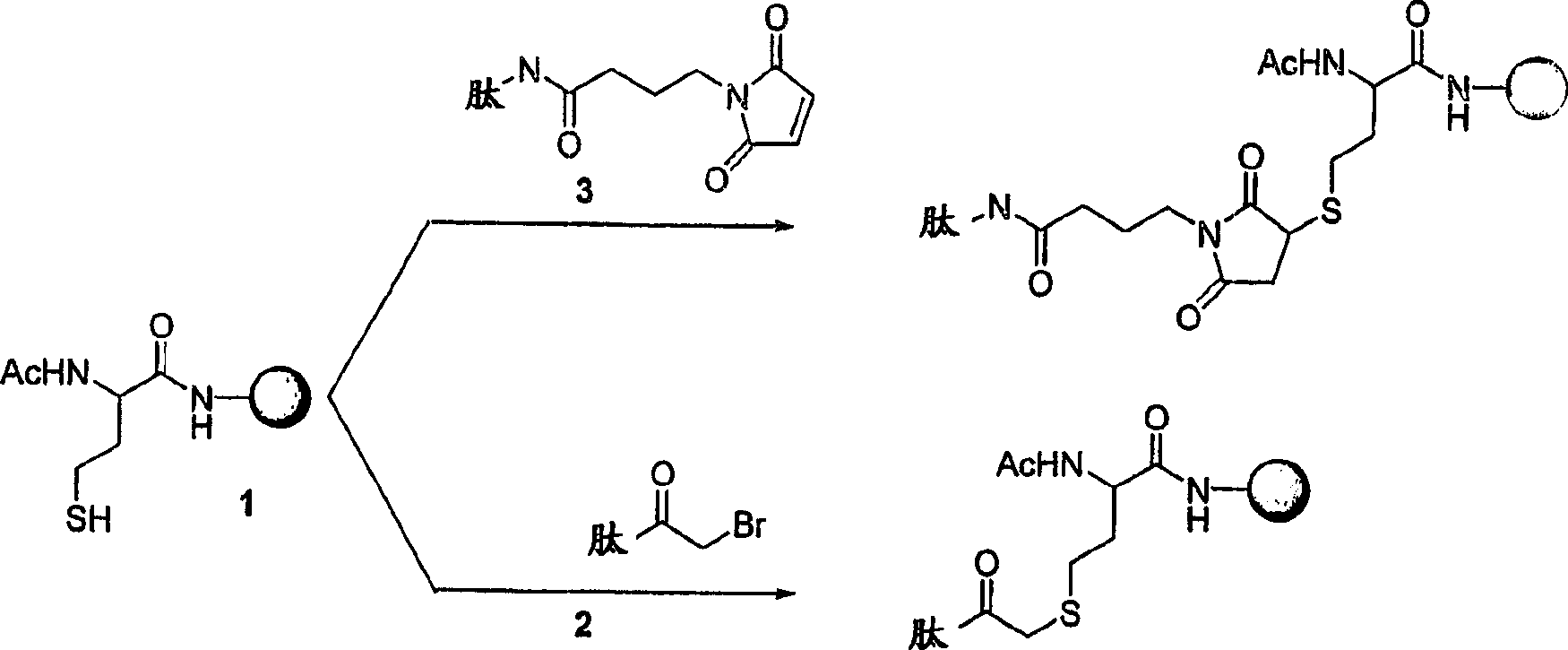
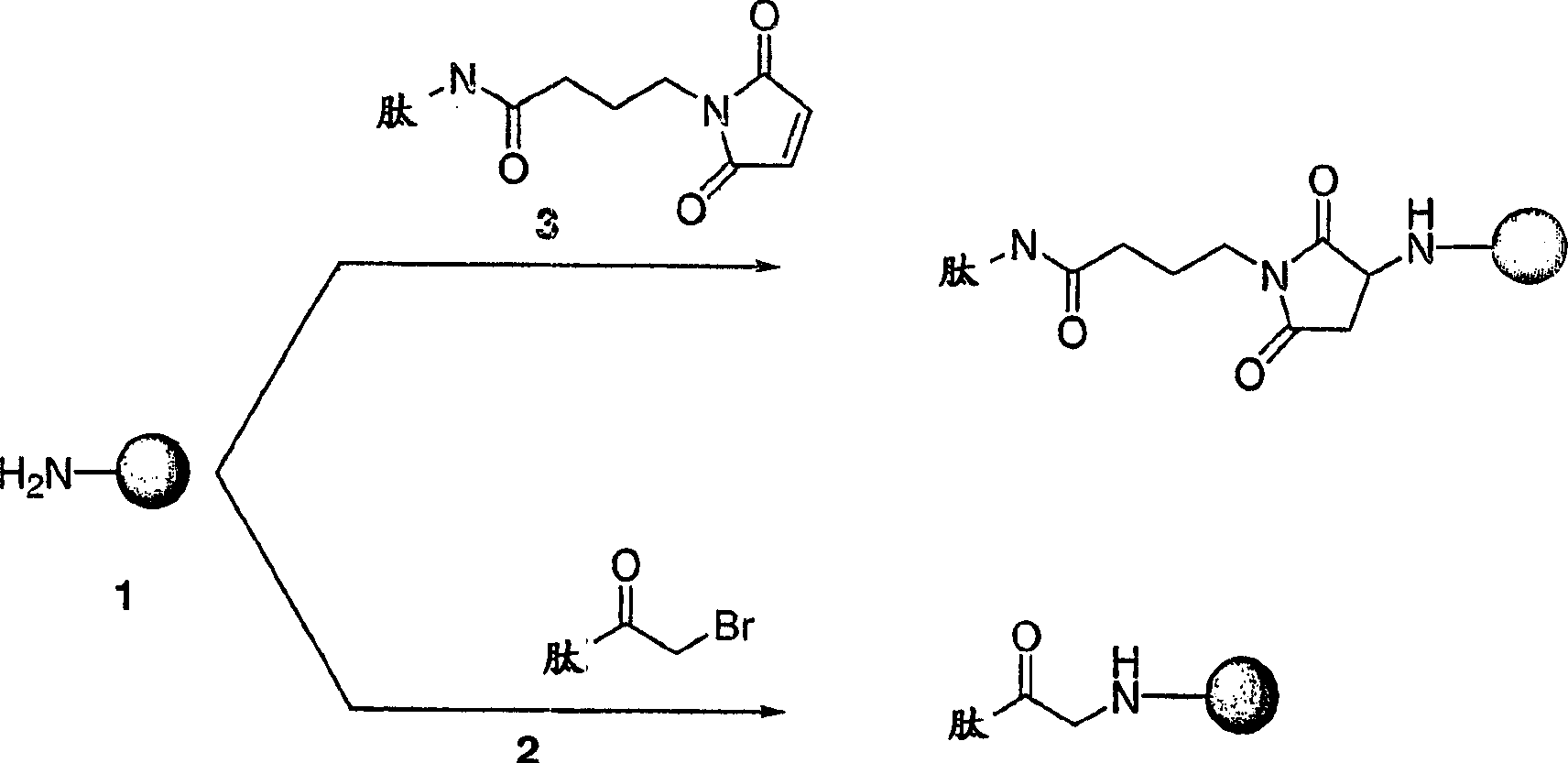
Patents
Literature
145 results about "Influenza virus vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza virus vaccines and uses thereof
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
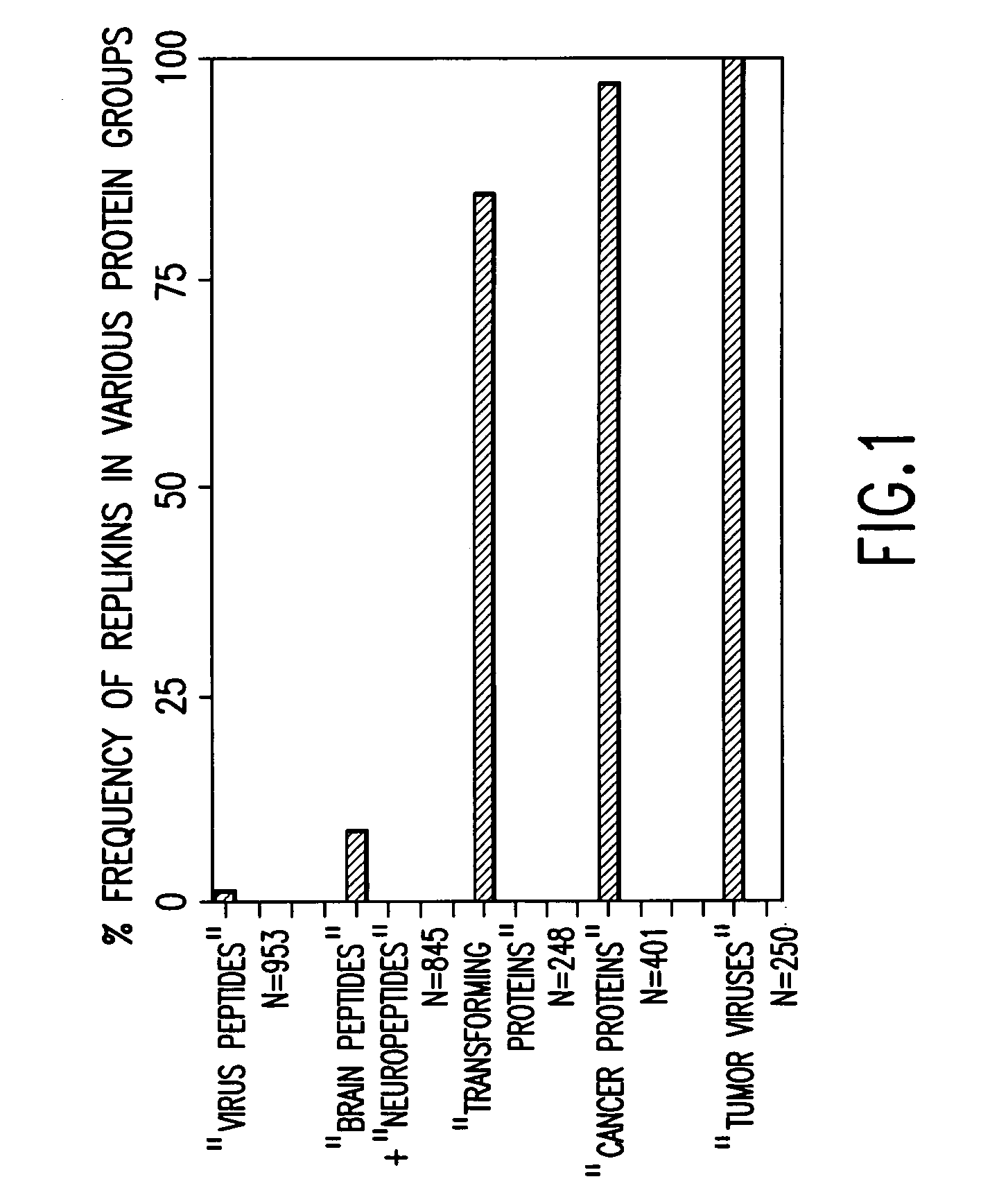
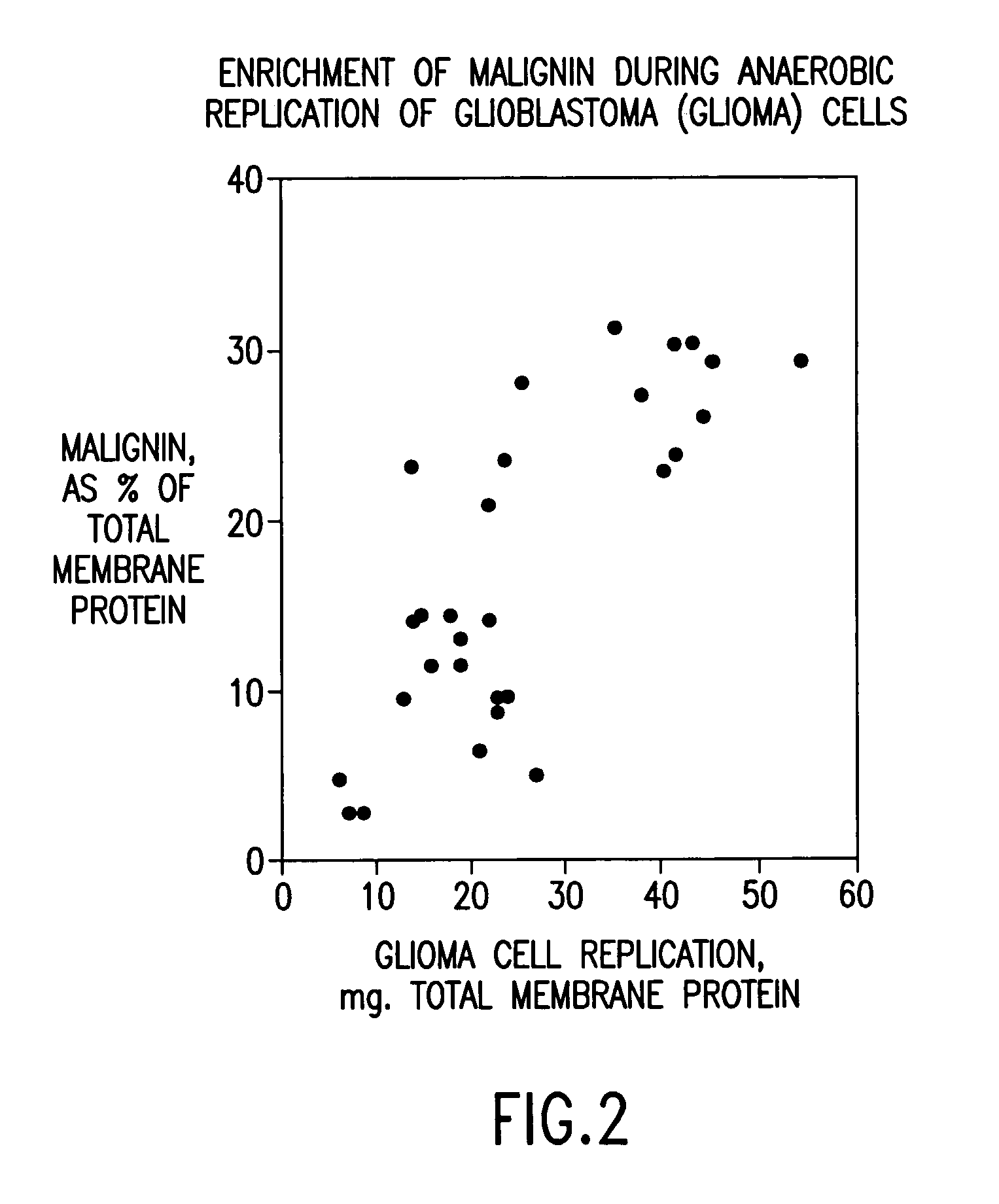
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
Vaccines Including Antigen From Four Strains of Influenza Virus
InactiveUS20140178429A1Reduce in quantityFacilitate matterSsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:SEQIRUS UK LTD
Influenza virus vaccine composition and methods of use
InactiveUS20070286869A1Enhance transfection expression efficacyLow costSsRNA viruses negative-senseOrganic active ingredientsEpitopeInfluenza virus vaccine
The present invention is directed to enhancing the immune response of a human in need of protection against IV infection by administering in vivo, into a tissue of the human, at least one polynucleotide comprising one or more regions of nucleic acid encoding an IV protein or a fragment, a variant, or a derivative thereof. The present invention is further directed to enhancing the immune response of a human in need of protection against IV infection by administering, in vivo, into a tissue of the human, at least one IV protein or a fragment, a variant, or derivative thereof. The IV protein can be, for example, in purified form or can be an inactivated IV, such as those present in inactivated IV vaccines. The polynucleotide is incorporated into the cells of the human in vivo, and an immunologically effective amount of an immunogenic epitope of an IV, or a fragment, variant, or derivative thereof is produced in vivo. The IV protein (in purified form or in the form of an inactivated IV vaccine) is also administered in an immunologically effective amount.
Owner:VICAL INC
Live Attenuated Influenza Virus Vaccines Comprising Microrna Response Elements
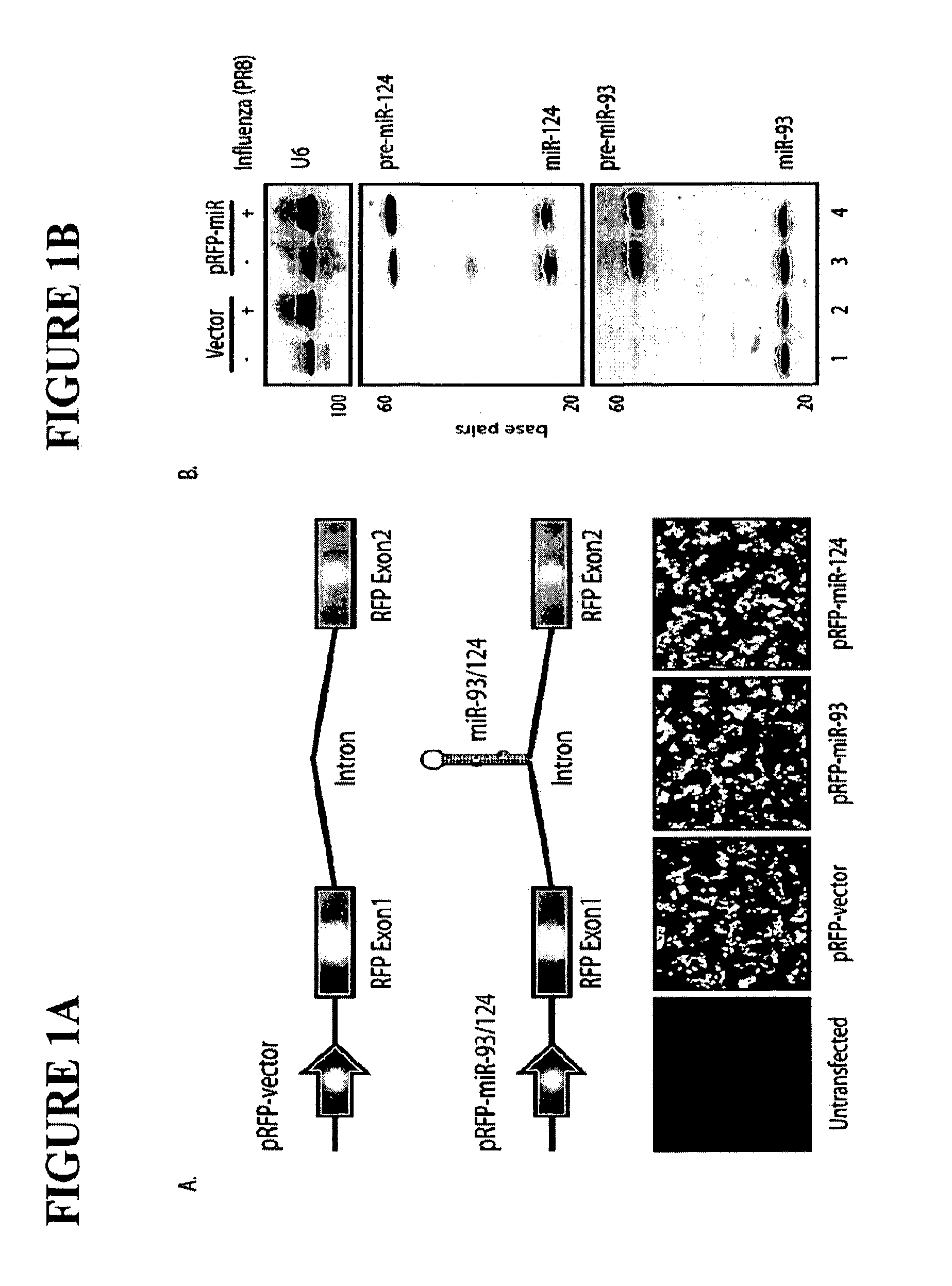
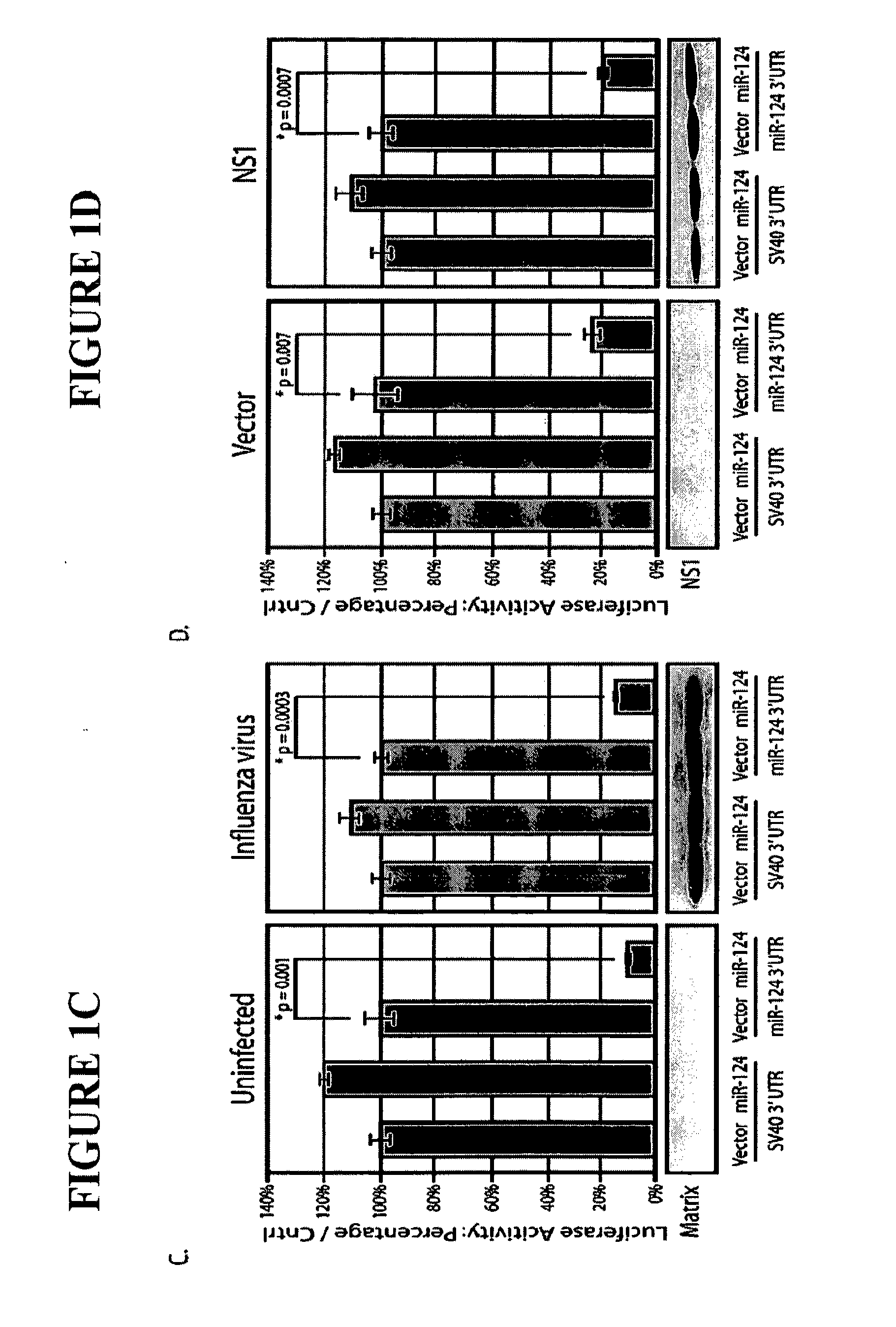
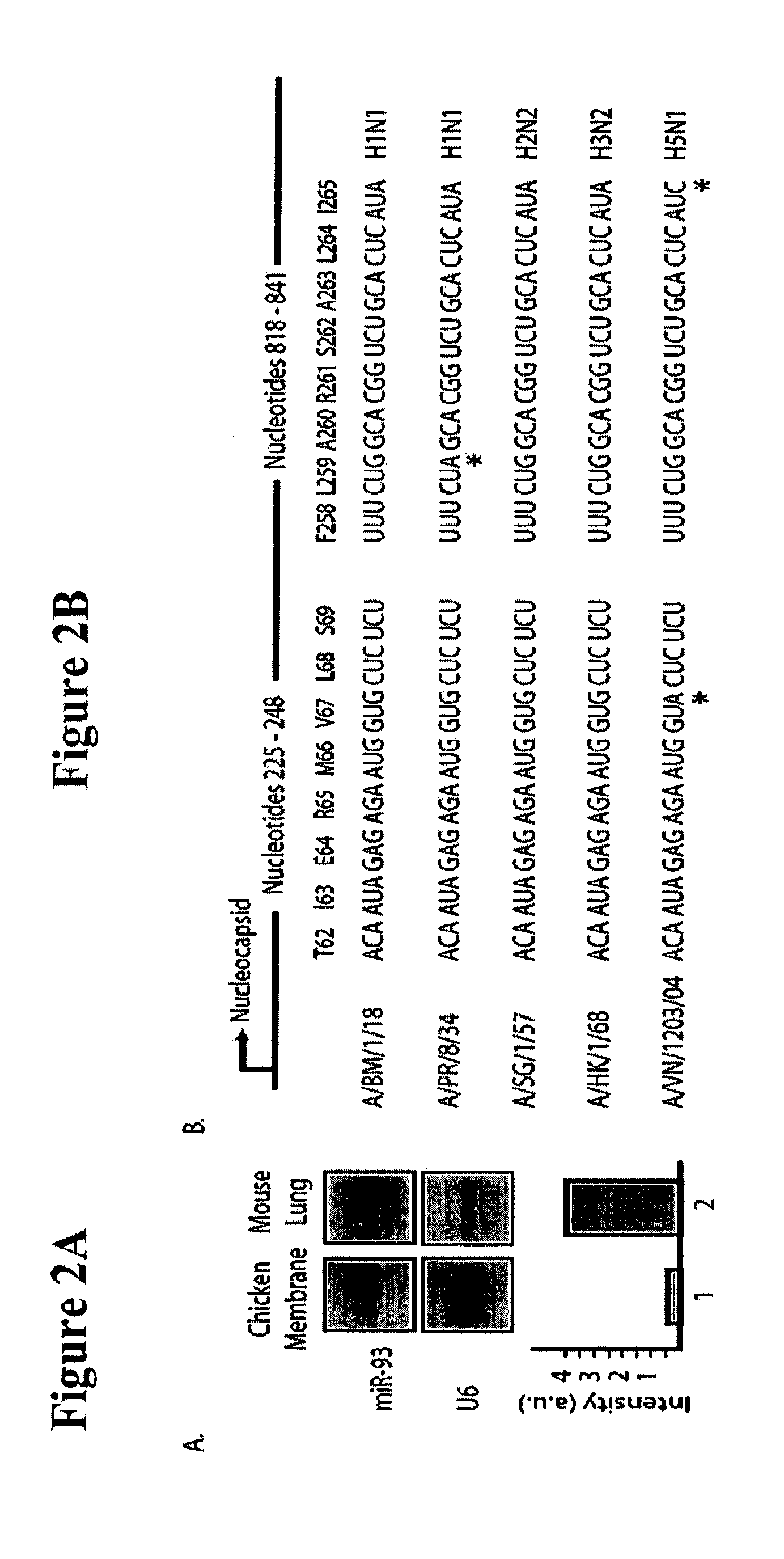
ActiveUS20120148622A1Increase vaccine safetyQuick buildSsRNA viruses negative-senseSugar derivativesUltrasound attenuationInfluenza virus vaccine
The invention is directed to novel live attenuated influenza virus (LAIV) vaccines comprising one or more microRNA (miRNA) Response Element(s) (MRE) within an influenza virus genome. The MREs useful for the present invention can be derived from any miRNA which is highly expressed in influenza-targeted cells of an animal in need of vaccination but are not expressed or are expressed at very low levels in species (e.g., embryonated chicken eggs) or cell lines used for a large-scale vaccine production. This allows efficient vaccine production but renders the vaccine virus susceptible to attenuation in the influenza-targeted cells of vaccinated animals expressing a cognate miRNA.
Owner:MT SINAI SCHOOL OF MEDICINE
Novel use
InactiveUS20090028903A1Enhance antibody responseImproving CD T-cell responseSsRNA viruses negative-senseViral antigen ingredientsAntigenDisease
The present invention relates to influenza vaccine formulations and vaccination regimes for immunising against influenza disease. In particular the invention relates to vaccine formulations comprising an oil-in-water emulsion adjuvant and optionally 3D-MPL, their use in medicine, in particular their use in augmenting immune responses to influenza antigens, and to methods of preparation, wherein the oil in water emulsion comprises a sterol, a metabolisable oil and an emulsifying agent. The present invention also provides for new prime-boost vaccination regimes for immunising humans against influenza disease, and in particular for ensuring and ameliorating the immunre response to the booster administration, in which a first influenza virus vaccine is administered in the presence of an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Influenza virus vaccines and uses thereof
ActiveUS20140328875A1Highly potent and broadly neutralizing antibodiesStimulate immune responseSsRNA viruses negative-senseBacteriaHemagglutininInfluenza virus vaccine
Provided herein are chimeric influenza hemagglutinin (HA) polypeptides, compositions comprising the same, vaccines comprising the same, and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
Influenza Virus Vaccines
InactiveUS20080254065A1SsRNA viruses negative-senseViral antigen ingredientsFowlInfluenza virus vaccine
The invention provides a vaccine for protecting a human patient against infection by a human influenza virus strain, wherein the vaccine comprises an antigen from an avian influenza virus strain that can cause highly pathogenic avian influenza. The antigen can invoke an antibody response in the patient that is capable of neutralising said human influenza virus strain. Whereas the prior art used known non-pathogenic avian strains to generate antibodies in humans against known pathogenic avian strains, the invention uses known pathogenic avian strains to protect against emerging pathogenic human strains. Furthermore, whereas the prior art focused on achieving a close antigenic match between the vaccine strain and the target strain, the invention selects vaccine strains based on their pathogenicity, regardless of any perceived close antigenic relationship to the target strain. As the invention does not require detailed knowledge of an emerging strain, a vaccine can be provided further in advance to reduce the risk and potential effects of a human pandemic outbreak.
Owner:SEQIRUS UK LTD
Cell-based systems for producing influenza vaccines
ActiveUS20100021499A1Minimize and prevent virus infectionAvoid componentsSsRNA viruses negative-senseAnimal cellsHemagglutininBinding site
The present invention relates to a cell-based method for producing influenza virus vaccines by enriching the population of surface-bound α2,6-sialic acid receptors on a cell surface, such as on a Chinese Hamster Ovary (CHO) cell surface. The host cell therefore presents numerous binding sites to which an influenza virus can bind via its hemagglutinin spike protein and infect the host cell. In contrast to wild-type CHO cells, the surface of the mutated CHO cells of the present invention contains an enriched population of α2,6-sialic acid receptors which makes the inventive CHO cells highly susceptible to viral infection, and therefore safe, effective, and highly efficient cells for rapidly producing influenza vaccines.
Owner:FLUGEN
Influenza virus vaccines and uses thereof
ActiveUS9051359B2Elimination of glycosylationReduce severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Provided herein are influenza hemagglutinin stem domain polypeptides, compositions comprising the same, vaccines comprising the same and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
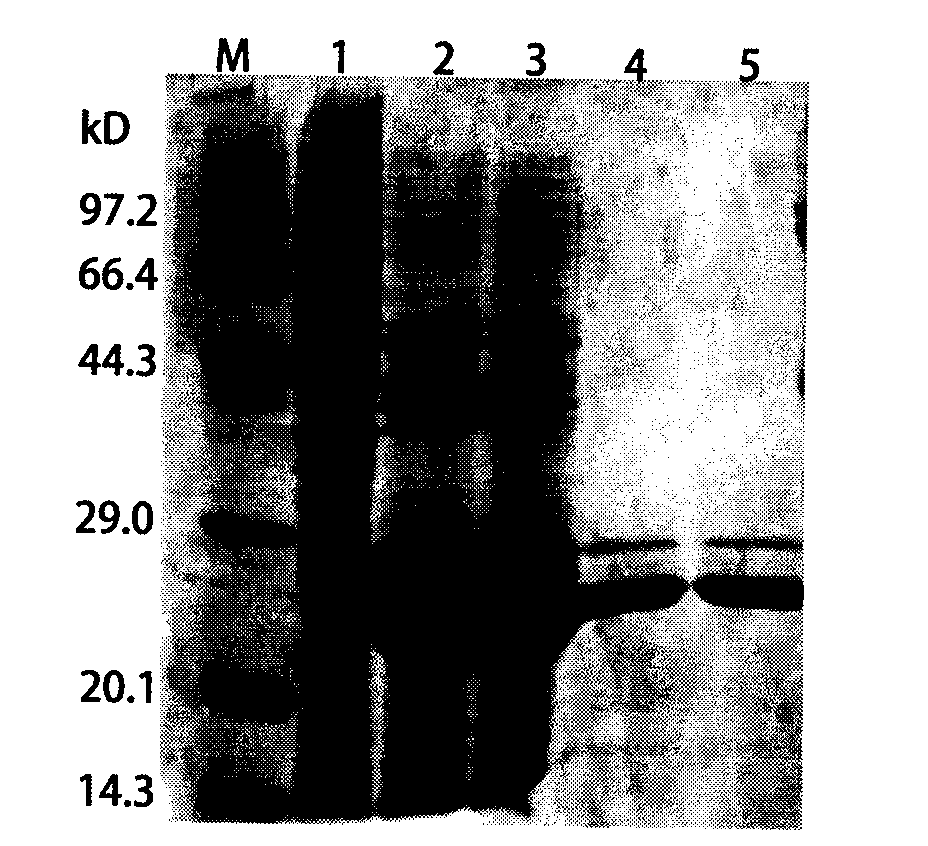
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant flu vaccines
InactiveUS20090117144A1Sufficient immunogenicityReduce loadSsRNA viruses negative-senseSsRNA viruses positive-senseInfluenza virus vaccinePlant virus
The present invention provides compositions for use as vaccines against the influenza virus, and rapid methods of producing such compositions. The composition include i) at least one peptide derived from an influenza virus, wherein the peptide is fused to a capsid protein derived from a plant virus forming a recombinant capsid fusion peptide and ii) at least one isolated antigenic protein or protein fragment derived from a human or avian influenza virus. The isolated antigenic protein or protein fragment derived from the human or avian influenza virus can be conjugated to the surface of the recombinant capsid fusion peptide.
Owner:PFENEX
High titer recombinant influenza viruses with enhanced replication in vero cells
ActiveUS20110110978A1Enhanced influenza virus replicationHigh pHSsRNA viruses negative-senseVirus peptidesDiseaseInfluenza virus vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, and genes from vaccine seed virus isolates which include a HA gene segment with a HA2 sequence encoding a HA2 that confers enhanced growth in cells in culture, such as Vero cells.
Owner:WISCONSIN ALUMNI RES FOUND
Production method for influenza virus vaccines
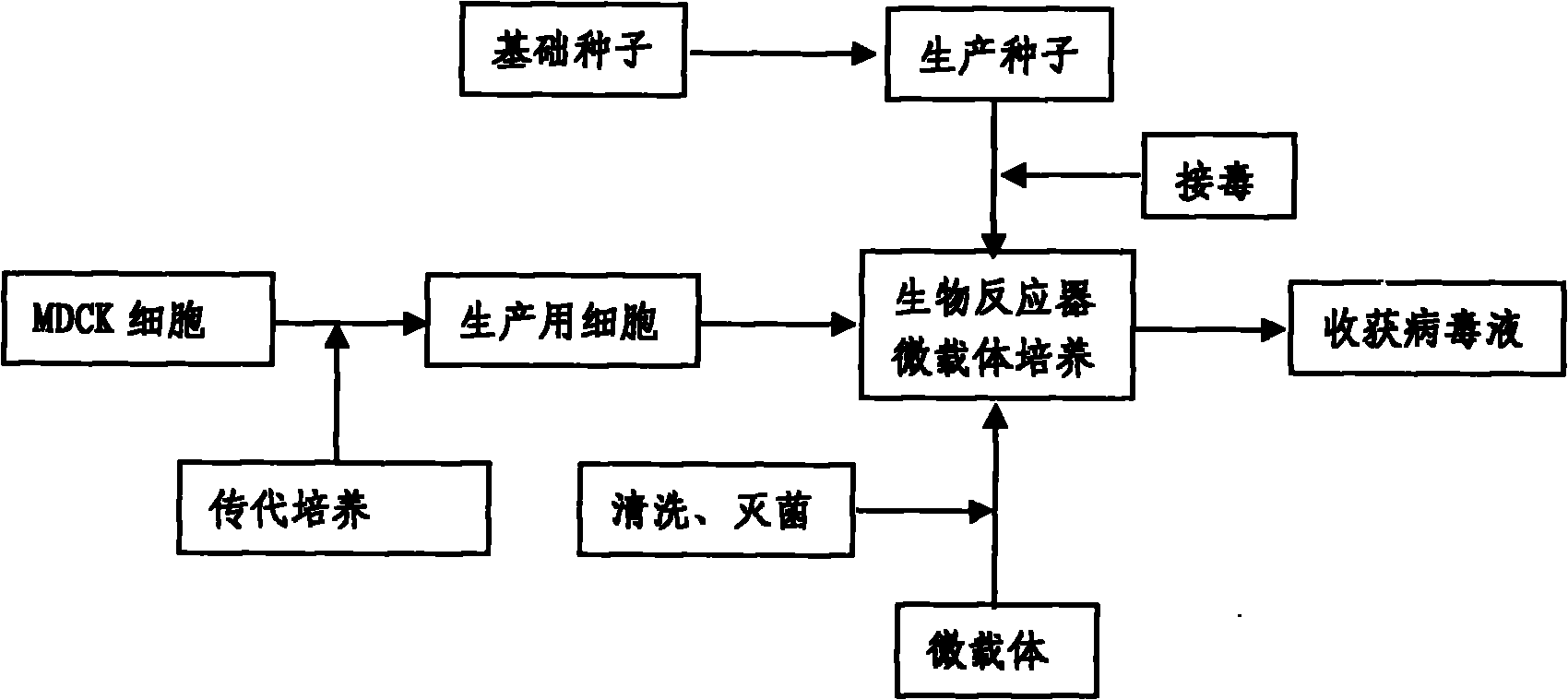
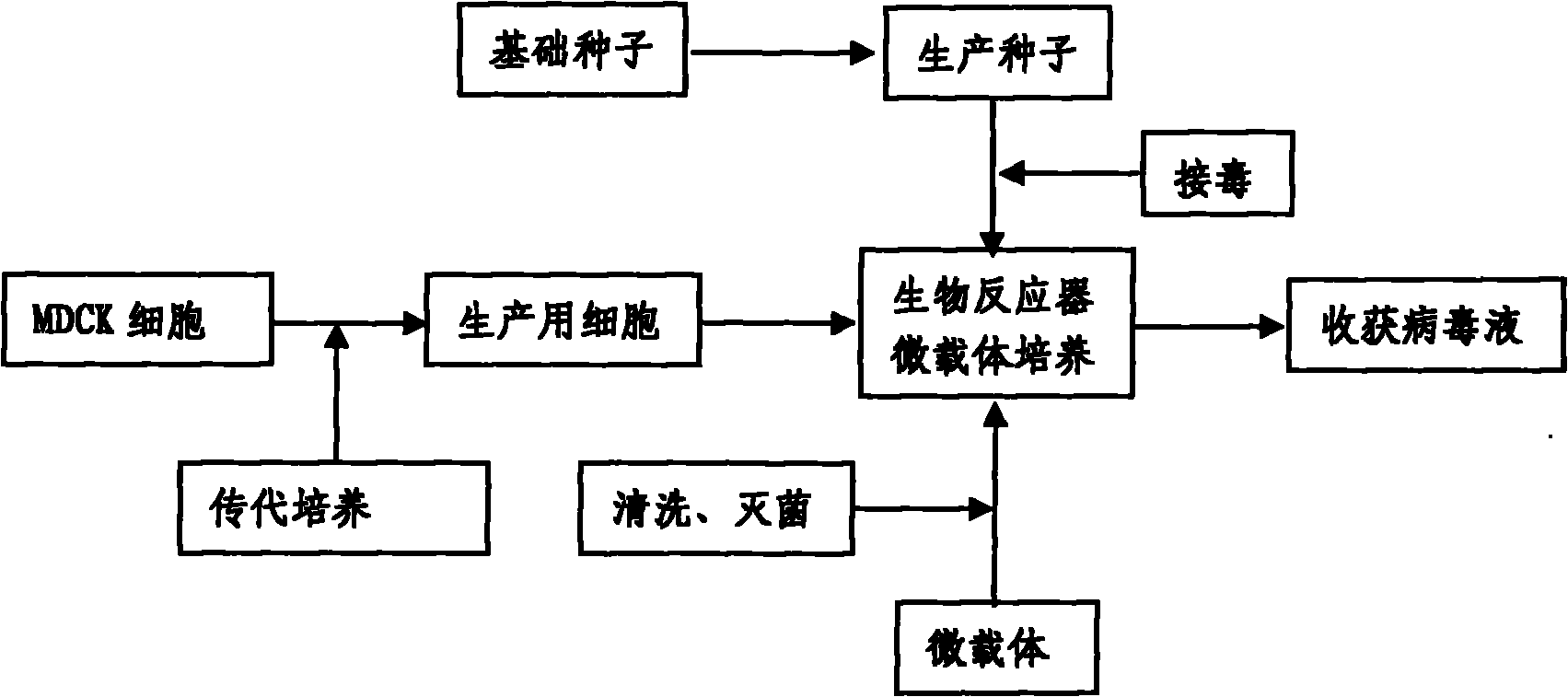
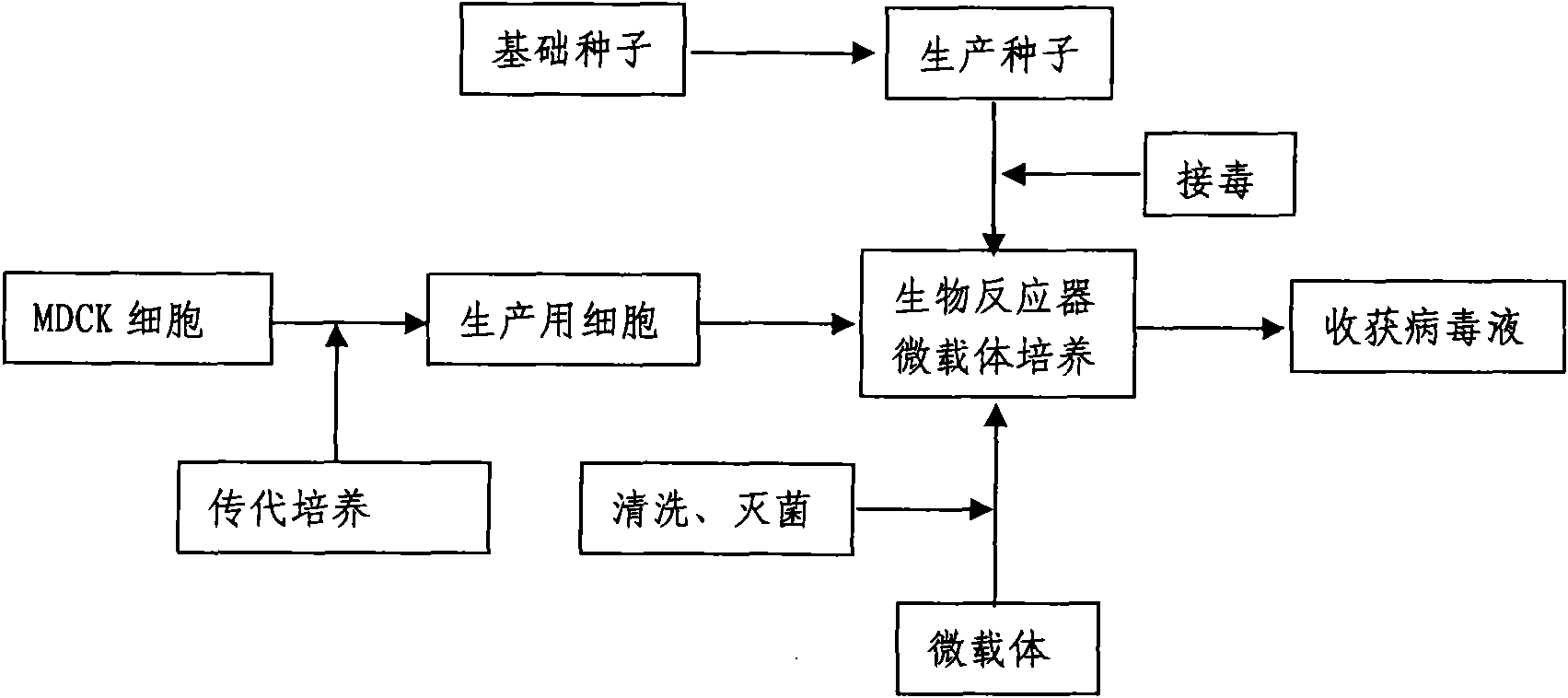
InactiveCN101818131ASolve yourselfSolve pollutionAntiviralsViruses/bacteriophagesEquine influenza vaccineBioreactor
The invention discloses a production method for vaccines of avian influenza virus and other influenza virus such as swine influenza, dog influenza and equine influenza, which comprises the following steps of: (1) transfer of culture and cultivation of vaccine-made cells; (2) reproduction of vaccine-made virus seeds; (3) microcarrier suspension culture of MDCK cells in a bioreactor; (4) reproduction of vaccine-made virus liquid; and (5) harvest of the virus liquid. The production method has the advantages of reducing the production cost greatly and improving the yield and quality of the vaccines obviously, along with short production period, no restriction to raw material supply, small occupied area, easy and quick expansion of production scale, light environmental pollution, easy processing, high automaticity, low employment and easy realization of balanced and stable quality.
Owner:成都史纪生物制药有限公司
Influenza virus vaccines and uses thereof
ActiveUS20150297712A1Elimination of glycosylationReduce severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Provided herein are influenza hemagglutinin stem domain polypeptides, compositions comprising the same, vaccines comprising the same and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
High titer recombinant influenza viruses with enhanced replication in mdck or vero cells or eggs
ActiveUS20170354730A1Improve scalabilitySignificantly higher viral titersSsRNA viruses negative-senseAntiviralsDiseaseEgg cell
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Influenza virus vaccine
InactiveCN1756562AAchieve desired propertiesViral antigen ingredientsFermentationDiseaseInfluenza virus vaccine
Owner:MERCK & CO INC +1
Influenza virus vaccine using nanometer emulsion as adjuvant and method for preparing same
InactiveCN101843901ALow toxicityMild in natureAntiviralsAntibody medical ingredientsSolubilityAdjuvant
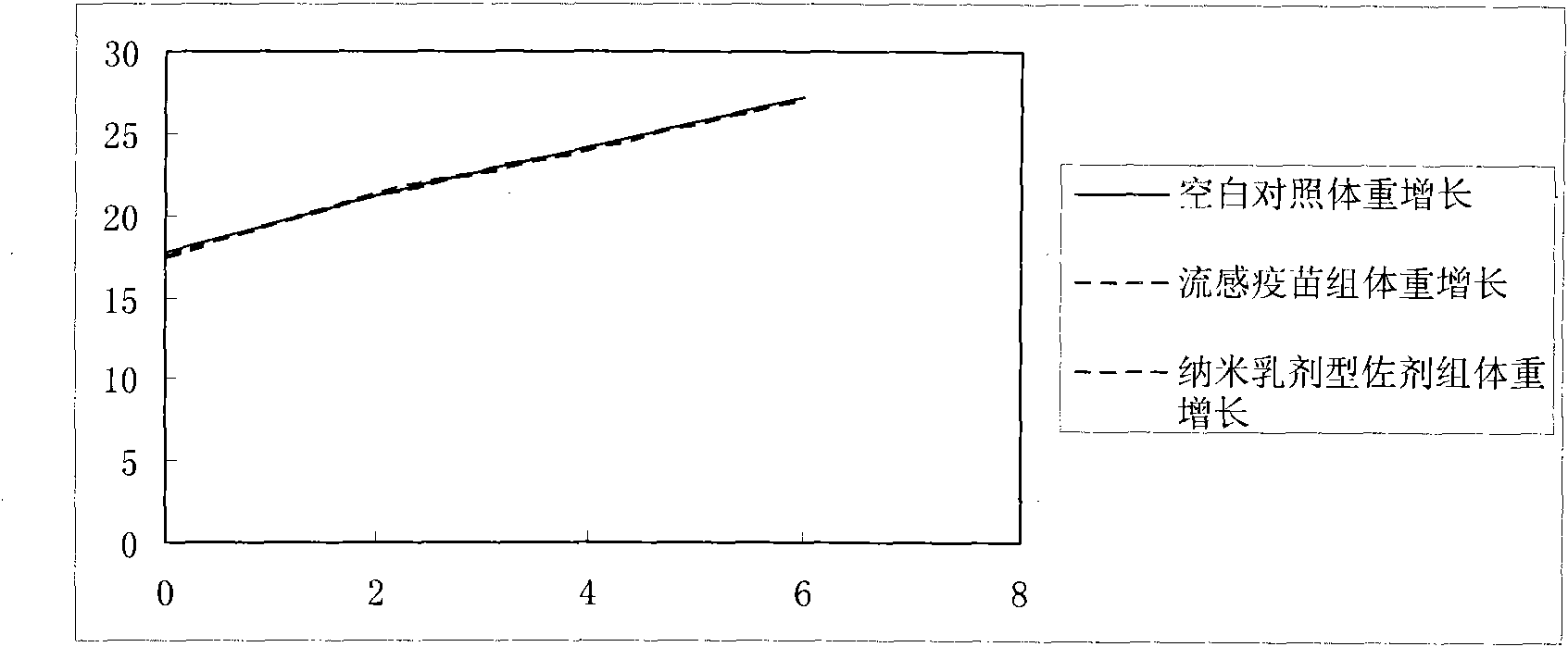
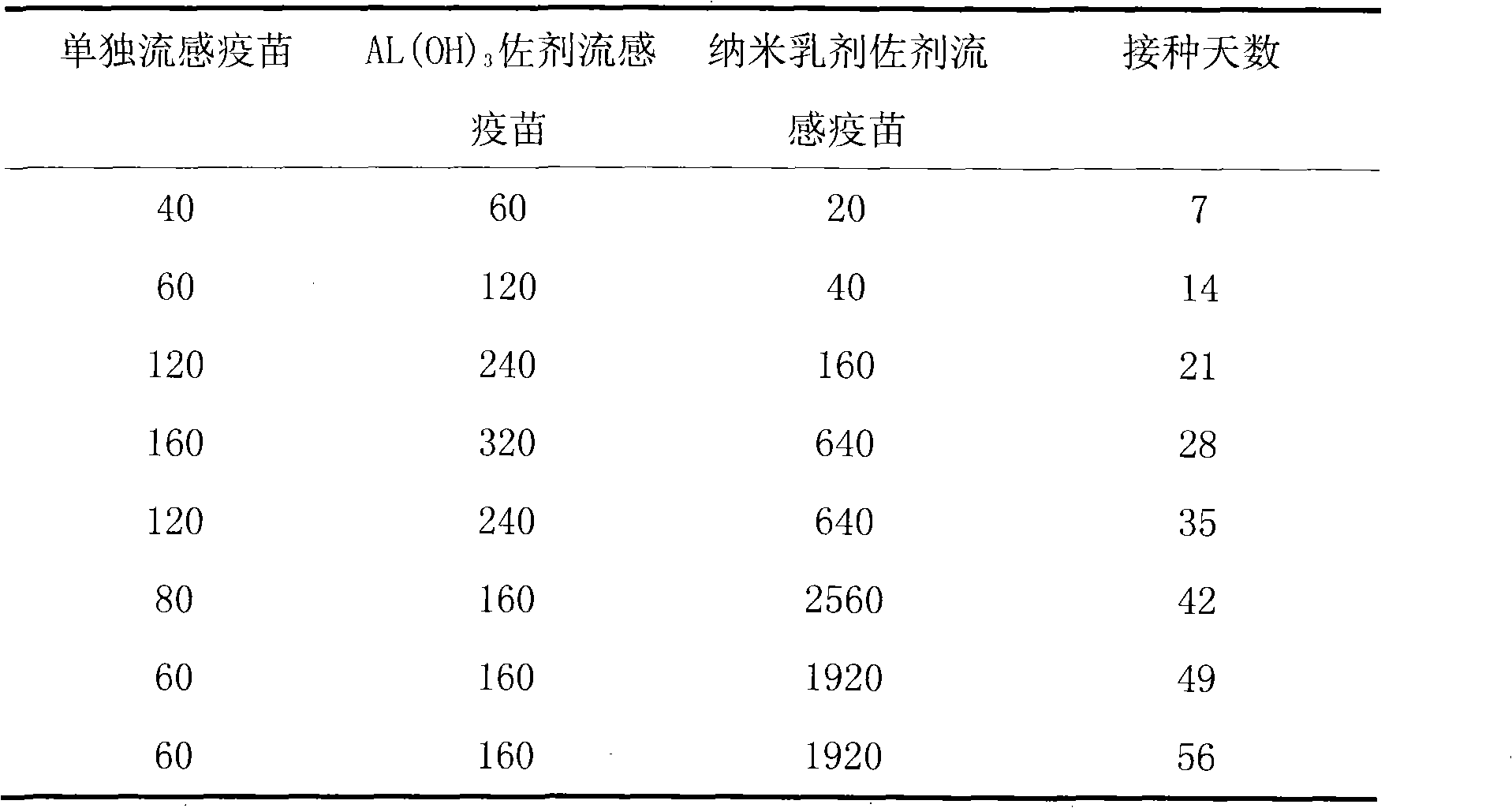
The invention provides an influenza virus vaccine using nanometer emulsion as an adjuvant and a method for preparing the same. The influenza virus vaccine consists of the following components in percentage by mass: 1 to 30 percent of diluted influenza virus vaccine, 1 to 50 percent of oil phase, 20 to 70 percent of emulsifying agent and 20 to 70 percent of auxiliary emulsifying agent. In the influenza virus vaccine using the nanometer emulsion as the adjuvant, the adjuvant, namely the nanometer emulsion, has the advantages of little toxicity, mild properties, obvious solubility-enhancing effect on medicaments, good fluidity, extremely low irritation on bodies and the like, so that the immunization effect of the influenza virus vaccine is greatly improved; compared with the adjuvant-free influenza virus vaccine, the influenza virus vaccine of the invention has the advantages of small dosage and high bioavailability; compared with other influenza virus vaccines using the adjuvant, the influenza virus vaccine of the invention has the advantages of greatly improving bioavailability, lowering irritation, reducing adverse reactions, prolonging vaccine-releasing time, improving the targeting property of the vaccine, reducing an attrition rate and the like.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
High titer recombinant influenza viruses with enhanced replication in vero cells
ActiveUS9109013B2Improve scalabilityEasy to copySsRNA viruses negative-senseVirus peptidesDiseaseInfluenza virus vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, and genes from vaccine seed virus isolates which include a HA gene segment with a HA2 sequence encoding a HA2 that confers enhanced growth in cells in culture, such as Vero cells.
Owner:WISCONSIN ALUMNI RES FOUND
Influenza a virus vaccines and inhibitors
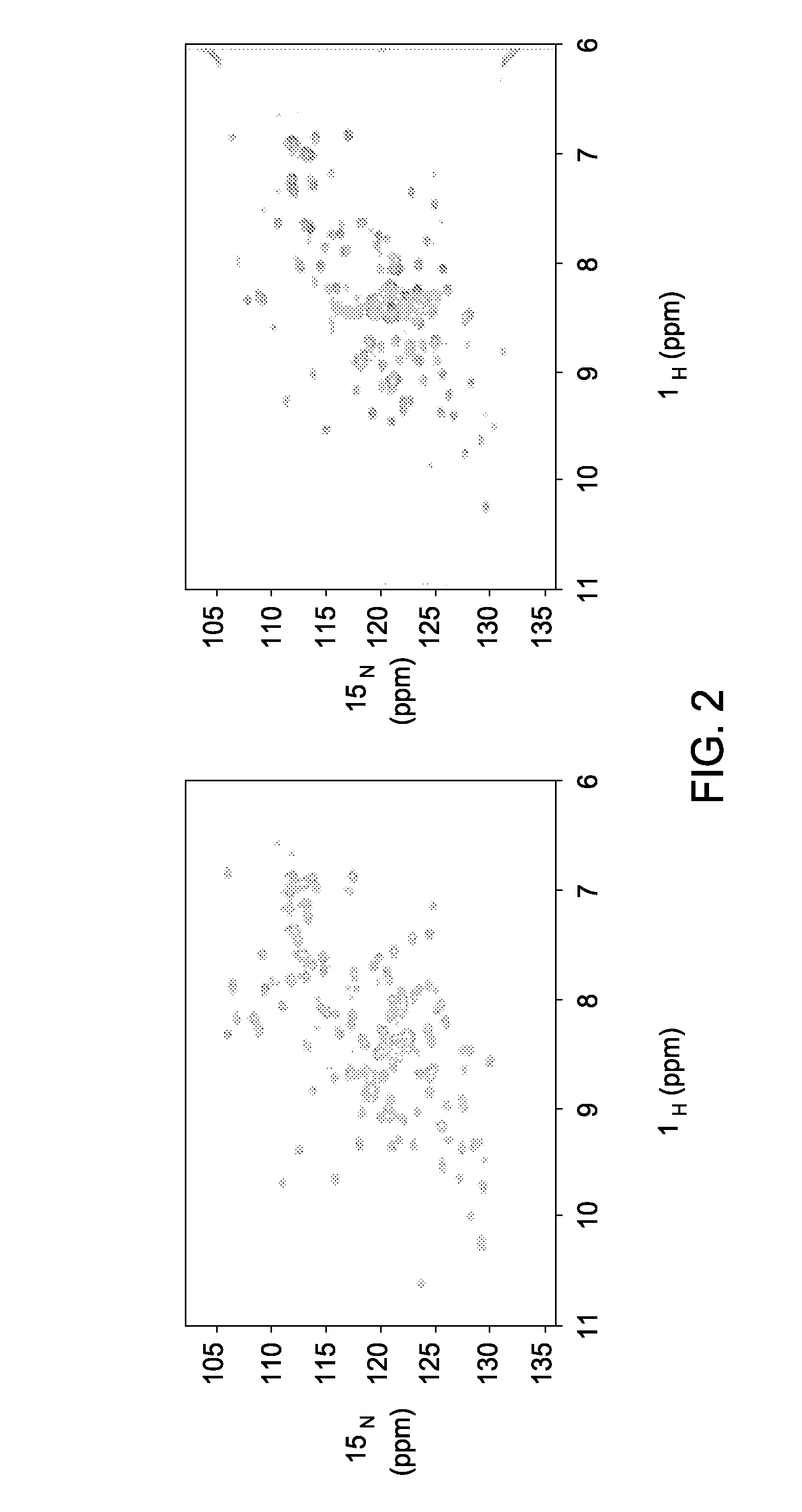
ActiveUS20080108050A1Decreases CPSF3 binding abilitySsRNA viruses negative-senseSurgical furniturePoly-A RNABinding site
The present invention includes compositions and methods related to the structure and function of the cellular polyadenylation and specificity factor 30 (CPSF30) binding site on the surface of the influenza A non-structural protein 1 (NS1). Specifically, critical biochemical reagents, conditions for crystallization and NMR analysis, assays, and general processes are described for (i) discovering, designing, and optimizing small molecule inhibitors of influenza A (avian flu) viruses and (ii) creating attenuated influenza virus strains suitable for avian and human flu vaccine development.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method for preparing Vero cell influenza virus vaccine
ActiveCN102078605ASimple purification processQuality standardsAntiviralsAntibody medical ingredientsInfluenza virus vaccineIon exchange
The invention provides a method for preparing a Vero cell influenza virus vaccine. Seasonal trivalent influenza vaccines and pandemic influenza storage vaccines with qualified quality can be prepared by improving a vaccine purification process, combining hydrophobic chromatography with ion exchange and auxiliarily adopting other purification methods.
Owner:吉林亚泰生物药业股份有限公司
Serum-free whole suspension MDCK cell strain and application thereof to production of influenza viruses
ActiveCN107460156AAchieve serum-free cultureOvercoming anoikisSsRNA viruses negative-senseCulture processSerum freeInfluenza virus vaccine
The invention discloses a serum-free whole suspension cultured MDCK cell strain domesticated by a one-step adaptation method and application thereof to preparation of influenza virus vaccines, and belongs to the technical field of veterinary biology. Adherent cells are domesticated into a single dispersed whole suspension MDCK cell line named MDCK-S within two months by the one-step adaptation method, and the strain preservation number is CGMCC No. 12256. The invention further provides a method of culturing influenza viruses using the cell line. The method for producing the H1N1 and H3N2 subtype swine influenza viruses through MDCK cell suspension culture can replace traditional chicken embryo production to significantly reduce the production cost and improve downstream purification efficiency, and can rapidly and steadily increase the production scale.
Owner:兆丰华生物科技(南京)有限公司北京生物医药科技中心 +2
Influenza virus replication for vaccine development
ActiveUS20170067029A1Increase productionImprove scalabilitySsRNA viruses negative-senseVirus peptidesInfluenza virus vaccineViral Vaccine
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Influenza virus vaccines and uses thereof
ActiveUS20160136262A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininInfluenza virus vaccine
Provided are influenza hemagglutinin stem domain polypeptides comprising (a) an influenza hemagglutinin HA1 domain that comprises an HA1 N-terminal stem segment comprising the amino acids from position 1 to position x, preferably from position p to position x, of the HA1 domain, covalently linked by a linking sequence of 0-50 amino acid residues to an HA1 C-terminal stem segment, comprising the amino acids from position y to and including the C-terminal amino acid of the HA1 domain; and (b) an influenza hemagglutinin HA2 domain, wherein the hemagglutinin stem domain polypeptide is resistant to protease cleavage at the junction between HA1 and HA2, and wherein one or more amino acid of the amino acids at positions 337, 340, 352, 353, 402, 406, 409, 413 and / or 416 have been mutated, as compared to the corresponding positions in wild-type influenza HA.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for producing influenza virus vaccine
ActiveCN101955915ASolve pollutionGuaranteed to be pureAntiviralsViruses/bacteriophagesCanine kidneyEquine influenza vaccine
The invention discloses a method for producing a vaccine for avian influenza viruses and other influenza viruses such as a swine influenza virus, a canine influenza virus and an equine influenza virus. The method comprises the following steps of: subculturing cells for preparing the vaccine; (2) reproducing cytotoxic varieties; (3) performing microcarrier suspension culture on darby canine kidney (MDCK) cells in a bioreactor; (4) reproducing venom for preparing the vaccine; and (5) harvesting virus liquid. The method has the advantages of great reduction in production cost, short production period, no restriction to raw material supply, small occupied area, easy and quick expansion in production scale, low and readily treated environmental pollution, high degree of automation, few labors, easy equilibrium and stabilization in quality and obvious improvement on the yield and the quality of the vaccines.
Owner:成都史纪生物制药有限公司
Influenza virus subunit vaccine and preparation method thereof
InactiveCN104888212AHigh purityQuality improvementAntiviralsAntibody medical ingredientsHemagglutininAdjuvant
The invention discloses an influenza virus subunit vaccine and a preparation method thereof. The influenza virus subunit vaccine is formed by further purifying cracked viral proteins through a cracking agent and a new purification method; each agent of subunit vaccine comprises more than 80% of H1N1, H3N2 and B type influenza hemagglutinin, and does not comprise adjuvant, thiomersal or other corrosion removers. The invention further provides the preparation method of the influenza virus subunit vaccine. The preparation method of the influenza virus subunit vaccine comprises the following steps of virus inoculation, virus multiplication culture, allantonic fluid harvesting, clarification, inactivation, ultrafiltration and concentration, cracking and overspeed centrifugal purification, gel filtration chromatography purification, mixture, filtration sterilization, subpackage, packaging and the like. The influenza virus subunit vaccine can improve safety, eliminate untoward effects caused by adjuvant and eliminate the toxic and side effects caused by thiomersalate.
Owner:ZHONGYI ANKE BIOTECH CO LTD
Method for cultivating recombinant avian influenza subtype virus through full-suspension cell
InactiveCN108220221AStable toxicityImprove performance and stabilitySsRNA viruses negative-senseArtificial cell constructsInfluenza virus vaccineAvian influenza virus
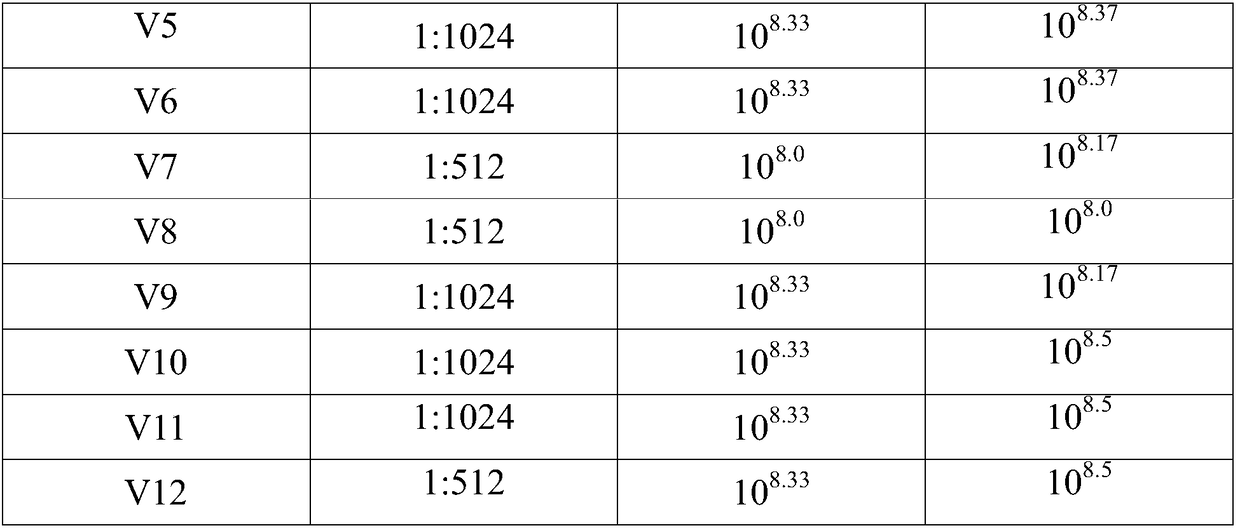
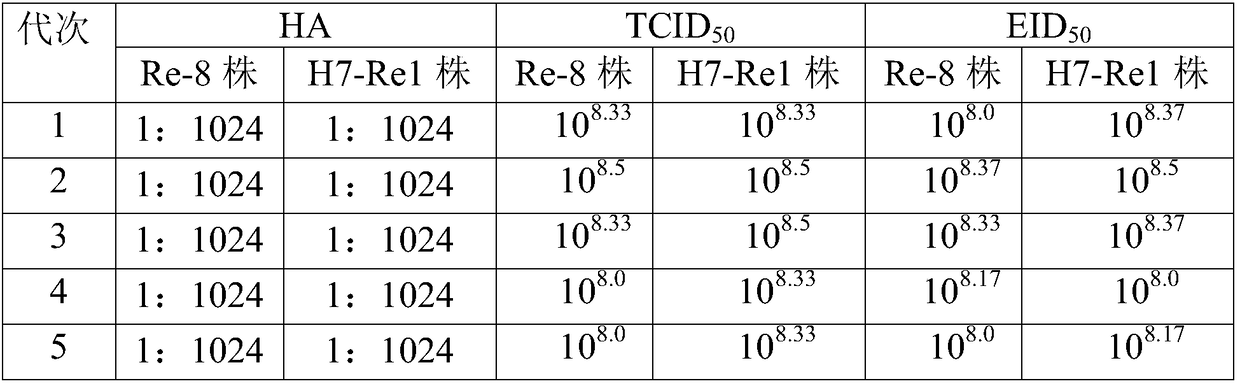
The invention relates to the preparation field of the avian influenza virus vaccine, and especially relates to a method for cultivating recombinant avian influenza subtype virus through full-suspension cell. The method comprises the following steps: inoculating the recombinant avian influenza subtype virus chick embryo virus into MDCK monolayer cell to acclimate and culture, inoculating the harvested culture, repeating the cultivating until the proliferation speed of the recombinant avian influenza subtype virus is stable, wherein the virus content is larger than or equal to 10<7.5>EID[50]; and then inoculating the suspension cell; acquiring virus liquid while cultivating until the cytopathy achieves 75% or above, namely obtaining the recombinant avian influenza subtype virus. The chick embryo virus is firstly inoculated into the MDCK monolayer cell to acclimate and cultivate, and then is inoculated to the suspension MDCK cell, thereby effectively increasing the performance stability of the suspension virus obtained through the production, wherein the obtained suspension virus HA is larger than or equal to 1 to 1024, each 0.1ml virus content is larger than or equal to 10<8.0>EID[50], and each 1ml virus content is larger than or equal to 10<8.0>TCID[50].
Owner:吉林冠界生物技术有限公司 +1
Acquisition method and adaptive sites of influenza A virus vaccine mammalian cell adaptive strain
InactiveCN104073513AFast growthDoes not increase lethalityVirus peptidesTransferasesMammalChick embryos
The invention relates to an acquisition method and adaptive sites of an influenza A virus vaccine mammalian cell adaptive strain. According to the invention, a conventional influenza A vaccine skeleton strain is subjected to continuous passage in mammalian cells so as to obtain the mammalian cell adaptive strain; four amino acid sites on the viral protein coded by a virogene in the adaptive strain undergo mutation, which contributes to adaptability to the skeleton strain. The adaptive strain does not has enhanced pathogenicity on living bodies like chick embryos and mice, but has substantially improved adaptability to mammalian cells.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Novel use
InactiveUS20110123568A1Enhance immune responseImprove responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseAntigen
The present invention relates to influenza vaccine formulations and vaccination regimes for immunizing against influenza disease. In particular the invention relates to vaccine formulations comprising an oil-in-water emulsion adjuvant and optionally 3D-MPL, their use in medicine, in particular their use in augmenting immune responses to influenza antigens, and to methods of preparation, wherein the oil in water emulsion comprises a sterol, a metabolizable oil and an emulsifying agent. The present invention also provides for new prime-boost vaccination regimes for immunizing humans against influenza disease, and in particular for ensuring and ameliorating the immune response to the booster administration, in which a first influenza virus vaccine is administered in the presence of an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Influenza virus replication for vaccine development
ActiveUS9890363B2Increase productionImprove scalabilitySsRNA viruses negative-senseVirus peptidesDiseaseEgg cell
The invention provides a composition useful to prepare high titer influenza viruses, e.g., in the absence of helper virus, which includes internal genes from an influenza virus vaccine strain or isolate, e.g., one that is safe in humans, for instance, one that does not result in significant disease, that confer enhanced growth in cells in culture, such as MDCK cells, or in eggs.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com