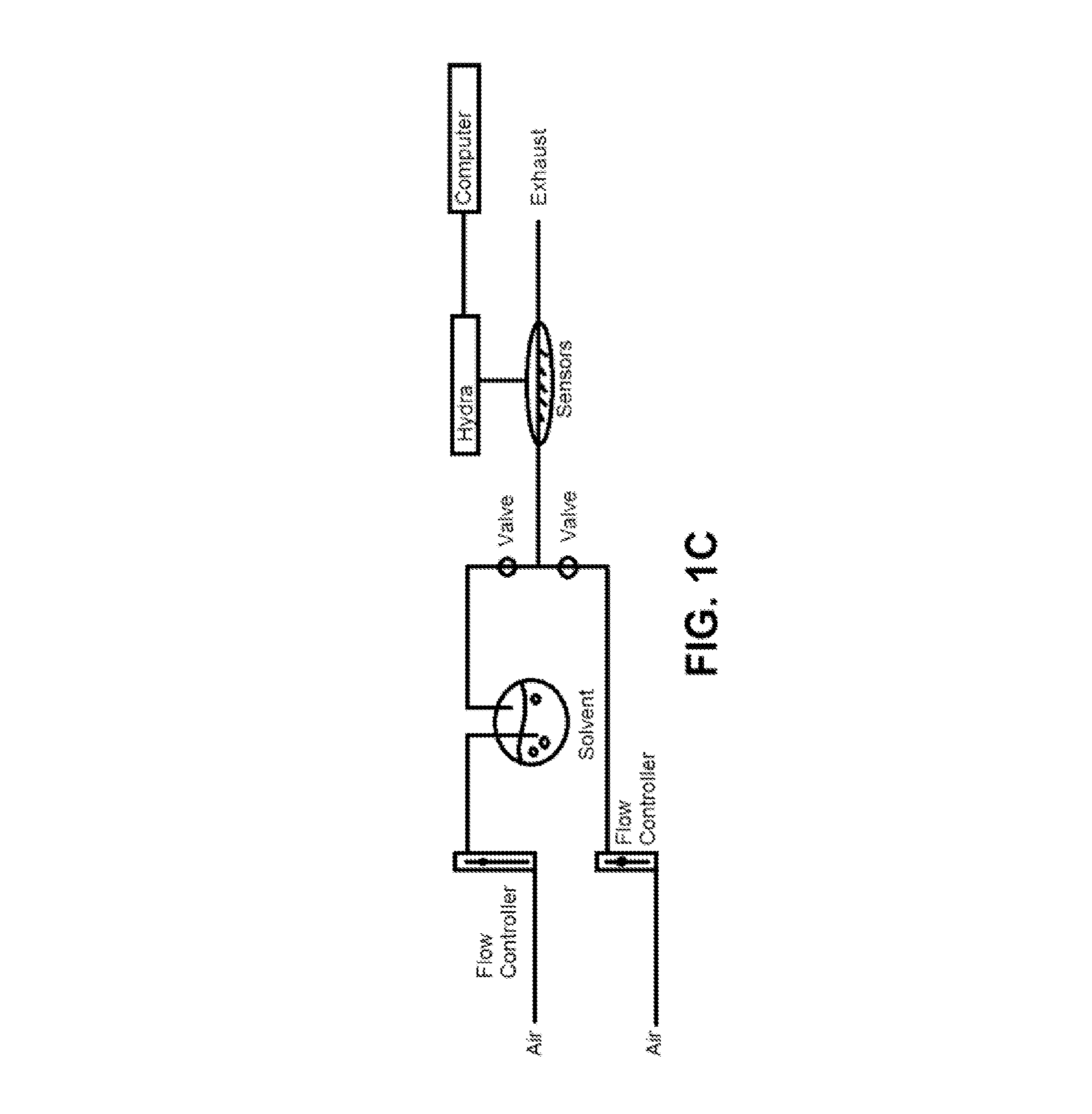
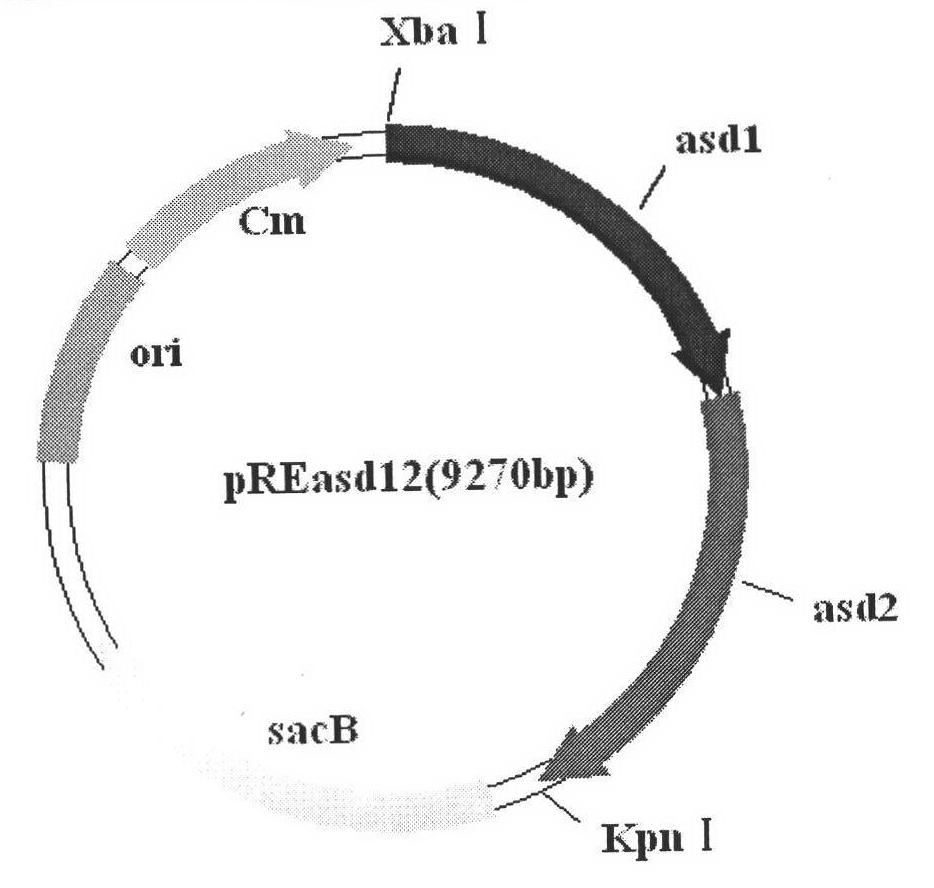
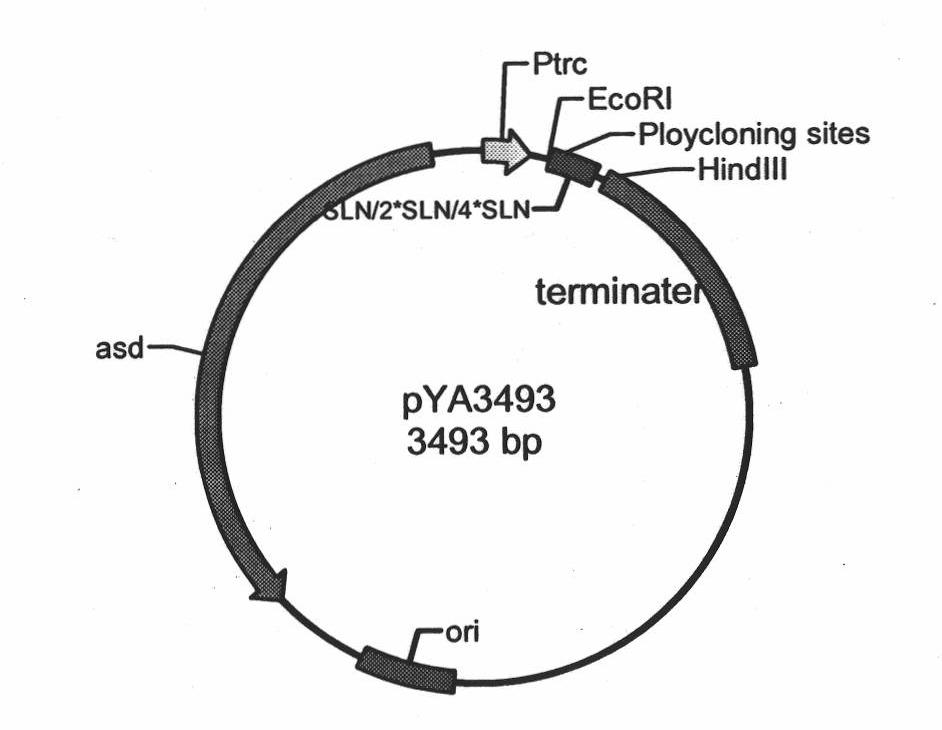
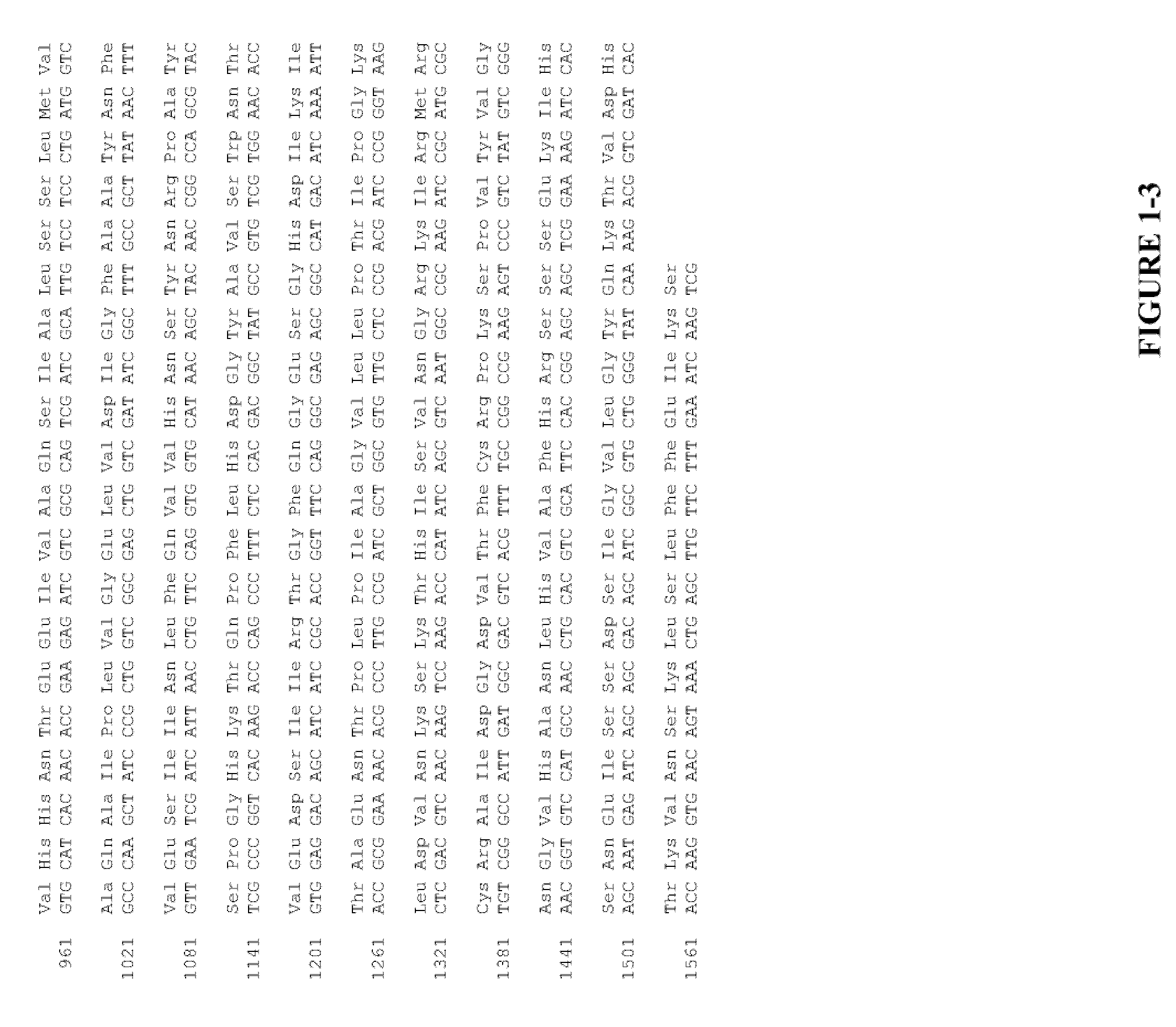Patents
Literature
371 results about "Cholera" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A bacterial infection which spreads through contaminated food and water.
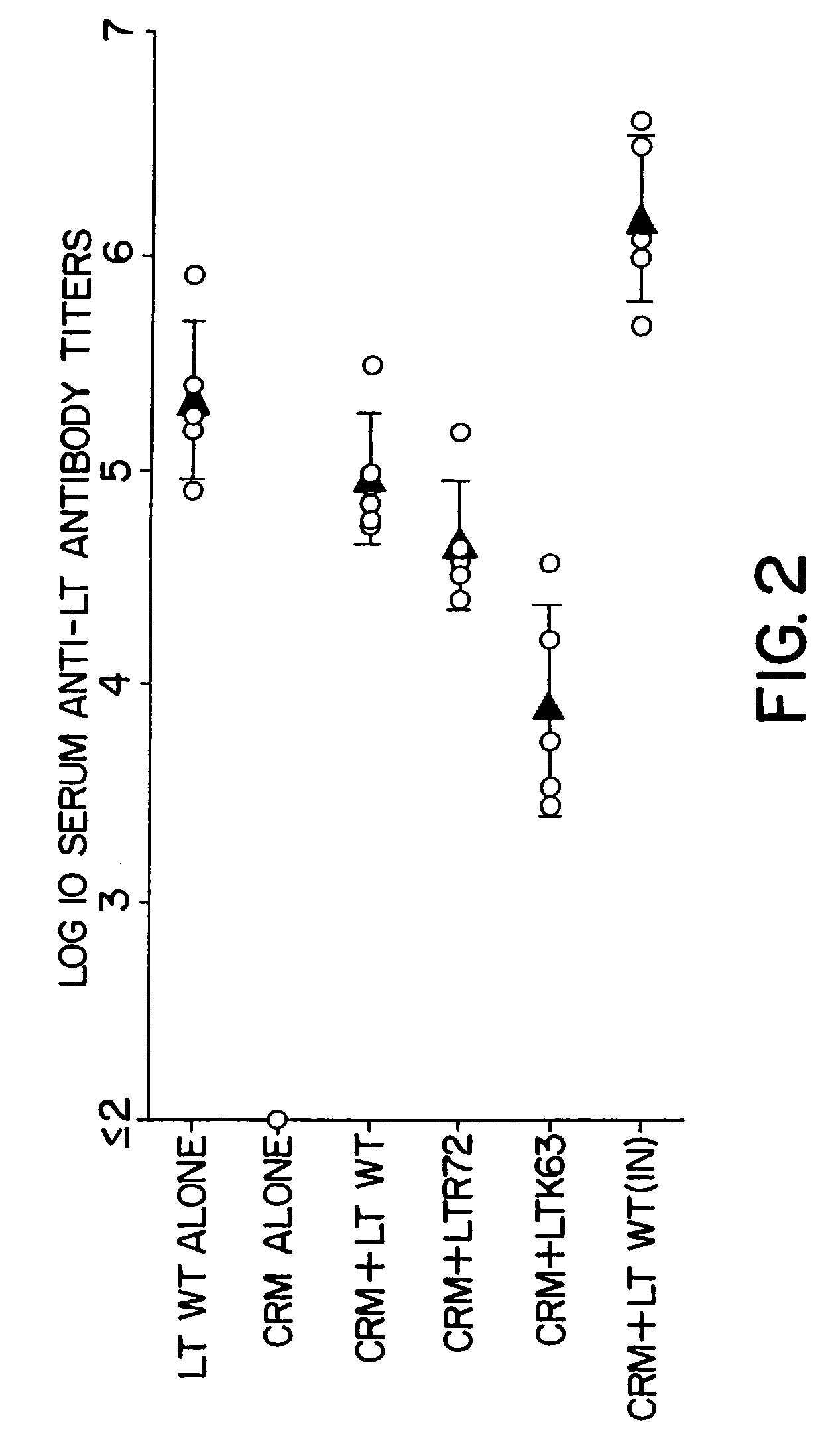
Mutant cholera holotoxin as an adjuvant
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
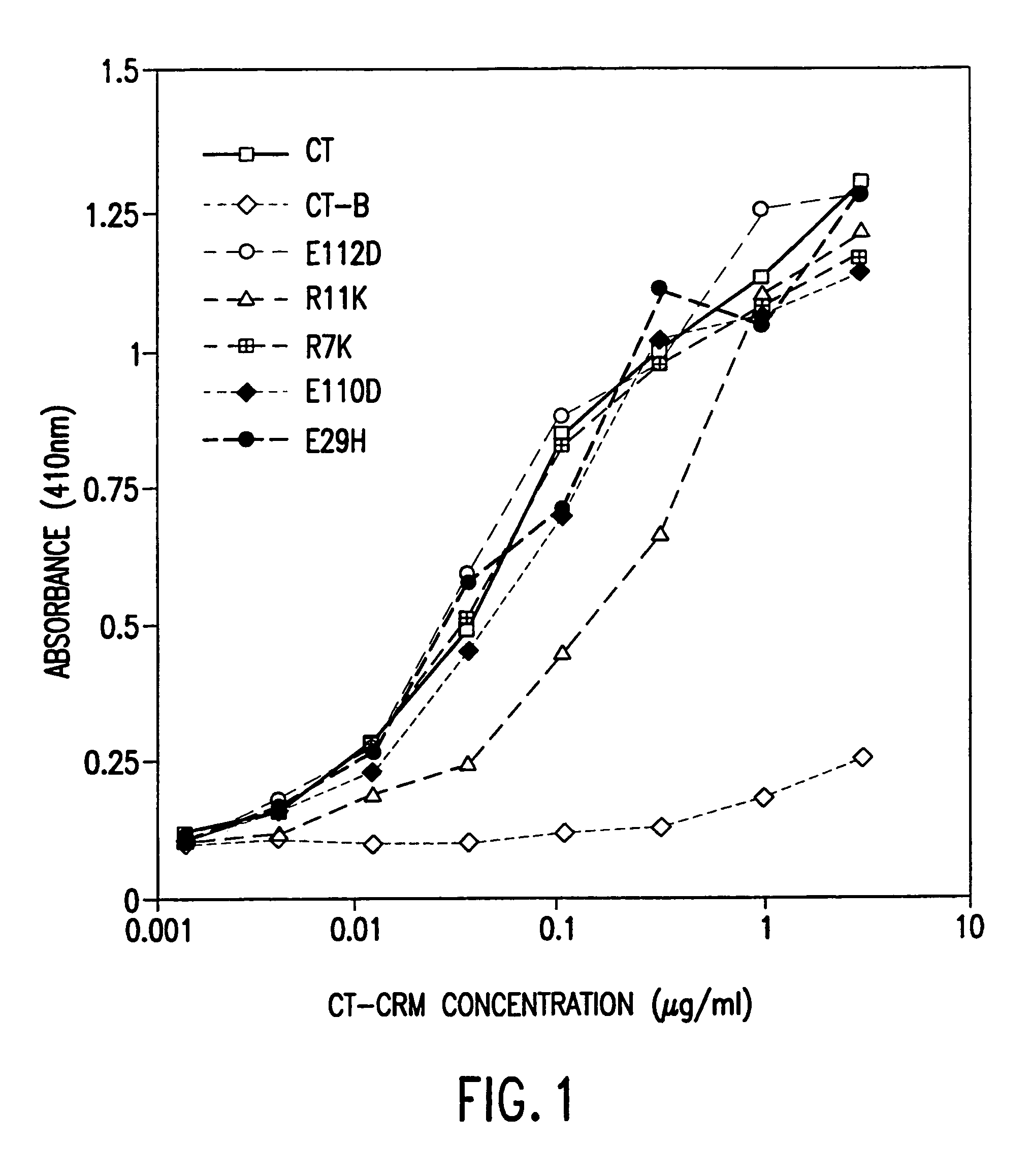
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
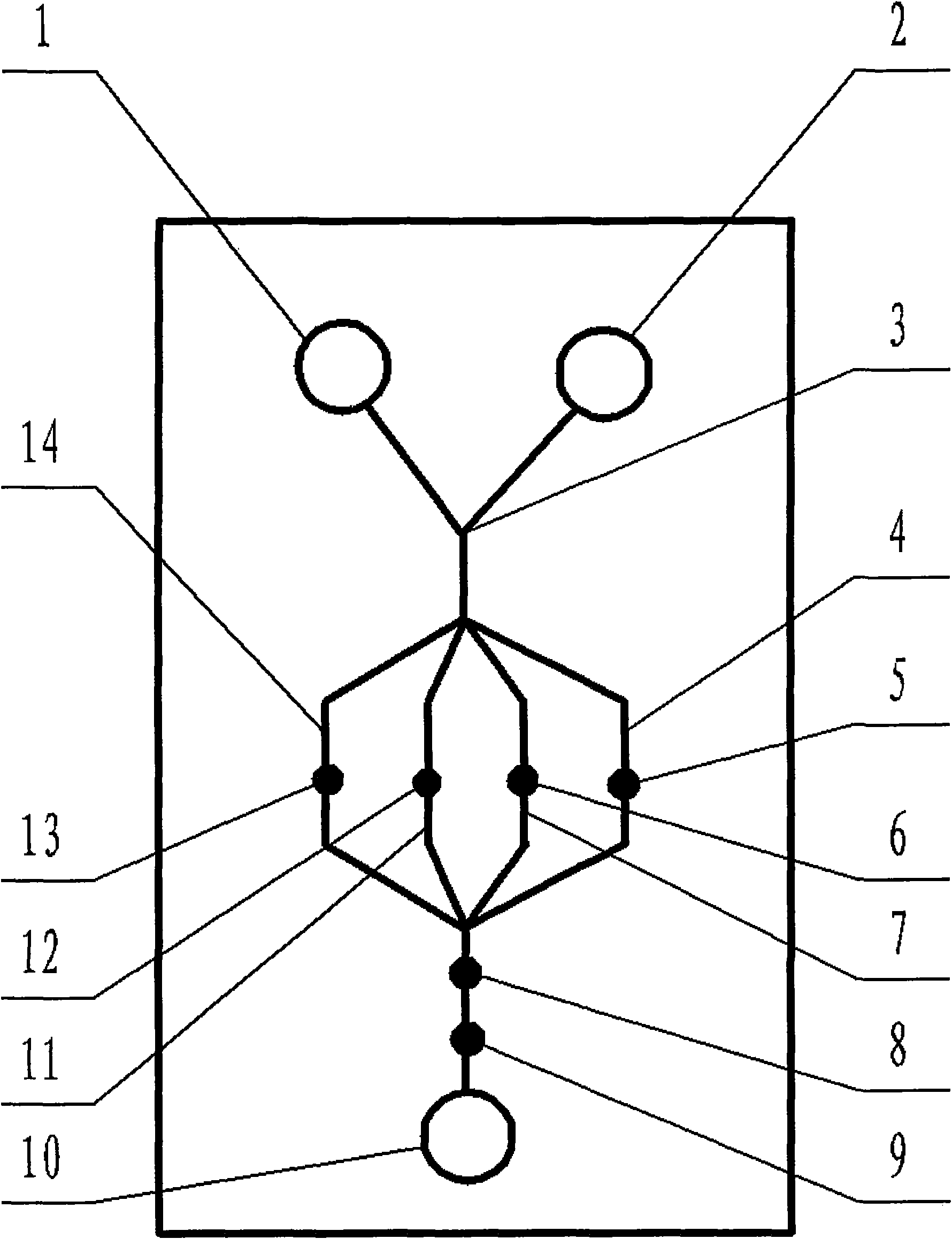
Special micro-fluidic chip for cholera diagnosis with one-dimensional self-assembly magnetic bead chain electrodes
InactiveCN101587123AImprove diagnostic efficiencyReduce dosageMaterial electrochemical variablesAgainst vector-borne diseasesMycoproteinMagnetic bead
The invention relates to a special micro-fluidic chip for cholera diagnosis with one-dimensional self-assembly magnetic bead chain electrodes, belonging to the field of testing. Rapid diagnosis of fulminating infectious disease cholera with low cost is one of many expectations in the development of medical technology, and the invention provides a micro-fluidic chip for simultaneously detecting and rapidly diagnosing cholera by using multiple characteristic antibodies of cholera. The chip is provided with three micro liquid storage tanks, and the invention is characterized in that capillary passages in parallel configuration contain in the chip, the parallel configuration contains four micro-passages that are mutually in parallel, four magnetic bead chain working electrodes are respectively installed inside the four micro-passages, the surface substances of the four magnetic bead chain working electrodes are respectively four antibody substances that are cholera TP0821 antibody, cholera TP0319 antibody, cholera TP0624 antibody and cholera O139 mycoprotein antibody. The integrated structure and the appearances of specific magnetic bead chain working electrodes facilitate to improve the diagnosis efficiency of cholera diseases.
Owner:NINGBO UNIV
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7332174B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAntigenAdjuvant
Mutant cholera holotoxins having single or double amino acid substitutions or insertions have reduced toxicity compared to the wild-type cholera holotoxin. The mutant cholera holotoxins are useful as adjuvants in antigenic compositions to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus, or parasite, a cancer cell, a tumor cell, an allergen, or a self-molecule.
Owner:WYETH HOLDINGS CORP +1
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7285281B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAdjuvantCancer cell
Owner:UNIV OF COLORADO FOUND +1
Multi-channel chip for cholera diagnosis based on structural conductive macromolecular material technology
InactiveCN101620227AReduce manufacturing costMechanically resistantMaterial analysisAgainst vector-borne diseasesCapillary channelLiquid storage tank
The invention relates to a multi-channel chip for cholera diagnosis based on a structural conductive macromolecular material technology, belonging to the test field. The invention aims to aid rapid and low-cost fulminating infectious disease cholera diagnosis which is one of medical technical progress expectations. The chip is provided with three micro liquid storage tanks and is characterized bycomprising capillary channels in parallel construction containing four micro channels mutually connected in parallel; four fibrous working electrodes are respectively mounted in the four micro channels; and four antibody substances, i.e. a cholera TP0821 antibody, a cholera TP0319 antibody, a cholera TP 0624 antibody and a cholera TP0139 antibody are respectively arranged on the surfaces of the four fibrous working electrodes which are originally made of a thermal-decomposition conductive macromolecular material. The chip has low cost and multiple channels.
Owner:NINGBO UNIV
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
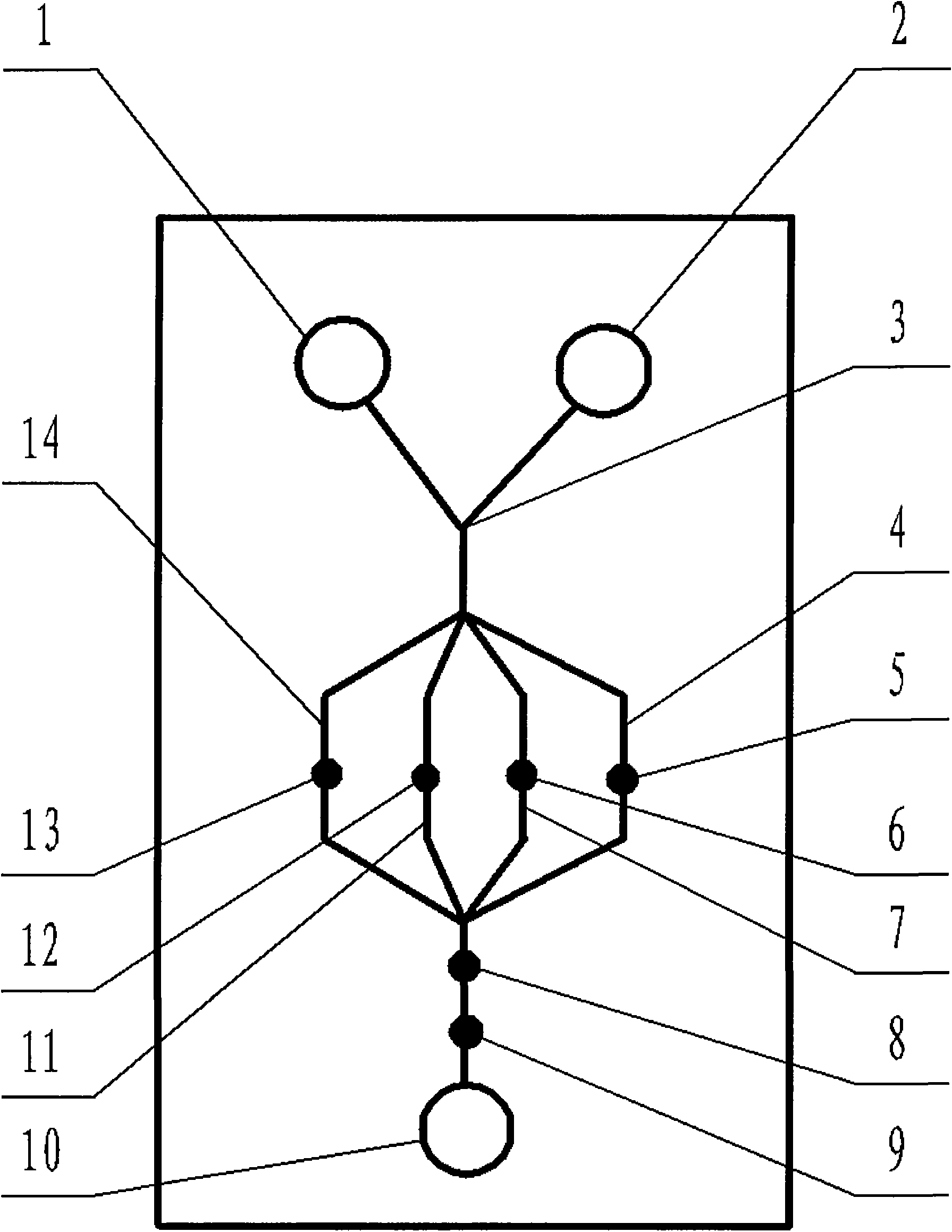
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
High level expression of recombinant toxin proteins
ActiveUS20110287443A1High yieldQuality improvementAntibody mimetics/scaffoldsBacteria peptidesBacteroidesHigh level expression
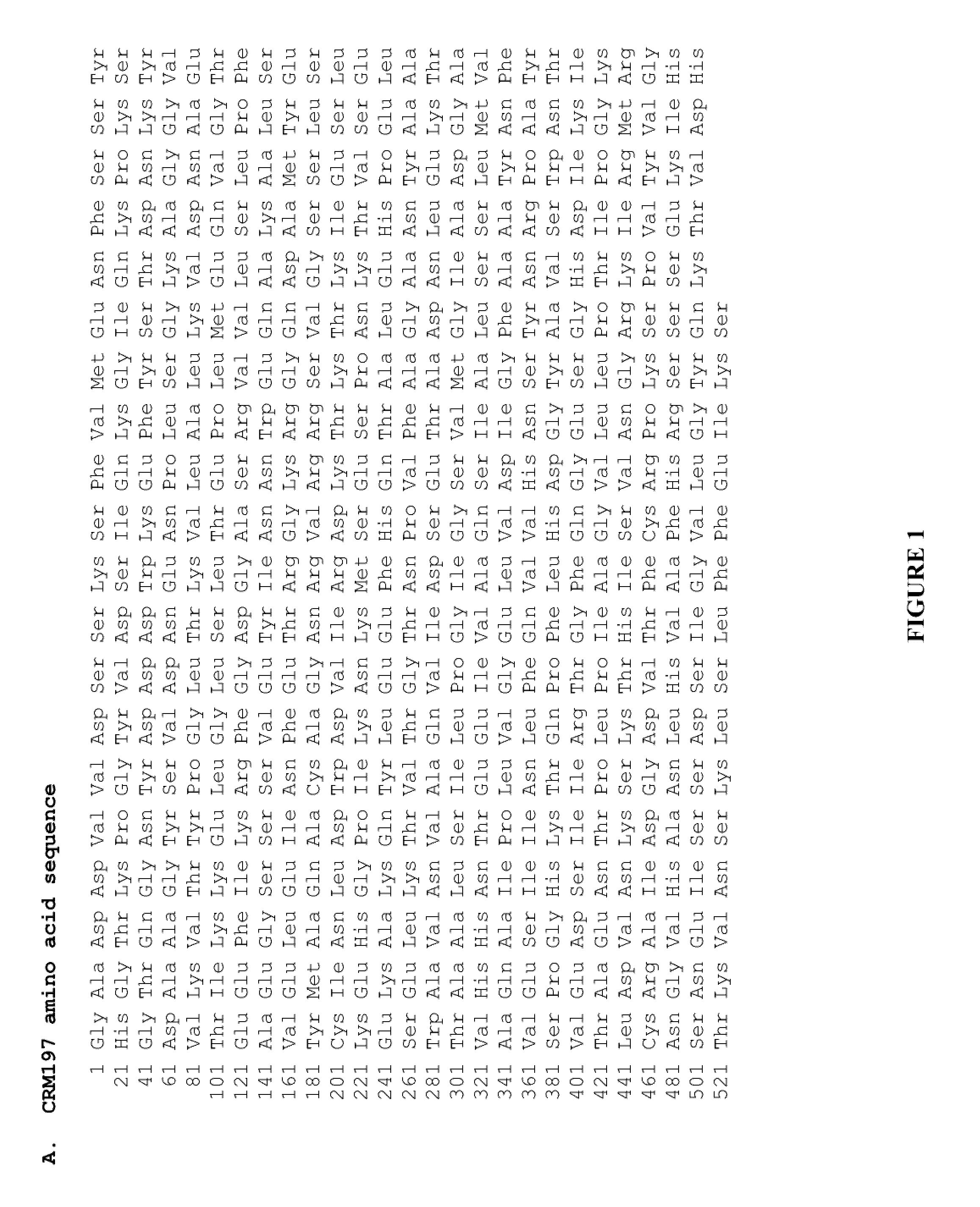
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PFENEX
Chinese medicinal feed additive for preventing and treating common diseases of chickens
InactiveCN101658247AImprove immunityImprove disease resistanceAnimal feeding stuffEscherichia coliCoccidiosis
The invention relates to a Chinese medicinal feed additive for preventing and treating common diseases of chickens. The Chinese medicinal feed additive is prepared from the following selected raw materials according to parts by weight: radix scutellariae, rhizoma coptidis, golden cypress, milk veteh, fructus forsythiae, honeysuckle, rhizoma belamcandae, rhizoma atractylodis, fried bighead atractylodes rhizome, hawthorn, barley malt, medicated leaven, codonopsis pilosula and liquorice. A preparation method of the Chinese medicinal feed additive comprises the steps of mixing and then grinding the raw materials, or respectively grinding and then mixing the raw materials. The feed additive has the effects of strengthening body immunity to eliminate pathogenic factors, clearing away heat and toxic material, diminishing inflammation and invigorating stomach, sterilizing and disinfesting; by being mixed into daily feed for feeding, the Chinese medicinal feed additive can increase body immunity and disease resistance of chickens; and the feed additive is excellent in preventing and treating such common diseases of chickens as bird flu, new-area plague, salpingitis, white diarrhea, cholera,ascariasis, laryngotracheitis, coccidiosis and colon bacillus. Application proves that the Chinese medicinal feed additive has good preventing and treating effects, easily obtained materials, low cost and no toxic side effect.
Owner:王直军
Detoxified mutants of bacterial ADP-ribosylating toxins as parenteral adjuvants
InactiveUS7056521B2Improving immunogenicityPromotes an increase in inductionSsRNA viruses negative-senseAntibacterial agentsEscherichia coliAdjuvant
The present invention provides parenteral adjuvants comprising detoxified mutants of bacterial ADP-ribosylating toxins, particularly those from pertussis (PT), cholera (CT), and heat-labile E. coli (LT).
Owner:CHIRON CORP
Feed additive prepared from traditional Chinese medicines and used for preventing and treating common diseases of chickens
InactiveCN102038092AImprove immunityImprove disease resistanceAnimal feeding stuffEscherichia coliCoccidiosis
The invention provides a feed additive prepared from traditional Chinese medicines and used for preventing and treating common diseases of chickens. The feed additive is prepared from the following raw medicines by weight: honeysuckle, rhizoma belamcandae, rhizoma atractylodis, hawthorns, baical skullcap root, rhizoma coptidis, amur corktree bark, radix astragali, radix isatidis, rabdosia rubescens and dandelions. A preparation method of the feed additive is characterized by mixing the above raw medicines and then grinding the mixture or respectively grinding the above raw medicines and then mixing the ground raw medicines. The feed additive has the functions of strengthening body resistance and eliminating evil, clearing away heat and eliminating toxins, diminishing inflammation and strengthening stomach and killing bacteria and pests. By being mixed into the daily feed, the feed additive enhances the immunity of the chicken bodies, improves the disease resistance and has good prevention and treatment effects on common diseases of chickens such as bird flu, newcastle disease, salpingitis, white diarrhea, cholera, ascariasis, laryngotracheitis, coccidiosis, escherichia coli and the like. Through applications, the feed additive has the advantages of good prevention and treatment effects, no toxic or side effect, easily obtained raw materials and low price.
Owner:吴艳霞
Culture medium for normal epithelial cell of human or mammal, culture methods, normal epithelial cell and application of normal epithelial cell
InactiveCN104694460ANormal differentiation physiological functionMicrobiological testing/measurementArtificial cell constructsMatrigelMammal
The invention relates to a culture medium for a normal epithelial cell of a human or mammal, primary separation culture, subculture, 3D gas-liquid culture and 3D matrigel culture methods, the normal epithelial cell generated by using the culture medium and the culture methods and application of the normal epithelial cell to a toxicological evaluation system. The culture medium is prepared by mixing DMEM and Ham's F-12NUTRIENT MIX according to the volume ratio of 3:1 and also adding 4-6% of fetal calf serum, 1-3nM triiodothyronine, 0.4-0.65% of insulin-transferrin-selenium reagent, 4-6mu g / ml transferrin, 9-11ng / mL epidermal growth factors, 0.3-0.5mu g / mL hydrocortisone, 0.5-1.5nM cholera toxin, 0.4-0.6mu g / mL amphoterrible B, 35-45mu g / mL gentamicin, 45-55nM calpeptin, 35-45ng / ml recombinant human IL-1RA and 3mu g / ml recombinant human R-Spondin-1. The culture medium disclosed by the invention can be used for carrying out separation culture or subculture on the normal epithelial cell of the human or the mammal and any other various tissue source, rapidly proliferating the normal epithelial cell in vitro and establishing a cell line; and the normal epithelial cell is a normal diploid cell and is applied to the toxicological evaluation system of the human or the mammal.
Owner:SHENZHEN RES INST OF WUHAN UNIVERISTY
Supression of allergic reactions by transdermal administration of allergens conjugated to cholera toxin or fragments thereof
InactiveUS20050074462A1Suppress allergic reactionsBacterial antigen ingredientsPeptide/protein ingredientsAdjuvantCo administration
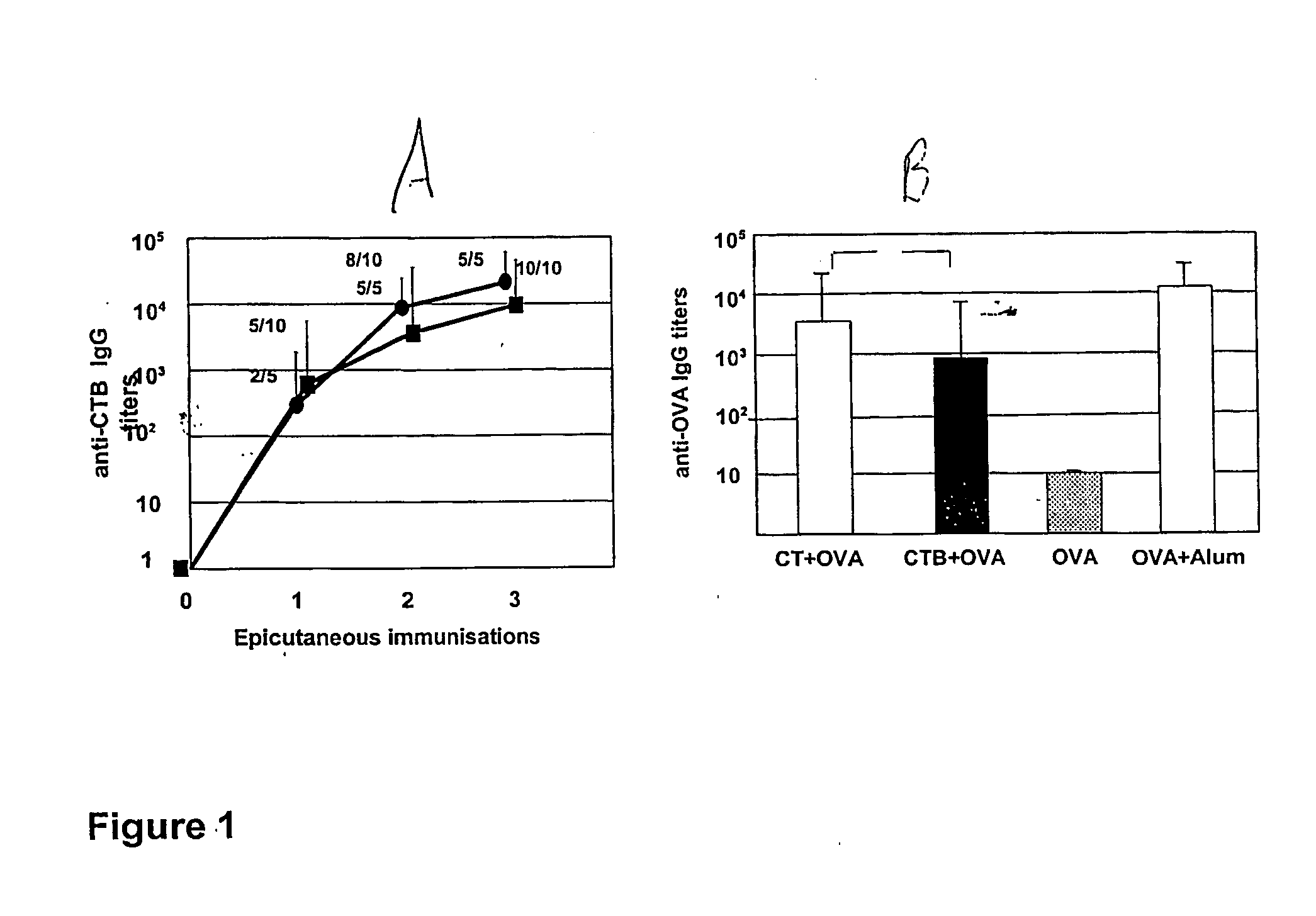
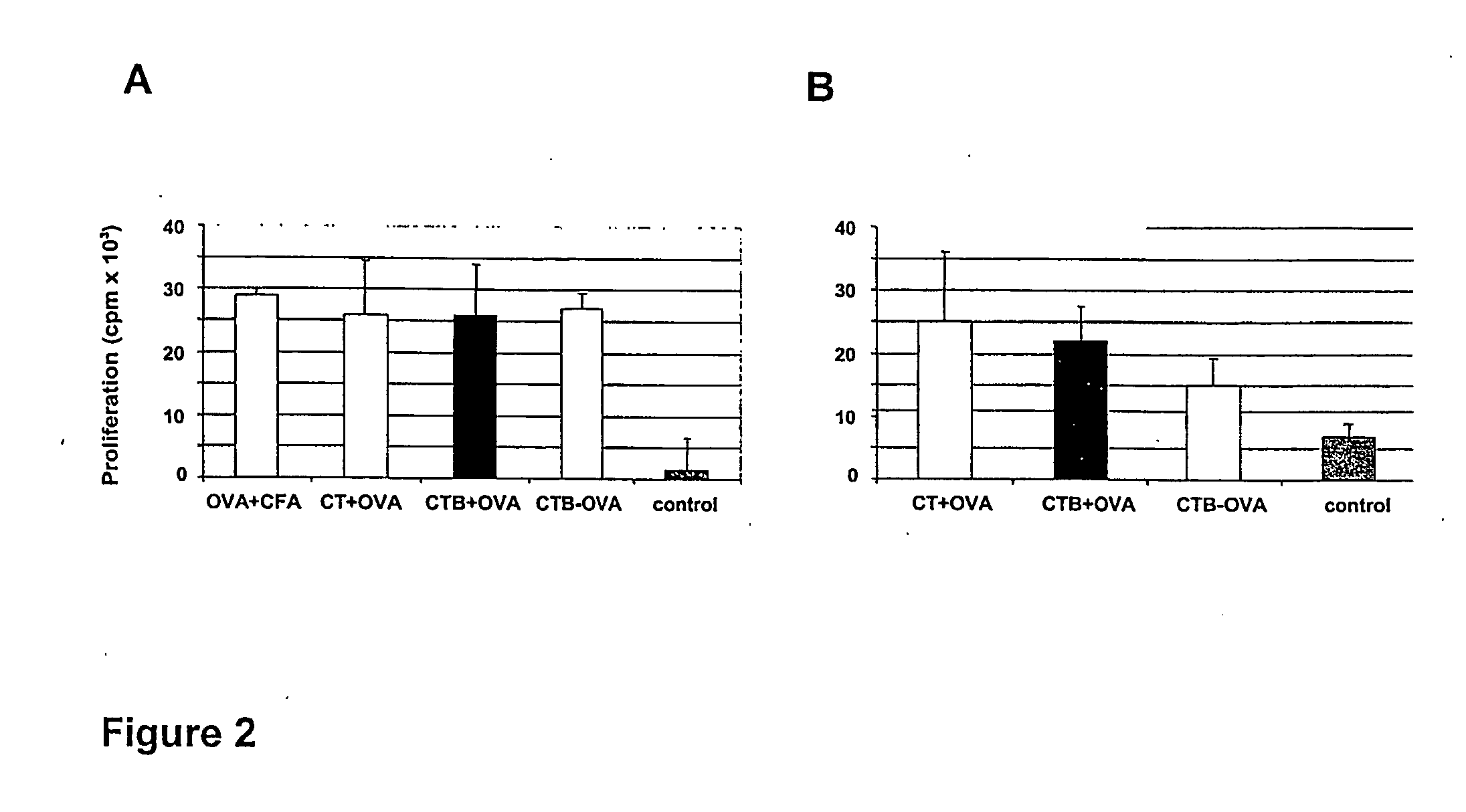
The present invention discloses the use of the non-toxic cell-binding B subunit of CT (CTB), and holotoxin CT that is devoid of ADP-ribosylating activity, as adjuvants for enhancing transcutaneous immune response to a co-administered protein allergen. It was found that topical administration of CTB to mice induced serum antibody response against itself comparable to those evoked by CT, but was inefficient at promoting systemic antibody responses against an admixed prototype protein allergen. To the contrary co-administration of either CT or CTB with allergen led to vigorous antigen-specific T cell proliferative responses in lymph nodes draining the cutaneous site of administration and at distant systemic sites. Consistent with these observations, it was found that CTB selectively potentiated Th1-driven responses without affecting Th2-dependent responses. Cutaneously applied CT enhanced serum IgE responses to a co-administered allergen, while CTB partially suppressed epicutaneously induced IgE responses to the same allergen.
Owner:DUOTOL
High level expression of recombinant toxin proteins
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PELICAN TECH HLDG INC
Pigeon feed and processing method thereof
InactiveCN104351519APromote growthImprove immunityFood processingAnimal feeding stuffCoccidiosisBiotechnology
The invention relates to a pigeon feed and a processing method thereof, belonging to the technical field of feeds and processing methods thereof. The pigeon feed is prepared from the following raw materials in parts by weight: 35-40 parts of corns, 15-20 parts of oats, 20-25 parts of green beans, 12-18 parts of barleys, 12-18 parts of wheat, 8-12 parts of sorghum, 8-10 parts of castor beans, 5-8 parts of houttuynia cordata, 5-8 parts of ajowan, 6-9 parts of momordica grosvenori, 4-9 parts of rhizoma atractylodis, 6-9 parts of orange peels, 3-7 parts of rhizoma alismatis and 4-8 parts of grifola. Due to the addition of dietary supplements formed by traditional Chinese medicines and water, the pigeon feed is capable of promoting the healthy and rapid growth of the pigeons, improving the immunity and the disease resistance of the pigeons, providing a certain function of preventing the common diseases such as cholera fowl and coccidiosis of the pigeons, making the edible pigeons be better in meat quality and making the ornamental pigeons be stronger in physique after feeding twice every day; the pigeon feed is particularly suitable for the pigeons in moulting period.
Owner:苗娥
Multiple PCR identification kit of salmonella and five serotypes of salmonella
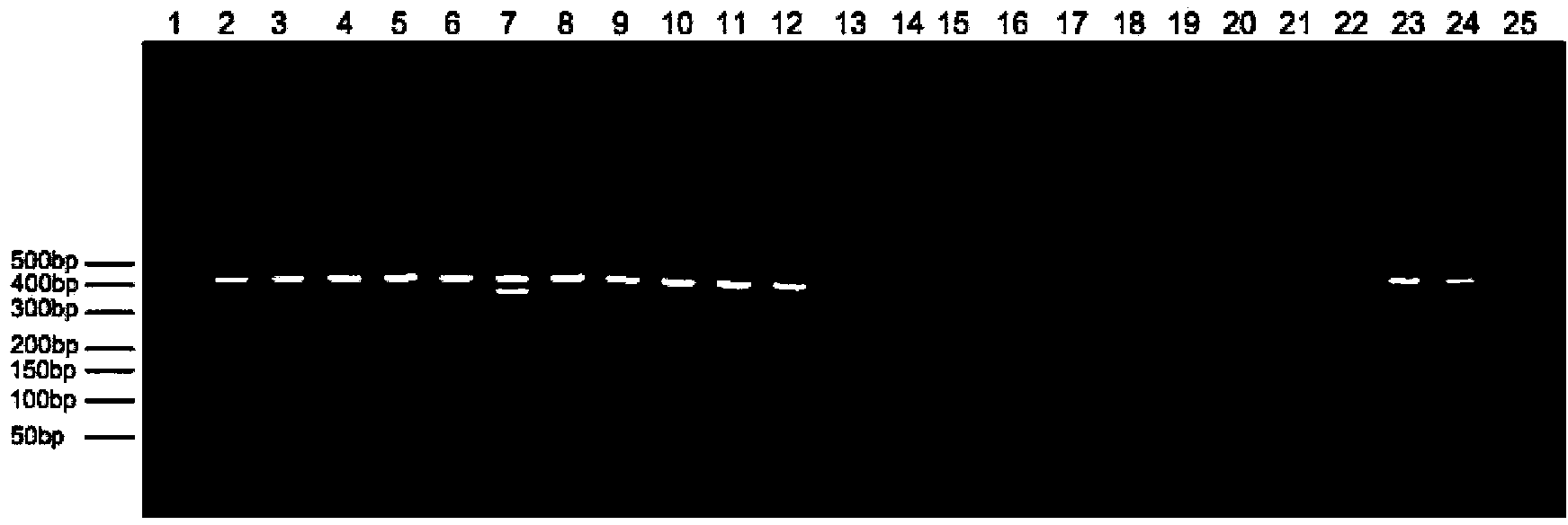
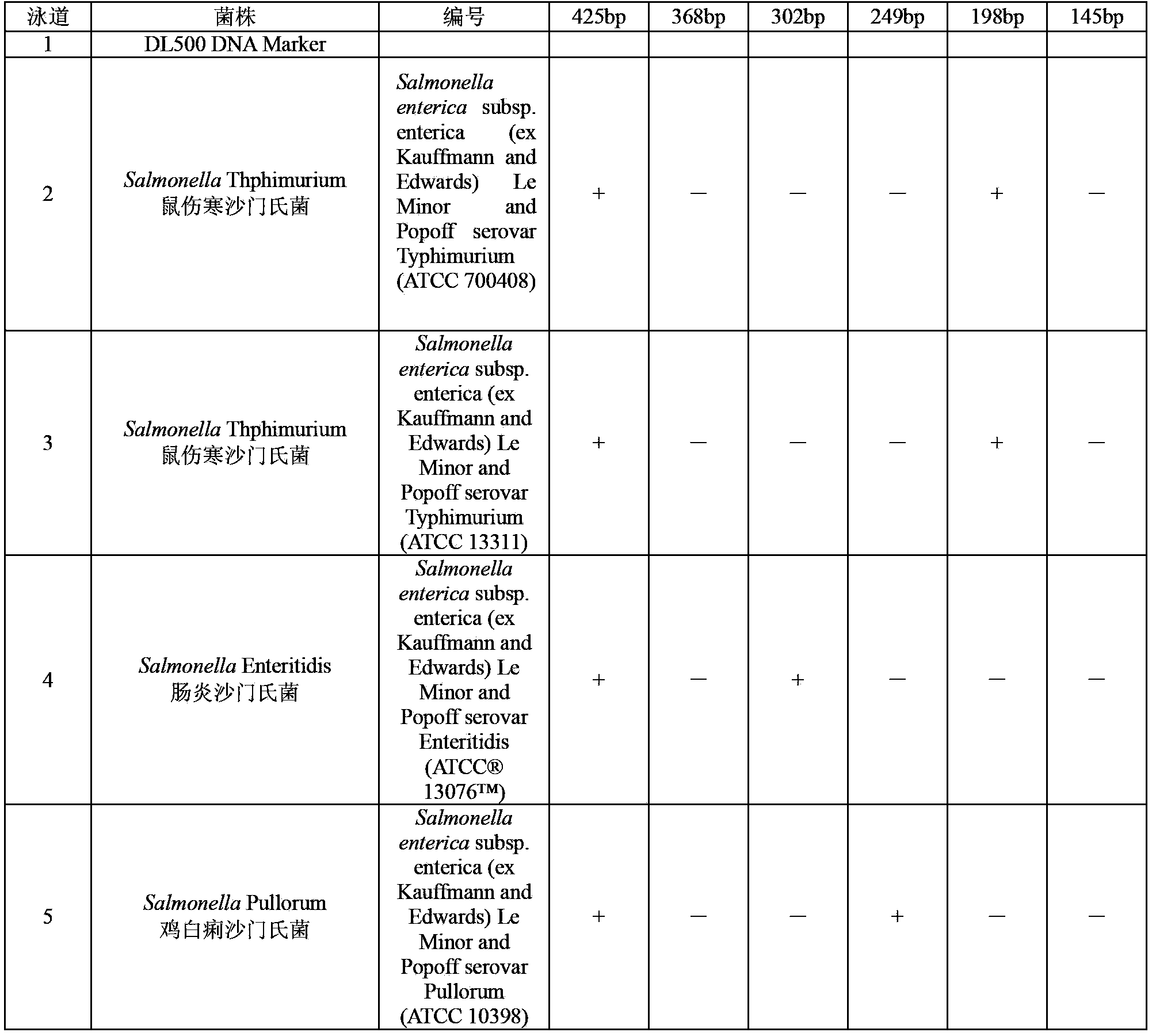
ActiveCN104087654ARapid identificationLow costMicrobiological testing/measurementMicroorganism based processesElectrophoresisSalmonella dan
The invention discloses a multiple PCR identification kit of salmonella and five serotypes of salmonella. The invention provides a primer group for detecting salmonella and five serotypes of salmonella. The primer group comprises a primer pair 1, a primer pair 2, a primer pair 3, a primer pair 4, a primer pair 5 and a primer pair 6. Aiming at specific fragments of salmonella and its five important serotypes (such as salmonella typhimurium, enteritidis, agona, choleraesuis and pullorum), the six pairs of the primers for specific amplification are designed, the amplified fragments comprise 425bp of salmonella, 198bp of salmonella typhimurium, 302bp of salmonella enteritidis, 145bp of salmonella agona, 368bp of salmonella choleraesuis and 249bp of salmonella pullorum, and a sample gene group can be accordingly determined by multiple PCR and electrophoresis detection. Through six pairs of the specific primers and one-step multiple PCR, the salmonella and its five important serotypes can be identified. The multiple PCR identification kit has the characteristics of fast identification rate, low cost, easy operation and easy result determination.
Owner:CHINA AGRI UNIV
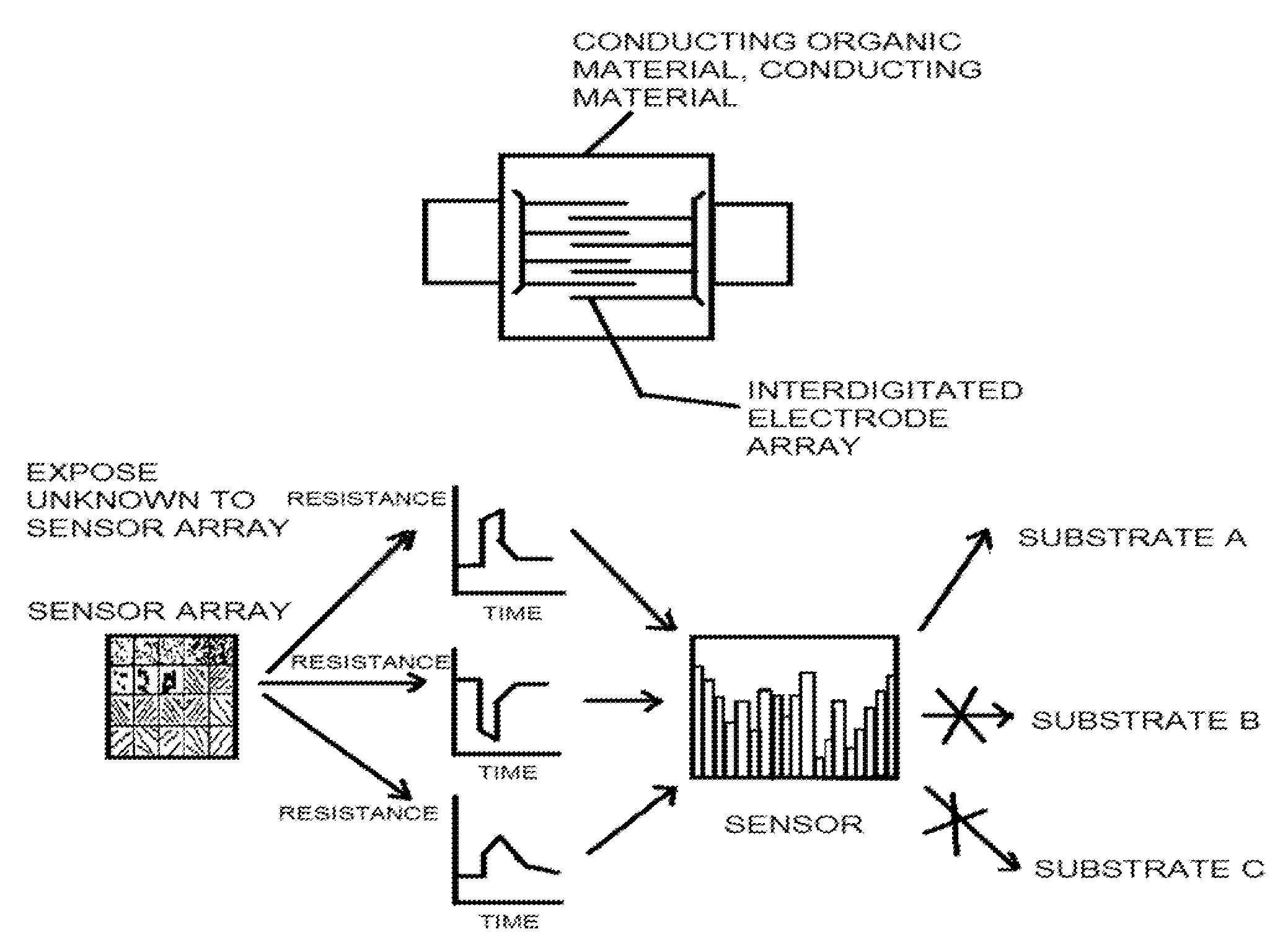
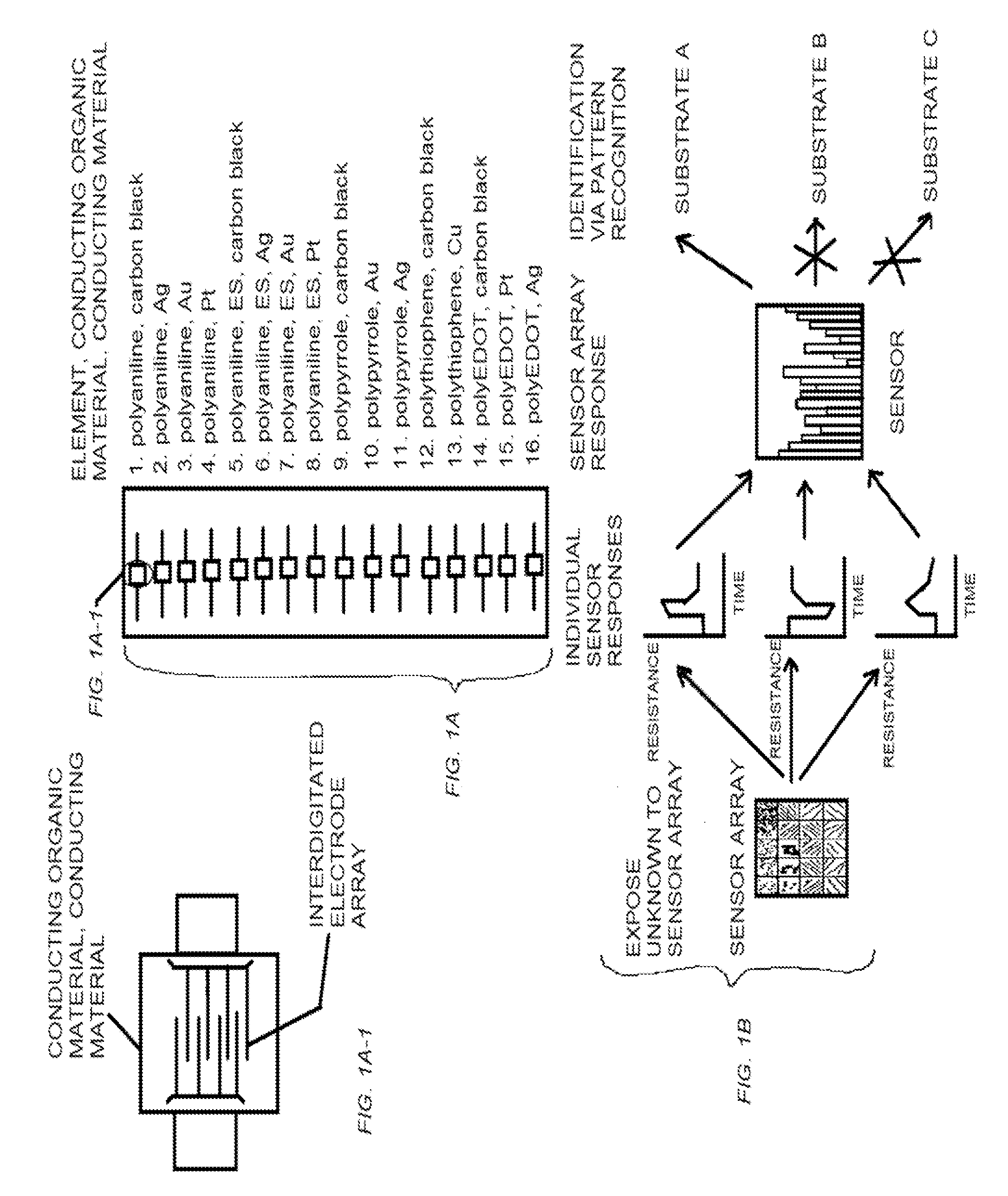
Conductive organic sensors, arrays and methods of use
InactiveUS8394330B1High sensitivityEnhanced informationImmobilised enzymesBioreactor/fermenter combinationsDiseaseLung cancer
The present invention provides a class of sensors prepared from regions of conducting organic materials and conducting materials that show an increase sensitivity detection limit for amines. The present class of sensors have applications in the detection of spoiled food products and in testing for diseases, such as cholera and lung cancer, which have amines as biomarkers.
Owner:CALIFORNIA INST OF TECH
Limbus corneae stem cell serum-free culture medium
InactiveCN101121926AMeet the nutritional needs of long-term expansion cultureArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsBiotechnologyPenicillin
The invention discloses a limbal stem cell serum-free medium, consisting of the following raw materials (calculated by weight percent): DMEM / F12 60ml-90ml, BSA 0.5g-4g, EGF 1Mug-4Mug, insulin 0.1mg-1mg, hydrogen 20ug-100ug, cholera toxin 1ug-10ug, transferrin 0.1mg-1mg, selenious acid 0.1ug-1ug, GCLCM 10ml-40ml, penicillin 5*103IU-2*104IU and streptomycin 5*103IU-2*104IU. The invention adopts the method that the culture medium is added with fibroblast conditioned medium, and the nutritive factors are supplemented with the hormone and cytokine combination, fibroblast conditioned medium and BSA. As a result, the nutritive needs for long-time in-vitro expansion and cultivation of the limbal stem cell serum-free medium are satisfied, and the medium is serum-free and suitable for commercial production.
Owner:NORTHWEST A & F UNIV
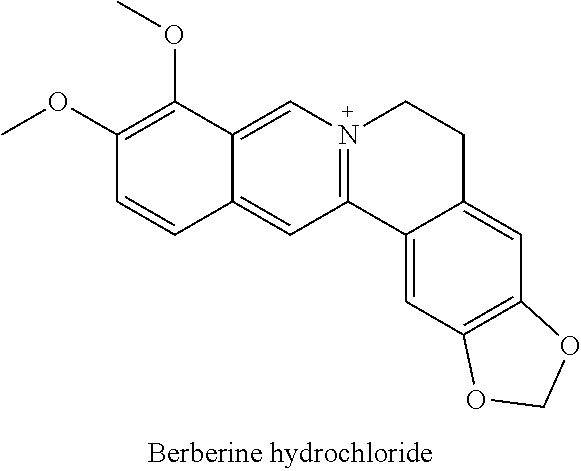
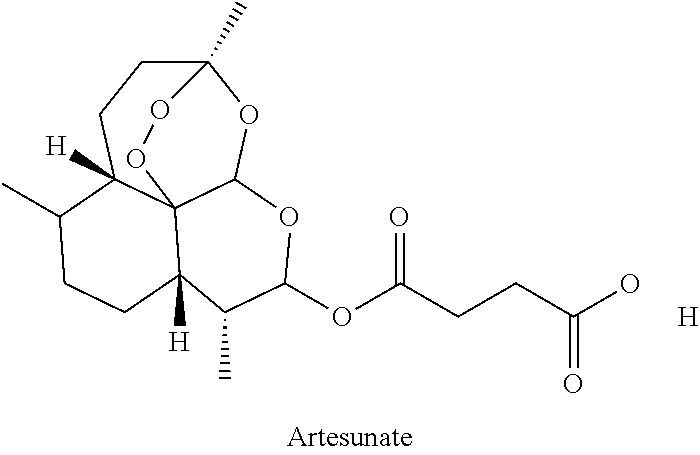
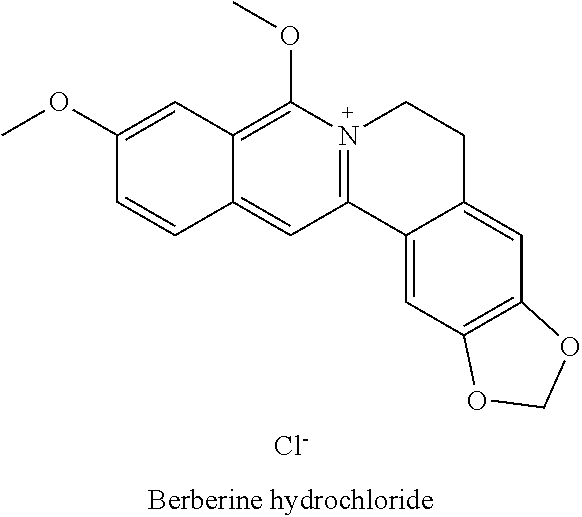
COMBINATIONS OF BERBERINE, ARTEMISININ, Loperamide AND THEIR DERIVATIVES TO TREAT MALARIA, DIARRHEA, TRAVELERS' DIARRHEA, DYSENTERY, DENGUE FEVER, PARASITES, CHOLERA AND VIRUSES
This invention relates to compositions and methods of combining berberine, artemisinin and loperamide or their derivatives in a therapeutic product for mammals suffering from malaria, diarrhea, travelers' diarrhea, dysentery, dengue fever, parasites, cholera and viruses by administration of a therapeutically effective amount of the composition.
Owner:SEUBERT KIRK MR +1
COMBINATIONS OF BERBERINE, ARTEMISININ, Loperamide AND THEIR DERIVATIVES TO TREAT MALARIA, DIARRHEA, TRAVELERS' DIARRHEA, DYSENTERY, DENGUE FEVER, PARASITES, CHOLERA AND VIRUSES
This invention relates to compositions and methods of combining berberine, artemisinin and loperamide or their derivatives in a therapeutic product for mammals suffering from malaria, diarrhea, travelers' diarrhea, dysentery, dengue fever, parasites, cholera and viruses by administration of a therapeutically effective amount of the composition.
Owner:SEUBERT KIRK MR +1
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Medicine for treating hog cholera
The invention discloses a medicine for curing hog cholera, which is composed of Melicope pteleifolia, Elephantopus Scaber, isatis root, Fructus Forsythiae, coptis root, rhubarb, aucklandia root, orientalis carbon, bark of tree peony root, figwort, rehmanniae radix, rhizoma anemarrhenae, Astragalus root, radix codonopsitis and Atractylodes macrocephaia according to certain ratio; the efficiency for curing the hog cholera can reach 94.7%, the recovery ratio can reach 90%, the medicine has no side effect and no recurrence after recovery, the action is immediate after using the medicine, the medicine material is easily obtained and the cost is low; thus being an ideal medicine for curing hog cholera.
Owner:田明军
Traditional Chinese medicine for treating bacterial diseases in poultry
ActiveCN102552502AGood curative effectFast playAntibacterial agentsAluminium/calcium/magnesium active ingredientsEscherichia coliEnterovirus
The invention discloses a traditional Chinese medicine for treating bacterial diseases in poultry, which is prepared from the following raw materials in parts by weight: 10-15 parts of wild chrysanthemum flower, 5-10 parts of Chinese goldthread, 8-10 parts of Scutellaria baicalensis, 8-10 parts of golden cypress, 10-15 parts of Chinese thorowax, 10-12 parts of Cape jasmine, 20-25 parts of Fagopyrum cymosum root, 25-30 parts of common andrographis herb, 8-10 parts of burdock, 25-30 parts of Chinese pulsatilla root and 30-35 parts of folium artemisiae argyi. The traditional Chinese medicine for treating the bacterial diseases in the poultry, disclosed by the invention, has the effects of preventing and treating the bacterial diseases caused by colibacillosis, salmonellosis, necrotizing enterocolitis, enterovirus syndromes, cholera fowls and the like in the poultry.
Owner:河南天纳图生物科技有限公司
Fluorescent quantitative PCR kit for rapid and quantitative detecting hog choleravirus and hog cholera lapinized vaccine and its use
InactiveCN1405328AQuantitatively accurateThe detection process is fastMicrobiological testing/measurementFluorescenceSwine Fever Virus
Owner:WUHAN UNIV
Preparation method of novel composite anti-coccidium, antibiotic preparation
ActiveCN101416973AImprove stabilityImprove securityAntibacterial agentsOrganic active ingredientsCoccidiosisTrimethoprim
The invention discloses a preparation method of a novel compound anti-coccidiosis and antibacterial preparation which is prepared by taking sulfachloropyrazine sodium and trimethoprim as raw materials. The preparation method comprises the steps of: taking 0.5kg to 0.7kg of trimethoprim, adding 10l to 14l of glacial acetic acid to form a mixture, heating the mixture slightly to dissolve the trimethoprim and the glacial acetic acid, dropping 200l to 280l of cyclodextrin saturated water solution with at temperature of 75 DEG C, stirring the mixture for 30min, stopping heating and continuously stirring for 5h to obtain a white sediment, filtering the mixture after being rested at room temperature for 12h, drying the sediment at 60 DEG C, sieving the sediment by an 80-mesh sieve, and drying the sediment by P2O5 in vacuum to obtain a clathrate. The clathrate is mixed with 2.5kg to 3.5kg of sulfachloropyrazine sodium and glucose of a proper amount up to 10kg, evenly mixed and made into particles by a fluidized drying granulator to obtain the finished product. The compound anti-coccidiosis and antibacterial preparation has efficient and quick therapeutic effect on explosive coccidiosis (caecal coccidiosis) of poultry, rabbits, and the like, and on mixed infections caused by various bacteria, such as chicken cholera, typhoid and the like.
Owner:PU LIKE BIO ENG +1
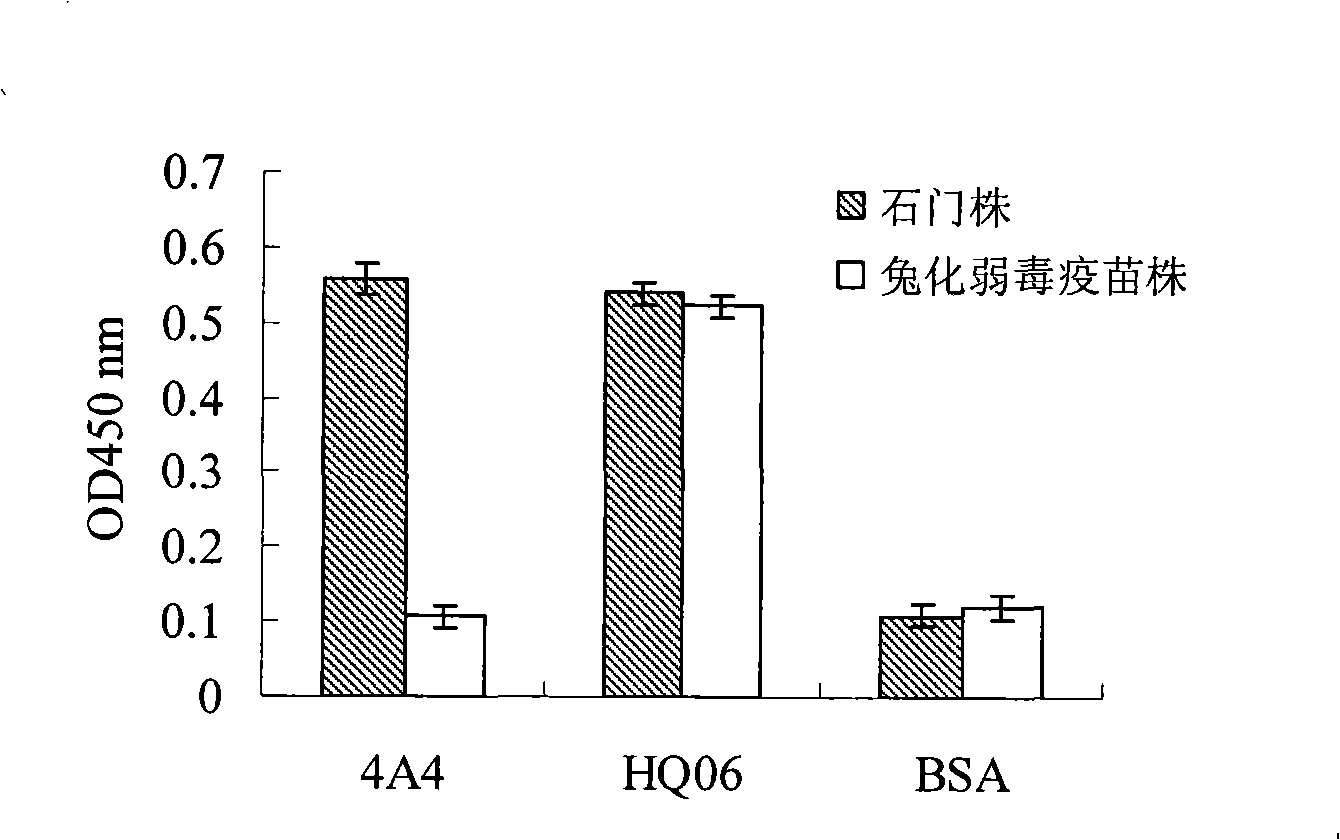
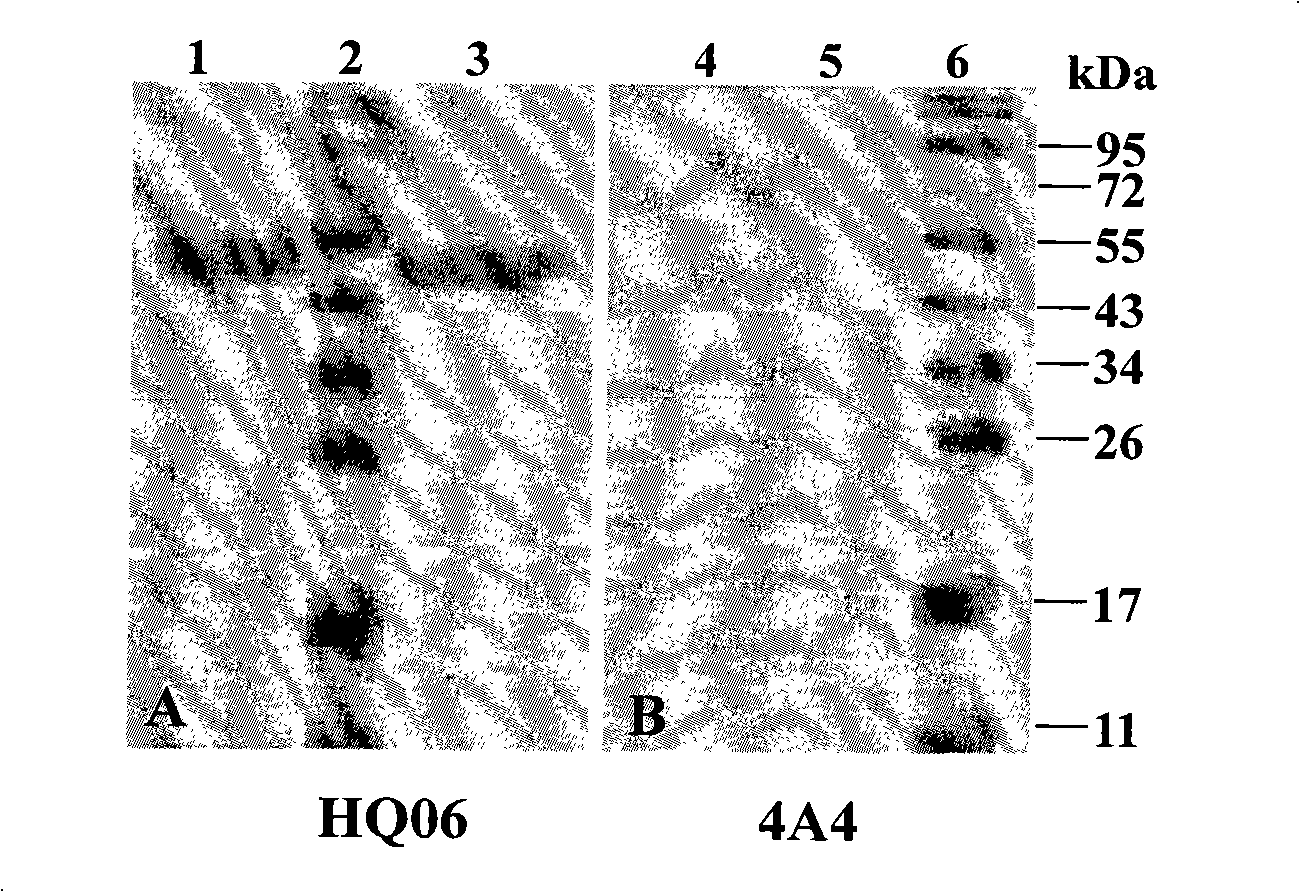
Recombinant Salmonella choleraesuis for expressing surface antigen gene sao of streptococcus suis type 2, vaccine and application
ActiveCN101979501AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesAntigen
The invention belongs to the field of animal bacterium gene engineering, and in particular relates to construction of resistance marker-free recombinant Salmonella choleraesuis for expressing surface antigen sao gene segment of streptococcus suis type 2, preparation of a vaccine and application. The resistance marker-free recombinant Salmonella choleraesuis for expressing the surface antigen sao gene segment of the streptococcus suis type 2, namely asd-C500 / Pya-saoA is obtained, and the collection number is CCTCC NO: M2010156. The asd gene of the Salmonella choleraesuis is deleted in the recombinant strain, and the recombinant strain contains plasmid pYA-saoA capable of expressing the asd and the sao gene segment of the streptococcus suis type 2. The invention also discloses a method for preparing the recombinant strain and the vaccine, and application in preparing Salmonella choleraesuis-streptococcus suis type 2 vaccines.
Owner:HUAZHONG AGRI UNIV +1
Recombinant salmonella choleraesuis, bivalent genetic engineering vaccine and application
InactiveCN101880647AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacteriaBacteroidesRecombinant vaccines
The invention belongs to the technical field of bacterial gene engineering of animals, and in particular relates to the construction of a recombinant salmonella choleraesuis strain which does not contain resistance markers and expresses major antigenic loci of porcine transmissible gastroenteritis virus, vaccine preparation and application. In the recombinant salmonella choleraesuis strain C500 (pYA-2SLN) which does not contain the resistance markers and expresses the major antigenic loci of the porcine transmissible gastroenteritis virus, the preservation No. is CCTCC NO: M 209189, and the strain misses asd genes which are necessary for the growth of salmonella choleraesuis and contains plasmid capable of expressing the asd genes, genes of an antigenic locus A, an antigenic locus D and an antigenic locus N321 of the porcine transmissible gastroenteritis virus in the strain. The invention also discloses a method for preparing the salmonella choleraesuis and porcine transmissible gastroenteritis vaccine by utilizing the recombinant strain and application thereof. The prepared recombinant vaccine can stimulate pigs to generate protective immune response for resisting the salmonella choleraesuis and the porcine transmissible gastroenteritis virus, and prevent the infection of the salmonella choleraesuis and the porcine transmissible gastroenteritis virus effectively.
Owner:HUAZHONG AGRI UNIV
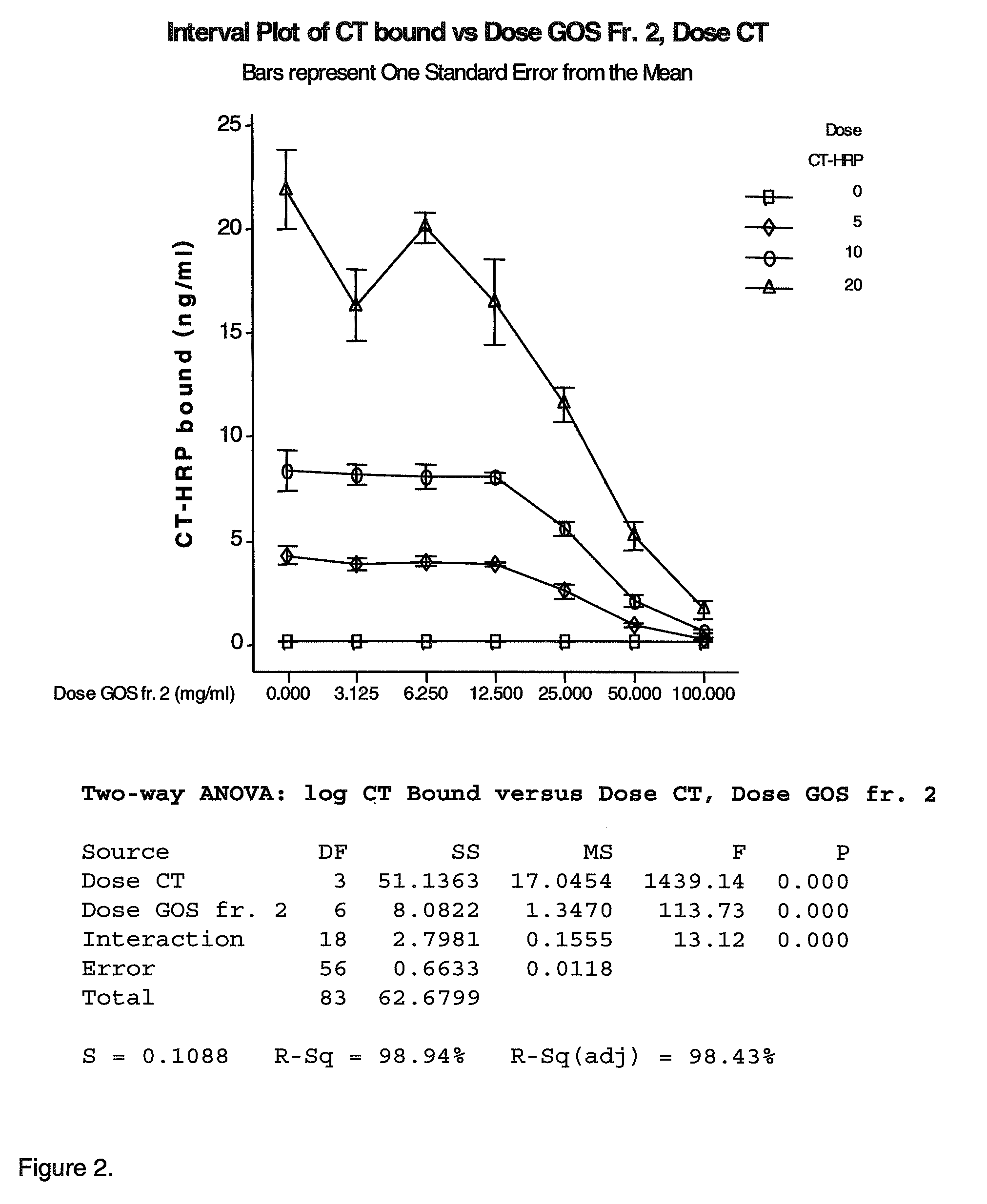
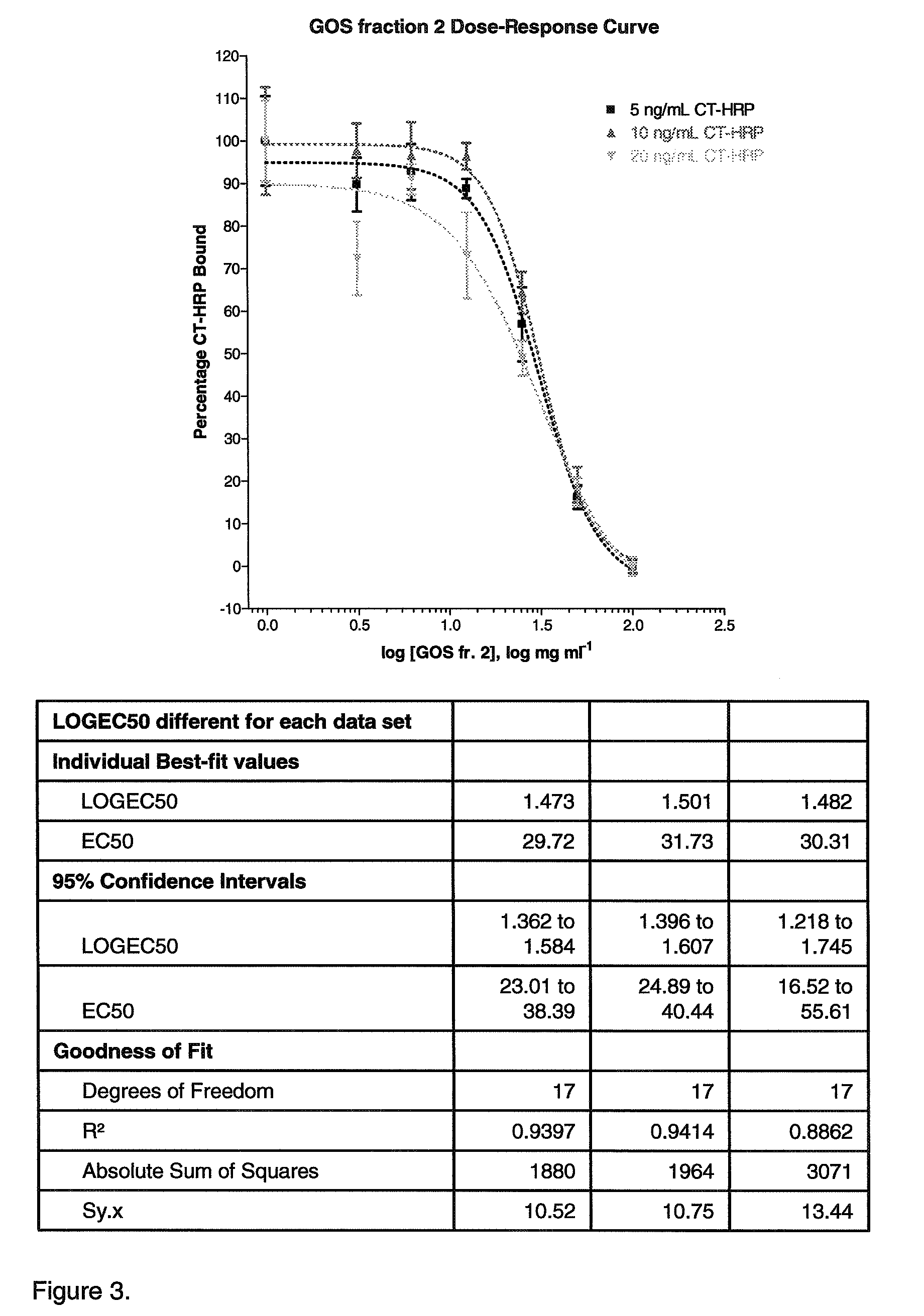
Inhibition of cholera toxins by galatooligosaccharides (GOS)
InactiveUS8202842B2Effective in prophylacticEffective inhibitorBiocideSugar derivativesDiseaseMedicine
The invention relates to nutritional and pharmaceutical compositions comprising non-digestible galactooligosaccharides (GOS) and uses thereof. In particular, it relates to the use of GOS species in preventing or treating disease caused by bacterial toxins. Provided is the use of GOS having a polymerization degree of 5 or higher, preferably 6 or higher, for the manufacture of a nutritional or pharmaceutical composition for the treatment or prevention of an acute or chronic disease associated with or caused by the adhesion and / or uptake of a cholera toxin family member. Also provided is a method for providing a GOS fraction capable of inhibiting cholera toxin (Ctx) binding to GM1 and fractions obtainable thereby.
Owner:FRIESLAND BRANDS BV
Preparation method of room air virus pathogenic-bacteria jinx
ActiveCN101491243AResolve inhibitionSolving the Killing ProblemBiocideGaseous substancesBacterial virusStaphylococcus aureus
The invention discloses a method for preparing an indoor air virus pathogen invincible opponent. The indoor air virus pathogen invincible opponent prepared by menthol, thymol, weeping forsythia oil and radix bupleuri oil has the killing rate of between 76 and 99.9 percent to idemic encephalitis, influenza viruses, hand-foot-and-mouth disease, avian influenza, legionnella, pneumoniae, hepatitis C viruses, hepatitis A viruses, Staphylococcus albus, Staphylococcus aureus, Klebsiella pneumoniae, Candida albicans, typhoid viruses, Escherichia coli, cholera viruses, infectious germs and bacteria, and harmful germs and bacteria. The indoor air virus pathogen invincible opponent is mainly applied to rooms, schools, hospitals, hotels, air conditioners, automobiles, public places, and the like, and has obvious effect on the control of diseases such as influenza, upper respiratory tract infection. The indoor air virus pathogen invincible opponent has outstanding substantive characteristics and broad application prospect.
Owner:上海钮爱环保科技有限公司
Traditional Chinese medicine feed additive for preventing and treating hog cholera
InactiveCN102771631AHeat-clearing and detoxifyingNo side effectsAnimal feeding stuffAntiviralsTreatment effectSide effect
The invention relates to a traditional Chinese medicine feed additive for preventing and treating hog cholera. The traditional Chinese medicine feed additive is characterized in that the following traditional medicine raw materials in parts by weight are selected: 150 parts of purple perilla, 50 parts of pinellia ternate, 180 parts of isatis root, 100 parts of platycodon grandiflorum, 80 parts of radix bupleuri and 100 parts of corydalis tuber. A preparation method of the feed additive comprises the following steps that 300 parts of radix saposhnikoviae, 150 parts of schizonepeta, 150 parts of isatis root, 150 parts of fructus forsythiae, 100 parts of radix bupleuri and 100 parts of burdock are adopted, the materials are mixed and then crushed, or are mixed after being respectively crushed, and the materials are packed. A use method comprises the following steps that the traditional Chinese medicine feed additive is fed in a mixed way according to a ratio that the feed: additive=100:(0.6-1.2). The raw material medicine adopted by the traditional Chinese medicine feed additive has the effects of clearing away the heat and the toxic materials and dispelling wind and heat, and has the beneficial effects of safety, no toxic or side effect and obvious and reliable treatment effect on hog cholera.
Owner:王志坚
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com