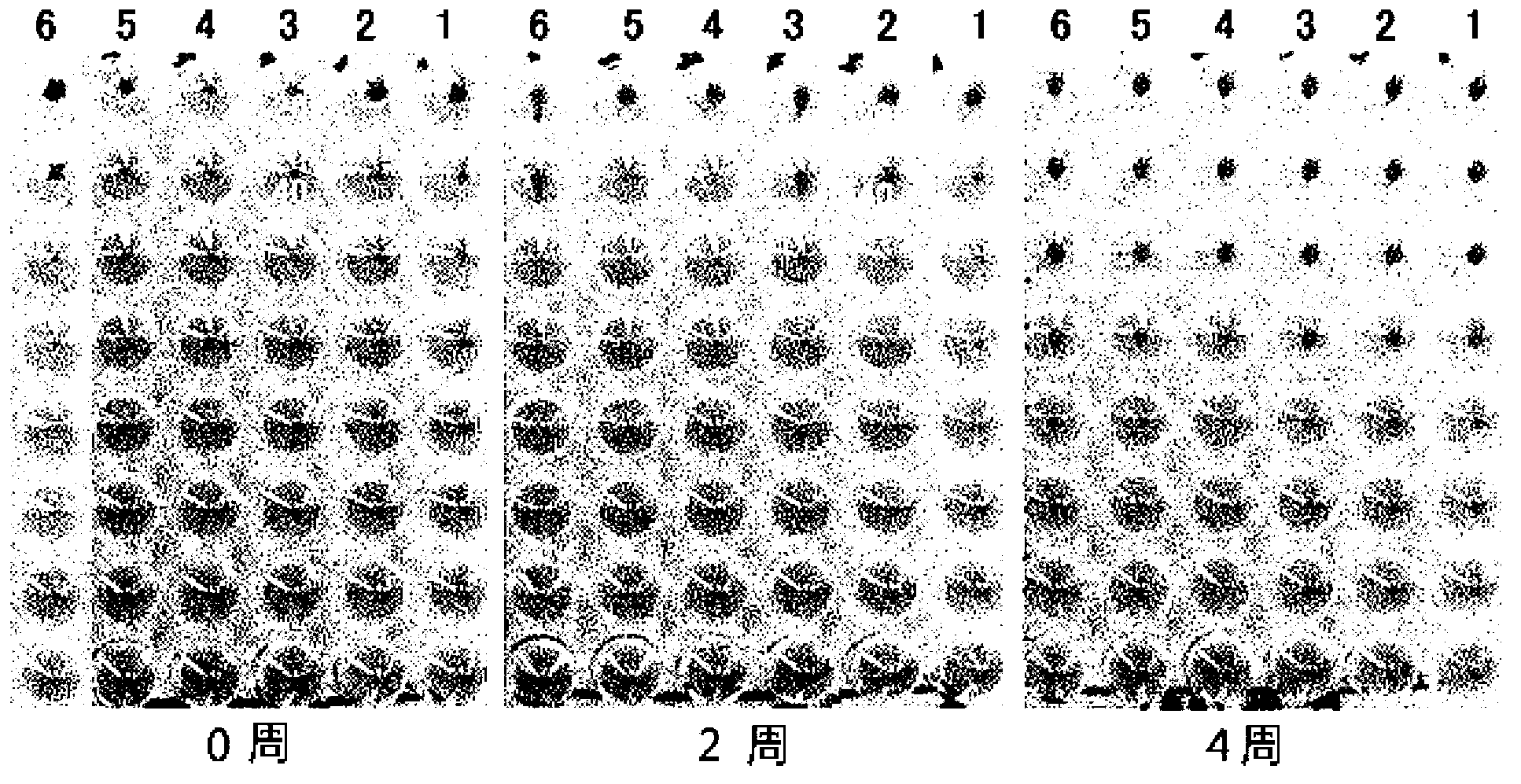
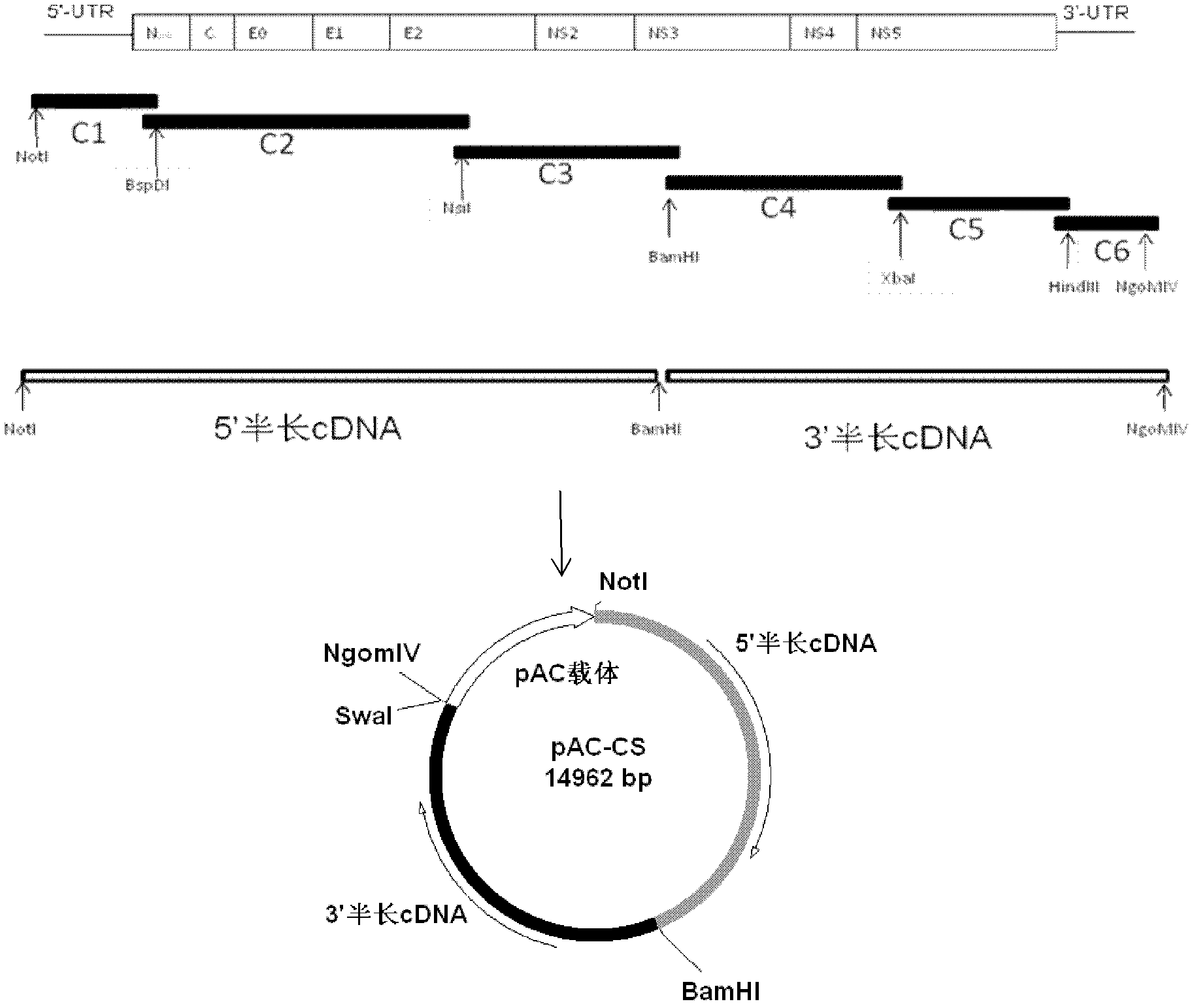
Patents
Literature
133 results about "Vaccine virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Live Attenuated Influenza Virus Vaccines Comprising Microrna Response Elements
ActiveUS20120148622A1Increase vaccine safetyQuick buildSsRNA viruses negative-senseSugar derivativesUltrasound attenuationInfluenza virus vaccine
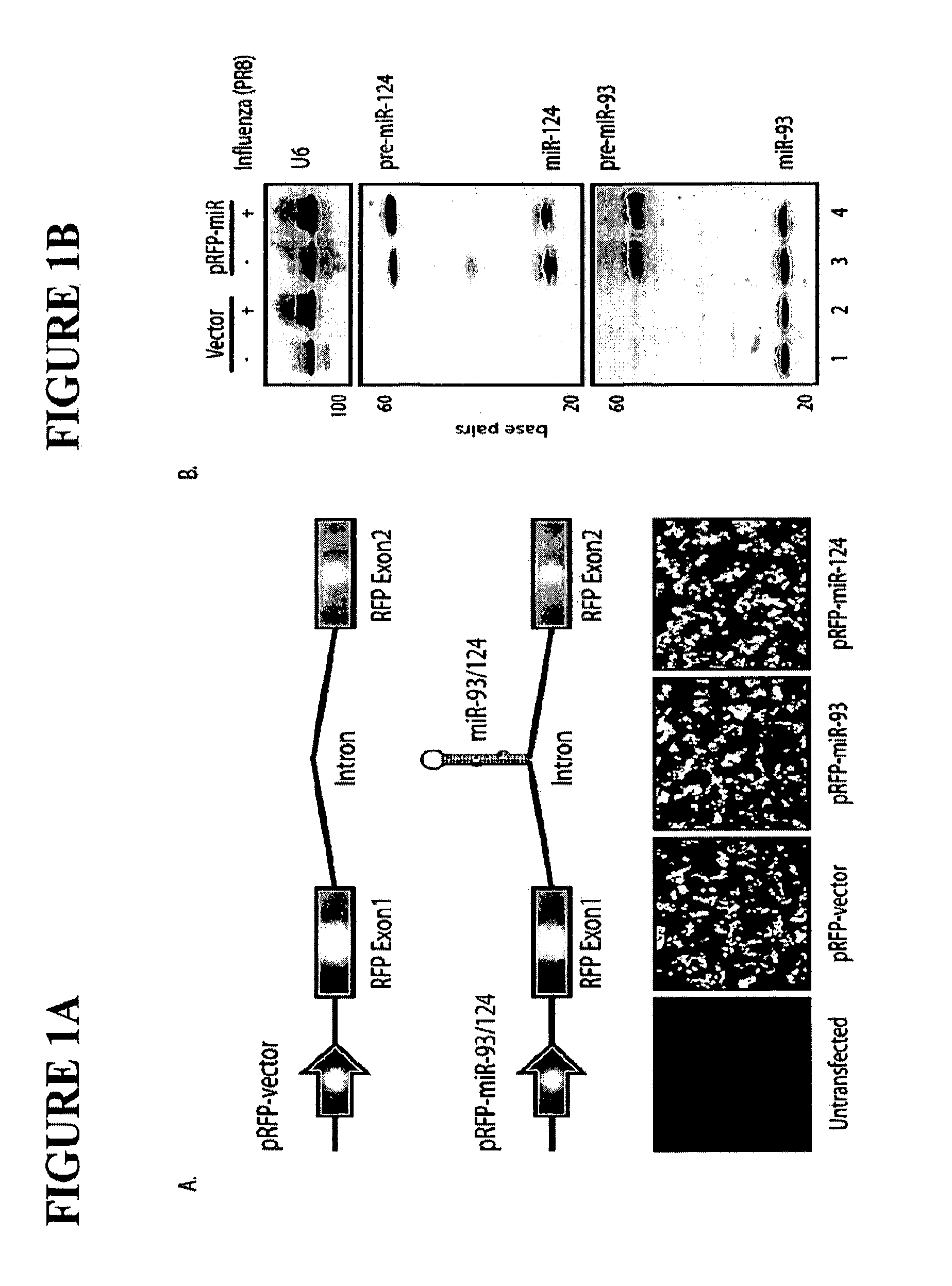
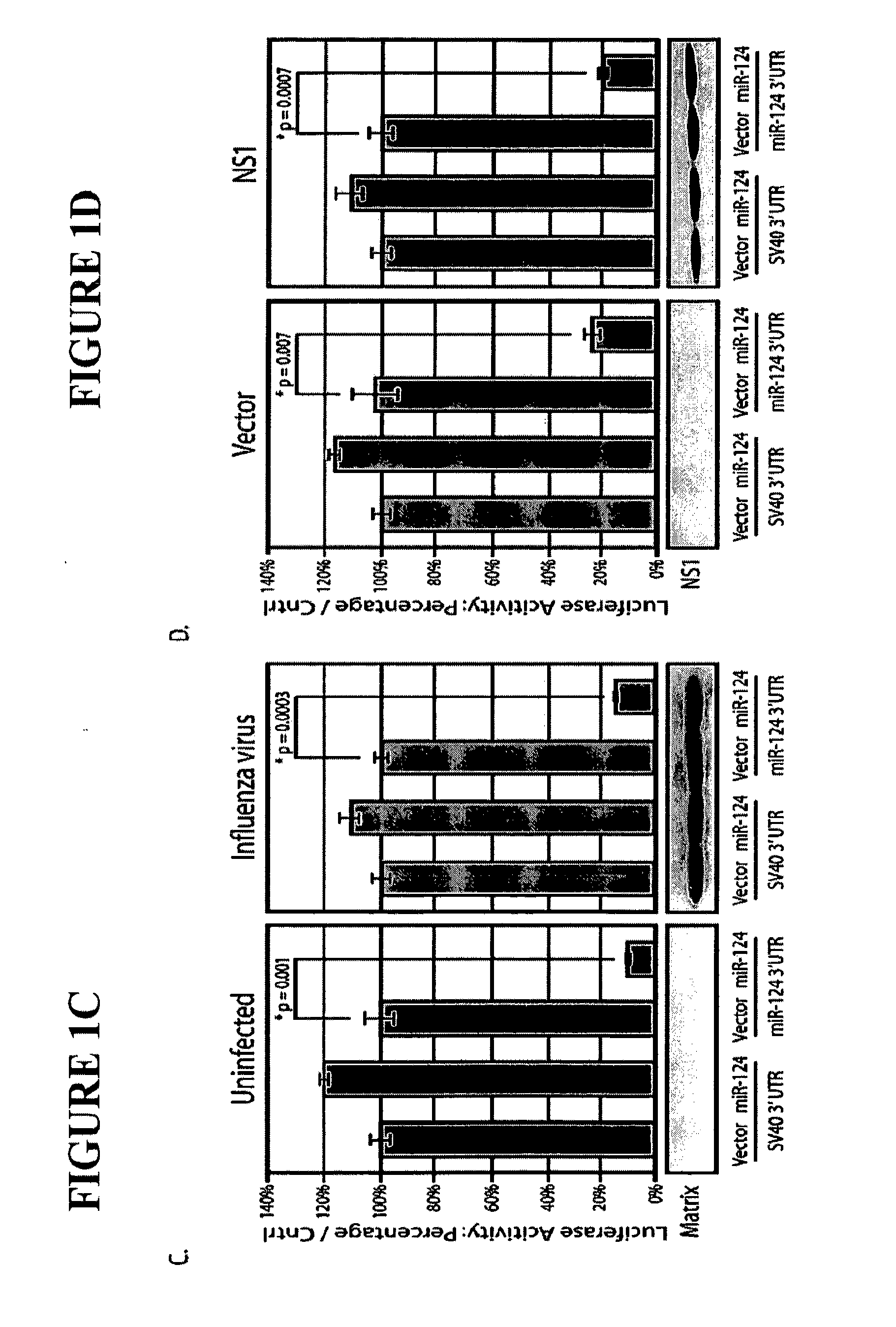
The invention is directed to novel live attenuated influenza virus (LAIV) vaccines comprising one or more microRNA (miRNA) Response Element(s) (MRE) within an influenza virus genome. The MREs useful for the present invention can be derived from any miRNA which is highly expressed in influenza-targeted cells of an animal in need of vaccination but are not expressed or are expressed at very low levels in species (e.g., embryonated chicken eggs) or cell lines used for a large-scale vaccine production. This allows efficient vaccine production but renders the vaccine virus susceptible to attenuation in the influenza-targeted cells of vaccinated animals expressing a cognate miRNA.
Owner:MT SINAI SCHOOL OF MEDICINE
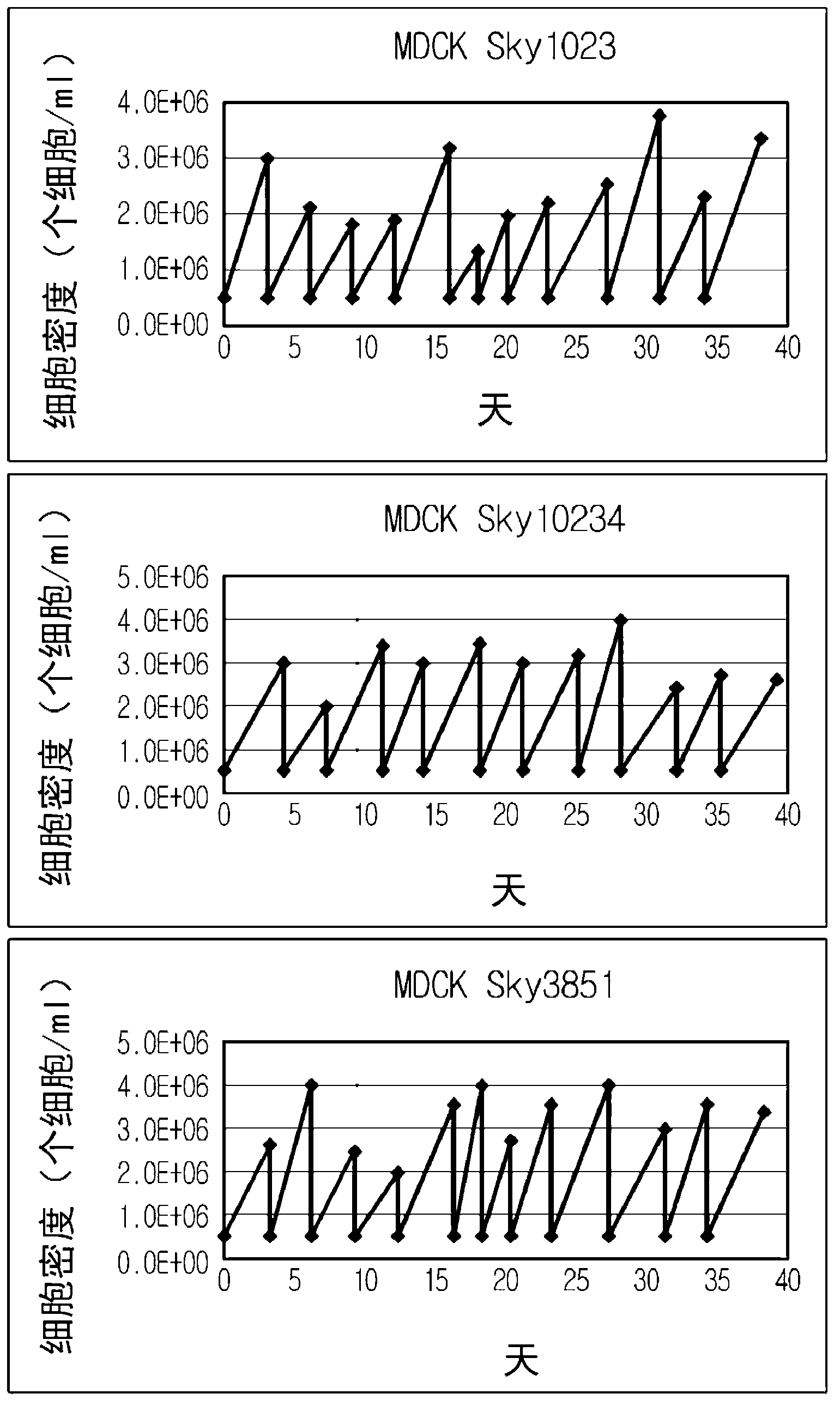
Mdck-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
InactiveUS20130183741A1Low and no tumorigenicityEasy to useSsRNA viruses negative-senseViral antigen ingredientsSerum igeSerum free
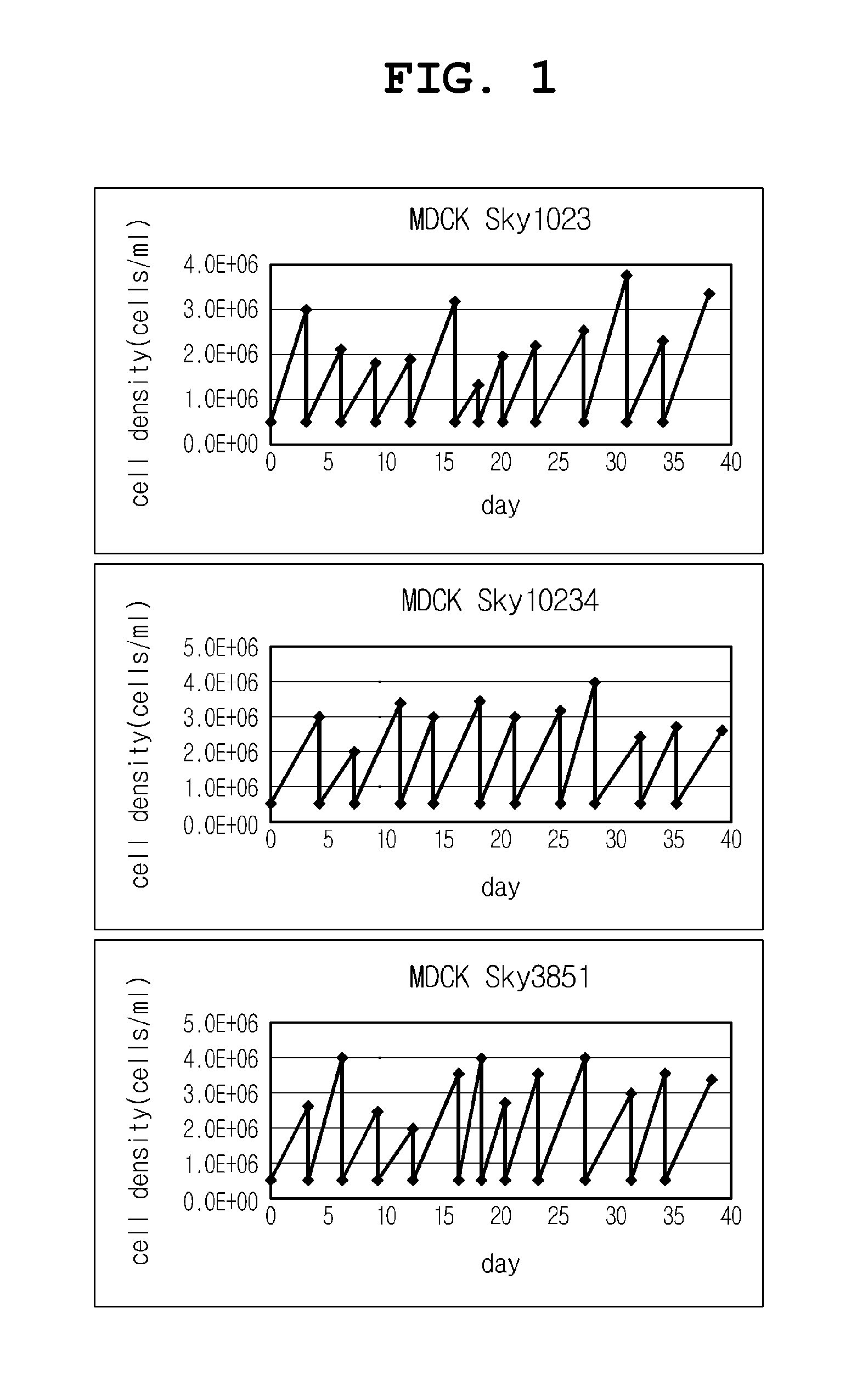
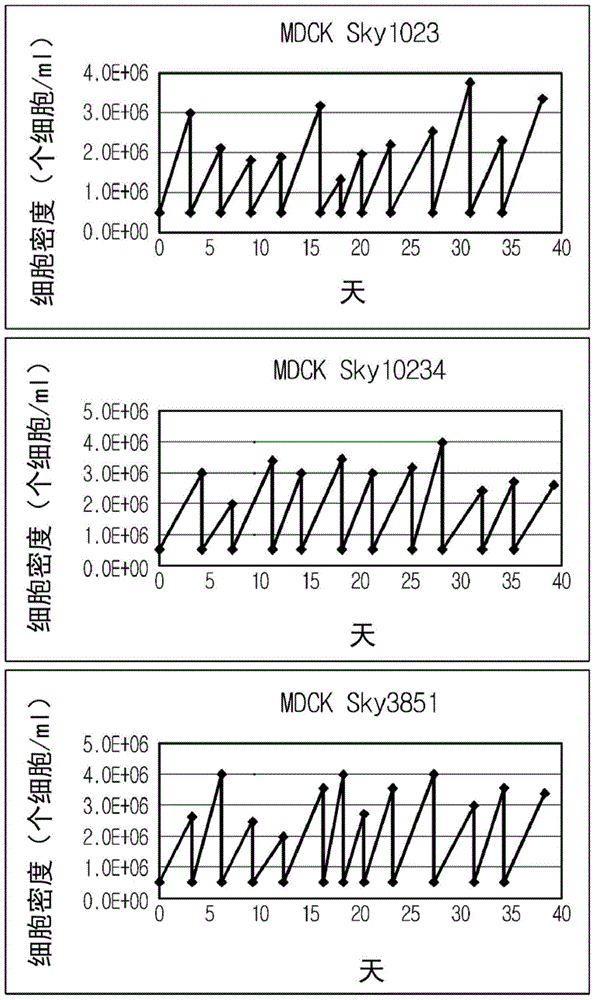
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK CHEM CO LTD
Use of flavivirus for the expression of protein epitopes and development of new live attenuated vaccine virus to immunize against flavivirus and other infectious agents
InactiveUS20060159704A1Stable expressionSafe and effectiveSsRNA viruses positive-senseVirus peptidesEpitopeSpecific immunity
The present invention relates to a vaccine against infections caused by flavivirus. More particularly to the use of the YF vaccine virus (17D) to express at the level of its envelope, protein epitopes from other pathogens which will elicit a specific immune response to the parental pathogen.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
Human cytomegalovirus antigens expressed in MVA and methods of use
ActiveUS7163685B2Improve immunitySugar derivativesViral antigen ingredientsModified vaccinia AnkaraVaccine virus
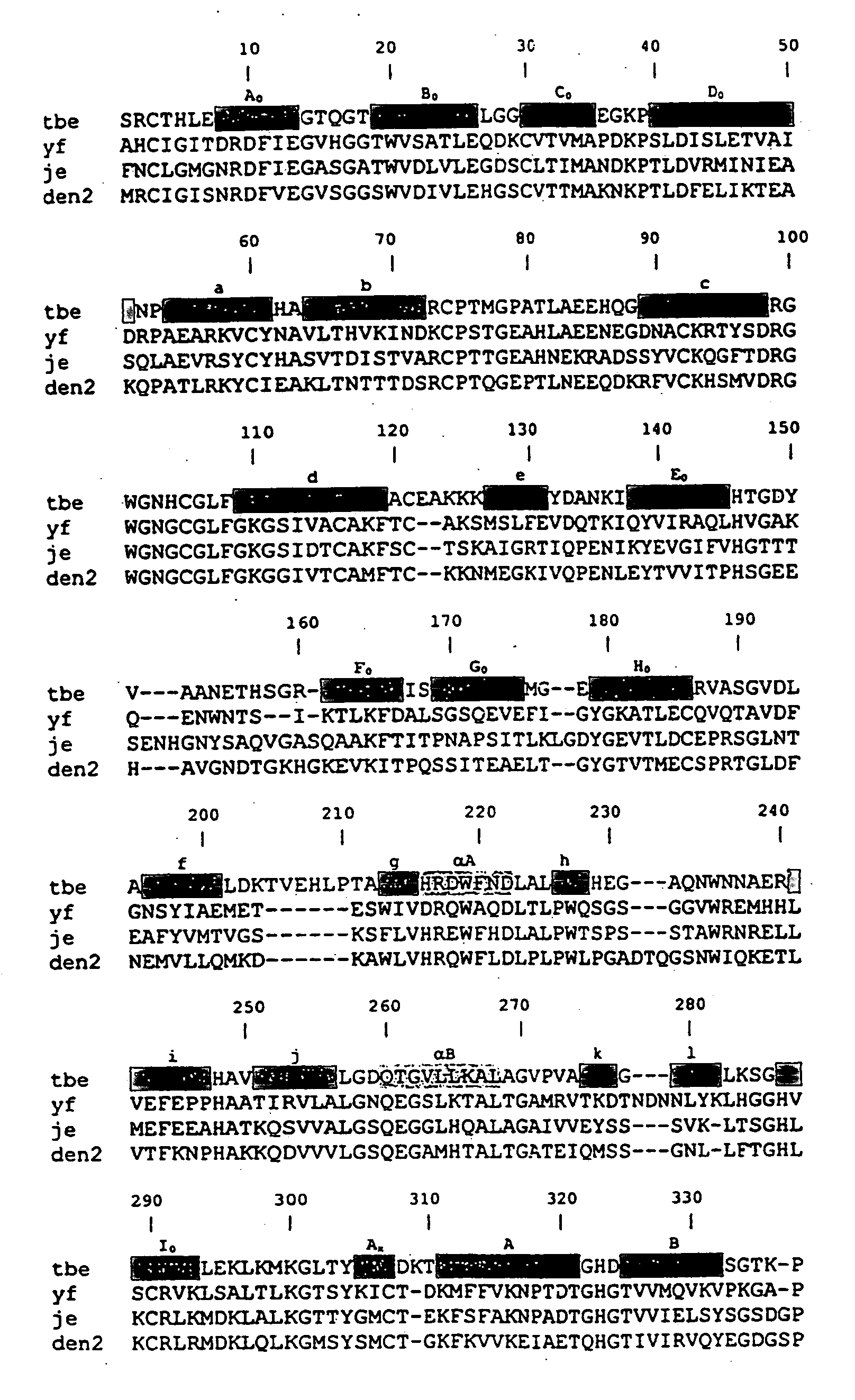
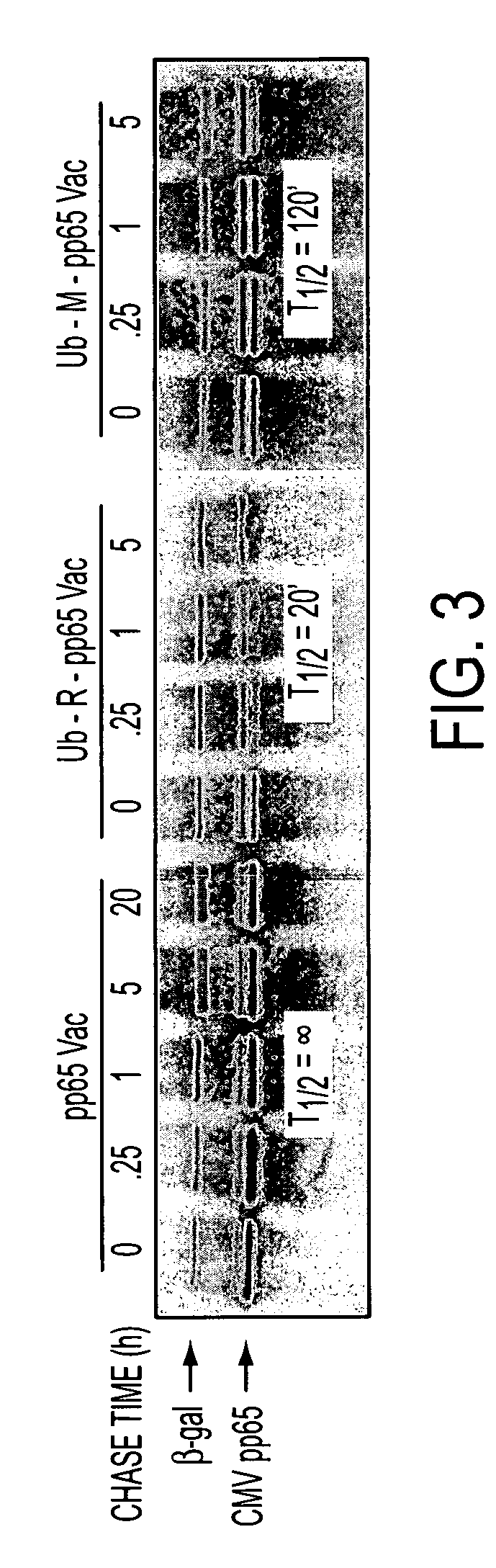
DNA and protein constructs useful in producing vaccines against human cytomegalovirus contain optionally N-end modified and N-terminal ubiquitinated human cytomegalovirus antigenic proteins, including pp65, pp150, IE1, gB and antigenic fragments thereof. Vaccine viruses, in particular poxviruses such as vaccinia and Modified Vaccinia Ankara, that express the constructs may be used as vaccines to augment the immune response to human cytomegalovirus, both prophylatically and in patients already carrying human cytomegalovirus, as well as to create and expand cytomegalovirus-reactive T cells for transfer of adoptive immunity.
Owner:CITY OF HOPE
ST cell adapting to full-suspension culture, and application thereof, and vaccine virus culturing method
ActiveCN105112352ASolve the requirements of cultivating large-scale industrializationHigh degree of automationMicroorganism based processesVertebrate cellsVaccine virusEngineering
The invention relates to an ST cell adapting to full-suspension culture, and an application in vaccine virus culturing, and belongs to biotechnical field. The ST cell adapting to full-suspension culture is named as a swine testicle cell suspension adaptation strain ST-2014S, and is preserved in China Center for Type Culture Collection on May 13, 2015 with the preservation number of CCTCC C201567. The invention also discloses a method for culturing a vaccine virus through using the full-suspension adaptation ST cell. Compared with vaccine viruses in the prior art, the full-suspension adaptation ST cell cultured vaccine virus has the advantages of high automation degree, no need of vector intervention, realization of suspension culture in a serum-free or low-serum medium, meeting of large-scale industrial requirements of virus culture, development of a large-scale production method meeting GMP production technology requirements, and very good industrial application prospect.
Owner:郑州爱科生物科技有限公司
Method of Immunization Against the 4 Serotypes of Dengue Fever
The invention relates to a method for inducing protection against the 4 serotypes of dengue fever in a patient, comprising:(a) the administration of a monovalent vaccine comprising a vaccinal virus of a first serotype of dengue fever, and(b) the administration of a tetravalent vaccine comprising vaccinal viruses of the four serotypes of dengue fever,in which administration (b) is made between at least 30 days and not more than 12 months following the first administration (a).
Owner:SANOFI PASTEUR SA
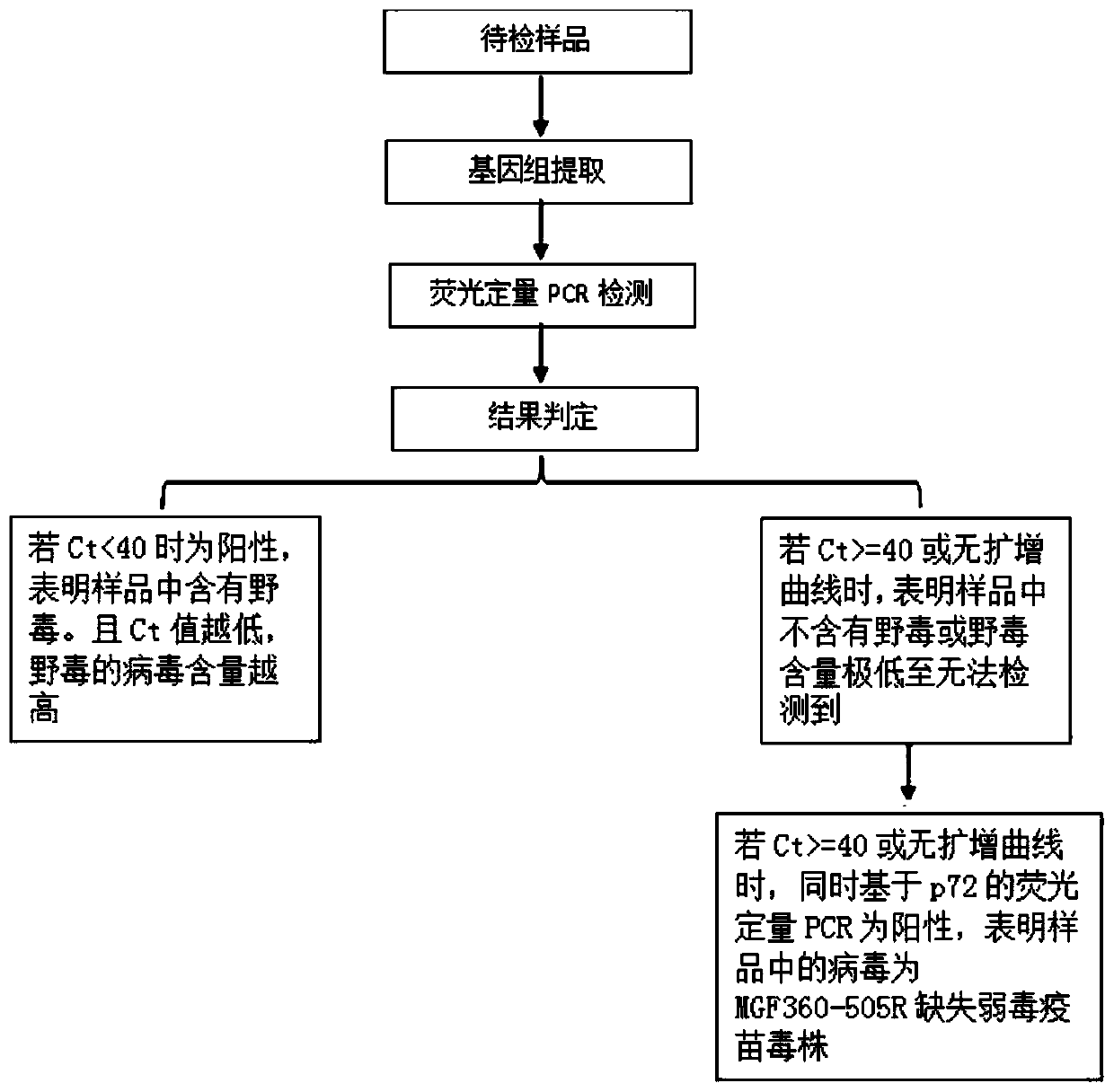
Real-time fluorescent PCR primer probe combination and kit for detecting African swine fever virus wild strains
ActiveCN110760617AEasy to controlImprove purification effectMicrobiological testing/measurementMicroorganism based processesAnimal virusAfrican swine fever
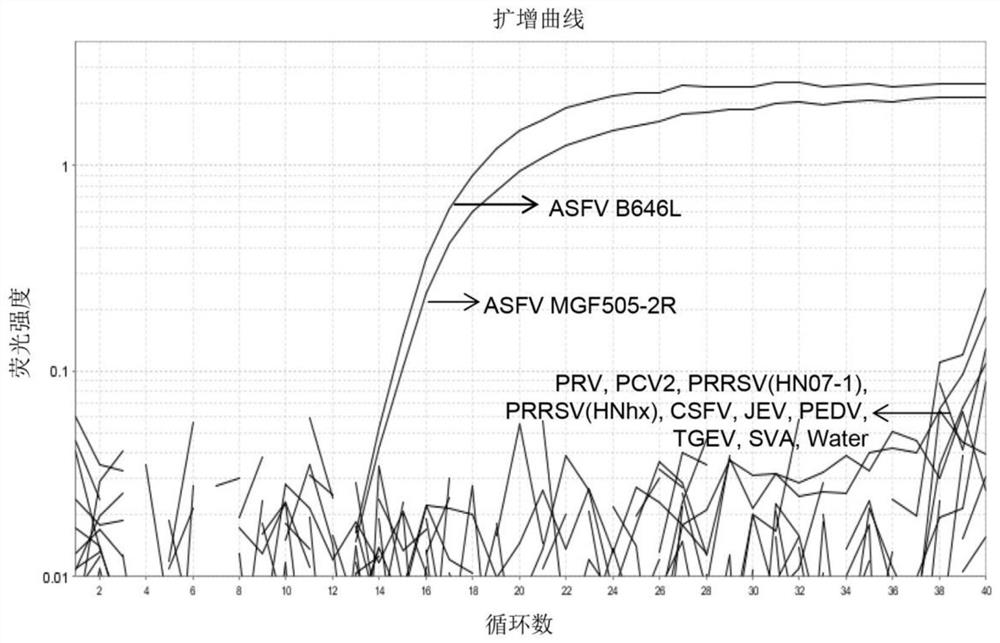
The invention relates to the technical field of animal virus detection in the field of veterinarians, and particularly discloses a real-time fluorescent PCR primer probe combination and a kit for detecting African swine fever virus wild strains. A PCR method based on the kit can not only specifically detect the African swine fever virus wild strains, but also serve as a method for distinguishing between the African swine fever virus wild strains and MGF360-505R loss attenuated vaccine virus strains when in use in combination with current p72 fluorescent PCR, therefore, important tools are provided for detecting the African swine fever virus wild strains and animals infected by the African swine fever virus wild strains, and control over African swine fevers and purification of the Africanswine fevers are promoted.
Owner:广纳达康(广州)生物科技有限公司
Production of attenuated negative stranded RNA virus vaccines from cloned nucleotide sequences
Attenuated, recombinant negative stranded RNA viruses suitable for vaccine use are produced from one or more isolated polynucleotide molecules encoding the virus. A recombinant genome or antigenome of the subject virus is modified to encode a mutation within a recombinant protein of the virus at one or more amino acid positions(s) corresponding to a site of an attenuating mutation in a heretologous, mutant negative stranded RNA virus. A similar attenuating mutation as identified in the heterologous negative stranded RNA virus is thus incorporated at a corresponding site within the recombinant virus to confer an attenuated phenotype on the recombinant virus. The attenuating mutation incorporated in the recombinant virus may be identical or conservative in relation to the attenuating mutation identified in the heterologous, mutant virus. By the transfer of mutations into recombinant negative stranded RNA viruses in this matter, candidate vaccine viruses are engineered to elicit a desired immune response against a subject virus in a host susceptible to infection thereby.
Owner:MURPHY BRIAN +3
Classical swine fever virus virulence determinant and a novel classical swine fever vaccine
InactiveUS7332170B1Readily apparentSsRNA viruses positive-senseViral antigen ingredientsHighly pathogenicVirulent characteristics
Transposon linker insertion mutagenesis of a full-length infectious clone of the highly pathogenic classical swine fever virus (CSFV) isolate Brescia (pBIC) was used to identify genetic determinants of CSFV virulence and host range. A virus mutant, RB-C22 (RB-C22v), possessing a 19-residue tag insertion at the carboxyl end of E1 was constructed. RB-C22v and the parental virus pBIC (pBICv) exhibited similar growth characteristics on primary porcine macrophage cell cultures although RB-C22v produced significantly smaller plaques on SK6 cell cultures. In vivo, RB-C22v was markedly attenuated in swine. In contrast with pBIC infection, where mortality was 100%, all RB-C22v-infected pigs survived infection remaining clinically normal. Additionally, chimeras of the Brescia strain and the attenuated vaccine strain CS were constructed and evaluated for viral virulence in swine. Chimeras 138.8v and 337.14v, chimeras containing the E2 glycoprotein of CS and chimeric virus 319.1v, which contained only the CS E2 glycoprotein in the Brescia background, were attenuated in swine. Chimeras encoding all Brescia structural proteins in a CS genetic background remained attenuated, indicating that additional mutations outside the structural region are important for CS vaccine virus attenuation. The combined results indicate a significant role for E1 glycoprotein and E2 glycoprotein in swine virulence.
Owner:UNITED STATES OF AMERICA AS RESPRESENTED BY THE SEC OF AGRI THE
Highly Safe Smallpox Vaccine Virus and Vaccinia Virus Vector
ActiveUS20070298054A1Improve securityAntibacterial agentsGenetic material ingredientsVaccine virusGene product
Objects of the present invention are to generate vaccine strains that undergo reversion (atavism) with difficulty and to provide smallpox vaccines with higher safety. The vaccine viruses are deficient in a part or the whole of the B5R gene of a vaccinia viral strain, LC16m8 or LC16mO, and produce no B5R gene products having normal functions. The vaccine viruses can be used as smallpox vaccines or vectors capable of expressing foreign genes. Hence, smallpox vaccines and vaccinia virus vectors are provided that produce no B5R gene products having normal functions due to reverse mutation.
Owner:SHIDA HISATOSHI
Method of immunization against the 4 serotypes of dengue fever
The invention relates to a method for inducing protection against the 4 serotypes of dengue fever in a patient, comprising:(a) the administration of a monovalent vaccine comprising a vaccinal virus of a first serotype of dengue fever, and(b) the administration of a tetravalent vaccine comprising vaccinal viruses of the four serotypes of dengue fever,in which administration (b) is made between at least 30 days and not more than 12 months following the first administration (a).
Owner:SANOFI PASTEUR SA
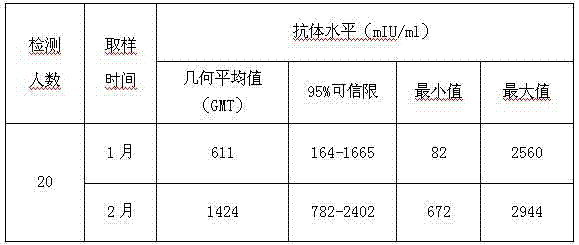
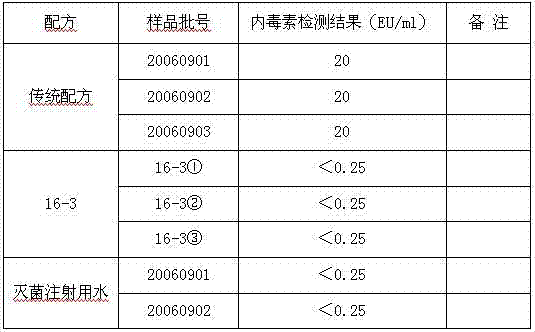
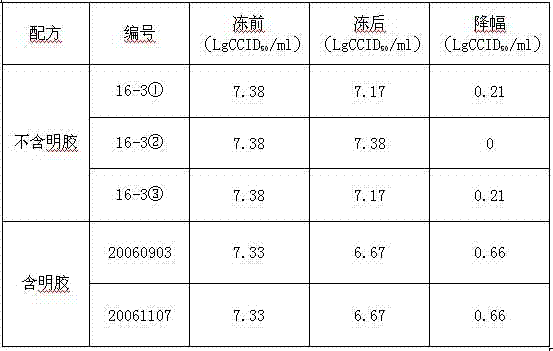
Freeze-dried live attenuated hepatitis A vaccine not containing gelatin or human albumin protective agent and preparation method for freeze-dried live attenuated hepatitis A vaccine
The invention belongs to the field of biological products, in particular to a freeze-dried live attenuated hepatitis A vaccine protective agent not containing gelatin or human albumin and used for preventing hepatitis A, and a preparation method for the freeze-dried live attenuated hepatitis A vaccine protective agent. The protective agent comprises trehalose, dextran 40, L-cysteine, arginine, glutamic acid, glycine, magnesium chloride, magnesium sulfate, sorbierite, mannitol and tris(hydroxymethyl)aminomethane. The protective agent with the formula is mixed with a hepatitis A vaccine stock solution to form a semi-finished product, the semi-finished product is packaged and freeze-dried, and virus infectious titers of the vaccine before and after freeze drying and the thermal stability after freeze drying are detected; after the formula is compared with the conventional production formula containing the gelatin, results show that the protective agent has good protective effect, the descent of the virus infectious titers of the vaccine in the freeze drying process is obviously decreased, and the endotoxin content in a finished product is obviously reduced (less than 0.25EU / ml); and after the freeze-dried vaccine with the formula is inoculated into a human body, results indicate that the vaccine has good immune effect and high safety.
Owner:ZHEJIANG PUKANG BIOTECH
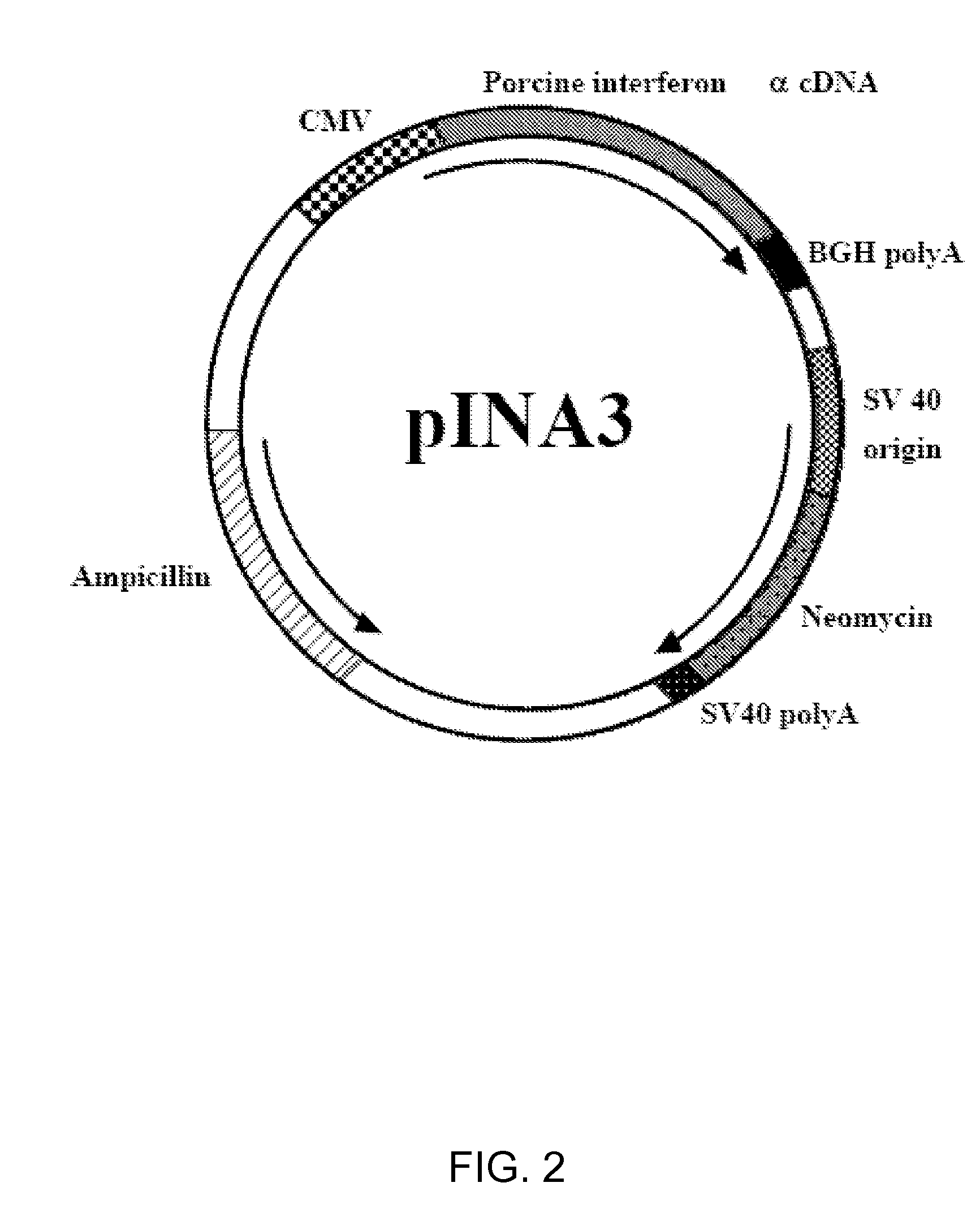
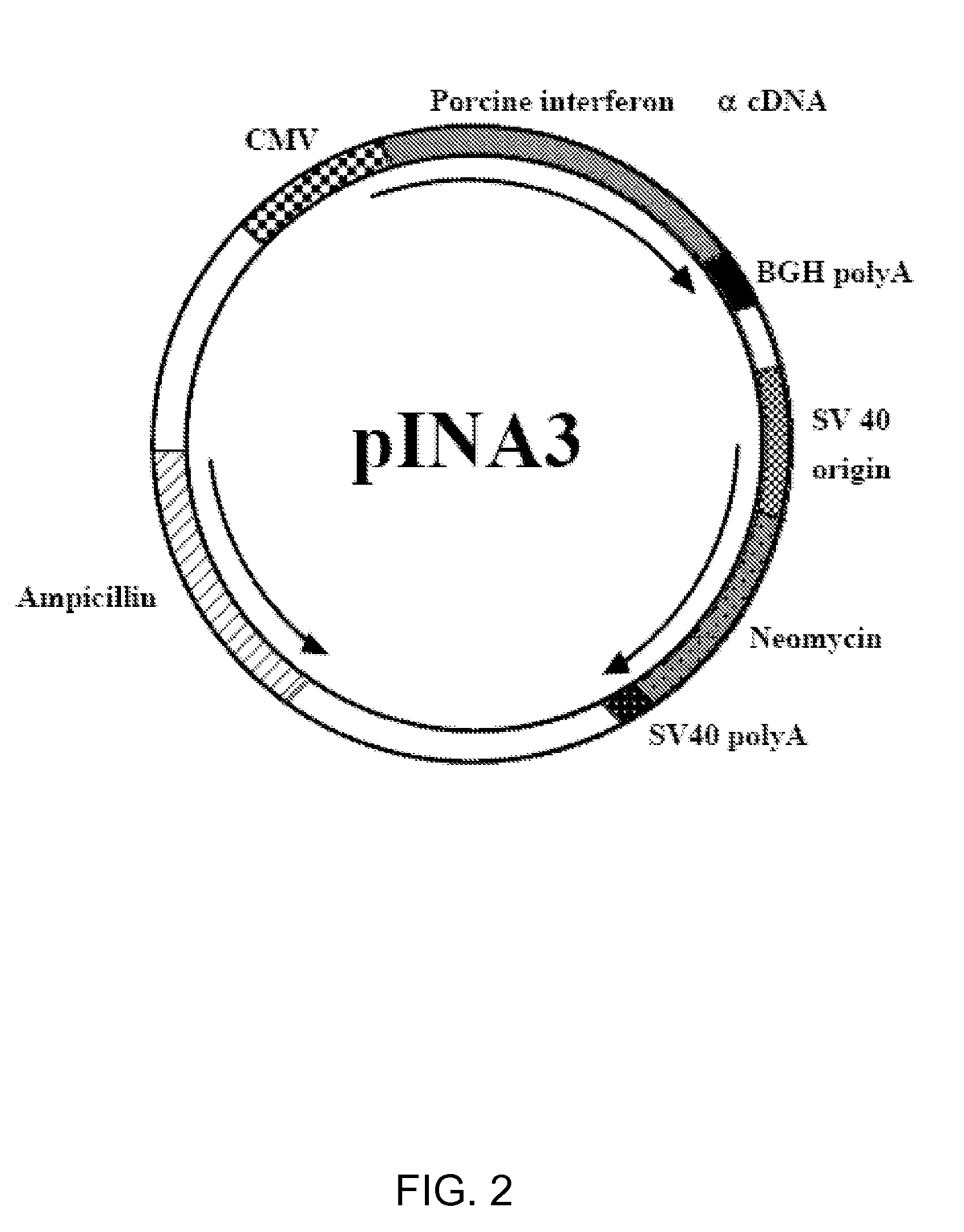
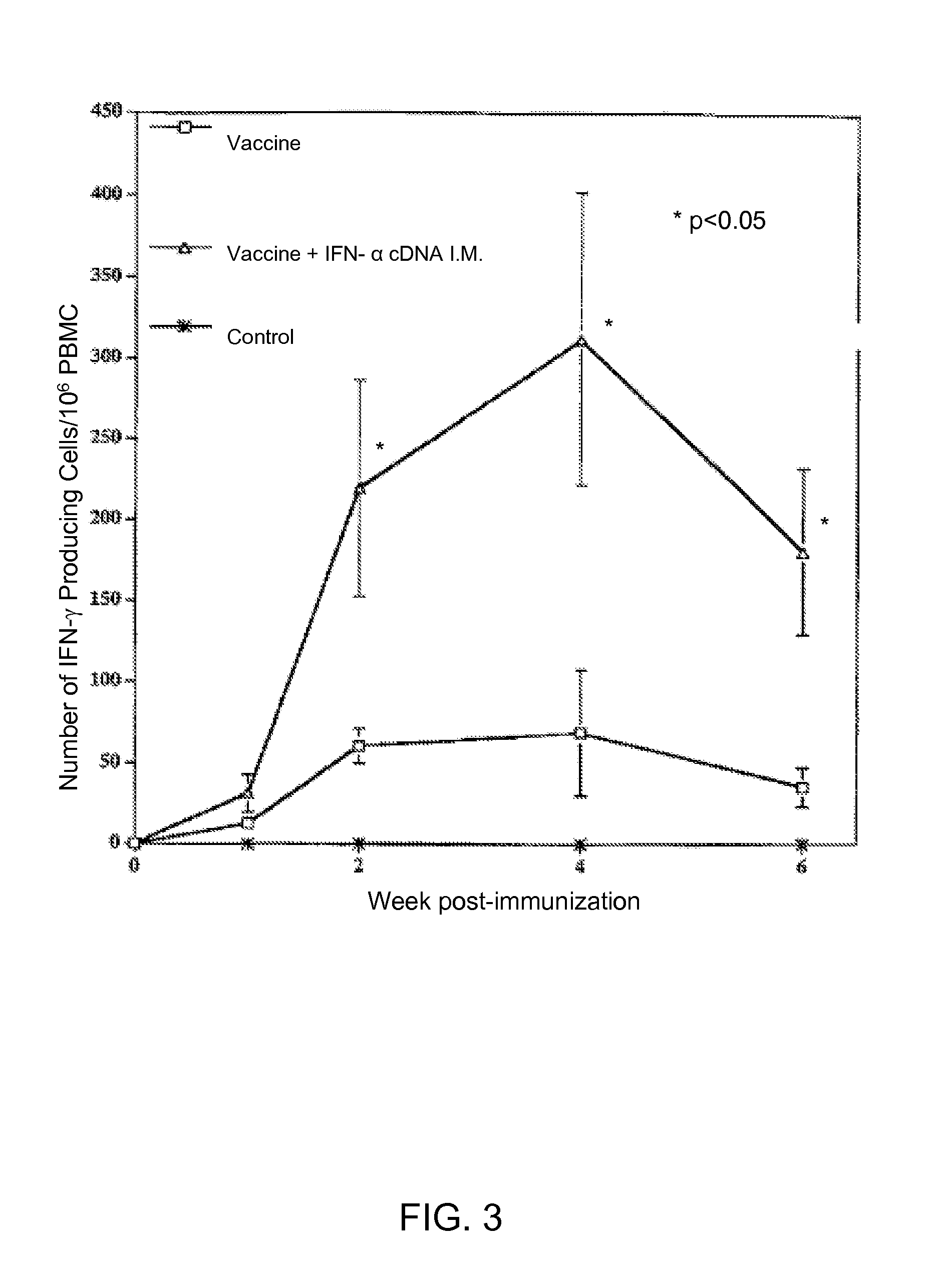
Enhancement of Immune Response to Vaccine by Interferon Alpha
InactiveUS20090022761A1Enhance immune responseSsRNA viruses positive-senseSugar derivativesDiseaseAdjuvant
Exogenous cDNA capable of expressing interferon″ activity, exogenous interferon″ protein, inducers of endogenous interferon″ protein activity, inducers of endogenous interferon $ protein activity, inducers of endogenous interferon′ activity, or inducers of other immune-enhancing activity can be combined with a vaccine to enhance an immune response. Specifically disclosed are adjuvant and vaccine combinations where the adjuvant comprises a cDNA capable of expressing interferon″ activity, a complex comprising polyriboinosinic-polyribocytidilic acid, or a complex comprising polyriboinosinic-polyribocytidilic acid, poly-L-lysine, and carboxymethylcellulose and where the vaccine is a live vaccine virus derived from a virus causing porcine reproductive and respiratory syndrome disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
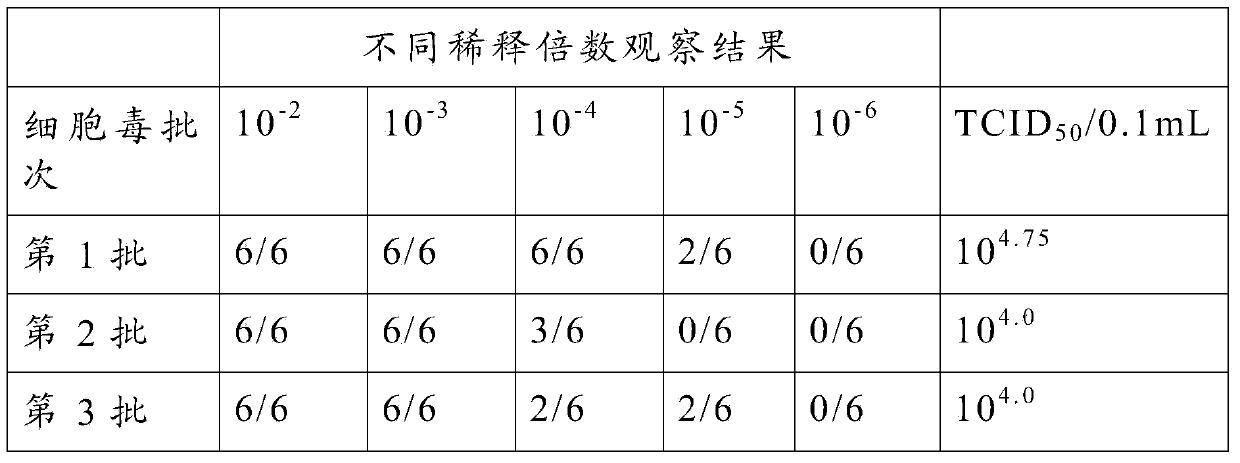
Method for detecting virus content of swine fever live vaccine through indirect immunofluorescence
InactiveCN103698518AThe test result is accurateShort detection timeFluorescence/phosphorescenceFluorescenceVaccine virus
The invention belongs to the technical field of biological quarantine and particularly relates to a method for detecting the virus content of a swine fever live vaccine through indirect immunofluorescence. The method for detecting the virus content of the swine fever live vaccine through indirect immunofluorescence comprises the following steps: (1) preparing subculture cells; (2) measuring the virus content of the swine fever live vaccine in the subculture cells; (3) fluorescently dyeing virus-inoculated cells. According to the method, the viruses of the live vaccine in the subculture cells are detected through indirect immunofluorescence so as to achieve the purpose of rapidly, accurately, simply and effectively detecting the virus content of the swine fever live vaccine.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
MDCK-derived cell lines adapted to serum-free culture and suspension culture and method for preparing vaccine virus using the cells
InactiveCN103154238AEffective proliferationNon-tumorigenicSsRNA viruses negative-senseViral antigen ingredientsCanine kidneySerum free
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK CHEM CO LTD
Method for establishing hog cholera lapinized virus labeled vaccine strain and preparing vaccine
ActiveCN102221618AImprove welfareReduce economic lossAntiviralsViruses/bacteriophagesHigh cellVaccine virus
The invention relates to a method for establishing a hog cholera lapinized virus labeled vaccine strain and preparing a vaccine. The method comprises the following steps: (1) establishing the overall-length infectious clone of the hog cholera lapinized virus strain (the C strain or the Chinese strain); (2) introducing labeled protein genes; (3) rescuing the hog cholera lapinized labeled vaccine virus; (4) breeding the virus; (5) inspecting a virus solution; (6) preparing a vaccine; and (7) inspecting the finished product of the vaccine, comprising safety inspection and efficacy inspection. The hog cholera lapinized virus labeled vaccine established by the invention maintains the characteristics of good safety, excellent immunogenicity and the like of the hog cholera lapinized virus strain; the virus strain has the advantages of good stability and high cell production virus content, can be used for industrial mass production and can generate a labeled protein specificity antibody aftera hog is immunized to distinguish immunization and natural infection and has important significances on hog cholera prevention, control and purification and emergent immunization.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Recombination Newcastle vaccine strain rAI4-S1 for expressing infectious bronchitis virus S1 protein and generating method thereof
InactiveCN103468651AMicroorganism based processesViruses/bacteriophagesInfectious bronchitisInfectious bronchitis virus
The invention discloses a recombination Newcastle vaccine strain rAI4-S1 for expressing infectious bronchitis virus S1 protein and a generating method of the recombination Newcastle vaccine strain rAI4-S1. The conservation number of the recombination Newcastle vaccine strain rAI4-S1 is CGMCC No: 8054. According to the generating method of the recombination Newcastle vaccine strain rAI4-S1, a built reverse genetic manipulation platform of a gene VII type NDV attenuated virus AI4 strain is utilized, the sequence revealed in the SEQ ID NO.7 is inserted into an AI4 strain genome full-length transcription carrier pNDV / AI4, and then the recombination Newcastle vaccine virus genome full-length cDNA clonal pNDV / AI4-S1 containing infectious bronchitis virus S1 genes is obtained. According to a recombination virus obtained through transfection, a chick embryo has high breeding titer, the S1 protein still can be expressed after continuous passage, and the generating method is suitable for large-scale production of vaccines and can be used for processing the vaccines.
Owner:YANGZHOU UNIV
CoxA16 virus strain and human CoxA16 inactivated vaccine
ActiveCN102839159AEffective immunogenicityGood growth and replication abilityMicroorganism based processesAntiviralsNucleotideVaccine virus
The invention provides a CoxA16 virus strain, named CoxA16 (GX-20K-D virus strain) with CGMCCNo.6350 and including three bases, namely an original seed base, a master seed base and a working seed base; in addition, the CoxA16 virus strain has a nucleotide sequence shown as SEQNo.1. The invention has the following obvious beneficial effects: 1, a virus strain is provided for preparing a human CoxA16 inactivated vaccine and has effective immunogenicity and good growth and replication capacity on cells; 2, a production and preparation method of the human CoxA16 inactivated vaccine which is prepared from KMB17 human diploid cell matrix is provided, and the vaccine product has excellent immunogenicity and safety; and a suspended absorption technical method is provided so as to ensure the effective infection of the CoxA16 inactivated vaccine virus strain on KMB17 cell and excellent growth of the CoxA16 inactivated vaccine virus strain on the matrix.
Owner:JIANGSU CONVAC BIO TECH CO LTD
Method of immunization against the 4 dengue serotypes
The invention relates to a method for inducing a homologous protection against the 4 dengue serotypes in a patient, comprising the sequential administration, to said patient, (i) of a dose of a vaccinal dengue virus of a first serotype and of a dose of a vaccinal dengue virus of a second serotype, and (ii) of a dose of a vaccinal dengue virus of a third serotype and of a dose of a vaccinal dengue virus of a fourth serotype, in which the vaccinal dengue viruses (ii) are administered at least 30 days and at most 1 year after administration of the vaccinal viruses (i).
Owner:SANOFI PASTEUR SA
Method for constructing PRRSV gene deletion vaccine toxin strain by using Nsp2 gene deletion and uses thereof
InactiveCN101220351AIncreased neutralizing antibody levelsOvercome inhibitory factorsGenetic material ingredientsInactivation/attenuationNucleotideVaccine virus
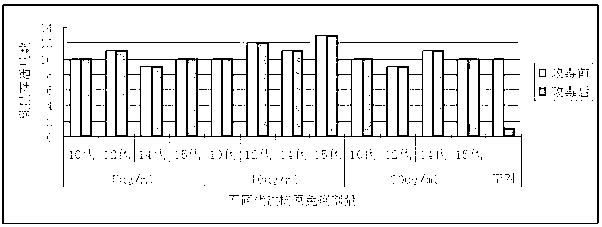
The invention discloses a method for utilizing Nsp2 gene deletion for constructing a PRRSV gene deletion vaccine virus strain and the application thereof. The method for lowering the toxicity of the PRRSV BJ-4 strain of the invention is that a nucleotide segment with the length of 69nt in a Nsp2 coding region of the PRRSV BJ-4 strain genome is continuously deleted to obtain the virus strain with reduced toxicity; the nucleotide sequence of the 69nt nucleotide segment is shown as the 2795 to 2863 sites from the 5' tail end of GenBank Accession Number AF331831. The Nsp2 of the virus rV63 which is prepared by the method for lowering the toxicity of the PRRSV BJ-4 strain deletes 21 amino acids, which has the potential to develop a marker vaccine and can identify the diagnosis method of the deleted polypeptide. When in the development process of vaccine, the invention can utilizes a reverse genetic technical platform which is already established for carrying out the transformation of GP5 glycosylation sites, thus overcoming the suppression factors which affect the neutralizing epitope and further improving the level of the vaccine-induced neutralizing antibody.
Owner:CHINA AGRI UNIV
Enhancement of immune response to vaccine by interferon alpha
Exogenous cDNA capable of expressing interferon α activity, exogenous interferon α protein, inducers of endogenous interferon α protein activity, inducers of endogenous interferon β protein activity, inducers of endogenous interfereon Γ activity, or inducers of other immune-enhancing activity can be combined with a vaccine to enhance an immune response. Specifically disclosed are adjuvant and vaccine combinations where the adjuvant comprises a cDNA capable of expressing interferon α activity, a complex comprising polyriboinosinic-polyribocytidilic acid, or a complex comprising polyriboinosinic-polyribocytidilic acid, poly-L-lysine, and carboxymethylcellulose and where the vaccine is a live vaccine virus derived from a virus causing porcine reproductive and respiratory syndrome disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
African swine fever virus wild virus infection and gene deletion virus strain dual fluorescence quantitative PCR detection composition, method and kit
ActiveCN111676327AStrong specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesClassical swine fever virus CSFVAfrican swine fever
The present invention relates to an African swine fever virus wild virus infection and gene deletion virus strain dual fluorescence quantitative PCR detection composition, method and kit. A TaqMan probe fluorescence quantitative detection method is used, the dual fluorescence quantitative PCR detection method for a B646L gene and a MGF505-2R gene is established, and the method can effectively distinguish a wild virus infection and vaccine strains containing MGF505 gene deletion. The method has good specificity, sensitivity and reproducibility and provides a good technical support for differential diagnosis of African swine fever virus wild viruses and vaccine viruses. Clinical sample testing shows that the method and a Thermo Fisher African swine fever detection kit have relatively good consistent results when detecting the wild virus infection. Besides, the method can distinguish the MGF505 gene deletion strain and has a good social application prospect.
Owner:HENAN ACAD OF AGRI SCI
Mdck-derived Cell Lines Adapted To Serum-free Culture And Suspension Culture And Method For Preparing Vaccine Virus Using The Cells
ActiveCN104862267AEffective proliferationNon-tumorigenicSsRNA viruses negative-senseViral antigen ingredientsCanine kidneySerum free
Disclosed is a Madin-Darby canine kidney (MDCK)-derived cell line. The MDCK-derived cell line is derived from MDCK cells deposited under accession number ATCC CCL-34. The MDCK-derived cell line can be prepared by serum-free culture and suspension culture. Preferably, the MDCK-derived cell line has low or no tumorigenicity. The MDCK-derived cell line is preferably selected from MDCK Sky1023, MDCK Sky10234 and MDCK Sky3851. Further disclosed are a culture method for growing the MDCK-derived cells and a method for producing a vaccine virus using the MDCK-derived cells.
Owner:SK BIOSCI CO LTD
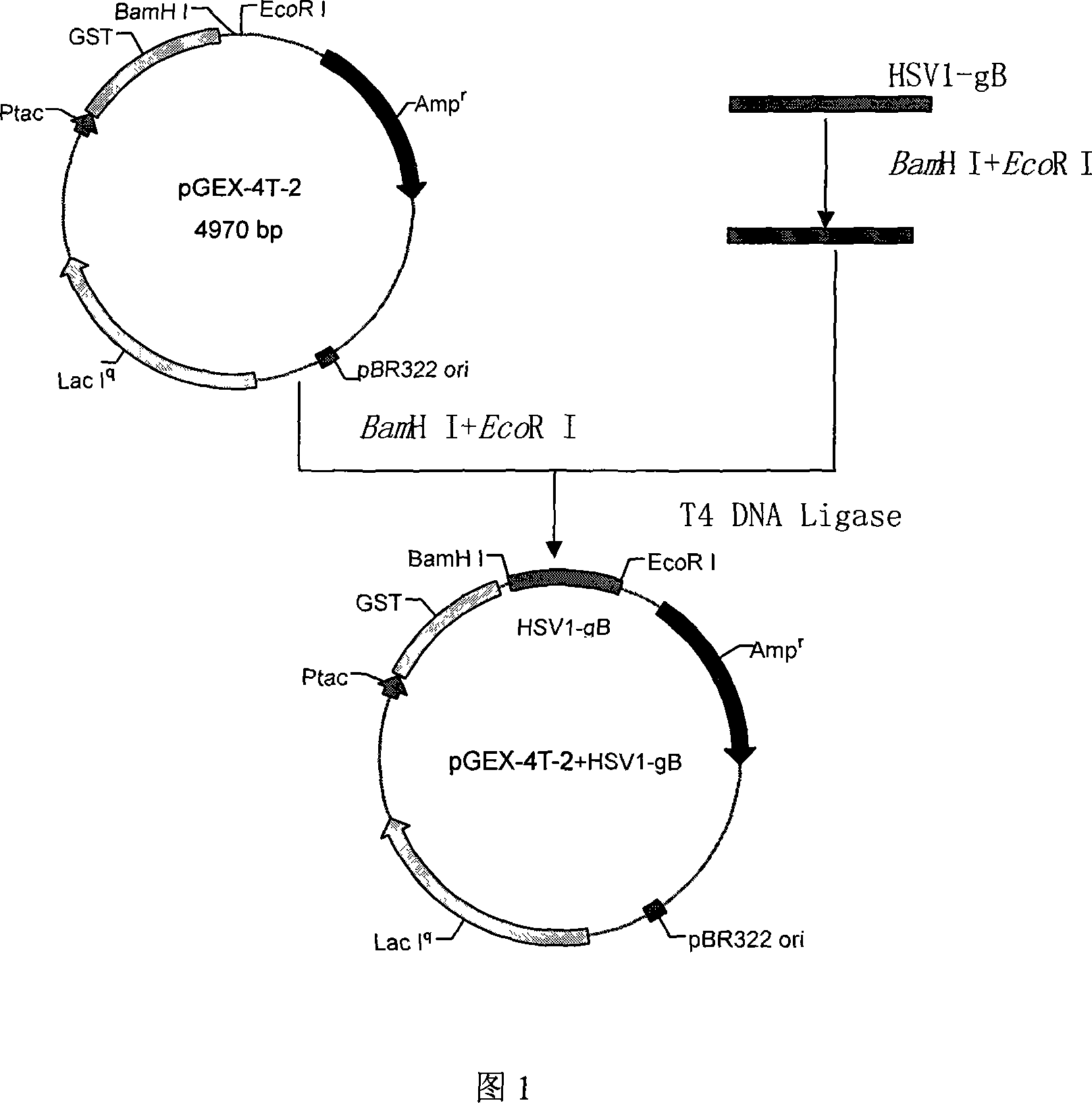
Chemically synthesized HSV1 virus gB glucoprotein extracellular region gene fragment, representation and application of the same
InactiveCN101173290APromote safe productionLow costGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsGenetic engineeringGlycoprotein
The chemically synthesized HSV1 viral gB glycoprotein extracellular region gene fragment and its expression and application relate to the fields of genetic engineering technology, vaccines and diagnostic reagents. The present invention screens out the strong epitope in the gB glycoprotein of HSV1 virus through computer analysis, from the first amino acid to the 696th amino acid, a total of 696 amino acids, selects codons favored by both eukaryotic and prokaryotic organisms, and chemically synthesizes The brand-new gene sequence of the antigenic epitope uses genetic engineering technology to express the gene fragment and prepare a strong antigenic epitope fragment of the gB glycoprotein of the HSV1 virus. The expressed strong antigenic epitope fragment of gB glycoprotein of HSV1 virus can be used for the detection of vaccine, HSV1 virus antibody or antigen, and for immunization preparation of anti-HSV1 virus monoclonal antibody and polyclonal antibody and the like.
Owner:李越希
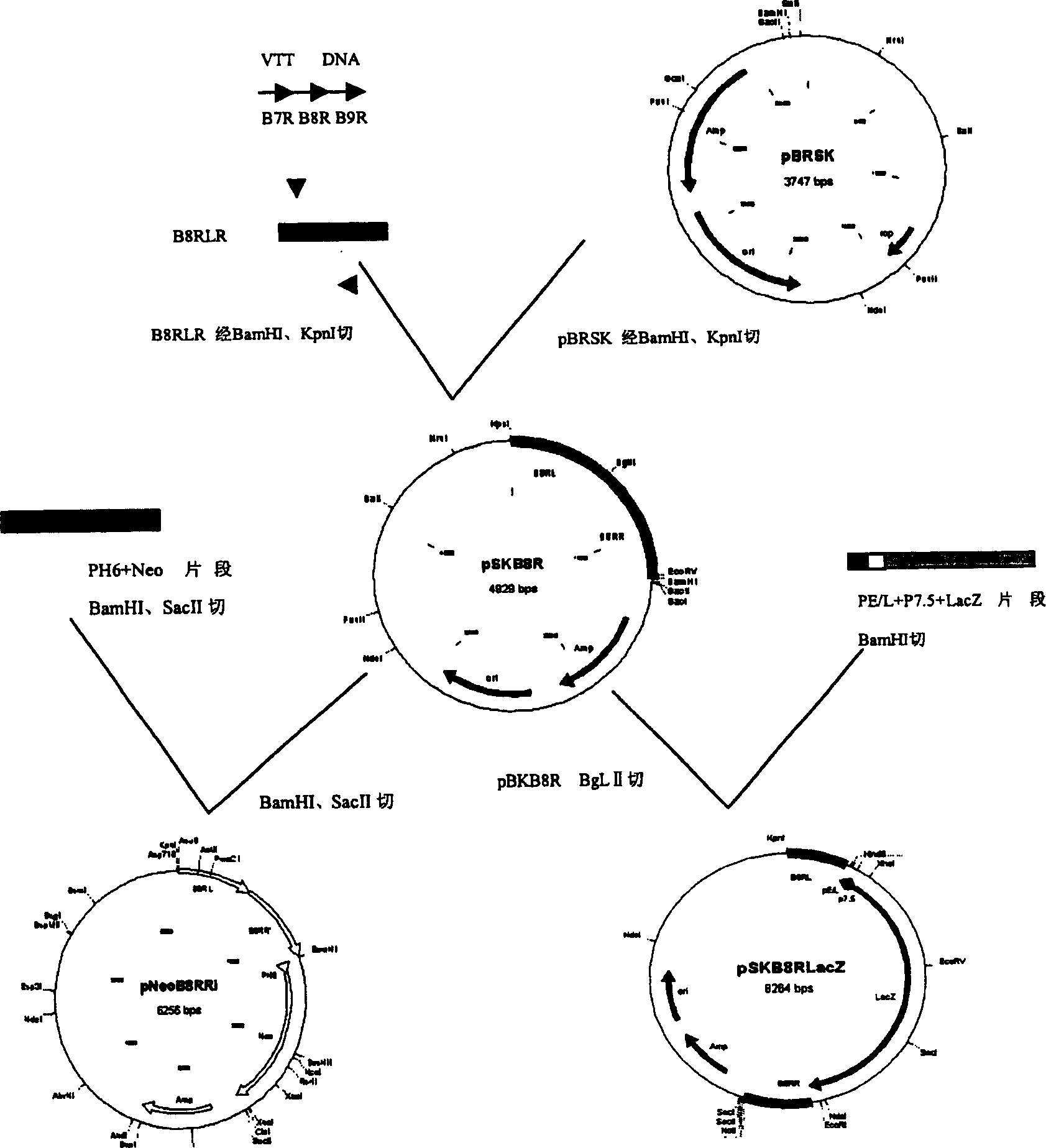
Tiantan remocined vaccine virus of IFN-alpha receptor gene (B8R) deletion and application thereof
InactiveCN1560248AImprove securityImproving immunogenicityViruses/bacteriophagesAntibody medical ingredientsIfn alphaHBsAg
The invention relates to an IFN-gama receptor gene (B8R) deleted attenuating carrier of Tiantan recombinant t vaccinia virus as well as a Tiantan recombinant t vaccinia virus AIDS vaccinum deleting IFN-gama receptor gene (B8R) and expressing various HIV-1 antigens, which is constructed based on this carrier, a Tiantan recombinant t vaccinia virus VTKgpe, recombinant t vaccinia virus VTKgpe CGMCC.No.1099 and another a hepatitis-B virus HBSAg antigen, and a hepatitis-B vaccinum of Tiantan recombinant t vaccinia virus of IL-2.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Establishment of hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody
InactiveCN103773736AImmunoglobulins against virusesMicroorganism based processesMolecular ImmunologyHN Protein
The invention relates to establishment of a hybridoma cell strain for secreting duck NDV (newcastle disease virus)-resisting isolate monoclonal antibody, which belongs to the technical field of molecular immunology and virology. The purified HN protein expressed by duck NDV isolate SDO3 pronucleus is used as immunogen, one hybridoma cell strain capable of secreting duck NDV-resisting isolate monoclonal antibody is researched, and the preservation number is CCTCC C2013173. The NDV specificity monoclonal antibody secreted by the hybridoma cell strain cannot be specially combined with the NDV strain but can be specifically combined with NDV clinical wild strain, so that the NDV specificity monoclonal antibody can be used as an identification reagent to be used for identifying the NDV vaccine virus and clinical wild strain, and the specificity is strong; the sensitivity is 1: 212 HAU; the hybridoma cell strain has the advantage of high sensitivity.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
RT-PCR detection for differential diagnosis of field isolates or lapinized vaccine strain of classical swine fever virus (CSFV) in samples
InactiveUS20060286551A1Quick distinctionIncreasingly sensitiveMicrobiological testing/measurementFermentationEnzymatic digestionConserved sequence
The present invention provides a rapid RT-PCR detection method and a diagnostic kit for differentiating field isolates of classical swine fever virus (CSFV) from lapinized CSF vaccine viruses in samples. In order to detecting different genotypes of CSF virus, this invention uses a pair (or pairs) of CSF virus specific primers designed from the conserved sequences within the 3′-untranslated region of CSFV which contains an insertion of 12˜13 nucleotides (poly T) in the region of the lapinized CSF vaccine virus, in comparison with the corresponding region of field isolates of CSFV. By the RT-PCR or the RT-PCR followed by a nest-PCR, field isolates of CSFV and lapinized CSF vaccine viruses in samples could be detected directly and quickly and / or differentiated in electrophoresis without further enzymatic digestion or DNA sequencing.
Owner:NAT INST FOR ANIMAL HEALTH COUNCIL AGRI EXECUTIVE YUAN
Primer, probe and kit for identifying serum I-type Mardivirus gene deletion vaccine, attenuation vaccine and wild virus
PendingCN108034767AClear statusAccurate differential diagnosisMicrobiological testing/measurementMicroorganism based processesSerum igeUltrasound attenuation
The invention discloses a primer, a probe and a kit for identifying serum I-type Mardivirus gene deletion vaccine, attenuation vaccine and wild virus. The primer and the probe comprise a first pair ofprimer and probe and a second pair of primer and probe, wherein the nucleotide sequence of the first pair of primer and probe is shown as SEQ ID NO. 1 to SEQ ID NO. 3; the nucleotide sequence of thesecond pair of primer and probe is shown as SEQ ID NO. 4 to SEQ ID No. 6. A fluorogenic quantitative PCR reaction is carried out by using the primers and the probes provided by the invention, the serum I-type Mardivirus gene deletion vaccine, attenuation vaccine and wild virus can be identified and diagnosed quickly, accurately, scientifically and objectively, and the immune state of chicken vaccines and the infection progress of wild virus are definite through accurate detection for vaccine virus and wild virus copy number.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Method for inactivating virus in vaccine production
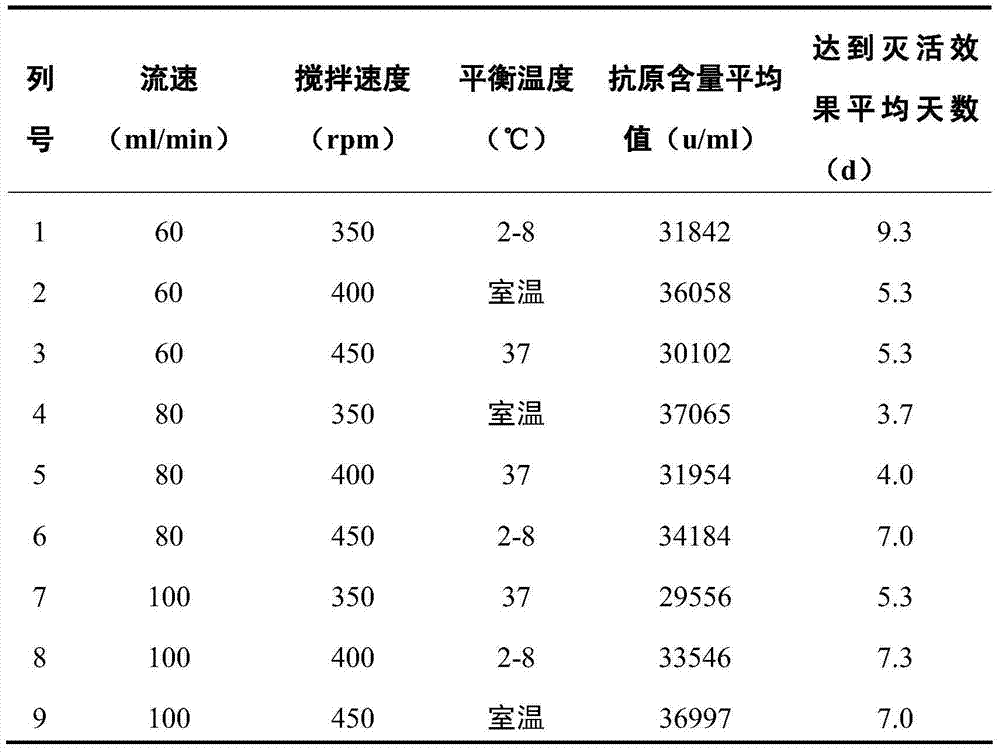
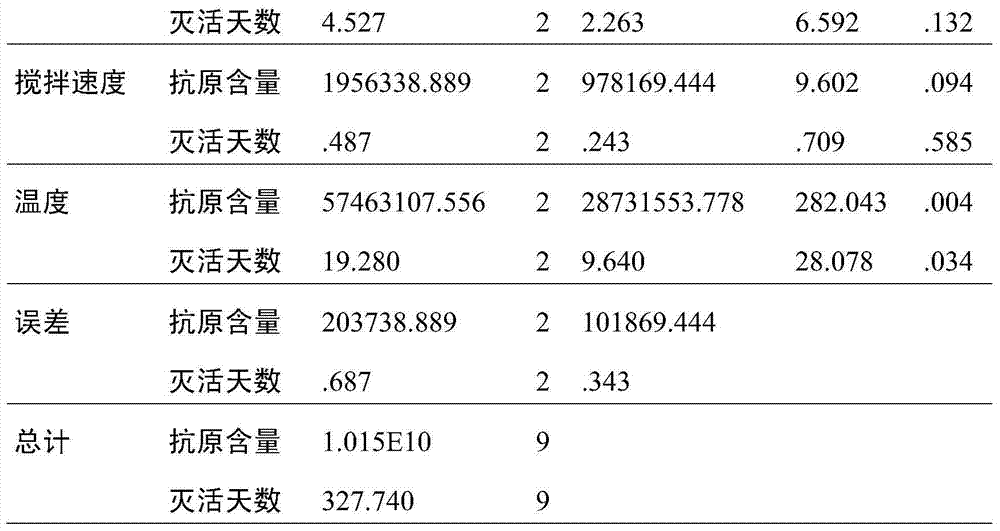
ActiveCN104498446AImproving immunogenicityGuaranteed stabilityInactivation/attenuationMicroorganism based processesTemperature controlVaccine Production
The invention relates to a method for inactivating virus in vaccine production, belonging to the field of preparation of biological products. According to the method, the conditional parameters of temperature control, stirring rate, the flow rate of adding formaldehyde solution and the like during the inactivation process of the vaccine production can be improved according to the factors influencing the antigen stability, immunogenicity and antigen recovery rate during the inactivation of vaccine virus liquid are improved to acquire optimal inactivation conditions, the antigen loss during inactivation can be reduced, the antigen structure can be stabilized and the immunogenicity can be improved. According to the method, the quality stability and uniformity of products can be guaranteed, and the production cost can be lowered; the inactivation time can be shortened within 2h from 2.5h, the fatigue of people can be reduced, the production efficiency is improved, the intermediate product during vaccine production is prevented from being stored at room temperature for long time, and the quality of vaccines can be improved indirectly.
Owner:SINOVAC BIOTECH
Vero E6 cell strain adapting to full-suspension culture and application thereof
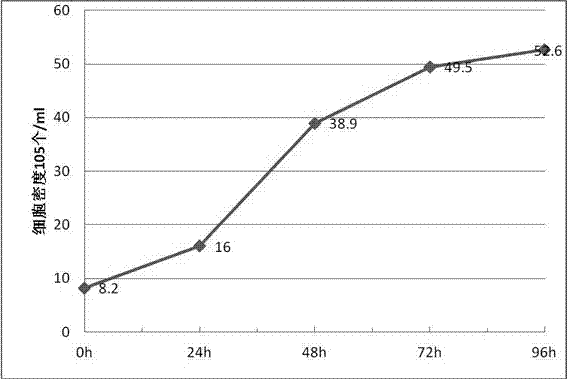
ActiveCN107267443AHigh degree of automationSolve the requirements of cultivating large-scale industrializationArtificial cell constructsViruses/bacteriophagesSerum freeVaccine virus
The invention discloses a vero E6 cell strain adapting to full-suspension culture, the vero E6 cell strain is named as vero E6-s, and is preserved in China Center for Type Culture Collection, the preservation number is CCTCC2017101, the vero E6 cell strain is classified and named as Vero cell suspension adaptive strain VeroE6-S, and the preservation date is June 29, 2017. Compared with the prior art, the vero E6 cell strain adapting to the full-suspension culture has high degree of automation for culture of vaccine viruses, can be suspended and cultured in a serum-free or low-serum medium without carrier intervention, and solves large-scale industrialization requirements of virus cultivation, a large-scale production method meeting GMP production technology requirements can be developed, and the large-scale production method has a good prospect of industrialization.
Owner:郑州爱科生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com