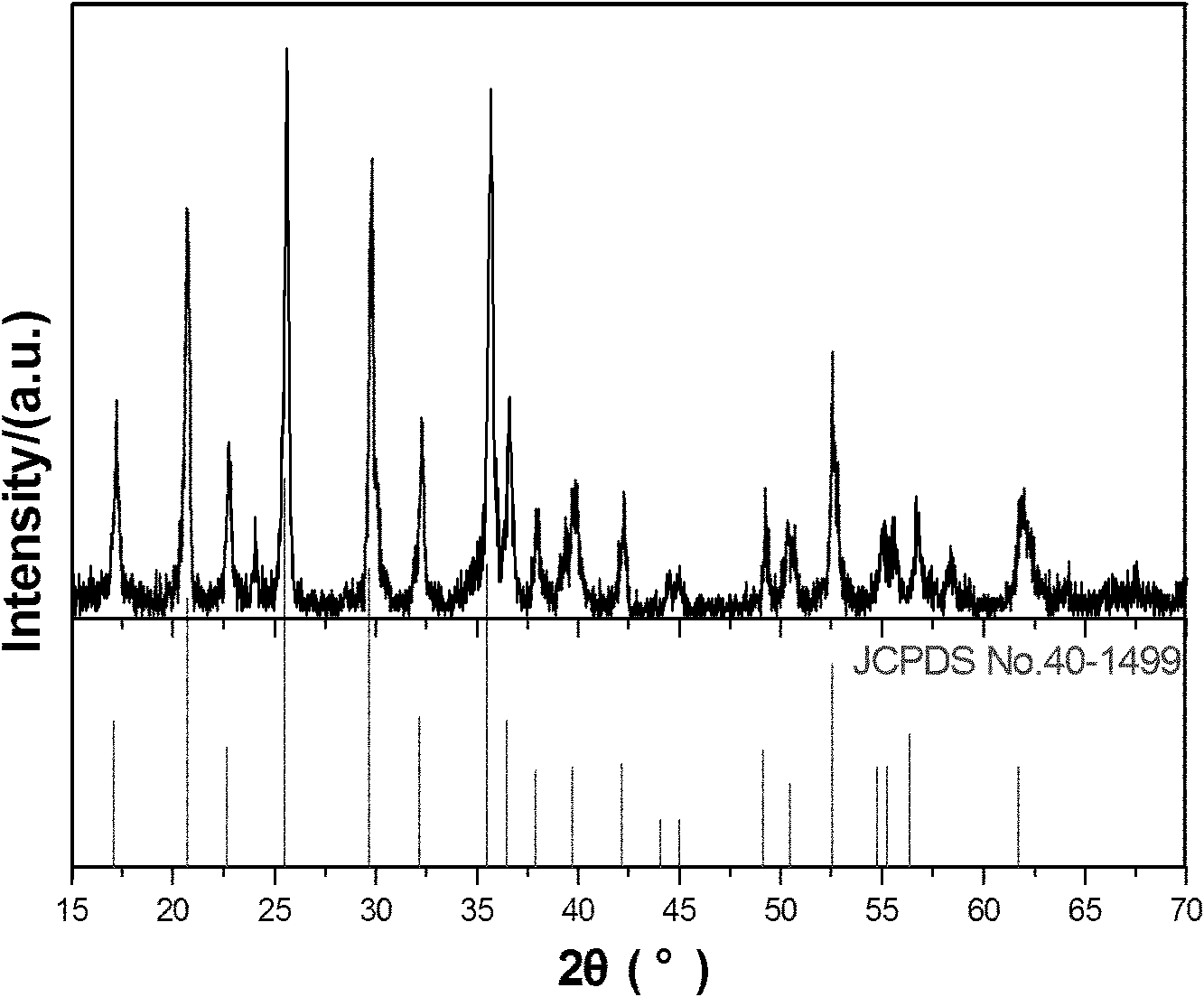
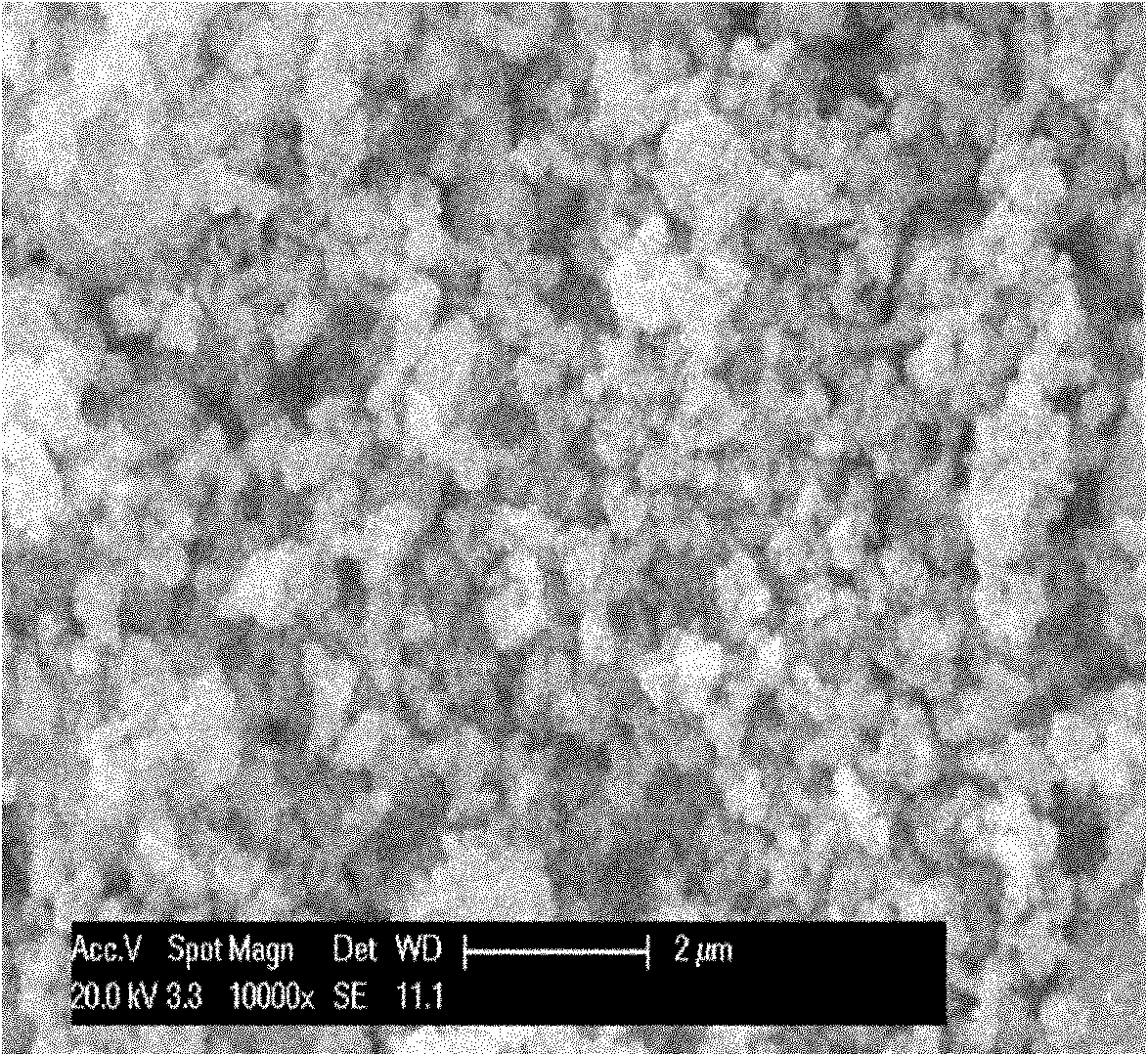
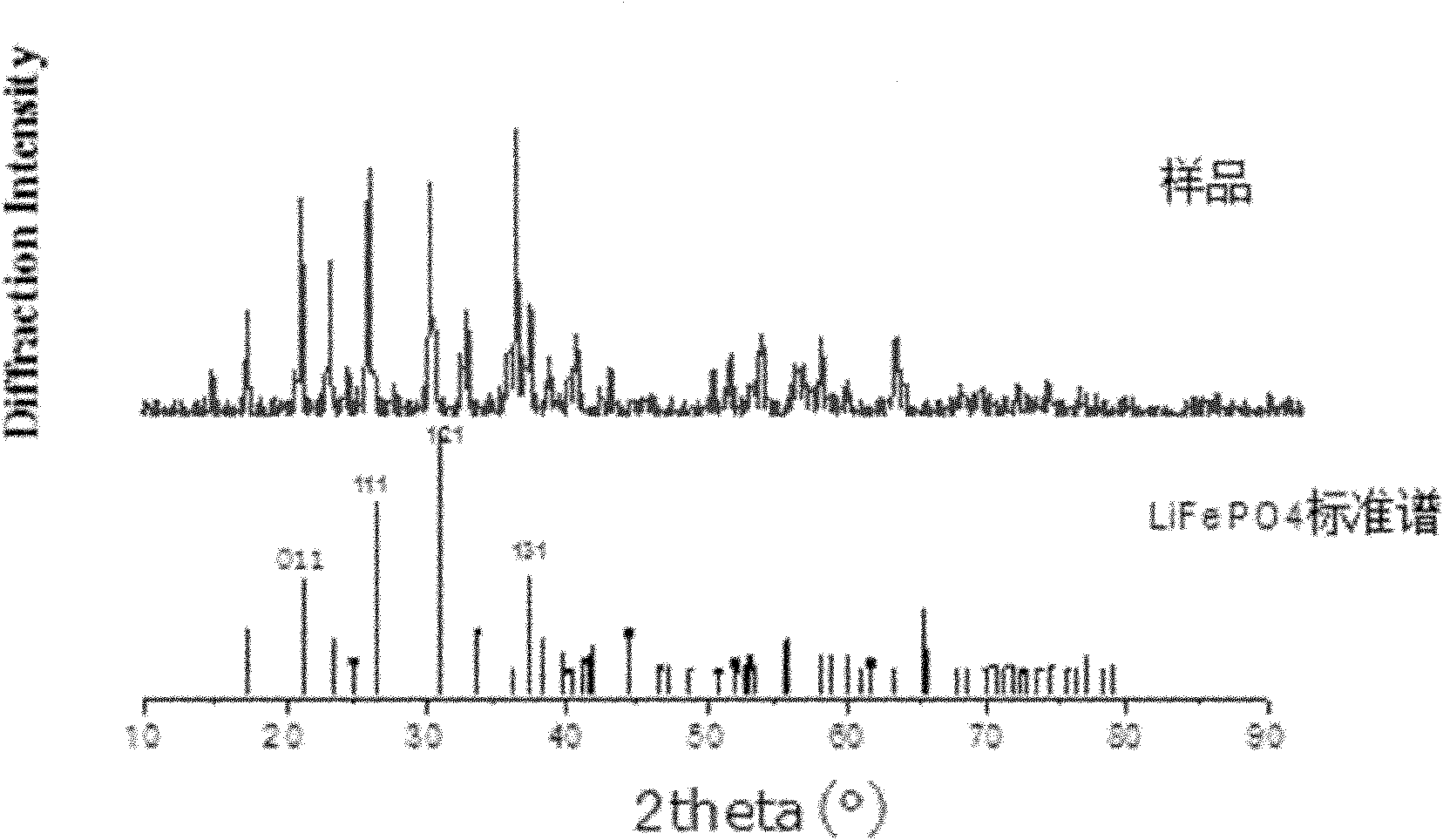
Hydrothermal synthesis method for preparing nano-scale carbon-coated lithium iron phosphate
A technology of carbon-coated lithium iron phosphate and hydrothermal synthesis, applied to electrical components, battery electrodes, circuits, etc., can solve the problems of poor ion conductivity of active materials, affecting high-rate discharge performance, slow migration and diffusion, etc. , to achieve the effects of easy industrial production, accelerated diffusion speed, and uniform particle distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] According to the molar ratio of Li:Fe:P=3:1:1, take 12.6 kg of lithium hydroxide monohydrate (LiOH·H 2 O) add 100 liters of deionized water, get 27.8 kilograms of ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O) add 200 liters of deionized water, get 11.5 kilograms of phosphoric acid (85% H 3 PO 4 ) and 30 liters of deionized water were added to prepare aqueous solutions. Expected target product 15 kilograms. First, add phosphoric acid solution in the reaction kettle, then add ferrous sulfate solution, then add 1.5 kg of carbon black, after fully stirring and mixing, add lithium hydroxide solution, and finally add 350 liters of glycerin. Nitrogen was blown from the bottom of the reactor for one hour, all the air in the reactor was exhausted, and then the reactor was sealed and heated to 120°C for 3 hours. After cooling, the product was filtered, washed, vacuum-dried and dried. Finally, put it into a sagger, and sinter it through a mesh belt furnace. Sintering conditio...
Embodiment 2
[0030] According to the molar ratio of Li:Fe:P=1:1:1, take 6.9 kg of lithium nitrate (LiNO 3 ) plus 100 liters of deionized water, get 12.7 kg of ferrous chloride (FeCl 2 ) plus 200 liters of deionized water and 11.5 kg of phosphoric acid (85% H3 PO 4 ) and 30 liters of deionized water were added to prepare aqueous solutions. Expected target product 10 kilograms. Take 0.5kg of cetyltrimethylammonium bromide, add 2 liters of deionized water at about 35°C and stir thoroughly to prepare a dispersant solution. First, add phosphoric acid solution into the reaction kettle, then add ferrous chloride solution, then add 0.5 kg of sucrose and 0.5 kg of carbon black, after fully stirring and mixing, add lithium nitrate solution, along with adding dispersant solution, finally add 350 liters of poly ethylene glycol. Nitrogen was blown from the bottom of the reactor for one hour, all the air in the reactor was exhausted, and then the reactor was sealed and heated to 150°C for 3 hours. A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com