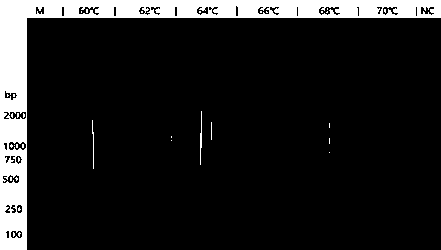Patents
Literature
48 results about "CagA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
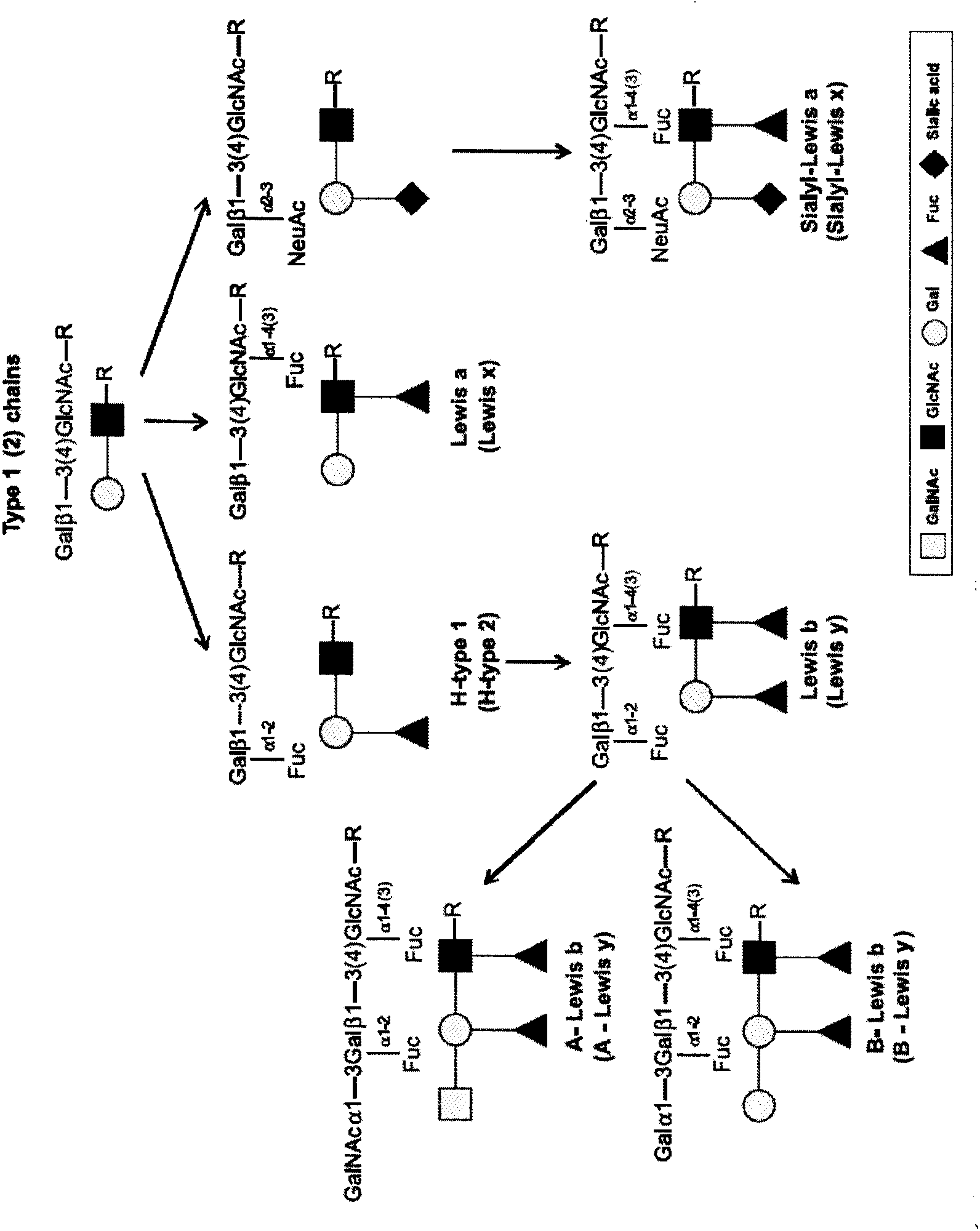
Helicobacter pylori virulence factor CagA (cytotoxin-associated gene A) is a 120–145kDa protein encoded on the 40kb cag pathogenicity island (PAI). H. pylori strains can be divided into CagA positive or negative strains.
Recombinant fusion protein vaccine and attenuated live vector vaccine for treating and preventing helicobacter pylori (Hp) infection
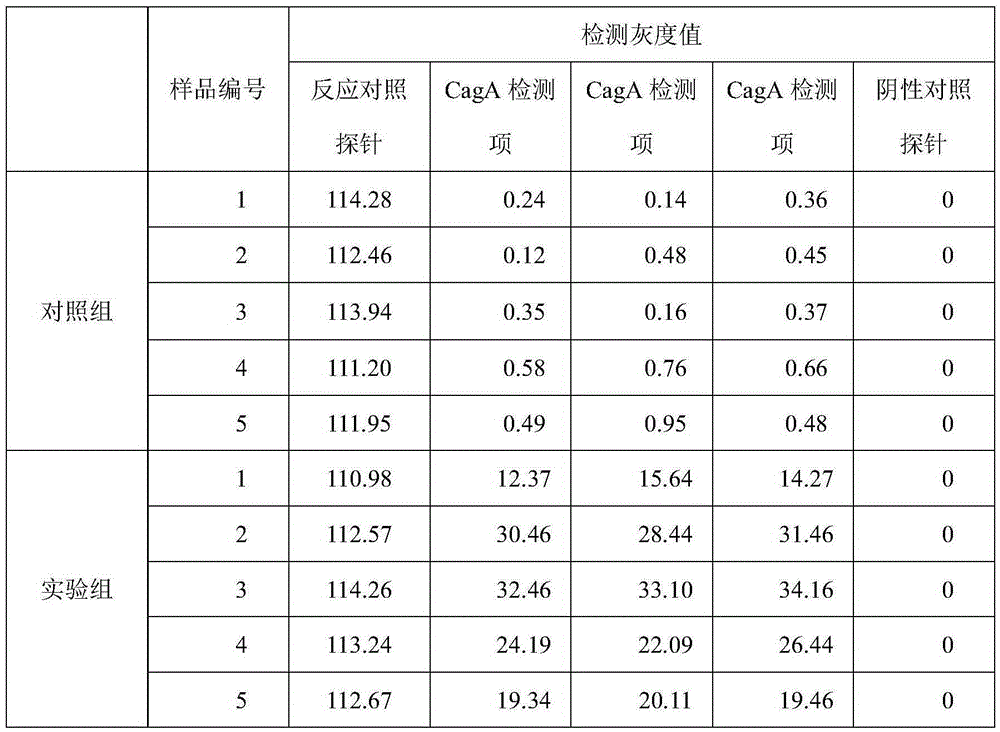
The invention relates to a recombinant fusion protein vaccine and an attenuated live vector vaccine for treating and preventing helicobacter pylori (Hp) infection, and belongs to the field of biopharmaceutics. The recombinant fusion protein vaccine and the attenuated live vector vaccine for expressing the recombinant fusion protein are characterized in that: the recombinant fusion protein is formed by connecting immune protective function fragments of helicobacter pylori cytotoxin relevant gene protein CagA from helicobacter pylori, vacuolization cytotoxin VacA and urease subunit UreB linearly, and the immunogenicity and immune protection of the recombinant fusion protein are verified through animal experiments. The live vaccine has the advantages that: 1, an Hp fusion protein gene can beexpressed stably; 2, mucosa and systemic immune response can be induced after immunity; and 3, the method is convenient, the cost is low, and the economic benefit is obvious. Therefore, the attenuated live vector vaccine can be used as candidate vaccines for treating and preventing the Hp infection.
Owner:ARMY MEDICAL UNIV
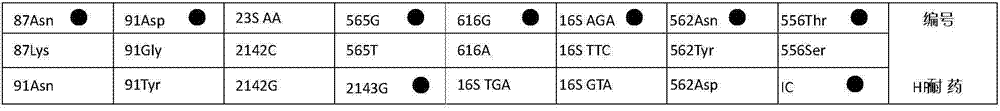
Helicobacter pylori (HP) type and drug-resistant mutation gene detection kit
ActiveCN104846097AQuick evaluationMicrobiological testing/measurementMicroorganism based processesHelicobacter pylori gastritisPcr dgge
The invention relates to a gene detection kit, in particular to a helicobacter pylori (HP) type and drug-resistant mutation gene detection kit. The kit comprises PCR (polymerase chain reaction) solutions and nucleic acid membrane strips for HP type and drug-resistant mutation gene detection; the PCR solutions comprise a PCR solution I, a PCR solution II, a PCR solution III and a PCR solution IV; the PCR solutions also contain a pair of internal control primers respectively. The HP type and drug-resistant mutation gene detection kit can be used for distinguishing two types of HP in one test, and can be used for detecting 15 mutation types of 9 hot spot mutational loci related with drug fastness of 5 therapeutics, a VacA gene and a CagA gene are used for typing, and a reference basis is provided for judgment of illnesses. Mutation types of drug-resistant mutation detection are more and more comprehensive, and the condition of genotypic resistance of HP from which a patient suffers is quickly and comprehensively evaluated.
Owner:杭州千基生物科技有限公司 +2
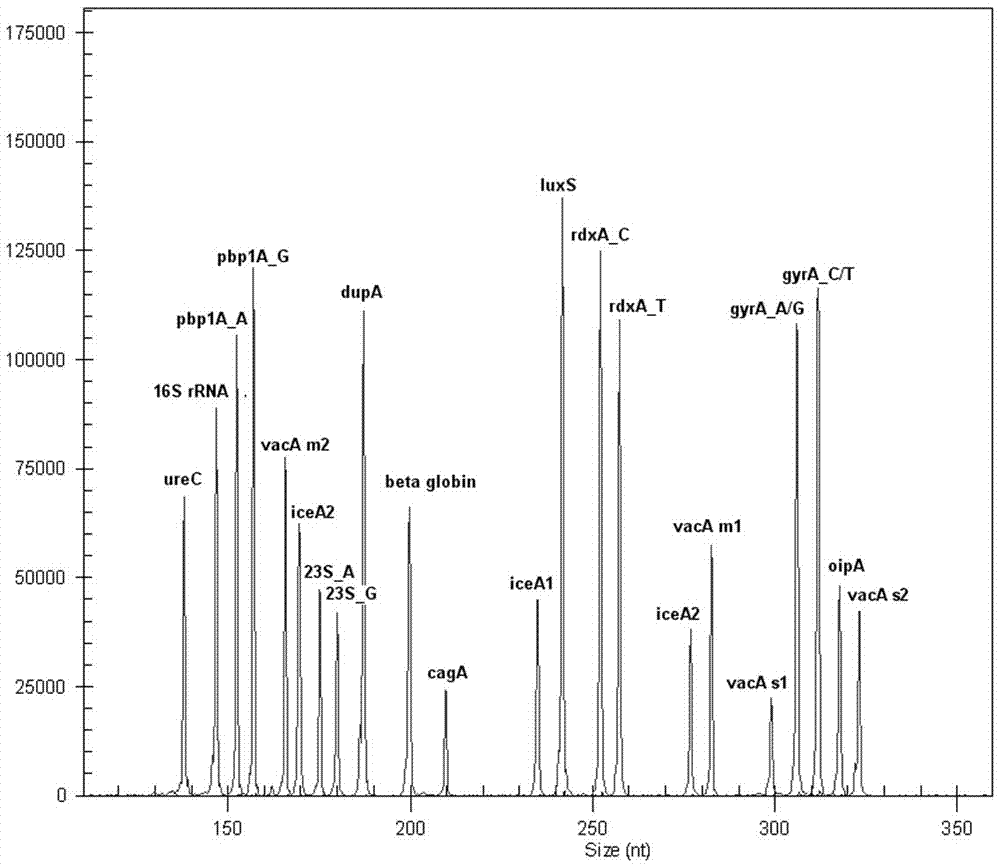
Multiple-gene helicobacter pylori detection system and kit and application thereof
ActiveCN105441583AThe test result has no impurityHigh sensitivityMicrobiological testing/measurementTissue sampleCagA
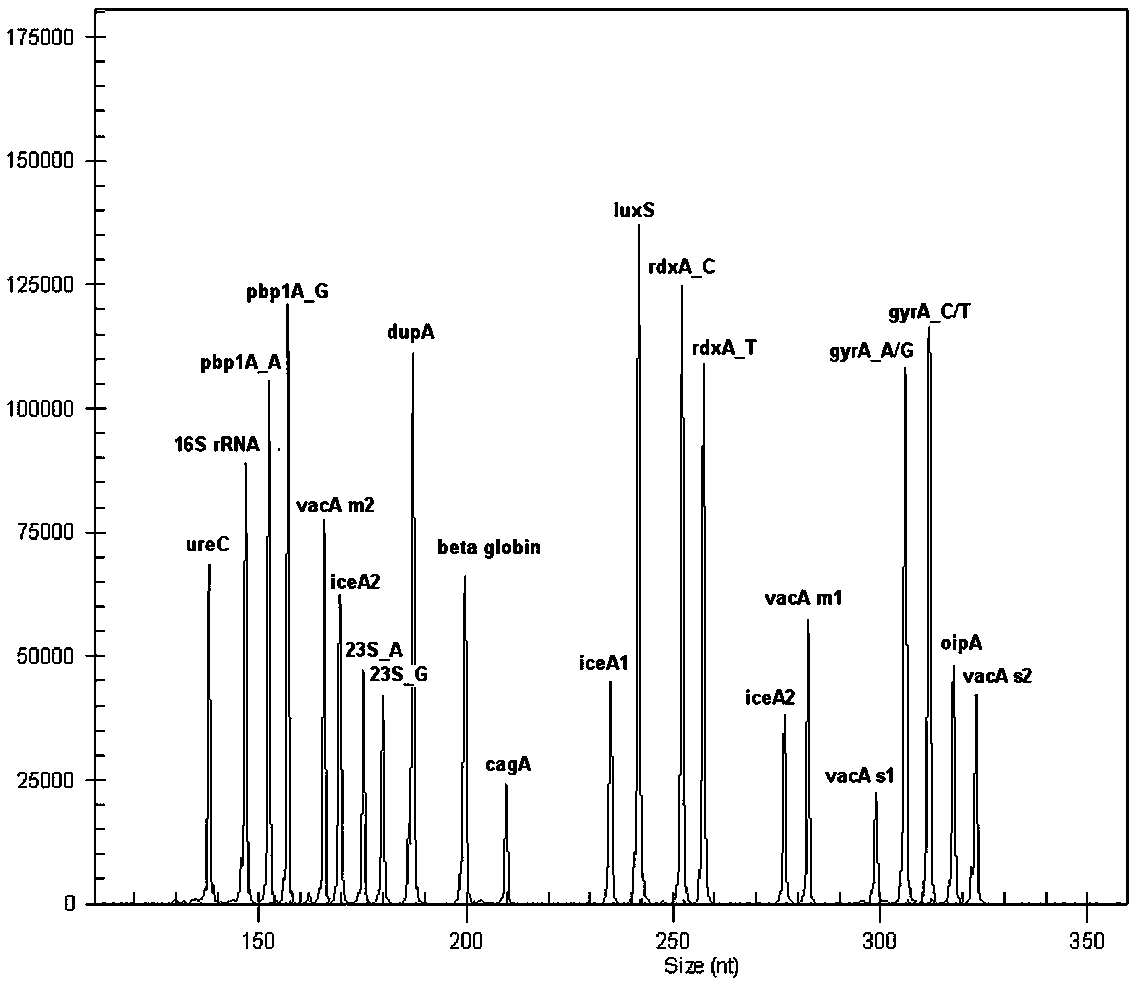
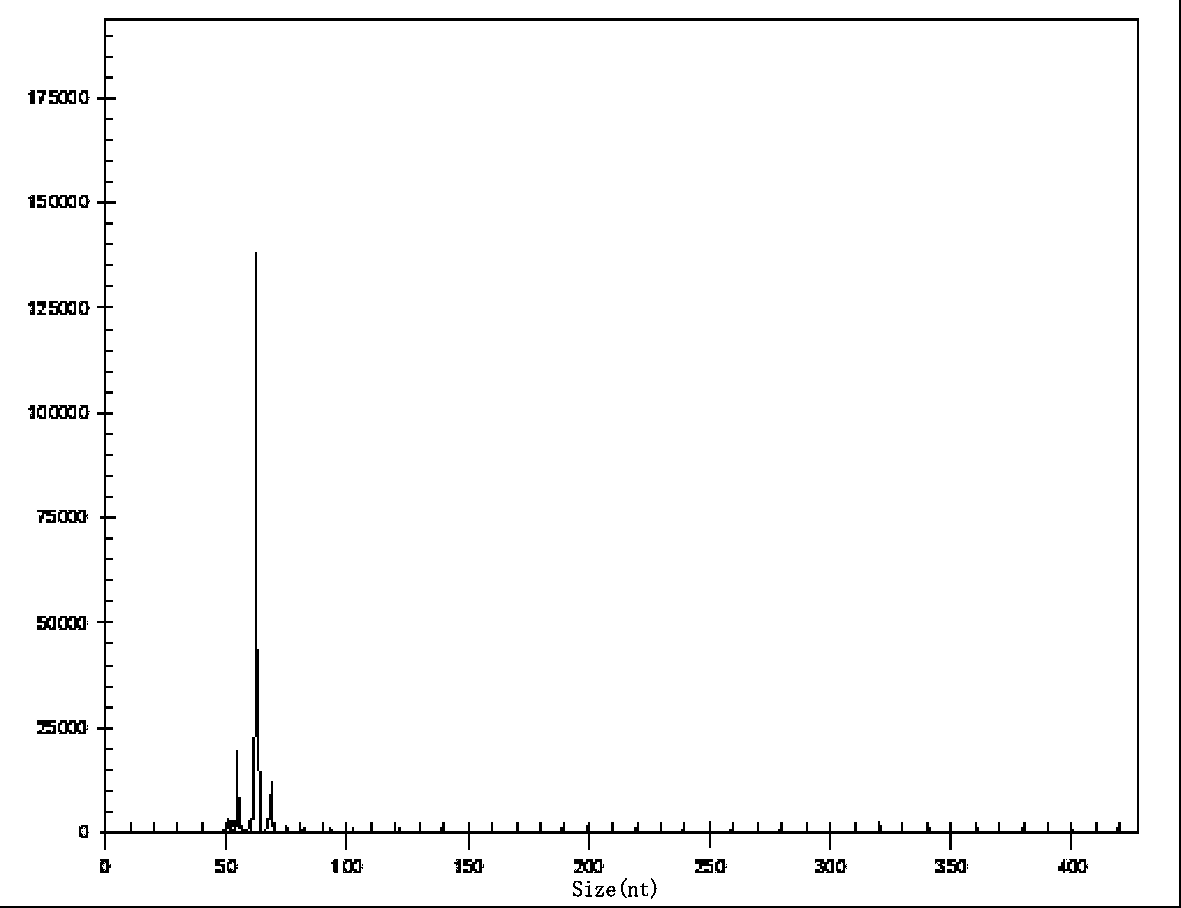
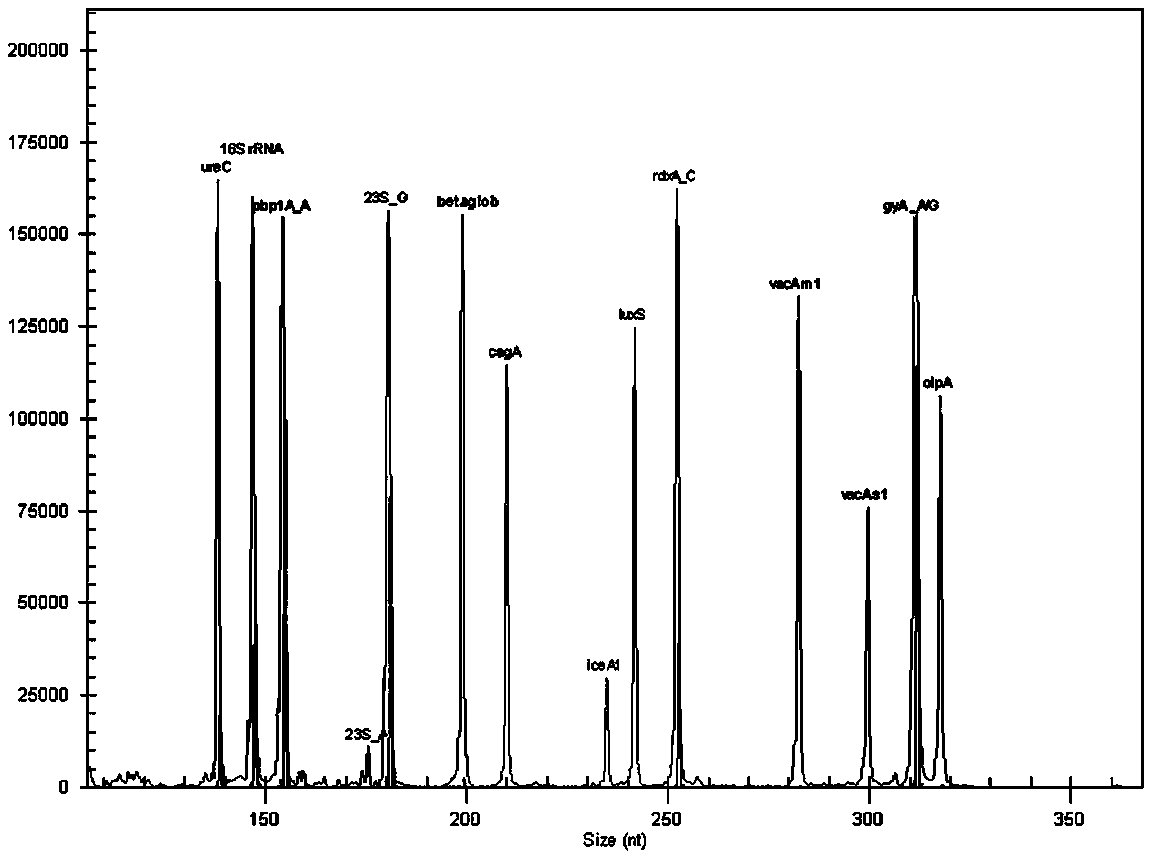
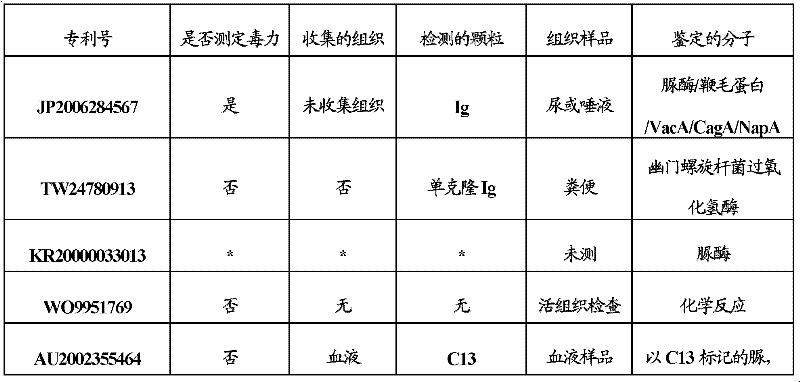
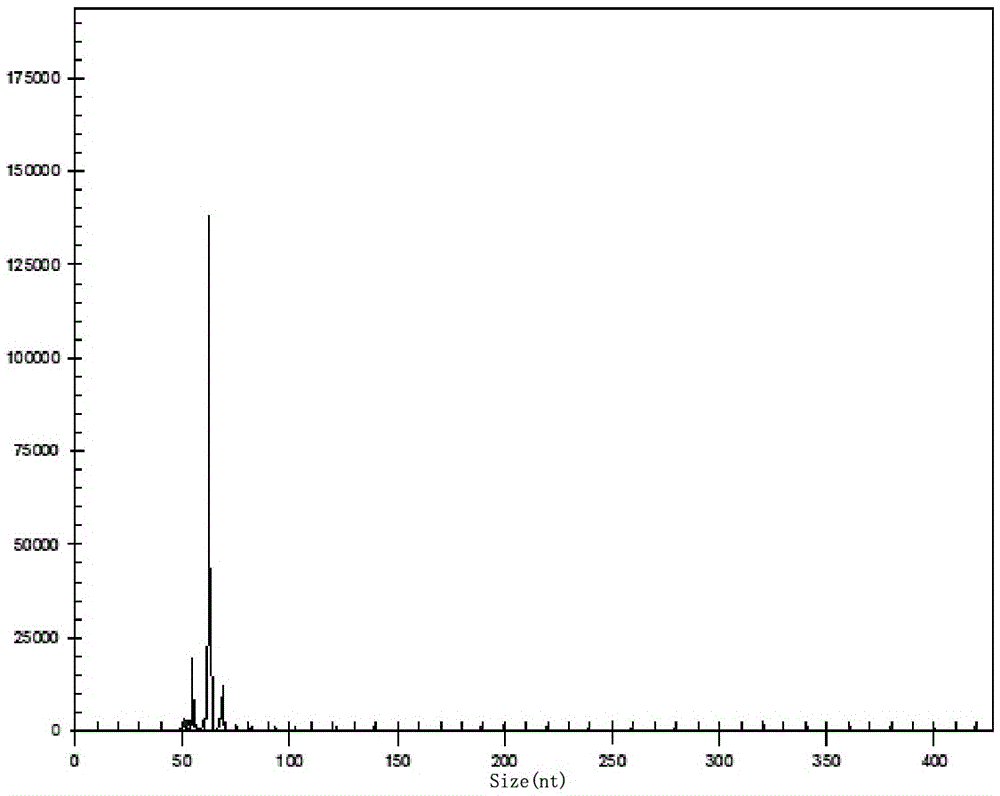
The invention relates to a multiple-gene helicobacter pylori detection system and a kit and application thereof. The helicobacter pylori detection system includes 21 pairs of primers respectively for a strain identification gene (16S rRNA), virulence genes (cagA, vacA-s1, vacA-s2, vacA-m1, vacA-m2, iceA1, iceA2, dupA, oipA and luxS), drug resistance genes (2143 locus of 23S rRNA, 148 locus of rdxA, 1777 locus of pbp1A and polymorphism of the 261 locus of gyrA) and quantitative analysis genes ureC and beta-globin of the helicobacter pylori. The multiple-gene helicobacter pylori detection system and the kit of the system do not need the steps including conventional isolated culture and the like, synchronous detection and analysis on strain identification, quantification, virulence and medicine resistance can be directly conducted on tissue samples in the same reaction system, the shortcomings of low flux, long consumed time, low detection rate and the like of a conventional detection method are overcome, a comprehensive, accurate and low-cost etiological diagnosis is clinically provided for the first time, and important references are provided for individualized diagnosis and accurate treatment of helicobacter pylori infection.
Owner:HUADONG HOSPITAL +1
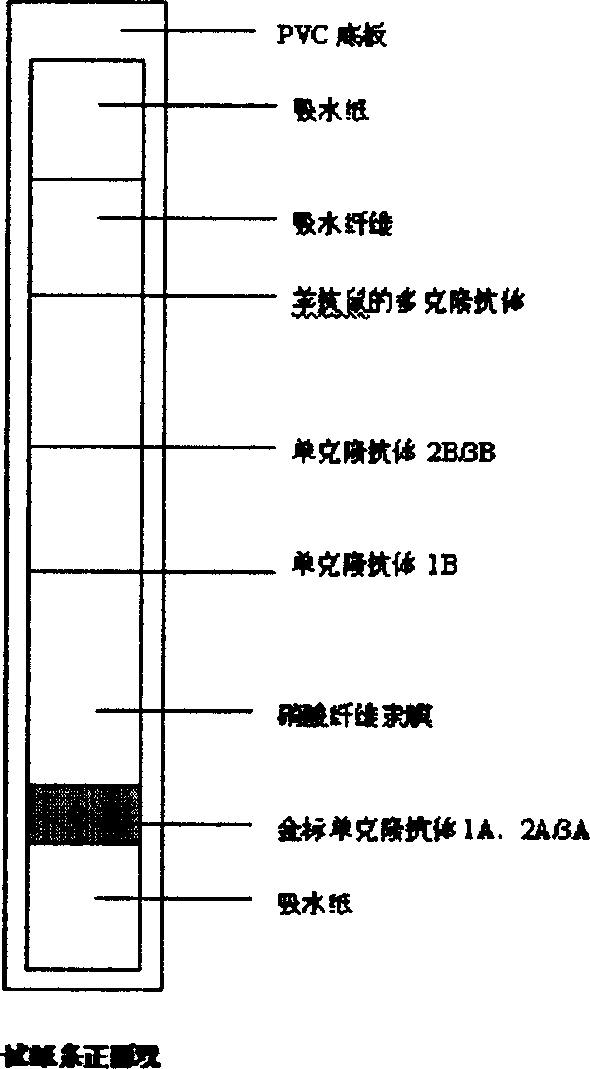
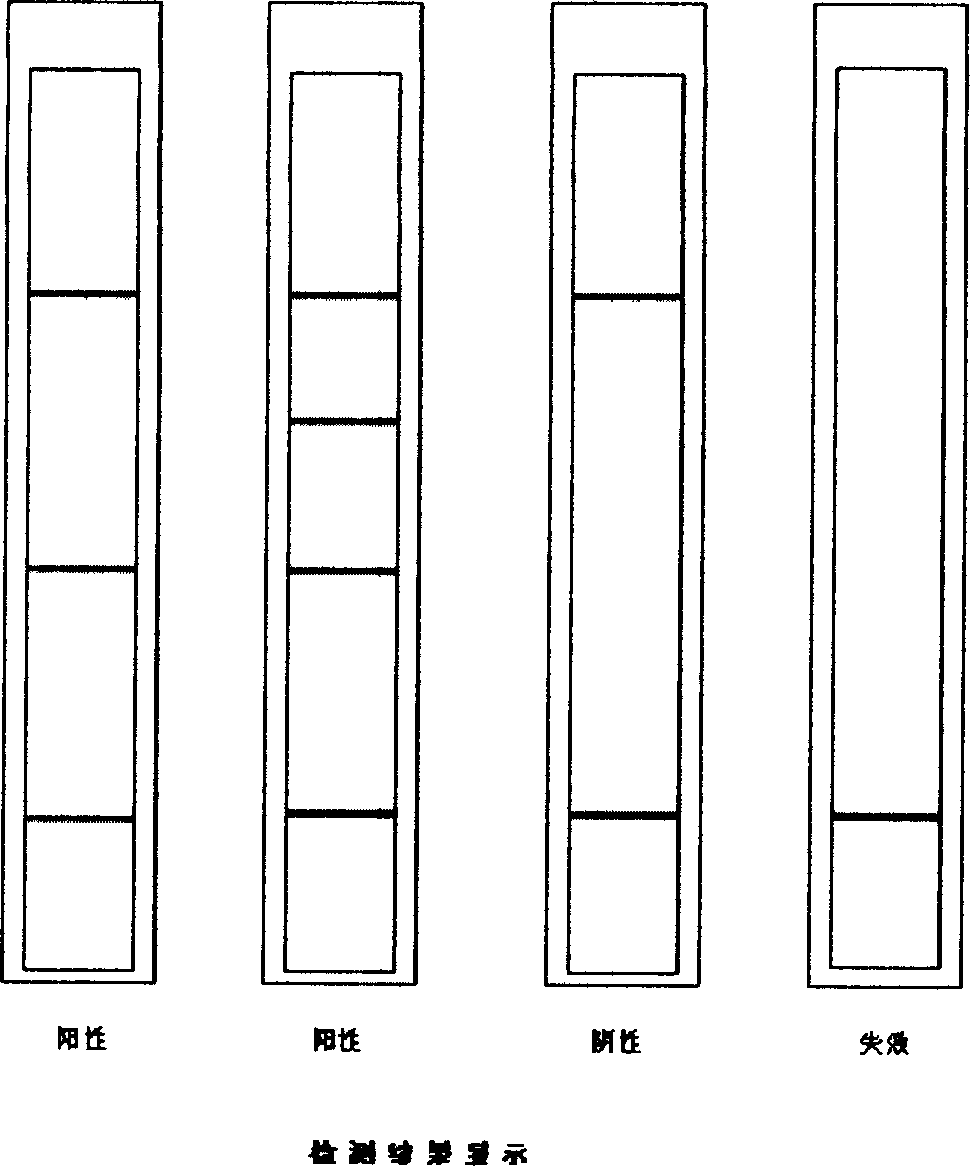
Immuo-colloidal gold test paper for detecting pyloric helicobacter antigen and its prepn process
The present invention relates to one kind of immuno-colloidal gold test paper for detecting pyloric helicobacter antigen and its preparation process. The test paper has coated urease monoclonal antibody, CagA or VacA monoclonal antibody and anti-mouse polyclonal antibody. It is used in detecting pyloric helicobacter related antigen existing in saliva, gastric juice, vomited matter, dental plaque and excrement of mammal so as to monitor the infection status of pyloric helicobacter, assist the diagnosis of gastritis, peptic ulcer and other diseases and predict gastric cancer probability. The present invention simple operation, high specificity and high sensitivity.
Owner:LANZHOU UNIVERSITY
Detection kit for helicobacter pylori virulence protein antibody and detection method by using the same
InactiveCN102721815AStrong specificityDifferentiate between strong and weak virulenceBiological testingAntigenPositive control
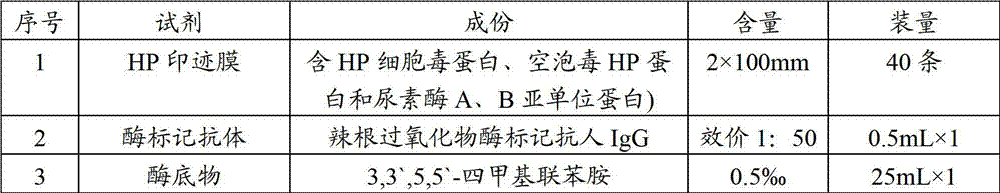
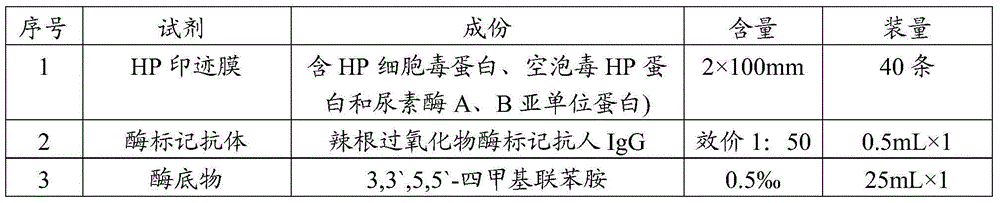
The invention relates to a detection kit for helicobacter pylori virulence protein antibody and a detection method by using the same. The kit comprises a microwell plate, an enzyme-labeled secondary antibody, a negative control serum, a positive control serum, a substrate, a stop buffer and a washing liquor, wherein the microwell plate is coated with purified recombinant helicobacter pylori virulence proteins, the substrate is a solution containing 1% m / m 3, 3', 5, 5'-tetramethyl benzidine and 30% v / v hydrogen peroxide, and the recombinant helicobacter pylori virulence proteins are cytotoxin-associated protein CagA, vacuolating toxin protein VacA, flagellum protein subunit A, flagellum protein subunit B, urease subunit A, and urease subunit B. According to the invention, six purified helicobacter pylori virulence proteins are used as antigens which itself have high conservatism and specificity and are related to the symptom of a patient, so the kit has characteristics of high specificity, identification capacity for helicobacter pylori clinical strain virulence strength, and semi-quantitative detection capacity.
Owner:TIANJIN TIANFOLUO BIOLOGICAL TECH
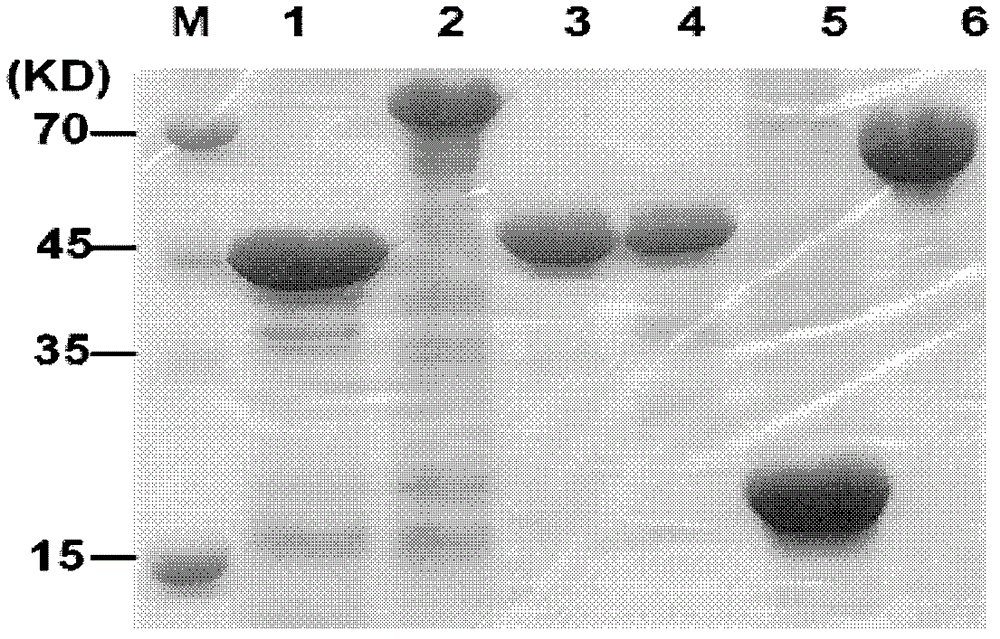
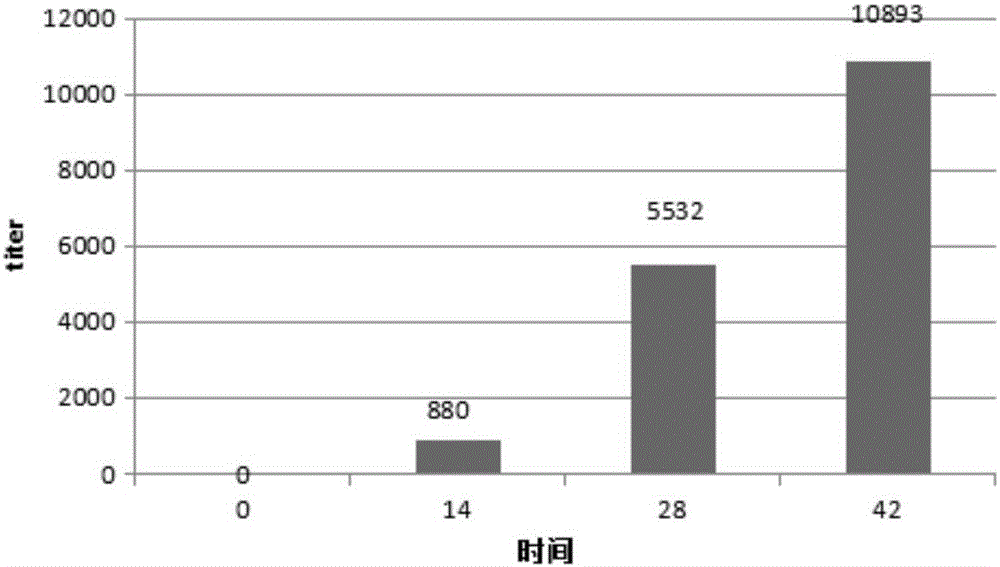
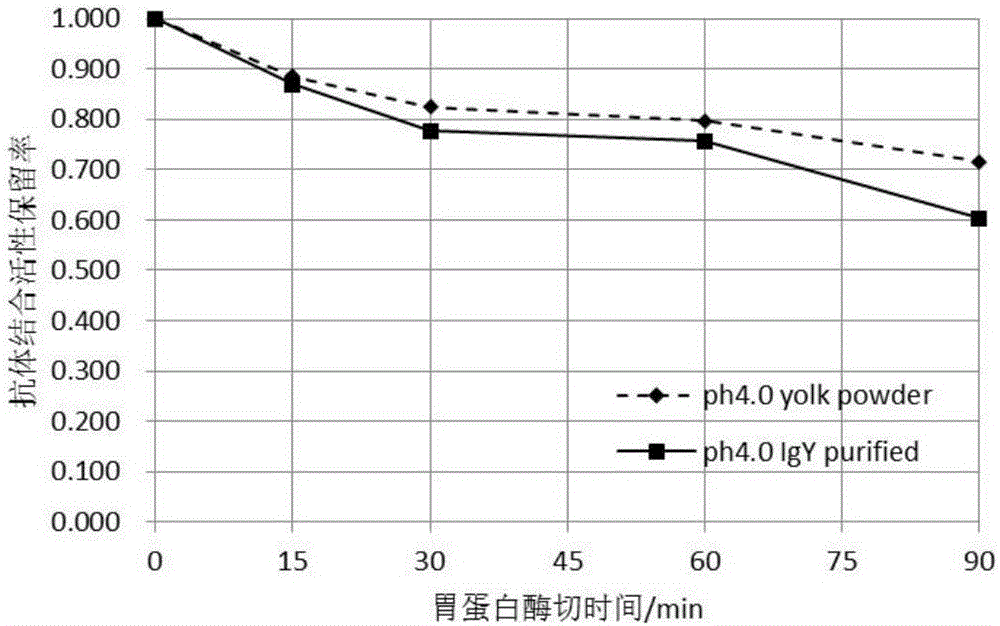
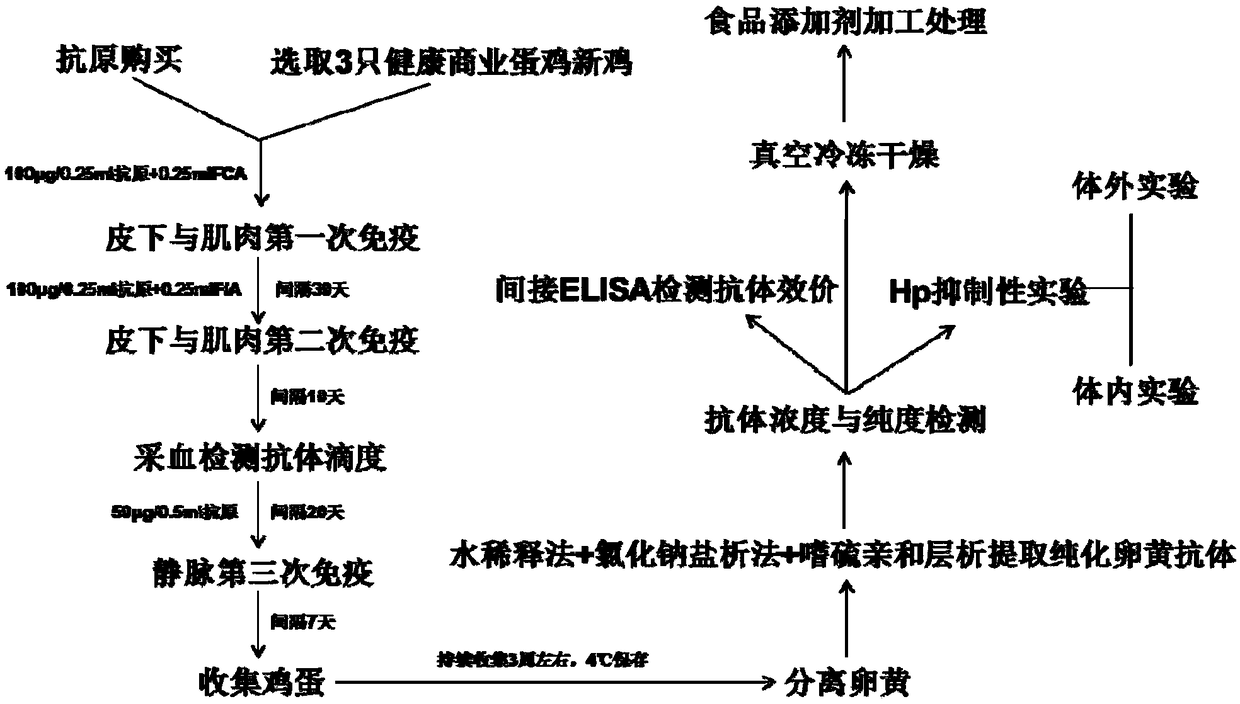
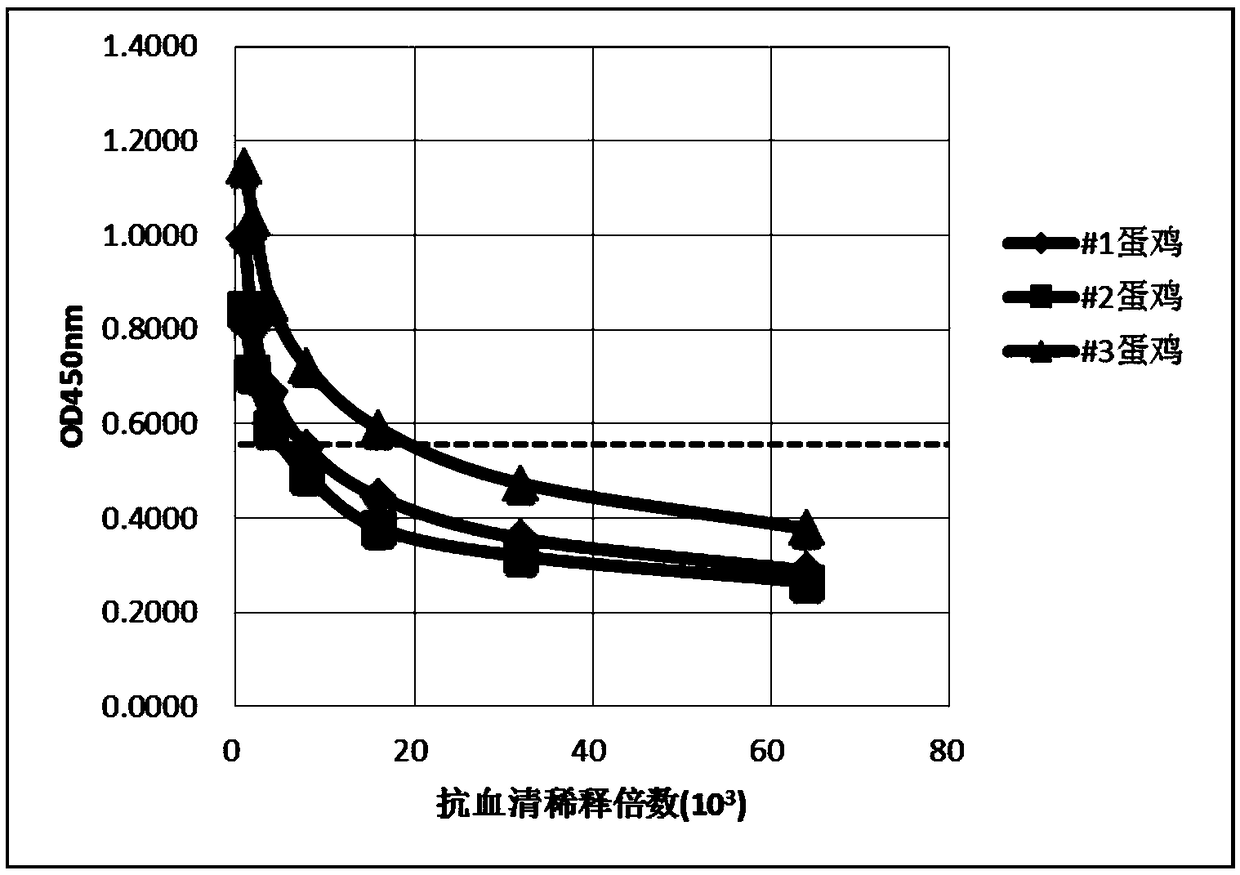
Preparation method of egg yolk antibody containing helicobacter pylori resisting IgY and application
The invention discloses a preparation method of an egg yolk antibody containing helicobacter pylori resisting IgY and an application of the egg yolk antibody. By the aid of a virulent helicobacter pylori immune laying hen with positive CagA genes, the egg yolk antibody is extracted from eggs laid by the laying hen and / or egg yolk powder is prepared. The egg yolk powder with the egg yolk antibody is added into food or prepared into health care food and can be used for preventing related diseases caused by infection of helicobacter pylori in an assisting manner, and the antibody can be prepared into medicines after being purified and is used for preventing the related diseases caused by infection of the helicobacter pylori.
Owner:徐州安替生物科技有限公司
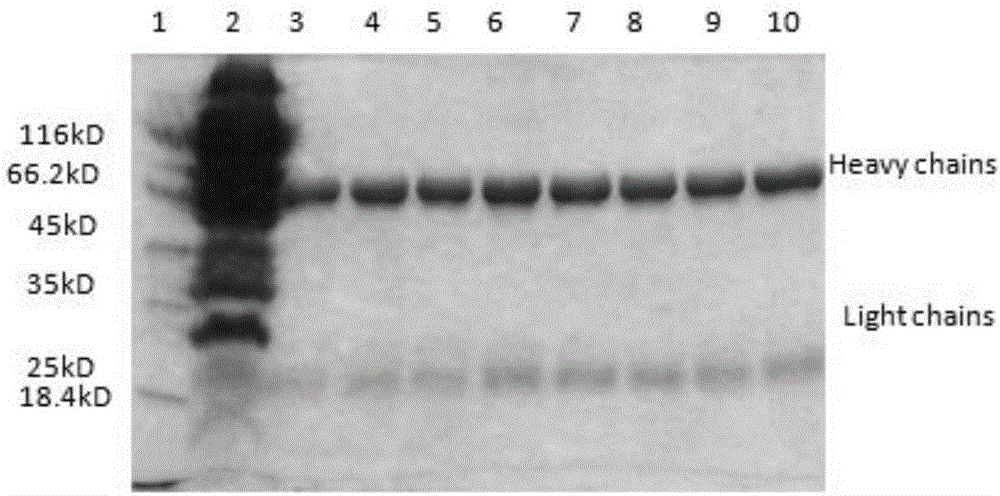
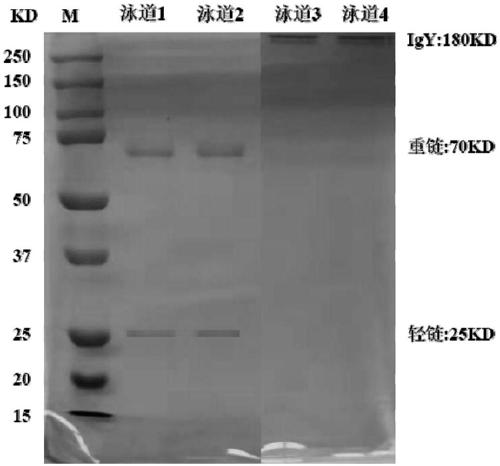
Anti-helicobacter pylori egg yolk antibody as well as preparation method and application thereof
InactiveCN109503710AHigh purityStrong neutralization of Helicobacter pylori activityAntibacterial agentsEgg immunoglobulinsYolkAnimal science
The invention discloses an anti-helicobacter pylori egg yolk antibody, which is prepared by immunizing an egg laying hen by immunizing an egg laying hen by injecting a mixture rich in three proteins,i.e., Ure, VacA and CagA separated from Hp lysate, wherein the mixture is used as an immunogen, and then extracting the purified antibody from egg yolk of the immunized hen by using a water dilution method, a sodium chloride salting-out method and a sulfurophilic affinity chromatography method. The egg yolk antibody prepared by the method is high in purity, has a stronger activity of neutralizinghelicobacter pylori, and can be applied to preparation of medicines for preventing and treating gastrointestinal diseases caused by infection of the helicobacter pylori.
Owner:ZHEJIANG BLUE SHIELD PHARM CO LTD
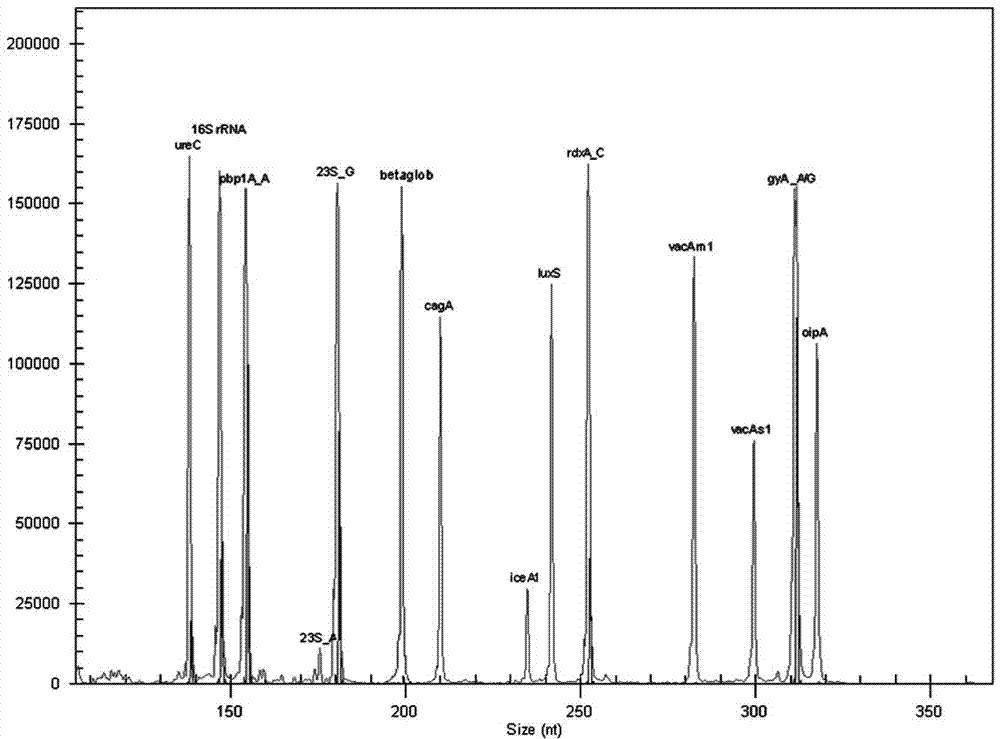
System for detecting multiple quantitative and virulent genes of H.pylori as well as kits and applications of system
ActiveCN105506160AThe test result has no impurityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBeta globinVirulent characteristics
The invention relates to a system for detecting multiple quantitative and virulent genes of H.pylori as well as kits and applications of the system. The system for detecting multiple quantitative and virulent genes of H.pylori comprises multiple pairs of primers, each for strain identification genes (16S rRNA), quantitative analysis genes ureCand Beta-globin, as well as virulent genes (cagA, vacA-s1, vacA-s2, vacA-m1, vacA-m2, iceA1, iceA2, dupA, oipA and luxS). The system for detecting multiple quantitative and virulent genes of H.pylori and the kits of the system can directly perform synchronous detection and analysis on the strain identification, quantification and virulence of a tissue sample in a same reaction system without adopting a conventional culture step or other steps, remedy defects that a conventional detection method is low in throughput, time-consuming and low in detection rate, obviously improve the accuracy of detection results, immediately provide a comprehensive, accurate and low-cost etiological diagnosis for clinic, and provide an important reference for the accurate diagnosis, differential diagnosis and disease prognosis of H.pylori infection.
Owner:HUADONG HOSPITAL +1
Helicobacter pylori serotype classification method and helicobacter pylori biochip construction method
InactiveCN104357552AQuick checkAccurate detectionMicrobiological testing/measurementBiological material analysisVirulent characteristicsClassification methods
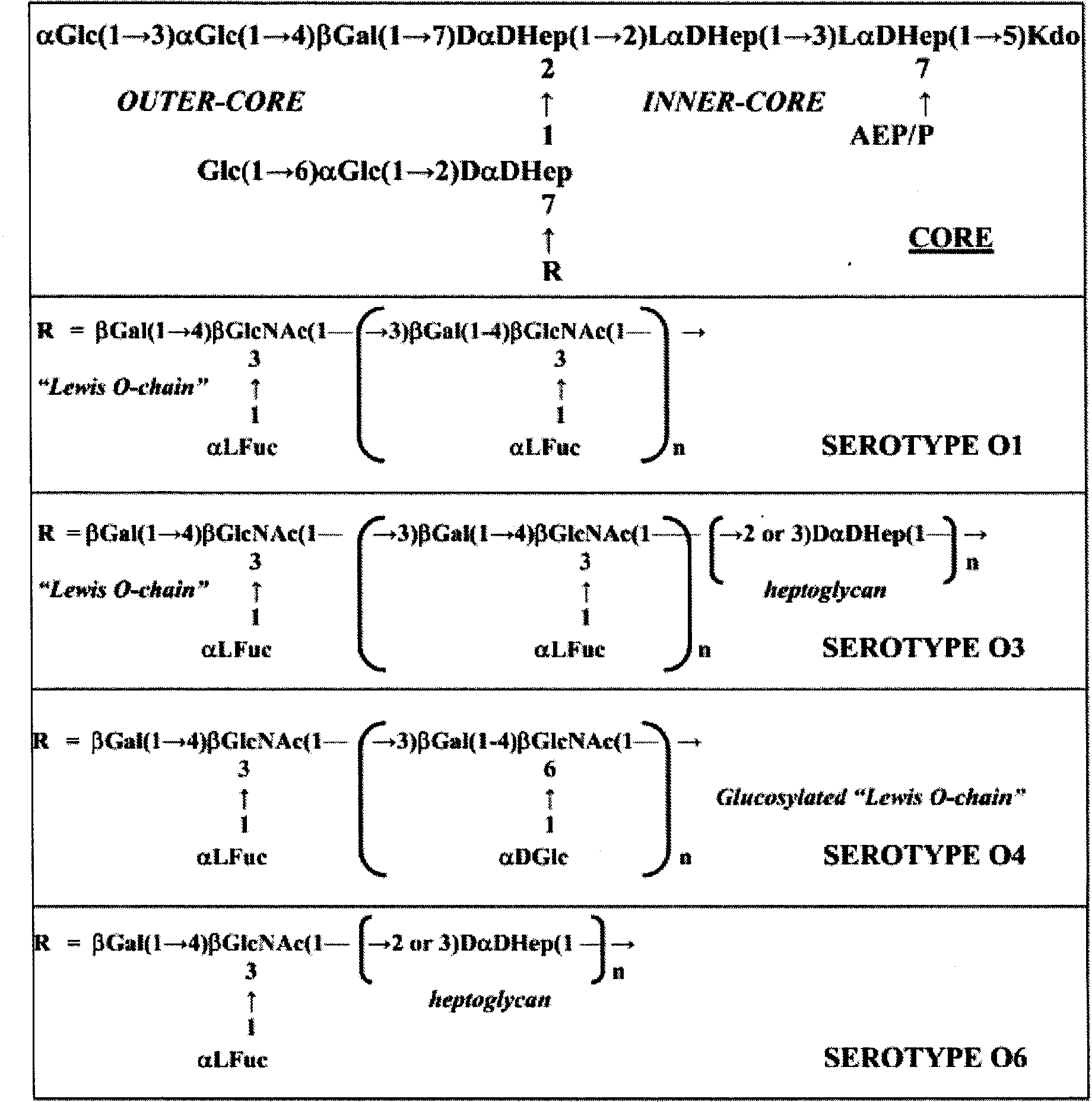
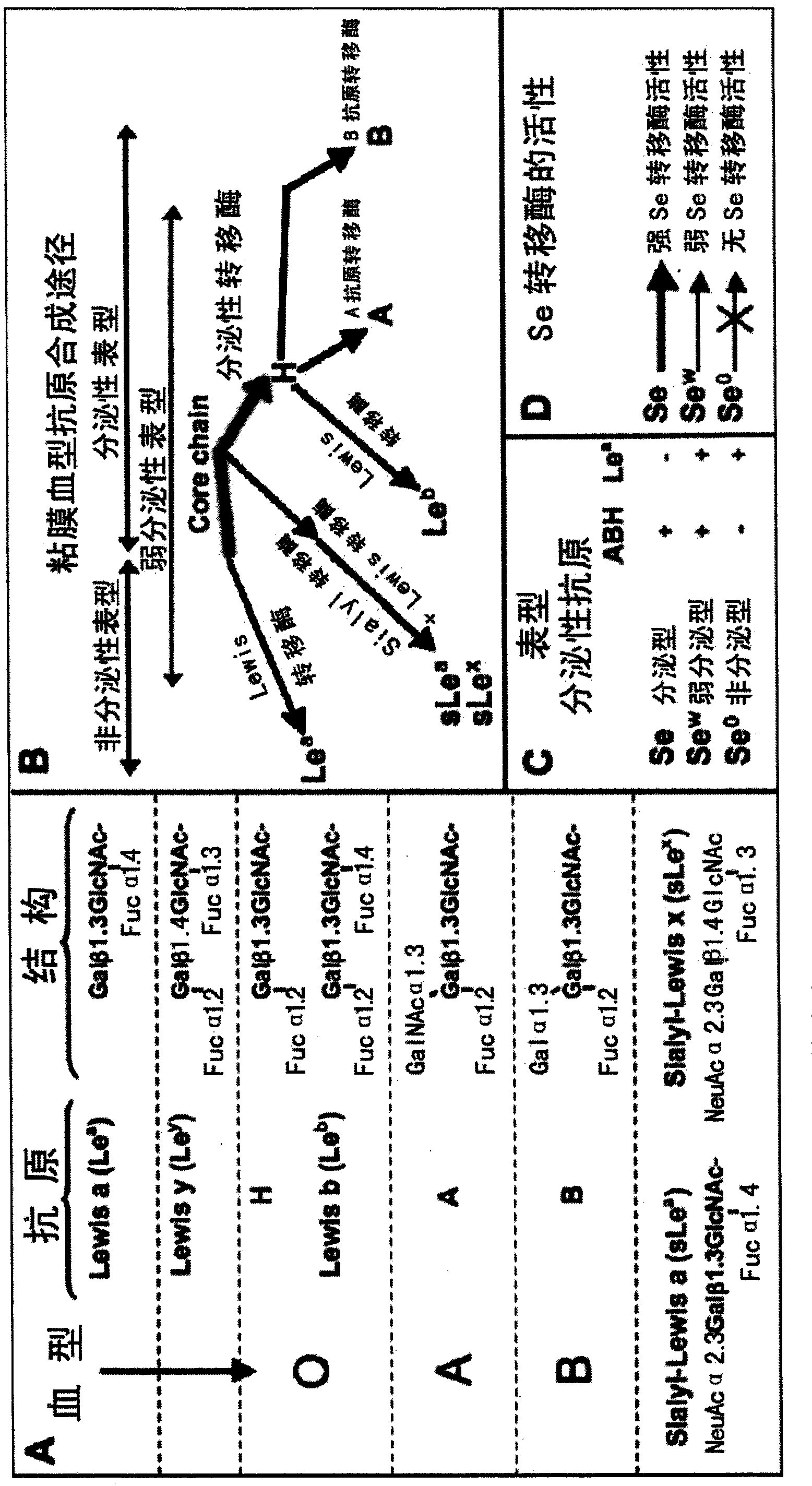
The invention discloses a helicobacter pylori serotype classification method and a helicobacter pylori biochip construction method. According to the methods, helicobacter pylori is extracted from a patient with helicobacter pylori; the extracted helicobacter pylori has PCR (polymerase chain reaction) of cagA and vacA and is sequenced; after sequencing for helicobacter pylori virulence genes cagA and vacA is finished, a regional virulence epidemic strain is screened out based on gene combination and is named; lipopolysaccharide and flagellum of the helicobacter pylori virulence epidemic strain are extracted and subjected to structural analysis, and serotype naming is performed according to virulence genes, the structure of the flagellum, the Lewis blood type structure of lipopolysaccharide and repeated oligosaccharide structure; and lipopolysaccharide and flagellum of the named strain are extracted to construct the biochip. The helicobacter pylori serotype classification method and the helicobacter pylori biochip construction method can greatly simplify detection means, has the characteristics of high accuracy, high throughput and high repeatability, and can meet requirements of clinical diagnosis, specific treatment and epidemiological investigation for helicobacter pylori.
Owner:FOURTH HOSPITAL OF HEBEI MEDICAL UNIV +2
Method for using lactoferrin for treatment or prevention of helicobacter pylori infection
The present invention provides a method for using lactoferrin for treatment or prevention of helicobacter pylori infection, and in particular, the present invention provides application of the lactoferrin in preparation of a composition for inhibiting helicobacter pylori and a composition for preventing or treating helicobacter-pylori-associated diseases. Tests show that recombinant lactoferrin can effectively inhibit the helicobacter pylori, and has a significant inhibiting effect on helicobacter pylori (Hp) virulence factor cagA, vacA and ure gene expression. In addition, when a lactoferrin preparation is compatibly used for a patient, the efficacy of conventional triple or quadruple therapy can be strengthened, so that the lactoferrin has a broad business prospect.
Owner:SHANGHAI GENON BIOENG +1
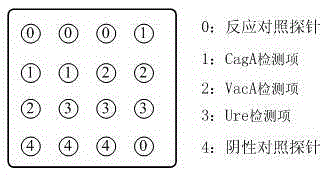
Detection reagent card for typing test of helicobacter pylori and preparation method of detection reagent card
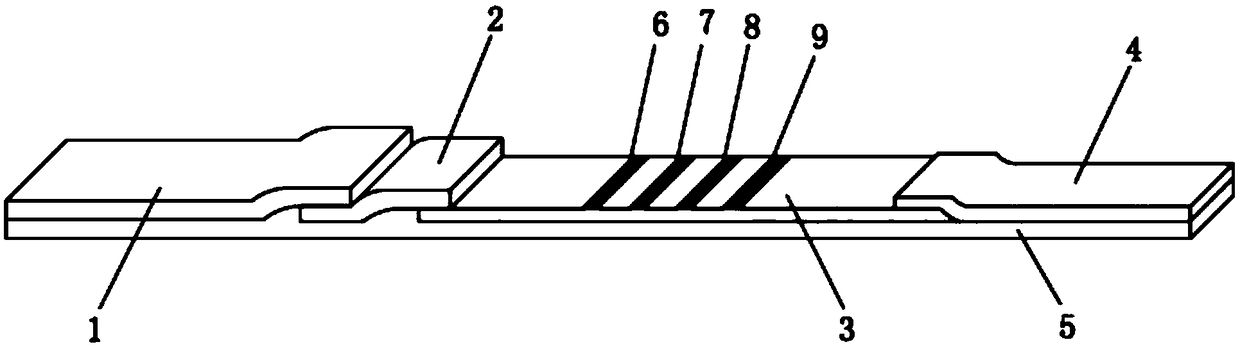
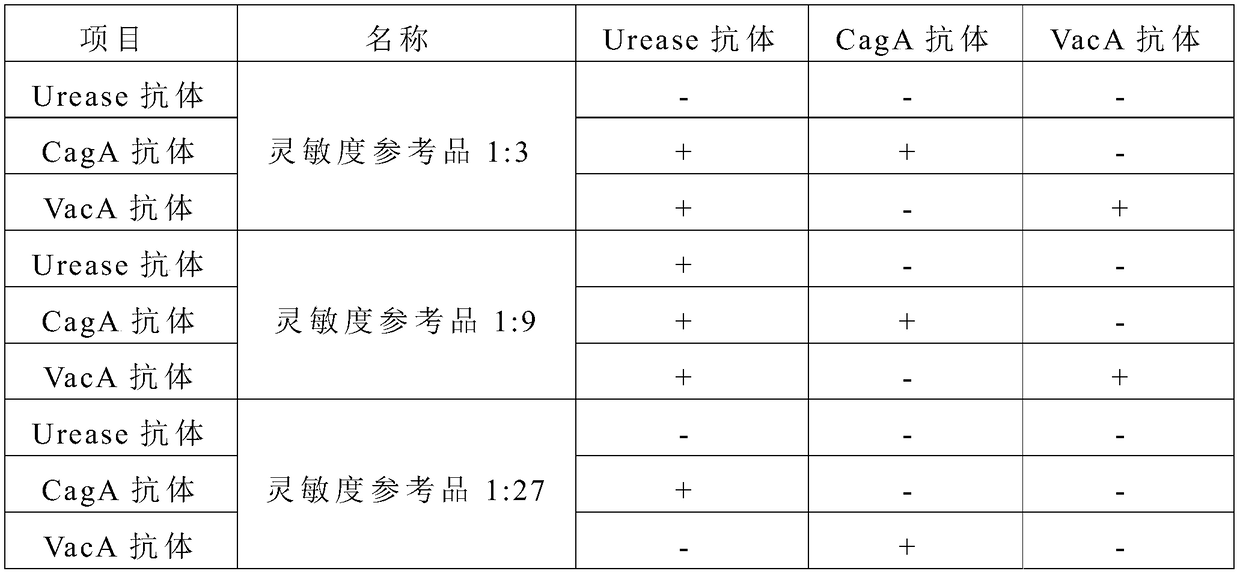
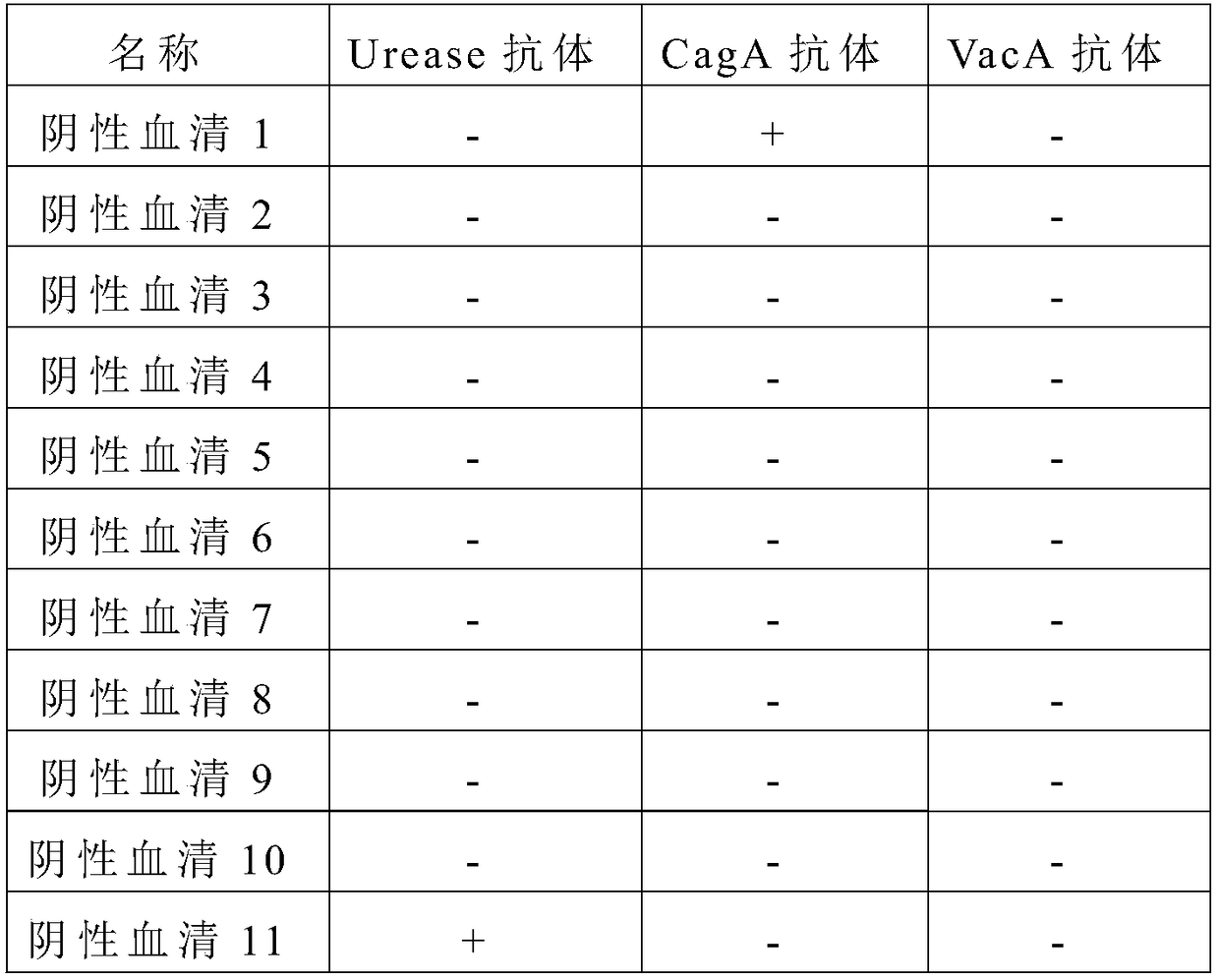
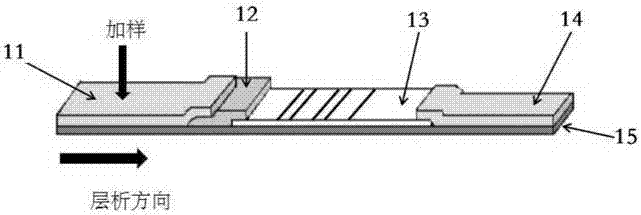
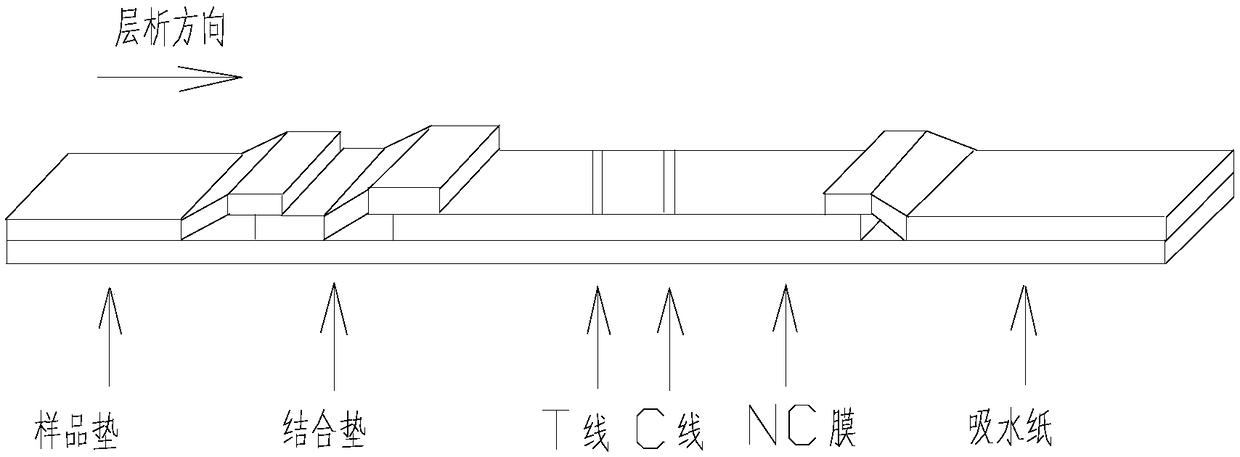
The invention relates to a detection reagent card for typing test of helicobacter pylori. The detection reagent card comprises a sample pad, a quantum pad, a nitrocellulose membrane, absorbent paper and a lining plate, wherein a quantum dot microsphere labeled antigen complex is coated on the quantum pad; the antigen complex comprises a Urease antigen, a CagA antigen, a VacA antigen and rate IgG;a first detection line, a second detection line, a third detection line and a quality control line are arranged on the nitrocellulose membrane; the VacA antigen is coated on the first detection line;the CagA antigen is coated on the second detection line; the Urease antigen is coated on the third detection line; and the goat anti-rat IgG is coated on the quality control line. The detection reagent card for typing test of helicobacter pylori provided by the invention is capable of rapidly and accurately obtaining the results, the infection type of the helicobacter pylori can be judged according to the detection result, and auxiliary diagnosis is further provided for clinical related diseases.
Owner:重庆新赛亚生物科技有限公司
Test strip and kit for helicobacter pylori colloidal gold typing detection
The invention provides a colloidal gold typing detection test strip for Helicobacter pylori. The test strip includes a support plate, and a sample pad, a gold standard pad, and nitrocellulose are arranged in sequence from the sample loading end on the surface of the support plate. These four parts are the plain film and the absorbent pad. The gold standard pad contains colloidal gold-labeled anti-human IgG Fc segment monoclonal antibody; Four detection lines and quality control lines for foamy toxin (VacA) antigen, HP urease subunit A protein antigen and HP urease subunit B protein antigen, and the above four detection lines and quality control lines do not overlap with each other. The test strip of the present invention belongs to the non-invasive detection technology, can detect 4 kinds of Helicobacter pylori antibodies of urease A, urease B, Cag / A and Vac / A at the same time, adopts the internationally recognized standard Helicobacter pylori strain and quality Control serum to ensure the reliability of detection. The operating method of the test strip is simple, fast and popular, and has application prospects and potential social value.
Owner:蔡长春 +2
Protein chip for typing detection on helicobacter pylori infection
InactiveCN105277722AReduce dosageBeneficial to clinical treatmentBiological material analysisBiological testingSpecific iggProtein C
The invention provides a protein chip for typing detection on helicobacter pylori infection. The protein chip comprises a basement membrane and antigens which are respectively dotted on the basement membrane in the form of dot matrix, wherein the antigens include three types, i.e. CagA (cytotoxin-associated gene) antigens, VacA (Vacuolating cytotoxin A) antigens and Ure (ureaplasma urealyticum) antigens. Due to the fact that the detection antigens used by the protein chip are three single proteins, i.e. Ure, CagA and VacA, specific IgG antibodies for the three antigens in the blood serum of a patient can be detected at the same time, I-type infection or II-type infection can be judged, and clinic treatment is better facilitated; furthermore, the protein chip has the advantages of high detection sensitivity, quick, simple and convenient detection, instant readability, small using amount of antigen and the like.
Owner:TAIZHOU SYNO GENE DIGITAL TECH CO LTD
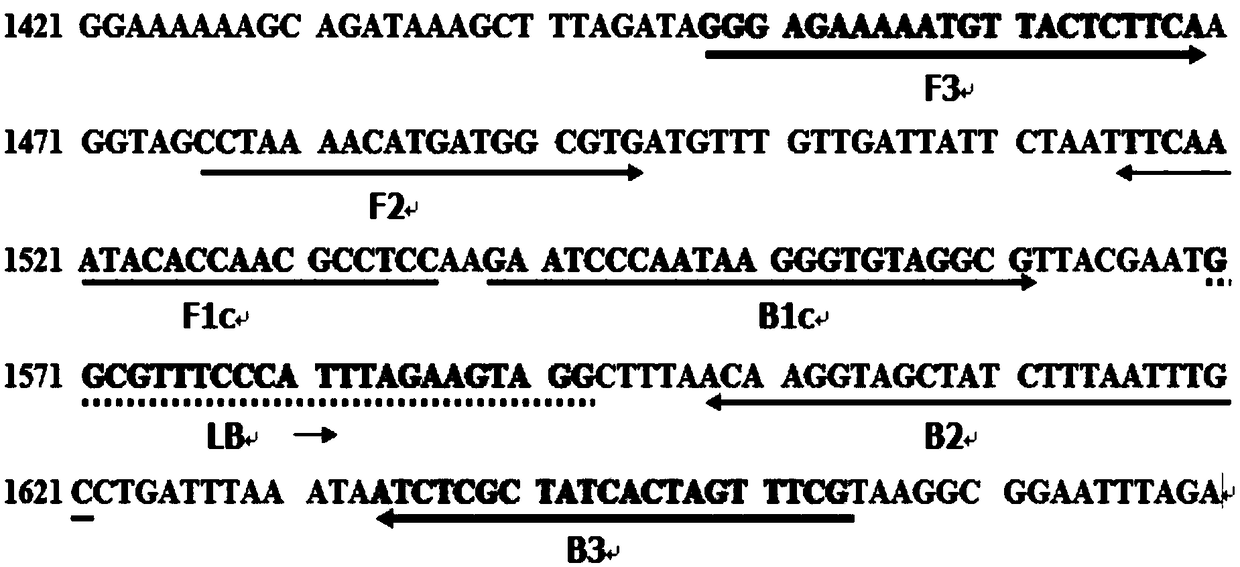
A loop-mediated isothermal amplification detection method and a kit of a high-virulence strain of Helicobacter pylori
PendingCN109182569AAvoid indiscriminate killingIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesVirulent characteristicsHelicobacter pylori
The invention provides a loop-mediated isothermal amplification detection method and a kit of a high-virulence strain of Helicobacter pylori. Five LAMP primers were designed for the nucleic acid sequence of Helicobacter pylori CagA gene, including external primers F3, B3; The internal primers FIP, BIP and loop primer LB ensured the high specificity of LAMP amplification. This technique is specificfor the detection of high virulence Helicobacter pylori strains, and has the characteristics of high speed, high efficiency, simple instrument requirements, simple operation, low cost, and easy to bepopularized and used in the grass roots. At the same time, the LAMP detection prim of the high-virulence strain of helicobacter pylori is designed and screen for the first time, and a LAMP detectionmethod of the high-virulence strain of helicobacter pylori is established, which fills a gap in the detection field at home and abroad.
Owner:JINAN CENTER HOSPITAL
Fluorescent immunochromatographic reagent kit for detecting human helicobacter pylori antibody and preparation method of reagent kit
InactiveCN109342740ARapid Quantitative DetectionSensitive Quantitative DetectionBiological testingFluorescence/phosphorescenceMicrosphereFluorescence
The invention belongs to the technical field of immunochromatographic diagnosis of in vitro diagnosis reagents, and particularly relates to a fluorescent immunochromatographic reagent kit for detecting a human helicobacter pylori antibody and a preparation method of the reagent kit. The reagent kit comprises a support, a blotting membrane fixed on the support, a water absorption pad fixed at one end of the blotting membrane, and a combining pad fixed at the other end of the blotting membrane, wherein a sample pad is fixed at the other end of the combining pad; a blood filter membrane is fixedat the other end of the sample pad; a detection line coated with a Caga A, urease and cell vacuolating cytotoxin recombinant antigen mixture and a control line coated with sheep anti-mouse IgG (immunoglobulin G) are arranged on the blotting membrane; and a mouse anti-human IgG fluorescent emulsion microsphere is sprayed onto the combining pad. The reagent kit achieves quick and sensitive quantitative detection of the helicobacter pylori antibody by a fluorescent immunochromatographic method through an indirect method principle and fluorescent characteristics of the fluorescent emulsion microsphere.
Owner:BIOHIT BIOTECH HEFEI
Kit for classificatory detection of helicobacter pylori
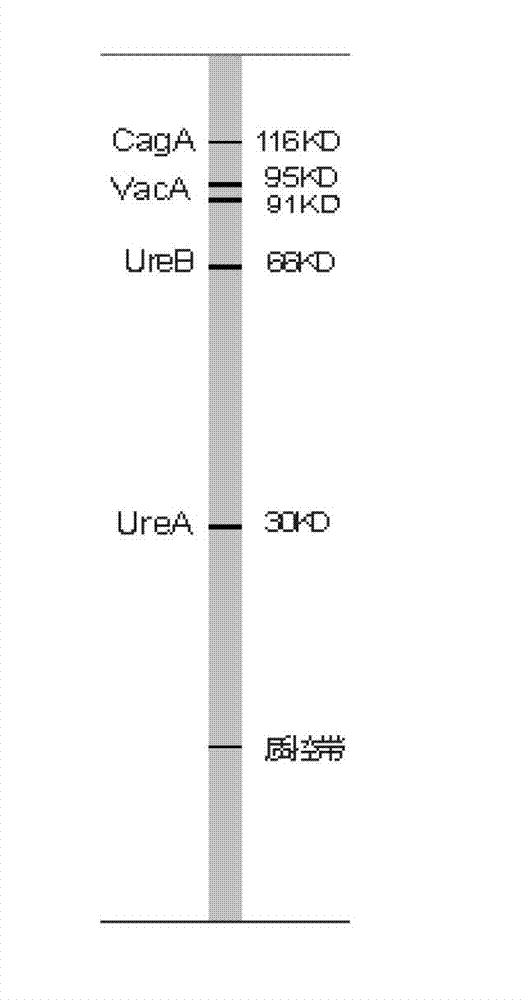
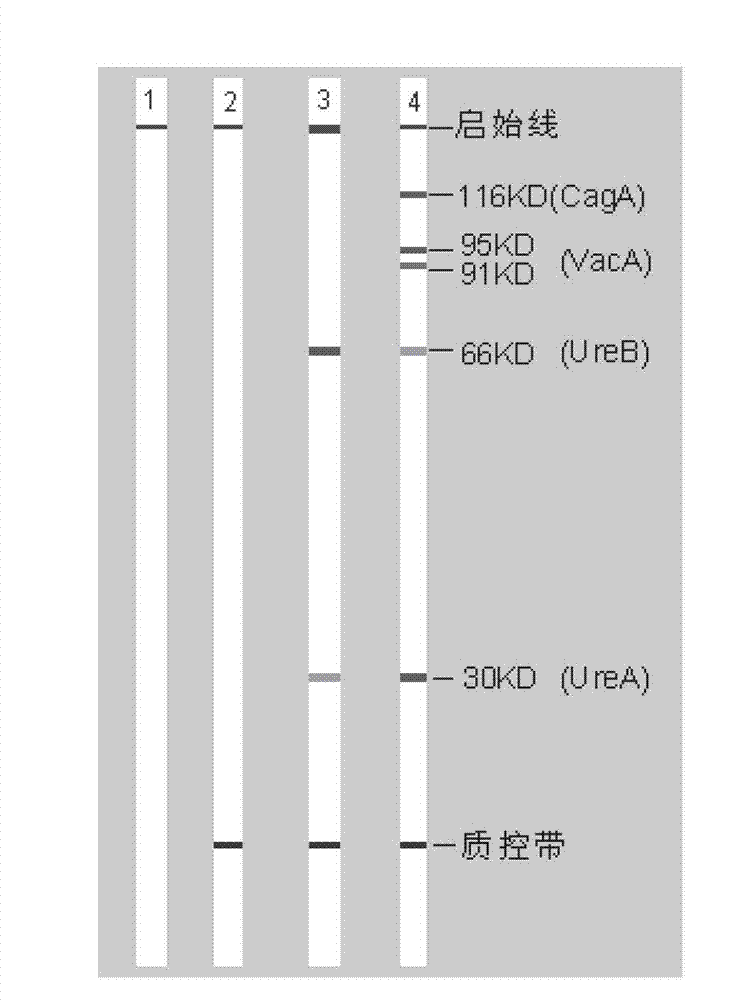
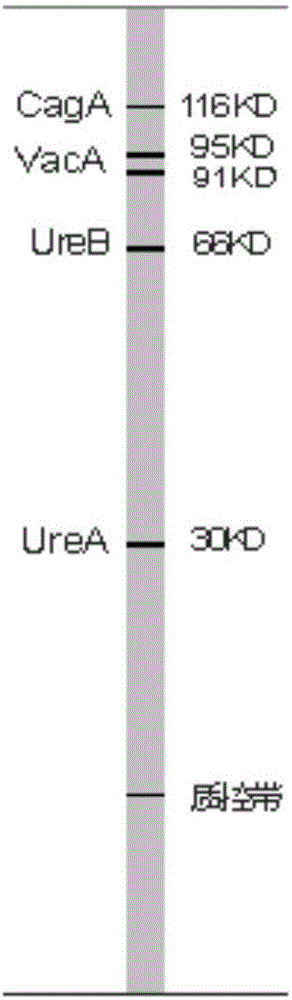
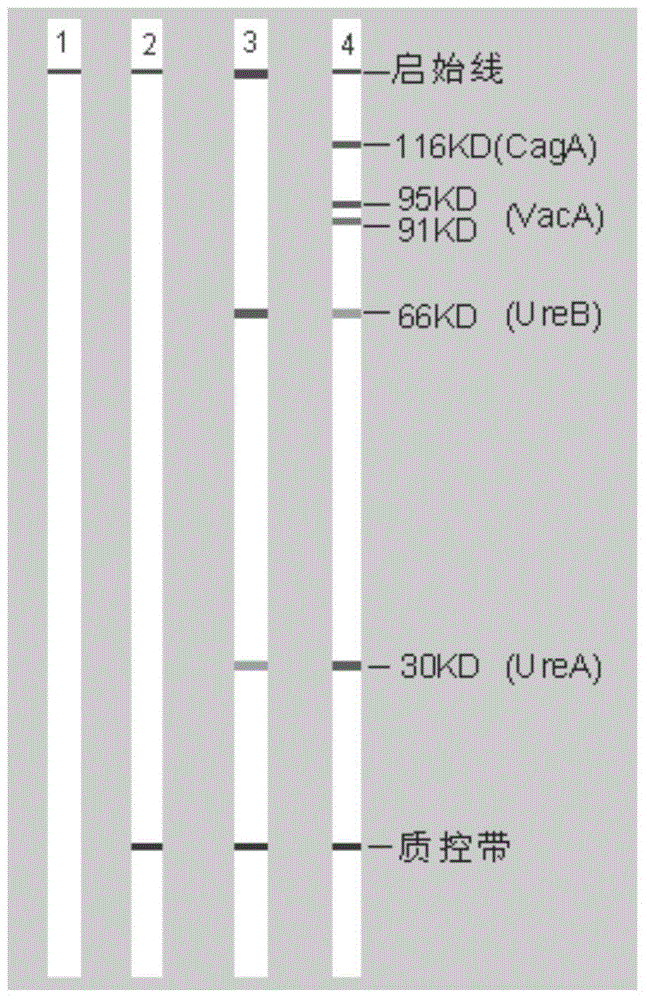
The invention belongs to the technical field of biology, and discloses a kit for classificatory detection of helicobacter pylori. The kit comprises a blotting membrane transferred with whole helicobacter pylori protein subjected to electrophoretic separation. The kit is used for detecting multiple Hp antibodies such as pathogenic factor cytotoxin (CagA), vacuolating cytotoxin (VacA) and urease subunit A and B antibodies in the serum of a helicobacter pylori infected person by adopting immuno-blotting, determining whether the infected Hp produces strains or not according to different Hp antibodies and further judging the serological types of the Hp strains infecting patients. The kit only requires an examinee to provide the serum, belongs to noninvasive examination, and is high in compliance of the patients, quick in detection (the whole time is only 2 hours), low in demand of samples (only 10 milliliters of examined serum is required), high in specificity, high in sensitivity, wide in application range and convenient to popularize and use in hospitals and hygiene departments.
Owner:SHENZHEN BLOT BIOTECH
Tjn molecular kit for diagnosing virulent strains of helicobacter pylori
InactiveCN102099486AFix long-standing problemsOvercome barriersMicrobiological testing/measurementFermentationHelicobacterMicrobiology
A kit in the form of a product and a method able to detect simultaneously four genes of Helicobacter pylori (rDNAl6S Hpy), i.e. one identification gene and three virulence genes (cagA, vacAml, dupA), is disclosed. Moreover, the kit envisages the association of primers which determine the quality of extraction of the DNA (Eub gene).
Owner:UNIV DE CONCEPCION
Helicobacter pylori identification and virulence multiplex gene detection system, kit adopting detection system and application of detection system
ActiveCN105463124AThe test result has no impurityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesEtiologyVirulent characteristics
The invention relates to a helicobacter pylori identification and virulence multiplex gene detection system, a kit adopting the detection system and the application of the detection system. The detection system comprises a plurality of pairs of primers aiming at strain identification genes (16S rRNA) and virulence genes (cagA, vacA-s1, vacA-s2, vacA-m1, vacA-m2, iceA1, iceA2, dupA, oipA and luxS) respectively. According to the helicobacter pylori identification and virulence multiplex gene detection system and the kit adopting the detection system, routine culture and other steps are not needed, helicobacter pylori identification and multiplex virulence analysis can be directly conducted on a tissue sample in the same reaction system, the defects of conventional detection methods of low flux, high time consumption and low detection rate are overcome, comprehensive, accurate and low-cost etiology basis is provided for clinical application quickly, and important reference is provided for helicobacter pylori infection accurate diagnosis and differential diagnosis and disease prognosis.
Owner:HUADONG HOSPITAL +1
Kit for typing detection of helicobacter pylori
ActiveCN104569389AReduce demandImprove complianceMaterial analysisPatient complianceWhole cell lysate
The invention belongs to the field of biotechnology, and discloses a kit for typing detection of helicobacter pylori. The kit comprises a blotting membrane transferred with helicobacter pylori whole cell lysate protein obtaind by electrophoretic separation. Various Hp antibodies including pathogenic factor cytotoxicity (CagA), vacuole (VacA) and urease subunit A and antibody B contained in the serum of the helicobacter pylori infected patients can be detected by adopting an immunoblotting method, whether infected HP produces strain can be determined according to different HP antibodies so as to further judge the serology type of the infected Hp strain of the patients. According to the kit, patients to be detected only needs to provide serum, tests belong to non-invasive examination, the compliance of the patients is good, the detection is fast as the whole time only lasts for 2 hours, the sample size is small as only 10mu L of to-be-detected serum is needed, the specificity is strong, the sensitivity is high, the application range is wide, and the kit is convenient for popularization and use in hospitals and hygiene departments at all levels.
Owner:SHENZHEN BLOT BIOTECH
Helicobacter pylori vaccination
InactiveUS20050175629A1Longer timescale of immunotherapyAntibacterial agentsAmpoule syringesAdjuvantVaccination
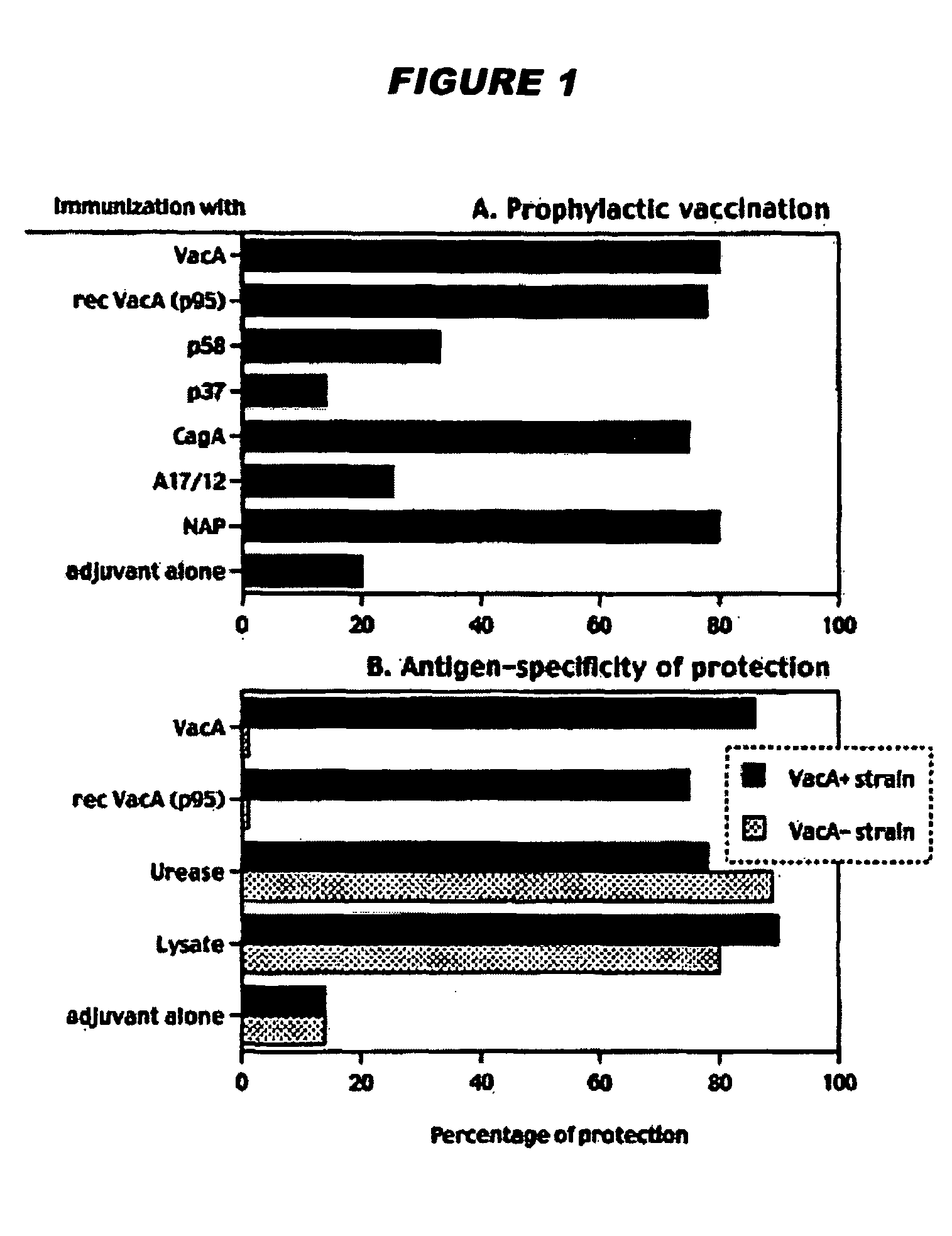
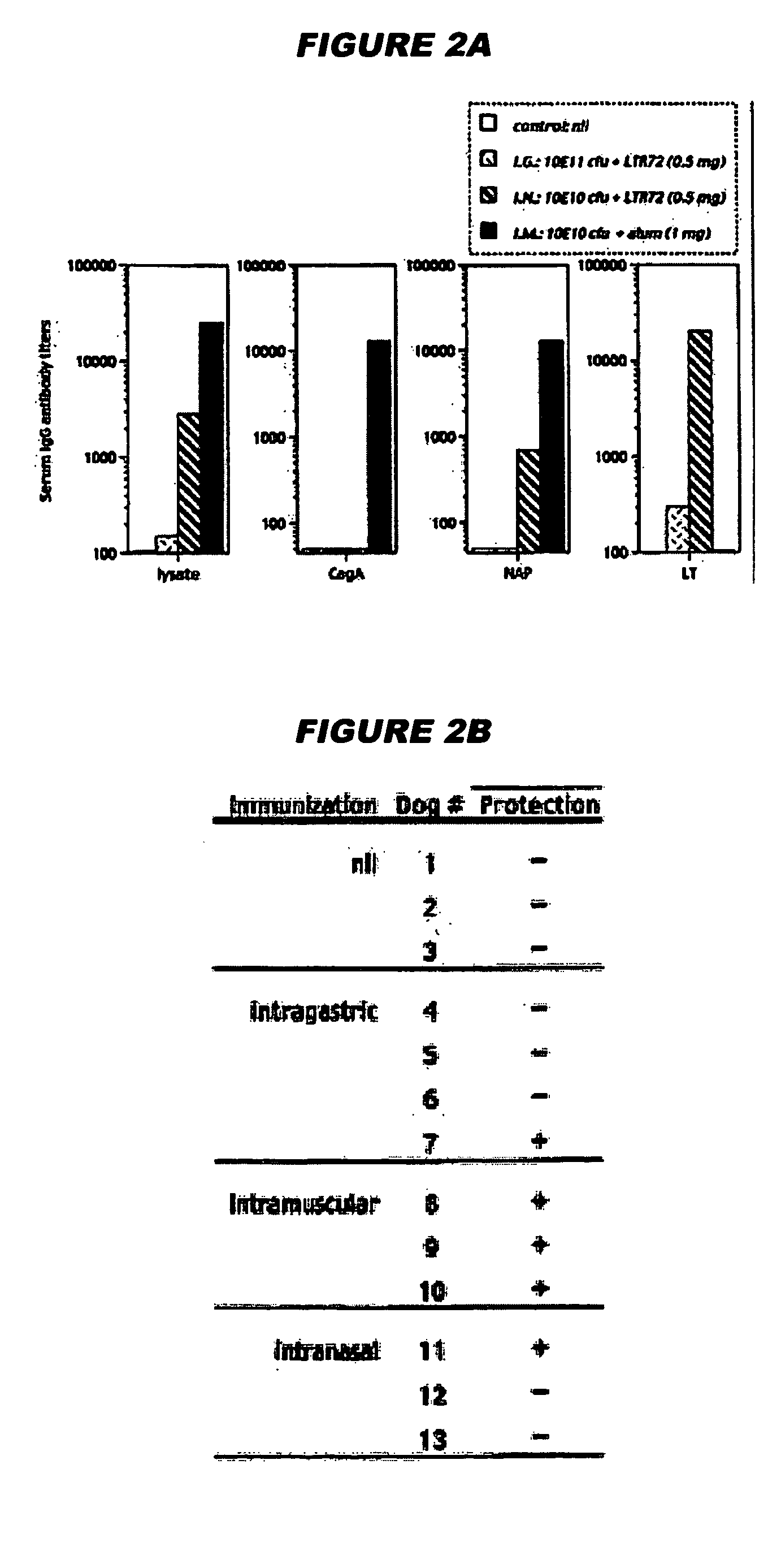
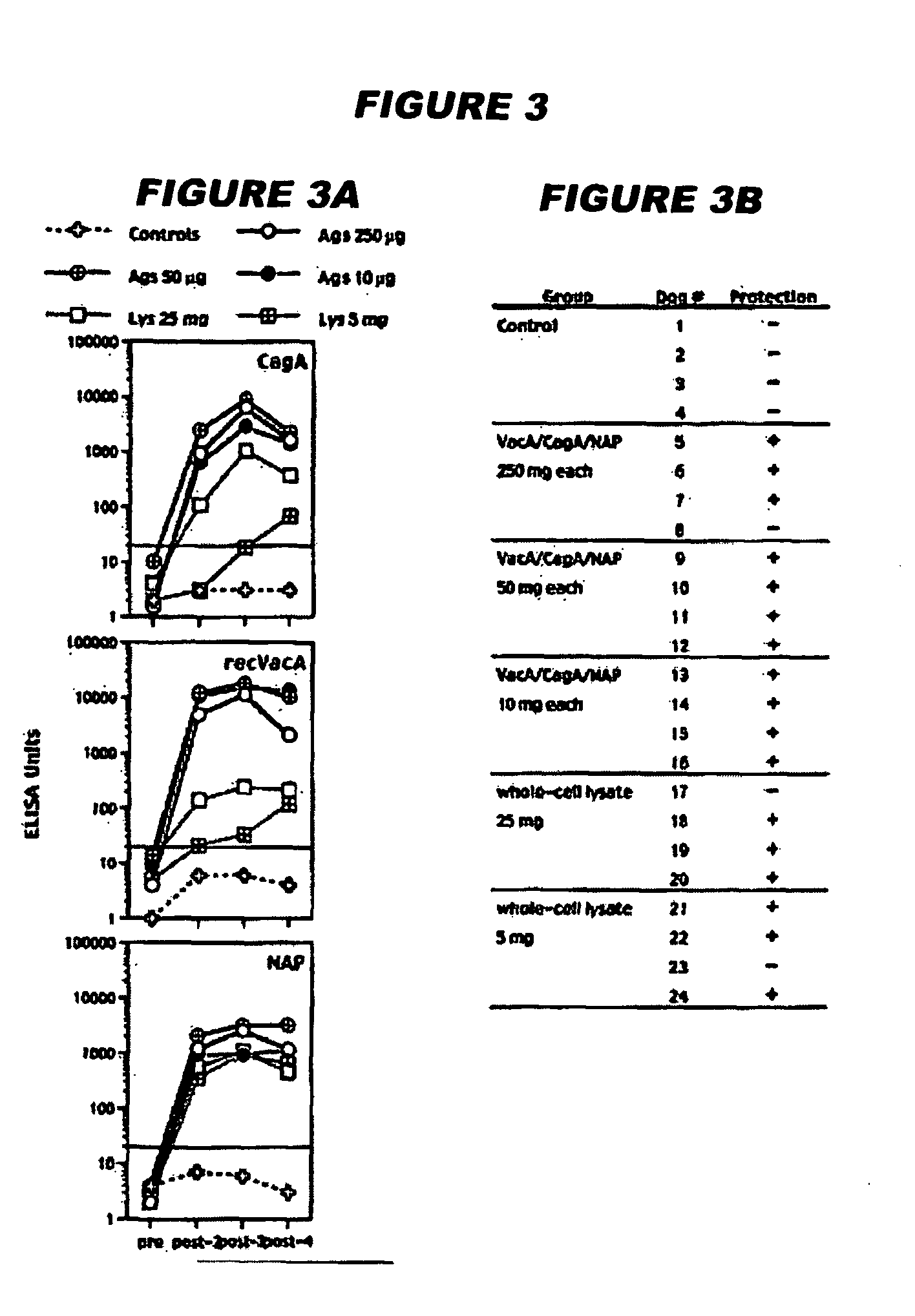
A sterile immunogenic preparation of three purified H. pylori antigens (CagA, VacA and NAP) adjuvanted with alum in an isotonic buffer solution for intramuscular injection. The antigens may be administered in conjunction with antibiotics and / or antisecretories. Urease breath testing, stool antigen testing, and / or immunological analysis may be used as correlate(s) of protection against H. pylori infection. Urea may be used to improve VacA solubility.
Owner:CHIRON CORP
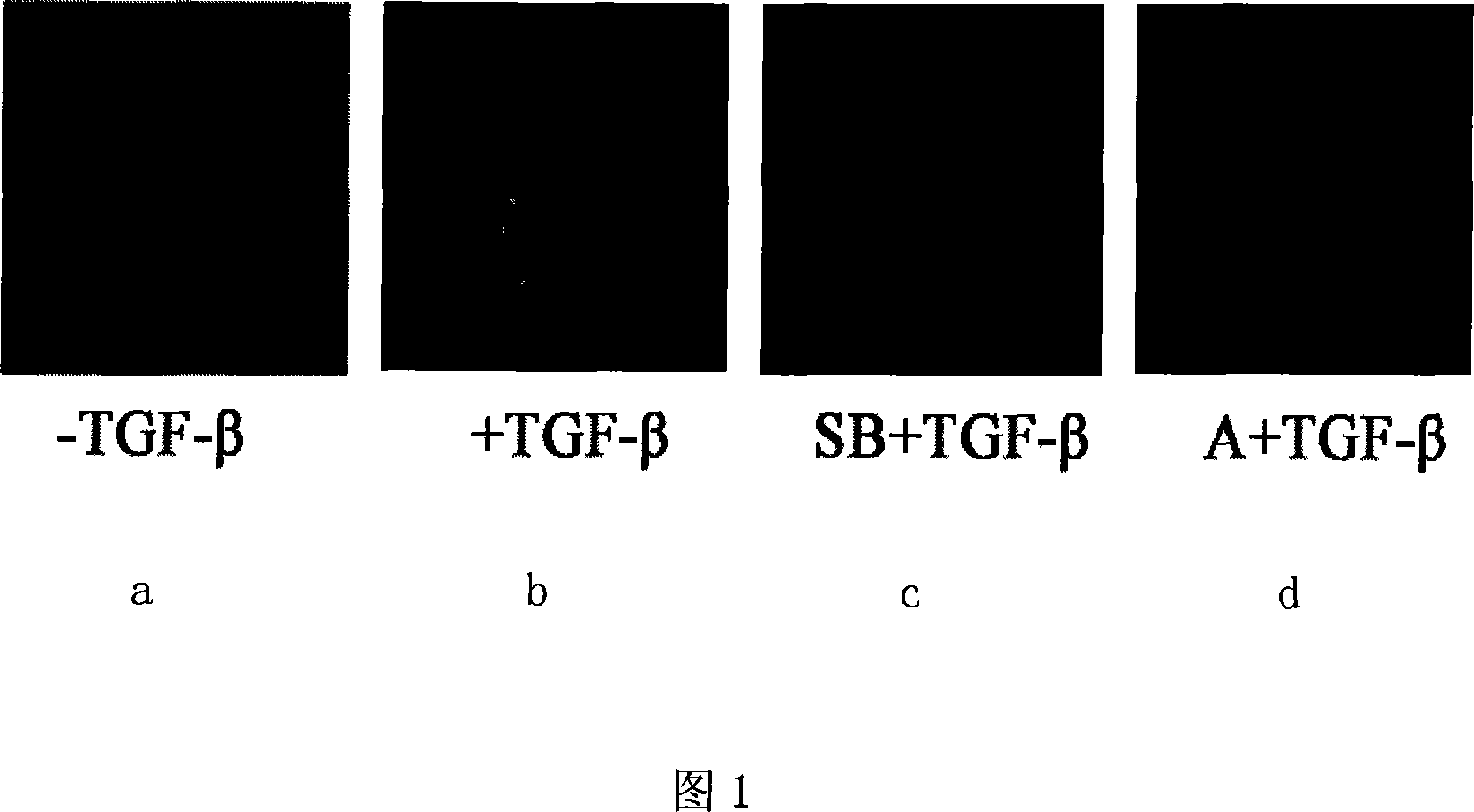
Screening model of medicaments related to TGF-Beta signal path
InactiveCN101182535ASimple and fast operationReduce dosageVector-based foreign material introductionCell systemBiology
The invention relates to a medicine selection model and a special plasmid, in particular to a medicine selection model and a special plasmid thereof relevant to the TGF-Beta signal channel. The reported plasmid provided by the invention is a recombinant expression vector obtained after a serial sequence consisting of 12 CAGA-boxes, an adenovirus major later promoter sequence and a reported protein code gene sequence is inserted into a plurality of cloning points of the mammal cell expression vectors. The selection model of medicines relevant to the TGF- Beta signal channel provided by the invention is a human liver cancer cell system which contains the reported plasmid. The selection model of the medicines relevant to the TGF- Beta signal channel of the invention is reliable and stable and is characterized by greatly-promoted selection efficiency, reduced selection cost, shortened selection cycle, reduced tested compound amount and reduced radioactive pollution. Therefore, the medicine selection model of the invention can be applied to the development of relevant medicines to reduce development cost and accelerate the development process; in this way, the invention has broad application prospects.
Owner:TSINGHUA UNIV
Method and compositions for detecting helicobacter pylori
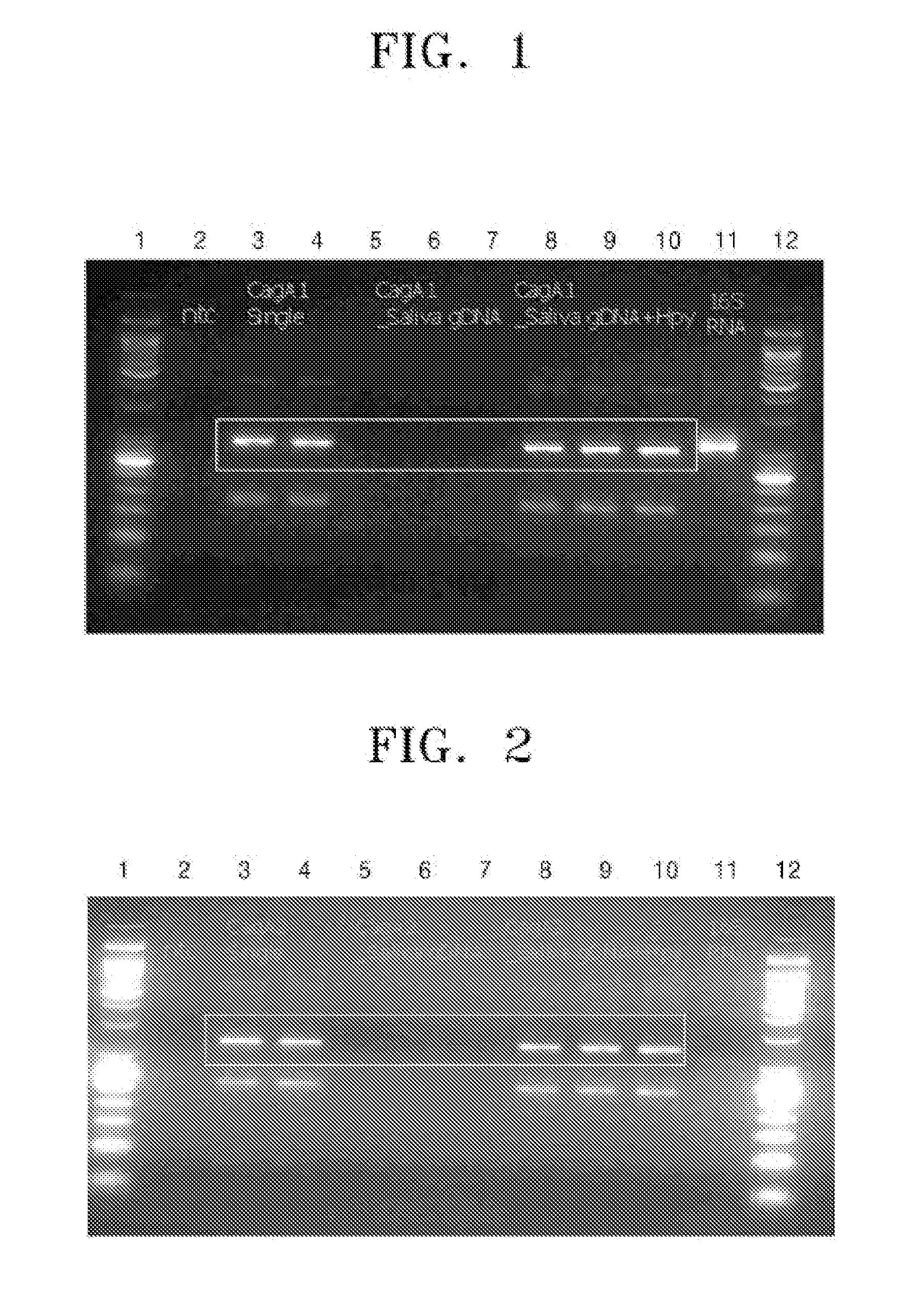
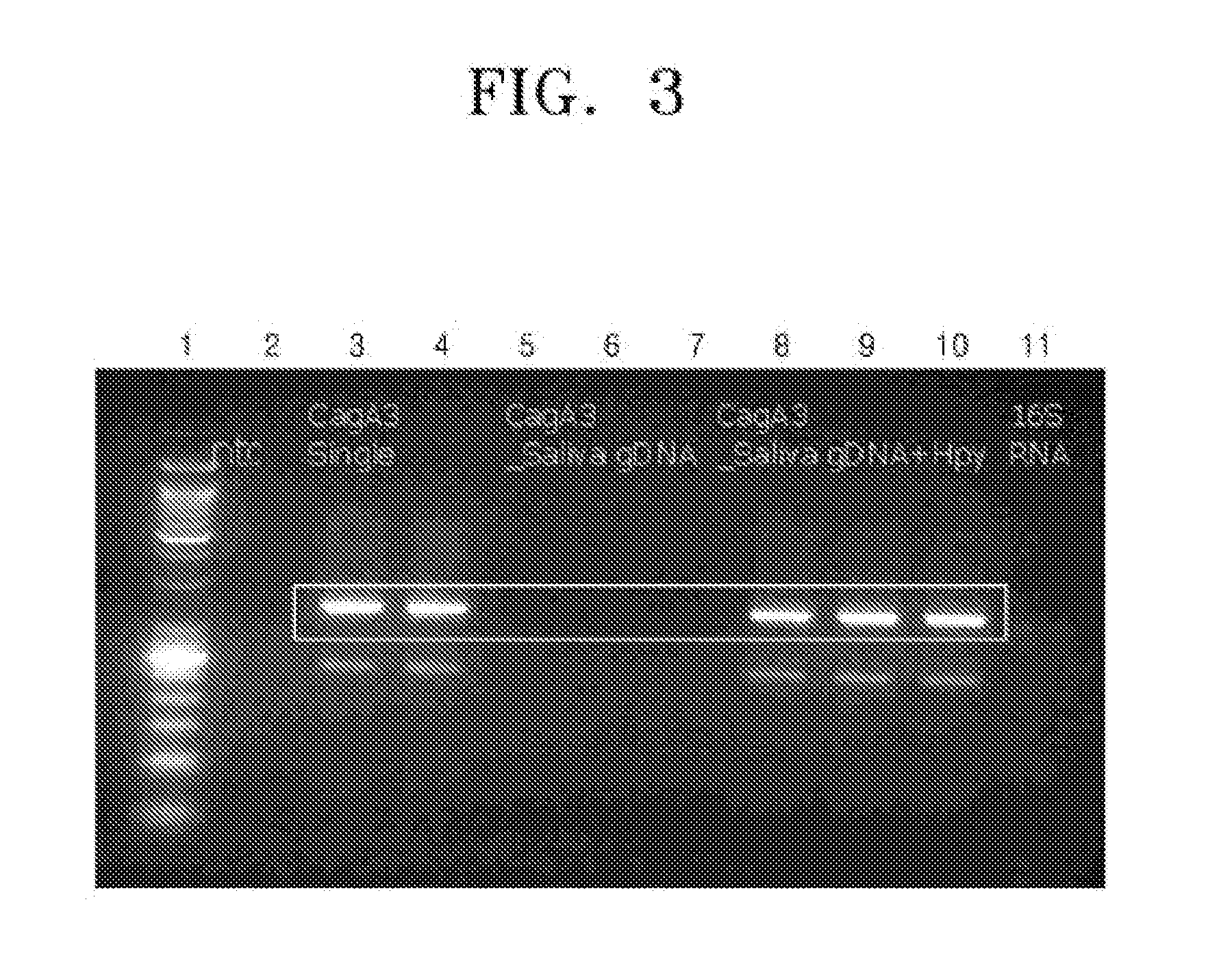
Provided are an oligonucleotide primer set for amplifying at least one target sequence of the cagA gene of Helicobacter pylori, a method of detecting Helicobacter pylori using the primer set, and a kit for detecting Helicobacter pylori, including the primer set.
Owner:SAMSUNG ELECTRONICS CO LTD
Immunofluorescence test reagent strip for measuring cytotoxin-related protein A antibodies as well as preparation method and application of immunofluorescence test reagent strip
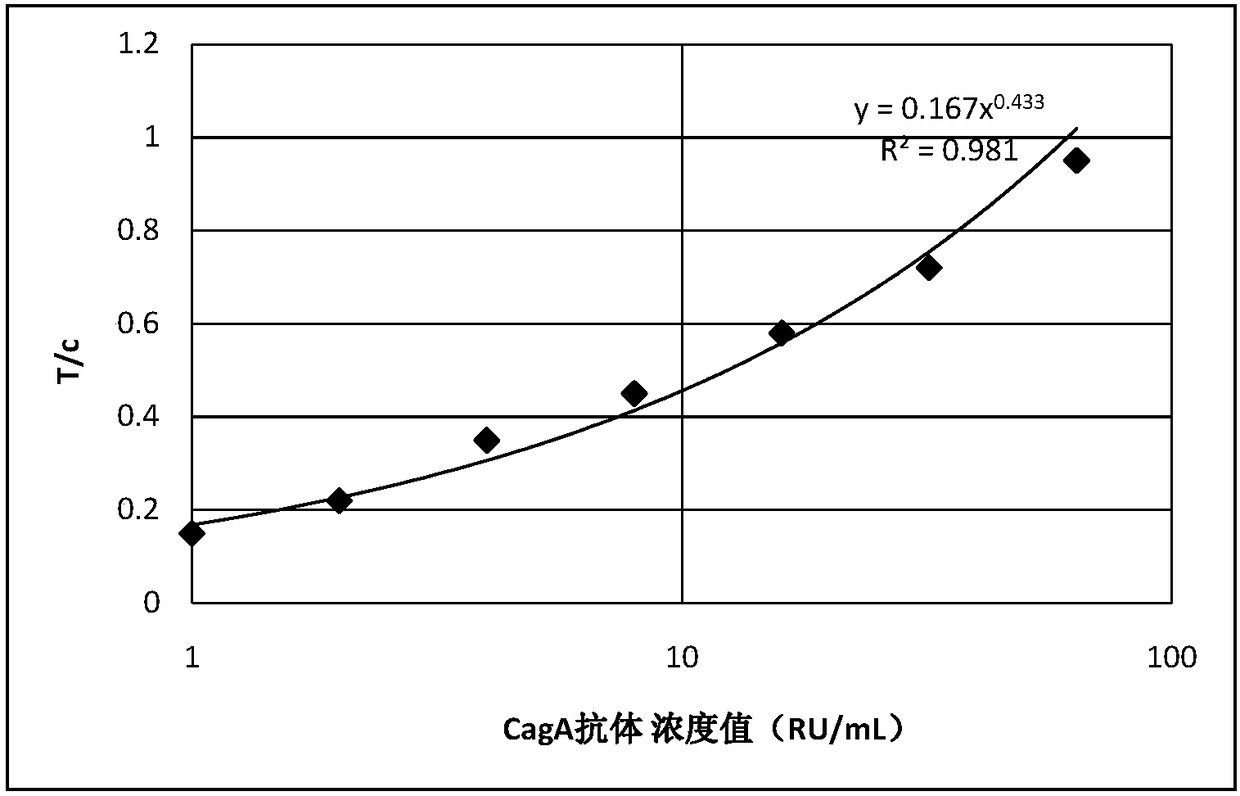
InactiveCN108061807ARealize quantitative detectionEasy to operateDisease diagnosisBiological testingReagent stripAntigen
The invention discloses an immunofluorescence test reagent strip for measuring cytotoxin-related protein A antibodies as well as a preparation method and application of the immunofluorescence test reagent strip. The immunofluorescence test reagent strip provided by the invention has three areas, i.e., a sample adding area, a binding area and a detection area. A water absorbing pad, a chromatographic membrane, a binding pad and a sample pad are separately arranged on the reagent strip from top to bottom. The reagent strip is characterized in that the binding pad is coated with a CagA antigen, labeled with a fluorescent substance CY3, in a membrane-spraying way, and the C line of the chromatographic membrane is coated with a monoclonal antibody coated with against CagA, so that the reagent strip has high specificity and can be used for detecting a CagA antibody in serum. The immunofluorescence test reagent strip can realize quantitative detection when being used together with a fluorescence analyzer, and is simple and convenient to operate. The immunofluorescence test reagent strip can be both used for laboratory detection and on-site quick testing, and only takes about 10min for detection.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
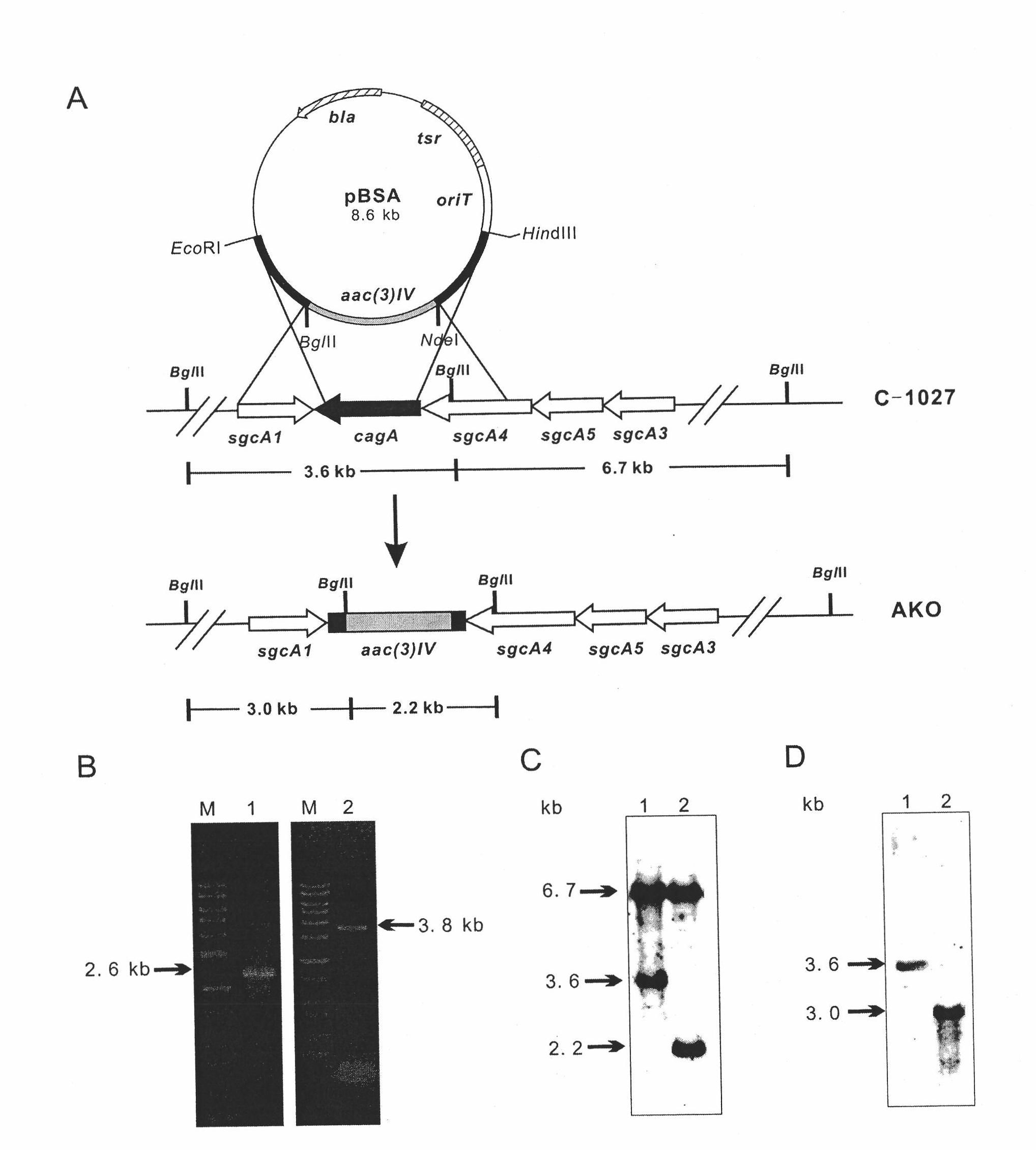
Method for preparing targeted lidamycin production strain by utilizing gene disruption strain and application of lidamycin production strain
InactiveCN102041268AStrategy does workBacteriaMicroorganism based processesTumor targetTumor targeting
The invention relates to a method for preparing a targeted lidamycin production strain by utilizing a gene disruption strain. The method comprises a step of introducing an expression plasmid of a fusion gene, which contains lidamycin apoprotein gene and a fusion gene consisting of different tumor targeted molecular coding sequences, into a lidamycin apoprotein gene disruption strain to obtain the lidamycin production strain with the target corresponding to the target of the tumor targeted molecule, wherein the gene interruption strain is a Streptomyces globisporus C-1027 lidamycin apoprotein gene (cagA) interruption strain, namely S.globisporus AKO; and the tumor targeted molecule includes, but not limited to tumor targeted antibody, tumor targeted peptide, cell factor and the like. The invention also relates to application of the production strain prepared by the method in producing targeted lidamycin.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
LAMP primer group for detecting helicobacter pylori cytotoxin related protein cagA and application thereof
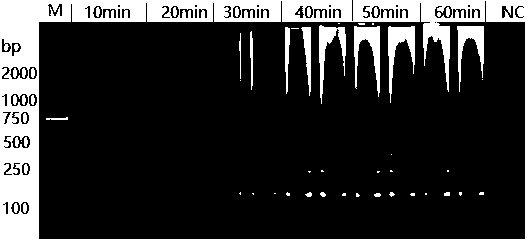
InactiveCN108531557AEasy to operateStrong specificityMicrobiological testing/measurementMicroorganism based processesWater bathsHelicobacter pylori
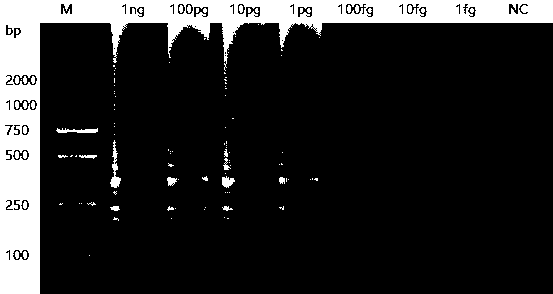
The invention discloses an LAMP primer group for detecting helicobacter pylori cytotoxin related protein cagA and application thereof. The LAMP primer group comprises an inner primer FIP / BIP, an outerprimer F3 / B3 and a loop primer LF / LB. The primer group disclosed by the invention has the technical effects and advantages that the operation is simple: only one water bath kettle is needed to complete the reaction, and no complicated instruments and professional operators are needed; the reaction is fast and efficient: the reaction only needs 30 min; the specificity is high: three pairs of primers are set for the cagA gene, whole gene sequences are fully covered, the triple guarantee enables the pertinence to be very high, and through the DNA verification of other eleven common intestinal strains, no false positive problem exists; and the sensitivity is high: the minimum detection limit is 100 fg DNA.
Owner:厦门蓝特生物科技有限公司
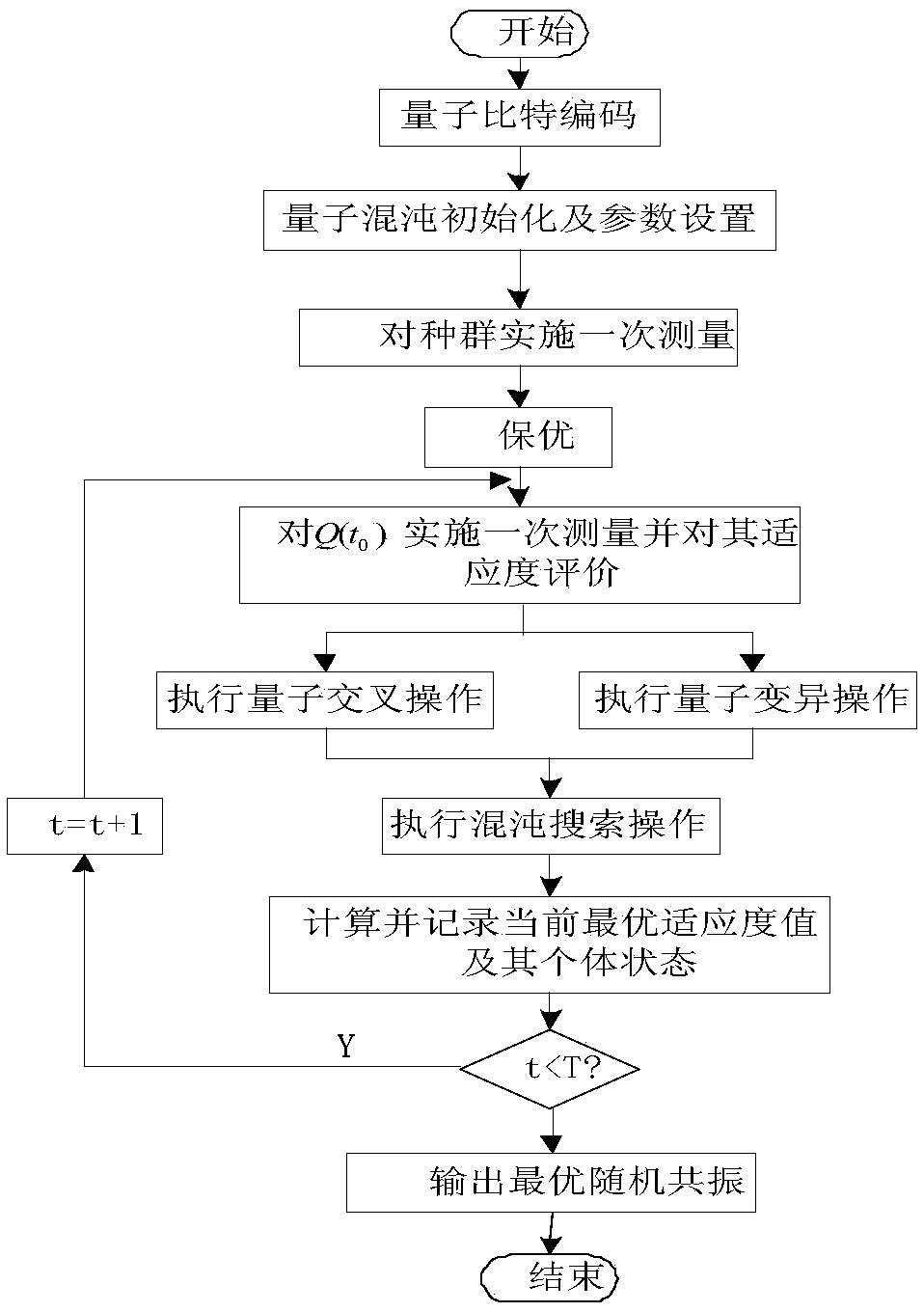
Stochastic Resonance Weak Signal Detection Method Based on Chaotic Quantum Genetic Algorithm
InactiveCN106372725BMeet high precision matching requirementsReduce computational complexityChaos modelsNon-linear system modelsStochastic resonanceQubit
The invention discloses a chaotic quantum genetic algorithm-based stochastic resonance weak signal detection method. Optimization parameters obtained by operation of a chaotic quantum genetic algorithm (CQGA) are substituted into an alpha stable noise-driven Duffing bistable stochastic resonance system, and weak signal detection is performed, wherein the CAGA is a quantum genetic algorithm improved by utilizing chaotic search; based on quantum operation, the probability amplitude of quantum bit is applied to genetic coding and expressed through a state vector of quantum; and the chaotic search operation is executed to update individual chromosome. According to the method, the signal energy is enhanced by utilizing the noise energy, the signal-to-noise ratio is greatly increased, the search and calculation can be performed without depending on an update table, the high-precision matching requirement is met, the application range of stochastic resonance is expanded based on an alpha stable noise background, and a new method is provided for small target detection in a non Gaussian noise background.
Owner:NANJING UNIV OF INFORMATION SCI & TECH
Nucleic acid composition for detecting virulence genes of helicobacter pylori, reagent and reagent kit including nucleic acid composition, and application of nucleic acid composition
ActiveCN111378771AQuick checkEfficient detectionMicrobiological testing/measurementMicroorganism based processesHelicobacter pyloriGenotype
The invention discloses a nucleic acid composition for detecting virulence genes of helicobacter pylori, a reagent and reagent kit including the nucleic acid composition, and an application of the nucleic acid composition, and relates to the technical field of biology. Specifically, the nucleic acid composition comprises one or more combinations of nucleic acid compositions 1-4, and the nucleic acid compositions 1-4 are respectively used for detecting virulence genes vacA s1 type, vacA m1 type, cagA and babA2 of the helicobacter pylori. The nucleic acid composition can detect the virulence genes of the helicobacter pylori quickly and efficiently, and has the advantages of being high in detection sensitivity and good in detection specificity.
Owner:重庆博利达医学科技有限公司
Preparation method of mouse chronic atrophic gastritis model
ActiveCN109758483AStable pathological changesEasy to operateOrganic active ingredientsBacteria material medical ingredientsAtrophic gastritisInflammation
Owner:右江民族医学院
Application of flavone compound in treating lung fibrosis
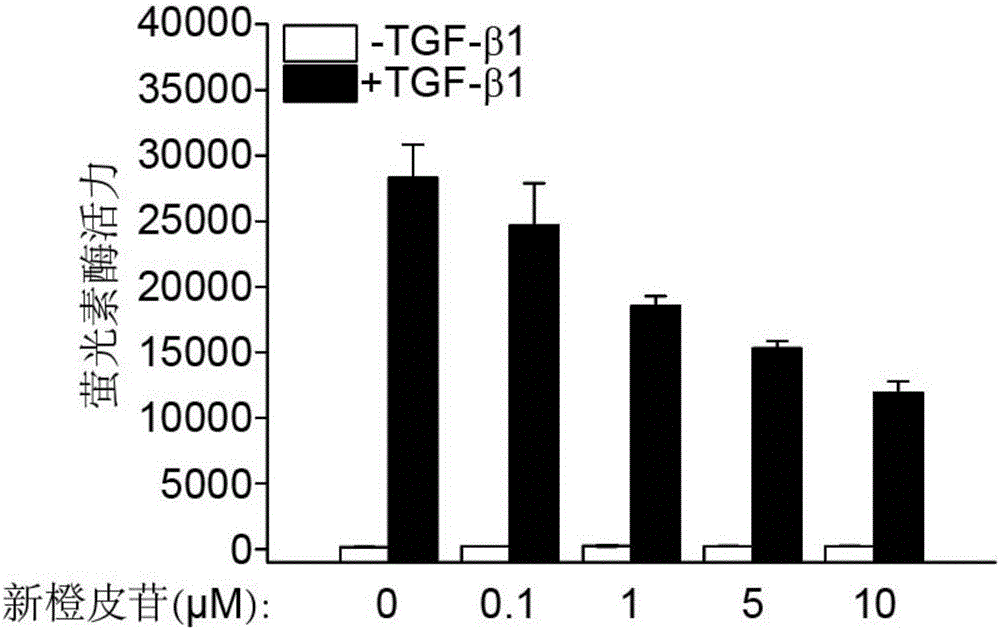
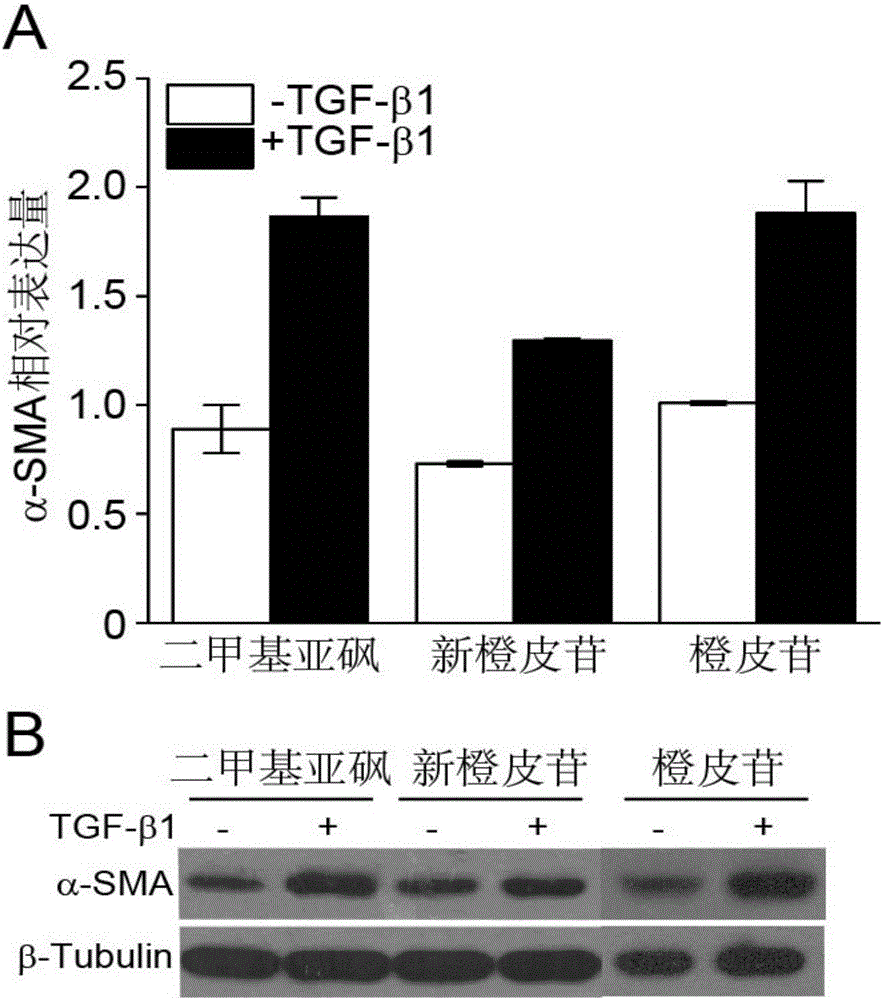
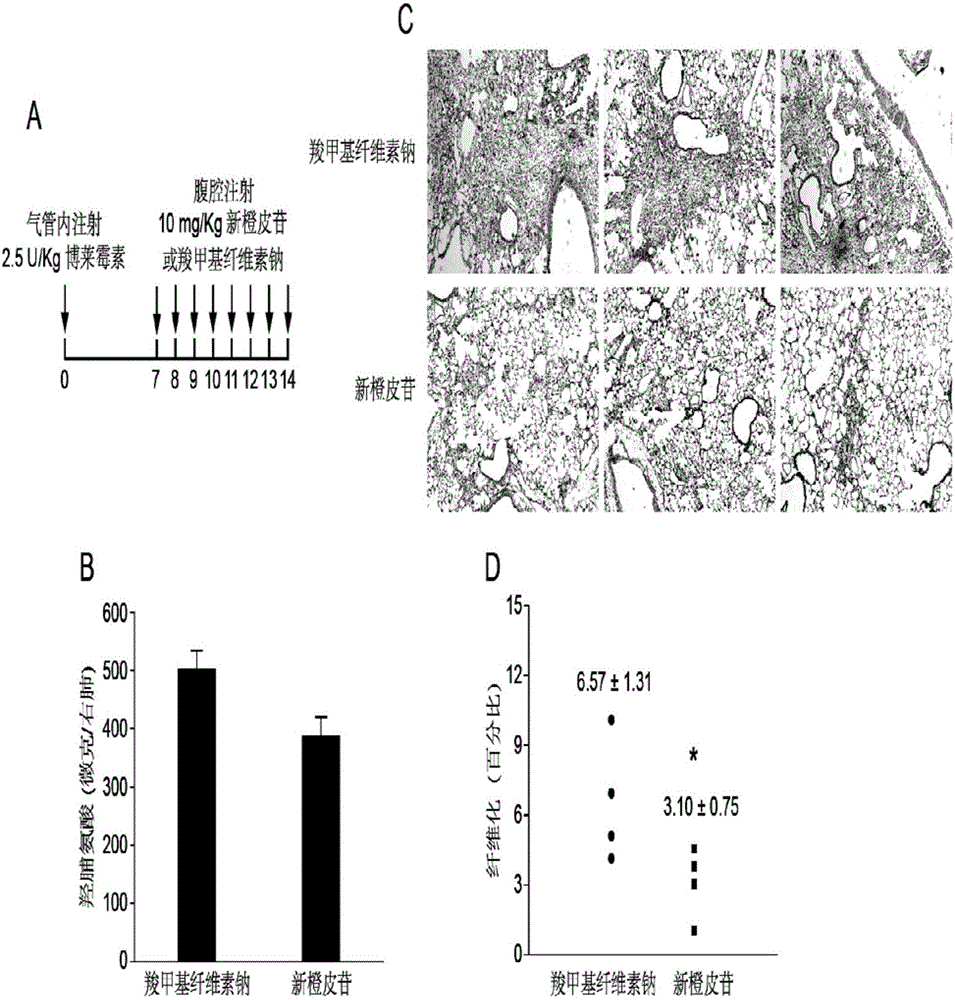
The invention relates to application of a flavone compound in treating lung fibrosis, particularly relates to application of neohesperidin in treating lung fibrosis, particularly relates to restraining of the neohesperidin on expression of a CAGA-luciferase reporter gene induced by TGF-beta1 by using dose dependency, particularly relates to application of the neohesperidin in restraining in muscle fiber mother cell differentiation induced by TGF-beta1, and particularly relates to application of the neohesperidin in alleviating murine lung fibrosis induced by bleomycin.
Owner:NANKAI UNIV
Construction method of helicobacter pylori cagA (cytotoxin-associated gene A) inactivated mutant strain
InactiveCN109897813AWell mixedGood conditionBacteriaMicroorganism based processesStart timeFhit gene
The invention relates to the technical field of genetic engineering, in particular to a construction method of a helicobacter pylori cagA (cytotoxin-associated gene A) inactivated mutant strain. The method comprises following steps: (1), H. Pylori 11639 thalli growing on Columbia agar are scraped and added to an electrotransformation buffer solution, the electrotransformation buffer solution is diluted until concentration of H. Pylori 11639 thalli is 1012 / L, and a solution A is obtained; (2), 100 mu L of the solution A is added to an ice-cold 0.2 cm BioRed electrotransformation cup, and 50 mug of pHG1 targeting vectors are added; (3), the electrotransformation cup is placed on ice cubes below a solution extracting device; (4), an adequate quantity of SOC media are added to an SOC medium storage box, and a solution extracting tube extends into the electrotransformation cup; (5), a Columbia blood plate is fixed with a barrel, a coating brush is contacted with the surface of the Columbiablood plate, and starting time input to a controller is 10-15 min; (6), after coating of the Columbia blood plate is completed, the Columbia blood plate is taken down. With the application of the solution extracting device, errors caused by manual operation can be reduced, and efficiency is improved.
Owner:GUIZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com