Preparation method of carbon dot having high fluorescent quantum yield
A fluorescence quantum yield, carbon dot technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problem of quantum yield not higher than 30%, and achieve good solubility, high output and broad application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 1.051g (5mmol) of citric acid solid powder (with a crystal water, Beijing Chemical Plant) and dissolve it in 10ml of deionized water, measure 335μL (5mmol) of ethylenediamine (Xilong Chemical Co., Ltd.) and add it to the citric acid solution , stir well with a glass rod. The liquid was transferred into a 20mL polytetrafluoroethylene-lined stainless steel reaction kettle, the lid of the kettle was tightened, and the reaction was carried out at 200°C for 5 hours. The reactor was naturally cooled to room temperature to obtain an aqueous solution of carbon dots.
[0031] Put the obtained carbon dot aqueous solution into a 3500 molecular weight dialysis bag for dialysis for 48 hours. The collected dialyzate was combined, and the liquid was spin-dried using a rotary evaporator, and the solid was dried in a vacuum oven to obtain 0.89 g of brown solid powder, and the reaction yield was close to 70%.
[0032] The prepared carbon dot solid powder does not have fluorescent...
Embodiment 2
[0040] Weigh 0.420g (2mmol) of citric acid solid powder and dissolve it in 10ml of deionized water, measure 268μL (4mmol) of ethylenediamine into the citric acid solution, and stir evenly with a glass rod. (At the same time, citric acid and ethylenediamine millimoles can be configured as 1:1, 2:2, 5:5, 10:10, 1:5, 5:1, 4:2, 1:10, 10:1 10 mL of each solution) Transfer the liquid into a 20 mL stainless steel reaction kettle lined with polytetrafluoroethylene, tighten the lid of the kettle, and react at 200°C for 5 hours. The reactor was naturally cooled to room temperature to obtain an aqueous solution of carbon dots.
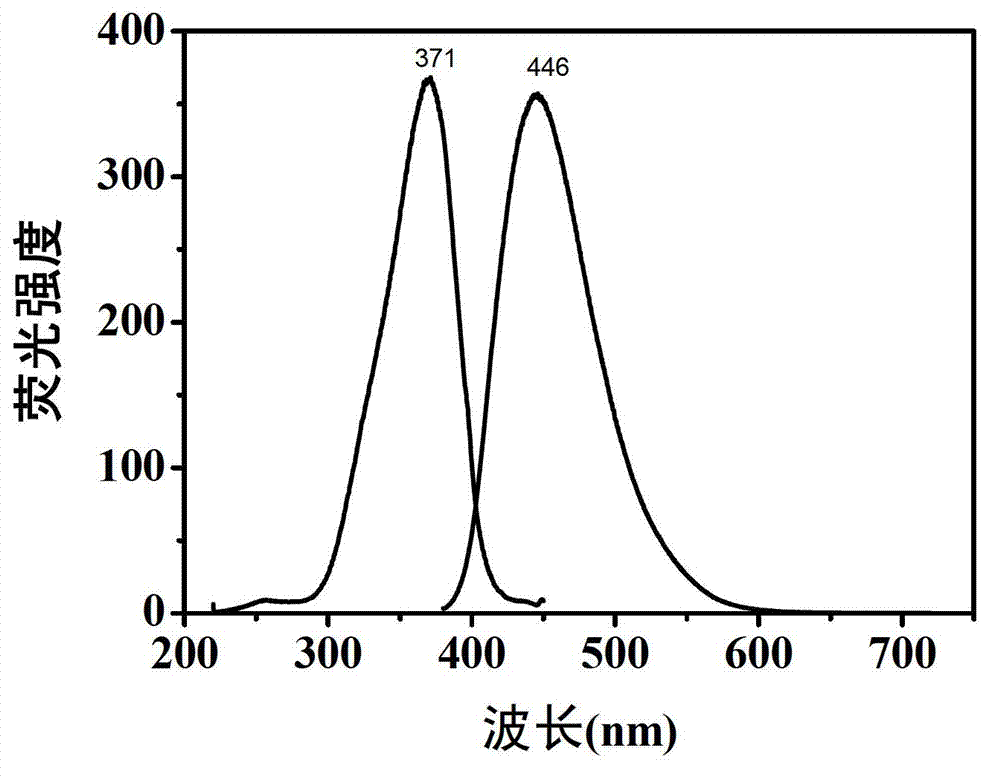
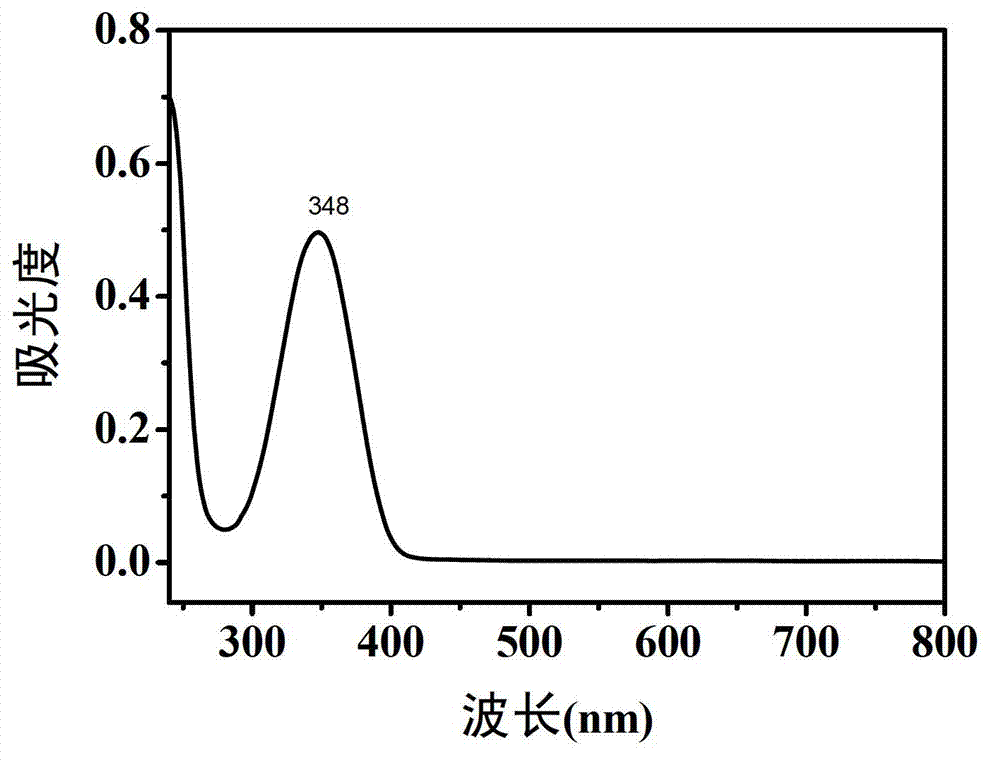
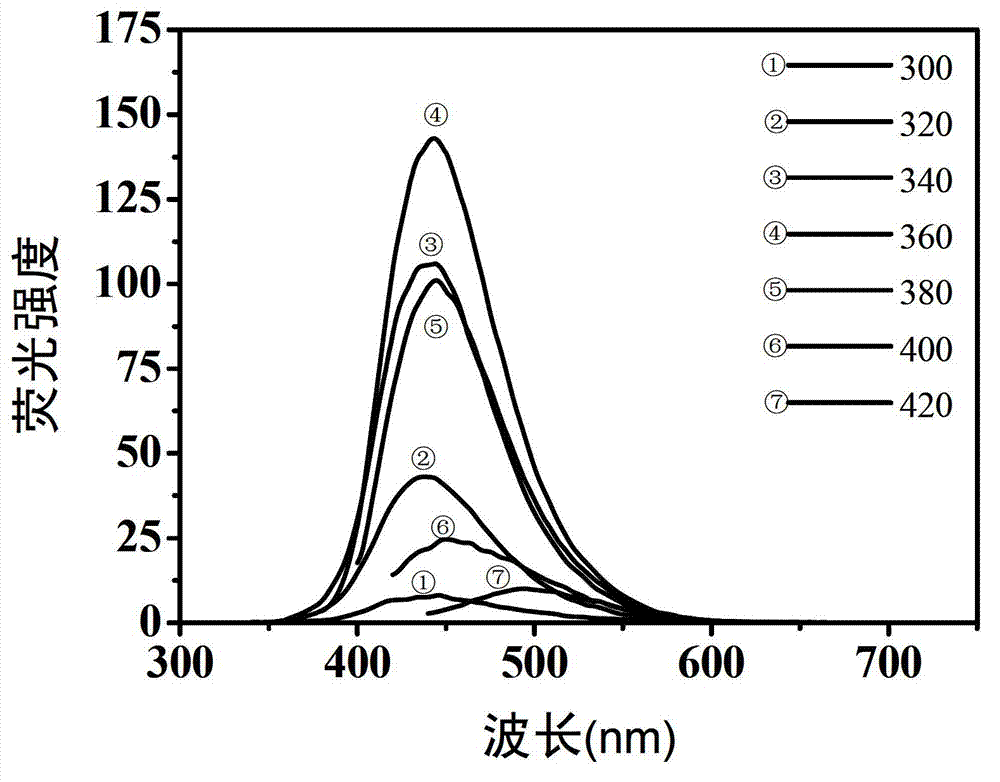
[0041] The fluorescent properties of carbon dot aqueous solutions prepared with different raw material ratios are similar, and the best excitation wavelength of each sample is around 370nm, and the best emission wavelength is around 445nm. We measured the solid content of each carbon dot aqueous solution and took quinine sulfate as a reference, measured the abso...
Embodiment 3
[0045] Weigh 1.051g (5mmol) of citric acid solid powder and dissolve it in 10ml of deionized water, measure 335μL of ethylamine and add it to the citric acid solution, and stir evenly with a glass rod. In addition, 335 μL of butanediamine, 600 μL of n-hexylamine, 0.303 g of urea, and 0.541 g of p-phenylenediamine were added to the citric acid solution of the same concentration. Each liquid was transferred into a 20mL polytetrafluoroethylene-lined stainless steel reaction kettle, the lid of the kettle was tightened, and the reaction was carried out at 200°C for 5 hours. The reactor was naturally cooled to room temperature to obtain an aqueous solution of carbon dots. .
[0046] The fluorescence properties of carbon dots prepared by different amine reactions are similar, and the fluorescence quantum yields are different. Among them, the fluorescence quantum yield of carbon dots using ethylenediamine as the starting material is higher than that of others (Table 3). In particula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com