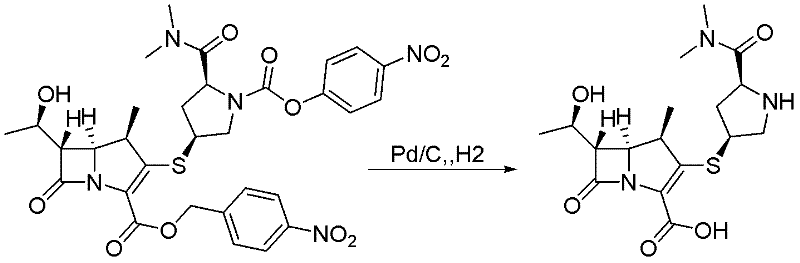
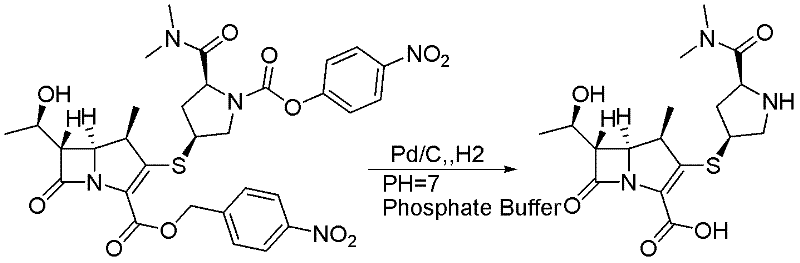
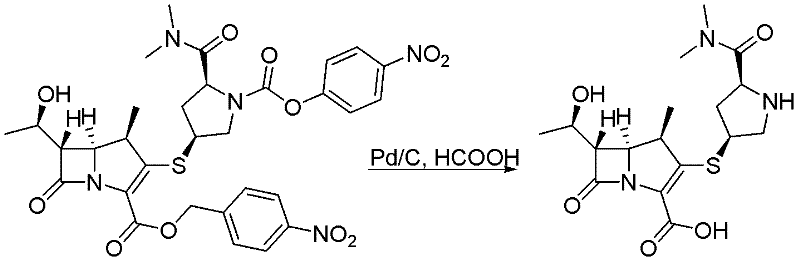
Patents
Literature
363 results about "Carbapenem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
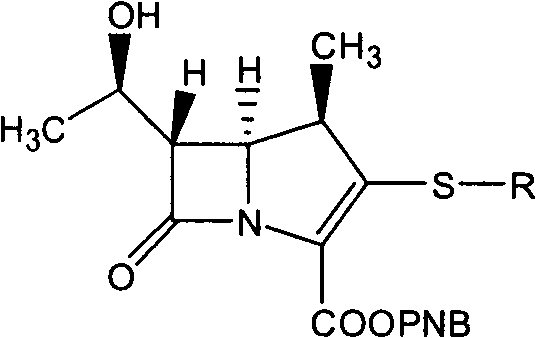
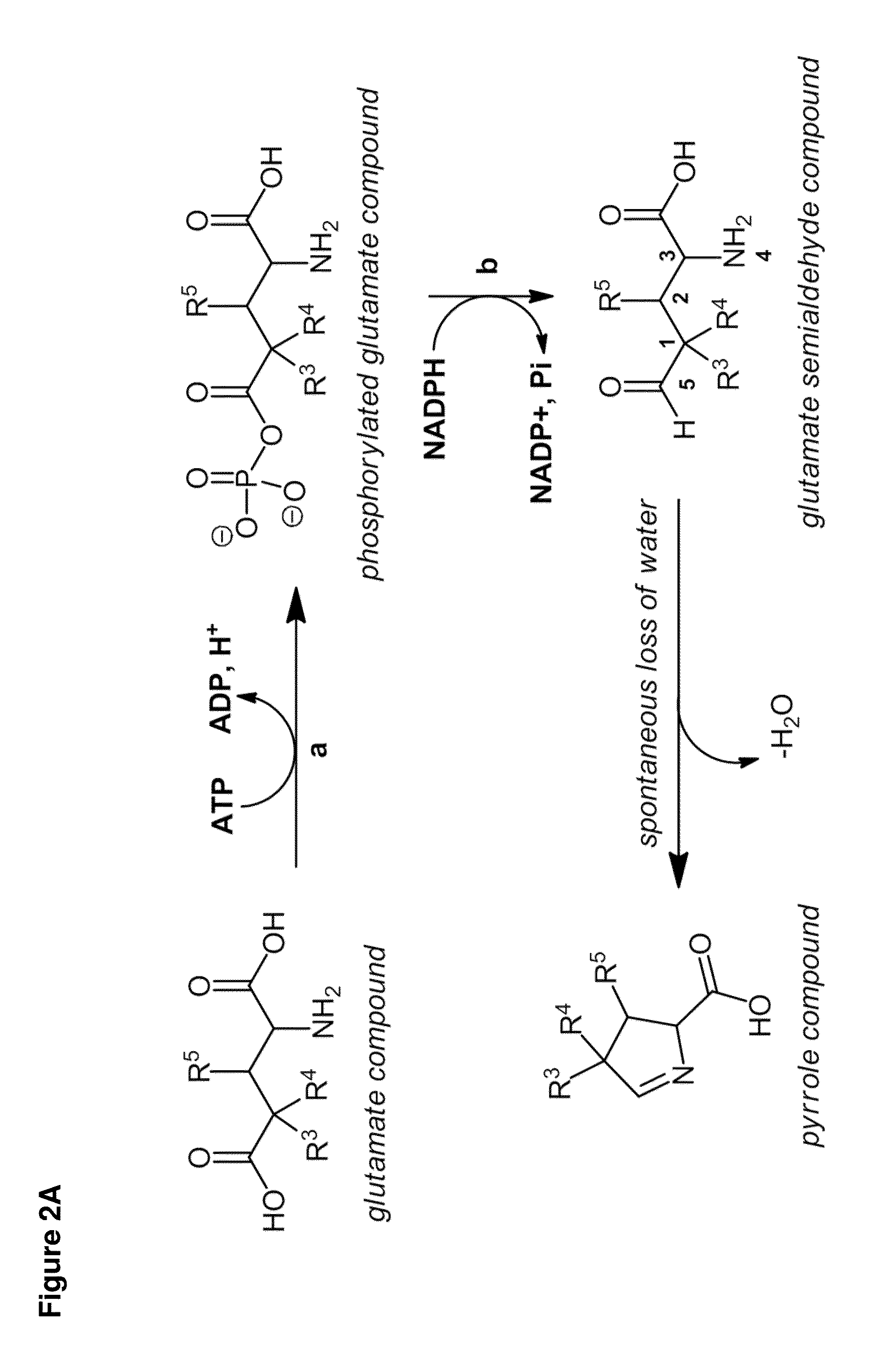
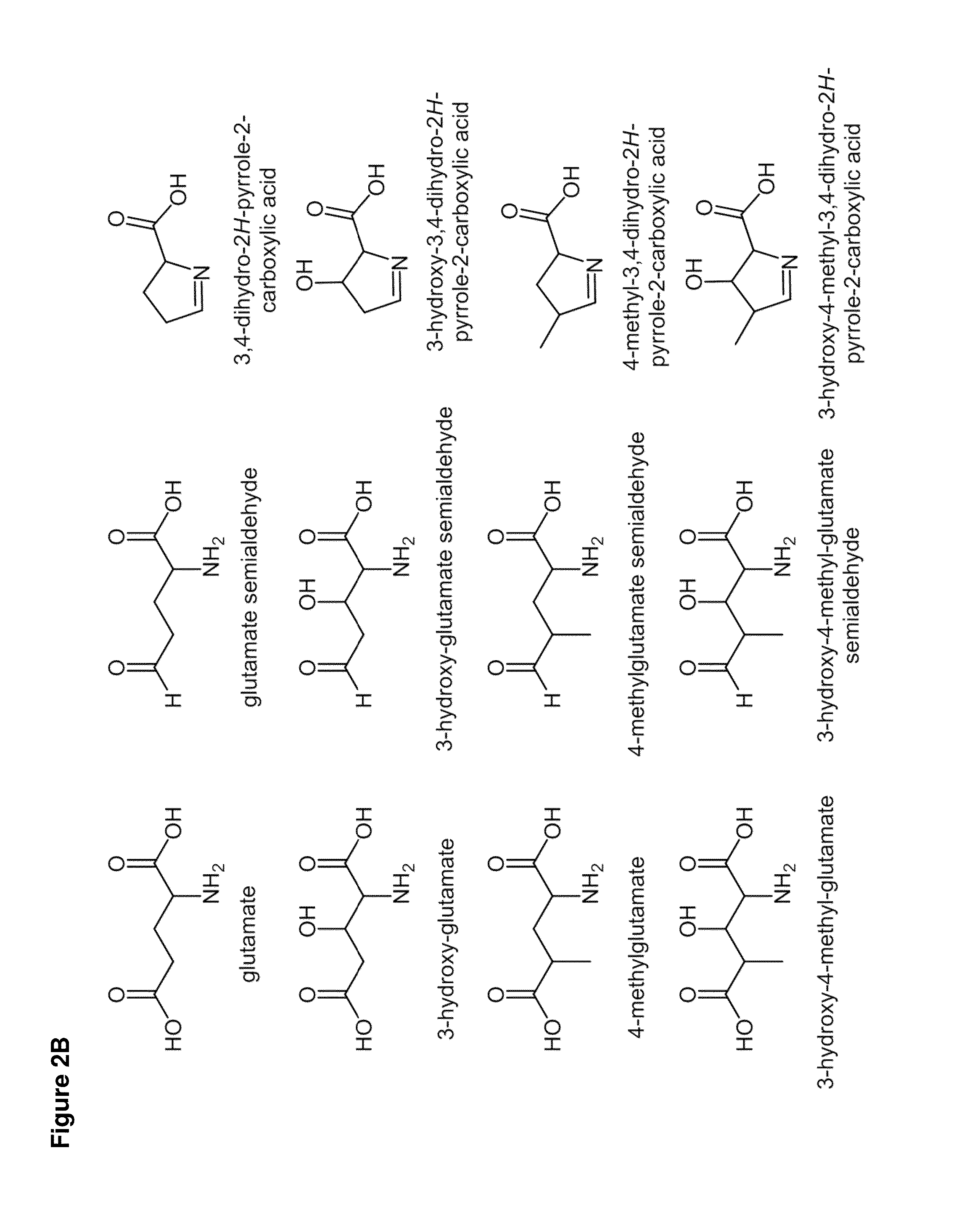
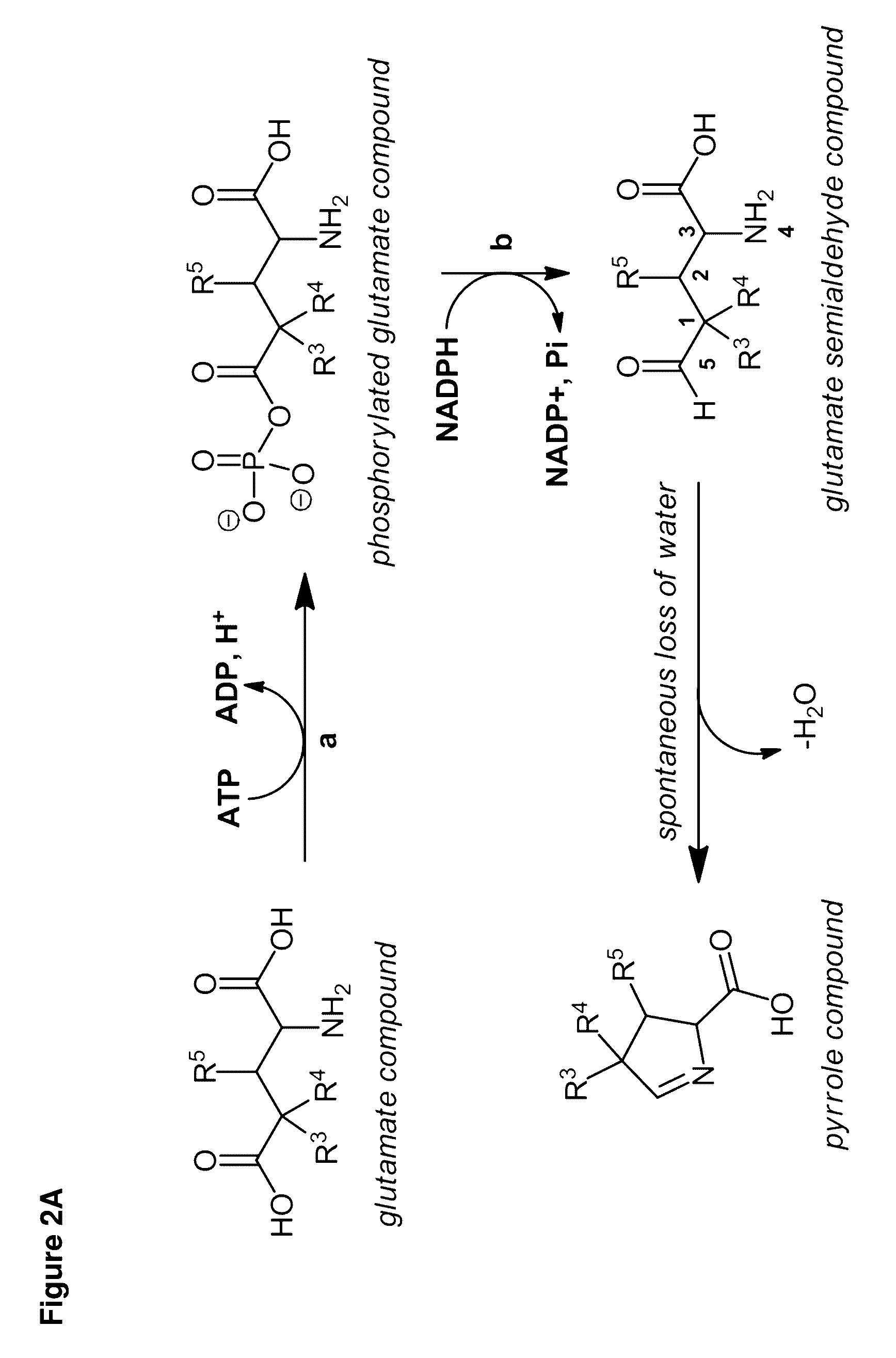
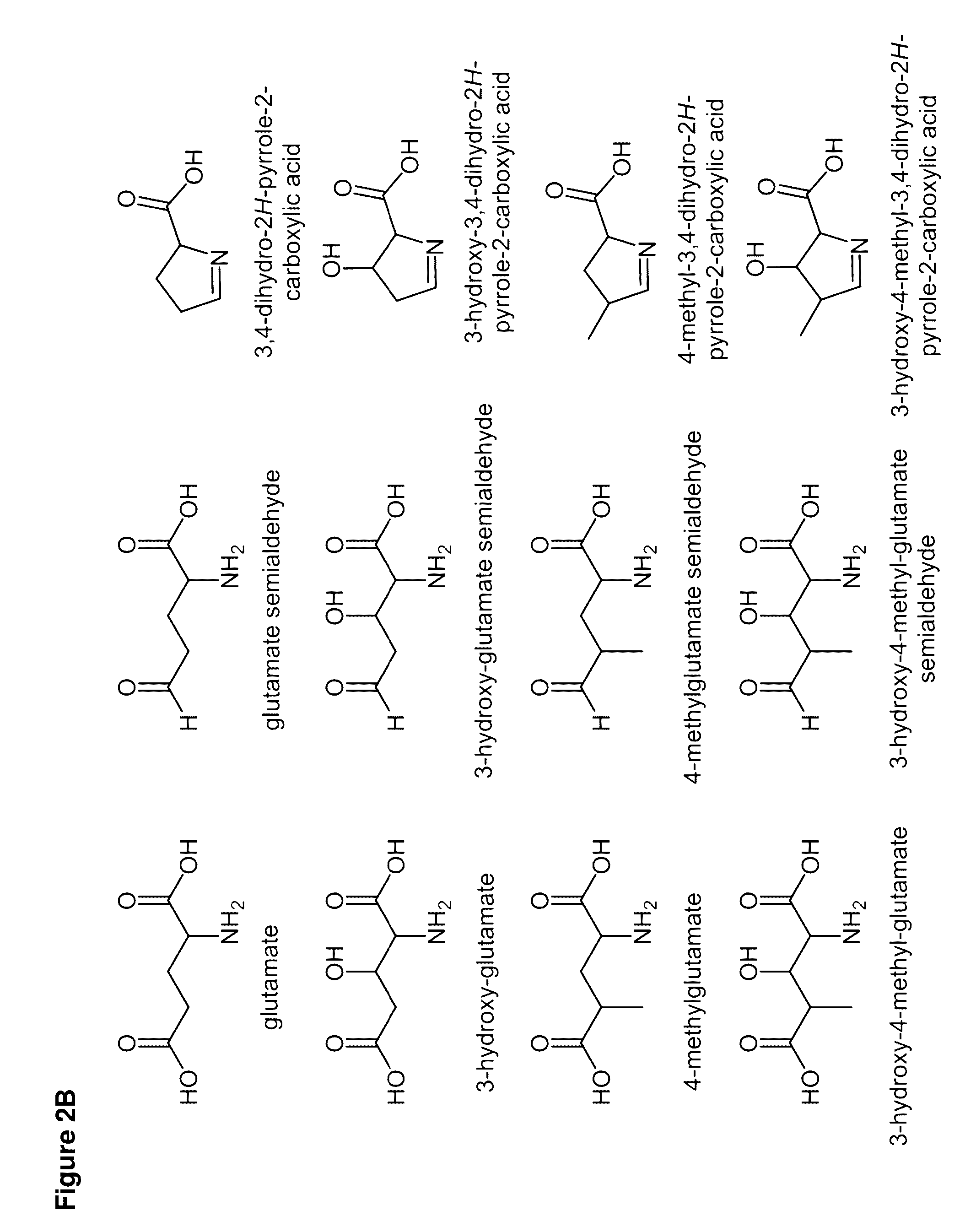
Carbapenems are a class of highly effective antibiotic agents commonly used for the treatment of severe or high-risk bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta lactam class of antibiotics, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams.
Malonic acid monoesters and process for producing the same
Owner:MEIJI SEIKA KAISHA LTD
Method for detecting the presence of carbapenemase-producing bacteria in a sample
ActiveUS20140134656A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMicrotiter plateCarbapenemase producing
The present invention relates to a method for detecting the presence of carbapenemase-producing bacteria in a sample, said method comprising the steps of: a) performing cell lysis on a test sample in order to obtain an enzymatic suspension; b) reacting a fraction of the enzymatic suspension obtained in step a) with a reagent kit, said reagent kit comprising —a carbapenemase substrate selected from the group consisting of carbapenems and cephamycins, —a pH color indicator which will change color when the pH of the reaction mixture is comprised between 6.4 and 8.4, wherein a color change after step b) indicates the presence of carbapenemase-producing bacteria in the test sample. The invention also relates to a reagent kit, to a microtiter plate and to their uses in detecting the presence of carbapenemase producers in a test sample.
Owner:UNIV PARIS SACLAY +2
Method for synthesizing 1 beta methyl carbapenem antibiotic
InactiveCN101935321AEasy to operateEase of mass productionOrganic chemistryBulk chemical productionOrganic baseReaction temperature
The invention relates to a method for synthesizing 1 beta methyl carbapenem antibiotic, comprising the following steps of: (1) adding an organic solvent, water, pivaloyloxymethyl ester, organic base and a catalyst to a reactor, introducing hydrogen, and then reacting for above 15 minutes, wherein the pressure of the hydrogen is controlled in 10.0 MPa, and the reaction temperature is controlled below 70 DEG C; (2) monitoring by using a HPLC (High Performance Liquid Chromatography), removing the hydrogen after finishing reaction, and filtering and recovering the catalyst (palladium charcoal or platinum charcoal); and (3) cooling a filtrate, adding a crystallizing solvent, stirring and devitrifying for above 10 minutes, and then filtering to obtain a corresponding target compound. The method has convenient operation, and a reaction liquid can be directly added to the solvent for crystallization without purification.
Owner:SHENZHEN HAIBIN PHARMA
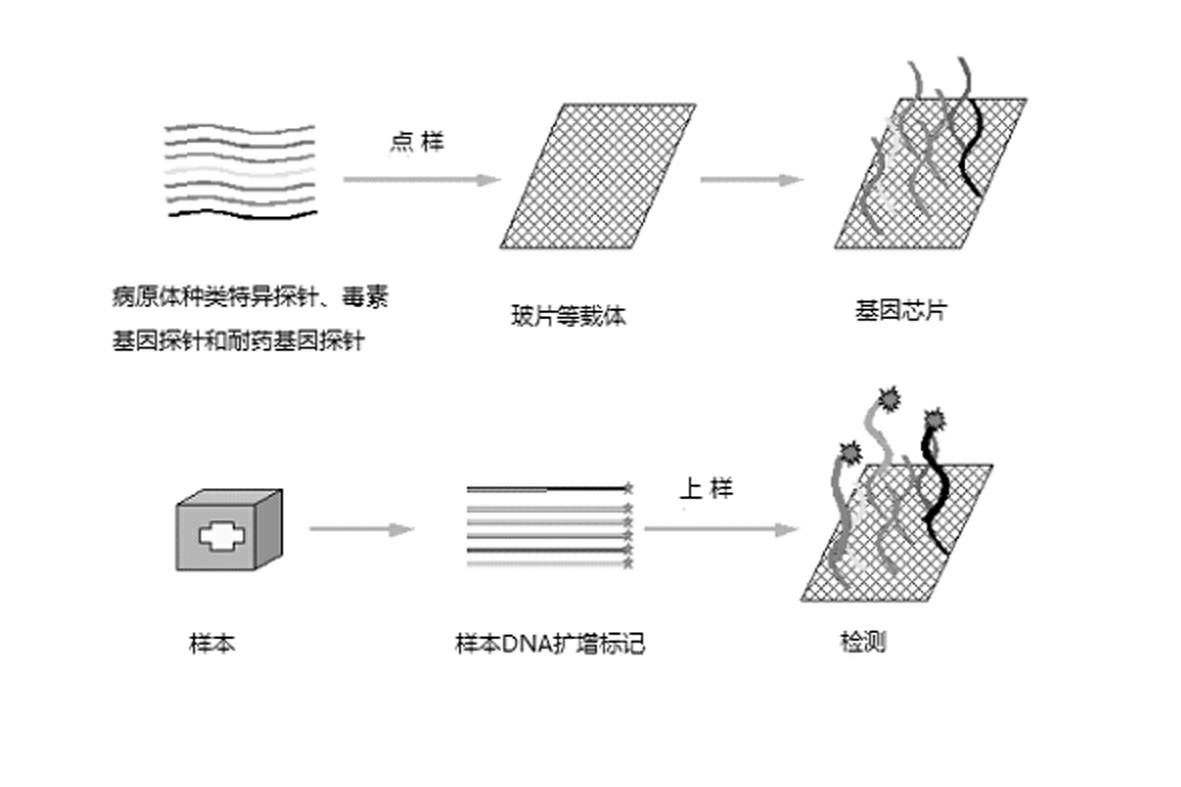
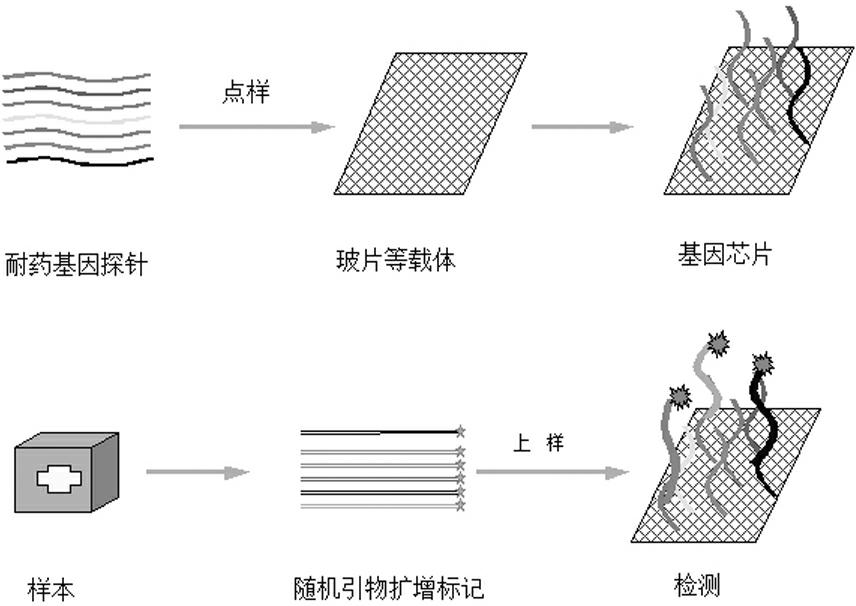
Gene chip for high-flux detection of pathogens and application thereof
InactiveCN102534013AStrong specificityDetermine the typeMicrobiological testing/measurementAgainst vector-borne diseasesYersinia pestisBrucella
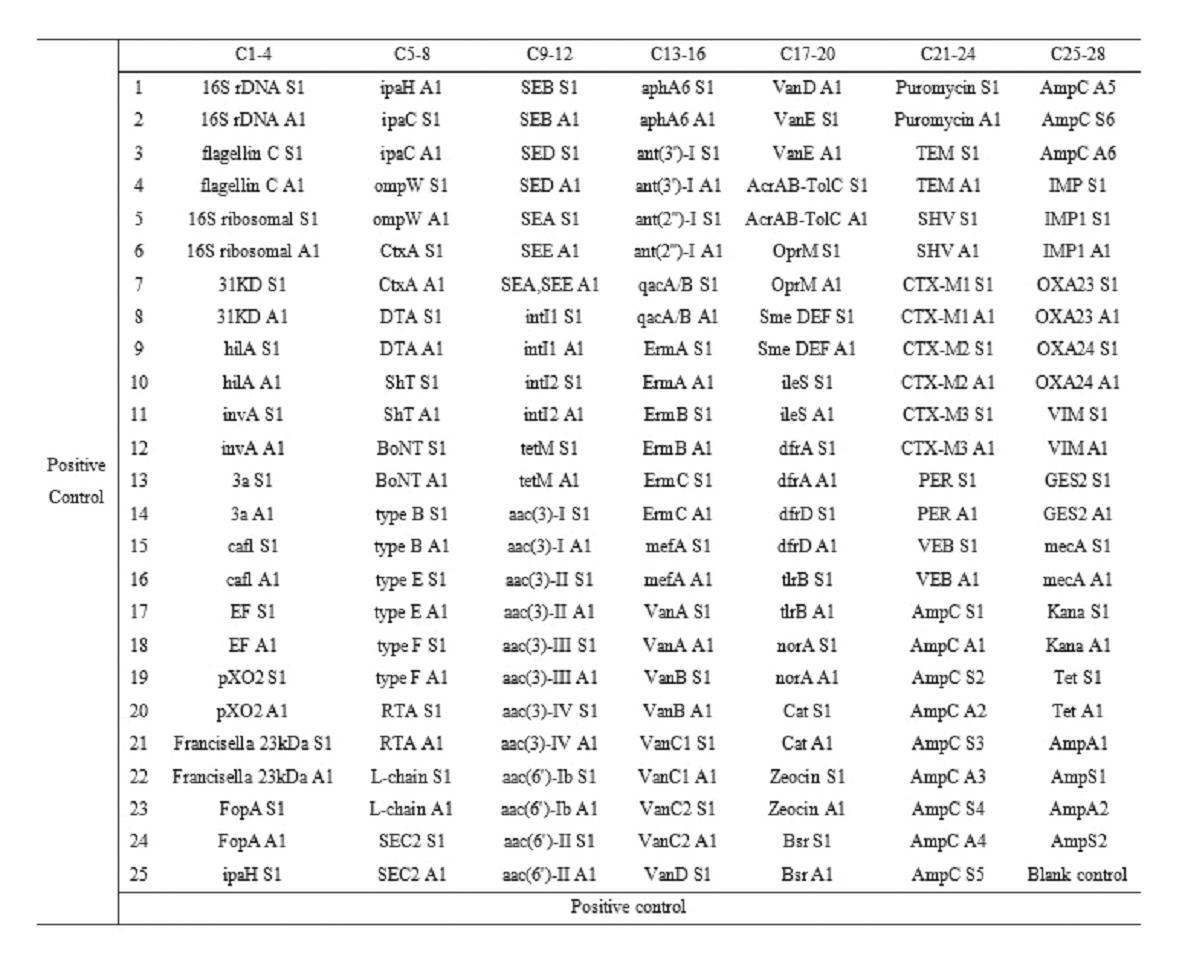
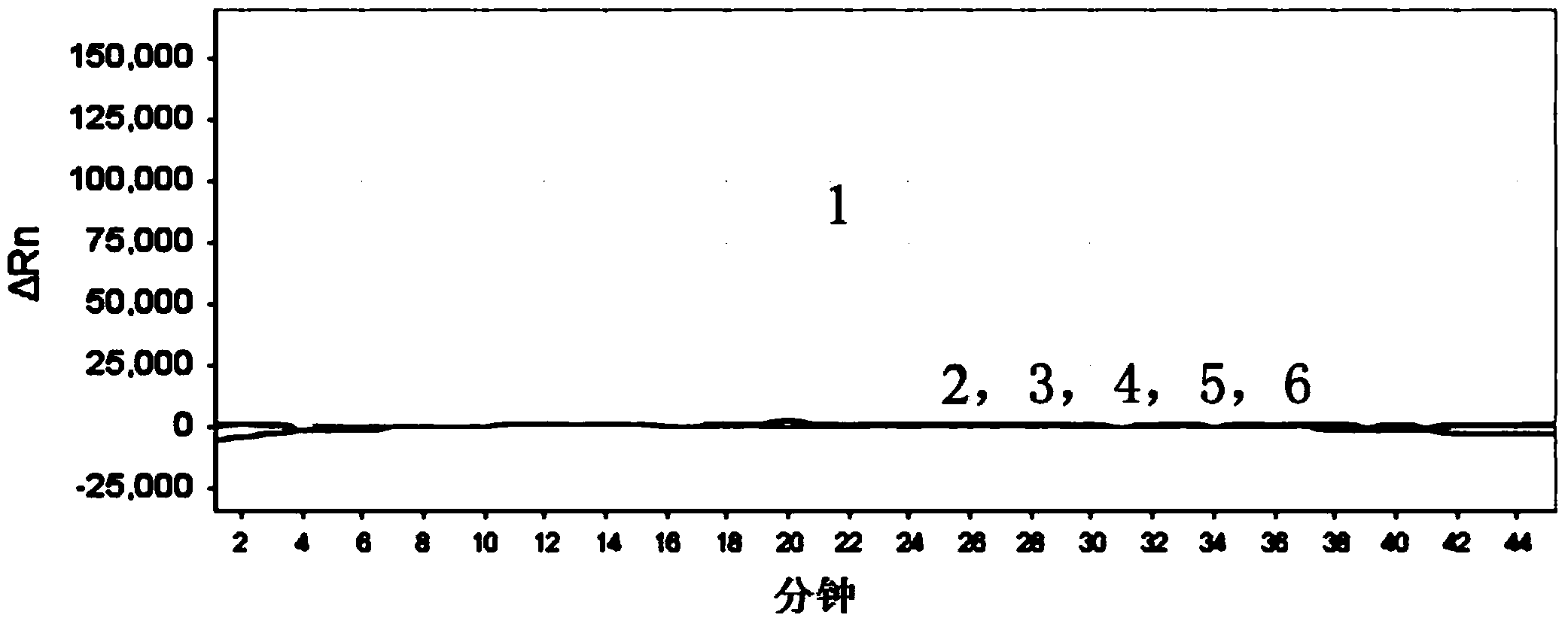
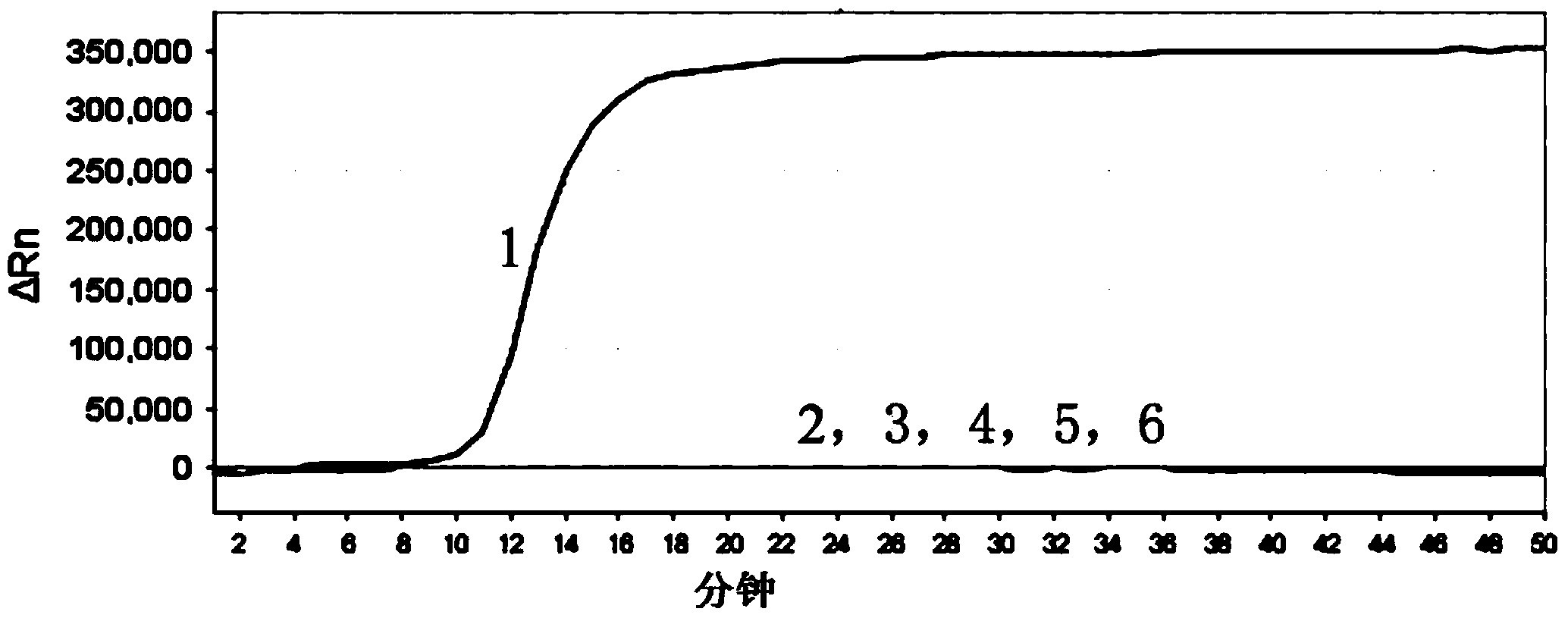
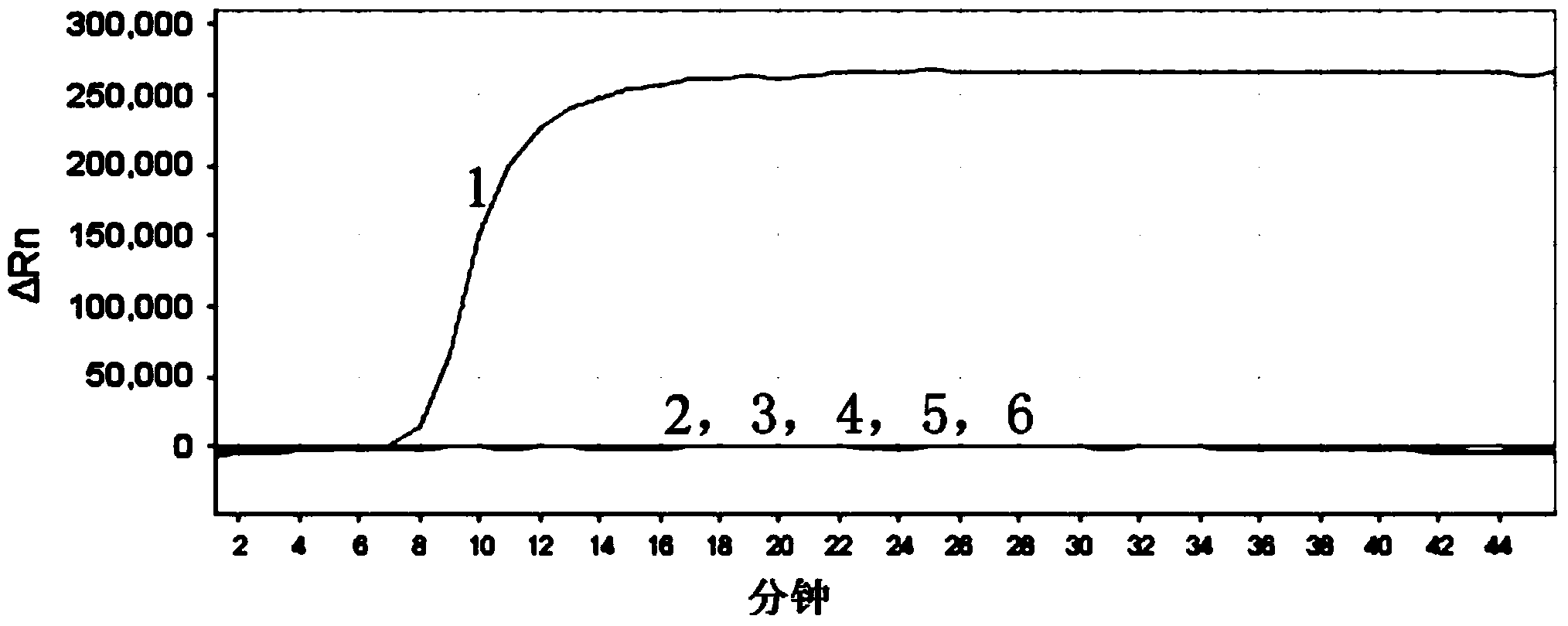
The invention relates to a gene chip for high-flux detection of pathogens and application thereof. The gene comprises (1) a combination of 174 oligonucleotide probes of pathogen variety specific genes, toxin genes and drug-resistant genes; and (2) a probe array, which is formed by curing the oligonucleotide probes on a carrier material by arm molecules. The gene chip comprises 174 gene probes, namely 32 pathogen variety specific gene probes of the following 8 pathogens of Burkholderia mallei, Burkholderia pseudomallei, Brucella, salmonella, Yersinia pestis, Bacillus anthracis, comma bacillus and the like, 25 toxin gene probe of the following 7 toxins of diphtheria toxin, Shiga toxin, staphylococcus enterotoxin, choleratoxin and the like, and 117 drug-resistant gene probes of 17 drug-resistant genes of extended-spectrum beta-lactamase, cephalosporinase, carbapenemase, integrase gene, common gene engineering carrier drug-resistant gene and the like. The gene chip can be used to detect multiple pathogen variety specific genes, toxin genes and drug-resistant genes.
Owner:李越希
Loop-mediated isothermal amplification (LAMP) primers, kit and detection method for detecting common carbapenemase genes of gram negative bacilli
ActiveCN103614465APrecise screeningSimple and fast operationMicrobiological testing/measurementAgainst vector-borne diseasesNucleotideEnterobacteriaceae bacterium
The invention discloses loop-mediated isothermal amplification (LAMP) primers, a kit and a detection method for detecting common carbapenemase genes of gram negative bacilli. In the LAMP primers disclosed by the invention, klebsiella pneumoniae carbapenemase (KPC) and new delhi metallo-b-lactamase (NDM) primer groups can detect all subtypes except for NDM-10; hypoxanthine nucleotide (IMP) and vimentin (VIM) primer groups can detect common subtypes at home and abroad. The LAMP kit built by the invention is applied to joint detection of KPC, NDM, IMP and VIM genes, can cover the common carbapenemase genes of non-fermentative bacteria and enterobacteriaceae, can accurately and quickly screen the common carbapenemase genes, and has great clinical significance for timely detecting and further controlling fulminant epidemic caused by propagation of the carbapenemase genes in enterobacteriaceae. The kit disclosed by the invention is high in detection sensitivity and the minimum detection limits of the KPC, NDM, IMP and VIM genes can reach 100 CFU / reaction.
Owner:SOUTHERN MEDICAL UNIVERSITY
Detection chip for drug resistance gene of bacteria, and application thereof
InactiveCN102321763AStrong specificityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationOligonucleotideThroughput
The present invention relates to a high-throughput detection chip for drug resistance gene of bacteria, and an application thereof. The detection chip comprises 117 gene probes, the drug resistance gene probes are selected from 17 categories of drug resistance genes, which comprise extended spectrum beta-lactamase, cephalosporinase, carbapenemase, integrase gene, tetracycline resistance gene, aminoglycoside resistance gene, disinfectant resistance gene, erythromycin resistance gene, macrolide efflux gene, vancomycin resistance gene, multidrug resistance efflux pump gene, mupirocin resistance gene, sulfanilamide resistance gene, tylosin resistance gene, fluoroquinolone resistance gene, chloramphenicol acetyltransferase and commonly-used genetic engineering vector resistance gene. The chip is adopted for detecting the resistance gene of the pathogenic bacteria. The chip is characterized in that: the chip comprises (1) 117-oligonucleotide probe composition and quality control probes of 17 categories of the drug resistance genes; (2) probe arrays, wherein the oligonucleotide probes are solidified on the vector material through arm molecules to form the probe arrays.
Owner:李越希
Carbapenems useful in treating and preventing pulmonary infections, pharmaceutical compositions thereof and modes of administration thereof
InactiveUS20050065141A1Satisfies needBiocideOrganic active ingredientsPulmonary infectionIndoschulzia
The present invention provides carbapenems to treat or prevent pulmonary infections, particularly in patients with cystic fibrosis, pneumonia, ventilator associated pneumonia, bronchitis or bronchiectstasis, pharmaceutical compositions of these carbapenems and methods of administering these carbapenems to treat or prevent pulmonary infections.
Owner:PENINSULA PHARMA
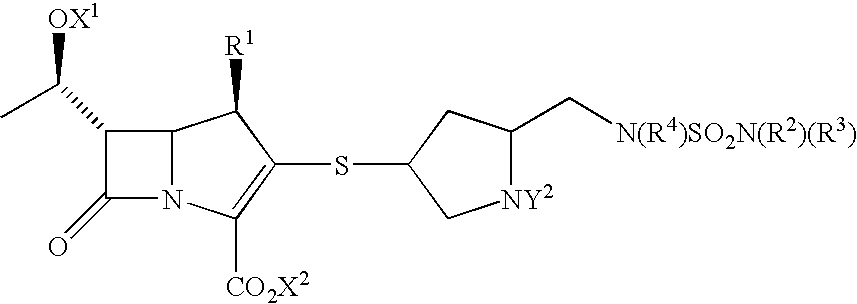
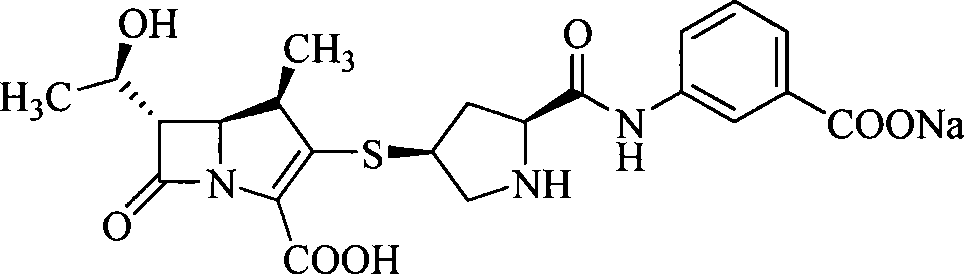
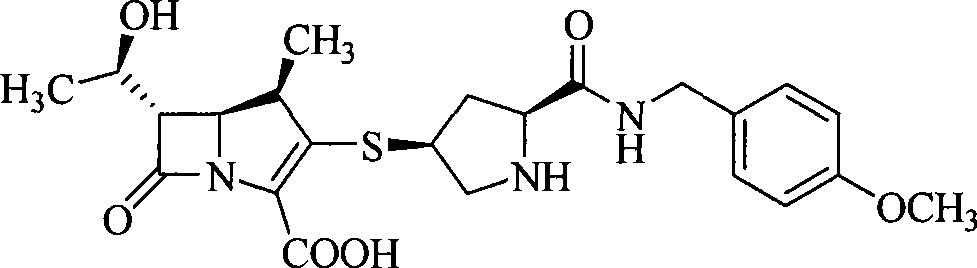
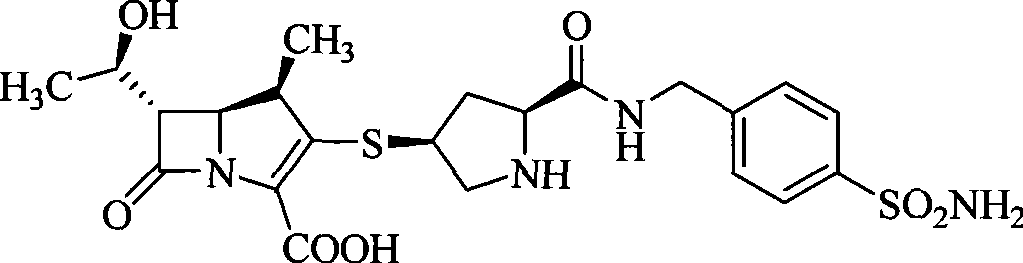
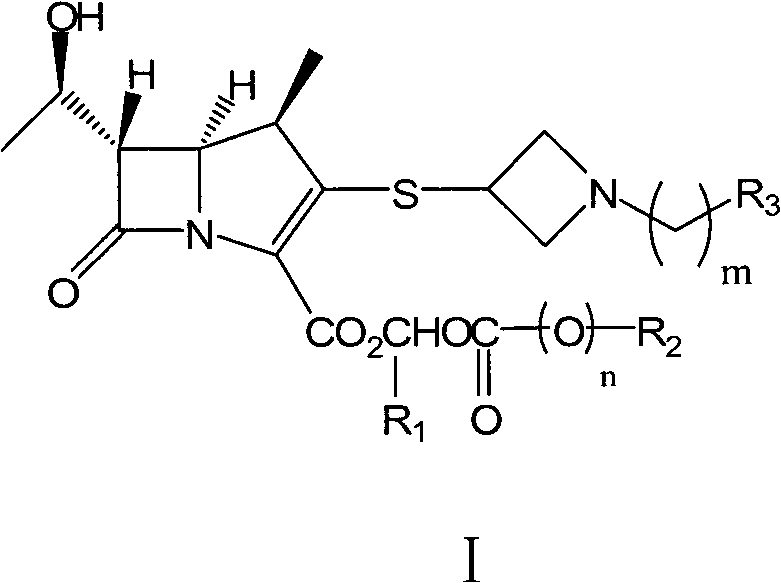
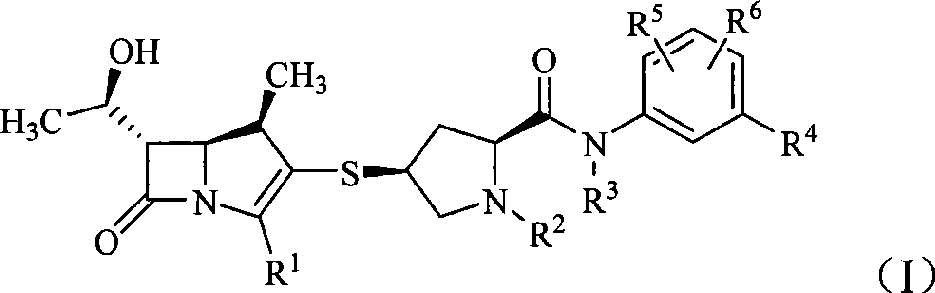
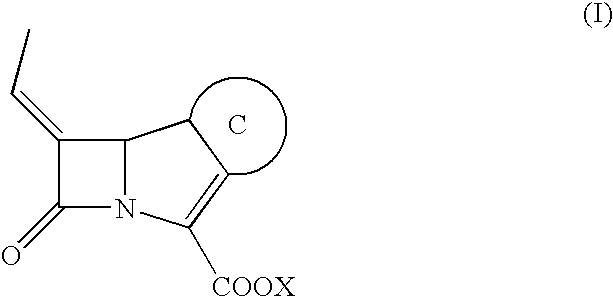
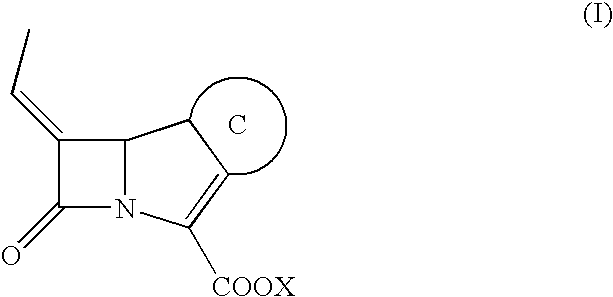
Carbapenem derivative containing sulfhydryl pyrrolidine formamide benzyl
ActiveCN101372489AHigh antibacterial activityLow toxicityAntibacterial agentsOrganic active ingredientsPyrrolidineFormamide
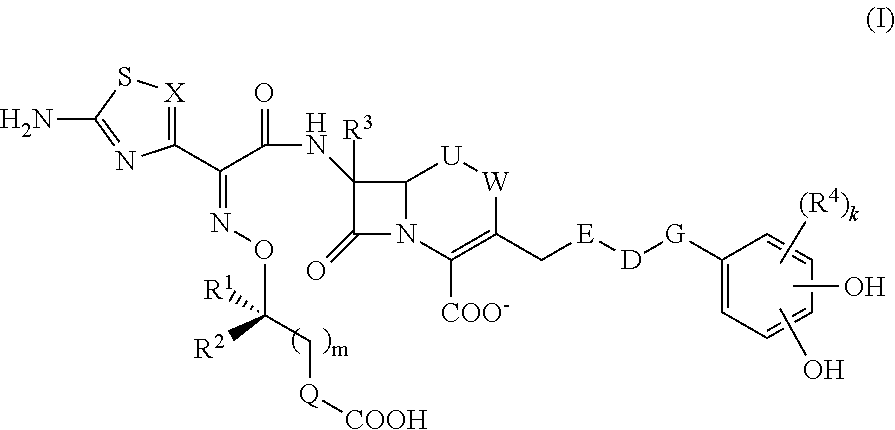
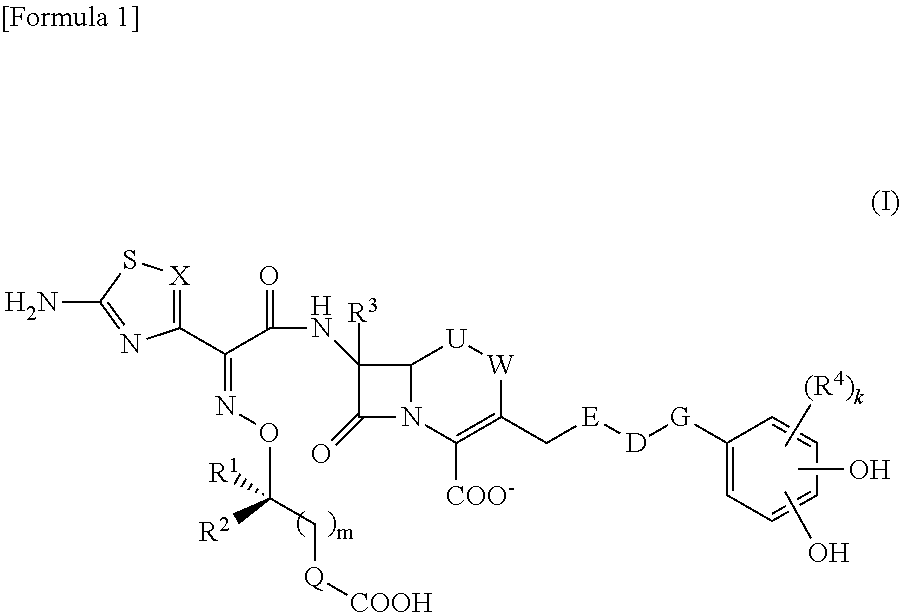
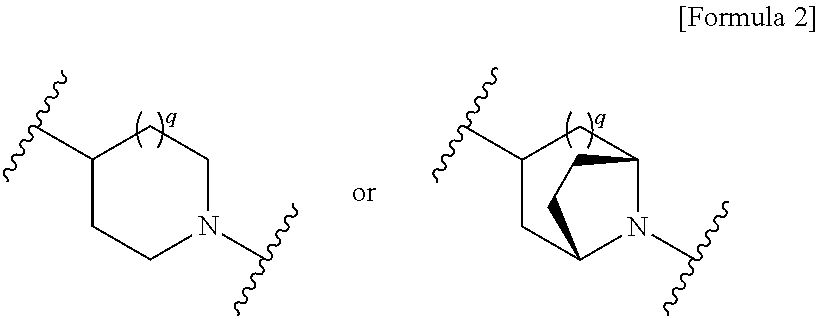
The invention belongs to the technical field of medicine, and more particularly relates to carbapenem derivatives which are shown in general formula (I) and contain hydrosulphonyl pyrrolidine methanamide phenmethyl, salt which can be accepted by the derivatives on pharmacy, ester of the derivatives that is easy to hydrolyze, isomer of the derivatives, hydrate of the derivatives, and hydrate of the ester or the salt of the derivatives; wherein, R1, R2, R3 and R4 are defined as instruction; the invention also relates to a method used for preparing the compounds, medicine combinations containing the compounds, and the application of the compounds in the preparation of the medicine for treating and / or preventing infectious diseases.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD
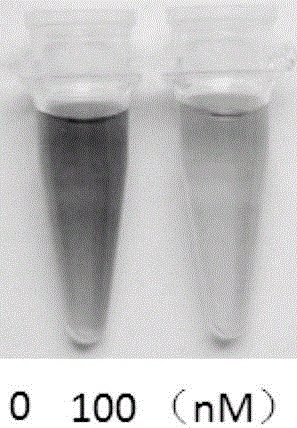
Fluorescent probe for carbapenem type antibioticbacteria and synthesis method and application thereof
ActiveCN106279178AHigh selectivityReduce usage costsOrganic chemistryFluorescence/phosphorescenceCarbamateSynthesis methods
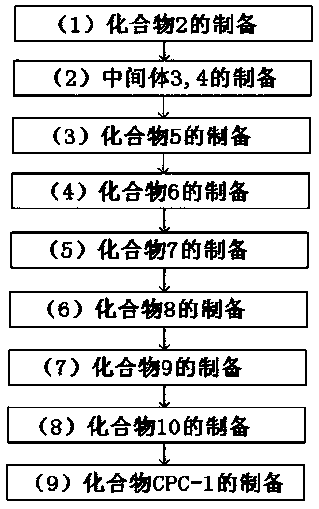
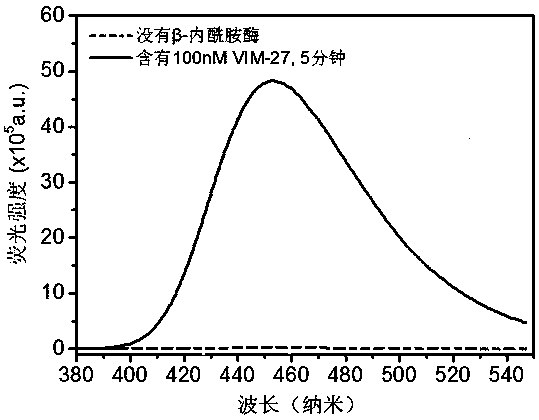
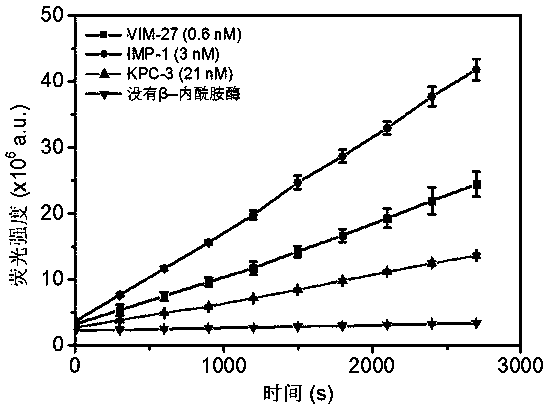
The invention provides a structural general formula (shown in the description) of a fluorescent probe for carbapenem type antibiotic bacteria. In the structural general formula, X represents carbon atoms or sulfur atoms; when the X represents CH, R1 represents methyl and can be of the configuration S or R, or the X represents CH2 or S; Y represents oxygen atoms, sulfur atoms or linkers on a fluorophorearomatic nucleus and comprises 4-hydroxymethylbenzyl or 4-hydroxyl or sulfydrylbenzyl or carbamate linkers. The synthesis method of the fluorescent probe comprises the following steps that 1, a compound 2 is prepared; 2, intermediates 3 and 4 are prepared; 3, a compound 5 is prepared; 4, a compound 6 is prepared; 5, a compound 7 is prepared; 6, a compound 8 is prepared; 7, a compound 9 is prepared; 8, a compound 10 is prepared;9, a compound CPC-1, namely a fluorescent probe compound is prepared. The fluorescent probe can be applied to detection of carbapenemases and drug-resistant bacteria containing the carbapenemases and has the important significance on antibiotic abandoning or few antibiotics adopted in medical treatment.
Owner:EAST CHINA UNIV OF SCI & TECH
Cell-free preparation of carbapenems
ActiveUS20130065878A1Improve propertiesGood water solubilityAntibacterial agentsBiocideCell freeChemical compound
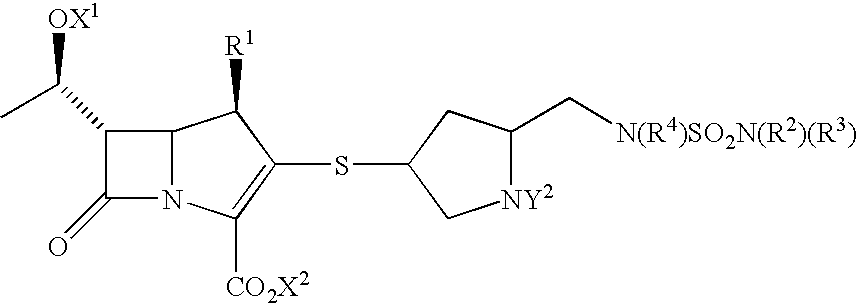
Provided herein are cell-free systems for generating carbapenems, e.g., a compound of the Formula (I):or salts thereof; wherein , R1, R2, R3, R4, R5, and R6 are defined herein. Also provided are pharmaceutical compositions comprising a compound generated by the inventive cell-free system, and use of these compounds and compositions for the treatment of bacterial infections.
Owner:GREENLIGHT BIOSCIENCES INC
Cell-free preparation of carbapenems
ActiveUS9469861B2Improve propertiesEnhance pharmaceuticallyAntibacterial agentsBiocideCell freePharmaceutical drug
Owner:GREENLIGHT BIOSCIENCES INC
Process for making carbapenem compounds
InactiveUS7022841B2Minimize degradationLevels of residual organic solvents are more readily reducedAntibacterial agentsOrganic active ingredientsArylOrganic solvent
The present invention relates to a process for reducing the levels of organic solvents to pharmaceutically acceptable levels in thermally unstable crystalline carbapenem solids represented by formula I: or a salt thereof, wherein R1 and R2, are the same or different, and are selected from H, alkyl, aryl, and heteroaryl, comprising washing a carbapenem solid containing organic solvent with an organic solvent containing water; and using vacuum and / or inert gas (hydrated or dry) at low temperature to produce a compound of formula I containing pharmaceutically acceptable levels of organic solvents, wherein the water content of the crystalline carbapenem solid, correcting for organic solvents, is maintained at about 13% to about 25% during the process.
Owner:MERCK SHARP & DOHME LLC
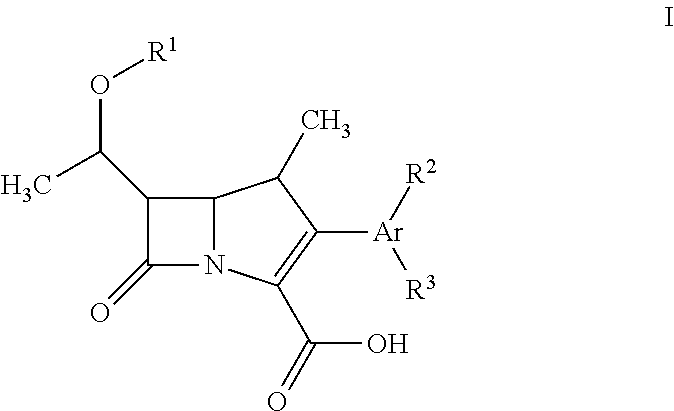
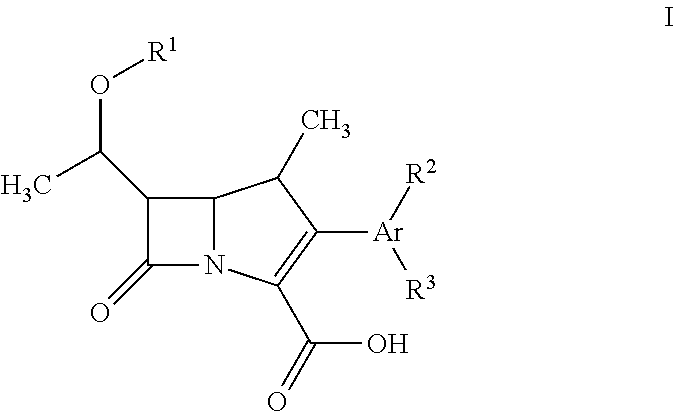
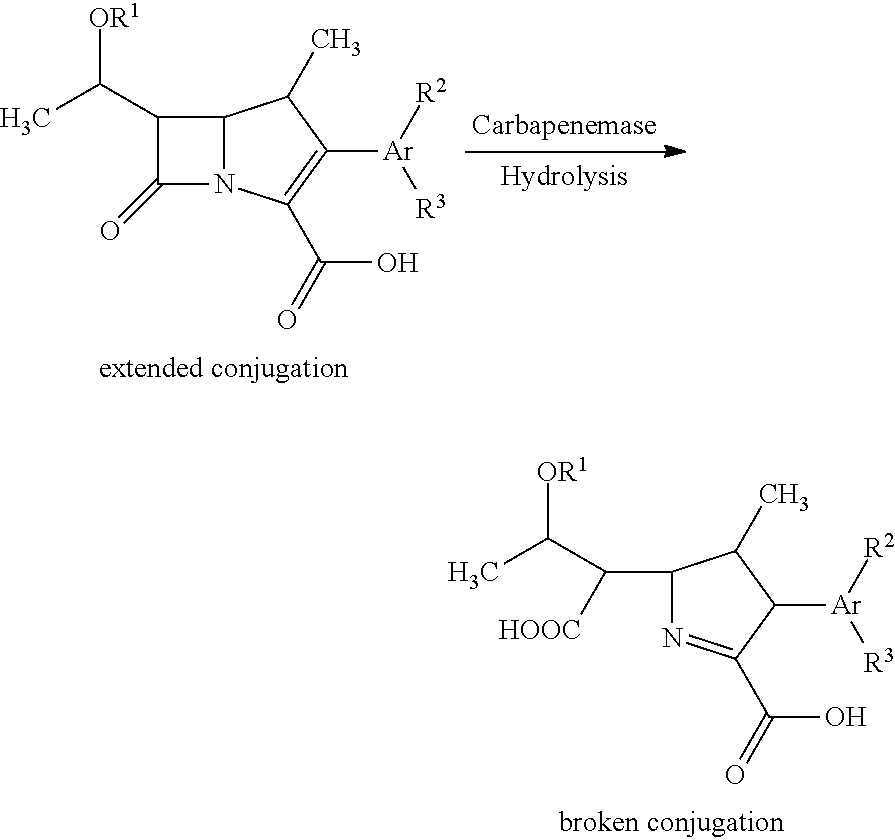
Fluorescent carbapenems
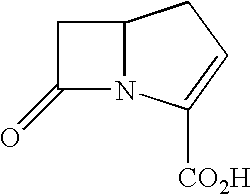
Chromogenic or fluorescent carbapenems according to formula I, wherein Ar is a mono or disubstituted carbocyclic aromatic group or an optionally mono or disubstituted heterocyclic aromatic group, are useful compounds for the detection of bacterial carbapenemase.
Owner:PFAENDLER HANS R
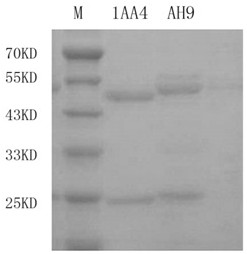
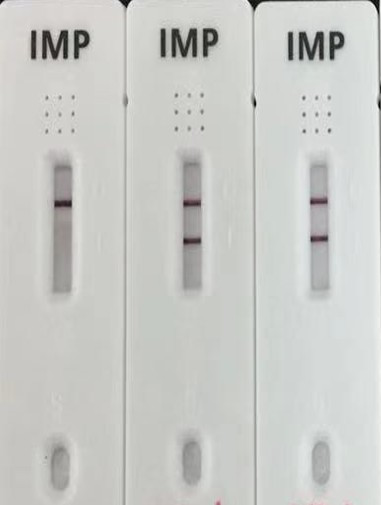
Anti-IMP type carbapenemase hybridoma cell strain, monoclonal antibody and application
ActiveCN112501131AAid clinical infection controlAdjuvant therapyBiological material analysisTissue cultureAntiendomysial antibodiesElisa method
The invention provides an anti-IMP type carbapenemase hybridoma cell strain, a monoclonal antibody and application. The hybridoma cell strain capable of stably secreting an anti-IMP type carbapenemaseantibody and a variable region sequence of the hybridoma cell strain are obtained by screening a mouse hybridoma monoclonal antibody and cloning an Ig variable region gene by an RT-PCR method, and the antibody binding specificity is identified by an ELISA method; the obtained anti-IMP type carbapenemase antibody can be used for detecting IMP type carbapenemase, the titer reaches 1:640000 or more,and the anti-IMP type carbapenemase antibody can be prepared into an in-vitro diagnostic kit or microfluidic chip for early typing of drug-resistant strains, guiding medication and assisted clinicalinfection control and treatment, and has important significance for improving the medical and health level of China.
Owner:TIANJIN ERA BIOLOGY TECH CO LTD +1
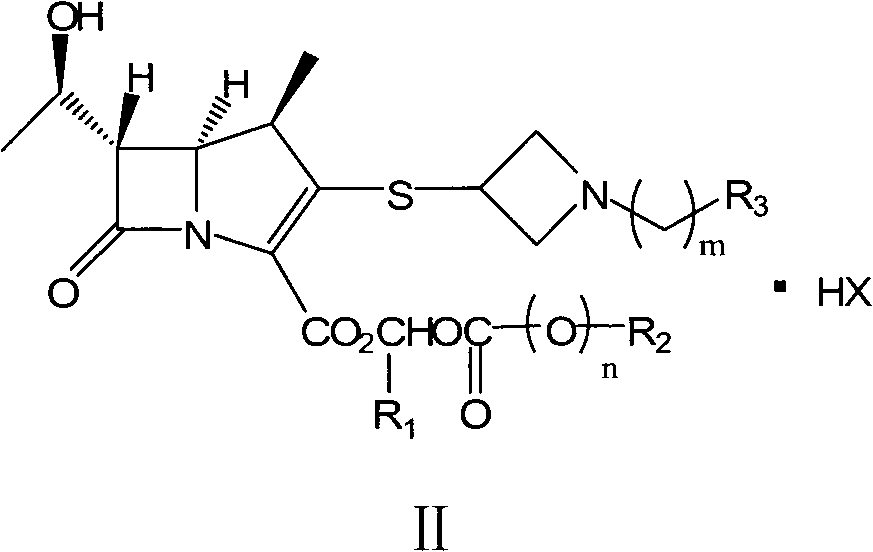
Preparation method of carbapenems
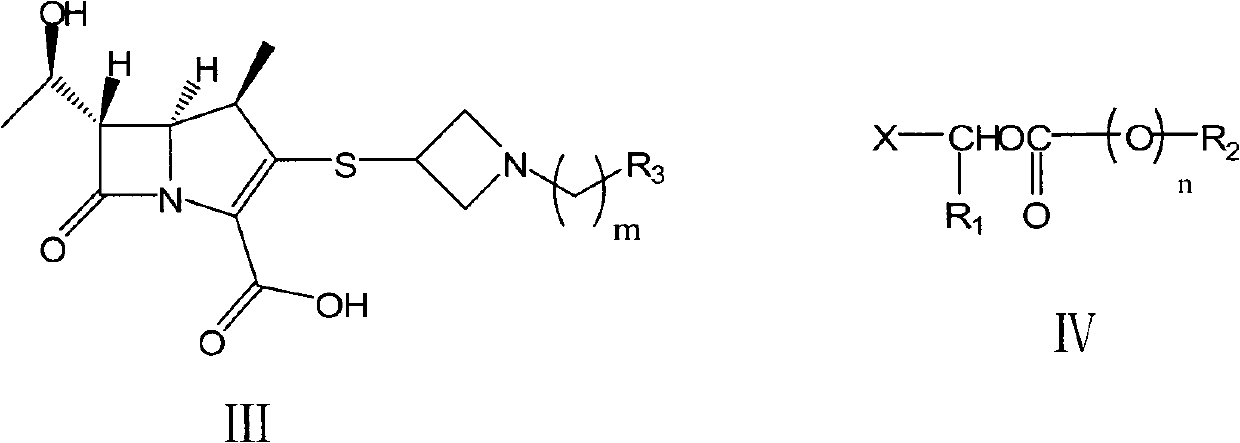
The invention relates to a preparation method of tebipenem pivoxil and analogue thereof. In the method, carbapenem compound salt with a formula II is used as a raw material to obtain carbapenem compound ester with a formula I under the action of alkali, wherein the carbapenem compound salt with the formula II is prepared by direct reaction of a carbapenem compound with a formula III and ester with a formula IV. According to the invention, high-yield and high-purity salt of tebipenem pivoxil and analogue thereof can be prepared, so that high-yield and high-purity tebipenem pivoxil or analogue thereof can be obtained without column chromatography. Thus, the method provided by the invention is more suitable for industrial production in a large scale.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
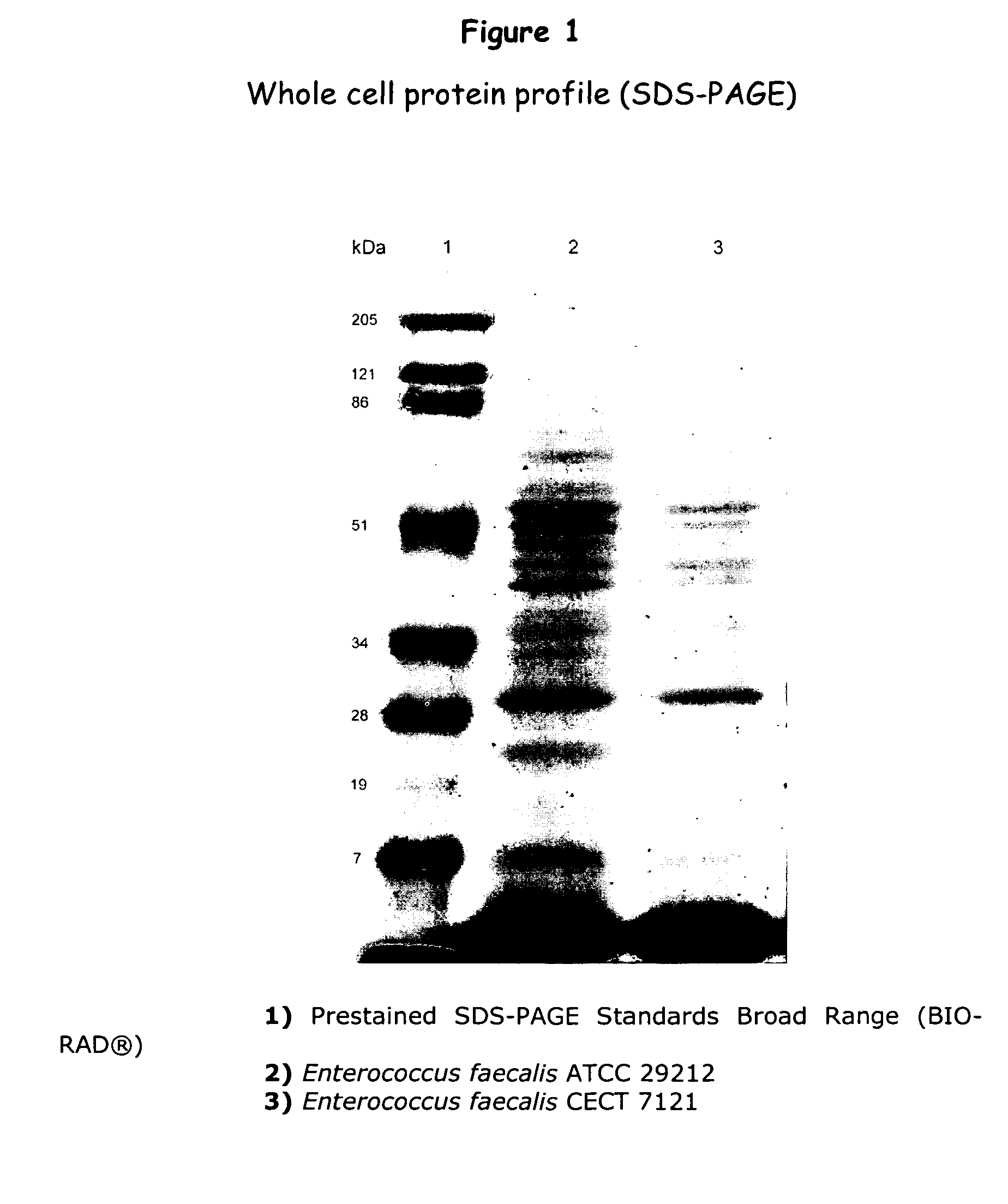
New lactic bacteria useful as probiotics
InactiveUS20080063666A1Pleasant flavorHealthy digestion and intestineBiocideBacterial antigen ingredientsDiseaseAmpicillin
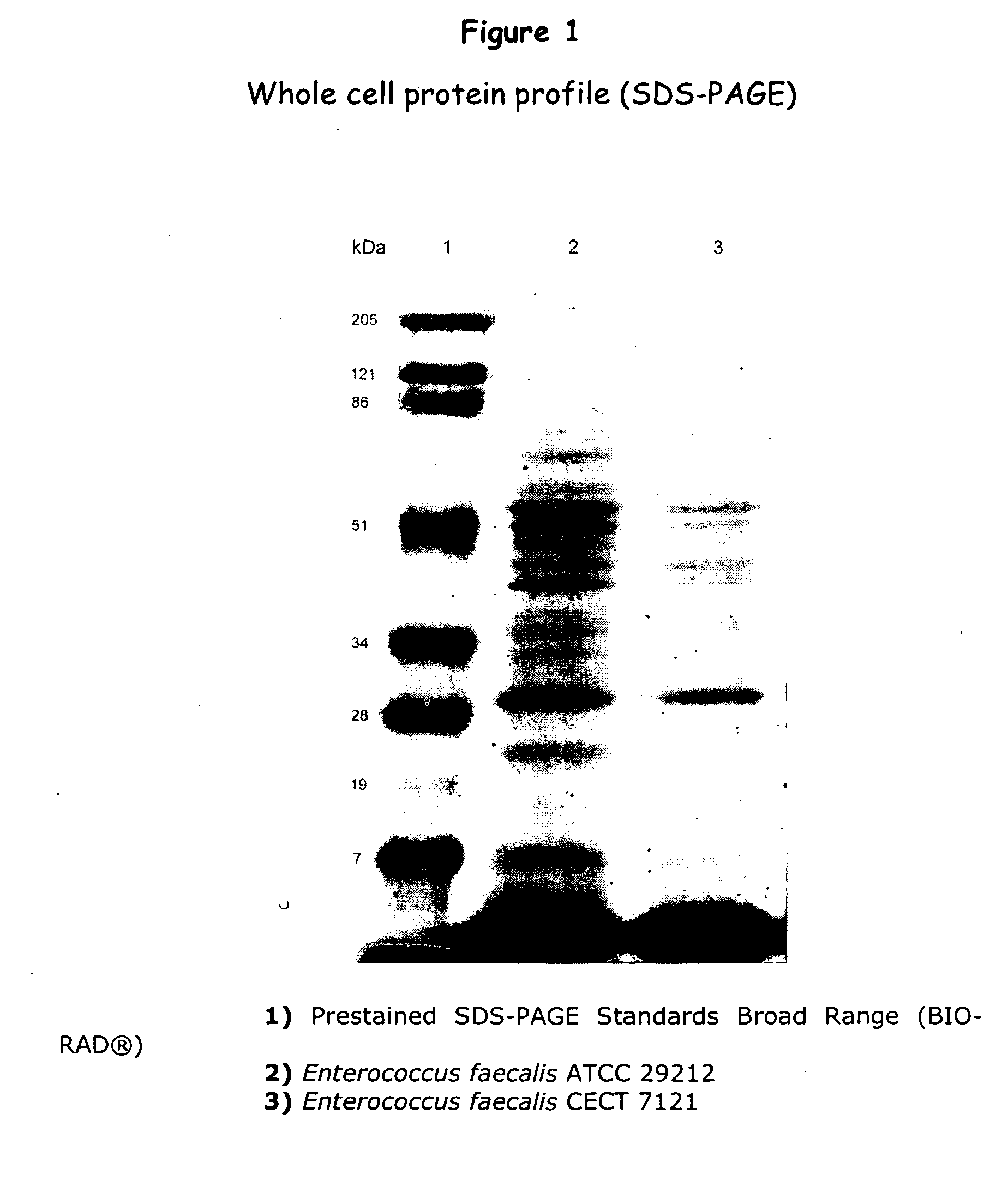
An isolated strain of Enterococcus faecalis GALT deposited under number C E C T 7121 of the group of lactic bacteria is disclosed, which is capable of surviving and colonizing the gastrointestinal tract of humans and / or animals and showing beneficial probiotic activity for the health of humans and animals. The strain E. faecalis GALT and / or a culture supernatant and / or metabolites thereof shows no in vitro multiresistance to antibiotics of common use in human clinics as glycopeptides, such as vancomycin, teicoplanine; carbapenemes, such as impipenem, meropenem; and ampicillin. The strain E. faecalis GALT contains no red blood cell-destroying hemolysins of human, ovine and equine origin; and it does not produce any gelatinase, DNase and decarboxylases. The strain E. faecalis GALT is useful for the preparation of a composition intended for the treatment and / or prophylaxis of disorders associated with colonization by pathogenic microorganisms of the gastrointestinal tract; for use as a regulator of the immune response in human and animals, as well as for the preparation of a composition. The invention is also directed to methods and uses of the strain E. faecalis GALT.
Owner:ALLENDE MIGUEL ANGEL GARCIA
Kit for quickly detecting drug resistance gene of pneumophila pathogenic bacteria
ActiveCN107475422AEasy to operateNucleotide librariesMicrobiological testing/measurementMethicillin resistanceAntibiotic Y
The invention relates to a kit for quickly detecting a drug resistance gene of pneumophila pathogenic bacteria, which comprises a gene chip capable of detecting 24 main drug resistance genes of pneumophila pathogenic bacteria and a reaction system for multiple asymmetric PCR reactions. The 24 drug resistance genes are derived from aminoglycosides, quinolones, extended spectrum beta-lactamases (ESBLs), cephalosporins (AmpC), carbapenems and drug resistance genes with vancomycin resistance and methicillin resistance causing membrane permeability transition. The kit can be used for directly detecting drug resistance genes of pneumophila pathogenic bacteria in clinical samples, is simple and quick to operate, does not need culture or drug sensitive tests, gains valuable time for reasonable application of antibiotics to a great extent, and is worthy of clinical popularization and application.
Owner:GENERAL HOSPITAL OF PLA +1
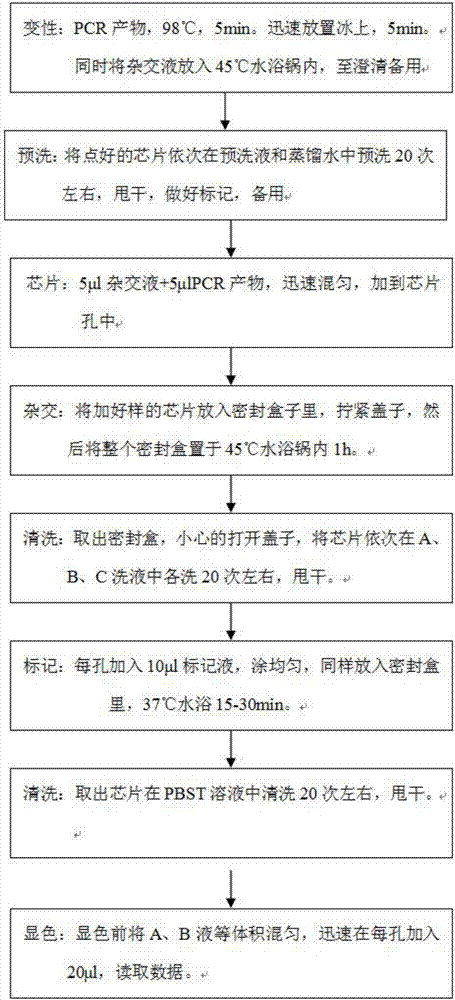
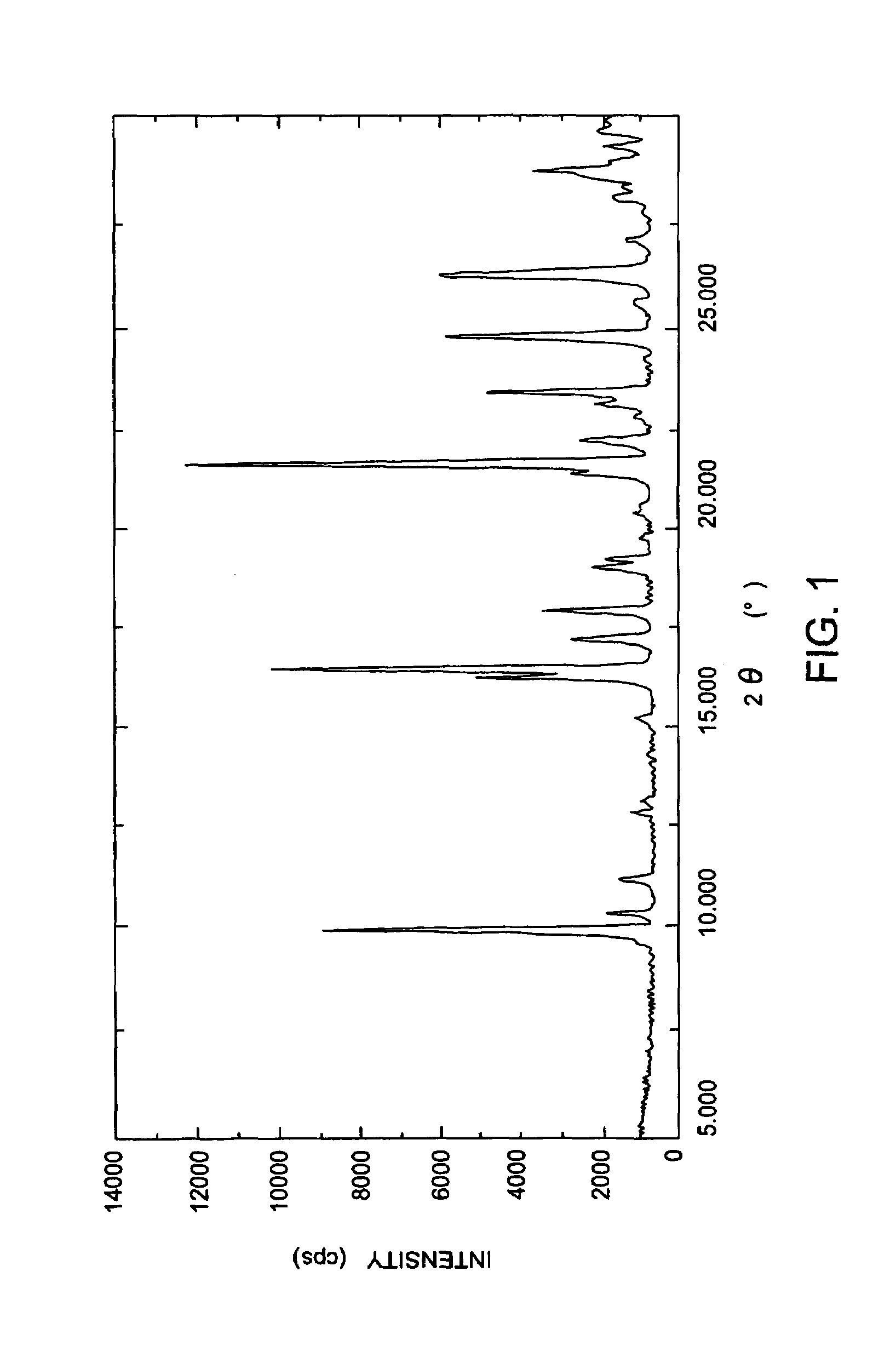
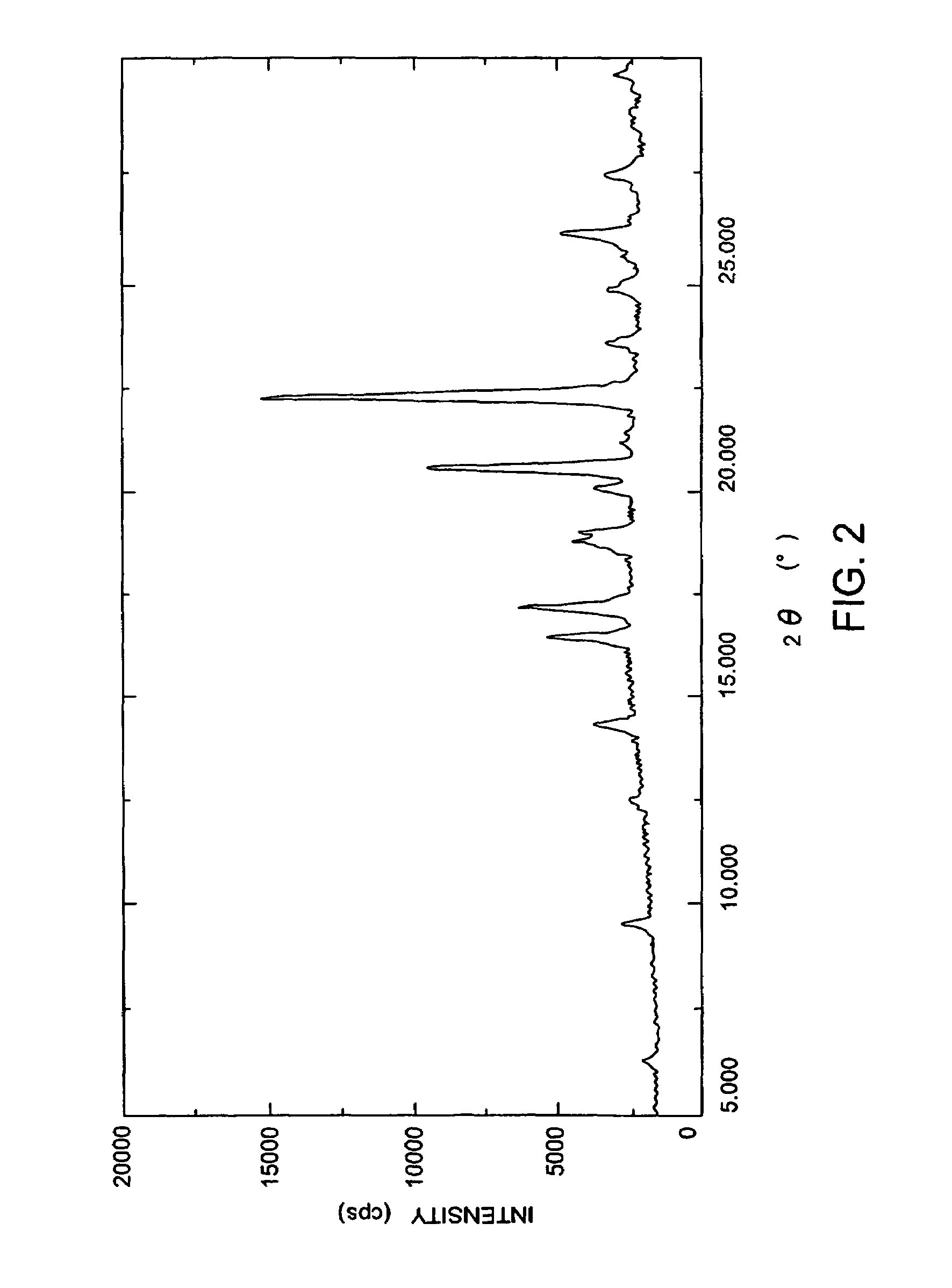
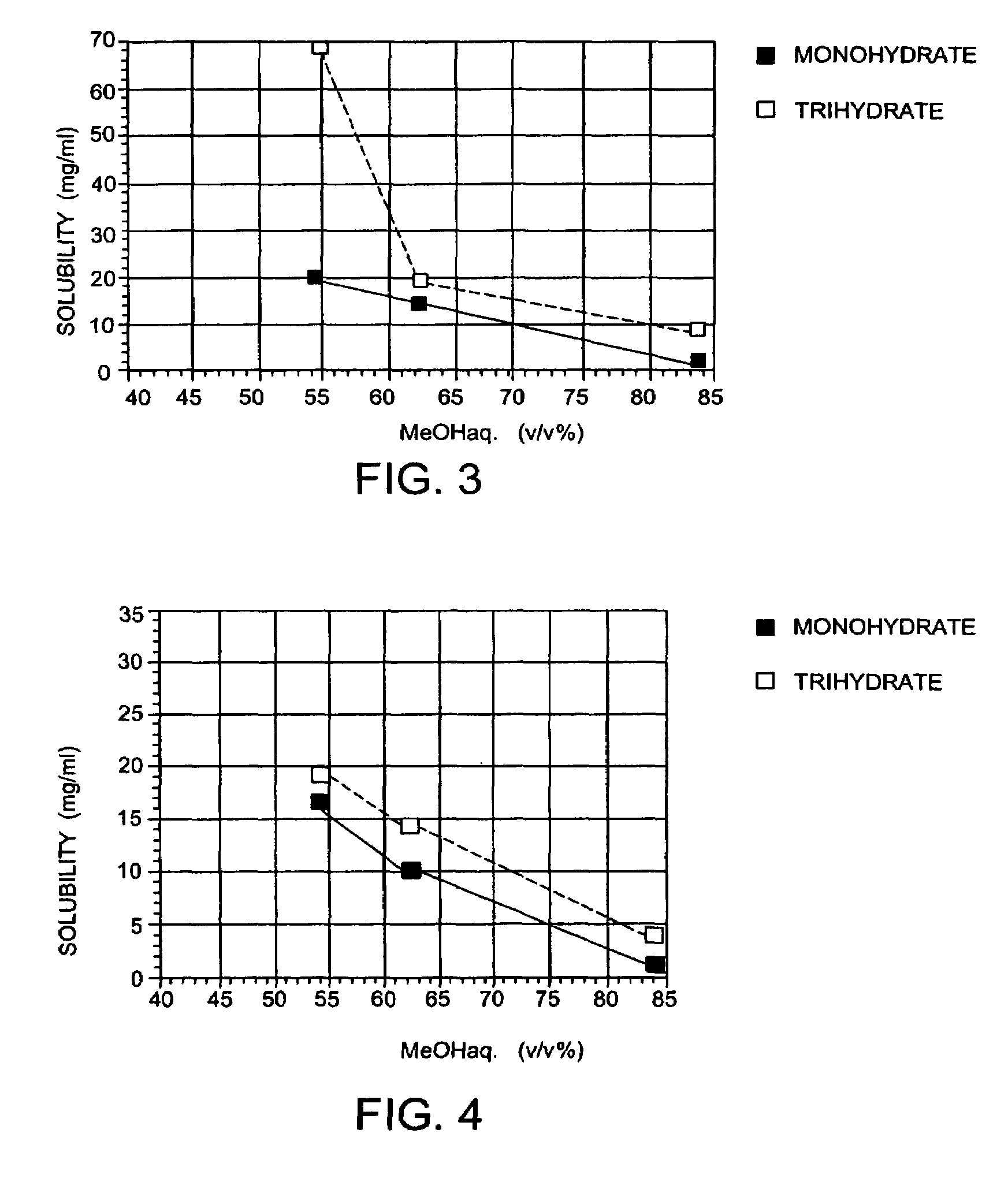
Carbapenem compound crystals and interjection preparations
InactiveUS7084268B1Easy maintenanceKeep longAntibacterial agentsOrganic active ingredientsMedicinal chemistryHydrochloride
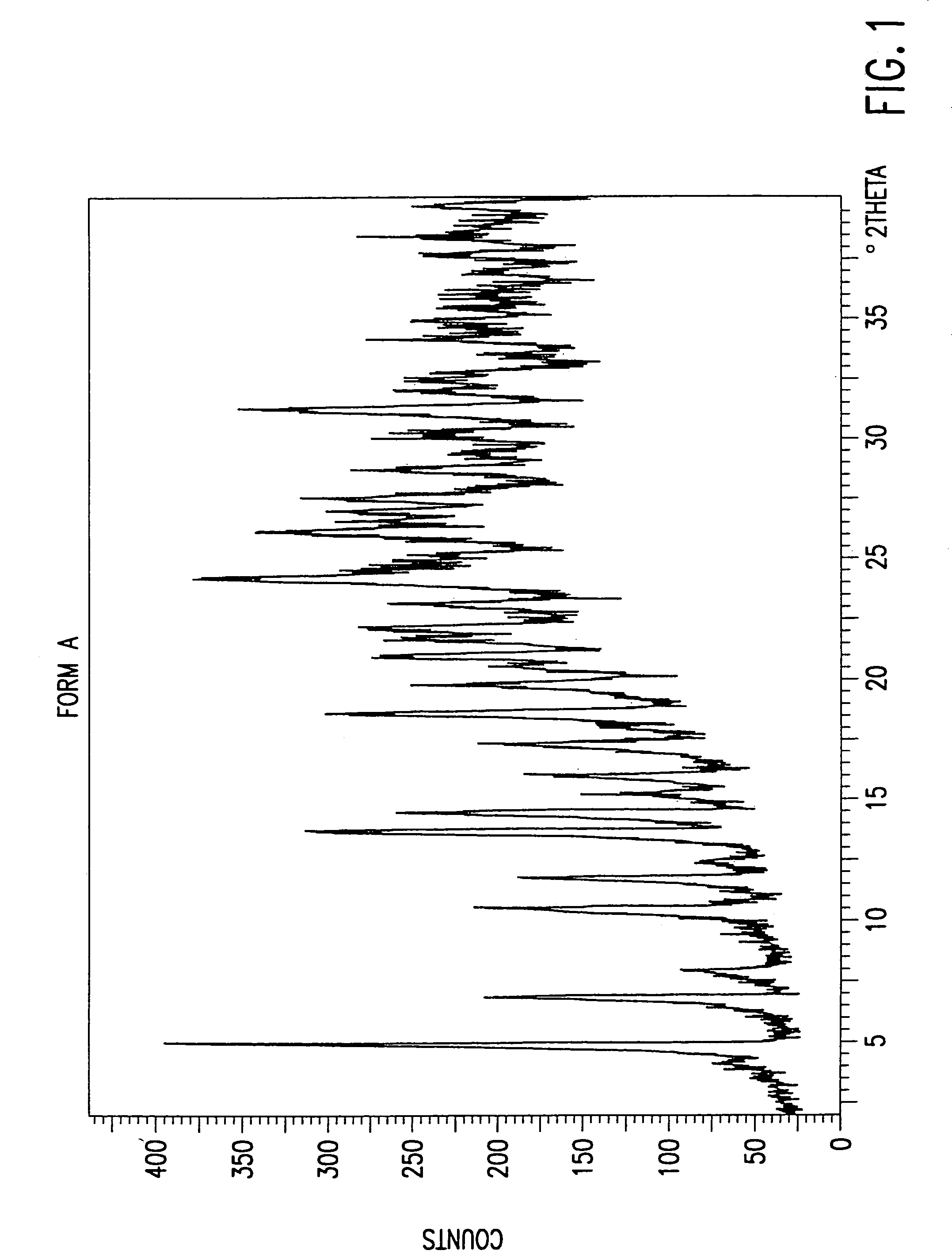
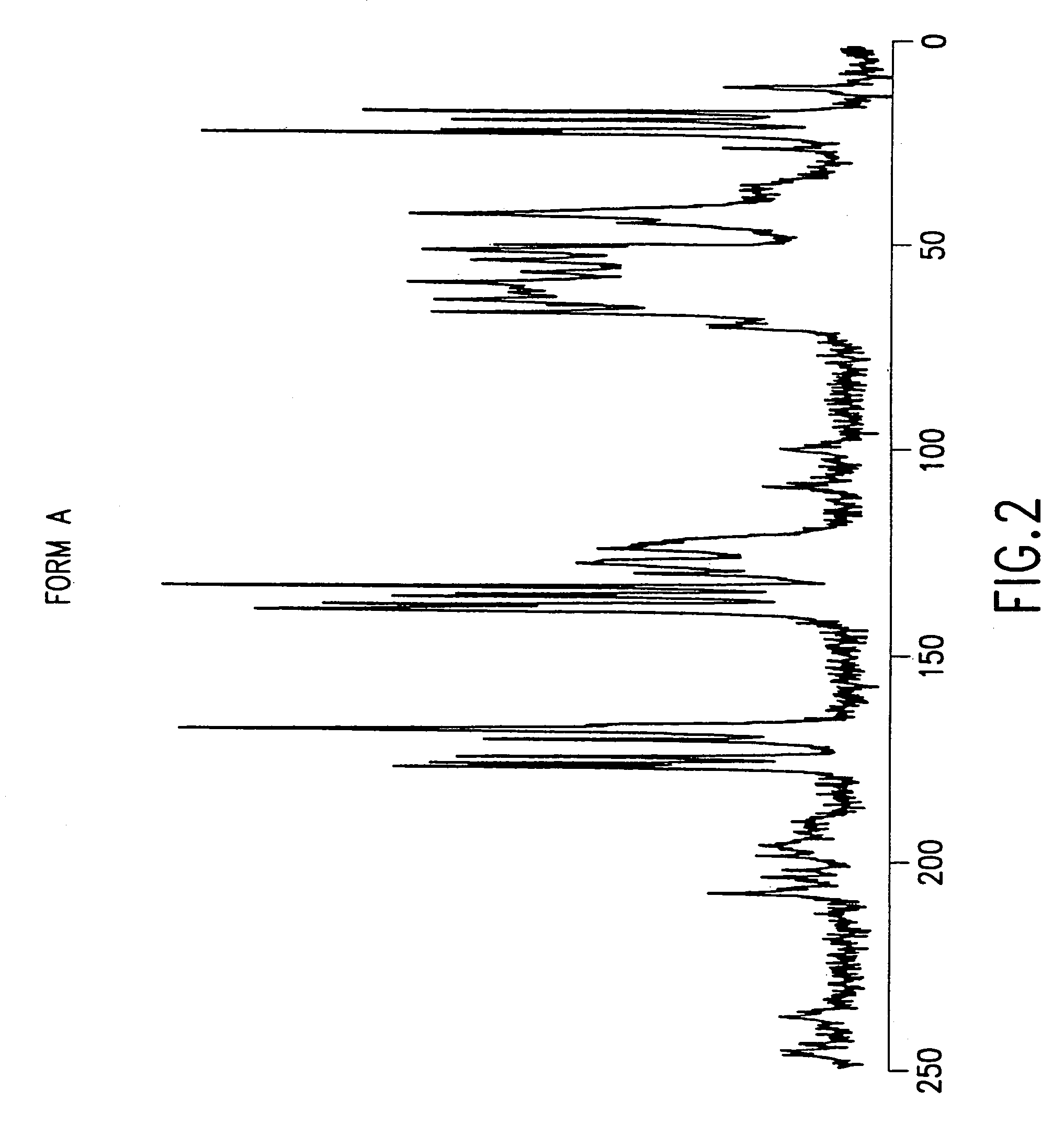
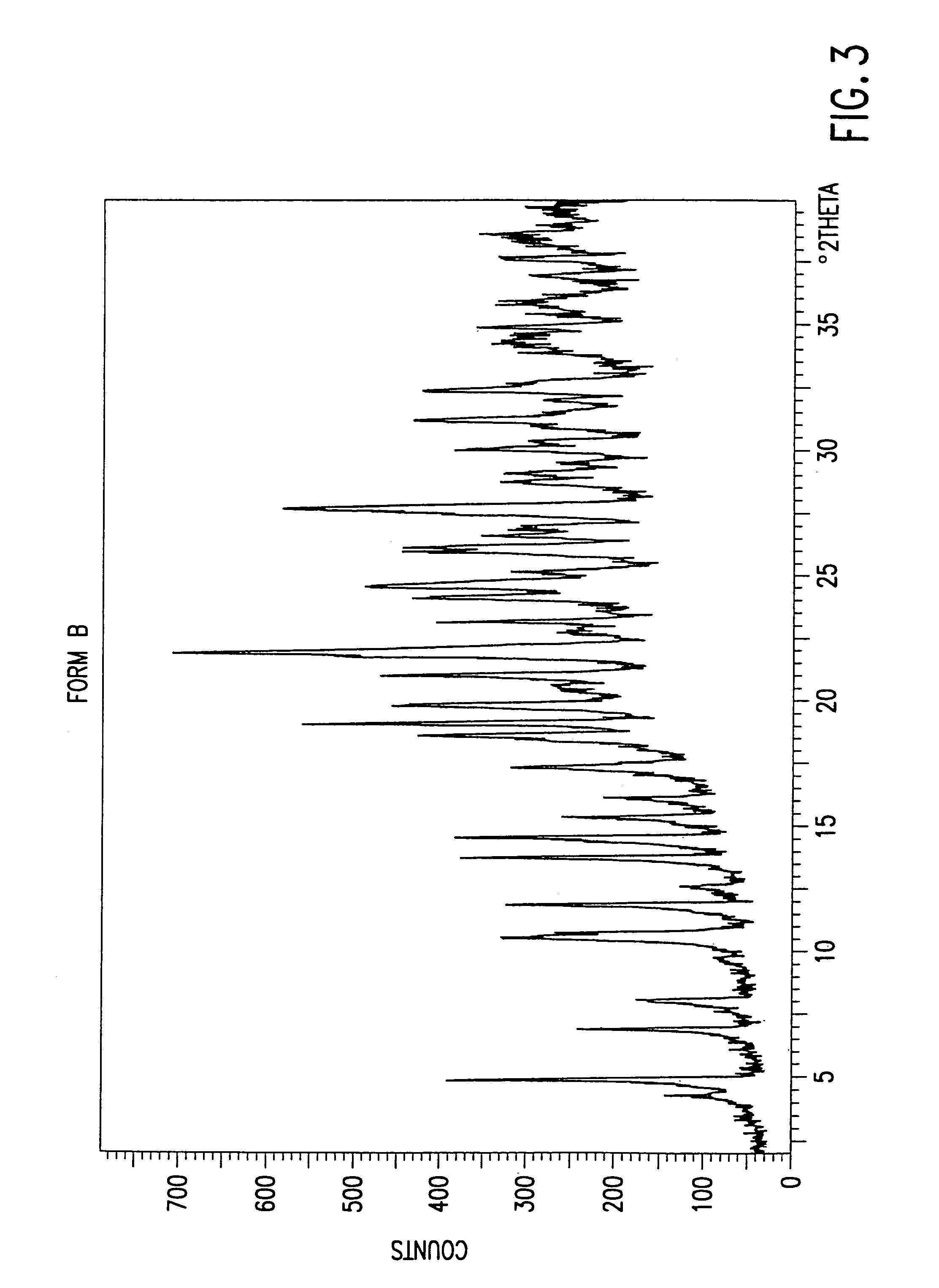
The present invention provides carbapenem hydrochloride trihydrate crystals, which are chemically stable, easily purified and useful as antimicrobial agents, a process for producing them, and a powdery charged preparation for injection containing them. That is, it provides carbapenem hydrochloride trihydrate crystals having a powdery X-ray diffraction pattern containing lattice distances (d) of 9.0, 4.1 and 2.8 Å, a process for producing them, and a powdery charged preparation for injection containing them.
Owner:EISIA R&D MANAGEMENT CO LTD
Cephem compound having catechol group
InactiveUS9145425B2Broad antimicrobial spectrumAntibacterial agentsOrganic active ingredientsSide chainPharmaceutical medicine
This invention provides Cephem compounds having the formula:or an ester, a protected compound at the amino on the ring in the 7-side chain, a pharmaceutically acceptable salt, or a solvate thereof, a pharmaceutical composition thereof, and a method for treating a bacterial infectious disease with the compound, the ester, the protected compound, the salt, or the solvate thereof, wherein the symbols in the formula are defined in the specification. The compounds exhibit potent antimicrobial spectrum against a variety of bacteria including Gram negative bacteria and / or Gram positive bacteria, preferably beta-lactamase producing Gram negative bacteria, more preferably, multi-drug resistant microbials, in particular, Class B type metallo-beta-lactamase producing Gram negative bacteria, and still preferably extended-spectrum beta-lactamase (ESBL) producing bacteria. The compounds most preferably do not exhibit cross-resistance against known Cephem drugs or Carbapenem drugs.
Owner:SHIONOGI & CO LTD
Compositions and methods for the identification of a carbapenemase gene
Owner:BECTON DICKINSON & CO
Gram-positive carbapenem antibacterials and processes for their preparation
The present invention provides β-methyl carbapenem compounds and pharmaceutical compositions useful in the treatment of bacterial infections and methods for treating such infections using such compounds and / or compositions. The invention includes administering an effective amount of a carbapenem compound or salt and / or prodrug thereof to a host in need of such a treatment. The present invention is also in the field of synthetic organic chemistry and is specifically provides an improved method of synthesis of β-methyl carbapenems which are useful as antibacterial agents.
Owner:FOB SYNTHESIS INC
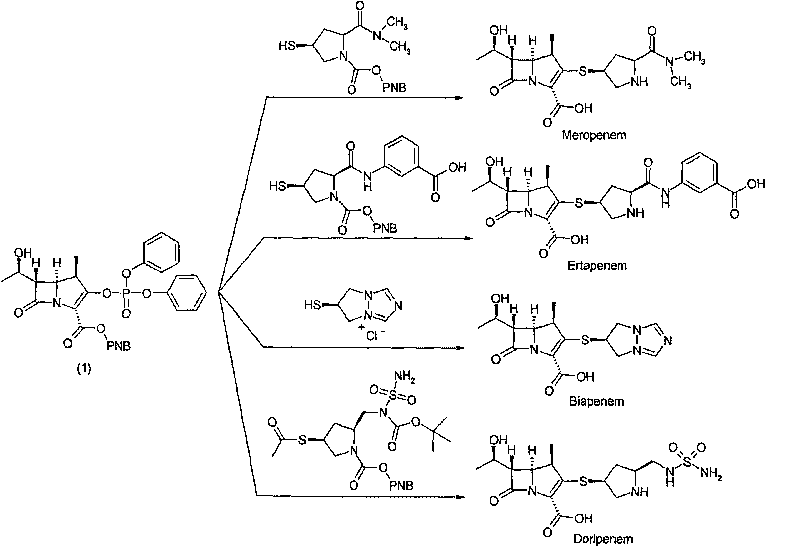
Method for preparing meropenem
The invention relates to a method for preparing meropenem. The method comprises the following step of: reducing beta-methyl carbapenem protected by a meropenem precursor into meropenem under the transferring and catalyzing actions of hydrogen. The method has the advantages of mild chemical reaction conditions, stable process conditions, easiness for operating, high transformation rate, high yield, product purity stabilized over 99 percent, recycling of solvents and catalysts in the entire process, and great saving in the production cost, and is a practicably synthesizing process suitable for large-scale production; and a novel thought and a novel method for a penem compound are provided.
Owner:ASYMCHEM LAB TIANJIN +4
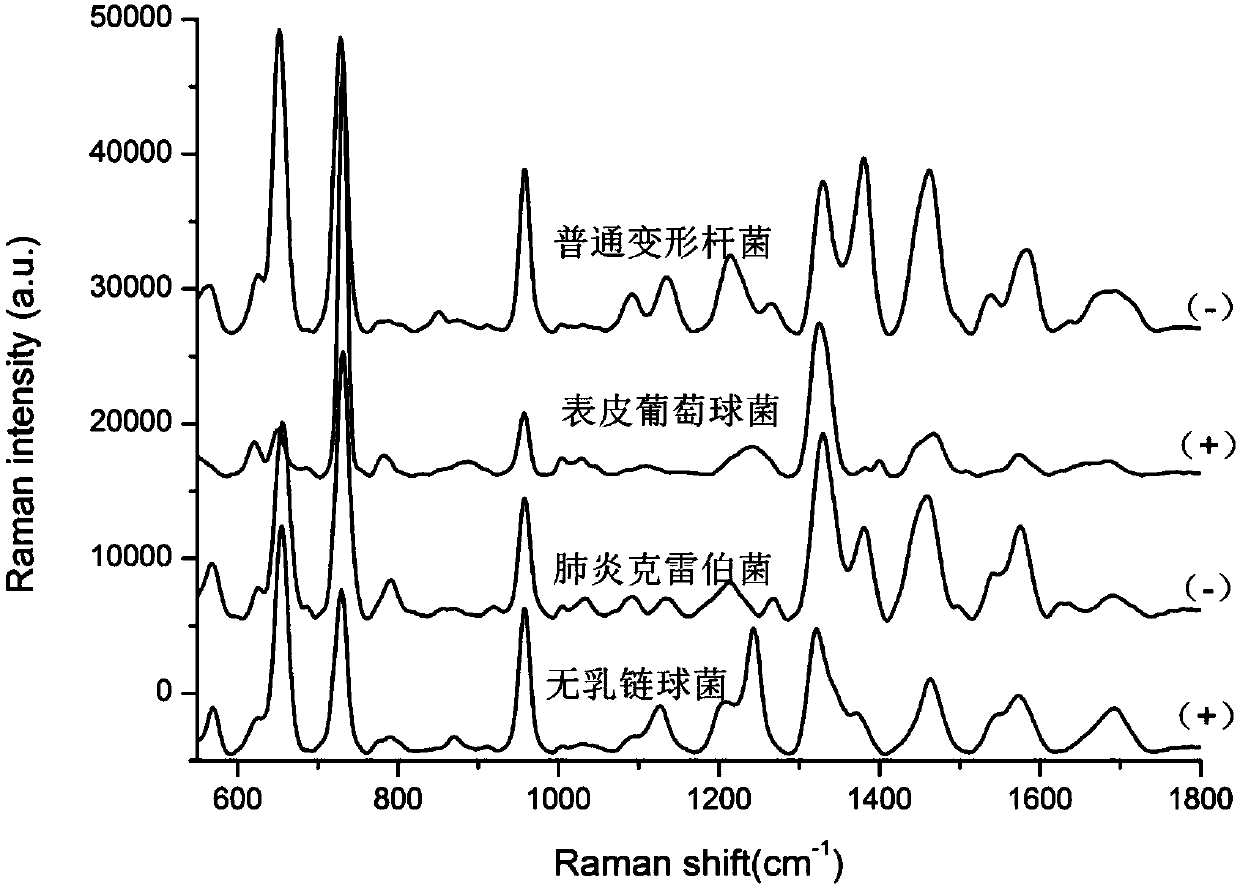
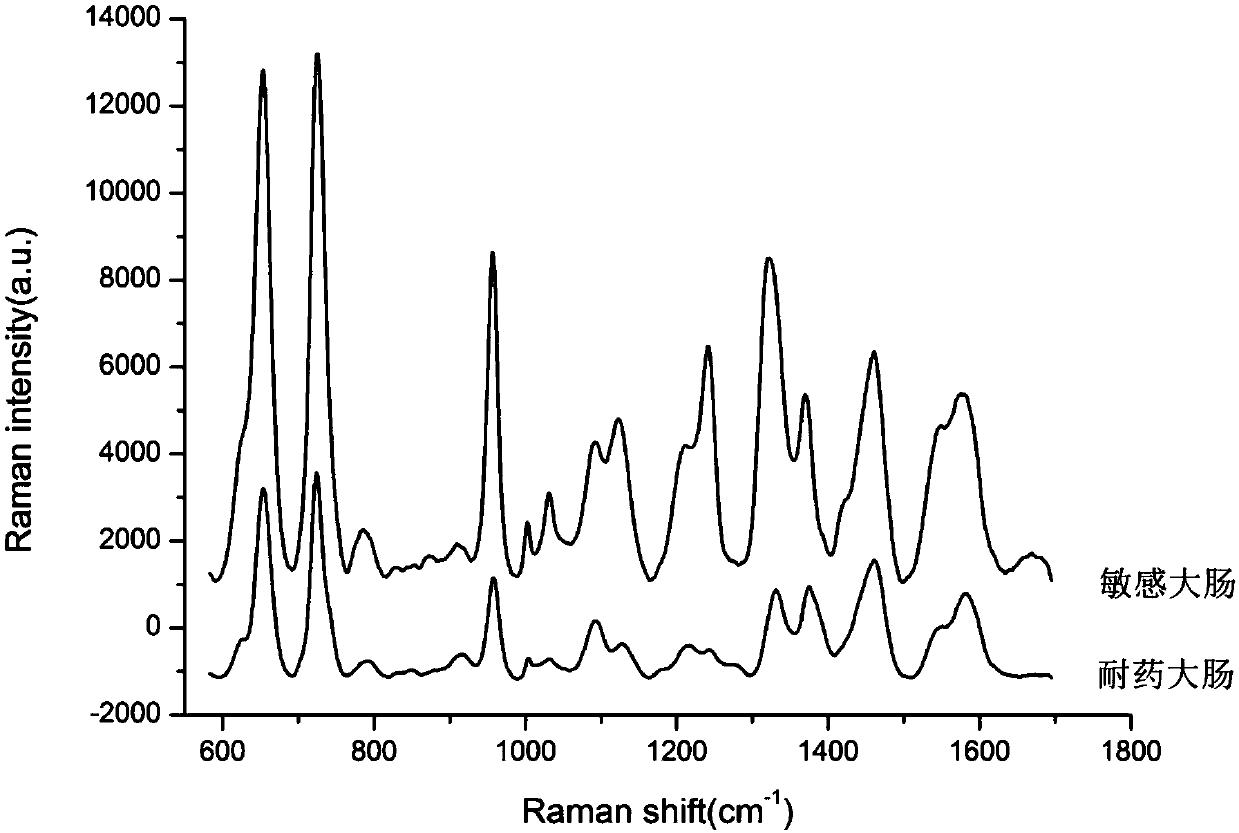
Rapid identification method for carbapenem drug susceptibility, based on Raman spectra technology
InactiveCN107586823AReduce dosageShort detection timeMicrobiological testing/measurementRaman scatteringStructure analysisRapid identification
The invention relates to a rapid identification method for carbapenem drug susceptibility, based on a Raman spectra technology. According to the method, a portable Raman detector is adopted, pathogenic bacteria to be detected is irradiated through laser, different types of nanostructures are taken as detection substrates, and scattered spectrum, the frequency of which is different from the frequency of incident light, is analyzed, so that information, such as molecular vibration, of a sample to be detected is obtained, corresponding molecular structure analysis is performed, obtained spectroscopic data of carbapenem drug sensitive bacteria and drug-resistance bacteria is analyzed through a chemometrics method, so that drug-resistance bacteria and sensitive bacteria are distinguished, and the method becomes an ultrasensitive pathogenic bacteria rapid detection tool. Compared with the clinically traditional drug sensitivity pathogenic microorganism detection method, the detection technology has the advantages that the quantity of samples is less, the detection time is short, the sensitivity is high, the detection time of clinical samples is shortened, and particularly as for detection samples with complex chemical and biochemical components, the detection efficiency is improved on the basis that the detection quality is guaranteed.
Owner:XUZHOU MEDICAL UNIV
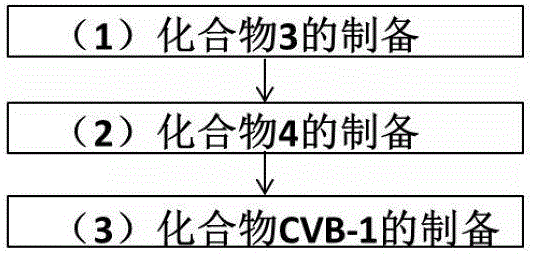
Carbapenem antibiotic resistant bacterium fluorescent probe and synthesis method and application thereof
ActiveCN106811192AHigh detection sensitivityWide detection rangeMicrobiological testing/measurementBiological material analysisSynthesis methodsAntibiotic Y
The invention discloses a carbapenem antibiotic resistant bacterium fluorescent probe. In a structural formula as shown in the specification, X refers to carbon atoms or sulfur atoms; when X is CH, R1 is methyl and can be R or S configuration, or X is CH2 or S; a dye is any one of boron-dipyrromethene, naphthalimides, coumarin, fluorescein or rhodamine. A synthesis method of the fluorescent probe includes steps: (1) preparation of a compound 3; (2) preparation of a compound 4; (3) preparation of a fluorescent probe CVB-1. The fluorescent probe can be made into test paper, kits or detection chips to be applied to detection of carbapenemases and carbapenem drug-resistant bacteria, detection or distinguishing of carbapenemases is realized by determining whether fluorescence intensity or color of the fluorescent probe changes or not, and accordingly pathogenic drug-resistant bacteria with expression of carbapenemases can be detected quickly, reasonable utilization of antibiotics in treatment or clinical application can be guided, and important significance to avoidance or low consumption of antibiotics is achieved.
Owner:EAST CHINA UNIV OF SCI & TECH
1beta-methyl carbapenem compound
ActiveCN101362760AHigh antibacterial activityLow toxicityOrganic active ingredientsOrganic chemistryMethyl carbonMedicinal chemistry
The invention pertains to the technical field of medicine and particularly relates to 1Beta-methyl carbon-substituted penem compound shown as general formula (I), and pharmaceutically acceptable salts, easily hydrolysable ester and isomers of the compound; wherein, R<1>, R<2>, R<3>, R<4>, R<5> and R<6> are defined as the specification. The invention also relates to a preparation method of the compounds, medicine composition containing the compounds as well as application of the compounds to the preparation of medicines treating and / or preventing infectious diseases.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD
Lactic bacteria useful as probiotics
InactiveUS7927584B2Pleasant flavorHealthy digestion and intestineBiocideBacterial antigen ingredientsDiseaseMetabolite
Owner:ALLENDE MIGUEL ANGEL GARCIA
Antibacterial combination and its use
The invention relates to a pharmaceutical composition comprising an ethylene derivatives of tricyclic carbapenems of the general Formula (I) in the form of pure diastereoisomers and in the form of pure geometric isomers or a salt, ester or amide derivate thereof and an antibiotic and the use of this composition as a broad band spectrum β-lactamase inhibitor.
Owner:LEK PHARMA D D
Preparation method of 1Beta-methyl carbapenem antibiotic bicyclic mother nucleus
ActiveCN101723971AHigh yieldImprove qualityGroup 5/15 element organic compoundsWhite powderAntibiotic Y
The invention relates to a preparation method of 1Beta-methyl carbapenem antibiotic bicyclic mother nucleus, comprising the following steps of: preparing a compound (6) with good stereoselectivity by the condensation between a compound (5) and a compound (4), and further hydrolyzing to obtain a compound (3); carrying out condensation reaction, deprotection reaction and diazotization reaction on the compound (3) by adopting a 'one-pot' method, then carrying out the posttreatment and directly crystallizing to obtain a compound (2), finally cyclizing the compound (2) under the catalyst effect and converting into bicyclic ketonicester, and further carrying out active esterification reaction to obtain a white powder solid compound (1). The invention has stable process, simple and convenient operation, easy reaction control and product separation, less three wastes, low cost and up to 46 percent of overall yield, and is suitable for industrialized mass production.
Owner:ZHEJIANG HUABANG MEDICAL & CHEM
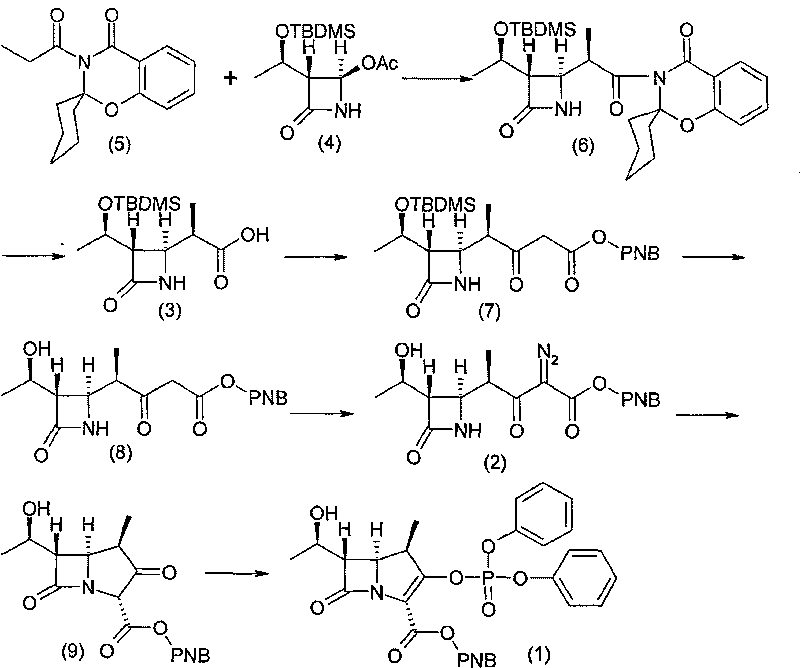
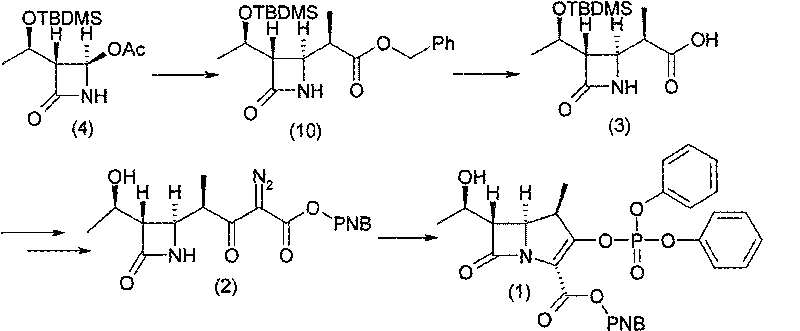
Preparation of carbapenem penicillin ertapenem intermediate
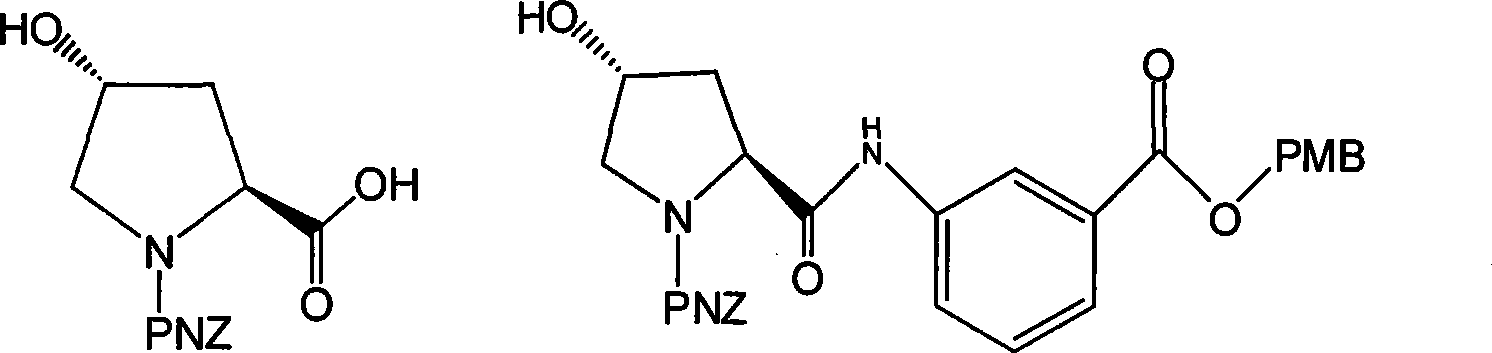
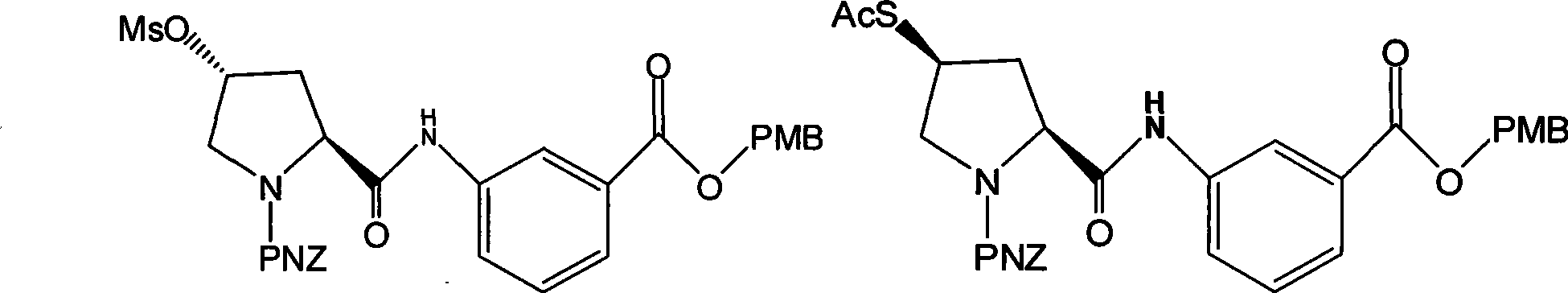
The invention discloses a preparation method of an intermediate for synthesizing carbapenems penicillin etapenem having the formula of VII. The preparation method comprises the following steps: allowing a compound having the formula of I and 4-methoxybenzyl chloride to react to obtain a product; reacting under the action of stannous chloride dehydrate, and regulating pH to 7; performing condensation reaction of above product and activated ester of PNZ L-hydroxyproline; reacting with methylsulfonyl chloride; reacting with potassium thioacetate; and hydrolyzing in acidic or alkaline condition, wherein PMB is shown in figure (1), PNZ is shown in figure (2), Ms is mesyl, and Ac is acetyl. The preparation method can be carried out at the room temperature, with the advantages of mild reaction condition, low cost, and easily realized industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Carbapenem compounds
A compound or its pharmaceutically acceptable salt represented by the following formula:The invention is a carbapenem compound which has a potent antibacterial activity over a broad range of Gram negative and Gram positive bacteria, especially penicillin-resistant Streptococcus pneumoniae (PRSP) which has been isolated at an elevated frequency in recent years and thus causes a serious clinical problem, and Haemophilus influenzae which has acquired resistance against the existing β-lactam antibiotics over a wide scope due to penicillin-binding protein (PBP) mutations such as β-lactamase non-producing ampicillin-resistant (BLNAR) Haemophilus influenzae, and has excellent oral absorbability.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com