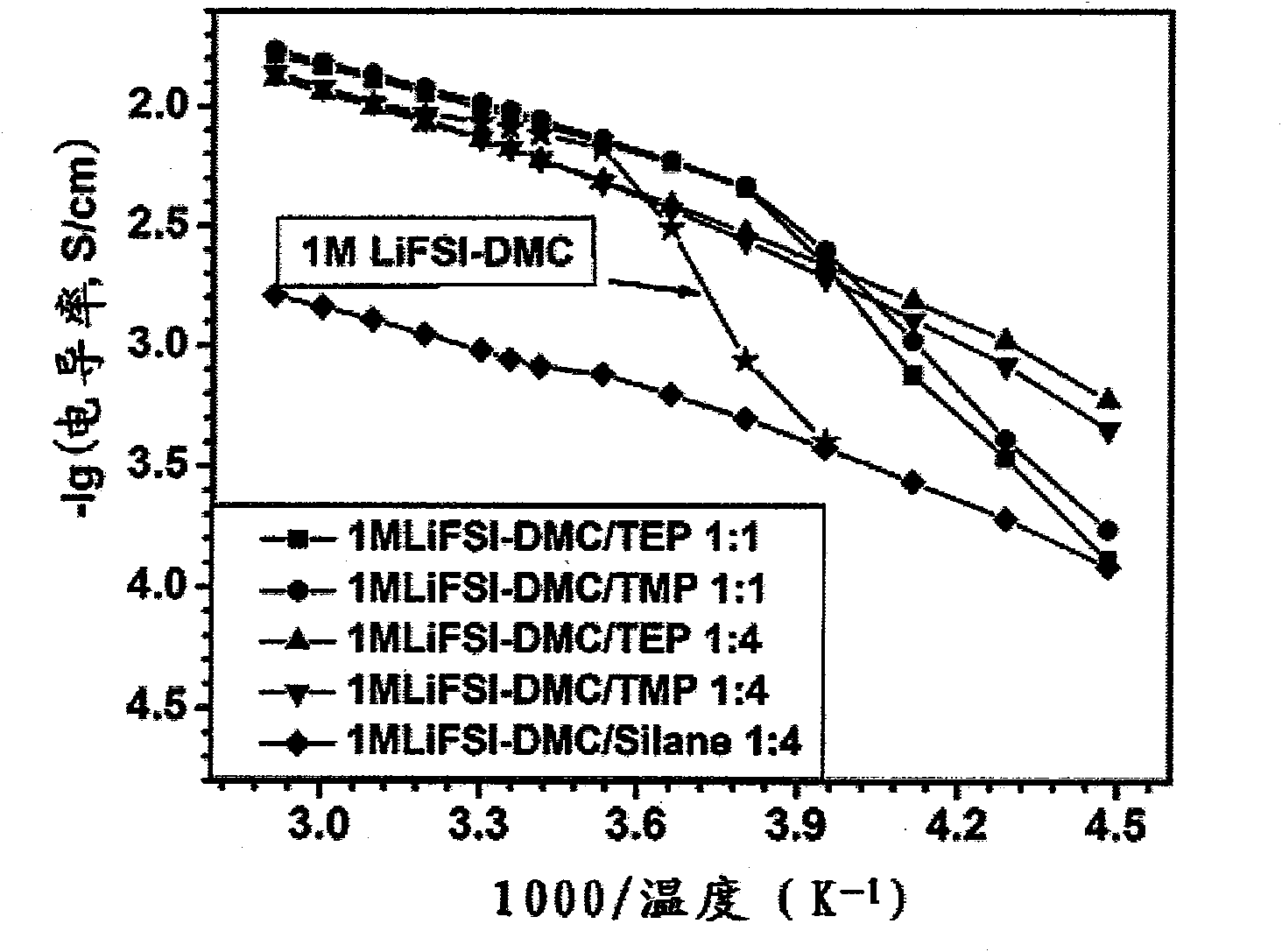
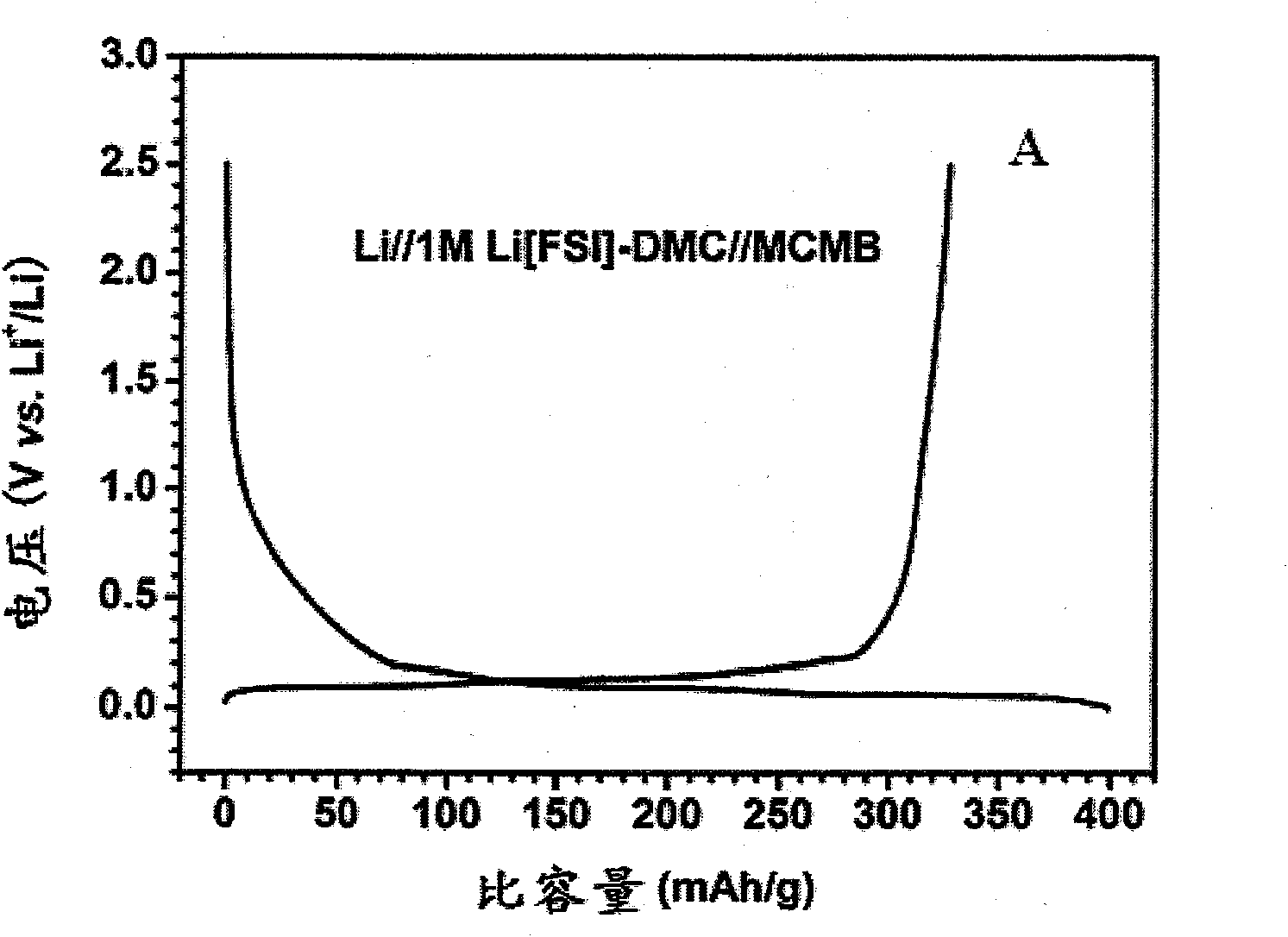
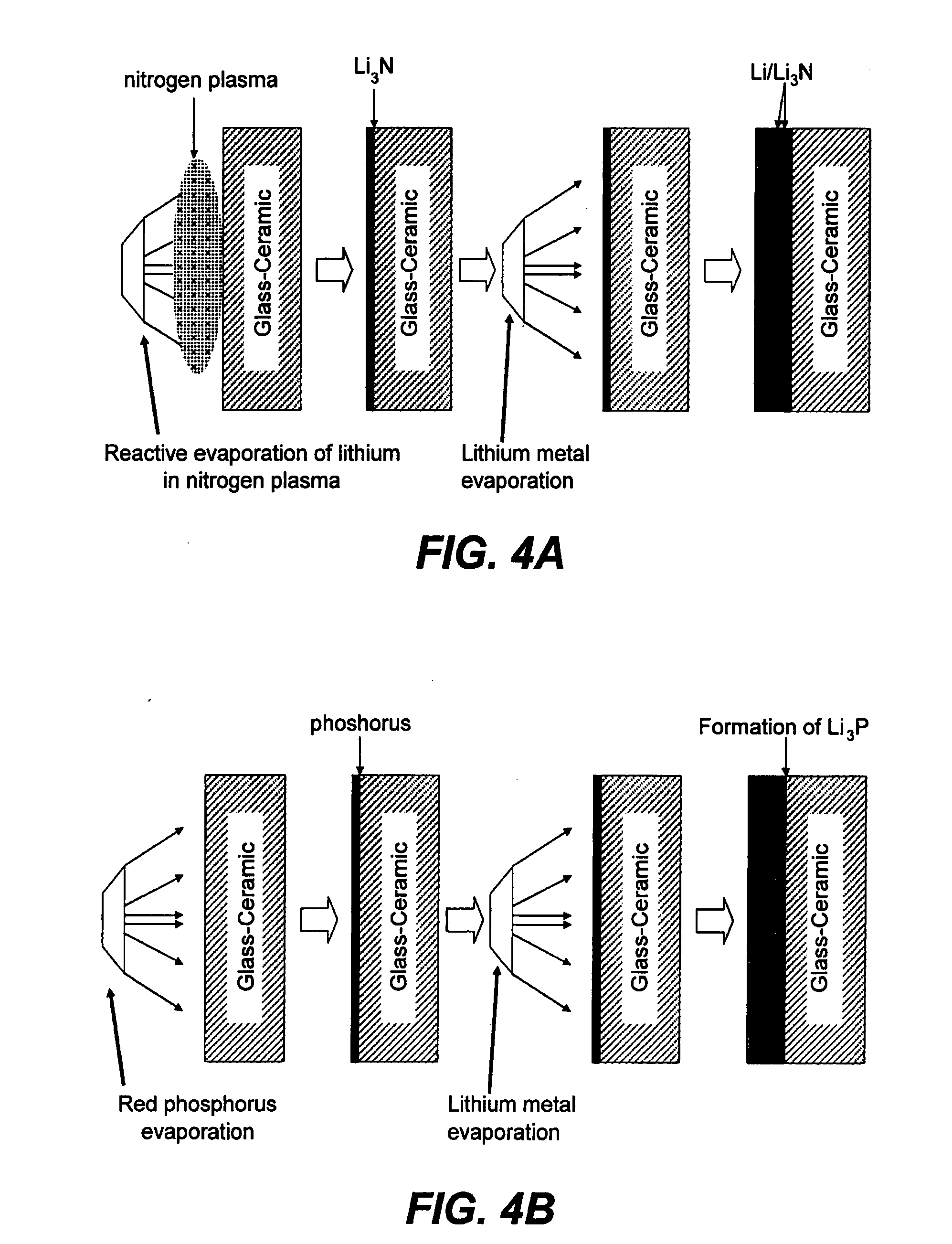
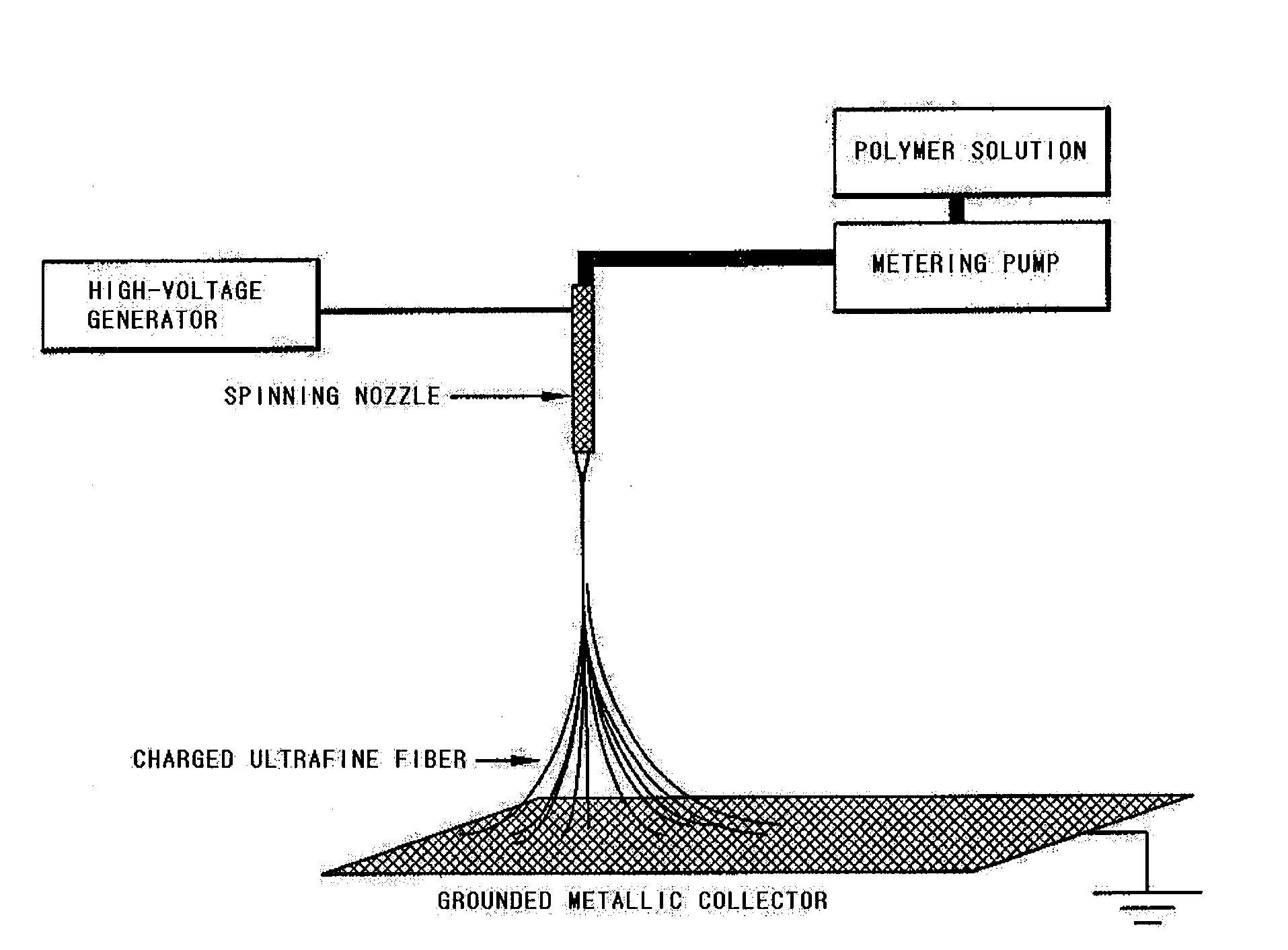
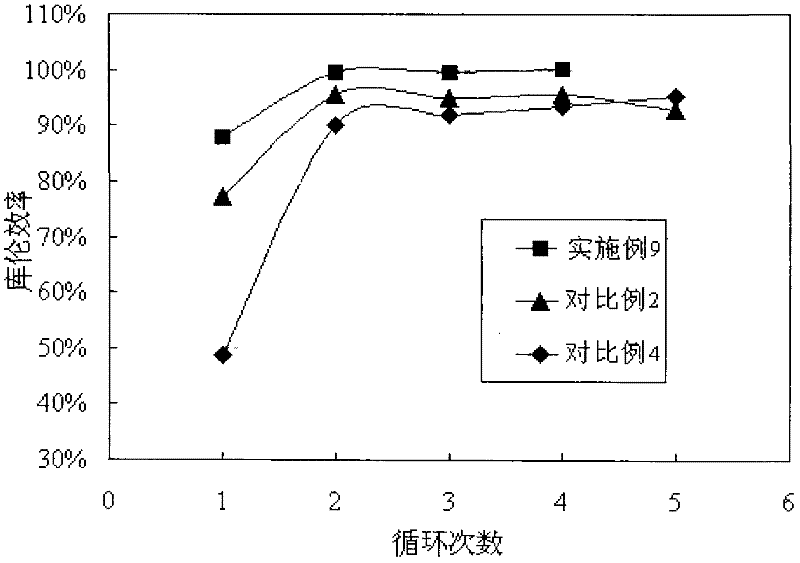
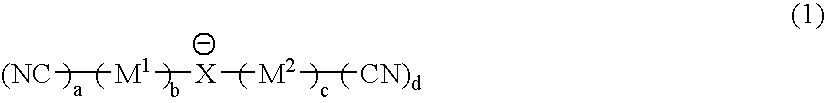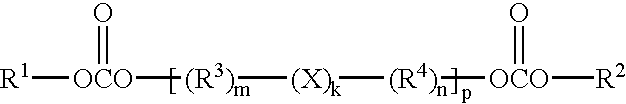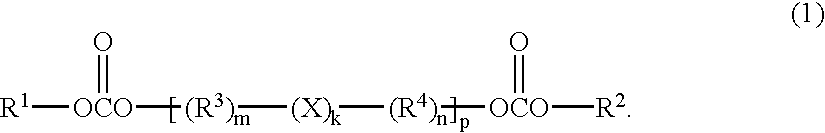Patents
Literature
4173results about How to "Improve ionic conductivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
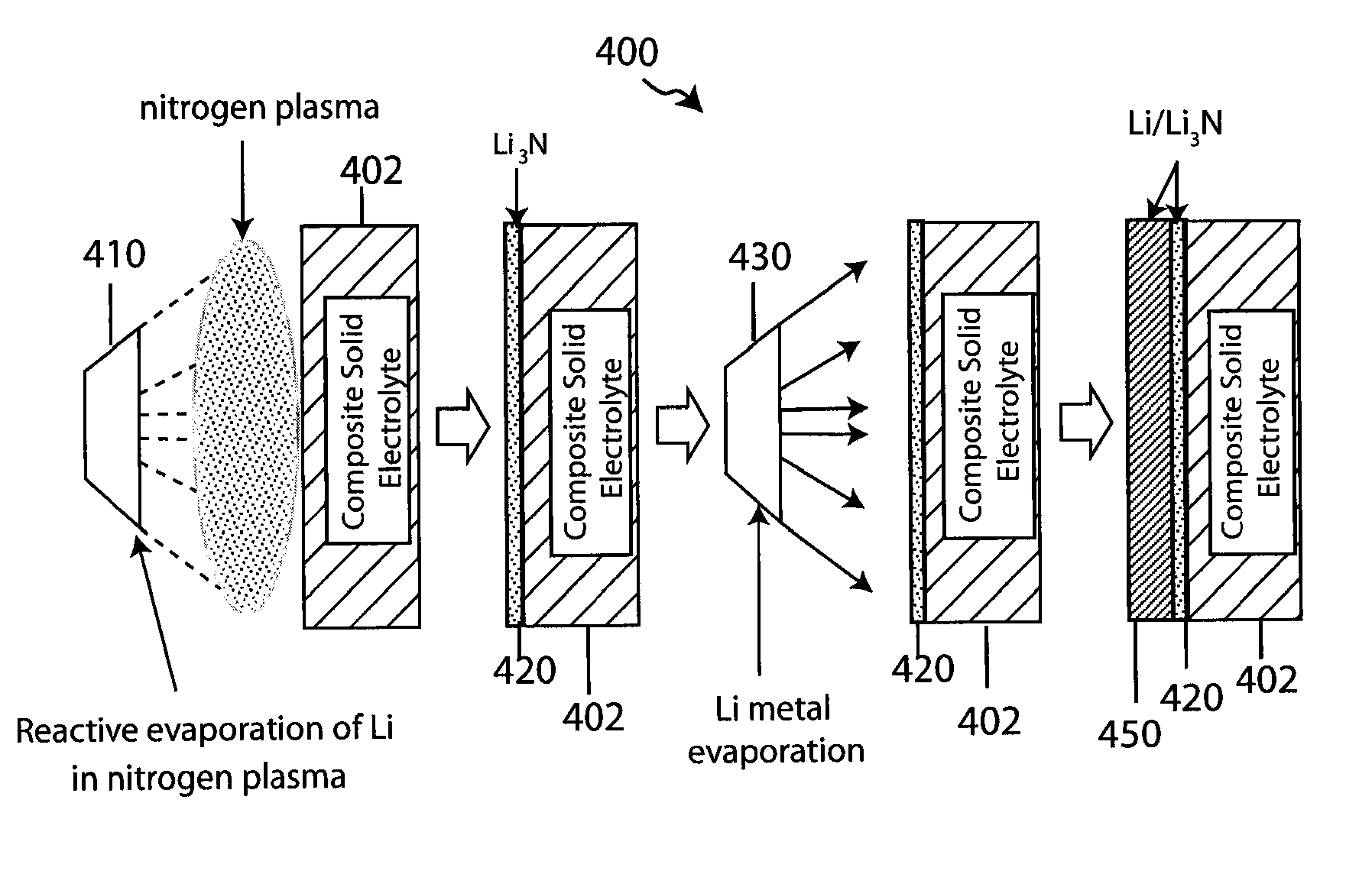
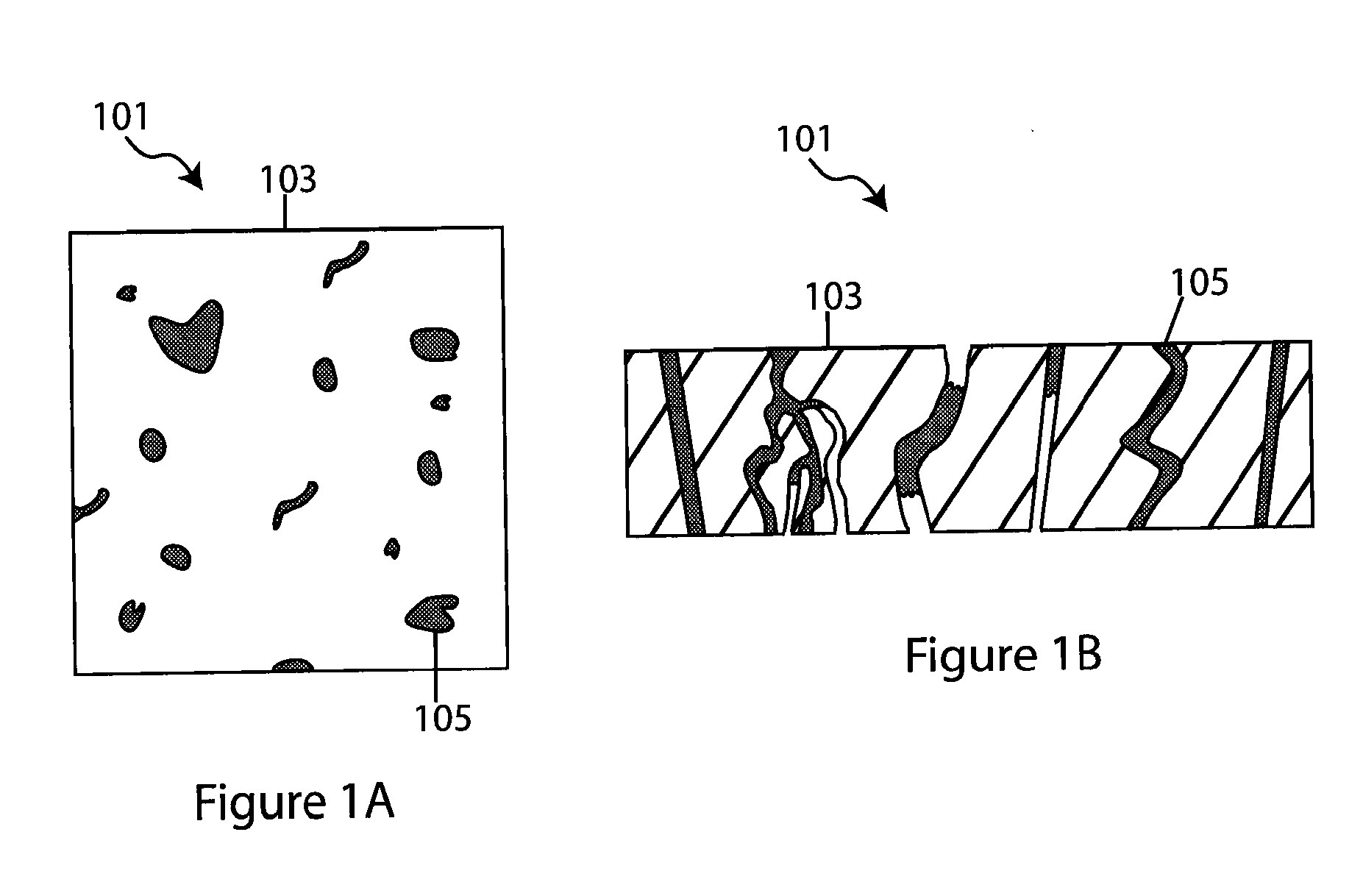
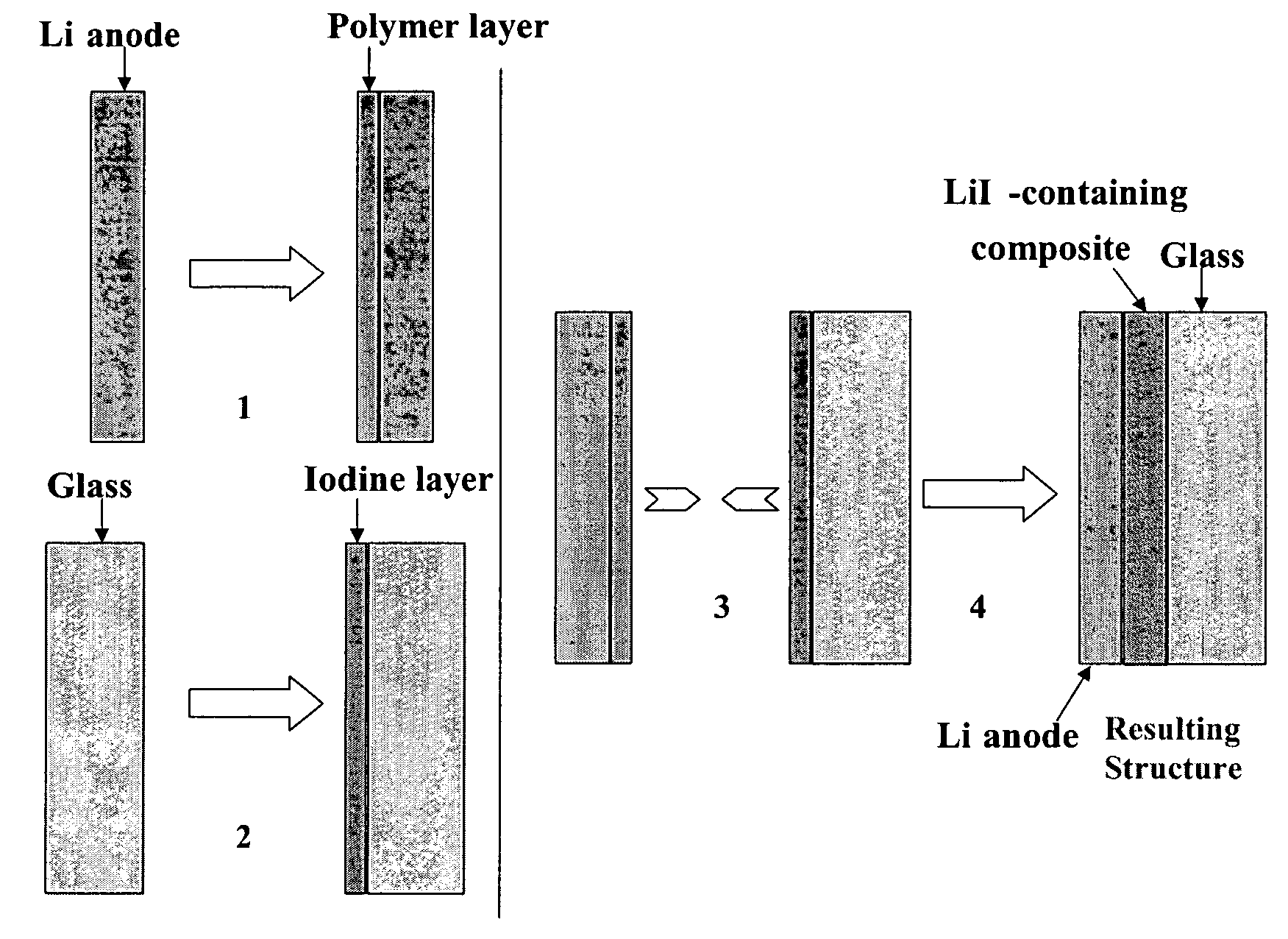
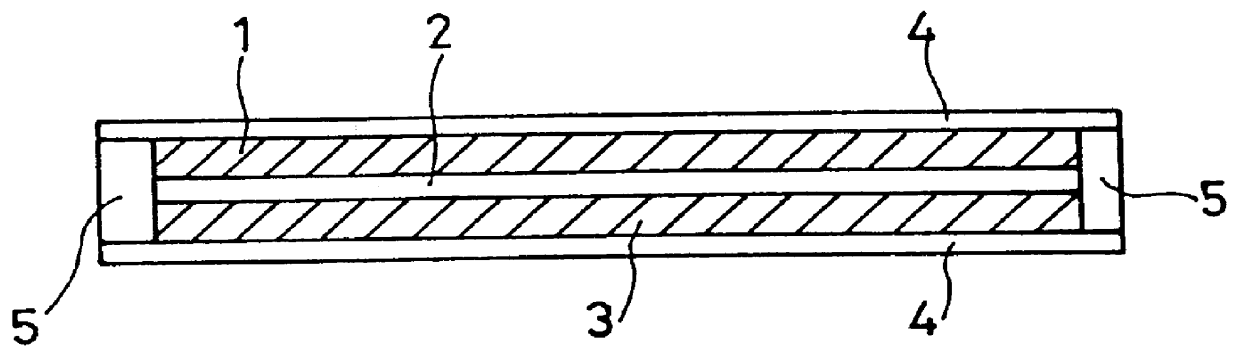
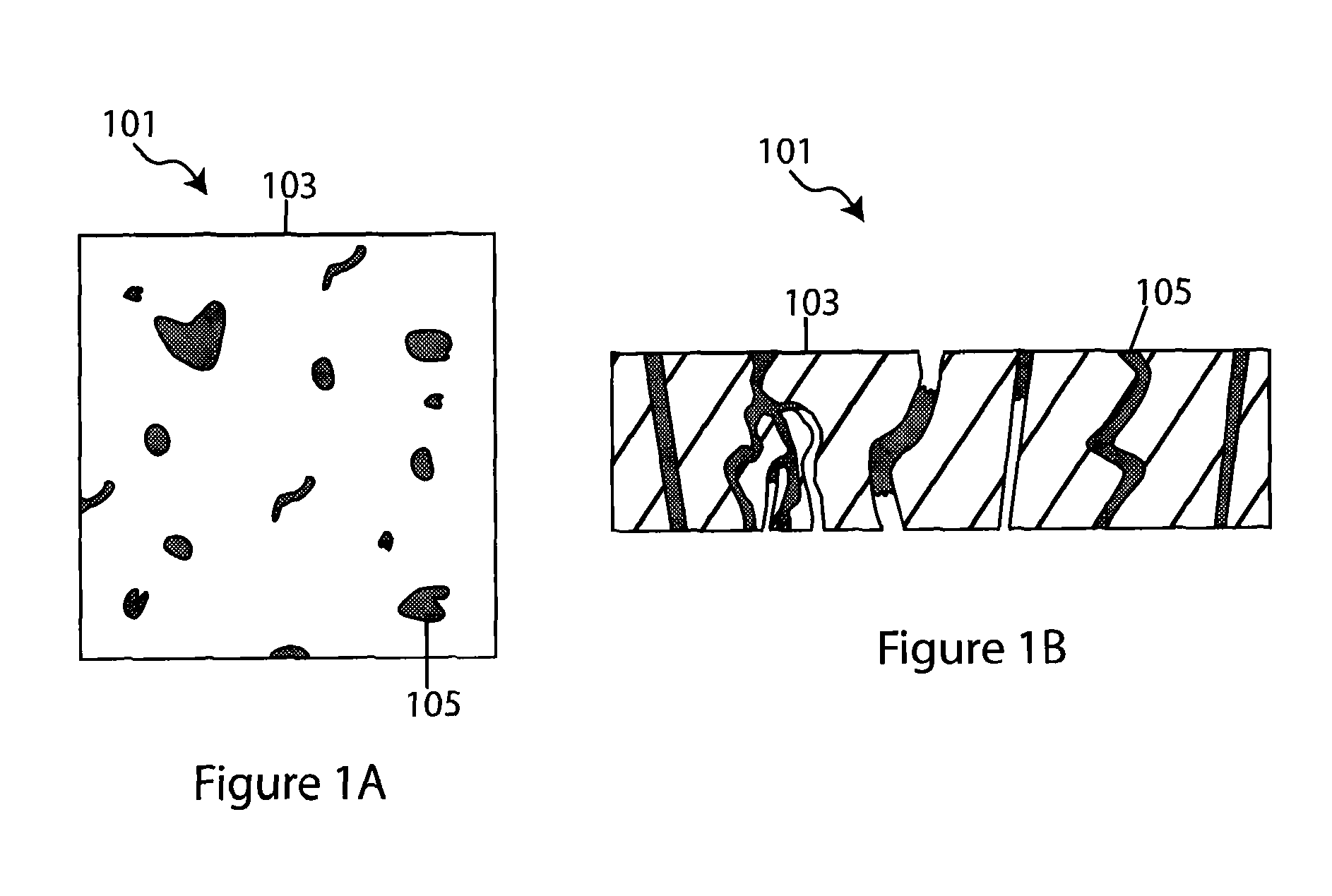
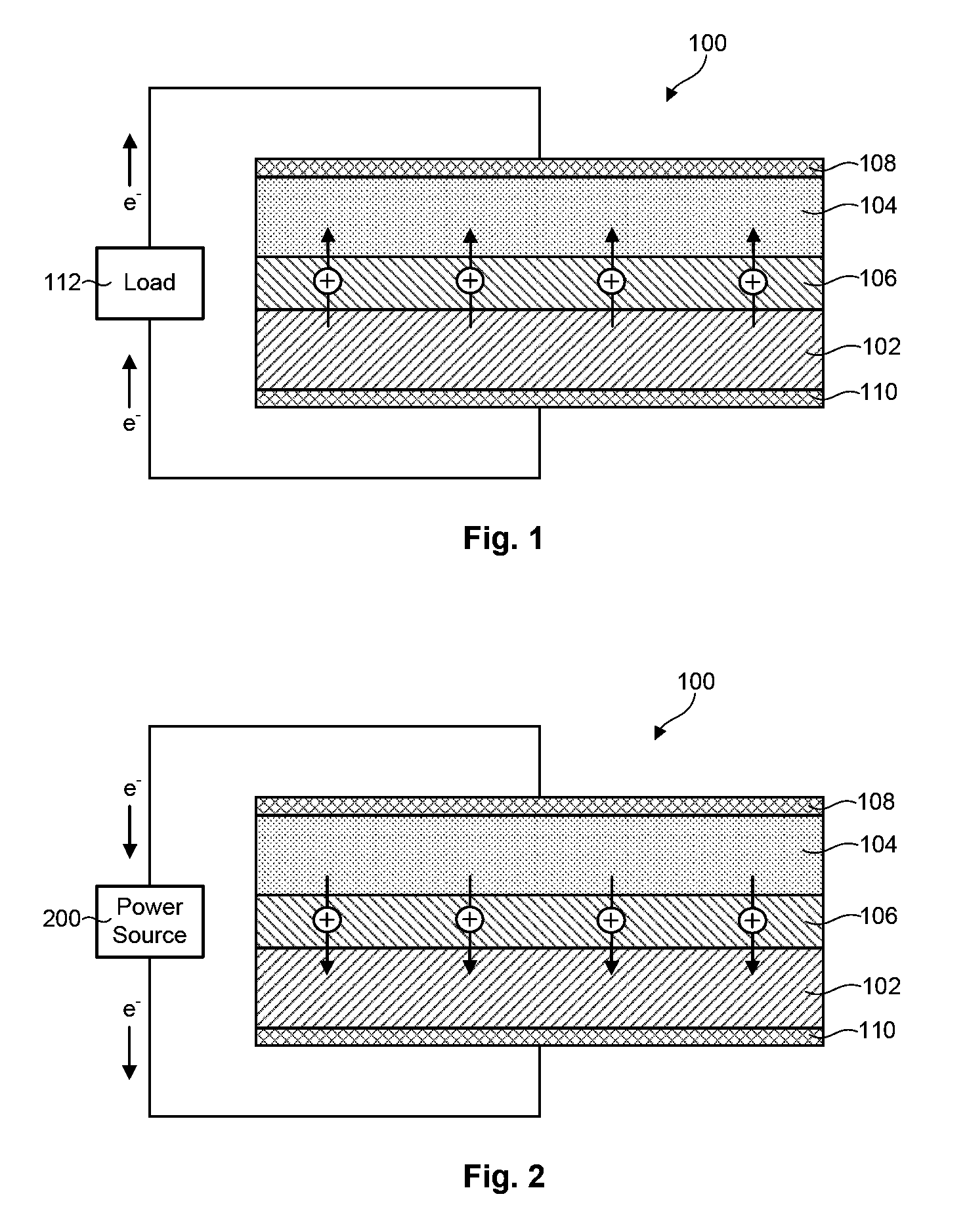
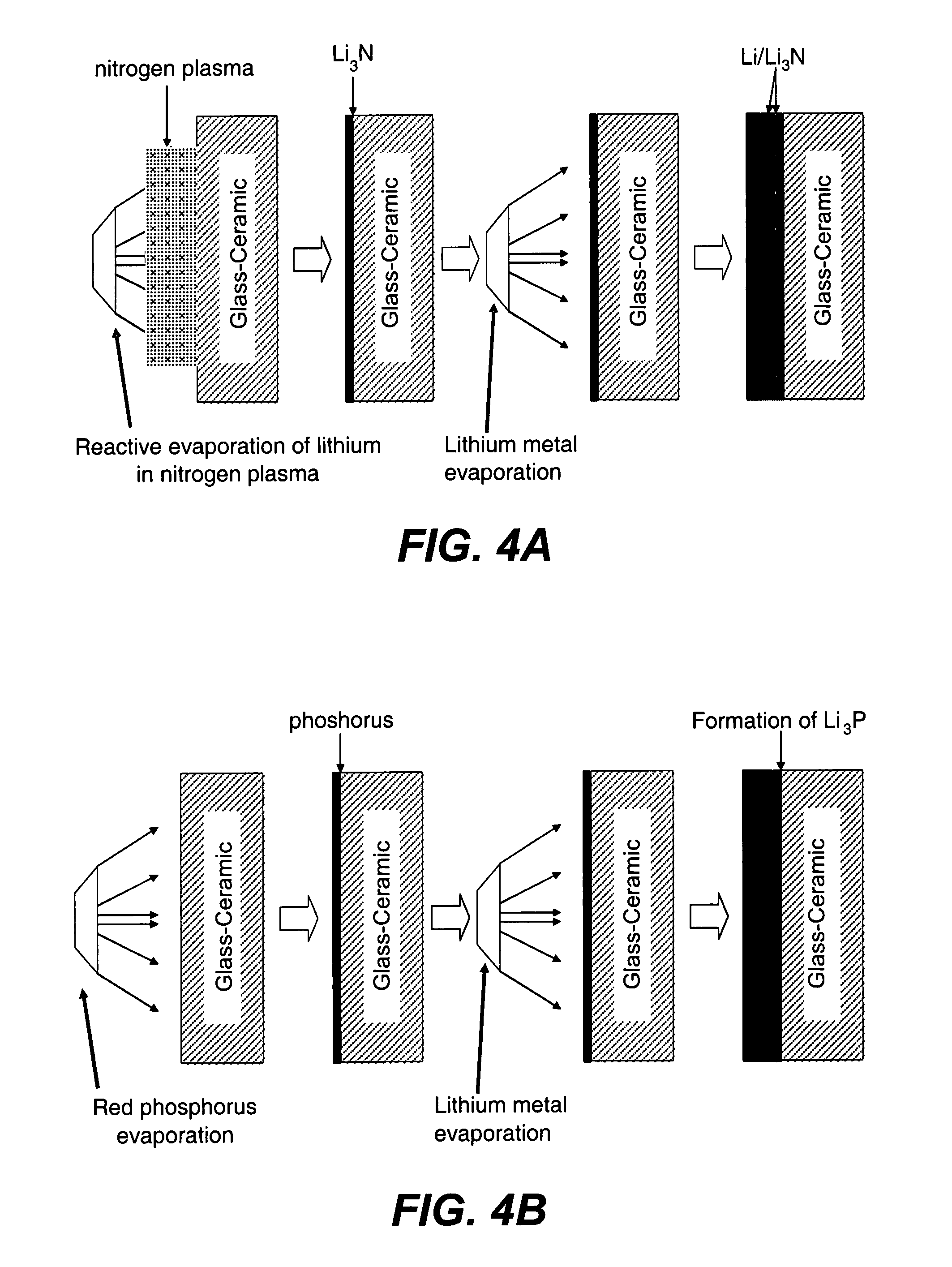
Composite solid electrolyte for protection of active metal anodes
ActiveUS20070172739A1Eliminate through-porosityHigh metal ion conductivityCell electrodesPrimary cellsPorosityTectorial membrane
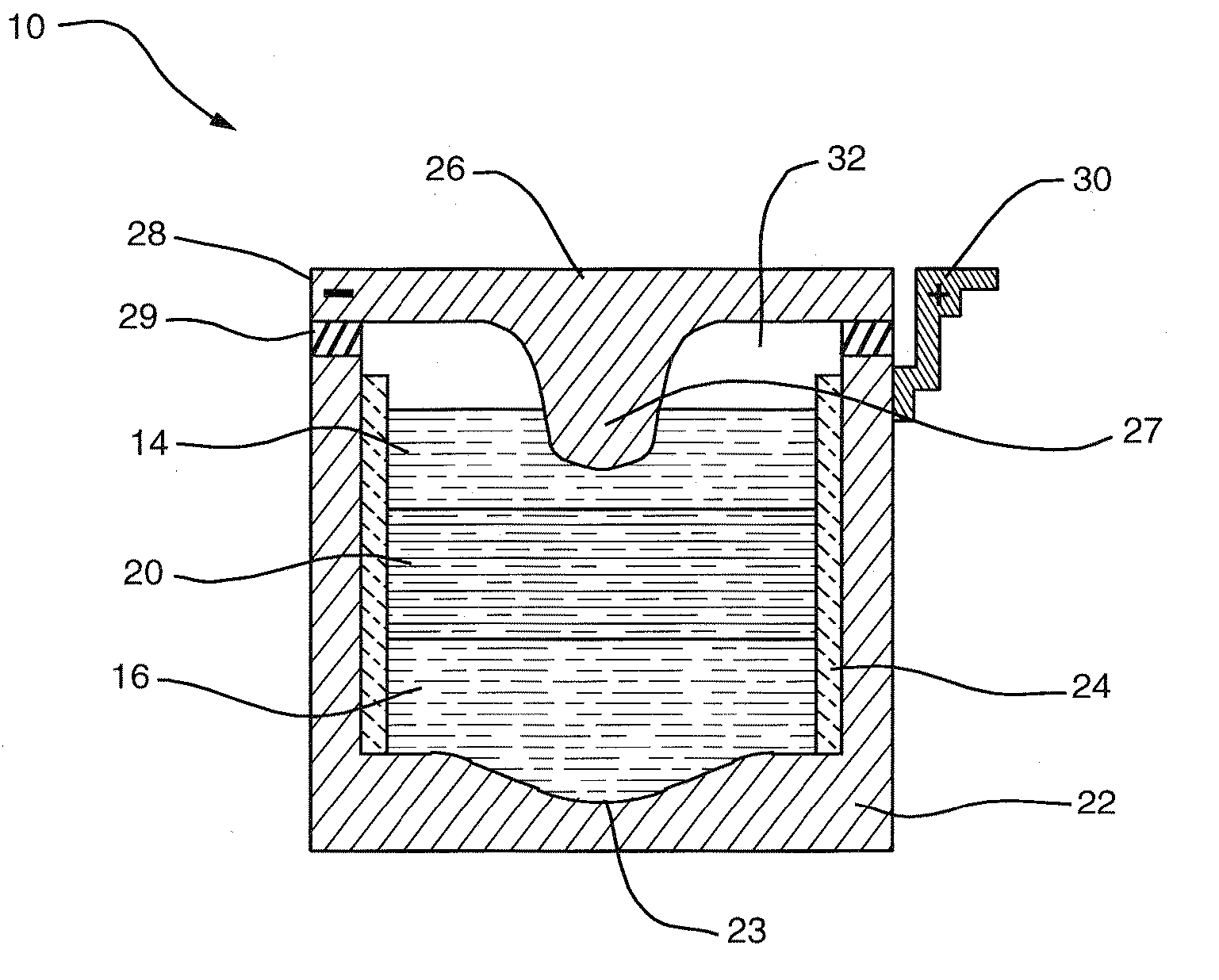
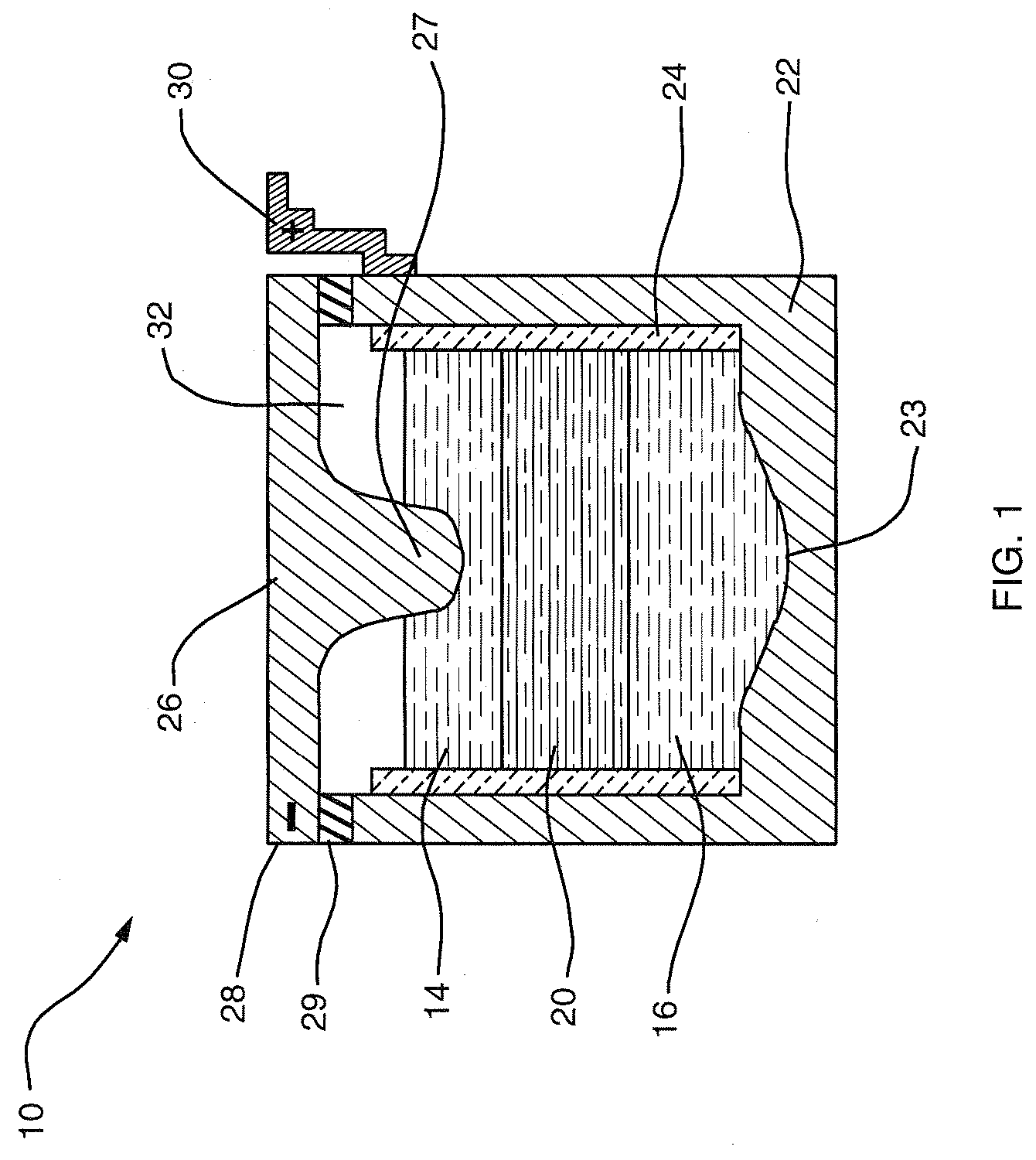
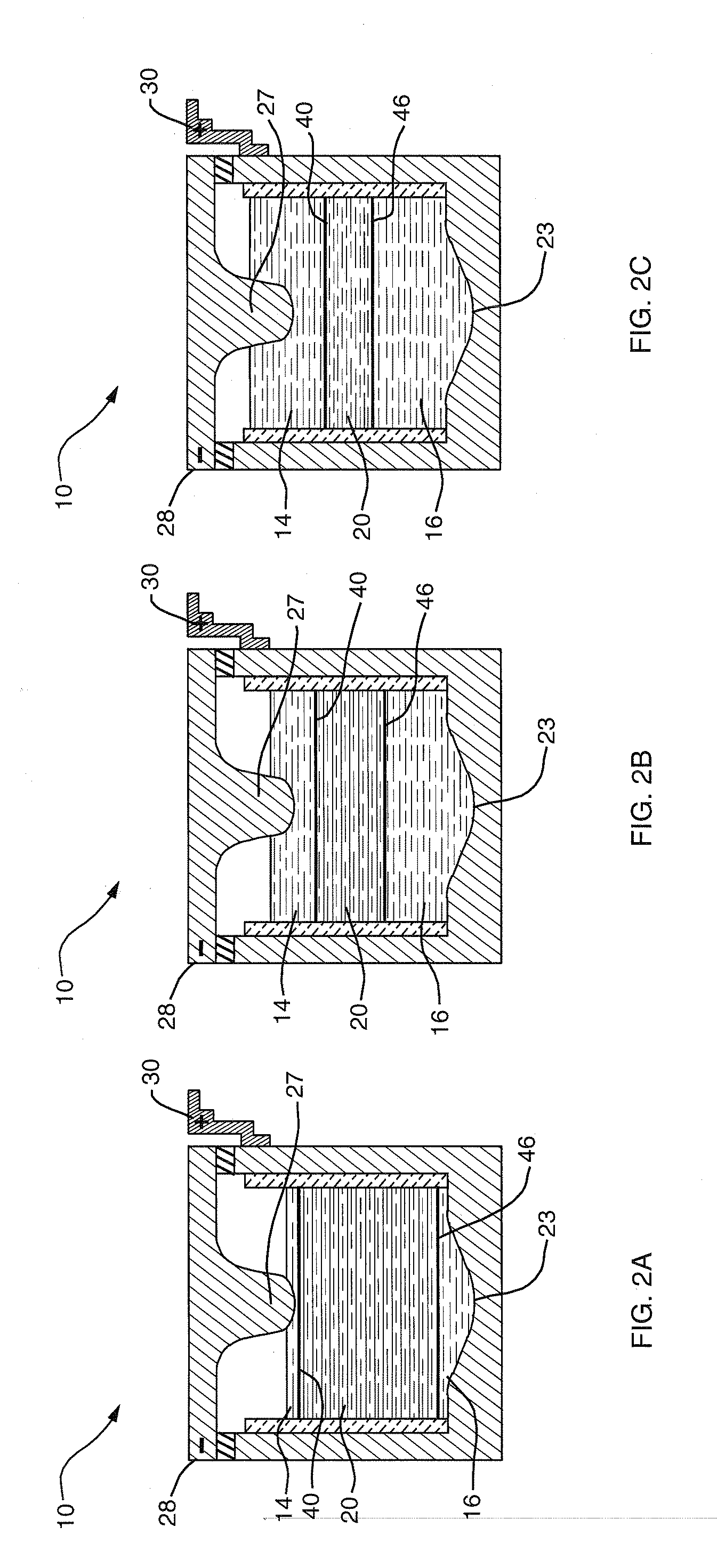
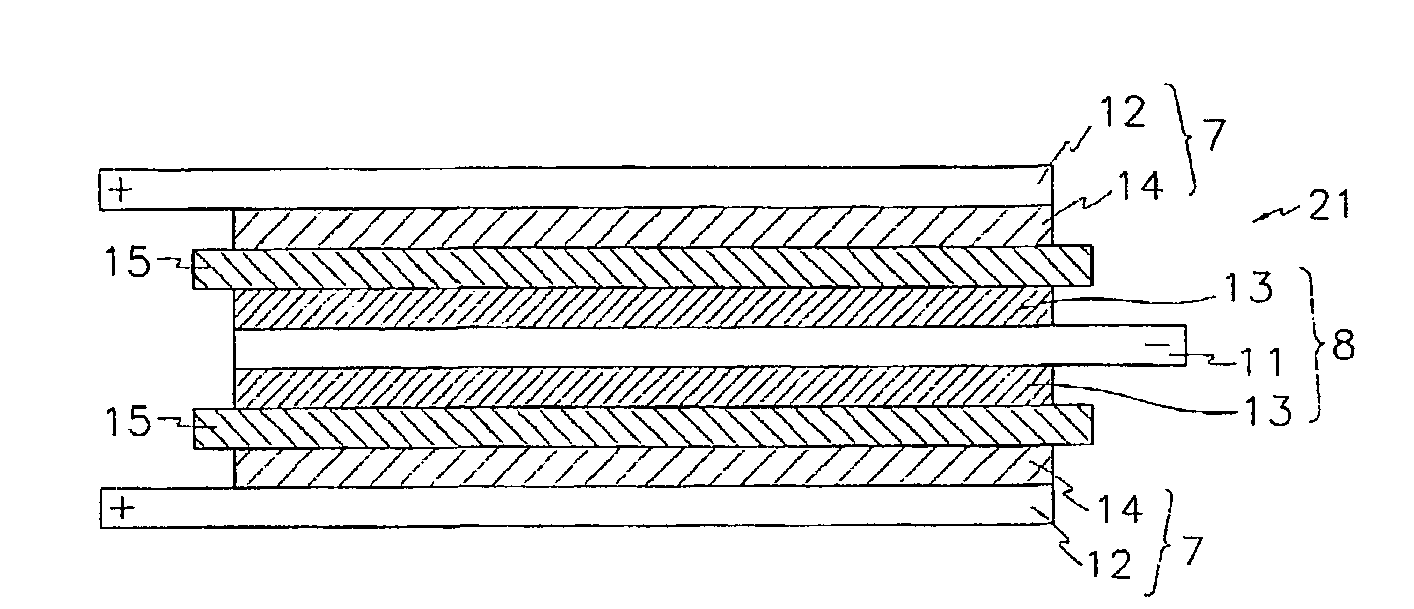
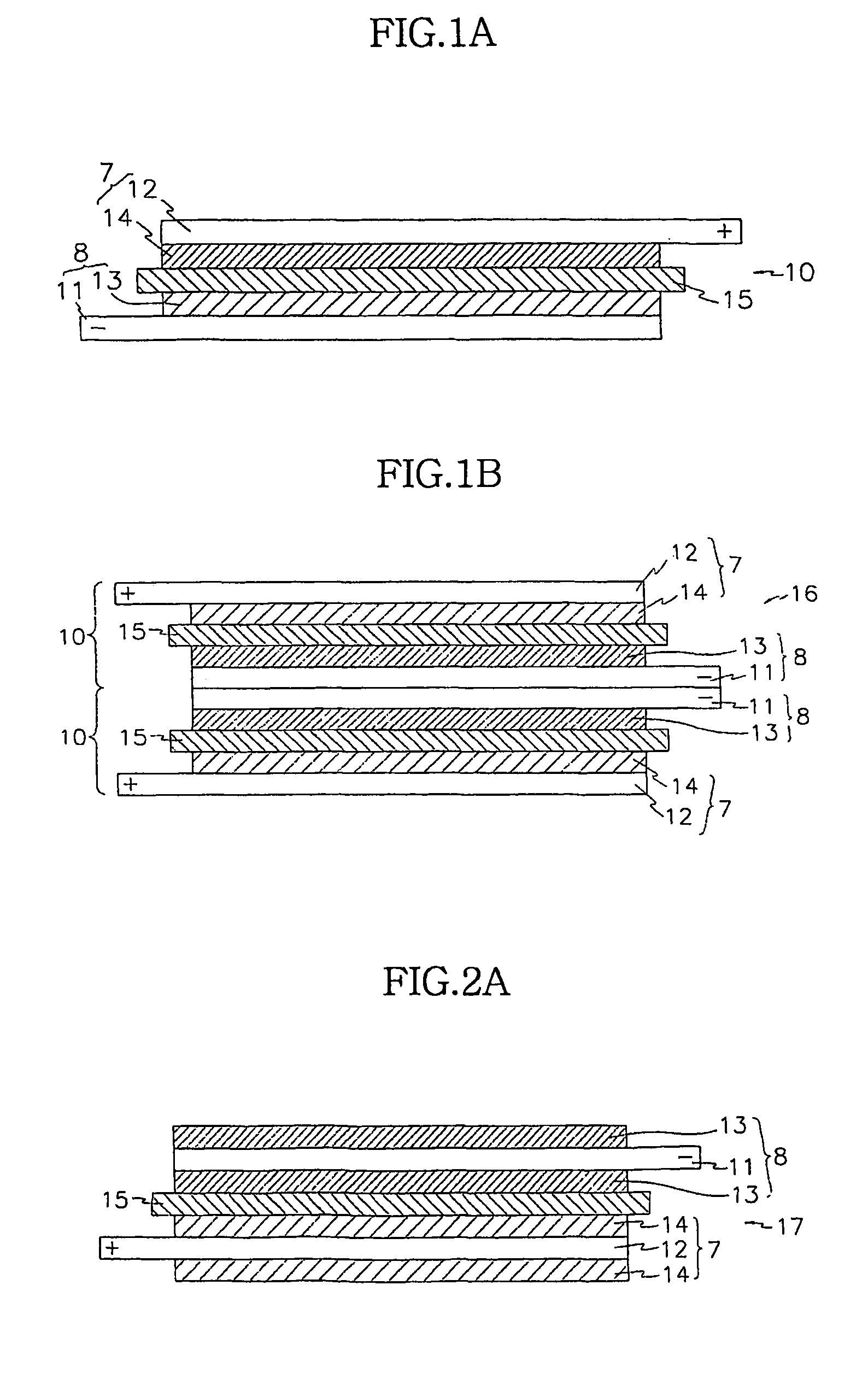
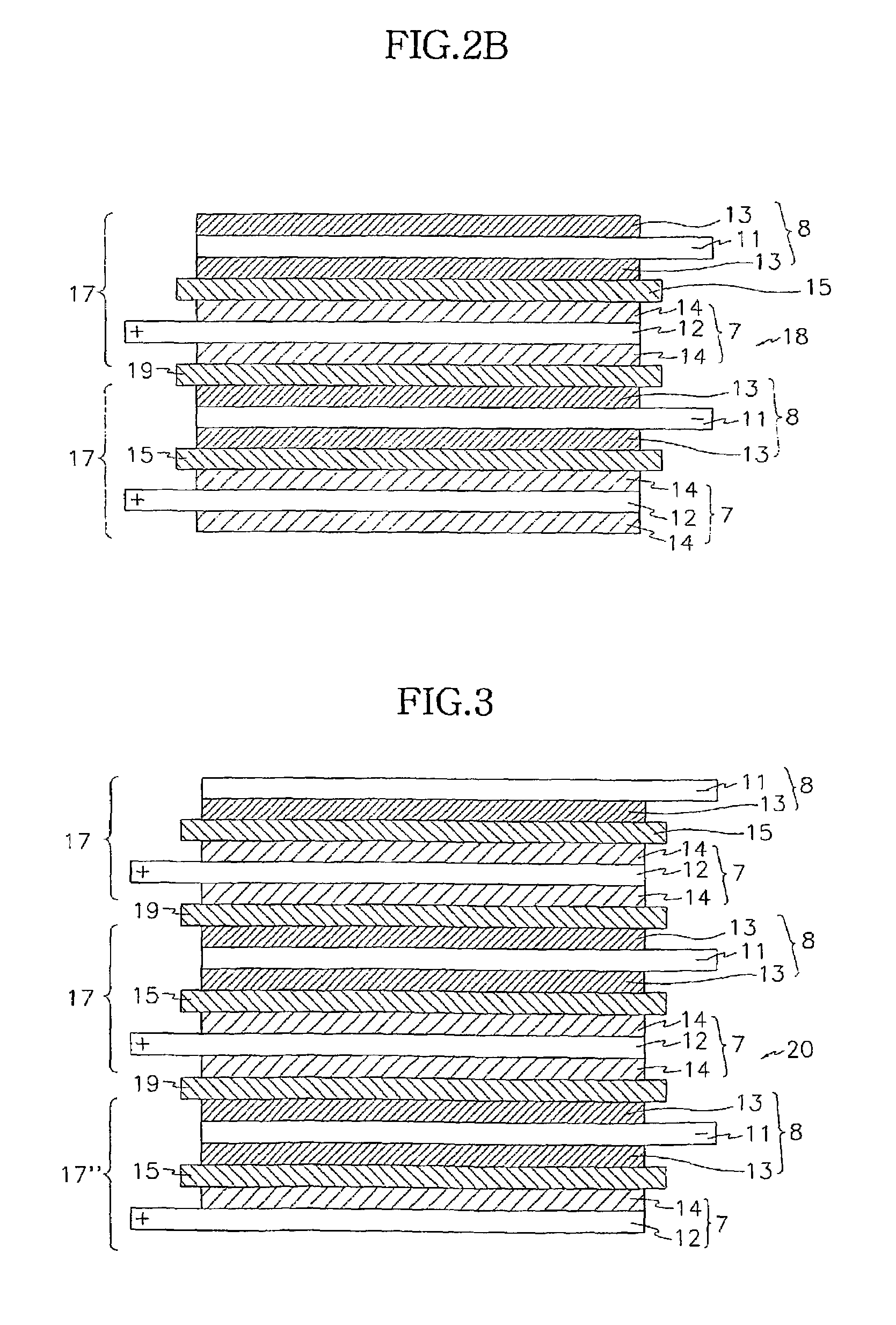
A composite solid electrolyte include a monolithic solid electrolyte base component that is a continuous matrix of an inorganic active metal ion conductor and a filler component used to eliminate through porosity in the solid electrolyte. In this way a solid electrolyte produced by any process that yields residual through porosity can be modified by the incorporation of a filler to form a substantially impervious composite solid electrolyte and eliminate through porosity in the base component. Methods of making the composites is also disclosed. The composites are generally useful in electrochemical cell structures such as battery cells and in particular protected active metal anodes, particularly lithium anodes, that are protected with a protective membrane architecture incorporating the composite solid electrolyte. The protective architecture prevents the active metal of the anode from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air and organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
Composite solid polymer electrolyte membranes
InactiveUS7550216B2Improve performanceLow costElectrolyte holding meansMembranesPolymer electrolytesFuel cells
Owner:FOSTER-MILLER
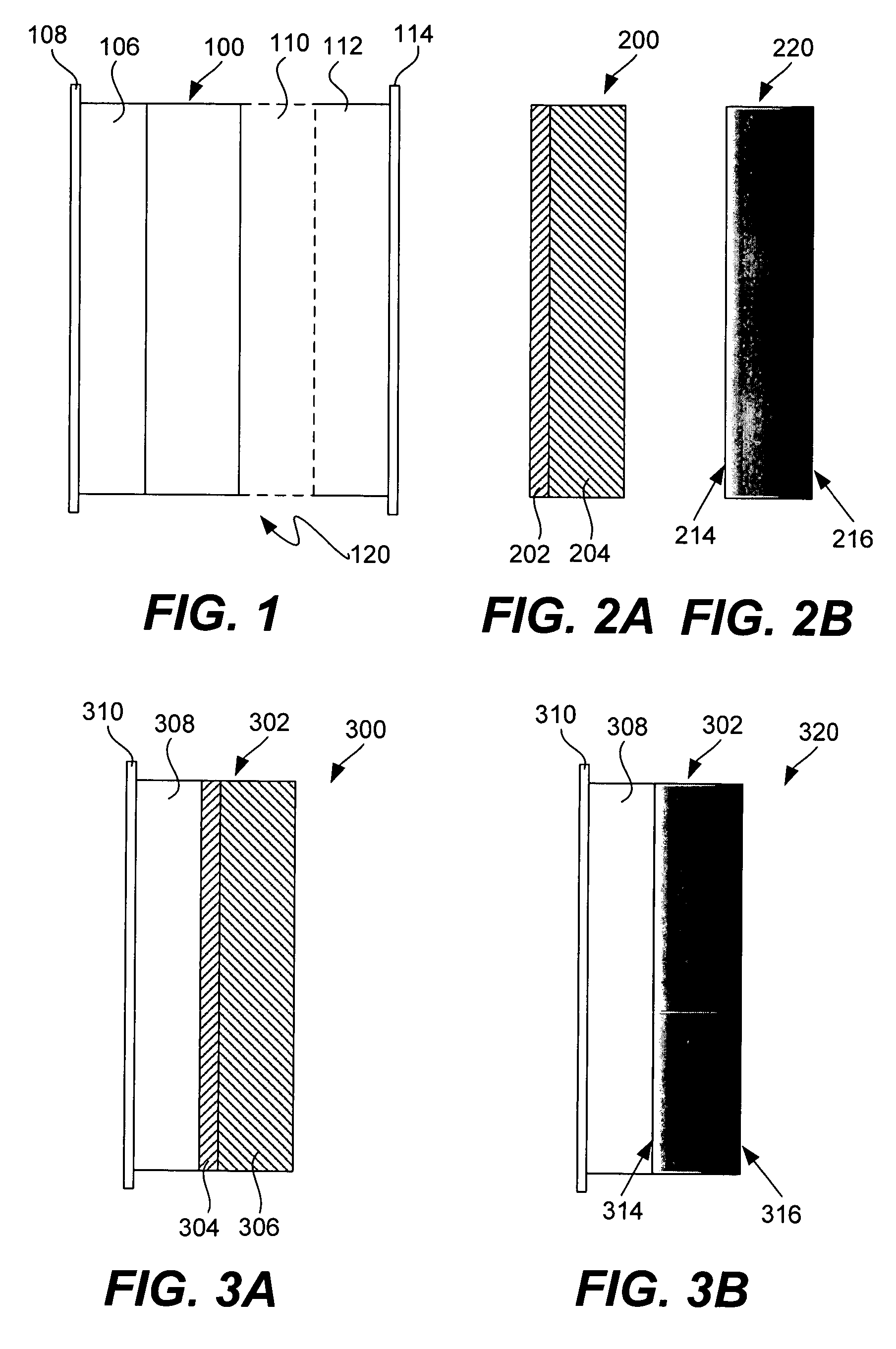
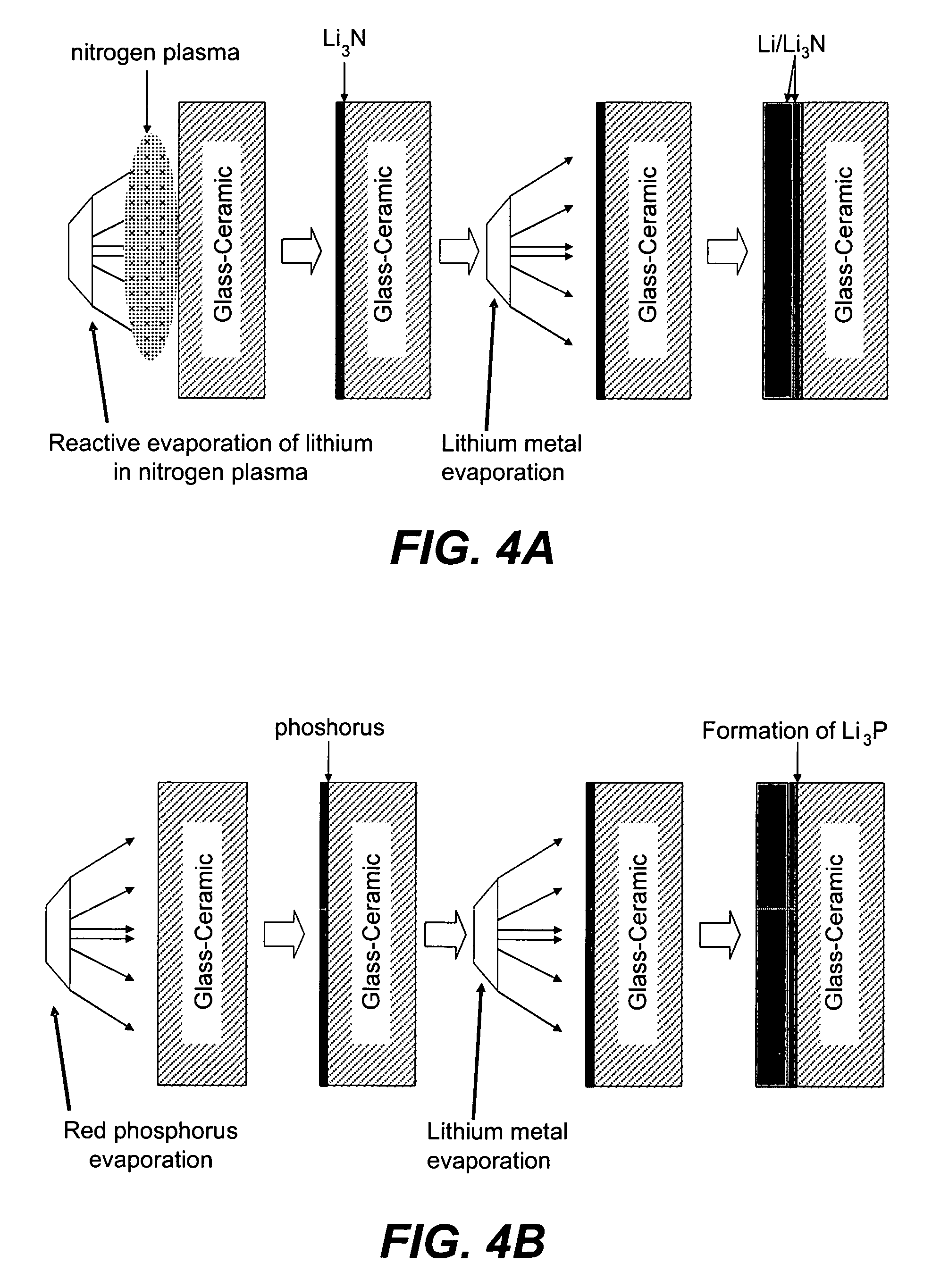
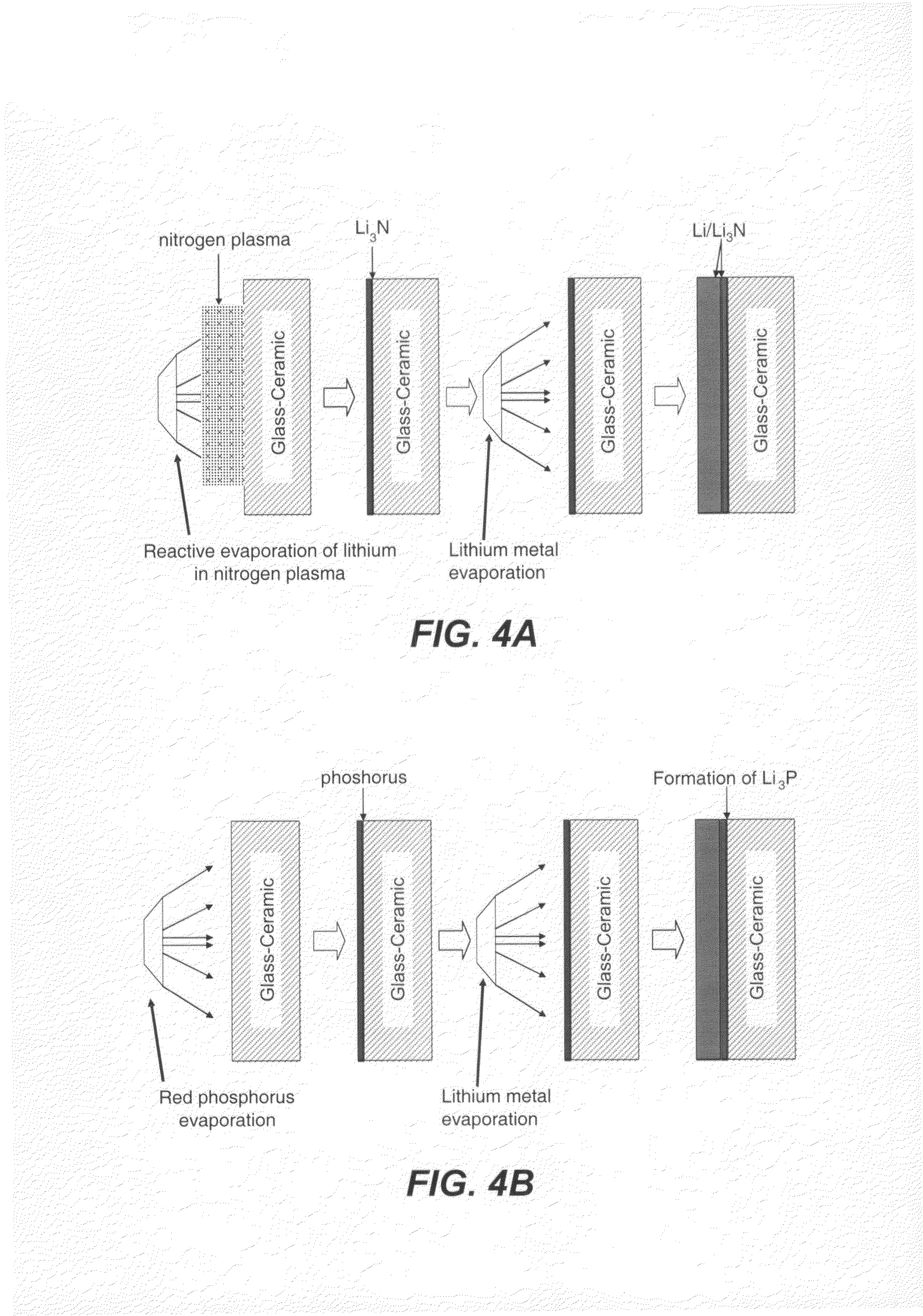
Ionically conductive membranes for protection of active metal anodes and battery cells
InactiveUS20040191617A1Improve propertiesImprove ionic conductivitySolid electrolytesSolid electrolyte cellsChemical stabilityBattery cell
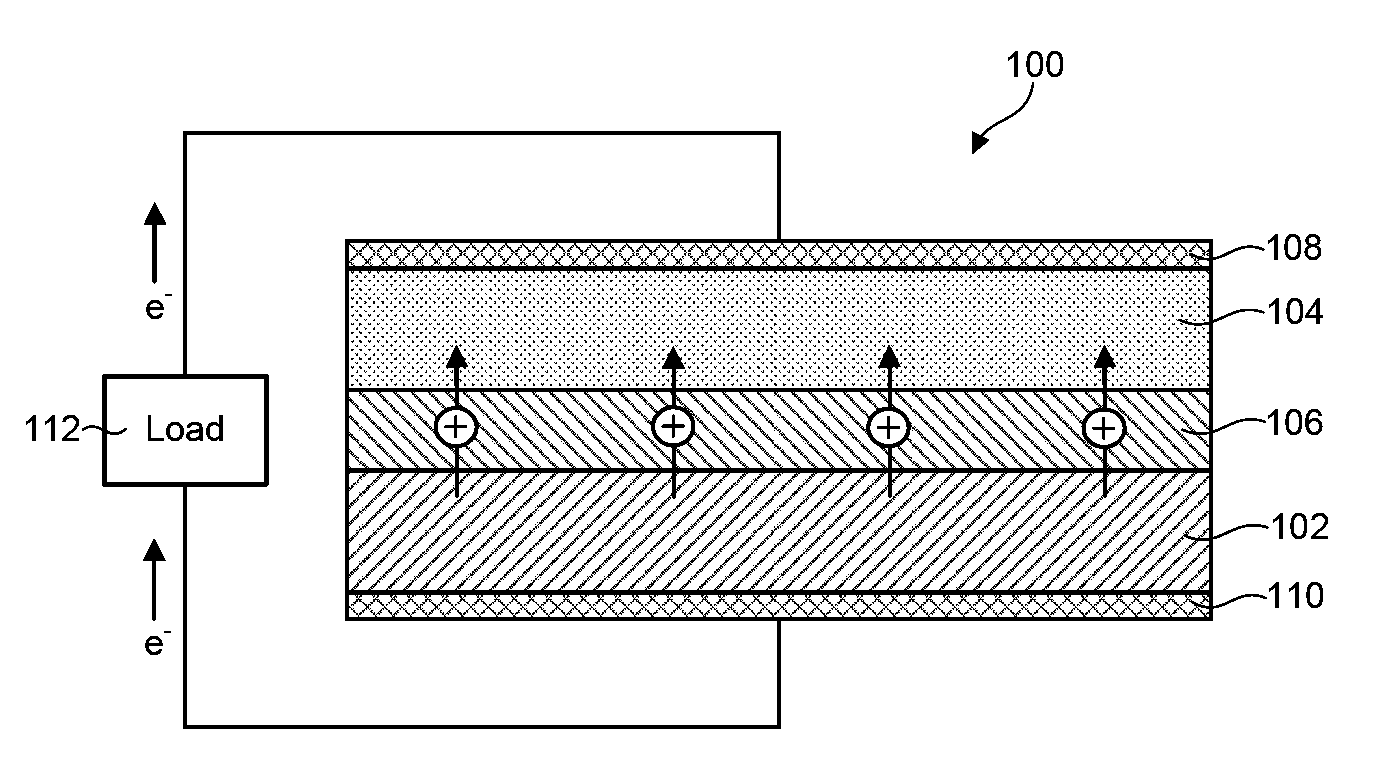
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
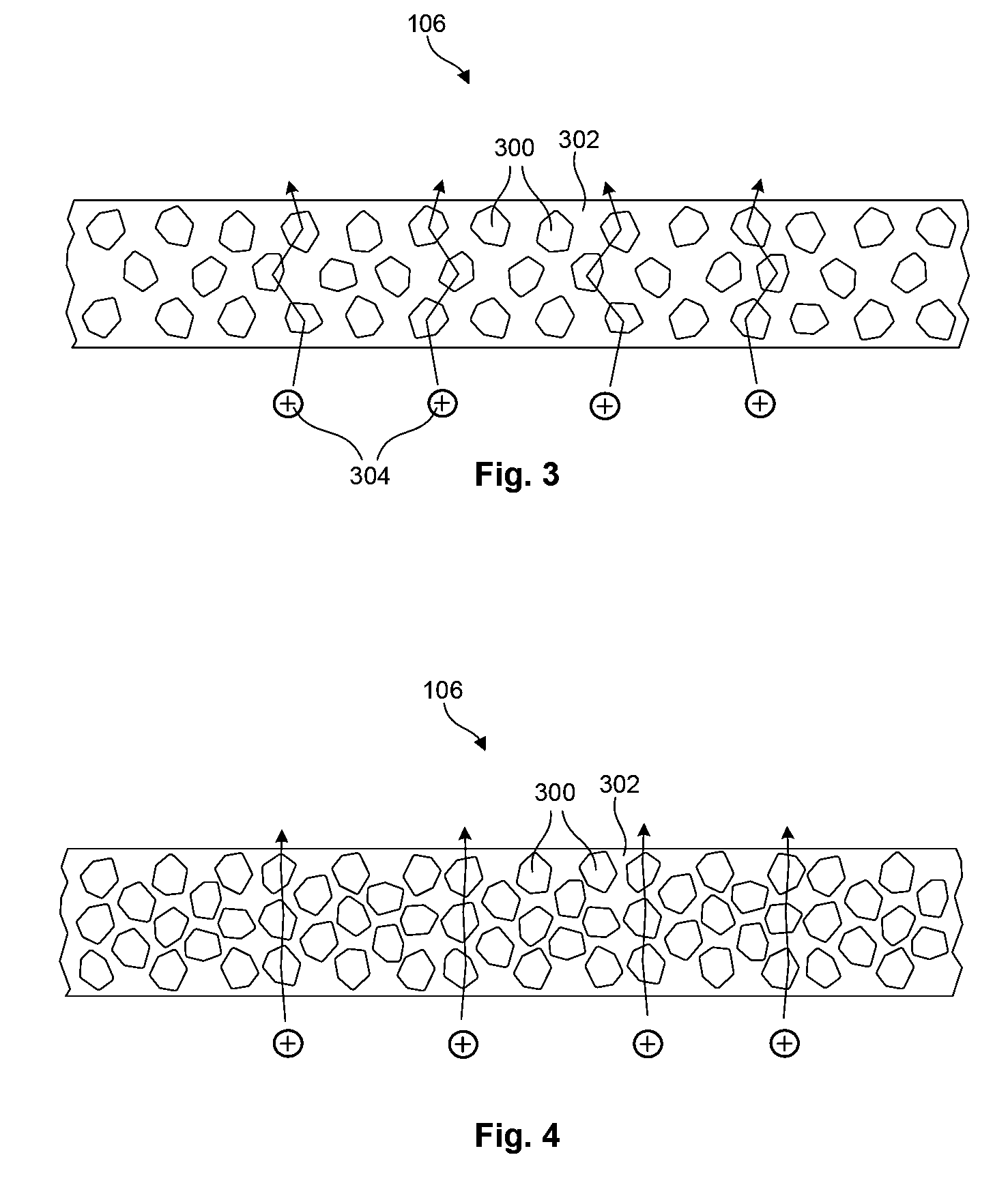
Ionically conductive composites for protection of active metal anodes
ActiveUS7282296B2Easy to manufactureImprove battery performanceFinal product manufactureElectrode carriers/collectorsChemical stabilityIonic conductivity
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
High-amperage energy storage device and method
ActiveUS20080044725A1Power capacity is large and scalableImprove ionic conductivityPrimary cell to battery groupingCell electrodesElectrical energy storageElectric energy
An electrochemical method and apparatus for high-amperage electrical energy storage features a high-temperature, all-liquid chemistry. The reaction products created during charging remain part of the electrodes during storage for discharge on demand. In a simultaneous ambipolar electrodeposition cell, a reaction compound is electrolyzed to effect transfer from an external power source; the electrode elements are electrodissolved during discharge.
Owner:MASSACHUSETTS INST OF TECH
Material for electrolytic solutions and use thereof
InactiveUS20040002002A1Avoid corrosionImprove ionic conductivityHybrid capacitor electrolytesAlkaline accumulatorsOrganic linkingElectrical conductor
It is an object of the present invention to provide a material for electrolytic solutions suited for use as material in electrolytic solutions serving as ionic conductors in electrochemical devices, such as large-capacity cells or batteries. The present invention is related to a material for electrolytic solutions which comprises, as an essential constituent, an anion represented by the general formula (1): wherein X represents at least one element selected from among B, N, O, Al, Si, P, S, As and Se; M<1 >and M<2 >are the same or different and each represents an organic linking group; a is an integer of not less than 1, and b, c and d each independently is an integer of not less than 0.
Owner:NIPPON SHOKUBAI CO LTD
Ionically conductive membranes for protection of active metal anodes and battery cells
InactiveUS7390591B2Improve conductivityEasy to manufactureSolid electrolytesSolid electrolyte cellsChemical stabilityBattery cell
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
High performance ultracapacitors with carbon nanomaterials and ionic liquids
InactiveUS20080192407A1Excellent electrolyte accessibilityImprove performanceHybrid capacitor electrolytesElectrolytic capacitorsSupercapacitorCarbon nanomaterials
The present invention is directed to the use of carbon nanotubes and / or electrolyte structures comprising ionic liquids in various electrochemical devices, such as ultracapacitors.
Owner:ADA TECH
Film for a separator of electrochemical apparatus, and production method and use thereof
InactiveUS6096456AHigh film strengthEasily and uniformly processedElectrolytic capacitorsSolid electrolyte cellsElectrolytic agentCarbamate
This invention provides a film comprising a cross-linked polymer having an oxyalkylene group or a cross-linked polymer having an oxyalkylene group through a urethane bond, as a constituent component, a production method of the film, and an electrochemical apparatus using the film as a separator. The film for separator of an electrochemical apparatus can be easily and uniformly processed, can include an electrolytic solution, exhibits good film thickness and ensures excellent safety and reliability. The electrochemical apparatus is free of leakage of the solution.
Owner:SHOWA DENKO KK
Composite solid electrolyte for protection of active metal anodes
ActiveUS8182943B2Eliminate through-porosityImprove ionic conductivityCell electrodesPrimary cellsPorosityLithium
A composite solid electrolyte include a monolithic solid electrolyte base component that is a continuous matrix of an inorganic active metal ion conductor and a filler component used to eliminate through porosity in the solid electrolyte. In this way a solid electrolyte produced by any process that yields residual through porosity can be modified by the incorporation of a filler to form a substantially impervious composite solid electrolyte and eliminate through porosity in the base component. Methods of making the composites is also disclosed. The composites are generally useful in electrochemical cell structures such as battery cells and in particular protected active metal anodes, particularly lithium anodes, that are protected with a protective membrane architecture incorporating the composite solid electrolyte. The protective architecture prevents the active metal of the anode from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air and organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
Electrochemical device using multicomponent composite membrane film
InactiveUS7014948B2Easy to manufactureImprove adhesionMembranesSemi-permeable membranesPolymer scienceElectrochemistry
The present invention provides a electrochemical element, wherein a multi-component composite film comprising a) polymer support layer film and b) a porous gellable polymer layer which is formed on either or both sides of the support layer film of a), wherein the support layer film of a) and the gellable polymer layer of b) are unified with each other without an interface between them.
Owner:TORAY BATTERY SEPARATOR FILM +1
Organic-inorganic all-solid-state composite electrolyte as well as preparation and application methods thereof
PendingCN106785009AIncrease transport activityImprove wettabilitySolid electrolytesSecondary cellsElectrical conductorComposite electrolyte
The invention relates to organic-inorganic all-solid-state composite electrolyte as well as preparation and application methods thereof, belonging to the field of lithium ion batteries. According to the organic-inorganic all-solid-state composite electrolyte, a highly-ordered three-dimensional connection network skeleton is formed by an inorganic fast lithium ion conductor, and a three-dimensional connection network is filled with a polymer and lithium salt. The organic-inorganic all-solid-state composite electrolyte which is flexible and has a controllable three-dimensional connection network structure is prepared. The electrolyte is high in lithium ion conductivity, wide in electrochemical window, good in mechanical property and stable to lithium metal. A lithium ion secondary battery assembled by a composite electrolyte membrane prepared by the method is high in capacity, stable in cycle performance, low in interface impedance and good in interface stability.
Owner:UNIV OF SCI & TECH BEIJING
Substantially Solid, Flexible Electrolyte For Alkili-Metal-Ion Batteries
ActiveUS20090136830A1Increase ionic conductivityImprove ionic conductivitySolid electrolytesFinal product manufactureIonCeramic particle
An alkali-metal-ion battery is disclosed in one embodiment of the invention as including an anode containing an alkali metal, a cathode, and an electrolyte separator for conducting alkali metal ions between the anode and the cathode. In selected embodiments, the electrolyte separator includes a first phase comprising poly(alkylene oxide) and an alkali-metal salt in a molar ratio of less than 10:1. The electrolyte separator may further include a second phase comprising ionically conductive particles that are conductive to the alkali metal ions. These ionically conductive particles may include ionically conductive ceramic particles, glass particles, glass-ceramic particles, or mixtures thereof.
Owner:FIELD UPGRADING USA INC
Composite solid electrolyte membrane, preparation method and lithium-ion battery
ActiveCN106654362AGuaranteed membrane flexibilityHigh mechanical strengthSecondary cellsChemistryMembrane configuration
The invention provides a composite solid electrolyte membrane, a preparation method and a lithium-ion battery. The composite solid electrolyte membrane comprises a porous support material, first compound adhesive layers coating two side surfaces of the porous support material, and second compound adhesive layers coating the first compound adhesive layers, wherein the inorganic proportion of the second compound adhesive layers is smaller than that of the first compound adhesive layers. The porous support material is added to the electrolyte membrane, so that the flexibility of the membrane is ensured, the mechanical strength of the membrane is strengthened, the interfacial compatibility can be improved by the electrolyte membrane of a sandwich structure and the cycle performance of the battery is improved.
Owner:重庆冠宇电池有限公司
Nonaqueous electrolyte material of fluorosulfonylimide lithium and application thereof
ActiveCN101882696AImprove solubilityNot easy to crystallizeOrganic chemistryCapacitor electrolytes/absorbentsDielectricPhosphate
The invention provides a nonaqueous electrolyte material, which consists of fluorosulfonylimide lithium and organic solvent with dielectric constant less than 30, and the organic solvent is selected from one or more of chain-like carbonate solvent, phosphate solvent, siloxane solvent, boroxane solvent, acetate solvent, propionate solvent, butyrate solvent, CF3OCH2CH2OCF3 solvent, C2H5OCH2CH2OCH3 solvent, C2F5OCH2CH2OCF3 solvent, 1,3-dioxolane solvent and aliphatic nitrile solvent with more than two carbon atoms. The ionic conductivity of the nonaqueous electrolyte material is 0.01mS / cm to 18mS / cm, the lithium ion transference number is tLi plus equal to 0.2 to 0.8, and the applicable temperature range is 80DEG C below zero to 60DEG C below zero. The invention also provides an application of the nonaqueous electrolyte material in the preparation of lithium batteries and super capacitors. Furthermore, the invention provides a lithium battery and a super capacitor which contain the nonaqueous electrolyte material of fluorosulfonylimide lithium.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Polymer electrolyte membrane fuel cells
InactiveUS6946211B1Improve performanceImprove toleranceSolid electrolytesPrimary cellsThermoplasticGas diffusion electrode
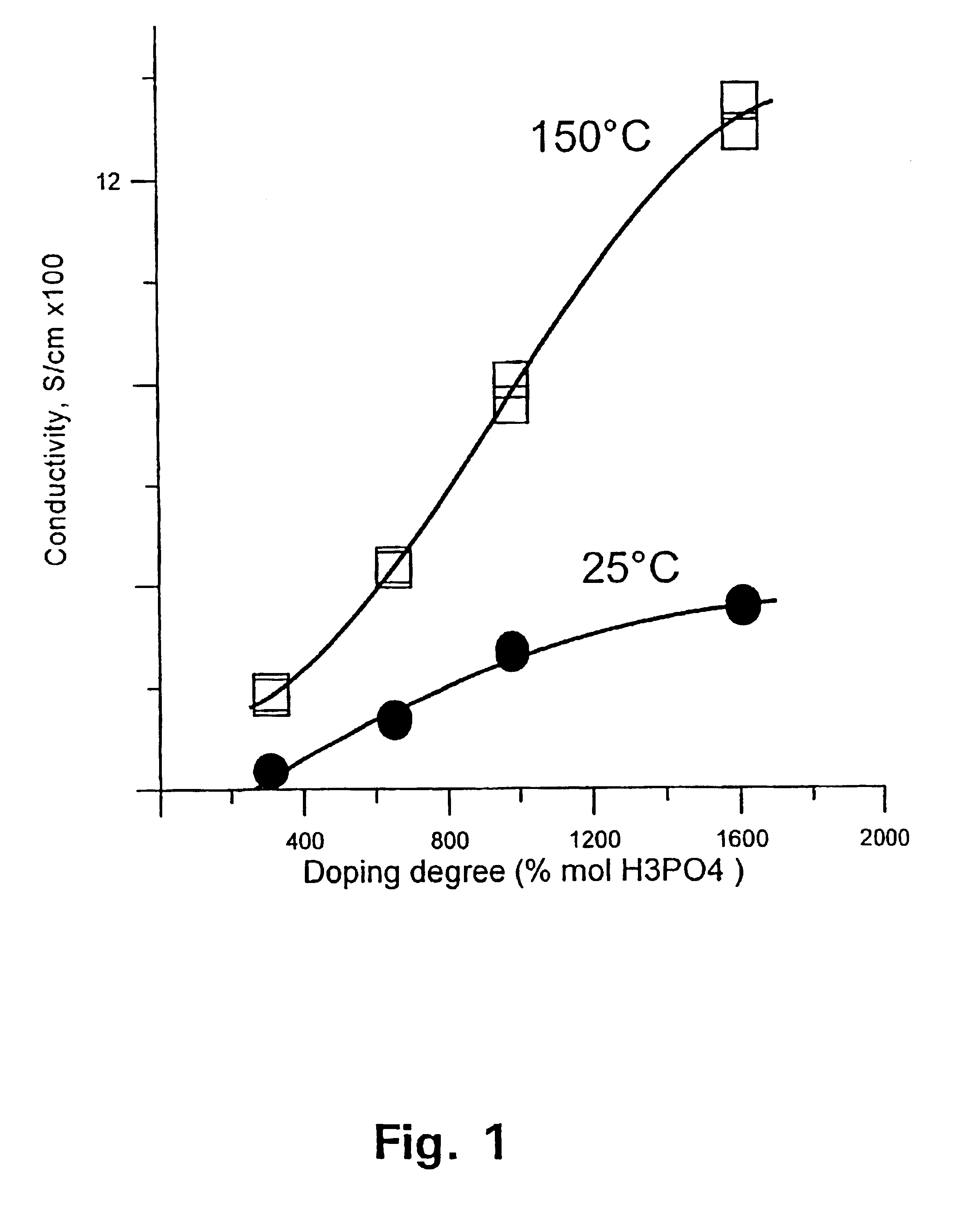
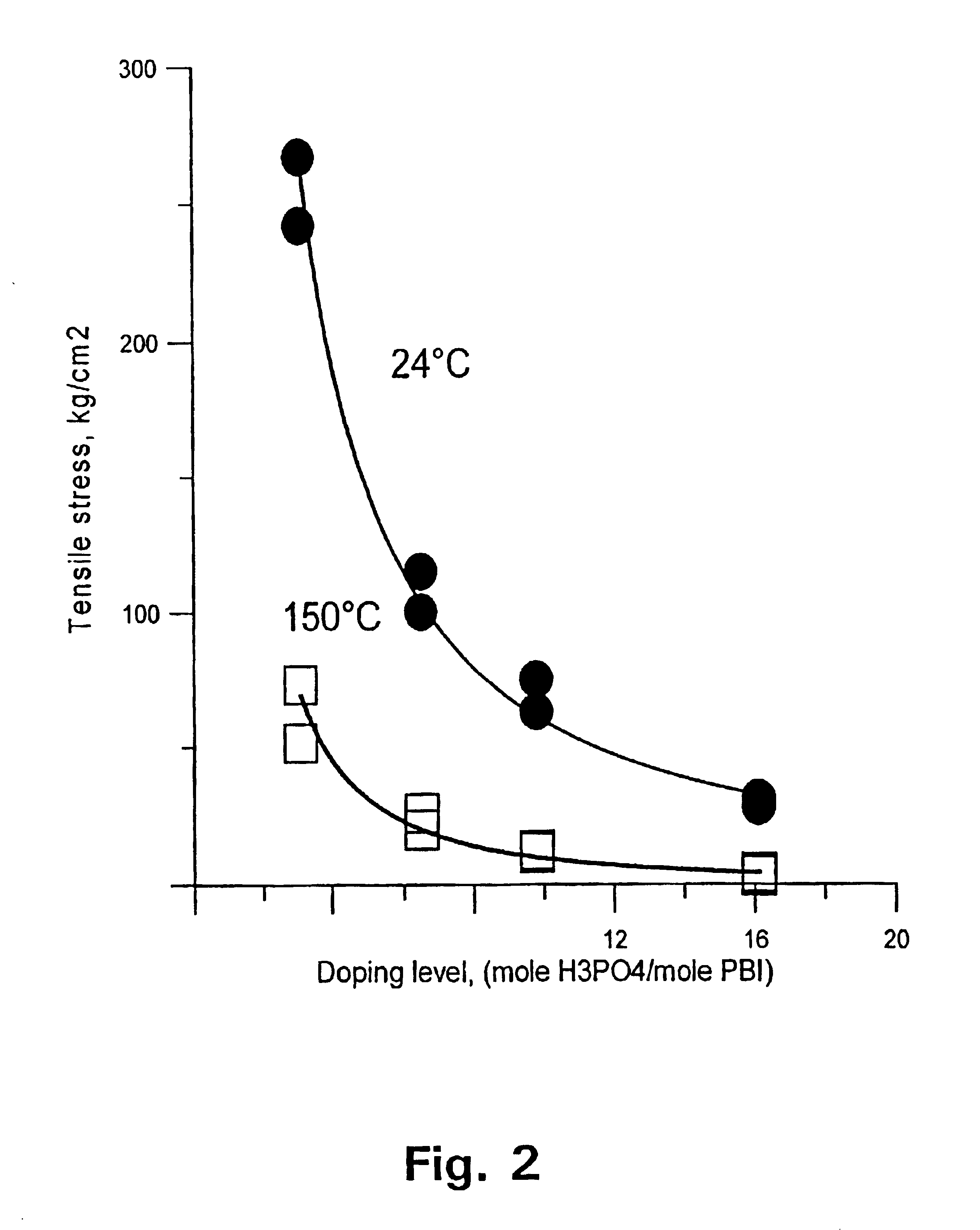
A method for preparing polybenzimidazole or polybenzimidazole blend membranes and fabricating gas diffusion electrodes and membrane-electrode assemblies is provided for a high temperature polymer electrolyte membrane fuel cell. Blend polymer electrolyte membranes based on PBI and various thermoplastc polymers for high temperature polymer electrolyte fuel cells have also been developed. Miscible blends are used for solution casting of polymer membranes (solid electrolytes). High conductivity and enhanced mechanical strength were obtained for the blend polymer solid electrolytes. With the thermally resistant polymer, e.g., polybenzimidazole or a mixture of polybenzimidazole and other thermoplastics as binder, the carbon-supported noble metal catalyst is tape-cast onto a hydrophobic supporting substrate. When doped with an acid mixture, electrodes are assembled with an acid doped solid electrolyte membrane by hot-press. The fuel cell can operate at temperatures up to at least 200° C. with hydrogen-rich fuel containing high ratios of carbon monoxide such as 3 vol % carbon monoxide or more, compared to the carbon monoxide tolerance of 10-20 ppm level for Nafion®-based polymer electrolyte fuel cells.
Owner:DANISH POWER SYST
Ionically conductive membranes for protection of active metal anodes and battery cells
InactiveUS20080057386A1Improve conductivityFacilitates cell fabricationSolid electrolytesCell seperators/membranes/diaphragms/spacersChemical stabilityIonic conductivity
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
Electrochemical device component with protected alkali metal electrode
InactiveUS7858223B2Easy to manufactureImprove battery performanceSolid electrolytesCell seperators/membranes/diaphragms/spacersElectrochemistryChemical stability
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Polymer gel electrolyte, secondary cell, and electrical double-layer capacitor
InactiveUS20020102464A1Large capacityWide operating temperature rangeNon-metal conductorsHybrid capacitor electrolytesPolymer capacitorCapacitance
A polymer gel electrolyte includes an electrolyte solution composed of a plasticizer with at least two carbonate structures on the molecule and an electrolyte salt, in combination with a matrix polymer. Secondary batteries made with the polymer gel electrolyte can operate at a high capacitance and a high current, have a broad service temperature range and a high level of safety, and are thus particularly well-suited for use in such applications as lithium secondary cells and lithium ion secondary cells. Electrical double-layer capacitors made with the polymer gel electrolyte have a high output voltage, a large output current, a broad service temperature range and excellent safety.
Owner:NISSHINBO IND INC
Ionically conductive composites for protection of active metal anodes
InactiveUS20080057399A1Easy to manufactureImprove battery performanceFinal product manufactureCell electrodesChemical stabilityIonic conductivity
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Ceramic and polymer composite coated lithium ion diaphragm and preparation method thereof
ActiveCN107275550AImprove ionic conductivityHigh bonding strengthCell component detailsSecondary cells servicing/maintenanceLithiumPolyolefin
The invention discloses a ceramic and polymer composite coated lithium ion diaphragm and a preparation method thereof. The ceramic and polymer composite coated lithium ion diaphragm comprises a polyolefin porous diaphragm, a ceramic coating coated on one side or the two sides of the diaphragm surface and a polymer coating coated on the ceramic surface or the diaphragm surface. The composite diaphragm prepared by the preparation method disclosed by the invention has the advantages that the heat resistance of the diaphragm is greatly improved, and the infiltration of an electrolyte is improved; the bonding strength of the diaphragm and a positive and negative pole piece is improved, so that the internal short-circuit caused by staggering between the diaphragm and an electrode is prevented; meanwhile, the hardness of a battery is improved, so that the safety performance of the battery is greatly improved. In the production process, the ceramic coating and the polymer coating are uniform in thickness and good in uniformity and facilitate continuous and large-scale production.
Owner:SHENZHEN SENIOR TECH MATERIAL
Heat resisting separator having ultrafine fibrous layer and secondary battery having the same
InactiveUS20100304205A1High suppression characteristicsImprove ionic conductivityHybrid capacitor separatorsProtecting/adjusting hybrid/EDL capacitorPolyolefinFuel cells
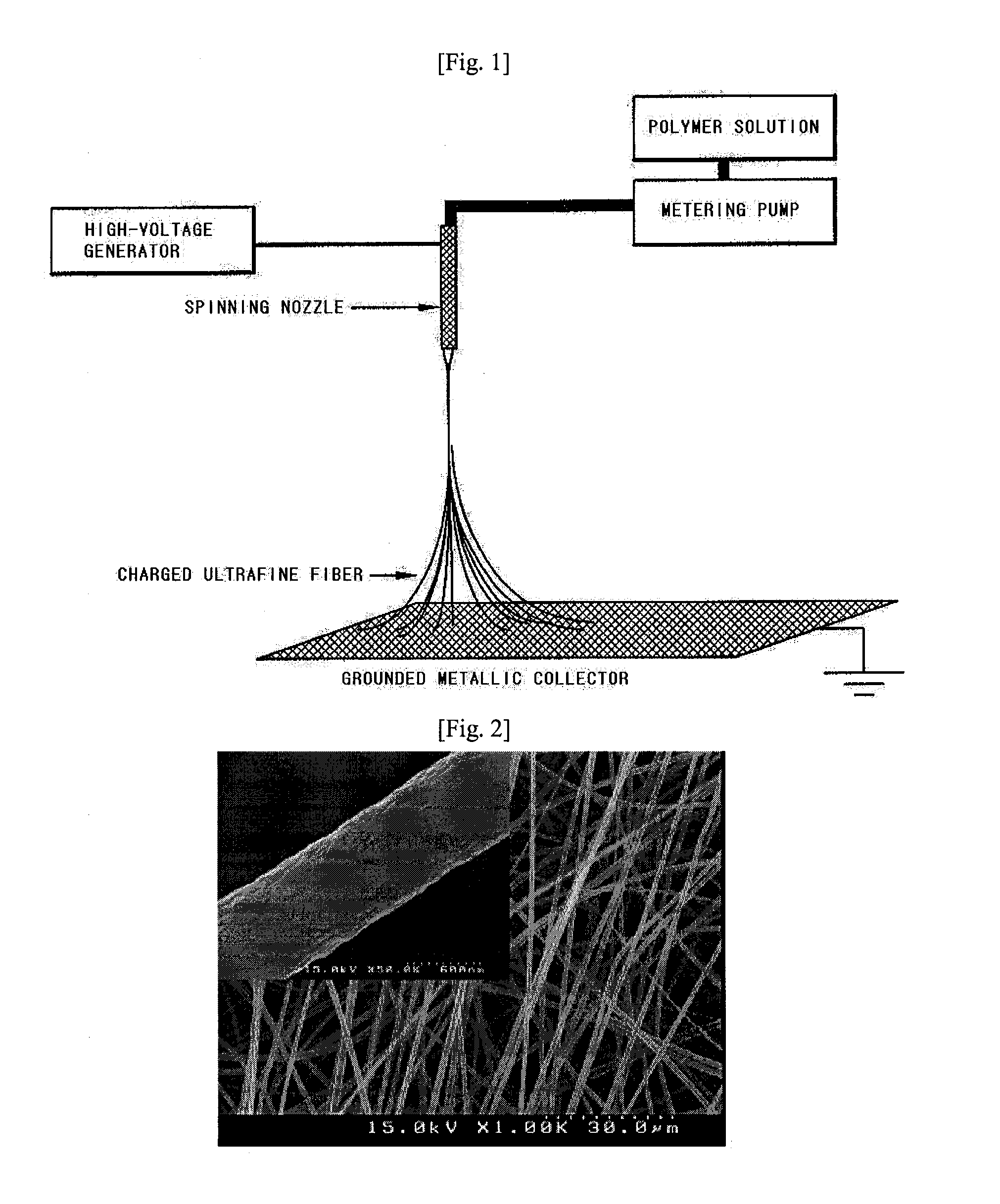
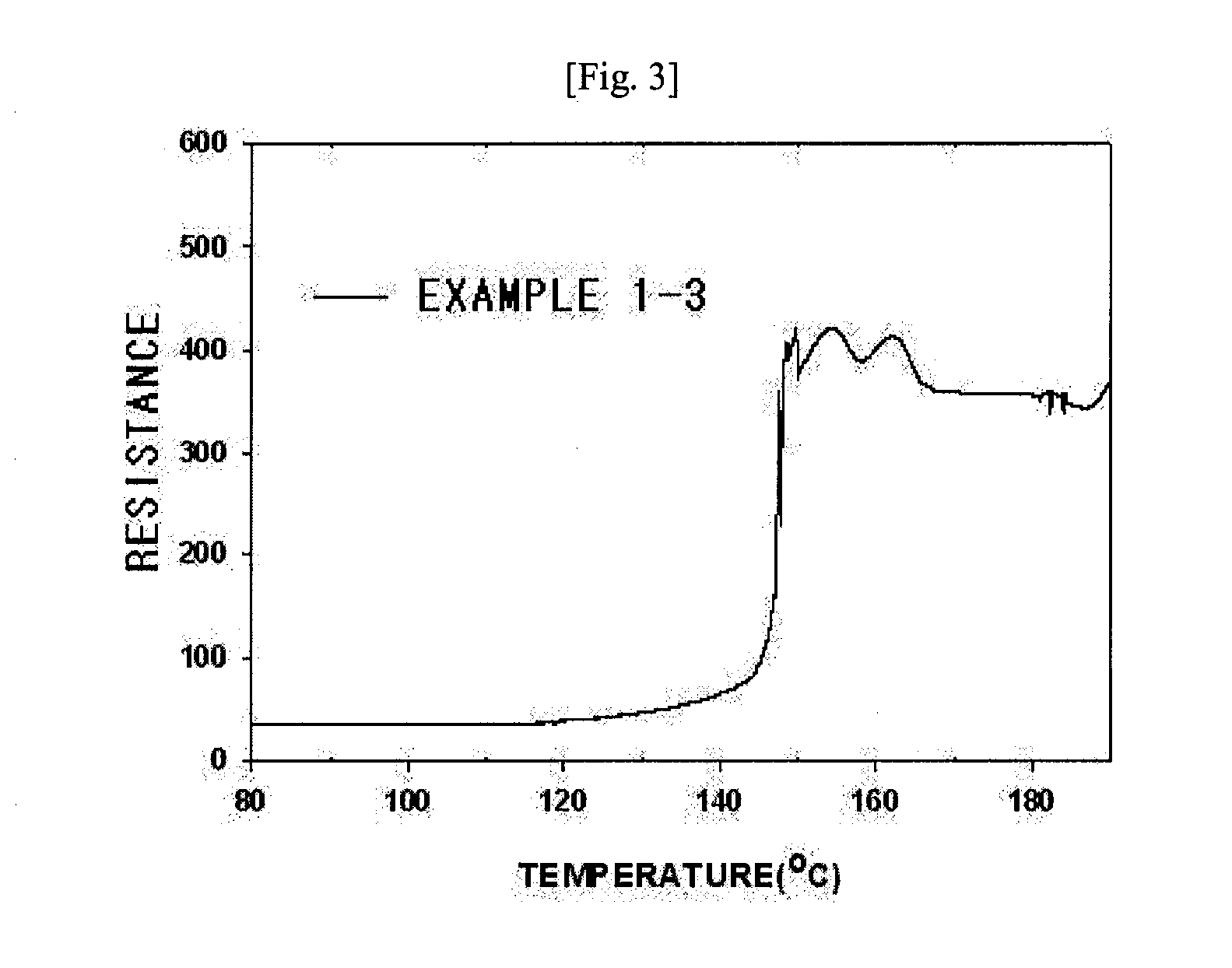
A polyolefin separator having an heat-resistant ultrafine fibrous layer and a secondary battery using the same, in which the separator has a shutdown function, low thermal contraction characteristics, thermal endurance, excellent ionic conductivity, excellent cycling characteristics at the time of battery construction, and excellent adhesion with an electrode. The present N invention adopts a very simple and easy process to form an ultrafine fibrous layer through an electrospinning process, and at the same time, to remove solvent and to form pores. Accordingly, the separator of the present invention is useful particularly for electrochemical devices used in a hybrid electric automobile, an electric automobile, and a fuel cell automobile, requiring high thermal endurance and thermal stability.
Owner:KOREA INST OF SCI & TECH
Polymer Solid Electrolyte
InactiveUS6162563AEasy to processGood moldabilityHybrid capacitor electrolytesCell electrodesLithium metalBackbone chain
PCT No. PCT / JP97 / 02854 Sec. 371 Date Mar. 11, 1999 Sec. 102(e) Date Mar. 11, 1999 PCT Filed Aug. 19, 1997 PCT Pub. No. WO98 / 07772 PCT Pub. Date Feb. 26, 1998A polymer solid electrolyte obtained by blending (1) a polyether copolymer having a main chain derived form ethylene oxide and an oligooxyethylene side chain, (2) an electrolyte salt compound, and (3) a plasticizer of an aprotic organic solvent or a derivative or metal salt of a polyalkylene glycol having a number-average molecular weight of 200 to 5,000 or a metal salt of the derivative is superior in ionic conductivity and also superior in processability, moldability and mechanical strength to a conventional solid electrolyte. A secondary battery is constructed by using the polymer solid electrolyte in combination with a lithium metal negative electrode and a lithium cobaltate positive electrode.
Owner:OSAKA SODA CO LTD
Lithium ion battery and anode strip thereof and stabilization lithium metal powder
ActiveCN102642024AImprove lithiation efficiencyImprove electronic conductivitySecondary cellsNon-aqueous electrolyte accumulator electrodesElectrical conductorElectrical battery
The invention belongs to the technical field of a lithium ion battery, and particularly relates to stabilization lithium metal powder. The stabilization lithium metal powder has a core shell structure; and the core layer is formed by lithium metal and is a composition consisting of an electron good conductor and a lithium ion good conductor. Compared with the prior art, the stabilization lithium metal powder provided by the invention has the advantages that: in the process of performing pre-lithiation of the anode-active material by use of the stabilization lithium metal powder, no limitation is imposed on the pressure of the cold pressing process, the 'dead lithium' disabling lithiation reaction is not produced, and the lithiation efficiency of the lithium metal powder is improved; and moreover, since the shell layer left on the electrode surface has good electron and lithium ion conductivity after the pre-lithiation, the electron and ion conductivity of the anode can be effectively improved so as to improve the electrochemical performance of the battery. In addition, the invention also discloses an anode strip performing pre-lithiation by use of the stabilization lithium metal powder, and a lithium ion battery comprising the anode strip.
Owner:NINGDE AMPEREX TECH +1
Membrane-electrode assembly, electrolytic cell employing the same, electrolytic-water sprayer, and method of sterilization
InactiveUS20090127128A1Improving bactericidal activityImproving bactericidal activity and feelingCellsWater treatment compoundsBiomedical engineeringElectrolytic cell
The present invention provides a membrane-electrode assembly which comprises: at least one rod-form or tubular electrode; a tubular diaphragm disposed around the periphery of the electrode; and a wire-form counter electrode disposed around the periphery of the diaphragm, the diaphragm being fixed to the rod-form or tubular electrode with the wire-form counter electrode to thereby form an electrode chamber having a gas / liquid passage between the diaphragm and the rod-form or tubular electrode.
Owner:DE NORA PERMELEC LTD +1
Cluster ion exchange membrane and electrolyte membrane electrode connection body
InactiveUS20050095486A1High mechanical strengthImprove ionic conductivityIon-exchange process apparatusSolid electrolytesIon-exchange membranesMembrane configuration
The present invention provides a composite ion exchange membrane which has high mechanical strength and is suitable for use as a solid polymer electrolyte membrane excellent in ionic conductivity and a method for its production. The invention is achieved by a composite ion exchange membrane including a composite layer comprising a support membrane with continuous voids formed of polybenzazole polymer, the support membrane being impregnated with ion exchange resin, and surface layers formed of ion exchange resin free of support membranes, the surface layers being formed on both surfaces of the composite layer so as to sandwich the composite layer therebetween.
Owner:TOYO TOYOBO CO LTD
Ambient temperature, rechargeable cells with metal salt-based electrodes and a system of cell component materials for use therein
InactiveUS6187479B1Improve battery performanceImprove performanceLead-acid accumulatorsNon-aqueous electrolyte cellsHalogenRechargeable cell
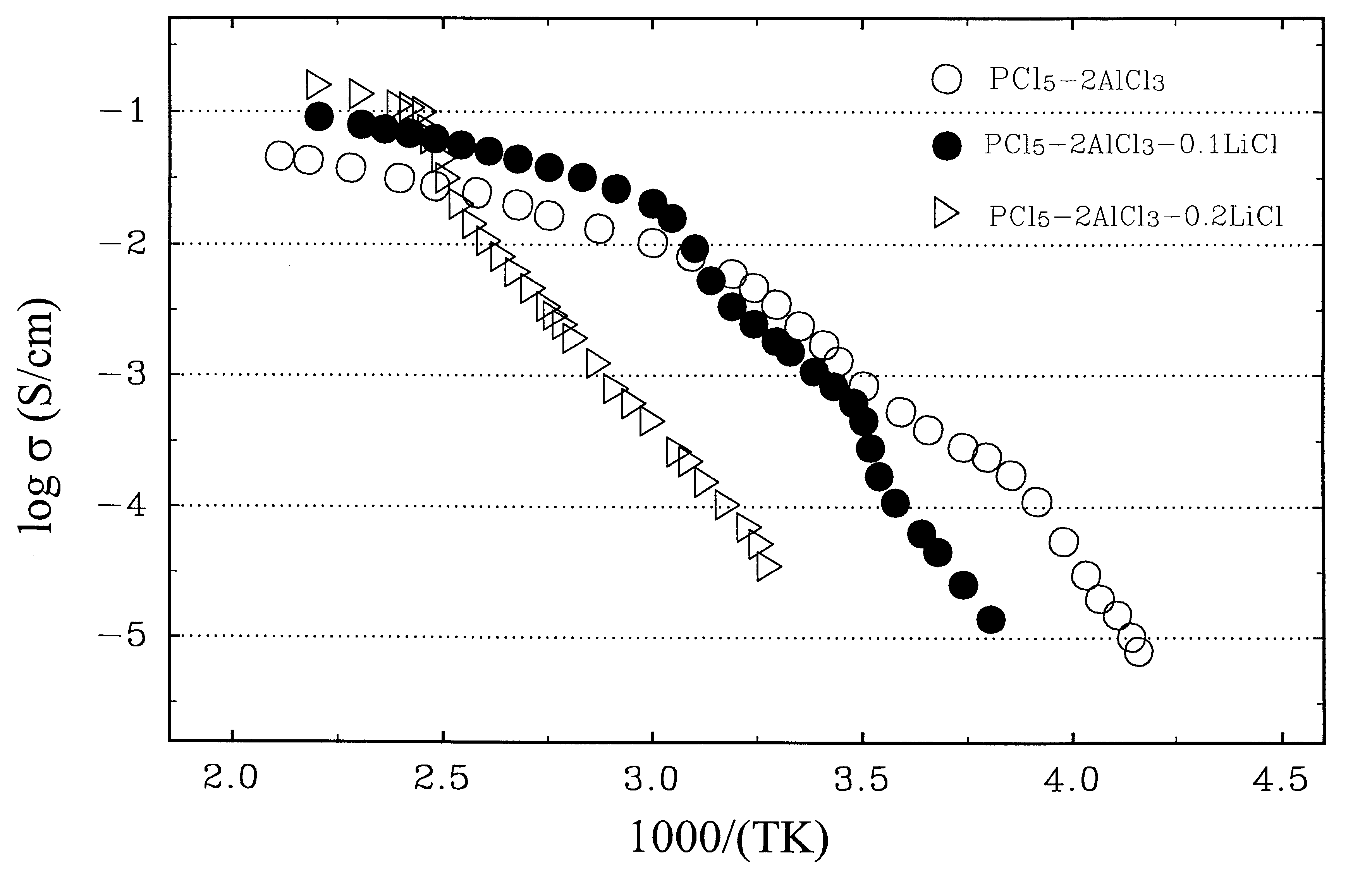
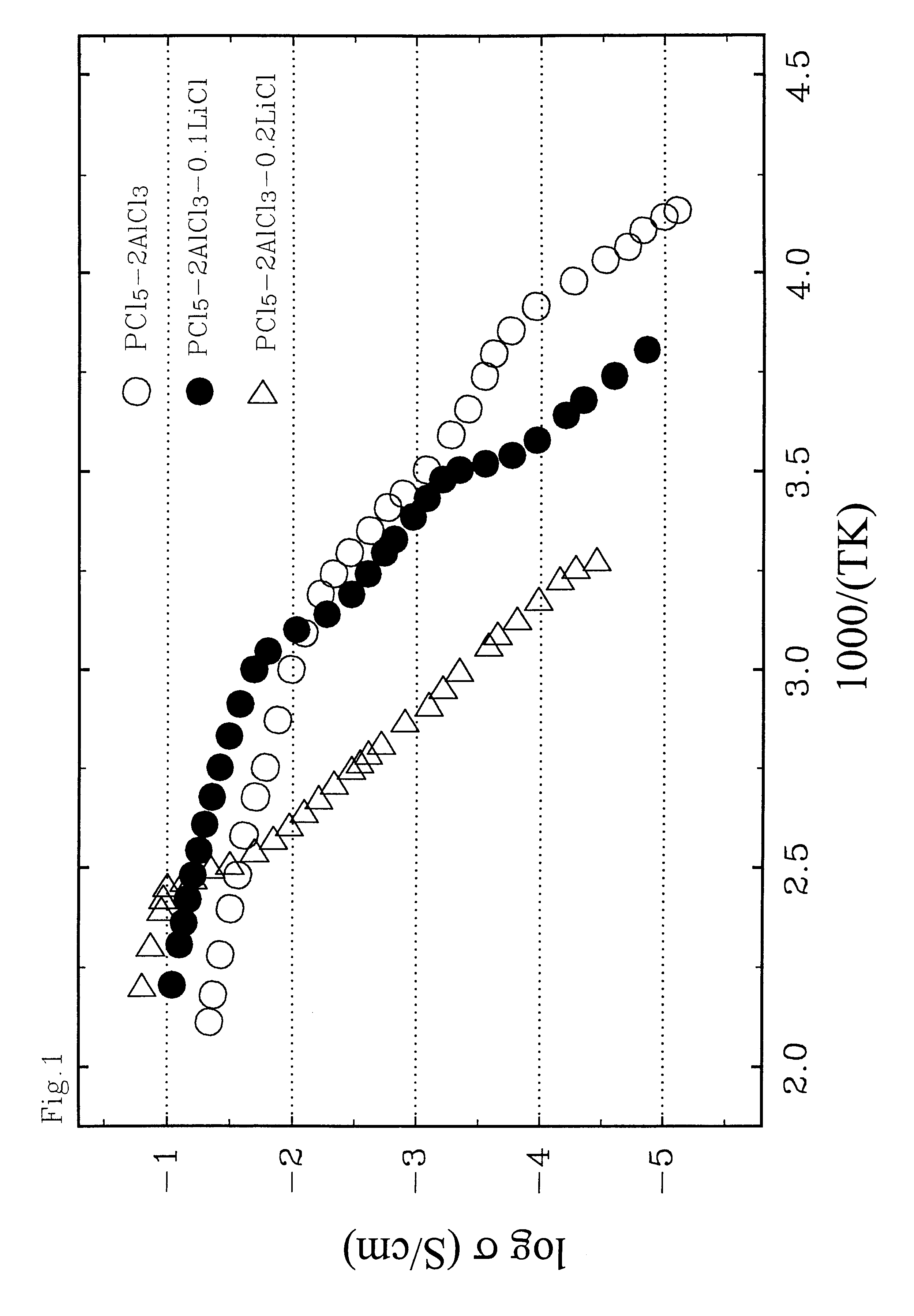
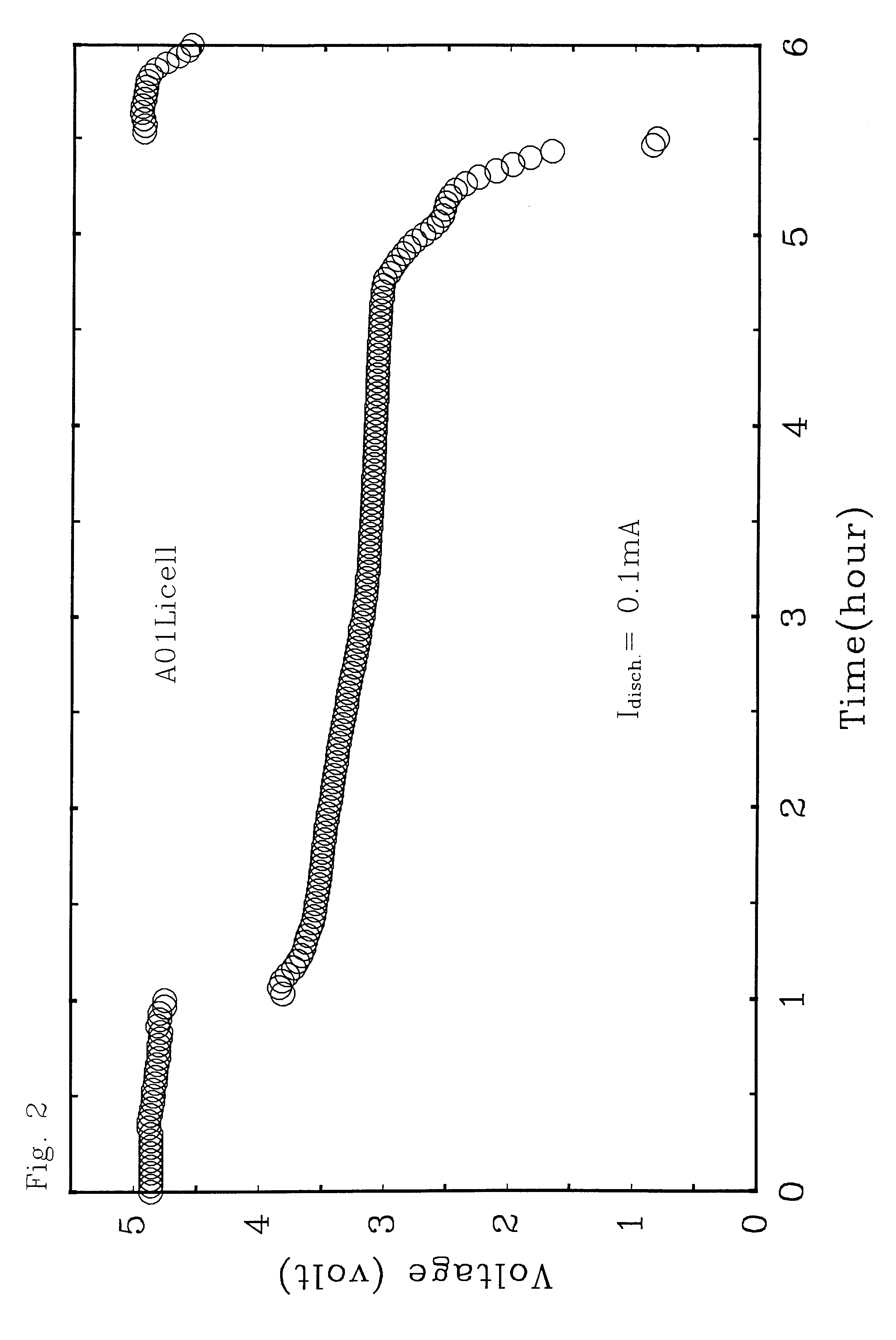
A rechargeable battery or cell is disclosed in which the electrode active material consists of at least one nonmetallic compound or salt of the electropositive species on which the cell is based, and the electrolyte or electrolyte solvent consists predominantly of a halogen-bearing or chalcogen-bearing covalent compound such as SOCl2 or SO2Cl2. Also disclosed are cell component materials which include electrodes that consist primarily of salts of the cell electropositive species and chemically compatible electrolytes. These latter electrolytes include several newly discovered ambient temperature molten salt systems based on the AlCl3-PCl5 binary and the AlCl3-PCl5-PCl3 ternaries.
Owner:LIU CHANGLE
Polycarbonate all-solid-state polymer electrolyte, all-solid-state secondary lithium battery made of same and preparation and application thereof
The invention relates to solid-state electrolytes, in particular to a polycarbonate all-solid-state polymer electrolyte, an all-solid-state secondary lithium battery made of the same and preparation and application thereof. The all-solid-state polymer electrolyte is prepared from polycarbonate macromolecules, lithium salt and a porous supporting material; the thickness is 20-800 micrometers, the mechanical strength is 10-80 MPa, the room temperature ion conductivity is 2*10<-5>S / cm-1*10<-3>S / cm, and the electrochemical window is higher than 4V. The all-solid-state polymer electrolyte is easy to prepare and form, good in mechanical property, high in ion conductivity and wide in electrochemical window; meanwhile, the solid-state electrolyte effectively inhibits growth of negative electrode lithium dendrites and improves interface stability and long circulation performance.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Lithium ion battery with electrolyte-embedded separator particles
InactiveUS20130052509A1Efficient use ofSuitable ionic conductivityFinal product manufacturePrimary cellsLithium-ion batteryViscosity
A lithium ion battery in which electrically-non conducting ceramic particles are interposed between the anode and cathode to enforce separation between them and prevent short circuits is described. The particles, preferably equiaxed or monodisperse, may be generally uniformly dispersed in a non-aqueous gelled or high viscosity electrolyte. The electrolyte may be applied to one or both of the anode and cathode in suitable thickness to deposit the particles with the electrolyte and form a layered composite with substantially uniformly spaced particles suitable for holding the opposing anode and cathode faces in spaced-apart relation. The thickness of the applied electrolyte layer will be selected to enable deposition of the particles substantially as a fractional monolayer, a monolayer, or a multilayer as required for the application.
Owner:GM GLOBAL TECH OPERATIONS LLC
Electrolyte for lithium ion secondary battery and lithium ion secondary battery comprising the same
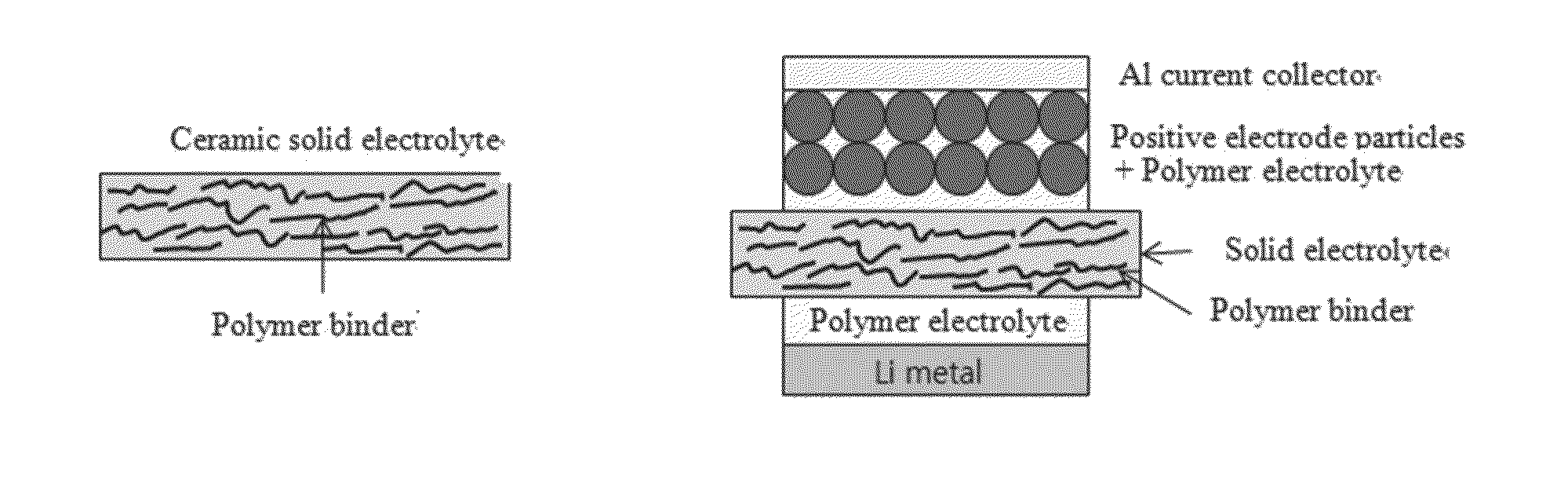
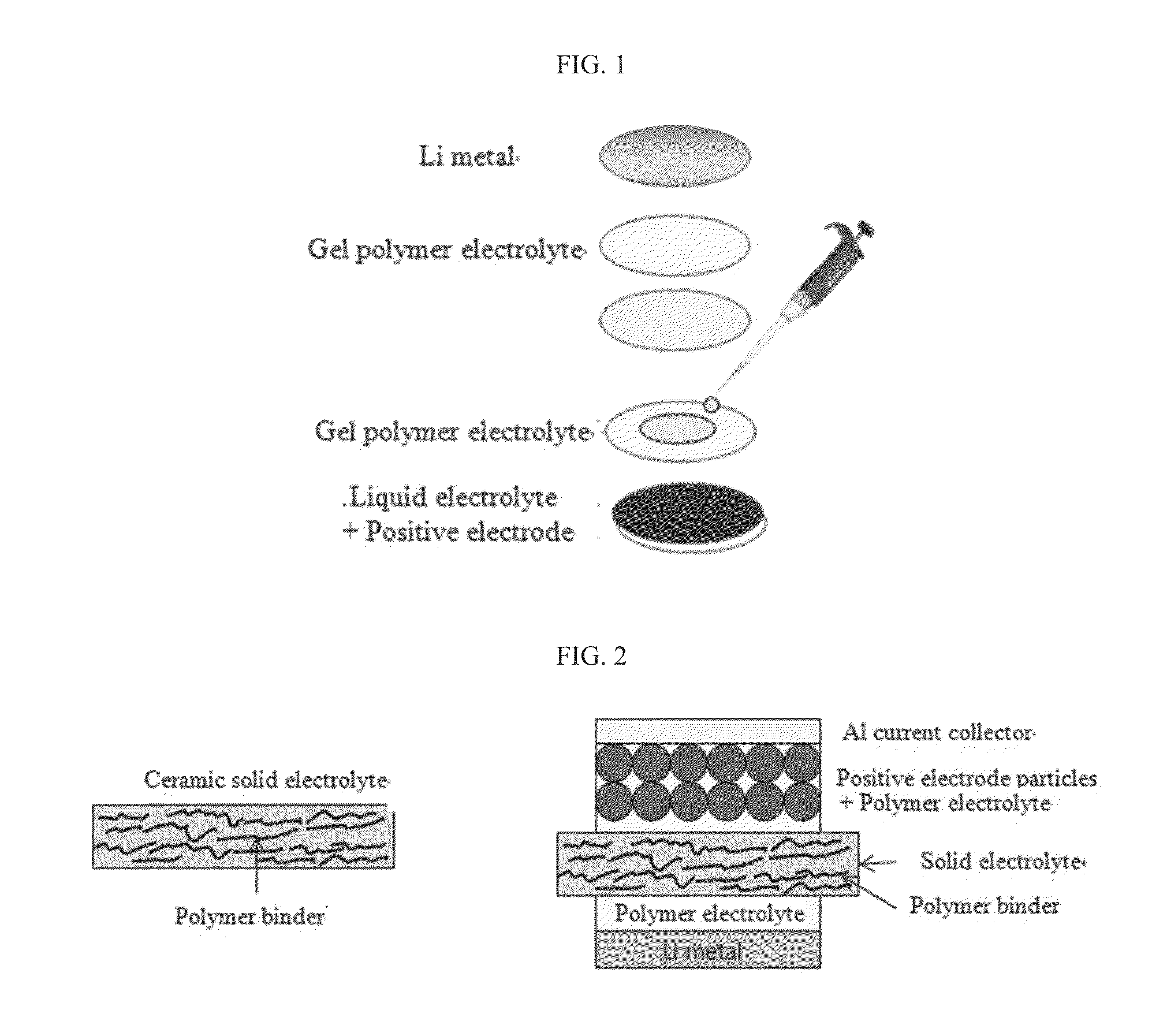
ActiveUS20130260257A1Improve ionic conductivityIncrease flexibilitySolid electrolytesNon-aqueous electrolyte cellsIonLayered structure
Provided are a multi-layered structure electrolyte including a gel polymer electrolyte on opposite surfaces of a ceramic solid electrolyte, for a lithium ion secondary battery including positive and negative electrodes capable of intercalating / deintercalating lithium ions, and a lithium ion secondary battery including the electrode. The electrolyte includes a gel polymer electrolyte on opposite surfaces of a ceramic solid electrolyte.
Owner:SAMSUNG SDI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com