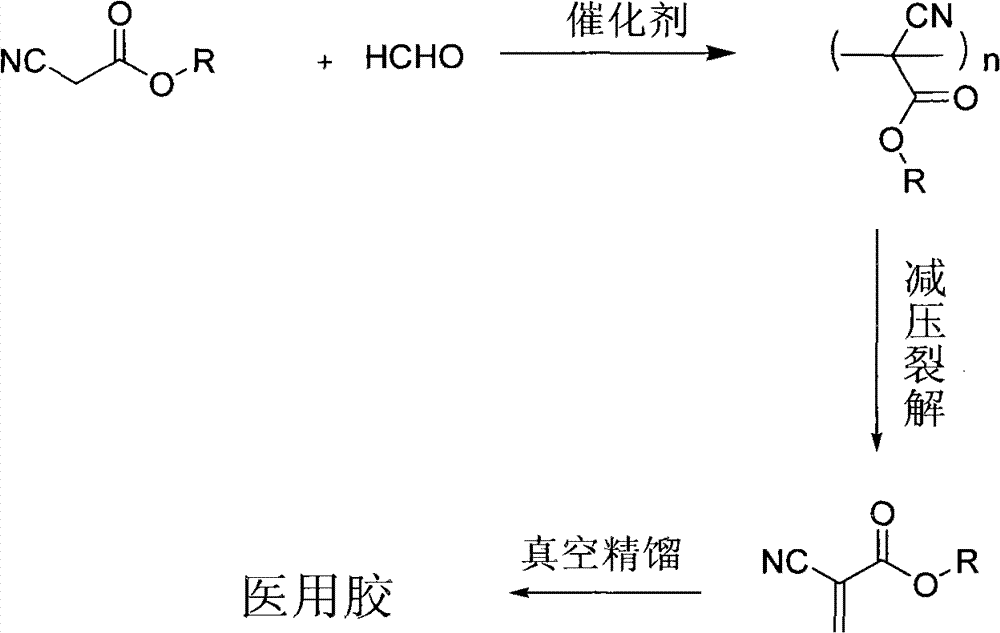
[0031] The present invention provides an environmentally friendly preparation method of α-cyanoacrylate compounds with less environmental pollution, less harm to production personnel and high biological safety. The three-step method comprises the following steps:
[0032] 1) Formaldehyde solution and composite catalyst A are added in a low-toxicity solvent, and a mixture of α-cyanoacetate compounds and catalyst B is added dropwise under stirring to generate prepolymers and oligomers of α-cyanoacrylate compounds. mixture;
[0033] 2) Add dilute acid solution, stir for a period of time and separate the liquid, remove the water layer, so that the remaining catalyst in the prepolymer and oligomer mixture of α-cyanoacrylate can be removed, and then add plasticizer and dehydrating agent and a polymerization inhibitor, heated to remove water, distilled at high temperature to depolymerize the prepolymer or oligomer to obtain the crude product of α-cyanoacrylic acid compound;
[0034]...
Embodiment 1
[0055] Embodiment 1, formaldehyde aqueous solution method prepares n-butyl α-cyanoacrylate
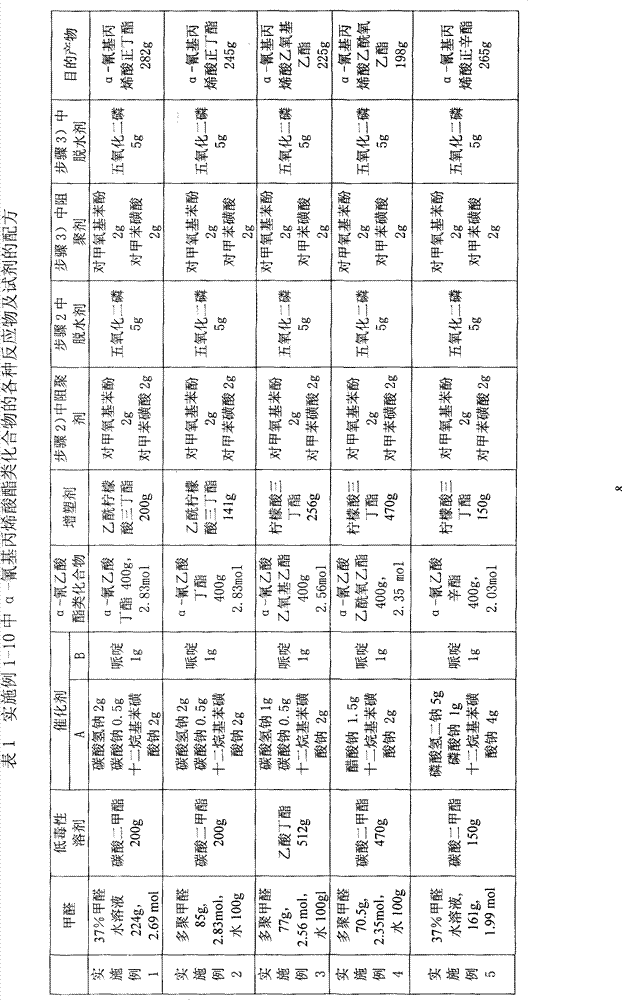
[0056] Prepare α-n-butyl cyanoacrylate with the method of the present invention, formula is shown in Table 1, specifically comprises the following steps:
[0057] 1) In a 2000mL four-neck flask, add 224g 37% formaldehyde solution, 200g methyl carbonate, 4.5g composite catalyst A, stir evenly, heat to about 60°C (50-75°C is acceptable), add dropwise 400g α-cyanide The mixture of butyl acetate and 1.0g piperidine, after the dropwise addition, continue to heat and keep the reaction under reflux state and temperature 50-85°C for 1.5-2 hours to obtain the prepolymer or oligomer of n-butyl cyanoacrylate thing.
[0058]2) stop heating, add 50g dilute hydrochloric acid, leave standstill layering after stirring evenly, after removing supernatant, add 200g acetyl tributyl citrate, assemble water separator on the right side port of four-neck flask, be warming up to 80 At about ℃, reflux begins ...
Embodiment 2
[0062] Embodiment 2, paraformaldehyde method prepares n-butyl α-cyanoacrylate
[0063] Using the same operation as in Example 1, using the materials and amounts listed in Table 1, step 2) collected about 350 g of crude product, and step 3) obtained about 245 g of refined monomer, with a yield of 55.5%. Product testing results: gas chromatography purity of 99.6%, 1 H-NMR (600MHz, CDCl 3 , δ / ppm): 6.99(s, 1H), 6.61(s, 1H), 4.04(t, 2H), 1.73(m, 2H), 1.51(m, 2H), 0.95(t, 3H), confirmation sheet The body is n-butyl α-cyanoacrylate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com