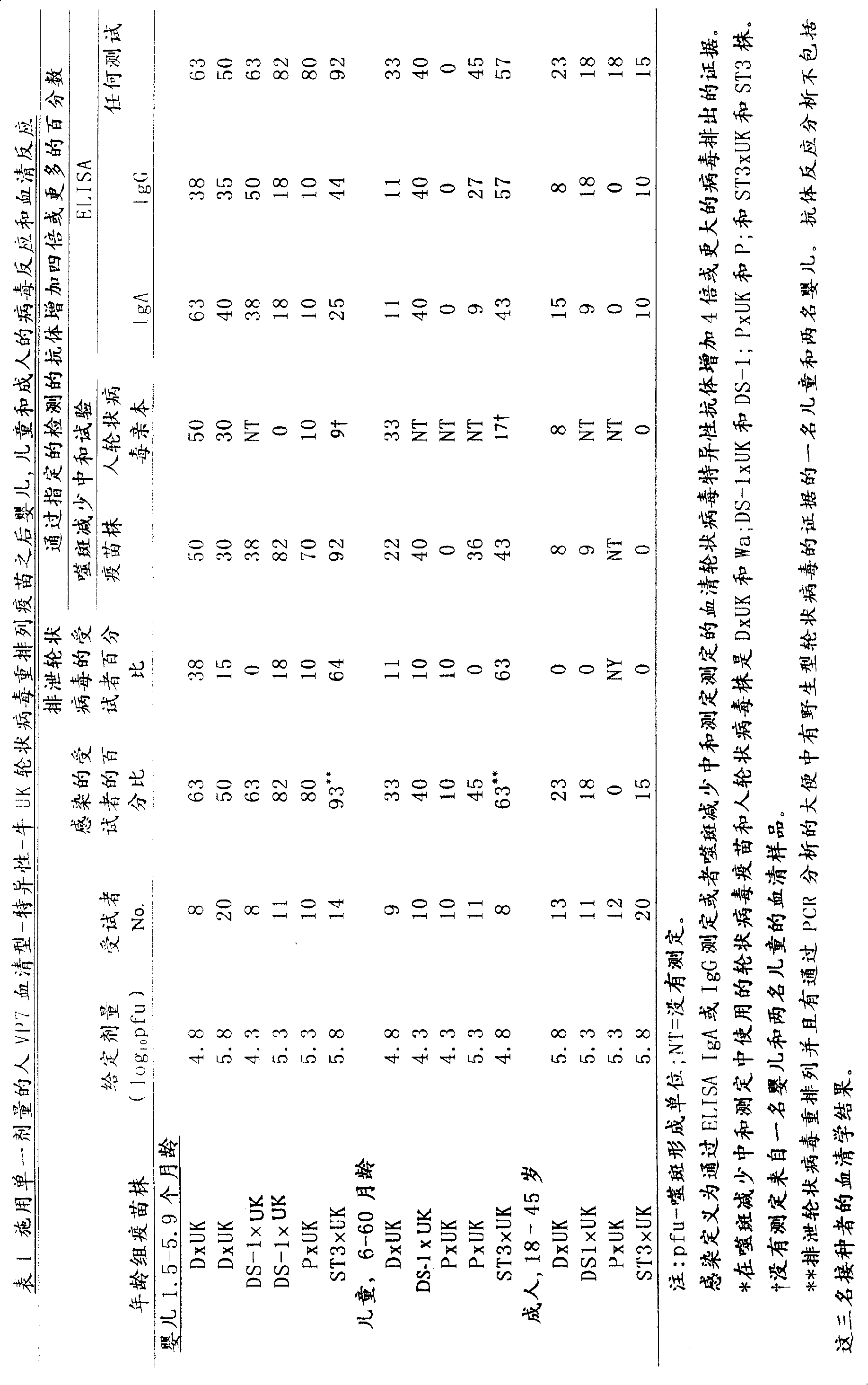
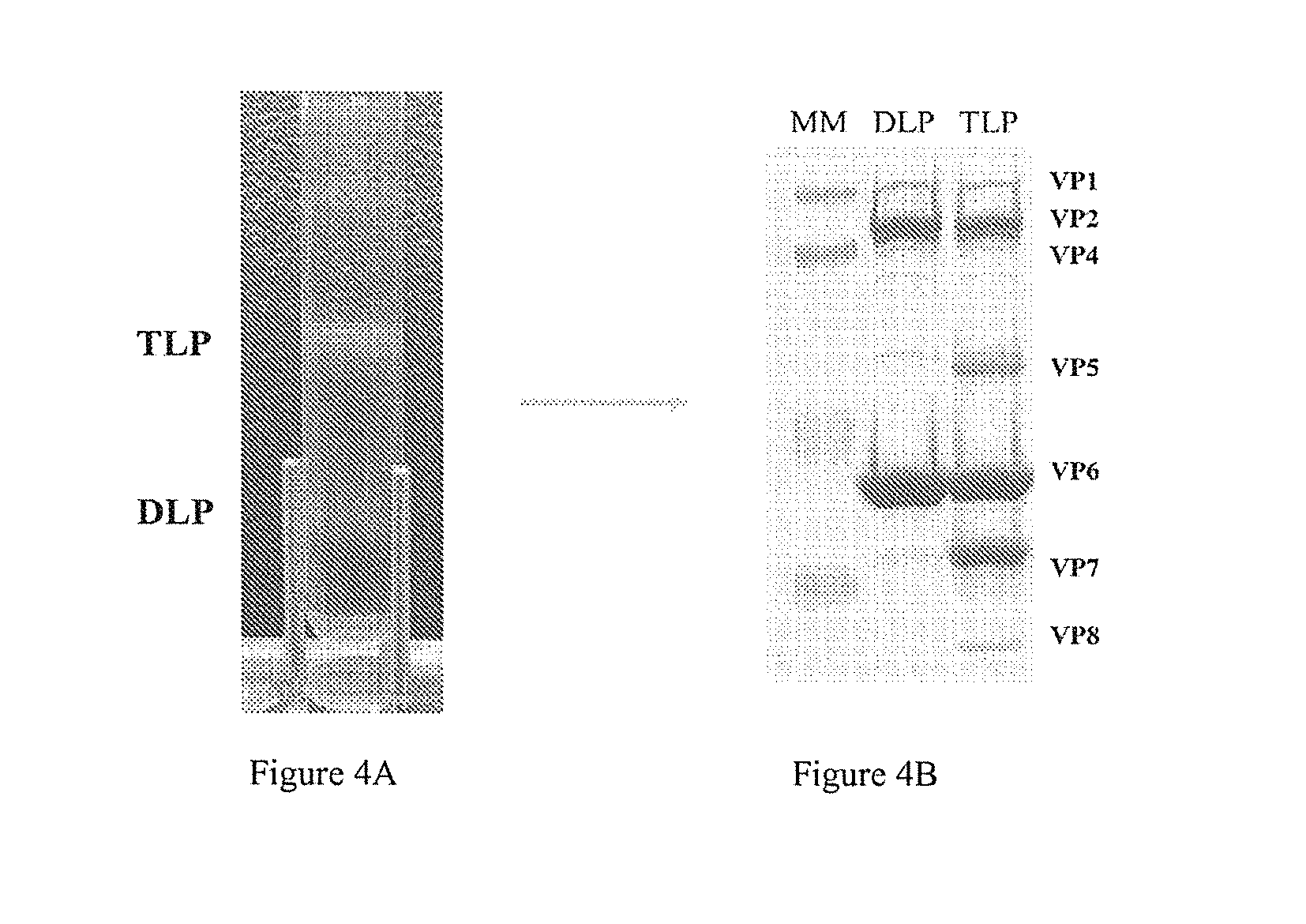
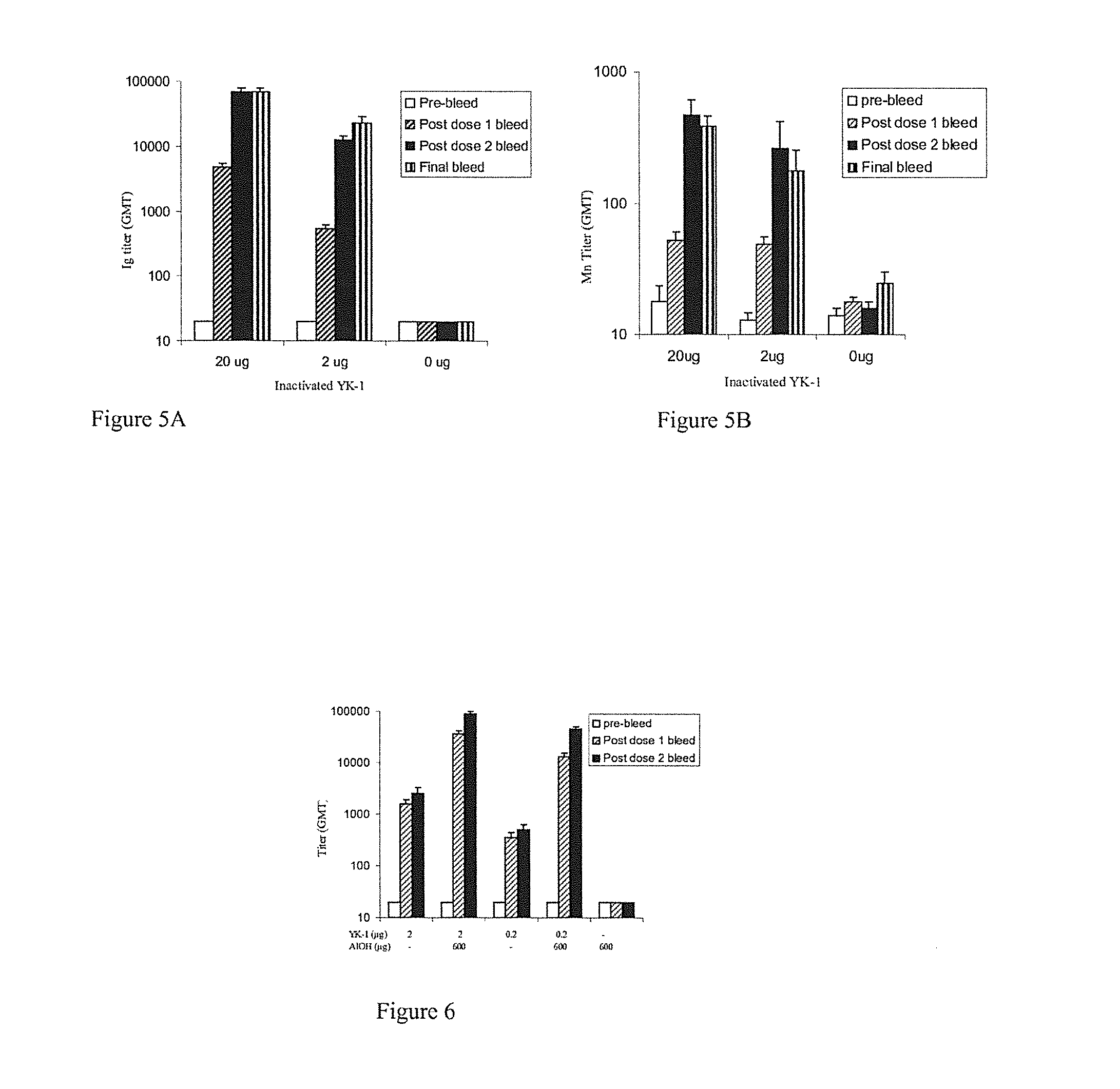
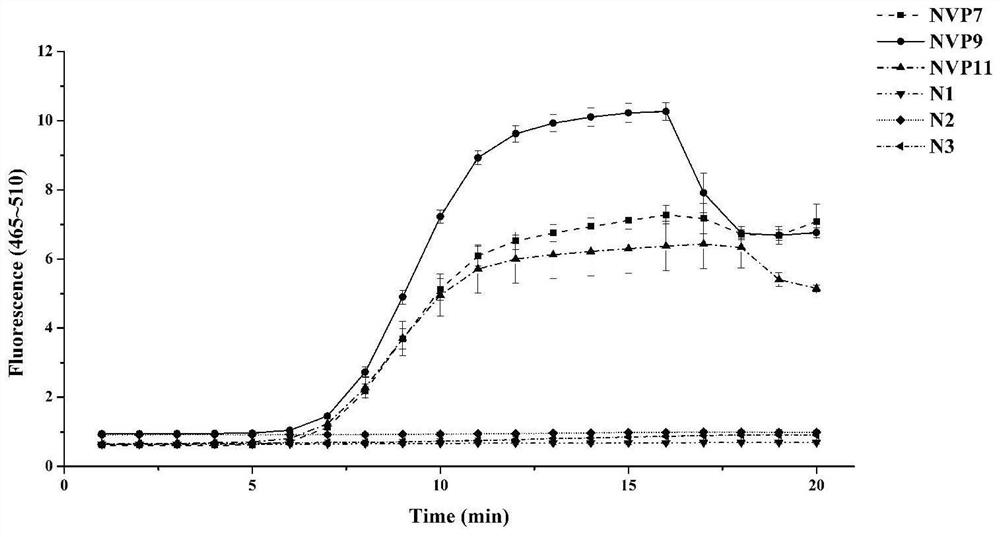
Patents
Literature
56 results about "Human rotavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Human rotavirus vaccine strains and diagnostics
A vaccine composition and method of vaccination are provided useful for immunizing a subject against a rotavirus. The vaccines include rotavirus strains CDC-9 and CDC-66, fragments thereof, homologues thereof, or combinations thereof. Inventive vaccines may include a fragment of CDC-9, CDC-66, homologues thereof, or combinations thereof. Methods of inducing an immunological response are provided by administering an inventive vaccine.
Owner:THE GOVERNMENT OF THE US SEC THE DEPT OF HEALTH & HUMAN SERVICES CENT FOR DISEASE CONTROL & PREVENTION
Human rotavirus strain and separation, culture and identification method thereof
InactiveCN102618506AMicrobiological testing/measurementInactivation/attenuationMicroorganismHuman rotavirus
The invention discloses a human rotavirus strain. The human rotavirus strain is characterized in that the strain is a Rotavirus A ZTR-5 strain, and is collected to CGMCC (China General Microbiological Culture Collection Center of Microbiological Culture Management Committee) on 27th, June 2011, and the number is CGMCCNo.4977. The strain is a wild type single purified strain of human rotaviruses, and is strong in virus replication capability and good in genetic stability; and the virus seed can be applied as a production virus seed for developing human rotavirus vaccines, and is applicable to attenuated type rotavirus oral vaccines and inactivated human rotavirus inject vaccines. The invention provides a separation, culture and identification method of the human rotavirus strain.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Attenuated human rotavirus vaccine
InactiveUS7150984B2Enhance immune responseMaximum efficacyViral antigen ingredientsInactivation/attenuationRotavirus RNACold adapted
The present invention provides vaccine compositions of attenuated human rotavirus. More particularly, the attenuated human rotavirus is produced by cold passage and thus contains attenuating mutations which produce virus having a cold-adapted (ca) and temperature sensitive (ts) phenotype. The attenuated strains are used in methods for stimulating the immune system of an individual to induce protection against human rotavirus by administration of the ca attenuated rotavirus.
Owner:GOVERNMENT UNITED STATES OF AMERICA THE
Purification preparation method of human rotavirus inactivated vaccines by utilizing ion exchange chromatography
ActiveCN102552898AImprove securityImproving immunogenicityViral antigen ingredientsMicroorganism based processesVirus inactivationIon exchange
A purification preparation method of human rotavirus inactivated vaccines by utilizing ion exchange chromatography relates to a preparation method of virus inactivated vaccines. The purification preparation method comprises concentrating virus harvest liquid, performing chromatographic purification on the concentrated virus harvest liquid through a Qsepharose Fast Flow ion exchange column, performing dialysis desalting on a purified product, and filtering, degerming, inactivating and the like to obtain the human rotavirus inactivated vaccines; and further detecting the purification, the totalprotein removal rate and the infectious titer, and then detecting the antigenicity, the immunogenicity and the genome banding pattern stability. The total protein removal rate of the purified virus harvest liquid is 99.69%, and the infectious titer of the virus before purification and the infectious titer of the virus after purification are respectively 4.25 lg CCID 50 / ml and 7.0 lg CCID 50 / ml, the genome banding pattern of purified virus after inactivation does not mutate, and antigenicity and immunogenicity are kept to be good.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
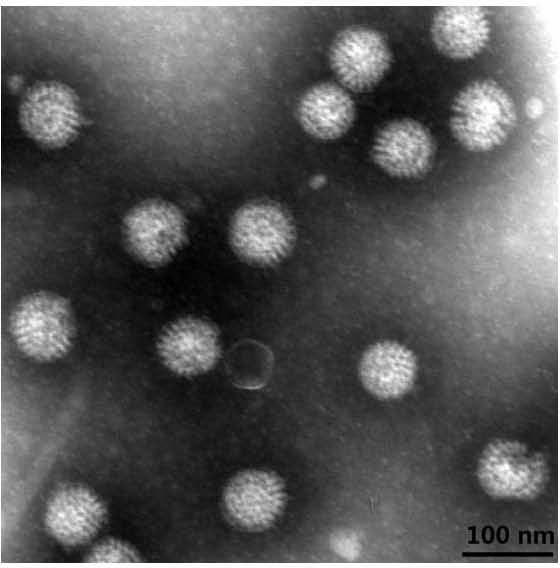
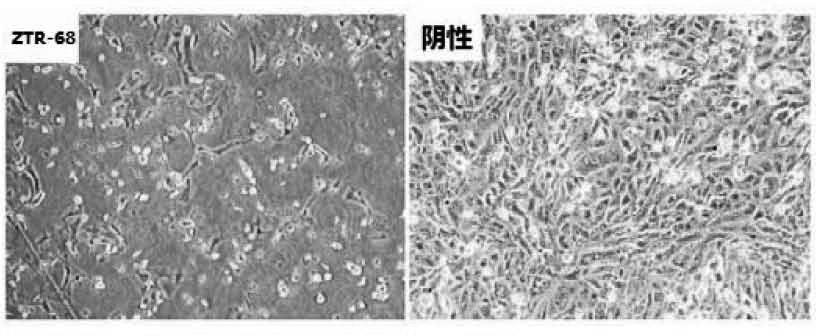
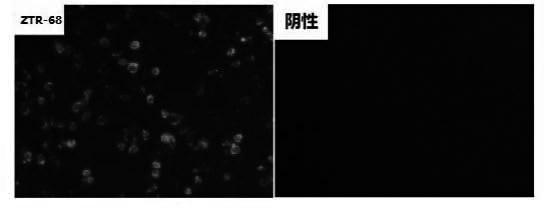
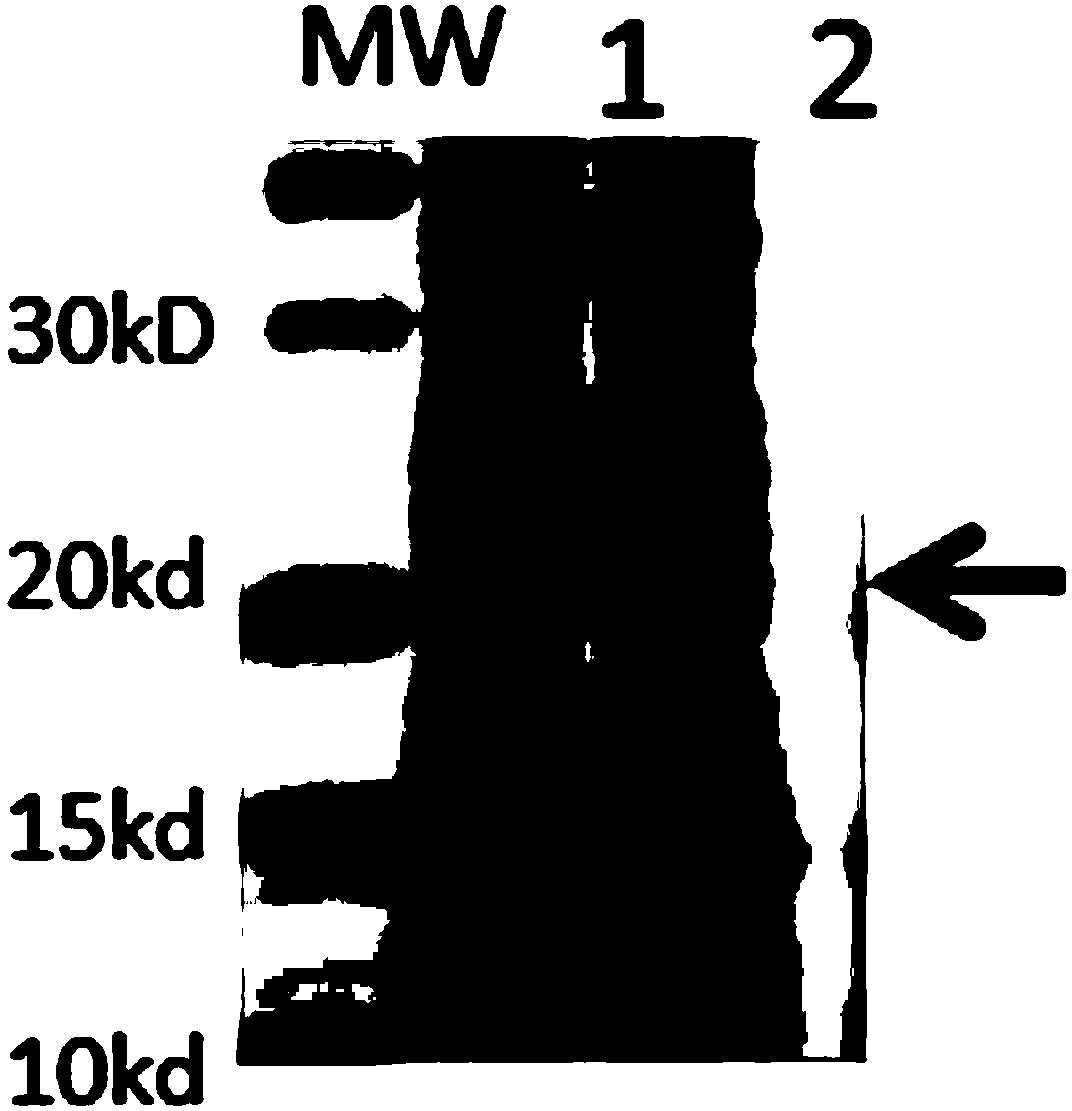
Human rotavirus-A seed strain ZTR-68, and isolation, culture and identification thereof
ActiveCN102618505AMicrobiological testing/measurementInactivation/attenuationBiotechnologyMicroorganism
A human rotavirus-A seed strain ZTR-68 is submitted to CGMCC (china general microbiological culture collection center) for preservation in September 22th, 2011, and encoded as CGMCC No.5265. The strain is a human rotavirus wide-type single purified strain, which is high in virus replication and fine in genetic stability. The human rotavirus-A seed strain ZTR-68 is applicable to developing and producing virus seeds for human rotavirus vaccines, and applicable to attenuated rotavirus oral vaccines and inactivated human rotavirus injection vaccines. The invention further provides a method for isolation, culture and identification of the human rotavirus-A seed strain ZTR-68.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Human rotavirus VP8 recombinant protein and human rotavirus vaccine using same
The invention provides a human rotavirus VP8 recombinant protein. The amino acid sequences of the VP8 recombinant protein include an amino acid sequence of a human rotavirus VP8 protein (hereinafter referred to as VP8 protein) and an amino acid sequence of exogenous polypeptide. The isoelectric point of the exogenous polypeptide is less than or equal to 5. The invention also provides a human rotavirus vaccine prepared based on the VP8 recombinant protein. Compared with the prior art, the VP8 recombinant protein provided by the invention can be expressed by fusing the VP8 protein with negativecharge-containing amino acid polypeptide fragments in tetanus toxin and / or diphtheria toxin proteins, and the water solubility and yield of the rotavirus VP8 protein expressed by escherichia coli areimproved; and moreover, the obtained fused protein has good immune ability, and the prepared vaccine has good effect.
Owner:CHENGDU MAXVAX BIOTECHNOLOGY LLC
Pichia pastoris for expressing rotavirus expression particles as well as preparation method and application of pichia pastoris
The invention relates to pichia pastoris for expressing rotavirus expression particles as well as a preparation method and application of the pichia pastoris, and discloses a human rotavirus structural protein expression cassette, comprising the following elements: (a) an initial signal element AOX; (b) a rotavirus structural protein gene; and (c) a termination signal element TT. The invention further discloses a yeast cell transfected by using the expression cassette, a method for preparing the rotavirus viroid particles by using the yeast cell, viroid particles prepared by using the method, and application of the viroid particles for preparing vaccine compositions for preventing or treating rotavirus infection. The invention also discloses a preparation method of the yeast cell.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604BImprove immune efficiencyFast titerBacteriaViral antigen ingredientsAntiendomysial antibodiesTetanus
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
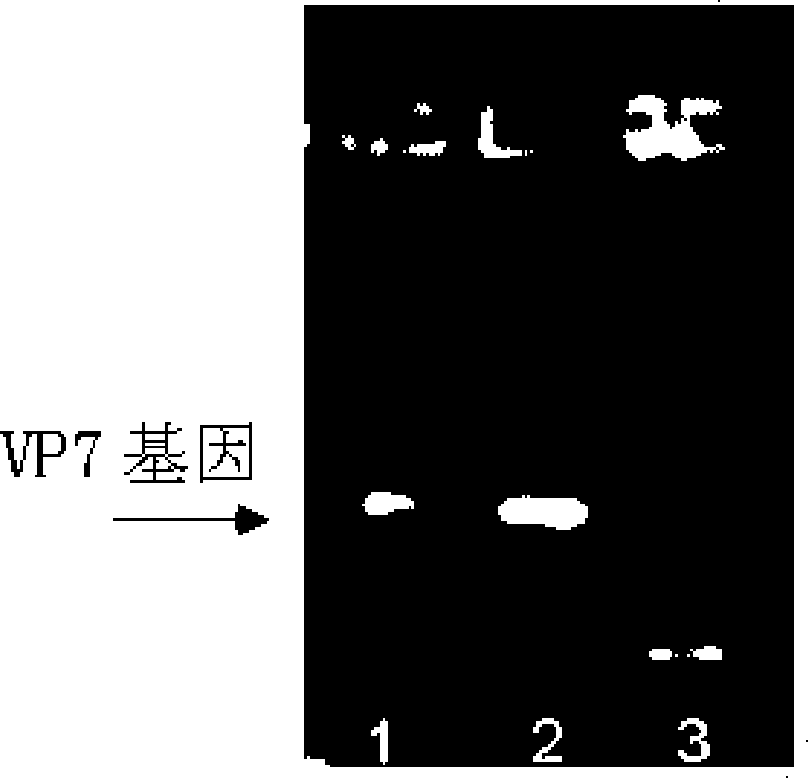
VP7 gene of human rotavirus and composition for diagnosis of human rotavirus infection comprising primer or probe specific to thereof
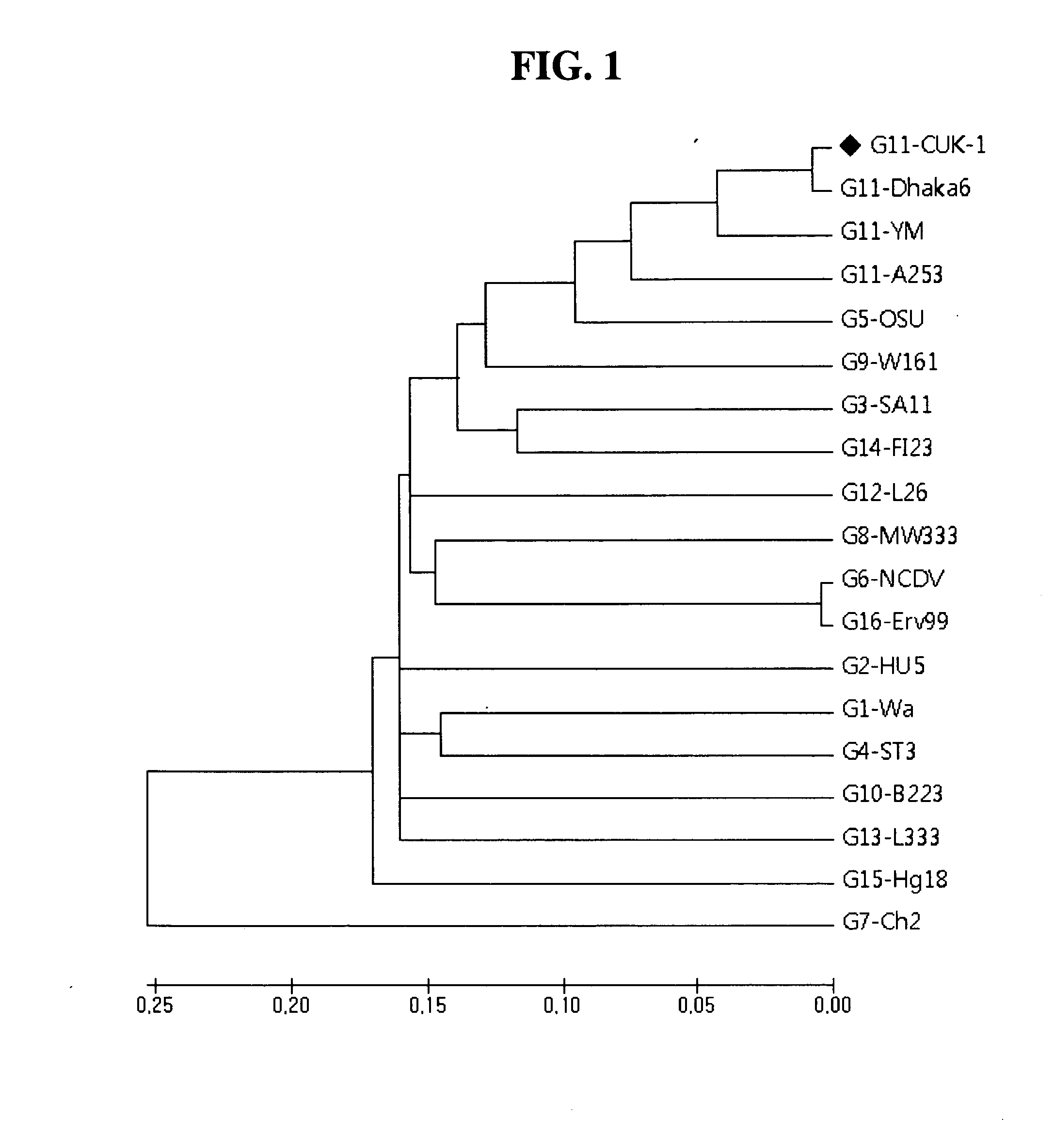
The present invention relates to a VP7 gene of human rotavirus and a composition for diagnosis of human rotavirus infection comprising primer or probe specific to thereof, and more particularly to a VP7 gene encoding the amino acid sequence set forth in SEQ ID NO:2 and a composition for diagnosis of human rotavirus infection comprising primer or probe specific to thereof. The human rotavirus VP7 gene according to the present invention will be useful for diagnosis of novel G11 type human rotavirus infection, and will be used for development of rotavirus vaccine.
Owner:MSD KOREA
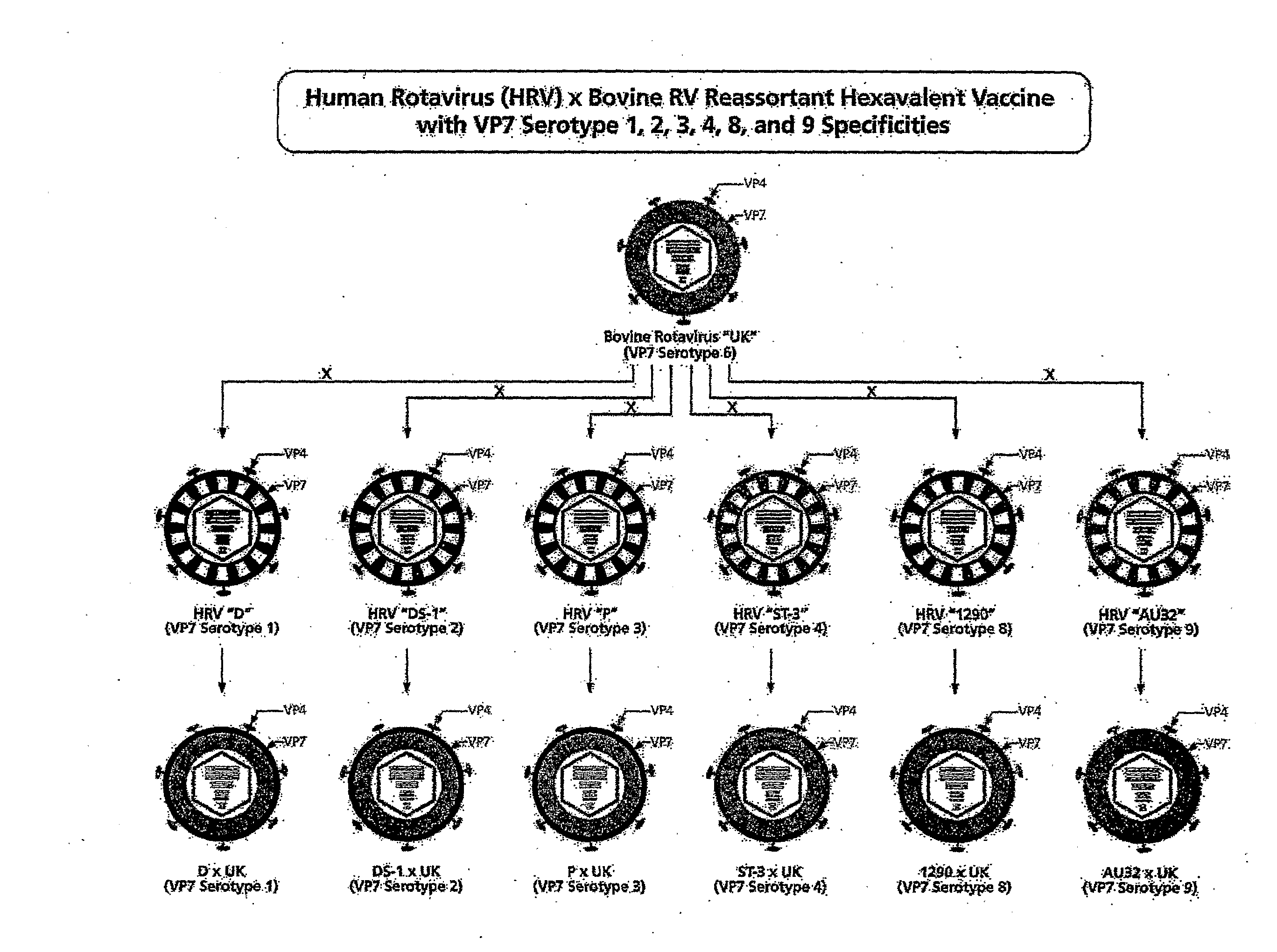
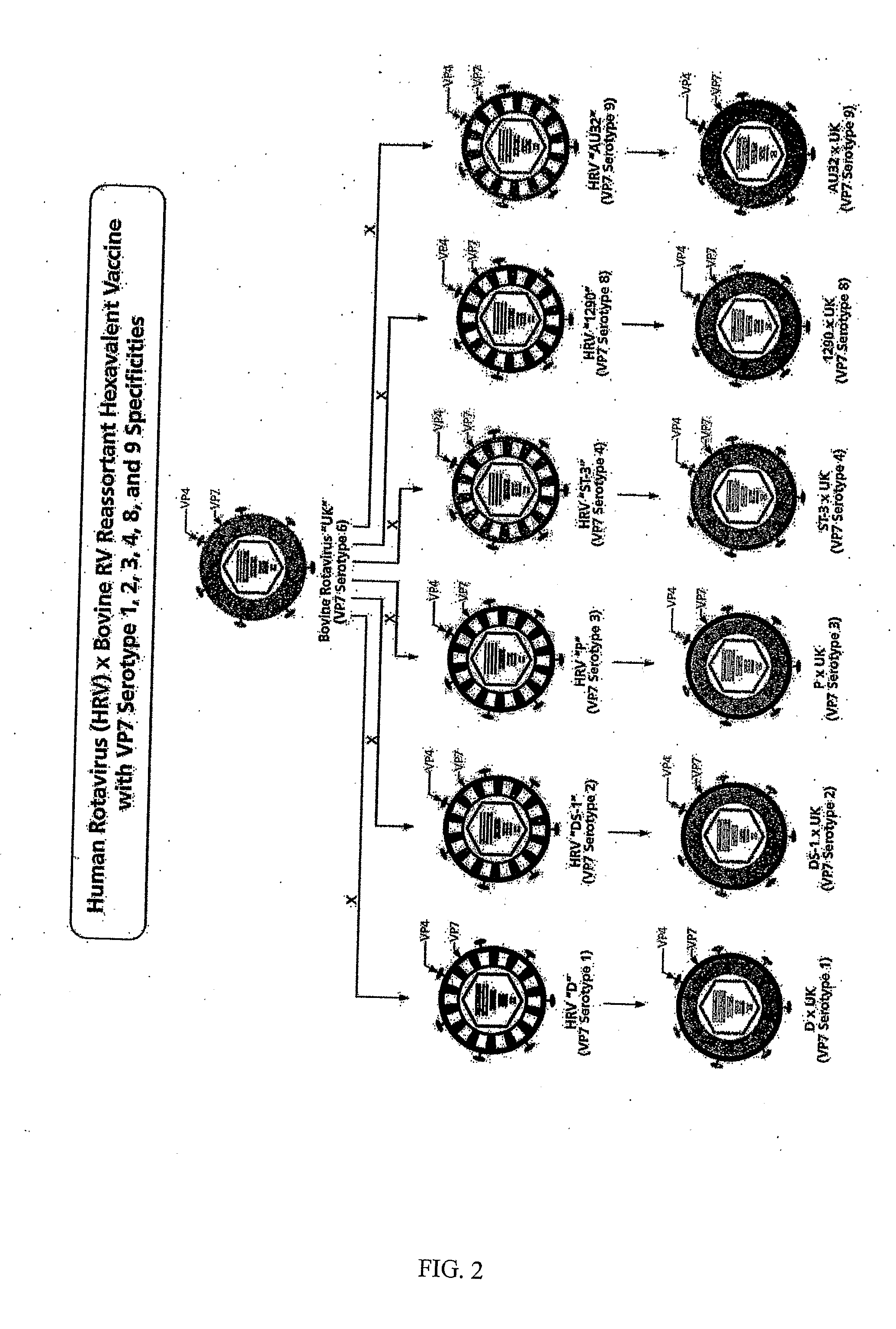
Hexavalent Bovine Rotavirus Reassortant Composition Designed for Use in Developing Countries
ActiveUS20080292660A1Improving immunogenicityHigh degree of infectivityViral antigen ingredientsSnake antigen ingredientsDiseaseBovine rotavirus
The present invention provides vaccine compositions for protection against human rotaviral disease designed for use in particular areas of the world. Human×bovine reassortant rotavirus comprising each of the four clinically most important VP7 serotypes of human rotavirus are combined with other VP7 serotypes typically found in the area of interest into a multivalent formulation which provides a high degree of infectivity and immunogenicity. Methods and an administration protocol for producing an immunogenic response without producing an increased risk of intussusception are also provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Multivalent huma-bovine retavirus vaccine
The present invention provides vaccine compositions for protection against human rotaviral disease without significant reactogenicity. Human×bovine reassortant rotavirus comprising each of the four clinically most important VP7 serotypes of human rotavirus are combined in a multivalent formulation which provides a high degree of infectivity and immunogenicity without producing a transient febrile condition. Methods for producing an immunogenic response without producing a transient febrile condition are also provided.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF HEALTH & HUMAN SERVICES THE
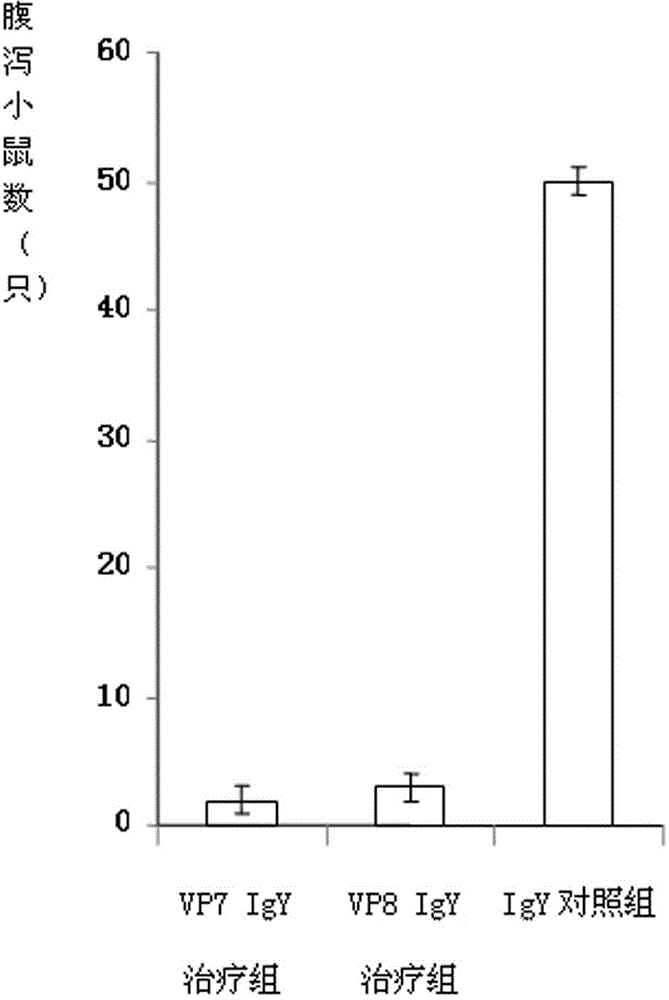
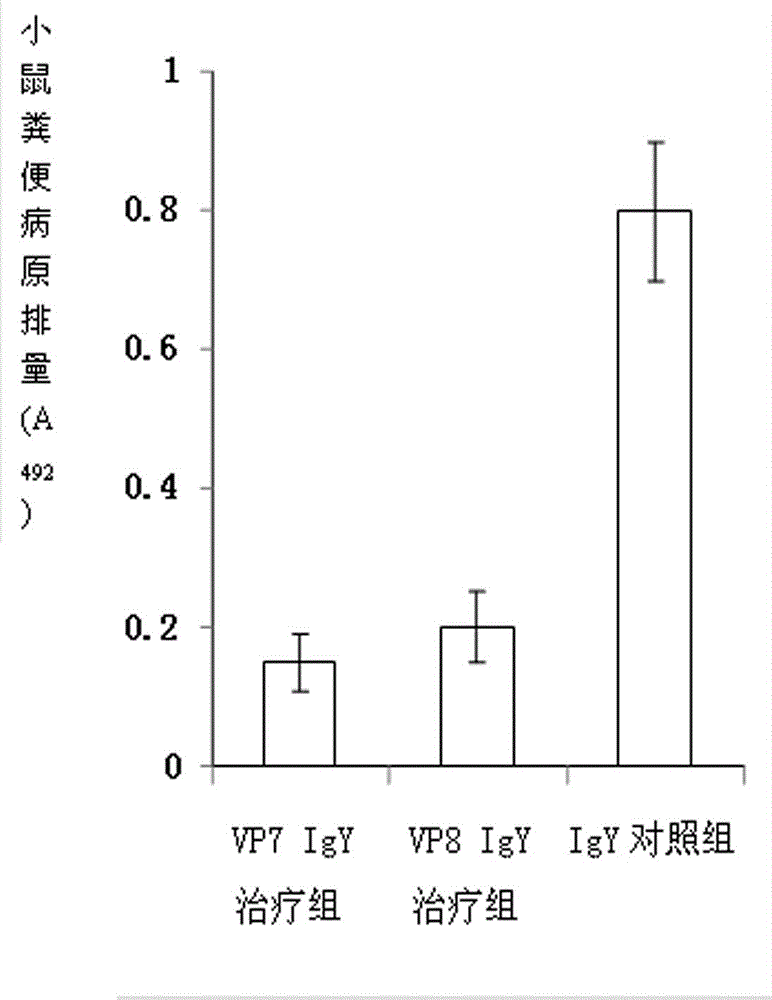
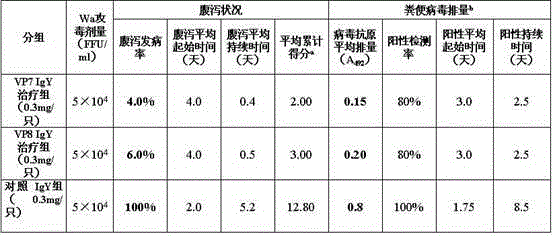
Chicken egg yolk antibody resisting human A rotavirus as well as preparation method and application thereof
InactiveCN103554254AClear genetic backgroundMature and perfect structureEgg immunoglobulinsImmunoglobulins against virusesAntigenRotavirus RNA
The invention discloses a chicken egg yolk antibody resisting human A rotavirus as well as a preparation method and application thereof. The method comprises steps of expressing and purifying structural protein VP7 or VP8 of human rotavirus in vitro, immunizing laying hens, collecting eggs, and separating and purifying egg yolk antibody of VP7 or VP8. The prepared chicken egg yolk antibody can be freeze dried and prepared into antibody dry powder for preventing and treating rotavirus. The preparation method has the beneficial effects that a large quantity of VP7 and VP8 antigens are expressed and recombined in vitro by a genetic engineering technology, the benefits are high, the cost is low, small field is required, and the method is environment-friendly; the potential risk of scattering virus caused by inactivation by rotavirus or attenuated vaccine in the traditional preparation process of egg yolk antibody is avoided, and the method meets the requirement of animal welfare. The egg yolk antibody prepared by the method can be prepared into oral antibody dry powder biological agent after sterility test, has obvious effect in treating rotavirus infectious diarrhea, takes effect rapidly and has low cost, and the prevention rate reaches over 95%.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Membrane gene chip for simultaneous detection of three groups, A, B and C, of human rotaviruses and its prepn and application
InactiveCN1810991ARapid diagnosisSuitable for useMicrobiological testing/measurementDiseaseHuman rotavirus
The membrane chip for simultaneous detection of three groups, A, B and C, of human rotaviruses includes positively charged nylon membrane, and dotted coating with oligonucleotide probes, contrasts and blanks distributed in array on glass substrate. The dotted coating has 6 specific detecting probes of different groups of human rotaviruses, 1 positive contrast probe with digoxin marker in its 5í» end, and 4 negative contrast probes and blank sample applying liquid contrast. The present invention also discloses the preparation process and application as clinical diagnosis reagent of the chip. The chip of the present invention operates in multiple PCR amplification mode to make the detected nucleic acid carry digoxin marker and obtain hybridized result through immunological coloration, can complete gene detection and group identification of several groups of human rotaviruses, and has wide application foreground in food detection, disease prevention and clinical application.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Human Rotavirus and Vaccine Composition Comprising Same
ActiveUS20100047278A1Prevent and treat diseaseViral antigen ingredientsMicrobiological testing/measurementDiseaseVp4 gene
Owner:CHUNG ANG UNIV IND ACADEMIC COOP FOUND
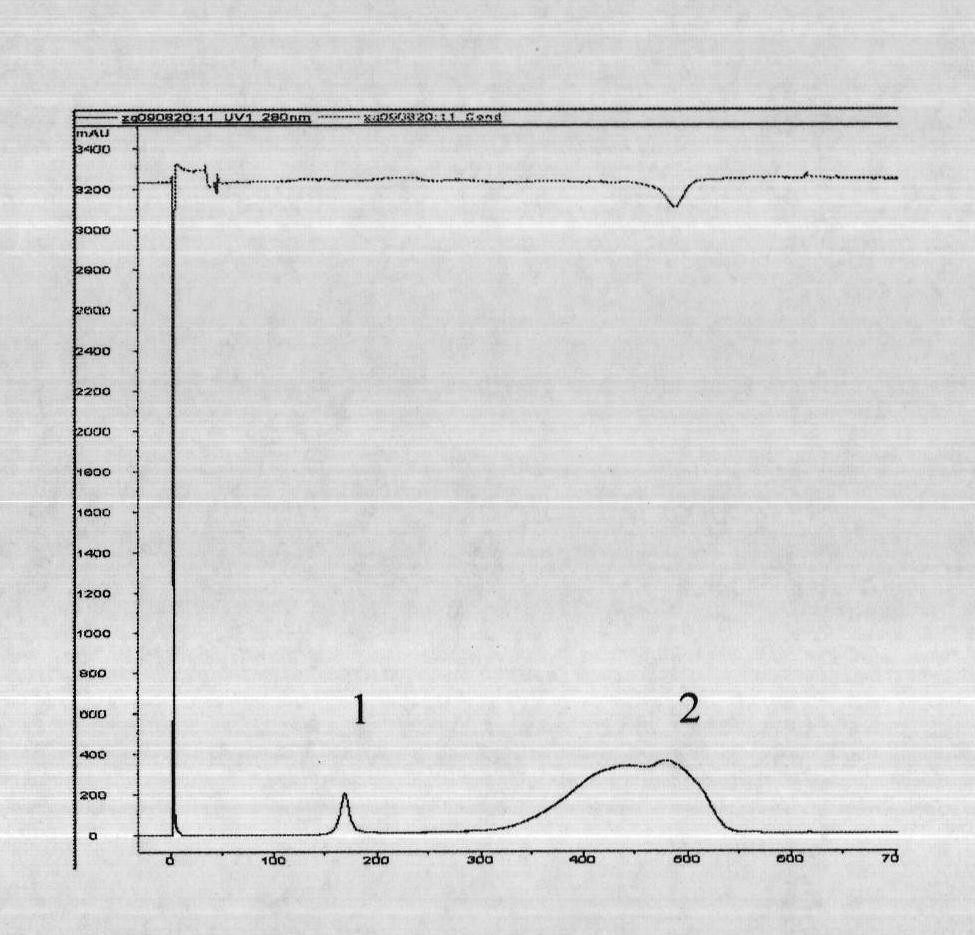
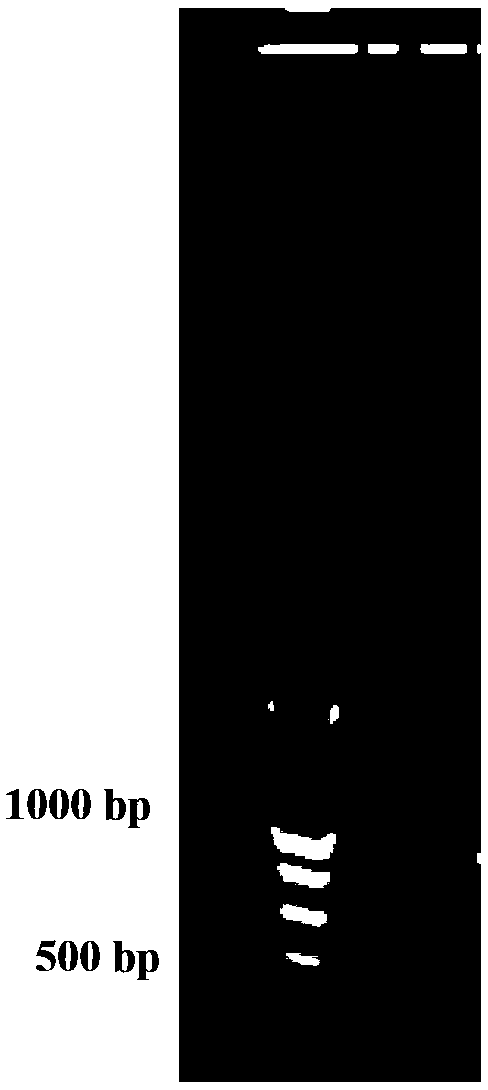
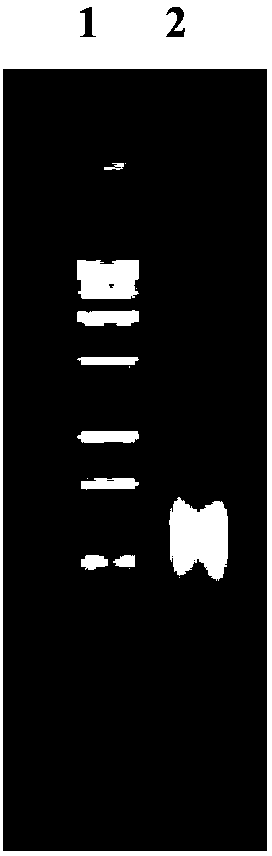
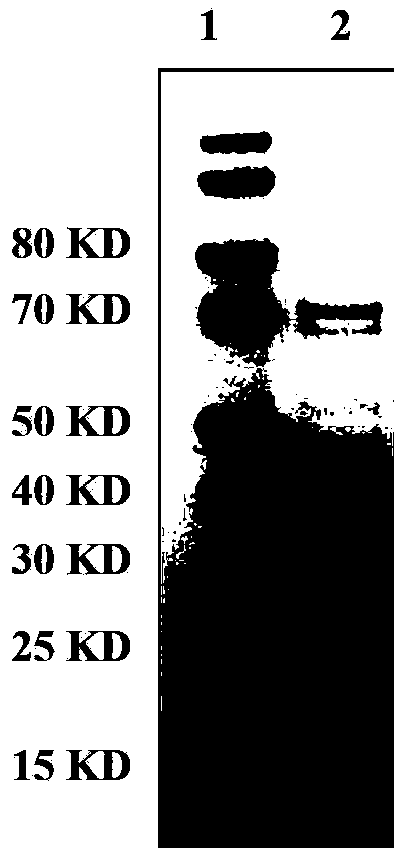
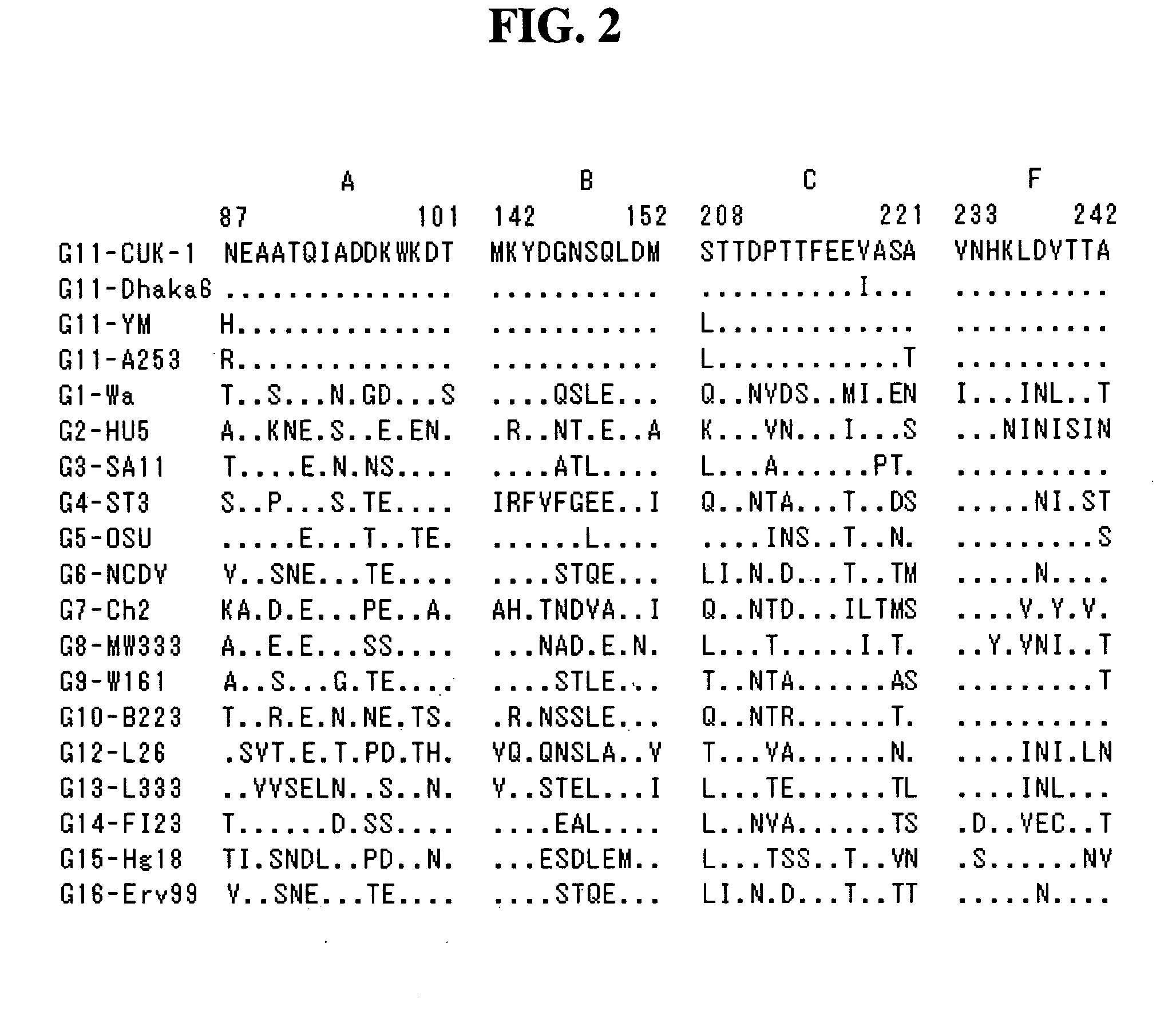
Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof
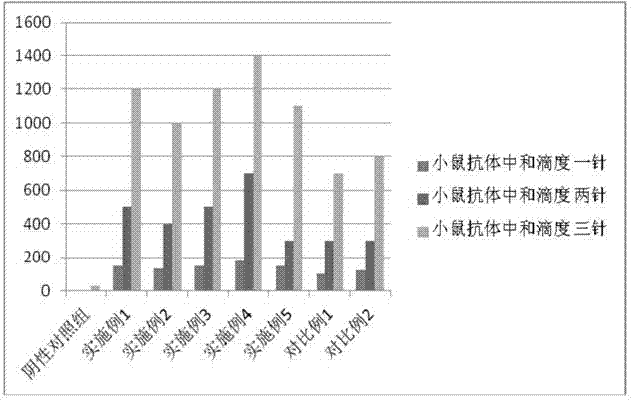
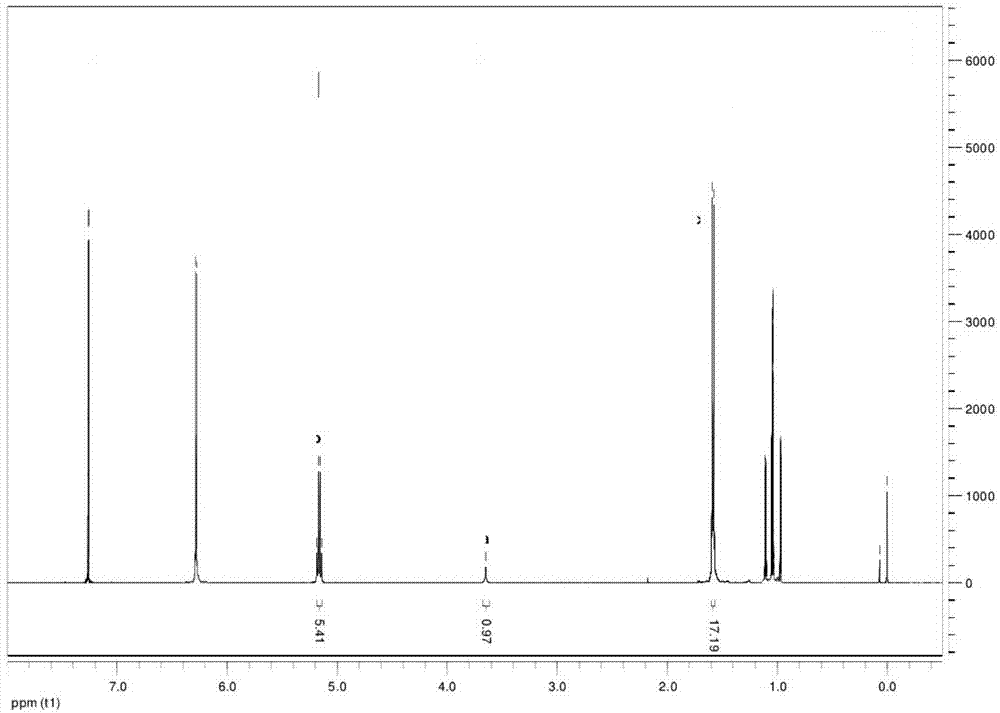
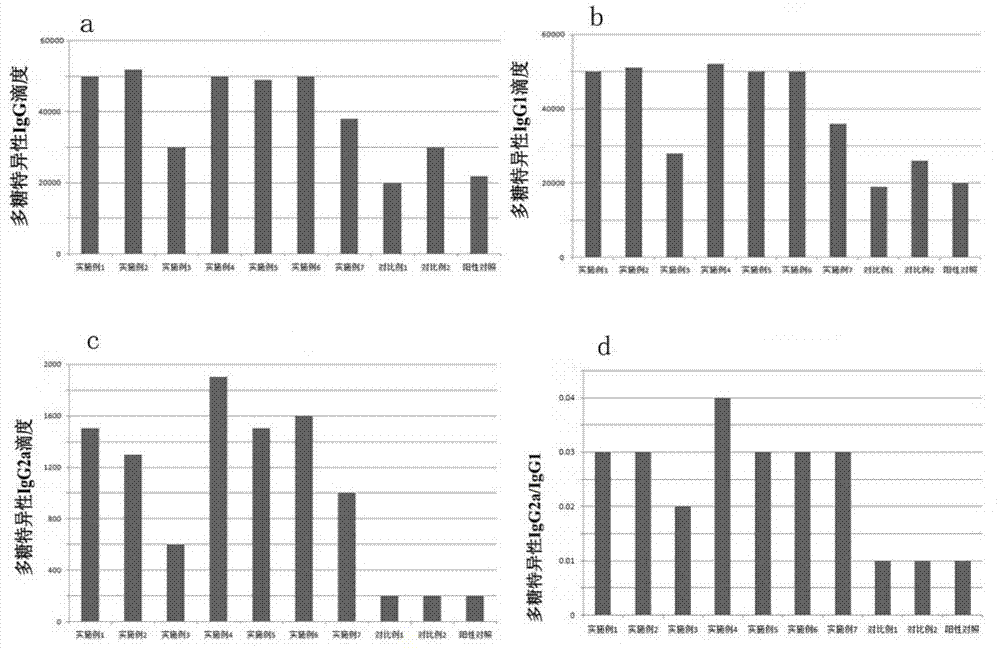
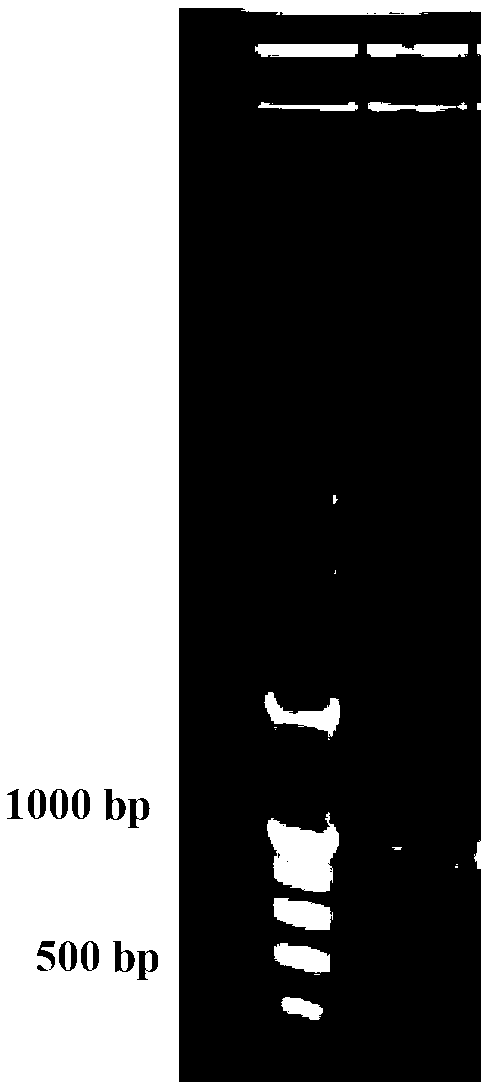
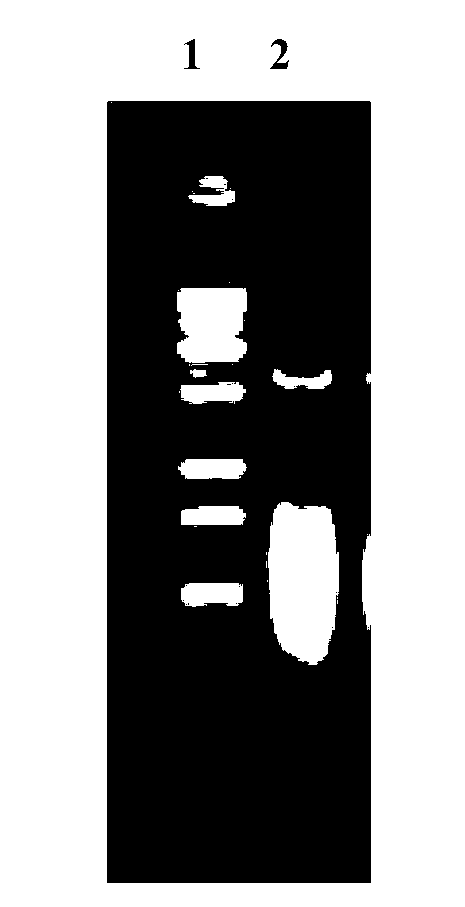
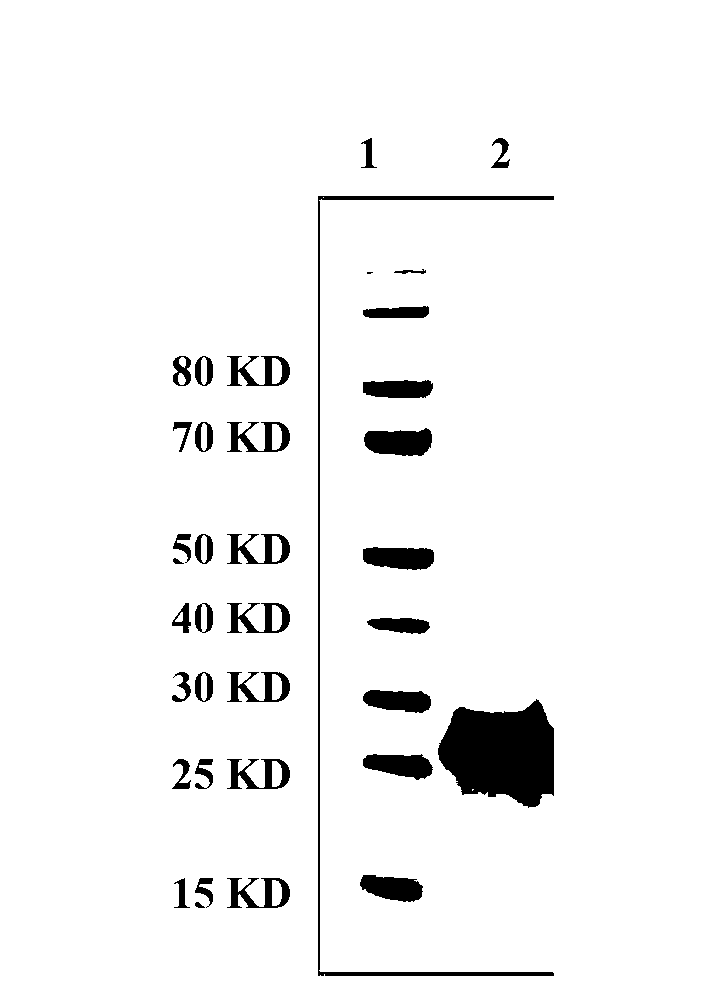
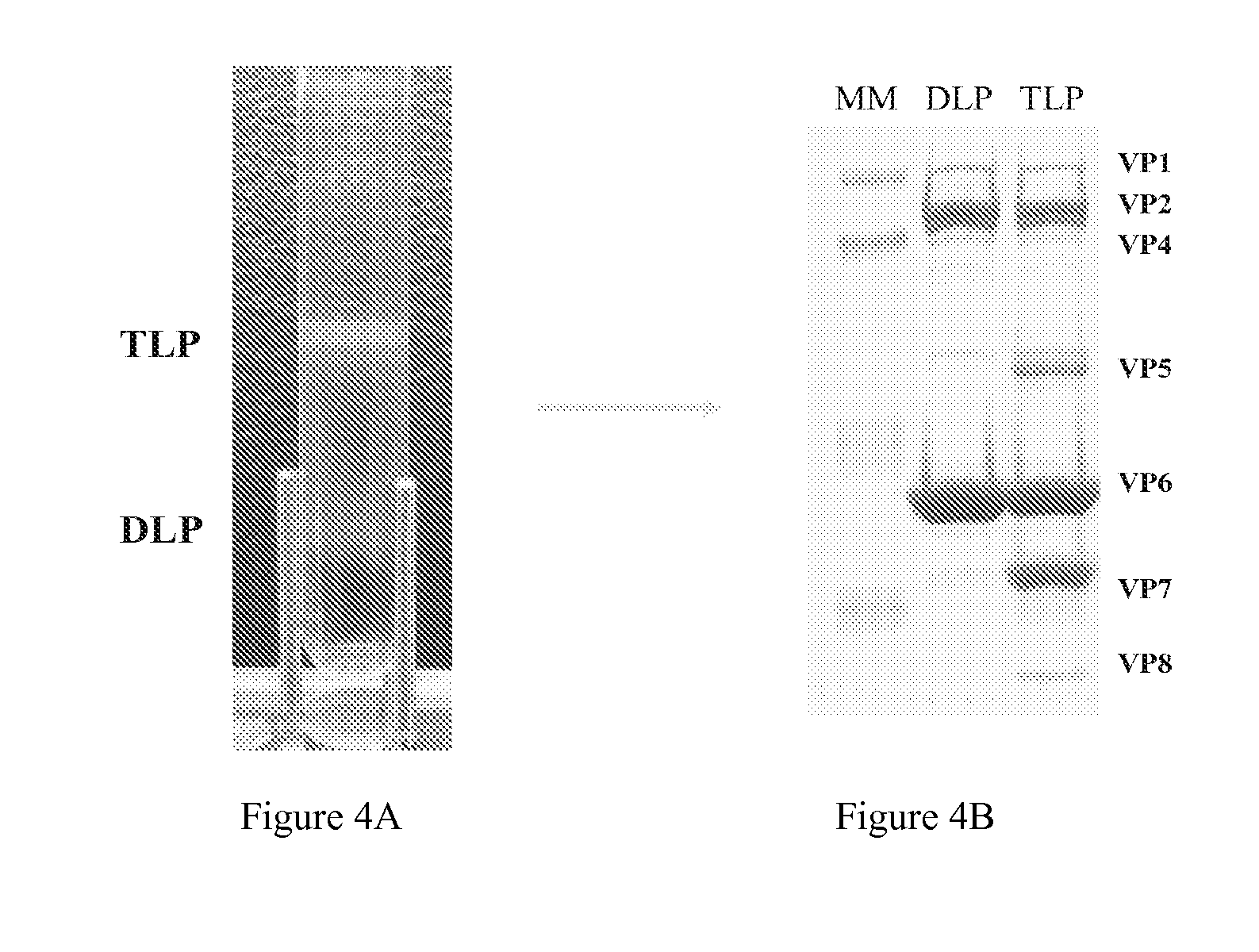
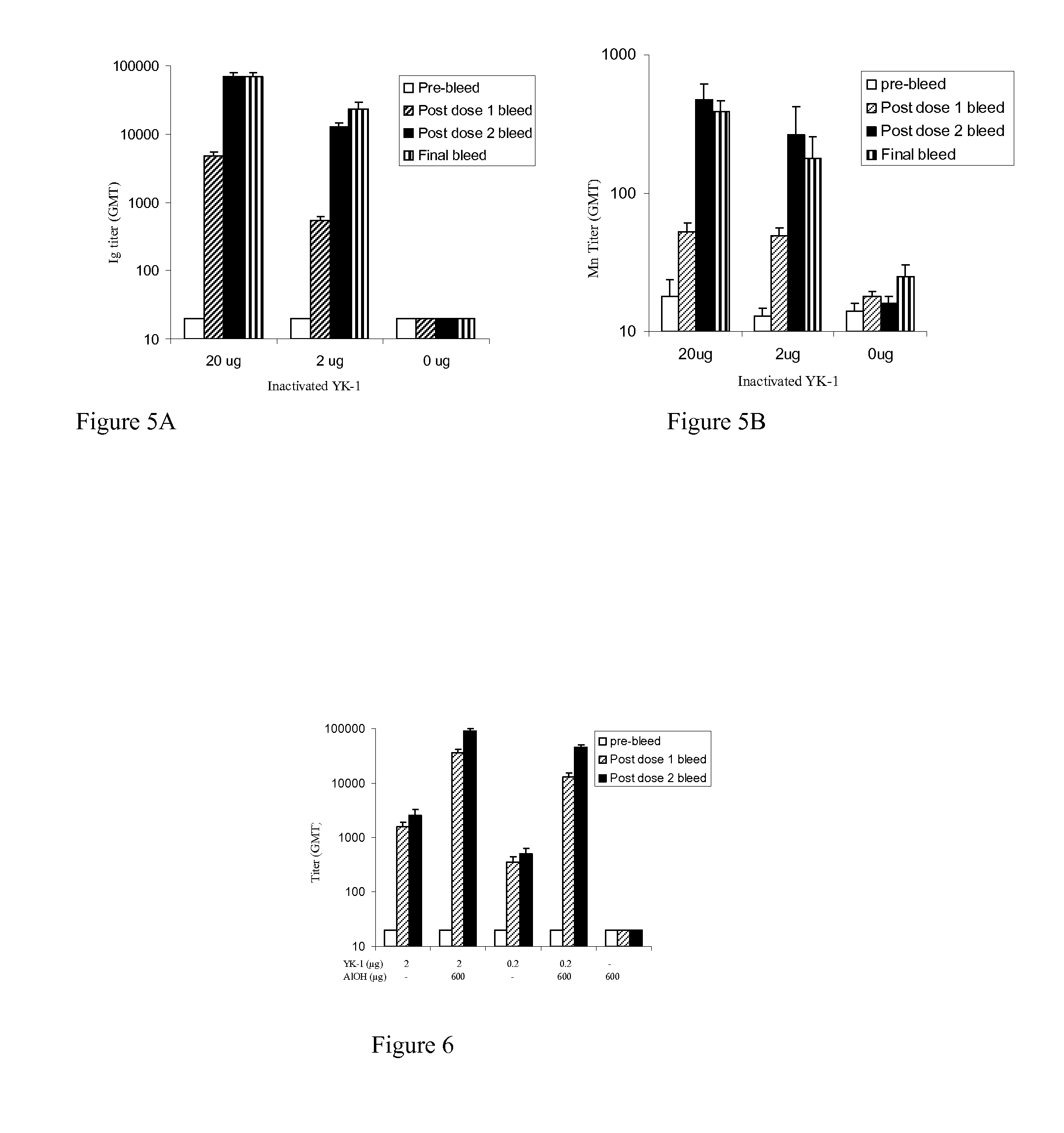
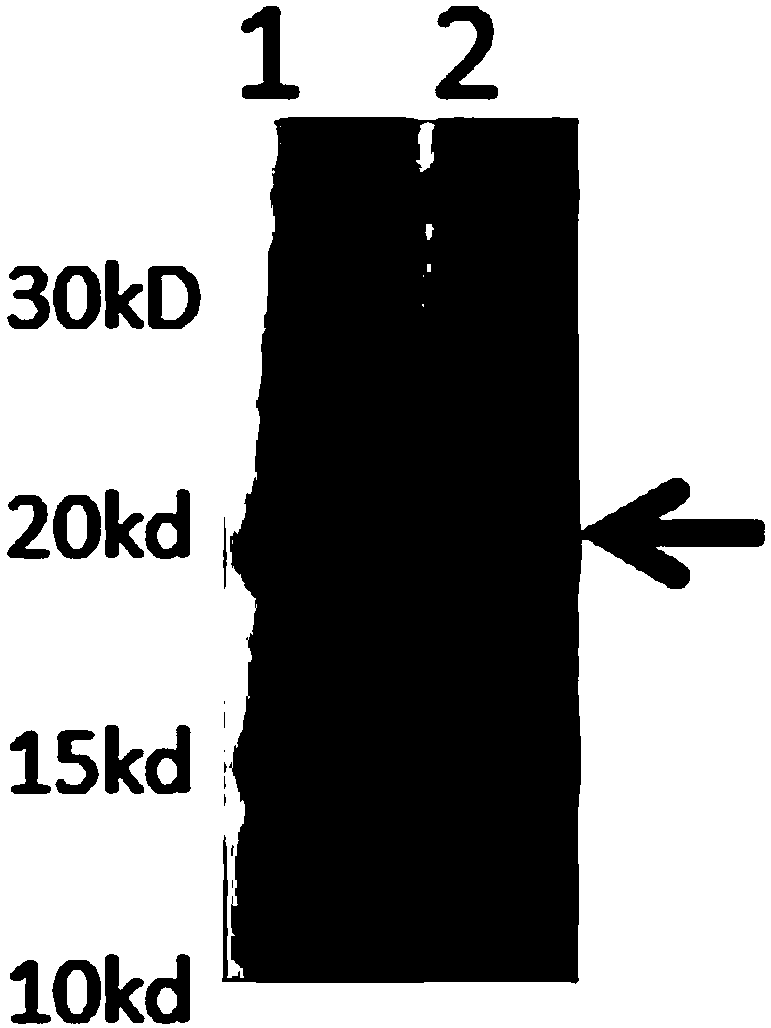
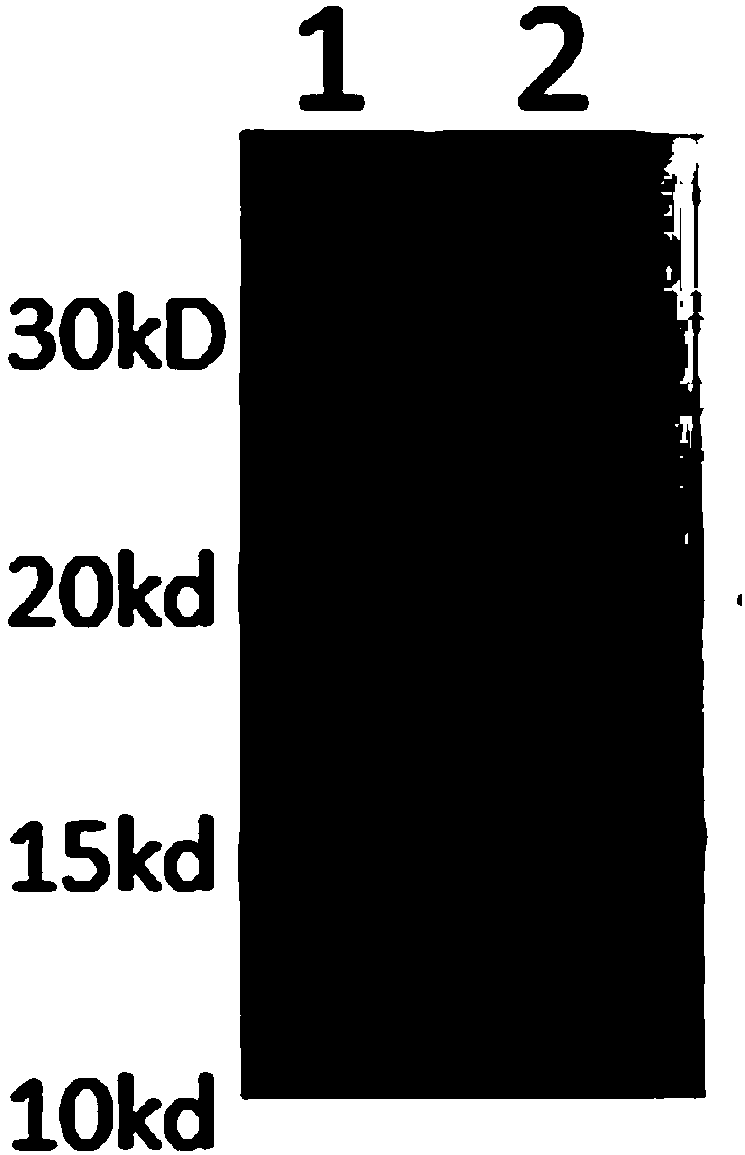
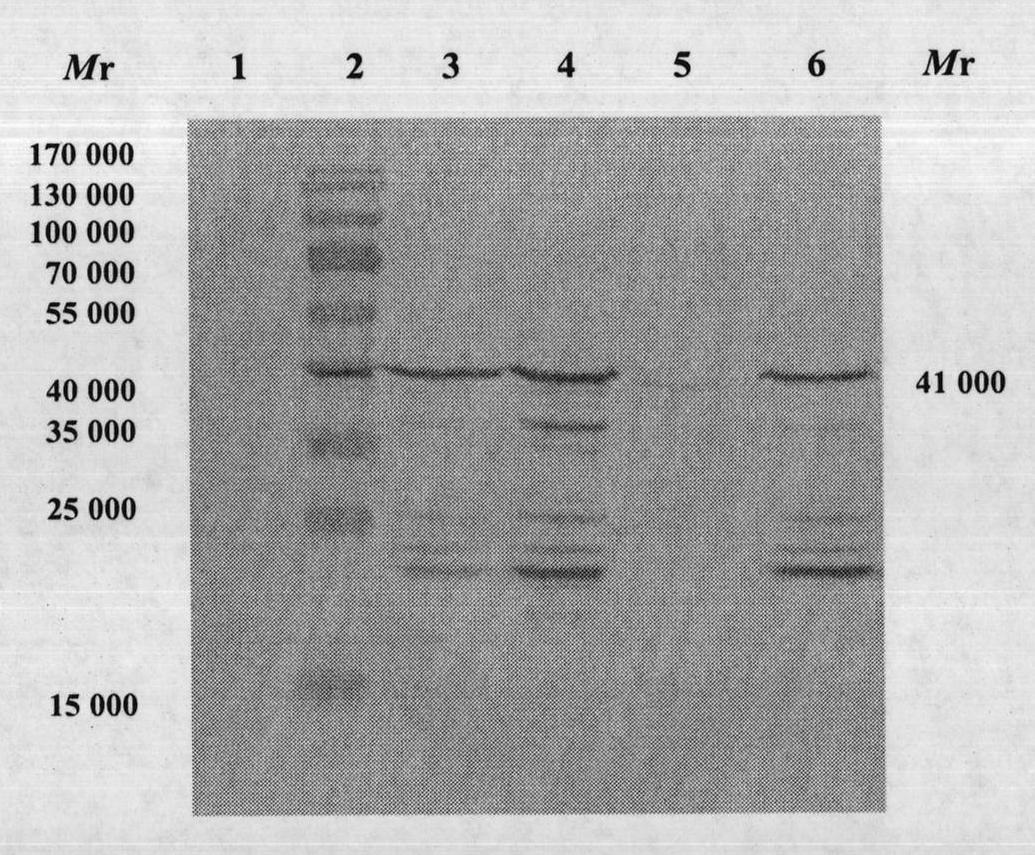
InactiveCN103304671AOvercoming the potential risk of intussusceptionImprove immune efficiencyViral antigen ingredientsMicroorganism based processesViral VaccineGenotype
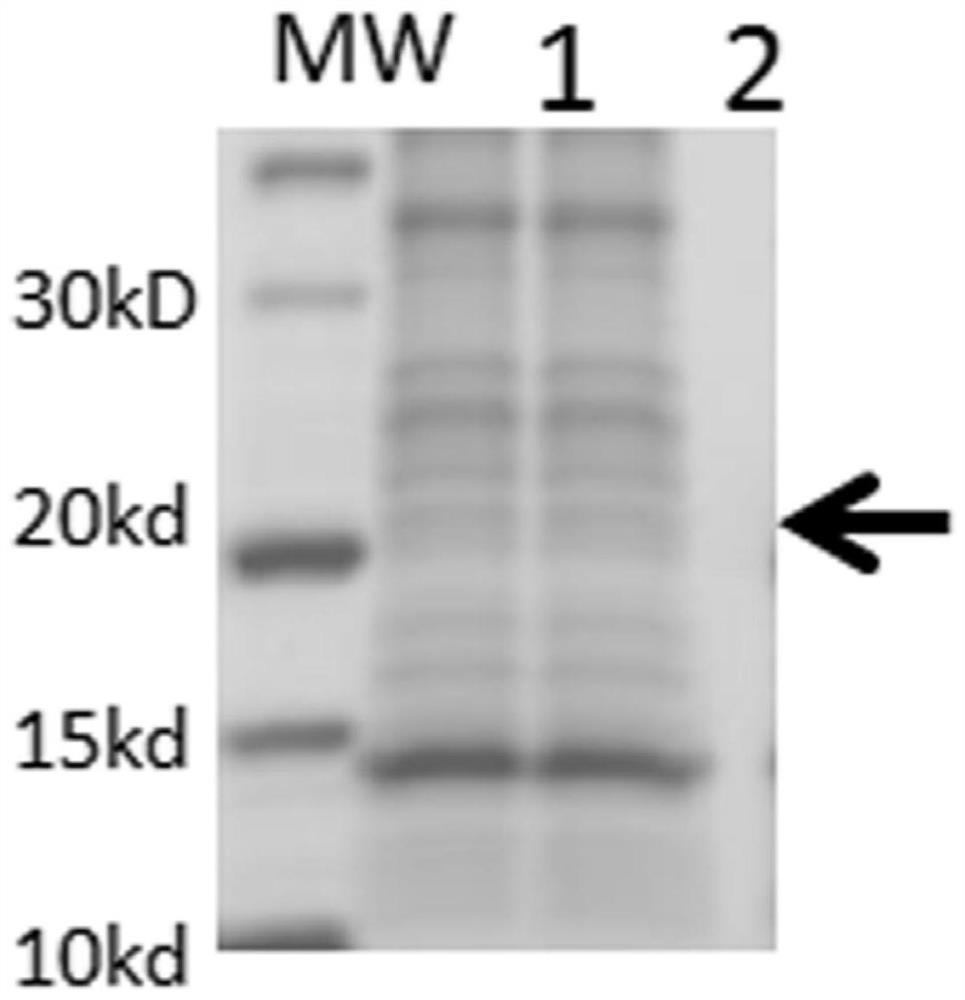
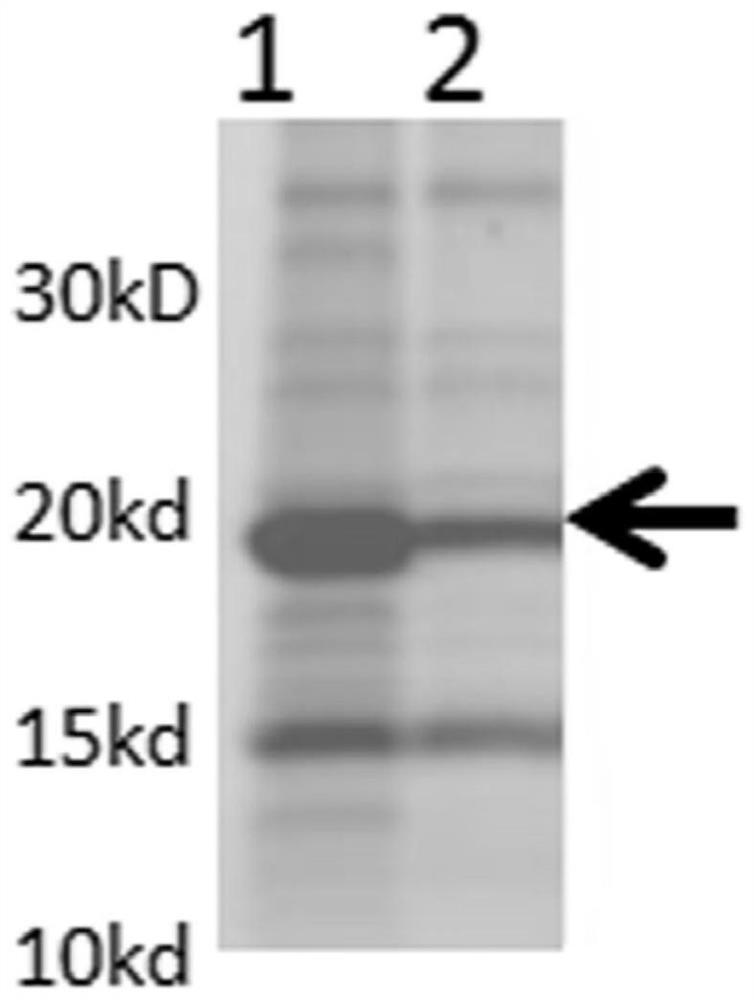
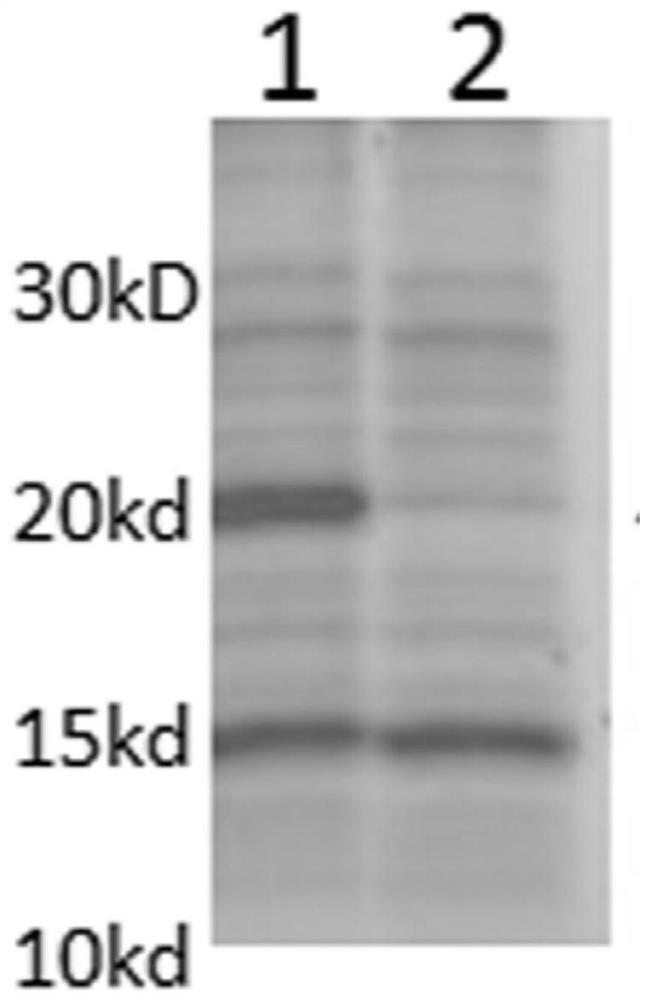
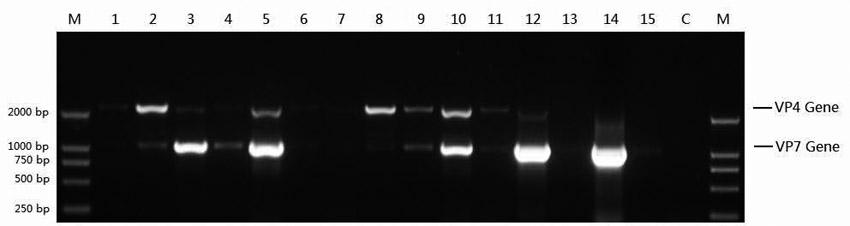
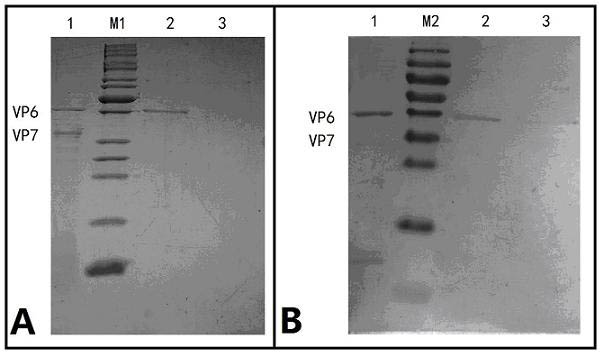
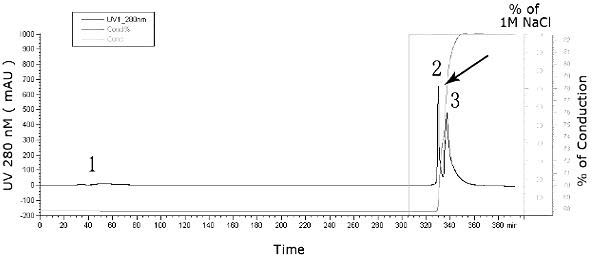
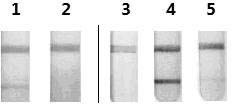
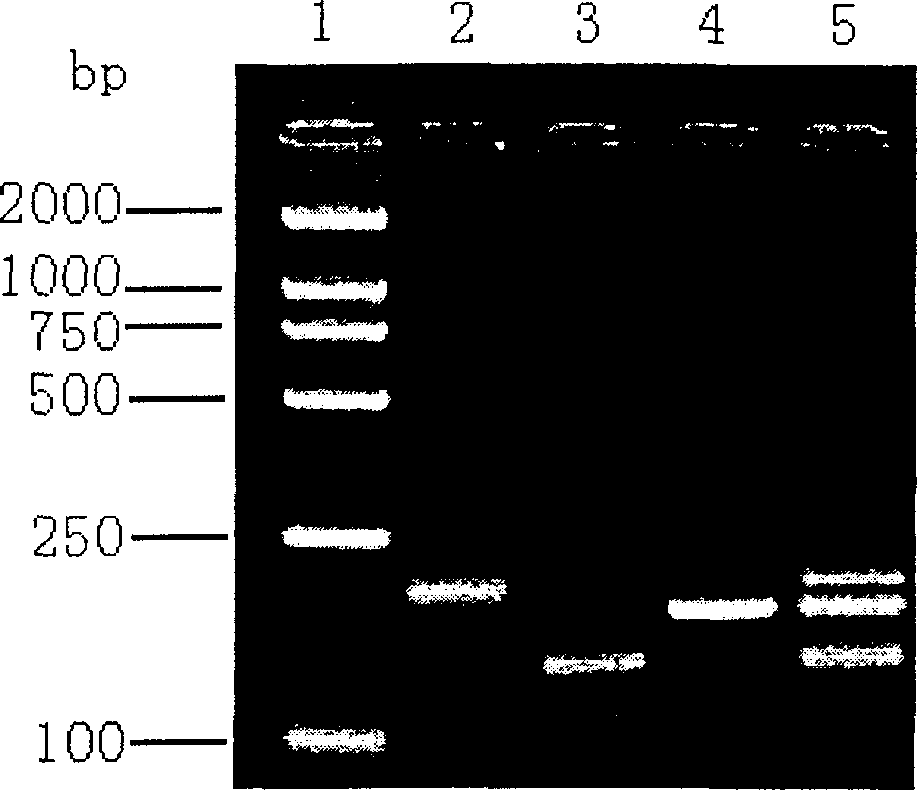
The invention relates to a human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein, wherein the amino acid sequence of the protein is shown in SEQ ID NO.2. Furthermore, the invention provides a coding gene and a preparation method of the protein as well as an application of the protein in preparation of a rotavirus vaccine. Two segments P[8]deltaVP8* and P[6]deltaVP8* are connected in serial to be expressed into the human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein; the protein can simultaneously induce neutralizing antibodies of major P genotypes P [8], P[4] and P[6] of the human rotavirus, overcome the shortcoming of an existing vaccine which can induce the generation of an antibody only capable of resisting one genotype and greatly enhance immune efficiency; and meanwhile, the protein can avoid a potential risk that oral administration of attenuated vaccine rotavirus possibly induces intestinal invagination, and is applicable to preparation of rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Expression and assembly of human group C rotavirus-like particles and uses thereof
Group C rotaviruses are a cause of acute gastroenteritis in children and adults that is distinct from group A RV. However, human group C rotaviruses cannot be grown in culture, resulting in a lack of tools for detection and treatment of GrpC RV disease. Consequently, the burden of GpC RV disease has not been clearly established. Isolated recombinant human rotavirus group C virus-like particles are provided according to embodiments of the present invention along with methods of their production and use in, inter alia, detection of Grp C RV infection, diagnostic assays and immunogenic compositions.
Owner:UNITED STATES OF AMERICA
Recombined bifidobacteria -hRV/VP7 expression vector and oral administration vaccine thereof
InactiveCN101220372AAvoid infectionTo achieve the active effect of changing strainsBacterial antigen ingredientsBacteriaAdjuvantRotavirus RNA
The invention relates to a recombinant bifidobacterium hRV-VP7 expression vector and the oral vaccine. The invention pertains to the field of genetic engineering. The technical problem to be solved is to construct a new bifidobacterium expression vector of hRV virus VP7 protein. The recombinant bifidobacterium hRV-VP7 expression vector of the invention is loaded with a virus protein VP7 coding gene sequence of a human group A rotavirus. The expression vector of the invention can be transfected into the bifidobacterium and can be further prepared into an hRV / VP7 transgenic bifidobacterium-hRV-VP7 recombinant vector oral live vaccine preparation after fermentation culture. The invention is mainly used for the prevention and adjuvant treatment of infantile diarrhea which is caused by human rotavirus, thus having great market prospect.
Owner:CHONGQING MEDICAL UNIVERSITY
Multivalent huma-bovine retavirus vaccine
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF HEALTH & HUMAN SERVICES THE
New human rotavirus vaccine strains and diagnostics
A vaccine composition and method of vaccination are provided useful for immunizing a subject against a rotavirus. The vaccines include rotavirus strains CDC-9 and CDC-66, fragments thereof, homologues thereof, or combinations thereof. Inventive vaccines may include a fragment of CDC-9, CDC-66, homologues thereof, or combinations thereof. Methods of inducing an immunological response are provided by administering an inventive vaccine.
Owner:THE GOVERNMENT OF THE US SEC THE DEPT OF HEALTH & HUMAN SERVICES CENT FOR DISEASE CONTROL & PREVENTION
Human rotavirus vaccine strains and diagnostics
A vaccine composition and method of vaccination are provided useful for immunizing a subject against a rotavirus. The vaccines include rotavirus strains CDC-9 and CDC-66, fragments thereof, homologues thereof, or combinations thereof. Inventive vaccines may include a fragment of CDC-9, CDC-66, homologues thereof, or combinations thereof. Methods of inducing an immunological response are provided by administering an inventive vaccine.
Owner:UNITED STATES OF AMERICA
Primer probe group, kit and method for detecting G II type norovirus
PendingCN113215327AHigh sensitivityShort timeMicrobiological testing/measurementMicroorganism based processesFluoProbesNucleotide
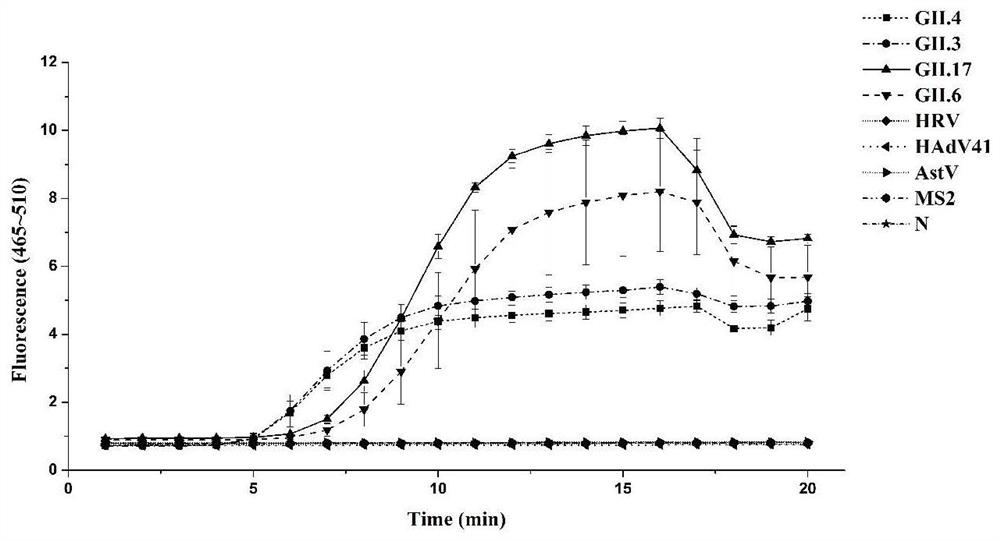
The invention provides a primer probe group, a kit and a method for detecting G II type norovirus, and belongs to the technical field of norovirus detection. The primer probe group comprises an upstream primer NVF6, a downstream primer NVR6 and a fluorescent probe NVP9, wherein the nucleotide sequence of the upstream primer NVF6 is shown as SEQ ID No. 1; the nucleotide sequence of the downstream primer NVR6 is shown as SEQ ID No. 2; and the nucleotide sequence of the fluorescent probe NVP9 is shown as SEQ ID No. 3. The primer probe group provided by the invention can be used for specifically detecting G II type norovirus including G II. 4, G II. 3, G II. 6 and G II. 17, and has no cross reaction with astrovirus, MS2 bacteriophage, human rotavirus and human adenovirus. The primer probe group for detecting G II type norovirus provided by the invention is high in sensitivity, and the lowest detection limit is 1.22 copies / microliter.
Owner:INST OF ENVIRONMENTAL MEDICINE & OCCUPATIONAL MEDICINE ACAD OF MILITARY MEDICINE ACAD OF MILITARY SCI
A human rotavirus vp8 recombinant protein and a human rotavirus vaccine using the vp8 recombinant protein
The present invention provides a human rotavirus VP8 recombinant protein, the amino acid sequence of the VP8 recombinant protein includes the amino acid sequence of the human rotavirus VP8 protein (hereinafter referred to as VP8 protein) and the amino acid sequence of an exogenous polypeptide, the exogenous polypeptide The isoelectric point of is less than or equal to 5. The invention also provides a human rotavirus vaccine prepared based on the VP8 recombinant protein. Compared with the prior art, the VP8 recombinant protein provided by the application is expressed through the fusion expression of the VP8 protein and the polypeptide fragment containing negatively charged amino acids in the tetanus toxin and / or diphtheria toxin protein, which improves the expression of the rotavirus VP8 protein expressed by Escherichia coli. Water solubility and yield, and the obtained fusion protein has good immunity, and the prepared vaccine has good effect.
Owner:CHENGDU MAXVAX BIOTECHNOLOGY LLC
Human rotavirus vaccine and preparation method thereof
ActiveCN104258387AImproving immunogenicityHigh levels of polysaccharide antibodiesAntibacterial agentsViral antigen ingredientsRotavirus RNAMicrosphere
The invention discloses a human rotavirus vaccine. The human rotavirus vaccine is a combined vaccine which is formed by polyvalent pneumococcal polysaccharide and two or more than two of carrier proteins by virtue of connectors, wherein the connectors are magnetic nano-microspheres; one of the carrier proteins is a rotavirus protein. A preparation method of the human rotavirus vaccine comprises a step of respectively coupling the polyvalent pneumococcal polysaccharide and two or more than two of carrier proteins (one of the carrier proteins is the rotavirus protein) with the magnetic nano-microspheres. The human rotavirus vaccine is simple in preparation process; by adopting the human rotavirus vaccine using the nano-microspheres as the connector, the mice Th1-type immune response, and the immune persistence, specificity and affinity of the polysaccharide specific antibody can be increased; in addition, the mice can be induced so as to produce rotavirus antibodies; the human rotavirus vaccine has the prevention effect of two vaccines; therefore, the human rotavirus vaccine has very wide application prospect.
Owner:BRAVOVAX +1
Glass gene chip for simultaneous detection of Group A, B and C human rotaviruses and the prepn and application
InactiveCN1772924AAccurate and Rigorous DataRealize detectionMicrobiological testing/measurementDiseaseHuman rotavirus
The glass gene chip for simultaneous detection of Group A, B and C human rotaviruses includes aldehydo treated glass chip, oligonucleotide probe array on the glass chip, contrast and blank spot coating, which includes 6 specific human rotavirus detecting probes in different groups, 1 positive contrast probe, 4 negative contrast probes and black sample application liquid contrast. The detecting probes and the contrast probes are connected with poly(dT)15 as connecting arm, and the probes have C6 radical with amino group in the 5í» end. The present invention also discloses the preparation process and clinical application of the chip. The chip of the present invention adopts multiple PCR amplification mode, may be used in the gene detection and group identification of several groups of human rotaviruses, and has wide latent application foreground in sanitary detection of food, disease prevention and control, and clinical medicine.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Method for producing dustless aseptic environment-friendly gertrude
The invention provides a method for producing a dustless aseptic environment-friendly gertrude, which comprises the steps of preparing finished underclothes from cotton cloth, and is characterized by also comprising three steps of washing the garment, performing ultraviolet sterilization and packaging under vacuum. In the method, ozone liquid for sterilizing the dustless aseptic underclothes has the high oxidation capacity, and oxygen atoms can oxidize cell walls of bacteria and even penetrate through the cell walls to perform chemical combination with unsaturated bonds in the cell walls to kill the bacteria, so the bacteria are killed by the high reduction potential of the ozone liquid. Therefore, the method has the advantages of removing and inhibiting rickettsia bacteria, chlamydia pathogenic bacteria, mycoplasma pathogenic bacteria, spirochete pathogenic bacteria and the like effectively, killing various pathogenic virus such as influenza virus, hepatitis C virus, human immunodeficiency virus (HIV), human rotavirus (HRV) and the like and reducing pathophoresis effectively.
Owner:郭翰祥
Product of transegenic plant for expressing coat protein of human rotavirus and application for preventing infant diarrhea
InactiveCN1661009AStable expressionThe result is stableCell receptors/surface-antigens/surface-determinantsDigestive systemAntigenRotavirus RNA
A transgenic plant able to express the coat protein of humanized rotavirus, its preparing process, and its application for preventing infantile diarrhea are disclosed.
Owner:INST OF IMMUNOLOGY P L A
Preparation method of multivalence egg-yolk immunoglobulin for resisting diarrheas
InactiveCN101921334ALow costIncrease productionEgg immunoglobulinsDigestive systemYolkEscherichia coli
The invention relates to a preparation method of multivalence egg-yolk immunoglobulin for resisting diarrheas, comprising the following steps of: (1) laying hen immunization: simultaneously immunizing laying hens by using a vaccine prepared from Escherichia coli and human rotaviruses in an immunology method; and (2) separation and purification of the multivalence egg-yolk immunoglobulin for resisting the diarrheas: degreasing the yolks of eggs collected in the step (1) in a water dilution method to obtain a multivalence egg-yolk immunoglobulin solution with the function of diarrhea resistance, concentrating the solution by 4-8 times by using 5 thousand membranes, then salting out the concentrated solution at the ammonium sulfate saturation of 45%-50% and the pH value of 6.0-7.0, sequentially ultrafitering the dissolved sediment by using 50 thousand membranes and 5 thousand membrane, carrying out chromatographic treatment to the salted and ultrafiltered product and then further purifying the product to obtain the multivalence egg-yolk immunoglobulin. Compared with the prior art, the preparation method has the advantages of low cost, high yield, wide application range and the like.
Owner:SHANGHAI WANWUCHUN BIO TECH
Application of lactoferrin in preparing medicament for preventing and controlling disease caused by human rotavirus
The invention discloses an application of lactoferrin in the drug to prevent human rotavirus, which is characterized by the following: the medium effective dose of lactoferrin is 278. 45+-12. 88mu g / mL; the medical treating index is 17. 98; the resource is wide and the cost is inexpensive, which can prevent HRV, therefore paving basis for new drug sieving.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Rotavirus vaccine and preparation method thereof
InactiveCN103182078ALess side effectsFast immune responseViral antigen ingredientsAntiviralsAdjuvantRotavirus RNA
The invention provides an inactivated vaccine for a human rotavirus, wherein the vaccine contains capsid protein produced from cracking of rotavirus totivirus particles, and the viral protein in each dose of the inactivated vaccine for the human rotavirus is 100-200 [mu]g, and the aluminum phosphate adjuvant is 0.5 mg per dose. Compared with an existing virus purification stoste, the impure protein in the inactivated vaccine for the human rotavirus is greatly reduced, and the rotavirus vaccine has higher immunogenicity and potency, is safer to use and is more applicable to large-scale production.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka.patsnap.com/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100011.PNG)
![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka.patsnap.com/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100012.PNG)
![Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof Human rotavirus P[8]deltaVP8*-P[6]deltaVP8* recombinant chimeric protein and application thereof](https://images-eureka.patsnap.com/patent_img/b30dd372-e6d6-40c8-84cc-007c9162170e/HDA00003309229100013.PNG)