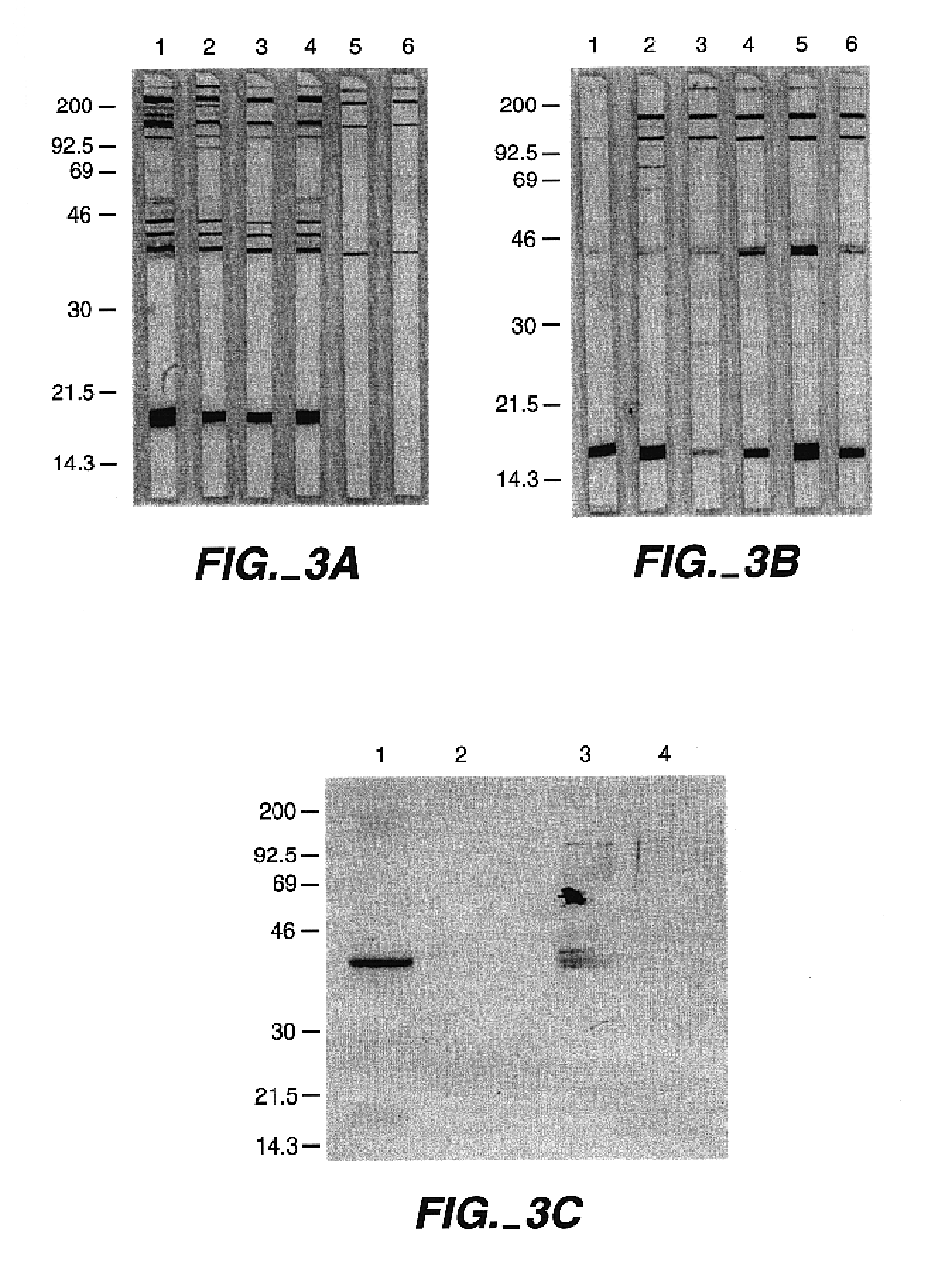
Patents
Literature
178 results about "Sapovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sapovirus is a genetically diverse genus of single-stranded positive-sense RNA, non-enveloped viruses within the Caliciviridae family. Together with norovirus, sapoviruses are the most common cause of acute gastroenteritis (commonly called the "stomach flu" although it is not related to influenza) in humans and other animals. It is a monotypic taxon, containing only one species, Sapporo virus.
Therapy of cancer by insect cells containing recombinant baculovirus encoding genes
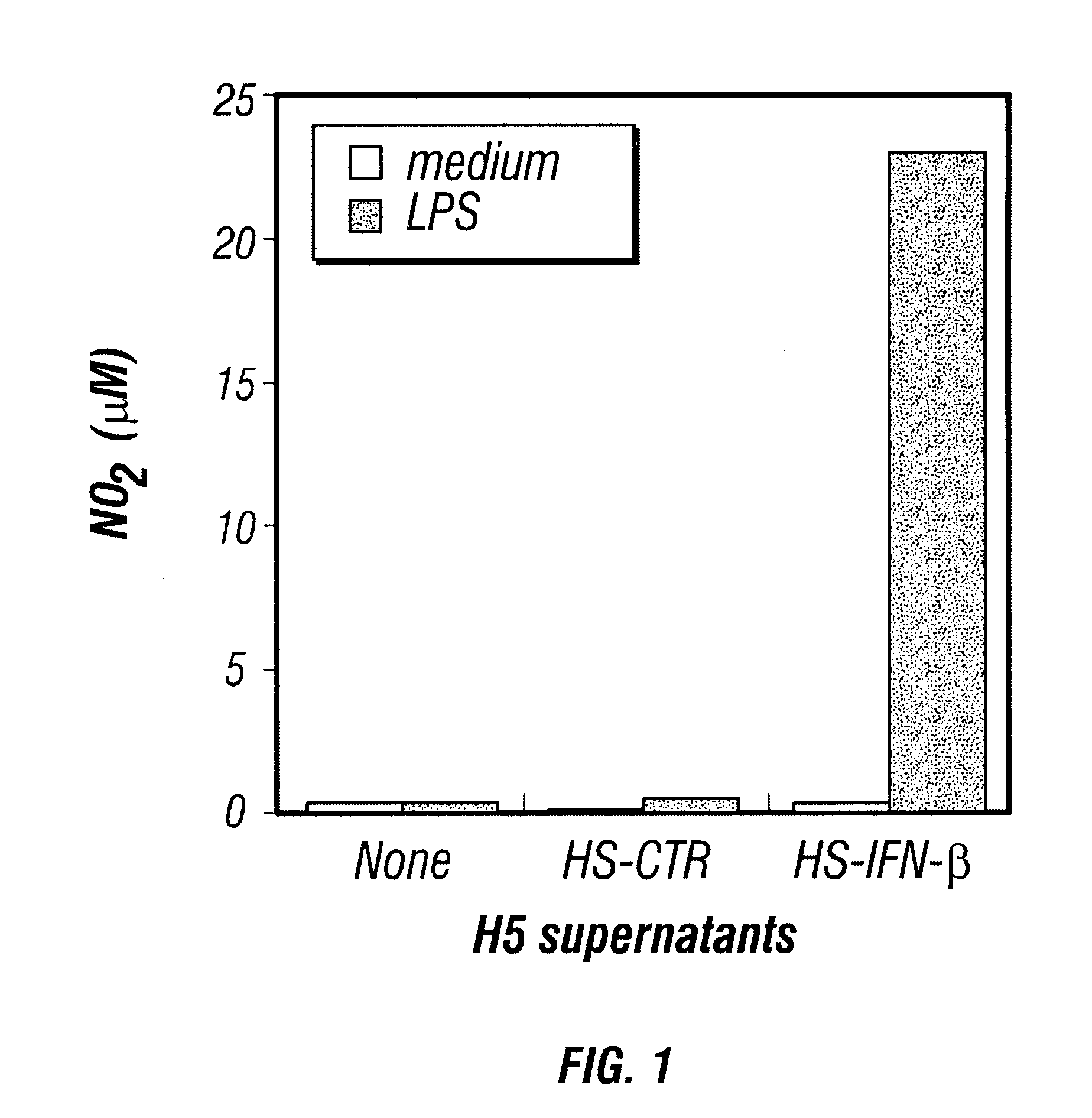
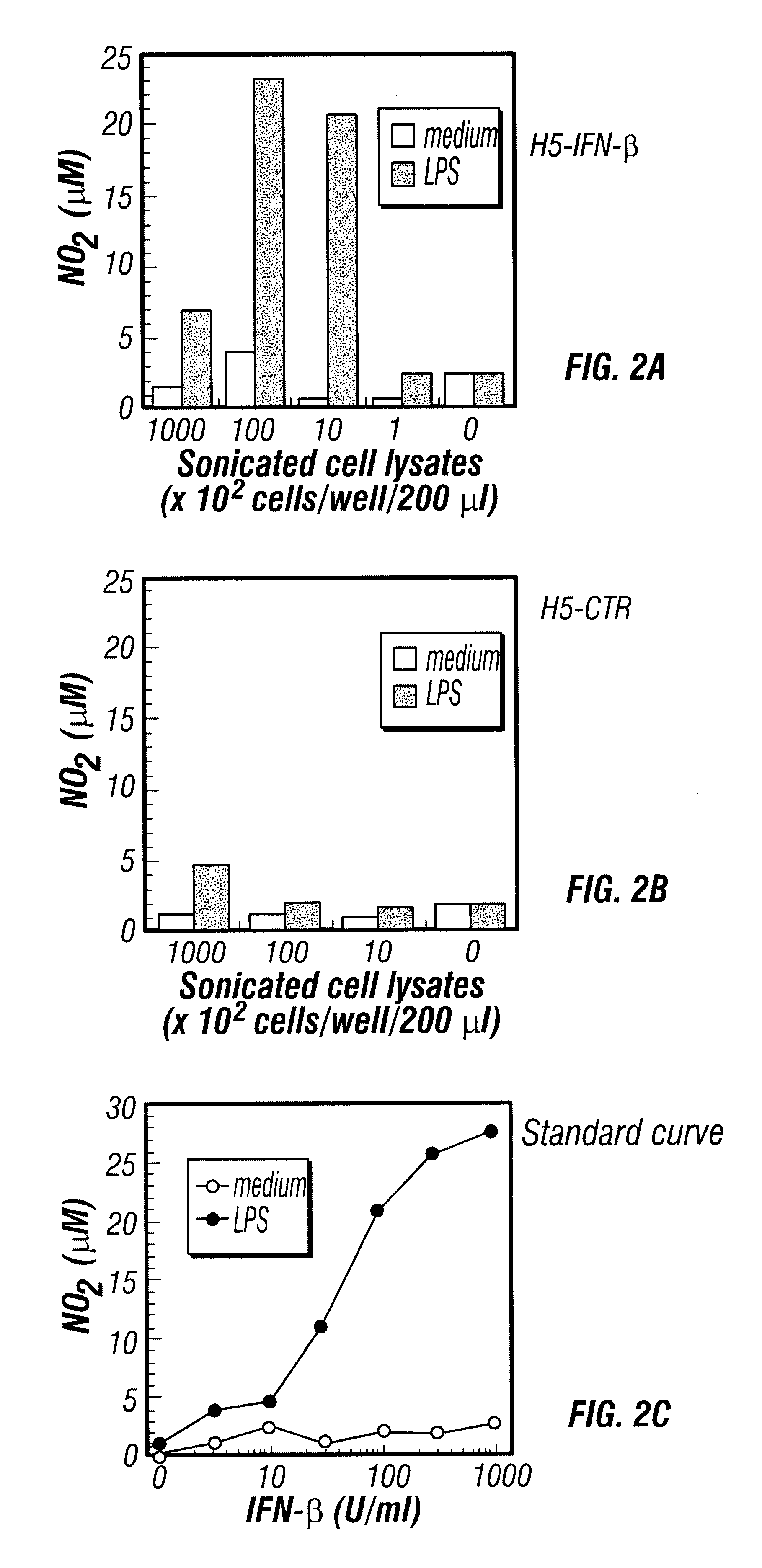
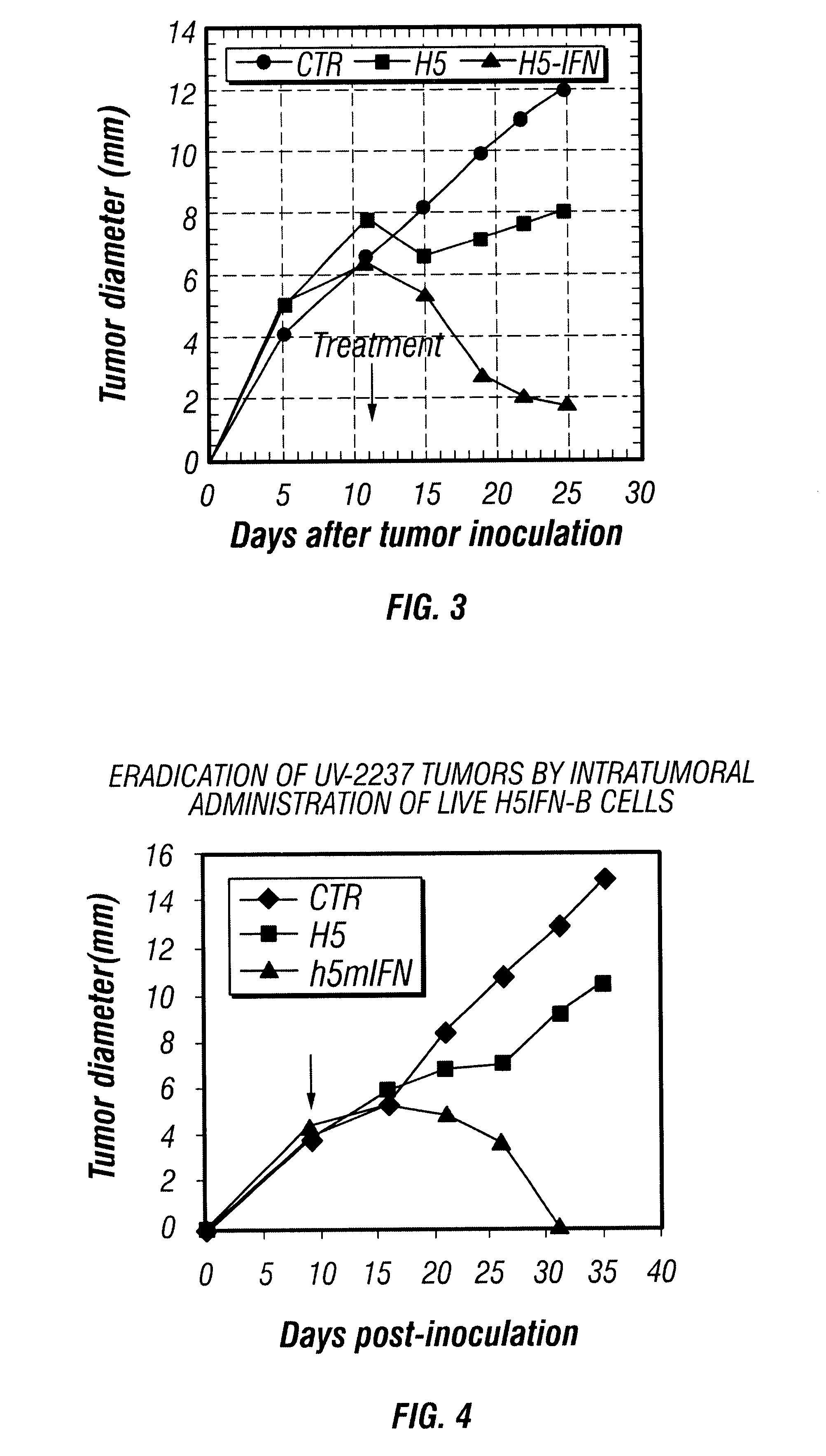
Provided are compositions and methods of use for insect cells comprising baculovirus encoding non-surface expressed proteins and peptides. The claimed invention particularly relates to compositions comprising insect cells containing baculovirus that express cytokines. Such compositions may be administered by, for example, direct intratumoral injection into tumors in mammals, resulting in tumor reduction or recission. Another aspect of the claimed invention concerns methods of promoting resistance to the reoccurence of tumors in mammals who have undergone such tumor recission. In a specific aspect of the claimed invention, the mammals are human subjects presenting with various forms of cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Porcine circovirus II-type recombinant baculovirus as well as preparation method and application thereof
ActiveCN103122352AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliSpecific immunity
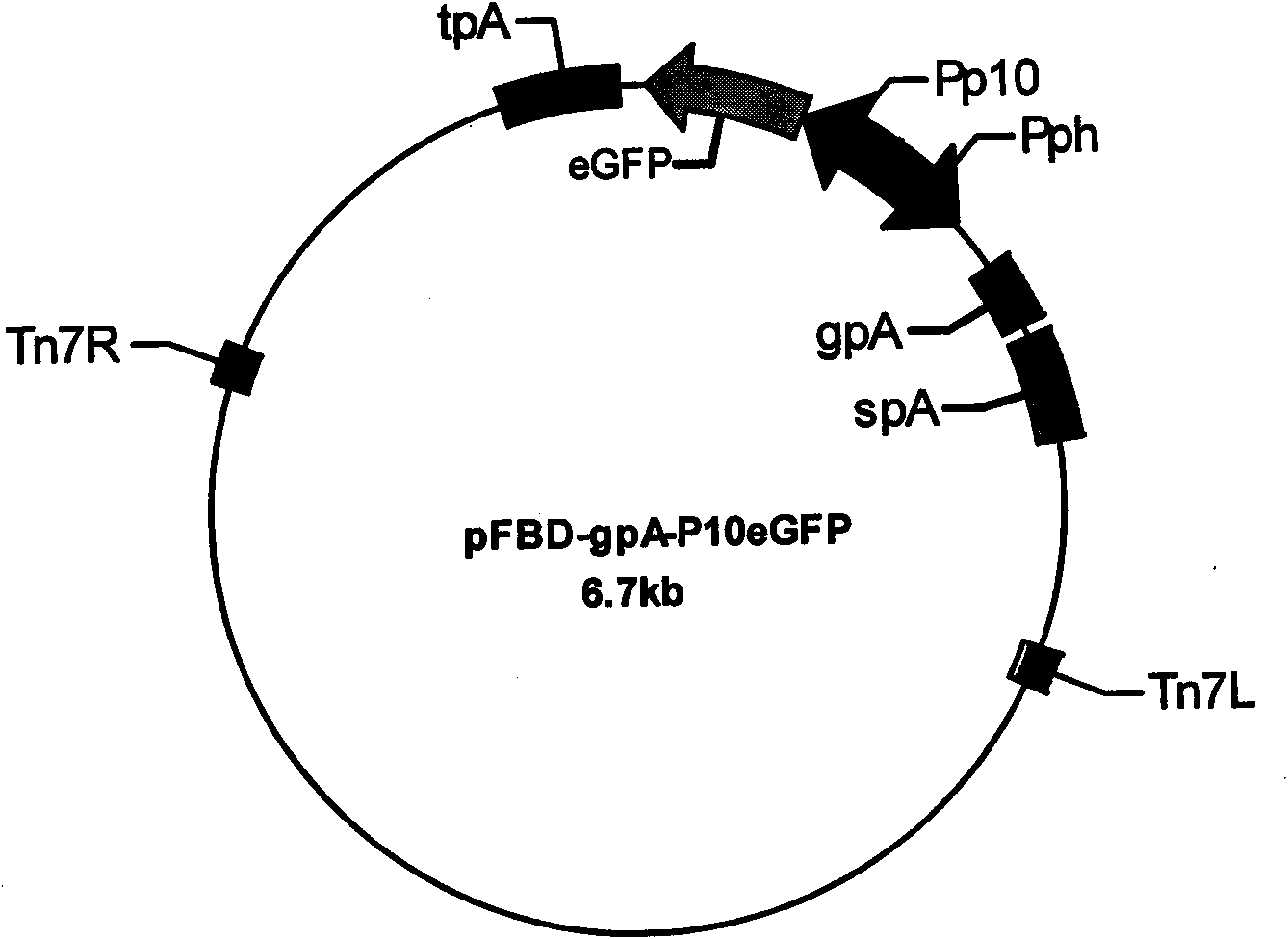
The invention discloses porcine circovirus II-type recombinant baculovirus as well as a preparation method and application thereof. ORF2 gene is artificially synthesized by referring to a PCV2b isolated strain ORF2 gene sequence; the synthesized ORF2 gene is connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHm 30RF2 is obtained. The baculovirus transfer vector pFBDPHm30RF2 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant rod granule rBac-PVR30RF2; the rod granule is transferred with a sf9 cell to obtain the recombinant baculovirus QP-Ac-30RF2. The recombinant baculovirus can be used for efficiently expressing the PCV20RF2 protein and forming virus-like particles. The VLP which is expressed and packaged by the recombinant baculovirus disclosed by the invention is used for preparing inactivated vaccine, and the organism is induced to generate specific immunity response after a 28-day-aged piglet is immunized, and the pig body can be completely protected from virulent attacks of the porcine circovirus.
Owner:HUAZHONG AGRI UNIV
Recombinant alpha-galactosidase A therapy for Fabry disease
InactiveUS7011831B2Peptide/protein ingredientsGenetic material ingredientsBaculovirus expressionInsect cell culture
Fabry disease results from an X-linked deficiency in the enzyme α-galactosidase A. The present invention is directed to recombinant human α-galactosidase A and provides baculovirus expression vectors and recombinant virus that provide stable expression of extracellular and intracellular levels of this enzyme in an insect cell culture. The recombinant-derived enzyme can be used in enzyme replacement therapy to treat Fabry patients. Composition useful in therapeutic administration of α-galactosidase A are also provided.
Owner:SHELBYZYME
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
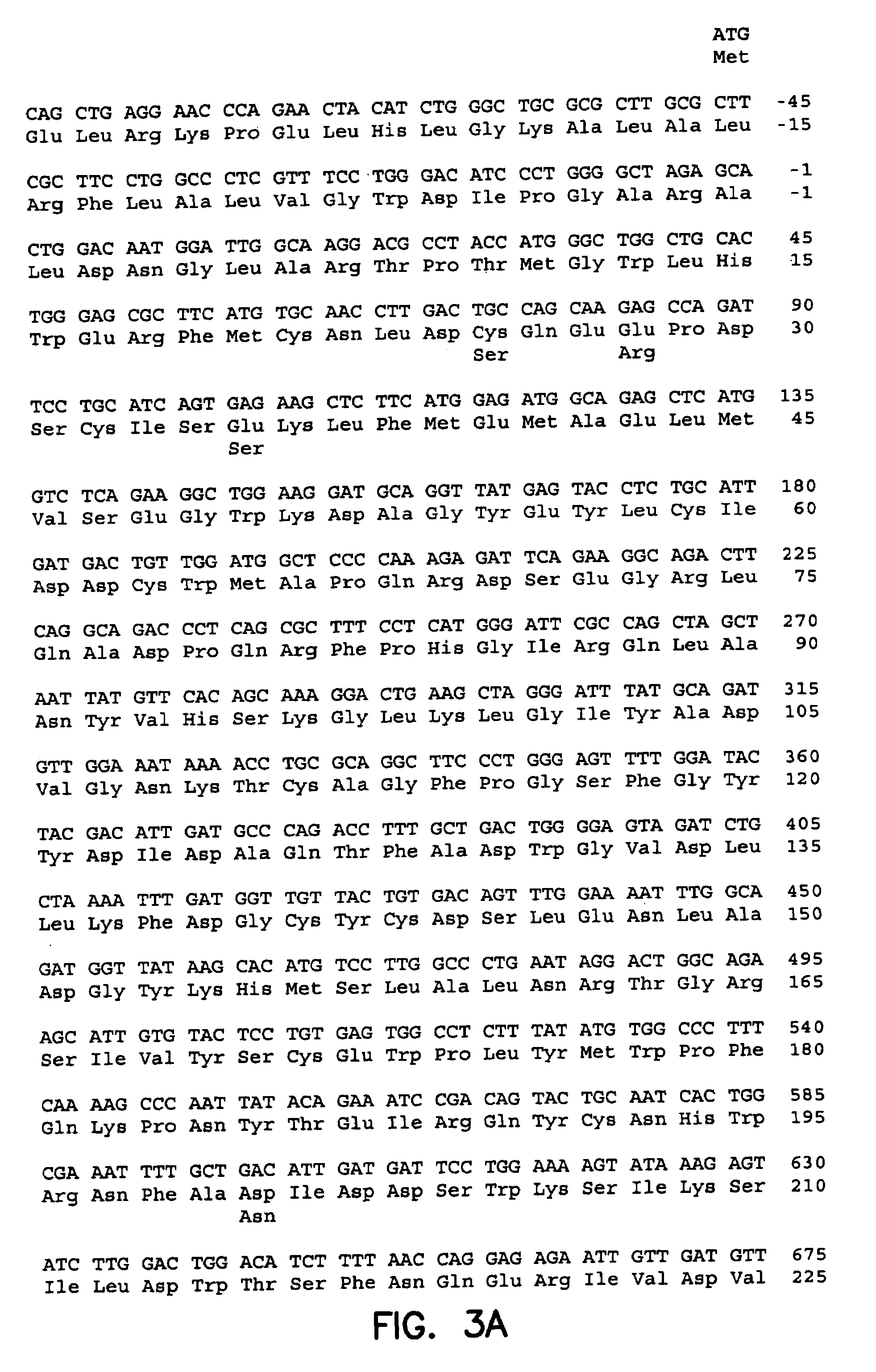
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Use of recombinant bovine CD14 in the treatment and prevention of coliform mastitis in dairy cows
InactiveUS6984503B1High affinityEnhanced ability to enhance activationBacteriaSugar derivativesBaculovirus expressionInduced infections
Studies in mice and humans indicate that membrane CD14 (mCD14) on the cell surface of monocytes, macrophages, and PMN mediates the activation of these cells by LPS. The soluble CD14 (sCD14) present in the circulation also binds to LPS and blocks LPS binding to mCD14. To determine the role of a recombinant bovine soluble CD14 polypeptide in cellular activation by LPS, a recombinant bovine soluble CD14 polypeptide, rbosCD14, was cloned and expressed in a baculovirus expression system. Results indicated that rbosCD14 inhibited the LPS-induced increase in CD18 expression and TNFα mRNA in vitro and reduced mortality in mice injected with LPS. Further, rbosCD14 sensitized mammary epithelial cells to low concentrations of LPS resulting in recruitment of white blood cells and prevention of LPS-induced infection.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
Subunit coronavirus vaccine for dimerization-based receptor binding domains
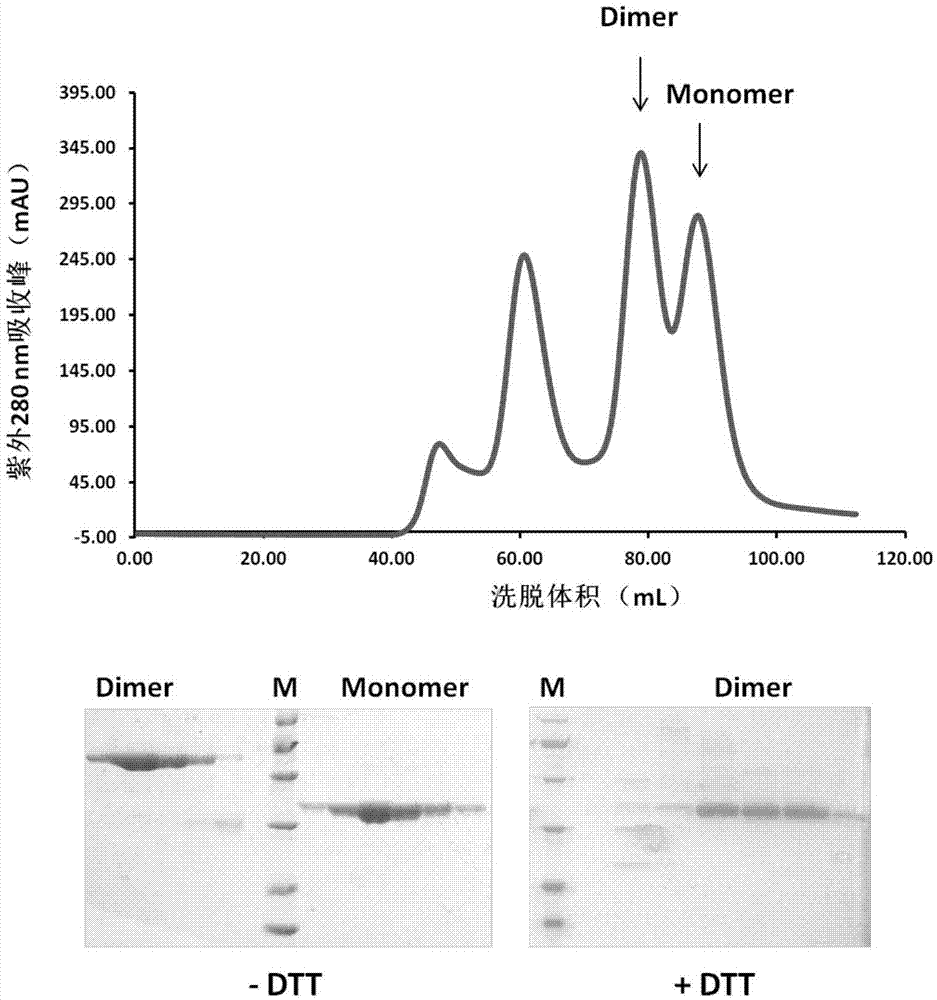
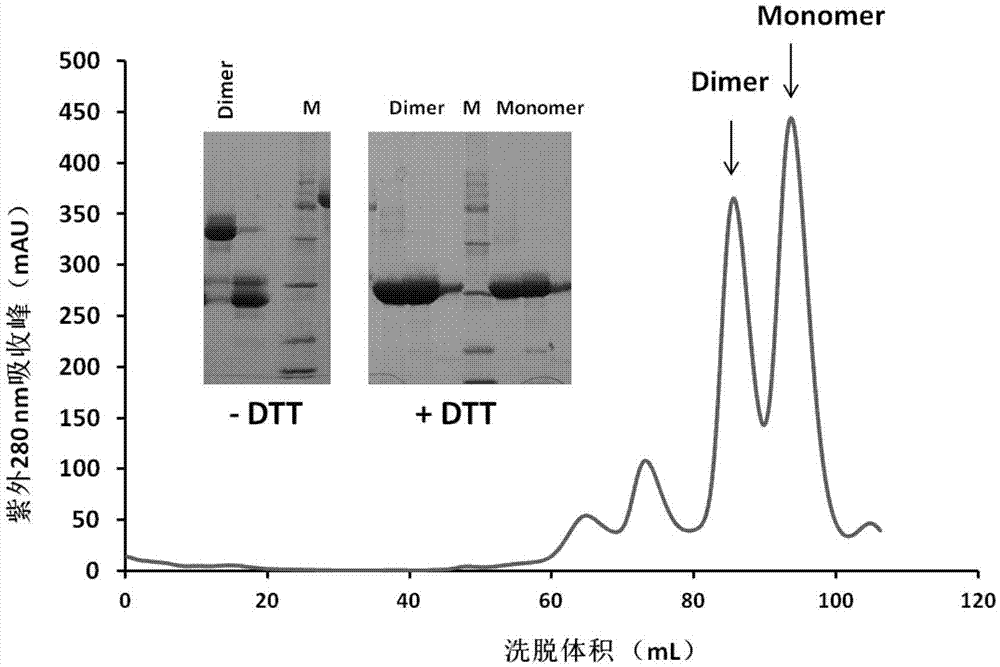
ActiveCN106928326AOvercoming the disadvantage of insufficient immunogenicityIncrease neutralizing antibody productionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationMiddle East respiratory syndrome coronavirus
The invention discloses a subunit coronavirus vaccine for dimerization-based receptor binding domains and belongs to the technical field of medicine. A baculovirus expresses RBD (receptor binding domain (E367-Y606) of MERS-CoV (middle east respiratory syndrome coronavirus) protein and RBD (R294-F515) of SARS-CoV (severe acute respiratory syndrome coronavirus) in insect cells, the RBDs may form a dimer through cysteine residue at 603 of S (spike) protein or form a dimer through cysteine residue at 512 of the S protein, and purified RBD protein dimer and monomer are used respectively to immunize Bald / c mice. The dimerized RBDs have the advantages that the defect that RBD monomers have poor immunogenicity is overcome and the generation of neutralizing antibodies in against MERS-CoV is increased greatly.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as preparation method and application thereof
InactiveCN103122353AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliShuttle vector
The invention discloses porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as a preparation method and application thereof. Sequences of VP0, VP1 and VP3 genes are artificially synthesized by referring to an FMDV (Foot And Mouth Disease Virus) O-type epidemic strain gene sequence; the VP0, VP1 and VP3 genes are connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHmVP013 is obtained. The baculovirus transfer vector pFBDPHmVP013 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant shuttle vector Bacmid; the shuttle vetcor Bacmid is transferred with a sf9 cell, and the recombinant baculovirus QP-Ac-FVLP is obtained by collecting the cell supernatant. The recombinant baculovirus can be used for efficiently expressing FMDVVP0, Vp1 and Vp3 proteins and forming virus-like particles. And the virus-like particles are used for preparing subunit vaccine, so that the organism is induced to generate specific immunity response after the mouse is immunized.
Owner:HUAZHONG AGRI UNIV
Humanized monoclonal antibody and application thereof
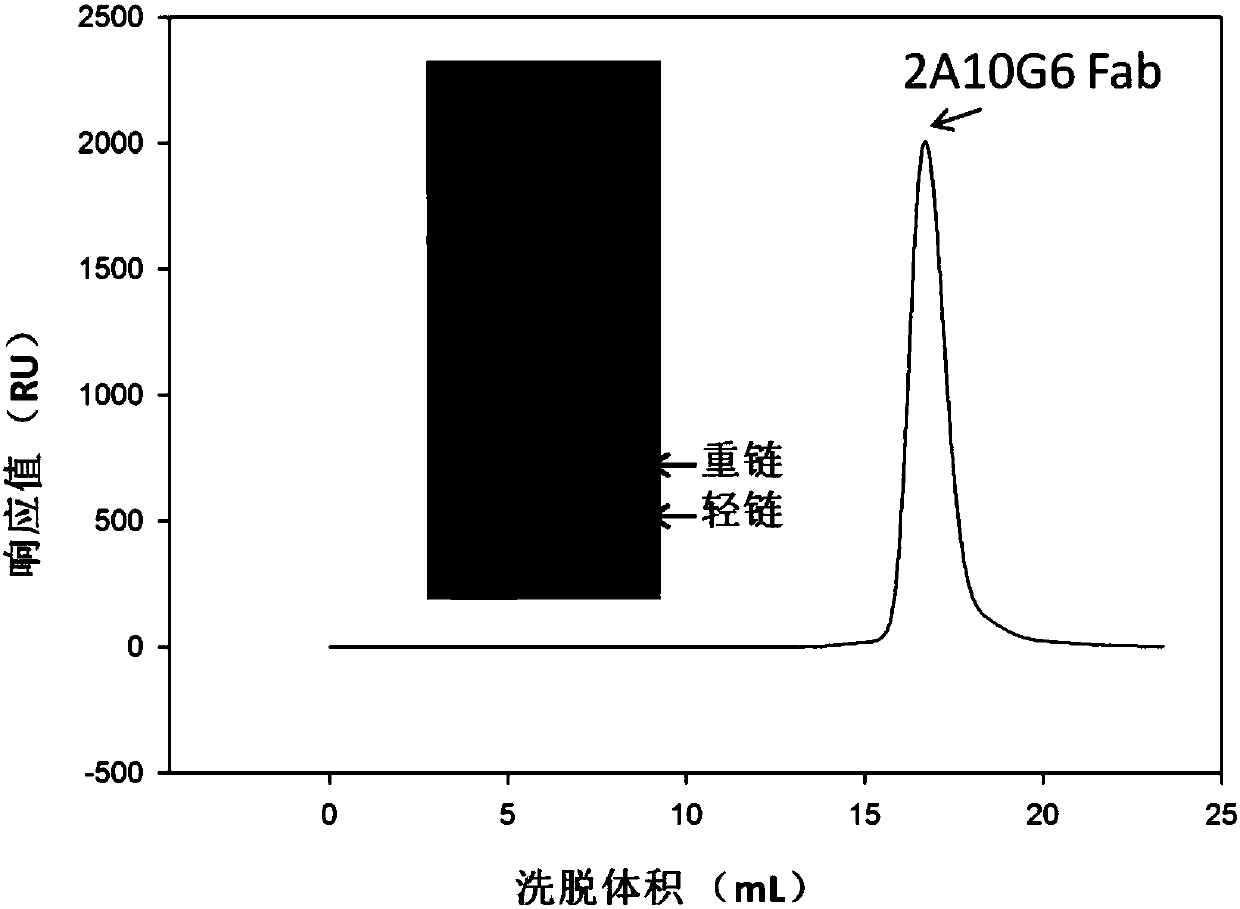
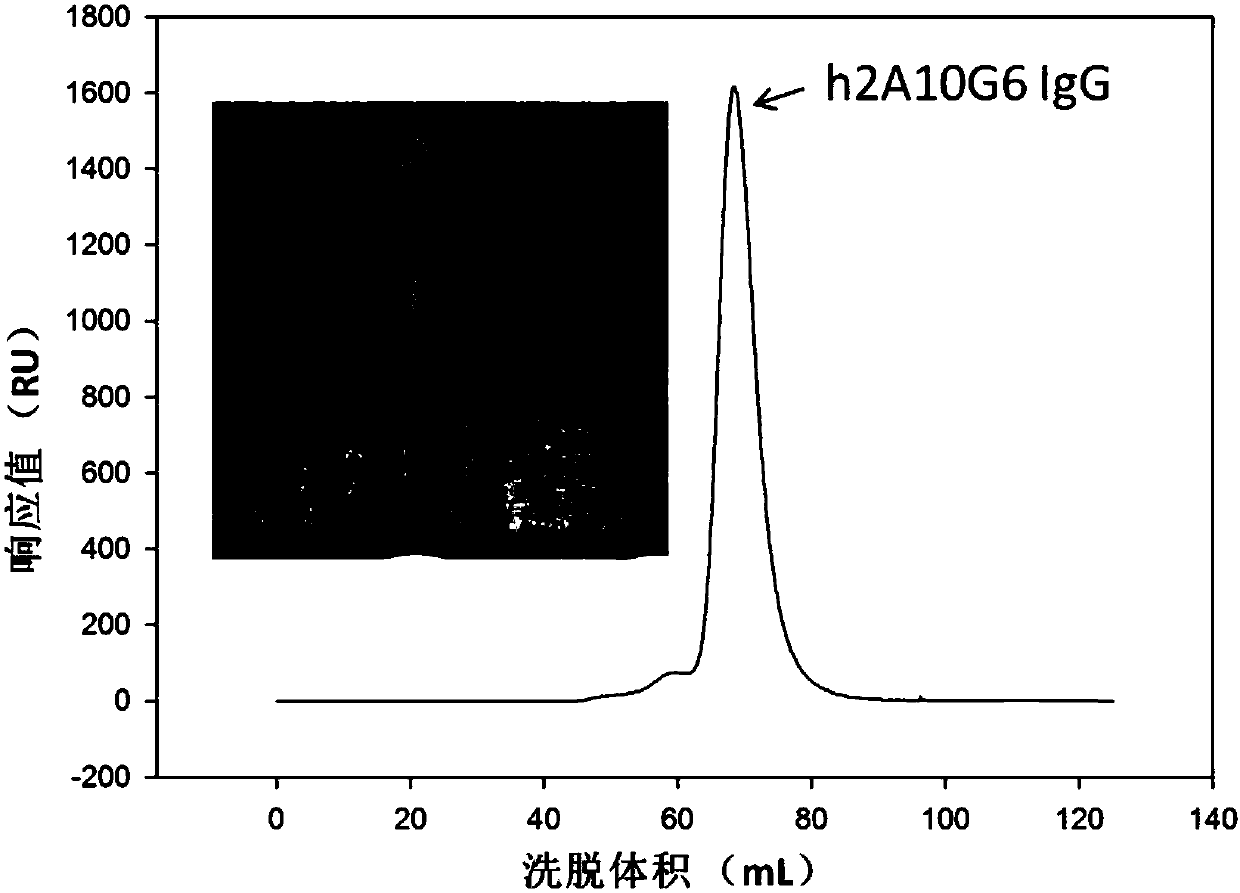
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Method for preparing rabies virus antigen
ActiveCN101307317AReduce consumptionImprove securityAntiviralsDepsipeptidesAntigenBaculovirus expression
The invention provides a method for making rabies virus antigen. The method comprises the following steps that: the antigen gene of rabies virus or the combined expression combination of the antigen gene is respectively cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the transfer expression carrier and baculovirus undergo cotransfection so as to carry out homologous recombination or transposition, thereby obtaining recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding rabies antigen; and the expressed antigen is ingathered and purified so as to obtain rabies virus antigen. The method adopts a baculovirus expression system to make safe and efficient rabies virus antigen in a domestic silkworm bioreactor; moreover, due to having extremely high safety, the made antigen can be directly used to make injection vaccine and oral vaccine used for animal immunization. The method can substantially reduce the production cost of rabies virus antigen, and has the advantages of safety, high efficiency, less energy consumption and low cost, etc.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
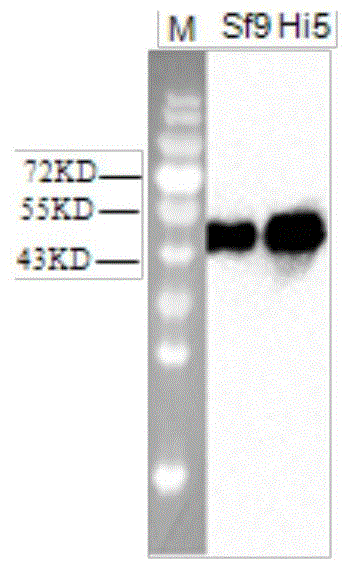
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Method for preparing foot-and-mouth disease antigen
ActiveCN101121938APromote safe productionReduce consumptionSsRNA viruses positive-senseVirus peptidesAntigenTransfer vector
The invention provides a method for expressing foot-and-mouth disease antigens in insects using recombinant baculoviruses, which includes: cloning different gene combinations of foot-and-mouth disease into baculovirus delivery vectors to construct transfer vectors; using the constructed transfer vectors to transfer Infect the baculovirus and perform DNA recombination to obtain the recombinant baculovirus; infect the insect host with the recombinant baculovirus; culture the infected insect host to express the foot-and-mouth disease antigen; collect and purify the expressed foot-and-mouth disease antigen. The method of the present invention uses a baculovirus expression system to safely and efficiently produce foot-and-mouth disease antigens in a silkworm bioreactor. The prepared antigens are extremely safe and can directly produce vaccines to immunize animals. The method of preparing foot-and-mouth disease antigen of the present invention does not require investment in building a factory, has no three wastes, consumes very little energy such as electricity and water resources, and its production cost is also significantly lower than the traditional method of preparing foot-and-mouth disease antigen. It is safe, efficient, has low energy consumption and low cost. Low and many advantages.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Recombinant baculovirus expressing manually modified and synthesized influenza A H1N1 virus HA-NA-M1 gene
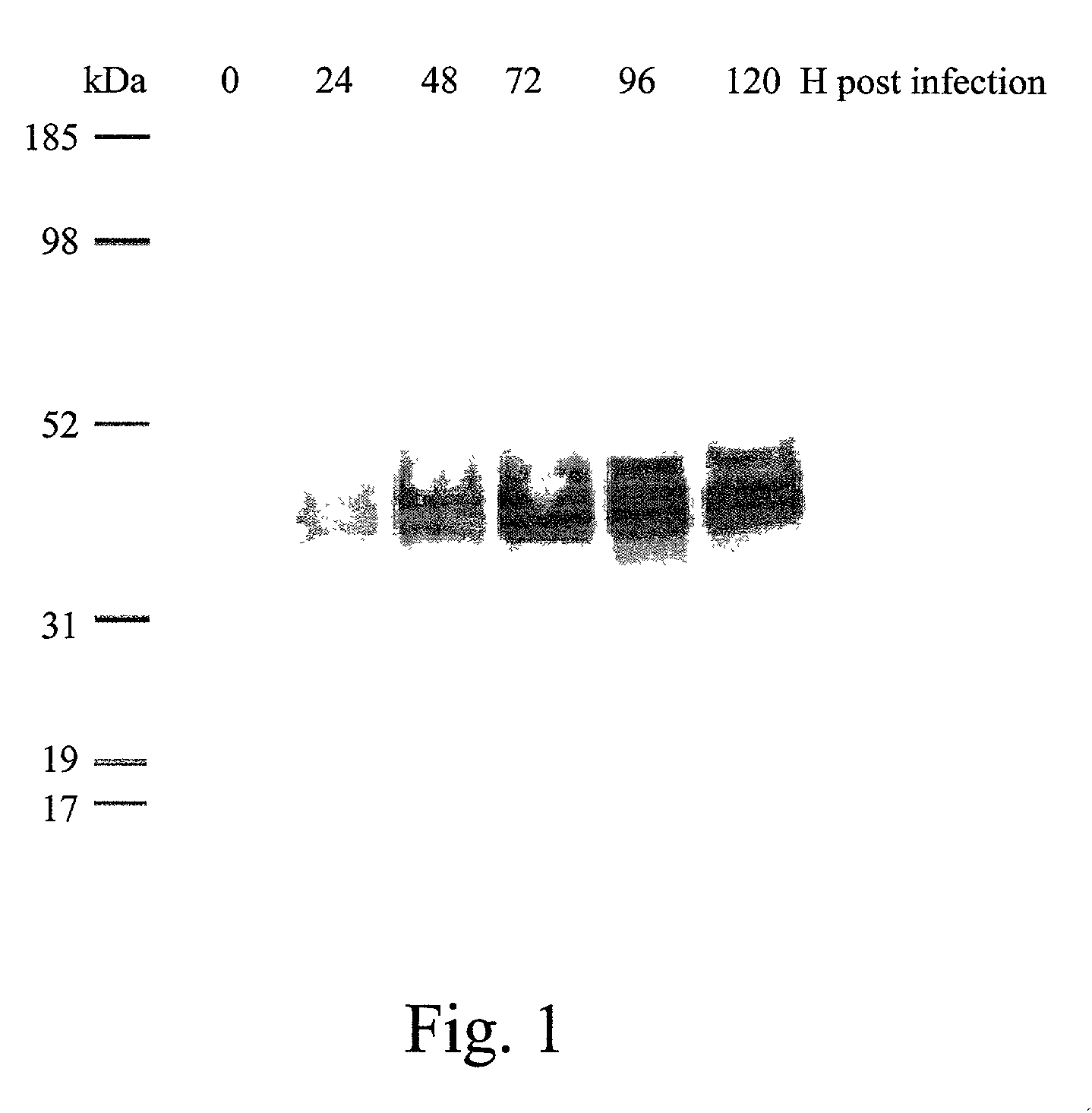
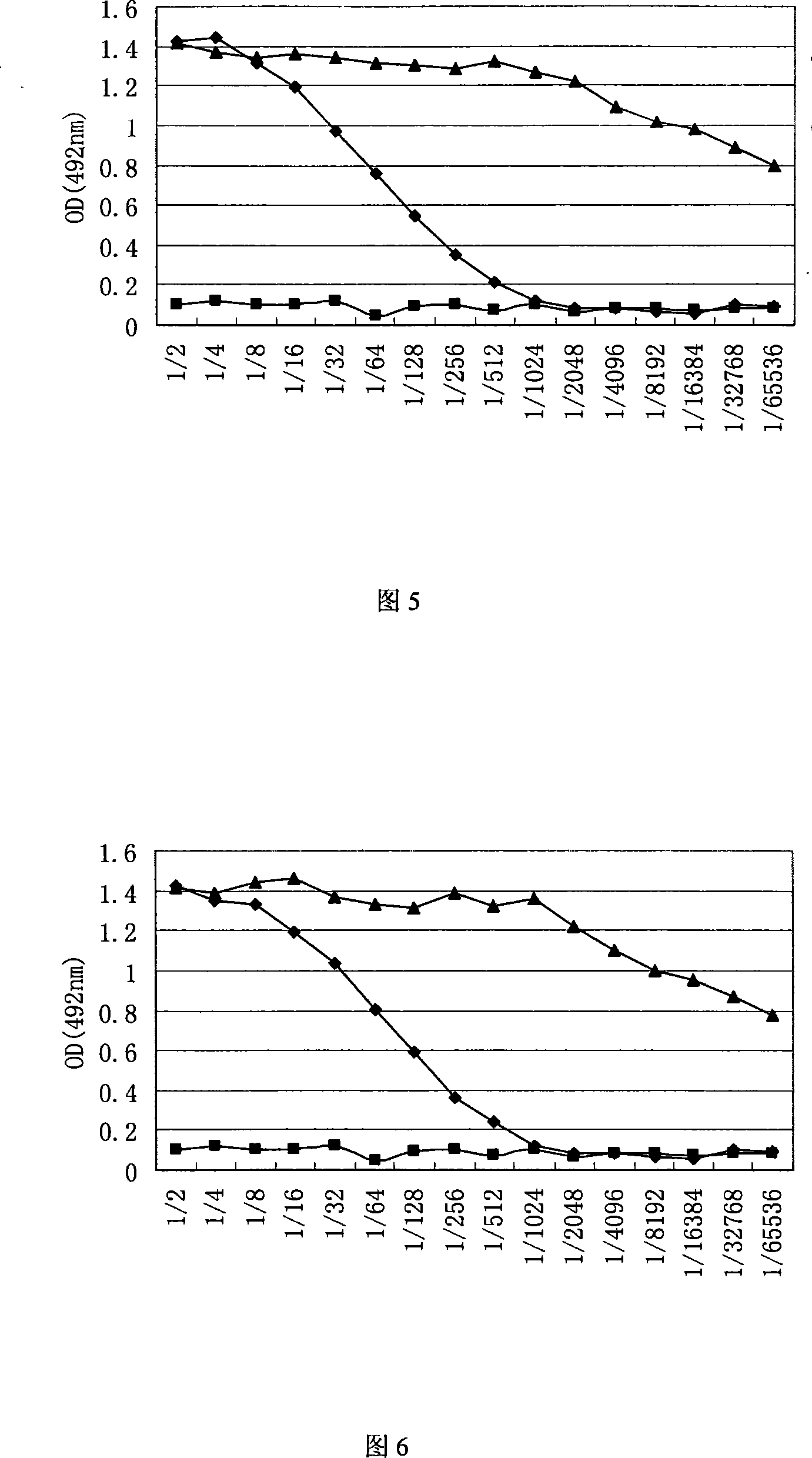
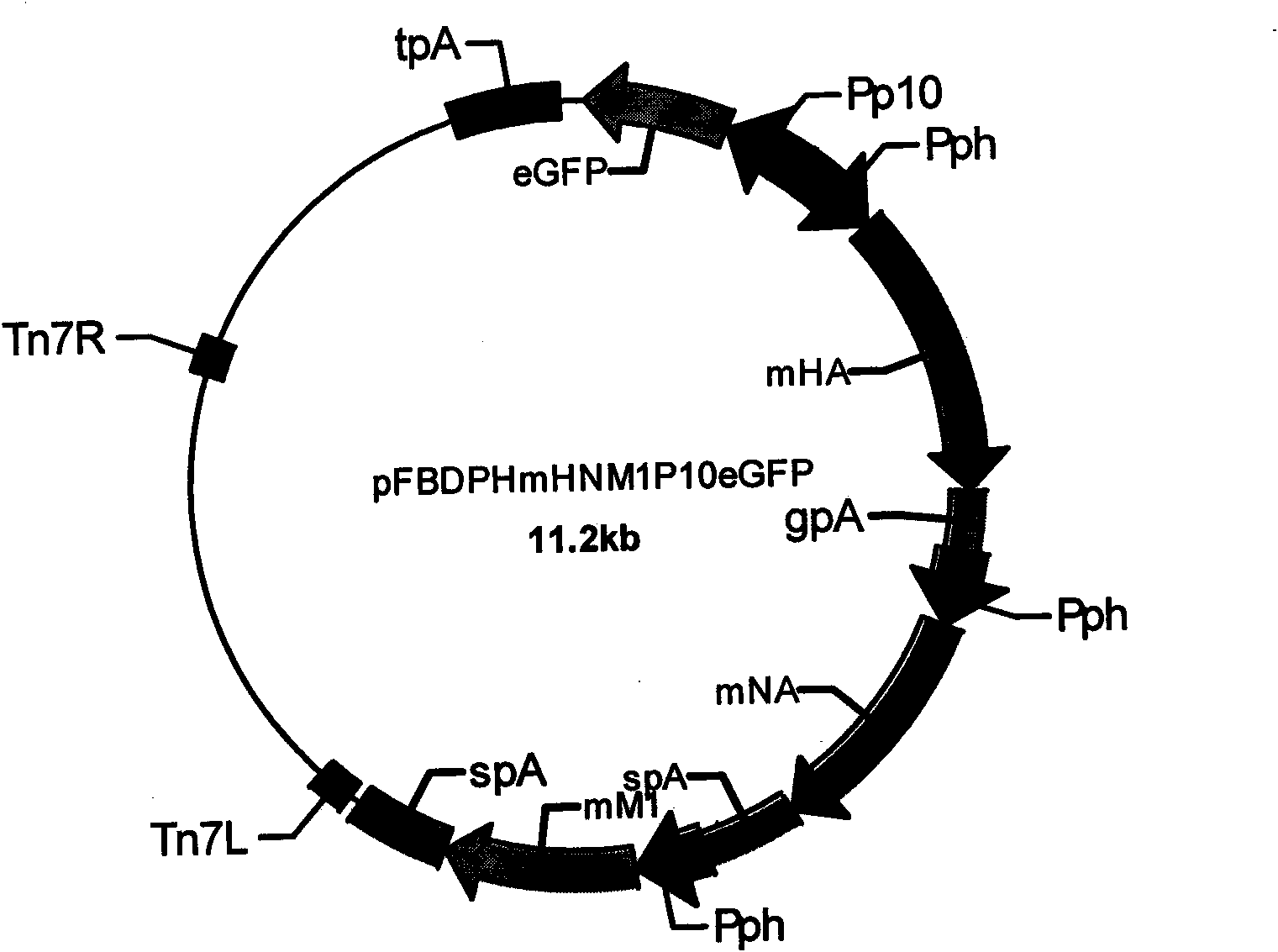
InactiveCN101624580AImprove expression levelImprove screening efficiencyGenetic material ingredientsVirus peptidesInfluenza A (H1N1) virusSapovirus
The invention relates to the field of virology, in particular to a recombinant baculovirus which is manually modified and synthesized and contains a main immunogenic gene HA-NA-M1 of an influenza A H1N1 virus. The strain QP-Ac-HNM1 belongs to the baculovirus (Baculovirus) and is preserved in the China Center for Type Culture Collection (CCTCC) with the preserving number of CCTCC-V200912. The recombinant virus is capable of synchronously expressing the HA and NA of the influenza A H1N1 virus and M1 proteins to form virus particles which can be used for developing vaccines so as to prevent human beings and swine from being infected with the influenza A H1N1 virus.
Owner:HUAZHONG AGRI UNIV
Baculovirus produced Plasmodium falciparum vaccine
Owner:UNIV OF HAWAII
Diluent for norovirus or sapovirus specimen and method for detecting virus
ActiveUS20060216695A1High detection sensitivityEliminating non-specific factorsMicrobiological testing/measurementImmunoglobulinsFecesDiluent
The present invention relates to a diluent for a Norovirus or Sapovirus specimen, the diluent containing an alkaline buffer of pH 9.0 to 10.0, and to a method for detecting a Norovirus or a Sapovirus through use of the diluent. According to the present invention, a Norovirus or a Sapovirus can be detected in a Norovirus- or Sapovirus-containing specimen such as stool, vomit, body fluid, blood, body tissue, or food, in a convenient manner without use of a special machine such as a centrifuge, with an improved detection sensitivity, and with complete elimination of non-specific factors.
Owner:DENKA CO LTD
Classical swine fever E2 subunit vaccine and application thereof
InactiveCN106139139AThe effective amount of antigen is stableLess side effectsViral antigen ingredientsVirus peptidesImmune effectsAdjuvant
The invention provides a classical swine fever E2 subunit vaccine and a preparation method thereof. The preparation method specifically comprises the steps that a Baculovirus Carrier Expression System is used for expressing a large amount of recombination classical swing fever E2 protein in insect cells, and the classical swine fever E2 subunit vaccine with a good immune effect is developed. The Bac-to-Bac Baculovirus Carrier Expression System is used for expressing the classical swine fever E2 protein. The classical swine fever E2 protein and an adjuvant 201R are emulsified into the novel subunit vaccine, and by means of piglet immune challenge tests, it is verified that the subunit vaccine has a good immune protection effect.
Owner:北京大北农科技集团股份有限公司动物医学研究中心 +3
Diarrhea virus detection kit and method
ActiveCN103215379AImprove detection efficiencySimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceRotavirus RNAFluorophore
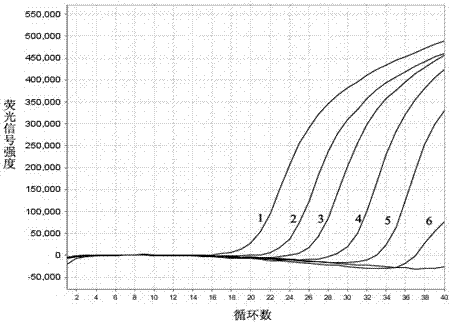
The invention is applicable to the technical field of biotechnology and medical detection and provides a diarrhea virus detection kit and method. The detection kit comprises PCR (polymerase chain reaction) liquid which comprises a PCR buffer solution, MgCl2, dNTP, DNA polymerase, primer pairs, probe sequences and Homo-tag, wherein the primer pairs and the probe sequences respectively aim at type I, type II and type IV of sapovirus, type I and type II of norovirus, rotavirus, adenovirus and astrovirus, the 5' ends of the probe sequences are connected with fluorophore, and the 3' ends of the probe sequences are connected with quencher. The detection kit provided by the invention can detect seven viruses (subtype) related to diarrhea at one time, and compared with a detection method in the prior art, the detection method provided by the invention has the advantages that operation is simple and convenient, a result is quick to read, and false positive is avoided.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
Recombinant porcine circovirus type 2 virus-like particle, and preparation method and application thereof
InactiveCN103555746AImproving immunogenicityStimulationViral antigen ingredientsGenetic material ingredientsAdjuvantPorcine circovirus
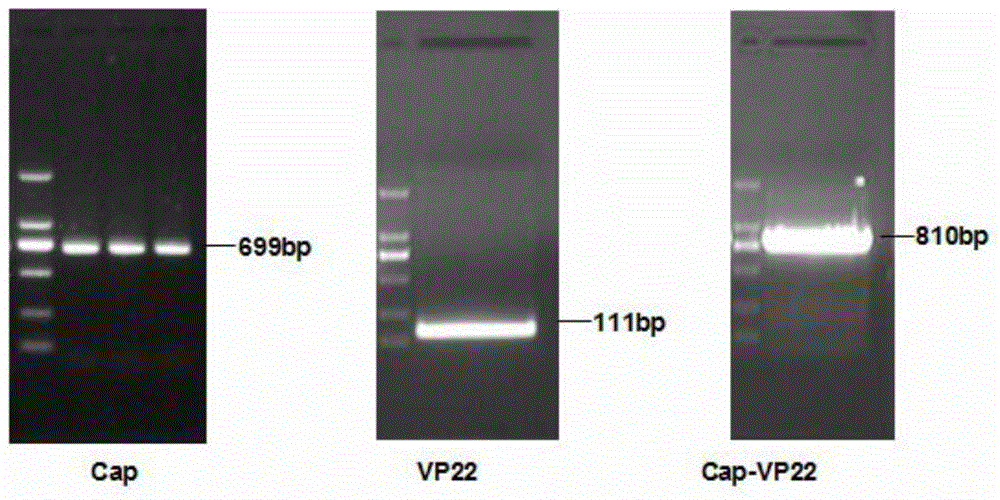
The invention discloses a recombinant porcine circovirus type 2 (PCV2) virus-like particle (VLP). The VLP is prepared by connecting a protein transduction domain originated from HSV-1VP22 with an antigen Cap gene needed for formation of PCV2 hollow capsid protein by using an SOE-PCR method, then carrying out cloning to a baculovirus transporter so as to obtain a homologous recombinant vector, carrying out encapsulation so as to produce recombinant baculovirus containing PCV2Cap protein and the protein transduction domain of HSV-1VP22, infecting an insect cell and expressing recombinant Cap-VP22 protein in which PCV2Cap protein and the protein transduction domain of HSV-1VP22 are fused. According to results of research, the VLP prepared in the invention can be individually used or used with adjuvant for inoculation to an animal and effectively stimulates generation of a specific antibody to PCV2. Thus, the VLP provided by the invention can be applied to development of a novel high-efficiency PCV2 subunit vaccine with a good immune effect.
Owner:CHANGCHUN SR BIOLOGICAL TECH +1
Hog cholera virus inhibition ELISA antibody detection kit and application thereof
ActiveCN104483490AAchieve mass productionIncreased sensitivitySsRNA viruses positive-senseVirus peptidesPositive controlHorse radish peroxidase
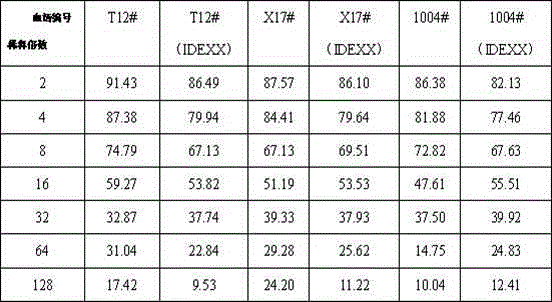
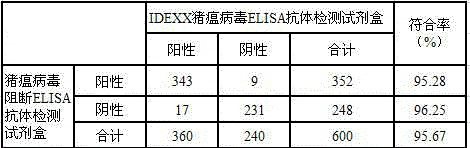
The invention discloses a hog cholera virus inhibition ELISA antibody detection kit and application thereof. The kit comprises hog cholera virus E2 protein which is expressed by virtue of a baculovirus expression system and a cell suspension culture process; the hog cholera virus E2 protein is purified to serve as a coated plate antigen and an immunogen to prepare a monoclonal antibody of the hog cholera virus E2 protein, and horse radish peroxidase (a hybridoma cell strain 4A7 CCTCC NO.C2014229) is marked. The kit further comprises an antigen coated plate, a positive control, a negative control, sample diluent, a scrubbing solution, a developing solution A, a developing solution B and a stop solution. According to the application of the hog cholera virus inhibition ELISA antibody detection kit in the detection of the hog cholera virus antibody, the kit is used for detecting other positive serums except hog cholera viruses, the cross reaction is avoided, the specificity is strong, the sensitivity is high, the detection time is short, and the repeatability is good; compared with an import kit, the detection coincidence rate reaches above 95%.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Triple fluorescent quantitative RT (Reverse Transcription)-PCR (Polymerase Chain Reaction) kit for detecting human calicivirus
ActiveCN103031386ALess consumablesShorten detection timeMicrobiological testing/measurementMicroorganism based processesCalicivirusSapovirus
The invention discloses a triple fluorescent quantitative RT (Reverse Transcription)-PCR (Polymerase Chain Reaction) kit for detecting human calicivirus, which consists of a quantitative RT-PCR reaction liquid tube, an enzyme mixed liquid tube, a primer probe mixed liquid tube, a negative control sample tube, three standard sample tubes, three positive control sample tubes and a kit body. The standard sample is GI type norovirus, GII type norovirus and sapovirus standard samples. The control samples are positive samples and negative samples. According to the invention, specific primers and probes are designed on the basis of the sequence of a conserved region of the GI type norovirus, GII type norovirus and sapovirus and a one-step triple real-time fluorescent quantitative RT-PCR technique is adopted to accurately detect the GI type norovirus, GII type norovirus and sapovirus in a specimen. The kit is reasonably designed, simple and convenient to operate, quick and accurate.
Owner:ZHEJIANG UNIV
Engineered Baculoviruses and Their Use
InactiveUS20090176660A1Improve the display effectEasy and fast productionAnimal cellsVectorsHeterologousGene product
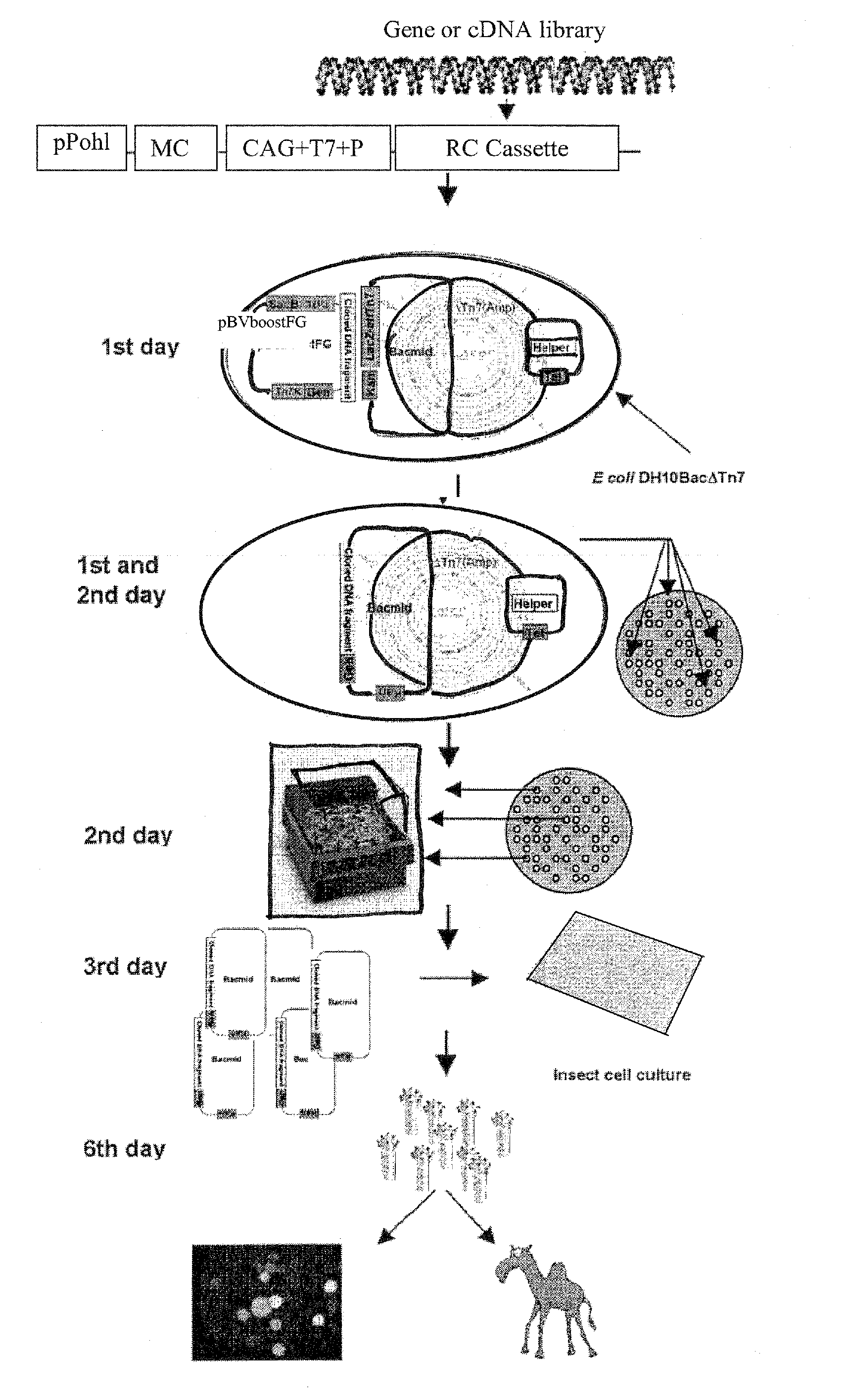
Baculovirus is engineered so that the capsid displays one or more heterologous peptides or protein. Such baculovirus can be used to deliver therapeutics, and in functional genomics.Also a method for generating recombinant baculoviruses comprises:(i) incorporating a lethal gene into a donor plasmid comprising an expression cassette;(ii) transposing the expression cassette from the donor plasmid into a bacmid in E. coli cells to form a recombinant bacmid, wherein the lethal gene product kills the cells still harbouring the donor vector;(iii) extracting the recombinant bacmids; and(v) transfecting insect cells with recombinant bacmids to form recombinant baculoviruses.
Owner:ARK THERAPEUTICS
Nucleic acid composition and kit for detecting diarrhea viruses as well as use method of kit
InactiveCN109593890AMicrobiological testing/measurementMicroorganism based processesRotavirus RNASapovirus
The invention relates to nucleic acid composition and a kit for detecting diarrhea viruses as well as a use method of the kit. The nucleic acid composition for detecting diarrhea viruses comprises anamplification primer pair and detection probes corresponding to amplification primers. The nucleic acid composition for detecting diarrhea viruses can at least detect three of group A rotavirus, groupB rotavirus, group C rotavirus, norovirus, norovirus I type, norovirus II type, sapovirus, astrovirus and adenovirus simultaneously and has high detection sensitivity and good specificity.
Owner:深圳市刚竹医疗科技有限公司
Triple fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR) detection kit and use thereof
ActiveCN102206713AQuantitatively accurateReduce mortalityMicrobiological testing/measurementMicroorganism based processesAvastrovirusBiochemistry
The invention provides a triple fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR) detection kit, which comprises quantitative RT-PCR reaction liquid, standard substances and reference substances. A kit body is provided with container holes in which quantitative RT-PCR reaction liquid tubes, a Sapovirus standard substance tube, an astrovirus standard substance tube, an adenovirus standard substance tube, a positive reference substance tube and a negative reference substance tube are arranged respectively, wherein the quantitative RT-PCR reaction liquid tubes are separately arranged and arrayed in a matrix; the standard substances are standard substances for Sapovirus, astrovirus and adenovirus; and the reference substances are positive and negative reference substances. By a real-time fluorescence quantitative RT-PCR technology, three kinds of virus specific primers and specific fluorescent probes are adopted, the design is reasonable, the kit can detect the Sapovirus, astrovirus and adenovirus from a sample through PCR reaction, and the detection method is simpler, quicker and more accurate.
Owner:ZHEJIANG UNIV
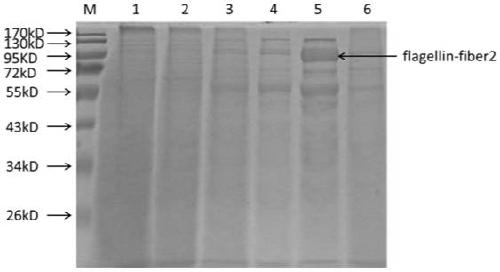
Flaggelin-fiber2 fusion protein, and preparation method and application thereof
ActiveCN109867727AEnhance immune responseStrong immune adjuvant effectAntiviralsAntibody medical ingredientsWestern blotFlagellin
The invention provides a flaggelline-fiber2 fusion protein as well as a preparation method and application thereof. The fusion protein is a fusion protein of a fowl adenovirus type 4 fiber2 protein with relatively high immunoprotection and a salmonella typhimurium flagellin. The preparation method comprises the following steps: cloning an artificially coded flaggin-fiber2 gene into a pFastBac-HA expression vector through fusion; carrying out gene transposition to form recombinant Bacmid, transfecting the recombinant Bacmid into Sf9 insect cells, expressing the fusion protein by utilizing a baculovirus system, and conducting identifying by virtue of indirect immunofluorescence IFA and Western blot. The period of obtaining the recombinant baculovirus through the system is short, and when SPFchicken are immunized by the flaggin-fibe2 fusion protein, results show that the flaggin-fibe2 fusion protein expressed by the baculovirus system has high immunoprotection capability.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Pseudotyped baculovirus to stimulate immunogenicity against avian influenza
The current invention relates to vaccines that use baculovirus vectors to expose a host organism to an immunogen, thereby eliciting an immune response. A pseudo-typed baculovirus is used to display hemagglutinin on the cell membrane in order to increase host immunogenicity.
Owner:NATIONAL TSING HUA UNIVERSITY
Human papillomavirus type bivalent virus-like particle mixed protein antigen and construction method
InactiveCN1683010AImproving immunogenicityImprove responseAntiviralsRecombinant DNA-technologyVaccine ImmunogenicityIndividual animal
The present invention relates to human papillomavirus type bivalent virus-like particle mixture, and human papillomavirus type bivalent virus-like particle mixing protein antigen and its construction method. The present invention includes one kind of cervical carcinoma virus HPV16 L1 antigen and one kind of condyloma acuminata virus HPV11 L1 antigen. The HPV11 L1 gene is first cloned from condyloma acuminata tissue through molecular geological method, and both HPV16 L1 gene cloned from cervical carcinoma tissue and the HPV11 L1 gene are then expressed in the insect cell expression system of baculovirus, so as to constitute recombinant virus strain Bacmid / HPV16-HPV11L1. Animal immunizing test shows that the constituted recombinant protein has excellent immunogenicity and immunoreactivity, and may be used as candidate antigen of gene engineering multivalent vaccine for preventing condyloma acuminata and cervical carcinoma.
Owner:HARBIN MEDICAL UNIVERSITY
Recombinant PRRSV virus-like particles having immunogenicity and preparation thereof
ActiveCN109385435ABroad-spectrum cross-immunogenicityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsSpecific immunityTransfer vector
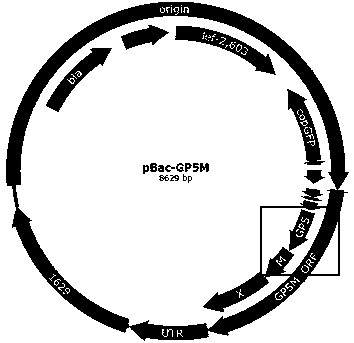
The invention discloses recombinant porcine reproductive and respiratory syndrome virus (PRRSV) virus-like particles (VLP) and a preparation method and an application thereof. Based on comparative analysis of GP5 of a PRRSV epidemic strain and an M gene sequence, a GP5 and M tandem sequence GP5M is synthesized artificially, the synthesized GP5M gene sequence is cloned into a vector with a pBAC5 plasmid as a skeleton, the baculovirus transfer vector pBAC-PRRSVGP5M is obtained, the recombinant bacmid rBacmid-GP5M is obtained, sf9 cells are transfected with the bacmid, and the recombinant baculovirus Ac-PRRSVGP5M is obtained. The PRRSV GP5 and M protein are expressed efficiently by the recombinant baculovirus, and the virus-like particles are formed. A subunit vaccine prepared by the proteinexpressed by the recombinant baculovirus can induce a body to produce a specific immune response after immunizing animals and can protect the pig body against the strong poison attacking of porcine reproductive and respiratory syndrome virus.
Owner:陕西诺威利华生物科技有限公司
Recombinant baculovirus expressing porcine parvovirus VP2 protein as well as preparation method and application
InactiveCN104561049AHigh expressionGenetic material ingredientsAntiviralsVp2 geneVirus-like particle
The invention discloses recombinant baculovirus expressing porcine parvovirus VP2 protein as well as a preparation method and an application. The method comprises the following steps: artificially synthesizing VP2 gene by referring to the VP2 gene sequence of a porcine parvovirus (PPV) isolate; with pFBDPHmHNM1P10eGFP plasmid as a skeleton, connecting the synthesized VP2 gene to the plasmid to obtain a baculovirus transfer vector pFBDPHm3VP2 and then obtain recombinant bacmid rBacmid-PPVP2; and transfecting the bacmid with sf9 cell to obtain recombinant baculovirus Ac-PPVP2. The recombinant baculovirus Ac-PPVP2 efficiently expresses PPV VP2 protein and successfully forms virus-like particles. The protein expressed by the recombinant baculovirus disclosed by the invention is used for preparing a subunit vaccine; and after the subunit vaccine immunizes an animal, the body can be induced to generate a specific immunoreaction, and the porcine body can be fully protected from the attack of strong poison of parvovirus.
Owner:HUAZHONG AGRI UNIV
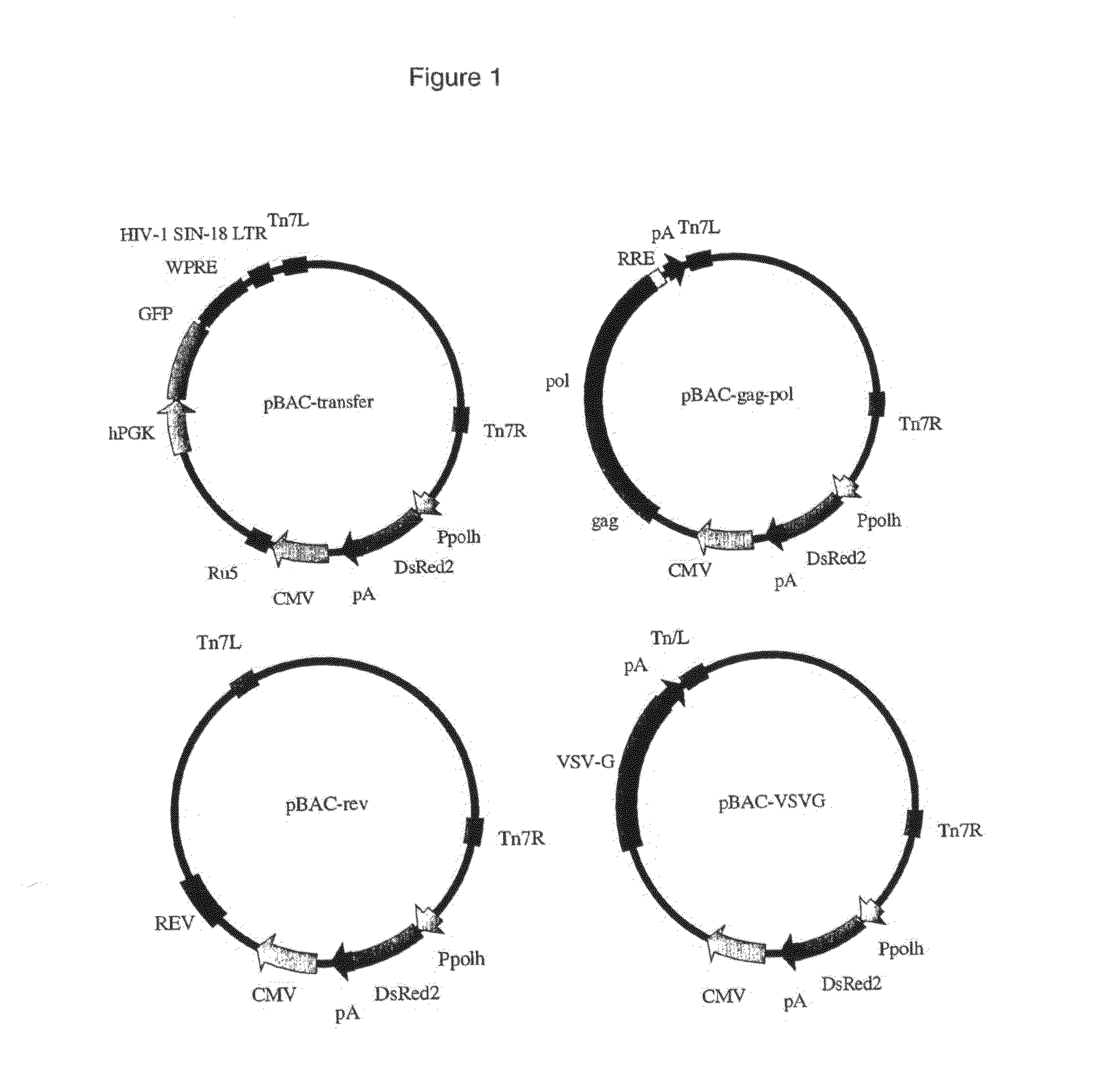
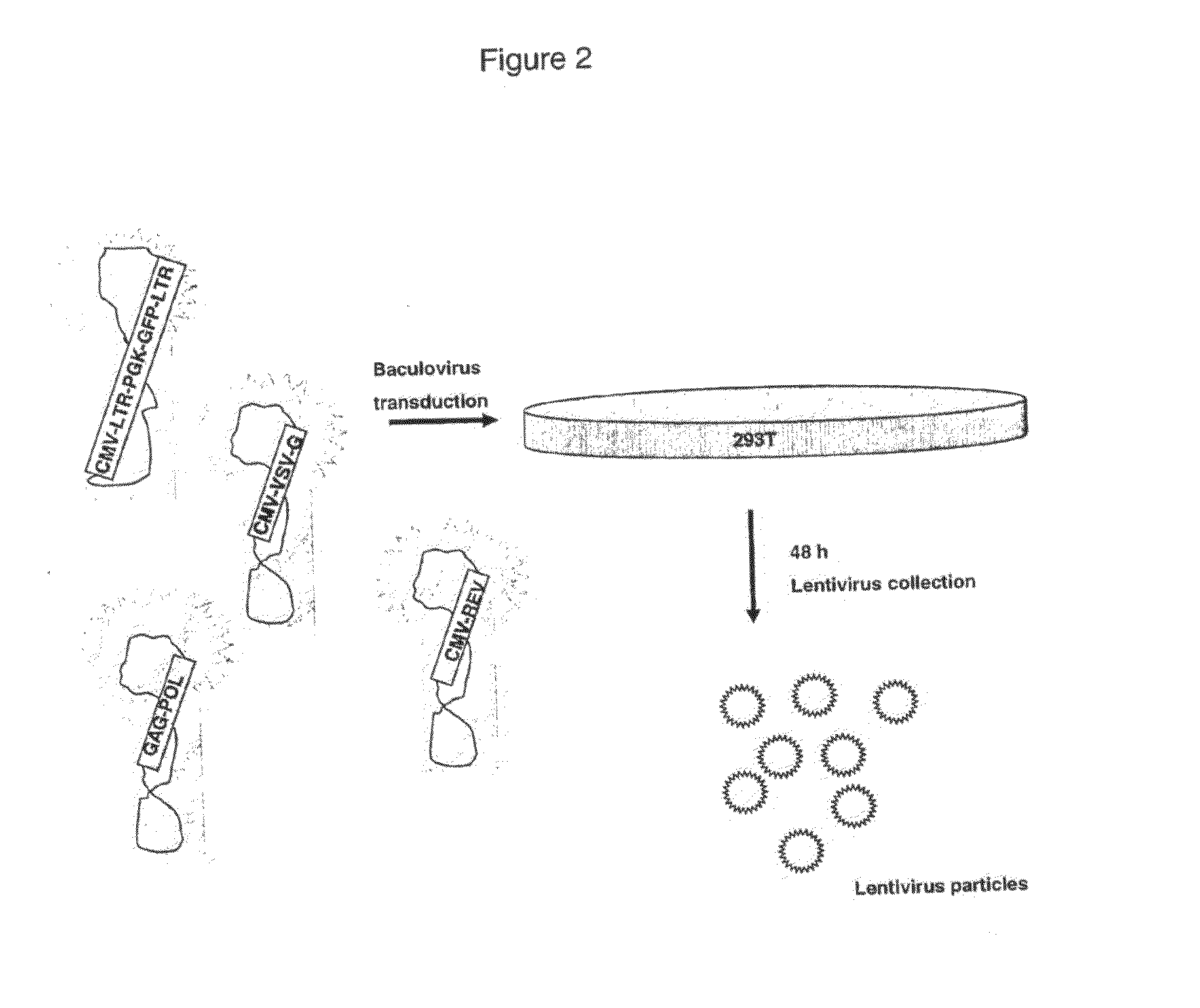
Production of Lentiviral Vectors
The present invention is a method of generating a lentivirus vector, comprising cloning each of a leotivimus transfer construct, gag, pol, an envelope protein and rev respectively into the same or different baculoviruses, and transducing a producer cell with the or each baculovirus.
Owner:TRIZELL LTD
Production of papillomavirus capsid protein and virus-like particles
ActiveUS8062642B1High expressionPeptide/protein ingredientsViral antigen ingredientsBaculovirus expressionVirus-like particle
The present invention is directed to a method of expressing the papillomavirus capsid protein coding sequence in a cell using an expression system under conditions facilitating expression of the protein in the cell.In another aspect of the invention, it has been discovered that virus-like particle(s) (VLPs), fragment(s), capsomer(s) or portion(s) thereof are formed from the papillomavirus capsid protein. It was further discovered that the virus-like particle(s) comprises antigenic characteristics similar to those of native infectious papillomavirus particles.In an embodiment of the invention, there is provided a method of expressing the L1 major capsid protein of human papillomavirus type-11 (HPV-11) in Sf-9 insect cells using the baculovirus expression system, and the production of HPV-11 virus-like particles.
Owner:UNIVERSITY OF ROCHESTER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com