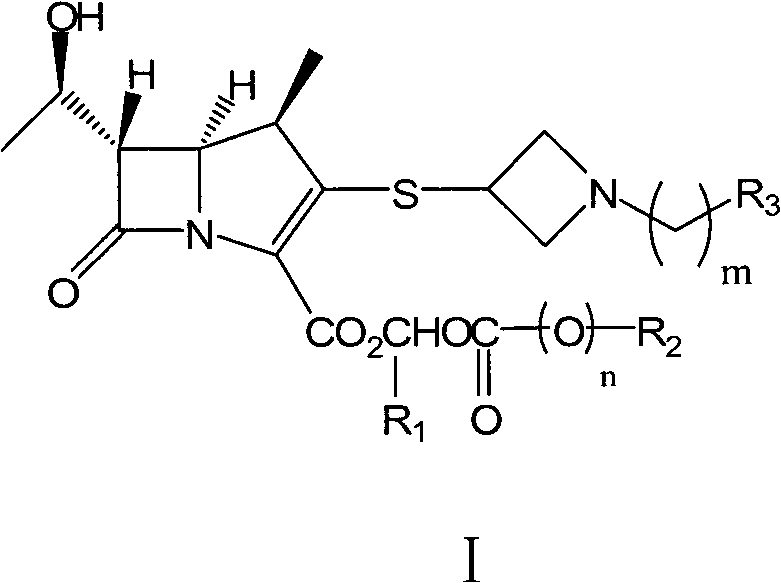
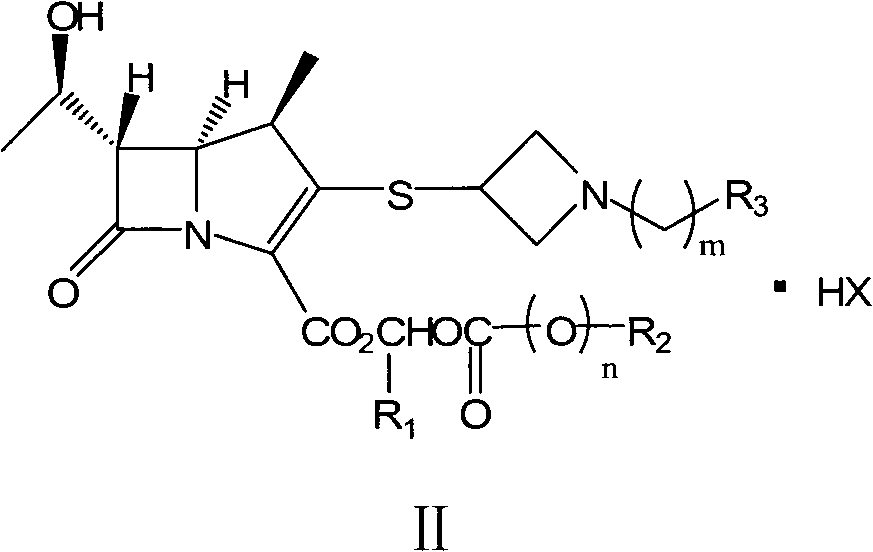
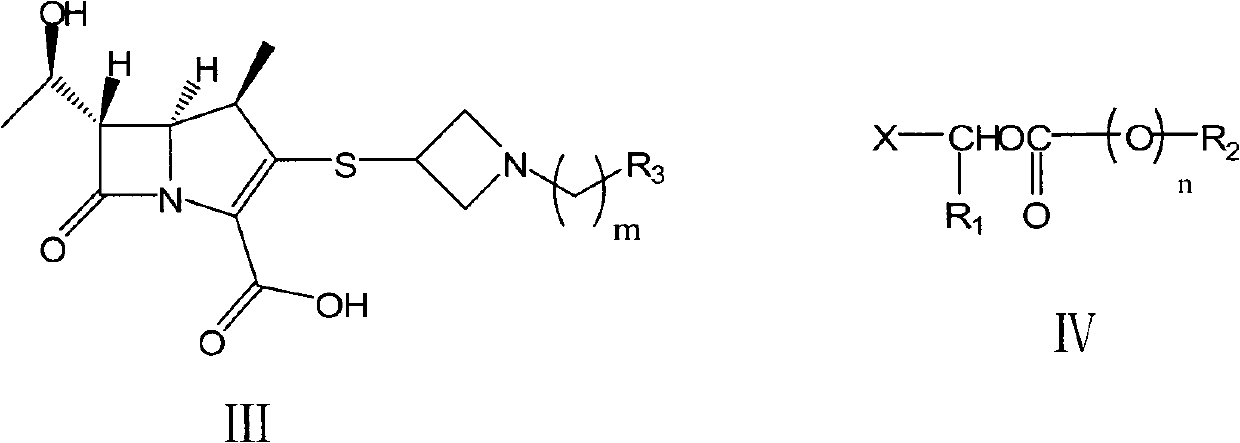
Preparation method of carbapenems
A technology of carbapenem and carbapenem, applied in the field of preparation of carbapenem antibiotics, can solve the problems of unavailable raw materials, unfavorable industrial production, and long cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Pivaloyloxymethyl (1R, 5S, 6S)-1-methyl-6-[(R)-1-hydroxyethyl]-2-[1-(1,3-thiazoline- Preparation of 2-base) azetidin-3-yl] thio-1-carbapenem-2-ene-3-carboxylate (tybipenem ester, compound of formula I-1)
[0112] Method A:
[0113] (1) Preparation of tibipenem ester hydrochloride (compound of formula II-1)
[0114] Add tibipenem (compound of formula III-1) 100.0 g (0.26 mol), NaHCO 3 32.9g (0.39mol), chloromethyl pivalate (compound of formula IV-1) 56.3mL (0.39mol), DMF 500mL, stirred at 60°C for 4h. DMF was distilled off under reduced pressure, and 200 mL of acetone was added and stirred for 0.5 h. Filter, wash the filter cake with 50 mL of acetone × 2, and vacuum-dry to obtain 140.8 g of off-white solids of tibipenem ester hydrochloride (compound of formula II-1), with a molar yield of 86.3% and a chromatographic purity of 99.6%.
[0115] 1 H NMR (DMSO, 500MHz) δ: 10.80(s, 1H), 5.89(d, J=6.0Hz, 1H), 5.76(d, J=6.0Hz, 1H), 5.14(s, 1H), 4.76~4.79 (m, 2H...
Embodiment 2
[0129] Example 2: 1-methylcyclohexyloxymethyl (1R, 5S, 6S)-1-methyl-6-[(R)-1-hydroxyethyl]-2-[1-(1,3 -thiazoline-2-yl) azetidin-3-yl] the preparation of thio-1-carbapenem-2-ene-3-carboxylate (formula I-2 compound)
[0130] Method A:
[0131] (1) Preparation of formula I-2 compound hydrochloride (II-2)
[0132] Add tibipenem (compound of formula III-1) 120.0g (0.31mol), triethylamine 86.2g (0.62mol), 1-methylcyclohexyloxymethyl chloride (formula III-1 compound) successively into a 1L four-neck flask Compound IV-2) 118.2g (0.62mol), DMF 600mL, stirred at 50°C for 4h. DMF was distilled off under reduced pressure, and 200 mL of acetone was added and stirred for 0.5 h. After filtration, the filter cake was washed with 50 mL of acetone x 2, and dried in vacuo to obtain 158.6 g of the title compound as an off-white solid, with a molar yield of 88.3% and a chromatographic purity of 98.6%.
[0133] (2) preparation of formula I-2 compound
[0134] Add 180.0 g of the compound of for...
Embodiment 3
[0145] Example 3: Pivaloyloxymethyl (1R, 5S, 6S)-1-methyl-6-[(R)-1-hydroxyethyl]-2-[1-(4-fluorobenzyl)nitrogen The preparation of heterocyclobutan-3-yl]thio-1-carbapenicill-2-ene-3-carboxylate (compound of formula I-5)
[0146] Method A:
[0147] (1) Preparation of formula I-5 compound hydrochloride (formula II-5 compound)
[0148] Add (1R, 5S, 6S)-1-methyl-6-[(R)-1-hydroxyethyl]-2-[1-(4-fluorobenzyl)azepine sequentially into a 1L four-necked flask Cyclobutane-3-yl]thio-1-carbapenem-2-ene-3-carboxylic acid (compound of formula III-2) 80.0g (0.20mol), DIPA 56.2mL (0.40mol), pivalic acid Chloromethyl ester (compound of formula IV-1) 57.7mL (0.40mol), DMF 650mL, stirred at 50°C for 8h. DMF was distilled off under reduced pressure, and 200 mL of acetone was added and stirred for 1 h. After filtering, the filter cake was washed with 100 mL of acetone × 2, and dried under vacuum to obtain 83.3 g of the title compound as an off-white solid, with a molar yield of 64.4% and a chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com