Patents
Literature
51 results about "Recombinant Chimeric Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
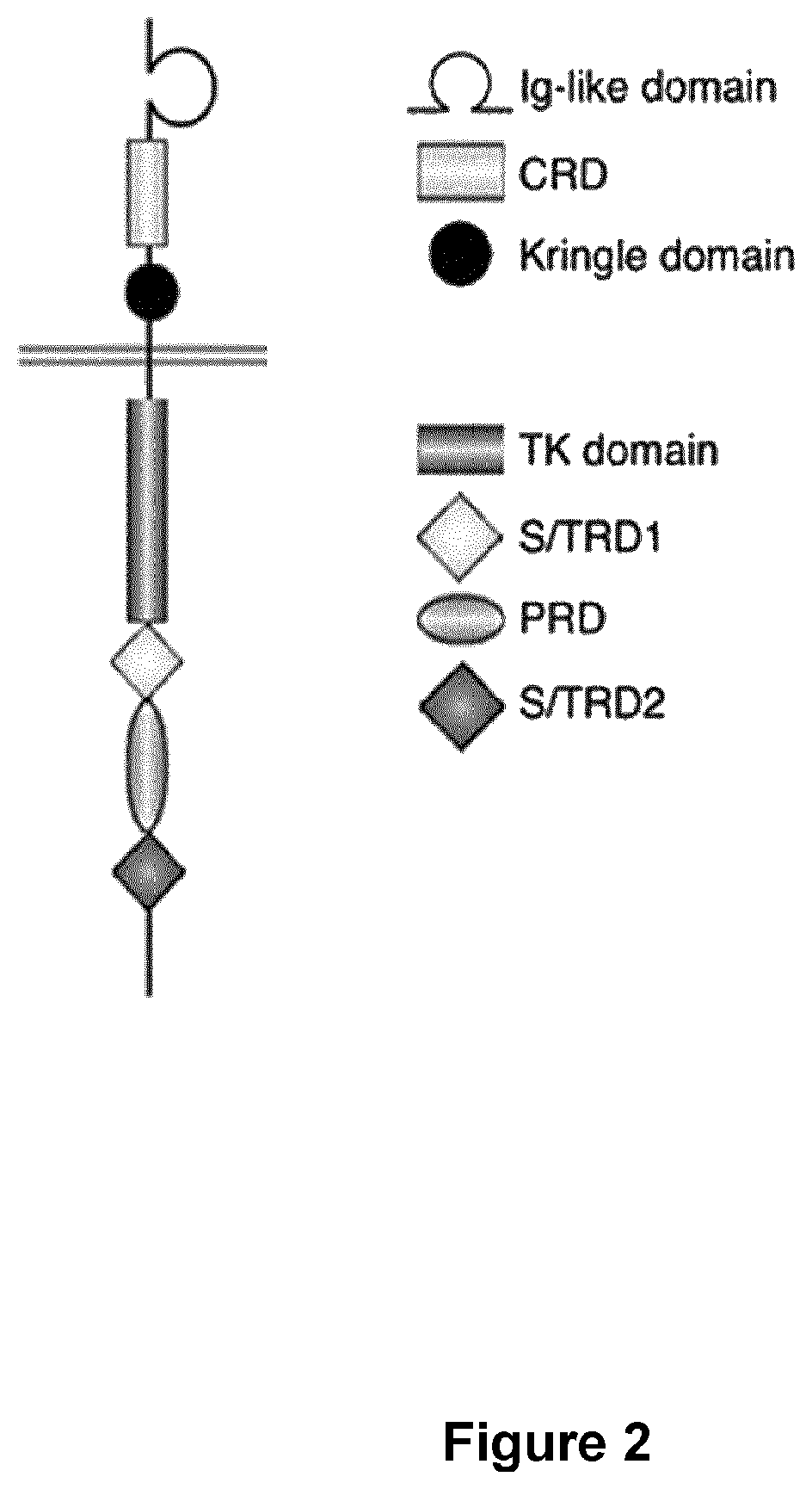
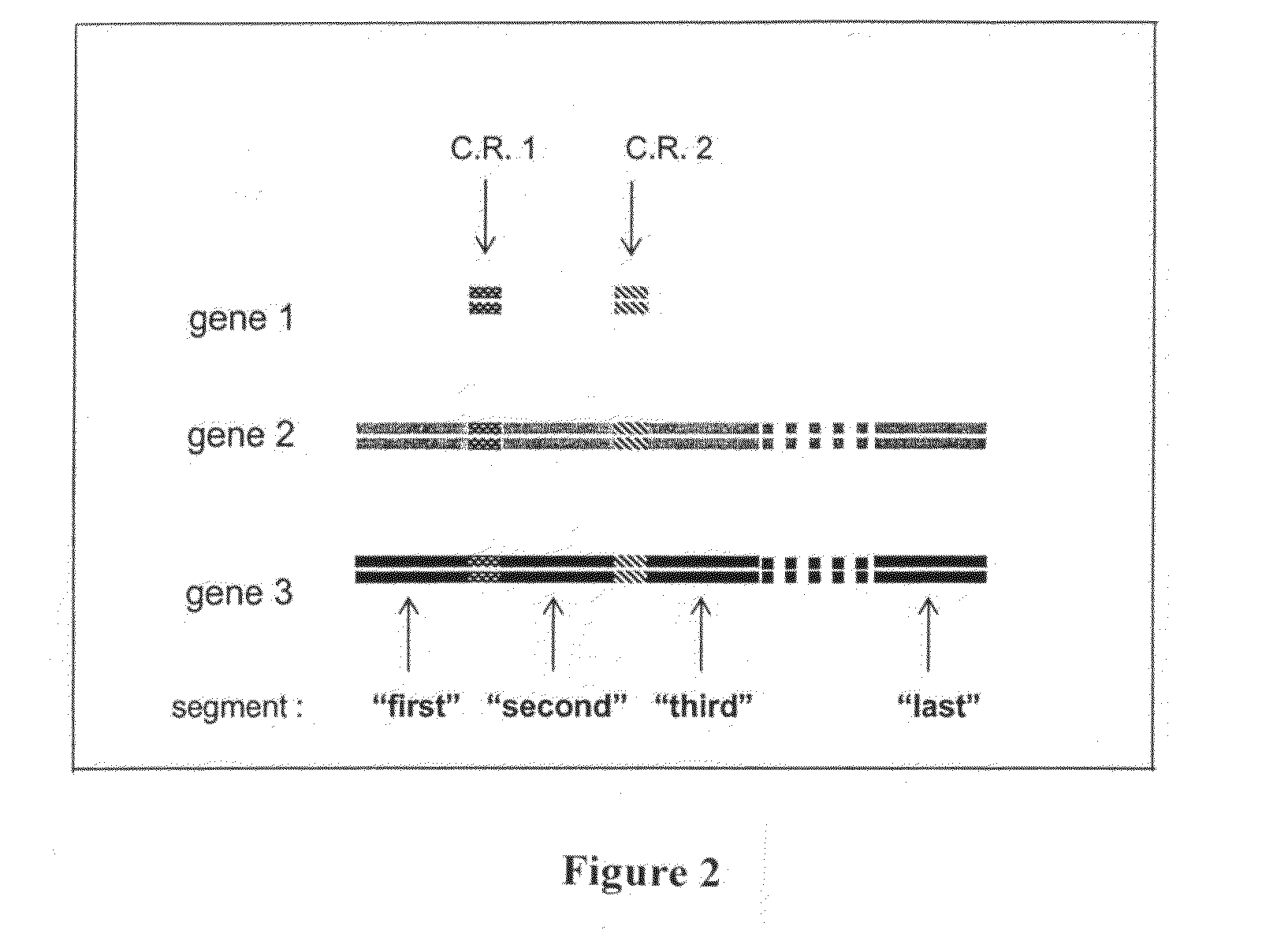
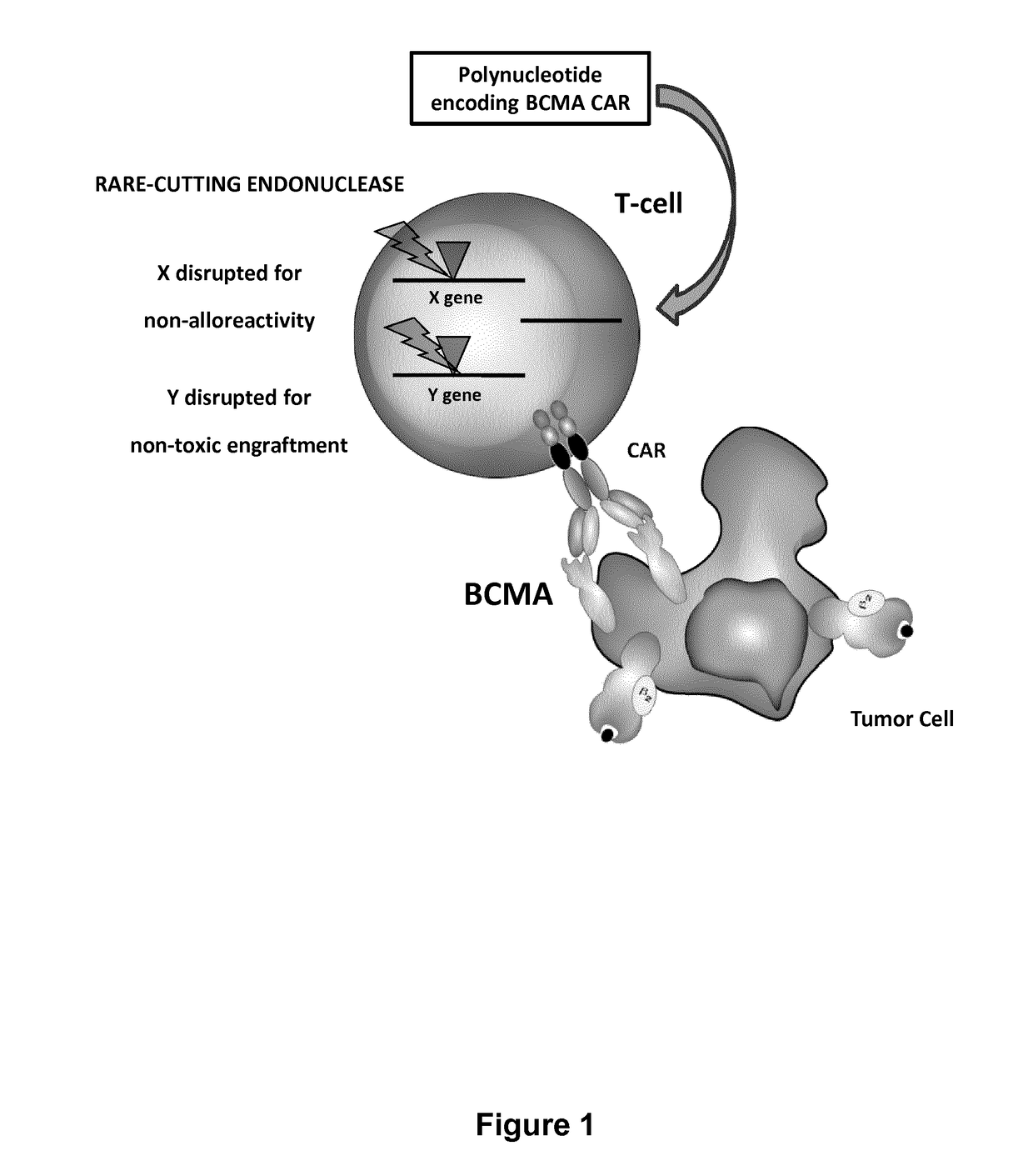
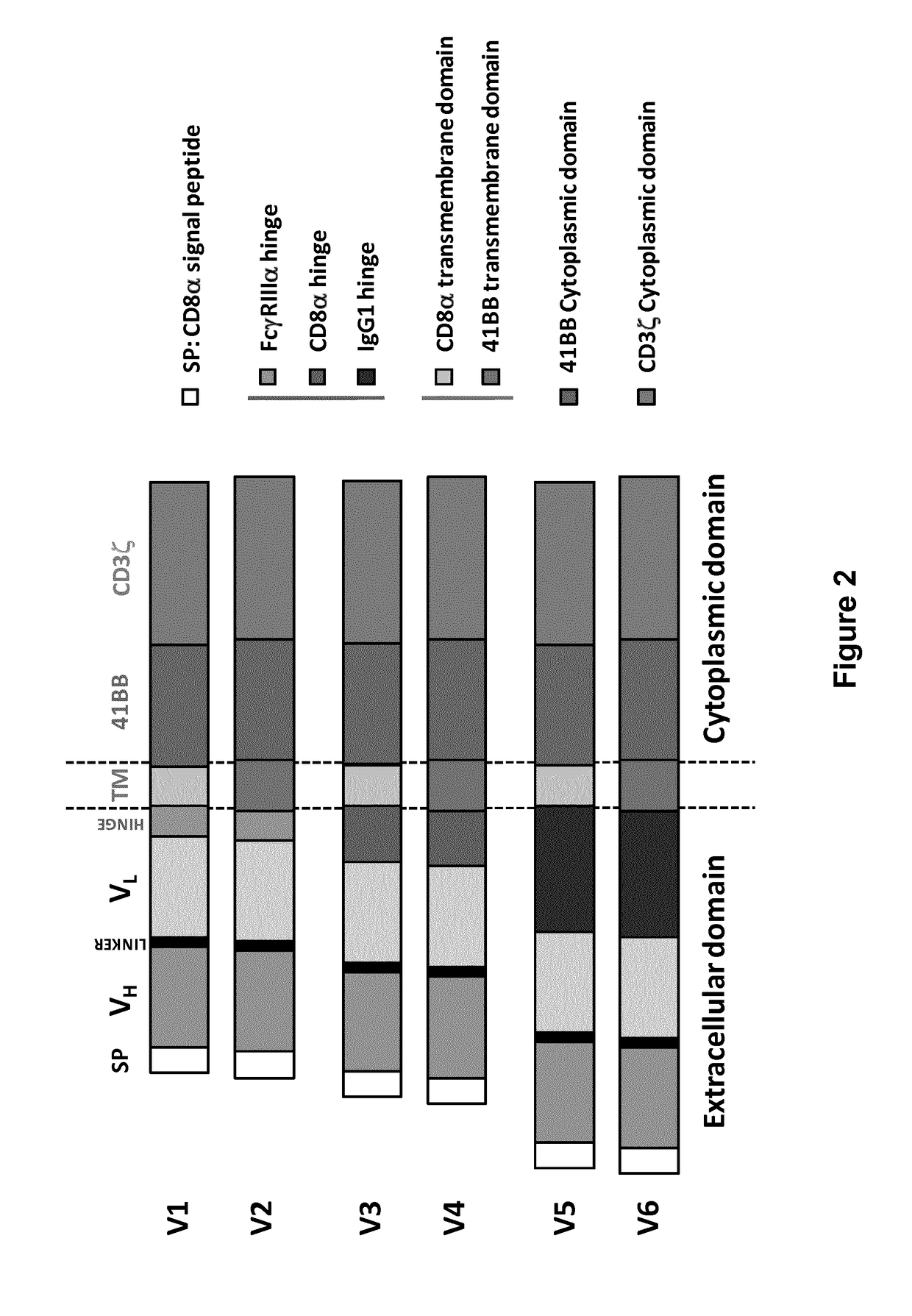
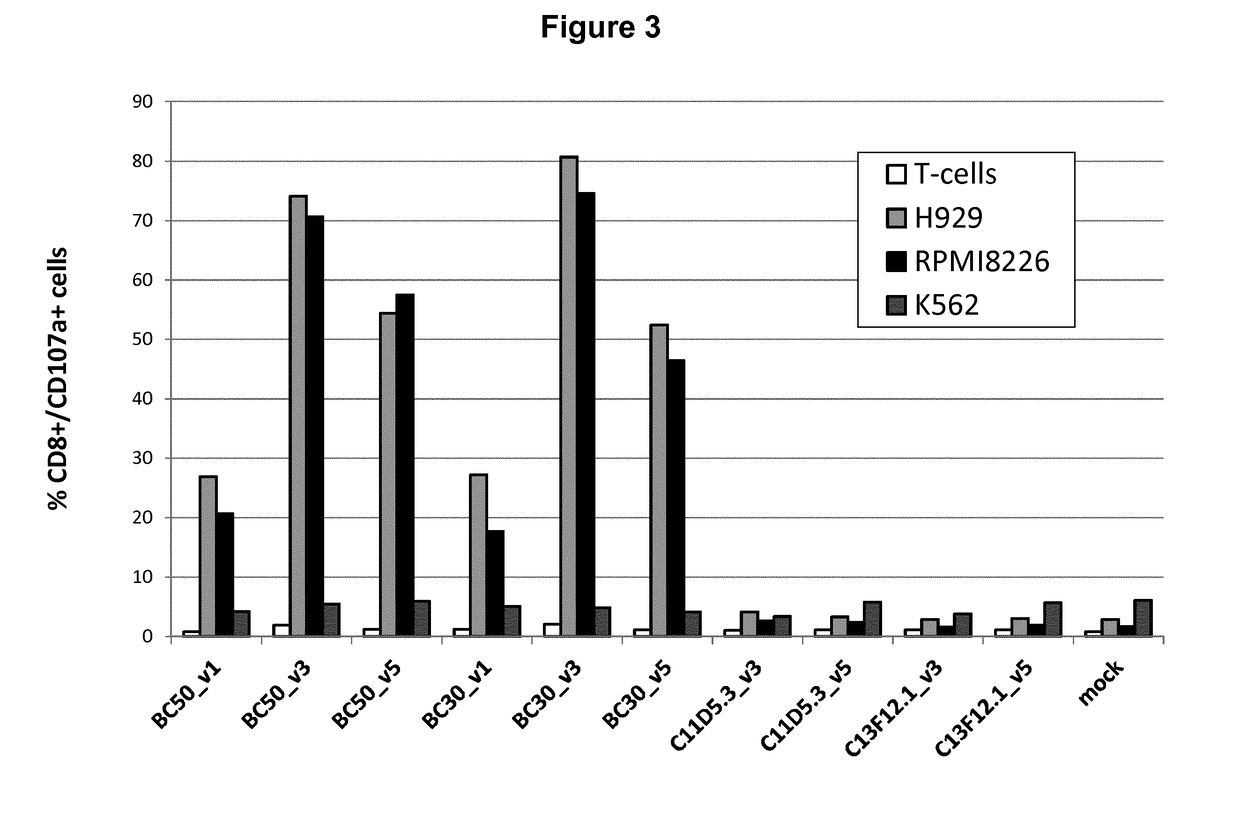
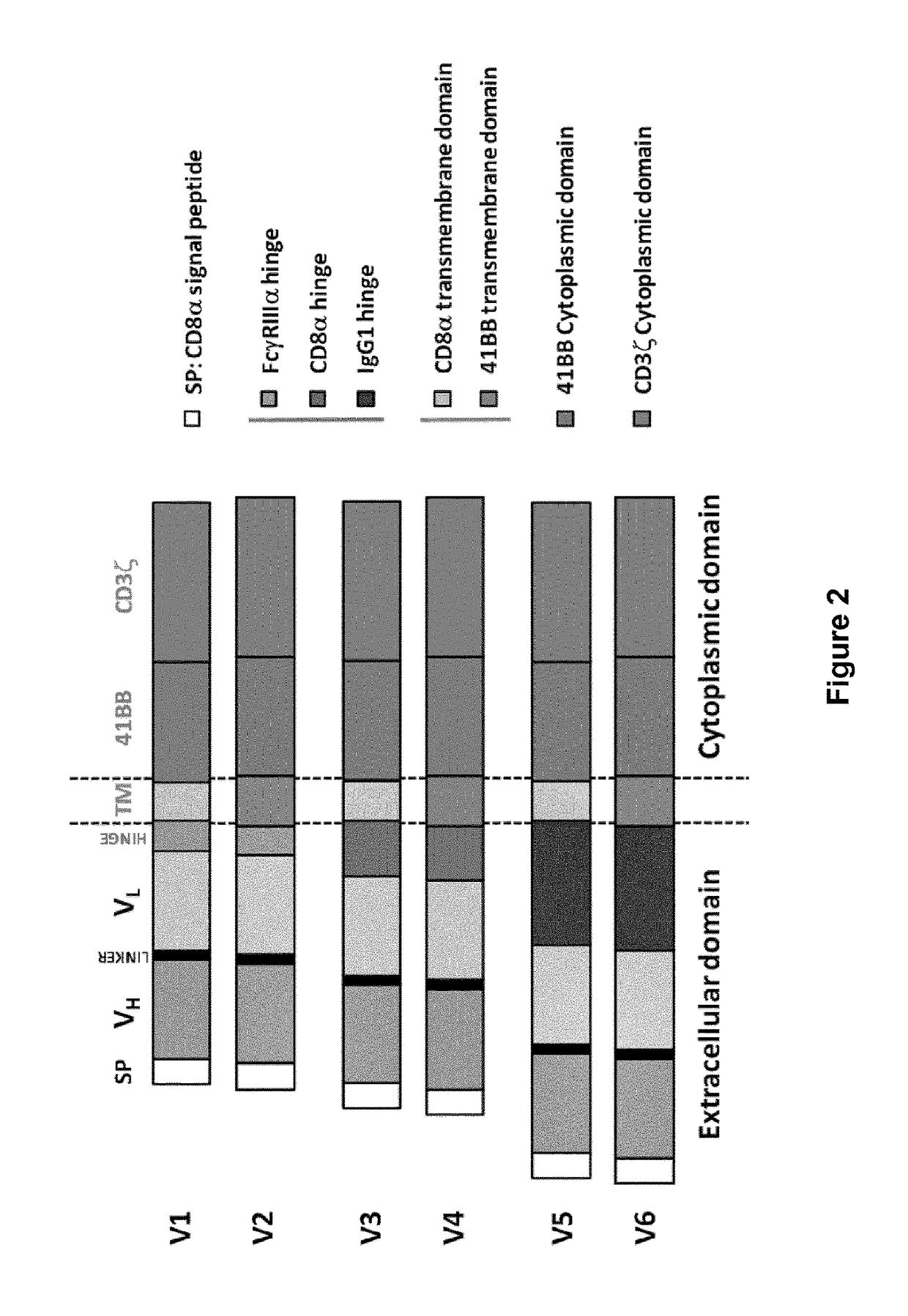
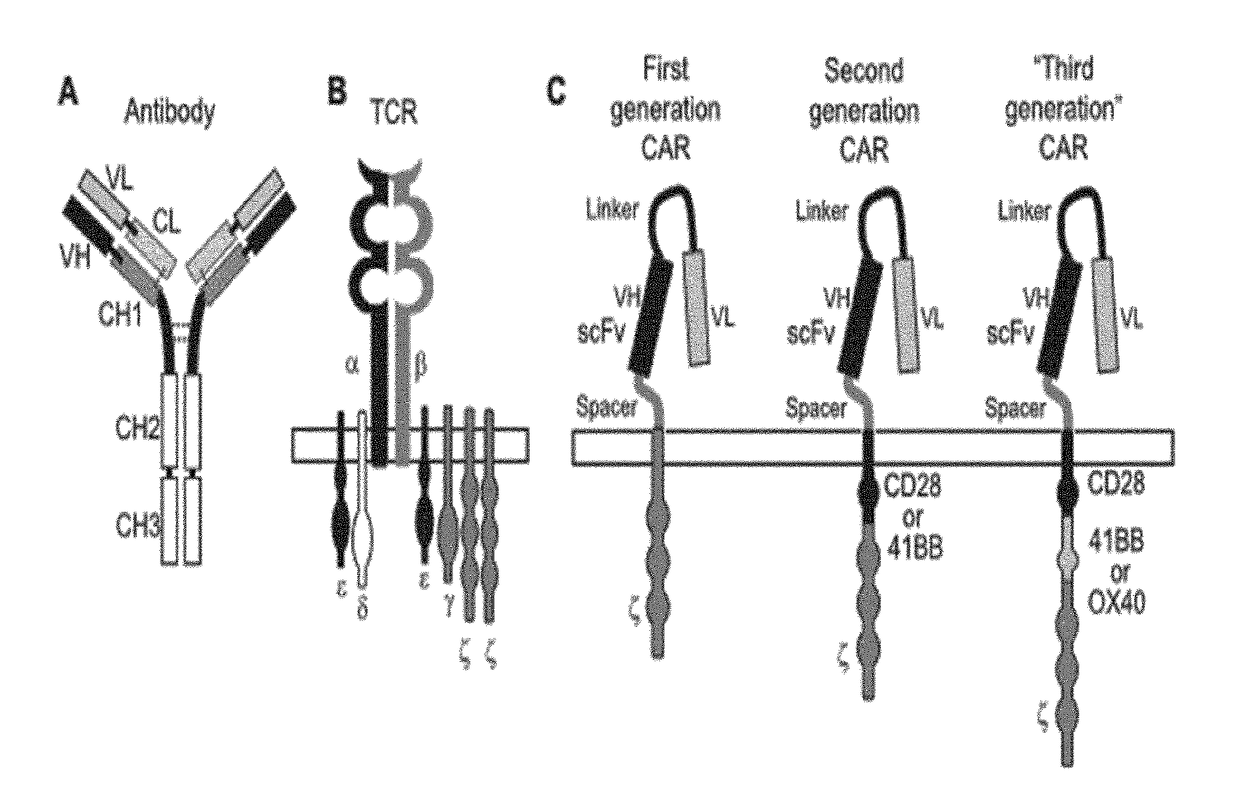
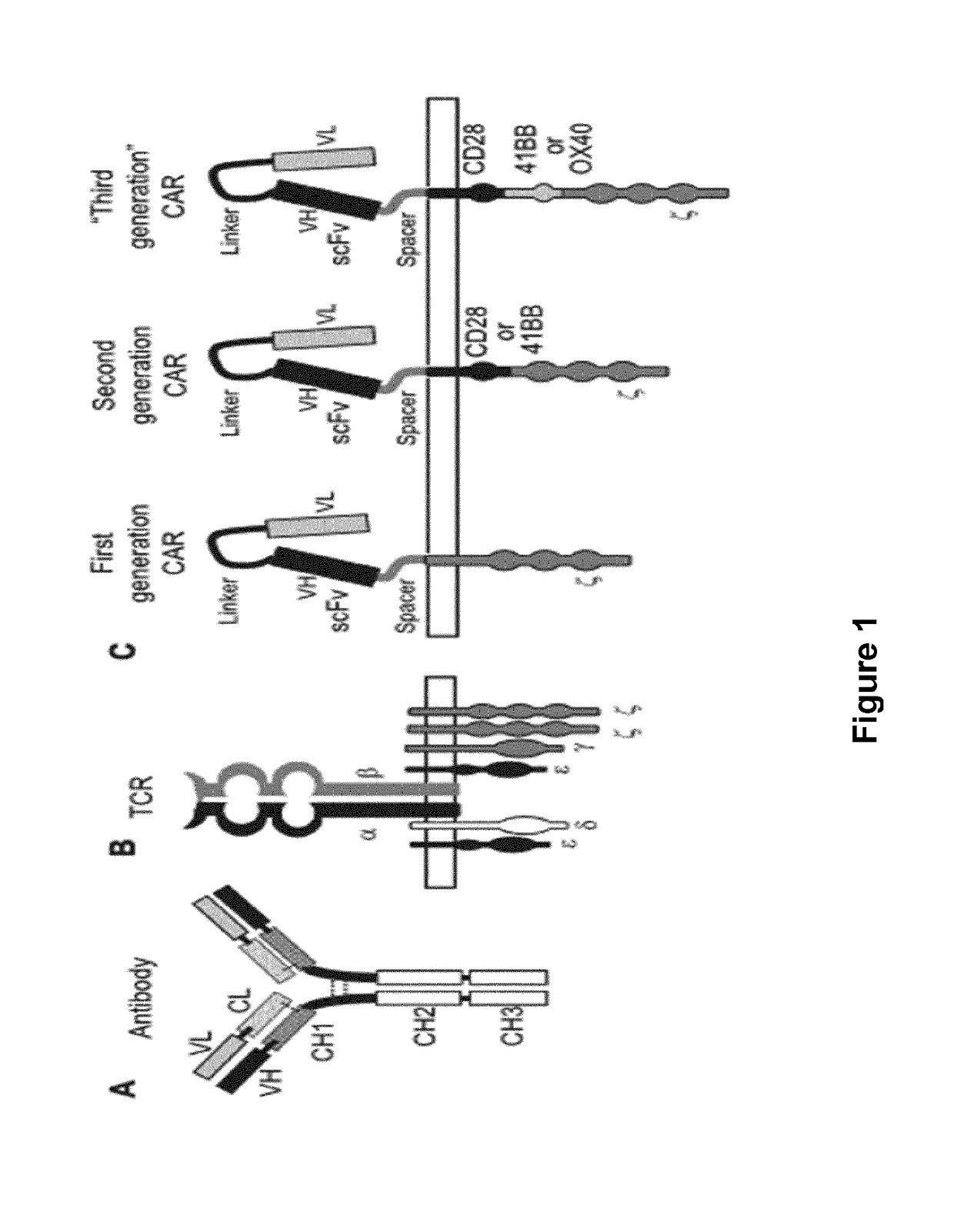
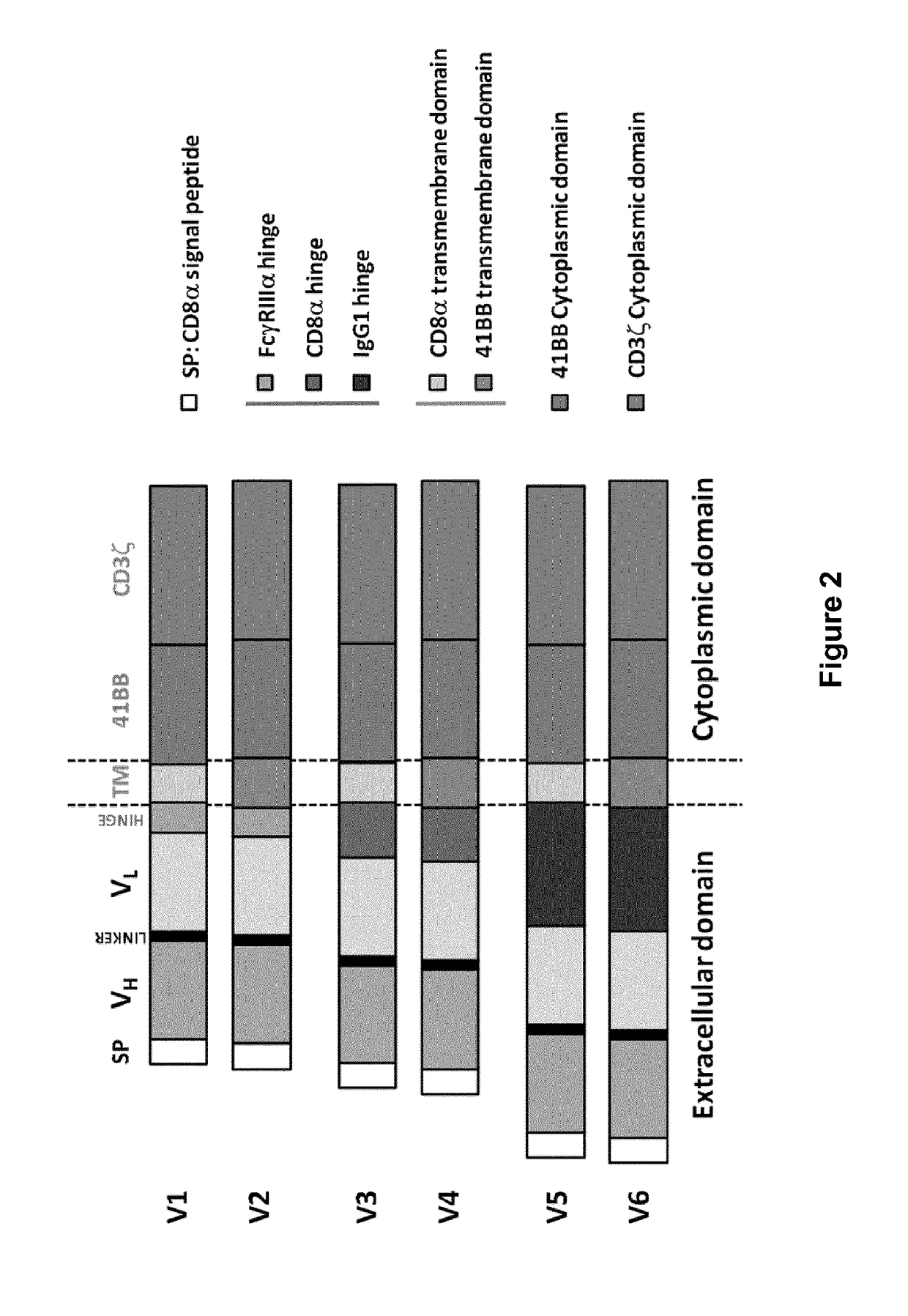
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
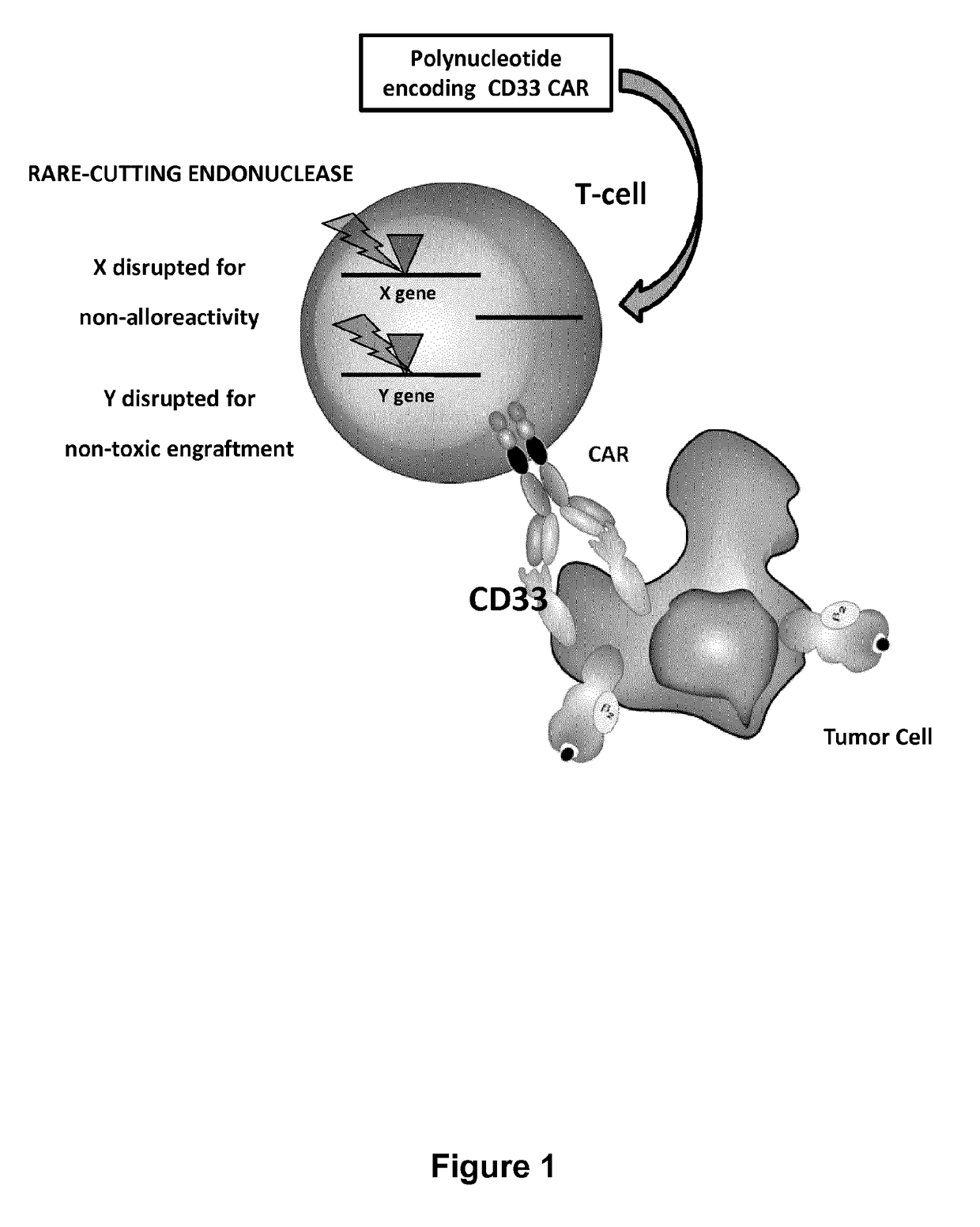
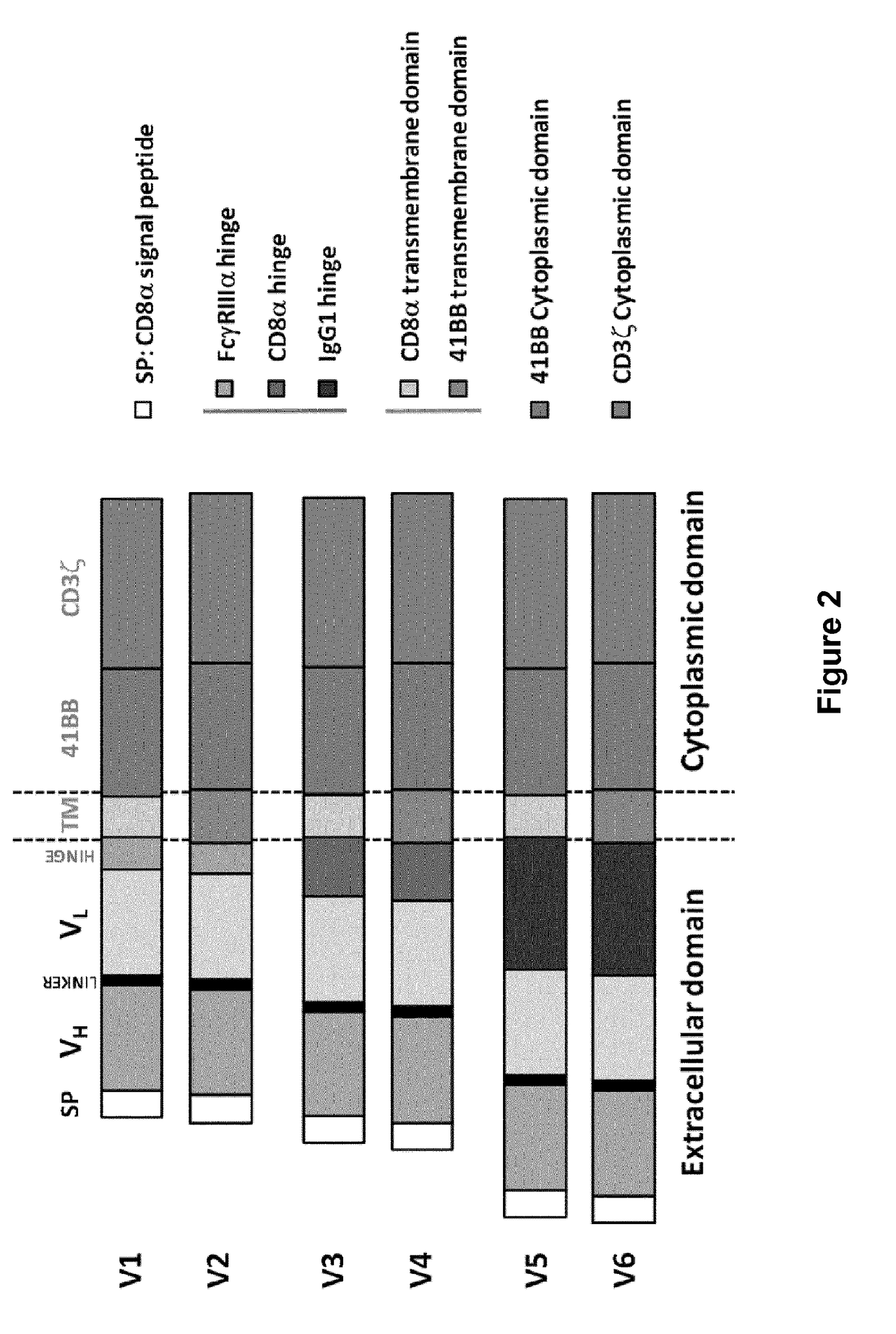
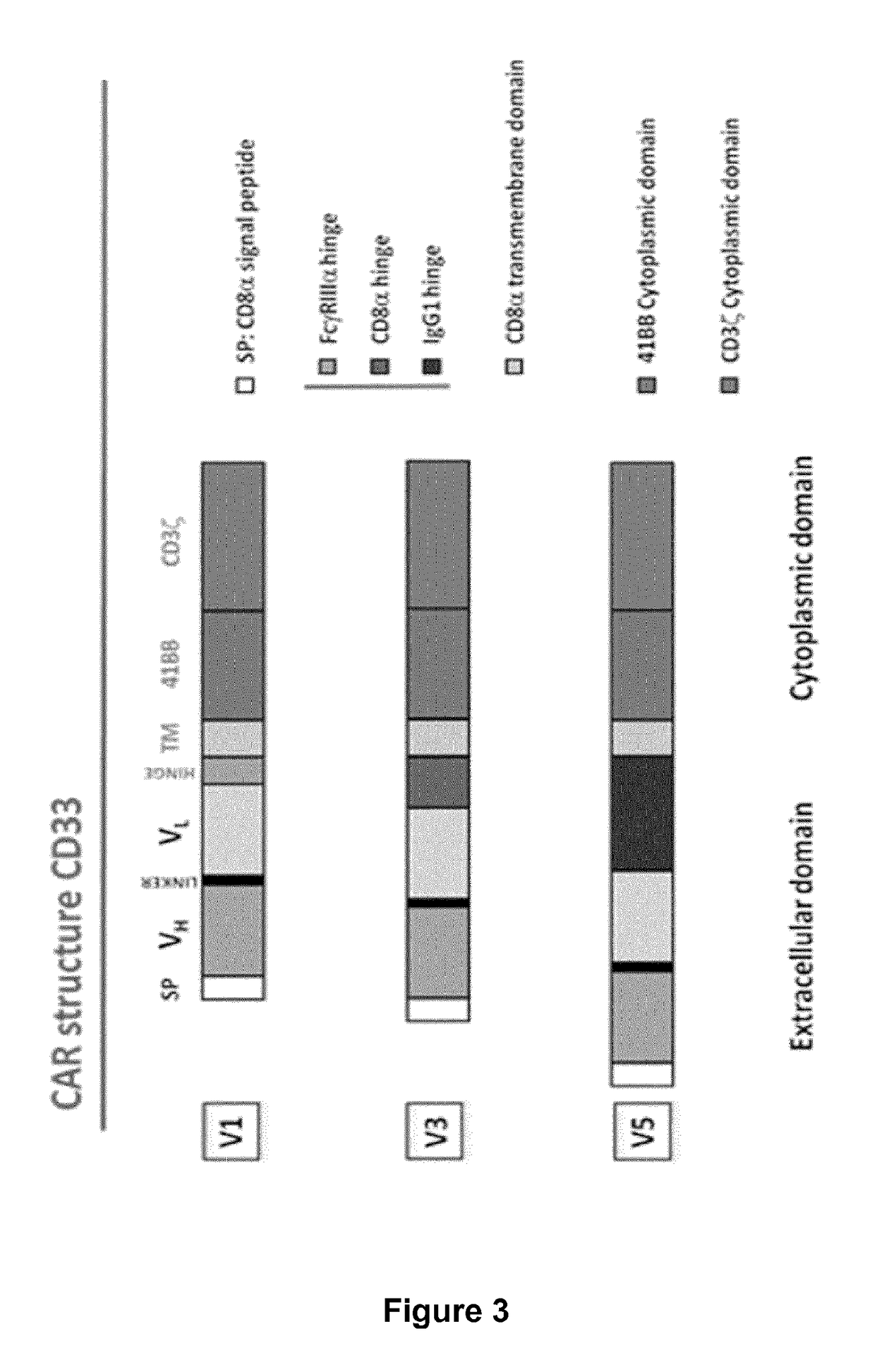
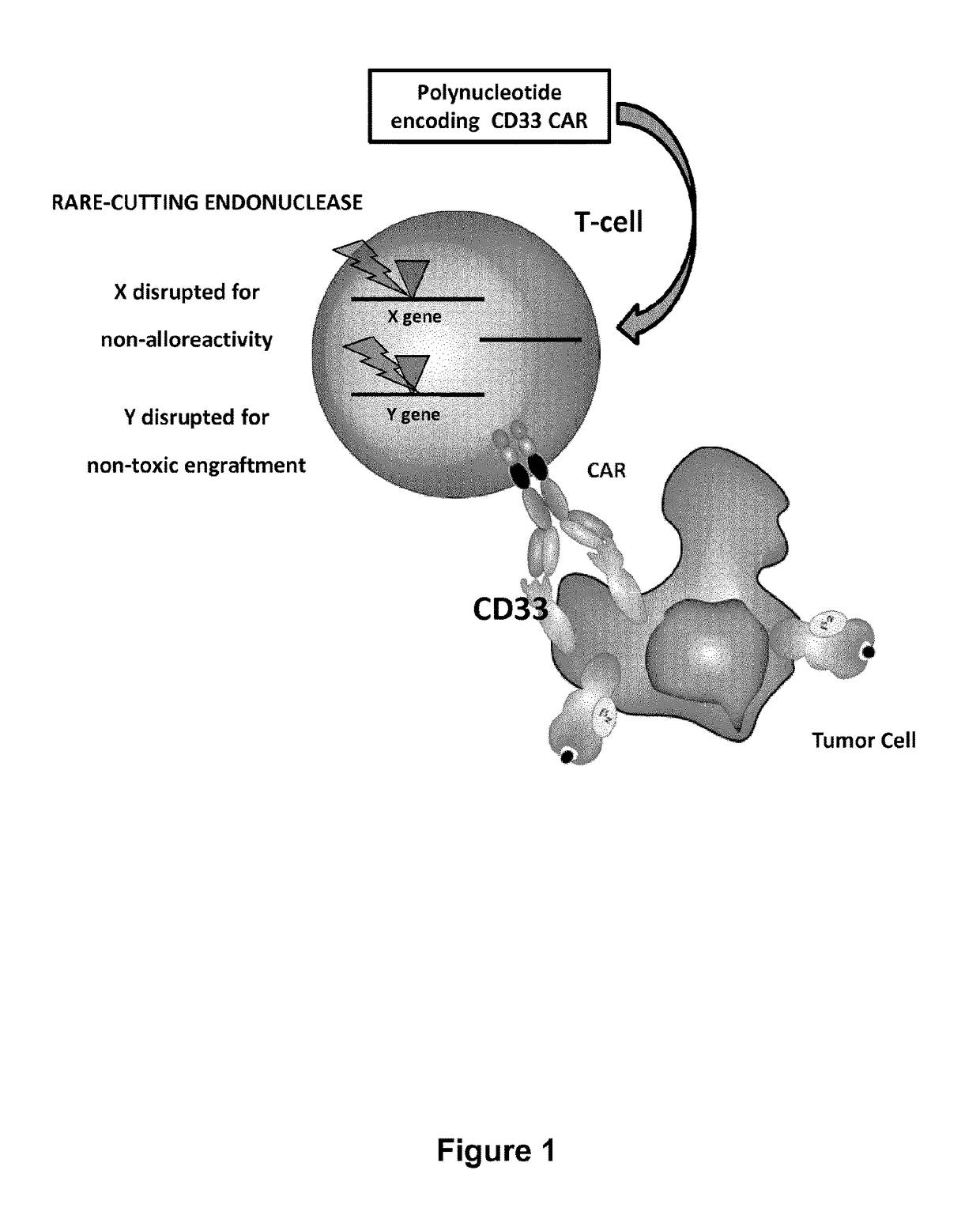
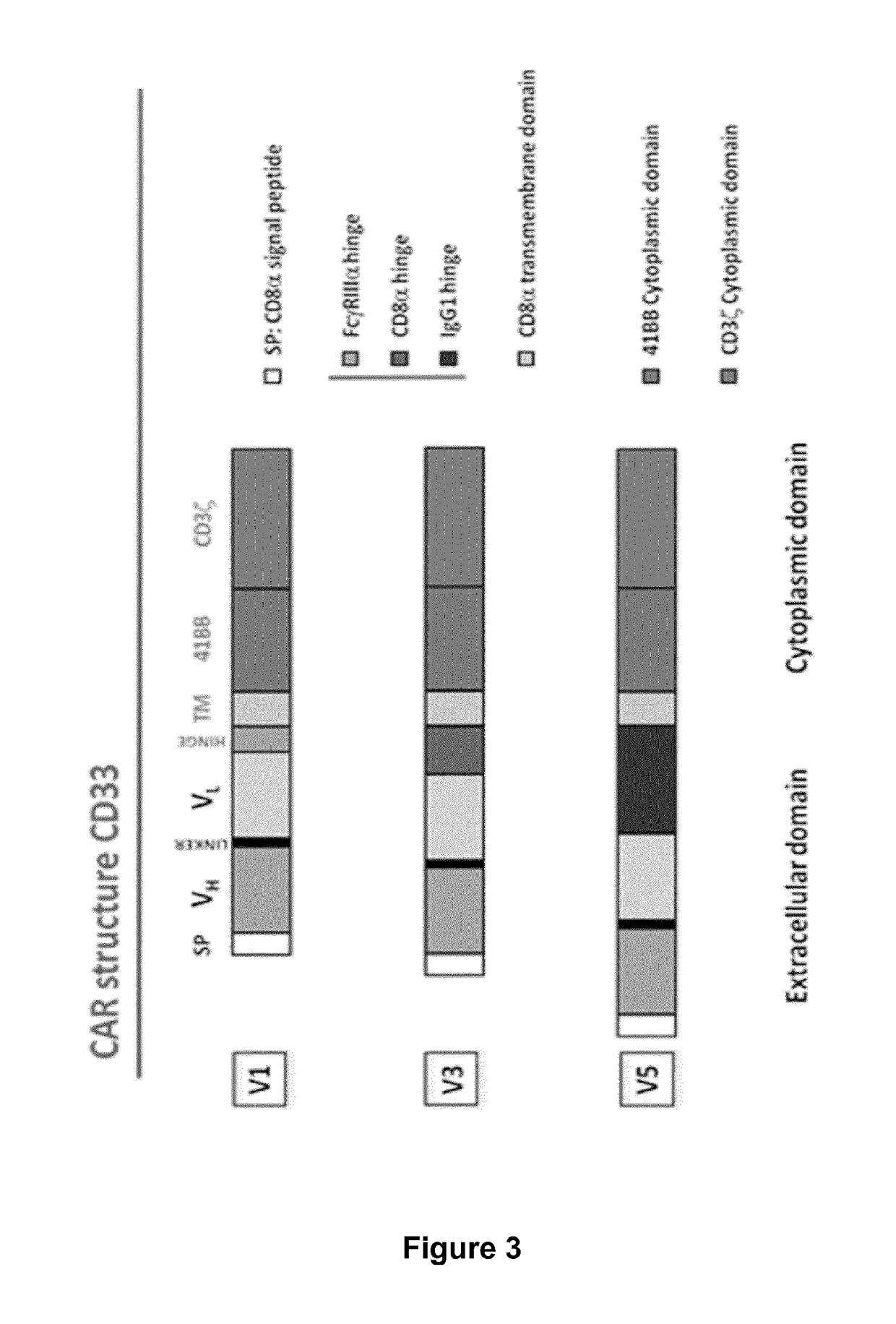
Cd33 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170145094A1Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD33
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD33 monoclonal antibody, conferring specific immunity against CD33 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
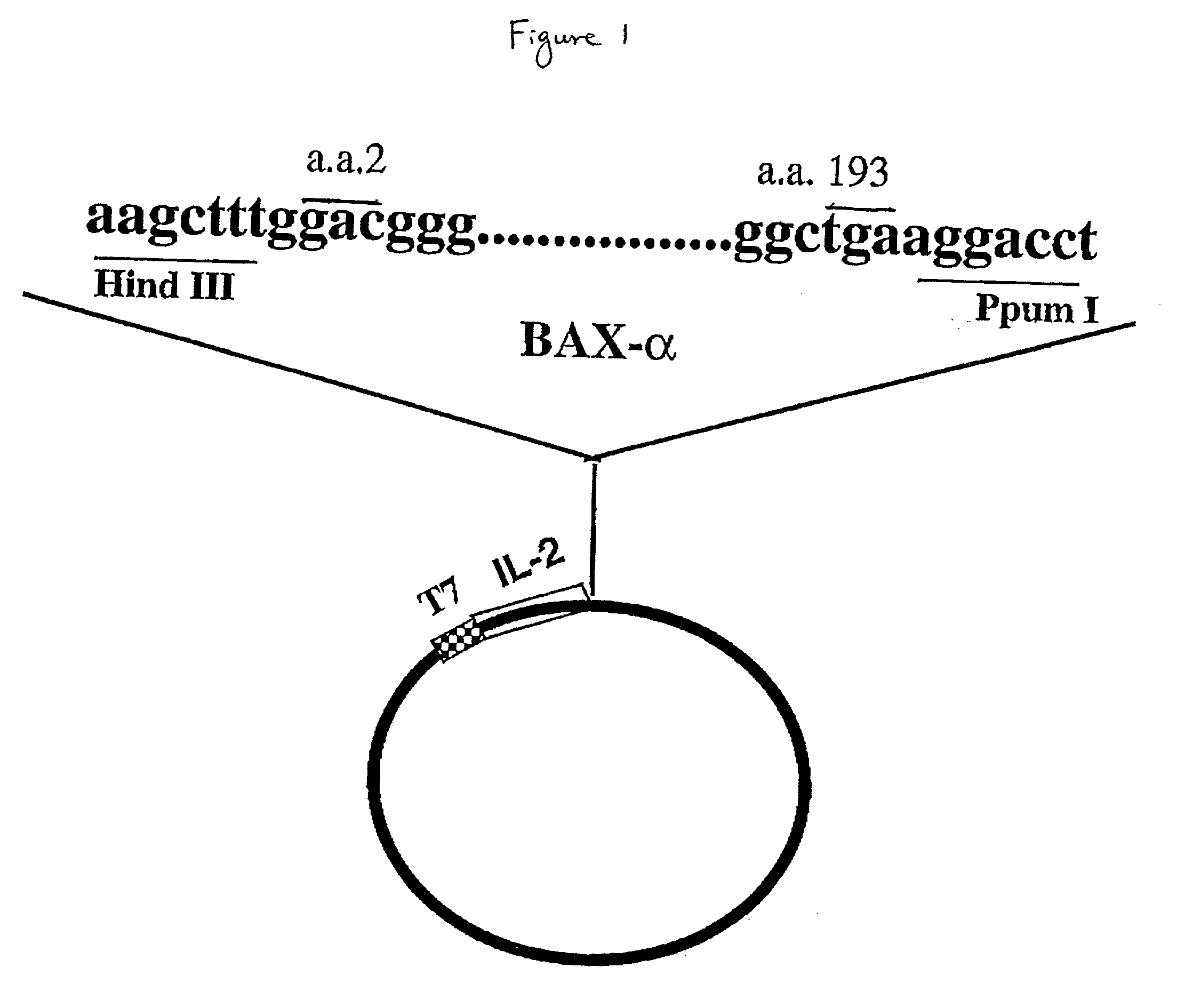
Chimeric proteins with cell-targeting specificity and apoptosis-inducing activities
InactiveUS20020090374A1Peptide/protein ingredientsAntibody mimetics/scaffoldsApoptosisInterleukin II
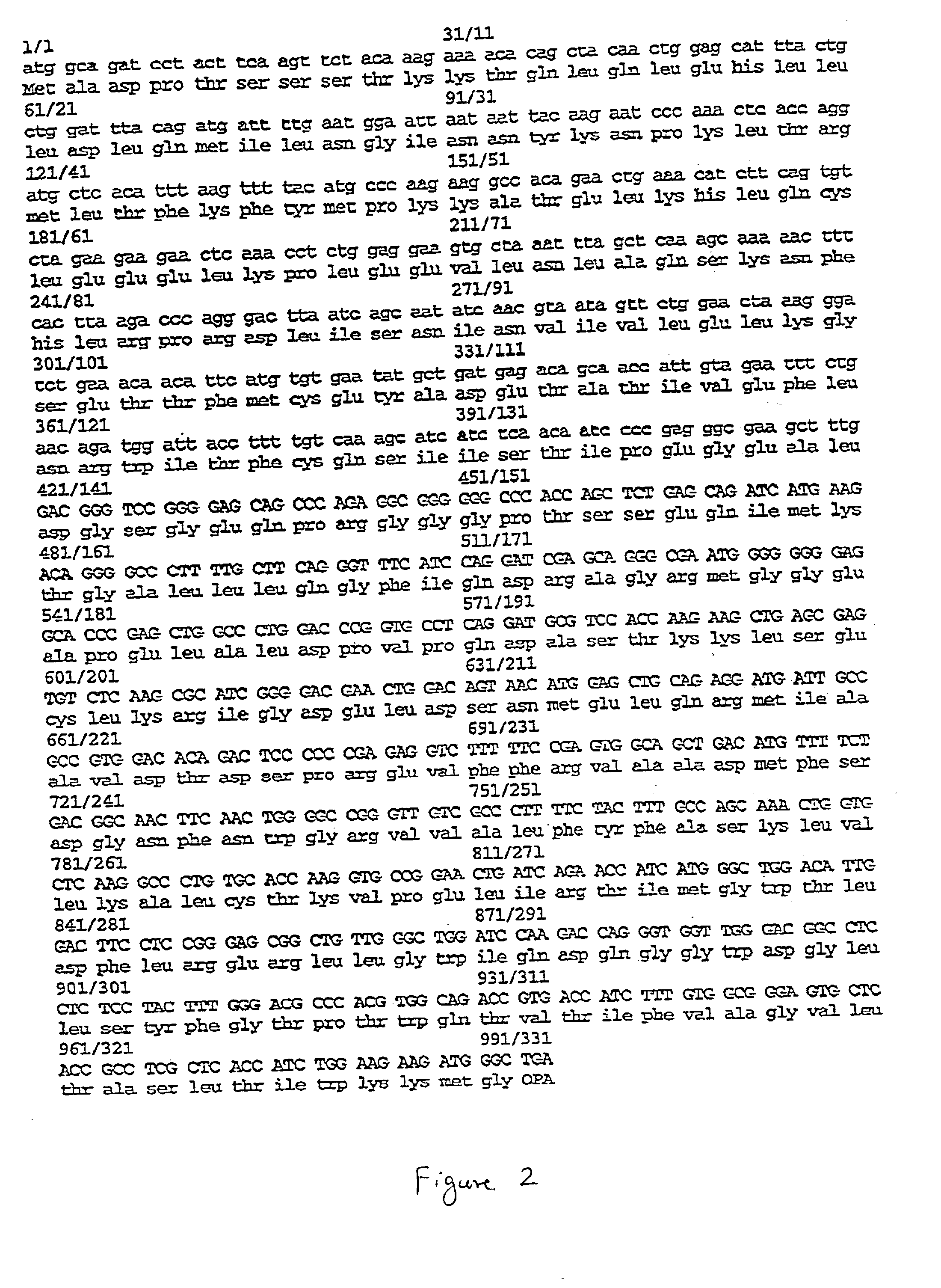
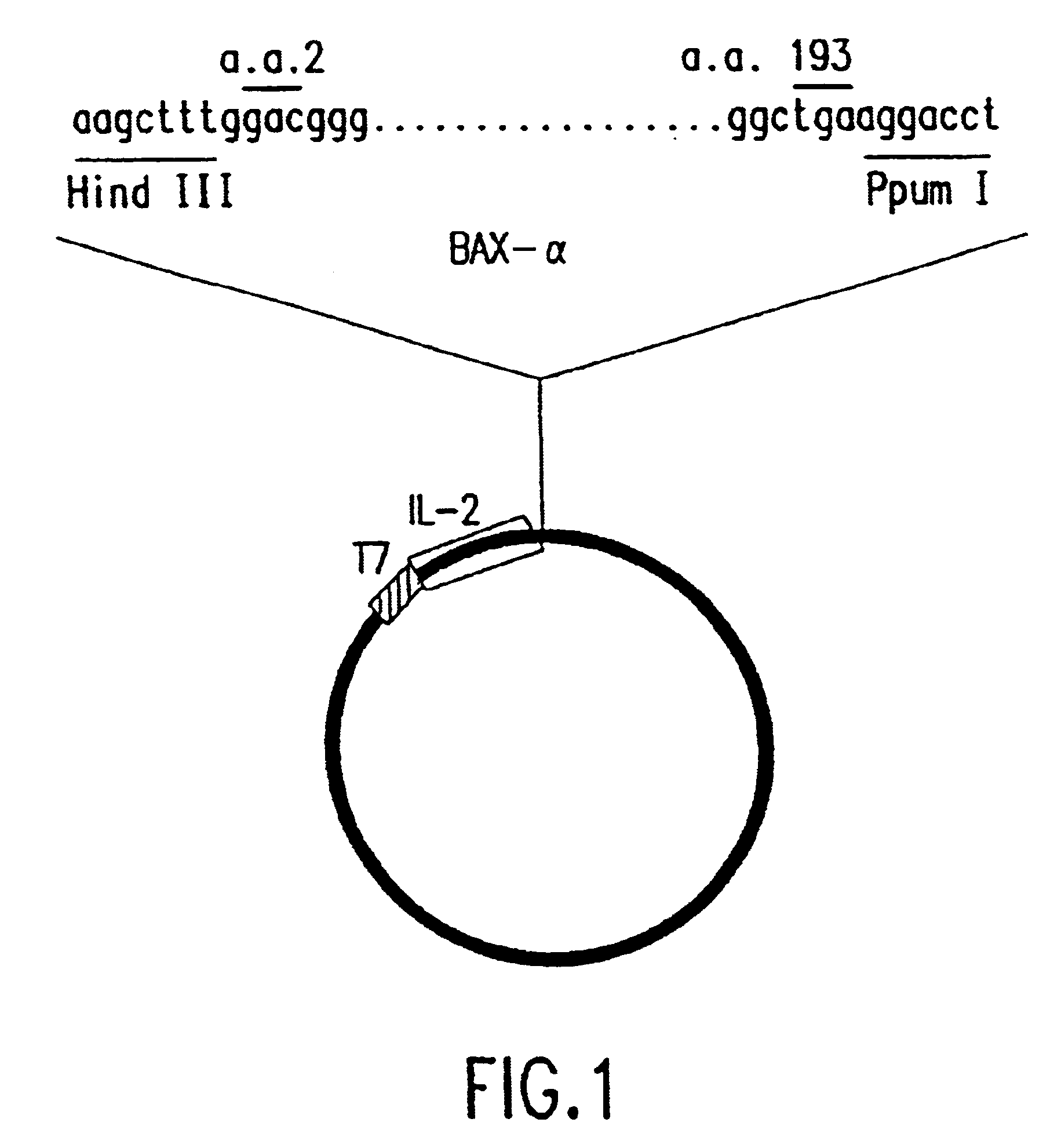
The present invention relates to chimeric proteins with cell-targeting specificity and apoptosis-inducing activities. In particular, the invention is illustrated by a recombinant chimeric protein between human interleukin-2 (IL2) and Bax. The chimeric protein specifically targets IL2 receptor (IL2R)-expressing cells and induces cell-specific apoptosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Chimeric proteins with cell-targeting specificity and apoptosis-inducing activities
The present invention relates to chimeric proteins with cell-targeting specificity and apoptosis-inducing activities. In particular, the invention is illustrated by a recombinant chimeric protein between human interleukin-2 (IL2) and Bax. The chimeric protein specifically targets IL2 receptor (IL2R)-expressing cells and induces cell-specific apoptosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
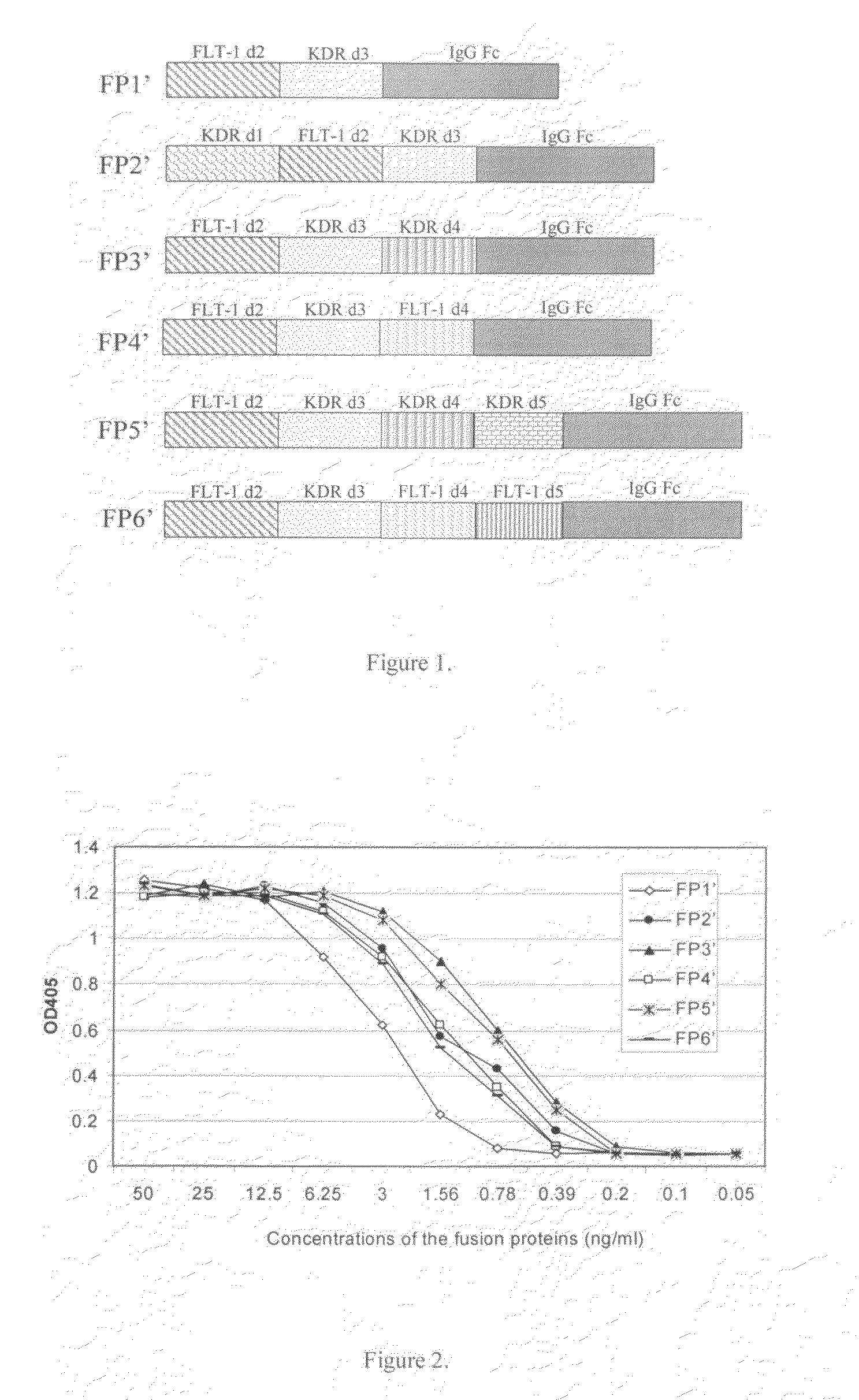
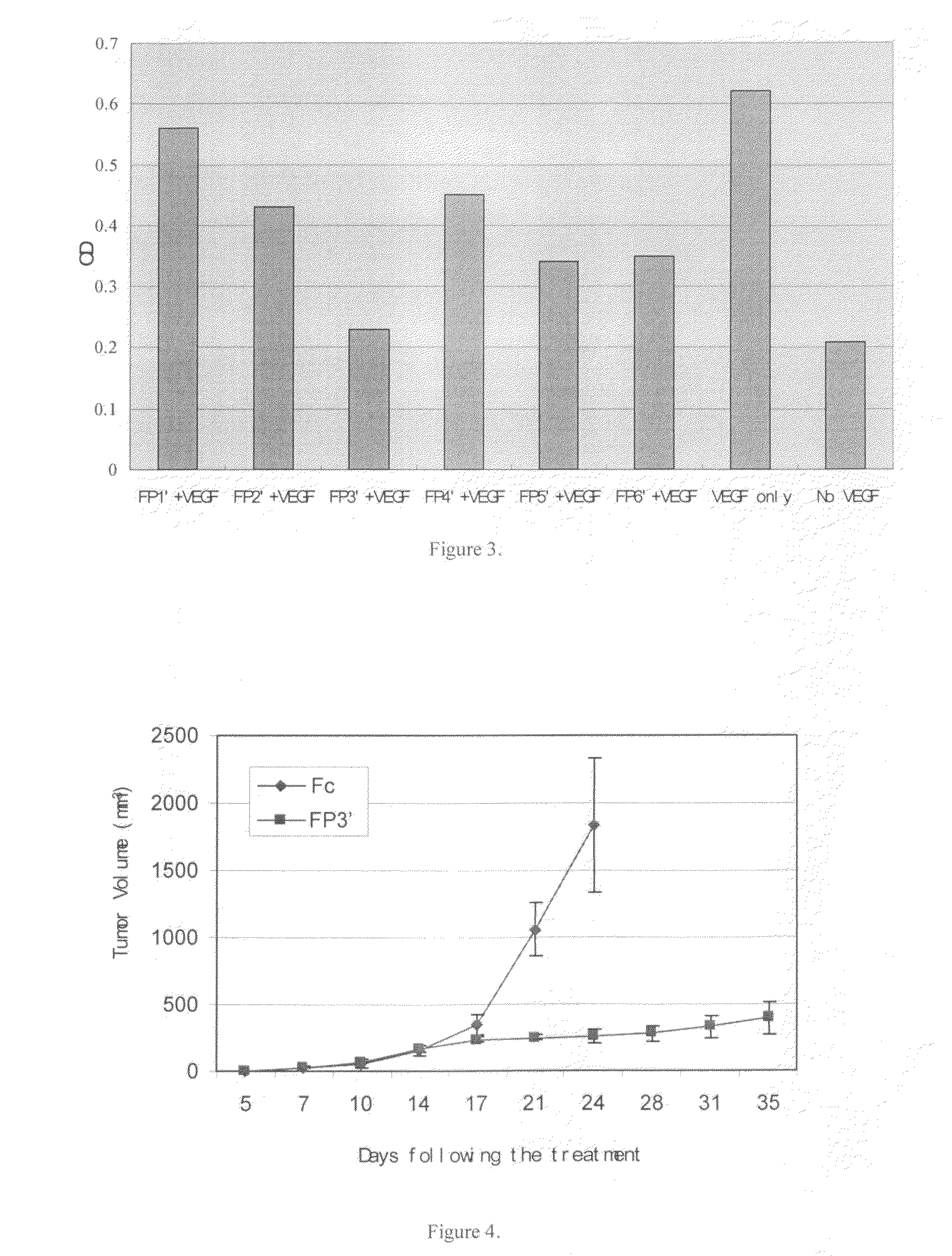
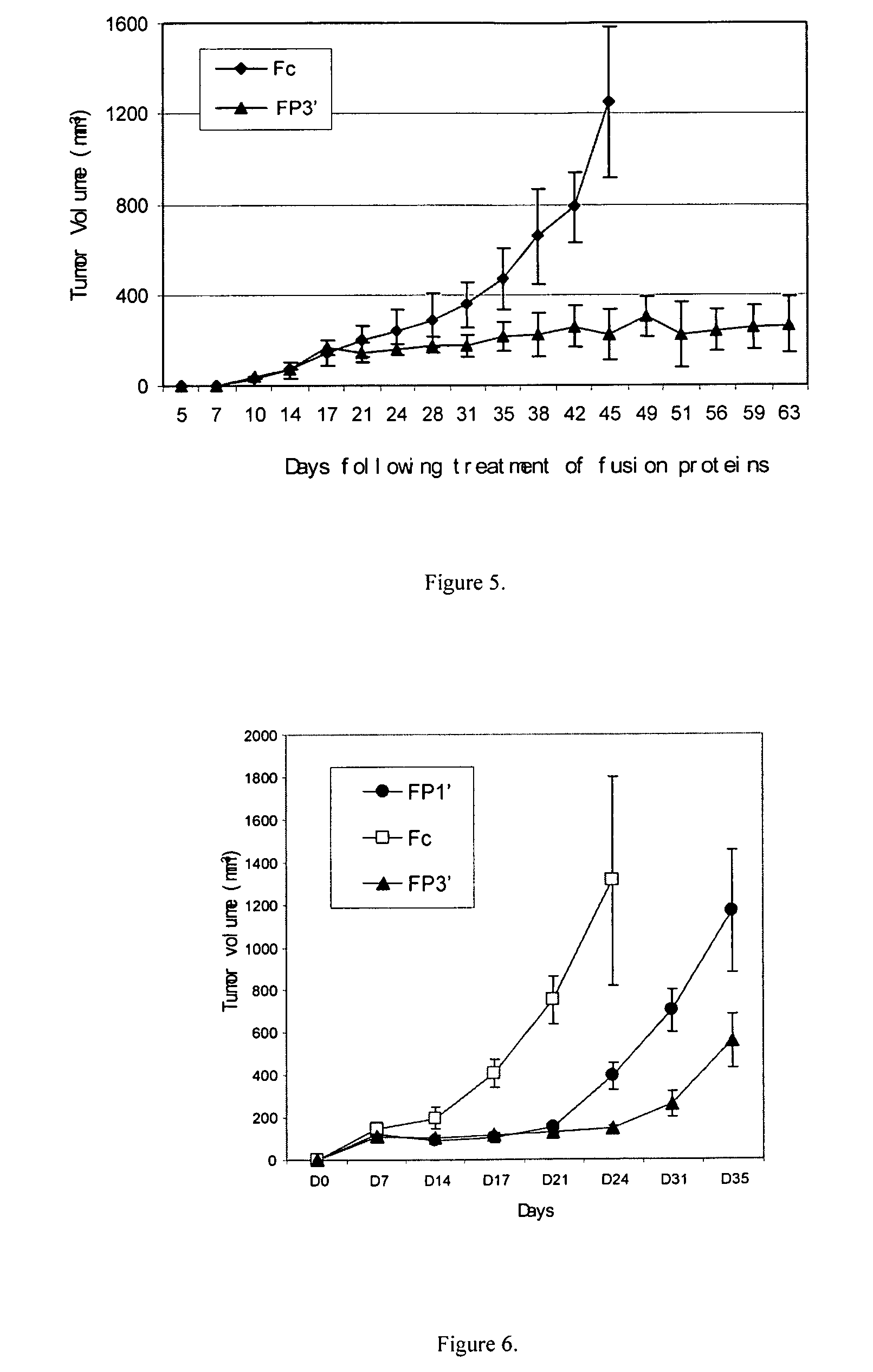
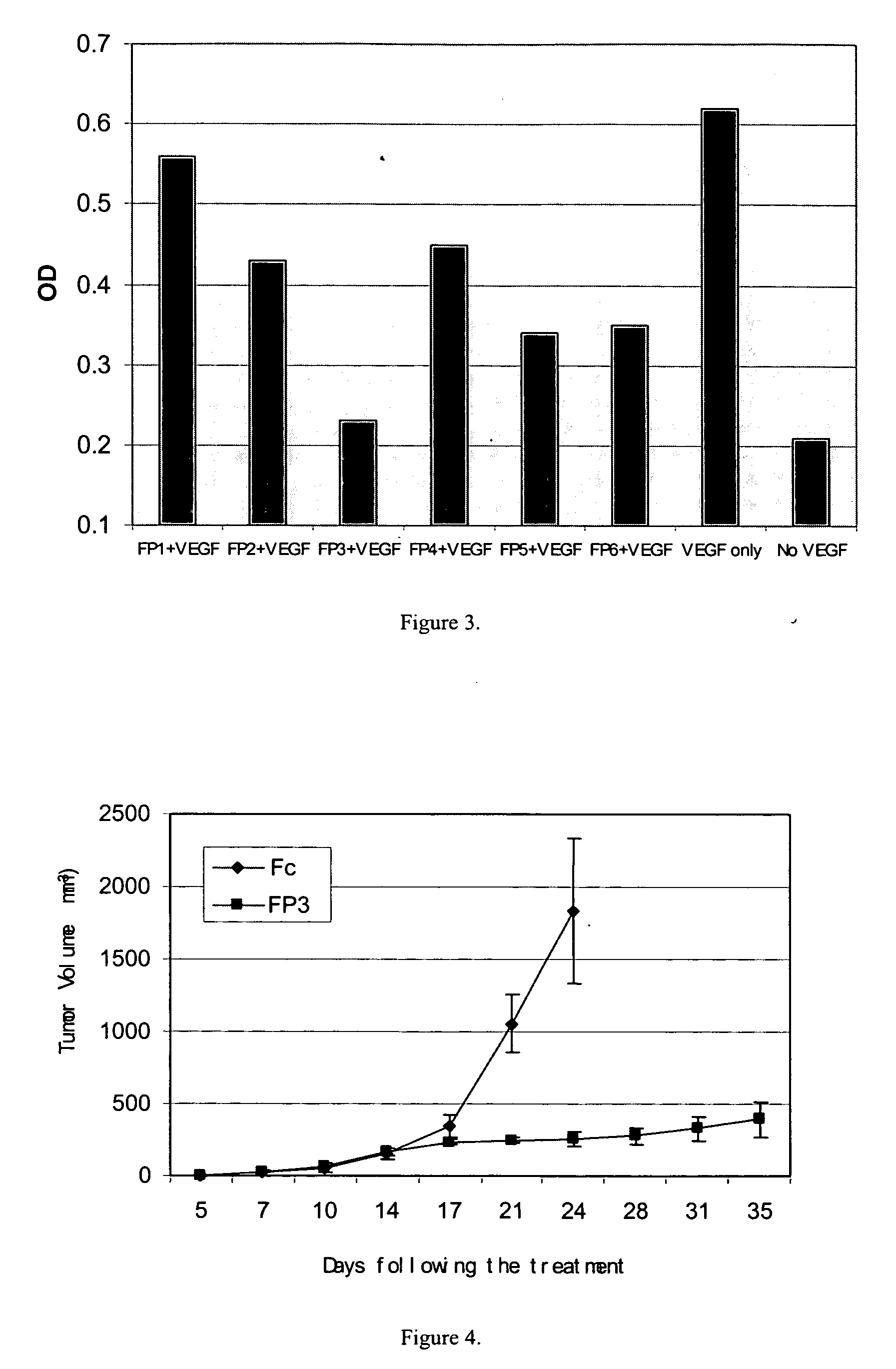
Angiogenesis-inhibiting chimeric protein and the use
ActiveUS7750138B2Increase binding siteHigh affinitySenses disorderPeptide/protein ingredientsAngiogenesis growth factorChimera Protein
Owner:CHENGDU KANGHONG BIOTECH
CD33 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS9944702B2Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD33
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD33 monoclonal antibody, conferring specific immunity against CD33 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
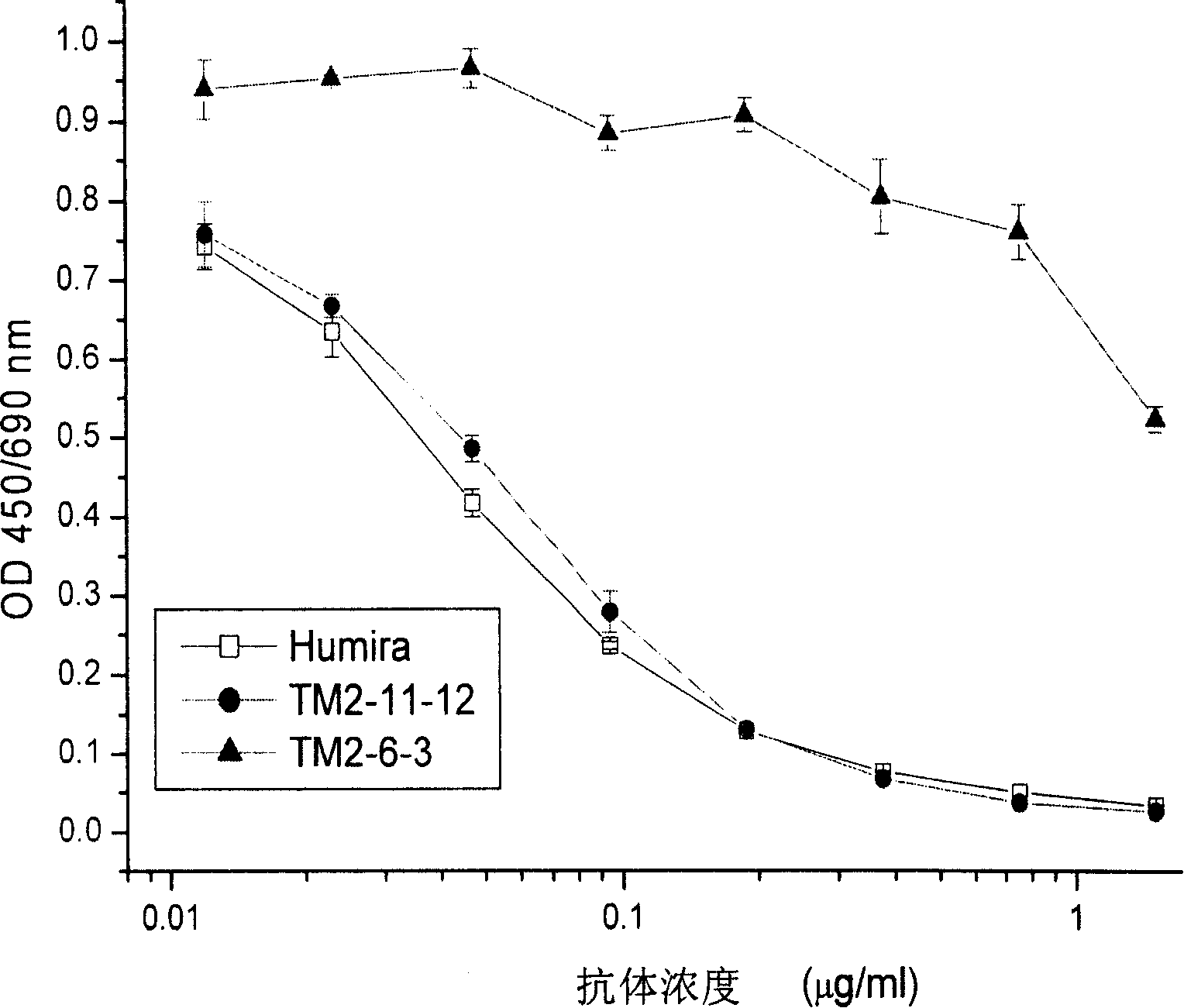
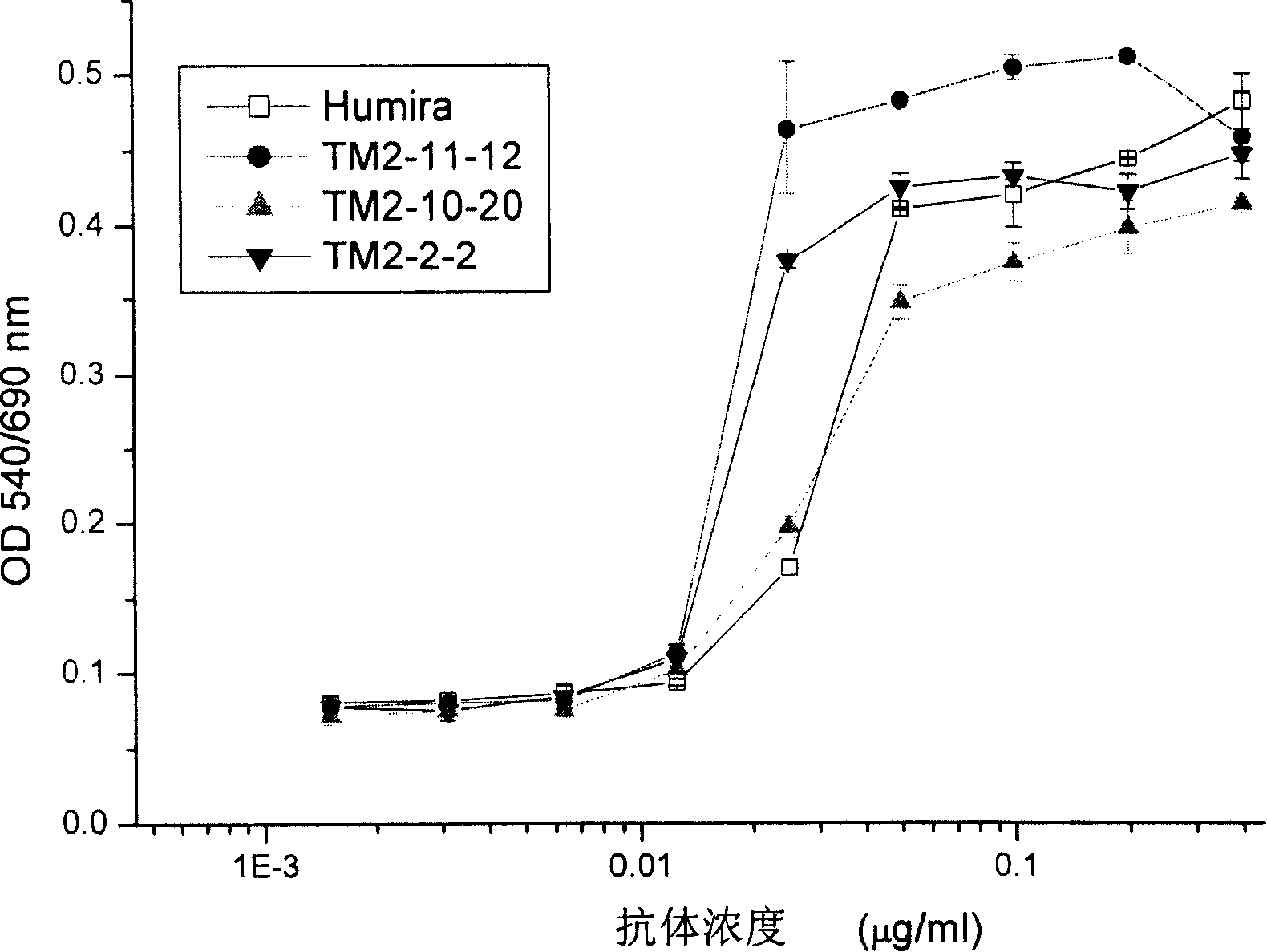
Recombined chimeric antibody against human tumor necrosis factor alpha
ActiveCN101177453AExtended half-lifeFunctionalPeptide/protein ingredientsTumor necrosis factorDiseaseHuman tumor
Described herein are antibodies that bind to human tumor necrosis factor alpha (hTNFα). And list the nucleotide sequence of antibody heavy chain and light chain variable region and its derived amino acid sequence. The heavy chain and light chain variable region genes are respectively connected with the human immunoglobulin gamma 1 (hIgG1) heavy chain constant region and human kappa (k) light chain constant region genes to form a chimeric gene. Then, the vector containing the chimeric gene is introduced into the host cell line to express the antibody protein. This recombinant chimeric antibody protein can neutralize the activity of hTNFα in vitro, and is suitable for treating patients with excessive secretion of harmful hTNFα, including certain inflammations, such as rheumatoid arthritis and Crohn's disease.
Owner:PHARMAB
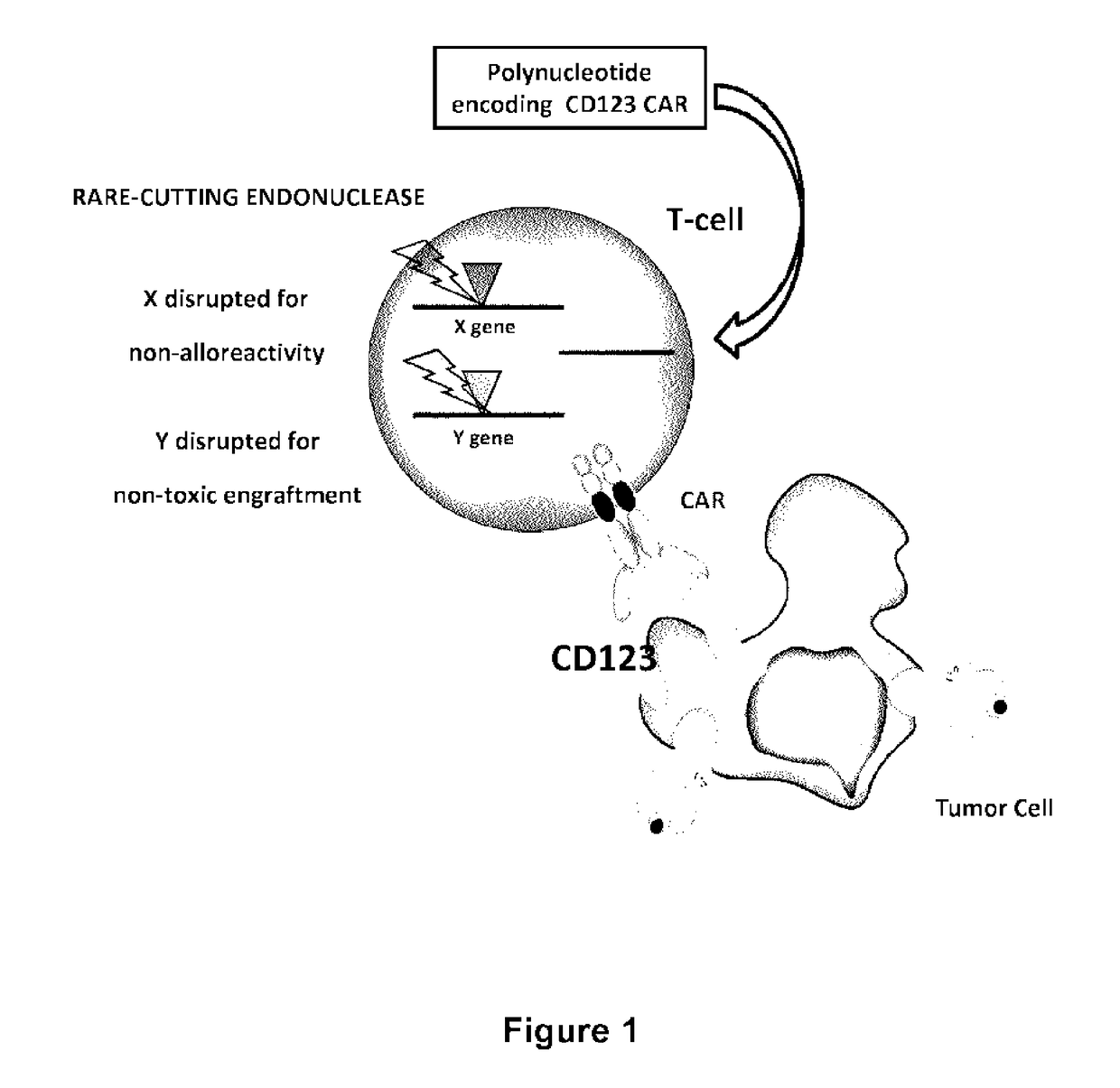
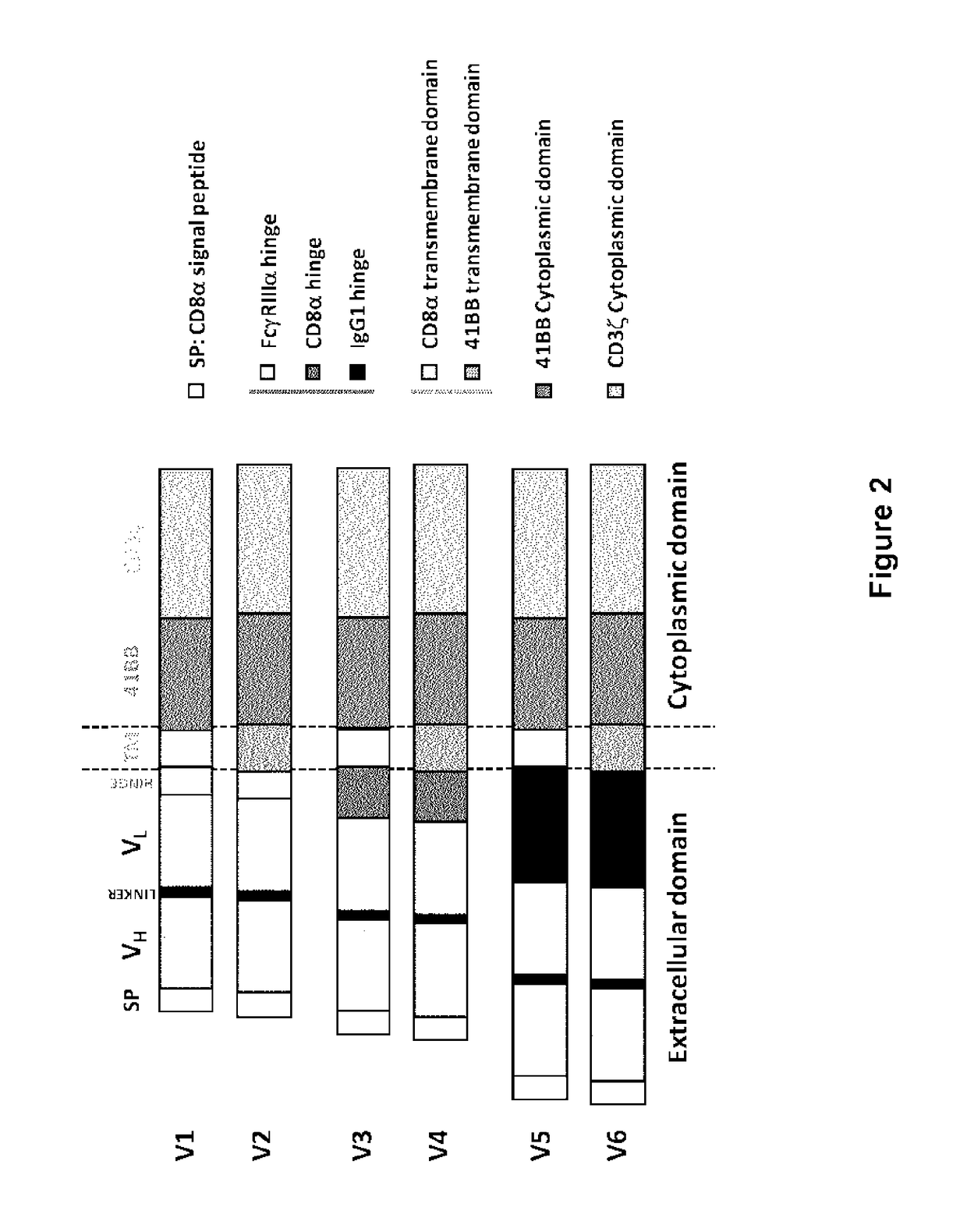
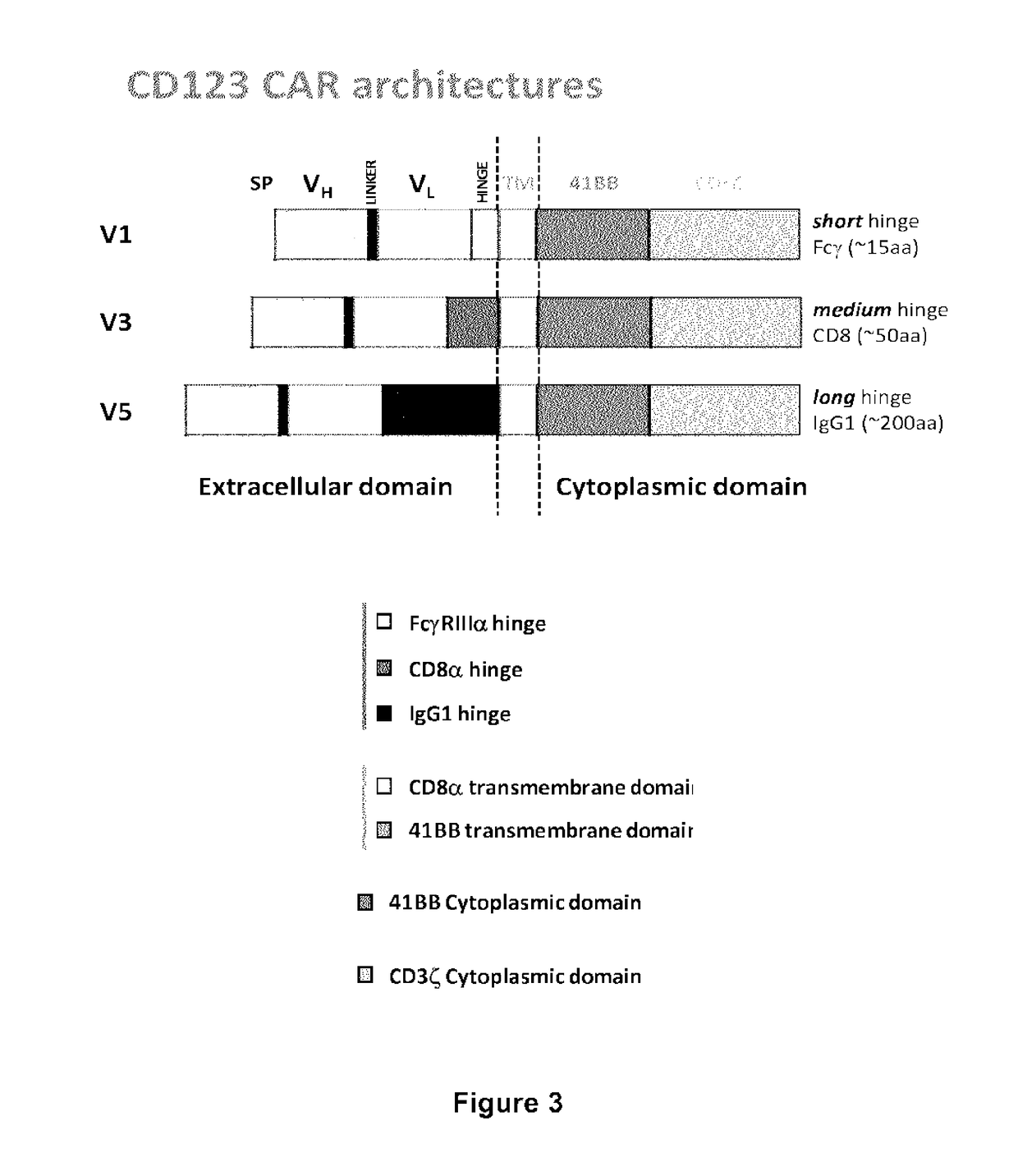
CD123 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS9944709B2Useful for immunotherapyReduce in quantityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenAntigen receptor
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD123 monoclonal antibody, conferring specific immunity against CD123 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
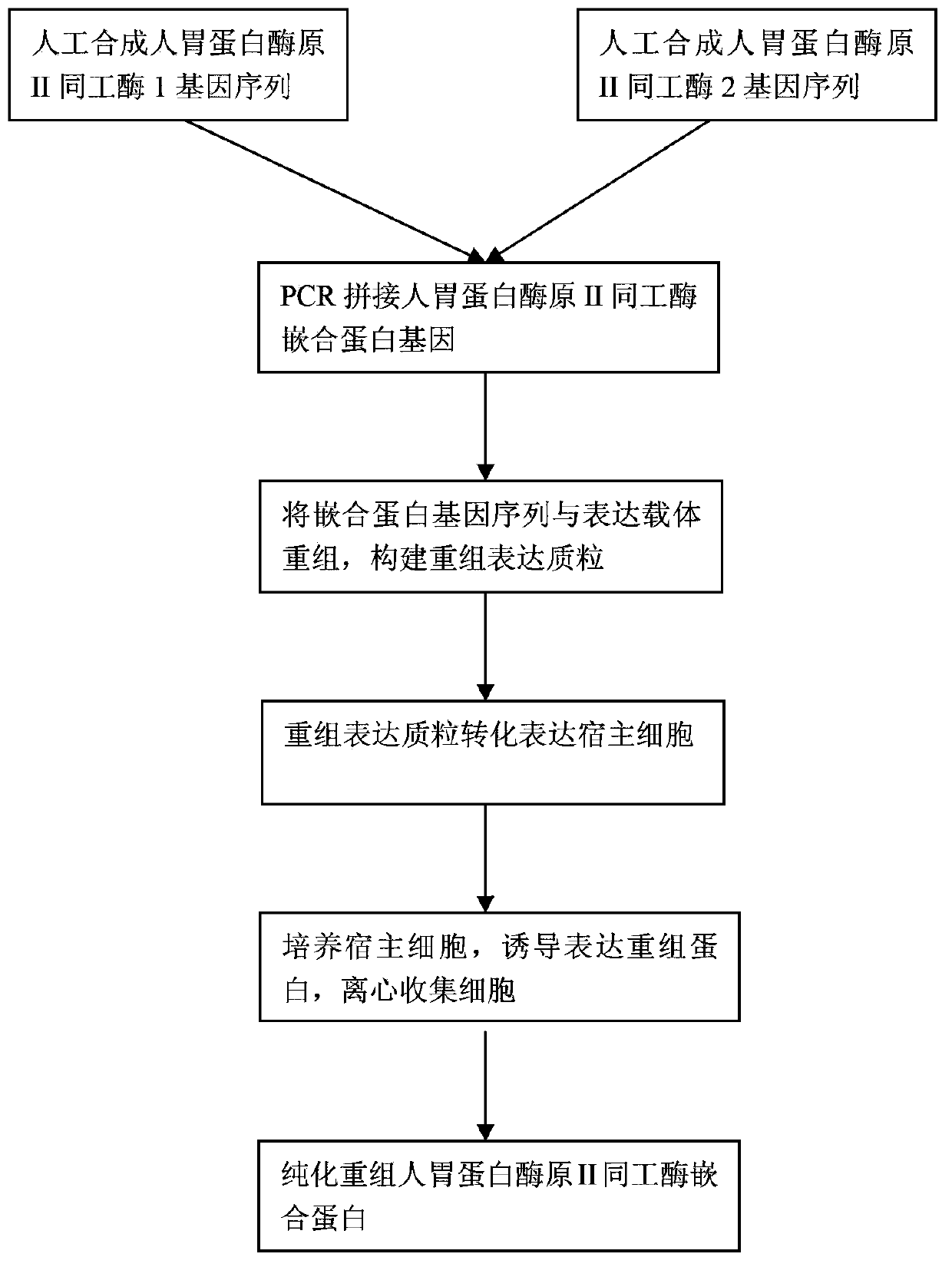
Recombinant human pepsinogen II isozyme chimeric protein, and preparation method and applications thereof
ActiveCN103387971AHigh sensitivityIncrease catch rateHydrolasesSerum immunoglobulinsPepsinogen IIIsozyme
The invention discloses a recombinant human pepsinogen II isozyme chimeric protein, and a preparation method and applications thereof. The preparation method comprises the steps as follows: by taking gene sequences of two isozymes of recombinant human pepsinogen II as a template, carrying out PCR (polymerase chain reaction) splicing to obtain a chimeric protein coding gene sequence, constructing recombinant expression plasmids, transforming the screened positive clone plasmids into expression host cells, screening an efficiently-expressed recombinant chimeric protein strain, carrying out enlargement culture on the cells and inducing expression chimeric protein, and purifying to obtain the recombinant human pepsinogen II isozyme chimeric protein. The invention further discloses the applications of the recombinant human pepsinogen II isozyme chimeric protein in preparing monoclonal antibodies and multiresistant serums or pepsinogen II kit calibration products. A great amount of stably expressed recombinant human pepsinogen II isozyme chimeric protein can be produced by utilizing a genetic engineering technology, and one protein has two chimeric protein sequences of the human pepsinogen II.
Owner:常州爱复康生物科技有限公司
Ror1 (ntrkr1) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170283497A1Good effectUseful for immunotherapyGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a ROR1 monoclonal antibody, conferring specific immunity against ROR1 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia, and for solid tumors such as breast, colon, lung, and kidney tumors
Owner:CELLECTIS SA
Novel coronavirus vaccine based on chimeric virus-like particles
PendingCN111620952AEnhance immune responseGood immune protectionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationReceptor
Owner:SUZHOU MIDI BIOTECH CO LTD
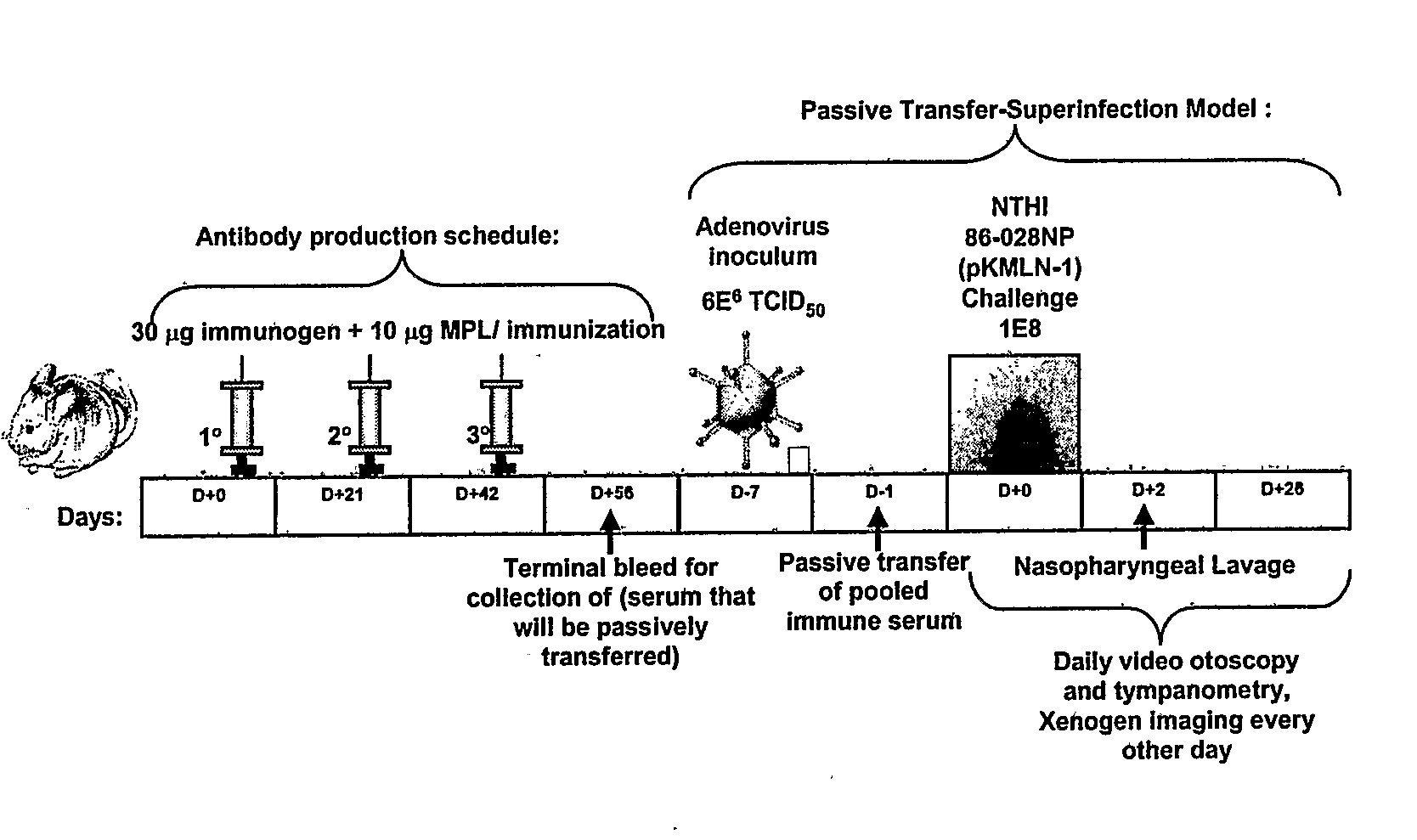
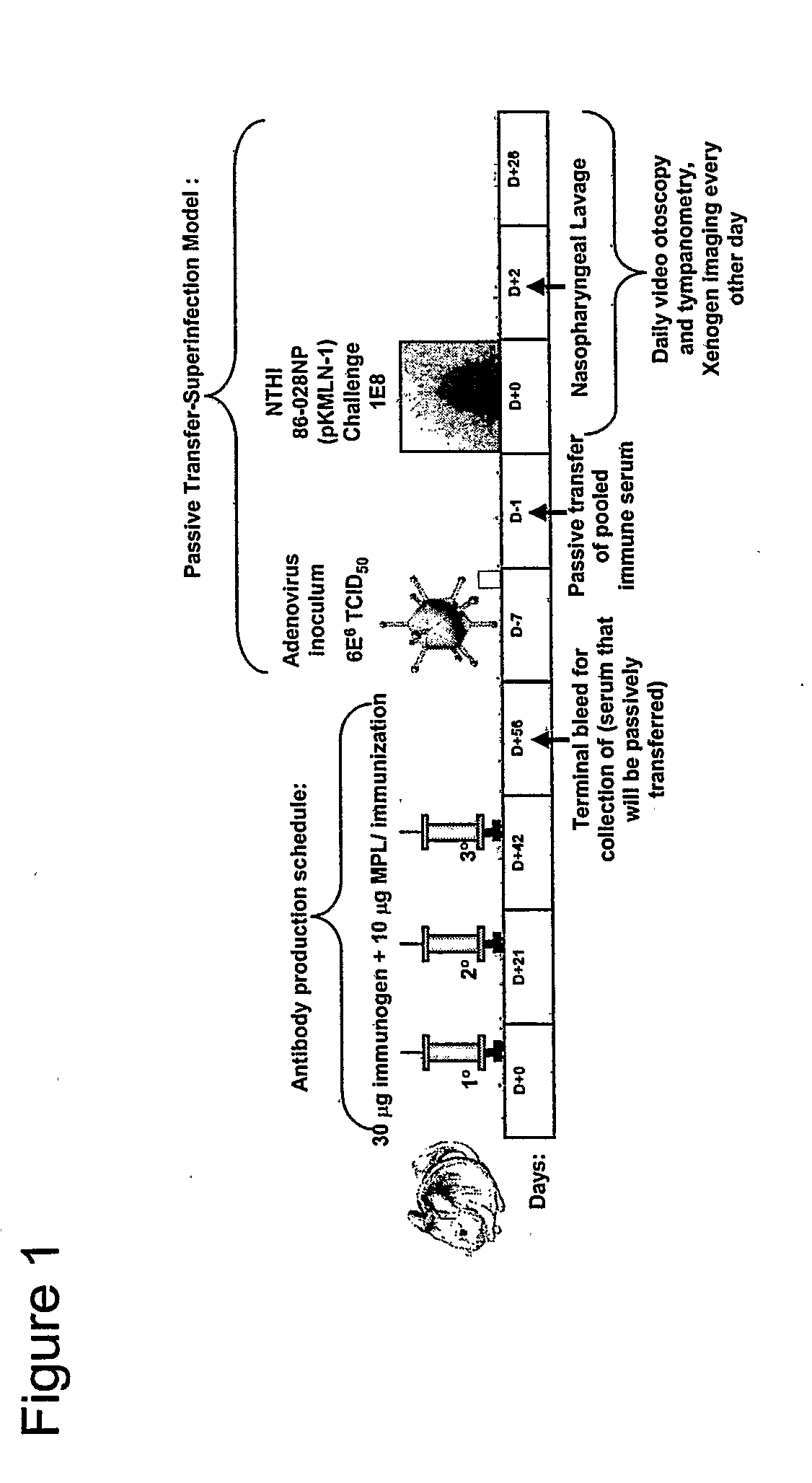
Chimeric vaccine for haemophilus influenzae-induced disease
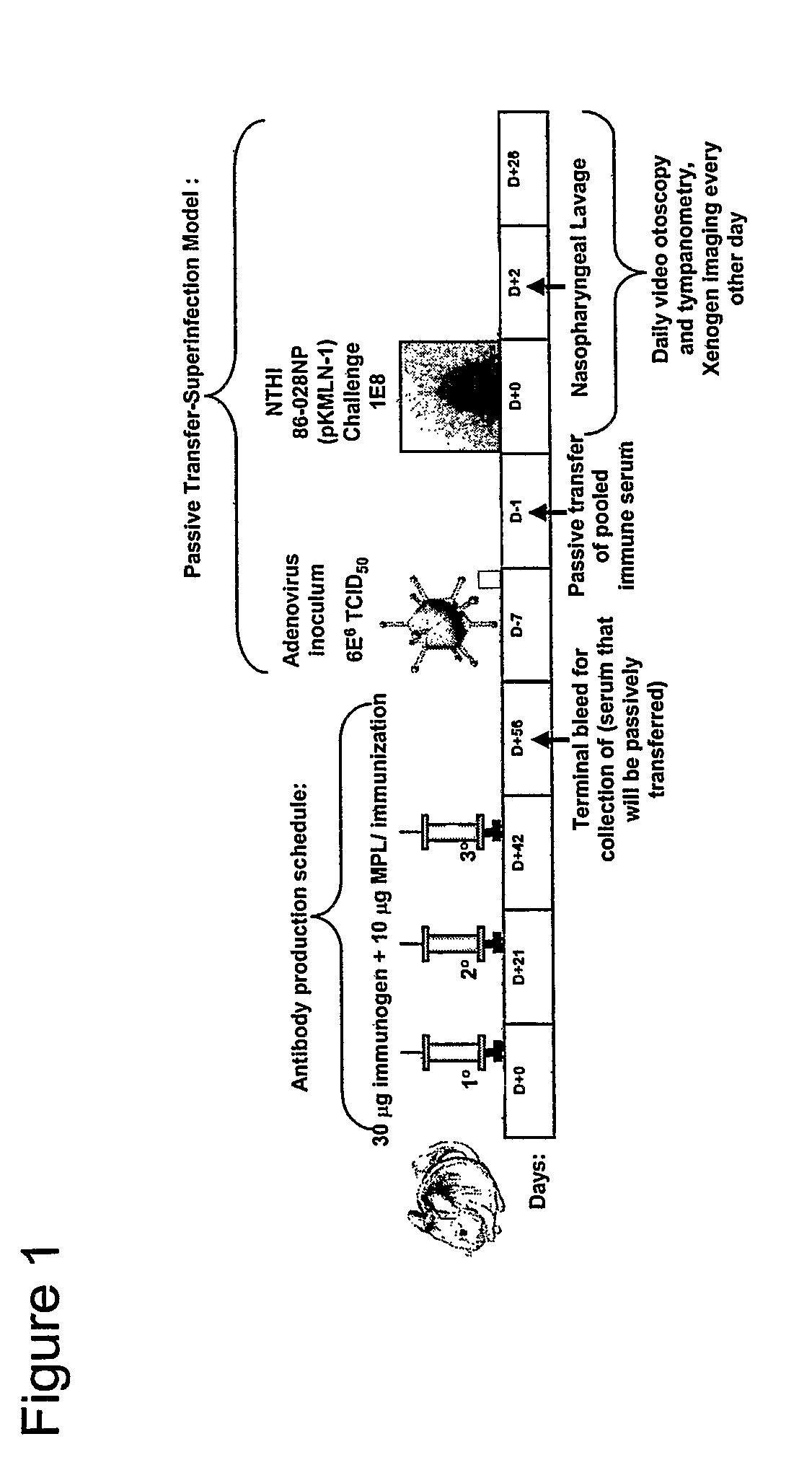
ActiveUS7811591B2Strong responseAssist in protein folding and/or antigen presentationAntibacterial agentsSenses disorderHaemophilus influenzaeRecombinant Chimeric Proteins
The invention described herein relates to a chimeric protein comprising the NTHi twitching pilus major subunit protein (PilA) presenting a portion of the NTHi OMP P5 protein. The invention provides for vaccine compositions comprising the recombinant chimeric protein and methods of eliciting an immune response using the recombinant chimeric proteins of the invention.
Owner:NATIONWIDE CHILDRENS HOSPITAL
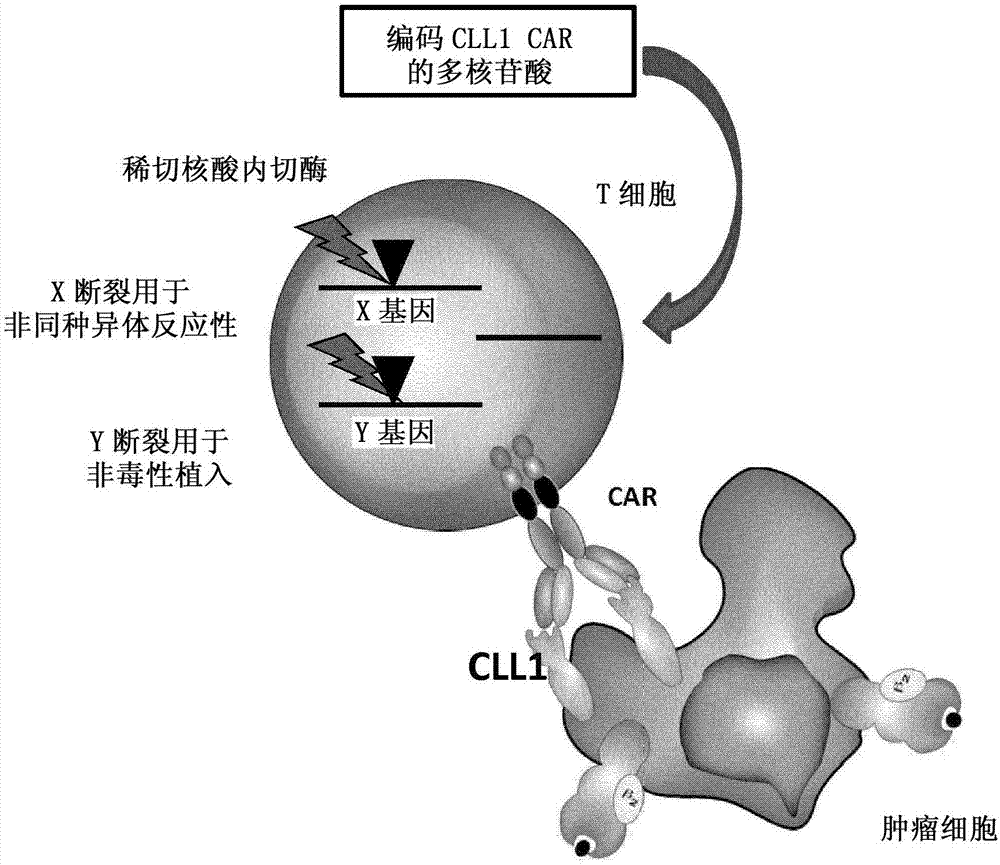
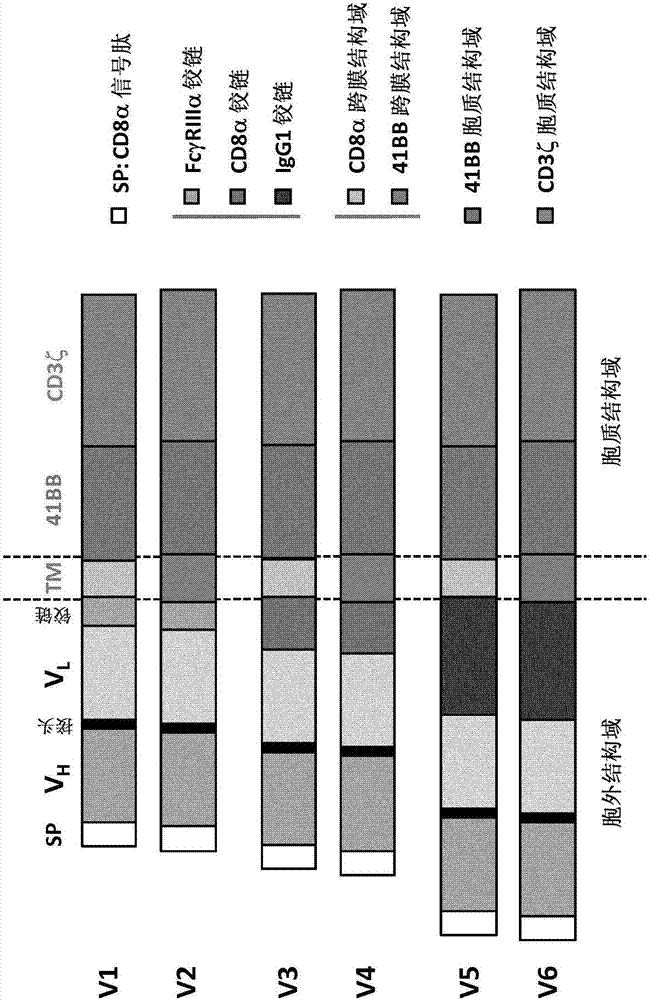
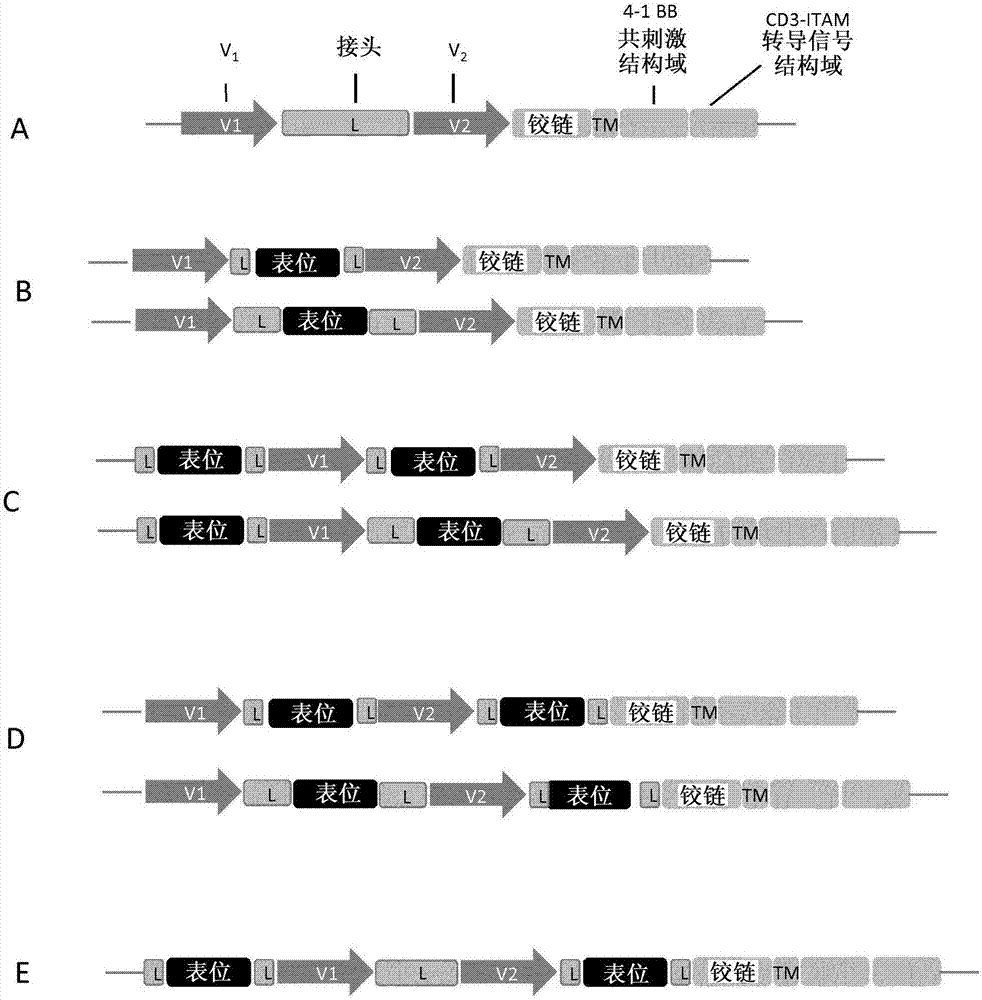
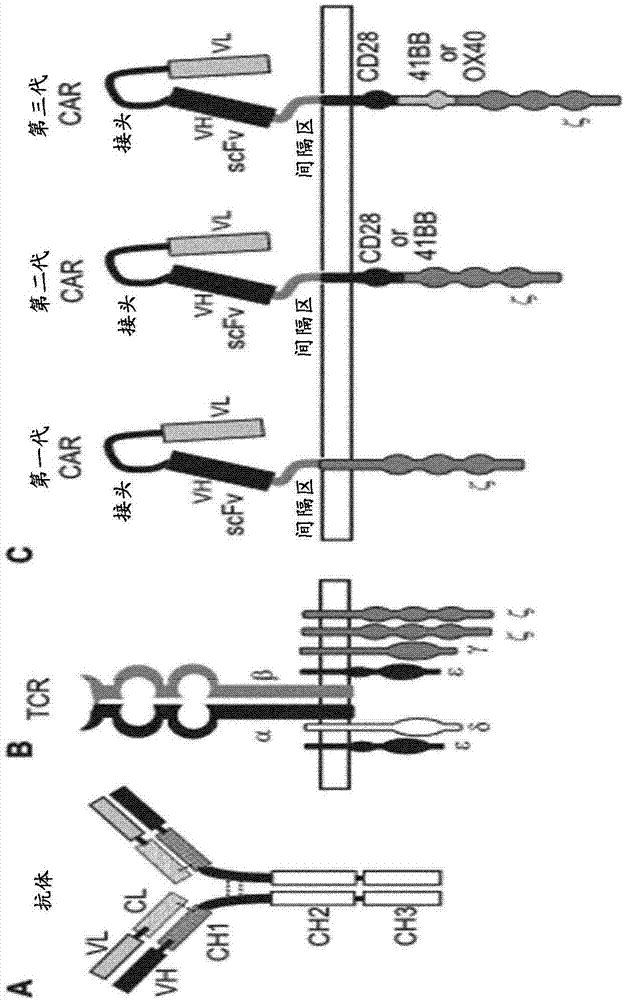
Anti-CLL1 specific single-chain chimeric antigen receptors (scCARs) for cancer immunotherapy
ActiveCN107406516AAnimal cellsAntibody mimetics/scaffoldsAntigen receptorsRecombinant Chimeric Proteins
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward CLL1 positive cells. The engineered immune cells endowed with such CARs are particularly suited for immunotherapy for treating cancer, in particular leukemia.
Owner:CELLECTIS SA
Ror1(ntrkr1)specific chimeric antigen receptors for cancer immunotherapy
ActiveCN106922148AStrong cytotoxicitySignificant clinical advantageGenetic material ingredientsMammal material medical ingredientsAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a ROR1 monoclonal antibody, conferring specific immunity against ROR1 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia, and for solid tumors such as breast, colon, lung, and kidney tumors
Owner:CELLECTIS SA
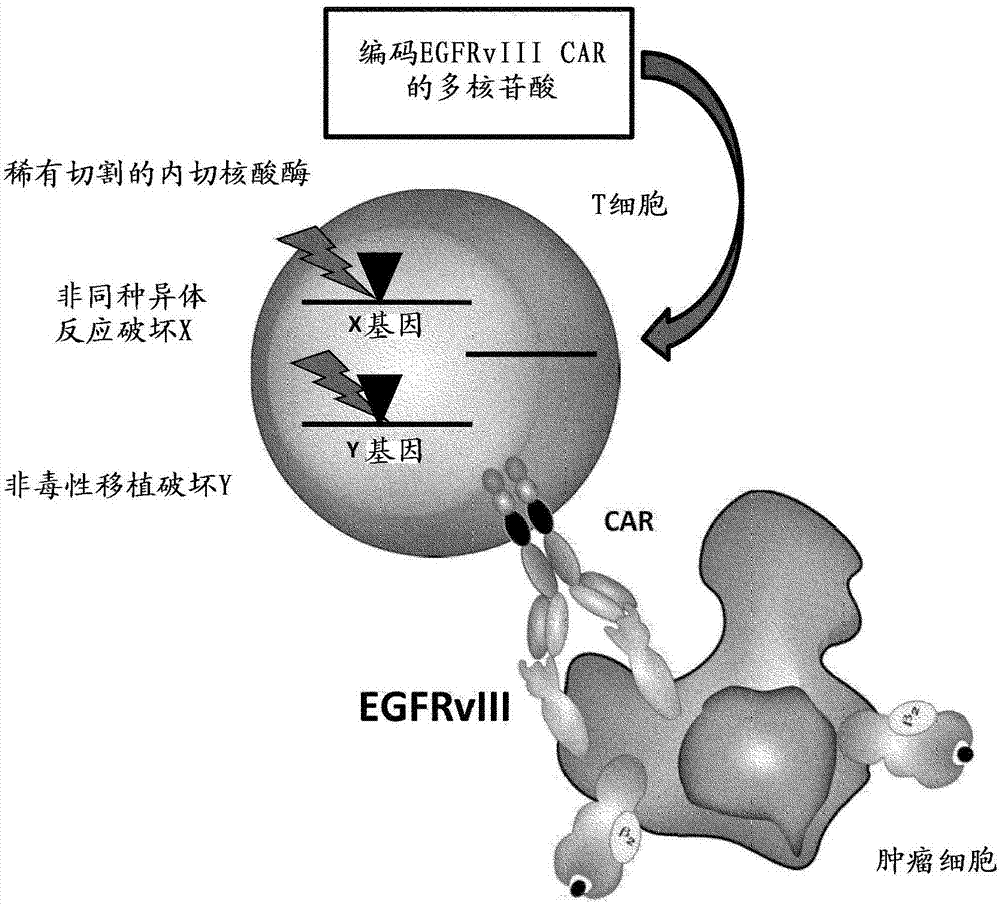
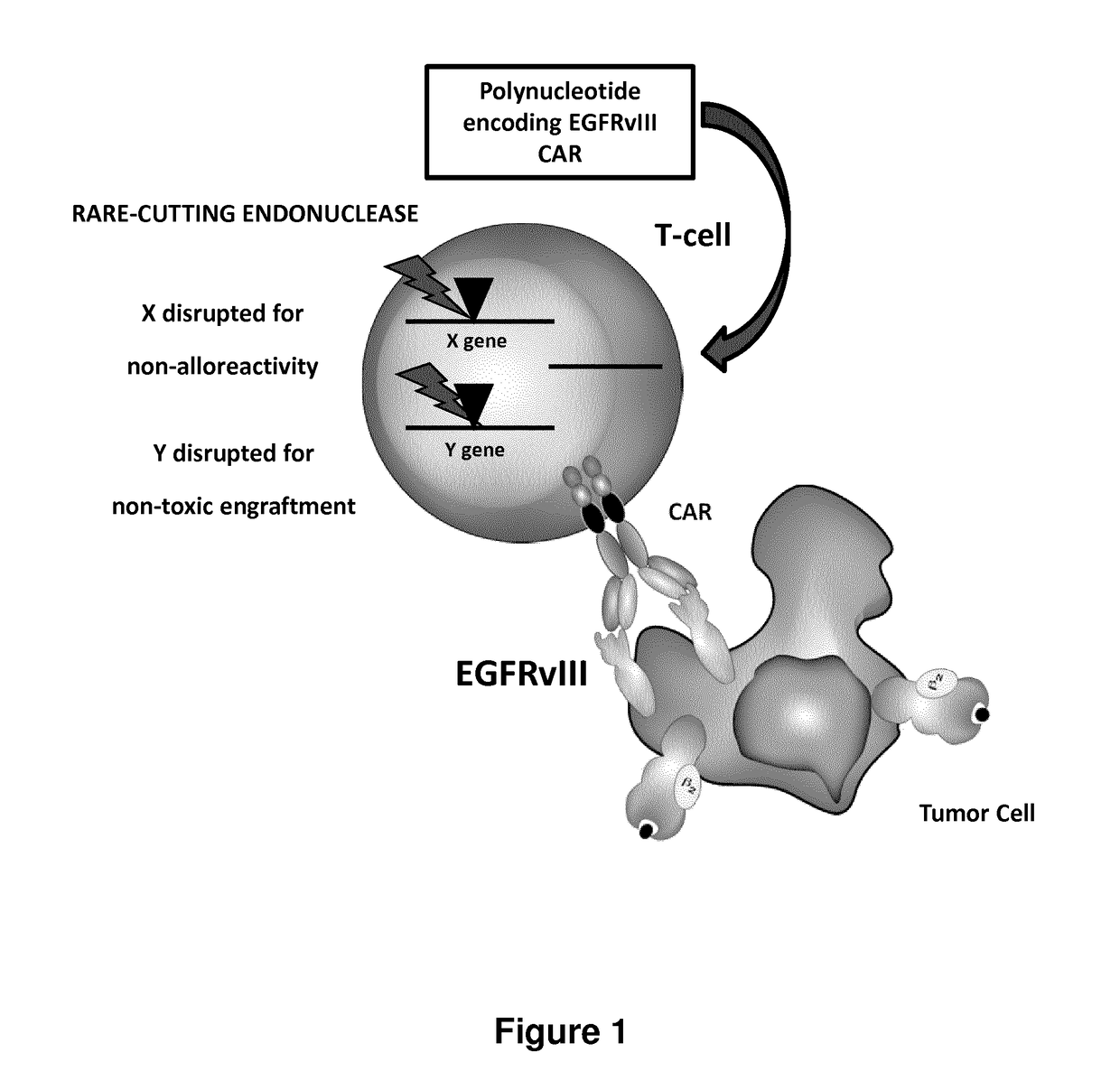
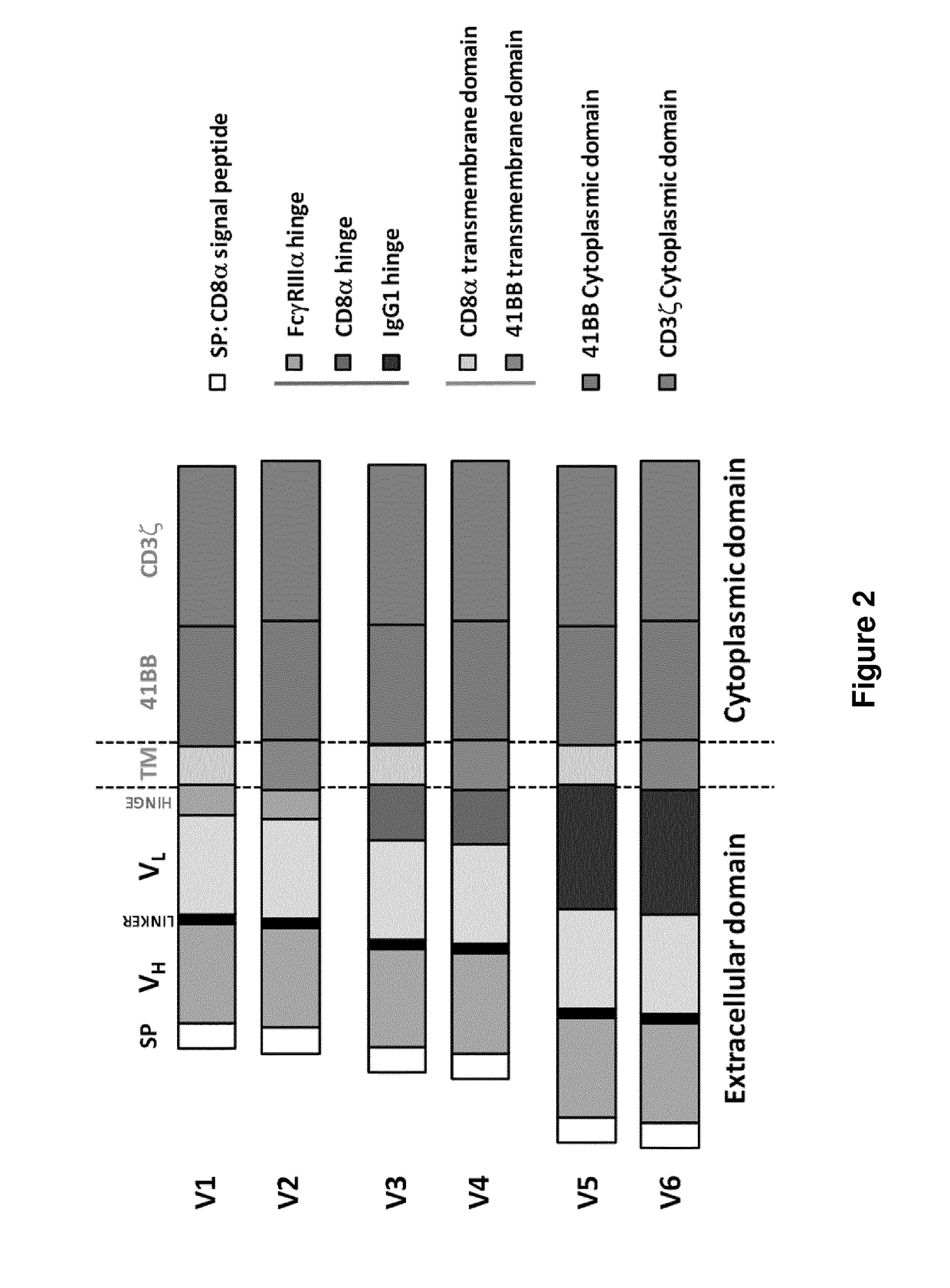
EGFRvIII specific chimeric antigen receptors for cancer immunotherapy
InactiveCN107074973AEasy to implantBetter and Safer ImplantationGenetic material ingredientsMammal material medical ingredientsAntigenGlioblastoma
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from an EGFRvIII monoclonal antibody, conferring specific immunity against EGFRvIII positive cells. The TCR KO engineered immune cells endowed with such CARs are particularly suited for treating lung cancer, anal cancers and glioblastoma multiforme.
Owner:ALLOGENE THERAPEUTICS INC
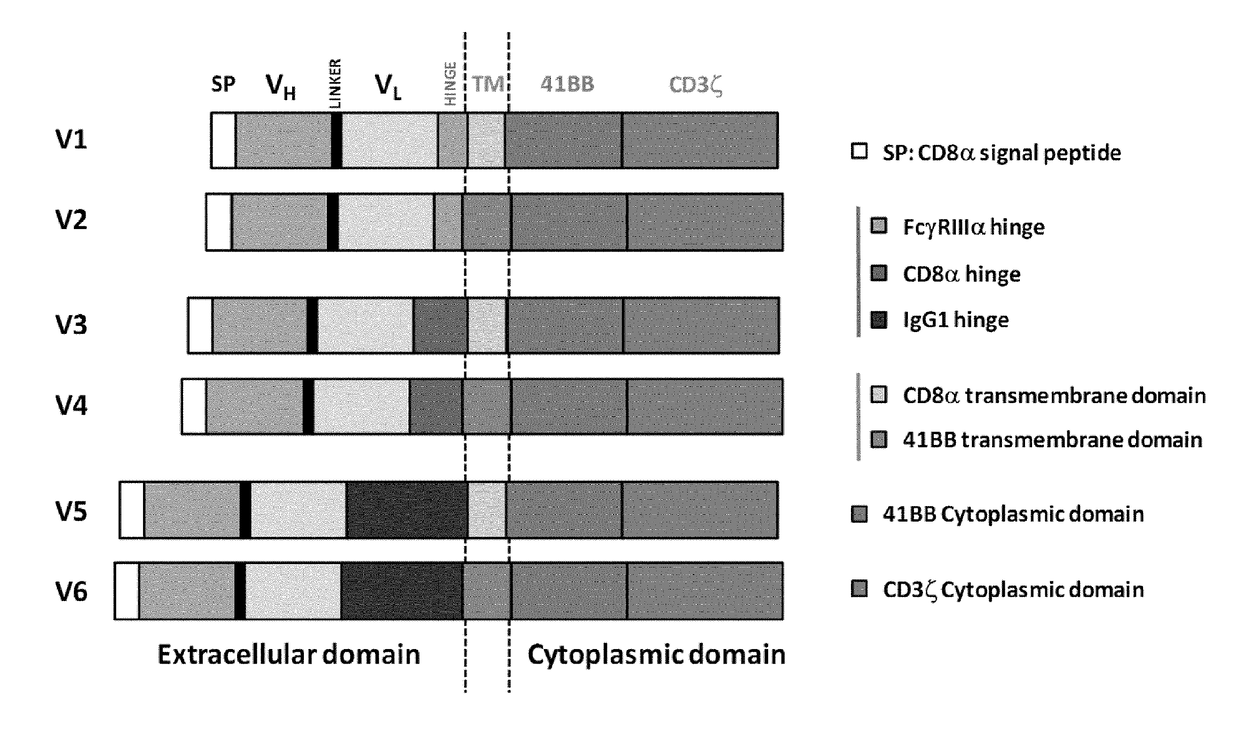
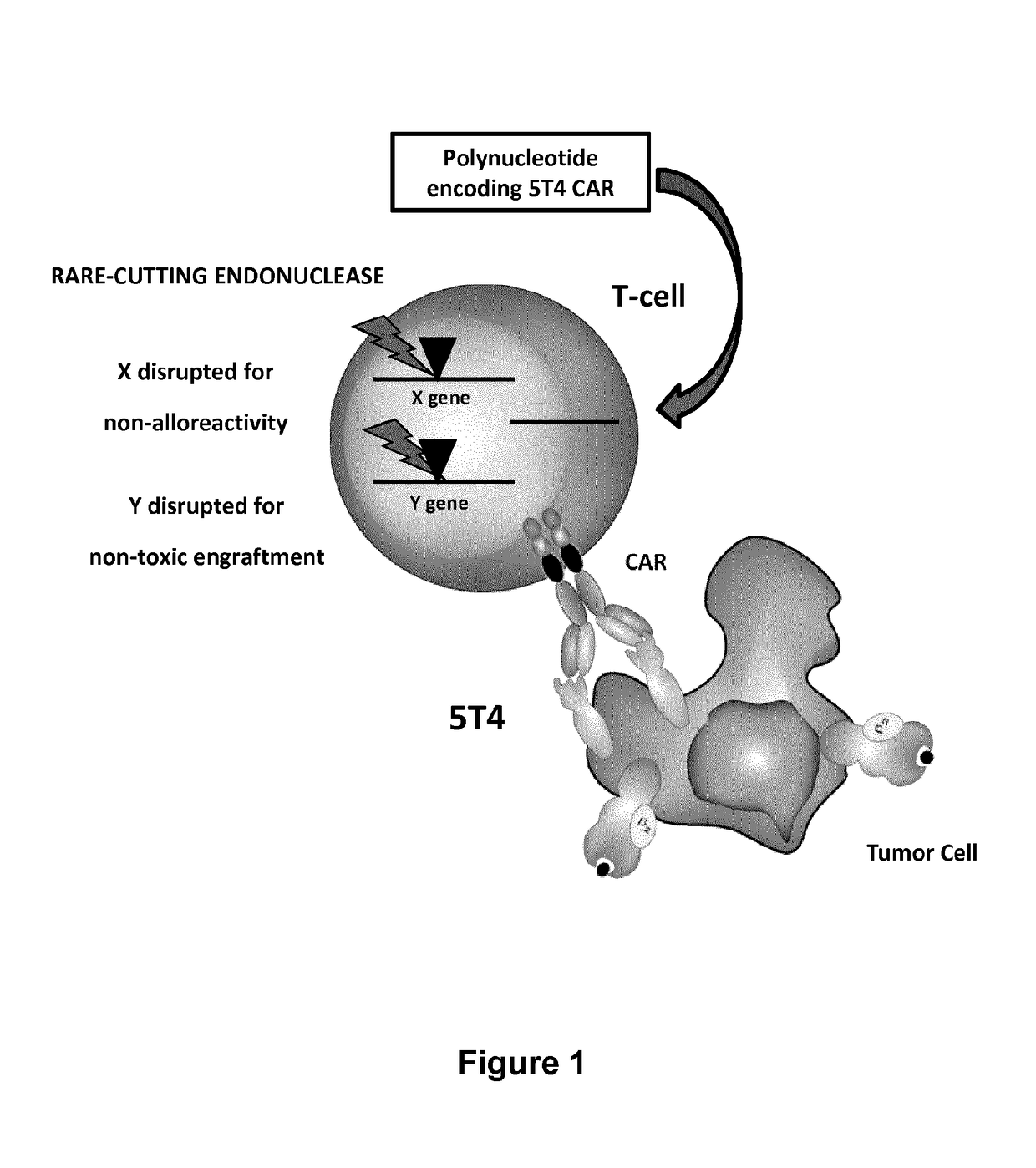
Trophoblast glycoprotein (5t4, tpbg) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170275374A1Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a 5T4 monoclonal antibody, conferring specific immunity against 5T4 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia, and for solid tumors such as colon, stomach, and ovarian tumors.
Owner:CELLECTIS SA
BCMA (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS10316101B2Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
Angiogenesis-Inhibiting Chimeric Protein and the Use
ActiveUS20080206238A1Inhibit tumor growthProlonged animal lifeSenses disorderPeptide/protein ingredientsAngiogenesis growth factorChimera Protein
The present invention is directed to DNA sequence encoding angiogenesis-inhibiting recombinant chimeric protein, the chimeric protein per se, the pharmaceutical use of the chimeric protein, and to the pharmaceutical composition containing the recombinant protein and the formulation thereof.
Owner:CHENGDU KANGHONG BIOTECH
Process for the production of monoclonal antibodies
InactiveUS7919314B2Highly efficient immunogenEfficient productionAnimal cellsAntibody mimetics/scaffoldsForeign proteinVirus-like particle
Owner:BIOTECHJOS INSTS
EGFRvIII Specific Chimeric Antigen Receptor For Cancer Immunotherapy
InactiveUS20170275366A1Improve survivalUseful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenAnal cancer
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from an EGFRvIII monoclonal antibody, conferring specific immunity against EGFRvIII positive cells. The TCR KO engineered immune cells endowed with such CARs are particularly suited for treating lung cancer, anal cancers and glioblastoma multiforme.
Owner:ALLOGENE THERAPEUTICS INC
Recombinant chimeric protein carrying hepatitis B virus epitope and preparation thereof
ActiveCN102643349AReactiveImmunogenicHybrid peptidesVector-based foreign material introductionHepatitis B Virus AntigenEpitope
The invention discloses a recombinant chimeric protein carrying a hepatitis B virus epitope and the preparation thereof, particularly a recombinant chimeric protein respectively carrying a hepatitis B virus S82, C19, S125, S2[8], S1[93] or C117 epitope, and a recombinant chimeric protein simultaneously carrying hepatitis B virus S82, C19, S125, S2[8], S1[93] and C117 epitopes, and through the immunology WesternBlot detection, each recombinant protein not only has the antigen reactivity and the immunogenicity of a VP6F protein, but also has the antigen reactivity and the immunogenicity of the HBV (hepatitis B virus) epitope, does not have infectivity and the side effects of virulence atavism, the intussusception and the like, and has important value for developing new generation RV (Rota Virus) / HBV recombinant chimeric vaccine and reagent.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Chimeric Vaccine for Haemophilus Influenzae-Induced Disease
ActiveUS20080311110A1Assist in protein foldingAssist in antigen presentationAntibacterial agentsSenses disorderDiseaseHaemophilus influenzae
The invention described herein relates to a chimeric protein comprising the NTHi twitching pilus major subunit protein (PilA) presenting a portion of the NTHi OMP P5 protein. The invention provides for vaccine compositions comprising the recombinant chimeric protein and methods of eliciting an immune response using the recombinant chimeric proteins of the invention.
Owner:NATIONWIDE CHILDRENS HOSPITAL
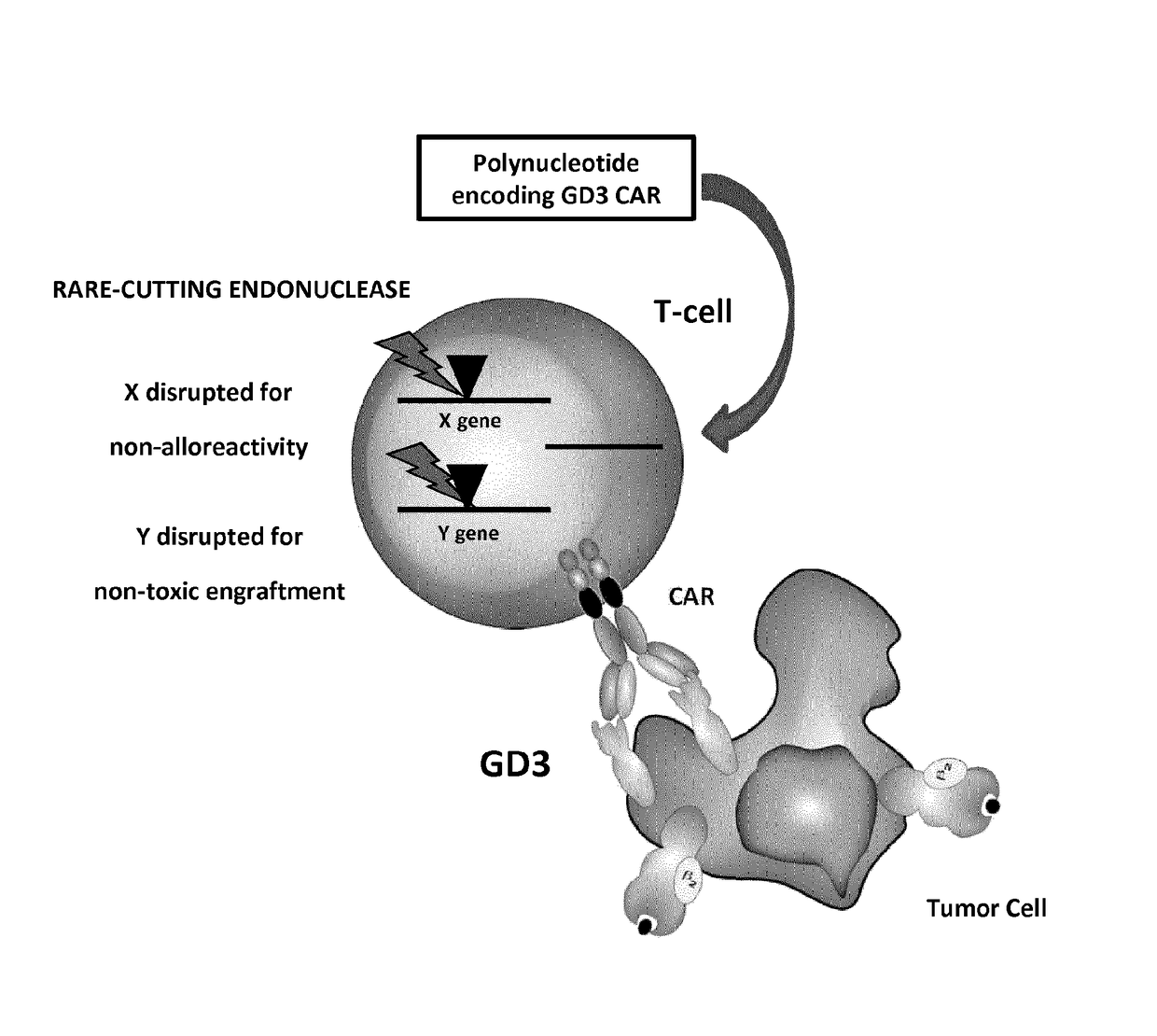
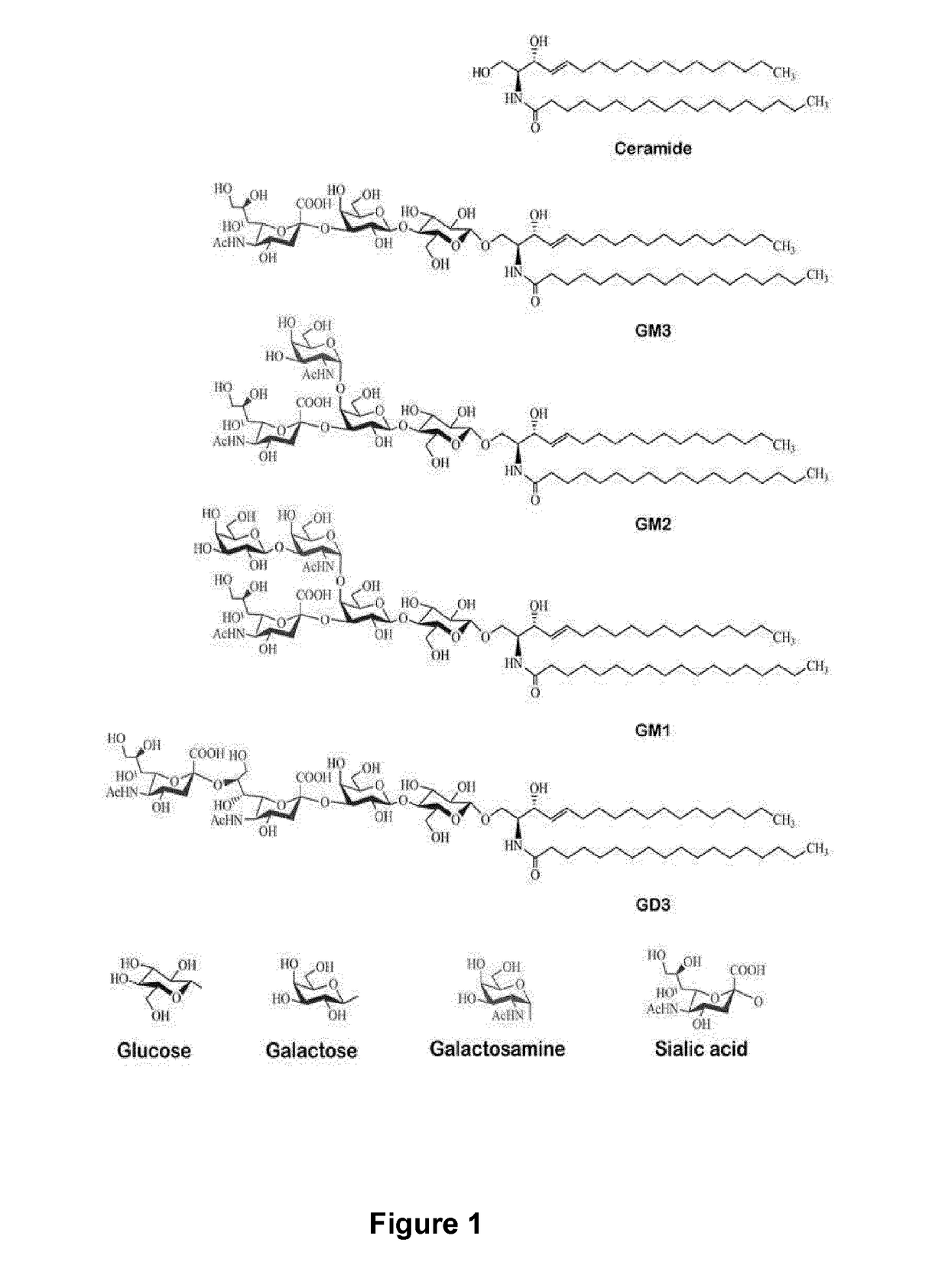
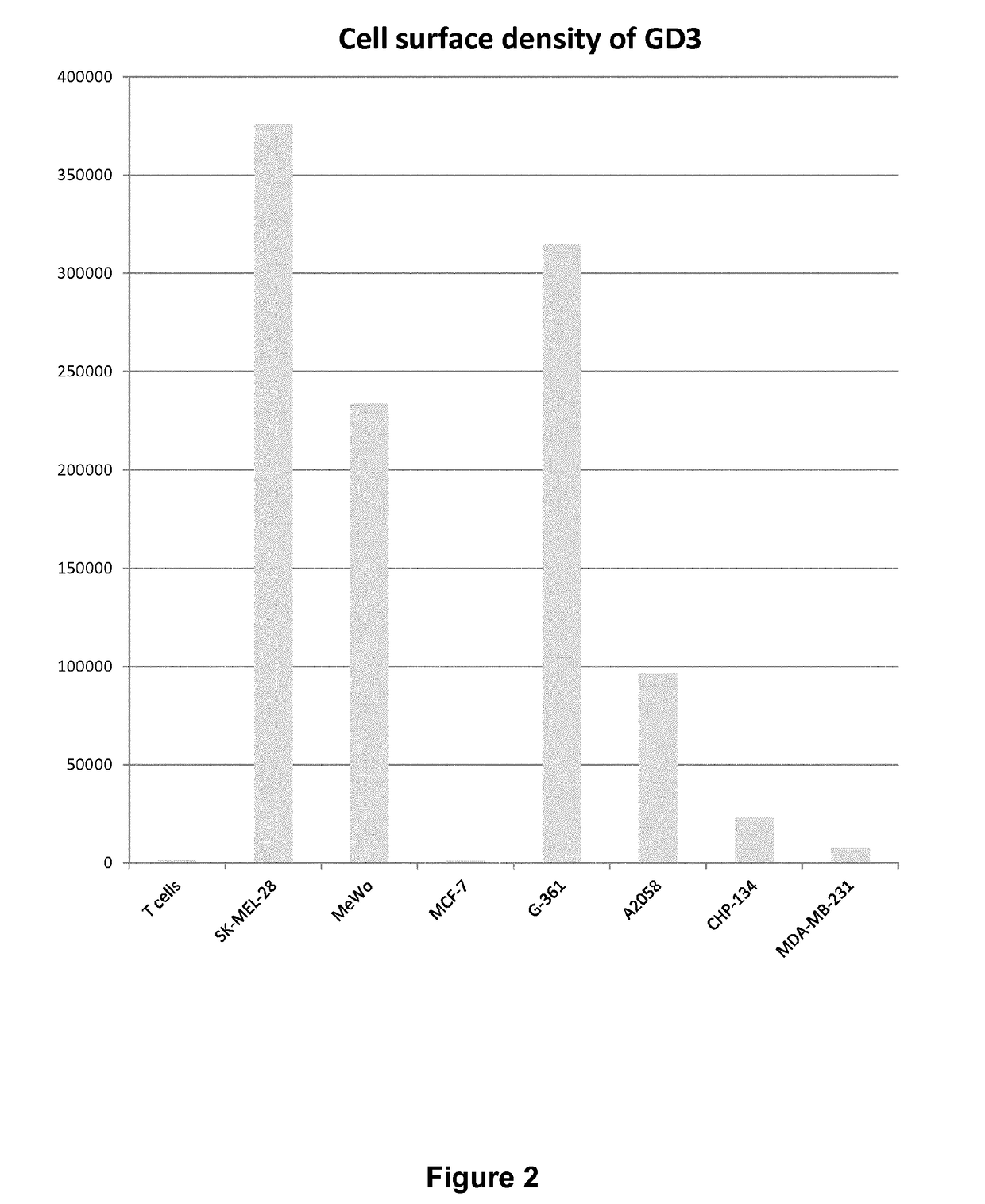
Anti-gd3 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20180291079A1Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD19-specific chimeric antigen receptor
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a GD3 monoclonal antibody, conferring specific immunity against GD3 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating solid tumors such as melanomas, carcinomas or liquid tumor such as T-cell lymphoblastic leukemia.
Owner:CELLECTIS SA
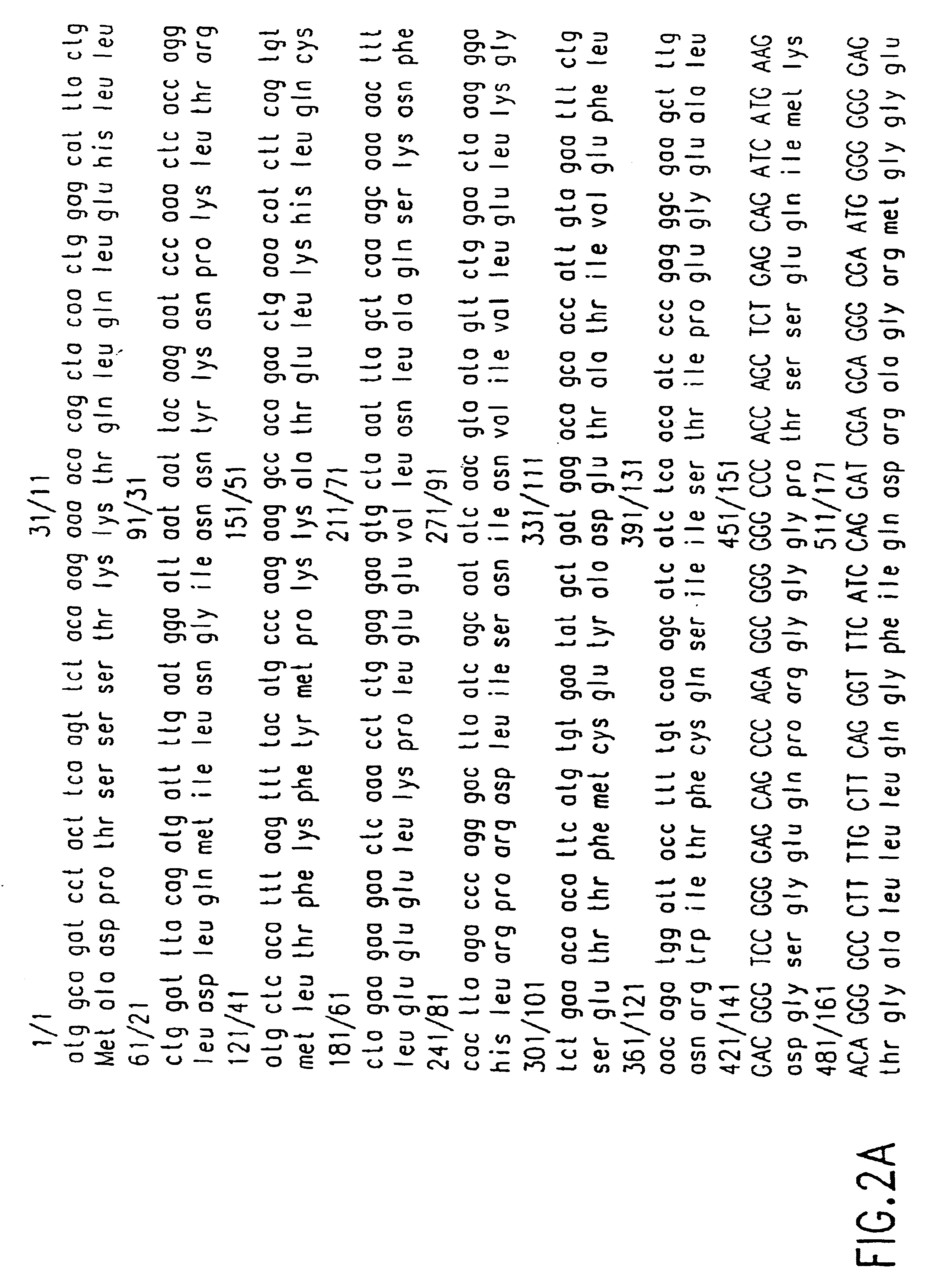
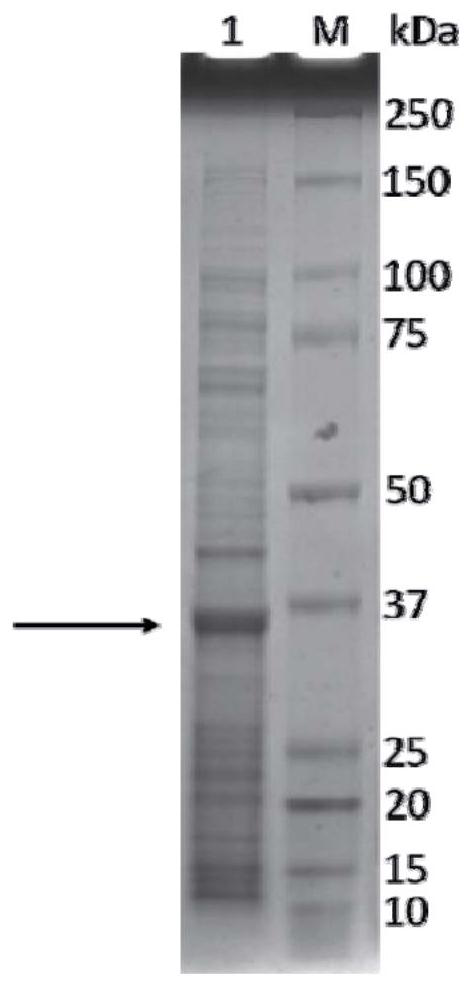
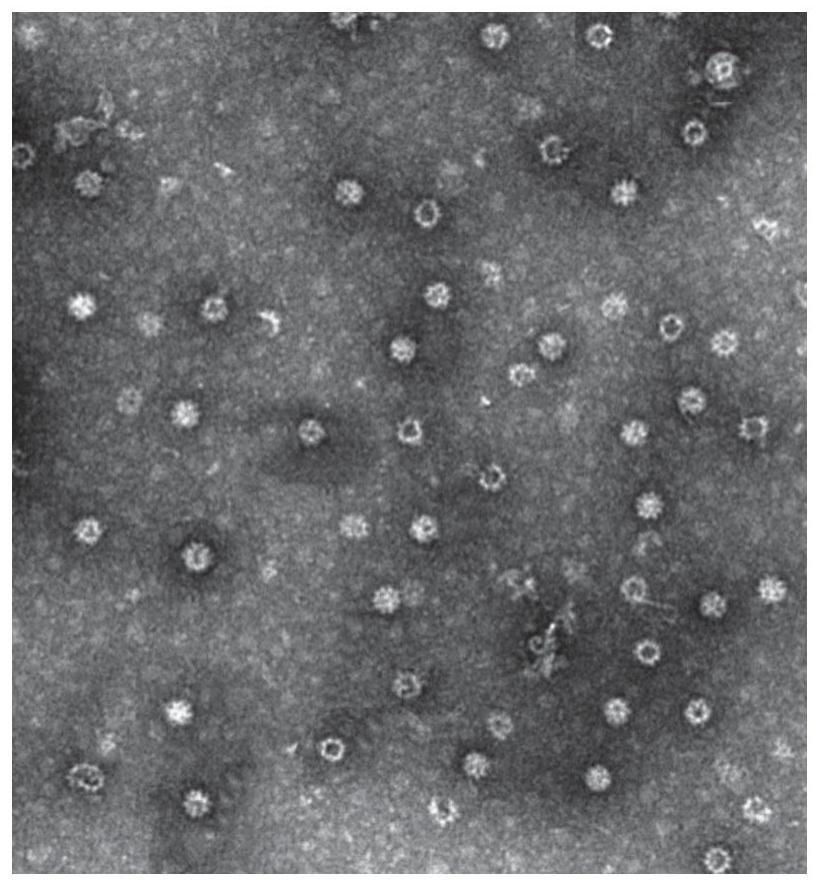
Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof
InactiveCN103304671AOvercoming the potential risk of intussusceptionImprove immune efficiencyViral antigen ingredientsMicroorganism based processesViral VaccineGenotype
The invention relates to a human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein, wherein the amino acid sequence of the protein is shown in SEQ ID NO.2. Furthermore, the invention provides a coding gene and a preparation method of the protein as well as an application of the protein in preparation of a rotavirus vaccine. Two segments P[8]deltaVP8* and P[6]deltaVP8* are connected in serial to be expressed into the human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein; the protein can simultaneously induce neutralizing antibodies of major P genotypes P [8], P[4] and P[6] of the human rotavirus, overcome the shortcoming of an existing vaccine which can induce the generation of an antibody only capable of resisting one genotype and greatly enhance immune efficiency; and meanwhile, the protein can avoid a potential risk that oral administration of attenuated vaccine rotavirus possibly induces intestinal invagination, and is applicable to preparation of rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Bovine rotavirus VP8* subunit recombinant chimeric protein and application thereof
ActiveCN104479028AImprove immune efficiencyReduce economic lossViral antigen ingredientsAntiviralsBovine rotavirusGenotype
The invention provides a bovine rotavirus VP8* subunit recombinant chimeric protein. According to the bovine rotavirus VP8* subunit recombinant chimeric protein, a T cell epitope polypeptide P2 in a tetanus toxin is introduced into an epitope vaccine of a bovine rotavirus VP8* subunit multi-copy chimeric gene recombinant protein, so that the immune efficacy of the epitope vaccine can be greatly enhanced, cross neutralizing immune protection can be induced, higher neutralizing antibody titer can be induced, and a high-titer anti-P[5] and P[11] genotype-specific rotavirus neutralizing antibody can be induced. The bovine rotavirus VP8* subunit recombinant chimeric protein provided by the invention can be used for preventing and controlling both G6 and G10 type rotaviruses which are mainly pandemic within a world range, and is suitable for preparing a safe and low-cost bovine rotavirus vaccine; in addition, the bovine rotavirus vaccine can be used for effectively reducing the economic loss brought by rotavirus gastroenteritis to cattle rearing by being matched with other rotavirus vaccines researched and developed at present, and has wide market prospects.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Serogroup B meningococcus recombinant chimeric protein vaccine and preparation method thereof
ActiveCN107823638AImprove the effect of preventionEffective protectionAntibacterial agentsNervous disorderPericarditisSalmonella serotype typhi
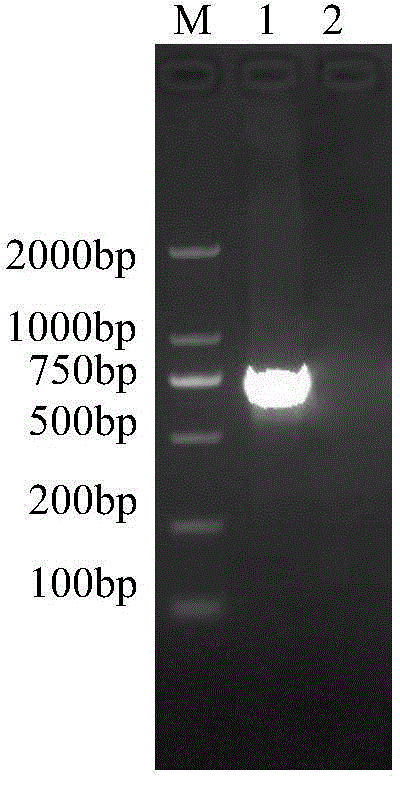
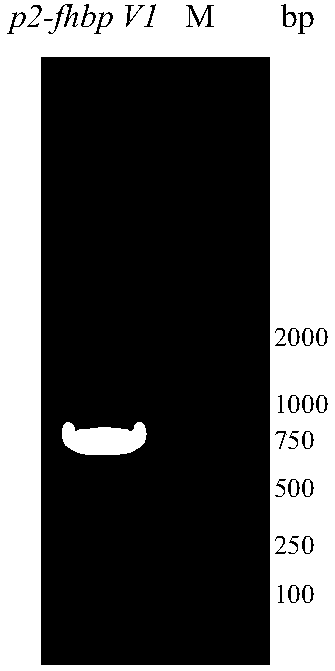
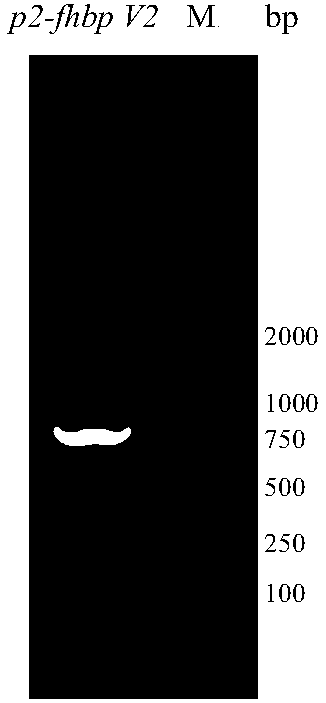
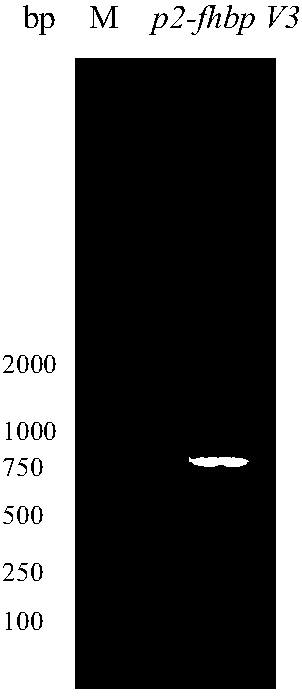
The invention provides a serogroup B meningococcus recombinant chimeric protein vaccine and a preparation method thereof. The vaccine contains three recombinant chimeric proteins of P2-fHBP V1, P2-fHBP V2 and P2-fHBP V3, wherein amino acid sequences of the recombinant chimeric proteins are SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3 respectively; the contents of all the proteins are 30 to 50mu g / dose; optimally, a vaccine preparation also contains a cryoprotectant or vaccine adjuvant. The invention also provides the preparation method of the three recombinant chimeric proteins. By using the recombinant chimeric proteins formed by linking P2 with serogroup B meningococcus fHBP protein, humoral immune response can be effectively induced, and immunogenicity of fHBP is obviously improved. The vaccine provided by the invention can effectively cover all serogroup B meningococcus strains, thereby providing a broad-spectrum preventing effect on invasive diseases such as cerebrospinal meningitis,bacteremia, pneumonia and pericarditis caused by the serogroup B meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Bovine infectious rhinotracheitis virus multi-epitope recombinant chimeric protein and application thereof
ActiveCN110003343AStatistical analysis Significant differenceImprove immunityBacteriaViral antigen ingredientsAntigen epitopeInfectious bovine rhinotracheitis virus
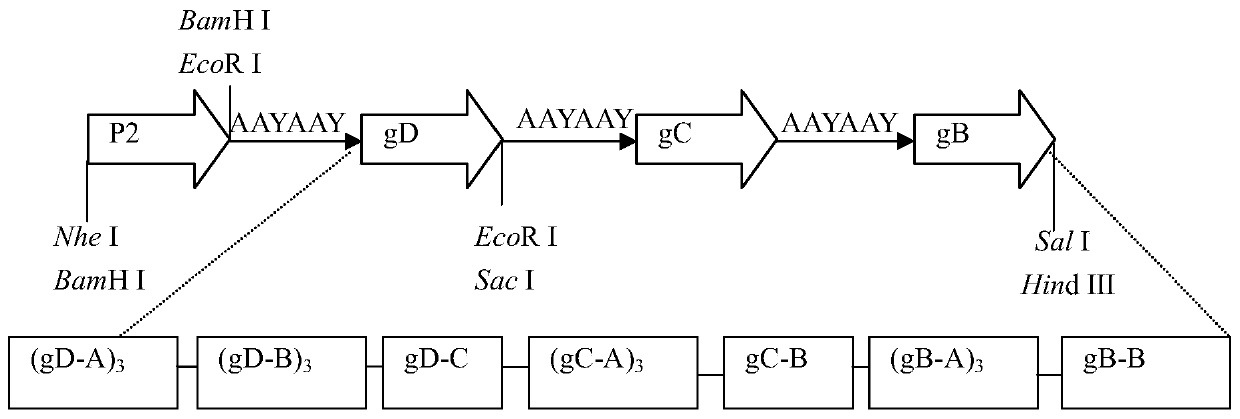
The invention provides a bovine infectious rhinotracheitis virus multi-epitope recombinant chimeric protein and an application thereof. The recombinant chimeric protein is formed by rigid linker series connection of a tetanus toxin general T cell epitope polypeptide P2, a bovine infectious rhinotracheitis virus gB antigen epitopes gB-A and gB-B, bovine infectious rhinotracheitis virus gC antigen epitopes gC-A and gC-B, bovine infectious rhinotracheitis virus gD antigen epitopes gD-A, gD-B and gD-C and bovine IL-6. The anti-bovine infectious rhinotracheitis virus antibody level induced by the bovine infectious rhinotracheitis virus multi-epitope recombinant chimeric protein provided by the invention is significantly higher than that of other control groups, and the recombinant chimeric protein is indicated to have good immunogenicity.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
ROR1 (NTRKR1) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS10752684B2Good effectUseful for immunotherapyGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a ROR1 monoclonal antibody, conferring specific immunity against ROR1 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia, and for solid tumors such as breast, colon, lung, and kidney tumors.
Owner:CELLECTIS SA
Recombinant DNA, plasmid, transformed microorganism and vaccine protein for prevention and therapy of urinary tract infection
Disclosed is a novel vaccine against Escherichia coli (E. coli) responsible for urinary tract infections. The vaccine is a recombinant chimeric protein which is prepared by linking by genetic recombination a gene encoding an antigenic determinant of uropathogenic E. coli to a CTXA2B gene encoding nontoxic A2 and B subunits of Vibrio cholerae cholera toxin (CTX) or a LTXA2B gene encoding nontoxic A2 and B subunits of E. coli heat-labile enterotoxin, wherein a translation product of the CTXA2B or LTXA2B gene serves as an immunogenic adjuvant stimulating mucosal immune responses, expressing the resulting recombinant gene in E. coli, and isolating and purifying an expressed recombinant fusion protein. The recombinant chimeric protein is useful as an oral vaccine with mild side effects and excellent vaccination efficiency against uropathogenic E. coli. Thus, the chimeric vaccine protein can remarkably reduce recurrence of urinary tract infections, prevent occurrence of antibiotic-resistant bacteria, and replace the conventional chemotherapy for urinary tract infections. Also, the chimeric vaccine protein has other advantages of being capable of being produced and commercialized in a short period with relatively low costs, and being easily modified by replacing its genetic constituents with other genes to provide various vaccines.
Owner:SUNGKYUNKWAN UNIVERSITY
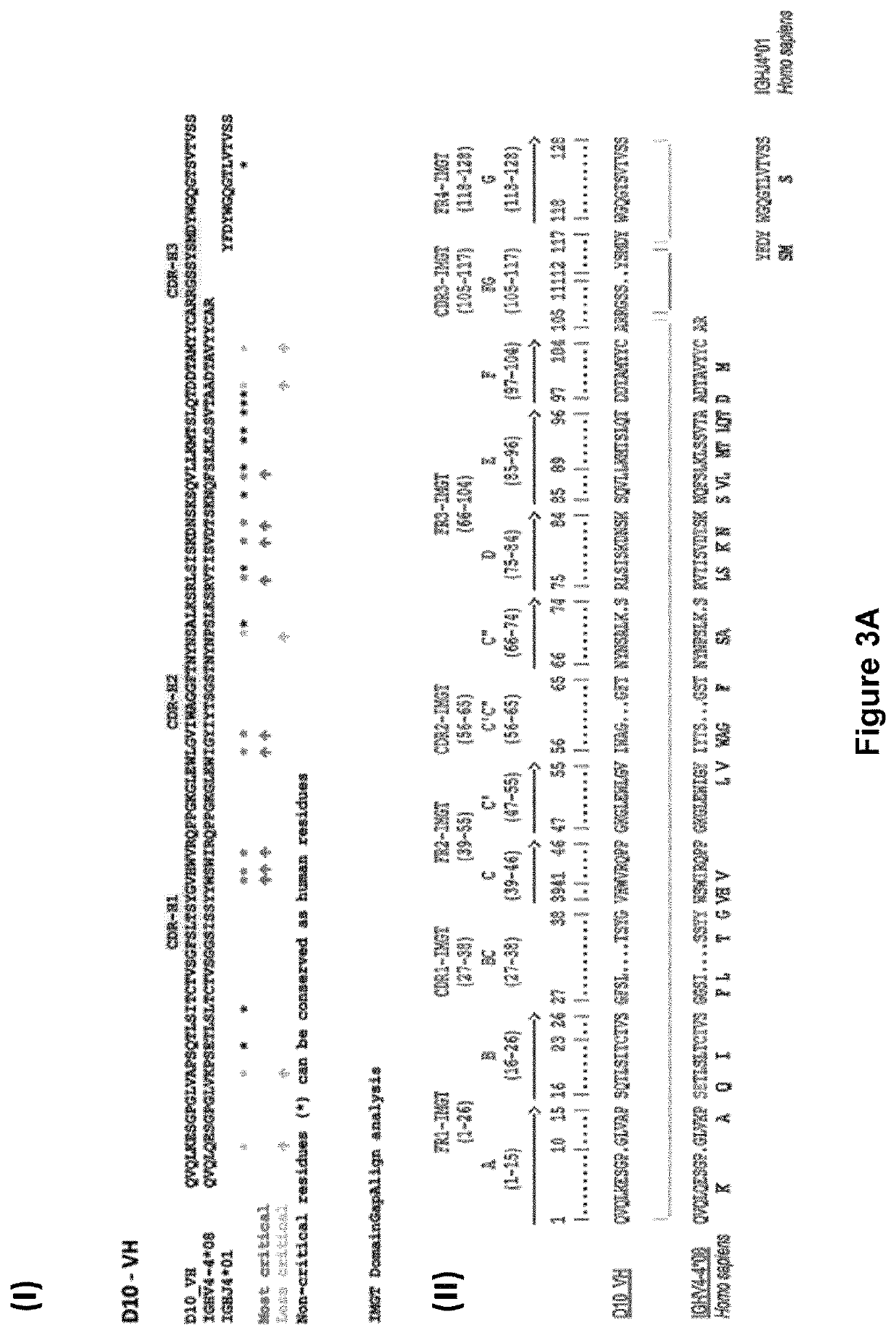
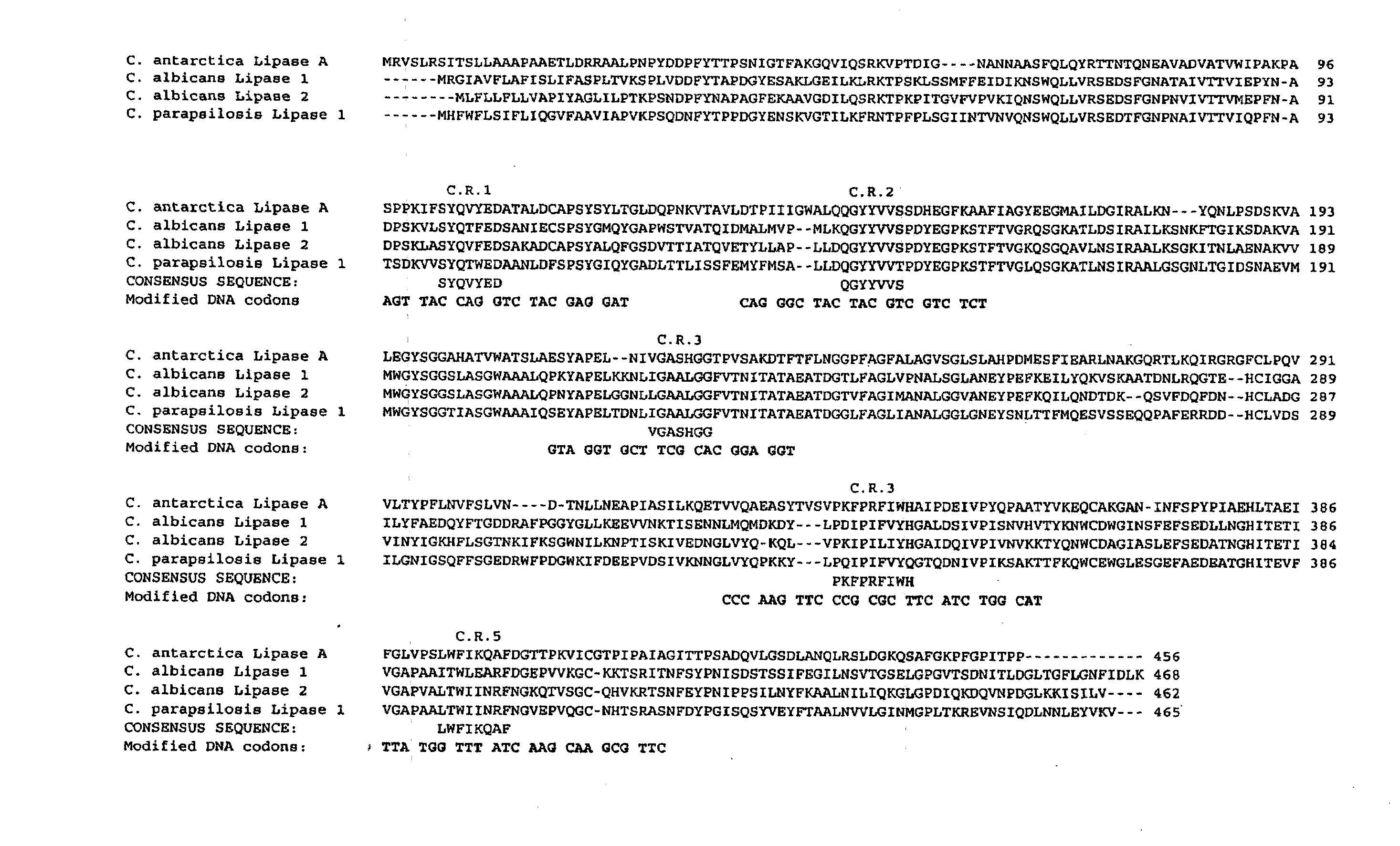
Libraries of recombinant chimeric proteins
InactiveUS20100069264A1Improve featuresLow improvement potentialBiological testingLibrary creationNucleotideActive protein
The provides methods for generating divergent libraries of recombinant chimeric proteins, comprising identifying a plurality of conserved amino acid sequences, selecting a plurality of consensus amino acid sequences as a backbone corresponding to said conserved amino acid sequences to serve as sites of recombination and as a backbone for recombinant chimeric proteins created, generating overlapping polynucleotides, inducing recombination between said polynucleotides to produce divergent libraries of chimeric polynucleotides wherein the recombinations intentionally take place between the sequences that correspond to the full length consensus amino acids. The advantage is that shuffling between variable regions, while maintaining the consensus backbone, increases the production of active proteins with high diversity, and better properties.
Owner:PROTEREC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka.patsnap.com/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100011.PNG)
![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka.patsnap.com/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100012.PNG)
![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka.patsnap.com/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100013.PNG)