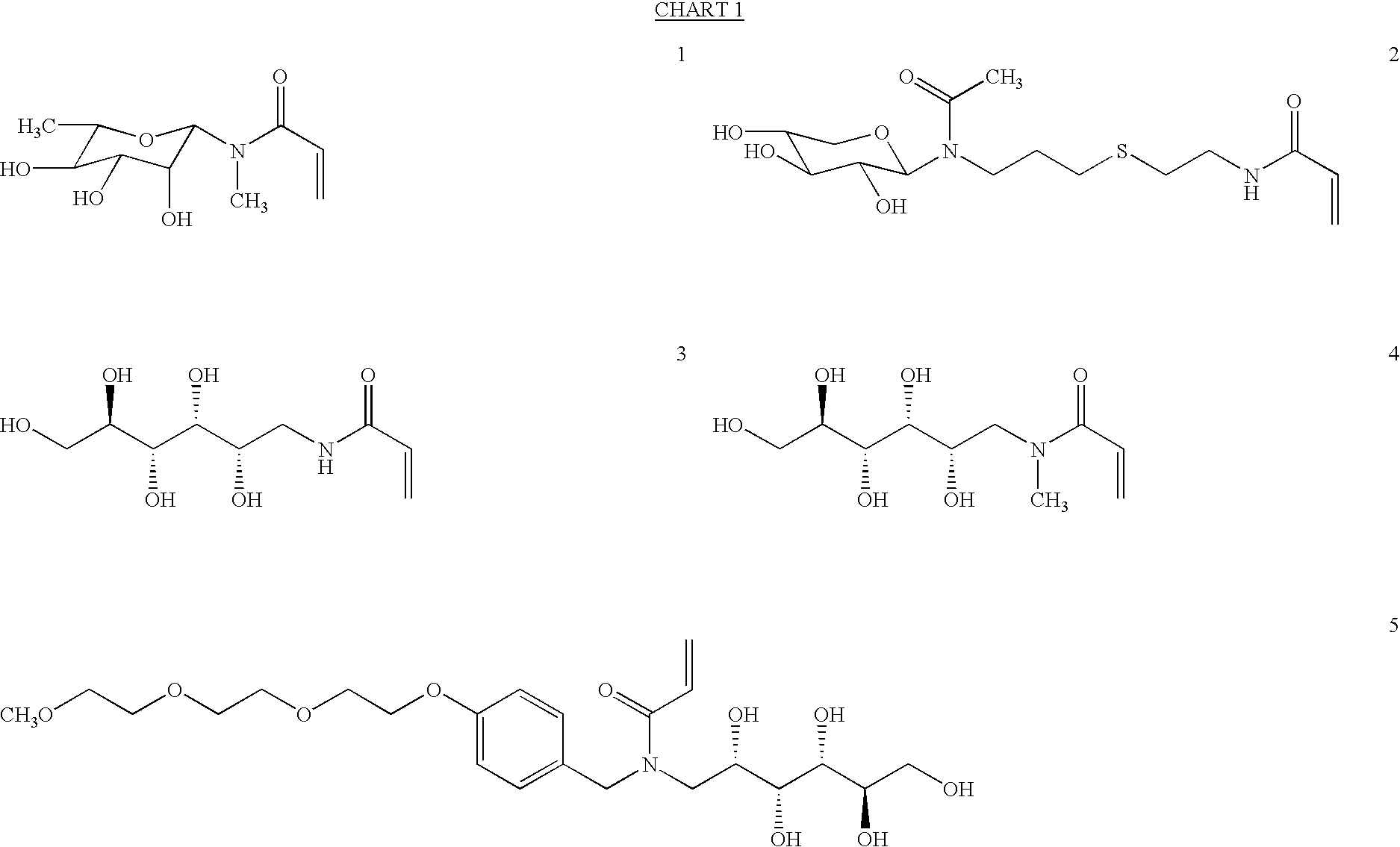
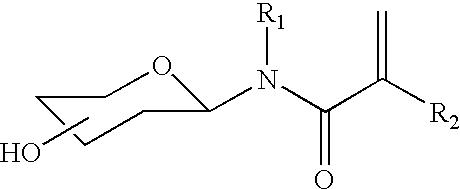
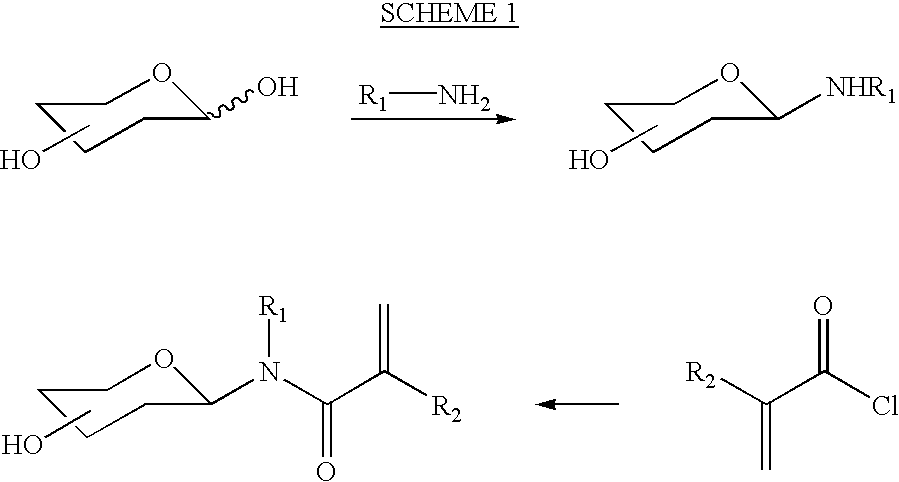
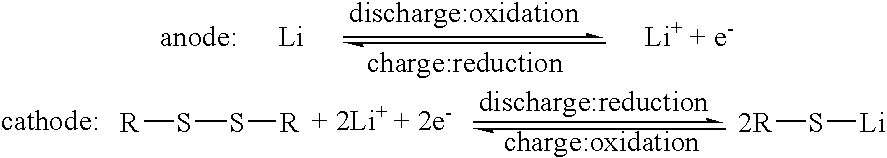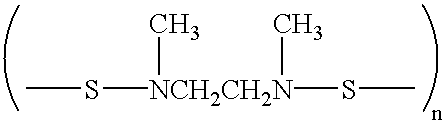Patents
Literature
6295 results about "Sulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S²⁻ or a compound containing one or more S²⁻ ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H₂S) and bisulfide (SH⁻) are the conjugate acids of sulfide.
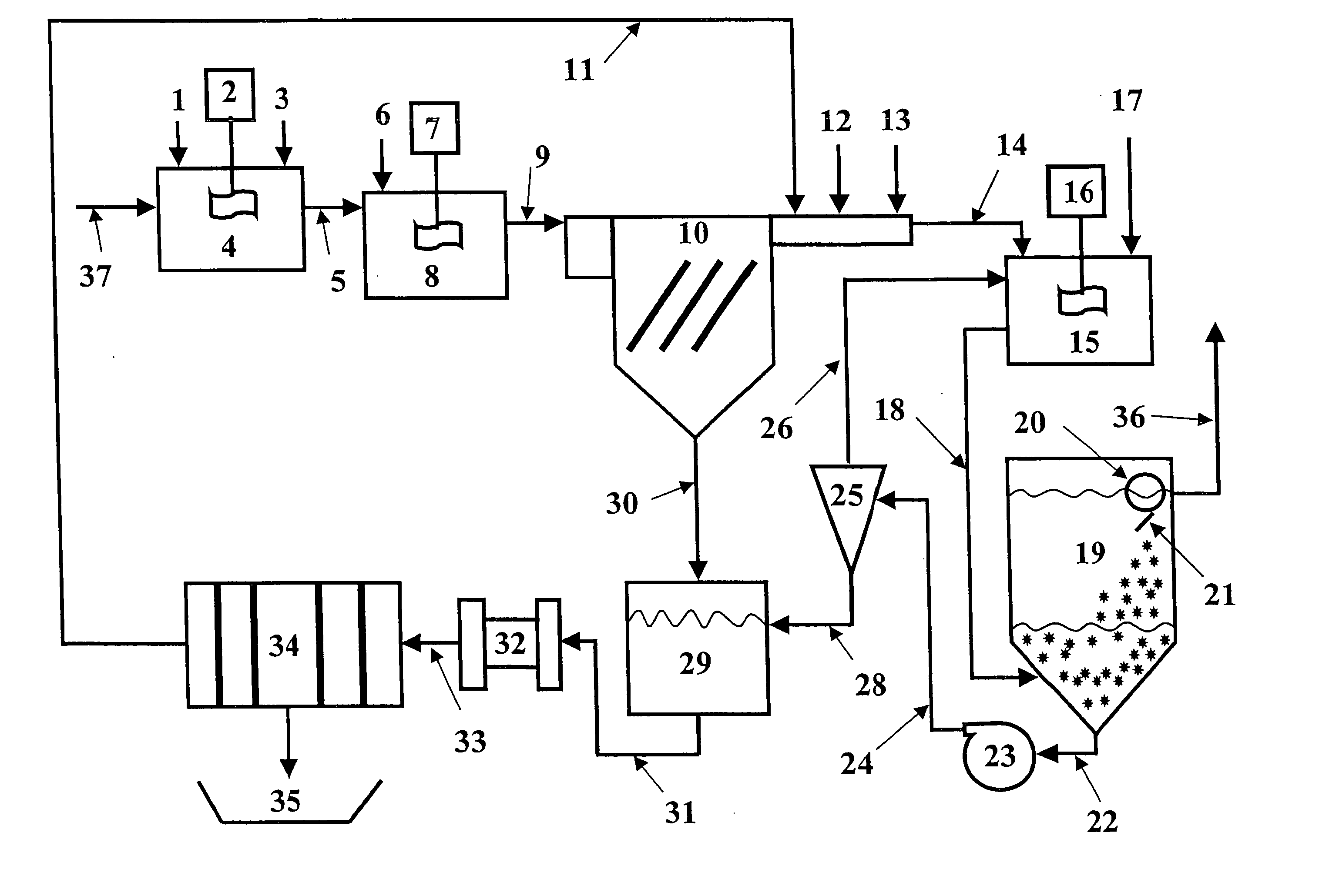
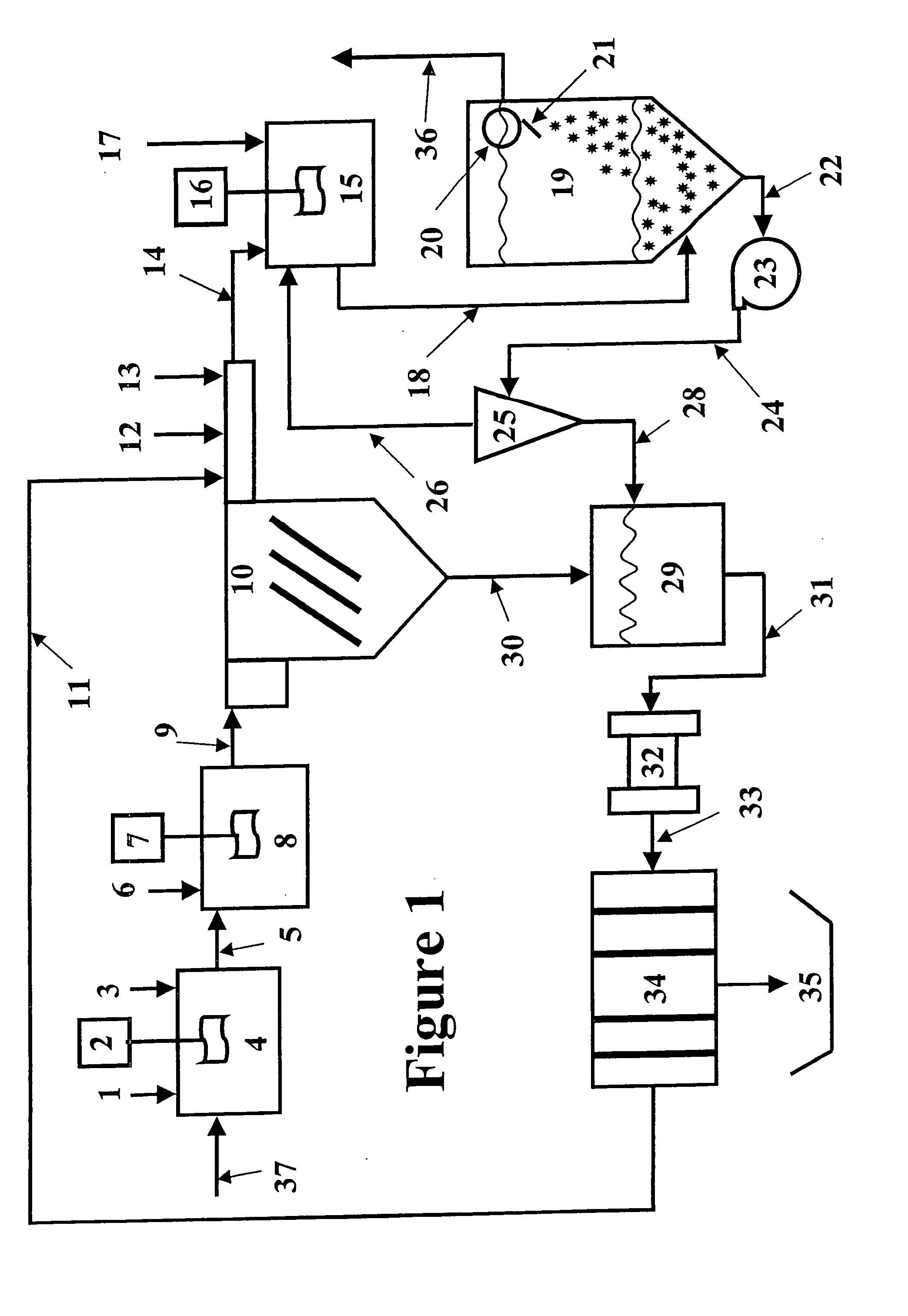
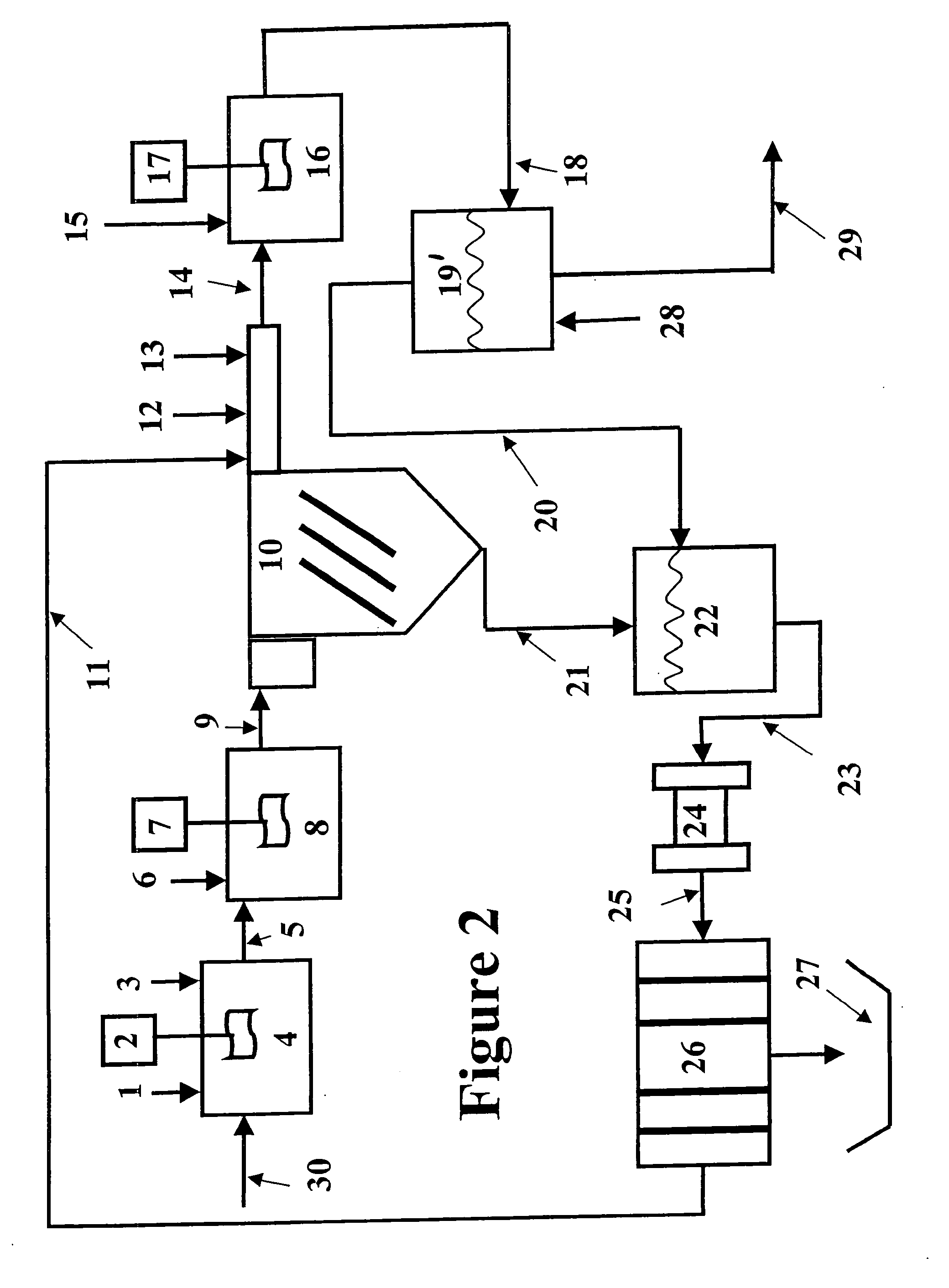
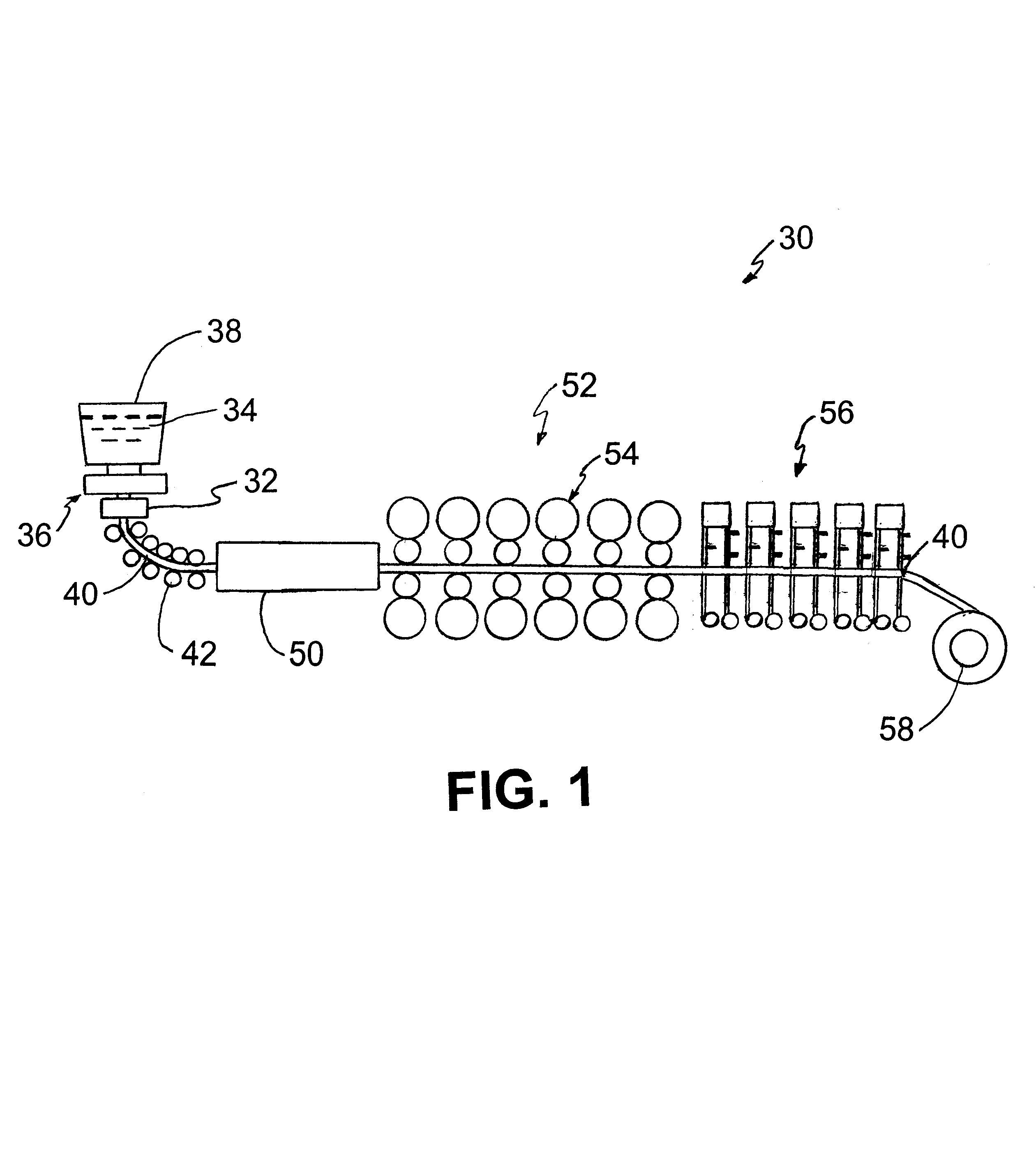
Vegetable oil hydroconversion process
InactiveUS20060186020A1Improved cetane indexAvoid excessive densityMolecular sieve catalystsBiofuelsVegetable oilHydrogen
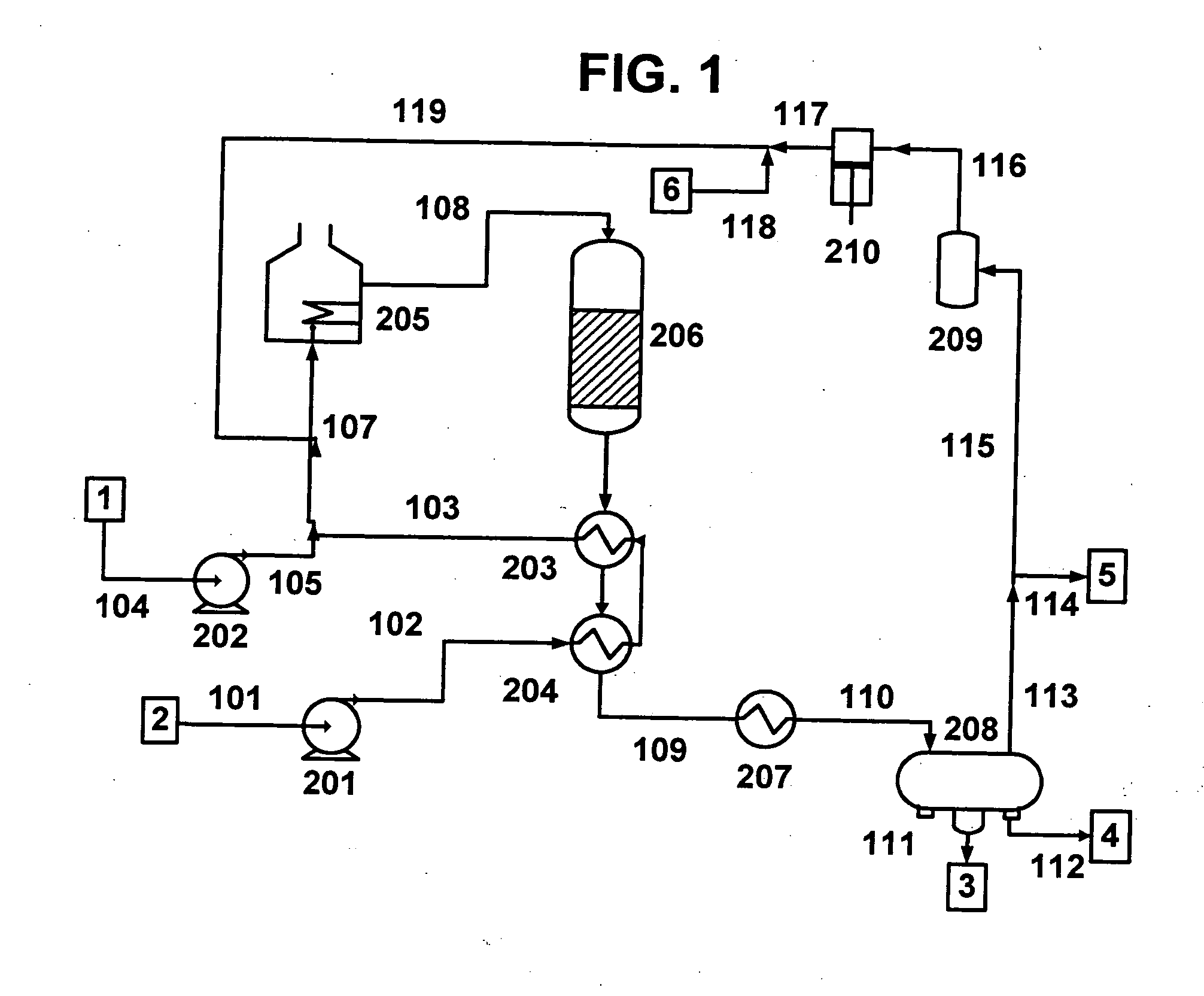
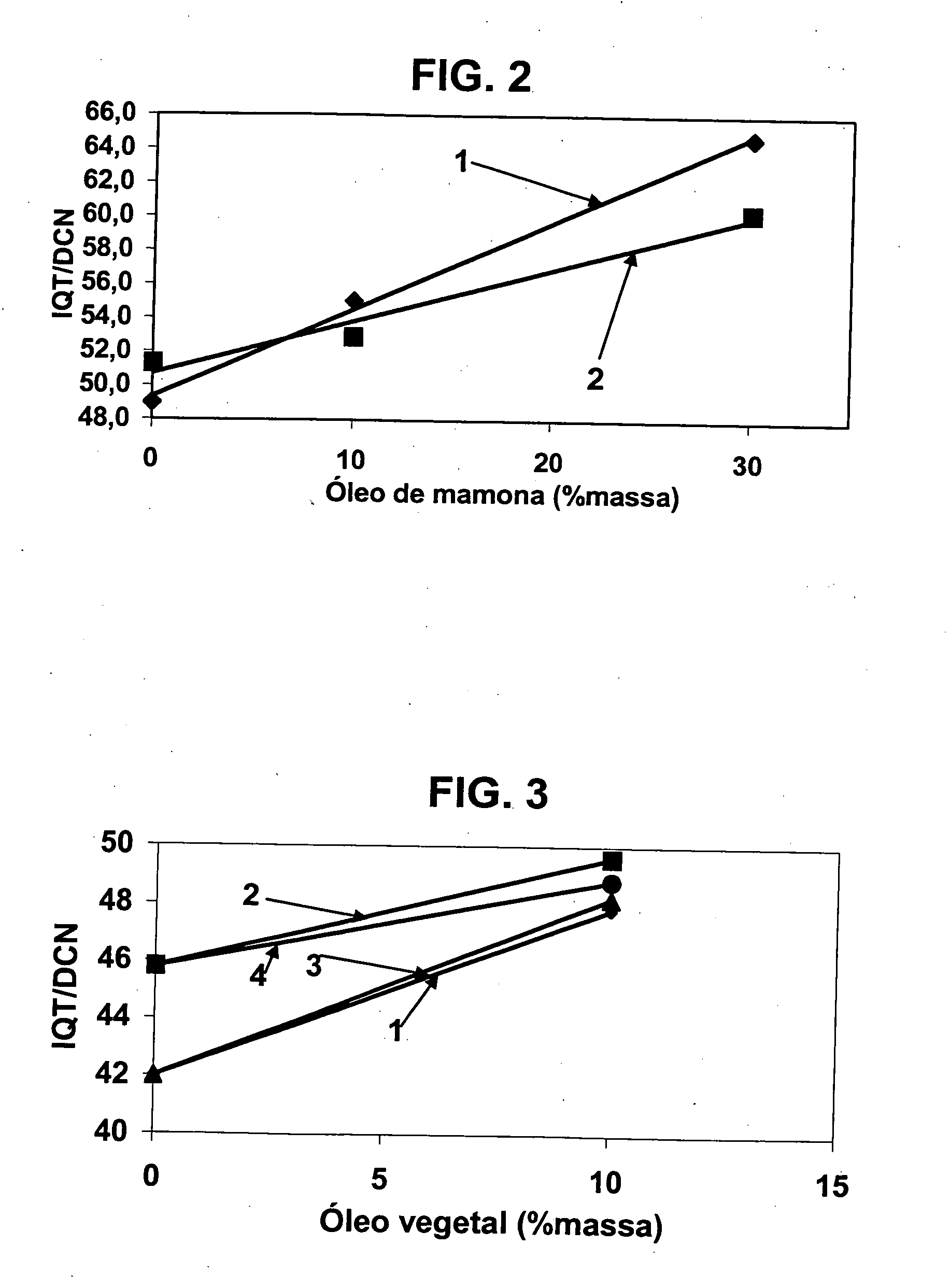
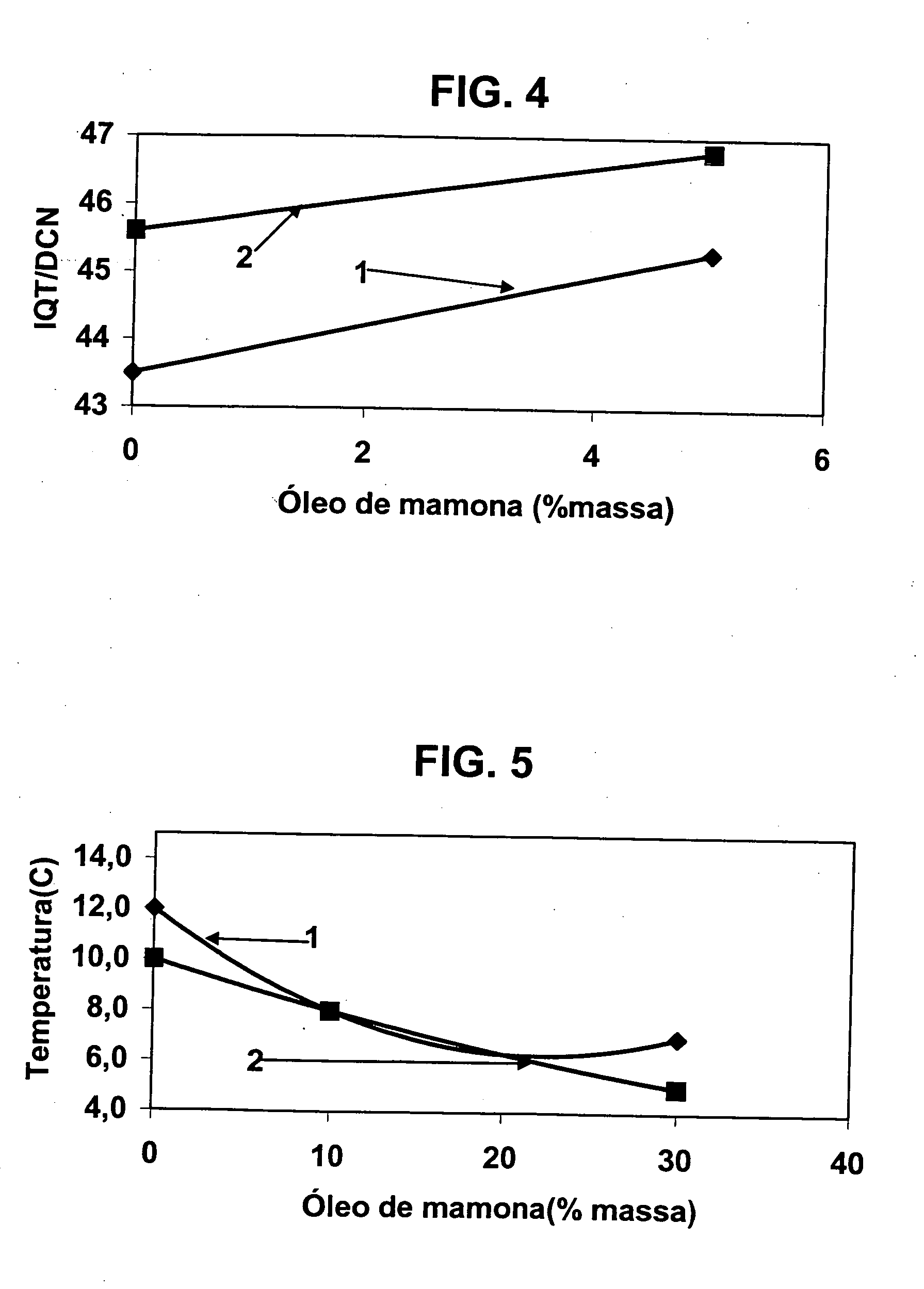
A vegetable oil hydroconversion process is described for hydroconverting a mixture between 1 to 75% in mass of oil or natural fat (1) and the rest mineral oil (2), hydroconverted in a reactor (205) under conditions of pressure, temperature, hydrogen (flow 119) and sulfide catalyst of Groups VIII and VIB, obtaining, after sour water separation (flow 111) and rectification (flow 112), a specified diesel product (4). The product (4) has an ITQ / DCN (cetane number) higher than a product obtained from a pure mineral based oil would have, lower density than from a base oil and a plugging point depending on the mineral oil flow, as well as greater oxidation stability than the base oil.
Owner:PETROLEO BRASILEIRO SA (PETROBRAS)
Pre-passivation process for a continuous reforming apparatus, and passivation process for a continuous reforming apparatus during the initial reacation
ActiveUS20100282645A1Reduce operational riskReduce contentThermal non-catalytic crackingPhysical/chemical process catalystsLiquid productReaction temperature
The present invention relates to a pre-passivation process for a continuous reforming apparatus prior to the reaction, or a passivation process for a continuous reforming apparatus during the initial reaction, comprising loading a reforming catalyst into the continuous reforming apparatus, starting the gas circulation and raising the temperature of a reactor, injecting sulfide into the gas at a reactor temperature ranging from 100-650° C., controlling the sulfur amount in the recycle gas within a range of 0.5-100×10−6 L / L so as to passivate the apparatus.The process of the present invention may also comprise the following steps:(1) loading a reforming catalyst into the continuous reforming apparatus, starting the gas circulation and raising the temperature of a reactor, feeding the reforming feedstock into the reaction system when the temperature of the reactor is increased to 300-460° C., introducing sulfide into the reaction system while or after the reforming feedstock is fed, controlling the ratio of the total sulfur amount introduced into the system to the reforming feedstock within the range of 0.5 μg / g-50 μg / g, reducing the content of sulfide introduced into the system when hydrogen sulfide concentration in the recycle gas reaches to 2.0 μL / L˜30 μL / L; and(2) maintaining the reforming reactor at a temperature of 460-490° C., controlling the ratio of the total sulfur amount introduced into the system to the reforming feedstock within the range of 0.2 μg / g-0.5 μg / g, adjusting the amount of the reforming feedstock to the design value of the apparatus, increasing the reforming reaction temperature to 490-545° C. according to the requirements on the octane number of the liquid product, and letting the reforming apparatus run under normal operating conditions.
Owner:CHINA PETROCHEMICAL CORP +1
All-solid lithium battery
ActiveUS20090081554A1Improve output performanceLayer formationSolid electrolytesElectrode carriers/collectorsRedoxSulfide
Owner:NAT INST FOR MATERIALS SCI
Zinc Ion-Exchanging Energy Storage Device
ActiveUS20160301096A1Quick releaseRapid depositionHybrid capacitor electrolytesAlkaline accumulatorsChemical treatmentZinc metal
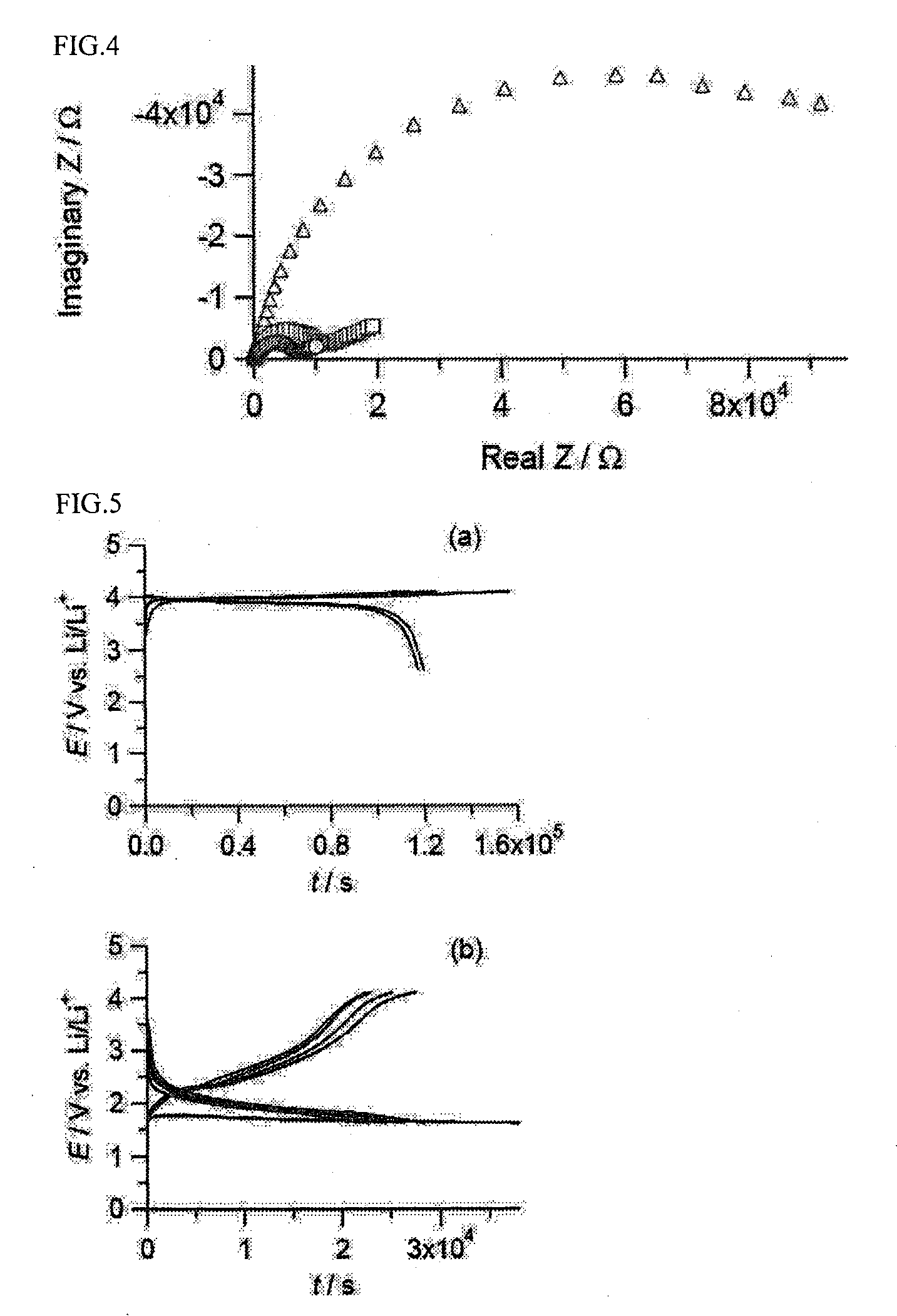
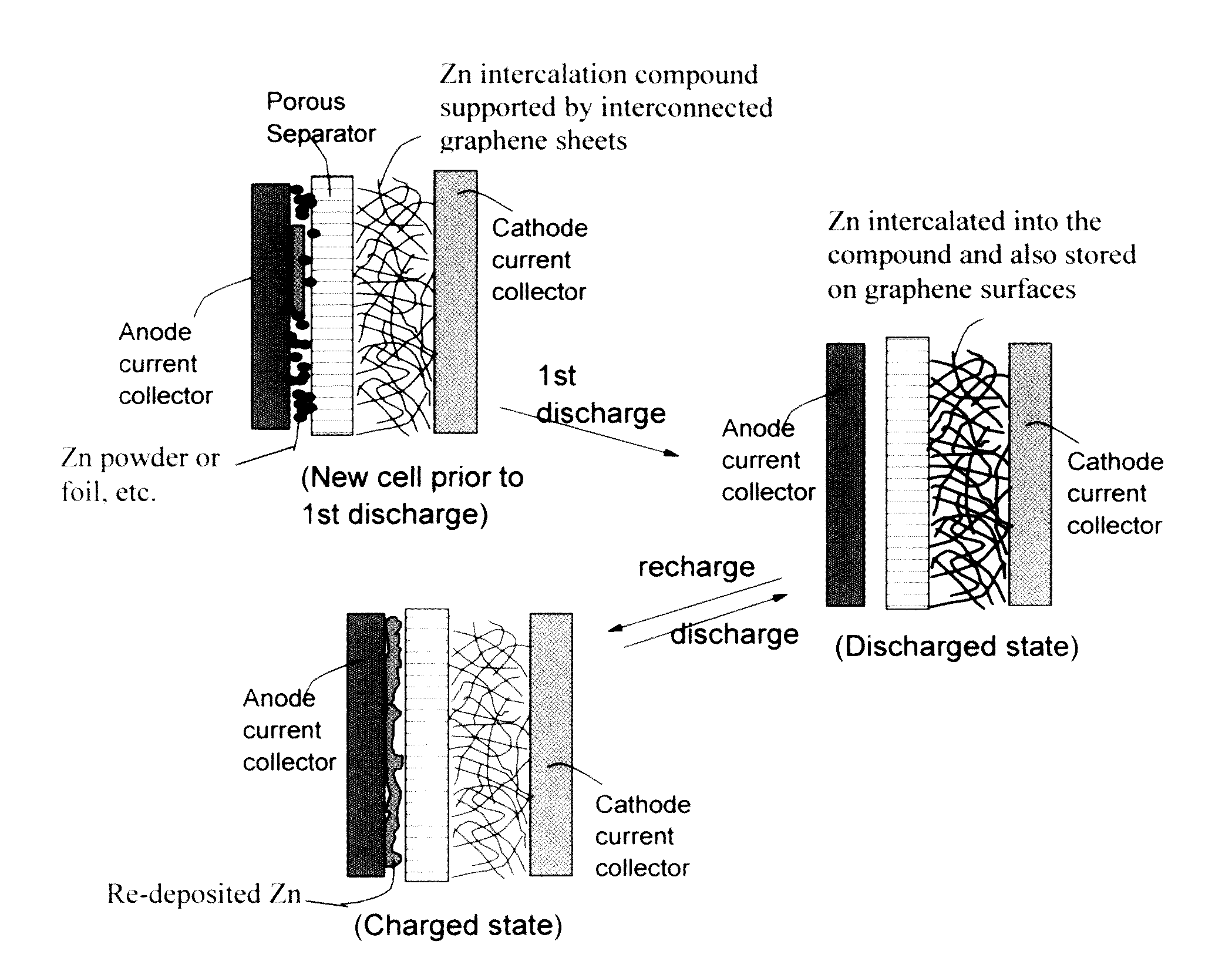
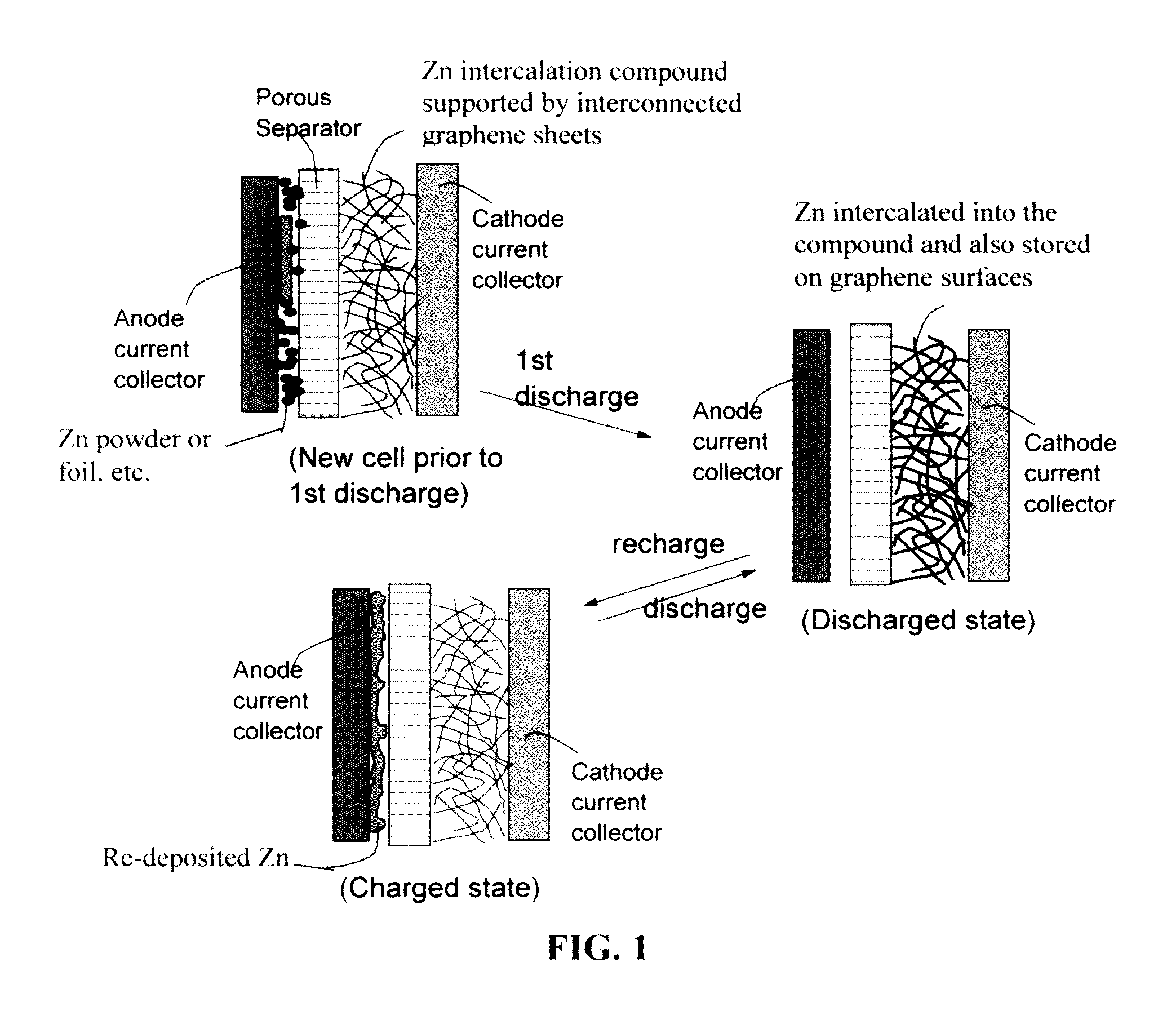
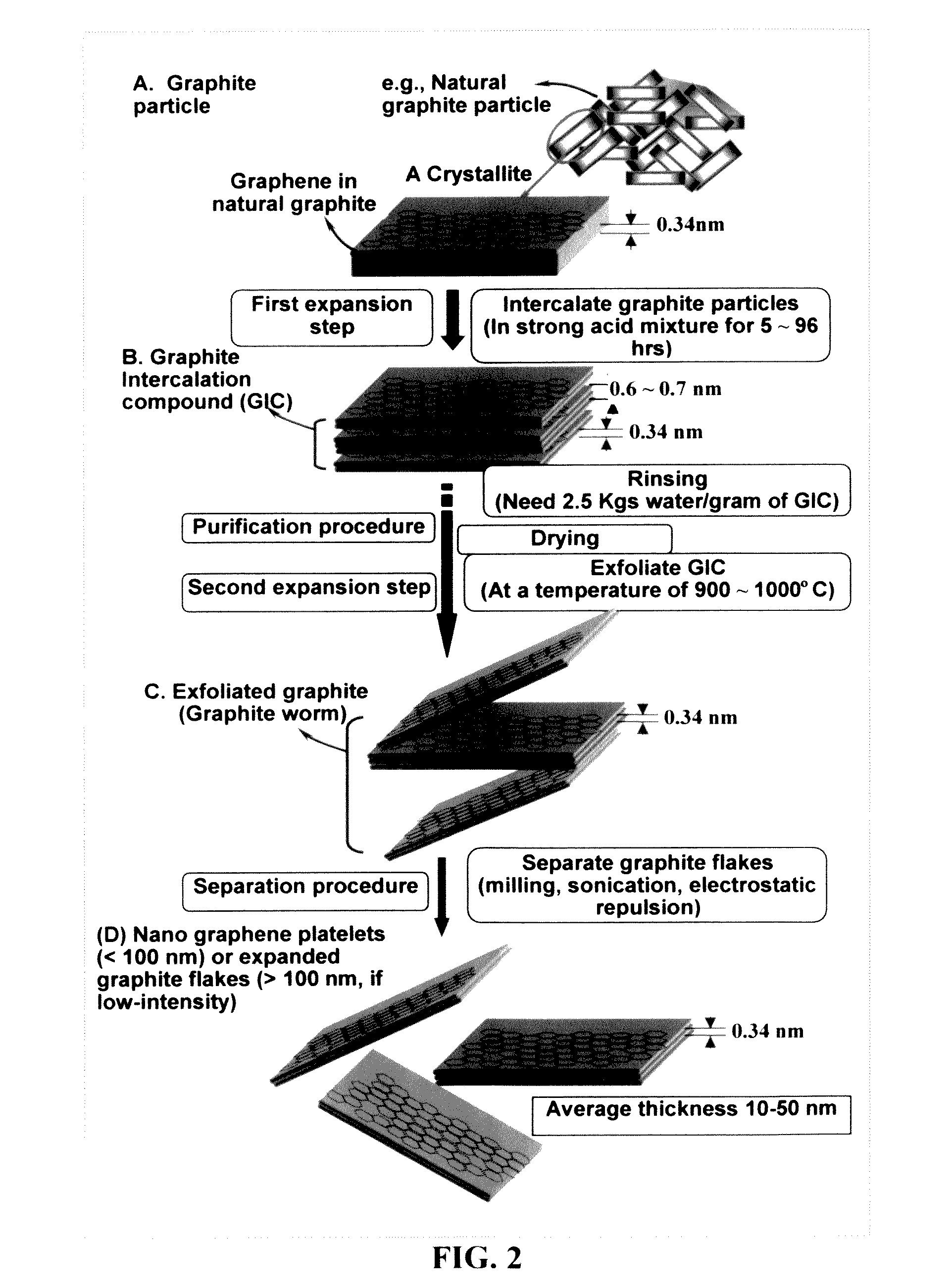
A zinc ion-exchanging battery device comprising: (A) a cathode comprising two cathode active materials (a zinc ion intercalation compound and a surface-mediating material); (B) an anode containing zinc metal or zinc alloy; (C) a porous separator disposed between the cathode and the anode; and (D) an electrolyte containing zinc ions that are exchanged between the cathode and the anode during battery charge / discharge. The zinc ion intercalation compound is selected from chemically treated carbon or graphite material having an expanded inter-graphene spacing d002 of at least 0.5 nm, or an oxide, carbide, dichalcogenide, trichalcogenide, sulfide, selenide, or telluride of niobium, zirconium, molybdenum, hafnium, tantalum, tungsten, titanium, vanadium, chromium, cobalt, manganese, iron, nickel, or a combination thereof. The surface-mediating material contains exfoliated graphite or multiple single-layer sheets or multi-layer platelets of a graphene material.
Owner:GLOBAL GRAPHENE GRP INC
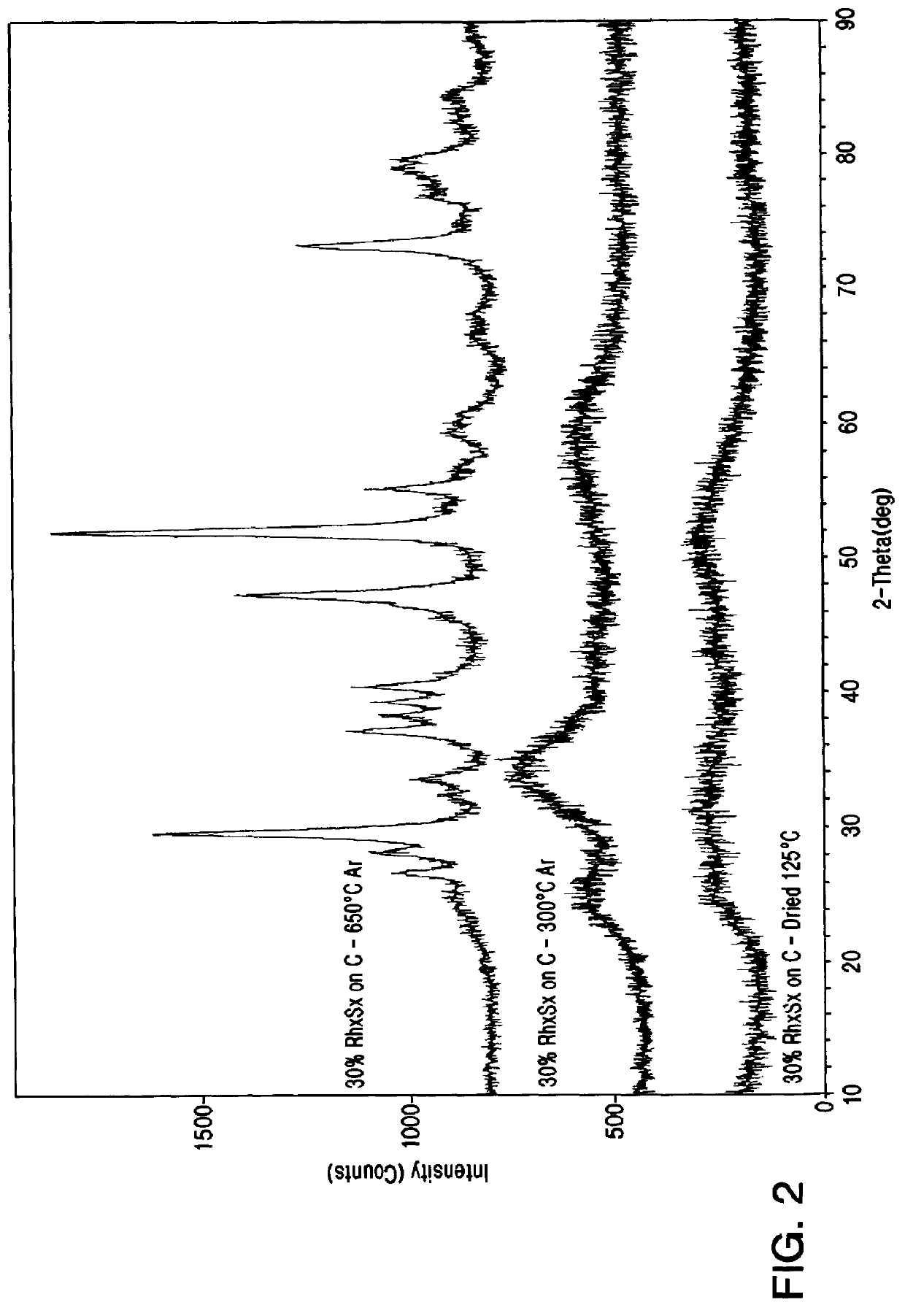
Rhodium electrocatalyst and method of preparation
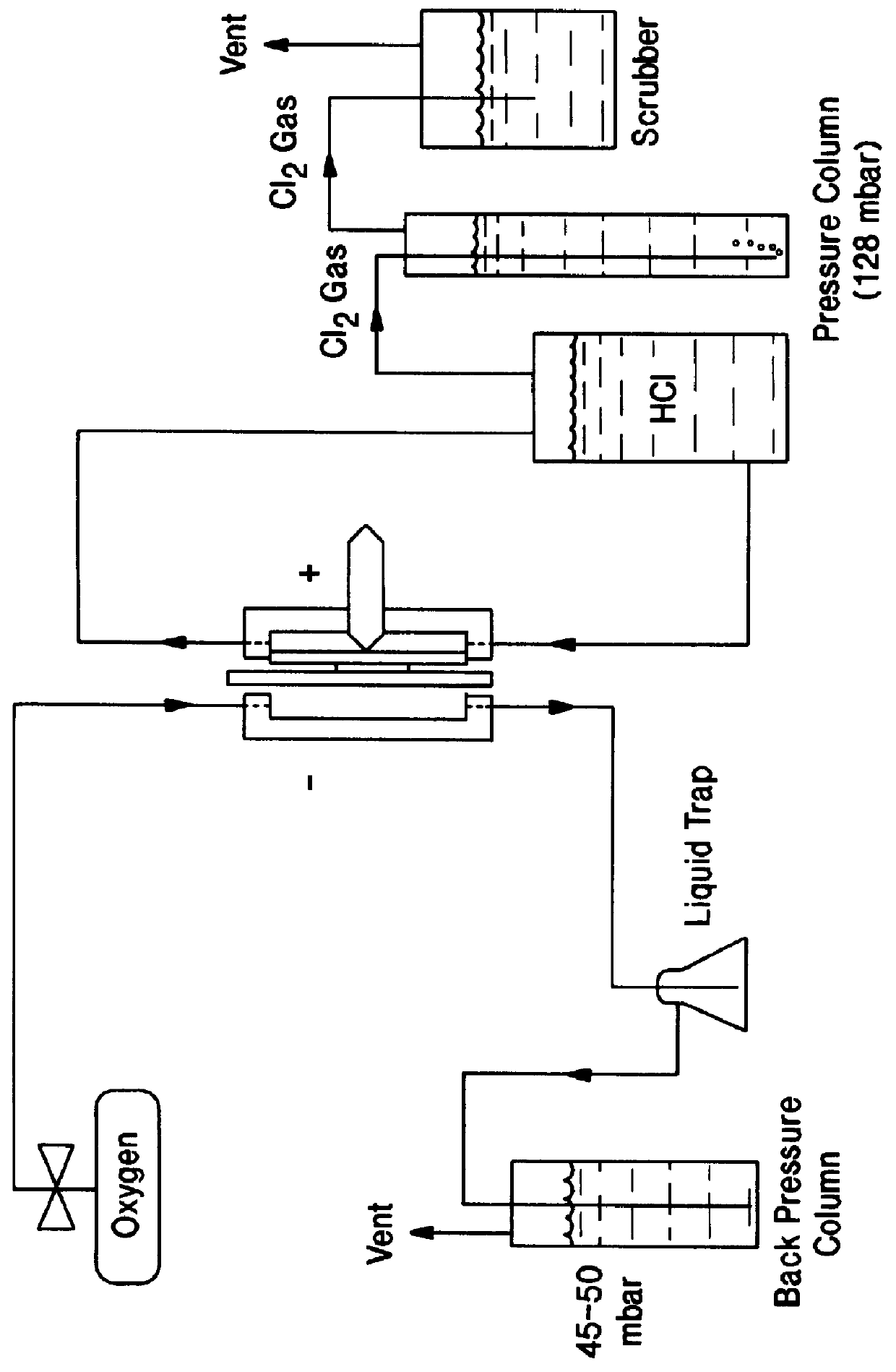
The invention relates to a novel rhodium sulfide catalyst for the reduction of oxygen in industrial electrolyzers. The catalyst is highly resistant towards corrosion and poisoning by organic species, thus resulting particularly suitable for use in aqueous hydrochloric acid electrolysis, when technical grade acid containing organic contaminants is employed.
Owner:DE NORA SPA
Process for the recovery of value metals from base metal sulfide ores
InactiveUS20050118081A1Simple gas/liquidReduce the amount requiredSulfur compoundsSolid sorbent liquid separationSulfideDissolution
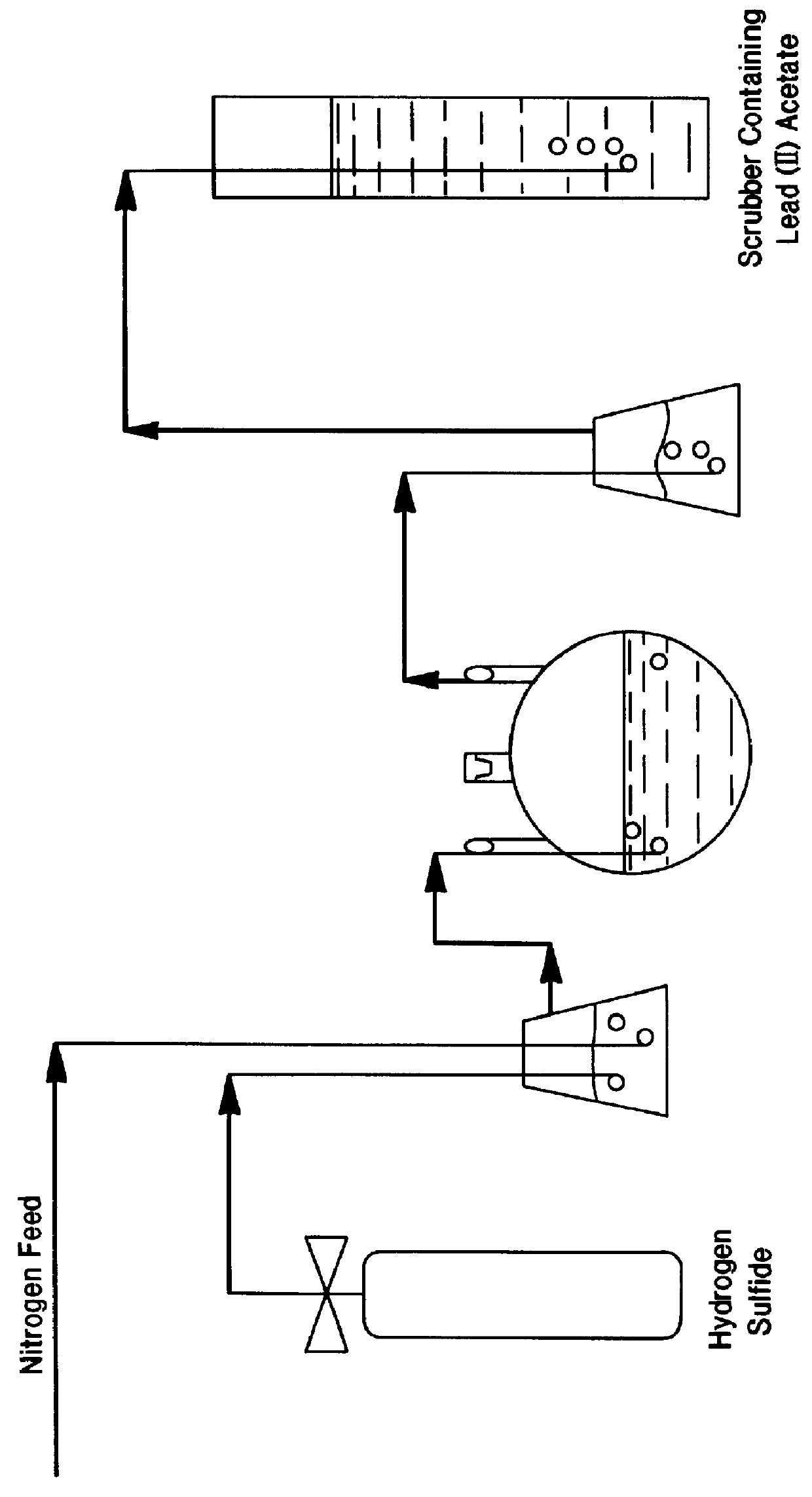
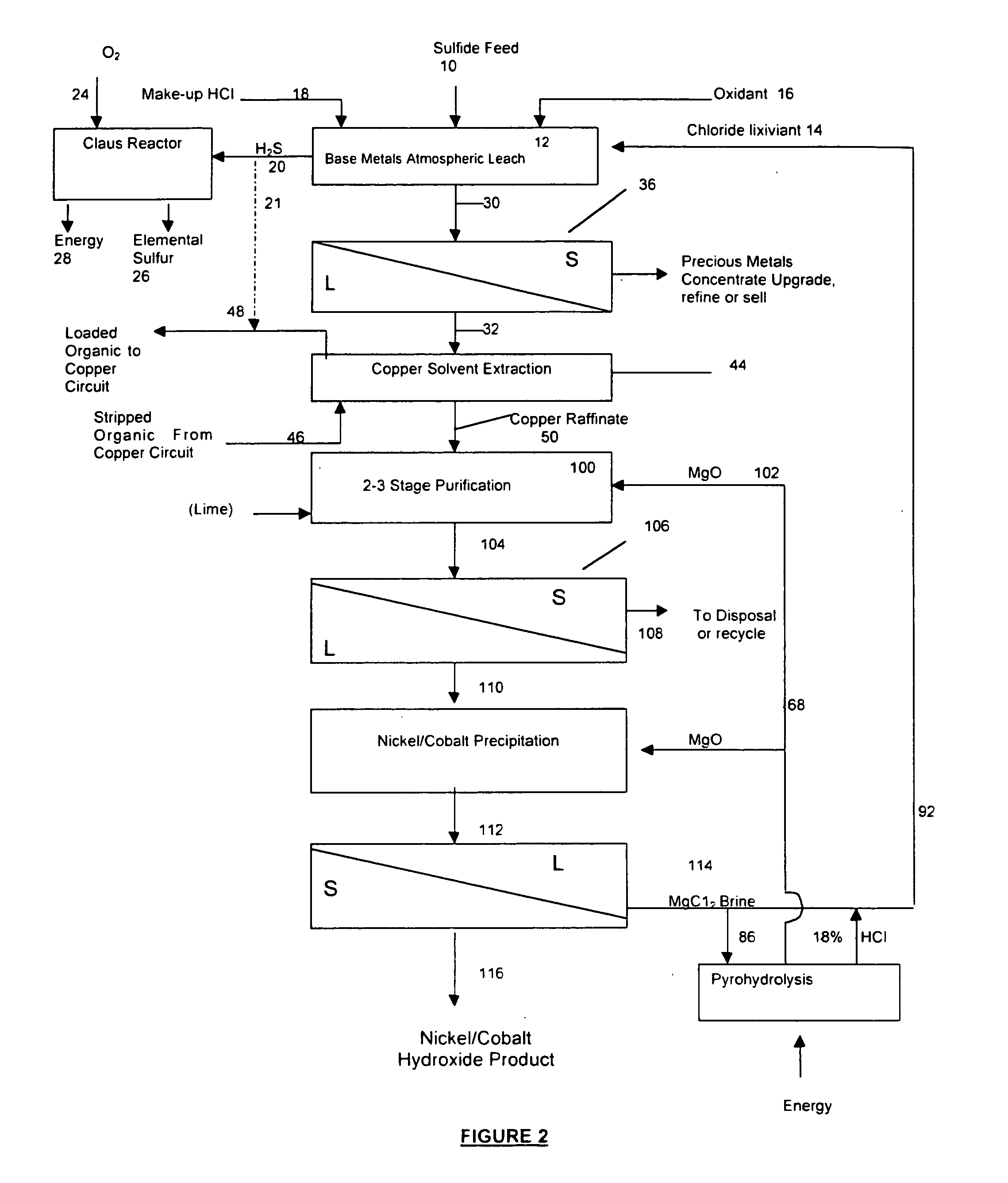
A process for leaching a value metal from a base metal sulfide ore, comprising the step of leaching the ore with a lixiviant comprising a chloride, an oxidant and hydrochloric acid is disclosed. The leaching is controlled, by use of low concentrations of hydrochloric acid and a redox potential, to effect formation of hydrogen sulfide from the base metal sulfide ore. The hydrogen sulfide is stripped from the leach solution, thereby reducing the amount of sulfate generated in the leach to very low levels. The leaching may also be conducted to limit the co-dissolution of platinum group metals and gold with the base value metals. The leach forms a value metal-rich leachate and a solids residue. The solids residue may be subsequently leached to recover the platinum group metals and gold. The value metal-rich leachate can be is oxidized and neutralized to recover the value base metals. In an embodiment, the chloride is magnesium chloride and lixiviant solution is regenerated.
Owner:JAGUAR NICKEL INC
Mixture of alkaline earth metal thiogallate green phosphor and sulfide red phosphor for phosphor-converted LED
InactiveUS20060082296A1Discharge tube luminescnet screensElectroluminescent light sourcesAlkaline earth metalSulfide
A device and method for emitting output light of a desired color utilizes green-emitting Thiogallate phosphor material and red-emitting SrCaS:Eu phosphor material to convert some of the original light emitted from a light source of the device to a longer wavelength light in order to produce the desired output light. The green-emitting Thiogallate phosphor material includes at least one of CaGa2S4:Ce phosphor and BaGa4S7:Eu phosphor. The device and method can be used to produce white light or other mixed color light using the light source, which may be a blue-green light emitting diode (LED) die.
Owner:AVAGO TECH ECBU IP (SINGAPORE) PTE LTD
Biomimetic hydrogel materials
Novel biomimetic hydrogel materials and methods for their preparation. Hydrogels containing acrylamide-functionalized carbohydrate, sulfoxide, sulfide or sulfone copolymerized with a hydrophilic or hydrophobic copolymerizing material selected from the group consisting of an acrylamide, methacrylamide, acrylate, methacrylate, vinyl and a derivative thereof present in concentration from about 1 to about 99 wt %. and methods for their preparation. The method of use of the new hydrogels for fabrication of soft contact lenses and biomedical implants.
Owner:RGT UNIV OF CALIFORNIA
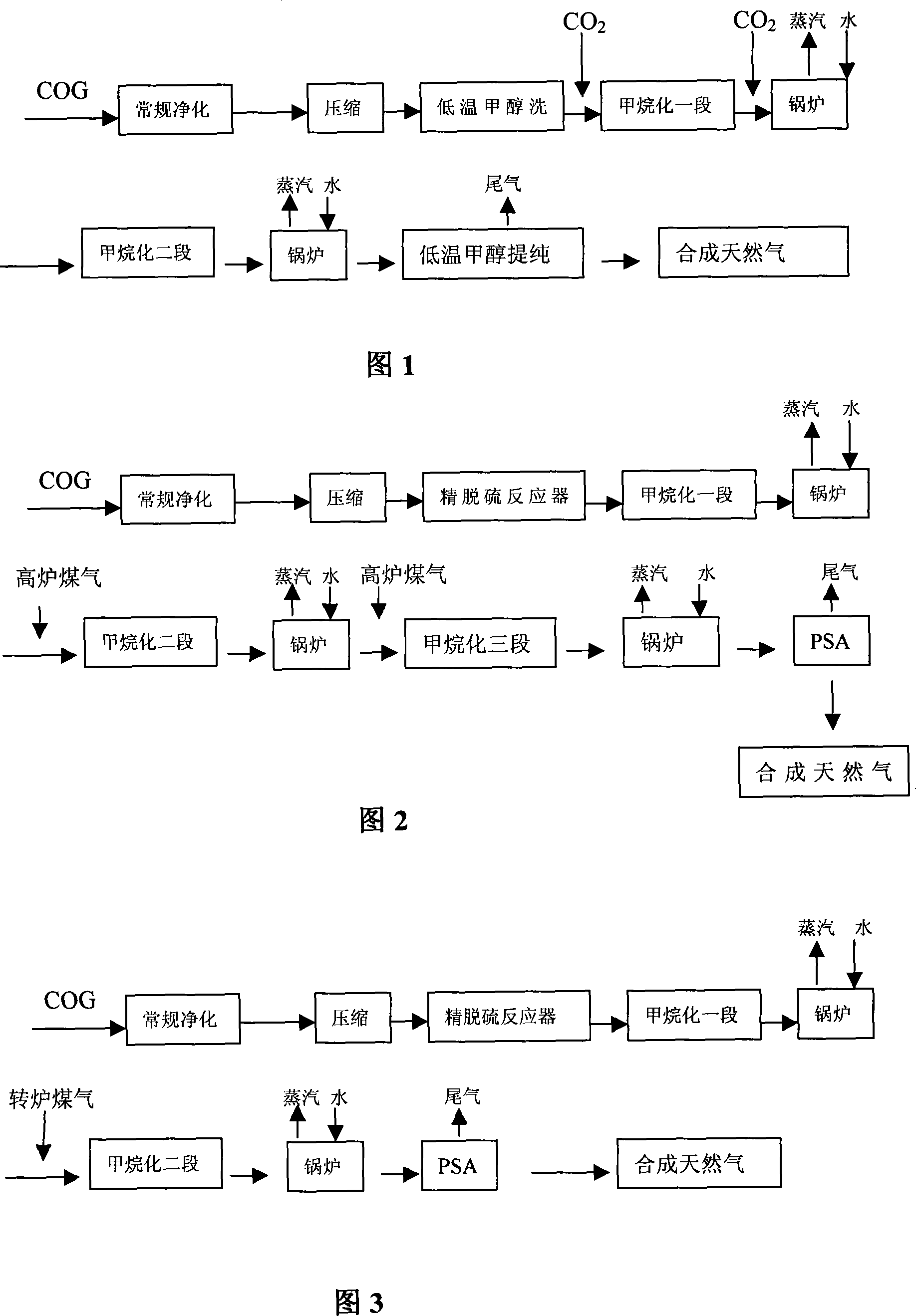
Method and device for synthesizing natural gas by using coke oven gas
A method for synthesizing and producing natural gas by hydrogen of coal gas and its synthesizer are disclosed. The process is carried out by purifying tar, crude removing sulfur, ammonia, benzene and naphthalene, compressing to 0.5-5.0 MPa, removing sulfur impurities, supplementing carbon 5-20 wt%, and methanation reacting to obtain final product. It saves energy resources and has no environmental pollution.
Owner:张文慧 +1
Methods for removing heavy metals from water using chemical precipitation and field separation methods
InactiveUS20050258103A1Efficient removalEasy to disassembleSedimentation separationDifferential sedimentationParticulatesSulfide
A two-step chemical precipitation process involving hydroxide precipitation and sulfide precipitation combined with “field separation ” technology such as magnetic separation, dissolved air flotation, vortex separation, or expanded plastics flotation, effectively removes chelated and non-chelated heavy metal precipitates and other fine particles from water. In the first-step, the non-chelated heavy metals are precipitated as hydroxides and removed from the water by a conventional liquid / solids separator such as an inclined plate clarifier to remove a large percentage of the dissolved heavy metals. The cleaned water is then treated in a second precipitation step to remove the residual heavy metals to meet discharge limits. In the second precipitation step, any metal precipitant more effective than hydroxide for metal precipitation can be used. The invention improves metal removal, lowers cost because fewer chemicals are used, produces less sludge, and reduces the discharge of toxic metals and metal precipitants to the environment. Magnetic separation is preferred for the separation of particles precipitated in the second stage. Similar methods can be employed for separation of other particulates from water. Particulates can also be removed by causing them to adhere to particles of expanded plastic, forming a floc lighter than water, so that the floc can be removed by flotation.
Owner:CORT CHERYL J
Lithium-enriched anti-perovskite sulfides, solid electrolyte material containing lithium-enriched anti-perovskite sulfides and application of solid electrolyte material
ActiveCN104466239AIncrease the carrier concentrationIncrease charge and discharge rateSecondary cellsElectrolytesWorking temperatureOperating temperature range
The invention discloses lithium-enriched anti-perovskite sulfides and a solid electrolyte material. The general formula of the lithium-enriched anti-perovskite sulfides is (LimMn)3-xS1-y(XaYb)1-z, wherein m is more than 0 and less than or equal to 1, n is more than or equal to 0 and less than to 0.5, (m+n) is less than or equal to 1, a is more than 0 and less than or equal to 1, b is more than or equal to 0 and less than 1, (a+b) is less than or equal to 1, x is more than or equal to 0 and less than or equal to 0.5, y is more than or equal to 0 and less than or equal to 0.5, z is more than or equal to 0 and less than or equal to 0.5 and x=2y+z; M is H, Na, K, Rb, Mg, Ca, Sr, Ba, Y, La, Ti, Zr, Zn, B, Al, Ga, In, C, Si, Ge, P, S or Se; and X is Fe, Cl, Br or I, and Y is a negative ion. The solid electrolyte material has high ion conductivity and thermal stability and a wide working temperature range, and can be applied to lithium ion batteries, rechargeable metal lithium batteries, lithium liquid flow batteries or lithium ion capacitors.
Owner:BEIJING WELION NEW ENERGY TECH CO LTD
Lithium battery and electrode
InactiveUS20020061441A1Material nanotechnologyElectrode manufacturing processesCarbon nanotubeSulfide
An electrode includes an electrically conductive matrix containing a disulfide group, wherein an S-S bond of the disulfide group is cleaved by electrochemical reduction and reformed by electrochemical oxidation. A plurality of carbon nanotubes is dispersed in the electrically conductive matrix. The electrode can be used as a cathode of a lithium battery.
Owner:CELANESE VENURES GMBH
Passivating agent for governing heavy metal contaminated soil and preparation and use method of passivating agent
ActiveCN104845626AGood stability and good performanceLong-term stabilization/passivationOther chemical processesContaminated soil reclamationClay mineralsSoil science
The invention discloses a passivating agent for governing heavy metal contaminated soil and a preparation and use method of the passivating agent. The passivating agent comprises, in weight percent, 30%-50% of clay minerals, 10%-20% of phosphate, 10%-15% of reducing agents, 10%-15% of sulfide and 15%-20% of alkaline substances. The preparation method includes a step: mixing the clay minerals, the phosphate, the reducing agents, the sulfide and the alkaline substances to mix to obtain the passivating agent. The use method includes the steps: firstly, crushing the heavy metal contaminated soil; secondly, adding the passivating agent into the crushed heavy metal contaminated soil; thirdly, adding water into the crushed heavy metal contaminated soil added the passivating agent to uniformly mix, maintaining the uniformly mixed soil on site, and enabling the passivating agent and heavy metal in the soil to sufficiently react. The passivating agent can effectively meet the urgent requirements of current Chinese heavy metal contaminated soil for high stability, no secondary pollution, durable and stable effect and low cost.
Owner:CHANGSHA HASKY ENVIRONMENTAL PROTECTION TECH DEV CO LTD
Methods for removing heavy metals from water using chemical precipitation and field separation methods
InactiveUS7255793B2Cost- and chemically-effectiveSedimentation separationWater/sewage treatment by neutralisationParticulatesSulfide
A two-step chemical precipitation process involving hydroxide precipitation and sulfide precipitation combined with “field separation ” technology such as magnetic separation, dissolved air flotation, vortex separation, or expanded plastics flotation, effectively removes chelated and non-chelated heavy metal precipitates and other fine particles from water. In the first-step, the non-chelated heavy metals are precipitated as hydroxides and removed from the water by a conventional liquid / solids separator such as an inclined plate clarifier to remove a large percentage of the dissolved heavy metals. The cleaned water is then treated in a second precipitation step to remove the residual heavy metals to meet discharge limits. In the second precipitation step, any metal precipitant more effective than hydroxide for metal precipitation can be used. The invention improves metal removal, lowers cost because fewer chemicals are used, produces less sludge, and reduces the discharge of toxic metals and metal precipitants to the environment. Magnetic separation is preferred for the separation of particles precipitated in the second stage. Similar methods can be employed for separation of other particulates from water. Particulates can also be removed by causing them to adhere to particles of expanded plastic, forming a floc lighter than water, so that the floc can be removed by flotation.
Owner:CORT CHERYL J
Apparatus to establish and optimize sedimentation and methane fermentation in primary wastewater ponds
InactiveUS6923906B2Raise the pHIncreases the rate of die-away of pathogenic bacteriaLiquid degasificationMixing methodsSludgeIncrease ph
Owner:GREEN FRANKLIN BAILEY +2
Methods for removing heavy metals from water using chemical precipitation and field separation methods
InactiveUS6896815B2Small sizeChemical cost reductionSolid sorbent liquid separationGold compoundsWater useSludge
A two-step chemical precipitation process involving hydroxide precipitation and sulfide precipitation combined with “field separation” technology such as magnetic separation, dissolved air flotation, vortex separation or expanded plastics flotation, effectively removes chelated and non-chelated heavy metal precipitates and other fine particles from water. In the first-step, the non-chelated heavy metals are precipitated as hydroxides and removed from the water by a conventional liquid / solids separator such as an inclined plate clarifier to remove a large percentage of the dissolved heavy metals. The cleaned water is then treated in a second precipitation step to remove the residual heavy metals to meet discharge limits. In the second precipitation step, any metal precipitant more effective than hydroxide for metal precipitation can be used. The invention improves metal removal, lowers cost because fewer chemicals are used, produces less sludge, and reduces the discharge of toxic metals and metal precipitants to the environment.
Owner:CORT STEVEN L
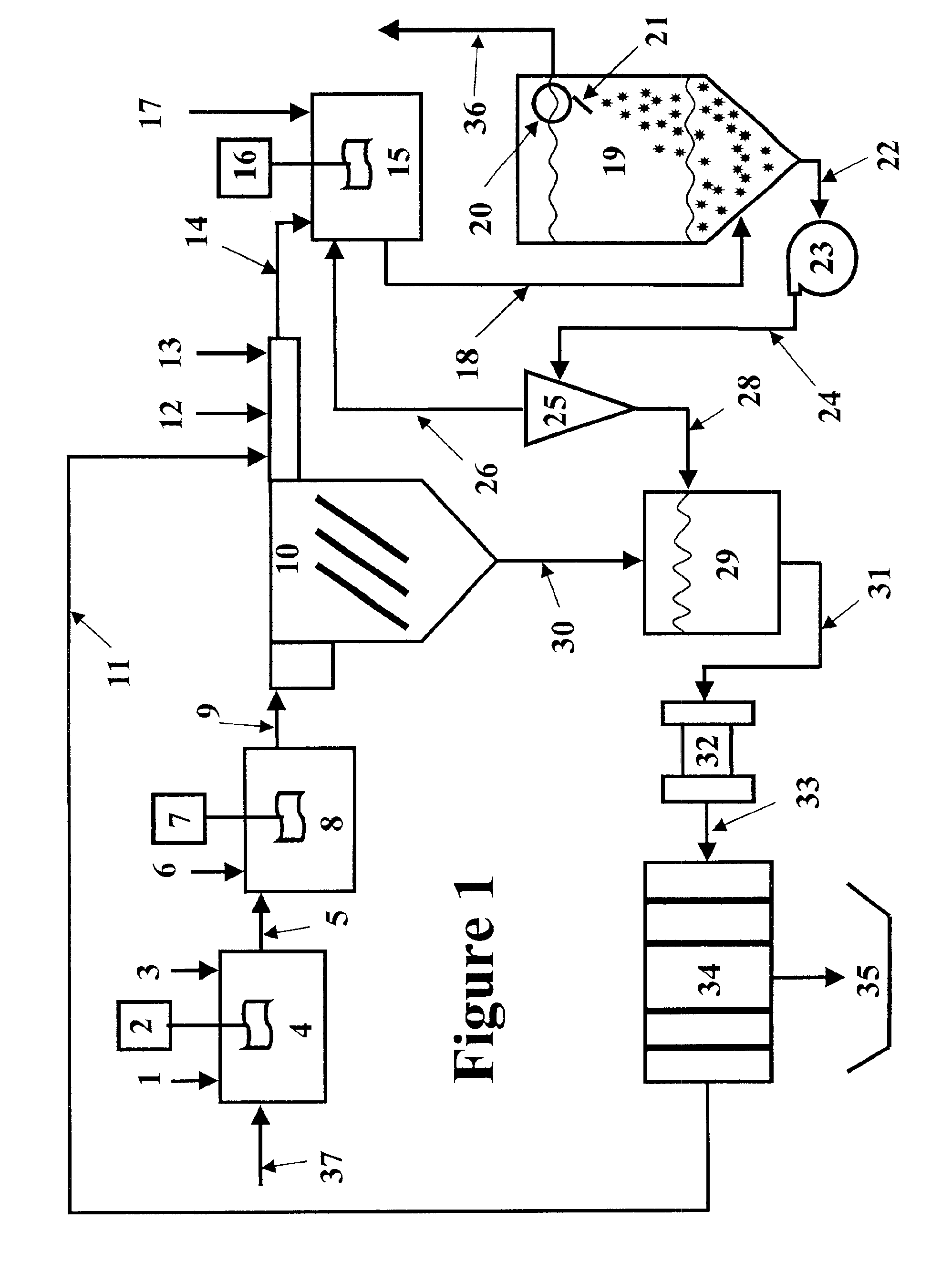
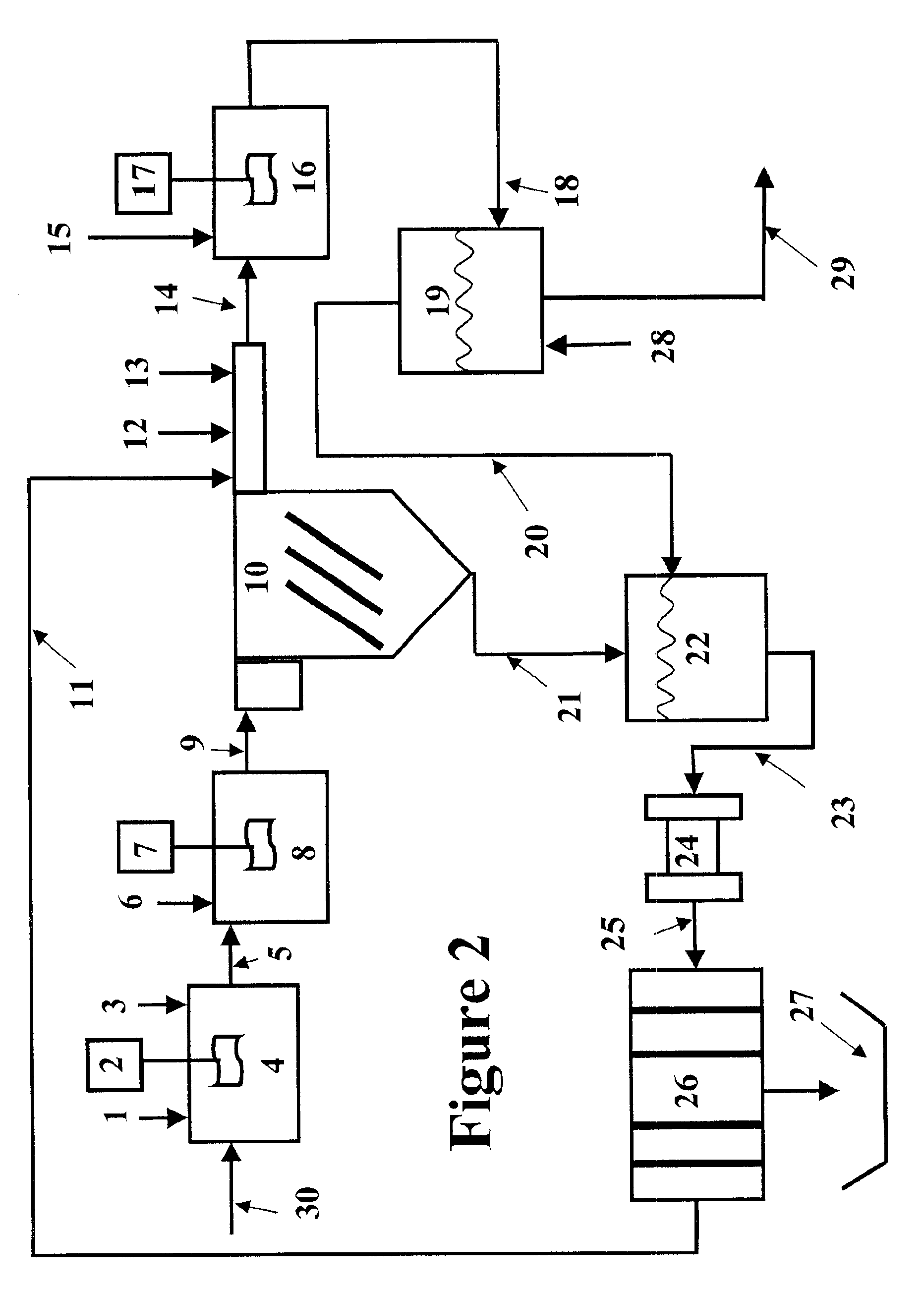
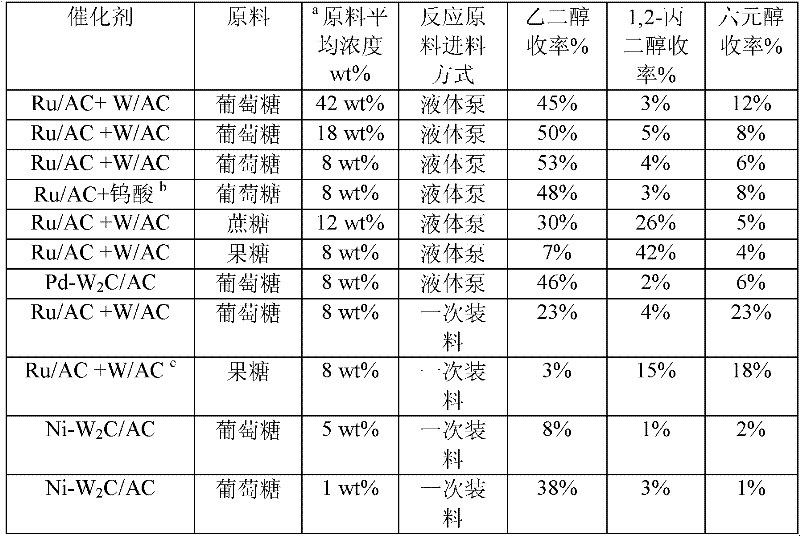
Method for preparing ethylene glycol and 1,2-propylene glycol by using saccharide solution
ActiveCN102675045AIncrease concentrationReduce distillation energy consumptionOrganic compound preparationHydroxy compound preparationHydrogen pressurePolyethylene glycol
The invention provides a method for preparing ethylene glycol and 1,2-propylene glycol by using a high-concentration saccharide solution. Reaction raw materials comprise cane sugar, glucose, fructose, fructosan, xylose, soluble lower polyxylose and soluble starch. According to the method, high-concentration saccharide is used as a reaction raw material, and a high-pressure pump feeding mode is used in a reaction process which is performed in a high-pressure reaction kettle; iron, cobalt, nickel, ruthenium, rhodium, palladium, iridium and platinum which serve as transition metal in eighth, ninth and tenth groups are used as hydrogenation active ingredients; the hydrogenation active ingredients form a composite catalyst together with metal tungsten, tungsten carbide, tungsten nitride, tungsten phosphide, tungsten oxide, tungsten sulfide, tungsten chloride, tungsten hydroxide, tungsten bronze, tungstic acid, tungstate, metatungstic acid, metatungstate, paratungstic acid, paratungstate, peroxotungstic acid, peroxotungstate and tungsten-containing heteropolyacid which serve as catalytic active ingredients; and the high-concentration saccharide solution can be efficiently prepared into the ethylene glycol and the propylene glycol at high selectivity and high yield in a one-step catalytic conversion process under the hydrothermal condition that the temperature is 120 to 300 DEG C and the hydrogen pressure is 1 to 13MPa. By the method, the problem of coking of the high-concentration saccharide in the catalytic conversion process can be effectively solved, and high-concentration ethylene glycol and propylene glycol can be prepared by the high-concentration saccharide.
Owner:中科柏易金(郑州)新能源科技有限责任公司
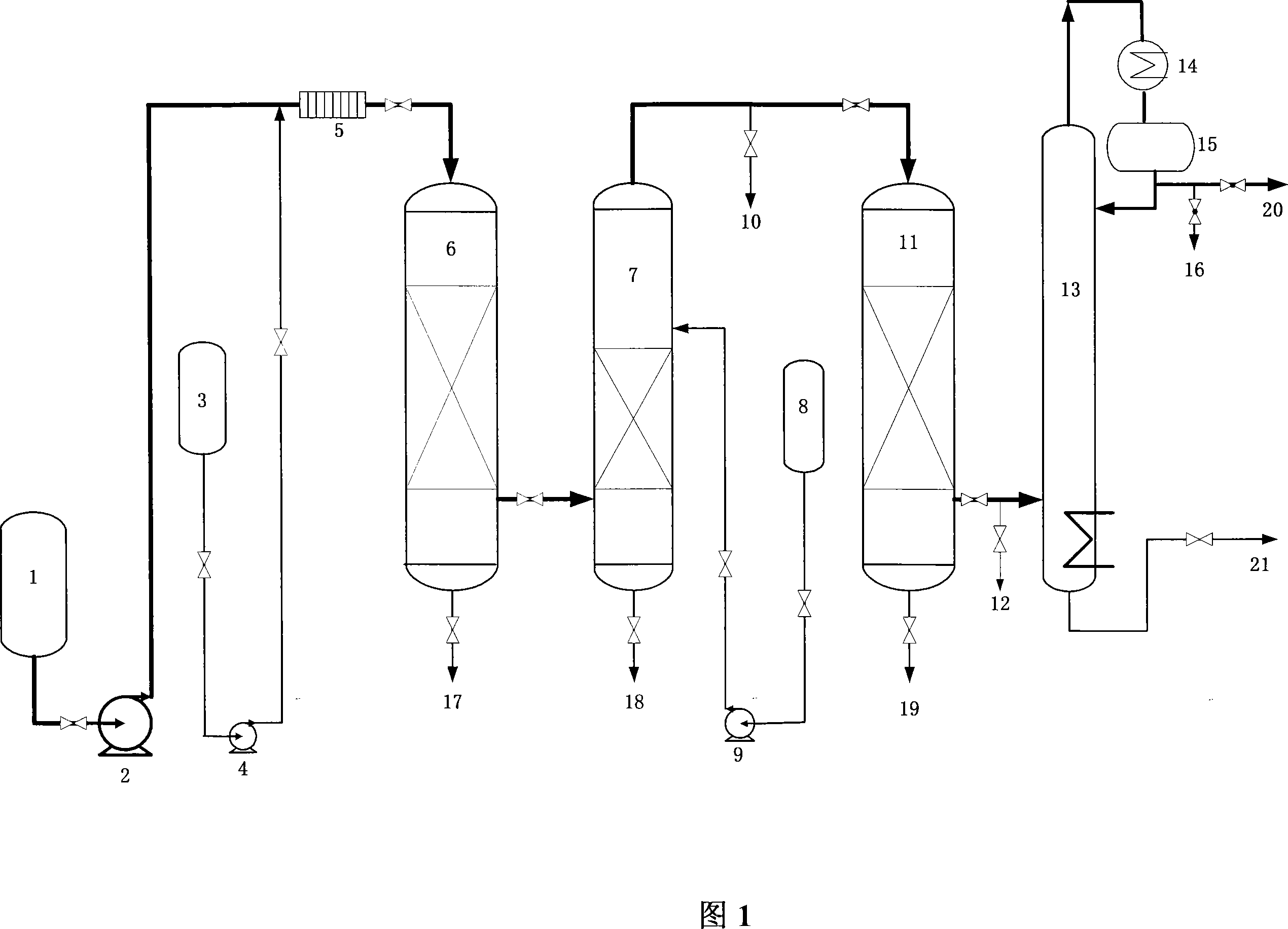
Deep desulfurization method for liquefied petroleum gas
The liquefied petroleum gas deep desulfurizing process includes the following steps: mixing alcohol amine treated liquefied petroleum gas with desulfurizer aqua, feeding the mixture into a reactor with carbonyl sulfide hydrolyzing catalyst for hydrolyzing carbonyl sulfide into hydrogen sulfide and CO2 and eliminating hydrogen sulfide, regenerating the used desulfurizer, water washing the liquefied petroleum gas to eliminate residual desulfurizer with water containing dissolved oxygen or hydrogen peroxide in a water washing tower with hydrogen peroxide decomposing catalyst in the stuffing layer, eliminating mercaptan from the liquefied petroleum gas through oxidizing mercaptan into disulfide in a mercaptan eliminating reactor with catalyst, and final rectifying in a rectifying tower to eliminate disulfide. The process is simple and practical, and can lower the total sulfide content in liquefied petroleum gas to below 5 ppm.
Owner:北京石大世通科技发展有限公司
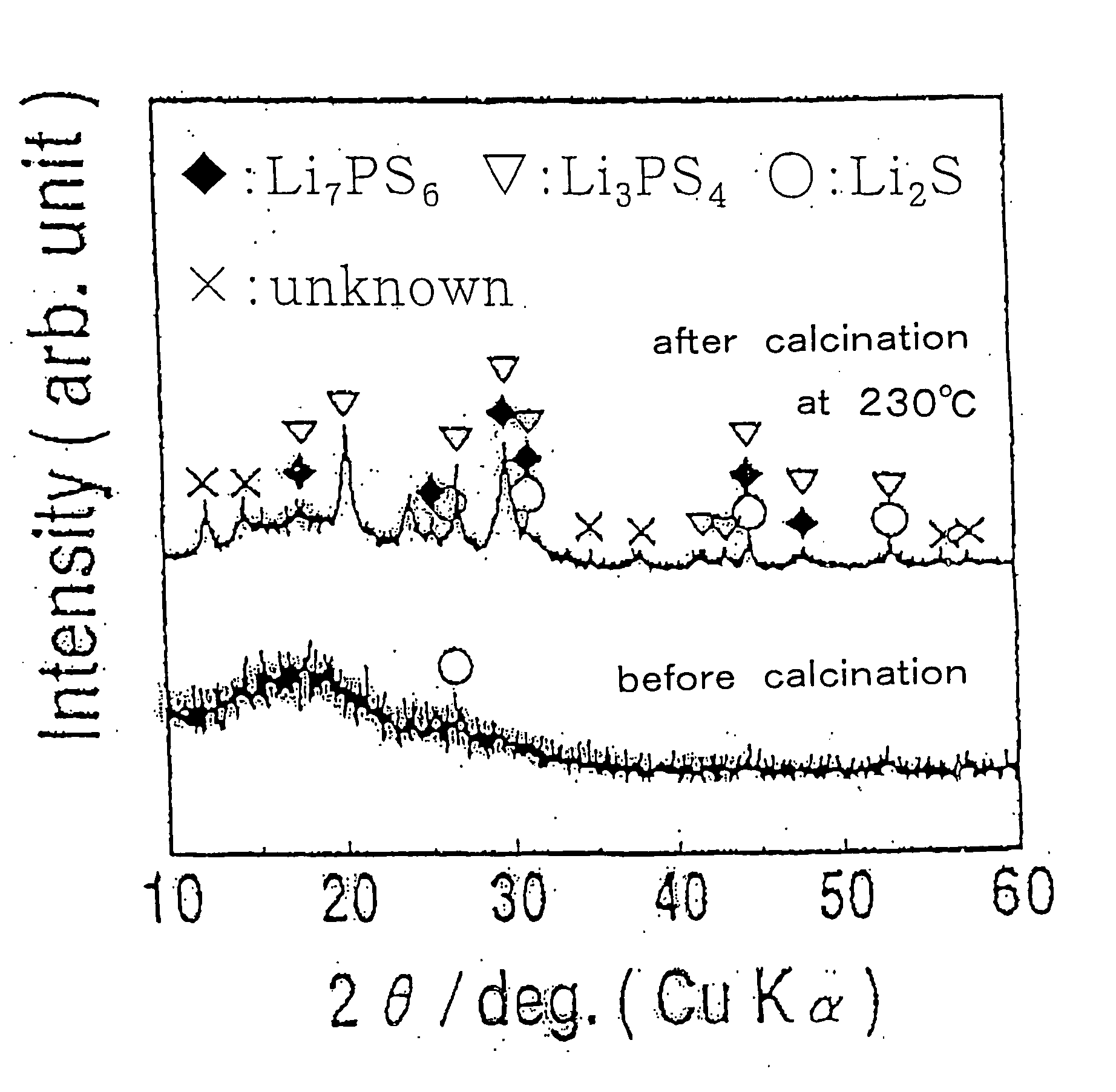
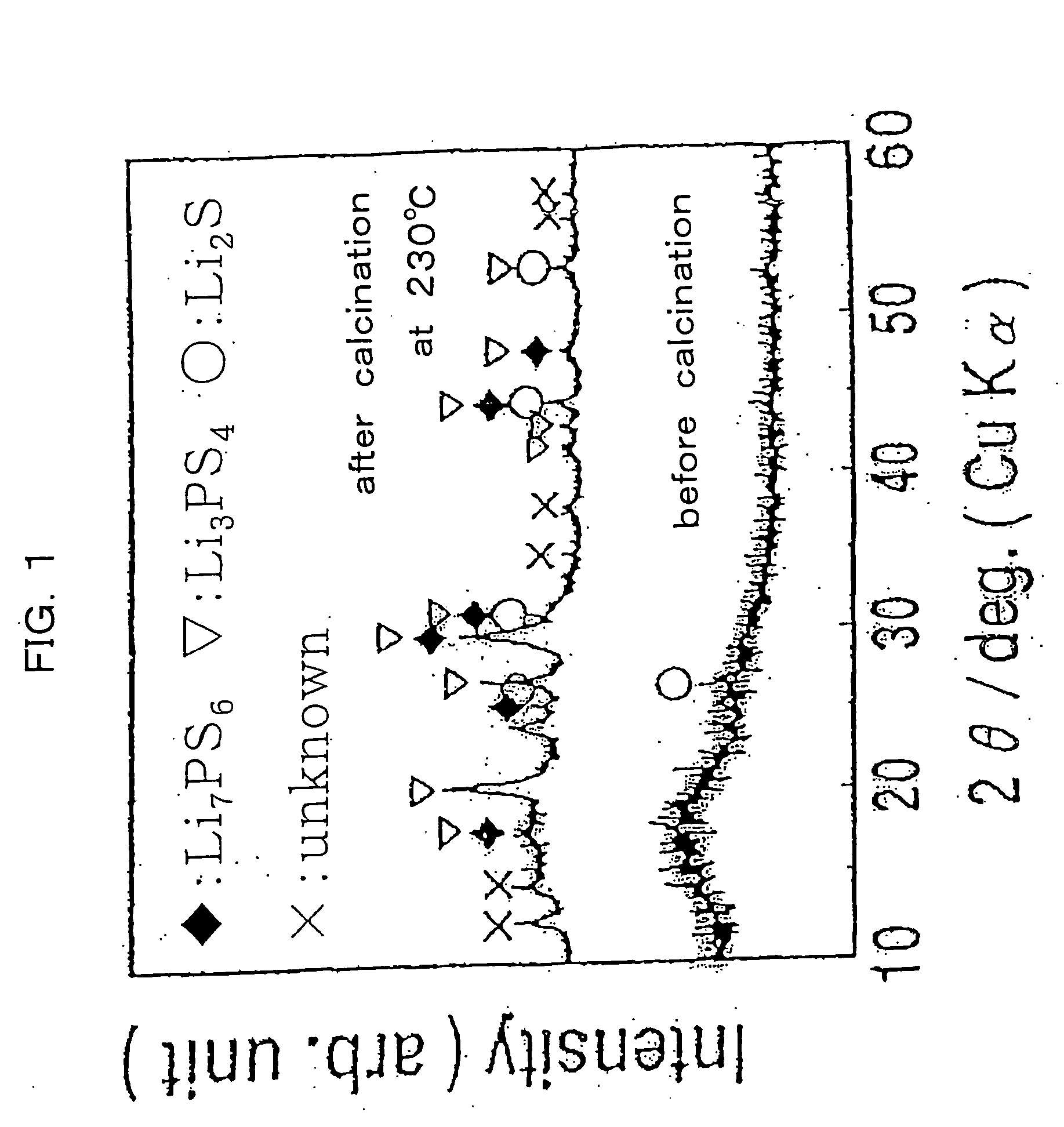
Method for producing sulfide glass or sulfide glass ceramic capable of conducing lithium ion, and whole solid type cell using said glass ceramic
InactiveUS20050107239A1Simple and convenient and easily availableSolid electrolytesConductive materialMetallic lithiumSulfur
The present invention relates to a process for producing sulfide glass or sulfide glass ceramic each capable of conducting a lithium ion, comprising subjecting metallic lithium, sulfur as a simple substance and phosphorus as a simple substance as starting raw materials, which constitute the sulfide glass and sulfide glass ceramic, to mechanical milling to thereby convert them into sulfide glass or sulfide glass ceramic; and a whole solid type cell using the above-mentioned sulfide glass ceramic as a solid electrolyte. According to the present invention, it is made possible to produce sulfide glass and sulfide glass ceramic which are each capable of conducting a lithium ion and which have high electroconductivity at room temperature by a simple and advantageous process from starting raw materials being easily available and inexpensive.
Owner:IDEMITSU KOSAN CO LTD +1
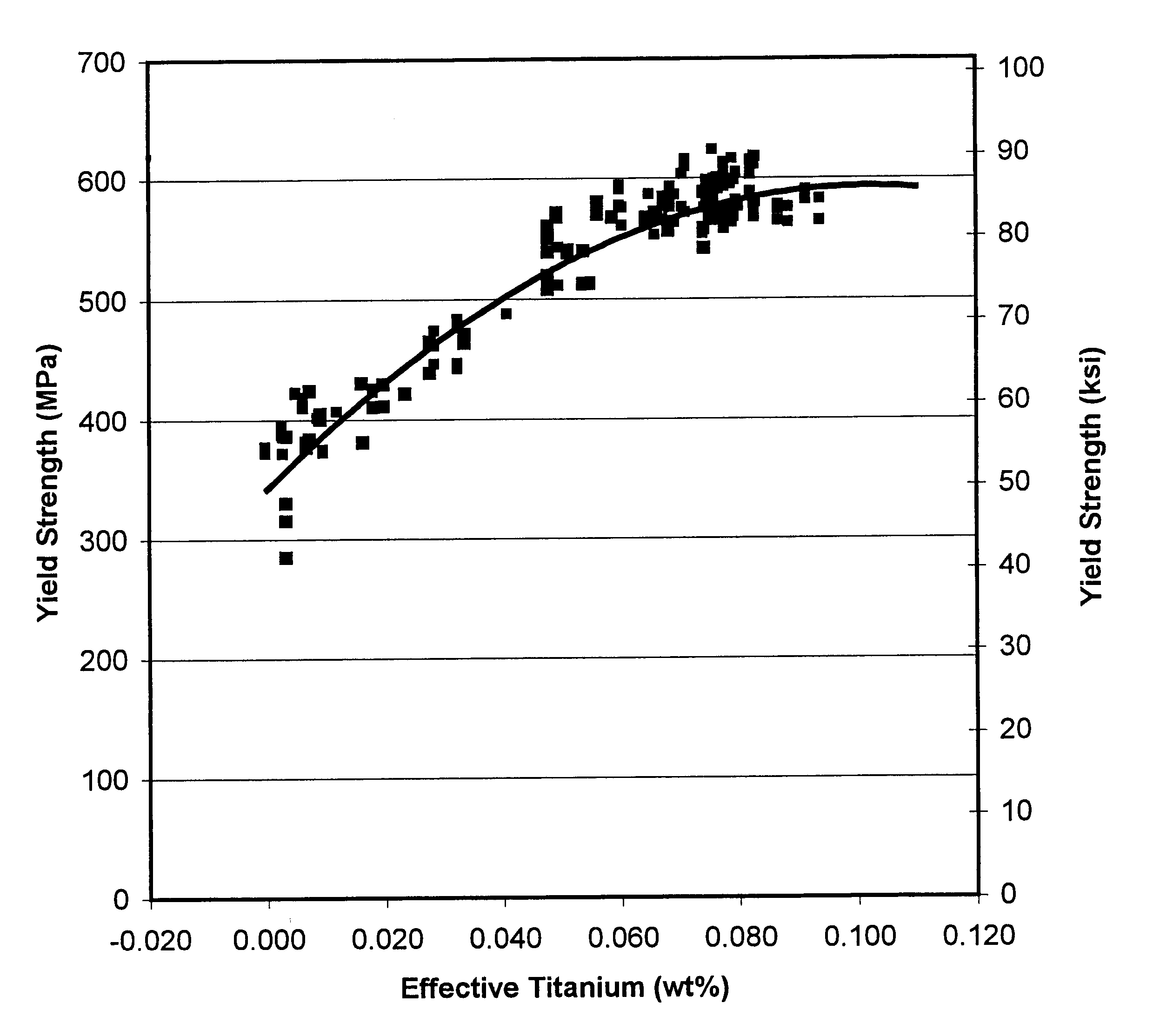
Method for producing titanium-bearing microalloyed high-strength low-alloy steel
A composition and method of making a high-strength low-alloy hot-rolled steel sheet, strip, or plate bearing titanium as the principal or only microalloy strengthening element. The steel is substantially ferritic and has a microstructure that is at least 20% acicular ferrite. The steel has a minimum yield strength of at least 345 MPa (50 ksi) and even over 621 MPa (90 ksi) adding titanium as the lone microalloy element for strengthening, with elongation of 15% and more. Addition of vanadium, niobium, or a combination thereof can result in yield strengths exceeding 621 MPa (90 ksi). Effective titanium content, being the content of titanium in the steel not in the form of nitrides, oxides, or sulfides, is in the range of 0.01 to 0.12% by weight. The manufacturing process includes continuously casting a thin slab and reducing the slab thickness using thermomechanical controlled processing, including dynamic recrystallization controlled rolling.
Owner:NUCOR CORP
Anode active material, preparation method of anode active material, high-performance anode slurry containing anode active material, and all-solid-state lithium ion battery
ActiveCN106784798AImprove mobilityImprove electrochemical performanceCell electrodesSecondary cellsInorganic compoundSulfide
The invention relates to an anode active material, a preparation method of the anode active material, high-performance anode slurry containing the anode active material, and an all-solid-state lithium ion battery. The anode active material is a nickel-rich type core-shell structure particle or a nickel-rich type core-shell structure particle coated with an inorganic compound coating layer at the surface; an inner core of the nickel-rich type core-shell structure particle is LiNixCoyMn1-x-yO2; the shell is nickel cobalt lithium aluminate. The invention also provides the high-performance anode slurry, which comprises the anode active material, a composite conductive agent, a composite bonding agent, an additive and an organic solvent, wherein the additive is sulfide solid electrolyte; the anode slurry is used for preparing an anode plate consisting of an anode current collector, an anode slurry layer and a modification layer; the anode plate, the sulfide solid electrolyte and a cathode plate are assembled into the all-solid-state lithium ion battery. The all-solid-state lithium ion battery has the prominent advantages of high mass specific energy, high volumetric specific energy, good rate capability, good cycle performance, high safety and the like, and has wide application prospects.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Lithium battery
InactiveUS20090068563A1Suppression problemOvercome lack of heat resistanceFinal product manufactureElectrode carriers/collectorsSulfideVapor phase
A lithium battery includes a substrate, a positive electrode layer, a negative electrode layer, and a sulfide solid electrolyte layer disposed between the positive electrode layer and the negative electrode layer, the positive electrode layer, the negative electrode layer, and the sulfide solid electrolyte layer being provided on the substrate. In this lithium battery, the positive electrode layer is formed by a vapor-phase deposition method, and a buffer layer that suppresses nonuniformity of distribution of lithium ions near the interface between the positive electrode layer and the sulfide solid electrolyte layer is provided between the positive electrode layer and the sulfide solid electrolyte layer. As the buffer layer, a lithium-ion conductive oxide, in particular, LixLa(2-x) / 3TiO3 (x=0.1 to 0.5), Li7+xLa3Zr2O12+(x / 2) (−5≦×≦3, preferably −2≦×≦2), or LiNbO3 is preferably used.
Owner:SUMITOMO ELECTRIC IND LTD
Gel-type polymer electrolyte used for lithium-sulfur secondary battery system and preparation method
InactiveCN102130364AHigh conductivity at room temperatureImprove securityFinal product manufactureElectrolyte accumulators manufacturePolymer electrolytesPolymer science
The invention relates to a gel-type polymer electrolyte used for a lithium-sulfur secondary battery system, consisting of a polymer support body, an ionic liquid, an organic solvent, a mixed lithium salt and dioxide silicon particles. A preparation method comprises the following steps of: preparing an imidazolium-based ionic liquid, dioxide silicon and composite lithium salt into a gel liquid in a carbonic ester solution dissolved with macromolecular polymers, then coating, drying and obtaining the gel-type polymer electrolyte film. In the gel-type polymer electrolyte prepared by the invention, sulfides in the lithium-sulfur battery system can be effectively prevented from dissolving in a liquid electrolyte solution, the ionic conductivity is high, and noninflammability and no leakage are achieved; and for the polymer electrolyte, the preparation process is simple, and the raw material source is wide, so that the gel-type polymer electrolyte is suitable for industrial production.
Owner:CENT SOUTH UNIV
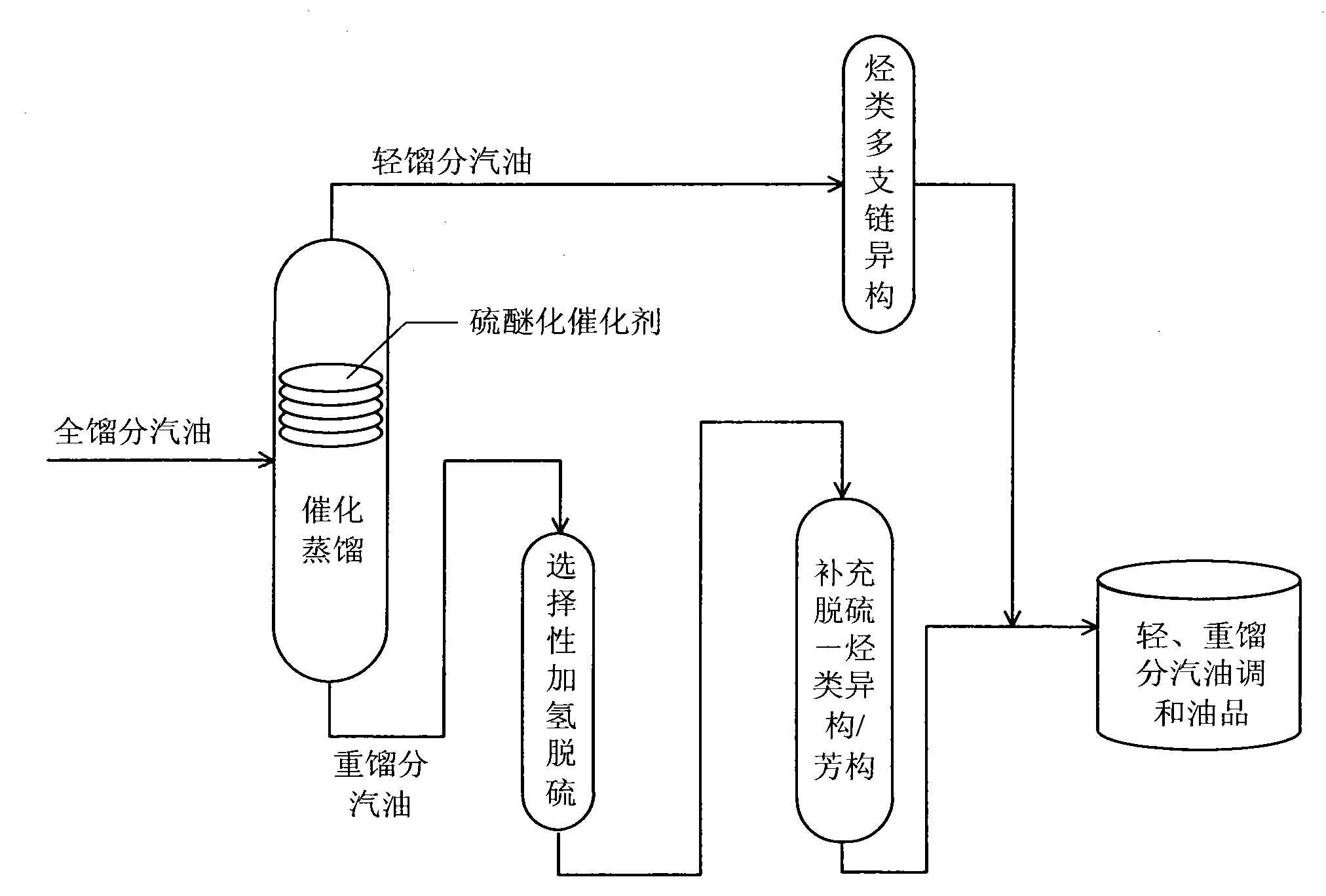
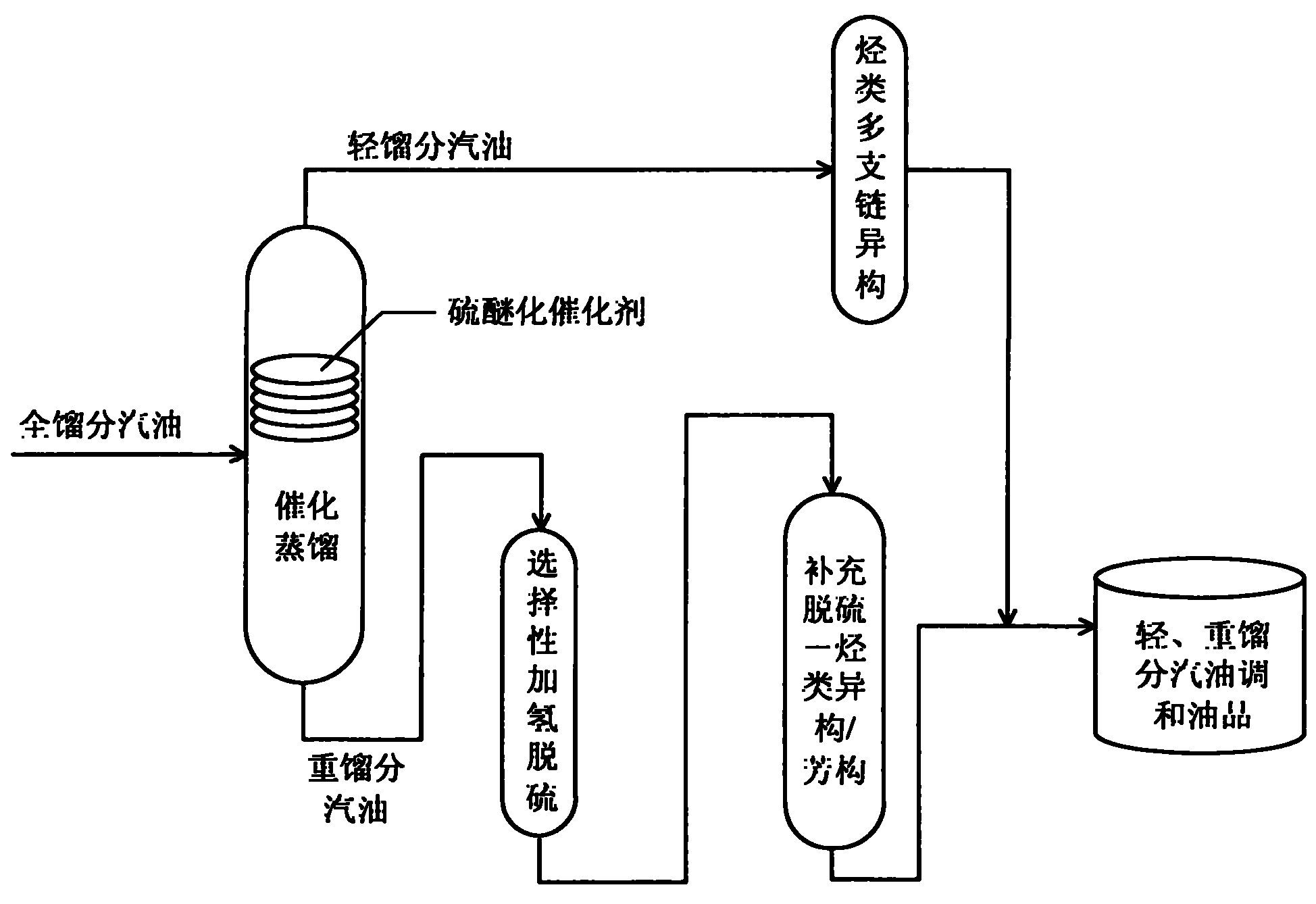
Production method for ultra-low sulfur and high-octane number gasoline
ActiveCN101885985ATake advantage ofIncrease temperatureTreatment with hydrotreatment processesIsomerizationHydrodesulfurization
The invention relates to a production method for ultra-low sulfur and high-octane number gasoline. The method comprises the following steps of: filling a poor-quality full range gasoline raw material in a reaction distillation column to contact the material with a sulfoether catalyst to perform a sulfur ether reaction and fraction cutting so that low-boiling point sulfides, such as thiol and thiophene, are converted into high-boiling point sulfoether which is then transferred into heavy fraction gasoline, wherein the cutting fractionation temperature of light fraction gasoline and the heavy fraction gasoline is 50 to 90 DEG C; contacting the light fraction gasoline with a hydrocarbon highly branched isomerization catalyst; contacting the heavy fraction gasoline with a selective hydrodesulfurization catalyst and a desulfurization-hydrocarbon isomerization / aromatization catalyst; and mixing the treated light fraction gasoline and the heavy fraction gasoline to obtain the ultra-low sulfur and high-octane number gasoline. The method is suitable for modifying poor-quality gasoline, can reach better desulfurization and olefin reduction effects on ultra-high sulfur and high-olefin poor-quality catalytic gasoline, and can maintain or increase the octane number of the product and keep a higher product yield after reaction.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
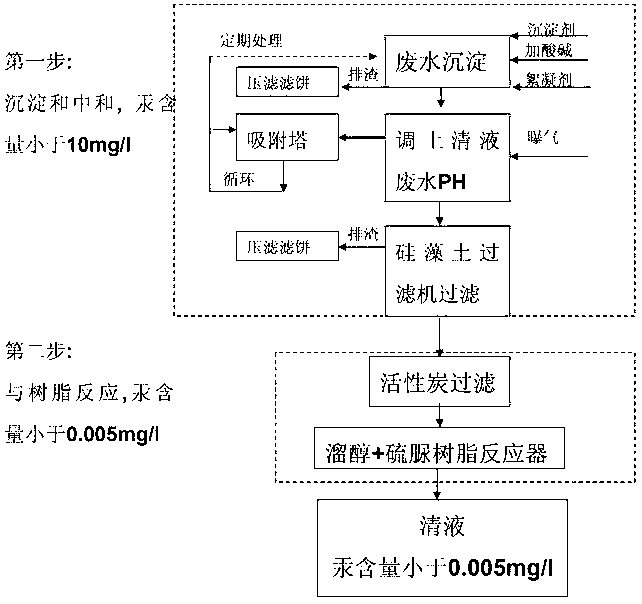
Method for treating mercury-containing wastewater during PVC (Polyvinyle Chloride) production through two-step process
InactiveCN102936070AEnsuring a circular economyImprove processing efficiencyMultistage water/sewage treatmentNature of treatment waterSlagGas phase
The invention discloses a method for treating mercury-containing wastewater during PVC (Polyvinyle Chloride) production through a two-step process. The method comprises the following steps of: firstly, completing primary removal of mercury, copper, ferrous and ferric iron, cadmium, zinc, manganese, lead and suspending impurities of above 10mg / l by using a precipitator and a flocculating agent, regulating PH to 6-8 and then adding the flocculating agent and stirring for 30 minutes, standing for above 1 hour, discharging slag and aerating, absorbing mercury carried away by a gas phase through a sulfide adsorber, purifying water by using a plate type kieselguhr filter, removing residual suspended matters; and 2, carrying out complete reaction on the mercury in the wastewater by using an active carbon and a mercaptan and thiourea resin combining method again for being removed, and finally reaching the standard that the content of the mercury is less than 0.005mg / l. The treated wastewater can be recycled to salt melting or an acetylene generator, so that zero emission of the treated wastewater is achieved; and the mercury-containing waste acid water of hydrochloric acid desorption in the industry of chlor-alkali can be treated, the consumption of acid is reduced, and the great popularization significance is achieved in the industry of chlor-alkali.
Owner:赵建军
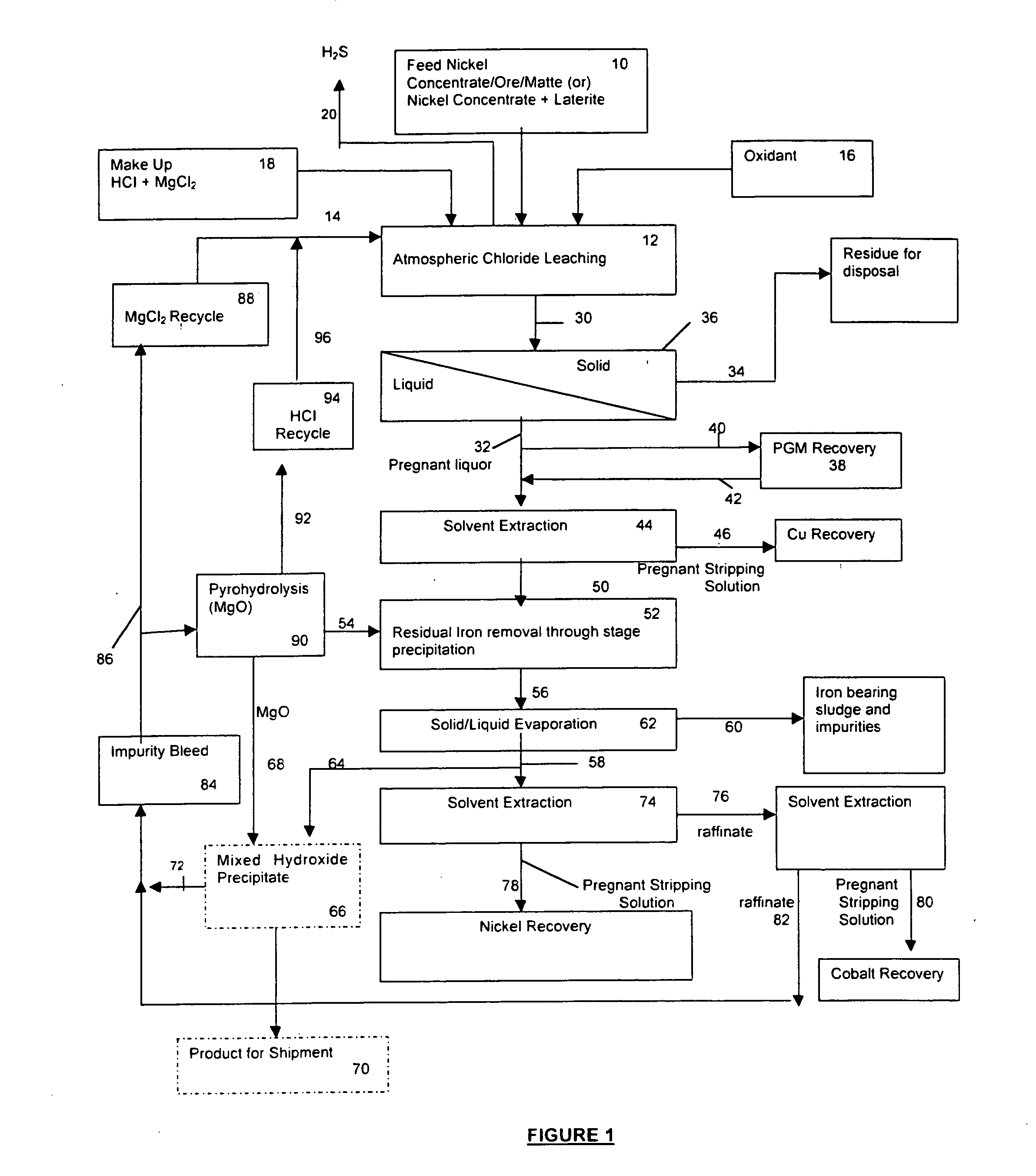
Hydrometallurgical process of nickel oxide ore
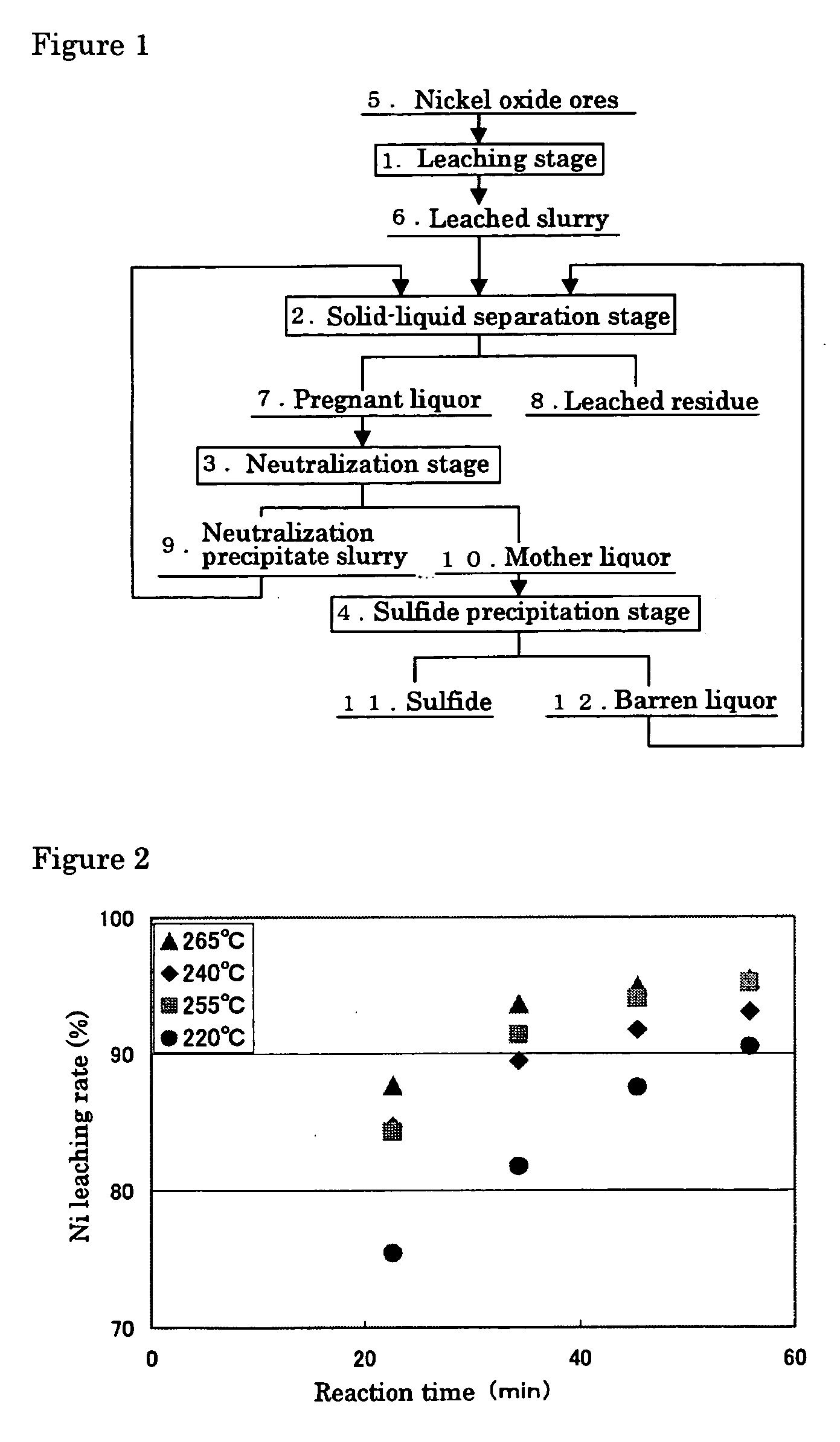
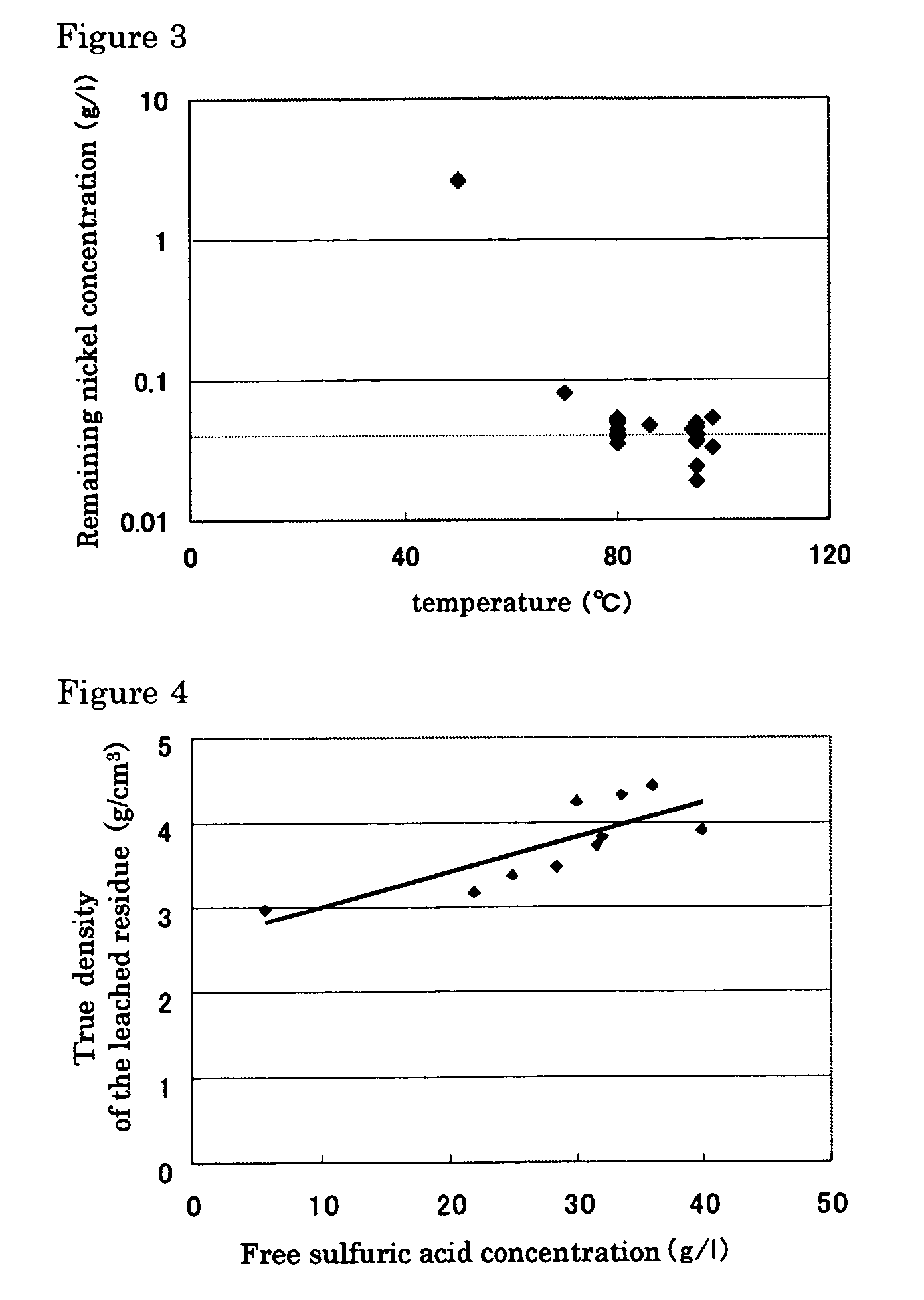
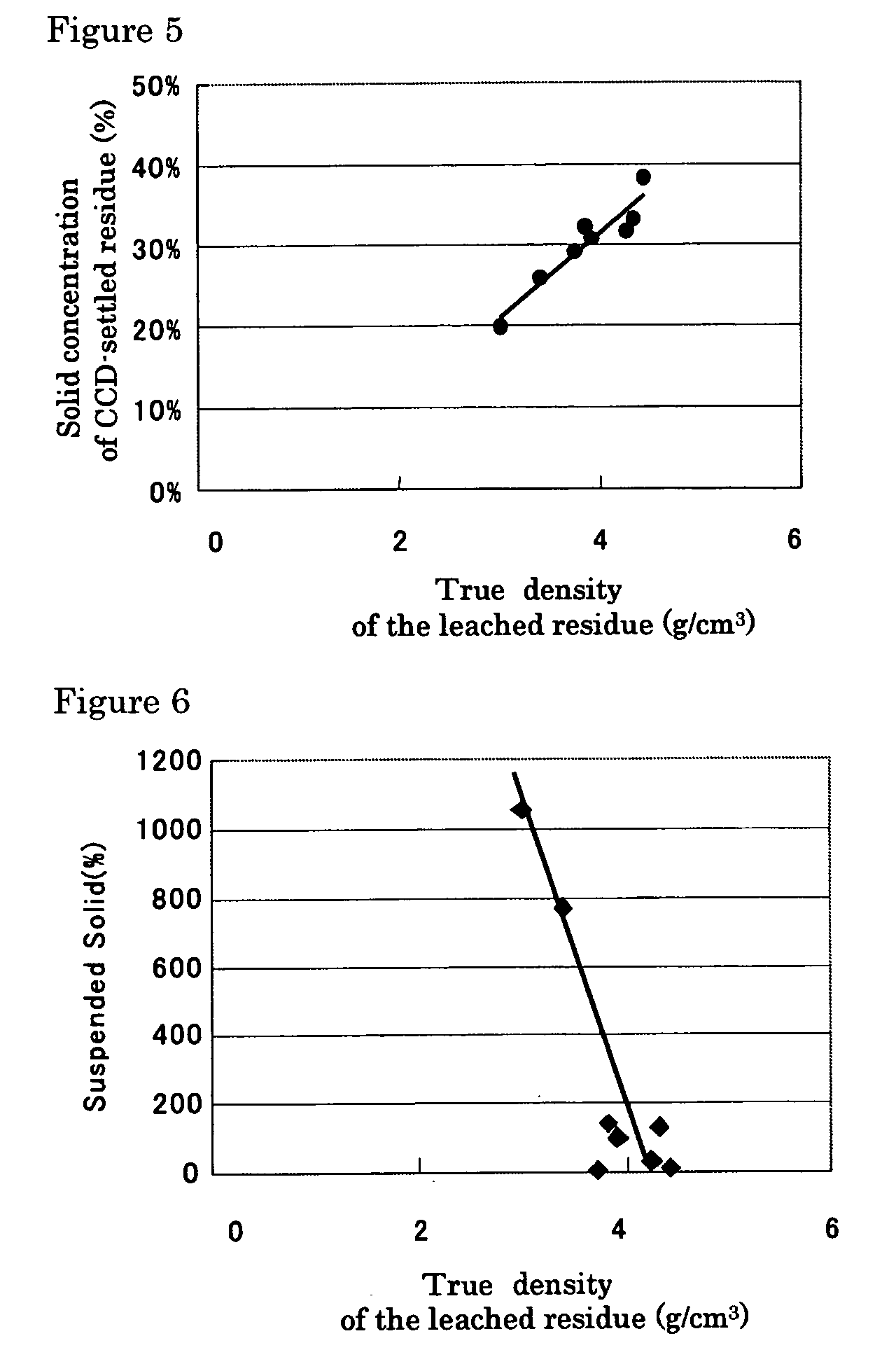
InactiveUS20050265910A1Simple and efficient processSimplified leaching stageSolvent extractionIron compoundsSlurryHydrometallurgy
A hydrometallurgical process based on pressure leaching at elevated temperature for recovering nickel from nickel oxide ores, characterized by a simplified and efficient process as a whole, realizing a simplified leaching stage, reduced neutralizer consumption and precipitate production in the neutralization stage, and efficient use of recycled water. The hydrometallurgical process of the present invention, comprising a leaching stage which stirs the slurried ore in the presence of sulfuric acid at 220 to 280° C. to produce the leached slurry; solid-liquid separation stage which washes the leached slurry in multi-stages to produce the pregnant liquor and leached residue, the former containing nickel and cobalt; neutralization stage which treats the pregnant liquor in the presence of calcium carbonate incorporated to keep the pH level at 4 or less, while suppressing oxidation of the liquor, to produce the neutralization precipitate slurry and mother liquor for nickel recovery, the former containing trivalent iron; and a sulfide precipitation stage which blows hydrogen sulfide gas into the mother liquor to produce the sulfide solution and barren liquor, the former containing nickel and cobalt.
Owner:SUMITOMO METAL MINING CO LTD
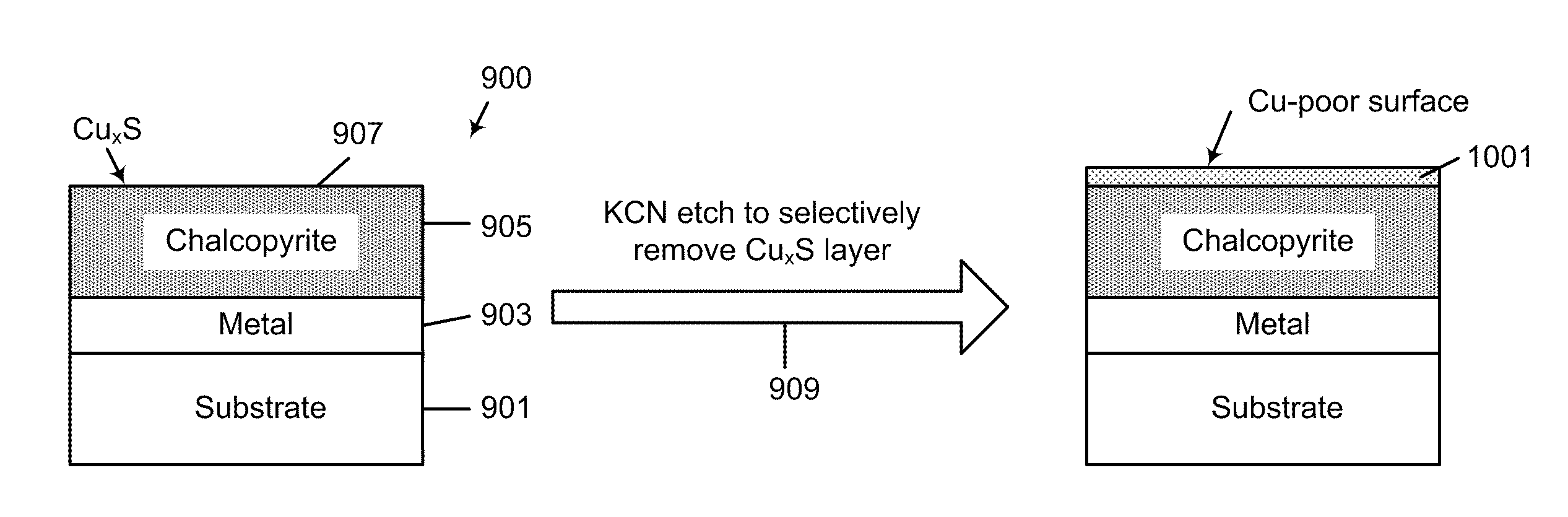
Sulfide species treatment of thin film photovoltaic cell and manufacturing method
ActiveUS8003430B1Toxic reductionAchieve benefitsSemiconductor/solid-state device manufacturingPhotovoltaic energy generationIndiumSulfur
A method for forming a thin film photovoltaic device. The method includes providing a transparent substrate comprising a surface region, forming a first electrode layer overlying the surface region, forming a copper layer overlying the first electrode layer and forming an indium layer overlying the copper layer to form a multi-layered structure. The multi-layered structure is subjected to a thermal treatment process in an environment containing a sulfur bearing species to forming a copper indium disulfide material. The copper indium disulfide material comprising a copper-to-indium atomic ratio ranging from about 1.2:1 to about 2:1 and a thickness of substantially copper sulfide material having a copper sulfide surface region. The thickness of the copper sulfide material is selectively removed to expose a surface region having a copper poor surface comprising a copper to indium atomic ratio of less than about 0.95:1. The method subjects the copper poor surface to a sulfide species to convert the copper poor surface from an n-type semiconductor characteristic to a p-type semiconductor characteristic. A window layer is formed overlying the copper indium disulfide material.
Owner:CM MFG
Lithium battery and electrode
An electrode includes an electrically conductive matrix containing a disulfide group, wherein an S-S bond of the disulfide group is cleaved by electrochemical reduction and reformed by electrochemical oxidation. A plurality of carbon nanotubes is dispersed in the electrically conductive matrix. The electrode can be used as a cathode of a lithium battery.
Owner:CELANESE VENURES GMBH
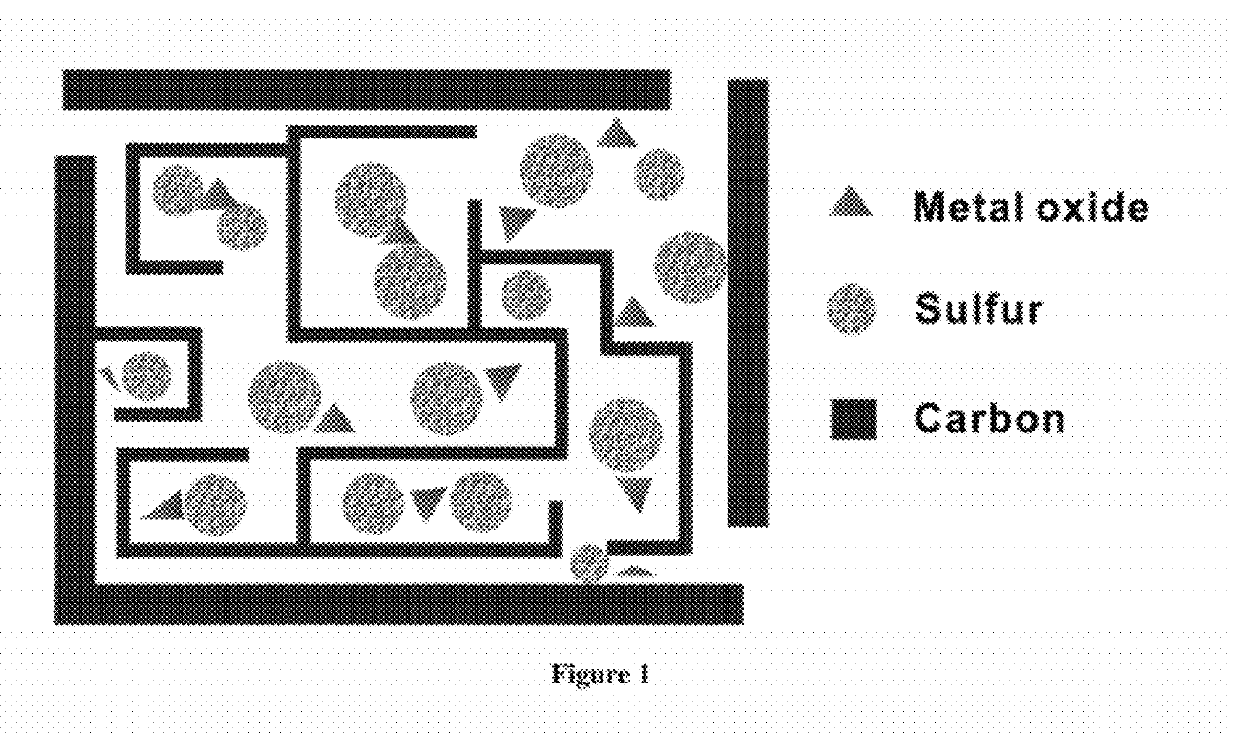
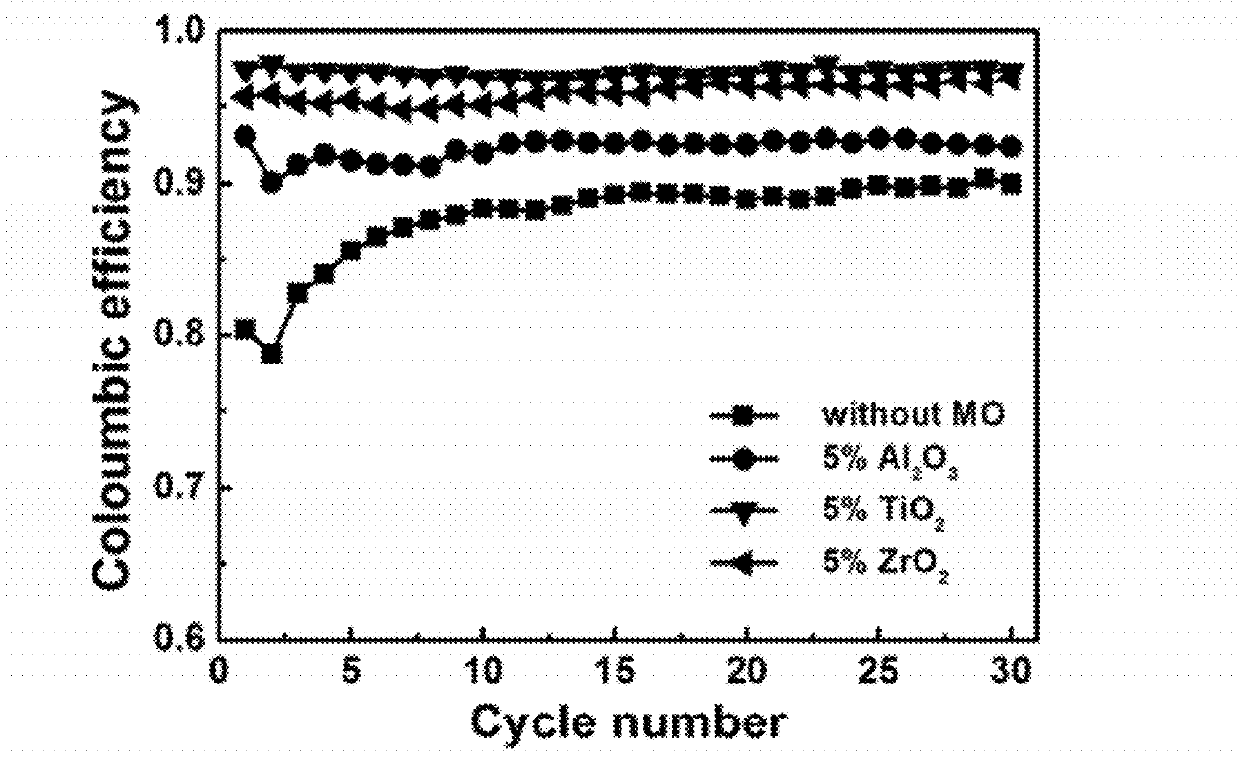
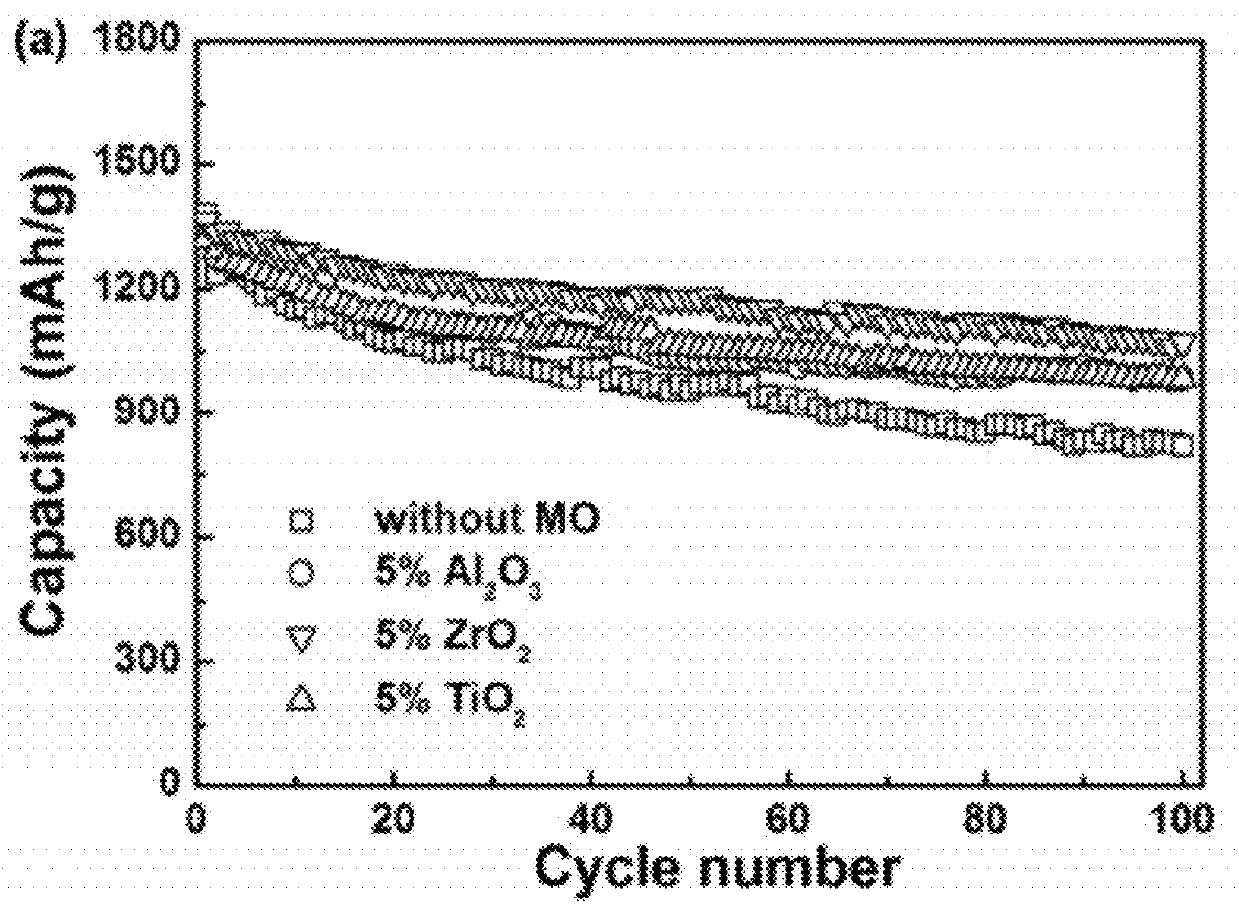
Carbon-metal oxide-sulfur cathodes for high-performance lithium-sulfur batteries
InactiveUS20120207994A1Improve performanceImprove Coulombic efficiencyNon-metal conductorsLayered productsPorous carbonLithium–sulfur battery
Embodiments presented herein provide a new approach for high-performance lithium-sulfur battery by using novel carbon-metal oxide-sulfur composites. The composites may be prepared by encapsulating sulfur particles in bifunctional carbon-supported metal oxide or other porous carbon-metal oxide composites. In this way, the porous carbon-metal oxide composite confines sulfur particles within its tunnels and maintain the electrical contact during cycling. Furthermore, the uniformly embedded metal oxides in the structure strongly adsorb polysulfide intermediates, avoid dissolution loss of sulfur, and ensure high coulombic efficiency as well as a long cycle life.
Owner:PENN STATE RES FOUND
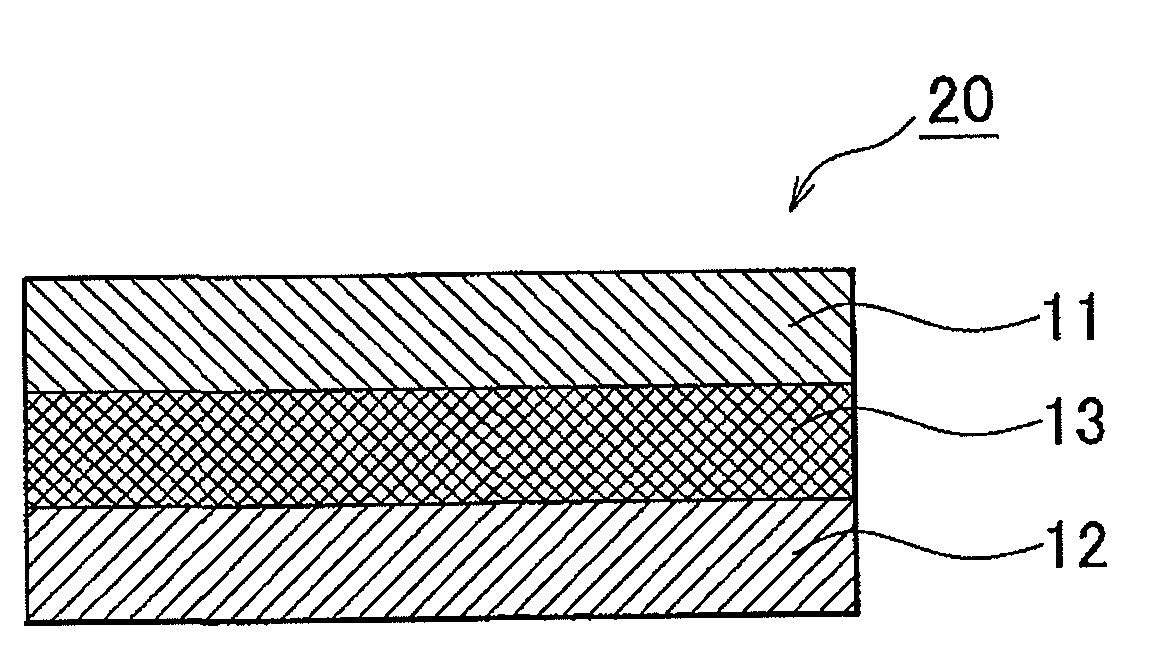
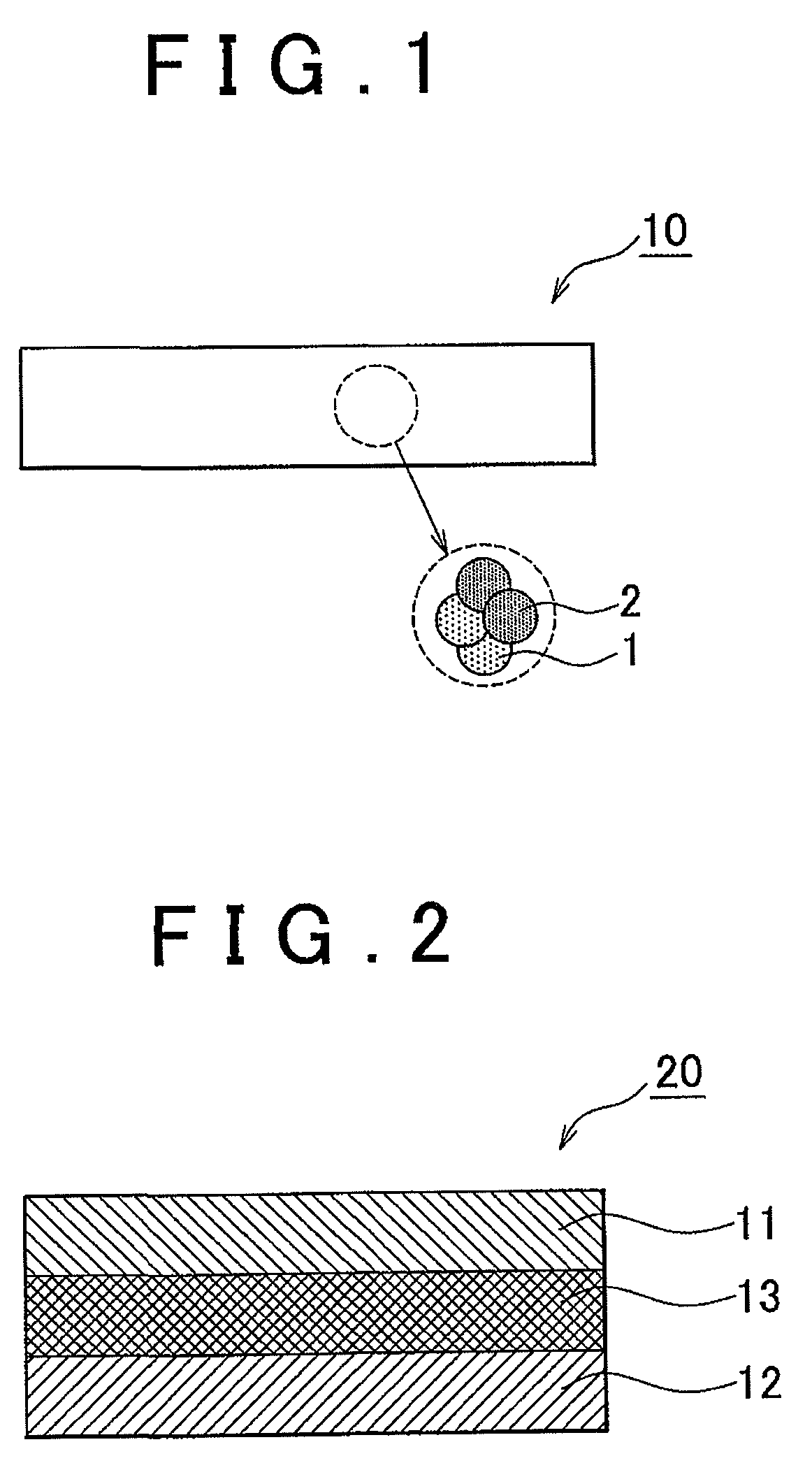
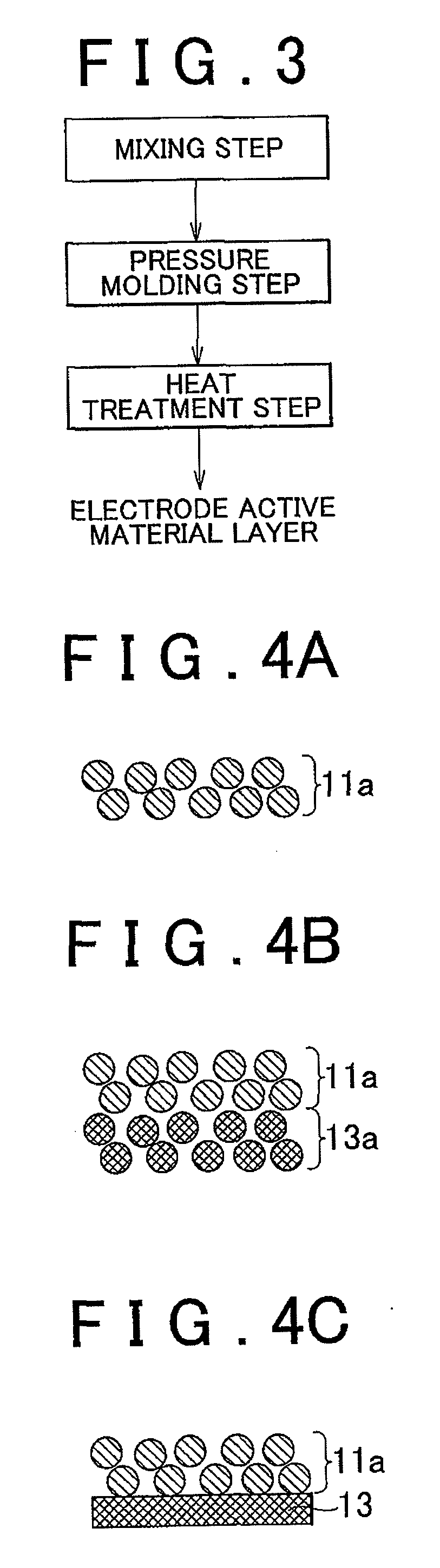
Electrode active material layer, all solid state battery, manufacturing method for electrode active material layer, and manufacturing method for all solid state battery
InactiveUS20110065007A1Reduce interface resistanceSuppress generationSolid electrolytesSolid electrolyte cellsAll solid stateSolid state electrolyte
Owner:TOYOTA JIDOSHA KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com