Humanized monoclonal antibody and application thereof
A monoclonal antibody and humanized technology, applied in the field of medicine, can solve the problems of antibody concept change, affinity decrease, disappearance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
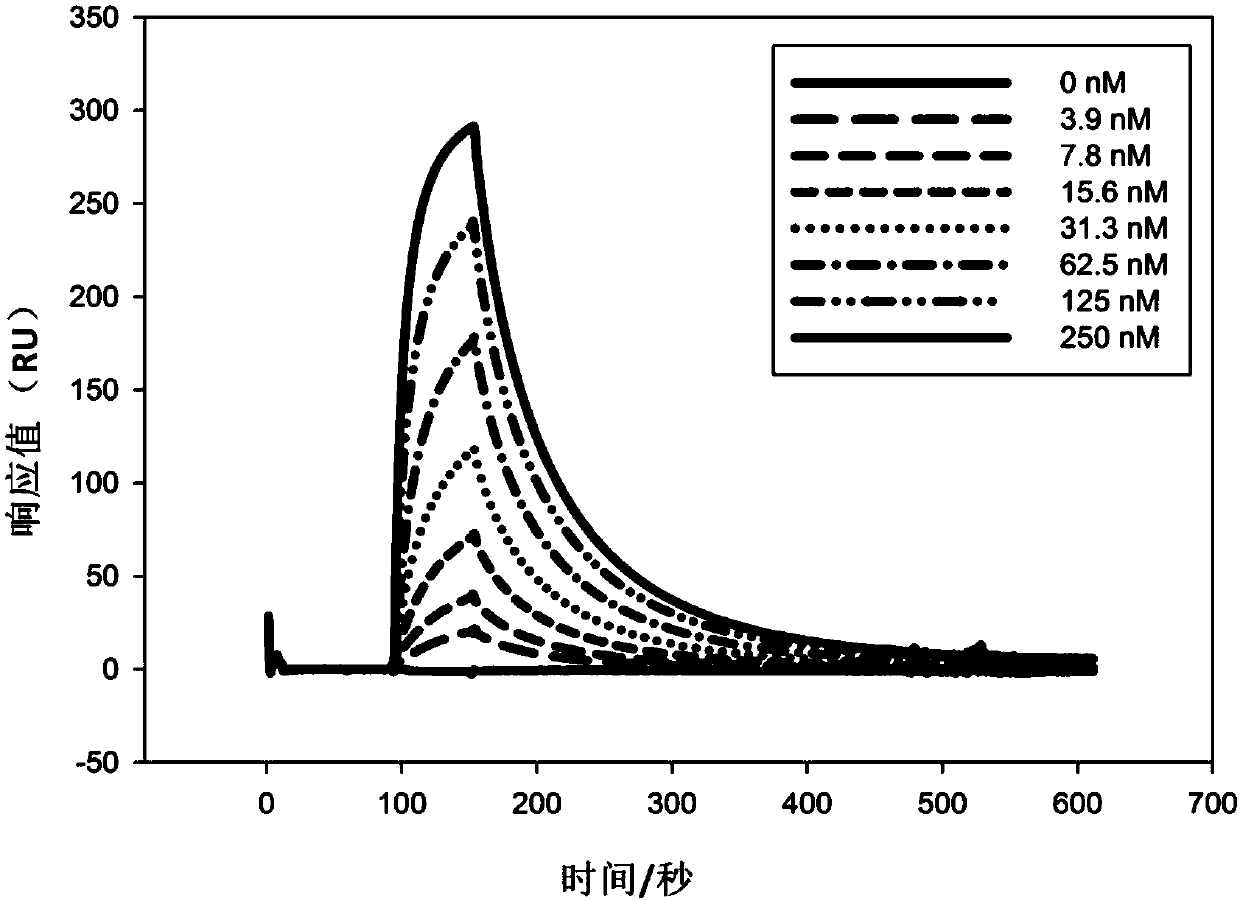
[0026] Example 1: Humanized transformation of mouse monoclonal antibody 2A10G6
[0027] 2A10G6 murine antibody Fab design strategy is as follows:
[0028] PH promoter-BamH1-GP67-EcoR1-L chain-Hind3
[0029] P10 promoter-Sma1-HBM SP-Nhe1-H chain-His-Kpn1
[0030] The Fab heavy chain amino acid sequence (VH-CH1) of the mouse monoclonal antibody 2A10G6 is shown in SEQ ID NO: 7, the nucleotide sequence is shown in SEQ ID NO: 8, the mouse monoclonal antibody 2A10G6mFab light chain amino acid sequence (VL- CL) is shown in SEQ ID NO:9, and the nucleotide sequence is shown in SEQ ID NO:10.
[0031] Compare the amino acid sequences of the 2A10G6 heavy chain and light chain V regions with the reported human antibody sequences, and sort them according to the amino acid homology, select 10 known antibodies with the top rankings of the light and heavy chains, and then compare them according to the difference in CDR length. Whether the minimum, light and heavy chain interaction amino aci...
Embodiment 2
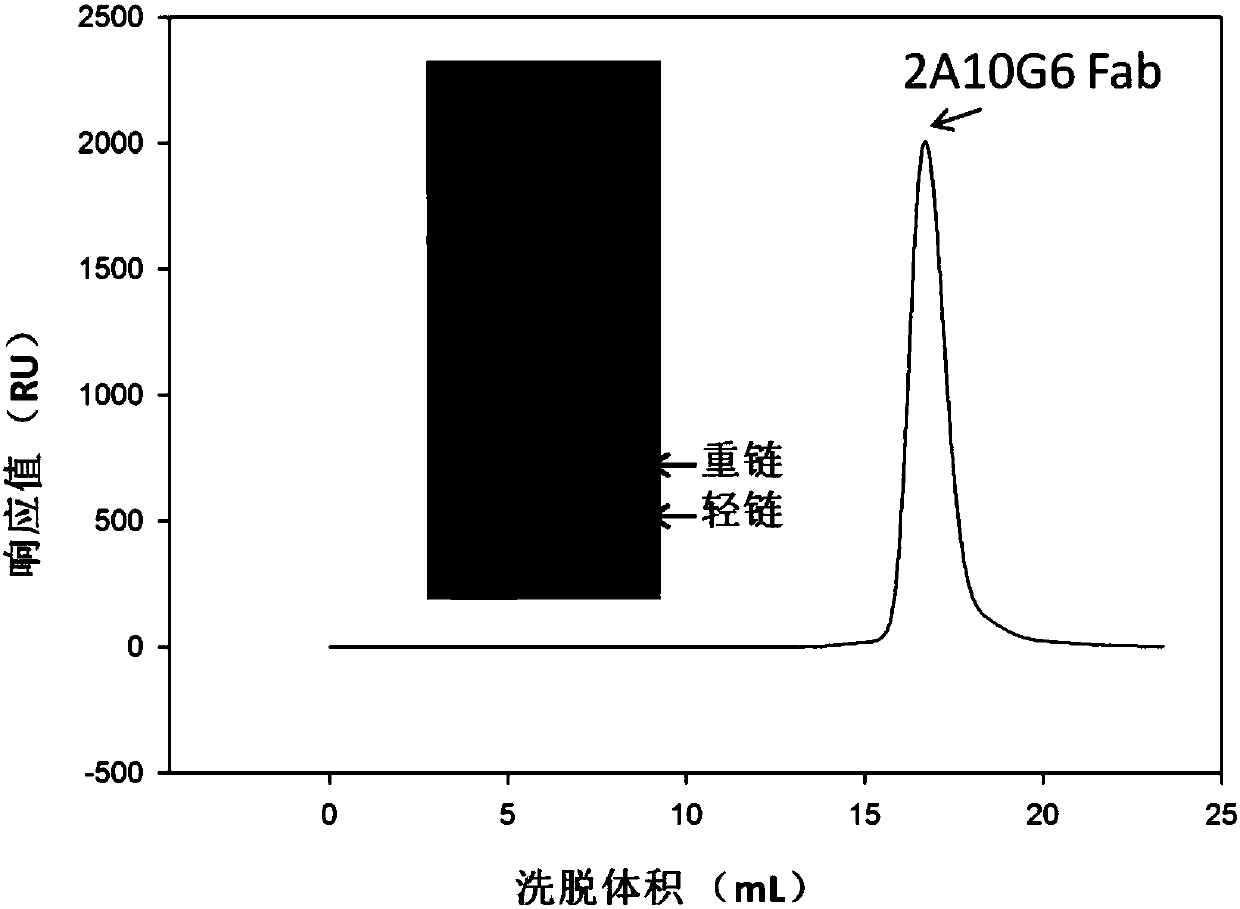
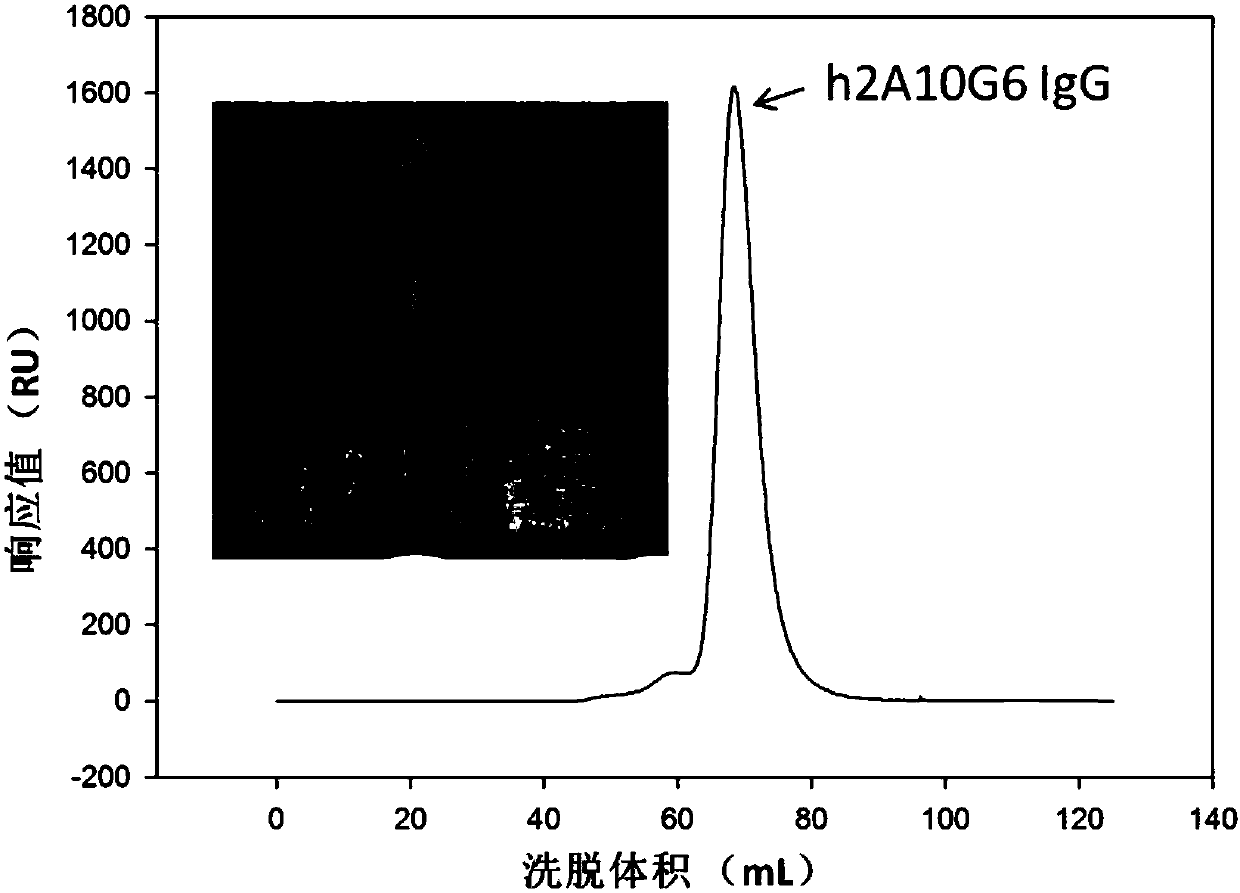
[0038] Example 2: Preparation and purification of monoclonal antibodies
[0039] The preparation methods of mouse antibody 2A10G6 and humanized antibody IgG h2A10G6 are as follows:
[0040] i) Plasmid construction: Insert the Fab gene sequence of the murine antibody 2A10G6 into the pFastBac-Dual vector, and add the name pFastBac-Dual-2AmFab behind the heavy chain gene. The humanized antibody IgG gene of the murine antibody 2A10G6 was inserted into the pFastBac-Dual vector, and a 6-histidine tag was added behind the gene, named pFastBac-Dual-2AhIgG. The recombinant plasmids were confirmed by PCR, enzyme digestion and sequencing to confirm that the inserted foreign fragments were completely correct.
[0041] ii) Preparation and purification of Fab fragment 2AmFab protein of murine monoclonal antibody 2A10G6 and humanized antibody h2A10G6 IgG protein:
[0042]First, the recombinant plasmid was transformed into DH10Bac competent cells, cultured overnight at 37°C, positive clones...
Embodiment 3
[0047] Example 3: Expression of Flavivirus Proteins
[0048] Expression of yellow fever E protein, dengue virus E protein, and West Nile fever E protein:
[0049] The DNA fragment of the extracellular region of YFV_E (the amino acid sequence is shown in SEQ ID NO: 11, and the nucleotide sequence is shown in SEQ ID NO: 12) was digested with NdeI and XhoI, and connected to the pET21a vector. The 3' end of the YFV_E protein coding region is connected with the coding sequence of 6 histidine tags (hexa-His-tag) and the translation stop codon. Then the ligation product was transformed into BL21 Escherichia coli competent cells. Single clones were inoculated into 40mL LB medium and cultured for 6-8 hours. Inoculate into 4L of LB medium and culture to OD at 37°C 600 =0.4-0.6, add IPTG to a final concentration of 1 mM, and continue culturing at 37°C for 4-6 hours. Inclusion bodies were harvested by refolding the inclusion bodies in the dilution method. The refolding solution (100m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com