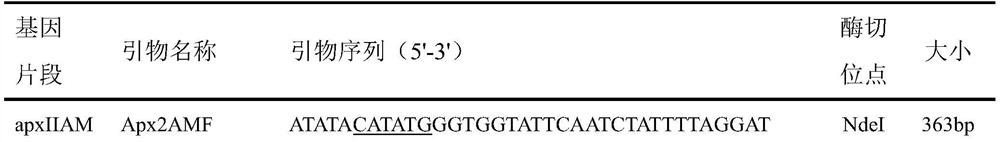Patents
Literature
127 results about "Truncated protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The truncated protein, the fusion protein, the conjugate, the pharmaceutical compositions and the vaccines can be used for preventing, relieving or treating rotavirus infection and diseases caused by rotavirus infection, for example, rotavirus gastroenteritis and diarrhea. Also disclosed are uses of the truncated protein,...
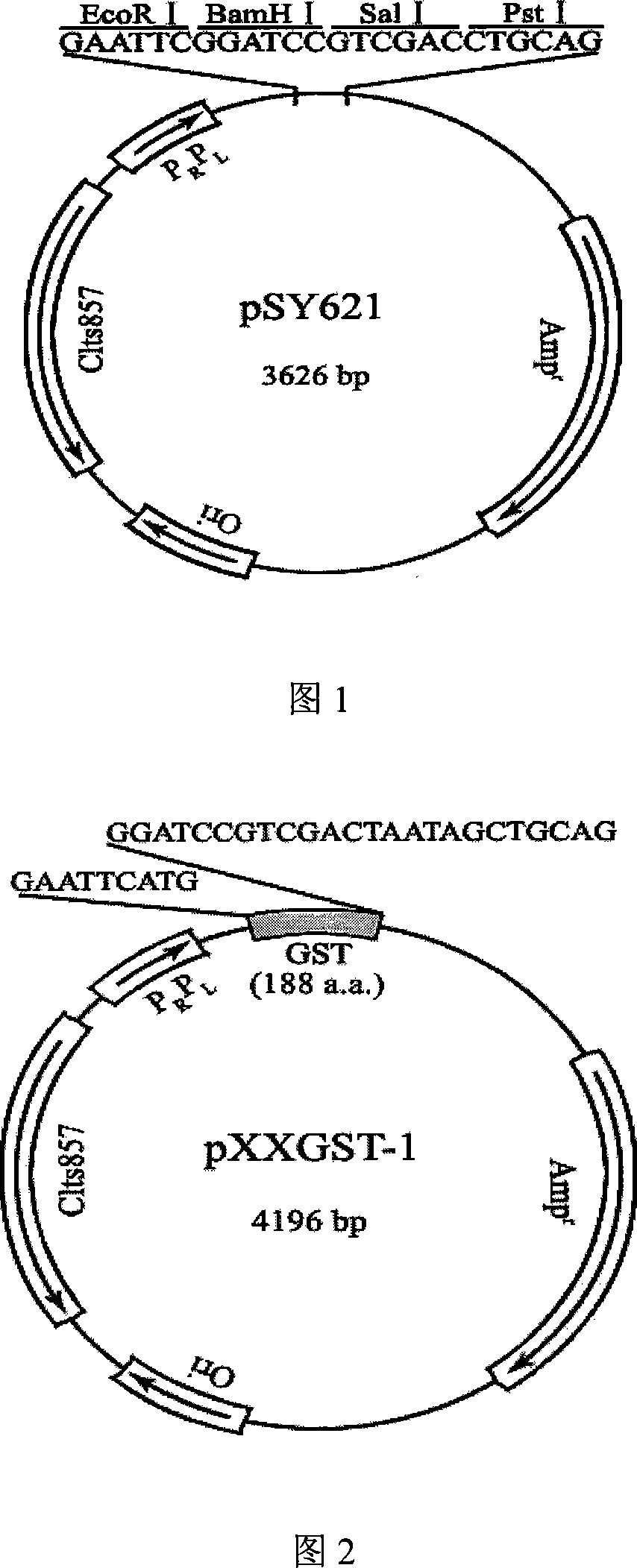
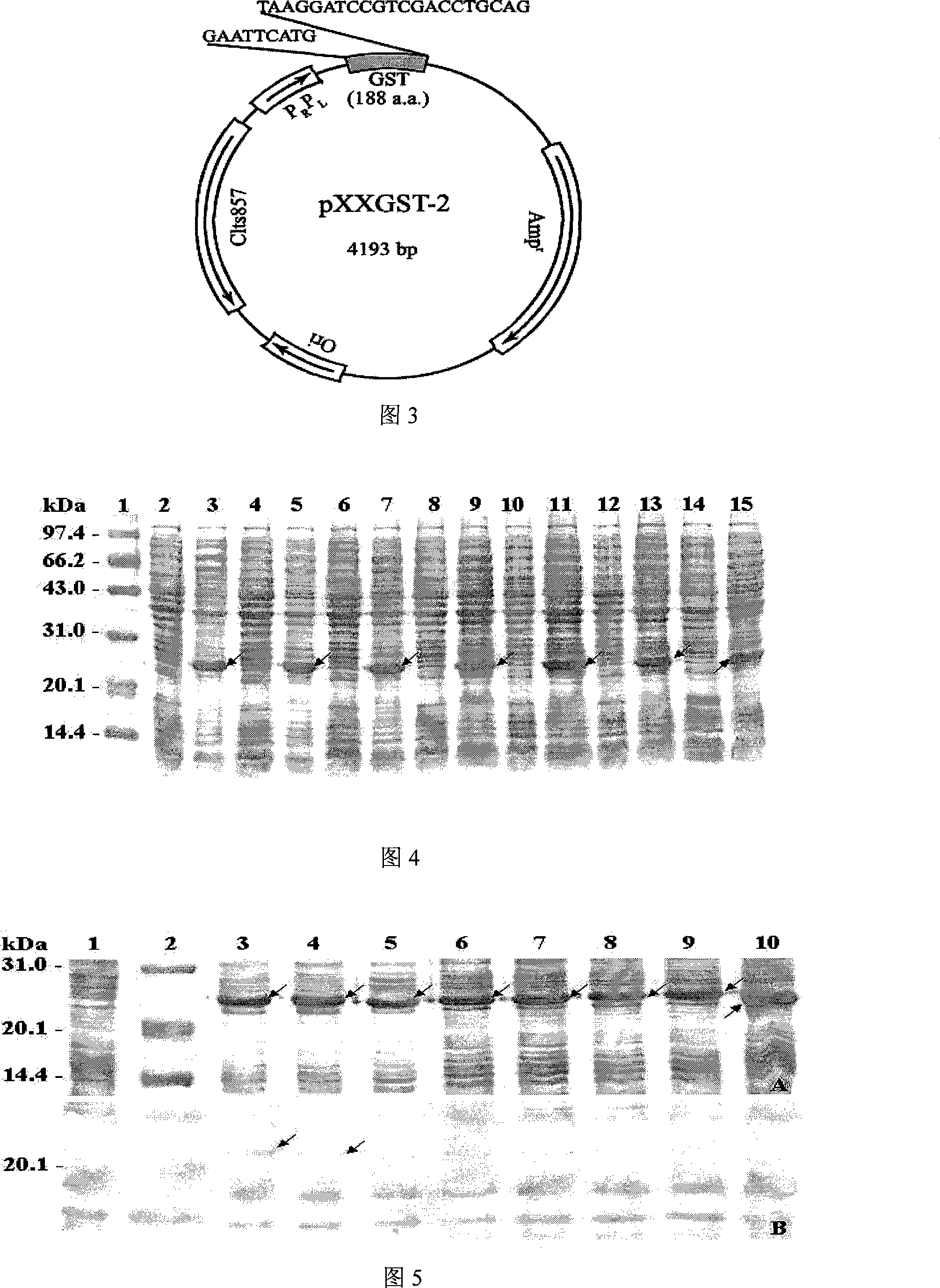
Cropping GST protein thermal induction fusion expression plasmid and preparation method thereof
InactiveCN101215573ASuitable for western blot experimentsWill not affect judgmentVector-based foreign material introductionDNA/RNA fragmentationEscherichia coliProtein target
The invention belongs to the technical field of biological engineering and in particular is a heat-induced fusion expression recombinant plasmid of truncated GST protein and a process for preparation. The invention selects utility truncated GST188 protein which is composed of 188aa as a carrier, a pXXGST-1 recombinant plasmid which expresses short peptide fusion protein in an escherichia coli heat-induced expression system and a heat-induced type pXXGST-2 recombinant plasmid which is used to express comparison to realize the aim of synthesizing short peptide creatures, which is especially provided for scanning, drawing, positioning antigen linear epitopes and identifying epitope motif. Experiments of the invention prove the adaptability and the applicability of the GST188 core protein which is used as the short peptide creatures expression vector, in antigen epitope scanning identification, and in particular when anti- recombinant target protein antiserum is used to indentify the epitope motif.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +2
Truncated recombinant major outer membrane protein antigen (R56) of orientia tsutsugamushi strains Karp, Kato and gilliam and its use in antibody based detection assays and vaccines
A recombinant, refolded non-fusion polypeptide expressed from a truncated r56 gene of the causative agent of scrub typhus, Orientia tsutsugamushi for the Karp, Kato and Gilliam strains has been produced. The invention is useful for detecting prior exposure to scrub typhus, screening for and / or identification of at least one infectious strain-similarity (i.e. a Karp-like, Kato-like or Gilliam-like strain) based on its strength of reaction toward a truncated protein and as a component in vaccine formulations and production of immune globulins for passive prophylaxis and immunity in subjects.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY NAVAL RES LAB WASHINGTON
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
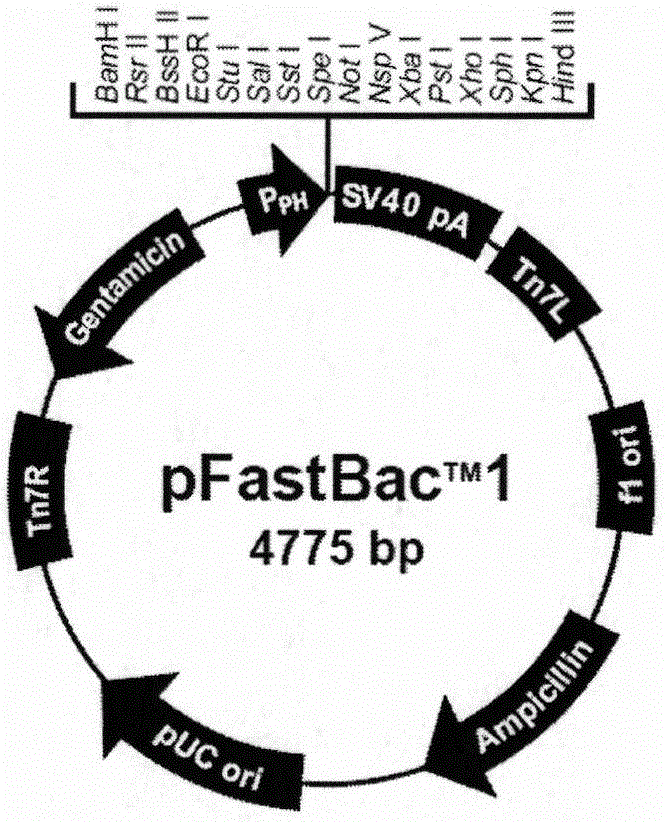
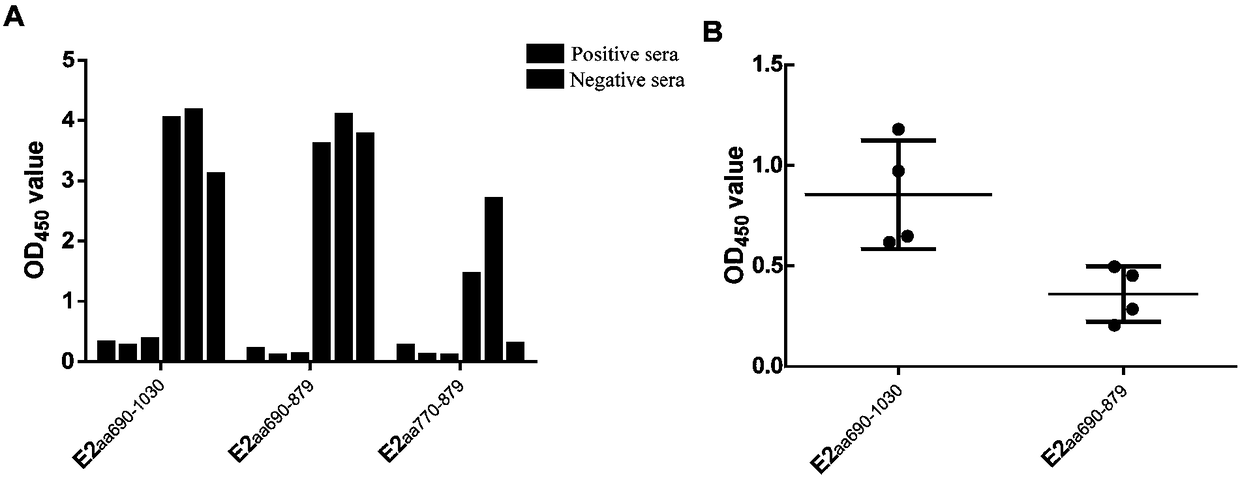
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Cell
ActiveUS20190023761A1Prevent and treat diseasePolypeptide with localisation/targeting motifImmunoglobulin superfamilyHeterologousPhosphorylation
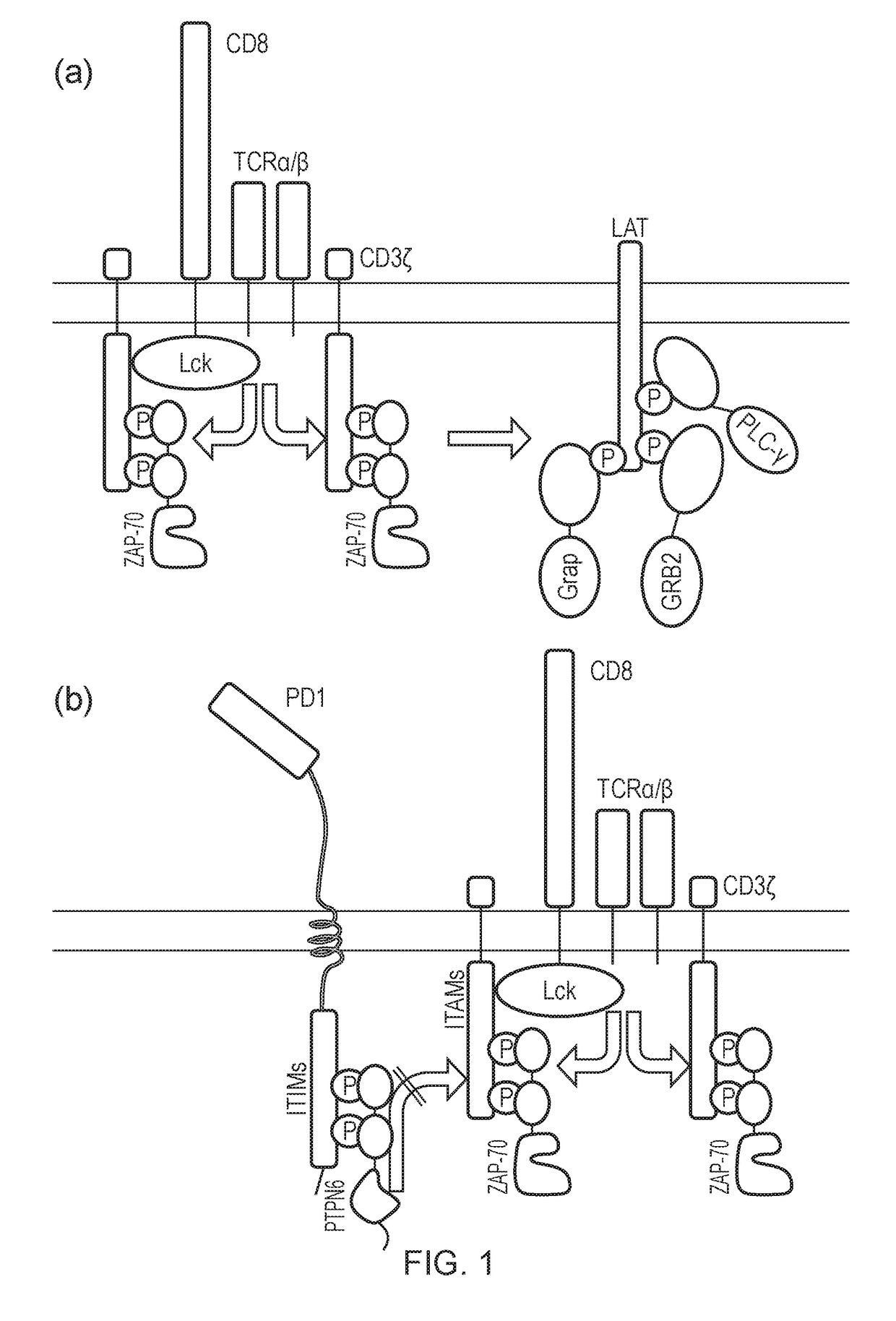
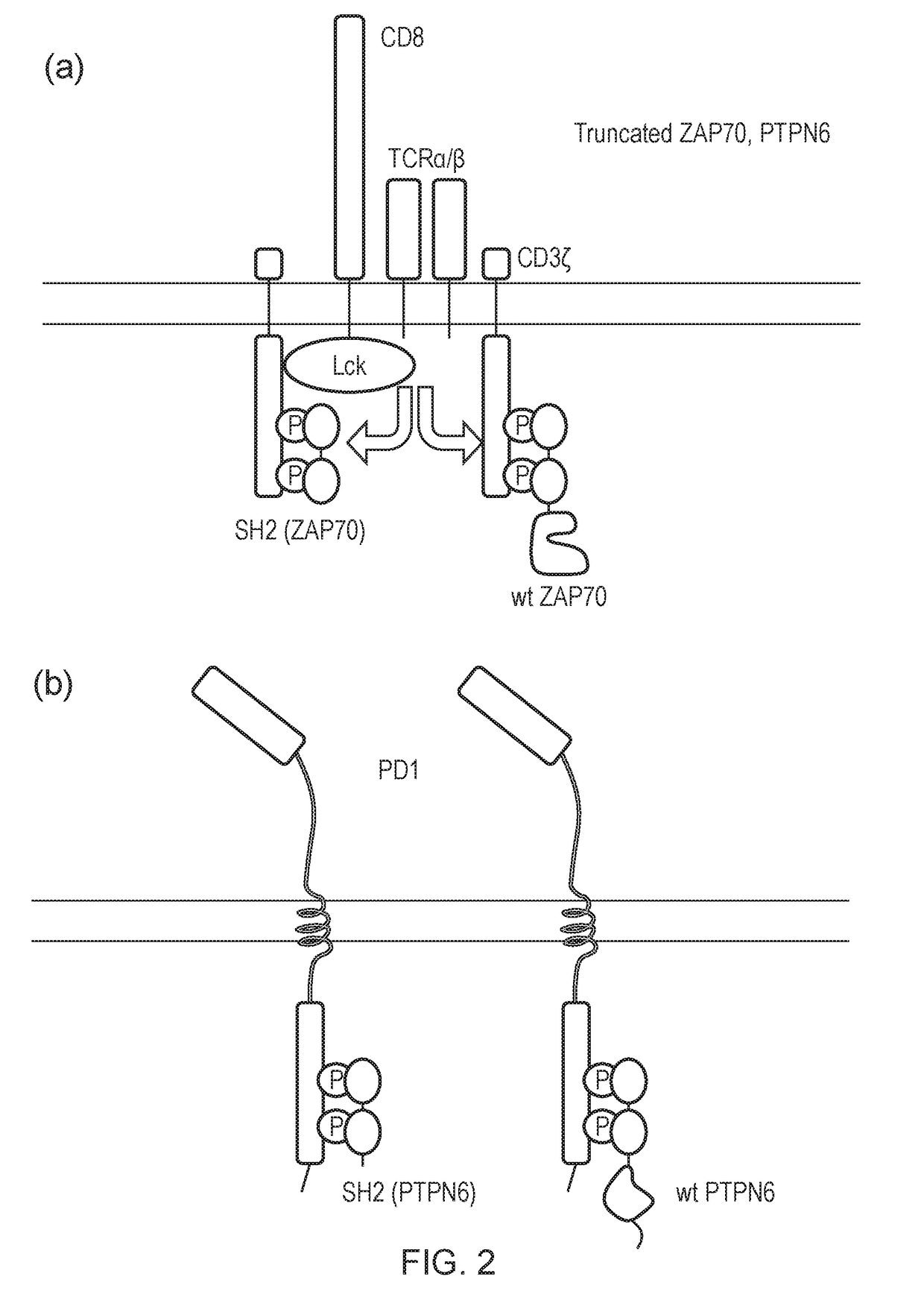
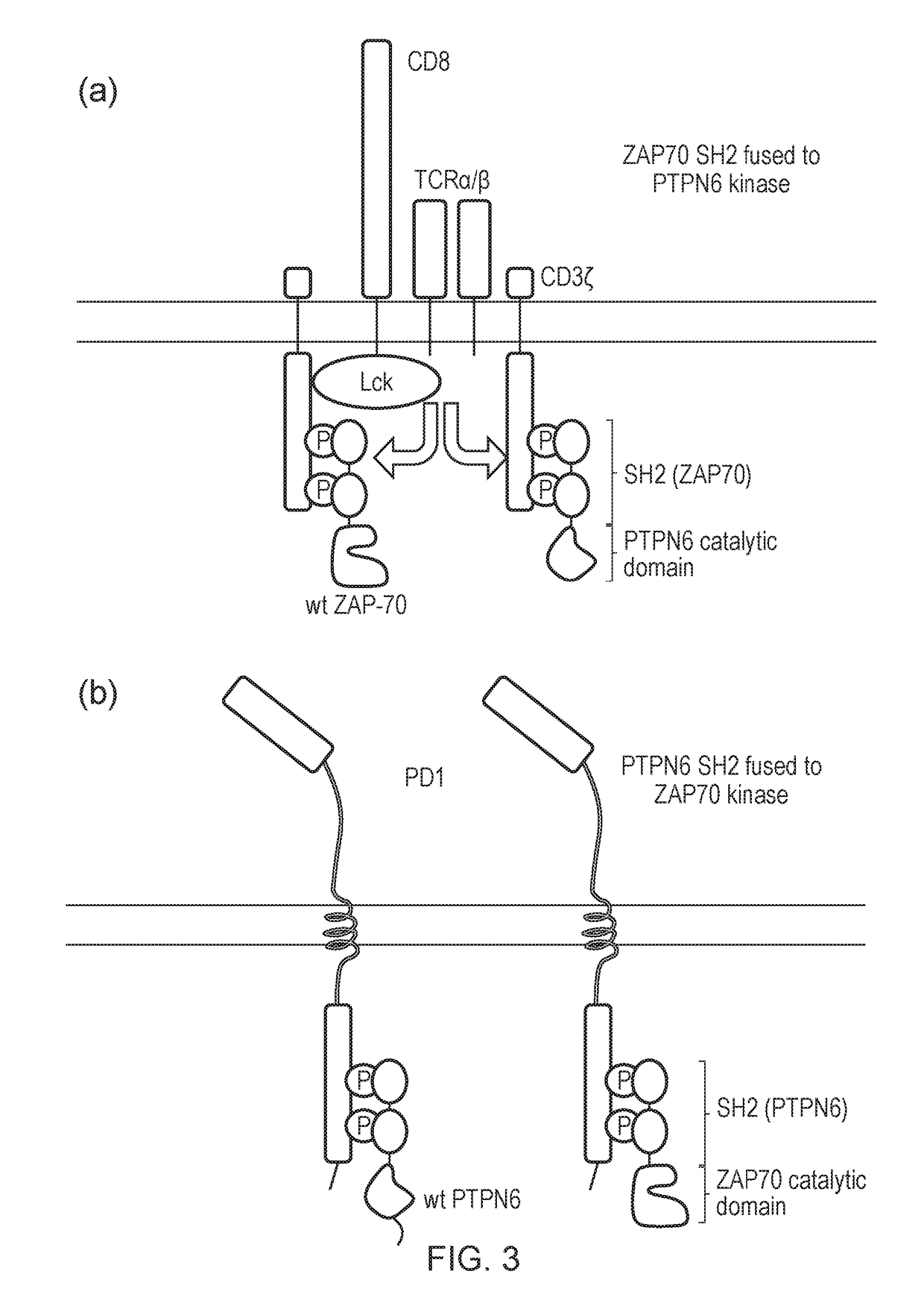
The present invention relates to a cell which comprises a chimeric antigen receptor (CAR) and a signal transduction modifying protein, selected from one of the following: (i) a truncated protein which comprises an SH2 domain from a protein which binds a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM), but lacks a kinase domain; (ii) a truncated protein which comprises an SH2 domain from a protein which binds a phosphorylated immunoreceptor tyrosine-based inhibition motif (ITIM) but lacks a phosphatase domain; (iii) a fusion protein which comprises (a) an SH2 domain from a protein which binds a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) or from a protein which binds a phosphorylated immunoreceptor tyrosine-based inhibition motif (ITIM); and (ii) a heterologous domain.
Owner:AUTOLUS LIMIED
Classic swine fever virus (CSFV) C strain E2 truncated protein and its preparation method and use
ActiveCN103588864ASimplifyEasy to purifySsRNA viruses positive-senseVirus peptidesInclusion bodiesElisa kit
The invention discloses a classic swine fever virus (CSFV) C strain E2 truncated protein and its preparation method and use. The main antigen region of the E2 gene is expressed and three pairs of disulfide bonds for maintaining the antigen space structure are expressed simultaneously so that the expressed protein has a certain space structure and satisfies natural protein characteristics and the inclusion body is conducive to protein purification. The E2 truncated protein obtained by the invention is used for building a CSFV IgG antibody ELISA detection method. A result shows that the recombinant protein has good antigenicity. The built ELISA kit adopting the CSFV C strain E2 truncated protein has good specificity, accuracy, sensitivity and repeatability after use. A substrate coloration solution used in the invention has an indication effect, and after several hours to 4 nights after the reaction, the detection result still has credibility. Therefore, the coloration system can reduced human factor influences, avoids false positive appearance, makes a negative result stable and guarantees good detection on CSFV antibodies.
Owner:SOUTH CHINA AGRI UNIV
Hog cholera virus truncated E2 protein and application of same
ActiveCN108107217APreserve antigenicityReduce manufacturing costBiological testingBovine Viral Diarrhea VirusesBiology
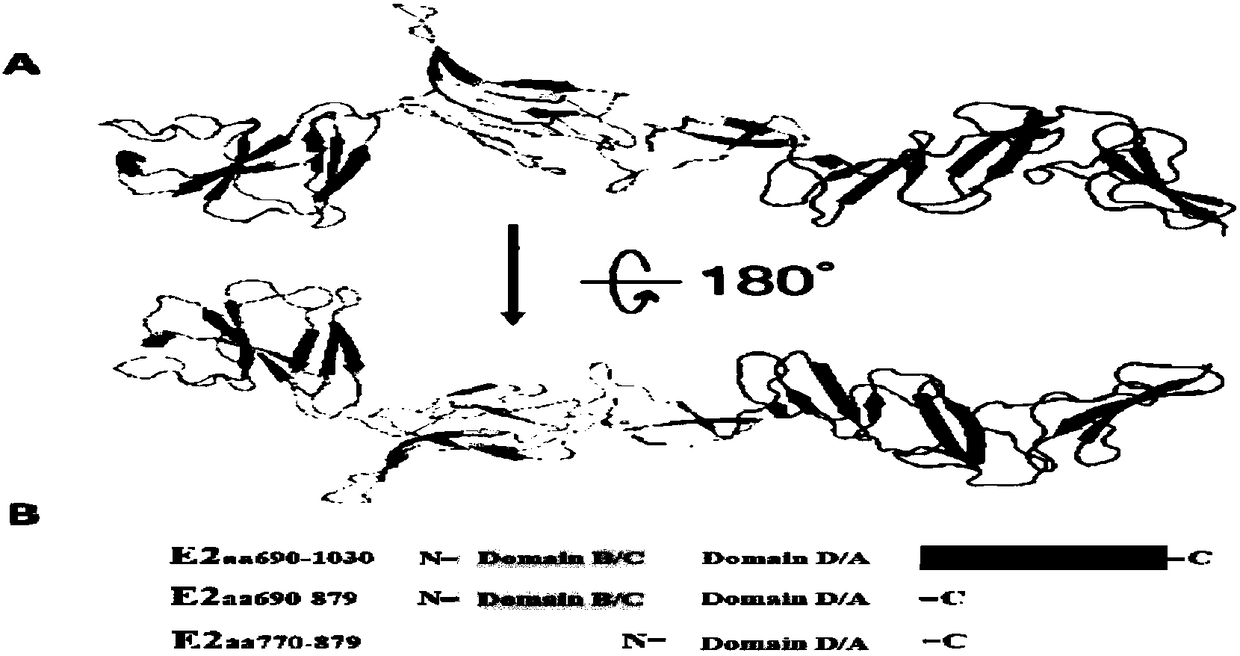
The invention discloses a hog cholera virus truncated E2 protein which is designed on the basis of protein spatial structure, and an application of the same. In the invention, according to the crystalstructure of bovine viral diarrhea virus E2 protein, the spatial structure of the hog cholera virus E2 protein is simulated, and then the hog cholera virus E2 protein is subjected to truncated expression, wherein the amino acid sequence of the truncated protein E2B / C / D / A is represented as the SEQ ID No.1. The truncated protein can maintain the complete antigenicity of the E2 protein, and has no cross reaction with a bovine viral diarrhea virus antibody. The invention further constructs a CHO cell line which stably expresses the truncated protein E2B / C / D / A and is assigned the accession numberof CGMCC No.14722. The invention also discloses an indirect ELISA kit which is used for detection of a hog cholera virus antibody, wherein the enveloped antigen is the hog cholera virus truncated protein E2B / C / D / A. The kit is used for specifically detecting the hog cholera virus antibody with high specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Methods for enriching subpopulations
InactiveUS20090264298A1Easy to reuseDirected macromolecular evolutionLibrary creationBiologyTruncated protein
Methods of enriching a subpopulation of beads are described. In one embodiment, first beads comprise immobilized first amplified product, said first amplified product encoding a truncated version of a first protein, and second beads comprise immobilized second amplified product, said second amplified product encoding an untruncated version of said first protein. Both first and second beads are exposed to a translation system under conditions such that said truncated and untruncated versions of said first protein are generated from at least a portion of said first and second immobilized amplified products, and these protein products are captured on the first and second beads, respectively. Using a ligand (e.g. with affinity for the untruncated version of said first protein), a portion of the second beads is separated from the mixture, thereby enriching a subpopulation of beads comprising truncated protein.
Owner:AMBERGEN
Expressing TGF-beta proteins in plant plastids
InactiveUS20060162026A1Easy to integrateEasy to identifyBiocideAnimal repellantsReceptorProstate cancer
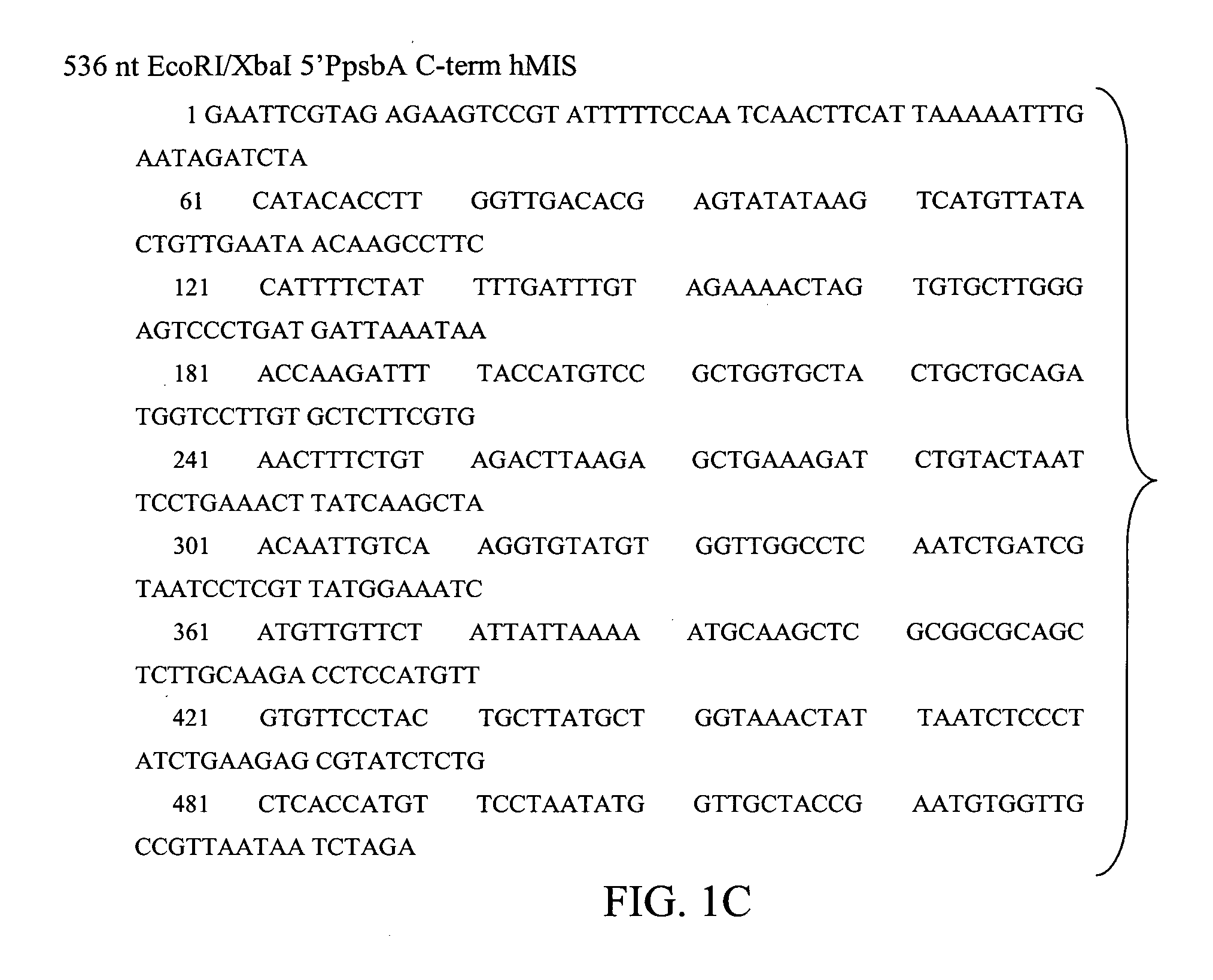
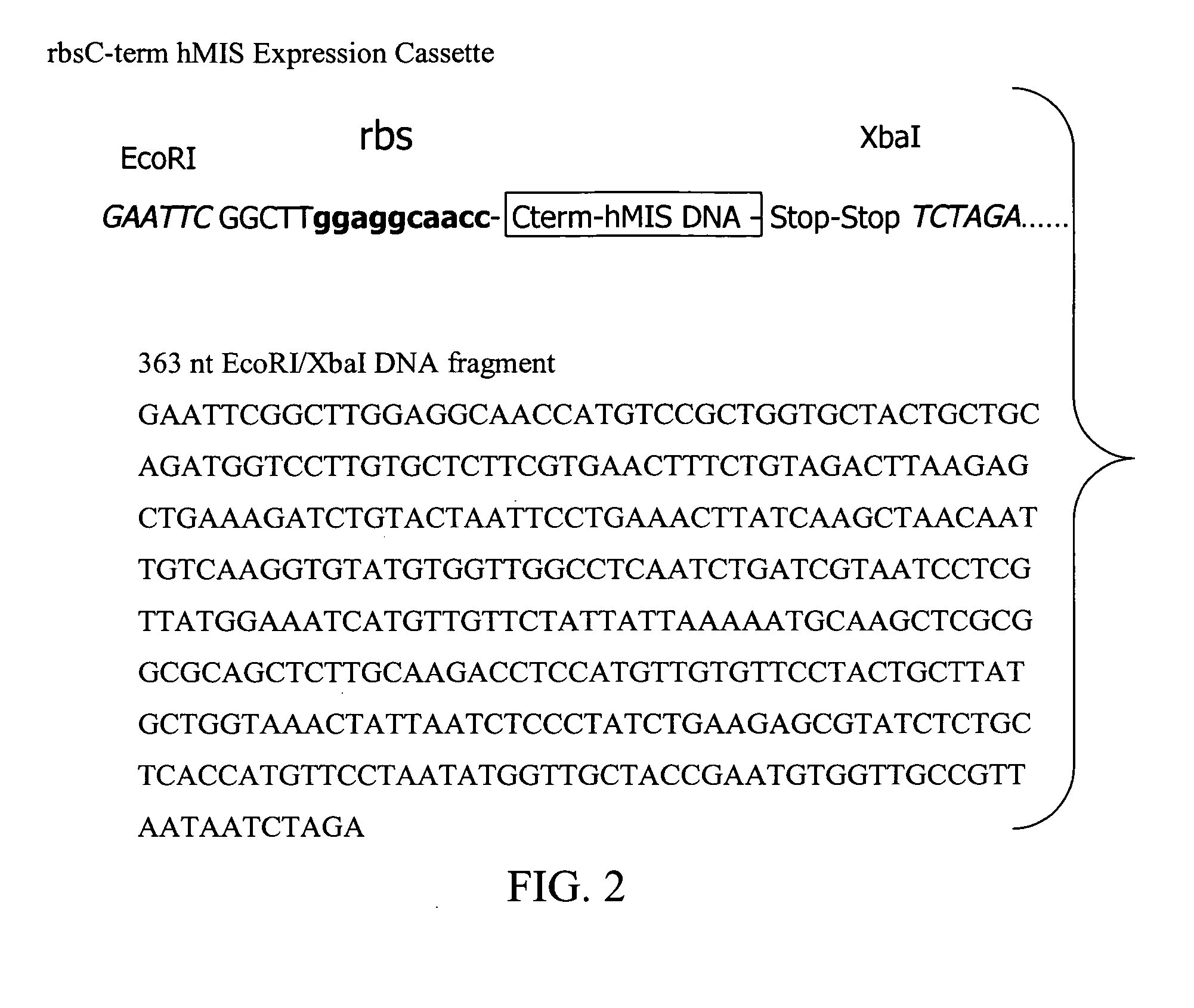
Bioactive TGF-β proteins are expressed in transgenic plastids. The TGF-β proteins are used for therapeutic and diagnostic purposes. In particular, Mullerian Inhibitor Substance (MIS), either full length or truncated proteins, are expressed in plastids and used for treating various cancers that contain MIS receptors such as ovarian cancer, breast cancer and prostate cancer.
Owner:CHLOROGEN
Duck tembusu virus E truncated protein and application
ActiveCN107656066AStrong specificityHigh compliance rateSsRNA viruses positive-senseVirus peptidesYolkDisk diffusion susceptibility test
The invention belongs to the technical field of detection of animal virology and animal infectious diseases, and particularly discloses a duck tembusu virus E truncated protein and application. Protein of an amino acid sequence as shown in SEQ ID NO. 2 is used as a coating antigen, a duck tembusu virus serum detection kit which is prepared from a hybridoma cell strain under the accession number CCTCC NO: C2017169 is used for detecting duck serum and an egg yolk antibody which are infected by wild type virus, and has good specificity; and the sensitivity is high, and after being diluted to be 1: 12800, duck tembusu virus positive serum can still be positive when detected. Compared with the traditional agar diffusion test, the duck tembusu virus E truncated protein is high in coincidence rate, simple and convenient to operate, short in detection time and suitable for simultaneously detecting a large number of samples.
Owner:HUAZHONG AGRI UNIV
Truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi strains Karp, Kato and Gilliam and its use in antibody based detection assays and vaccines
A recombinant, refolded non-fusion polypeptide expressed from a truncated r56 gene of the causative agent of scrub typhus, Orientia tsutsugamushi for the Karp, Kato and Gilliam strains has been produced. The invention is useful for detecting prior exposure to scrub typhus, screening for and / or identification of at least one infectious strain-similarity (i.e. a Karp-like, Kato-like or Gilliam-like strain) based on its strength of reaction toward a truncated protein and as a component in vaccine formulations and production of immune globulins for passive prophylaxis and immunity in subjects.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY NAVAL RES LAB WASHINGTON
Group I type 4 avian adenovirus subunit protein as well as preparation method and application thereof
The invention belongs to the technical field of biology, and discloses group I type 4 avian adenovirus subunit protein with an immunoprotection function. The subunit protein is group I type 4 avian adenovirus short fiber mutation protein spherical region truncated protein; the antigen from amino acid 283 site to amino acid 479 site determines the cluster amino acid sequence; better antigenicity and immunogenicity are realized. The group I type 4 avian adenovirus subunit protein and pharmaceutically acceptable adjuvant is prepared into vaccine; a preparation method of the vaccine mainly comprises the steps that firstly, the group I type 4 avian adenovirus short fiber mutation protein spherical region truncated protein nucleotide sequence subjected to codon optimization is cloned into the carrier; then, a recombinant vector is subjected to conversion, inducible expression and purification to obtain antigen protein; finally, the antigen protein and the adjuvant are emulsified to obtain the vaccine. The vaccine is safe and effective; the method for preparing the subunit vaccine is simple; the cost is low; the amplification is easy.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Establishment of method for preparing recombinant protein vaccine of type A H1N1 influenza virus
InactiveCN101716340AEngineering antibody technology is simpleLow costAntiviralsAntibody medical ingredientsEscherichia coliAntigenic analysis
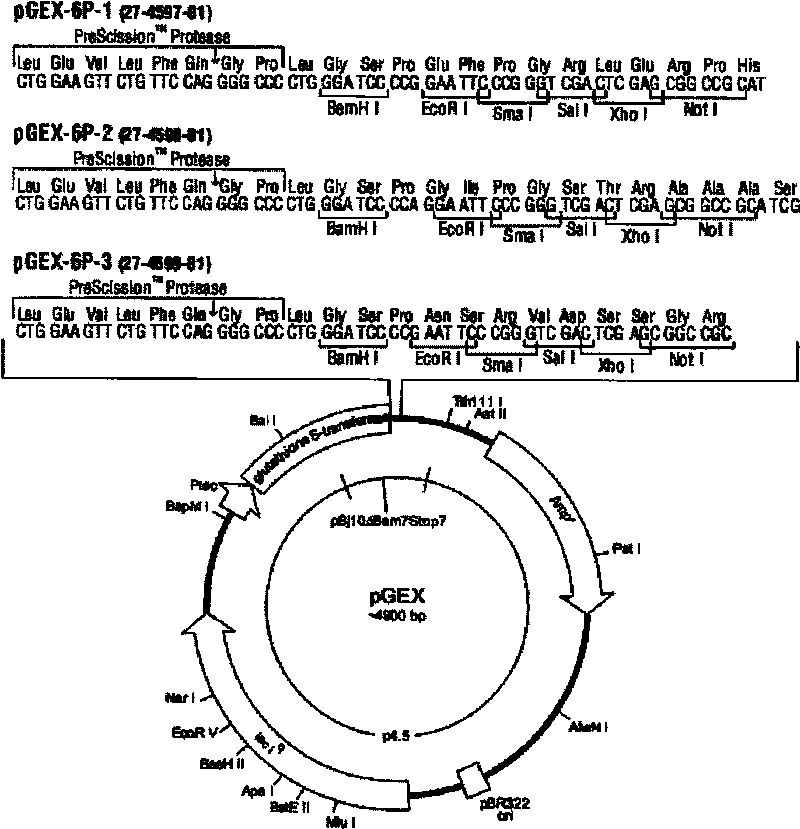
The invention belongs to the field of zoonosis researches, and relates to a method for preparing a vaccine of zoonosis. In the method, an HA gene in a cDNA sequence of a type A H1N1 California virus strain (A / California / 08 / 2009(H1N1)) is selected as a research content; the method comprises the following steps of: firstly, analyzing a sequence of the part of an HA protein leaked outside an envelope according to structural biology software; secondly, selecting a part with high immunogenicity according to an antigenic analysis, and constructing a prokaryotic expression vector pGEX-6P-1-HA by using a genetic fragment obtained through a gene synthesis method; and thirdly, converting a masculine recombinant plasmid into Escherichia coli to obtain a recombinant strain (Escherichia coli BL21 rosseta / pGEX-6P-1-HA truncated protein). Through a plurality of chromatography methods, a purified HA truncated protein of the H1N1 is obtained; and by utilizing the expression vector and an adjuvant together to immune organisms, the protective effect on the H1N1 virus is achieved.
Owner:CUSABIO TECH LLC
Materials and Methods for Producing Animals With Short Hair
ActiveUS20160081313A1Improve productivityImprove embryo survivalAnimal cellsSugar derivativesAnimal scienceProviding material
The subject invention provides materials and methods for producing animals with short hair length. In a preferred embodiment, this is accomplished by altering in the animal the nucleotide sequence that encodes the prolactin receptor (PRLR) protein such that a truncated version of the protein is produced. Advantageously, and surprisingly, the truncated protein produced according to the subject invention retains lactogenic functionality, but causes the animal to have a short-hair coat.
Owner:ACCELIGEN INC
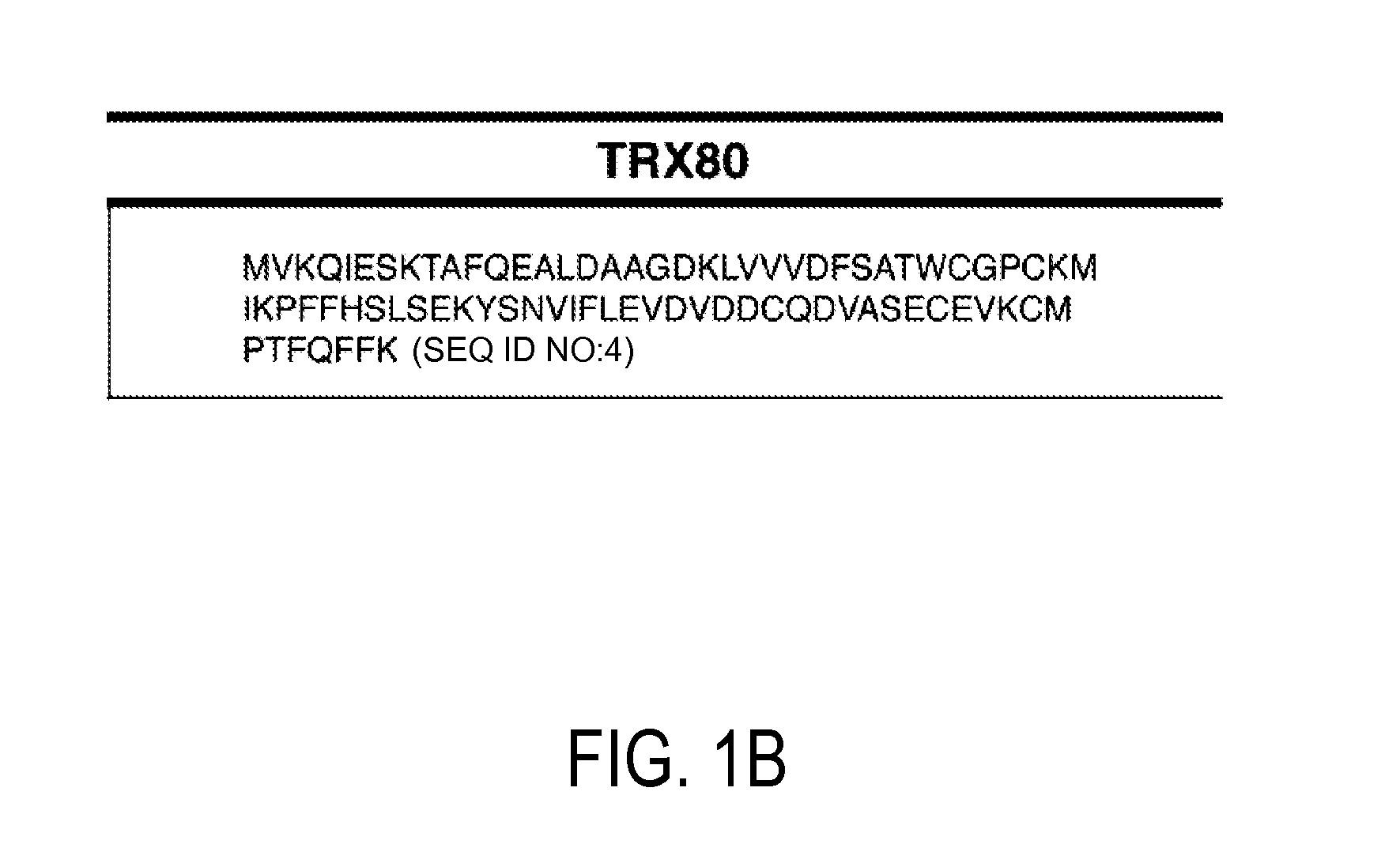
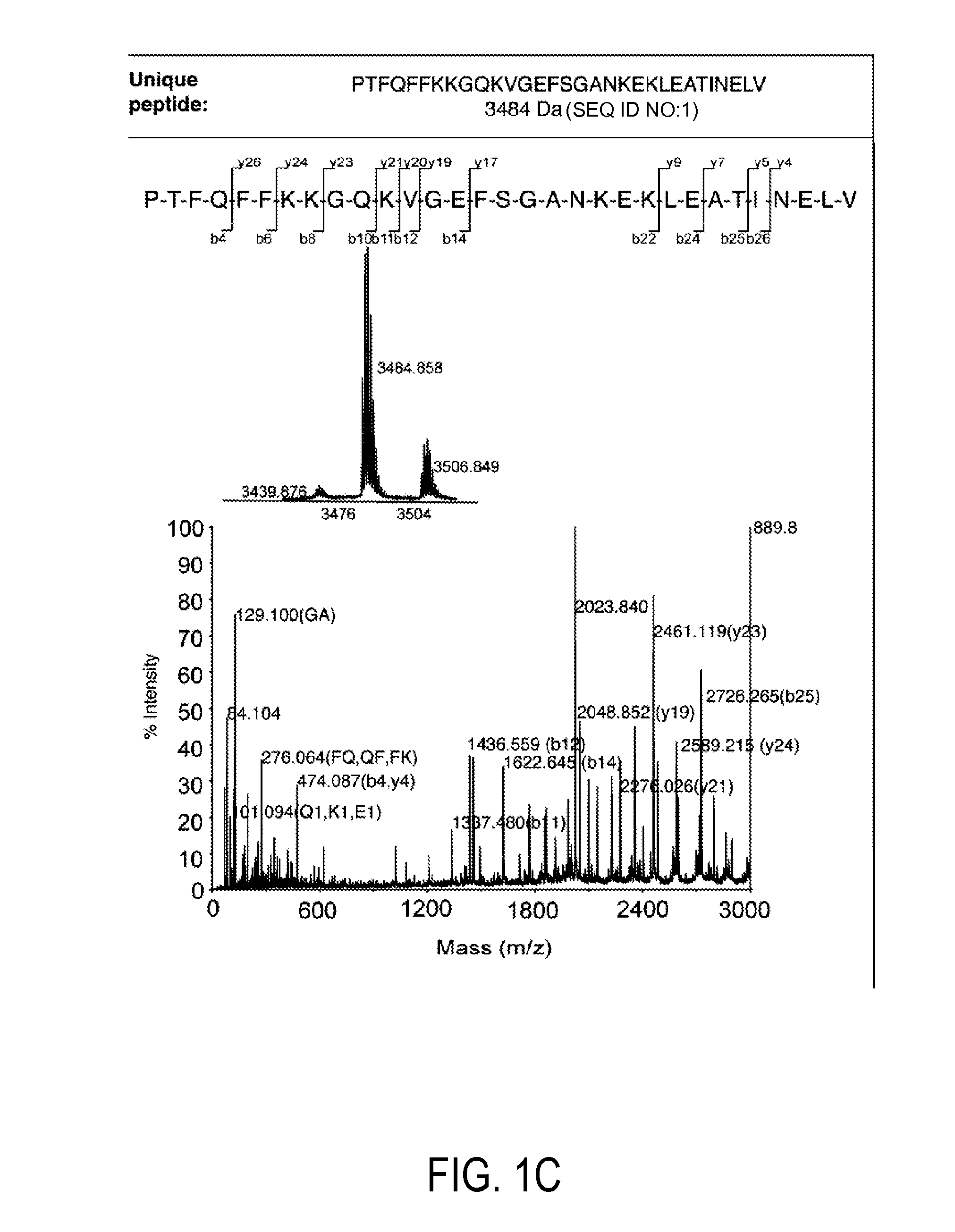
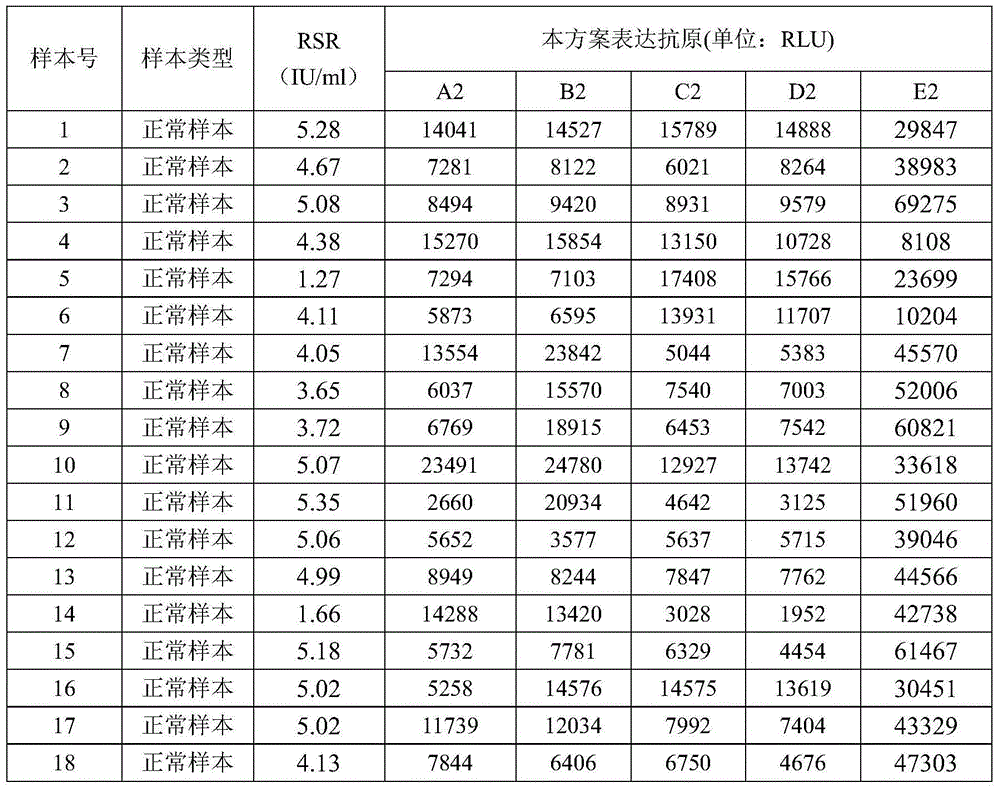
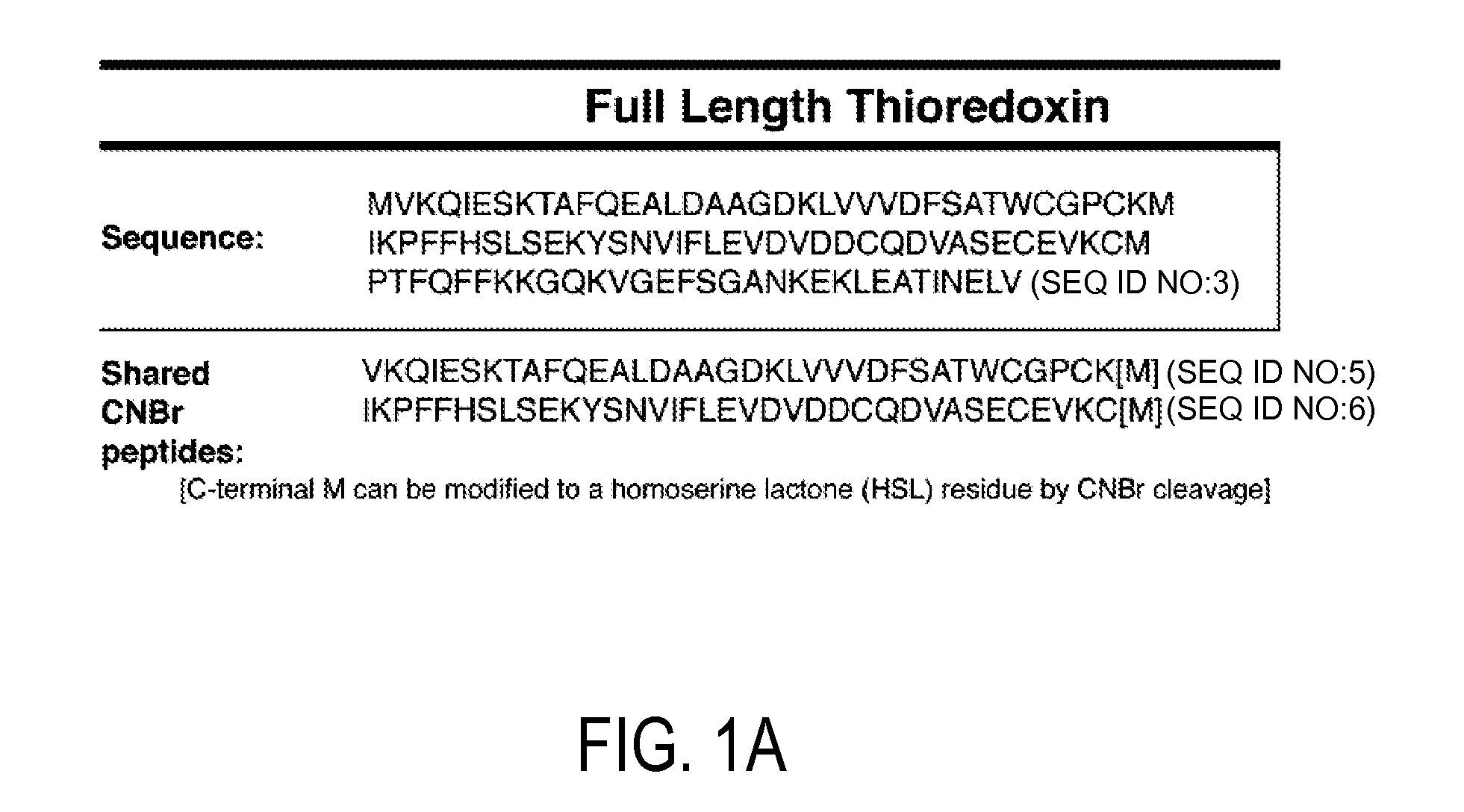
Detection and quantitation of full-length thioredoxin (TRX) and truncated thioredoxin (TRX 80) in complex samples
The present invention relates, e.g., to a method for detecting a full-length protein and a truncated form (e.g., a naturally occurring cleavage product) thereof, in a sample, comprisingoptionally denaturing and reducing proteins in the sample,cleaving the proteins into smaller peptides, anddetecting a unique peptide identifier for the full-length protein and / or a unique peptide identifier for the truncated protein, in the sample.In one embodiment of the invention, the full-length protein is thioredoxin (TRX), and the truncated form thereof is its biologically active, C-terminal truncated, 10 kDa cleavage product, TRX 80.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Indirect ELISA kit for detecting porcine transfusion transmitted virus 2 (TTV2) antibody
InactiveCN103235121AFast detection methodThe detection method is simpleMaterial analysisTransfusion transmitted virusPorcine serum
The invention relates to an indirect ELISA kit for detecting a porcine transfusion transmitted virus 2 (TTV2) antibody and belongs to the field of biotechnology. The invention comprises antigen recombinant protein preparation, indirect ELISA establishing, and use of determination standard and clinical serological test. Through pcoldI prokaryotic expression vectors, a gene engineering bacterium pcoldI-ORF1 for expression of a porcine TTV2ORF1 truncated protein is constructed and the expressed antigen recombinant protein is purified and is used as an antigen so that an indirect ELISA detection method is established. The indirect ELISA detection method is used for detecting a TTV2 antibody level of porcine serum, has good repeatability and high singularity, can be used for porcine TTV2 serology investigation and is a fast and simple serological test method for prevention, treatment and prevalence state control of porcine transfusion transmitted diseases. The indirect ELISA detection method utilizes the porcine TTV2ORF1 recombinant protein to detect the porcine TTV2 antibody first in China.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Subunit vaccine for bovine fusobacterium necrophorum and preparation method of subunit vaccine
ActiveCN110613842AImprove the level ofGood immune protectionAntibacterial agentsBacterial antigen ingredientsTGE VACCINEBiology
The invention relates to a subunit vaccine for bovine fusobacterium necrophorum. The subunit vaccine comprises three proteins: an outer membrane protein 43kDa OMP of bovine fusobacterium necrophorum,a leukotoxin truncated protein PL-4 of fusobacterium necrophorum and a hemolysin truncated protein H2 of fusobacterium necrophorum, wherein the dosage ratio of the three proteins is 1:1:1. The invention also relates to a preparation method of the subunit vaccine for bovine fusobacterium necrophorum. The subunit vaccine for bovine fusobacterium necrophorum comprises the three proteins: the outer membrane protein 43kDa OMP of bovine fusobacterium necrophorum, the leukotoxin truncated protein PL-4 of fusobacterium necrophorum and the hemolysin truncated protein H2 of fusobacterium necrophorum that are used in combination to produce a high level of specific antibody after immunity, thereby provoking cellular immunity and humoral immunity well in mice and effectively protecting the mice from attack of the fusobacterium necrophorum. The subunit vaccine has a better immune protection effect comparable to that of a whole-bacteria inactivated vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
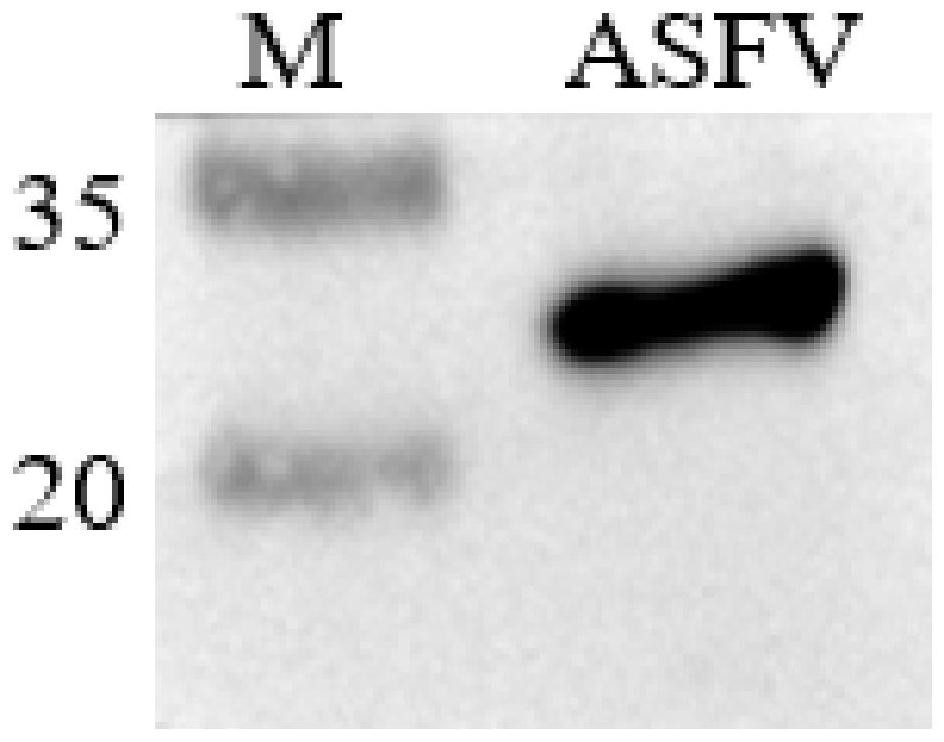
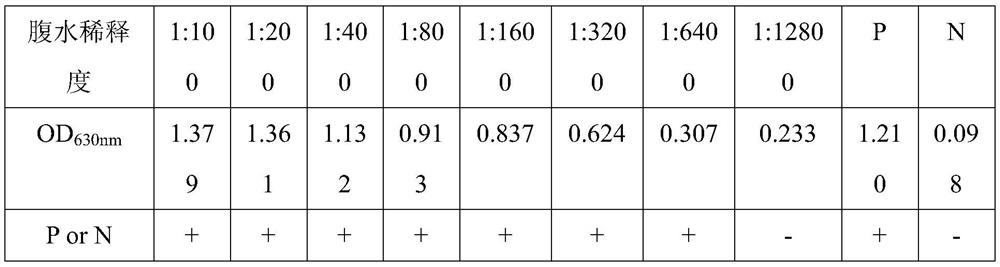
Monoclonal antibody prepared from African swine fever virus truncated protein p54 and application of monoclonal antibody
ActiveCN111849922AImproving immunogenicityImprove biological characteristicsBiological material analysisImmunoglobulins against virusesImmunologic TechniqueTruncated protein
The invention belongs to the technical field of clinical immunology, and discloses a monoclonal antibody prepared from African swine fever virus truncated protein p54 and application of the monoclonalantibody. The monoclonal antibody clone antibody 4-4C is finally obtained by immunizing the effectively truncated African swine fever virus protein p54 and systematically screening hybridoma. The obtained monoclonal antibody can react with the African swine fever totivirus to meet the basic requirements of the monoclonal antibody; in addition, the monoclonal antibody is good in specificity and has no cross reaction with other proteins of the African swine fever virus; in addition, after the monoclonal antibody is detected by Elisa, the titer of the monoclonal antibody reaches up to 1600000; finally, the monoclonal antibody has a blocking effect, the established blocking Elisa detection method has a detection effect equivalent to that of a Spanish detection kit recommended by OIE, the detection time is shortened, and the monoclonal antibody has the capacity of detecting the antibody level of African swine fever for pig farms.
Owner:HUAZHONG AGRI UNIV
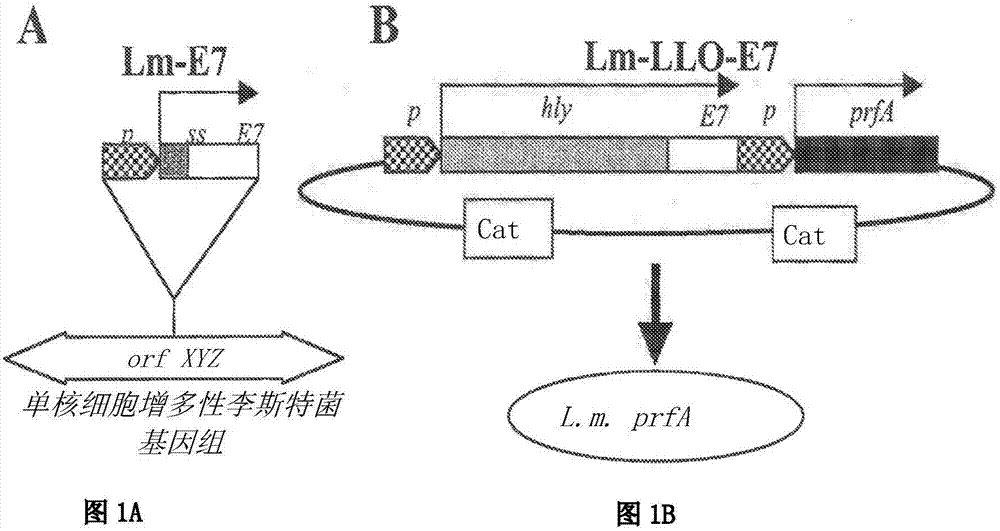
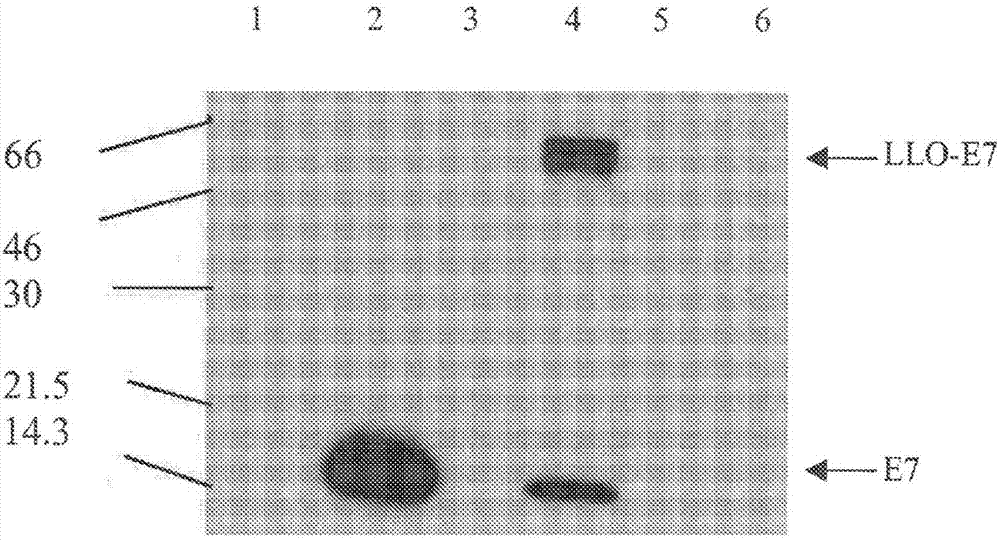
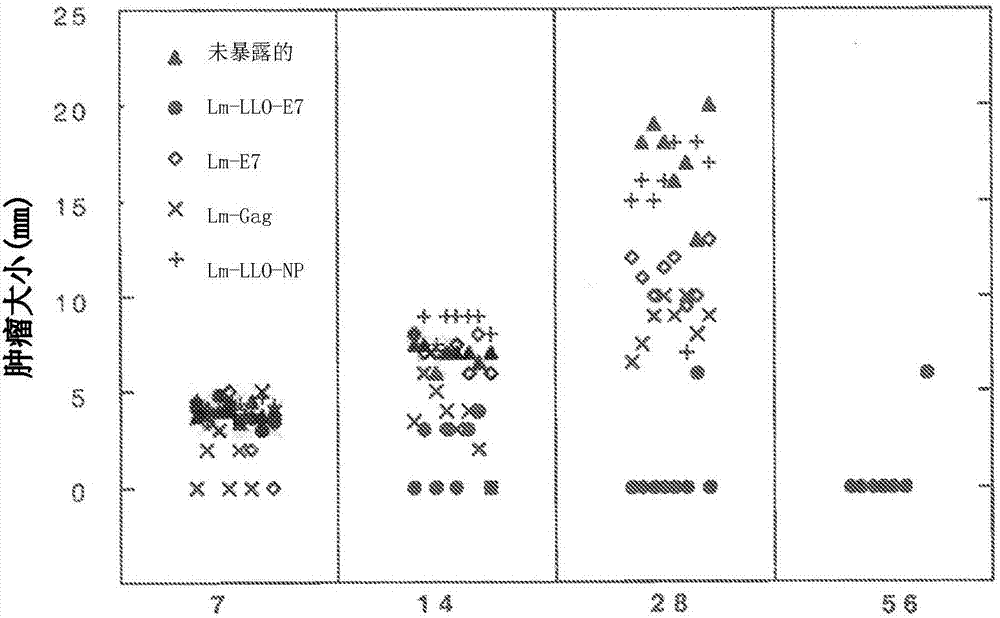
Combination of listeria-based vaccine with anti-OX40 or anti-GITR antibodies
InactiveCN107206060AAntibody mimetics/scaffoldsFusion with degradation motifListeriolysin OGenus Listeria
Disclosed herein are compositions comprising use of compositions comprising a live attenuated recombinant Listeria strain comprising a fusion protein of a truncated listeriolysin O (LLO) protein, a truncated ActA protein, or a PEST amino acid sequence fused to a heterologous antigen, including a tumor-associated antigen, wherein the compositions further comprise or are co-administered with an antibody or fragment thereof. Also disclosed are combination therapies comprising use of these compositions comprising live attenuated recombiant Listeria strains, in conjuction with an antibody or fragment thereof for use in treating, protecting against, and / or inducing an immune response against a tumor, especially wherein the treating, protection against and / or inducing an immune response increases percent survival in a subject.
Owner:ADVAXIS +1
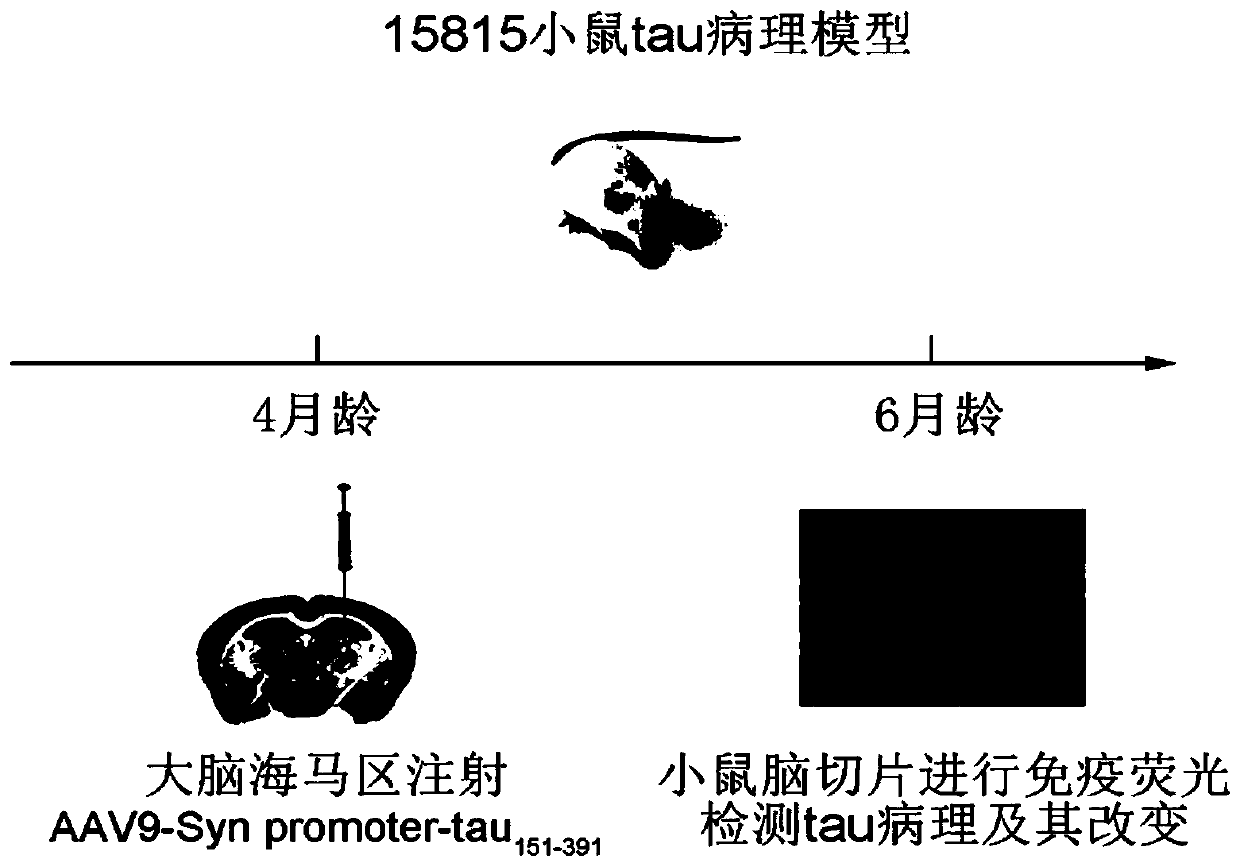
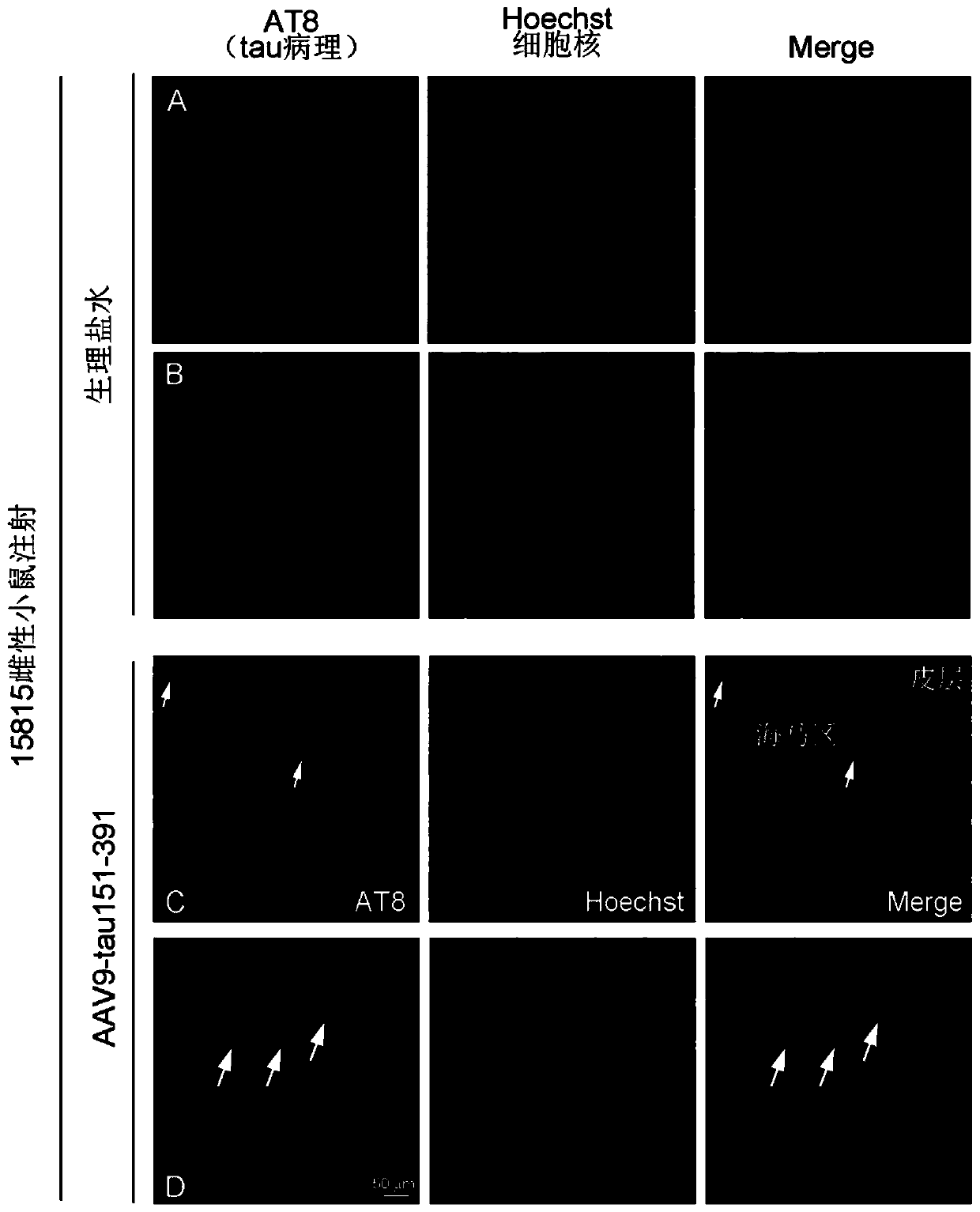
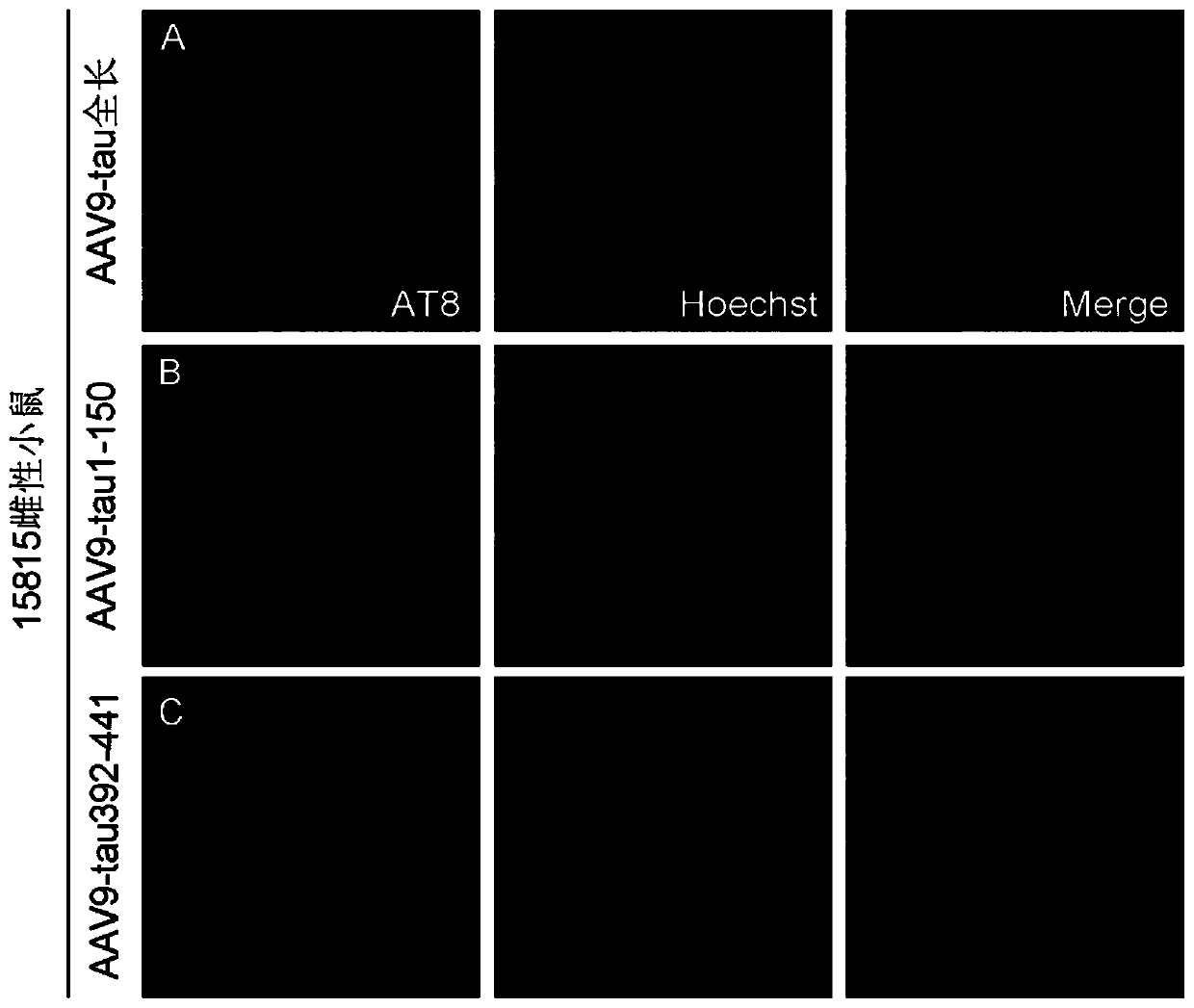
Method for constructing Alzheimer disease mouse model
The invention discloses a method for constructing an Alzheimer disease mouse model. A genotype FVB-Fgf14Tg (tetO-MAPT* P301L) 4510 Kha / JlwsJ female mouse is adopted, a truncated tau protein or a vector expressing the truncated tau protein is injected into the brain of the mouse at the age of 4 months and then the mouse is fed for 2-4 months, and the amino acid sequence of the truncated tau proteinis as shown in SEQ ID NO: I. The Alzheimer disease mouse model is successfully constructed, tau pathology only appears in the hippocampus region of the constructed mouse model, and does not appear inother brain regions, the construction period is shortened to 6 months, and more than 50% of modeling time is saved.
Owner:NANTONG UNIVERSITY
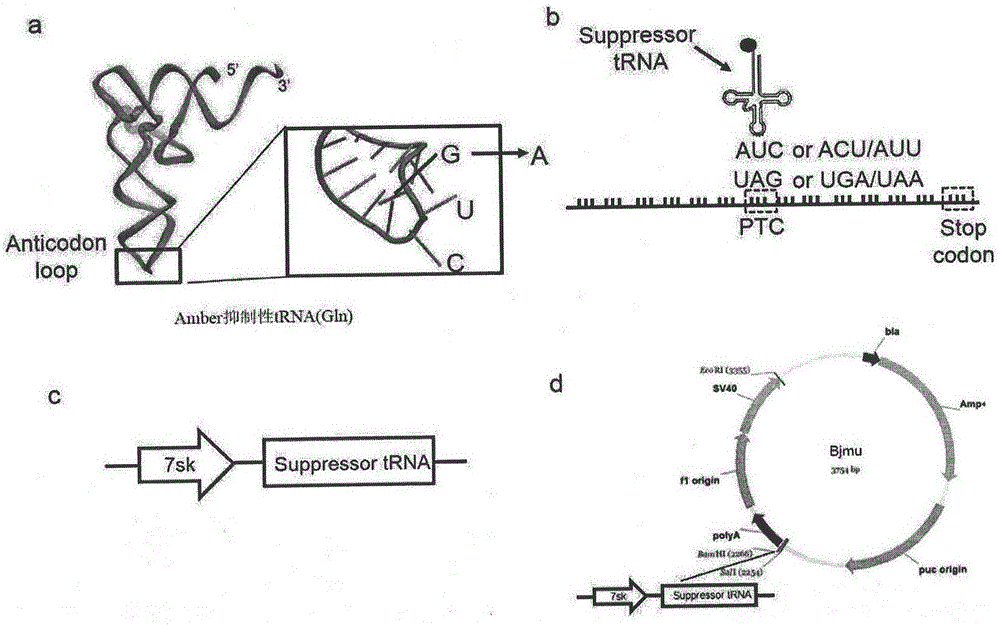
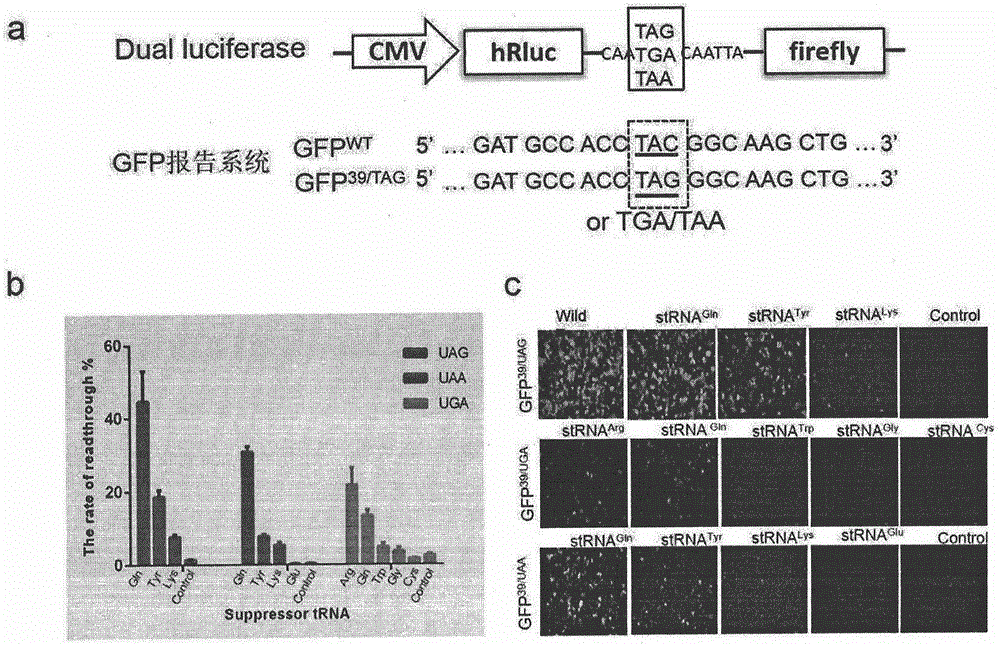
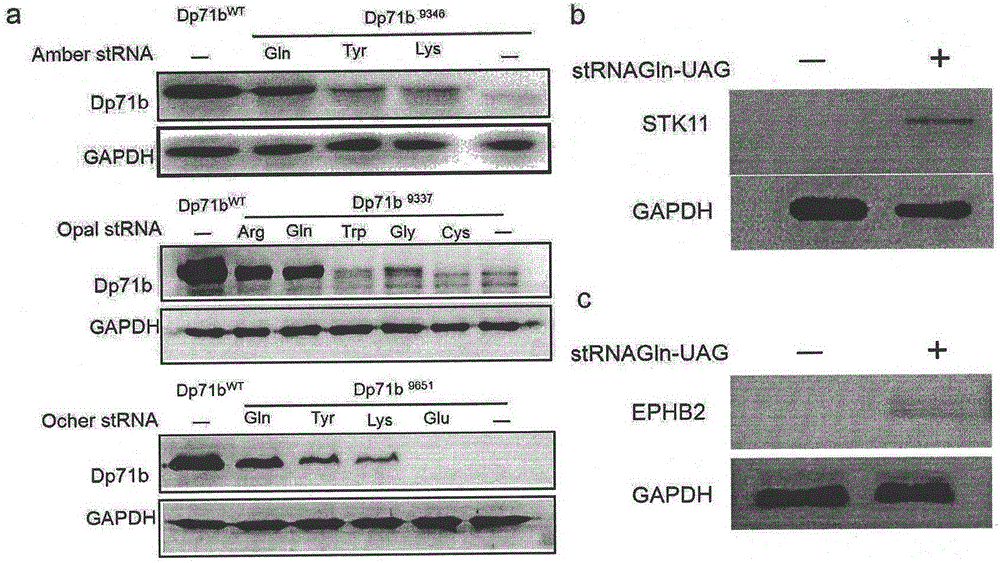
Truncated protein for reading through premature termination codons diseases by using inhibitory transfer ribonucleic acids (tRNAs)
The invention relates to a truncated protein for prolonging a disease-causing gene nonsense mutant by establishing inhibitory transfer ribonucleic acids (tRNAs) so as to enable full-length functional proteins to be produced in mammalian cells and further restore the normal structure and function of the mutant. A nonsense mutation system related in the invention comprises disease-causing genes of single gene inheritance diseases and tumor suppressor genes in tumor cells. The invention mainly relates to constructing of nineteen inhibitory tRNAs corresponding three termination codons, a dystrophin protein is efficiently read through in the mammalian cells, and a nonsense mutant protein is read through in the tumor cells, so that a remarkable effect is achieved.
Owner:PEKING UNIV
Dual fluorescent microsphere immunological detection method for pseudorabies virus gE and gB IgG antibodies
ActiveCN109307772AGood repeatabilityStrong specificityBiological material analysisBiological testingWild typeDifferential diagnosis
The invention discloses a dual fluorescent microsphere immunological detection method for pseudorabies virus gE and gB IgG antibodies. The detection method is based on a liquid protein chip technology-based carboxylated fluorescent microsphere group for detecting pseudorabies virus antibodies; the group comprises carboxylated fluorescent microspheres coupled with gE truncated proteins and gB truncated proteins, respectively; and the amino acid sequences of gE and gB truncated proteins are shown in SEQ ID NOs: 2 and 4, respectively. The dual fluorescent microsphere immunological detection method for simultaneously detecting PRV gE and gB IgG antibodies is good in reproducibility, high in sensitivity and good in specificity, and has no cross-reaction with other common porcine virus-positiveserums. The method can be used for the rapid differential diagnosis of pseudorabies virus wild-type infected pigs and vaccine-vaccinated pigs and the detection of protective antibodies, thereby providing an important method for the monitoring of swine virus diseases, having a great application value and being worthy of large-scale promotion.
Owner:SOUTH CHINA AGRI UNIV
Antigen protein specifically bound with tyrosine phosphatase antibody
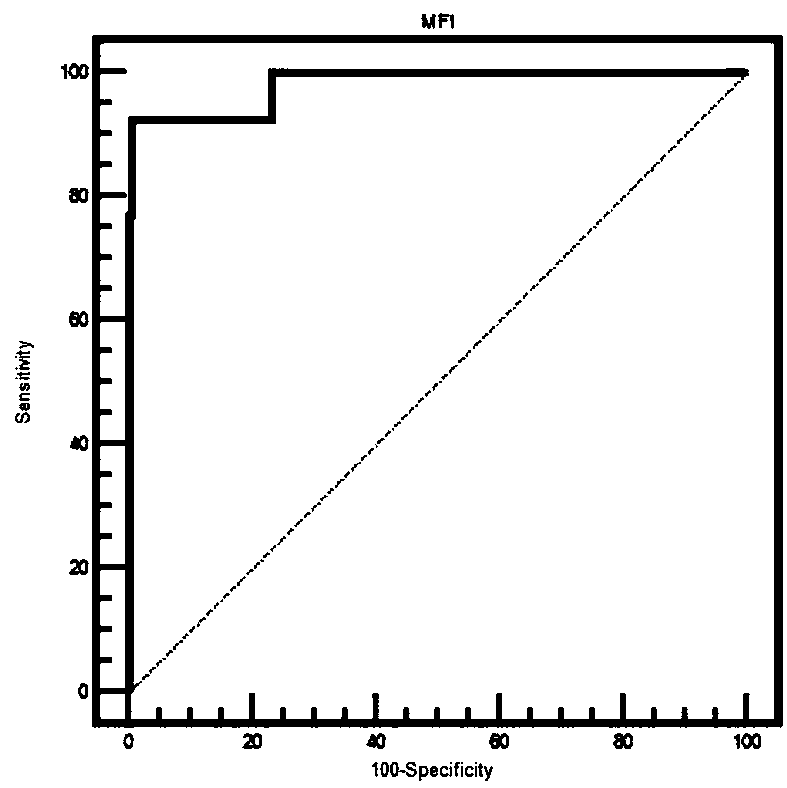
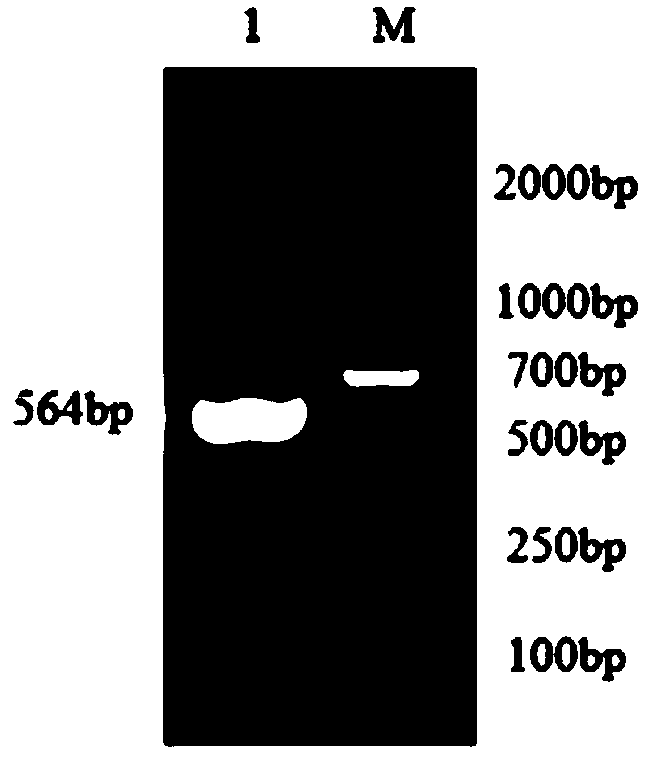
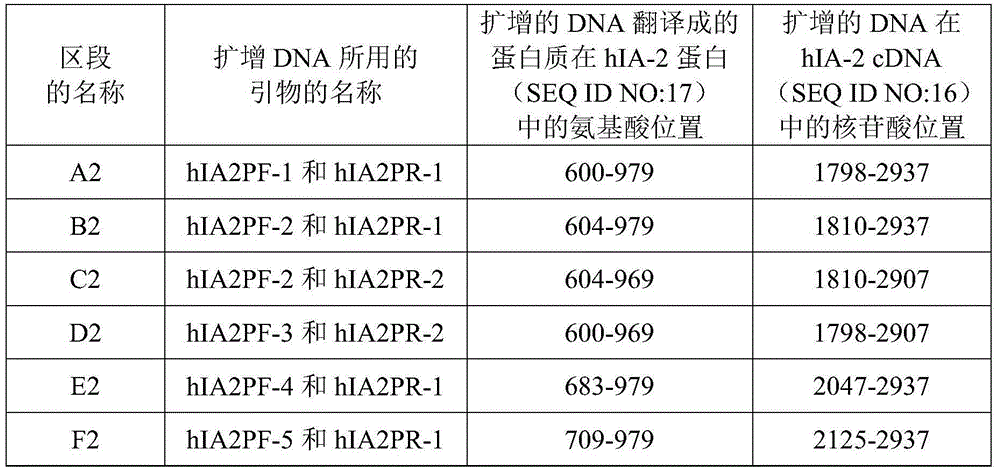
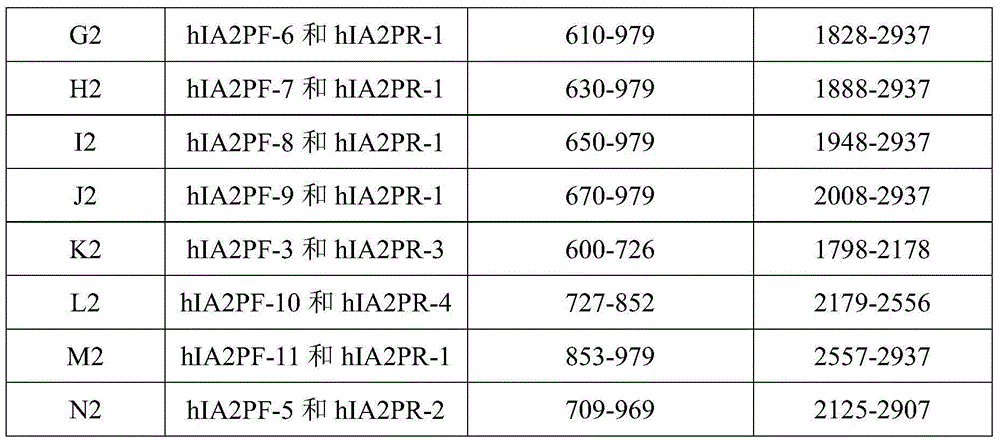
The invention relates to the technical field of antigen-antibody protein, and particularly relates to the technical field of truncated tyrosine phosphatase (IA-2) for detecting diabetes mellitus. One aim of the invention is to screen a truncated antigen protein specifically bound with IA-2 antibody, and the other aim of the invention is to develop antigen protein which is capable of fulfilling a chemiluminiscence closed system and applicable to the chemiluminiscence closed system. According to the antigen protein specifically bound with the IA-2 antibody, the antigen protein is one of 600th-979th, 604th-979th, 604th-969th, 600th-969th and 709th-969th truncated proteins. The invention further provides the truncated protein or IA-2 full-length protein which is coated with nano-magnetic beads, and a kit comprising the protein.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Detection and quantitation of full-length thioredoxin (TRX) and truncated thioredoxin (trx 80) in complex samples
ActiveUS20110143379A1Microbiological testing/measurementBiological testingSmall peptideADAMTS Proteins
The present invention relates, e.g., to a method for detecting a full-length protein and a truncated form (e.g., a naturally occurring cleavage product) thereof, in a sample, comprisingoptionally denaturing and reducing proteins in the sample,cleaving the proteins into smaller peptides, anddetecting a unique peptide identifier for the full-length protein and / or a unique peptide identifier for the truncated protein, in the sample.In one embodiment of the invention, the full-length protein is thioredoxin (TRX), and the truncated form thereof is its biologically active, C-terminal truncated, 10 kDa cleavage product, TRX 80.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Recombinant Newcastle disease heat-resistant vaccine strain for expression of signal peptide-replaced H5 subtype avian influenza virus HA protein and preparation method thereof
InactiveCN106085970AImprove thermal stabilityEnhanced secretory expressionSsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVaccine Immunogenicity
The invention discloses a recombinant Newcastle disease heat-resistant vaccine strain for expression of a signal peptide-replaced H5 subtype avian influenza virus HA protein and a preparation method thereof. The heat-resistant vaccine strain is named as rTS-tHA / H5, is preserved in the Wuhan university China center for type culture collection on April 19, 2016 and has an accession number of CCTCC NO: V201628. After infection of animals or cells with the vaccine strain, the vaccine strain can highly express a H5 subtype avian influenza virus truncated HA protein and the tPAs of the vaccine strain can improve secretory expression and immunogenicity of the truncated HA protein. The vaccine strain can be used for preparation of a chicken Newcastle disease and H5 subtype avian influenza bivalent heat-resistant living vaccine.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
African swine fever virus truncated protein and application thereof in preparation of ELISA detection kit
ActiveCN111848748AHigh sensitivityImmunogenicVirus peptidesMicroorganism based processesClassical swine fever virus CSFVAfrican swine fever
The invention belongs to the technical field of biology, and discloses an African swine fever virus truncated protein and application thereof in preparation of an ELISA detection kit, and the truncated protein is shown as SEQ ID NO. 1. When the indirect ELISA kit prepared by taking the protein as a coating antigen is used for detecting the African swine fever virus, the coincidence rate of the kitis equivalent, but the sensitivity of the kit is higher than that of a Spanish detection kit, the test time is shortened, and the operation steps are simpler. Therefore, the African swine fever virusindirect ELISA detection kit prepared from the truncated protein is very suitable for clinical detection of large samples and is suitable for large-scale popularization.
Owner:HUAZHONG AGRI UNIV
Sensitization polystyrene nano microsphere for detection of canine parvo virus structural protein VP2 antibody, and preparation method and application thereof
ActiveCN109212230AGood antigenicityImprove hydrophilicityBiological material analysisBiological testingMicrospherePolystyrene
The invention discloses a sensitization polystyrene nano microsphere for detection of a canine parvo virus structural protein VP2 antibody, and a preparation method and application thereof. A surfaceof the sensitization polystyrene nano microsphere is coupled with a canine parvo virus recombinant VP2 protein, the recombinant VP2 protein is a truncated protein of a canine parvo virus VP2 protein and located at the 365-486 digits of the canine parvo virus VP2 protein, and an amino acid sequence of the recombinant VP2 protein is shown as the SEQ ID NO.3. The protein contains a main epitope domain of the VP2 protein and has high antigenicity, excellent hydrophilicity and relatively strong specificity and immunogenicity. With the recombinant protein used as an antigen, a sensitization color polystyrene nano microsphere is prepared, and the sensitization color polystyrene nano microsphere is used for detecting a canine parvo virus serum antibody. Experiments show that the prepared sensitization microsphere cannot generate an autoagglutination phenomenon and has excellent repeatability and stable property. A detection method provided by the invention is suitable for clinical fast detection of single or multiple dog serum samples for pets, and compared with a commercial detection kit, the detection method is simple and convenient to operate, and a detection result has relatively highaccuracy and coincidence rate.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Recombination recin toxin B chain truncated protein and expressing method and application thereof
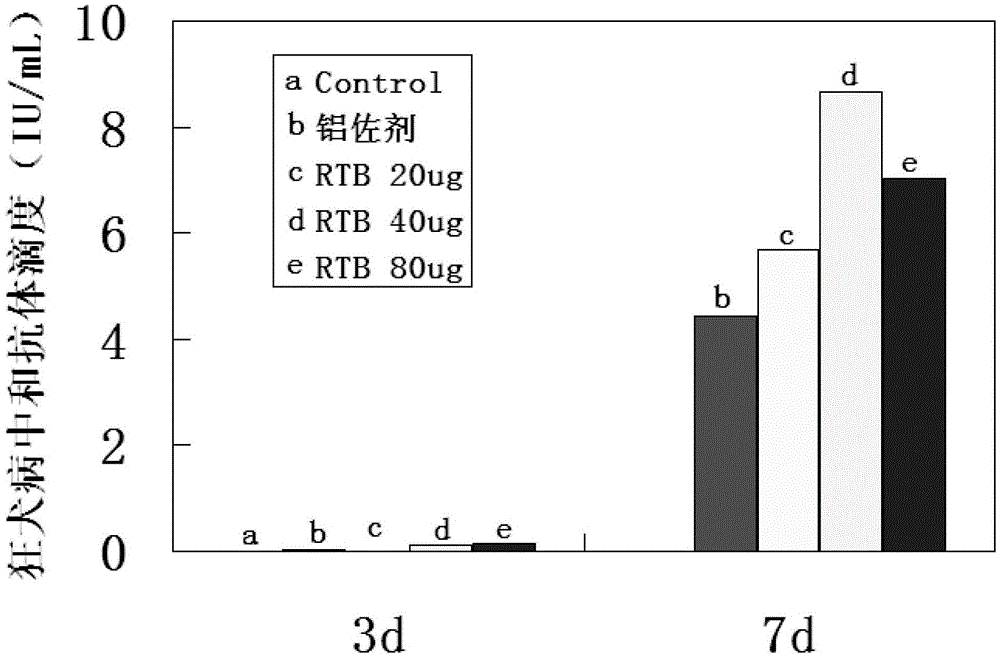
ActiveCN105481956AIncreased chain annealing rateImprove stabilitySsRNA viruses negative-senseViral antigen ingredientsEscherichia coliAdjuvant
The invention discloses a recombination recin toxin B chain truncated protein and an expressing method and application thereof, and relates to the field of bioengineering. The technical problems that an existing recombination recin toxin B chain protein is low in renaturation, poor in renaturation protein stability and the like are solved. The nucleotide sequence of the recombination recin toxin B chain truncated protein is shown in the SEQ ID NO:1 in a sequence table. The amino acid sequence of the recombination recin toxin B chain truncated protein is shown in the SEQ ID NO:2 in the sequence table. The expressing method of the recombination recin toxin B chain truncated protein comprises the step of expressing the recombination recin toxin B chain truncated protein through an escherichia coli expression system. According to the recombination recin toxin B chain truncated protein, the renaturation rate is improved, the stability is enhanced, the immunogenicity is good, the vaccine immunity effect is enhanced, the vaccine consumption is lowered, and the recombination recin toxin B chain truncated protein can be used as adjuvants of various vaccines such as inactivated vaccines, activity reduction vaccines, split vaccines, DNA vaccines, recombinant vaccines, subunit vaccines and polypeptide protein vaccines.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
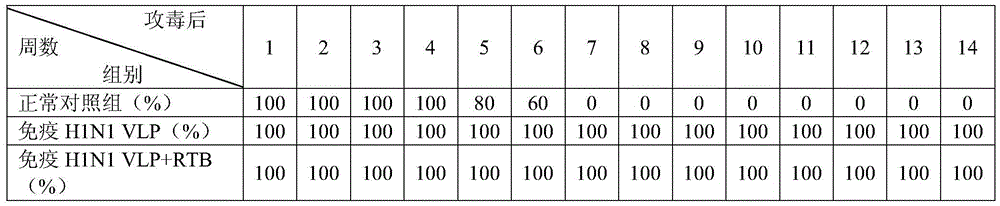
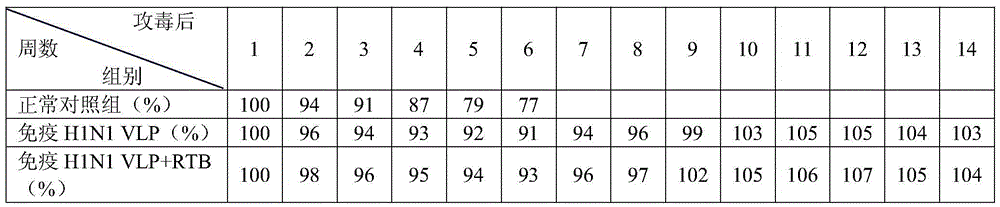
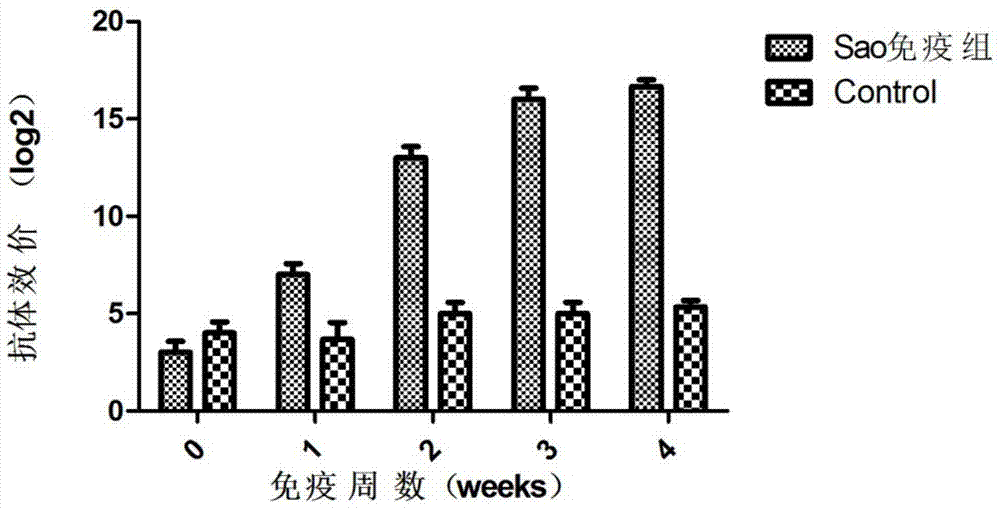
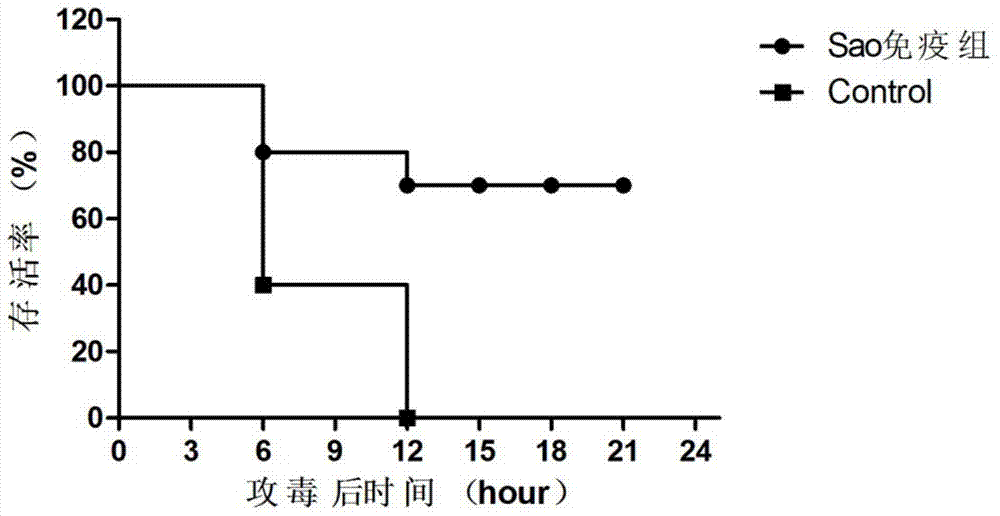
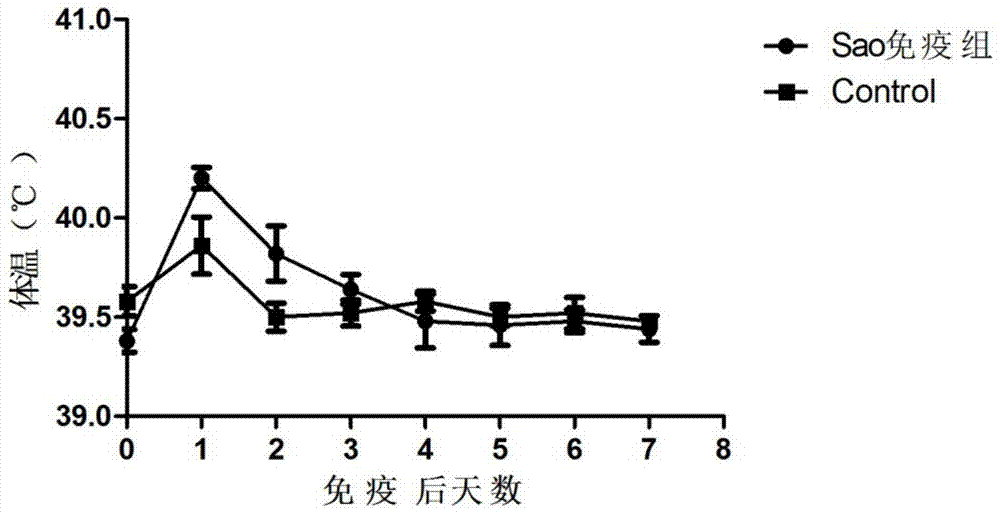
Streptococcus suis truncated protein Sao and application thereof
ActiveCN106995489AStable in natureEasy to degradeAntibacterial agentsBacterial antigen ingredientsLarge doseToxic material
The invention discloses streptococcus suis truncated protein Sao and application thereof. Amino acid sequence of the streptococcus suis truncated protein Sao is as shown in the SEQ ID NO.2. The protein stability is better than stability of full-length protein, and the protein of the invention is not easy to degrade. After the Sao protein is used for preparation of a vaccine, the vaccine can effectively protect piglets. By the use of large dose of virulent strain SC19 for counteracting toxic substances, the protecting force still reaches 60%. The Sao protein is safe and nontoxic. After immunization of piglets and pregnant sows, body temperature, breathe, appetite and metal state are all normal during the whole observation period, farrowing of pregnant sows is normal, and there is no abortion, fetal death and the like. The Sao protein can be combined with other proteins to prepare multi-vaccine and is suitable for mass production.
Owner:HUAZHONG AGRI UNIV
Porcine infectious actinobacillus pleuropneumoniae subunit vaccine
ActiveCN112704732AAchieve immune protectionEasy to produceAntibacterial agentsBacterial antigen ingredientsAntigenTGE VACCINE
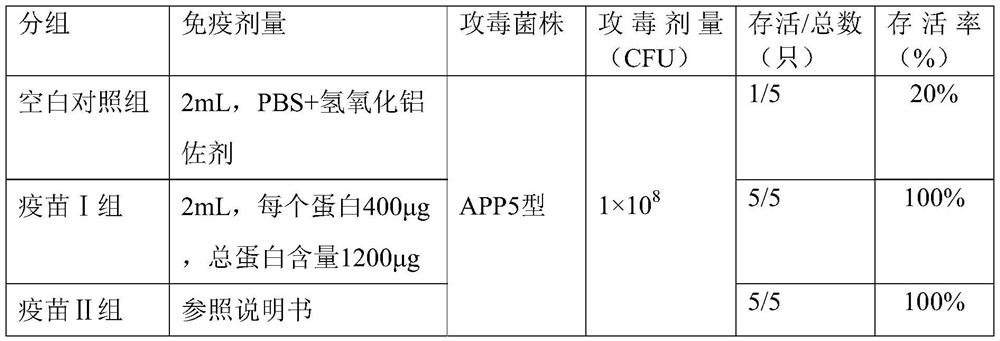
The invention discloses a porcine infectious actinobacillus pleuropneumoniae subunit vaccine, and belongs to the field of veterinary vaccines. The porcine infectious actinobacillus pleuropneumoniae subunit vaccine comprises protein 1-3 combinations with amino acid sequences shown as SEQ ID NO. 1-3 respectively. Truncated proteins selected in the antigen protein combinations are highly homologous in all serotypes of APP, and the subunit vaccine prepared from the truncated proteins can provide cross protection for porcine infectious actinobacillus pleuropneumoniae of different serotypes; and the truncated proteins are expressed in a soluble form, and are easy to produce and low in cost.
Owner:HUAZHONG AGRI UNIV +1
Cyclin-C variants, and diagnostic and therapeutic uses thereof
InactiveUS6306648B1VirusesPeptide/protein ingredientsPTK InhibitorsCyclin-Dependent Kinase Inhibitor 1B
The present invention includes alternatively and partially spliced cyclin C mRNAs, recombinant DNA and the truncated protein (a truncated cyclin C) they encode. The alternatively spliced mRNAs result from an insertion of unique exons containing premature termination codons. The partially spliced mRNAs result from an insertion of additional coding sequence derived from exons. One aspect of the present invention is the demonstration that at least one of the alternatively spliced cyclin C mRNAs is produced in a cell cycle dependent fashion, as is the novel truncated cyclin box protein that it encodes. Truncated cyclin C acts as an endogenously encoded cyclin C inhibitor by negatively regulating cyclin C / cdk8 complex activity, in much the same way as the cyclin dependent protein kinase inhibitors that inhibit the D-type cyclins, cyclin A and cyclin E.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com