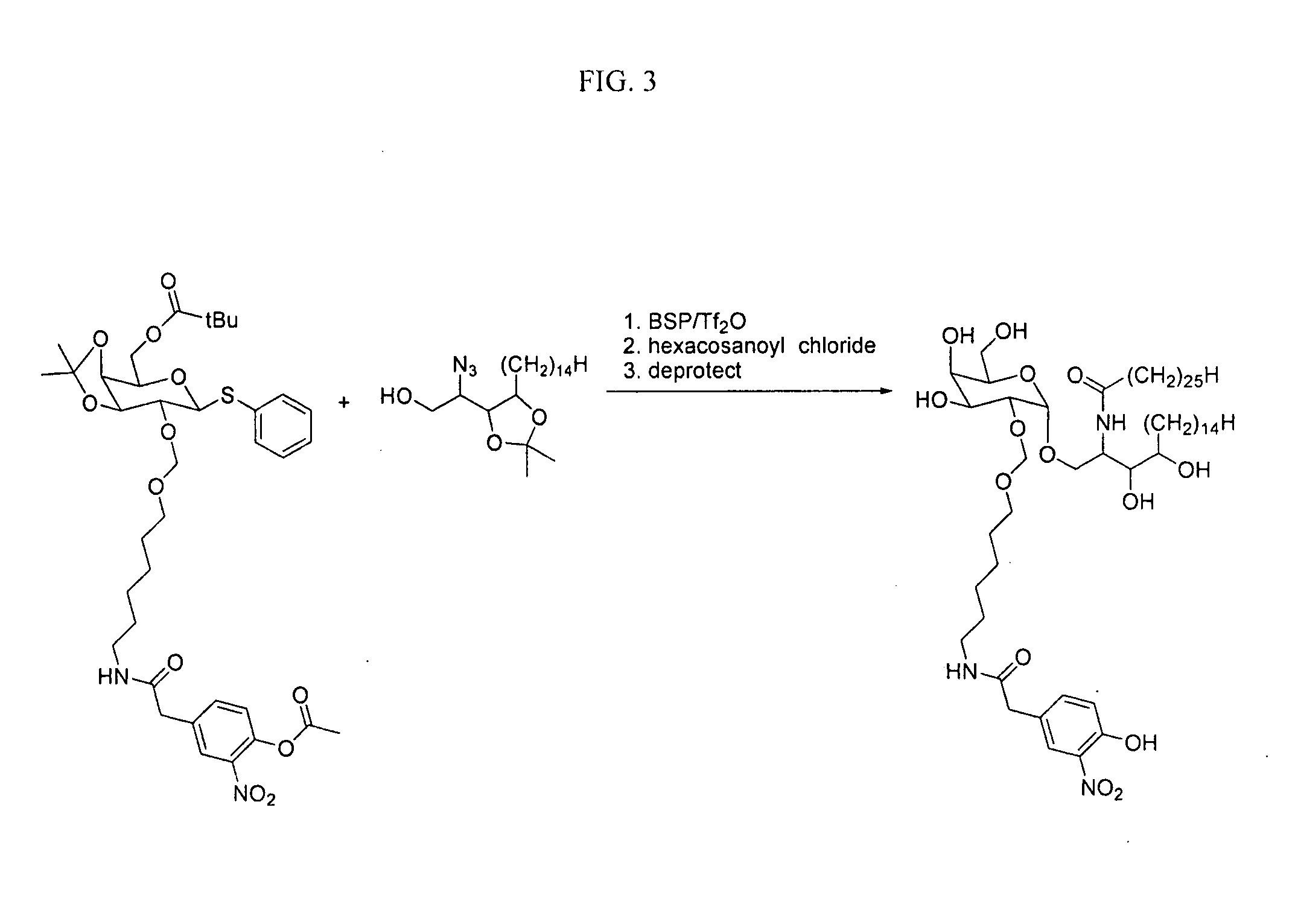
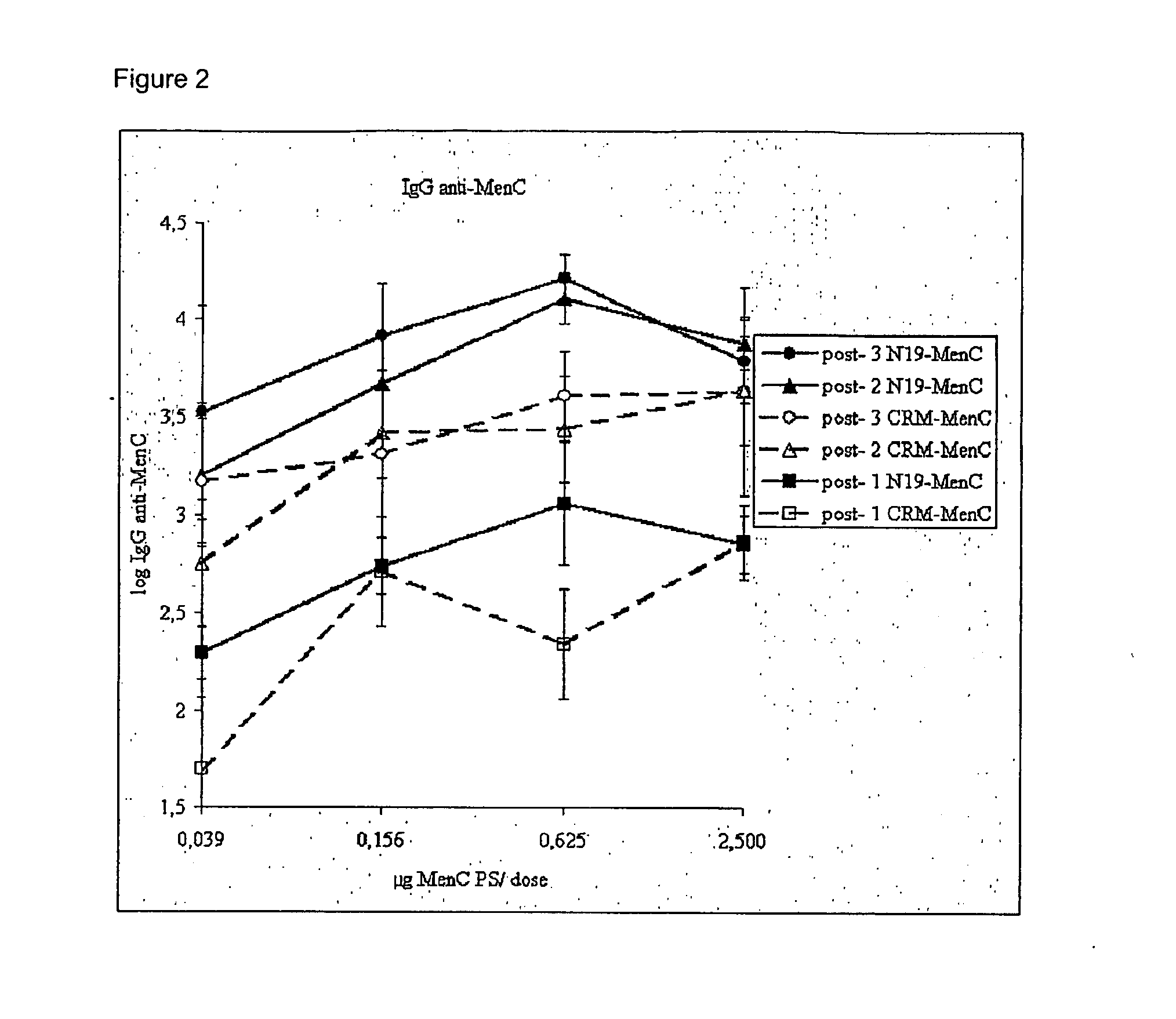
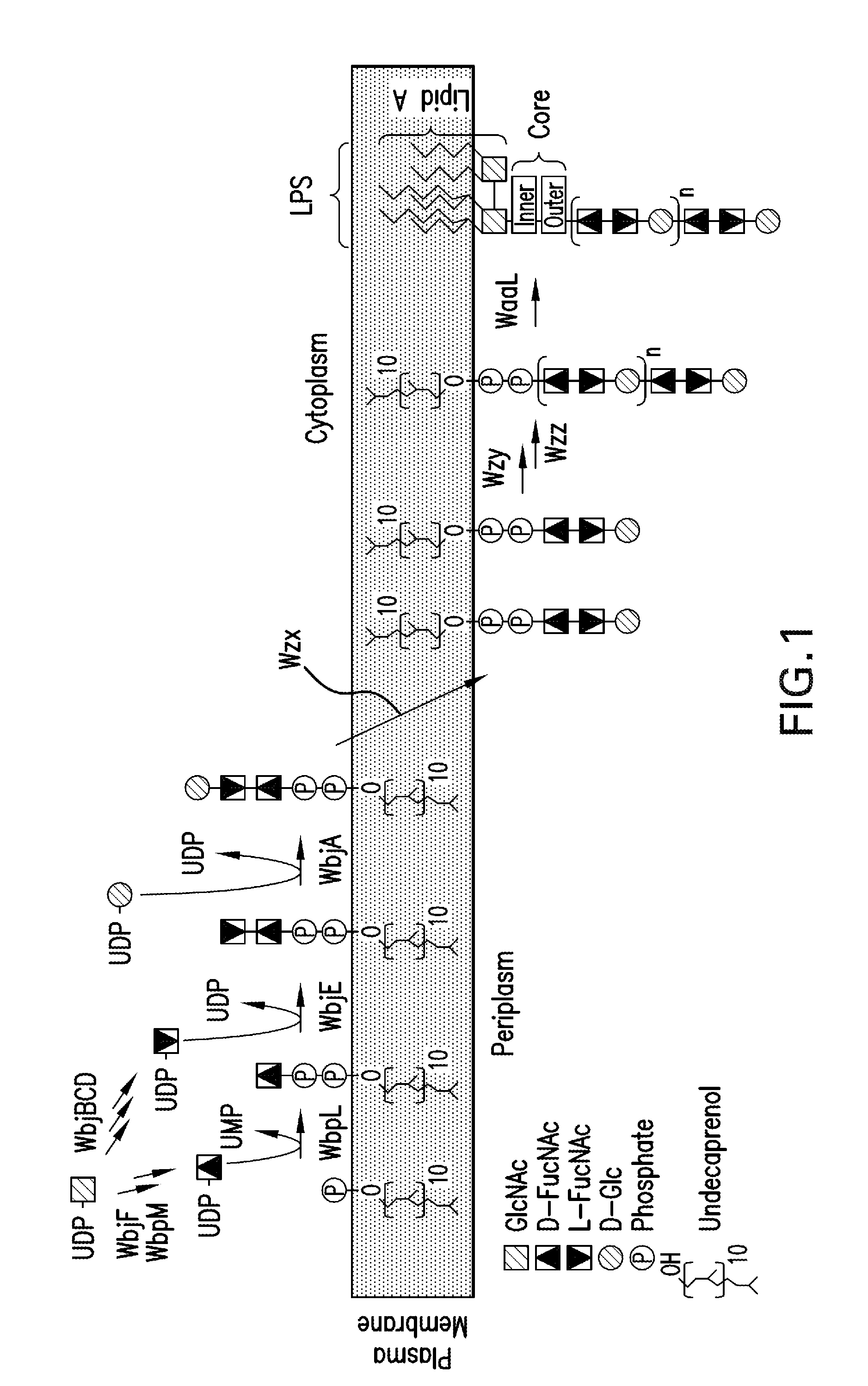
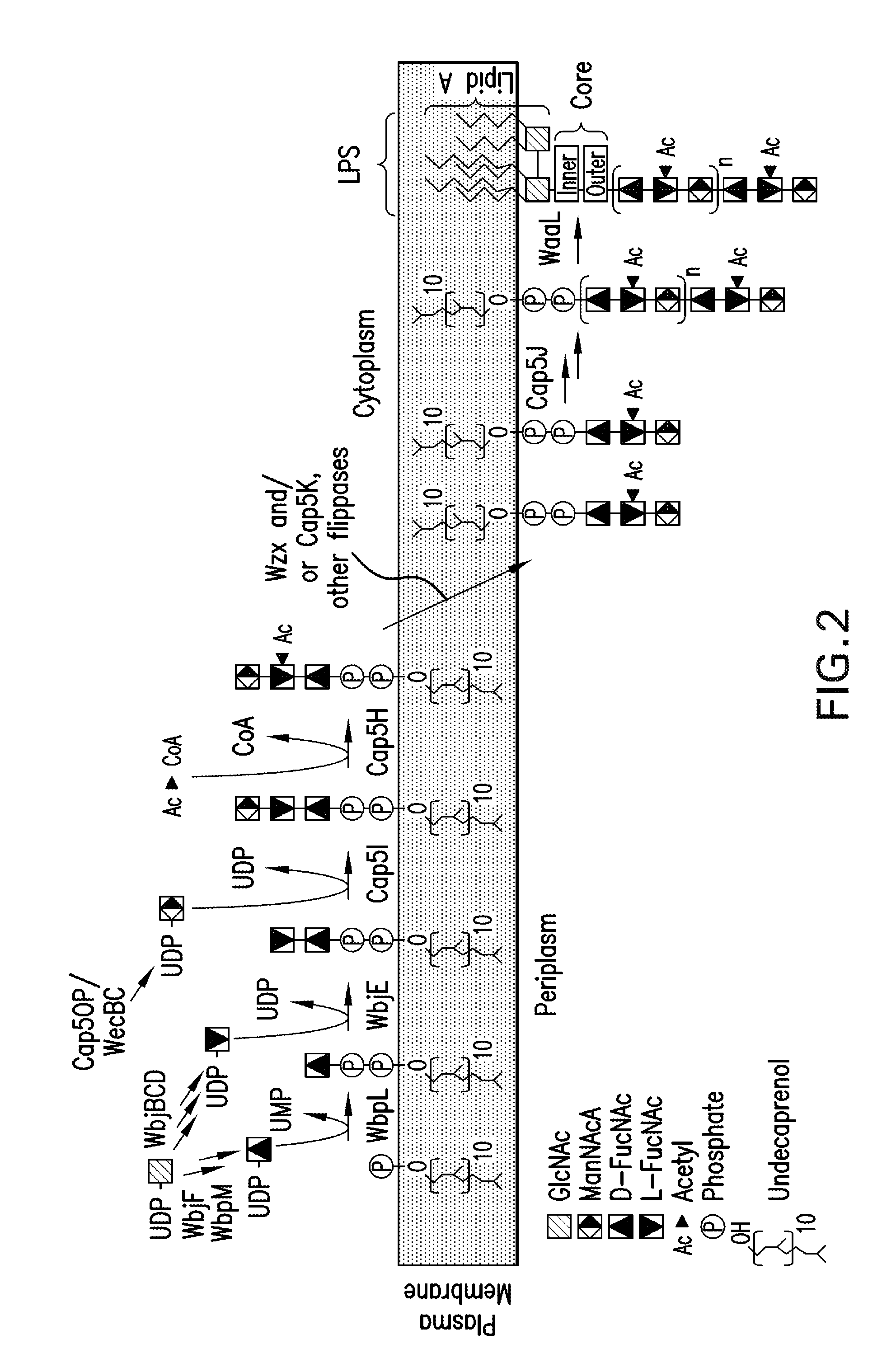
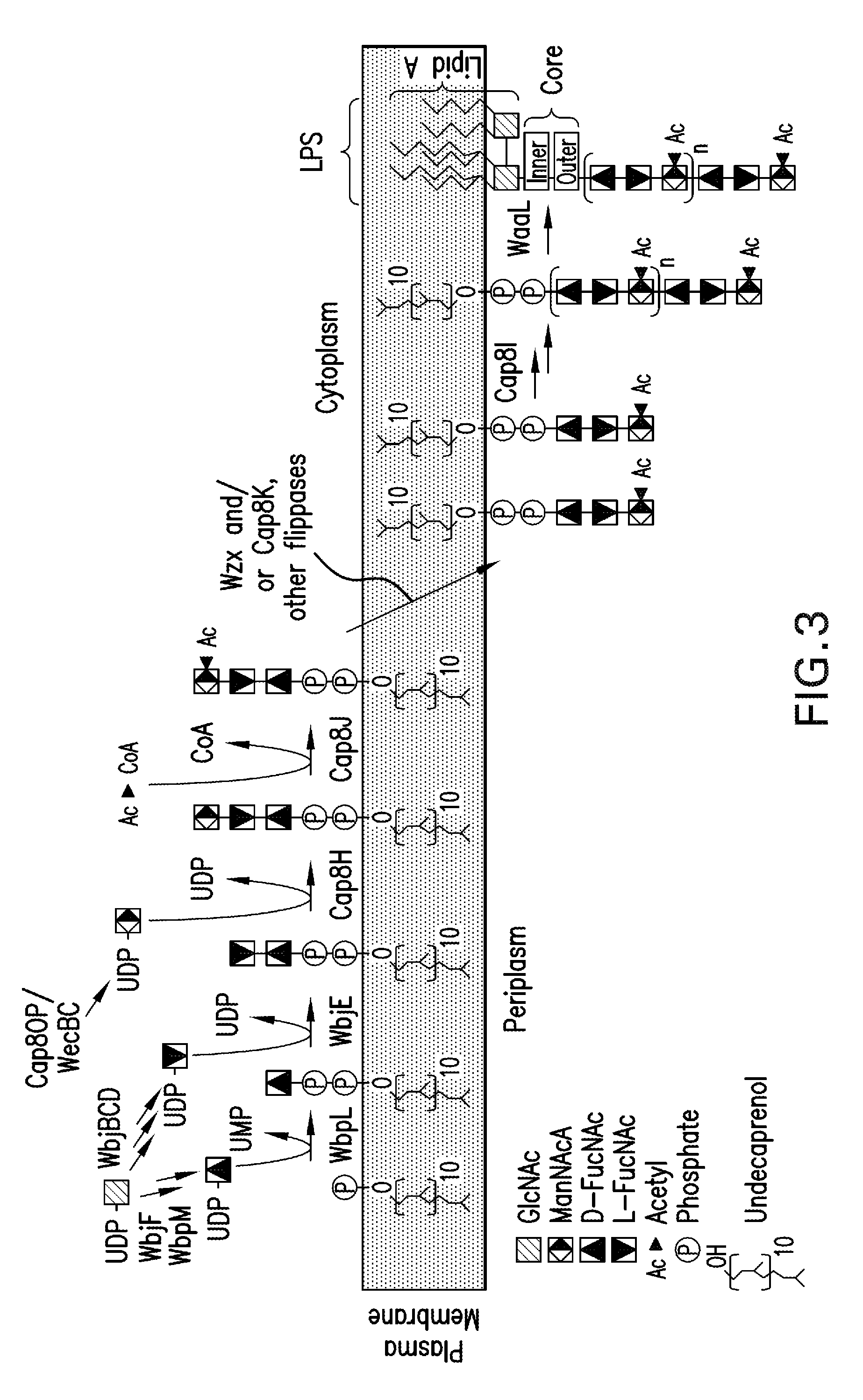
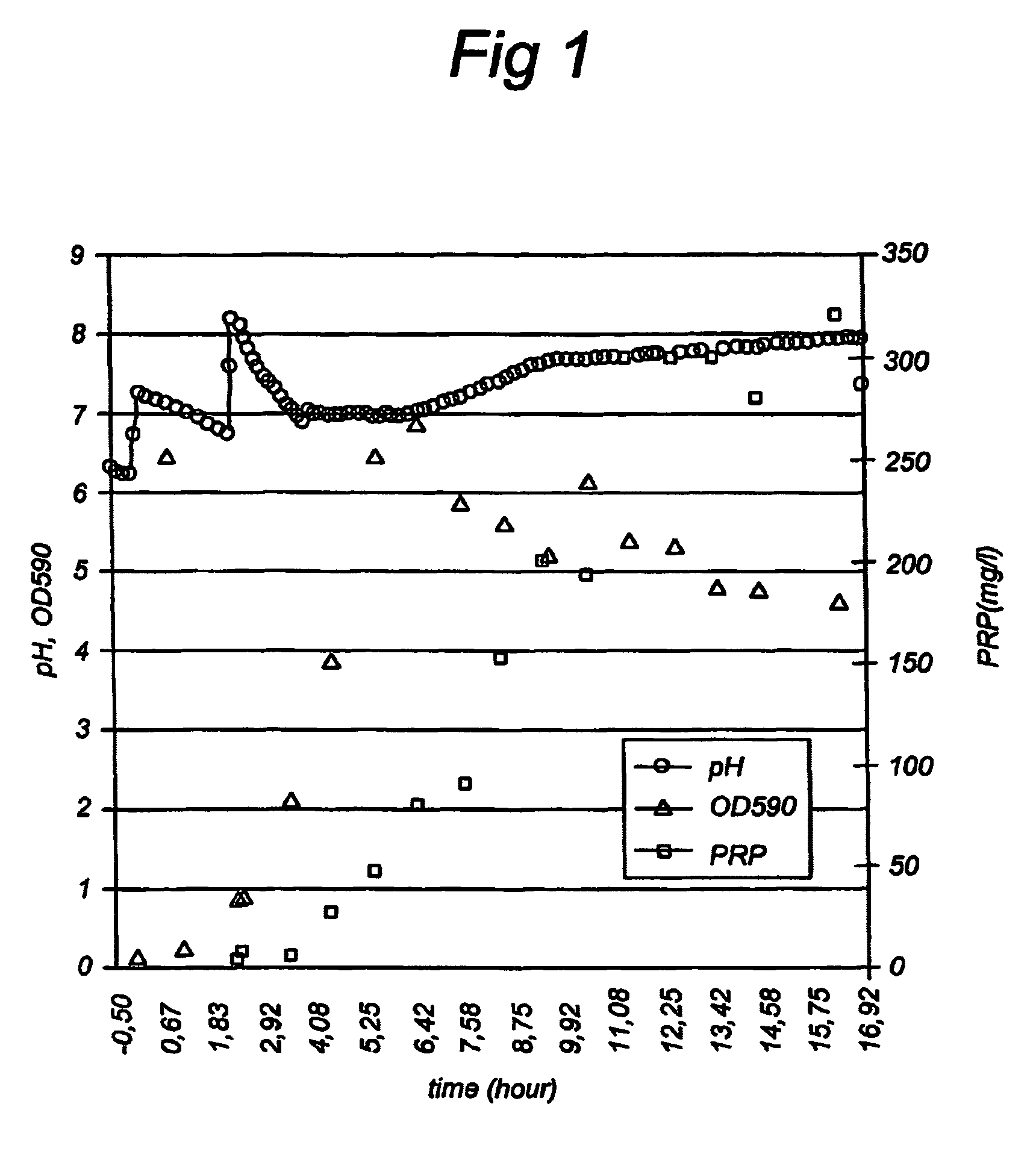
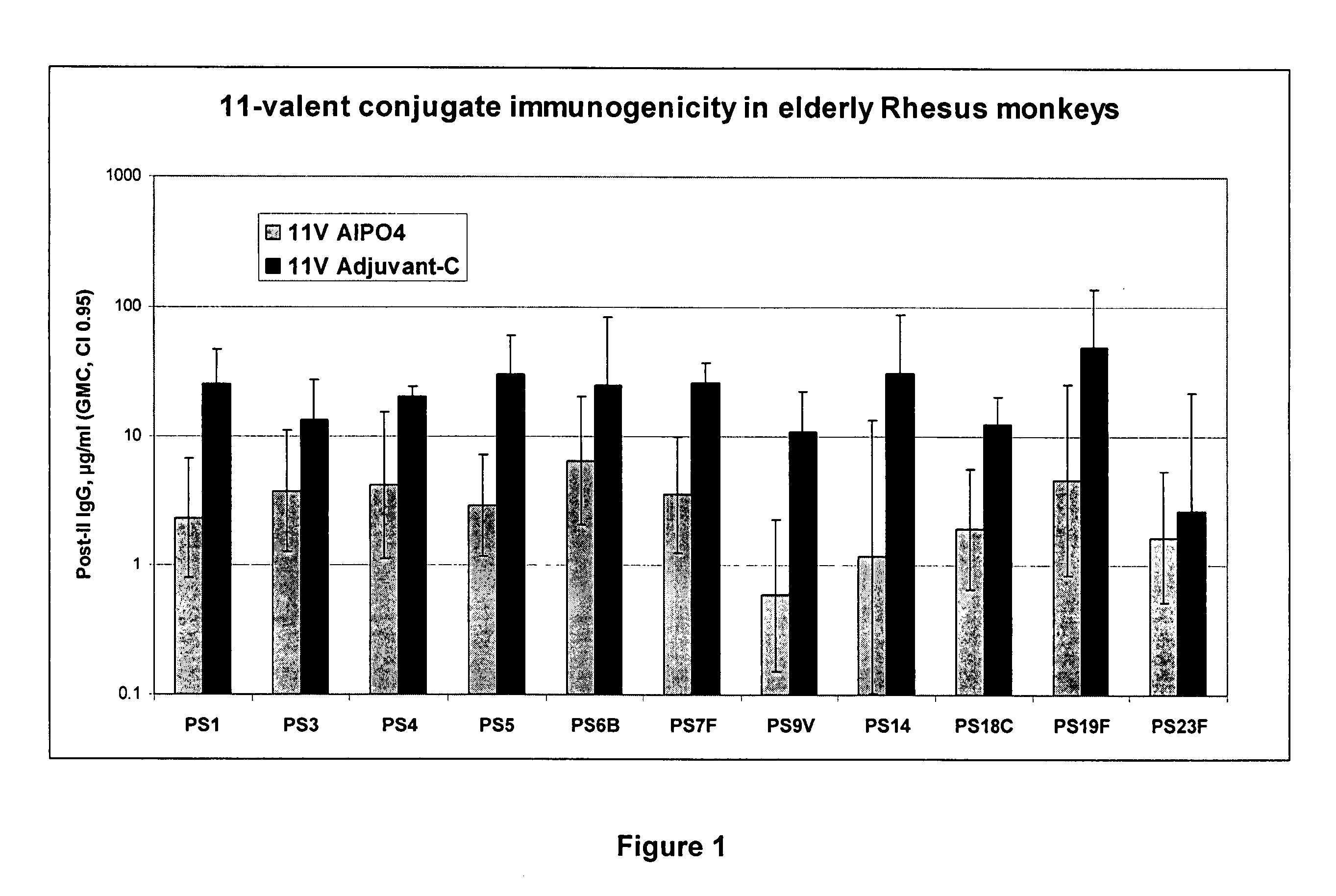
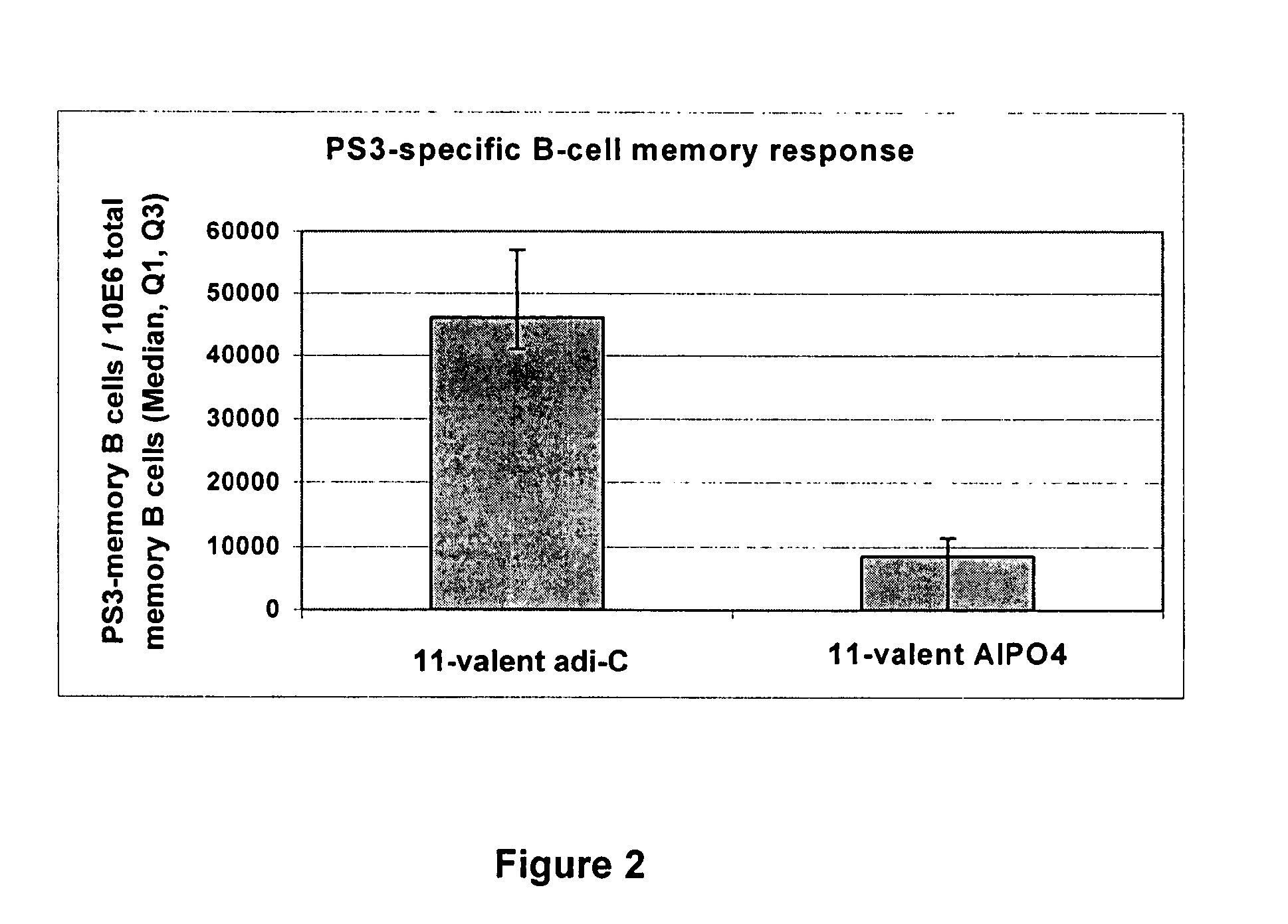
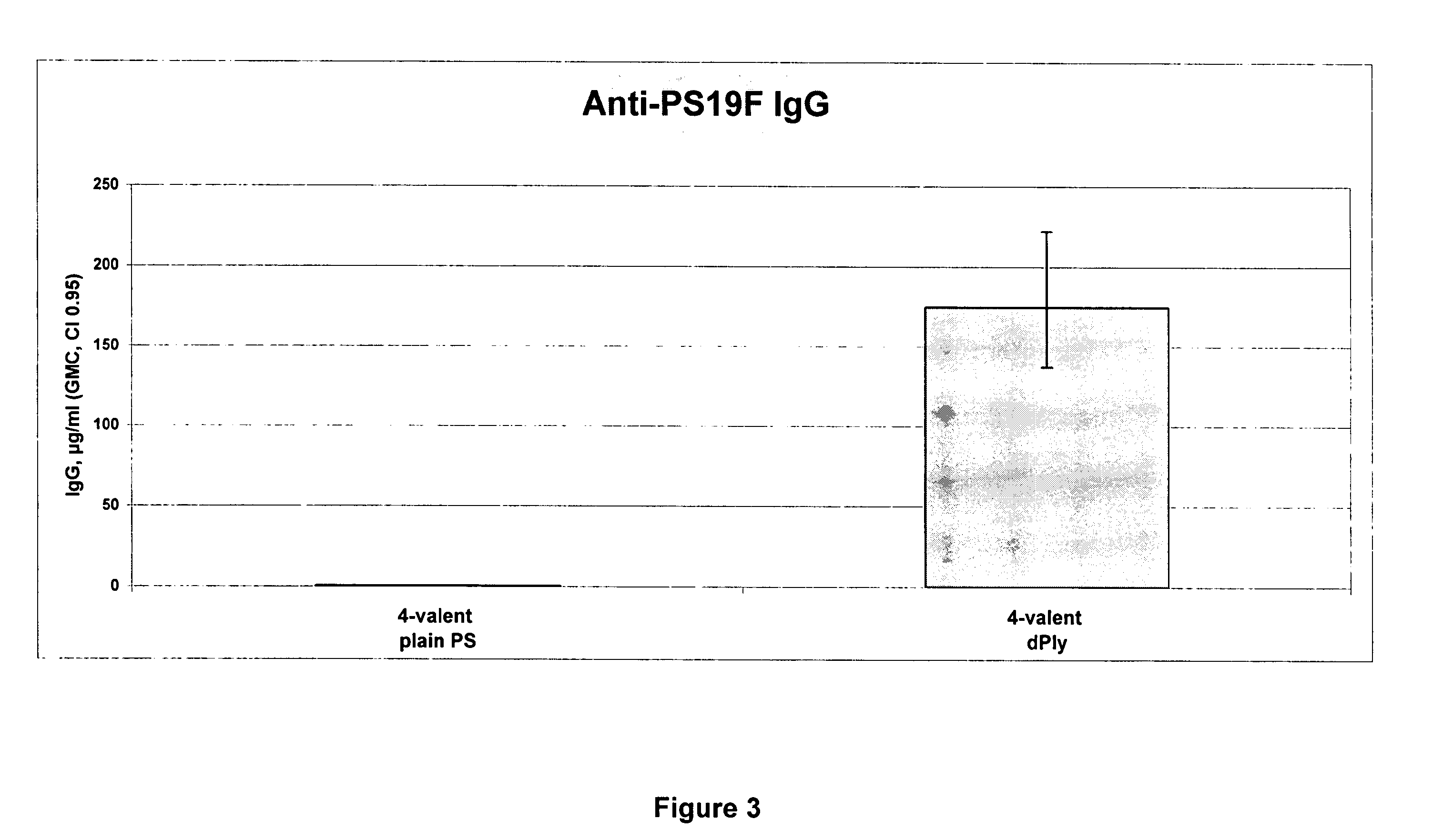
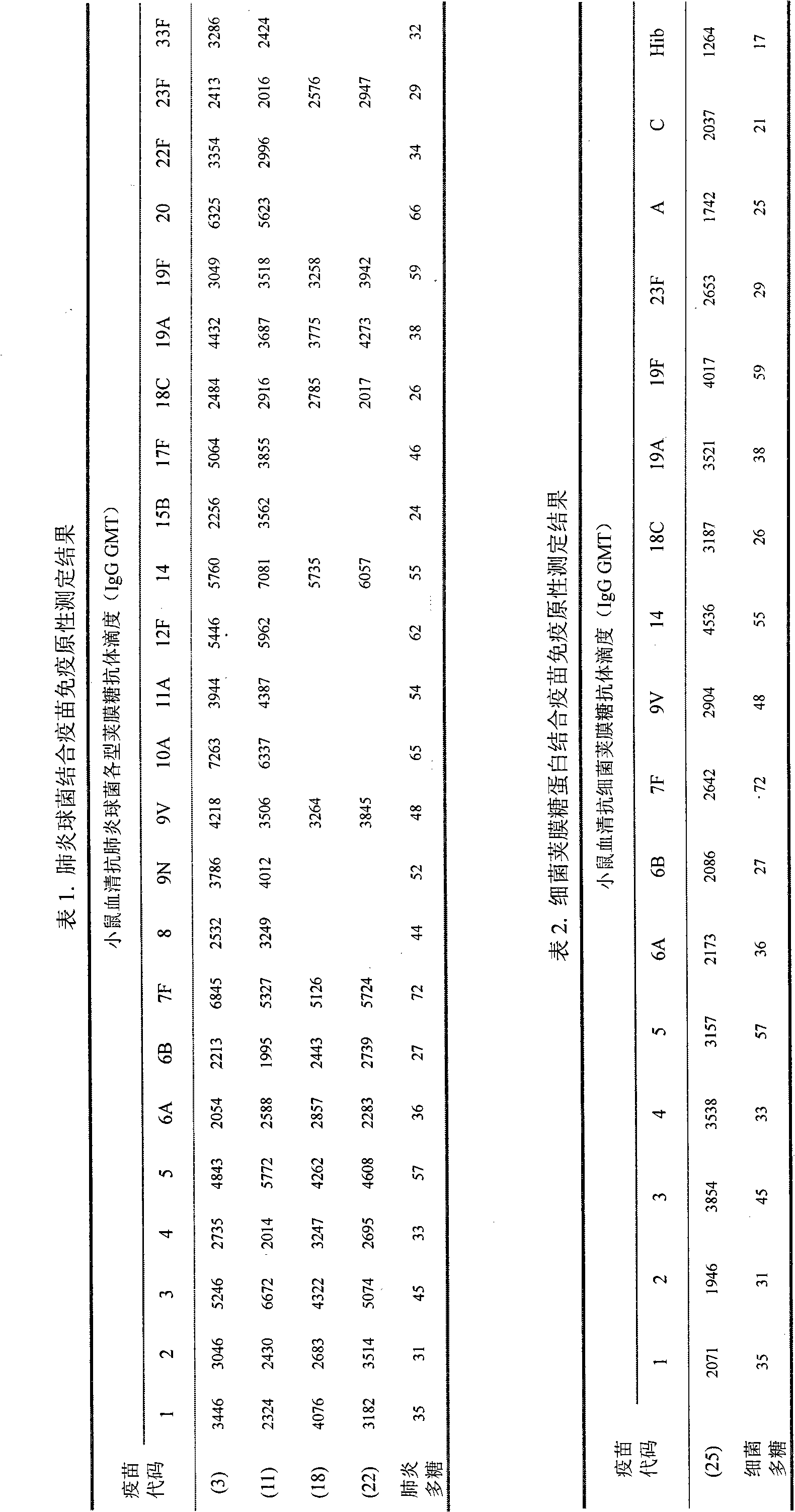
Patents
Literature
160 results about "Conjugate vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Conjugate vaccines combine a weak antigen with a strong antigen as a carrier so that the immune system has a stronger response to the weak antigen. Vaccines are used to prevent diseases by invoking an immune response to an antigen, the foreign part of a bacteria or virus that the immune system recognizes. This is usually accomplished with an attenuated or dead version of a pathogenic bacterium or virus in the vaccine, so that the immune system can recognize the antigen later in life. Many vaccines contain a single antigen that the body will recognize.
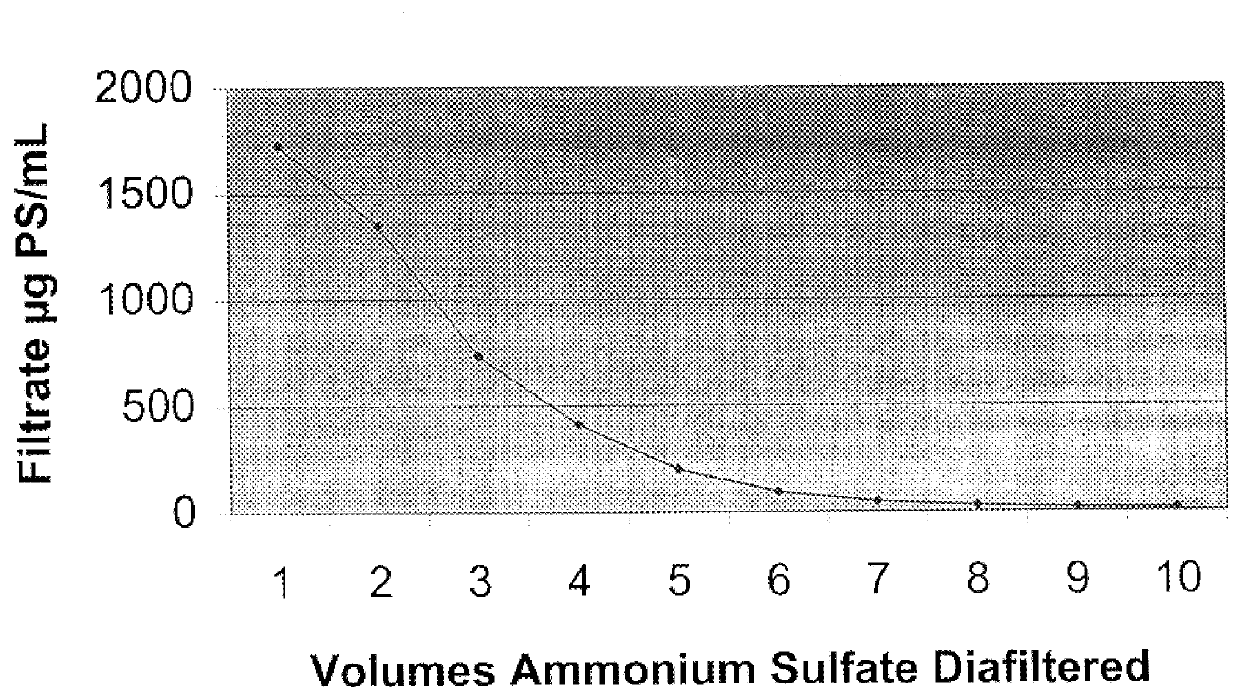
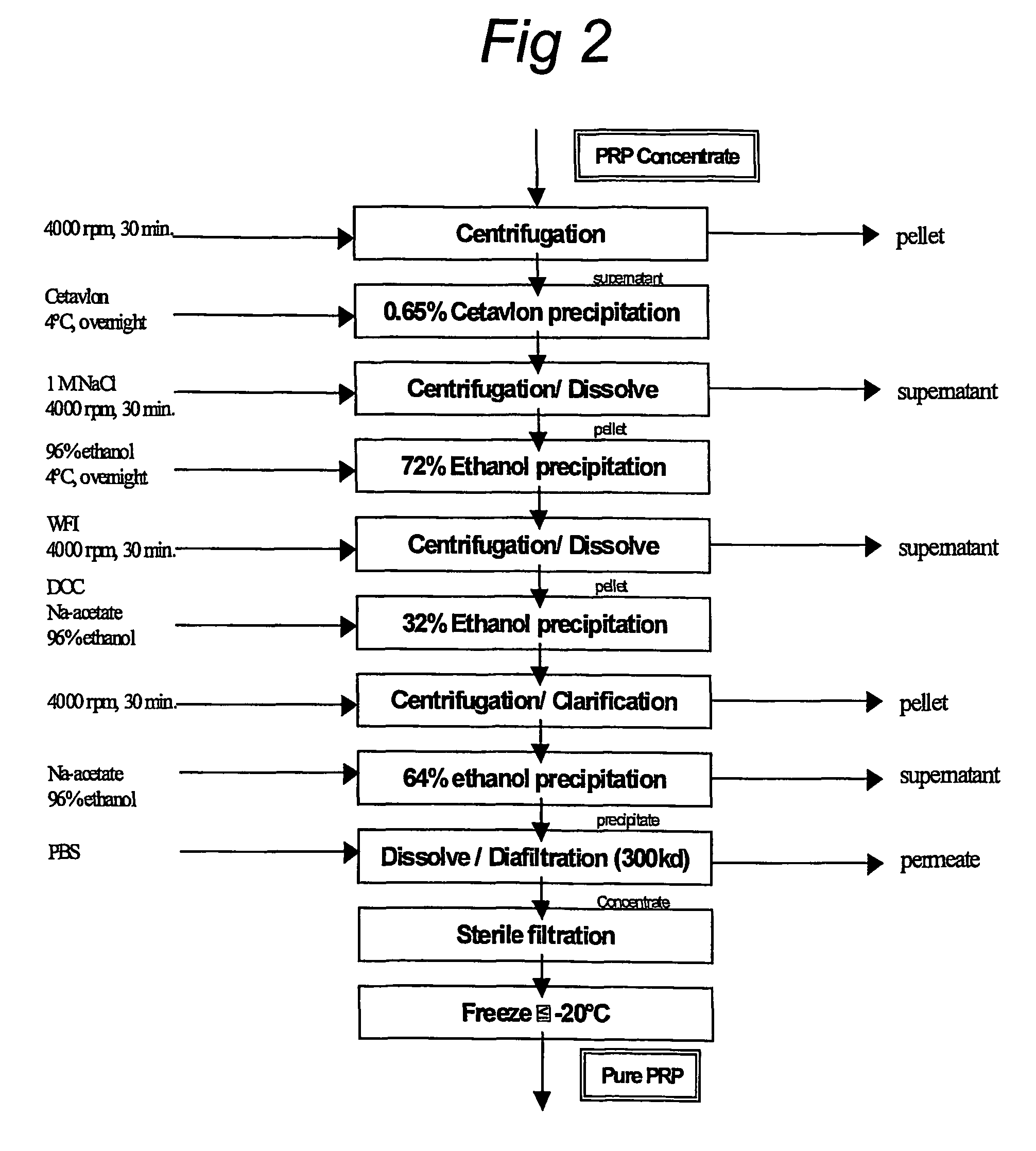
Purification of polysaccharide-protein conjugate vaccines by ultrafiltration with ammonium sulfate solutions
InactiveUS6146902AImprove scalabilityLevel of purityAntibacterial agentsSugar derivativesConjugate vaccineUltrafiltration
Disclosed and claimed are a method for the purification of polysaccharide-protein conjugate vaccines by ultrafiltration in a saturated solution of ammonium sulfate. The ultrafiltration method of the present invention provides an efficient, readily scalable method for removal of unbound polysaccharides from polysaccharide-protein vaccines, thereby improving the purity and consistency of the polysaccharide-protein vaccines.
Owner:AVENTIS PASTUER LTD
Conjugate vaccine for Neisseria meningitidis
InactiveUS6531131B1Bacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsConjugate vaccineLacto-N-tetraose
A conjugate vaccine for Neisseria meningitidis comprising lipooligosaccharide which does not contain a lacto-N-tetraose antigen from which at least one primary O-linked fatty acid has been removed conjugated to an immunogenic carrier. The vaccine is useful for prevention of meningitis and septic shock in mammals.
Owner:THE GOVERNMENT OF THE UNITED STATES REPRESENTED BY SEC DEPT OF HEALTH & HUMAN SERVICES
Use of amino-oxy functional groups in the preparation of protein-polysaccharide conjugate vaccines
InactiveUS20050169941A1Antibacterial agentsBacterial antigen ingredientsConjugate vaccineCompound (substance)
The invention relates to a process for preparing a conjugate comprising combining an amino-oxy homofunctional or heterofunctional reagent with an entity chosen from polysaccharides, oligosaccharides, carbohydrates, and carbohydrate-containing molecules containing at least one carbonyl group, to form a polysaccharide, oligosaccharide, carbohydrate, or carbohydrate-containing molecule functionalized via at least one oxime linkage. The functionalized compound is then reacted either directly or indirectly with a protein moiety to form a protein-carbohydrate conjugate that may be used as a vaccine.
Owner:FINA BIOSOLUTIONS
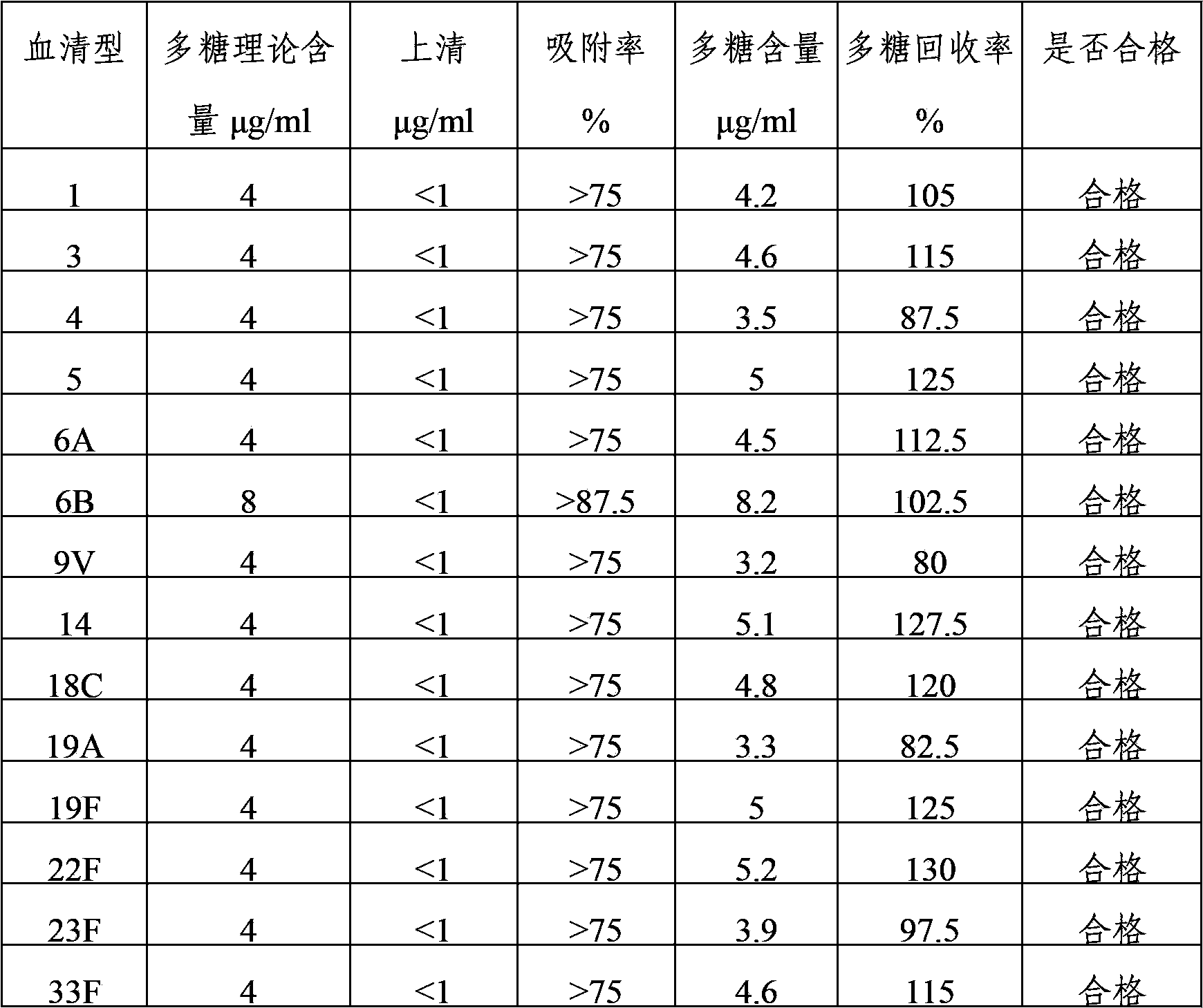
15-valent pneumococcal polysaccharide-protein conjugate vaccine composition
ActiveUS20110195086A1Antibacterial agentsBacteria material medical ingredientsConjugate vaccineDisease
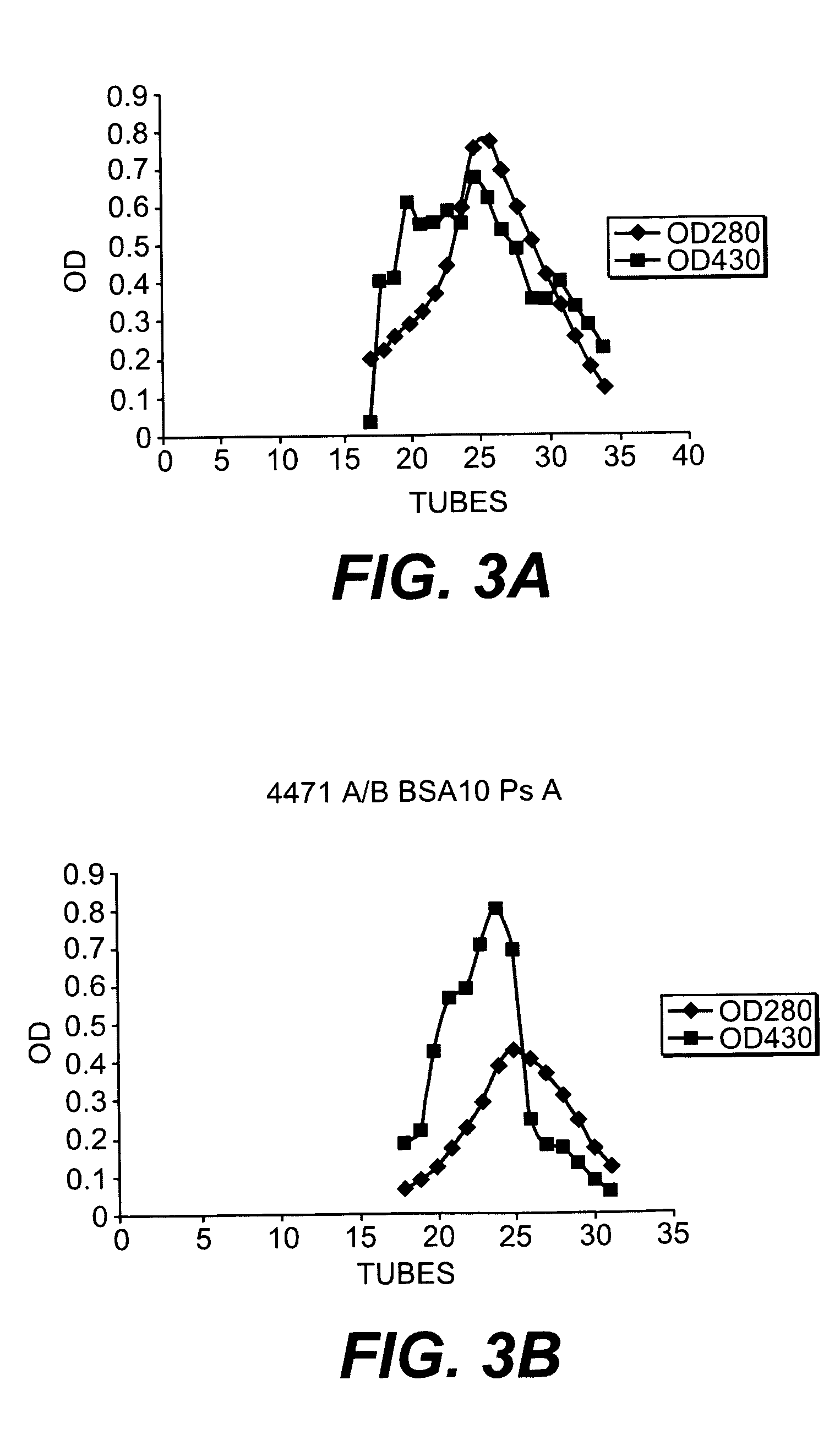
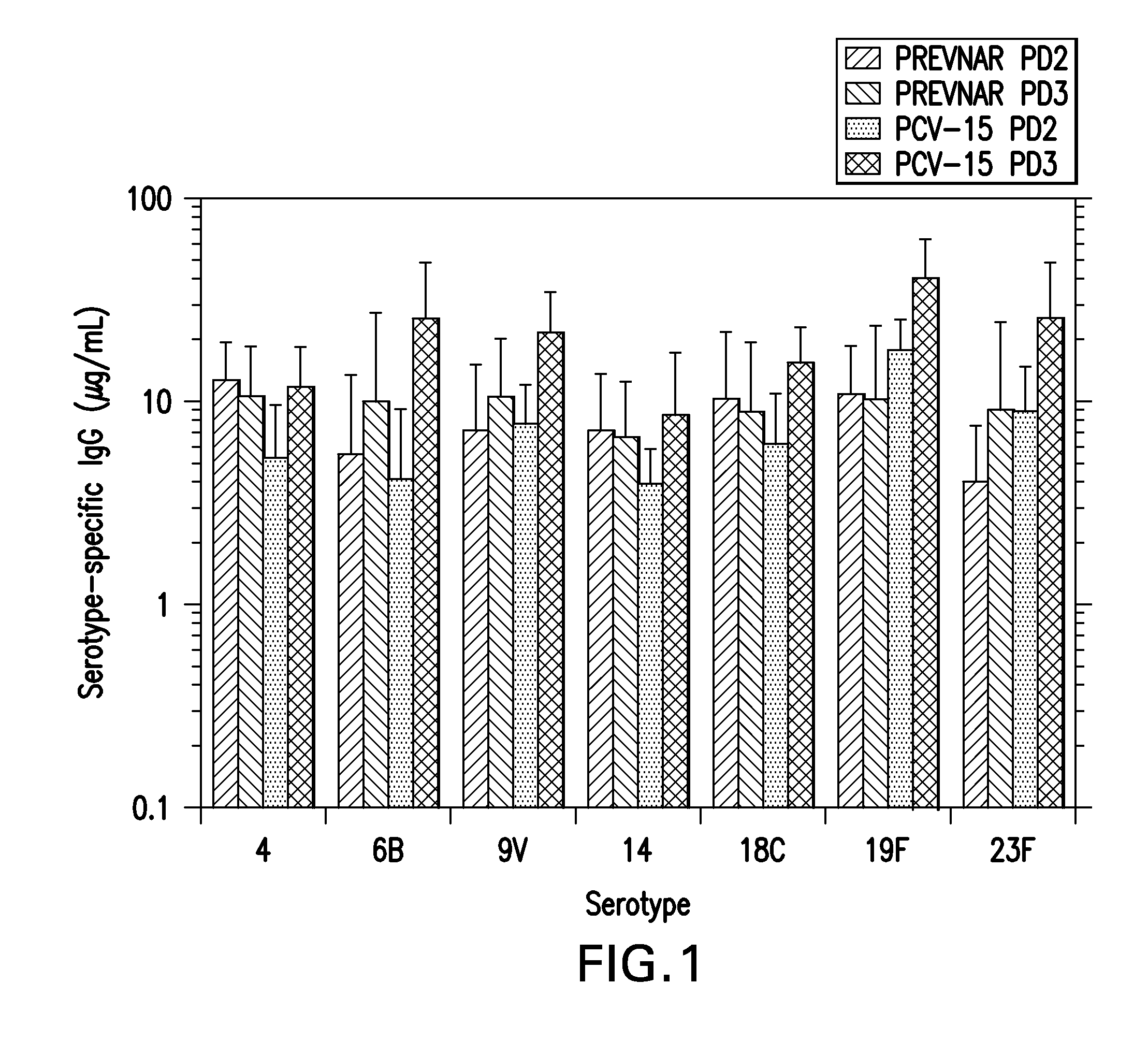
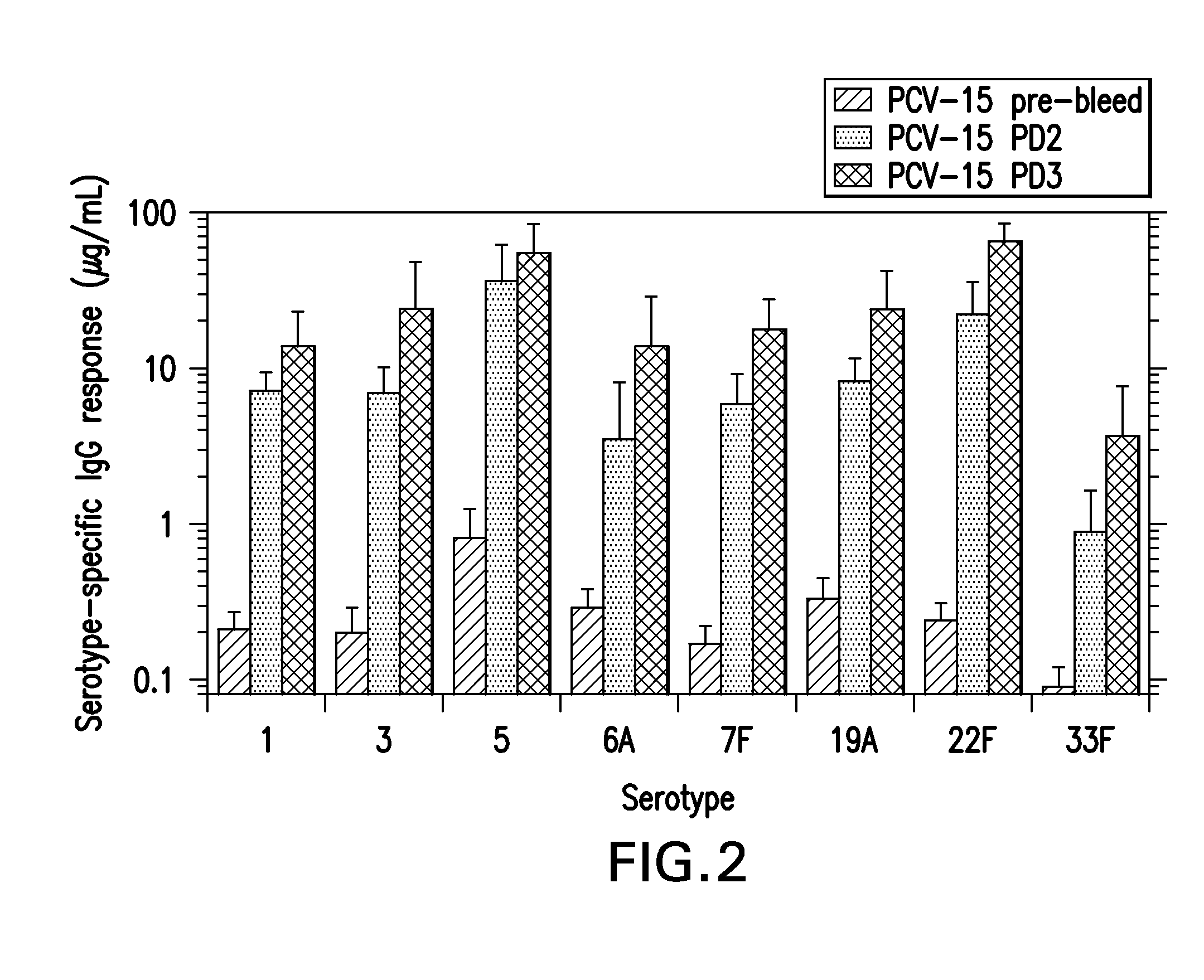
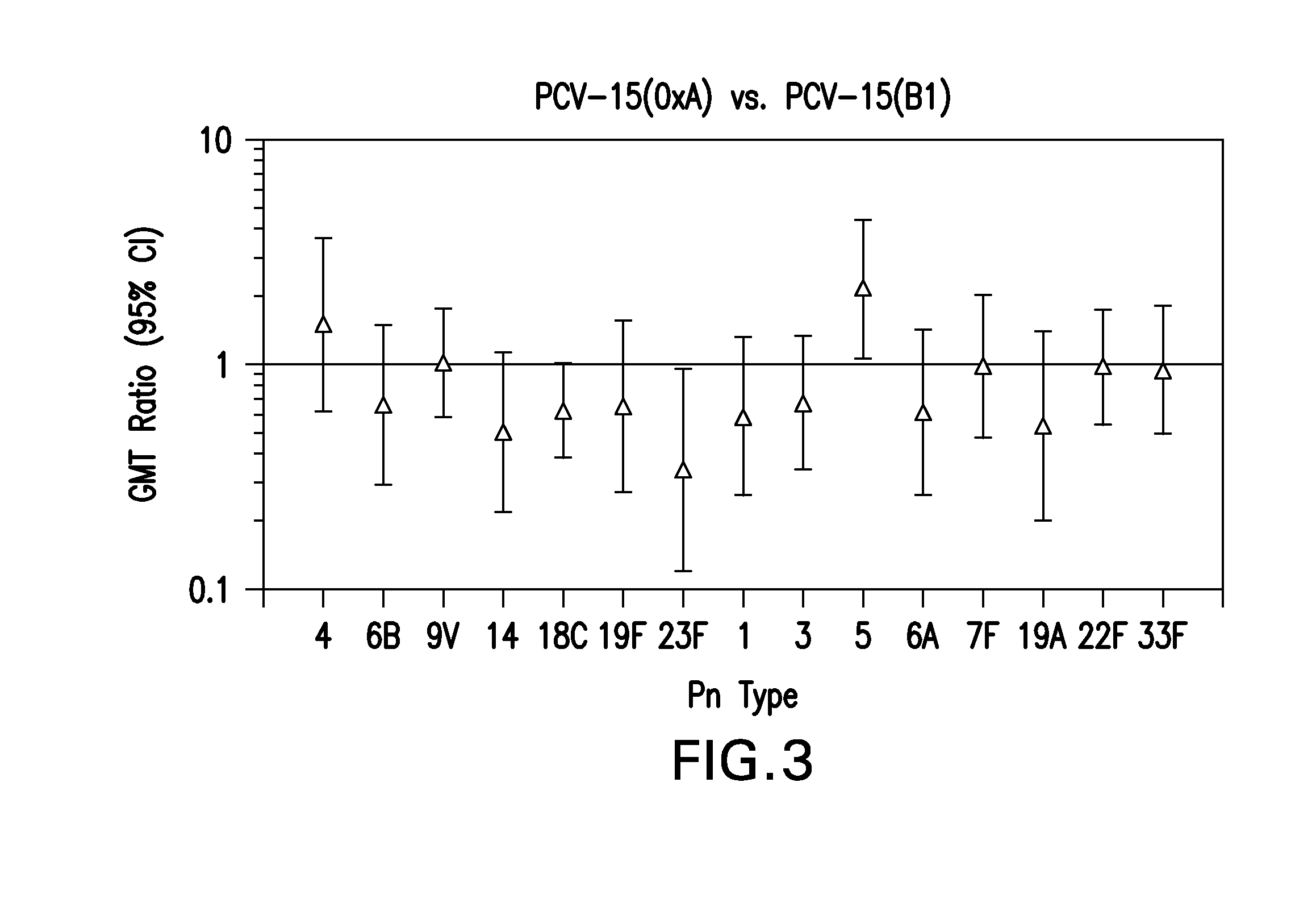
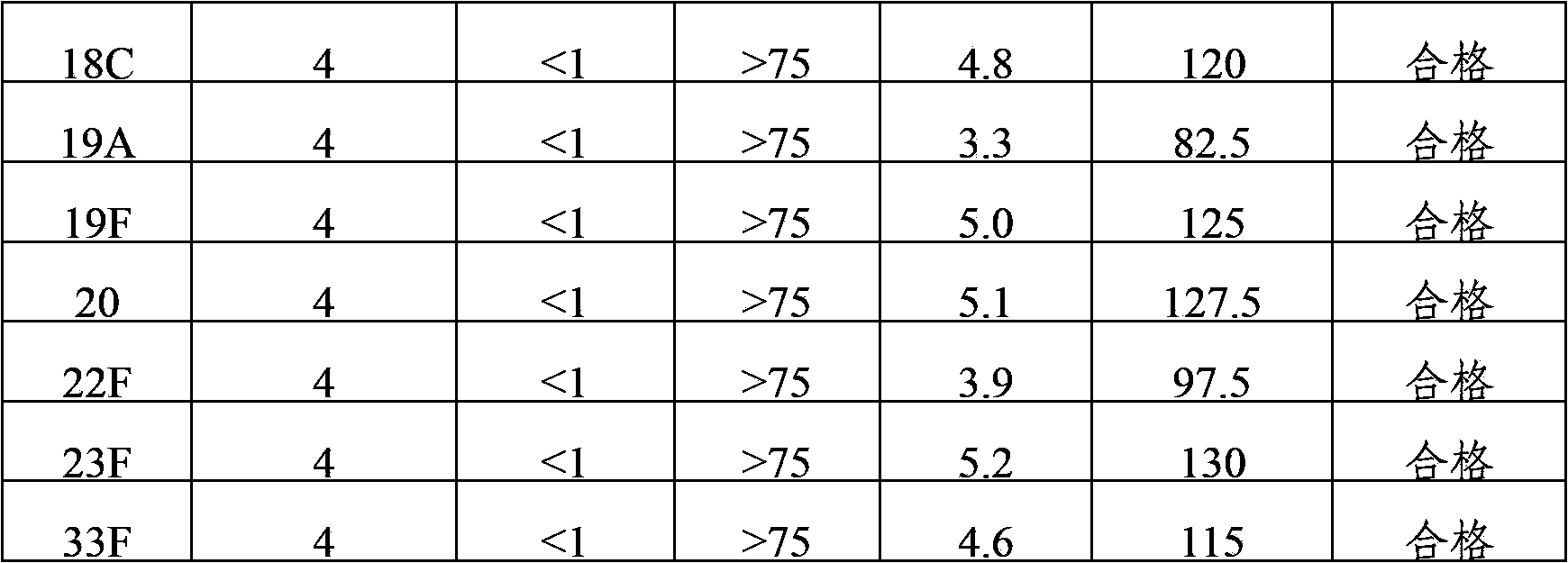
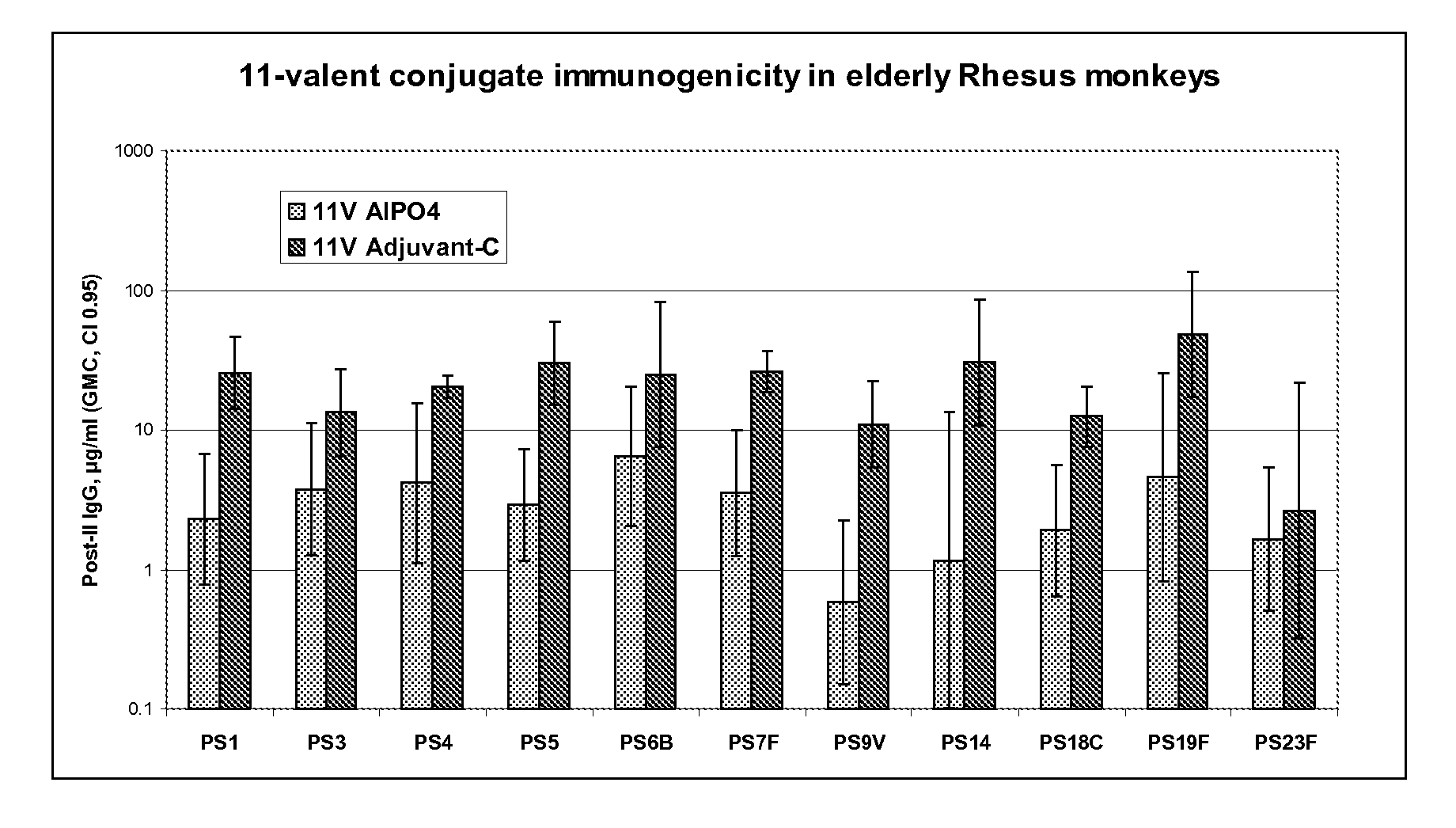
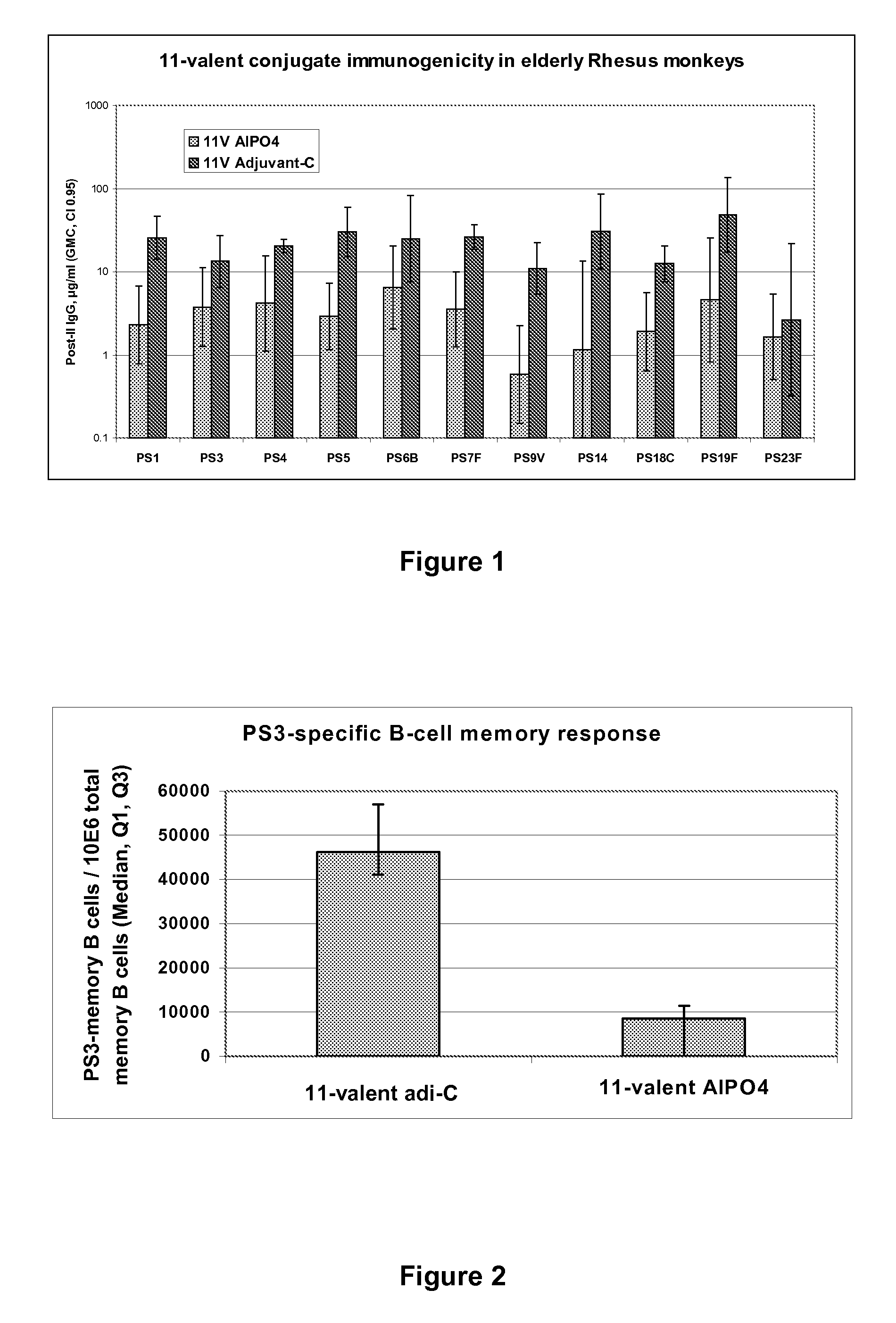
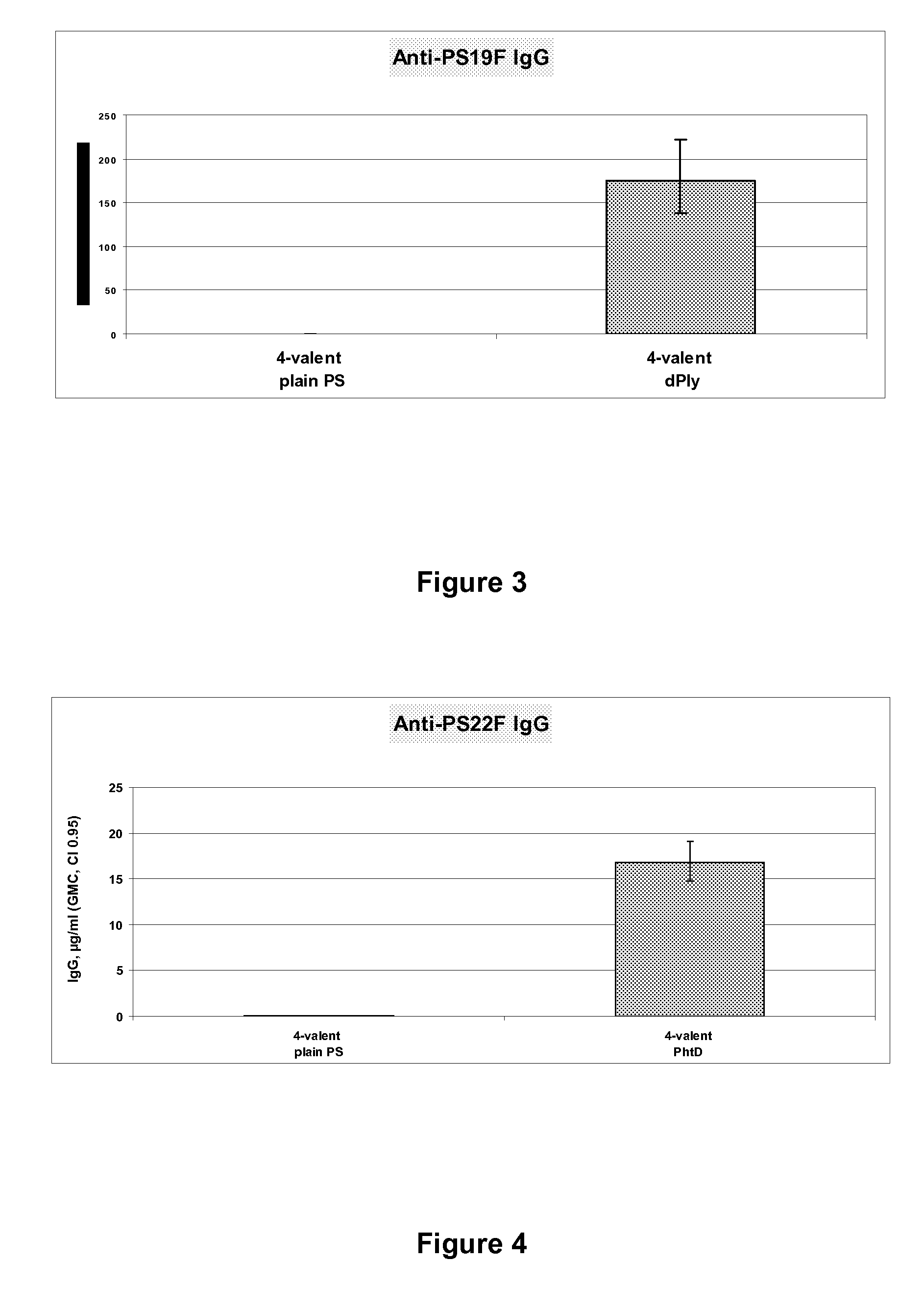
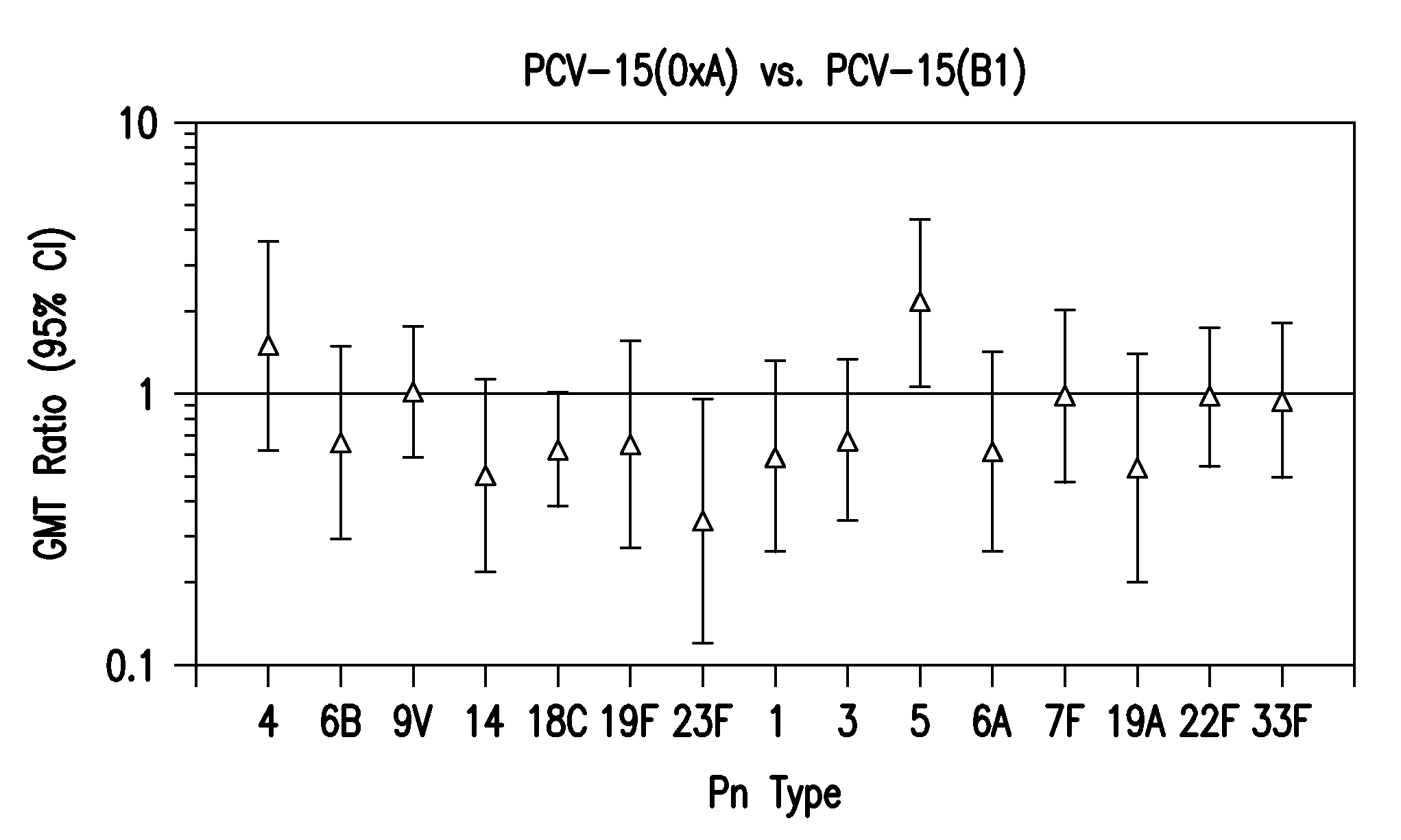
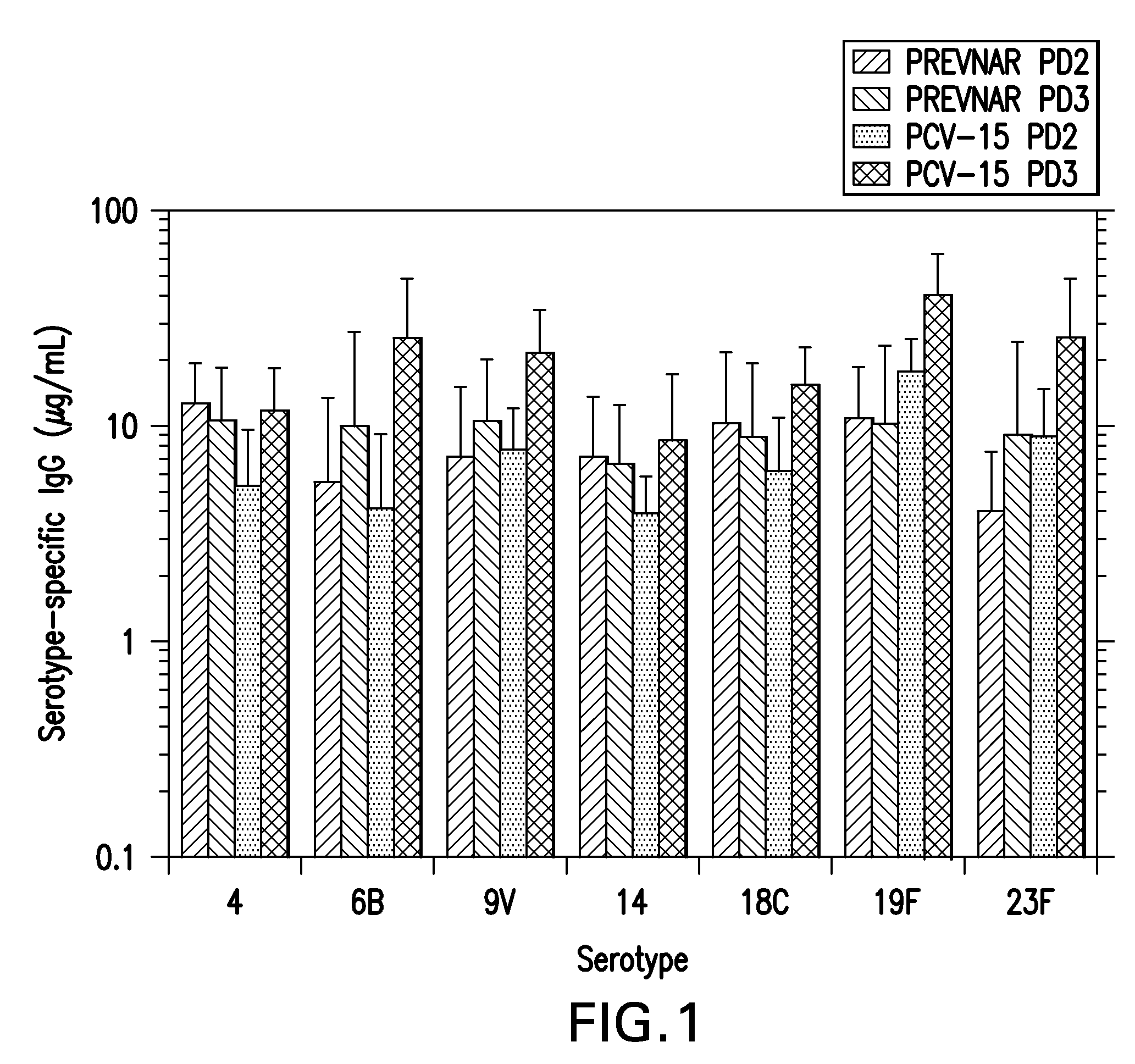
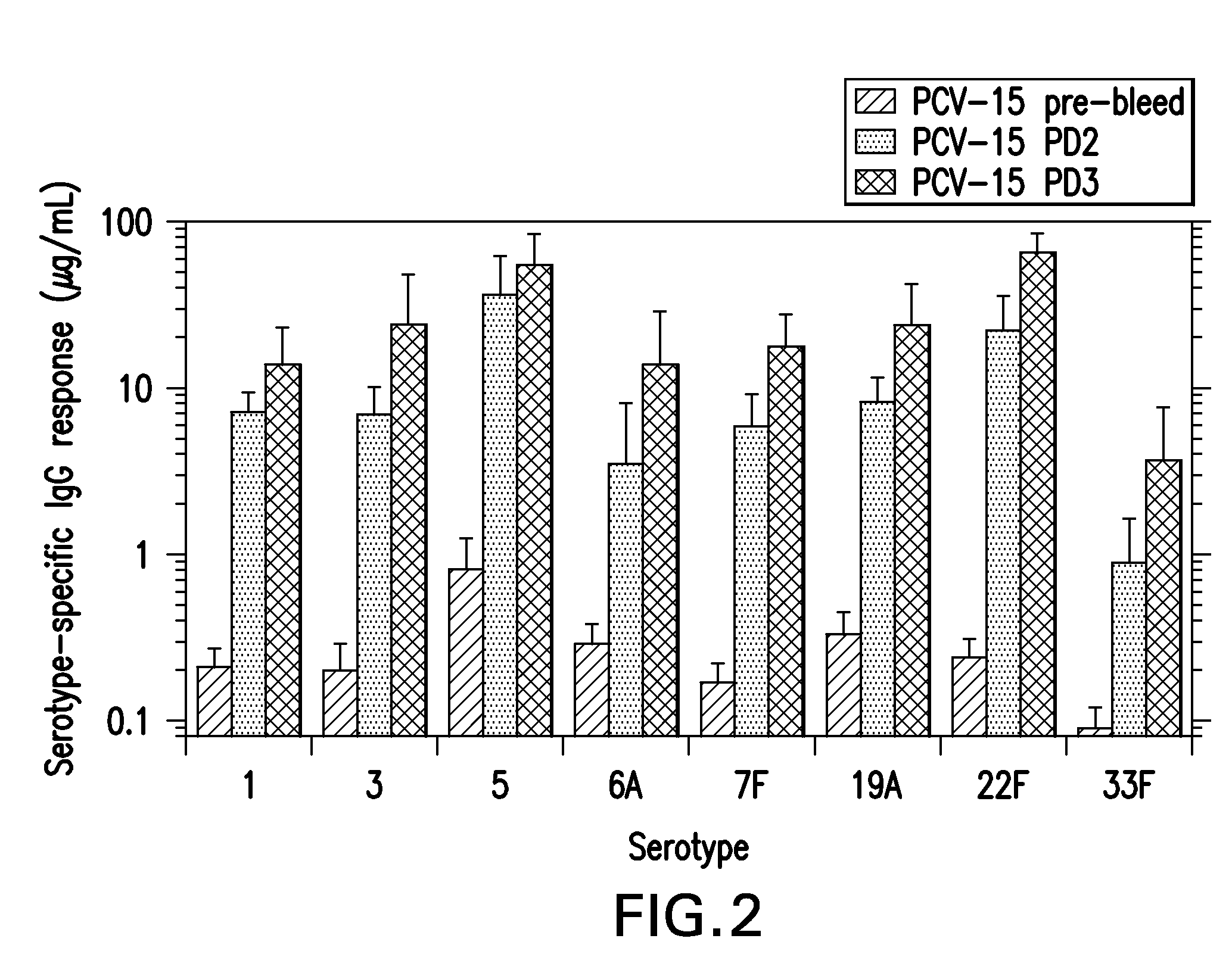
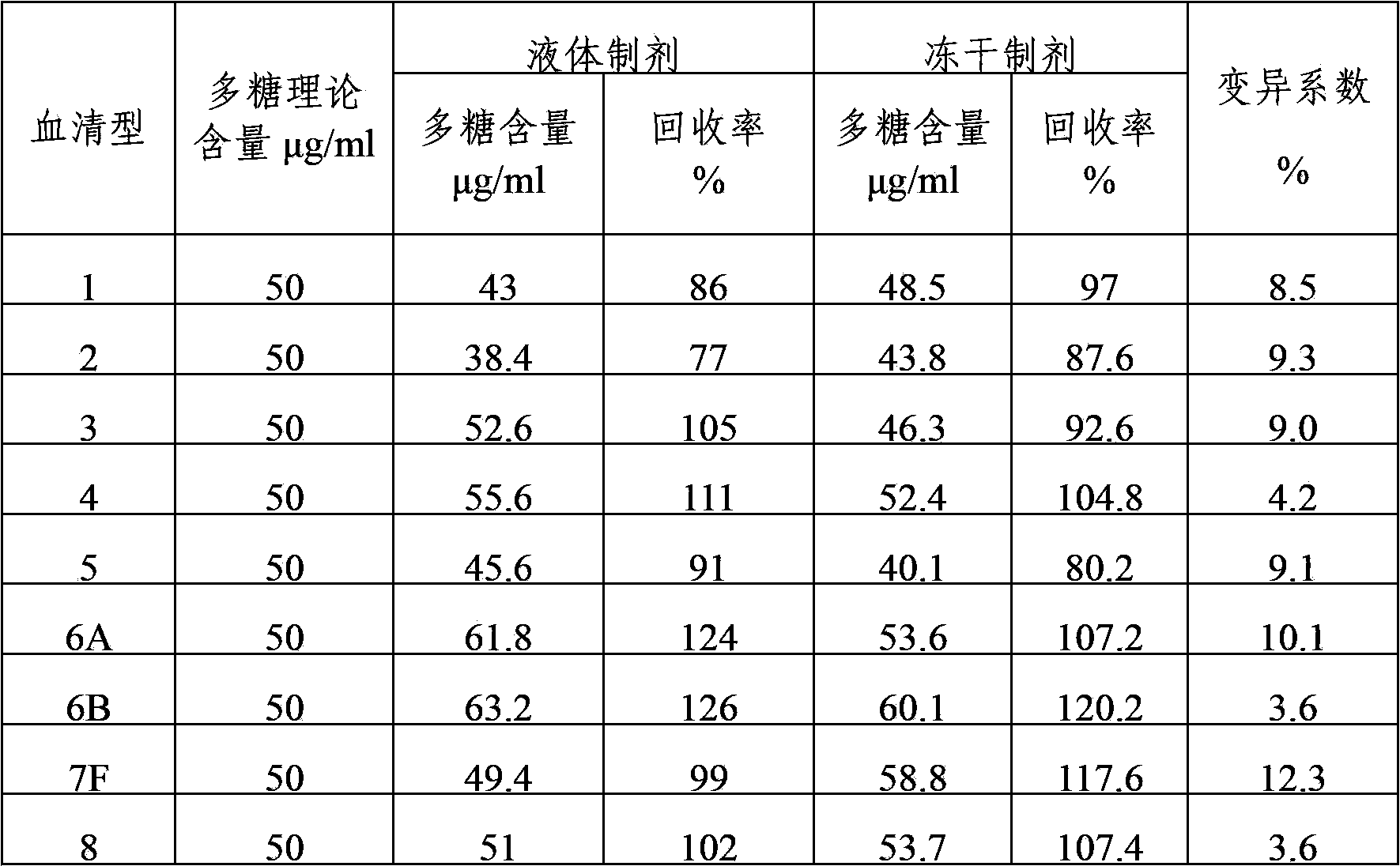
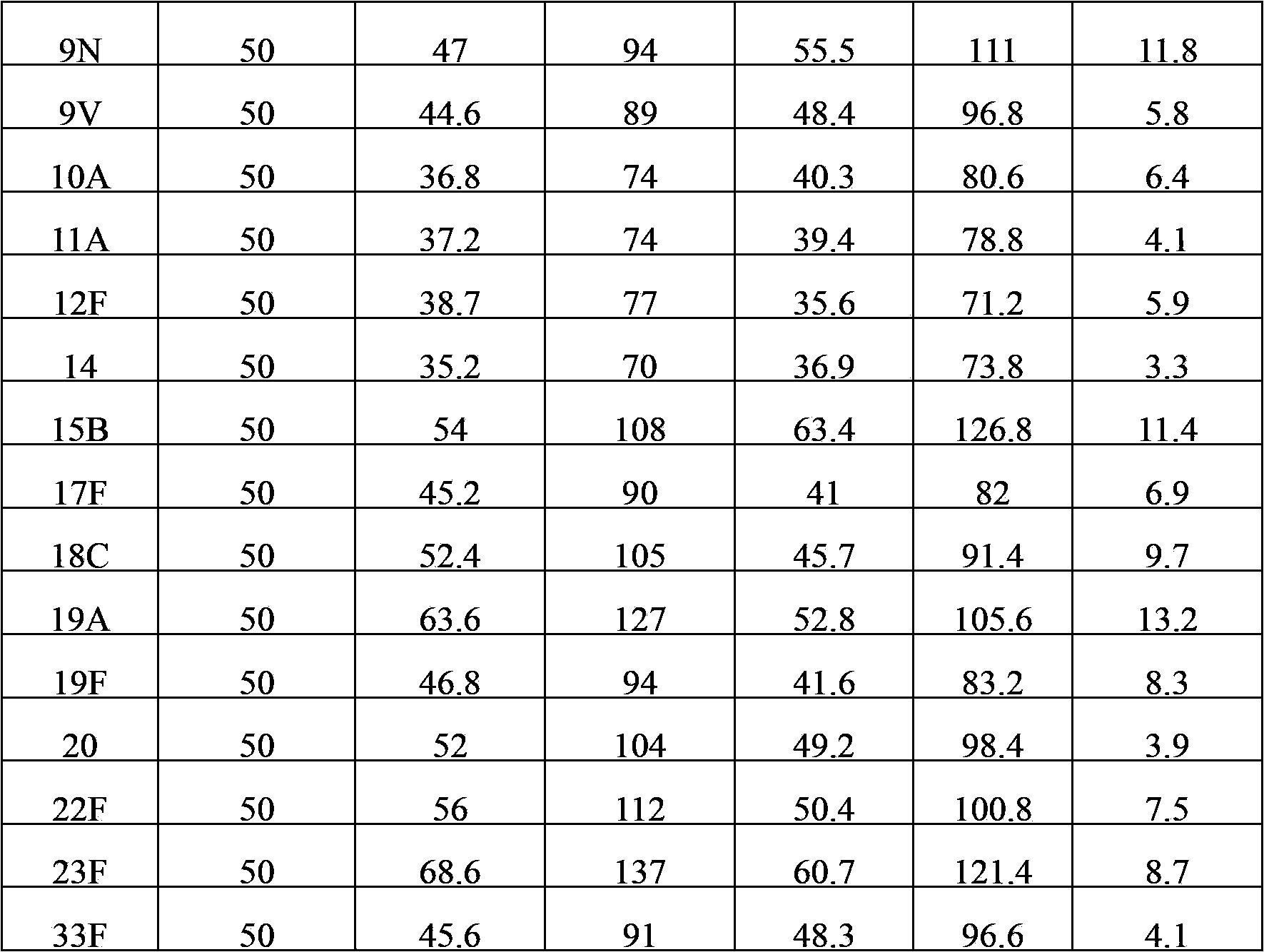
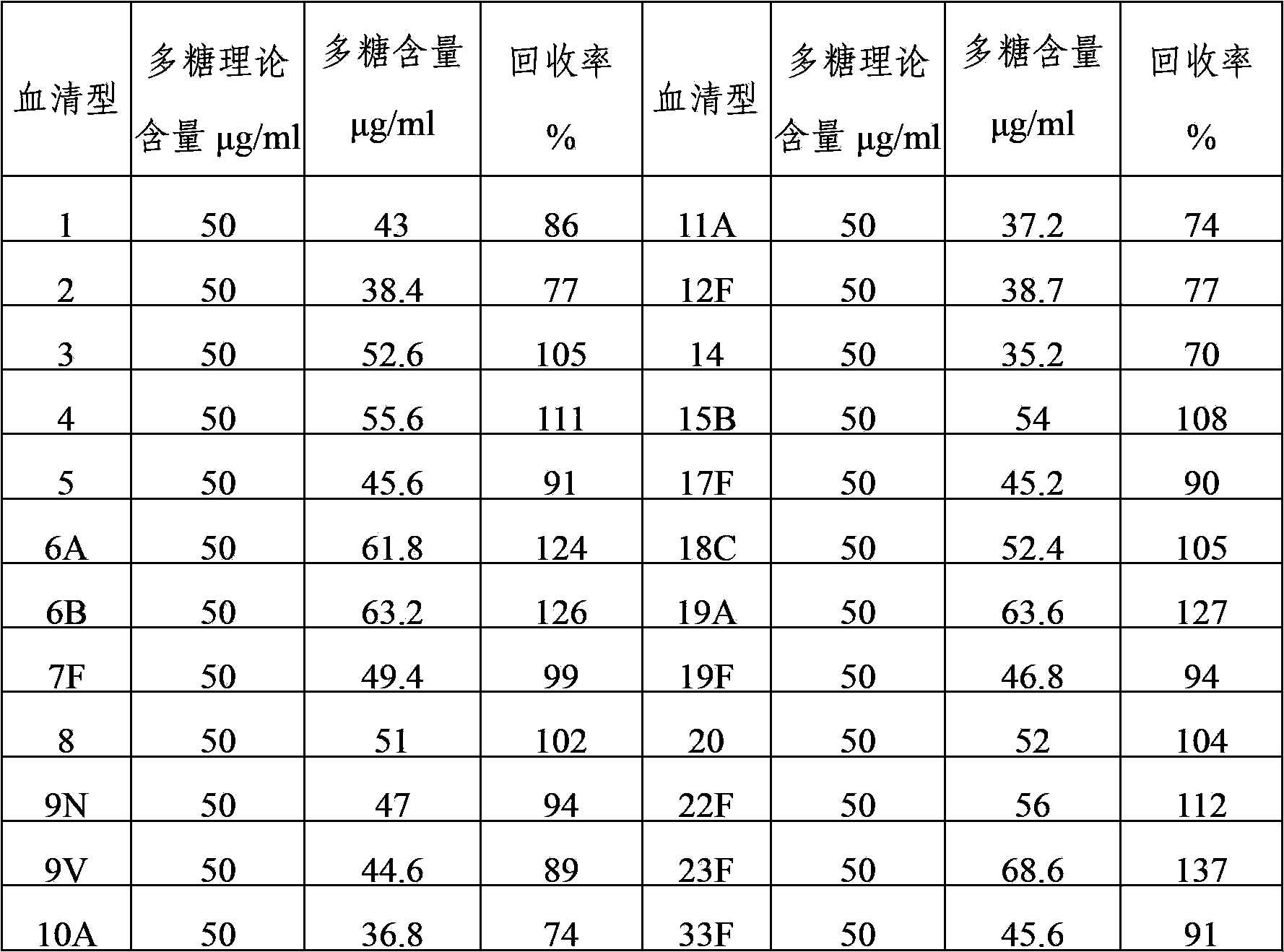
The present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197. The immunogenic composition, preferably formulated as a vaccine on an aluminum-based adjuvant, provides broad coverage against pneumococcal disease, particularly in infants and young children.
Owner:MERCK SHARP & DOHME LLC
Pneumococcal Polysaccharide Conjugate Vaccine
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid (DT) or CRM197, optionally wherein 19F is the only saccharide in the composition conjugated to diphtheria toxoid (DT) or CRM197.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Polysaccharide-protein conjugate vaccines
ActiveUS20070141084A1High yieldReduce productionAntibacterial agentsBacterial antigen ingredientsConjugate vaccineHydrazide
Abstract of the Disclosure Methods for synthesis and manufacture of polysaccharide-protein conjugate vaccines at high yield are provided. The methods involve reaction of a hydrazide group on one reactant with an aldehyde or cyanate ester group on the other reactant. The reaction proceeds rapidly with a high conjugation efficiency, such that a simplified purification process can be employed to separate the conjugate product from the unconjugated protein and polysaccharide and other small molecule by-products.
Owner:UNITED STATES OF AMERICA
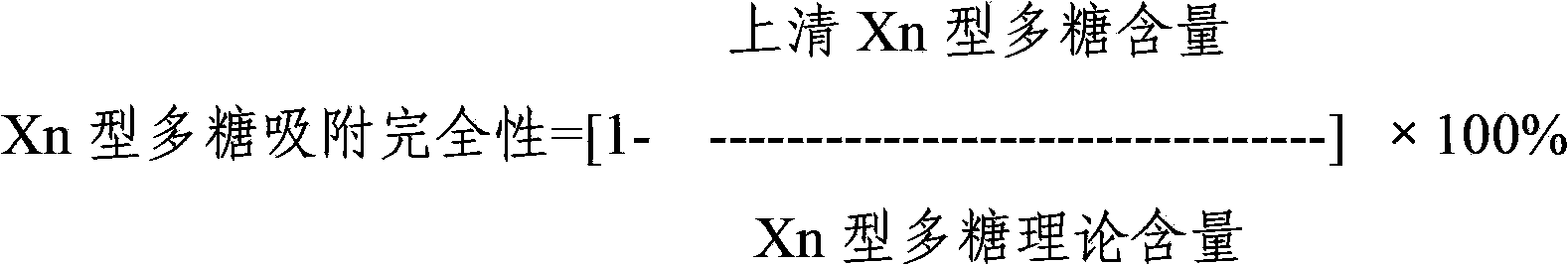
Multivalence pneumococcus capsular polysaccharide-protein conjugate composition and preparation method thereof
ActiveCN103656631AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineDisease
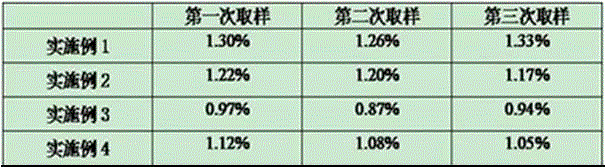
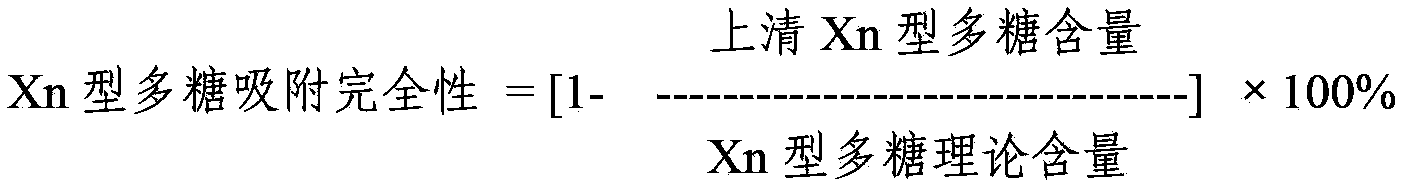
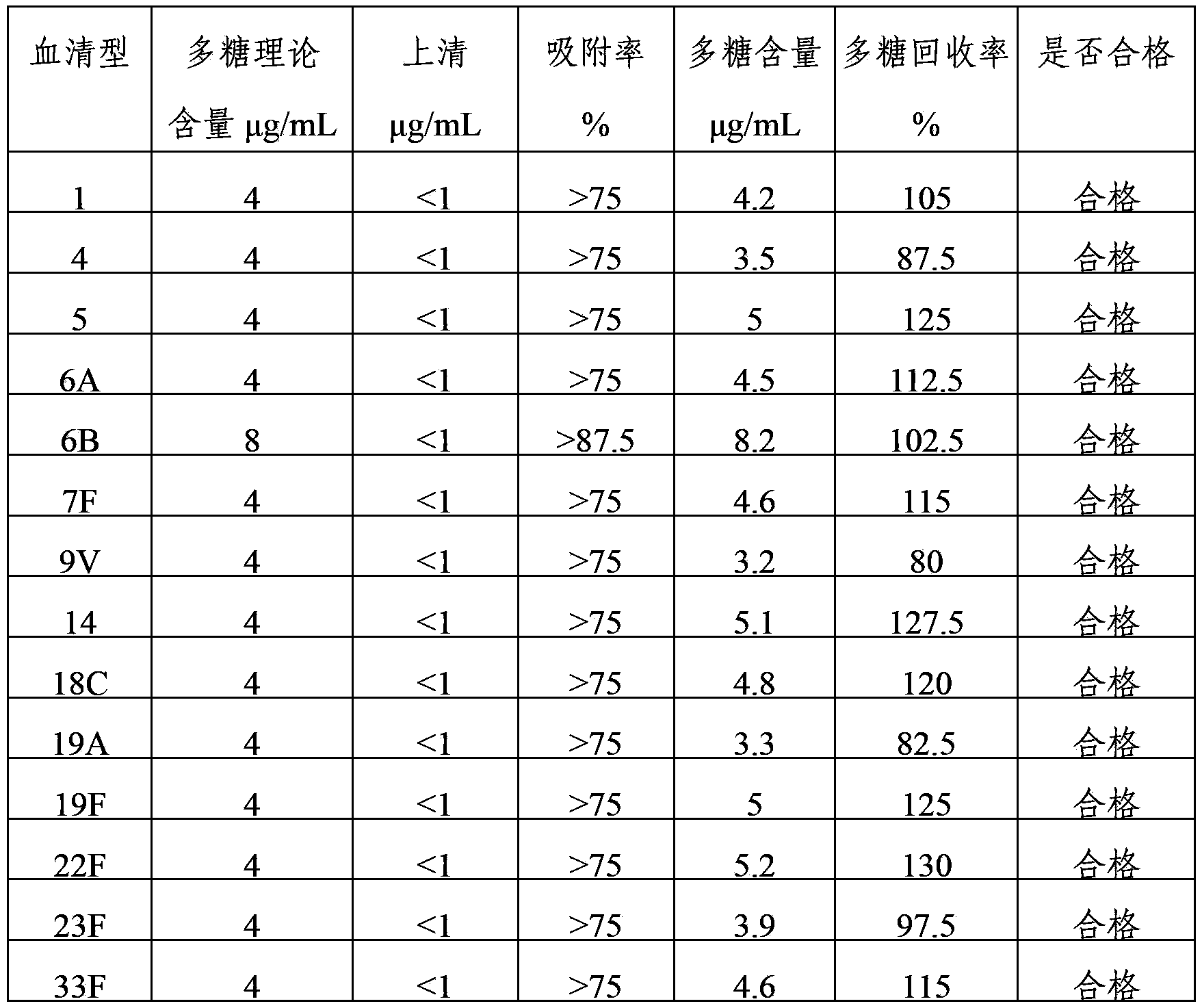
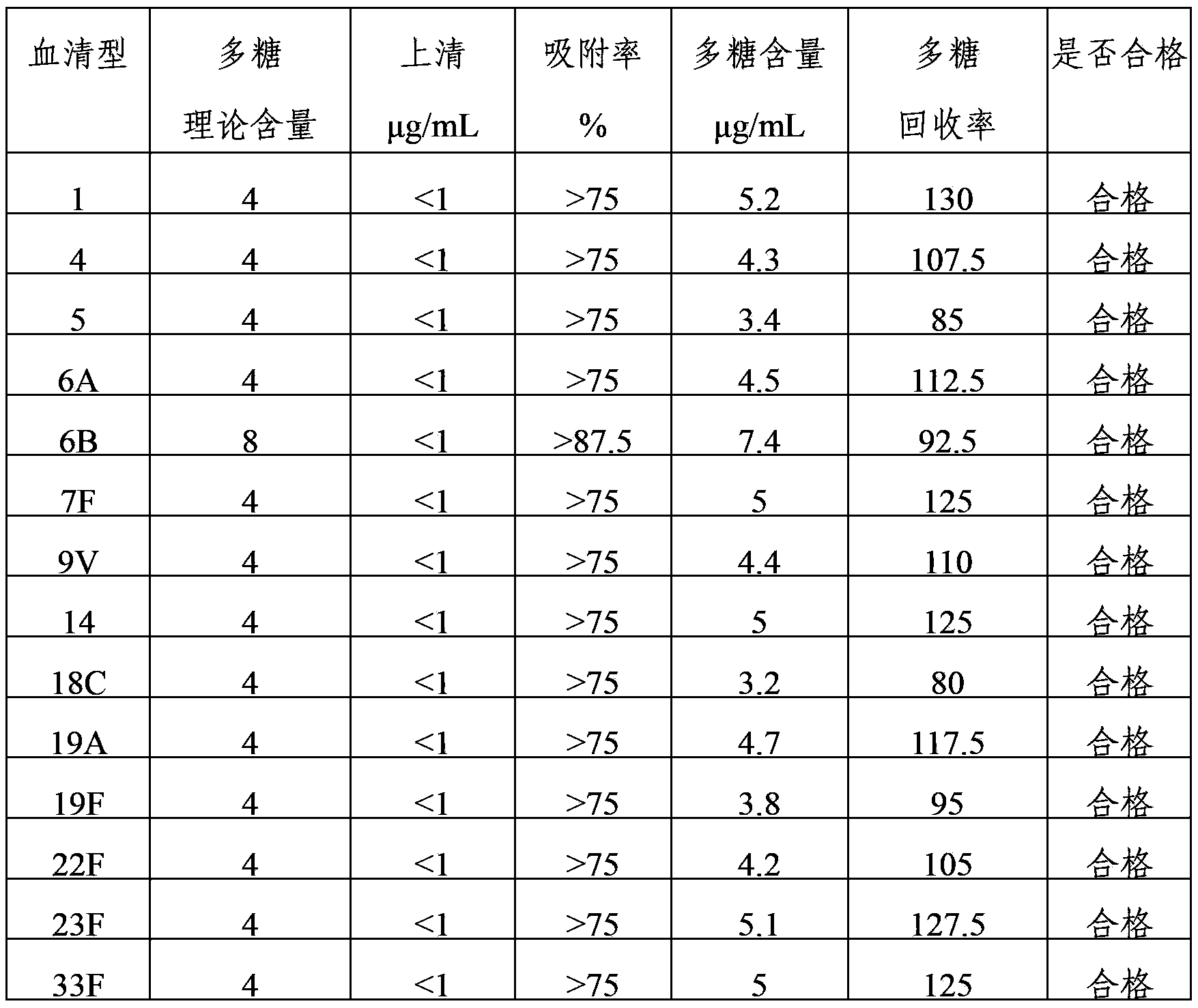
The invention provides a multivalence pneumococcus capsular polysaccharide-protein conjugate composition and a preparation method thereof. The conjugate composition is prepared from capsular polysaccharides of pneumococcus of 24 different serotypes and a carrier protein in a covalence connection manner, wherein the 24 different serotypes are 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The conjugate composition is good in adsorption effect and stability, has multiple immunogenicity and protection properties aiming at invasion of pneumococcus of the 24 serotypes, and is superior to the low-valence pneumonia composition in the market, and the immune response is higher than that of uncombined compositions. By inoculating a multivalence pneumococcus capsular polysaccharide conjugate vaccine prepared from the conjugate composition, the inoculation injection times can be reduced, the immunization procedure can be simplified, and meanwhile human beings and animals can be effectively prevented from diseases resulted from the pneumococcus of the 24 serotypes, and the conjugate composition is wide in coverage range and good in immune effect.
Owner:SINOVAC RES & DEV
Vaccine comprising streptococcus pneumoniae capsular polysaccharide conjugates
InactiveUS20100209450A1Antibacterial agentsSenses disorderConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid. Methods of making and uses thereof are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
15-valent pneumococcal polysaccharide-protein conjugate vaccine composition
Owner:MERCK SHARP & DOHME LLC
Process for preparing polysaccharide-protein conjugate vaccines
ActiveUS20070110762A1High yieldReduce productionAntibacterial agentsAntipyreticConjugate vaccineHydrazide
Methods for the manufacture of polysaccharide-protein conjugate vaccines at high yield are provided. The methods involve reaction of a hydrazide group on one reactant with an aldehyde group on the other reactant. The reaction proceeds rapidly with a high conjugation efficiency. Simplified purification processes can be employed to separate the conjugate product from the unconjugated protein and polysaccharide and other small molecule by-products.
Owner:DEPT OF HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC THE +1
Conjugate vaccines for non-proteinaceous antigens
InactiveUS20070231344A1Strong and rapid effectStrong and rapid immune responseBacterial antigen ingredientsLipid/lipoprotein ingredientsAntigenConjugate vaccine
The present invention is directed to pharmaceutical compositions that can be used to immunize subjects using, for example, lipid, glycan, or nucleic acid antigens. These antigens are conjugated to a glycosphingolipid.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Saccharide Conjugate Vaccines
InactiveUS20080260773A1Improve bioavailabilityImprove efficacyAntibacterial agentsPeptide/protein ingredientsConjugate vaccineCarrier protein
The invention provides compositions comprising a combination of two or more monovalent conjugates, each of said two or more monovalent conjugates comprising a carrier protein comprising T cell epitopes from two or more pathogens conjugated to saccharide antigen. The invention also provides a multivalent conjugate comprising two or more antigenically distinct saccharide antigens conjugated to the same carrier protein molecule, wherein the carrier protein comprises T cell epitopes from two or more pathogens. Further compositions comprise one or more of said monovalent conjugates and one or more of said multivalent conjugates. The invention further provides methods for making said compositions and uses for said compositions.
Owner:NOVARTIS AG
Capsular gram-positive bacteria bioconjugate vaccines
The present invention encompasses a novel S. aureus bioconjugate vaccine. More generally, the invention is directed to Gram-positive and other bioconjugate vaccines containing a protein carrier, at least one polysaccharide such as a capsular Gram-positive polysaccharide, and, optionally, an adjuvant or pharmaceutically acceptable carrier. The instant invention also includes methods of producing Gram-positive and other bioconjugate vaccines. An N-glycosylated protein is also provided that contains one or more polysaccharides such as Gram-positive polysaccharides. The invention is additionally directed to engineered prokaryotic organisms comprising nucleotide sequences encoding a glycosyltransferase of a first prokaryotic organism and a glycosyltransferase of a second prokaryotic organism. The invention further includes plasmids and prokaryotic cells transformed with plasmids encoding polysaccharides and enzymes which produce an N-glycosylated protein and / or bioconjugate vaccine. Further, the invention is directed to methods of inducing an immune response in a mammal comprising administering said bioconjugate vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
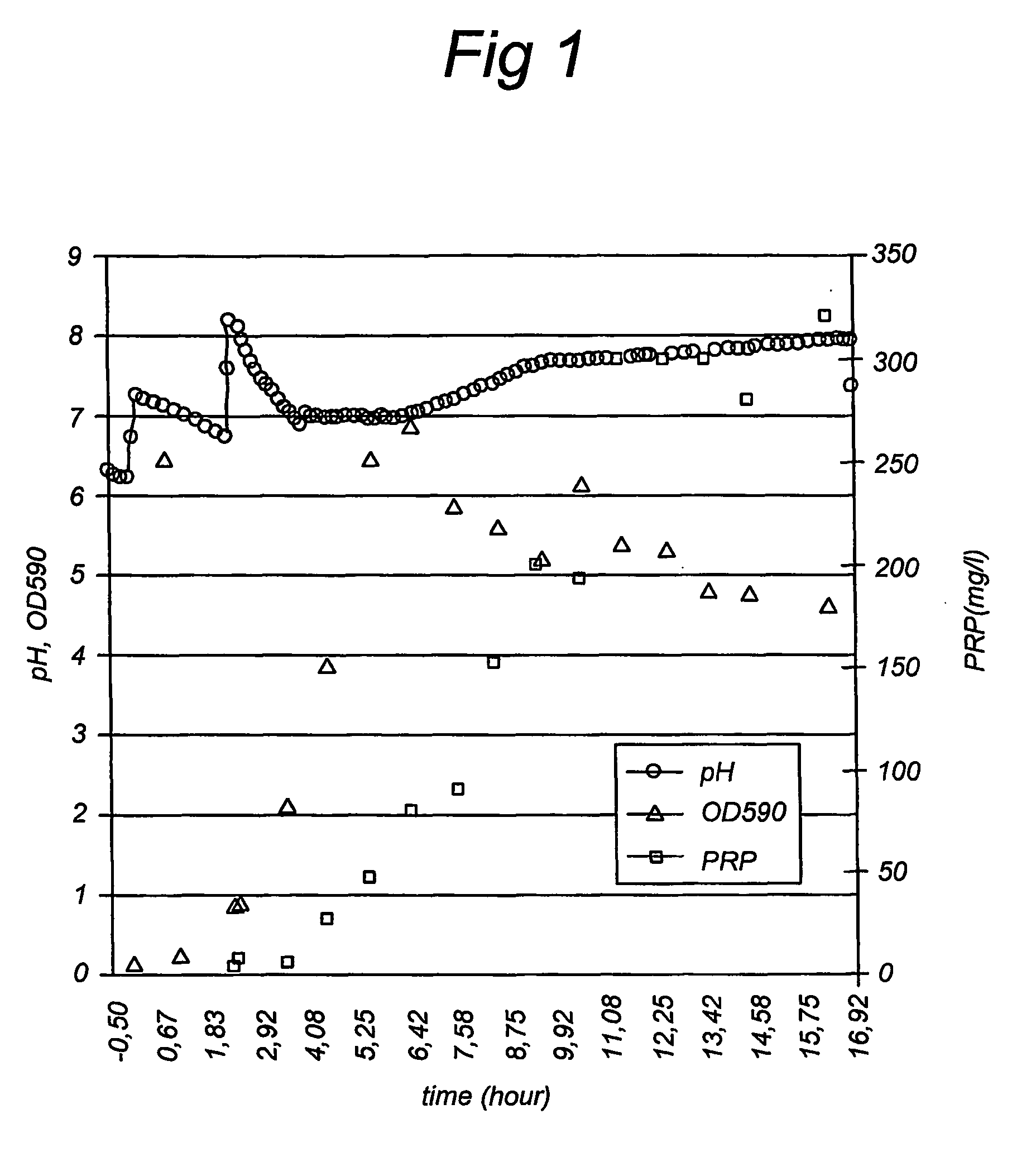
Process for producing a capsular polysaccharide for use in conjugate vaccines
ActiveUS7582459B2Good yieldSimple processCarrier-bound antigen/hapten ingredientsPharmaceutical non-active ingredientsConjugate vaccineCost effectiveness
A method for producing a polysaccharide and a conjugate vaccine including the polysaccharide produced according to the method. A characteristic step in the method is that the pH of the culture medium is kept at a constant value with base or acid until adjustment with respectively base or acid is not possible anymore. Using the method, capsular polysaccharide may be obtained in a high yield in a relatively short time. The method is straightforward, reproducible and cost-effective.
Owner:DE STAAT DER NEDERLANDEN VERT DOOR DE MINIST VAN VWS
Bacterial polysaccharide-protein conjugate vaccine and preparation method thereof
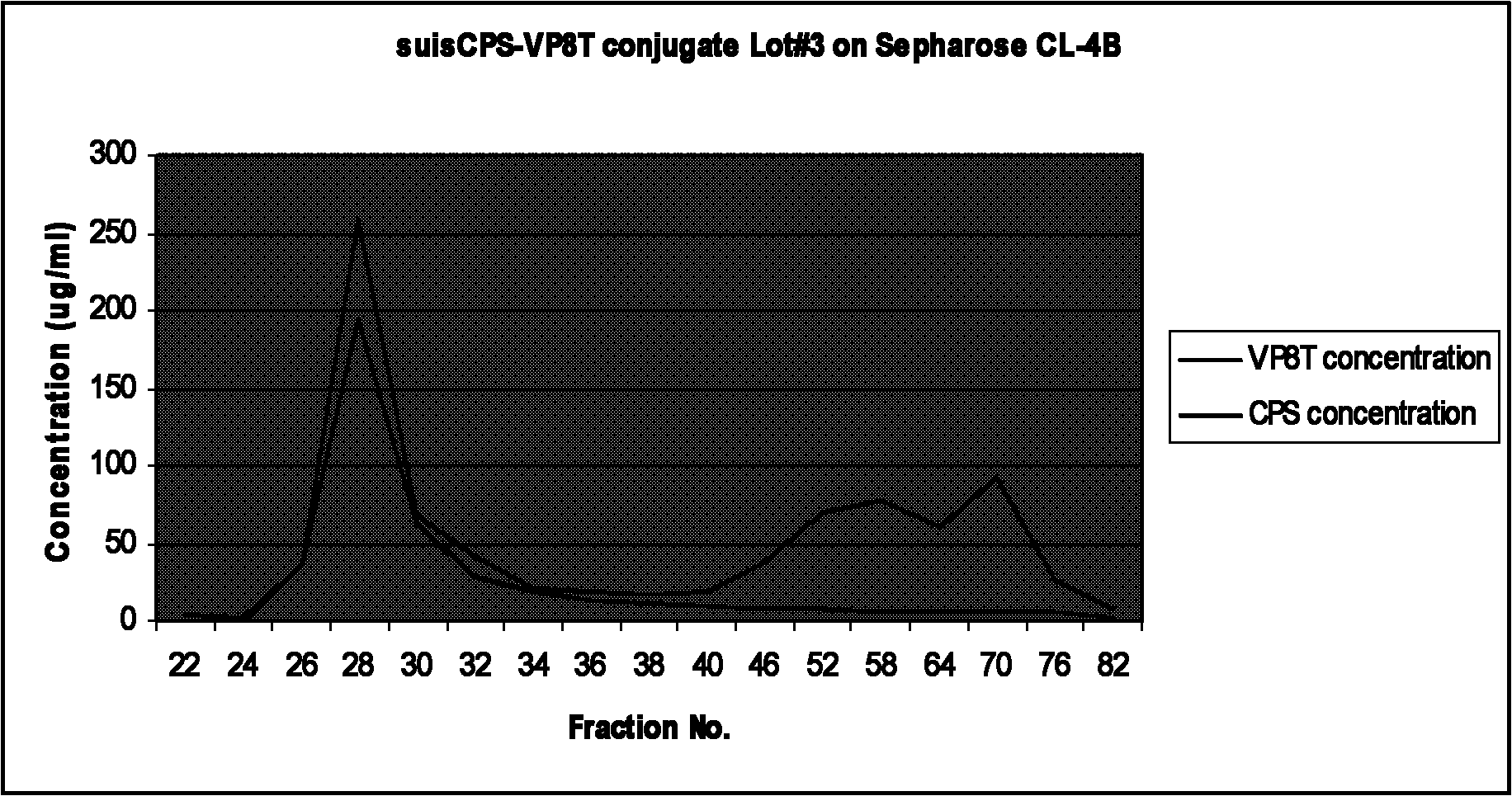
The invention relates to a bacterial polysaccharide-protein conjugate vaccine with immunogenicity, in particular to a conjugate vaccine which is formed by connecting a recombinant rotavirus protein with a bacterial polysaccharide by using a covalent bond, a nucleotide sequence for coding the recombinant rotavirus protein, a recombinant expression system, a protein expressed by the recombinant expression system, a preparation method of the conjugate vaccine and a pneumococcus polysaccharide-recombinant rotavirus protein conjugate vaccine. The bacterial polysaccharide is connected with a recombinant rotavirus surface protein through a covalent bond. The recombinant rotavirus protein is selected from a partial or complete amino acid sequence of a P-gene rotavirus protein and a partial or complete amino acid sequence of a G-gene rotavirus protein.
Owner:普大生物科技(泰州)有限公司
Vaccine comprising streptococcus pneumoniae capsular polysaccharide conjugates
InactiveUS20100183662A1Antibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, an immunogenic composition is provided comprising a multivalent Streptococcus pneumoniae vaccine comprising 13 Streptococcus pneumoniae capsular saccharide conjugates. Methods of making and uses thereof are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel pneumococcal conjugate vaccine and preparation method thereof
ActiveCN101785857AImproving immunogenicityReduce immune competitionAntibacterial agentsBacterial antigen ingredientsConjugate vaccineSugar
The invention relates to a novel pneumococcal conjugate vaccine and a preparation method thereof. The novel pneumococcal conjugate vaccine comprises a dual-valent serum type pneumococcal capsular sugar-protein combination and / or a multi-valent serum type pneumococcal capsular sugar-protein combination. The dual-valent serum type capsular sugar-protein combination is a combination formed by combining two different serum types of capsular sugar with a protein carrier through chemical bonds, and the two different serum types of capsular sugar form links in the structure according to the combination with the protein carrier through the chemical bonds. The multi-valent serum type capsular sugar-protein combination is a combination formed by combining two different serum types of capsular sugar with a protein carrier through chemical bonds, and the two different serum types of capsular sugar form links in the structure according to the combination with the protein carrier through the chemical bonds. In addition, the invention also provides a method of preparing the bacterial capsular sugar-protein conjugate vaccine.
Owner:复星安特金(成都)生物制药有限公司
Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
ActiveCN103656632AStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Pneumococcal polysaccharide and protein conjugated vaccine and preparation method thereof
ActiveCN103893751AEnhance immune responseImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
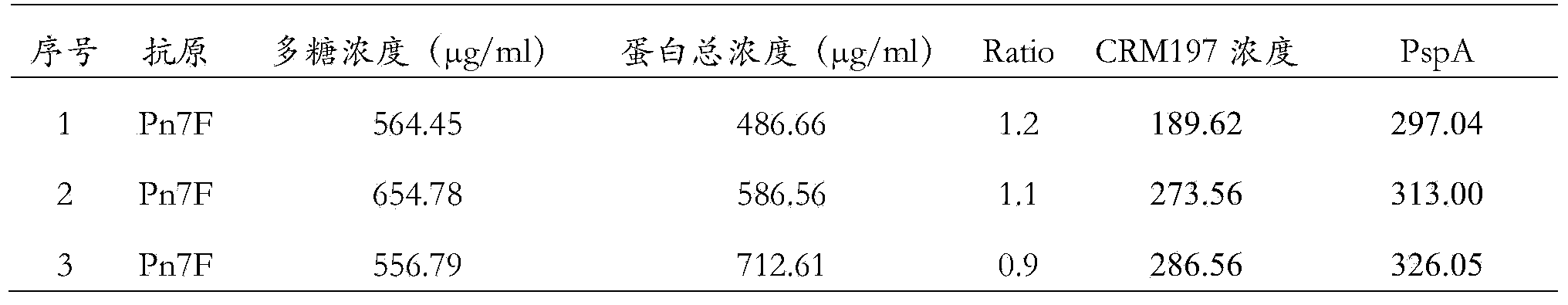
The invention provides a pneumococcal polysaccharide and protein conjugated vaccine and a preparation method thereof. The pneumococcus polysaccharide protein conjugated vaccine comprises one or more streptococcus pneumoniae capsular polysaccharide and protein coupled immune conjugates, and at least one of the immune conjugates is formed by coupling a single streptococcus pneumoniae capsular polysaccharide with two or more proteins. Compared with the existing pneumococcal conjugated vaccine, the pneumococcal polysaccharide and protein conjugated vaccine has stronger immunogenicity, and can cause immune response in a wider crowd; meanwhile, the two carrier proteins are covalently coupled through the polysaccharide, the protective protein antigen epitope induces higher immune response compared with the mixed injection of the two proteins, and through mutual synergy, the immunogenicity of the carrier protein is further improved and the immune response of the body to the polysaccharide is improved; the preparation method is simple, and meets the requirement of large-scale industrial production.
Owner:CANSINO BIOLOGICS INC
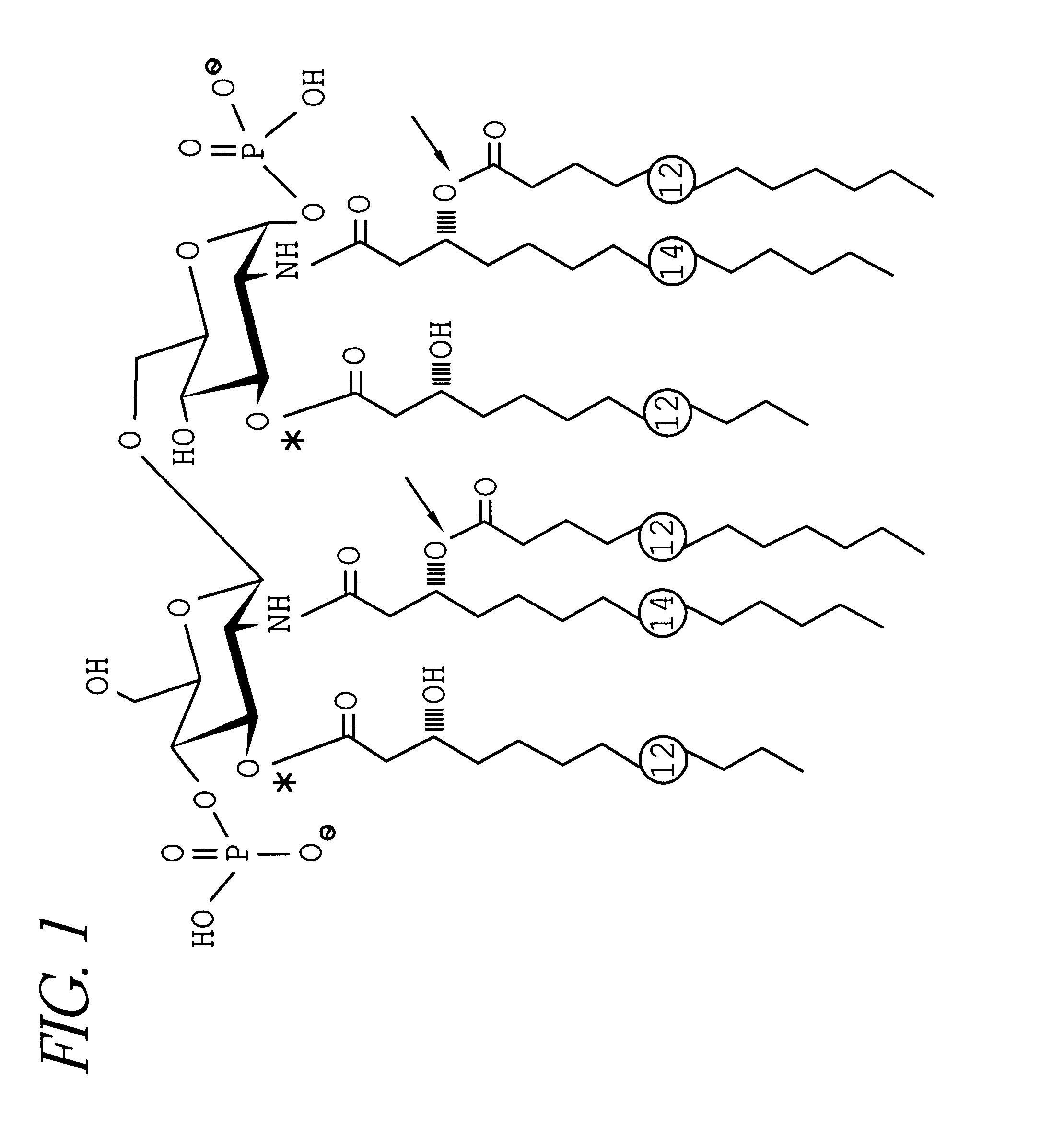
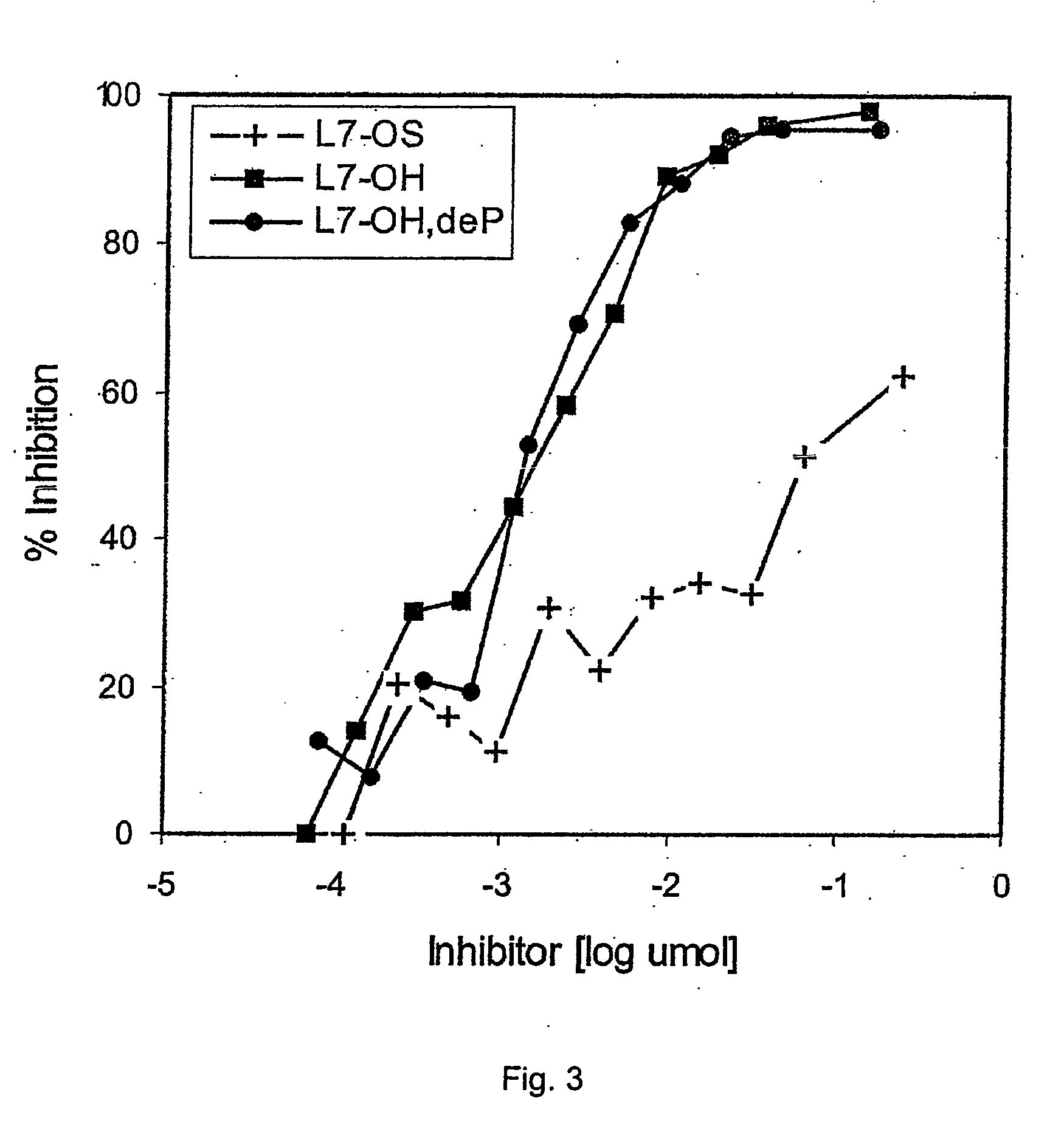
Synthesis of lipopolysaccharide-protein conjugate vaccines via the lipid a region following removal of the glycosidic phosphate residue
InactiveUS20050147624A1Avoid bacterial infectionImproving immunogenicityBacterial antigen ingredientsPeptide preparation methodsConjugate vaccinePhosphorylation
This invention relates to lipopolysaccharide-protein conjugate vaccines with appropriate presentation of conserved inner core oligosaccharide epitopes having improved immunogenic properties. These are based upon antigenic, detoxified bacterial lipopolysaccharides which optimally present an inner core oligosaccharide epitope following removal of at least a glycosidic phosphate of the lipid A region. These partially or completely dephosphorylated antigenic, detoxified bacterial lipopolysaccharides are linkable to an immunologically acceptable carrier and can be used in polyvalent or multivalent vaccines.
Owner:THE CHANCELLOR MASTERS & SCHOLARS OF THE UNIV OF OXFORD +1
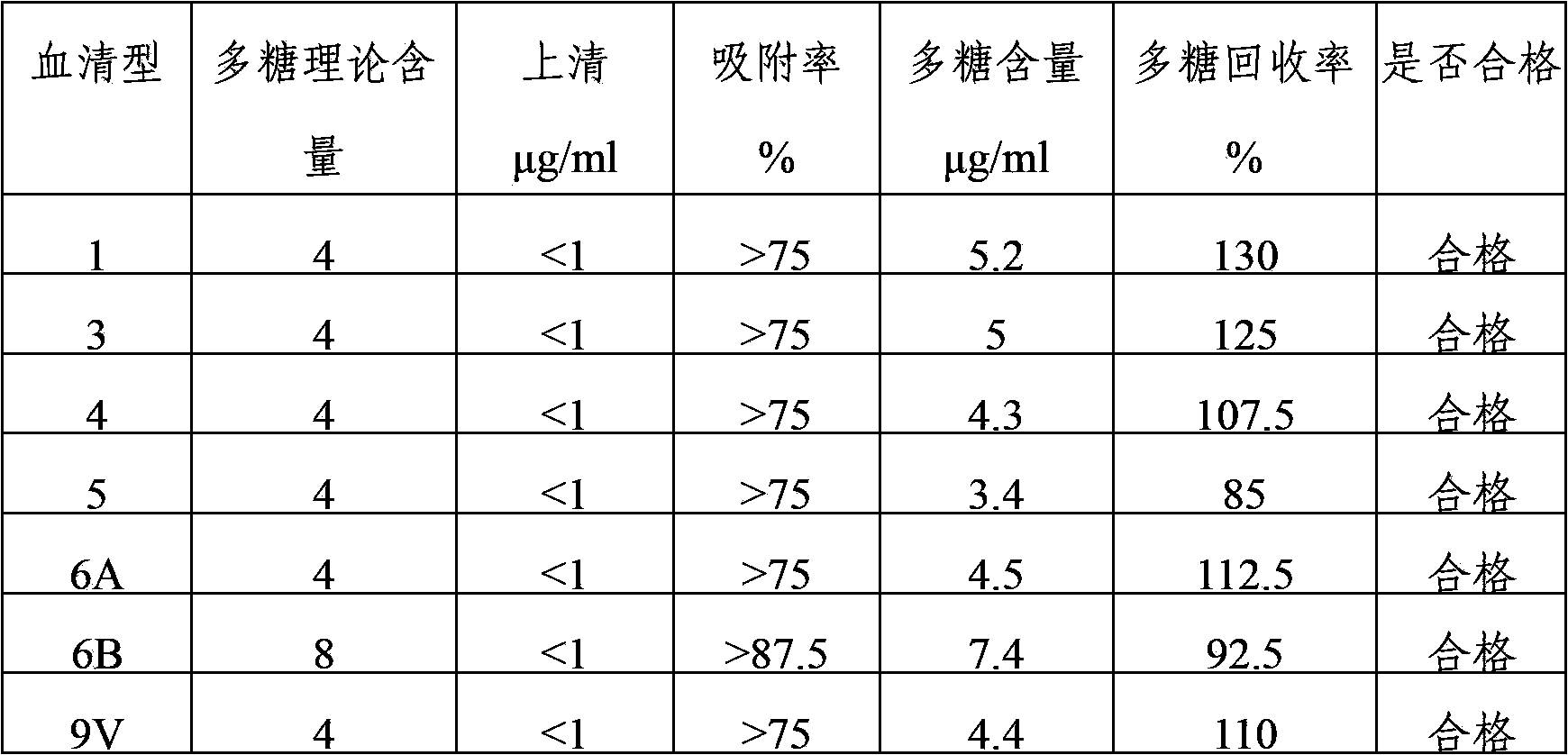
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
InactiveCN103623401AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineDisease
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed through covalent linkages between pneumococcus capsular polysaccharides of 14 different serotypes and a carrier protein, wherein the 14 different serotypes are: 1, 3, 4, 5, 6A, 6B, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F. The conjugated composition has a good absorption effect and stability, and has multiple immunogenicity and protective property aiming at the invasion of pneumococcus of the 14 serotypes; the effect of the conjugated composition is better than that of the low price and low quality pneumonia-treating compositions on the market, and moreover the immune response of the conjugated composition is higher than that of the composition that has not been combined. By using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the inoculation times can be reduced, the immune procedure is simplified, diseases of humans and animals caused by the pneumococcus of the 14 serotypes can be effectively prevented, moreover the disease coverage is wider, and the immune effect is better.
Owner:SINOVAC RES & DEV
Methods of assaying vaccine potency
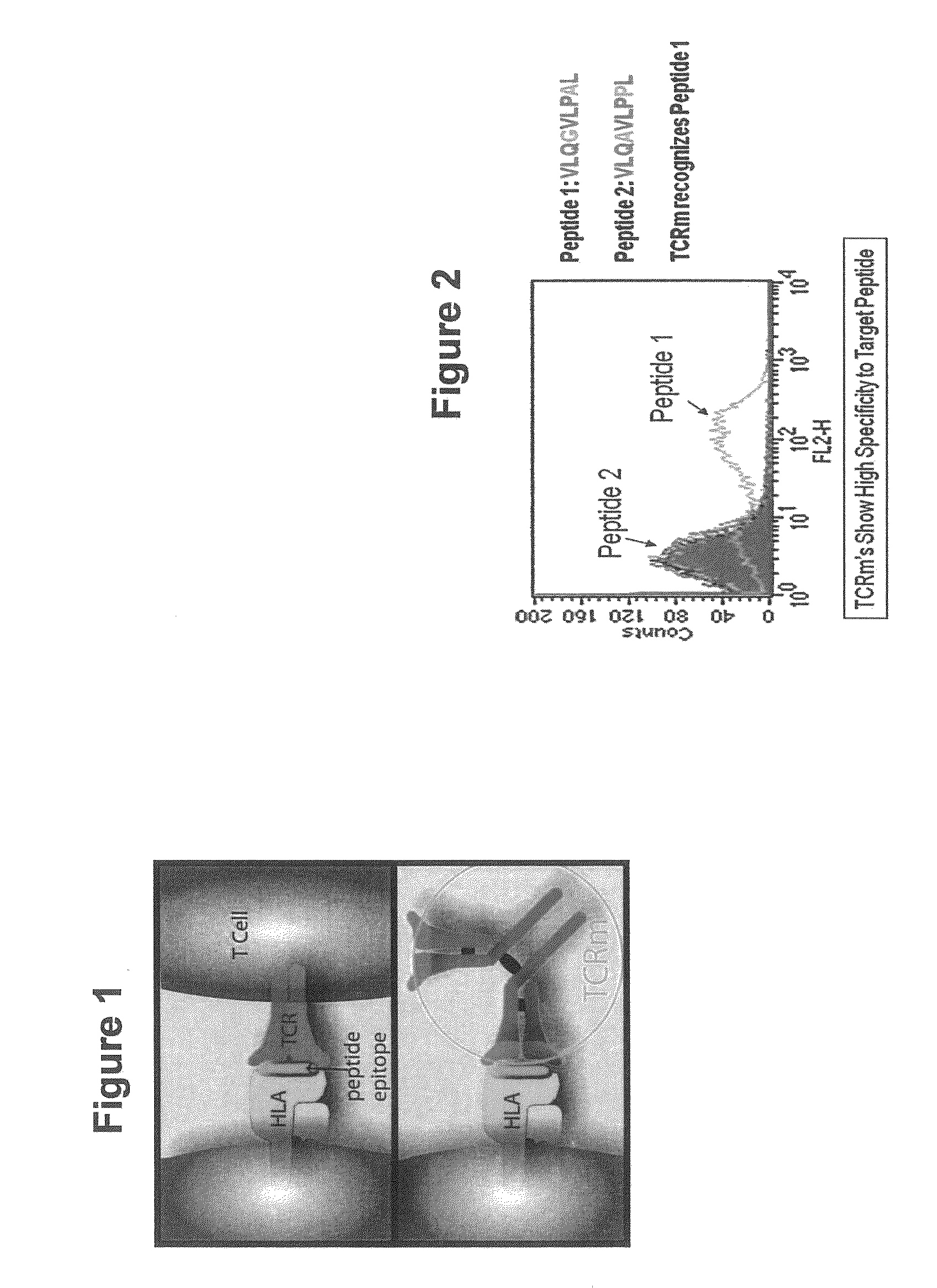
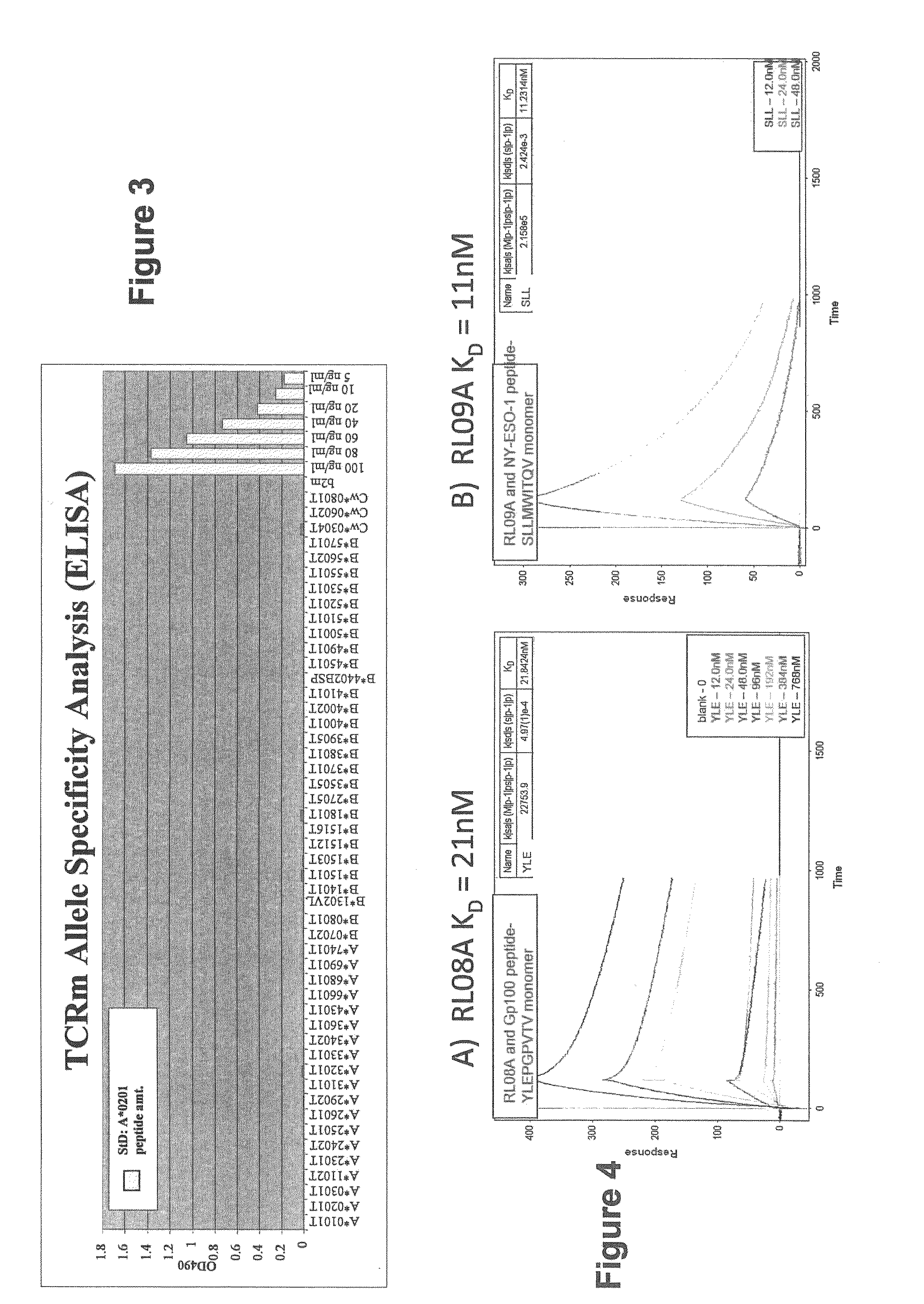
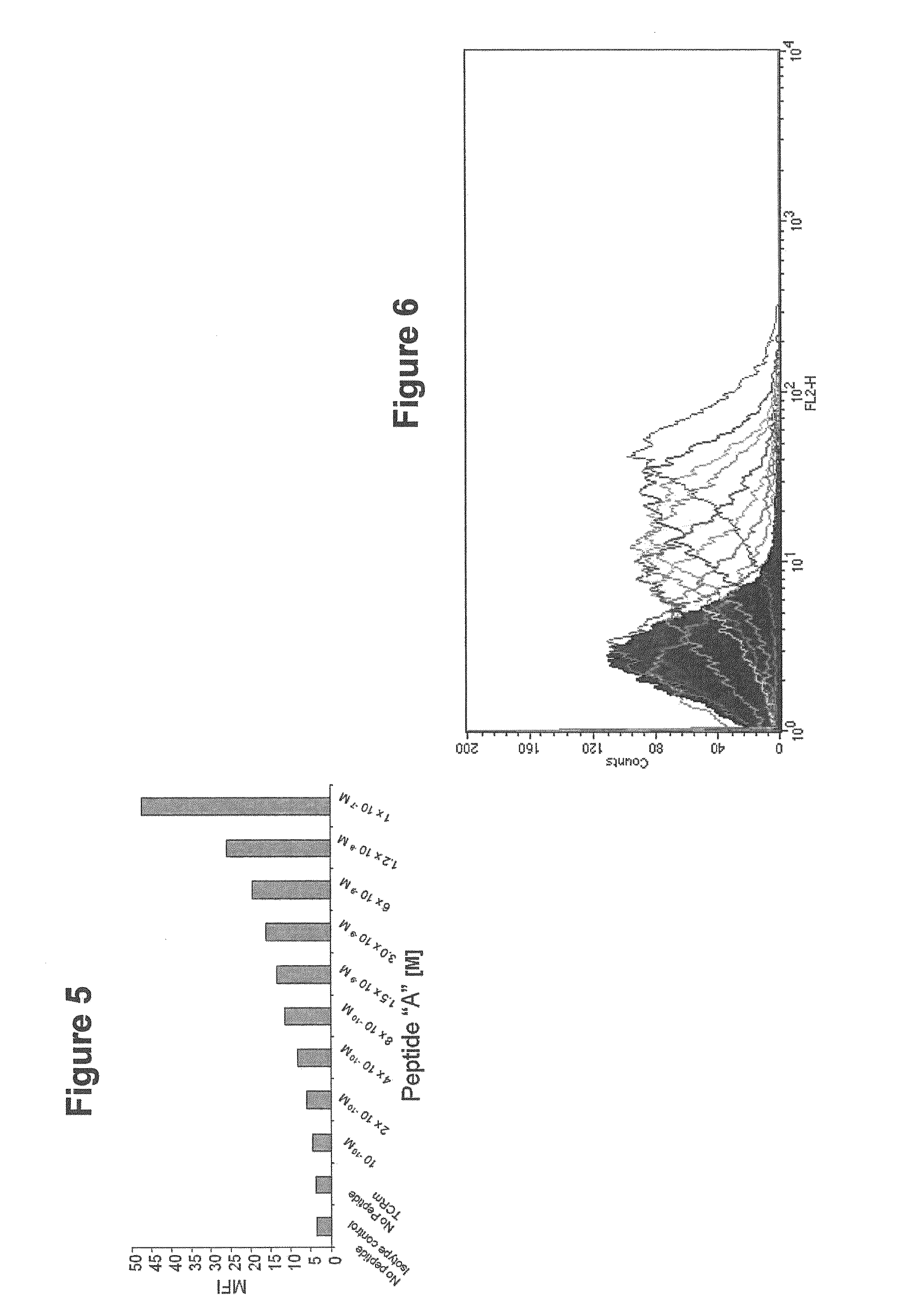
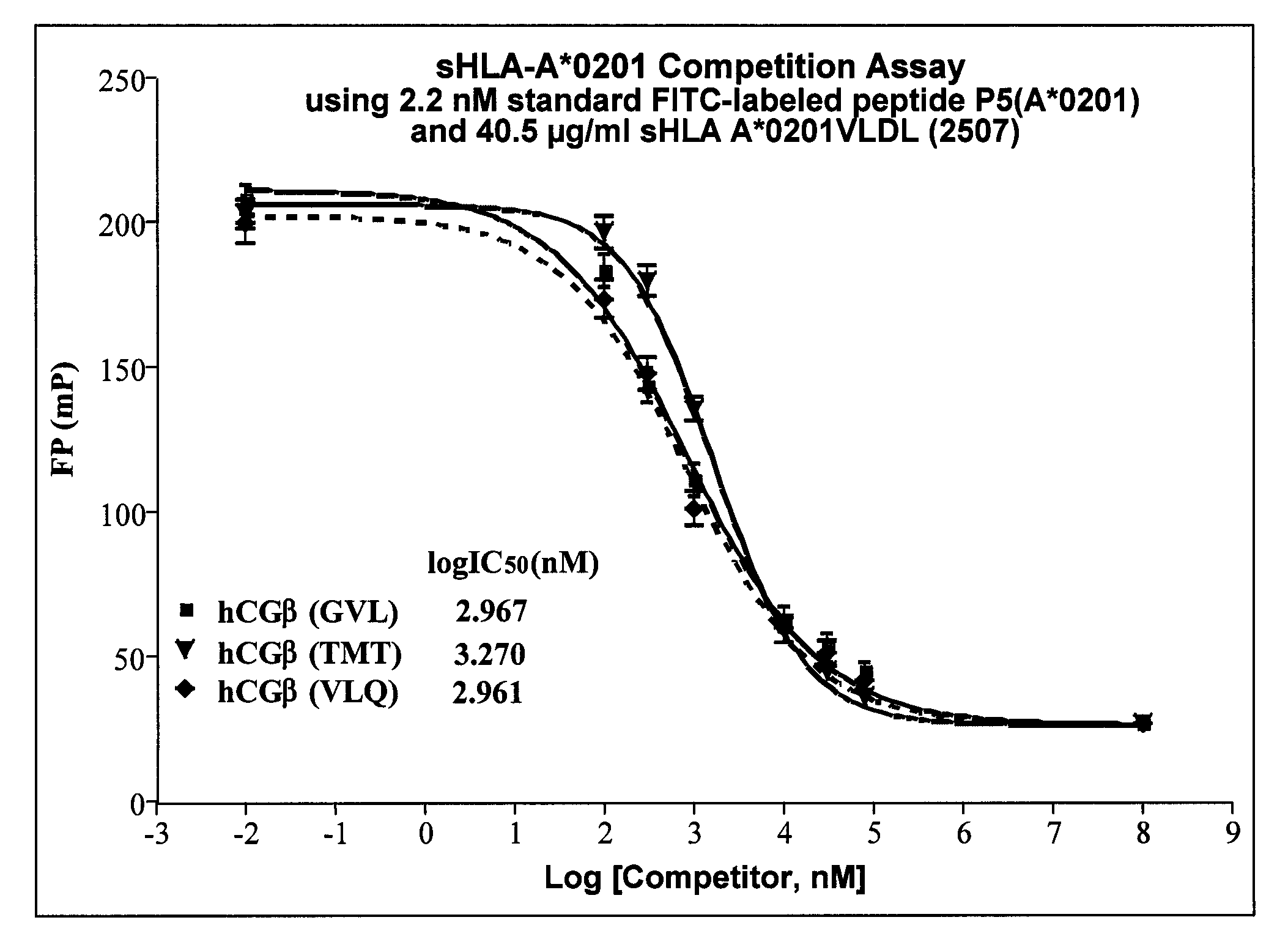
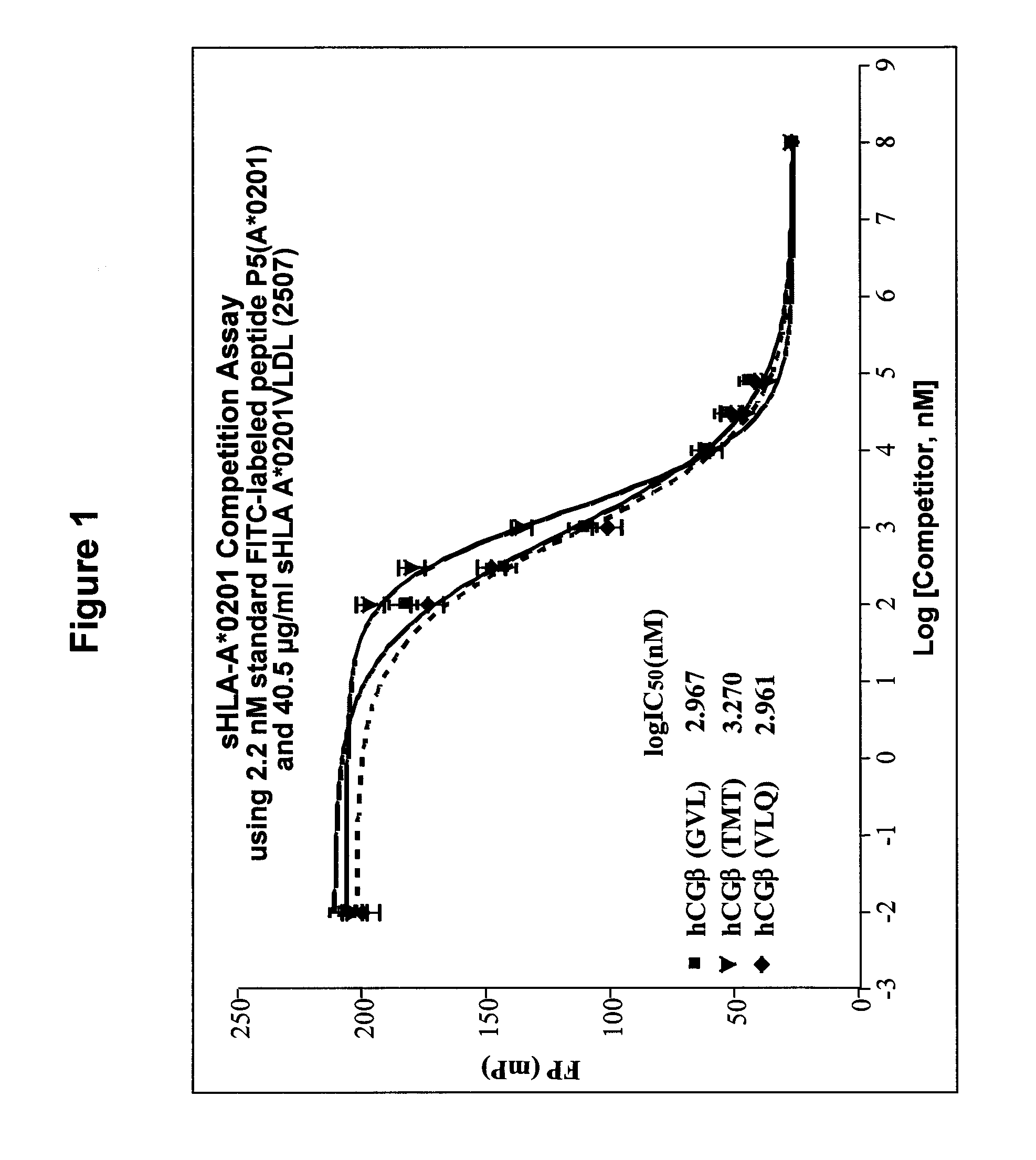
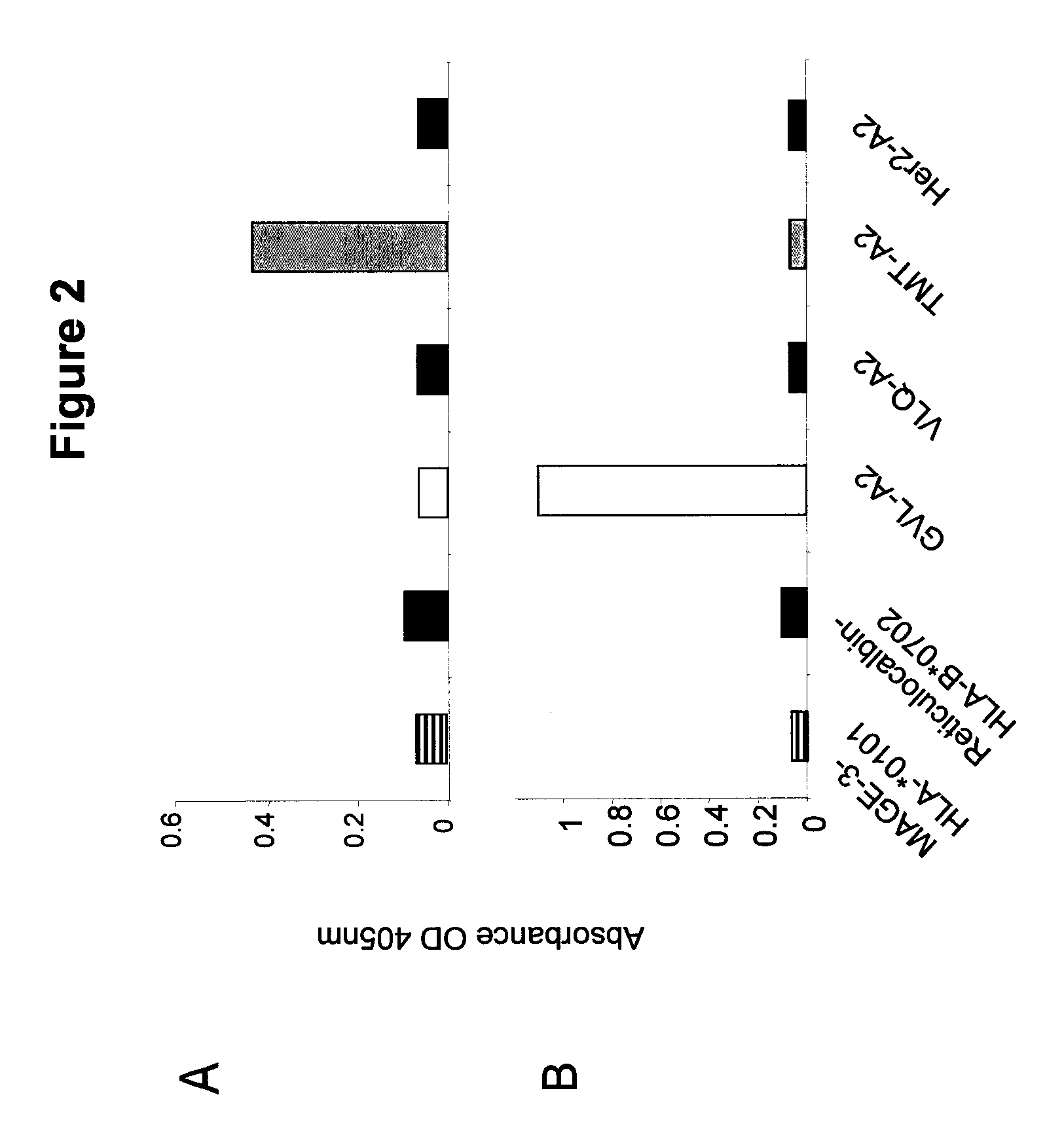
InactiveUS20090233318A1Immunoglobulin superfamilyCancer antigen ingredientsConjugate vaccineVaccine Potency
The present invention is related to methods of assaying potency of a vaccine composition, wherein the potency is a pre-defined minimum level of potential biological activity for the vaccine composition. The method includes providing a vaccine composition and delivering same to an antigen presenting cell, wherein the vaccine composition is processed into peptides and the peptides are presented by MHC complexes on the cell surface. An agent, such as a T cell receptor mimic, that is reactive against a specific peptide / MHC complex is provided and reacted with the vaccine-treated antigen presenting cell, whereby the agent binds to the cell surface of the vaccine-treated antigen presenting cell if the specific peptide / MHC complex recognized by the agent is present on the cell surface. A density of the specific peptide / MHC complex on the surface of the vaccine-treated antigen presenting cell is measured by agent binding. The potency of the vaccine is then determined based upon the measured density of specific peptide / MHC complex present on the surface of the vaccine-treated antigen presenting cell.
Owner:RECEPTOR LOGIC
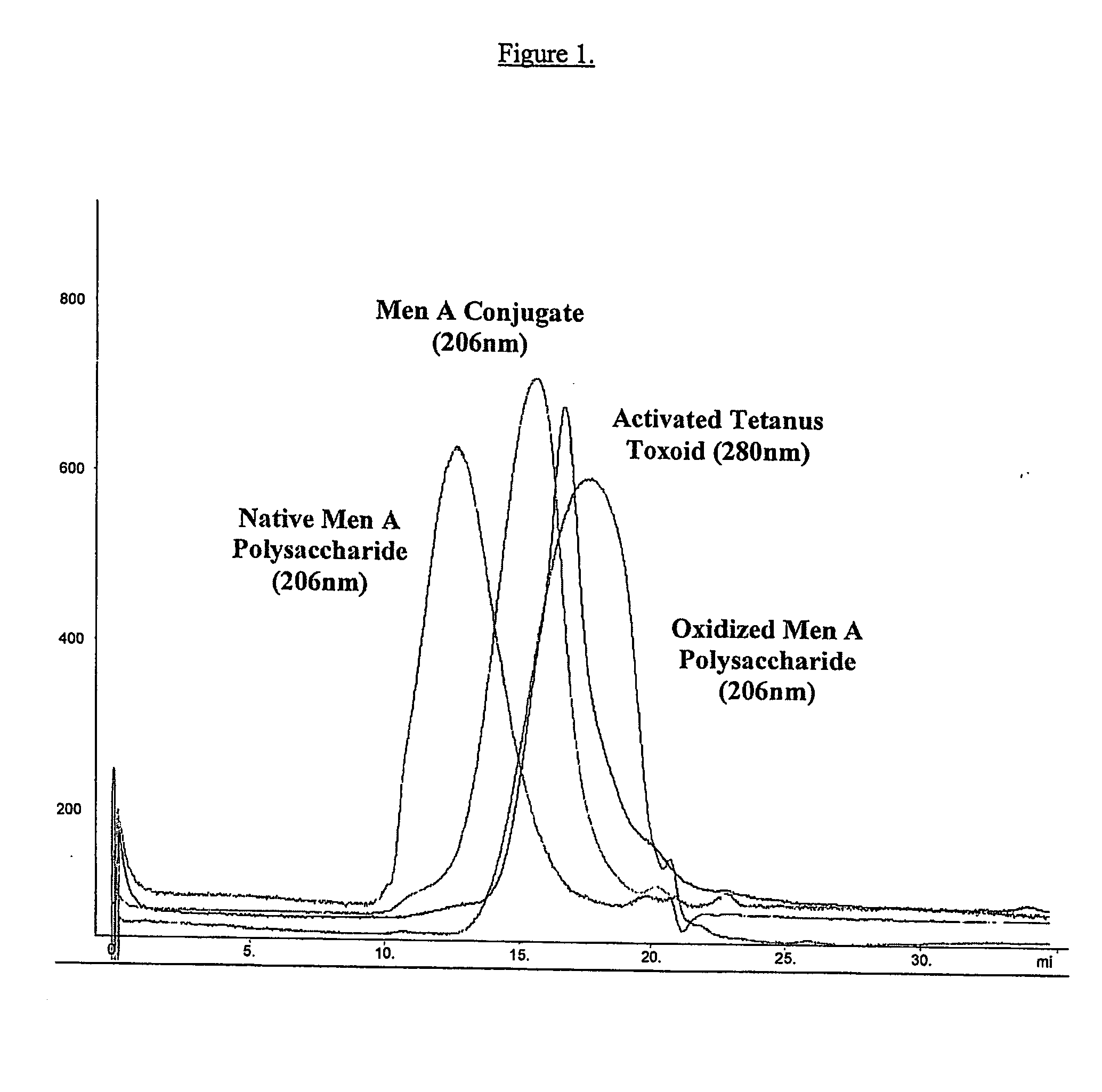
Epidemic meningitis polyose-protein binding vaccine
A polyose-protein vaccine for epidemic cerebrospinal meningitis is prepared through chemically binding the two Neisseria capsular polyoses of cluster-A and-C epidemic cerebrospinal meningitis with protein carrier by covalent bonds.
Owner:BEIJING LUZHU BIOTECH
Process for producing a capsular polysaccharide for use in conjugate vaccines
ActiveUS20070065460A1Good yieldSimple processBacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsConjugate vaccineCost effectiveness
A method for producing a polysaccharide and a conjugate vaccine including the polysaccharide produced according to the method. A characteristic step in the method is that the pH of the culture medium is kept at a constant value with base or acid until adjustment with respectively base or acid is not possible anymore. Using the method, capsular polysaccharide may be obtained in a high yield in a relatively short time. The method is straightforward, reproducible and cost-effective.
Owner:DE STAAT DER NEDERLANDEN VERT DOOR DE MINIST VAN VWS
Polysaccharide-protein conjugate vaccines
ActiveUS8048432B2High yieldReduce productionAntibacterial agentsBacterial antigen ingredientsConjugate vaccineHydrazide
Abstract of the Disclosure Methods for synthesis and manufacture of polysaccharide-protein conjugate vaccines at high yield are provided. The methods involve reaction of a hydrazide group on one reactant with an aldehyde or cyanate ester group on the other reactant. The reaction proceeds rapidly with a high conjugation efficiency, such that a simplified purification process can be employed to separate the conjugate product from the unconjugated protein and polysaccharide and other small molecule by-products.
Owner:UNITED STATES OF AMERICA
A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process
InactiveCN105031634AKeep healthyAvoid pollutionAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineVaccine manufacturing
The invention discloses an A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process. The problem that highly-toxic chemical reagents, such as cyanogen bromide, are used in the vaccine manufacturing process in the prior art and accordingly are harmful to protection of human health and environment is solved. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process comprises the following steps of 1 cultivating Neisseria meningitidis, 2 utilizing the cultivated Neisseria meningitidis to extract crude polysaccharide, 3 purifying the crude polysaccharide to prepare refined Neisseria meningitidis polysaccharide and 4 activating and deriving the refined polysaccharide. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process has the advantages that the extraction rate of the polysaccharide from a capsule is higher, the precipitation rate of the polysaccharide is higher, the recovery rate and purity of the refined polysaccharide are high, the derivation effect is better and the like.
Owner:CHENGDU OLYMVAX BIOPHARM
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
InactiveCN104069488AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed by covalent linkage of multivalent pneumococcus capsular polysaccharides of 14 different serotypes and carrier protein, wherein the 14 serotypes include 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F. The conjugated composition has good adsorption effect and good stability, has multiple immunogenicity and protective performance against invasion of the pneumococcus of 14 serotypes, is superior to on-sale low-valent pneumonia compositions, and the immune response of the conjugated composition disclosed by the invention is higher than that of an uncombined composition. The inoculating injection frequency can be reduced by using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the immune process can be simplified, and diseases of human and animals caused by the 14 serotypes of pneumococcal bacteria can be effectively prevented. The conjugated composition has wider coverage and better immune effect.
Owner:SINOVAC RES & DEV
Method of immunizing humans against Salmonella typhi using a Vi-rEPA conjugate vaccine
InactiveUS6797275B1Reduce severityPrevent typhoid feverAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
This invention relates to conjugates of the Vi polysaccharide of S. typhi with the carrier Pseudomonas aeruginosa recombinant exoprotein A (rEPA), and compositions thereof, and to methods of using of these conjugates and / or compositions thereof for eliciting an immunogenic response in humans, including responses which provide protection against, or reduce the severity of, S. typhi bacterial infections. The conjugates, and compositions thereof, are useful as vaccines to induce serum antibodies againt S. typhi and are useful to prevent and / or treat illnesses caused by S. typhi.
Owner:UNITED STATES OF AMERICA
Conjugate vaccines for the prevention of dental caries
InactiveUS20070065465A1Organic active ingredientsBacterial antigen ingredientsConjugate vaccineEpitope
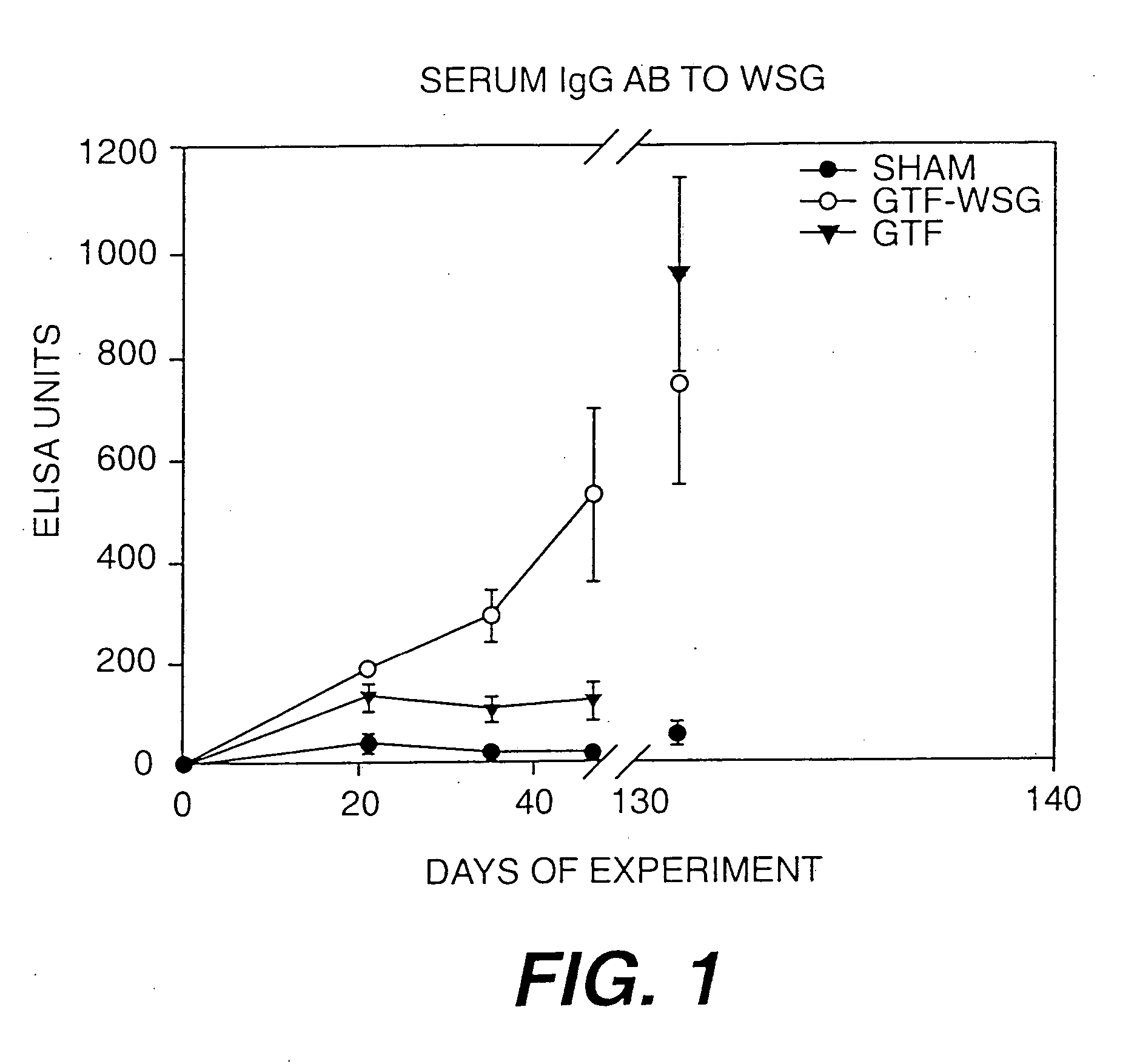
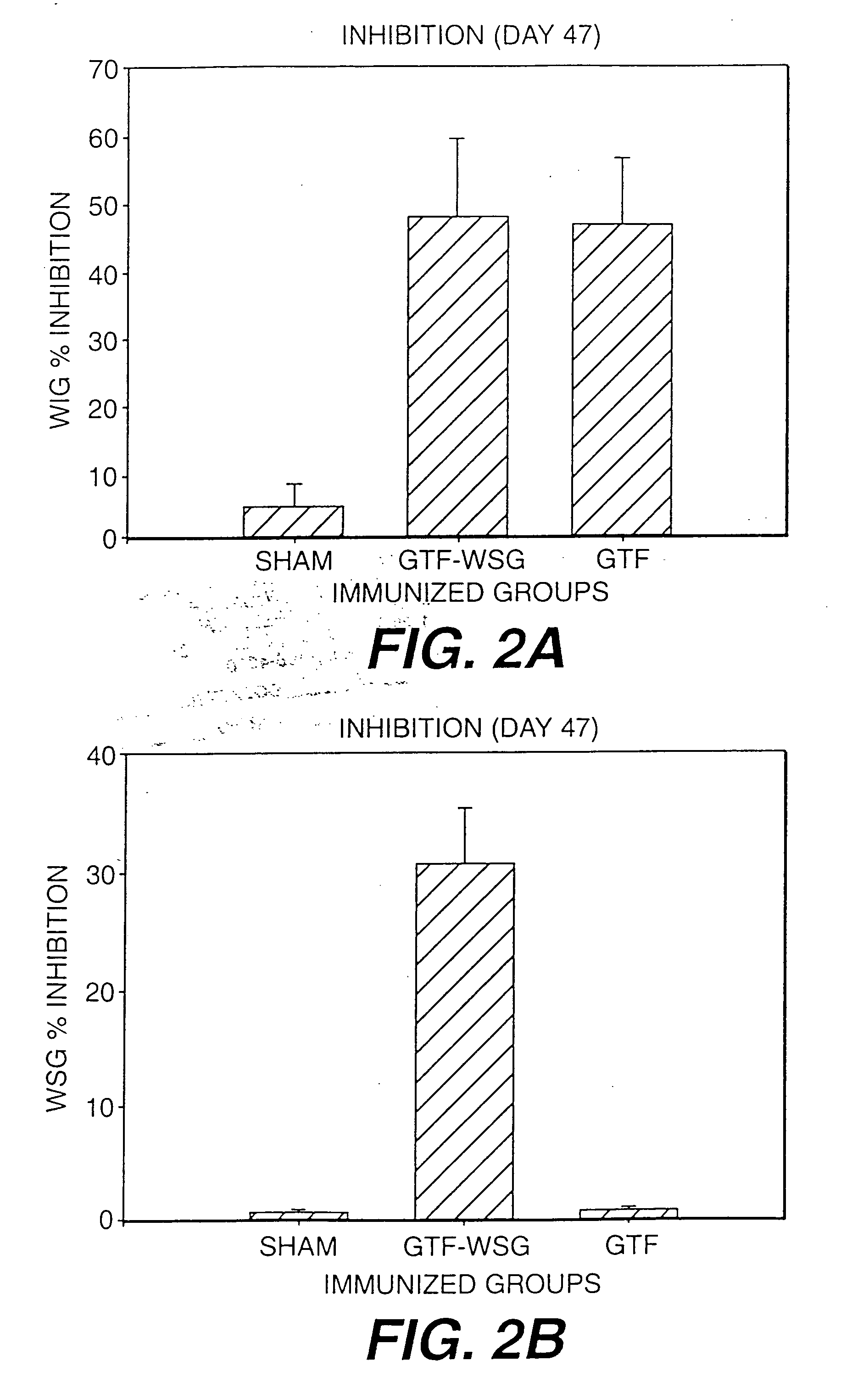
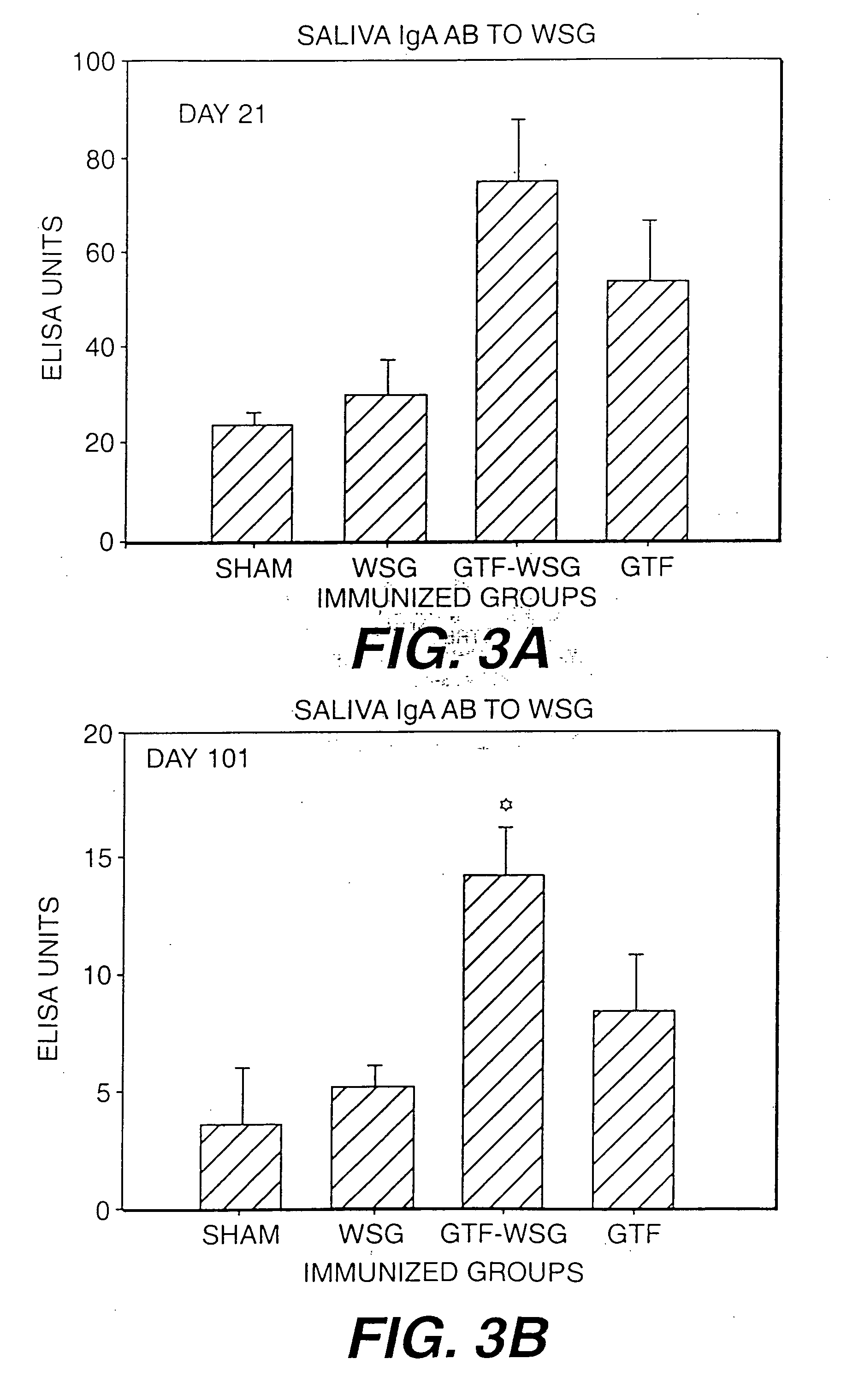
The present invention provides glucan-based compositions and methods for stimulating an immune response against mutans streptococci components and vaccines and methods for the treatment and prevention of dental caries. In a preferred embodiment, a glucan polymer, preferably WSG, is covalently bound to one or more T cell-dependent antigens to form a conjugate vaccine. The T cell-dependent antigen preferably contains epitopes of one or more mutans streptococcal proteins, such as a glucosyltransferase. Moreover, one or more moieties, including haptens, may be conjugated to the glucan or to the glucan-T cell-dependent composition. In a preferred embodiment, these moieties are peptides which contain immunogenic epitopes corresponding to components of a mutans streptococcus.
Owner:LEES ANDREW +2
Methods of assaying vaccine potency
InactiveUS20090075304A1Immunoglobulin superfamilyCancer antigen ingredientsConjugate vaccineVaccine Potency
The present invention is related to methods of assaying potency of a vaccine composition, wherein the potency is a pre-defined minimum level of potential biological activity for the vaccine composition. The method includes providing a vaccine composition and delivering same to an antigen presenting cell, wherein the vaccine composition is processed into peptides and the peptides are presented by MHC complexes on the cell surface. An agent, such as a T cell receptor mimic, that is reactive against a specific peptide / MHC complex is provided and reacted with the vaccine-treated antigen presenting cell, whereby the agent binds to the cell surface of the vaccine-treated antigen presenting cell if the specific peptide / MHC complex recognized by the agent is present on the cell surface. A density of the specific peptide / MHC complex on the surface of the vaccine-treated antigen presenting cell is measured by agent binding. The potency of the vaccine is then determined based upon the measured density of specific peptide / MHC complex present on the surface of the vaccine-treated antigen presenting cell.
Owner:RECEPTOR LOGIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com