Patents
Literature
32908 results about "Citric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Citric acid is a weak organic acid that has the chemical formula C₆H₈O₇. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
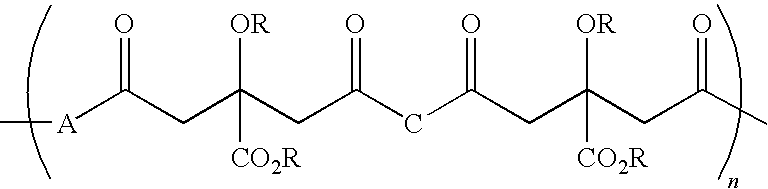
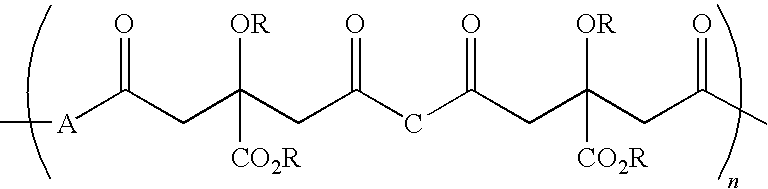
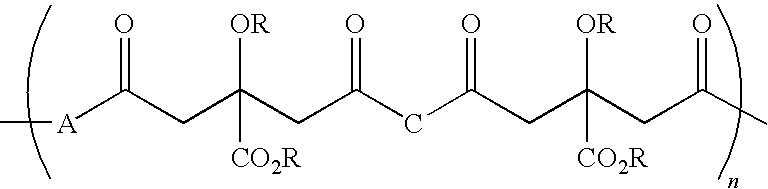
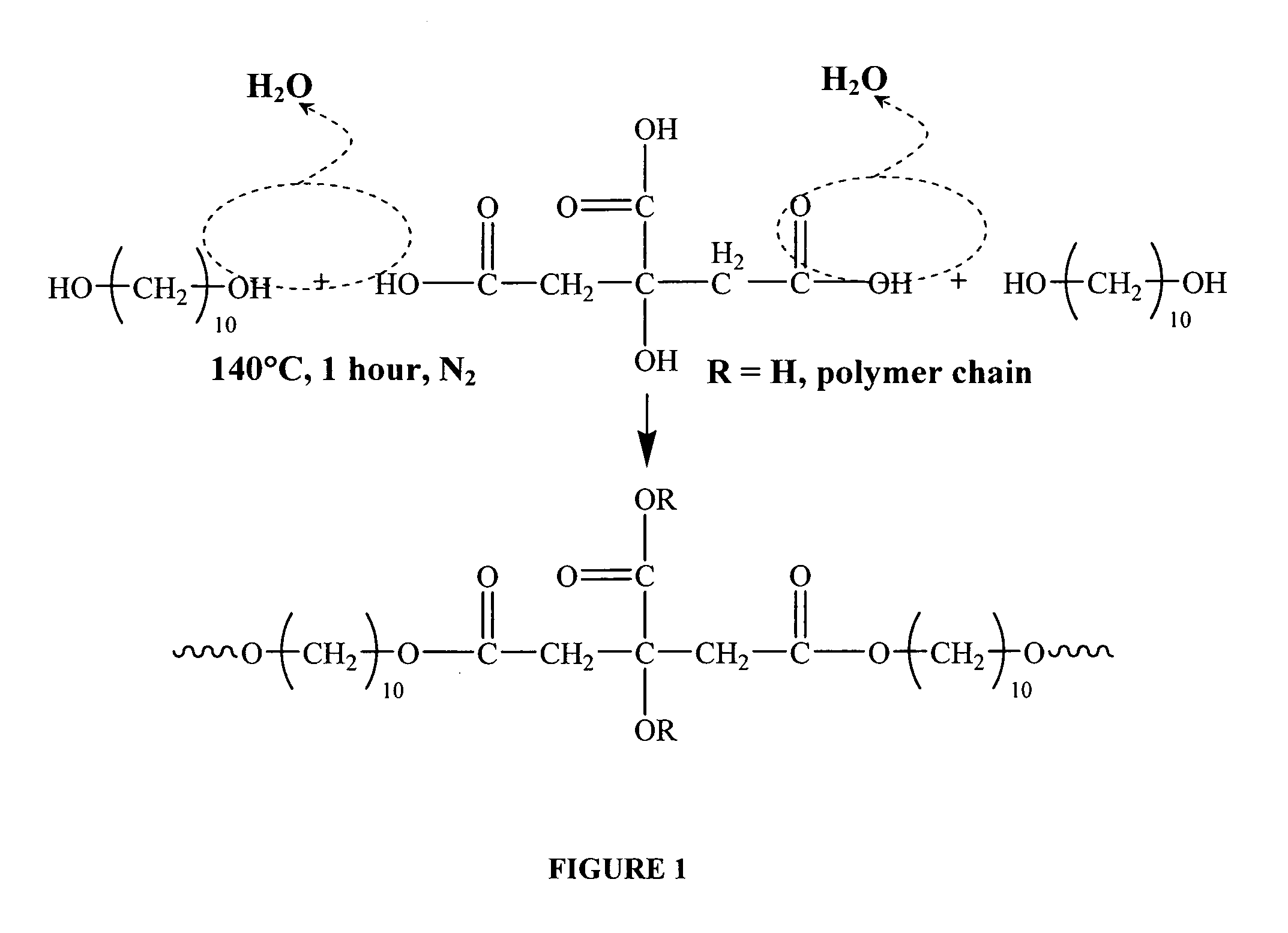
Citric acid polymers
InactiveUS20090325859A1Peptide/protein ingredientsGenetic material ingredientsPoly(ethylene glycol) dimethyl etherPolyethylene glycol
The present invention provides polymers (e.g., elastomeric citric acid polymers) and methods of making and using these polymers (e.g., as a biologically active molecule delivery platform). In certain embodiments, the polymer has adsorbed biologically active molecules. In particular embodiments, the polymer comprises pores that are between about 7 and 15 nanometers in diameter. In other embodiments, the polymer comprises poly(1,8 octanediol-co-ctric acid). In certain embodiments, the polymers are made by employing polyethylene glycol dimethyl ether (PEGDM).
Owner:NORTHWESTERN UNIV
Method for producing carbon coated nano stage lithium iron phosphate by precipitation
InactiveCN101393982AAvoid synthetic stepsEasy to controlElectrode manufacturing processesIron saltsPhosphate
The invention discloses a precipitation method for preparing nanometer level iron phosphate lithium coated with carbon. The method comprises the following steps: firstly, weighing iron salt, deionized water and a compound of metallic elements; after the stirring and the mixing are performed, adding a phosphorous compound and citric acid diluted with water to the mixture; after the stirring is performed again, adding a precipitation agent to the mixture and controlling to the neutrality; stirring to react in a container, and after the static placement, respectively adding the deionized water, a carbon source and lithium salt to mix uniformly after the precipitate is filtered and washed; stirring again to react, and drying the water at 30 to 160 DEG C and warming up at the heating rate under the protection of non-oxidized gas after a product is crashed; baking at a constant temperature of 450 to 850 DEG C, cooling down to a room temperature at a cooling rate or with a stove, and finally obtaining the nanometer level ferric phosphate lithium coated with the carbon after crashing is performed. The precipitation method has the advantage that the raw material cost and the processing cost are low because bivalent iron is taken as the raw material. The iron phosphate lithium prepared by using the process has the characteristics of good physical processing performance and good electrochemistry performance, and is suitable for industrialized production.
Owner:南京海泰纳米材料有限公司
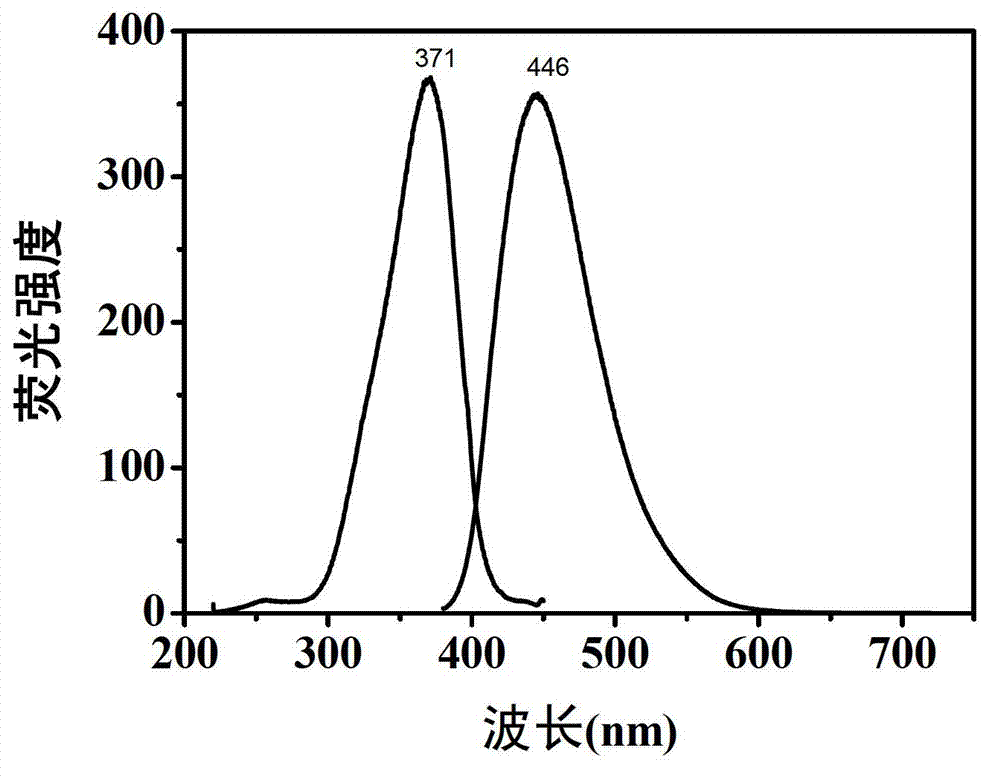
Preparation method of carbon dot having high fluorescent quantum yield
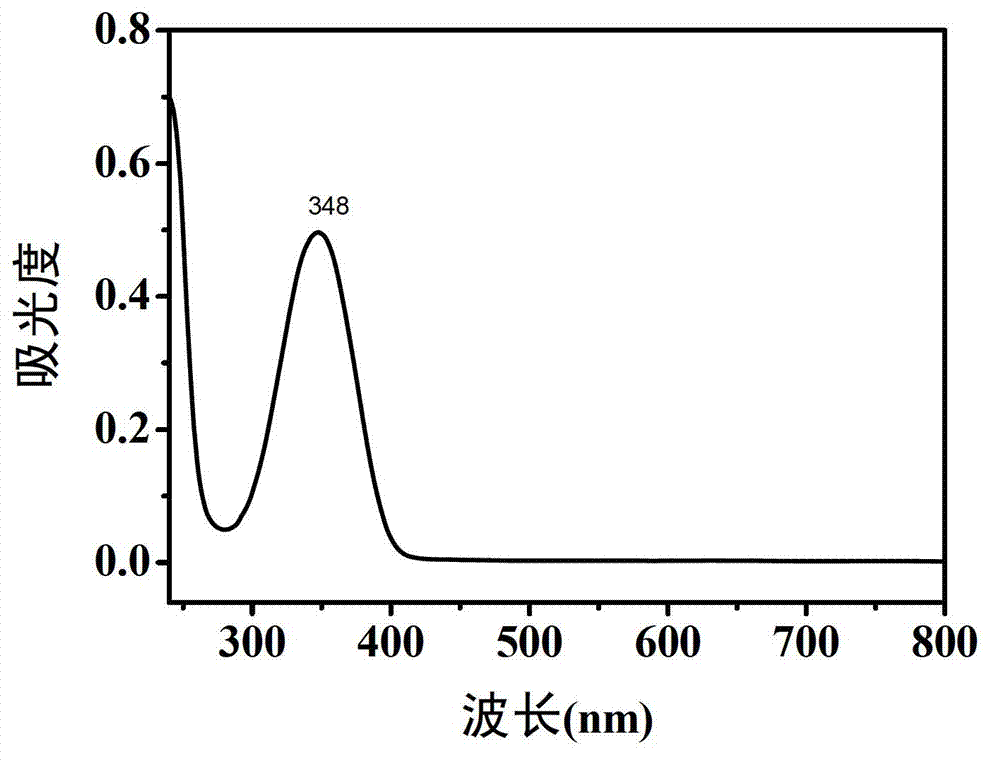
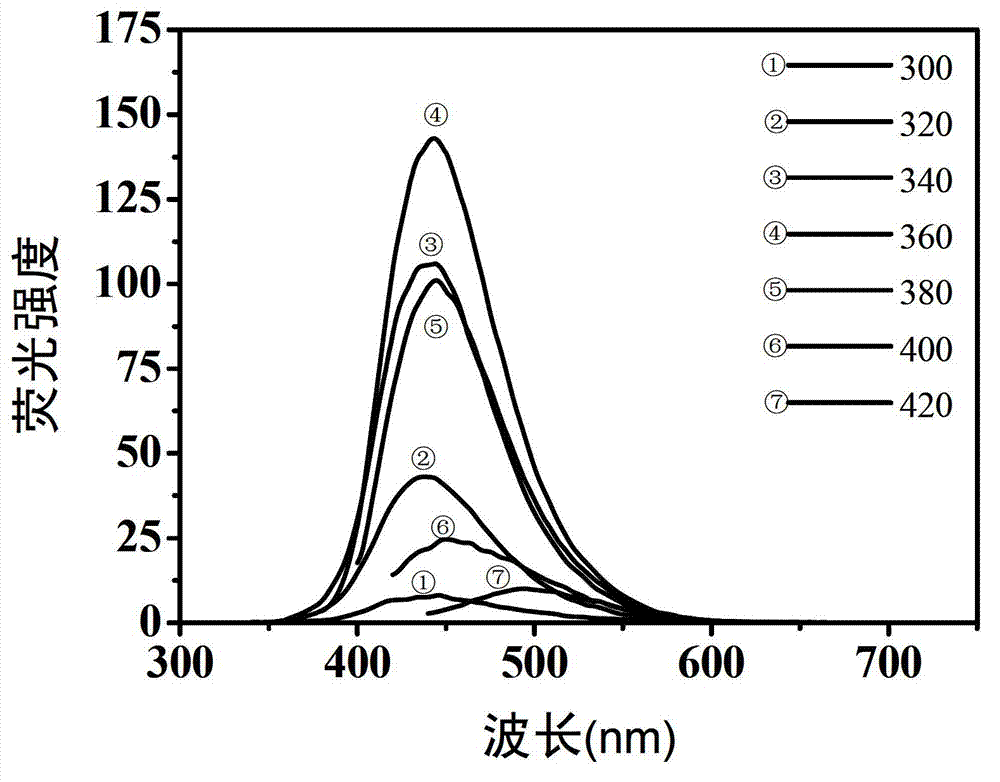
The invention belongs to the technical field of preparation of a carbon nano material, and particularly relates to a method for preparing a carbon dot having a high fluorescent quantum yield from citric acid and different nitrogen-containing molecules. The method comprises the following steps: weighing 1-10 mmol of solid citric acid, and dissolving in 10 ml of deionized water; weighing 1-10 mmol of ethylenediamine, ethylamine, propylamine, butanediamine, n-hexylamine, p-phenylene diamine or urea, adding into the citric acid solution, and uniformly stirring; transferring the solution into a hydrothermal or high-pressure microwave reaction kettle, reacting under hydrothermal or microwave conditions, and naturally cooling the reaction kettle to room temperature, thus obtaining a yellow or brown carbon dot water solution; and finally, purifying the carbon dot water solution, evaporating, and drying to obtain pure carbon dot solid powder. The carbon dot solution can send out bright blue fluorescence under the irradiation of a handheld ultraviolet lamp. The invention has wide application prospects in the fields of biological imaging, fluorescent printing and the like.
Owner:JILIN UNIV
Poly(ethylene chlorotrifluoroethylene) membranes
InactiveUS7247238B2Improve permeabilityImprove integrityMembranesSemi-permeable membranesFiberGlycerol
Porous polymeric membranes including Halar (poly (ethylene chlorotrifluoroethylene)) and related compounds and the methods of production thereof which avoid the use of toxic solvents. Preferred solvents, coating agents and pore forming agents are citric acid ethyl ester or glycerol triacetate. The membranes may be in the form of a hollow fiber or flat sheet, and may include other agents to modify the properties of the membrane, such as the hydrophilic / hydrophobic balance. Leachable agents may also be incorporated into the membranes.
Owner:EVOQUA WATER TECH LLC
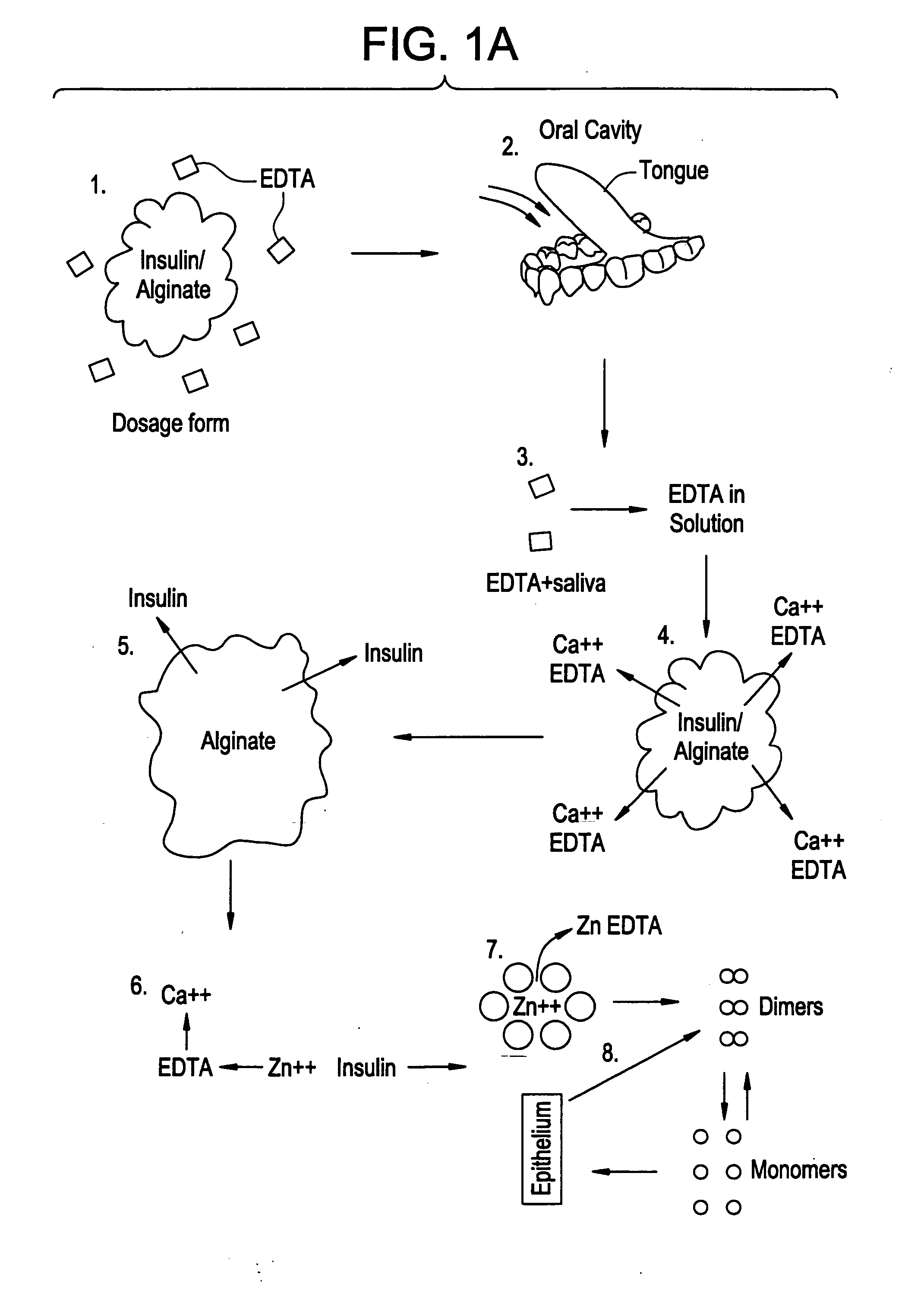
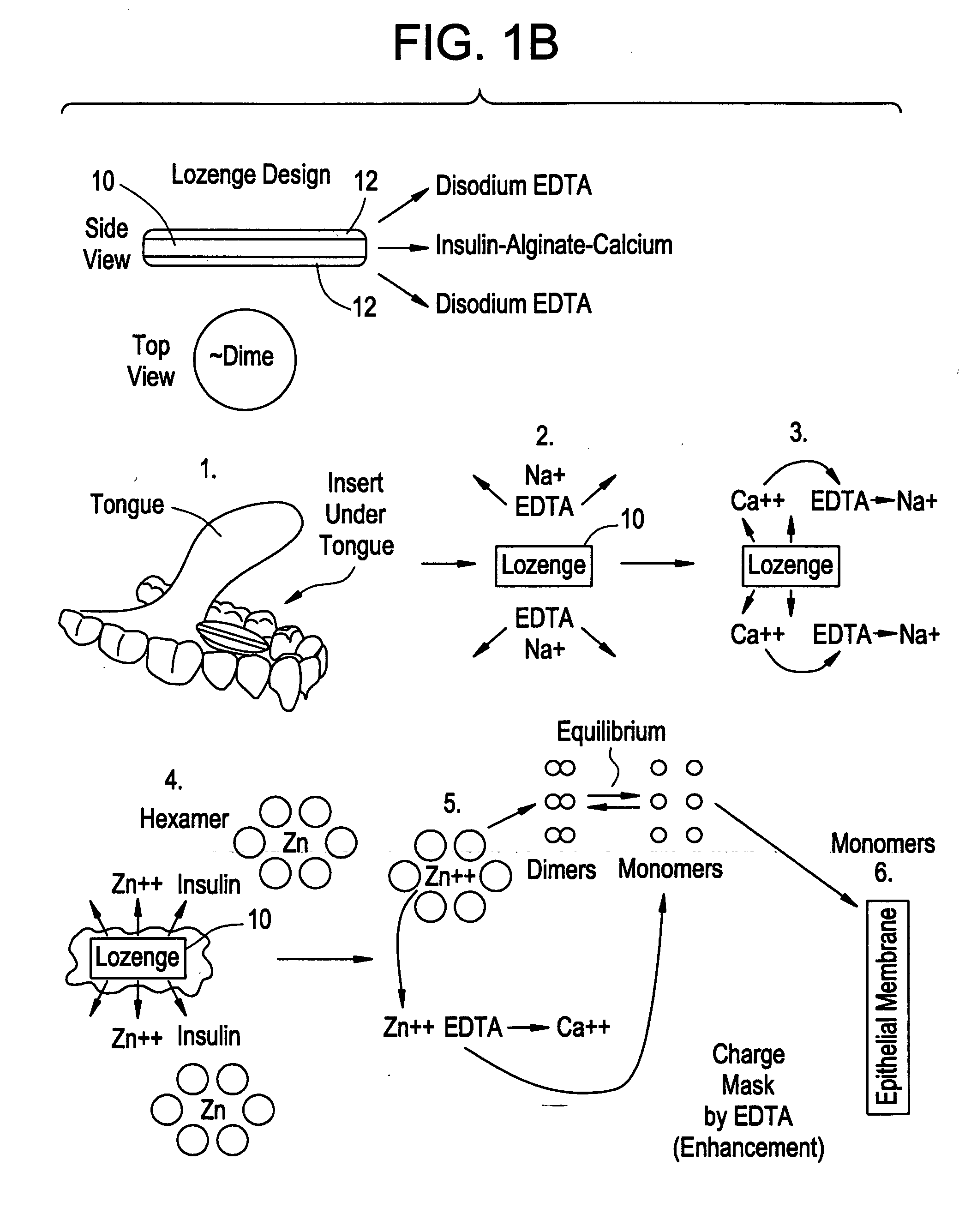
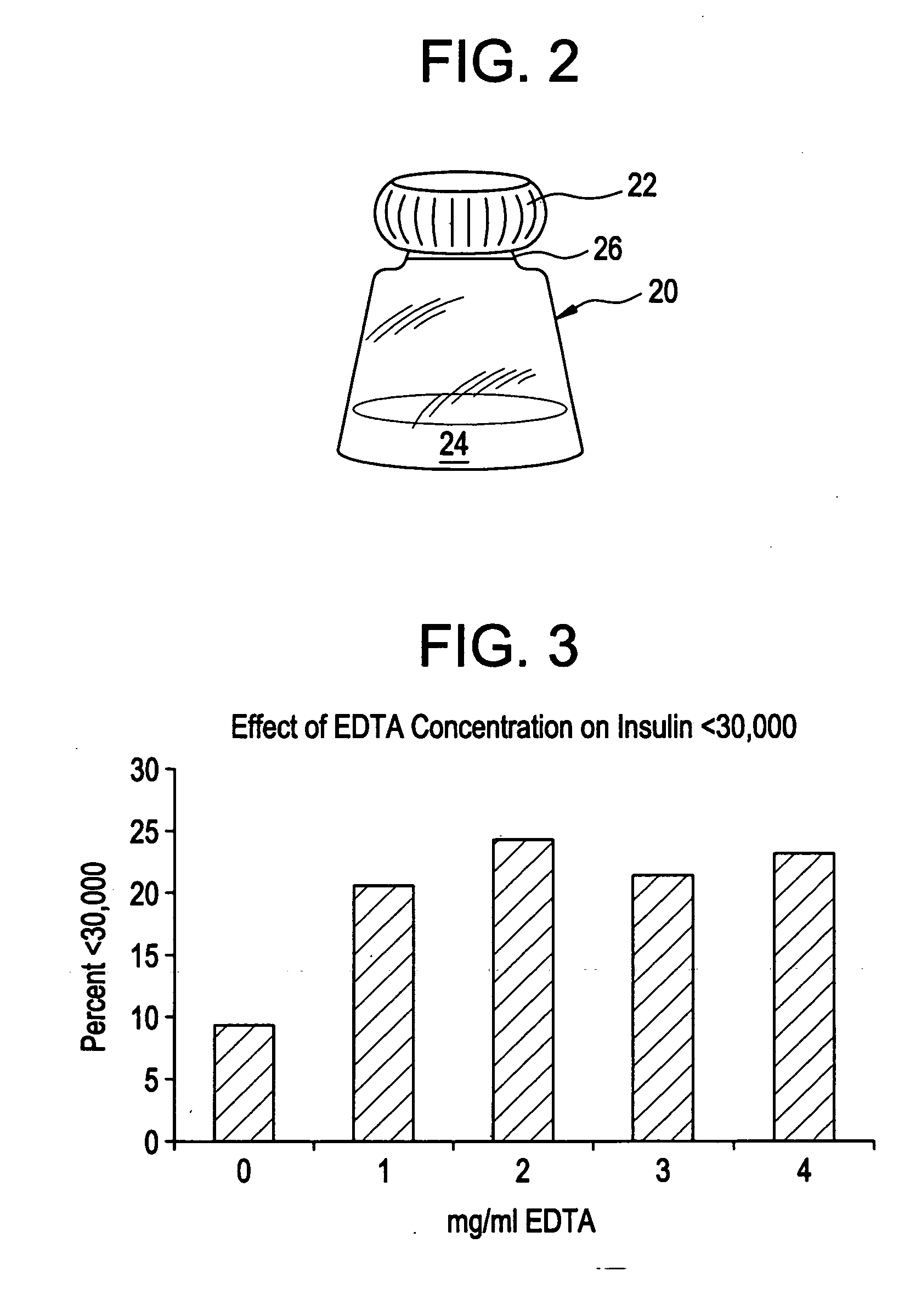
Rapid acting drug delivery compositions
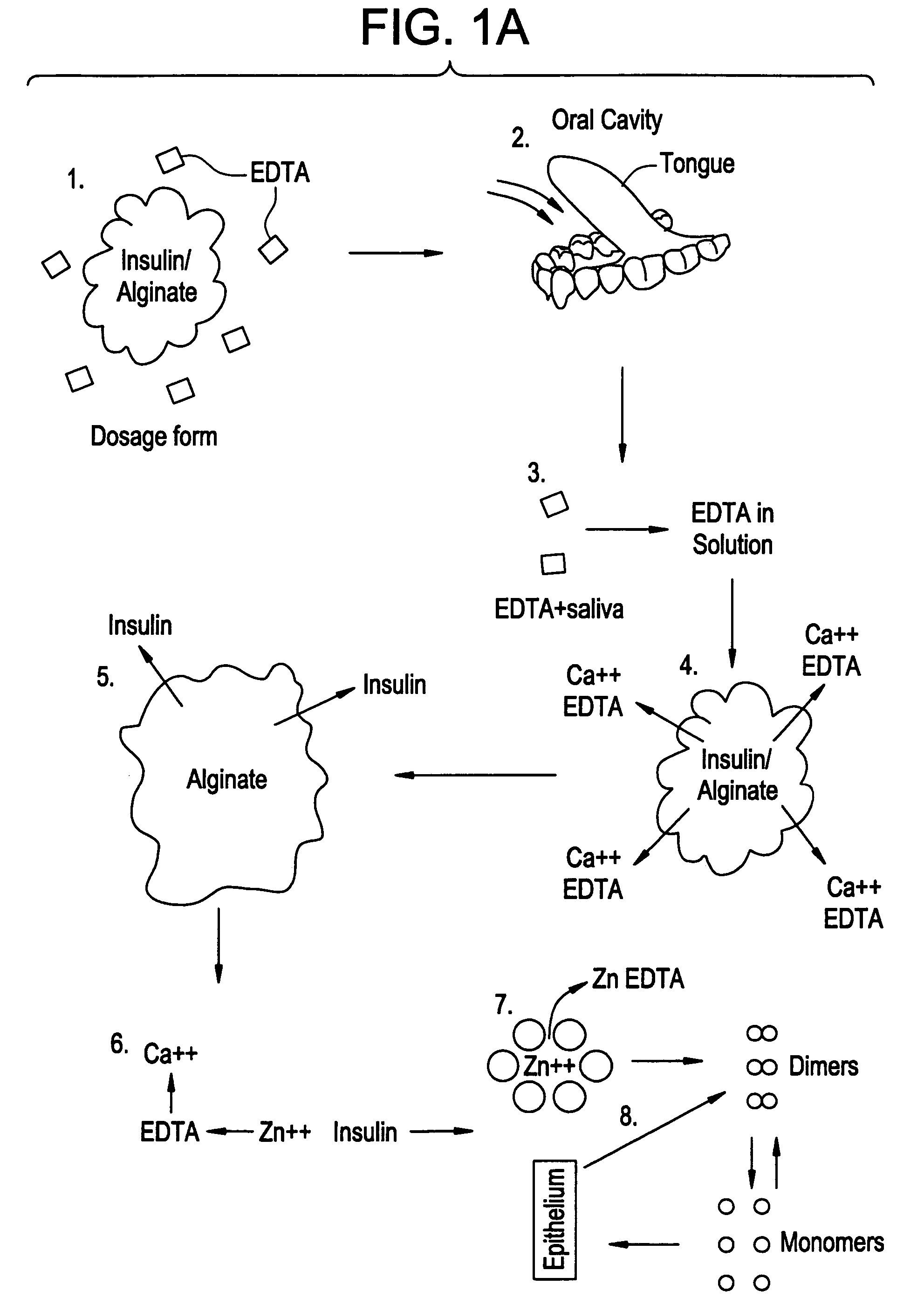
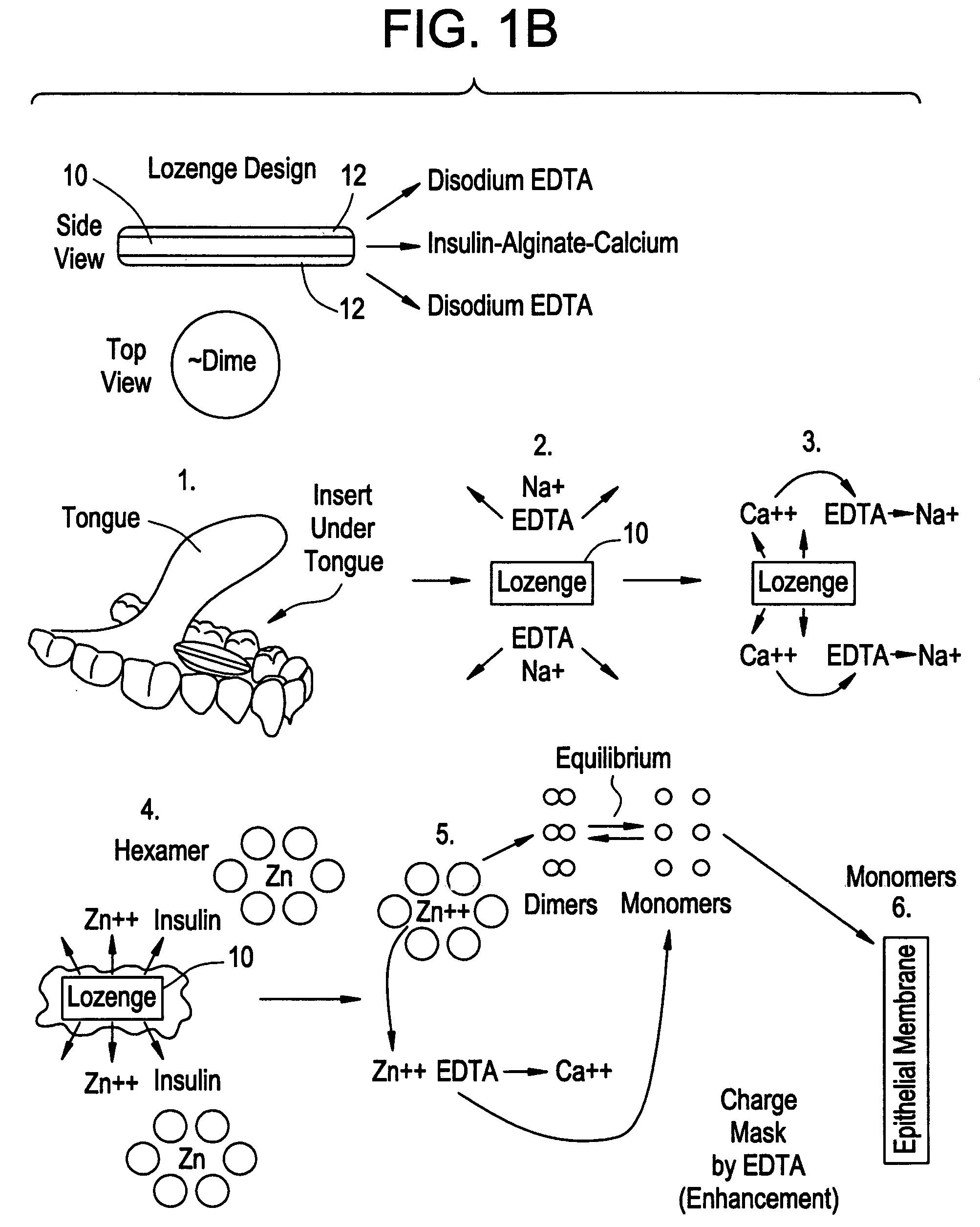
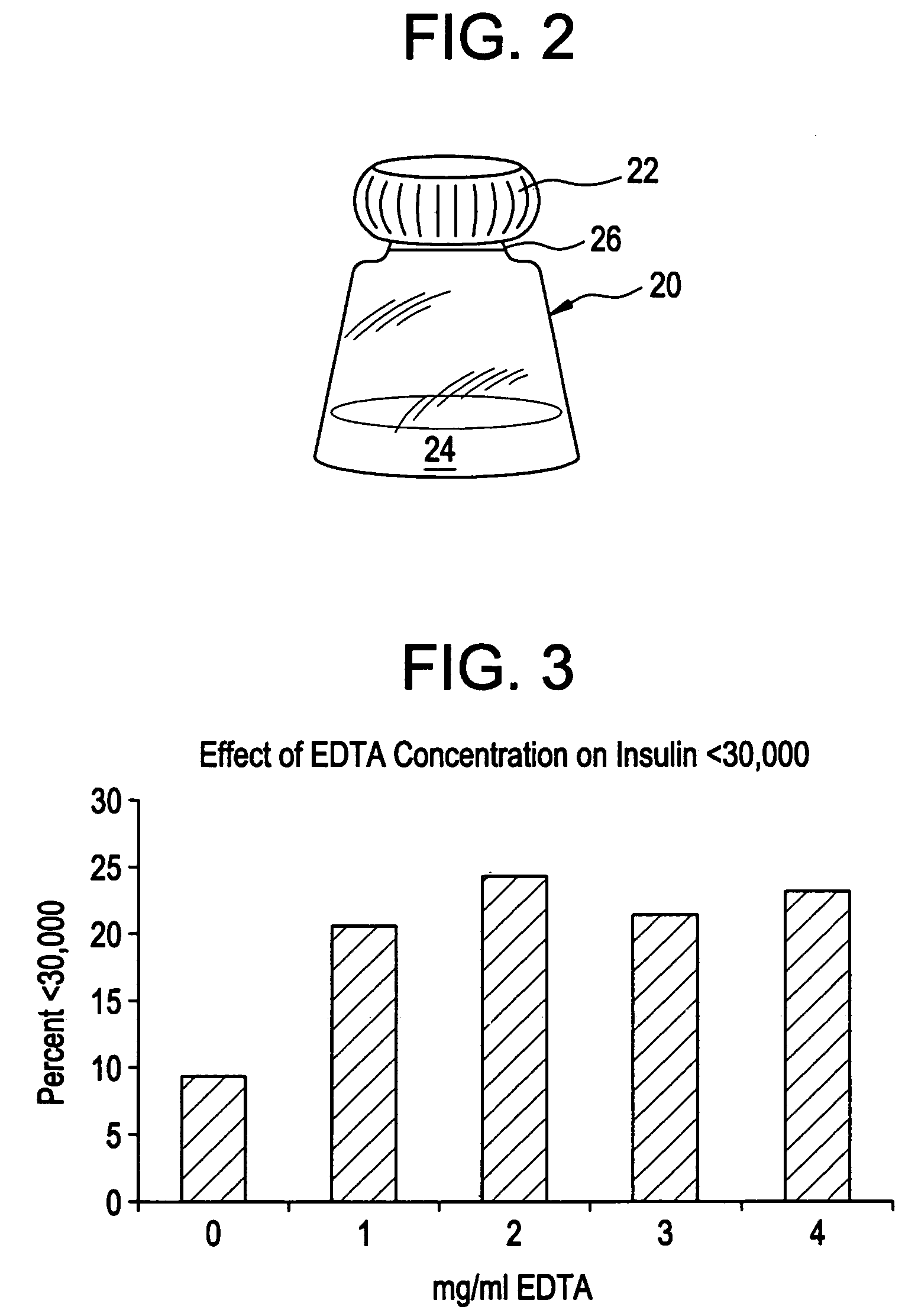
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Bio-based binders for insulation and non-woven mats
ActiveUS20110086567A1Readily availableLow costStarch dervative coatingsStarch adhesivesFiberProcedure Agents
An aqueous binder composition is provided that includes a carbohydrate and a crosslinking agent. In exemplary embodiments, the carbohydrate-based binder composition may also include a catalyst, a coupling agent, a process aid, a crosslinking density enhancer, an extender, a moisture resistant agent, a dedusting oil, a colorant, a corrosion inhibitor, a surfactant, a pH adjuster, and combinations thereof. The carbohydrate may be natural in origin and derived from renewable resources. Additionally, the carbohydrate polymer may have a dextrose equivalent (DE) number from 2 to 20. In at least one exemplary embodiment, the carbohydrate is a water-soluble polysaccharide such as dextrin or maltodextrin and the crosslinking agent is citric acid. Advantageously, the carbohydrates have a low viscosity and cure at moderate temperatures. The environmentally friendly, formaldehyde-free binder may be used in the formation of insulation materials and non-woven chopped strand mats. A method of making fibrous insulation products is also provided.
Owner:OWENS CORNING INTELLECTUAL CAPITAL LLC
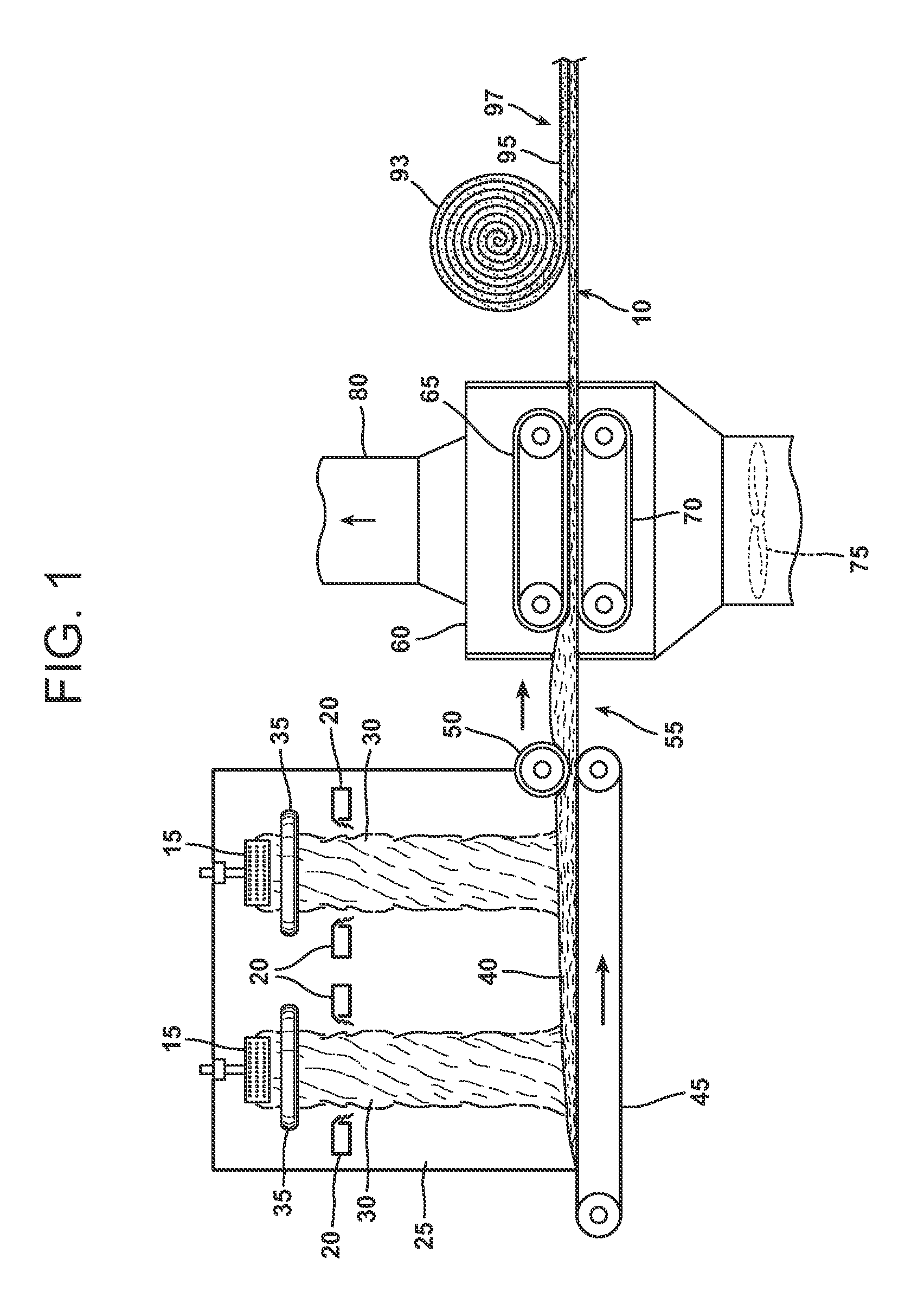
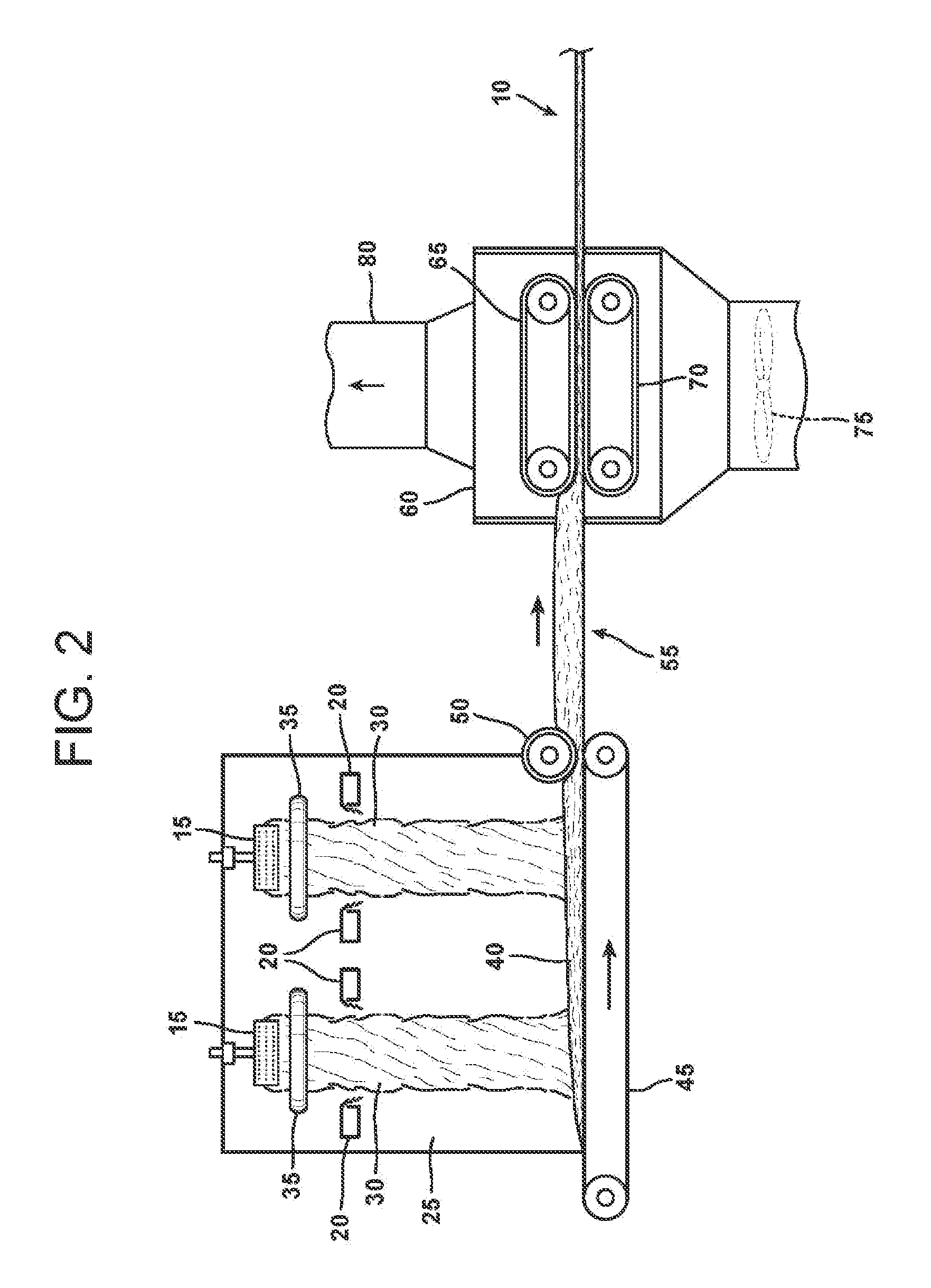
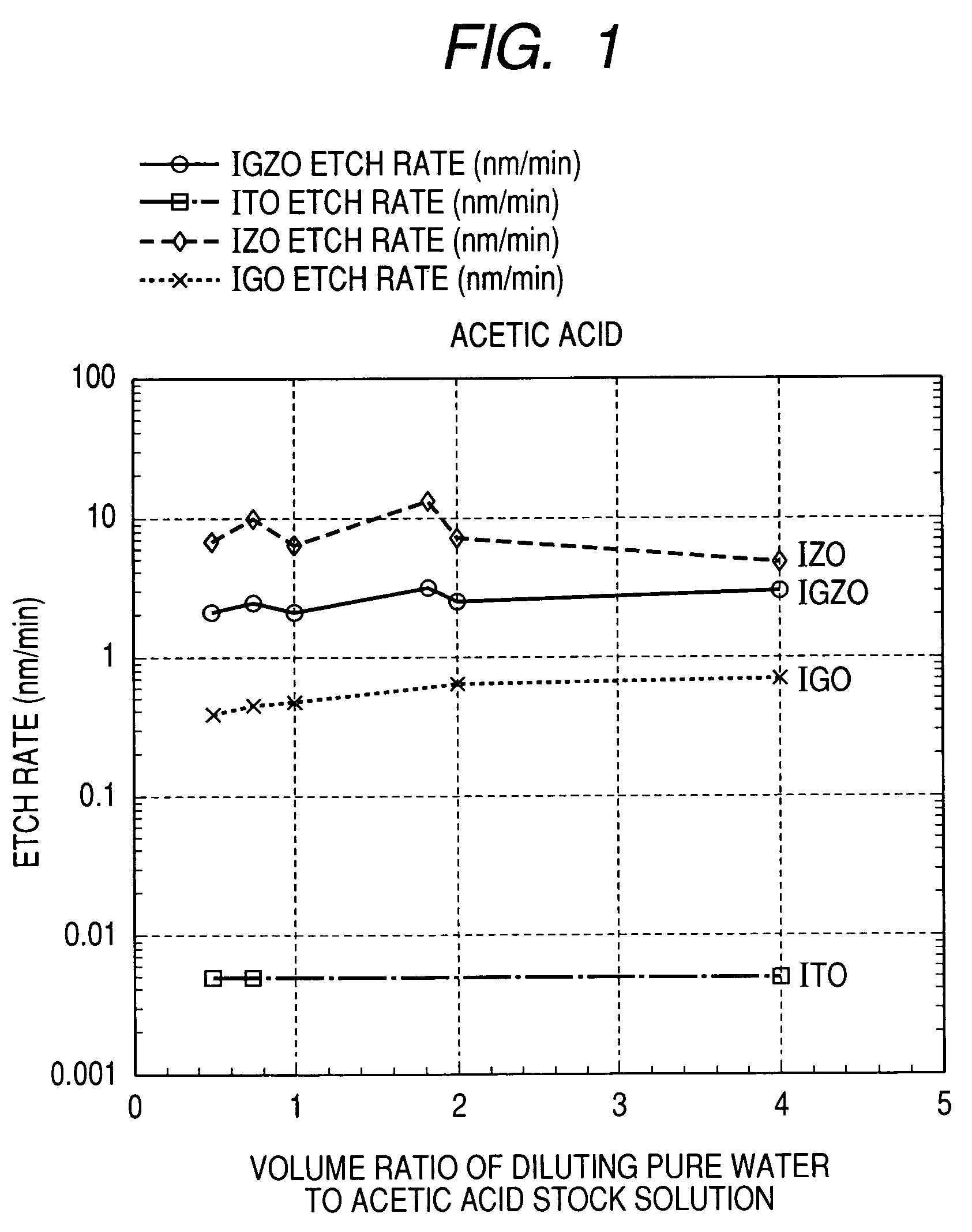
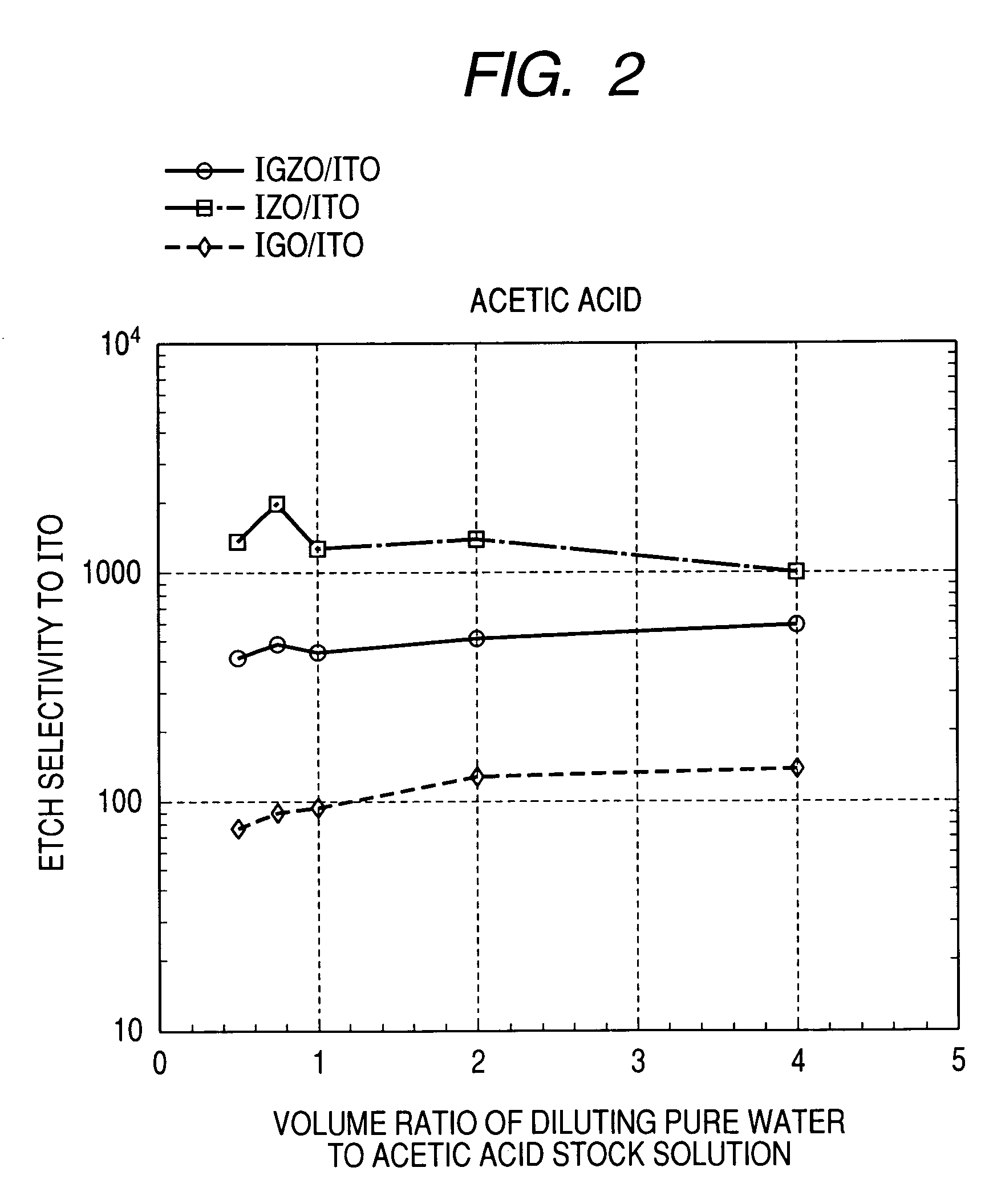
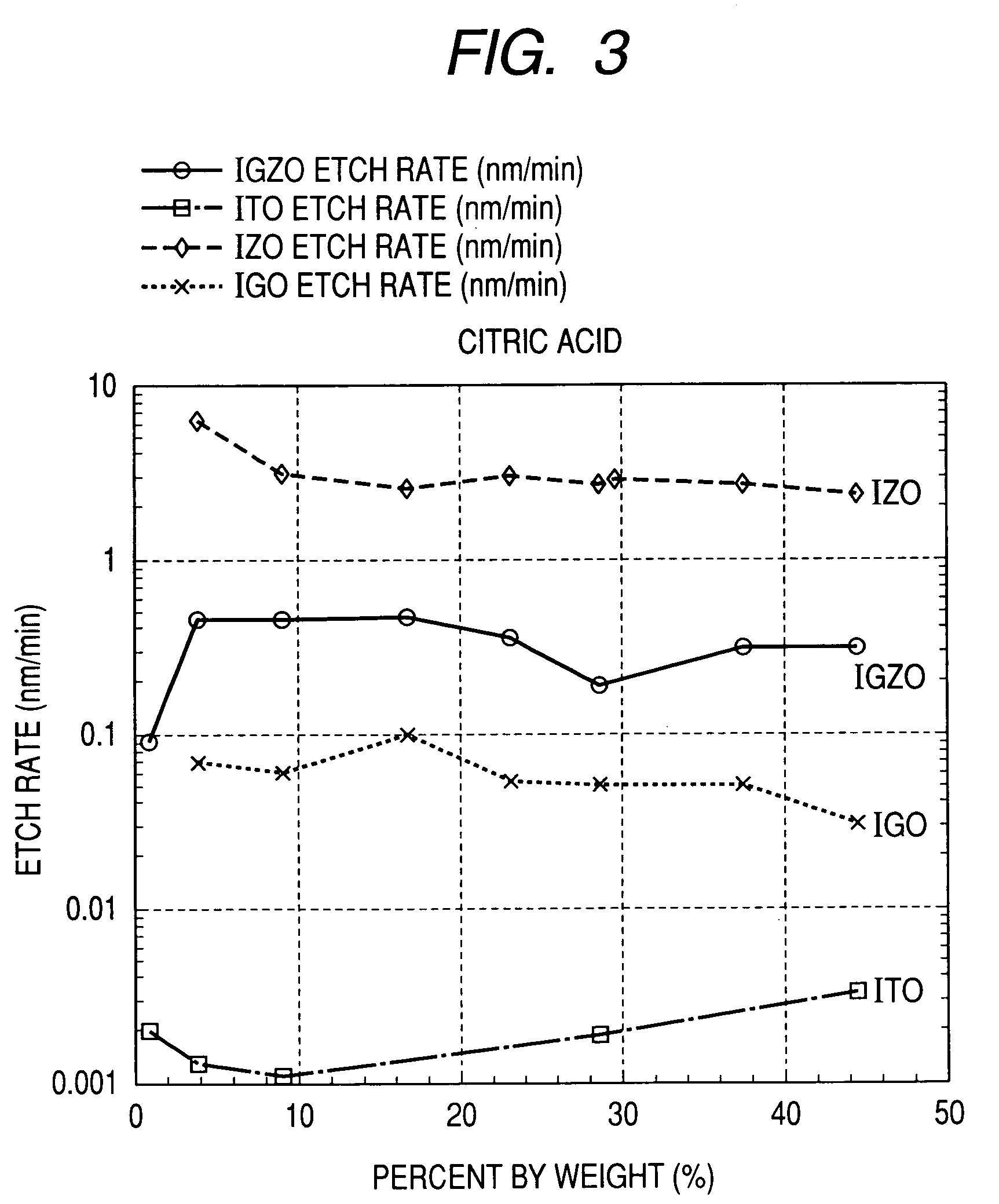
Oxide etching method
InactiveUS8168544B2Precise and easy etchingPrecise and highly selective wet etchingDecorative surface effectsFinal product manufactureAcetic acidEtching
There is provided an etching method of an amorphous oxide layer containing In and at least one of Ga and Zn, which includes etching the amorphous oxide layer using an etchant containing any one of acetic acid, citric acid, hydrochloric acid, and perchloric acid.
Owner:CANON KK
Novel multifunctional jam
The invention provides novel multifunctional jam, which comprises fruit flesh particles, wherein the jam comprises the following ingredients through being calculated according to the weight: 70 to 80 parts of fruit flesh, 0.1 to 0.3 parts of vitamin C, 0.3 to 0.8 parts of citric acid, 80 to 120 parts of sweeteners and 0.5 to 1.2 parts of thickeners. The novel multifunctional jam has the beneficial effects that the pigment-free, essence-free and preservative-free green healthy jam integrating nutrition, delicacy and multifunctional eating is provided, the use additives of the jam are few, meanwhile, the jam can be made into fruit tea, fruity milk or fruity yoghourt by warm water, milk, yoghourt and the like, and the requirements of different user groups can be met.
Owner:TIANJIN PENGFANCHENG FOOD
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Fly ash based lightweight cementitious composition with high compressive strength and fast set
InactiveUS20100071597A1Quick SetupEnhanced early and final compressive strengthSolid waste managementPolymer scienceCompressive strength
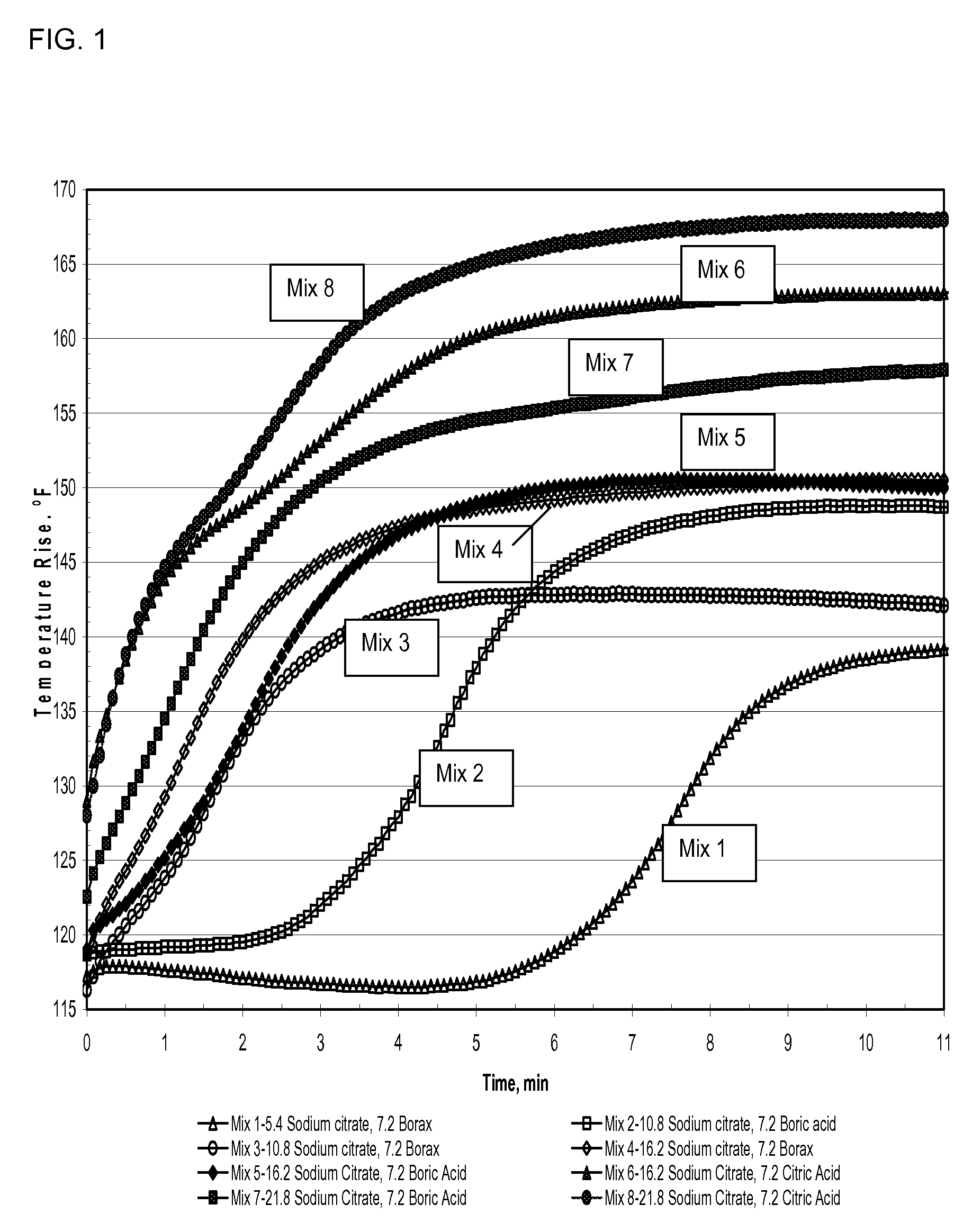
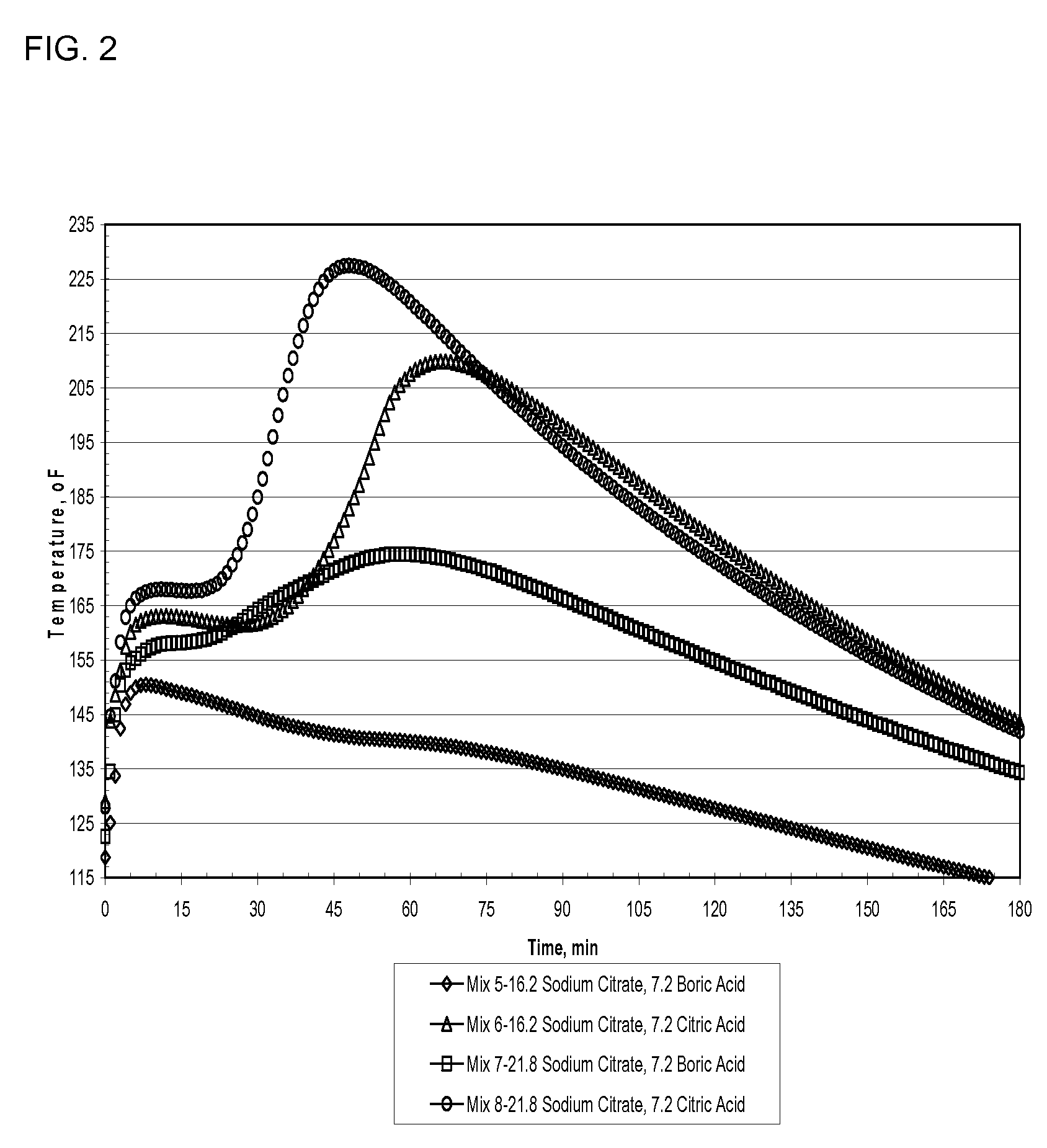
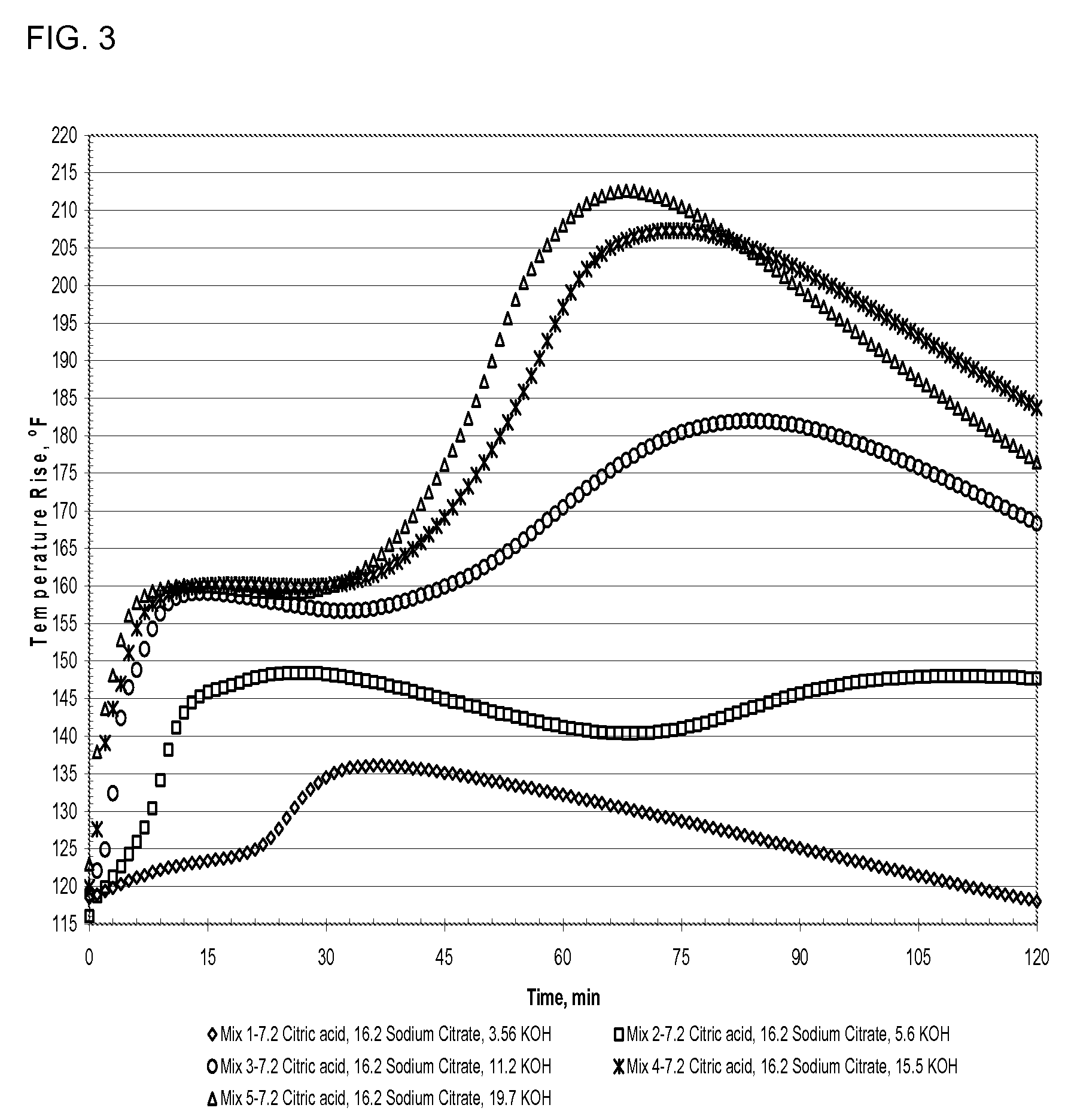
A method of making a rapid setting lightweight cementitious composition with improved compressive strength for products such as boards is disclosed. The method mixes fly ash, alkali metal salt of citric acid and lightweight aggregate with water. Compositions which include fly ash, alkali metal salts of citric acid and lightweight aggregate are also disclosed.
Owner:UNITED STATES GYPSUM CO
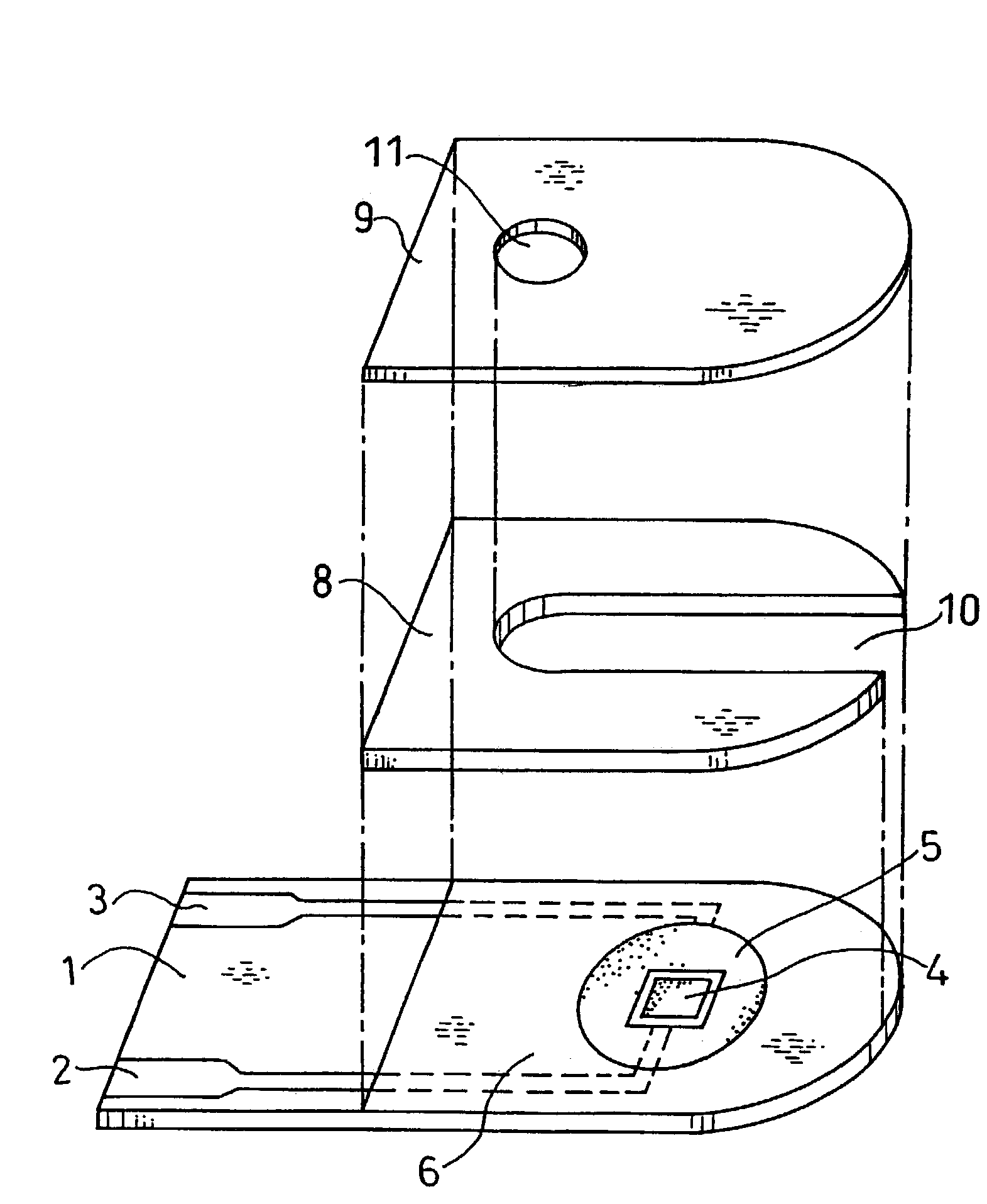
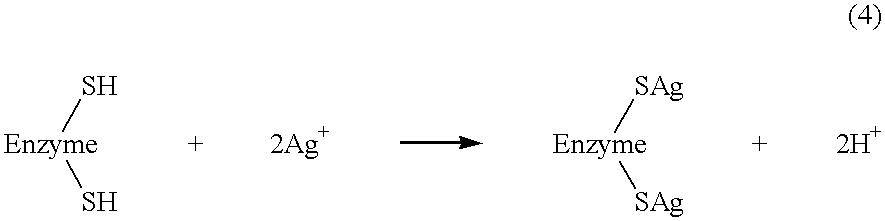
Biosensor
InactiveUS7235170B2Immobilised enzymesBioreactor/fermenter combinationsGluconolactonaseOxidoreductase
A biosensor that is highly responsive and capable of rapid and highly sensitive quantification of a specific component contained in a sample is provided. The biosensor of this invention comprises: an electrically insulating base plate; an electrode system comprising a working electrode and a counter electrode disposed on the base plate; and a reagent system comprising an oxidoreductase which catalyzes the oxidation reaction of glucose, gluconolactonase and a buffer. The buffer is selected from the group consisting of phthalic acid and its salts, maleic acid and its salts, succinic acid and its salts, phospholic acid and its salts, acetic acid and its salts, boric acid and its salts, citric acid and its salts, glycine, tris(hydroxymethyl)aminomethane, piperazine-N,N′-bis(2-ethane sulfonic acid) and the like.
Owner:PHC HLDG CORP
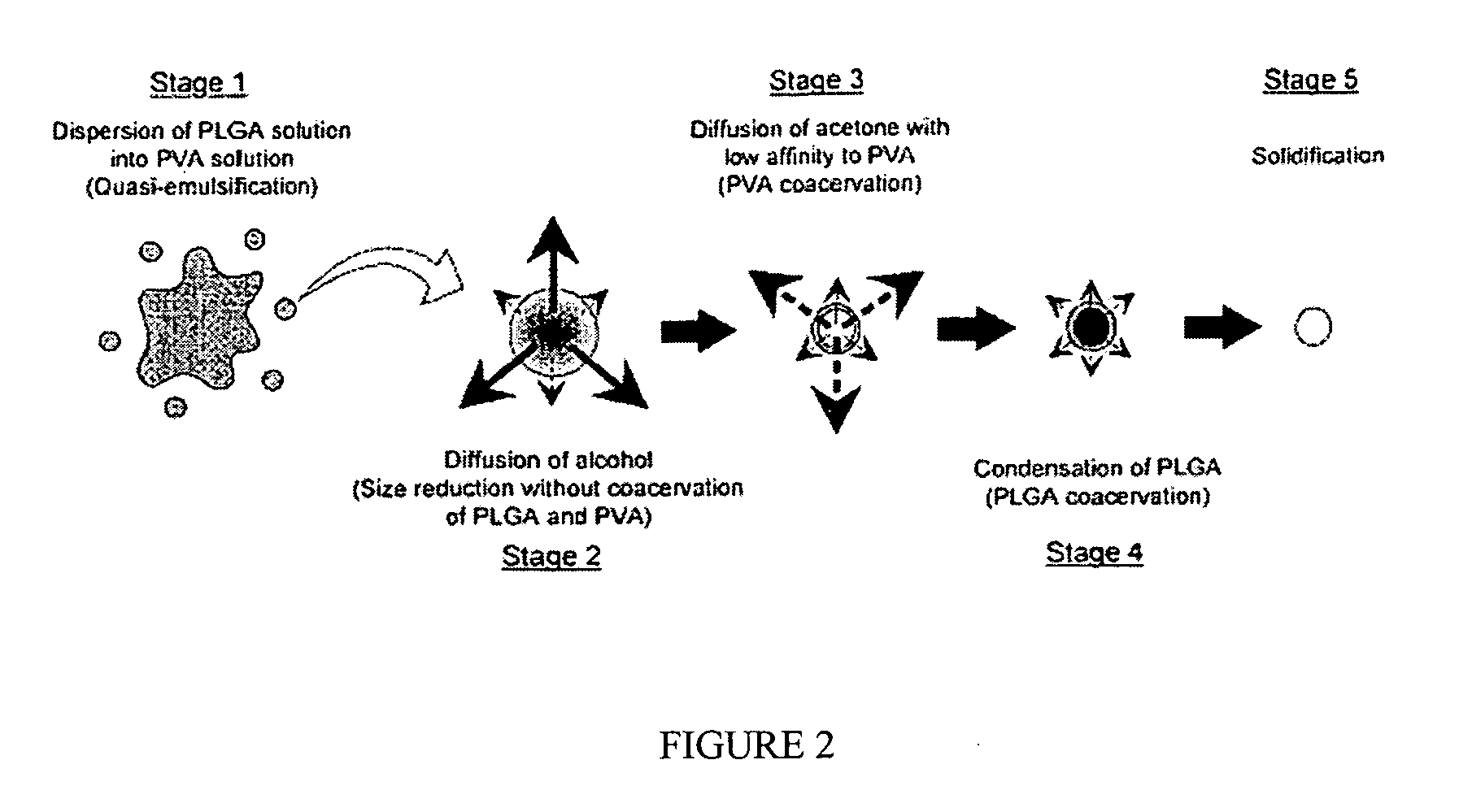
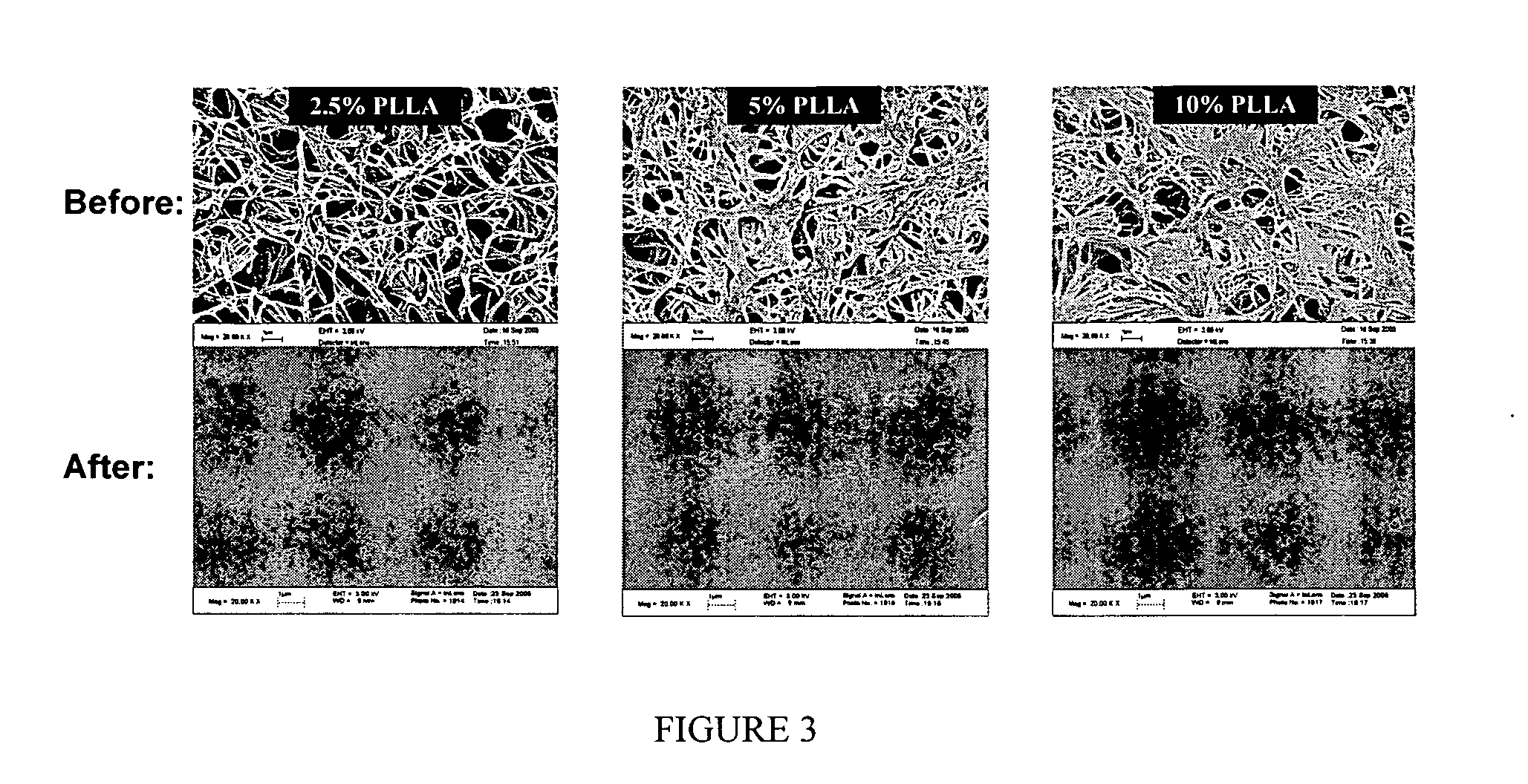
Biodegradable nanocomposites with enhance mechanical properties for soft tissue
InactiveUS20070071790A1Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsMechanical propertyNanocomposite
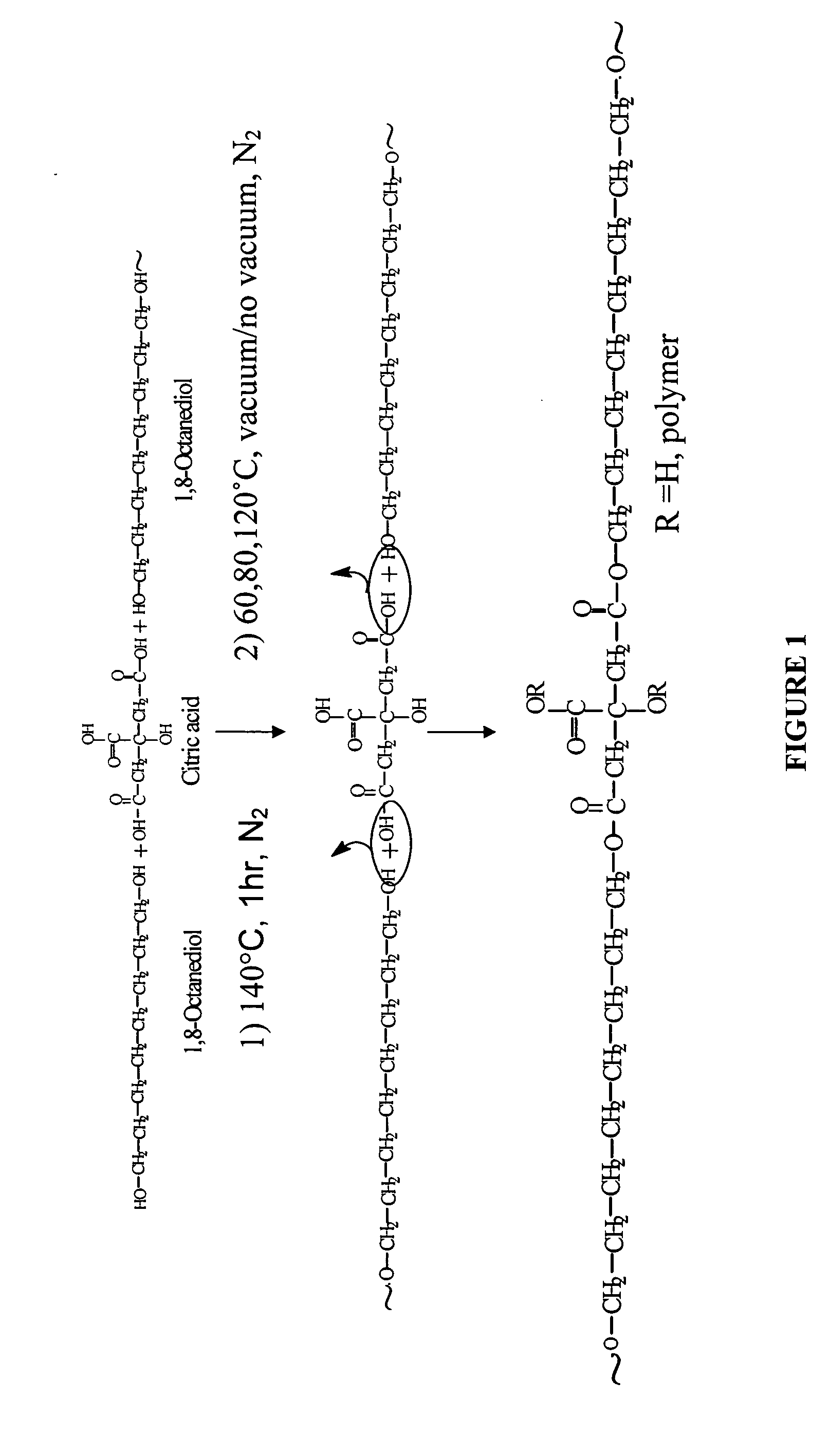
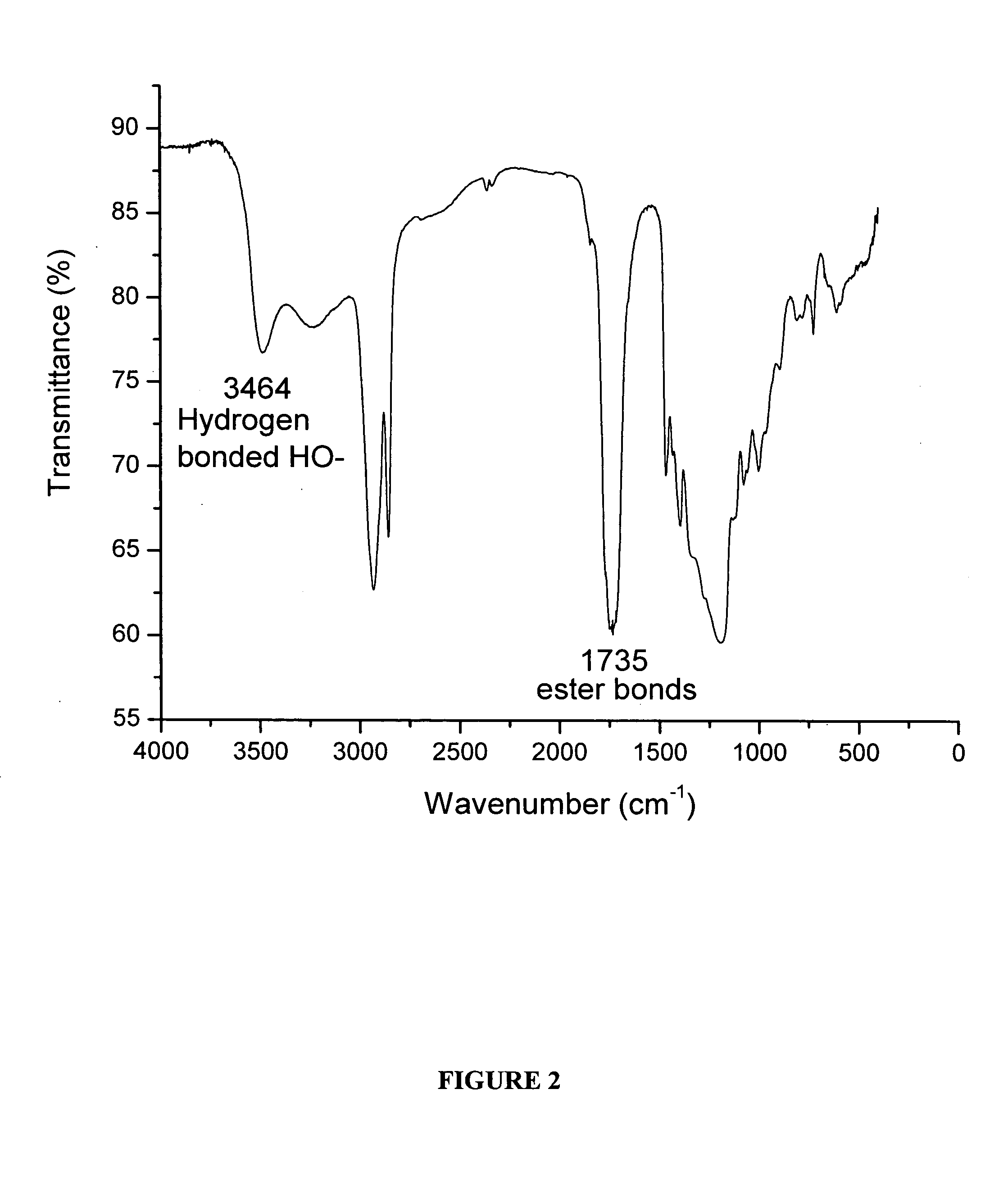
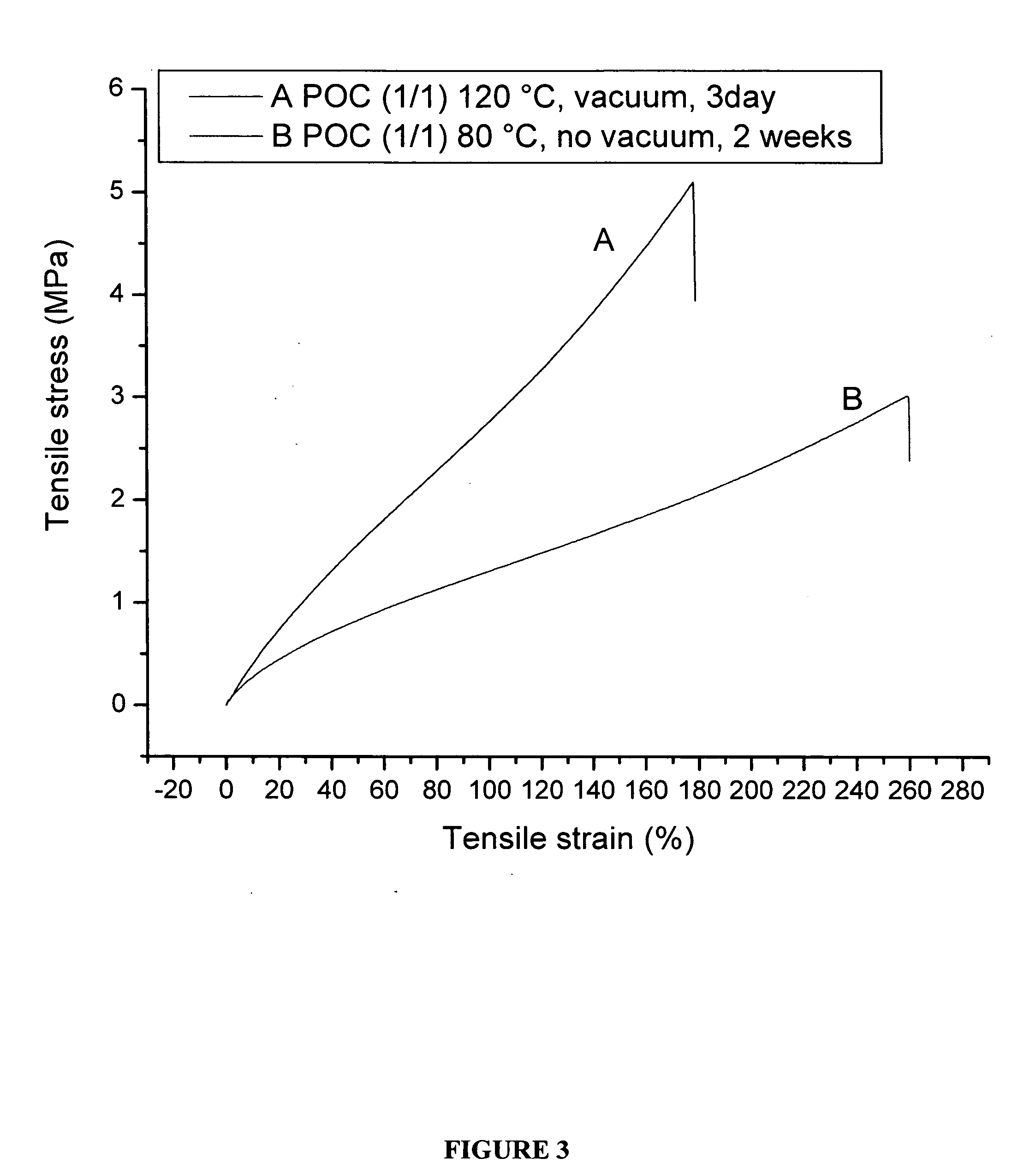
The present invention is directed to a novel poly(diol citrates)-based nanocomposite materials created using completely biodegradable and biocompatible polymers that may be used in tissue engineering. More specifically, the specification describes methods and compositions for making and using nanocomposites comprised of citric acid copolymers and polymers including but not limited to poly(L-lactic acid) (PLLA) and poly(lactic-co-glycolic acid) (PLGA).
Owner:NORTHWESTERN UNIV
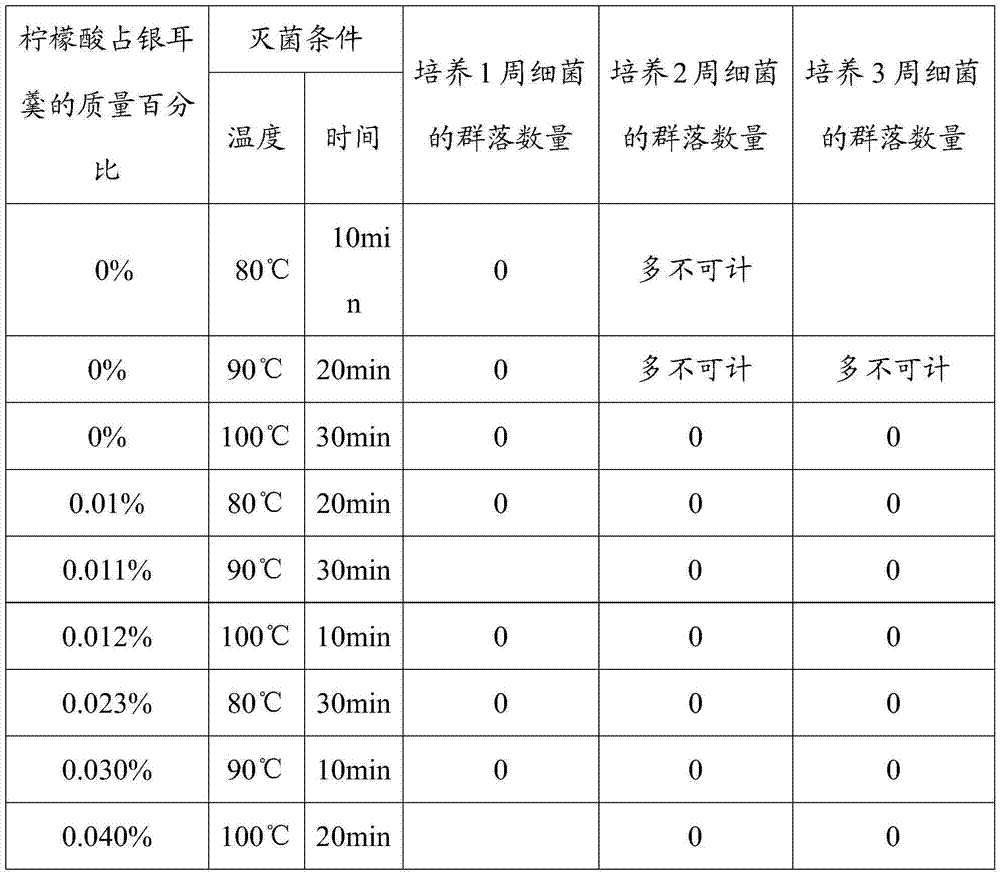
Ready-to-eat white fungus soup and preparation method thereof
The invention belongs to the technical field of food processing, and particularly relates to ready-to-eat white fungus soup and a preparation method thereof. The ready-to-eat white fungus soup is prepared from the following components in percentage by weight: 12.1-20.0% of fresh white funguses, 3.0-10.0% of crystal sugar, 0.011-0.040% of citric acid, 3.0-10.0% of red jujubes, 3.0-10.0% of lily bulbs, and the balance being water. The method for preparing the ready-to-eat white fungus soup comprises the following steps of preparing white fungus pulp, preparing mixed liquor and preparing finished products. The white fungus soup in which the fresh white funguses are used as main raw materials is rich in nutrition, has the efficacies of nourishing yin, nourishing the lung, nourishing the stomach, nourishing the heart and soothing the nerves, is fragrant and sweet in taste, and refreshing and smooth in mouth feel. The defects that in the prior art, white fungus soup products are single in nutrient components, and the taste of the conventional white fungus soup products cannot meet the requirements of different crowds for taste are overcome.
Owner:谢勇 +1
Disinfection of dead-ended lines in medical instruments
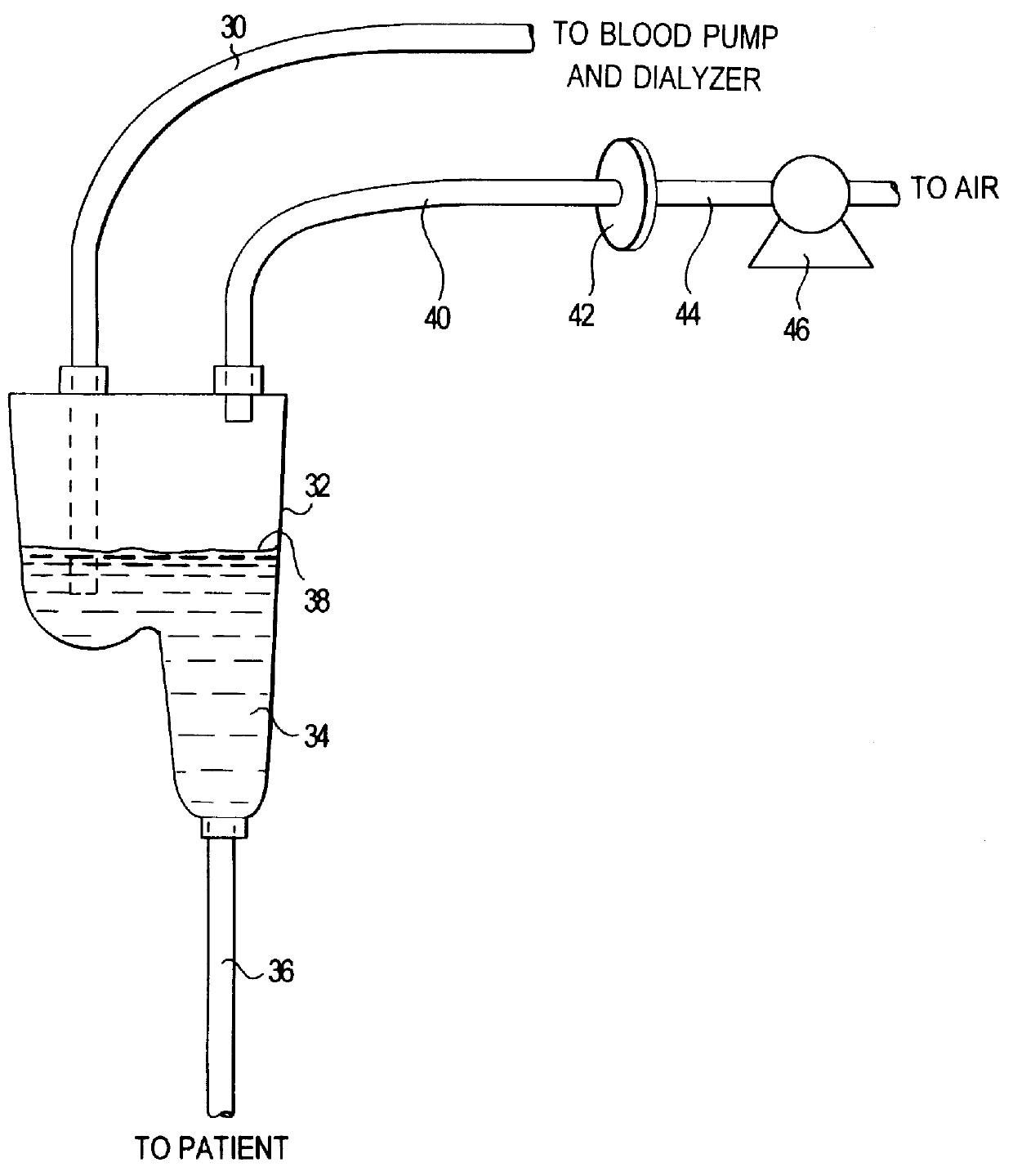
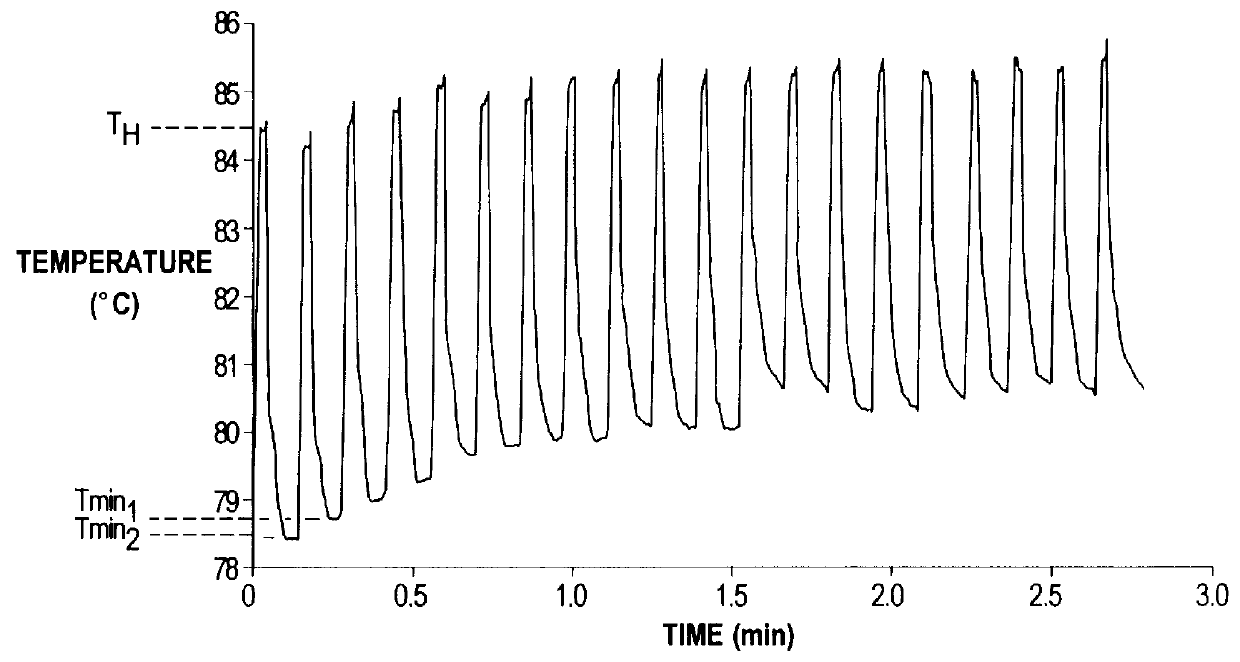
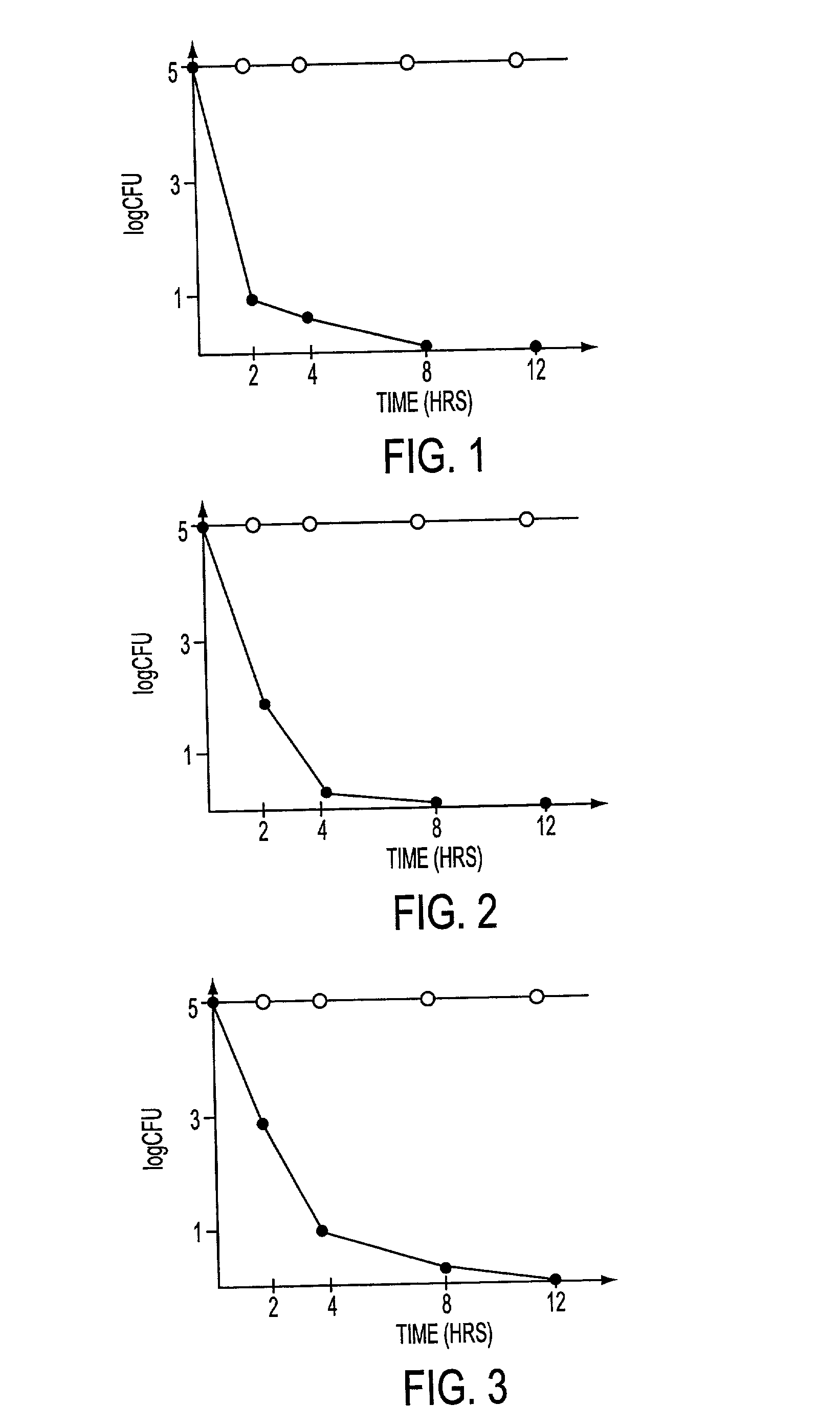
A method of disinfection of a dead-ended fluid line in a medical instrument such as a dialysis machine is described. The method comprises introducing a heated fluid into the fluid line, allowing the fluid to remain in the line for an experimentally determined optimal dwell period, removing the fluid from the fluid line, and then repeating the cycle for a time period sufficient to achieve a disinfection of the fluid line. The optimum dwell period and frequency for exchanging the heated fluid is determined so that the heated fluid is left resident in the line to exert a cidal effect but not so long that the it cools to the point of being ineffective, nor changed so frequently that that the time spent with no hot water resident in the line begins to detract (e.g., unduly prolong) the disinfection process. A representative cycle is introducing water at a temperature of about 85 degrees C, allowing it to reside in the fluid line for about 10 seconds, withdrawing the water, and then reintroducing water at 85 degrees C. The process continues for 1-2 hours. Variation from the representative cycle will be expected based on parameters such as the degree to which disinfection is to be achieved, the length and diameter of the fluid line, the temperature of the fluid, the ambient temperature, the presence of elements in the fluid line that contribute to heat loss, the material used for fluid line tubing, and whether the fluid comprises water or a disinfection solution such as a dilute citric acid solution. The optimum dwell period and frequency of the cycles can be determined experimentally from the teachings described herein.
Owner:BAXTER INT INC +2
Methods and kits for locking and disinfecting implanted catheters
InactiveUS6685694B2Reduce riskInhibiting fouling and plugging of the lumenDialysis systemsMedical devicesTriclosanThrombus
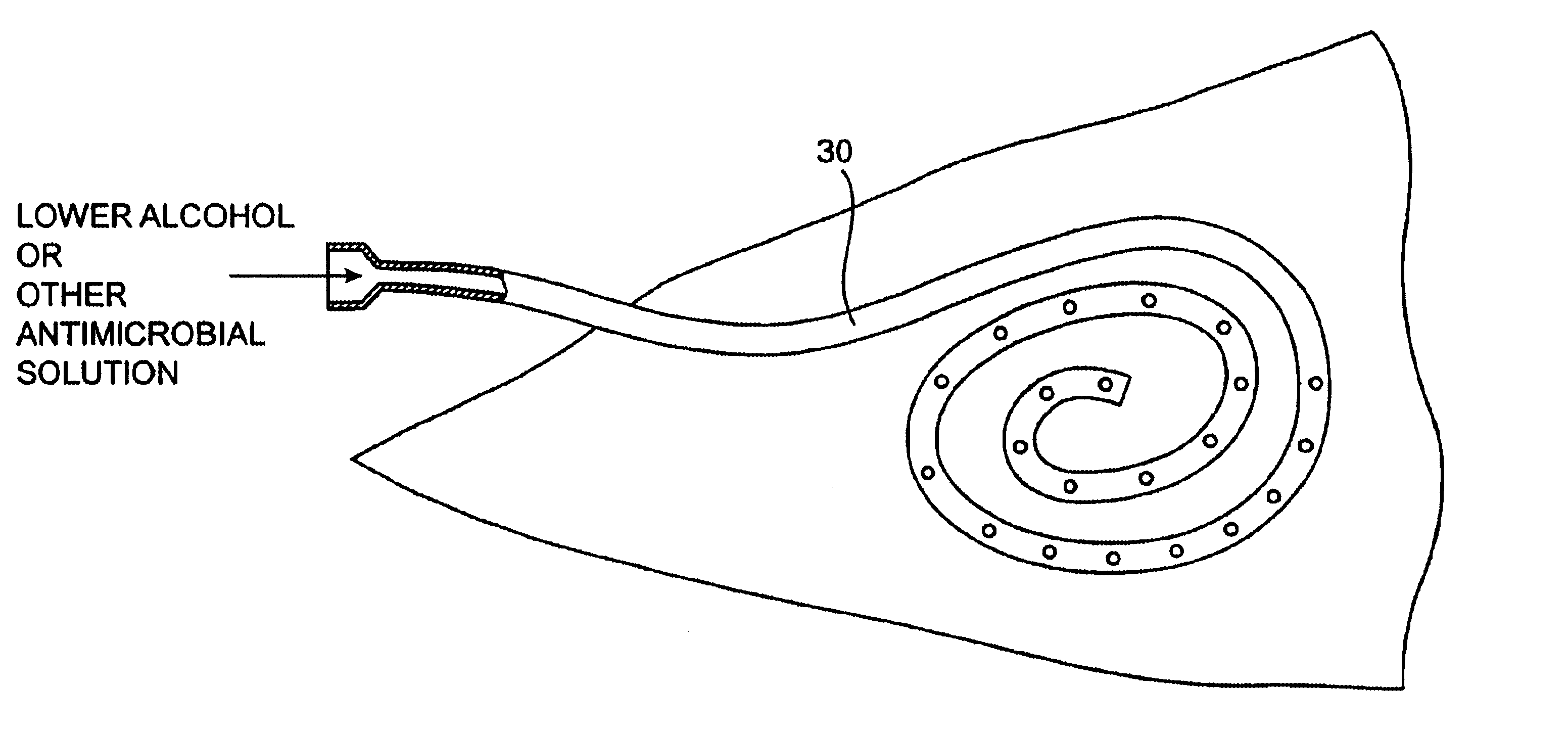
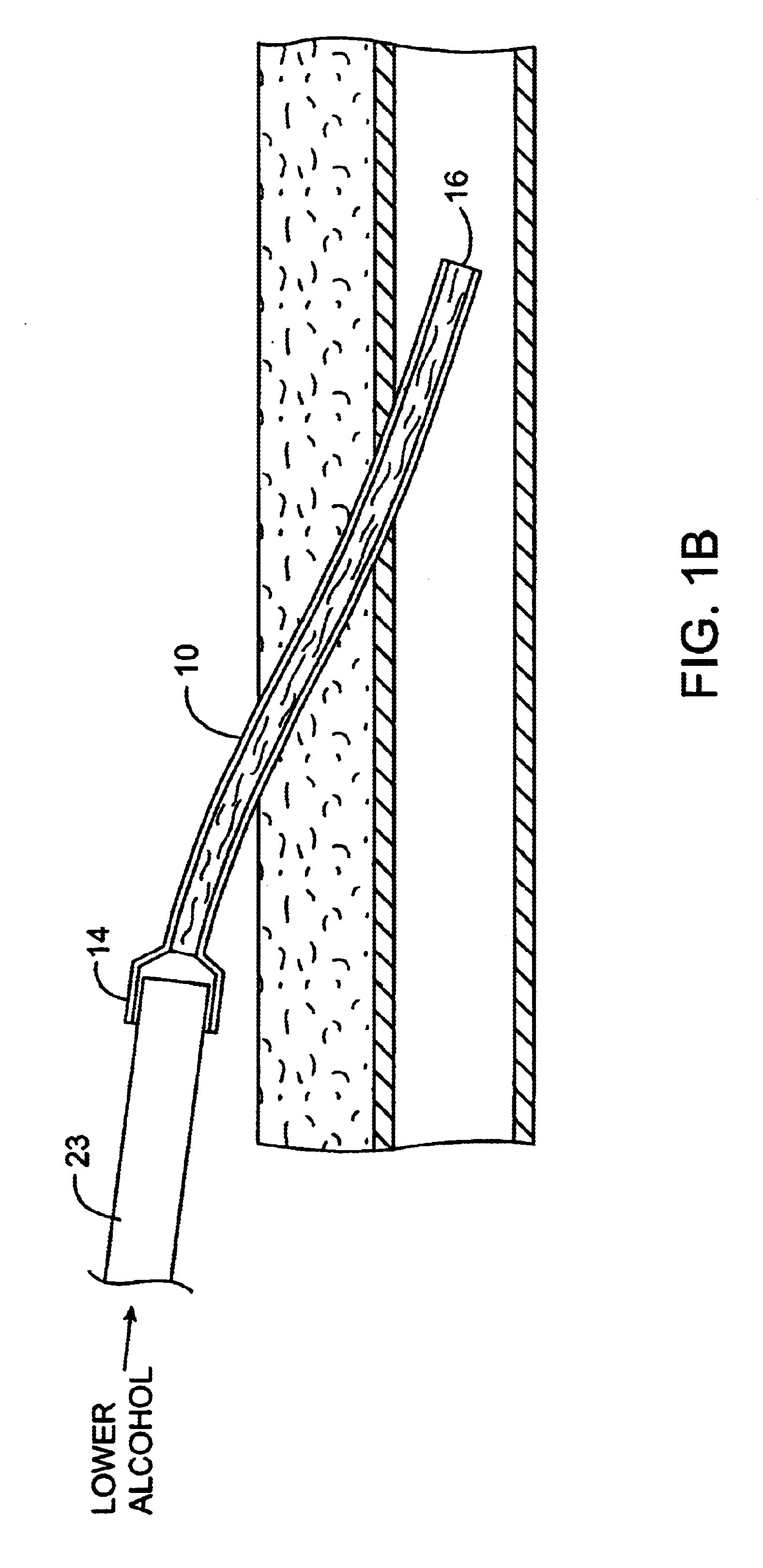
Implanted catheters are locked with a solution comprising a lower alcohol, typically ethanol, propanol, or butanol, in a range from 1% to 99% by volume, and an additive in a range from 1% to 99% by volume, the additive comprising an anti-microbial, typically taurolidine or triclosan, or an anti-coagulant, typically riboflavin, sodium citrate, ethylene diamine tetraacetic acid, or citric acid. The use of an alcohol and additive solution can effectively reduce fouling of the catheter, particularly clotting and thrombus in intravascular catheters, as well as eradicate existing infections and / or reduce the risk of potential infections. Existing infections and / or potential infections can be further reduced by employing a catheter body which permits an anti-microbial solution to penetrate into the catheter body and preferably through the catheter into tissue surrounding the implanted catheter.
Owner:EXCELSIOR MEDICAL
Pharmaceutical-grade ferric citrate
The present invention relates to pharmaceutical-grade ferric citrate. Pharmaceutical-grade ferric citrate contains a definite composition and a definite hydrate. The present invention also relates to a method using a solid—solid reaction to produce pharmaceutical-grade ferric citrate. The present invention also relates to a pharmaceutical composition comprising a therapeutically effective amount of pharmaceutical-grade ferric citrate and a food comprising pharmaceutical-grade ferric citrate.
Owner:PANION & BF BIOTECH INC
Composite oxide catalyst for cryogenic selective catalystic reductic oxide nitrogen
InactiveCN101028594AEasy to makeEasy to operateCatalyst carriersDispersed particle separationManganese oxideNitrogen gas
A composite oxide catalyst for removing NOx from the tail gas generated by combustion through low-temp selective catalytic reduction reaction in which ammonia gas or urea is used as reducing agent for reducing the NOx into N2 and H2O is prepared from a composite metallic oxide (whose main component is manganese oxide) and a porous inorganic oxide as carrier through citric acid complexing method.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Novel biodegradable elastomeric scaffold for tissue engineering and light scattering fingerprinting methods for testing the same
The present invention is directed to a novel biocompatible polymer that may be used in tissue engineering. More specifically, the specification describes methods and compositions for making and using a citric acid copolymers.
Owner:NORTHWESTERN UNIV
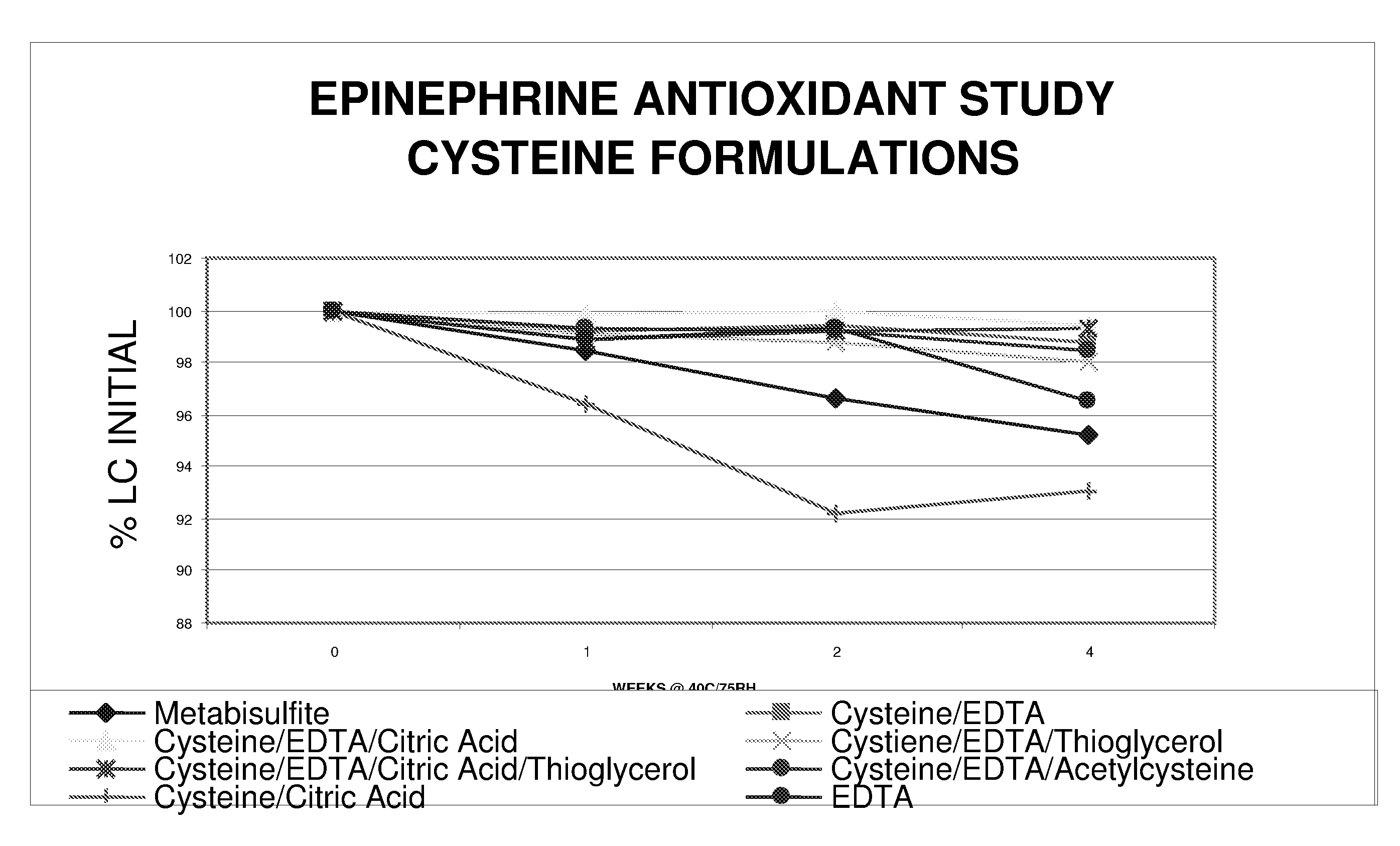
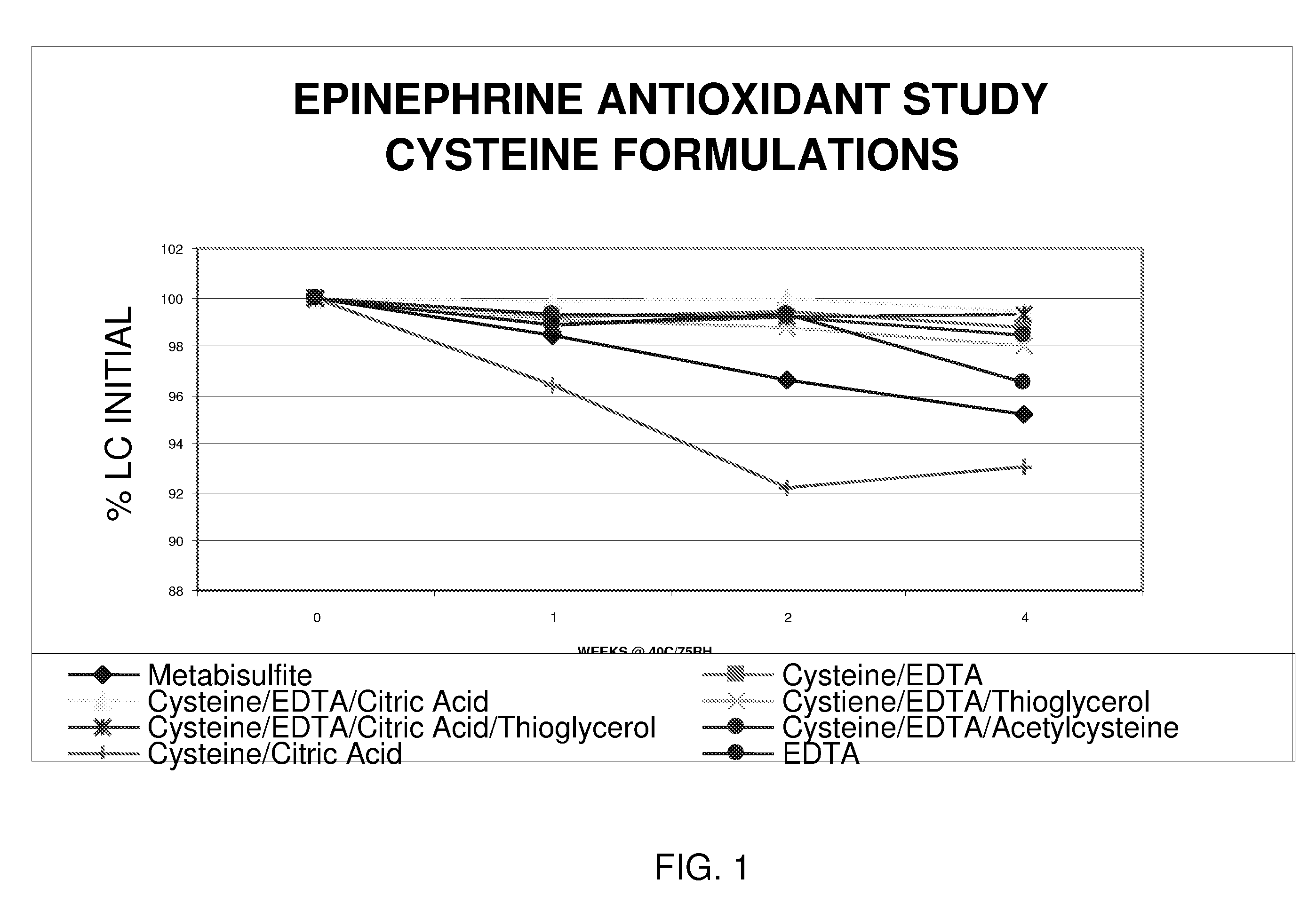
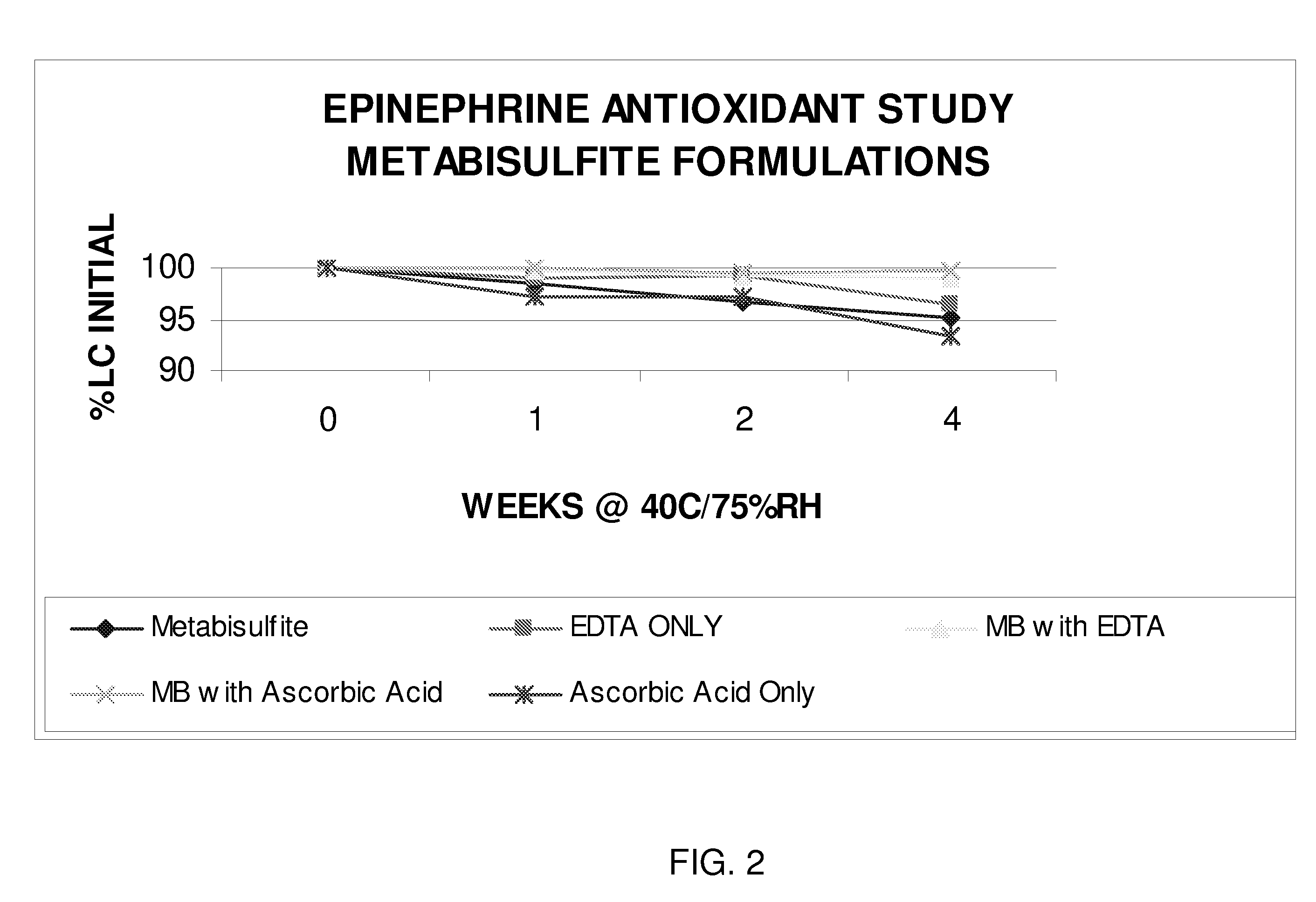
Epinephrine formulations
InactiveUS20080269347A1Improve stabilityBiocideOrganic active ingredientsCardiorespiratory arrestAntioxidant
The present invention generally concerns an epinephrine formulation that has enhanced stability. In particular embodiments, the formulation is an injectable formulation. In specific aspects, the formulation comprises epinephrine, EDTA, and one or more of an antioxidant such as cysteine, citric acid, acetylcysteine, or thioglycerol. The formulations are suitable for any medical condition that is in need of epinephrine, although in specific embodiments the medical condition is anaphylaxis, asthma, or cardiac arrest.
Owner:UNION SPRINGS PHARMA
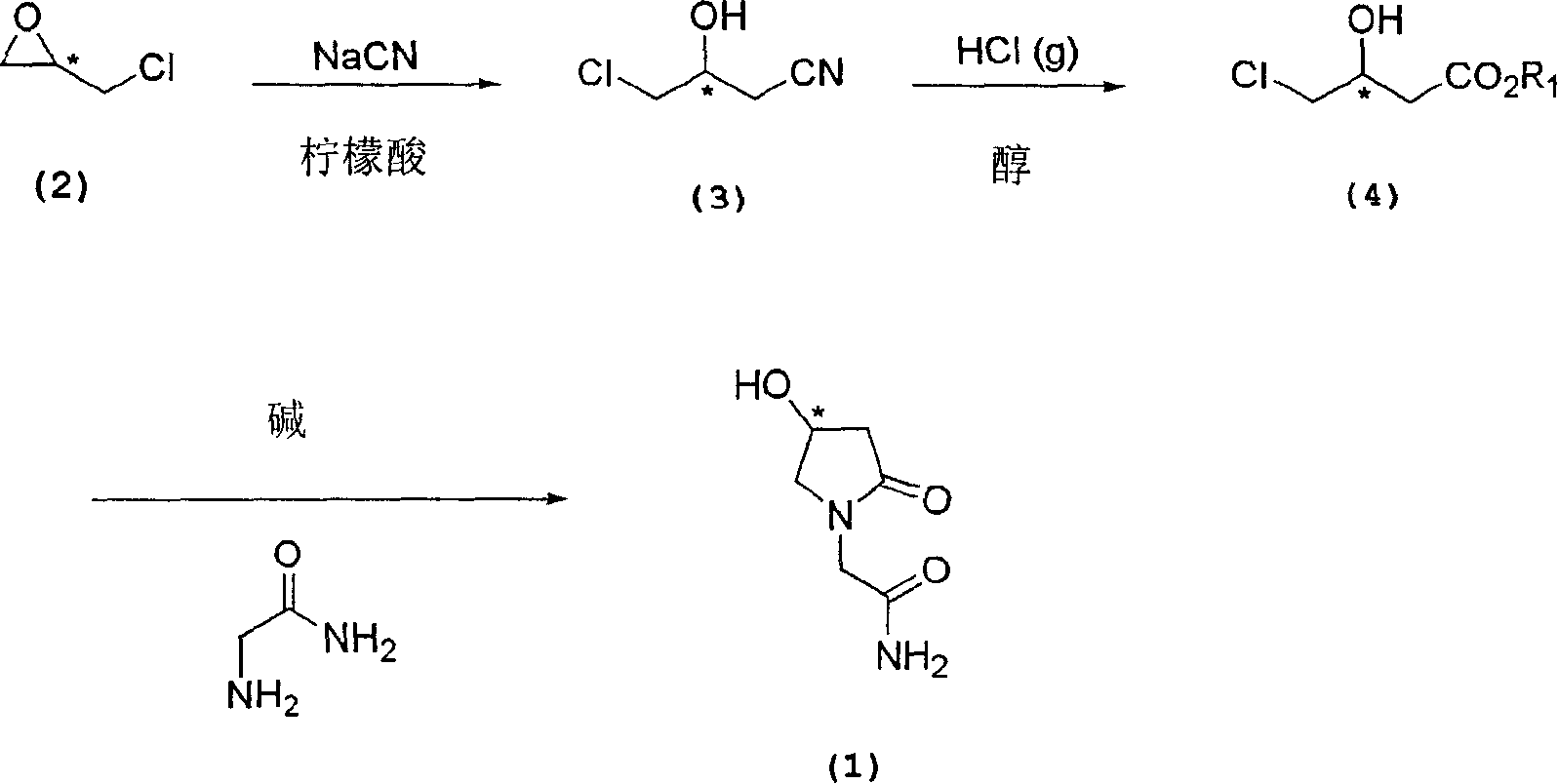
Process for the preparation of optically pure 4-hydroxy-2-oxo-1-pyrrolidine acetamide
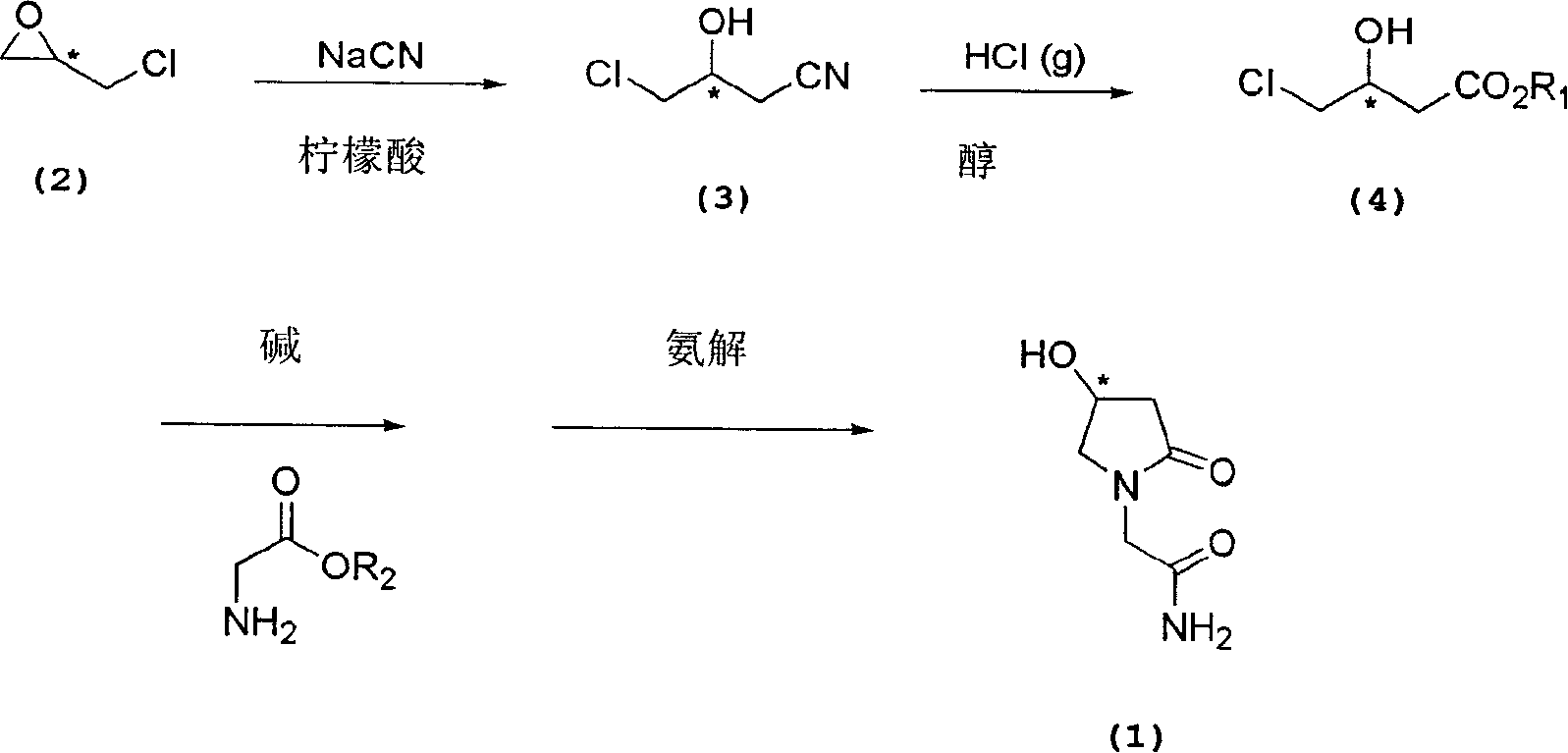
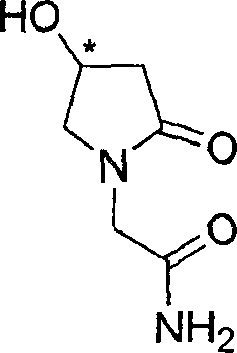
The present invention relates to a process for the preparation of chiral 4-hydroxy-2-oxo-1-pyrrolidine acetamide. The process comprises adding sodium cyanide together with citric acid to a solution of chiral epichlorohydrin to obtain chiral 3-chloro-2-hydroxypropionitrile by ring opening reaction of the chiral epichlorohydrin, reacting the obtained product with an alcohol containing hydrochloride gas to obtain chiral 4-chloro-3-hydroxybutyric acid ester, and reacting the obtained product in a presence of a base with glycinamide or with glycine ester accompanied by ammonolysis with ammonia to produce the targeted chiral 4-hydroxy-2-oxo-1-pyrrolidine acetamide. The process according to the present invention provides optically pure 4-hydroxy-2-oxo-1-pyrrolidine acetamide in high yield and in high purity, which is suitable for industrial mass-production.
Owner:AHN GOOK PHARMA CO LTD +1
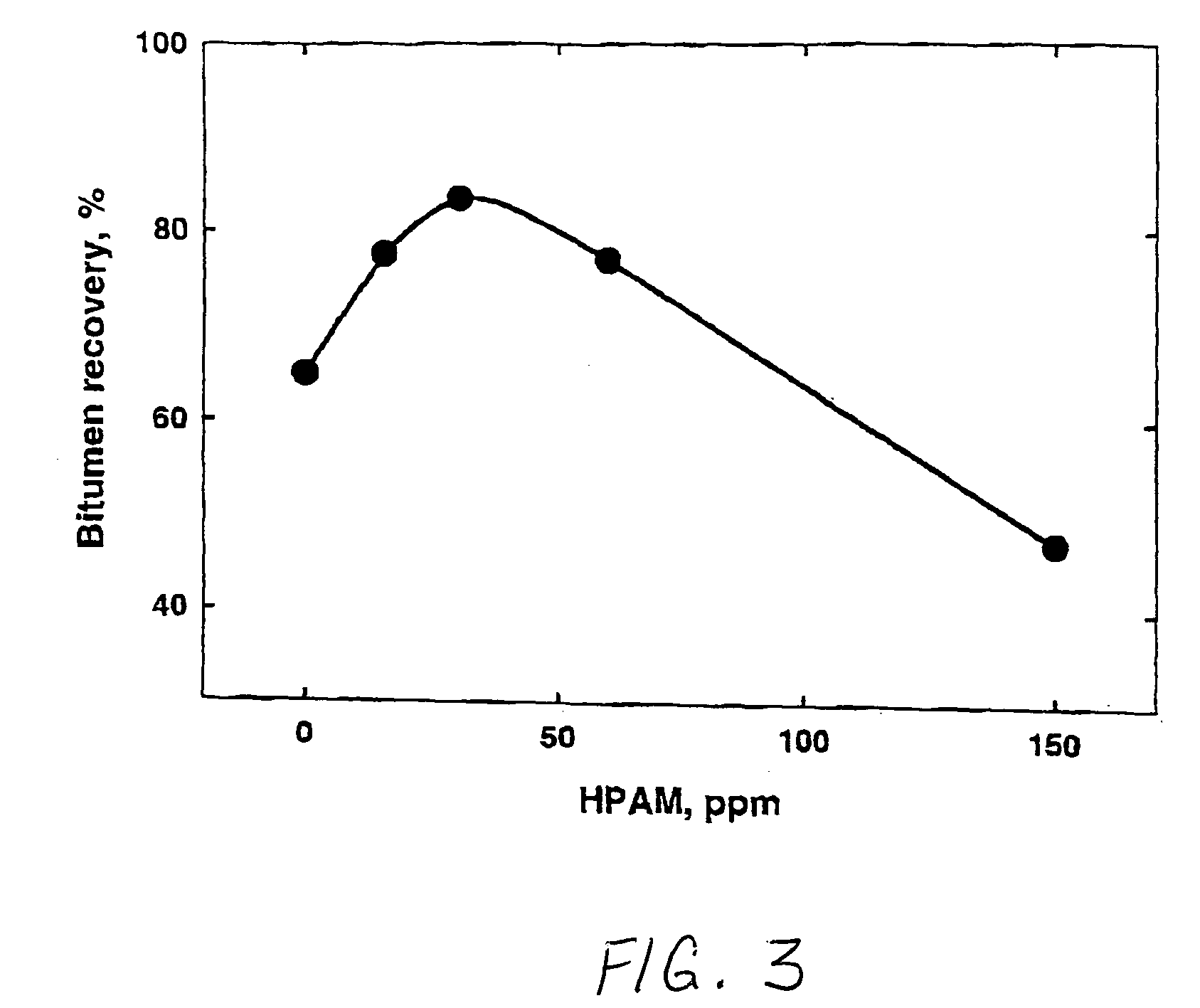
Processing aids for enhanced hydrocarbon recovery from oil sands, oil shale and other petroleum residues
InactiveUS20050194292A1Liberation of additionalPromote recoveryDewatering/demulsification with chemical meansLiquid hydrocarbon mixture productionHydrocotyle bowlesioidesSlurry
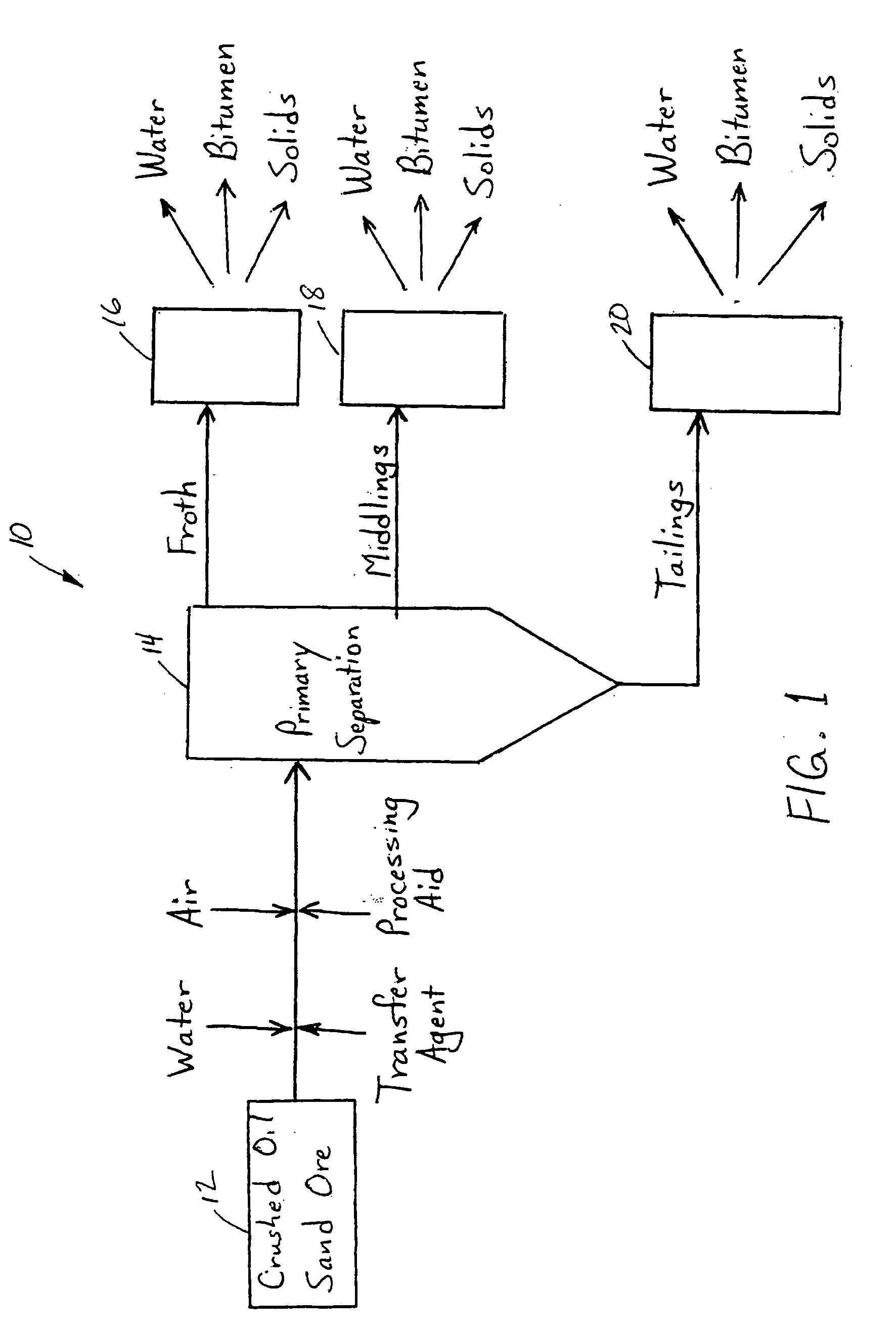
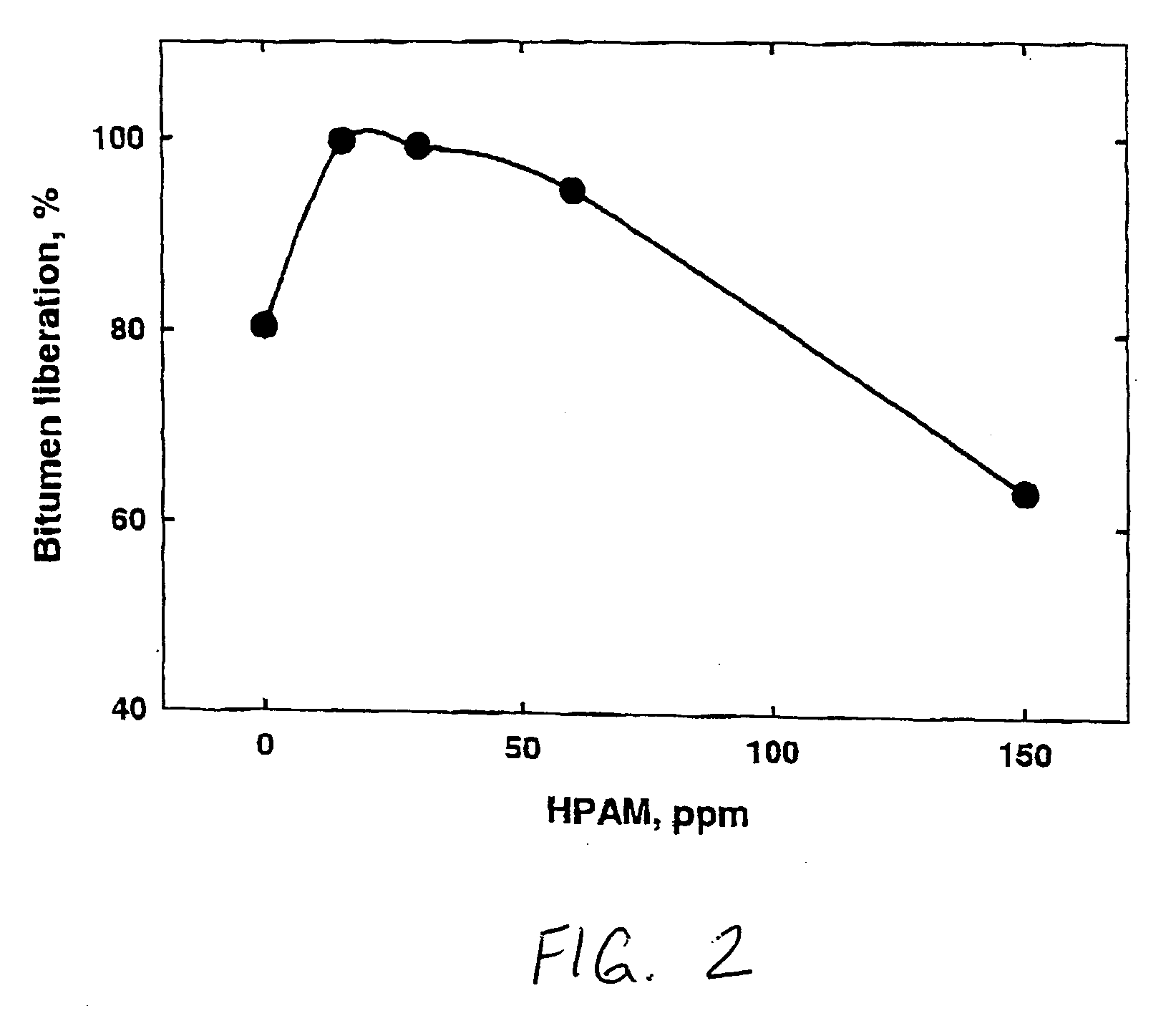
A method of improving hydrocarbon recovery from oil sands, oil shale, and petroleum residues includes adding a polymeric or nonpolymeric processing aid capable of sequestering cations, such as the multivalent calcium, magnesium and iron cations. The hydrocarbons are preferably contacted with the processing aid before a primary separation of the hydrocarbons in order to increase bitumen recovery. A processing aid is provided in an effective amount to increase the liberation of the hydrocarbons from inorganic solids, particularly when the source is a poor processing ore. Preferred processing aids include citric acid or a polymeric acid selected from polyacrylic acid, polymethacrylic acid, salts of these acids, partial salts of these acids, and combinations thereof. The processing aids significantly increase the hydrocarbon recovery typically with concentrations less than 50 ppm and the polymeric processing aids can also provide beneficial flocculation of solids in tailings slurry.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Antimicrobial salt solutions for food safety applications
ActiveUS7090882B2Reduce in quantityInhibit microorganismsMilk preservationDough treatmentBiologyFood processing
Owner:CARGILL INC
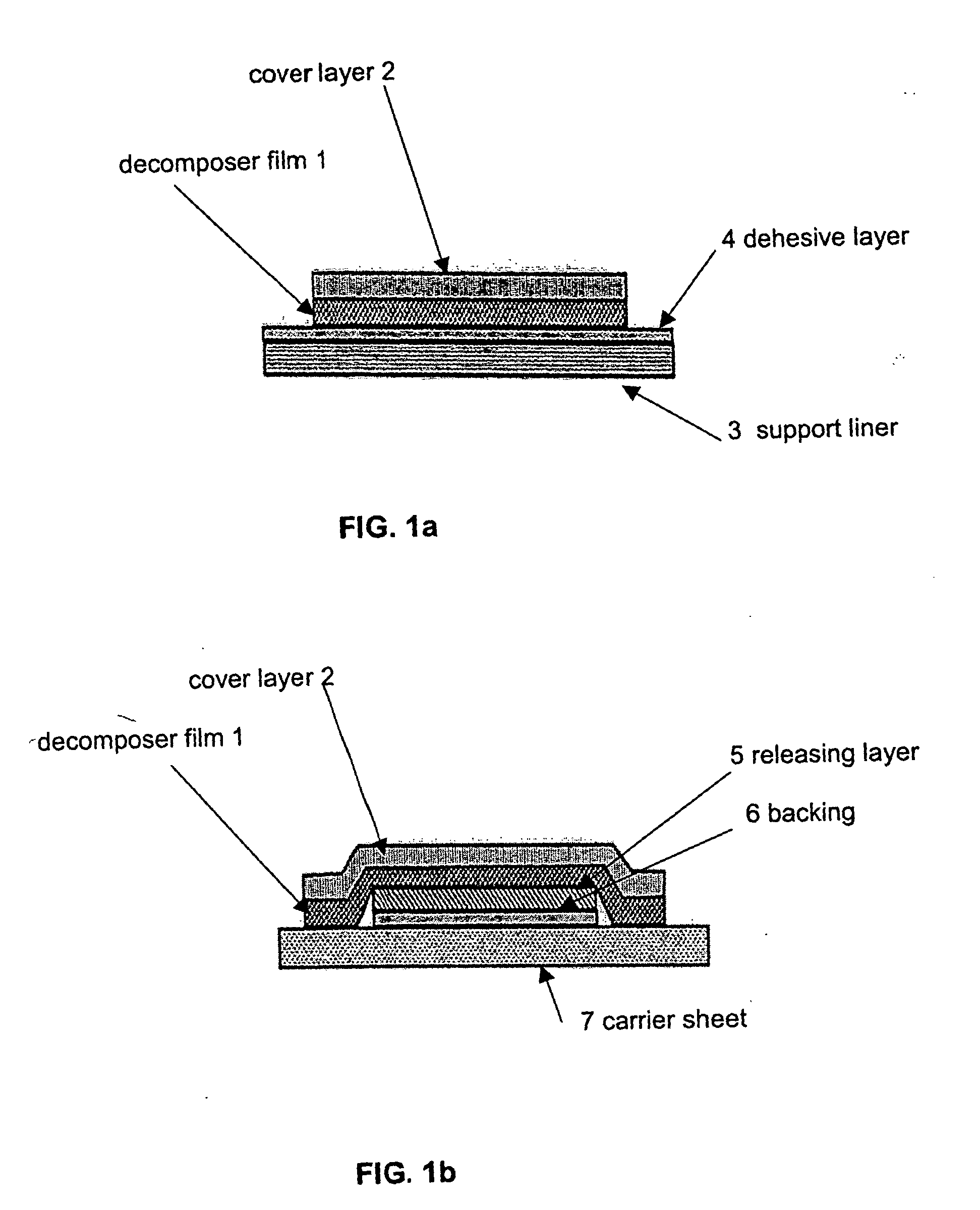
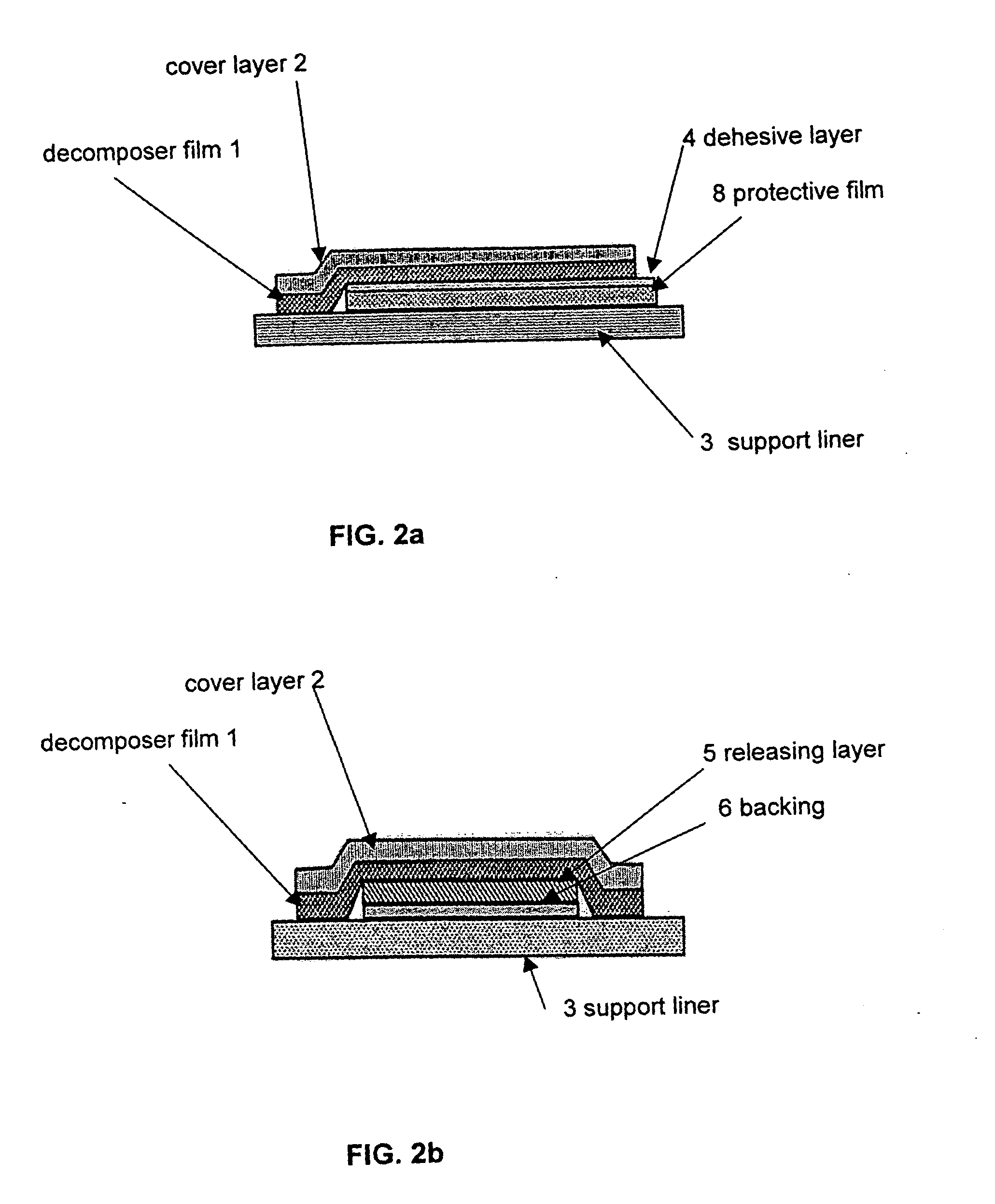
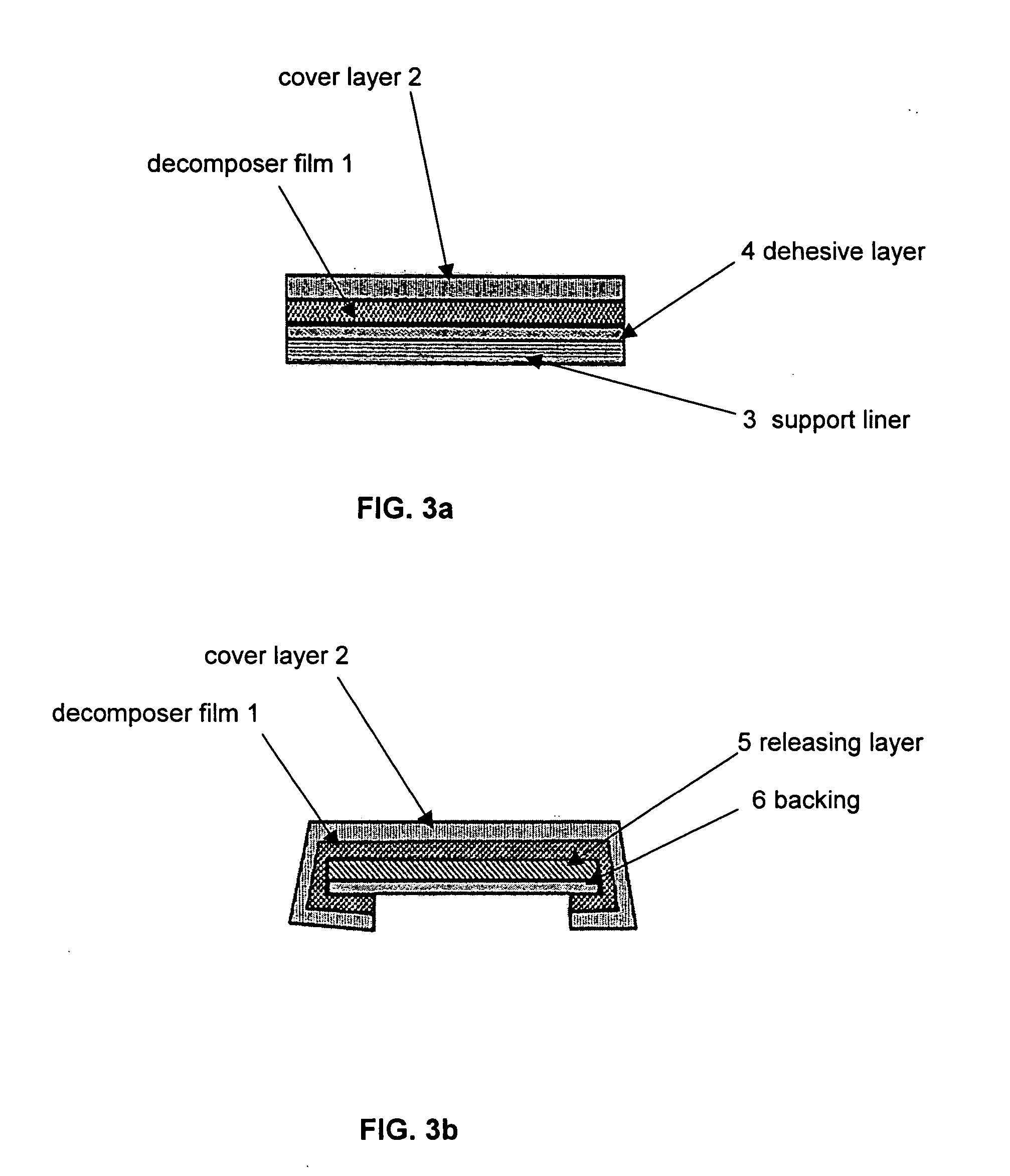
Decomposer film for transdermal patches
InactiveUS20070014839A1Increased internal surface areaAccelerate decomposition reactionOrganic active ingredientsAntipyreticTransdermal patchPolyvinyl alcohol
The decomposer film product has a polymeric decomposer layer, a cover layer for protecting the decomposer film from the surroundings and a releasable support liner, which is removed prior to use. The polymeric decomposer film contains a water-soluble or water-insoluble polymeric adhesive material and a decomposition accelerator, which acts to decompose an effective ingredient, such as a steroid hormone, of a worn or unused transderamal patch, when the effective ingredient releasing layer of the patch adheres to the polymer film, so that the pharmaceutical effective ingredient comes into contact with the decomposition accelerator by diffusion. The decomposition accelerator includes a chemically oxidizing substance, preferably urea peroxide, manganese (III) acetate or iron (III) citrate. The water-insoluble polymeric adhesive material is preferably an acrylates adhesive. The water-soluble polymeric adhesive material is preferably polyvinyl alcohol, polyvinyl pyrrolidone, a cellulose derivative or a polyacrylic acid.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Compositions and polymer composites prepared from the same
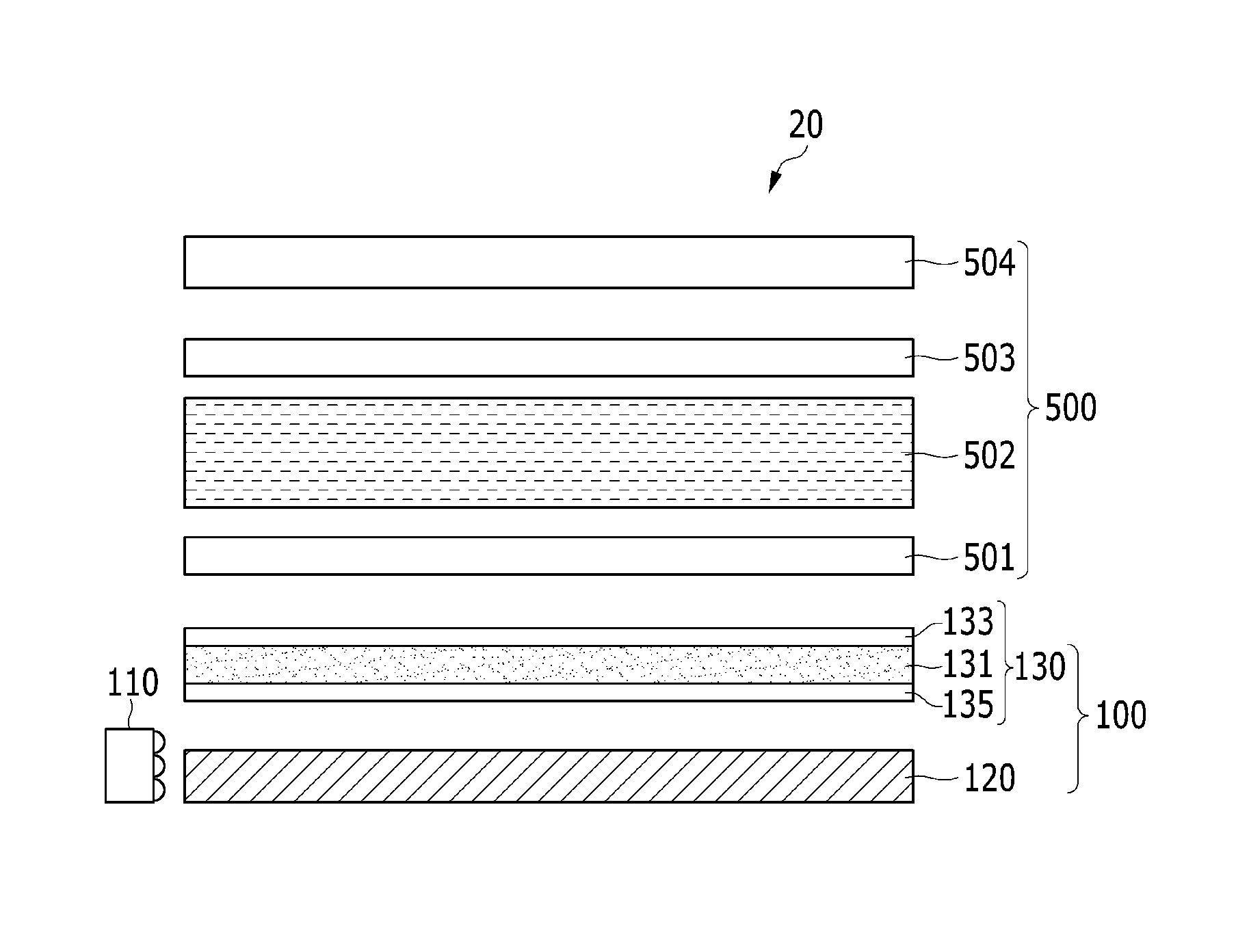
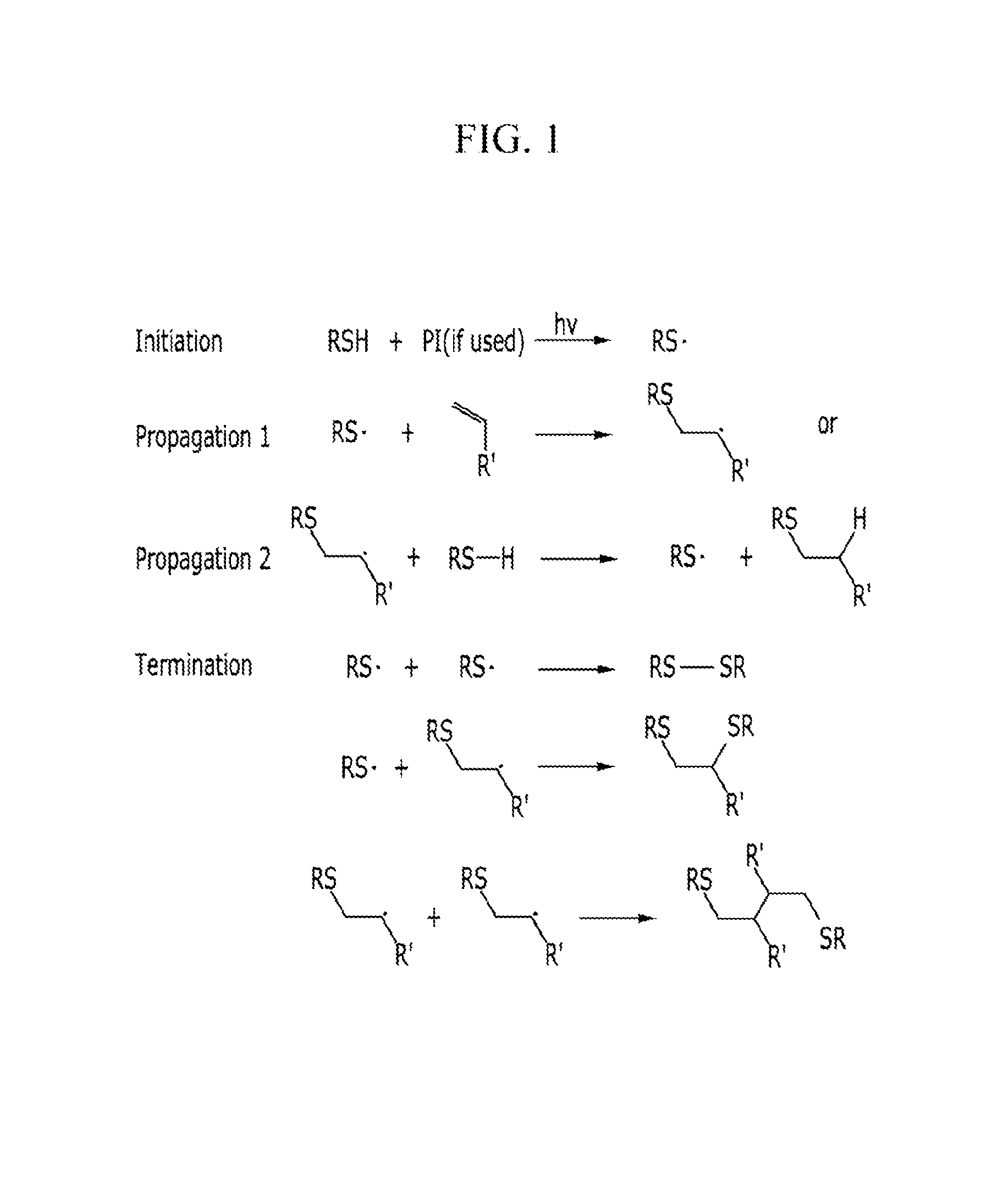
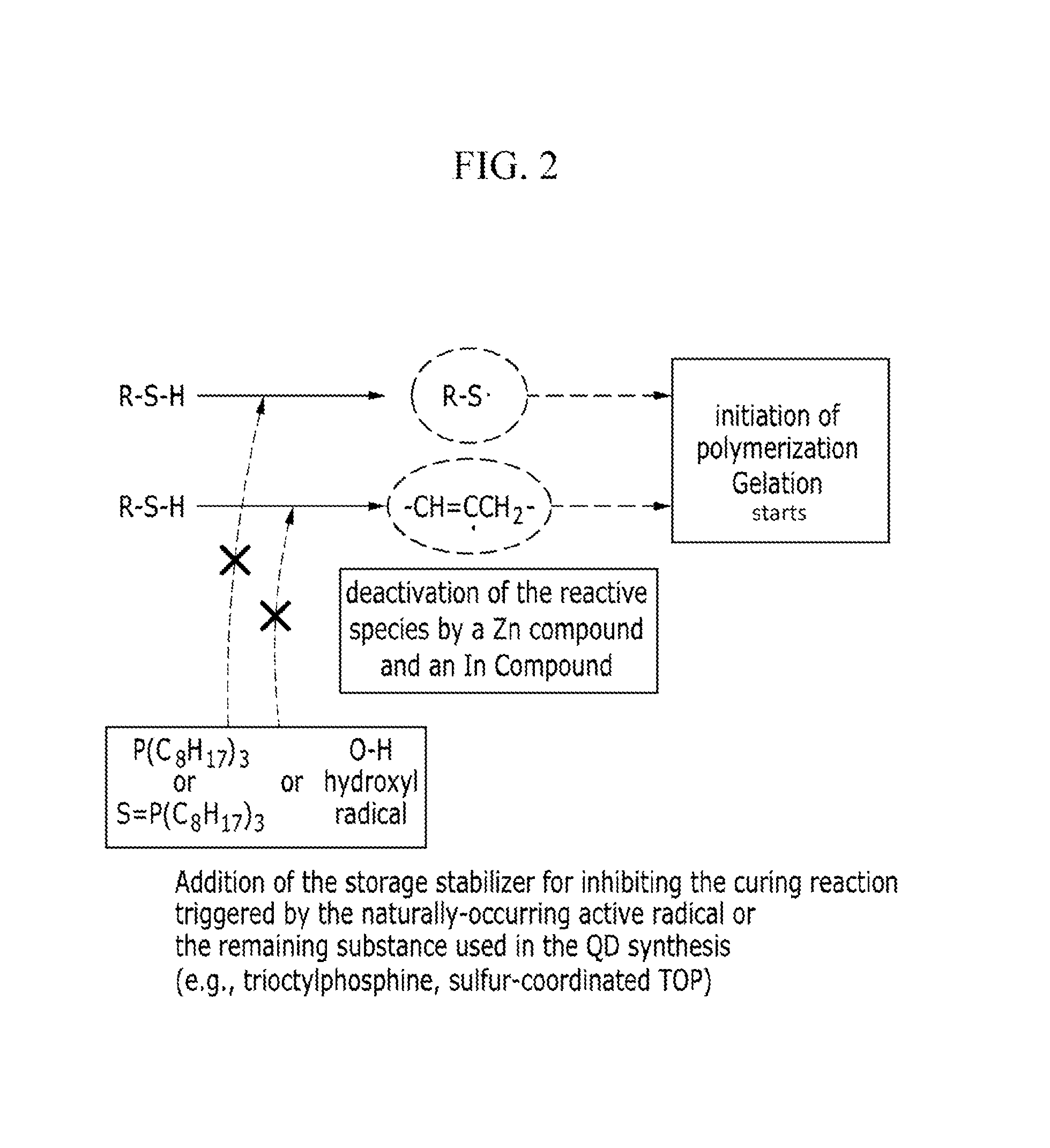
ActiveUS20160005932A1Good storage stabilityOther chemical processesSolid ballsZinc compoundsPolymer composites
A composition including: a monomer mixture including a first monomer having at least two thiol groups at its terminal end and a second monomer having at least two carbon-carbon unsaturated bond-containing groups at its terminal end; and at least one additive selected from a zinc compound, an indium compound, ascorbic acid or a salt thereof, citric acid or a salt thereof, a tocopherol, and a tocotrienol.
Owner:SAMSUNG ELECTRONICS CO LTD
Electrolyte composition and treatment for electrolytic chemical mechanical polishing
Owner:APPLIED MATERIALS INC
Biosensor
InactiveUS20030175841A1Avoid influenceReduce solubilityImmobilised enzymesBioreactor/fermenter combinationsGluconolactonaseSuccinic acid
A biosensor that is highly responsive and capable of rapid and highly sensitive quantification of a specific component contained in a sample is provided. The biosensor of this invention comprises: an electrically insulating base plate; an electrode system comprising a working electrode and a counter electrode disposed on the base plate; and a reagent system comprising an oxidoreductase which catalyzes the oxidation reaction of glucose, gluconolactonase and a buffer. The buffer is selected from the group consisting of phthalic acid and its salts, maleic acid and its salts, succinic acid and its salts, phospholic acid and its salts, acetic acid and its salts, boric acid and its salts, citric acid and its salts, glycine, tris(hydroxymethyl)aminomethane, piperazine-N,N'-bis(2-ethane sulfonic acid) and the like.
Owner:PHC HLDG CORP
Amino acid compound leaf fertilizer and preparation method thereof
InactiveCN102219610APromote absorptionNutritional diversityFertilizer mixturesMonopotassium phosphateAmino acid
The invention discloses an amino acid compound leaf fertilizer and a preparation method thereof, relating to the technical field of production of agricultural fertilizer. The amino acid compound leaf fertilizer is prepared by the following components according to parts by weight: 1-2 parts of zinc sulphate, 1-2 parts of borax, 2-3 parts of magnesium sulfate, 1-1.5 parts of surfactant, 2-3 parts of monopotassium phosphate, 15-20 parts of compound amino acid, 1-2 parts of potassium humate, 1-2 parts of ammonium molybdate, 3-5 parts of calcium chloride, 5-8 parts of urea, 10-15 parts of citric acid, and 15-20 parts of chelant; the amino acid compound leaf fertilizer has the characteristics of being complete in nutrient, high in utilization rate, good in absorption, fast in effect and the like, and can be widely applied to crops such as tobacco, oranges and tangerines, vegetables, melon and fruits and the like.
Owner:HUNAN ZHONGKE AGRI
Gas propelled munitions Anti-fouling system
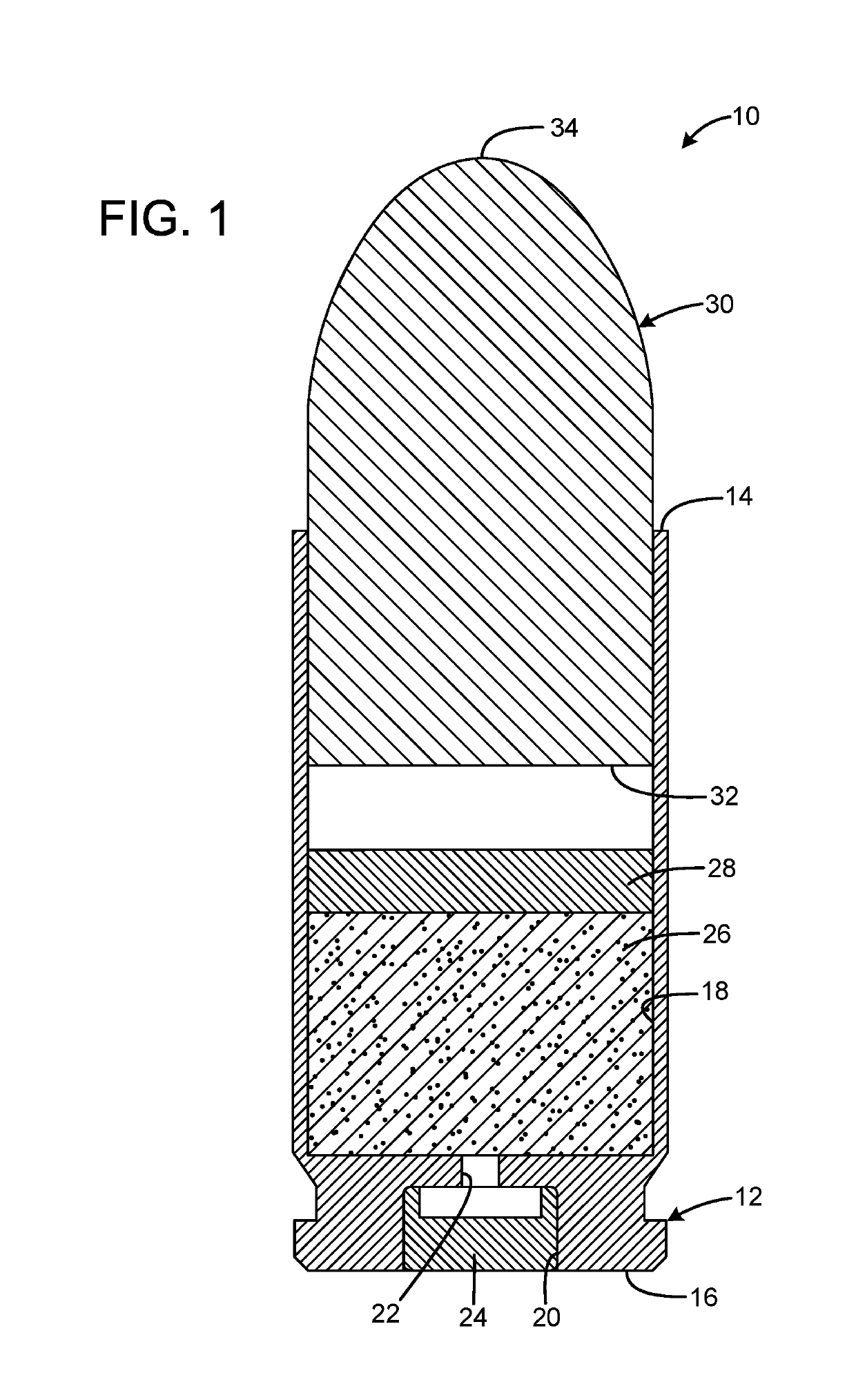
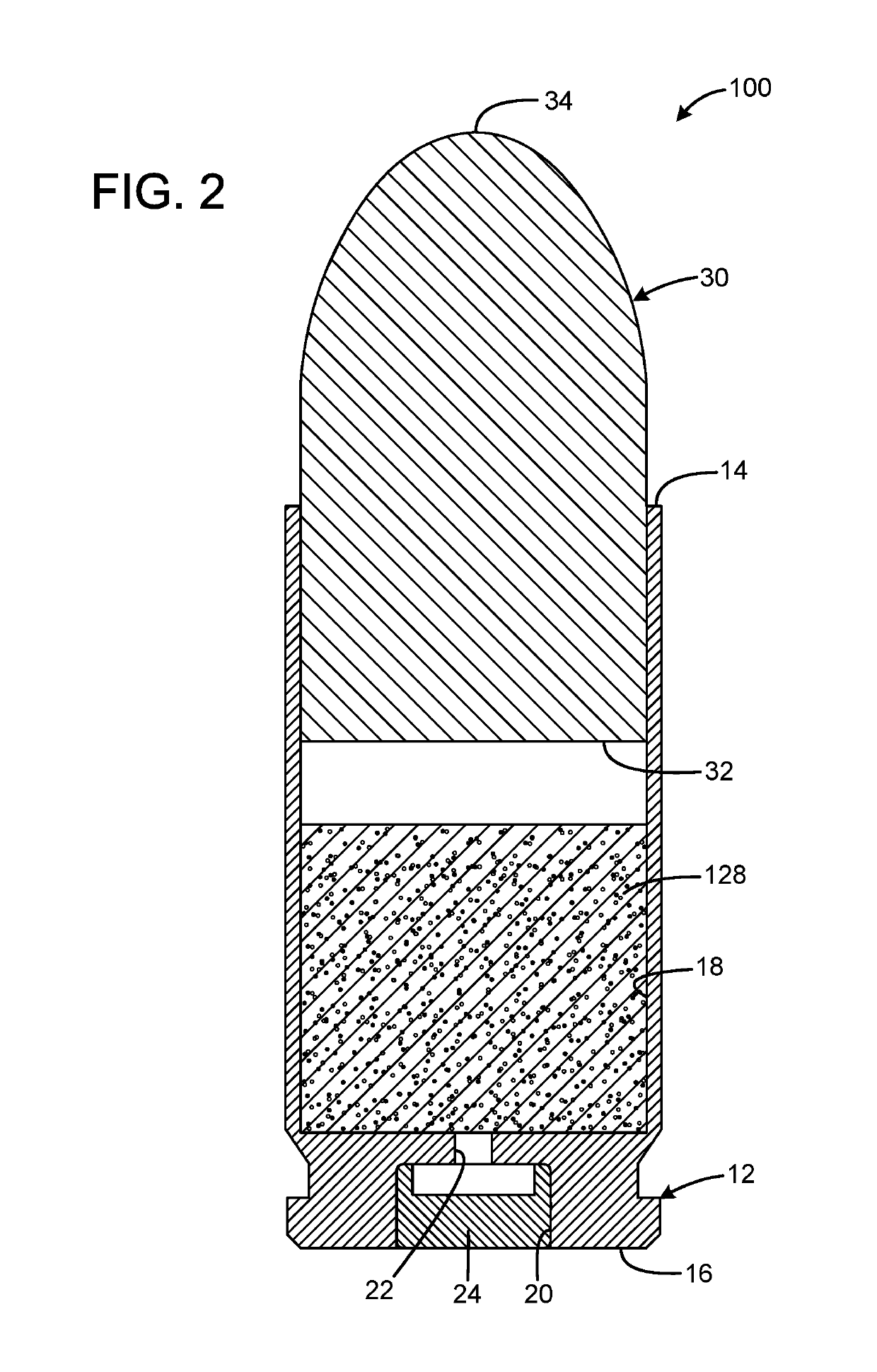
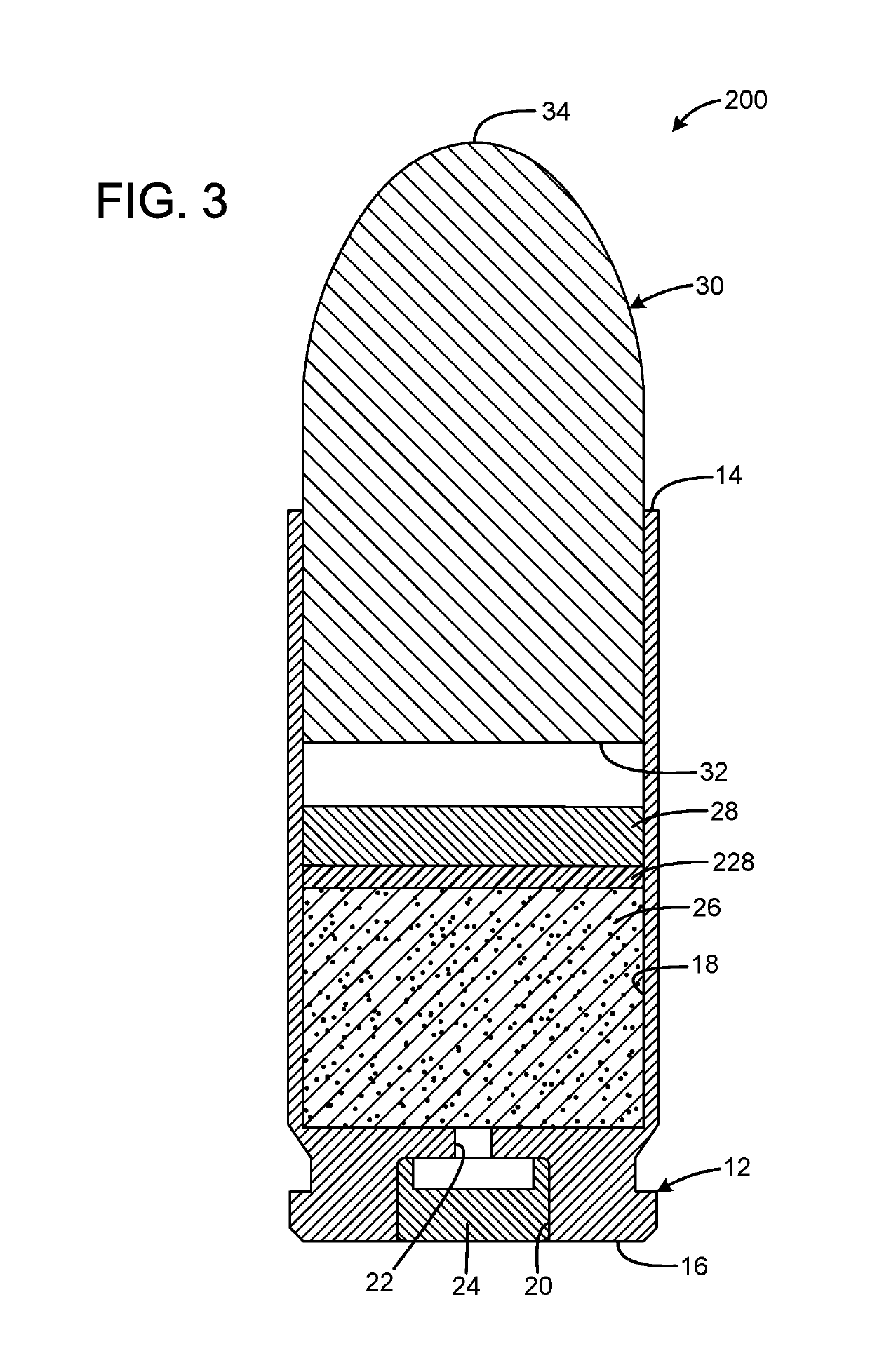
InactiveUS20190106364A1Great contributionShotgun ammunitionNon-explosive/non-thermic compositionsEngineeringCitric acid
A gas propelled munitions anti-fouling system has a case having an open forward mouth end, a rear end, and an interior, the rear end defining a pocket that receives a primer and a passage communicating between the pocket and the interior of the case, a quantity of propellant received within the interior of the case, a quantity of anti-fouling composition received within the interior of the case, and a bullet having a rear portion inserted into the open forward mouth end of the case. The anti-fouling composition may have at least one salt and at least one acid. The acid may be anhydrous. The salt may be sodium chloride or sodium nitrate. The acid may be anhydrous citric acid. The anti-fouling composition may have at least one abrasive. The abrasive may be stannic acid. The anti-fouling composition may be 50% salt by weight and 50% acid by weight.
Owner:ADLER CAPITAL LLC
Compositions containing pipercillin and tazobactam useful for injection
InactiveUS6900184B2Reduce formationMaintain stabilityBiocidePeptide/protein ingredientsParticulatesDepressant
The invention pertains to pharmaceutical compositions of Zosyn® piperacillin with tazobactam in the presence of a buffer, preferably citrate, a particulate formation inhibitor, preferably EDTA optionally an aminoglycoside which when frozen and thawed or lyophilized and reconstituted reform a solution which has decreased particulate formation.
Owner:MEDICAL SAFETY PROD
Nanosilver-containing antibacterial and antifungal granules and methods for preparing and using the same
The present invention relates to nanosilver-containing antibacterial and antifungal granules ("NAGs"). The NAGs have longlasting inhibitory effect on a broad-spectrum of bacteria and fungi, which include, but are not limited to, Escherichia coli, Methicillin resistant Staphylococcus aureus, Chlamydia trachomatis, Providencia stuartii, Vibrio vulnificus, Pneumobacillus, Nitrate-negative bacillus, Staphylococcus aureus, Candida albicans, Bacillus cloacae, Bacillus allantoides, Morgan's bacillus (Salmonella morgani), Pseudomonas maltophila, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bacillus subtilis, Bacillus foecalis alkaligenes, Streptococcus hemolyticus B, Citrobacter, and Salmonella paratyphi C. The NAGs contain ground stalk marrow of the plant Juncus effuses L. which has been dispersed with nanosilver particles. The nanosilver particles are about 1-100 mn in diameter. Each of the nanosilver particles contain a metallic silver core which is surrounded by silver oxide. The present invention also provides a process for making the NAGs. The NAGs can be used in a variety of healthcare and industrial products. Examples of the healthcare products include, but are not limited to, ointments or lotions to treat skin trauma, soaking solutions or cleansing solutions for dental or women hygiene, medications for treating gastrointestinal bacteria infections, sexual related diseases, and eye diseases. Examples of industrial products include, but are not limited to, food preservatives, water disinfectants, paper disinfectants, construction filling materials (to prevent mold formation).
Owner:LEGEND WIN FINANCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com