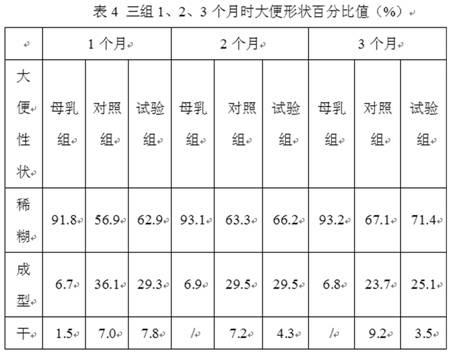
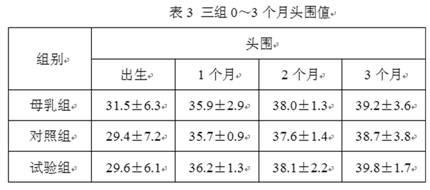
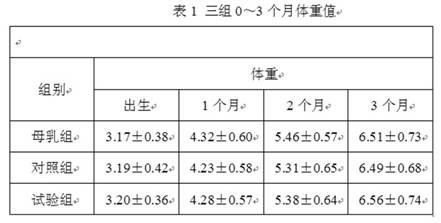
Patents
Literature
943 results about "Lactoferrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
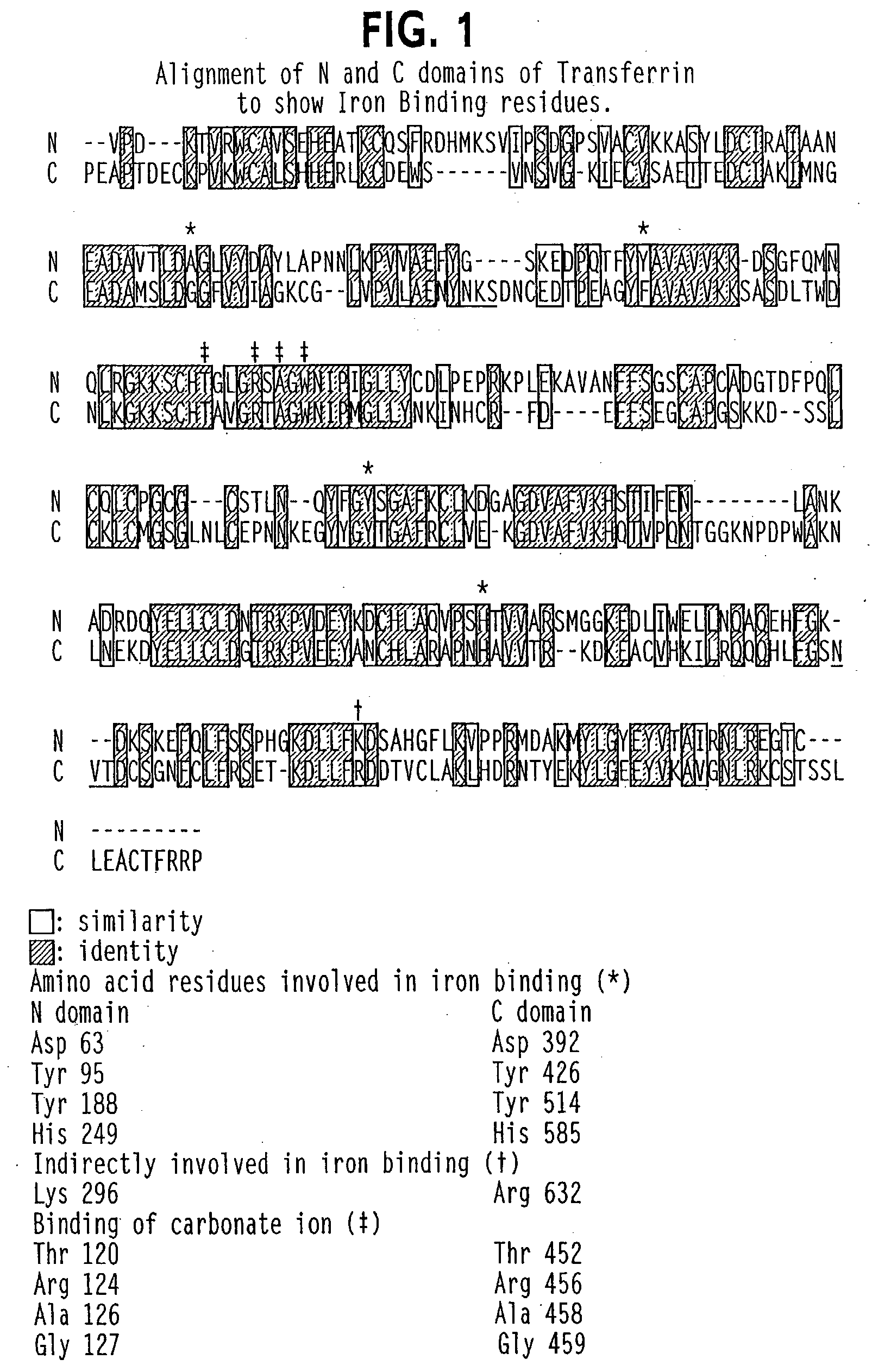
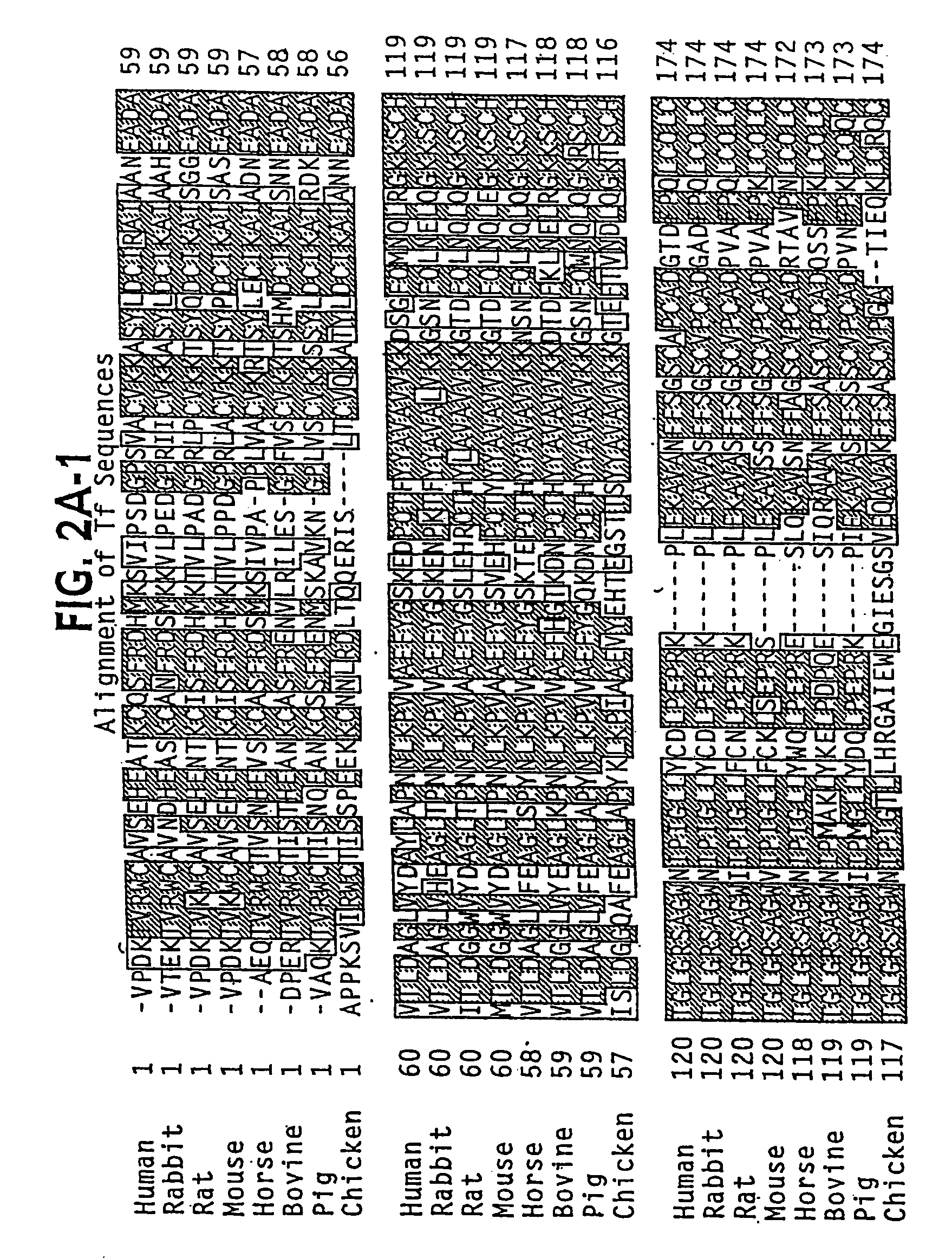
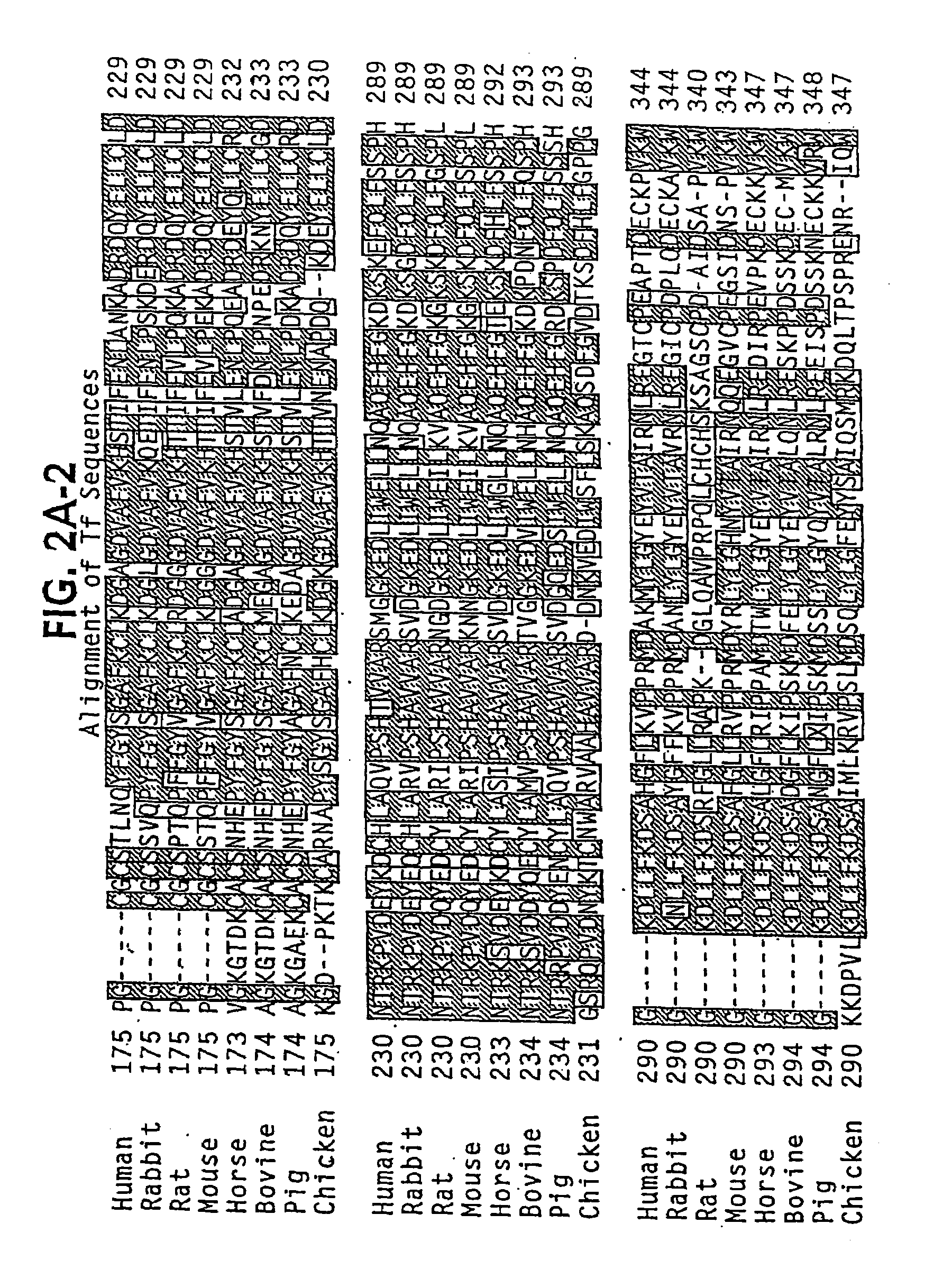
Lactoferrin (LF), also known as lactotransferrin (LTF), is a multifunctional protein of the transferrin family. Lactoferrin is a globular glycoprotein with a molecular mass of about 80 kDa that is widely represented in various secretory fluids, such as milk, saliva, tears, and nasal secretions. Lactoferrin is also present in secondary granules of PMNs and is secreted by some acinar cells. Lactoferrin can be purified from milk or produced recombinantly. Human colostrum ("first milk") has the highest concentration, followed by human milk, then cow milk (150 mg/L).
Modified transferrin fusion proteins
InactiveUS7176278B2Increased serum half-lifeImprove bioavailabilityAntibody mimetics/scaffoldsVirus peptidesDiseaseSerum ige
The present invention discloses fusion proteins comprising transferrin, lactoferrin or melanotransferrin fused to glucagon-like peptide 1 (GLP-1). In one embodiment of the invention, the fusion protein displays increased serum half-life as compared to a GLP-1 peptide in an unfused state. The invention includes a pharmaceutical composition comprising the GLP-1 fusion protein of the invention and a carrier. The fusion protein of the invention can be administered to a subject for treatment of diseases or conditions treatable by GLP-1, including, but not limited to, diabetes, obesity, congestive heart failure and inflammatory bowel syndrome.
Owner:BIOREXIS TECH INC
Coenzyme Q10, lactoferrin and angiogenin compositions and uses thereof
ActiveUS20070253941A1Efficient assimilationReduce frequencyOrganic active ingredientsAntimycoticsAngiogeninFunctional health
Methods of enhancing the bio-availability of coenzyme Q10, and methods of supporting the cardiovascular system to accommodate the increase in cellular energy synthesis as a result of the bio-availability of coenzyme Q10 are described. Compositions which include coenzyme Q10, lactoferrin and / or angiogenin are described for use in the related methods, for multi-functional health applications.
Owner:NAIDU LP
Enriched infant formulas
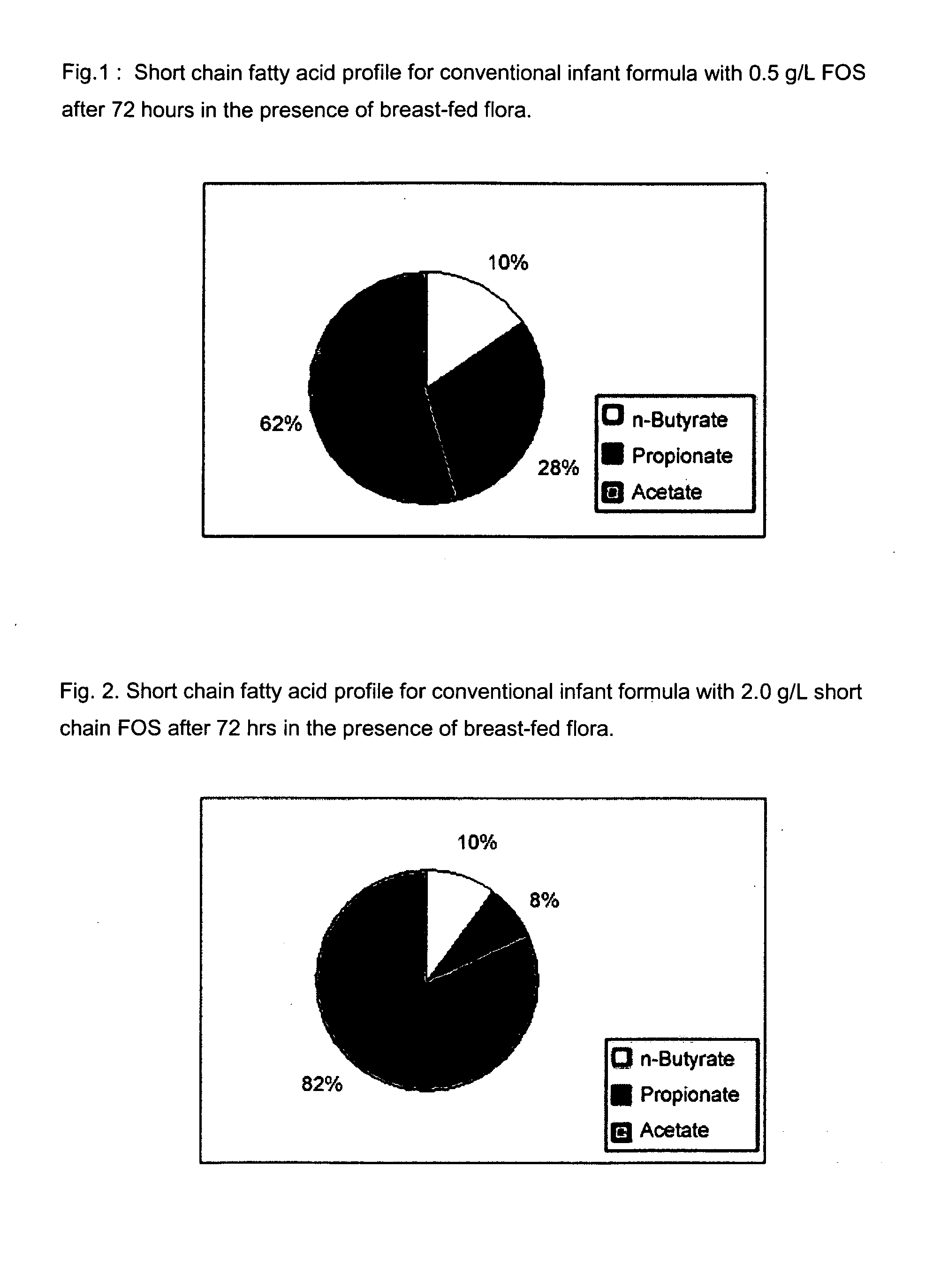
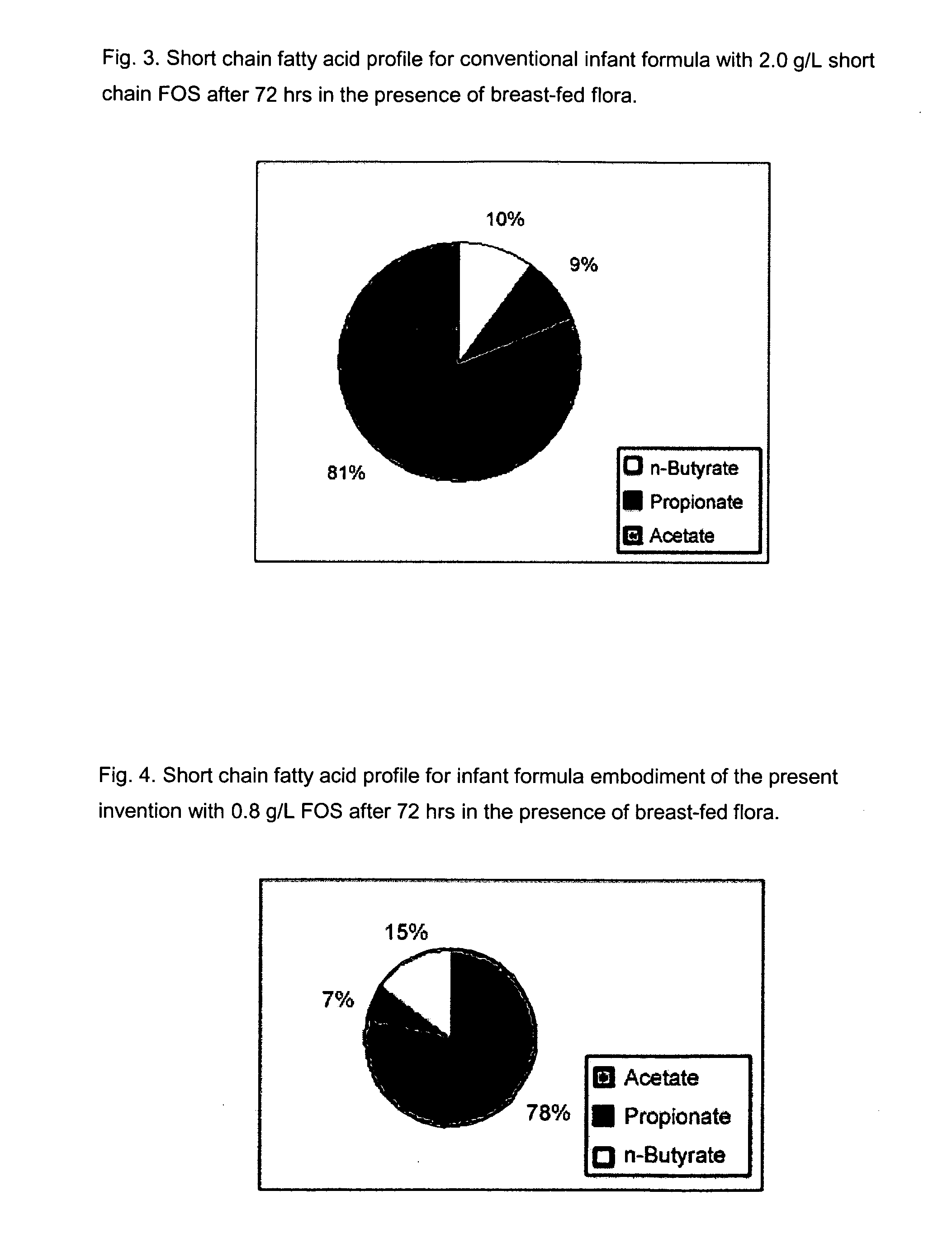
InactiveUS20080003329A1Reduce riskShorten the construction periodFood preparationSialic acidPhospholipid
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis (A) at least about 5 mg / L of gangliosides, (B) at least about 150 mg / L of phospholipids, and (C) lactoferrin, and (D) at least about 70 mg / L of sialic acid, with at least about 2.5% by weight of the sialic acid as lipid-bound sialic acid. Also disclosed are methods of using the formula to reduce the risk of diarrhea infants, and to produce a gut microflora profile similar to that of breast-fed infants.
Owner:ABBOTT LAB INC
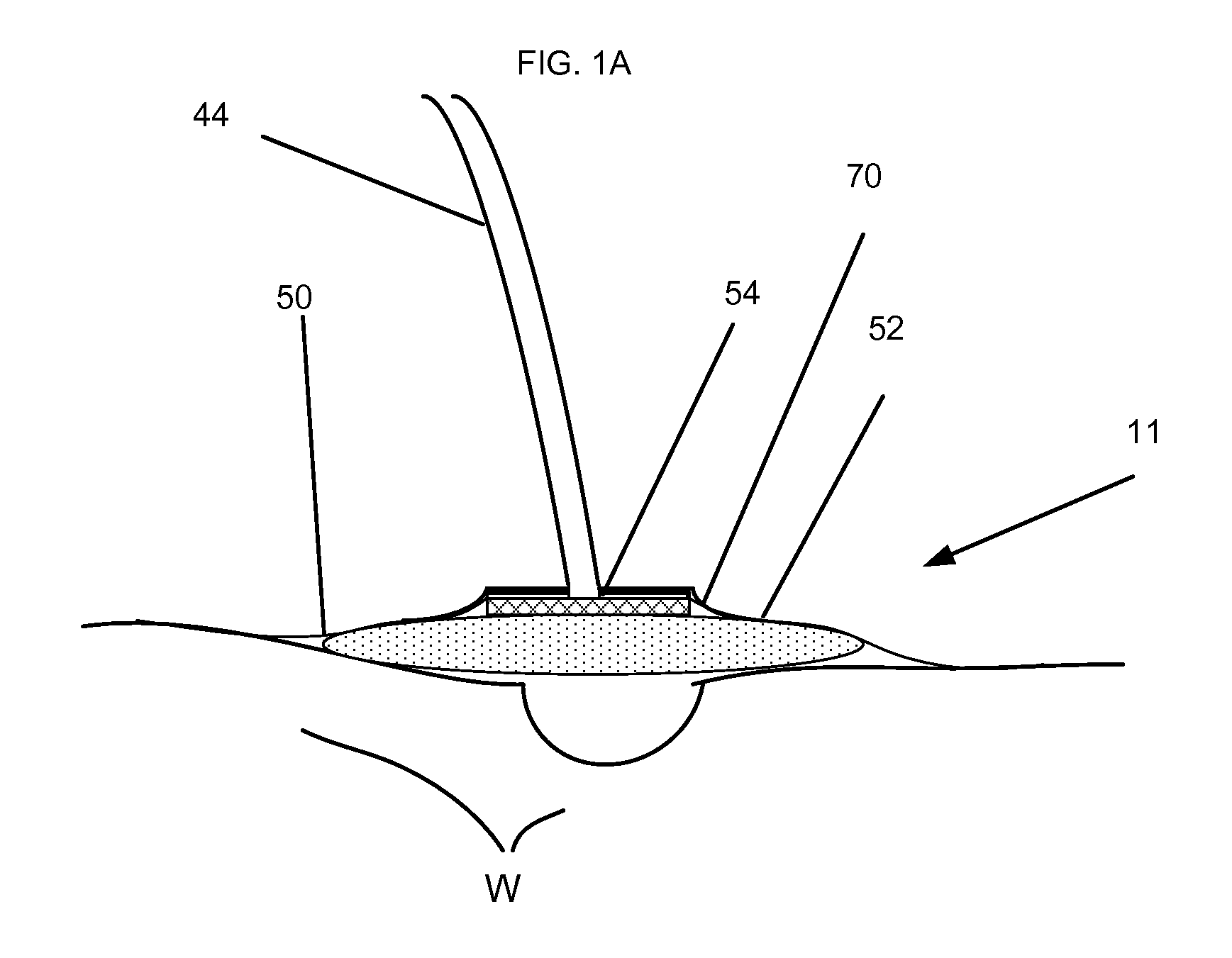
Irrigation Device and Method Using Same
InactiveUS20110015587A1Promote wound healingEasy treatmentMedical devicesMedical applicatorsWound healingDakins Solution
A disposable therapeutic device for the promotion of wound healing providing fluid irrigation and vacuum drainage of a wound includes a housing containing a controller and fluid moving device in a waterproof manner, a fluid mover capable of raising, compressing, or transferring fluid, a controller equipped to restrict fluid moving device in accordance with a predetermined treatment plan or duration, a chargeable power source removably connected to the housing, an optional therapeutic member of a compressible dressing or inflatable cuff to provide hemostasis, an identification member for regulating the operation of the device in accordance with a predetermined treatment plan, a disposable container, a pressure sensor and a control display panel. The fluid includes, but is not limited to, Lactoferrin, Xylitol, Dakins Solution, Polyhexanide and Hypochlorous Acid.
Owner:VOGEL RICHARD C MR
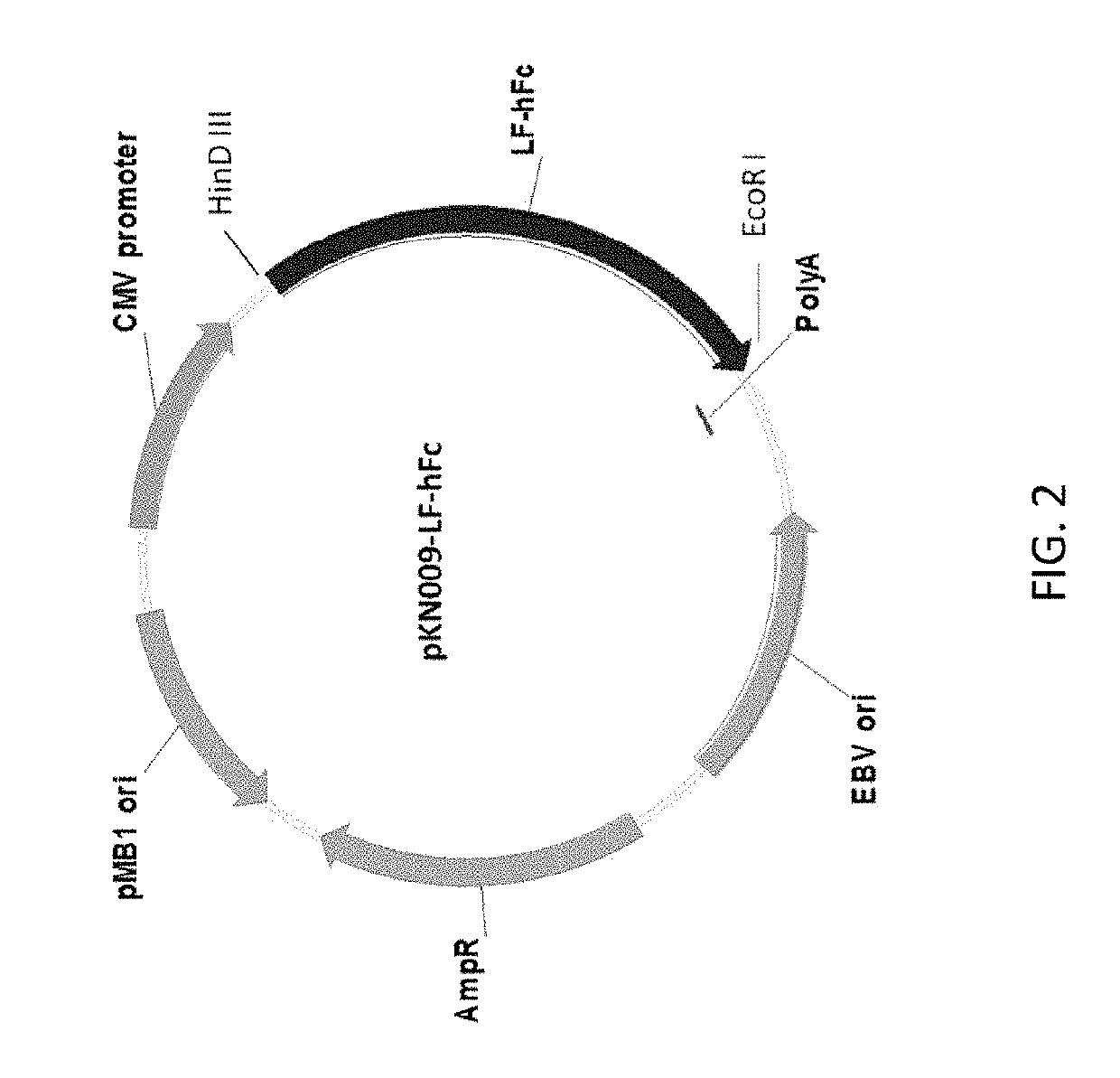
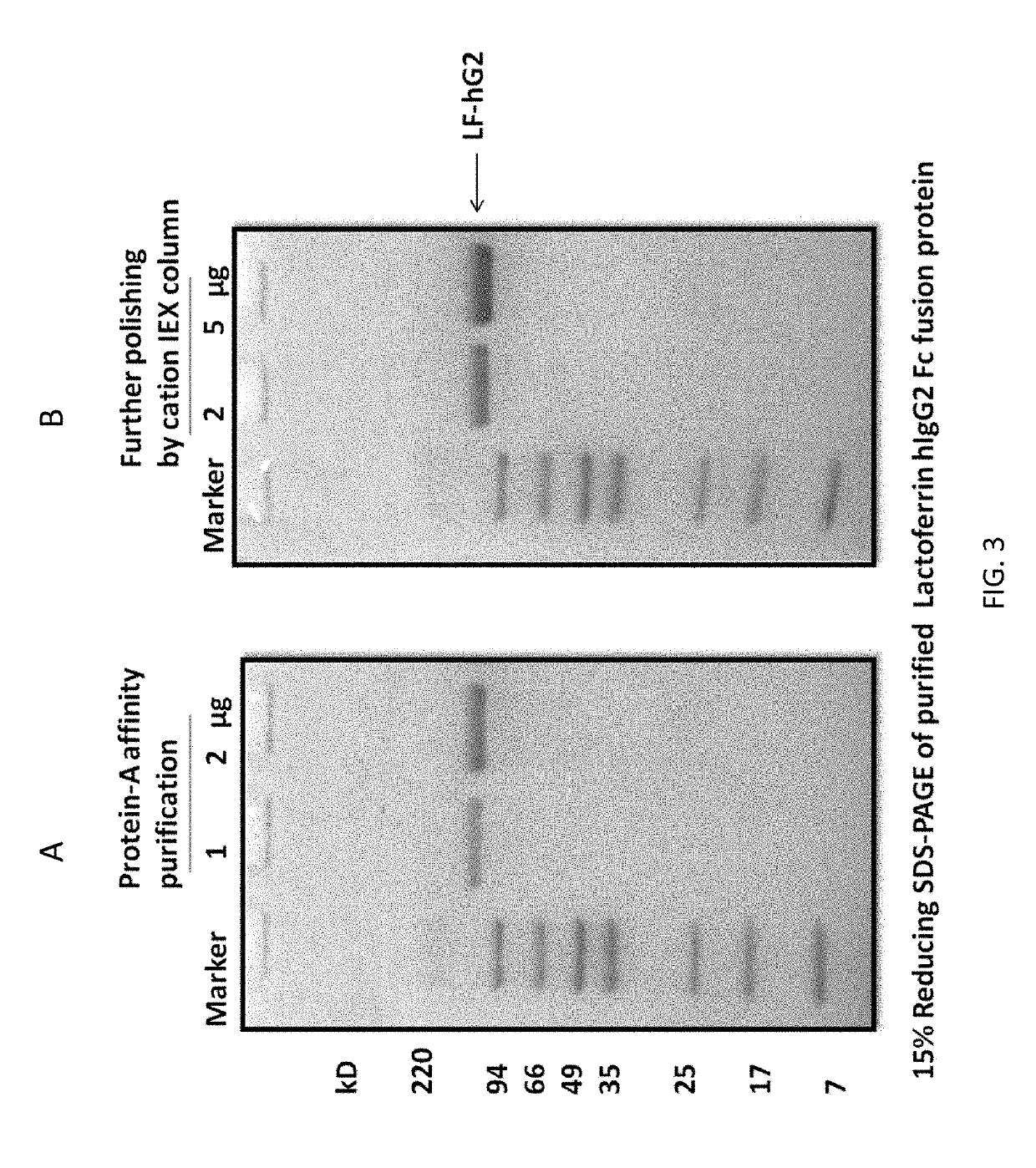
Methods and compositions for treatment of symptoms associated with intracranial hemorrhage
ActiveUS10442852B2Good treatment effectAvoid symptomsPeptide/protein ingredientsTransferrinsMedicineFc domain
Methods and compositions are provided for the treatment of a patient with intracranial hemorrhage (ICH). Methods include the use of the products of recombinant constructs such as those that contain lactoferrin, as well as fusion protein constructs of lactoferrin and Fc domain for IgG.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Bioactive factors in wound healing topical compositions and methods
InactiveUS20060240116A1Promote repairPeptide/protein ingredientsUnknown materialsCutaneous woundMicrobial agent
A topical composition for treating a cutaneous wound includes whole colostrum or one or more growth factors, as well as a base by which the whole colostrum or one or more growth factors are carried. The topical composition may also include, without limitation, immunomodulators, lactoferrin, enzymes, vitamins, minerals, amino acids, or combinations thereof. Other components may also be included in the topical composition, including, but not limited to, antiseptics, antimicrobial agents, and anesthetics. When applied to a wound, the bioactive topical composition promotes healing of the treated wound.
Owner:JOLLEY DAVID
A kind of infant formula milk powder that does not get angry and its preparation process
ActiveCN102283289ANot ediblePromote digestion and absorptionMilk preparationVegetable oilFructooligosaccharide
The invention relates to an anti-inflaming infant formula milk powder and a preparation process thereof. The anti-inflaming infant formula milk powder comprises the following components in percentage by weight: 22%-50% of lactose, 10%-20% of goat milk whey protein concentrate, 9.8%-20% of structural grease 1,3-dioleoyl 2-palmitoyl triglyceride, 10%-13.5% of non-fat goat milk powder, 8%-10% of vegetable oil, 2%-8% of beta-casein, 0.8%-1.6% of fructooligosaccharides, 0.5%-1.5% of mineral premix, 0.3%-1% of galactooligosaccharide, 0.2%-1% of immunoglobulin G, 0.2%-0.5% of lactulose, 0.10%-0.45% of arachidonic acid, 0.1%-0.6% of docosahexaenoic acid, 0.06%-0.12% of vitamin premix, 0.04%-0.06% of lactoferrin, and 0.01%-0.06% of nucleotide. The invention also includes the preparation process ofthe infant formula milk powder. The nutrition constituents and the functions of the infant formula milk powder are close to those of breast milk, the infant formula milk powder is easy to assimilate,and infants do not get inflamed after eating the milk powder.
Owner:AUSNUTRIA DAIRY CHINA
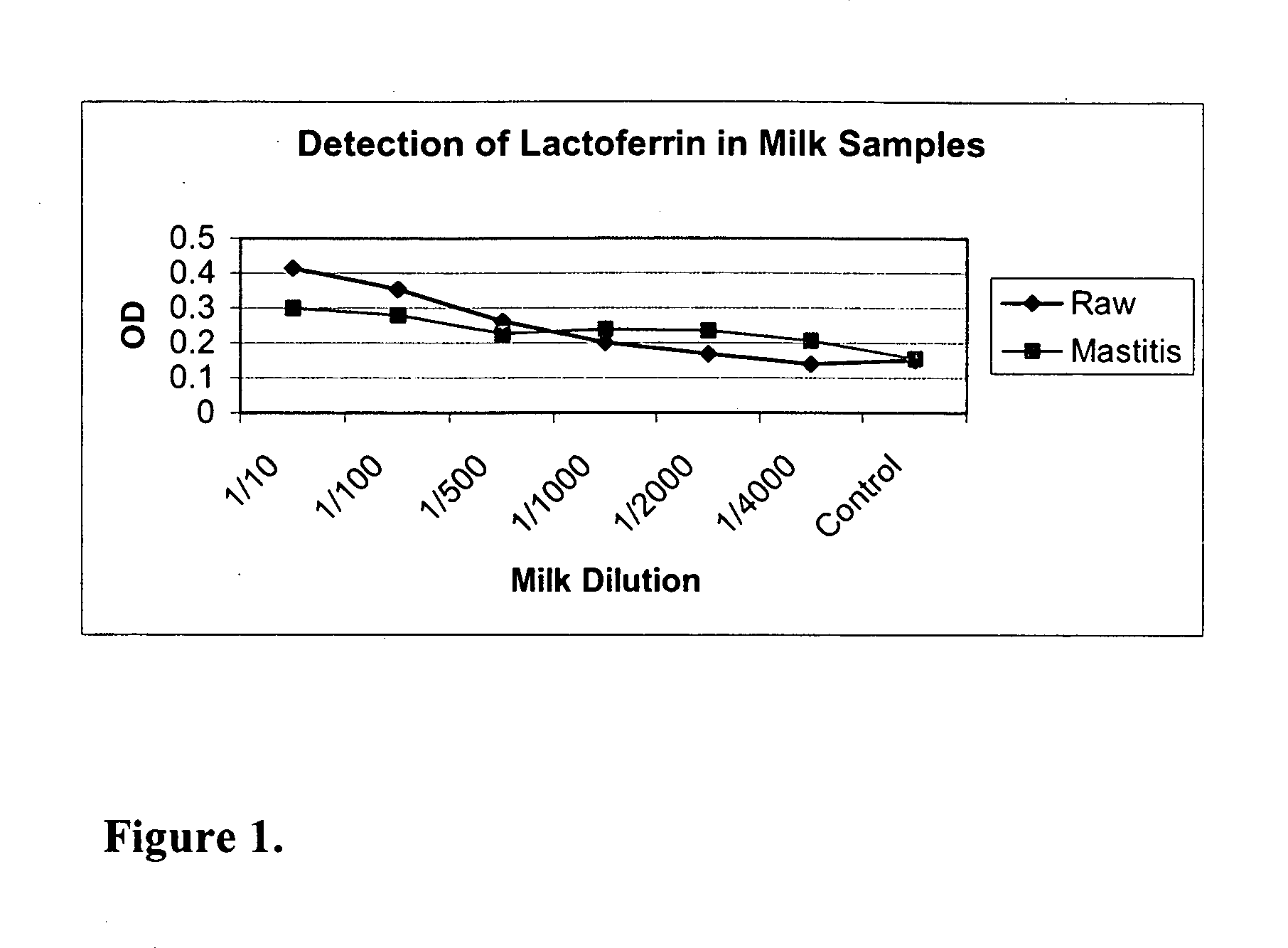
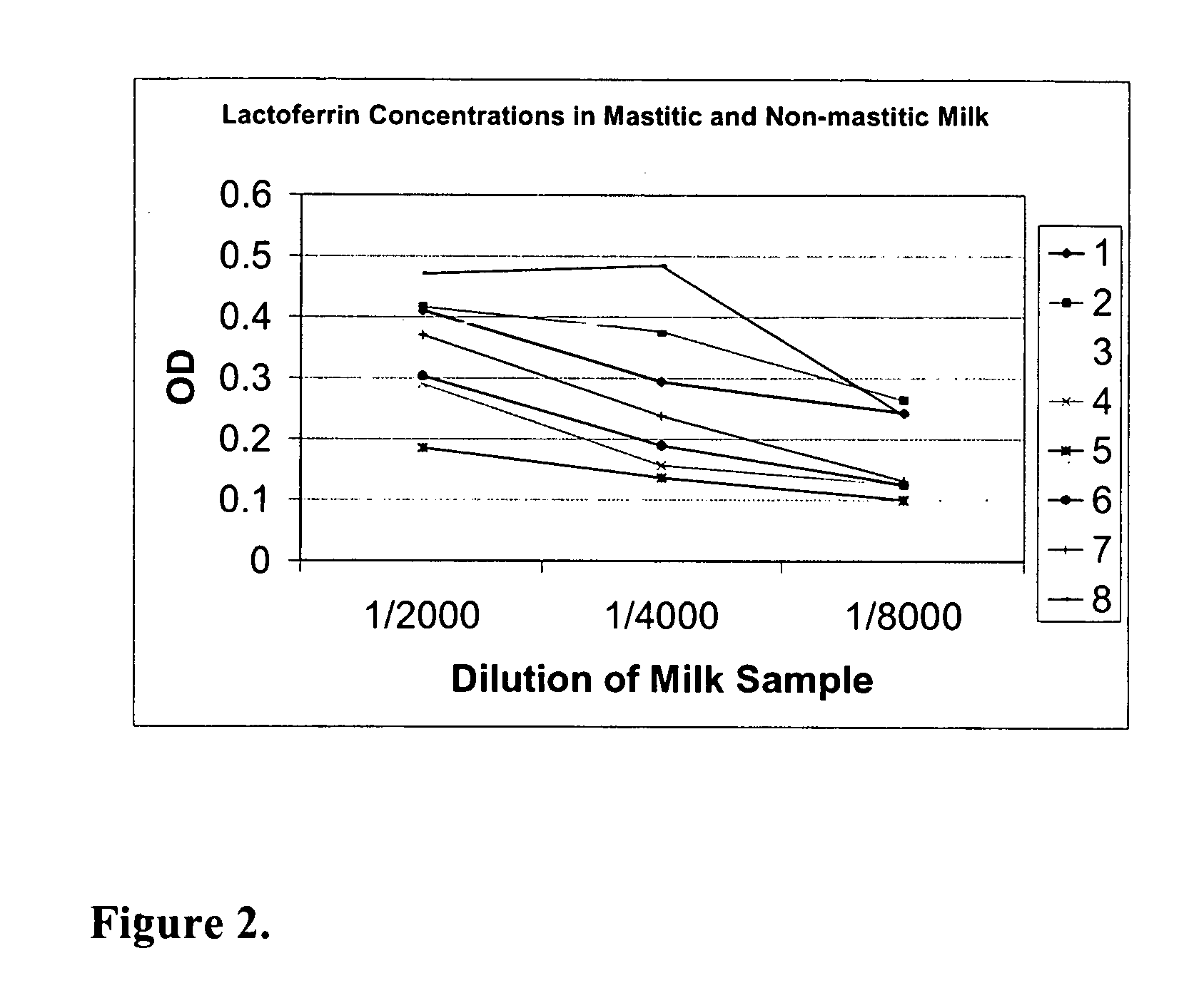
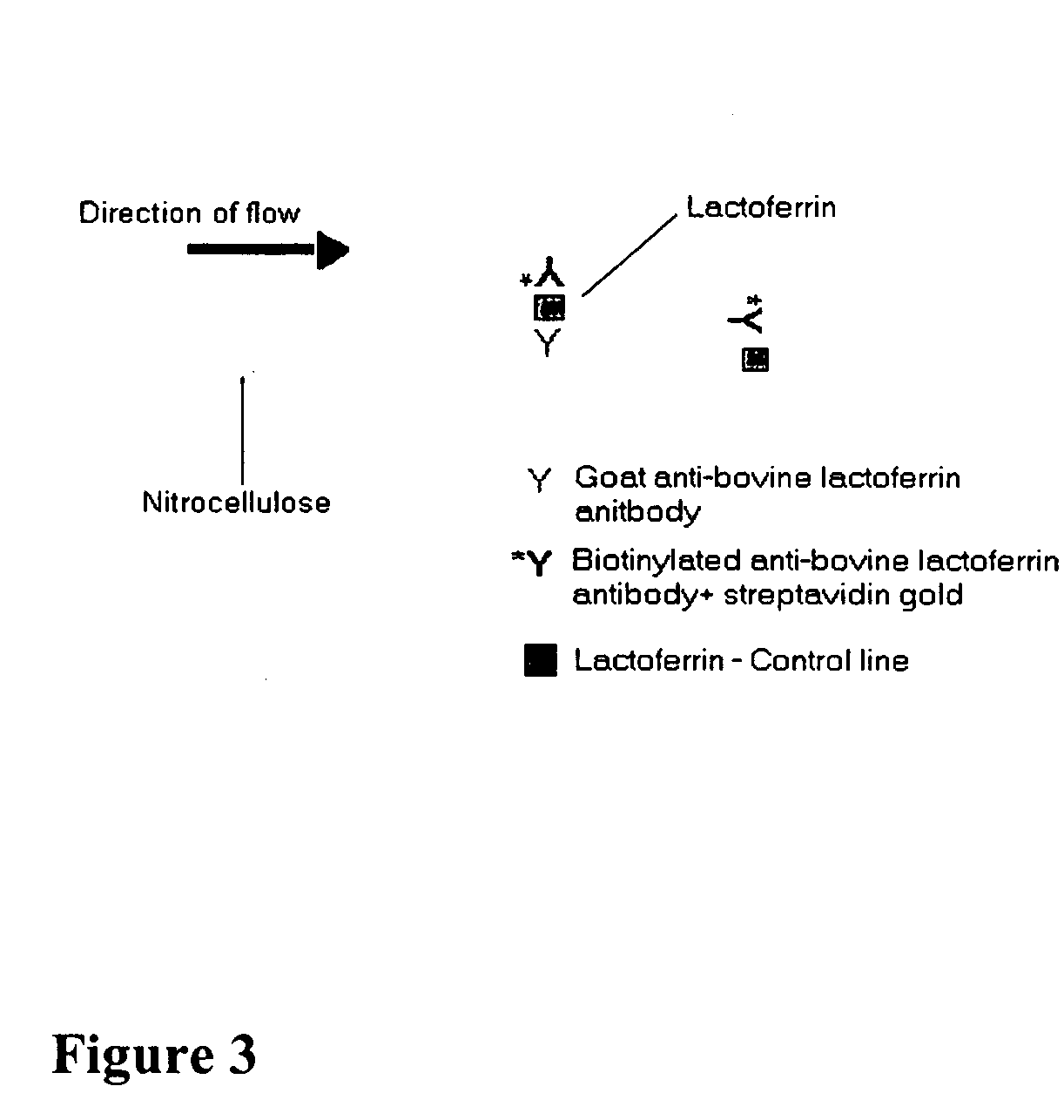
Methods, compositions, devices, and kits for detecting mastitis
InactiveUS20050260695A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMilk sampleMonoclonal antibody
The present invention includes compositions, kits and methods useful for the detection of mastitis in an animal. These agents and methods are primarily directed to a method of detecting the presence of mastitis, including sub-clinical mastitis, in cows, involving incubating a sample of milk from the cow with an agent that binds to lactoferrin such as, e.g., a monoclonal antibody specific for lactoferrin, and then detecting bound lactoferrin. The invention includes lateral-flow immunoassay methods and devices for assessing the presence of lactoferrin in milk samples.
Owner:GENPRIME
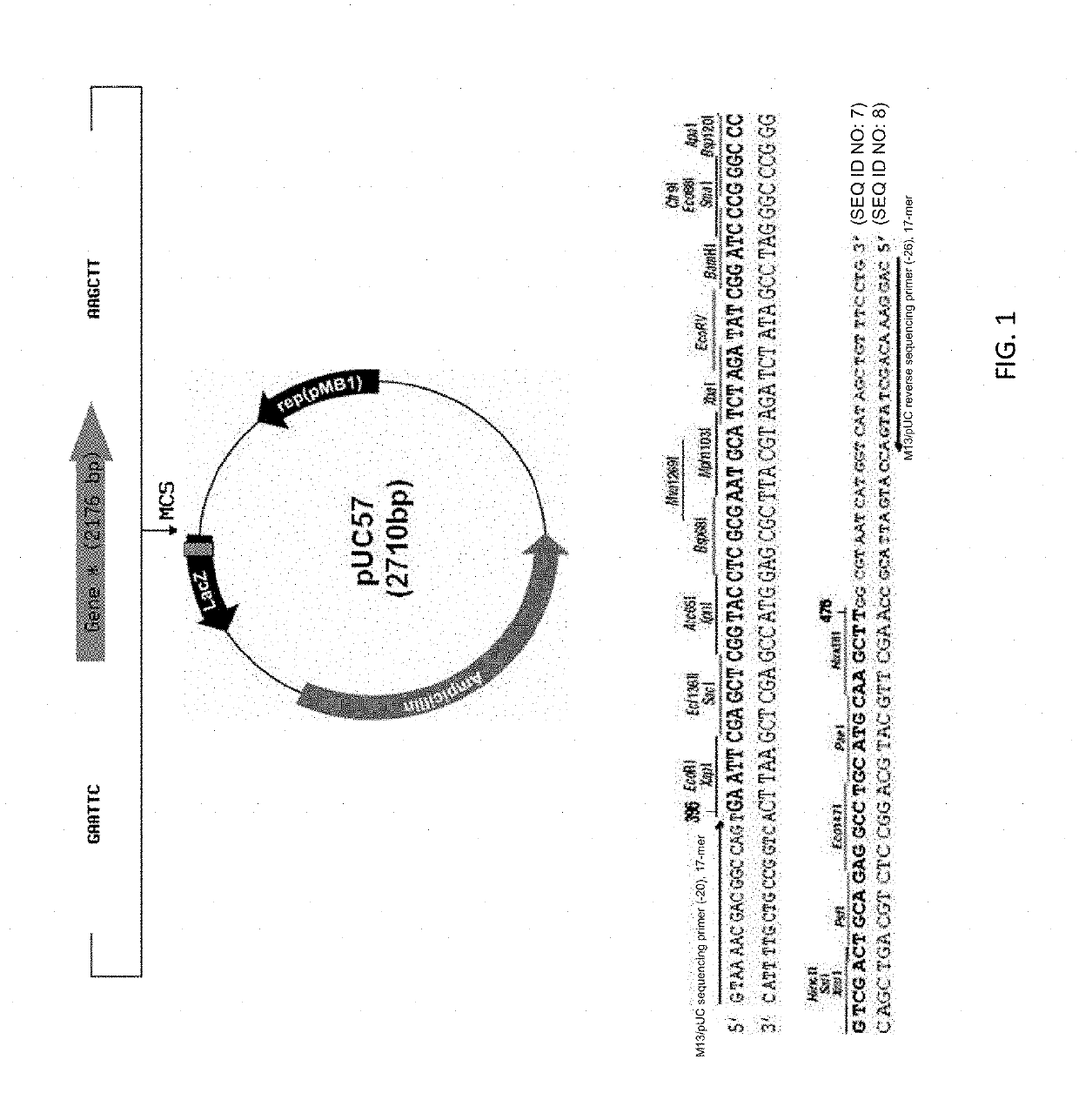
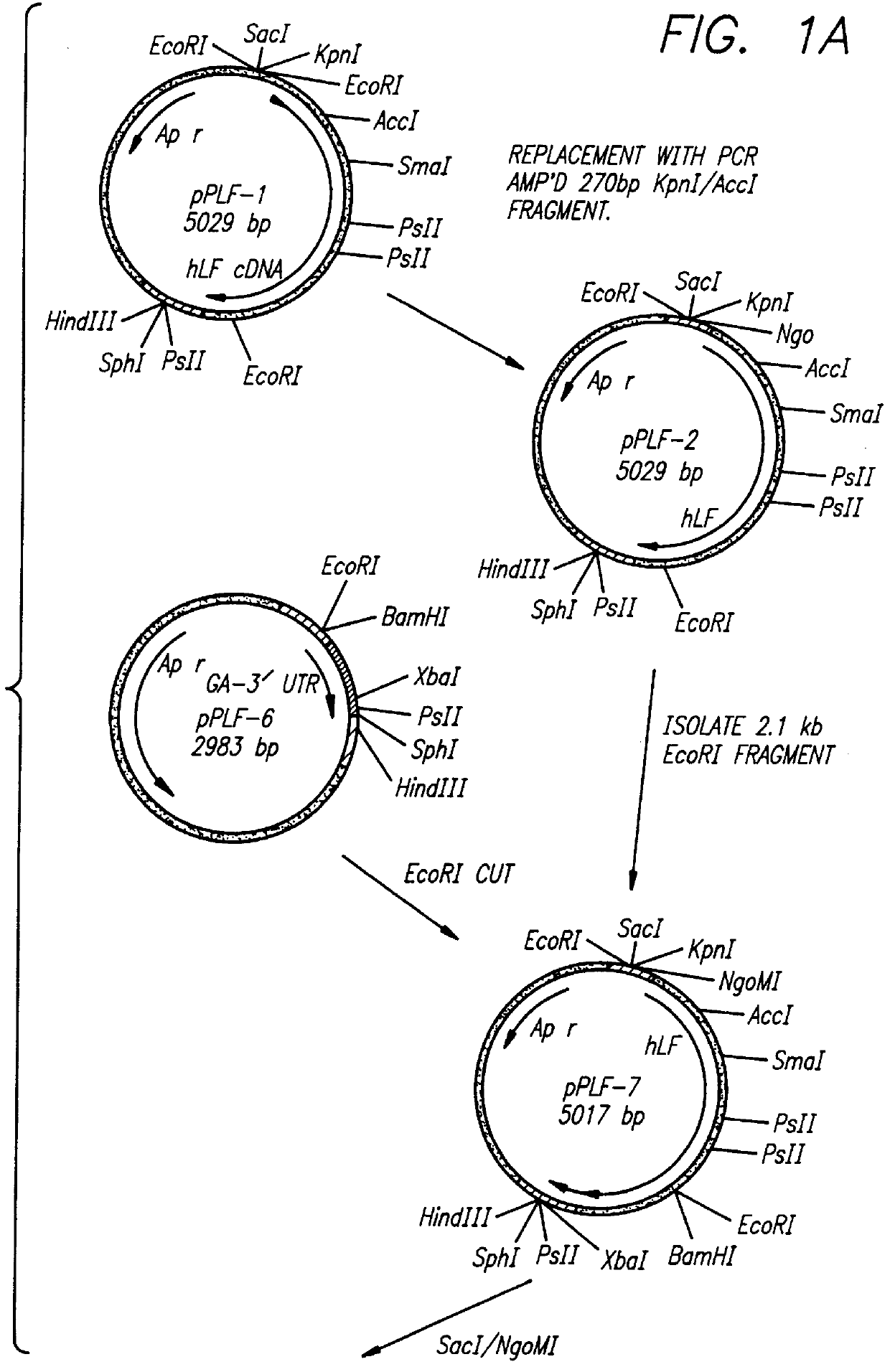
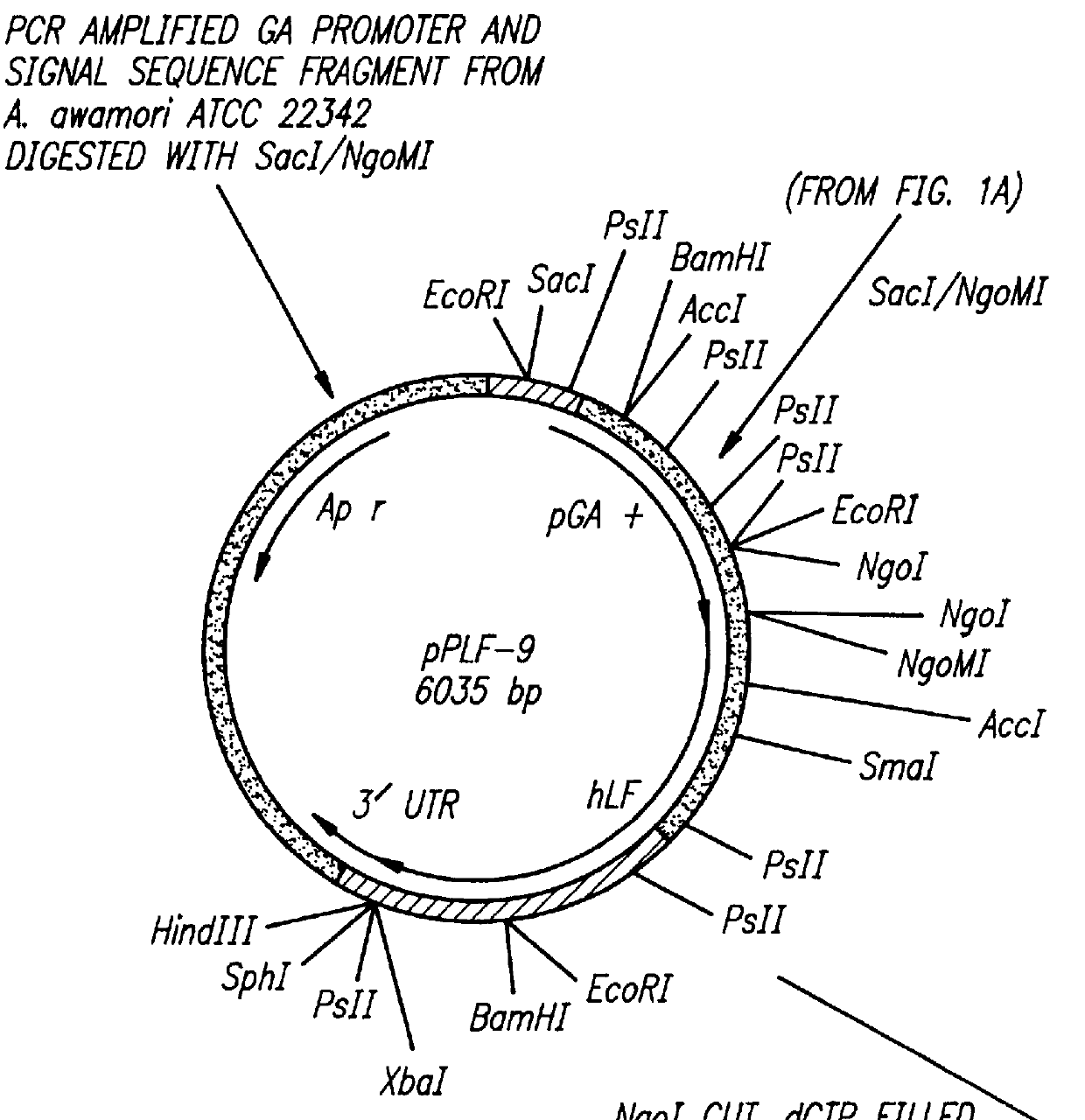
Expression of processed recombinant lactoferrin and lactoferrin polypeptide fragments from a fusion product in Aspergillus
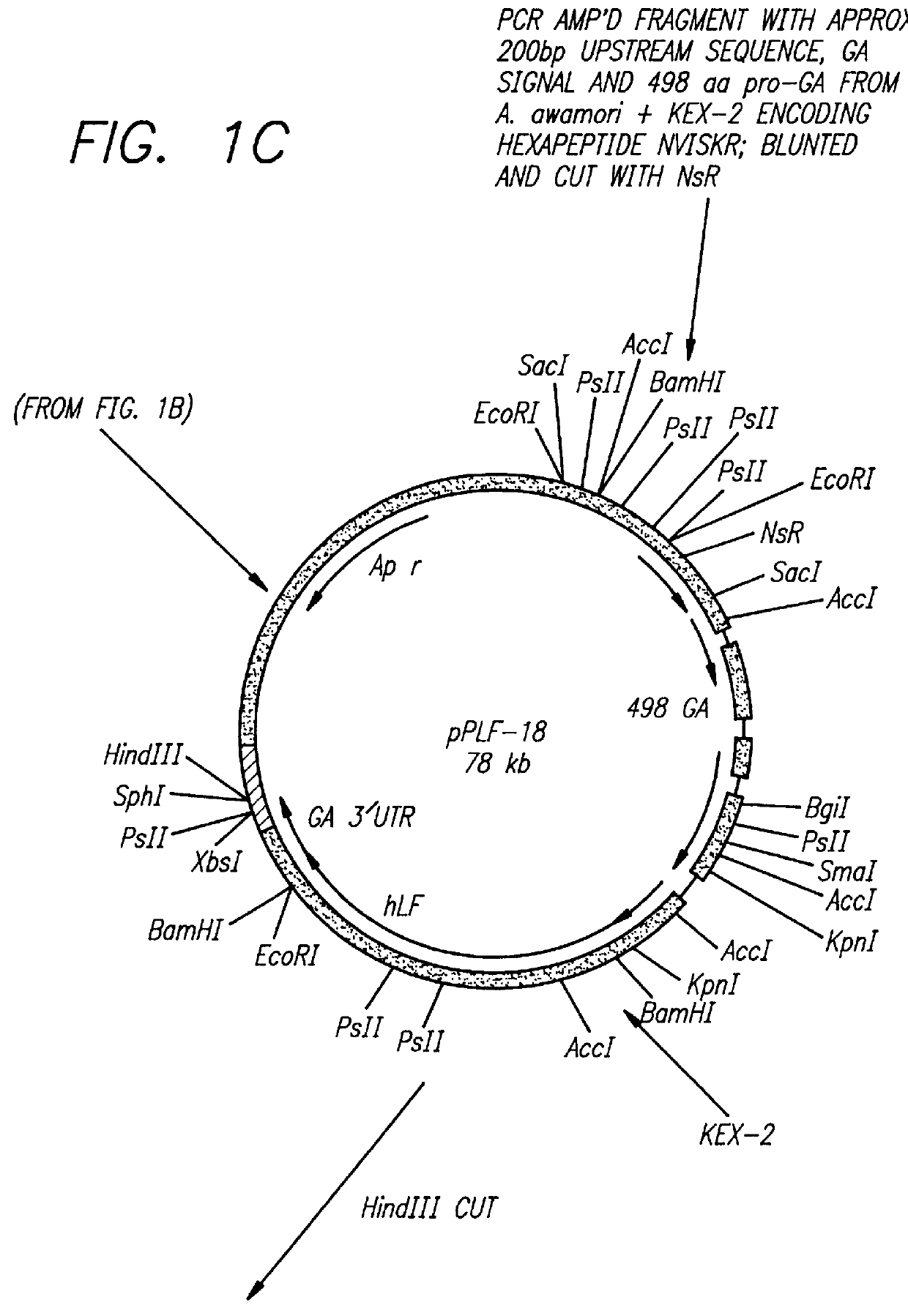
The subject invention provides for the production of lactoferrins and lactoferrin polypeptide fragments using the host cells Aspergillus in combination with novel plasmid constructs. More specifically, the subject invention provides novel vector constructs capable of producing lactoferrins and lactoferrin polypeptide fragments in Aspergillus host cells. More particularly, the subject invention provides for novel plasmid constructs suitable for use with Aspergillus and especially Aspergillus awamori, niger and oryzae host cells, which enables them to produce large amounts of recombinant lactoferrins and lactoferrin polypeptide fragments.
Owner:AGENNIX
Medicaments for healing skin conditions in humans
InactiveUS20040214750A1Short timeQuick cureBiocideCosmetic preparationsOral medicationAdditive ingredient
The present invention is directed to a novel progression of topical and oral administration or use of lactoferrin or lactoferrin and other biological ingredients to prevent and treat dermatological conditions or disorders in humans. More specifically, the present invention is directed to the administration of lactoferrin in a topical material such as an ointment or cream to the skin of humans and also including a oral administration, together the topical and oral administration has a synergetic effect in the treatment of the skin. The progression of medicaments of the present invention are: 1) lactoferrin; 2) lactoferrin and conjugated linoleic acids; 3) lactoferrin, a plant or animal derived lipid and oblepicha; 4) lactoferrin, a plant or animal derived lipid, oblepicha, and an omega fatty acid; and 5) lactoferrin, a plant or animal derived lipid, oblepicha, an omega fatty acid and a whey protein.
Owner:GEORGIADES IZOLDA M
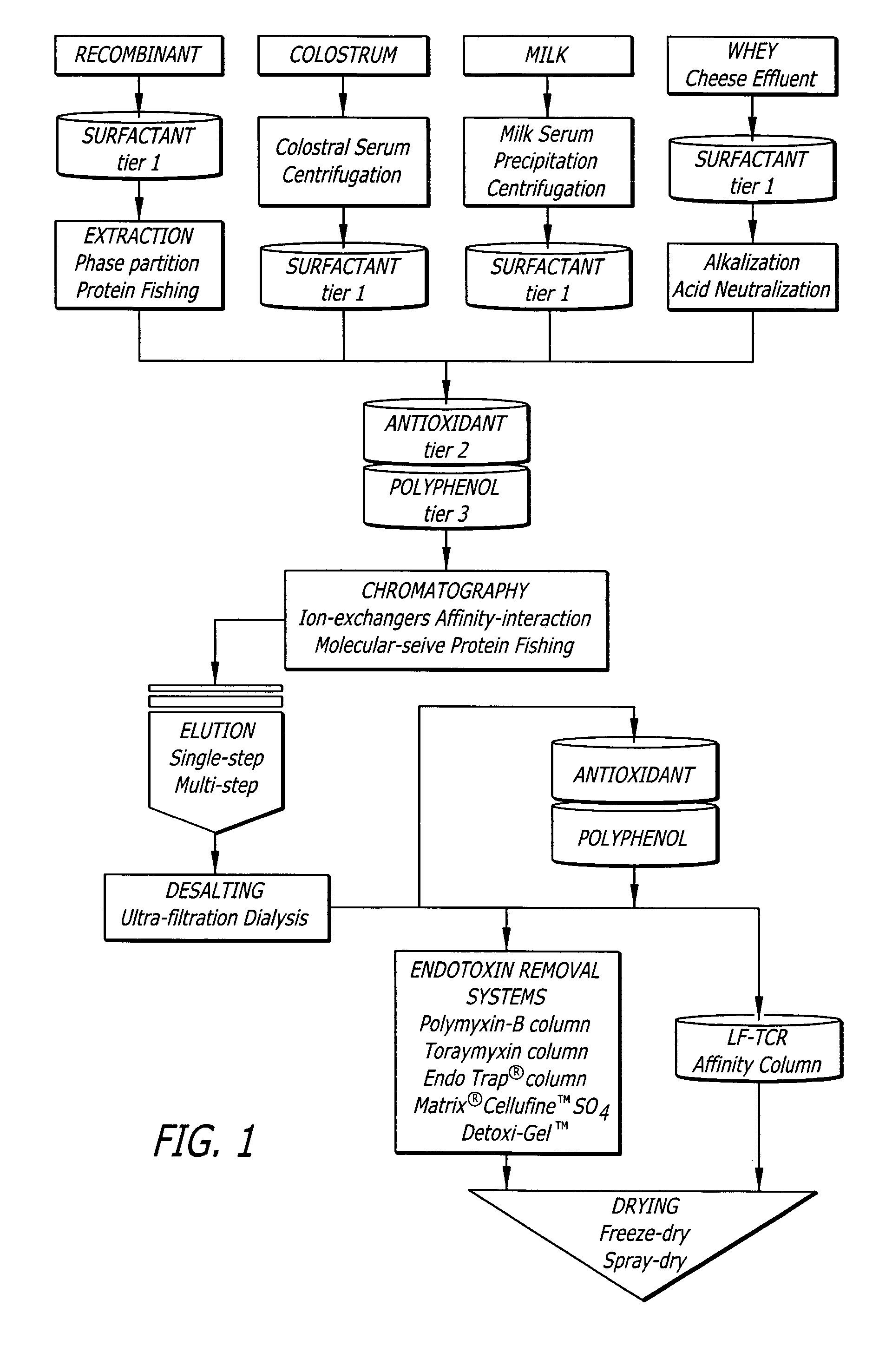
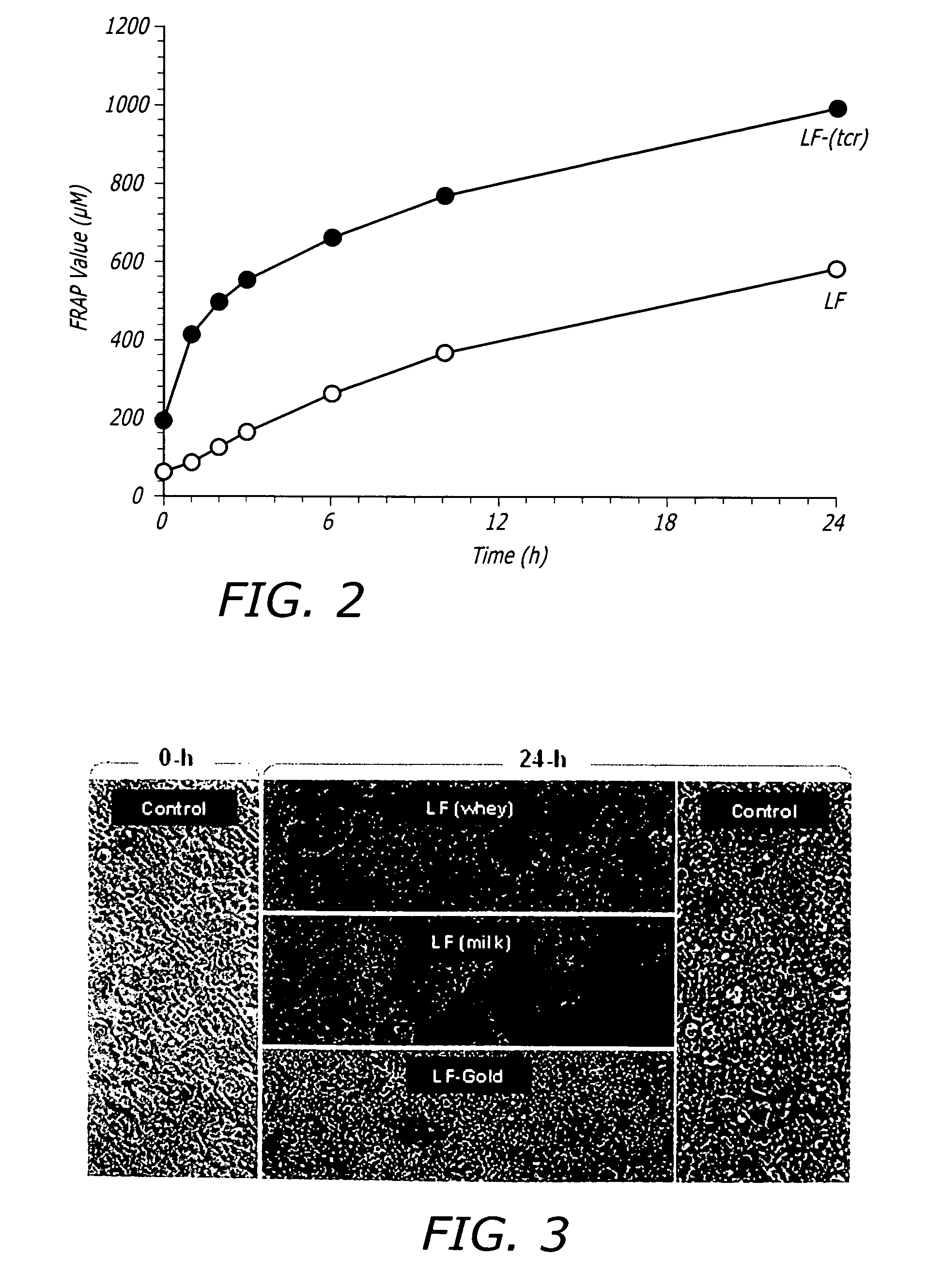
Treatments for contaminant reduction in lactoferrin preparations and lactoferrin containing compositions
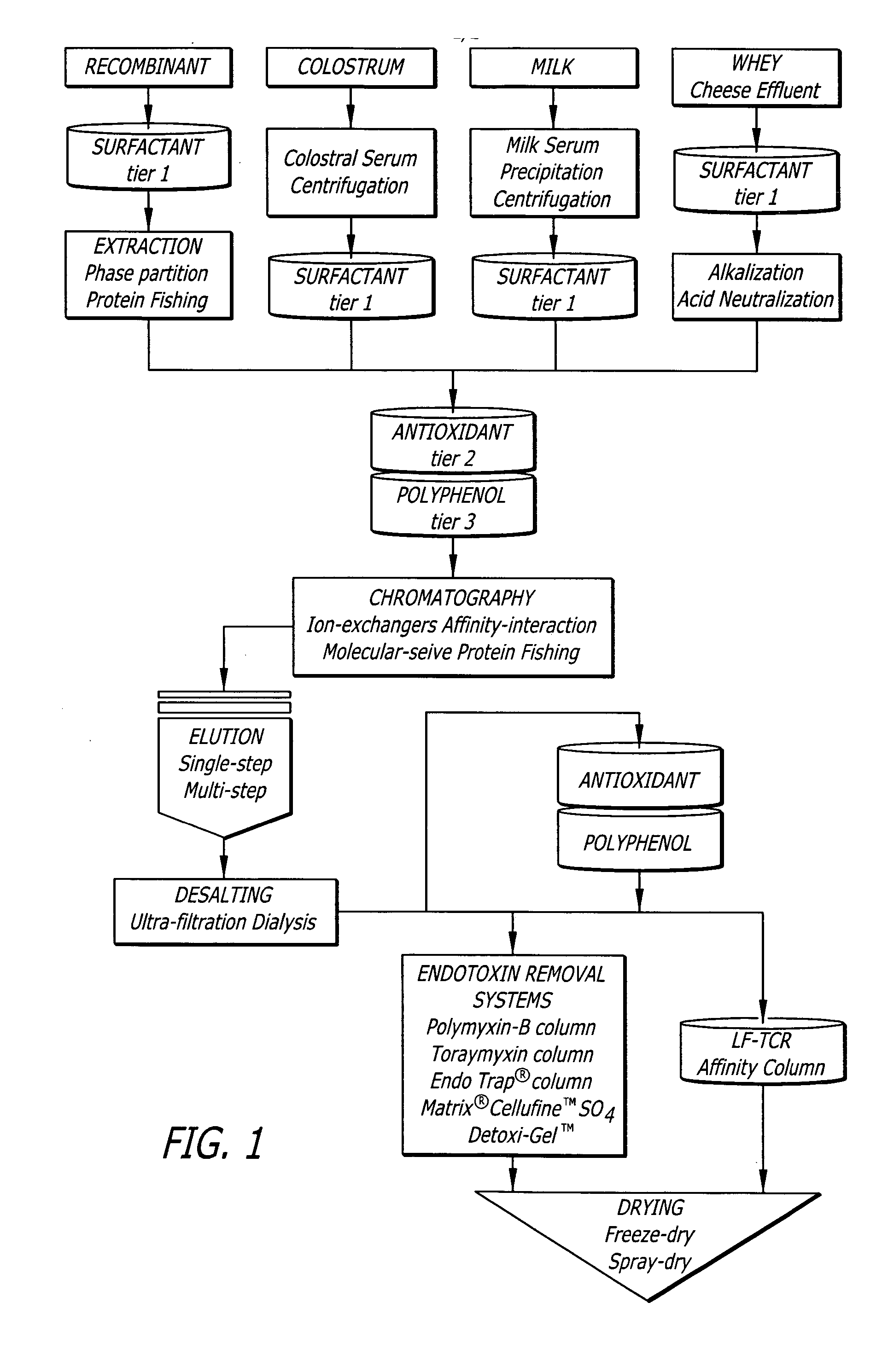
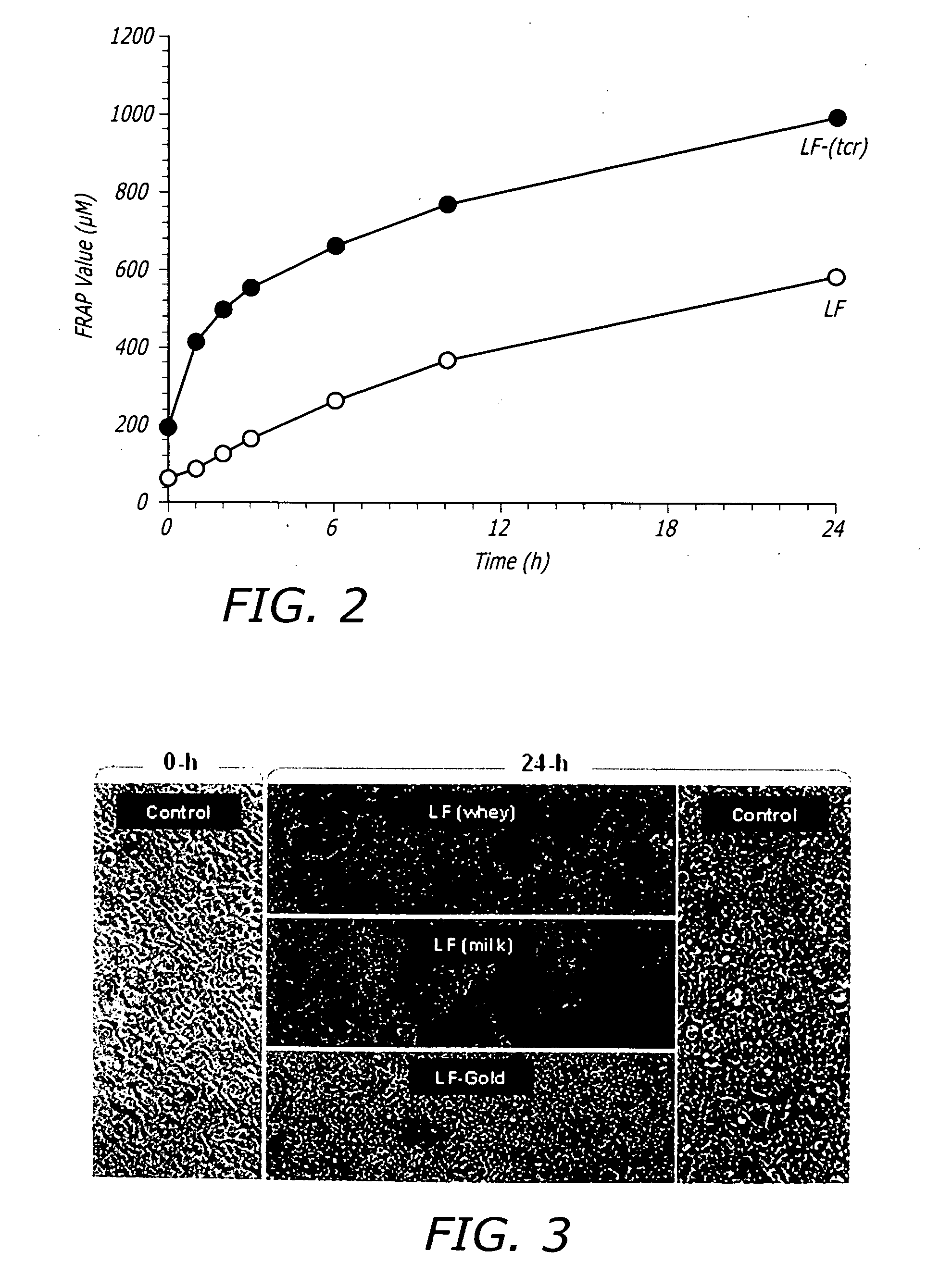
A method of preparing an ultra-cleansed lactoferrin preparation, termed treatment for contaminant reduction (TCR) is provided which includes the steps of treating commercial lactoferrin preparation with at least one each of surfactants, antioxidants and polyphenols to form purified lactoferrin (LF-TCR) and drying the LF-TCR. Additionally a therapeutic lactoferrin composition is provided which contains LF-TCR and optionally surfactants, antioxidants, polyphenols, tissue / membrane diffusion facilitating agents and anionic compounds. The therapeutic lactoferrin composition can additionally contain bioactive agents, dietary supplements, nutraceuticals / functional foods, prophylactic agents, therapeutic agents and probiotic lactic acid bacteria.
Owner:NAIDU LP
Infant nutrition immunity formula milk powder and method of preparing the same
InactiveCN101278688APromote complexationPromote absorptionMilk preparationMilk preservationFluidized bed dryingNervous system
The invention relates to an infant nutrition immunity formula powdered milk and a production method thereof, wherein, the production method of the infant nutrition immunity formula powdered milk comprises the following steps: milk which is raw material, is preprocessed, the mixed solution of docosahexaenoic acid and arachidonic acid is prepared, ingredients are mixed, preheating, homogenizing and heating are carried out for sterilizing, decompression is carried out for concentrating, and spray-drying under negative pressure and low temperature and drying on a fluidized bed are carried out, then cooling, packing and inspecting are carried out. The infant nutrition immunity formula powdered milk manufactured by the method of the invention mainly comprises casein phosphoeptide, vitamin D3, lactoferrin, nucleotide, linoleic acid, linolenic acid, the docosahexaenoic acid, the arachidonic acid, fructo-oligosaccharide and galacto-oligosaccharide, which has the advantage of reasonable trophic structure and also ensures that various nutrient substance to be absorbed and deposited to the utmost extend, so as to promote the development of brain and nervous system, improve active immunity, improve intestinal tract micro-ecological environment and strengthen the absorption of calcium, iron and zinc as well as effectively maintain every active nutrient component during the production process.
Owner:AUSTRALIA DIVINE DAIRY
Liquid state milk suitable for baby from newborn to six months old
ActiveCN101233873AGuaranteed stabilityEnsure safetyMilk preparationFood preparationVegetable oilGalactooligosaccharide
The invention relates to milk and a method for preparing thereof, in particular to fluid milk applicable for infants below six months old, which belongs to a milk technical field. The milk comprises 18.5-33 percent of fresh milk or reconstituted milk, 0.8-1.6 percent of concentrated lactoalbumin, 3.1-8.5 percent of sugar, 0.35-2.5 percent of vegetable oil, 0.01-0.6 percent of emulsifier (0.1-0.4 percent is preferable), 3-10 mg / 100g of ethylamine sulfonic acid, 1-7mg / 100 g of nucleotide, 20-30mg / 100 g of casein phospho peptides, 1-21mg / 100 g of DHA, 4-30mg / 100 g of ARA, 4-15mg / 100 g of lactoferrin, 50-70mg / 100g of GOS, 40-60mg / 100 g of FOS, 5-28mg / 100 g of choline, 5-28mg / 100 g of phaseomannite, 0.007-0.03 percent of Vitamin B complex, 0.00005-0.08 percent of complex mineral element (by elements) and soft water for the rest percentage.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Liquid state milk suitable for baby from 12 to 36 months old
ActiveCN101233874AGuaranteed stabilityEnsure safetyMilk preparationFood preparationVegetable oilGalactooligosaccharide
The invention relates to milk and a method for preparing thereof, in particular to fluid milk applicable for infants of 12-36 months old, which belongs to a milk technical field. The milk comprises 5.5-16 percent of full-cream milk powder, 0.12-3.3 percent of dried skimmed milk, 0.065-7.26 percent of sugar, 0.35-2.5 percent of vegetable oil, 0.01-0.6 percent of emulsifier, 3-10mg / 100 g of ethylamine sulfonic acid, 1-12mg / 100 g of nucleotide, 20-30mg / 100 g of casein phospho peptides, 0.3-10mg / 100g of DHA, 0.6-22mg / 100g of ARA, 4-15mg / 100g of lactoferrin, 20-130mg / 100g of GOS, 30-500mg / 100g of FOS, 5-35mg / 100g of choline, 3-28mg / 100g of phaseomannite, 0.007-0.08 percent of Vitamin B complex, 0.00005-0.08 percent of complex mineral element (by elements) and water for the rest percentage.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Milk-based nutritional compositions containing lactoferrin and uses thereof
The present disclosure relates to milk-based nutritional compositions comprising lactoferrin and / or a prebiotic component, wherein, when combined, the lactoferrin and prebiotic component may exhibit additive or synergistic beneficial effects on the health and development of a pediatric subject. The disclosure further relates to methods comprising the administration of said milk-based nutritional compositions to pediatric subjects.
Owner:MEAD JOHNSON NUTRITION
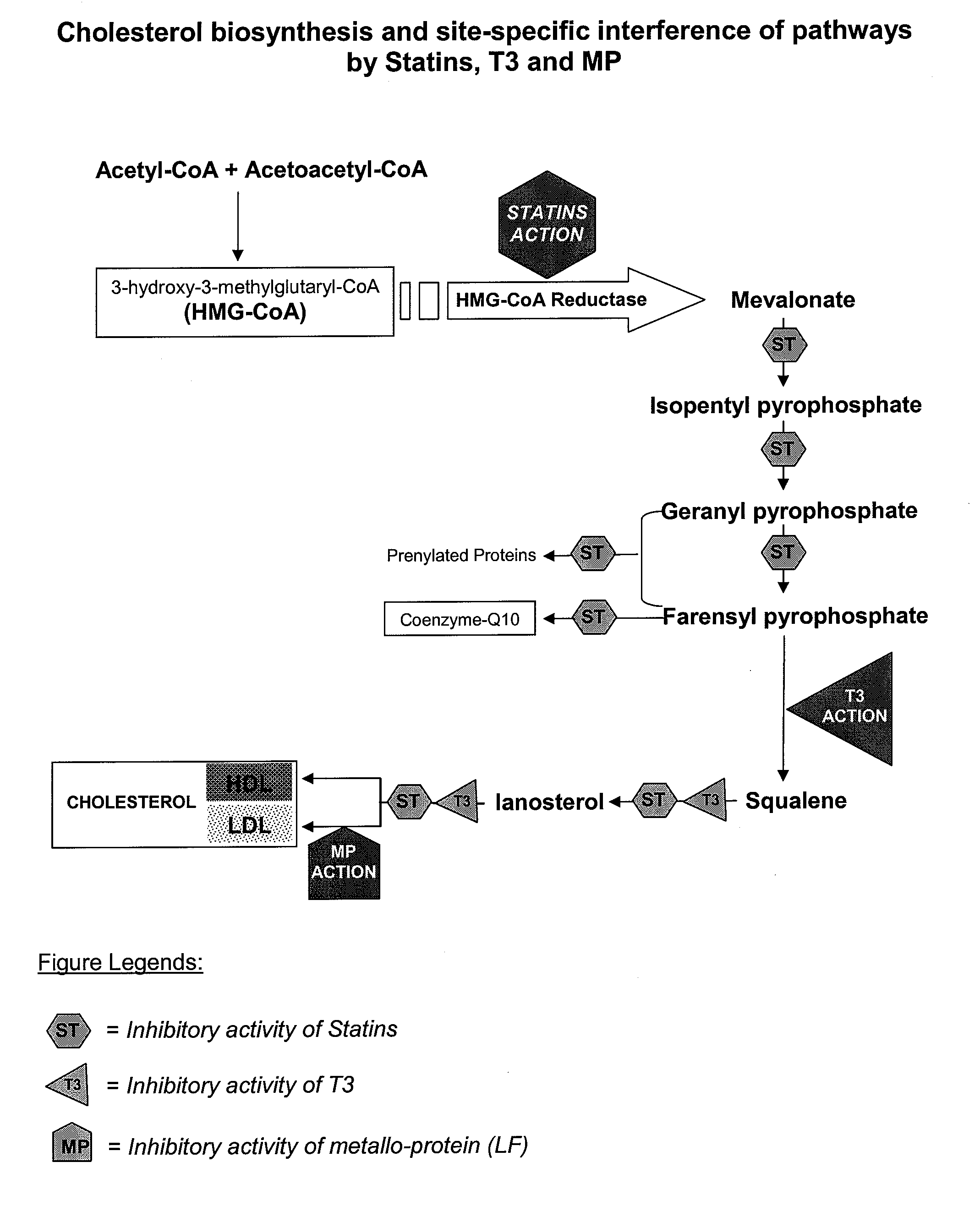
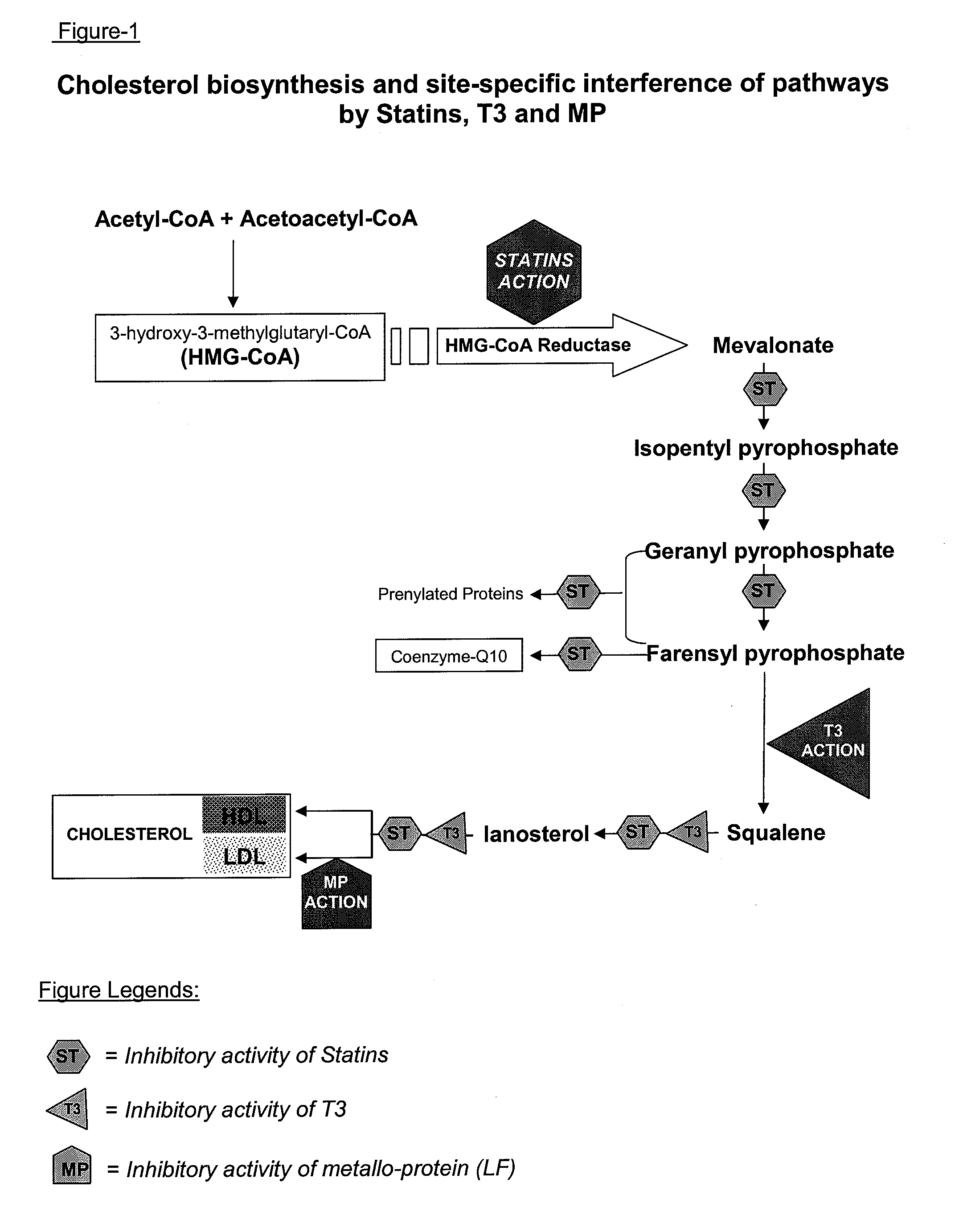
Metallo-protein and tocotrienol (mp-t3) compositions and uses thereof
Metallo-proteins including but not limited to lactoferrin (LF), transferrin (TF) and ovotransferrin (OTF) (all members of transferrin family), ceruloplasmin (CP) and metallo-thionein (MT) were found to stabilize and enhance the bio-functional activity of tocotrienol (T3), T3 mixtures or derivates. The synergism between MP and T3 also promote the intestinal transfer and the ultimate bio-availability of T3 and T3-derivatives for physiological functions. Such functional synergism includes hypocholesterolemic, anti-thrombotic, antioxidant, anti-athermogenic, anti-inflammatory and immuno-regulatory activities of T3 agents. These T3 compositions are useful as pharmaceuticals, in cosmetics, in foods and as nutritional supplements.
Owner:NAIDU LP
Novel fourth-generation infant formula and preparation method thereof
InactiveCN104351356AImprove absorption and utilizationPromote absorptionWhey manufactureNutritionAdditive ingredient
The invention provides a novel fourth-generation infant formula. The novel fourth-generation infant formula comprises the following ingredients: lactose, desalted whey powder, 1,3-dioleoyl 2-palmitoyl triglyceride, hydrolyzed whey protein, fructo-oligosaccharide, galactooligosaccharide, mineral substance premix compound, casein hydrolysate, whey protein concentrate, soyabean lecithin, vitamin premix, lactoferrin, L-tyrosine, nucleotide, L-tryptophan, L-methionine and the like. The infant formula is added with appropriate amount of 1,3-dioleoyl 2-palmitoyl triglyceride, appropriate amount of protein hydrolysate, monomer amino acid and the like, mineral contents such as phosphorus, sodium and the like in milk powder are reduced, therefore, the fat structure, the amino acid composition, the amino acid content and the osmotic pressure in the milk powder are close to those of the breast milk, the infant formula is balanced and comprehensive in nutrition and is appropriate in composition, all substances have synergistic effect, the bioavailability of protein and the fat is effectively improved, and the infant formula can promote digestive absorption and skeletal development of babies and can reduce the visceral organ burden.
Owner:AUSNUTRIA DAIRY CHINA
Infant formula milk powder added with secretory immunoglobulin A and preparation method thereof
ActiveCN101953403AMeet the needs of healthy growth and developmentRich in nutrientsWhey manufactureBiotechnologyImmunologic substance
The invention discloses infant formula milk powder added with secretory immunoglobulin A and a preparation method thereof. The infant formula milk powder consists of demineralized whey powder, defatted milk powder, mixed vegetable oil, milk sugar, mineral substance premix, lecithin, arachidonic acid, lactulose, fructooligosaccharides, oligosaccharide, water soluble vitamin premix, secretory immunoglobulin A, calcium hydroxide, citric acid, docosahexenoic acid, milk phosphatide, lactoferrin, soyabean lecithin, liposoluble vitamin premix, nucleotide and L-carnitine. The invention also discloses a method for preparing the infant formula milk powder. The infant formula milk powder has important effects of improving infant immunologic function, preventing allergy and preventing mucous membrane infection. The provided secretory immunoglobulin A fluidized bed low-temperature addition technology contributes to effectively guaranteeing the activity of immunity substances.
Owner:AUSNUTRIA DAIRY CHINA
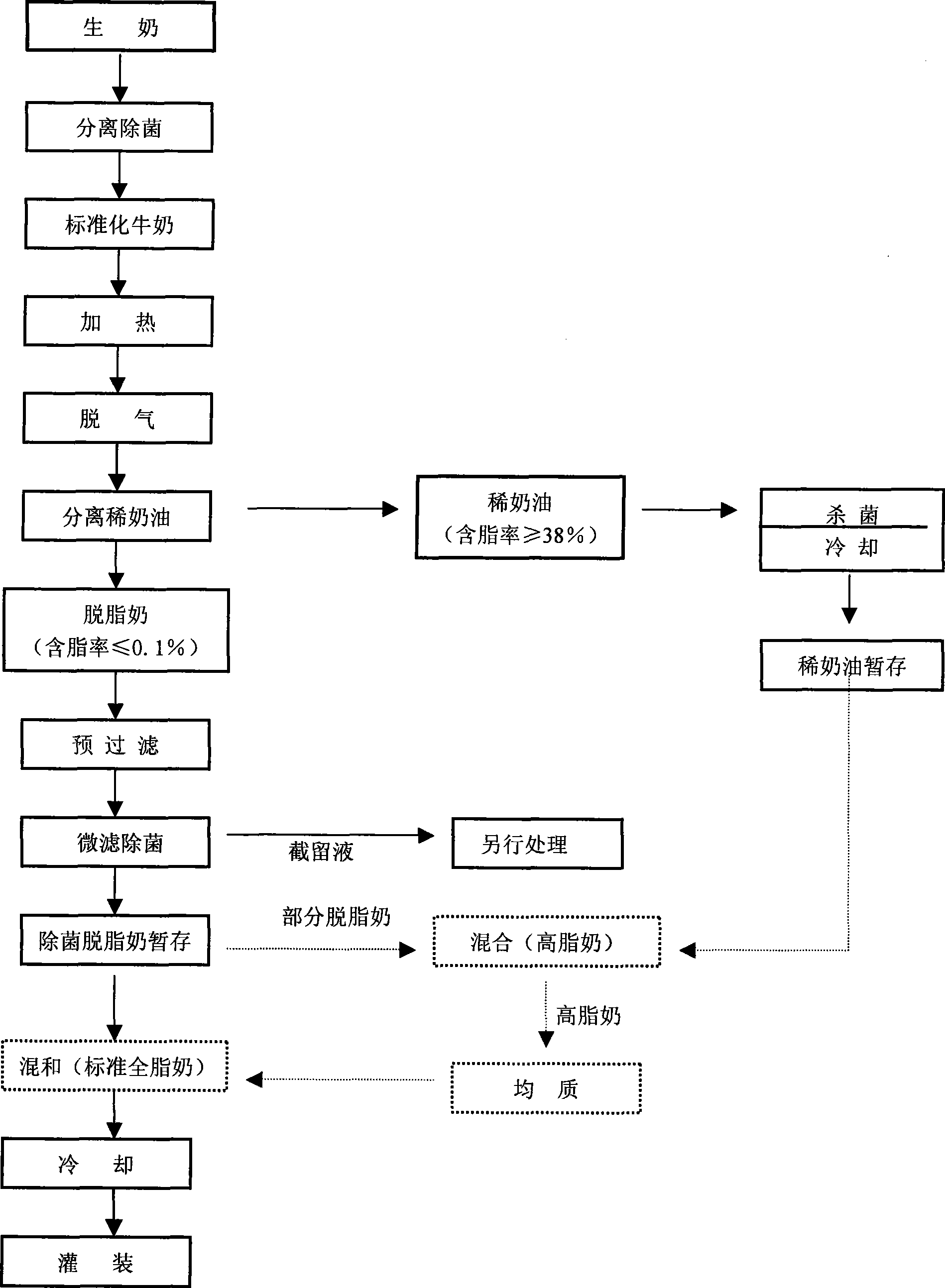
Production method of milk and milk produced by the method
Owner:BRIGHT DAIRY & FOOD
Use of lactoferrin in prophylaxis against infection and/or inflammation in immunosuppressed subjects
InactiveUS20050075277A1Reduce infectionReduce inflammationOrganic active ingredientsBiocidePharmaceutical formulationLactoferrin
The present invention relates to a use of lactoferrin in prophylaxis against infection and / or inflammation in immunosuppressed subjects or subjects whose immune systems are expected to be suppressed. Specifically, the invention provides a method of preventing infection and / or inflammation in individuals by administrating an effective amount of pharmaceutical formulation comprised of a lactoferrin product.
Owner:AGENNIX
Treatments for contaminant reduction in lactoferrin preparations and lactoferrin containing compositions
A method of preparing an ultra-cleansed lactoferrin preparation, termed treatment for contaminant reduction (TCR) is provided which includes the steps of treating commercial lactoferrin preparation with at least one each of surfactants, antioxidants and polyphenols to form purified lactoferrin (LF-TCR) and drying the LF-TCR. Additionally a therapeutic lactoferrin composition is provided which contains LF-TCR and optionally surfactants, antioxidants, polyphenols, tissue / membrane diffusion facilitating agents and anionic compounds. The therapeutic lactoferrin composition can additionally contain bioactive agents, dietary supplements, nutraceuticals / functional foods, prophylactic agents, therapeutic agents and probiotic lactic acid bacteria.
Owner:NAIDU LP
Formula milk powder assisting development of height of children and production method
The invention discloses formula milk powder assisting development of height of children. The formula milk powder consists of the following components of raw milk or whole milk powder, desalinated wheypowder, skimmed milk powder, solid corn syrup ( or maltodextrin ), whey protein powder, crystallized fructose, vegetable oil, fructo-oligosaccharide, anhydrous butter, galactooligosaccharide, docosa-hexaenoic, bovine coloctrum powder, milk mineral salt, hydrolyzed dried egg yolk, lactoferrin, phosphatide, vitamin A, vitamin D, vitamin E, vitamin K, vitamin B1, vitamin B2, vitamin B6, vitamin B12,vitamin C, magnesium, calcium, iron, zinc, selenium and the like. According to the formula milk powder assisting the development of the height of the children and a production method disclosed by theinvention, the products have the characteristics of ''supplementing, promoting and improving'', namely supplementing nutrition required by growth and development of Chinese children, promoting absorption of key nutrition for the development of the height, and improving immunity of the children, so that the height of the Chinese children is assisted to quickly grow.
Owner:上海育博营养食品有限公司
Amino acid humanized infant formula milk powder and preparation method thereof
ActiveCN104012657APromote absorptionPromote growth and developmentMilk preparationVegetable oilTyrosine
The present invention relates to amino acid humanized infant formula milk powder and a preparation method thereof. The amino acid humanized infant formula milk powder comprises the following materials by a certain weight percent: lactose, demineralised whey powder, vegetable oil, skimmed milk powder, hydrolyzed whey protein, galactooligosaccharides, fructo oligosaccharide, a mineral premix, ARA, casein hydrolysate, whey protein concentrate, soybean phospholipids, DHA, a water-soluble vitamin premix, casein phosphopeptides, lactoferrin, L-tyrosine, nucleotide, a lipid soluble vitamin premix, L-tryptophan, L-methionine and taurine. The present invention also includes a method for preparing the amino acid humanized infant formula milk powder. The present invention can effectively solve the problem of pool protein absorption and digestion effect and the low protein utilization rate of infants during infant period.
Owner:AUSNUTRIA DAIRY CHINA
Prostaglandin f2alpha analogs and their use in combination with antimicrobial proteins for the treatment of glaucoma and intraocular hypertension
InactiveUS20060264353A1High indexReduce morbidityAntibacterial agentsBiocideProstaglandin analogPreservative
An ophthalmic formulation for the treatment of glaucoma and intraocular pressure with a prostaglandin compound of the F-series (PGF), and particularly a prodrug form of a PGF2α analog, such as an ester, amide, or internal lactone, wherein preservative is an antimicrobial peptide, such as lactoferrin. In particularly preferred embodiments, the prostaglandin compound is a macrocyclic internal 1,15-lactone, such as the 16-aryloxy prostaglandin analogs, illustratively fluprostenol or cloprstenol.
Owner:CAYMAN CHEMICAL COMPANY
Lactoferrin as an adjuvant in cancer vaccines
The present invention relates to methods of treating cancer by administering a composition of lactoferrin (LF) in combination with cancer vaccines.
Owner:AGENNIX
Nutritional compositions containing synergistic combination and uses thereof
A composition and method for enhancing brain development in a pediatric subject, the method including administering to the pediatric subject a nutritional composition having up to about 7 g / 100 kcal of a fat or lipid source, wherein the fat or lipid source includes at least about 0.5 mg / 100 kcal of milk or non-milk polar lipids; up to about 5 g / 100 kcal of a protein source; at least about 15 mg / 100 kcal of lactoferrin from a non-human source; about 0.015 g / 100 kcal to about 0.15 g / 100 kcal of a prebiotic composition including polydextrose and / or galactooligosaccharide; and at least about 5 mg / 100 kcal of a source of long chain polyunsaturated fatty acids.
Owner:MEAD JOHNSON NUTRITION
Composition for preparing camel milk facial mask and preparation method thereof
ActiveCN105106098ARetain propertiesRetain the advantageCosmetic preparationsToilet preparationsCuticleL glutamate
The invention discloses a composition for preparing a camel milk facial mask, and particularly a composition for preparing a camel milk moisturizing skin-calming facial mask, a camel milk moisturizing clear facial mask, a camel milk moisturizing compact facial mask and a camel milk moisturizing conditioning facial mask according to functions. By virtue of the synergism of camel milk, water, sodium carbomer, glycerinum, xanthan gum, butanediol, sodium hyaluronate, poly(sodium L-glutamate), skin conditioner and preservative, the characteristics and advantages of the camel milk are maximally maintained, so that the composition for preparing the cameral milk facial mask has the advantages of the camel milk, namely, proteins, vitamins, minerals and fatty acid rich in the composition can provide rich nutrient substances for the skin and can improve the face skin; particularly, the camel milk contains lactoferrin and lysozyme which are short in cow milk, so that the composition has the effects of sterilizing, killing demodex, removing toxicity, softening stratum corneum epidermidis, moisturizing and tendering the skin, preventing wrinkles and aging resistance, and delaying senescence.
Owner:新疆旺源生物科技集团有限公司
Infant formula goat milk powder and preparation method thereof
The invention discloses infant formula goat milk powder and a preparation method thereof. Alpha-lactalbumin and hydrolytic lactalbumin peptide, malt sugar (corn syrup), glucose, pure colostrum powder, lactoferrin, living bifidobacterium and the like are added in the infant formula goat milk powder. The method comprises the steps of: obtaining goat milk, performing pasteurization (85 DEG C), cutting material mixing tanks with high speed (the material in the tank includes malt sugar, glucose, decavitamin, mineral nutrient, hydrolytic lactalbumin peptide and plant oil), using a homogenizer, performing pasteurization, vacuum concentration and spray drying, cooling a fluid bed (adding the lactoferrin, the pure colostrum powder IgG and the plant oil), mixing the added materials (adding the lactoferrin, the pure colostrum powder IgG and the living bifidobacterium), and canning. The infant formula goat milk powder is easy to digest and absorb, and muting sensitive; and the formula powder is more humanized, and can enhance organism immunity.
Owner:BAOJI ANEED NUTRITION DAIRY
Use of lactoperoxidase, a peroxide donor and thiocyanate for the manufacture of a medicament for treating Helicobacter pylori infection
InactiveUS6149908AHigh frequencyKeep for a long timeAntibacterial agentsMilk preparationMedicineIn vivo
PCT No. PCT / SE97 / 00098 Sec. 371 Date Jul. 22, 1998 Sec. 102(e) Date Jul. 22, 1998 PCT Filed Jan. 22, 1997 PCT Pub. No. WO97 / 26908 PCT Pub. Date Jul. 31, 1997Use of an antibacterial system comprising lactoperoxidase and a peroxide donor for preparing a preparation for prophylactic or therapeutic treatment "in vivo" of an infection caused by the bacteria Helicobacter pylori existing in the stomach, which preparation is completed by the presence of thiocyanate in an antibacterial level, and eventually in the presence of lactoferrin. A daily dose for human treatment is 1.2-1.6 grams of the system taken 3 times a day.
Owner:MJOLKKANNAN FORVALTNING
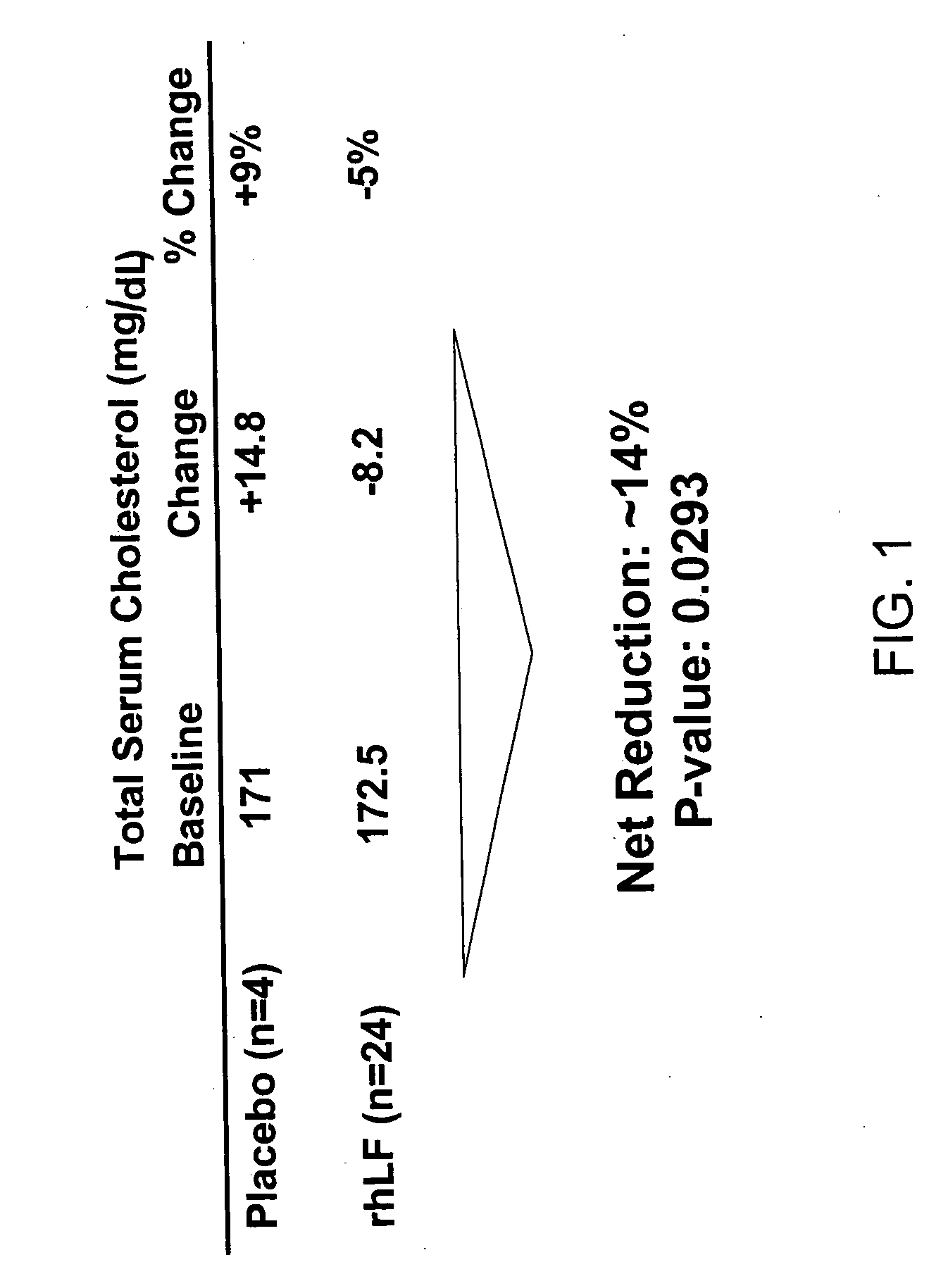
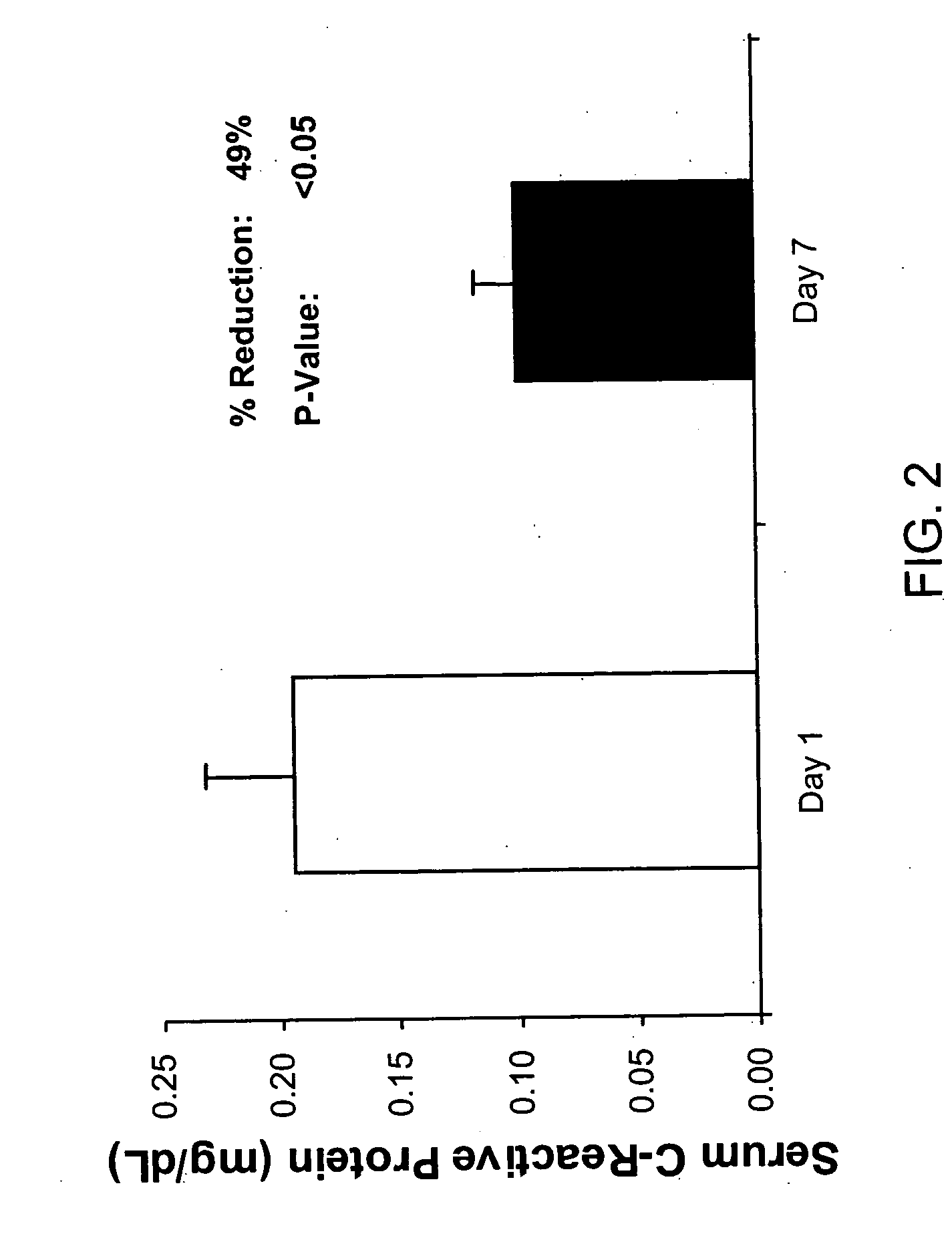
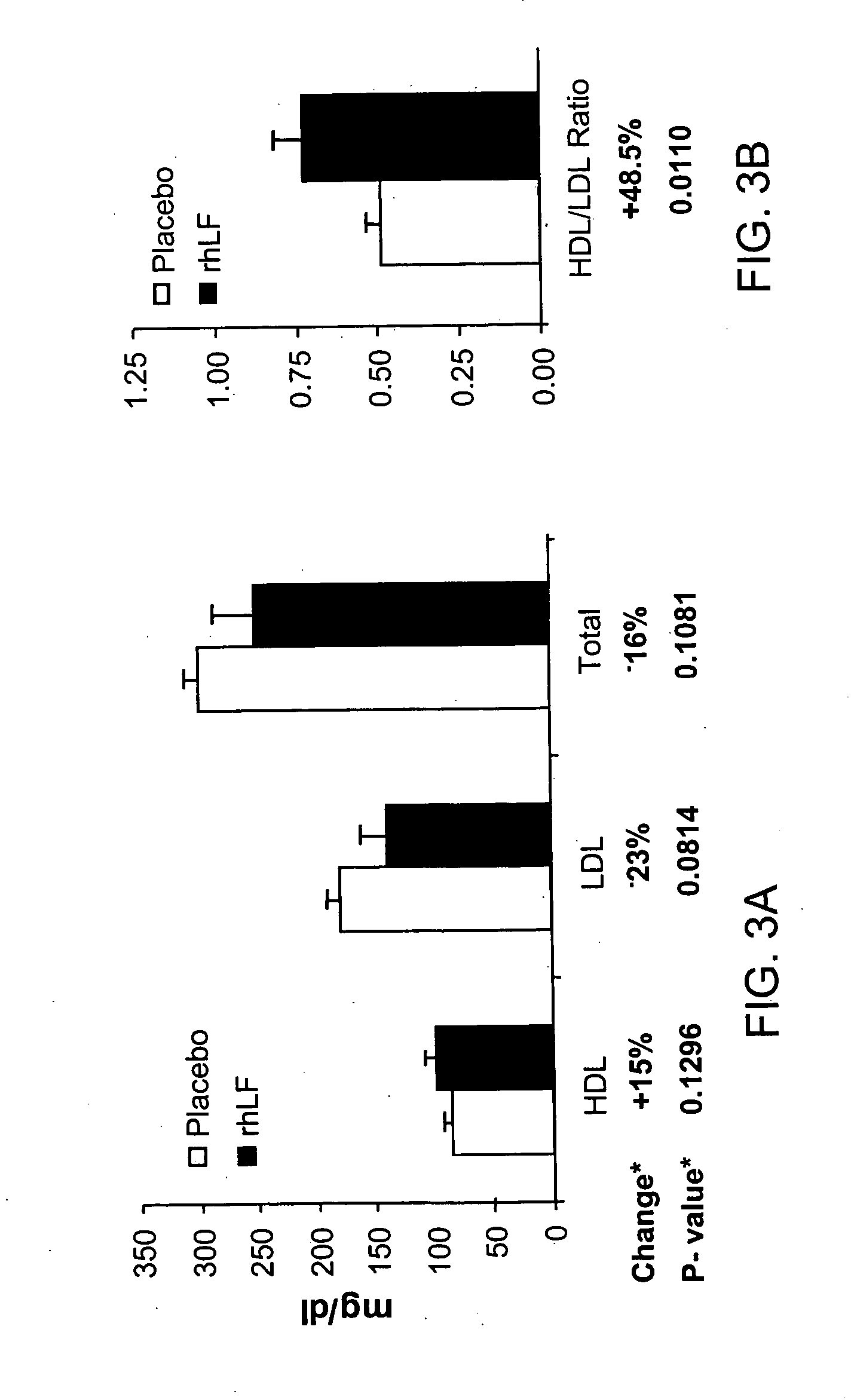
Lactoferrin in the reduction of circulating cholesterol, vascular inflammation, atherosclerosis and cardiovascular disease
The present invention relates to methods of using lactoferrin (LF) to reduce circulating levels of cholesterol and vascular inflammation, in order to treat, prevent or reduce the incidence of atherosclerosis and cardiovascular disease.
Owner:VARADHACHARY ATUL +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com