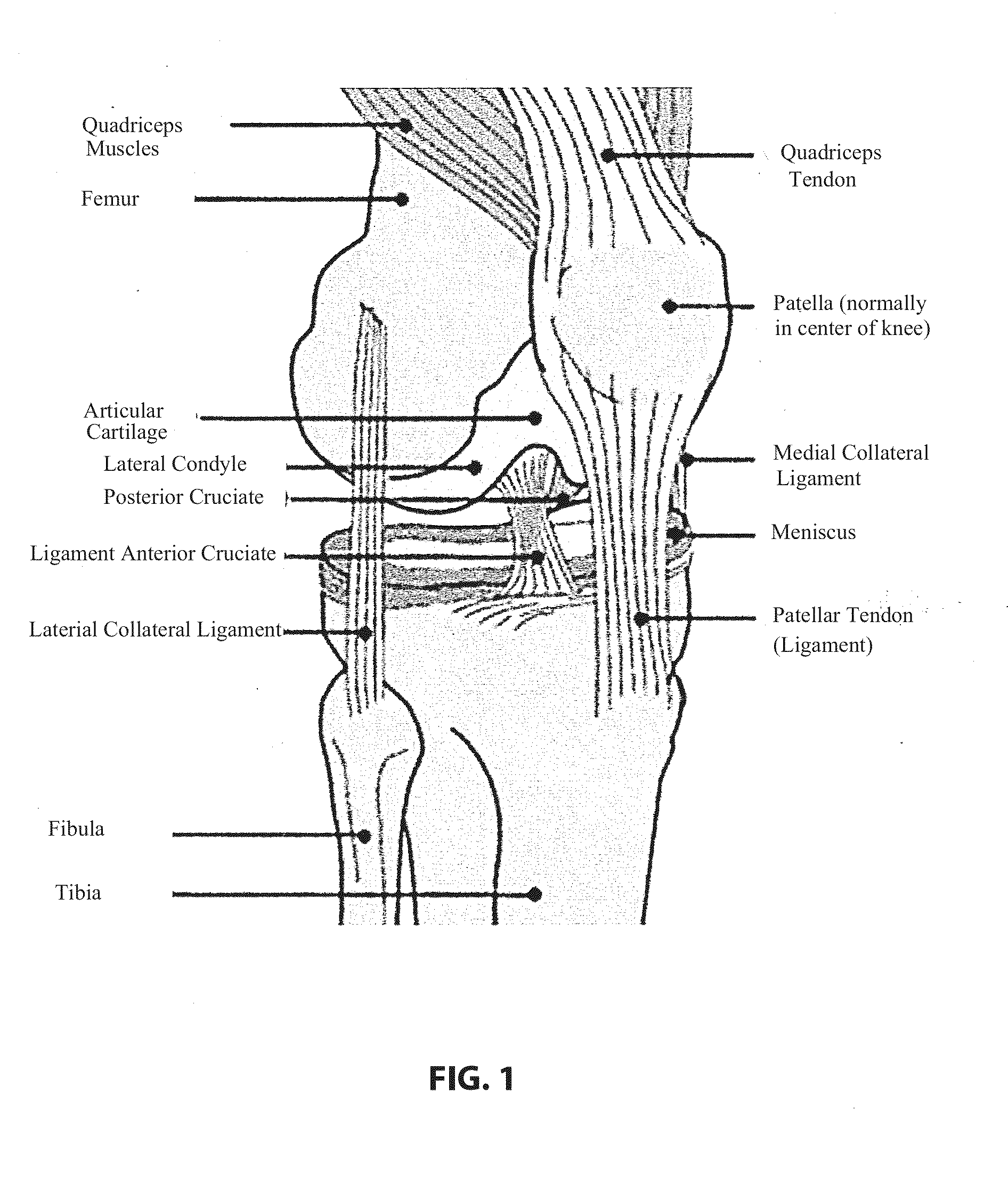
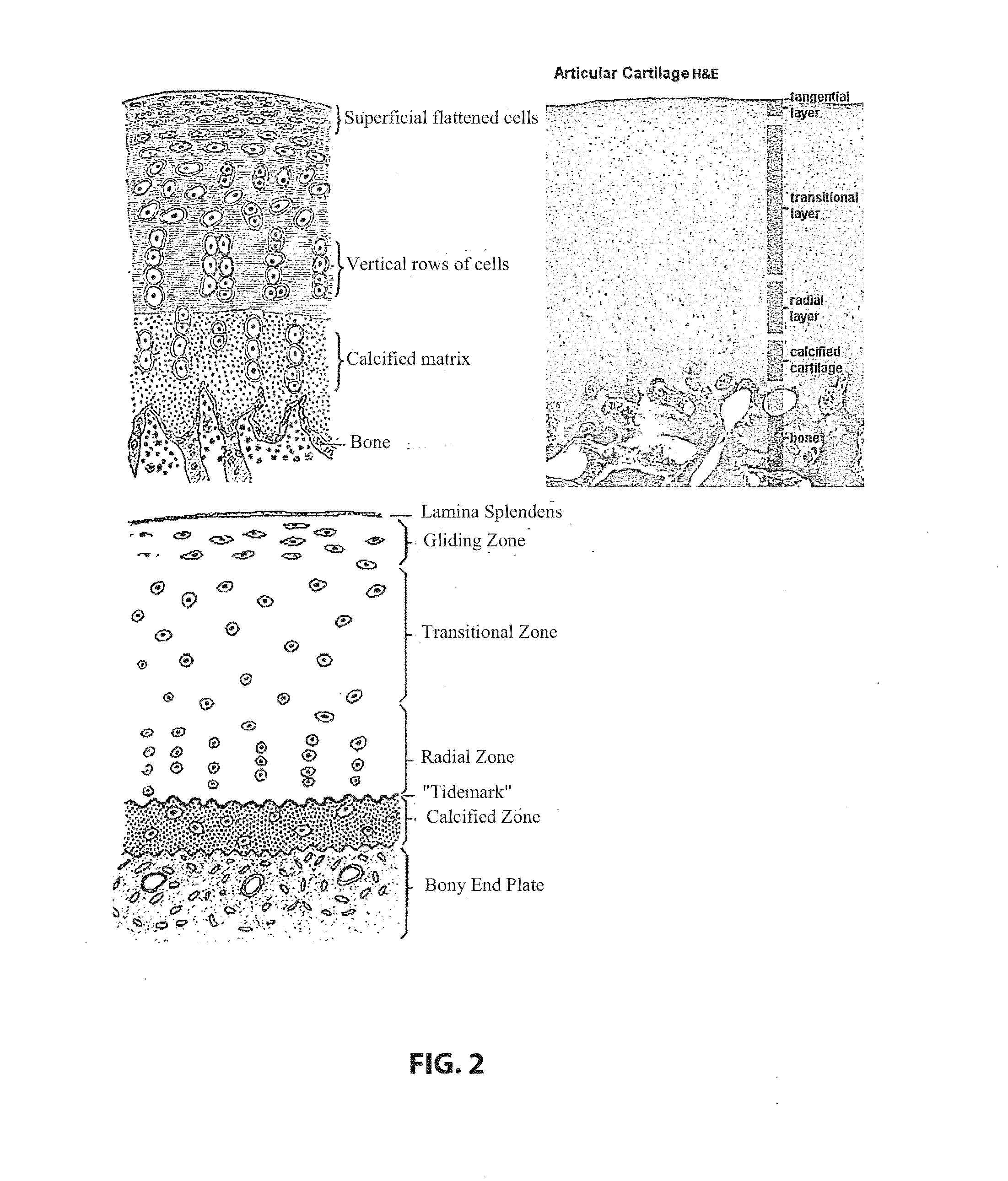
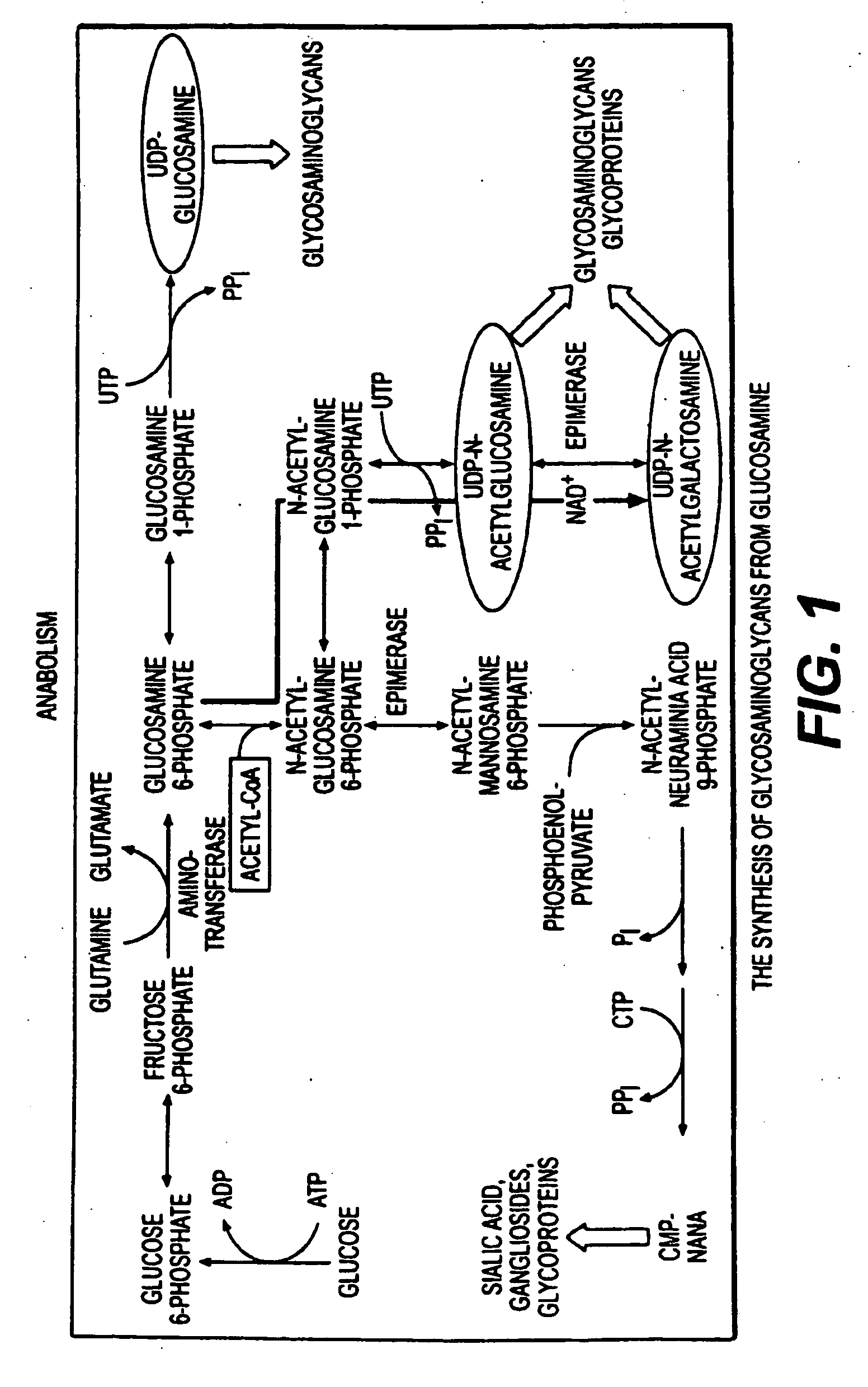
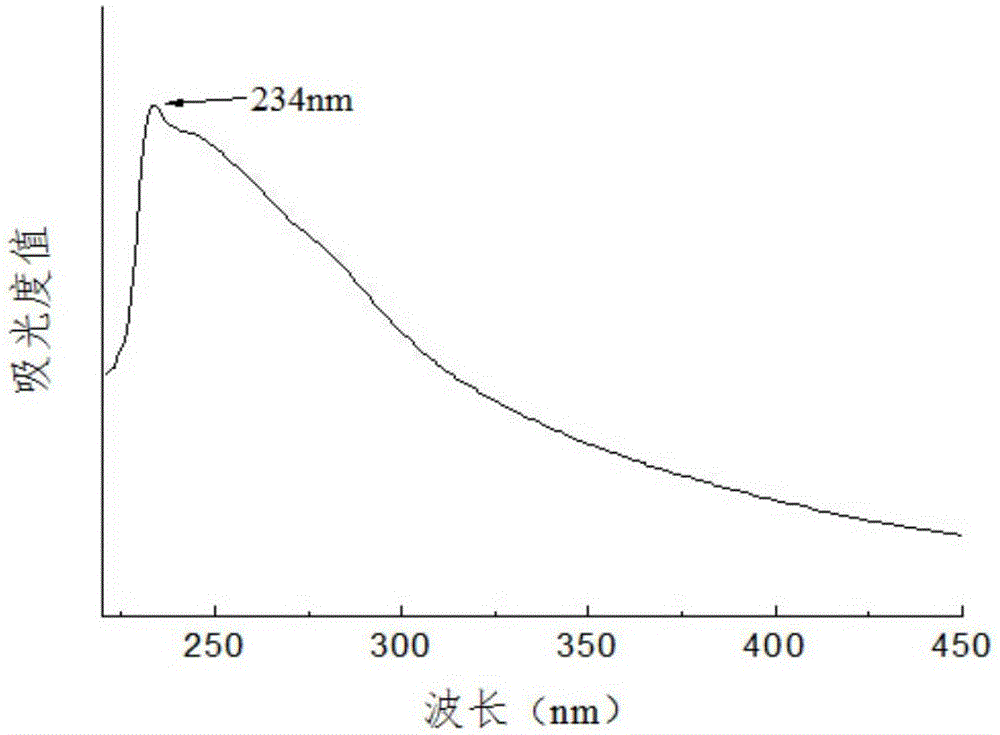
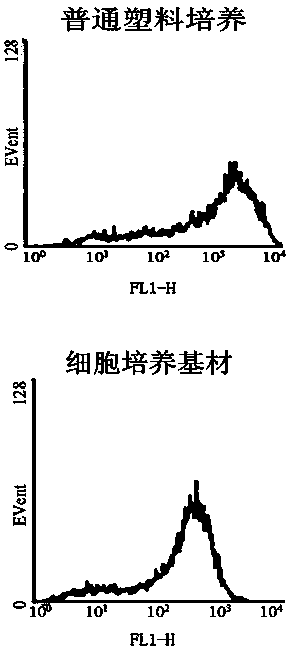
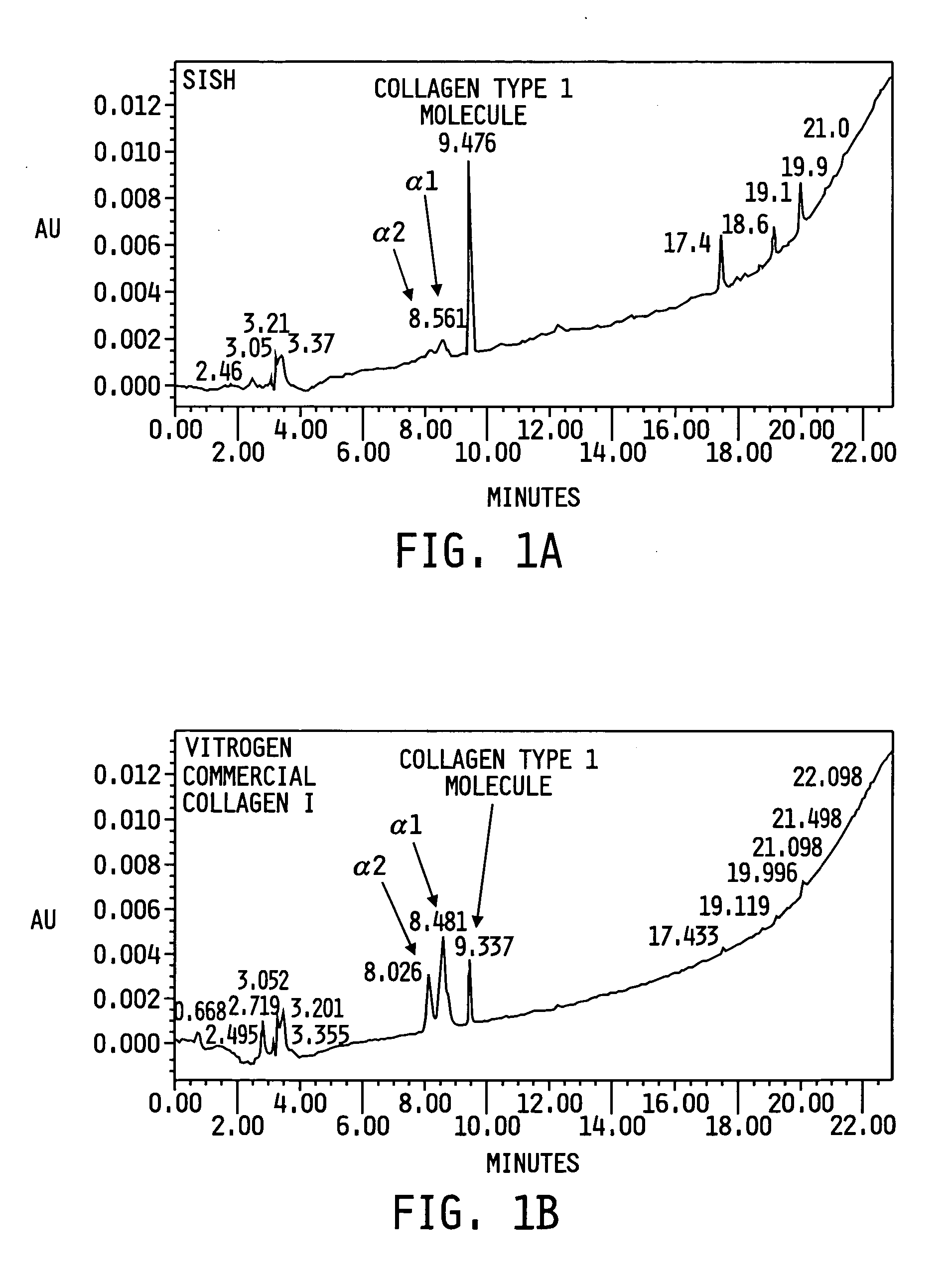
Patents
Literature
116 results about "Collagen type" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biomaterials with enhanced properties and devices made therefrom
ActiveUS20120059487A1Reduce thicknessLow profile insertionHeart valvesSurgeryCell membraneLong term durability
Biomaterials with enhanced properties such as improved strength, flexibility, durability and reduced thickness are useful in the fabrication of biomedical devices, particularly those subjected to continuous or non-continuous loads where repeated flexibility and long-term durability are required. These enhanced properties can be attributed to elevated levels of elastin, altered collagen types, and other biochemical changes which contribute to these enhanced properties. Examples of devices which would be improved by use of such tissue include heart valves, including percutaneous heart valves, and vascular grafts, patches and the like. Such enhanced materials can be sourced from specific populations of animals, such as neonatal calves, or in range-fed adult cattle, or can be fabricated or created from cell populations exhibiting such properties. In one embodiment, glutaraldehyde-fixed neonatal pericardial tissue is used to create leaflets in a percutaneous heart valve, and may be used without chemical fixation, with or without processes to remove residual cellular membranes, and utilized as a scaffold material for tissue engineering.
Owner:SOUTHERN LIGHTS VENTURES 2002
Collagen type I and type III compositions for use as an adhesive and sealant
InactiveUS20030032143A1Reduce riskInherently hemostatic propertiesFibrinogenSurgical adhesivesWound healingCollagen i
Polymerized type I and / or III collagen based compositions for medical use as adhesives and sealants and preparation thereof are described. Prior to polymerization, the collagen monomers are prepared recombinantly whereby chemical modifications of the collagen are not needed to form such monomers. The type I and / or III collagen compositions are useful as medical adhesives for bonding soft tissues or in a sealant film for a variety of medical uses. In a further aspect of the present invention, the polymerized type I and / or III collagen composition includes agents which induce wound healing or provide for additional beneficial characteristics desired in a tissue adhesive and sealant.
Owner:NEFF THOMAS B +1
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm<3>, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsilon-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Human stem cells originating from human amniotic mesenchymal cell layer
InactiveUS20060281178A1Stable supplyEffective drug deliveryNervous system cellsArtificial cell constructsCell layerOsteocyte
Owner:SAKURAGAWA NORIO +1
Chitosan collagen and calcium alginate compounded spongy biological dressing and its preparation process
The composite spongy biological dressing contains chitosan, collagen and calcium alginate with weigh tmixing ratio o f 0.5-8:0.5-8:0.1-8. Its preparation method includes the following steps: selecting chitosan and collagen type I, adding calcium alginate, compounding and cross-linking, using buffer solution to make neutralization, emulsifying, prefreezing and one-step freeze-drying so as to obtain the invented dressing with good biological compatibility and strong adhesion property. Said invented dressing possesses active function of promoting wound healing and hemostatic action, can be combined with anti-bacterial medicine to obtain gene engineeirng dressing for curing wound surface infection, also can be combined with active growth factor or active cell to form gene engineering dressingfor curing intractable ulcer and burn wound surface.
Owner:JIANGXI RUIJI BIOTECH CO LTD
Animal chew toy containing solid food
InactiveUS20080314333A1Easy to processImprove and protect healthPeptide/protein ingredientsBoron compound active ingredientsVitamin CManganese
An animal toy containing food having a first portion with food delivery means integrated therein adapted to securely house solid food treats, including a nutritional pet supplement, to be removed by the animal during play or through chewing action, and a second side with a plurality of gum stimulation teeth integrated within and projecting therefrom that act to massage the gums of the animal. Food delivery means may be provided as a plurality of cavities integrated within the first portion (and optionally, the second portion), preferably having a grooved or threaded interior for securely holding food pieces securely therein. Alternatively, the food delivery means is provided as a food portion composing the first portion. A pet supplement may be utilized, including joint preserving and joint rebuilding compositions comprising chicken collagen type II, glucosamine hydrochloride and chondroitin sulfate, a vitamin composition comprising vitamins C, D and K, a mineral composition comprising calcium, magnesium, zinc, copper, manganese and boron, a herbal anti-oxidant cofactor blend comprising citrus bioflavonoids, red grapes anthocyanins, turmeric rhizome, boswellia resin and fennel seed.
Owner:I DID IT
Carrier with solid fibrinogen and solid thrombin
InactiveUS7052713B2Safety and efficacyShorten hemostasis timePowder deliverySurgical adhesivesNatural sourceFiber
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibers. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin. The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
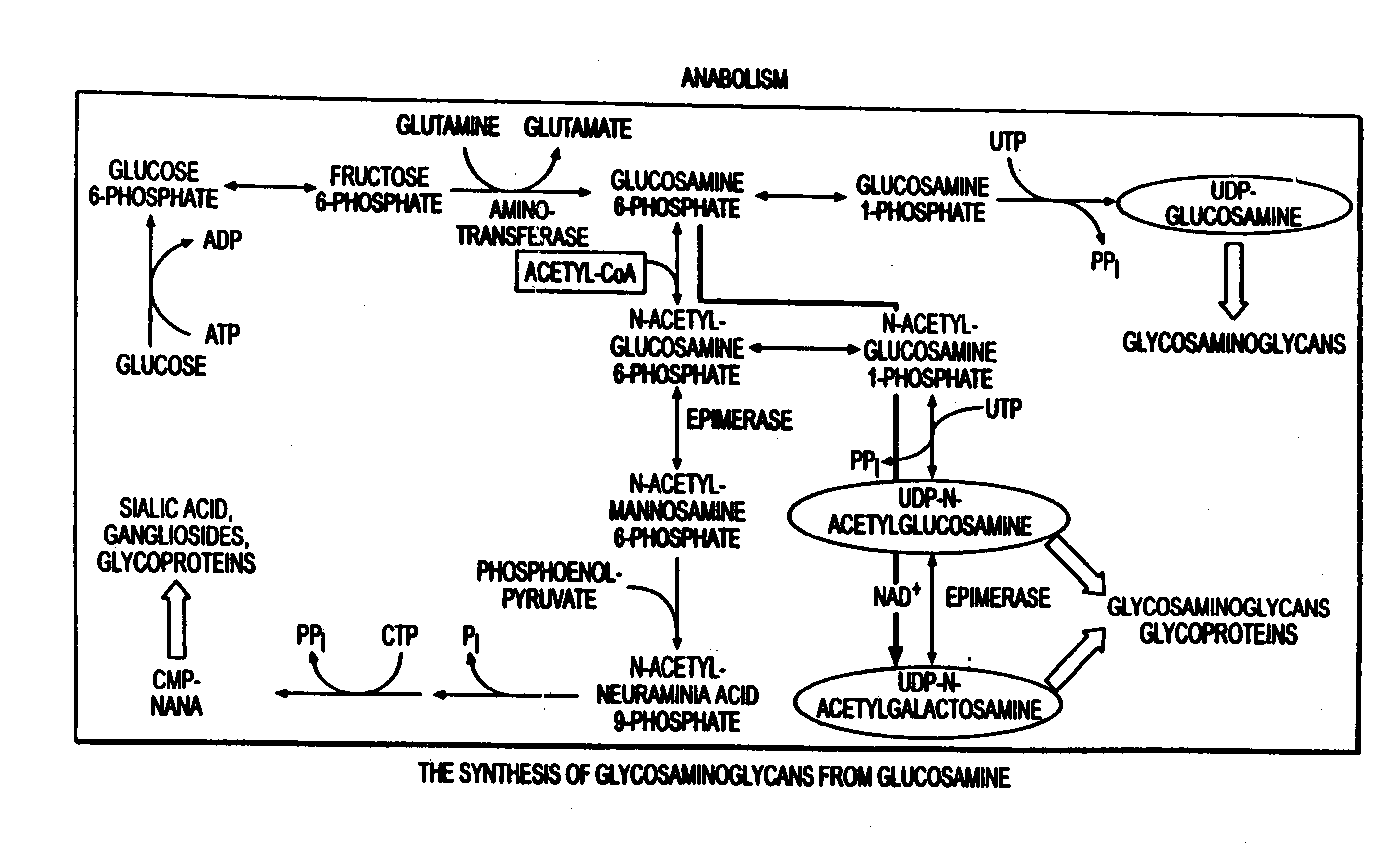
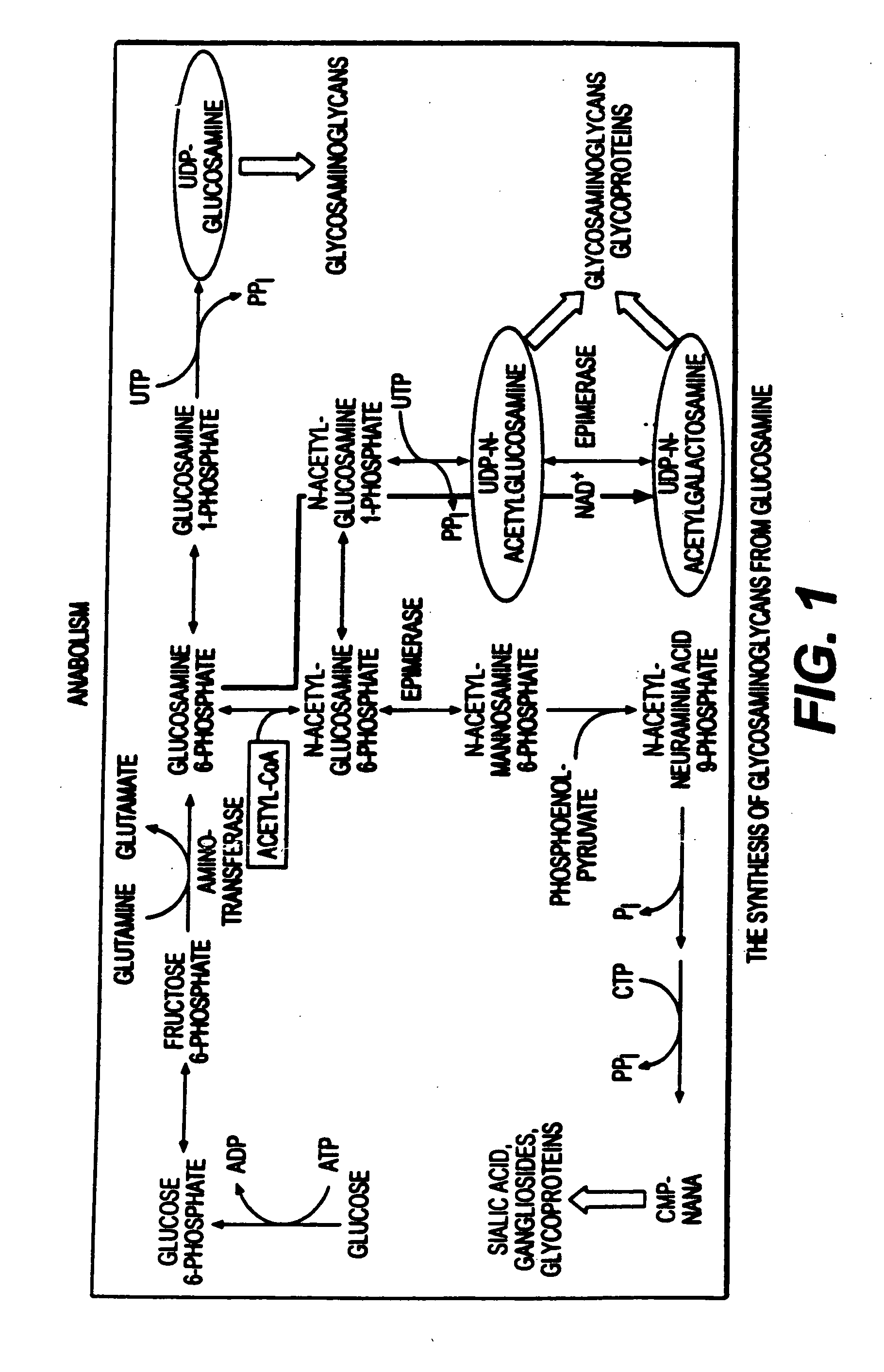
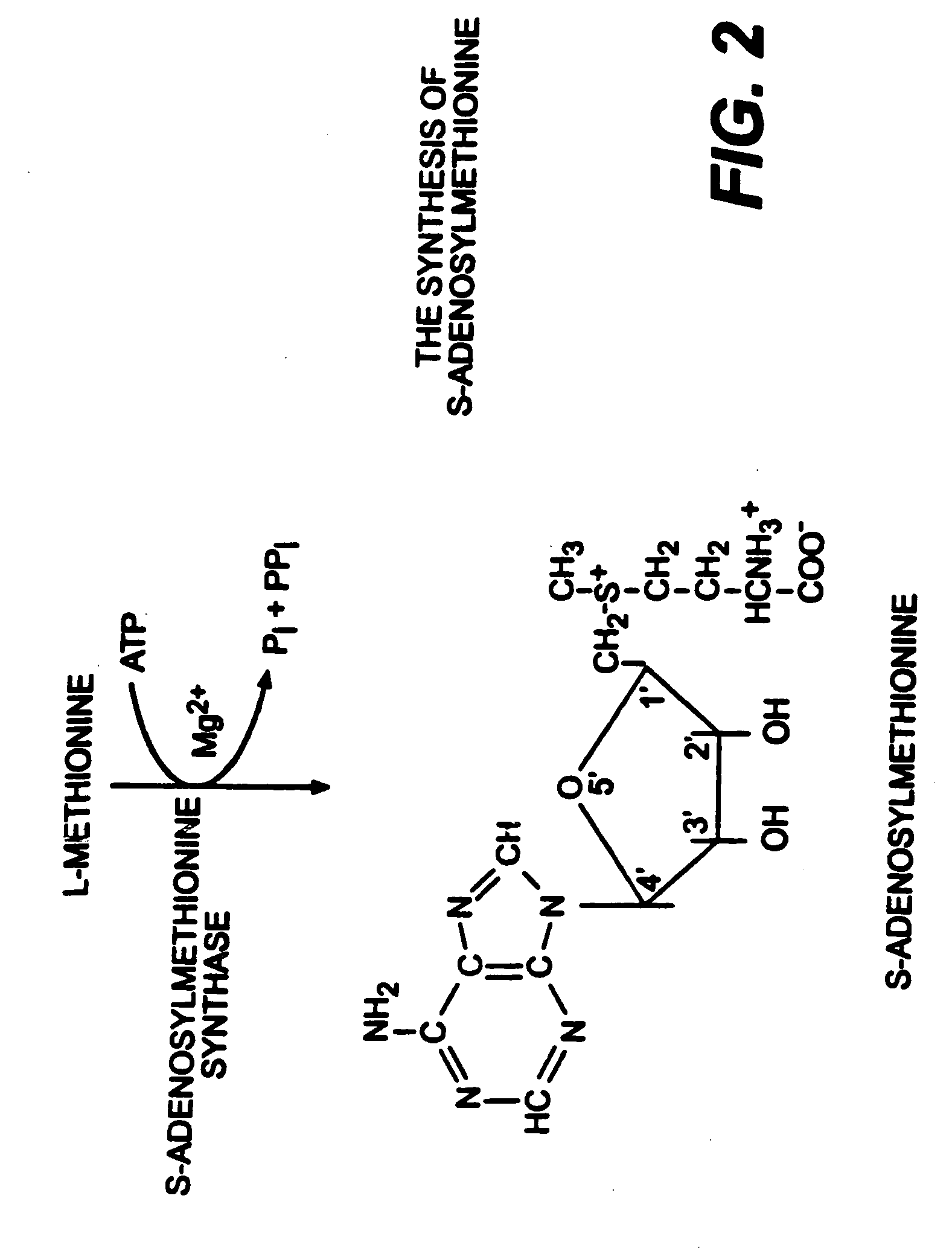
Use of anabolic agents, anti-catabolic agents, antioxidant agents and analgesics for protection, treatment and repair of connective tissues in humans and animals
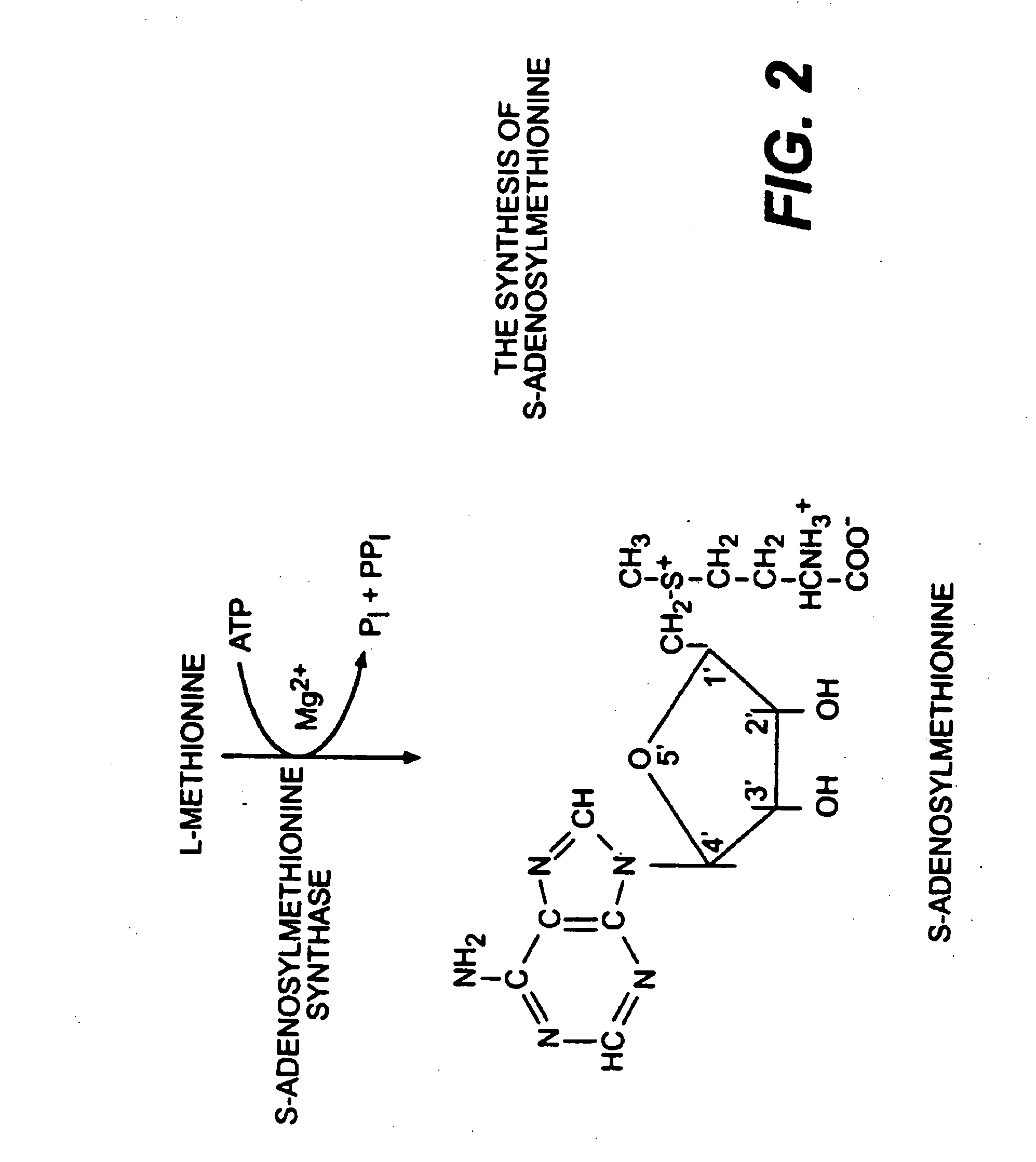
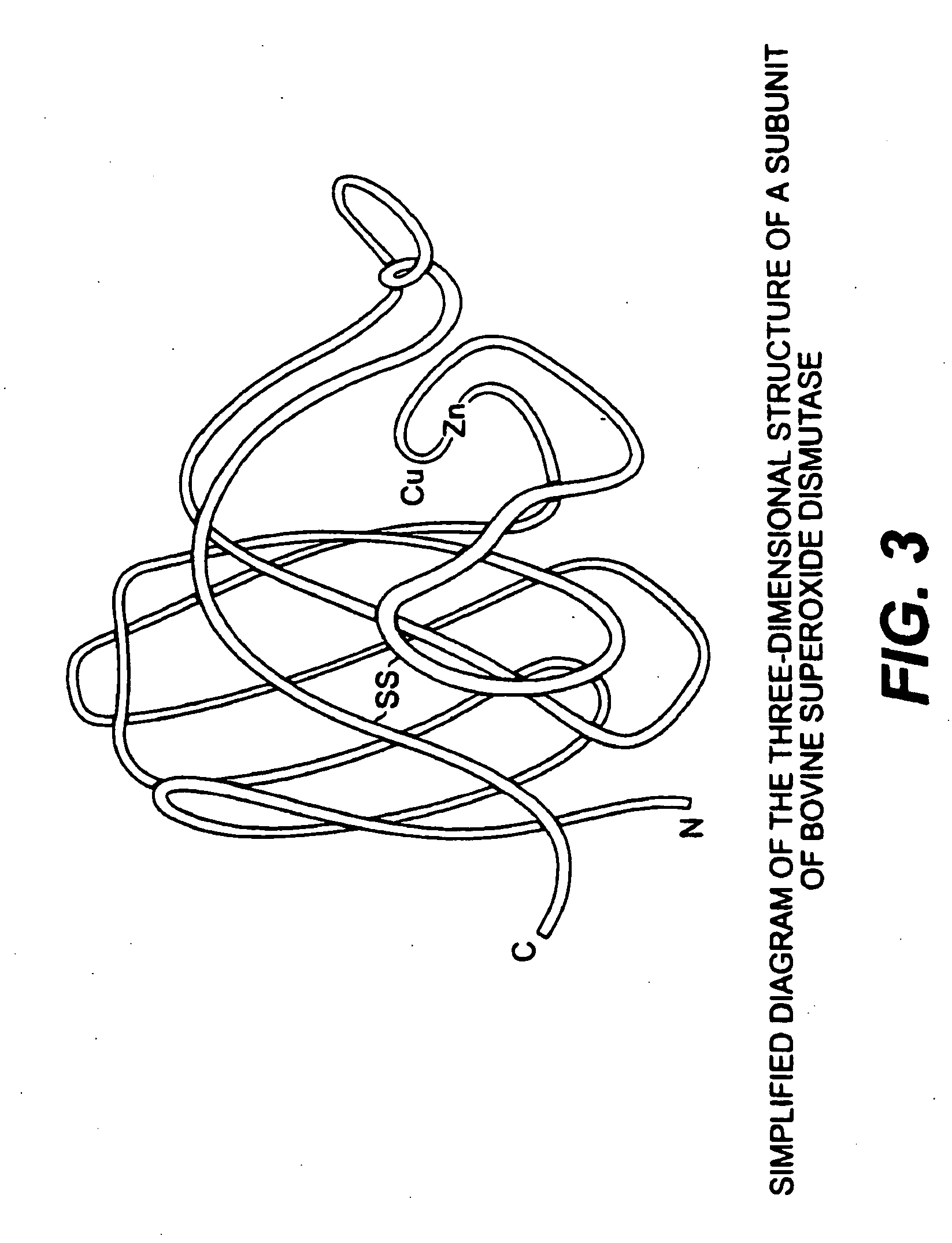
InactiveUS20090087503A1Repair and treat and prevent damageBiocideElcosanoid active ingredientsSuper oxide dismutaseS-Adenosyl methionine
The present invention relates to compositions for the protection, treatment and repair of connective tissues in humans and animals comprising any or all of anabolic, anti-catabolic, anti-oxidant and analgesic agents, including aminosugars, S-adenosylmethionine, arachadonic acid, GAGs, including pentosan, collagen type II, tetracyclines or tetracycline-like compounds, diacerin, super oxide dismutase, L-ergothionine, one or more avocado / soybean unsaponifiables, and an analgesic, e.g., acetaminophen, and to methods of treating humans and animals by administration of these novel compositions to humans and animals in need thereof.
Owner:NUTRAMAX LABORATORIES INC
Disrupted cartilage products
InactiveUS20140030309A1Increase flexibilityBiocidePeptide/protein ingredientsMedicineCollagen matrices
This invention provides disrupted cartilage products, methods of manufacturing disrupted cartilage products, and methods of treating a subject comprising administering a cartilage product. The cartilage products are manufactured by a method comprising disrupting a collagen matrix, e.g. to produce a flexible cartilage product. Optionally, the cartilage products comprise viable chondrocytes, bioactive factors such as chondrogenic factors, and a collagen type II matrix. Optionally, the cartilage products are non-immunogenic.
Owner:OSIRIS THERAPEUTICS
Human stem cells originating from human amniotic mesenchymal cell layer
InactiveUS20100330672A1Stable supplyEfficient deliveryNervous system cellsSkeletal/connective tissue cellsCell layerOsteocyte
Neural stem cells which can be provided stably and which are free from the problem of compatibility in transplantation are disclosed. The stem cells are separated from human amniotic mesenchymal cell layer and express vimentin, nestin and BrdU which are markers of neural stem cells. The stem cells can also be differentiated to cells expressing alkaline phosphatase, that is, osteocytes, and to cells expressing collagen type II, that is, chondrocytes.
Owner:SAKURAGAWA NORIO +1
Methods and compositions for growing corneal endothelial and related cells on biopolymers and creation of artifical corneal transplants
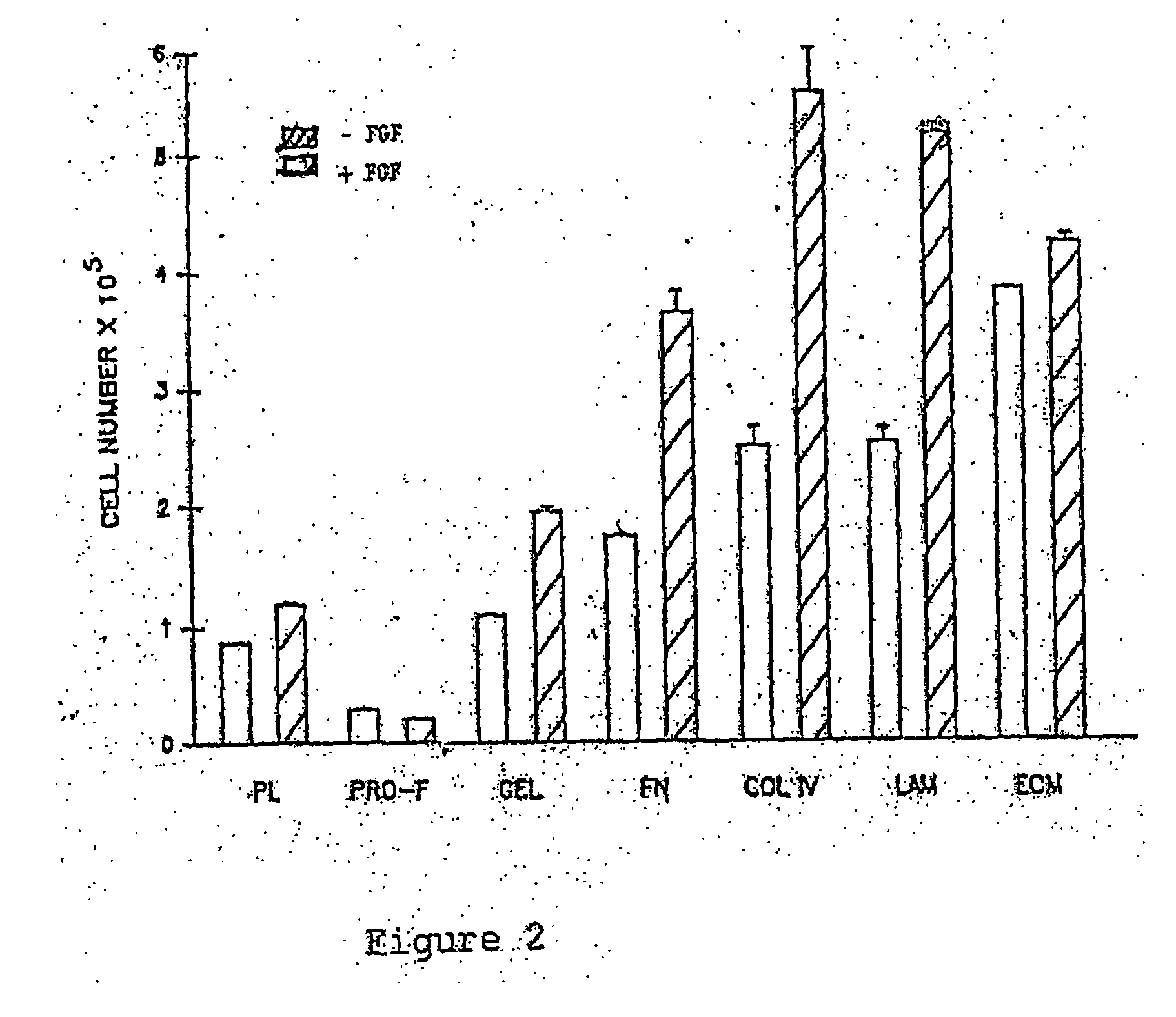
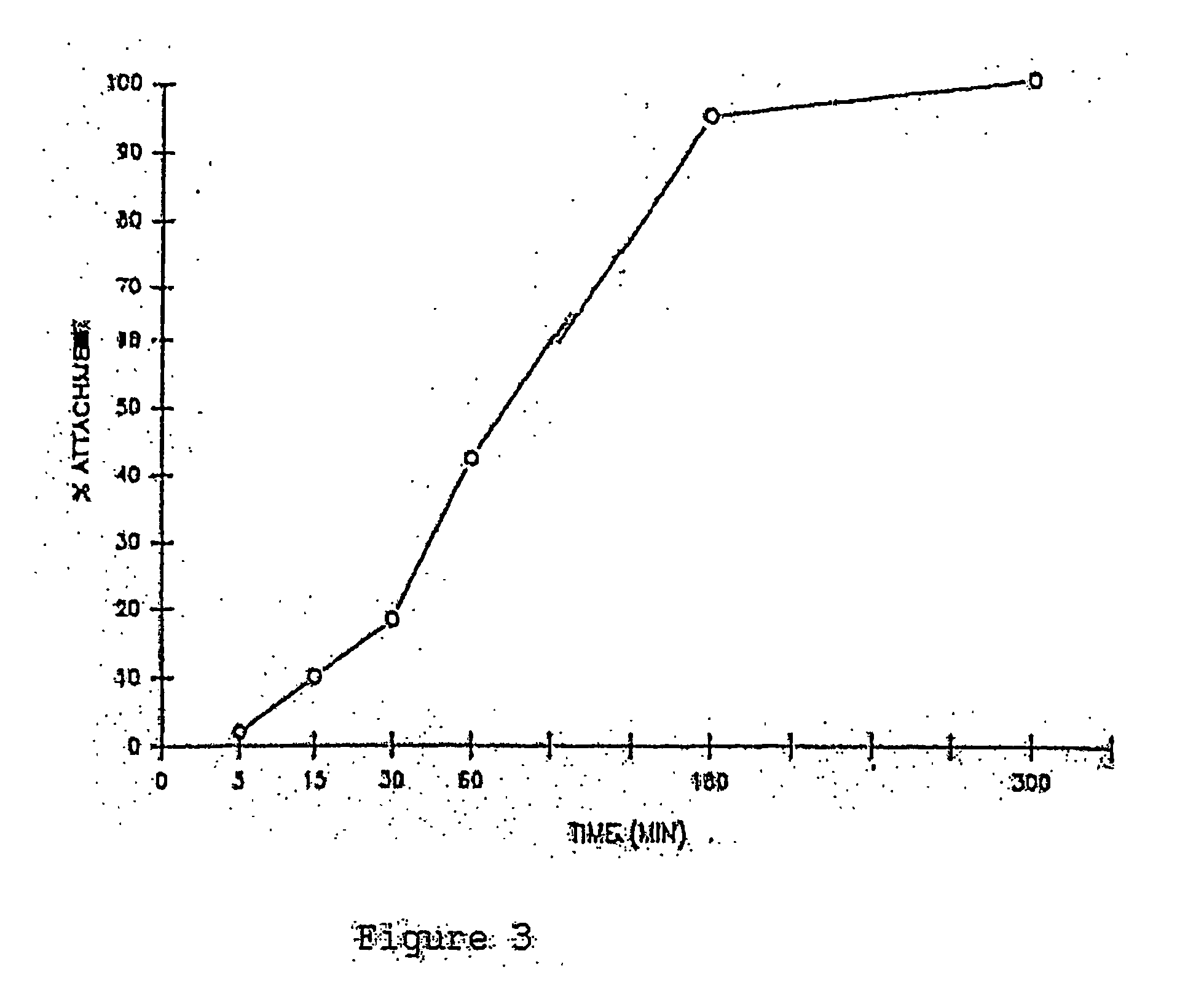
This invention discloses methods to attach and grow a monolayer of cultured human corneal endothelial cells onto the endothelial side of the stroma synthesized from biopolymer to generate a more bio-equivalent artificial cornea. The approaches will include the use of attachment and growth promoting agents such as fibronectin, laminin, RGDS, collagen type IV, bFGF conjugated with polycarbophil, and EGF conjugated with polycarbophil. The patent also describes a method to create a self-sustaining polymer containing adhesive molecules and growth factors to support the attachment and proliferation of cultured human corneal endothelial cells for corneal transplantation either as a half-thickness device or full-thickness button replacement. An approach for the implantation of cultured retinal pigment epithelial (RPE) cells into the sub-retinal space for treatment of age-related macular degeneration (ARMD) is disclosed in this invention. This method will enable the delivery of the transplanted RPE in a sheet of monolayer cells and will be better suited to perform their physiological function.
Owner:CELLULAR BIOENG
Beautifying and nursing essence and preparation method thereof
InactiveCN103908424AAvoid allergic reactionsNo side effectsCosmetic preparationsToilet preparationsCuticleUmbilical cord tissue
The invention discloses a beautifying and nursing essence and a preparation method thereof. The essence is prepared from an epidermis cell nutrient solution and contains a plurality of cell growth factors, amino acids and vitamins. The essence mainly comprises an epidermal growth factor, a fibroblast growth factor, collagen type I, a stem cell growth factor, a horn cell growth factor and amino acids and vitamins including gamma-aminobutyric acid, coenzyme Q, medical vitamin C, medical vitamin B5 and medical vitamin E. The preparation method for the essence comprises the following steps: acquisition of epidermis of umbilical cord tissue of a newborn; cell culture; collection of a cell nutrient solution; filtration sterilization; condensation of a volume; addition of active components; addition of glycerin and hyaluronic acid; etc. The beautifying and nursing essence provides a plurality of endogenous nutritional ingredients with physiological activity for the skin, can promote metabolism of epidermis cells and repair damaged cells, has a moisture retention effect and is applicable to skin beautifying and nursing.
Owner:奥思达干细胞有限公司
Porated cartilage products
This invention provides porated cartilage products, methods of producing porated cartilage products, and methods of treating subjects by administering cartilage products. Optionally, the cartilage products are sized, porated, and digested to provide a flexible cartilage product. Optionally, the cartilage products comprise viable chondrocytes, bioactive factors such as chondrogenic factors, and a collagen type II matrix. Optionally, the cartilage products are non-immunogenic.
Owner:OSIRIS THERAPEUTICS
Use of anabolic agents, anti-catabolic agents, antioxidant agents, and analgesics for protection, treatment and repair of connective tissues in humans and animals
InactiveUS20070141181A1Repair and treat and prevent damageBiocideAntipyreticSuper oxide dismutaseS-Adenosyl-l-methionine
The present invention relates to compositions for the modulation of inflammation in connective tissues in humans and animals and the modulation of markers of such inflammation, including COX-2, TNF-α, IL-1β, iNOS, p38, and chemokines, comprising any or all of anabolic, anti-catabolic, anti-oxidant and analgesic agents, including aminosugars, S-adenosylmethionine, arachadonic acid, GAGs, including pentosan, collagen type II, tetracyclines or tetracycline-like compounds, diacerin, super oxide dismutase, L-ergothioneine, methylsulfanylmethane, one or more avocado / soybean unsaponifiables, and an analgesic, e.g., acetaminophen, and to methods of treating humans and animals by administration of these novel compositions to humans and animals in need thereof.
Owner:NUTRAMAX LABORATORIES INC
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin. The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsi-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
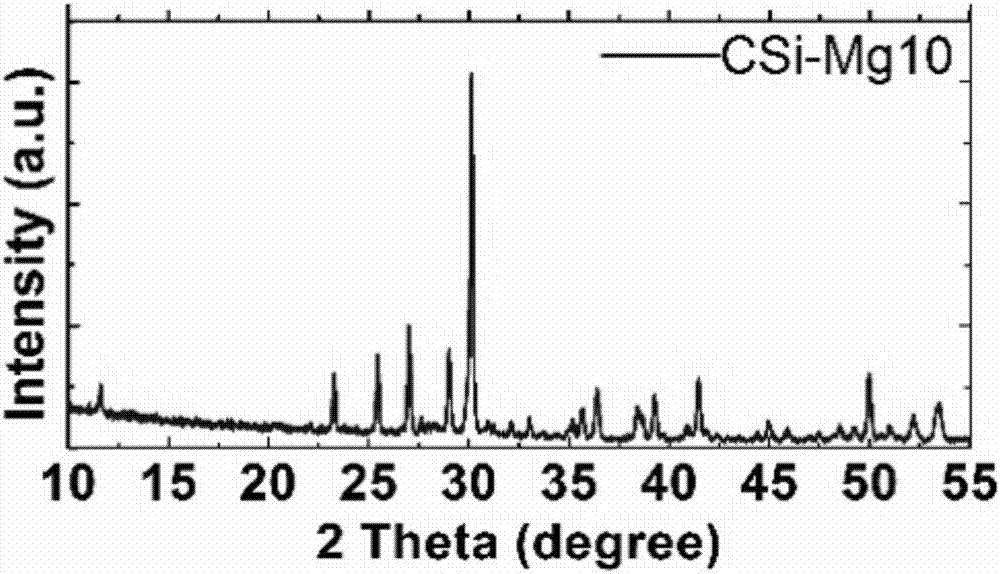
Method for inducing bionic calcification in collagen fibers through polymer polyelectrolyte, and applications thereof
ActiveCN107224609AObvious superiorityHigh mechanical strengthPharmaceutical delivery mechanismTissue regenerationFiberCationic polyelectrolytes
The present invention relates to a method for inducing the bionic calcification in collagen fibers through a polymer polyelectrolyte, and applications thereof. The method comprises: treating different types of collagen scaffolds in a calcium phosphate mineralized solution containing a cationic polyelectrolyte or anionic polyelectrolyte to obtain the bionic calcification collagen scaffold material having the inside-fiber mineralization characteristic, wherein the collagen scaffold is recombinant collagens type I having different sources, completely demineralized bone collagen, completely demineralized dentin collagen, or rat tail collagen, and the mineralization liquid is a calcium phosphate solution added with a cationic polyelectrolyte or anionic polyelectrolyte. According to the present invention, through the stabilizing effect of the polyelectrolyte on the calcium phosphate solution, the novel bionic calcified collagen scaffold material with characteristics of similar natural bone tissue structure, good mechanical property, good biocompatibility and suitable biodegradability is constructed.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Scar repair material
The invention discloses a scar repair material which is prepared by the following method: dissolving 0.5-5 parts by weight of water-soluble chitosan or chitosan the deacetylation degree of which is 75-97% into 10-100 parts by weight of water or an acetic acid aqueous solution the mass concentration of which is 1-5% while stirring; adding 1-3 parts by weight of polyoxyethylene Sorbitan lauric acid monoester which has emulsifying action and 5-10 parts by weight of humectant glycerol to obtain a transparent gelatinous solution; adding 20-90 parts by weight of polydimethylsiloxane into the gelatinous solution under homogeneity; and evenly mixing to obtain the scar repair material. The scar repair material disclosed in the invention can actively and passively moisturize skin, has the efficacy on actively resisting and inhibiting bacteria, has the action on quickening the healing of skin wound surface, has better repair and protection actions on tiny flaws and infection on the scar surface as being used on the scar surface, and can inhibit the secretion of collagen type I in cheloid skin so as to lower the ratio of collagen type I / collagen type III.
Owner:TIANJIN JIASHITANG SCI & TECH
Large-scale preparation method of fish scale type I collagen peptides
ActiveCN104140992AAvoid pollutionAvoid consumptionChemical industryPeptide preparation methodsCollagen VIImpurity
The invention relates to a large-scale preparation method of fish scale type I collagen peptides. The preparation method comprises the following steps: firstly, performing alkali and acid pretreatment on marine fish scales or freshwater fish scales serving as raw materials to remove impurities, and directly performing directed enzymatic hydrolysis on collagen type I in the fish scales by adopting a one-step biological enzymatic hydrolysis technology to form collagen peptides type I with relatively centralized molecular weight distribution; secondly, separating, purifying and concentrating directed enzyme liquid at the normal temperature by adopting a continuous centrifugal separation and membrane separation combined technology to obtain a fish scale type I collagen peptide solution with purity of not less than 95% and molecular weight of less than 1,000 Dalton; finally, quickly drying finished fish scale type I collagen peptides by adopting a spray-drying technology. The method is simple and feasible in overall process, efficient, energy-saving, short in production cycle and suitable for large-scale production; a fish scale type I collagen peptide product produced by adopting the method is high in purity and good in quality, and the content of peptides with molecular weight of less than 1,000 Dalton is more than 97%.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
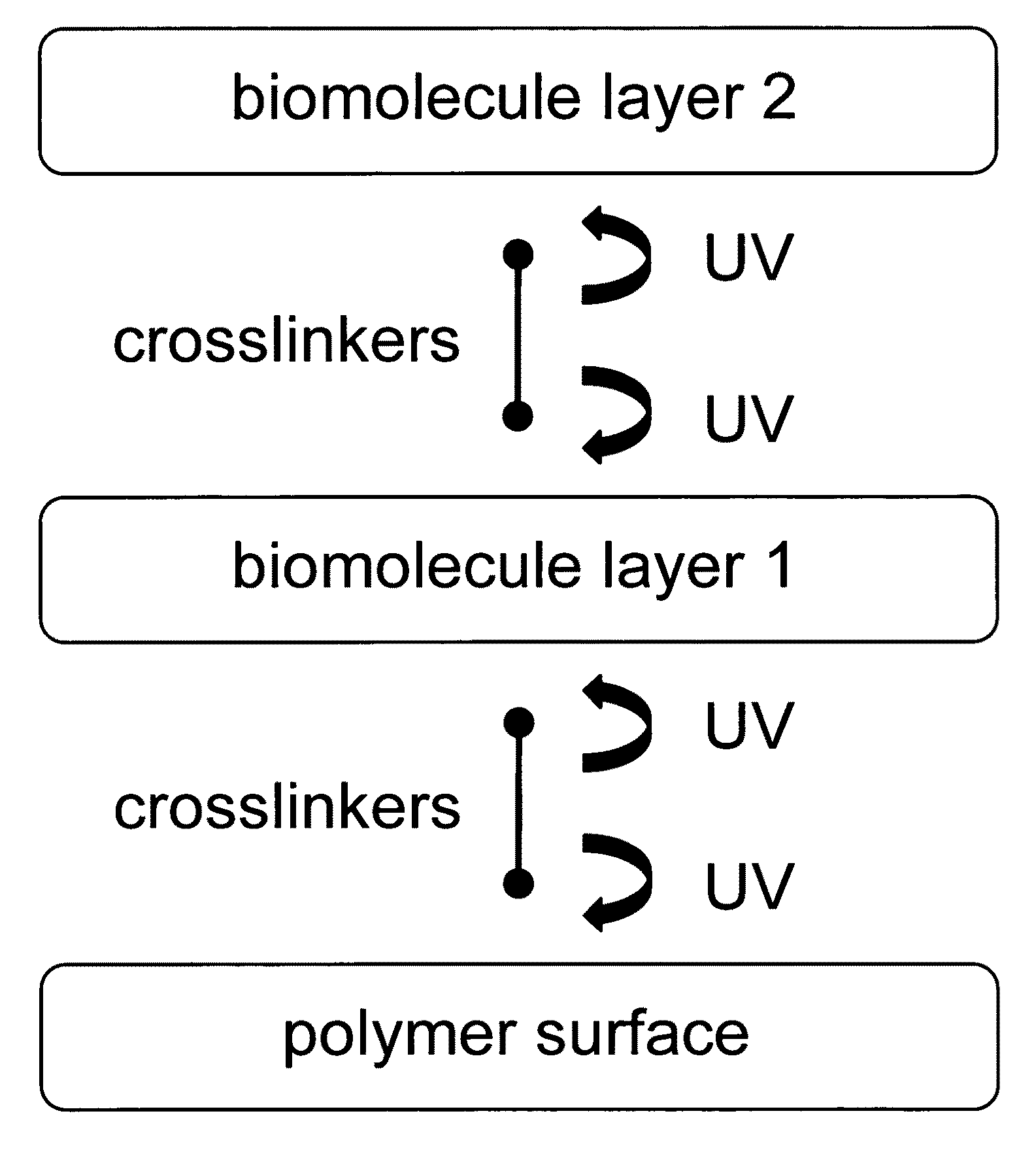
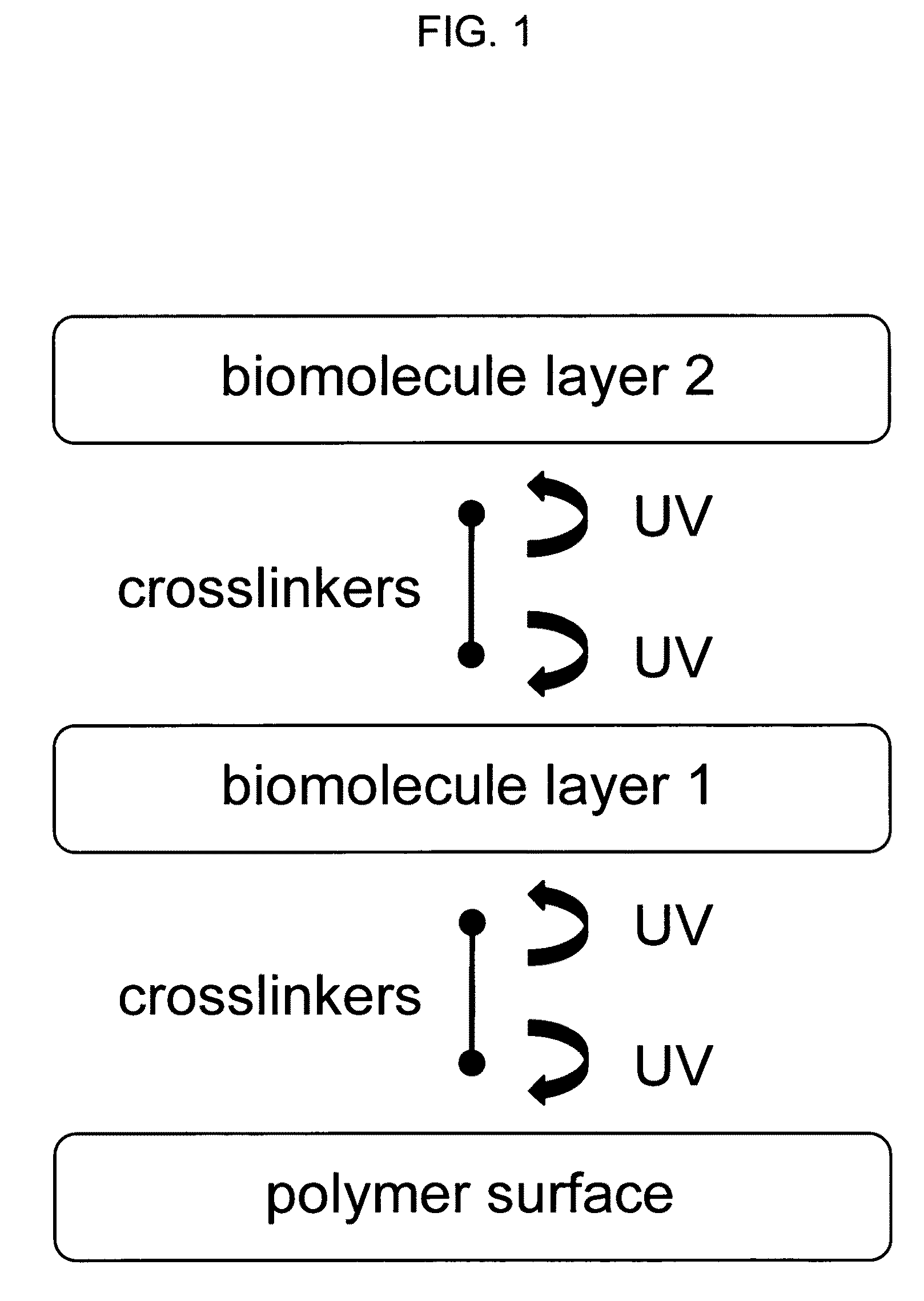
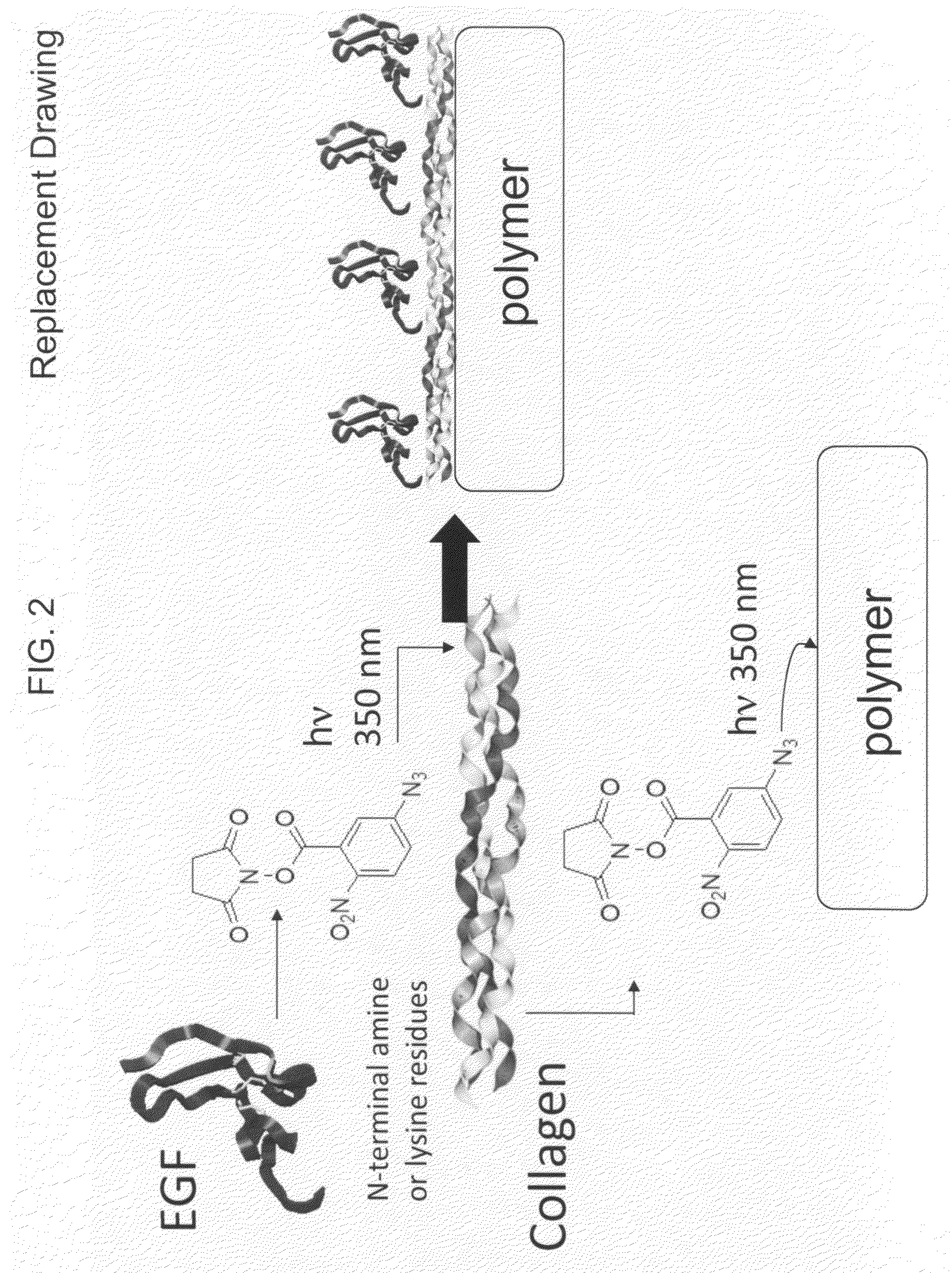
Sequential coupling of biomolecule layers to polymers
InactiveUS20090117166A1Used in fieldPowder deliveryPeptide/protein ingredientsCell-Extracellular MatrixECM Protein
A bio-mimetic or bio-implantable material based on a sequential process of coupling biomolecule layers to a polymer layer is provided. In general, the material could be based on two or more biomolecule layers starting with one of the layers covalently linked to the polymer layer via cross-linkers and the other layers sequentially and covalently linked using cross-linkers to the previously added layer. The polymer layer could be a hydrogel or an interpenetrating polymer network hydrogel. The first layer of biomolecules could be a collagen type, fibronectin, laminin, extracellular matrix protein, or any combinations thereof. The second layer of biomolecules typically is a growth factor, protein or stimulant. The cross-linkers are either water soluble or insoluble bifunctional cross-linkers or azide-active-ester crosslinkers. The material and process as taught in this invention are useful in the field of tissue engineering and wound healing.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
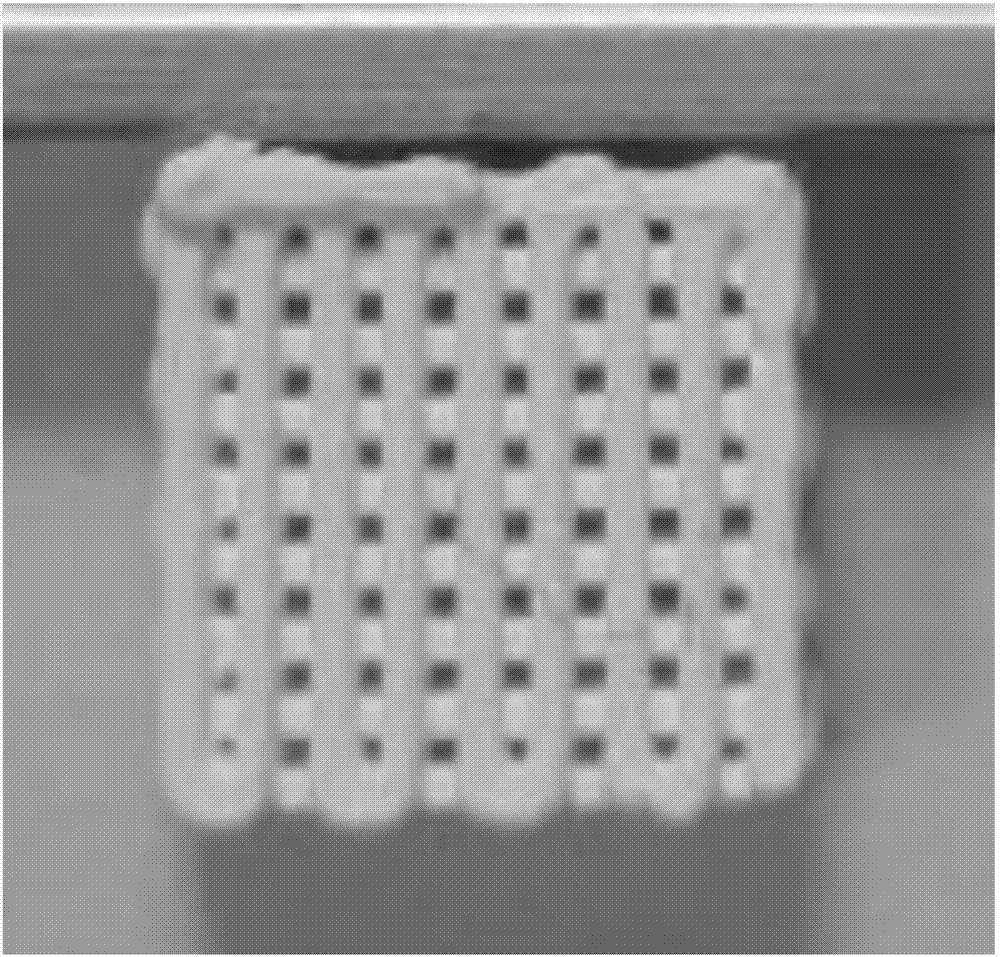
3D printed sodium alginate-collagen type I-ceramic composite scaffold and preparation method and application thereof
ActiveCN107376017AIncreased proliferationHigh activityAdditive manufacturing apparatusTissue regenerationCeramic compositeMass ratio
The invention discloses a 3D printed sodium alginate-collagen type I-ceramic composite scaffold and a preparation method and application thereof. The composite scaffold is mainly composed of sodium alginate, collagen type I and calcium magnesium silicate according to a mass ratio of (1-6): 2: (0-10) and utilizes calcium chloride as a crosslinking agent, wherein a mass fraction of calcium chloride in the composite scaffold is 1 to 10%. The preparation method comprises dispersing the calcium magnesium silicate powder in deionized water, fully mixing the solution, compounding the solution, collagen type I and sodium alginate to obtain uniform hydrogel, placing the hydrogel in a 3D printer, carrying out crosslinking with calcium chloride and printing a porous scaffold which is the composite scaffold. The composite scaffold can promote chondrocyte proliferation and ALP activity, has excellent biological activity and has an application value in tissue engineering.
Owner:ZHEJIANG UNIV
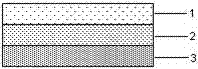
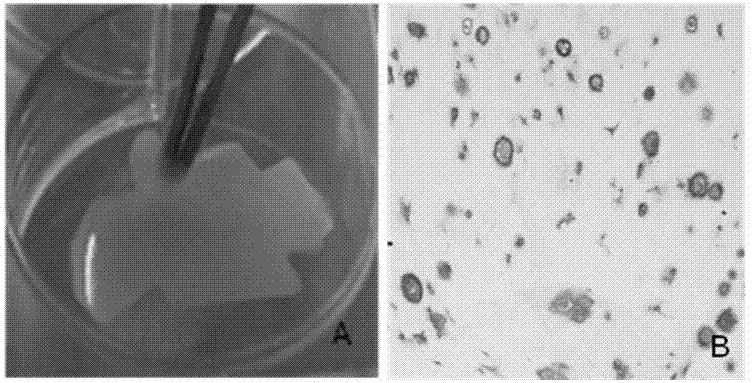
Articular cartilage graft and preparation method thereof
ActiveCN103920190AAvoid allergiesLower immune responseJoint implantsCell-Extracellular MatrixCartilage lesion
An articular cartilage graft and a preparation method thereof are provided. The prepared articular cartilage graft is composed of a superficial layer, a middle layer and a deep layer from outside to inside; the thickness, shape and size of each layer are all matched with a cartilage injury part, and thus preoperative shaping is not required; after grafting, the articular cartilage graft has layer distribution corresponding to distribution of each layer of surrounding normal cartilages, is conducive to intercellular signal transmission, transduction and regulation, and can be better integrated with surrounding normal cartilage tissues; the articular cartilage graft has structure characteristics consistent with those of the natural cartilages, the collagen type II content is gradually decreased from the superficial layer to the deep layer, the GAG content is increased gradually from the superficial layer to the deep layer, and compression resistance and wear resistance are good; and chondrocytes in the cartilage graft are wrapped with an extracellular matrix, have low immunogenicity, allow generation of immunologic rejection to be avoided after grafting, can survive for a long term and exert functions, and improve cartilage repair long-term curative effects.
Owner:西安博鸿生物技术有限公司
Cardiac fibroblast-derived extracellular matrix and injectable formulations thereof for treatment of ischemic disease or injury
InactiveUS20160354447A1Effective treatmentReduce injuriesAntibacterial agentsPowder deliveryDiseaseExtremity injury
Compositions and methods using an engineered cardiac fibroblast-derived 3-dimensional extracellular matrix (ECM) are disclosed. The ECM includes the structural proteins fibronectin, collagen type I, collagen type III, and elastin, and from 60% to 90% of the structural proteins present in the engineered extracellular matrix are fibronectin. The compositions, which can be used to treat cardiac disease or ischemic disease or injury, are injectable compositions, where the ECM is diced into small fragments or lyophilized into a powder. The disclosed methods include a method of treating ischemic disease or injury by contacting a cell free patch made from the ECM with the injured tissue, without the concomitant delivery of therapeutic cells, and a method of treating ischemic limb injury by contacting a patch, either by itself or seeded with therapeutic cells, with the injured limb tissue.
Owner:WISCONSIN ALUMNI RES FOUND
Methods and devices for treating dural puncture
A device for repairing a dural puncture, including: a patch formed of collagen type of material that includes a bulbous head portion that is larger than the puncture opening, and a neck portion that is proportioned to fit through the puncture opening.
Owner:PERPER YAKOV +1
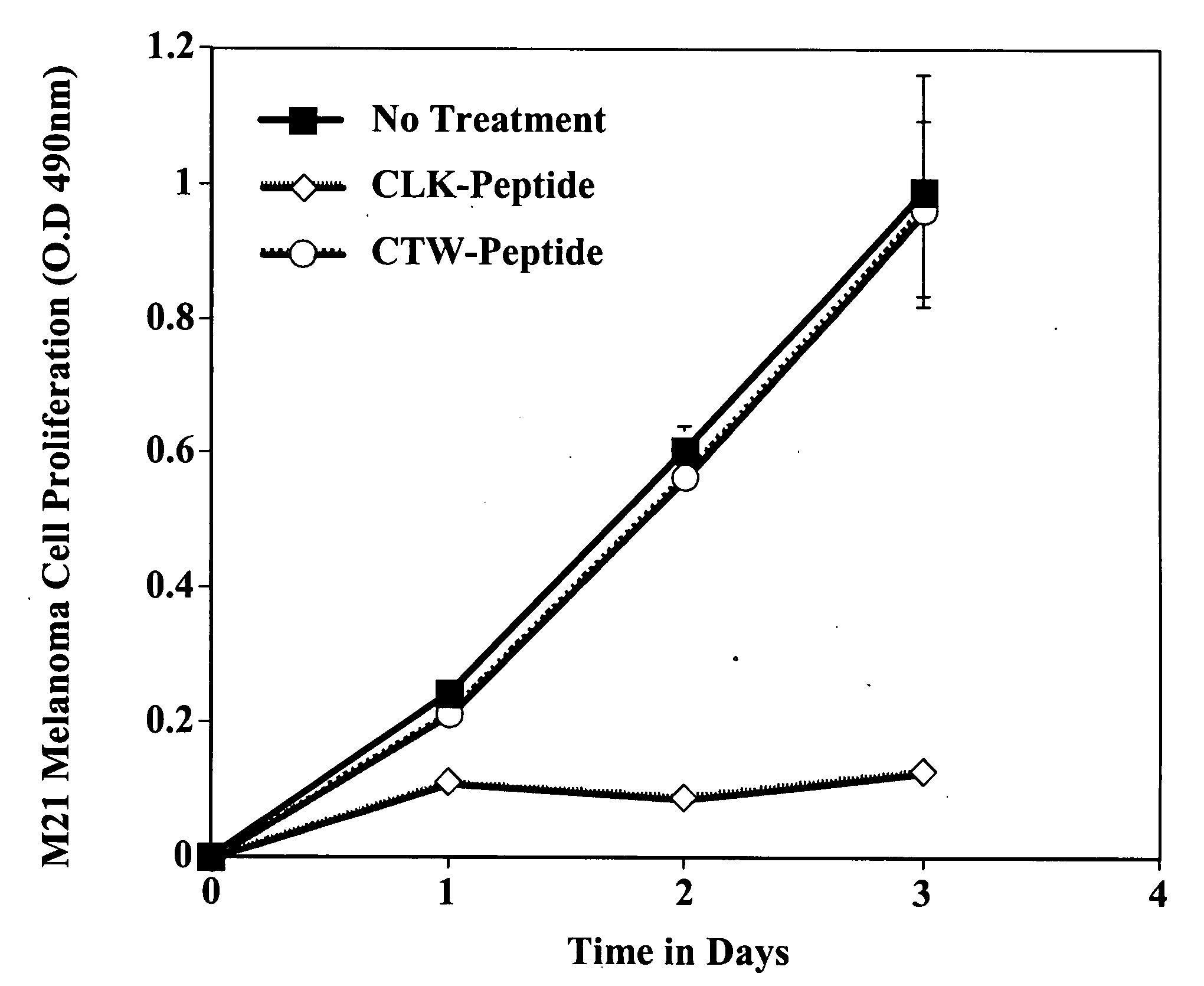
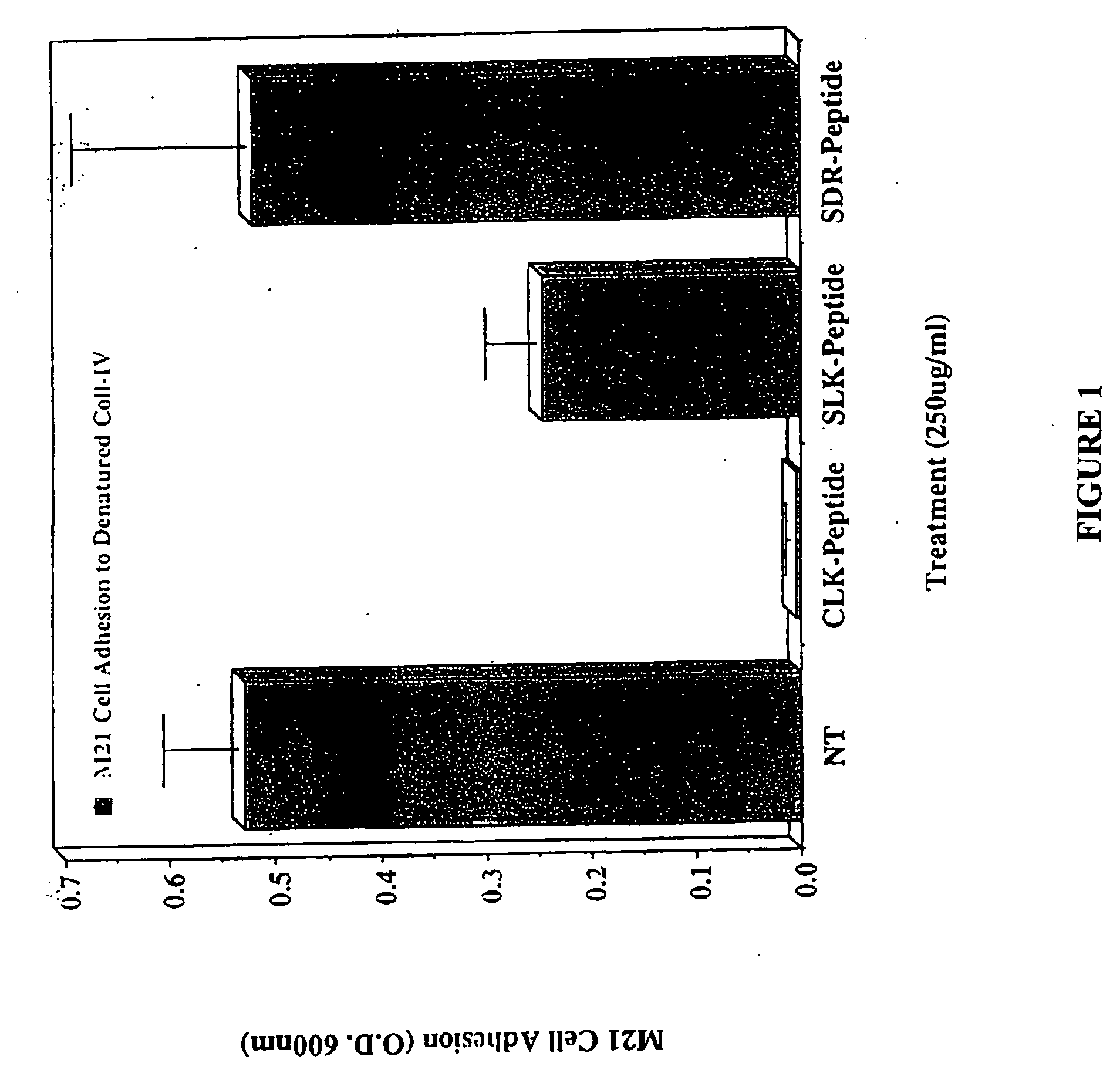
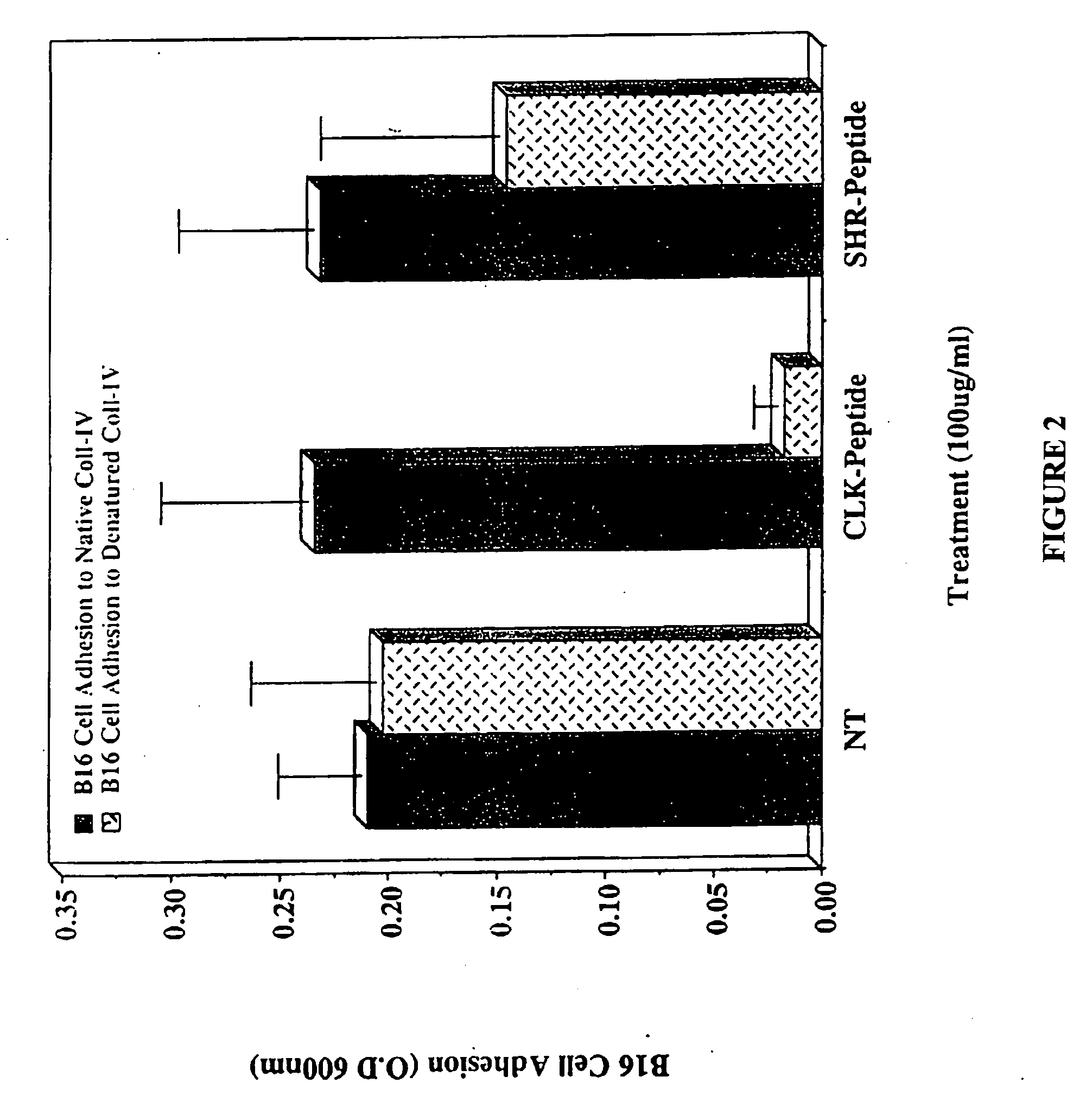
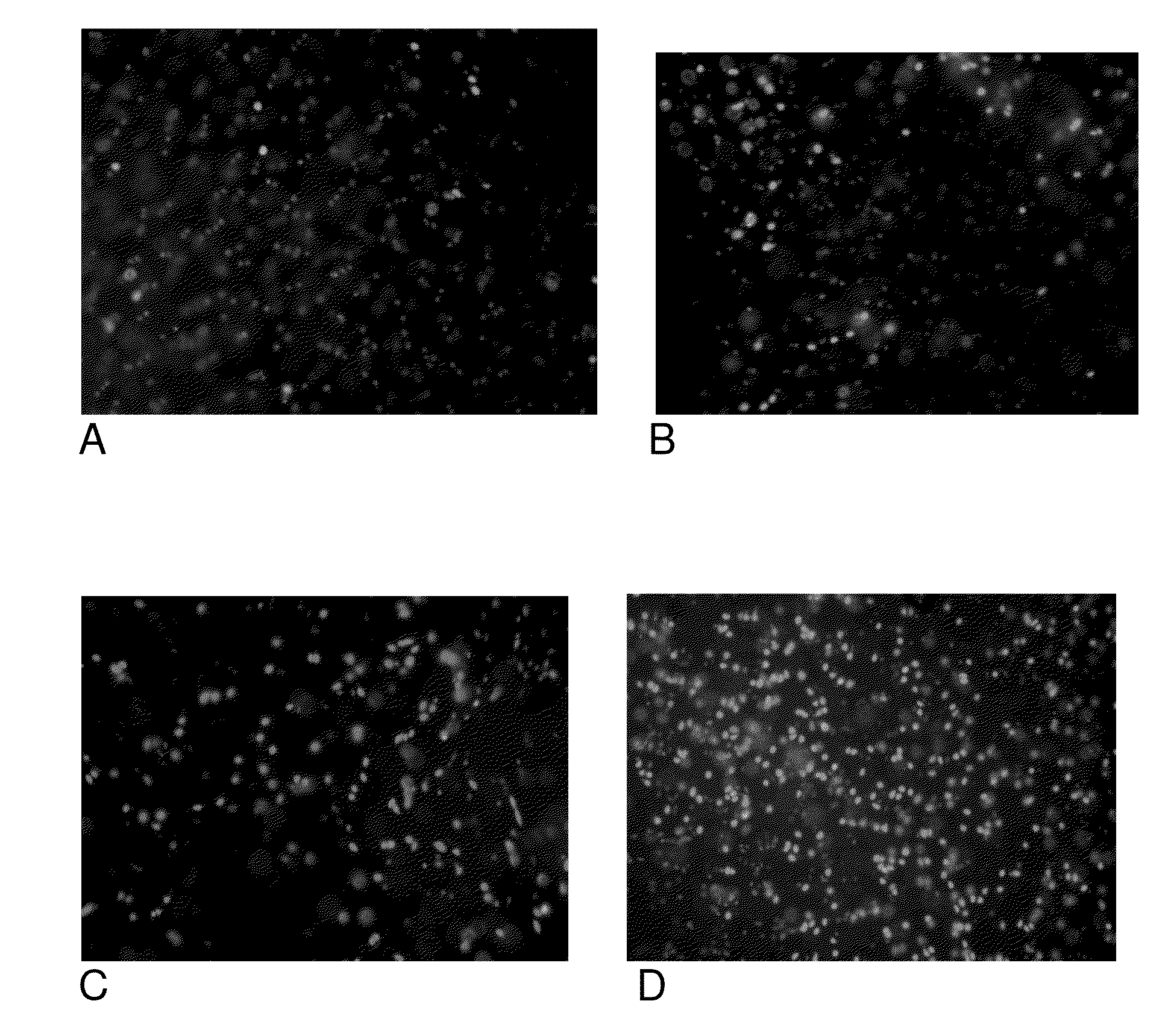
CLK-peptide and SLK-peptide
InactiveUS20070259817A1Improve efficiencyElectrotherapyPeptide/protein ingredientsAbnormal tissue growthLymphatic Spread
The invention describes methods for inhibiting angiogenesis in a tissue by administering an antagonist that specifically binds to a proteolyzed or denatured collagen type-IV with substantially greater affinity than to the native triple helical form of collagen type-IV. Methods utilizing such antagonists for therapeutic treatment of tumor growth, tumor metastasis or of restenosis also are described, as are methods to use such antagonists as diagnostic markers of angiogenesis in normal or diseased tissues both in vivo and ex vivo. The invention further describes methods for treating tumors using said antagonists in combination with radiation therapy and therapies comprising the the antagonists and radiation treatment.
Owner:NEW YORK UNIV
Methods of manufacturing cartilage products
This invention provides porated cartilage products and methods of producing porated cartilage products. Optionally, the cartilage products are sized, porated, and digested to provide a flexible cartilage product. Optionally, the cartilage products comprise viable chondrocytes, bioactive factors such as chondrogenic factors, and a collagen type II matrix. Optionally, the cartilage products are non-immunogenic.
Owner:OSIRIS THERAPEUTICS
Method for producing human collagen type II
ActiveCN104313053AImprove stabilityImprove hydrophilicityConnective tissue peptidesPeptide preparation methodsProtein structureCollagen VI
The invention discloses a method for producing human collagen type II, and belongs to the technical field of gene engineering. The method is characterized in that a baculovirus multi-gene expression system is adopted, insect cells are infected by the bacteria converting the baculovirus multi-gene expression system to produce baculovirus with multiple genes expressed, the virus liquid is infected in the insect cells or injected into silkworm larva bodies to produce recombinant human collagen type II full-length protein, the protein structure is that 3 peptide chains with (G-X-Y) n repetitive sequences form a three-strand helical structure, to both keep the conformation of the natural collagen type II and have the activity of the natural collagen type II. By adopting the method disclosed by the invention, multiple genes can be expressed to facilitate the production of then collagen type II full-length protein; the method disclosed by the invention is simple in process method, simple and convenient to operate and short in process route.
Owner:SOUTH CHINA AGRI UNIV +2
Method for extracting pure type II collagen from chicken cartilage
The invention discloses a method for extracting pure type II collagen from chicken cartilage. The method is characterized by including, using the fresh chicken cartilage as raw material, removing other non-cartilage components, performing degreasing pretreatment, using mixed solution of 4mol / L NaCl and 0.05mol / L Tris-HCl to remove soluble impure protein, using mixed solution (pH 7.5) of 4mol / L guanidine hydrochloride and 0.05mol / L Tris-HCl to remove insoluble impure protein, using acetic acid solution containing 1-2% pepsin for solid matter enzymolysis, then centrifuging to take supernate, adding NaCl until final concentration is 3-4.2mol / L, 1.2-1.8mol / L and 0.8-1.0mol / L, successively salting out to remove other types of collagen, repeatedly adding NaCl until the final concentration is 0.8-1.0mol / L, salting out for 2-5 times, and dialyzing to obtain the pure type II collagen. The type II collagen produced by the method is single in type, high in bioactivity, stable in physical and chemical property. Meanwhile, the method is simple to operate and capable of benefiting large-scale production.
Owner:SICHUAN UNIV
Cell culture substrate, and preparation method and application thereof
InactiveCN103497892AHigh amplification efficiencyMaintain immunophenotypeCell culture supports/coatingSkeletal/connective tissue cellsCell culture mediaCollagen Type III
The invention belongs to the technical field of biological material of tissue engineering. The invention discloses a cell culture substrate, a preparation method thereof, and an application thereof. According to the invention, umbilical cord fibroblast cell self-secretion substrate is adopted; and through decellularization treatment and drying and sterilization, a natural tissue engineering material is obtained. The material comprises collagen type I, collagen type III, fibronectin, laminin, decorin, and the like, and can be used in in-vitro amplification of target mesenchymal stem cells. With the cell culture substrate provided by the invention, mesenchymal stem cell in-vitro amplification efficiency can be substantially improved, special immune phenotype of stem cells can be maintained, and stem cell active oxygen content can be substantially reduced. Also, raw material source is wide, and no ethic and moral problem is caused.
Owner:SUN YAT SEN UNIV
Self-assembling protein matrix prepared from natural extracellular matrices
The present invention provides a method for preparing a biomaterial involving extracting Collagen Type I (Col I) protein from small intestine submucosa (SIS) under conditions that preserve the native helical configuration. The extract of the present method contains a substantial amount of the alpha helical form of Col I. Further the present method includes inducing the Col I protein to form a polymer material in solution or on a target surface.
Owner:DEPUY PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com