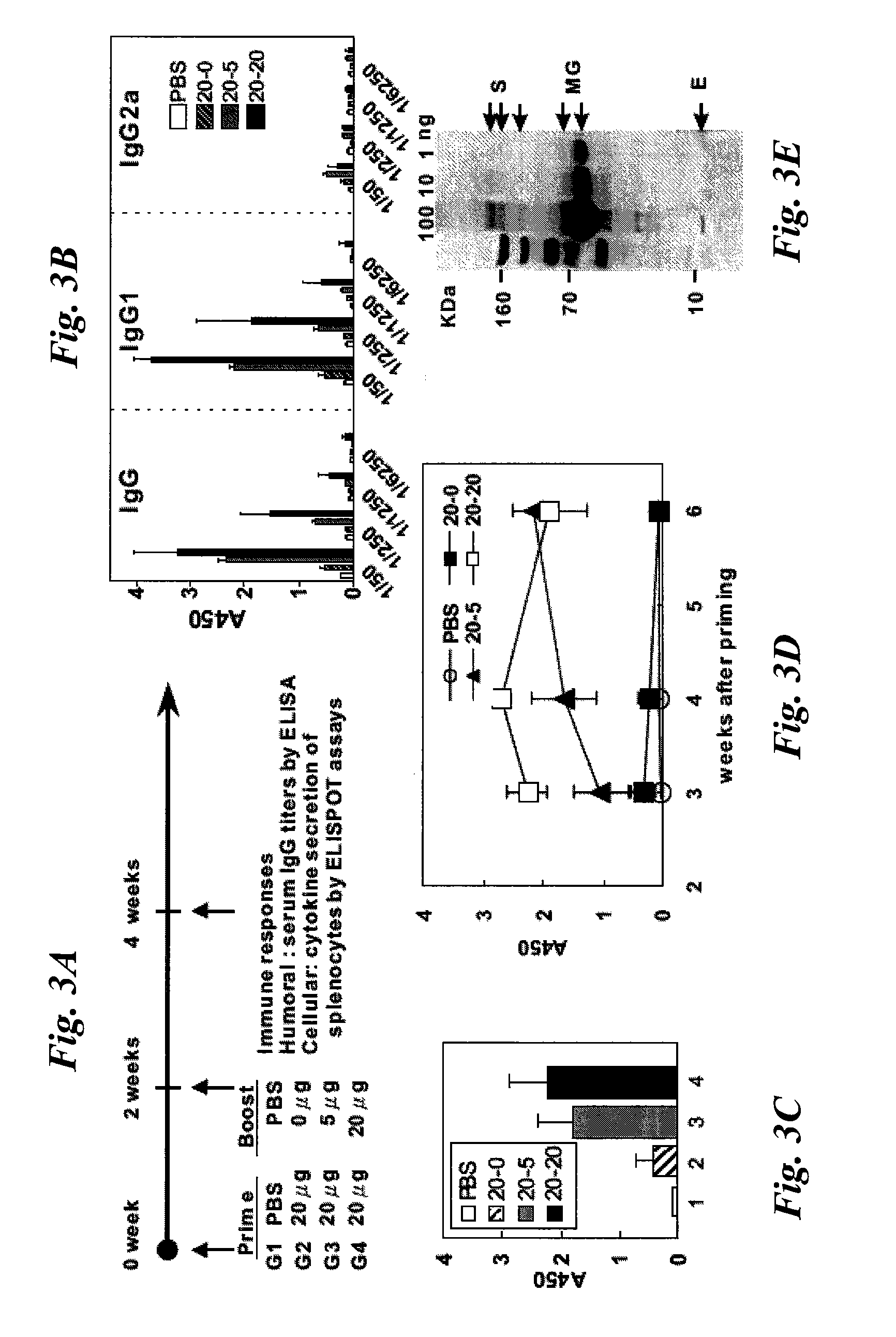
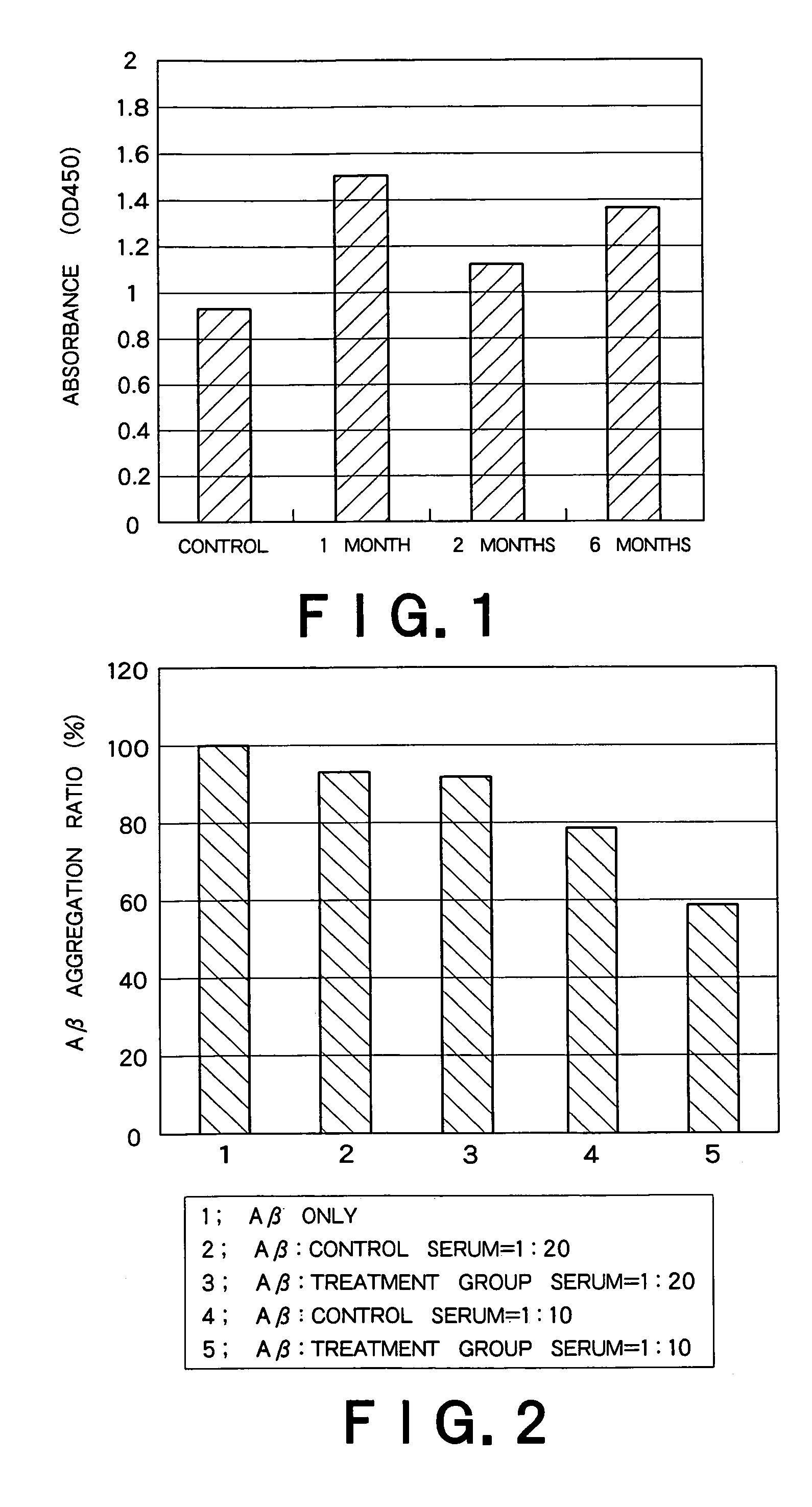
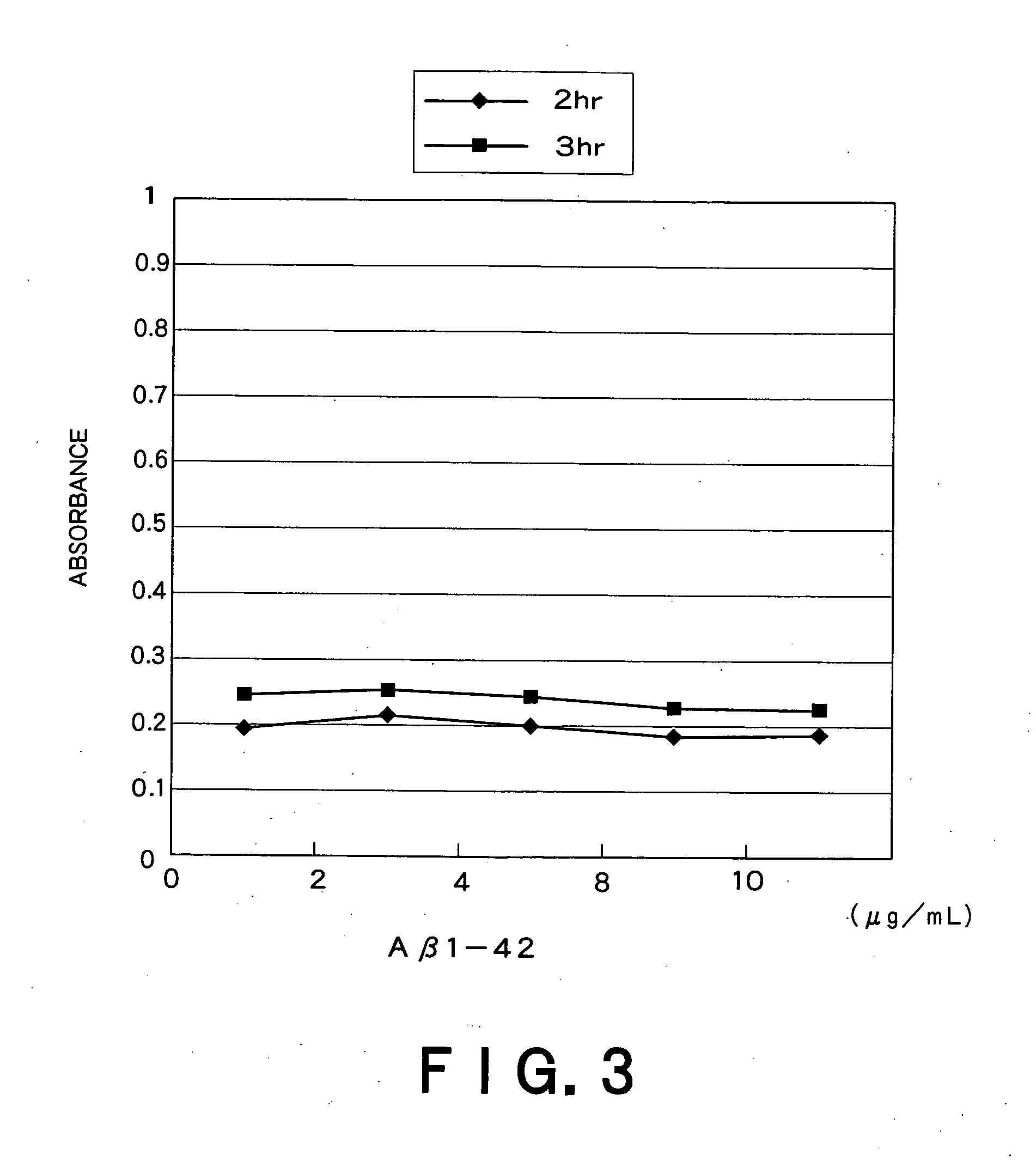
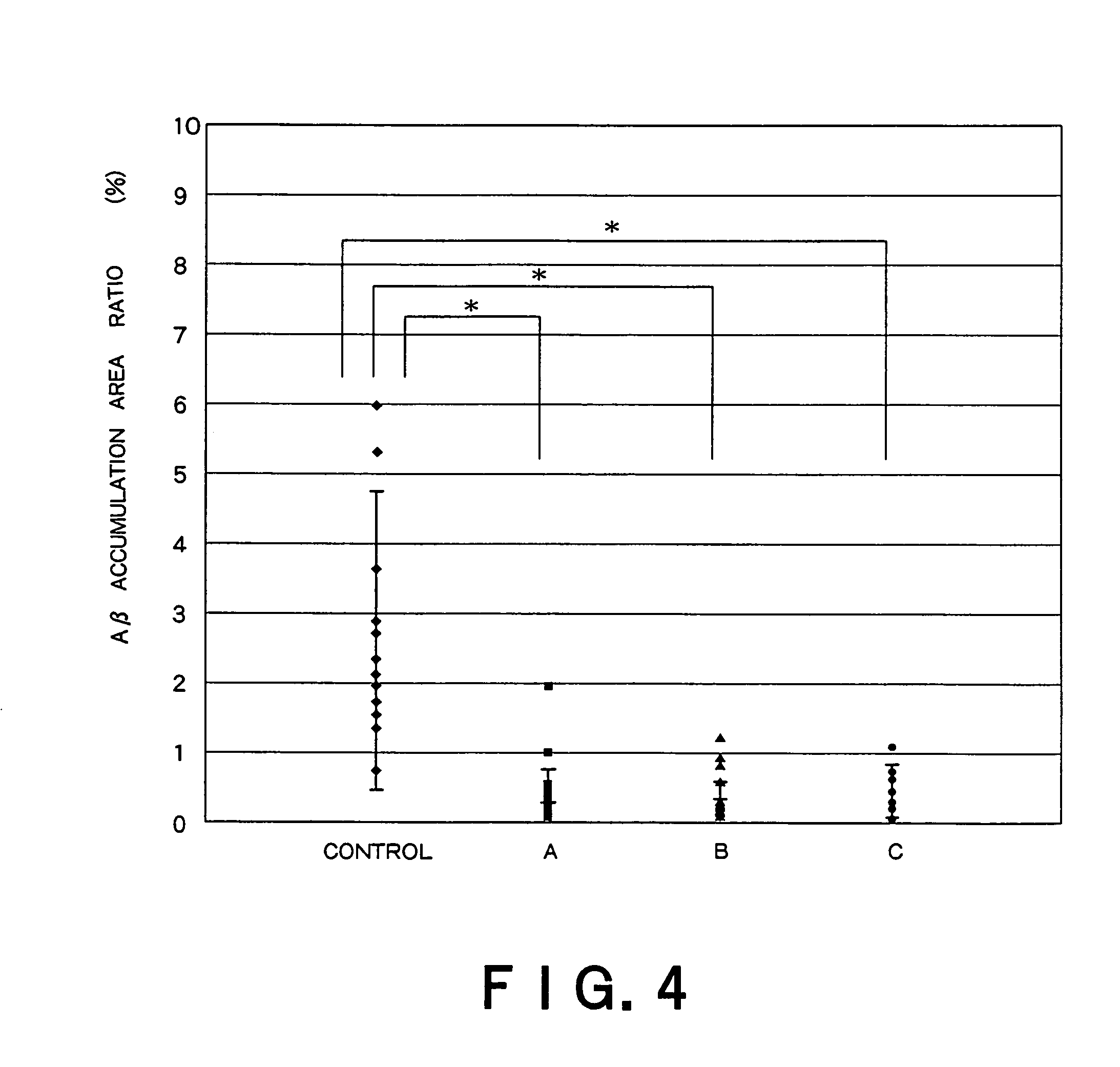
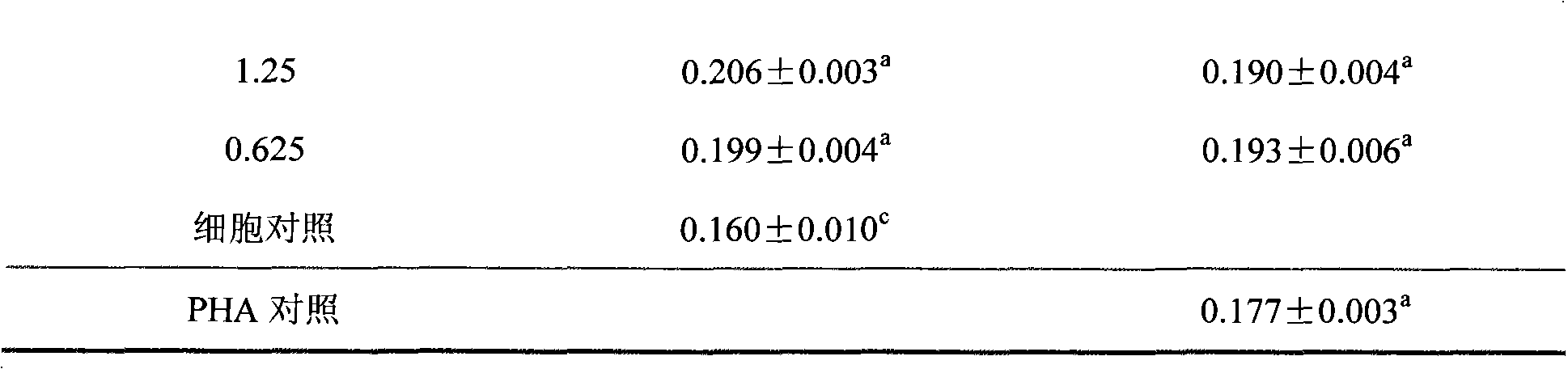Patents
Literature
605 results about "Humoral immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Humoral immunity or humoural immunity is the aspect of immunity that is mediated by macromolecules found in extracellular fluids such as secreted antibodies, complement proteins, and certain antimicrobial peptides. Humoral immunity is so named because it involves substances found in the humors, or body fluids. It contrasts with cell-mediated immunity. Its aspects involving antibodies are often called antibody-mediated immunity.
Methods and compositions using immunomodulatory compounds for the treatment of immunodeficiency disorders
Methods of treating, preventing and / or managing an immunodeficiency disease or disorder are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active agent. Methods of boosting humoral immunity are also disclosed.
Owner:CELGENE CORP
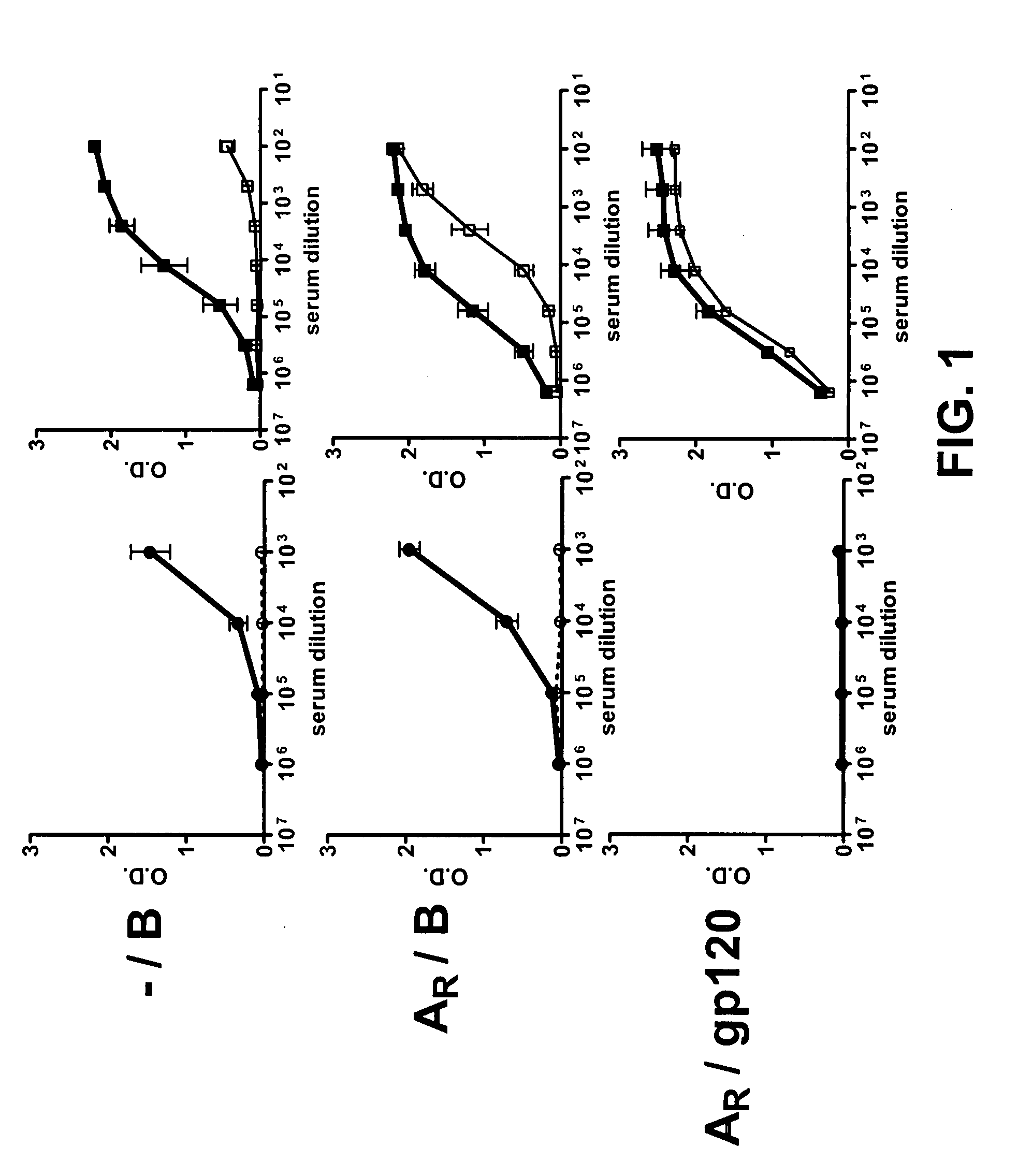
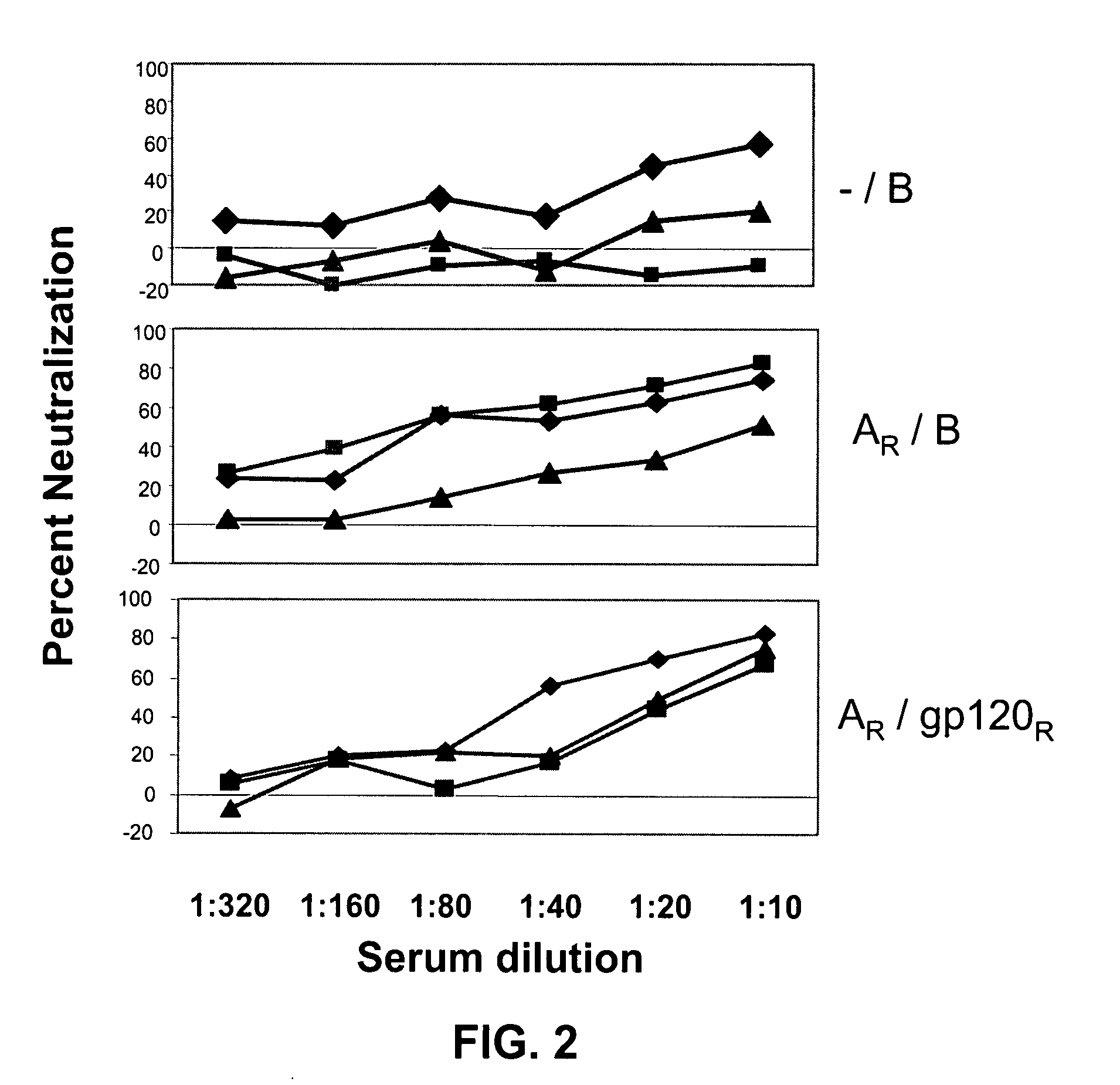
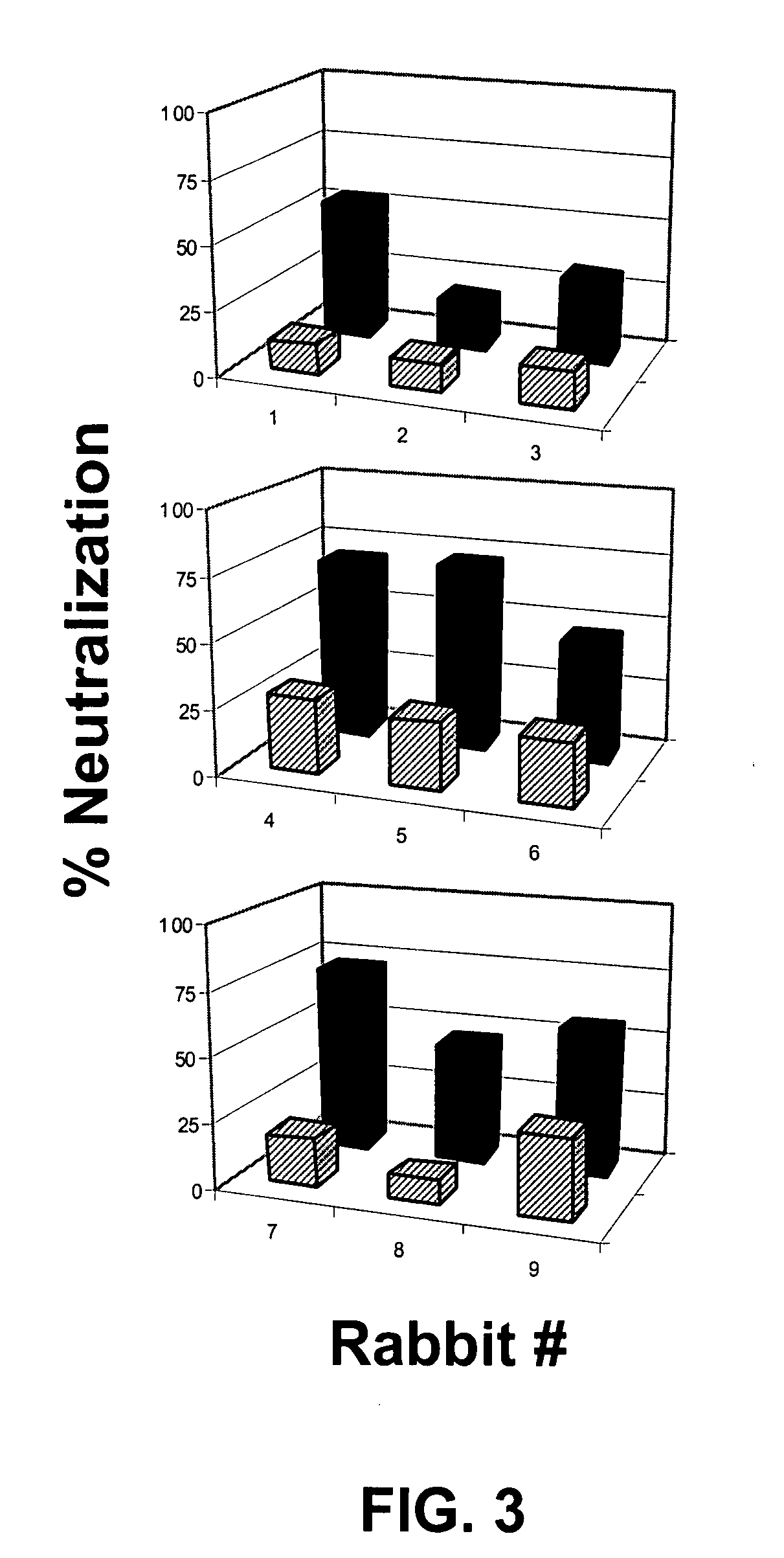
INDUCTION OF BROADLY REACTIVE NEUTRALIZING ANTIBODIES BY FOCUSING THE IMMUNE RESPONSE ON V3 EPITOPES OF THE HIV-1 gp120 ENVELOPE
InactiveUS20080279879A1Vigorous Ab responseViral antigen ingredientsAntibody mimetics/scaffoldsHeterologousNeutralizing antibody
Compositions, kits and methods for boosting, or for priming and boosting, high titer broadly neutralizing cross-clade antibody responses focused on single HIV-1 neutralizing epitopes are disclosed. gp120 DNA plasmids comprising HIV env genes are used to prime the antibody response. Primed subjects are immunized with recombinant fusion proteins that comprise a “carrier” protein fusion partner, preferably a truncated form of the MuLV gp70 Env protein, and a desired HIV neutralizing epitopes. Preferred epitopes are epitopes of V3 from one or more HIV clades. Immune sera from such immunized subjects neutralized primary isolates from virus strains heterologous to those from which the immunogens were constructed. Neutralizing activity was primarily due to V3-specific antibodies and cross-clade neutralizing Abs were present. This approach results in more potent and broader neutralizing antibody levels, a result of “immunofocusing” the humoral immune response on neutralizing epitopes such as V3.
Owner:NEW YORK UNIV
High-yield Transgenic Mammalian Expression System for Generating Virus-like Particles
ActiveUS20100166769A1Improving immunogenicityStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMammalVirus-like particle
Virus-like particles (VLPs) of mammalian-hosted viruses, such as SARS-CoV and influenza viruses, have been recombinantly produced from Vero cells. The VLPs closely emulate the exterior of authentic virus particles and are highly immunogenic. They can elicit not only humoral but also cellular immune responses in a mammal. Compositions and methods related to the VLPs are also described.
Owner:ACAD SINIC
Recombinant adeno-associated virus vector for treatment of Alzheimer disease
ActiveUS8318687B2Highly safe treatmentReduce concentrationNervous disorderVirusesAdeno associate virusDna encoding
Owner:TABIRA TAKESHI +1
Treating neoplasms with neurotoxin
InactiveUS7709440B2Inhibit transferSuppression of squeeze effectBiocidePeptide/protein ingredientsCancer cellAutoimmune responses
The present invention provides a method of treating a cancer using a neurotoxin, preferably Botulinum toxin (“BTX”). The application of a neurotoxin around a cancer acts to decrease the contractile forces of the muscles surrounding a neoplasm which normally squeeze cancer cells through efferent channels leaving the cancer vicinity to distant sites. Also, the application of the toxin at sites distant from the cancer enhances cellular and humoral immunologic functions which further contributes to cancer cell death and spread. Following administration of botulinum toxin around and distant to a cancer, it is noticed that local, regional, and distant spread is reduced or eliminated. Immunomodulation with botulinum toxin is also valuable in treating other disease that may or may not be associated with cancers, such as viral-induced growths, viral conditions, fungal disease, chronic wounds, graft versus host disease, autoimmune disease, and HIV.
Owner:TOXCURE
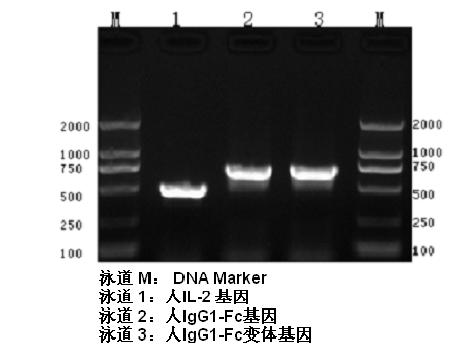
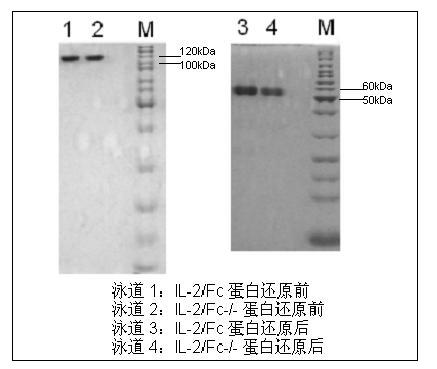
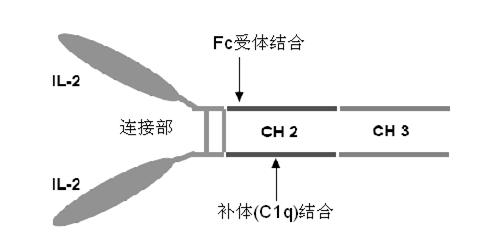
Human interleukin-2 (IL-2)/Fc fusion protein
ActiveCN102174111AEnhance humoral immune responseImprove immunityPeptide/protein ingredientsDigestive systemRegulatory T cellHalf-life
The invention provides human interleukin interleukin-2 (IL-2) / Fc fusion protein. The human IL-2 of the fusion protein comprises all sequences of a human IL-2 extracellular region; the Fc fragments comprise a hinge region, a CH2 region and a CH3 region; the human IL-2 / Fc sequences are fused directly or through a connection sequence; and the Fc fragments are human or animal IgG, IgM, IgD and IgA orsubtypes thereof. The ADCC and CDC effective factor action can be eliminated, and in addition, the human IL-2 / Fc fusion protein has the compatibility with a recombinant IL-2 receptor so that the half-life period is obviously prolonged and also has all the biological activity of the IL-2 receptor. The IL-2 / Fc obviously improves the humoral immune response stimulated by the hepatitis B vaccine and the immunity of the CD8+T cells targeted to the hepatitis B vaccine. Moreover, the balance immune (suppression) of the effective T cells and the regulatory t cells can be adjusted under the action of the cyclosporine A so that the pancreatic islet transplantation immune tolerance is induced.
Owner:上海百英生物科技股份有限公司
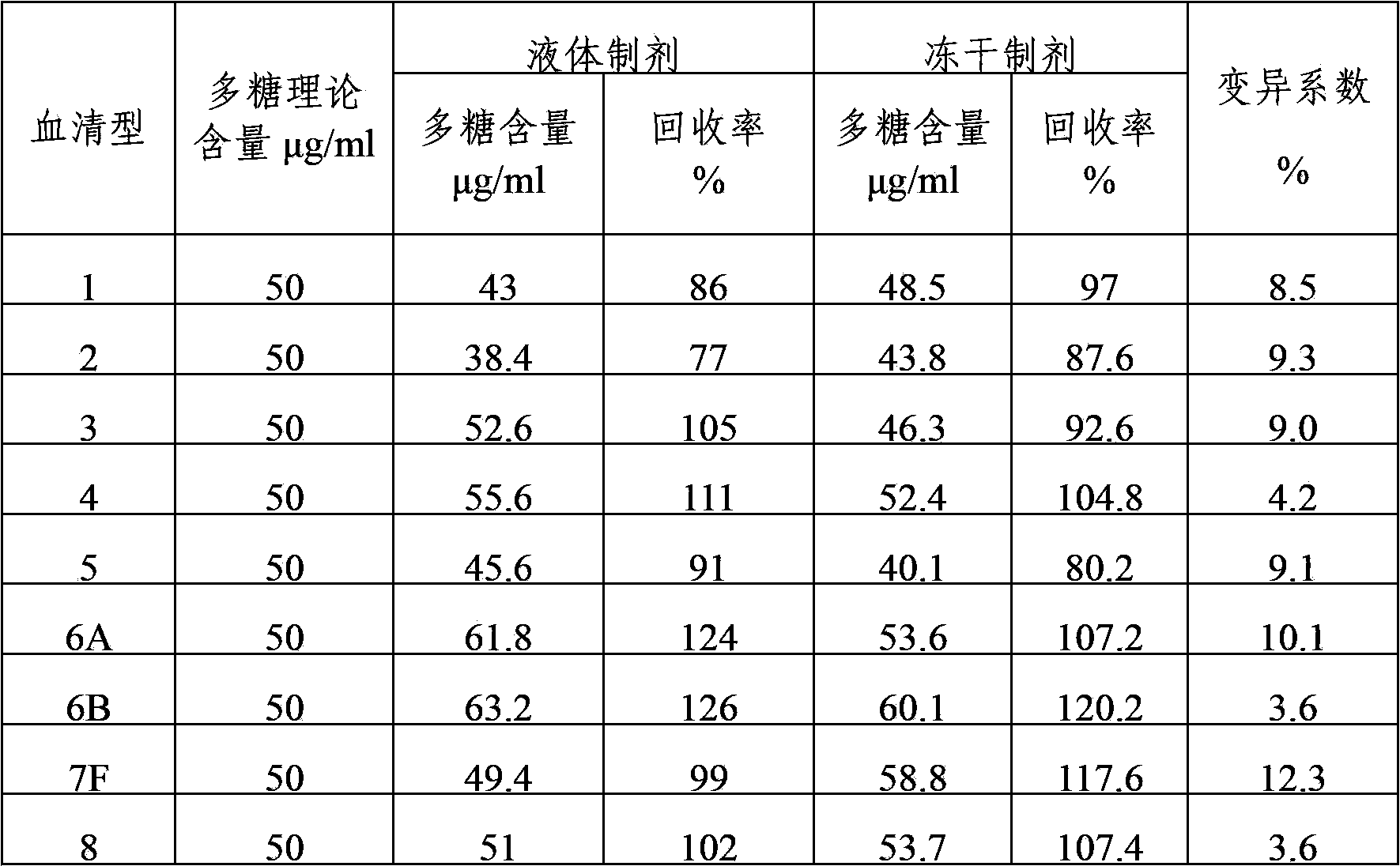
Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
ActiveCN103656632AStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
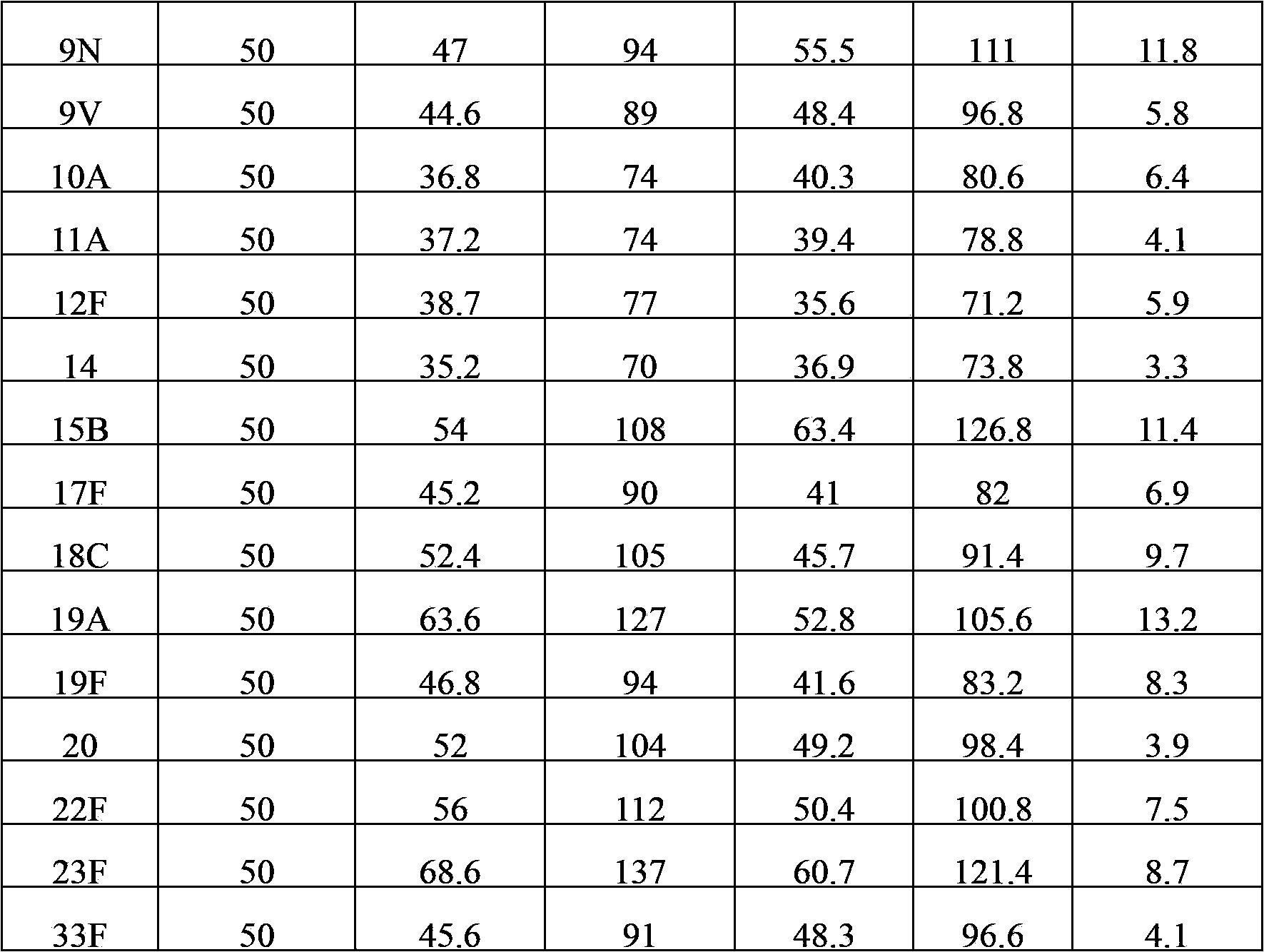
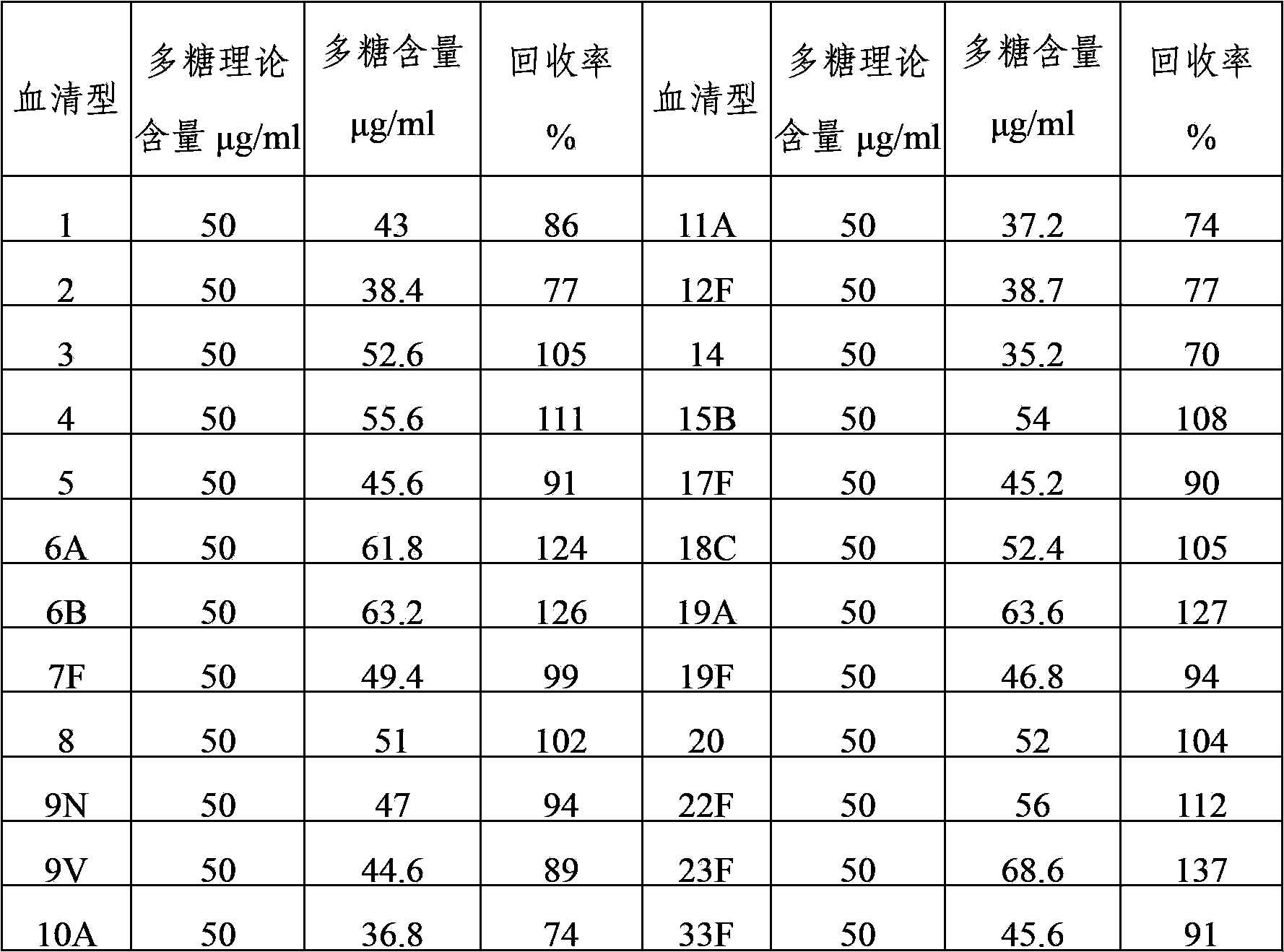
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Plant extract feed additive for piglets
ActiveCN102813064AExtensive sources of raw materialsWide variety of sourcesDigestive systemAnimal feeding stuffBiotechnologyGrape seed
The invention provides a compound feed additive which is prepared by compounding hawthorn powder, portulaca oleracea herb micro powder, honeysuckle extract, dandelion extract, grape seed extract, echinacea extract, astragalus extract, common andrographis herb extract, numerous types of plant materials subjected to ultramicro grinding or extracting. The plant extract feed additive is prepared by selecting natural plants through the processes of micro grinding, extracting, concentrating and the like, and has the characteristics of wide raw material sources, high biological utilization rate, no toxicity and no residue, and antibiotics are prevented from being accumulated in the livestock body at the source. The compound feed additive can obviously reduce the piglet diarrhea which is caused by various reasons, the daily increased weight of the piglet is increased, and the humoral immunity and cell immunity functions of the piglet are improved.
Owner:申亚生物科技股份有限公司
MntC recombinant protein of staphylococcus aureus and preparation method and application thereof
ActiveCN103694323AHigh expressionEasy to separate and purifyAntibacterial agentsBacteriaPurification methodsStaphylococcus aureus
The invention belongs to the field of biotechnology, and relates to a MntC recombinant protein of staphylococcus aureus (SA), a carrier comprising the recombinant protein, a host, a composition or a kit, application, preparation, fermentation and purification method of the protein. The MntC recombinant protein prepared by the method has strong immunogenicity, is safe and non-toxic, and is proved by animal tests to be able to effectively stimulate an organism to generate high efficient humoral immune response and good immune protection.
Owner:CHENGDU OLYMVAX BIOPHARM +1
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151AStrengthen cellsEnhance humoral immune stimulationAntibacterial agentsBacterial antigen ingredientsCholesterolVaccine antigen
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
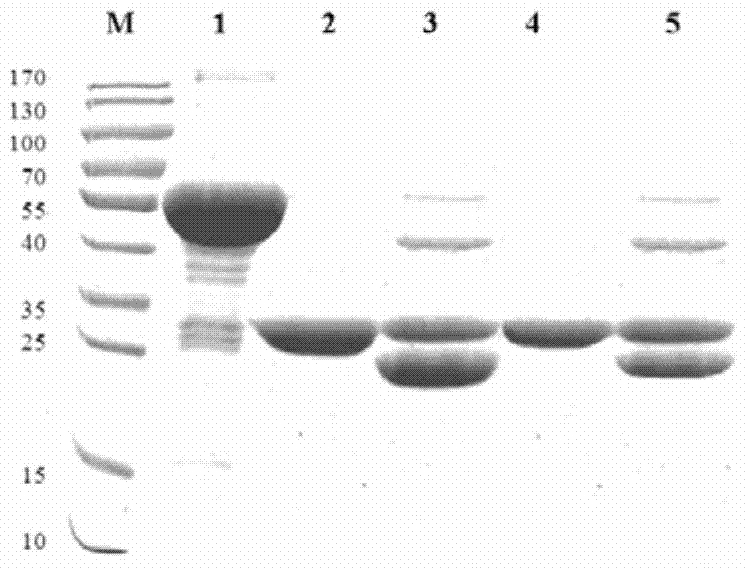
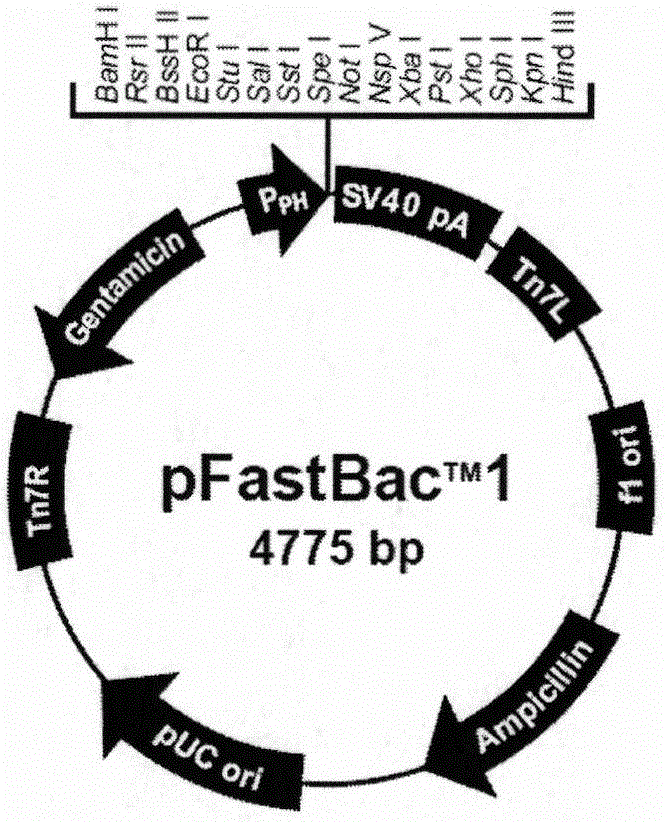
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Methods and compositions for manipulation of the immune response using anti-metallothionein antibody
InactiveUS20030007973A1Easy to separateEnhance humoral immune responseImmunoglobulins against animals/humansAntibody ingredientsHumoral immunityMetallothionein
Metallothionein is a stress response protein responsible for some forms of stress-related immunosuppression. An anti-metallothionein antibody can stimulate the humoral immune response to T-dependent antigens. An anti-metallothionein antibody is particularly useful when administered with an antigen to stimulate the immune response in a subject. An anti-metallothionein antibody can be administered to a subject with a vaccine to immunize the subject.
Owner:UNIV OF CONNECTICUT
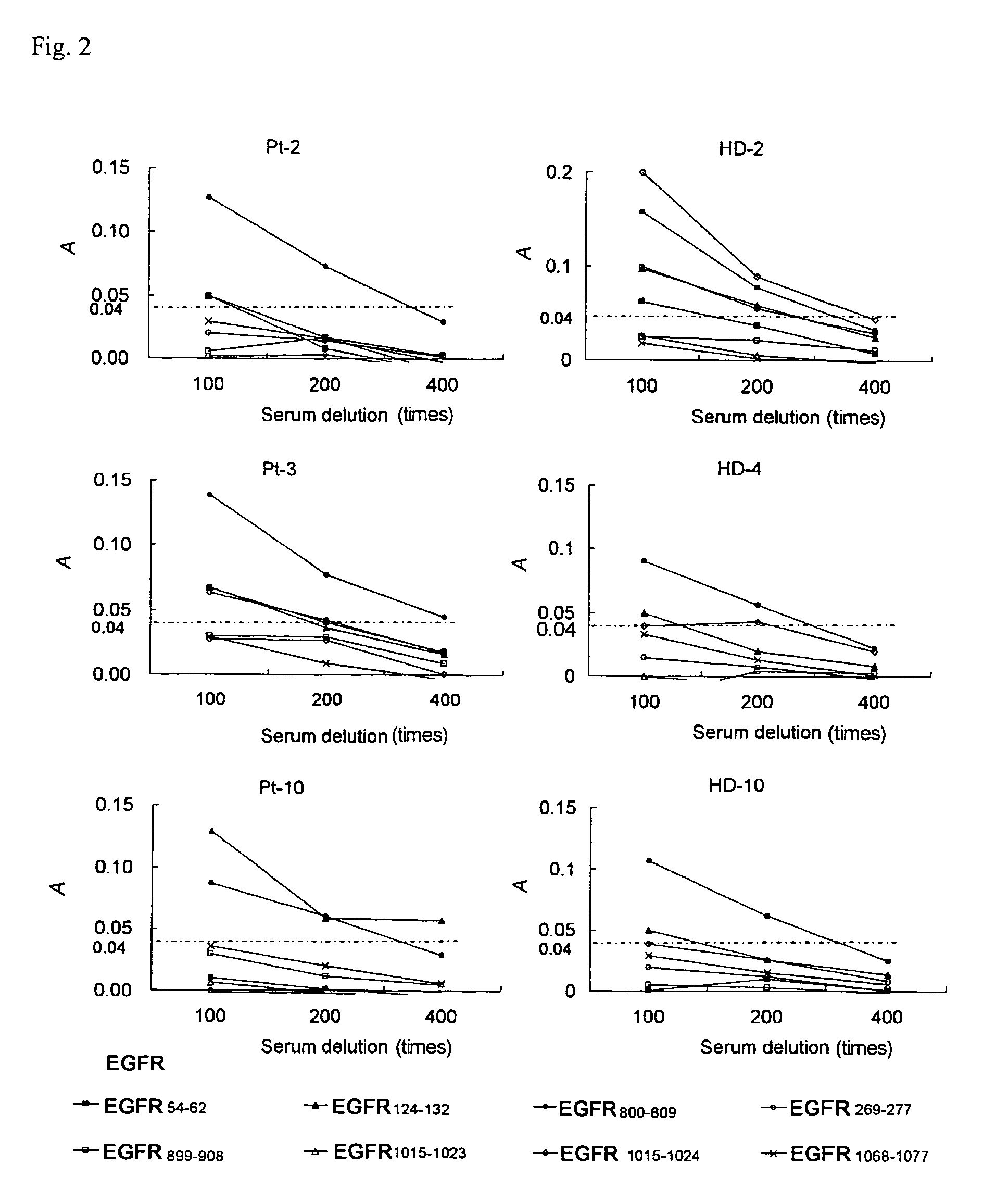
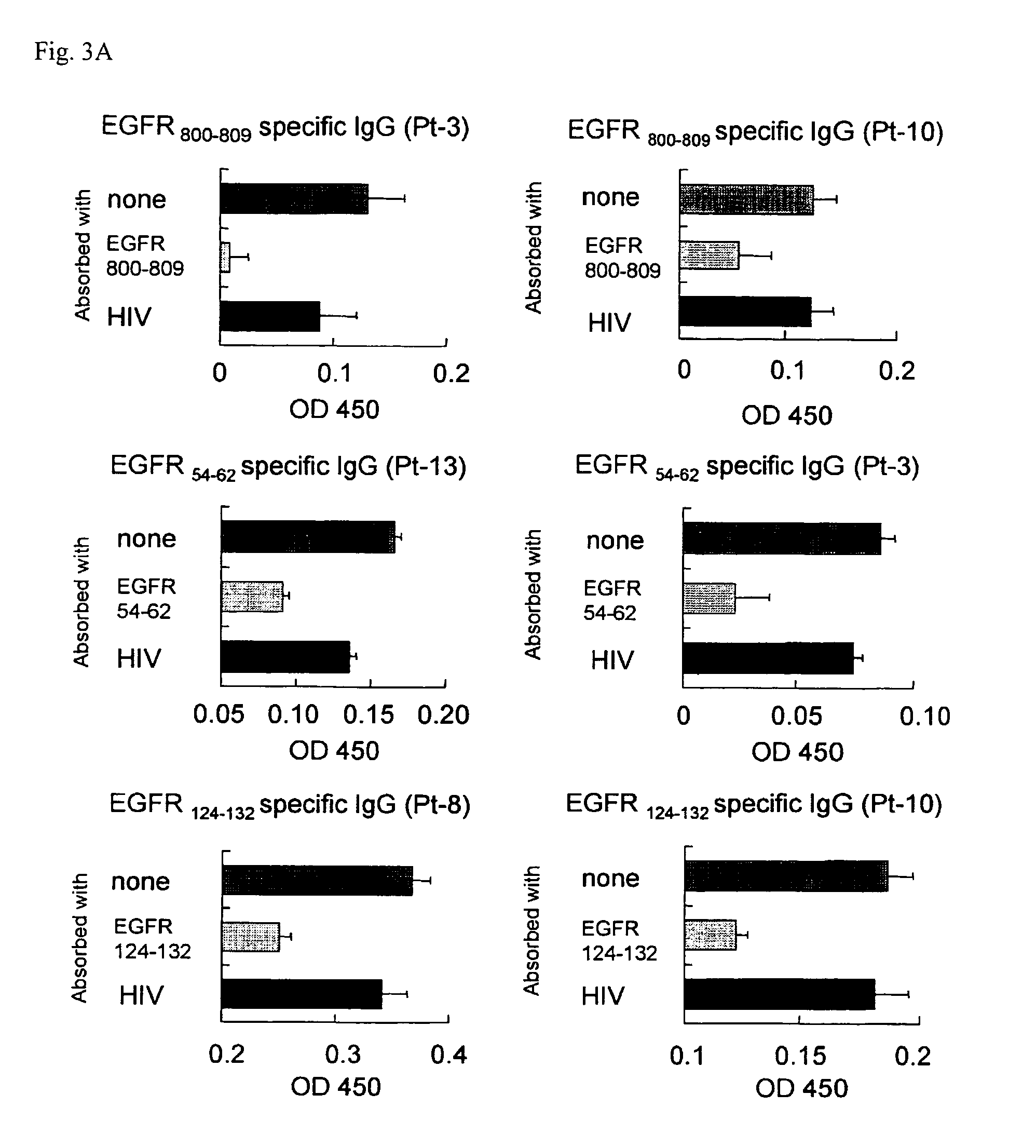
Epidermal Growth Factor Receptor-Derived Peptides
ActiveUS20080119399A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman epidermal growth factor receptorTransit Peptide
The object of the invention is to provide an EGFR-derived peptide useful for EGFR-based immunotherapy.The invention provides an EGFR-derived peptide capable of inducing both cellular and humoral immune responses and mutant peptide thereof and a polypeptide comprising said peptide, a nucleic acid molecule encoding the same, and a pharmaceutical composition comprising the same.
Owner:BRIGHTPATH BIOTHERAPEUTICS CO LTD
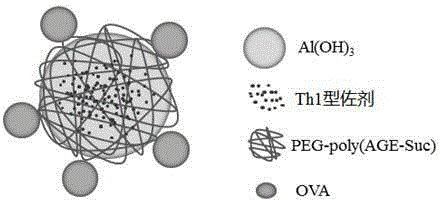
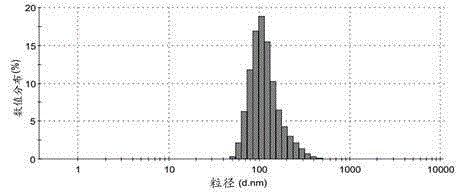
Vaccine vector based on aluminum hydoxide nano-particles
ActiveCN104587464AReduce aluminum contentNo inflammationPharmaceutical non-active ingredientsImmunological disordersDendritic cellTGE VACCINE
The invention provides an aluminum adjuvant used as a vaccine vector. The adjuvant is characterized in that a PEG derivative bio-material and aluminum are compounded to form nano-particles, so that the property of strong Th2 body fluid immunologic adjuvant of aluminum salt is maintained, and the adjuvant can be efficiently transferred to draining lymph nodes in the body and can be easily ingested by dendritic cells to perform effective cross-presentation and induce cellular immunologic response. The aluminum adjuvant has strong Th1 immunologic response.
Owner:SICHUAN UNIV
African swine fever virus vaccine and preparation method thereof
ActiveCN112876570AAntibody mimetics/scaffoldsVirus peptidesAntiendomysial antibodiesAfrican swine fever
The invention discloses an African swine fever vaccine and a preparation method thereof. According to the invention, the structural proteins P72, P30, P54 or CD2v-AC of the African swine fever virus are respectively displayed on the surface of the cage-shaped structure of the self-assembled ferritin, so that the humoral immune efficacy and width of the vaccine are improved, and the immunogenicity of the structural proteins of the African swine fever virus is improved. Structural proteins P30, P54 and CD2v are recombined to obtain recombinant proteins, and the recombinant proteins are connected with ubiquitin to obtain two recombinant proteins, so that the cellular immune effect of the structural proteins of the African swine fever virus is further improved, and better immune protection is provided for animals. The invention also provides a method for preparing the recombinant protein or the African swine fever vaccine. The African swine fever vaccine provided by the invention can initiate a wide neutralizing anti-African swine fever antibody, not only improves the immune efficacy, but also expands the immune range, provides effective immune protection for virulent infection, and has the potential of becoming a universal safe vaccine with multiple protection effects.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Nano-element traditional Chinese medicinal fabric antibacterial underwear for preventing and treating psoriasis
InactiveCN102078029AAmphibian material medical ingredientsElectrotherapyHigh morbidityHumoral immune reaction
The invention discloses nano-element traditional Chinese medicinal fabric antibacterial underwear for preventing and treating psoriasis, which belongs to the technical field of functional healthy fabric underwear production. Psoriasis is a skin disease which has high morbidity and stubborn disease process, is easy to relapse, is difficult to cure and does serious harm to the physical and psychological health of people. Far infrared heat effect takes effect in deep subcutaneous tissues through medical stone, tourmaline and a nano silver (Ag) antimicrobial agent, so the underwear expands blood vessels, quickens blood flow, increases cell activity and blood oxygen content, makes human bodies absorb more negative ions and trace elements, promotes body metabolism, enhances cellular immunity and humoral immunity of the human bodies, promotes the balance of elements in the human bodies, strengths the regeneration activity of cells and resists bacteria, cancers and inflammation. The underwear prevents and treats psoriasis when a user wears the underwear.
Owner:成进学
Recombinant porcine epidemic diarrhea virus (PEDV) lactococcus lactis and its construction method and use
InactiveCN103074291AGood immune protectionPromote absorptionBacteriaMicroorganism based processesProtective antigenWestern blot
Owner:DALIAN UNIVERSITY
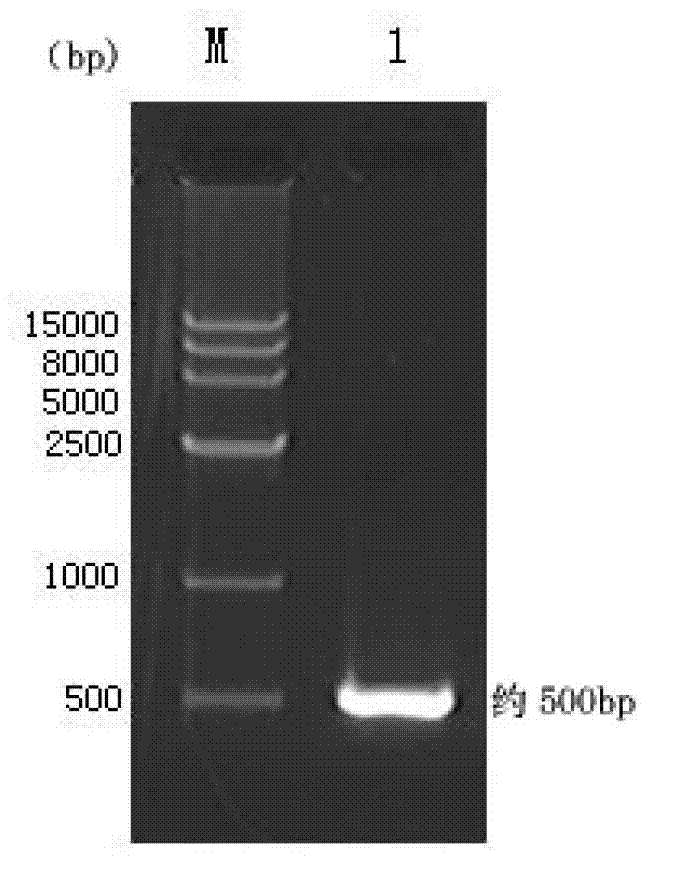
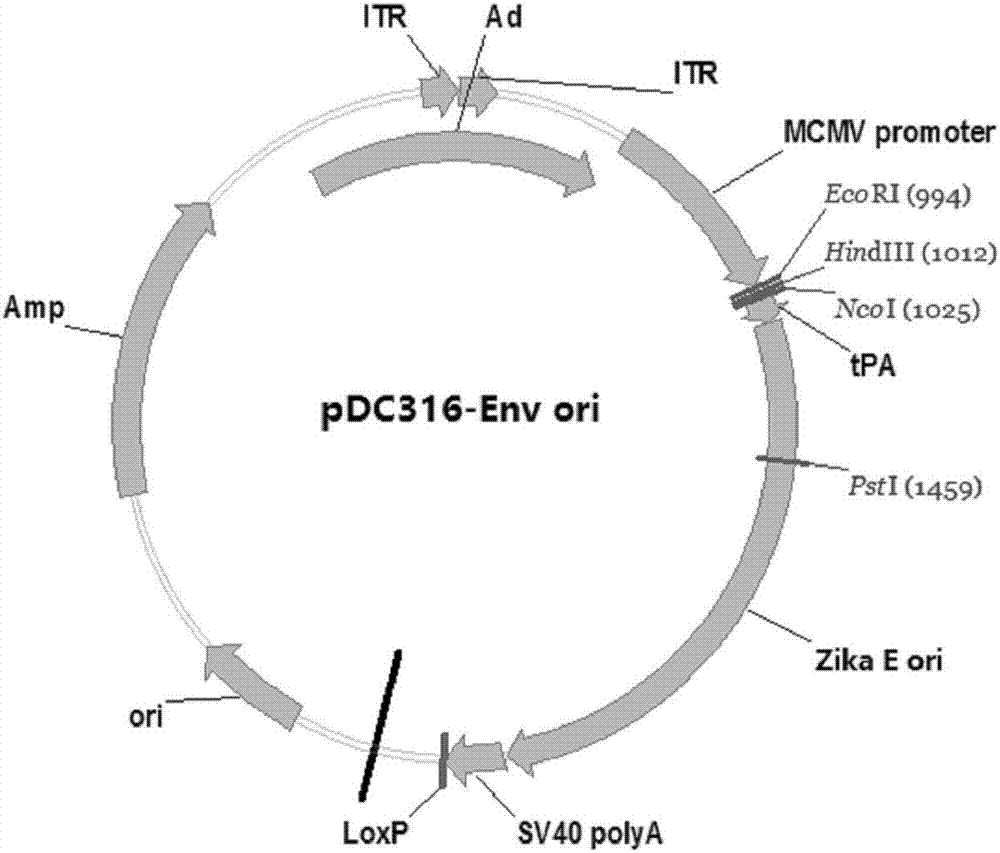
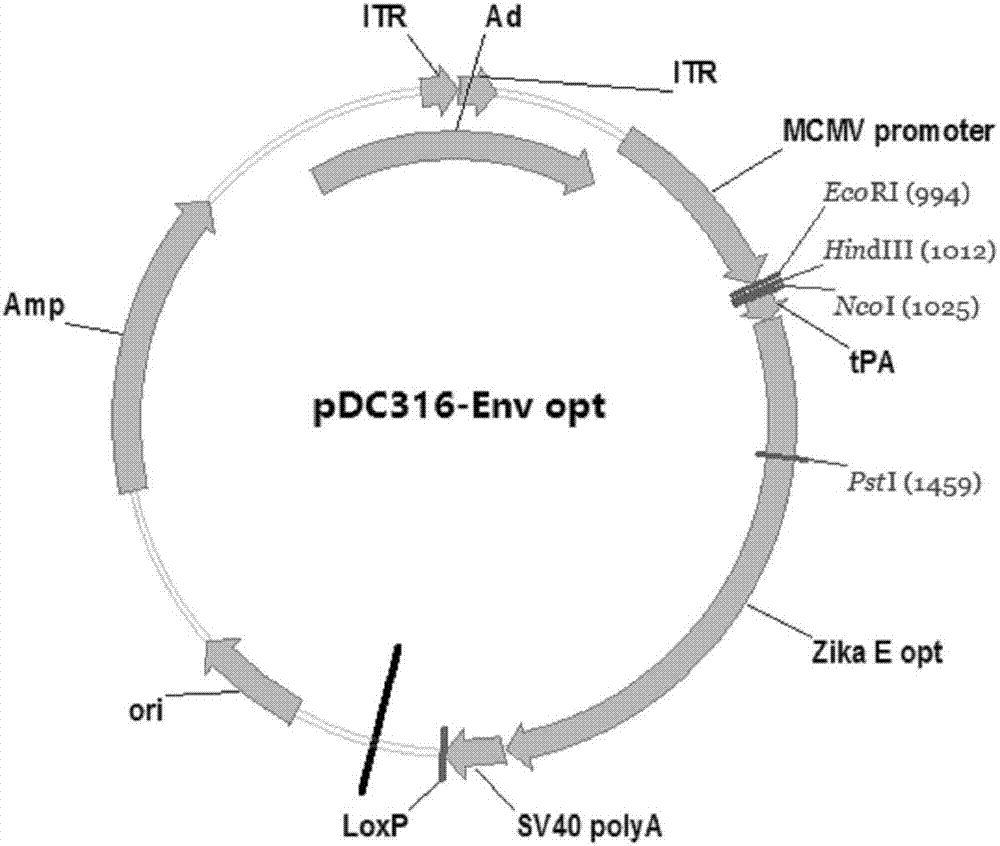
Zika virus disease vaccine taking human Ad5 replication-defective adenovirus as vector
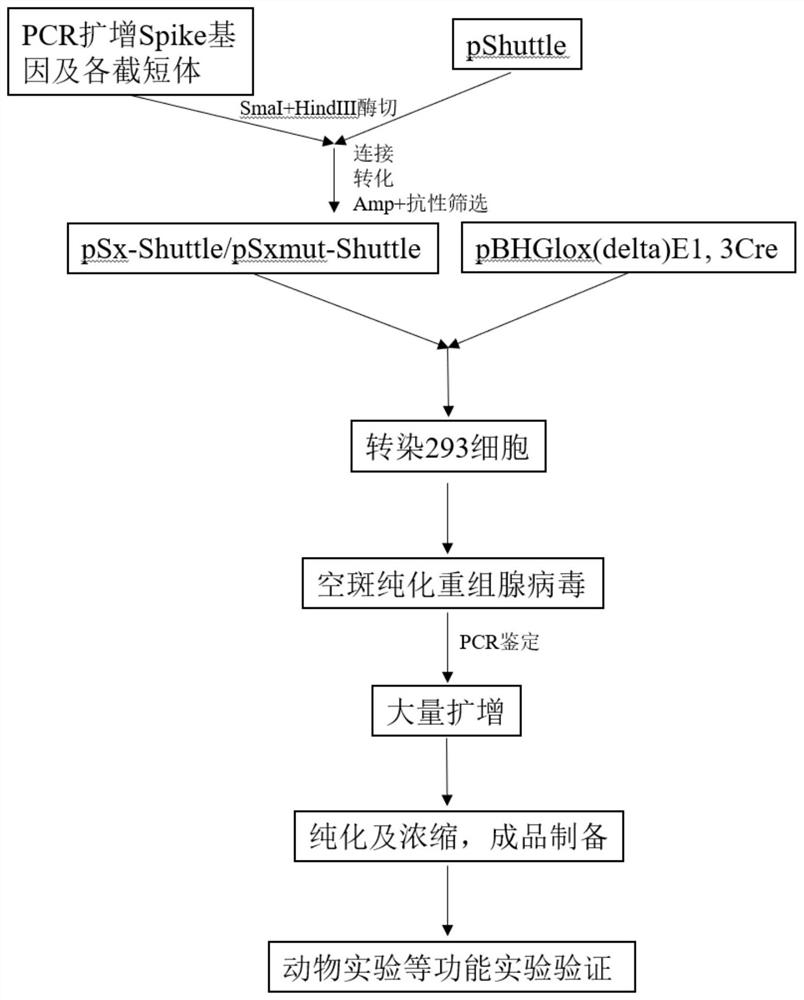
ActiveCN107190013ARapid responseRapid immune responseSsRNA viruses positive-senseViral antigen ingredientsInfected cellShuttle vector
The invention discloses a codon-optimized nucleotide sequence capable of expressing an Env protein of a zika virus. The sequence can be fused with a protein prM and a protein prM endogenous signal peptide of a codon-optimized zika virus; after the sequence is inserted into a shuttle vector pDC316, the sequence and an auxiliary vector pBHGlox_E1, 3Cre realize cotransfection of a cell HEK293, so as to package an E1 and E3 combined missing-recombinant adenovirus taking a replication-defective human type-5 adenovirus as a vector; the recombinant adenovirus vector can efficiently express an envelope protein of the zika virus in an infected cell. After the nucleotide sequence-inserted recombinant adenovirus serving as a vaccine is immune to an animal for single time, strong humoral immune and cellular immune responses can be quickly induced. A zika vaccine taking the recombinant adenovirus as the vector is suitable for large-scale and rapid preparation and can be used for emergent vaccination for a large scale of people in a zika outbreak and prophylactic immunization for people at ordinary times.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Nucleotide sequence for encoding novel coronavirus antigen and application of nucleotide sequence
ActiveCN112626090AIncrease GC contentRaise the ratioSsRNA viruses positive-senseViral antigen ingredientsWild typeCell immune response
The invention provides a nucleotide sequence for encoding a novel coronavirus antigen and application of the nucleotide sequence. According to the nucleotide sequence, a wild-type DNA sequence for encoding SARS-CoV-2 virus surface protein Spike is optimized, a wild-type gene signal peptide is optimized and then inserted into an eukaryotic expression vector, the eukaryotic expression vector is introduced into a host cell, a virus Spike antigen is efficiently expressed in the host cell, and an antiviral humoral immune response and a cellular immune response are systematically activated after antigen presentation. The antibody generated by the activated humoral immune response can prevent invasion of the SARS-CoV-2 virus, and the activated cellular immune response can further remove cells infected by the virus and adjust adverse reactions caused by potential side effects of ADE.
Owner:ADVACCINE SUZHOU BIOPHARMACEUTICALS CO LTD
Polyepitope tuberculosis gene vaccine and its prepn process
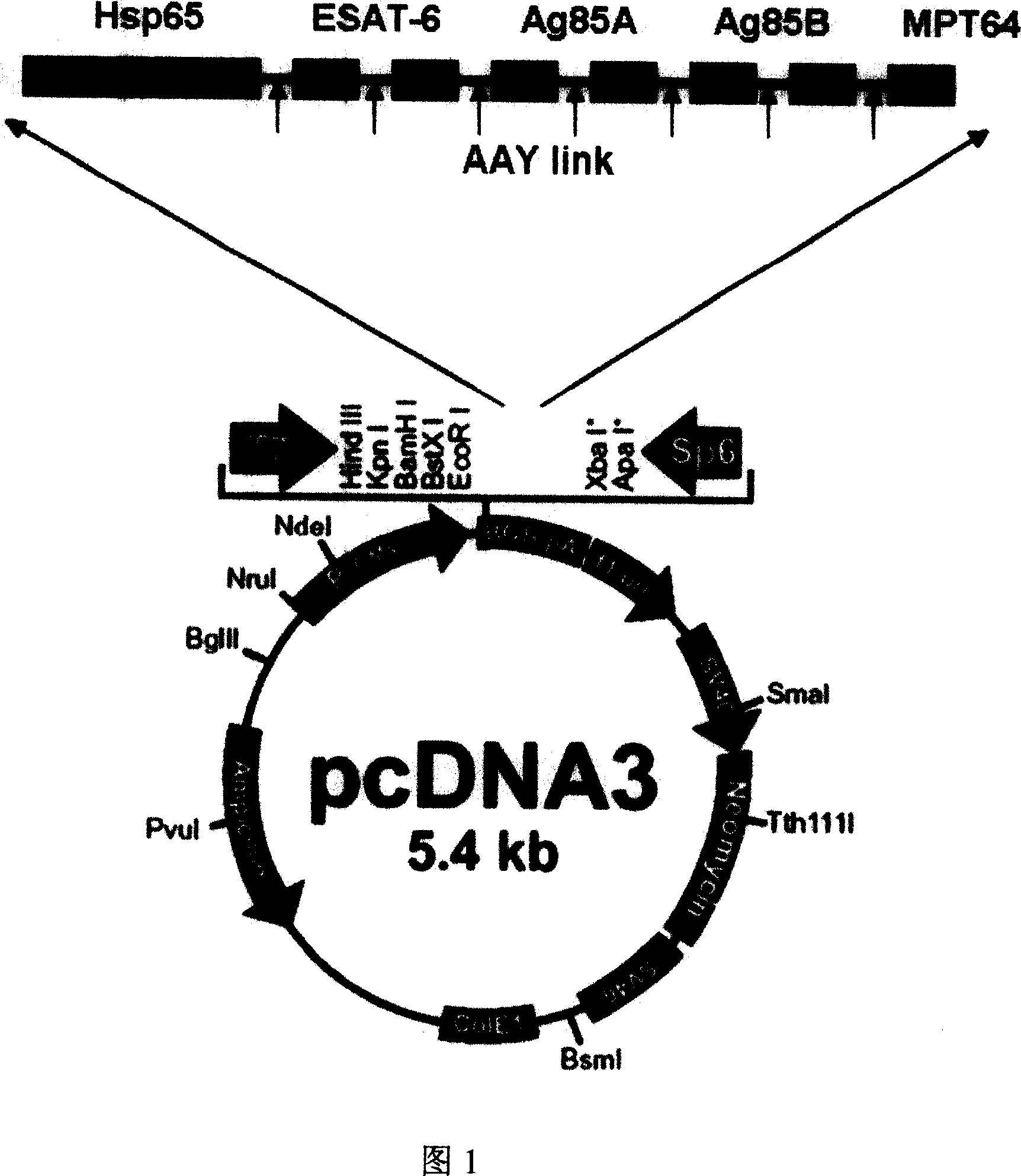
InactiveCN101088559ARaise the level of immune responseStimulate immune responseAntibacterial agentsBacterial antigen ingredientsEpitopeGenetic vaccine
The present invention discloses one kind of polyepitope tuberculosis gene vaccine comprising one kind of carrier and one inserted segment of target gene, which has in the 5' end one whole length HSP65 gene, and in the 3' end serially arranged selected epitope genes, including ESAT-6Á1-20 positions genes and 61-81 positions genes of ESAT-6, 62-84 positions genes of Ag85A, 121-155 positions genes of Ag85B, 143-166 positions genes of Ag85A, 234-256 positions genes of Ag85B and 177-228 positions genes of MPT64C. The present invention discloses the application of the polyepitope tuberculosis gene vaccine. Mouse immunizing experiment shows that the gene vaccine can well induce humoral immunity response. The gene vaccine of the present invention can well induce Th1 type immunity response to mycobacterium tuberculosis and enhance the anti-mycobacterium tuberculosis immunity response level.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
Fusion protein of tetanus toxin T cell expressing bit polypeptide and human bata lymph cell stimulating factor and preparing thereof
InactiveCN1793178AImprove protectionOvercome the limitations of self-toleranceHybrid peptidesVector-based foreign material introductionEscherichia coliDisease
The invention discloses tetanus toxin T cell epi position peptide and human B lymphocyte activate and gene interfusing albumen. It could activate CD4+T cell to take cell action, promote body liquid immunity level. It adopts step clone to construct TT and BLyS interfusing gene and gain high efficiency expression in coliform bacteria. It conquers the immunity tolerate of the body.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
SARS-CoV-2 coronavirus vaccine and preparation method thereof
ActiveCN112618707AMaintain native conformationImprove biological activitySsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationImmunogenicity
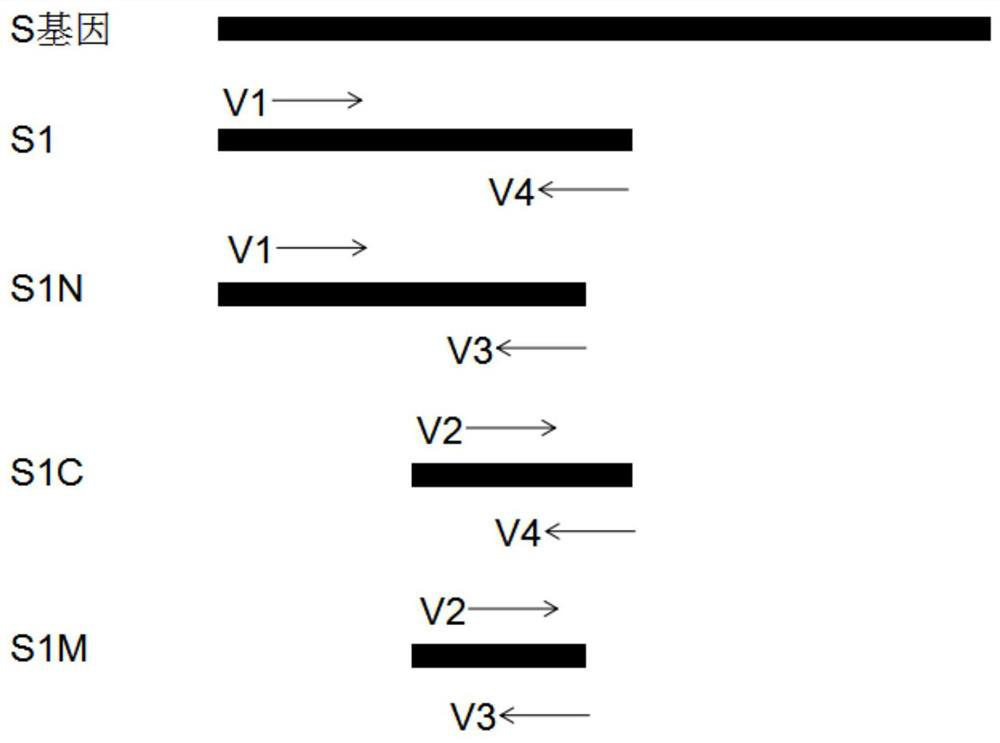
The invention discloses a SARS-CoV-2 coronavirus vaccine and a preparation method thereof. An S gene of a SARS-CoV-2 coronavirus is subjected to codon optimization, truncated body and mutant sequences of the S gene are introduced into a secretory defective adenovirus vector, and packaging is conducted to obtain a corresponding recombinant adenovirus; and the recombinant adenovirus can express SARS-CoV-2 virus related protein in vivo, processing, folding, glycosylation and other modifications are completed, the native conformation of S protein is basically maintained, and the characteristics of high biological activity, long half-life period, lasting immunogenicity and the like are achieved. According to the vaccine and the method, by adopting the defective adenovirus vector carrying secretory peptide, a recombinant adenovirus vaccine can be secreted out of cells after being expressed in vivo, so that the humoral immunity is activated.
Owner:GUANGZHOU DOUBLLE BIOPRODUCT CO LTD
Porcine circovirus-like particle, and vaccine and preparation method thereof
InactiveCN103436499ASafe infectionEffective infectionViral antigen ingredientsInactivation/attenuationAdjuvantStructural protein
The invention discloses a porcine circovirus-like particle, a vaccine and a preparation method thereof. The virus-like particle is composed of a main structural protein-nucleocapsid protein of 2-type porcine circovirus, can excite cell and humoral immune response, can be used as a virus-like particle vaccine (VLP vaccine) to immune different fauna and can safely and effectively prevent PCV-2 infections after being used. The VLP can be made into injections, nose drops and drinking preparations by adding adjuvants or not. An ideal vaccine is provided for security of different populations of sows, piglets, fattening pigs and for effective immune prevention and control of PCV-2 infections.
Owner:CHONGQING AULEON BIOLOGICALS
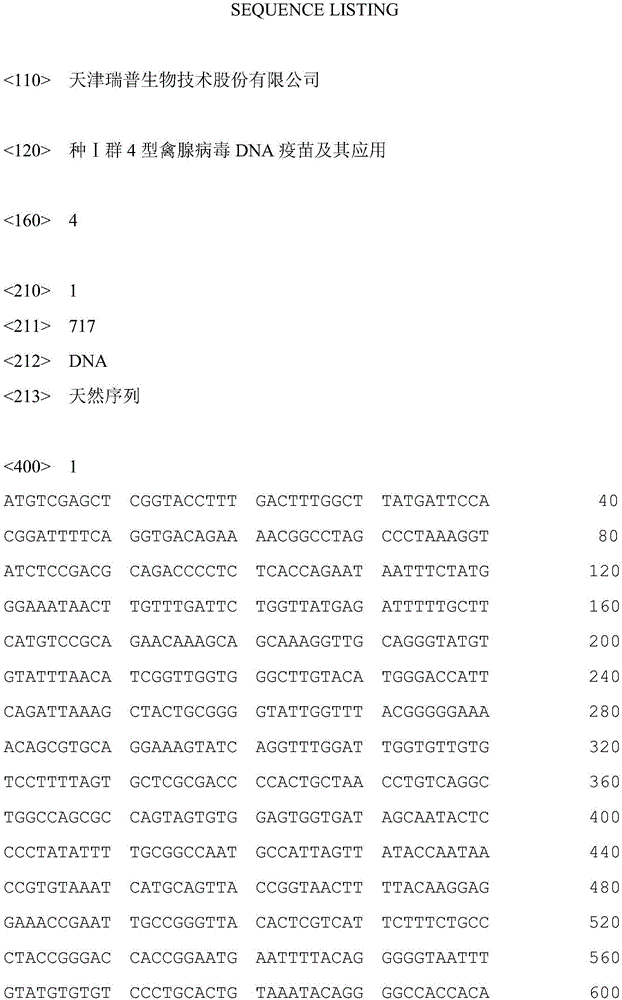
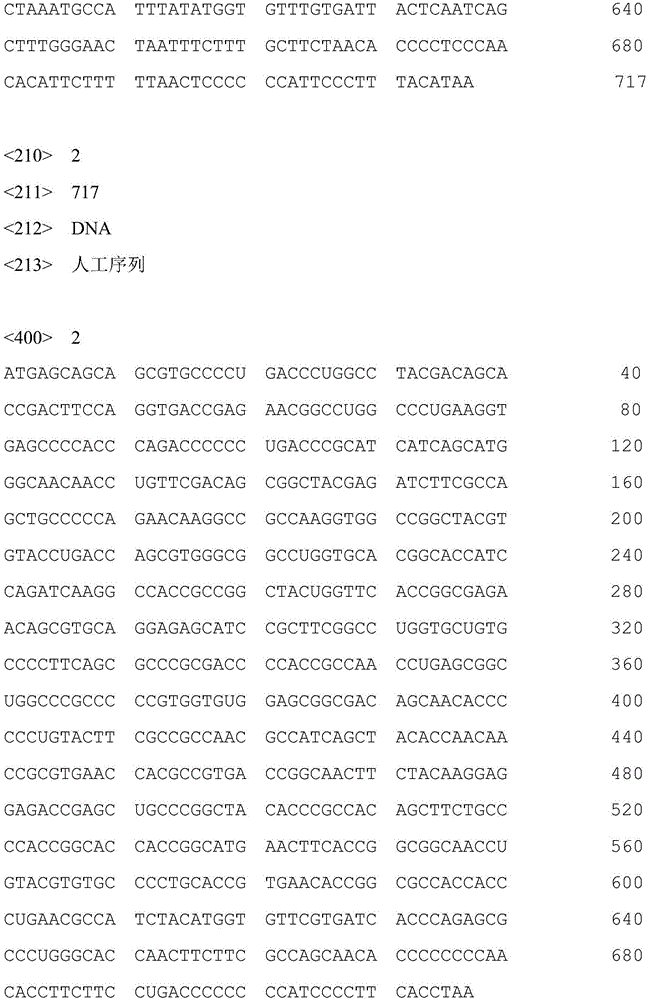
I-group 4-type fowl adenovirus DNA vaccine and application thereof
InactiveCN106729694AEffective immune protectionGood prospects for commercial developmentDigestive systemAntiviralsFiberRecombinant vaccines
The invention provides an I-group 4-type fowl adenovirus DNA vaccine and application thereof. According to the technical scheme, based on experimental means, research finds and shows that fiber protrusions have good immunity prototypes, in this way, with 4-type fowl adenovirus fibrous protein C-terminal genes being antigen substances, codon optimization is carried out on the basis of a natural sequence, an eukaryotic expression vector pCAGGS is cloned, and the DNA vaccine pCAGoptiFAV4C is constructed. According to the researched fowl adenovirus NDA vaccine, a method of gene engineering fermentation is adopted for preparing antigens, and therefore the I-group 4-type fowl adenovirus DNA vaccine is low in cost, pure in antigen and safe to use. By the utilization of a serology method and an immunity virus attack method, the immunity effect of the vaccine is evaluated. The result shows that the vaccine can provide effective immune protection for fowl, and has good commercial development prospects. Compared with a traditional vaccine, the DNA vaccine achieves the safety of subunit vaccines and inactivated vaccines, and also has the features of simultaneous induction of humoral immunity and cellular immune response, wherein the features only belong to attenuated vaccines or recombinant vaccines.
Owner:TIANJIN RINGPU BIO TECH
Vaccine adjuvant
InactiveCN101444623AExcellent allergenicityExcellent securityAntibody medical ingredientsZinc hydroxideBody fluid
The invention relates to a vaccine adjuvant, in particular to the application of compound zinc hydroxide in the aspect of vaccine adjuvant preparations and further the application in the aspect of VAOTA adjuvant preparation.s The zinc hydroxide has a chemical formula of Zn(OH)2 and a chemical formula weight of 99.4046 Dalton. The zinc hydroxide working as an adjuvant is jointly applied with vaccine, so as to effectively strengthen body fluid immune response of the vaccine, and the immune strength effect is superior to that of aluminum adjuvant. In addition, according to the animal experiment result, both the sensitization and the safety of zinc hydroxide are superior to aluminum adjuvant.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Vaccine Composition Comprising Alpha-Galactosylceramide as an Adjuvant For Intranasal Administration
InactiveUS20080317769A1Improve responseViral antigen ingredientsSnake antigen ingredientsNasal cavityAdjuvant
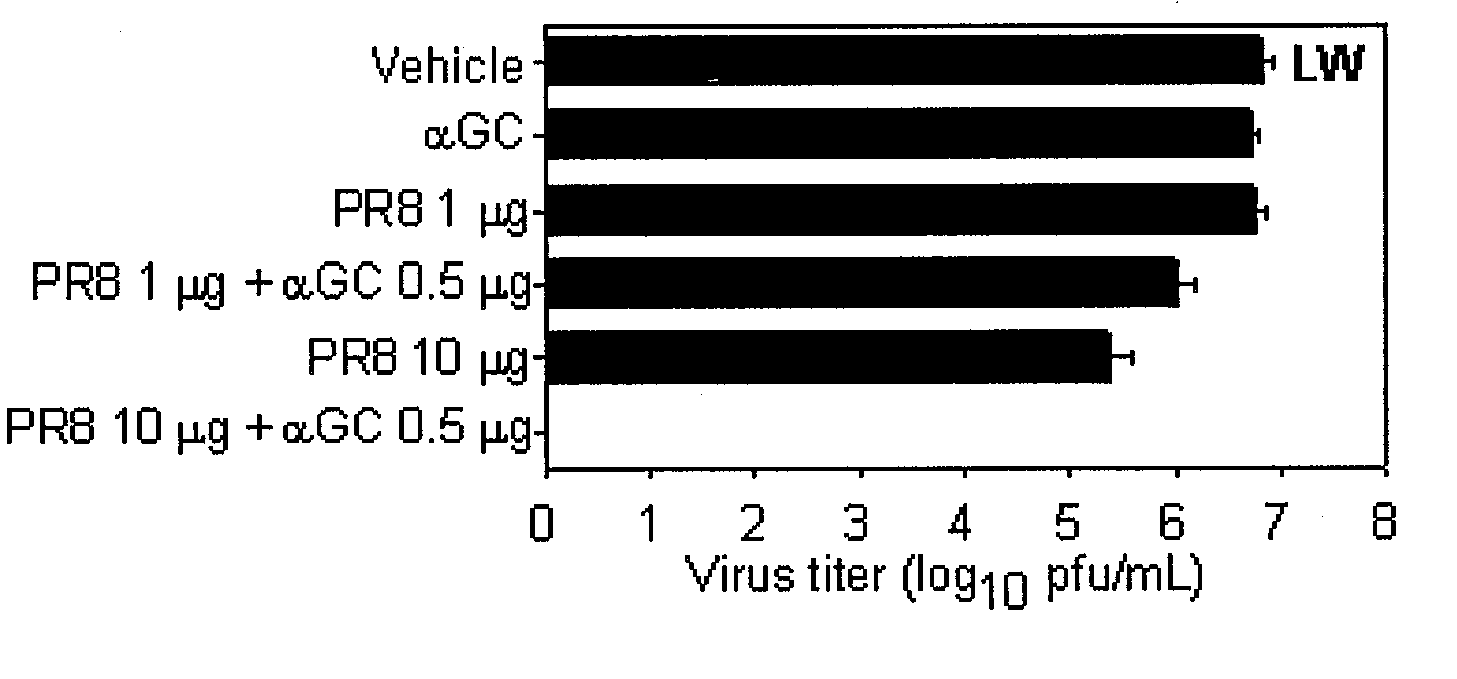
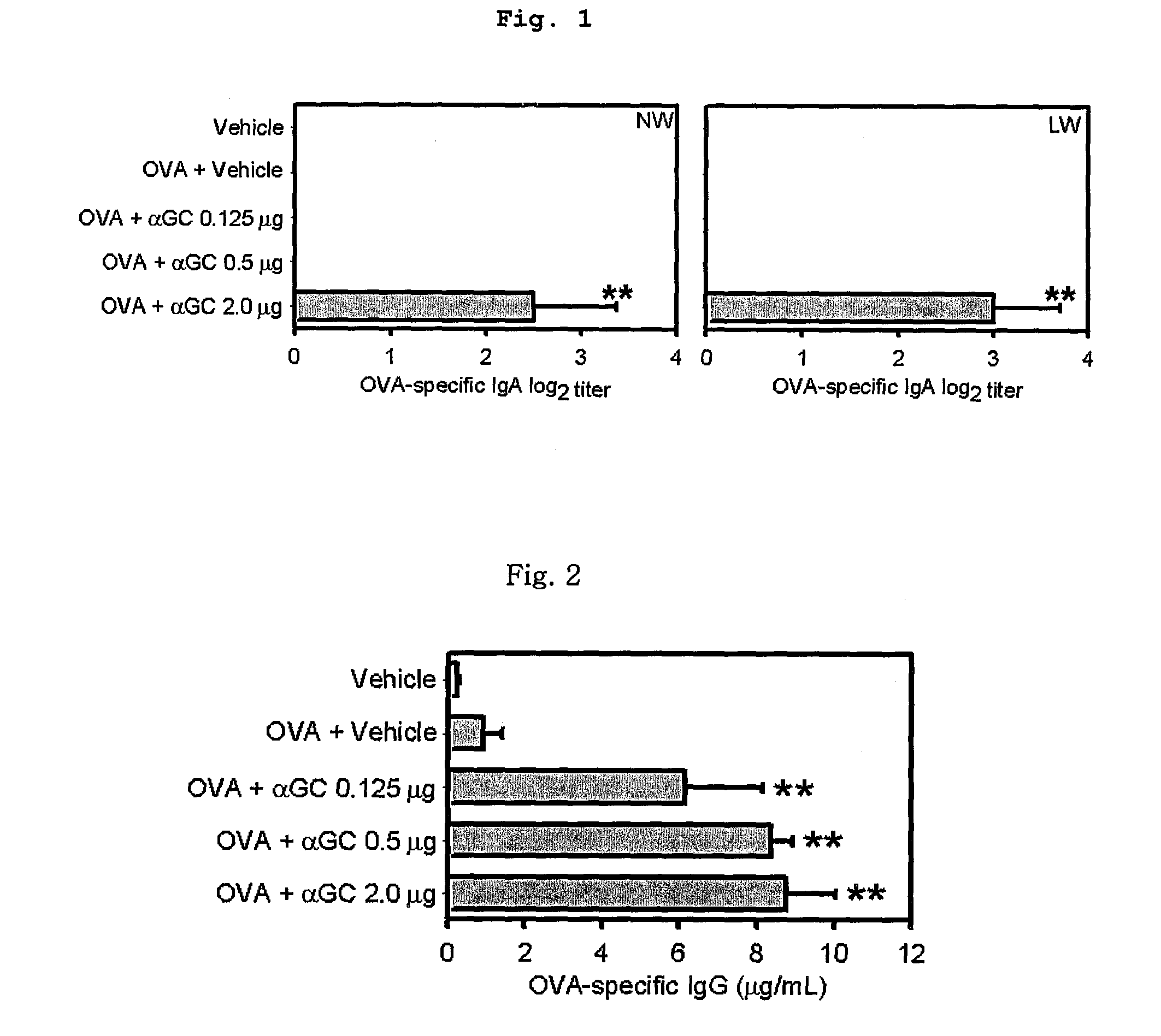
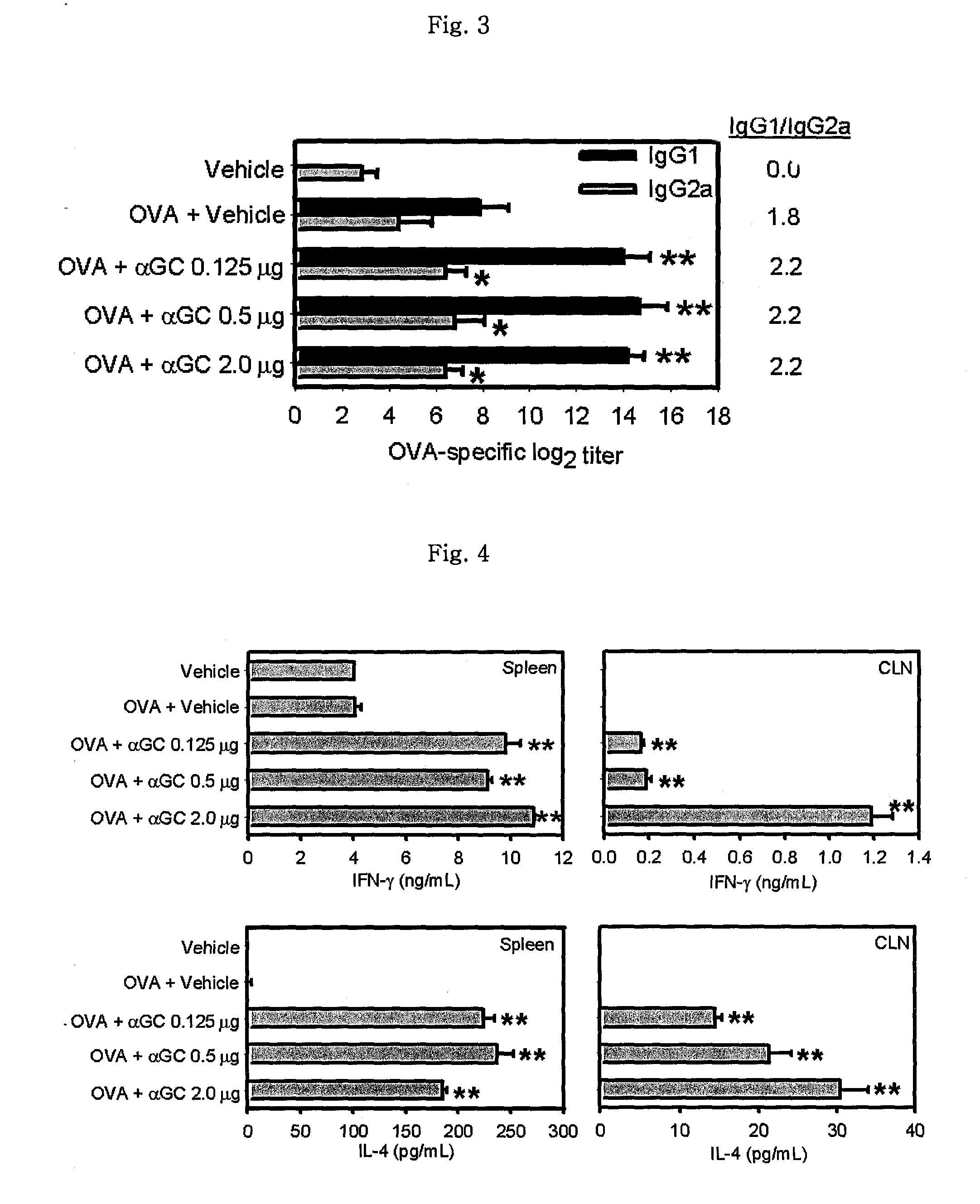
The present invention related to a vaccine composition comprising alpha-galactosylceramide (αGalCer) as an adjuvant for the intranasal administration. The present inventors administered αGalCer together with a tumor cell antigen or a virus antigen to the nasal cavity of a mouse and then confirmed that the αGalCer effectively induced not only humoral immunity but also cell-mediated immunity. Thus, the αGalCer can be effectively used as an adjuvant for a vaccine by the intranasal administration for the prevention and treatment of virus infection and cancer.
Owner:SEOUL NAT UNIV R&DB FOUND
Recombinant fusion protein and use thereof
InactiveCN104341506AAntiviralsAntibody medical ingredientsHepatitis B virus core AntigenAntigen epitope
The invention belongs to the biological medicine field, relates to recombinant fusion protein and use thereof, and in particular relates to recombinant fusion protein carrying hepatitis B virus therapeutic antigen epitopes which are inserted into hepatitis B core antigen protein particles or truncated fragments and use thereof. The recombinant fusion protein contains multiple epitope antigens of hepatitis B virus (HBV) and other immune stimulating epitope antigens and hepatitis B core antigen virus-like particles or truncated fragments thereof for preparation of chimeric antigen, the multiple antigen epitopes can be inserted into same or different sites of hepatitis B virus core antigen HBc or truncated fragments thereof in the manner of single epitope or multi epitope combination, and by combination with different adjuvants, HBV specific humoral and cellular immune functions can be strengthened.
Owner:FUDAN UNIV
Preparation and application of CpG DNA molecule anti-infection and immunity prepn
InactiveCN1692943ARaise the level of immune responseReduce plasmid usageSugar derivativesGenetic material ingredientsBiotechnologyMolecular Immunology
An anti-infectious immunopotentiator for pig, ox, yak, etc is prepared through artificially synthesizing the CpG oligonucleotide sequce able to excite the reproductive activity of immune cell, preparing the chitosan nanoparticles from deacetyl chitosan, and using said chitosan nanoparticles for molecular packaging of CpG oligonucleotide. It can be used to immunize the experimental animal by intramuscular injection or oral applialtion.
Owner:SICHUAN UNIV
Traditional Chinese medicine astragalus polysaccharide immunopotentiator
ActiveCN101884788AGood effectNo side effectsAntibody medical ingredientsCellular immunityNewcastle disease vaccine
The invention relates to a traditional Chinese medicine immunopotentiator (an astragalus polysaccharide immunopotentiator for short) prepared from astragalus extracts and sulfated epimedium polysaccharides, which belongs to the field of immunological adjuvants of livestock and poultry. 1,000ml of the liquid medicine is prepared from 40g of astragalus and 120g of epimedium herb. The preparation method of the immunopotentiator comprises the following steps of: decocting the astragalus with water for three times, then merging the obtained filter liquor and condensing the filter liquor into 500ml of astragalus solution; extracting the epimedium polysaccharides by using the epimedium herb water decoction and ethanol precipitate method, then decorating the polysaccharides by using the chlorosulfonic acid-pyridine method, and preparing the polysaccharides into 500ml of sulfated epimedium polysaccharide solution after carrying out distilled water dialysis on the polysaccharides; and mixing the astragalus solution and the sulfated epimedium polysaccharide solution, and then filtering, sub-packaging and sterilizing to obtain the traditional Chinese medicine astragalus polysaccharide immunopotentiator. The immune experiment shows that the astragalus polysaccharide immunopotentiator has the advantage of obviously improving the proliferation of peripheral blood lymphocyte of chicken and enhancing the cellular immunity, obviously improving the potency of a serum antibody, promoting the lymphocyte proliferation, enhancing the cellular immunity and humoral immunity, and improving the immune response of a vaccine by coordinately immunizing chickling by using the Newcastle disease vaccine.
Owner:NANJING AGRICULTURAL UNIVERSITY
Bisphosphonate-containing vaccine pharmaceutical composition for humoral immunity
ActiveUS20170281759A1Improve complianceImprove the quality of lifeOrganic active ingredientsAntibody ingredientsHumoral immunityAntibody production
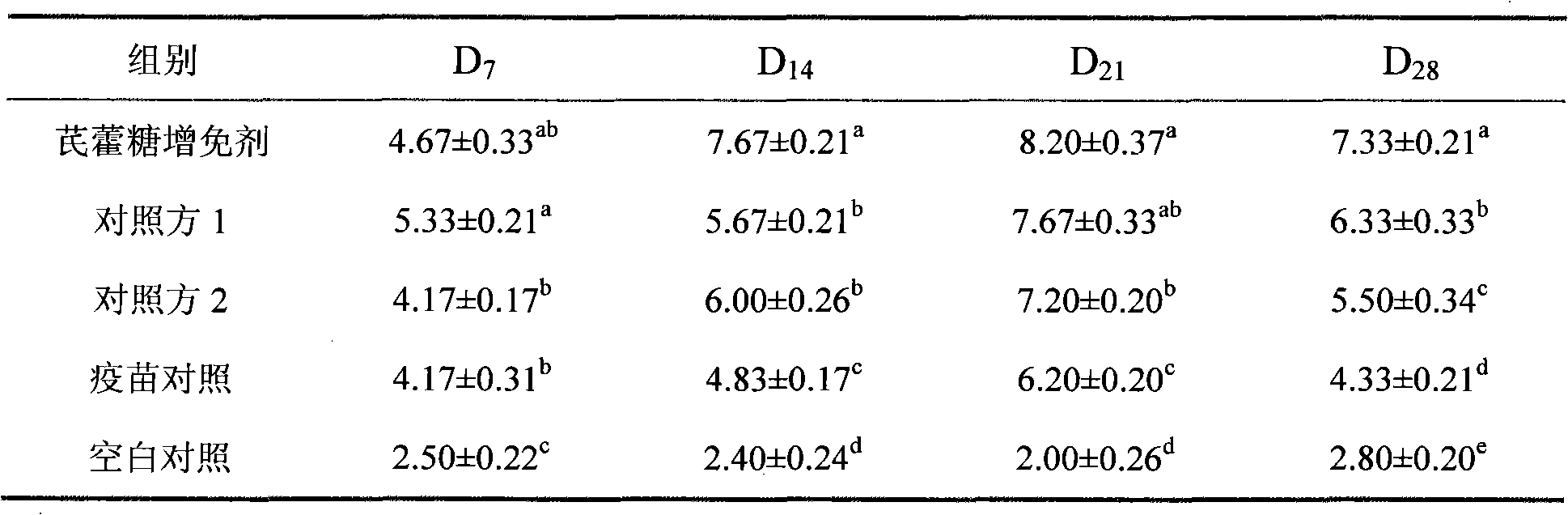
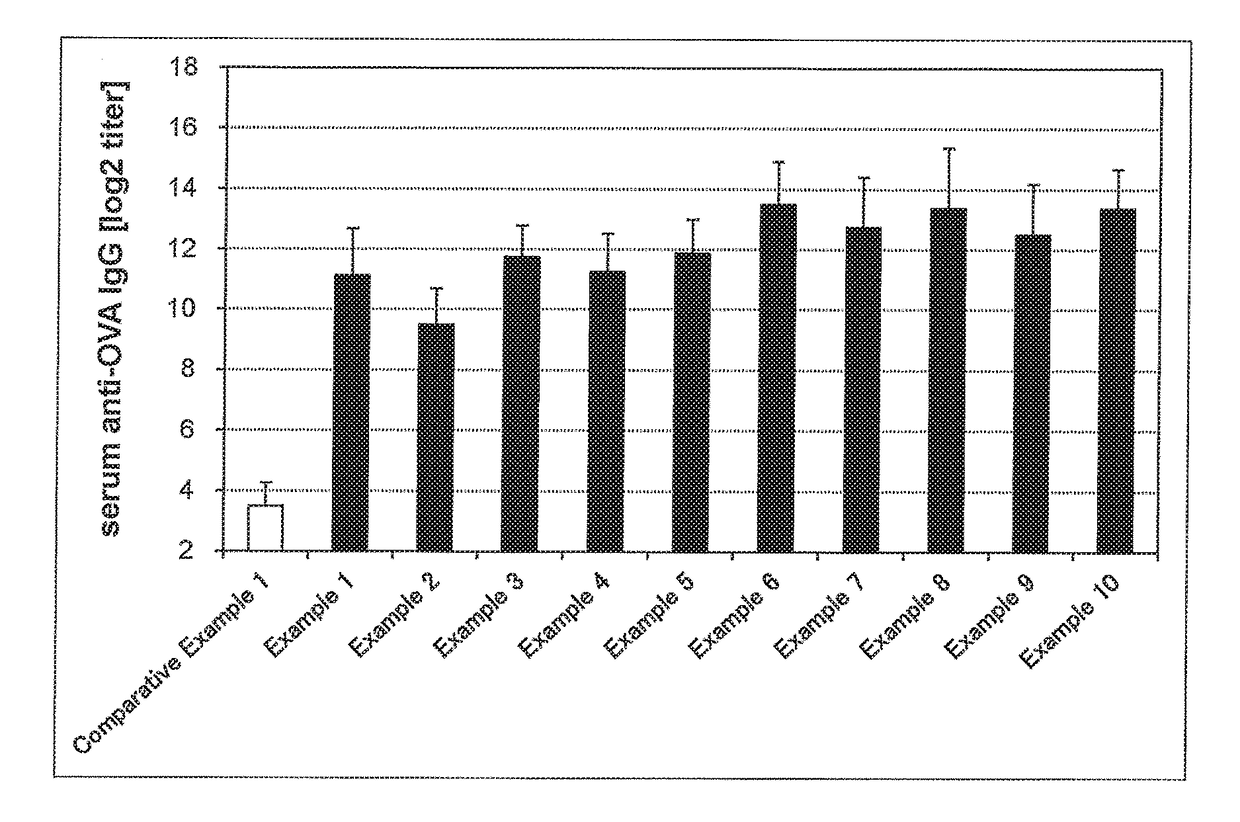
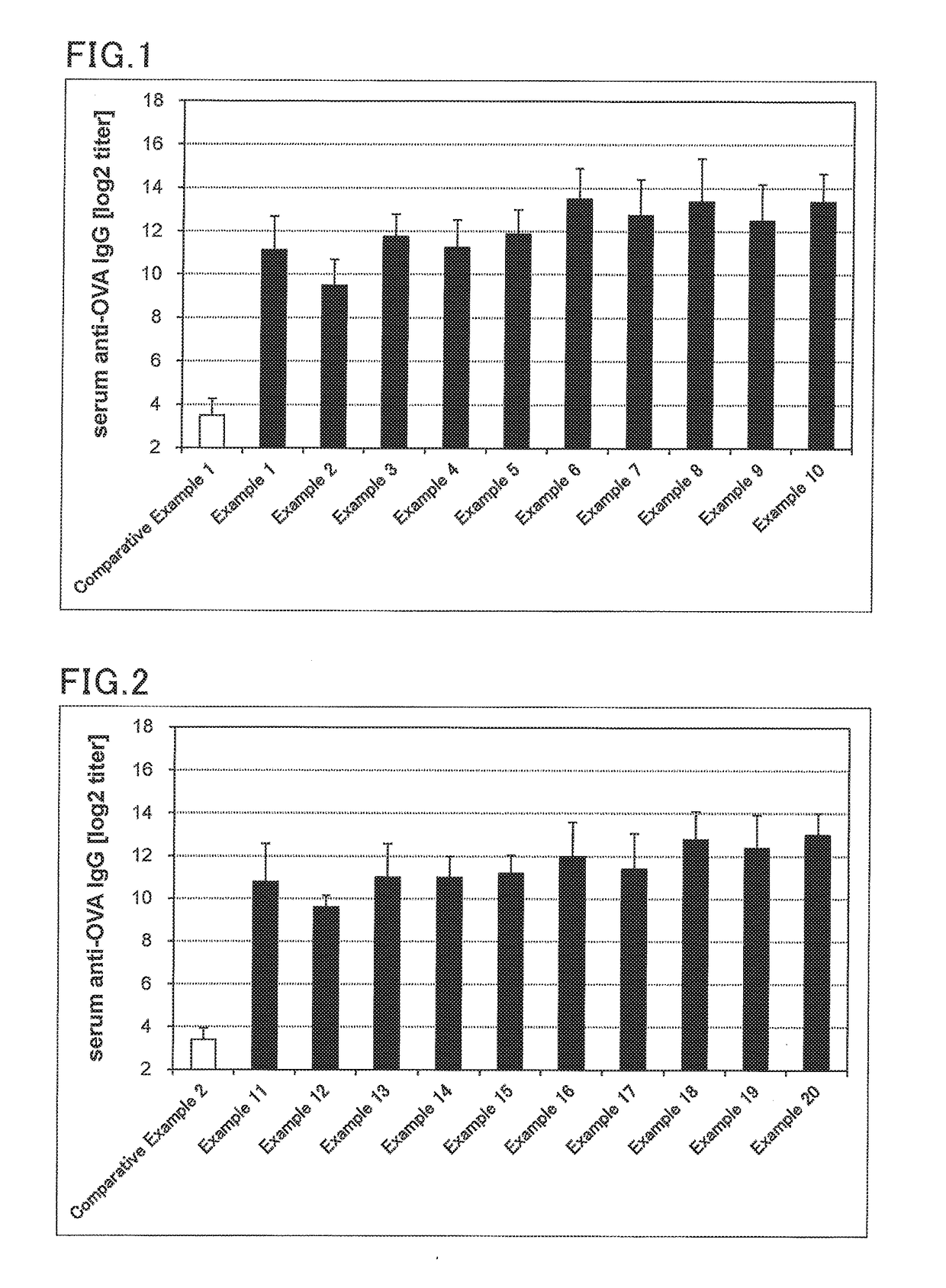
The present invention aims to provide a vaccine pharmaceutical composition universally usable for induction of humoral immunity against various antigens and exerting a high antibody production inducing effect. The present invention relates to a vaccine pharmaceutical composition for inducing humoral immunity, including: an antigen; and an immunity induction promoter that is a bisphosphonate.
Owner:NITTO DENKO CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com