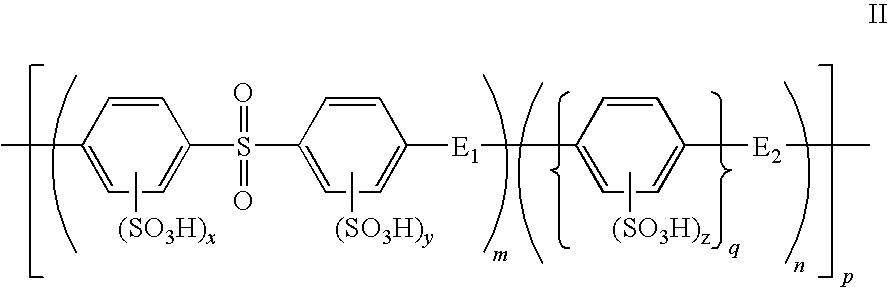
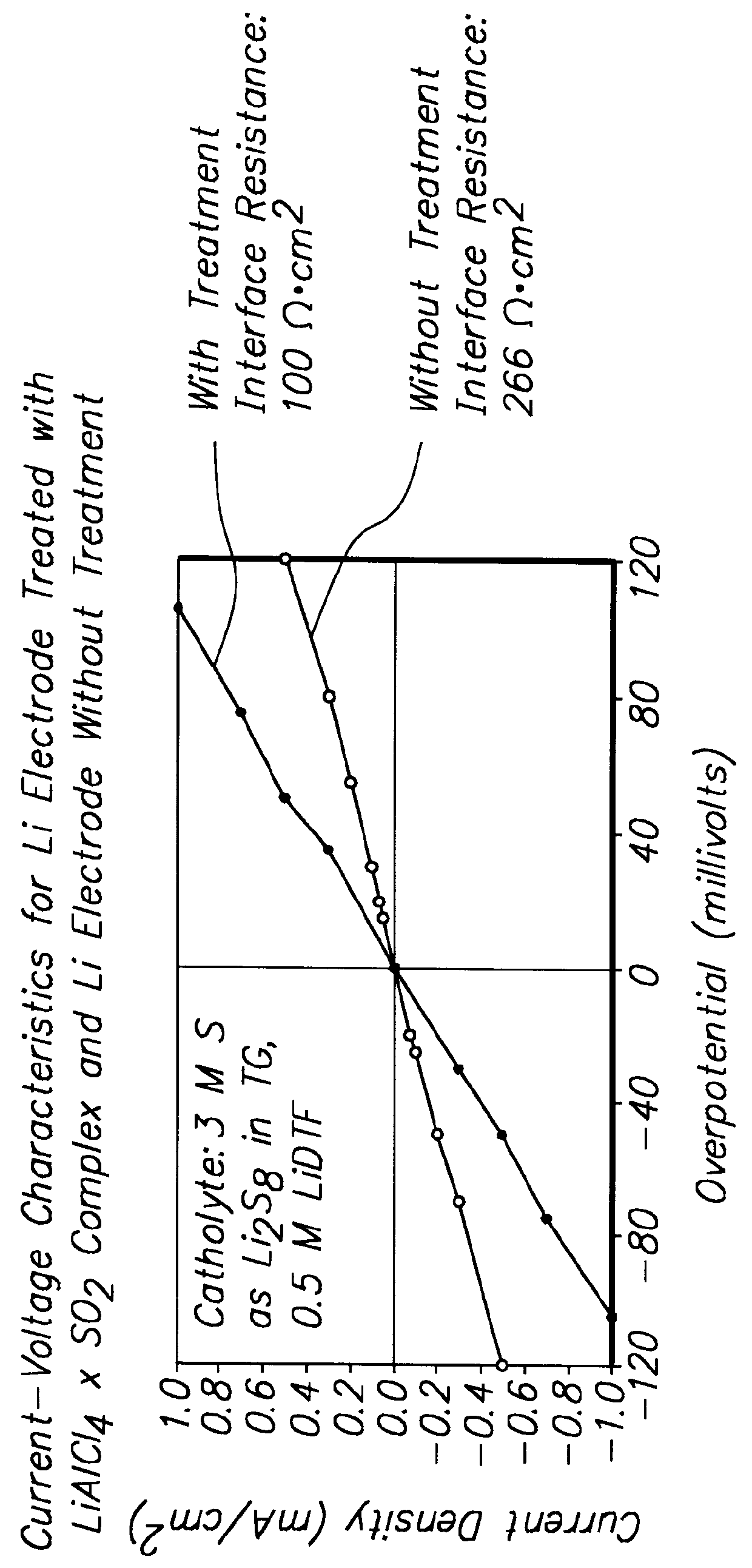
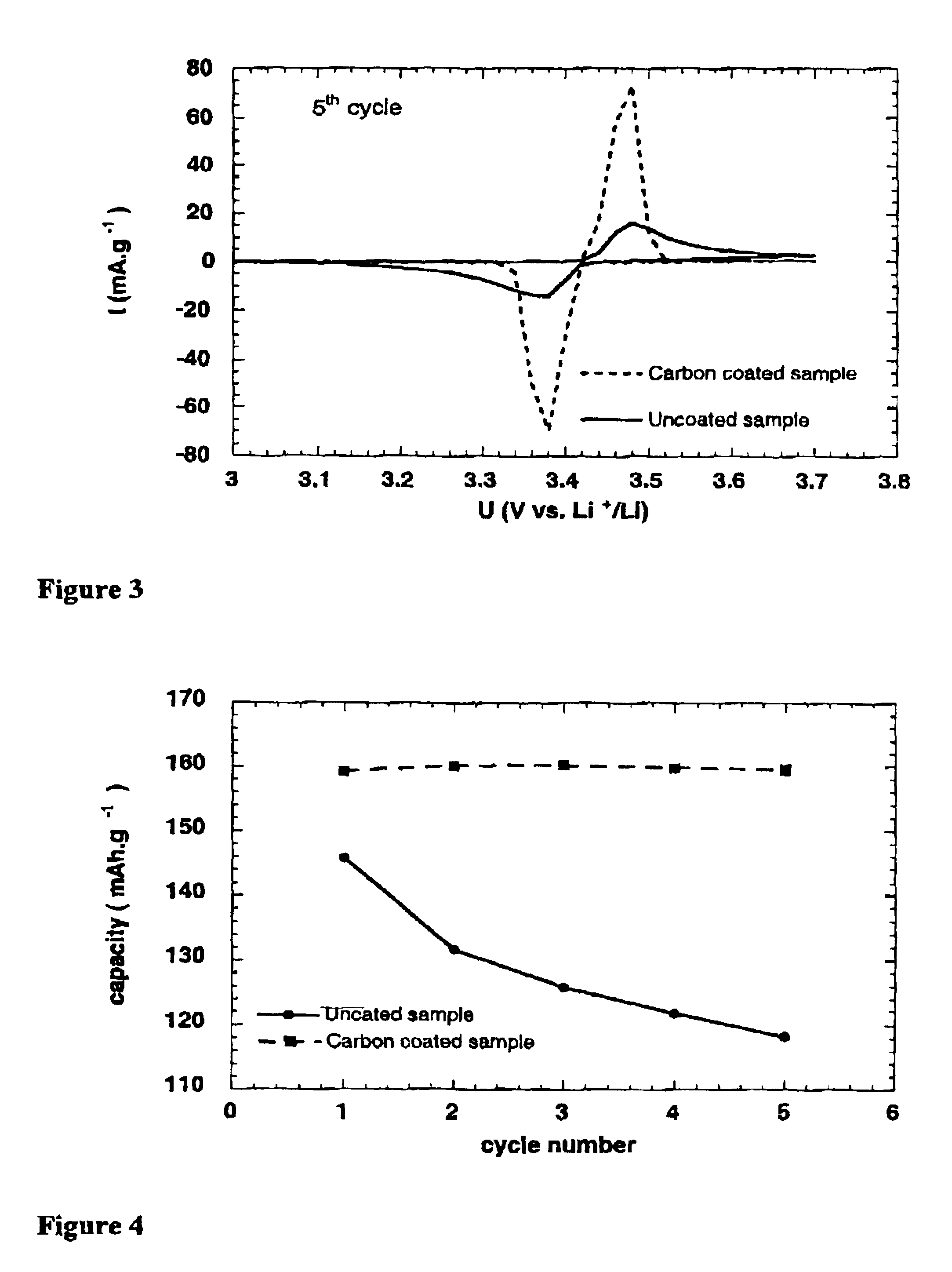
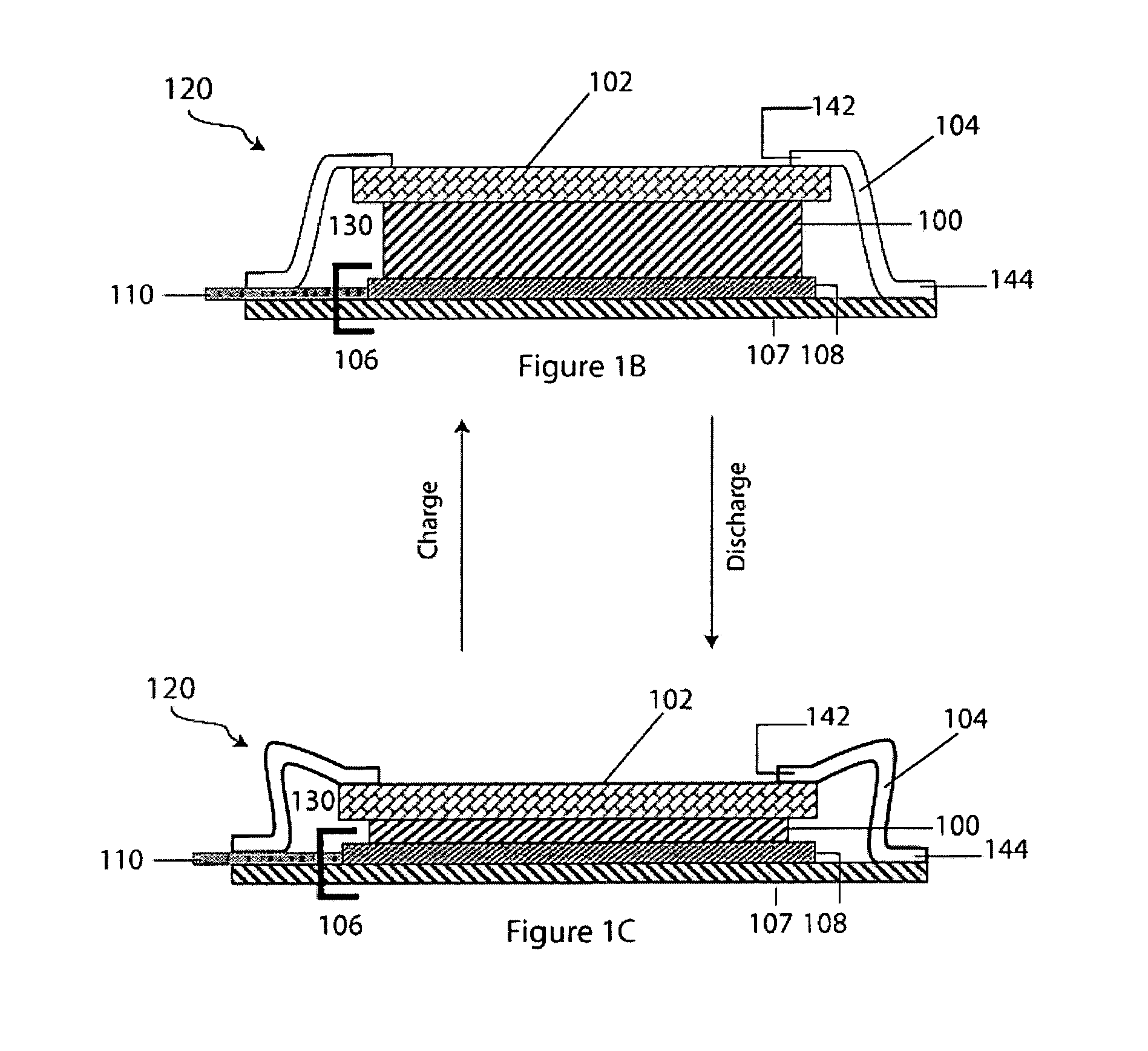
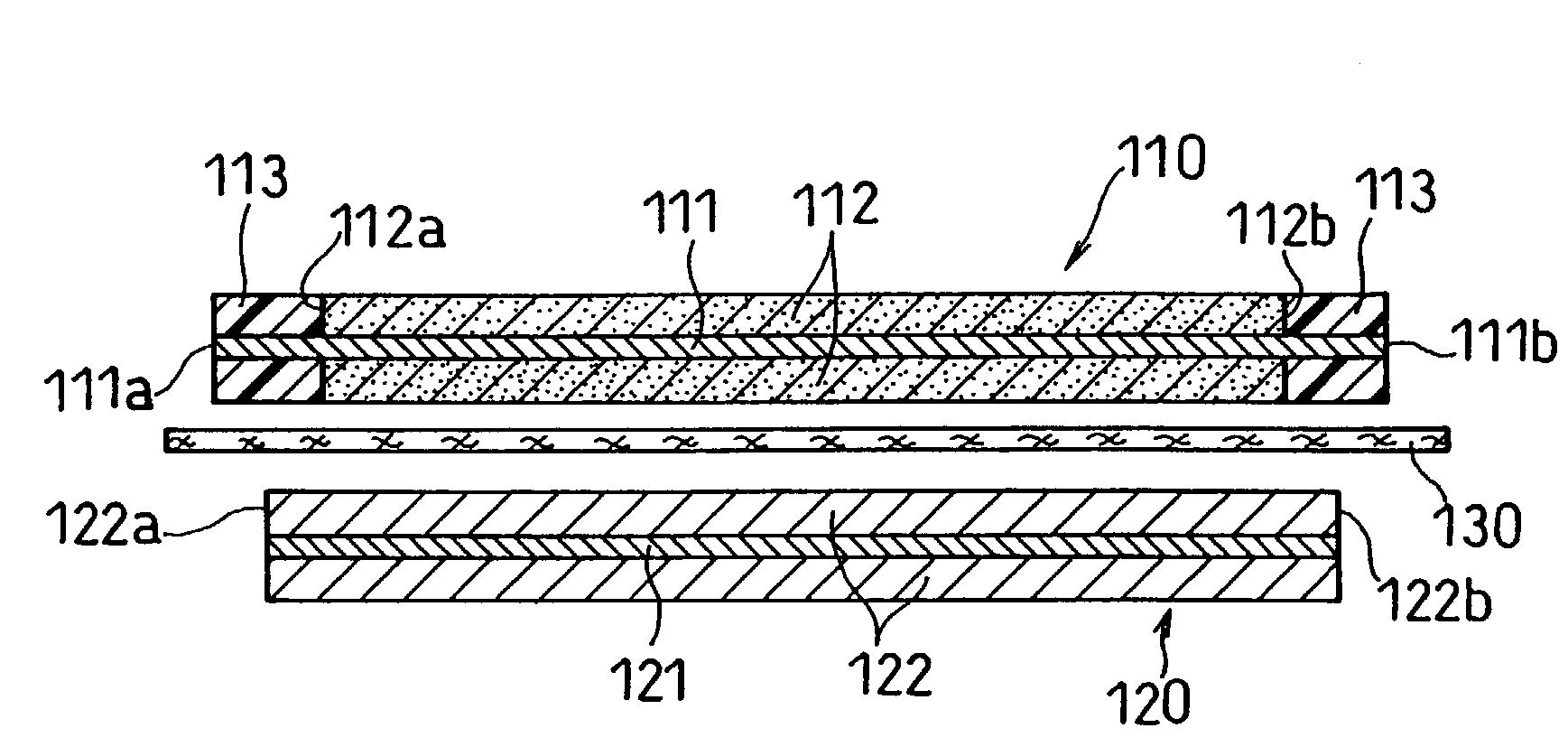
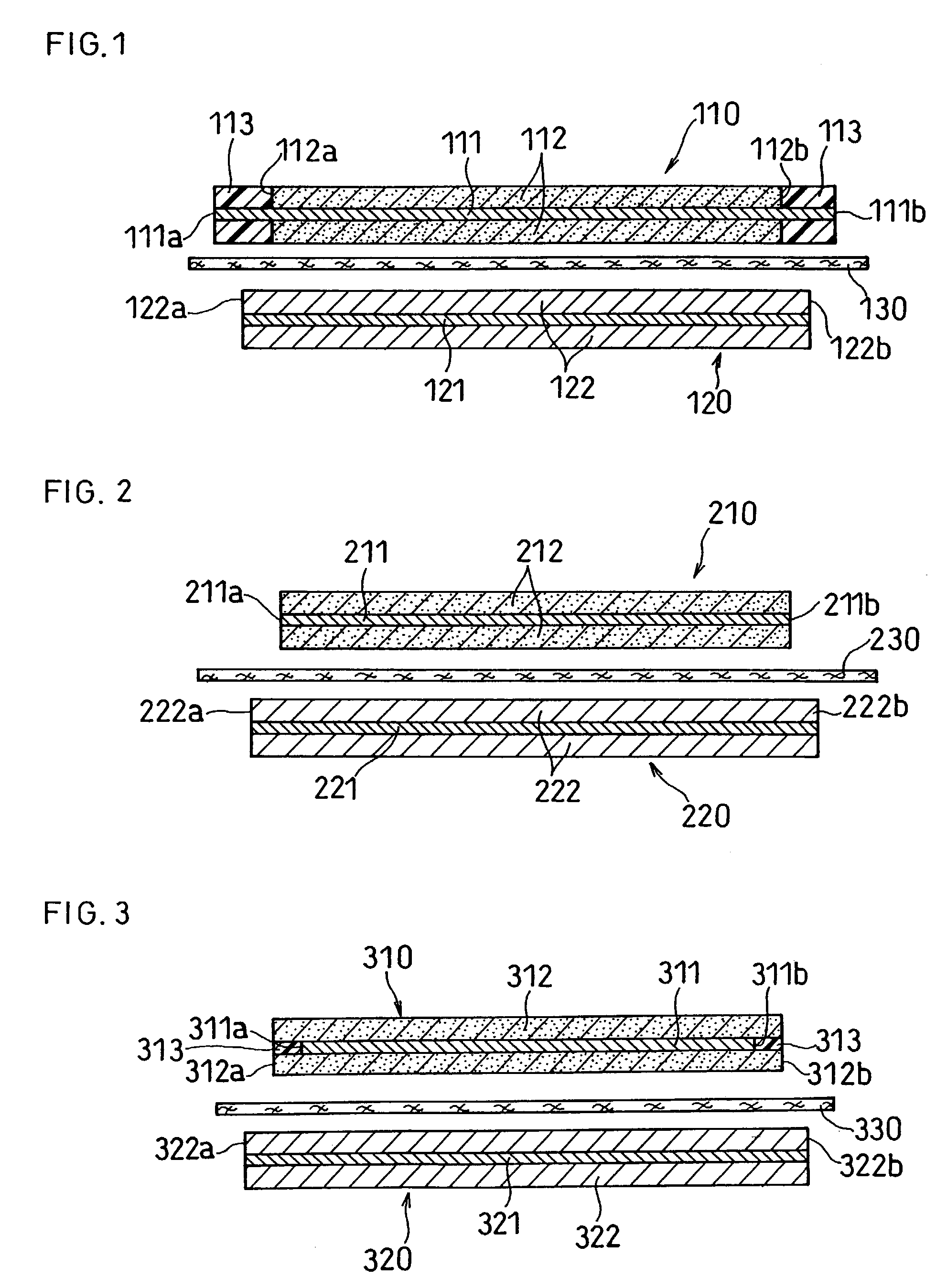
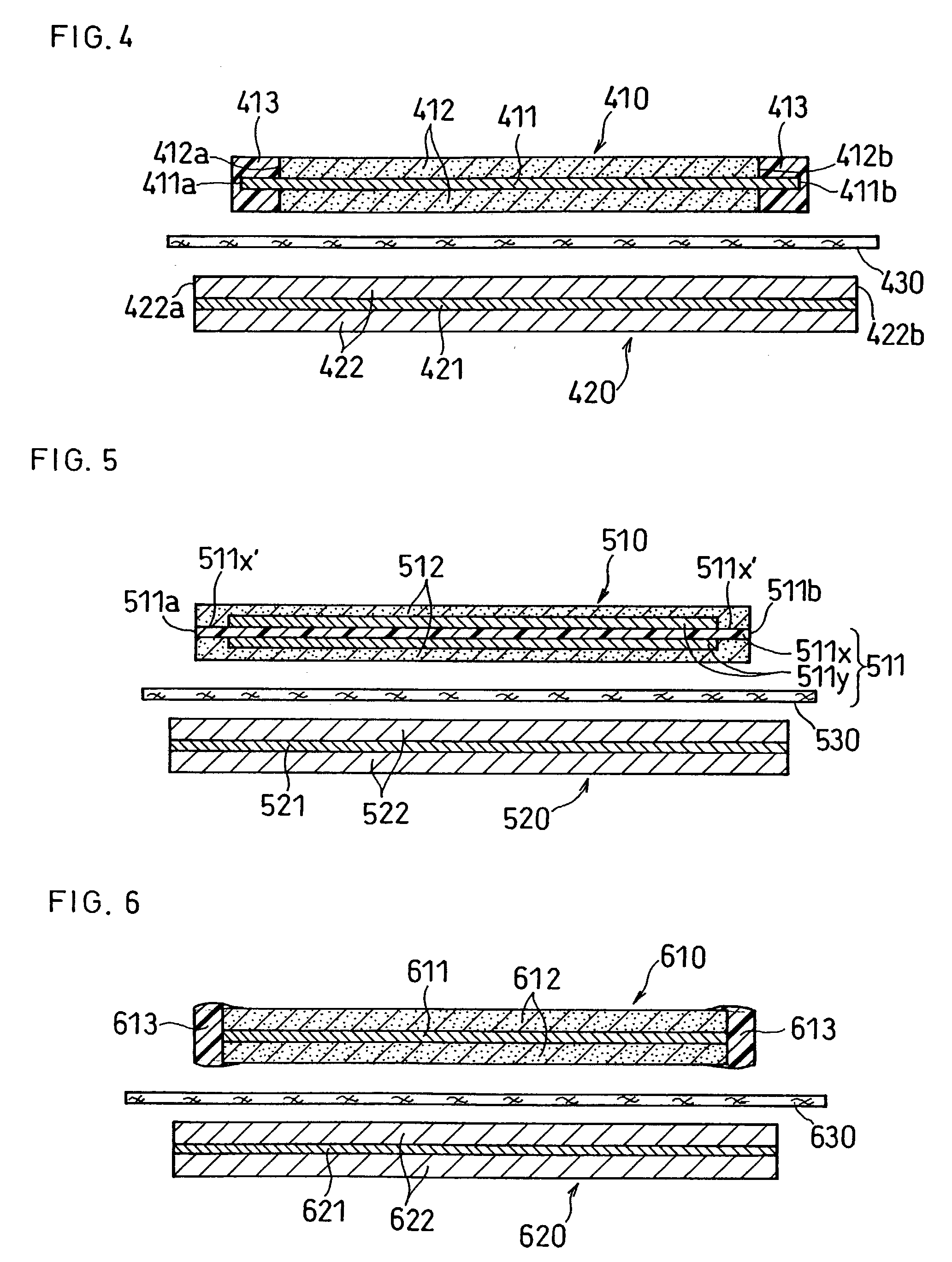
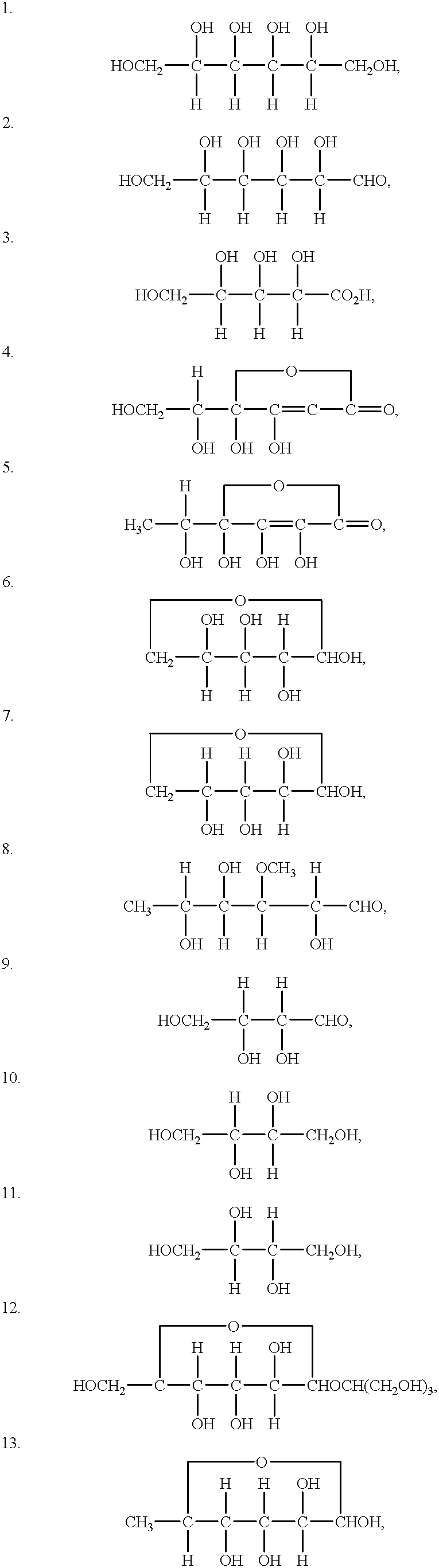
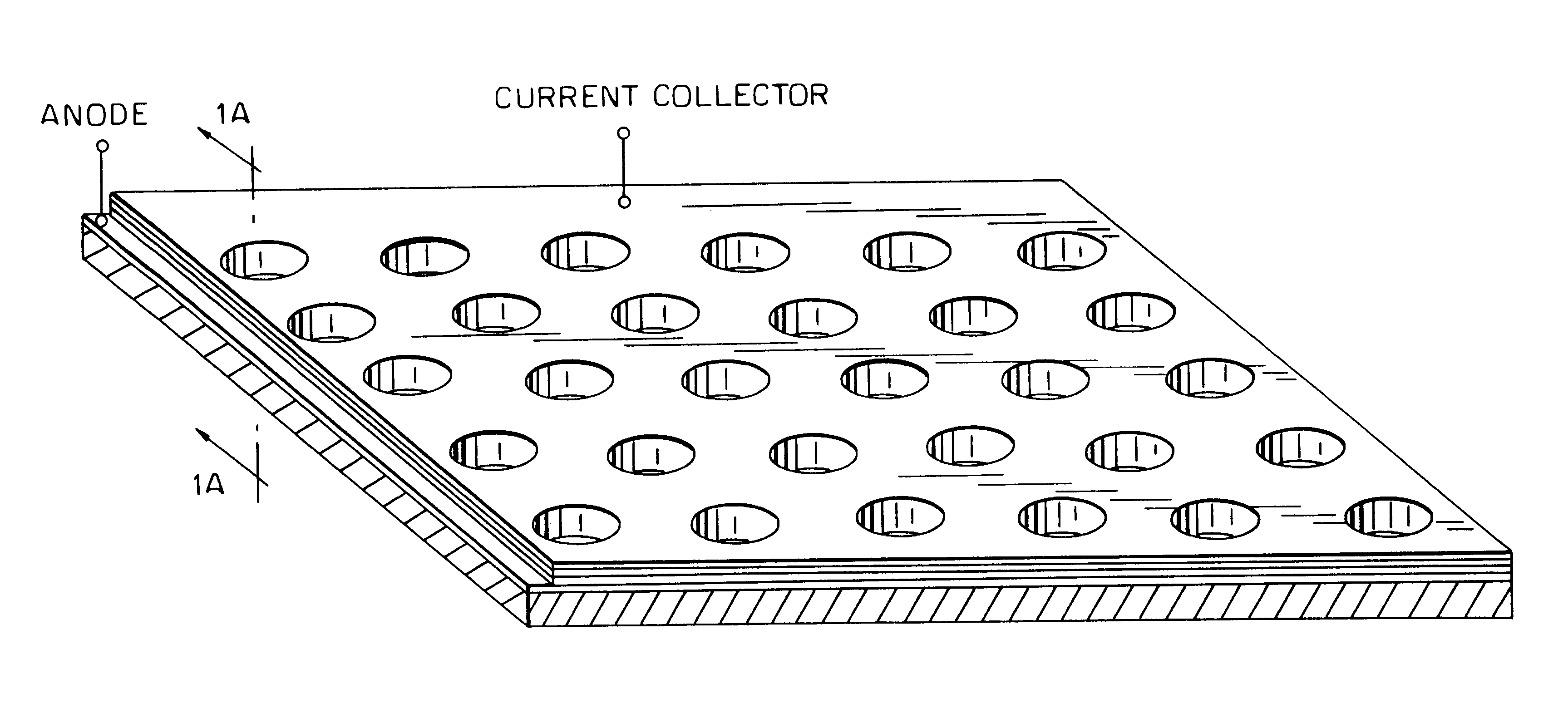
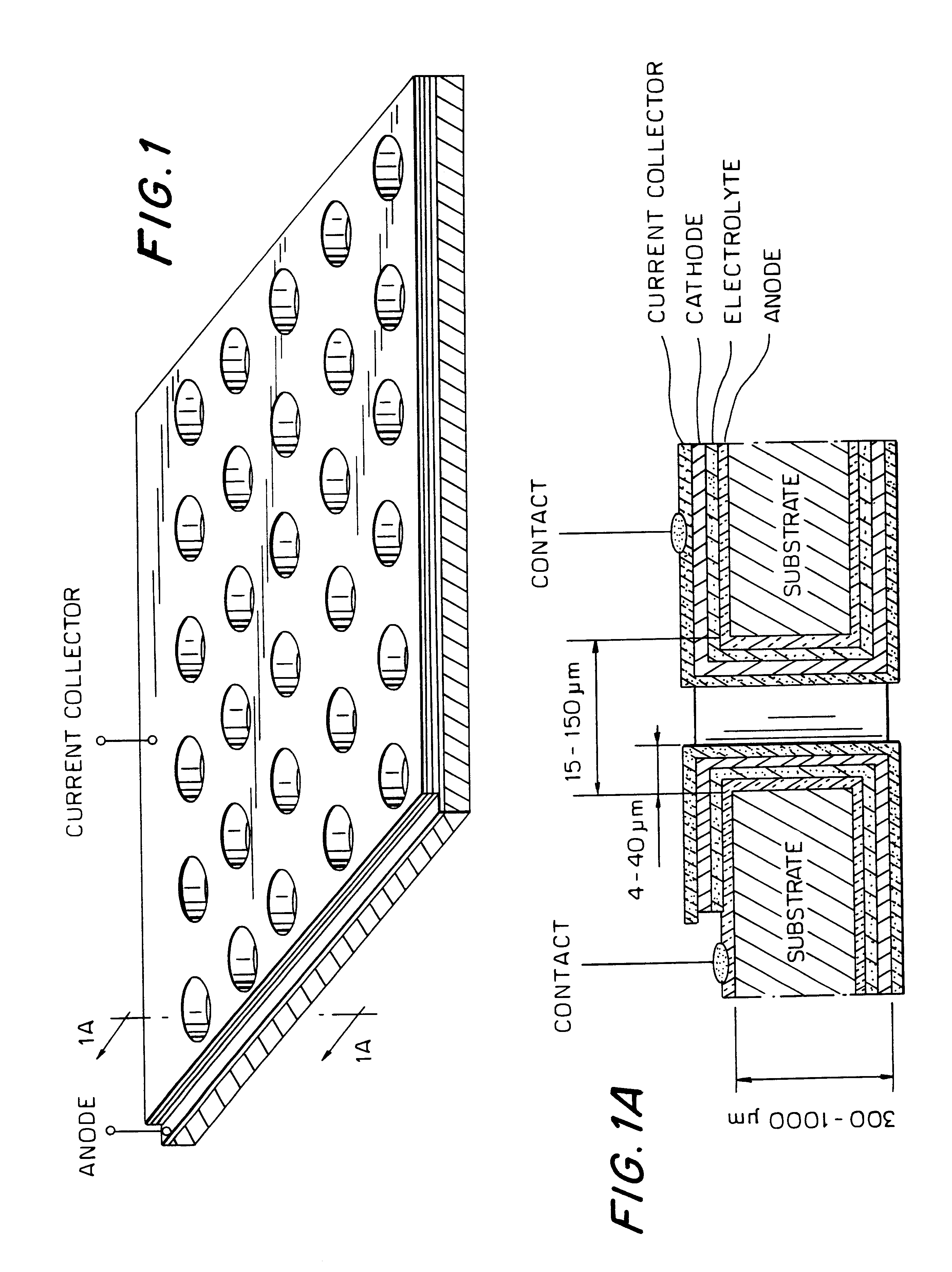
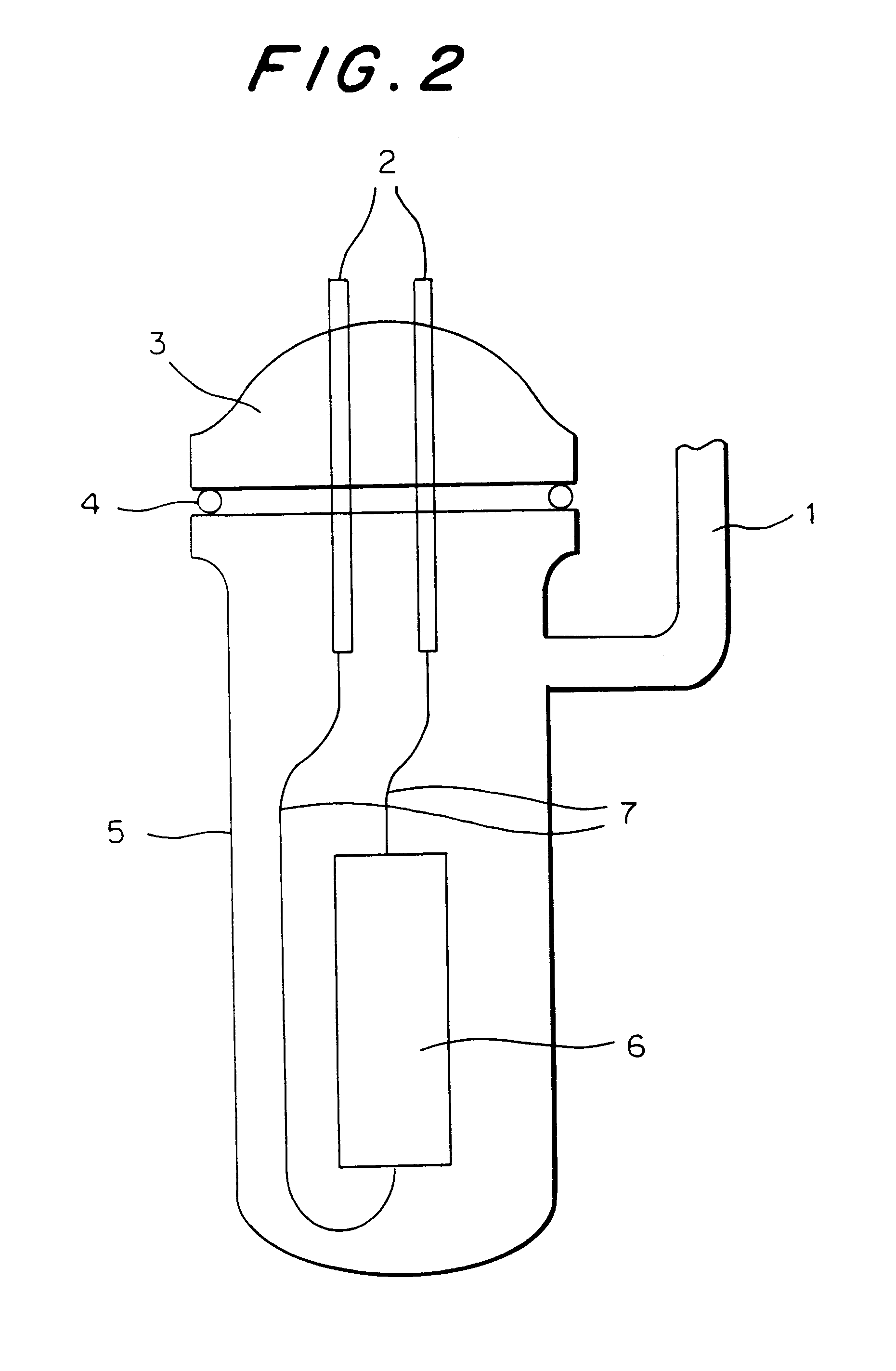
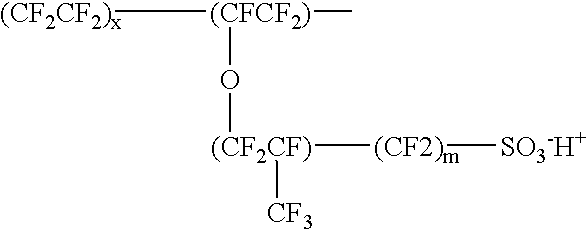
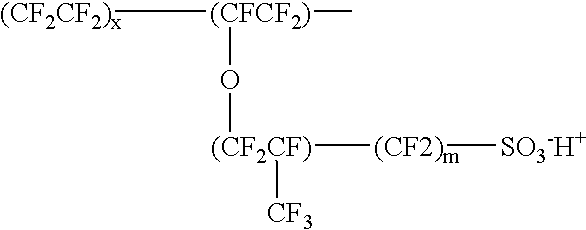
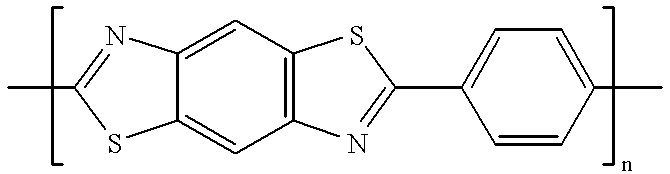
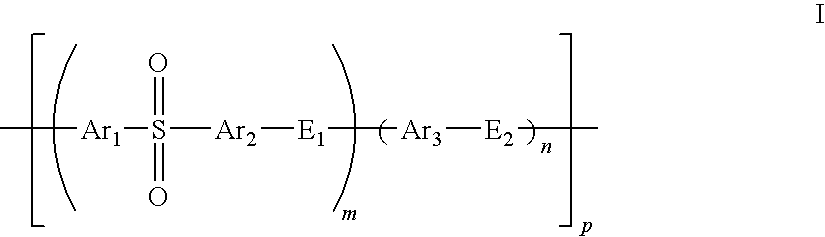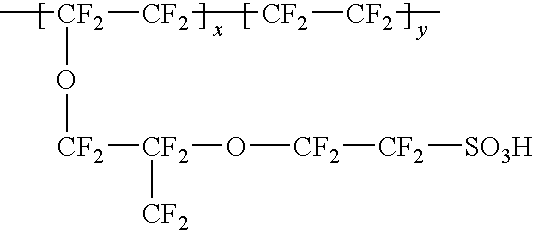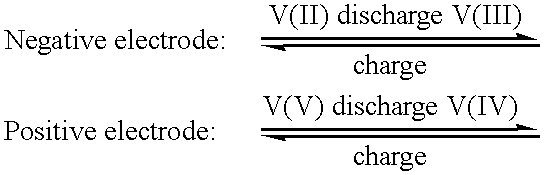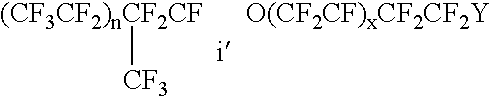Patents
Literature
2426results about "Solid electrolyte cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
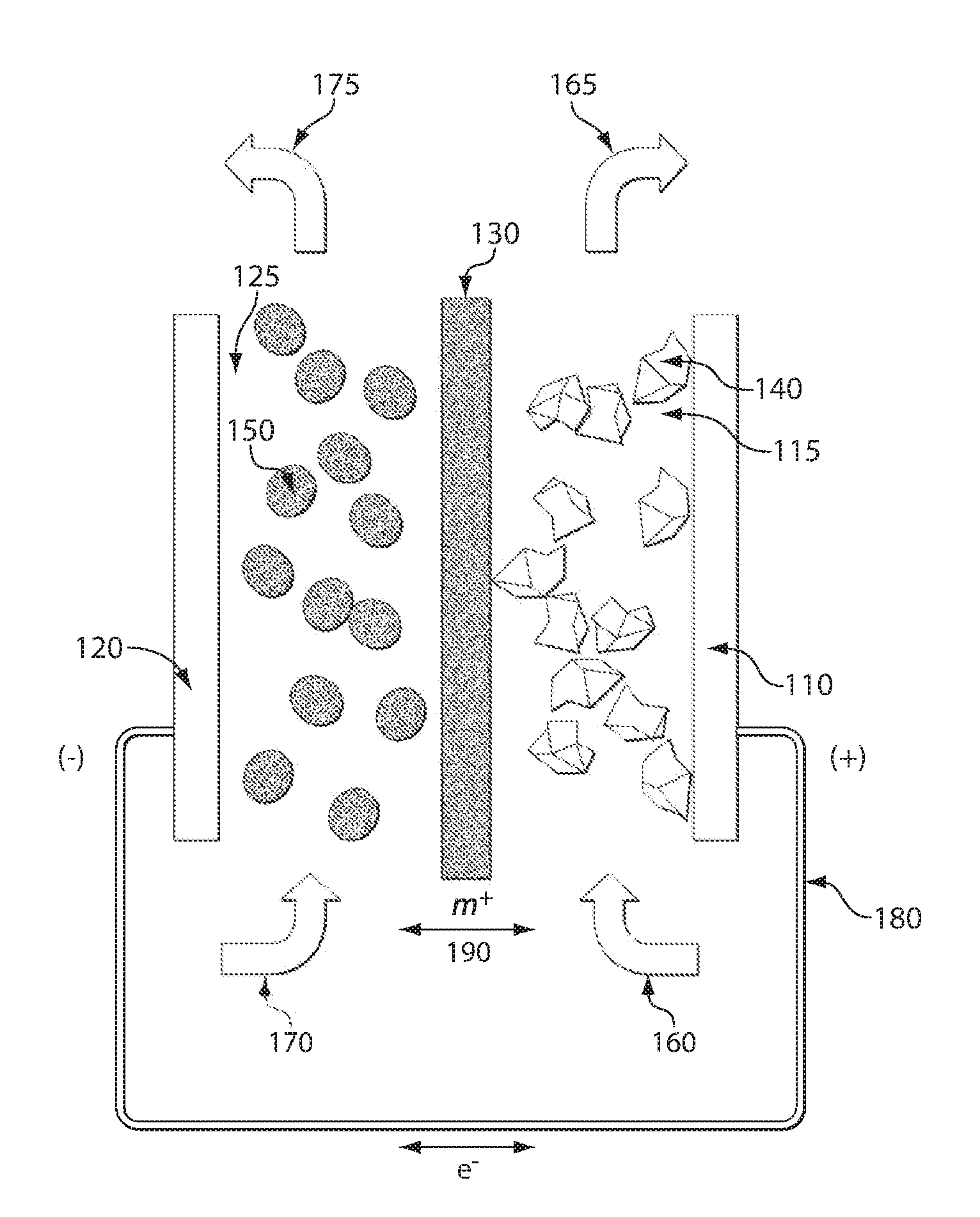
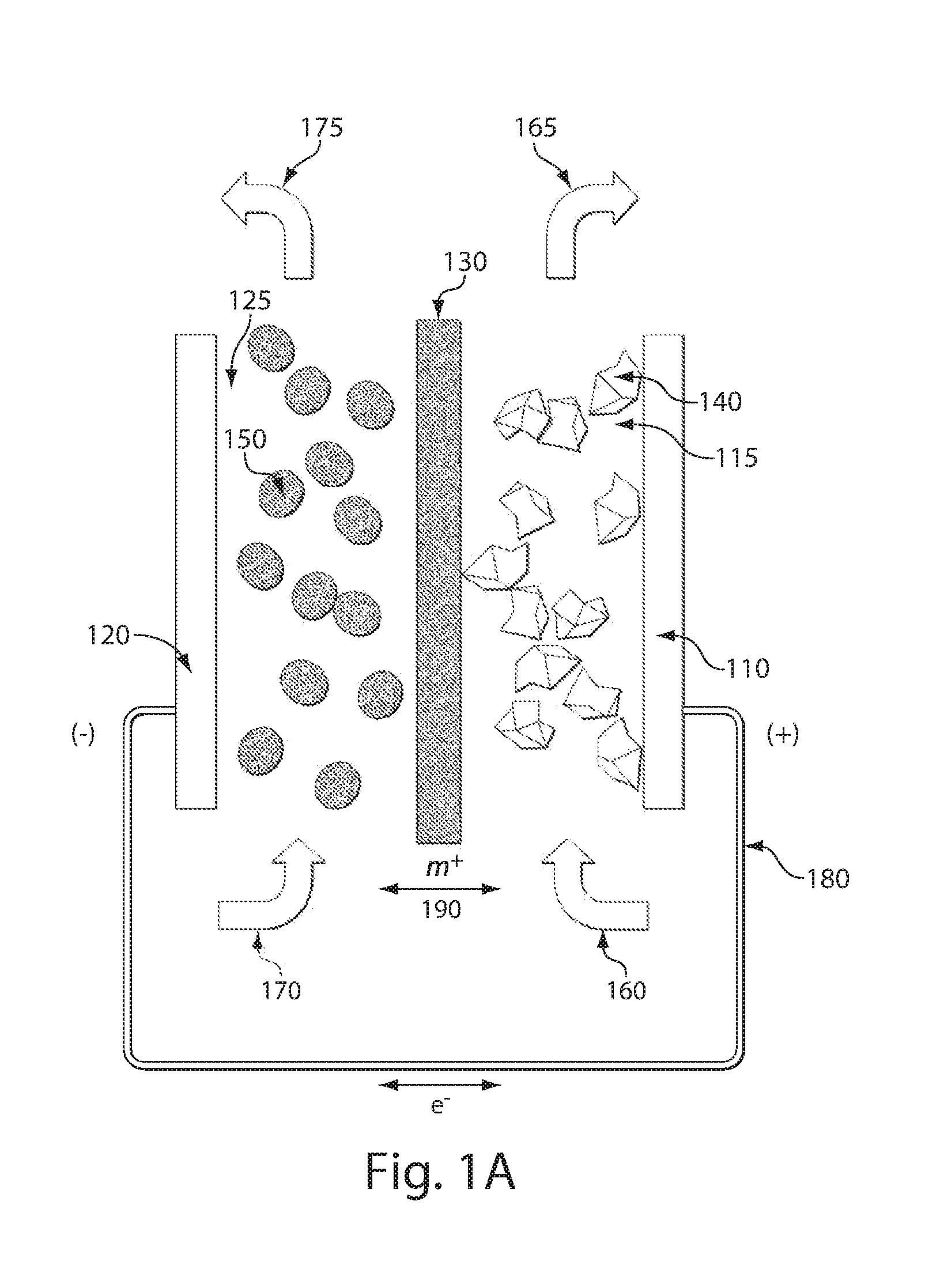
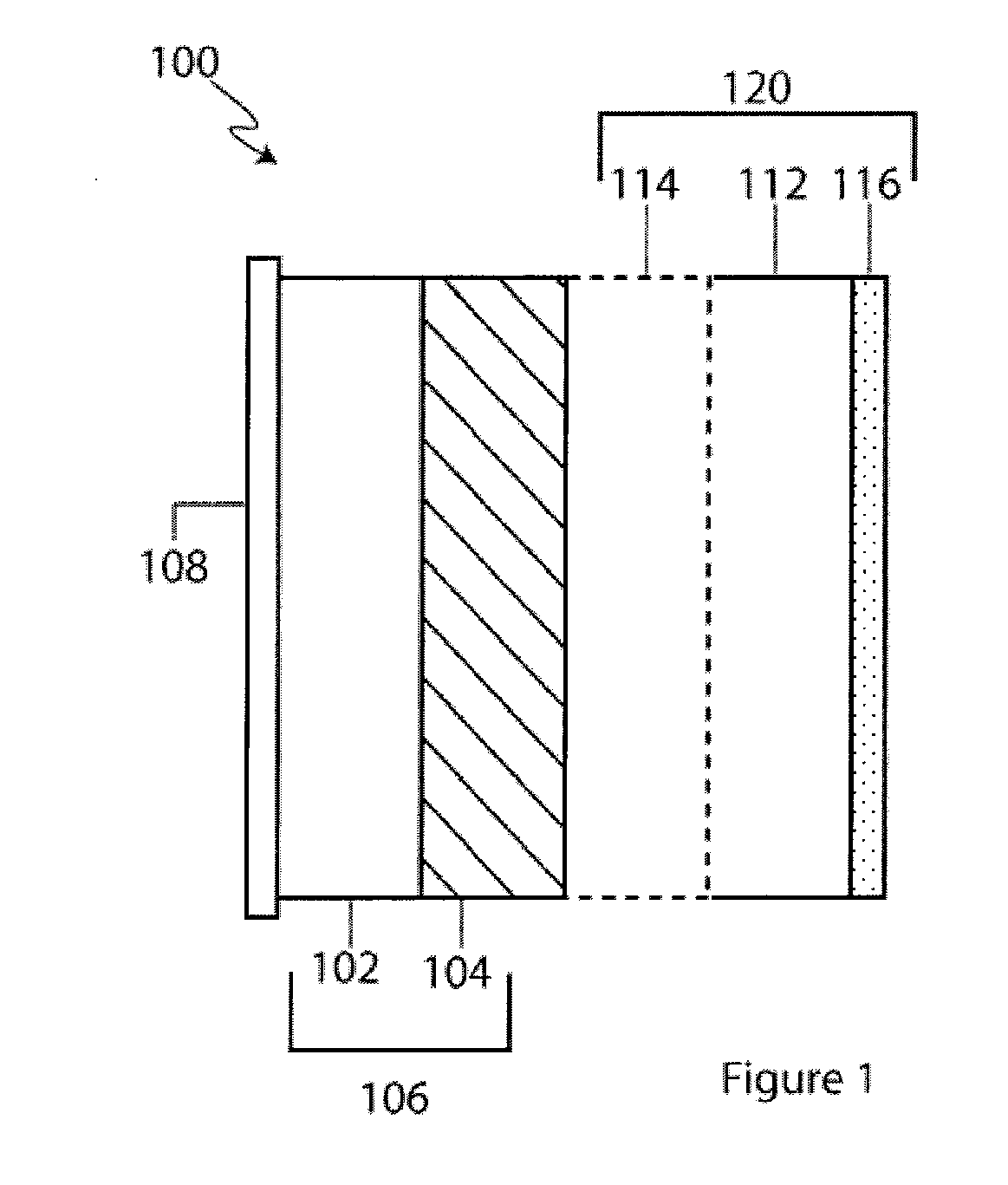
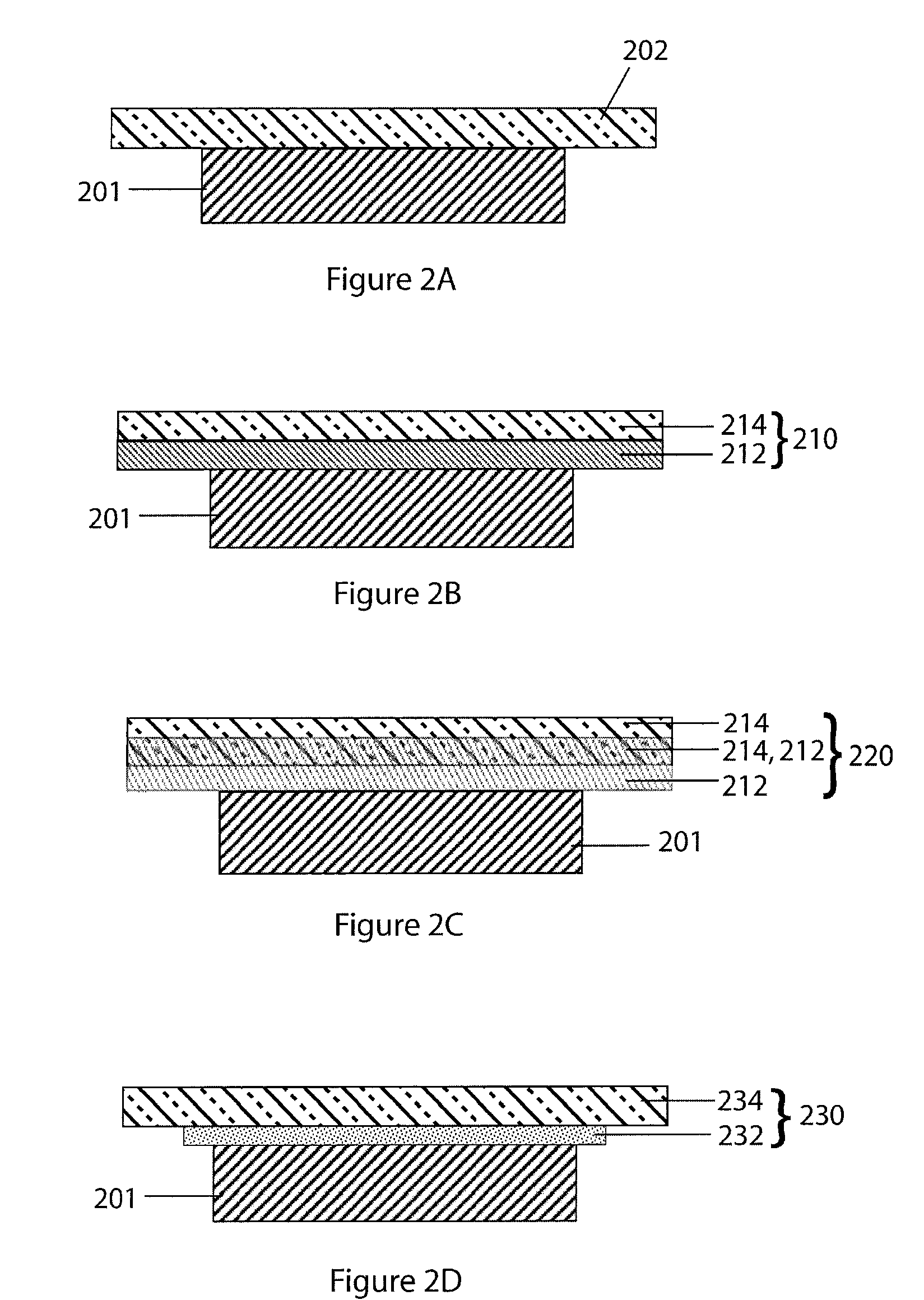
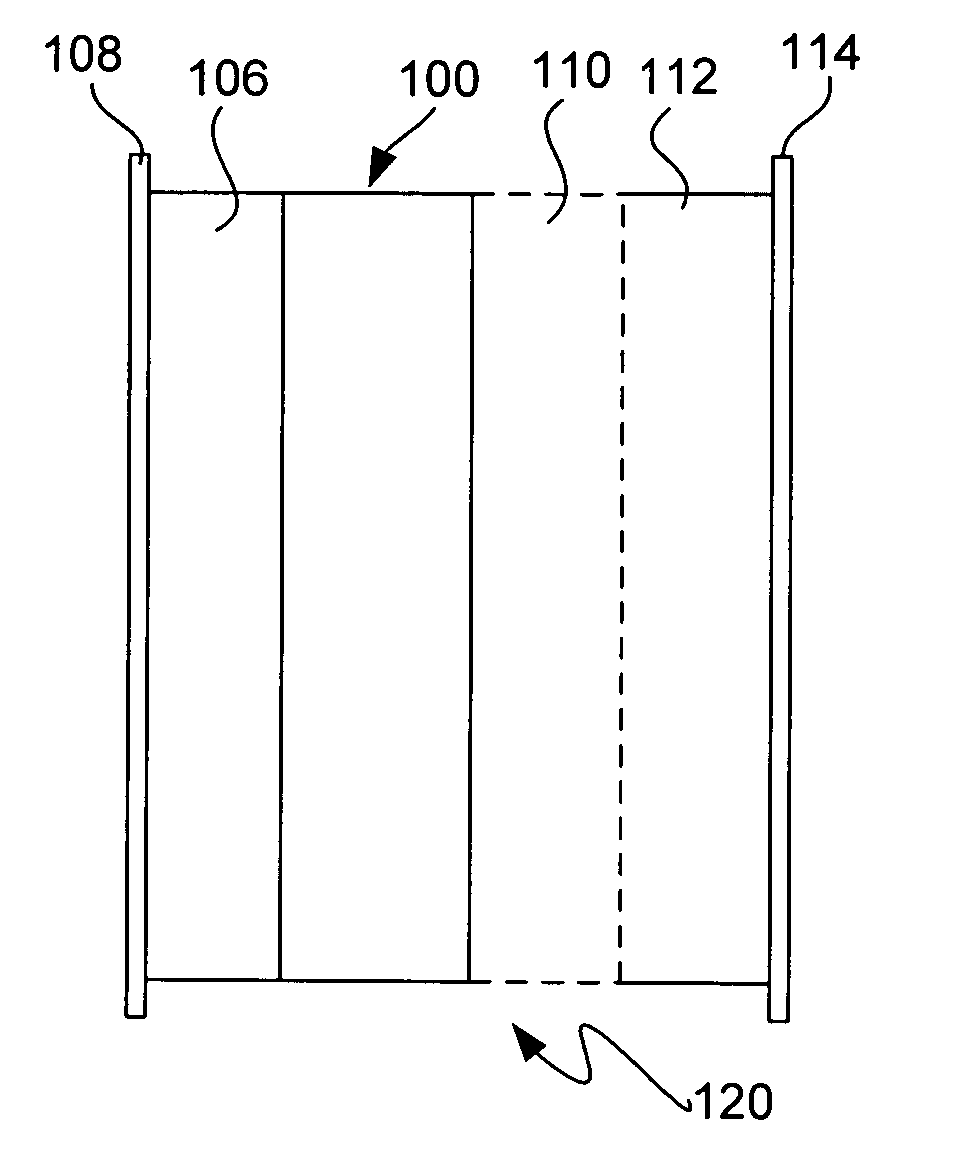
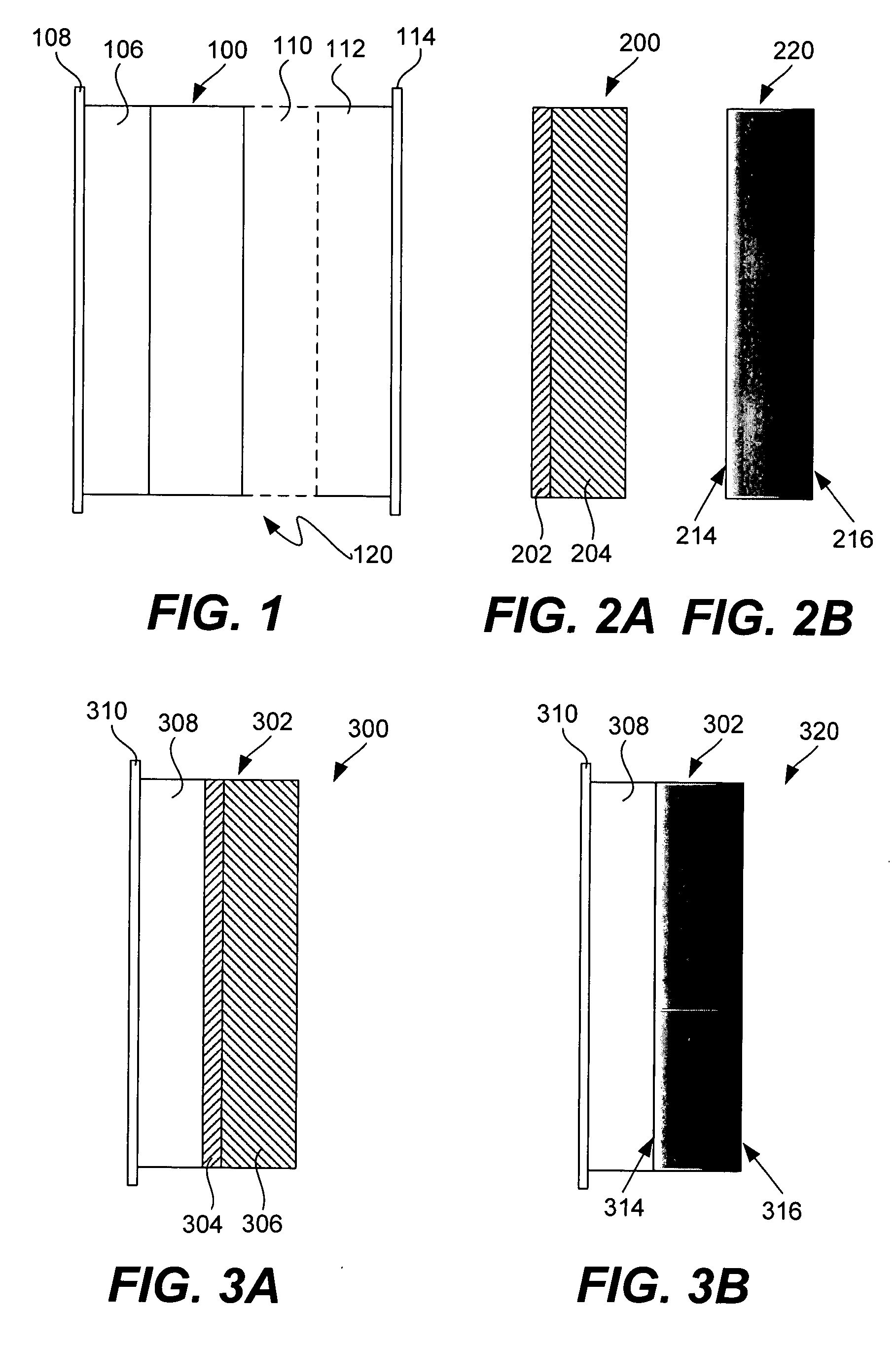
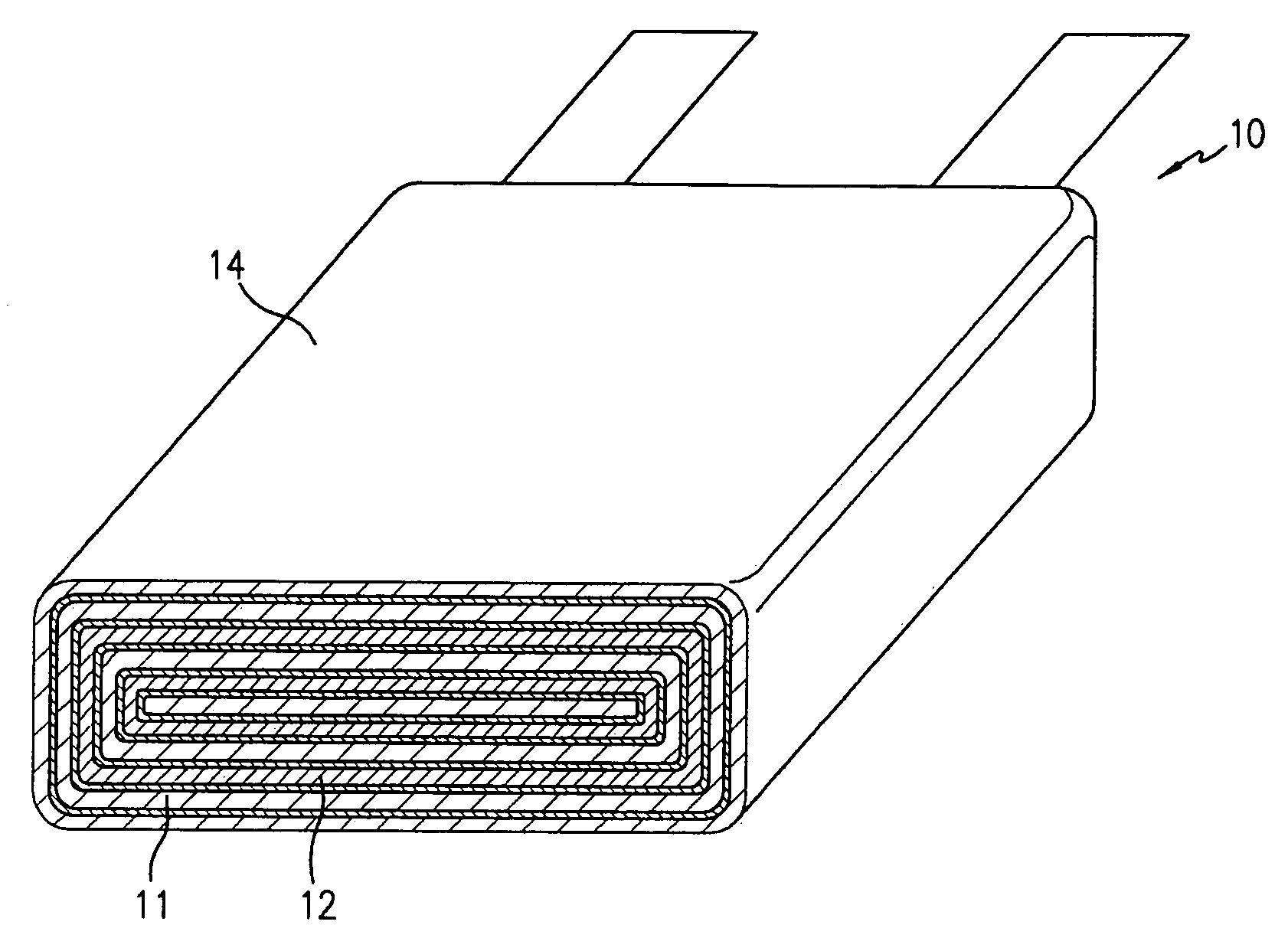
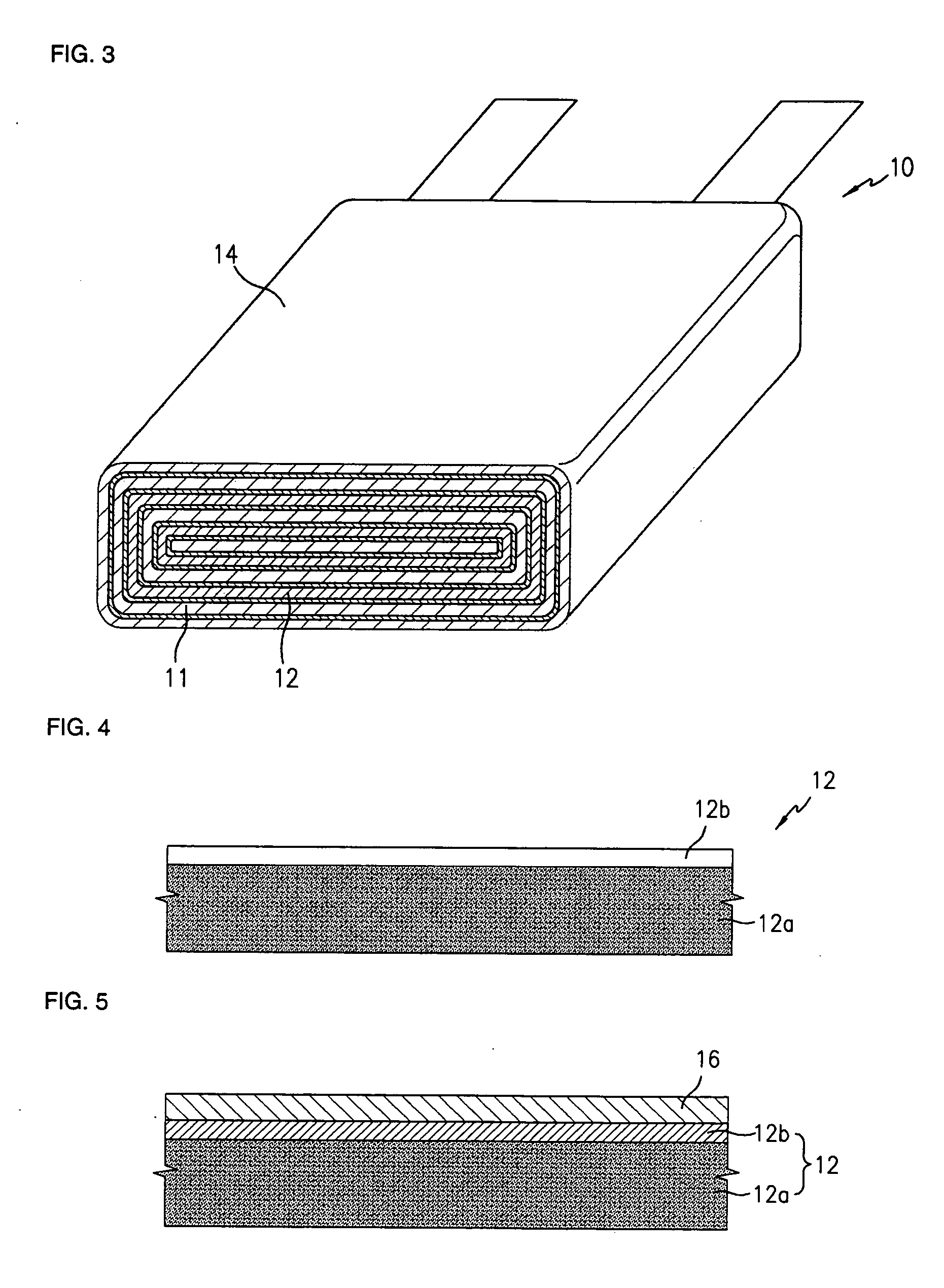
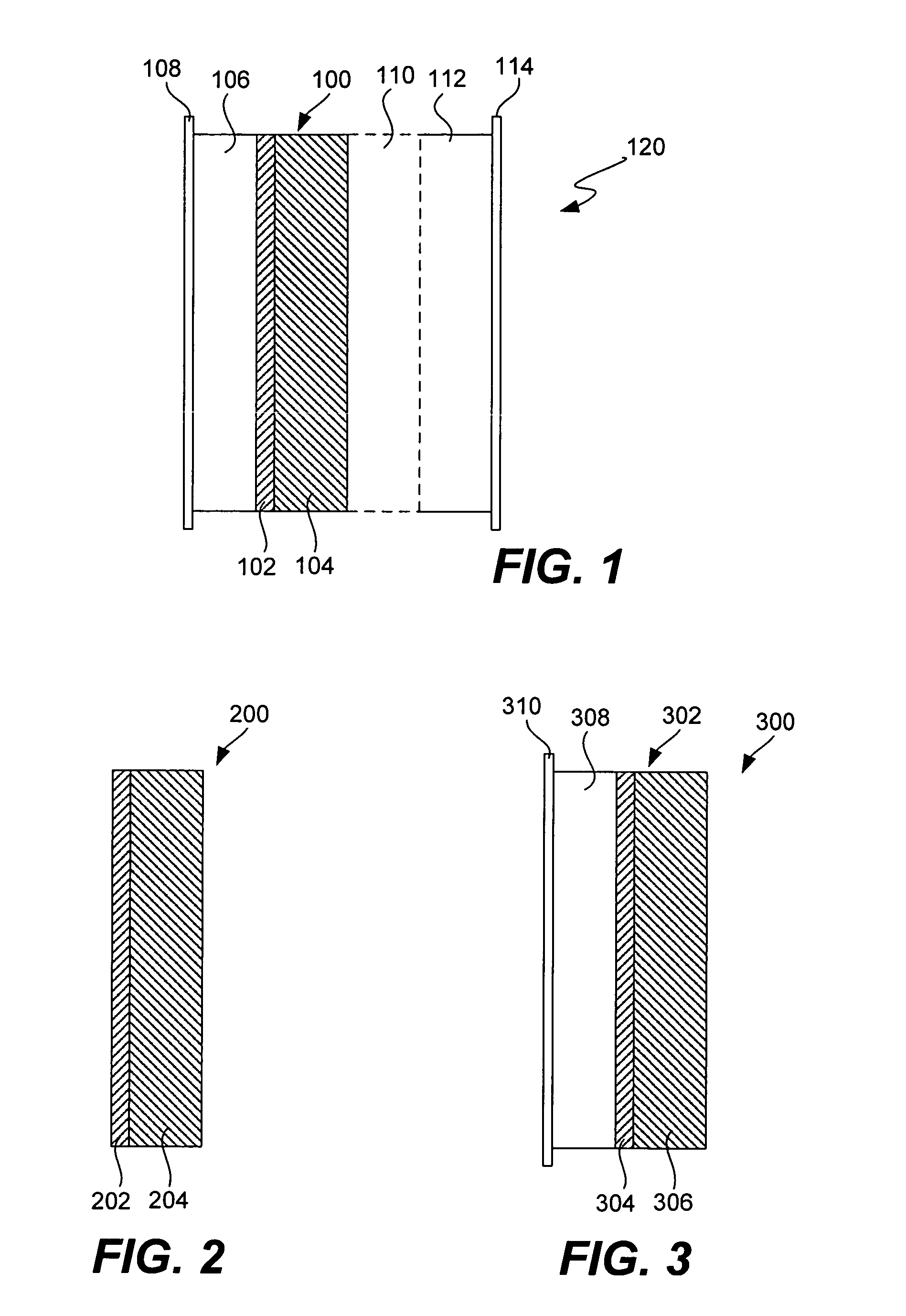
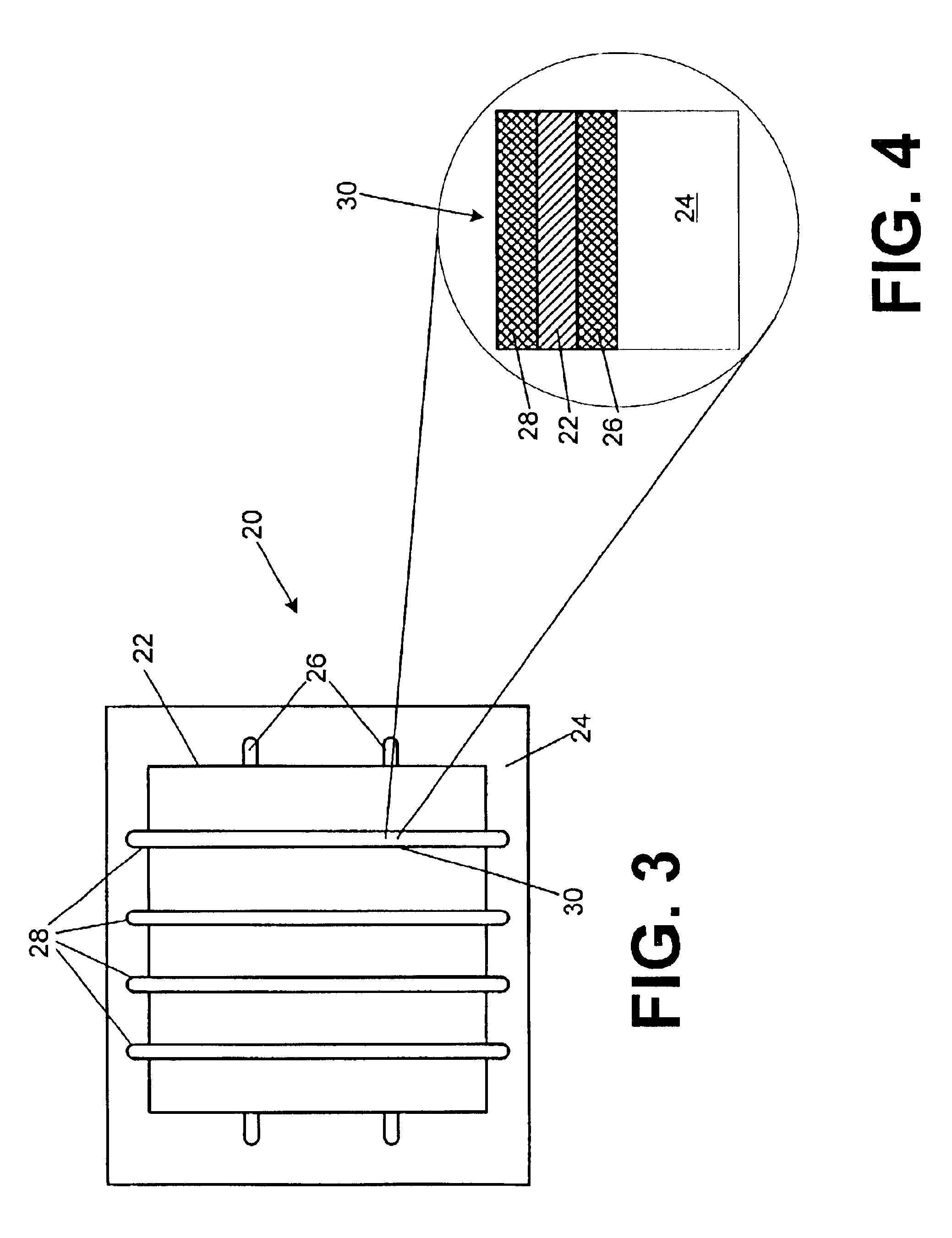
Lithium anodes for electrochemical cells
InactiveUS7247408B2Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
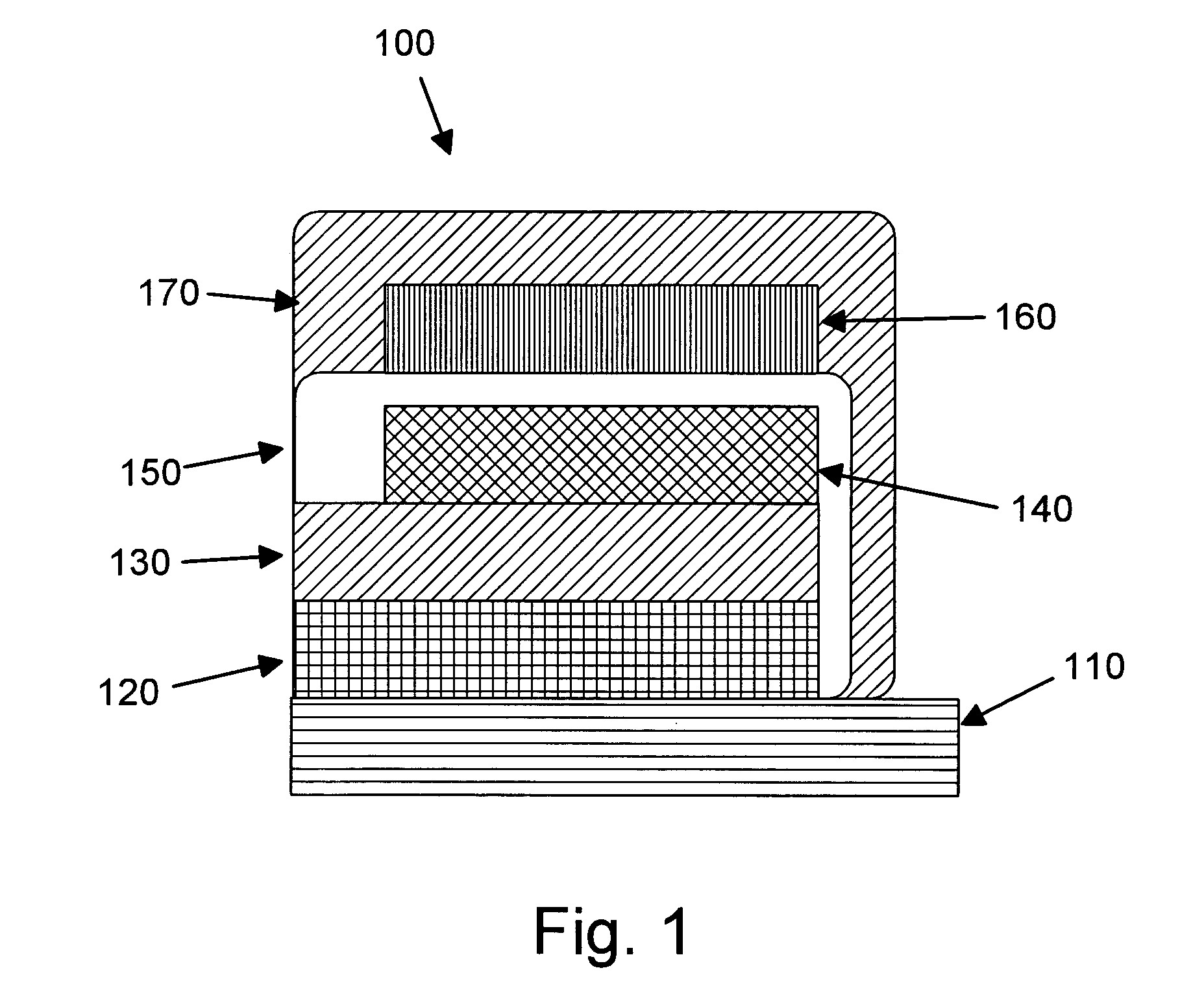
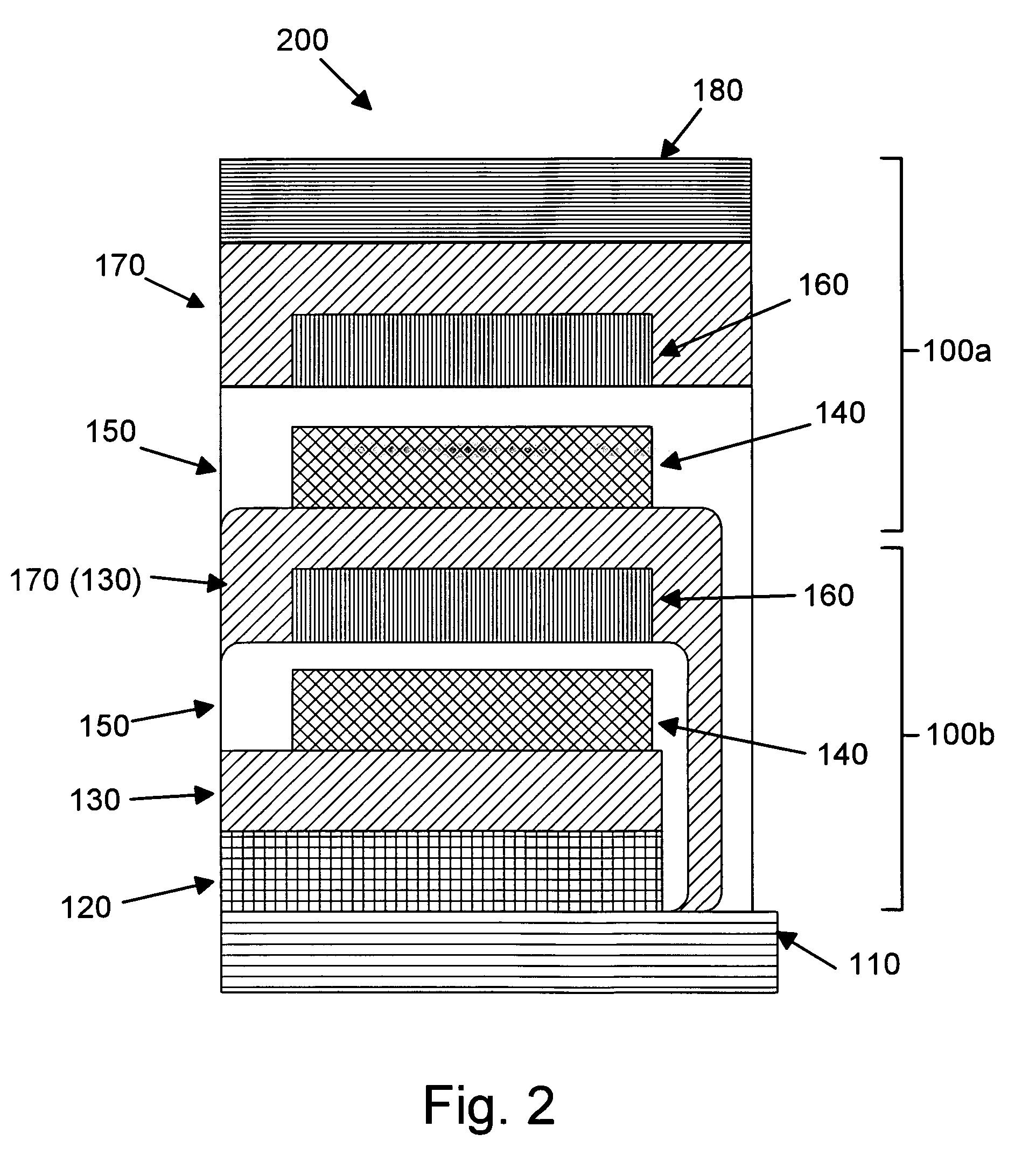
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Rechargeable thin film battery and method for making the same
InactiveUS6982132B1Improve lithium ion mobilityHigh voltageElectrode thermal treatmentFinal product manufactureElectrical batteryHigh energy
A rechargeable, stackable, thin film, solid-state lithium electrochemical cell, thin film lithium battery and method for making the same is disclosed. The cell and battery provide for a variety configurations, voltage and current capacities. An innovative low temperature ion beam assisted deposition method for fabricating thin film, solid-state anodes, cathodes and electrolytes is disclosed wherein a source of energetic ions and evaporants combine to form thin film cell components having preferred crystallinity, structure and orientation. The disclosed batteries are particularly useful as power sources for portable electronic devices and electric vehicle applications where high energy density, high reversible charge capacity, high discharge current and long battery lifetimes are required.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Electroactive high storage capacity polyacetylene-co-polysulfur materials and electrolytic cells containing same
InactiveUS6117590AHigh storage capacity per unit weightFacilitates electron transportElectrode manufacturing processesNon-aqueous electrolyte accumulatorsElectrochemical cellElectrode material
The present invention relates to novel electroactive energy storing polyacetylene-co-polysulfur (PAS) materials of general formula (C2Sx)n wherein x is greater than 1 to about 100, and n is equal to or greater than 2. This invention also relates to novel rechargeable electrochemical cells containing positive electrode materials comprised of said polyacetylene-co-polysulfur materials with improved storage capacity and cycle life at ambient and sub-ambient temperatures.
Owner:THE BANK OF NEW YORK +1
Composite solid polymer electrolyte membranes
InactiveUS7550216B2Improve performanceLow costElectrolyte holding meansMembranesPolymer electrolytesFuel cells
Owner:FOSTER-MILLER
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Electrode materials with high surface conductivity
InactiveUS6855273B2Electrode manufacturing processesDouble layer capacitorsSurface conductivityIon exchange
The present invention concerns electrode materials capable of redox reactions by electrons and alkaline ions exchange with an electrolyte. The applications are in the field of primary (batteries) or secondary electrochemical generators, super capacitors and light modulating system of the super capacitor type.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Electrochemical device separator structures with barrier layer on non-swelling membrane
InactiveUS7070632B1Avoid harmful reactionsElectrode carriers/collectorsSolid electrolyte cellsElectrochemistryFluid electrolytes
Disclosed are electrochemical device separator structures which include a substantially impervious active metal ion conducting barrier layer material, such as an ion conducting glass, is formed on an active metal ion conducting membrane in which elongation due to swelling on contact with liquid electrolyte is constrained in at least two of three orthogonal dimensions of the membrane. The non-swelling character of the membrane prevents elongation in the x-y (or lateral, relative to the layers of the composite) orthogonal dimensions of the membrane when it is contacted with liquid electrolyte that would otherwise cause the barrier layer to rupture. Substantial swelling of the membrane, if any, is limited to the z (or vertical, relative to the layers of the composite) dimension.
Owner:POLYPLUS BATTERY CO INC
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
Li/air non-aqueous batteries
ActiveUS20070117007A1Improve battery performanceLarge capacityFuel and primary cellsFuel and secondary cellsLithiumOxygen
Non-aqueous alkali metal (e.g., Li) / oxygen battery cells constructed with a protected anode that minimizes anode degradation and maximizes cathode performance by enabling the use of cathode performance enhancing solvents in the catholyte have negligible self-discharge and high deliverable capacity. In particular, protected lithium-oxygen batteries with non-aqueous catholytes have this improved performance.
Owner:POLYPLUS BATTERY CO INC
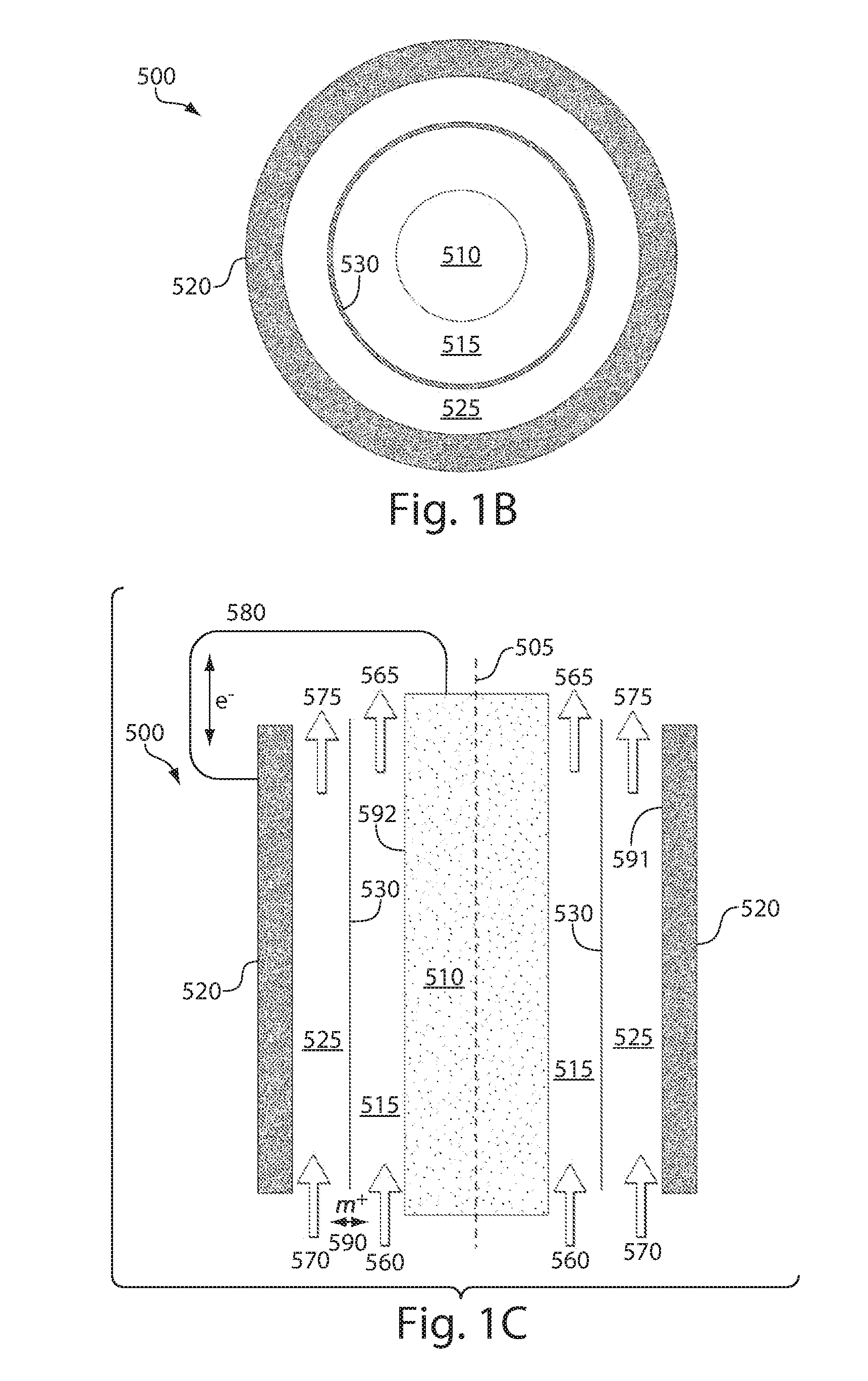
Ionically conductive membranes for protection of active metal anodes and battery cells
InactiveUS20040191617A1Improve propertiesImprove ionic conductivitySolid electrolytesSolid electrolyte cellsChemical stabilityBattery cell
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
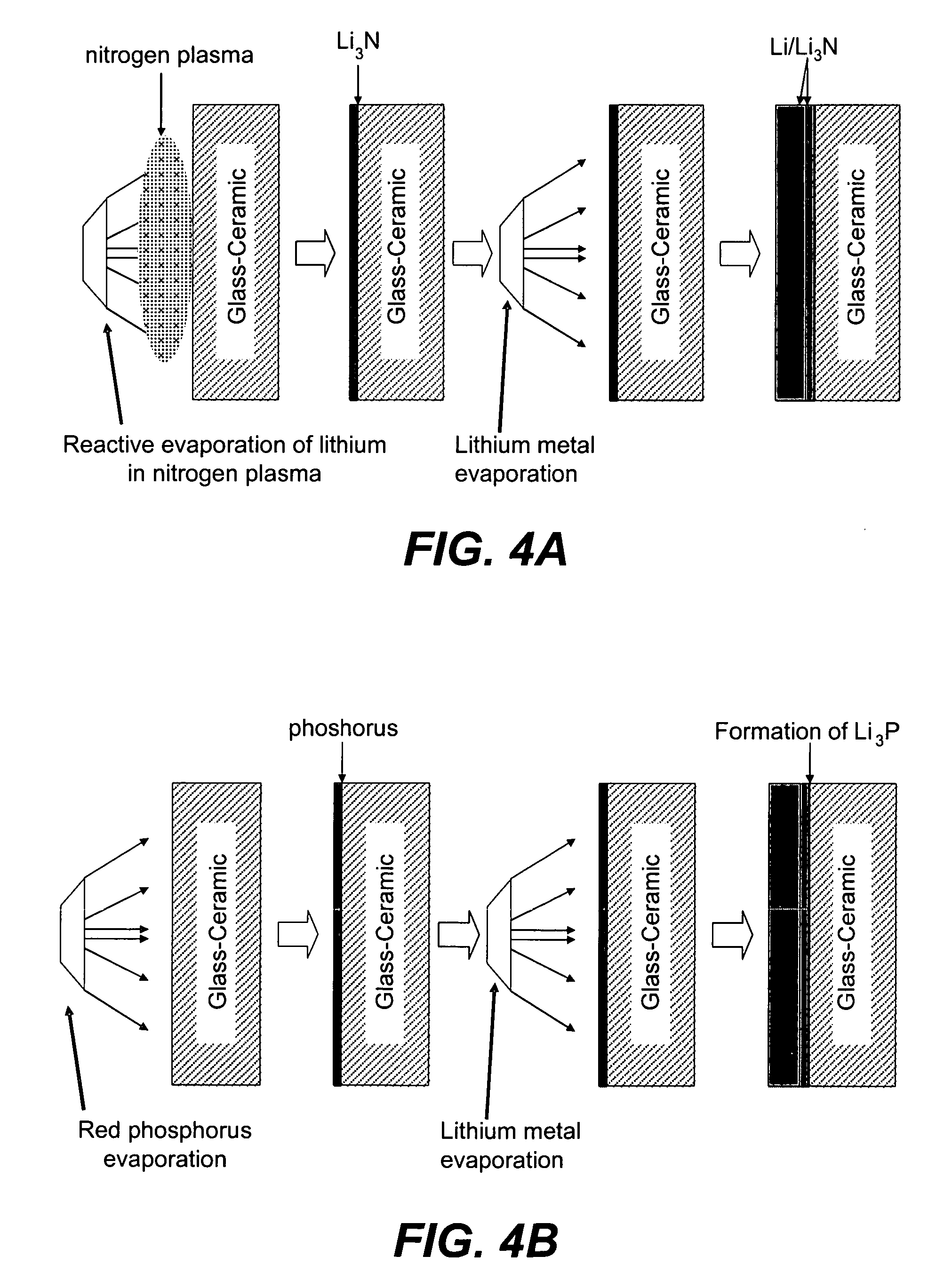
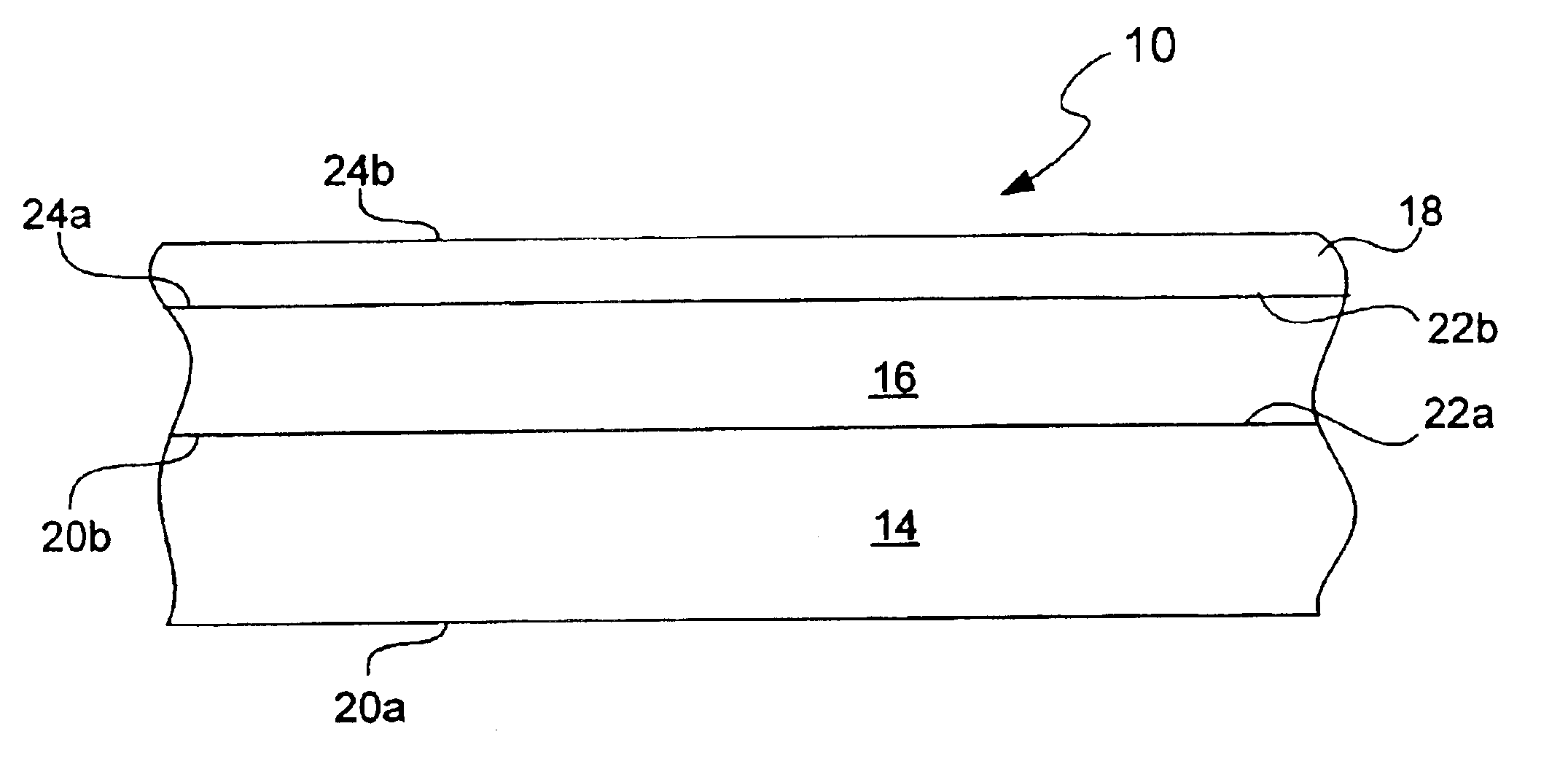
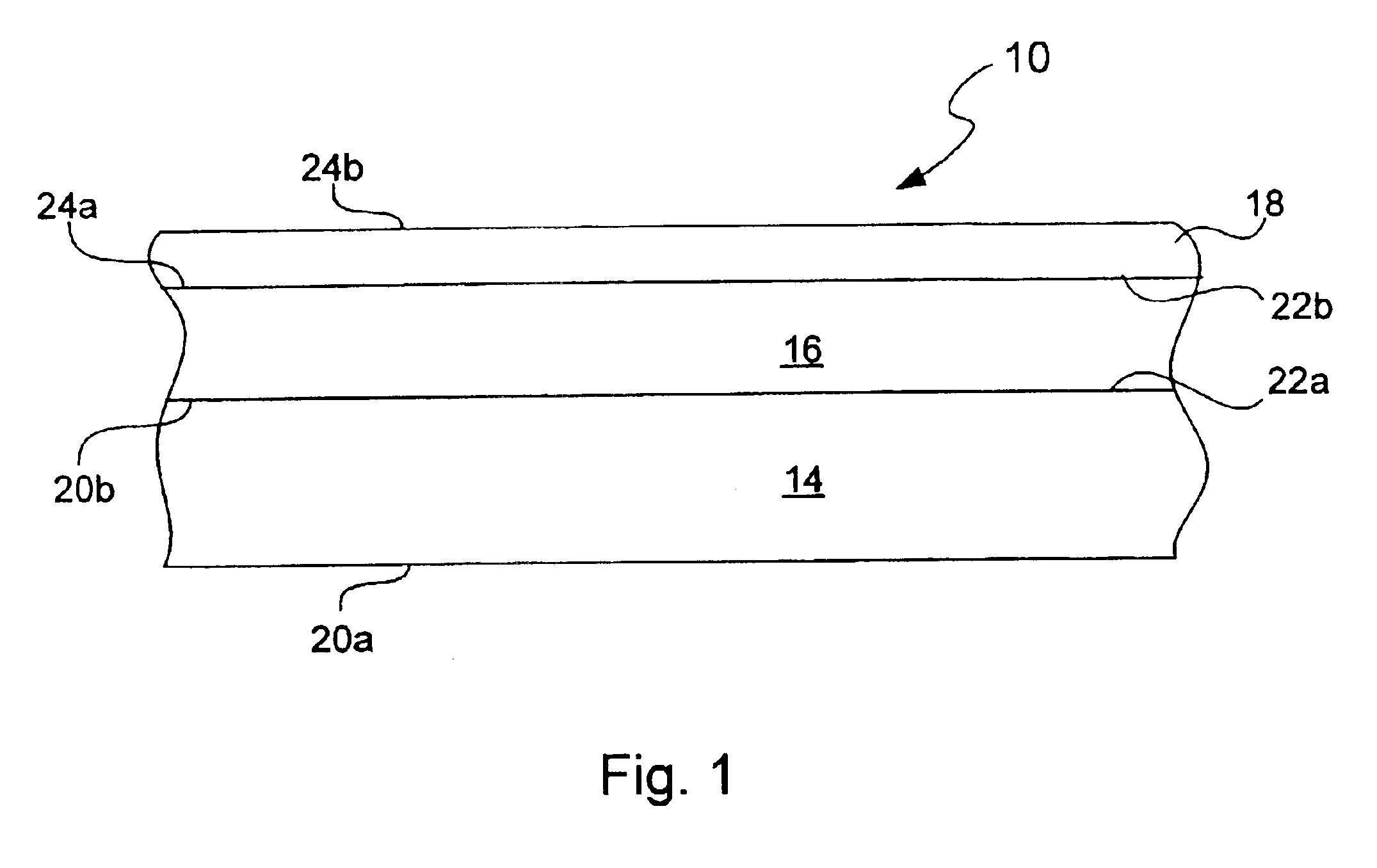
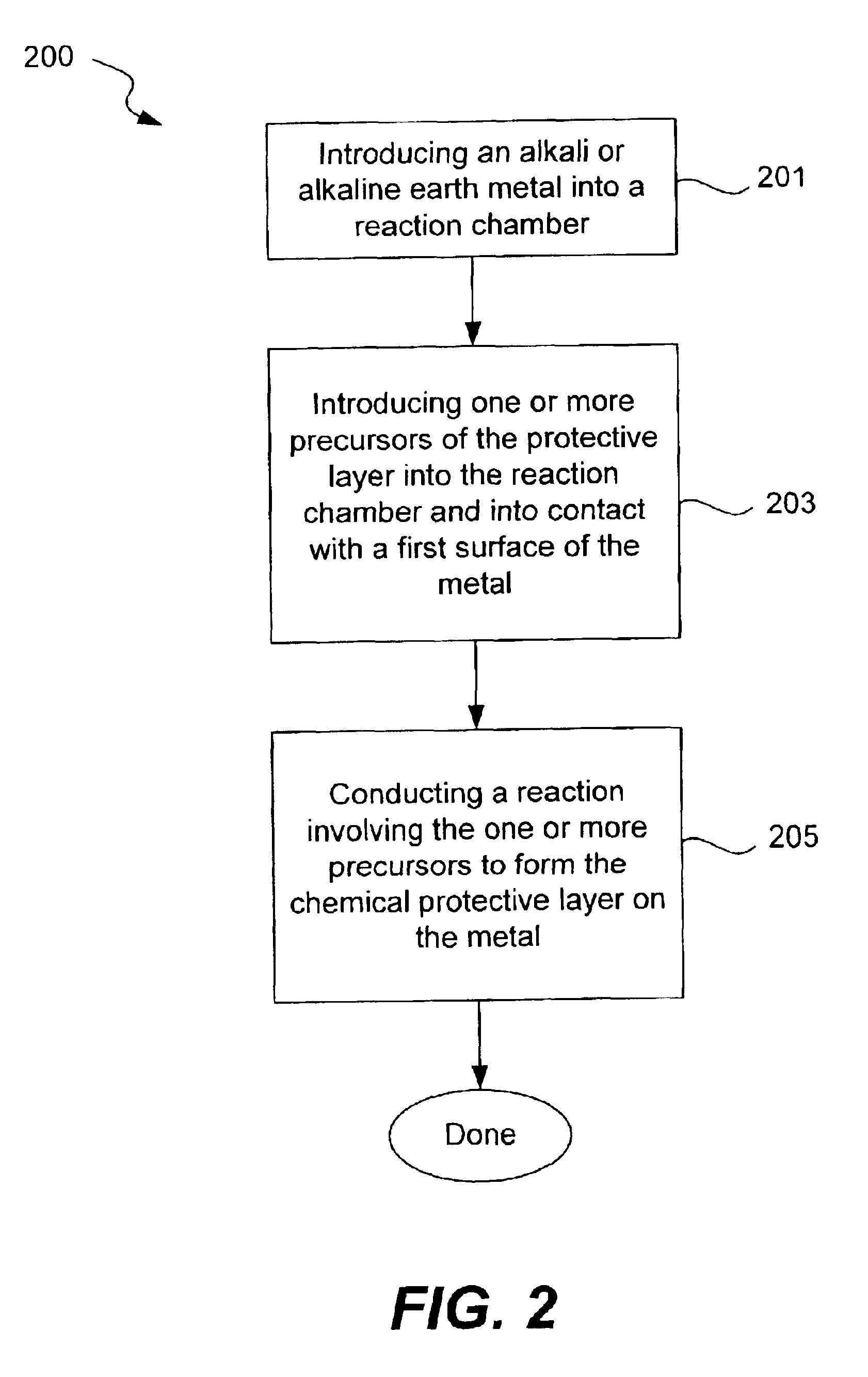
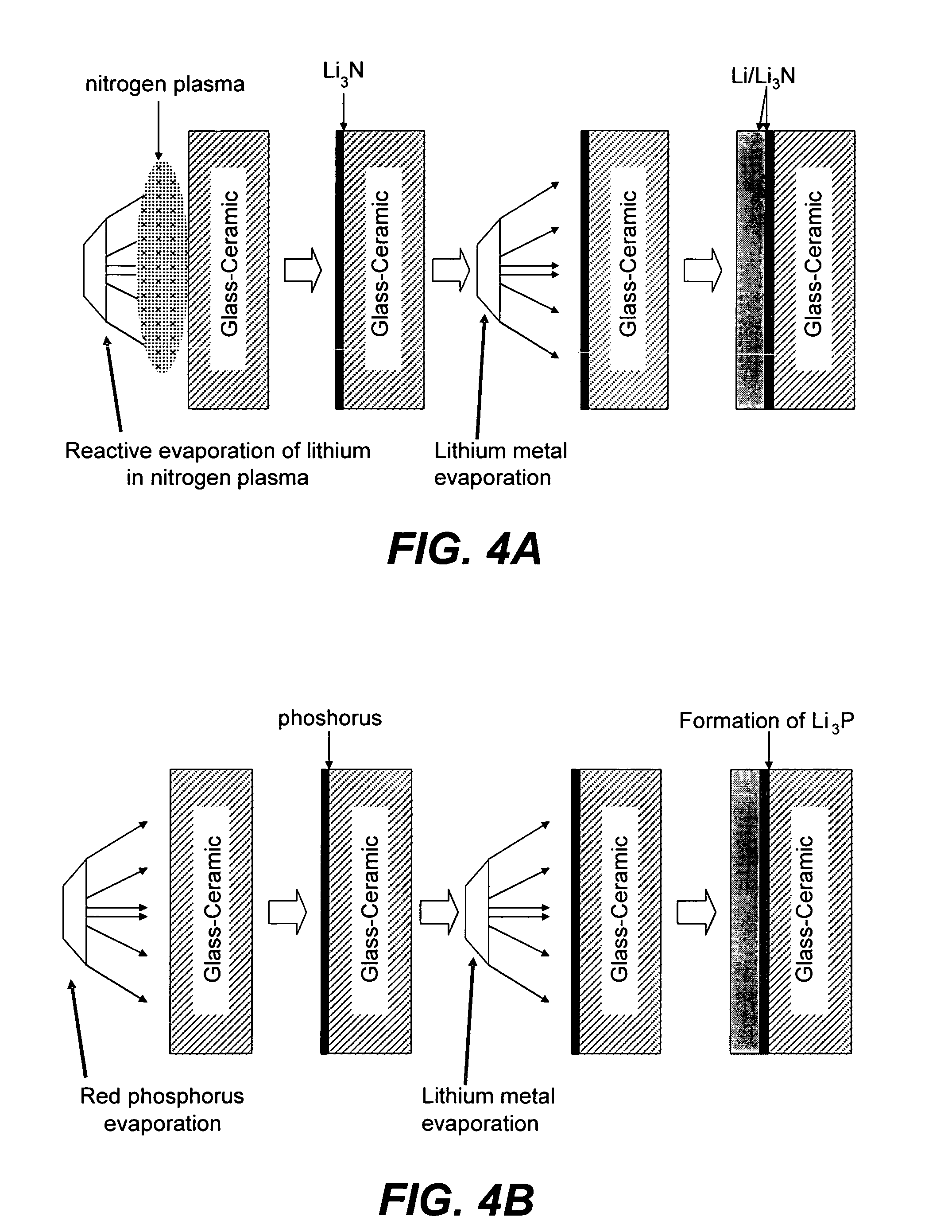
Chemical protection of a lithium surface
InactiveUS6911280B1Easy to produceSimple processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
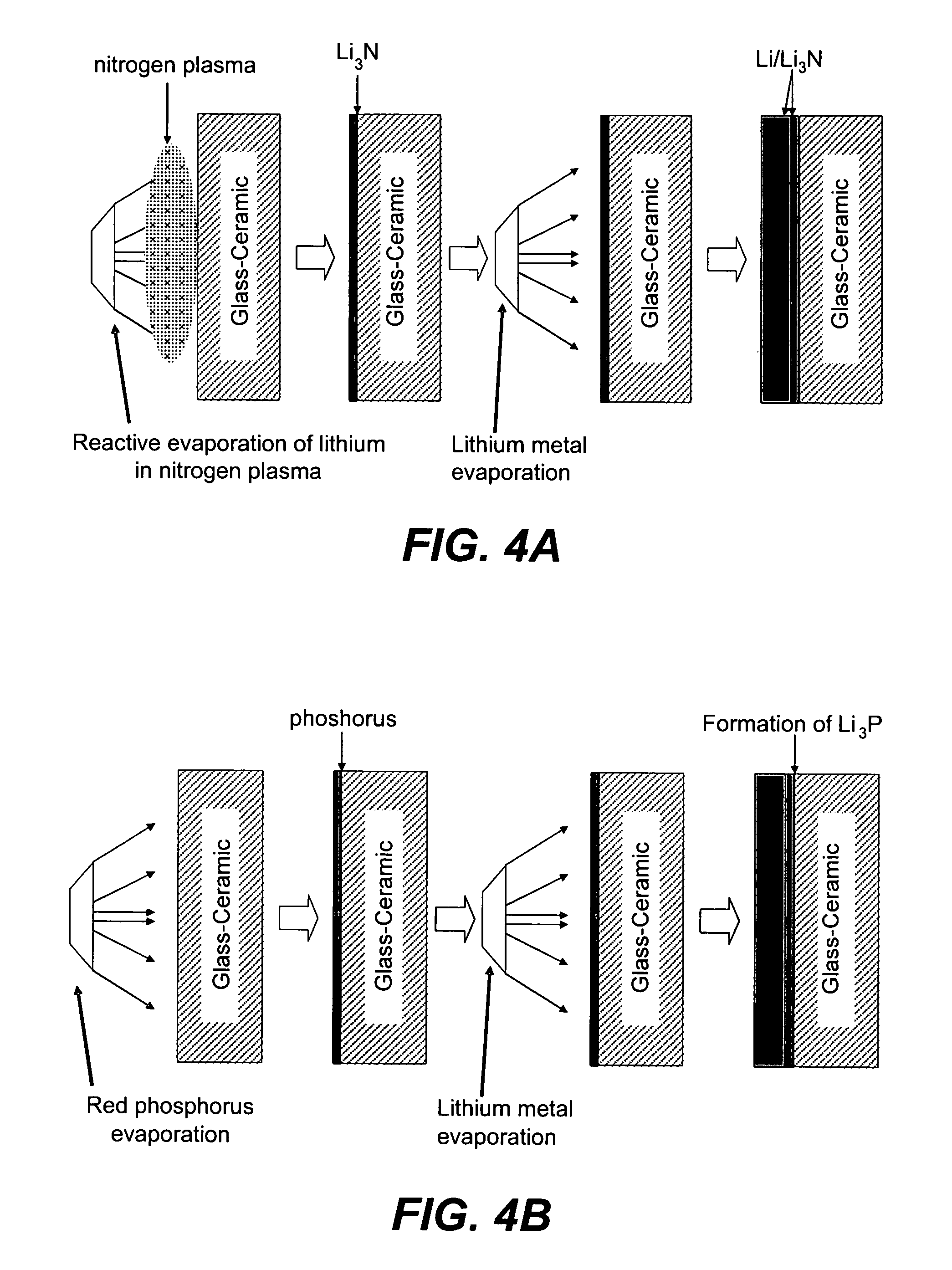
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process in the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Negative electrode for lithium metal battery and lithium metal battery comprising the same
InactiveUS20050095504A1Improving life-cycle characteristicInhibit side effectsFinal product manufacturePretreated surfacesCross-linkMetallic lithium
The present invention relates to a negative electrode for a lithium metal battery and a lithium metal battery comprising the same. The negative electrode of the present invention comprises a negative active material layer of metallic lithium or a lithium alloy, and a passivation layer formed on the negative active material layer. The passivation layer has a structure comprising a 3-dimensionally cross-linked polymer network matrix penetrated by linear polymers. The passivation layer formed on the surface of the negative electrode reduces reactivity of the negative electrode and stabilizes the surface, so that it offers a lithium metal battery having superior life cycle characteristics.
Owner:SAMSUNG SDI CO LTD
Ionically conductive composites for protection of active metal anodes
ActiveUS7282296B2Easy to manufactureImprove battery performanceFinal product manufactureElectrode carriers/collectorsChemical stabilityIonic conductivity
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Electrolyte composition, photoelectric conversion device and photo-electrochemical cell
InactiveUS6376765B1Improve rendering capabilitiesIncreased durabilityLight-sensitive devicesOrganic chemistryPhotoelectrochemical cellHydrogen atom
Owner:FUJIFILM HLDG CORP +1
Long cycle-life alkali metal battery
InactiveUS6203947B1Improve cycle lifeFast charging rateElectrochemical processing of electrodesElectrode carriers/collectorsHigh pressureElectrochemical cell
The present invention provides a cathode for use in a secondary electrochemical cell, such cathode being coated with a very thin, protective film, permeable to ions. The protective film of the cathode usually has a thickness of up to about 0.1 mum and it provides protection against high voltage charging and overdiscbarging. The present invention further provides a secondary electrochemical cell comprising such a cathode.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Compliant seal structures for protected active metal anodes
ActiveUS20070037058A1Reduced ionic contact areaReduce the total massPrimary cell to battery groupingElectrode manufacturing processesOptoelectronicsAnodic protection
Protected anode architectures have ionically conductive protective membrane architectures that, in conjunction with compliant seal structures and anode backplanes, effectively enclose an active metal anode inside the interior of an anode compartment. This enclosure prevents the active metal from deleterious reaction with the environment external to the anode compartment, which may include aqueous, ambient moisture, and / or other materials corrosive to the active metal. The compliant seal structures are substantially impervious to anolytes, catholyes, dissolved species in electrolytes, and moisture and compliant to changes in anode volume such that physical continuity between the anode protective architecture and backplane are maintained. The protected anode architectures can be used in arrays of protected anode architectures and battery cells of various configurations incorporating the protected anode architectures or arrays.
Owner:POLYPLUS BATTERY CO INC
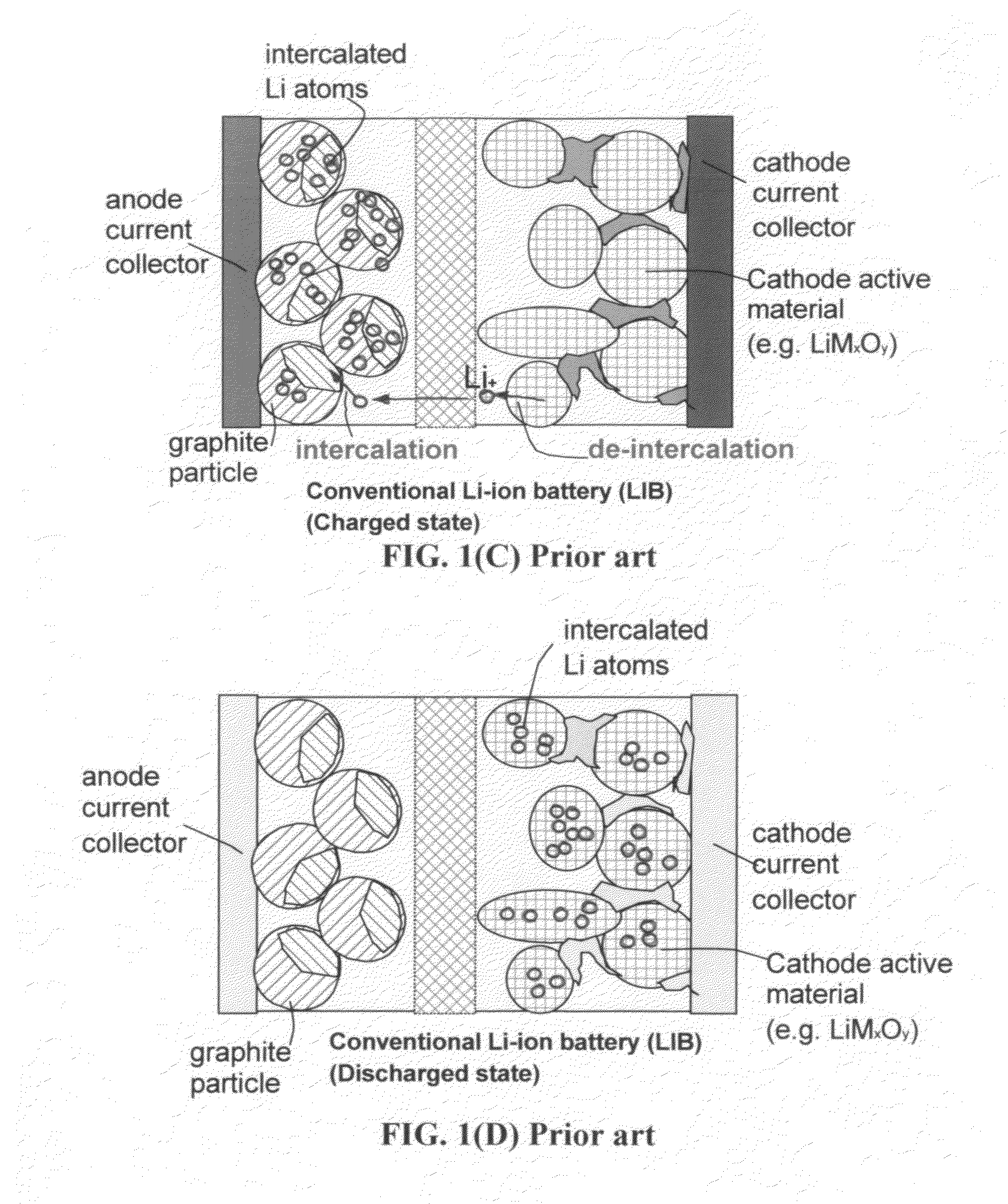
Lithium ion secondary battery
InactiveUS7335448B2Improve securityFinal product manufactureElectrode carriers/collectorsLithiumEngineering
A lithium ion secondary battery includes: (a) a positive electrode plate comprising an active material part and a current collector carrying the active material part, the active material part comprising a positive electrode active material capable of absorbing or desorbing a lithium ion during charge and discharge; (b) a negative electrode plate comprising an active material part and a current collector carrying the active material part, the active material part comprising a negative electrode active material capable of absorbing or desorbing a lithium ion during charge and discharge; (c) a separator interposed between the positive and negative electrode plates; (d) an electrolyte; and (e) a battery case accommodating the positive and negative electrode plates, the separator, and the electrolyte. The positive and negative electrode plates are wound with the separator interposed therebetween, thereby to form an electrode plate assembly. The electrode plate assembly is so configured that each lengthwise edge of the positive electrode current collector is positioned on an outer side of each lengthwise edge of the negative electrode active material part.
Owner:GK BRIDGE 1
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS20010028977A1Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
All-solid lithium battery
ActiveUS20090081554A1Improve output performanceLayer formationSolid electrolytesElectrode carriers/collectorsRedoxSulfide
Owner:NAT INST FOR MATERIALS SCI
Lithium ion conductive solid electrolyte and production process thereof
InactiveUS20070231704A1Increase battery capacitySimple and convenient manufactureSecondary cellsSolid electrolyte cellsPorosityLithium metal
A lithium ion conductive solid electrolyte formed by sintering a molding product containing an inorganic powder and having a porosity of 10 vol % or less, which is obtained by preparing a molding product comprising an inorganic powder as a main ingredient and sintering the molding product after pressing and / or sintering the same while pressing, the lithium ion conductive solid electrolyte providing a solid electrolyte having high battery capacity without using a liquid electrolyte, usable stably for a long time and simple and convenient in manufacture and handling also in industrial manufacture in the application use of secondary lithium ion battery or primary lithium battery, a solid electrolyte having good charge / discharge cyclic characteristic in the application use of the secondary lithium ion battery a solid electrolyte with less water permeation and being safe when used for lithium metal-air battery in the application use of primary lithium battery, a manufacturing method of the solid electrolyte, and a secondary lithium ion battery and a primary lithium battery using the solid electrolyte.
Owner:OHARA
Ionically conductive composites for protection of active metal anodes
ActiveUS7282302B2Good chemical stabilityImprove ionic conductivitySolid electrolytesCell seperators/membranes/diaphragms/spacersChemical stabilityBattery cell
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Garnet-type lithium ion-conducting oxide and all-solid-state lithium ion secondary battery containing the same
ActiveUS20110244337A1Improve lithium ion conductivitySmall rate of changeSolid electrolyte cellsElectrolytesAll solid stateIntensity normalization
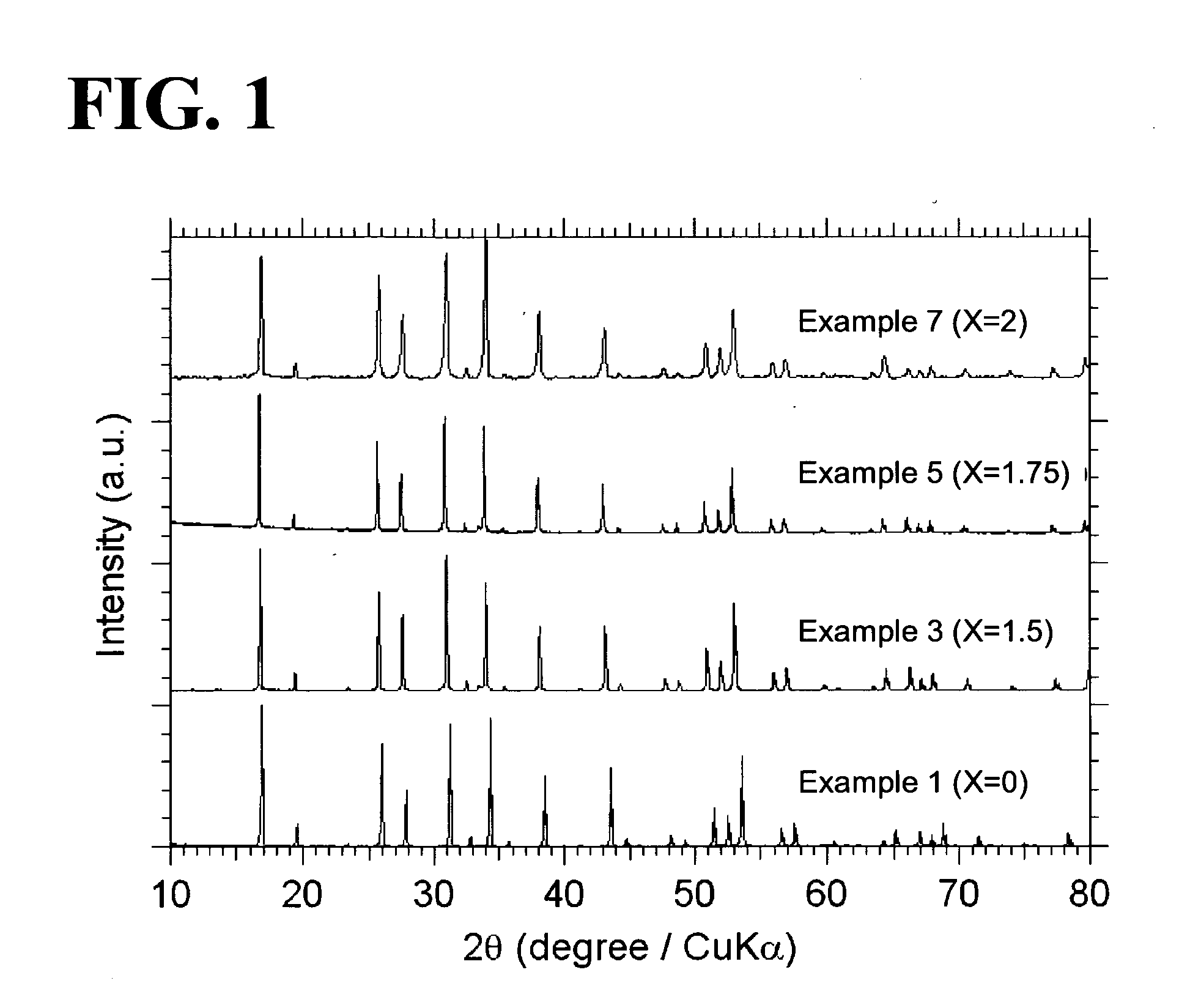
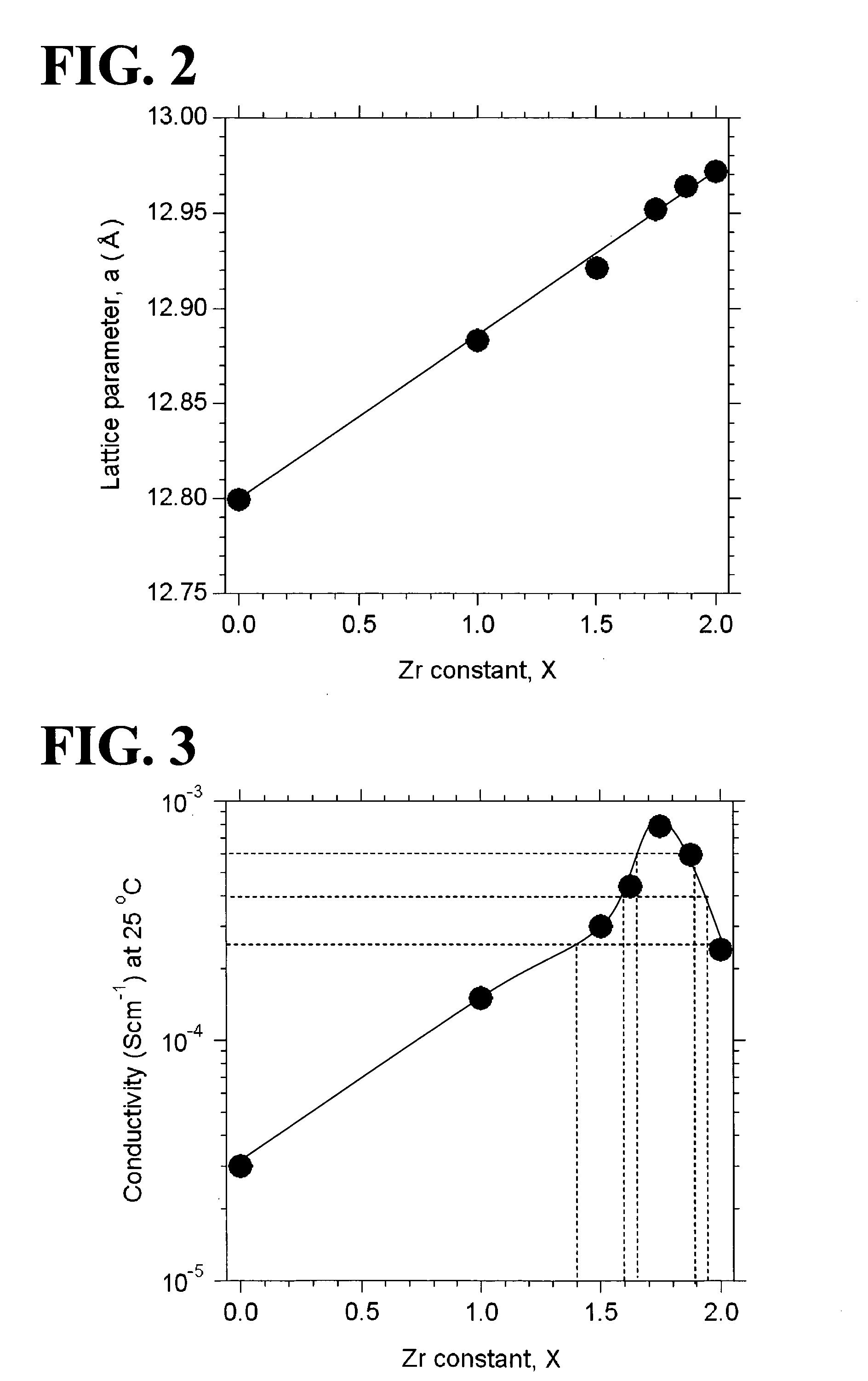
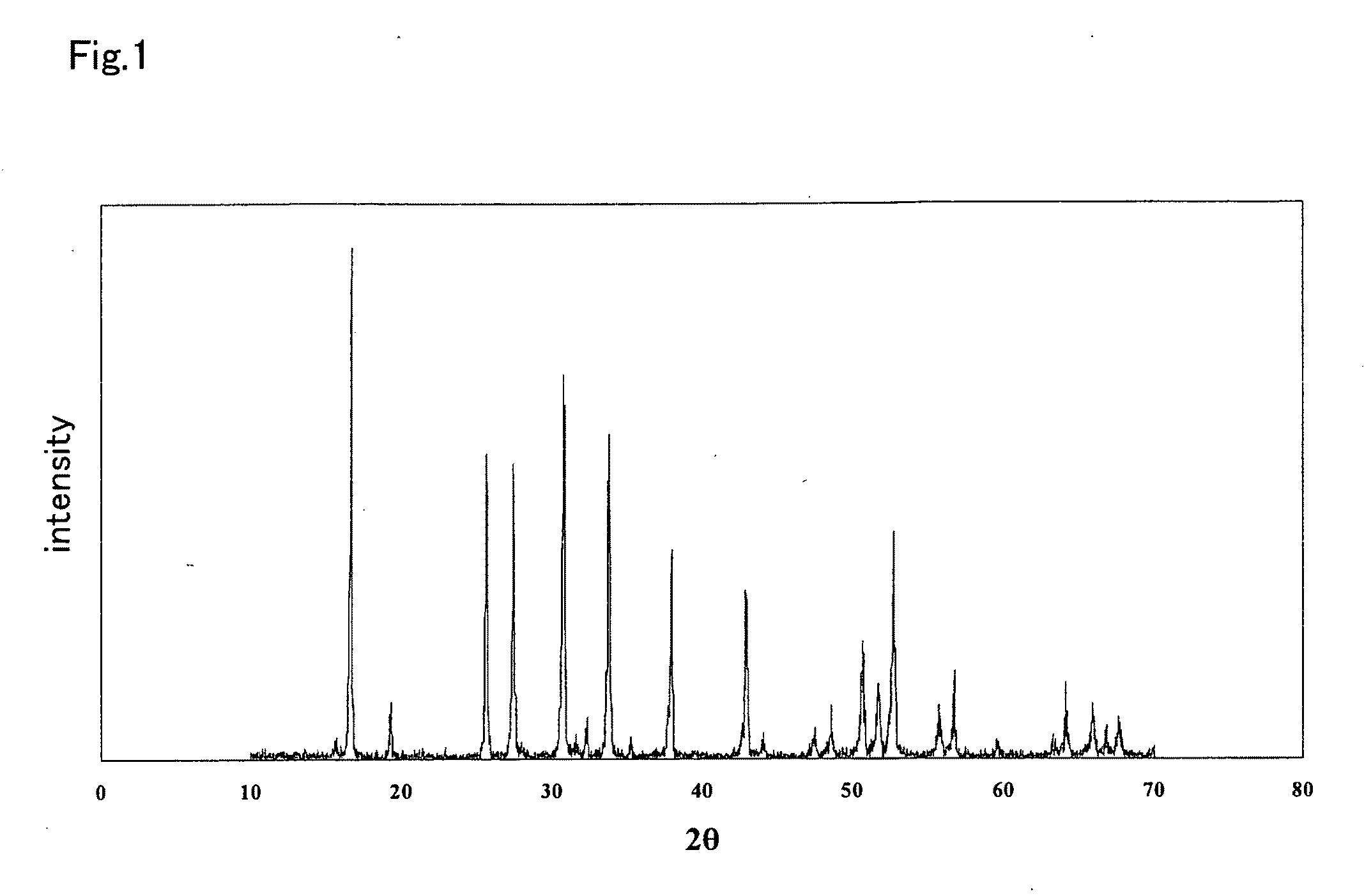
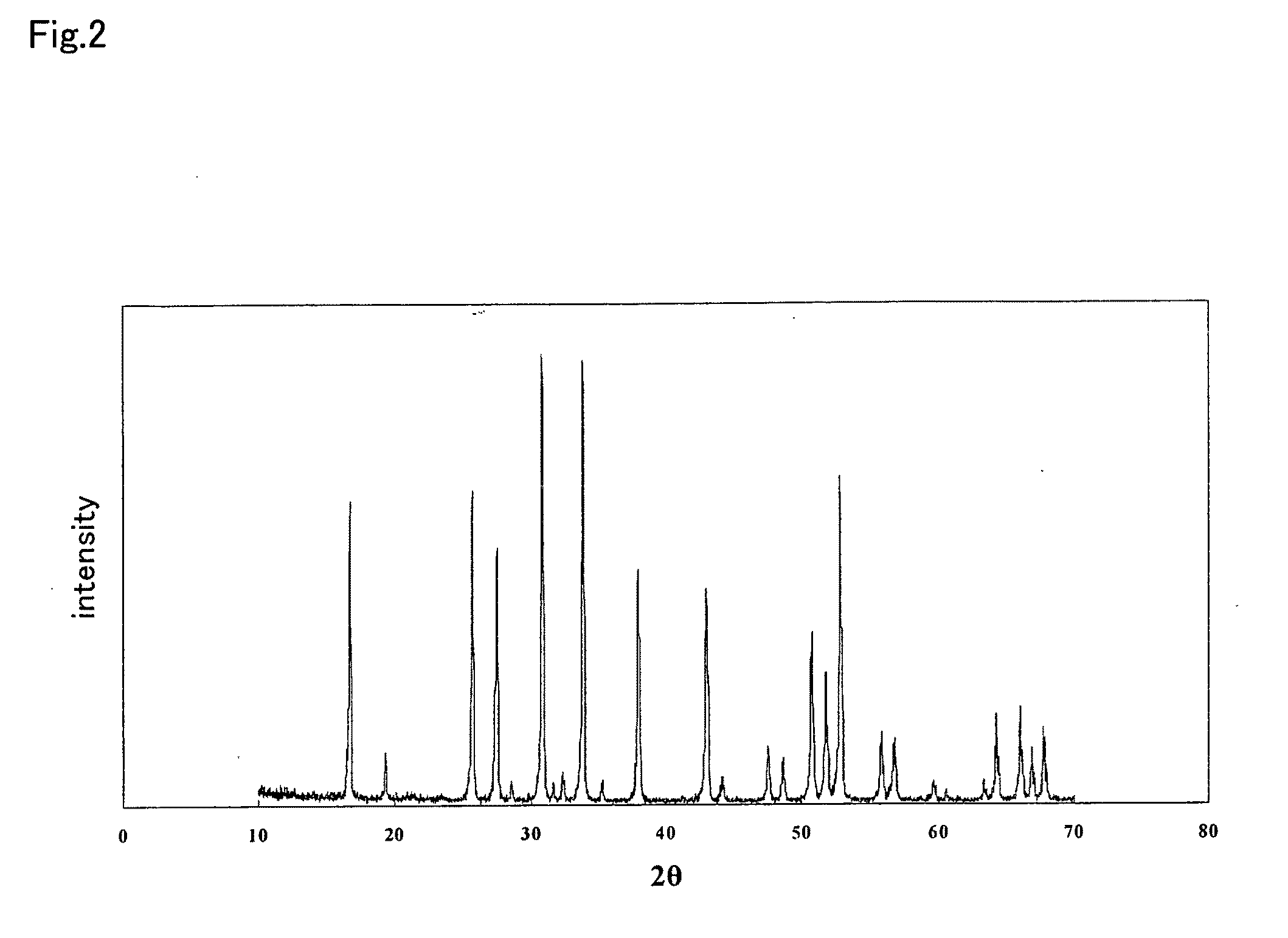
An all-solid-state lithium ion secondary battery containing a novel garnet-type oxide serving as a solid electrolyte. The garnet-type lithium ion-conducting oxide is one represented by the formula Li5+XLa3(ZrX, A2-X)O12, wherein A is at least one selected from the group consisting of Sc, Ti, V, Y, Nb, Hf, Ta, Al, Si, Ga, Ge, and Sn and X satisfies the inequality 1.4≦X<2, or is one obtained by substituting an element having an ionic radius different from that of Zr for Zr sites in an garnet-type lithium ion-conducting oxide represented by the formula Li7La3Zr2O12, wherein the normalized intensity of an X-ray diffraction (XRD) pattern with a diffraction peak, as normalized on the basis of the intensity of a diffraction peak, is 9.2 or more.
Owner:TOYOTA CENT RES & DEV LAB INC
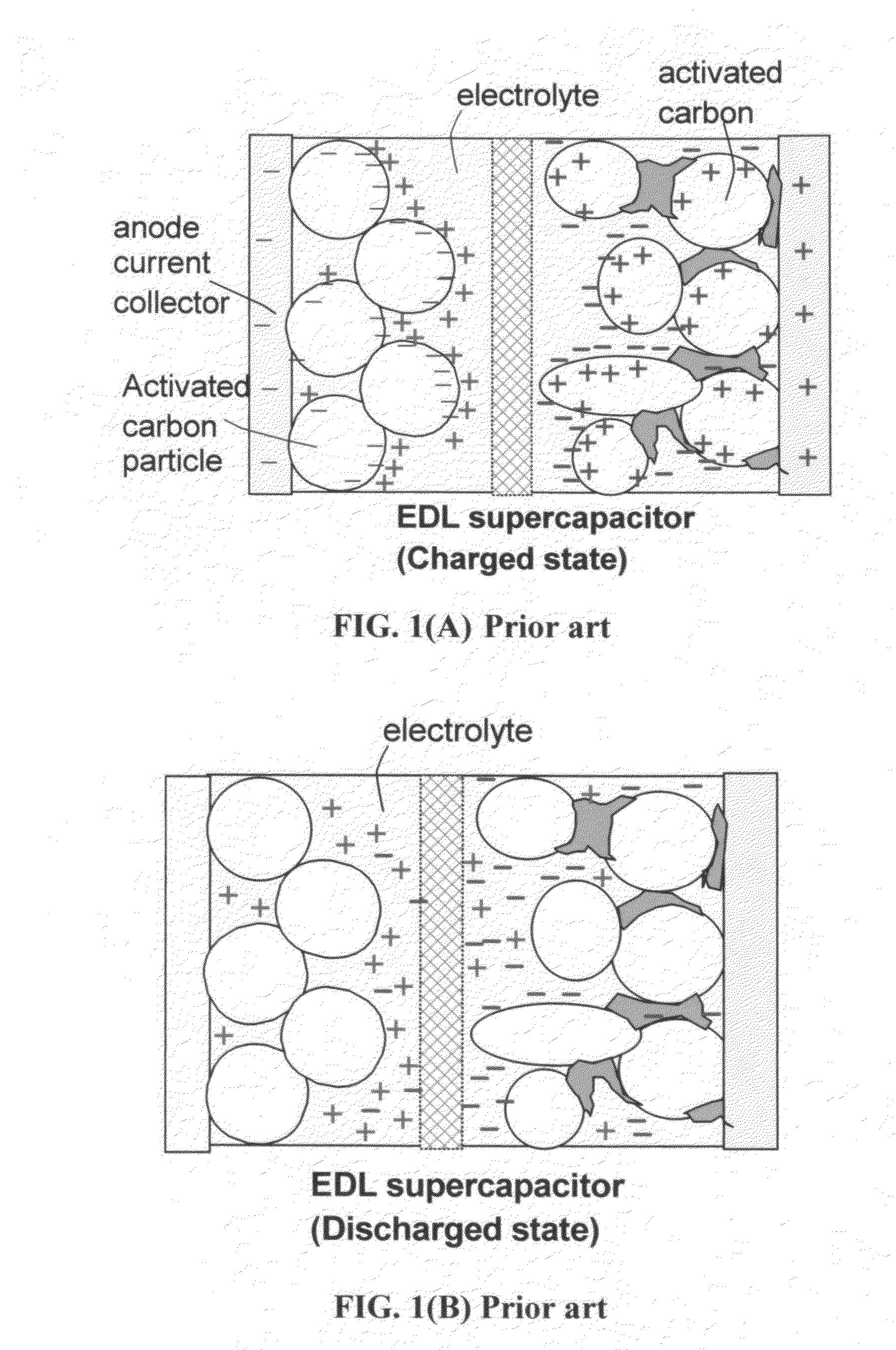
Micro electrochemical energy storage cells
InactiveUS6197450B1Improve performanceIncrease capacityPrimary cell to battery groupingFinal product manufactureThin layerOptoelectronics
Thin-film micro-electrochemical energy storage cells (MEESC) such as microbatteries and double-layer capacitors (DLC) are provided. The MEESC comprises two thin layer electrodes, an intermediate thin layer of a solid electrolyte and optionally, a fourth thin current collector layer; said layers being deposited in sequence on a surface of a substrate. The MEESC is characterized in that the substrate is provided with a plurality of through cavities of arbitrary shape, with high aspect ratio. By using the substrate volume, an increase in the total electrode area per volume is accomplished.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Proton-selective conducting membranes
InactiveUS20020127474A1Easy to operateSelective operationIon-exchanger regenerationSolid electrolyte cellsHydrophobic polymerProton
A membrane comprising: (a) a hydrophobic matrix polymer, and (b) a hydrophilic non-ionic polymer, wherein the hydrophobic polymer and the hydrophilic polymer are disposed so as to form a dense selectively proton-conducting membrane. The microstructure of such a membrane can be tailored to specific functionality requirements, such as proton conductivity vs. proton selectivity, and selectivity to particular species.
Owner:E C R ELECTRO CHEM RES
Thin film battery and electrolyte therefor
A solid amorphous electrolyte composition for a thin-film battery. The electrolyte composition includes a lithium phosphorus oxynitride material containing a sulfide ion dopant wherein the atomic ratio of sulfide ion to phosphorus ion (S / P) in the electrolyte ranges greater than 0 up to about 0.2. The composition is represented by the formula: where 2x+3y+2z=5+w, x ranges from about 3.2 to about 3.8, y ranges from about 0.13 to about 0.46, z ranges from greater than zero up to about 0.2, and w ranges from about 2.9 to about 3.3. Thin-film batteries containing the sulfide doped lithium oxynitride electrolyte are capable of delivering more power and energy than thin-film batteries containing electrolytes without sulfide doping.
Owner:OAK RIDGE MICRO ENERGY
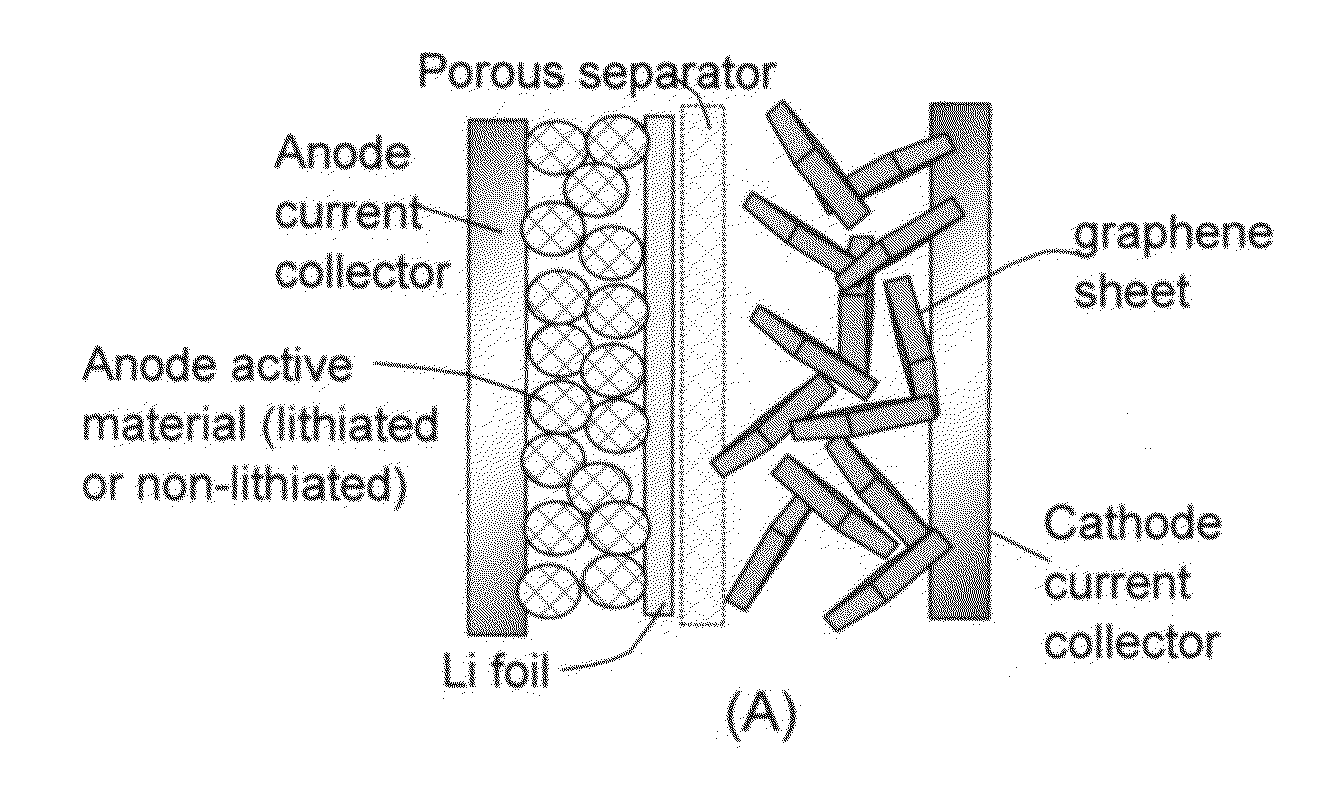
Lithium-ion cell having a high-capacity anode and a high-capacity cathode
ActiveUS20130224603A1Easy dischargeImprove power densityMaterial nanotechnologyHybrid capacitor electrodesLithiumHigh energy
A lithium-ion cell comprising: (A) a cathode comprising graphene as the cathode active material having a surface area to capture and store lithium thereon and wherein said graphene cathode is meso-porous having a specific surface area greater than 100 m2 / g; (B) an anode comprising an anode active material for inserting and extracting lithium, wherein the anode active material is mixed with a conductive additive and / or a resin binder to form a porous electrode structure, or coated onto a current collector in a coating or thin film form; (C) a porous separator disposed between the anode and the cathode; (D) a lithium-containing electrolyte in physical contact with the two electrodes; and (E) a lithium source disposed in at least one of the two electrodes when the cell is made. This new Li-ion cell exhibits an unprecedentedly high energy density.
Owner:GLOBAL GRAPHENE GRP INC
Lithium anodes for electrochemical cells
InactiveUS20060222954A1Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Composite solid polymer elecrolyte membranes
InactiveUS20020045085A1Optimize swellingOptimize fuel crossover resistanceElectrolyte holding meansFinal product manufacturePolymer electrolytesPolymer science
The present invention relates to composite solid polymer electrolyte membranes (SPEMs) which include a porous polymer substrate interpenetrated with an ion-conducting material. SPEMs of the present invention are useful in electrochemical applications, including fuel cells and electrodialysis.
Owner:FOSTER-MILLER
Ceramic material and process for producing the same
ActiveUS20100047696A1Compactness sufficientOvercome lack of conductivitySolid electrolyte cellsCapacitor electrolytes/absorbentsLithiumCrystal structure
A ceramic material that can exhibit sufficient compactness and lithium (Li) conductivity to enable the use thereof as a solid electrolyte material for a lithium secondary battery and the like is provided. The ceramic material contains aluminum (Al) and has a garnet-type crystal structure or a garnet-like crystal structure containing lithium (Li), lanthanum (La), zirconium (Zr) and oxygen (O).
Owner:NGK INSULATORS LTD
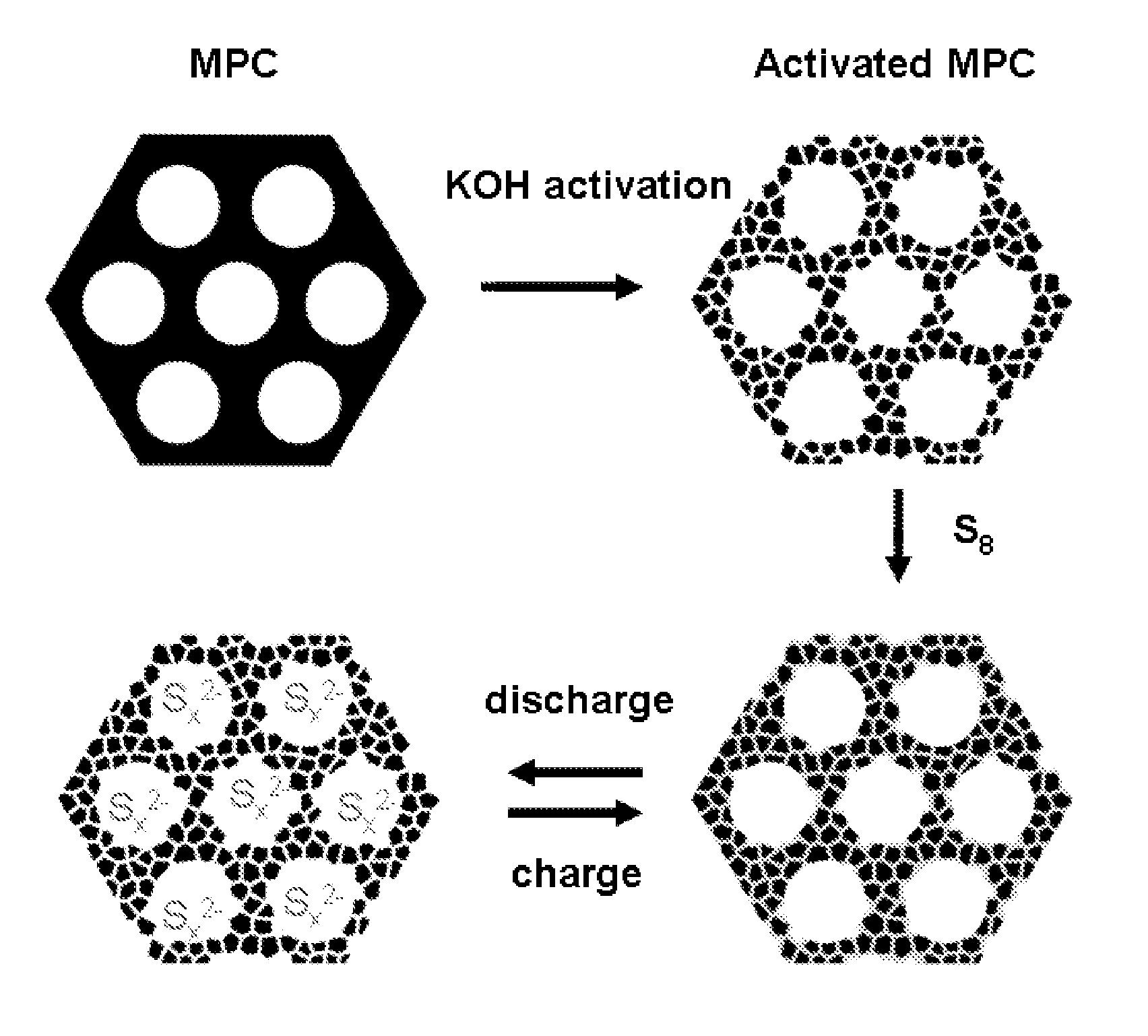
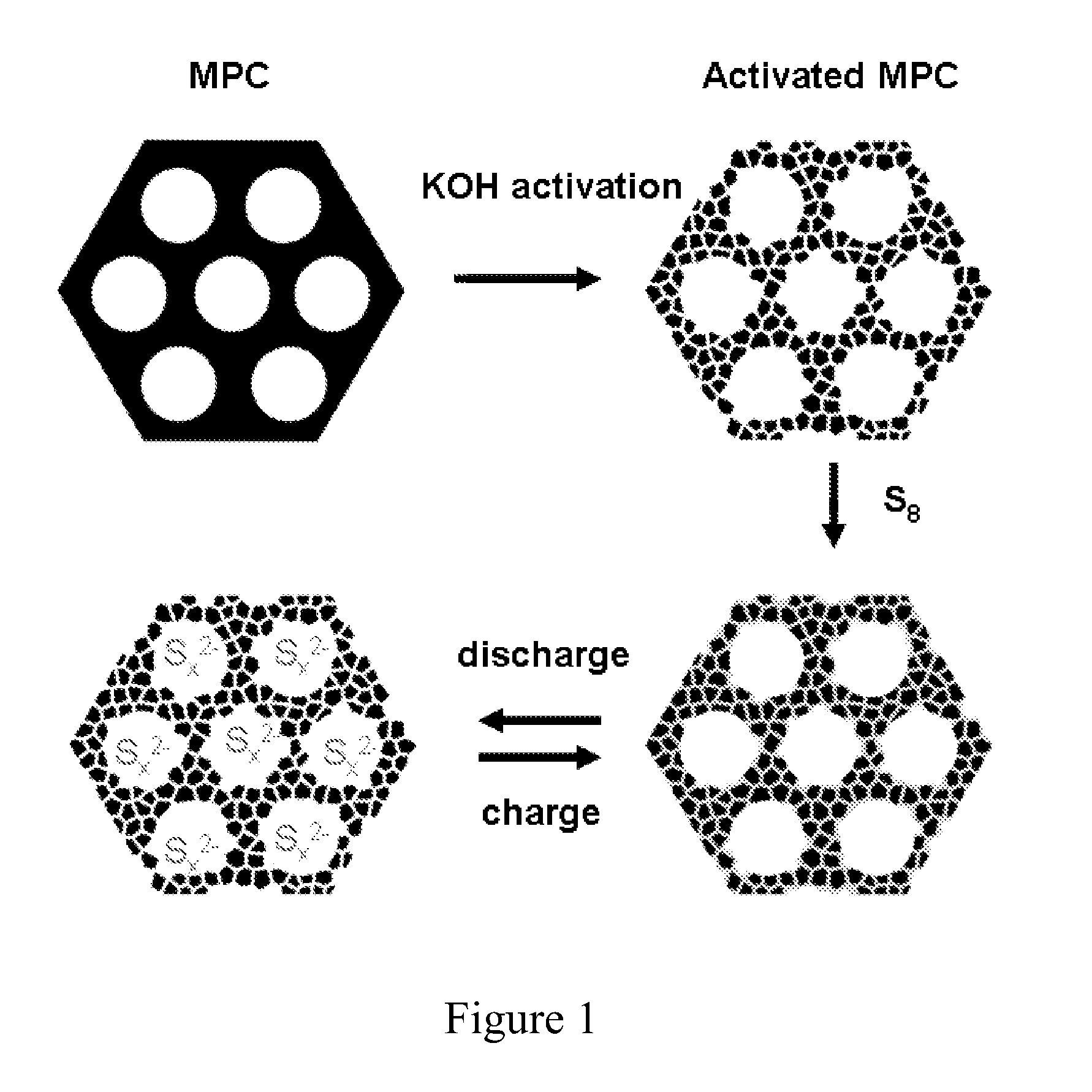
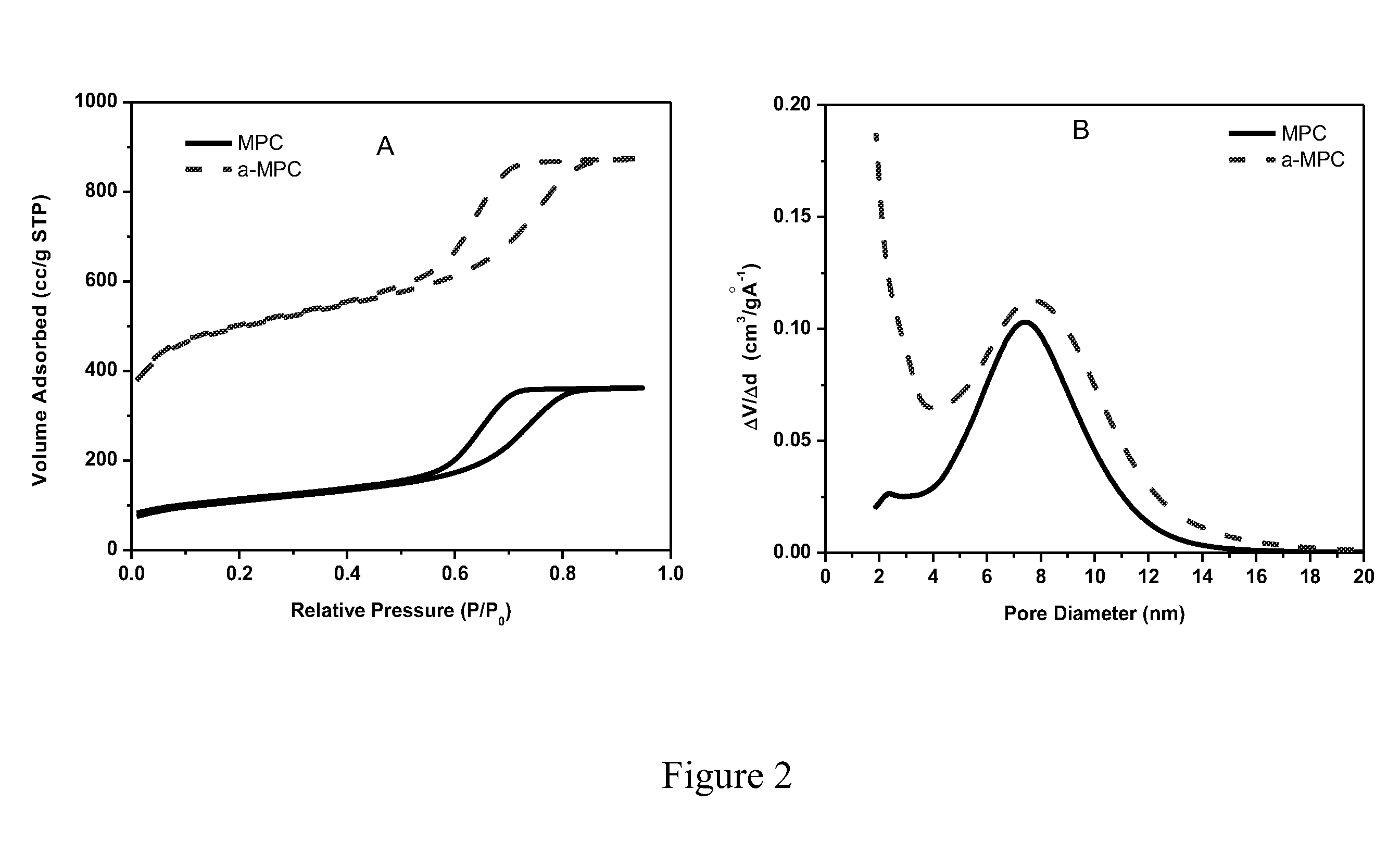
Sulfur-carbon nanocomposites and their application as cathode materials in lithium-sulfur batteries
ActiveUS20110052998A1Minimize formationEasy to transportAlkaline accumulatorsElectrode thermal treatmentCarbon compositesPorous carbon
The invention is directed in a first aspect to a sulfur-carbon composite material comprising: (i) a bimodal porous carbon component containing therein a first mode of pores which are mesopores, and a second mode of pores which are micropores; and (ii) elemental sulfur contained in at least a portion of said micropores. The invention is also directed to the aforesaid sulfur-carbon composite as a layer on a current collector material; a lithium ion battery containing the sulfur-carbon composite in a cathode therein; as well as a method for preparing the sulfur-composite material.
Owner:UT BATTELLE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com