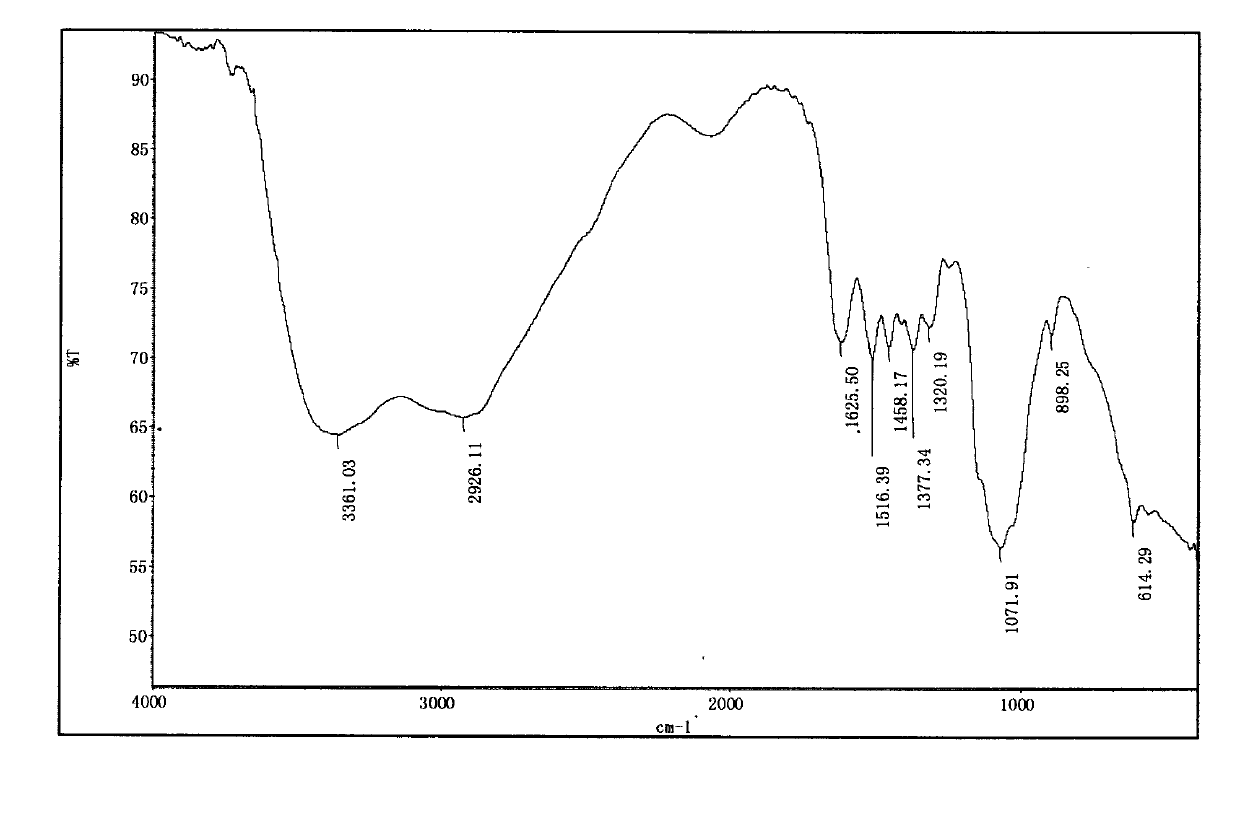
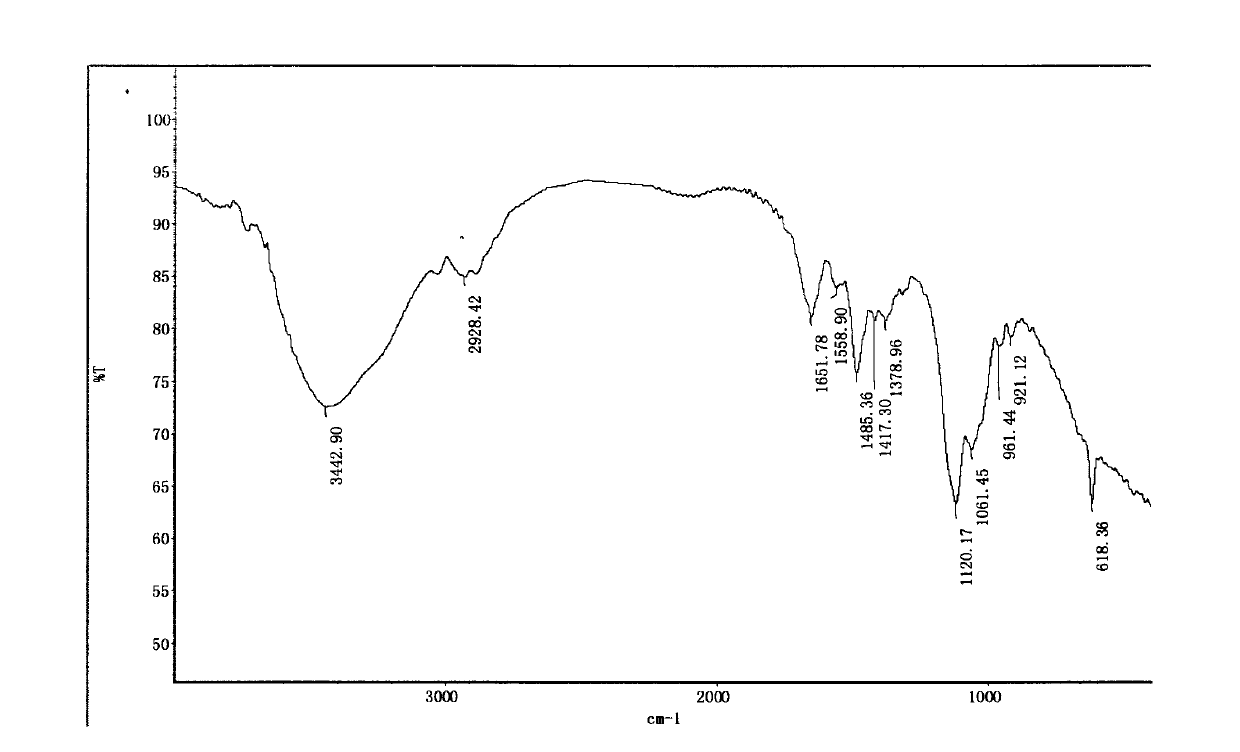
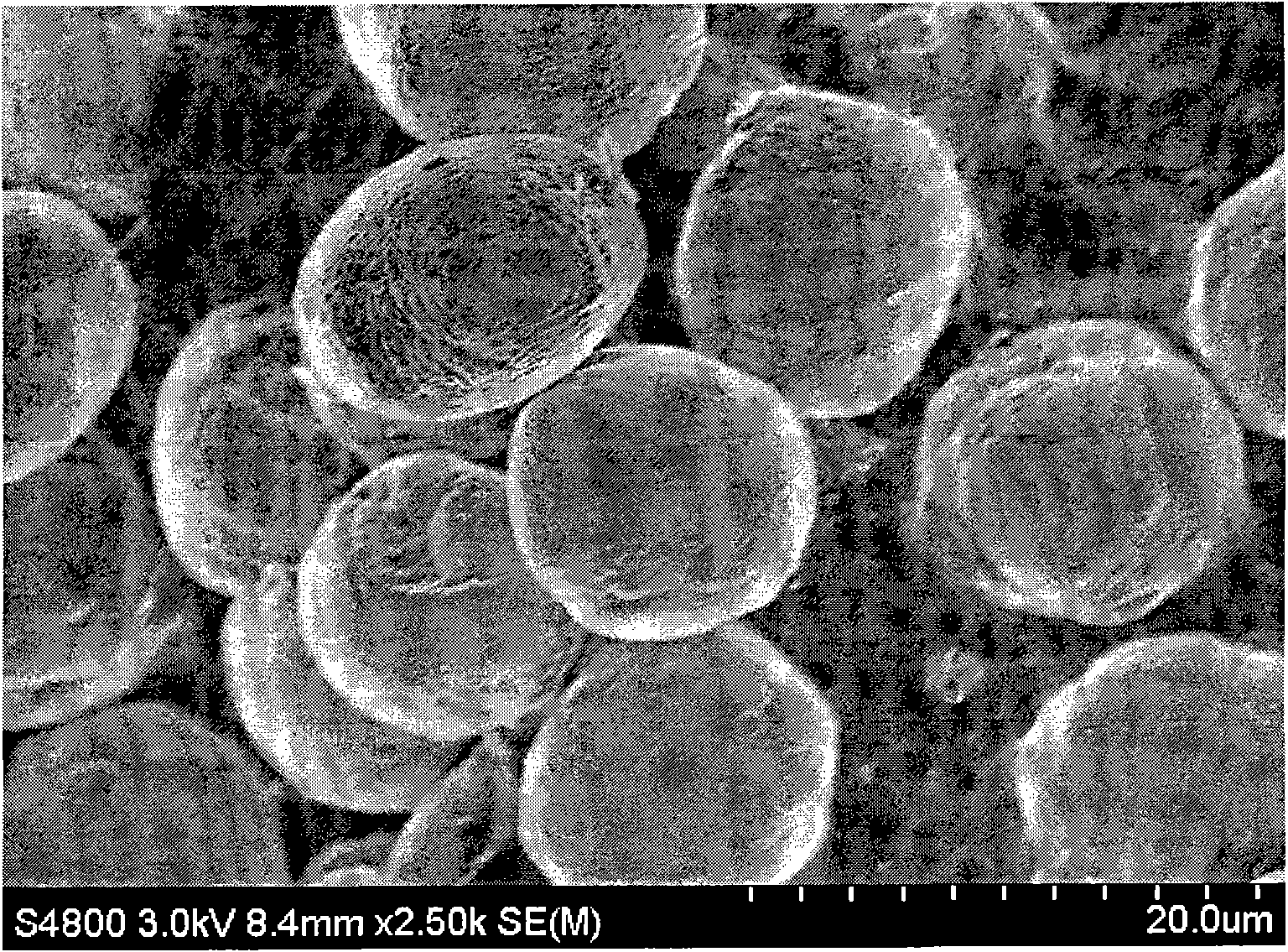
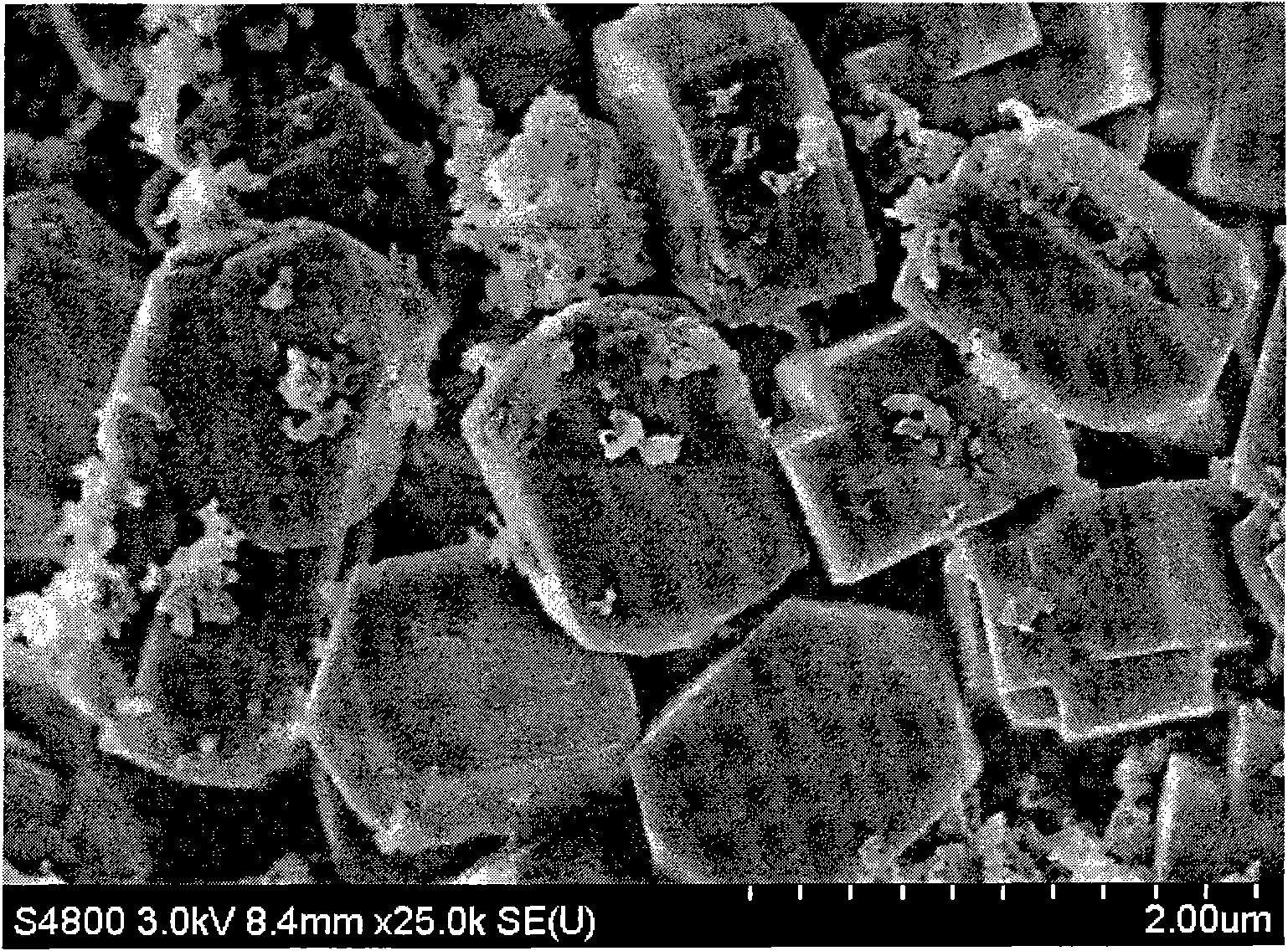
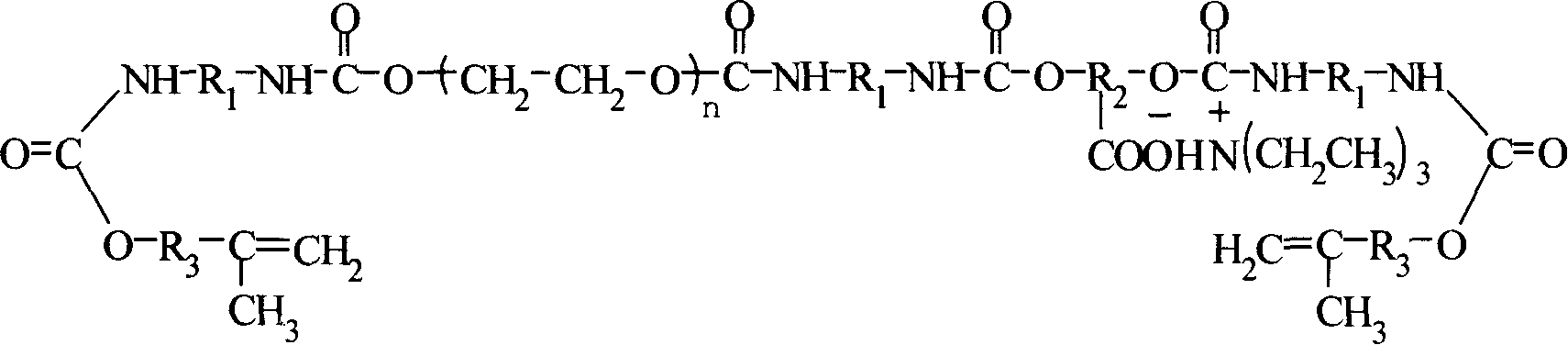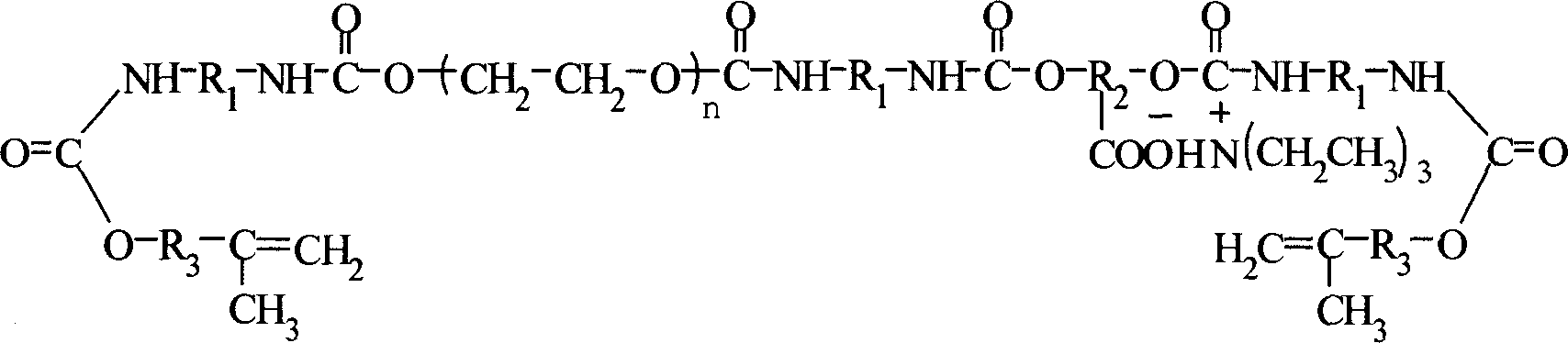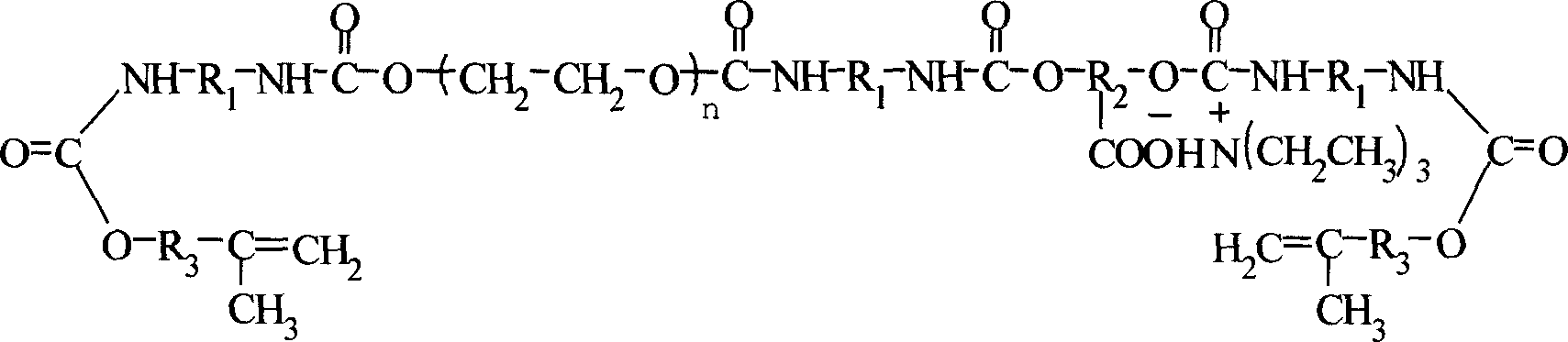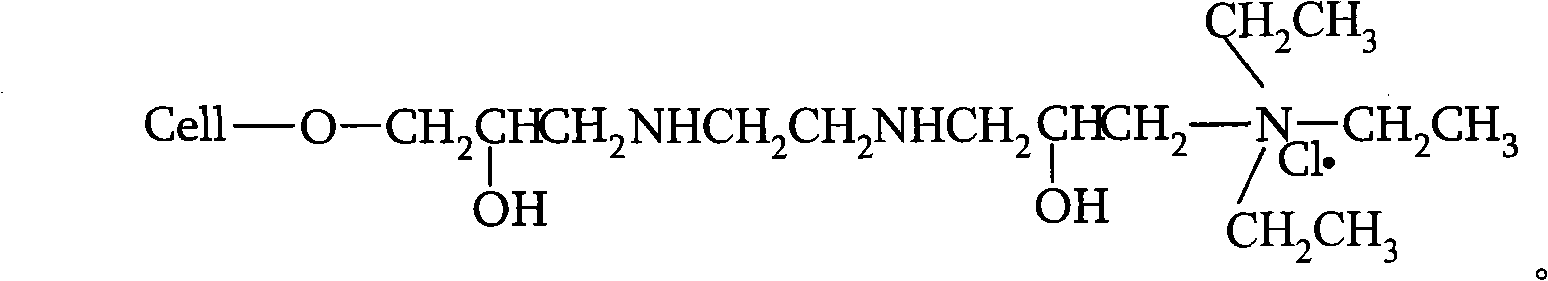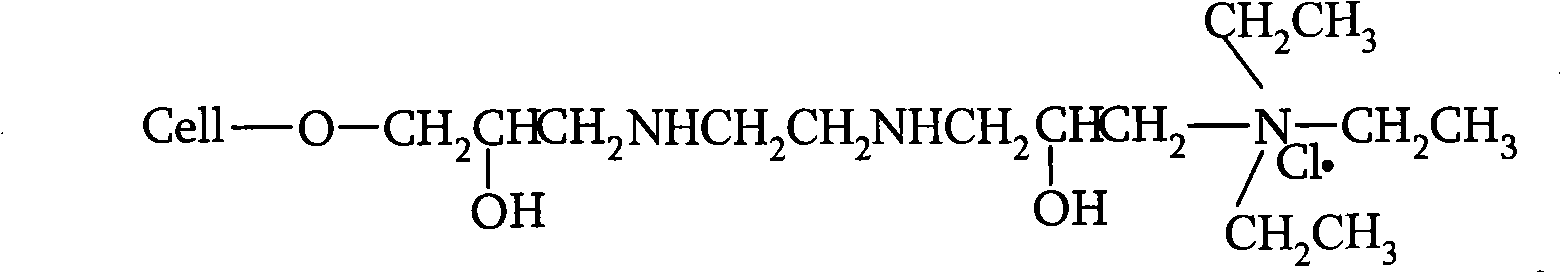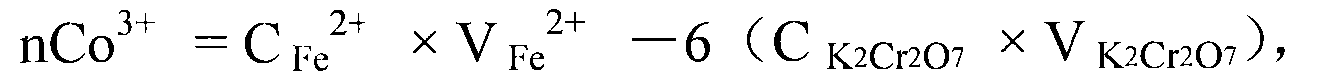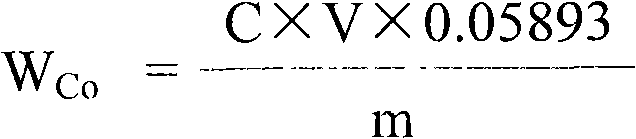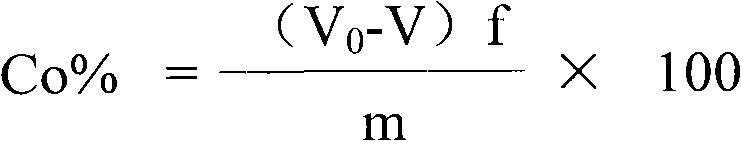Patents
Literature
679 results about "Ethylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethylamine is an organic compound with the formula CH₃CH₂NH₂. This colourless gas has a strong ammonia-like odor. It is miscible with virtually all solvents. It is a nucleophilic base, as is typical for amines. Ethylamine is widely used in chemical industry and organic synthesis.
Composition Containing Statins and Omega-3 Fatty Acids
InactiveUS20080089876A1Hydroxy compound active ingredientsPeptide/protein ingredientsFatty acidStatine
A combination is described comprising at least one omega-3 fatty acid, optionally esterified or salified, at least one statin, Coenzyme Q10, resveratrol, at least one policosanol, pantethine, selenium, and zinc. This combination is endowed with a synergistic effect and is useful in the treatment of disease forms due to insulin resistance and in cardiovascular diseases.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Preparation method of carbon dot having high fluorescent quantum yield
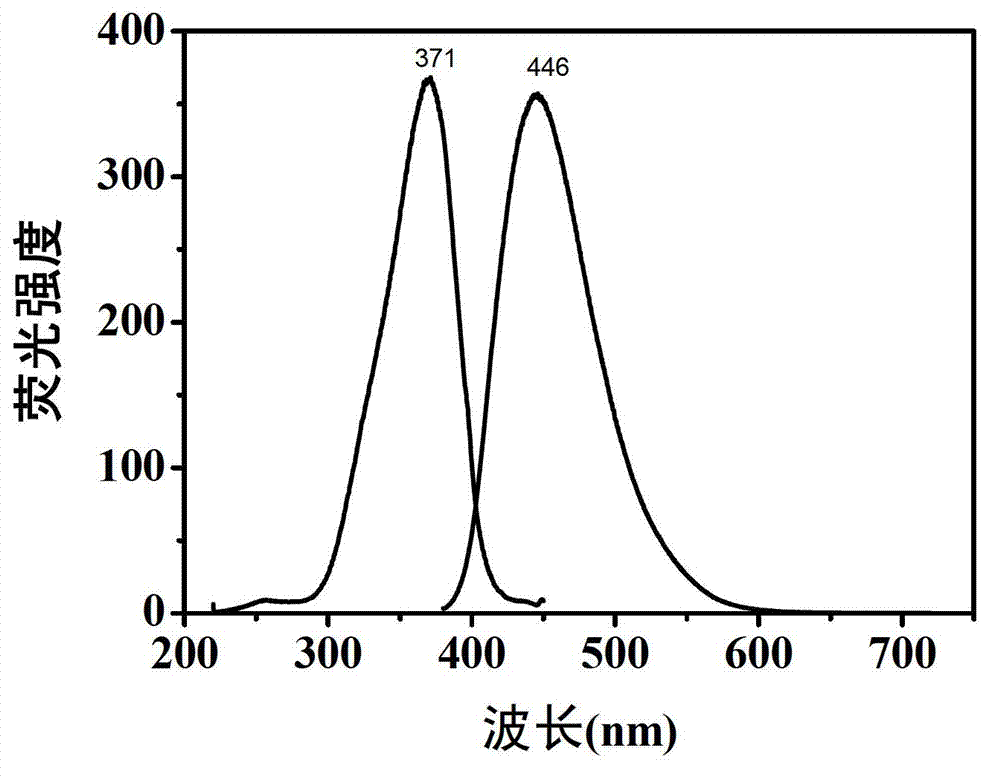
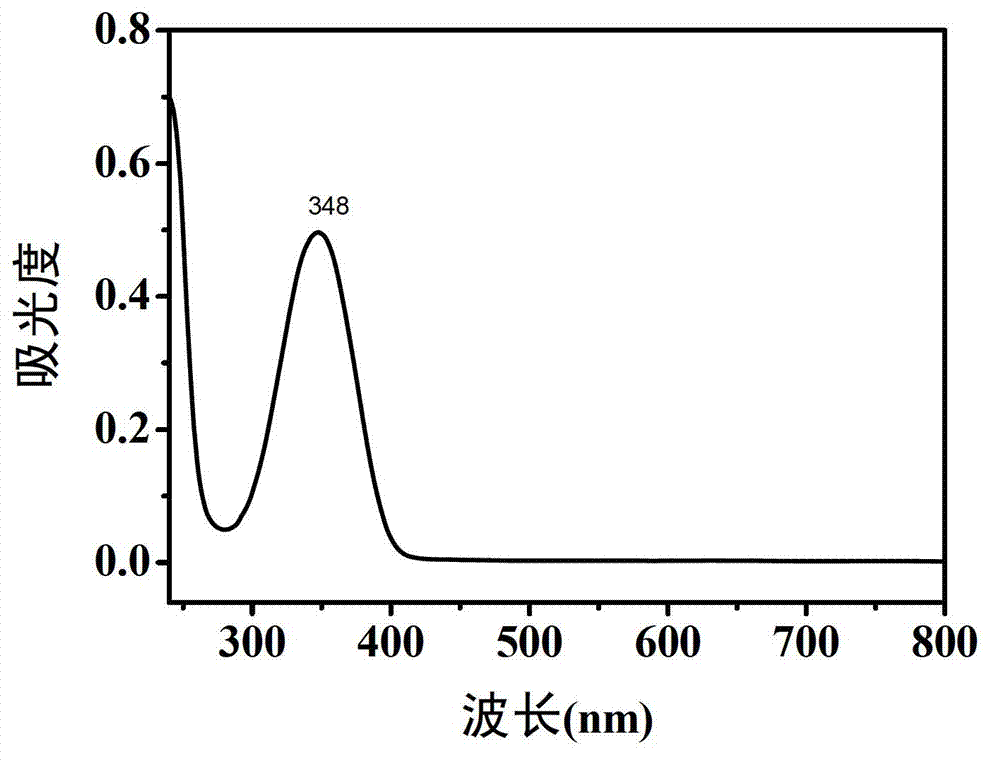
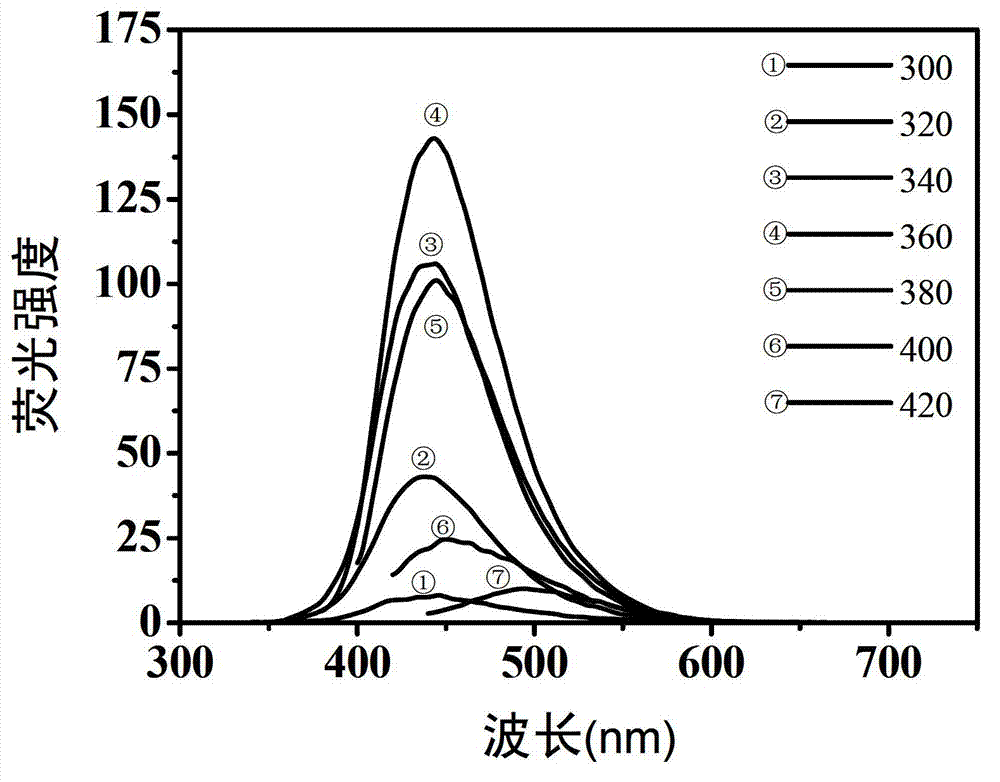
The invention belongs to the technical field of preparation of a carbon nano material, and particularly relates to a method for preparing a carbon dot having a high fluorescent quantum yield from citric acid and different nitrogen-containing molecules. The method comprises the following steps: weighing 1-10 mmol of solid citric acid, and dissolving in 10 ml of deionized water; weighing 1-10 mmol of ethylenediamine, ethylamine, propylamine, butanediamine, n-hexylamine, p-phenylene diamine or urea, adding into the citric acid solution, and uniformly stirring; transferring the solution into a hydrothermal or high-pressure microwave reaction kettle, reacting under hydrothermal or microwave conditions, and naturally cooling the reaction kettle to room temperature, thus obtaining a yellow or brown carbon dot water solution; and finally, purifying the carbon dot water solution, evaporating, and drying to obtain pure carbon dot solid powder. The carbon dot solution can send out bright blue fluorescence under the irradiation of a handheld ultraviolet lamp. The invention has wide application prospects in the fields of biological imaging, fluorescent printing and the like.
Owner:JILIN UNIV
Sensor with improved shelf life
InactiveUS20080121533A1Weather/light/corrosion resistanceVolume/mass flow measurementMetal electrodesEthylamine
Owner:LIFESCAN INC
Ultraviolet light solidfication water polyurethane acrylate paint resin and its preparation method
InactiveCN1869139AThe molecular structure is well controlledImprove performancePolyurea/polyurethane coatingsSolubility(Hydroxyethyl)methacrylate
The invention relates to an ultraviolet light solidified water urethane acrylate coating resin. It is mainly made up of aromatic diisocyanate, polyethylene glycol, polyhydroxy-carboxylic acid, dihydric alcohol, hydroxyethyl methacrylate ethyl ester, and tri-ethylamine. It is prepared by adding the above materials orderly. It has the advantages of good water solubility, pliability, and stability.
Owner:SHANTOU UNIV +1
Method for preparing quaternary amines salt cationic adsorption agent with plant of high cellulose content
InactiveCN101342485AGood effectReduce secondary pollutionOther chemical processesWater/sewage treatment by sorptionCelluloseEutrophication
The invention discloses a method for preparing quaternary amine salt ion adsorbent by using a plant with high cellulose content. Straw stalks with high cellulose content, epichlorohydrin and tri-ethylamine are taken as raw materials, ethylene diamine, diethylene triamine pentacetic acid (DTPA) or triethylenetetramine, etc are used as crosslinking agent to prepare the adsorbent under the condition of existence of N, N-dimethyl formamide. The method has the advantages of simple process, low cost, economic and practical, reproducible, etc. The adsorbent prepared by the method has the advantages of high liveweight growth rate, low secondary pollution, high stability, sound adsorption effect, wide application scope, etc; and the adsorbent can be extensively applied to the treatment of eutrophication of waters.
Owner:SHANDONG UNIV
Method for racemate splitting of 2-hydroxypropionic acids
InactiveUS6559338B1Easy to carryOrganic compound preparationOptically-active compound separationEthylaminePhotochemistry
A process for racemate resolution of 2-hydroxypropionic acids by reacting the racemic acid with an optically active base and subsequently separating off a diastereomeric salt of acid and base comprises using 1-(4-chlorophenyl)ethylamine as optically active base.
Owner:ROYALTY PHAMA COLLECTION TRUST
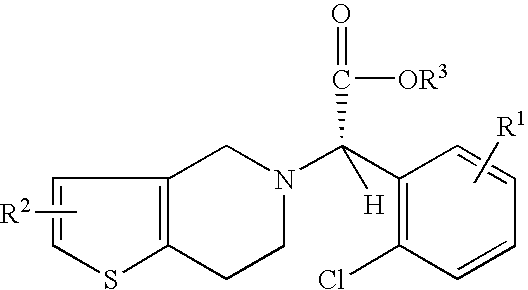
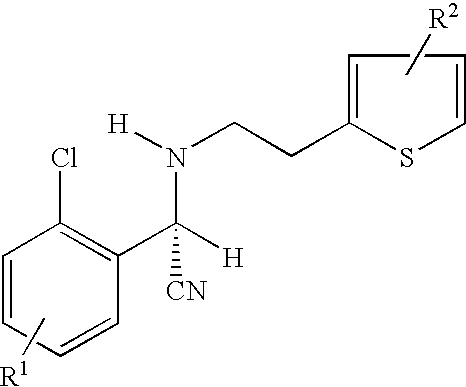
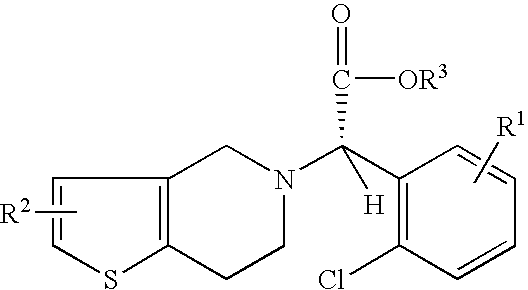
Preparation of (S)-Clopidogrel and related compounds
A process for producing enantiomerically enriched (S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5 (4H)-acetic acid hydrocarbyl ester, represented by the formula: Is provided, wherein R1 and R2 are hydrogens and R3 is methyl (i.e., (S)-Clopidogrel). The process includes the steps of: (a) contacting N-2-chlorobenz-aldehyde-ylidene-1-ethylamine-2(2-thiophenyl)imine and an HCN source, in the presence of a non-metallic asymmetric Strecker catalyst to form enantiomerically enriched (S)-α,α-(2-thiophenylethylamino)(2-chlorophenyl)acetonitrile; (b) contacting the enantiomerically enriched (S)-α,α-(2-thiophenylethylamino)(2-chlorophenyl)acetonitrile and a formaldehyde equivalent, in the presence of an acid catalyst to form enantiomerically enriched α-5(4,5,6,7-tetrahydro[3,2-c]thienopyridyl)(2-chlorobenzyl)-nitrile; and (c) contacting the enantiomerically enriched α-5(4,5,6,7-tetrahydro[3,2-c]thienopyridyl)(2-chlorobenzyl)-nitrile and a reagent capable of converting a cyano group into an ester group to form enantiomerically enriched hydrocarbyl ester of (S)-α-(2-chlorophenyl)-6,7-dihydrothieno-[3,2-c]pyridine-5(4H)-acetic acid.
Owner:SHASUN PHARMA SOLUTIONS LTD
Preparing method for titanium dioxide fibre
The producing method of two-oxidation titanium fibre belongs to function fibre material technology scopes. Compose poly-acetamide acetone titanium to be the van-substance. Let chemic dosage of four-chloridize titanium, distilled water, acetamide acetone and three-ethylamine dilute in methanol respectively. Mix them and react when mixing round at 0-25 deg.C. Compose poly-acetyl acet, titanium. Remove hydrochloric three-ethylamine by using four-hydrogen furan. Dissolve poly-acetyl acet, titanium in methanol to make filature liquid. Obtain van-substance short fibre by using acentric swing method. Obtain van-substance long fibre by using dry filature method. And we can obtain two-oxidation titanium fibre by using high pressure or normal pressure vapor heat treatment technique agglomeration. The intension of extend of this invention is 100 MPa-1.2GPa. The diameter is 3um-20um. The one-thread series length is 1 cm-50 cm or higher than 1 m. The crystal diameter is 5 nm-100 nm. The appearance is two-oxide ation short fibre or consecutive fibre with titanium droff-titanium-mine posture or rutile posture or coexistence.
Owner:SHANDONG UNIV
Room-temperature efficient ozone decomposition catalyst and preparation method thereof
ActiveCN102513106AGuaranteed conditions that generate the desired valenceRich in channelsDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsWater vaporDecomposition
The invention discloses a room-temperature efficient ozone decomposition catalyst and a preparation method thereof. The catalyst is prepared by the following steps of: preparing manganese nitrate, cerous nitrate and silver nitrate into a solution according to a mol ratio of 1: (0.3-0.5): (0.01-0.1); taking potassium carbonate and / or potassium bicarbonate as a precipitant, taking potassium chlorate as an oxidant, and precipitating to prepare active components; and then, adding N,N-diethyl ethylamine and crystallizing in a reaction kettle at a temperature in a range of 180-220 DEG C; after the crystallization, carrying out filter pressing to obtain sediments with the water content of 70-80 wt%; and then, adding a binding agent and a pore-forming agent and uniformly agitating; conveying the mixture into a banded extruder to be extruded and molded into cylindrical grains; drying the cylindrical grains at a temperature in a range of 80-100 DEG C and adding the dried cylindrical grains intoa muffle furnace to be roasted at a temperature in a range of 450-550 DEG C. The ozone decomposition catalyst provided by the invention has the advantages of capabilities of completely purifying thousands of ppm of ozone at the room temperature and resisting the violent fluctuation of the ozone concentration, good water vapor resistance and long service life.
Owner:SUZHOU IND PARK ANZEWEN ENVIRONMENTAL PROTECTION TECH
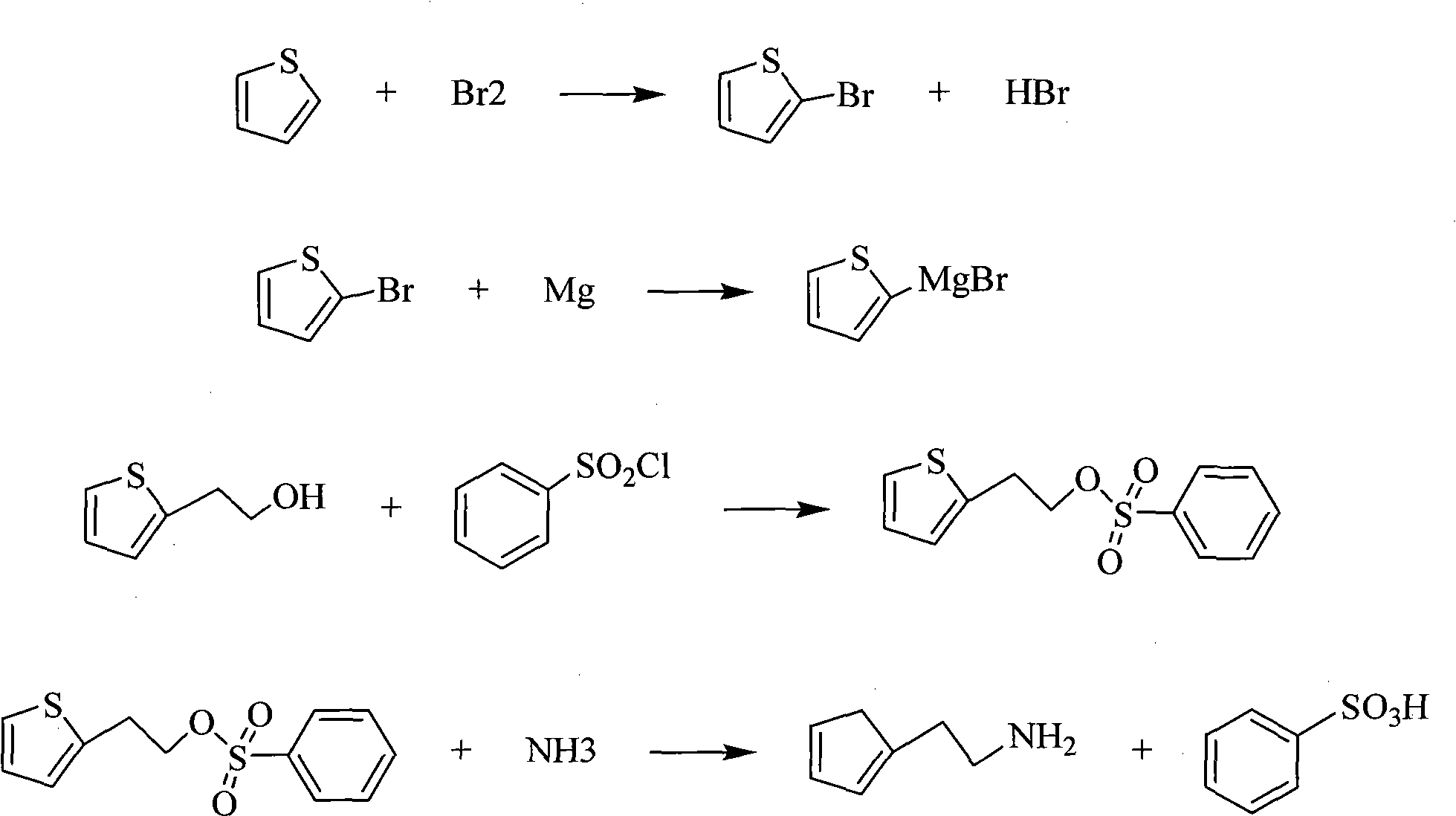
Method for synthesizing 2-thiophene ethylamine
ActiveCN101885720AReduce manufacturing costMild reaction conditionsOrganic chemistryTerthiopheneEthylene oxide
The invention relates to a method for synthesizing 2-thiophene ethylamine, which is characterized in that thiophene is taken as a raw material, intermediate 2-thiophene ethanol is firstly prepared, and then the 2-thiophene ethylamine is obtained through pressurization and ammonolysis. The method mainly comprises the following steps: bromizing thiofuran at low temperature to obtain 2-bromo thiophene; carrying out Grignard reaction on the 2-bromo thiophene and magnesiumchips and reacting with ethylene oxide to generate the 2-thiophene ethanol; and preparing 2-thiophene ethylamine from the 2-thiophene ethanol through the two steps of esterification and ammonolysis. The invention takes cheap and easily obtained thiophene as the raw material which is subjected to the fives reactions of bromination, Grignard, addition, esterification and ammonolysis, greatly reduces the production cost, has mild reaction condition, simple process and small pollution, facilities the realization of the industrialized production, does not use the reducing agent and avoids the utilization of expensive or toxic materials.
Owner:LIANYUNGANG DIPU CHEM
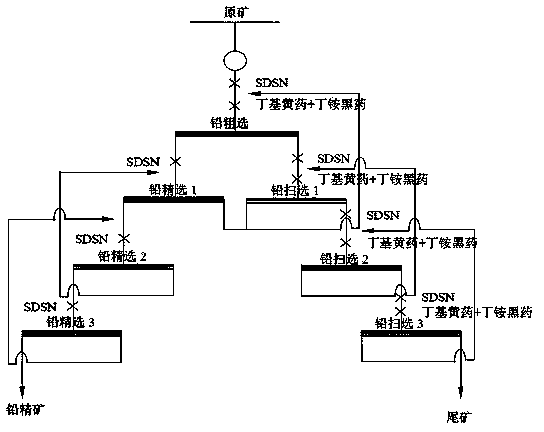
Flotation separation inhibitor and separation method of galena, pyrite and sphalerite
InactiveCN103909020AEfficient separationPlay a synergistic roleFlotationEcological environmentSeparation technology
The invention discloses a flotation separation inhibitor and separation method of galena, pyrite and sphalerite, and belongs to the technical field of mineral processing. The method includes adopting raw ores of sulfide lead-zinc mine as raw material, and adding SDSN (dimethyl dithiocarbamate : 2-(methylthio)ethylamine = 1-3:1)to serve as inhibitor of the pyrite and the sphalerite so as to perform lead and zinc sulphur flotation separation after the raw ores are grinded. Compared with an existing lead and zinc sulphur separation technology, the composite inhibitor SDSN has the advantages that selectivities of the pyrite and the sphalerite are high, inhibiting capability is high, usage is fewer, and adding is facilitated. The the pyrite and the sphalerite can be inhibited well, efficient separation of the galena and the zinc sulphur is implemented, the problems that according to the traditional lime or cyanide method, the recovery rate of associated gold, silver and other precious metals in the lead-zinc sulfide mine is low and ecological environment is seriously damaged are overcome, and an efficient and environment-friendly flotation separation method is provided for complex sulfide lead-zinc mine separation.
Owner:HUNAN RES INST FOR NONFERROUS METALS
Method for synthesizing core-shell type zeolite molecular sieve
ActiveCN101723402APromote formationEvenly dispersedMolecular sieve catalystsPentasil aluminosilicate zeoliteSoluble glassPropylamine
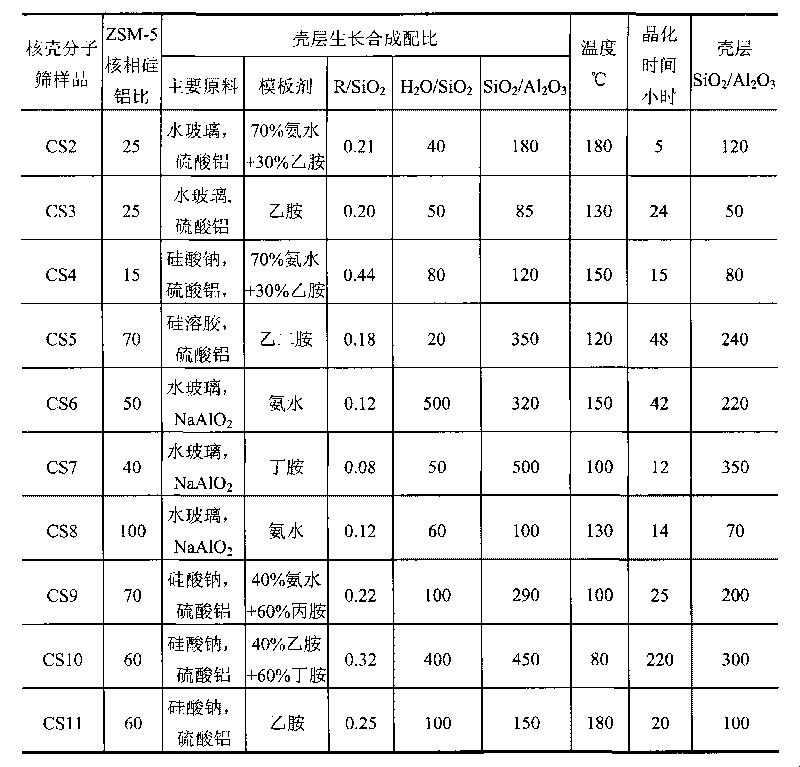
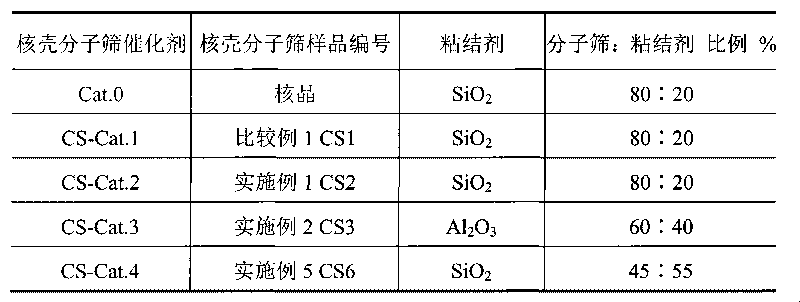
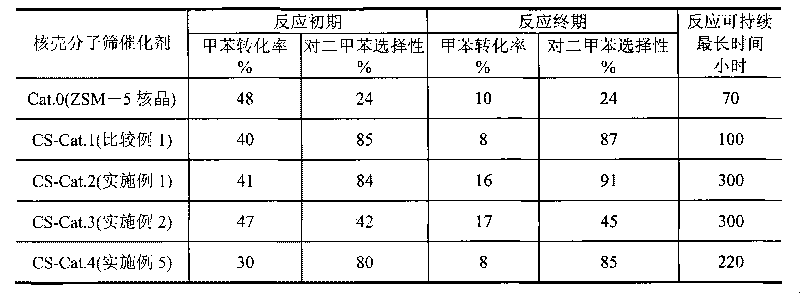
The invention relates to a method for synthesizing ZSM-5 / ZSM-5 core-shell type zeolite molecular sieve, mainly solving the technical problems of expensive used raw materials, great environment pollution of template agent, high difficulty of industrial amplification and synthesis, difficult industrial production and the like of the core-shell type zeolite molecular sieve in the prior art. The invention adopts the following technical scheme: the core-shell zeolite molecular sieve is prepared by adopting ZSM-5 core phase molecular sieve with a relatively low silica alumina ratio as a seed crystal, using at least one of soluble glass, silica solution, sodium silicate, white carbon black or atlapulgite which are cheap and easily obtained as a silicon source, and taking at least one organic amine from ethylamine, ethanediamine, propylamine and butylamine or the mixture of the organic amine and inorganic amine as a template agent so as to better solve the above technical problems. The invention can be used in industrialized synthetic production of core-shell type molecular sieve.
Owner:CHINA PETROLEUM & CHEM CORP +1
Novel nano-composite double network hydrogel and preparation method thereof
The invention relates to a novel nano-composite double network hydrogel and a preparation method thereof. The preparation method of the hydrogel comprises the steps of: performing a condensation (polycondensation) reaction of amines and epichlorohydrin or polyalcohol and aldehydes in clay solution to obtain a condensate (polycondensate) / clay composite first; and adding a hydrophilic monomer under the action of an initiator and a cross-linking agent to obtain the nano-composite double network hydrogel through free radical crosslinking polymerization, wherein in the condensation (polycondensation) reaction, the amines are selected from dimethylamine, methylamine, ethylamine, propylamine, diethylamine, quadrol, propylene diamine, diethylenetriamine, triethylenetetamine and tetraethylenepentamine, the polyalcohol is selected from glycol, propanediol, glycerol, butanediol, neopentyl glycol, pentaerythritol, polyvinyl alcohol, polyethylene glycol, glucose, sucrose, sorbierite, soluble starch and chitosan, the aldehydes are selected from formaldehyde, malonaldehyde and glutaraldehyde, clay is selected from laponite, bentonite or hydrophilic modified bentonite, and the hydrophilic monomer is selected from acrylic acid, acrylamide, N-Isopropylacrylamide, methacrylic acid, acrylonitrile and propene sulfonic acid. The novel hydrogel has the excellent mechanical strength under the condition of high moisture content.
Owner:XINJIANG UNIVERSITY
Method for synthesizing theanine
InactiveCN1415599ANo cost increaseOrganic compound preparationCarboxylic acid amides preparationL-theanineCarboxylic acid
A process for synthesizing theanine features that L-pyrrolidone carboxylic acid and anhydrous ethylamine directly take part in reaction in inert gas atmosphere at 60-100 deg.c and 6-12 MPa to obtain L-theanine. Its advantages are high output rate up to 38%, and short reaction time.
Owner:JINAN UNIVERSITY
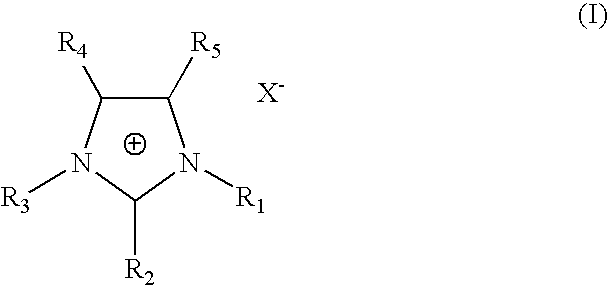
Chitosan aminoethyl quaternary ammonium salt derivative and preparation method thereof
The invention belongs to ocean chemical engineering technology, and specifically relates to a chitosan aminoethyl quaternary ammonium salt derivative and a preparation method thereof. The chitosan aminoethyl quaternary ammonium salt derivative is shown as a formula (I), wherein R1 represents methyl or -H, and R2 represents -CH3, linear alkyl, branched alkyl or -Ar, and n equals to 4-4000. According to the invention, 2-chloroethylamine is subjected to an electrophilic substitution reaction with -NH2 on C2 and -OH on C6 of the chitosan to generate aminoethyl chitosan, amino on which reacts directly with iodomethane to generate N-trimethyl quaternary ammonium salt derivative, or which is subjected to a condensation reaction with aromatic aldehyde or fatty aldehyde to generate Schiff base which is reduced by sodium borohydride and quaternized under effect of iodomethane to obtain chitosan aminoethyl quaternary ammonium salt derivative. According to infrared spectroscopic analysis on the obtained derivative, chitosan and the grafted groups are effectively combined to generate aminoethyl quaternary ammonium salt. According to the invention, aminoethyl quaternary ammonium salt group is introduced into the chitosan structure to increase positive charge level of the chitosan, substantially enhance biological activity of the chitosan, such as antibiosis and sterilization, etc.
Owner:水母娘娘海洋生物科技有限公司
Solid phase polypeptide synthesis preparation method for leuprorelin
ActiveCN1865280AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionHydrogen fluorideLeuprorelin
The invention discloses a bright-ala-ruilin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Wang resin or CTC resin as original material to connect amino with protective group to produce protective nonapeptide resin; removing Fmoc-protective group sequently; proceeding side-chain protective group synchronizingly and cutting peptide; connecting ethylamine through ethylamine-to-HOBT to produce crude product; proceeding separation and purifying through C18 (or C8) pillar to produce fine bright-ala-ruilin. The invention avoids the utility of poisonous agent, which improves the purifying, peptide connecting and obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
Rapid preparation method of environment-friendly metal-organic framework material
ActiveCN105693750ALarge specific surface areaHigh porosityGroup 1/11 organic compounds without C-metal linkagesOther chemical processesPorosityFiltration
The invention discloses a rapid preparation method of an environment-friendly metal-organic framework material. Amino acid is dissolved in deionized water containing sodium hydroxide, an ethylamine derivative with the volume percentage being 3%-10% is added as a catalyst, a metal salt solution with the concentration being 0.5 mol / L is slowly dropwise added under the action of stirring, and the mixture continues to react for 1-5 h after the solution is dropwise added; the mixture is dried for at least 24 h at the temperature of 80 DEG C after being treated multiple times through filtration, water washing and ethanol washing, and the metal-organic framework material is obtained. The synthesized metal-organic framework material has a large specific surface area and high porosity; the preparation is operated at the room temperature, and energy consumption is reduced; water is taken as a solvent, and the metal-organic framework material is environment-friendly; when the metal-organic framework material is used in dye wastewater, the metal-organic framework material has a high adsorption rate for dye and has high adsorption efficiency; the framework material adsorbing the dye is easily recovered and can be repeatedly used.
Owner:QINGDAO UNIV
Self-emulsifying aqueous nitrocellulose emulsion and preparation thereof
InactiveCN101486798AImprove stabilityReduced Possibility of ContaminationPolyurea/polyurethane coatingsWater basedNitrocellulose
The invention relates to the nitro coating field, in particular to a self-emulsifying water-based nitrocellulose emulsion and a preparation method thereof; and the raw materials of the invention comprise the following components by weight percentage: 0.89%-8.60% of nitrocellulose or nitro coating paint film, 1.80%-2.00% of dimethylolpropionic acid, 2.90%-3.25% of N-methyl pyrrolidone, 4.15%-4.40% of isophorone diisocyanate, 0.01%-0.03% of dibutyl tin dilaurate, 23.80%-26.90% of butanone, 1.40%-1.60% of triethylamine and 57.00%-62.00% of water.
Owner:XI AN JIAOTONG UNIV
Synthetic method of high silica ZSM-5 zeolite
InactiveCN101898767AReduce dosageShape is easy to controlPentasil aluminosilicate zeoliteTetramethylammonium bromideAdhesive
The invention discloses a synthetic method of high-silica ZSM-5 zeolite, the method comprises the steps of adopting water glass as a silica source, adopting aluminum salts of aluminum sulfate, aluminum nitrate, aluminum chloride and the like, as well as organic aluminum compounds of sodium metaaluminate, isopropanol and the like as aluminum sources, using sulfuric acid or hydrochloric acid to regulate alkalinity, adding an organic template, simultaneously adding a guide adhesive, carrying out high-temperature crystallization at 120-200 DEG C, and then synthesizing the high-silica ZSM-5 zeolite with the SiO2 / Al2O3 ratio which is greater than 100; and the molar ratio of raw materials is as follows: SiO2 / Al2O3 is equal to 100-infinity, Na2O / SiO2 is equal to 0.05-0.20, and H2O / SiO2 is equal to 10-100. The organic template comprises tetrapropylammonium bromide, tetrapropylammonium hydroxide, tetraethylammonium bromide, tetraethylammonium hydroxide, n-butylamine, ethylamine, hexamethylene diamine and any other organic matters which can synthesize the ZSM-5 zeolite or the mixture thereof, wherein the using amount of the organic template is that R / SiO2 is equal to 0.005-0.50; and the amount of silicon dioxide in the guide adhesive is 0-5% of the total weight of the silicon dioxide in a synthetic system. The synthetic method can synthesize the high-silica ZSM-5 zeolite with the SiO2 / Al2O3 ratio which is greater than 100 and has the advantages of cheap raw materials, a small using amount of the organic template, and capability of realizing the control of product appearance and particle size by changing the relative using amount of the template and the guide adhesive.
Owner:EAST CHINA NORMAL UNIV
Symmetrical double-rhodamine B fluorescent probe for detecting mercury ion and preparation method of fluorescent probe
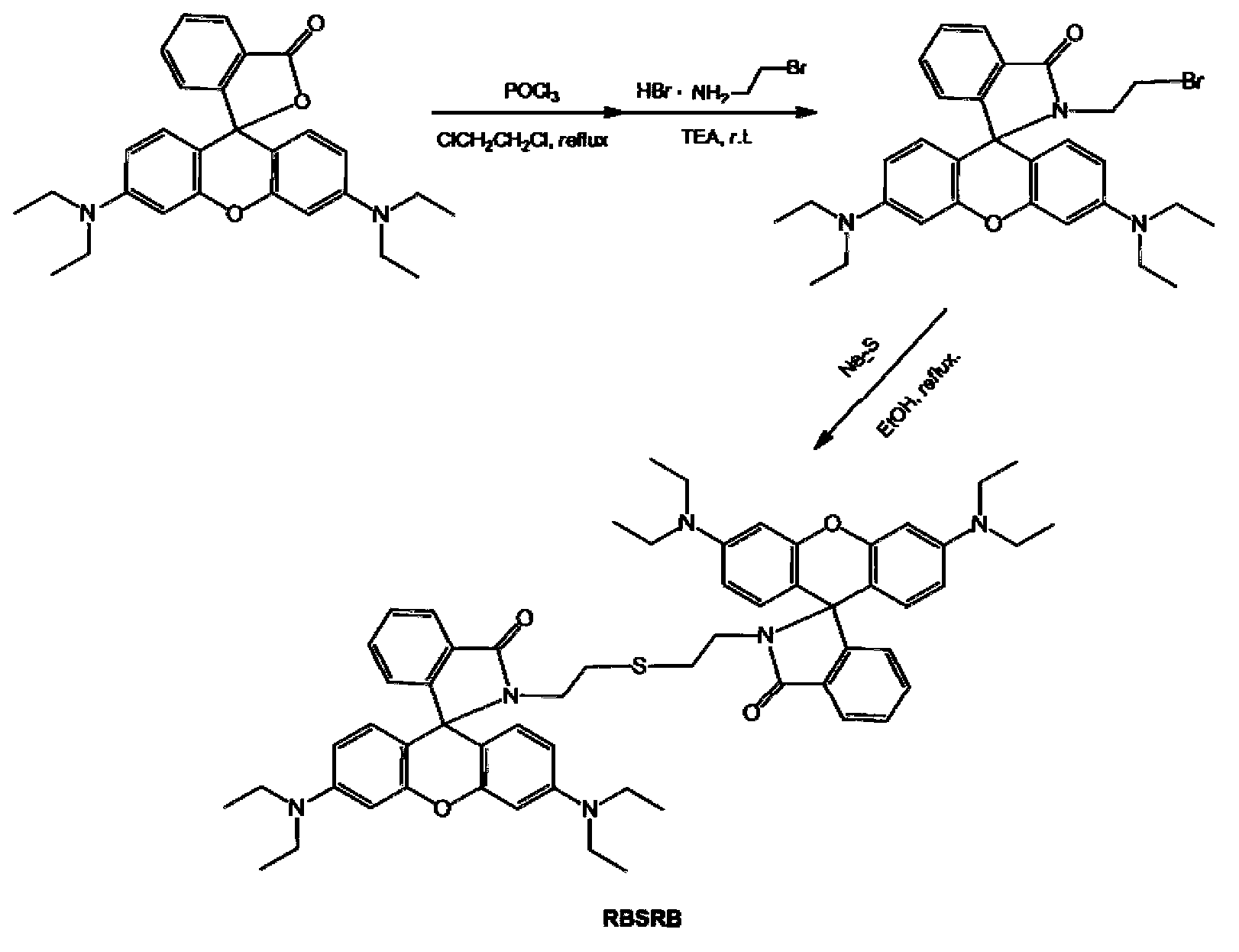
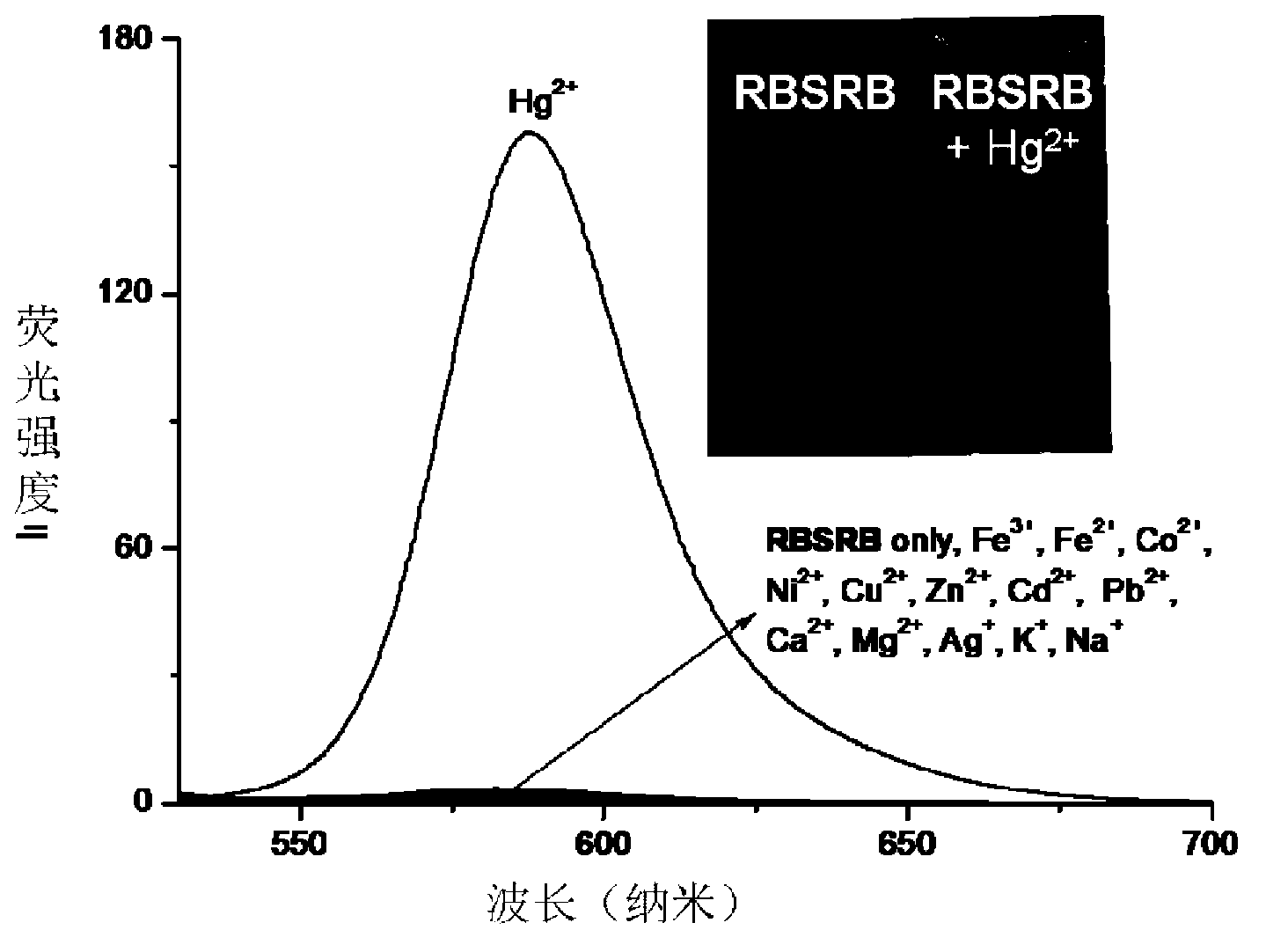
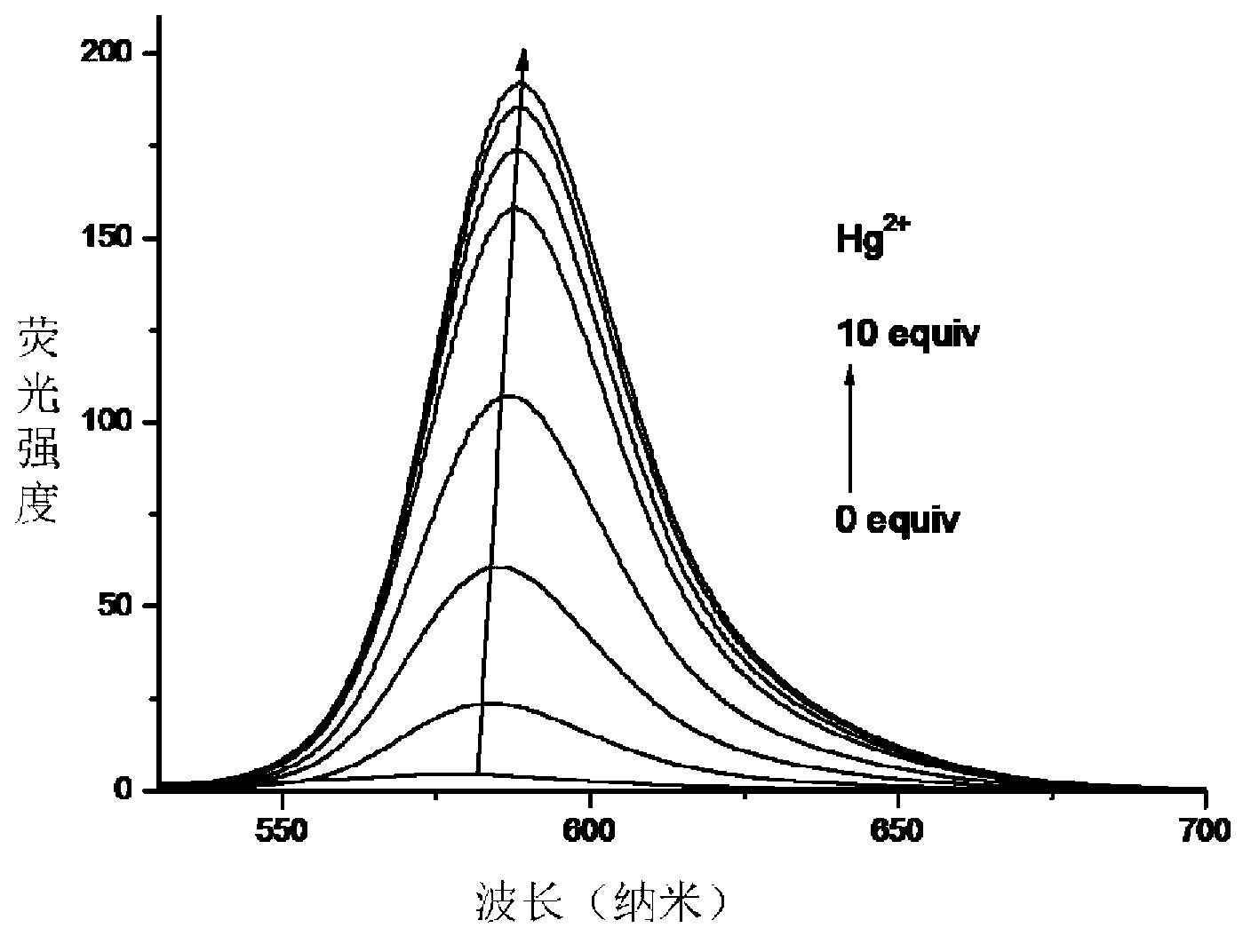
InactiveCN103254893AEnables visualization of fluorescence imagingFluorescent signal enhancementOrganic chemistryFluorescence/phosphorescenceFluorescenceAcyl group
The invention relates to a symmetrical double-rhodamine B fluorescent probe for detecting a mercury ion and a preparation method of the fluorescent probe. The structural formula of the symmetrical double-rhodamine B fluorescent probe RBSRB (rhodamine B-symmetrical-rhodamine B) is as shown in the specification. The preparation method comprises the following steps: adding rhodamine B and phosphorus oxychloride into a solvent for reflux reaction for 20-24h at 85-90 DEG C, obtaining rhodamine B acyl chloride, dissolving rhodamine B acyl chloride and 2-bromine ethylamine in the solvent, stirring for reaction for one night at room temperature, purifying to obtain rhodamine B acyl-2-bromine ethylamine, adding rhodamine B acyl-2-bromine ethylamine and sodium sulfide into the solvent for reflux reaction for 5-8h at 80-85 DEG C, and purifying to obtain the fluorescent probe. The fluorescent probe is symmetrical double-rhodamine B amide connected by a sulphur atom; only alcohol under 1% is introduced as a cosolvent during an experiment; the fluorescent probe is high in selectivity, and has high selectivity to the mercury ion; other common ions do not interfere with the fluorescent probe obviously; and the fluorescent probe has an application prospect in bioluminescence imaging.
Owner:DONGHUA UNIV
Disinfectant health-care aromatic hand paper towel and preparation method thereof
ActiveCN104846685ALow costReduce manufacturing costCosmetic preparationsToilet preparationsAntibacterial agentAntibacterial property
The invention discloses a disinfectant health-care aromatic hand paper towel and a preparation method thereof. The hand paper towel comprises the following components: softwood sulfite pulp, bamboo-pulp paper fiber, straw pulp, a cationic starch solution, a wet strength agent, a powerful antibacterial agent, an aromatic agent and titanium dioxide, wherein the wet strength agent is polyethylene ethylamine resin, the powerful antibacterial agent is composed of pure-oxygen-based quaternary ammonium salt, Sophora flavescens, common cnidium fruit, honeysuckle, borneol, isatis root, ethanol and distilled water, and the aromatic agent is composed of chitosan, ethanol, essence extract, deionized water, glycerin and triphosphoric acid. The prepared disinfectant health-care aromatic hand paper towel in the invention has the characteristics of no toxicity, harmlessness, environmental protection, highly-efficient antibacterial property and emission of aroma, can efficiently avoid contact-type cross infection with bacteria in public places and is safer and more secure to use.
Owner:ZHEJIANG HUACHUAN IND GRP
Measuring method of divalent cobalt content in lithium cobalt oxide
InactiveCN101685067AEasy to judgeImprove electrochemical performanceMaterial analysis by observing effect on chemical indicatorAcetic acidAmmonium ferrous sulfate
The invention belongs to a measuring method of divalent cobalt content in lithium cobalt oxide in the metallic ion quantitative detection field; the measuring method is characterized in that: total cobalt content and trivalent cobalt ion content in the lithium cobalt oxide are respectively measured, and then the total cobalt content subtracts the trivalent cobalt ion content for obtaining the divalent cobalt content in lithium cobalt oxide; wherein, the measuring method of the total cobalt content in the lithium cobalt oxide adopts ethylenediamine tetraacetic acid (EDTA) chelatometrie volumetric method, an iodometry method or a potassium ferricyanide oxidimetry method, and the measuring method of the trivalent cobalt ion content in the lithium cobalt oxide adopts an ammonium ferrous sulfate oxidimetry method. The measuring method in the invention makes up the disadvantage that the prior art has no measuring method of divalent cobalt content in lithium cobalt oxide and provides a measuring method of divalent cobalt content in lithium cobalt oxide in the metallic, wherein the method has simple operation, easy judgment of a finishing point and accurate measuring result, thereby providing powerful reference for judging the purity of the lithium cobalt oxide products and ensuring the lithium cobalt oxide products to have good electro-chemical performance.
Owner:SHENZHEN BAK BATTERY CO LTD
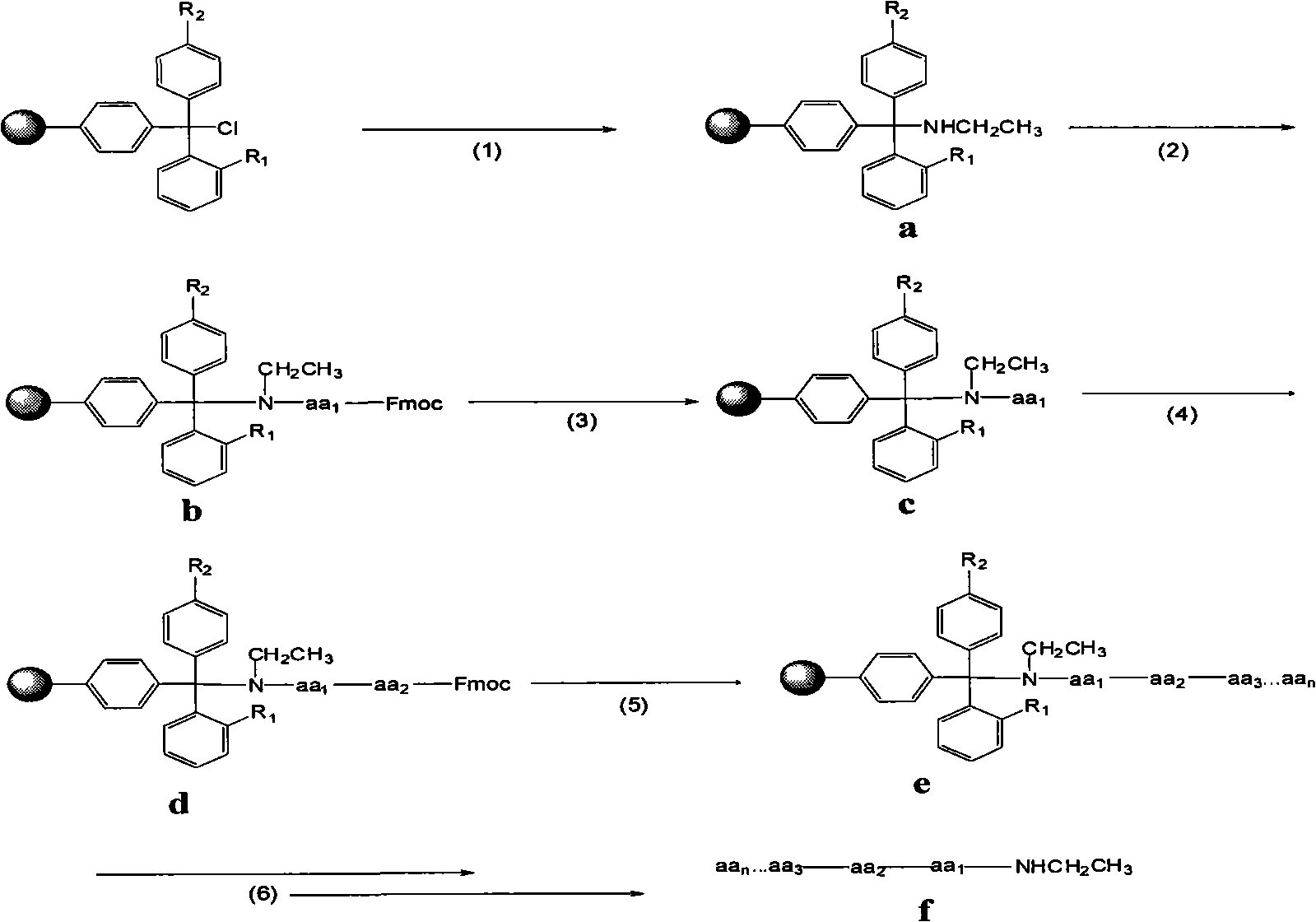
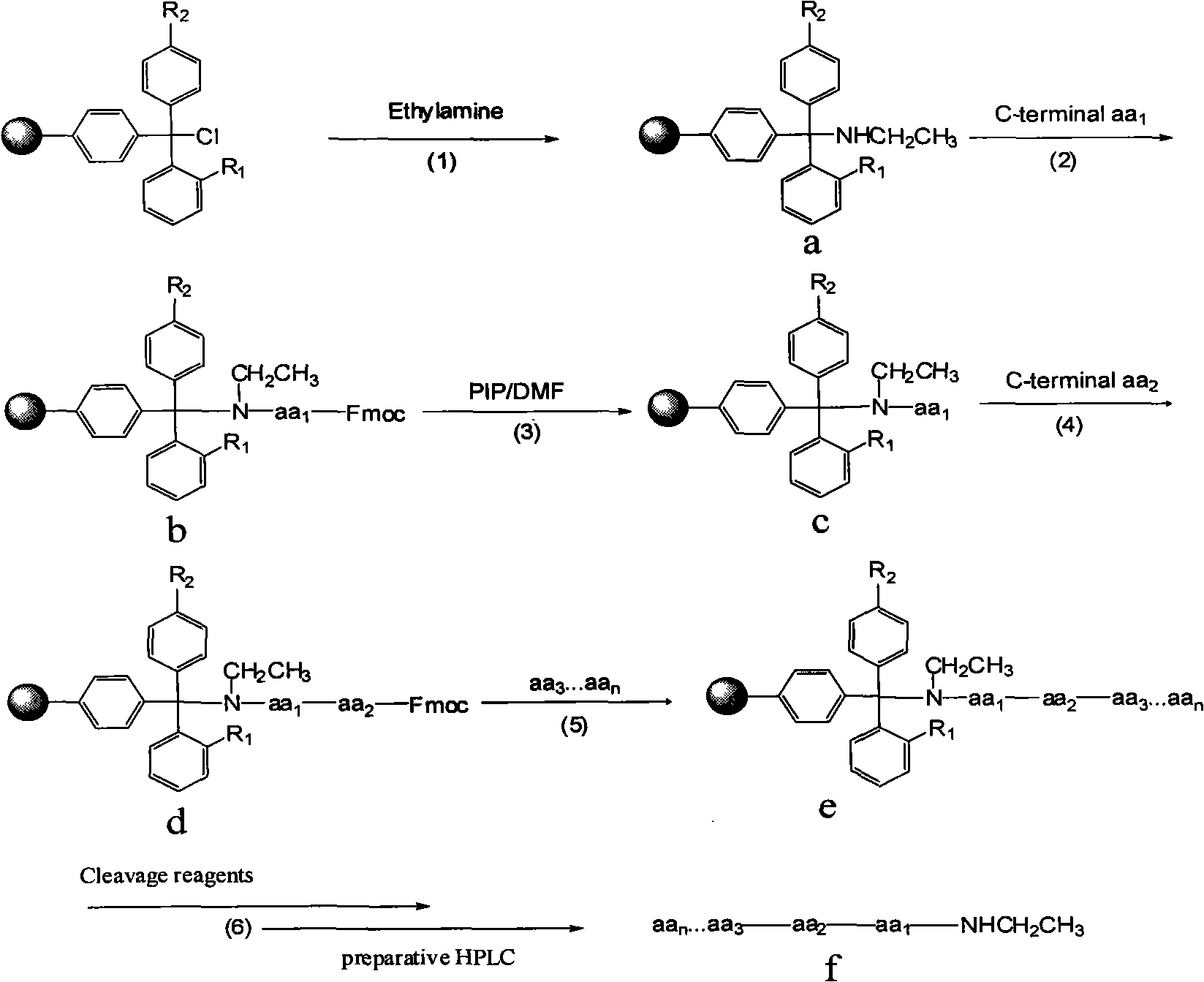
Preparation of C-terminal ethylamine polypeptides and derivates thereof
ActiveCN101279998ASolve the problem of localizationSolve the economyPeptide preparation methodsBulk chemical productionCombinatorial chemistryEthylamine
The invention relates to a method to prepare a polypeptide and in particular relates to a method to prepare C terminal ethylaminated polypeptide, solving the problems of long period in liquid phase synthesis, the use of poisonous reagent in Boc solid-liquid method and high cost in Fmoc method. The preparation method includes the following steps(1)preparing solid pahse carrier; (2)connecting terminal amino acids at the C terminal with the solid pahse carrier; (3) desorbing the amido protecting groups of the amino acids at the C terminal; (4)condensating the second amino acid and the first amino acid at the C terminal; (5)repeating the processes in step(3)and step(4) according to the sequence from C terminal to N terminal of the polypeptide of the derivative thereof to synthesize the polypeptide or the derivative peptide chain e thereof; (6)adding reagent to cleave the peptide resin and purifying through high efficiency liquid chromatography; in this way the target product f is obtained. The method is suitable for producing C terminal ethylaminated polypeptide with low cost and high efficiency.
Owner:GL BIOCHEM SHANGHAI
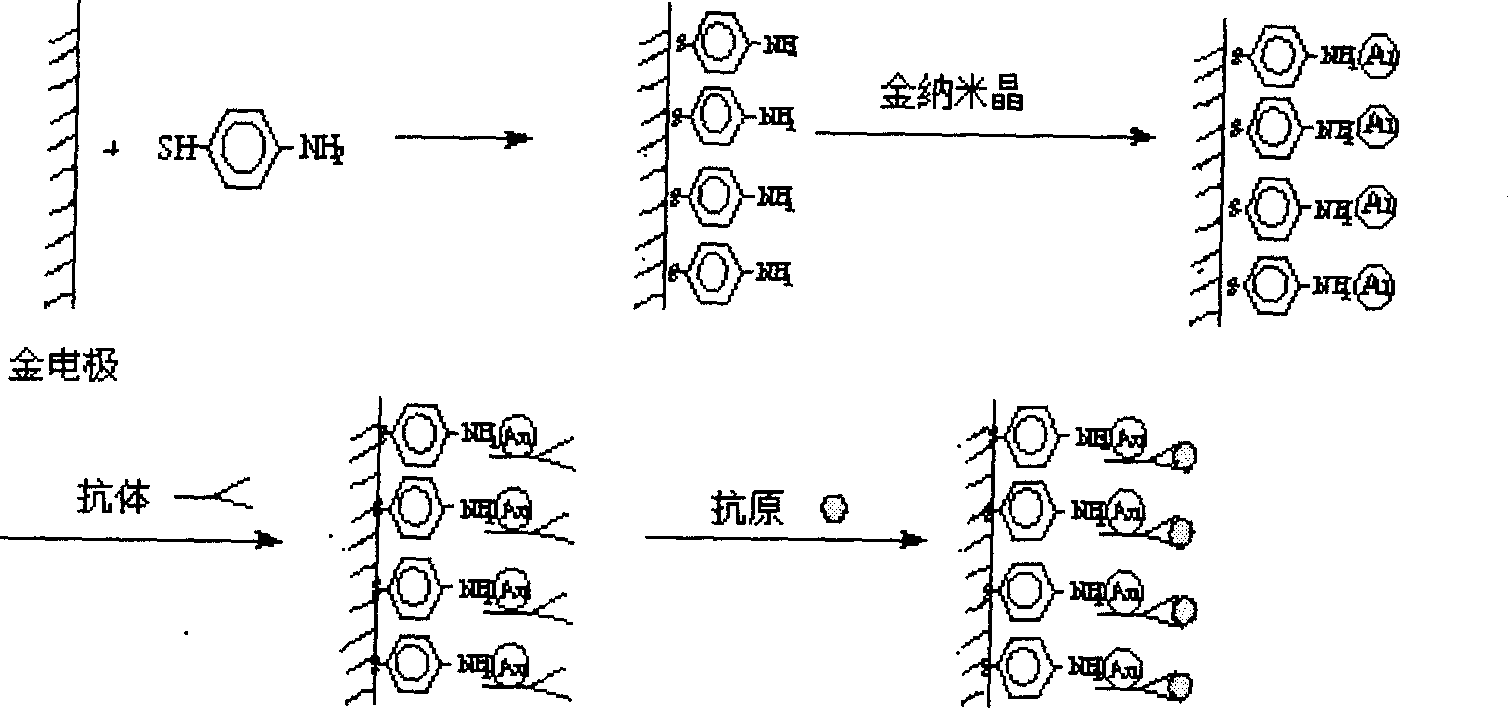
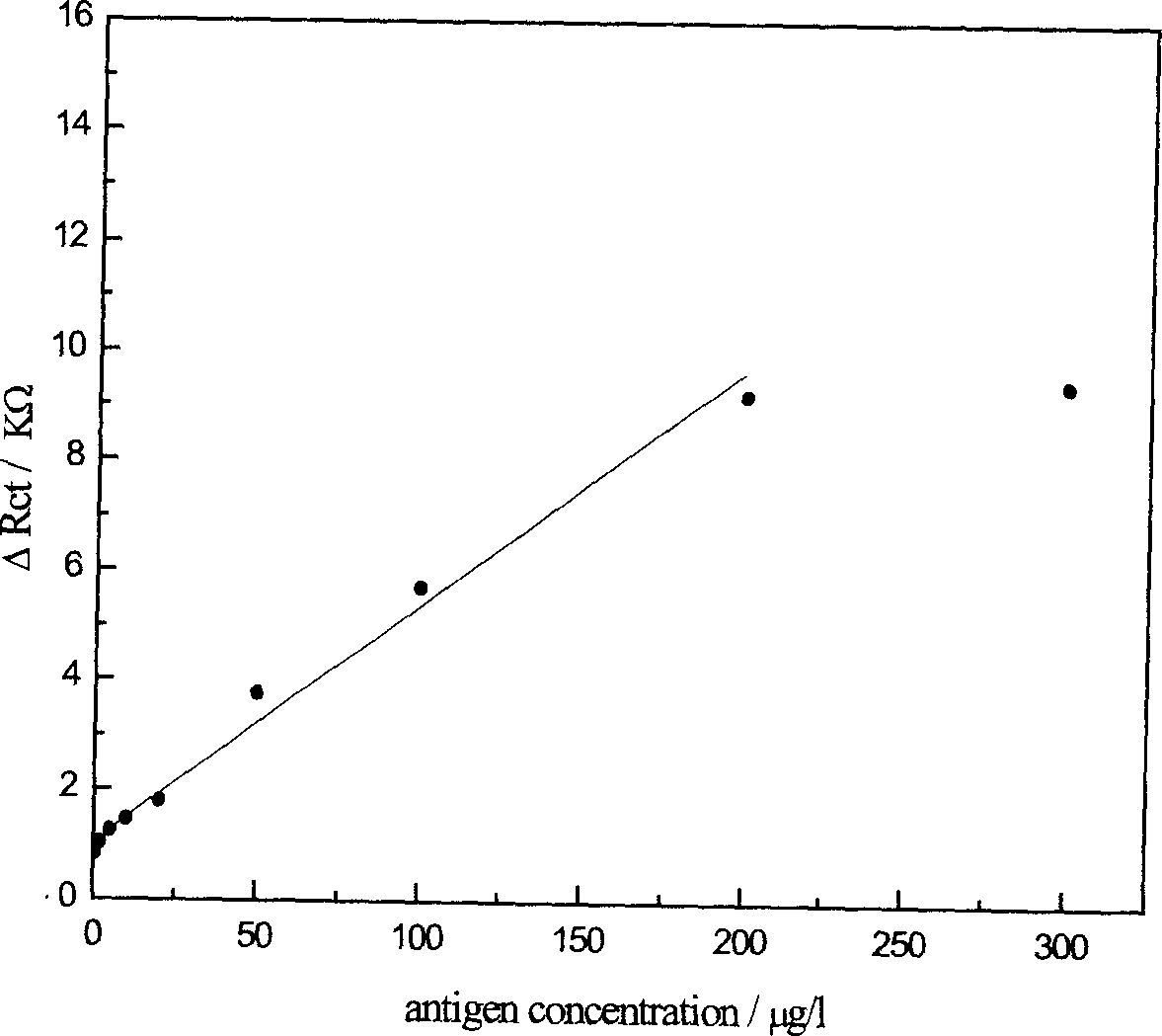
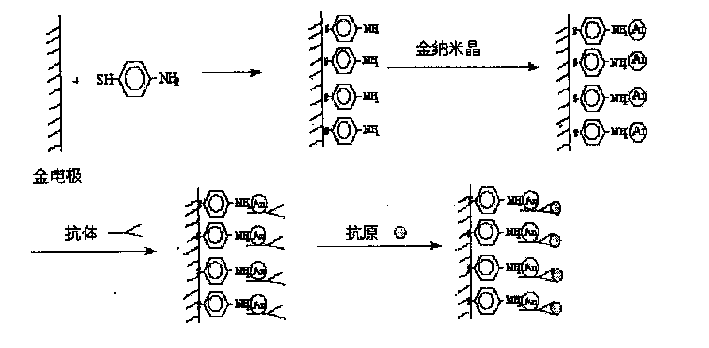
Nano immunological biosensor
InactiveCN1523346AEasy to prepareKeep aliveBiological testingMaterial impedanceAntiendomysial antibodiesMercaptoethylamines
The invention chemically decorates the gold electrode surface with mercapto compound, like mercapto aniline, mercapto ethylamine, etc. It uses the mercapto end of the mercapto compound to combine with the gold electrode, chemically, and fixes the mercapto end on the gold electrode to make the amido on the other end exposed on the electrode surface. On a certain condition, the exposed amido can combine with the nano gold crystal by chemical bond, thus fixing the nano gold crystal on the gold electrode surface. The surface of the nano gold crystal has charges and can strongly absorb the antibody on the neutral or physiological pH condition, making the antibody fixed on the surface of the nano gold crystal, and thus making the nano immune biosensor.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Synthesis process of theancine
InactiveCN1560025AEasy to crystallize and separateReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationAcetic acidRoom temperature
The invention discloses a theanine synthesizing method, using phthalic anhydride to react with excessive L-glutamic acid at 120-180 deg.C to generate phthalyl L-glutamic acid, which is processed by azeotropism with acetic acid to form phthalyl L-glutamic anhydride, at room temperature, making the phthalyl L-glutamic anhydride directly react with ethylamine water solution to generate phthalyl L-theanine, adding in hydrated hydrazine solution and placing for 2 days to eliminate protective group, so as to obtain L-theanine. And it has the advantages of low price of fast reaction, high selectivity, high yield, generally over 50%, etc.
Owner:NANJING UNIV
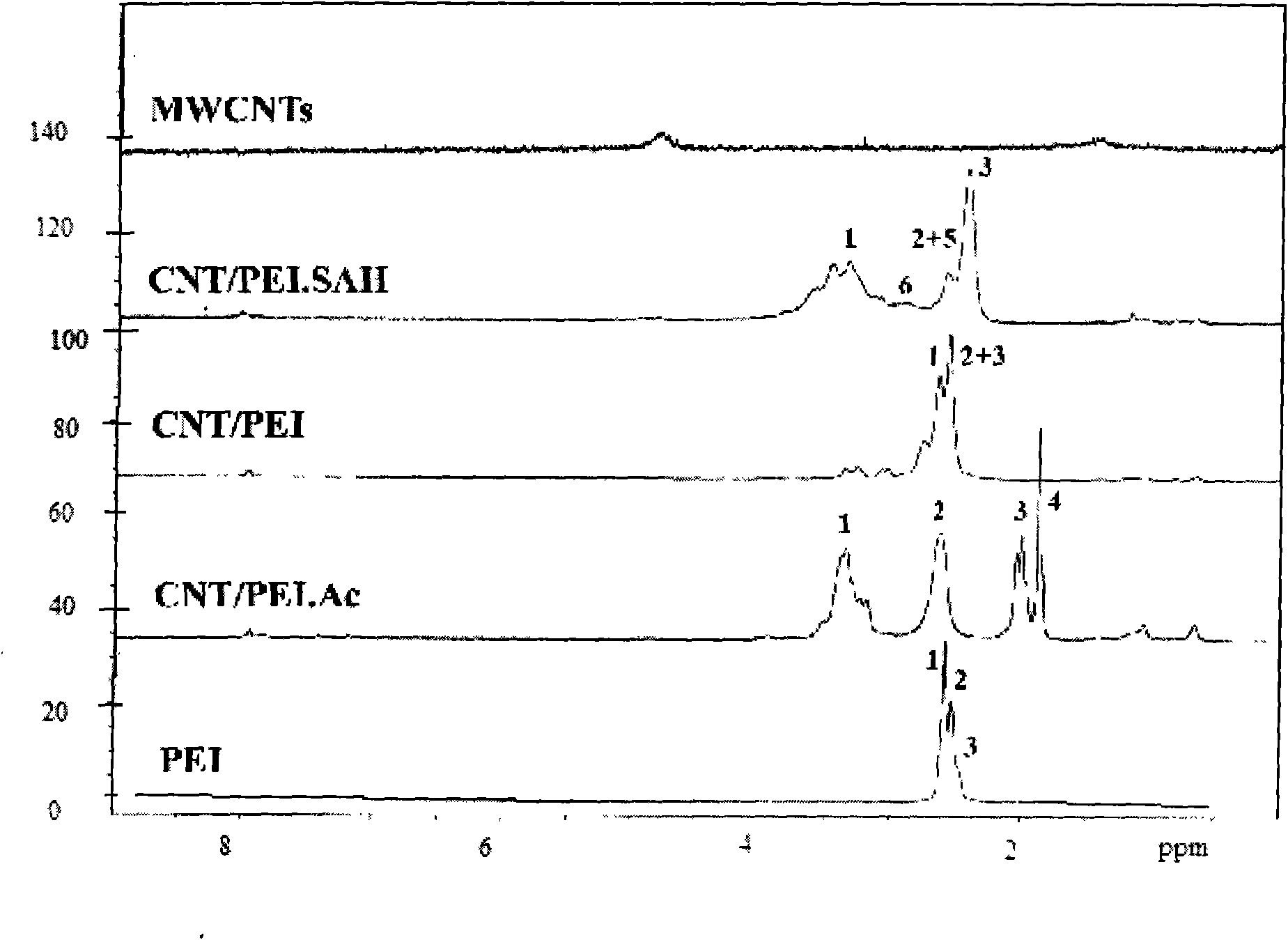
Method for preparing functionalized carbon nanotube based on polyethyleneimine
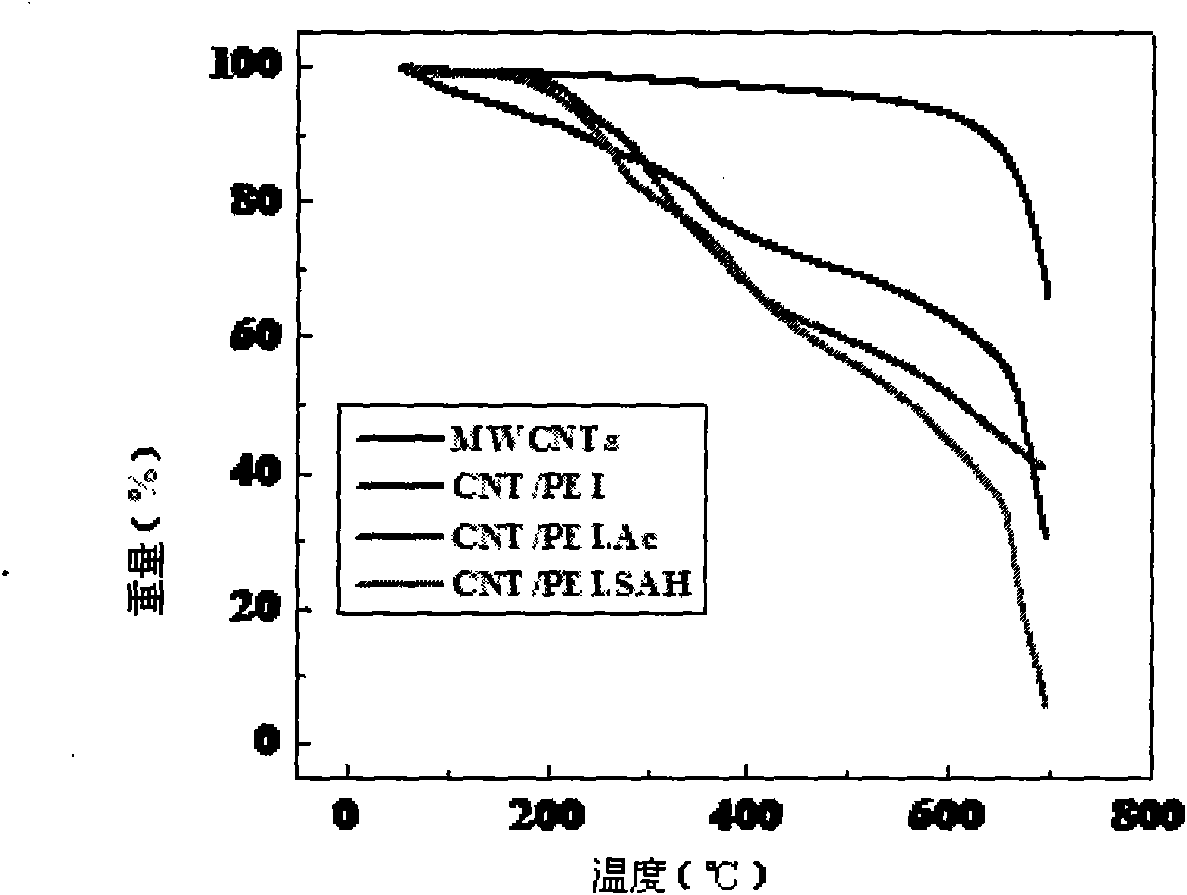
The invention relates to a method for preparing a functionalized carbon nanotube based on a polyethyleneimine, comprising the following steps of: (1) dipping the carbon nanotube in a strong acid for 2-4 hours, subsequently filtering, drying and storing the carbon nanotube; (2) conducting the circumfluence operation in the DMF of SOCl2 and introducing acyl chloride on the surface of the carbon nanotube and subsequently centrifuging and washing the carbon nanotube by the DMF; (3) dispersing the carbon nanotube in the DMF solvent and adding the DMF solution with PEI dissolved wherein; mixing the mixed solution by strong magnetic force; subsequently adding tri-ethylamine in the solution; repeatedly conducting a plurality of pure water dialysis after reaction; subsequently obtaining the CNT / PEI by freezing and drying; and (4) conducting acetylation reaction on the amino functional group on the surface of the CNI / PEI; or conducting carboxyl reaction on the amido on the surface of the CNT / PEI. The method has simple preparation process, moderate reaction condition, easy operation and industrial executive prospect; and the carbon nanotube prepared by the method can be dispersed in the solution for long, the agglomeration phenomenon does not occur and the carbon nanotube has excellent biocompatibility and safety in bio-medicals.
Owner:DONGHUA UNIV
Method for preparing gold nano particles and application of particles in detection of cyhalothrin
InactiveCN101892043AThe preparation method is simple and feasibleProcess conditions are stableMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsEthylamineNanometer particle
The invention provides a method for preparing gold nano particles. The method particularly comprises the following steps of: at room temperature, adding perchloric acid gold into distilled water to obtain 10<-2>mol / L aqueous solution of the perchloric acid gold; adding sodium citrate and sodium borohydride while stirring the aqueous solution; continuously stirring the mixed solution for 0.5 to 2 hours; and adding 3*10<-5>mol / L aqueous solution of mercaptoethylamine to obtain mercaptoethylamine-modified gold nano particles, wherein the weight ratio of the distilled water, the aqueous solution of the perchloric acid gold, the sodium citrate, the sodium borohydride to the aqueous solution of the mercaptoethylamine is 1:(0.008-0.01): (0.032-0.04):(0.04-0.12):(0.005-0.05). The method is simple and has high operability; and by the change of visible color of the gold nano particles prepared by the method, cyhalothrin in esbiothrin pesticides can be quickly identified and detected, and the detection limit reaches national standard value.
Owner:HUAZHONG NORMAL UNIV
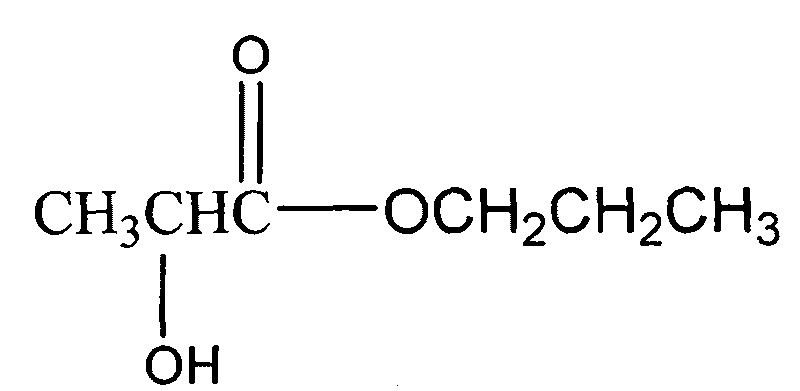
Method for preparing lactic acid n-propyl ester with low water content, low acidity and high purity
InactiveCN101759559AReduce utilizationOrganic compound preparationCarboxylic acid esters preparationN-Propyl alcoholOrganic synthesis
The invention relates to a method for preparing lactic acid n-propyl ester with low water content, low acidity and high purity, which is an alcohol ester preparation method belonging to the field of synthesis of fine organic chemicals. The method comprises the following steps of: adding lactic acid, n-propyl alcohol, a water-carrying agent and an esterification catalyst in the mass ratio of 90:(30-300):(45-450):(0.1-50) into a reactor, and reflux-reacting for 0.5-24 h; in situ removing water and low-boiling acid in an azeotropic mixture which is a water-carrying agent in an external circulating water-separating device by using the reactivity of anhydrous magnesium sulfate, anhydrous sodium sulfate and anhydrous zinc sulfate or calcium oxide with water; and removing a little amount of lactic acid remained in reacted material through the salification between an organic amine mixture and the lactic acid remained in the reacted material, wherein the organic amine mixture comprises ethylamine, diethylamine, triethylamine, propylamine, dipropyl amine and tripropyl amine in the mass ratio of (0.001-5):(0.001-8):(0.003-6):(0.0001-7):(0.001-10):(0.003-3). After being treated by an activated 4A and / or 5A molecular sieve, the lactic acid n-propyl ester product has the ester content more than 98.5 percent, the water content less than 0.1 percent, and the acidity (represented by OH<->) less than 0.5.
Owner:盐城市益泰化工有限公司
One-step process for the preparation of halide-free hydrophobic salts
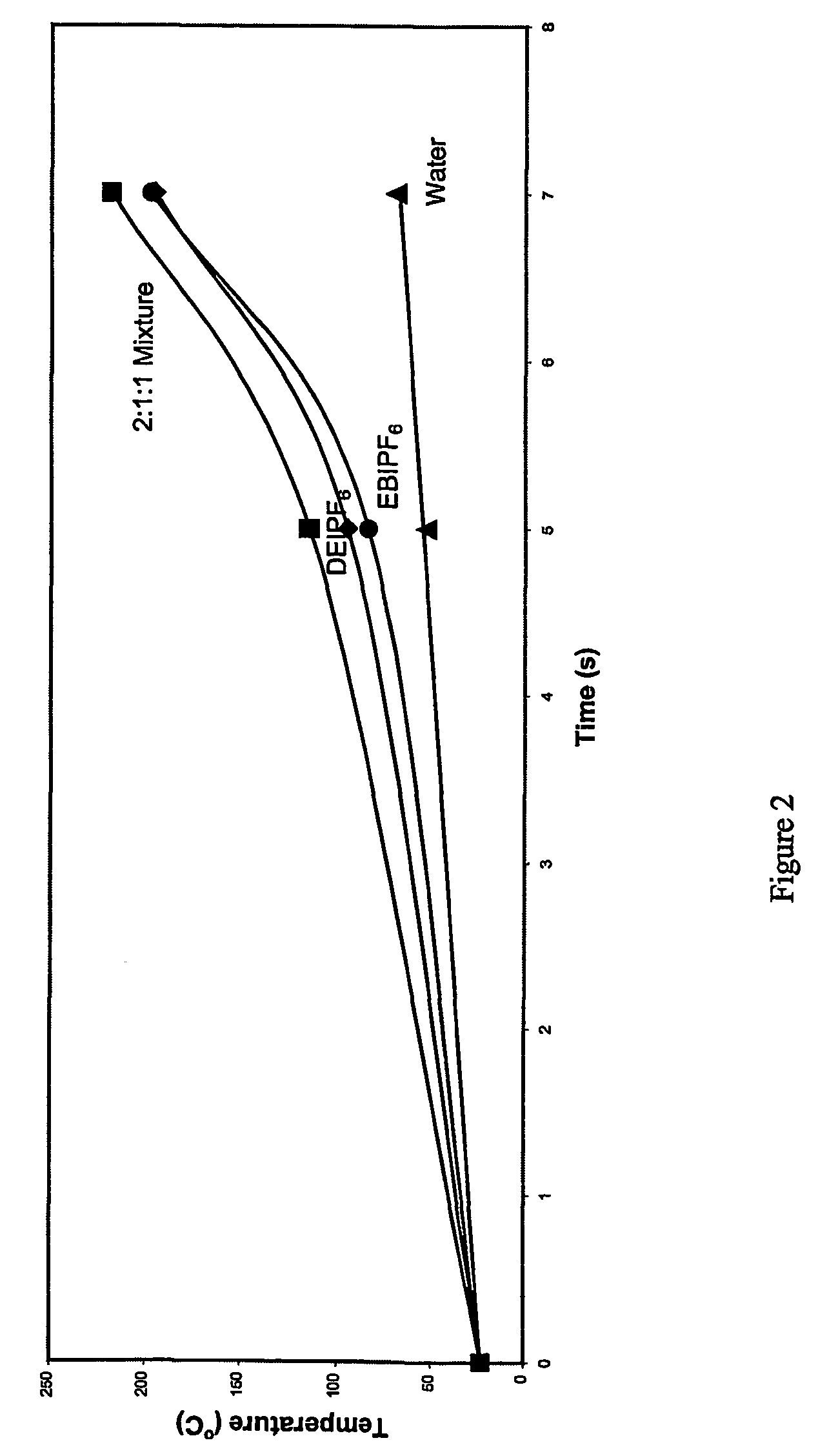
InactiveUS7253289B2Conveniently separatedImprove ionic conductivityGroup 5/15 element organic compoundsPropylamineSolvent
This invention describes a one pot, single-step process for the preparation of halide-free hydrophobic salts comprising polyalkylated imidazolium cations and various anions in accordance with the following structure, where R1 and R3 represent the either the same or different alkyl groups, and R2, R4, and R5 represent either hydrogen atoms, or the same or different alkyl group substituents; X represents a polyatomic anion that is the conjugate base of an acid. By simply mixing aqueous formaldehyde with an alkyl amine such as methylanune, ethylamine, n-propyl oriso-propylamine, or n-butyl-, iso-butyl, or t-butylamine, or by mixing aqueous formaldehyde with two alkyl amines (preferably one being methylamine, ethylamine, n-propyl- or iso-propylamine, or n-butyl-, isobutyl, or t-butylamine) and another being n-propyl- or isopropylaine, or n-butyl-, isbutyl, or t-butylamine), an acid (such as hexafluorophosphoric acid, trifluoroacetic acid, pentafluoropropionic, heptafluorobutyric acid, or the free acid of a bis(perfluoroalkylsulfonyprnide or tris(perfluoroalkylsulfonyl)methide as the source of the anion) and aqueous glyoxal solution, the hydrophobic ionic salts or mixtures thereof thus formed may be conveniently separated directly from the aqueous byproduct layer. Like the single cation hydrophobic salts, these mixed hydrophobic ionic liquids are non-flammable and manifest no detectable vapor pressure up to their decomposition temperature of greater than 300° C. We have also discovered that, surprisingly, ternary mixtures of dialkylated ionic liquids manifest higher ionic conductivities than a single ionic liquid of the mixture alone. This property benefits electrochemical power source applications such as batteries and capacitors. Furthermore, we have discovered that ternary mixtures of dialkylated ionic liquids absorb microwave radiation more efficiently than a single ionic liquid of the mixture alone. This property benefits microwave-induced synthetic reactions. Such physical and chemical properties make it possible to employ inexpensive mixtures of polyalkylated imidazolium cations in an advantageous manner as thermal transfer fluids, high temperature lubricants, and plasticizers, and as solvents in the areas of electrochemistry, synthetic chemistry, catalysis, and separations chemistry.
Owner:COVALENT ASSOCS
Method for preparing high-purity high-compactness battery-grade ferric phosphate
ActiveCN108840317AAvoid introducingEmission reductionNanotechnologyPhosphorus compoundsPhosphatePhosphoric acid
The invention provides a method for preparing high-purity high-compactness battery-grade ferric phosphate. The method comprises the following steps: firstly, enabling a certain amount of iron and 15-35% phosphoric acid to react at 40-100 DEG C so as to generate a phosphoric acid solution with ferrous ions, further heating the material liquid to 60-100 DEG C, adding a complexing agent, introducingozone to carry out oxidation for 3.5-7.5 hours so as to obtain ferric phosphate slurry, filtering the ferric phosphate slurry, repeatedly washing filter cakes, filtering till the filtrate is neutral,drying the collected solid for 2-4 hours, and calcining for 2-4 hours at 400-600 DEG C, thereby obtaining expected ferric phosphate, wherein the complexing agent is one or more of ethidene diamine, triethanolamine, 2-hydroxyl ethylamine, 1,2-propane diamine, 1,3-propane diamine, succinamide and malonamide. The method provided by the invention is simple in process, safe and environmental-friendly,high in atom utilization rate, low in cost and possible in continuous production, and the ferric phosphate prepared by using the method has an iron-phosphorus ratio of 0.96-0.99, a D95 granularity of78-150 nanometers and a compact density of 2.40-2.50g / cm<3>, and is particularly applicable to application as a raw material of lithium battery anode material lithium iron phosphate.
Owner:GUANGDONG GUANGHUA SCI TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com