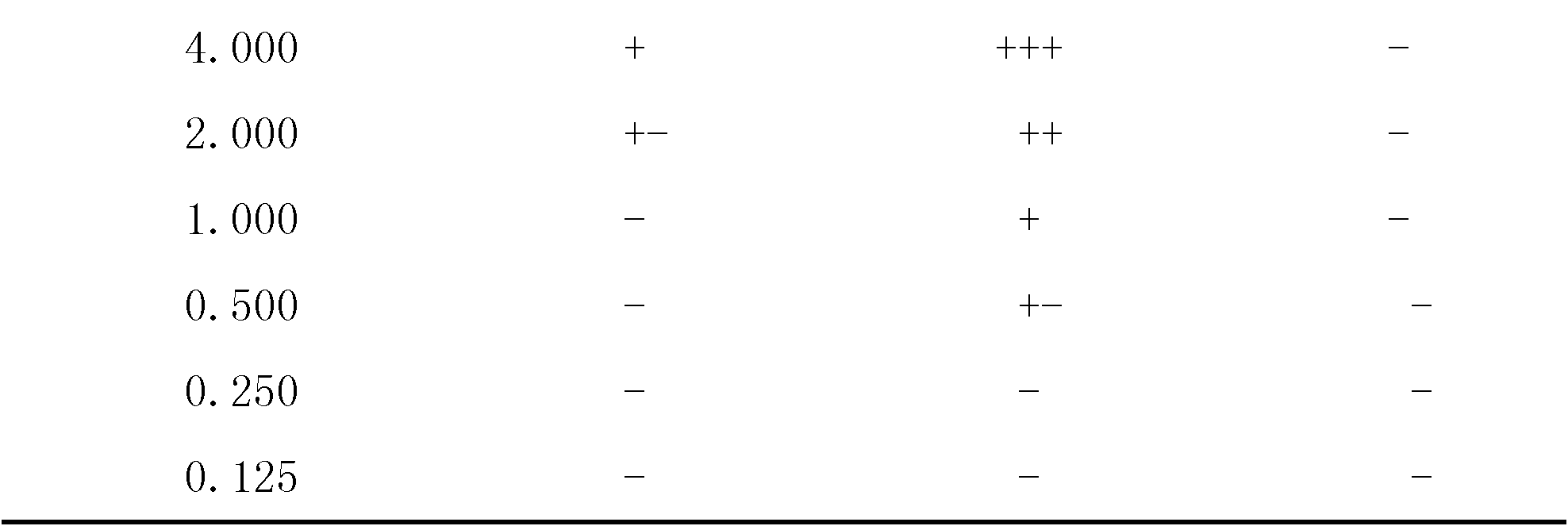Patents
Literature
38 results about "Cefoperazone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
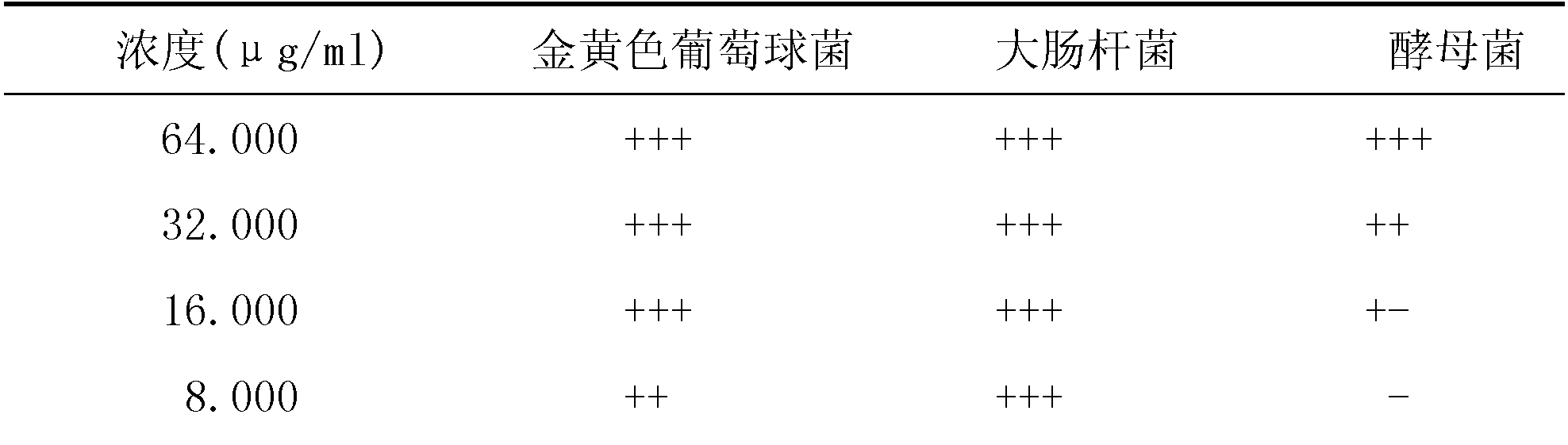
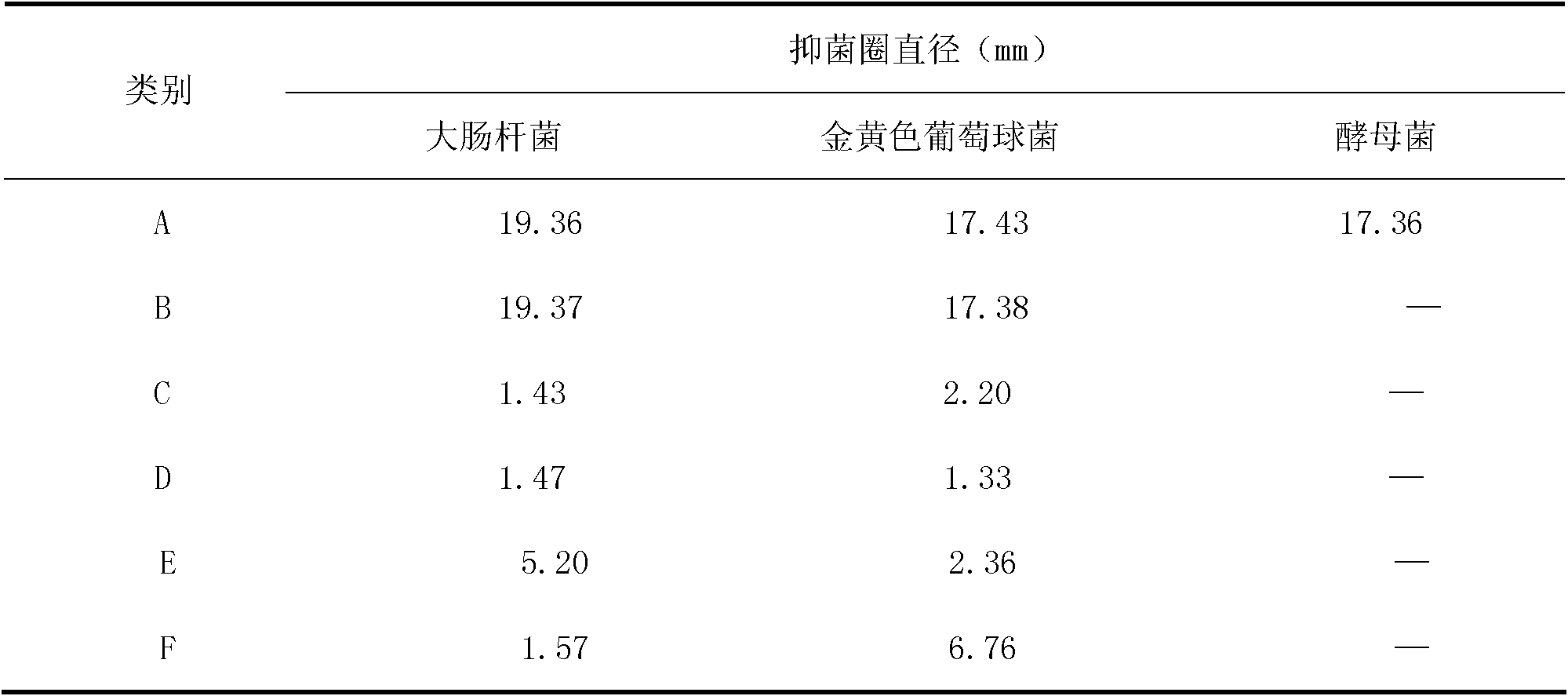
Cefoperazone is a third-generation cephalosporin antibiotic, marketed by Pfizer under the name Cefobid. It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics.
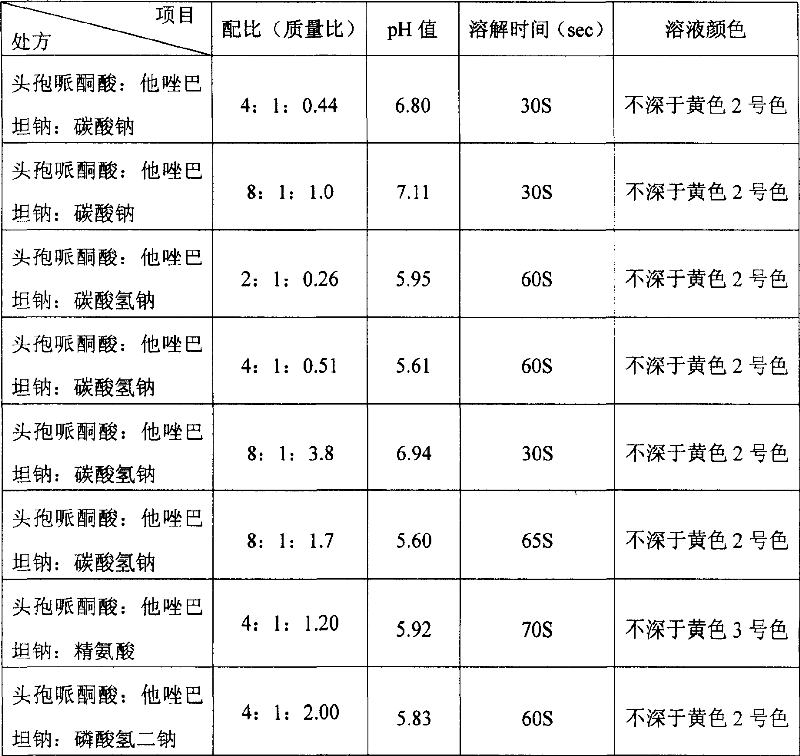
Stable cefoperazone tazobactam medicine compound preparation
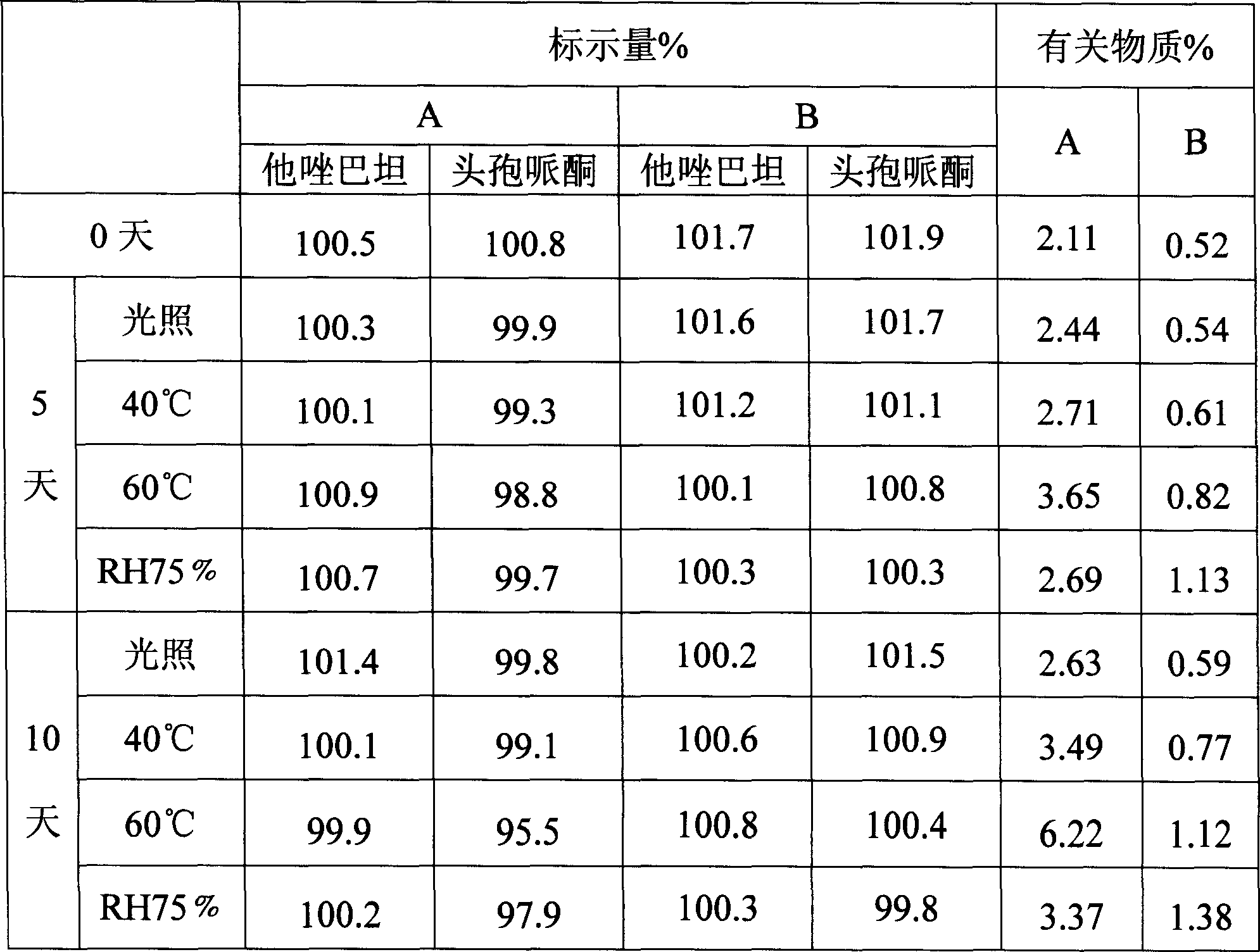
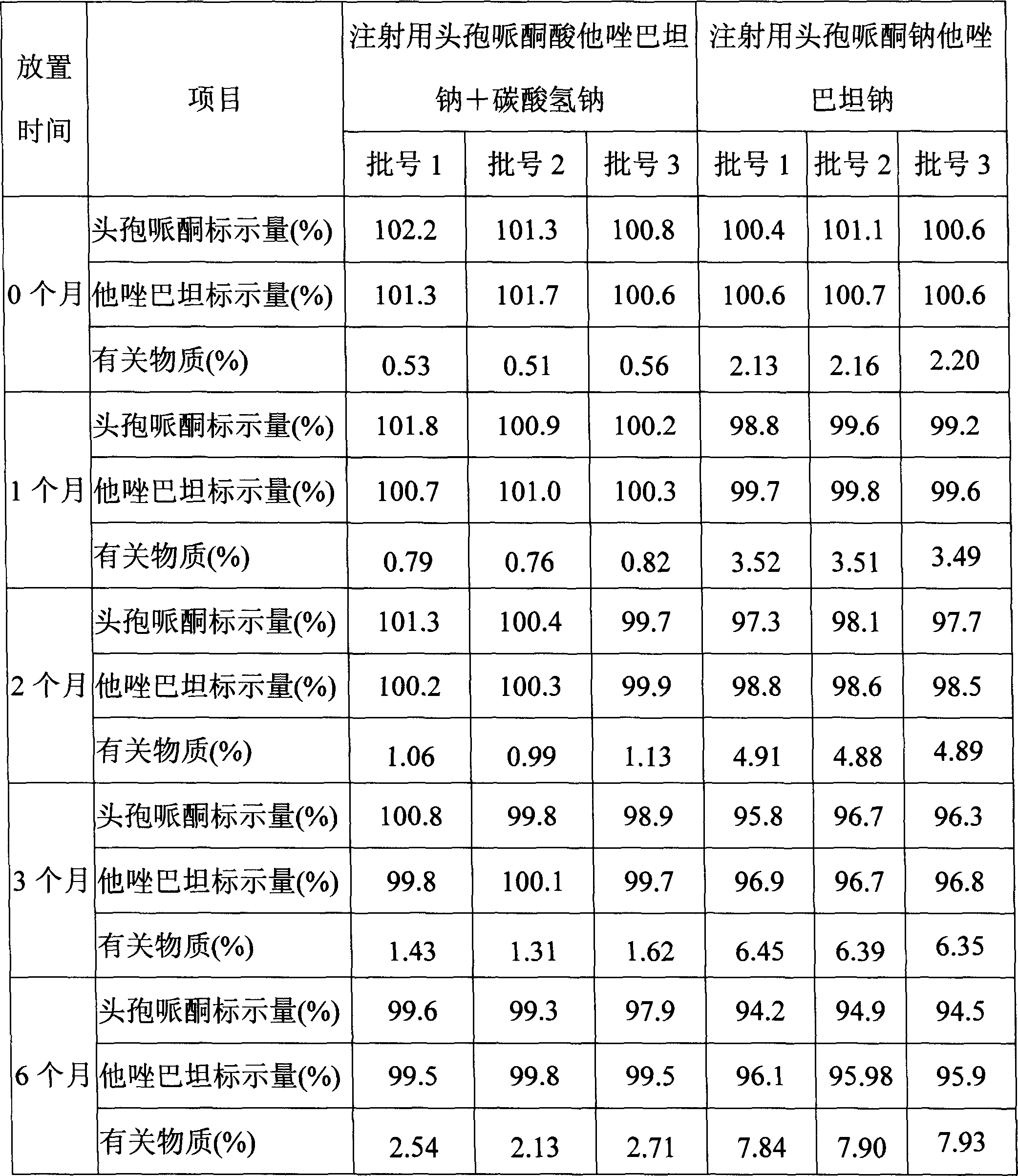
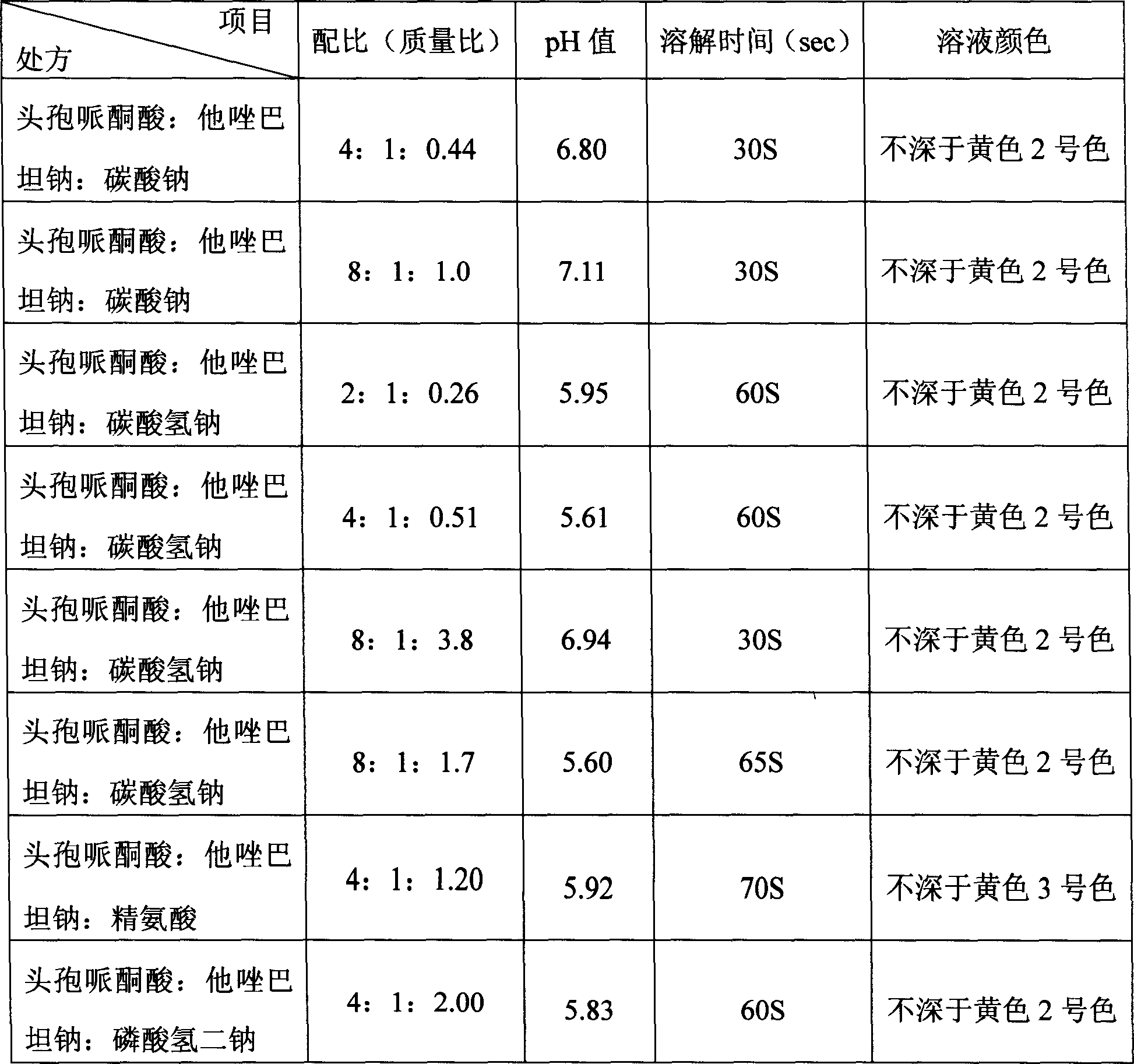
The invention discloses a stable compound preparation of cefoperazone-tazobactam drug, which is comprised by cefoperazone acid, tazobactam and latent solvent, which weight ratio is 8~1:1:5.6~0.06. The latent solvent is preferred selected from sodium carbonate and sodium bicarbonate. Related substances of the compound preparation in the invention are lower than standard of Chinese pharmacopoeia (2005 edition) in influencing factor and accelerated test in 40 DEG C, labelled content accords with the standard of the pharmacopoeia and changes very little in the experiment, and the product quality is stable.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Multi-detection method of residual of cephalo-type drugs in milk product
ActiveCN105116063AGuaranteed SensitivityAvoid interferenceComponent separationCefotaximeRelative standard deviation
The invention belongs to the technical field of detection of residual of drugs in an animal source food, and relates to a method for determining the residual quantity of cephalo-type drugs in a milk product through a high performance liquid chromatograph. The method comprises the steps of sample pre-extraction, standard curve drafting and apparatus detection analysis, the detection method is a multi-detection method carried out by using high performance liquid chromatography, and detected drugs comprise cefoperazone, cefotaxime, ceftriaxone and cephalothin. The cefoperazone detection limit of the method is 0.26mg / Kg, the cefotaxime detection limit of the method is 0.01mg / Kg, the ceftriaxone detection limit of the method is 0.10mg / Kg, and the cephalothin detection limit of the method is 0.07mg / Kg; the method has good linear relationship in an addition concentration range of 1-50mg / kg, and the recovery rate is 90-105%; and the intra-batch relative standard deviation is not greater than 15%, and the inter-batch relative standard deviation is not greater than 20%. The method has the advantages of short analysis time, low detection limit, high precision and multi-detection, and is of great significance to accurately monitor the residual quantity of the cephalo-type drugs in milk.
Owner:山东世通检测评价技术服务有限公司
Lung-targeting cefoperazone microsphere for animal and birds and its preparing method
InactiveCN1985832AReduce releaseGood curative effectAntibacterial agentsOrganic active ingredientsSide effectMicrosphere
The present invention belongs to the field of veterinary medicine technology, and is especially lung targeting cefoperazone microsphere for animal and its preparation process. The cefoperazone microsphere is prepared with cefoperazone as medicine component and gelatin as carrier in the weight ratio of 1 to 2, and through dissolving cefoperazone in gelatin solution and adding Span-80 and liquid paraffin through stirring to obtain emulsion; cooling in icy bath to below 5 deg.c and adding glutaraldehyde through stirring for cross-linking and curing; dewatering with isopropyl alcohol and suction filtering; washing with isopropyl alcohol and ethyl ether to eliminate glutaraldehyd, washing with petroleum ether to eliminate liquid paraffin in the surface of microsphere and vacuum drying at room temperature to obtain cefoperazone microsphere. The medicine has raised tissue selectivity, delayed release, raised curative effect and lowered toxic side effect.
Owner:TIANJIN RINGPU BIO TECH
Broad spectrum and efficient composite antibacterial agent and preparation method thereof
InactiveCN102488693AHigh antibacterial activityAvoid drug resistanceAntibacterial agentsHeterocyclic compound active ingredientsAntibacterial activityBULK ACTIVE INGREDIENT
The invention discloses a broad spectrum and efficient composite antibacterial agent and a preparation method thereof. The preparation method comprises the following steps of: taking cefoperazone or physiologically-acceptable salt thereof, cefradine or physiologically-acceptable salt thereof, sulbactam or physiologically-acceptable salt thereof as active ingredients; adding acceptable carriers orminor ingredients; and adopting a certain preparation technology to prepare into various clinically applicable preparations. Compared with other antibacterial agents, according to the antibacterial agent disclosed by the invention, the antibacterial activity against G+ bacteria is 2-8 times of that of the traditional cephalosporin antibacterial agent, and the antibacterial activity against G-bacteria is 2-4 times of that of the traditional cephalosporin antibacterial agent and is 2.5-10 times of other traditional antibacterial agents. The antibacterial agent can overcome the defect of drug resistance of bacteria caused by unreasonable application of the clinical antibacterial drug, and has obvious advantages showed by wide antibacterial spectrum when being used for treating pathogen infection and infection with unclear sensitivity condition, in particular severe infections or mixed infections caused by fungi. The preparation cost of the broad spectrum and efficient composite antibacterial agent is economical, the preparation method is simple and easy to operate, and the preparation technology is environmentally-friendly.
Owner:HENAN UNIVERSITY OF TECHNOLOGY +1
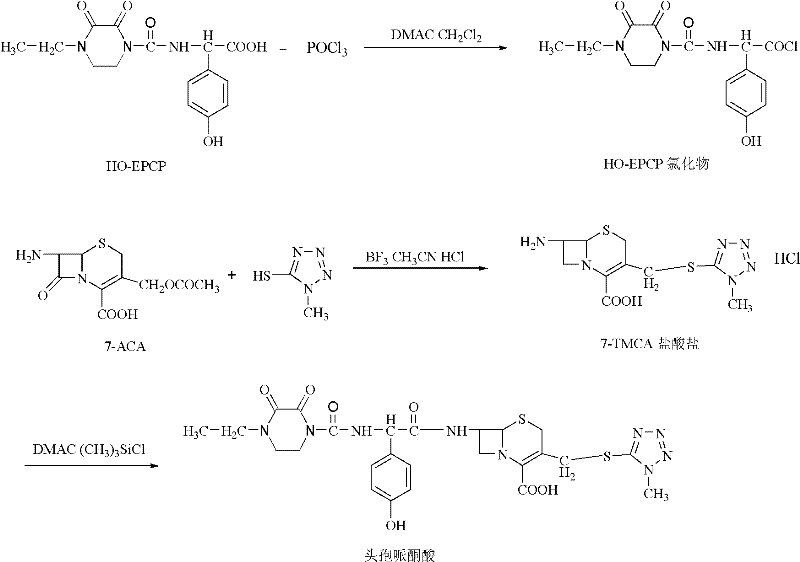
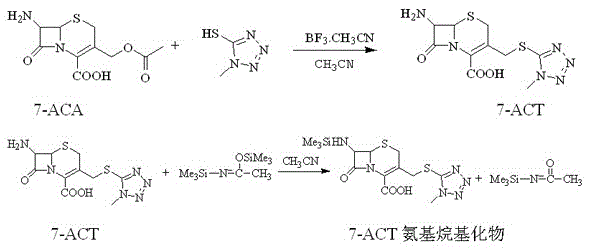
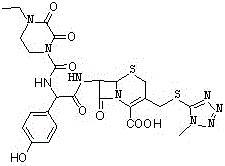
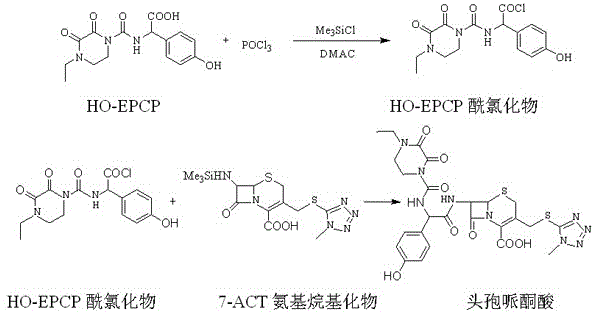
Synthesis method of cefoperazone acid
InactiveCN102532168AHigh yieldReduce generationOrganic chemistrySynthesis methodsPolyethylene glycol
The invention relates to a synthesis method of cefoperazone acid, and belongs to the technical field of medicines. The method comprises the following steps: (1) adopting 7-ACA (7-aminocephalosporanic acid) and 1-methyl-5-mercapto-1,2,3,4-tetrazole as raw materials, allowing reaction between the two raw materials in the catalysis of a boron trifluoride-acetonitrile solution to obtain 7-TMCA (7-amino-3-methyl tetrazolyl cephalosporanic acid) hydrochloride, and performing the protection of carboxyl group and amino group on 7-TMCA hydrochloride with trimethylchlorosilane; (2) allowing reaction of HO-EPCP (2-[(4-ethyl-2,3-dioxopiperazinyl)carbonylamino]-2-(4-hydroxyphenyl)acetic acid) and phosphorus oxychloride in a DMAC (dimethylacetamide) and dichloromethane solution in the protection of nitrogen gas to obtain HO-EPCP chloride; and (3) allowing N-acylation reaction of the 7-TMCA hydrochloride subjected to the protection of carboxyl group and amino group obtained by the step (1) and HO-EPCP chloride obtained by the step (2) with polyethylene glycol 800 as a phase transfer catalyst in a dichloromethane-water mixed solution, regulating pH with a hydrochloric acid solution, and crystallizing to obtain cefoperazone acid. In the invention, the yield of 7-TMCA hydrochloride under the catalysis of boron trifluoride is improved by 3%. The addition of the phase transfer catalyst reduces occurrence of side reaction, improves the reaction yield by 5%, makes the final product yield reach above 69.0%, and improves purity to above 99%.
Owner:YIYUAN XINQUAN CHEM
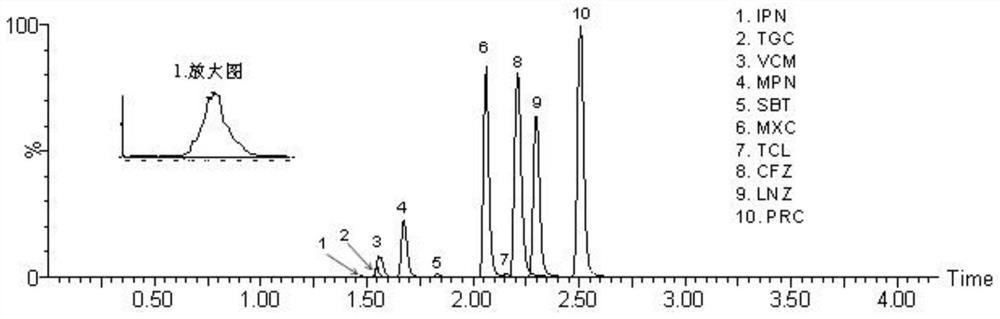
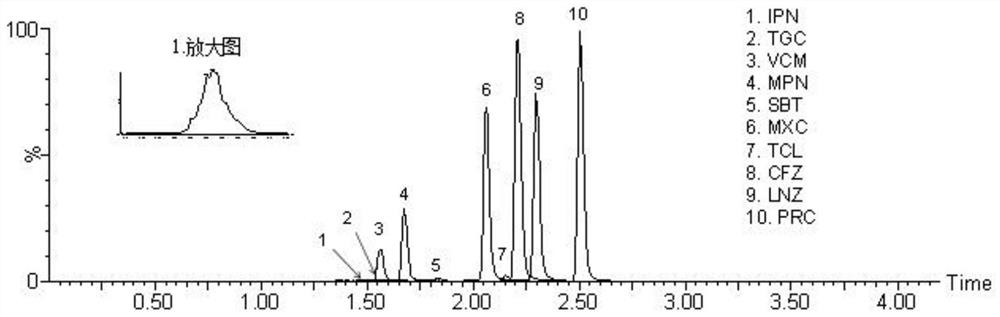
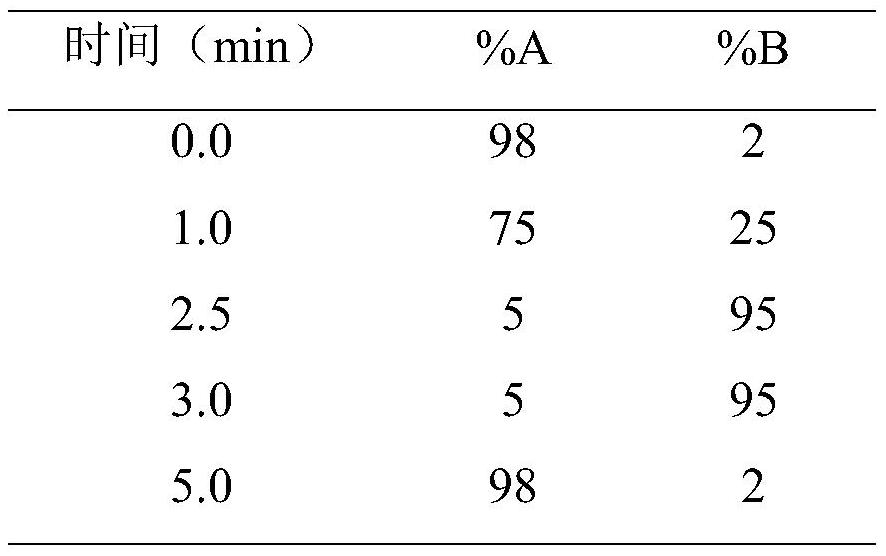
Method for detecting antibacterial agent in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
The invention discloses a method for detecting an antibacterial agent in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antibacterial agent containssulbactam (SBT), imipenem (IPN), linezolid (LNZ), melopenem (MPN), moxifloxacin (MXC), piperacillin (PRC), tigecycline (TGC), cefoperazone (CFZ), vancomycin (VCM) and teicoplanin (TCL). The method comprises the steps: detecting the content of the antibacterial drug in the pretreated serum by adopting an ultra-high performance liquid chromatography tandem mass spectrometry method, quantifying by utilizing a mass spectrometry isotope internal standard method, establishing a calibration curve by taking the concentration ratio of a standard substance to an internal standard substance as an X axisand the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the concentration of a target drug in the serum. According to the method, the pretreatment process is simple, the sensitivity is high, the specificity is high, separation and detection of the antibacterial agent are completed within 5 min, and a reliable detection method is provided for monitoring the treatment concentration of the antibacterial agent clinically.
Owner:南京品生医学检验实验室有限公司
Quantitative detection method of campylobacter in food
ActiveCN101824462AEasy to trainCulture specificMicrobiological testing/measurementAgainst vector-borne diseasesBacteroidesSeparation technology
The invention relates to the bacterium separation technology, in particular to a direct quantitative counting method of campylobacter in food. The method comprises the following steps: culturing samples on a solid CCDA culture medium containing growth promoters and six kinds of antibiotics such as polymyxin B, trimethoprim, rifampicin, actidione, cefoperazone and fungizone B; then, directly carrying out campylobacter counting and separation. Compared with a bacterium culture, separation and certification method of a traditional method, the invention has the advantages of simplicity, convenience, specificity and accuracy.
Owner:YANGZHOU UNIV
Amino butanetriol salt of cephalosporin compounds and preparing method
InactiveCN101012235AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefuroximeCefazolin
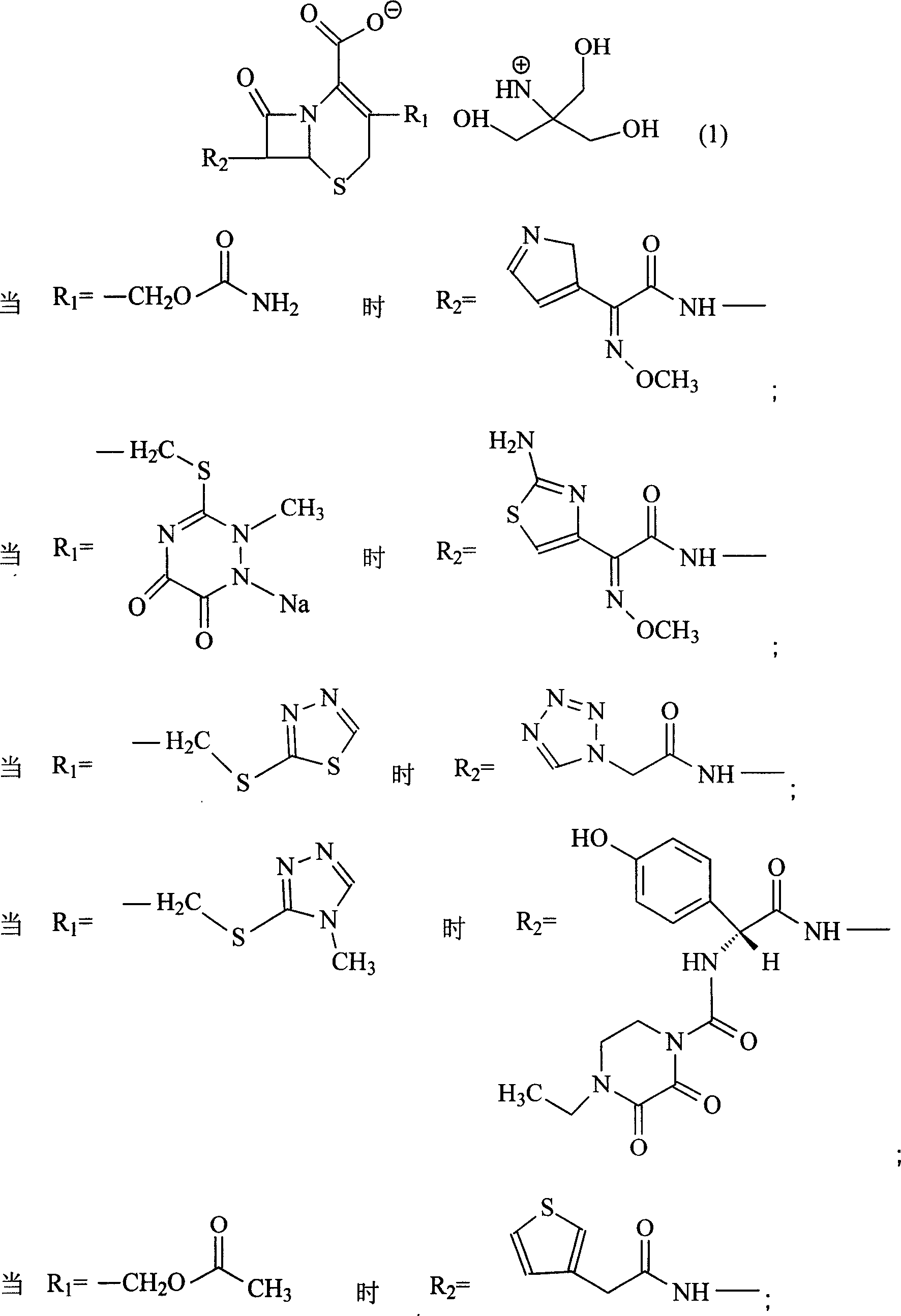
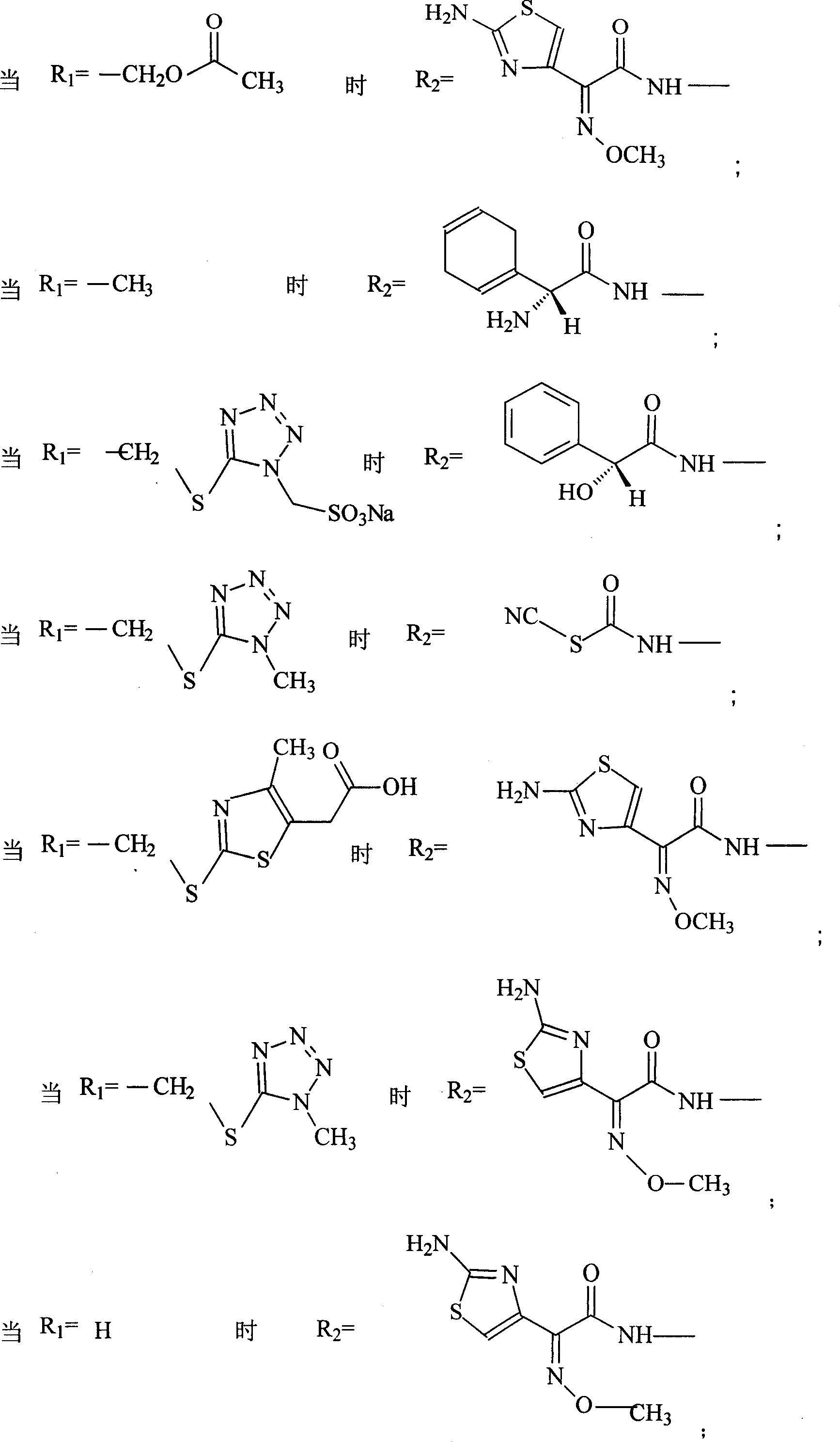
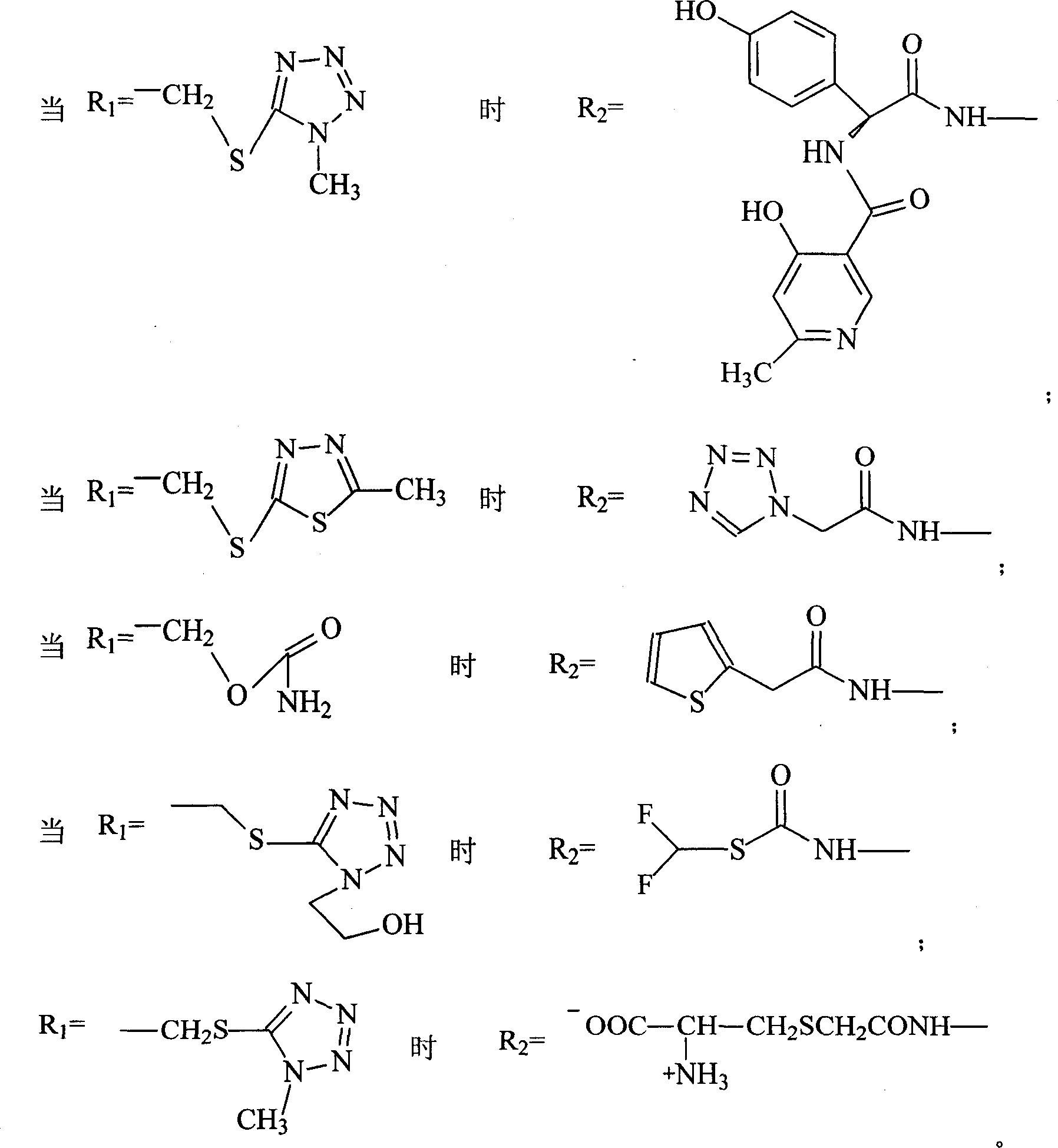
The invention discloses a pehanorm salt or hydrate with chemical formula as picture (1) and drug composition and application to treat bacterial infection, which comprises the following parts: cefuroxime oxtatromethane, cepham qusong tromethane, cepham thiotepa tromethane, cefoperazone tromethane, cephalothin tromethane, cefotaxime tromethane, cefolading tromethane, cefonixin tromethane, cefameizin tromethane, cefadizine tromethane, cefuroxime tromethane, cefazolin tromethane, cefapamine tromethane, cefazoline tromethane, cefaadid tromethane, cefaoxofluoride tromethane, cefaminol tromethane and their hydrate.
Owner:GUANGDONG ZHONGKE DRUG R&D
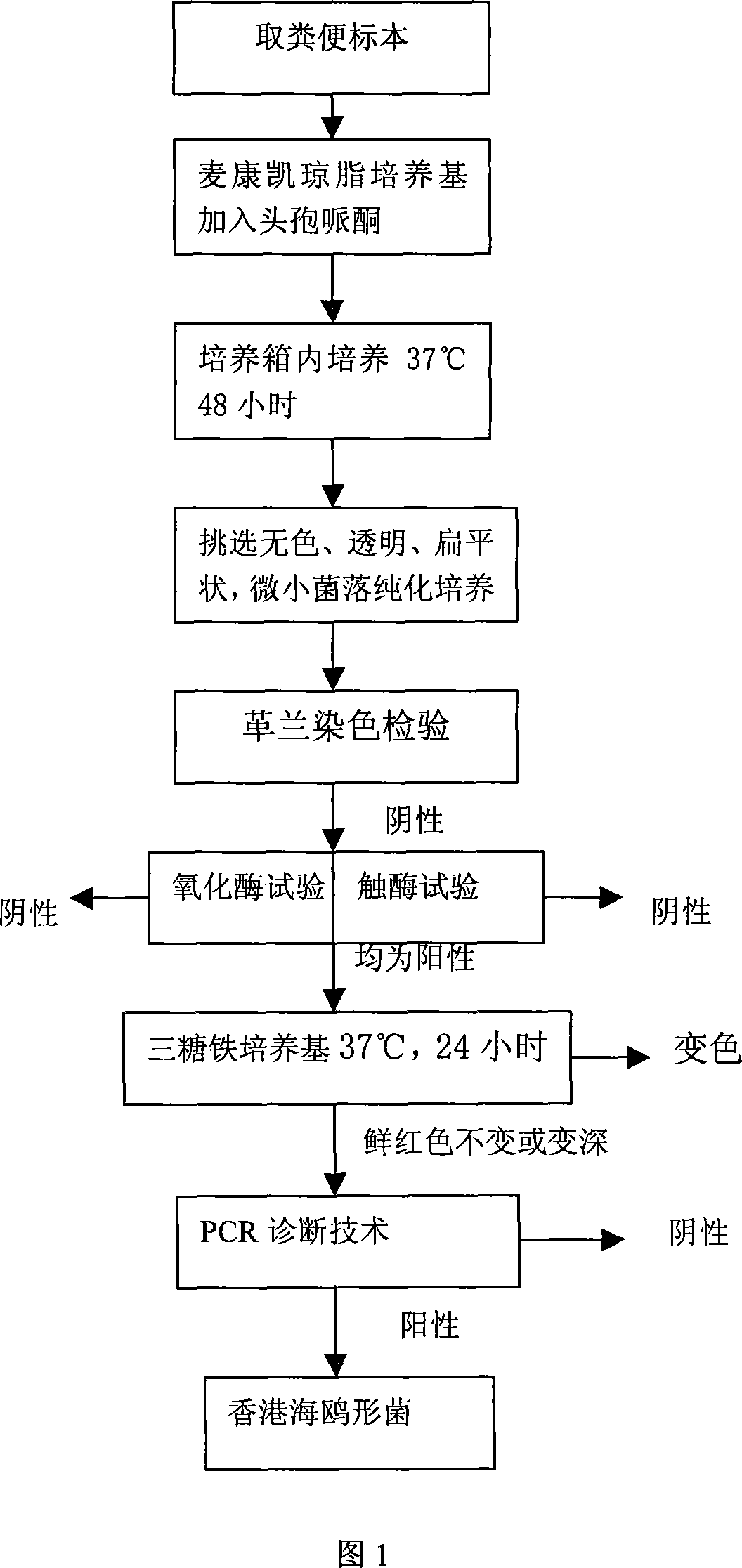
Hongkong sea-gull shape bacterium inspection technology
InactiveCN101063164AShorten the timeSave human effortMicrobiological testing/measurementGramClinical therapy
The invention discloses a testing technique of HongKong sea-gull shape bacteria, which comprises the following steps: fetching faecal specimen; seeding on MaiKangKai medium with cefoperazone; putting into 37 deg. c box for 48 h; taking-up; sorting colourless, transparent and flat dwarf colony with diameter at 0. 5-1. 0mm; proceeding purified culture; testing as negative bacillus brevis or vibrio with Gram's stain; checking as positive with oxidase and catalase; seeding in triose metal medium tube; placing in 37 deg. c box; culturing for 24 h; taking-up; keeping the colour of fresh red medium; or changing more red; diagnosing fitting result bacteria with PCR technique; diagnosing as HongKong sea-gull shape bacteria when PCR result as positive. This invention provides criterion for prevention and cure or clinical therapy of HongKong sea-gull shape bacteria disease, which is convenient, practical and rapid.
Owner:杭州市疾病预防控制中心 +1
Cefmenoxime hydrochloride compound and synthesizing method thereof
ActiveCN102731531AQuality assuranceHigh purityOrganic chemistryCefmenoxime HydrochlorideMedicinal chemistry
The invention relates to the field of pharmacy, and especially related to a cefmenoxime hydrochloride compound and a synthesizing method thereof. The cefmenoxime hydrochloride compound has a formula shown below, and is prepared with a method comprising the steps that: (1) a cefoperazone precursor 7-ATCA.HCl and AE active ester are subjected to a condensation reaction under the existence of CH2Cl2and an alkalizing agent; and the obtained product is subjected to extraction, decolorization, press-filtration, neutralization, rejection filtration, and drying, such that cefmenoxime acid CMX-H is prepared; (2) the cefmenoxime acid is completely dissolved by using sodium carbonate; the obtained product is subjected to decolorization, press-filtration, neutralization, decolorization, filtration, crystallization, washing, and drying, such that a cefmenoxime hydrochloride half-finished product is prepared; a jet mill is started; and the material is crushed and is filled in aluminum bottles. According to the invention, cefmenoxime acid is prepared into crystals, such that the product purity is greatly improved, and the product quality is ensured. Also, the method provided by the invention is advantaged in small three-waste volume, simple preparation process, and low cost. Therefore, the method is suitable for the industrialized productions of our nation.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Medicine for treating osteomyelitis and preparation method thereof
InactiveCN109045065ASimple recipeEasy accessAntibacterial agentsSkeletal disorderTreatment effectCefamandole
The invention discloses a medicine for treating osteomyelitis and a preparation method thereof. The medicine comprises the following raw materials in parts by weight: 1.0 to 20.0 parts of calcium hydroxide powder, 0.3 to 4.0 parts of antibiotics (one or two of cefoperazone, ceftazidime, cefamandole, imipenem, tobramycin, gentamicin and vancomycin), 1.0 to 20.0 parts of normal saline, and 0.1 to 3.0 parts of an iodophor solution. The adopted calcium hydroxide powder, antibiotics, normal saline and iodophor solution are medical grades. When in use, the raw materials are weighed according to theweight, mixed in an aseptic condition, sufficiently stirred, and prepared into paste. The paste is injected into osteomyelitis medullary canals subjected to debridement and blocked through bone cement, and wounds are sutured. The medicine has the advantages that the raw material the formula is simple, the raw materials are easy to get, the preparation method is simple, and convenient to operate, and the treatment effect is obvious.
Owner:彭磊
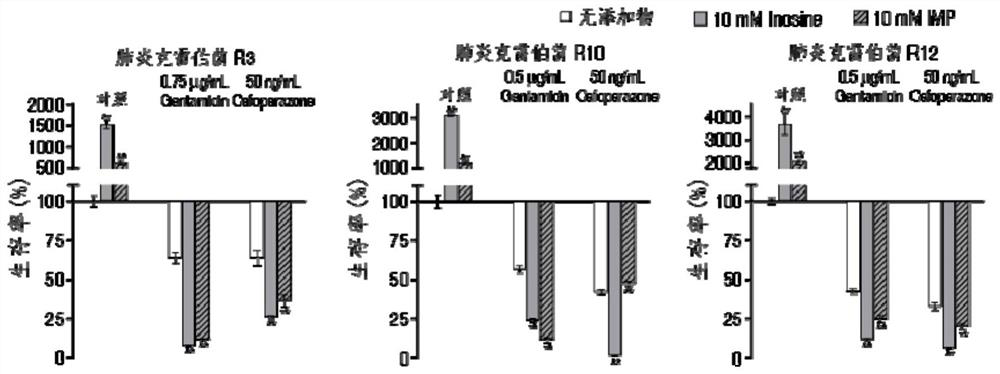
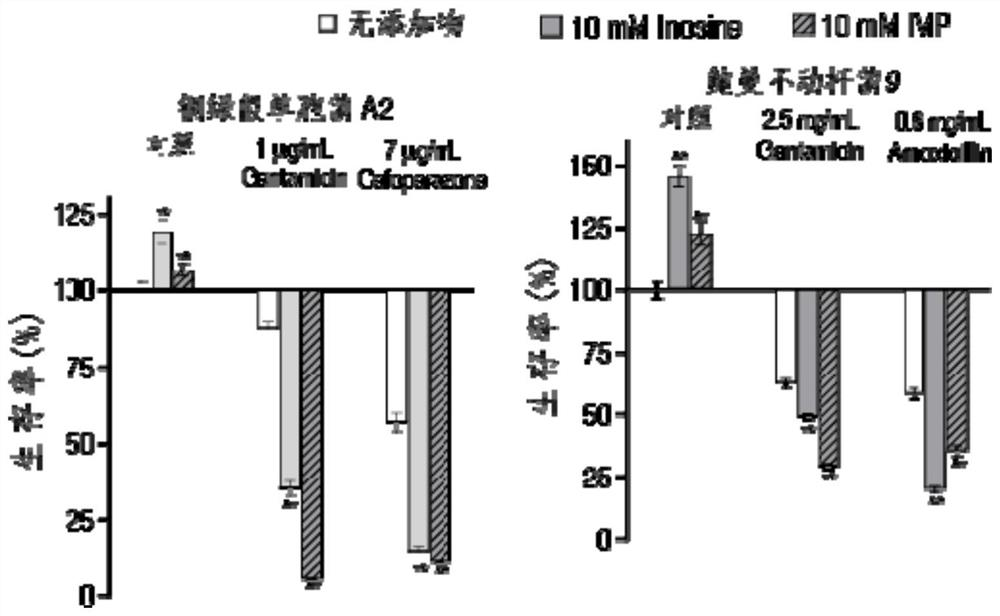
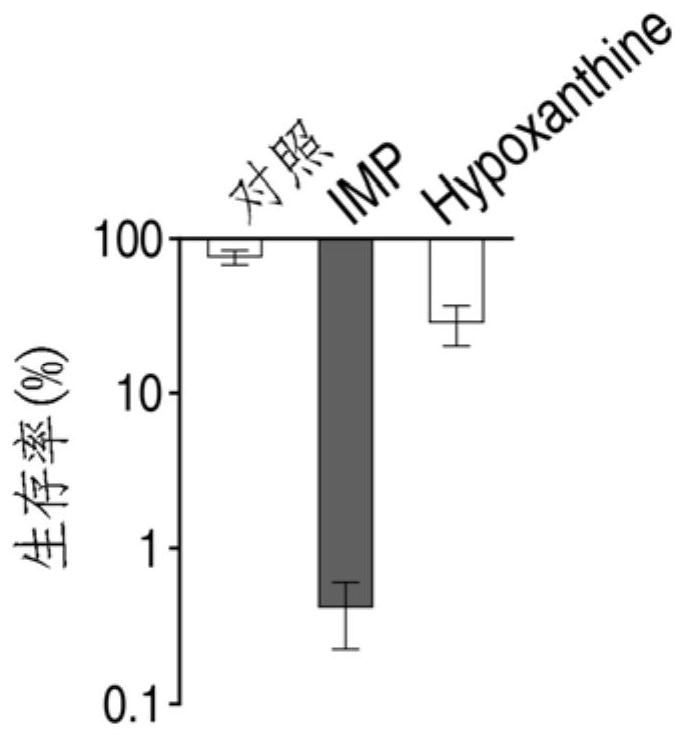
Application of hypoxanthine nucleotide in preparation of anti-infective drugs
PendingCN112569251AGood effectDelay drug resistanceAntibacterial agentsOrganic active ingredientsMulti resistant bacteriaK pneumoniae
The invention belongs to the technical field of medicine, and particularly relates to application of hypoxanthine nucleotide in preparation of anti-infective drugs. Experiments prove that the hypoxanthine nucleotide can significantly improve the sensitivity of Escherichia coli, Klebsiella pneumoniae, staphylococcus aureus multi-drug-resistant bacteria, pseudomonas aeruginosa and other bacteria toamoxicillin, cefoperazone, meropenem, gentamicin and other antibiotics, so that the hypoxanthine nucleotide can be used as an anti-infective drug together with antibiotics. Bacteria are killed under the condition of low-concentration antibiotics, a good anti-infection effect is achieved, and meanwhile bacterial drug resistance is reduced.
Owner:SUN YAT SEN UNIV
Synthetic method of cefoperazone acid
ActiveCN105566350AImprove conversion rateEasy to prepareOrganic chemistrySodium bicarbonateReaction temperature
The invention discloses a synthetic method of cefoperazone acid. The method comprises the steps of: reacting raw materials of 7-ACA and 1-methyl-5-mercapto tetrazole under the catalysis of boron trifluoride acetonitrile to obtain 7-ACT; dissolving the 7-ACT in a mixed solution of acetonitrile and N,O-(bis) trimethylsilyl acetamide to obtain a 7-ACT amino alkylate solution; mixing HO-EPCP, an organic solvent and a catalyst, adding phosphorus oxychloride for reaction at the temperature of -25 to -20 DEG C, wherein when the residue amount of HO-EPCP in the reaction solution is no more than 0.5%, the reaction is complete, so as to prepare a HO-EPCP acyl chloride solution; mixing and reacting 7-ACT amino alkylate solution with HO-EPCP acyl chloride solution, adding an aqueous solution of sodium bicarbonate after the reaction, standing foe stratification, filtering a lower layer material, adding water, crystallizing, filtering, washing and drying to obtain the cefoperazone acid. The method has the advantages of simpleness, high yield and stable intermediate.
Owner:HENAN KANGDA PHARMA
Selective growth media for campylobacter bacteria and plating media with said growth media
InactiveUS20130059320A1BacteriaMaterial analysis by observing effect on chemical indicatorGrowth retardantCefsulodin sodium
A selective growth media for Campylobacter bacteria comprising nutrients, growth inhibitors for organisms other than Campylobacter including bile salts, Irgasan DP 300, sodium cefsulodin hydrate, cycloheximide and sodium cefoperazone, and one or more ingredients for absorbing oxygen. Also, selective plating media for Campylobacter bacteria comprising said growth media, a thickening agent and Aldol acetate.
Owner:RESTAINO LAWRENCE
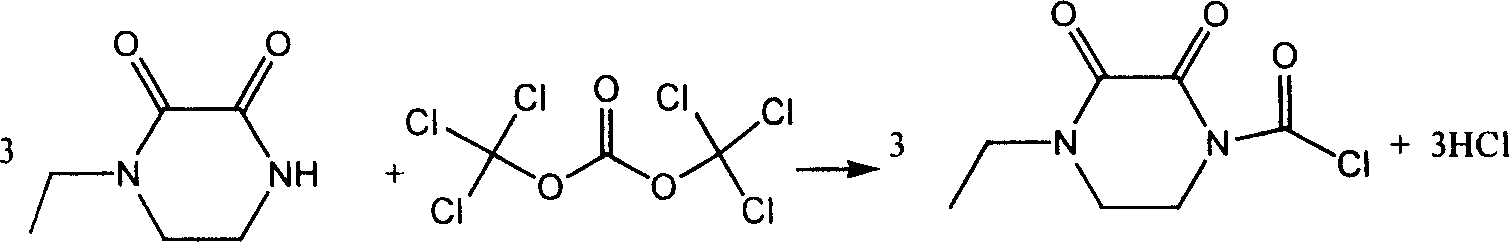
Chemosynthesis method of 1-chloroformyl-4-ethyl-2, 3-dioxopiperazine
InactiveCN1218944CAdvanced process routeReasonable process conditionsOrganic chemistryChemical synthesisOrganic solvent
A process for chemically synthesizing 1-chloroformacyl-4-ethyl-2,3-dioxypiperazine used to synthesize oxypiperazine penicillin and cefoperazone features the reaction between organic alkali as acid capturing agent, bis (trichloromethyl) carbonate and N-ethyl-2,3-dioxypiperazine. Its advantages are high output rate and basically no environmental pollution.
Owner:ZHEJIANG UNIV OF TECH
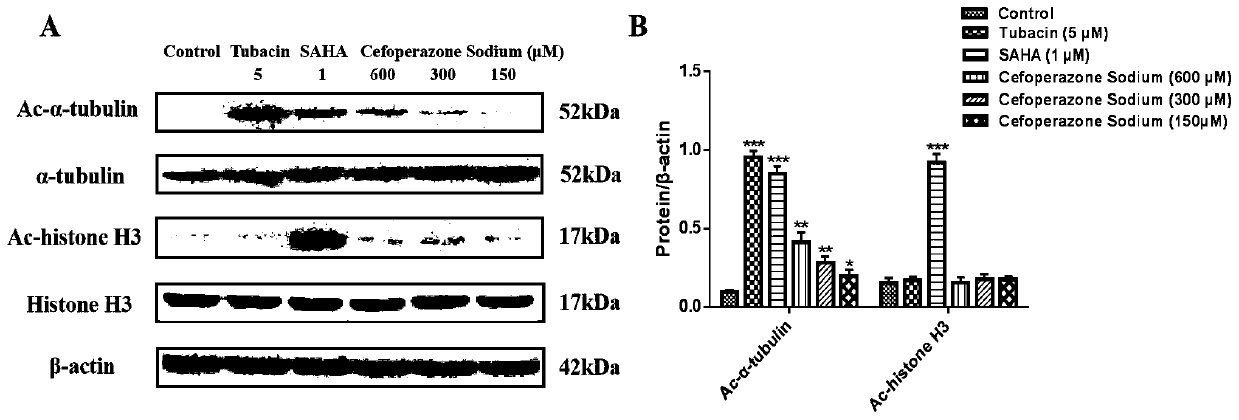
Application of beta-lactam compounds
InactiveCN111184726AInhibition of metastatic progressionOrganic active ingredientsAntineoplastic agentsChemical compoundEnzyme binding
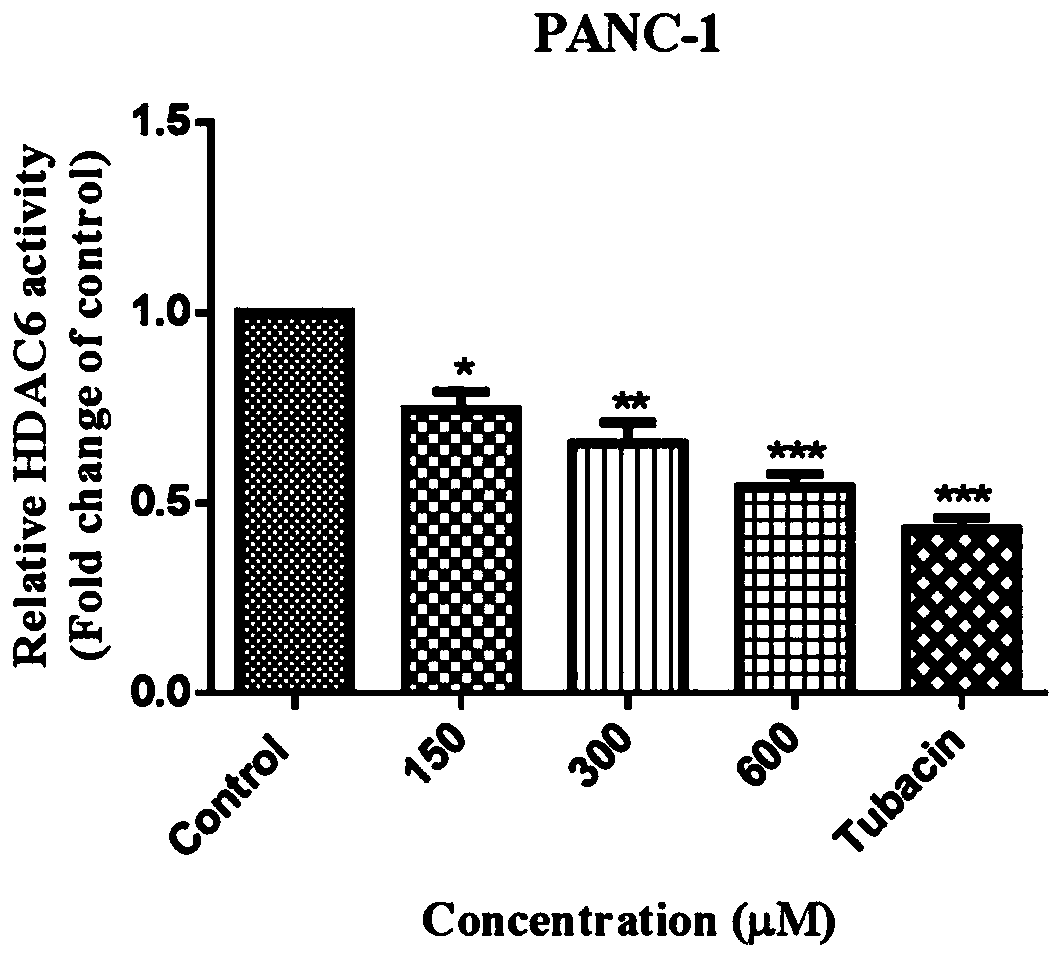
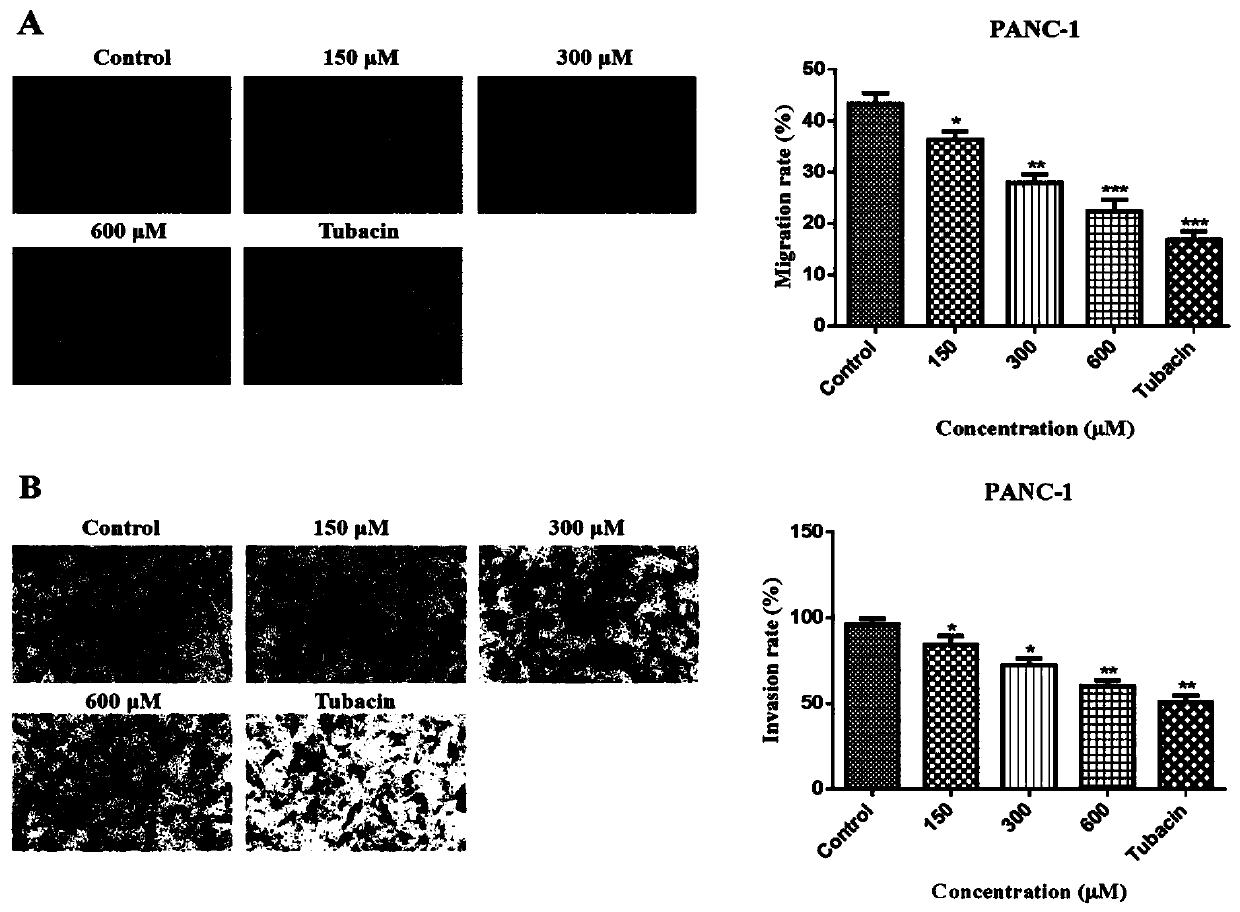
The invention belongs to the technical field of medicines, and relates to application of beta-lactam compounds, in particular to application of beta-lactam compounds in production of histone deacetylase inhibitors and application of the beta-lactam compounds in production of anti-tumor-metastasis medicines. According to application of the beta-lactam compounds in production of the histone deacetylase inhibitors and application of the beta-lactam compounds in production of the anti-tumor-metastasis medicines, it is found by research through a substrate-enzyme binding test, immunoblotting, cellenzyme activity detection, a scratch test and a Transwell test that cefoperazone has a significant inhibiting effect on malignant tumor metastasis by inhibiting the activity of HDAC6. Therefore, the beta-lactam compounds represented by the cefoperazone can be used for production of the anti-tumor-metastasis medicines, and have excellent application prospects in prevention and inhibition of postoperative metastasis of malignant tumors.
Owner:SHENYANG PHARMA UNIVERSITY
Composition containing piperacillin, its pharmaceutical preparation and its application
ActiveCN107875154BPlay a therapeutic effectAntibacterial agentsHeterocyclic compound active ingredientsDrugs preparationsTherapeutic effect
The invention provides a piperacillin-containing composition. The composition contains piperacillin and a certain proportion of ampicillin and sulbactam. The invention also provides its pharmaceutical preparation and application. Compositions and pharmaceutical preparations of the present invention can significantly inhibit drug-resistant Acinetobacter baumannii, especially to carbapenem antibiotics or cefoperazone-sulbactam-resistant Acinetobacter baumannii infection can play a significant role. Good therapeutic effect, with significant clinical advantages.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Cefmenoxime hydrochloride compound and synthesizing method thereof
ActiveCN102731531BQuality assuranceHigh purityOrganic chemistryCefmenoxime HydrochlorideMedicinal chemistry
The invention relates to the field of pharmacy, and especially related to a cefmenoxime hydrochloride compound and a synthesizing method thereof. The cefmenoxime hydrochloride compound has a formula shown below, and is prepared with a method comprising the steps that: (1) a cefoperazone precursor 7-ATCA.HCl and AE active ester are subjected to a condensation reaction under the existence of CH2Cl2 and an alkalizing agent; and the obtained product is subjected to extraction, decolorization, press-filtration, neutralization, rejection filtration, and drying, such that cefmenoxime acid CMX-H is prepared; (2) the cefmenoxime acid is completely dissolved by using sodium carbonate; the obtained product is subjected to decolorization, press-filtration, neutralization, decolorization, filtration, crystallization, washing, and drying, such that a cefmenoxime hydrochloride half-finished product is prepared; a jet mill is started; and the material is crushed and is filled in aluminum bottles. According to the invention, cefmenoxime acid is prepared into crystals, such that the product purity is greatly improved, and the product quality is ensured. Also, the method provided by the invention is advantaged in small three-waste volume, simple preparation process, and low cost. Therefore, the method is suitable for the industrialized productions of our nation.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
A multiple detection method for cephalosporin residues in milk products
ActiveCN105116063BGuaranteed SensitivityAvoid interferenceComponent separationCefotaximeRelative standard deviation
The invention belongs to the technical field of detection of residual of drugs in an animal source food, and relates to a method for determining the residual quantity of cephalo-type drugs in a milk product through a high performance liquid chromatograph. The method comprises the steps of sample pre-extraction, standard curve drafting and apparatus detection analysis, the detection method is a multi-detection method carried out by using high performance liquid chromatography, and detected drugs comprise cefoperazone, cefotaxime, ceftriaxone and cephalothin. The cefoperazone detection limit of the method is 0.26mg / Kg, the cefotaxime detection limit of the method is 0.01mg / Kg, the ceftriaxone detection limit of the method is 0.10mg / Kg, and the cephalothin detection limit of the method is 0.07mg / Kg; the method has good linear relationship in an addition concentration range of 1-50mg / kg, and the recovery rate is 90-105%; and the intra-batch relative standard deviation is not greater than 15%, and the inter-batch relative standard deviation is not greater than 20%. The method has the advantages of short analysis time, low detection limit, high precision and multi-detection, and is of great significance to accurately monitor the residual quantity of the cephalo-type drugs in milk.
Owner:山东世通检测评价技术服务有限公司
An indirect competition ELISA kit for detecting cephalosporin antibiotics in food of animal origin and its application
ActiveCN107014993BIncreased cross-reactivityIncreased sensitivityMaterial analysisElisa kitCefotaxime
The invention discloses an indirect-competitive ELISA (Enzyme-linked Immunosorbent Assay) kit for detecting cephalosporin antibiotics in animal derived foods and application of the kit. The kit comprises an ELISA plate coated with a coating antigen, a cephalosporin antibiotic standard substance, a cephalosporin antibiotic general antibody, an enzyme labeled secondary antibody, a dilution buffer, a washing buffer, a substrate developing solution and a stop solution, wherein the cephalosporin antibiotic general antibody is capable of specifically identifying cefalexin, cefradine, cefadroxil, cefoperazone, cefazolin or cefotaxime and cannot identify penicillin sodium. According to the cephalosporin antibiotic general antibody prepared in the invention, the general antibody and most of cephalosporin antibiotics have high cross reaction rates, while hardly have any cross reaction for the analogue penicillin sodium containing a beta-lactam ring; and therefore, the general antibody has high specificity. The kit prepared by the general antibody has the advantages of high sensitivity, capability of detecting many types of drugs, low cost, simple operation and short detection time.
Owner:HEBEI AGRICULTURAL UNIV.
Preparation method of Chinese medicine irrigation solution for treating blood stasis type senile vaginitis
InactiveCN102743611BEasy to makeSmall side effectsSexual disorderPlant ingredientsAngelica Sinensis RootDrug fever
The invention discloses a preparation method of a Chinese medicine irrigation solution for treating blood stasis type senile vaginitis, belonging to the technical field of preparation method of Chinese medicines. At present, generally, antibiotics and sulfanilamide medicines are used for treating blood stasis type senile vaginitis, if the treatment is conducted by using cefoperazone, intramuscular injection can cause local pains, occasionally skin rash, drug fever and other allergic reactions are observed, and individual patients have pancytopenia, elevation of transaminase, prothrombin time extension or hemorrhage, etc. The Chinese medicine irrigation solution disclosed herein is prepared by putting 20 kinds of herbs consisting of jujube, polyporus lucidus, donkey-hide glue, polygonum multiflorum, Angelica sinensis, prepared rehmannia root, white paeony root, longan pulp, gentian, Polygonum hydropiper, sugarcane, HlblSClls llutabilis, sophora flavescens, balsam pear, ilex latifolia thumb, Elephantopus scaber, polygonum cuspidatum, saxifrage, bird's-nest and liquorice in water for soaking, then decocting with slow fire, filtering and removing the residues to obtain a liquid medicine. The Chinese medicine irrigation solution disclosed herein has the advantages of small toxic and side effect, short treatment course and high cure rate.
Owner:张国建
Preparation method of Chinese medicinal lotion for treating spleen deficiency ecthyma
InactiveCN102526690BEasy to makeSmall side effectsDermatological disorderPlant ingredientsMonkshoodsSerum rash
The invention discloses a preparation method of a Chinese medicinal lotion for treating spleen deficiency ecthyma, belonging to the technical field of Chinese medicine preparation methods. At present, antibiotics and sulfanilamide are generally adopted for treating spleen deficiency ecthyma. If cefoperazone is adopted, partial intramuscular pain can be caused, allergic reactions such as erythra and medicament fever occur occasionally, and reduction in complete blood cells, prolonging in prothrombin time or bleeding and the like can occur on individual patients. According to the technical scheme of the invention, the method comprises the following steps of: putting 31 medicaments including cabbage, dried longan pulp, large-head atractylodes rhizome, white paeony root, American ginseng, Chinese angelica, lotus seed, dangshen, sharpleaf galangal fruit, astragalus, sealwort, polished round-grained rice, dried ginger, wild pepper, medicinal evodia fruit, common monkshood mother root, aconitum carmichaeli debx, prepared common monkshood daughter root, wild ginger, pepper, long pepper, piper cubeba, fennel, chive, Chinese ginger, selinum japenious seed, hot pepper, caraway, resurrection lily rhizome, black-bone chicken and liquoric root into water for soaking; and decocting with soft fire to obtain a decocted medicinal liquid which serves as a Chinese medicinal lotion for treating spleen deficiency ecthyma. The Chinese medicinal lotion has the advantages of small toxic and side effects of a prepared Chinese medicine, short treatment course and high curative rate.
Owner:刘春美
Slow released capsule of cefoperazone and bletilla tuber glue for animal and birds
InactiveCN1985948AExtended stayGood treatment effectAntibacterial agentsOrganic active ingredientsDiseaseBletilla striata
The present invention belongs to the field of veterinary medicine technology, and is especially slow released veterinary medicine capsule prepared with cefoperazone and bletilla tuber as material. The preparation process includes the following steps: water extracting and alcohol precipitation to extract bletilla tuber glue; mixing cefoperazone and bletilla tuber in the ratio of 1 to 3 and 10 % concentration alcohol solution of PVP as adhesive; making pellet of 20 mesh; and encapsulating. The slow released veterinary medicine capsule has long medicine retaining time in the disease focus, high local medicine concentration, excellent bioadhesion and obvious medicine slow releasing characteristic.
Owner:TIANJIN RINGPU BIO TECH
Quantitative detection method of campylobacter in food
ActiveCN101824462BEasy to separateRapid Quantitative AnalysisMicrobiological testing/measurementAgainst vector-borne diseasesBacteroidesSeparation technology
The invention relates to the bacterium separation technology, in particular to a direct quantitative counting method of campylobacter in food. The method comprises the following steps: culturing samples on a solid CCDA culture medium containing growth promoters and six kinds of antibiotics such as polymyxin B, trimethoprim, rifampicin, actidione, cefoperazone and fungizone B; then, directly carrying out campylobacter counting and separation. Compared with a bacterium culture, separation and certification method of a traditional method, the invention has the advantages of simplicity, convenience, specificity and accuracy.
Owner:YANGZHOU UNIV
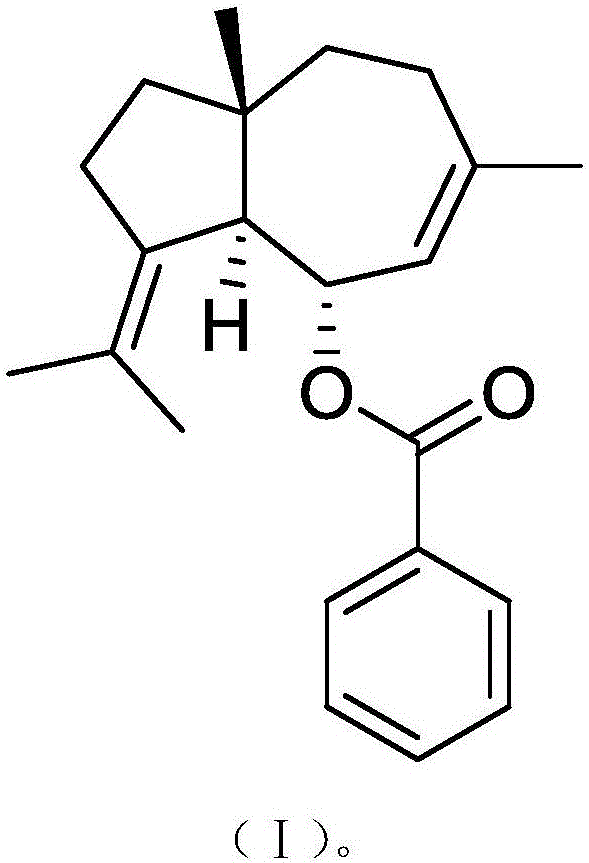
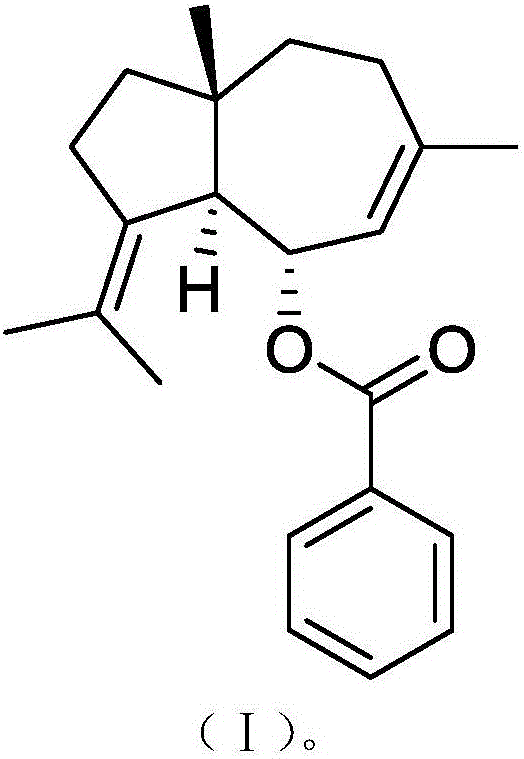
Applications of compound and cefoperazone pharmaceutical composition in preparing drugs used for treating periodontitis
InactiveCN106265623AHas therapeutic effectGood treatment effectOrganic active ingredientsOrganic compound preparationNatural productPharmaceutical drug
The invention relates to novel applications of cefoperazone, and more specifically relates to applications of a compound and a cefoperazone pharmaceutical composition in treating periodontitis The cefoperazone pharmaceutical composition contains cefoperazone and a natural product compound (I) with a novel structure. Independent application of cefoperazone, and independent application of the compound (I) both possess treatment effects on periodontitis, and cefoperazone and the compound (I) can be used for preparing drugs used for treating periodontitis. Curative effect is improved further via combination of cefoperazone with the compound (I), and the drugs used for treating periodontitis can be developed preferably. Compared with the prior art, the applications possess following advantages: substantive distinguishing features and significant progress are achieved.
Owner:朱正直
Slow released capsule of cefotaxime sodium and bletilla tuber glue for animal and birds
InactiveCN1985950AExtended stayGood treatment effectAntibacterial agentsOrganic active ingredientsDiseaseBletilla striata
The present invention belongs to the field of veterinary medicine technology, and is especially slow released veterinary medicine capsule prepared with cefotaxime and bletilla tuber as material. The preparation process includes the following steps: water extracting and alcohol precipitation to extract bletilla tuber glue; mixing cefotaxime and bletilla tuber in the ratio of 1 to 3 and 10 % concentration alcohol solution of PVP as adhesive; making pellet of 20 meshes and encapsulating. The slow released veterinary medicine capsule has long medicine retaining time in the disease focus, high local medicine concentration, excellent bioadhesion and obvious medicine slow releasing characteristic.
Owner:TIANJIN RINGPU BIO TECH
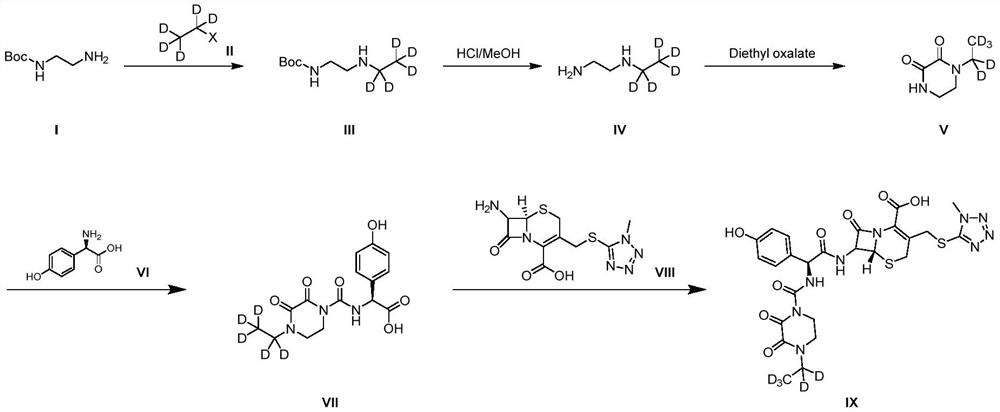
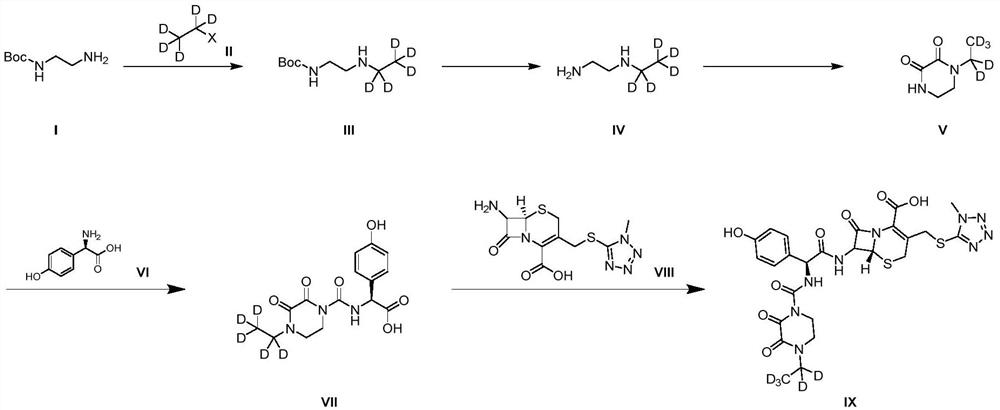
A kind of preparation method of cefoperazone deuterated internal standard substance
ActiveCN110746445BLow priceSimple and fast operationOrganic chemistry methodsGlycineChemical compound
The invention provides a method for preparing a cefoperazone deuterated internal standard substance. The method comprises the following steps: (1) subjecting Boc-ethylenediamine to alkylation and deprotection, so as to obtain an intermediate IV; (2) subjecting the intermediate IV to cyclization, so as to obtain an intermediate V; (3) subjecting the intermediate V and an intermediate VI to condensation, so as to obtain an intermediate VII, wherein the intermediate VI is D(-)-p-hydroxybenzene glycine; and (4) subjecting the intermediate VII and an intermediate VIII to condensation, so as to obtain cefoperazone-D5 target compound IX, wherein the intermediate VIII is a cefmenoxime intermediate. According to the method provided by the invention, the process design is reasonable, the employed deuterating reagent is cheap, the operation is simple and convenient, an experimentation process is controllable, and the purity of the prepared target product is high and reaches 99% or more.
Owner:FOOD INSPECTION CENT OF CIQ SHENZHEN +1
Preparation process of medicine for treating chronic pulmonary heart disease
InactiveCN109394772AGood treatment effectThe composition ratio is simpleRespiratory disorderCardiovascular disorderAmpicillinTreatment effect
The invention relates to a preparation process of a medicine for treating chronic pulmonary heart disease. The medicine is prepared from 0.5-1 g of midecamycin, 0.03-0.2 g of compound sulfamethoxazole, 0.5 g of cefalexin, 0.3-0.5 g of ciprofloxacin, 4-6 g of ampicillin, 0.5-1 g of cefazolin, 2-4 g of cefoperazone each time and 2-4 g of cefodizime. 100 parts of the medicine are matched with a normal saline injection for intravenous drip once every day. The medicine has the benefits as follows: the treatment effect is good, the component ratio is simple, and the cost is low.
Owner:GUANGDONG HONGRUI TECH CO LTD
Stable cefoperazone tazobactam medicine compound preparation
The invention discloses a stable compound preparation of cefoperazone-tazobactam drug, which is comprised by cefoperazone acid, tazobactam and latent solvent, which weight ratio is 8~1:1:5.6~0.06. The latent solvent is preferred selected from sodium carbonate and sodium bicarbonate. Related substances of the compound preparation in the invention are lower than standard of Chinese pharmacopoeia (2005 edition) in influencing factor and accelerated test in 40 DEG C, labelled content accords with the standard of the pharmacopoeia and changes very little in the experiment, and the product quality is stable.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Oral preparation containing cefoperazone and its making method
InactiveCN1939265AQuick effectAntibacterial agentsOrganic active ingredientsAdditive ingredientCurative effect
An orally absorbed medicine in the form of buccal lozenge, sweets, or dripping pill dissolved by saliva features that it contains cefoperazone. Its preparing process is also disclosed.
Owner:刘凤鸣
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com