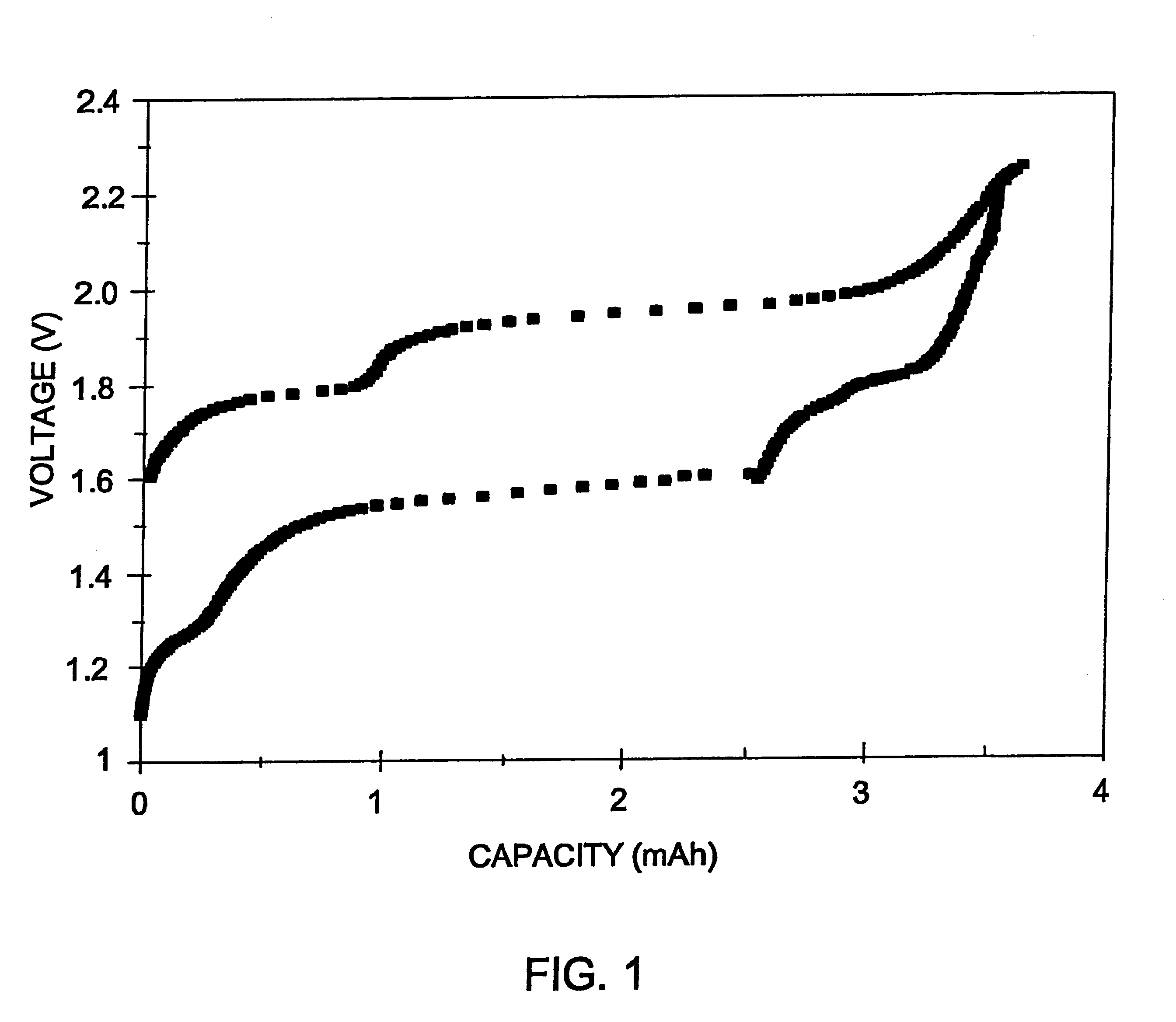
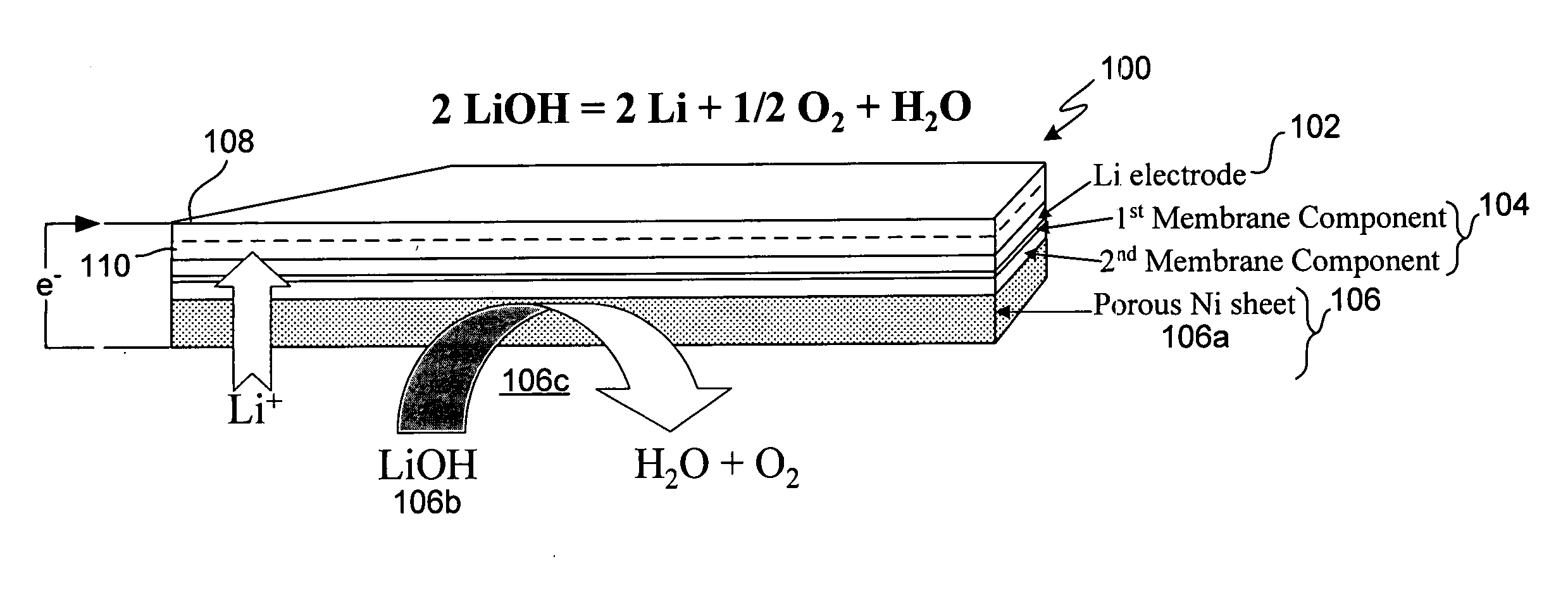
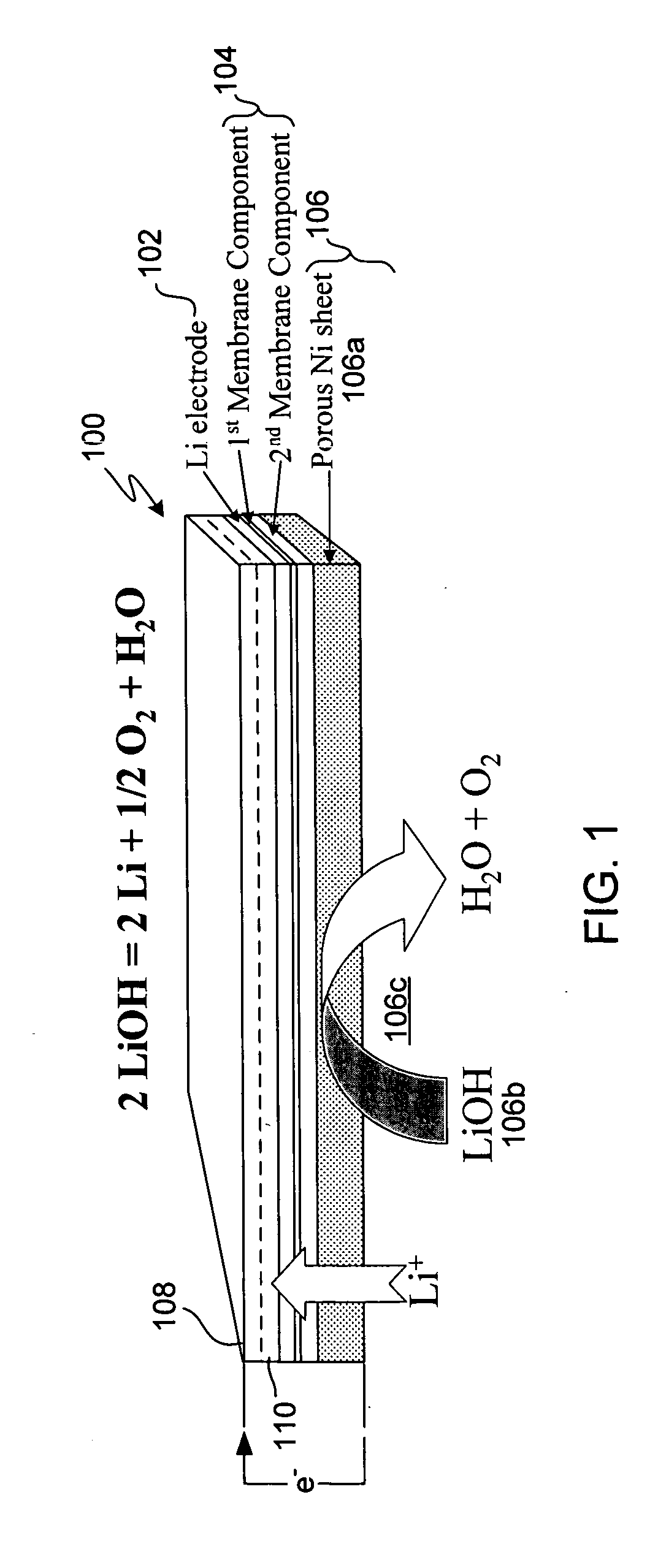
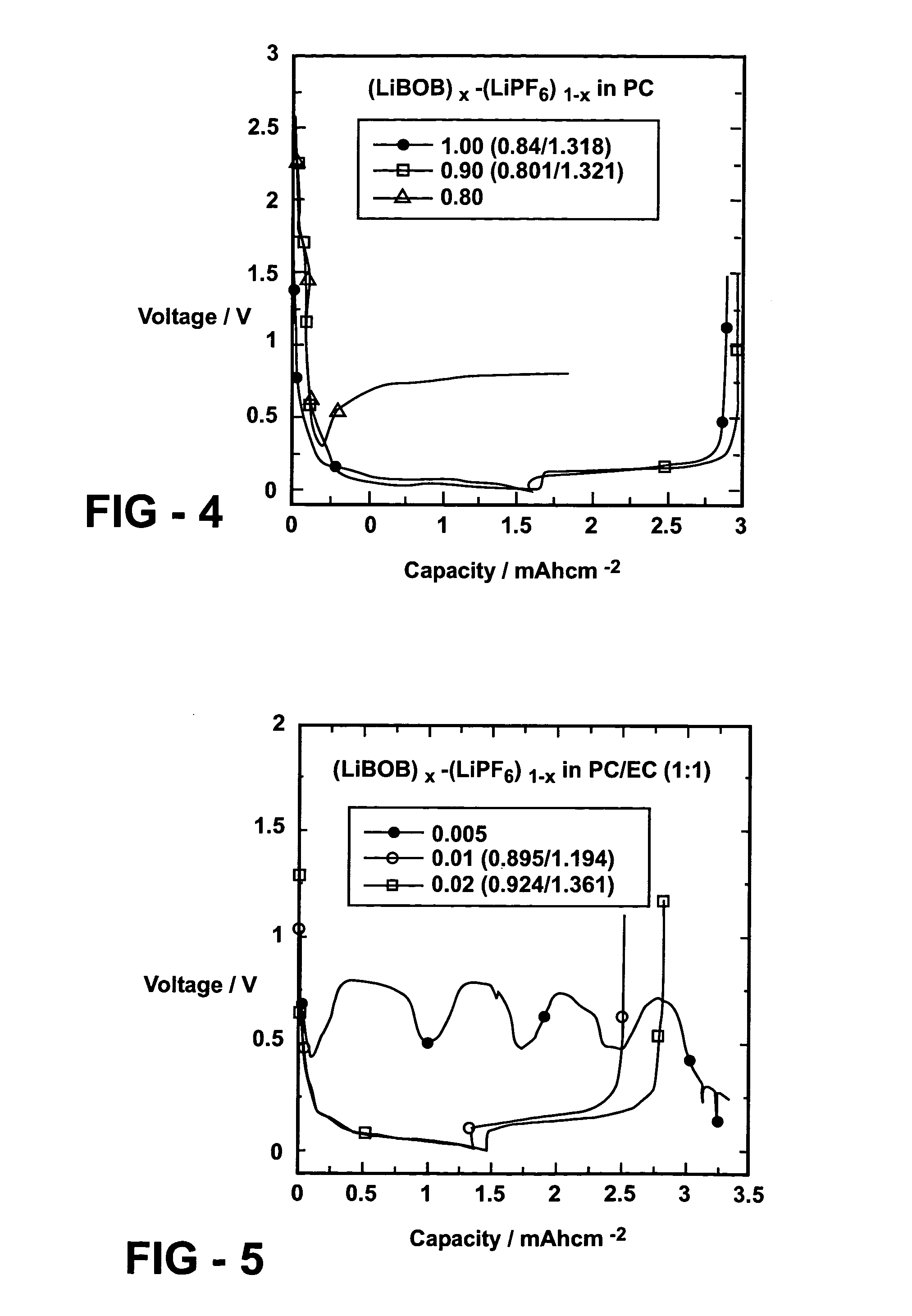
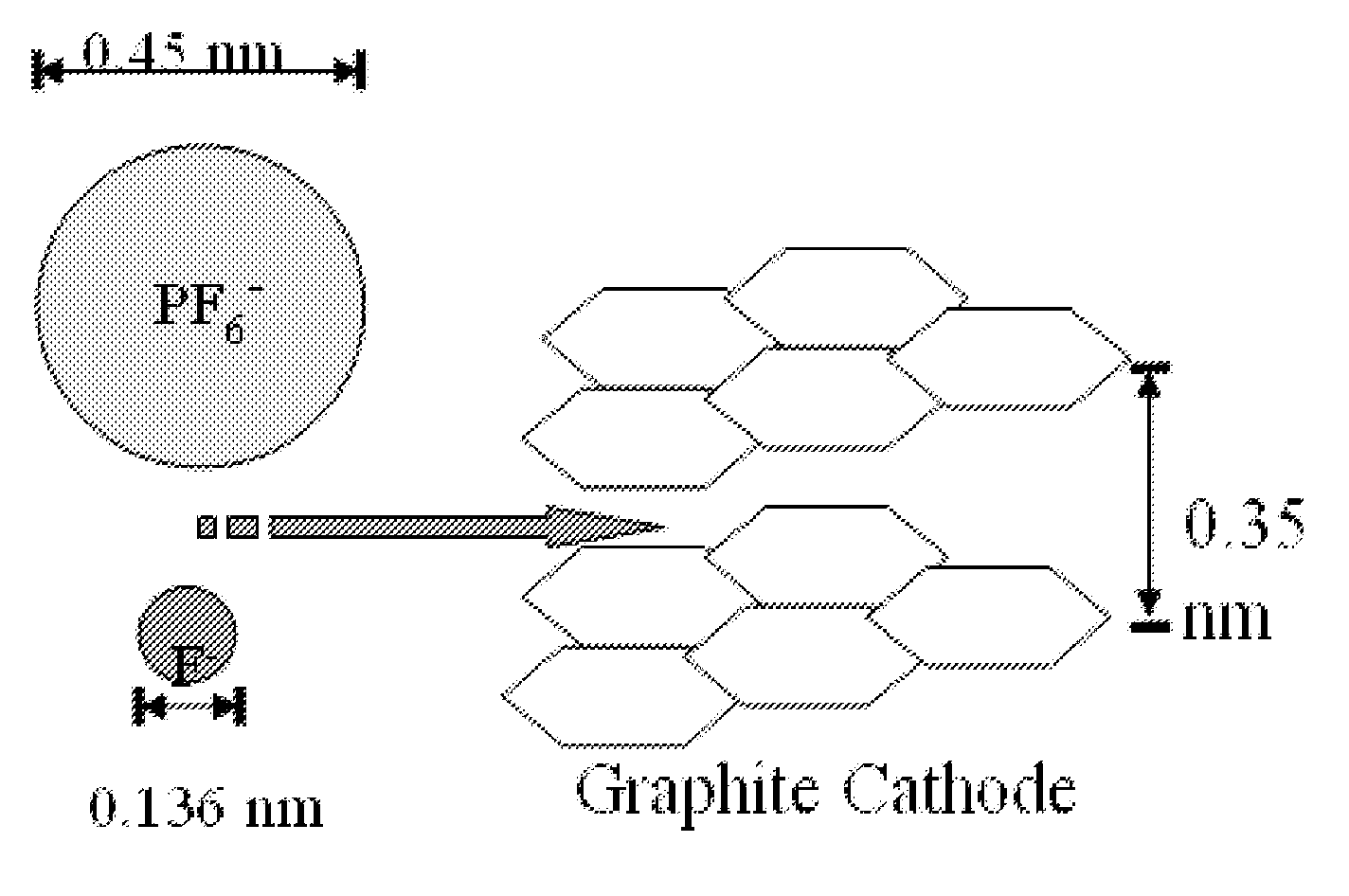
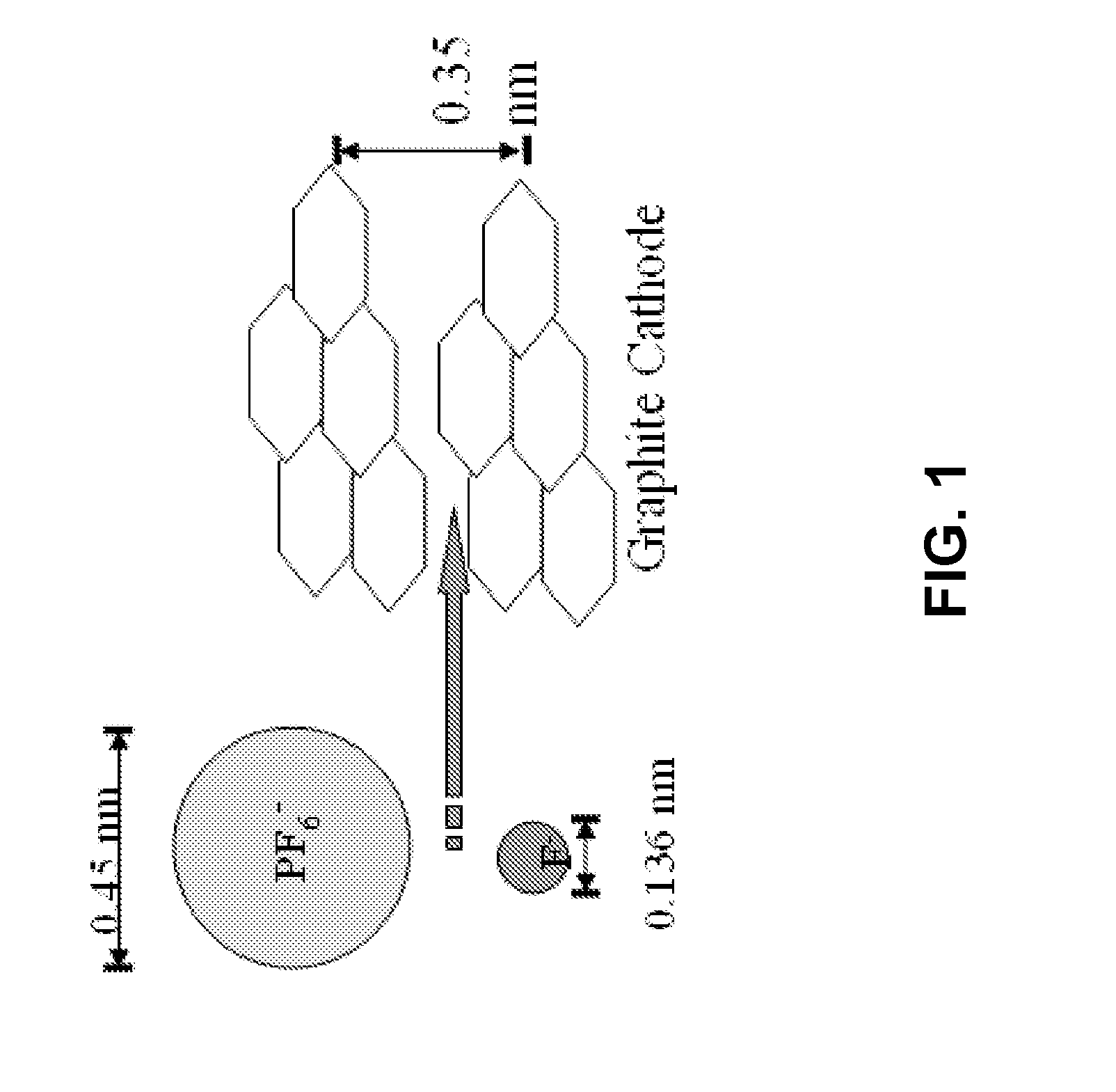
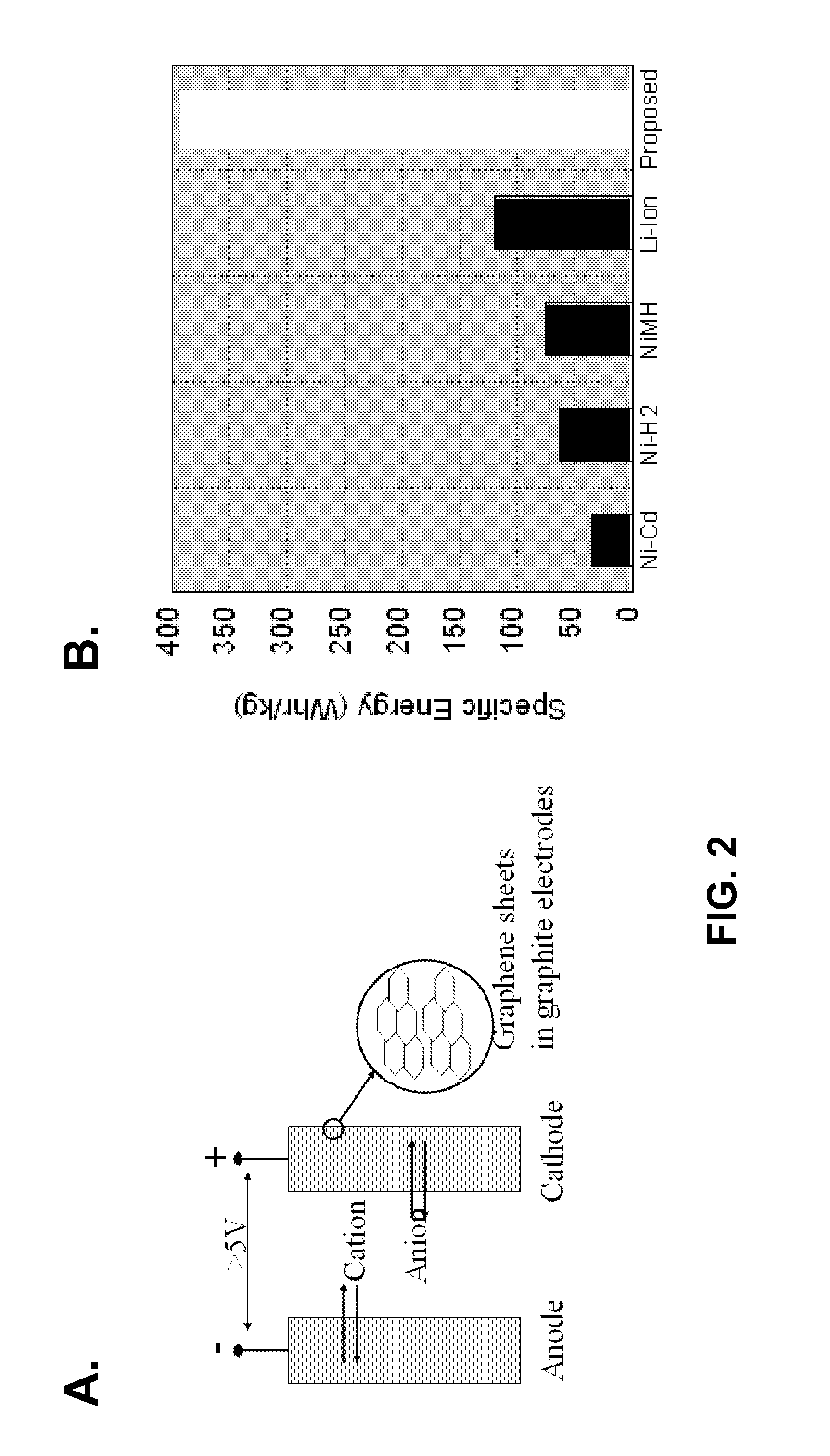
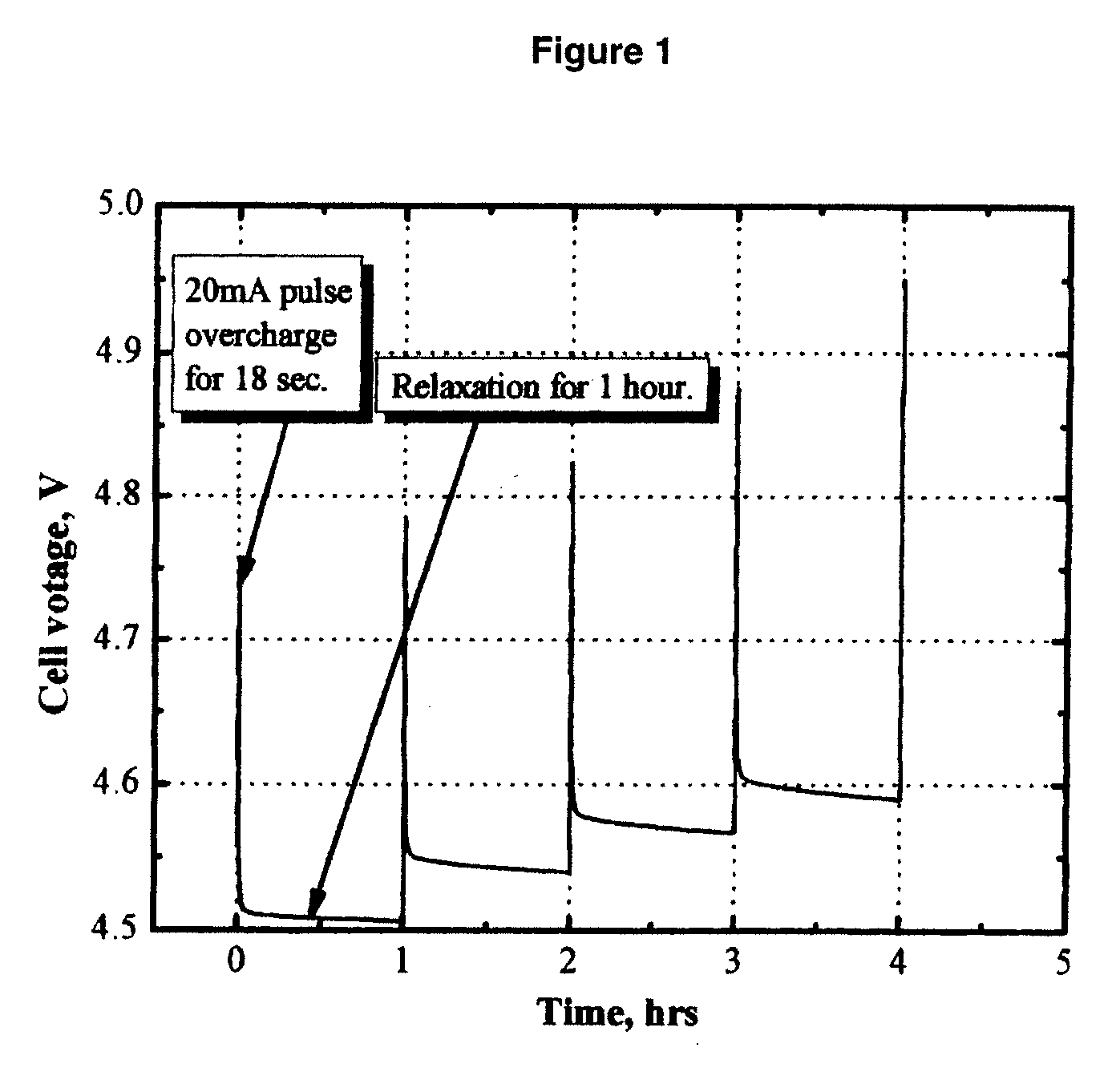
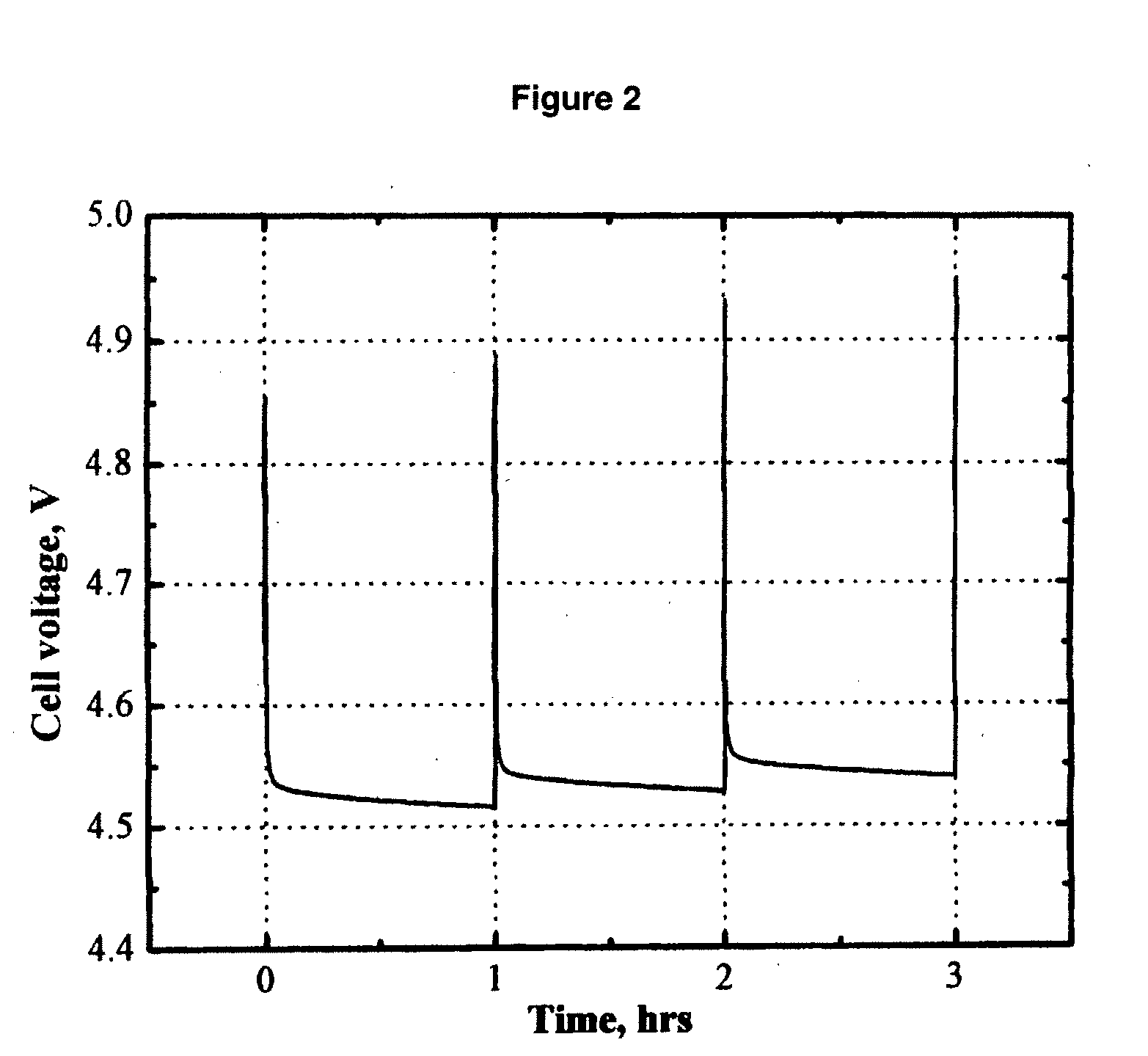
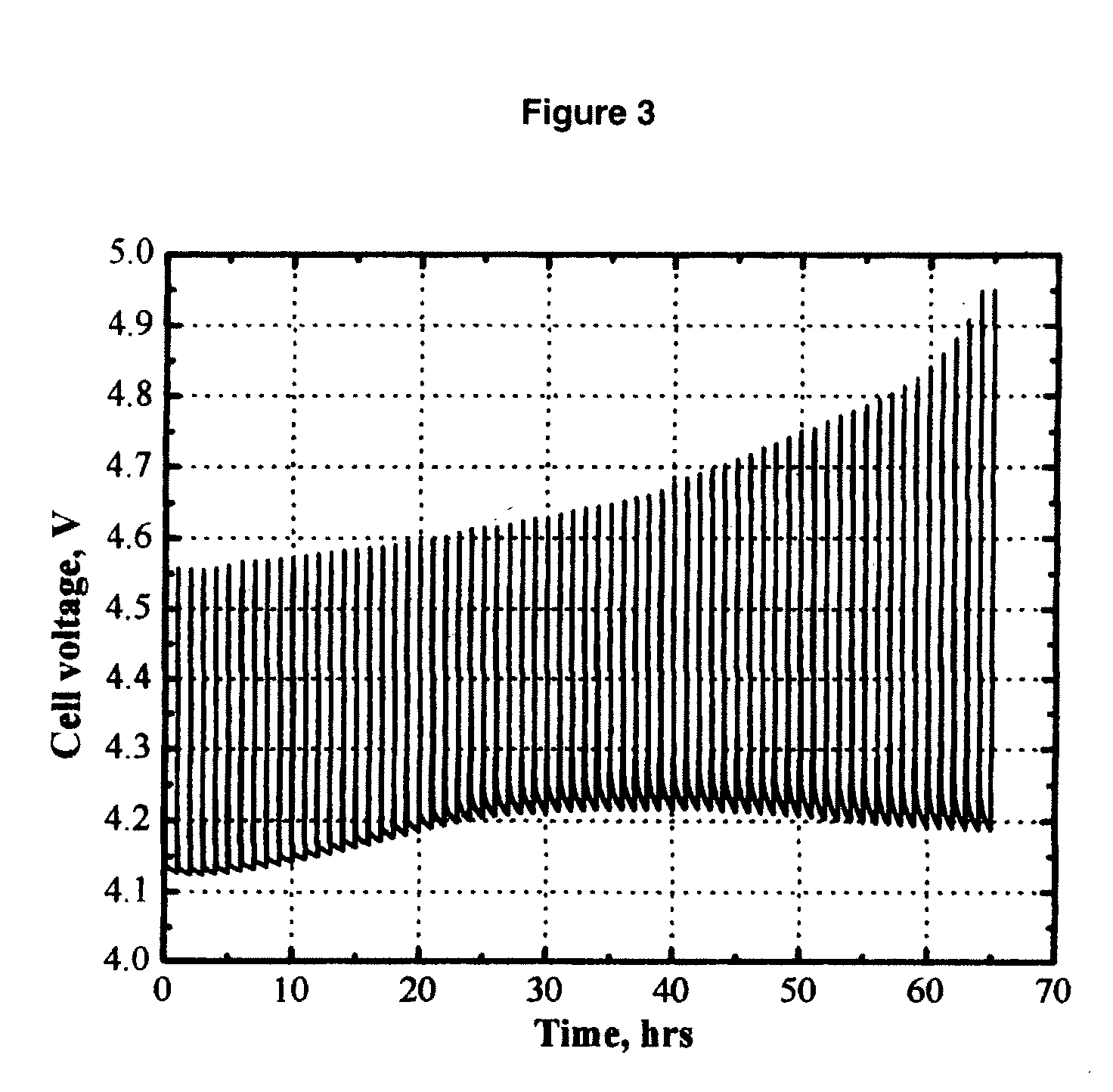
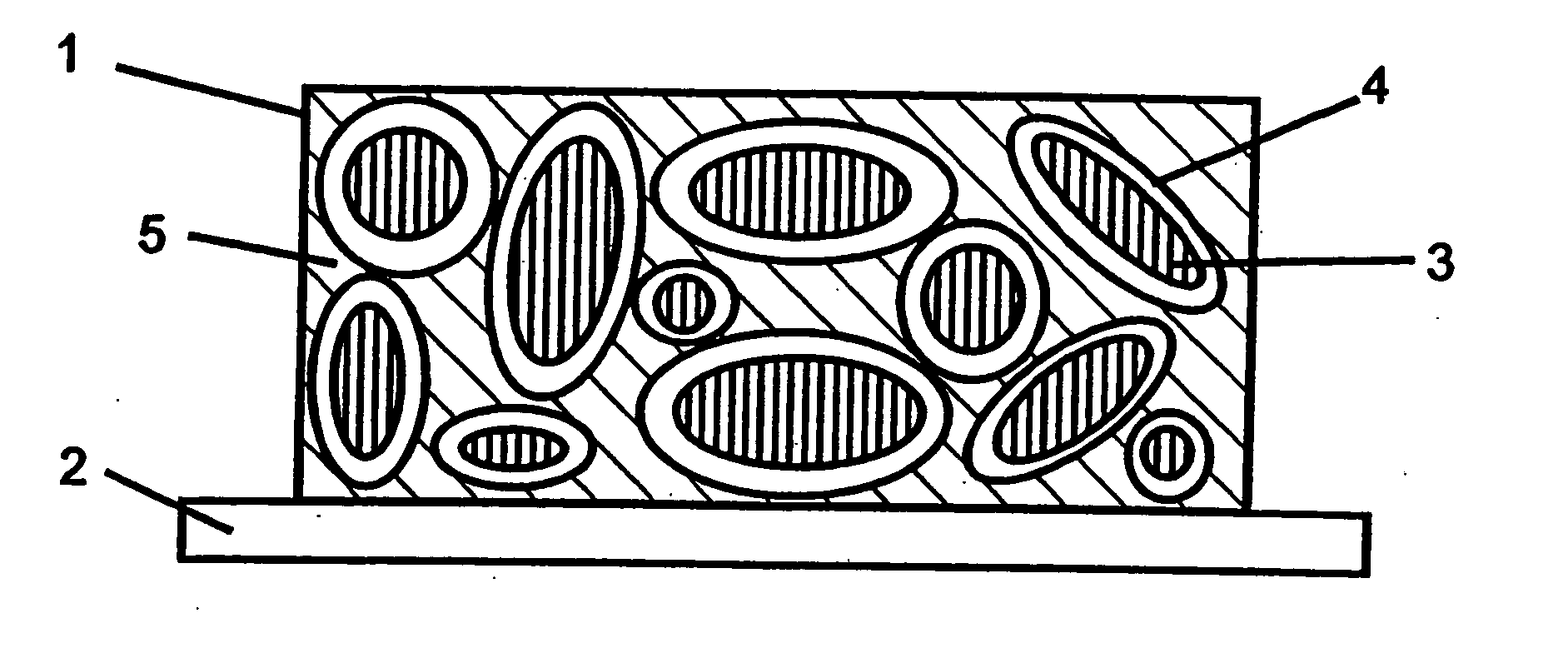
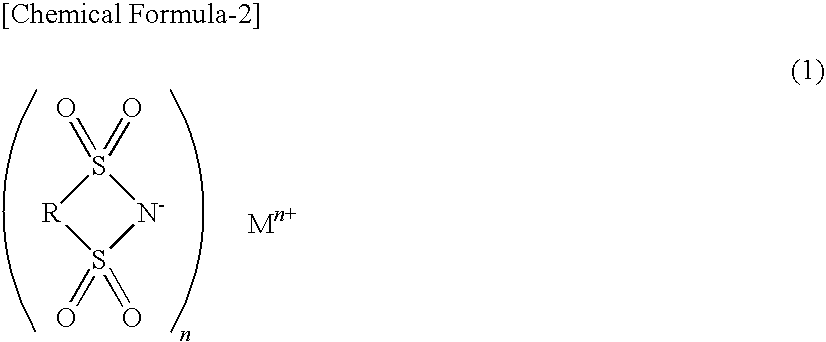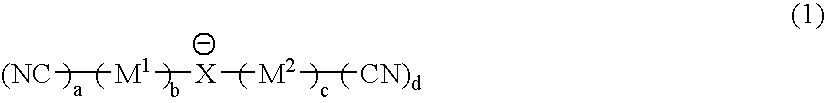Patents
Literature
3190results about "Organic electrolyte cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium anodes for electrochemical cells
InactiveUS7247408B2Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
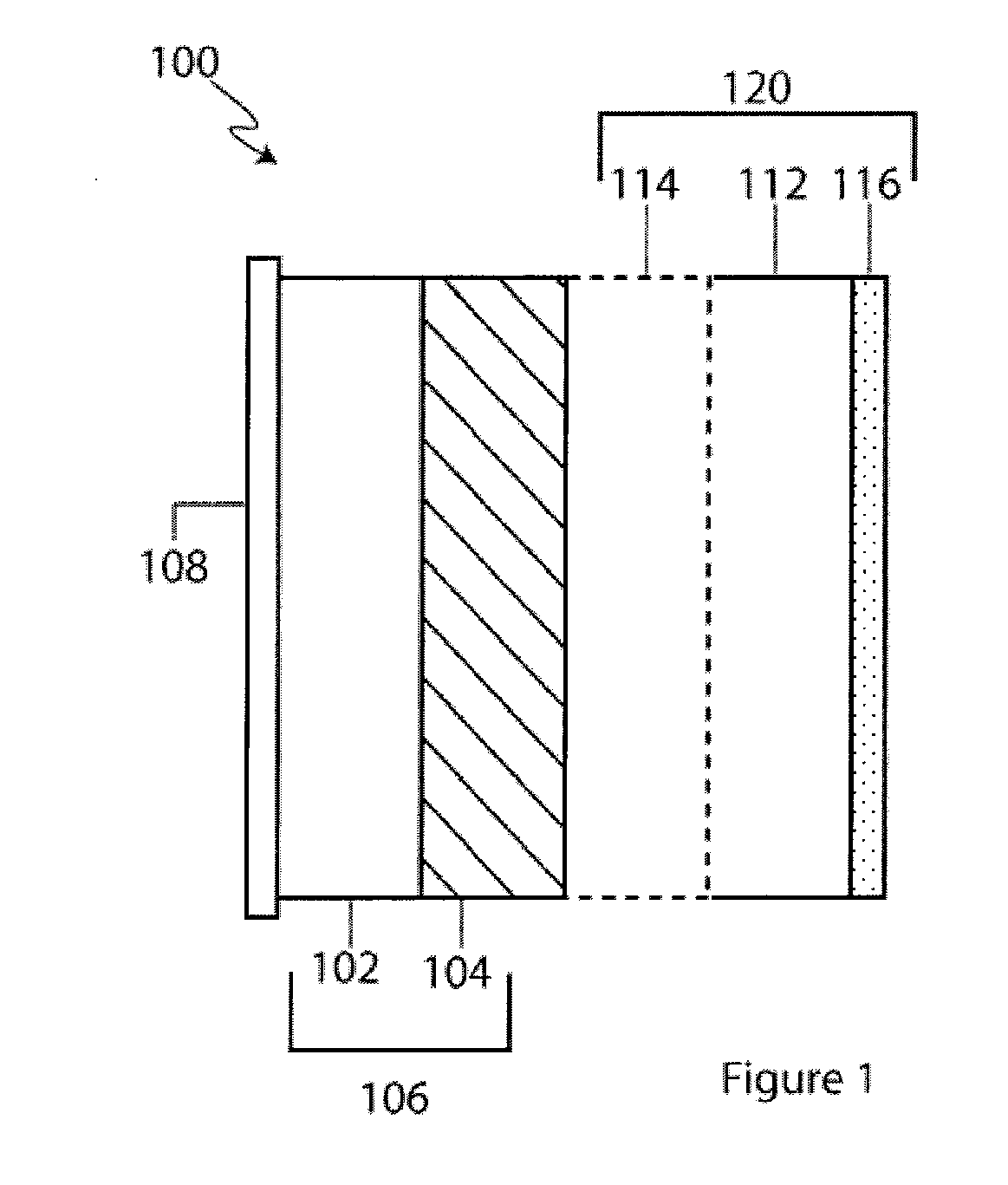
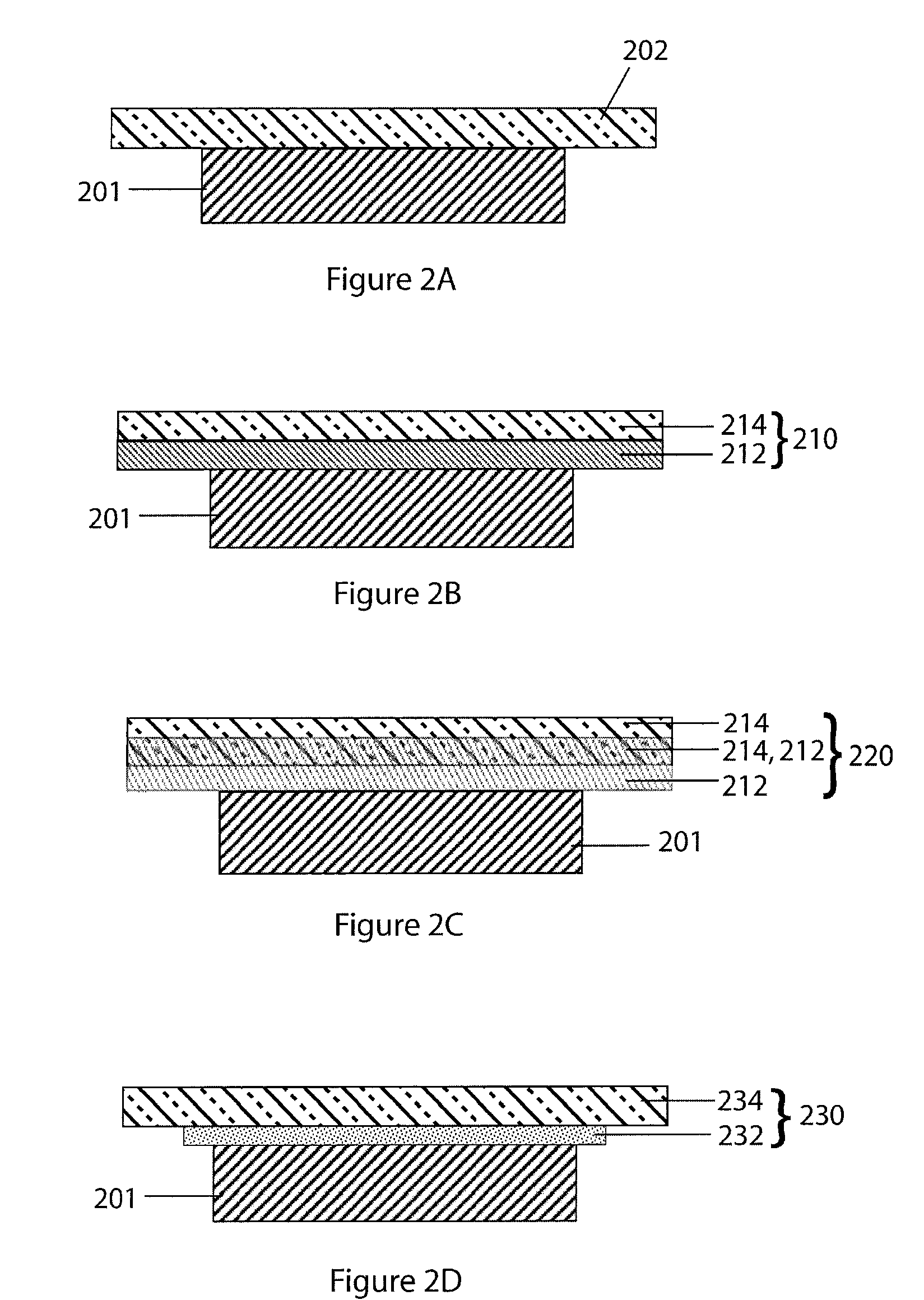
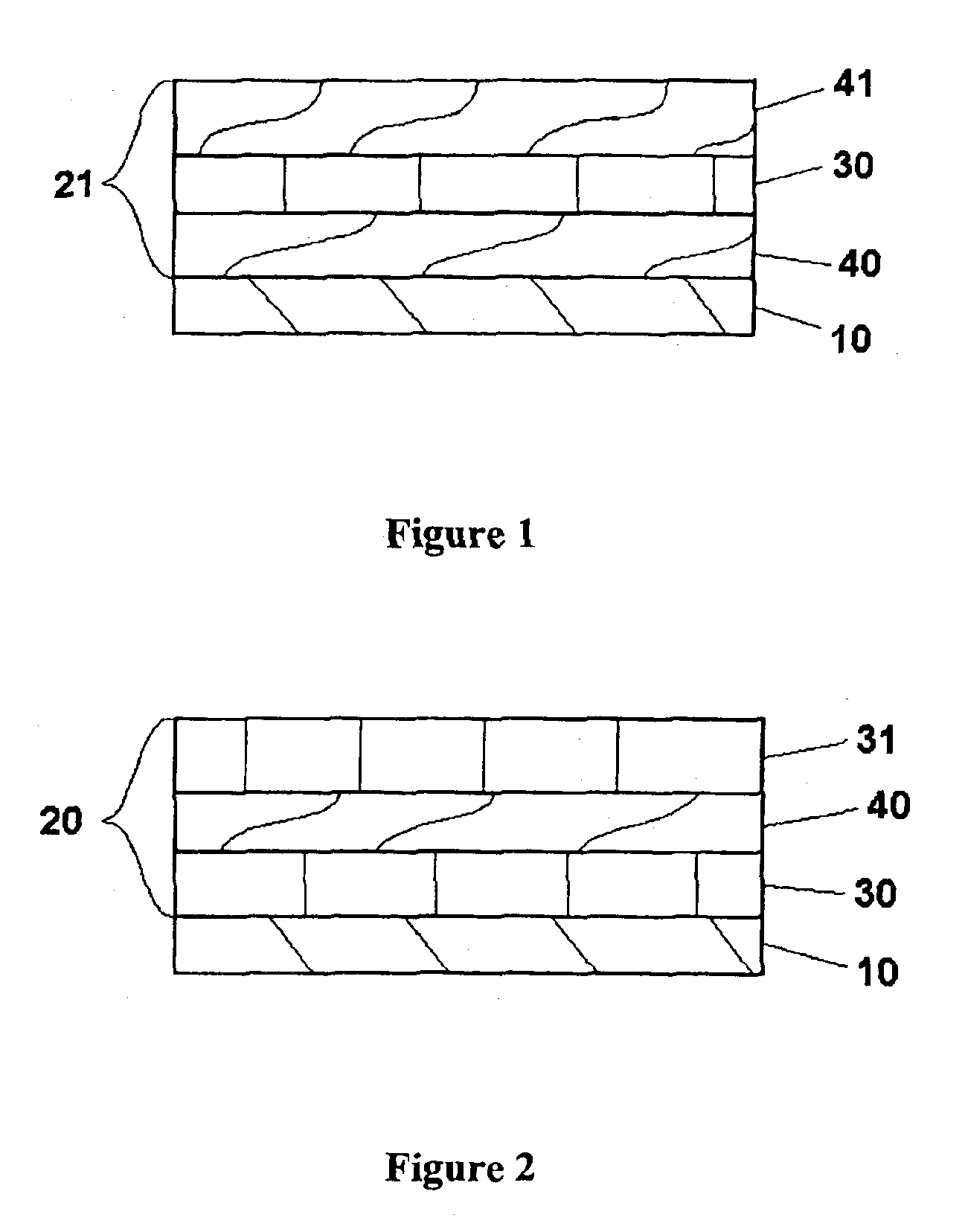
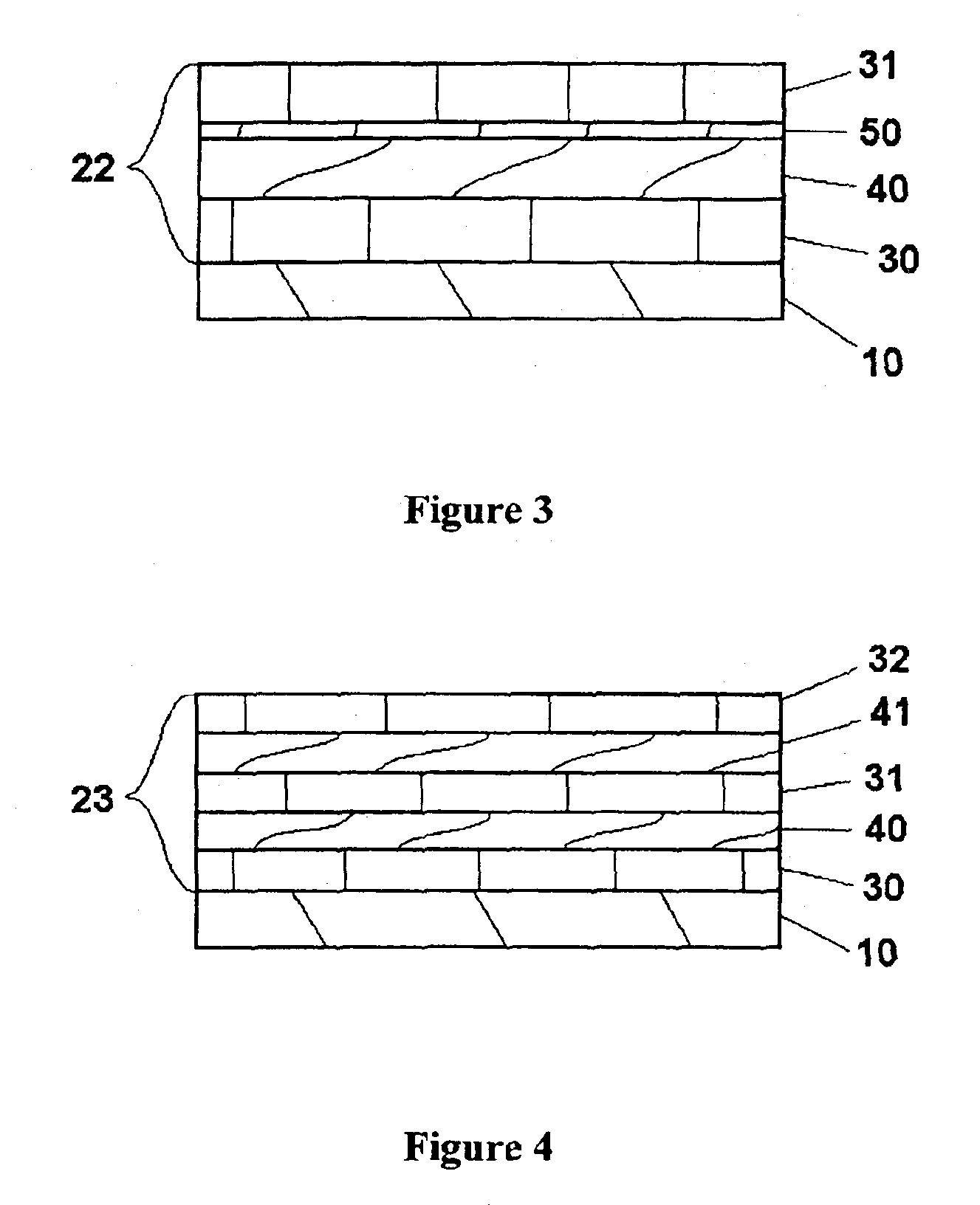
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
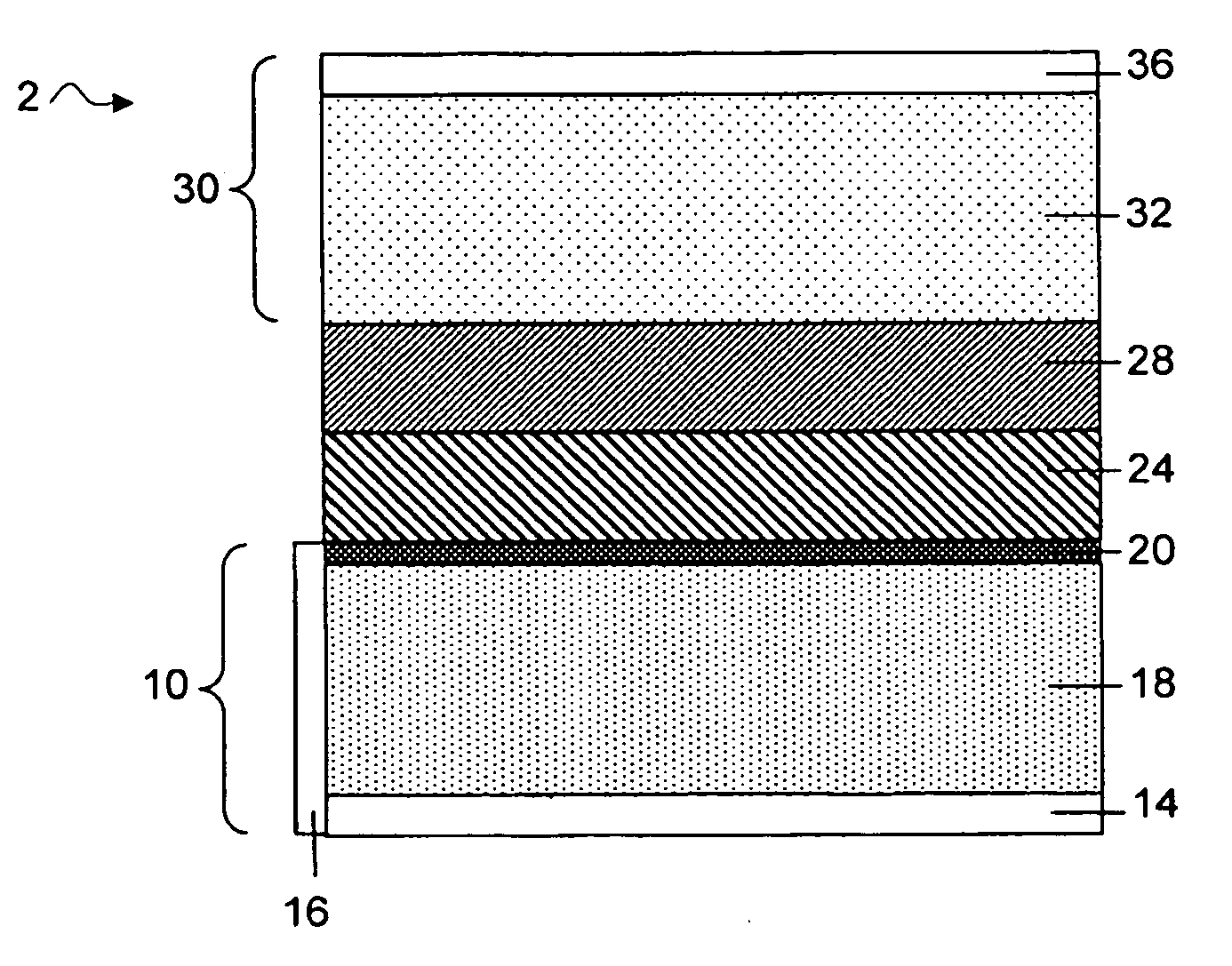
Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
ActiveUS7282295B2Avoid harmful reactionsFinal product manufactureElectrode carriers/collectorsMetal electrodesBattery cell
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Electrode materials with high surface conductivity
InactiveUS6855273B2Electrode manufacturing processesDouble layer capacitorsSurface conductivityIon exchange
The present invention concerns electrode materials capable of redox reactions by electrons and alkaline ions exchange with an electrolyte. The applications are in the field of primary (batteries) or secondary electrochemical generators, super capacitors and light modulating system of the super capacitor type.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Conductive lithium storage electrode
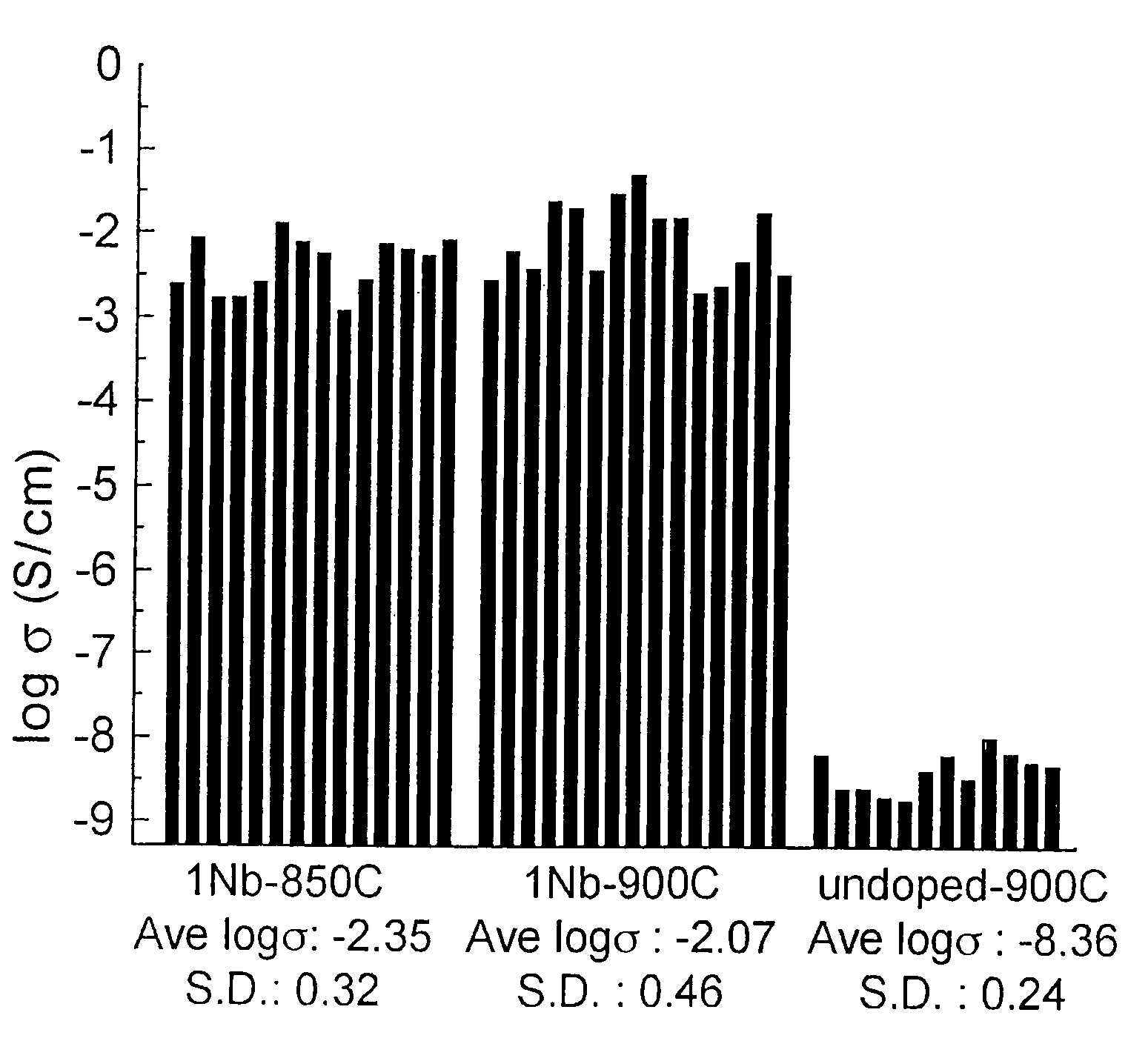
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
Li/air non-aqueous batteries
ActiveUS20070117007A1Improve battery performanceLarge capacityFuel and primary cellsFuel and secondary cellsLithiumOxygen
Non-aqueous alkali metal (e.g., Li) / oxygen battery cells constructed with a protected anode that minimizes anode degradation and maximizes cathode performance by enabling the use of cathode performance enhancing solvents in the catholyte have negligible self-discharge and high deliverable capacity. In particular, protected lithium-oxygen batteries with non-aqueous catholytes have this improved performance.
Owner:POLYPLUS BATTERY CO INC
Electrolyte composition, photoelectric conversion device and photo-electrochemical cell
InactiveUS6376765B1Improve rendering capabilitiesIncreased durabilityLight-sensitive devicesOrganic chemistryPhotoelectrochemical cellHydrogen atom
Owner:FUJIFILM HLDG CORP +1
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Long cycle-life alkali metal battery
InactiveUS6203947B1Improve cycle lifeFast charging rateElectrochemical processing of electrodesElectrode carriers/collectorsHigh pressureElectrochemical cell
The present invention provides a cathode for use in a secondary electrochemical cell, such cathode being coated with a very thin, protective film, permeable to ions. The protective film of the cathode usually has a thickness of up to about 0.1 mum and it provides protection against high voltage charging and overdiscbarging. The present invention further provides a secondary electrochemical cell comprising such a cathode.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Batteries including improved fine fiber separators
Alkaline and lithium batteries are disclosed that advantageously include separators comprising at least one porous layer of fine fibers having a diameter of between about 50 nm and about 3000 nm that provide improved combinations of reduced thickness, dendritic barrier against short-circuiting and low ionic resistance as compared with known battery separators.
Owner:DUPONT SAFETY & CONSTR INC
Active metal electrolyzer
InactiveUS20050100793A1Effective isolationReduce environmental pollutionPhotography auxillary processesElectrode manufacturing processesElectrolysisAqueous electrolyte
Electro-winning of active metal (e.g., lithium) ions from a variety of sources including industrial waste, and recycled lithium and lithium-ion batteries is accomplished with an electrolyzer having a protected cathode that is stable against aggressive solvents, including water, aqueous electrolytes, acid, base, and a broad range of protic and aprotic solvents. The electrolyzer has a highly ionically conductive protective membrane adjacent to the alkali metal cathode that effectively isolates (de-couples) the alkali metal electrode from solvent, electrolyte processing and / or cathode environments, and at the same time allows ion transport in and out of these environments. Isolation of the cathode from other components of a battery cell or other electrochemical cell in this way allows the use of virtually any solvent, electrolyte and / or anode material in conjunction with the cathode. The electrolyzer can be configured and operated to claim or reclaim lithium or other active metals from such sources.
Owner:POLYPLUS BATTERY CO INC
Nonaqueous electrolyte secondary battery and battery module
ActiveUS20050069777A1Excellent in battery capacity characteristic and cycle performanceEasy dischargeFinal product manufactureElectrode carriers/collectorsAlloyAluminum foil
A nonaqueous electrolyte secondary battery includes a case, a nonaqueous electrolyte provided in the case, a positive electrode provided in the case, and a negative electrode provided in the case, the negative electrode comprising a negative electrode current collector and a negative electrode layer that is carried on the negative electrode current collector and contains negative electrode active material particles, and the negative electrode current collector comprising an aluminum foil having an average crystal grain size of 50 μm or less or an aluminum alloy foil having an average crystal grain size of 50 μm or less.
Owner:KK TOSHIBA
Three dimensional free form battery apparatus
A battery apparatus comprising a casing, at least two stacked lithium ion cells a member for maximizing the utilization of the casing and a member for precluding inadvertent deformation of the casing. The casing includes a non-uniform inner periphery. Each of at least two stacked lithium ion cells is positioned within the casing. The utilization maximizing member maximizes the utilization of the inner periphery of the casing by facilitating the independent shaping of each of the at least two stacked lithium ion cells to confirm to the inner periphery. As a result, the shape of one cell does not limit or dictate the shape of any other cell. The deformation precluding member is associated with each of the at least two lithium ion cells, and, substantially precludes inadvertent deformation of the casing by the at least two lithium ion cells, during cell cycling and storage. The invention further includes a process for fabricating a battery apparatus.
Owner:MITSUBISHI CHEM CORP
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
High discharge capacity lithium battery
InactiveUS20050233214A1Improve discharge performanceIncrease energy densityFinal product manufactureOrganic electrolyte cellsHigh rateIron disulfide
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The iron disulfide of the positive electrode has a controlled average particle size range which allows the electrochemical cells to exhibit desired properties in both low and high rate applications. In various embodiments, the iron disulfide particles are wet milled, preferably utilizing a media mill or milled utilizing a non-mechanical mill such as a jet mill, which reduces the iron disulfide particles to a desired average particle size range for incorporation into the positive electrode.
Owner:EVEREADY BATTERY CO INC
Lithium anodes for electrochemical cells
InactiveUS20060222954A1Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Nonaqueous electrolyte for secondary battery and nonaqueous-electrolyte secondary battery employing the same
ActiveUS20100119956A1Reduce impactLower performance requirementsCell electrodesOrganic electrolyte cellsHigh current densityDifluorophosphate
An object is to provide a nonaqueous electrolyte and a nonaqueous-electrolyte secondary battery which have excellent discharge load characteristics and are excellent in high-temperature storability, cycle characteristics, high capacity, continuous-charge characteristics, storability, gas evolution inhibition during continuous charge, high-current-density charge / discharge characteristics, discharge load characteristics, etc. The object has been accomplished with a nonaqueous electrolyte which comprises: a monofluorophosphate and / or a difluorophosphate; and further a compound having a specific chemical structure or specific properties.
Owner:MU IONIC SOLUTIONS CORP +1
Lithium anodes for electrochemical cells
InactiveUS6936381B2Cell seperators/membranes/diaphragms/spacersElectrode carriers/collectorsLithium metalCrosslinked polymers
Owner:SION POWER CORP
Sulfur-carbon nanocomposites and their application as cathode materials in lithium-sulfur batteries
ActiveUS20110052998A1Minimize formationEasy to transportAlkaline accumulatorsElectrode thermal treatmentCarbon compositesPorous carbon
The invention is directed in a first aspect to a sulfur-carbon composite material comprising: (i) a bimodal porous carbon component containing therein a first mode of pores which are mesopores, and a second mode of pores which are micropores; and (ii) elemental sulfur contained in at least a portion of said micropores. The invention is also directed to the aforesaid sulfur-carbon composite as a layer on a current collector material; a lithium ion battery containing the sulfur-carbon composite in a cathode therein; as well as a method for preparing the sulfur-composite material.
Owner:UT BATTELLE LLC
Lithium ion battery with high voltage electrolytes and additives
ActiveUS20110136019A1Final product manufactureElectrode carriers/collectorsMethyl carbonateHigh pressure
Desirable electrolyte compositions are described that are suitable for high voltage lithium ion batteries with a rated charge voltage at least about 4.45 volts. The electrolyte compositions can comprise ethylene carbonate and solvent composition selected from the group consisting of dimethyl carbonate, methyl ethyl carbonate, γ-butyrolactone, γ-valerolactone or a combination thereof. The electrolyte can further comprise a stabilization additive. The electrolytes can be effectively used with lithium rich positive electrode active materials.
Owner:IONBLOX INC
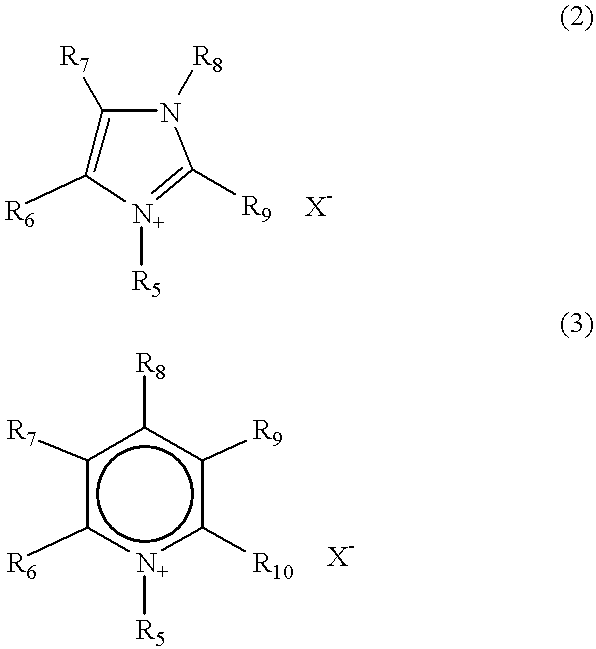
Material for electrolytic solutions and use thereof
InactiveUS20040002002A1Avoid corrosionImprove ionic conductivityHybrid capacitor electrolytesAlkaline accumulatorsOrganic linkingElectrical conductor
It is an object of the present invention to provide a material for electrolytic solutions suited for use as material in electrolytic solutions serving as ionic conductors in electrochemical devices, such as large-capacity cells or batteries. The present invention is related to a material for electrolytic solutions which comprises, as an essential constituent, an anion represented by the general formula (1): wherein X represents at least one element selected from among B, N, O, Al, Si, P, S, As and Se; M<1 >and M<2 >are the same or different and each represents an organic linking group; a is an integer of not less than 1, and b, c and d each independently is an integer of not less than 0.
Owner:NIPPON SHOKUBAI CO LTD
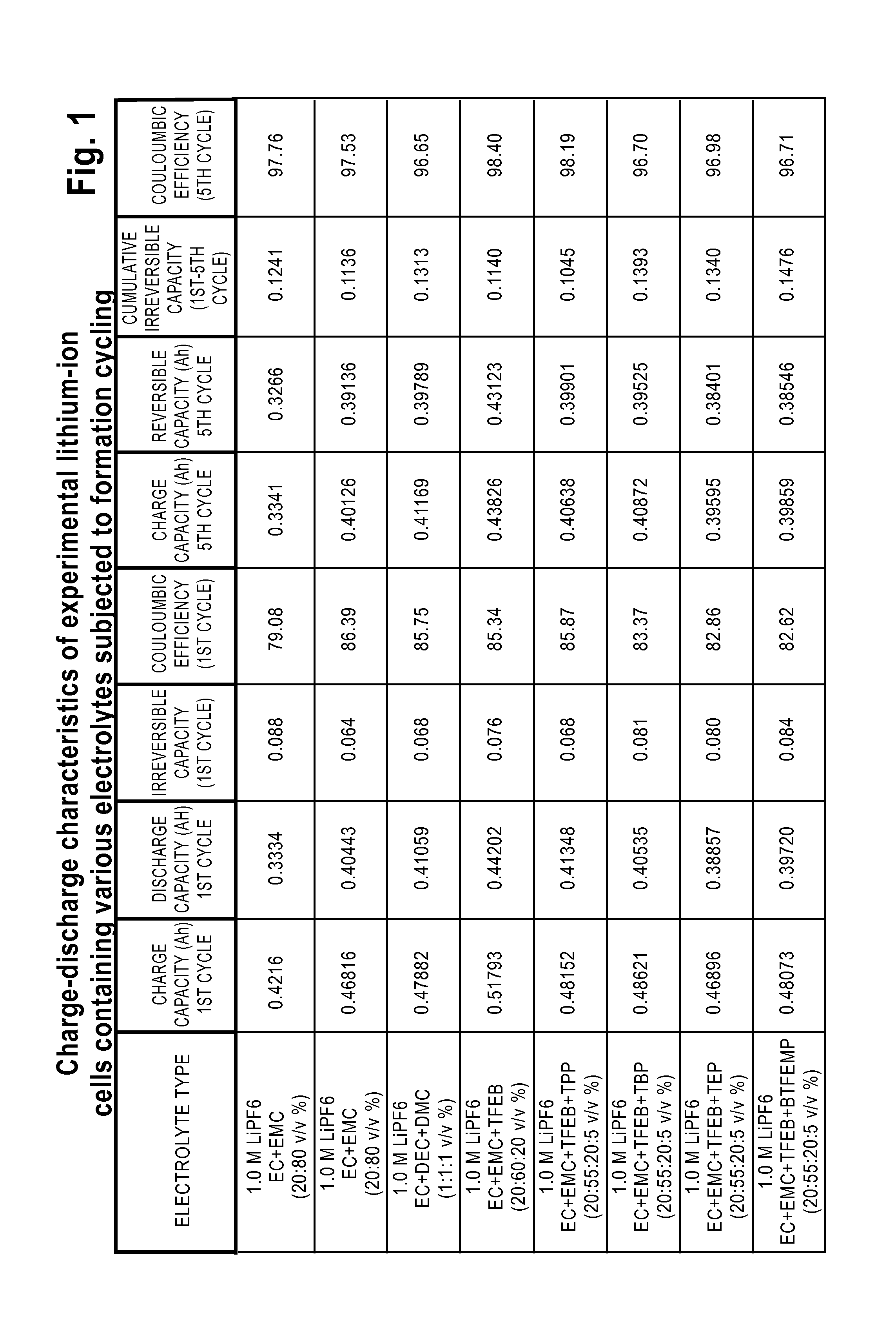
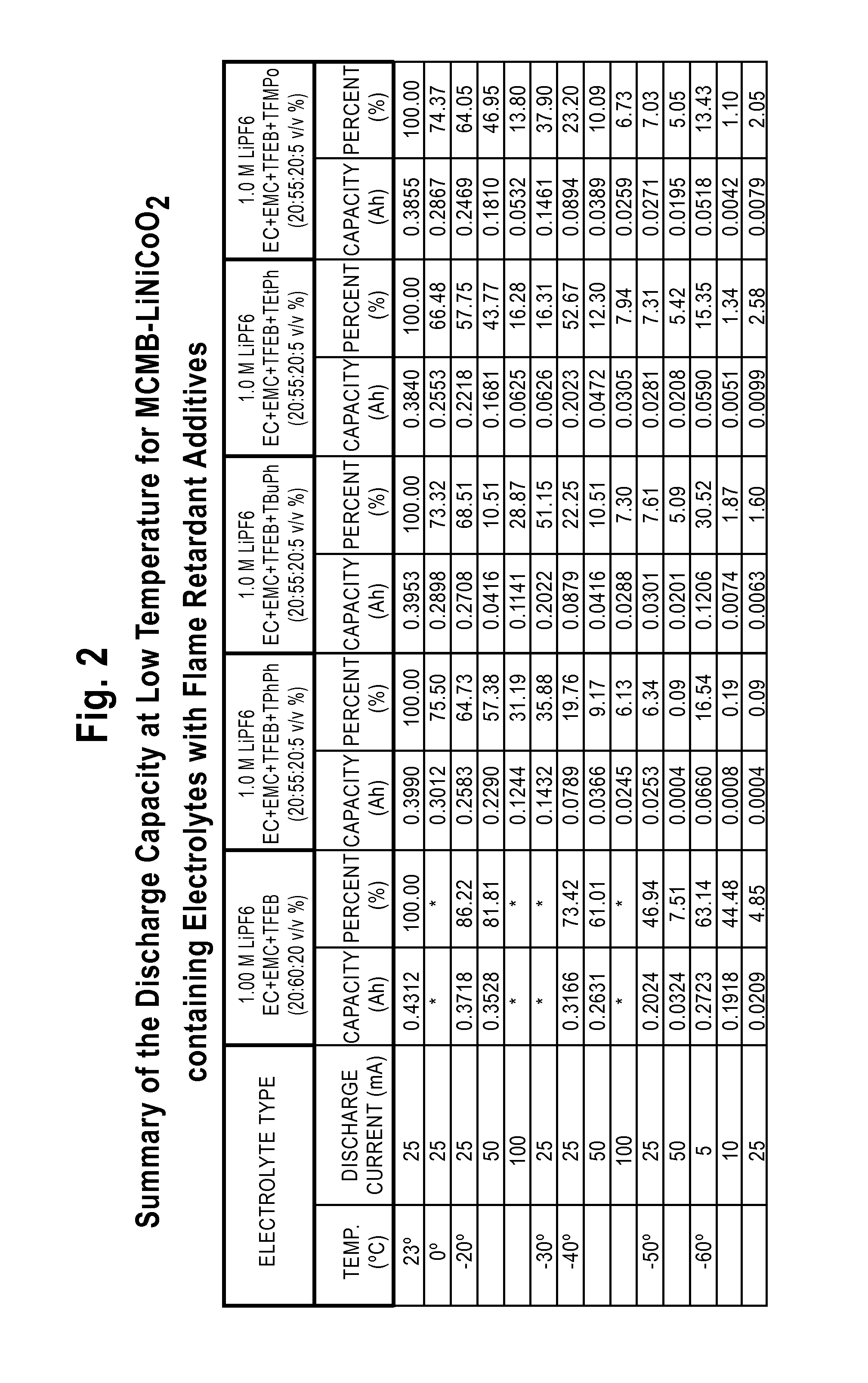
Lithium-Ion Electrolytes Containing Flame Retardant Additives for Increased Safety Characteristics
InactiveUS20100047695A1Improve performanceImprove security featuresOrganic electrolyte cellsSecondary cellsMethyl carbonatePhysical chemistry
The invention discloses various embodiments of Li-ion electrolytes containing flame retardant additives that have delivered good performance over a wide temperature range, good cycle life characteristics, and improved safety characteristics, namely, reduced flammability. In one embodiment of the invention there is provided an electrolyte for use in a lithium-ion electrochemical cell, the electrolyte comprising a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), a fluorinated co-solvent, a flame retardant additive, and a lithium salt. In another embodiment of the invention there is provided an electrolyte for use in a lithium-ion electrochemical cell, the electrolyte comprising a mixture of an ethylene carbonate (EC), an ethyl methyl carbonate (EMC), a flame retardant additive, a solid electrolyte interface (SEI) film forming agent, and a lithium salt.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Nonaqueous electrolyte
ActiveUS20050221188A1Excellent characteristicsNon-aqueous electrolyte accumulatorsElectrode carriers/collectorsLithiumMaterials science
A nonaqueous electrolyte battery includes a positive electrode containing an active material, a negative electrode, and a nonaqueous electrolyte, the negative electrode including a current collector and a negative electrode active material supported by the current collector, the negative electrode active material having a Li insertion potential not lower than 0.2V (vs. Li / Li+) and an average primary particle diameter not larger than 1 μm, and a specific surface area of the negative electrode, excluding a weight of the current collector, as determined by the BET method falls within a range of 3 to 50 m2 / g.
Owner:KK TOSHIBA
Non-aqueous electrolyte secondary battery
ActiveUS20050147889A1Reduce the possibilityIncrease resistanceFinal product manufactureElectrode carriers/collectorsHigh rateCobalt
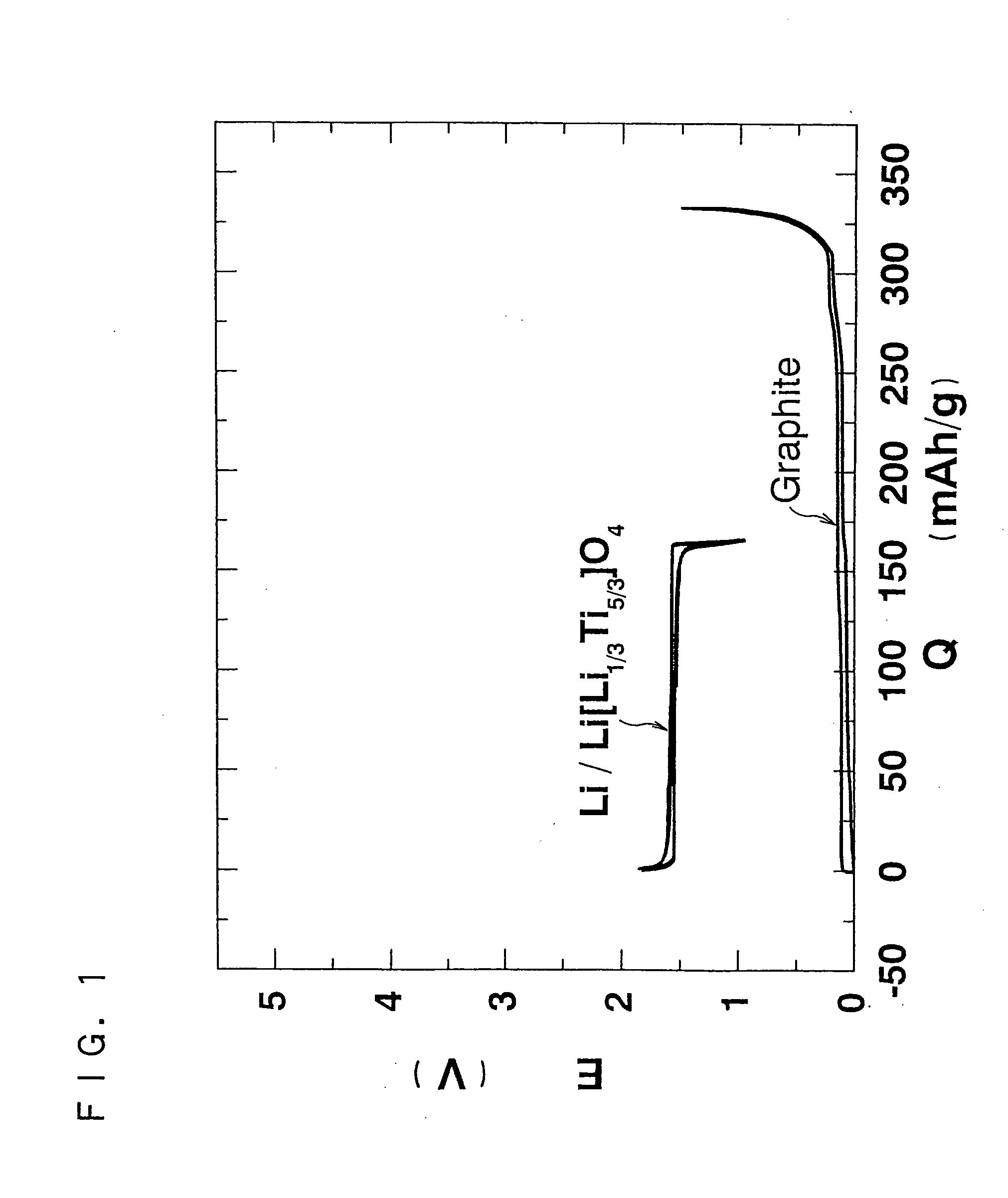
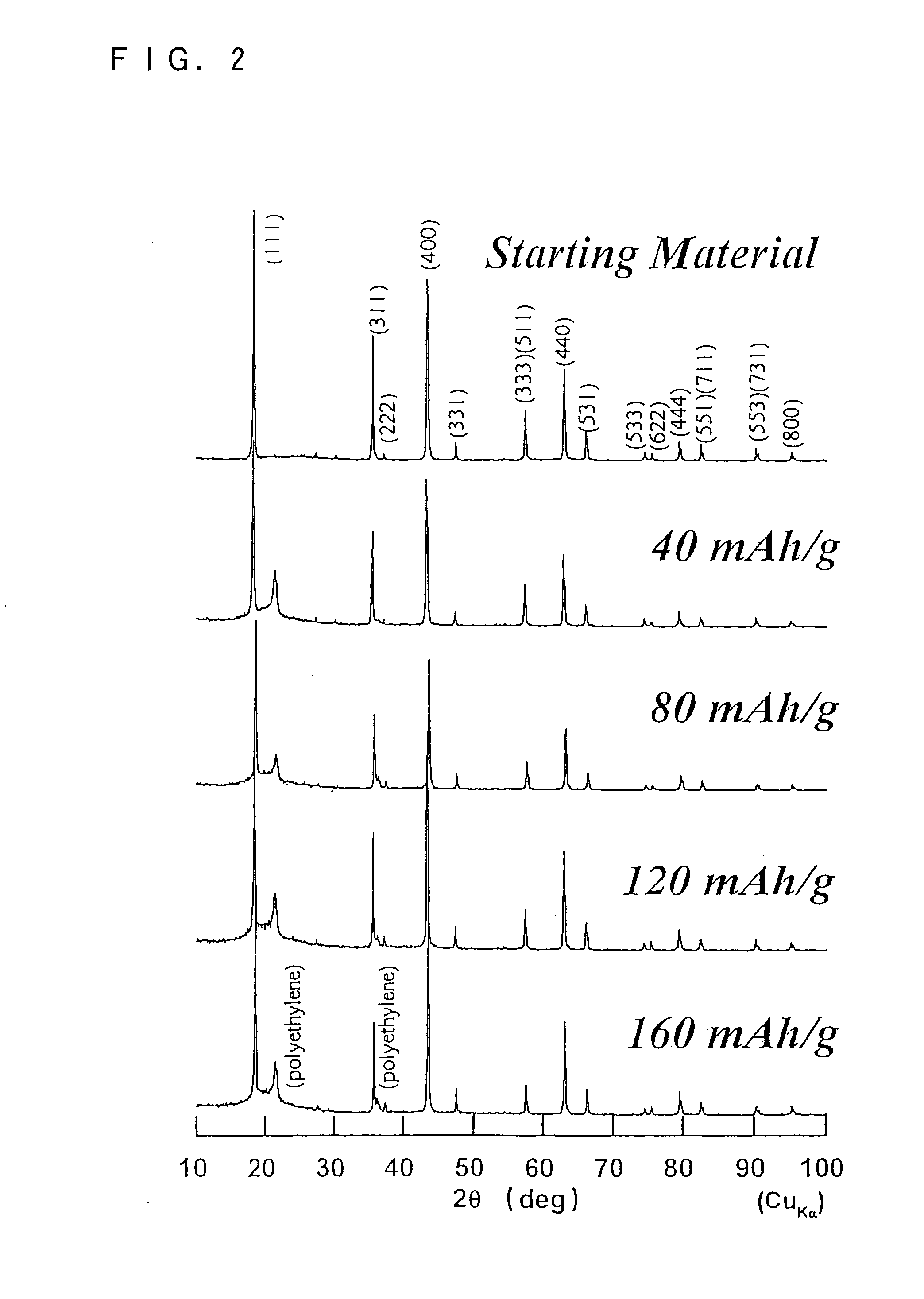
As an alternative technique to lead-acid batteries, the present invention provides an inexpensive 2 V non-aqueous electrolyte secondary battery having excellent cycle life at a high rate by preventing volume change during charge and discharge. The non-aqueous electrolyte secondary battery uses: a positive electrode active material having a layered structure, being represented by chemical formula Li1±α[Me]O2, where 0≦α<0.2, and Me is a transition metal including Ni and at least one selected from the group consisting of Mn, Fe, Co, Ti and Cu, and including elemental nickel and elemental cobalt in substantially the same ratio; and a negative electrode active material including Li4Ti5O12(Li[Li1 / 3Ti5 / 3]O4).
Owner:OSAKA CITY UNIV +1
Nonaqueous electrolytes and nonaqueous-electrolyte secondary batteries employing the same
ActiveUS20100099031A1Large capacityImprove cycle performanceCell electrodesOrganic electrolyte cellsHigh current densityChemical structure
A subject is to provide a nonaqueous electrolyte excellent in cycle performances such as capacity retention after cycling, output after cycling, discharge capacity after cycling, and cycle discharge capacity ratio, output characteristics, high-temperature storability, low-temperature discharge characteristics, heavy-current discharge characteristics, high-temperature storability, safety, high capacity, high output, high-current-density cycle performances, compatibility of these performances, etc. Another subject is to provide a nonaqueous-electrolyte secondary battery employing the nonaqueous electrolyte. The subjects have been accomplished with a nonaqueous electrolyte which contains a monofluorophosphate and / or a difluorophosphate and further contains a compound having a specific chemical structure or specific properties.
Owner:MU IONIC SOLUTIONS CORP +1
Additive for enhancing the performance of electrochemical cells
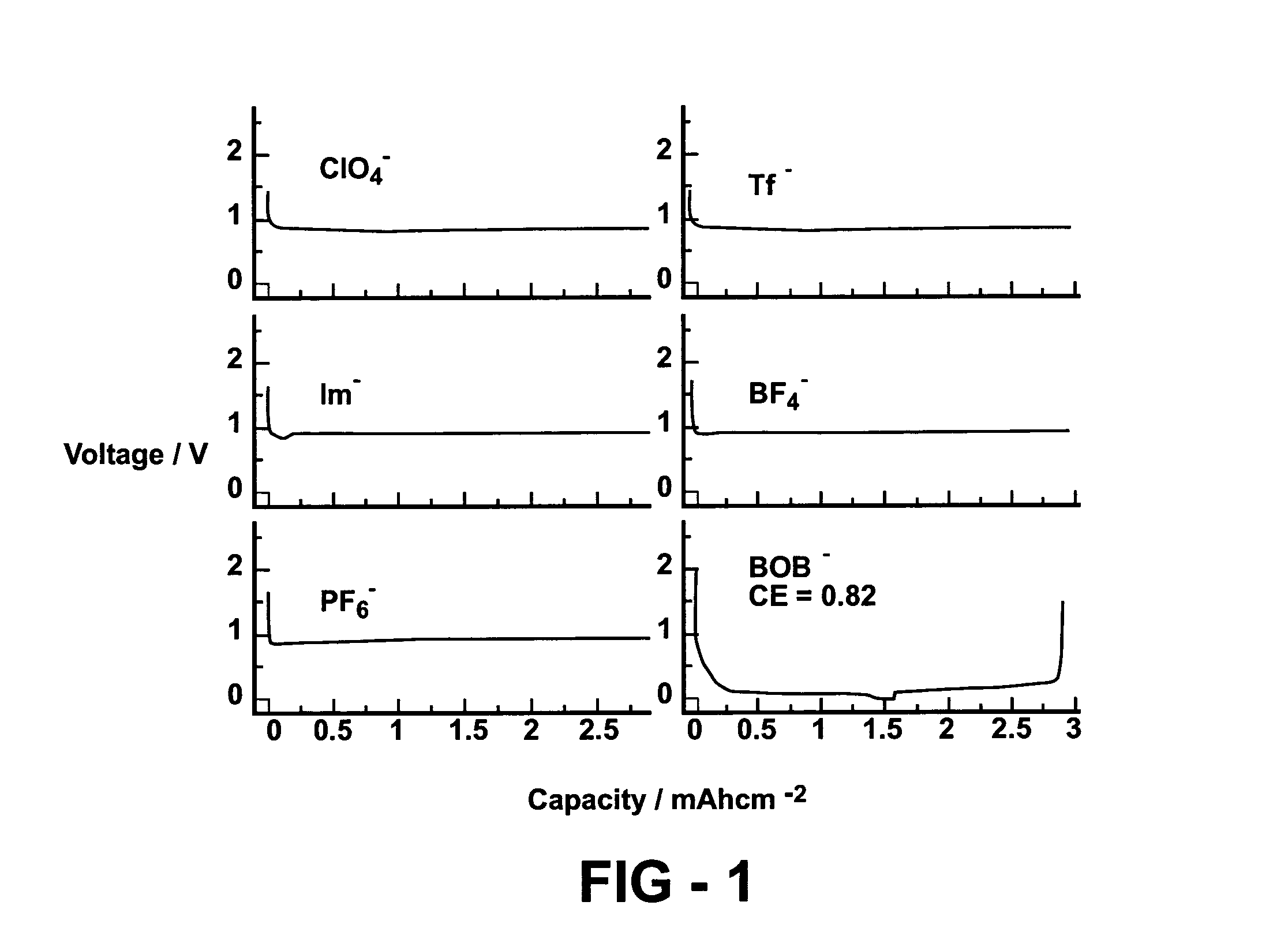
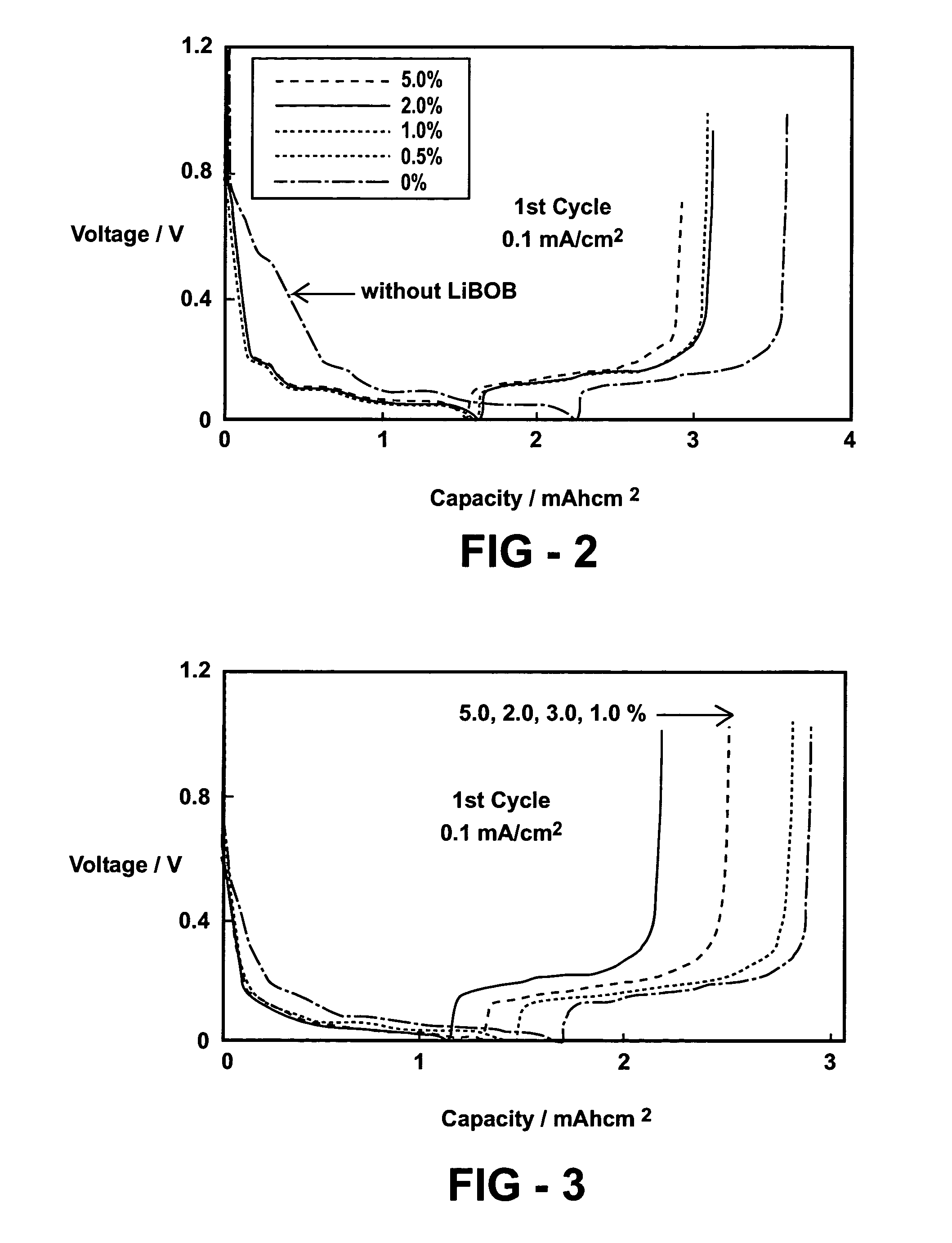
InactiveUS7172834B1Prevent peelingReduce solubilityConductive materialOrganic electrolyte cellsOxalateSalt content
A lithium battery includes an electrolyte comprised of a non-aqueous solvent, and a salt mixture. The salt mixture includes an alkali metal electrolyte salt and an additive salt having an anion of a mixed anhydride of oxalic acid and boric acid. Specific additive salts include lithium bis(oxalato) borate and lithium oxalyldifluoroborate. Particular electrolyte salts comprise LiPF6 and LiBF4. The additive salt is present in an amount of 0.1–60 mole percent of the total of the additive salt and electrolyte salt content of the electrolyte. Also disclosed is a method for enhancing the performance characteristics of a lithium battery through the use of the electrolyte composition. Also disclosed is the compound lithium oxalyldifluoroborate.
Owner:ARMY US SEC THE
High Voltage and High Specific Capacity Dual Intercalating Electrode Li-Ion Batteries
ActiveUS20060269834A1High voltageHigh energyElectrode carriers/collectorsOrganic electrolyte cellsPresent methodElectrode pair
The present invention provides high capacity and high voltage Li-ion batteries that have a carbonaceous cathode and a nonaqueous electrolyte solution comprising LiF salt and an anion receptor that binds the fluoride ion. The batteries can comprise dual intercalating electrode Li ion batteries. Methods of the present invention use a cathode and electrode pair, wherein each of the electrodes reversibly intercalate ions provided by a LiF salt to make a high voltage and high specific capacity dual intercalating electrode Li-ion battery. The present methods and systems provide high-capacity batteries particularly useful in powering devices where minimizing battery mass is important.
Owner:CALIFORNIA INST OF TECH
Separator for nonaqueous electrolyte batteries, nonaqueous electrolyte battery using it, and method for manufacturing separator for nonaqueous electrolyte batteries
InactiveUS6511774B1High tear strengthEnhanced penetration strengthNon-aqueous electrolyte accumulatorsCell seperators/membranes/diaphragms/spacersHigh energyEngineering
The present invention provides a separator for non-aqueous electrolyte batteries which neither breaks nor slips off at the time of fabrication of battery, gives excellent battery fabricability, causes no internal short-circuit caused by contact between electrodes even if the electrodes are externally short-circuited, can inhibit ignition of battery and produces high energy density and excellent cycle life, and further provides a non-aqueous electrolyte battery using the separator and a method for manufacturing the separator. That is, the present invention relates to a separator for non-aqueous electrolyte batteries which comprises a porous base containing at least one member selected from a porous film, a woven fabric or nonwoven fabric containing an organic fiber and a paper and an organometallic compound applied to the porous base; a method for the manufacture of the separator for non-aqueous electrolyte batteries which comprises allowing said porous base to contact with a solution of organometallic compound by impregnation, coating or spraying, followed by drying or curing with heating to apply the organometallic compound to the porous base; and a non-aqueous electrolyte battery using the separator.
Owner:MITSUBISHI PAPER MILLS LTD
Electrolytes, cells and methods of forming passivaton layers
InactiveUS20080026297A1Improve thermal stabilityImprove stabilityAlkaline accumulatorsHybrid capacitor electrolytesSolventPhotochemistry
An electrolyte comprising at least one organic aprotic solvent, at least one salt and at least one chelatoborate additive. A method of forming an SEI layer in a cell comprising a positive electrode, a negative electrode and an electrolyte, said method comprising the step of overcharging the electrolyte prior to fabricating the cell, or said cell during the formation cycle.
Owner:AIR PROD & CHEM INC
Novel composite cathodes, electrochemical cells comprising novel composite cathodes, and processes for fabricating same
The present invention pertains to composite cathodes suitable for use in an electrochemical cell, said cathodes comprising: (a) an electroactive sulfur-containing cathode material, wherein said electroactive sulfur-containing cathode material, in its oxidized state, comprises a polysulfide moiety of the formula —Sm—, wherein m is an integer equal to or greater than 3; and, (b) an electroactive transition metal chalcogenide composition, which encapsulates said electroactive sulfur-containing cathode material, and which retards the transport of anionic reduction products of said electroactive sulfur-containing cathode material, said electroactive transition metal chalcogenide composition comprising an electroactive transition metal chalcogenide having the formula MjYk(OR)l wherein: M is a transition metal; Y is the same or different at each occurrence and is oxygen, sulfur, or selenium; R is an organic group and is the same or different at each occurrence; j is an integer ranging from 1 to 12; k is a number ranging from 0 to 72; and l is a number ranging from 0 to 72; with the proviso that k and l cannot both be 0. The present invention also pertains to methods of making such composite cathodes, cells comprising such composite cathodes, and methods of making such cells.
Owner:SION POWER CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com