Patents
Literature
124 results about "West Nile virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The West Nile virus is spread by insects, most often mosquitoes. The West Nile virus can infect humans, birds, mosquitoes, horses, and some other mammals.
Screening for west nile virus antiviral therapy
InactiveUS20050058987A1Improve efficiencySsRNA viruses positive-senseVectorsHigh-Throughput Screening MethodsImmunogenicity
The instant invention provides stable and novel lineage I WNV reverse genetics systems, and methods for making the reverse genetics systems, specifically, a fully-infectious lineage I WNV cDNA or replicon system engineered with one or more nucleotide sequences each encoding a reporter gene to be used in high throughput cell-based screening assays for the identification of novel antiflaviviral chemotherapeutics and / or vaccines effective to treat and / or immunize against infections by WNV and other emerging flaviviruses, such as, for example, JEV, SLEV, AV, KV, JV, CV, YV, TBEV, DENV-1, DENV-2, DENV-3, DENV-4, YFV and MVEV. The present invention further provides methods of high throughput screening of antiflaviviral compounds or improved derivatives thereof using novel lineage I WNV reverse genetics systems and / or cell lines stably containing the reverse genetics systems. Also, the invention provides novel pharmaceutical compositions comprising an attenuated lineage I WNV that is less virulent but similarly immunogenic as the parent WNV and is capable of providing a protective immune response in a host.
Owner:HEALTH RES INC
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
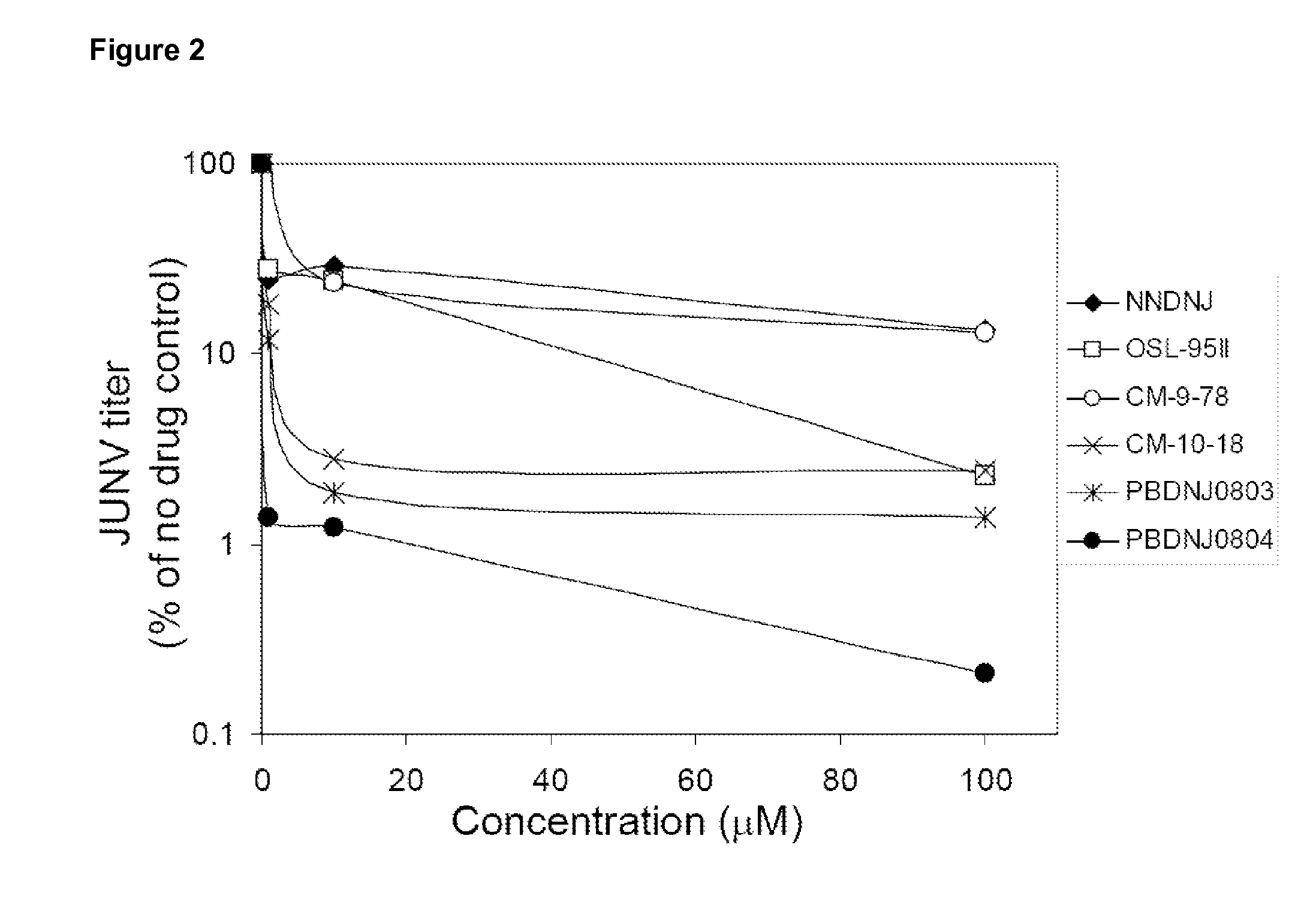
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
Diagnostic test for west nile virus
ActiveUS20060115896A1More sensitiveEasy to useAnimal cellsMicrobiological testing/measurementDiagnostic testFlavivirus
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result. The invention also provides monoclonal antibodies against WNV NS5 and DENV NS5 antigen and their use in detecting WNV and DENV infections in a biological sample.
Owner:HEALTH RES INC
Recombinant vaccine against West Nile Virus
ActiveUS20050255127A1Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD
Vaccines Against Japanese Encephalitis Virus and West Nile Virus
ActiveUS20070269458A1Decrease viscerotropism/viremiaHigh genetic stabilitySsRNA viruses positive-senseSugar derivativesViral VaccineWest Nile virus RNA
The invention provides attenuated Flavivirus vaccines, such as vaccines against Japanese encephalitis virus and West Nile virus, as well as methods of making and using these vaccines.
Owner:SANOFI PASTEUR BIOLOGICS CO
Modified nucleosides for the treatment of viral infections and abnormal cellular proliferation
The disclosed invention is a composition for and a method of treating a Flaviviridae (including BVDV and HCV), Orthomyxoviridae (including Influenza A and B) or Paramyxoviridae (including RSV) infection, or conditions related to abnormal cellular proliferation, in a host, including animals, and especially humans, using a nucleoside of general formula (I)-(XXIII) or its pharmaceutically acceptable salt or prodrug.This invention also provides an effective process to quantify the viral load, and in particular BVDV, HCV or West Nile Virus load, in a host, using real-time polymerase chain reaction (“RT-PCR”). Additionally, the invention discloses probe molecules that can fluoresce proportionally to the amount of virus present in a sample.
Owner:GILEAD SCI INC
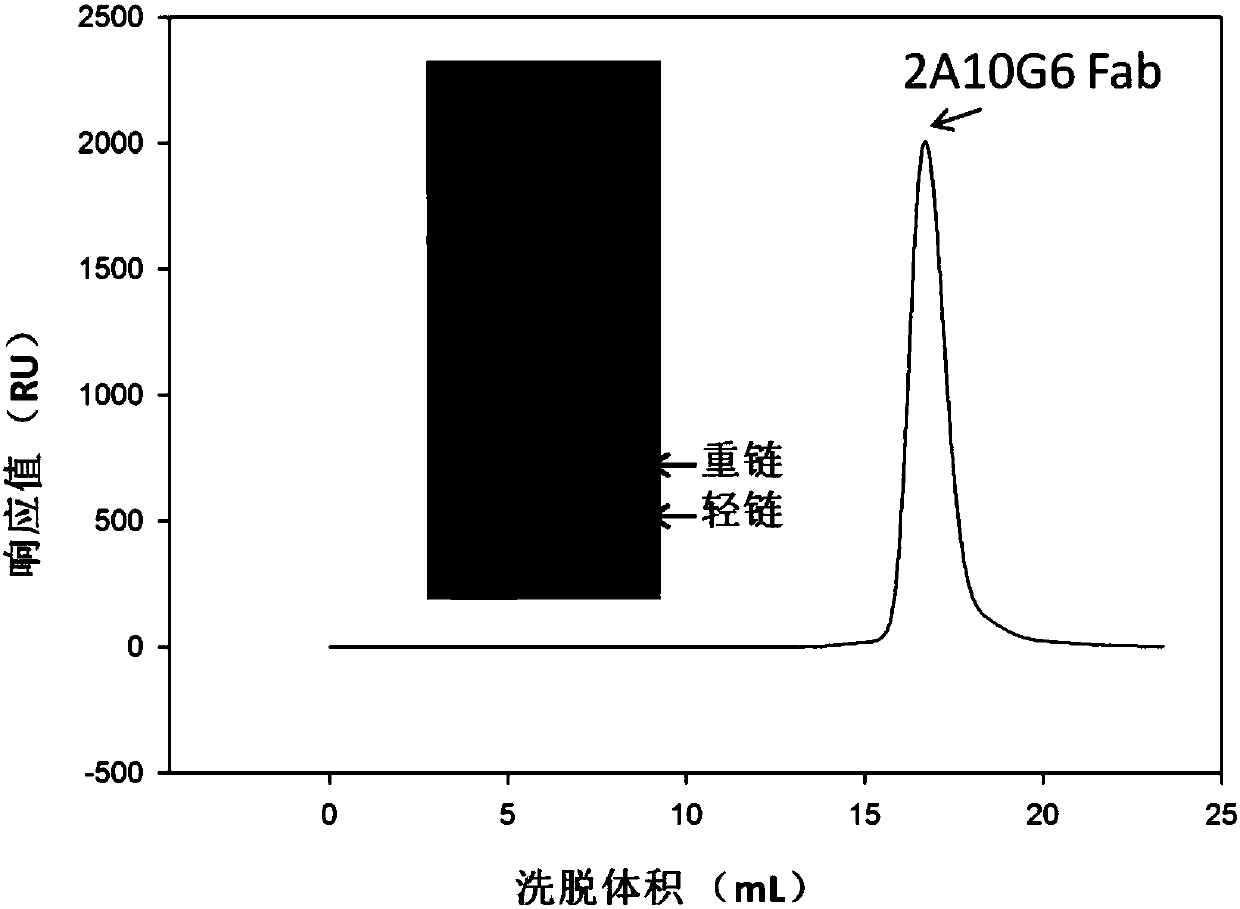
Humanized monoclonal antibody and application thereof
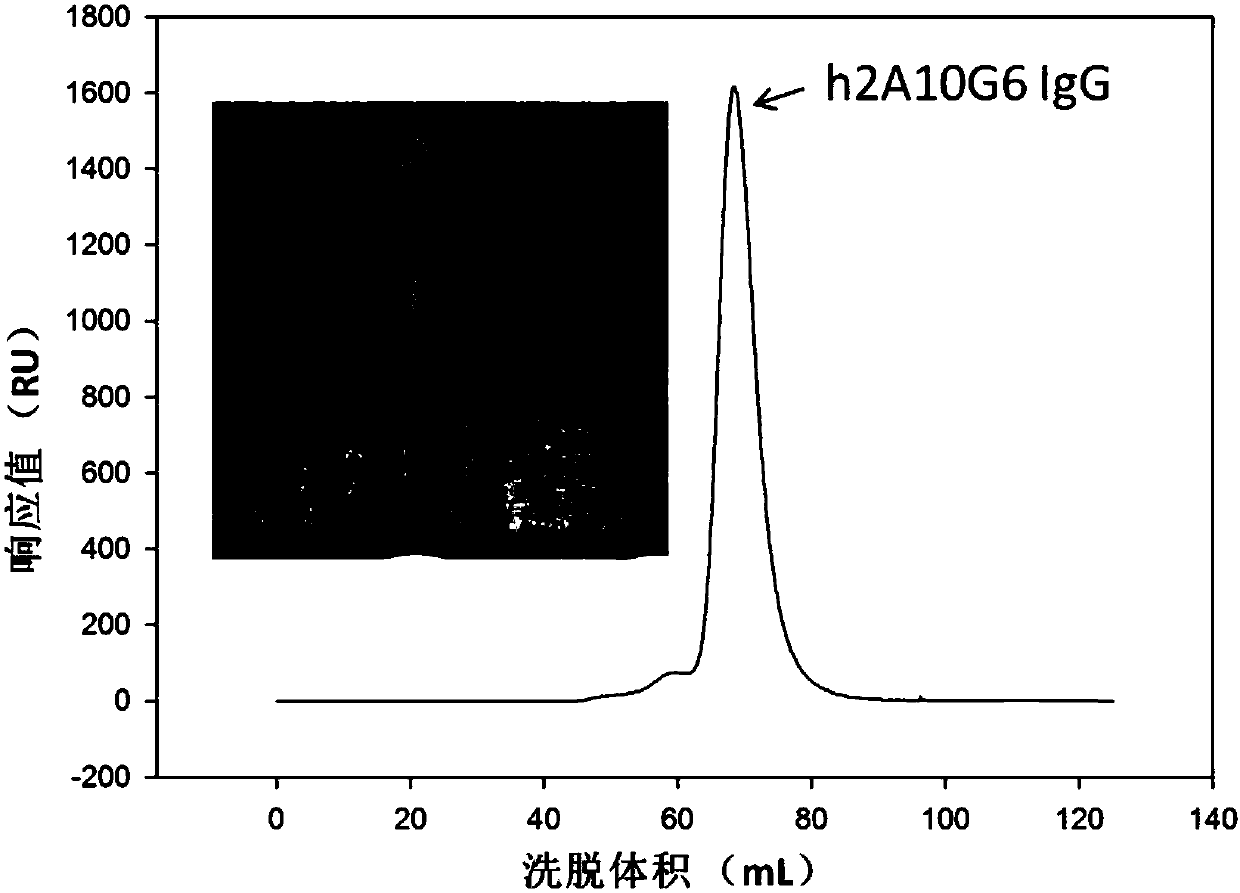
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
West nile vaccine
InactiveUS7153513B2Safe and effectiveSuitable for useAntibacterial agentsSsRNA viruses negative-senseEquidaeDisease
The present invention provides a safe and effective vaccine composition against West Nile virus disease. An immunogenically active component of West Nile virus or plasmid DNA, an adjuvant such as a metabolizable oil, and a pharmacologically acceptable carrier are formulated into an immunizing vaccine. The invention also provides a method for the prevention or amelioration of West Nile disease, such as encephalitis, in equidae by administering the vaccine composition herein set forth.
Owner:ZOETIS SERVICE LLC
Compositions enriched in phenolic compounds and methods for producing the same
Provided are processes for the preparation of compositions enriched in phenolic compounds from a crude plant extract. One process includes a novel column purification step using a polymer resin that releasably adsorbs the phenolic compounds but does not retain polar non-phenolic compounds, wherein the resin comprises aromatic rings substituted with one or more electron-withdrawing groups. This invention also includes compositions enriched in phenolic compounds. This invention encompasses methods of using the phenolic-enriched compositions for treating warm-blooded animals, including humans, infected with paramyxovaridae such as respiratory syncytial virus, orthomyoxovaridae such as influenza A, B, and C, parainfluenza, Herpes viruses such as HSV-1 and HSV-2, and Flaviviruses such as West Nile Virus, and for treating inflammation such as caused by arthritis, stress and digestive disease. The compositions are also useful as meat additives to inhibit food-borne pathogens.
Owner:PHENOLICS LLC
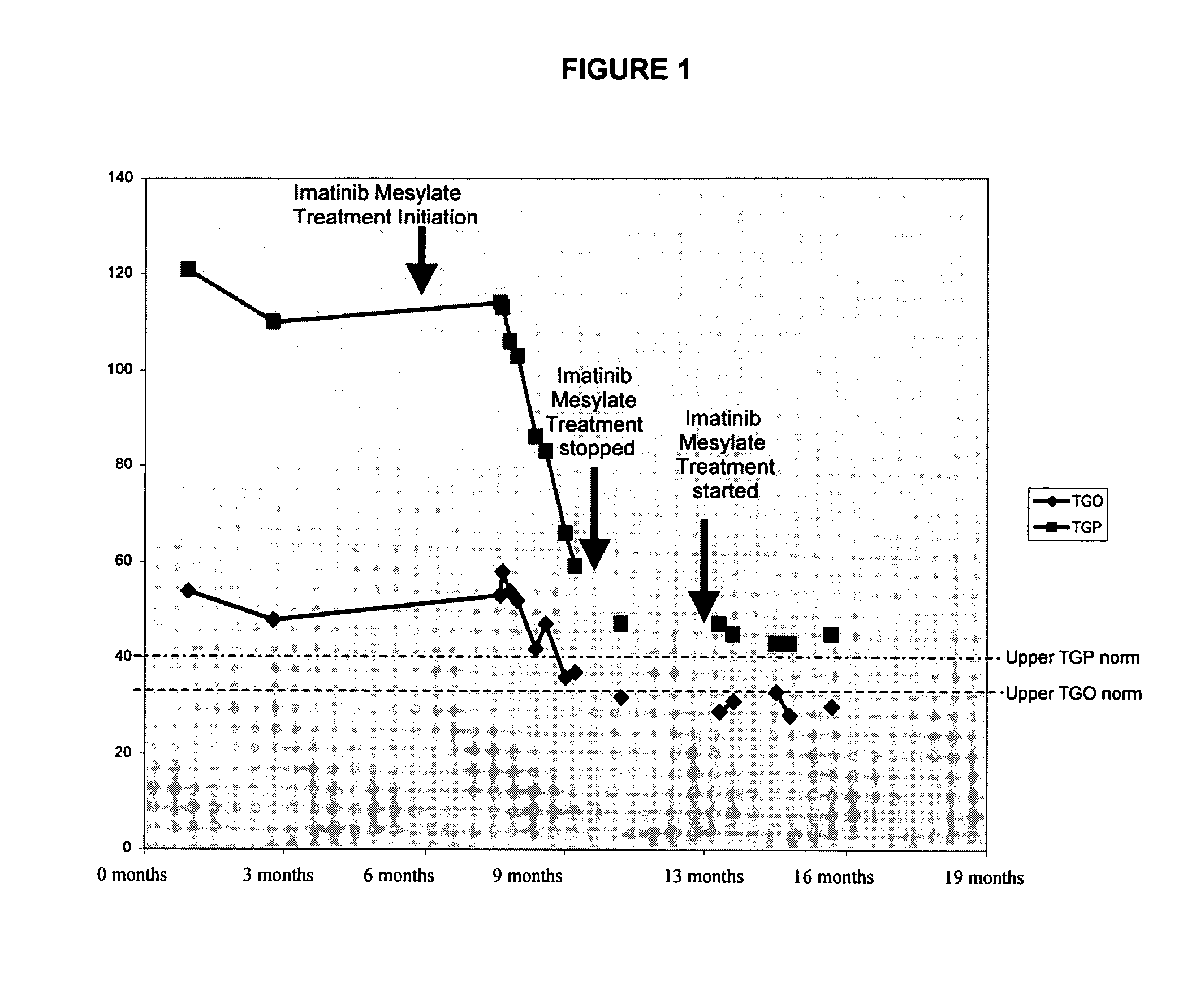
Use of imatinib to treat liver disorders and viral infections
The present invention relates to the use of imatinib for treating viral liver diseases and in particular for viral hepatitis. The invention provides the use of imatinib for inhibiting replication, transmission or both of hepatitis viruses. The invention further relates to the use of imatinib for inhibiting replication, transmission or both of other viruses including herpes virus, poxvirus, influenza virus, para influenza virus, respiratory syncytial virus, rhinovirus, yellow fever virus, west nile virus, and encephalitis virus.
Owner:BIONICHE LIFE SCI
Efficient method for producing compositions enriched in total phenols
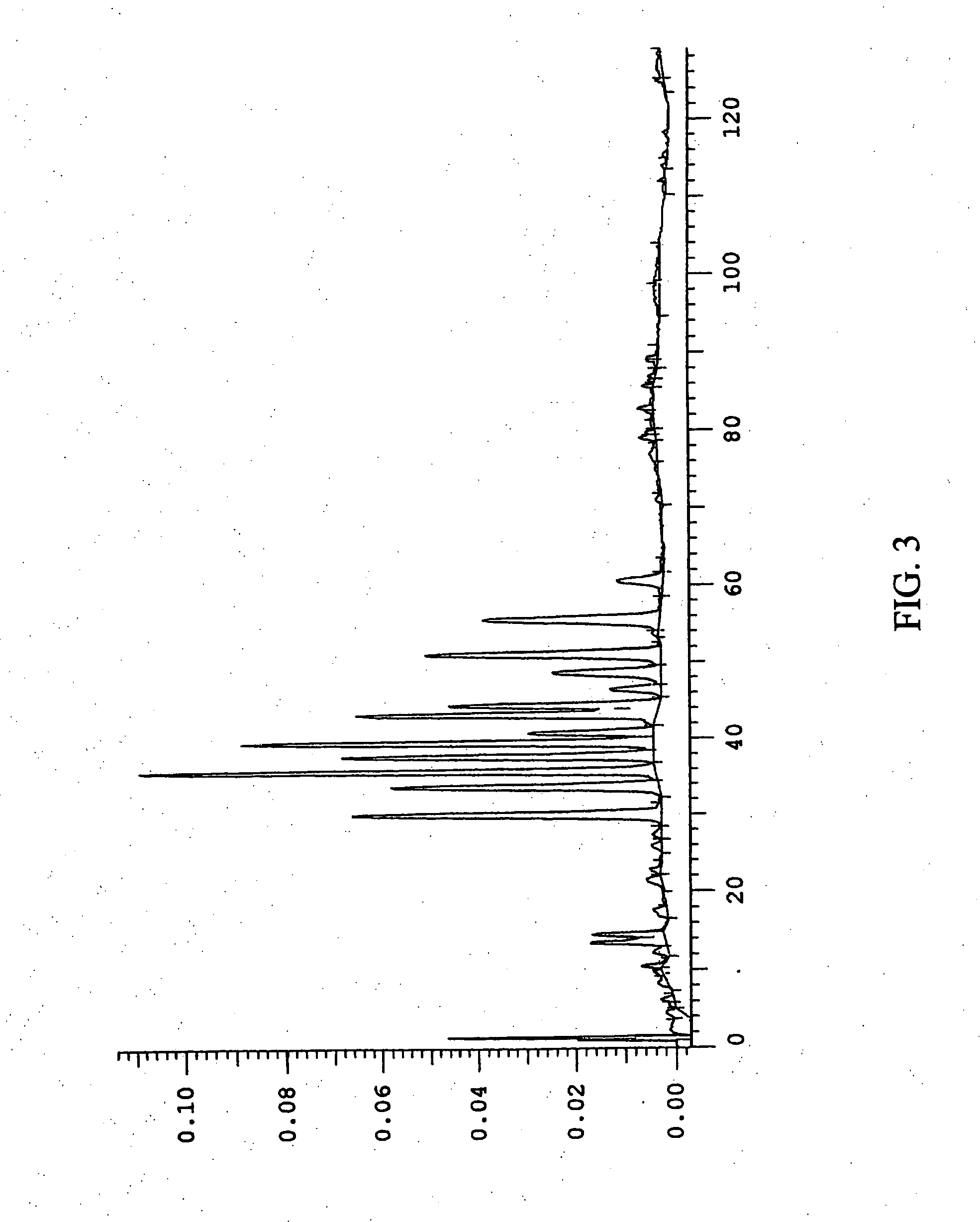
This invention provides a process for the preparation of compositions enriched in total phenols from a crude plant extract. The process includes a novel column purification step using a brominated polystyrene resin. This invention also includes compositions enriched in total phenols. The enriched compositions are characterized as containing monomeric, oligomeric and polymeric phenols and having HPLC chromatograms substantially as set forth in FIGS. 10-13. This invention encompasses methods of using the total phenol-enriched compositions for treating warm-blooded animals, including humans, infected with paramyxovaridae such as respiratory syncytial virus, orthomyoxovaridae such as influenza A, B, and C, parainfluenza, Herpes viruses such as HSV-1 and HSV-2, and Flaviviruses such as West Nile Virus, and for treating inflammation such as caused by arthritis, stress and digestive disease.
Owner:PHENOLICS LLC
Infectious DNA as a vaccine against west nile and other flavivirues
InactiveUS20050276816A1Easy to prepareStable and safe vaccineSsRNA viruses positive-senseViral antigen ingredientsDna encodingInfectious agent
A vaccine for West Nile virus that protects a subject against West Nile infection comprising an a pharmaceutically acceptable carrier and a therapeutically effective does of an infectious agent selected from the group consisting of: a live attenuated infectious (+) RNA virus designated as WN1415, a vector comprising infectious DNA encoding an infectious (+) RNA molecule encoding the West Nile virus, and the West Nile (+) RNA virus designated as WN956D117B3 (GenBank #M12294).
Owner:UNIVERSITY OF KANSAS
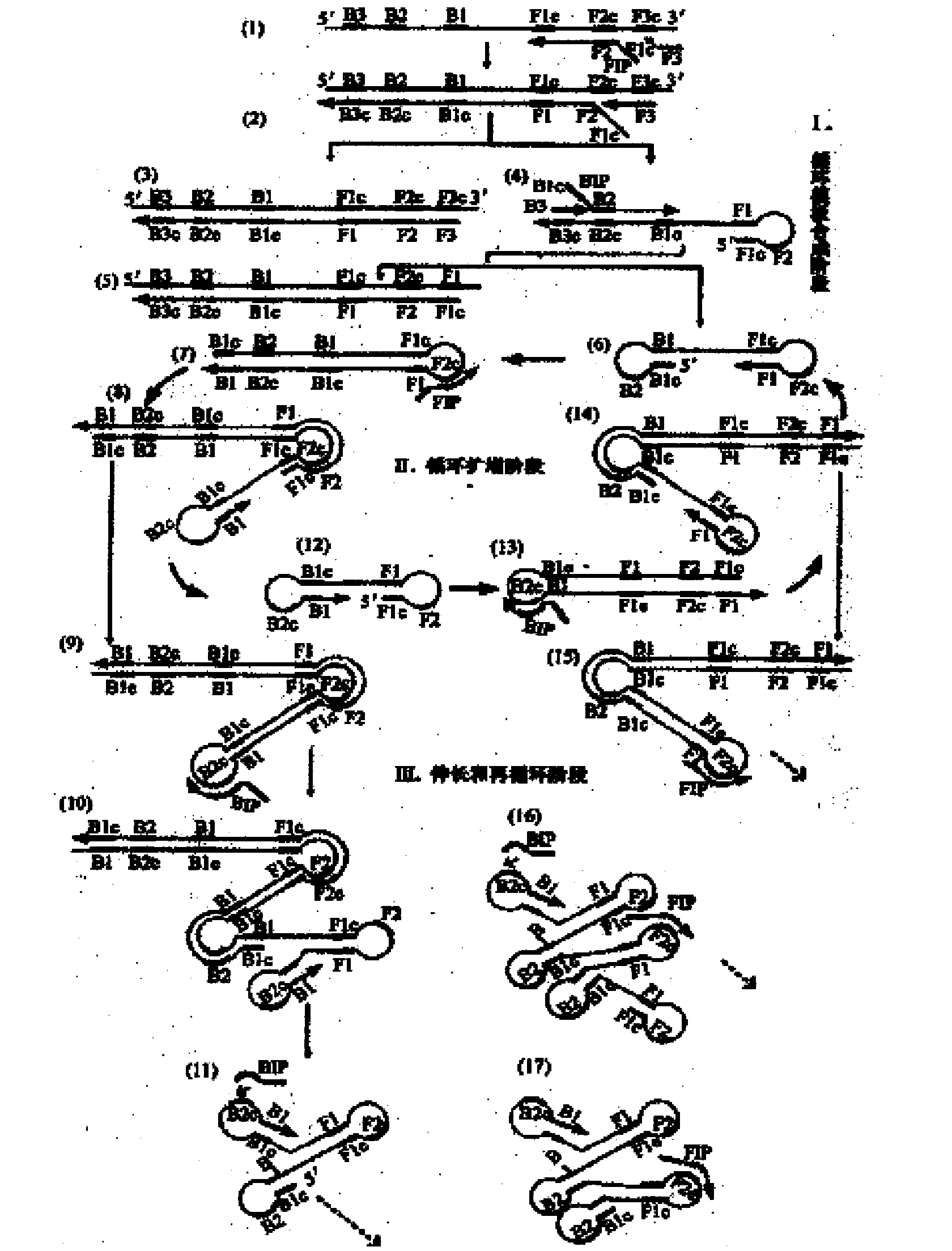
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Kit for detecting mosquito borne pathogens and detection method thereof
InactiveCN103911463AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic encephalitisMultiplex polymerase chain reaction
The invention discloses a kit for detecting a plurality of mosquito borne pathogens and a detection method thereof. The plurality of mosquito-borne infectious pathogens are detected by using multiplex polymerase chain reaction (PCR) combined with a liquid chip, and a yellow fever virus (YFV), dengue fever viruses (DV) I-IV type, epidemic encephalitis B (japanese encephalitis) virus (JEV), plasmodium (Plasmodium), (including plasmodium vivax (PV), plasmodium falciparum (PF), plasmodium malariae (PM), plasmodium ovale (PO)), a west nile virus (MNV) and a chikungunya virus (CHIKV) can be detected in parallel one time. The kit has the advantages of being large in flux, high in specificity and sensitivity, stable in result, good in repeatability, simple to operate and fast in detection speed.
Owner:河北国际旅行卫生保健中心
Recombinant vaccine against West Nile Virus
InactiveUS20050031641A1Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD A CO LTD BY SHARES +1
Detection of West Nile Virus
A method and device for detecting a West Nile Virus (WNV) infection including contacting a biological sample from a subject with an anti-IgM antibody and a recombinant WNV E polypeptide and detecting whether the polypeptide substantially binds to an antibody in the sample. The invention also includes a method and device for determining whether an animal is infected with West Nile Virus (WNV), or is either not infected or is vaccinated with a WNV vaccine.
Owner:IDEXX LABORATORIES
Infectious DNA as a vaccine against west nile and other flaviviruses
InactiveUS7459163B2High infection rateReduce mortalitySsRNA viruses positive-senseGenetic material ingredientsTGE VACCINERNA virus
A vaccine for West Nile virus that protects a subject against West Nile infection comprising an a pharmaceutically acceptable carrier and a therapeutically effective dose of an infectious agent selected from the group consisting of: a live attenuated infectious (+) RNA virus designated as WN1415, a vector comprising infectious DNA encoding an infectious (+) RNA molecule encoding the West Nile virus, and the West Nile (+) RNA virus designated as WN956D117B3 (GenBank #M12294).
Owner:UNIVERSITY OF KANSAS
Production of a Monoclonal Antibody Therapeutic Against West Nile Virus in Plants
InactiveUS20120329994A1Rapid productionMaintain good propertiesHybrid immunoglobulinsAntibody ingredientsTherapeutic antibodyAnti-West Nile virus IgG
The present invention describes the plant-based production of a therapeutic antibody against West Nile Virus.
Owner:ARIZONA STATE UNIVERSITY
Binding molecules capable of neutralizing west nile virus and uses thereof
ActiveUS20070122801A1Enhance phagocytosisAssisting in the lysis of WNVPeptide librariesSsRNA viruses positive-senseWest Nile virus RNAPost-exposure prophylaxis
The invention provides human binding molecules specifically binding to West Nile virus and having West Nile virus neutralizing activity, nucleic acid molecules encoding the human binding molecules, compositions comprising the human binding molecules and methods of identifying or producing the human binding molecules. The human binding molecules can be used in the diagnosis, post-exposure prophylaxis and / or treatment of a condition resulting from West Nile virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Multiple polymerase chain reaction (PCR) kit and method for detecting mosquito-borne pathogens
InactiveCN101979665AReliable informationEffective early warning dataMicrobiological testing/measurementAgainst vector-borne diseasesTissue fluidMultiplex pcrs
The invention provides a multiple polymerase chain reaction (PCR) kit for detecting mosquito-borne pathogens. The kit comprises six pairs of specific primers. The invention also provides a method for detecting the mosquito-borne pathogens. Electrophoresis is performed on a PCR-amplified product. Whether pathogens, such as encephalitis B virus, dengue fever virus, yellow fever virus, plasmodium falciparum, plasmodium vivax, plasmodium knowlesi, plasmodium ovale, plasmodium malariae, wuchereria malayi, wuchereria bancrofti and the like, exists or not is detected and identified according to the length of a PCR-amplified fragment. By the method, various reported mosquito-borne pathogens, and the yellow fever virus and west nile virus which come from other countries can be detected quickly, accurately and sensitively at the same time, and can be applied to the detection of various samples, such as mosquitoes, blood of patients, tissue fluid and the like. The invention provides a low-cost and high-efficient method for early monitoring and finding mosquito-borne disease prevalence for prevention and control work of mosquito-borne diseases in China.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Recombinant vaccine against West Nile Virus
InactiveUS7740863B2Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD
West nile virus monoclonal antibody and kit
InactiveCN104498438AStrong specificityIncreased sensitivityMicroorganism based processesImmunoglobulins against virusesCell strainHybridoma cell
The invention provides a west nile virus monoclonal antibody and a kit. A hybridomas cell strain secreting west nile virus monoclonal antibody is obtained, and has the preservation number of CGMCC No.8508. The obtained west nile virus monoclonal antibody possesses the advantages of good sensitivity, strong specificity, high titer and the like, and is applicable to identify whether a to-be detected virus is west nile virus and indentify whether a to-be detected sample contains west nile virus. The monoclonal antibody is applicable to prepare immunological diagnostic reagents of west nile virus, for example, an enzyme-linked diagnostic reagent, a colloidal gold immunochromatographic strip and the like.
Owner:北京世纪元亨动物防疫技术有限公司
Primer and probe of west nile virus and real time RT-PCR detection reagent kit with one-step method
ActiveCN101245394AQuick checkReduce false positive resultsMicrobiological testing/measurementDNA/RNA fragmentationReference genesReal-Time PCRs
The invention relates to a group of primers and probes and a corresponding kit, including a pair of primers which can simultaneously detect West Nile virus and the reference gene and two TaqMan probes against the West Nile virus and the reference gene, so as to reduce the number of the primers and the probes in a PCR reaction system from the usual 6 to 4, further to realize the high efficient amplification of the West Nile virus and the reference gene in the same tube, thus realizing the detection of the target genes of the different species, having strong universality, reducing the number of the primers and the probes, the cost of the reagents and the operation steps, and reducing the pollution chances which need to be avoided generally in a PCR experiment. The kit of the invention is applicable to the detection of the West Nile virus in the scientific research or the clinical aspect, especially the monitoring of the West Nile virus in the prevention and the control works of viral diseases.
Owner:谢鹏 +1
Identification and detection method for yellow fever, Japanese encephalitis, chikungunya fever and west Nile fever, primers and probes
InactiveCN103911462AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesChikungunya feverRepeatability
The invention discloses an identification and detection method for yellow fever, Japanese encephalitis, chikungunya fever and west Nile fever, primers and probes. The identification and detection method comprises the step of rapidly, qualitatively and quantitatively detecting a yellow fever virus, a Japanese encephalitis virus, a chikungunya virus and a west Nile virus by combining polymerase chain reaction (PCR) and a liquid chip. The four pairs of primers and probes with high specificity, high sensitivity and good repeatability are provided in the method.
Owner:河北国际旅行卫生保健中心
Vaccines against West Nile Virus
The present invention relates to novel vaccines containing (whole-inactivated) West Nile Viruses and / or West Nile viral proteins derived therefrom, produced on human cells, wherein the human cells comprise a sequence encoding at least one early region-1 (E1) gene product of an adenovirus. The cells are preferably cultured in suspension to very high densities and under serum-free conditions. Herein, it is disclosed that use of such cells results in high titers of West Nile Virus produced.
Owner:JANSSEN VACCINES & PREVENTION BV
Compounds for the treatment of flaviviridae infections
InactiveUS20050049204A1Lower Level RequirementsLower levelBiocideDigestive systemBovine Viral Diarrhea VirusesL-Aspartate
The disclosed invention is a composition for and a method of treating a Flaviviridae infections, such as bovine viral diarrhea virus (“BVDV”), Dengue Virus (DENV), West Nile Virus (WNV) and hepatitis C virus (HCV), as well as abnormal cellular proliferation, in a host, including animals, and especially humans, using a nucleoside of general formula (I)-(V) or N-(phosphonoacetyl)-L-aspartate (PALA), or a pharmaceutically acceptable salt or prodrug thereof.
Owner:PHARMASSET
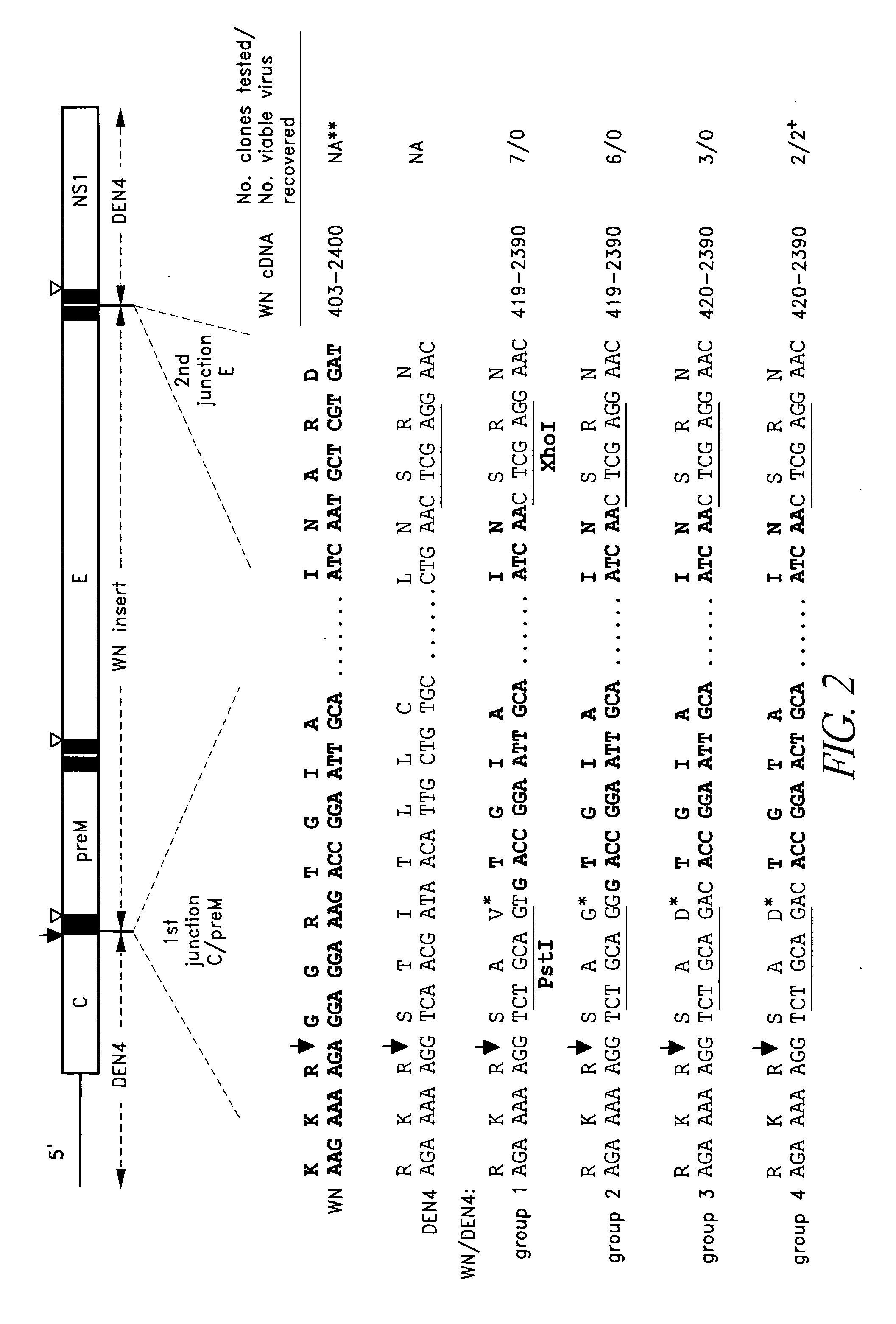
Construction of West Nile virus and dengue virus chimeras for use in a live virus vaccine to prevent disease caused by West Nile virus
ActiveUS20050100886A1Organic active ingredientsSsRNA viruses positive-senseViral VaccineImmunogenicity
The present invention relates to attenuated, immunogenic West Nile virus chimeras built on a dengue virus backbone for the production of immunogenic, live, attenuated West Nile virus vaccines.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
Kit and detection method for rapidly detecting three flaviviruses in combined manner
InactiveCN103361443AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesLoop-mediated isothermal amplificationJapanese encephalitis viruses
The invention relates to the technical field of medical biological detection, and provides a kit for rapidly detecting three flaviviruses in a combined manner. The kit is characterized in that firstly, a loop-mediated isothermal amplification (LAMP) is utilized to detect whether the flaviviruses are positive, then, Japanese encephalitis virus (JEV), dengue pathogen (DEV) and west nile virus (WNV) are simultaneously detected and distinguished. The invention also provides a method for detecting by utilizing the kit for rapidly detecting three flaviviruses in the combined manner. The kit provided by the invention has the advantages that the detection is simple and convenient, rapid and sensitive, and the combined rapid detection for the three flaviviruses is realized.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Fluorescence quantitative RT-PCR detection reagent for West Nile virus and preparation method and use thereof
InactiveCN1840702AConvenient and efficientImprove featuresMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceGene
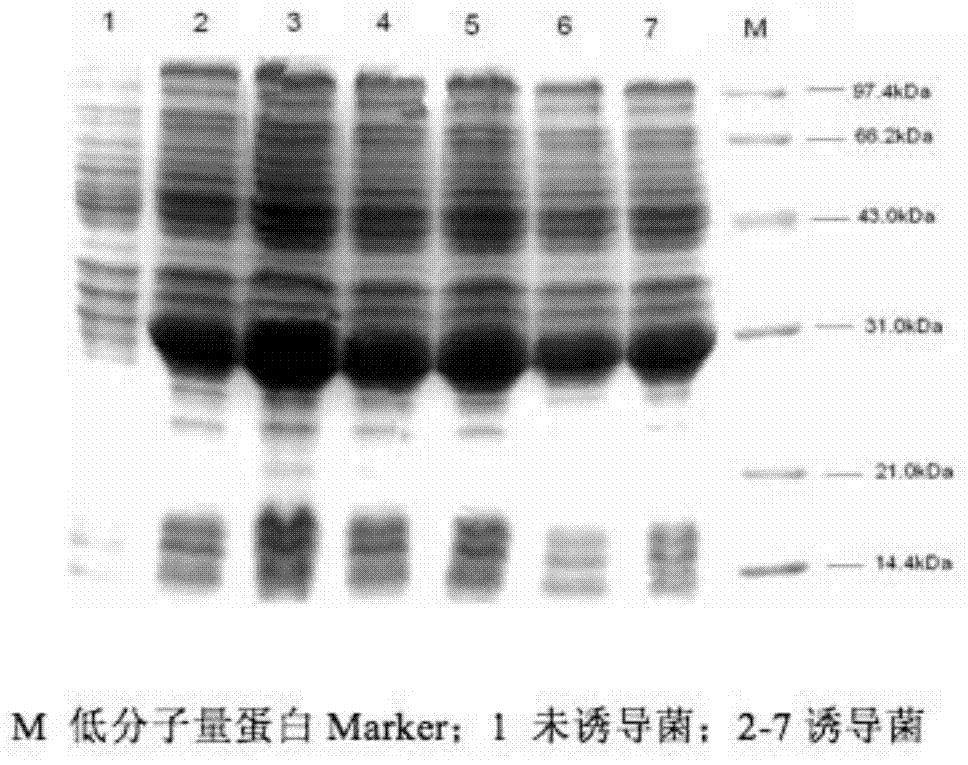
The related bio-reagent is designed by: with conservative West Nile virus E gene fragment as target object, designing and composing carefully for large quantities of primers and probes, taking optimal pairing screen test on different conditions to obtain the most proper primer and probe. The fluorescence quantitative RT-PCR testing reagent includes one couple of specific primers and one specific fluorescent probe, and the amplification fragment length is 71bp.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com