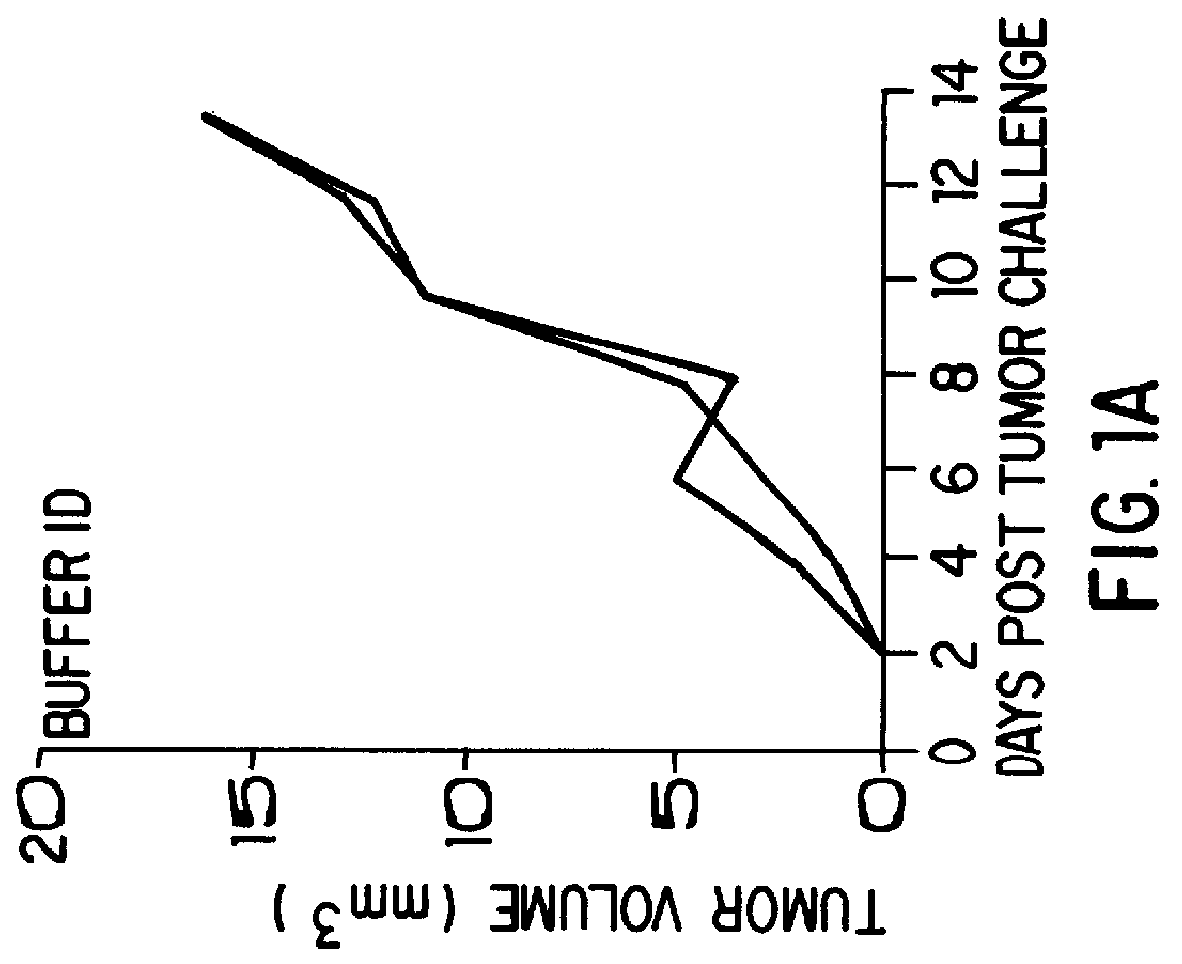
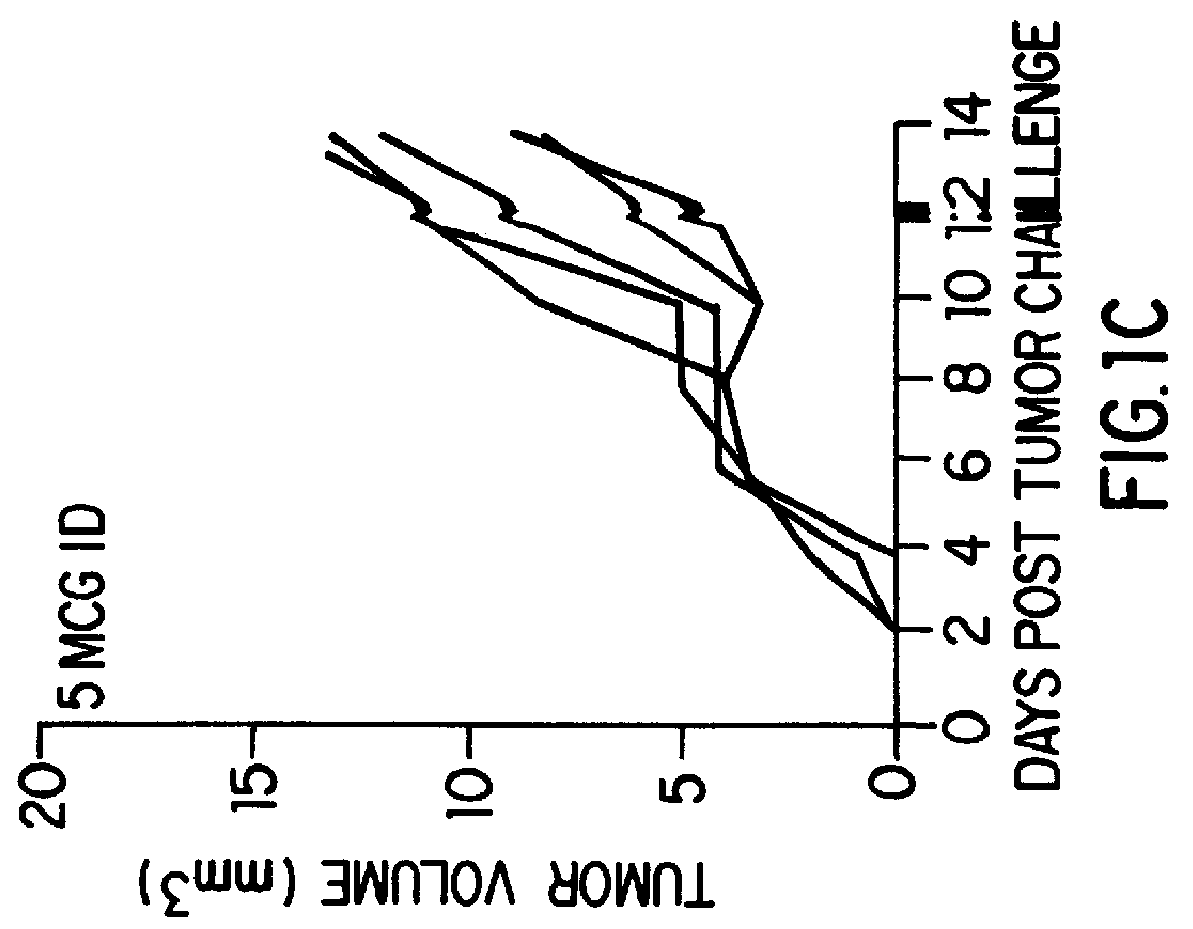
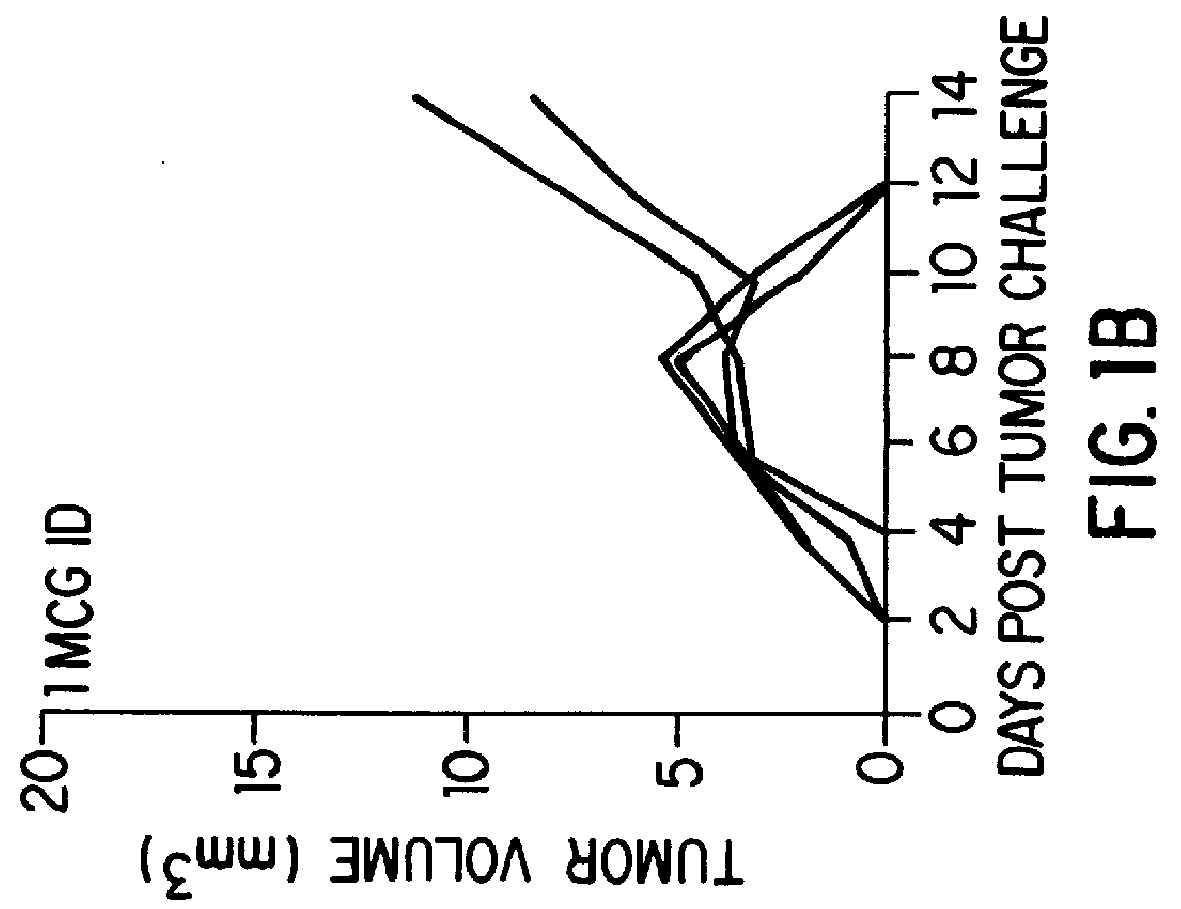
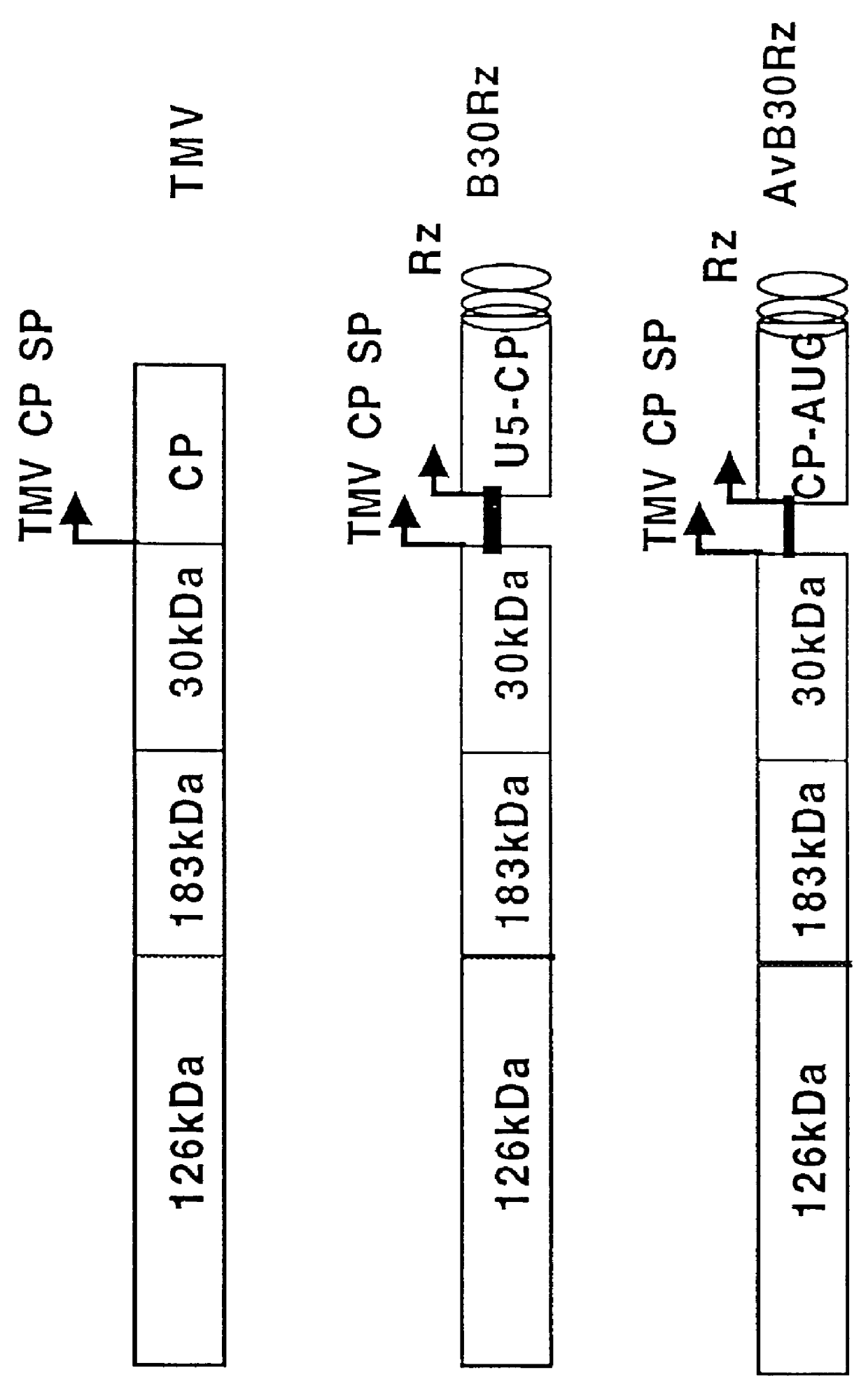
Patents
Literature
1221 results about "Antigenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antigenicity is the capacity of a chemical structure (either an antigen or hapten) to bind specifically with a group of certain products that have adaptive immunity: T cell receptors or antibodies (a.k.a. B cell receptors). Antigenicity was more commonly used in the past to refer to what is now known as immunogenicity, and the two are still often used interchangeably. However, strictly speaking, immunogenicity refers to the ability of an antigen to induce an adaptive immune response. Thus an antigen might bind specifically to a T or B cell receptor, but not induce an adaptive immune response. If the antigen does induce a response, it is an 'immunogenic antigen', which is referred to as an immunogen.
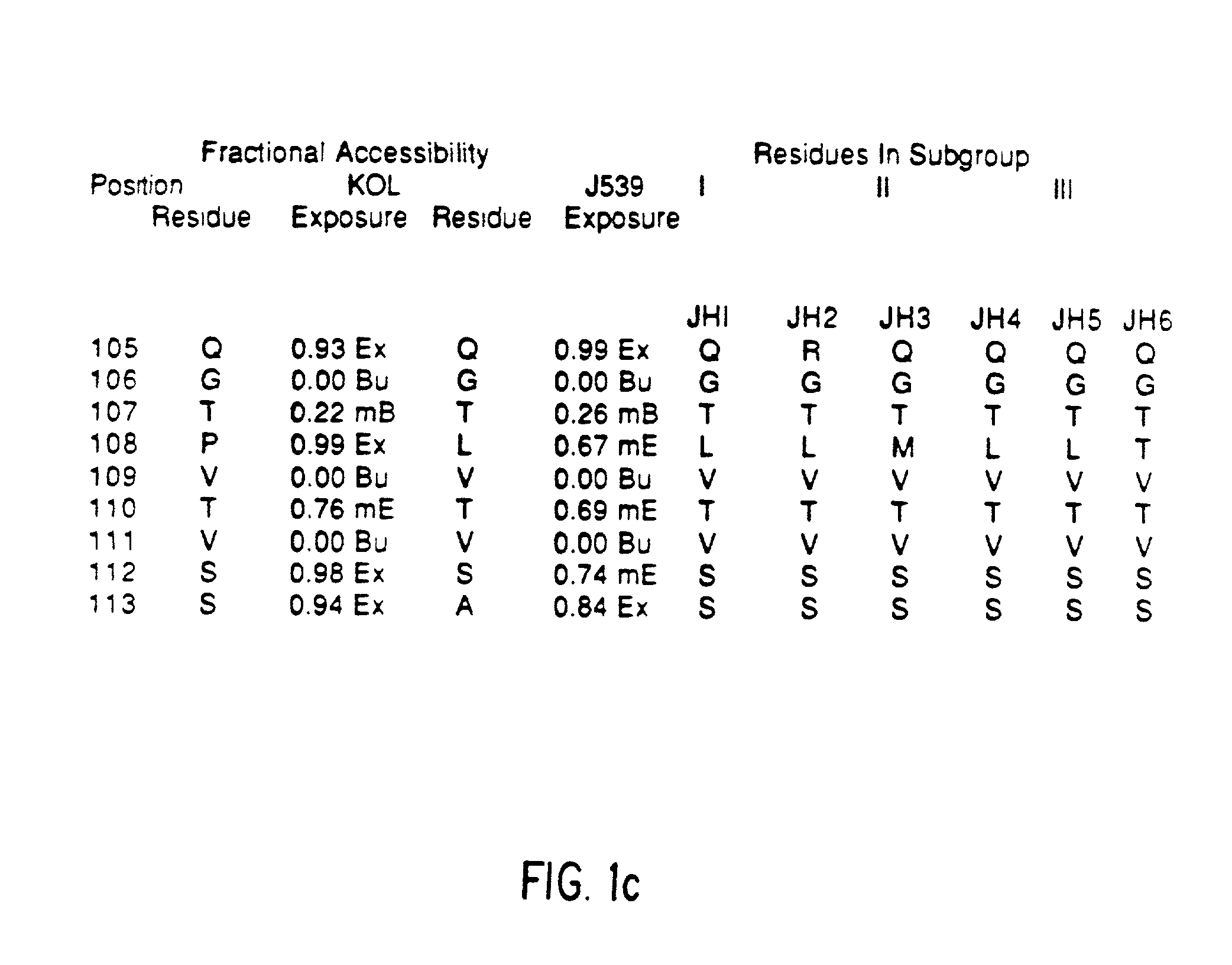
Method for reducing the immunogenicity of antibody variable domains
A unique method is disclosed for identifying and replacing immunoglobulin surface amino acid residues which converts the antigenicity of a first mammalian species to that of a second mammalian species. The method will simultaneously change immunogenicity and strictly preserve ligind binding properties. The judicious replacement of exterior amino acid residues has no effect on the ligind binding properties but greatly alters immunogenicity.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE +1
Natural human antibody
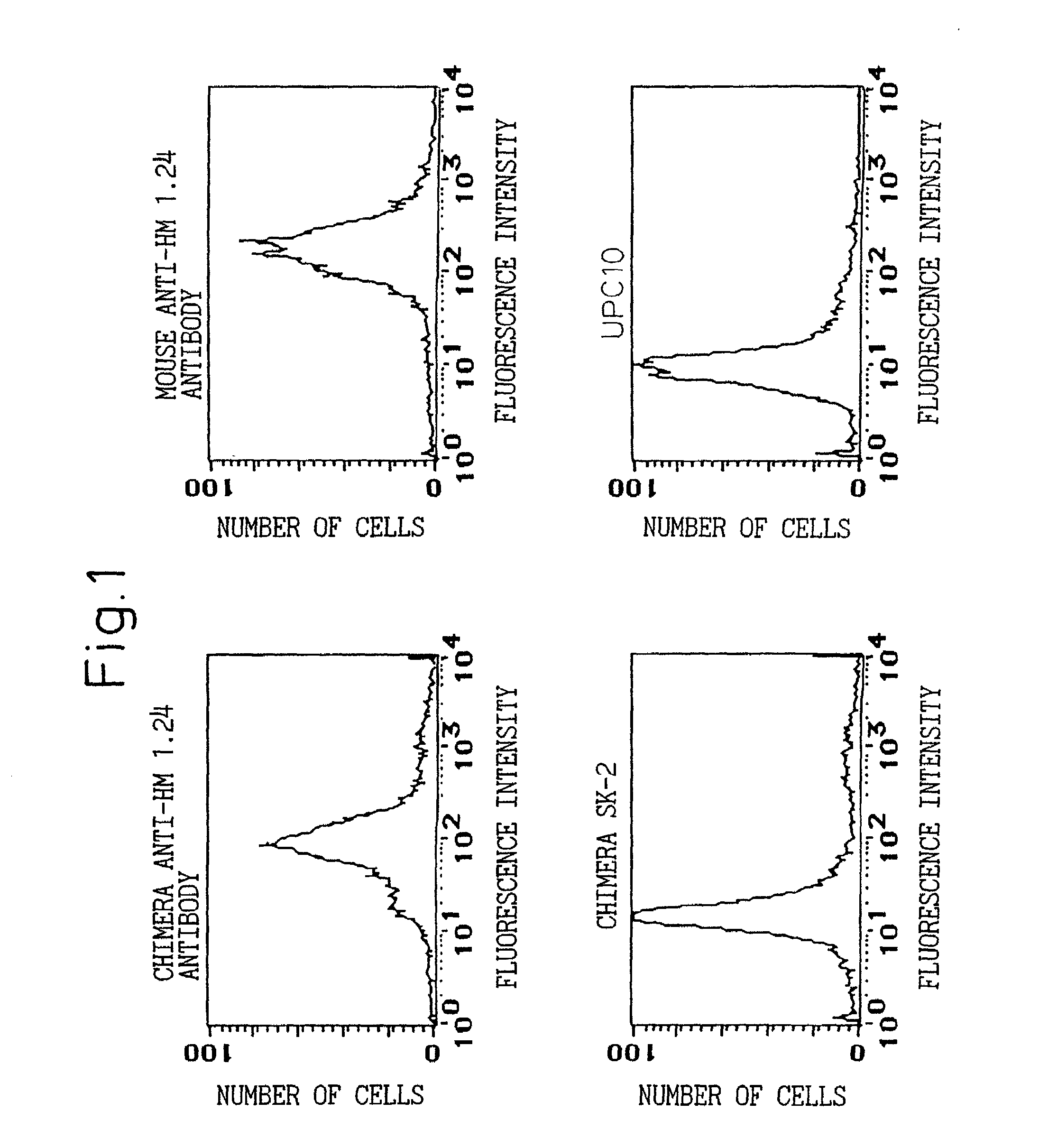
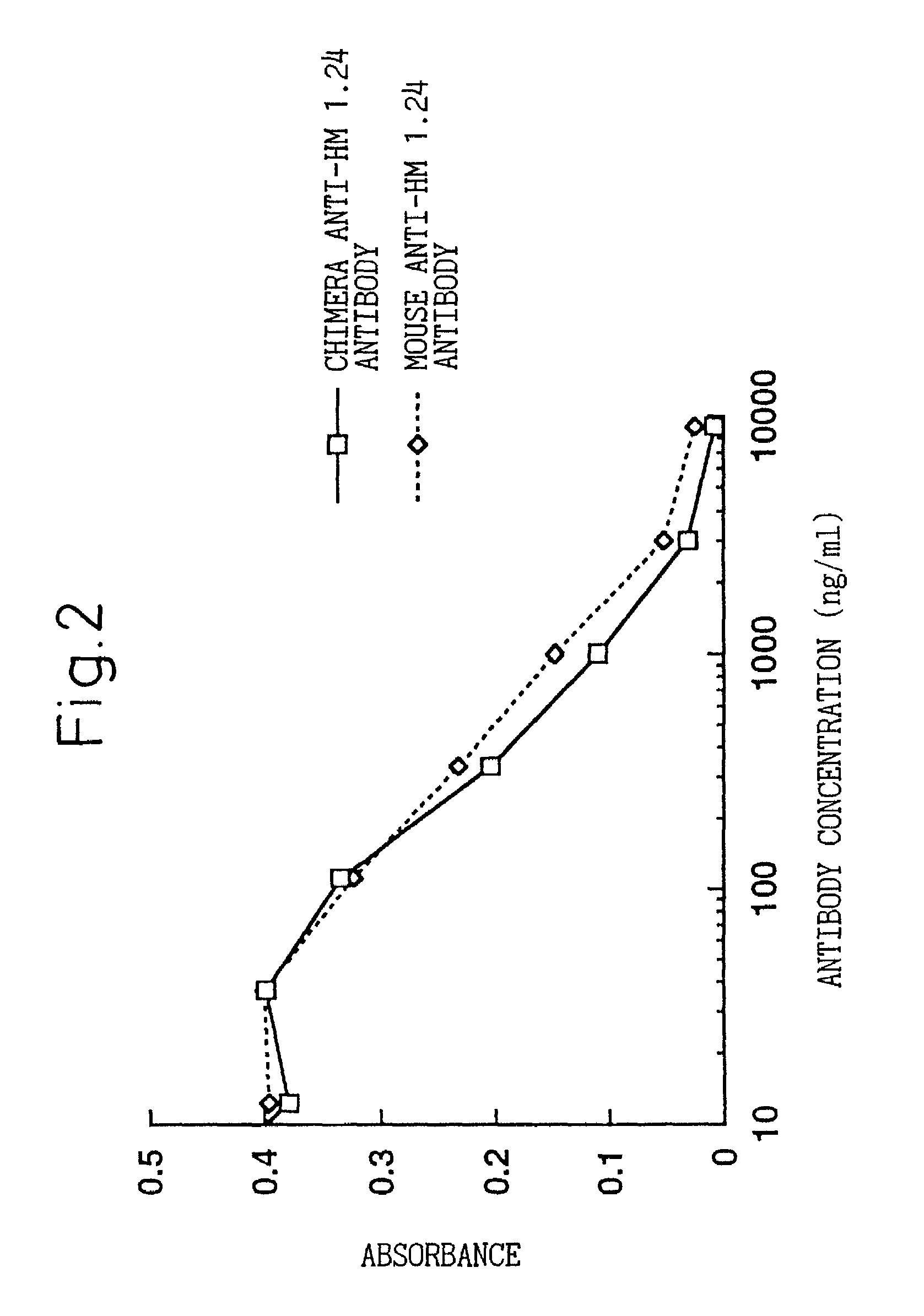
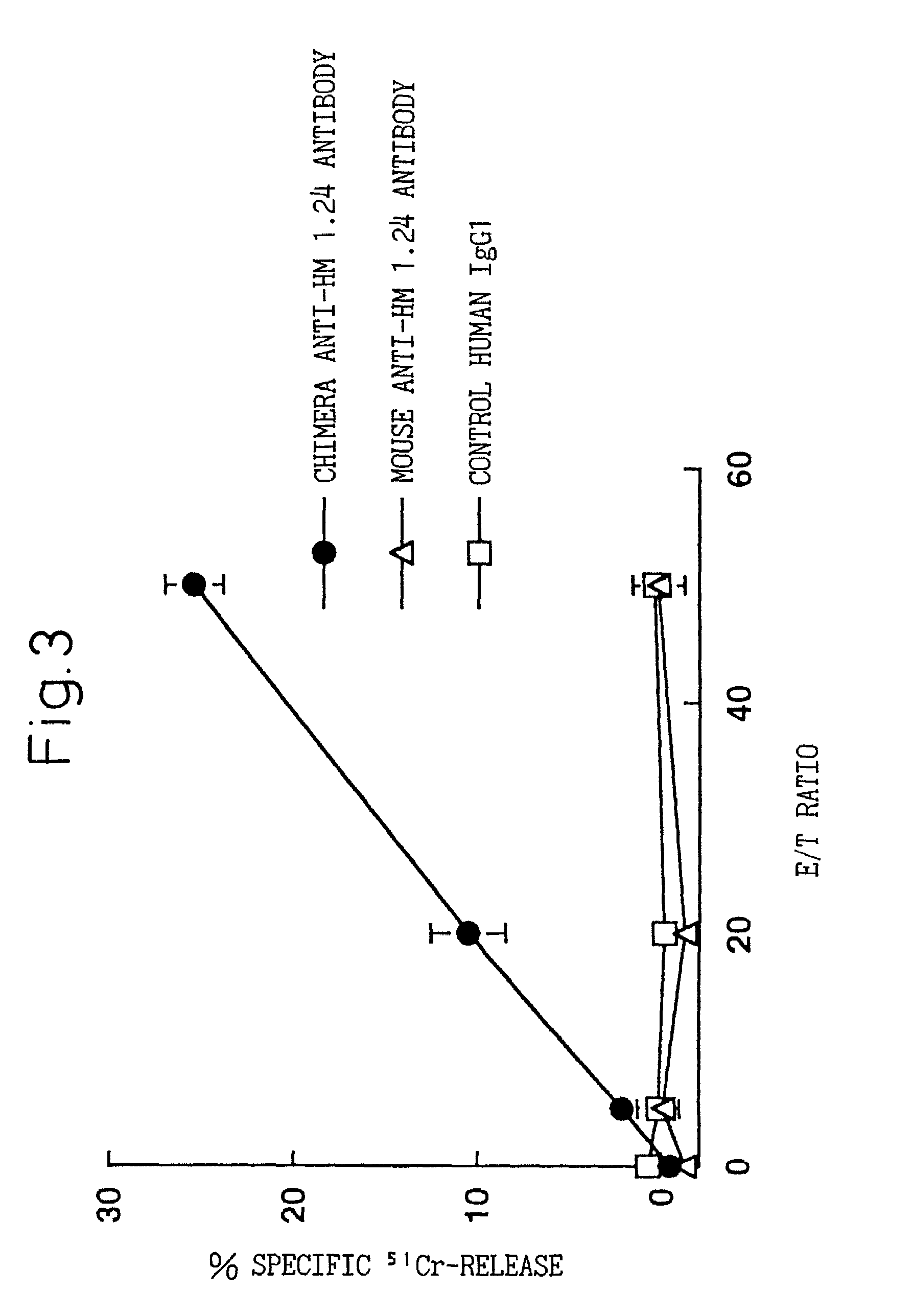
A reshaped human anti-HM1.24 antibody comprising:(A) L chains each comprising (1) a constant region of a human L chain, and (2) FRs of a human L chain, and CDRs of L chain of mouse anti-HM1.24 monoclonal antibody; and(B) H chains each comprising (2) a constant region of a human H chain, and (2) FRs of a human H chain, and CDRs of H chain of mouse anti-HM1.24 monoclonal antibody. Since the majority of the reshaped human antibody is derived from human antibody and the CDR has a low antigenicity, the reshaped human antibody of the present invention has low antigenicity and therefore is very promising in medical and therapeutic applications.
Owner:CHUGAI PHARMA CO LTD
Insect cells or fractions as adjuvant for antigens
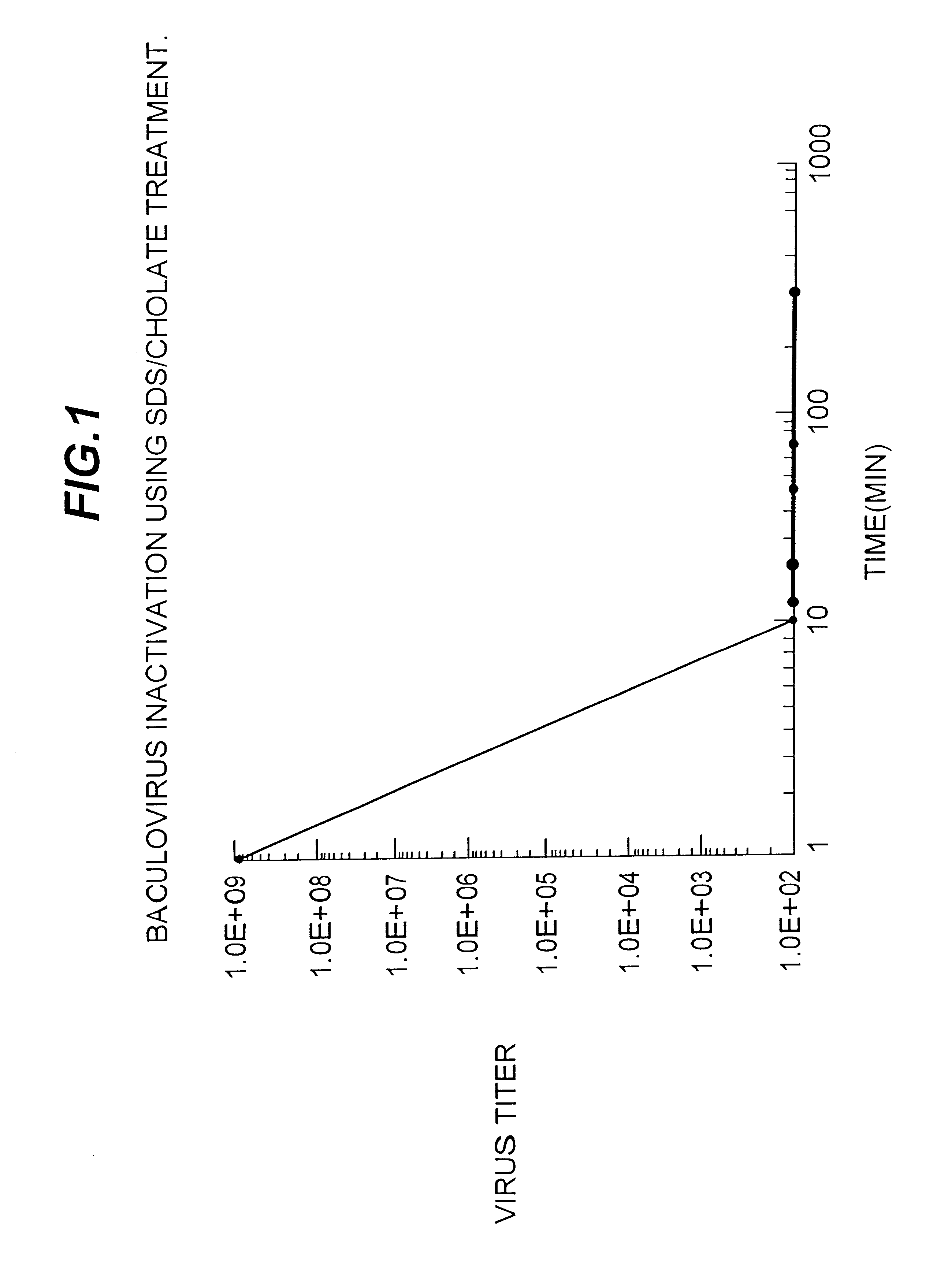
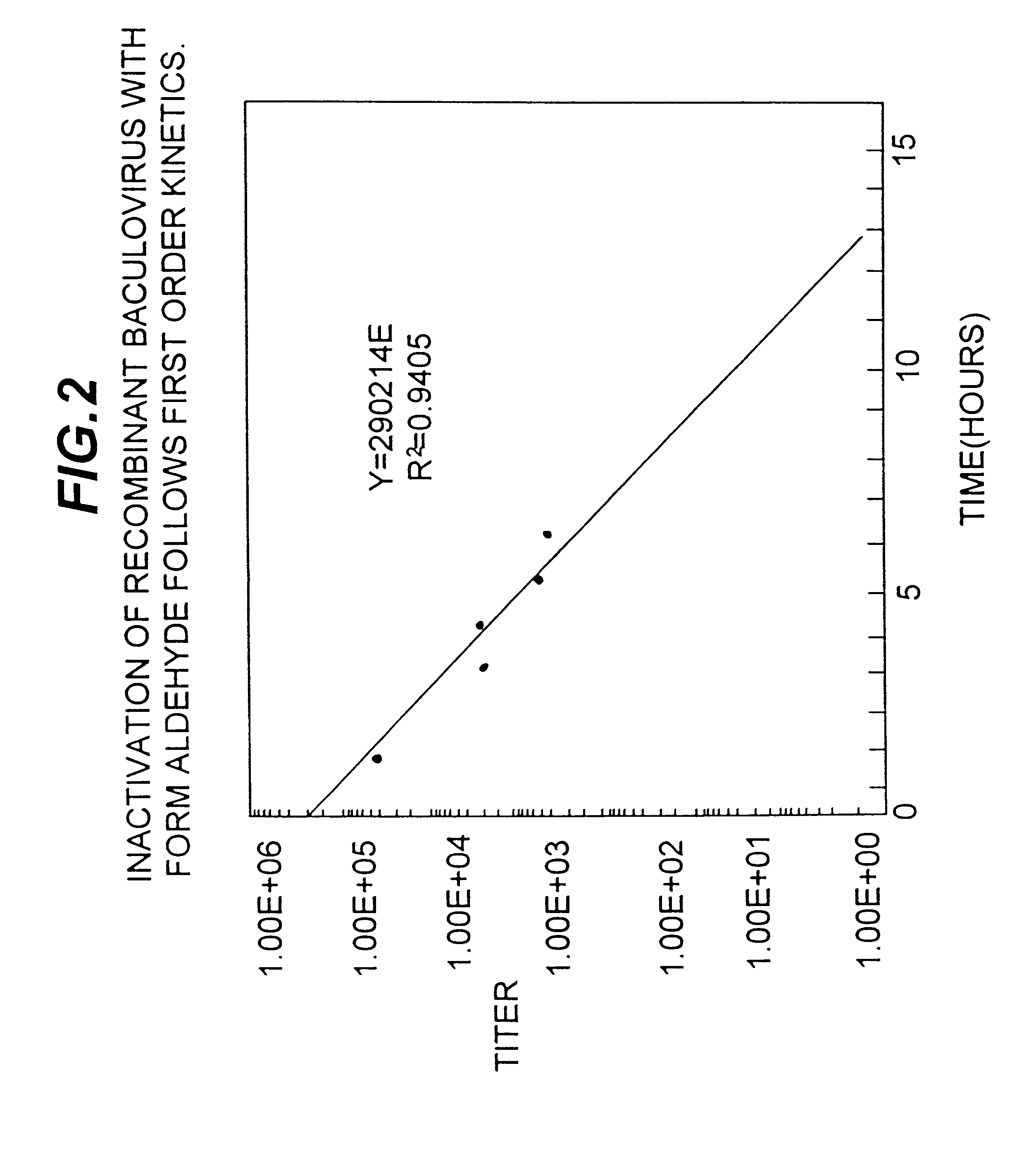
Disclosed and claimed is an adjuvant for immunogenic, immunological, antigenic or vaccine compositions. The adjuvant is composed of insect cells or fractions thereof. Disclosed and claimed are also methods for preparing and using the adjuvant and compositions containing the adjuvant. Advantageously, a recombinant baculovirus containing DNA encoding and expressing an epitope of interest or antigen can be infected into insect cells such as insect cells derived from a Lepidopteran species such as S. frugiperda for expression, and the infected insect cells or a fraction thereof can be used with the expressed epitope of interest or antigen as an inventive antigen or in an inventive immunological, antigen or vaccine composition.
Owner:MERIAL LTD
Prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases with heat shock/stress protein-peptide complexes
InactiveUS6017540AEnhancing host 's immunocompetenceHigh activityBiocidePeptide/protein ingredientsStress ProteinsIn vivo
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. Optionally, the methods further comprise administering antigen presenting cells sensitized with complexes of hsps noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. In a specific embodiment, the effective amounts of the complex are in the range of 0.1 to 9.0 micrograms for complexes comprising hsp70, 5 to 49 micrograms for hsp90, and 0.1 to 9.0 micrograms for gp96.
Owner:FORDHAM UNIVERSITY
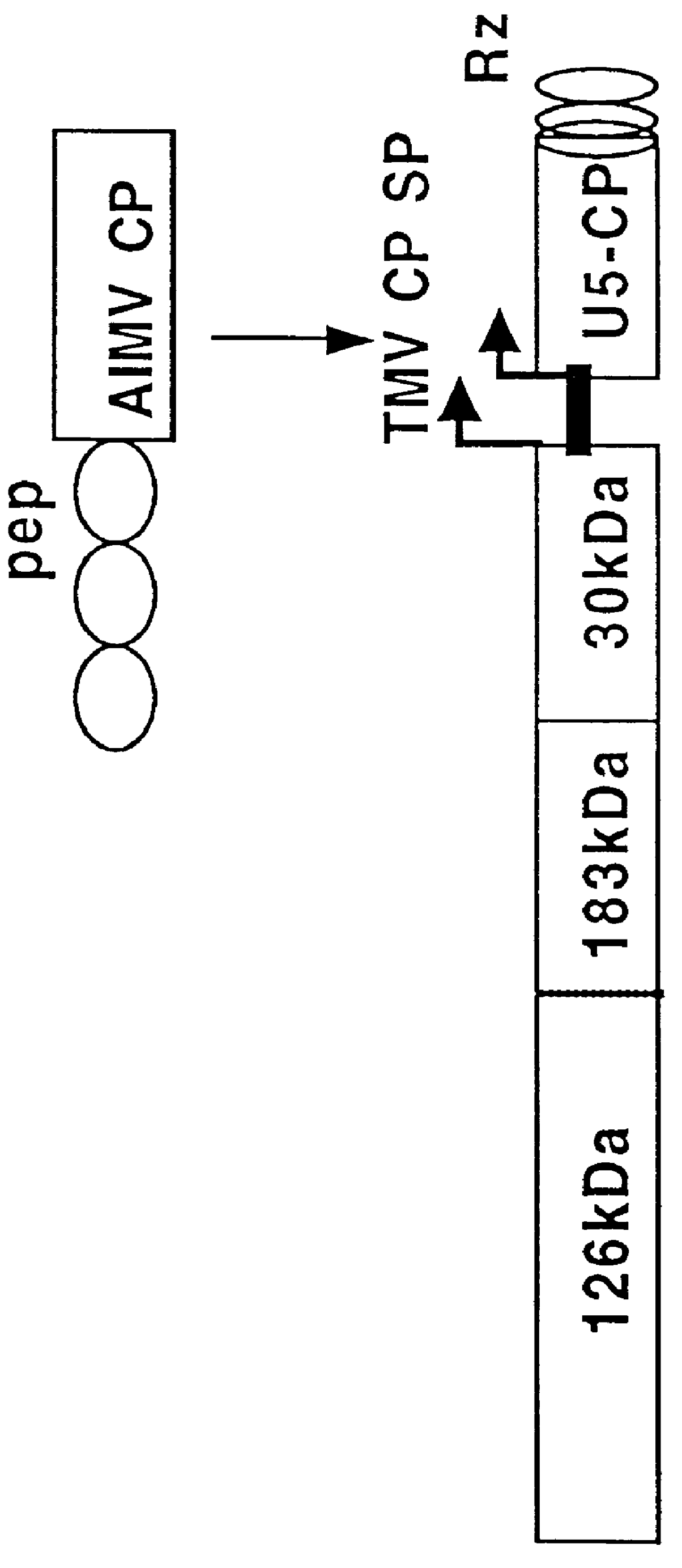
Polypeptides fused with alfalfa mosaic virus or ilarvirus capsid proteins
InactiveUS6042832AEfficient presentation of antigenImprove protectionSsRNA viruses negative-senseSsRNA viruses positive-senseAntigenIlarvirus
Owner:THOMAS JEFFERSON UNIV
Method for reducing the immunogenicity of antibody variable domains
InactiveUS20020034765A1Altered immunogenicityLow immunogenicityHybrid immunoglobulinsBiological testingBound propertyVariable domain
A unique method is disclosed for identifying and replacing immunoglobulin surface amino acid residues which converts the antigenicity of a first mammalian species to that of a second mammalian species. The method will simultaneously change immunogenicity and strictly preserve ligind binding properties. The judicious replacement of exterior amino acid residues has no effect on the ligind binding properties but greatly alters immunogenicity.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE +1
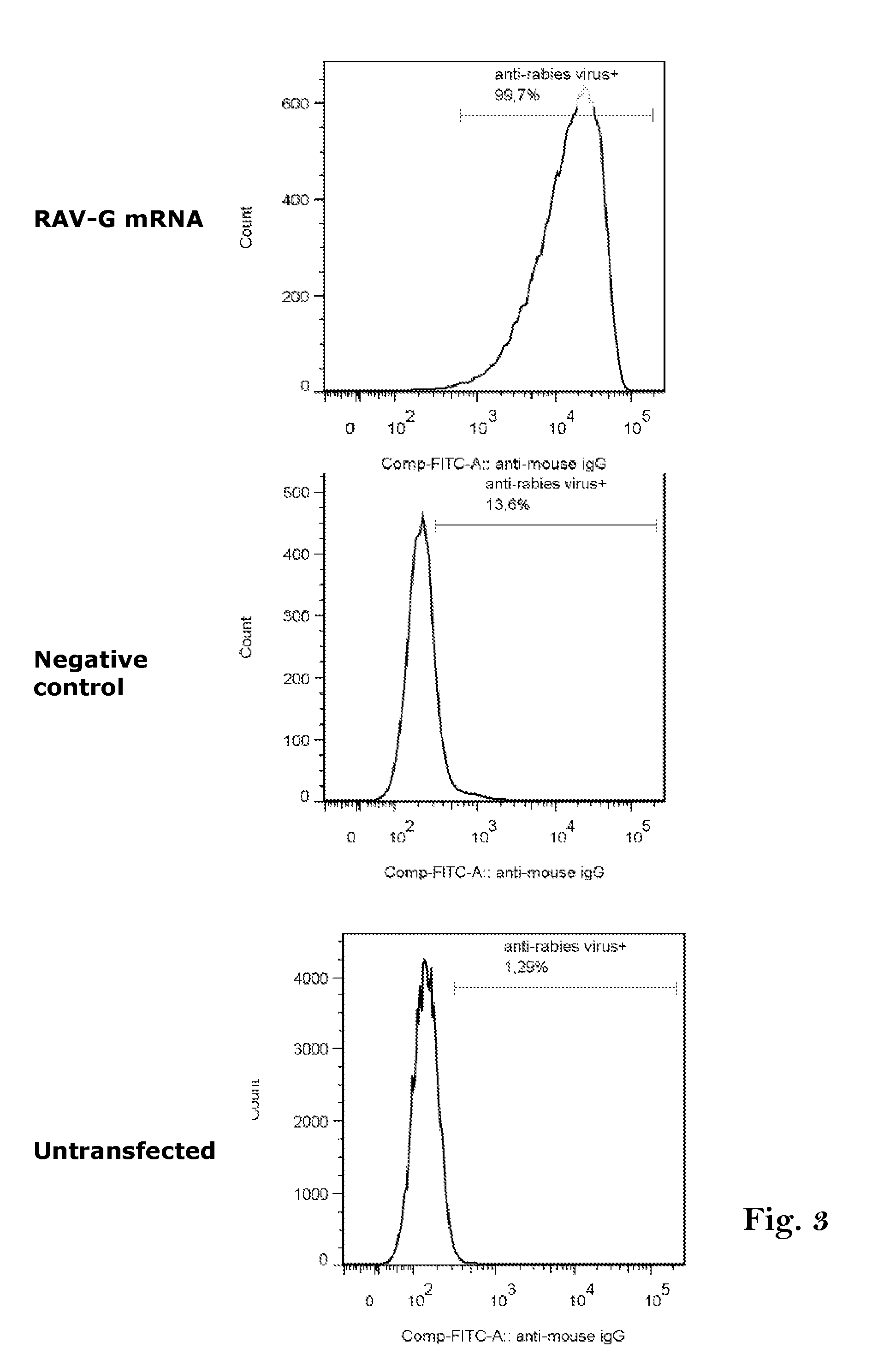
Rabies vaccine
ActiveUS20160166711A1Easy to identifyFaster and strong attackSsRNA viruses negative-senseVirus peptidesCoding regionRabies vaccine
The present invention relates to an mRNA sequence, comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof. Additionally the present invention relates to a composition comprising a plurality of mRNA sequences comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof.Furthermore it also discloses the use of the mRNA sequence or the composition comprising a plurality of mRNA sequences for the preparation of a pharmaceutical composition, especially a vaccine, e.g. for use in the prophylaxis or treatment of Rabies virus infections. The present invention further describes a method of treatment or prophylaxis of rabies using the mRNA sequence.
Owner:CUREVAC AG
Isolating biological modulators from biodiverse gene fragment libraries
InactiveUS6994982B1Increase diversityEasy to controlPeptide-nucleic acidsPeptide librariesExpression LibraryNucleotide
The present invention provides a method for identifying a modulator or mediator of a biological activity, which activity includes antigenicity and or immunogenicity, said method comprising the step of:(i) producing a gene fragment expression library derived from defined nucleotide sequence fragments; and(ii) assaying the expression library for at least an amino acid sequence derived from step (i) for a biological activity wherein that activity is different from any activity the amino acid sequence may have in its native environment.
Owner:PHYLOGICA
Respiratory syncytial virus (RSV) vaccine
ActiveUS20160168207A1Easy to identifyFaster and strong attackSsRNA viruses negative-senseSugar derivativesRespiratory syncytial virus BRSV Infections
The present invention relates to an mRNA sequence, comprising a coding region, encoding at least one antigenic peptide or protein of RSV infections Respiratory syncytial virus (RSV) or a fragment, variant or derivative thereof. Additionally the present invention relates to a composition comprising a plurality of mRNA sequences comprising a coding region, encoding at least one antigenic peptide or protein of RSV infections Respiratory syncytial virus (RSV) or a fragment, variant or derivative thereof. Furthermore it also discloses the use of the mRNA sequence or the composition comprising a plurality of mRNA sequences for the preparation of a pharmaceutical composition, especially a vaccine, e.g. for use in the prophylaxis or treatment of RSV infections Respiratory syncytial virus (RSV) infections. The present invention further describes a method of treatment or prophylaxis of RSV infections using the mRNA sequence.
Owner:CUREVAC SE
Compositions and methods using complexes of heat shock protein 90 and antigenic molecules for the treatment and prevention of neoplastic diseases
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp7o, 50-1000 micrograms for hsp9o, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in viva in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp7o-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Method for preparing SIS tissue repair material
The invention provides a method for preparing an SIS tissue restoring material. The method comprises the following steps: carrying out sterilizing treatment, degreasing treatment, cell free treatment and absterging treatment according to an arbitrary sequence at the lower layer of small intestinal mucosa; and finally preparing the material through freezing and drying. The SIS tissue restoring material can be used as an independent and common support vector for a growth factor, a cell, a drug and the like, and becomes a tissue engineering material through in-vitro culture. The material is a natural and nontoxic biological material without antigenicity and immunity. The SIS tissue restoring material can be used for restoring hernia, defect of skin, urinary system tissue defect, mucosa defect and other periphery and internal parenchyma defects.
Owner:BEIJING DATSING BIO TECH
Viruses and virus-like particles for multiple antigen and target display
InactiveUS20060121468A1Improve effectivenessImprove responseSsRNA viruses positive-senseVectorsTissue targetingVaccine Immunogenicity
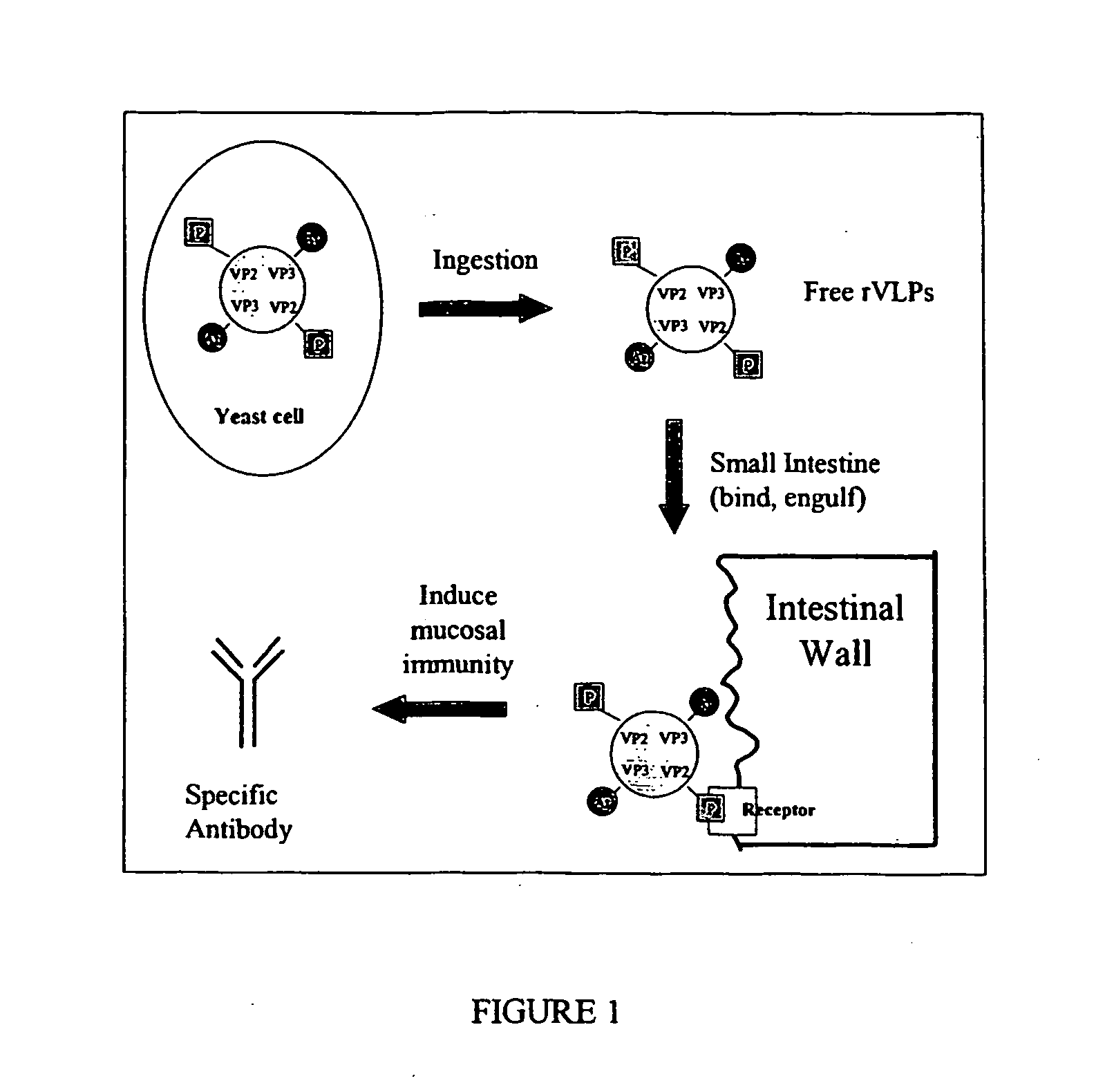
The present invention relates to the display of antigenic or allergenic components along with a tissue-targeting component on viruses or virus-like particles. Capsid protein genes are recombinantly modified to contain the specified components then expressed within a host organism, such as yeasts, bacteria, or algae, and allowed to spontaneously form active virus particles or virus-like particles. The recombinant complexes (virus or virus-like particle) can then be purified or used in situ as a therapeutic tool for disease or allergy prevention. The expression of multivalent and multifunctional components to increase the immunogenicity of the recombinant complexes, especially on oral administration, is provided.
Owner:ADVANCED BIONUTRITION CORP
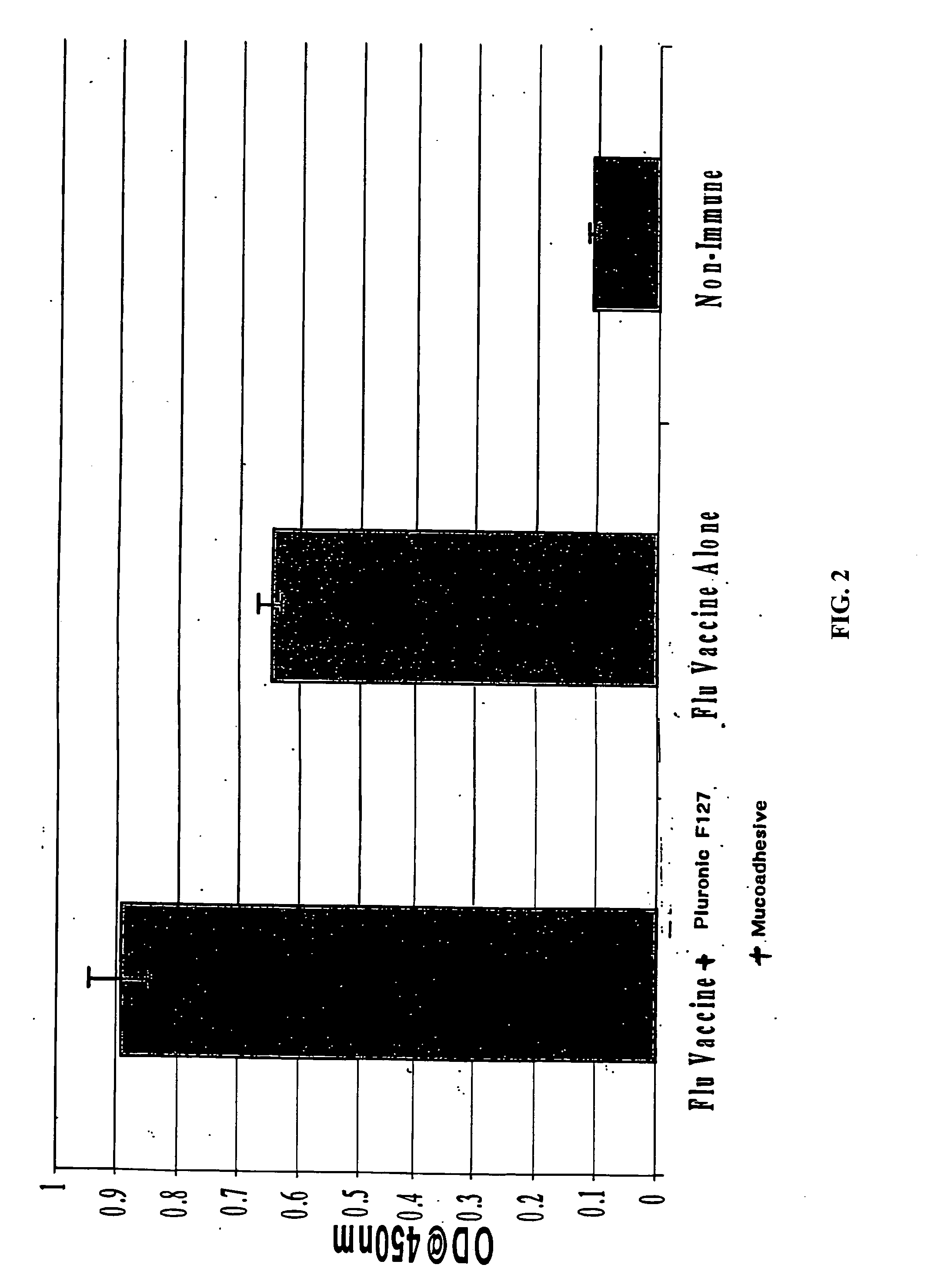
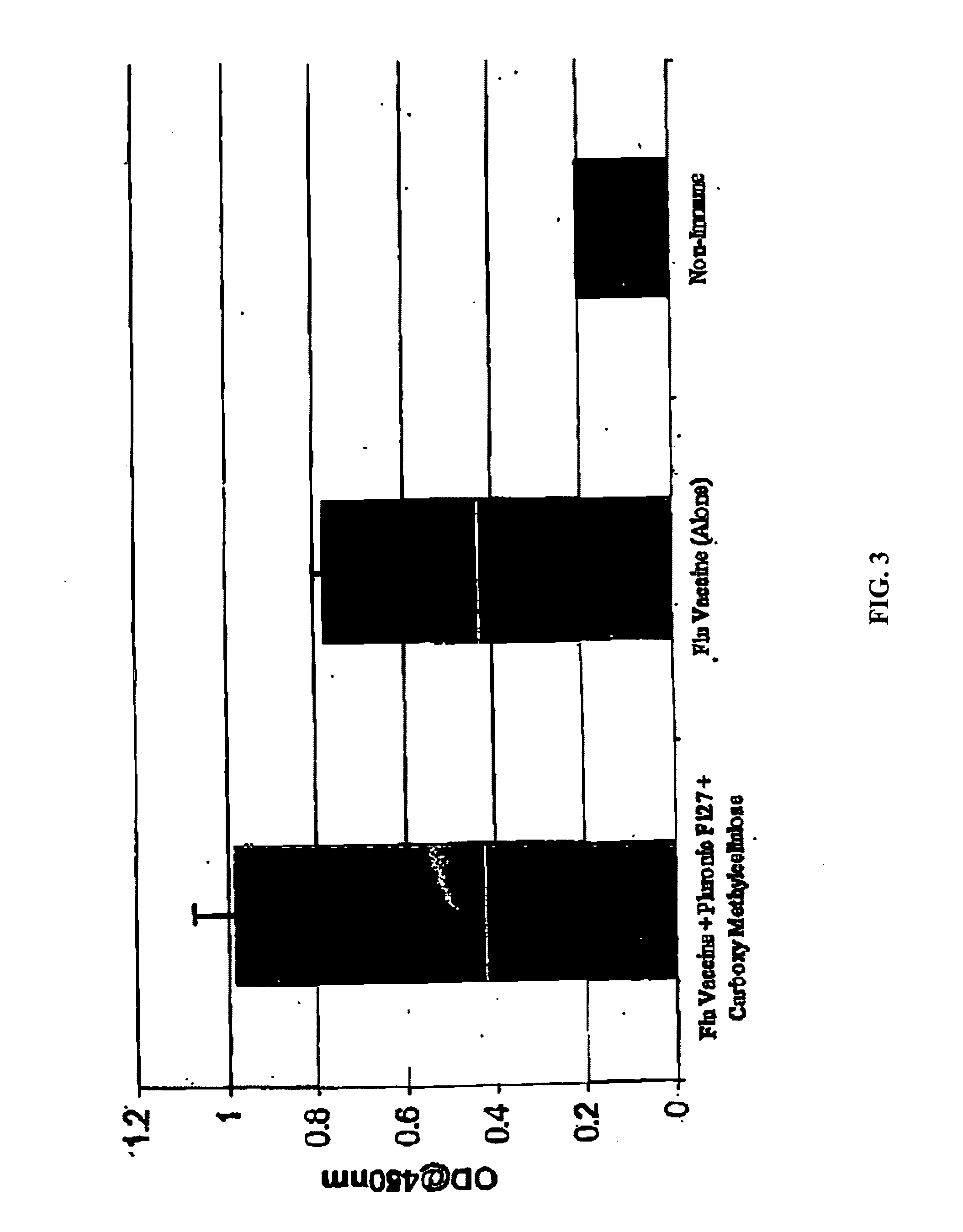
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050123550A1Enhances therapeutic efficacy and protective immune responseGood curative effectSsRNA viruses negative-senseOrganic active ingredientsInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
Thiepane compounds
Tri- and tetra substituted thiepane compositions having use as immunogens, therapeutics, diagnostics and for other industrial purposes are disclosed. The compositions inhibit proteolytic activity of viral enzymes and are useful for the inhibition of such enzymes as well as in assays for the detection of such enzymes. Embodiments in which antigenic polypeptides are bonded to the compositions are useful in raising antibodies against the thiepane haptens or the polypeptide. Labeled thiepanes of this invention are useful as diagnostic reagents.
Owner:GILEAD SCI INC
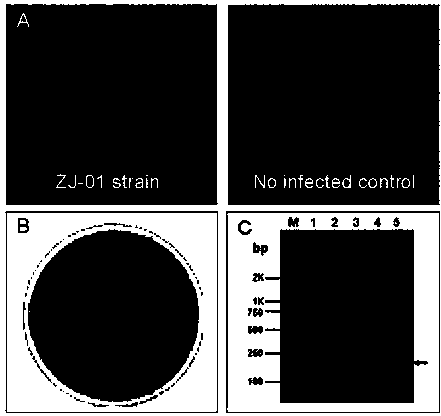
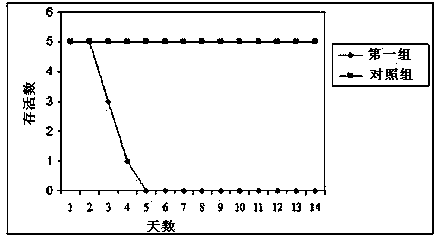
Porcine pseudorabies virus (PRV) variant PRV-ZJ01 and application thereof
ActiveCN103627678AImprove securityImproving immunogenicityMicroorganism based processesAntiviralsRabiesEngineering
The invention relates to the technical field of porcine pseudorabies viruses (PRVs) and in particular relates to a porcine PRV variant PRV-ZJ01 with collection number of CGMCCNo.8170 and an application of the porcine PRV variant PRV-ZJ01 in preparation of vaccines. The porcine PRV variant PRV-ZJ01 has the beneficial effects that a water-soluble inactivated vaccine is prepared by adopting a PRV-ZJ01 variant virus solution and is subjected to a swine immune protection test with live vaccines of Bartha-K61, Bucharest and HB-98 strains and the results show that the inactivated vaccine of the ZJ01 strain has relatively high safety and has the immune protection efficiency obviously higher than that of immunity groups of the live vaccines of the Bartha-K61, Bucharest and HB-98 strains, and the live vaccines of the Bartha-K61, Bucharest and HB-98 strains can not provide full protection for the ZJ01 very virulent strain; the inactivated vaccine of ZJ01 has relatively good immune protection effects on the PRV variant and the traditional strains; infected with 10<6.0>TCID50 (Tissue culture infectious dose 50) / ml nasal drops of the PRV-ZJ01 variant, all the 85-day-old non-immune swine can become ill and die; results prove that the virulence of the virus strain is obviously enhanced, the antigenicity is varied and the virus strain has relatively good immunogenicity after being inactivated and can be used for research and development of the vaccine of the virus strain and the diagnostic methods.
Owner:NANJING AGRICULTURAL UNIVERSITY
Compositions comprising heat shock proteins or alpha(2) macroglobulin, antigenic molecules and saponins, and methods of use thereof
InactiveUS20020037290A1Growth inhibitionEffective amountBiocideSenses disorderAutoimmune ReactionsAutoimmune responses
The present invention relates to pharmaceutical compositions and methods for the prevention and treatment of autoimmune diseases, infectious diseases, neurodegenerative diseases, and primary and metastatic neoplastic diseases. In the practice of the invention, the compositions are employed comprising: (a) a heat shock protein (hsp) or an alpha(2)macroglobulin (alpha2M); (b) a saponin; and, optionally, (c) an antigenic molecule. The antigenic molecule displays the antigenicity of an antigen of: (a) a cell that elicits an autoimmune response; (b) an agent of an infectious disease; (c) a cancerous cell; or (d) a cell or structure associated with a neurodegenerative or amyloid disease. The hsps that can be used in the practice of the invention include but are not limited to hsp70, hsp90, gp96, calreticulin, hsp 110, grp 170, and PDI, alone or in combination with each other. The antigenic molecule can be covalently or noncovalently bound to the hsp or alpha2M, free in solution, and / or covalently bound to the saponin. The compositions of the invention can be administered alone or in combination with the administration of antigen presenting cells sensitized with an hsp- or alpha2M-antigenic molecule complex.
Owner:ANTIGENICS
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050255121A1Good antigenicityEnhance immune responseSsRNA viruses negative-senseBiocideInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
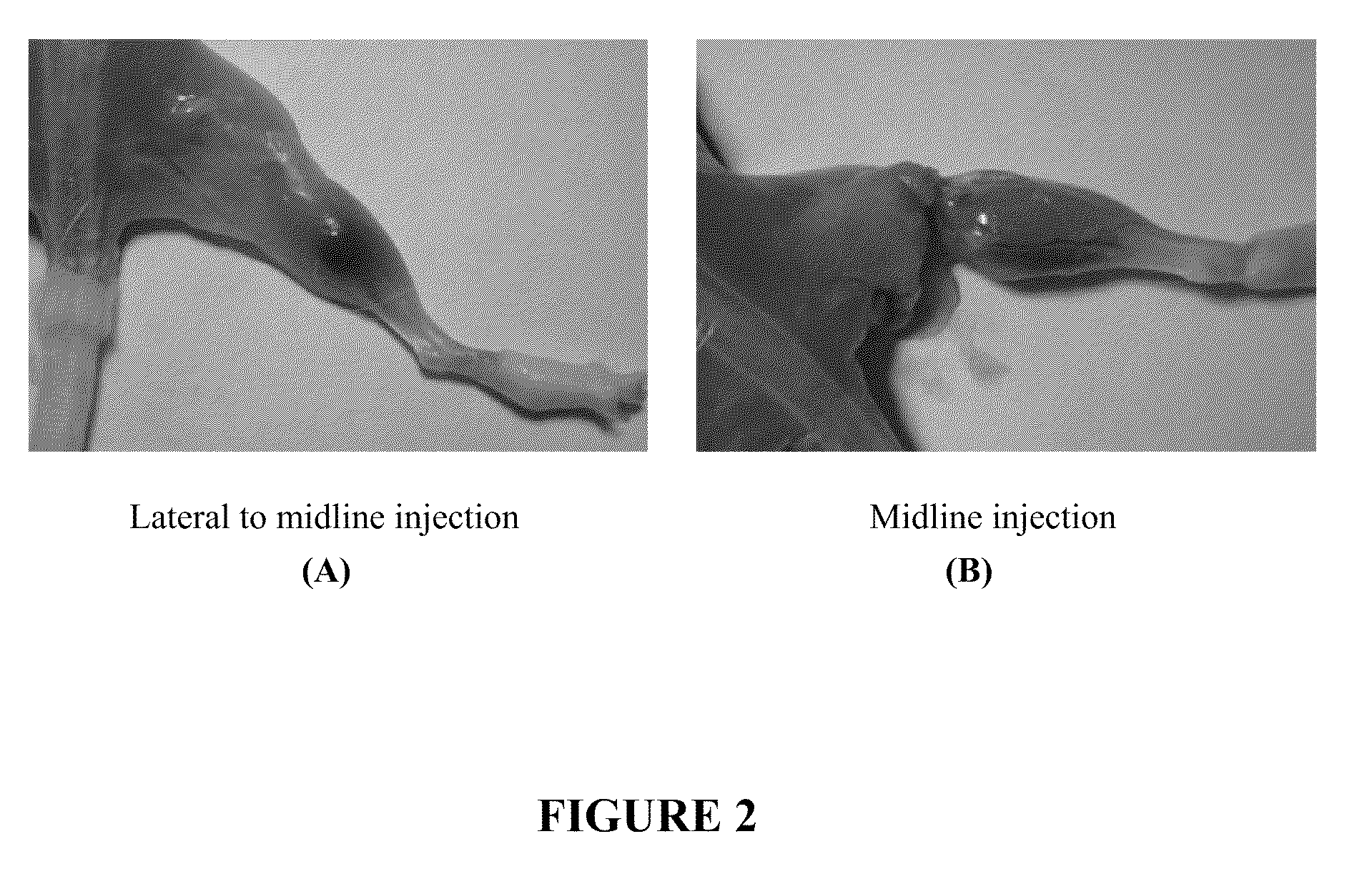
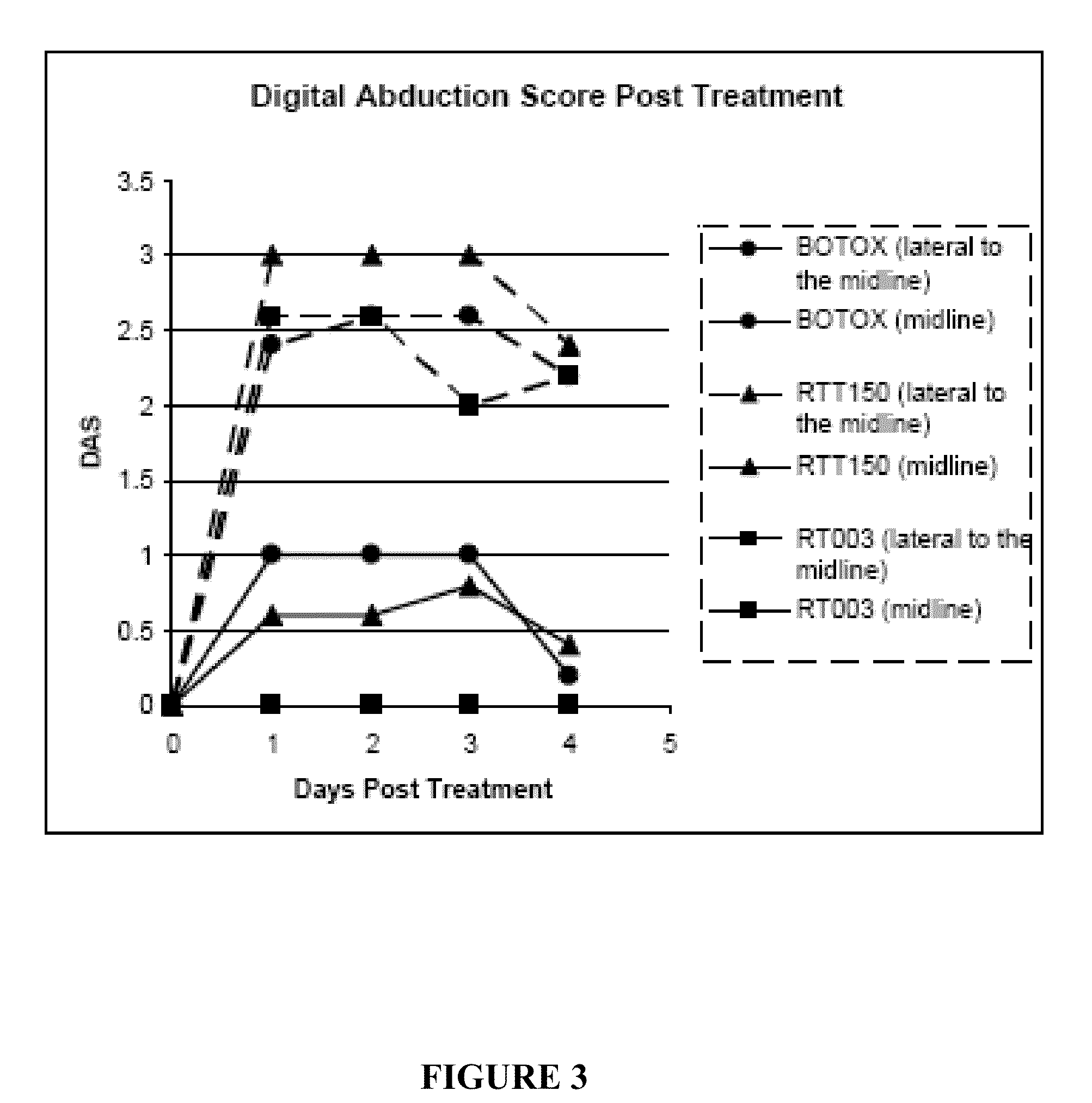
Injectable Botulinum Toxin Formulations
InactiveUS20100168023A1Low antigenicityExtended durationCosmetic preparationsSenses disorderClinical efficacyBotulinum toxin type
This invention provides novel injectable compositions comprising botulinum toxin that may be administered to a subject for various therapeutic, aesthetic and / or cosmetic purposes. The injectable compositions contemplated by the invention exhibit one or more advantages over conventional botulinum toxin formulations, including reduced antigenicity, a reduced tendency to undergo unwanted localized diffusion following injection, increased duration of clinical efficacy or enhanced potency relative, faster onset of clinical efficacy, and / or improved stability.
Owner:REVANCE THERAPEUTICS INC
Saccharide Conjugate Vaccines
InactiveUS20080260773A1Improve bioavailabilityImprove efficacyAntibacterial agentsPeptide/protein ingredientsConjugate vaccineCarrier protein
The invention provides compositions comprising a combination of two or more monovalent conjugates, each of said two or more monovalent conjugates comprising a carrier protein comprising T cell epitopes from two or more pathogens conjugated to saccharide antigen. The invention also provides a multivalent conjugate comprising two or more antigenically distinct saccharide antigens conjugated to the same carrier protein molecule, wherein the carrier protein comprises T cell epitopes from two or more pathogens. Further compositions comprise one or more of said monovalent conjugates and one or more of said multivalent conjugates. The invention further provides methods for making said compositions and uses for said compositions.
Owner:NOVARTIS AG
Pressure-assisted molecular recovery (PAMR) of biomolecules, pressure-assisted antigen retrieval (PAAR), and pressure-assisted tissue histology (PATH)
ActiveUS8288122B2Facilitate re-hydrationEasy to usePreparing sample for investigationDead animal preservationCross-linkHigh pressure
A method is disclosed for reversing fixation-induced cross-linking in tissue specimens that have been preserved for histological examination. The method involves placing the fixed tissue in a liquid under elevated temperature and pressure conditions that are sufficient to reverse the fixation-induced cross-linking, restore antigenicity to proteins, and permit improved molecular and proteomic analysis of the preserved tissue specimen. Methods are also disclosed for processing tissues for histological examination under elevated pressure conditions that enhance the perfusion of liquid reagents into the tissue and reduce overall processing times.
Owner:AMERICA REGISTRY OF PATHOLOGY +2
Bracket material for bone tissue engineer and preparation method thereof
InactiveCN101417145AImprove mechanical propertiesGood biocompatibilityProsthesisDrug biological activityProtein C
The invention relates to a bracket material used for an osseous tissue project, and a preparation method thereof. Firstly, the bracket of a type I collagen is extracted from a small fresh pigskin by a mature extracting technique. Then, the bracket is further expanded in a Tris cushion liquid, the pH of which is equal to 8.8 to obtain a natural porous collagen bracket by the treatments of freezing and drying. The bracket is respectively and repeatedly mineralized in a CaCl2 liquid and in (NH4)2HPO4 liquid or mineralized in simulated body fluid for a long period to lead the weakly crystallized HA to be uniformly settled into the collagen bracket; and then a pigskin collagen-hydroxyapatite ossein is obtained by the treatments of freezing and drying to replace the natural bracket material. The invention not only maintains the natural bracket structure of the collagen in an organism, but also has the advantages of low material cost, simple devices, short period and easy operation. The obtained compound bracket material used for the pigskin collagen-hydroxyapatite osseous tissue project has the characteristics of high intensity, large toughness, non-antigenicity, higher biological activity as well as degradation and releasing control.
Owner:SHANDONG UNIV
Cartilage cell epimatrix three-dimensional porous sponge stent for tissue engineering and preparation method thereof
ActiveCN101496913AFacilitate in vitro constructionPromotes regeneration in the bodyBone implantCartilage cellsCell-Extracellular Matrix
The invention discloses a three-dimensional porous cartilage extracellular matrix sponge scaffold made of natural cartilage, which can compound cells further to construct tissue engineered cartilage, and can be used for clinically repairing cartilage defects. In the invention, conditions which can fully perform cell extraction and form a porous scaffold matrix are provided by processing cartilage into cartilage microfilaments, and then the cell extraction and solidification and / or strengthening treatment are performed to obtain the three-dimensional porous cartilage extracellular matrix sponge scaffold which is completely decellurized. The antigenicity and cell components are removed in the natural cartilage, and an extracellular matrix component of the cartilage is retained to obtain the scaffold with appropriate pore diameter and porosity, suitable degradation rate, good biocompatibility and certain biomechanical strength. The scaffold has the advantages of broad material sources, low cost, simple and feasible preparation technology, and good repetitiveness; and the scaffold can be widely applied in the field of tissue engineering and has good clinical application prospect.
Owner:GENERAL HOSPITAL OF PLA
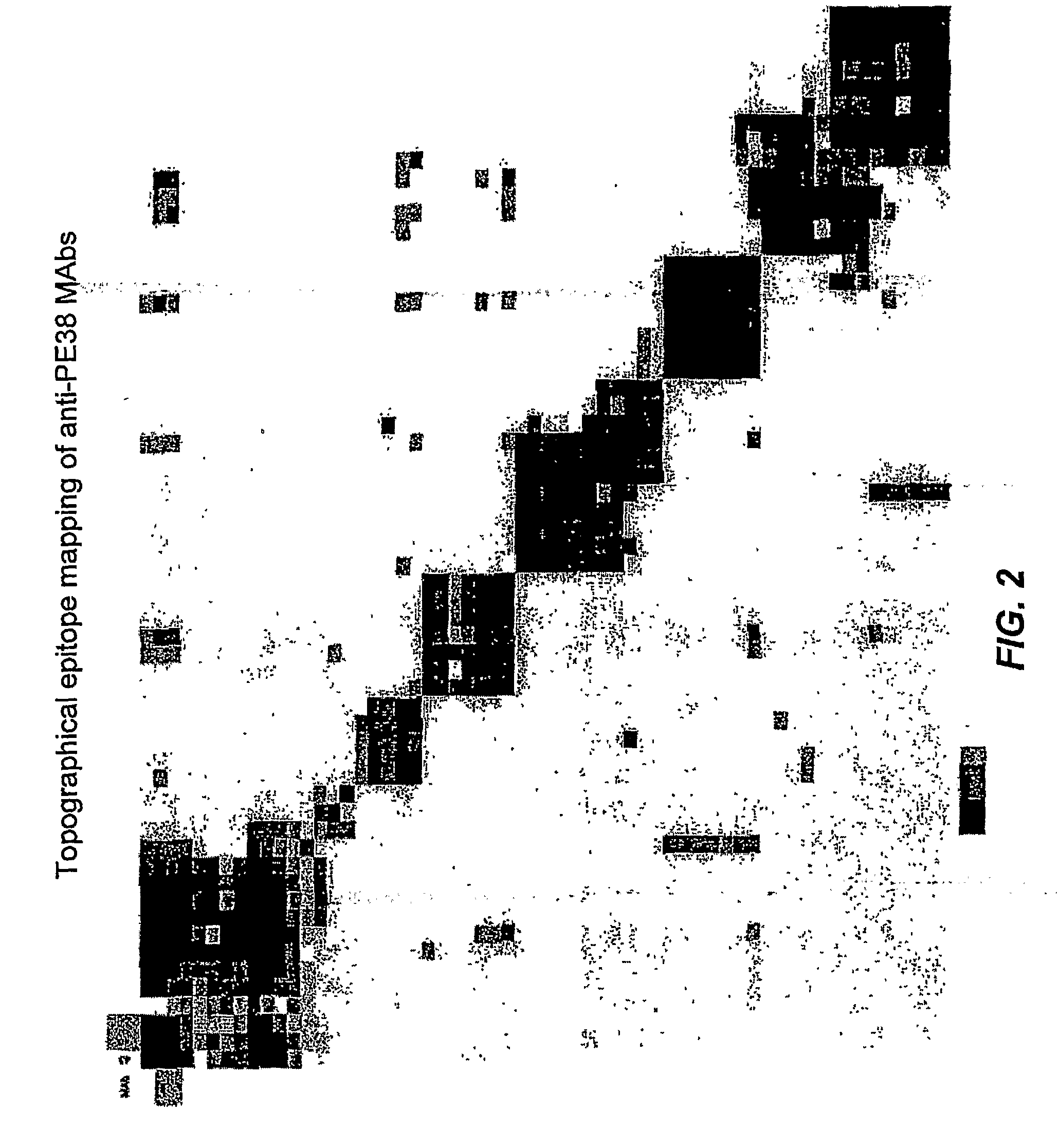
Mutated pseudomonas exotoxins with reduced antigenicity
ActiveUS20090142341A1Peptide/protein ingredientsBacteria peptidesPseudomonas aeruginosa exotoxin AEpitope
The invention provides mutated Pseudomonas exotoxins (PE) that have reduced immunogenicity compared to PEs containing the native sequence. The PEs of the invention have one or more individual mutations of positions of the native sequence of PE that reduce antibody binding to one or more PE epitopes. Nucleic acids encoding the mutated PEs, chimeric molecules comprising them, compositions comprising the chimeric molecules and methods of using them, are also provided.
Owner:UNITED STATES OF AMERICA
Guided tissue regeneration membrane and its preparation method
The invention relates to a guided tissue regeneration membrane, which comprises a compact coating and a loose coating, wherein the compact coating is composed of collagen-I or collagen-I composite hyaluronic acid; and the loose coating is composed of collagen-I or collagen-I composite calcium salt. A preparation method of the guided tissue regeneration membrane comprises the following steps of: preparing a collagen solution or a collagen and hyaluronic acid mixed solution by the use of acetic acid; injecting the solution into a self-made die, and drying it in the air to prepare the compact coating; injecting the collagen solution or a mixed liquor of the collagen solution and calcium salt onto the compact coating, and uniformly spreading; precooling the die at ultralow temperature, followed by freeze-drying to prepare the guided membrane with the compact coating and loose coating structure; carrying out vacuum high-temperature crosslinking and chemical crosslinking; and finally cleaning. The regeneration membrane has good histocompatibility and mechanical strength, low antigenicity and strong guided tissue regeneration capability. Its degradation rate in vivo is 3-8 months. The method is easy to operate, is suitable for automatic and continuous large-scale production, and solves the problems of large size of collagen membrane and material uniformity during industrial production.
Owner:SHENZHEN LANDO BIOMATERIALS
Tissue electro-sectioning apparatus
InactiveUS20050220674A1Increase the sectionIntense and localized fieldAutomatic control devicesAnalysis using chemical indicatorsFresh TissueGene and protein expression
An apparatus for sectioning fresh unfixed tissue into very thin layers with preserved tissue architecture, antigenicity, mRNA content, and amenable to 3-D computer reconstruction without mechanical or thermal damage by employing a sectioning tool having an electrode with an intense focused electrical field at an edge. A computer controlled x-y-z translation stage moves the sectioning tool through the tissue as defined by a predetermined program. The sectioning tool produces consecutive thin sections of fresh tissue for immunohistochemical and nucleic acids analyses without mechanical or thermal damage, ultimately allowing high-resolution volumetric reconstruction of gene and protein expression patterns of large tissue specimens. The geometry of the sectioning tool is selected so as to produce a spatially localized electrical field of sufficient intensity to sever molecular bonds or propagate flaws in tissue without mechanical cutting.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
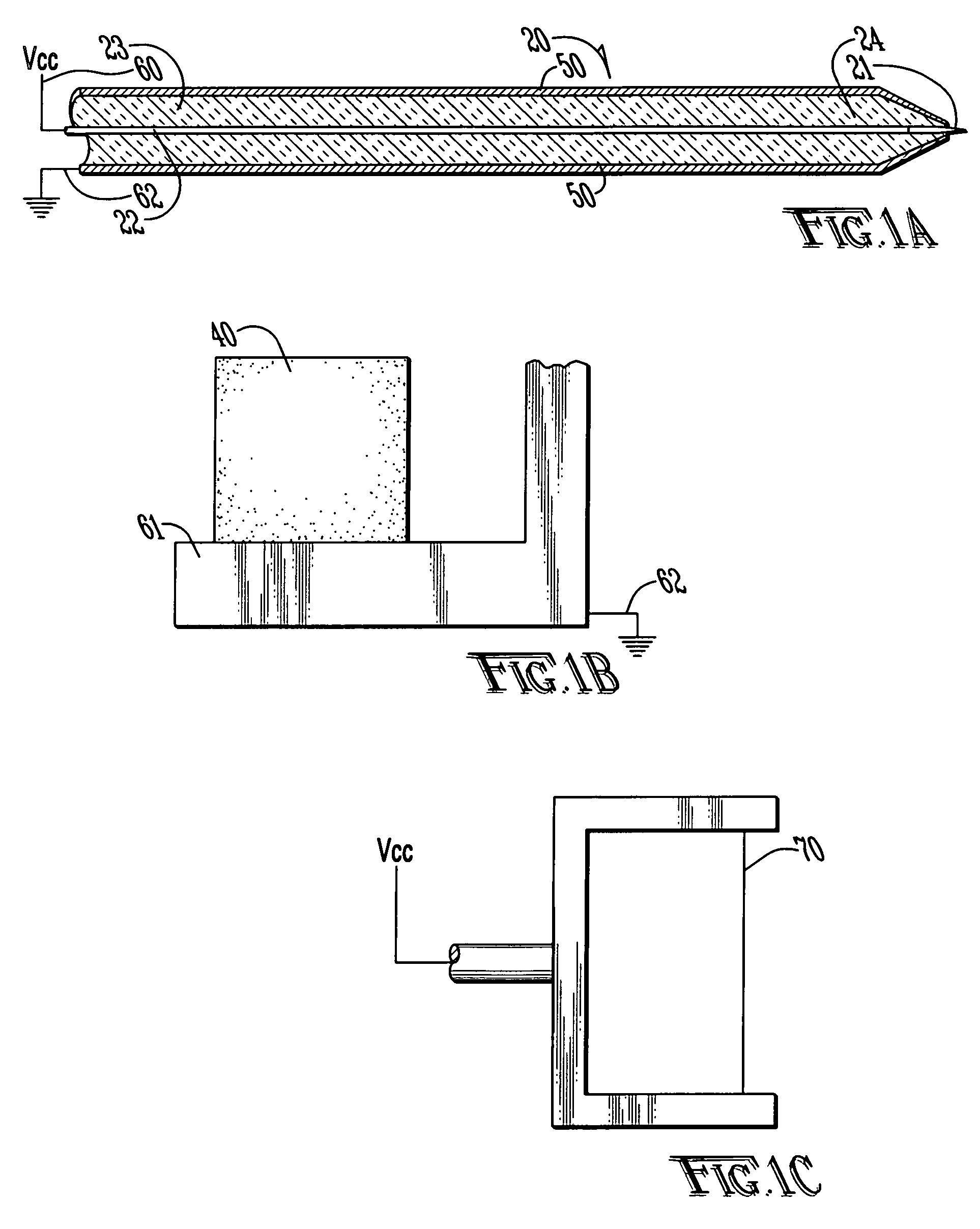
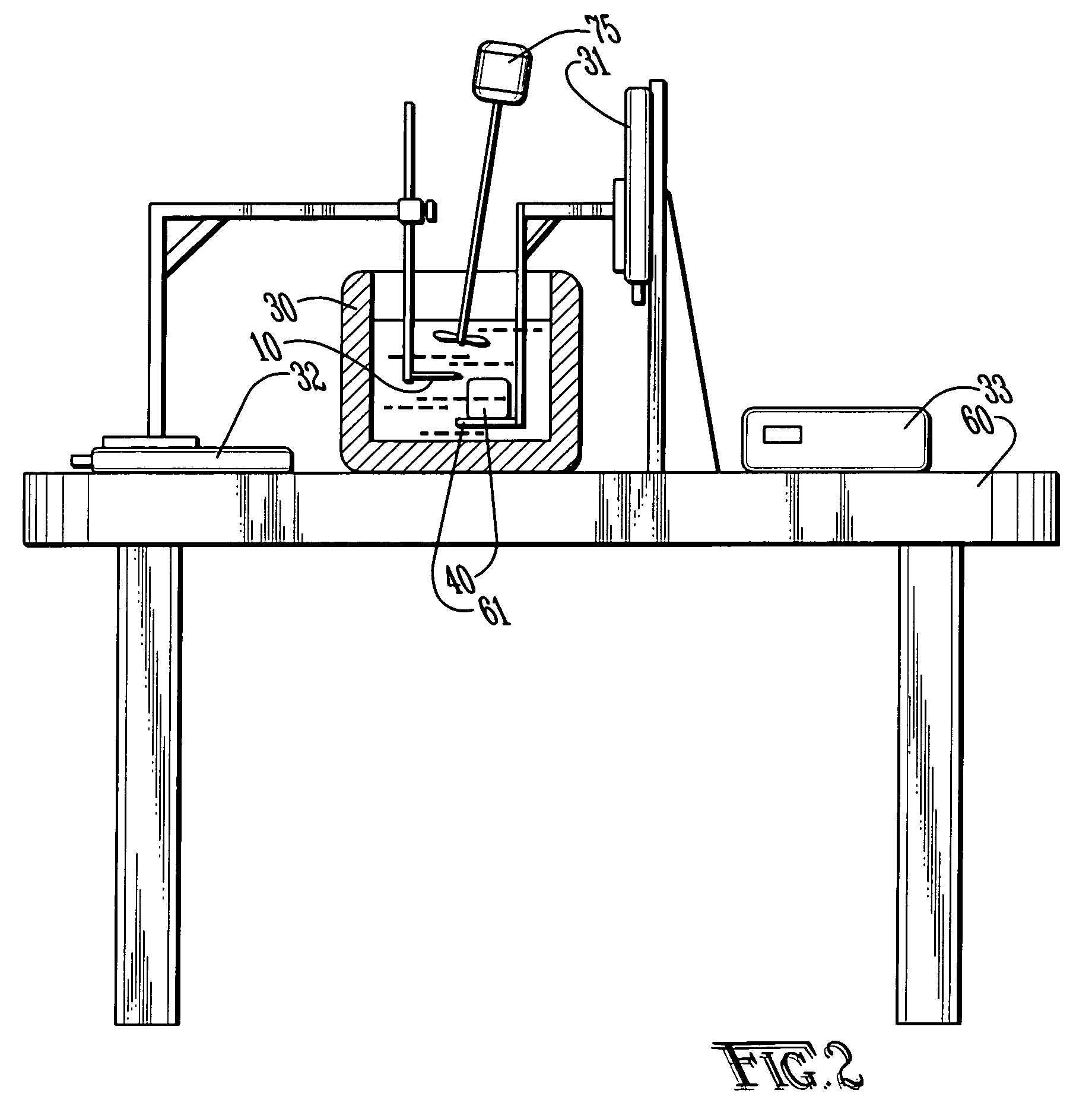
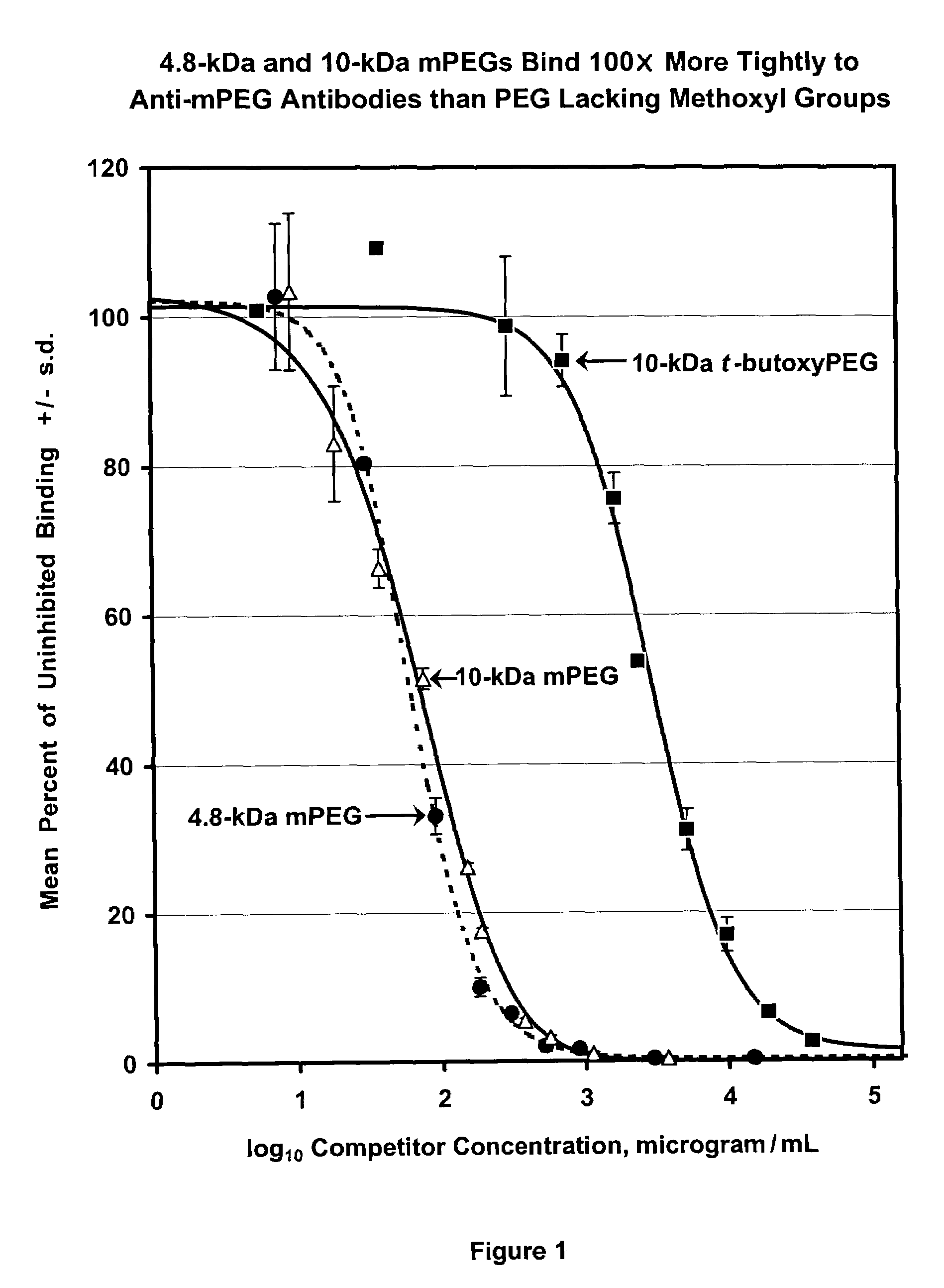
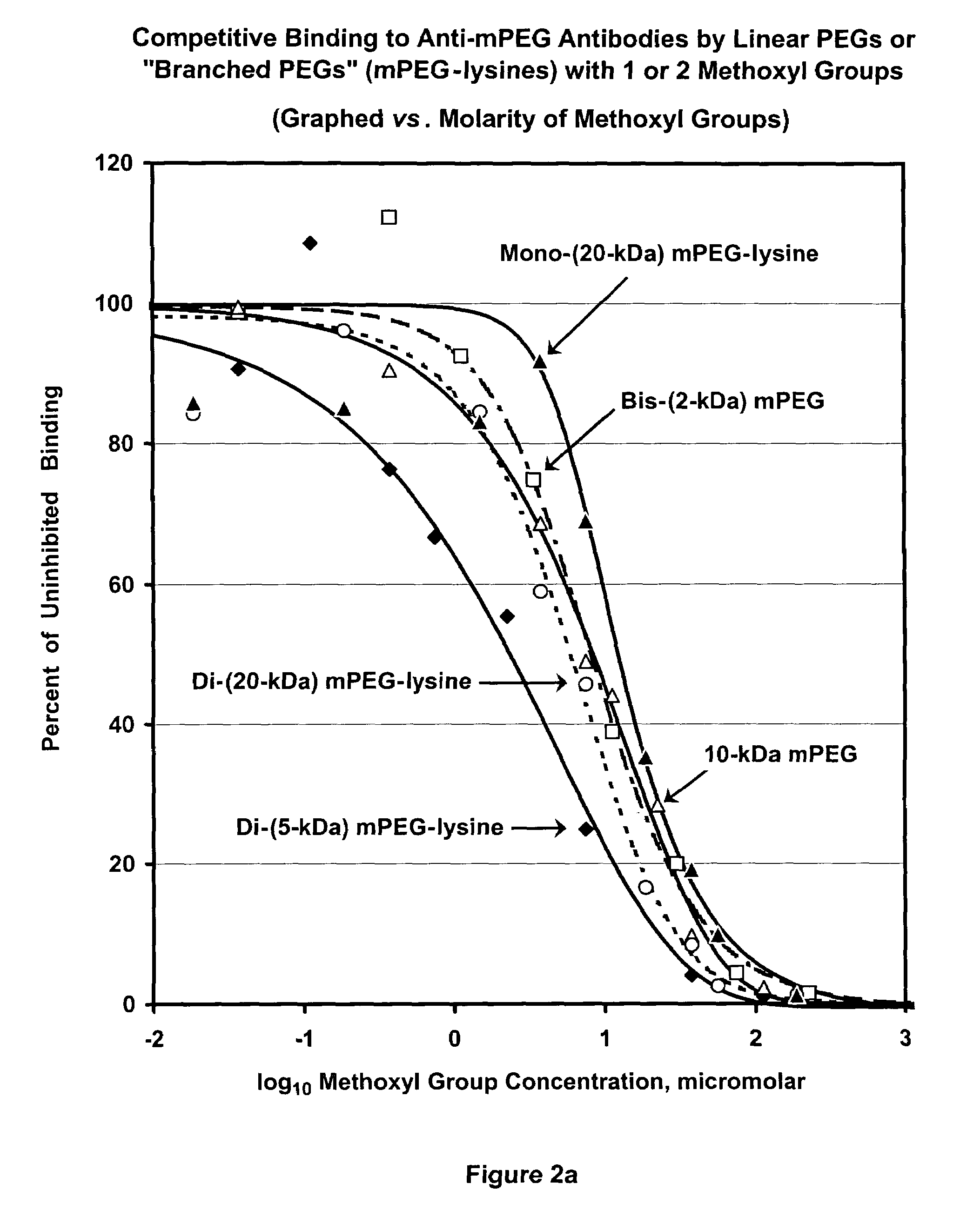
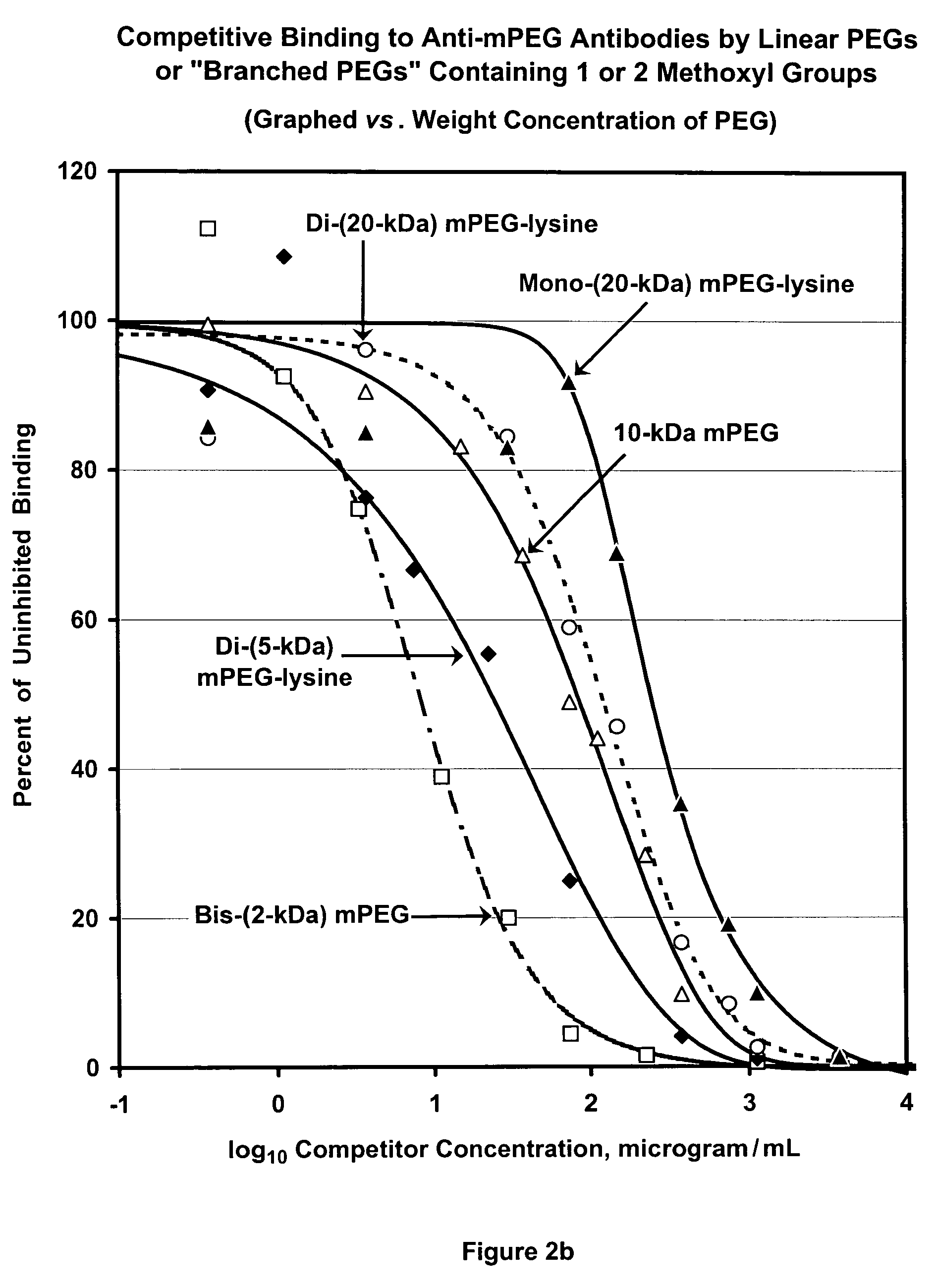
Polymer conjugates with decreased antigenicity, methods of preparation and uses thereof
ActiveUS8129330B2Reduced and substantially reduced antigenicityReduced and substantially reduced and undetectable antigenicity and immunogenicityAntibacterial agentsNervous disorderWater solubleImmunogenicity
Methods are provided for the preparation of conjugates of a variety of bioactive components, especially proteins, with water-soluble polymers (e.g., poly(ethylene glycol) and derivatives thereof), which conjugates have reduced antigenicity and immunogenicity compared to similar conjugates prepared using poly(ethylene glycol) containing a methoxyl or another alkoxyl group. The invention also provides conjugates prepared by such methods, compositions comprising such conjugates, kits containing such conjugates or compositions and methods of use of the conjugates and compositions in diagnostic and therapeutic protocols.
Owner:MOUNTAIN VIEW PHARMA
Vaccine formulations for intradermal delivery comprising adjuvants and antigenic agents
InactiveUS20060171917A1Improve therapeutic efficacyEnhance immune responseSsRNA viruses negative-senseBacterial antigen ingredientsAdjuvantImmunogenicity
The present invention relates to compositions for intradermal delivery of an antigenic or immunogenic agent in combination with one or more adjuvants. The immunogenic compositions of the invention comprise an antigenic or immunogenic agent and at least one adjuvant, which enhances the immune response to the antigenic or immunogenic agent, once delivered to the intradermal compartment of a subject's skin. The immunogenic compositions of the invention have enhanced efficacy as the adjuvants of the composition promote recruitment of antigen presenting cells to the intradermal compartment and thus enhance presentation and / or availability of the antigenic or immunogenic agent to the antigen presenting cells. The enhanced efficacy of the immunogenic compositions of the invention results in a therapeutically and / or prophylactically effective immune response after a single intradermal dose, with lower doses of adjuvant than conventionally used, achieving therapeutic efficacy from a single administration.
Owner:BECTON DICKINSON & CO
Porcine circovirus type 2 subunit vaccine and preparation method thereof
InactiveCN101884787AImprove biological activityHighly species-specificViral antigen ingredientsVirus peptidesOpen reading frameAntigenicity
The invention mainly aims to provide a porcine circovirus type 2 (PCV2) subunit vaccine and a preparation method thereof. Particularly, a baculovirus vector expression system is utilized to express a large amount of recombinant open reading frame type 2 (ORF2) protein in insect cells, so that the subunit vaccine with good immunity effect is developed. A bac-to-bac baculovirus expression system is adopted to perform whole gene amplification on the porcine circovirus type 2 ORF2 gene, and a melittin signal peptide nucleotide sequence is introduced into a terminal 5', so that the recombinant baculovirus of an open reading frame containing the melittin signal peptide nucleotide sequence and a PVC2ORF2 gene sequence is established, wherein the infected insect cell expresses the recombinant ORF2 protein with efficient and high PCV2 antigenicity; and thus the subunit vaccine containing the PCV2 recombinant ORF2 protein is prepared. The inoculation experiments of piglets show that the subunit vaccine has a good immunity protection effect.
Owner:PU LIKE BIO ENG
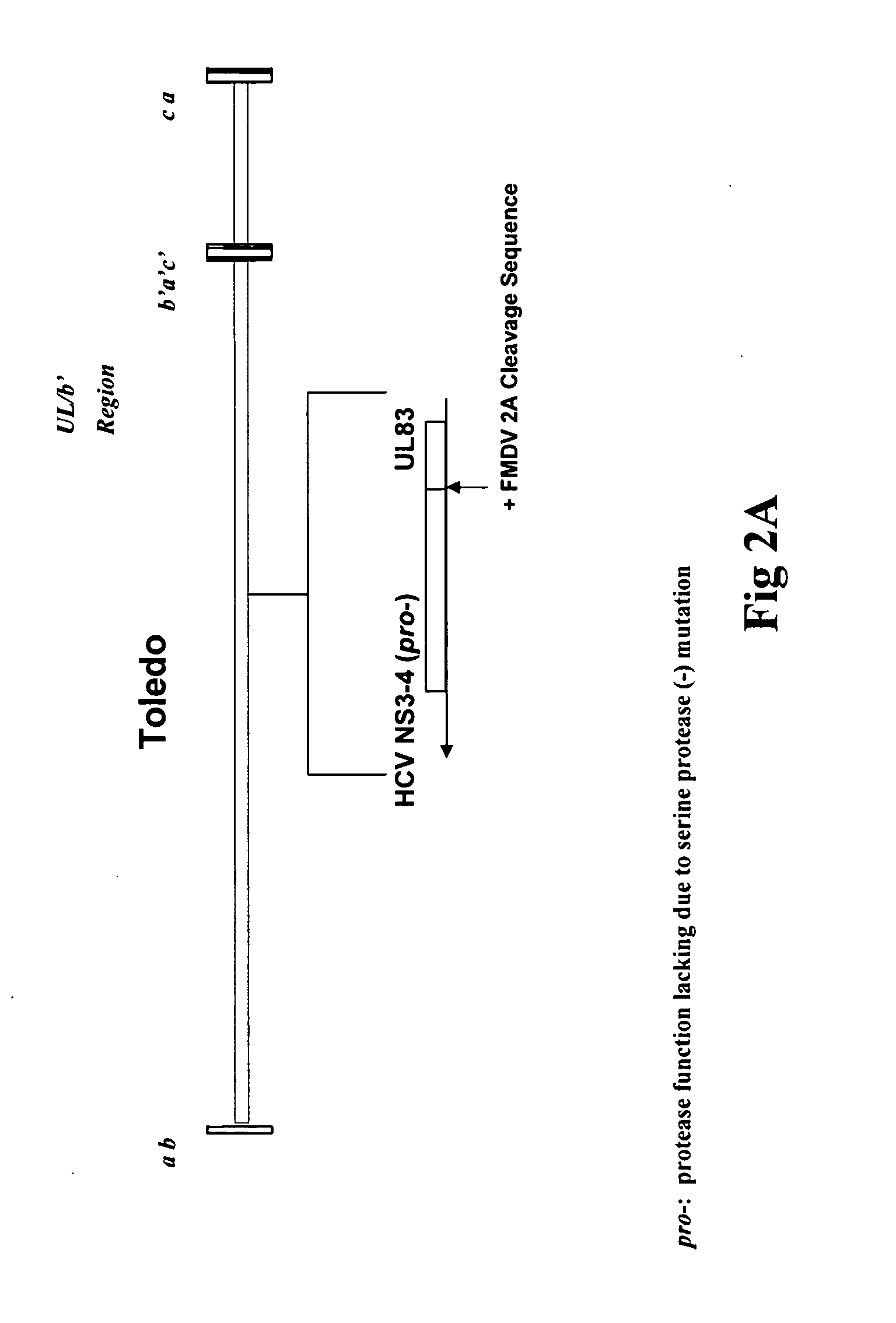
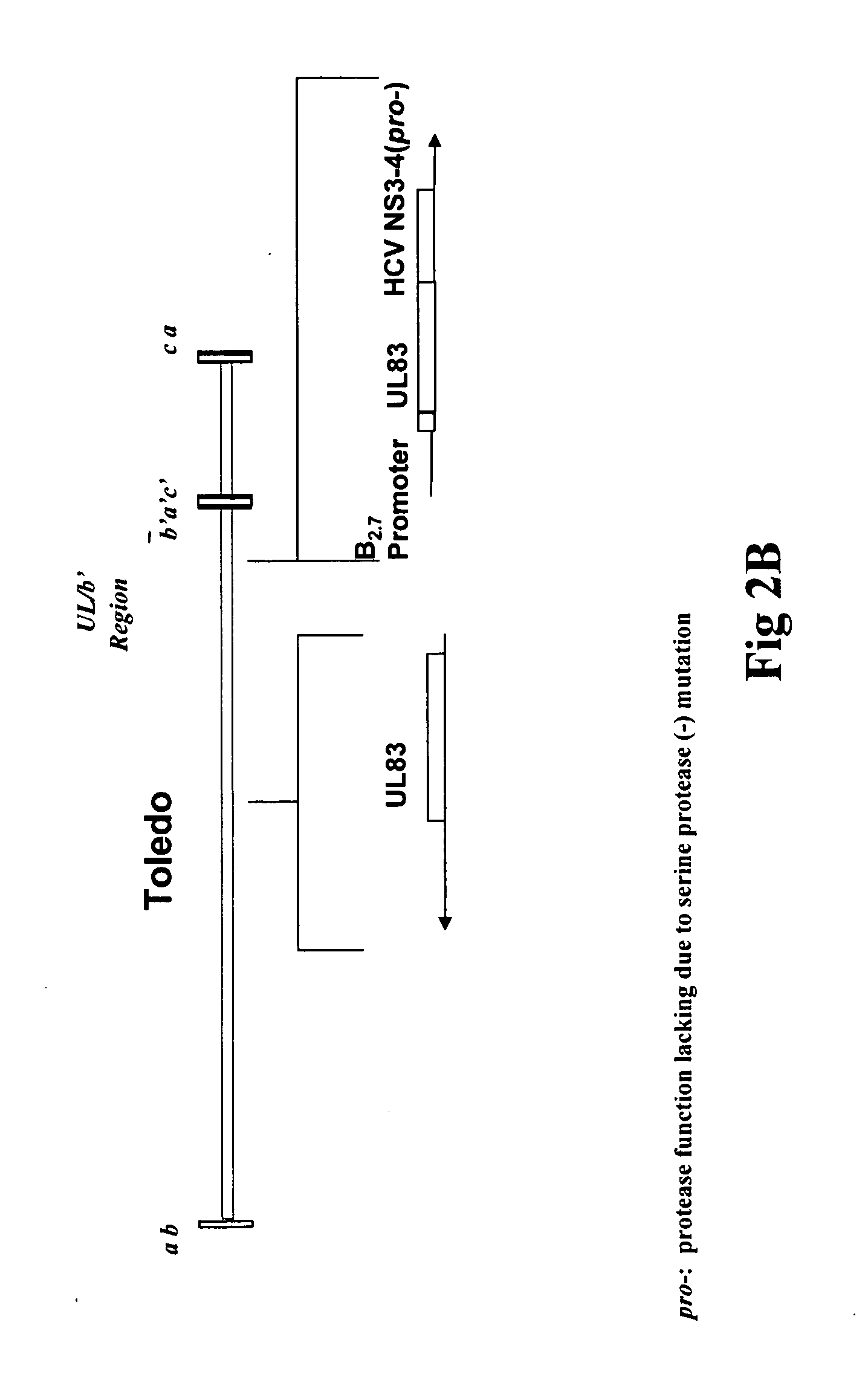
Recombinant human cytomegalovirus and vaccines comprising heterologous antigens
InactiveUS20090297555A1SsRNA viruses positive-senseViral antigen ingredientsHeterologousHeterologous Antigens
The present invention relates to recombinant HCMV (human cytomegalovirus) expressing a pp65 polypeptide or fragment thereof fused to a heterologous or non-native polypeptide, in particular immunogenic and / or antigenic polypeptides. In particular, the heterologous gene products include antigenic or immunogenic polypeptides from a variety of pathogens, cellular genes, tumor antigens, and viruses. The recombinant viruses may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Amino acid sequence of recombined human papilloma virus L1 capsid protein and uses thereof
InactiveCN101245099ABreak through the bottleneckControl the degree of aggregationBacteriaViral antigen ingredientsPentamerNucleotide
The invention relates to an amino acid sequence of a recombinant human papillomavirus L1 capsid protein, a nucleotide sequence which encodes the amino acid sequence and a recombinant vector and an expression host which contain the nucleotide sequence. The invention further relates to an application of a HPV L1 protein which is composed of the amino acid sequence in the preparation of vaccines, drug combination and diagnostic antigens or antibodies. The invention allows the recombinant HPV L1 capsid protein which is expressed in a prokaryotic system to be dissolvable in water by the modification of the HPV L1 wild-type sequence, and an L1 pentamer which has the same immunogenicity and antigenicity with the VLP of HPV L1 is obtained by expression. The invention allows the industrial production of the HPV L1 capsid protein by utilizing the prokaryotic expression system to become a reality, compared with the currently used eukaryotic expression system, the invention has the advantages of more stable quality of the products, higher yield, low cost and convenient quality control, which has great economic benefits and social effects.
Owner:马润林 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com