Purification preparation method of human rotavirus inactivated vaccines by utilizing ion exchange chromatography
An ion exchange chromatography and rotavirus technology, applied in the field of preparing virus inactivated vaccines, can solve the problems of virus particle damage, high cost, high equipment requirements, etc., and achieve the effects of good stability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation and concentration of virus liquid
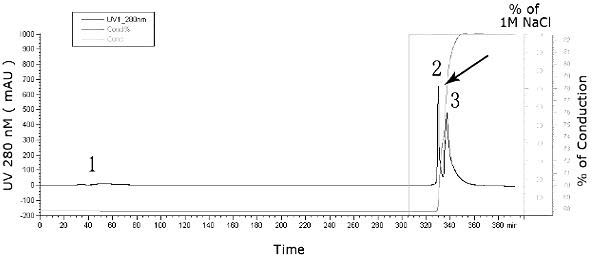
[0040] The method of spinner bottle culture was used to amplify the virus on Vero cells, and 45 spinner flasks were cultivated on a large scale. When the cells were a dense monolayer, the virus was seeded, and the cell density was as follows: 1.33×10 5 cells / cm 2 ×850cm 2 / Rotary bottle=1.1×10 8 cells / spinner bottle; harvest the virus when the virus in the Vero cell culture medium multiplies for 72 hours, after repeated freezing and thawing 3 times, centrifuge at 8000rpm for 20min to get the supernatant and harvest 9.8 L virus stock solution containing virus particles, the protein content of the virus stock solution is between 600ug / mL~700ug / mL, the virus infectivity titer is above 4.0lgCCID50 / mL; use the Pellicon tangential flow ultrafiltration system with a molecular weight cut-off of 100KDa to concentrate the 9.8L virus stock solution by ultrafiltration 36 times to 0.27L for further analysis. Purified by Q Sepharo...
Embodiment 2
[0053] Except for the following steps of detecting antigenicity and total protein removal rate before and after virus purification, the remaining steps are the same as in Example 1
[0054] unanimous.
[0055] (a) Detection of infectious titer and antigenicity before and after virus purification
[0056] Fluorescence focus assays (FFA) were used to measure the infectious titer and antigenicity before and after virus purification:
[0057] Take a 10-fold serial dilution of the virus and inoculate MA104 monolayer cells for CCID 50 detection. Divide MA104 cells into 1.5×10 4 cells / well were seeded on a 96-well plate at 37°C, 5% CO 2 Cultivate in the incubator until it grows into a dense monolayer, add 10-fold serially diluted virus, the dilution is from 10 -1 ~10 -8 , 10 wells were set for each dilution, after adsorption at 37°C for 1 hour, DMEM cell maintenance solution without calf serum was added, after incubation at 37°C for 16 hours, an equal volume of formaldehyde sol...
Embodiment 3
[0070] Except for the SDS-PAGE and Western-blot detection steps of the virus liquid in the following purification process, the remaining steps
[0071] Consistent with embodiment 1 or embodiment 2. The detection steps are as follows:
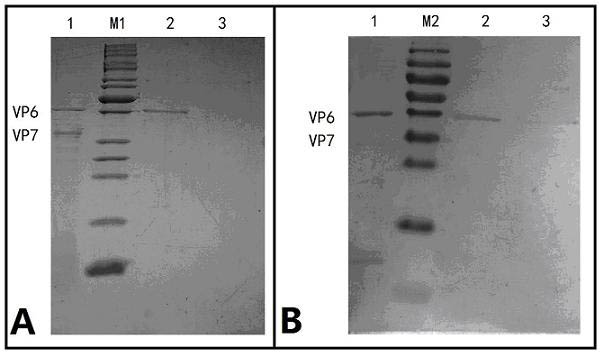
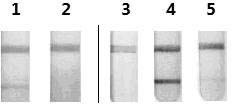
[0072] Sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis: 5% stacking gel, 10% separating gel. The purified rotavirus sample was mixed with 6× loading buffer, lysed by heating at 95°C for 5 min, loaded with 15 μl of sample, and electrophoresed at 90V. Coomassie brilliant blue staining was used to detect the purification effect.
[0073] Western-blot: Purified rotavirus protein was separated by 10% SDS-PAGE electrophoresis and transferred to nitrocellulose membrane. First, the membrane was blocked for 2 h with TBST solution containing 5% skimmed milk powder. Then use 1:500 dilution of guinea pig anti-rotavirus immune serum in TBST buffer to bind to the protein; finally use 1:2000 horseradish peroxidase-labeled goat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com