Subunit vaccine for bovine fusobacterium necrophorum and preparation method of subunit vaccine
A subunit vaccine, Necroptosis bacillus technology, applied in the fields of molecular biology and genetic engineering, can solve the problems of natural toxin side effects, difficulty in vaccine application, difficulty in bacterial culture, etc., and achieve high levels of specific antibodies, good cellular immunity and humoral Immunity, protection from attack effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] This example illustrates the cloning of 43kDa OMP, PL-4 and H2 genes and the construction of expression vectors.
[0048] According to the gene sequences of Necroptosis 43kDa OMP, leukotoxin lktA and hemolysin hly published on GenBank, specific primers were designed for PCR amplification using Primer5.0 software. The designed specific primers were all provided by Harbin Boshi Biotechnology Co., Ltd. synthesis. The primer sequences are as follows:
[0049] Table 1 Fusobacterium necroptosis specific amplification primers
[0050]
[0051] The genome of Necroptosis bacilli was extracted using Tiange Bacteria Genomic DNA Extraction Kit. The extracted genome was used as a template, and PCR amplification was performed according to conventional methods. The amplification system and reaction conditions are as follows:
[0052] Table 2 PCR reaction system and reaction conditions
[0053]
[0054] Continued Table 2 PCR reaction system and reaction conditions
[0055] ...
Embodiment 2
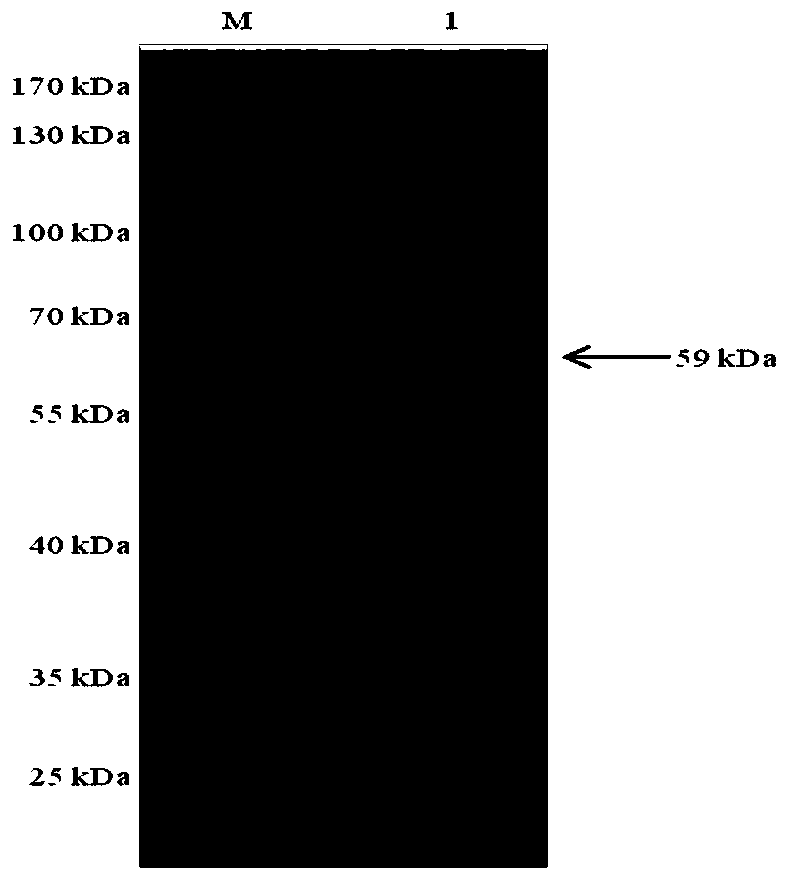
[0060] This example illustrates the induction of expression in recombinant E. coli.
[0061] Use pGEX-6p-1-PL-4-BL21, pET-32a-H2-BL21, pET-32a-43kDa OMP-BL21 positive bacteria and pET-32a, pGEX-6p-1 empty vector at a ratio of 1:1 000 Inoculated in LB (Amp + ) liquid medium, cultured on a constant temperature shaker at 37°C at 220r / min. When bacteria OD 600 When the value reaches 0.4-0.6, add IPTG to the bacterial solution to induce expression, the final concentration of IPTG is 0.5mmol / L, and the induction condition is 16°C, 160r / min for overnight culture. Collect the induced bacterial liquid, centrifuge and sonicate, as follows:
[0062] Bacterial precipitates were collected, and each induced bacterial solution was placed in a high-speed centrifuge for 10 min at 8 000 r / min, the supernatant was discarded to collect the precipitate, and the precipitate was washed with sterilized PBS buffer 3 times, each time at 5 000 r / min Centrifuge for 10 min, and finally suspend each in...
Embodiment 3
[0065] This example illustrates the purification of recombinant proteins.
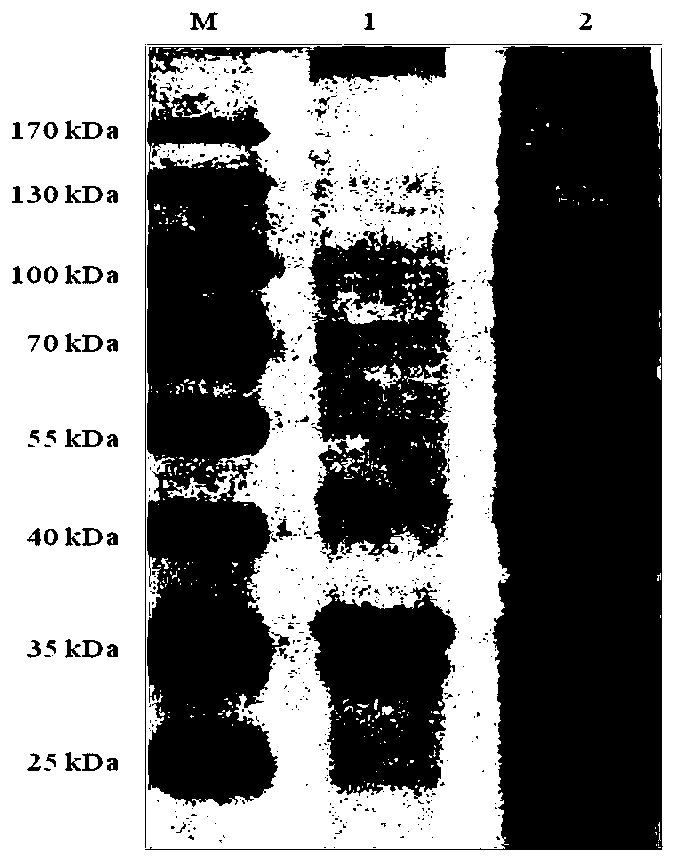
[0066] 1. Purification of pET-32a-43kDa OMP recombinant protein
[0067] Gently invert the column to resuspend the Ni-NTA Agarose while tapping repeatedly. Add resin to the purification column, the resin sinks completely due to gravity, suck out the supernatant, then add sterilized distilled water, turn over and tap the purification column again and again to resuspend the resin. The resin sinks completely due to gravity, and the supernatant is aspirated. Add Denaturing binding buffer, gently flip the column up and down to resuspend the resin, then allow the resin to sink naturally by gravity, suck out the supernatant, and repeat 2-3 times to make protein purification under denaturing conditions. Put the bacterial lysate in the Ni-NTA column, and gently stir it with a magnetic stirrer at room temperature, so that the lysate and the resin fully interact for 20-30min. Precipitate the resin naturally ag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com