Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
A monoclonal antibody and swine fever virus technology, applied in hybridoma cell lines, the application of monoclonal antibodies in clinical diagnosis of swine fever, the field of monoclonal antibodies, can solve the problems of differentiation, limited application, difficult natural infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 monoclonal antibody
[0021] 1. Purification and detection of recombinant protein
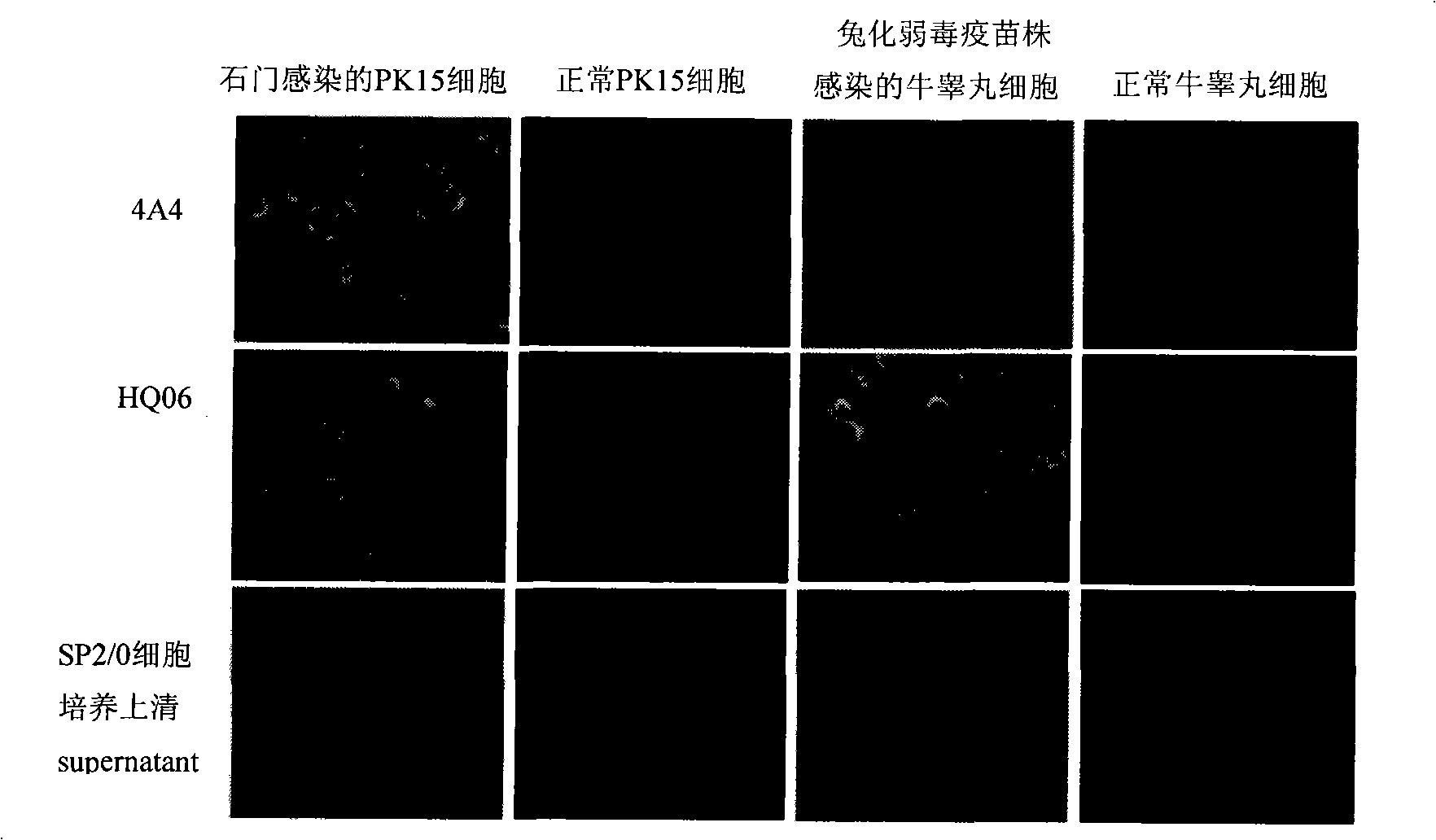
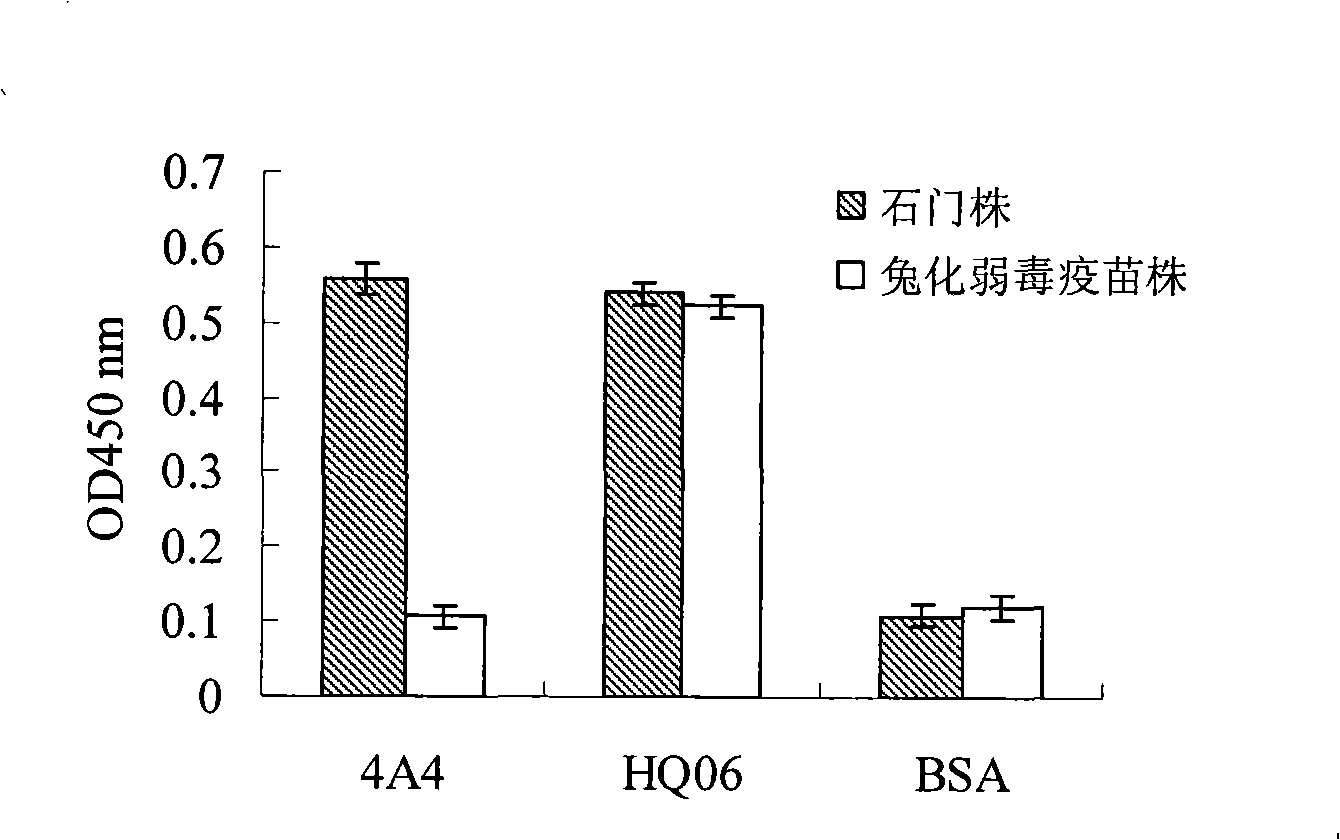
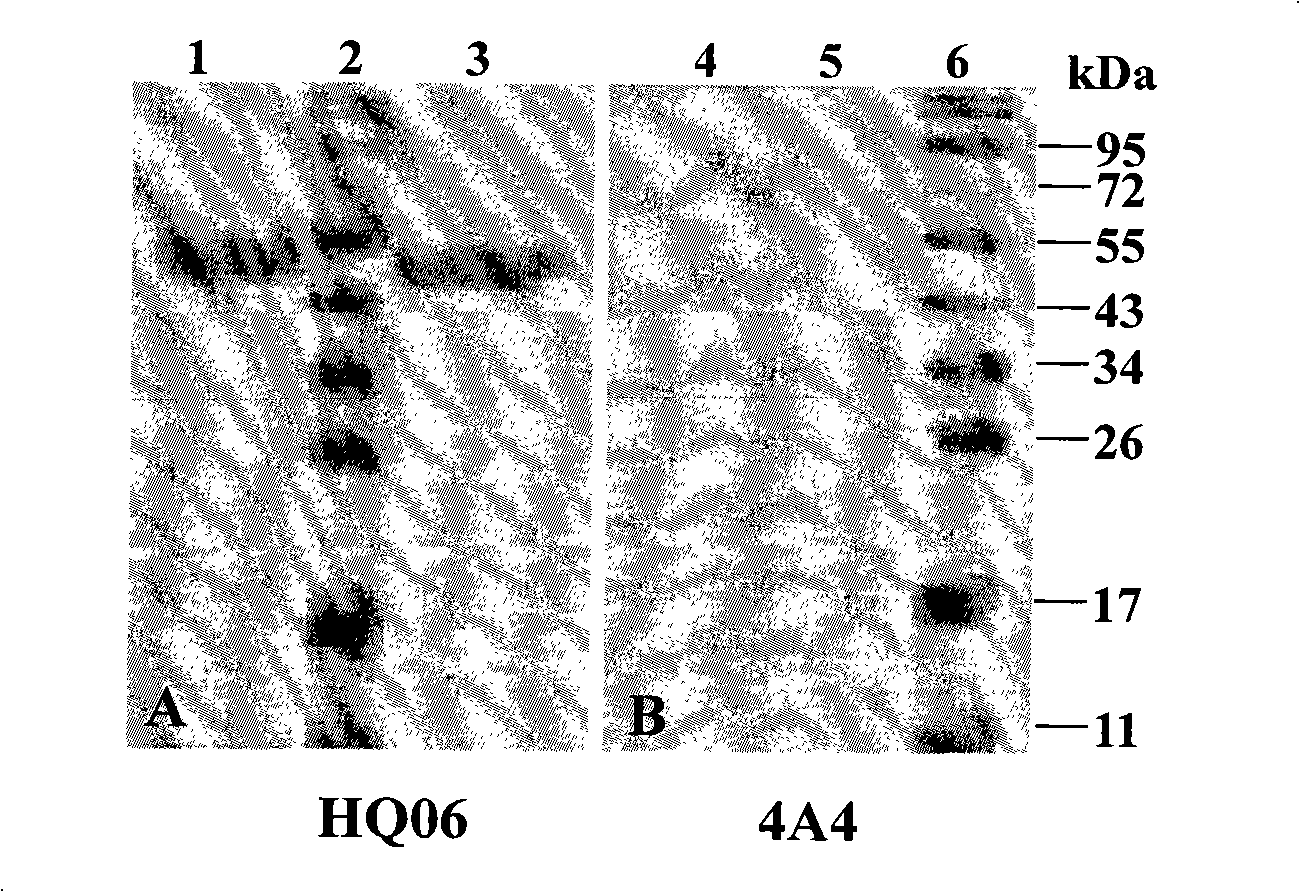
[0022] Sf9 cells in the logarithmic growth phase were infected with the 4th generation recombinant baculovirus rAcV-Shimen-E2 and rAcV-HCLV-E2 respectively, cultured at 27°C for 96h, and the supernatant of the cell culture medium was collected, centrifuged at 3000r / min, 4°C for 10min , to remove cell debris; then put the supernatant in a dialysis bag and concentrate it by embedding with PEG 8000; the concentrated supernatant was placed in Ni 2+ Ion affinity chromatography equilibrium solution (50mmol / L Na 3 PO 4 , 500mmol / LNaCl, pH7.0) and dialyzed at 4°C overnight. Then mix the dialyzed supernatant with the balanced resin, gently shake at 4°C for 1 hour, elute with 250mmol / L imidazole, collect the eluate, conduct SDS-PAGE electrophoresis analysis, and then transfer the target protein On the nitrocellulose membrane, with the anti-E2 protein monoclonal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com