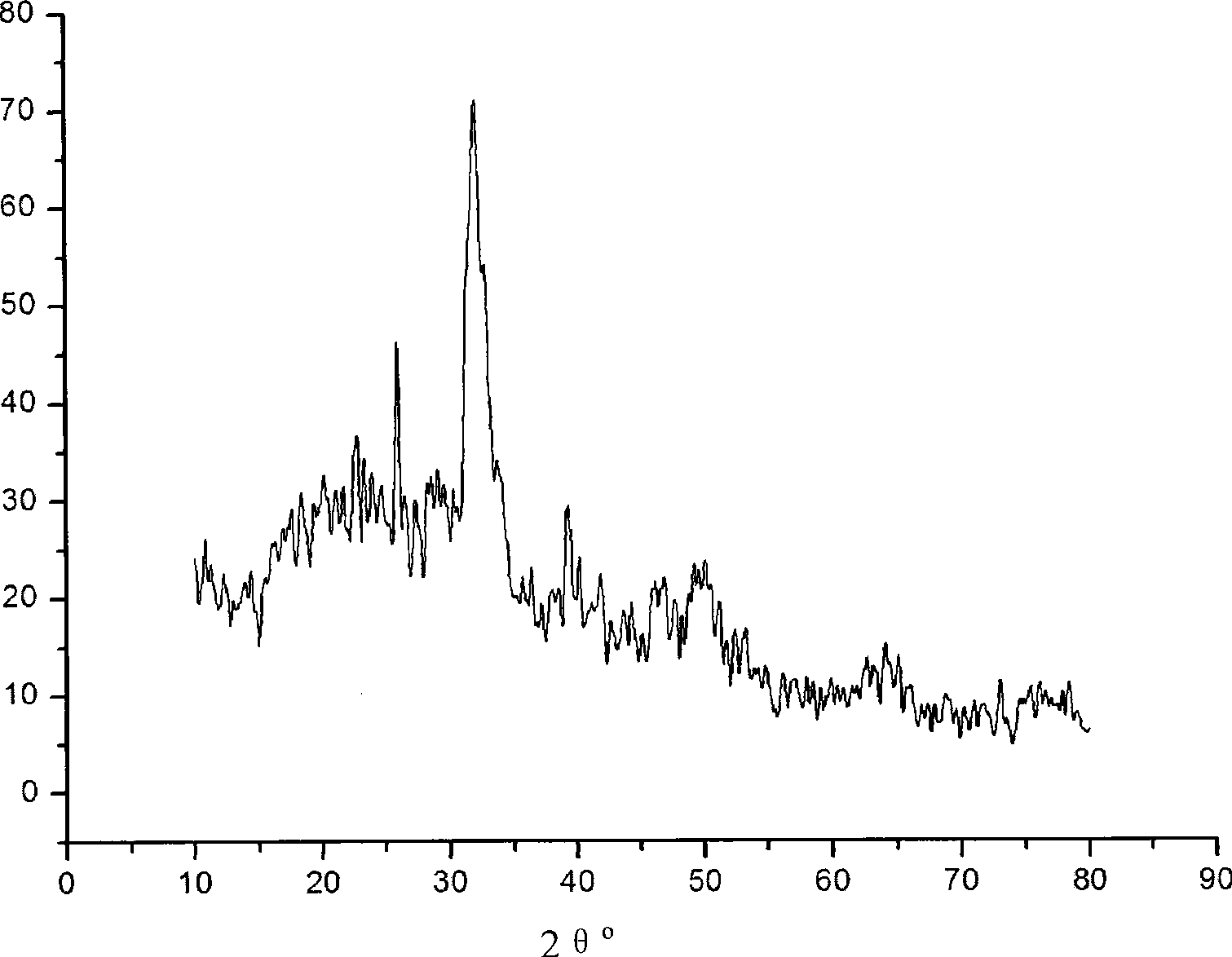
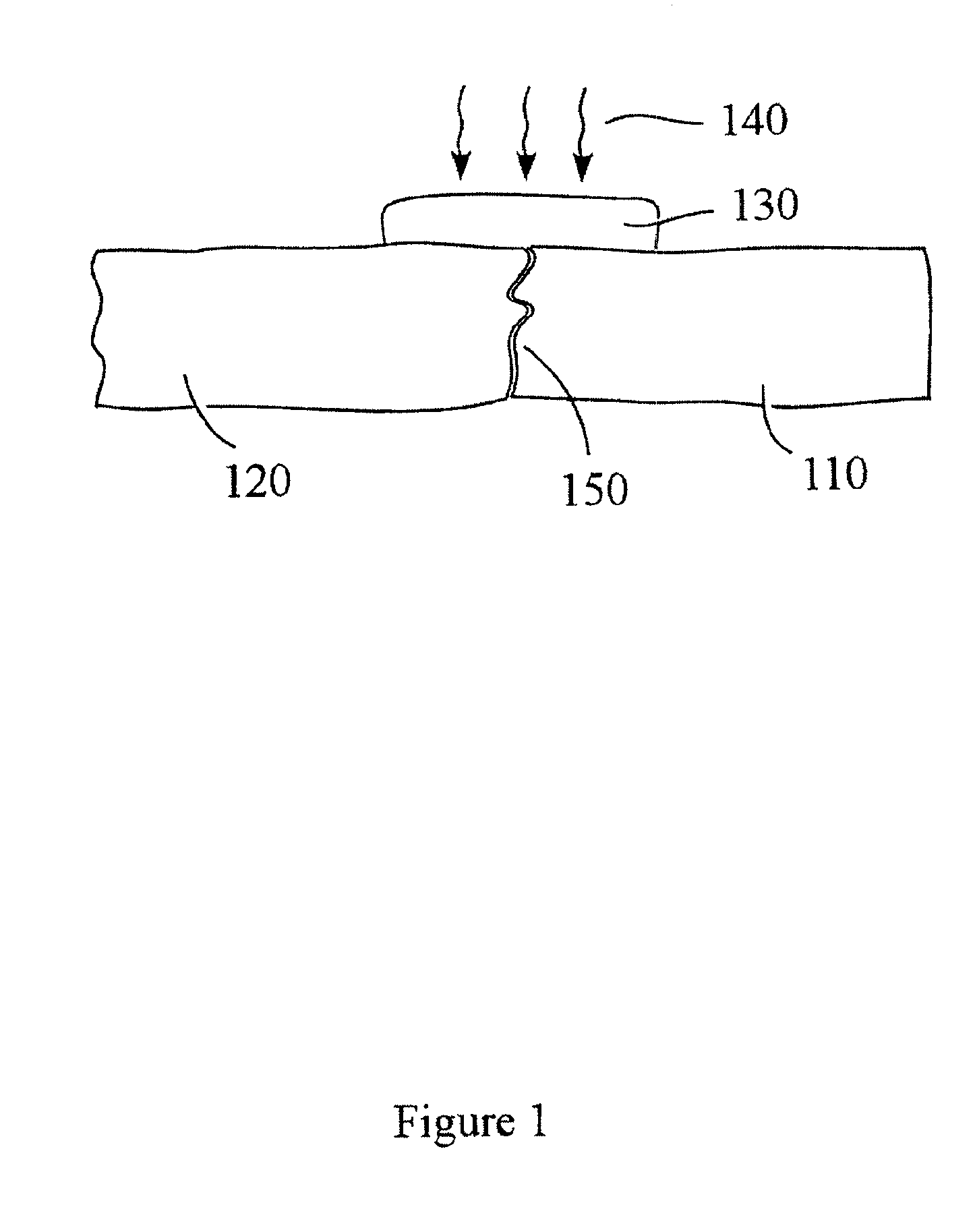
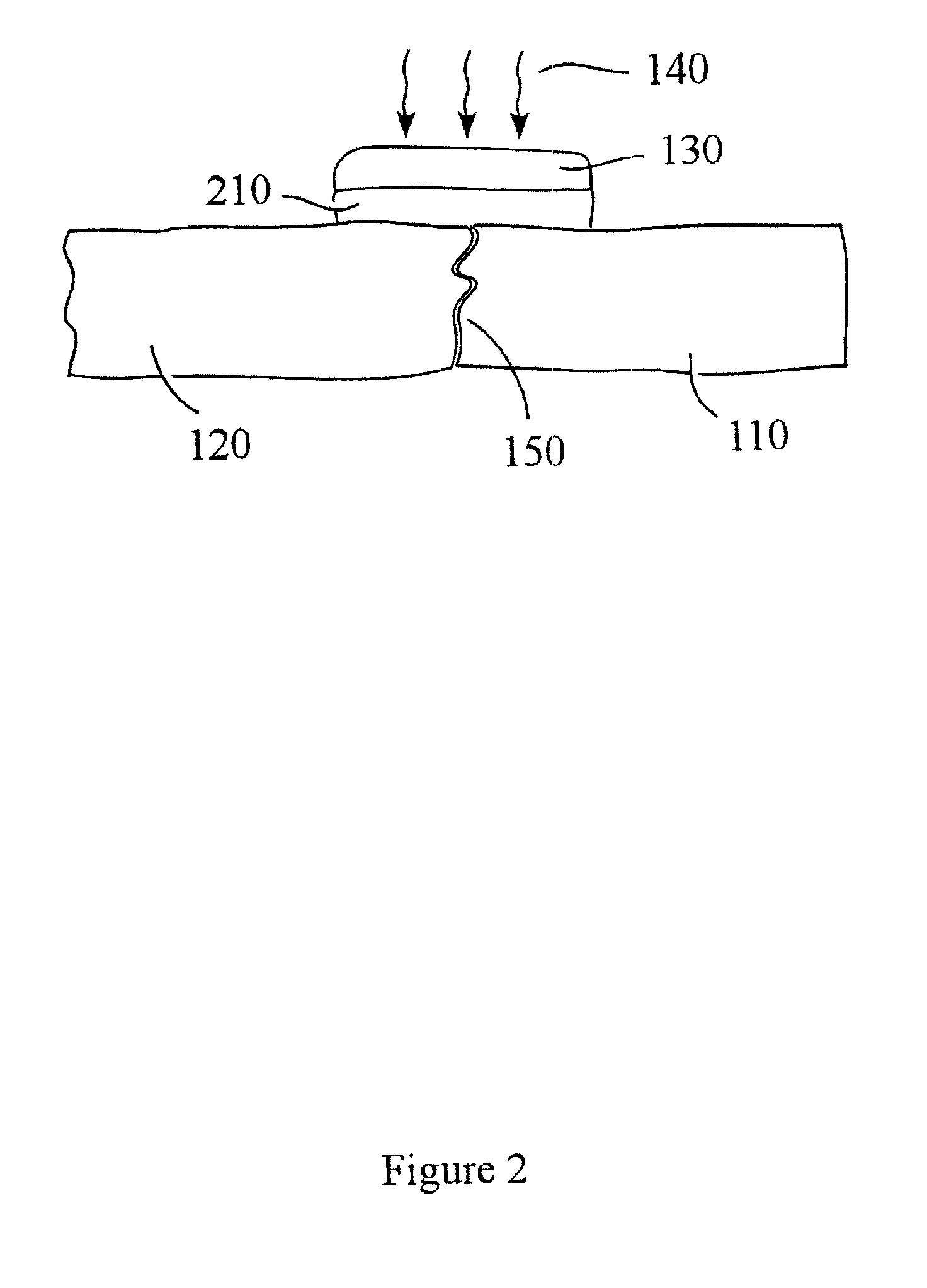
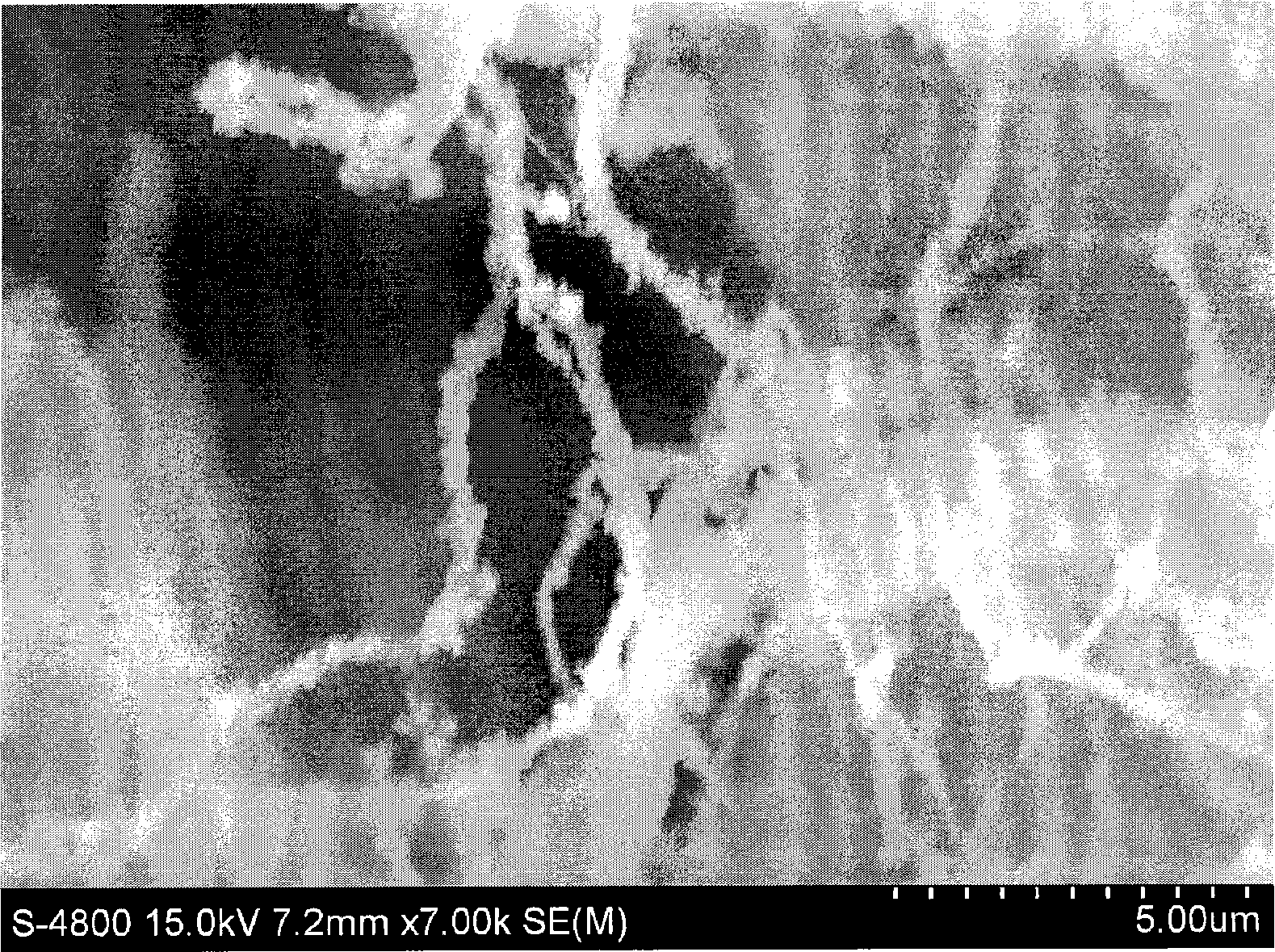Patents
Literature
361 results about "Type I collagen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Type I collagen is the most abundant collagen of the human body. It forms large, eosinophilic fibers known as collagen fibers. It is present in scar tissue, the end product when tissue heals by repair, as well as tendons, ligaments, the endomysium of myofibrils, the organic part of bone, the dermis, the dentin, and organ capsules.
Vertebral disc repair
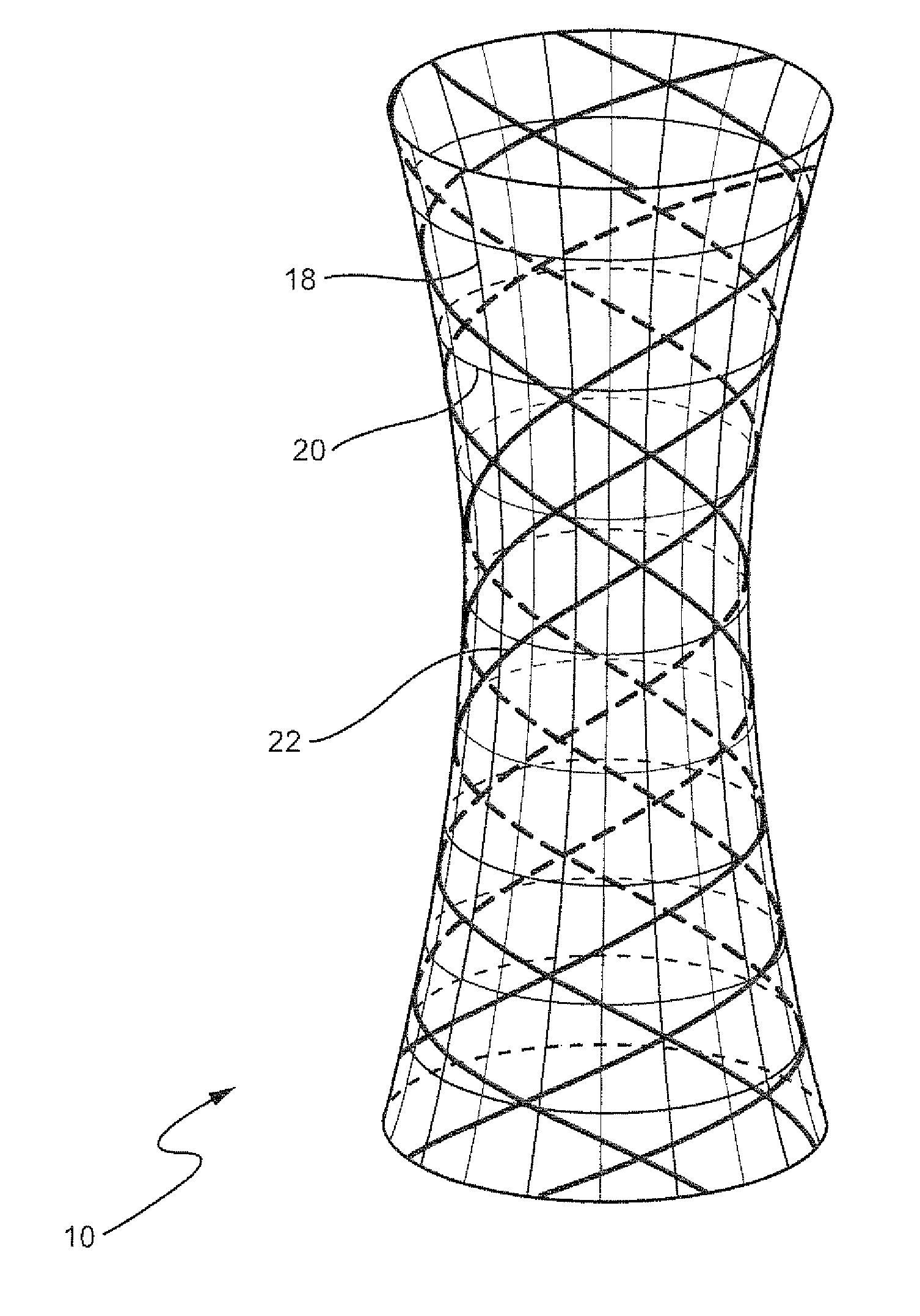
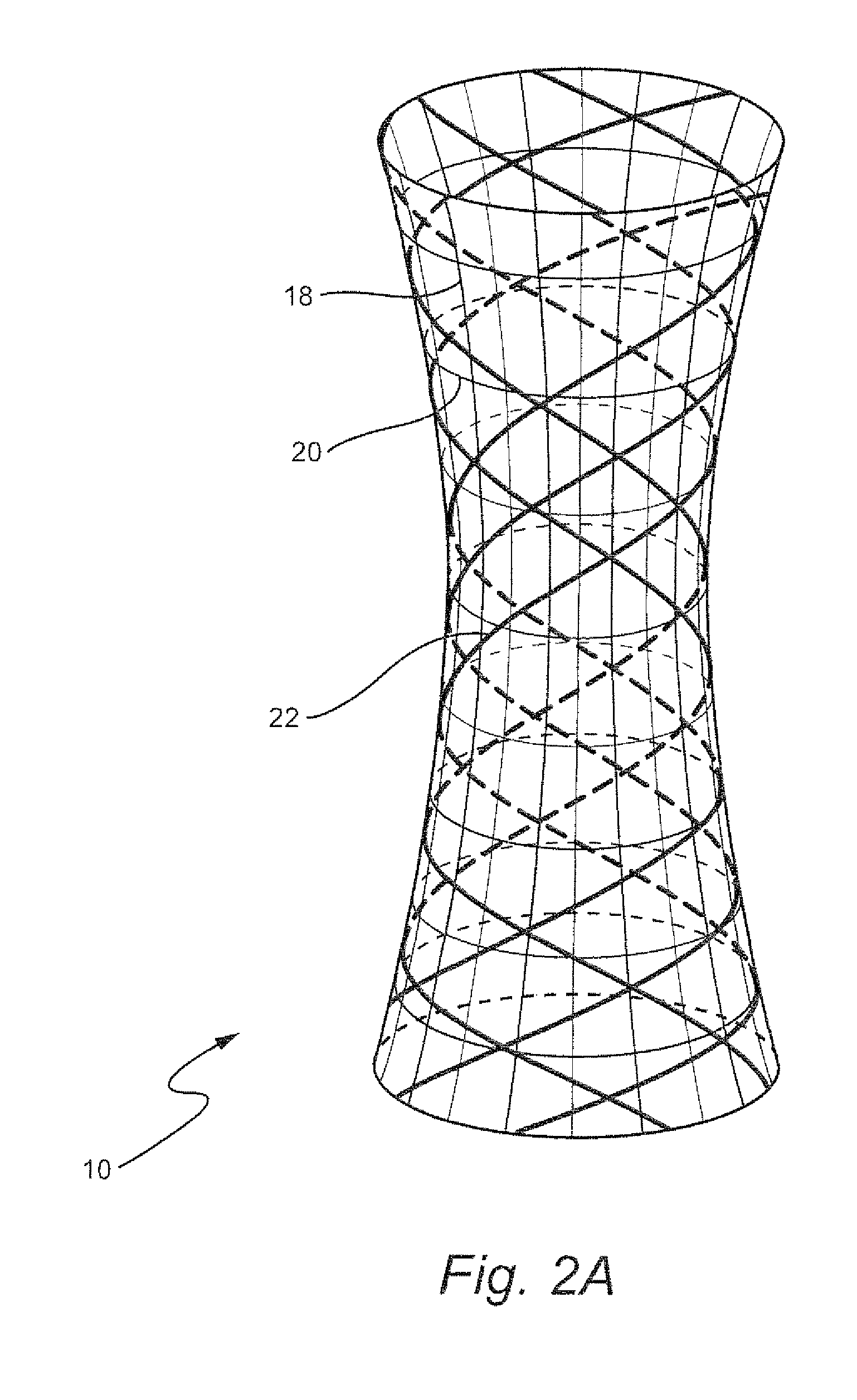
InactiveUS7879103B2Eliminate osteoinductivitySuture equipmentsBone implantShape-memory alloyBone Cortex
A sterile implant for treatment of a spinal disc defect comprising an allograft cortical bone demineralized to a Type I collagen having a specific shape which is treated to eliminate osteoinductivity. The implant is lyophilized and compressed into smaller first shape which 20 to 80% from its original shape in at least one dimension and hardened. The implant expanding when hydrated into a second shape having the shape memory of the first shape and expanded in dimensional size from the first compressed shape.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Composite tissue adhesive
InactiveUS6939364B1Precise temperature controlShorten treatment timeSurgical instrument detailsSurgical veterinaryThermal energyHigh concentration
Consistent with the present invention, tissue adhesive compositions and an associated laser exposure system are provided for bonding or sealing biological tissues. The compositions are comprised of chemically derivatized soluble collagen which is formulated to concentrations ranging from 300 mg / ml (30%) to 800 mg / ml (80%) collagen protein. In particular, Type I collagen, for example, is first prepared by extraction from bovine or porcine hide and purified. The collagen preparations are then chemically derivatized with sulfhydryl reagents to improve cohesive strength and with secondary derivatizing agents, such as carboxyl groups, to improve the adhesive strength of the solder to the tissue. The compositions are then formed into viscous solutions, gels or solid films, which when exposed to energy generated from an infrared laser, for example, undergo thermally induced phase transitions. Solid or semi-solid protein compositions become less viscous enabling the high concentration protein to penetrate the interstices of treated biological tissue or to fill voids in tissue. As thermal energy is released into the surrounding environment, the protein compositions again become solid or semi-solid, adhering to the treated tissue or tissue space.
Owner:CONVERSION ENERGY ENTERPRISES
Vertebral disc repair
InactiveUS20060235534A1Eliminate osteoinductivitySuture equipmentsBone implantBone CortexCortical bone
A sterile implant for treatment of a spinal disc defect comprising an allograft cortical bone demineralized to a Type I collagen having a specific shape which is treated to eliminate osteoinductivity. The implant is lyophilized and compressed into smaller first shape which 20 to 80% from its original shape in at least one dimension and hardened. The implant expanding when hydrated into a second shape having the shape memory of the first shape and expanded in dimensional size from the first compressed shape.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Integral engineering rack of interface osteochondro tissue with bionic function
InactiveCN101020083AIncrease connection areaImprove connection strengthJoint implantsSubchondral boneBiocompatibility Testing
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Nanofibrous biocomposite prosthetic vascular graft
The present invention provides a bioactive, small-diameter (typically less than 6 mm in internal diameter) vascular graft prosthesis, and is a textile conduit preferably manufactured using a novel electrospinning perfusion methodology. One preferred embodiment is a nanofibrous biocomposite textile conduit which comprises a prepared liquid admixture of polyester (Dacron), a biodurable implantable synthetic polymer, and Type IV collagen, an extracellular matrix protein. This prepared admixture and blending of diverse fibrous matter is utilized in a novel electrospinning perfusion process to form a small-diameter (less than 6 mm) fabricated textile conduit, a discrete article of manufacture, which then serves as an antecedent tangible workpiece for a subsequently-made prosthetic vascular graft construct.
Owner:BIOSURFACES +2
Composition for acceleration of type i collagen production
InactiveUS20090325885A1Good effectPromotes collagen productionOrganic active ingredientsCosmetic preparationsHuman skin fibroblastOrganic chemistry
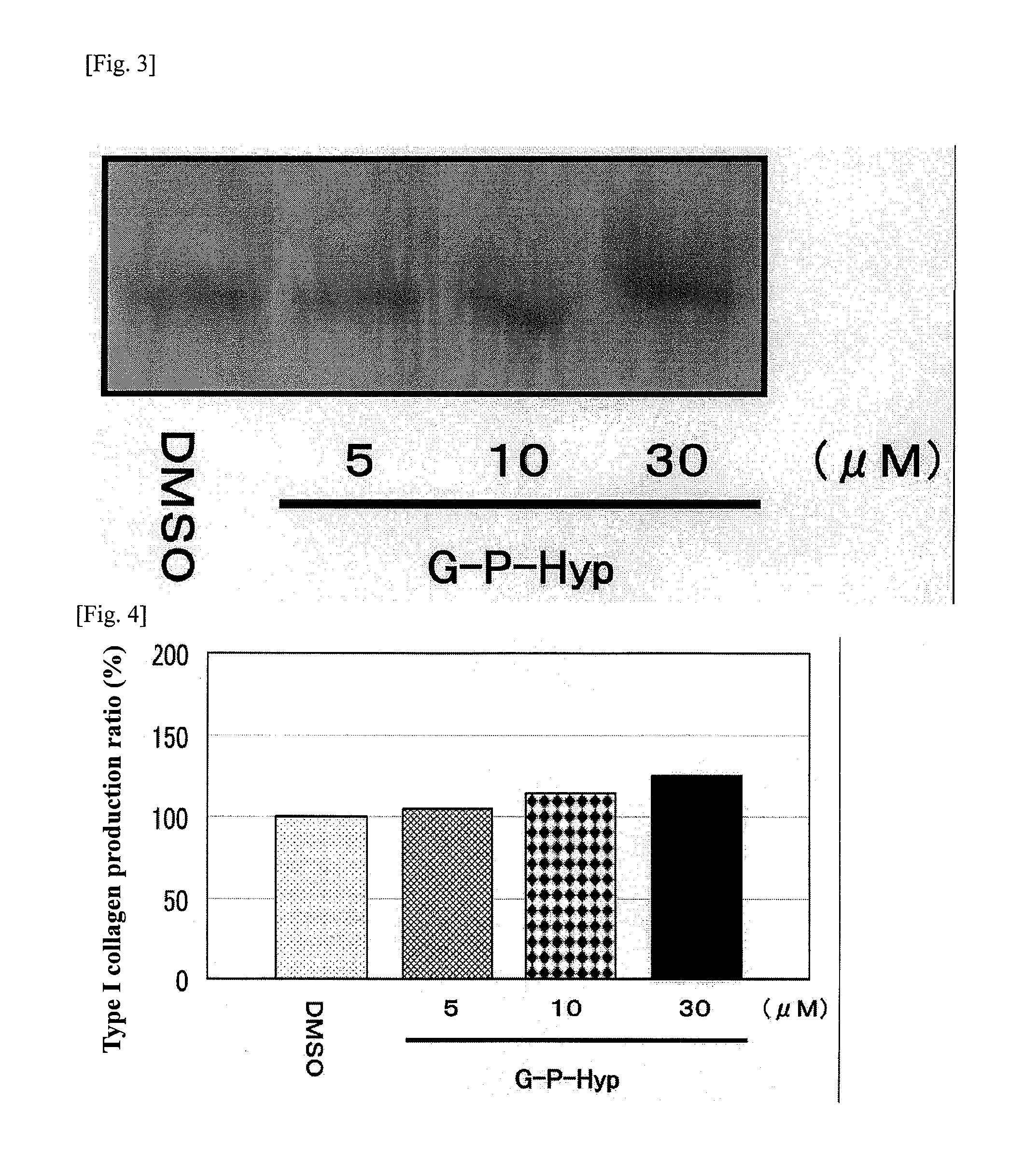
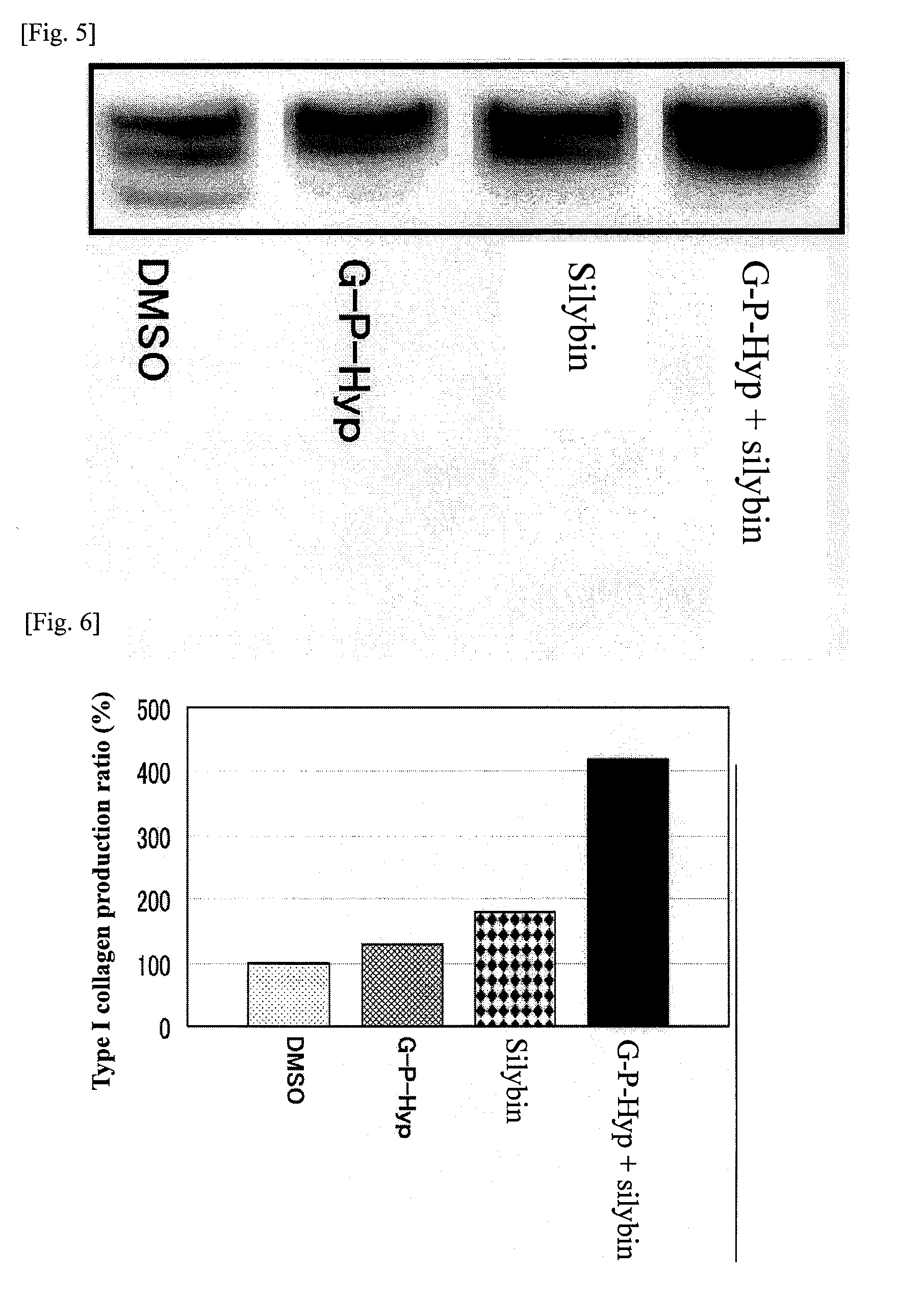
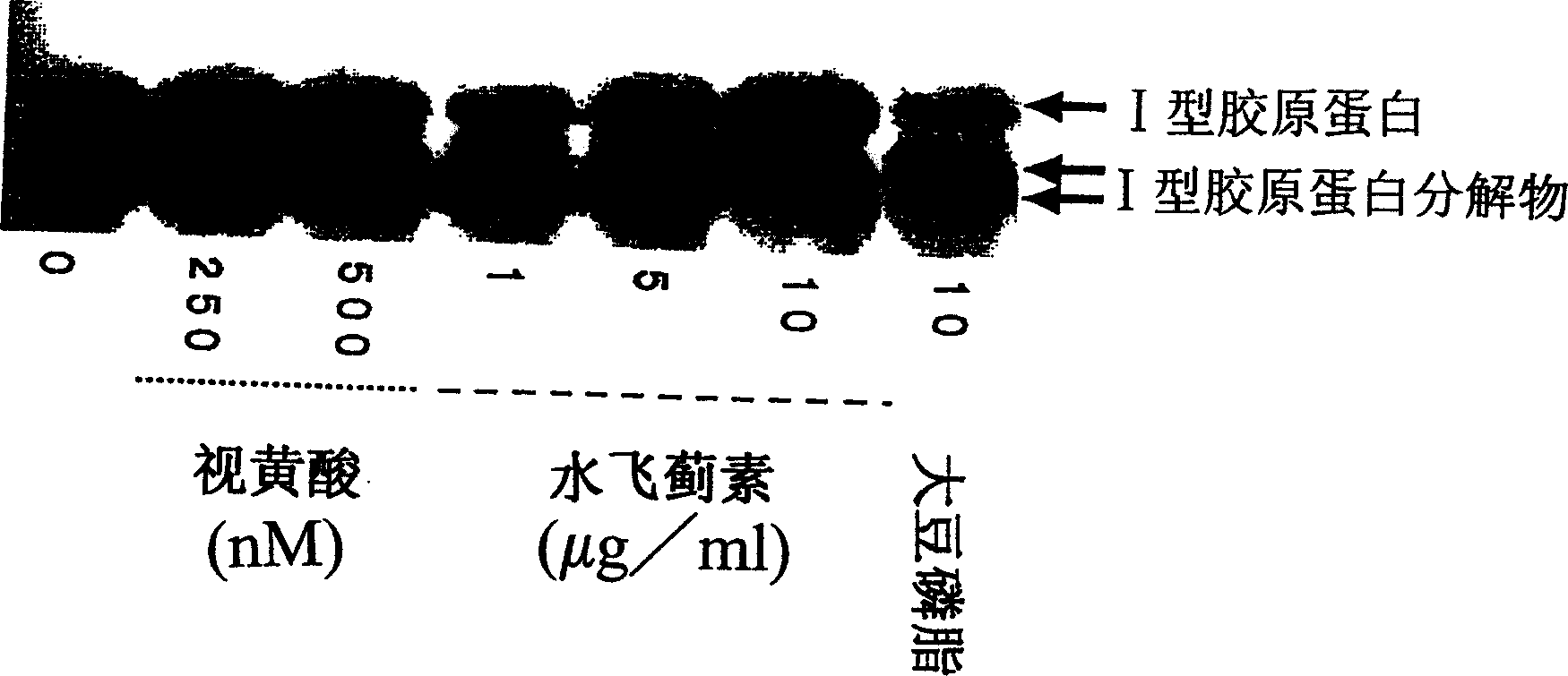
It is an object of the present invention to provide a composition for promoting the production of type I collagen by human skin fibroblasts, and the present invention relates to a composition for promoting type I collagen production containing silybin and a peptide that promotes collagen production (peptide having an amino acid sequence of Gly-Pro-Hyp, Gly-His-Lys, Lys-Thr-Thr-Lys-Ser or Gly-Glu-Pro-Arg).
Owner:FUAN KERU
Bracket material for bone tissue engineer and preparation method thereof
InactiveCN101417145AImprove mechanical propertiesGood biocompatibilityProsthesisDrug biological activityProtein C
The invention relates to a bracket material used for an osseous tissue project, and a preparation method thereof. Firstly, the bracket of a type I collagen is extracted from a small fresh pigskin by a mature extracting technique. Then, the bracket is further expanded in a Tris cushion liquid, the pH of which is equal to 8.8 to obtain a natural porous collagen bracket by the treatments of freezing and drying. The bracket is respectively and repeatedly mineralized in a CaCl2 liquid and in (NH4)2HPO4 liquid or mineralized in simulated body fluid for a long period to lead the weakly crystallized HA to be uniformly settled into the collagen bracket; and then a pigskin collagen-hydroxyapatite ossein is obtained by the treatments of freezing and drying to replace the natural bracket material. The invention not only maintains the natural bracket structure of the collagen in an organism, but also has the advantages of low material cost, simple devices, short period and easy operation. The obtained compound bracket material used for the pigskin collagen-hydroxyapatite osseous tissue project has the characteristics of high intensity, large toughness, non-antigenicity, higher biological activity as well as degradation and releasing control.
Owner:SHANDONG UNIV
Light energized tissue adhesive
InactiveUS6875427B1Lower requirementPrecise temperature controlBiocideSurgical adhesivesHigh concentrationThermal energy
Consistent with the present invention, tissue adhesive compositions and an associated laser exposure system are provided for bonding or sealing biological tissues. The compositions are comprised of chemically derivatized soluble collagen which is formulated to concentrations ranging from 300 mg / ml (30%) to 800 mg / ml (80%) collagen protein. In particular, Type I collagen, for example, is first prepared by extraction from bovine or porcine hide and purified. The collagen preparations are then chemically derivatized with sulfhydryl reagents to improve cohesive strength and with secondary derivatizing agents, such as carboxyl groups, to improve the adhesive strength of the solder to the tissue. The compositions are then formed into viscous solutions, gels or solid films, which when exposed to energy generated from an infrared laser, for example, undergo thermally induced phase transitions. Solid or semi-solid protein compositions become less viscous enabling the high concentration protein to penetrate the interstices of treated biological tissue or to fill voids in tissue. As thermal energy is released into the surrounding environment, the protein compositions again become solid or semi-solid, adhering to the treated tissue or tissue space.
Owner:CONVERSION ENERGY ENTERPRISES
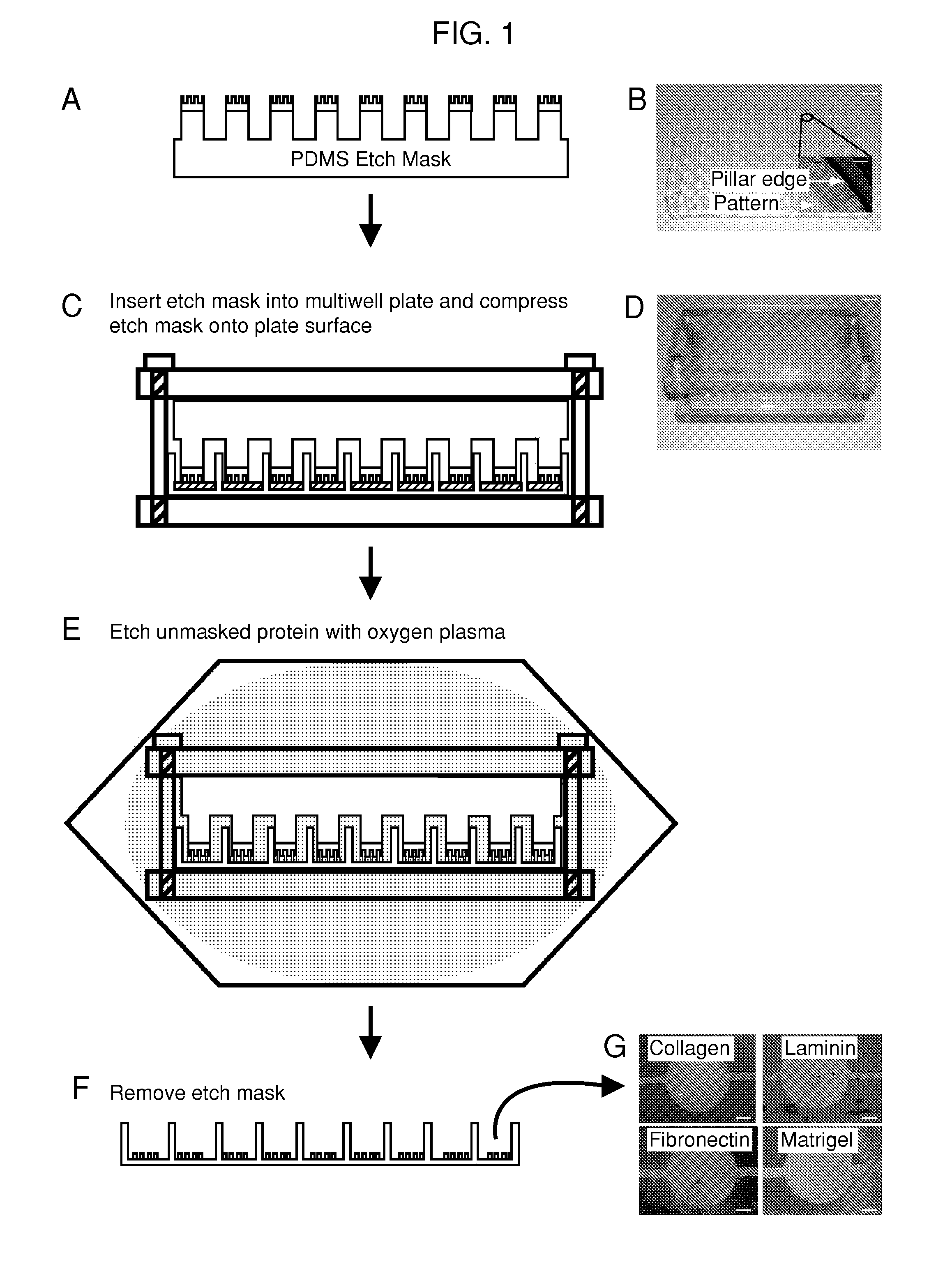
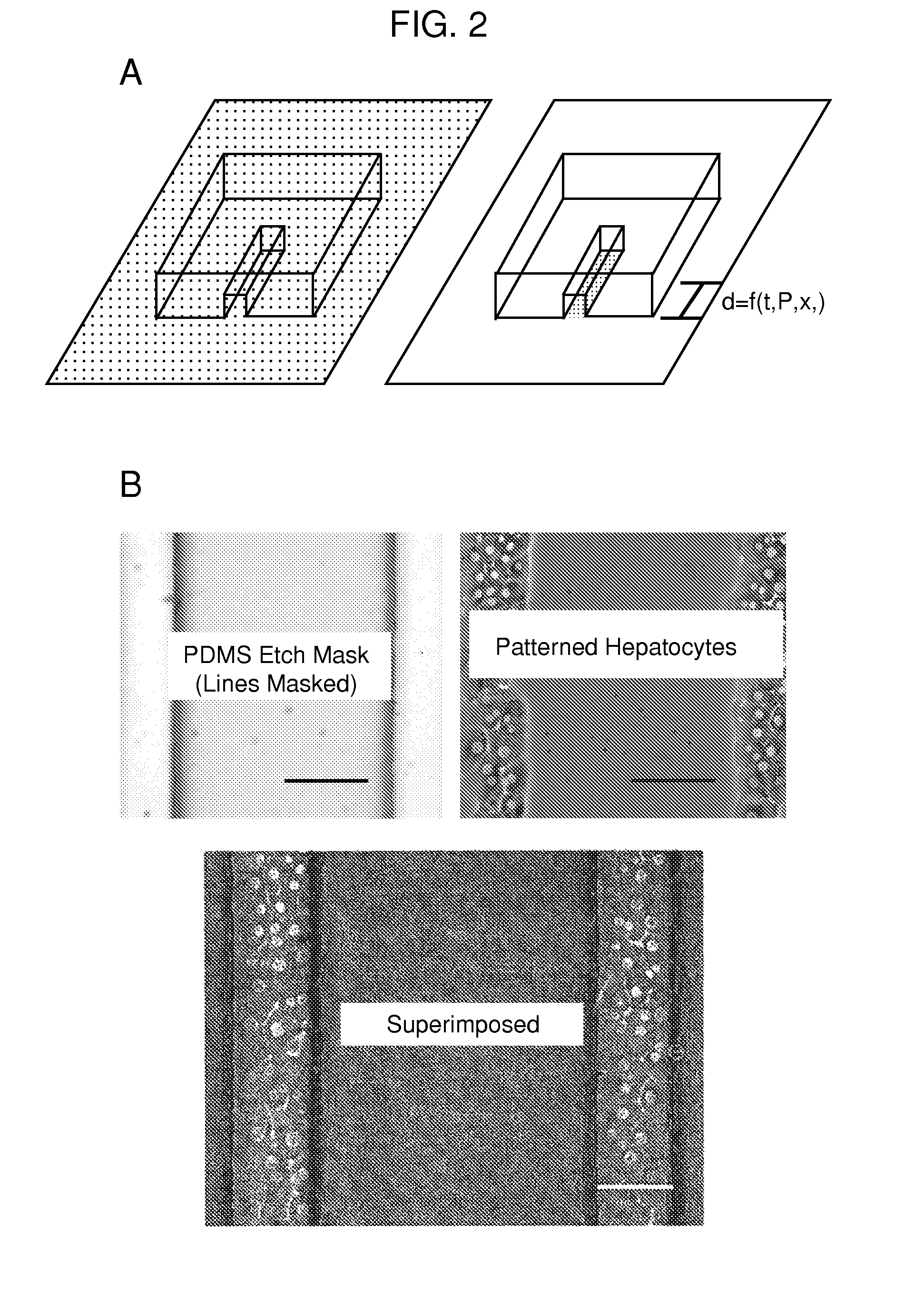
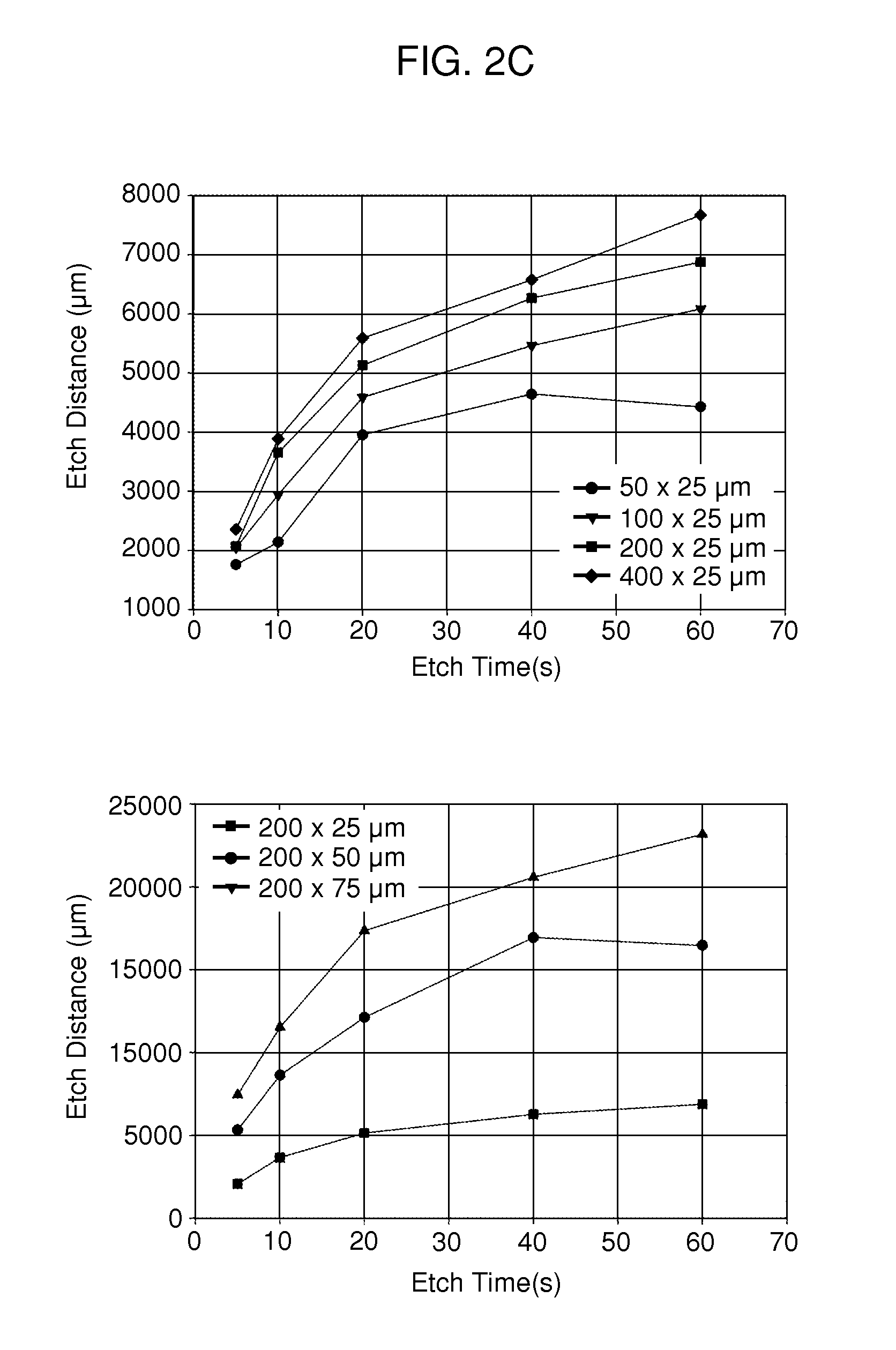
Multi-well micropatterning by ablation
InactiveUS20080220516A1Without increasing experimental complexitySolve the practicality is not highBioreactor/fermenter combinationsSequential/parallel process reactionsMatrigelAlternative methods
The present invention is drawn to the generation of micropatterns of biomolecules and cells on standard laboratory materials through selective ablation of a physisorbed biomolecule with oxygen plasma. In certain embodiments, oxygen plasma is able to ablate selectively physisorbed layers of biomolecules (e.g., type-I collagen, fibronectin, laminin, and Matrigel) along complex non-linear paths which are difficult or impossible to pattern using alternative methods. In addition, certain embodiments of the present invention relate to the micropatterning of multiple cell types on curved surfaces, multiwell plates, and flat bottom flasks. The invention also features kits for use with the subject methods.
Owner:MASSACHUSETTS INST OF TECH
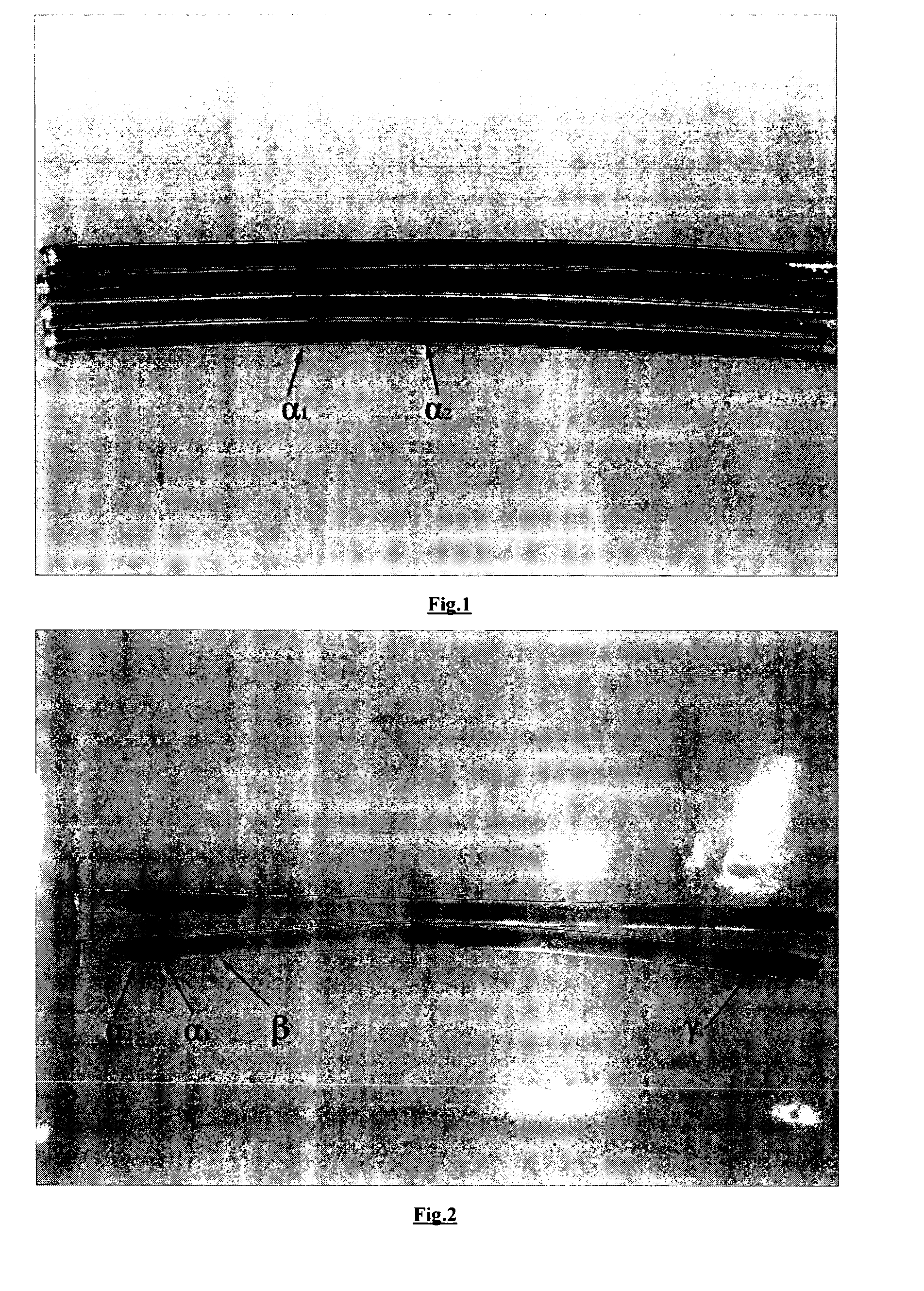
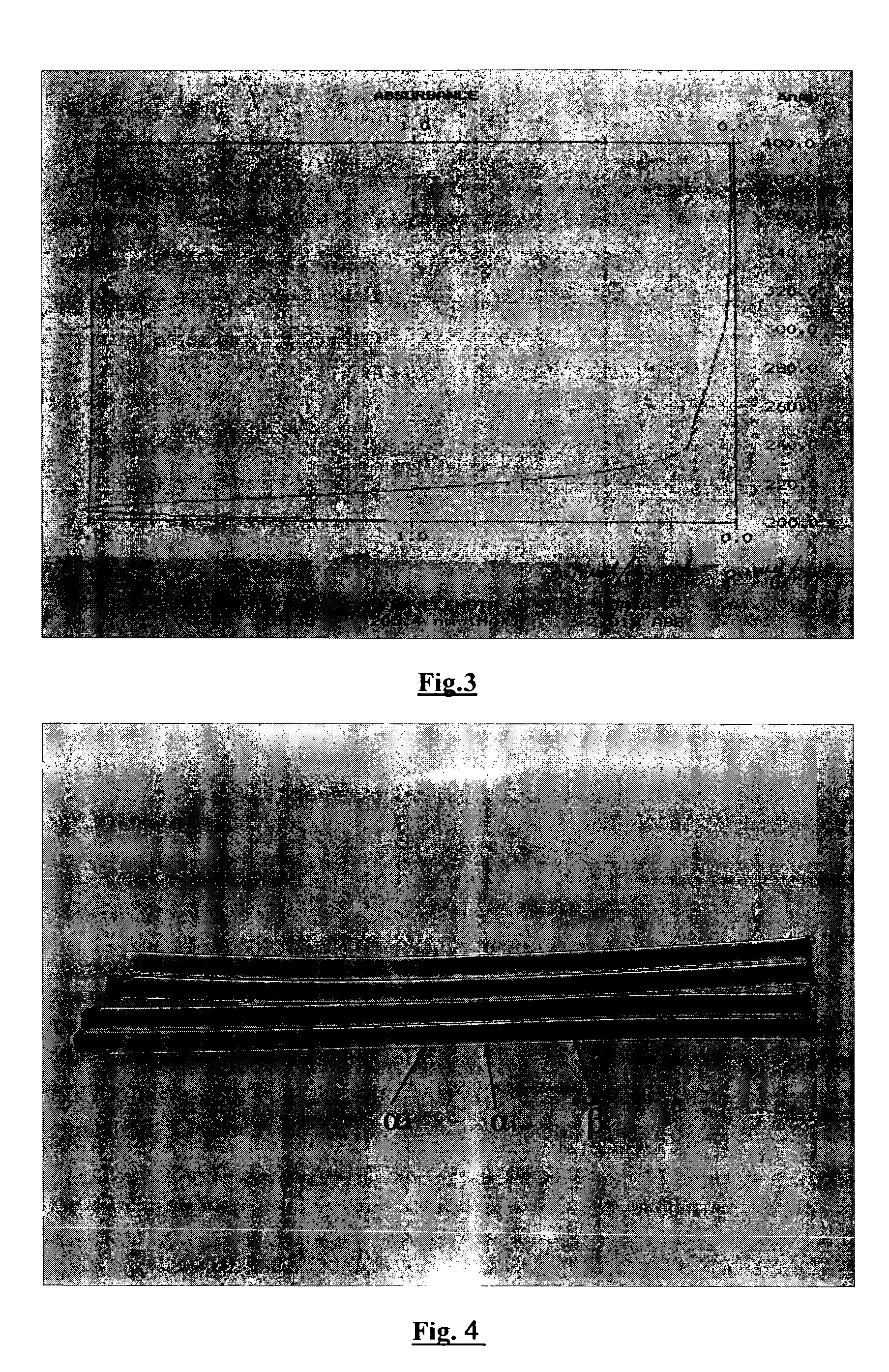
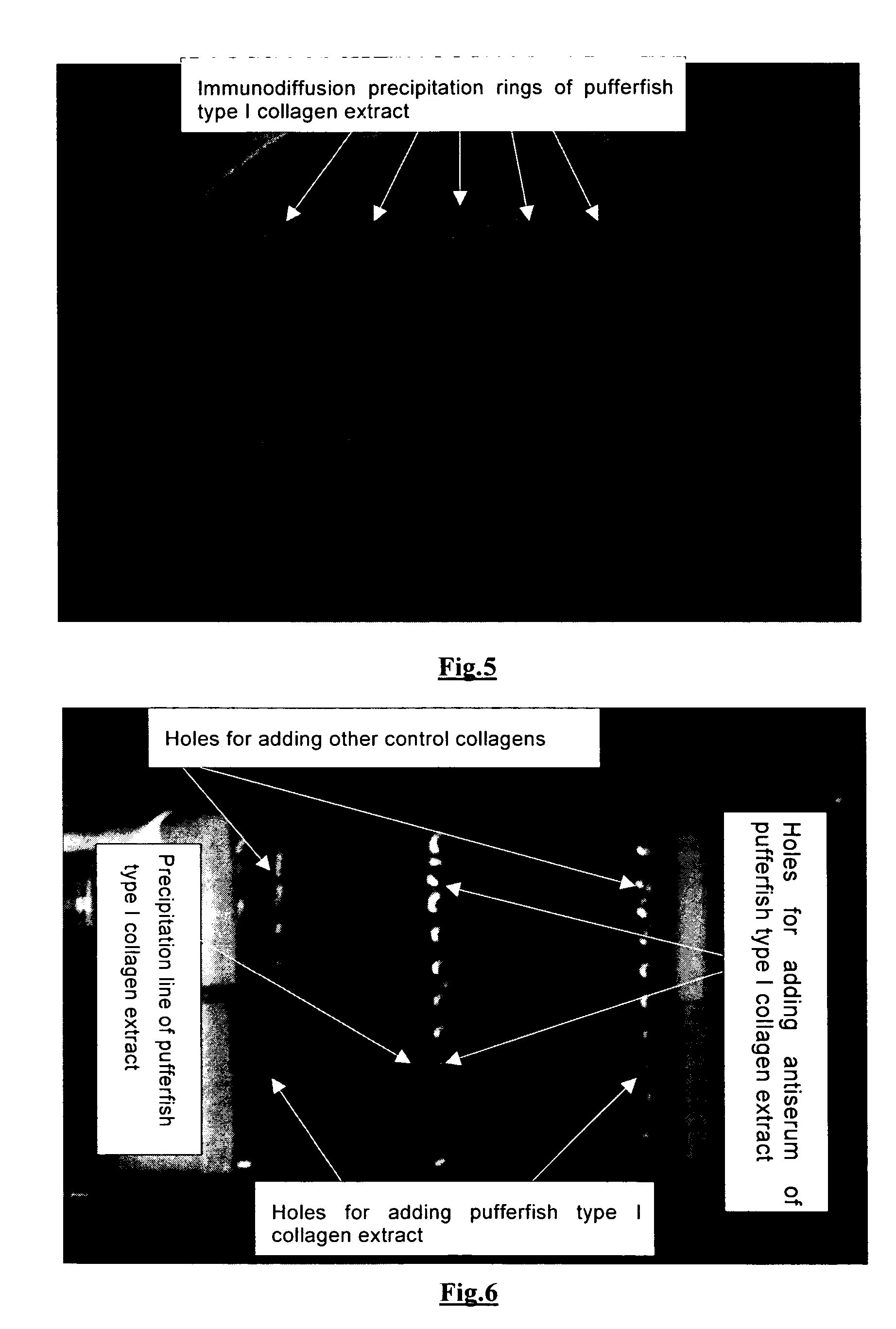
Medical and health-care uses of pufferfish type I collagen extract and processes for producing said extract
InactiveUS20070219128A1Maintain pharmacological activityStable pharmaceutical activityNervous disorderPeptide/protein ingredientsHydrolysateAdditive ingredient
The present invention relates to the use of pufferfish type I collagen extract as effective ingredient in the manufacture of medicaments and health-care foods for prevention and treatment of the following diseases, wherein the main chemical components and active components of the pufferfish type I collagen extract are natural pufferfish type I collagen or denatured pufferfish type I collagen extract and partial hydrolysates thereof. The present invention further relates to processes for the production of said pufferfish type I collagen extract, immunological assay methods of said extract, and uses of said extract as effective ingredient in treatment and health-care.
Owner:NANJING BESSON PHARMACY
Fabrication of a cartilage implant
InactiveUS6852331B2Efficient manufacturingSure easyBiocideBone implantΑ helicalMesenchymal stem cell
A method of fabricating a cartilage implant including embedding and growing chondrocytes or mesenchymal stem cells in a three-dimensional substrate. The substrate contains randomly rewound α-helical monomers of type I collagen.
Owner:TAIPEI BIOTECH
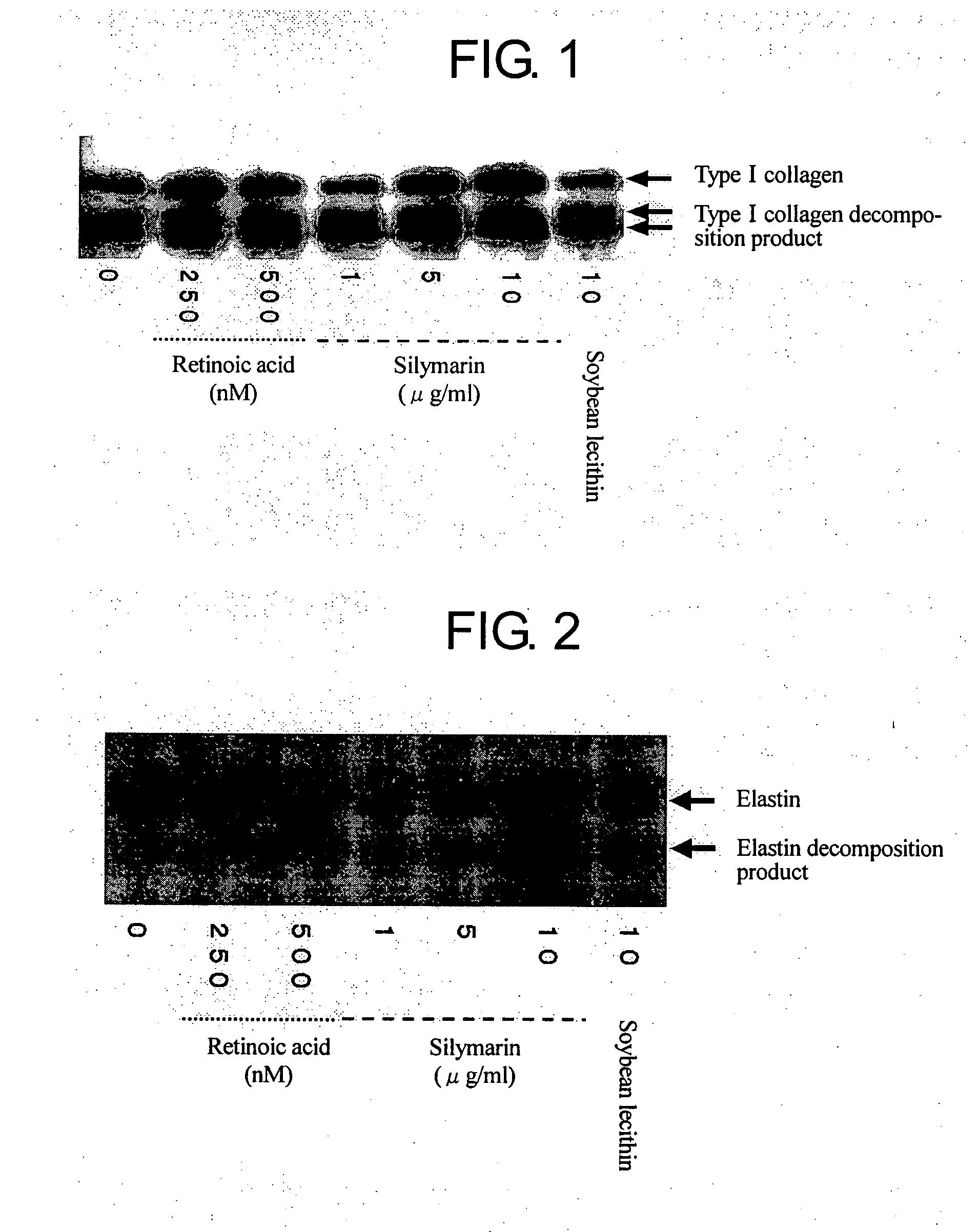
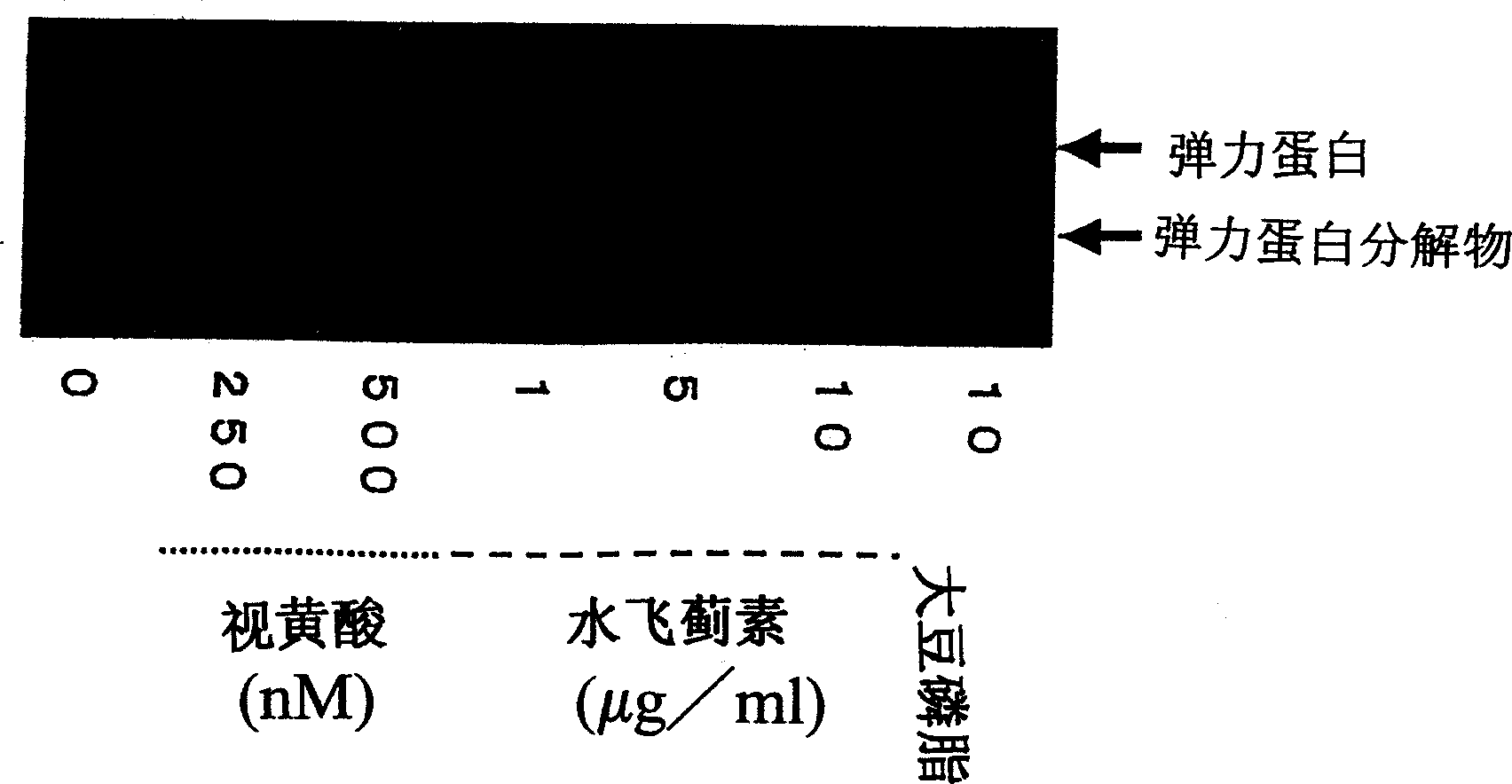
Composition for promoting production of type 1 collagen and/or elastin
InactiveUS20060233738A1Easy to produceImproves suppleness and elasticity of skinBiocideCosmetic preparationsWrinkle skinMedicine
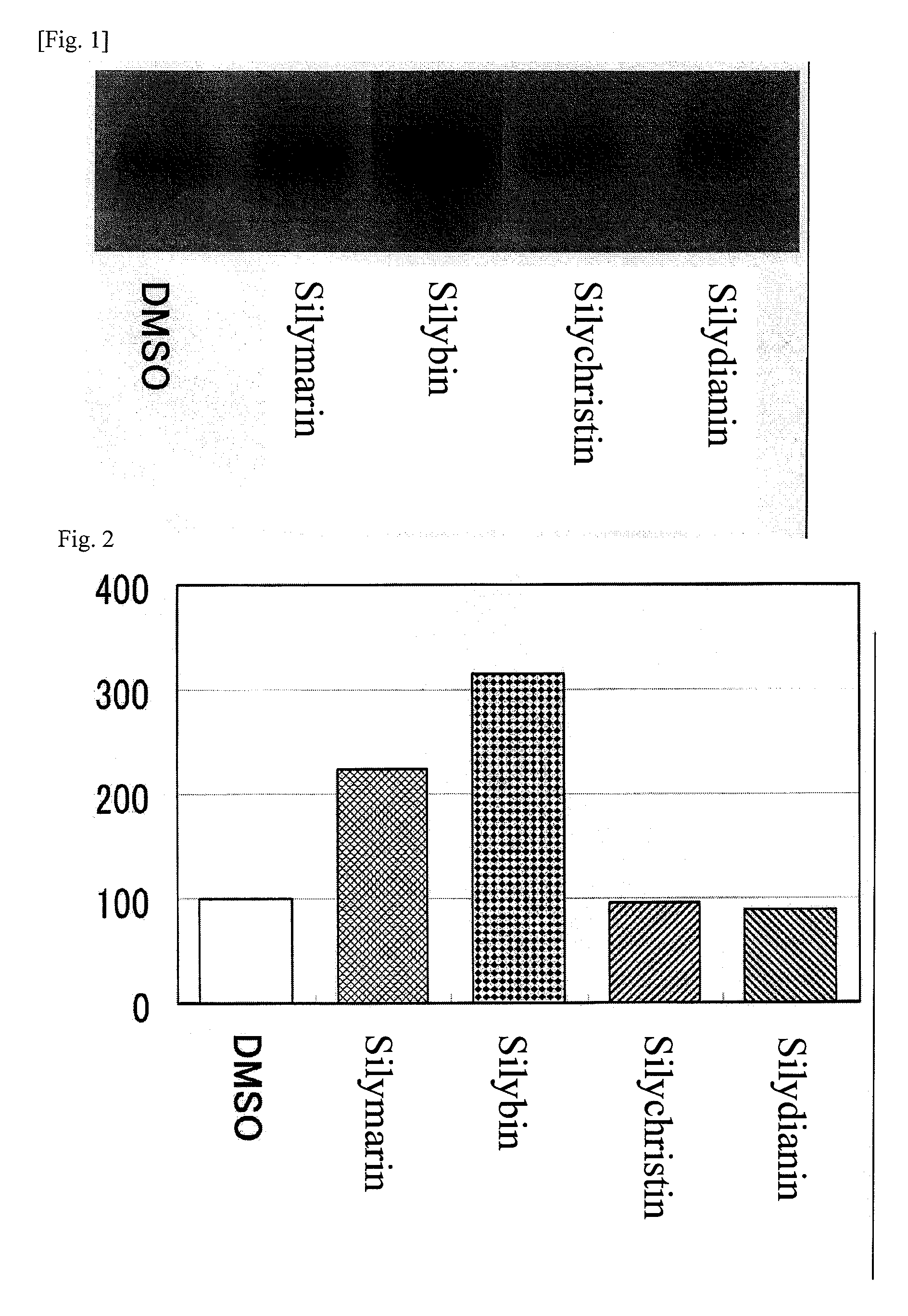
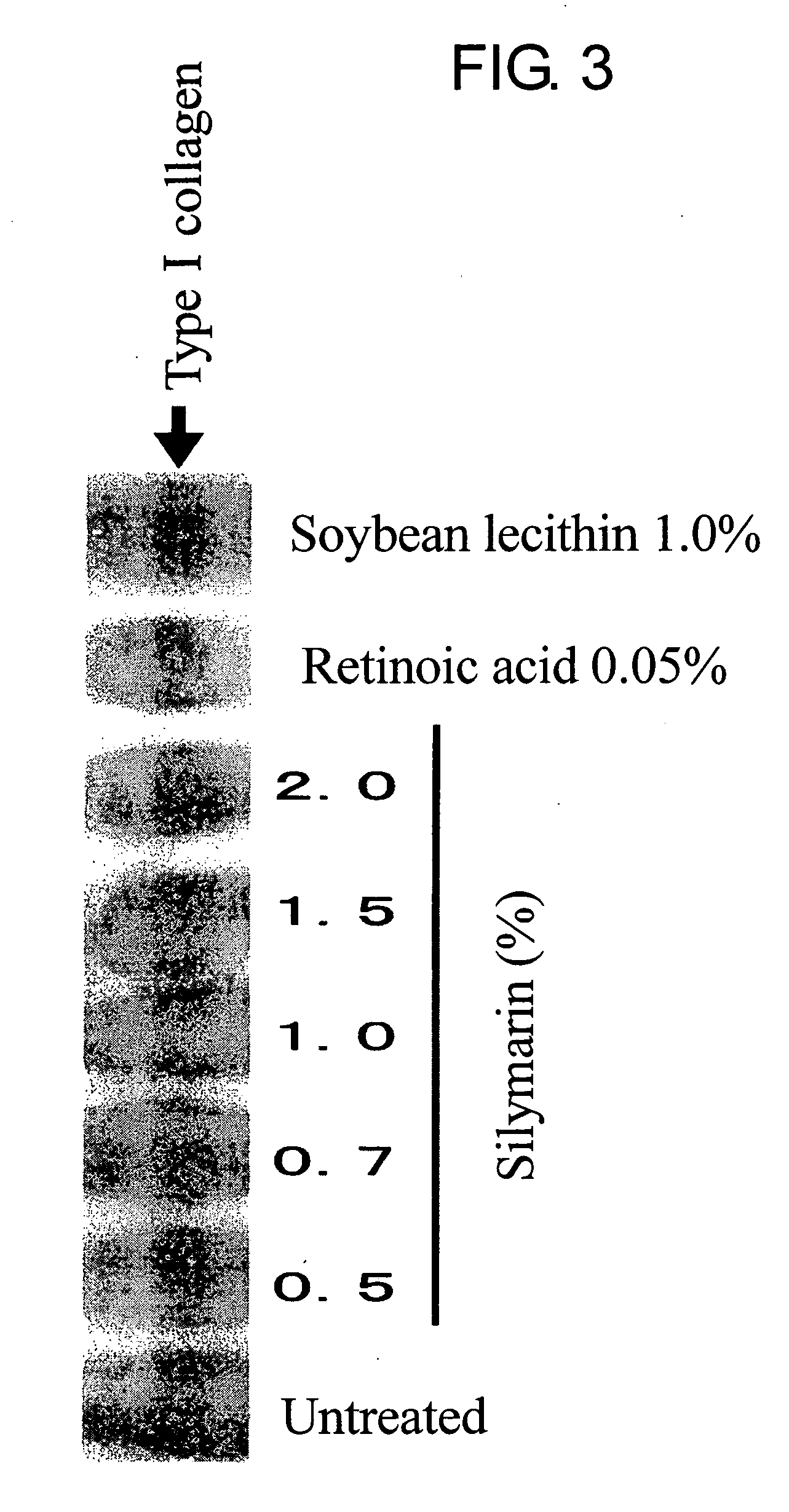
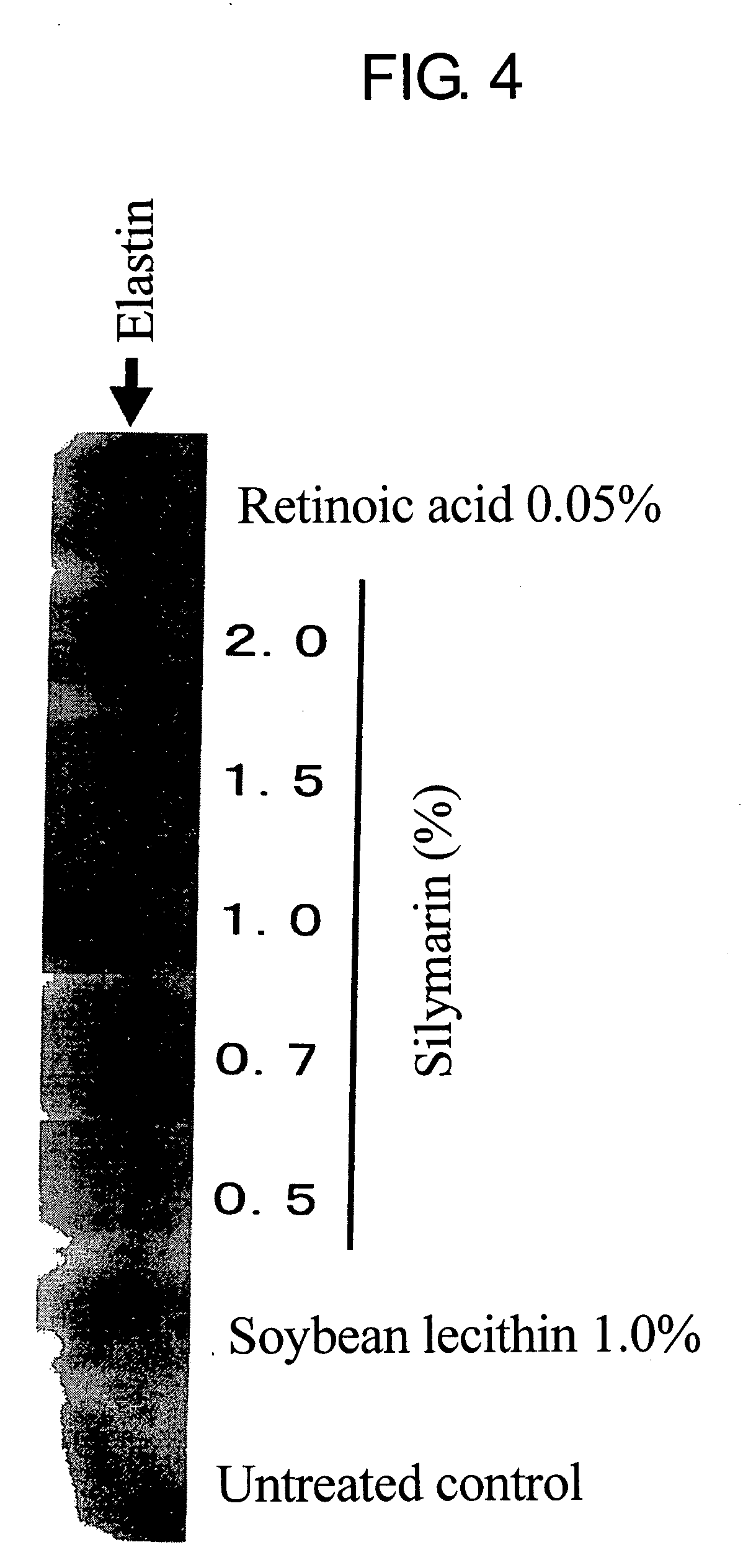
This invention aims to provide a composition that promotes the production of type I collagen and / or elastin in the human skin fibroblast cells, wherein the composition improves the suppleness and elasticity of the skin, is amply effective in preventing and improving wrinkles and sagging, and is also very safe to the skin. The present invention relates to a composition that contains silymarin, which is a general term for flavonolignans such as silybin, silydianin, silychristin and isosilybin, wherein the aforementioned composition has a property to promote the production of type I collagen and / or property to promote the production of elastin. It also relates to a composition containing silymarin derived from a silymarin-containing plant and / or extract of such plant, wherein the aforementioned composition also has a property to promote the production of type I collagen and / or property to promote the production of elastin.
Owner:FUAN KERU
Collagen/hydroxyapatite composite artificial bone and preparation method thereof
InactiveCN106620869AImprove toughnessNothing producedTissue regenerationProsthesisOrthopedics surgeryFreeze-drying
The invention relates to a collagen / hydroxyapatite composite artificial bone and a preparation method thereof. The artificial bone comprises type I collagen, hydroxyapatite and poly-p-dioxanone. The preparation method disclosed by the invention comprises the following steps: adding the type I collagen in a solvent to prepare a collagen solution, adding an appropriate amount of the hydroxyapatite and performing uniform stirring; placing the mixed slurry in a mold, performing pre-freezing, performing freeze-dried forming, and crushing the freeze-dried forming porous material to obtain blended powder; dissolving the poly-p-dioxanone in tetrafluoroacetic acid to prepare a solution, adding the blended powder in proportion, performing uniform stirring and performing cold press forming; and washing the formed pre-product with water, and drying the washed pre-product to obtain collagen / hydroxyapatite composite artificial bone. The preparation method disclosed by the invention is simple and low in the production cost. The collagen / hydroxyapatite composite artificial bone has compressive strength of 75-90MPa and favorable toughness, so that the collagen / hydroxyapatite composite artificial bone can meet requirements for bone filling and partial replacement in orthopedic surgery.
Owner:WUHAN YIJIABAO BIOMATERIAL CO LTD
Method for preparing bionic modified collagen tissue repair material
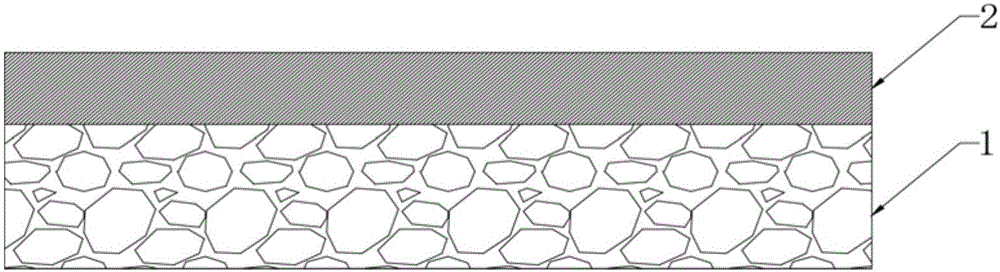
InactiveCN101954126AGuaranteed dense structureSimple molding processSurgeryTissue regenerationTissue repairDamages tissue
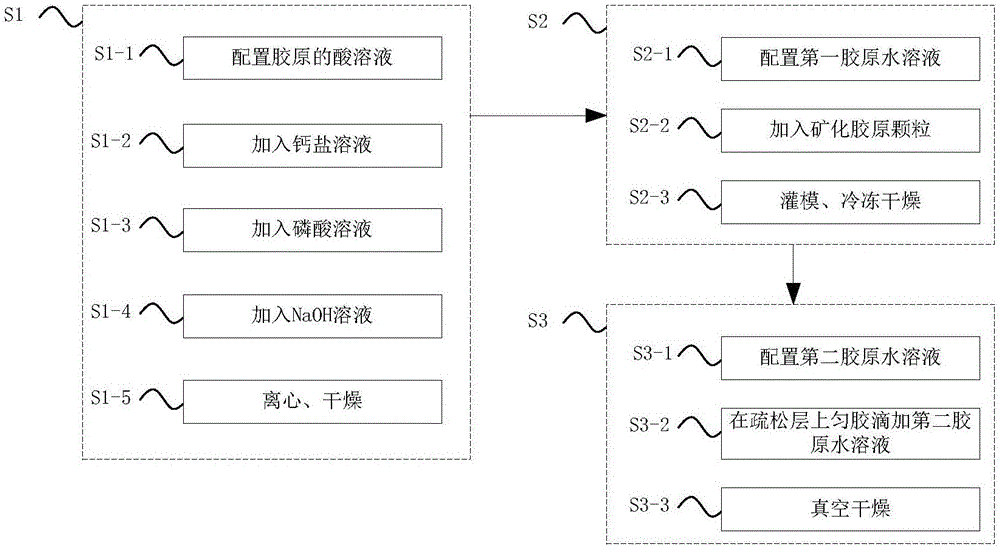
The invention discloses a method for preparing a bionic modified collagen tissue repair material. In the preparation method, the tissue repair material is prepared from type I collagen serving as a raw material, is formed by the processes of freeze-drying, pressing, coating, cross-linking, drying and the like and has a bionic structure, namely the structure of a compact layer and a loose layer is in graded distribution and does not have any obvious interface layer. The bionic modified collagen tissue repair material does not contain any chemical components, has high biocompatibility and can be completely absorbed by organisms, wherein the compact layer has barrier function and can effectively prevent cells at the periphery of defective tissues from growing into a damaged tissue area; and the loose layer can guide the adhesion and proliferation of the cells of the defective tissues so as to fulfill the aim of tissue repair.
Owner:SOUTH CHINA UNIV OF TECH
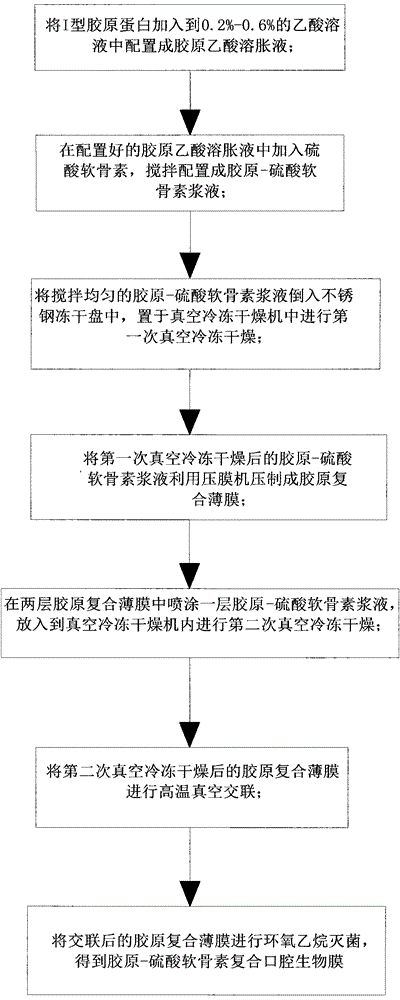
Preparation method of oral biofilm
The invention relates to a preparation method of an oral biofilm. The preparation method comprises the following steps: adding type I collagen protein into an acetic acid solution to prepare a collagen-acetic acid swelling solution; adding chondroitin sulfate and stirring to prepare a collagen-chondroitin sulfate serous fluid; carrying out vacuum freeze-drying; pressing to prepare a collagen composite film; spraying the collagen-chondroitin sulfate serous fluid between two layers of the collagen composite film, and carrying out vacuum freeze-drying; carrying out high temperature vacuum crosslinking; and sterilizing after crosslinking to obtain a collagen-chondroitin sulfate composite oral biofilm. The invention has the following beneficial effects: the oral biofilm prepared by the above method has good histocompatibility, suitable controllable degradability and absorbability, low antigenicity and good plasticity and mechanical property; the oral biofilm is used in oral operation; and the three-dimensional porous structure of the oral film provides a tissue engineering scaffold for repairing of wounded tissues, is beneficial to invasion of cells and differential growth and can induce reparative regeneration of defective tissues, and specific thickness of the biofilm can effectively prevent ingrowth of soft tissue.
Owner:TIANXINFU (BEIJING) MEDICAL APPLIANCE CO LTD
Materials for soft and hard tissue repair
Biomaterials and methods and uses for repair or augmentation of tissues are provided. In particular, the invention provides a multi-layered, naturally occurring multi-axial oriented biomaterial comprising predominately type I collagen fibers. The invention further provides methods and uses for repair or augmentation of tissues using biomaterials of the invention.
Owner:OBI BIOLOGICS
High-artificial tissue engineering nerve repair material NGCS and preparation method thereof
The invention relates to a high simulation tissue engineering nerve repairing material NGCS and a preparing method thereof which is characterized in that the NGCS material comprises one, two or three raw materials of type I collagen, chitosan and gelatin. The NGCS material is made of the following raw materials by weight portions: 1-10 portions of type I collagen, 1 portion of chitosan and 1 portion of gelatin. The NGCS can be applied to both the basic research of the nerve injury repairing and the bridge repairing of clinical human spinal and peripheral nerve injuries or defects. The NGCS material is beneficial for the growth of nerve regenerated fiber and can be applied to the repairing of spinal and peripheral nerve injuries.
Owner:SHENZHEN YINGPULAN MEDICAL DEVICE
Method for extracting type I collagen
InactiveCN106701879AImprove extraction yieldGood biocompatibilityConnective tissue peptidesPeptide preparation methodsEnzymatic digestionActive enzyme
The invention relates to a method for extracting type I collagen. The method comprises the following steps: cleaning bovine tendon, refrigerating and slicing; disinfecting and degreasing the sliced bovine tendon with 75-percent alcohol and normal hexane; adding a proper amount of active enzyme into homogenate for performing enzymatic digestion after removing impure proteins and smashing the homogenate; performing salting-out and dialysis treatment on supernatant; performing freeze drying and vacuum packaging on a collagen stock solution obtained after the dialysis to obtain a type I collagen product. The method is simple in preparation process, can be used for realizing large-scale production, and is an economical and effective extraction method. The type I collagen extracted by the method has superior biocompatibility and bioactivity, can be applied to biomedicine, and has an important application value in the field of makeups; meanwhile, epithelial cells can be activated, the hyperplasia of the epithelial cells and the generation of collagenase are facilitated, and the skin becomes tight and elastic.
Owner:WUHAN YIJIABAO BIOMATERIAL CO LTD
Bone implant and manufacturing method thereof
InactiveUS20110262486A1Readily availableHigh activityBiocideHeavy metal active ingredientsBiopolymerBone implant
The invention discloses a bone implant and a manufacturing method thereof. The manufacturing method of the bone implant comprises a step of coating or mixing type II collagen with at least one porous bone material comprising metals, bio-ceramics, natural biopolymers and synthetic polymers. Another manufacturing method of the bone implant comprises the steps of loading type II collagen with or without at least one porous bone material in a container, and lyophilizing the type II collagen to generate a type II collagen sponge construct with or without the porous bone material as the bone material. The manufactured bone implants are effective, with or without loading cells having differentiation tendency towards osteogenesis, to facilitate bone repair upon introduction of the bone implant into various osseous defects.
Owner:TAIPEI MEDICAL UNIV
Composite tissue-engineered intervertebral disc with self-assembled annular alignment
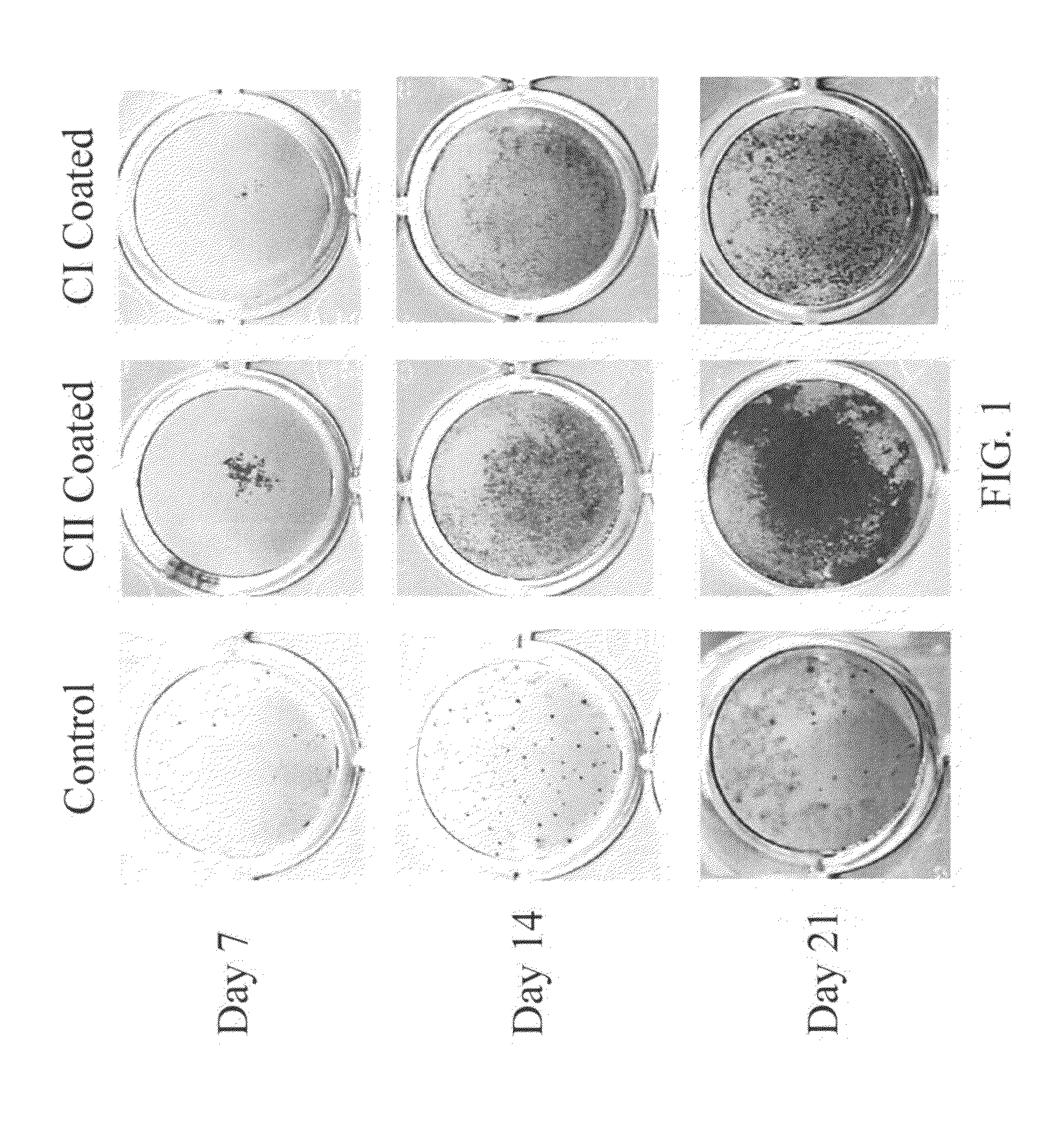
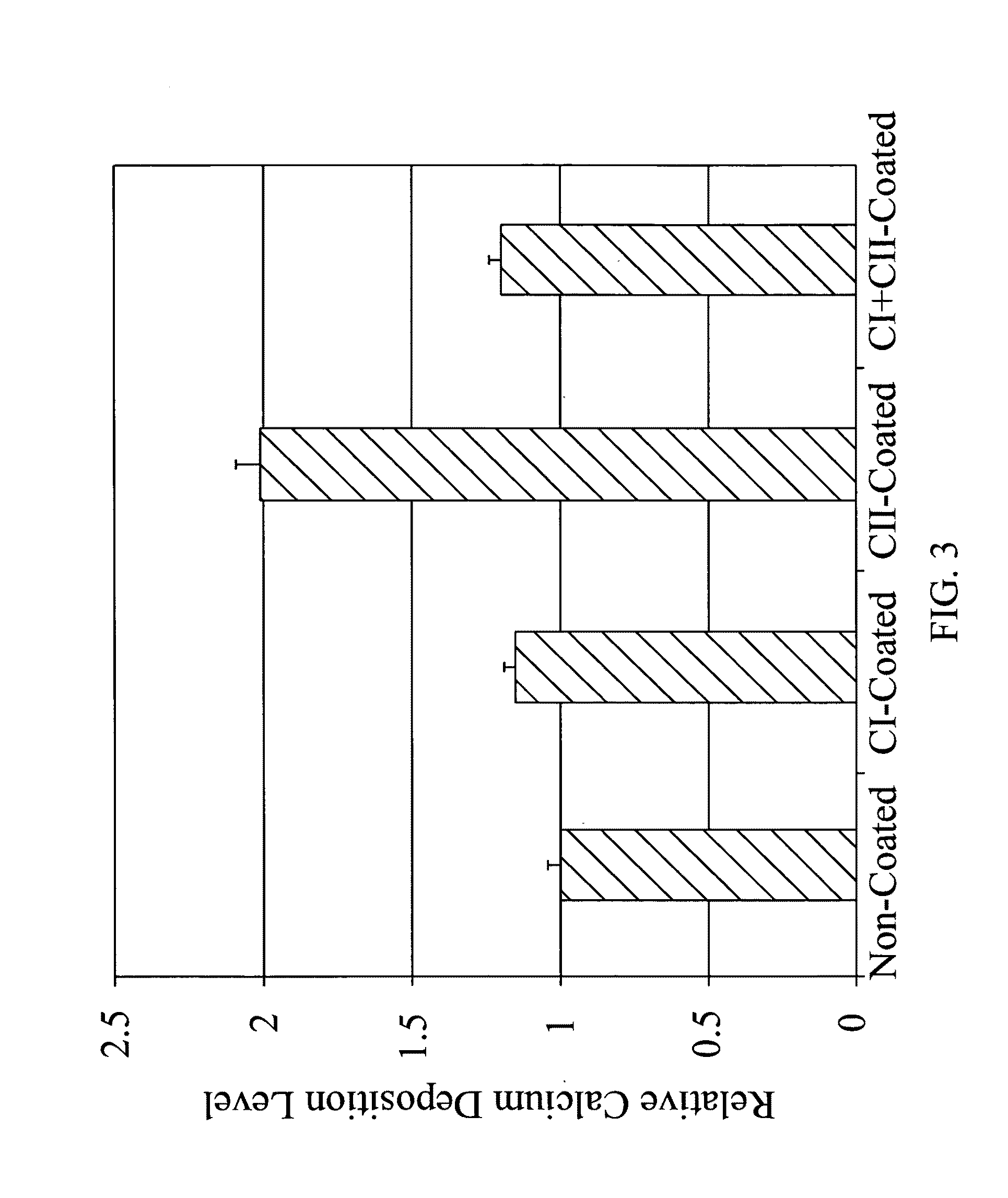
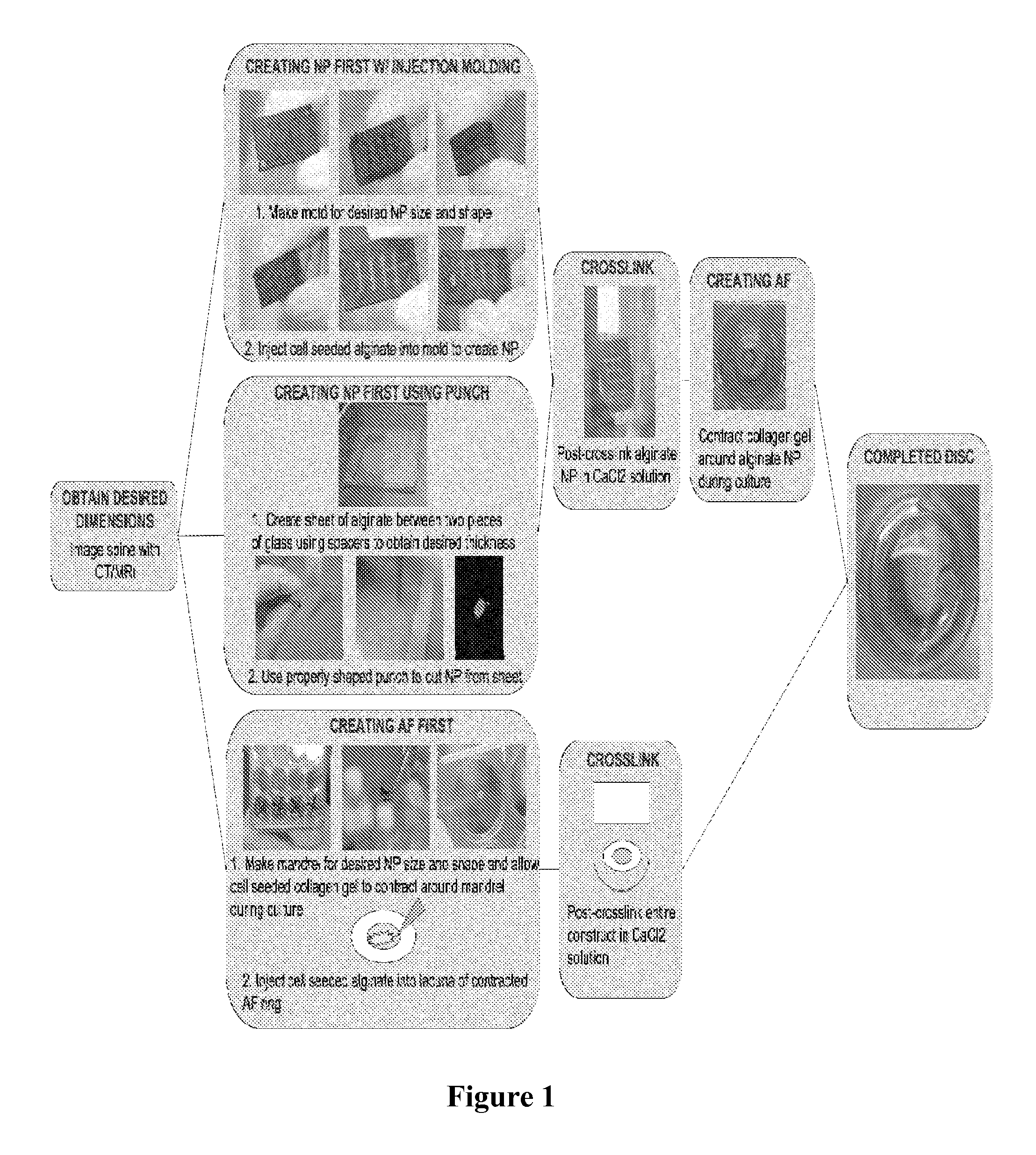
ActiveUS9044335B2Remarkable ability to restore functionUnknown materialsSpinal implantsFiberIntervertebral disc
The present invention relates to a tissue-engineered intervertebral disc (IVD) suitable for total disc replacement in a mammal and methods of fabrication. The IVD comprises a nucleus pulposus structure comprising a first population of living cells that secrete a hydrophilic protein and an annulus fibrosis structure surrounding and in contact with the nucleus pulposus structure, the annulus fibrosis structure comprising a second population of living cells and type I collagen. The collagen fibrils in the annulus fibrosis structure are circumferentially aligned around the nucleus pulposus region due to cell-mediated contraction in the annulus fibrosis structure. Also disclosed are methods of fabricating tissue-engineered intervertebral discs.
Owner:CORNELL UNIVERSITY
Stent for osteochondral defect repair and preparation method thereof
The invention discloses a stent for osteochondral defect repair. The stent consists of a cartilage layer with pores and a subchondral bone layer with pores, wherein the cartilage layer and the subchondral bone layer are tightly combined into a whole; the material of the cartilage layer is a mixture of type I collagen and chondroitin sulfate; and the material of the subchondral bone layer is porous titanium or porous titanium oxide or a porous titanium alloy. A preparation method of the stent comprises the following steps of: (1) processing the porous titanium or porous titanium oxide or porous titanium alloy to a shape and a size required by the subchondral bone layer; (2) preparing cartilage layer slurry; and (3) molding and treating a stent, putting the subchondral bone layer prepared in the step (1) into a mold, injecting the cartilage layer slurry prepared in the step (2) into the mold, putting the mold into a freeze dryer, freezing for at least 5 hours, molding, performing thermal crosslinking and chemical crosslinking on the molded stent, and putting the chemically-crosslinked stent into sterile water for rinsing to obtain the stent for osteochondral defect repair.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Biologic artificial bone
A biologic artificial bone includes an artificial fiber material formed from a synthetic polymer with mechanical properties similar to type I collagen. A biocompatible liquid substance is impregnated in the fiber material that hardens and stiffens the fiber material. A bone substitute is impregnated in the hardened and stiffened fiber material forming an artificial bone composite. Vascular channels are formed in the artificial bone composite to facilitate in-growth of vessels and bone forming cells. The construction and methods achieve an artificial composite structure that is similar to natural bone with comparable properties.
Owner:SPINAL ELEMENTS INC
Tissue-engineered neural tissues and construction method thereof
InactiveCN102228718AConducive to survivalLarge apertureNervous system cellsProsthesisMatrigelRegenerative medicine
The invention discloses tissue-engineered neural tissues and a construction method thereof, belonging to the technical field of tissue engineering and regeneration medicine. Subcultured and purified neural stem cells serve as seed cells of the tissue-engineered neural tissues, a liquid type I collagen and Matrigel matrix serves as stent materials, and the seed cells and the stent materials are cultured after being compounded in vitro. A stent system adopted in the invention can be used for effectively maintaining the survival and proliferation of the neural stem cells, promoting the differentiation of the neural stem cells, and forming engineered neural tissues consisting of mature neurons, astrocytes and oligodendrocytes. The tissue-engineered neural tissues have an important significance in treating neural system injury through the transplantation of the tissue-engineered neural tissues, and can also serve as an in-vitro model for the psychological research of neurodevelopment, cerebral trauma and neuroelectricity.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
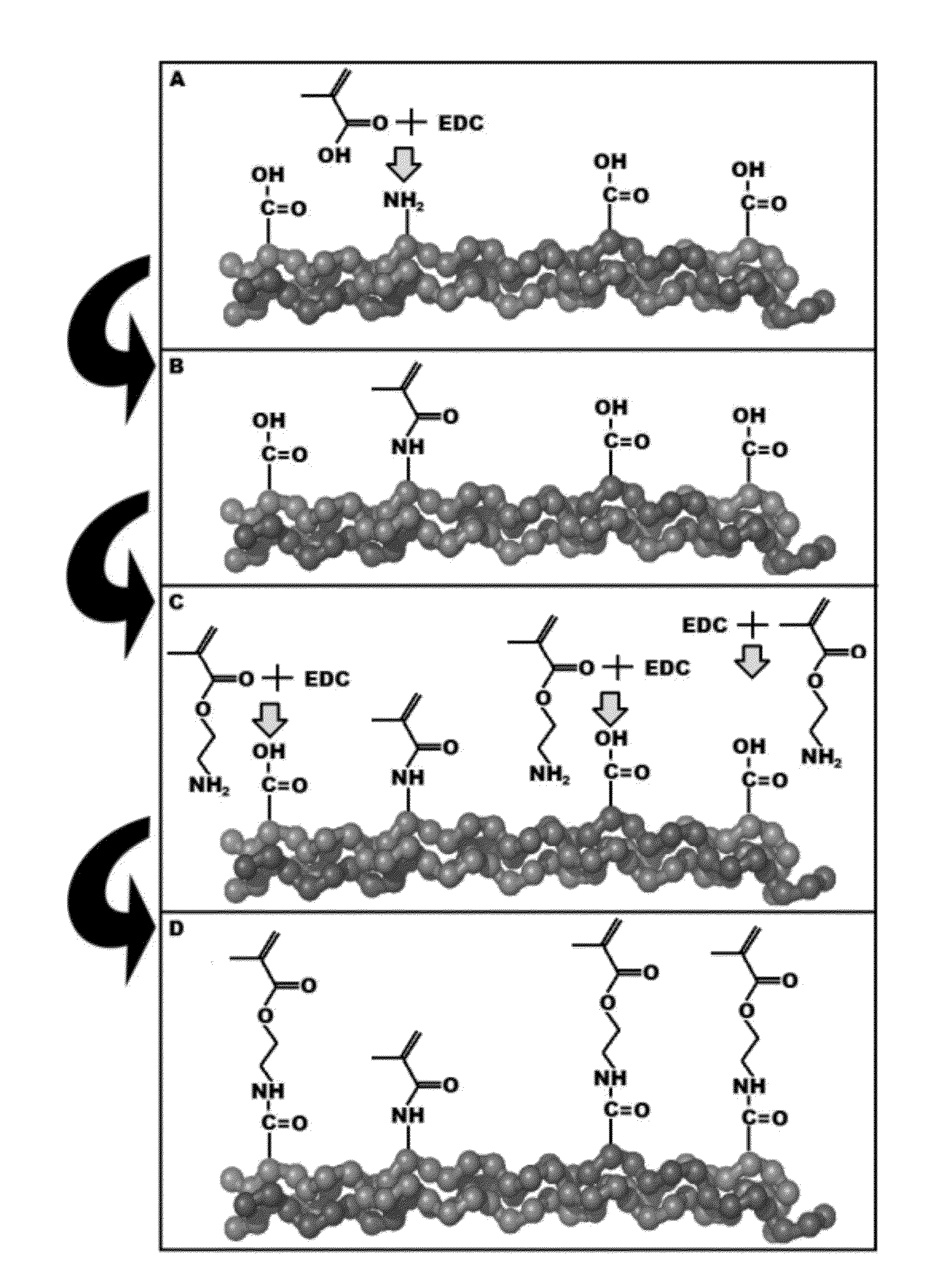
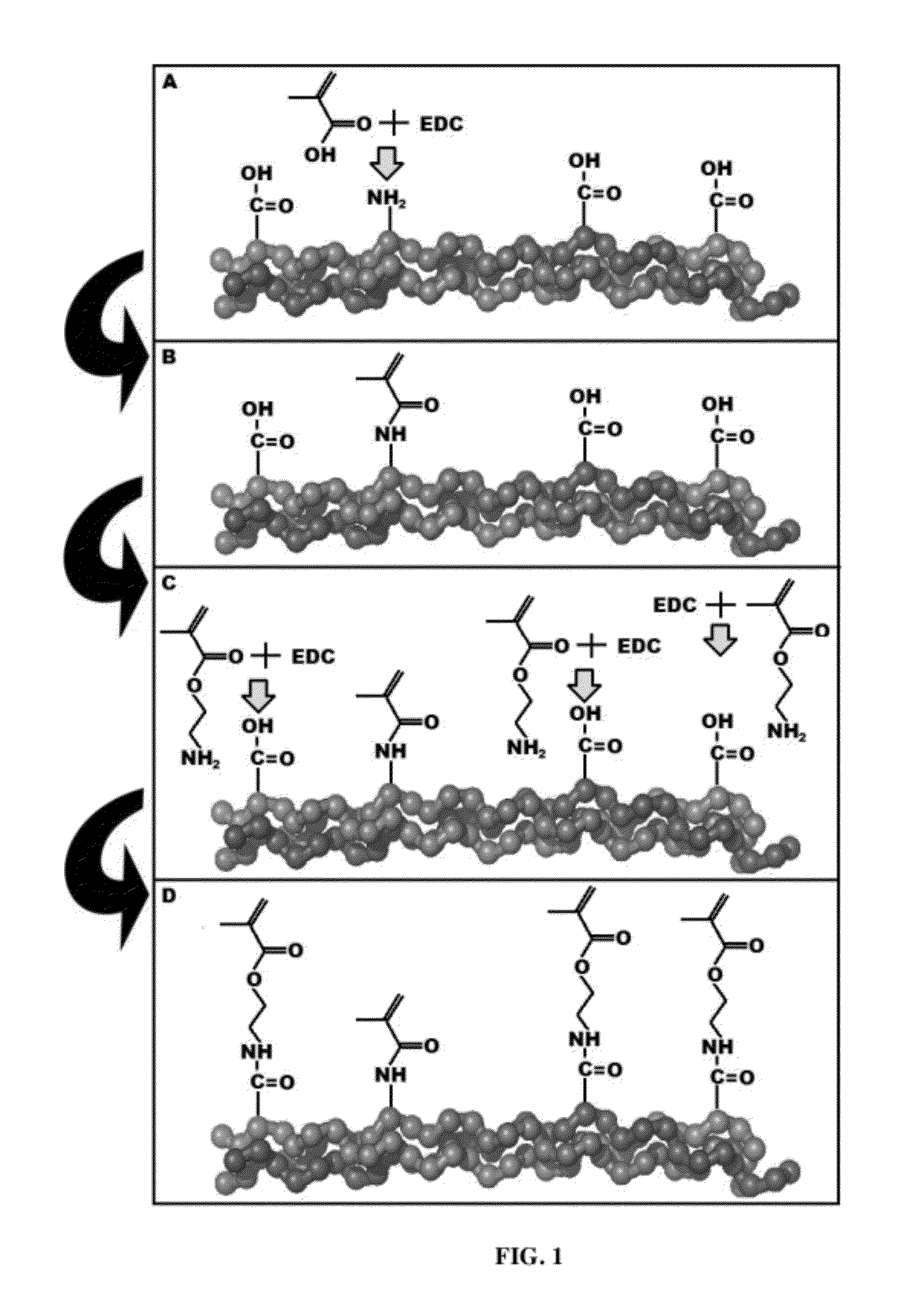
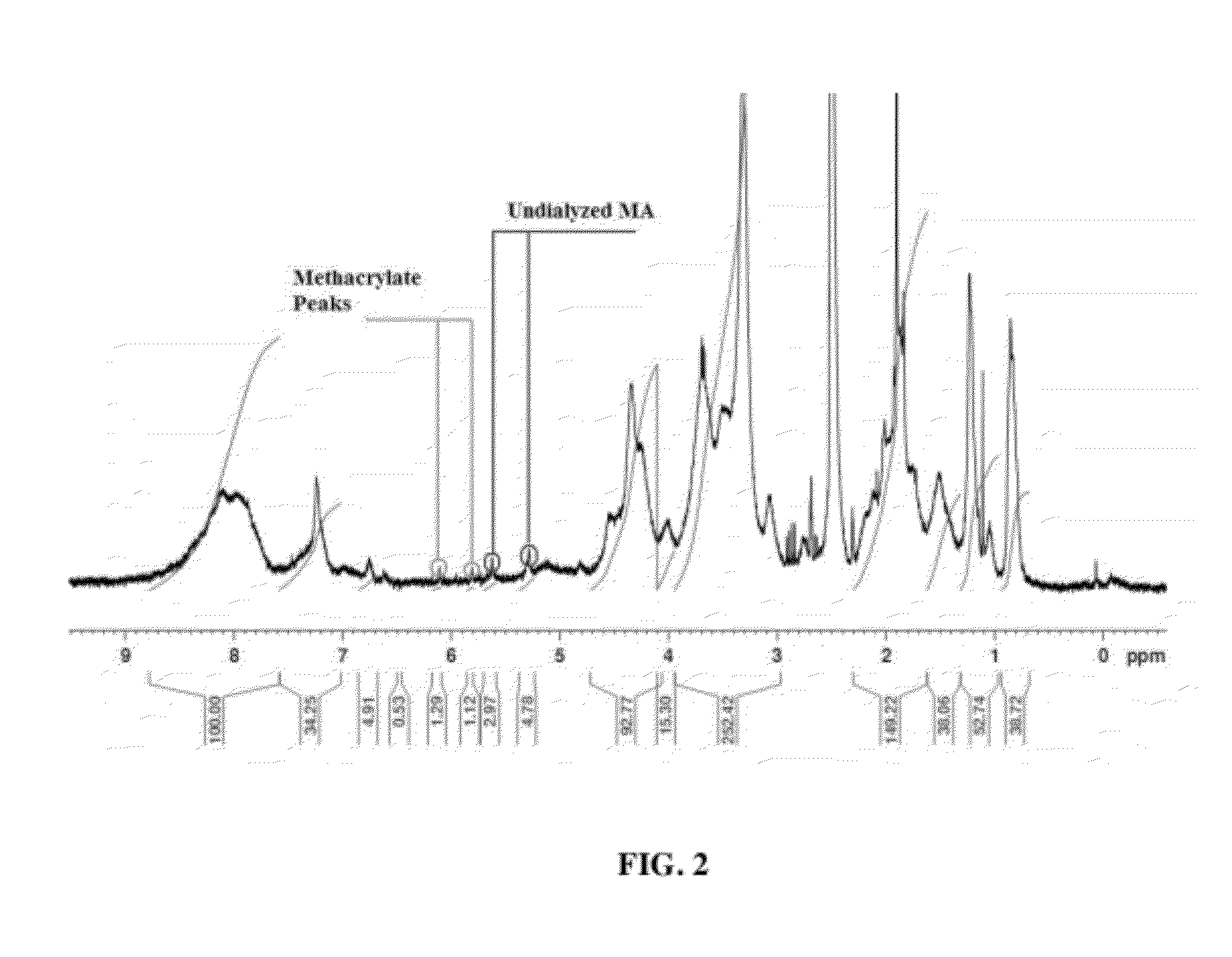
Process for the synthesis of methacrylate-derivatized type-1 collagen and derivatives thereof
ActiveUS20120220691A1Increase production capacityCosmetic preparationsImpression capsCross-linkMethacrylate
Methods for synthesizing a methacrylate-derivatized type-I collagen in which methacrylic acid is reacted with a carboxylic acid activating reagent in the presence of a carbodiimide to form a methacrylic acid with an activated carboxylic acid group, which is then reacted with free amino groups on type-I collagen to form a collagen methacrylamide. Methacrylate-derivatized collagen, cross-linked collagens formed therefrom and products containing the cross-linked collagen are also disclosed.
Owner:RUTGERS THE STATE UNIV
Preparation method of bionic mineralized collagen scaffold
The invention provides a preparation method of a bionic mineralized collagen scaffold. The preparation method comprises the following steps: 1), preparing a bionic mineralized liquid: adjusting the pHvalue of carboxymethyl chitosan-amorphous calcium phosphate (or amorphous strontium phosphate or amorphous strontium carbonate) nanocomposite liquid to be lower than an isoelectric point of CMC; 2),preparing the bionic mineralized collagen scaffold: mixing the bionic mineralized liquid and acid-dissolved type-I collagen, then putting into a dialysis bag, sealing, then putting into a PBS buffer liquid, self-assembling and mineralizing for 2-5 days, then adding an alkaline CMC / ACP (ASP or ASC) liquid into the dialysis bag, and changing the PBS buffer liquid; 1-3 days later, putting the dialysis bag into deionized water for dialyzing for 10-72h, changing the deionized water for several times, centrifuging the liquid in the bag to obtain collagen gel, and performing freeze drying to obtain the bionic mineralized collagen scaffold. By the preparation method, collagen self-assembly and mineralization are cooperatively performed, intra-collagen fiber mineralization is achieved at relativelyhigh efficiency, and osteogenic ability-promoting strontium is integrated into the collagen scaffold, so that the preparation method has a wide clinical application prospect.
Owner:博纳格科技(天津)有限公司
Method for preparing basic fibroblast growth factor sustained-release carrier
InactiveCN102228695AMaintain biological activityGood biocompatibilityPeptide/protein ingredientsPharmaceutical delivery mechanismMicrosphereFreeze-drying
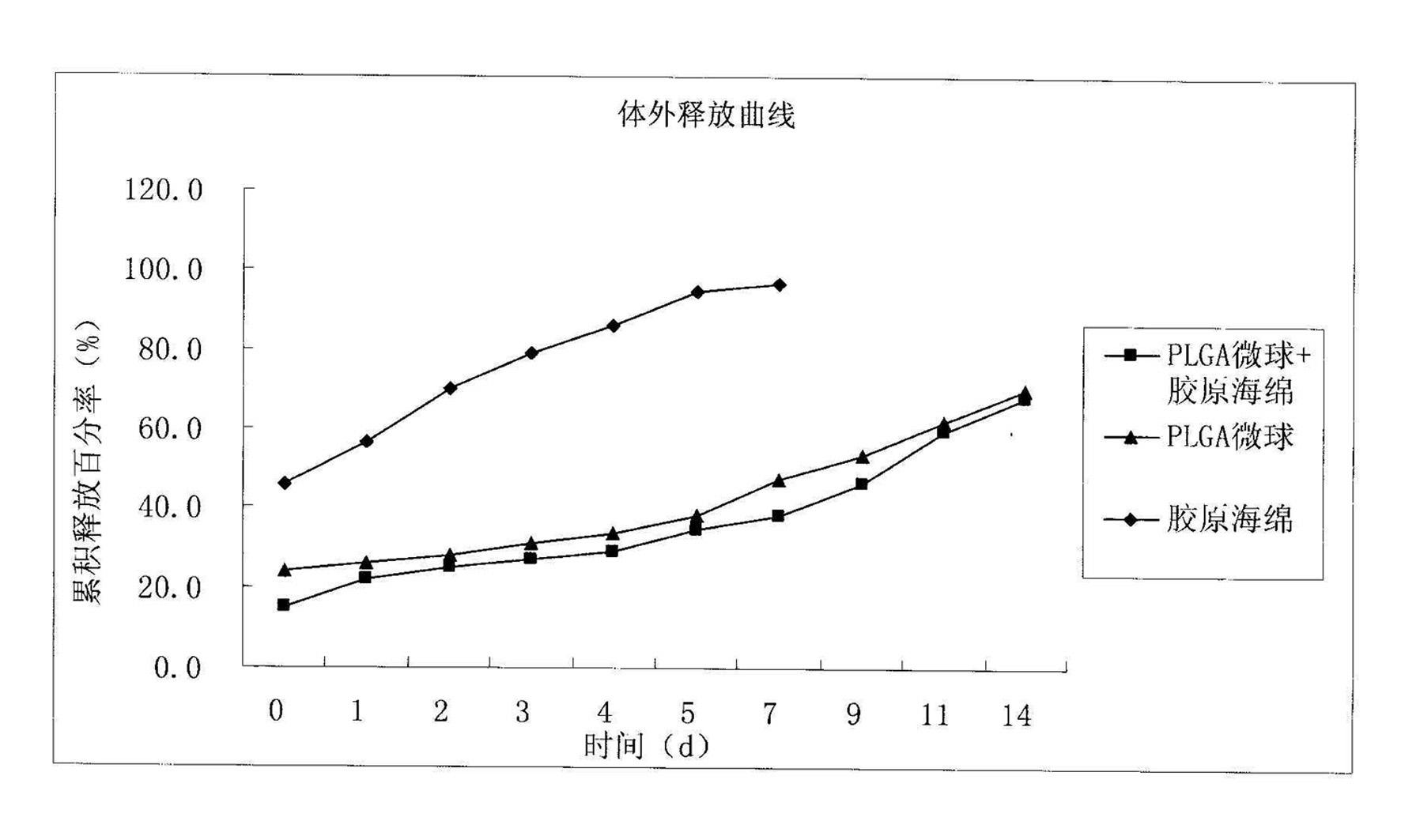
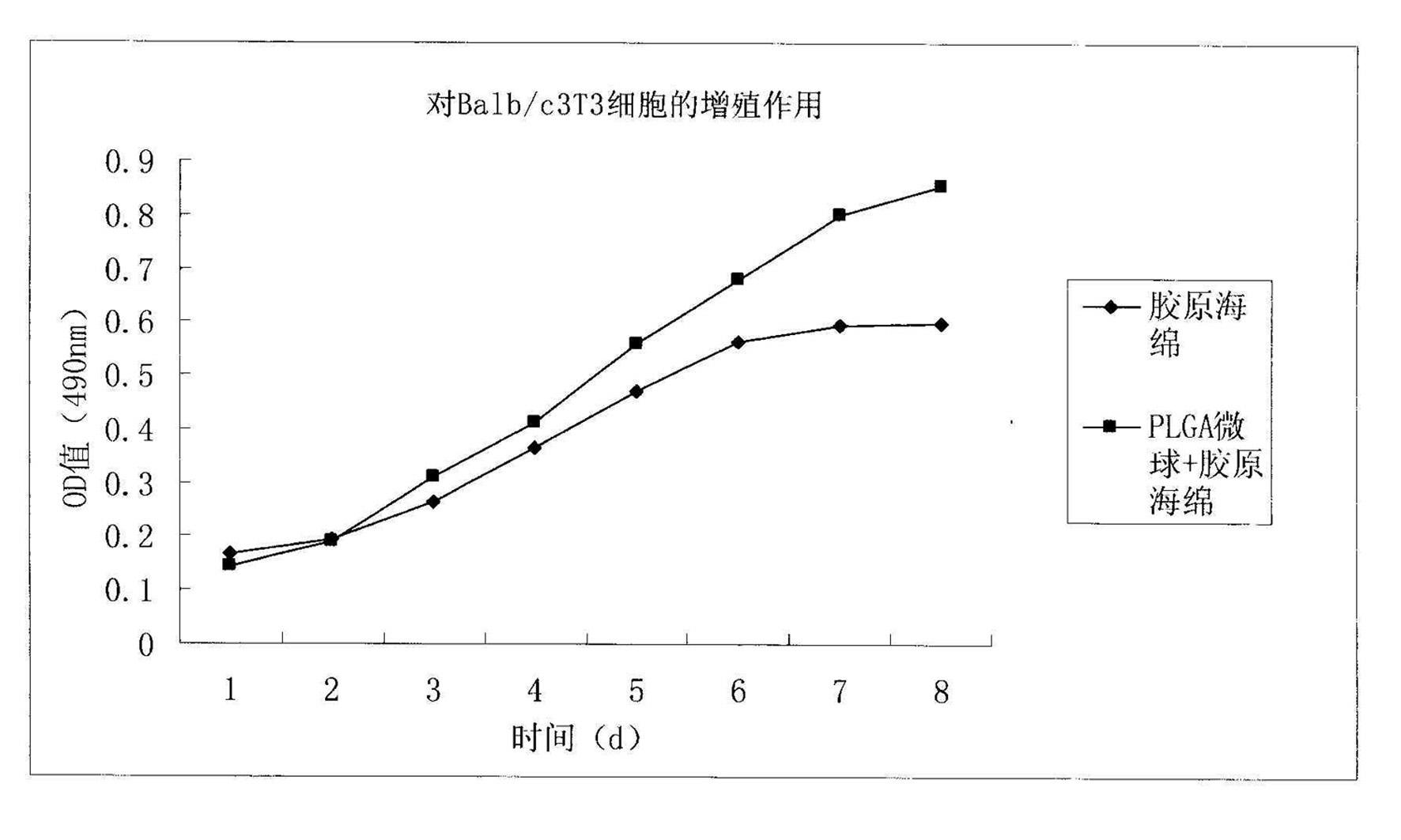
The invention discloses a method for preparing a basic fibroblast growth factor (bFGF) sustained-release carrier, which comprises the following steps of: 1, preparing bFGF-poly(lactide-co-glycolide) (PLGA) sustained-release microspheres encapsulating 0.00001 to 0.001 percent of bFGF; 2, dissolving type I collagen in an acetic acid solution to obtain a collagen solution; 3, mixing and stirring the bFGF-PLGA sustained-release microspheres and the collagen solution, performing freeze drying, solidifying, and performing freeze drying again to obtain a bFGF-PLGA collagen sustained-release carrier; and 4, performing in-vitro release experiments and Balb / c3T3 cell proliferation promotion experiments, wherein experimental results show that the sustained-release carrier has good biocompatibility, can slowly control the release of the bFGF and effectively promote Balb / c3T3 cell growth, and is an ideal bFGF sustained-release carrier.
Owner:舒泰经贸(广州)有限公司
Tissue engineered bone-cartilage complex tissue graft and preparation method thereof
The invention provides a tissue engineered bone-cartilage composite tissue transplant and a preparation method thereof, and belongs to the tissue engineering field. The method comprises the following steps: seed cells are implanted on a scaffold material and cultured in vitro to form the tissue engineered bone-cartilage composite tissue transplant; the seed cells are osteogenic stem cells; the scaffold material comprises an osteogenic part and a chondroblast part, and the osteogenic part is added with an osteoblast induction factor and modified by a type-I collagen in a preparation process; and the chondroblast part is added with a chondroblast induction factor and modified by a type-II collagen. Bone marrow mesenchymal stem cells are successfully induced into osteocytes and chondrocytes, and bone tissues and cartilage tissues are formed after the obtained transplant is transplanted in vivo. Satisfactory effect is obtained from the tissue engineered bone-cartilage composite tissue which is constructed by the transplant.
Owner:JINAN UNIVERSITY
Mineralization guided tissue regeneration membrane and preparation method and application thereof
ActiveCN106492283AGood tissue compatibilityHigh tensile strengthProsthesisPhysical BarrierNano hydroxyapatite
The invention relates to a mineralization guided tissue regeneration membrane and a preparation method and application thereof, wherein the mineralization guided tissue regeneration membrane comprises: a loose layer formed by compositing type I collagen and nano hydroxyapatite; a compact layer positioned on the loose layer and formed from type collagen. The double-layer mineralization guided tissue regeneration membrane is constructed herein, wherein the loose layer is consistent to autogenous bone in terms of composition, and the membrane may be attached to diseased oral bone defect surface to induce the generation of new bones; the surface of the compact layer is smooth, a diseased area may be isolated from surrounding tissues by making good use of physical barrier function of the membrane, regenerating capacity of a specific tissue is given to maximum play, and the prepared mineralization guided tissue regeneration membrane has high tensile strength and elasticity.
Owner:BEIJING ALLGENS MEDICAL SCI & TECH
Large-scale preparation method of fish scale type I collagen peptides
ActiveCN104140992AAvoid pollutionAvoid consumptionChemical industryPeptide preparation methodsCollagen VIImpurity
The invention relates to a large-scale preparation method of fish scale type I collagen peptides. The preparation method comprises the following steps: firstly, performing alkali and acid pretreatment on marine fish scales or freshwater fish scales serving as raw materials to remove impurities, and directly performing directed enzymatic hydrolysis on collagen type I in the fish scales by adopting a one-step biological enzymatic hydrolysis technology to form collagen peptides type I with relatively centralized molecular weight distribution; secondly, separating, purifying and concentrating directed enzyme liquid at the normal temperature by adopting a continuous centrifugal separation and membrane separation combined technology to obtain a fish scale type I collagen peptide solution with purity of not less than 95% and molecular weight of less than 1,000 Dalton; finally, quickly drying finished fish scale type I collagen peptides by adopting a spray-drying technology. The method is simple and feasible in overall process, efficient, energy-saving, short in production cycle and suitable for large-scale production; a fish scale type I collagen peptide product produced by adopting the method is high in purity and good in quality, and the content of peptides with molecular weight of less than 1,000 Dalton is more than 97%.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Composition for promoting production of type I collagen and/or elastin
It is intended to provide a composition promoting the production of type I collagen and / or elastin in human skin fibroblasts to thereby provide a composition for preventing the skin from aging which can improve skin tension and elasticity, is sufficiently efficacious in preventing, inhibiting or relieving wrinkles and sagging in the skin and yet has a high safety to the skin. A composition characterized by containing silymarins, (i.e., a general term for flavonolignans such as silybin, silydianin, silychristin and siosilybin) and having an effect of promoting the production of type I collagen and / or elastin; and a composition characterized in that silymarins contained therein originate from a silymarin-containing plant and / or an extract of the plant and having an effect of promoting the production of type I collagen and / or elastin.
Owner:FUAN KERU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com