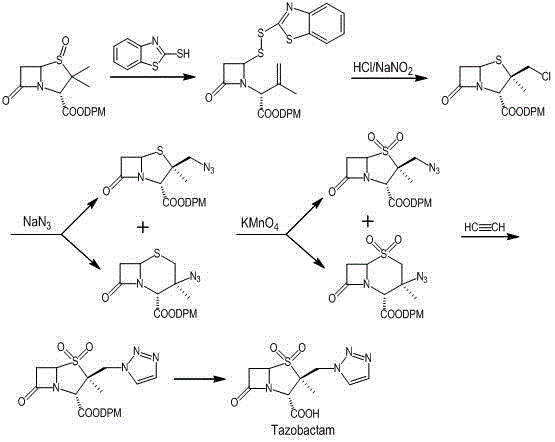
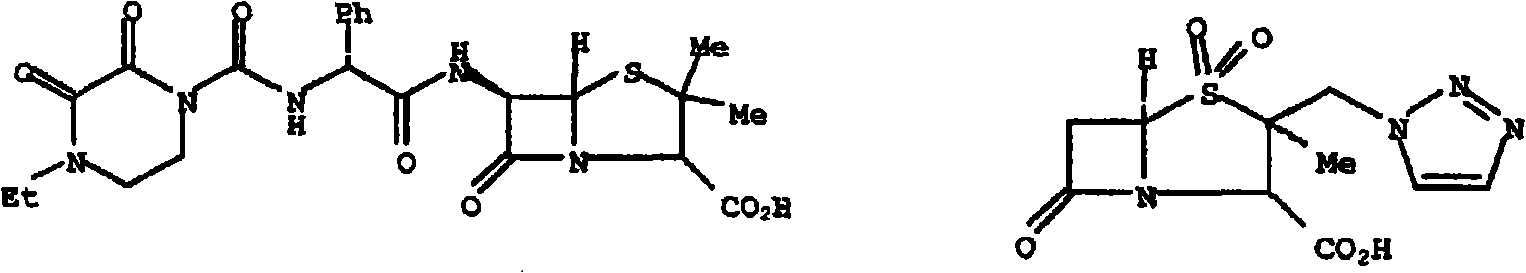
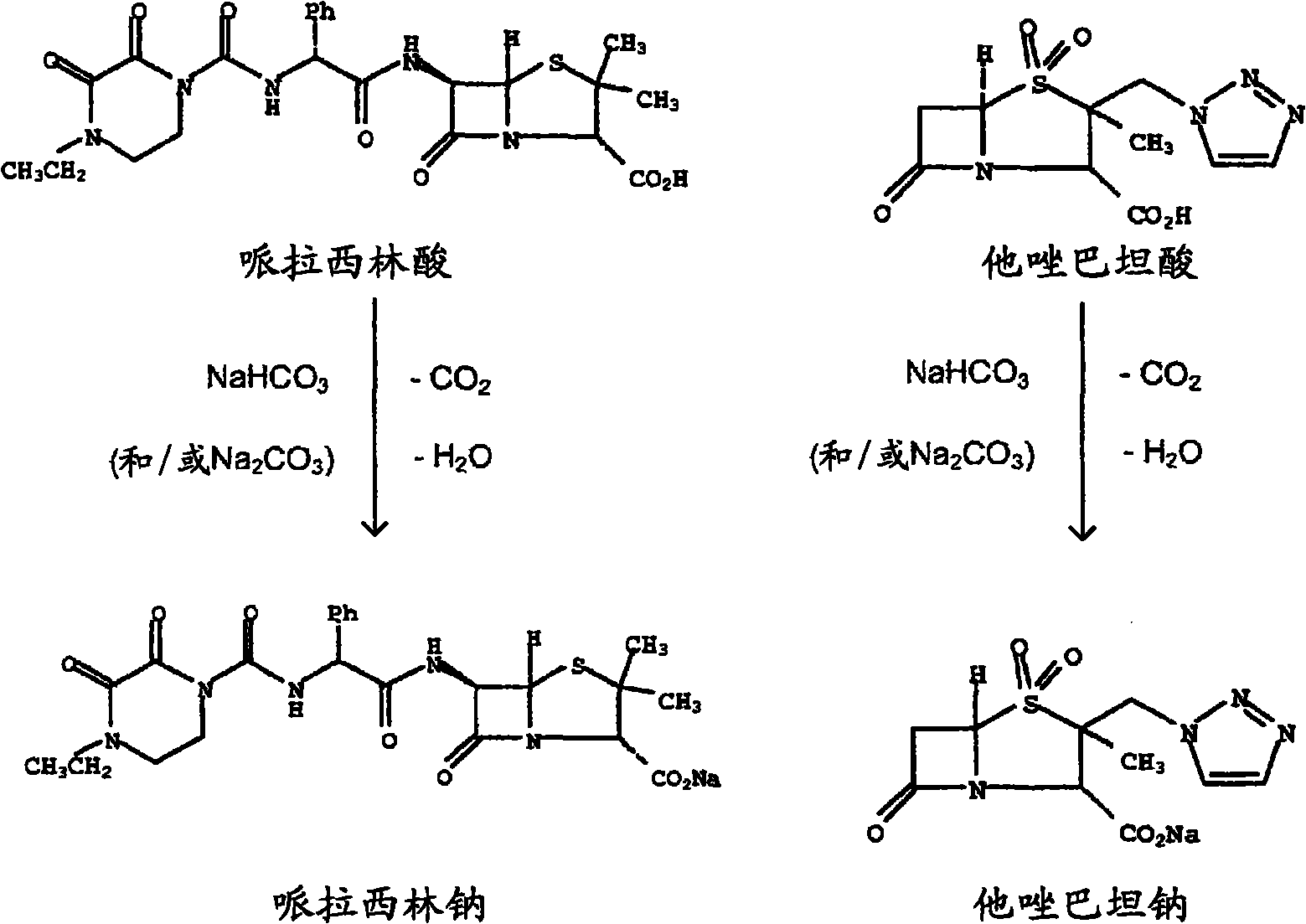
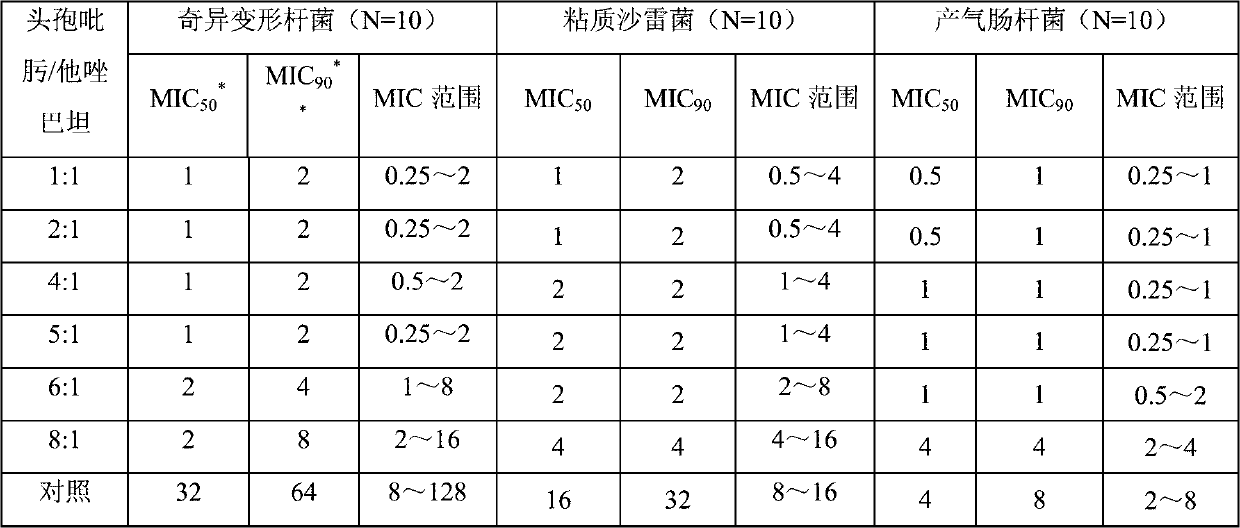
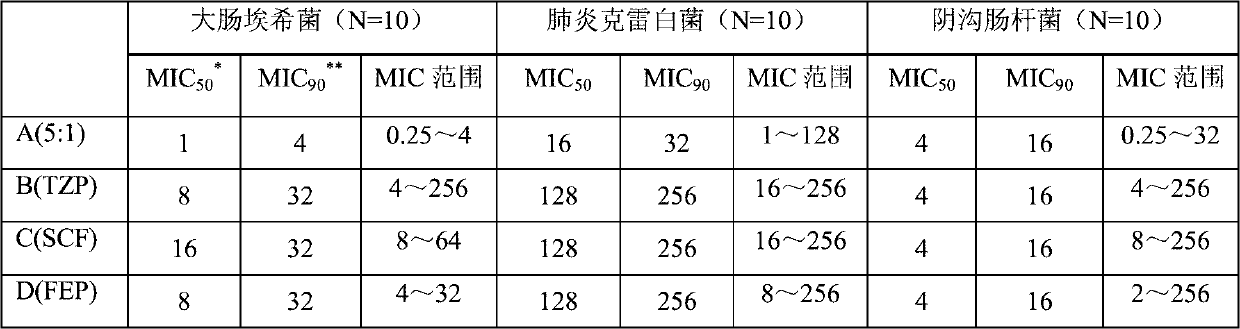
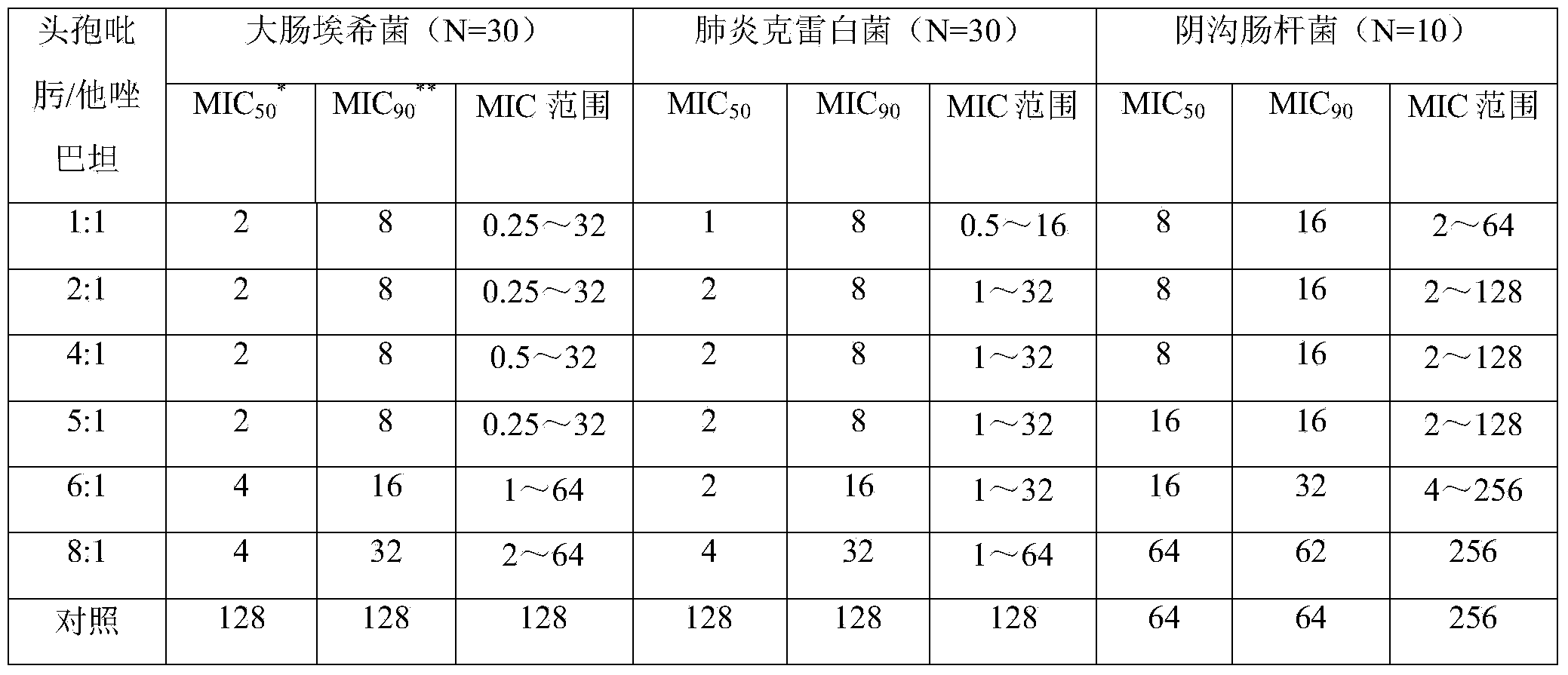
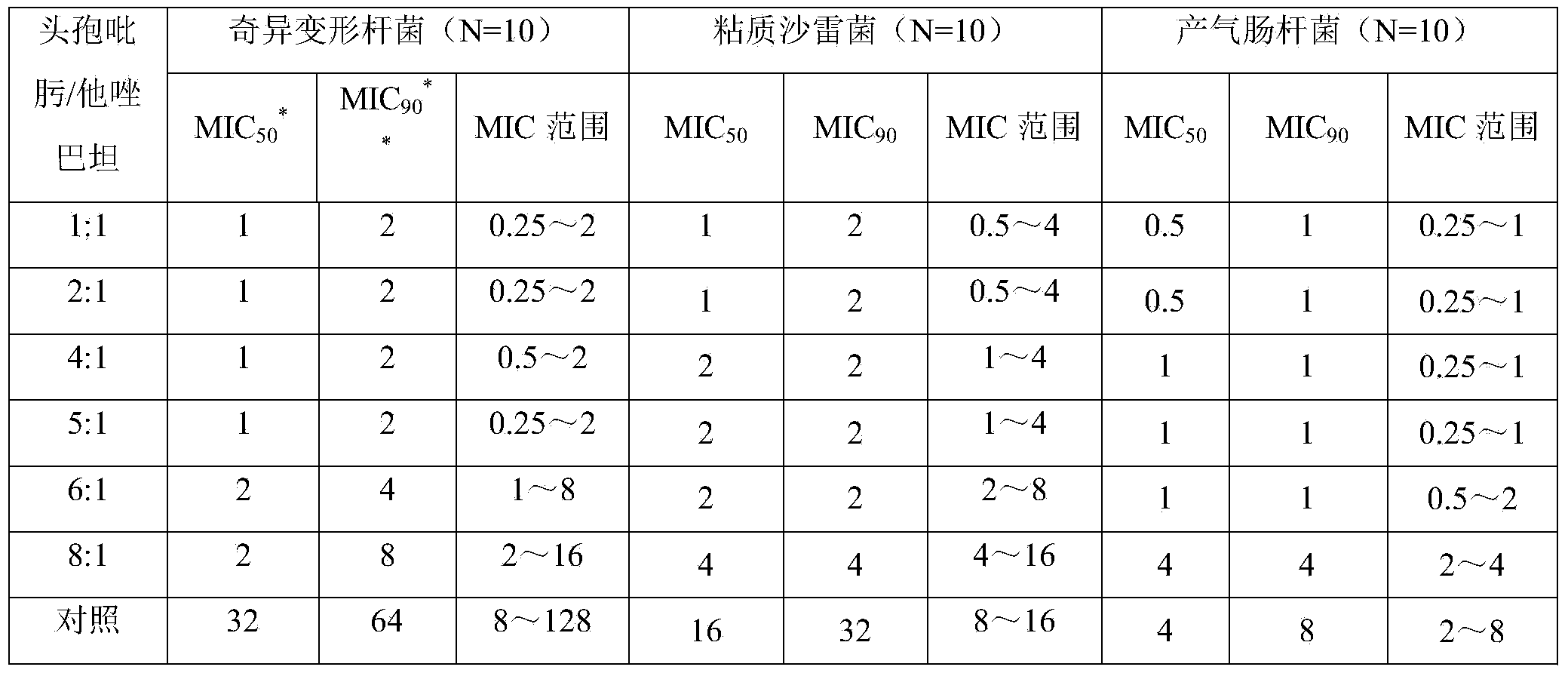
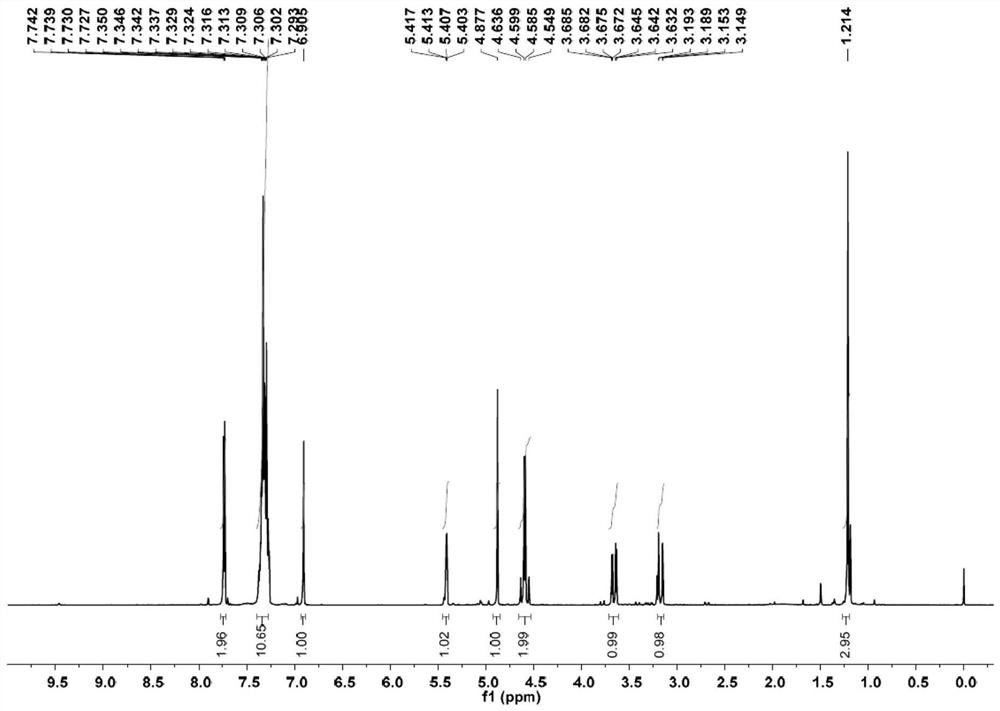
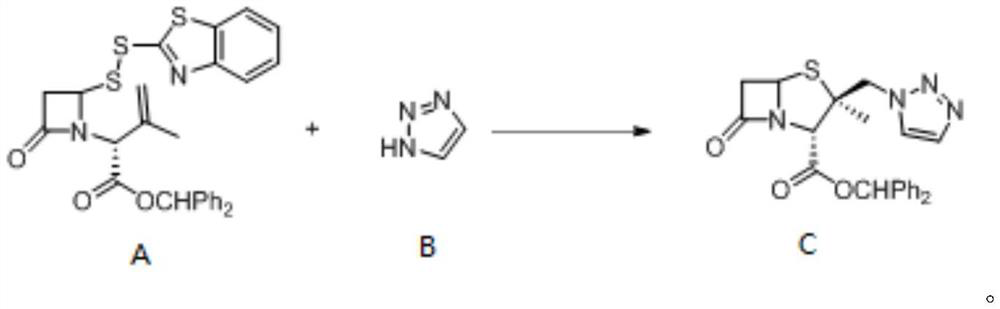
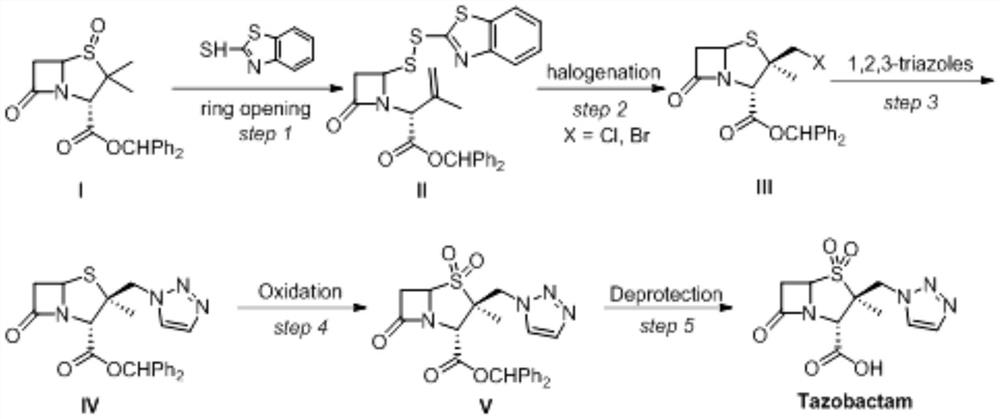
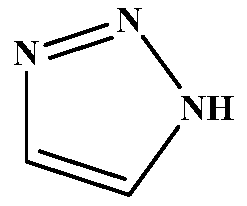
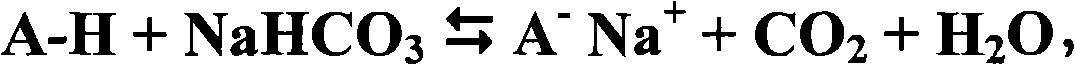Patents
Literature
124 results about "Tazobactam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
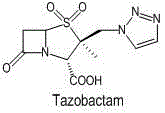
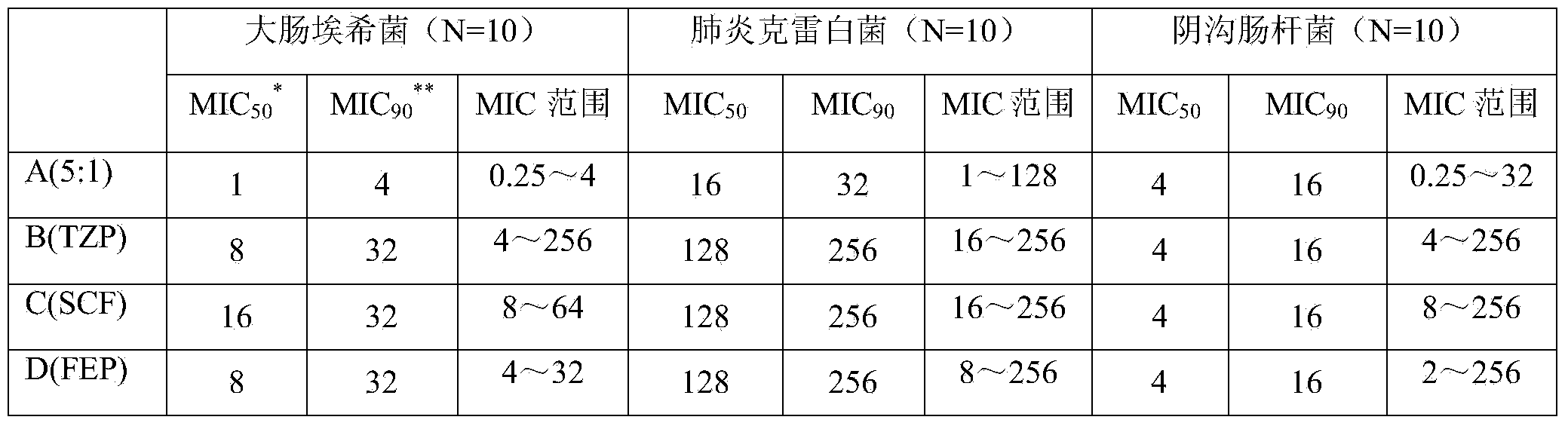
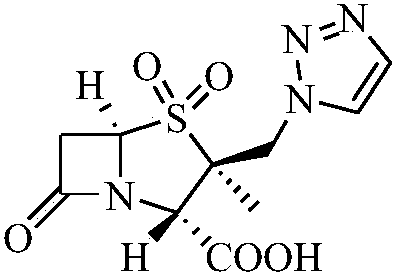
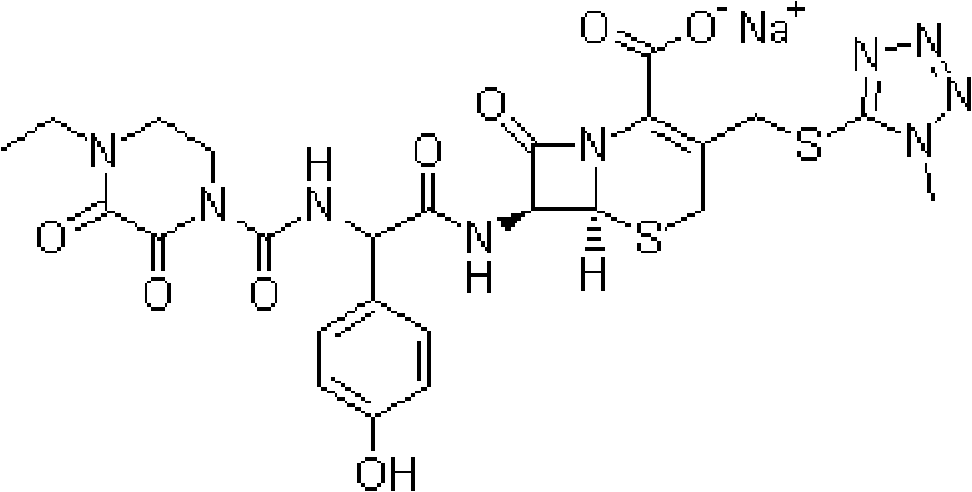
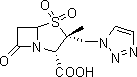
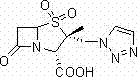
Tazobactam is a pharmaceutical drug that inhibits the action of bacterial β-lactamases, especially those belonging to the SHV-1 and TEM groups. It is commonly used as its sodium salt, tazobactam sodium. In simple terms, it is an ingredient that can be added to certain antibiotics to make them less vulnerable to bacteria's antimicrobial resistance.
Compositions containing pipercillin and tazobactam useful for injection
InactiveUS6900184B2Reduce formationMaintain stabilityBiocidePeptide/protein ingredientsParticulatesDepressant
The invention pertains to pharmaceutical compositions of Zosyn® piperacillin with tazobactam in the presence of a buffer, preferably citrate, a particulate formation inhibitor, preferably EDTA optionally an aminoglycoside which when frozen and thawed or lyophilized and reconstituted reform a solution which has decreased particulate formation.
Owner:MEDICAL SAFETY PROD
Anti beta-bactamase antibiotic compound prepn.
InactiveCN1513457ABroad spectrum antibacterialImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsCefuroximeSulbactam
A compound anti-beta-lactamase antibiotic is prepared from cefuroxime or its salt, sulbactam or its salt, and tazobactan or its salt. Its advantages are broad spectrum and high antibacterial effect.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
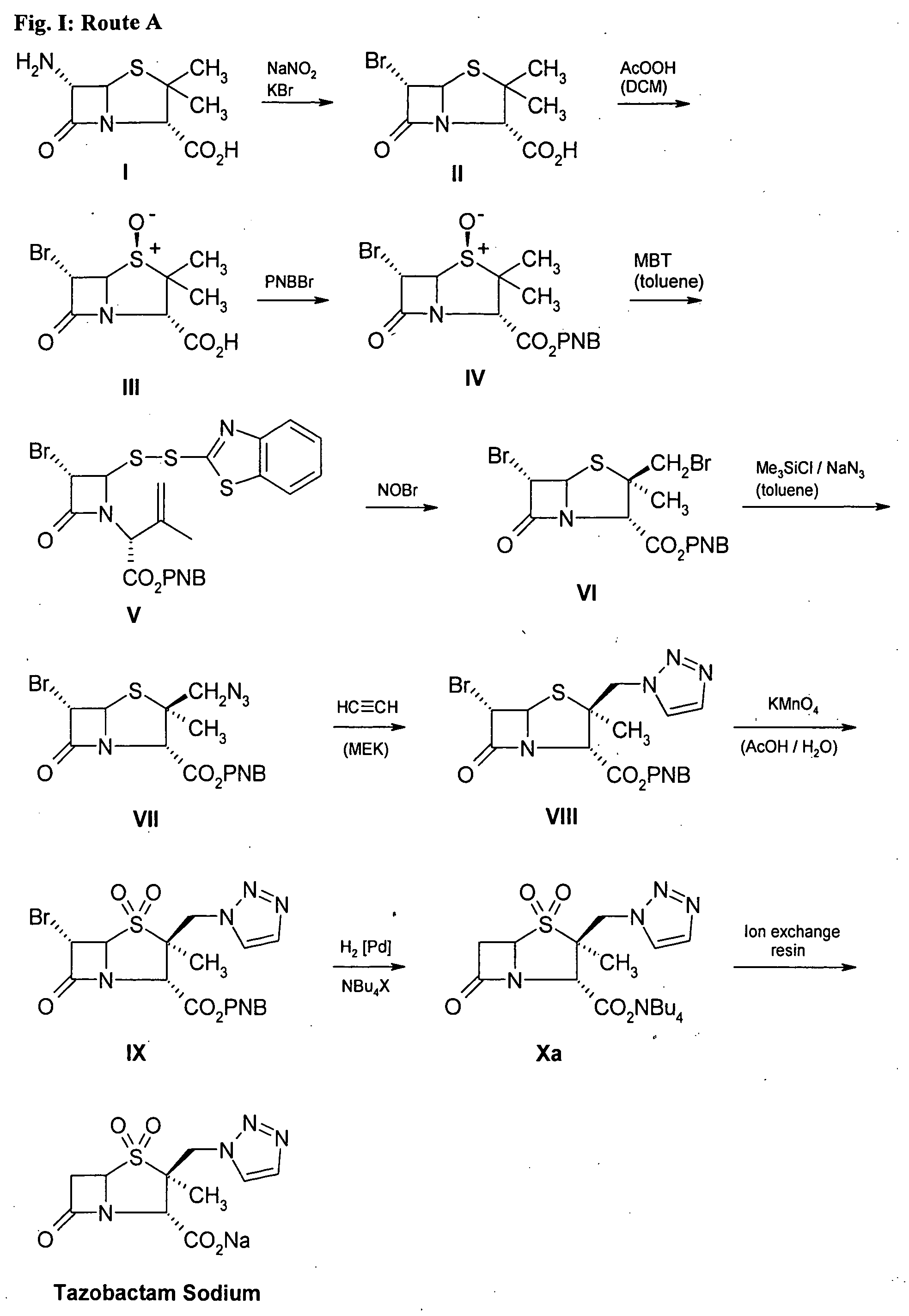
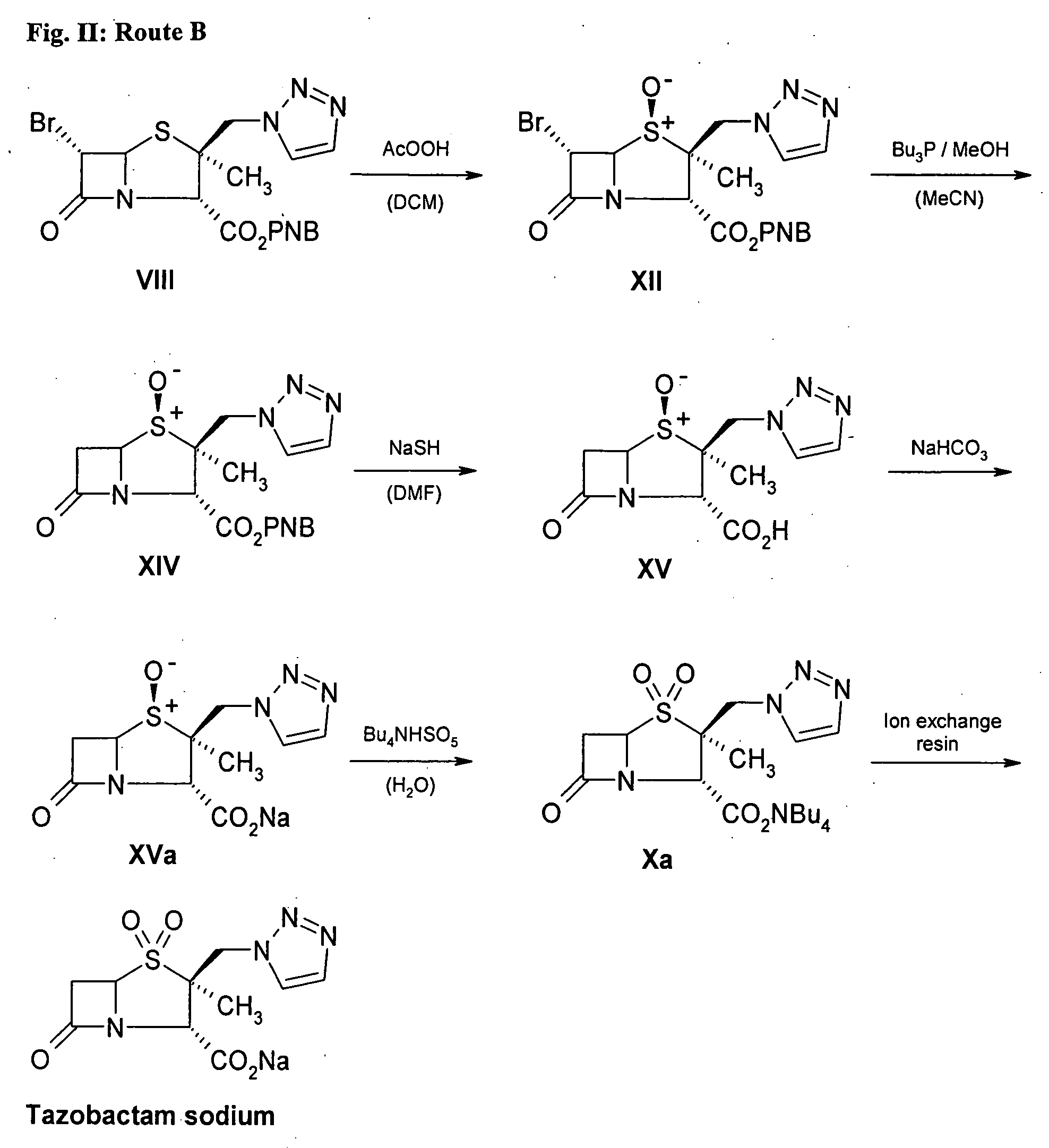
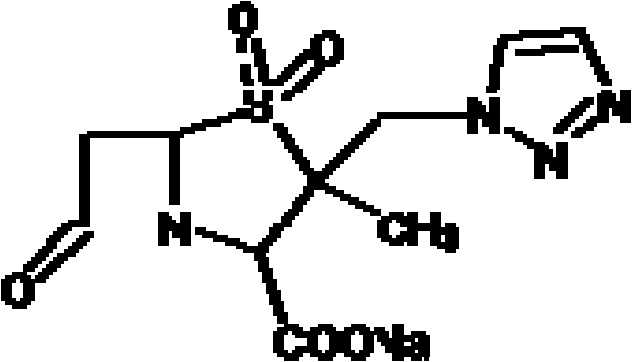
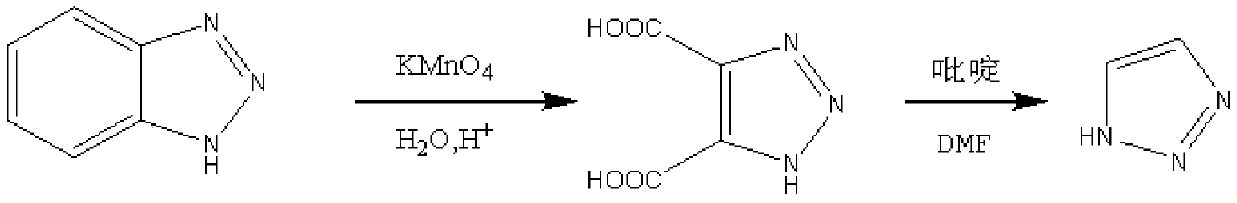
Tazobactam synthesis method
ActiveCN102020663AReduce usageWill not polluteOrganic chemistryChemical recyclingMetacresolSynthesis methods
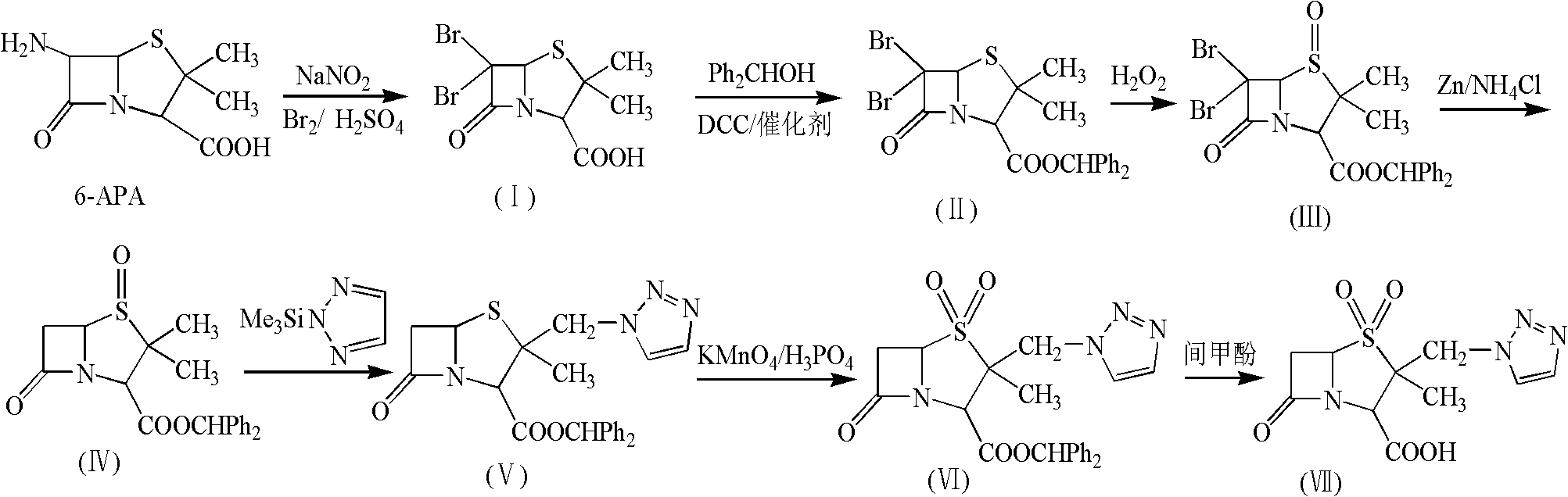
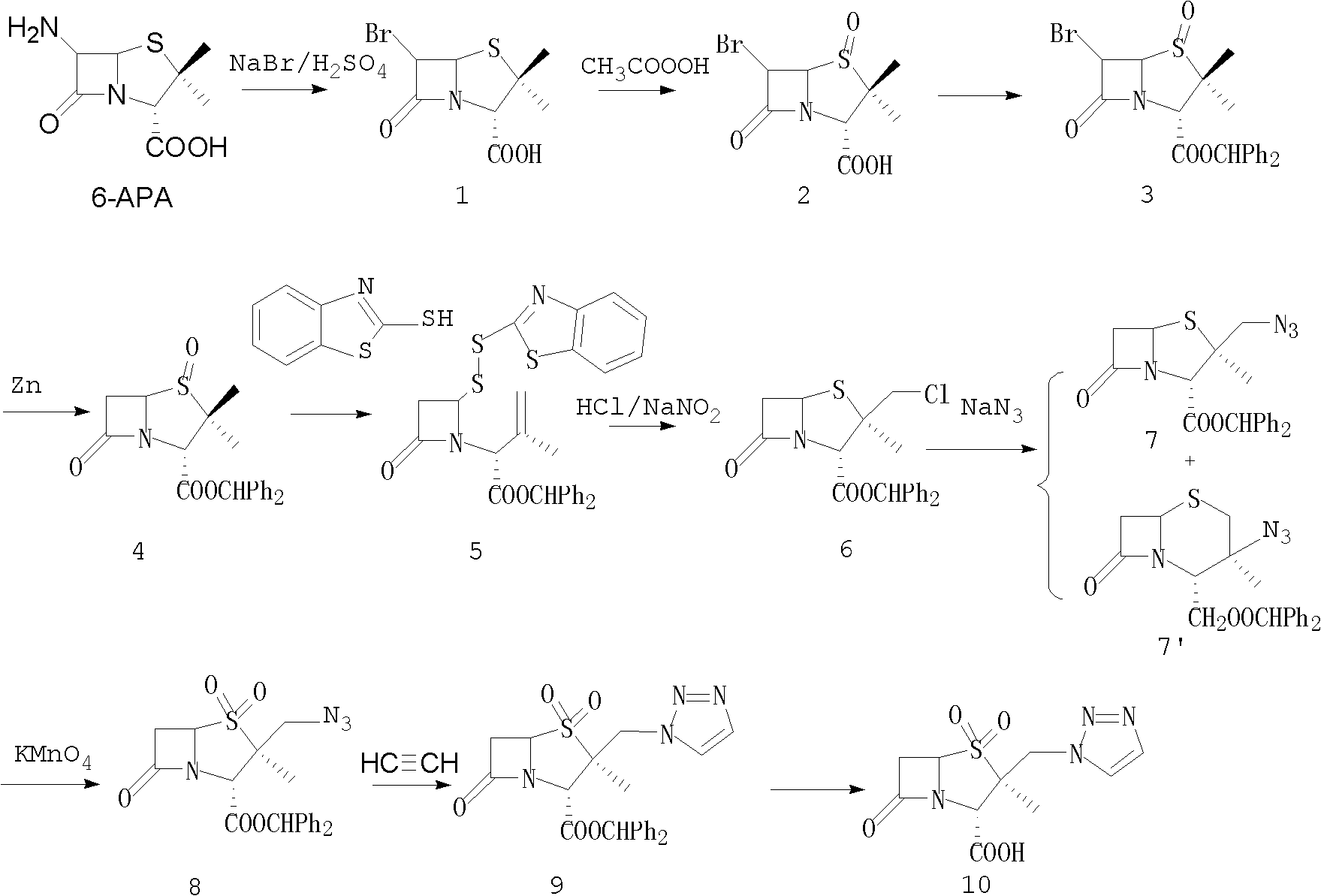
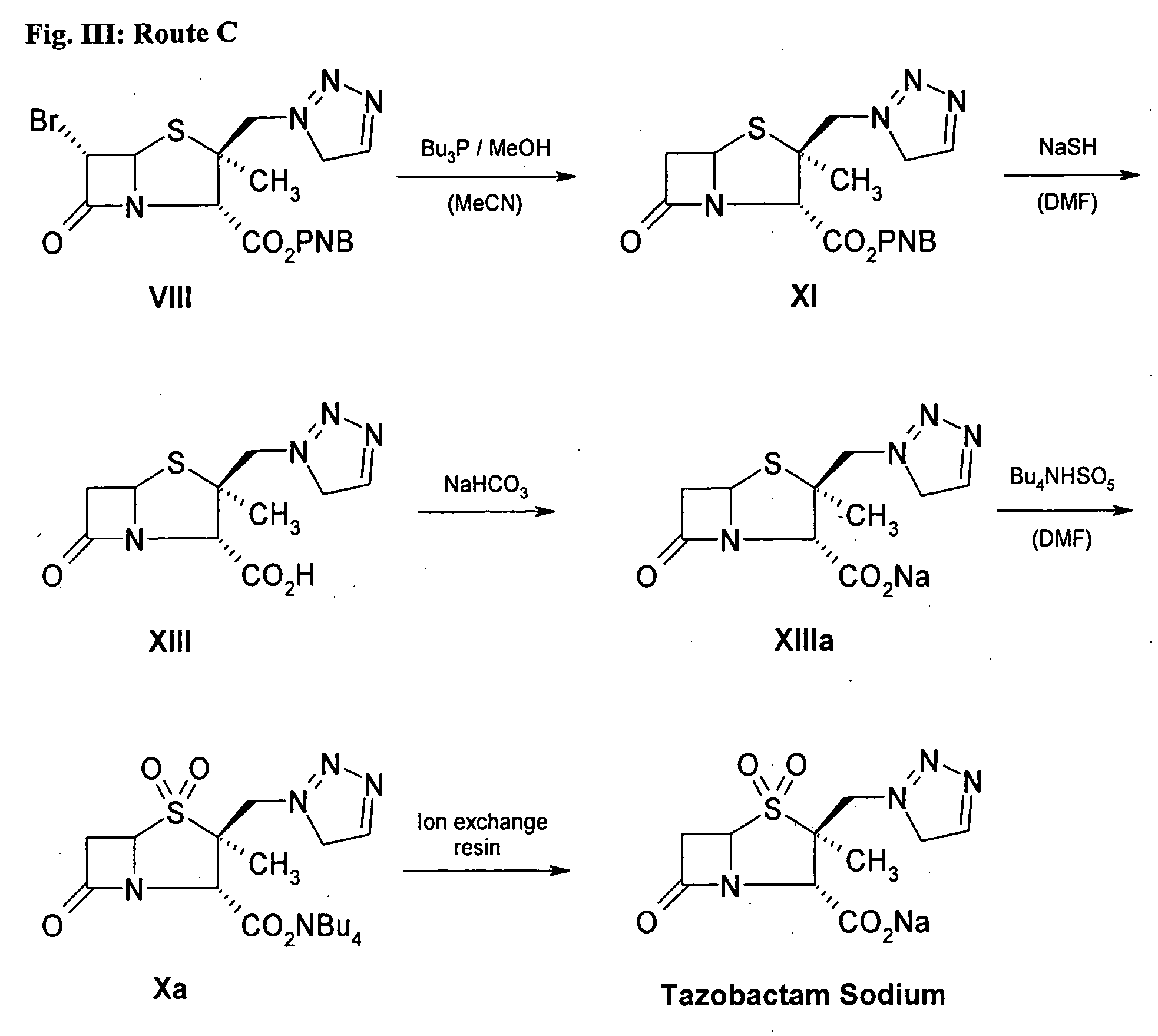
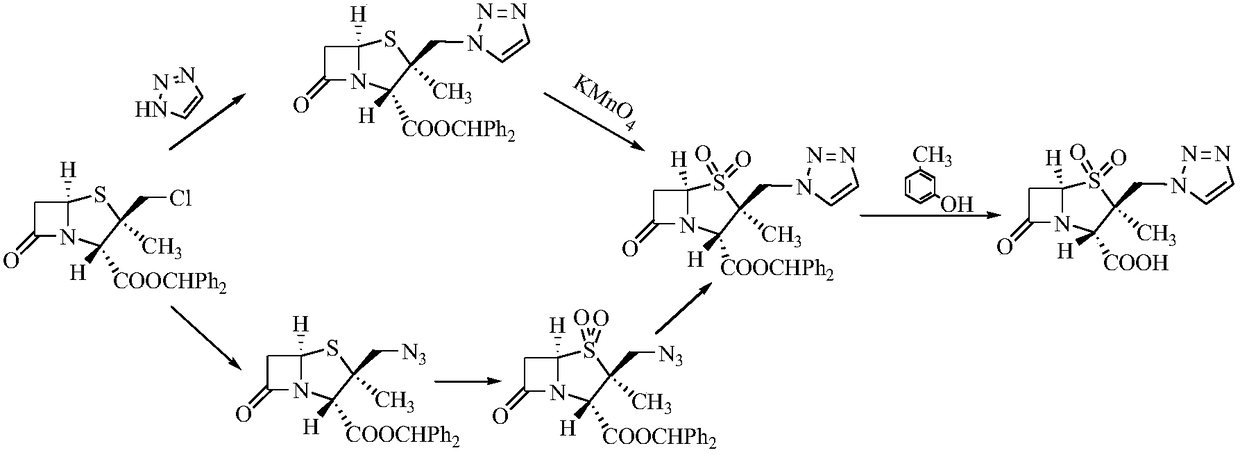
The invention relates to a tazobactam synthesis method which comprises the steps of: with 6-APA(Amino Penicillanic Acid) as raw material, preparing a key intermediate 6,6-dihydro penam sulphoxide acid diphenylcarbinol ester through successive reactions of esterification, oxidation, reduetive debromination and the like without separation; then, reacting with 2-triphenyl silicon-1,2,3-triazole; introducing a triazole ring; and finally obtaining the final product of tazobactam through potassium permanganate oxidation and metacresol deprotection. The tazobactam synthesis method is mainly characterized in that a phase transfer catalyst is introduced in the first step, therefore, the reaction rate and the product purity are improved; since an environment-friendly hydrogen peroxide-cobalt acetate catalytic oxidation system is adopted in the third step, the characteristics of good reaction selectivity, high yield, catalyst recyclability and the like are achieved; a method for synthesizing 2 alpha-methyl-2 beta-(1,2,3- triazole-1- radical) methyl penam-3 alpha-carboxylic acid diphenylcarbinol ester by using 2-triphenyl silicon-1,2,3-triazole is adopted in the fifth step, and the tazobactamsynthesis method is simple and convenient to operate, is safe and reliable, shortens the reaction route and improves the total yield. Compared with the traditional process, the tazobactam synthesis method greatly reduces the production cost and the environment pollution and has greater implementation value and economic benefits.
Owner:YIYUAN XINQUAN CHEM
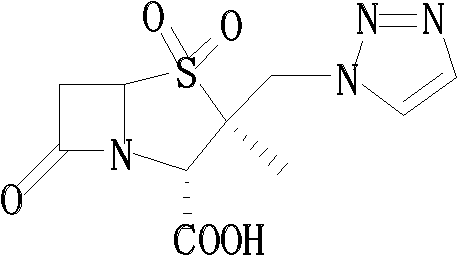
Penam iodide, preparation and use thereof
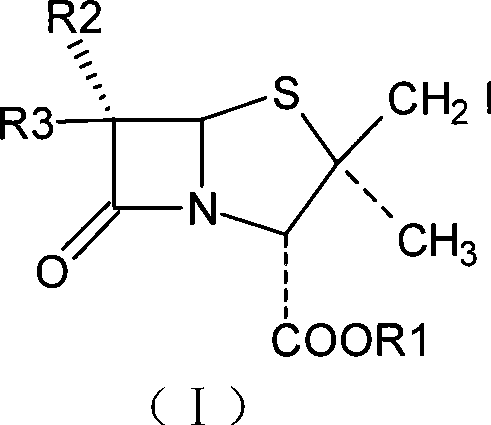
ActiveCN101434610AReduce pollutionLow production costOrganic chemistryBulk chemical productionIodideStructural formula
The invention relates to an intermediate iodide in the synthesis of Tazobactam, and a preparation method and the application thereof in preparing Tazobactam, and discloses the structural formula (I) of 2Beta-iodomethyl penicillanic acid ester. The invention has unique technique, stable product quality, mild technique condition and high yield, is easy to be controlled, requires relatively low production cost, reduces environmental pollution, needs no special equipment and is suitable for industrialized mass production.
Owner:QILU PHARMA HAINAN +1
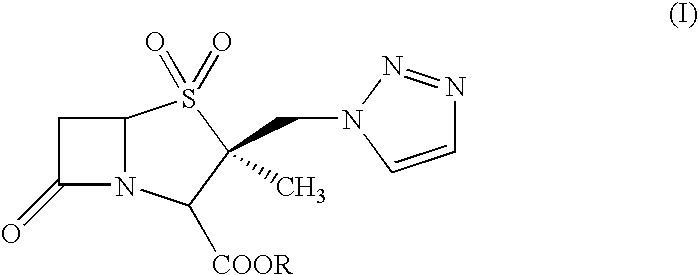
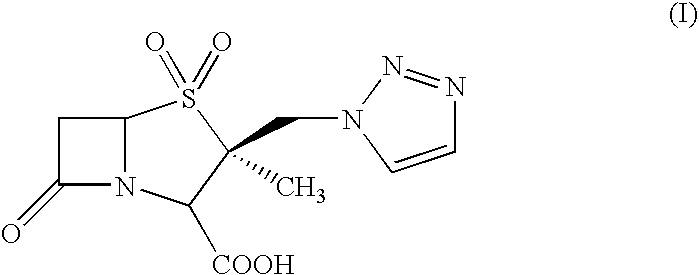
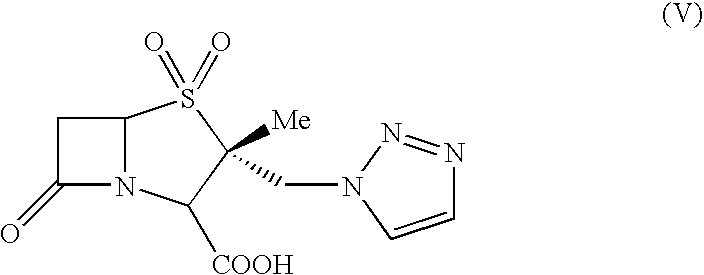
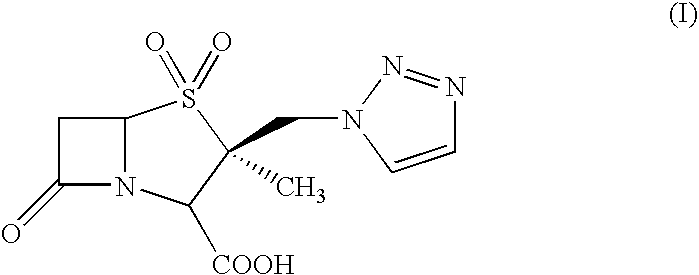
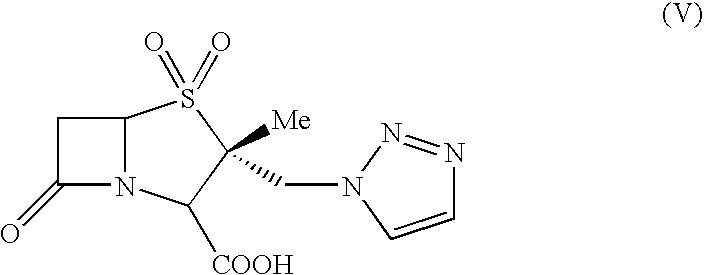
Process for the preparation of tazobactam in pure form
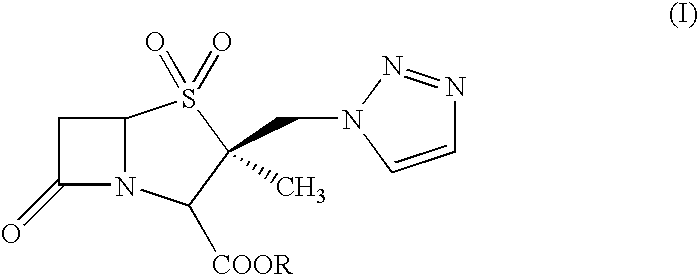
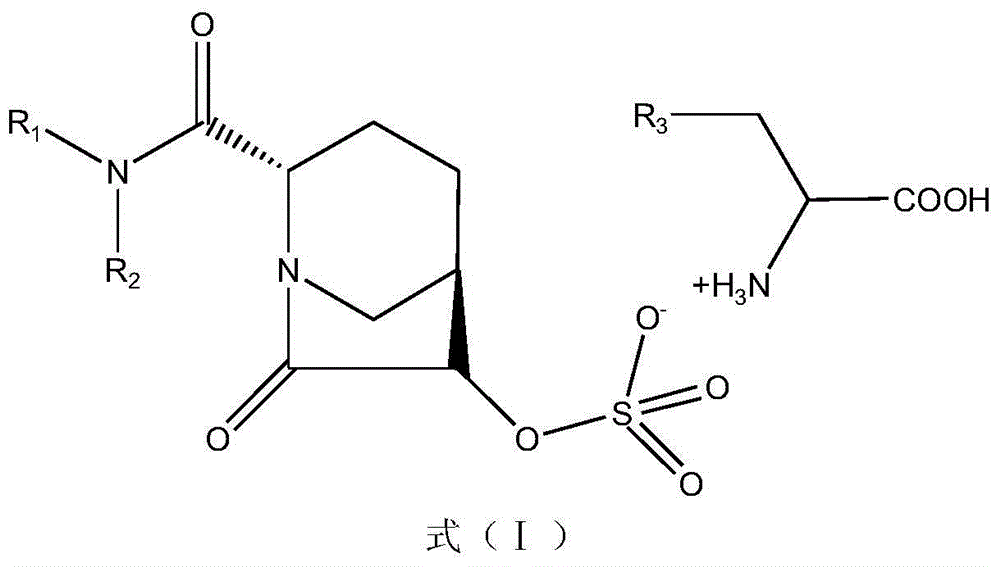
An improved process for the purification of tazobactam or its derivatives of the formula (I) wherein R represents hydrogen, C1-C6alkyl, p-methoxybenzyl, o-nitrobenzyl, p-nitrobenzyl, o-chlorobenzyl, benzyl or diphenylmethyl, which comprises the steps of: i) slurrying the compound of formula (I) containing the impurity of the formula (V) using a solvent in the presence or absence of tartaric acid with or without the presence of water at 20-50° C. and ii) isolating the compound of formula (I) in pure form
Owner:ORCHID CHEM & PHARM LTD
Tazobactam synthesis method
ActiveCN102643292ASteps to increase monoxidationBlocking affinityOrganic chemistryMetacresolSynthesis methods
The invention discloses a tazobactam synthesis method, which belongs to the technical field of medicines, and includes the steps: firstly, enabling 6,6-dihydropenam sulfoxide acid diphenylmethyl ester serving as raw materials to undergo thermal cracking and chloromethylation reaction to obtain 2beta-chloromethyl penicillanic acid diphenylmethyl ester; secondly, adding oxidizing agent to oxidize the 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide, enabling the oxidized 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide to react with sodium azide to generate 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1beta- oxide, and then generating 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1,1- dioxide by means of oxidization under the action of potassium permanganate and acetic acid; and finally, preparing the tazobactam by means of deprotection under the action of acetylene cyclization and metacresol. Compared with a past 6-APA (aminopenicillanic acid) route, the tazobactam synthesis method has the advantages that the step of sulfur atom single oxidization is added, so that possibility of ring expansion due to affinity of lone pair electrons on a sulfur atom is blocked, and transformation of five-membered ring products to six-membered ring by-products during hydrazoic reaction can be effectively controlled.
Owner:山东安信制药有限公司 +1
Preparation method for tazobactam
InactiveCN104031065AImprove stabilityInhibition of ring expansion reactionOrganic chemistryBulk chemical productionCatalytic oxidationM-Cresol
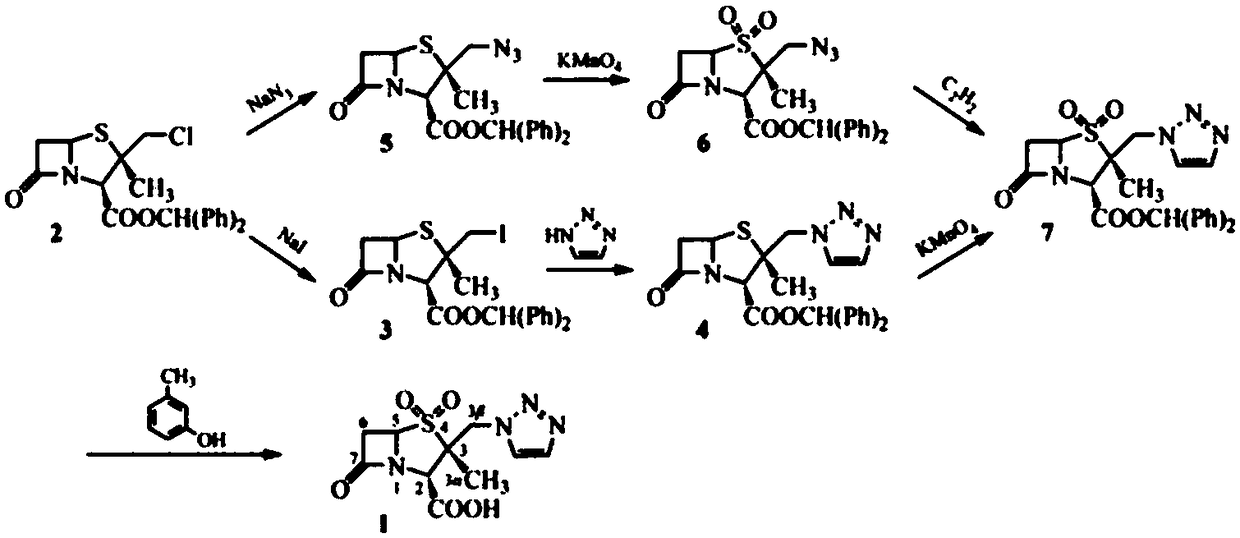
The invention discloses a preparation method for tazobactam. The preparation method comprises the following steps: with benzhydryl s-oxopenicillanate as a raw material, successively carrying out thermal cracking, bromination, catalytic oxidation and a reaction with 1H-1,2,3-triazole under the action of an anion resin carrier so as to obtain an important intermediate 2beta-(1H-1,2,3-triazolyl)-2alpha-methyl-benzhydryl penicillanate-1beta-oxide; and carrying out potassium permanganate oxidation and then protective group removal under the action of meta-cresol so as to obtain the target product tazobactam. The invention is characterized in that a sulfur atom is subjected to monooxidation so as to improve compound stability, then a nucleophilic substitution reaction with 1H-1,2,3-triazole is carried out so as to effectively control the possibility of ring enlargement during introduction of a triazole ring, and total yield is increased to 68%. The preparation method has the advantages of stable process, simple and convenient operation, easy separation and purification of the reaction product, a small amount of waste gas, waste water and industrial residues, high yield and suitability for clean, industrial and large-scale production.
Owner:JIANGXI HUABANG PHARMA
Compositions containing piperacillin and tazobactam
InactiveUS20050171077A1Increased health riskAvoid bacterial infectionAntibacterial agentsPowder deliveryPharmacologyGalactomannan
The invention pertains to pharmaceutical compositions of Zosyn® having substantially free or reduced levels of galactomannan and processes to prepare said pharmaceutical compositions.
Owner:WYETH LLC
Process for the preparation of lyophilized piperacilline sodium with improved stability after reconstitution
The invention discloses an improved process for the production of lyophilized Piperacillin alone or in combination with Tazobactam with improved pH adjustment, by degassing the solution of products to a controlled low carbon dioxide content prior to lyophilization.
Owner:SANDOZ LTD
Preparation technologies of tazobactam acid and tazobactam diphenylmethyl ester and application
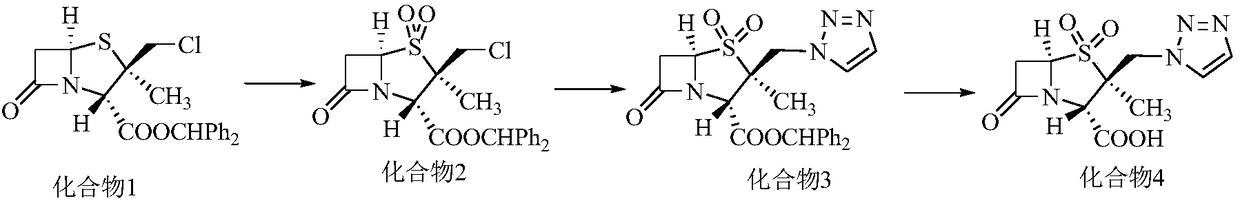
InactiveCN108164550ALow toxicityImprove stabilityAntibacterial agentsOrganic chemistryEthyl ChlorideMercaptobenzothiazole
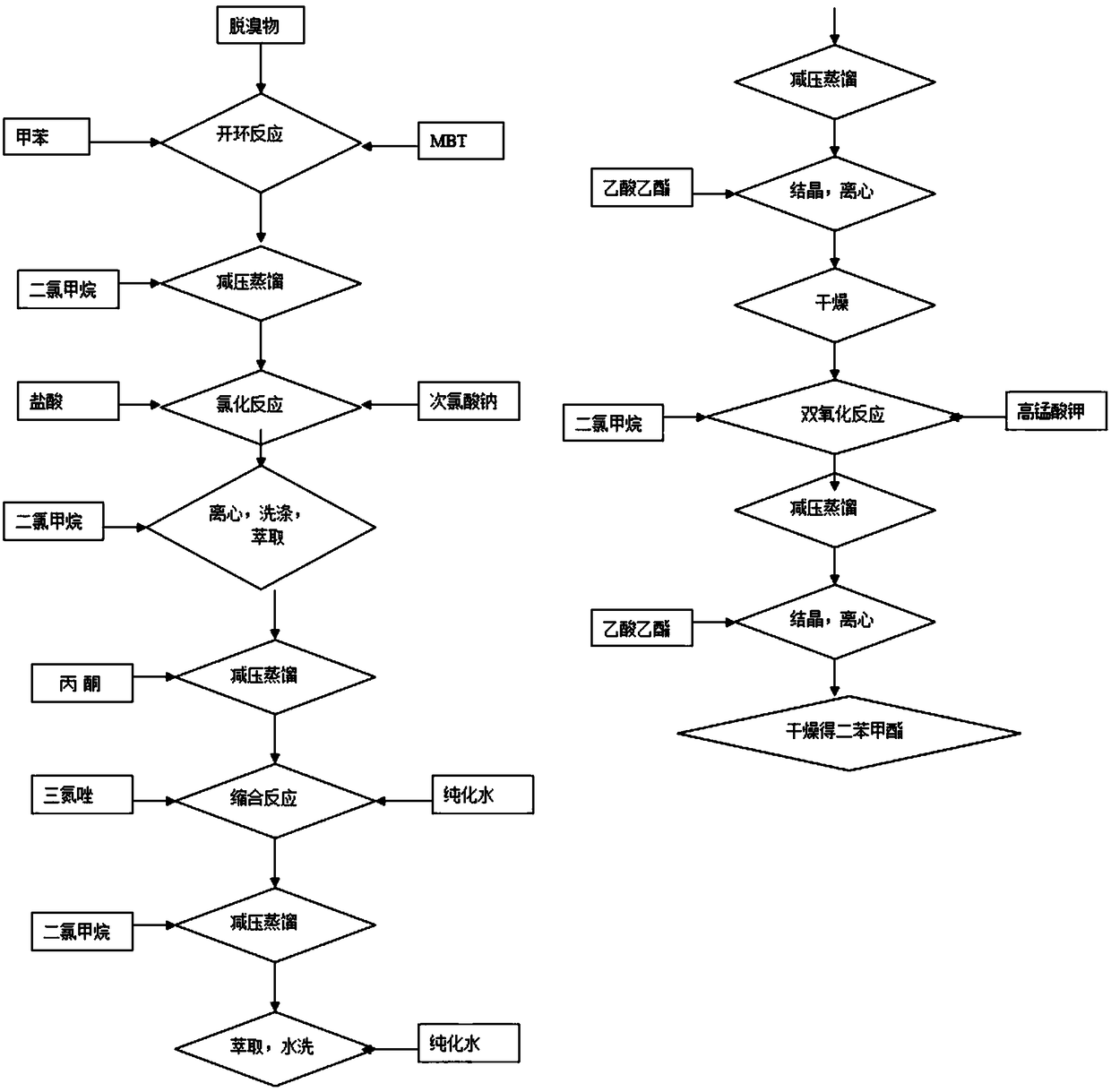
The invention relates to preparation technologies of tazobactam acid and tazobactam diphenylmethyl ester and application. A preparation route is as follows: with methylbenzene, 2-mercaptobenzothiazoleand a debrominated product as starting materials, the tazobactam diphenylmethyl ester is prepared after a ring opening reaction, a chlorination reaction, a condensation reaction and a double oxidation reaction, then the tazobactam diphenylmethyl ester is transformed into the tazobactam acid after a deprotection reaction; a preparation method in the scheme is improved on the basis of an iodinationreaction in the prior art, and the chlorination reaction is introduced, so that the reaction quality is optimized, the product purity is improved, and use of toxic, harmful, flammable and explosive substances in the reaction process is reduced, and thus the reaction is more environment-friendly; the product yield can be as high as 98-99.92%, and the preparation cost is also lower than that of thepreparation technology in the prior art.
Owner:常州红太阳药业有限公司
Compositions containing piperacillin and tazobactam useful for injection
The invention provides a pharmaceutical composition comprising piperacillin, tazobactam, an aminocarboxylic acid, and a buffer in a sodium lactate diluent. The invention further relates to a method of treating a bacterial infection and an LR condition in a human which comprises administering to said human an effective amount of a pharmaceutical composition comprising piperacillin, tazobactam, an aminocarboxylic acid, and a buffer in a sodium lactate diluent.
Owner:WYETH
Process for the preparation of Tazobactam in pure form
An improved process for the purification of tazobactam or its derivatives of the formula (I)wherein R represents hydrogen, C1-C6alkyl, p-methoxybenzyl, o-nitrobenzyl, p-nitrobenzyl, o-chlorobenzyl, benzyl or diphenylmethyl, which comprises the steps of:i) slurrying the compound of formula (I) containing the impurity of the formula (V) using a solvent in the presence or absence of tartaric acid with or without the presence of water at 20-50° C. andii) isolating the compound of formula (I) in pure form.
Owner:ORCHID CHEM & PHARM LTD
Process for preparation of penam derivatives
The invention relates to novel processes for preparing penam derivatives, such as Tazobactam and derivatives thereof. The processes according to the invention encompass procedures for the protection and deprotection of the carboxylic group as well as for the oxidation of the sulfur moiety of penam derivatives. Additionally, the present invention relates to new intermediates for the production of penam derivatives, allowing the desired penam-derivatives to be formulated with high purity and in good yields.
Owner:HELM AG
Lyophilization Process
InactiveUS20090186865A1Method of consistentlyAntibacterial agentsOrganic chemistryLow carbon dioxideChemistry
An improved process for the production of lyophilized Piperacillin alone or in combination with Tazobactam with improved pH adjustment, by degassing the solution of products to a controlled low carbon dioxide content prior to lyophilization.
Owner:SANDOZ AG
Beta- lactamase suppressing antibacterial compound drugs
InactiveCN1565457AHigh tissue contentWide distribution in the bodyAntibacterial agentsOrganic active ingredientsCompounding drugsCeftizoxime
The invention discloses a beta- lactamase suppressing antibacterial compound drugs, which comprises ceftizoxime, or cefodizime and beta-lactam enzyme inhibitor by the active acid weight ratio of 1-10:10-1, which are in the forms of alkali metal salts or free acid and assisting solvents, the beta-lactam enzyme inhibitor can be Tazobactam, or clavulanic acid, or tapazole or their derivatives.
Owner:张哲峰
Composition used for inhibiting bacteria generating novel beta lactamase
ActiveCN102743388AEnhanced inhibitory effectGood/or killing effectAntibacterial agentsHeterocyclic compound active ingredientsActive componentSocial effects
The invention discloses a composition used for inhibiting bacteria generating novel beta lactamase. The composition is advantaged in that the active components of the composition comprise cefepime and tazobactam with a weight ratio of 2-6:1. According to the invention, the composition used for inhibiting the bacteria generating novel beta lactamase has a good inhibiting and / or killing effect against the bacteria generating novel CTX-M enzyme and AmpC enzyme, and is especially effective against bacteria with drug resistance to CTX-M enzyme and AmpC enzyme hydrolysis effects. The composition has good practicality, and provides good economic and social effects.
Owner:NANJING YOKO PHARMA GRP CO LTD +1
Composition used for inhibiting bacteria generating novel beta lactamase
ActiveCN102743388BEnhanced inhibitory effectHigh antibacterial activityAntibacterial agentsHeterocyclic compound active ingredientsActive componentSocial effects
The invention discloses a composition used for inhibiting bacteria generating novel beta lactamase. The composition is advantaged in that the active components of the composition comprise cefepime and tazobactam with a weight ratio of 2-6:1. According to the invention, the composition used for inhibiting the bacteria generating novel beta lactamase has a good inhibiting and / or killing effect against the bacteria generating novel CTX-M enzyme and AmpC enzyme, and is especially effective against bacteria with drug resistance to CTX-M enzyme and AmpC enzyme hydrolysis effects. The composition has good practicality, and provides good economic and social effects.
Owner:NANJING YOKO PHARMA GRP CO LTD +1
Beta-lactamase inhibitor
InactiveCN105801579AExtended half-lifeLower minimum inhibitory concentrationAntibacterial agentsOrganic active ingredientsHalf-lifeAvibactam sodium
The invention discloses a beta-lactamase inhibitor. The beta-lactamase inhibitor can inhibit A-type beta-lactamases (such as SHV, TEM and CTX), C-type beta-lactamase (mainly comprising an AmpC enzyme) and D-type beta-lactamase (such as OXA), has good inhibition effects and can reduce the lowest ceftazidime bacteriostasis concentration through combination with ceftazidime. Compared with tazobactam, the beta-lactamase inhibitor has a longer half life. A part of the beta-lactamase inhibitor has a half life longer than that of avibactam sodium. The beta-lactamase inhibitor provides more possibility for antibiotic development.
Owner:卢来春
Diffluent antibacterial drugs composition pharmaceutical formulation, method of preparing the same and use thereof
The invention relates to an easily soluble antibacterial drug agent, making method and application, which consists of component A and component B with weight ratio at 100:0. 1 to 1:10, wherein the component A is piperacilliin or other physiological medicinal salt or hydrate, or tazobactam or other physiological medicinal salt or hydrate or the composition of piperacilliin or other physiological medicinal salt or hydrate and tazobactam or other physiological medicinal salt or hydrate, and the component B is pharmacy Lewis acid or Lewis base comprising soluble alkaline metals or salt of alkaline-earth metal, soluble organic base, organic acid or one or some kinds of salt of the both. The invention has merits of good water-solubility to solve fast and extend solubility, which is fit for anti-infectious treatment and precaution of bacillary diseases to human and animals.
Owner:刘力
Preparation method of tazobactam
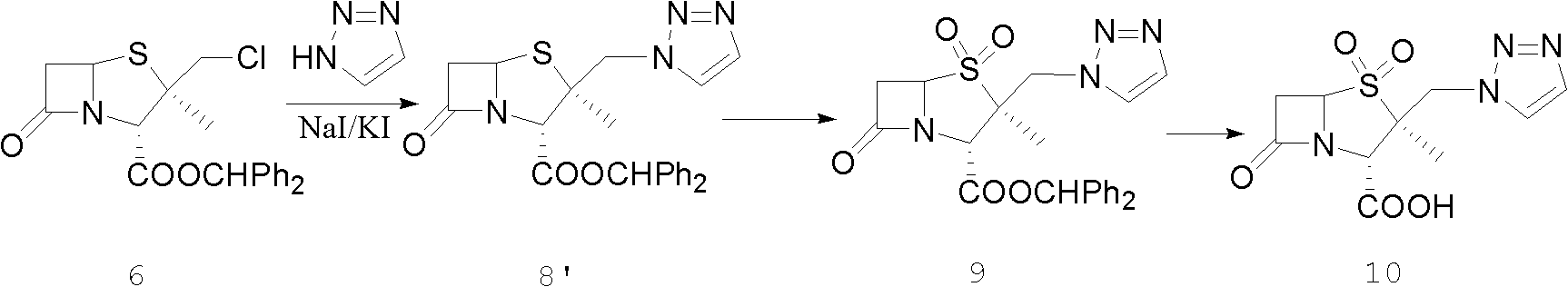
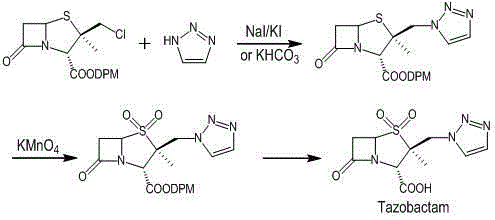
The invention discloses a preparation method of tazobactam. The preparation method comprises the following steps: performing double oxidization on 2beta-chloromethyl penicillanic acid diphenyl methylester by adopting a solution prepared from potassium permanganate, glacial acetic acid and concentrated sulfuric acid; then loading triazole by taking crown ether as a phase transfer catalyst and taking potassium iodide as a catalyst; then performing deprotection to obtain tazobactam. Compared with the prior art, the preparation method disclosed by the invention has the advantages that although sulfur atoms are oxidized into sulfone to lower chlorine atom activity, the use of the crown ether as the phase transfer catalyst and the potassium iodide as the catalyst compensates for the inactivation well. By adopting the method, the stability of the 2beta-chloromethyl penicillanic acid diphenyl methyl ester is improved, the reaction time is shortened, the reaction yield is improved, the operation risk is lowered, and the industrial production is facilitated.
Owner:山东安信制药有限公司 +1
Stable cefoperazone tazobactam medicine compound preparation
The invention discloses a stable compound preparation of cefoperazone-tazobactam drug, which is comprised by cefoperazone acid, tazobactam and latent solvent, which weight ratio is 8~1:1:5.6~0.06. The latent solvent is preferred selected from sodium carbonate and sodium bicarbonate. Related substances of the compound preparation in the invention are lower than standard of Chinese pharmacopoeia (2005 edition) in influencing factor and accelerated test in 40 DEG C, labelled content accords with the standard of the pharmacopoeia and changes very little in the experiment, and the product quality is stable.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Injection powder and injection preparation of cefoperazone sodium-tazobactam combination
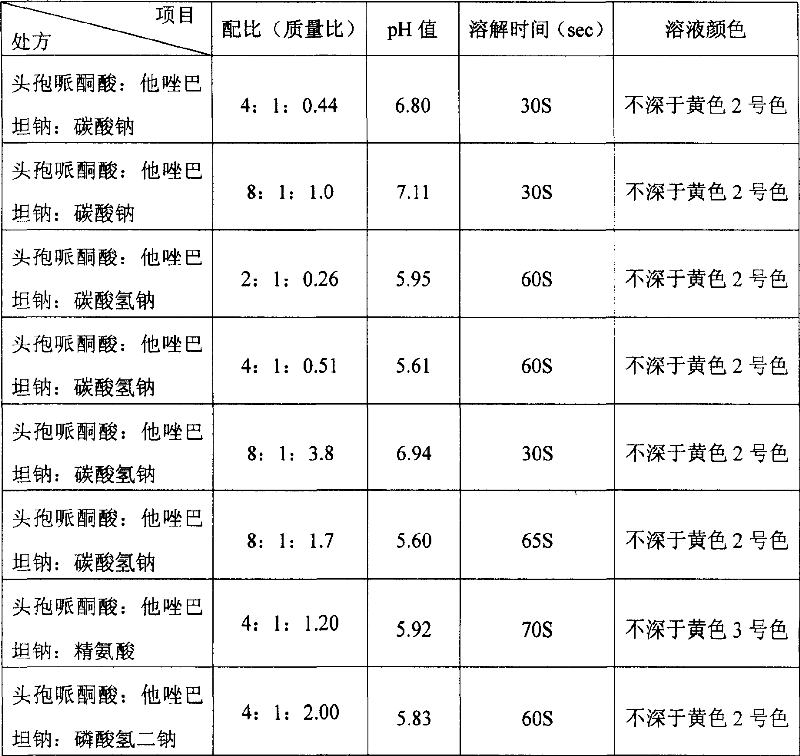
ActiveCN102552275AImprove efficacyAntibacterial agentsPowder deliveryCurative effectInjection powder
The invention relates to the technical field of medicine, in particular to an injection powder and injection preparation of a cefoperazone sodium-tazobactam combination. The injection powder and injection preparation comprises cefoperazone sodium, tazobactam combination and lignocaine hydrochloride, wherein the mass ratio of the cefoperazone sodium to the tazobactam combination to the lignocaine hydrochloride is 4:1:(0.01-0.05). Compared with a positive medicament control group, the injection powder and injection preparation of the cefoperazone sodium-tazobactam combination provided by the invention has the advantages of capability of relieving injection pain and remarkable improvement on the curative effect.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Antiseptic composition of mezlocillin
InactiveCN1485035AImprove antibacterial propertiesHigh antibacterial activityAntibacterial agentsHeterocyclic compound active ingredientsSolubilitySemisynthetic penicillin
An antibiotic composition of meloxine, which is composed of meloxine andª‰-lactamase inhibitor at the weight ratio of 1-10í†10-1 based on the amount of active acids. meloxine is in the form of the alkali salt of meloxine or in the form of its free acid and solubility promoter; theª‰-lactamase inhibitor is clavulanic acid or tazobactam or their derivatives. Besides mixing and preparing beforehand,the composition could be prepared by mixing meloxine andª‰-lactamase inhibitor proportionally in clinical applications, then is administered, ormeloxine andª‰-lactamase inhibitor are administered independently. meloxine andª‰-lactamase inhibitor are synergetic, which could solve the problem of meloxine resistance in clinical applications.
Owner:周宇
Compound prepn. contg. ceftazidime and tazobactam for injection use
InactiveCN1418632AEnhance clinical antibacterial effectImprove adverse reactionsAntibacterial agentsOrganic active ingredientsCeftazidimeResistant strain
Owner:海南瑞生堂制药有限公司
Antibiotic composition
InactiveCN1709263AHigh antibacterial activityPreservation of antimicrobial activityOrganic active ingredientsActive componentCefmenoxime
The present invention discloses an antibiotic composition. It includes the following components: cefmenoxime or cefmenoxime salt or hydrate of cefmenoxime salt or cefmenoxime ester or precursor medicine of cefmenoxime as active component, and beta-lactamase inhibitor as inhibitor; the described beta-lactamase inhibitor is clavulanic acid or sulbactam or tazobactam or clavulanic acid medicinal salt or its hydrate or precursor medicine of clavulanic acid or sulbactam medicinal salt or its hydrate or tazobactam medicinal salt or its hydrate or tazobactam ester or precursor medicine of tazobactam. The mixing ratio of both cefmenoxime and inhibitor (by weight portion) is 1:0.1-99.
Owner:黄文豪
Method for synthesizing deuterated tazobactam
ActiveCN103012431AHigh yieldCarbon-deuterium bonds are stableOrganic chemistryDecompositionSodium ascorbate
The invention discloses a method for synthesizing deuterated tazobactam. The method comprises the following steps: adding 2beta-azomethylpenicillanic acid-1beta-oxide, propiolic acid, a catalyst containing copper or cuprous ion and sodium ascorbate in a deuterated solvent in turn, stirring at 25-200 DEG C for reaction for 1 to 48 hours, and after the reaction, performing extraction, filtering and column chromatography to obtain the deuterated tazobactam. Through the method, according to the method for synthesizing the deuterated tazobactam, the deuterated tazobactam is a novel beta-lactamase inhibitor of the penicillanic sulfone type and can be used for treating various bacterial infections; the method has the characteristics of mild reaction condition, short synthesis procedure, simple process and high yield, the safety during the reaction is effectively improved by using propiolic acid as the raw material, and the carbon-deuterium chain in the obtained deuterated tazobactam molecule is so stable that the medicine decomposition process can be effectively retained, therefore, the deuterated tazobactam has a longer action time in the body and is better than the normal tazobactam.
Owner:SUZHOU ROEING BIOPHARMACEUTICALS CO LTD
Drug composition containing cefazolin and beta-lactamase inhibitor
InactiveCN1424039AAddressing drug resistanceHigh activityAntibacterial agentsHeterocyclic compound active ingredientsCefazolinAntibacterial action
An antibacterial composite medicine contains ancef or its salt and beta-lactamase inhibitor (tazobactam or clavulanic acid) in Wt ratio of (1-20):(1-5). Its advnatage is high synergistic antibacterial action.
Owner:YOUCARE PHARMA GROUP +1
Method for electrochemical synthesis of tazobactam key intermediate
ActiveCN113073348AHigh selectivityFew reaction stepsAnodisationElectrolysis componentsChemical synthesisCompound a
The invention provides a method for electrochemical synthesis of a tazobactam key intermediate. The method comprises the following steps: by taking a disulfide ring-opening compound A and 1, 2, 3-triazole B as reaction substrates, mixing the reaction substrates, electrolyte and a reaction solvent, and then performing electrochemical anodic oxidation to obtain the tazobactam key intermediate C. The method effectively solves the problems of low selectivity, low reaction efficiency or poor economical efficiency and the like during synthesis of 2 beta-triazole methyl penicillic acid diphenylmethyl ester in the prior art.
Owner:JINLIN ASYMCHEM PHARM CO LTD
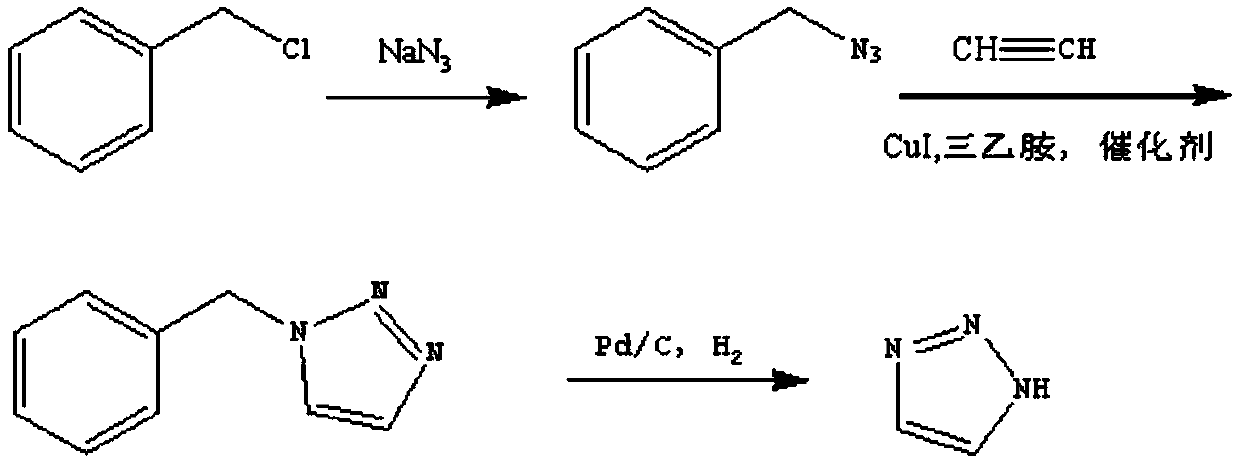
Method for preparing important intermediate 1H-1,2,3-triazole of Tazobactam
ActiveCN110240569AEasy to operateSimple post-processingOrganic chemistryNitriteHydroxylamine Hydrochloride
The invention discloses a method for preparing an important intermediate 1H-1,2,3-triazole of Tazobactam. The method comprises the steps: firstly, subjecting glyoxal to a reaction with hydroxylamine hydrochloride, so as to obtain an intermediate I; subjecting the intermediate I and an ammonium salt to cyclization in the presence of a catalyst, so as to obtain an intermediate II; and subjecting the intermediate II to a reaction with nitrite under acidic conditions to deaminate so as to obtain crude triazole, and carrying out further refining, thereby obtaining finished triazole. According to the method, the operation is simple, the production cycle is short, the aftertreatment is simple, few waste gases, waste water and waste residues are produced, and the obtained product is high in yield, good in purity and low in cost, so that the method is more applicable to industrial large-scale production.
Owner:山东安信制药有限公司 +1
Synthetic method for tazobactam
The invention discloses a synthetic method for tazobactam. The method comprises the following steps: adding propiolic acid, 2 beta-azidomethyl penicillanic acid-1 beta-oxide, sodium ascorbate and a catalyst containing cuprous ions or copper ions to a solvent in sequence; stirring and reacting these materials for 0.5-72 h at 20-180 DEG C; and after the reaction is finished, extracting a reactant and carrying out column chromatography on the reactant so as to obtain the tazobactam. Through the method, the synthetic method for the tazobactam, disclosed by the invention, has the advantages as follows: the tazobactam is a novel sulbactam type beta-lactamase inhibitor and can be used for treating a plurality of bacterial infections; and compared with a method with acetylene as raw material, the method has the advantages as follows: through using the propiolic acid as the raw material, the safety in the reaction process is enhanced and an electricity absorbing carboxyls on a molecule is beneficial for carrying out cycloaddition reaction and preferably compatible with a reaction substrate; the reaction condition is mild; the reaction can be conducted at normal temperature; the operation process is convenient and simple; the yield of obtained products is high; and industrial production can be carried out on a large scale.
Owner:SUZHOU ROEING BIOPHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com