Patents
Literature
31results about How to "Safety and efficacy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
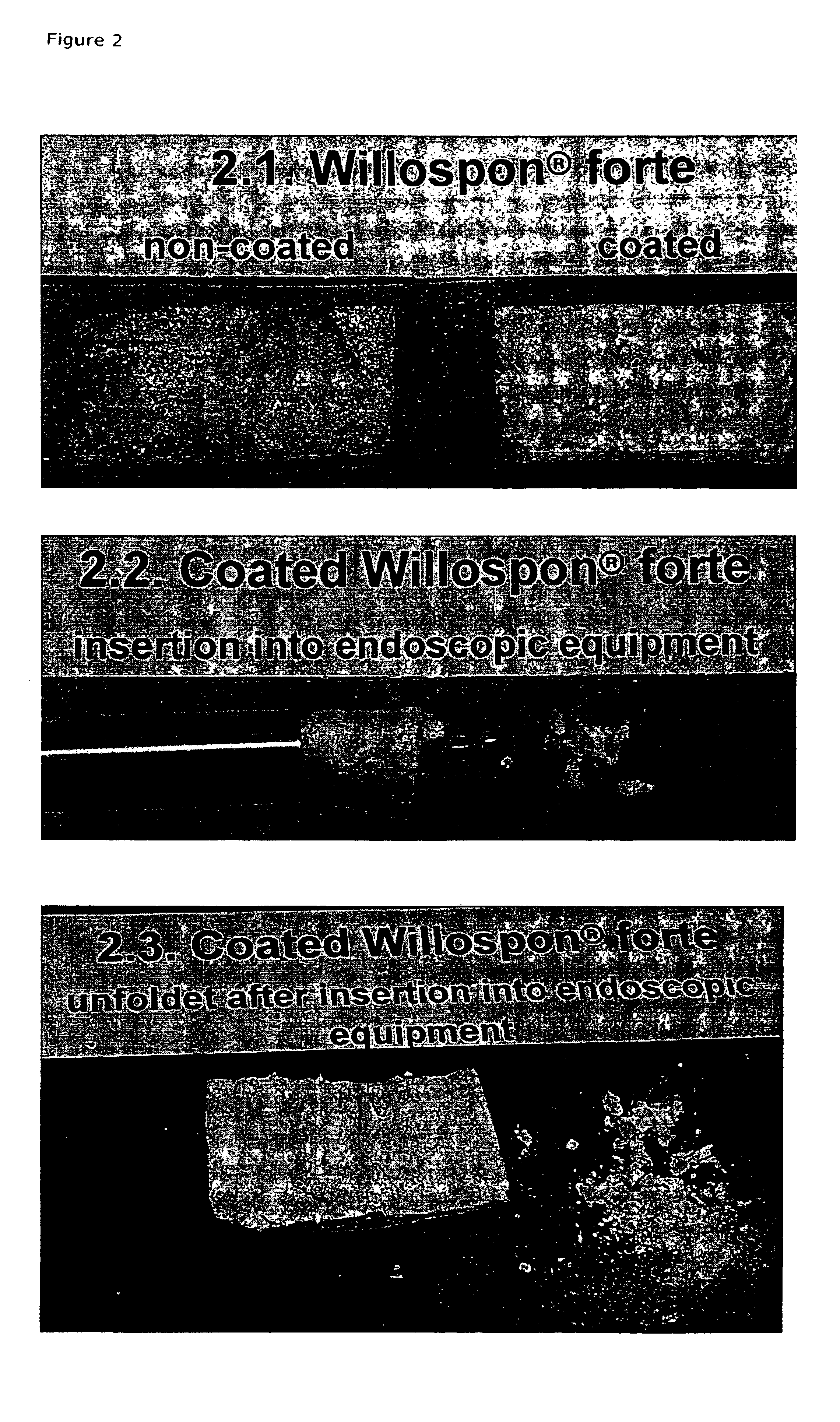
Carrier with solid fibrinogen and solid thrombin
InactiveUS7052713B2Safety and efficacyShorten hemostasis timePowder deliverySurgical adhesivesNatural sourceFiber
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibers. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine
InactiveUS20040175398A1Efficient responseSafety and efficacyViral antigen ingredientsMicrobiological testing/measurementHuman useDiagnostic test
This invention relates to methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. This invention is one that expands on current use of vaccinia virus propagation developed for gene therapy applications, and pharmaceuticals and nutraceuticals packaging and formualtion technologies. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete, lytic virus replication, or it may be derived from such a virus, contain additional immunogens, or be delivered as viral antigens. Furthermore, the invention establishes innovative methods for formulation and packaging and for preclinical testing of the vaccine invention for safety, efficacy and potency with the use of human intestinal and other test cells and diagnostic test systems and kits.
Owner:INCELLS
Methods and elements for identifying the appropriate patient populations who can safely use prescription chronic care drugs that are switched to an over-the-counter regulatory status
InactiveUS20130218586A1Safety and efficacyDrug and medicationsMedical automated diagnosisDiseaseDrugs label
A method of administering an over the counter drug for treatment of a chronic medical condition to a patient is provided wherein a drug label for over-the-counter treatment of a chronic medical condition is created which requires a new patient to take an initial point-of-service diagnostic test to determine whether the patient suffers from the condition treated by the drug and requires patients already receiving the drug on an over-the-counter basis to take one or more follow up point-of-service diagnostic tests to determine whether the patient is benefiting from the drug and are not experiencing undue side effects. Patients determined eligible to take the drug may then be provided with a random access code for use in association with a lockable, electromechanical cabinet by means of which the patient can receive an appropriate dose of the drug to treat the patient's chronic medical condition. In certain embodiments, a follow up point-of-service diagnostic test determines whether the patient is experiencing any undue side effects from the drug. In further embodiments, a national patient registry for containing the point of service diagnostic test results is created for access by appropriate health care professionals.
Owner:HUSER FREDERIC J
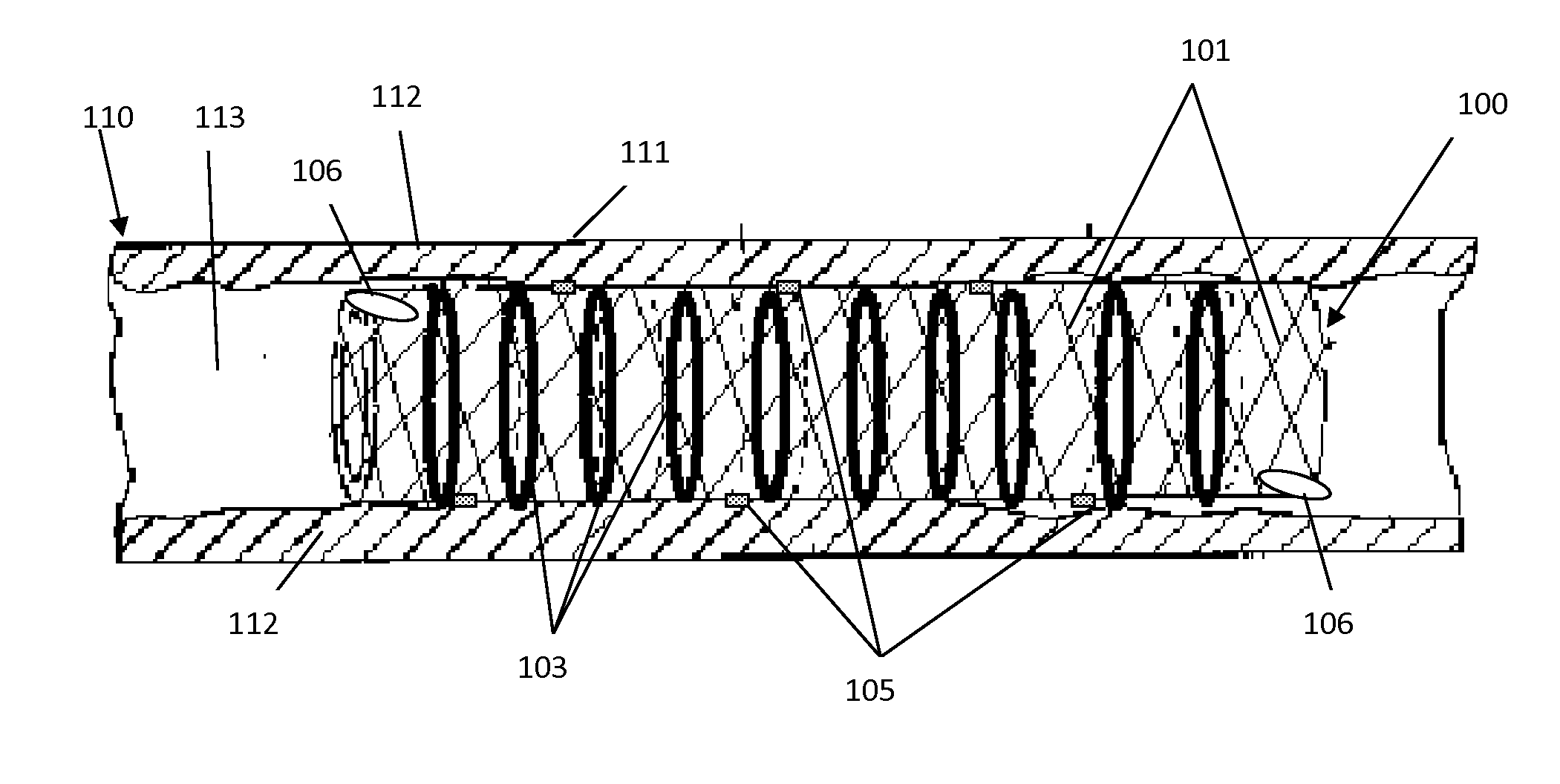
Sensor Actuated Stent
InactiveUS20130041454A1Avoid complicationsSafety and efficacyStentsBlood vesselsVascular lumenRadial position
The invention is an implantable medical device to expand a vascular lumen into various positions, internally or from a remote location. The device is designed as a stent having a framework for radial or longitudinal expansion, including one or more integrated shape memory materials. The shape memory materials, or halos, radially expand to fix the framework into a first radial position. Sensors integrated with the framework mechanically or electro-mechanically monitor and control the positioning of the framework to a fixed second position, or gradually expand the framework to various radial positions. Visualization and communications devices assist in the monitoring and controlling mechanisms to position the implantable device during surgery or catherization, post-surgery, or at follow-up. The device is biocompatible, alleviating complications. The device can be utilized as a substitute, or in combination with another stent. Devices may be utilized in cardiovascular, neurovascular surgery or other intervention.
Owner:ITRACKEVERYTHING LLC
Dry powder inhalation device for the simultaneous administration of more than one medicament
InactiveUS20090250056A1Enables the adjustment of the inhaler's resistanceLower resistanceRespiratorsSmall article dispensingAdditive ingredientBlisters
The present invention relates to a dry powder inhalation device which is suitable for the simultaneous administration of a combination of pharmaceutical ingredients, wherein each pharmaceutical ingredient is packed in a separate blister of the same single dose blister strip. The medicaments that form the combination come into contact just before their exit from the mouthpiece of the device.
Owner:PENTAFRAGAS DIMITRIOS
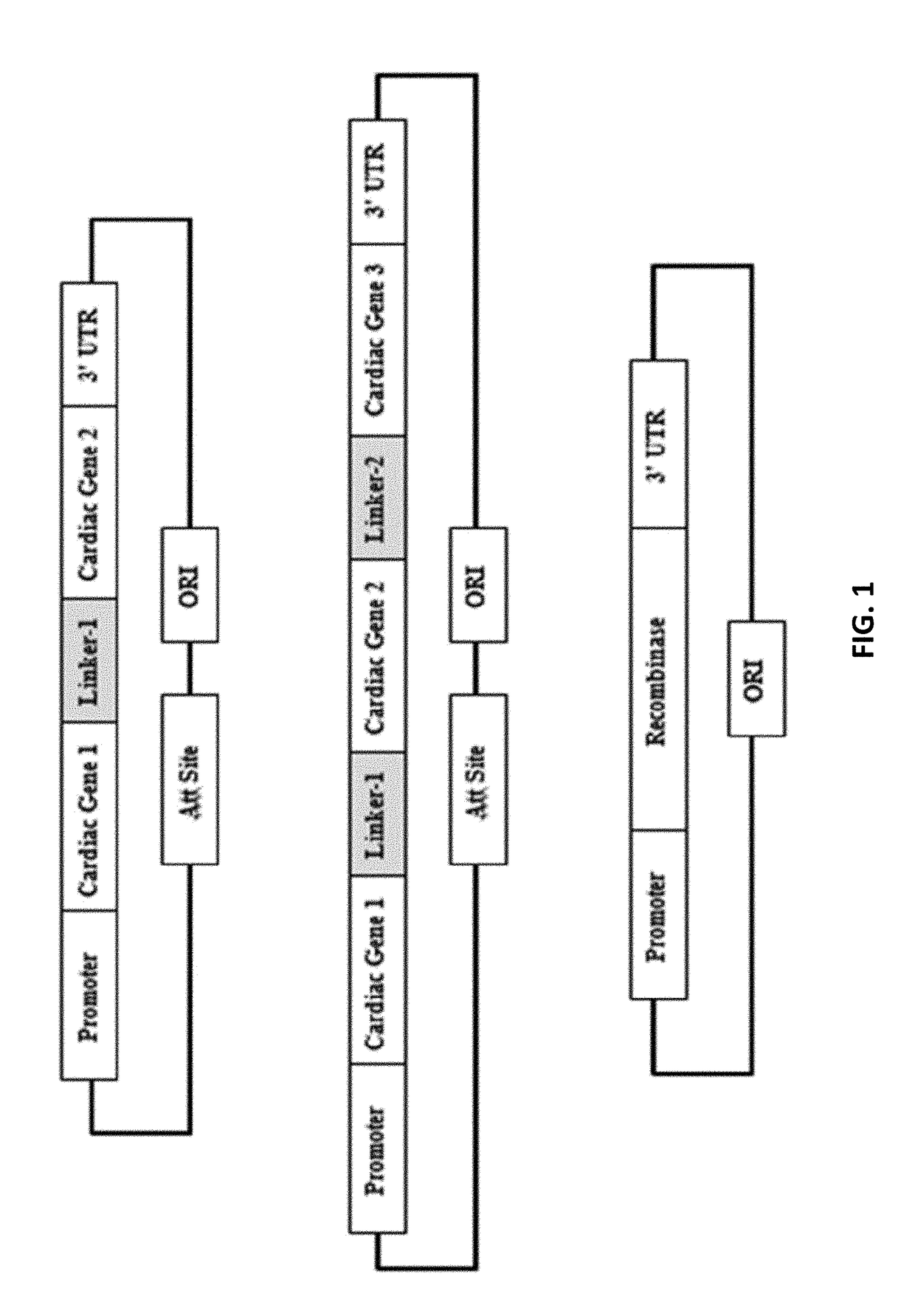
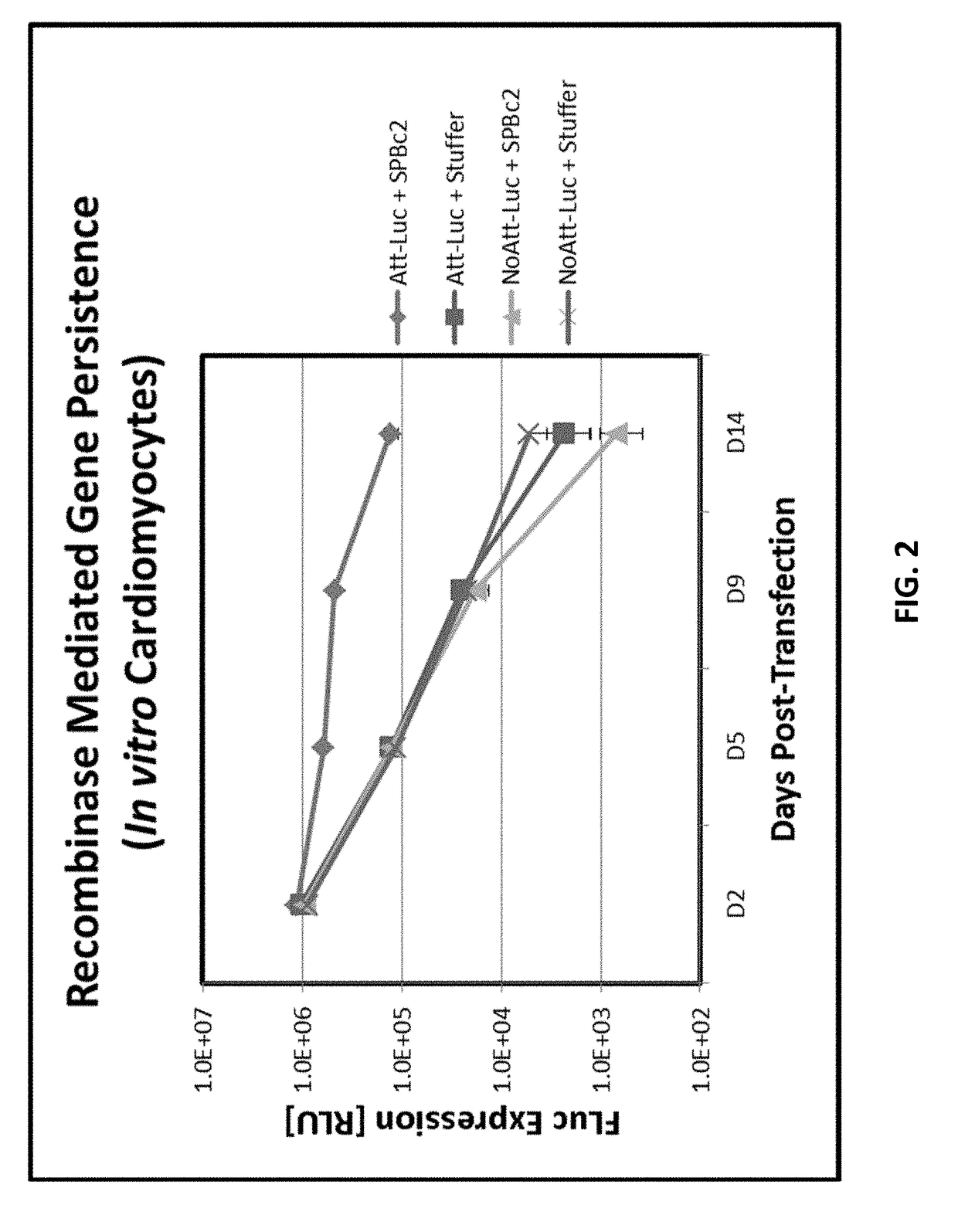
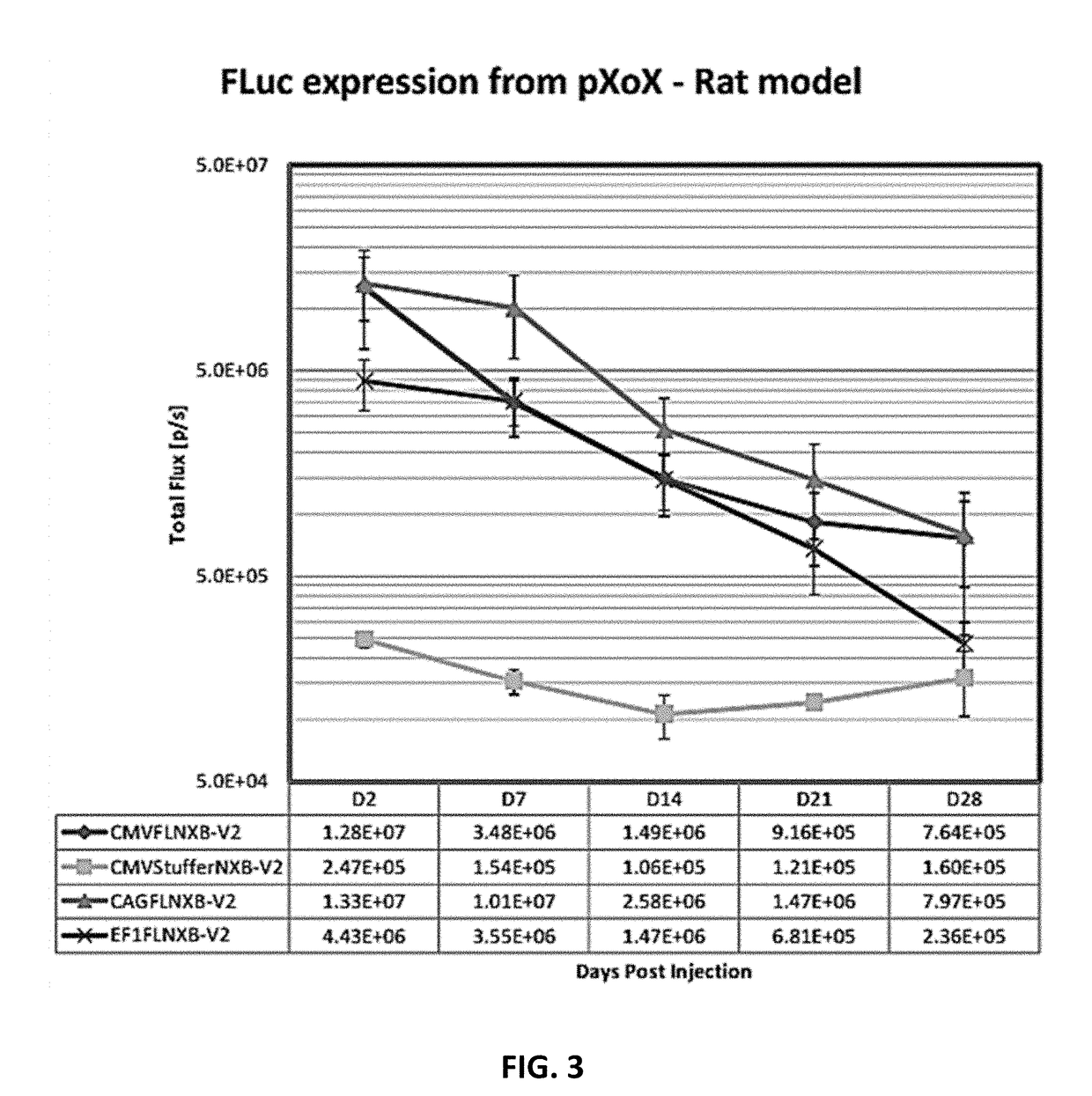
Compositions and methods for expression of multiple biologically active polypeptides from a single vector for treatment of cardiac conditions and other pathologies
ActiveUS20180360992A1Promote neovascularizationLocal broadeningPeptide/protein ingredientsAntibody mimetics/scaffoldsHeart diseaseCytokine
The present invention provides compositions and methods useful for treating disorders amenable to therapy via introduction of multigenic expression vectors. More particularly, the invention provides vectors and polynucleotides encoding polypeptides for treatment of cardiac disorders wherein said polypeptides may comprise a cytokine, a chemokine, and / or an angiogenic polypeptide, or functional derivatives thereof. Also provided, as compositions of the invention, are linkers useful for connecting and expressing functional (biologically active) polypeptides from single, multigenic-expression constructs.
Owner:PRECIGEN INC
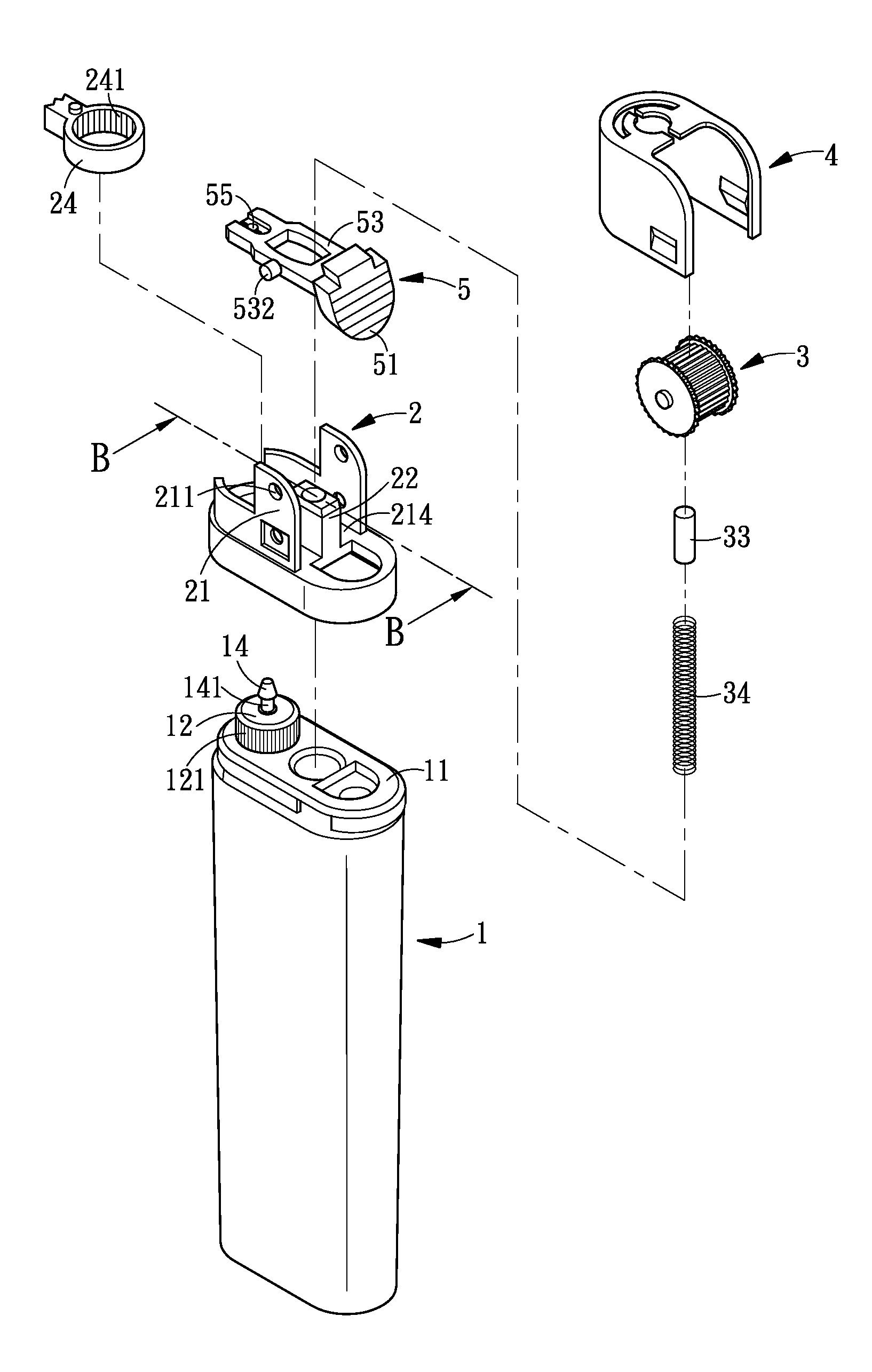
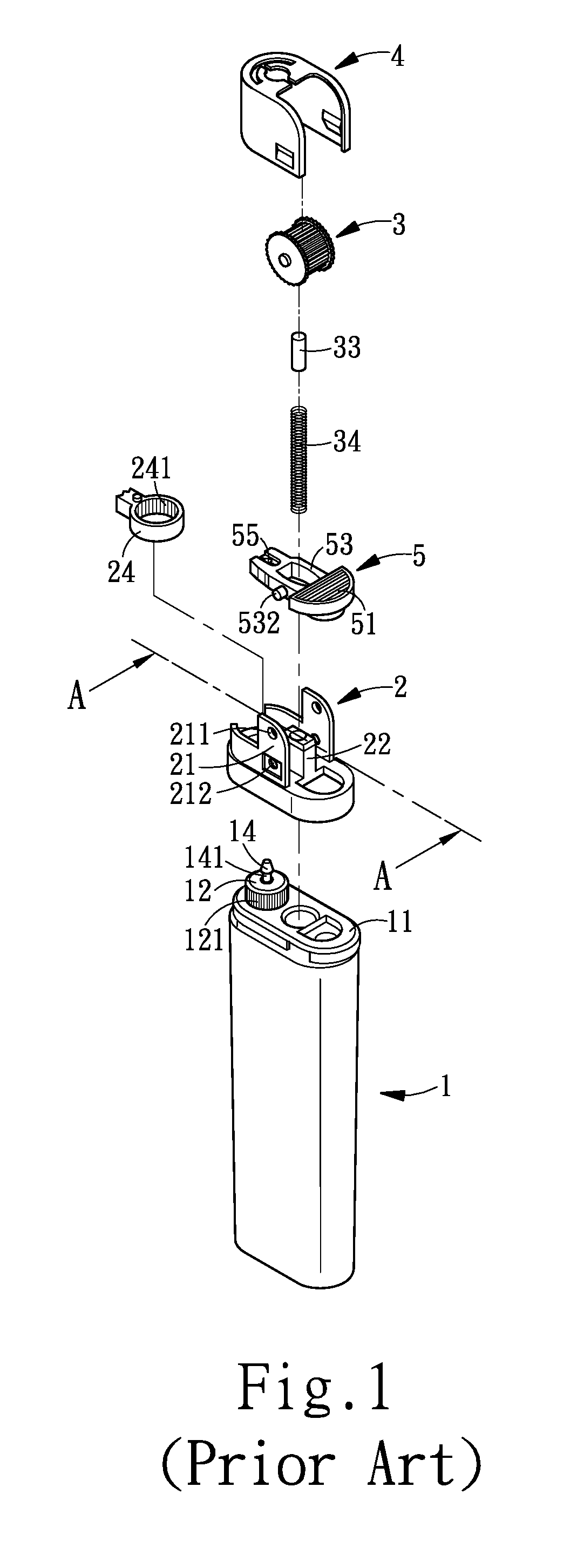
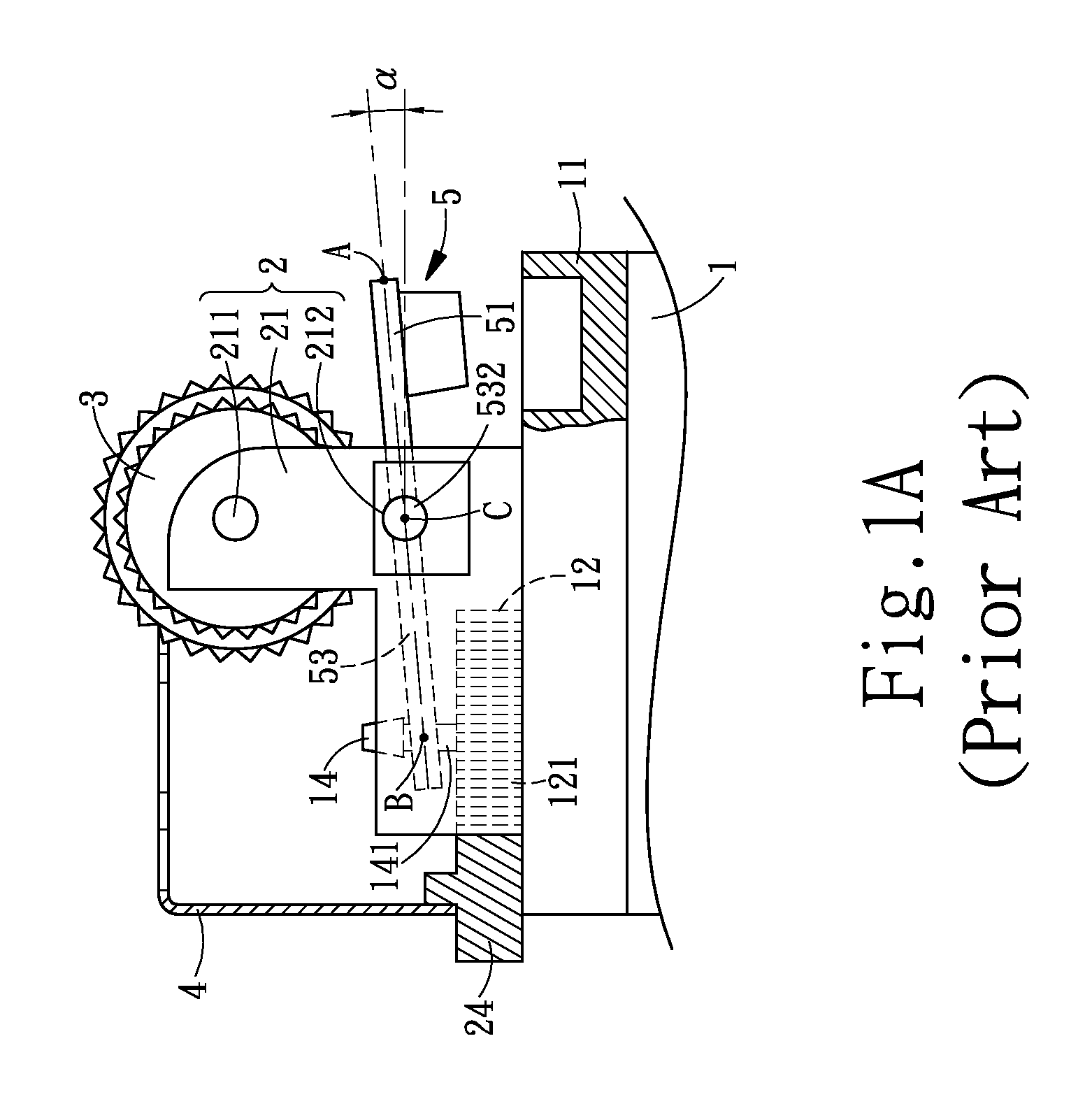
Child-resistant lighter
InactiveUS20090191496A1Provide usageGood curative effectFuel lightersMechanical ignitersSlant angleMechanical engineering
A child-resistant lighter includes a main body, a wheel racket, a file wheel, a metal cap, and a lever is disclosed. The lever having an upward slant angle of α is within the range 10°≦α≦20° further includes a pressing part, a base, and a slipping hole. Both sides of the base furnish a salient part for acting as the pivot point of the lever, and the salient part is fitted into a securing hole provided at the bottom portion of the file wheel. The securing holes are used as the pivotal bearing seat for bearing the salient part. The pressing part having a slant surface forms an angle θ with respect to the lever and has a horizontal downward slant angle(θ-α). When it comes to use, the user exerts a force P by pressing on the slant surface of the pressing part and the file wheel with his / her thumb making the lighter generate flames. The effective force acting on the lever is P cos θ where the effective range of θ is 27°≦θ≦60°, thereby the range of the effective force is 0.5P≦P cos θ≦0.9P. Therefore, the child-resistant lighter of the invention is capable of preventing the children from carelessness or accidental igniting, thereby improving the safety efficacy of the child-resistant lighter. As a result, the lighter of the invention is capable of protecting the children from causing fire accident to achieve the efficacy of safety usage of the lighter. In this way, the lighter of the invention is capable of improving the efficacy of safety usage. Nevertheless, the exerting force P is still within the range of an adult user.
Owner:HUMBOLDT STATE UNIVERSITY
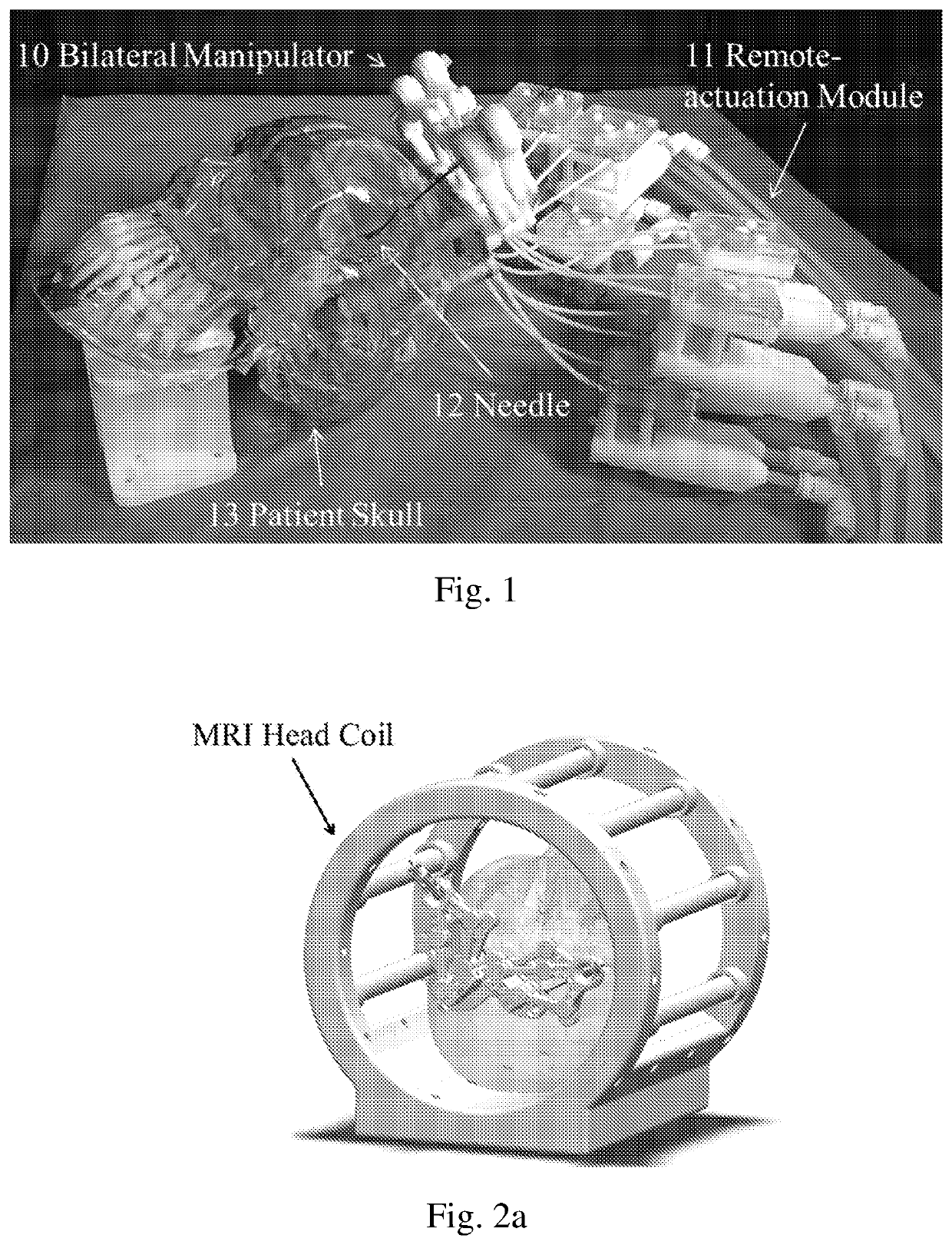
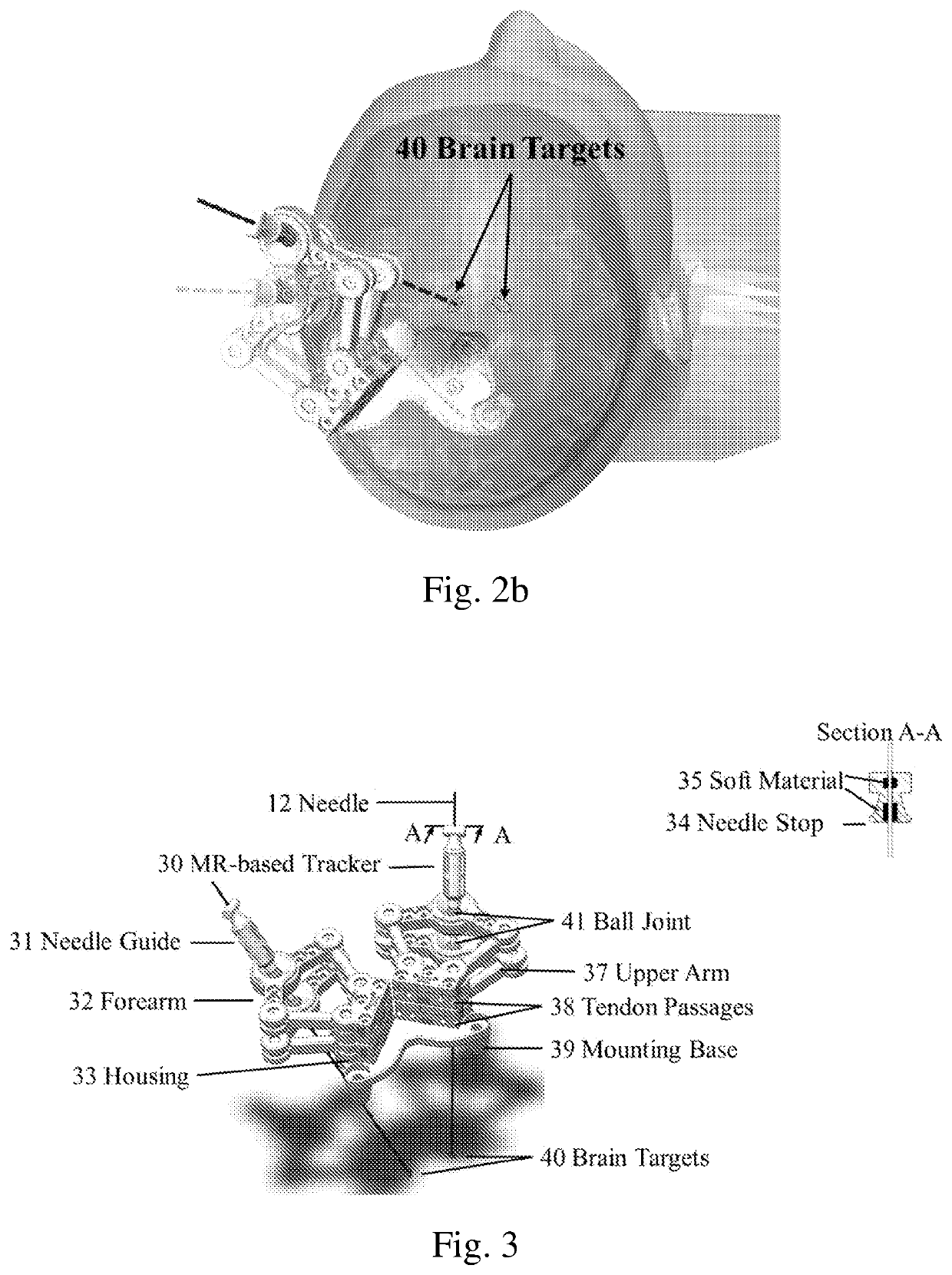
Robotic stereotactic system for mri-guided neurosurgery
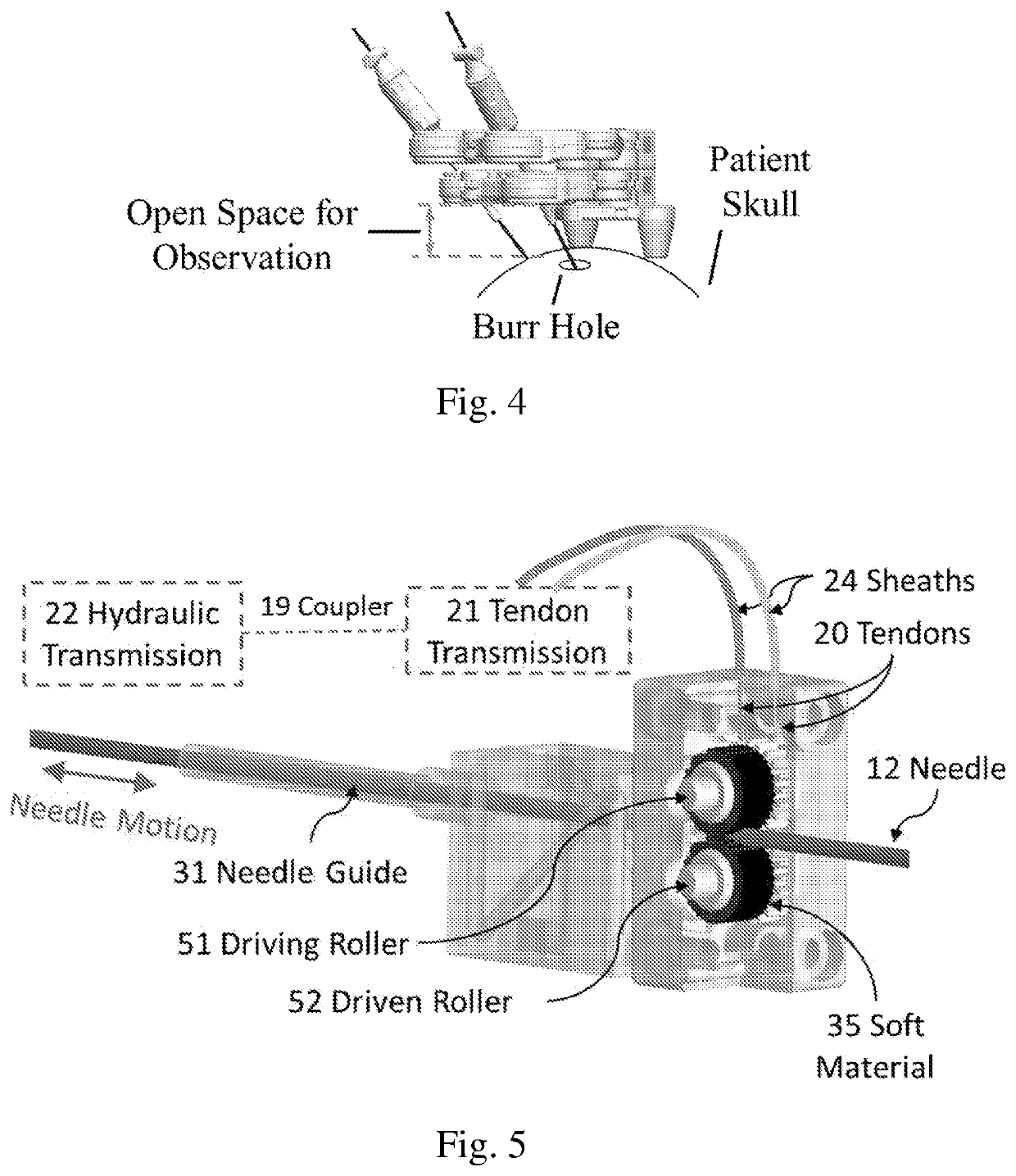
PendingUS20210015558A1Not noticeable image artifactSafety and efficacyDiagnosticsSurgical navigation systemsRobotic systemsNeedle guidance
A neurosurgical robotic system for bilateral stereotaxy that integrates intraoperative MRI guidance is provided. The robotic system can be implemented in regular diagnostic MRI facilities. Navigation for bilateral brain targets can be performed independently and simultaneously. The robotic system includes a plurality of manipulators, a needle guide (31), a needle (12) disposed within the needle guide (31); and a mounting base (39) with a plurality of screw holes for bone mounting.
Owner:THE UNIVERSITY OF HONG KONG
Methods of selecting and designing safer and more effective Anti-ctla-4 antibodies for cancer therapy
PendingUS20210047410A1Improve the level ofReduced Treg/TeffCompound screeningApoptosis detectionAntiendomysial antibodiesSide effect
The present invention relates to compositions of anti-CTLA-4 antibodies that bind to the human CTLA4 molecule and their use in cancer immunotherapy and for the reduction of autoimmune side effects compared to other immunotherapeutic agents.
Owner:ONCOC4 INC +1
Compositions and methods for activating nk cells
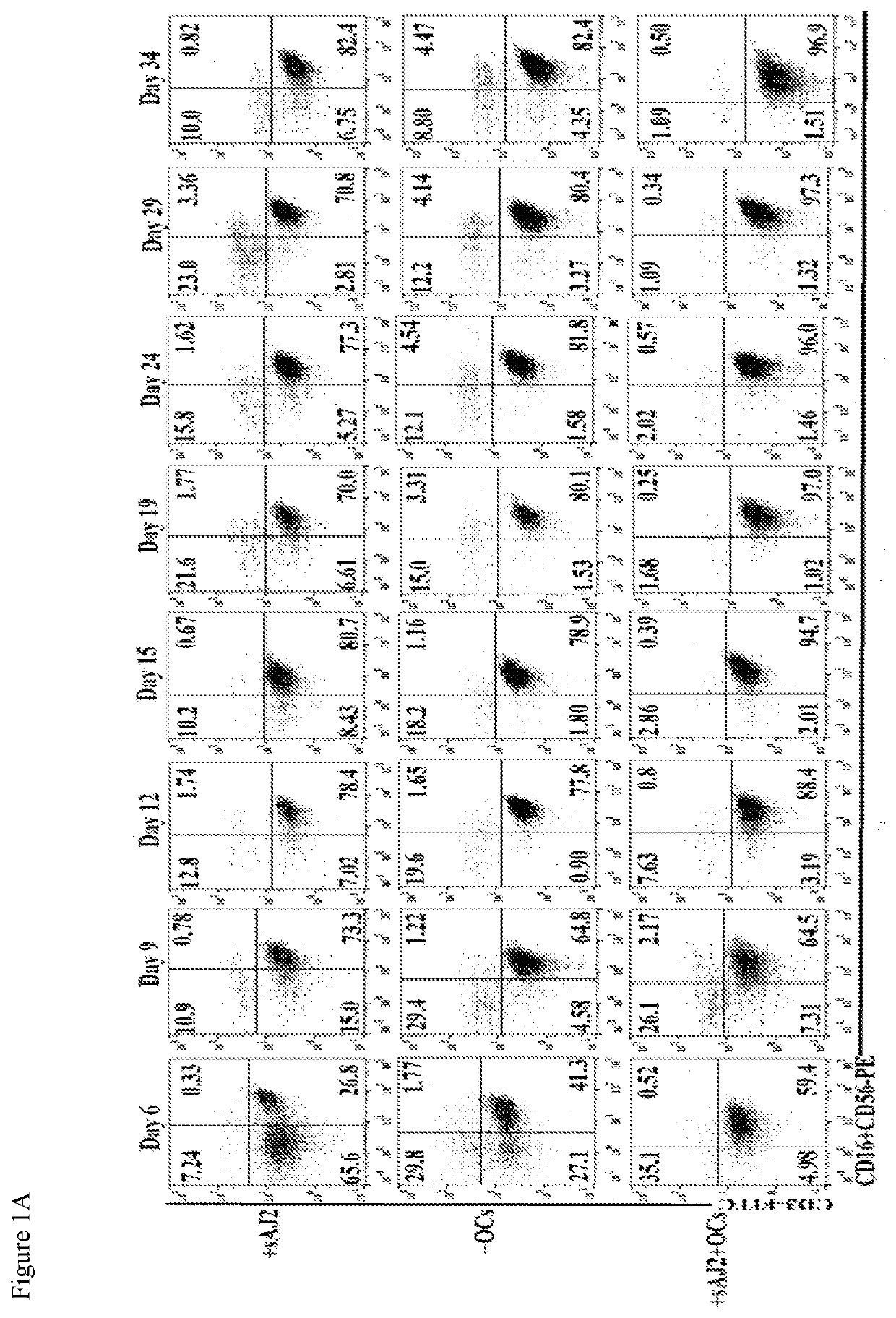
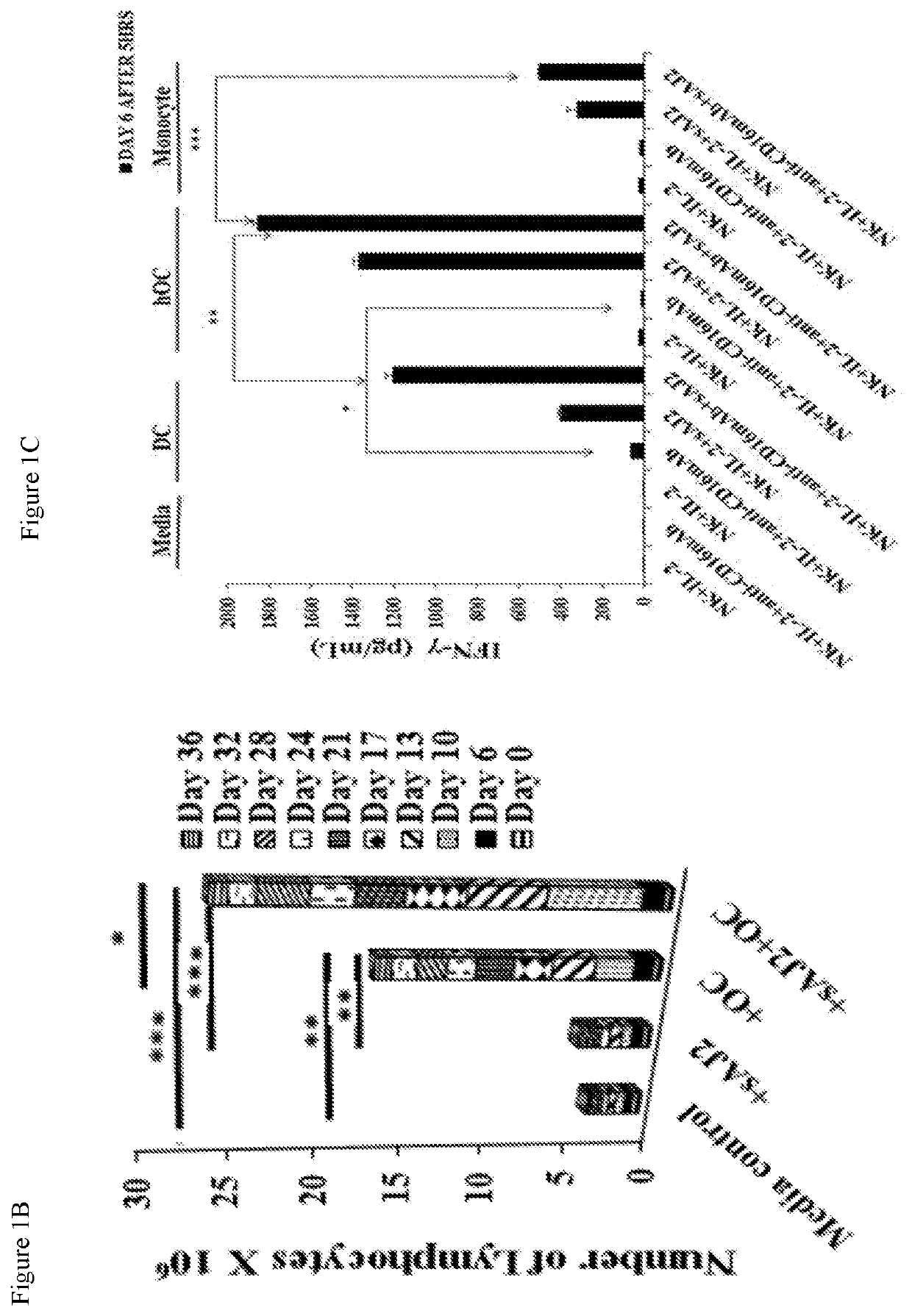
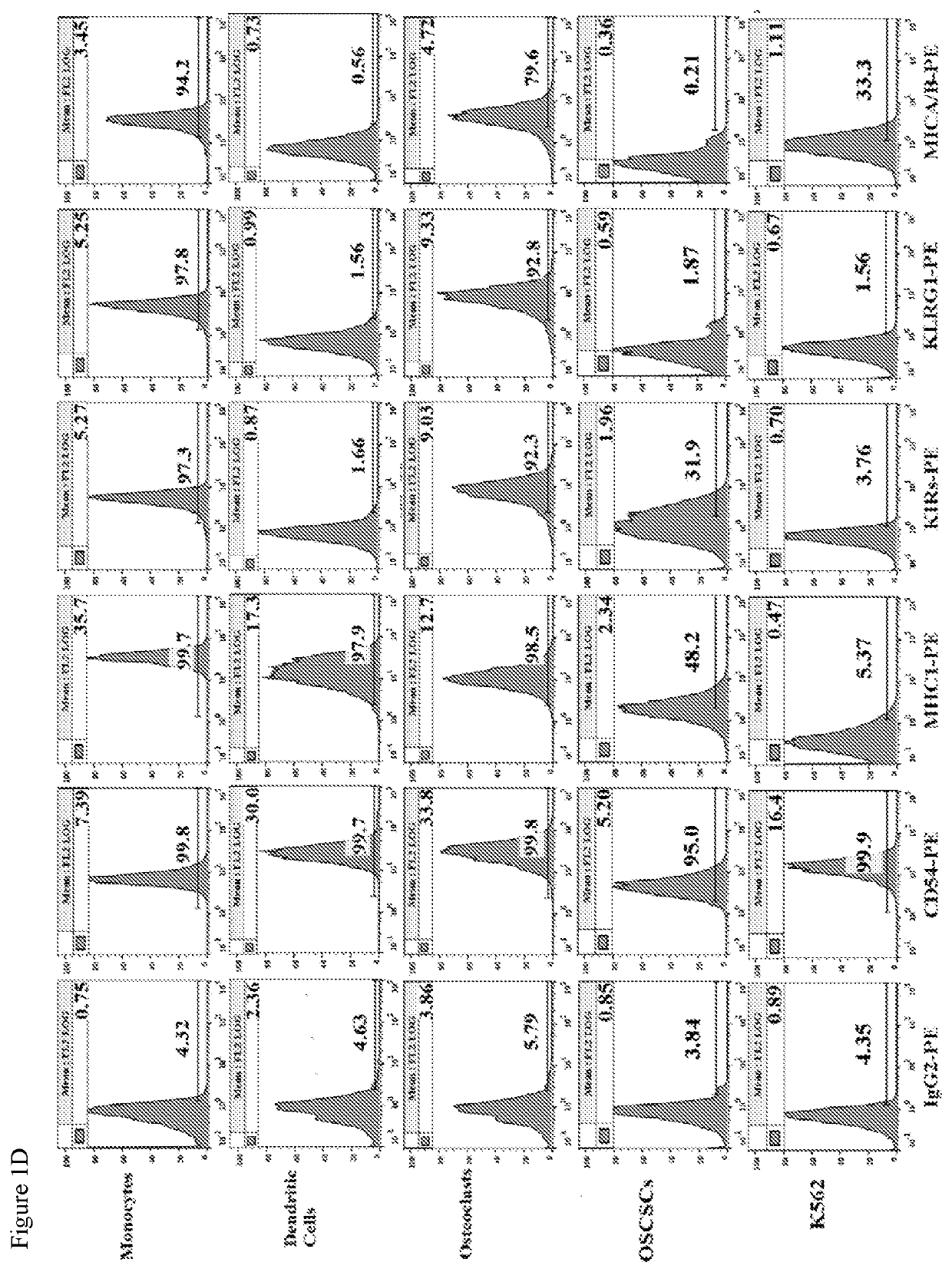
PendingUS20200306300A1Enhance expansionEnhance secretionBacteria material medical ingredientsCulture processMolecular biologyOsteocyte
The present application relates to methods of activating a NK cells in vitro, ex vivo, and / or in vivo by an osteoclast cell (OC) and / or a dendritic cell, and methods of treating disease using these activated NK cells.
Owner:RGT UNIV OF CALIFORNIA
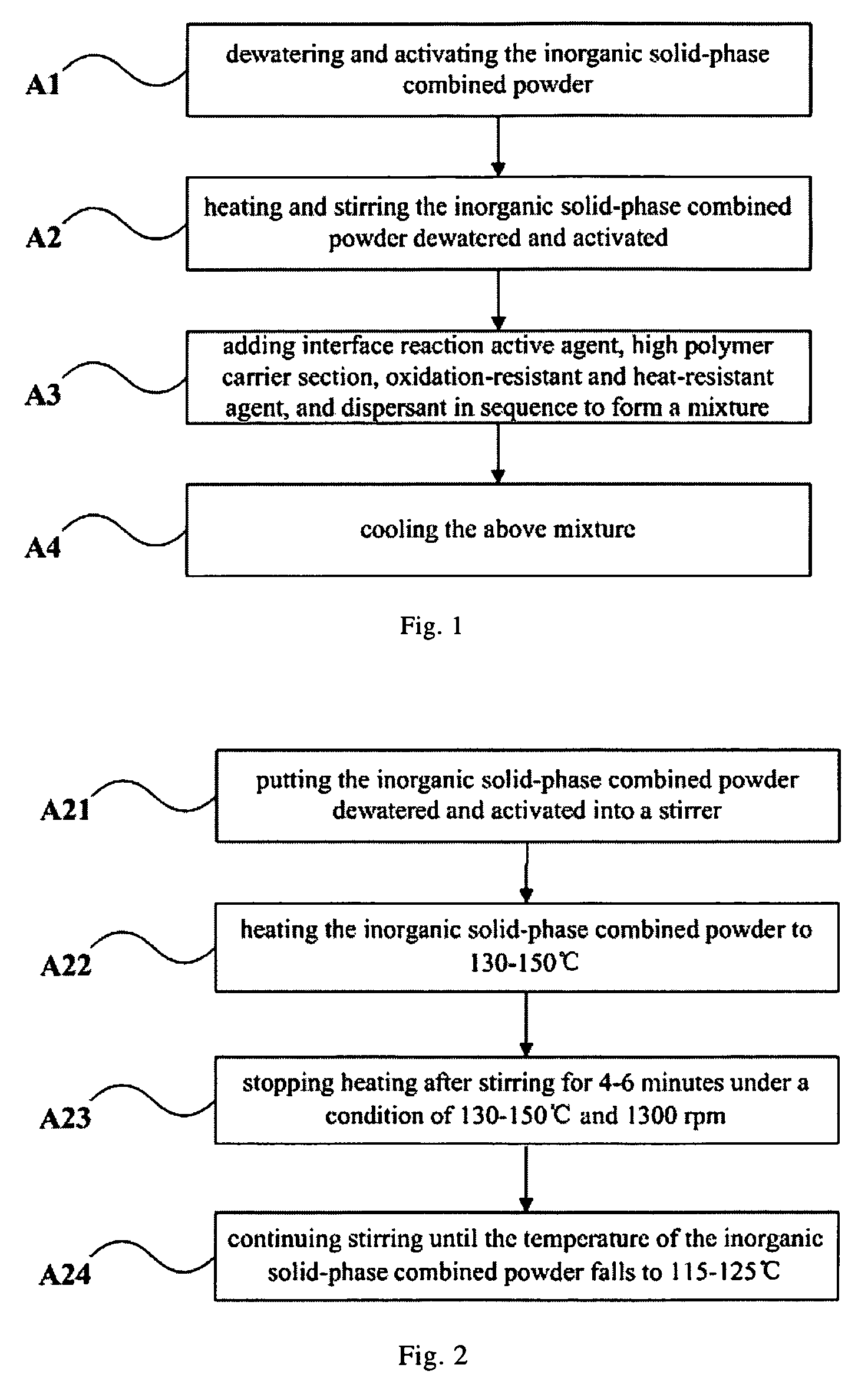
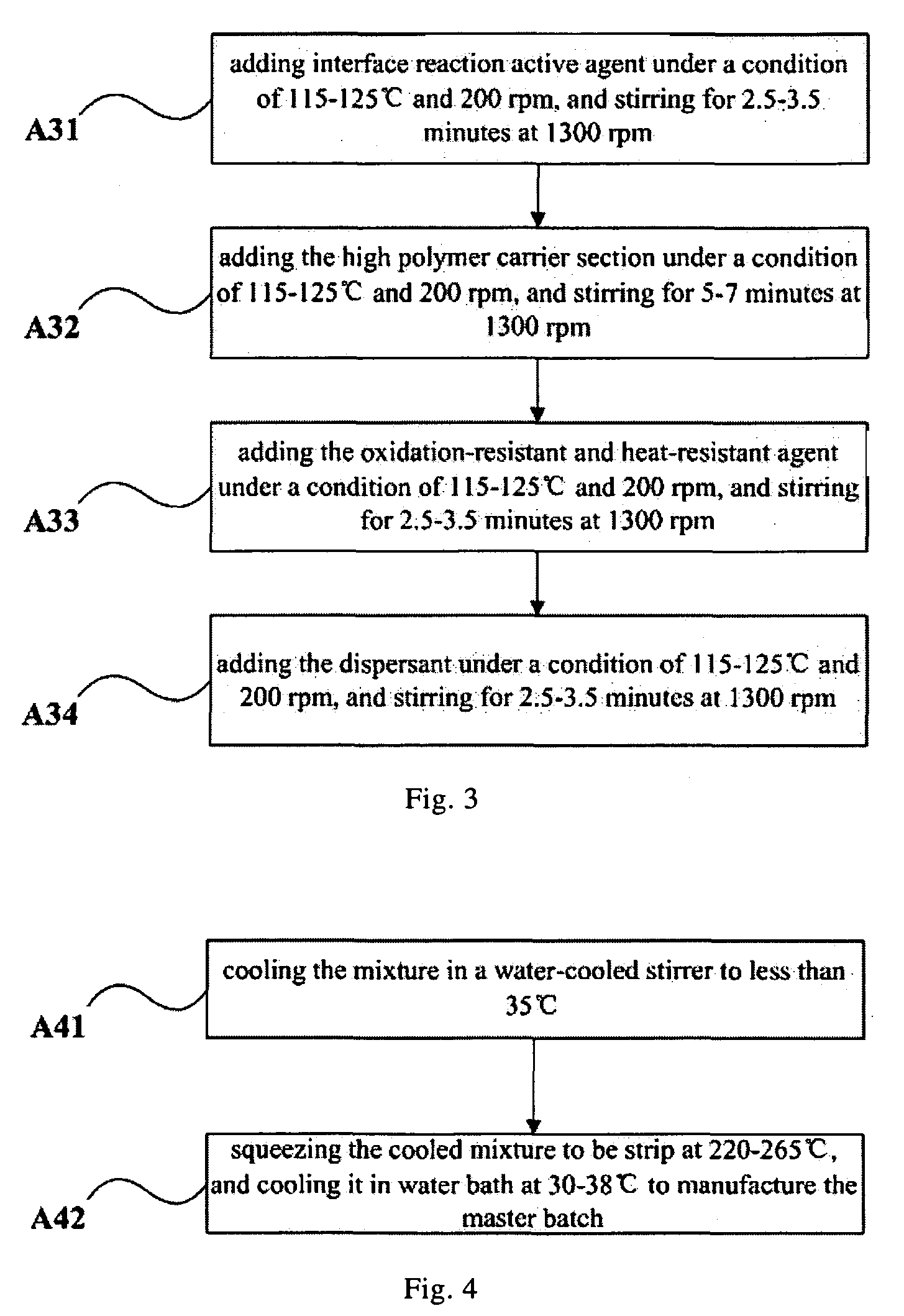
Inorganic solid-phase combined powder, master batch and method for manufacturing the same, fiber and method for manufacturing the same
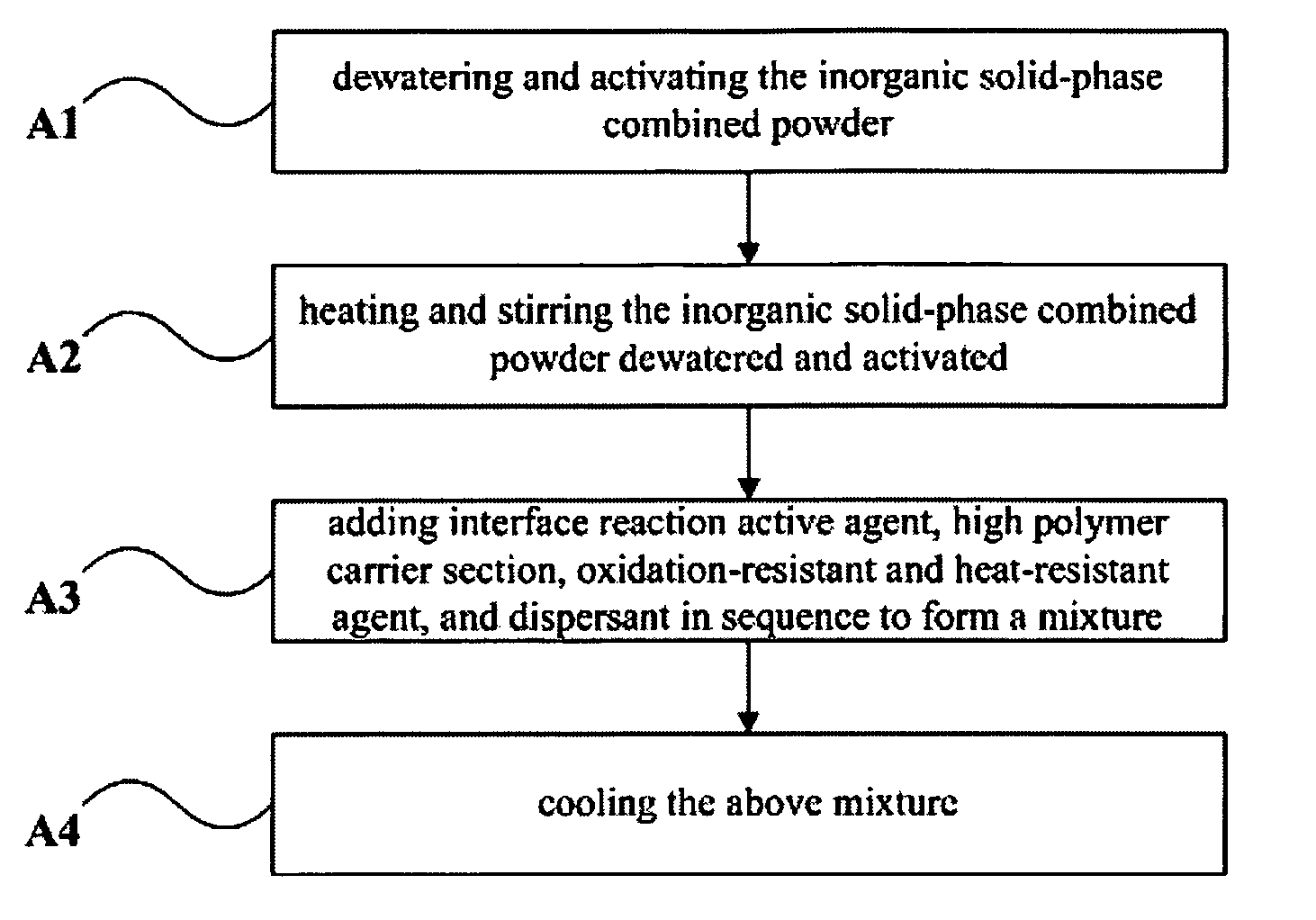
ActiveUS8105688B2Good chemical and thermal stabilityLong-lasting effectBiocideCosmetic preparationsFiberUltraviolet
The present invention relates to an inorganic solid-phase combined powder, a master batch and a method for manufacturing the same, a fiber and a method for manufacturing the same. The fiber manufactured by the above method includes the following constituents in percentage by weight: 10-20% the master batch and 80-90% the high polymer carrier section. Silver content in the fiber of the present invention can reach up to 3-10%, the bactericidal rate is high, the blocking rate against ultraviolet ray is high, the efficacy is perdurable and safe, and there is no toxic or side effects. The spinnability of the fiber is good. The water absorption, permeability, dyeing evenness and electrical conductivity of the fiber are improved greatly, and the drapability and comfortableness are increased when the textile of the fiber is worn.
Owner:LIU YANPING
Pharmaceutical compositions for treating anxiety
InactiveUS20100310682A1Avoid missingLess side effectsOrganic active ingredientsBiocideANXIETY COMPLEXDiazepam
Owner:KOREA AEROSPACE INDUSTRIES +2
Dry powder inhalation device for the simultaneous administration of more than one medicament
InactiveUS8286631B2Enables the adjustment of the inhaler's resistanceLower resistanceRespiratorsLiquid surface applicatorsAdditive ingredientBlisters
The present invention relates to a dry powder inhalation device which is suitable for the simultaneous administration of a combination of pharmaceutical ingredients, wherein each pharmaceutical ingredient is packed in a separate blister of the same single dose blister strip. The medicaments that form the combination come into contact just before their exit from the mouthpiece of the device.
Owner:PENTAFRAGAS DIMITRIOS
Combination drug formulations for treating patients with cardiovascular disease and associated conditions
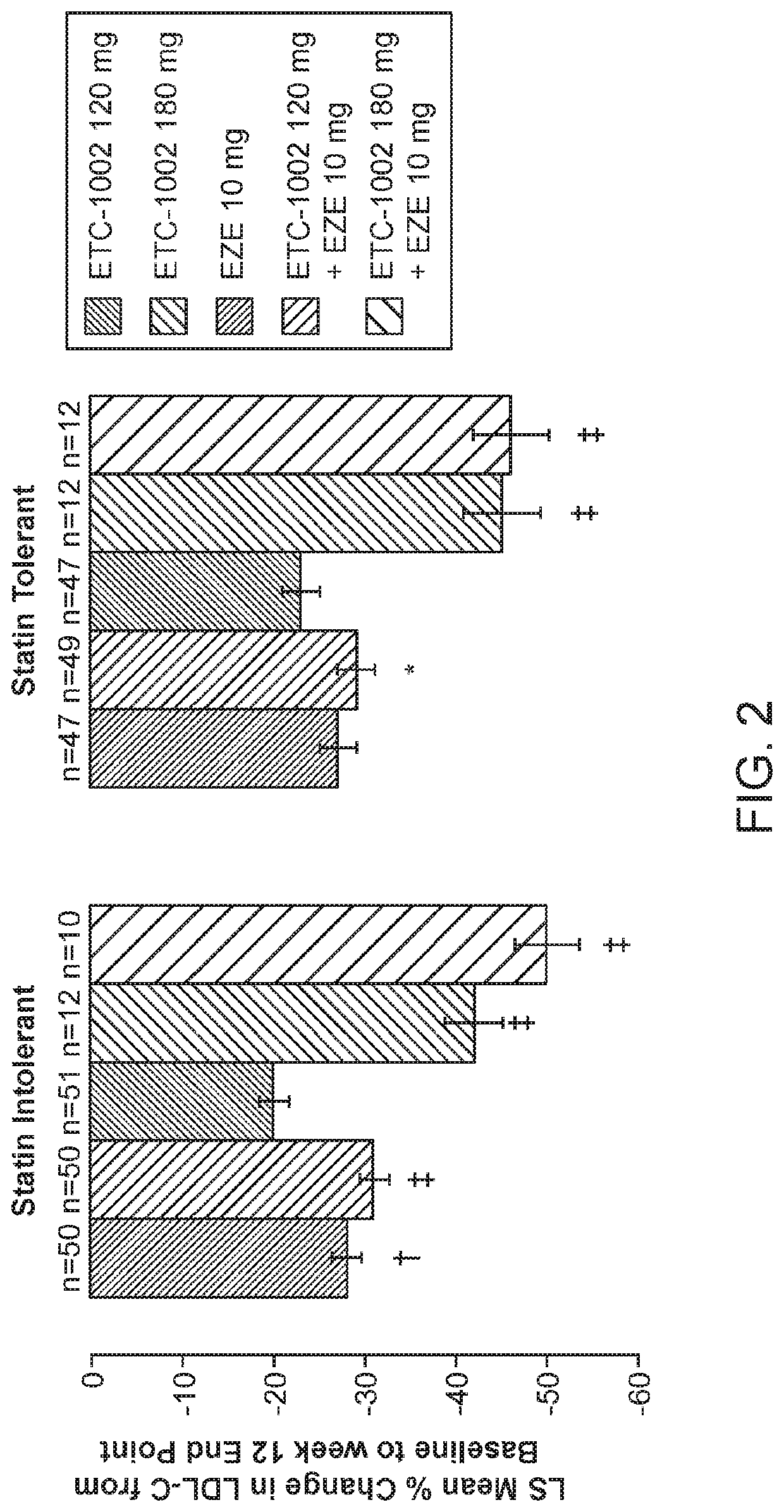
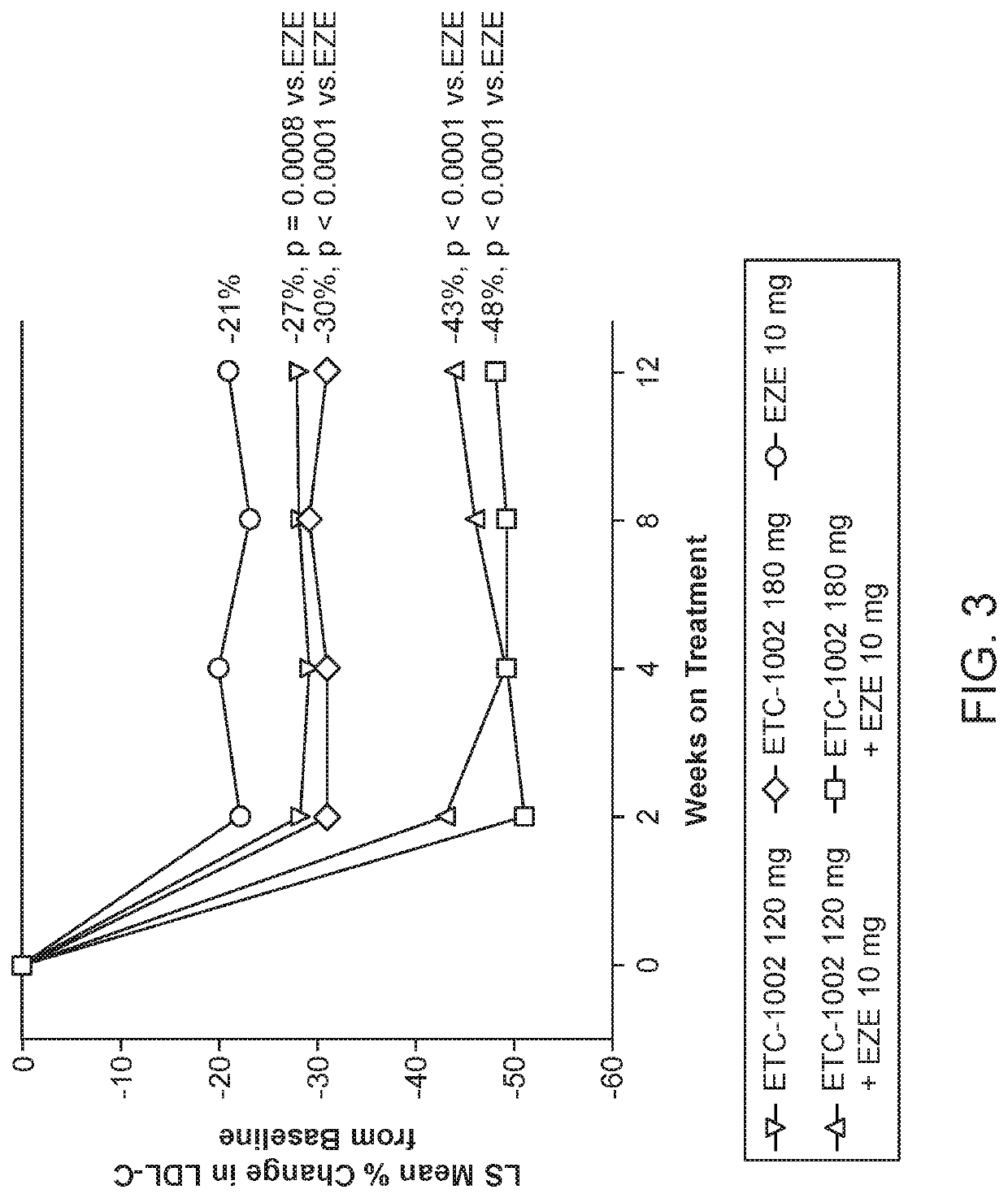
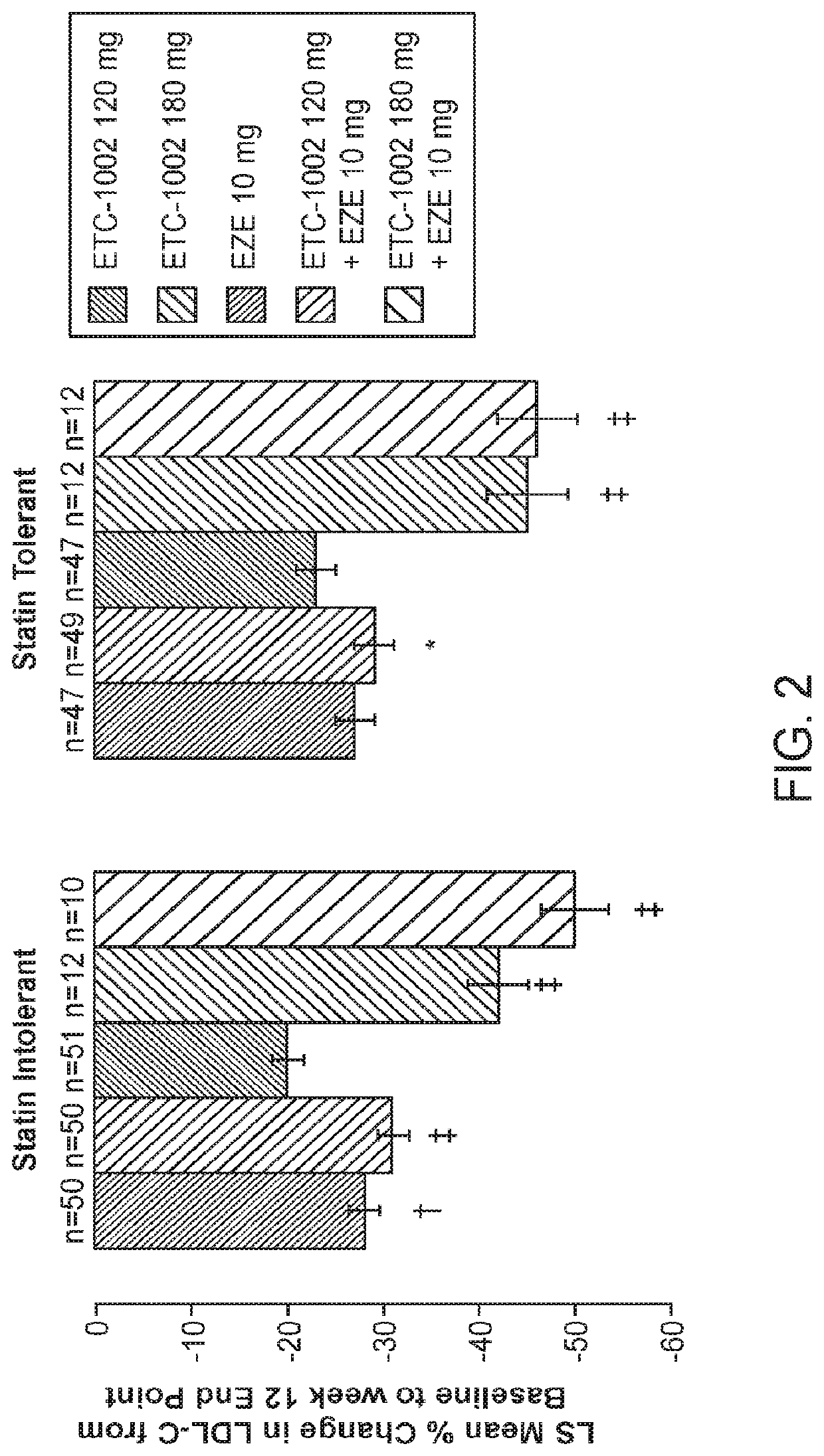
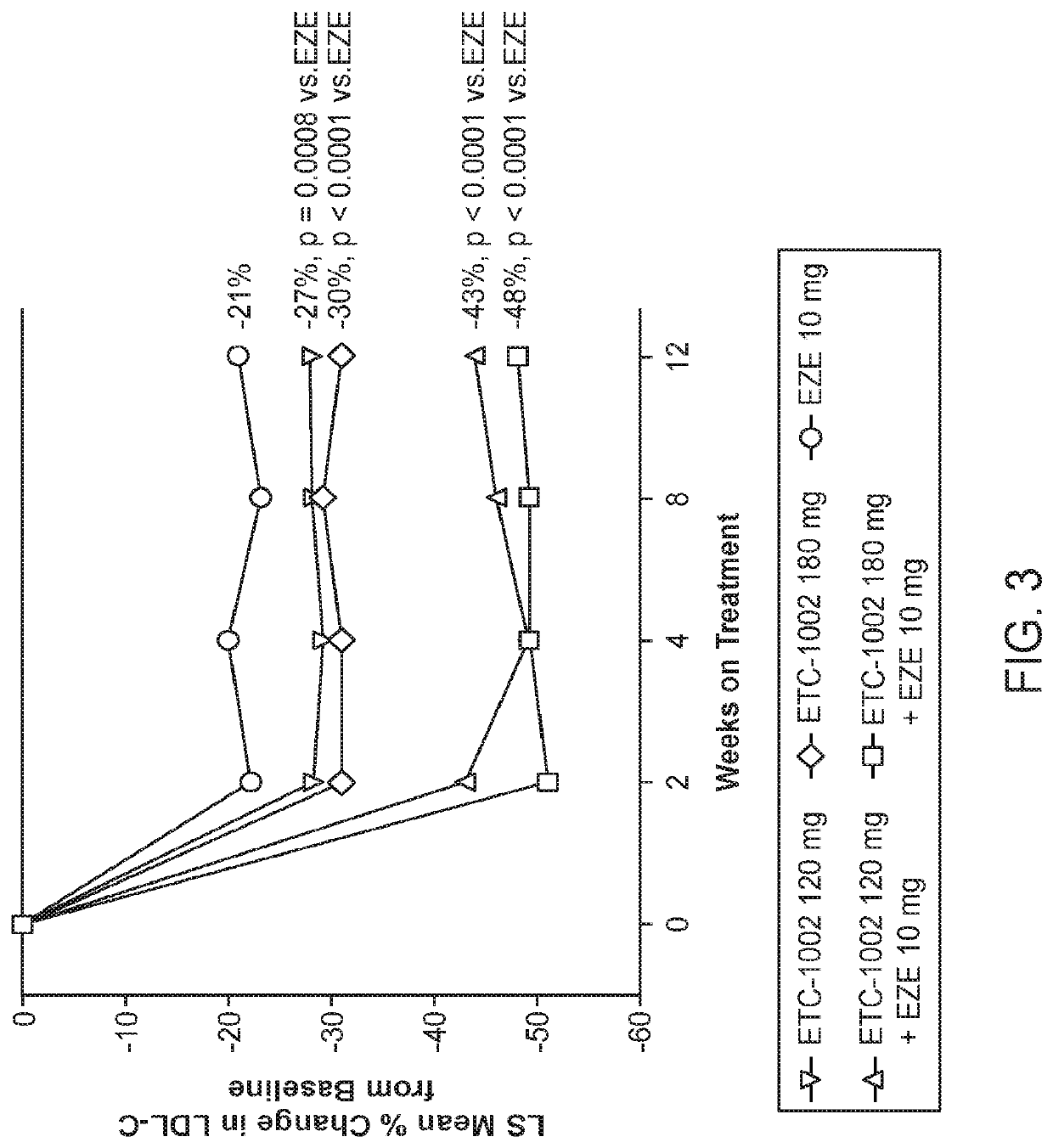
InactiveUS20210322375A1Reduce riskReduce additionalOrganic active ingredientsMetabolism disorderCholesterolCardio vascular disease
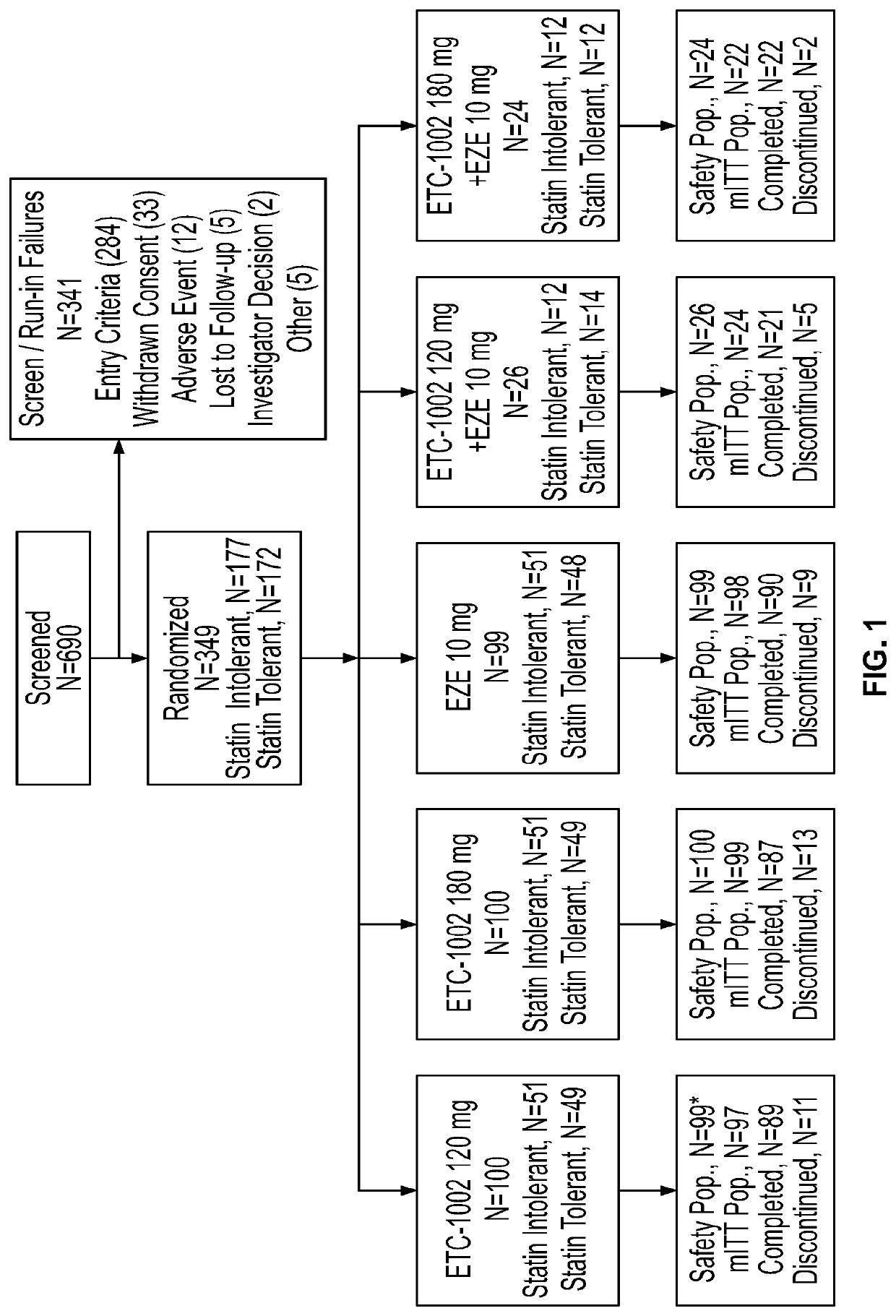
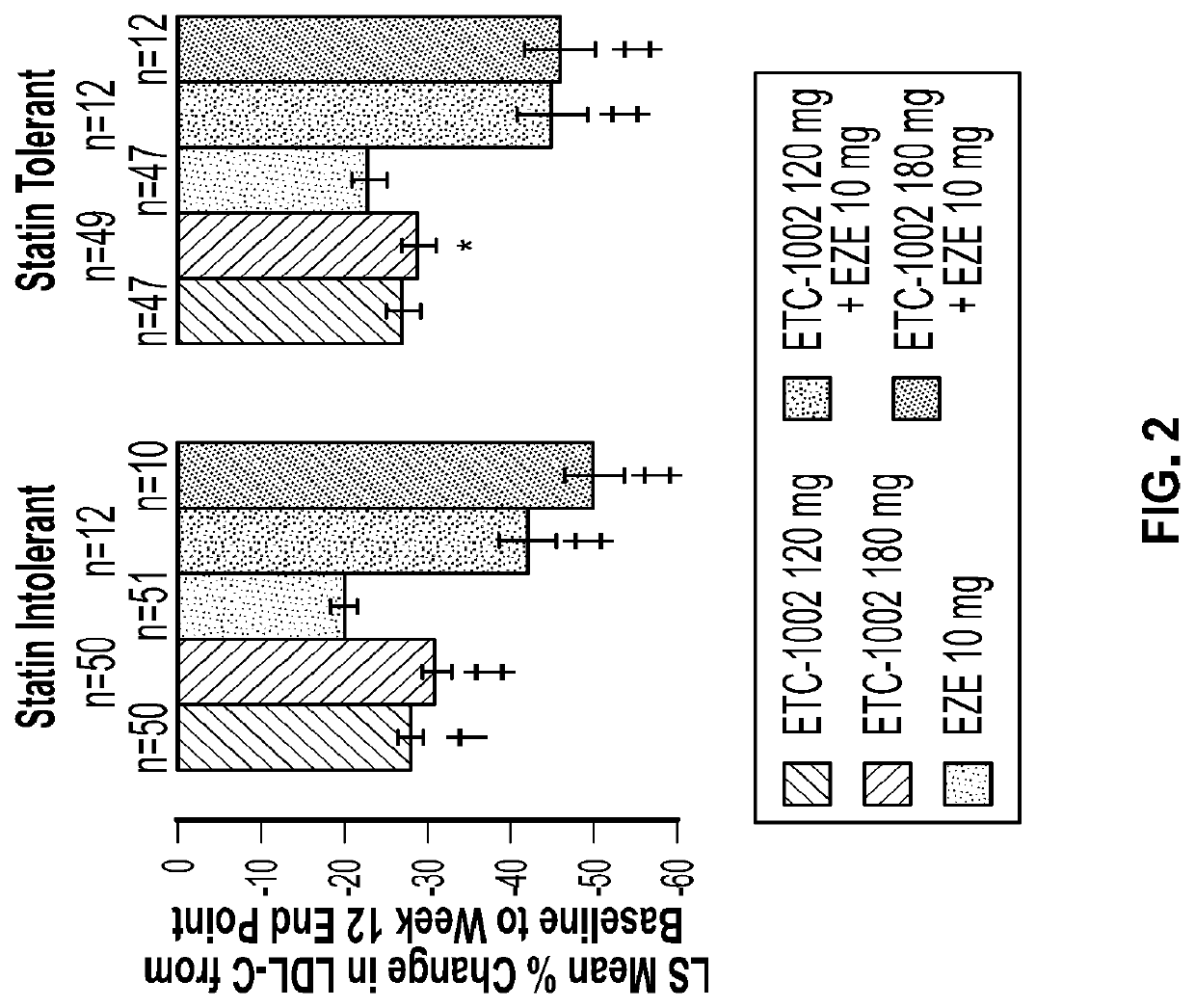
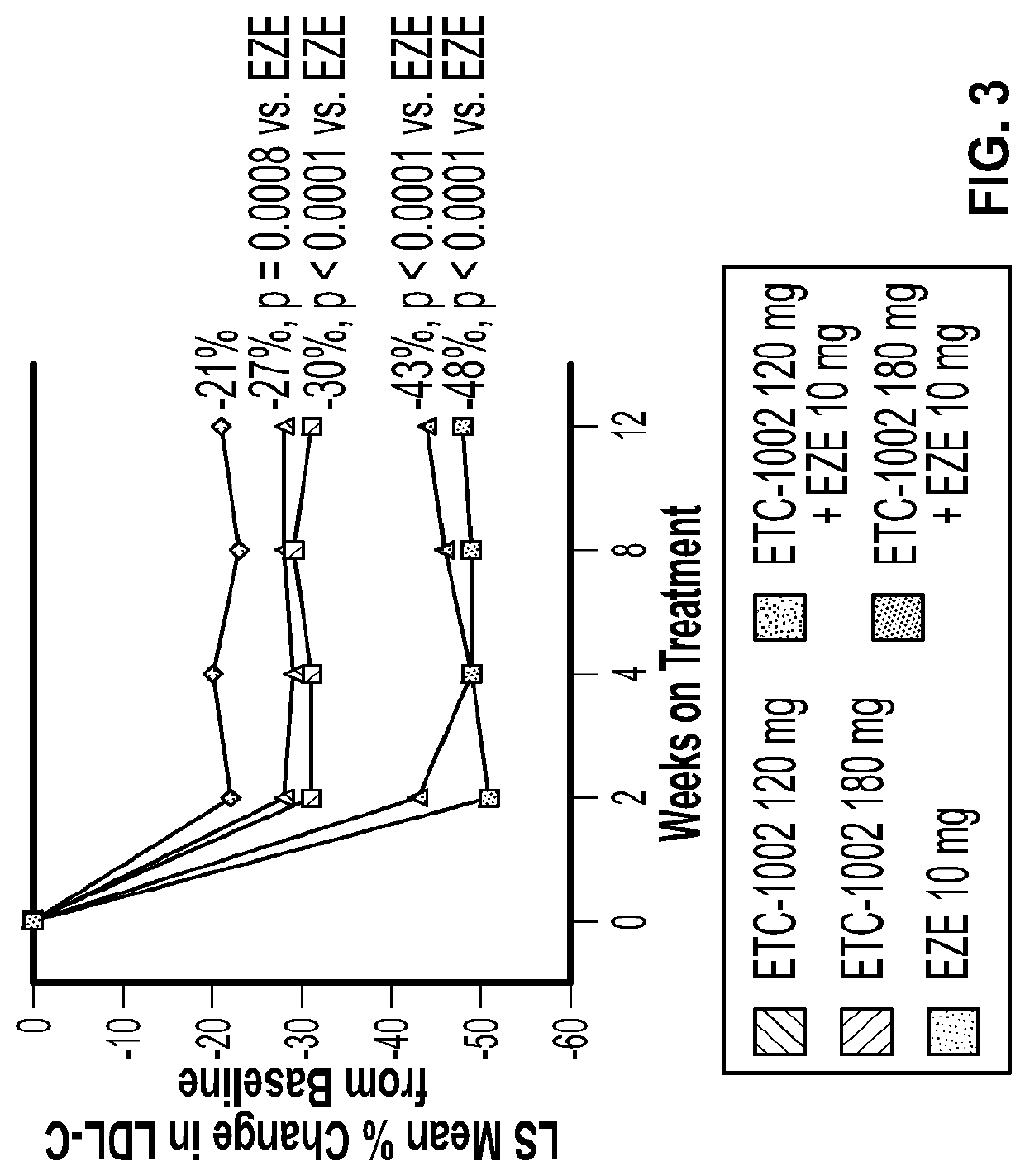
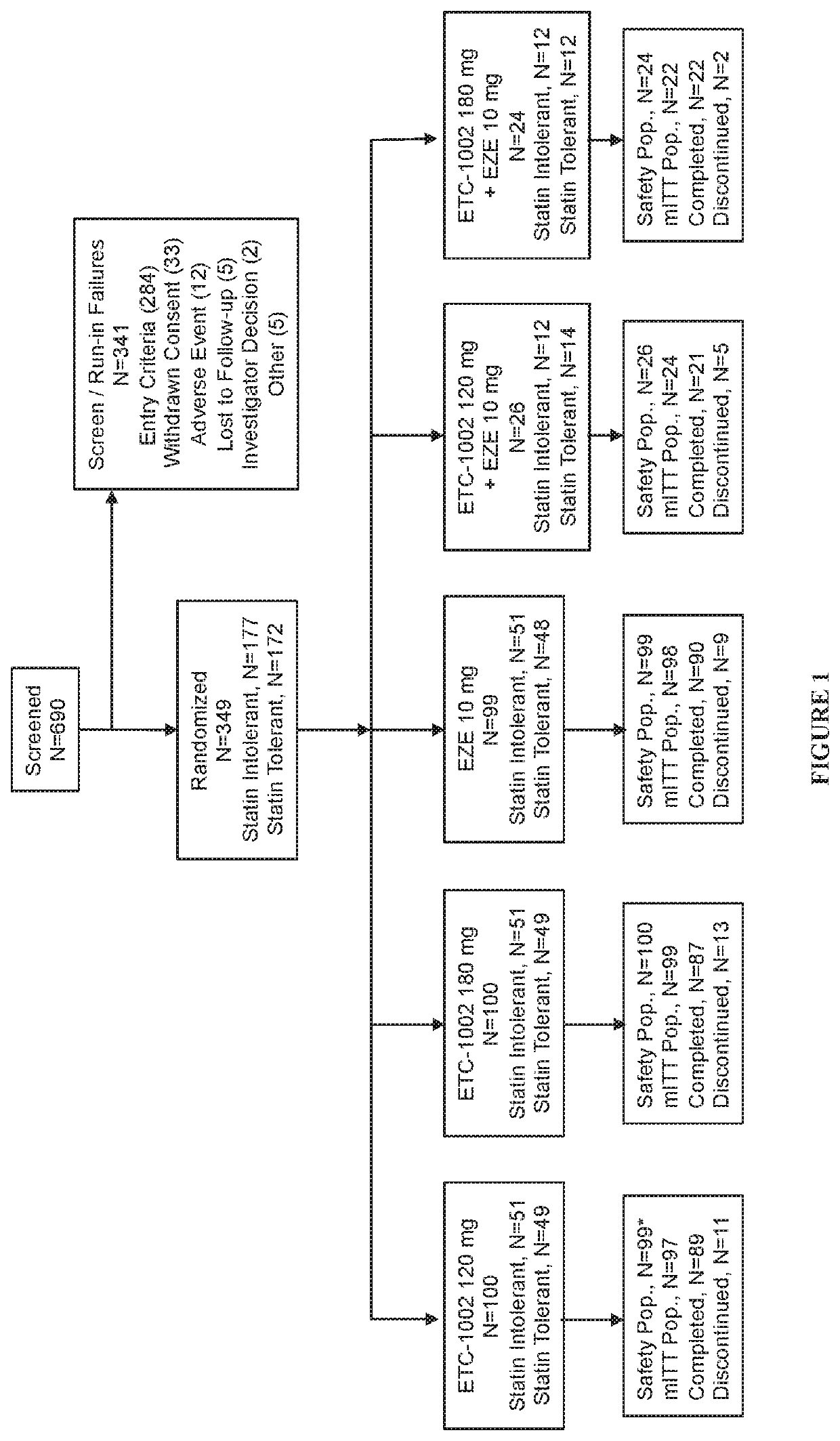
Disclosed herein are compositions comprising fixed doses of ETC-1002 and Ezetimibe. Also disclosed herein are methods for using fixed doses of ETC-1002 and Ezetimibe. Uses include methods of treating cardiovascular disease or reducing the risk of cardiovascular disease in a subject. Uses also include methods of treating hypercholesterolemia in a subject.
Owner:ESPERION THERAPEUTICS
Fixed dose combinations and formulations comprising ETC1002 and ezetimibe and methods of treating or reducing the risk of cardiovascular disease
ActiveUS10912751B2Reduce riskReduce additionalMetabolism disorderAnhydride/acid/halide active ingredientsCholesterolHypercholesteraemia
Disclosed herein are compositions comprising fixed doses of ETC-1002 and Ezetimibe. Also disclosed herein are methods for using fixed doses of ETC-1002 and Ezetimibe. Uses include methods of treating cardiovascular disease or reducing the risk of cardiovascular disease in a subject. Uses also include methods of treating hypercholesterolemia in a subject.
Owner:ESPERION THERAPEUTICS
Tumor targeted radionuclide therapy and molecular imaging of her2+ cancers and other neoplasms
ActiveUS20200085981A1Reduce spreadModulate pathologic angiogenesisImmunoglobulins against growth factorsRadioactive preparation carriersImmunooncologyTumor targeting
Methods and compositions for treating, diagnosing and staging cancers, in particular overexpressing the Human Epidermal growth factor Receptor 2 protein (HER2+) given rise to in breast, gastric, gastroesophageal, ovarian, pancreatic cancer and brain tumors, which may be metastatic to the brain or other site. More specifically, the invention provides for Targeted Radionuclide Therapy (TRNT) with a compound of the invention having a peptide that targets the HER2+ cells, a second component for combining metals into complexes through a ring structure (DOTA), and a third radioisotope component, Lu-177 and Ga-68, in which embodiments further include a companion diagnostic, and in which embodiments further include anti-integrin precision medicines for cancers expressing αvβ3 and αvβ5 integrins, HER2+, vascular endothelial growth factor, vitronectin, fibronectin, tenascin, reelin, kindlin and talin. TRNT may be administered alone or in combination with standard-of-care; an immunooncologic and / or chemotherapeutic, adjuvantly or neoadjuvantly.
Owner:SATZ STANLEY
Bivalent swine influenza virus vaccine
ActiveUS10029005B2Safety and efficacySsRNA viruses negative-senseViral antigen ingredientsInfluenza virus vaccineSwine influenza virus vaccine
The present invention relates to an immunogenic composition comprising: a) a modified live H3 virus of swine influenza, and b) a modified live H1 virus of swine influenza. Furthermore, the present invention relates to methods for immunizing a subject comprising administering to such subject the immunogenic composition of the present invention. Moreover, the present invention relates to methods of treating or preventing clinical signs caused by swine influenza virus in a subject of need, the method comprising administering to the subject a therapeutically effective amount of an immunogenic composition according to the present invention.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Precision medicine theranostics and diagnostics a combination thereof
InactiveUS20200085979A1Safety and efficacyHigh affinityImmunoglobulins against growth factorsRadioactive preparation carriersPharmaceutical drugCombinatorial chemistry
The component ‘drug’ new molecule for combining metals into complexes through a ring structure (DOTA) or linear structure, and a radionuclidic component, and chelating agent wherein embodiments may include a companion diagnostic, and in which embodiments further include anti-integrin precision medicines for cancers expressing αvβ3 and αvβ5 integrins, second component for combining metals into complexes through a ring or linear structure, and one or more radionuclidic components, a chelating agent for diagnosis and / or therapy (theranostic) in which the embodiments further include a anti-integrin peptidomimetic for precision medicine for cancers expressing αvβ3 and αvβ5 integrins.
Owner:SATZ STANLEY
Injection composition containing acetaminophen
ActiveUS20190380950A1Avoid it happening againReduce solubilityOrganic active ingredientsPharmaceutical delivery mechanismPhysiology
The present disclosure relates to the injection composition containing acetaminophen, the injection composition comprising acetaminophen mixed in 0.01-5 wt %, a solubilizer mixed in 0.01-10 wt %, a toxicity inhibitor mixed in 0.05-5 wt %, an isotonic agent mixed in 0.1-10 wt %, an antioxidant mixed in 0.001-5 wt %, and the balance sterile purified water for injection.
Owner:WOOSUNG PHARMA CO LTD
Fixed dose combinations and formulations comprising etc1002 and ezetimibe and methods of treating or reducing the risk of cardiovascular disease
ActiveUS20210361605A1Reduce cholesterolLow LDL-CMetabolism disorderAnhydride/acid/halide active ingredientsCholesterolHypercholesteraemia
Disclosed herein are compositions comprising fixed doses of ETC-1002 and Ezetimibe. Also disclosed herein are methods for using fixed doses of ETC-1002 and Ezetimibe. Uses include methods of treating cardiovascular disease or reducing the risk of cardiovascular disease in a subject. Uses also include methods of treating hypercholesterolemia in a subject.
Owner:ESPERION THERAPEUTICS
Pharmaceutical compositions for treating anxiety
InactiveUS20170274032A1Less side effectsEasy to useNervous disorderPlant ingredientsANXIETY COMPLEXDiazepam
Owner:ZHANG ZUOGUANG +2
Pharmaceutical combination and its use for treating synucleinopathties
ActiveUS11318122B2Safety and efficacyDelay onsetOrganic active ingredientsNervous disorderGene mutationPharmaceutical medicine
The present invention describes the combination of a 6-propylamino-4,5,6,7-tetrahydro-1,3-benzothiazole-2-amine or of a pharmaceutically acceptable salt or solvate thereof with fluoxetine or a pharmaceutically acceptable salt or solvate thereof, for use for treating a synucleinopathy such as Parkinson's disease, Lewy body disease, mutations in the glucocerebrosidase gene, or multiple system atrophy.
Owner:CHASE THERAPEUTICS CORP
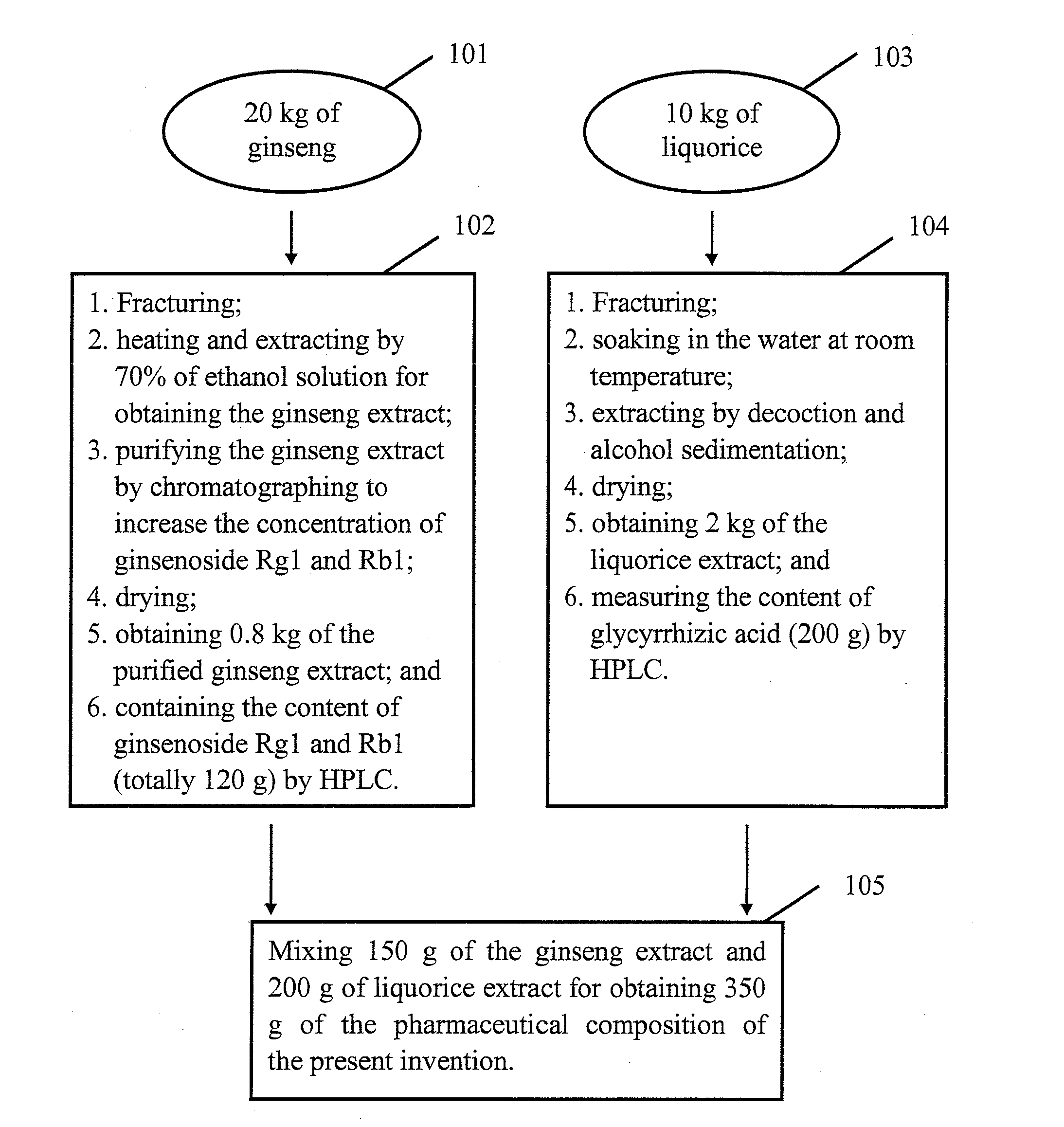
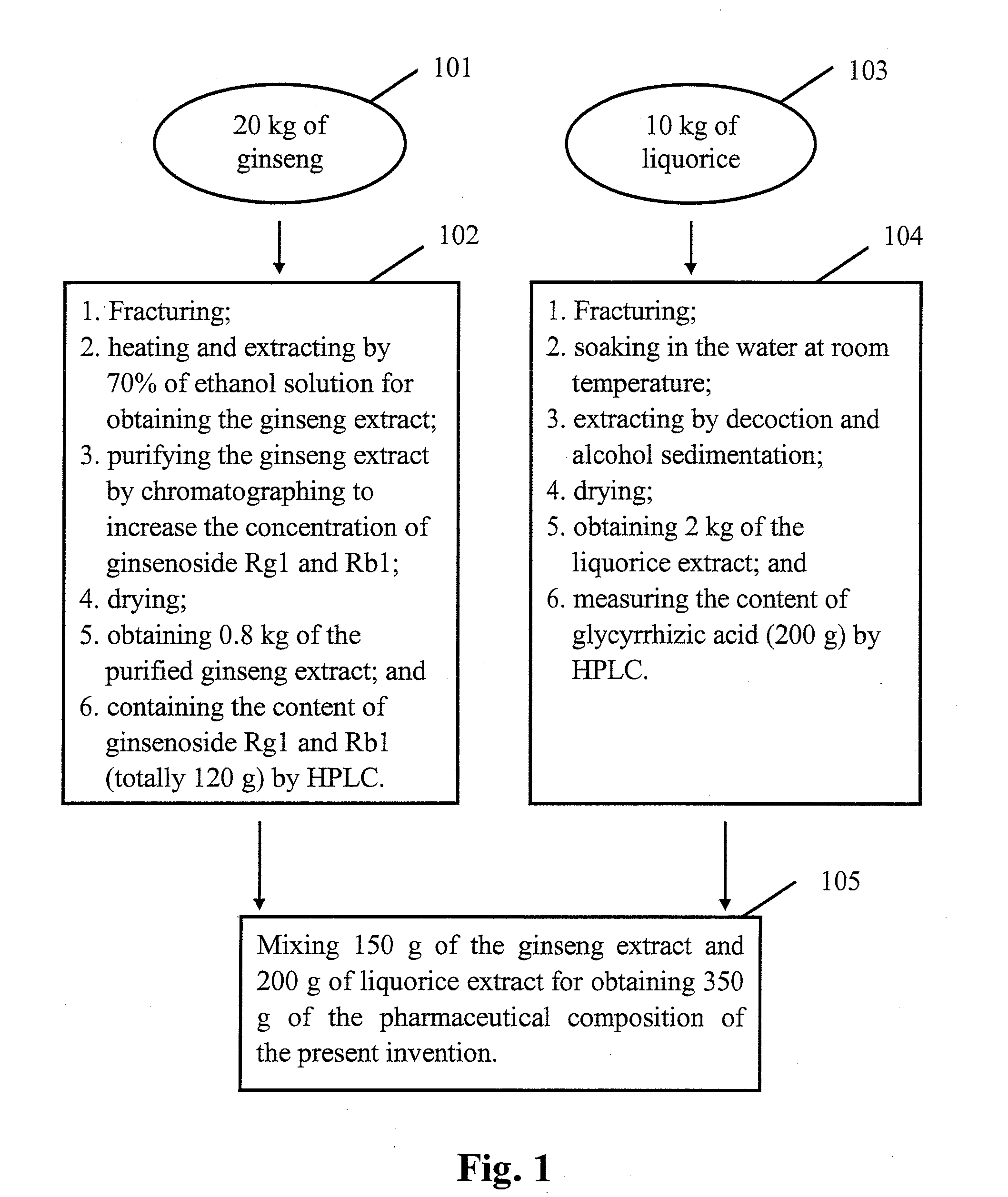
Busulfan composition, preparation method therefor and application thereof
PendingUS20210228527A1Reduce riskReduce use costPowder deliveryOrganic active ingredientsFreeze-dryingCyclodextrin
Busulfan composition contains Busulfan and cyclodextrin in a weight ratio of 1-20:100-2000. The Busulfan composition is preferably prepared by following steps of: dissolving Busulfan in an organic solvent to obtain a Busulfan solution with a concentration of 1-20 mg / mL; dissolving SBE-β-cyclodextrin with water for injection to obtain 10-40% (w / v) aqueous solution of SBE-beta-cyclodextrin; mixing the two solutions under nitrogen atmosphere by stirring for 1 hour, and removing the organic solvent; and filtering and freeze drying the mixed solution. According to the present invention, the method can be used for tablets and injections.
Owner:JIANGSU LINGHANG BIOLOGICAL TECH CO LTD
Composition and Methods of Controllable Co-Coupling Polypeptide Nanoparticle Delivery System for Nucleic Acid Therapeutics
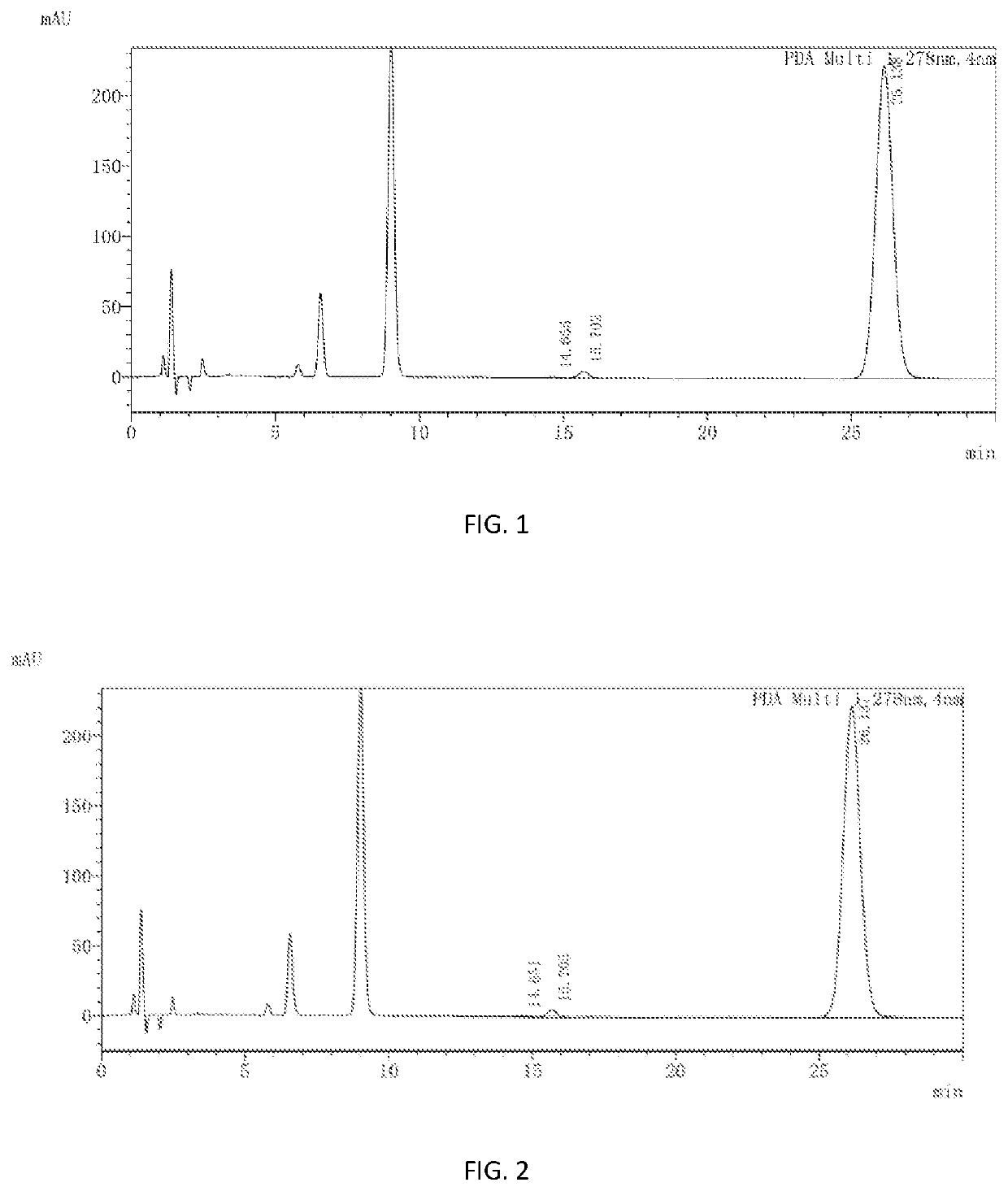
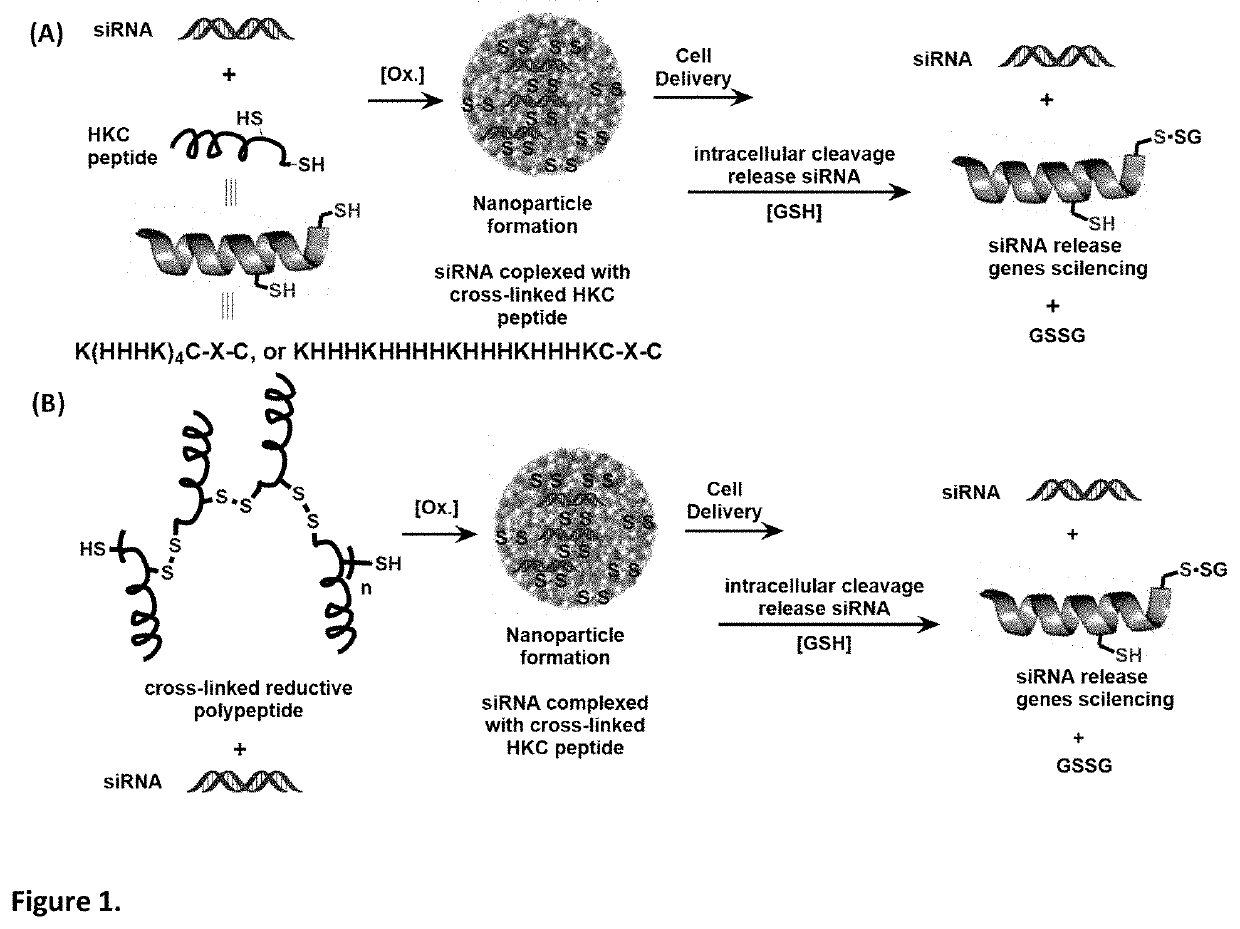
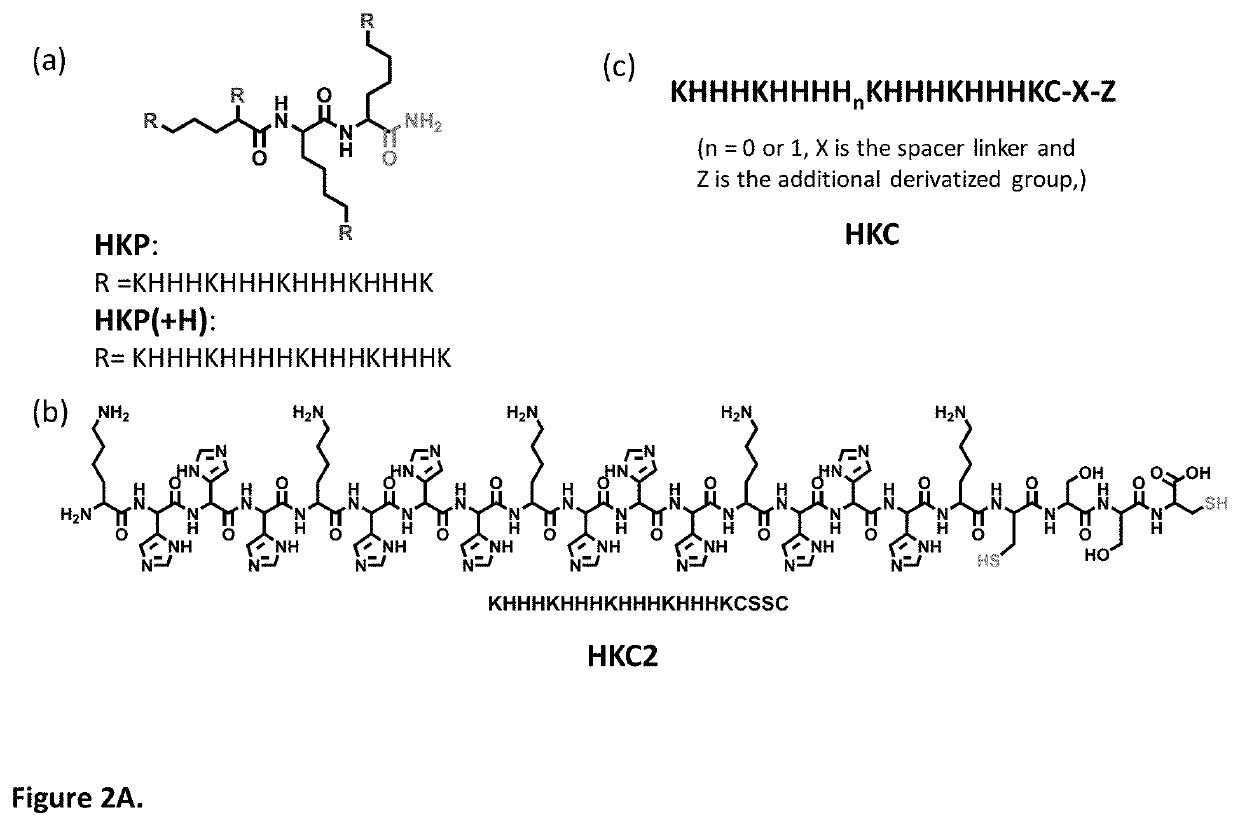
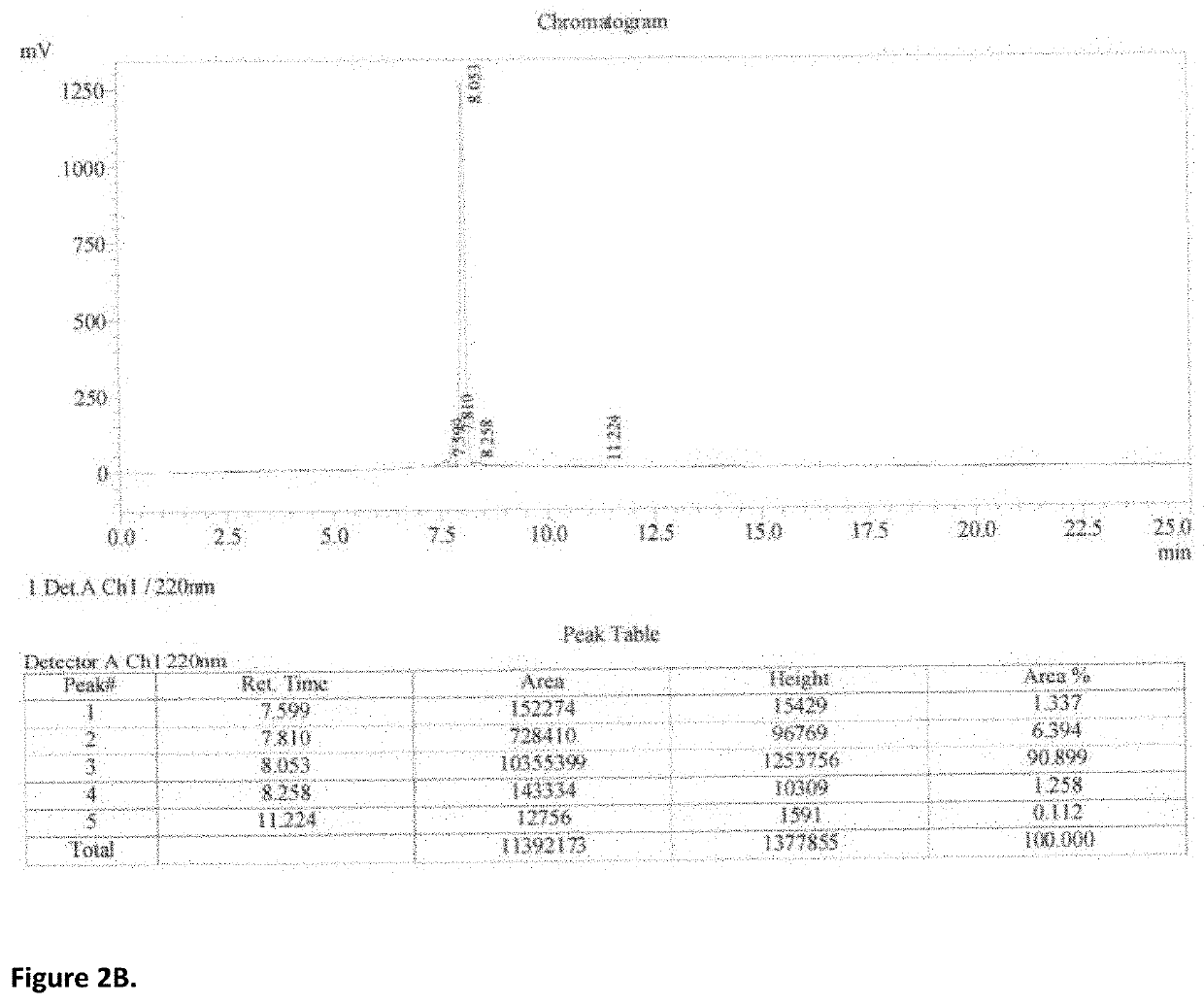
PendingUS20210162067A1Safety and efficacyLow costPowder deliveryOrganic active ingredientsNanoparticlePharmaceutical drug
Owner:SIRNAOMICS INC
Injection composition containing acetaminophen
ActiveUS11040007B2Avoid it happening againReduce solubilityOrganic active ingredientsPharmaceutical delivery mechanismAnti oxidantPurified water
The present disclosure relates to the injection composition containing acetaminophen, the injection composition comprising acetaminophen mixed in 0.01˜5 wt %, a solubilizer mixed in 0.01˜10 wt %, a toxicity inhibitor mixed in 0.05˜5 wt %, an isotonic agent mixed in 0.1˜10 wt %, an antioxidant mixed in 0.001˜5 wt %, and the balance sterile purified water for injection.
Owner:WOOSUNG PHARMA CO LTD
Bivalent swine influenza virus vaccine
ActiveUS20160250318A1Safety and efficacySsRNA viruses negative-senseViral antigen ingredientsInfluenza virus vaccineSwine influenza virus vaccine
The present invention relates to an immunogenic composition comprising: a) a modified live H3 virus of swine influenza, and b) a modified live H1 virus of swine influenza. Furthermore, the present invention relates to methods for immunizing a subject comprising administering to such subject the immunogenic composition of the present invention. Moreover, the present invention relates to methods of treating or preventing clinical signs caused by swine influenza virus in a subject of need, the method comprising administering to the subject a therapeutically effective amount of an immunogenic composition according to the present invention.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Artificial dna-binding proteins and uses thereof
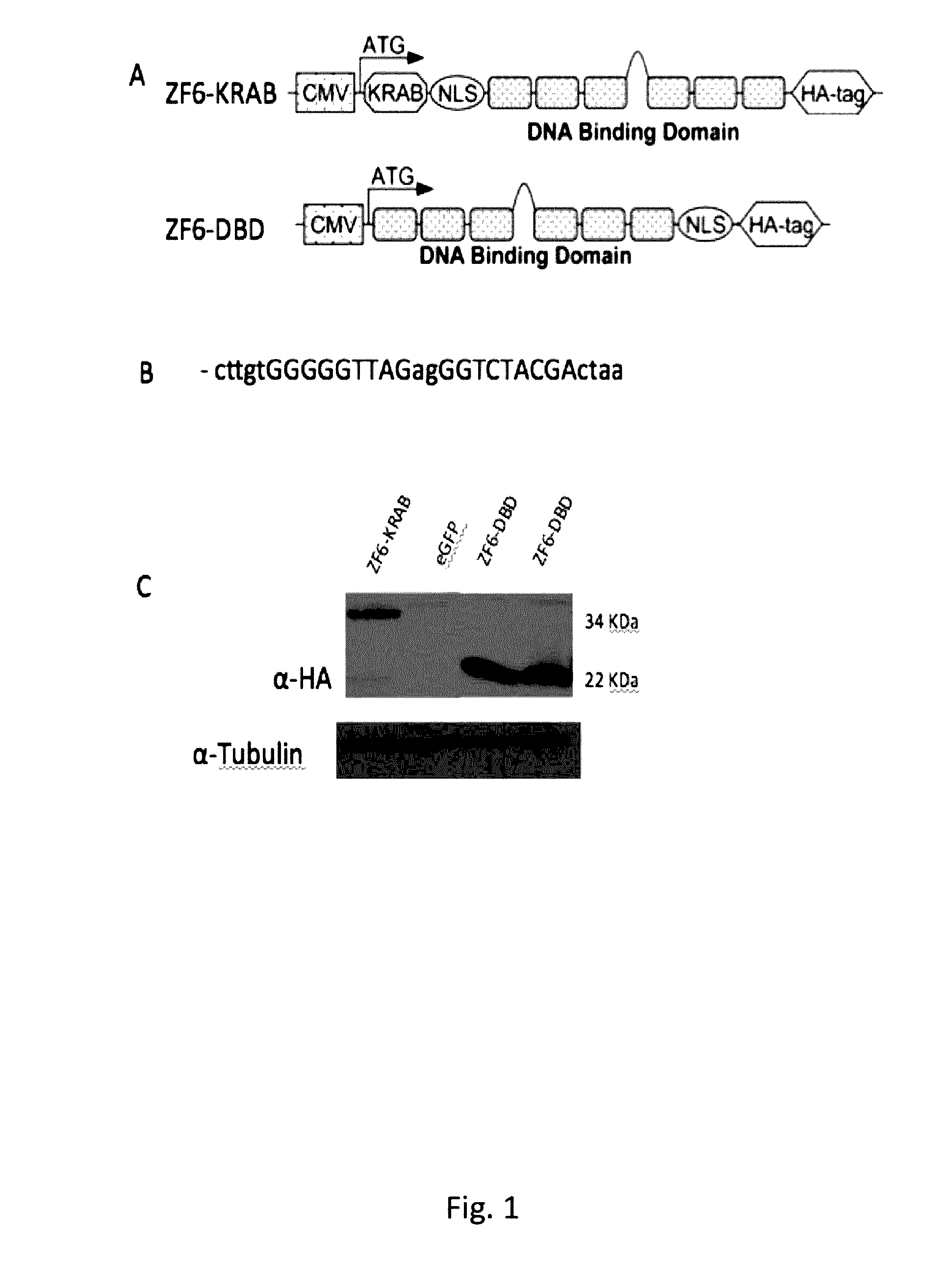
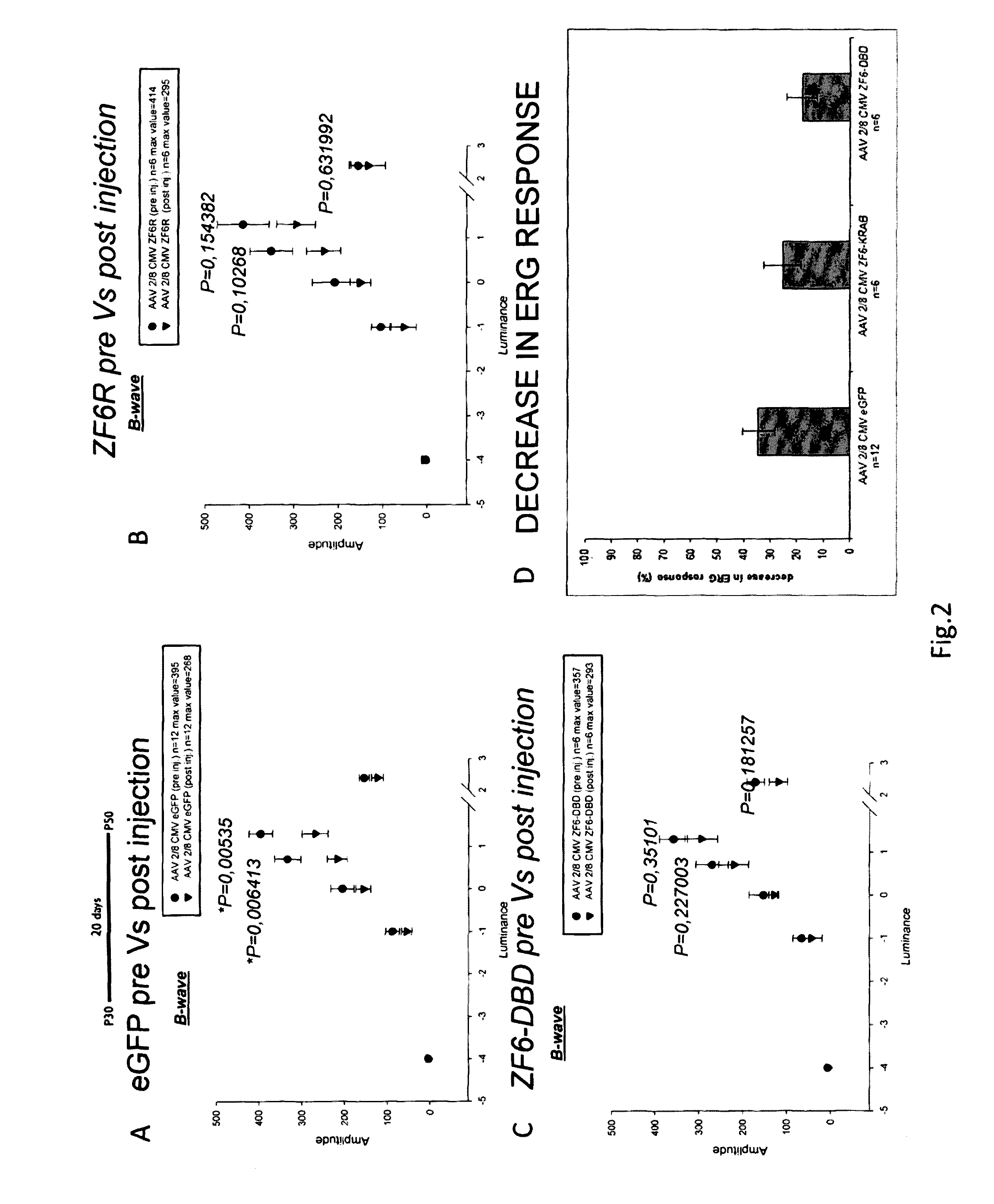
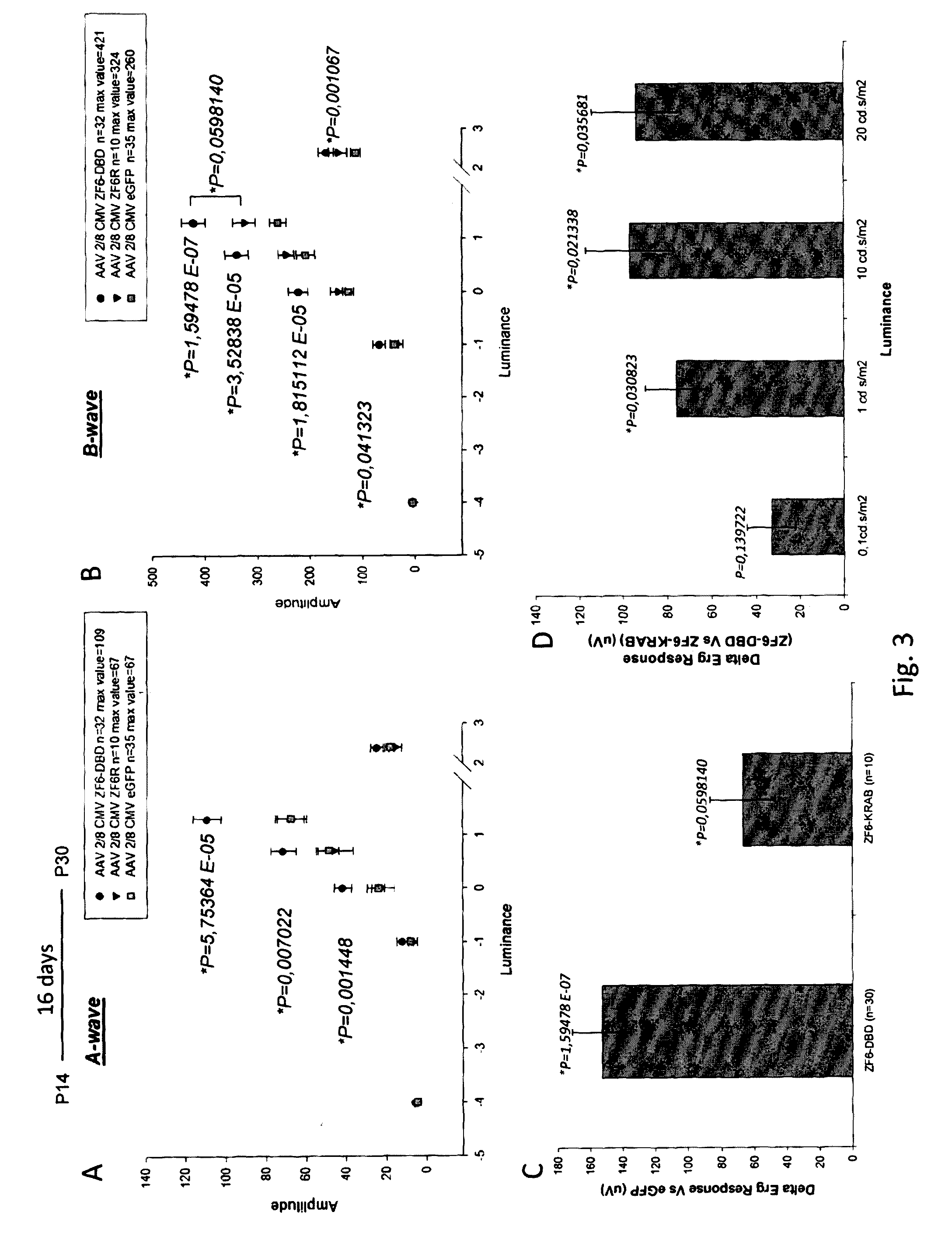
ActiveUS20160289284A1Safety and efficacyDramatic beneficial effectVectorsPeptide/protein ingredientsEye disorderDisease
The present invention relates to proteins consisting of an artificial DNA-binding domain (DBD) and related molecules and uses thereof. In particular, the proteins are ZF-DBD or TALE-DBD and are used for the treatment of eye disorders caused by gain of function mutation. The disorder may be ADRP, in particular ADRP caused by mutation in the rhodopsin gene. The present invention also relates to a method to identify cis-regulatory elements and to modulate them via DBDs.
Owner:UNIV DEGLI STUDI DI NAPOLI FEDERICO II
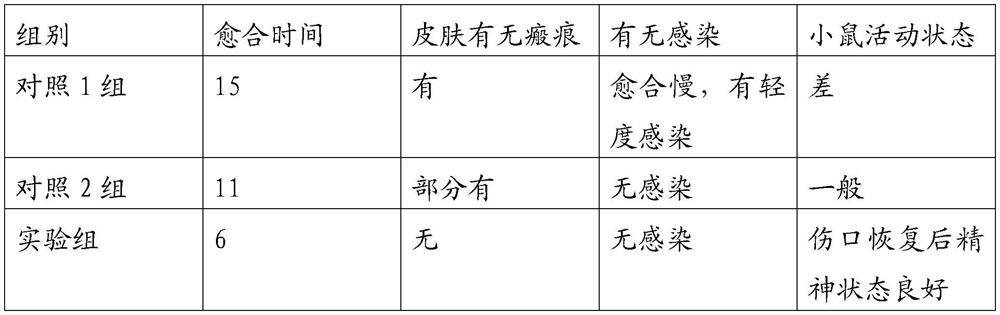
External traditional Chinese medicine preparation for treating scalds and burns and preparation method thereof
PendingCN114099421AImprove the immunityRestore elasticityAerosol deliveryOintment deliveryAngelica Sinensis RootPOLYGONUM CUSPIDATUM
The invention discloses an external traditional Chinese medicine preparation for treating scalds and burns and a preparation method thereof.The external traditional Chinese medicine preparation for treating scalds and burns is prepared from, by weight, 30-60 parts of spica prunellae, 20-30 parts of dragon's blood, 20-30 parts of flos sophorae, 20-30 parts of herba ecliptae, 20-30 parts of rhizoma imperatae and 20-30 parts of semen cassiae. The traditional Chinese medicine composition is prepared from 20-30 parts of polygonum cuspidatum, 10-20 parts of radix paeoniae rubra, 10-20 parts of cortex moutan, 30-40 parts of beewax, 20-35 parts of dog oil, 20-30 parts of ligusticum chuanxiong hort, 20-30 parts of herba violae, 20-30 parts of angelica sinensis and 60-80 The traditional Chinese medicine components in the formula cooperate with one another and play a role together, and the ointment is used for treating burns and scalds and has the advantages of being convenient to use, rapid in pain relieving effect, obvious in detumescence, convergence and tissue regeneration promoting effect, remarkable in curative effect, free of toxic and side effects, safe in medication and the like.
Owner:AFFILIATED HOSPITAL OF WEIFANG MEDICAL UNIV
Immune modulation by mesenchymal stem cells
PendingUS20220218817A1Enhance therapeutic benefitSafety and efficacyAntiviralsSkeletal/connective tissue cellsImmunomodulationsViral pneumonitis
A method is disclosed of treating a patient having viral pneumonia secondary to COVID-19 using IV infusion of mesenchymal stem cells in patients receiving COVID-19 vaccination. The vaccine may be Pfizer, Moderna, Johnson & Johnson or Astra Zenica COVID-19 vaccine, for example and the patient may be treated with an mRNA-based vaccine followed by IV-infusion of 100 million MSCs. MSCs can be derived from patients who have been exposed to the COVID-19 virus. Moreover, the MSCs may be derived from the umbilical cord of a donor.
Owner:VITRO BIOPHARMA INC
Acidulated surfactant compositions and methods of reducing microbial load
PendingUS20200093128A1Avoid cross contaminationReduce microbial loadBiocideAnimal repellantsBiotechnologyActive agent
Compositions and methods for reducing microbial loads on agricultural products are disclosed. The compositions include at least one acidulant and at least one surfactant, each present in an effective amount to reduce a microbial load on an agricultural product. The methods include applying compositions of at least one acidulant and at least one surfactant to agricultural products to reduce microbial loads and cross contamination of agricultural processing equipment.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com