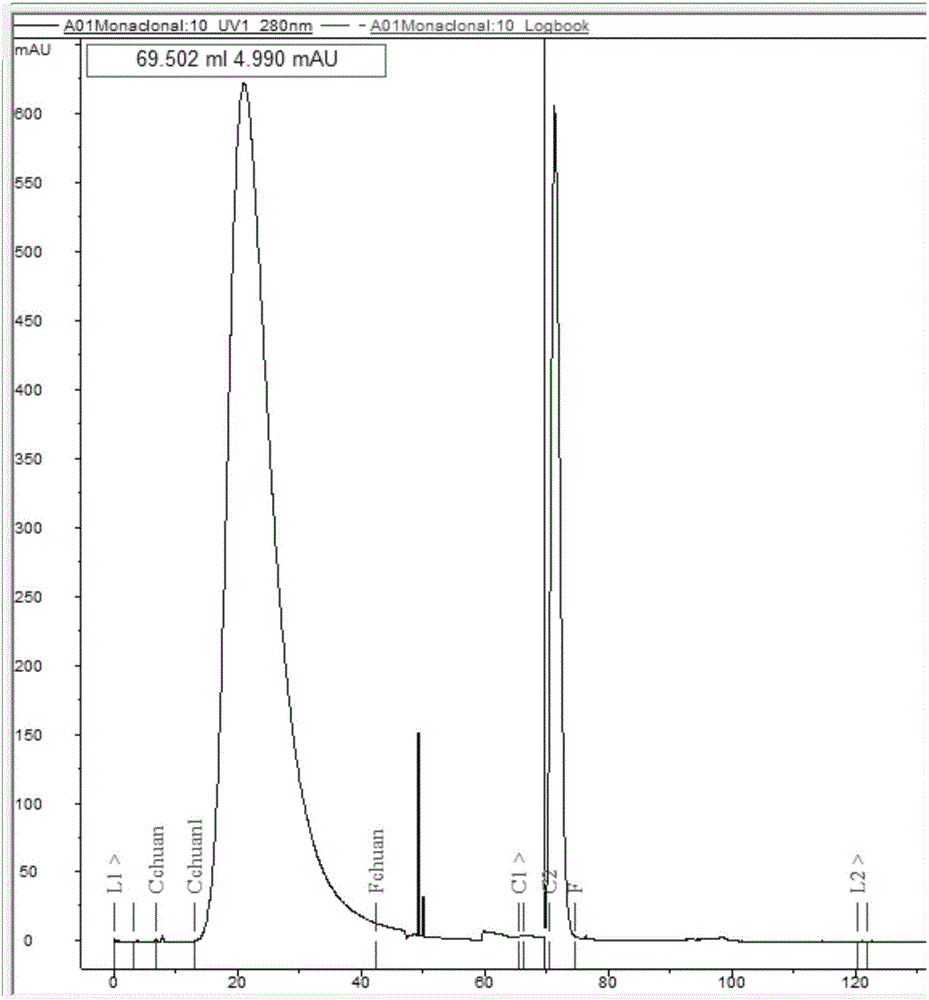
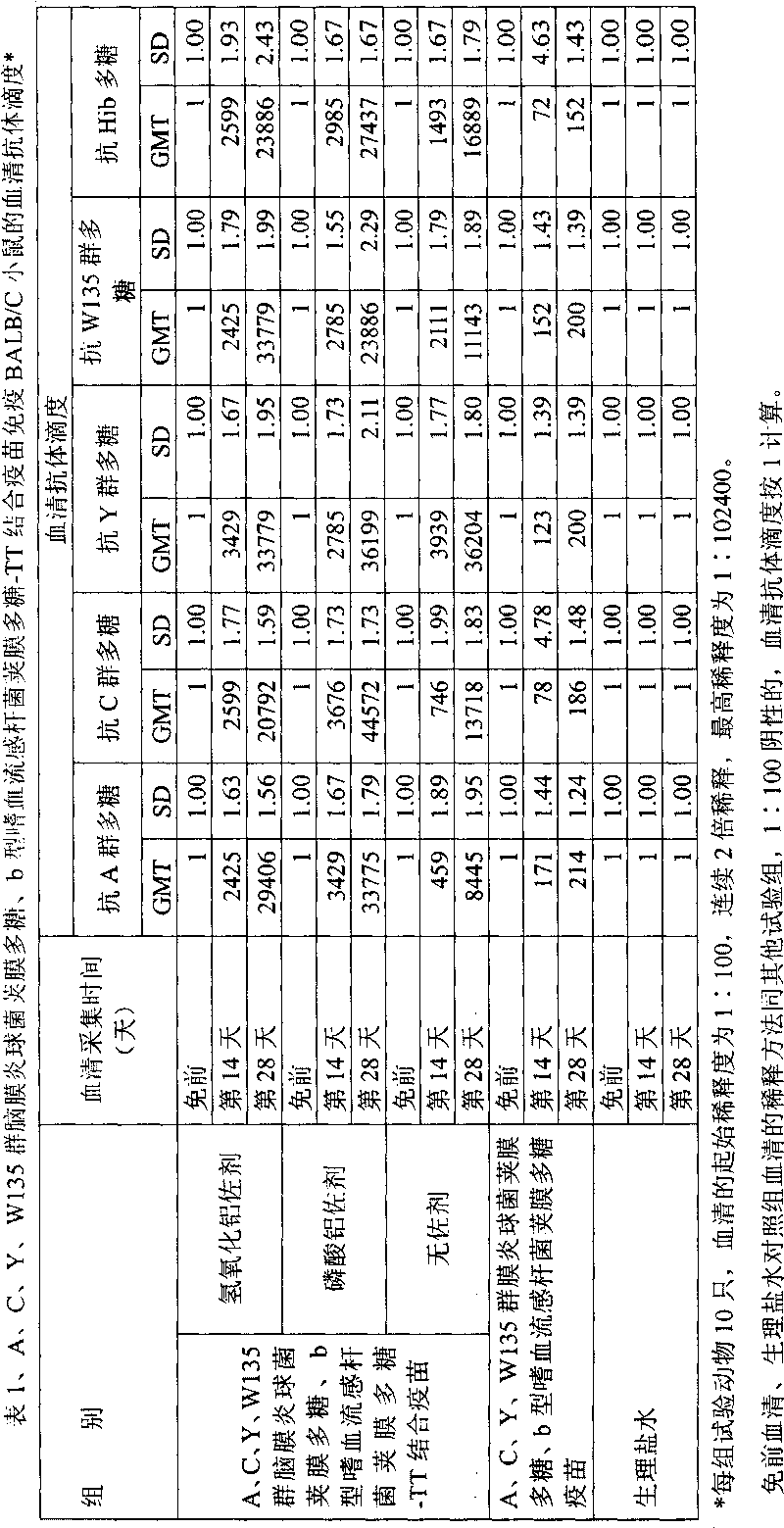
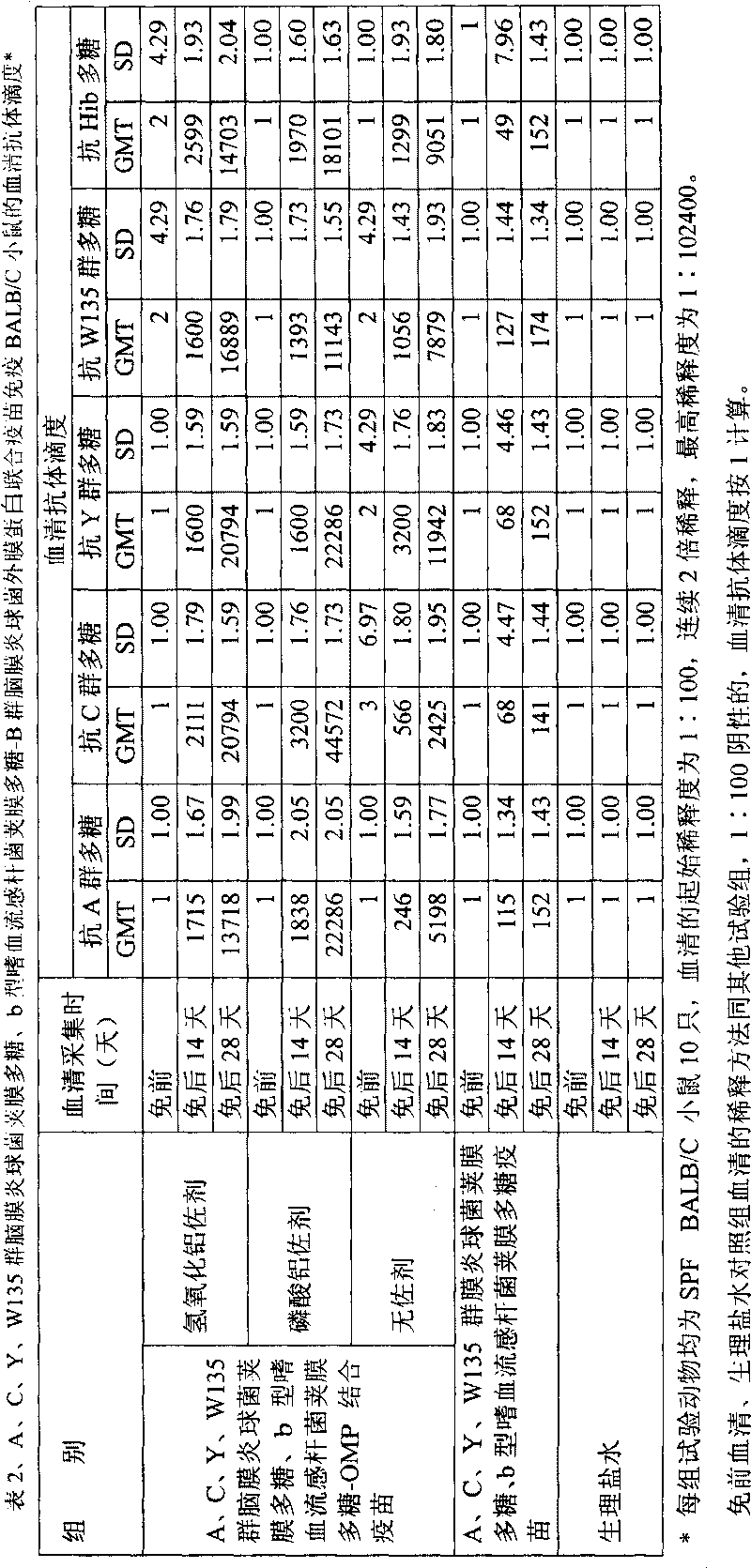
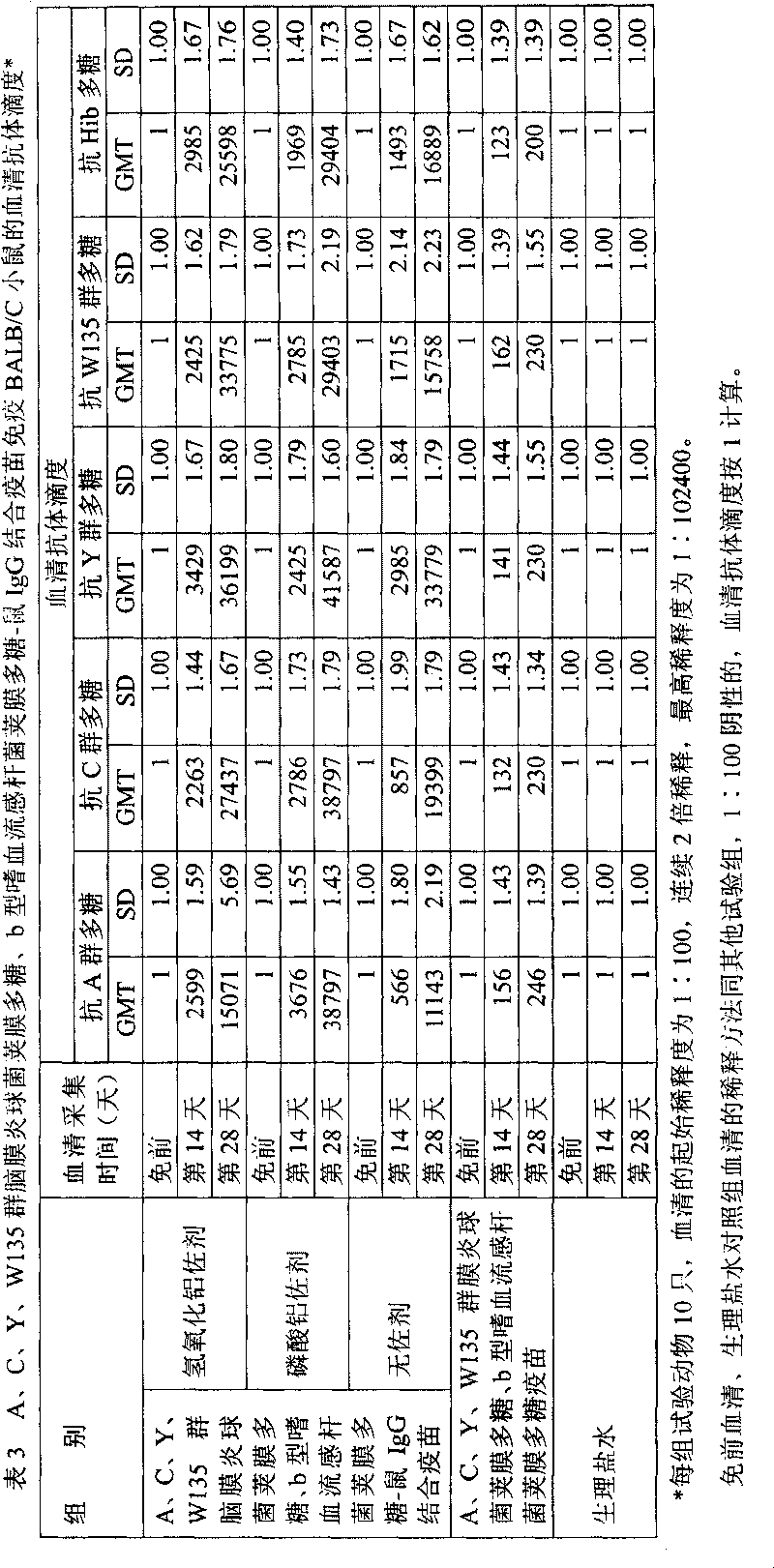
Patents
Literature
73 results about "Cerebrospinal meningitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
British Dictionary definitions for cerebrospinal meningitis. noun. an acute infectious form of meningitis caused by the bacterium Neisseria meningitidis, characterized by high fever, skin rash, delirium, stupor, and sometimes comaAlso called: epidemic meningitis.
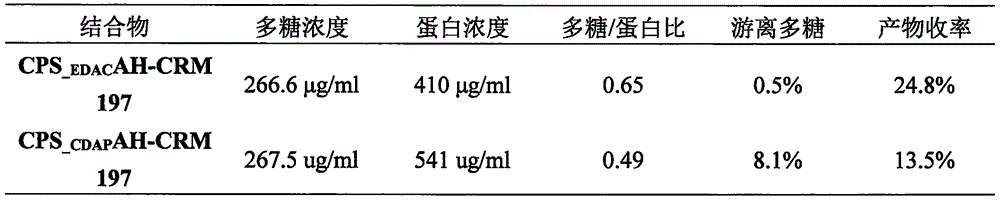
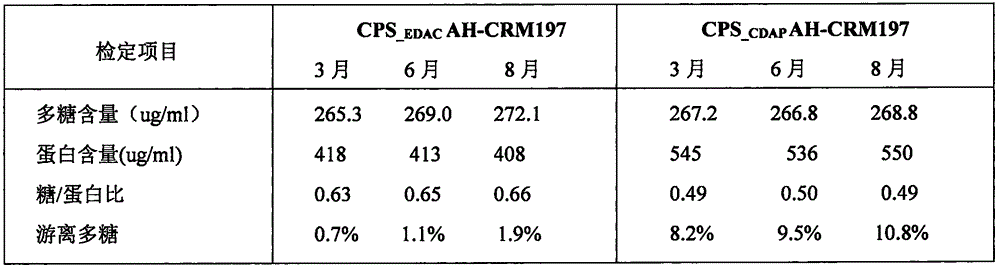
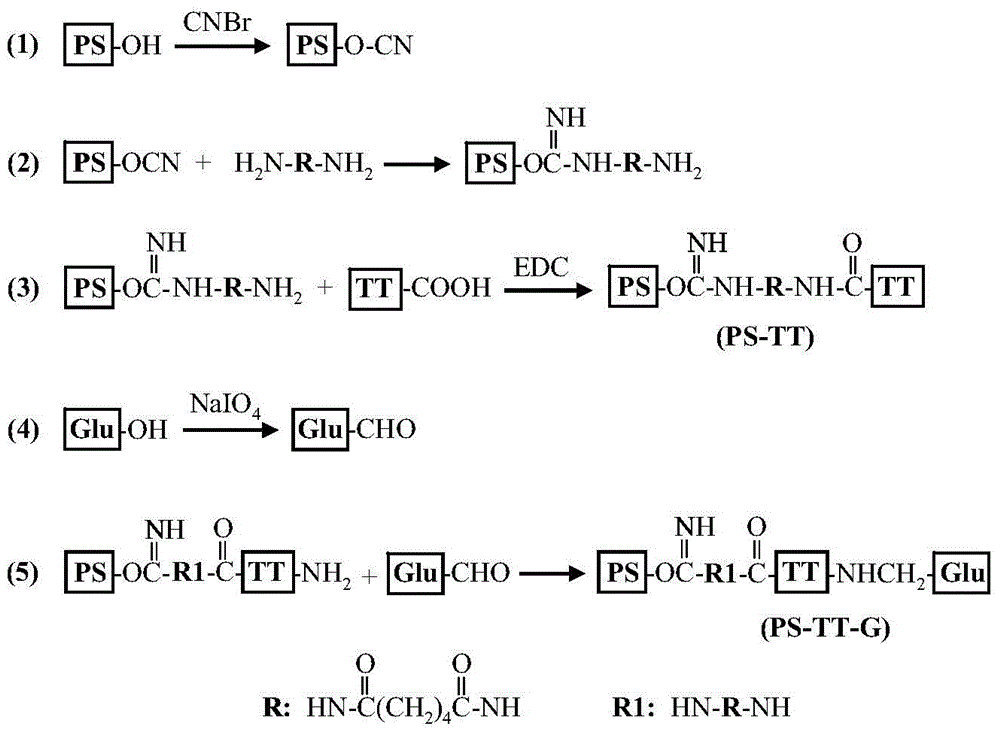
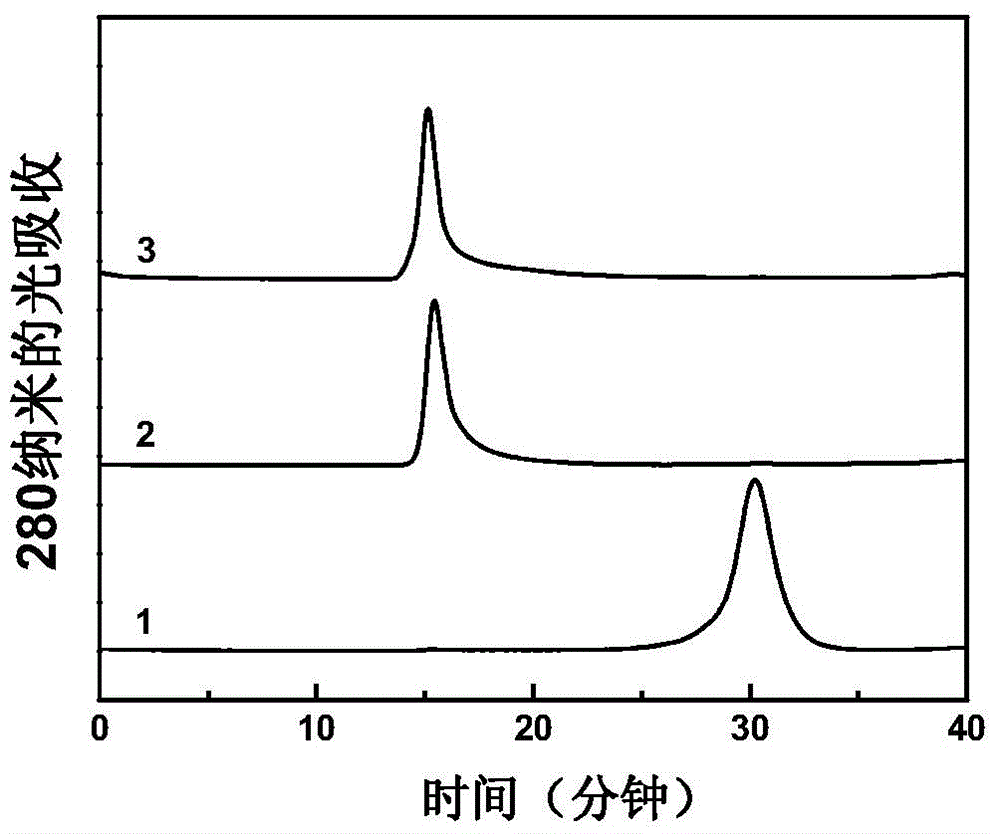
Poly saccharide-protein combination vaccine
InactiveCN1425465AImproving immunogenicityReduce the number of vaccinationsViral antigen ingredientsKetone active ingredientsMedicineNeisseria
Group A and Group C epidemuic cerebrospinal meningitis Neisseria capsular polysaccharide and type-B influenza Hemophilus polysaccharide are combined onto effective protein carrier chemically via covalent bond, so that combined polysacchairde-protein vaccine for immunologically inocualting human body is prepared to prevent infection caused by Group A and Group C epidemic cerebrospinal meningitis neisseria and type-B influenza Hemophilus.
Owner:BEIJING LUZHU BIOTECH +1
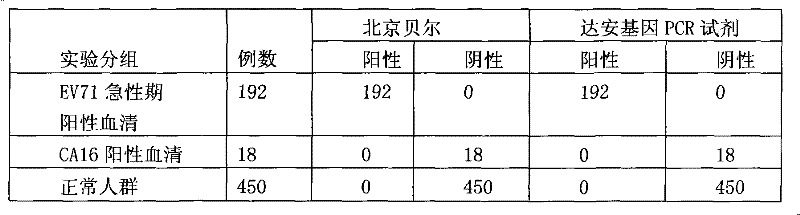
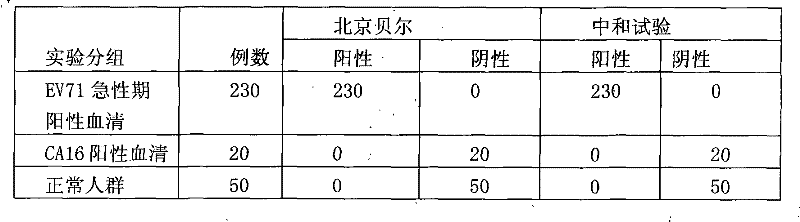
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
Polyvalent bacteria capsule polysaccharide-protein conjugate combined vaccine
ActiveCN1709505AImproving immunogenicityReduce the number of vaccinationsBacterial antigen ingredientsHaemophilusMedicine
The present invention relates to a polyvalent bacterial capsule polysaccharide-protein conjugate combined vaccine preparation, in particular, it is a combined vaccine containing group A, group C, group Y and group W135 epidemic cerebrospinal meningitis coccal capsule polysaccharide-protein conjugate and b type haemophilus influenzal capsule polysaccharide-protein conjugate.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM
Epidemic meningitis polyose-protein binding vaccine
A polyose-protein vaccine for epidemic cerebrospinal meningitis is prepared through chemically binding the two Neisseria capsular polyoses of cluster-A and-C epidemic cerebrospinal meningitis with protein carrier by covalent bonds.
Owner:BEIJING LUZHU BIOTECH
Traditional Chinese medicine composition for treating epidemic cerebrospinal meningitis
InactiveCN103830523ALittle side effectsGood curative effectAntibacterial agentsHeavy metal active ingredientsSide effectRegimen
The invention provides a traditional Chinese medicine composition for treating epidemic cerebrospinal meningitis. The composition is fewer in side effect, good in curative effect, short in course of treatment and low in cost. The traditional Chinese medicine composition for treating epidemic cerebrospinal meningitis comprises the following raw medicinal material in parts by weight: 10-15g of isatis roots, 3-5g of scutellaria baicalensis, 3-5g of golden cypress, 3-5g of jasmine, 10-15g of trumpet shells, 1.5-3g of leeches, 9-12g of corals, 5-9g of liquorice, 10-15g of rhizoma anemarrhenae, 10-15g of divaricate saposhnikovia roots, 10-15g of moutan barks, 9-12g of red ginseng, 9-12g of angelica sinensis, 9-12g of rhizoma atractylodis macrocephalae, 5-9g of pinellia ternate, 25g of hawthorn, 25g of akebiaquinata, 25g of rattletop and 3-5g of cinnabar.
Owner:王玉芳
Meningococcal vaccine and its preparing method
InactiveCN1457879ALess reactogenicityReduced reactogenicityAntibacterial agentsPeptide/protein ingredientsCerebrospinal meningitisMeningococcus vaccine
The present invention relates to meningococcal vaccine and its preparation process. Purified colony-A meningococcus polysaccharide and colony-C meningococcus polysaccharide are made to couple chemically with colony-B meningococcus outer membrane protein separately to form monovalent binders, and the monovalent binders are then mixed to compound the vaccine. The vaccine may be used for infant to prevent epidemic cerebrospinal meningitis caused by colony-A and colony-C meningococcus and ichorrhemia.
Owner:BEIJING SANROAD BIOLOGICAL PROD CO LTD
Traditional Chinese medicine composition for treating epidemic cerebrospinal meningitis
InactiveCN104042937AHeat-clearing and detoxifyingClearing away heat and cooling bloodAntibacterial agentsPlant ingredientsCerebrospinal meningitisToxin
The invention provides a traditional Chinese medicine composition for treating epidemic cerebrospinal meningitis. The traditional Chinese medicine composition comprises gypsum, radix gentianae, rhizoma anemarrhenae, licorice root, lalang grass rhizome and folium isatidis, has effects of removing heat and toxins, and clearing heat and cooling blood, and has a clinical cure rate of 95%.
Owner:余勇成
Application of nitidine chloride to preparation of medicament for resisting autoimmunity disease and graft versus host disease
InactiveCN102008474ARich sourcesLow cost of treatmentOrganic active ingredientsSenses disorderInterleukin 10Dendritic cell
The invention provides application of nitidine chloride to the preparation of a medicament for resisting autoimmunity disease and graft versus host disease. An experiment shows that the nitidine chloride can inhibit the proliferation of human dendritic cells in vitro, inhibit the human dendritic cells from promoting the proliferation reaction of allogeneic T cells and strengthen the capacity of secreting interleukin 10 of the human dendritic cells. Meanwhile, in an experimental autoimmunity disease cerebrospinal meningitis model, when used in vivo, the nitidine chloride can reduce the average clinical score of a mouse and inhibit the spinal marrow infiltration degree of monocytes and can obviously improve the secretion level of the interleukin 10 in the blood serum of the mouse. In a heart transplantation experiment, when used in vivo, the nitidine chloride can prolong the survival time of the transplanted heart. In a delayed type hypersensitivity reaction model, when used in vivo, the nitidine chloride can reduce the ear swelling degree of the mouse. Both in-vivo and in-vitro results show that the nitidine chloride has a certain effect of resisting the autoimmunity disease and the graft versus host disease. The medicament is a preparation which consists of the nitidine chloride serving as an active ingredient and a pharmaceutical carrier. The medicament can be prepared into an oral preparation, an injection, a suppository or an external preparation and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Group ABC meningococcus combined vaccine and preparing method thereof
PendingCN106215183AEase the pain of vaccinationEffective preventive effectAntibacterial agentsBacterial antigen ingredientsPericarditisMENINGOCOCCAL POLYSACCHARIDE
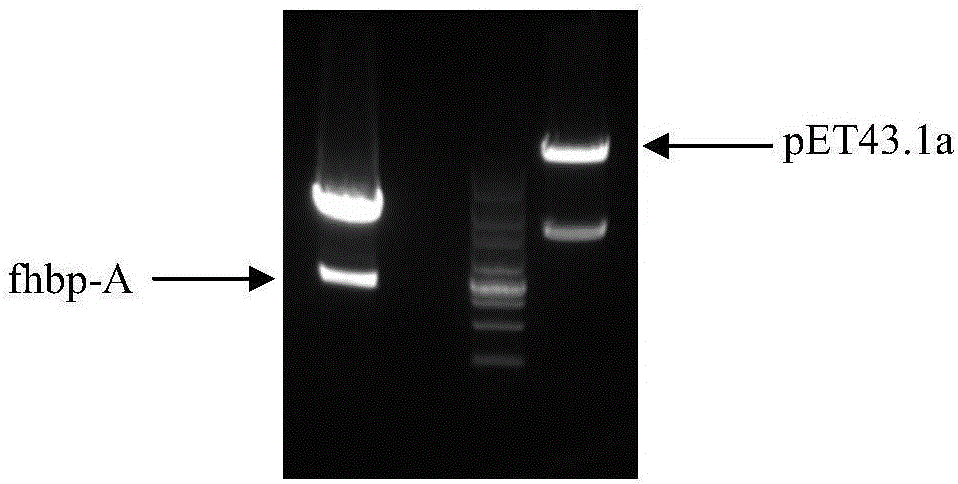
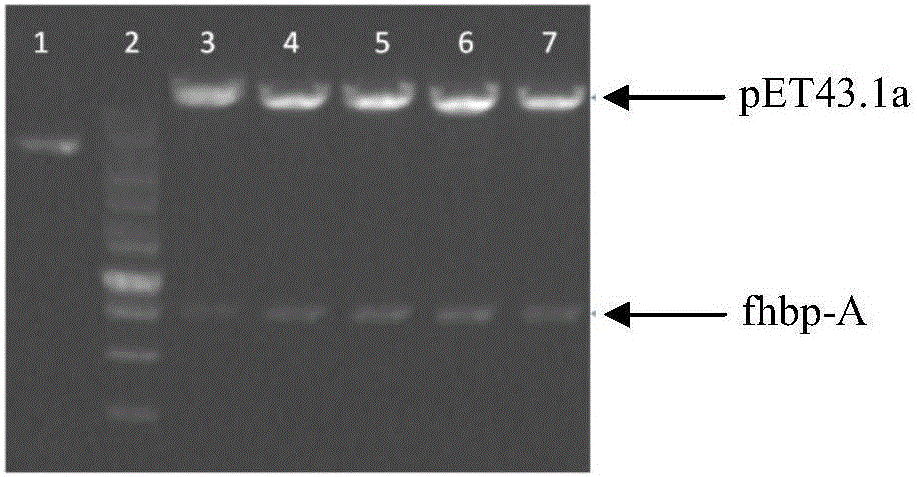
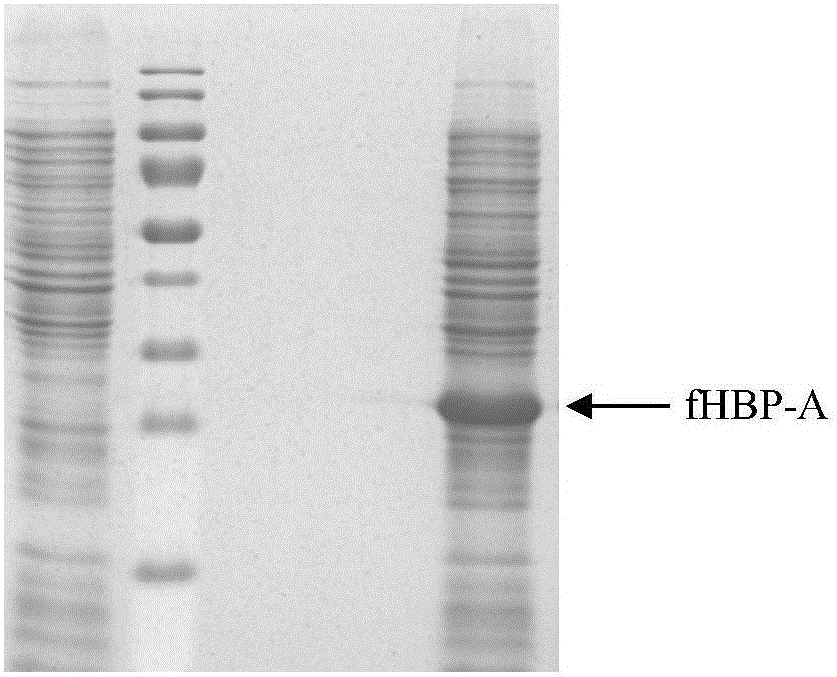
The invention provides a group ABC meningococcus combined vaccine and a preparing method thereof. The group ABC meningococcus combined vaccine is prepared from a group A and group C meningococcus polysaccharide-protein conjugate, and recombinant protein of a human H factor binding protein (fHBP) subgroup A and a human H factor binding protein (fHBP) subgroup B of group B meningococcus. The invention further provides a preparing method of the recombinant fHBP-A protein and fHBP-B protein. The combined vaccine is used for immunity of children 2 or more years old, and used for preventing invasive diseases such as cerebrospinal meningitis, bacteremia, pneumonia and pericarditis caused by group A or group B or group C meningococcus, and providing a better and wider protection effect on meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Preparation method of group C meningococcal capsular polysaccharide conjugate vaccine
InactiveCN105879020AHarm reductionReduce hydrolytic denaturationAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineMeningococcal carriage
Owner:北京成大天和生物科技有限公司
Beta-glucan modified meningitis polysaccharide conjugate vaccine and preparation method thereof
ActiveCN104548090AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsCarrier-bound antigen/hapten ingredientsCyanogen bromideMeningococcal meningitis
The invention relates to a beta-glucan modified meningitis polysaccharide conjugate vaccine and a preparation method thereof. The preparation method of the beta-glucan modified meningitis polysaccharide conjugate vaccine comprises the following steps: (1) activating meningococcus polysaccharide by cyanogen bromide, and then deriving by adopting adipic dihydrazide; (2) combining a derived meningococcus polysaccharide derivative with carrier protein; (3) activating beta-glucan; and (4) modifying polysaccharide-protein conjugate by the activated beta-glucan. By virtue of the steps, a novel and efficient meningitis polysaccharide conjugate vaccine can be prepared and can be used for preventing infection caused by epidemic cerebrospinal meningitis Neisseria gonorrhoeae.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Combined vaccine bonding bacterial polysaccharides and protein for human
InactiveCN101559222AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsBacterial antigen ingredientsBacterial polysaccharideHaemophilus influenzae
The invention discloses a bacterial polysaccharides and protein binding combined vaccine for human, wherein Group A and Group B epidemic cerebrospinal meningitis Neisser's coccus capsular polysaccharides and Type B haemophilus influenzae capsular polysaccharide are bonded on an effective protein carrier with covalent bonds by a chemical method to prepare a preventive combined vaccine bonding polysaccharides and protein for human. Three vaccinations are conducted on children with the age of three to eight months and each for one month, and one vaccination is conducted on children older than one year. More than 96.5 percent of vaccine inoculators can be immunized from the Group A and Group C epidemic cerebrospinal meningitis Neisser's coccus and 91.2 percent of the vaccine inoculators can obtain long-term immunity from the Type B haemophilus influenzae one month after immunization. The vaccine is applied to the prevention of the infection caused by the Group A and Group C epidemic cerebrospinal meningitis Neisser's coccus and the Type B haemophilus influenzae.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +1
Novel medicinal application of baicalein
InactiveCN102106849AEasy to takeEasy to controlOrganic active ingredientsNervous disorderDiseaseNervous system
The invention discloses novel medicinal application of baicalein, in particular to application of the baicalein to the preparation of medicament for treating demyelinative diseases of central nervous system. The baicalein shows favorable treatment functions on an experimental allergic cerebrospinal meningitis model of a mouse and the treatment functions are achieved by inhibiting microglia 12 / 15 lipoxygenase in the central nervous system and adjusting the expression condition of a group of molecules with nerve protecting functions, such as FIZZ1 (foundininflammatoryzone1), MRC1 (mannose receptor, C type 1) and Arg1 (arginase 1) on the mRNA (messenger ribonucleic acid) level, thereby inducing high-level M2 type microglia in the central nervous system. The M2 type microglia has the function of CNS (central nervous system) neuroendocrine nutrilit and can promote the repair of medullary sheath, thereby relieving the progress of disease conditions.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Phlegm eliminating and convulsion arresting powder and application thereof
InactiveCN102335357AReasonable formulaGood effectAntibacterial agentsInorganic boron active ingredientsConvulsionJapanese encephalitis
The invention discloses phlegm eliminating and convulsion arresting powder and application thereof. The phlegm eliminating and convulsion arresting powder is prepared from roasted scorpion, centipede, defatted croton seed powder, bezoar, borax, realgar, arisaema cum bile, unibract fritillary bulb, tabasheer and musk. The formula is reasonable, and the powder has a good treatment effect, and can effectively treat diseases such as Japanese encephalitis, pneumonia, epidemic cerebrospinal meningitis, toxic bacillary dysentery, pertussis encephalopathy and the like.
Owner:朱良春
Method for processing apple flavor water chestnut soft sweets
InactiveCN104431249AAdd cleaning functionBoth deliciousConfectionerySweetmeatsBiotechnologyCarrageenan
The invention discloses a method for processing apple flavor water chestnut soft sweets, and belongs to the field of food processing. The method is characterized by comprising the following steps: processing raw materials, including 100% of water chestnut, 25% of malt syrup, 35% of apple juice, 60-65% of glucose, 2-3% of carrageenan, 0.25-0.35% of citric acid and 0.2-0.4% of potassium sorbate, filtering, blending, cooling and molding, slitting, wrapping with glutinous rice paper, drying and packaging. The method has the beneficial effects that the apple flavor water chestnut soft sweets are thick in apple flavor, are full of elasticity and toughness, have mild sour sweetness, have the unique fresh and sweet flavor of the water chestnut and the sour and sweet flavor of an apple, are rich in multiple nutritional substances such as proteins and fibers, are easily assimilated by human bodies, have a good intestinal tract cleaning function, not only remove heat to promote salivation, but also supplement nutrition, also can be used for preventing epidemic cerebrospinal meningitis and flu, are convenient to eat, are simple to operate, and are healthy and environment foods with great taste and nutrition.
Owner:林静
Multi-component group B meningococcus vaccine and preparation method thereof
PendingCN108939061AAdequate and widespread preventionAdequate and widespread infection preventionAntibacterial agentsApolipeptidesPericarditisInvasive disease
The invention provides a multi-component group B meningococcus vaccine and a preparation method thereof. The multi-component group B meningococcus vaccine disclosed by the invention comprises a groupB meningococcus low-endotoxin mutant outer membrane vesicle (OMV), recombinant lipoproteins (fHbp-A, fHbp-B) of group B meningococcus subfamily A and subfamily B human factor H binding proteins (fHbp), a group B meningococcus neisserial adhesion A (NadA) recombinant protein, and a group B meningococcus neisseria heparin binding antigen (NHBA) recombinant protein. The vaccine disclosed by the invention is used for preventing cerebrospinal meningitis, bacteremia, pneumonia, pericarditis and other invasive diseases caused by the group B meningococcus, and provides wide effective protection effects to the group B meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Drug for preventing and treating porcine hemagglutinating encephalomyelitis viruses
The invention relates to a drug for preventing and treating porcine hemagglutinating encephalomyelitis viruses and belongs to the technical field of medicine. The drug is used for solving the problem in the treatment of porcine hemagglutinating encephalomyelitis. The drug is characterized by being prepared through combining the following traditional Chinese medicine in parts by mass: 15 parts of cornu bubali, 10 parts of raw rhubarb roots, 9 parts of mirabilite, 12 parts of immature bitter oranges, 8 parts of prepared pinellia tubers, 10 parts of areca catechu, 15 parts of rhizoma anemarrhenae, 15 parts of fructus forsythiae, 15 parts of folium isatidis, 15 parts of mung bean, 4 parts of radix puerariae, 3 parts of flos lonicerae japonicae, 15 parts of radix isatidis, 13 parts of giant typhonium rhizomes and 15 parts of caulis spatholobi.
Owner:毛华养
Application of lariciresinol to preparing medicament for resisting autoimmune disease and graft rejection disease
InactiveCN101991566AInhibitionInhibition of developmentOrganic active ingredientsSenses disorderInterleukin 10White blood cell
The invention provides application of lariciresinol to preparing a medicament for resisting an autoimmune disease and a graft rejection disease. An experiment discovers that: the lariciresinol can inhibit human dendritic cell proliferation in vitro and inhibit the capability of human dendritic cells of promoting allogeneic T cell proliferation reaction and enhancing the secretion of interleukin 10. Meanwhile, in an experimental autoimmune myelomeningitis model, the lariciresinol is applied in vivo, so that an average clinical score of mice can be reduced and the spinal infiltration of mononuclear cells is inhibited. In a heart transplant experiment, the lariciresinol is applied in vivo, so that the survival time of a transplanted heart is prolonged. In a delayed hypersensitivity model, the lariciresinol is applied in vivo, so that the auricle swelling degree of the mice can be reduced. In-vivo and in-vitro results both show that: the lariciresinol has the certain effect of resisting the autoimmune disease and graft rejection disease. The medicament is a preparation which consists of the lariciresinol serving as an active ingredient and a medical carrier, and comprises an oral preparation, an injection, a suppository, an external preparation and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
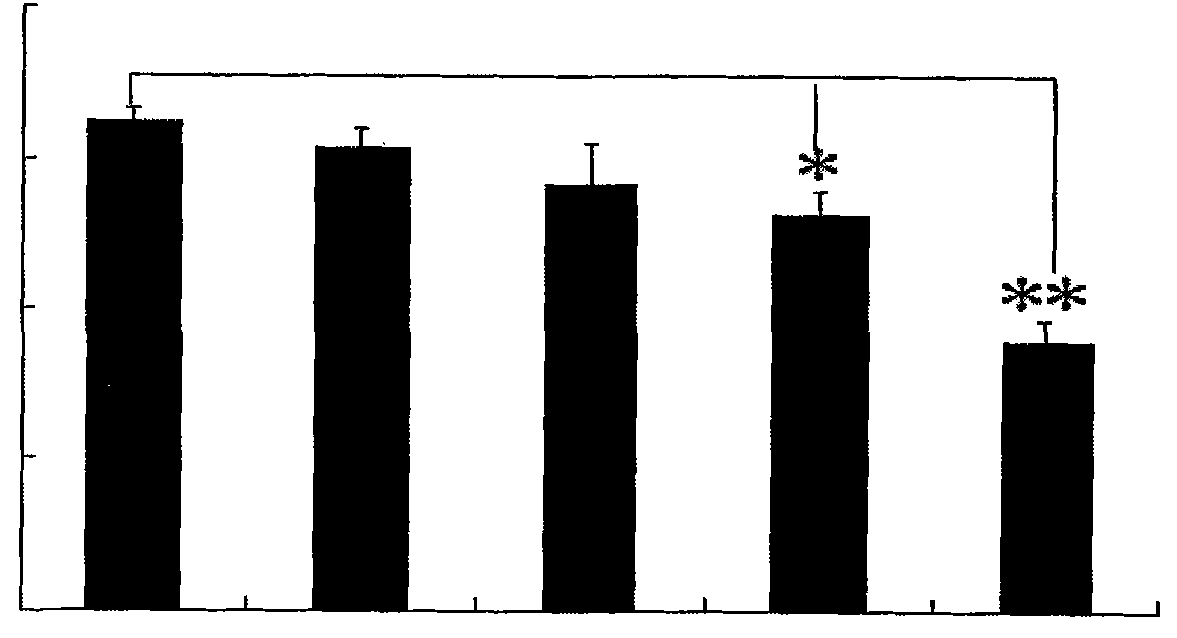
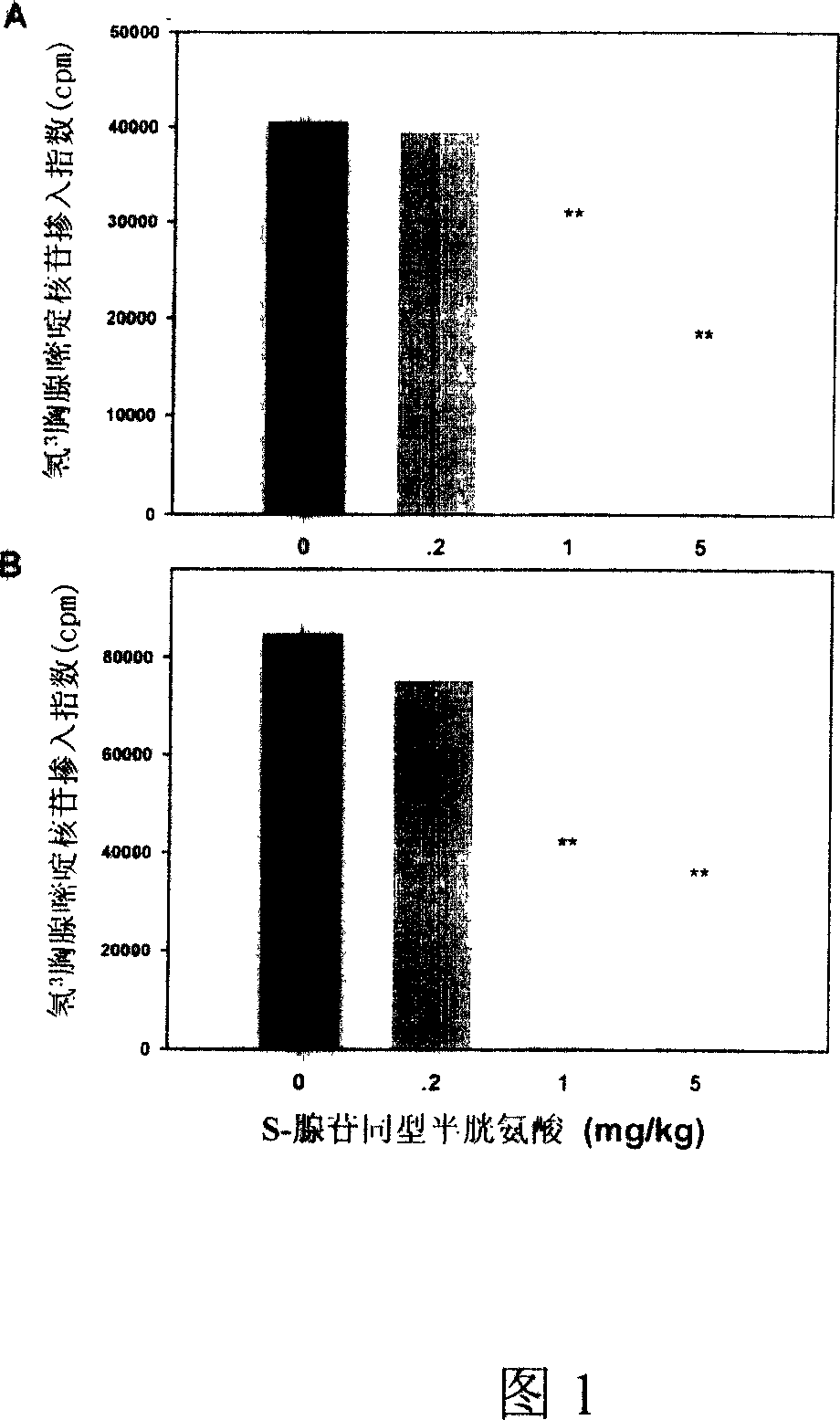
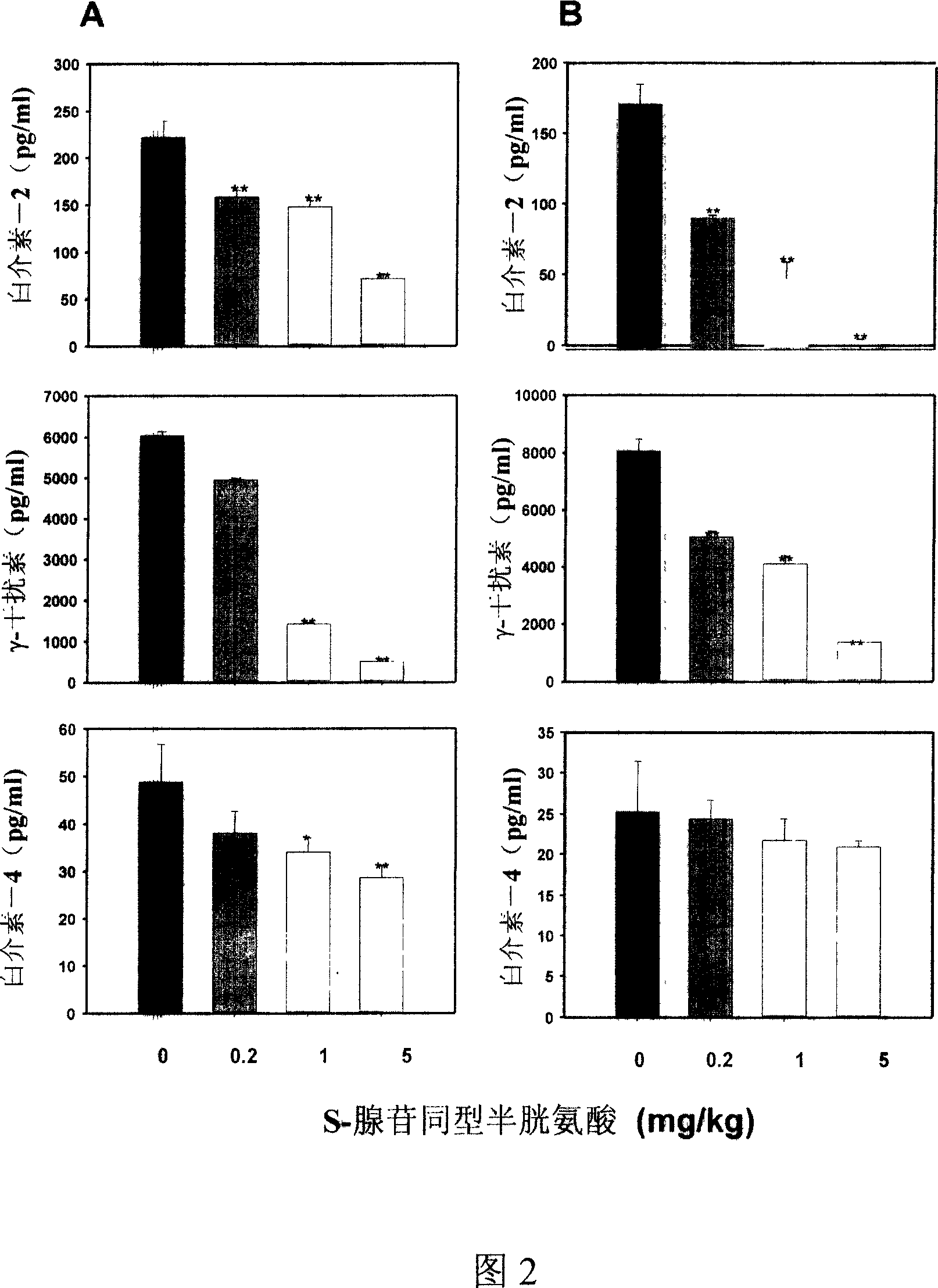
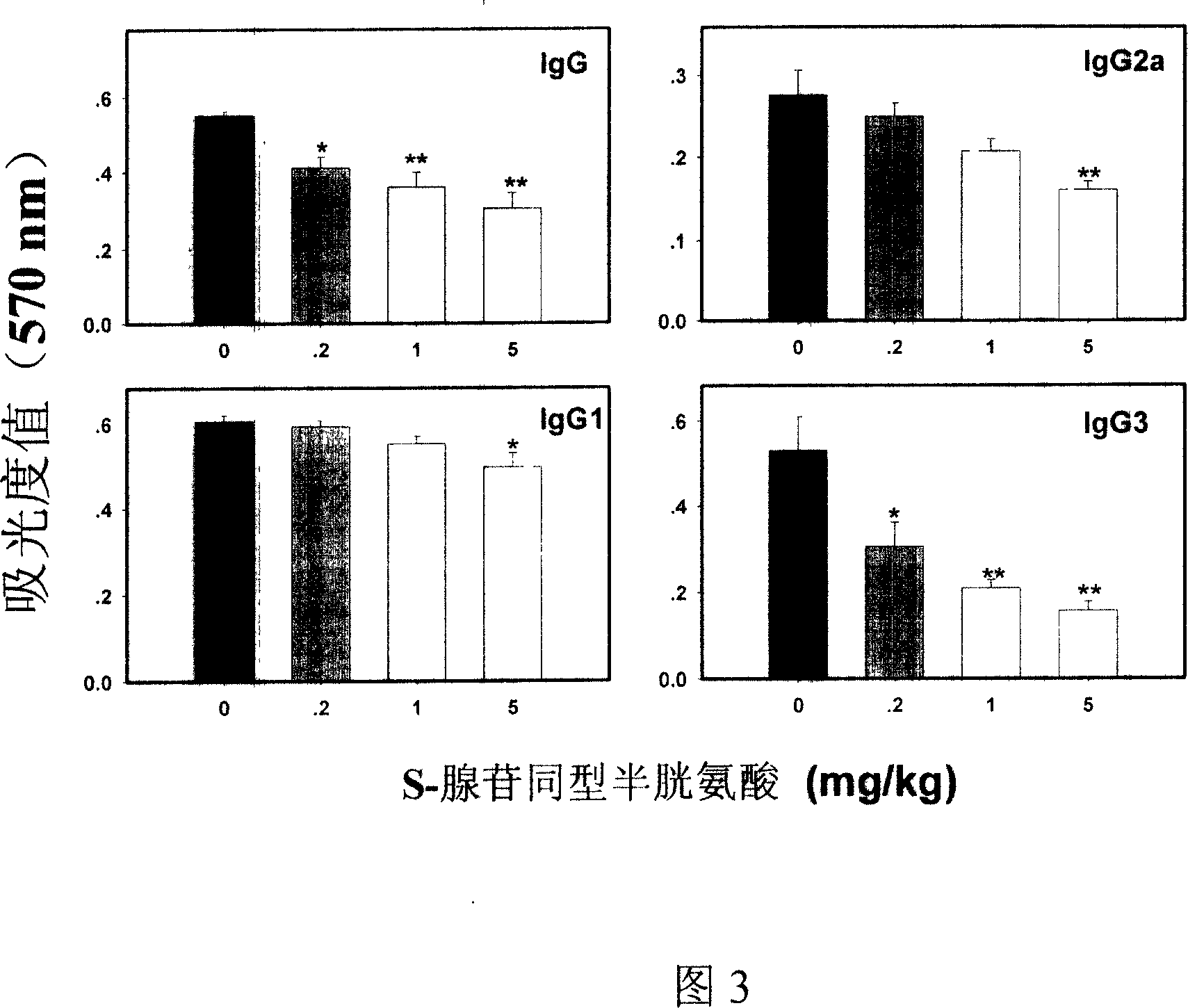
Medical usage of S-adenyhomotype cysteine
A medical application of S-adenosine homocysteine in preparing immunodepressant with high effect and low poison is disclosed.
Owner:NINGBO ZIYUAN PHARMA INC
Chinese herb agentia for treating epidemic cerebrospinal meningitis and preparation method thereof
InactiveCN104306679AEasy to makeHeat-clearing and detoxifyingAntibacterial agentsPowder deliveryBiotechnologyMeningococcal meningitis
The invention discloses Chinese herb agentia for treating epidemic cerebrospinal meningitis. The Chinese herb agentia is prepared mainly through, by weight, 0.5-1.2 parts of cyrtomium fortunei, 0.4-1.5 parts of fructus forsythiae, 0.8-1.6 parts of isatis roots, 0.3-0.8 part of galinsoga parviflora, 0.6-1.5 parts of rhizoma dioscoreae nipponicae, 0.8-1.6 parts of catharanthus roseus, 0.6-1.0 part of manglietia fordiana fruits, 1.0-1.8 parts of almonds, 1.5-2.0 parts of astragalus membranaceus, 0.3-0.5 part of stiff silkworms, 0.4-1.2 parts of stiff silkworm and 0.4-1.0 part of lilies. The Chinese herb agentia has the effects of clearing away heat and toxic materials, invigorating qi for strengthening superficies and reducing phlegm and resolving masses, the clinical application verifies that the treatment effect is good, no toxic and side effect exists, and patients can be effectively helped to eliminate disease trouble.
Owner:郝建友
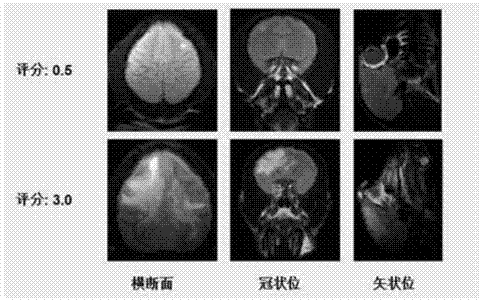
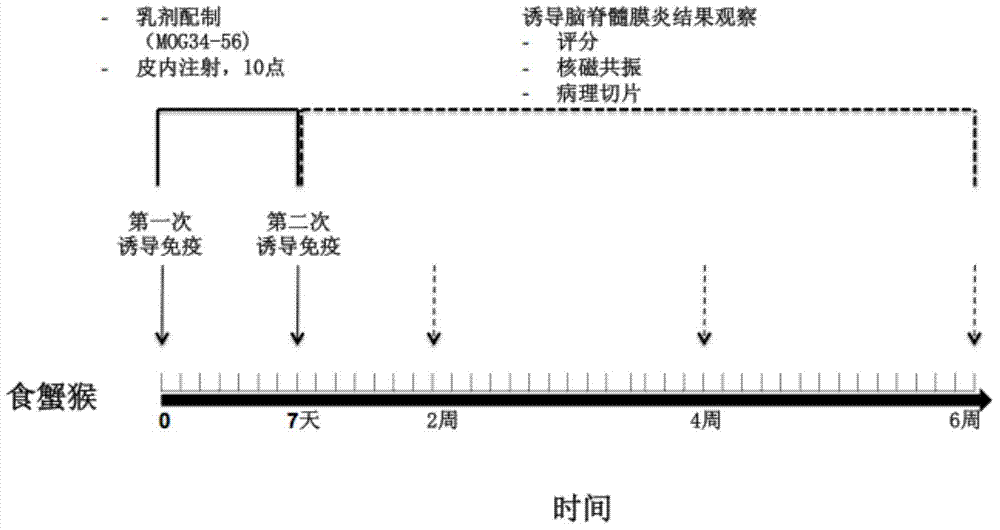
Method for establishing non-human primate autoimmune cerebrospinal meningitis model and application of model
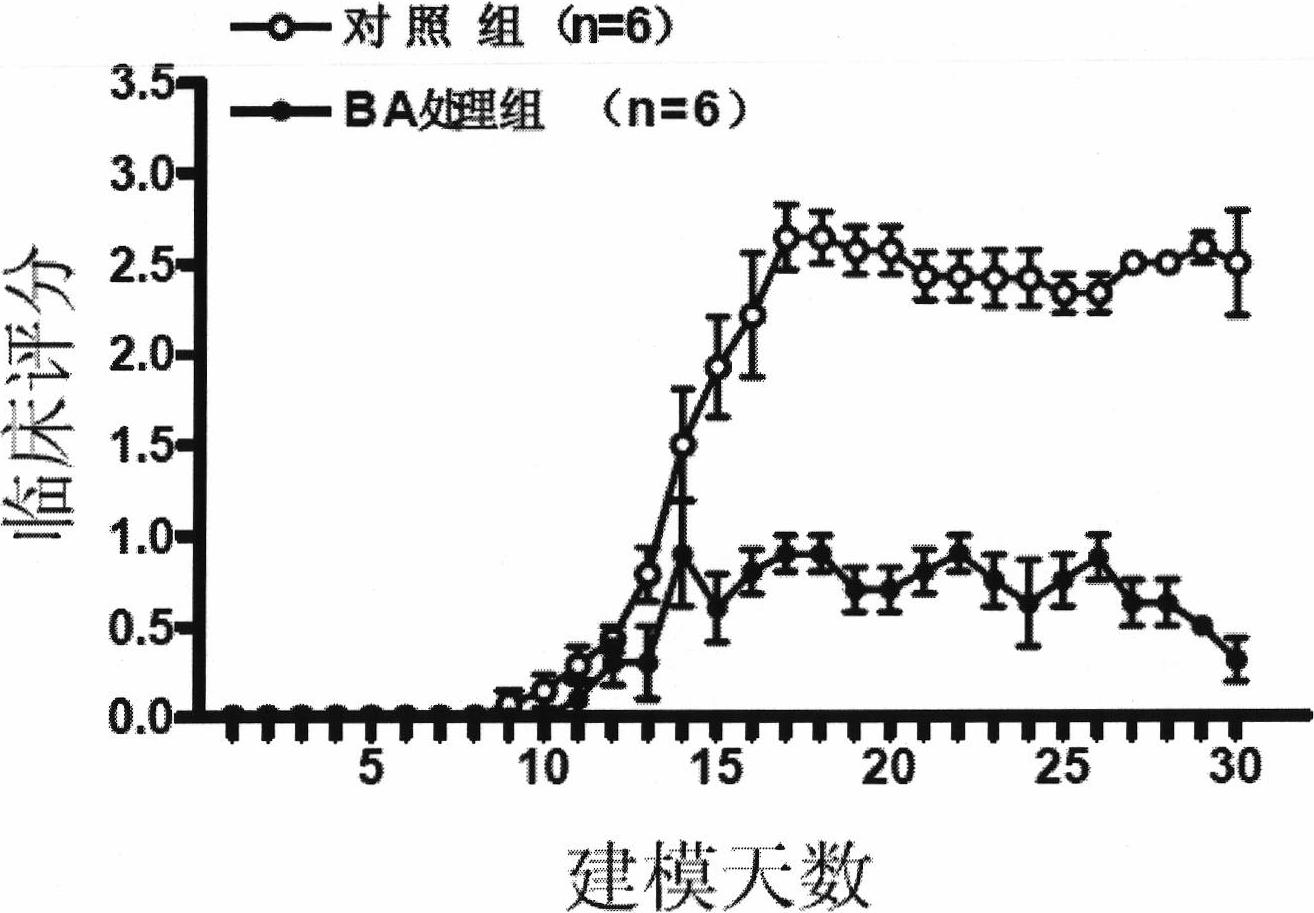
InactiveCN104758080AShorten the induction periodIndividual smallVeterinary instrumentsDiseaseMS multiple sclerosis
The invention discloses a method for establishing a non-human primate autoimmune cerebrospinal meningitis model and the application of the model. The specific technical scheme comprises the following steps; a machin is selected as the non-human primate experimental animals; MOG34-56(100Mug / ml) is prepared into an emulsion (MOG CFA=1; 1); after the experimental machin is anesthetized, the machin is intradermally injected with 1ml of prepared emulsion at 10 injection points; on the seventh day after the first injection, an MOG-CFA emulsion is prepared by use of the same method, and the second immune injection is performed at the same dosage and by use of the same method; the experimental autoimmune cerebrospinal meningitis model established by use of the method has the clinical characters of remission-relapse, and can be widely used to the related field of disseminated sclerosis diseases. Compared with the existing rhesus method and model, the method and the model also have the characteristics of short induction period, low cost, rich monkey resources and the like, and also have the application value which cannot be realized by rodent models.
Owner:上海浦灵生物科技有限公司
ABC group meningococcal combined vaccine and preparation method thereof
ActiveCN104001166BBest combinationGood prevention effectAntibacterial agentsBacterial antigen ingredientsGenotypeInvasive disease
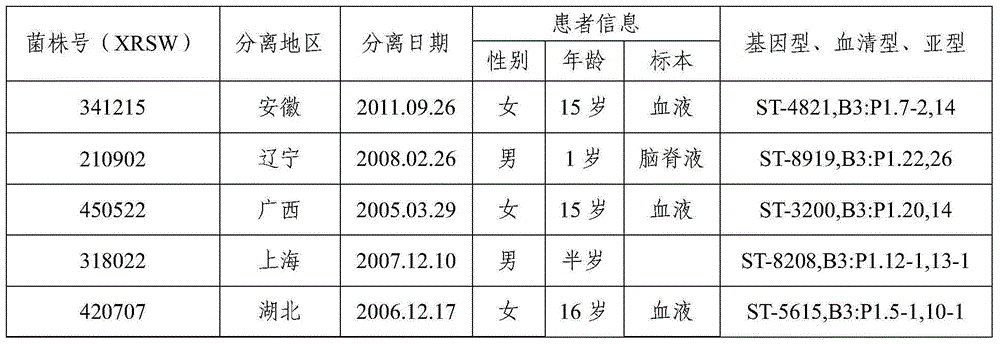
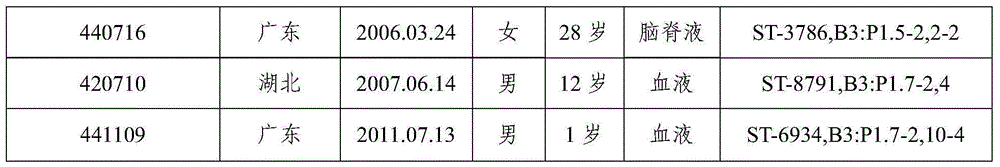
The invention provides a group A, group B and group C meningococcus combined vaccine which is composed of polysaccharide-protein conjugates and a group B ST-4821 genotype (xrsw341215) meningococcus outer membrane protein vesicle (OMV), wherein the polysaccharide-protein conjugates are obtained in a manner that a group A meningococcus capsular polysaccharide and a group C meningococcus capsular polysaccharide are each subjected to covalent binding with a group B serotype 4-type (CMCC29356) meningococcus outer membrane protein vesicle (OMV). The vaccine can simultaneously prevent group A, group B and group C meningococcus invasive diseases, has better protective effects especially on prevention aiming at epidemic cerebrospinal meningitis high-risk groups at the age of less than 2 years, and moreover, has better specificity and better protection effect aiming at nature group A, group B and group C meningococcus invasion.
Owner:CHENGDU OLYMVAX BIOPHARM
Preparation method for improving yield of epidemic cerebrospinal meningitis A group capsular polysaccharide
InactiveCN106282262AHigh yieldLow costMicroorganism based processesFermentationHeminFifth generation
The invention discloses a preparation method for improving the yield of epidemic cerebrospinal meningitis A group capsular polysaccharide. The method comprises the following steps of (1) starting strain and passage: starting the epidemic cerebrospinal meningitis A group strain passage onto a hemin epidemic cerebrospinal meningitis half-integral culture medium; putting the materials into a CO2 (carbon dioxide) cell culture box to perform culture by 8-percent CO2 at 36.5 DEG C; using a half-integral solid culture medium for culturing the second generation; using an epidemic cerebrospinal meningitis half-integral liquid culture medium for culturing the third generation in a constant-temperature oscillation oscillating table; (2) inoculating the materials into a seed tank to be cultured: inoculating the epidemic cerebrospinal meningitis A group seeds obtained through culture into the seed tank; performing constant temperature stirring and ventilation culture; (3) performing fermentation in a fermentation tank: after the epidemic cerebrospinal meningitis A group fifth-generation seeds obtained through culture are inoculated into the seed tank, maintaining the constant temperature of 36 DEG C; performing deep layer ventilation and 220rpm stirring culture; culturing the epidemic cerebrospinal meningitis A group bacteria to the later stage of the logarithmic phase or the early period of the still period (at the moment, the bacterium concentration reaches 10000 to 20000 million / mL); after the formaldehyde sterilization, removing the thalli, and collecting supernate; (4) obtaining polysaccharide after purification. The process provided by the invention has the advantage that the yield of the epidemic cerebrospinal meningitis A group capsular polysaccharide can be effectively improved.
Owner:CHENGDU OLYMVAX BIOPHARM
Serogroup B meningococcus recombinant chimeric protein vaccine and preparation method thereof
ActiveCN107823638AImprove the effect of preventionEffective protectionAntibacterial agentsNervous disorderPericarditisSalmonella serotype typhi
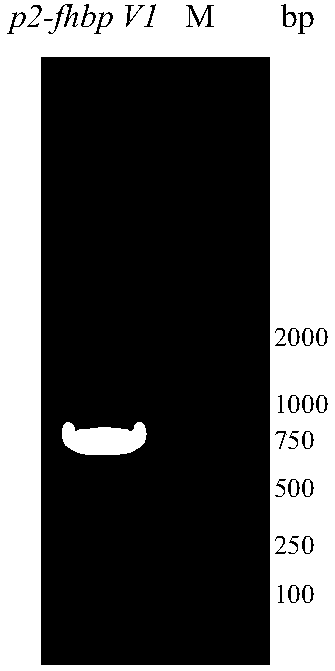
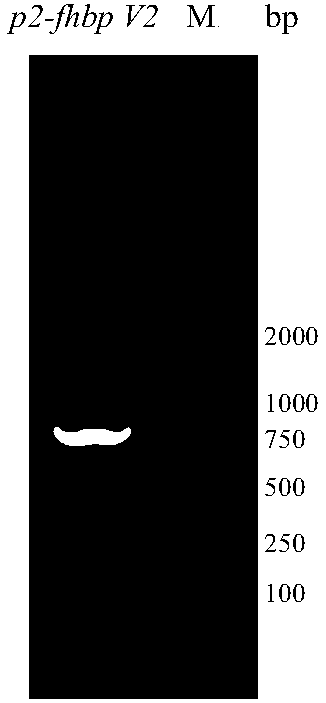
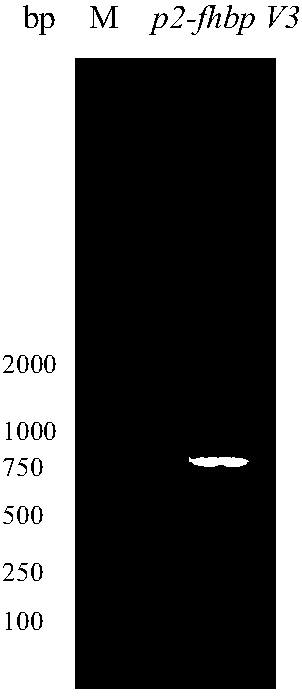
The invention provides a serogroup B meningococcus recombinant chimeric protein vaccine and a preparation method thereof. The vaccine contains three recombinant chimeric proteins of P2-fHBP V1, P2-fHBP V2 and P2-fHBP V3, wherein amino acid sequences of the recombinant chimeric proteins are SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3 respectively; the contents of all the proteins are 30 to 50mu g / dose; optimally, a vaccine preparation also contains a cryoprotectant or vaccine adjuvant. The invention also provides the preparation method of the three recombinant chimeric proteins. By using the recombinant chimeric proteins formed by linking P2 with serogroup B meningococcus fHBP protein, humoral immune response can be effectively induced, and immunogenicity of fHBP is obviously improved. The vaccine provided by the invention can effectively cover all serogroup B meningococcus strains, thereby providing a broad-spectrum preventing effect on invasive diseases such as cerebrospinal meningitis,bacteremia, pneumonia and pericarditis caused by the serogroup B meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Multivalent group b meningococcal protein vaccine and preparation method thereof
ActiveCN104127869BGuaranteed Protection CoverageGood cross-protection antibody titersAntibacterial agentsBacterial antigen ingredientsAdjuvantMeningococcal carriage
Owner:BEIJING SANROAD BIOLOGICAL PROD CO LTD
Monoclonal antibody capable of resisting group A meningococcal capsular polysaccharide conjugate, hybridoma cell strain and applications
PendingCN106399257AHigh titerHigh purityImmunoglobulins against bacteriaTissue cultureConjugate vaccineMeningococcal carriage
The invention discloses a monoclonal antibody capable of resisting group A meningococcal capsular polysaccharide conjugate, a hybridoma cell strain and applications. The hybridoma cell is named as WV-A-01, and is assigned with the accession number of CCTCC NO: C2015229. The monoclonal antibody is secreted by the hybridoma cell strain of claim 1. The monoclonal antibody can be used for the serotype typing diagnosis of group A epidemic cerebrospinal meningitis, and the quantitative determination for group A polysaccharide in the research and development processes of epidemic cerebrospinal meningitis multivalent polysaccharide vaccines and conjugate vaccines, is wide in applicability and high in specificity, and has a considerable practical value, and thus a foundation is laid for the quality control of epidemic cerebrospinal meningitis vaccines and the epidemiological investigation and prevention and control.
Owner:云南沃森生物技术股份有限公司
Polyvalent bacteria capsule polysaccharide-protein conjugate combined vaccine
ActiveCN1709505BImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsBacterial antigen ingredientsBacteroidesHaemophilus
The present invention relates to a polyvalent bacterial capsule polysaccharide-protein conjugate combined vaccine preparation, in particular, it is a combined vaccine containing group A, group C, group Y and group W135 epidemic cerebrospinal meningitis coccal capsule polysaccharide-protein conjugate and b type haemophilus influenzal capsule polysaccharide-protein conjugate.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM
Process beneficial for quality control of polysaccharide-SPH derivative
InactiveCN106318992AReduce dosageLow content of impuritiesMicroorganism based processesFermentationHeminPhenol
The invention discloses a process beneficial for quality control of a polysaccharide-SPH derivative. The process comprises the steps of culture, fermentation and purification of an epidemic cerebrospinal meningitis A-group strain and activation and derivation of epidemic cerebrospinal meningitis A-group capsular polysaccharide, wherein culture, fermentation and purification of the epidemic cerebrospinal meningitis A-group strain specifically comprise the steps that the epidemic cerebrospinal meningitis A-group strain is opened and passed to a hemin epidemic cerebrospinal meningitis semi-synthetic medium, and the medium is placed in a carbon dioxide cell culture box to be cultured at the temperature of 36.5 DEG C with 8% CO2; epidemic cerebrospinal meningitis A-group seeds obtained through culture are put in a seed tank in an inoculation mode and then put in a fermentation tank in an inoculation mode after constant-temperature stirring and aerobic culture, the constant temperature is 36.5 DEG C, deep-layer aeration is carried out for stirring culture at 240 rpm, epidemic cerebrospinal meningitis A-group bacteria are cultured till the later period of the logarithmic phase or the early period of the stationary phase, thalli are removed after formaldehyde sterilization, and supernate is collected; polysaccharide is obtained after purification, and the dosage of phenol is 3 / 4 (v / v) that of raw sugar. According to the optimized process, on the one hand, the component content of nucleic acid, protein and other impurities in epidemic cerebrospinal meningitis A-group fermentation liquor is controlled to be low, and on the other hand, the dosage of phenol is lowered.
Owner:CHENGDU OLYMVAX BIOPHARM
Elephantopus scaber health-caring tea
InactiveCN104642630AEffective health careCool bloodAntibacterial agentsSenses disorderDiseaseMedicine
The invention provides elephantopus scaber health-caring tea which is mainly prepared from following raw materials, in a certain weight ratio: elephantopus scaber, folium isatidis, herba houttuyniae and dandelion. The health-caring tea has effects of cooling blood, clearing heat, removing moisture, draining water and removing toxin, is low in cost, is good in effect, is reasonable in formula, is simple in preparation, is easy to store, is suitable in all seasons, can not only treat patients but also improving health of healthy people, is quite convenient to drink and is good in mouthfeel, is suitable for cold, heat dysentery, acute gastroenteritis, tonsillitis, conjunctivitis, furuncles and damp toxin, can prevent diseases such as epidemic cerebrospinal meningitis and the like, has an excellent health-caring effect on patients and is a quite excellent health-caring beverage.
Owner:叶崎峰
Antiinflammatory walnut oil
The invention relates to antiinflammatory walnut oil. The antiinflammatory walnut oil is prepared from the following raw materials in parts by weight: 300-320 parts of East China Chinese walnut kernels, 50-60 parts of peanut kernels, 50-60 parts of barley, 30-40 parts of laver, 20-30 parts of litchis, 20-30 parts of rhizoma coptidis, 20-30 parts of fructus gardeniae, 10-15 parts of radix scutellariae, 10-15 parts of phellodendron and the like. The invention discloses walnut oil and a preparation technology thereof. The laver and the litchis are added, so that the walnut oil has the functions of resisting ultraviolet rays, resisting oxidation, resisting ageing, and the like, and is beneficial for the health of human bodies; besides, the rhizoma coptidis which is bitter and cold is also added for purging the sthenic heart-fire, because the heart controls mental and emotional activities, the fire is mainly from the heart, in order to realize the purpose of purging fire, the heart needs to be firstly purged, the heart fire is purged, the fire from all meridian can be purged, and the walnut oil can also purge the fire of a middle warmer; the radix scutellariae which is added can purge the fire of an upper warmer; the phellodendron which is added can purge the fire of a lower warmer; the fructus gardeniae which is added can discharge heat from the three warmers by catharsis, conduct heat for enabling the fire to go down, so that the fire is purged from below, the effects of clearing heat and removing toxicity are realized, and the walnut oil is suitable for patients suffering from heat-toxicity diseases of blood poisoning, pyemia, dysentery, pneumonia, urinary infection, epidemic cerebrospinal meningitis, Japanese encephalitis, infection inflammation and the like to eat.
Owner:ANQING XIAOZHU FOOD TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com