Monoclonal antibody ZK7C3 and application
An antibody and sequence listing technology, applied in applications, antibodies, antiviral agents, etc., can solve problems such as aggravating the disease, increasing the virus entry, and aggravating the severity of the disease, and achieves far-reaching social significance and significant application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the discovery of antibody
[0041] 1. Protein preparation
[0042] 1. Construction of recombinant plasmids
[0043] (1) Synthesize the double-stranded DNA molecule shown in Sequence 2 of the Sequence Listing.
[0044] The double-stranded DNA molecule shown in sequence 2 of the sequence listing encodes the protein shown in sequence 1 of the sequence listing, wherein the open reading frame is nucleotides 13-1296 from the 5' end of sequence 2.
[0045]In sequence 1 of the sequence listing, the 1st to 19th amino acid residues from the N-terminal form the signal peptide, the 20th to 421st amino acid residues form the E protein of Zika virus, and the 422nd to 427th amino acid residues form the His 6 Label. Name the protein shown in Sequence 1 of the sequence listing as E-His 6 Fusion protein, expected molecular weight 50kDa.
[0046] (2) Double-digest the double-stranded DNA molecule obtained in step 1 with restriction endonucleases BamHI and NotI, and reco...
Embodiment 2
[0074] Example 2, Preparation of ZK7C3 Antibody
[0075] 1. Construction of recombinant plasmids
[0076] The double-stranded DNA molecule shown in sequence 4 of the sequence listing is inserted into the PMD18T vector to obtain a heavy chain expression vector. In sequence 4 of the sequence listing, nucleotides 1 to 888 form the CMV promoter, nucleotides 889 to 2301 encode the full-length heavy chain shown in sequence 3 of the sequence listing, nucleotides 2354 to 2499 for the ployA fragment.
[0077] The double-stranded DNA molecule shown in sequence 6 of the sequence listing is inserted into the PMD18T vector to obtain a light chain expression vector. In sequence 6 of the sequence listing, the 1st to 888th nucleotides constitute the CMV promoter, the 889th to 1590th nucleotides encode the full-length light chain shown in sequence 5 of the sequence listing, and the 1591st to 1736th nucleotides for the ployA fragment.
[0078] 2. Antibody preparation
[0079] 1. The heavy ...
Embodiment 3
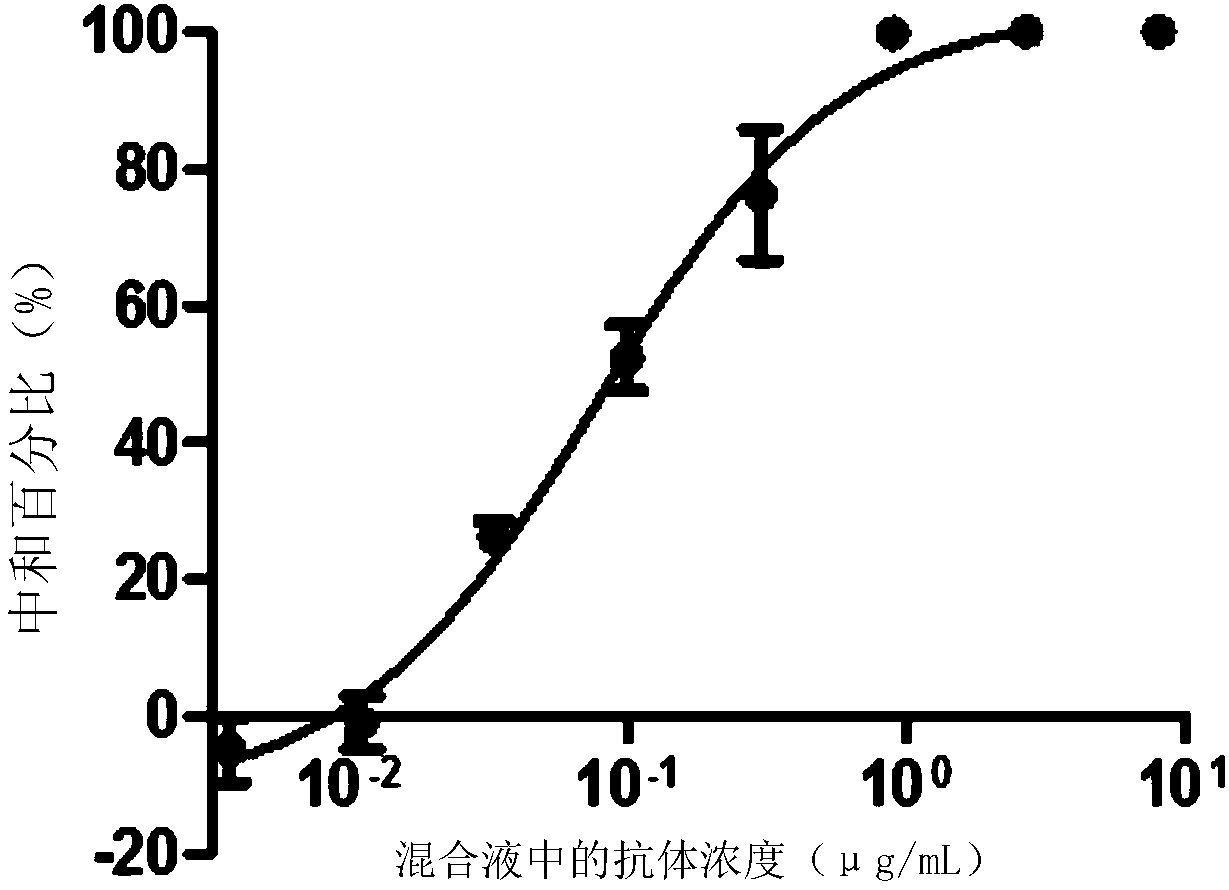
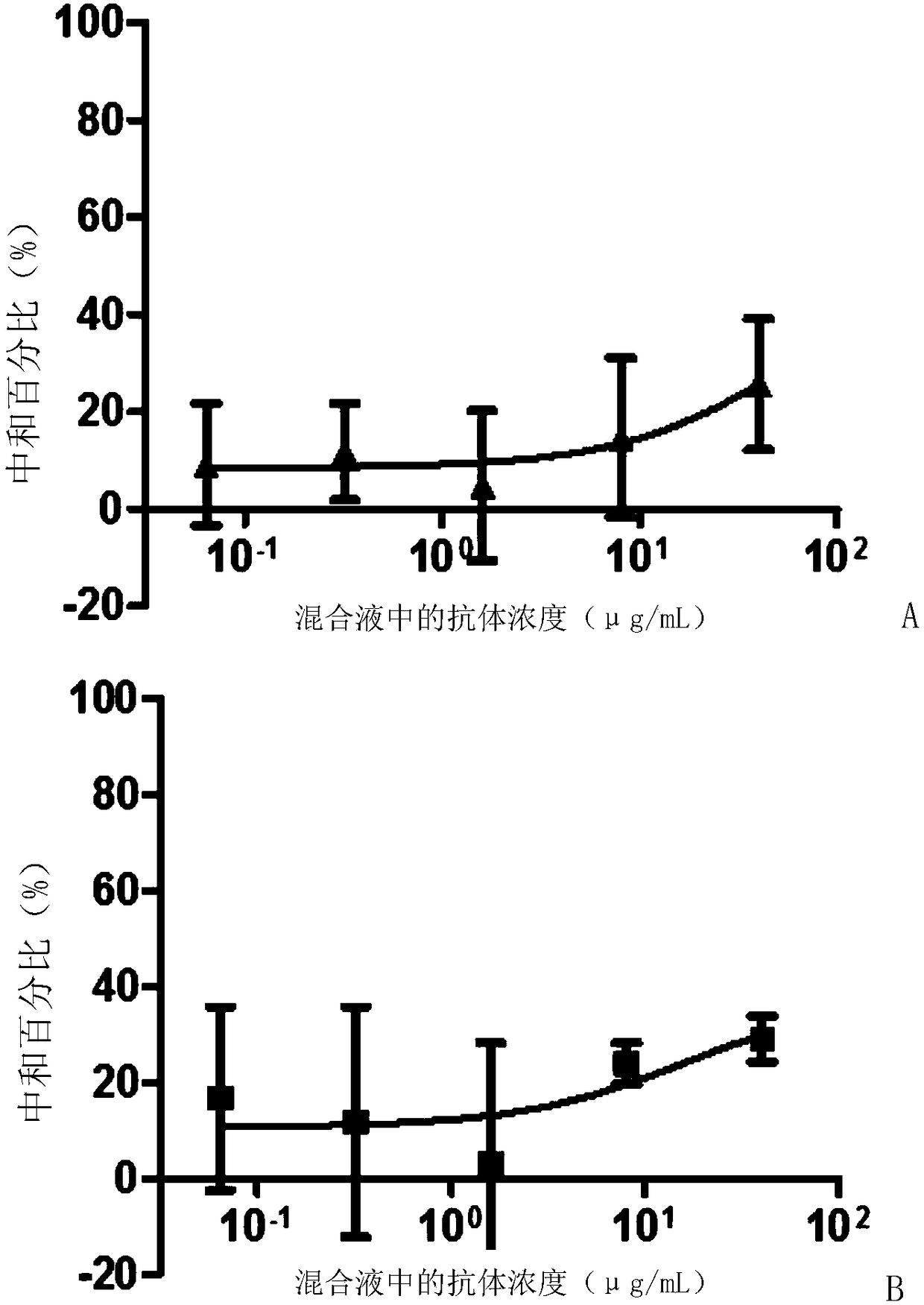
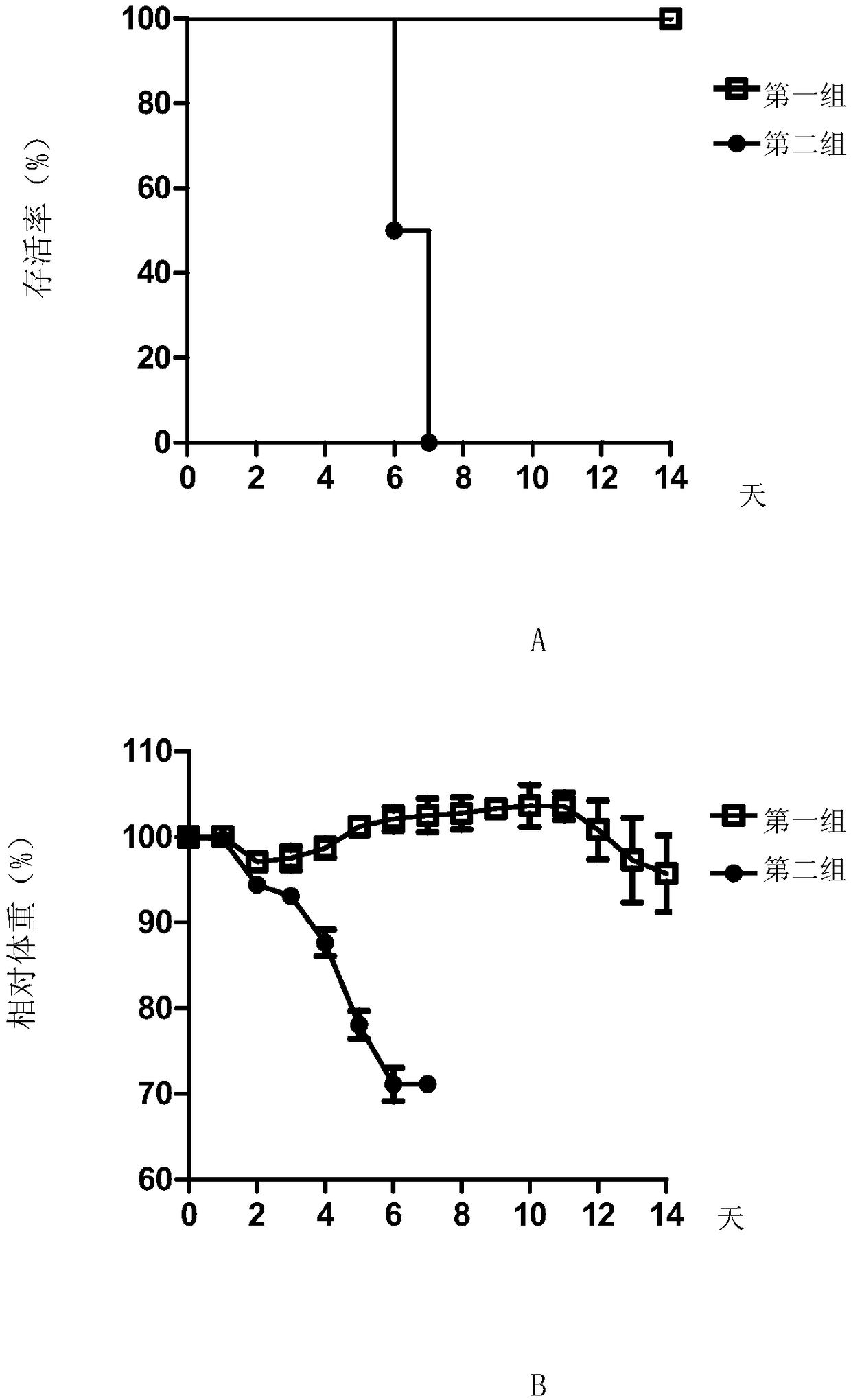
[0087] Embodiment 3, the neutralizing activity of ZK7C3 antibody to virus
[0088] 1. Preparation of virus
[0089] Zika virus was inoculated into C6 / 36 cells (using DMEM medium containing 10% FBS), placed at 28°C, 5% CO 2 Under the condition of static culture for 4 days, then centrifuged at 3000rpm for 5min, the supernatant was collected, which was the Zika virus liquid.
[0090] 2. Detection of neutralizing activity of monoclonal antibodies
[0091] 1. Take the ZK7C3 solution prepared in Example 2 and dilute it with PBS buffer (pH7.2, 10mM) to obtain antibody dilution.
[0092] 2. Inoculate Vero cells into a six-well plate (4 × 10 per well5 cells), cultured overnight until the cell density reached 90% and evenly covered the well plate.
[0093] 3. Mix the Zika virus solution prepared in step 1 with the antibody dilution prepared in step 1 to obtain each mixed solution (in each milliliter of mixed solution, the virus content is 100 pfu, and the antibody concentration is 8 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com