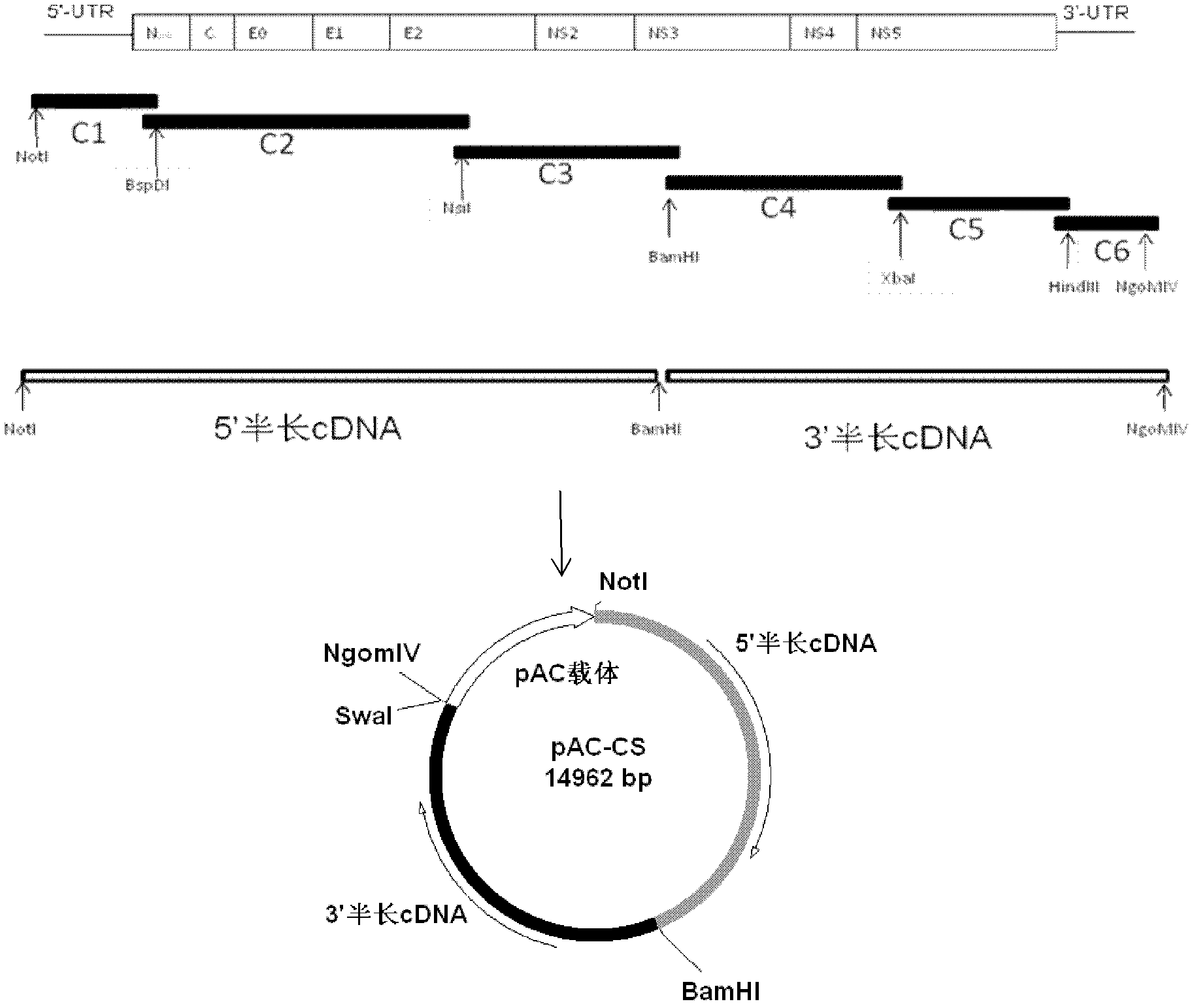
Method for establishing hog cholera lapinized virus labeled vaccine strain and preparing vaccine
A technology of swine fever rabbit and vaccine poison, applied in biochemical equipment and methods, medical preparations containing active ingredients, antiviral agents, etc., can solve problems such as biological safety hazards and unconfirmed immune effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] 1. Vaccine Preparation
[0097] Propagation of venom by using PK15 subcultured cells to produce seedlings
[0098] The transfected cells containing the CGMCC No.4975 strain passed to the 8th to 13th passages were used as seed cells, frozen in liquid nitrogen, and the cells were revived when the virus was propagated. They could be directly cultured or added with 50% negative SK6 cells (, ST and P Any one of the cells such as K15) is co-cultured for about 48-72 hours. After the cells grow into a dense monolayer, the supernatant (virus liquid) is harvested and the cells are passaged at the same time. The harvested virus liquid was frozen and stored below -20°C.
[0099] 2. Inspection of seedling venom:
[0100] (1) Sterility test: according to "Chinese Veterinary Pharmacopoeia" (Chinese Veterinary Pharmacopoeia Committee. People's Republic of China Veterinary Pharmacopoeia 2005 edition three China Agricultural Press, 2006, the present invention is called "Chinese Veterin...
Embodiment 2
[0106] Propagation of venom by using ST subcultured cells to produce seedlings
[0107] The transfected cells containing the CGMCC No.4975 strain passed to the 8th to 13th passages were used as seed cells, frozen in liquid nitrogen, and the cells were revived when the virus was multiplied. They could be directly cultured or co-cultured with 50% negative ST cells, and cultured for 48 After ~72 hours, after the cells grow into a dense monolayer, the supernatant (virus fluid) is harvested, and the cells are passaged at the same time. The harvested virus liquid was frozen and stored below -20°C.
[0108] Other processes of making seedlings are the same as in Example 1.
Embodiment 3
[0110] Propagation of venom by using PK15 subcultured cells to produce seedlings
[0111] The transfected cells containing the CGMCC No.4975 strain passed to the 8th to 13th passages were used as seed cells, frozen in liquid nitrogen, and the cells were revived when the virus was propagated. They could be directly cultured or co-cultured with 50% negative PK15 cells, and cultured for 48 After ~72 hours, after the cells grow into a dense monolayer, the supernatant (virus fluid) is harvested, and the cells are passaged at the same time. The harvested virus liquid was frozen and stored below -20°C.
[0112] Other processes of making seedlings are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com