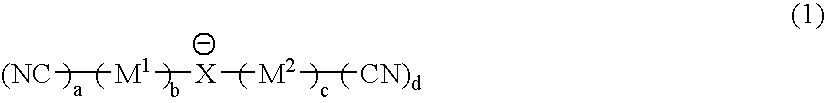Patents
Literature
1167results about "Alkaline accumulators" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High energy lithium ion batteries with particular negative electrode compositions
ActiveUS20090305131A1Degree of crystallinity of will decreaseAlkaline accumulatorsElectrode manufacturing processesHigh energyMetal alloy
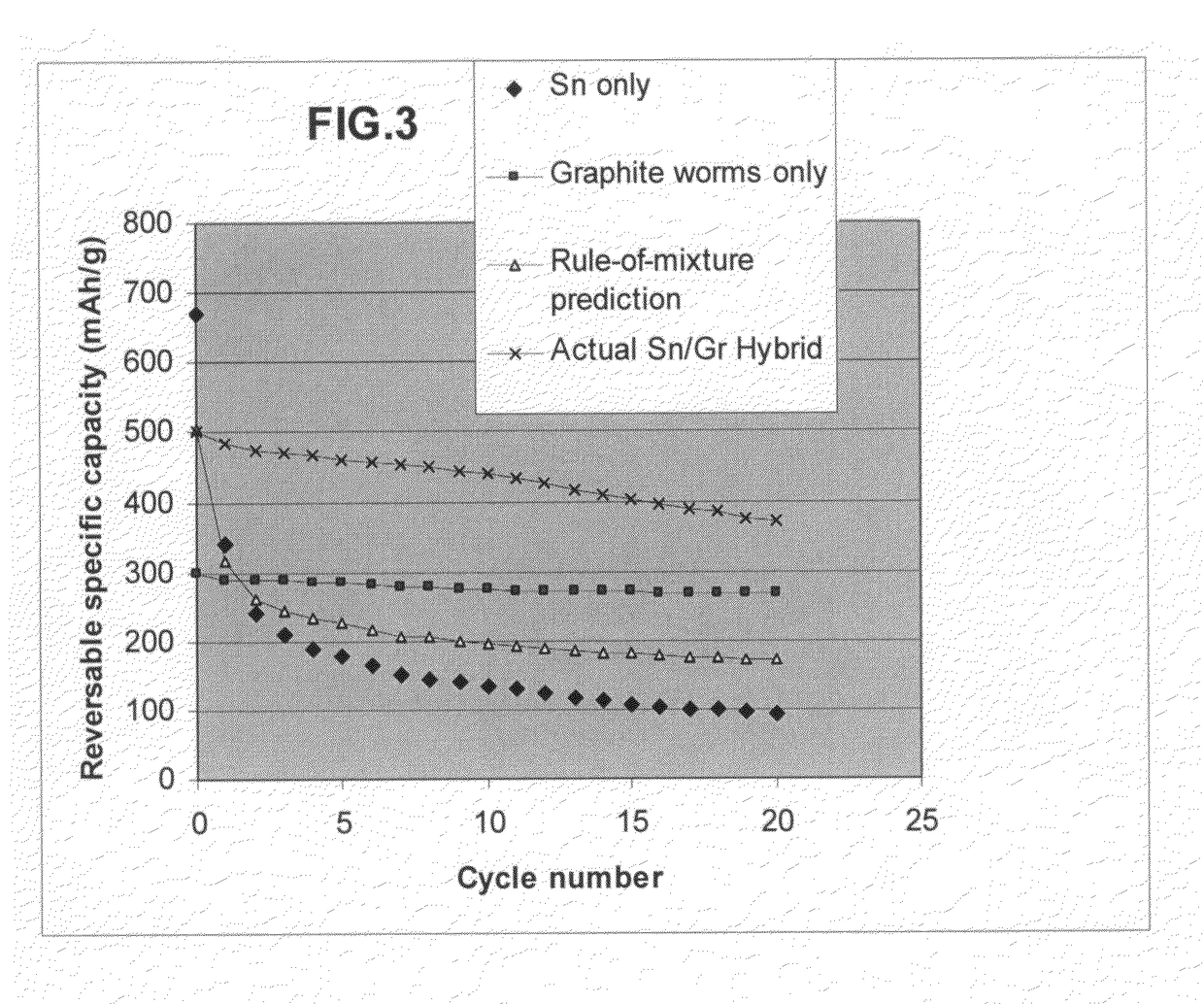
Combinations of materials are described in which high energy density active materials for negative electrodes of lithium ion batteries. In general, metal alloy / intermetallic compositions can provide the high energy density. These materials can have moderate volume changes upon cycling in a lithium ion battery. The volume changes can be accommodated with less degradation upon cycling through the combination with highly porous electrically conductive materials, such as highly porous carbon and / or foamed current collectors. Whether or not combined with a highly porous electrically conductive material, metal alloy / intermetallic compositions with an average particle size of no more than a micron can be advantageously used in the negative electrodes to improve cycling properties.
Owner:IONBLOX INC
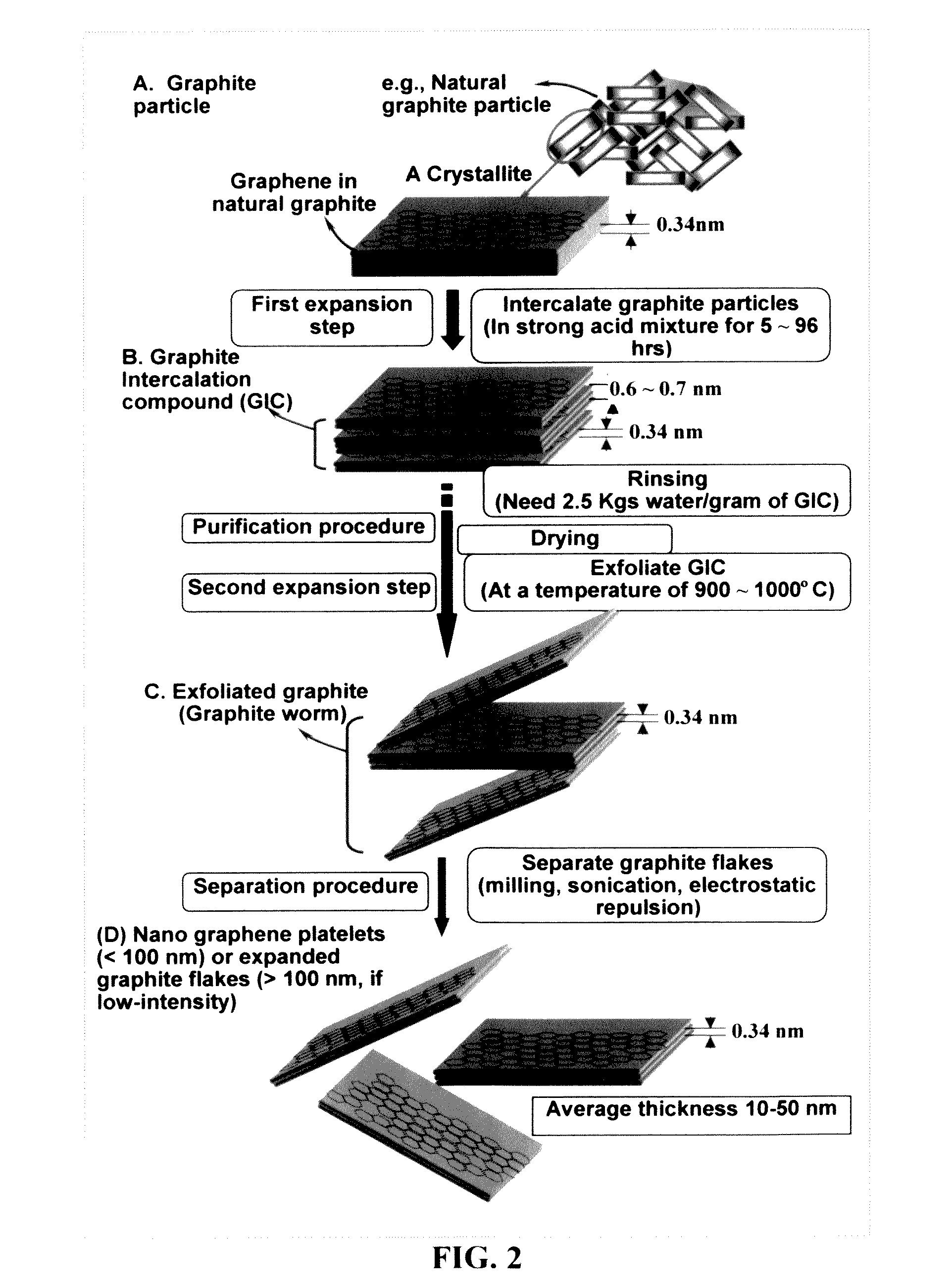
Nano graphene platelet-base composite anode compositions for lithium ion batteries
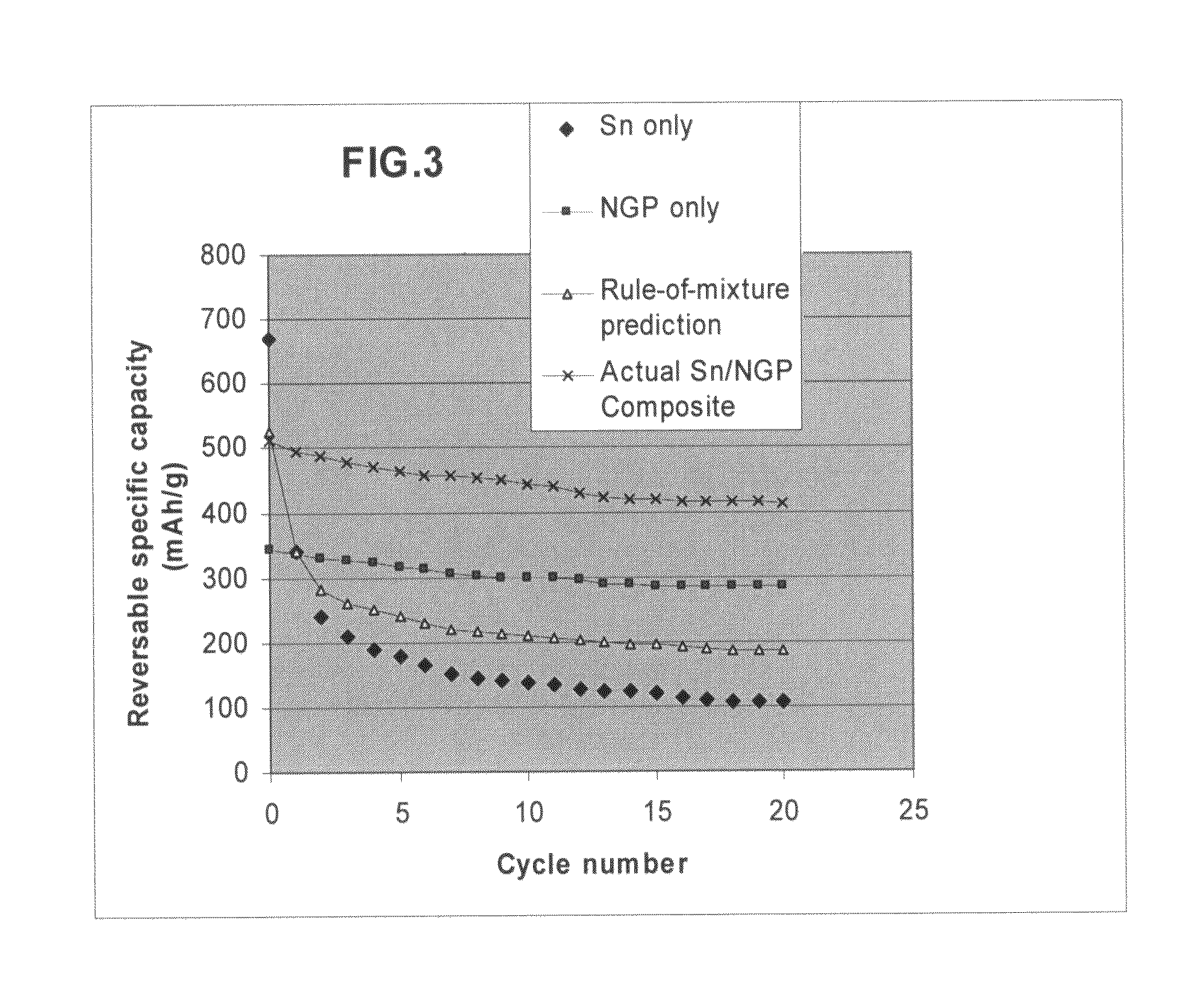
ActiveUS7745047B2Improve conductivityLower internal resistanceAlkaline accumulatorsElectrolytic capacitorsGraphiteGraphene
The present invention provides a nano-scaled graphene platelet-based composite material composition for use as an electrode, particularly as an anode of a lithium ion battery. The composition comprises: (a) micron- or nanometer-scaled particles or coating which are capable of absorbing and desorbing lithium ions; and (b) a plurality of nano-scaled graphene platelets (NGPs), wherein a platelet comprises a graphene sheet or a stack of graphene sheets having a platelet thickness less than 100 nm; wherein at least one of the particles or coating is physically attached or chemically bonded to at least one of the graphene platelets and the amount of platelets is in the range of 2% to 90% by weight and the amount of particles or coating in the range of 98% to 10% by weight. Also provided is a lithium secondary battery comprising such a negative electrode (anode). The battery exhibits an exceptional specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD
Hybrid anode compositions for lithium ion batteries
ActiveUS20090117466A1Superior multiple-cycle behaviorSmall capacity fadeAlkaline accumulatorsConductive materialHybrid materialSodium-ion battery
The present invention provides an exfoliated graphite-based hybrid material composition for use as an electrode, particularly as an anode of a lithium ion battery. The composition comprises: (a) micron- or nanometer-scaled particles or coating which are capable of absorbing and desorbing alkali or alkaline metal ions (particularly, lithium ions); and (b) exfoliated graphite flakes that are substantially interconnected to form a porous, conductive graphite network comprising pores, wherein at least one of the particles or coating resides in a pore of the network or attached to a flake of the network and the exfoliated graphite amount is in the range of 5% to 90% by weight and the amount of particles or coating is in the range of 95% to 10% by weight. Also provided is a lithium secondary battery comprising such a negative electrode (anode). The battery exhibits an exceptional specific capacity, excellent reversible capacity, and long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD
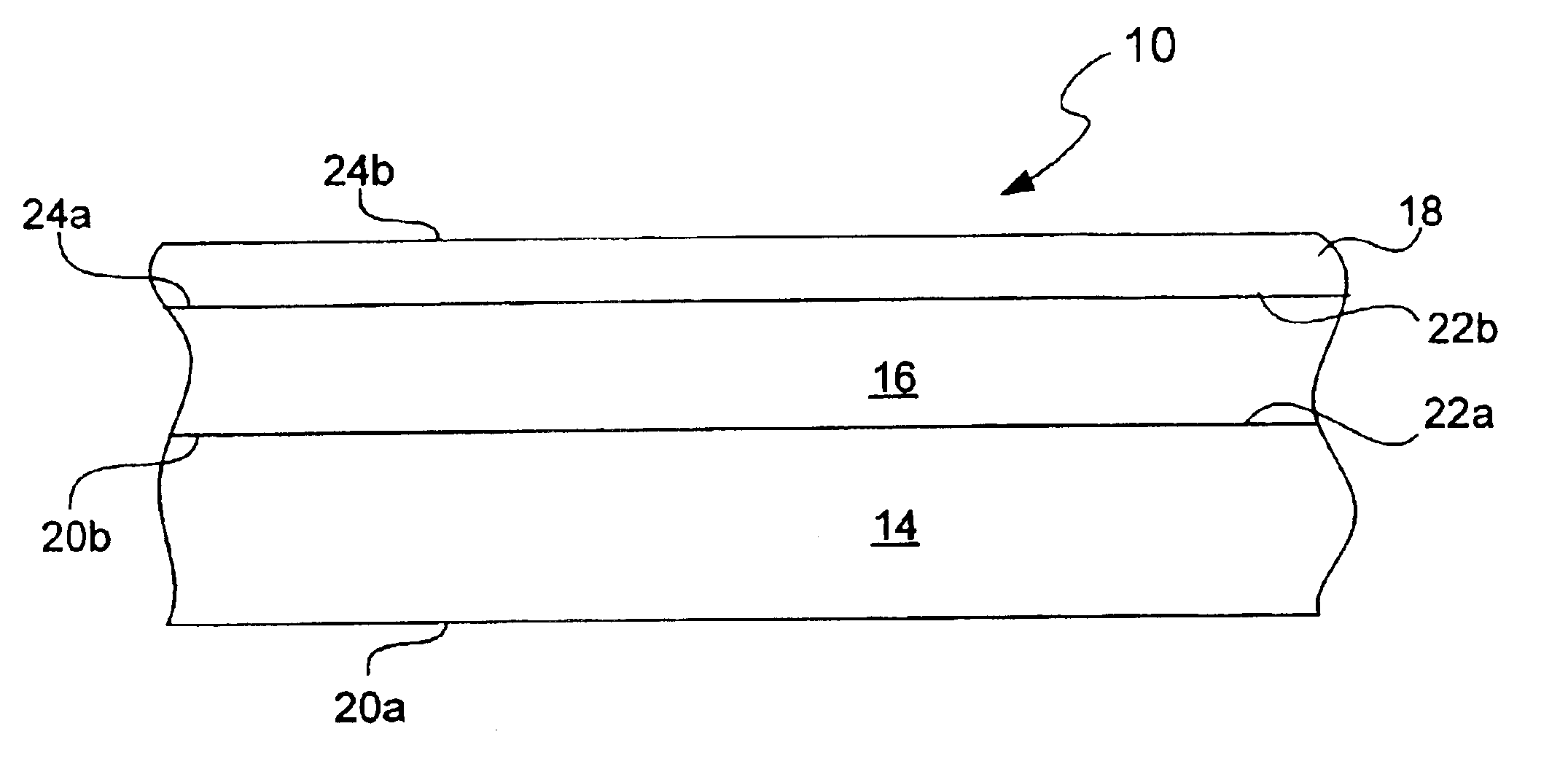
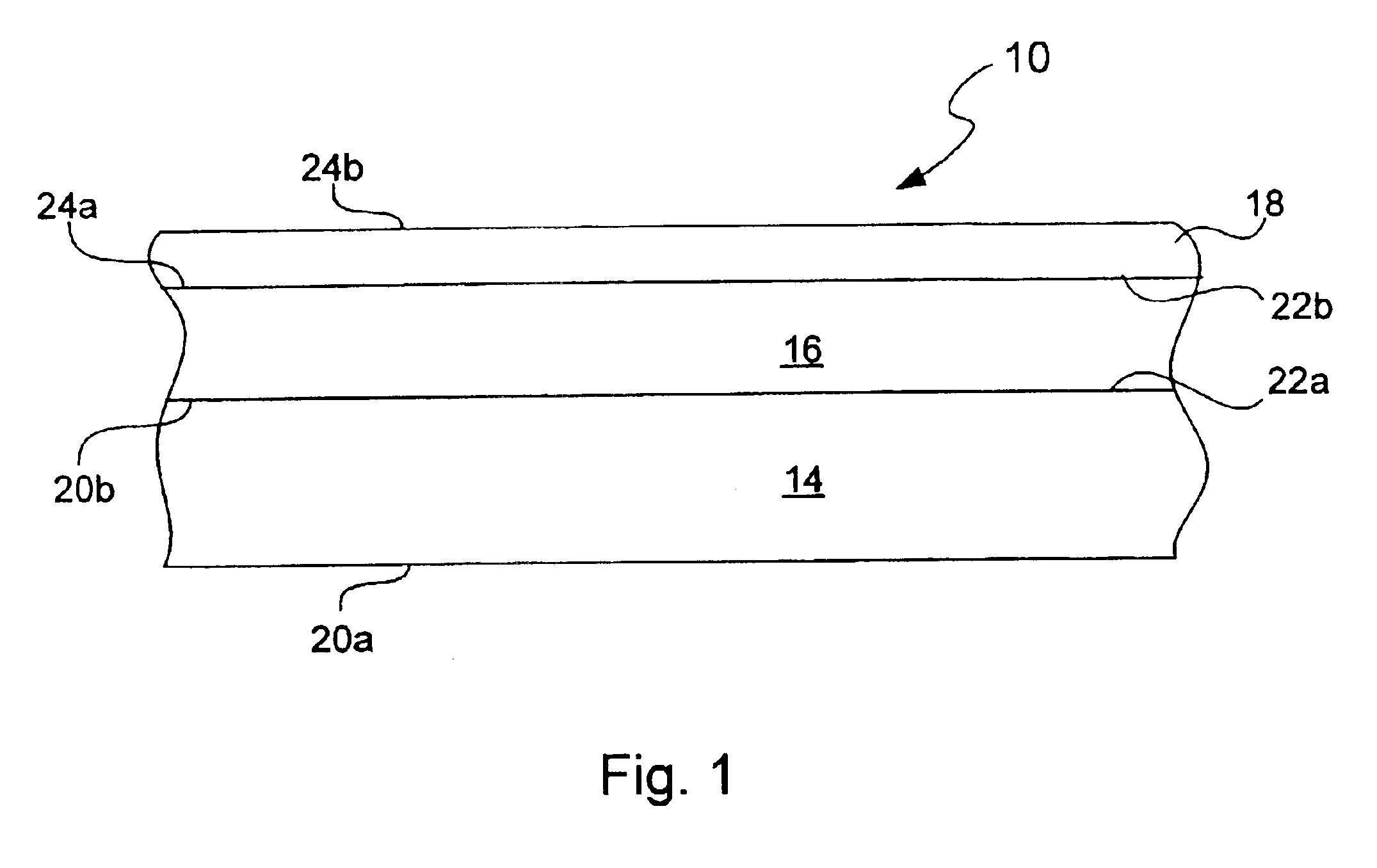
Chemical protection of a lithium surface
InactiveUS6911280B1Easy to produceSimple processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process in the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Hybrid electrode and surface-mediated cell-based super-hybrid energy storage device containing same
PendingUS20130171502A1Primary cell to battery groupingMaterial nanotechnologyHigh energyLithium metal
The present invention provides a multi-component hybrid electrode for use in an electrochemical super-hybrid energy storage device. The hybrid electrode contains at least a current collector, at least an intercalation electrode active material storing lithium inside interior or bulk thereof, and at least an intercalation-free electrode active material having a specific surface area no less than 100 m2 / g and storing lithium on a surface thereof, wherein the intercalation electrode active material and the intercalation-free electrode active material are in electronic contact with the current collector. The resulting super-hybrid cell exhibits exceptional high power and high energy density, and long-term cycling stability that cannot be achieved with conventional supercapacitors, lithium-ion capacitors, lithium-ion batteries, and lithium metal secondary batteries.
Owner:GLOBAL GRAPHENE GRP INC +1
Metal oxide coated positive electrode materials for lithium-based batteries
Positive electrode active materials are formed with various metal oxide coatings. Excellent results have been obtained with the coatings on lithium rich metal oxide active materials. Surprisingly improved results are obtained with metal oxide coatings with lower amounts of coating material. High specific capacity results are obtained even at higher discharge rates.
Owner:IONBLOX INC
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Batteries including improved fine fiber separators
Alkaline and lithium batteries are disclosed that advantageously include separators comprising at least one porous layer of fine fibers having a diameter of between about 50 nm and about 3000 nm that provide improved combinations of reduced thickness, dendritic barrier against short-circuiting and low ionic resistance as compared with known battery separators.
Owner:DUPONT SAFETY & CONSTR INC
Layer-layer lithium rich complex metal oxides with high specific capacity and excellent cycling
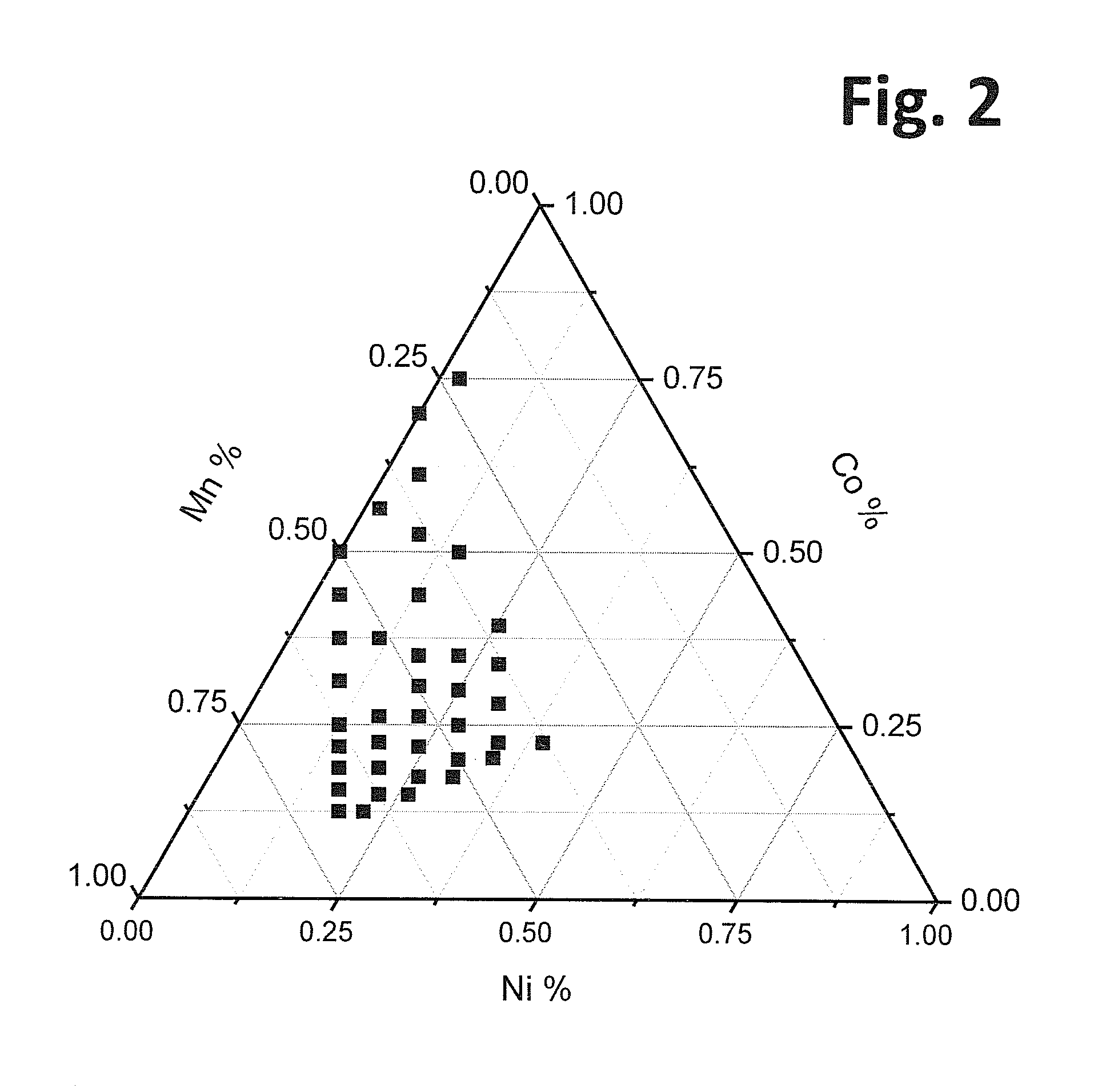
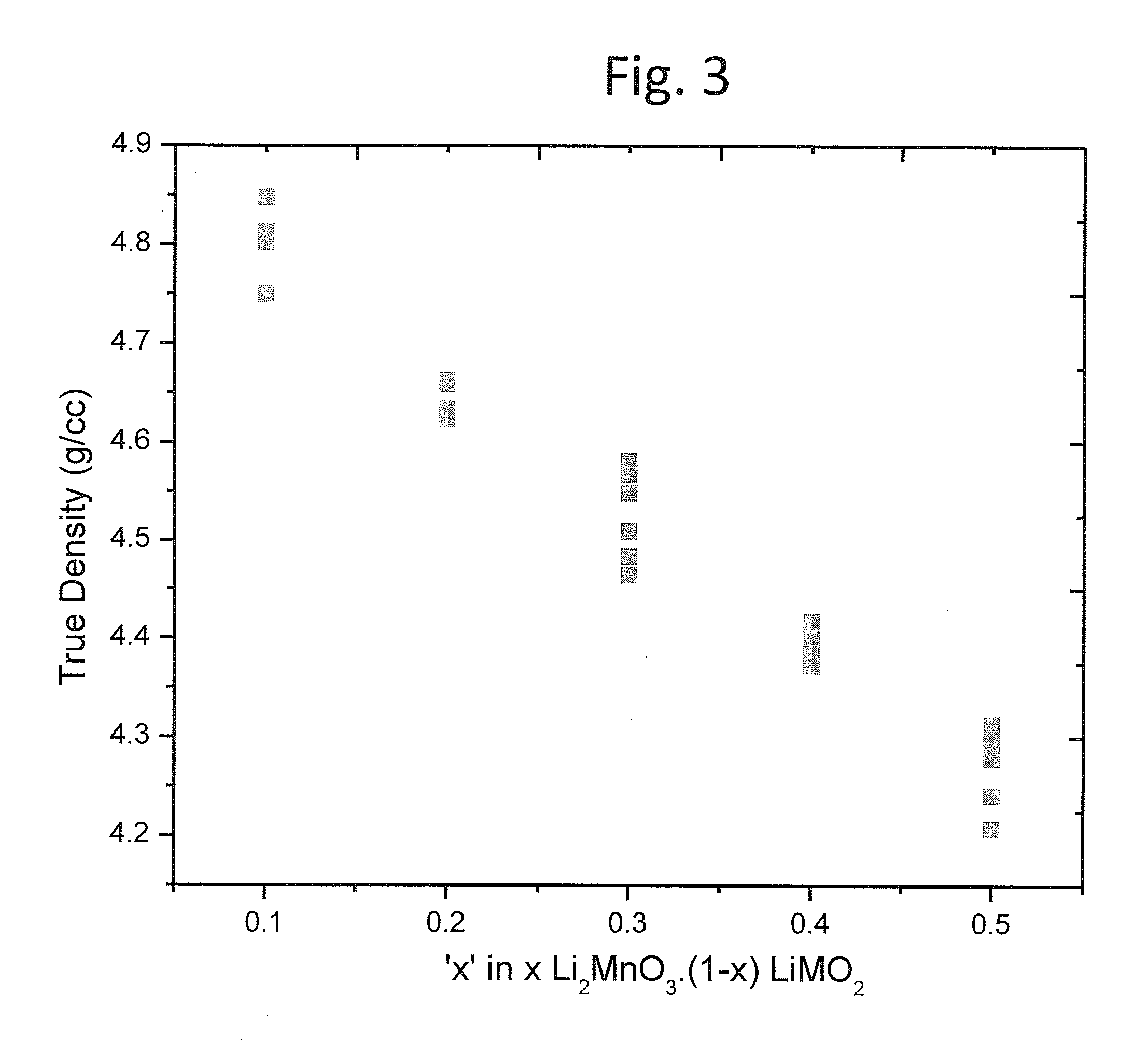
Lithium rich and manganese rich lithium metal oxides are described that provide for excellent performance in lithium-based batteries. The specific compositions can be engineered within a specified range of compositions to provide desired performance characteristics. Selected compositions can provide high values of specific capacity with a reasonably high average voltage. Compositions of particular interest can be represented by the formula, xLi2MnO3.(1−x)LiNiu+ΔMnu−ΔCowAyO2. The compositions undergo significant first cycle irreversible changes, but the compositions cycle stably after the first cycle.
Owner:IONBLOX INC
Carbonaceous material for electrode and non-aqueous solvent secondary battery using this material
InactiveUS6632569B1Large discharge capacityImprove propertiesAlkaline accumulatorsConductive materialX-rayPeak value
A carbonaceous material has a plane space d002 of a (002) plane less than 0.337 nm in an X-ray wide angle diffraction method, a crystallite size (Lc) of 90 nm or higher, an R value, as a peak intensity ratio of a peak intensity of 1360 cm<HIL><−1 < / SP><PDAT>to a peak intensity of 1580 cm<HIL><−1 < / SP><PDAT>in a Raman spectrum in use of an argon ion laser, of 0.20 or higher, and a tap density of 0.75 g / cm<HIL><3 < / SP><PDAT>or higher. Also disclosed is a multilayer structure carbonaceous material for electrode, which is manufactured by carbonizing some organic compounds where the carbonaceous material for electrode is mixed with the organic compounds. The battery using the carbonaceous material for electrode or the multilayer structure carbonaceous material for electrode has a large capacity, a small irreversible capacity admitted in the initial cycle, excellent capacity maintaining rate of the cycle, and particularly, largely improved quick charging and discharging characteristics.< / PTEXT>
Owner:MITSUBISHI CHEM CORP
Sulfur-carbon nanocomposites and their application as cathode materials in lithium-sulfur batteries
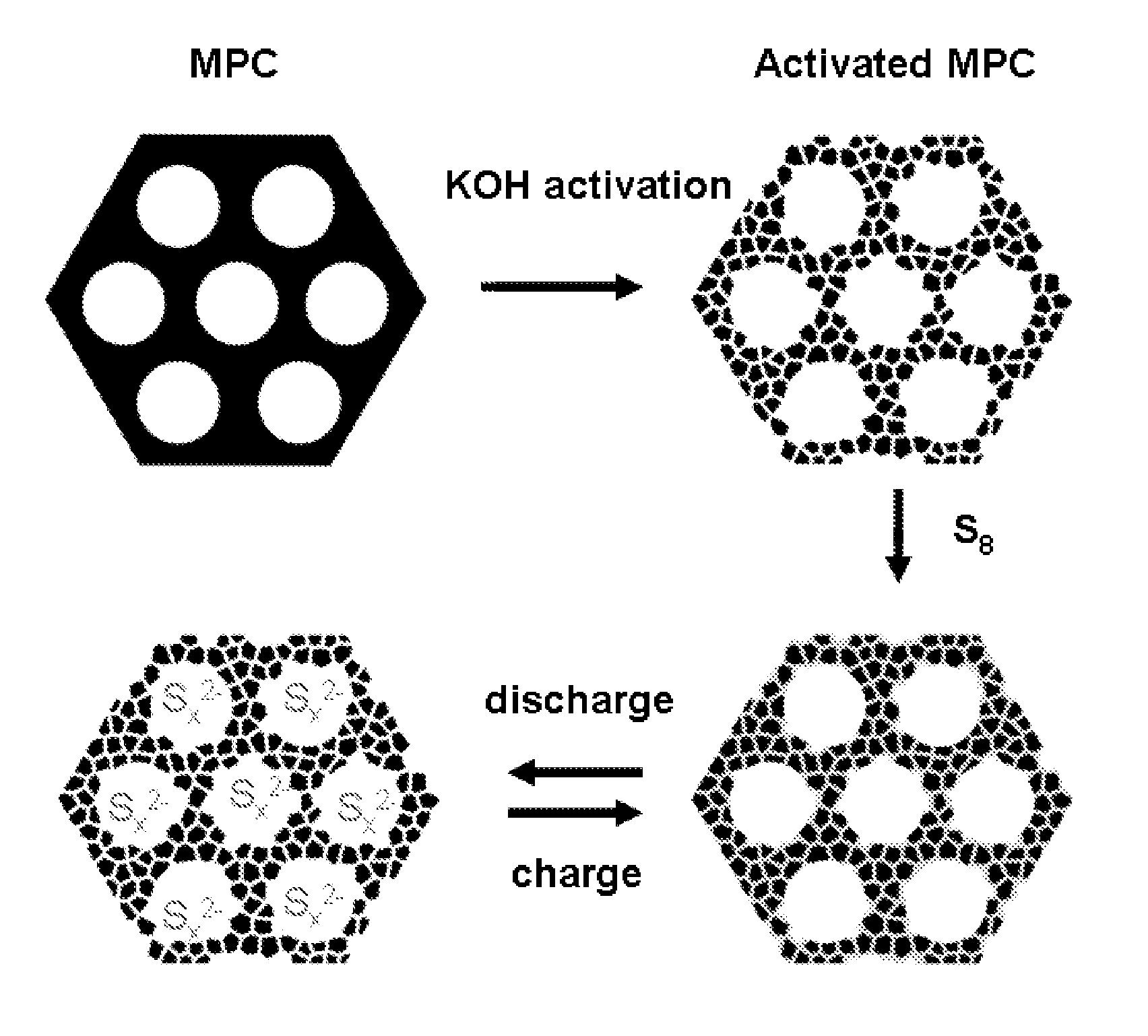
ActiveUS20110052998A1Minimize formationEasy to transportAlkaline accumulatorsElectrode thermal treatmentCarbon compositesPorous carbon
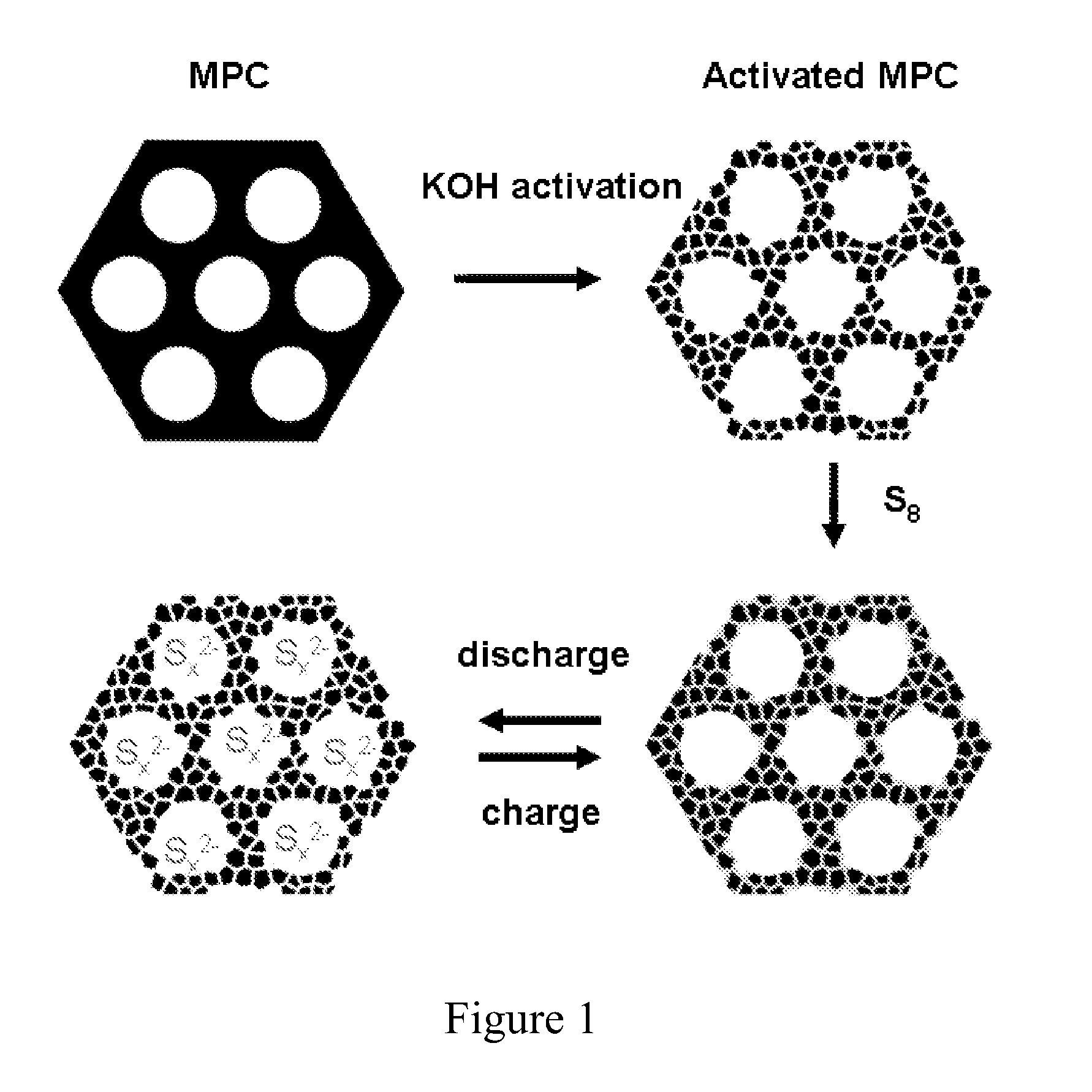
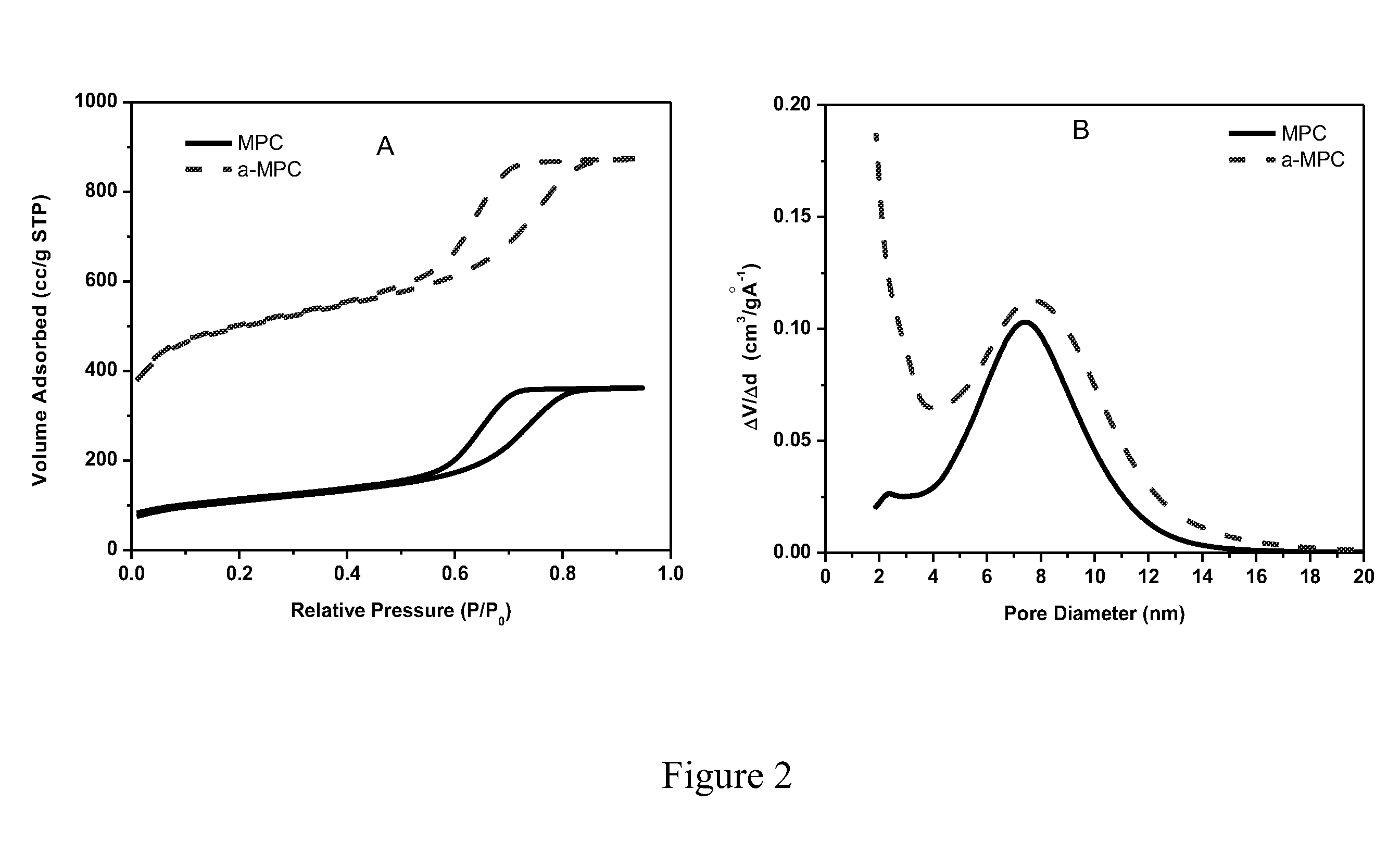
The invention is directed in a first aspect to a sulfur-carbon composite material comprising: (i) a bimodal porous carbon component containing therein a first mode of pores which are mesopores, and a second mode of pores which are micropores; and (ii) elemental sulfur contained in at least a portion of said micropores. The invention is also directed to the aforesaid sulfur-carbon composite as a layer on a current collector material; a lithium ion battery containing the sulfur-carbon composite in a cathode therein; as well as a method for preparing the sulfur-composite material.
Owner:UT BATTELLE LLC
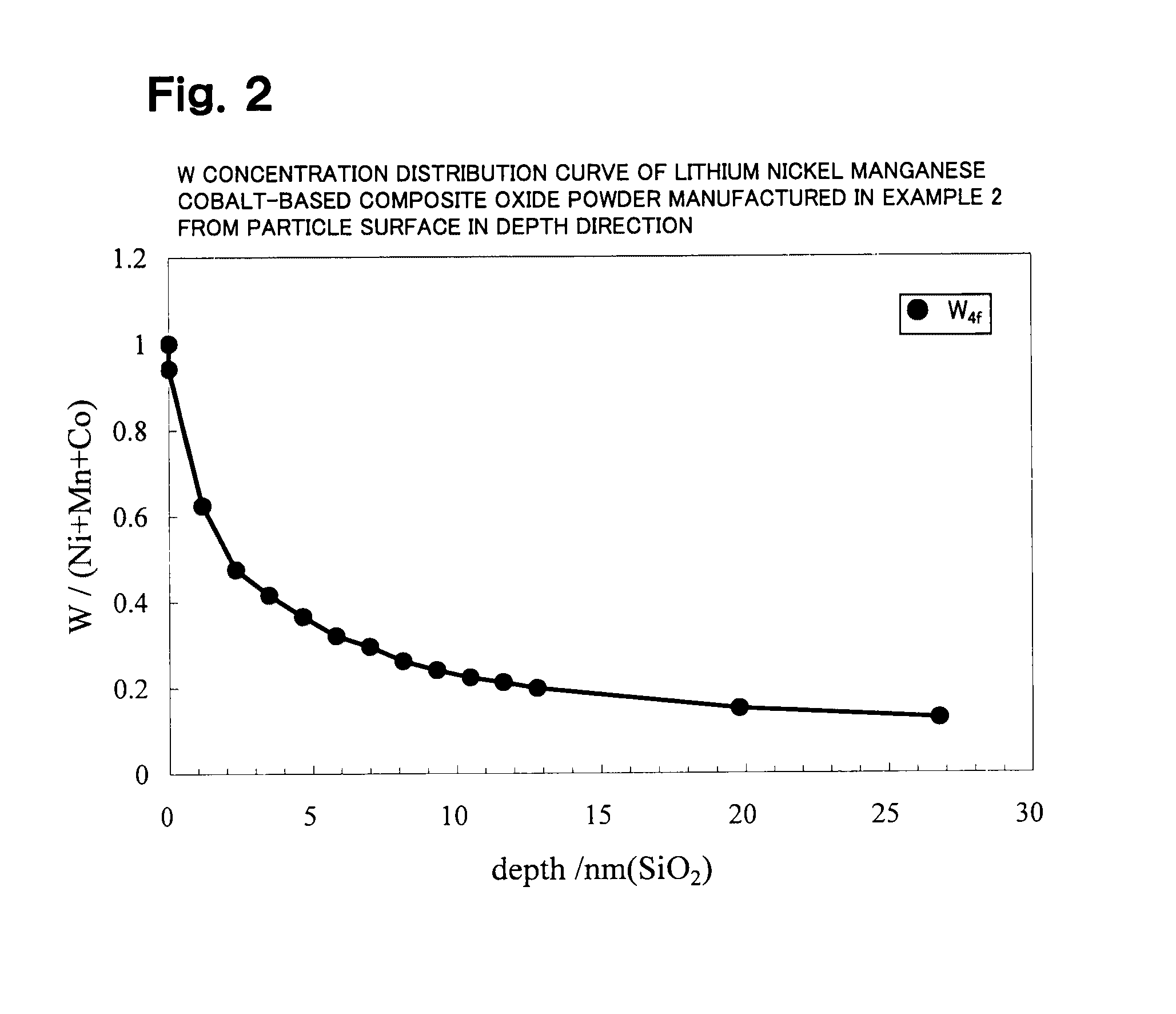
Lithium transition metal-based compound powder, method for manufacturing the same, spray-dried substance serving as firing precursor thereof, and lithium secondary battery positive electrode and lithium secondary battery using the same
ActiveUS20100209771A1Improve load characteristicsHigh densityMaterial nanotechnologyAlkaline accumulatorsLithiumHigh density
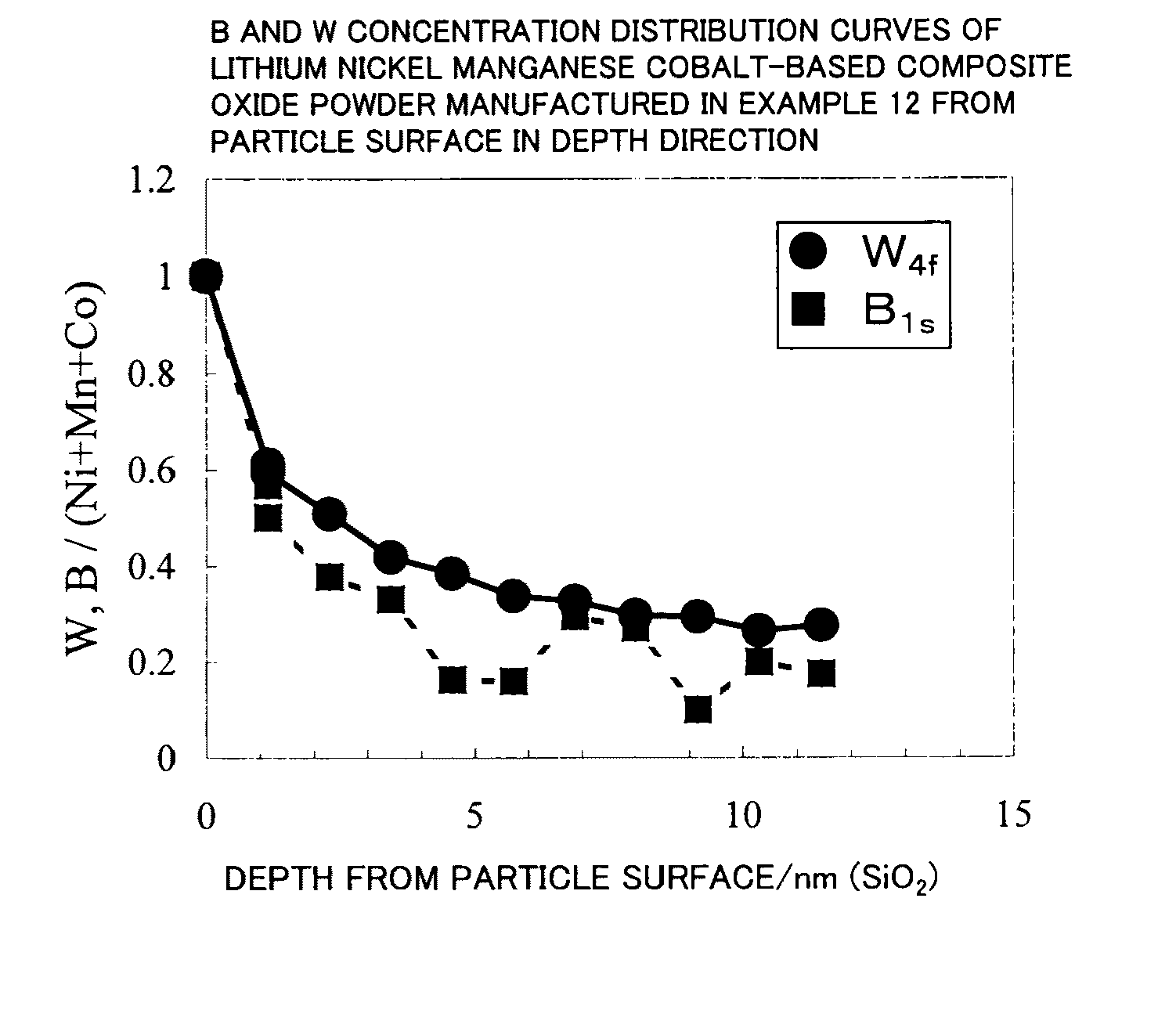
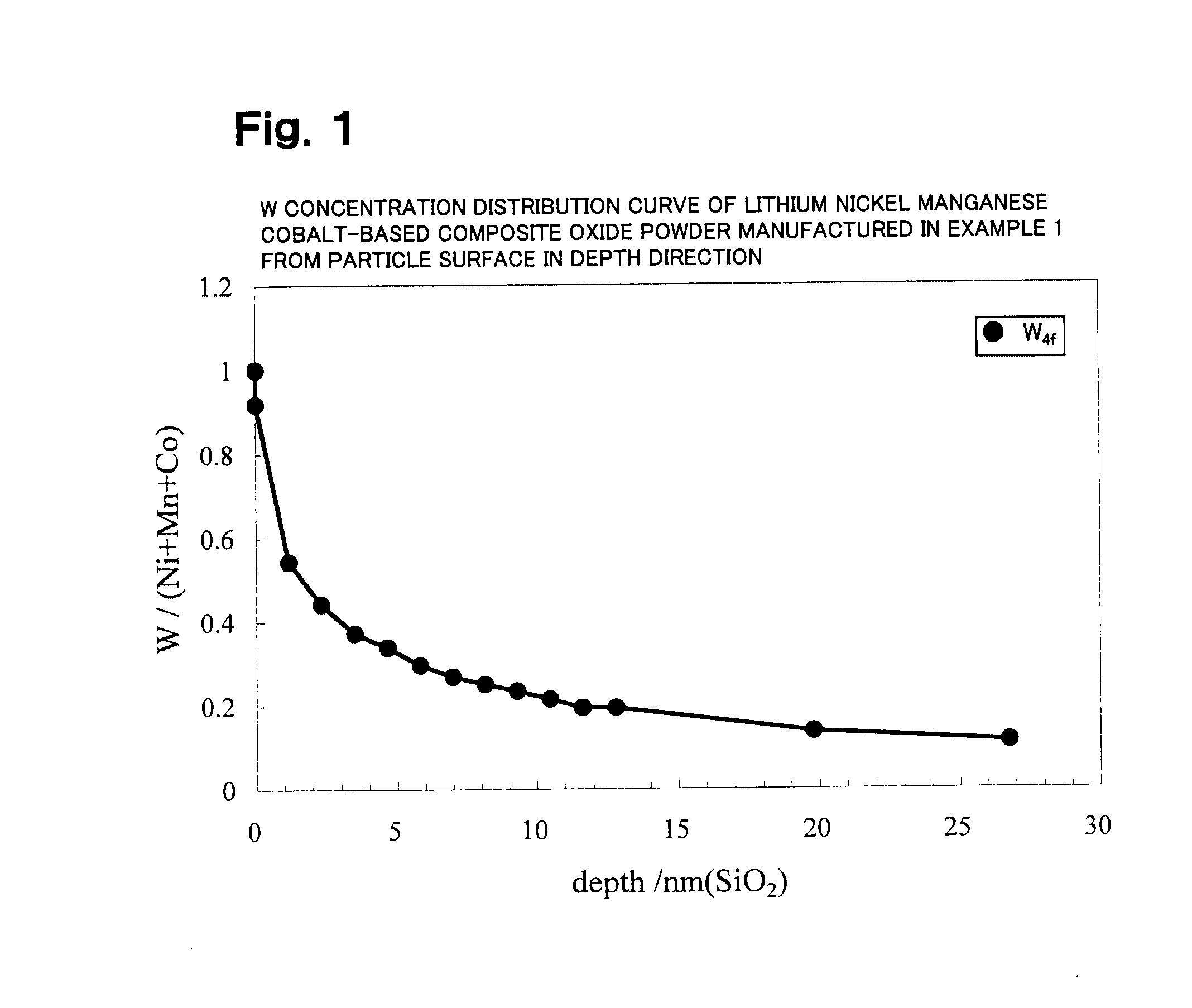
A lithium transition metal-based compound powder for a lithium secondary battery positive electrode material that can achieve both improvements of load characteristics such as rate and output characteristics and a higher density is a lithium transition metal-based compound powder containing, as a main component, a lithium transition metal-based compound that has a function of allowing elimination and insertion of lithium ions, and including a crystal structure belonging to a layer structure, wherein primary particles are aggregated to form secondary particles, the ratio A / B of a median diameter A of the secondary particles to an average diameter (average primary particle diameter B) is in the range of 8 to 100, and 0.01≦FWHM(110)≦0.5 where FWHM(110) is the half width of a (110) diffraction peak present near a diffraction angle 2θ of 64.5° in a powder X-ray diffraction analysis using a CuKα line.
Owner:MITSUBISHI CHEM CORP
Material for electrolytic solutions and use thereof
InactiveUS20040002002A1Avoid corrosionImprove ionic conductivityHybrid capacitor electrolytesAlkaline accumulatorsOrganic linkingElectrical conductor
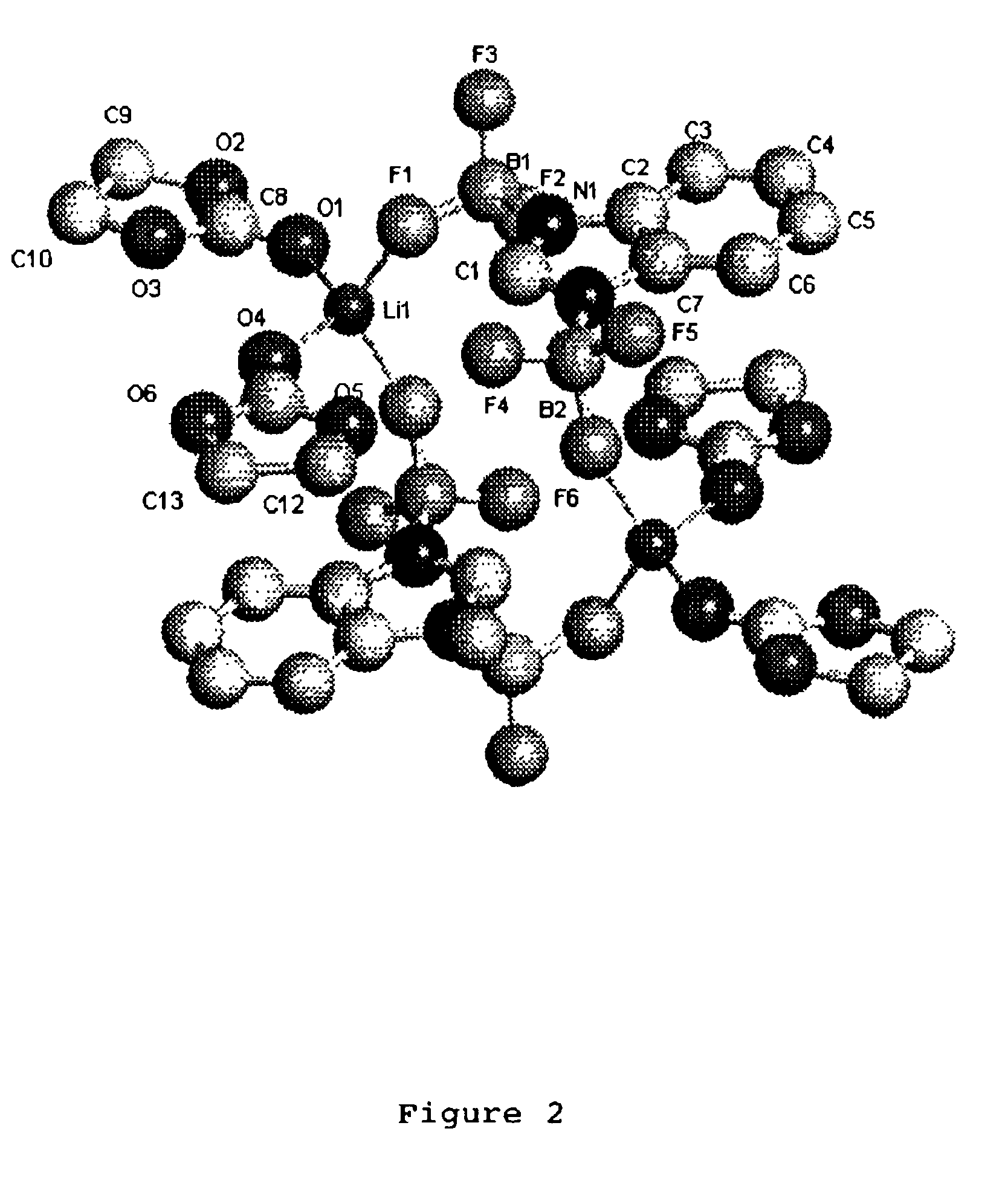
It is an object of the present invention to provide a material for electrolytic solutions suited for use as material in electrolytic solutions serving as ionic conductors in electrochemical devices, such as large-capacity cells or batteries. The present invention is related to a material for electrolytic solutions which comprises, as an essential constituent, an anion represented by the general formula (1): wherein X represents at least one element selected from among B, N, O, Al, Si, P, S, As and Se; M<1 >and M<2 >are the same or different and each represents an organic linking group; a is an integer of not less than 1, and b, c and d each independently is an integer of not less than 0.
Owner:NIPPON SHOKUBAI CO LTD
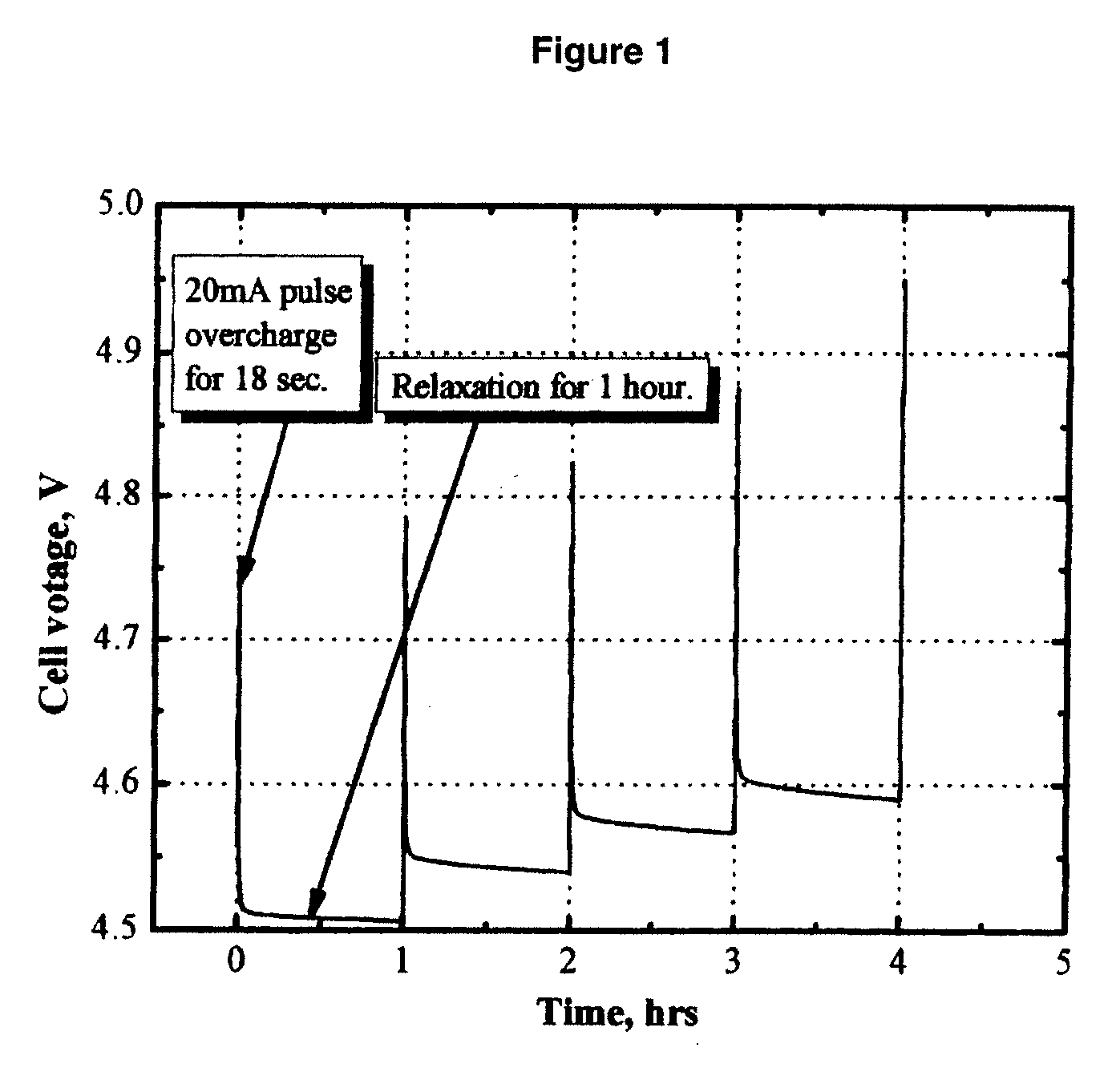
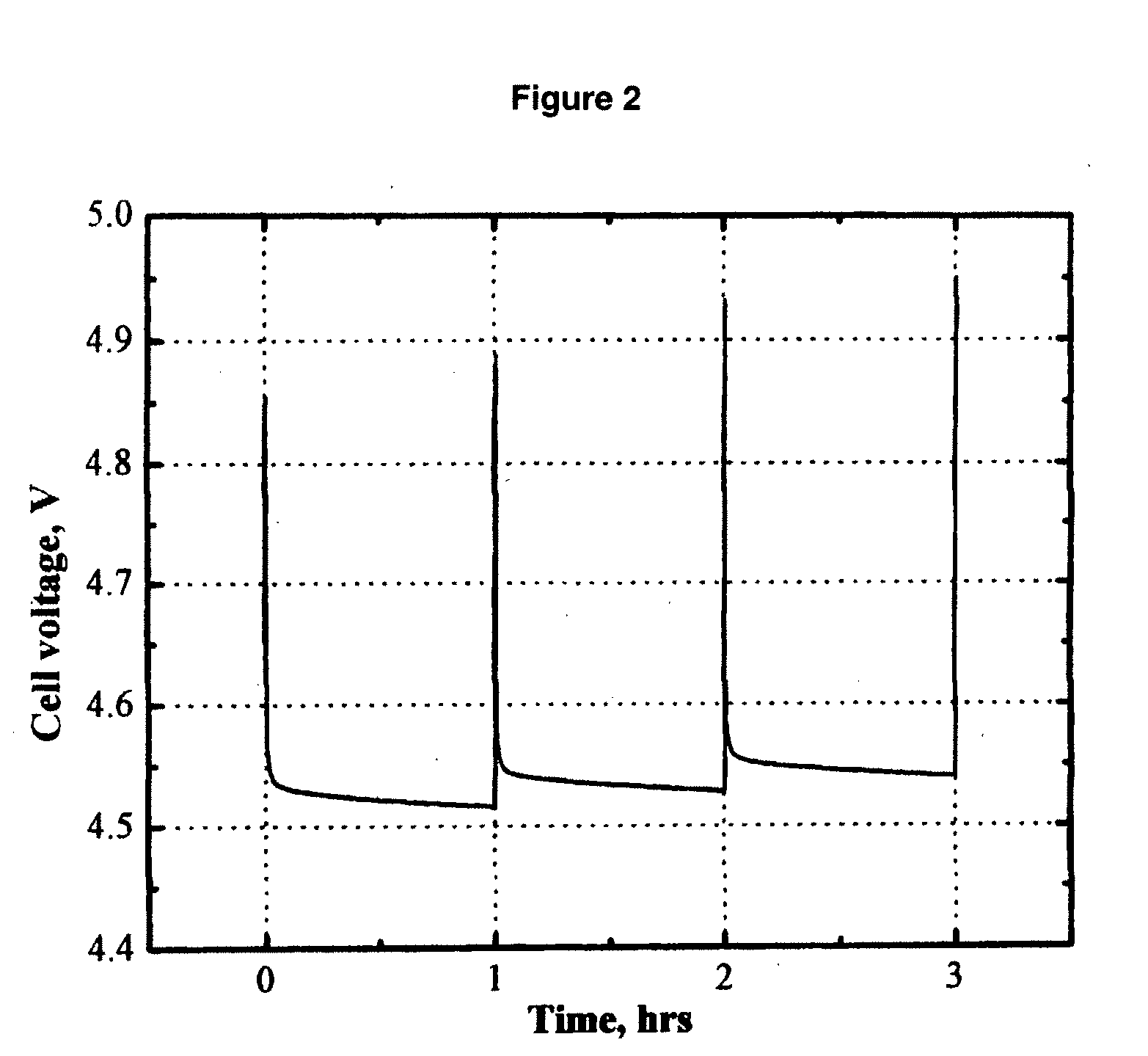
Electrolytes, cells and methods of forming passivaton layers
InactiveUS20080026297A1Improve thermal stabilityImprove stabilityAlkaline accumulatorsHybrid capacitor electrolytesSolventPhotochemistry
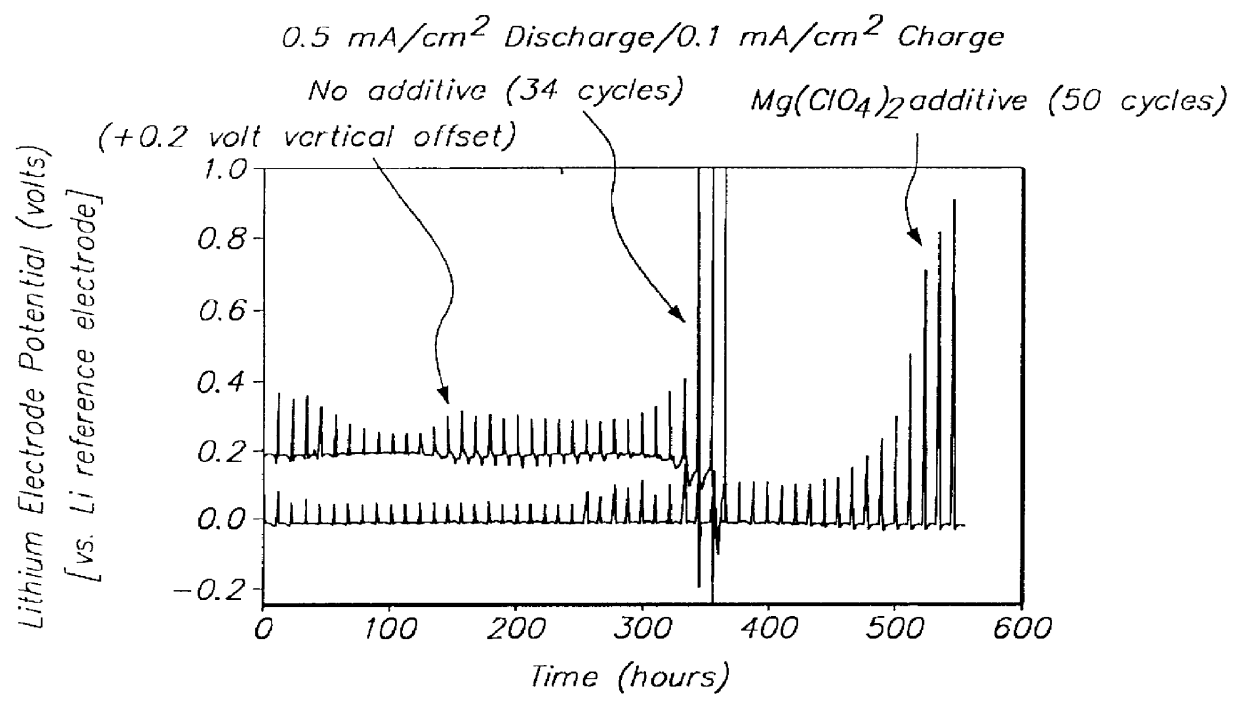
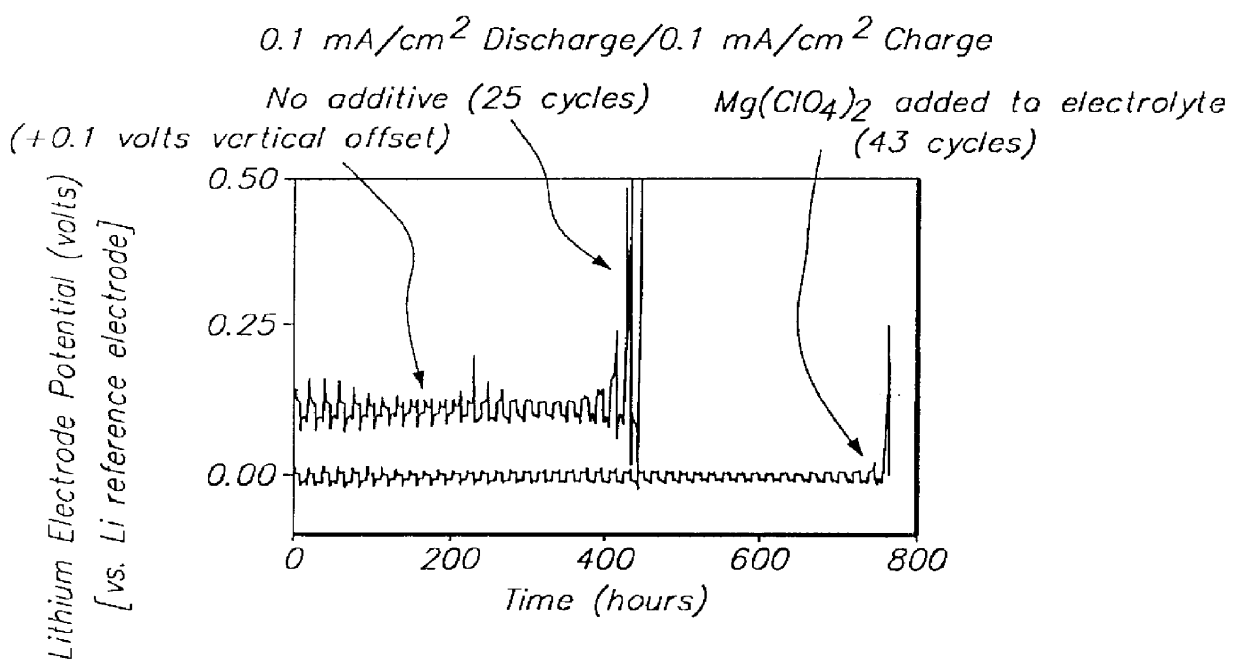
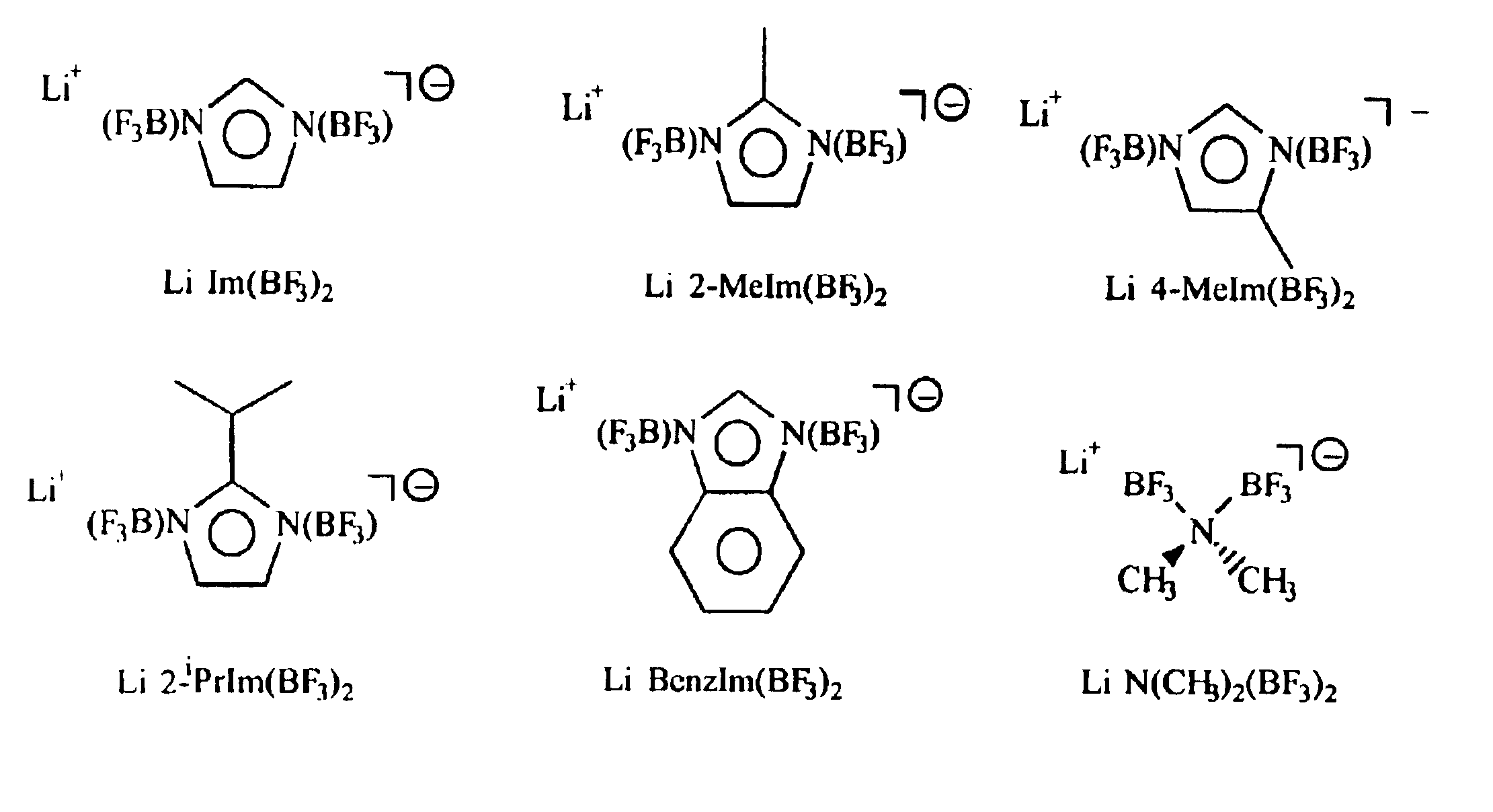
An electrolyte comprising at least one organic aprotic solvent, at least one salt and at least one chelatoborate additive. A method of forming an SEI layer in a cell comprising a positive electrode, a negative electrode and an electrolyte, said method comprising the step of overcharging the electrolyte prior to fabricating the cell, or said cell during the formation cycle.
Owner:AIR PROD & CHEM INC
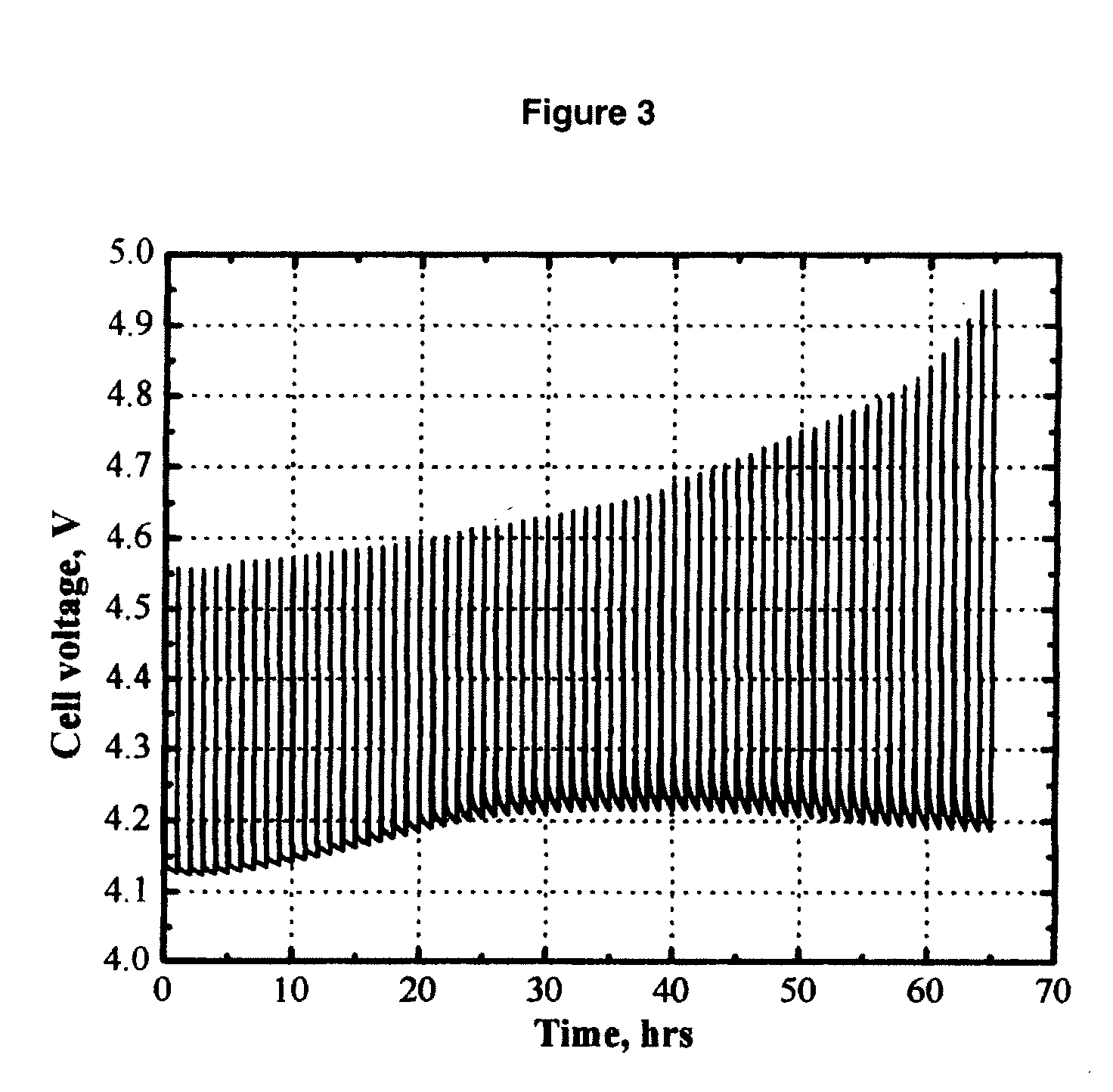
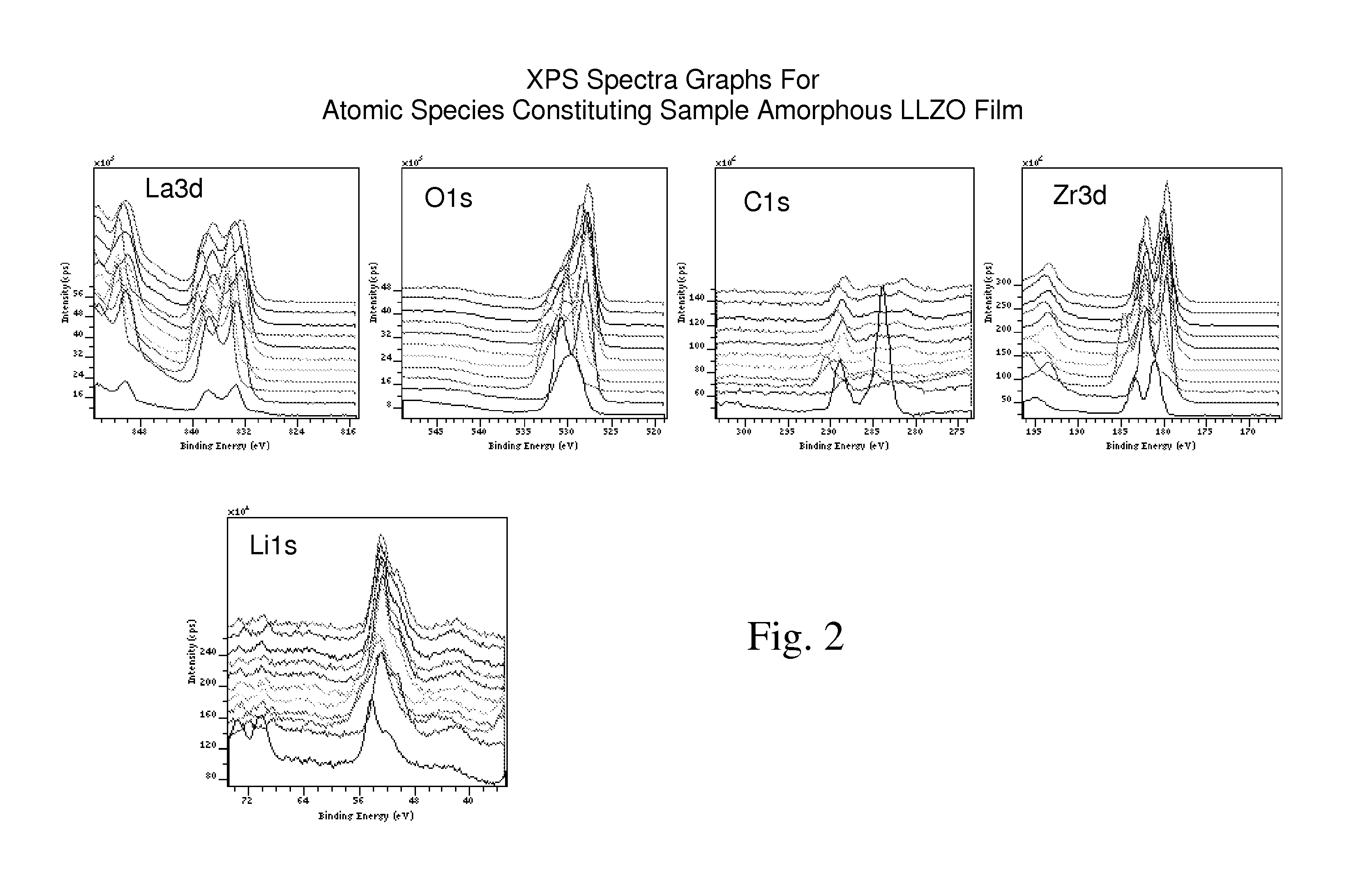
Ionically-conductive amorphous lithium lanthanum zirconium oxide
Amorphous lithium lanthanum zirconium oxide (LLZO) is formed as an ionically-conductive electrolyte medium. The LLZO comprises by percentage of total number of atoms from about 0.1% to about 50% lithium, from about 0.1% to about 25% lanthanum, from about 0.1% to about 25% zirconium, from about 30% to about 70% oxygen and from 0.0% to about 25% carbon. At least one layer of amorphous LLZO may be formed through a sol-gel process wherein quantities of lanthanum methoxyethoxide, lithium butoxide and zirconium butoxide are dissolved in an alcohol-based solvent to form a mixture which is dispensed into a substantially planar configuration, transitioned through a gel phase, dried and cured to a substantially dry phase.
Owner:JOHNSON IP HLDG LLC
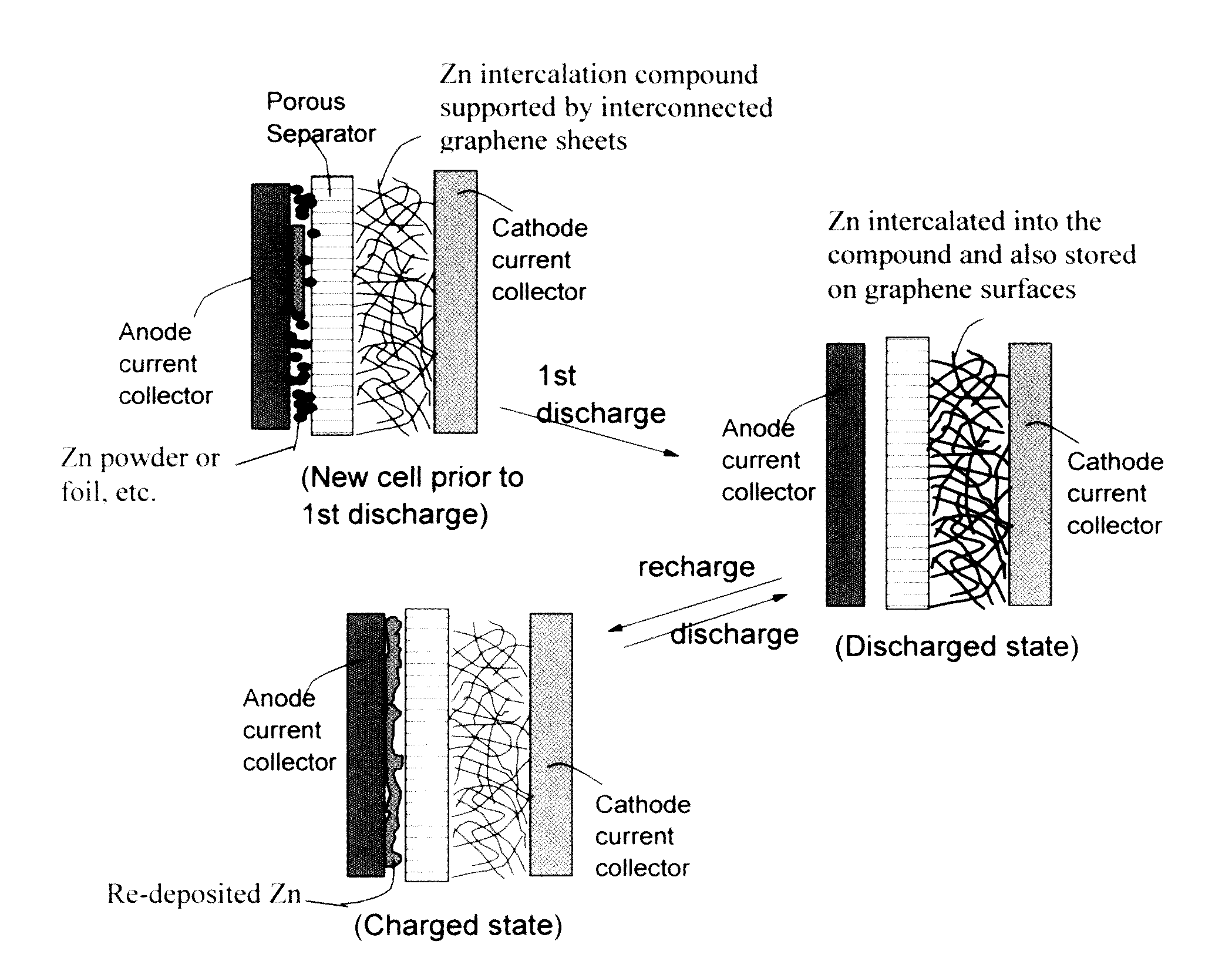
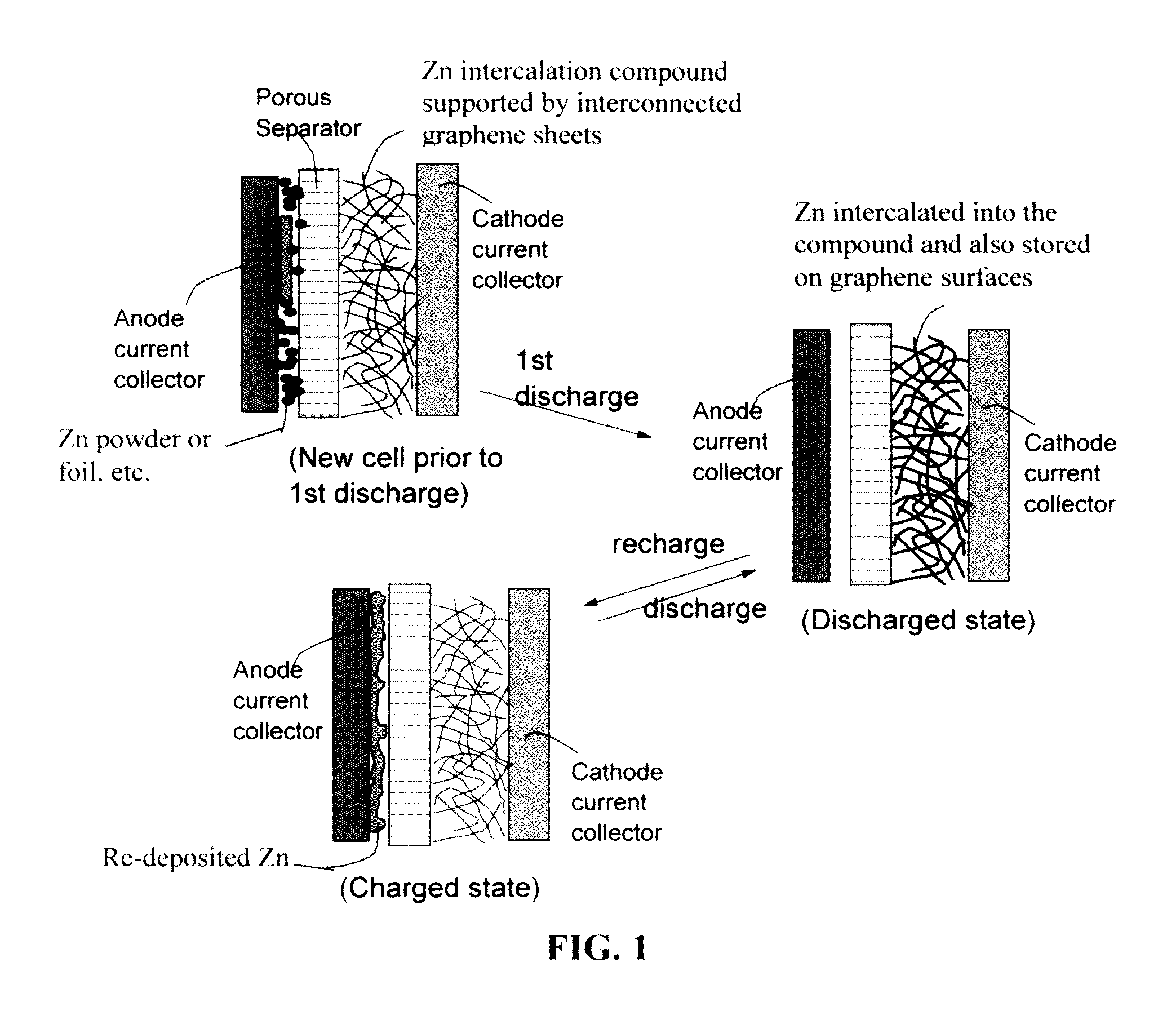
Zinc Ion-Exchanging Energy Storage Device
ActiveUS20160301096A1Quick releaseRapid depositionHybrid capacitor electrolytesAlkaline accumulatorsChemical treatmentZinc metal
A zinc ion-exchanging battery device comprising: (A) a cathode comprising two cathode active materials (a zinc ion intercalation compound and a surface-mediating material); (B) an anode containing zinc metal or zinc alloy; (C) a porous separator disposed between the cathode and the anode; and (D) an electrolyte containing zinc ions that are exchanged between the cathode and the anode during battery charge / discharge. The zinc ion intercalation compound is selected from chemically treated carbon or graphite material having an expanded inter-graphene spacing d002 of at least 0.5 nm, or an oxide, carbide, dichalcogenide, trichalcogenide, sulfide, selenide, or telluride of niobium, zirconium, molybdenum, hafnium, tantalum, tungsten, titanium, vanadium, chromium, cobalt, manganese, iron, nickel, or a combination thereof. The surface-mediating material contains exfoliated graphite or multiple single-layer sheets or multi-layer platelets of a graphene material.
Owner:GLOBAL GRAPHENE GRP INC
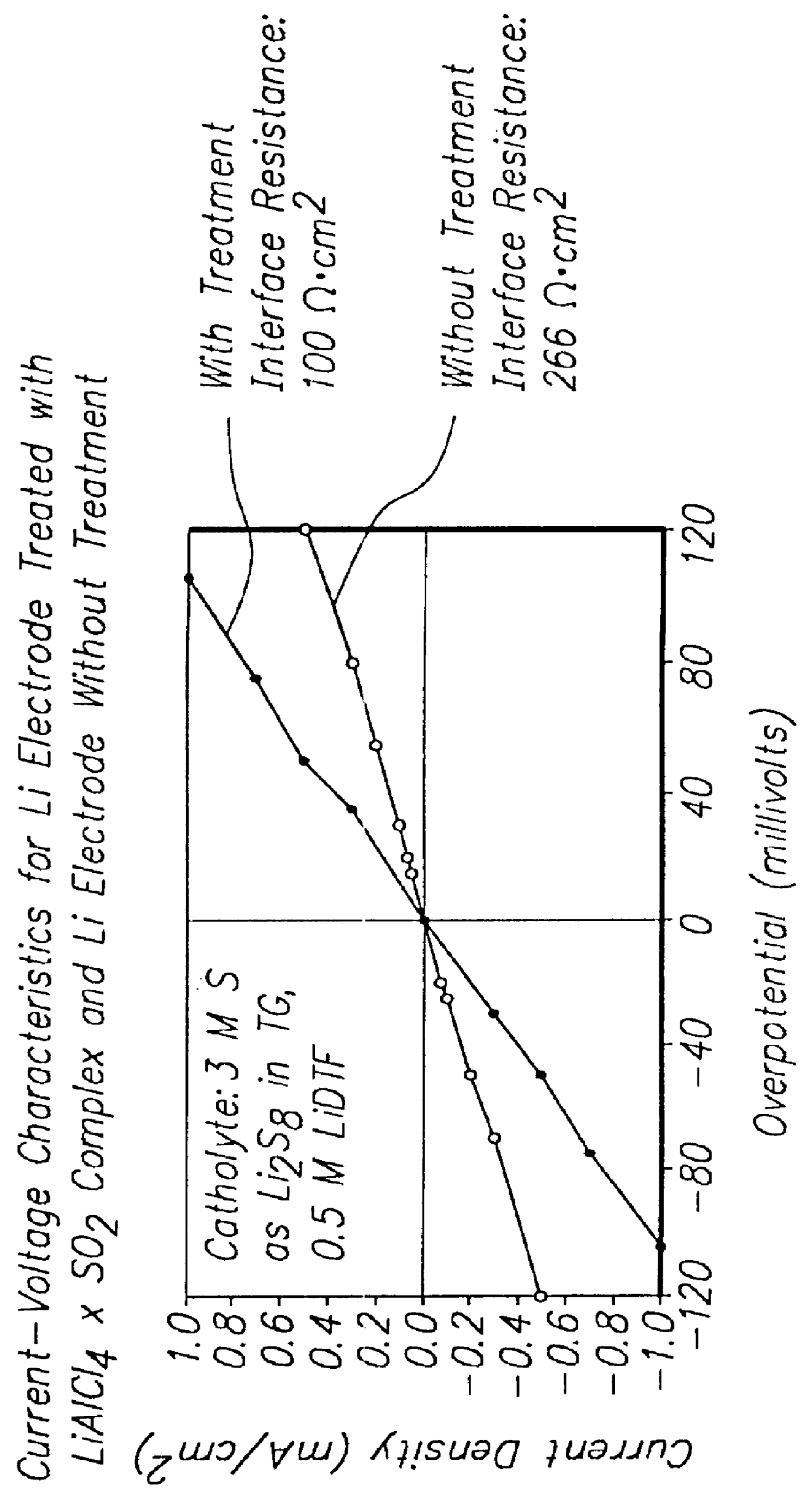
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6165644AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Non-aqueous electrolytes for lithium electrochemical cells
InactiveUS6852446B2Improve conductivityImprove stabilityAlkaline accumulatorsOrganic electrolyte cellsHydrogen atomHydrogen
A non-aqueous electric current producing electrochemical cell is provided comprising an anode and a cathode, an ionically permeable separator interposed between the anode and the cathode, and a non-aqueous electrolyte, the electrolyte comprising an ionically conducting salt in a non-aqueous medium, the ionically conducting salt corresponding to the formula:M+(Z*(J*)j(X*)x)−, wherein:M is a lithium atom,Z* is an anion group containing two or more Lewis basic sites and comprising less than 50 atoms not including hydrogen atoms,J* independently each occurance is a Lewis acid coordinated to at least one Lewis basic site of Z*, and optionally two or more such J* groups may be joined together in a moiety having multiple Lewis acidic functionality,X* independently each occurrence is selected from the group consisting of H, C1-C4 alkyl, alkoxide, halide and mixtures thereof,j is an integer from 2 to 12, andx is an integer from 0 to 4.
Owner:EAGLE PICHER TECH LLC
Active metal/aqueous electrochemical cells and systems
InactiveUS7645543B2Degree of flexibilityWithout performanceFuel and primary cellsAlkaline accumulatorsElectrochemical cellBattery cell
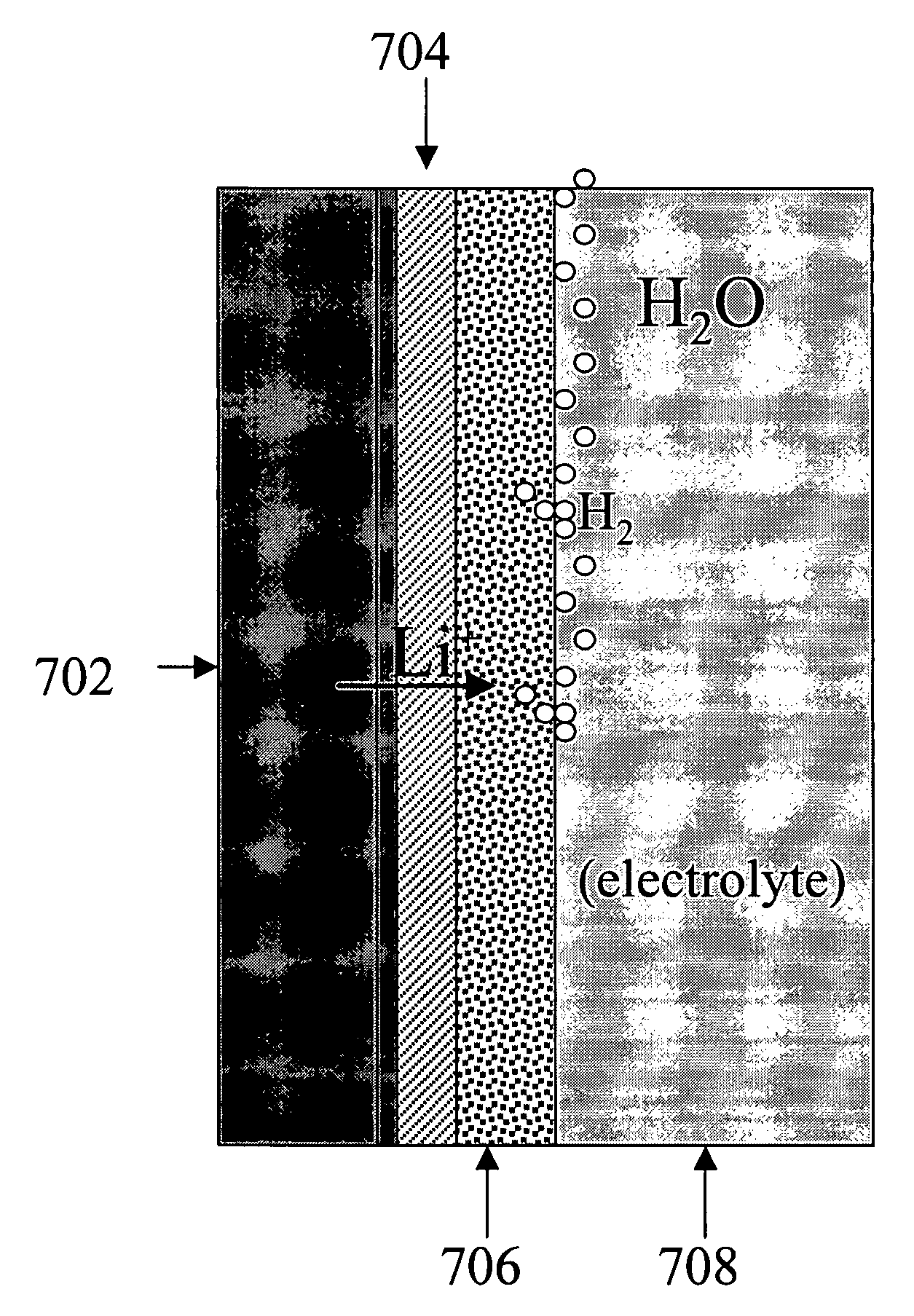
Alkali (or other active) metal battery and other electrochemical cells incorporating active metal anodes together with aqueous cathode / electrolyte systems. The battery cells have a highly ionically conductive protective membrane adjacent to the alkali metal anode that effectively isolates (de-couples) the alkali metal electrode from solvent, electrolyte processing and / or cathode environments, and at the same time allows ion transport in and out of these environments. Isolation of the anode from other components of a battery cell or other electrochemical cell in this way allows the use of virtually any solvent, electrolyte and / or cathode material in conjunction with the anode. Also, optimization of electrolytes or cathode-side solvent systems may be done without impacting anode stability or performance. In particular, Li / water, Li / air and Li / metal hydride cells, components, configurations and fabrication techniques are provided.
Owner:POLYPLUS BATTERY CO INC
Electrode structure and method for making the same
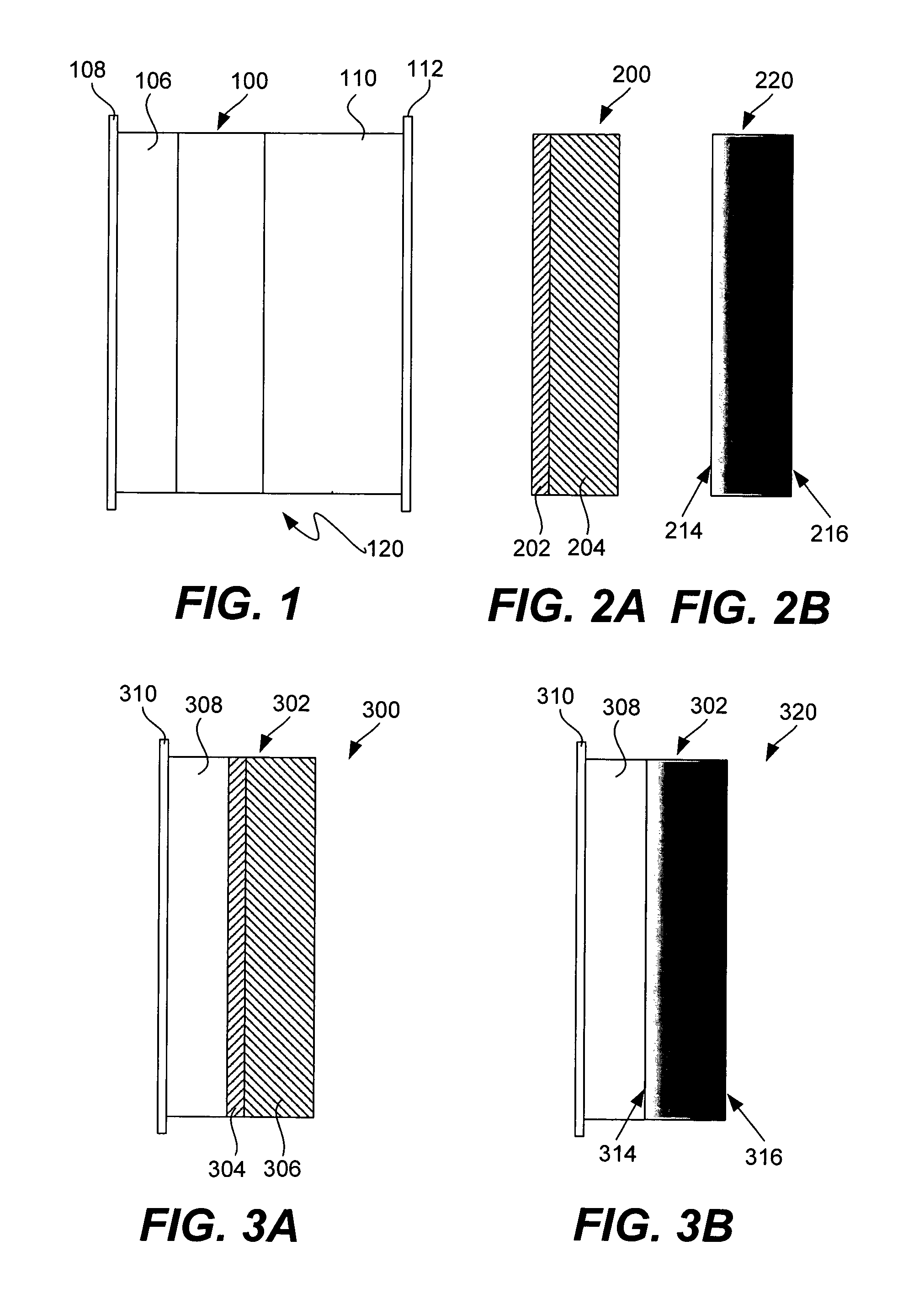
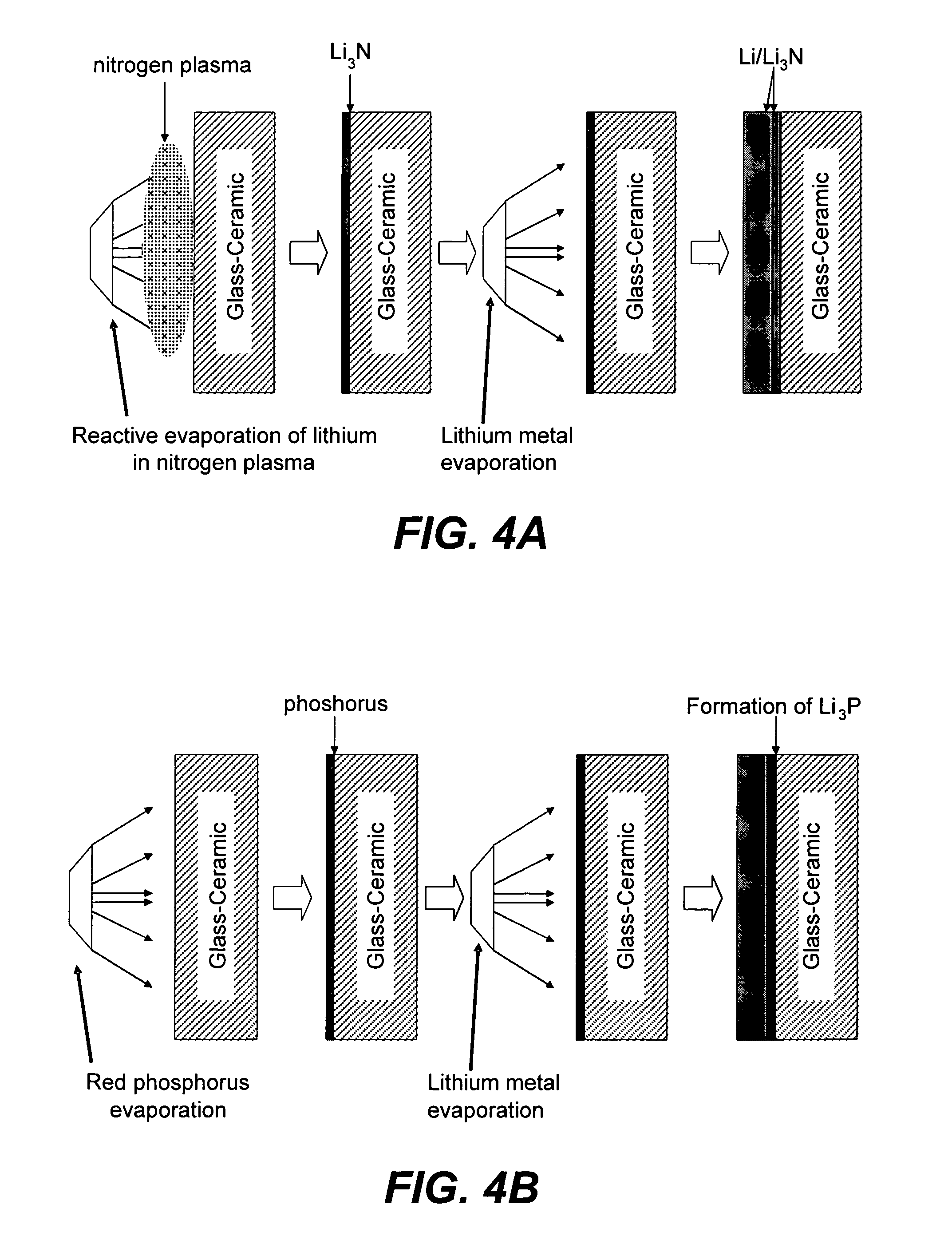
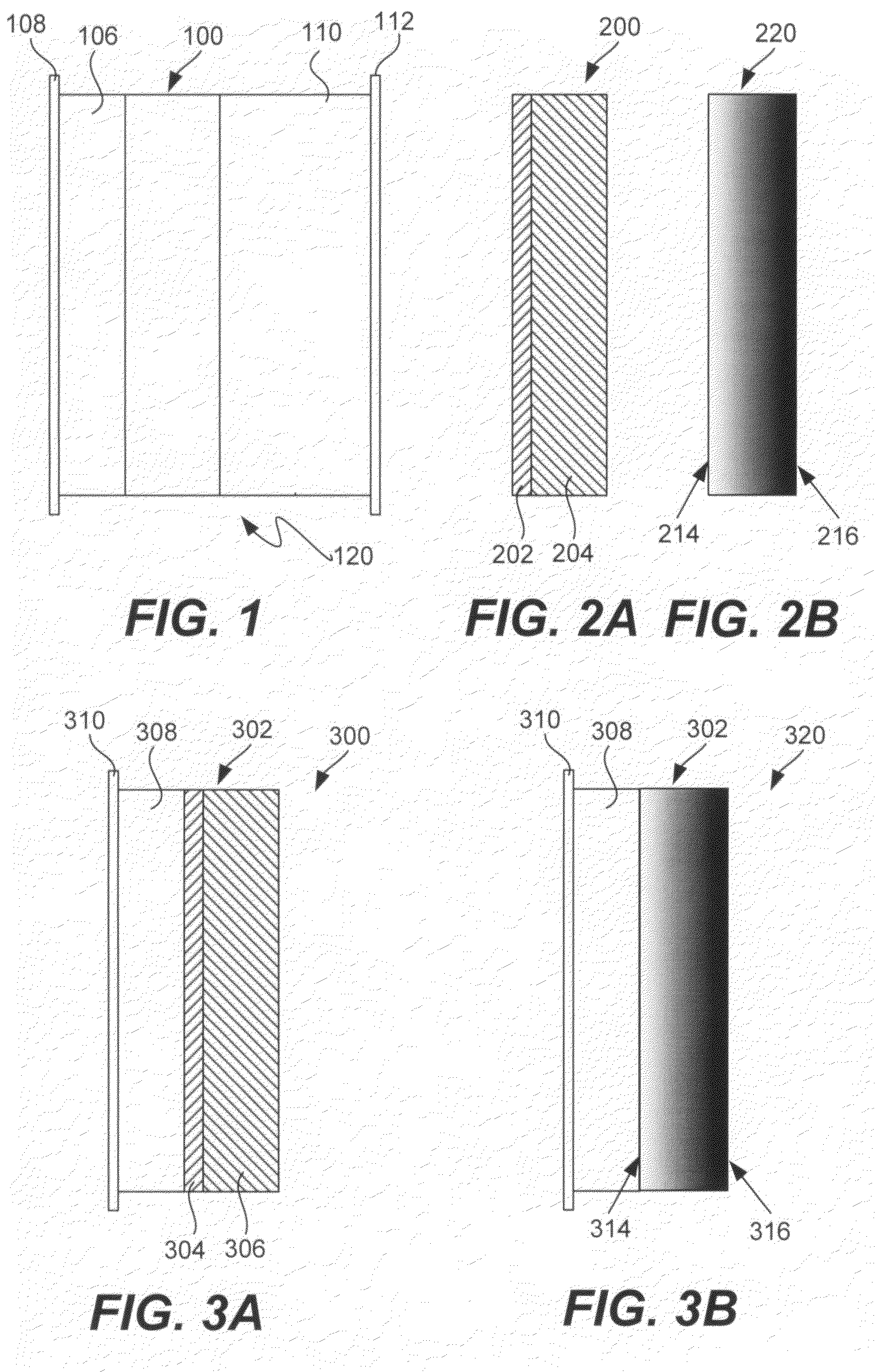
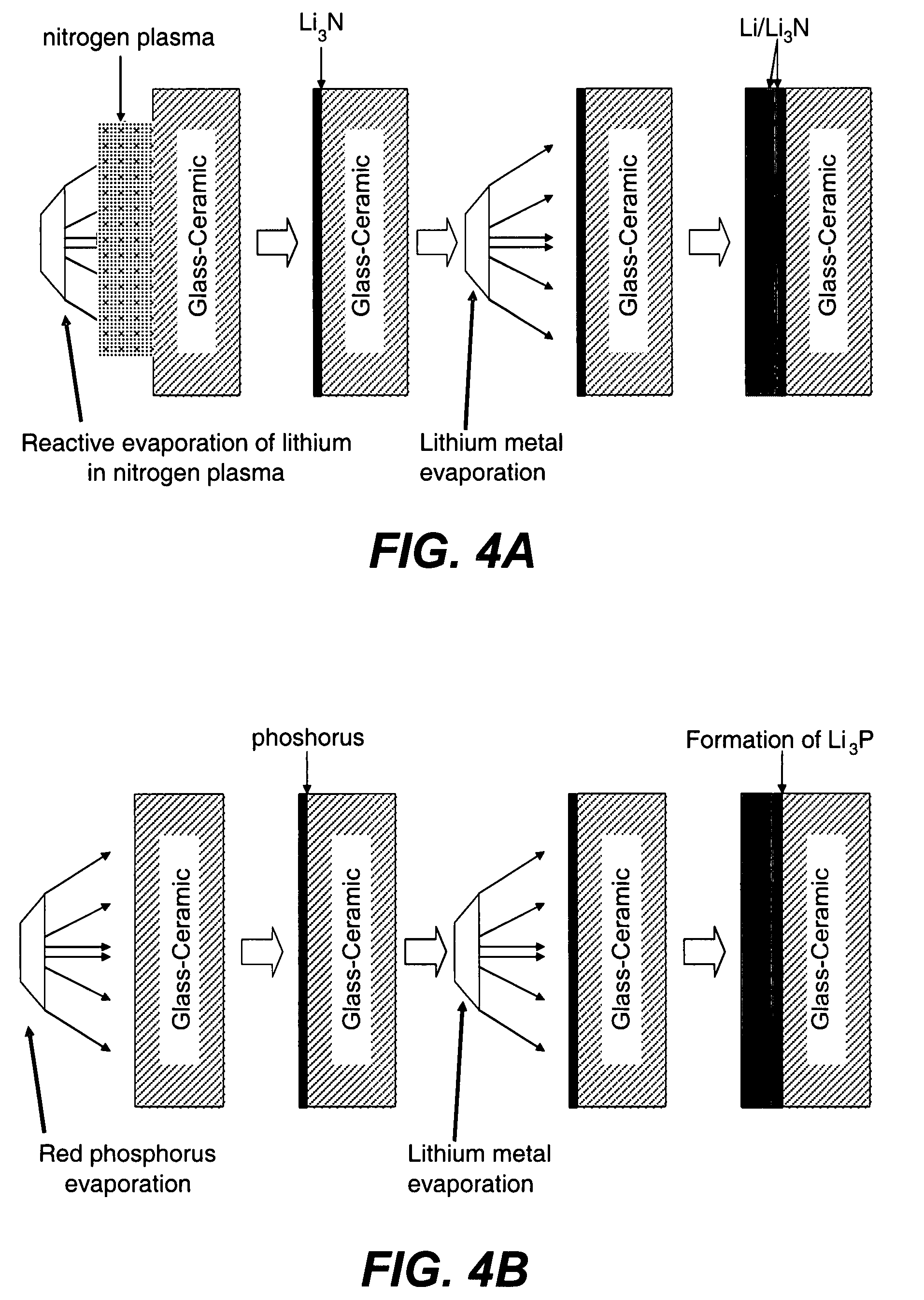
Electrode structures, and more specifically, electrode structures for use in electrochemical cells, are provided. The electrode structures described herein may include one or more protective layers. In one set of embodiments, a protective layer may be formed by exposing a lithium metal surface to a plasma comprising ions of a gas to form a ceramic layer on top of the lithium metal. The ceramic layer may be highly conductive to lithium ions and may protect the underlying lithium metal surface from reaction with components in the electrolyte. In some cases, the ions may be nitrogen ions and a lithium nitride layer may be formed on the lithium metal surface. In other embodiments, the protective layer may be formed by converting lithium to lithium nitride at high pressures. Other methods for forming protective layers are also provided.
Owner:SION POWER CORP
Hydrophilic polymer treatment of an activated polymeric material and use thereof
InactiveUS6830782B2Simple materialImprove nucleationRadiation applicationsFibre typesFiberMicroorganism
A method of modifying a polymeric material which comprises the steps of activation-treatment and a hydrophilic polymer-treatment, or comprises the steps of activation-treatment, a hydrophilic polymer-treatment, and monomer grafting in this order, or comprises the step of a solvent-treatment followed by these steps. Thus, the polymeric material, e.g., polyolefin, is improved in hydrophilicity, adhesion, etc. without lowering the practical strength thereof. The polymeric material thus improved in adhesion and other properties can be used in many applications where water absorption and adhesion are required, such as an absorption material, e.g., a wiping / cleansing material, a water retention material, a material for microorganism culture media, a separator for batteries (or cells), a synthetic paper, a filter medium, a textile product for clothing, a medical / sanitary / cosmetic supply, and reinforcing fibers for composite materials.
Owner:KB INT
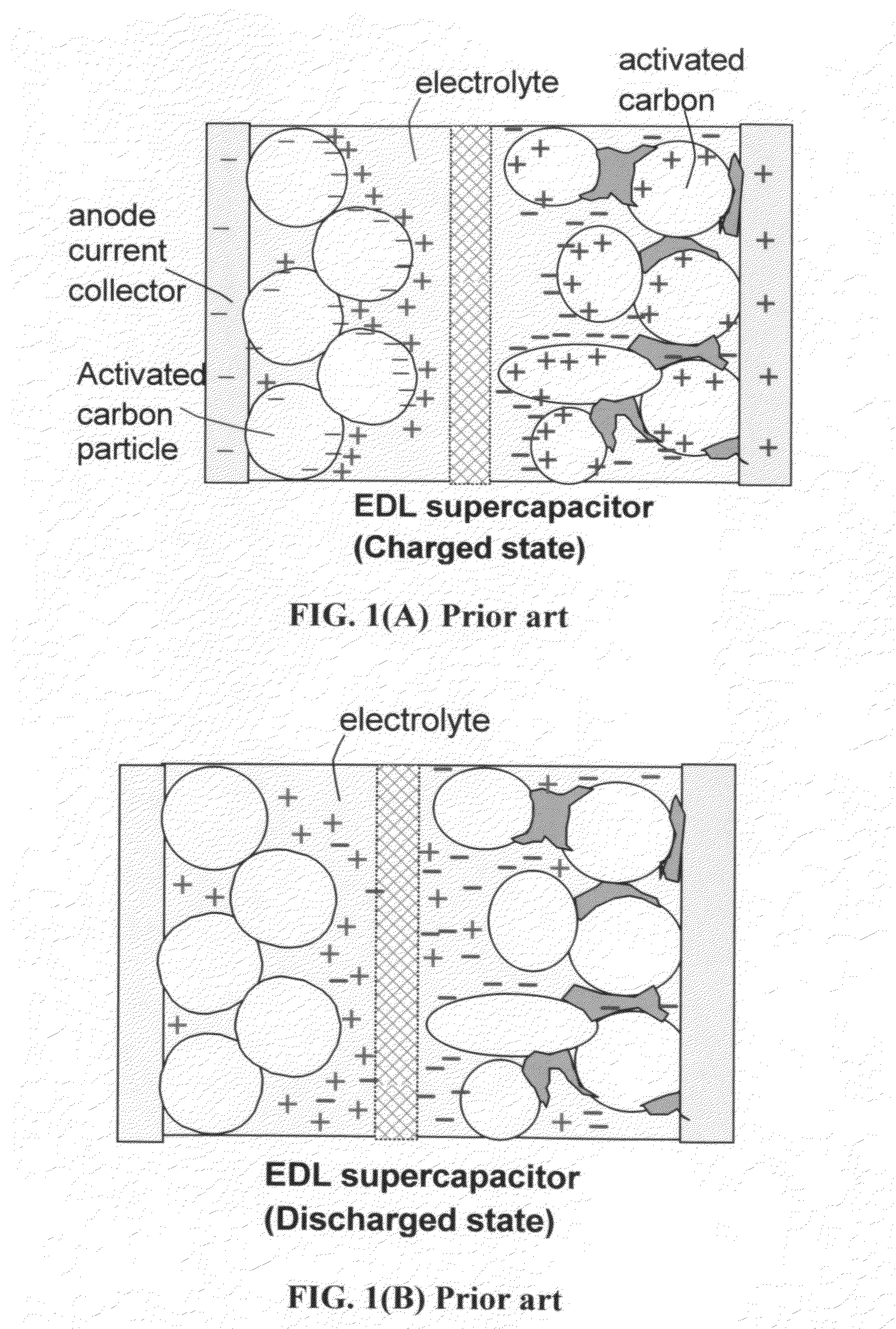
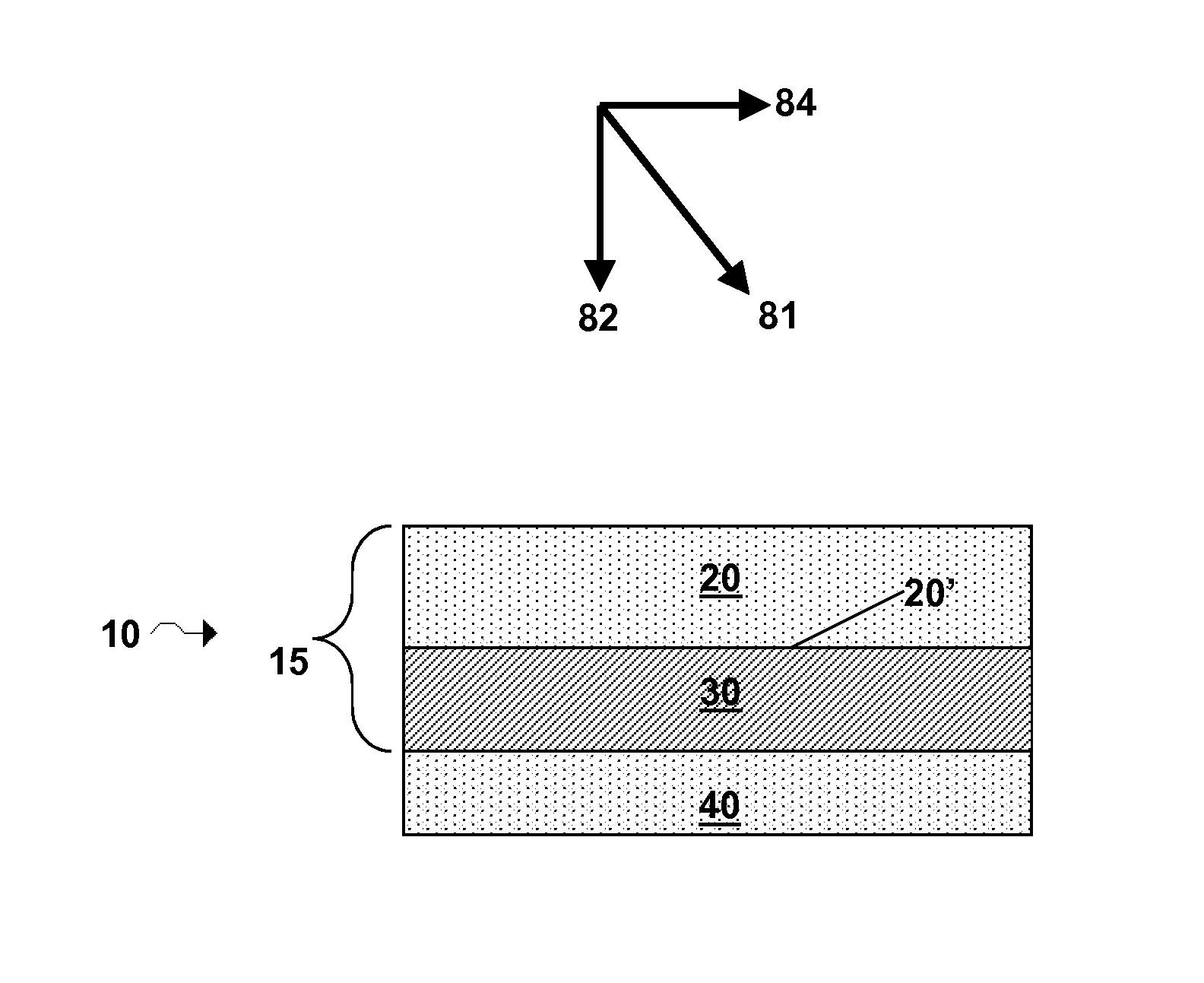
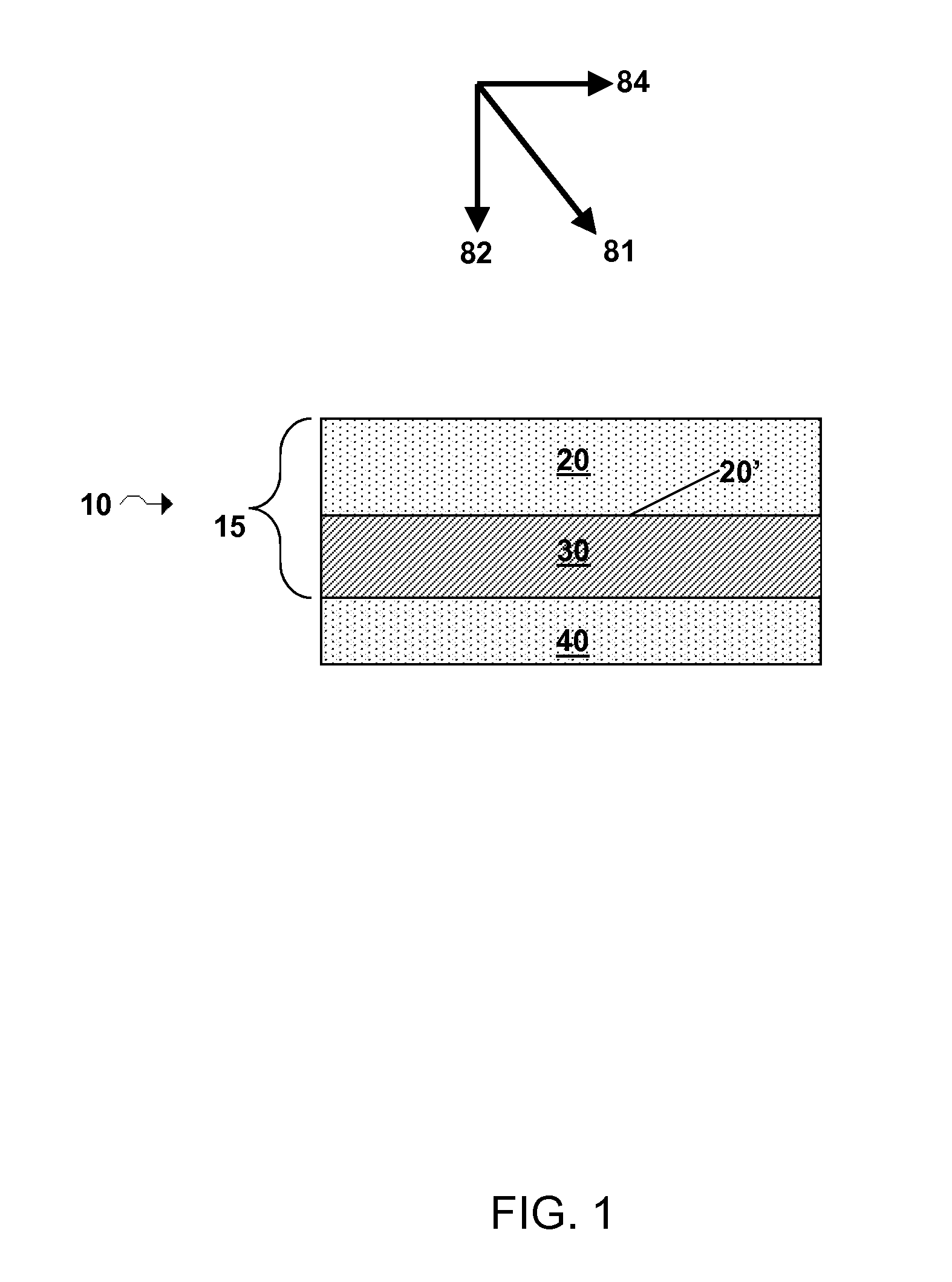
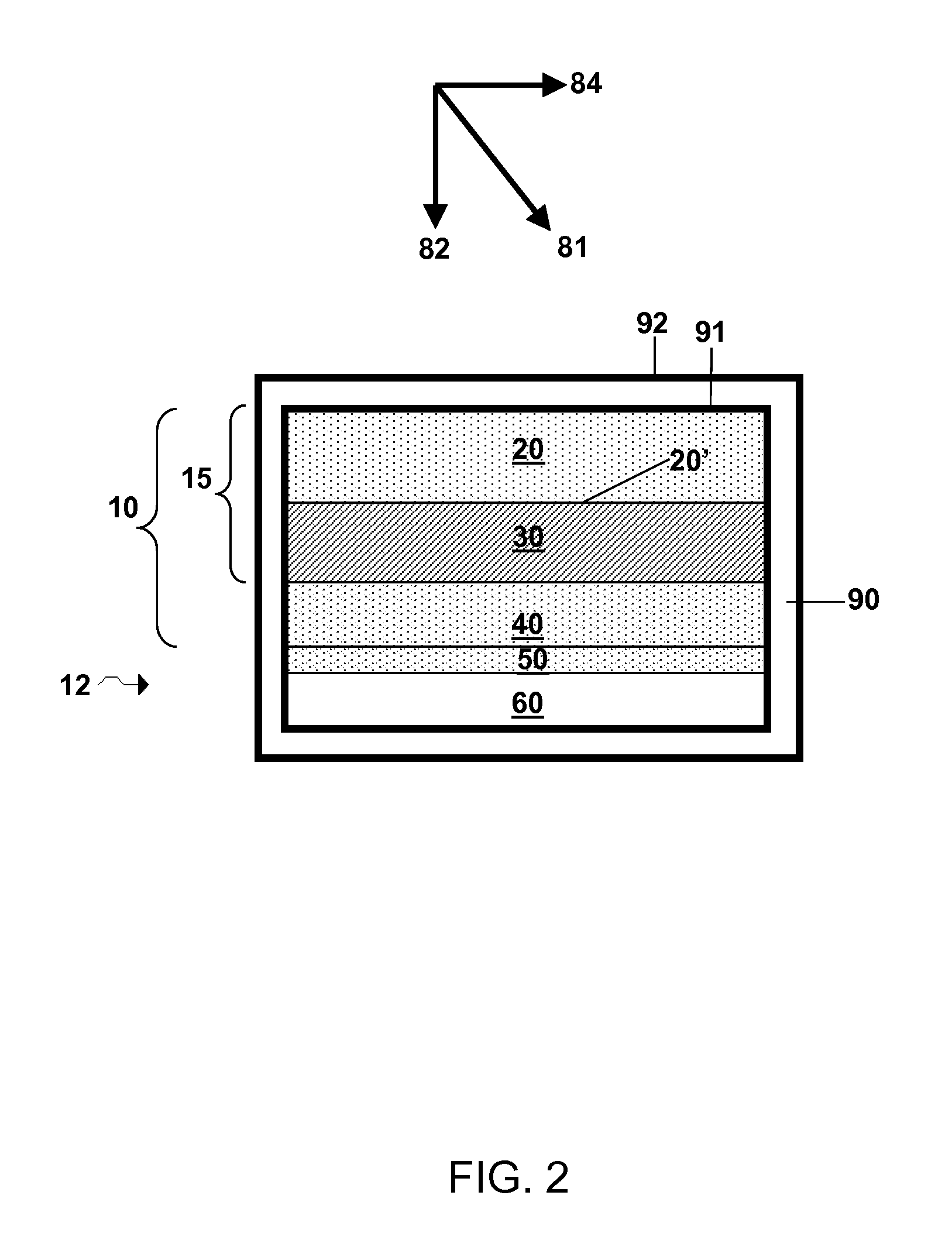
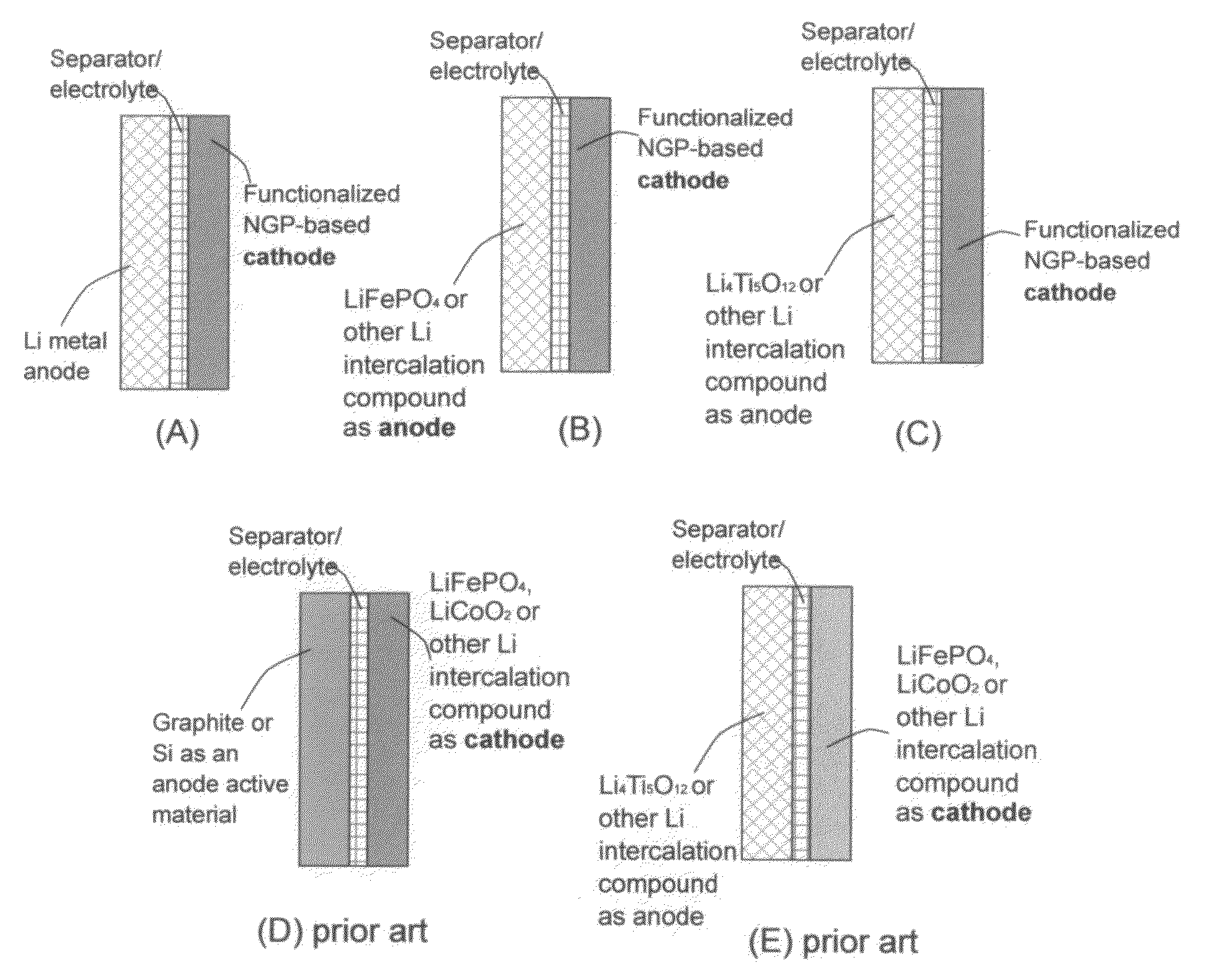
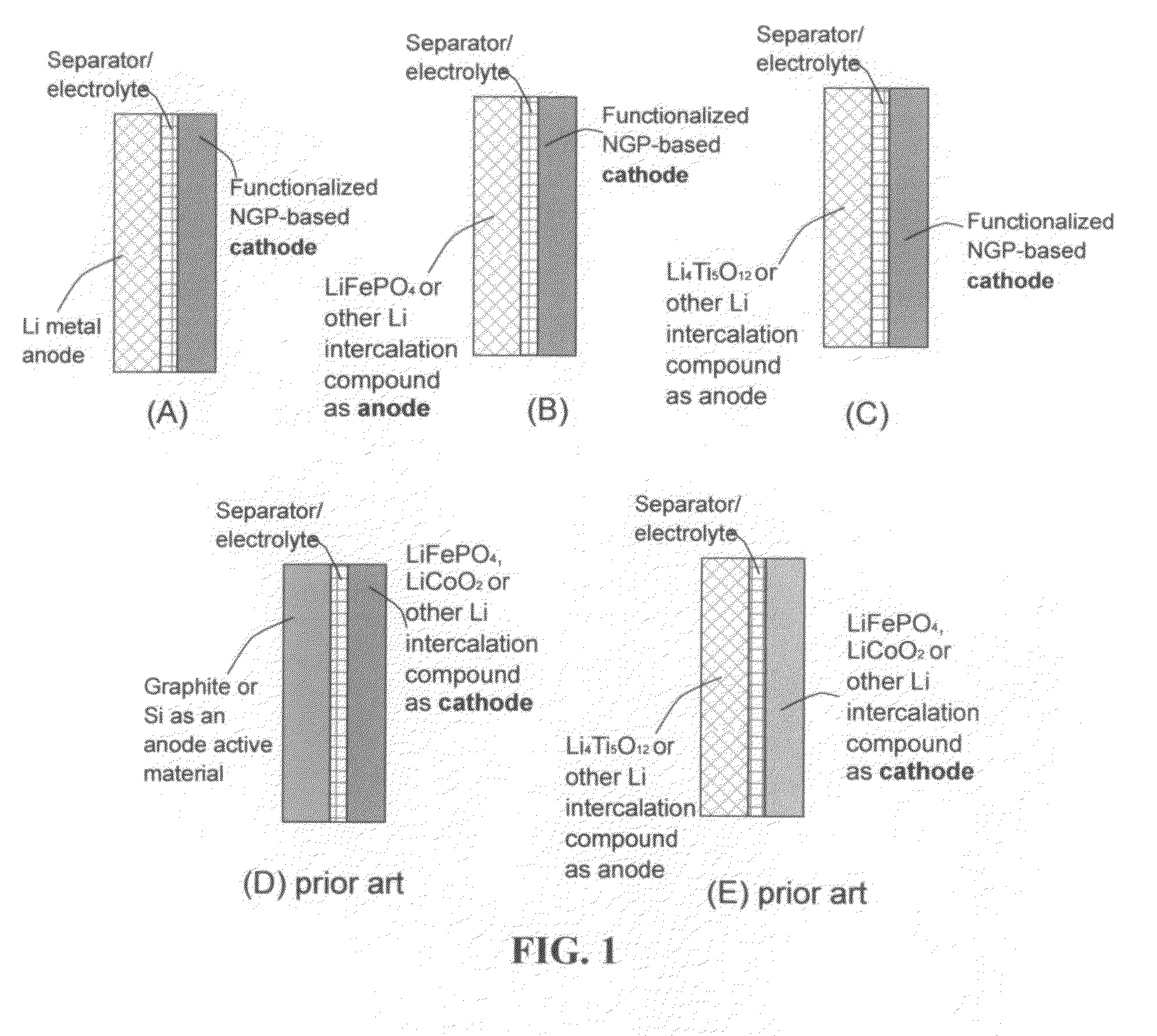
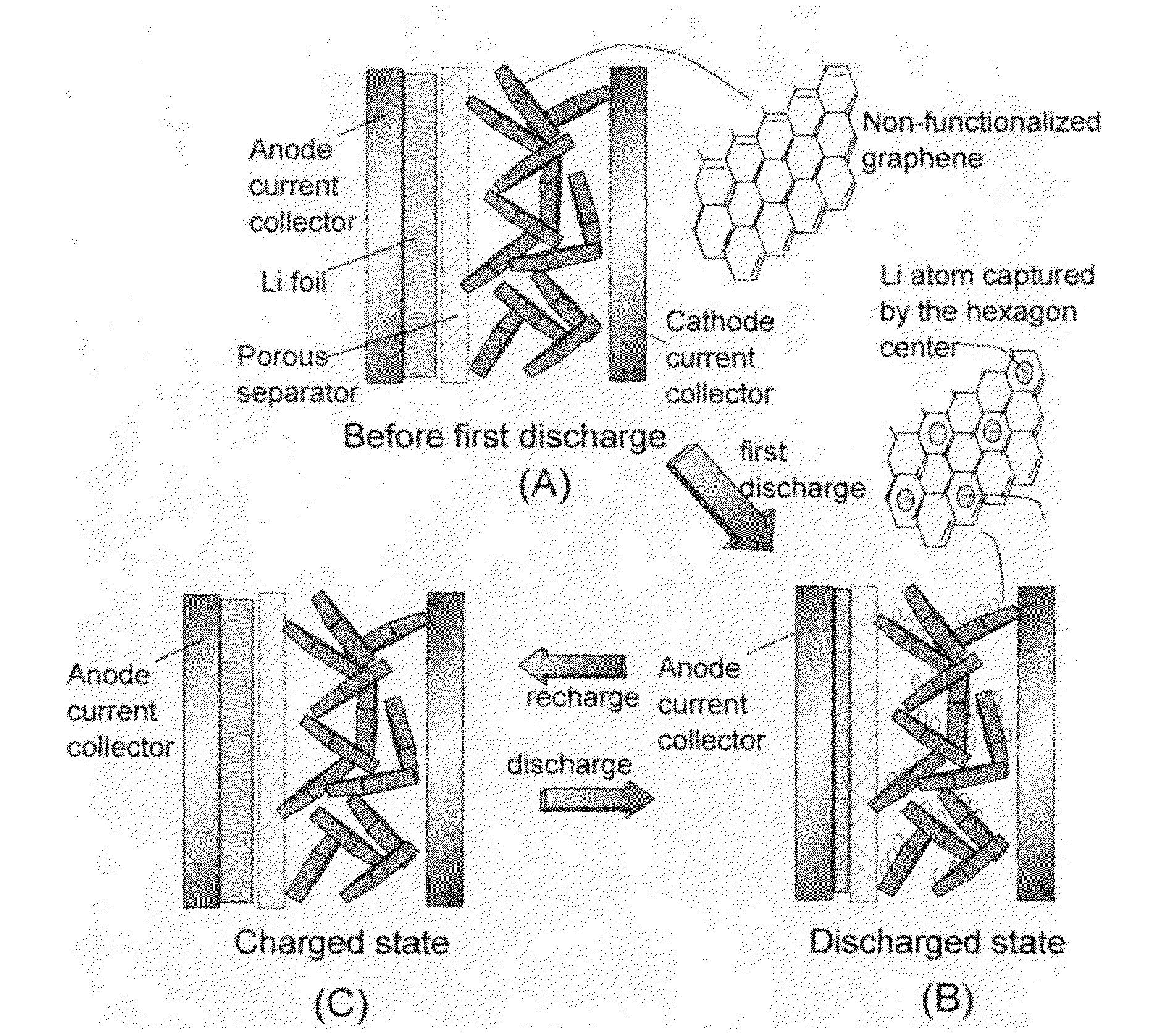
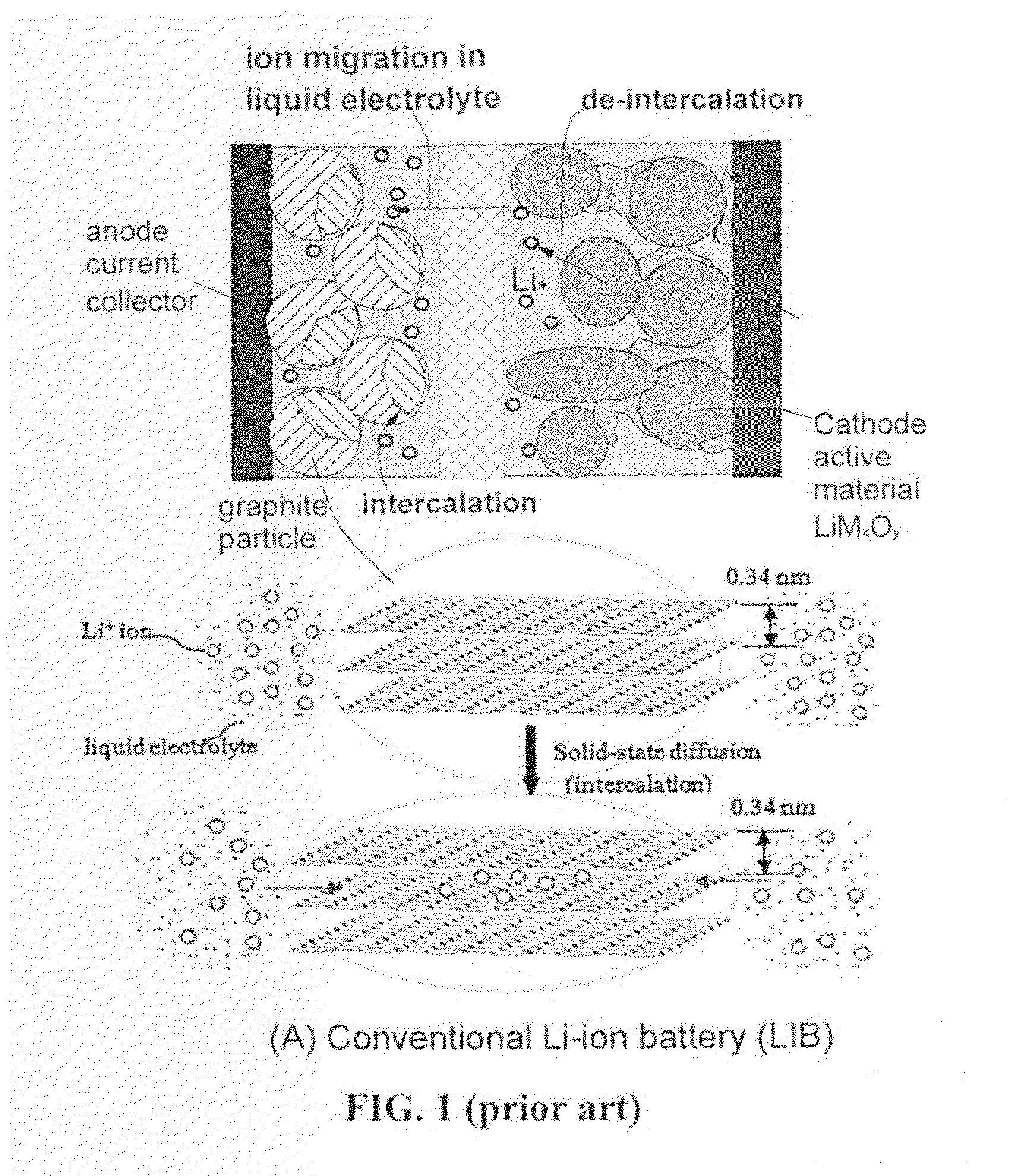
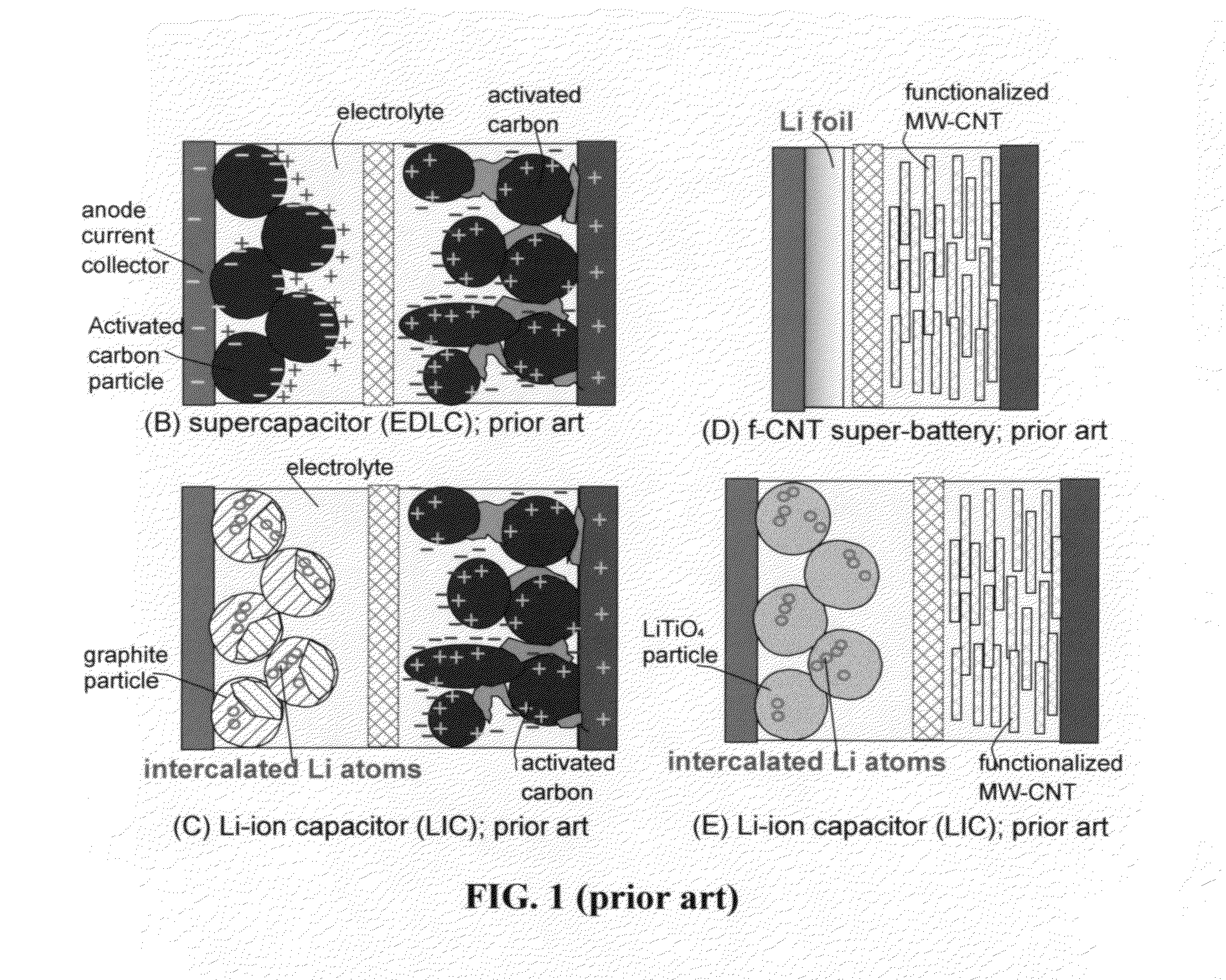
Lithium super-battery with a functionalized nano graphene cathode
An electrochemical energy storage device, lithium super-battery, comprising a positive electrode, a negative electrode, a porous separator disposed between the two electrodes, and a lithium-containing electrolyte in physical contact with the two electrodes, wherein the positive electrode comprises a plurality of chemically functionalized nano graphene platelets (f-NGP) or exfoliated graphite having a functional group that reversibly reacts with a lithium atom or ion. In a preferred embodiment, a lithium super-battery having a f-NGP positive electrode and Li4Ti5O12 negative electrode exhibits a gravimetric energy ˜5 times higher than conventional supercapacitors and a power density ˜10 times higher than conventional lithium-ion batteries. This device has the best properties of both the lithium ion battery and the supercapacitor.
Owner:GLOBAL GRAPHENE GRP INC +1
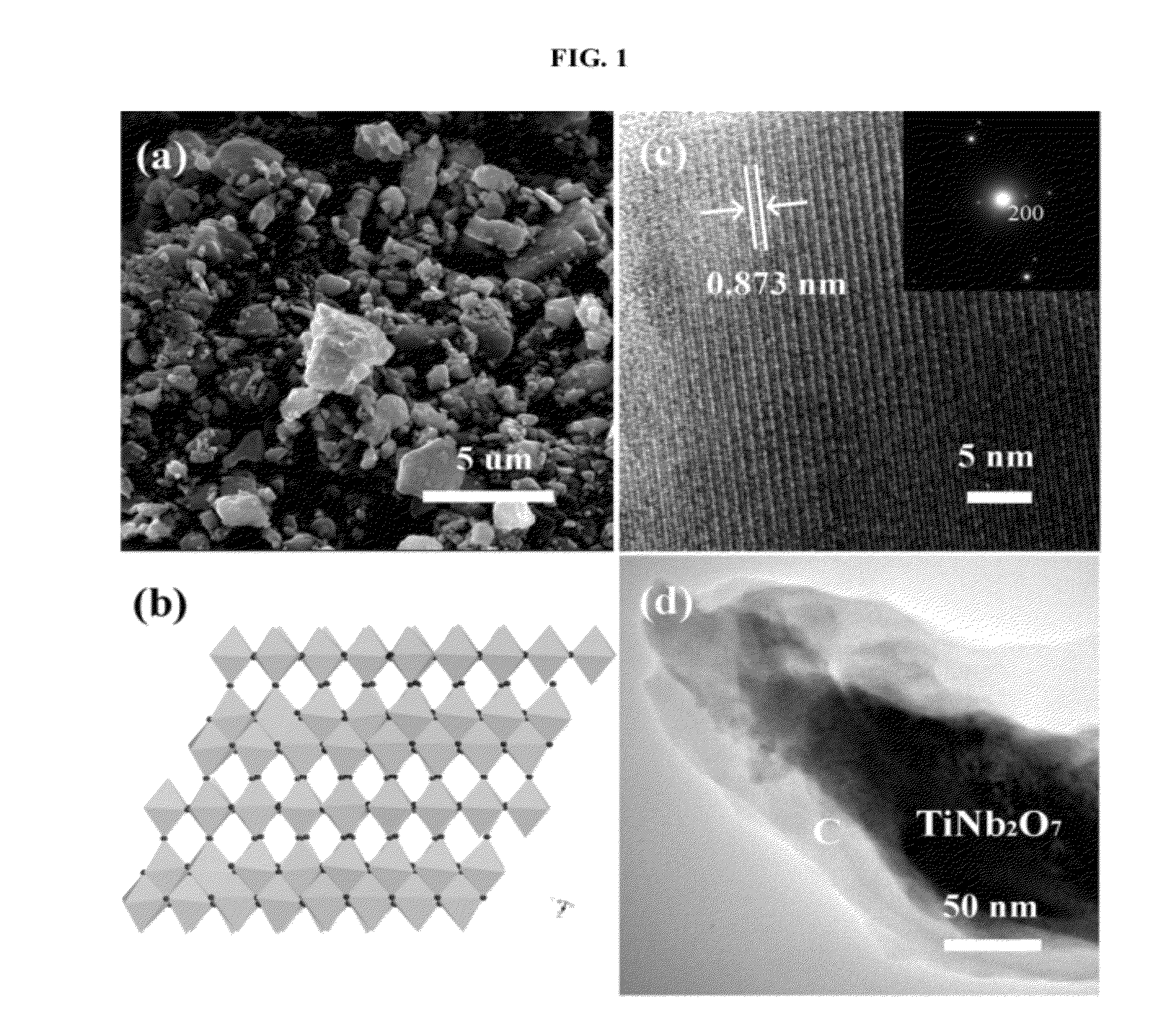
Niobium Oxide Compositions and Methods for Using Same
ActiveUS20120052401A1Alkaline accumulatorsElectric discharge heatingLithium-ion batteryCarbon coated
The disclosure relates a niobium oxide useful in anodes of secondary lithium ion batteries. Such niobium oxide has formula LixM1−yNbyNb2O7, wherein 0≦x≦3, 0≦y≦1, and M represents Ti or Zr. The niobium oxide may be in the form of particles, which may be carbon coated. The disclosure also relates to an electrode composition containing at least one or more niobium oxides of formula LixM1−yNbyNb2O7. The disclosure further relates to electrodes, such as anodes, and batteries containing at least one or more niobium oxides of formula LixM1−yNbyNb2O7. Furthermore, the disclosure relates to methods of forming the above.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Gradient cathode material for lithium rechargeable batteries
InactiveUS6921609B2Increase capacityImproved cyclabilityAluminium compoundsAlkaline accumulatorsManganesePotassium
A composition suitable for use as a cathode material of a lithium battery includes a core material having an empirical formula LixM′zNi1−yM″yO2. “x” is equal to or greater than about 0.1 and equal to or less than about 1.3. “y” is greater than about 0.0 and equal to or less than about 0.5. “z” is greater than about 0.0 and equal to or less than about 0.2. M′ is at least one member of the group consisting of sodium, potassium, nickel, calcium, magnesium and strontium. M″ is at least one member of the group consisting of cobalt, iron, manganese, chromium, vanadium, titanium, magnesium, silicon, boron, aluminum and gallium. A coating on the core has a greater ratio of cobalt to nickel than the core. The coating and, optionally, the core can be a material having an empirical formula Lix1Ax2Ni1−y1−z1Coy1Bz1Oa. “x1” is greater than about 0.1 a equal to or less than about 1.3. “x2,”“y1” and “z1” each is greater than about 0.0 and equal to or less than about 0.2. “a” is greater than 1.5 and less than about 2.1. “A” is at least one element selected from the group consisting of barium, magnesium, calcium and strontium. “B” is at least one element selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium.
Owner:TIAX LLC
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Non-aqueous liquid electrolyte and non-aqueous liquid electrolyte secondary battery
InactiveUS20090325065A1Improve charging capacityMaintain good propertiesAlkaline accumulatorsElectrode manufacturing processesLithiumCarbonate
A non-aqueous liquid electrolyte secondary battery using negative-electrode active material having Si, Sn and / or Pb, with high charge-capacity, superior characteristics including discharge-capacity retention rate over long is provided. Its non-aqueous liquid electrolyte contains carbonate having unsaturated bond and / or halogen and compounds like LiPF6 and / or LiBF4 (first lithium salt) and lithium salt different from said first one, represented by formula below (second lithium salt).Lil(αmXan)
Owner:MITSUBISHI CHEM CORP
Metal halide coatings on lithium ion battery positive electrode materials and corresponding batteries
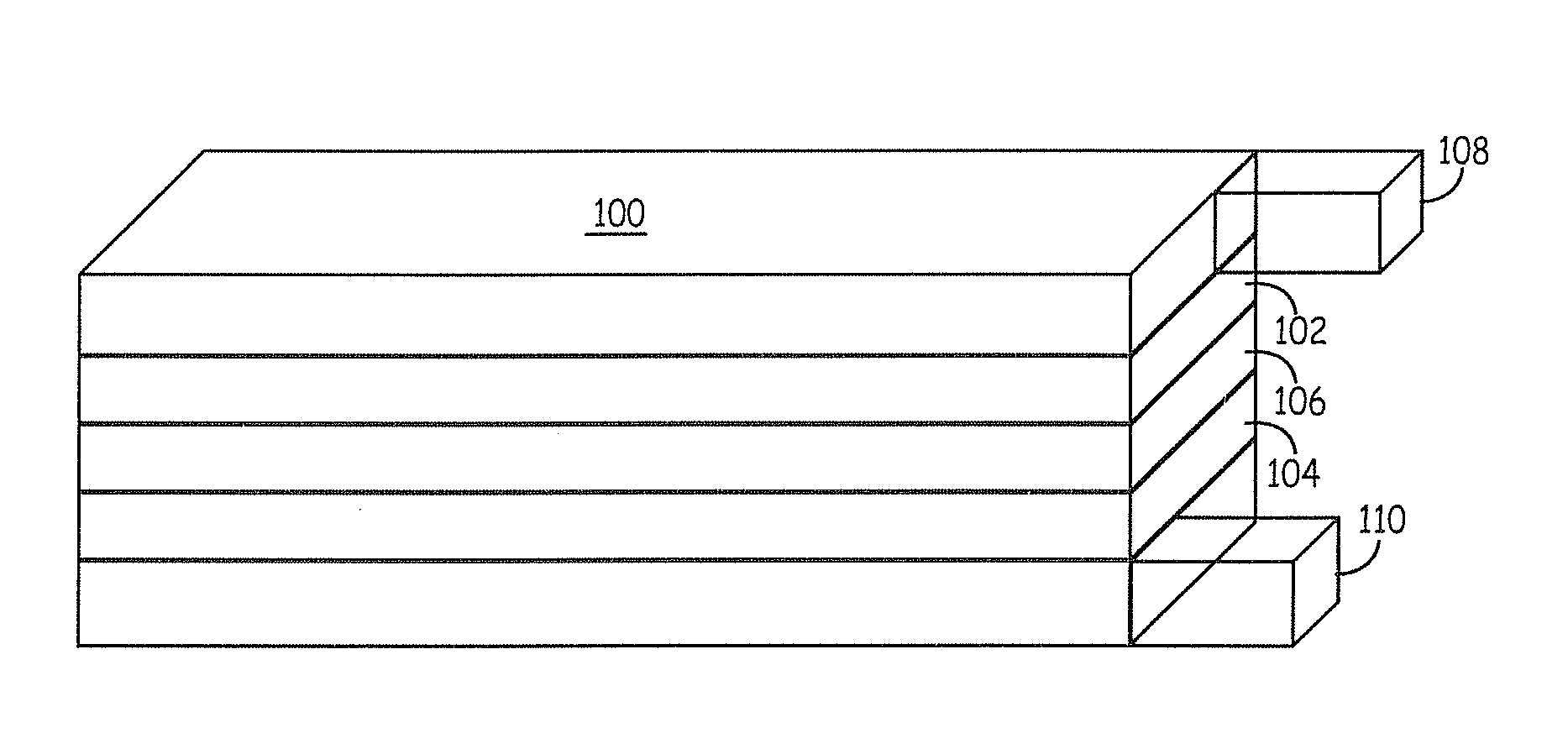
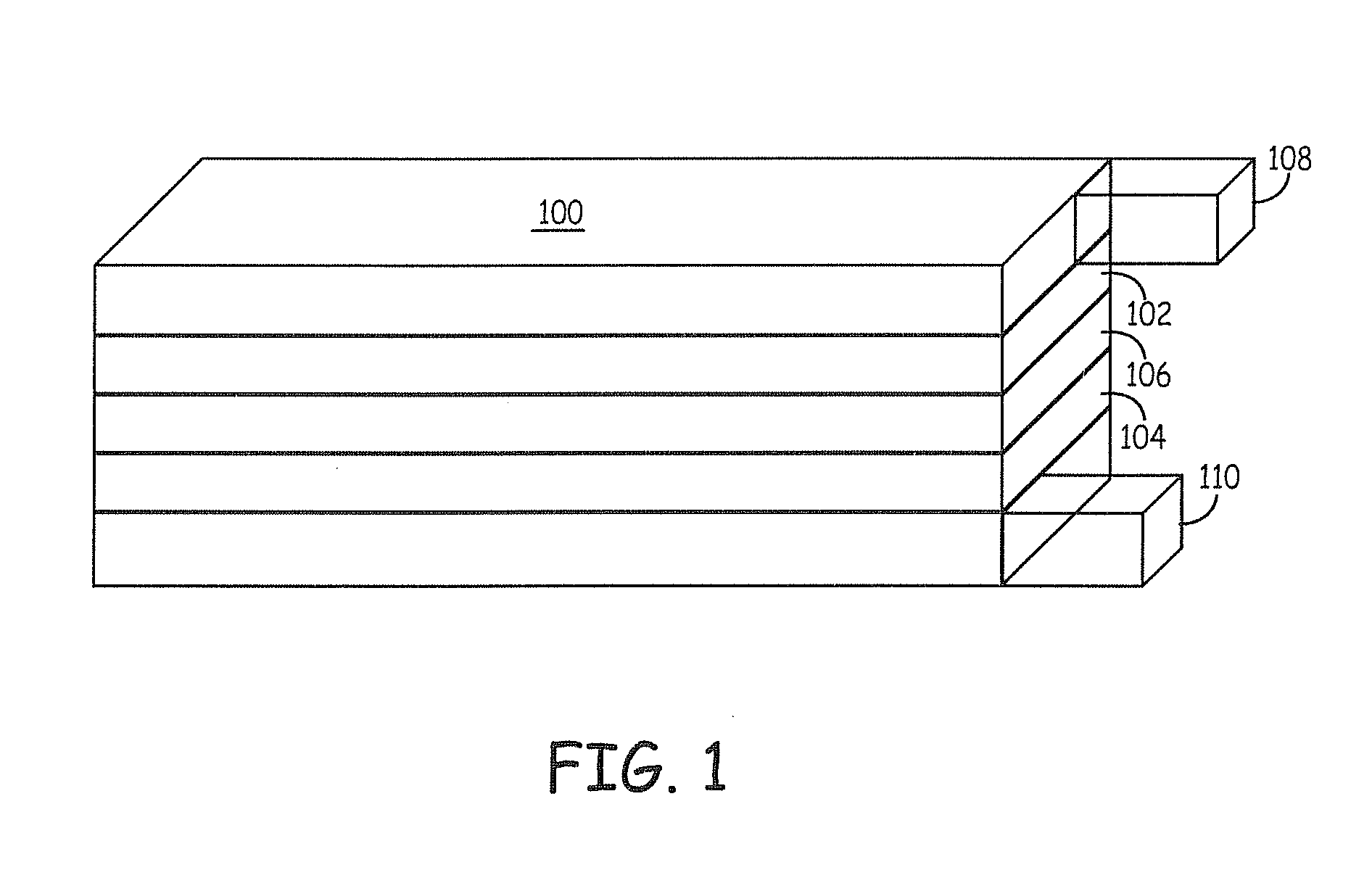
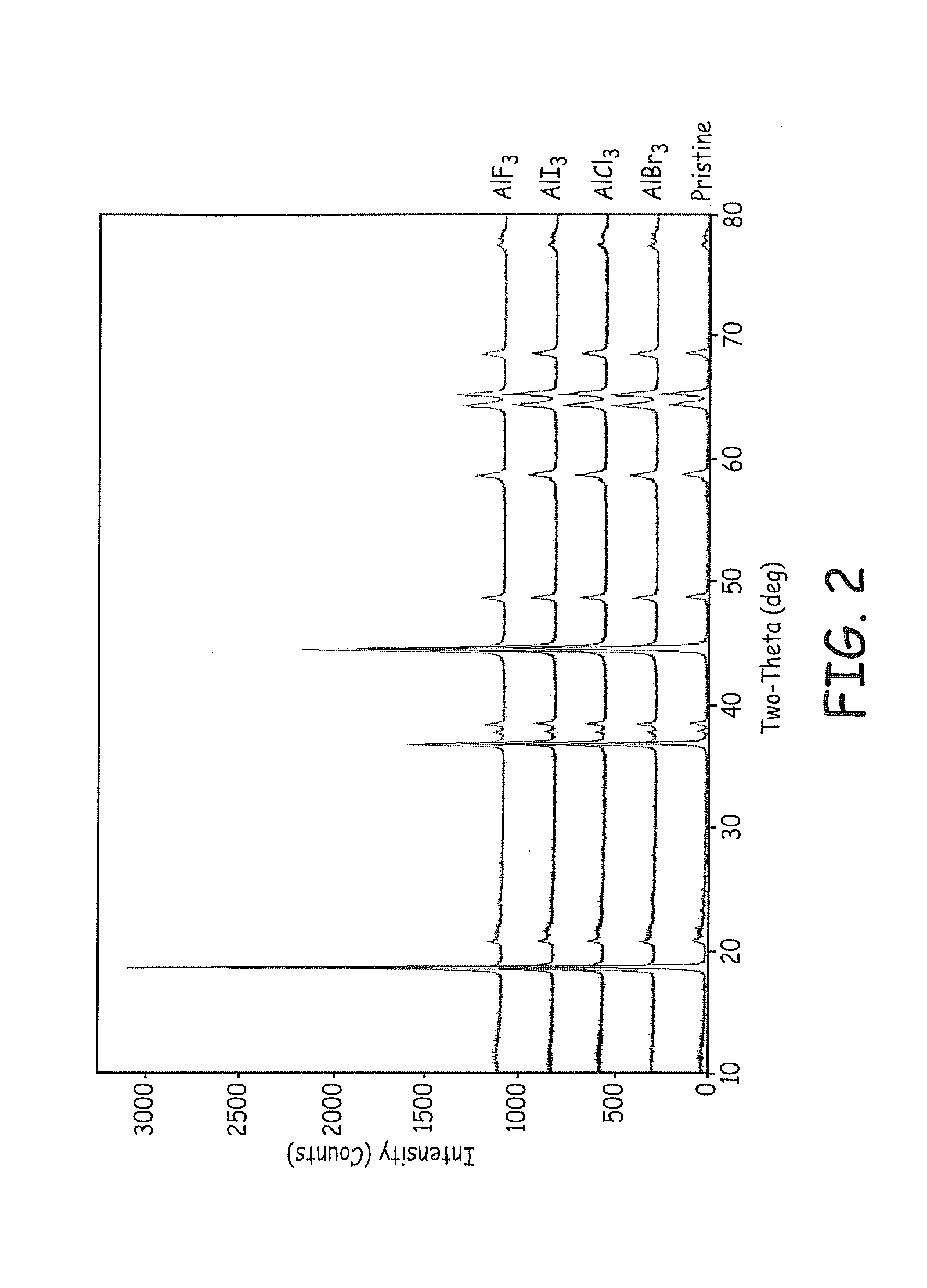
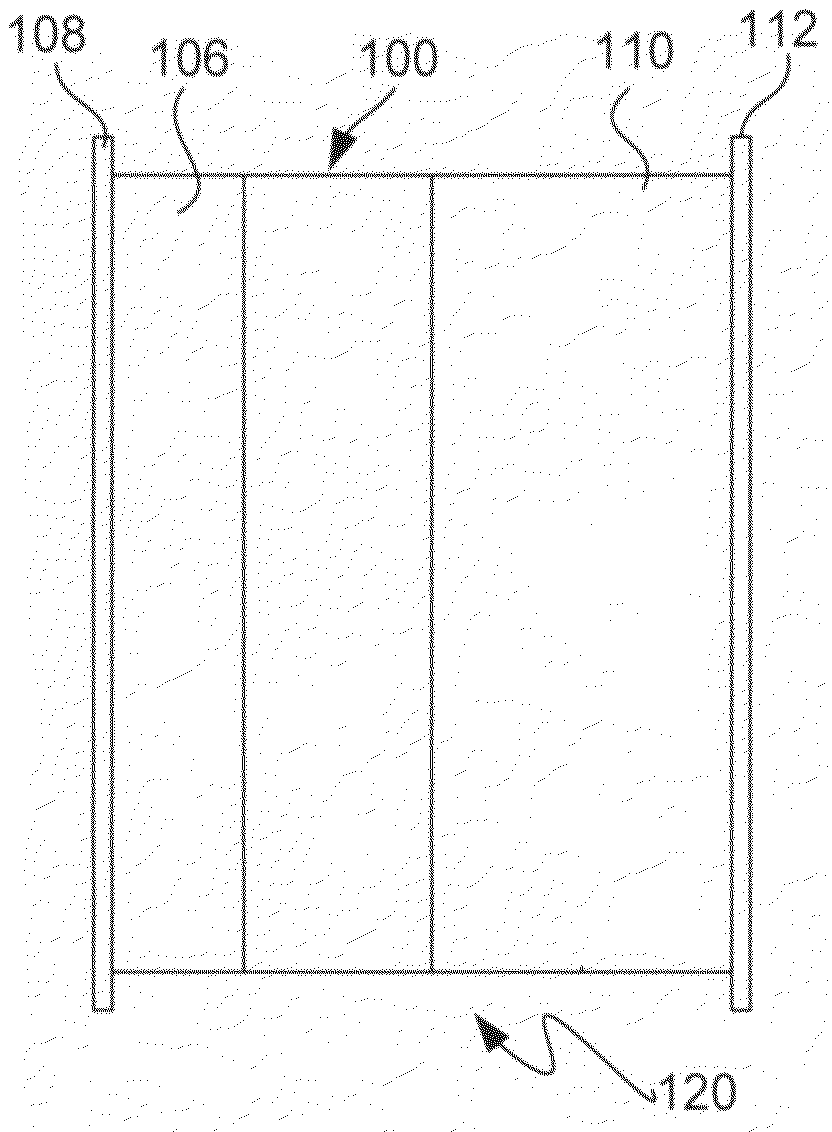
Lithium ion battery positive electrode material are described that comprise an active composition comprising lithium metal oxide coated with an inorganic coating composition wherein the coating composition comprises a metal chloride, metal bromide, metal iodide, or combinations thereof. Desirable performance is observed for these coated materials. In particular, the non-fluoride metal halide coatings are useful for stabilizing lithium rich metal oxides.
Owner:IONBLOX INC
Active metal/aqueous electrochemical cells and systems
InactiveUS7666233B2Degree of flexibilityWithout performanceFuel and primary cellsElectrode manufacturing processesManufacturing technologyElectrochemical cell
Owner:POLYPLUS BATTERY CO INC
Hybrid anode compositions for lithium ion batteries
ActiveUS8119288B2Improve conductivityLower internal resistanceAlkaline accumulatorsConductive materialPhysical chemistryHybrid material
The present invention provides an exfoliated graphite-based hybrid material composition for use as an electrode, particularly as an anode of a lithium ion battery. The composition comprises: (a) micron- or nanometer-scaled particles or coating which are capable of absorbing and desorbing alkali or alkaline metal ions (particularly, lithium ions); and (b) exfoliated graphite flakes that are substantially interconnected to form a porous, conductive graphite network comprising pores, wherein at least one of the particles or coating resides in a pore of the network or attached to a flake of the network and the exfoliated graphite amount is in the range of 5% to 90% by weight and the amount of particles or coating is in the range of 95% to 10% by weight. Also provided is a lithium secondary battery comprising such a negative electrode (anode). The battery exhibits an exceptional specific capacity, excellent reversible capacity, and long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD
Partially surface-mediated lithium ion-exchanging cells and method for operating same
ActiveUS20130059174A1Short charging timeEasy to handleHybrid capacitor electrolytesAlkaline accumulatorsIon exchangeSupercapacitor
A lithium super-battery cell comprising: (a) A cathode comprising a cathode active material having a surface area to capture or store lithium thereon, wherein the cathode active material is not a functionalized material and does not bear a functional group; (b) An anode comprising an anode current collector; (c) A porous separator disposed between the two electrodes; (d) A lithium-containing electrolyte in physical contact with the two electrodes, wherein the cathode active material has a specific surface area of no less than 100 m2 / g being in direct physical contact with the electrolyte to receive lithium ions therefrom or to provide lithium ions thereto; and (e) A lithium source implemented at one or both of the two electrodes prior to a first charge or a first discharge cycle of the cell. This new generation of energy storage device exhibits the best properties of both the lithium ion battery and the supercapacitor.
Owner:GLOBAL GRAPHENE GRP INC +1
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com