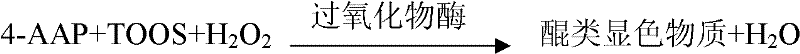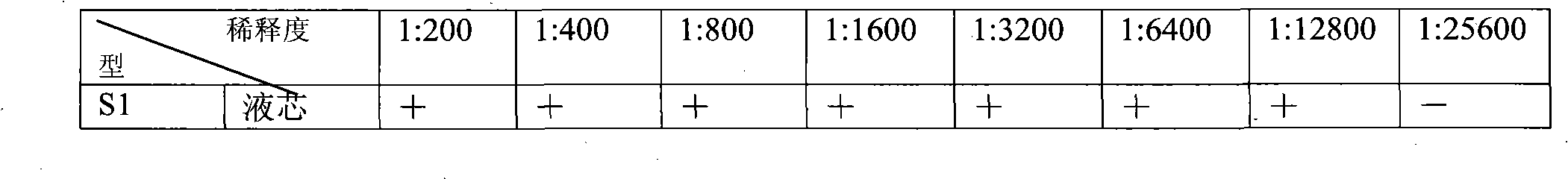Patents
Literature
40results about How to "Maintain native conformation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Magnetic composite microsphere immobilized laccase and preparation method thereof
InactiveCN102206624AAvoid chemical damageFacilitate recycling and reuseOn/in inorganic carrierChemistryCarbonyl group
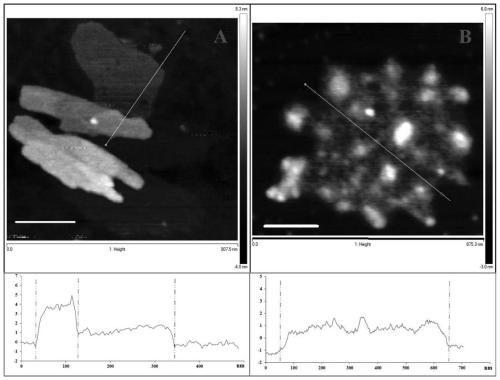
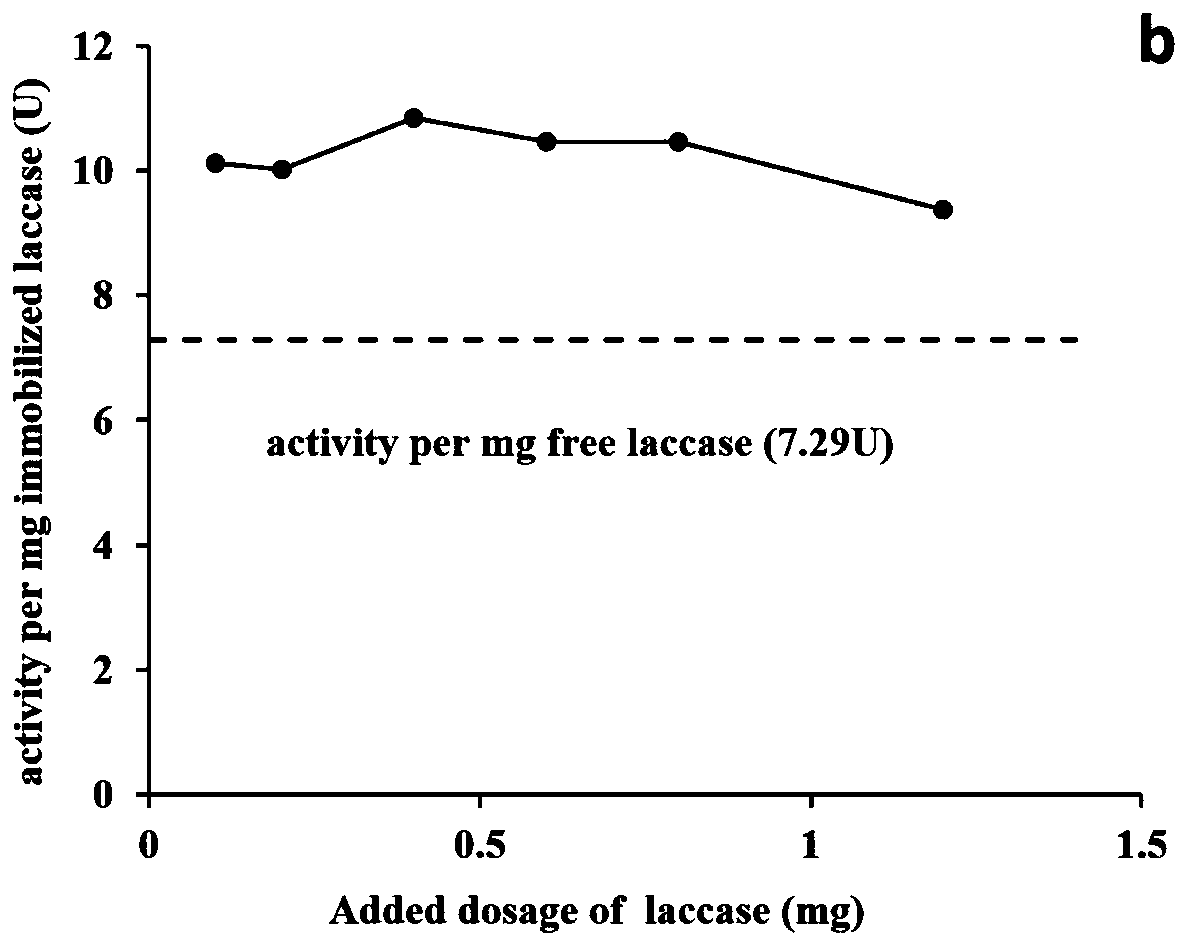
The invention relates to a magnetic composite microsphere immobilized laccase and a preparation method thereof, and belongs to the field of environmental microbiology. In the immobilized laccase, metal-chelated magnetic composite microspheres are taken as a carrier, and laccase is immobilized on the carrier through coordination bonding action. The preparation method comprises two steps of: preparing the carrier, namely synthesizing magnetic ferroferric oxide nanoparticles by using a chemical precipitation process, preparing core-shell magnetic silica by taking the nanoparticles as cores through a sol-gel method, grafting polymer containing carbonyl or amino on the surface of the magnetic silica by taking gamma-chloropropyltrimethoxysilane as a medium, and preparing a functional carrier capable of immobilizing the laccase by chelating transition metal ions (such as Cu2+. Zn2+, Ni2+ or Co2+); and immobilizing the laccase, namely performing coordination adsorption of the laccase on the surface of the carrier, washing, and drying to obtain the finished product of immobilized laccase. The immobilized laccase prepared by the method has high stability and activity and quick magnetic response, is easily recovered from a reaction system and can be repeatedly used; and the preparation process is simple and the cost is low.
Owner:BEIJING NORMAL UNIVERSITY
Method for preparing enzyme electrode with MWCNTs-TiO2/Nafion composite medium
InactiveCN101603940AExplanation of validityImprove catalytic abilityMaterial electrochemical variablesBiocompatibility TestingCore shell nanocomposites
The invention discloses a method for preparing an enzyme electrode with an MWCNTs-TiO2 / Nafion composite medium. In the method, MWCNTs-TiO2 core-shell nanocomposite materials are scattered into Nafion to prepare an organic-inorganic composite membrane as a fixed matrix for biological molecules for structuring a hemoglobin electrode, and a hemoglobin biosensor with fast response, high sensitivity and strong catalytic capacity is prepared by using the properties of large specific surface area, high surface reactivity, strong adsorption capacity, large electrical conductivity and the like of the MWCNTs-TiO2 core-shell nanocomposite materials and the properties of membrane forming, high chemical stability, high anti-interference capacity and the like of the Nafion. The biosensor has good biocompatibility, stability and repeatability, and has potential application in structuring biosensors. The sensor can be used for detecting the substances of hydrogen peroxide, trichloroacetic acid and the like, and has the advantages of low sensitivity, low detection limit and the like.
Owner:NANJING UNIV OF TECH
Method for preparing biological polysaccharide self-assembly modificatory chitosan antibacterial biological material
InactiveCN101905034AImprove surface biological propertiesInhibition of antimicrobial activityProsthesisLentinan sulfateSurface layer
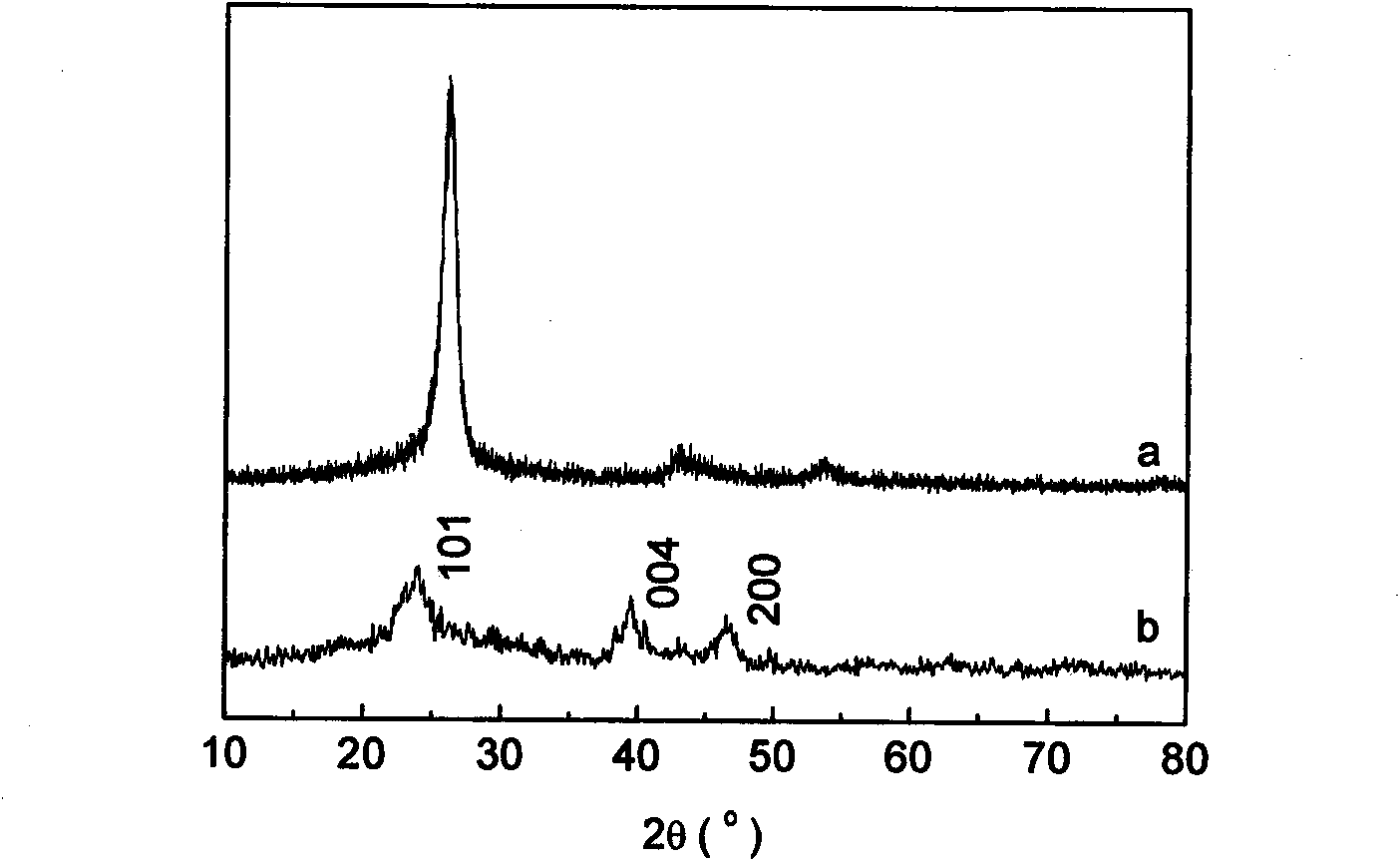
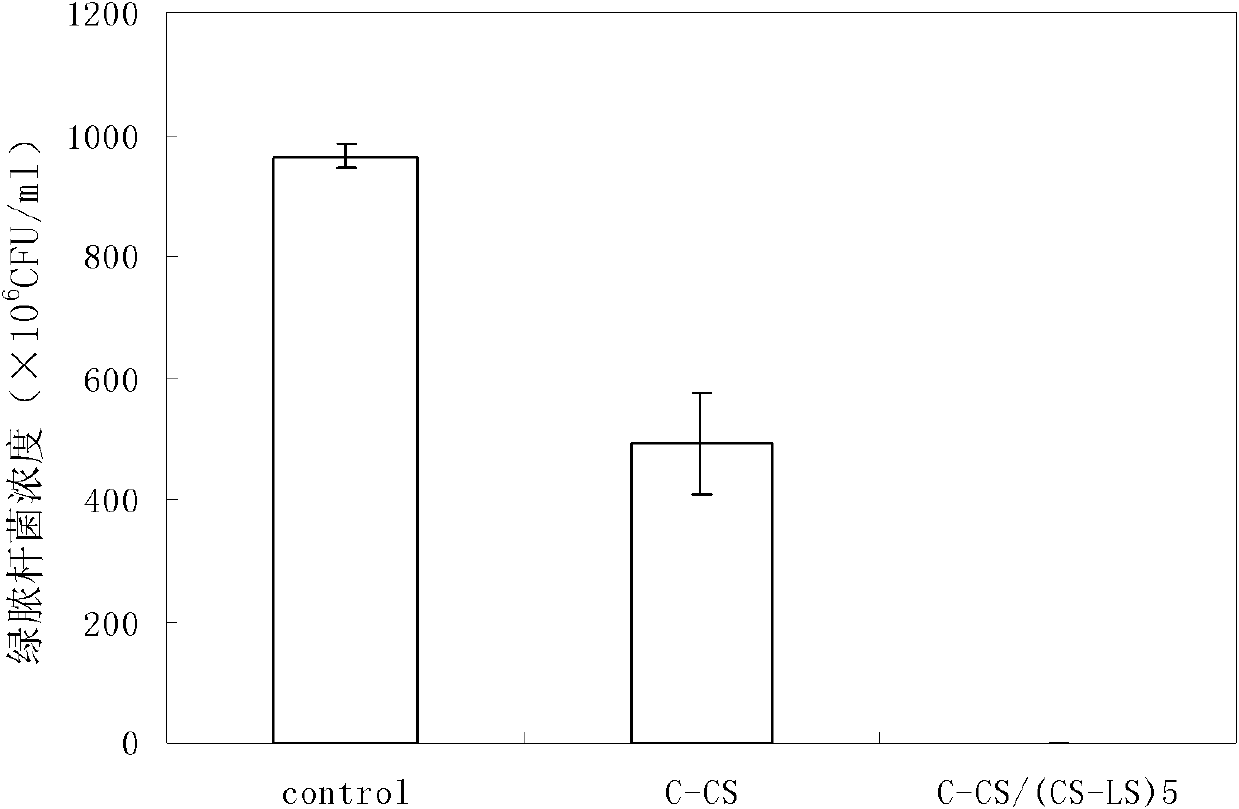
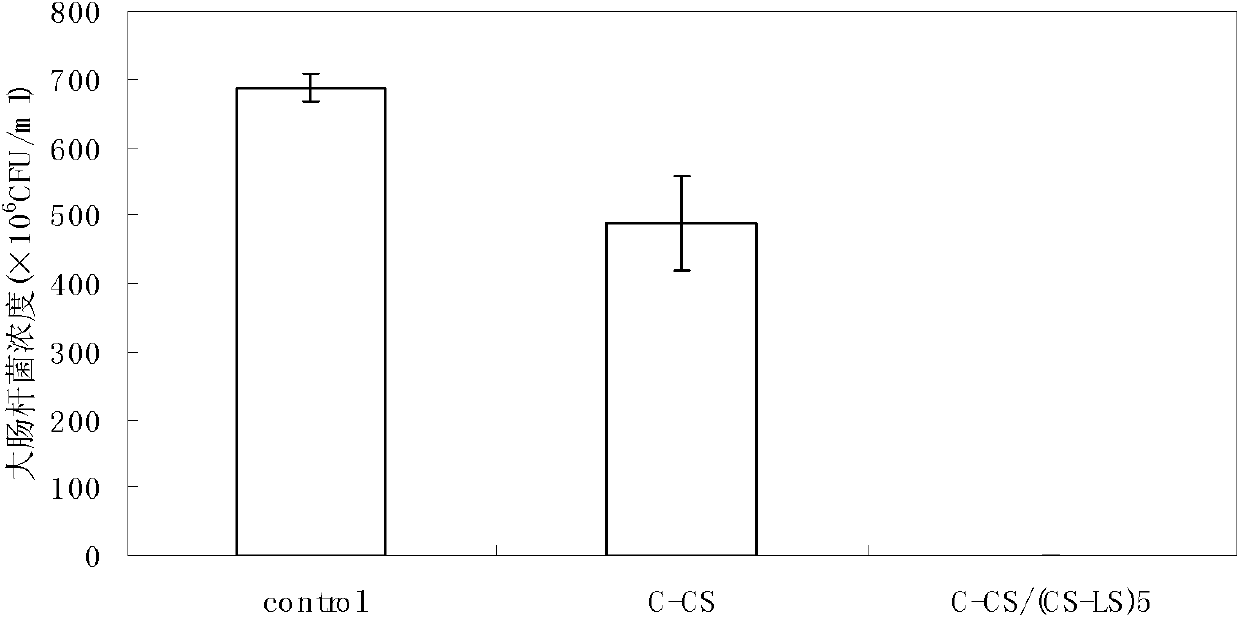
The invention relates to a method for preparing a biological polysaccharide self-assembly modificatory chitosan antibacterial biological material, which is characterized by comprising the following steps: (1) preparing a lentinan derivant, i.e. lentinan sulfate; (2) preparing a chitosan membrane base material; (3) preparing a lentinan sulfate solution and a chitosan solution; and (4) carrying outlayer-by-layer self-assembly of the lentinan sulfate and the chitosan on the surface of the base material: respectively carrying out alternative self-assembly on the chitosan membrane base material in the lentinan sulfate solution and the chitosan solution for 5 times to obtain a surface layer-by-layer self-assembly modificatory chitosan mebrane containing 5 bilayers with the outermost layer of the lentinan sulfate, washing and drying to obtain the biological polysaccharide self-assembly modificatory chitosan antibacterial biological material. The chitosan antibacterial biological material prepared by the method has excellent antibacterial activity; and the method has simple preparation process, easy control, mild preparation conditions and low cost.
Owner:WUHAN UNIV OF TECH
Aptamer EpCAM (epithelial cell adhesion molecule) Ccut of EpCAM and preparation method thereof
ActiveCN103387988AMaintain native conformationNo toxicityMicrobiological testing/measurementDNA preparationProtein targetNeural cell adhesion molecule
Owner:XIAMEN UNIV
SARS-CoV-2 coronavirus vaccine and preparation method thereof
ActiveCN112618707AMaintain native conformationImprove biological activitySsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationImmunogenicity
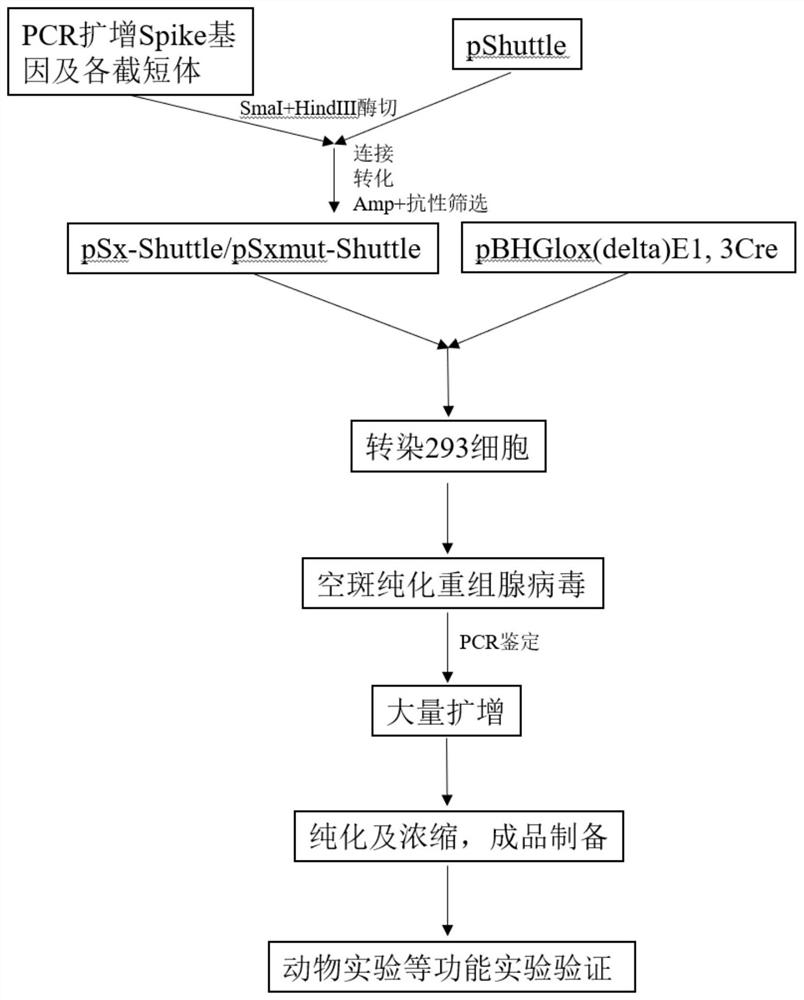
The invention discloses a SARS-CoV-2 coronavirus vaccine and a preparation method thereof. An S gene of a SARS-CoV-2 coronavirus is subjected to codon optimization, truncated body and mutant sequences of the S gene are introduced into a secretory defective adenovirus vector, and packaging is conducted to obtain a corresponding recombinant adenovirus; and the recombinant adenovirus can express SARS-CoV-2 virus related protein in vivo, processing, folding, glycosylation and other modifications are completed, the native conformation of S protein is basically maintained, and the characteristics of high biological activity, long half-life period, lasting immunogenicity and the like are achieved. According to the vaccine and the method, by adopting the defective adenovirus vector carrying secretory peptide, a recombinant adenovirus vaccine can be secreted out of cells after being expressed in vivo, so that the humoral immunity is activated.
Owner:GUANGZHOU DOUBLLE BIOPRODUCT CO LTD
Nucleic acid aptamer of epithelial cell adhesion molecule and preparation method thereof
ActiveCN102827845AMaintain native conformationNo toxicityMicrobiological testing/measurementBiological testingProtein targetSingle strand dna
The invention relates to a nucleic acid, in particular to a nucleic acid aptamer of the epithelial cell adhesion molecule and a preparation method thereof. The invention provides a high-specificity and high-affinity nucleic acid aptamer of the epithelial cell adhesion molecule EpCAM and a preparation method and applications thereof. The nucleic acid aptamer of the epithelial cell adhesion molecule EpCAM is of the G-quadruplex structure or the stem-loop structure. The preparation method comprises the following steps: designing and synthetizing a single-stranded DNA (deoxyribonucleic acid) random oligonucleotide library, screening the target oligonucleotide sequence, and identifying the binding specificity and affinity of the sequence to the target protein through the flow cytometry. The screened nucleic acid aptamer is non-toxic, has small molecular weight and good permeability, is easy in synthesis and marking and can only perform specific identification to the EpCAM protein and perform specific identification and specific expression to the cell line of the EpCAM protein; and the nucleic acid aptamer can not identify the cell line which does not express the protein.
Owner:苏州德运康瑞生物科技有限公司
Liquid phase chip used for detecting bone metabolism biochemical marker and its preparing method
InactiveCN101221171AImprove detection efficiencySmall sample sizeMaterial analysisMicrospherePhycoerythrin
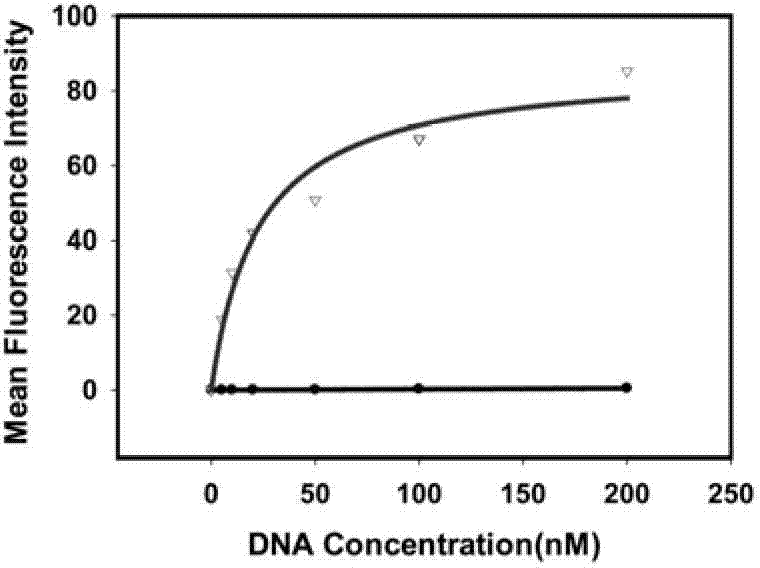
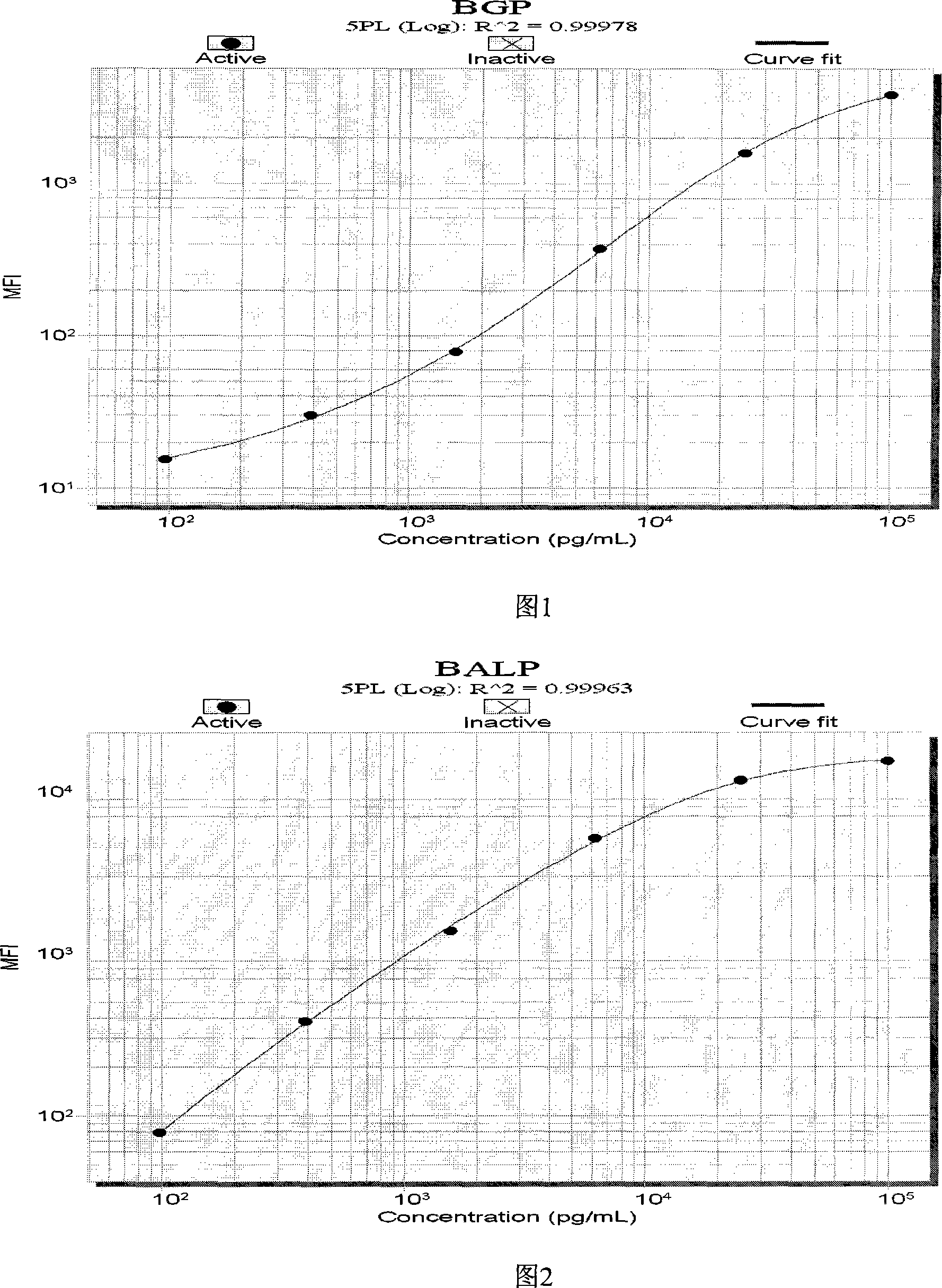
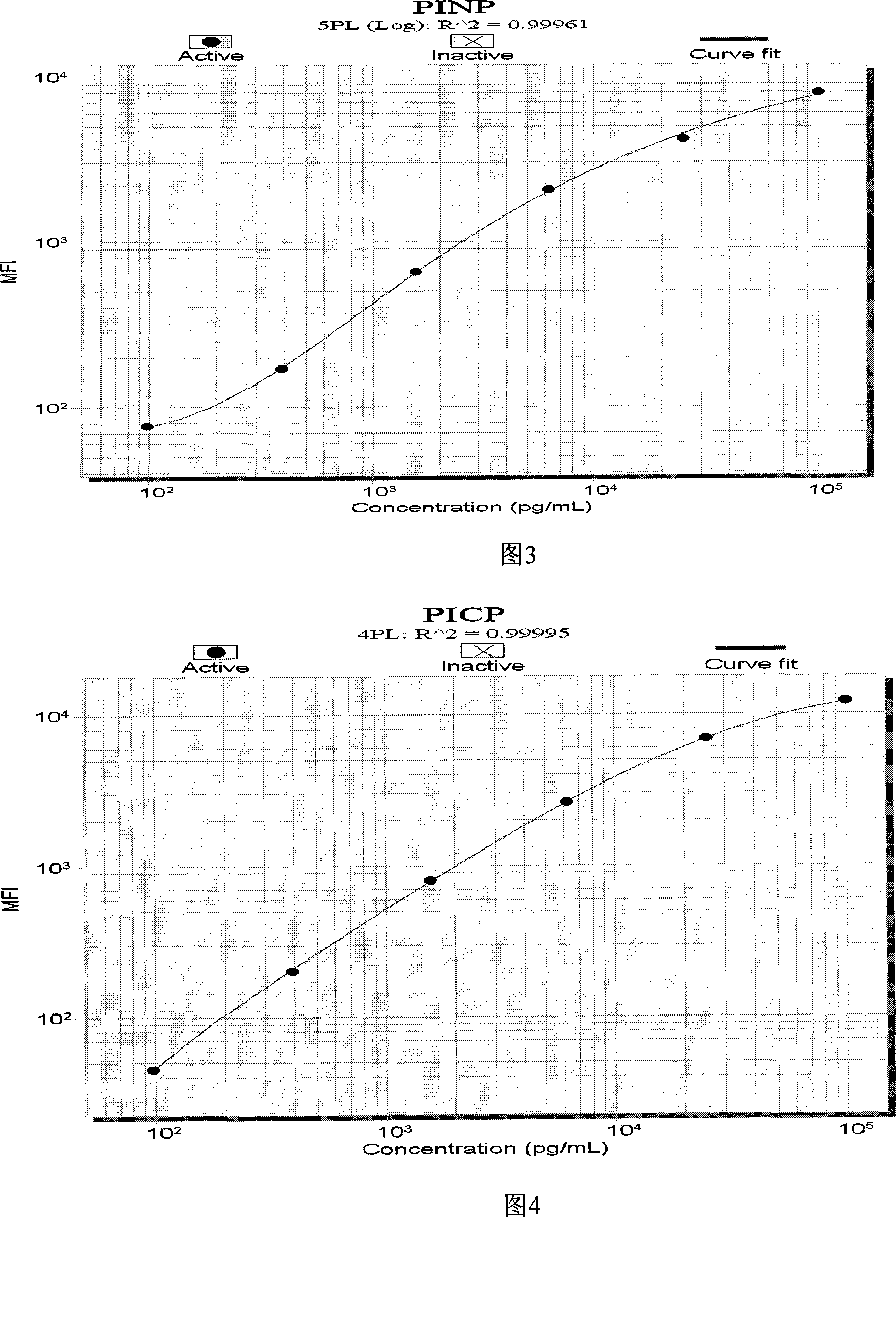
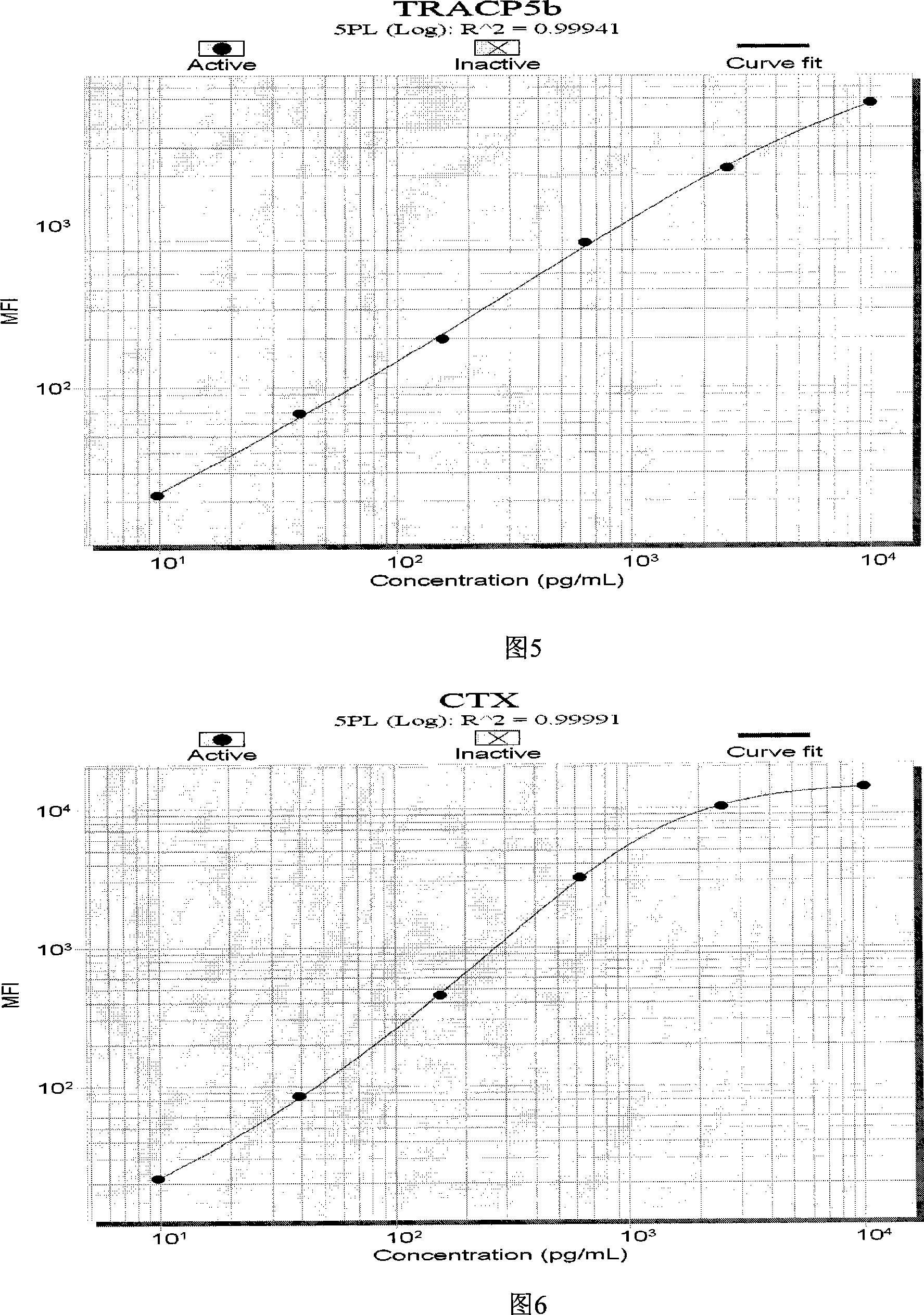
The invention discloses a liquid phase chip kit which is used for the parallel detection of a plurality of bone metabolism biochemical markers, and the invention mainly includes: coated microspheres, detection antibodies which are respectively labelled by biotin and streptavidin phycoerythrin. The liquid phase chip kit which is used for the parallel detection of a plurality of bone metabolism biochemical markers and is provided by the invention has the advantages of high detection efficiency, fewer required samples, strong specificity, high sensitivity, and so on. At the same time, all the bone metabolism biochemical markers can be combined freely, and the usage is convenient. At the same time, as all the reactions are positioned in the liquid phase environment, the invention is more conductive to the maintenance of the native conformation of protein, the reaction of a probe and the object to be detected is faster and more complete, while the detection sensitivity and the linear range are both greatly improved.
Owner:SUREXAM BIO TECH
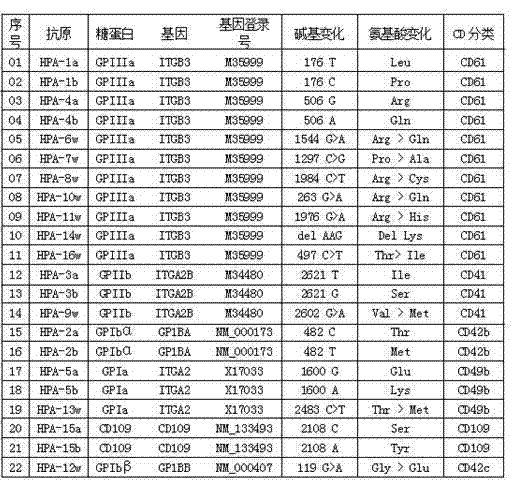
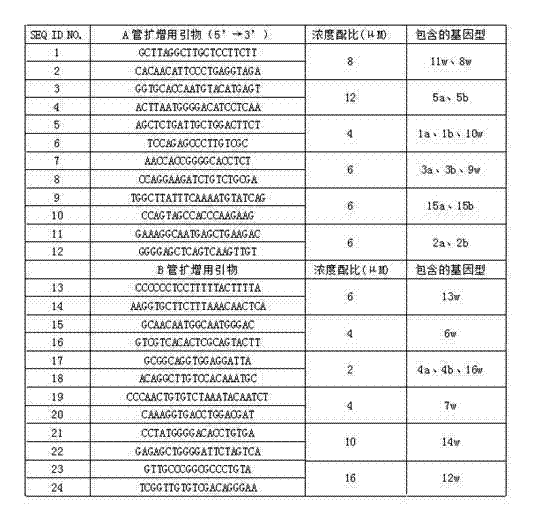
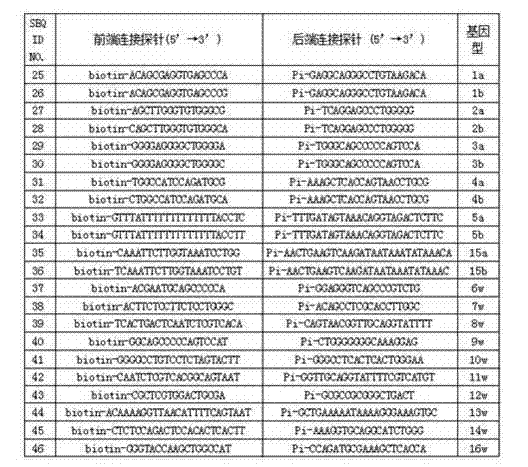
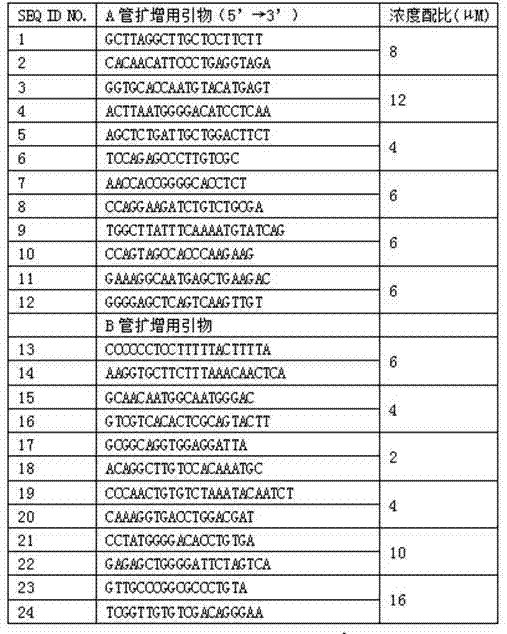
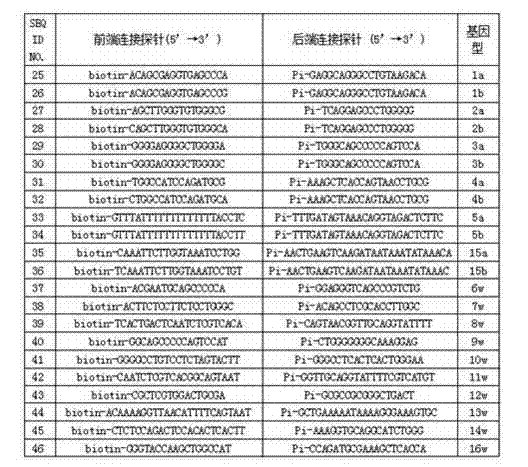
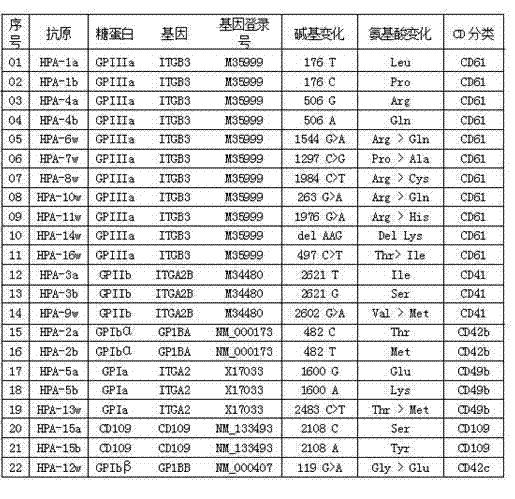
Human platelet antigen genotyping liquid chip and human platelet antigen genotyping detection method thereof
InactiveCN102230017AImprove throughputIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHigh fluxBiochemistry
The invention belongs to the technical field of molecular biology and particularly relates to a human platelet antigen genotyping liquid chip and a human platelet antigen genotyping detection method. A human platelet antigen genotyping technique is an important safe and efficient technical means of providing matching platelets for patients and promoting clinic platelet injection, but the conventional human platelet antigen genotyping technique has the problems of low flux, low accuracy, low sensitivity and other low indexes and the like. The liquid chip provided by the invention contains primers numbered from 1 to 24, connecting probes numbered from 25 to 46 and fluorescent coded microspheres coated with detection probes numbered from 47 to 68 and can accomplish human platelet antigen genotyping detection by amplification, chain connection and crossing reaction. The liquid chip and the detection method, which are adopted by the invention, have the advantages of high flux, high sensitivity, high specificity, high repeatability, wide linear range, simple operation, high flexibility and wide application range, and the method is an accurate, efficient and practical human platelet antigen genotyping detection method for use in clinic.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
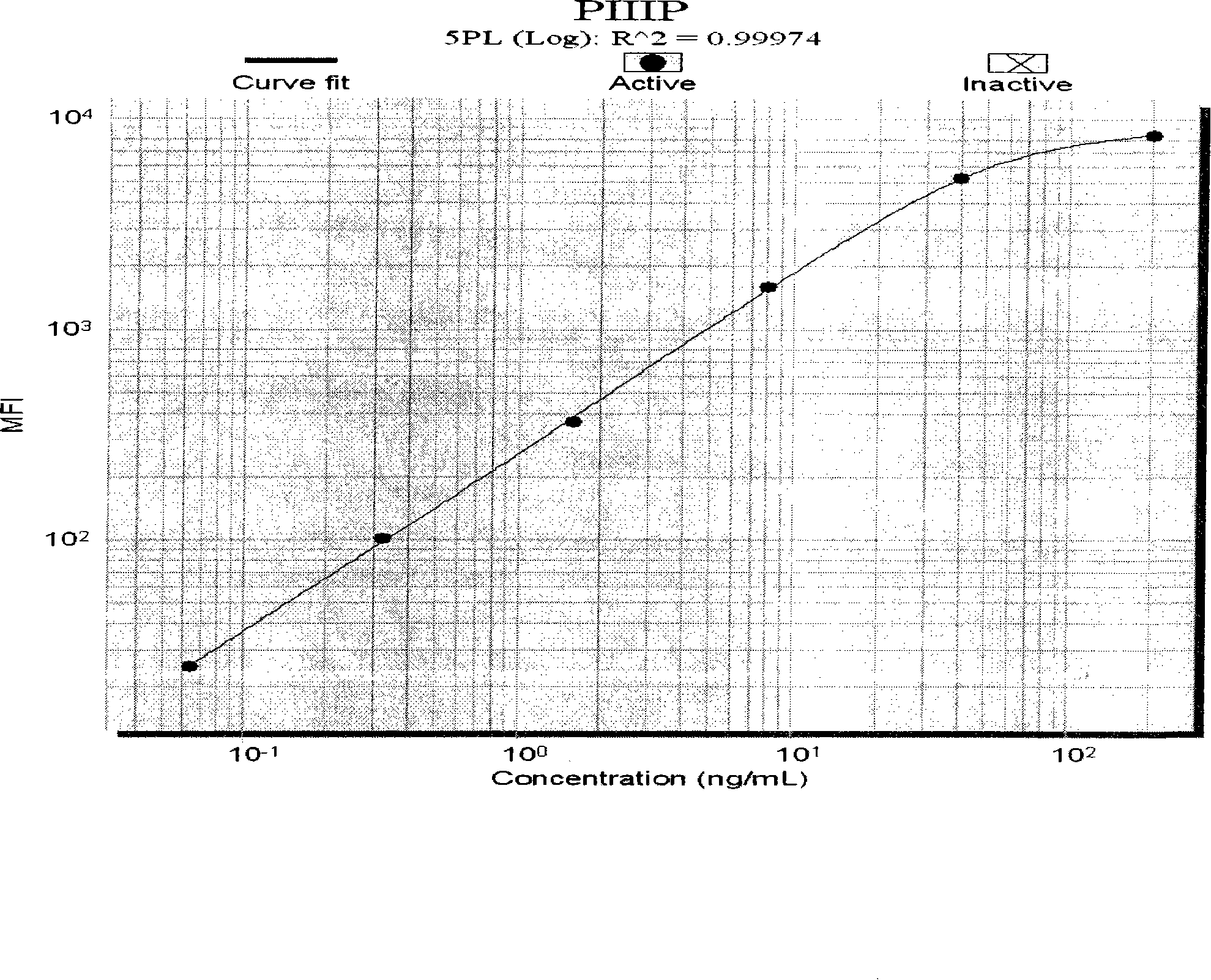
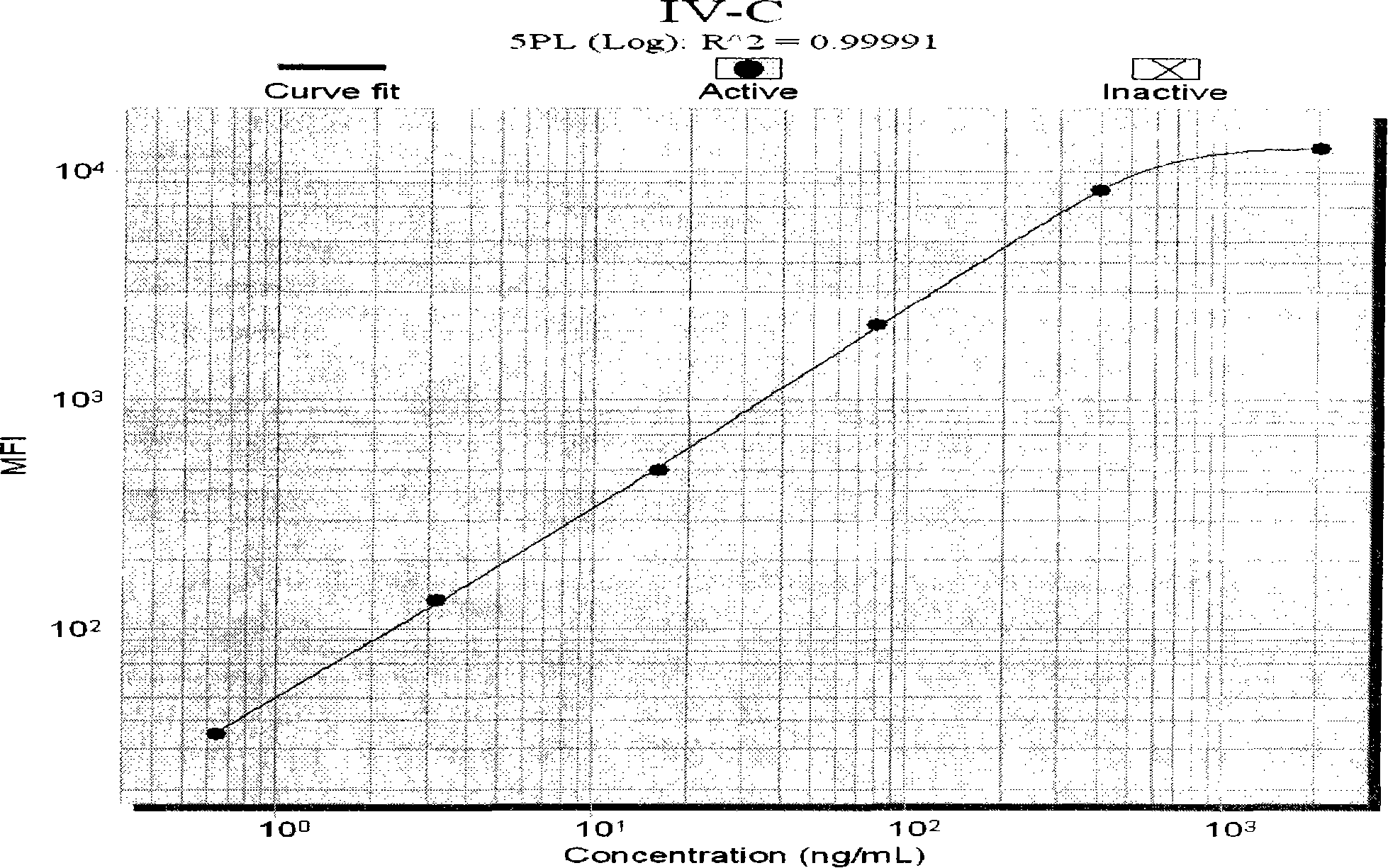
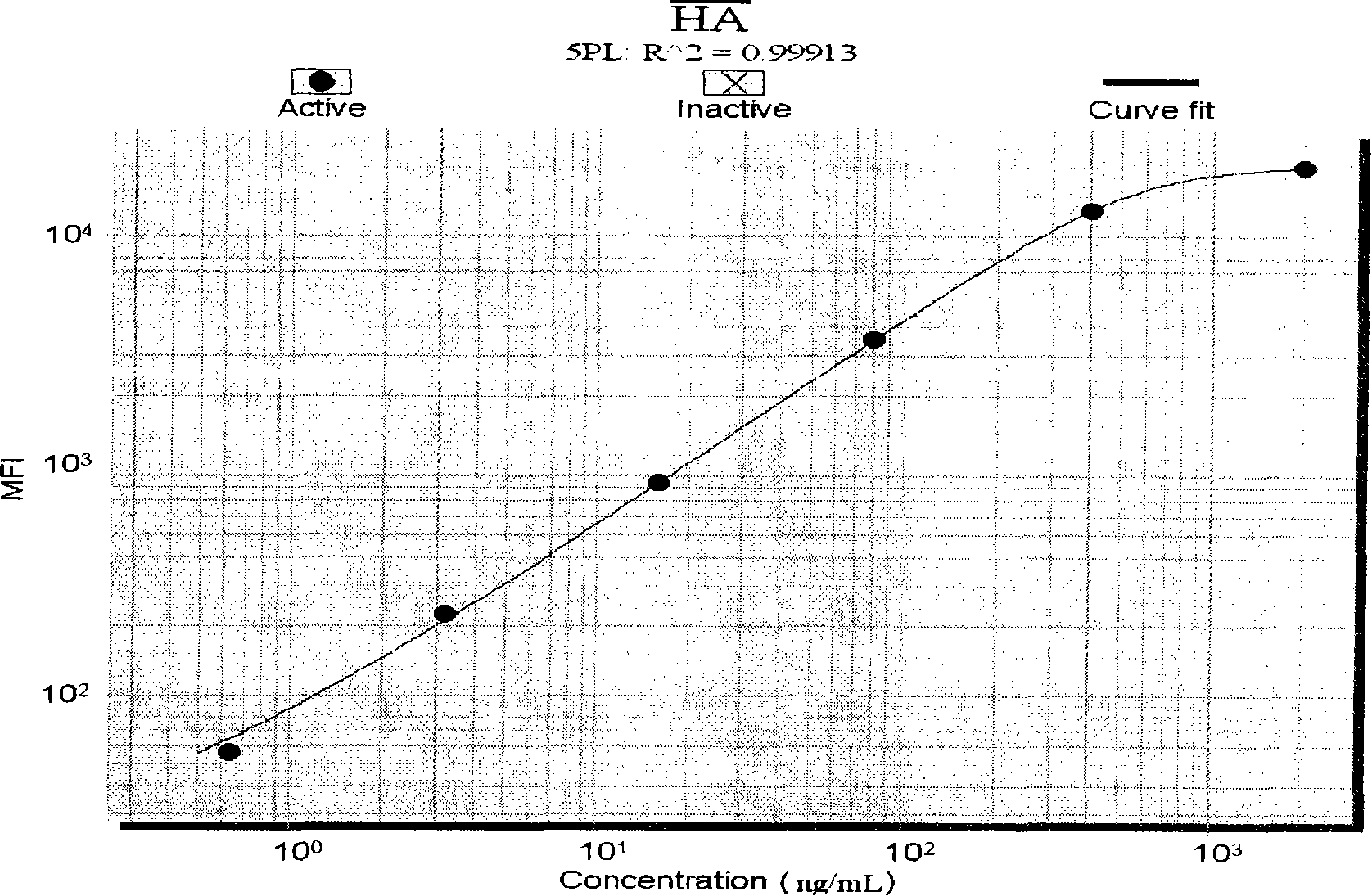
Liquid phase chip used for detecting liver fibrosis and method of producing the same
The invention discloses a liquid phase chip for detecting liver fibrosis, which mainly includes: (1) 4-plex coated microspheres: containing respectively coated type III procollagen (PIIIP) capture antibody, type IV collagen and its fragment (IV-C) capture antibody, laminin (LN) capture antibody and hyaluronidase (HA) capture antibody with different color-coded microspheres; (2) 4-plex biotin-labeled detection antibody: respectively Biotin-labeled detection antibodies of PIIIP, IV-C, LN, HA; and (3) streptavidin phycoerythrin. The invention also discloses a preparation method of the liquid phase chip. The liquid phase chip for liver fibrosis detection of the present invention has the advantages of no side effects, high throughput, multi-indicator parallel detection, high sensitivity, good repeatability, rapid and accurate detection, low cost and the like.
Owner:SUREXAM BIO TECH
Method for preparing fructose lysine enzyme and application of preparing fructose lysine enzyme
ActiveCN102559643AHigh specific vitalityEasy extractionHydrolasesMicrobiological testing/measurementFructoseFractional Precipitation
The invention provides a method for preparing fructose lysine enzyme and a glycated albumin detection kit, wherein the glycated albumin detection kit is prepared by utilizing the fructose lysine enzyme that is prepared by adopting the method. The method comprises the operation steps as follows: strains are screened so as to obtain 11 to 82 aspergillus strains of the high-activity fructose lysine enzyme; the 11 to 82 aspergillus strains are cultivated, and mycelia are collected; the mycelia are suspended in buffer solution, and then are processed through cell disruption and centrifugation, and supernatant fluid is collected; the supernatant fluid is processed through fractional precipitation by adopting ammonium sulfate solution, and deposits are collected so as to obtain crude extracts; the crude extracts are processed through hydrophobic chromatography, and eluant is collected; in addition, the collected eluant is processed through affinity chromatography, and then eluant is collected, that is, fructose lysine enzyme solution is obtained. The method has the advantages as follows: the preparation steps are simple, and the obtained fructose lysine enzyme achieves high purity, high activity and high reaction specificity.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Aptamer EpCAM (epithelial cell adhesion molecule) B of EpCAM and preparation method thereof
ActiveCN103387990AMaintain native conformationNo toxicityMicrobiological testing/measurementDNA preparationAptamerProtein target
The invention provides an aptamer EpCAM (epithelial cell adhesion molecule) B of an EpCAM as well as a preparation method and application thereof, relating to nucleic acids. The aptamer has high specificity and affinity. The aptamer has a G tetramer structure or a stem-loop structure. The preparation method comprises the steps of designing and synthesizing a single-stranded DNA (deoxyribonucleic acid) random oligonucleotide bank, screening a target oligonucleotide sequence, and identifying the target protein binding specificity and affinity of the target oligonucleotide sequence by a flow analysis method. The screened aptamer does not have toxicity, has small molecular weight and good permeability, is easy to synthesize and label, only specifically identifies EpCAM proteins and cell lines expressing the EpCAM proteins, and does not have a function of identifying the cell lines which do not express the proteins.
Owner:XIAMEN UNIV
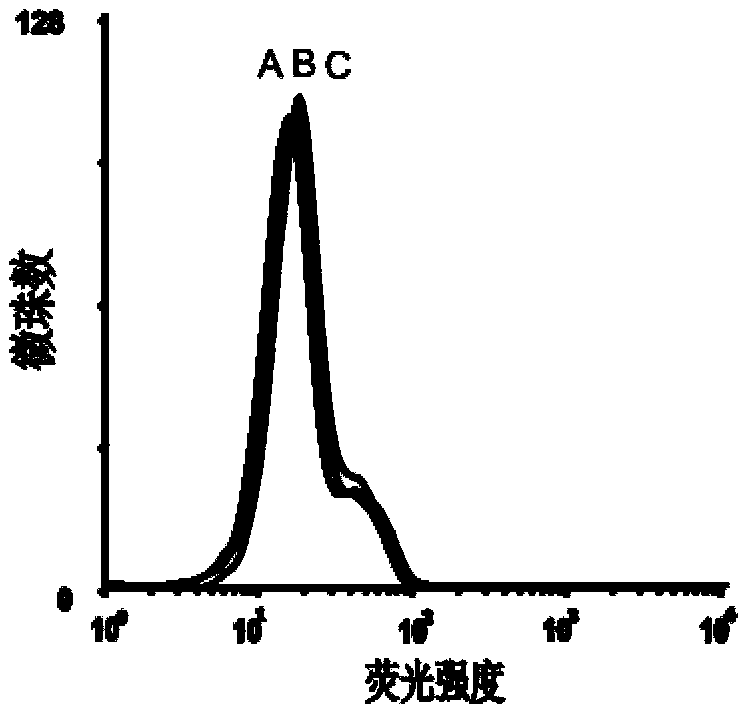
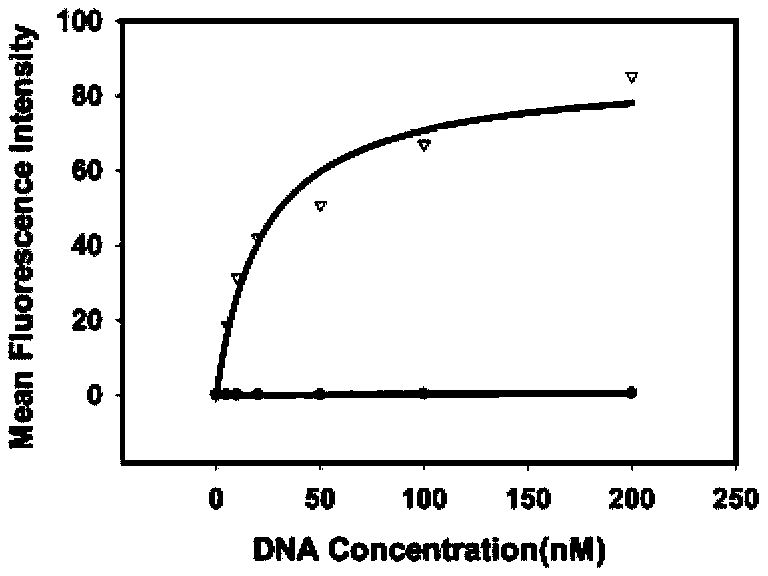
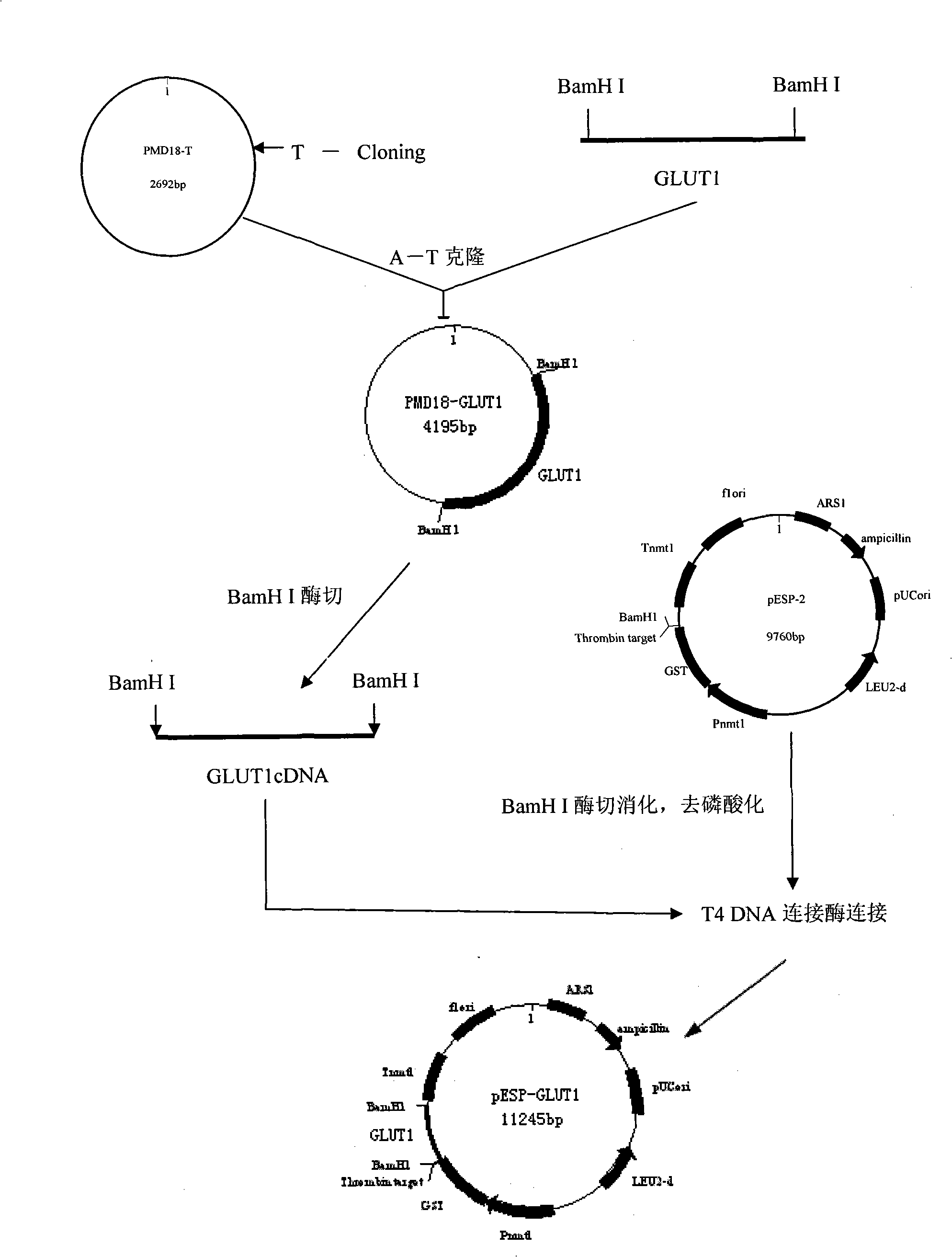
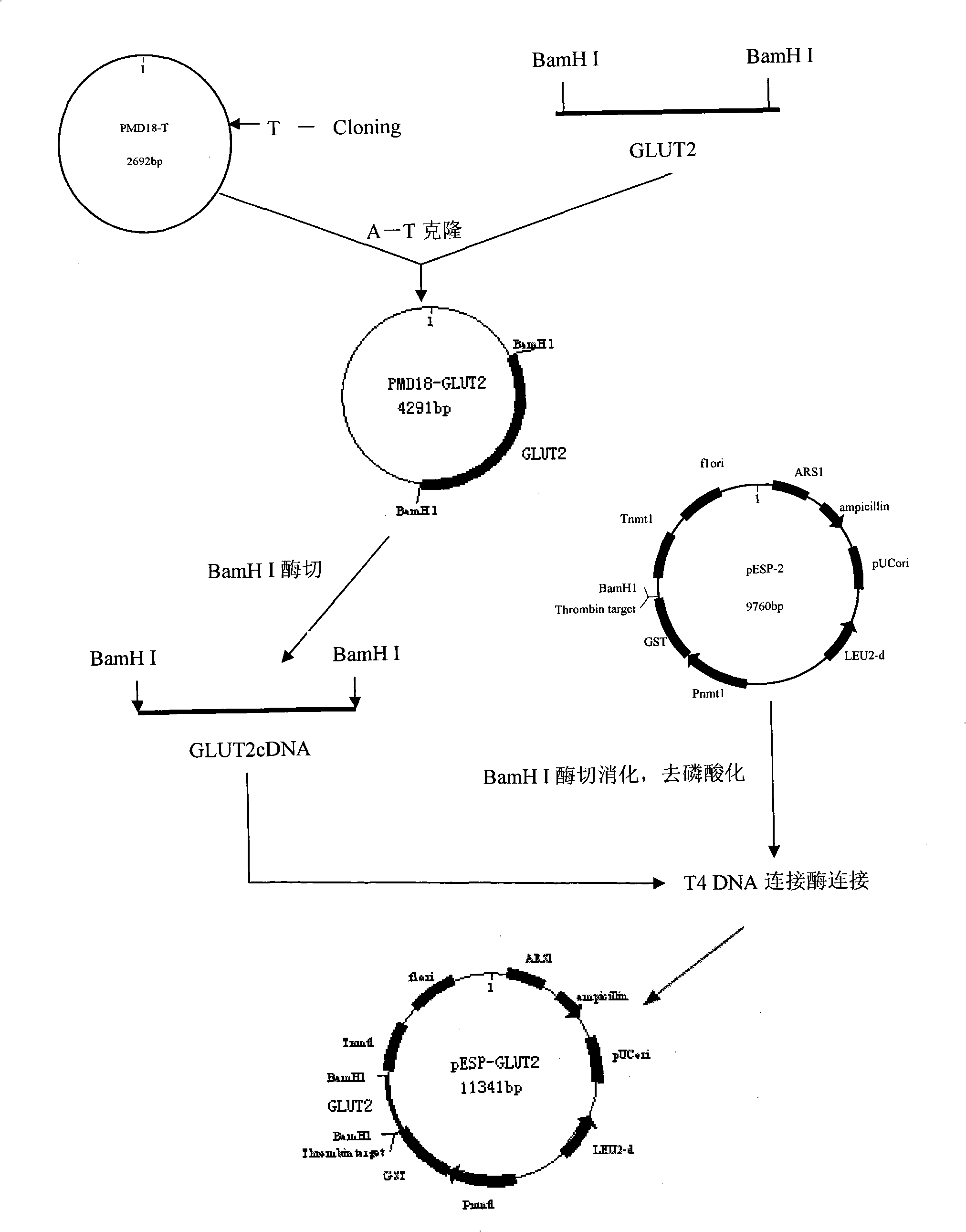
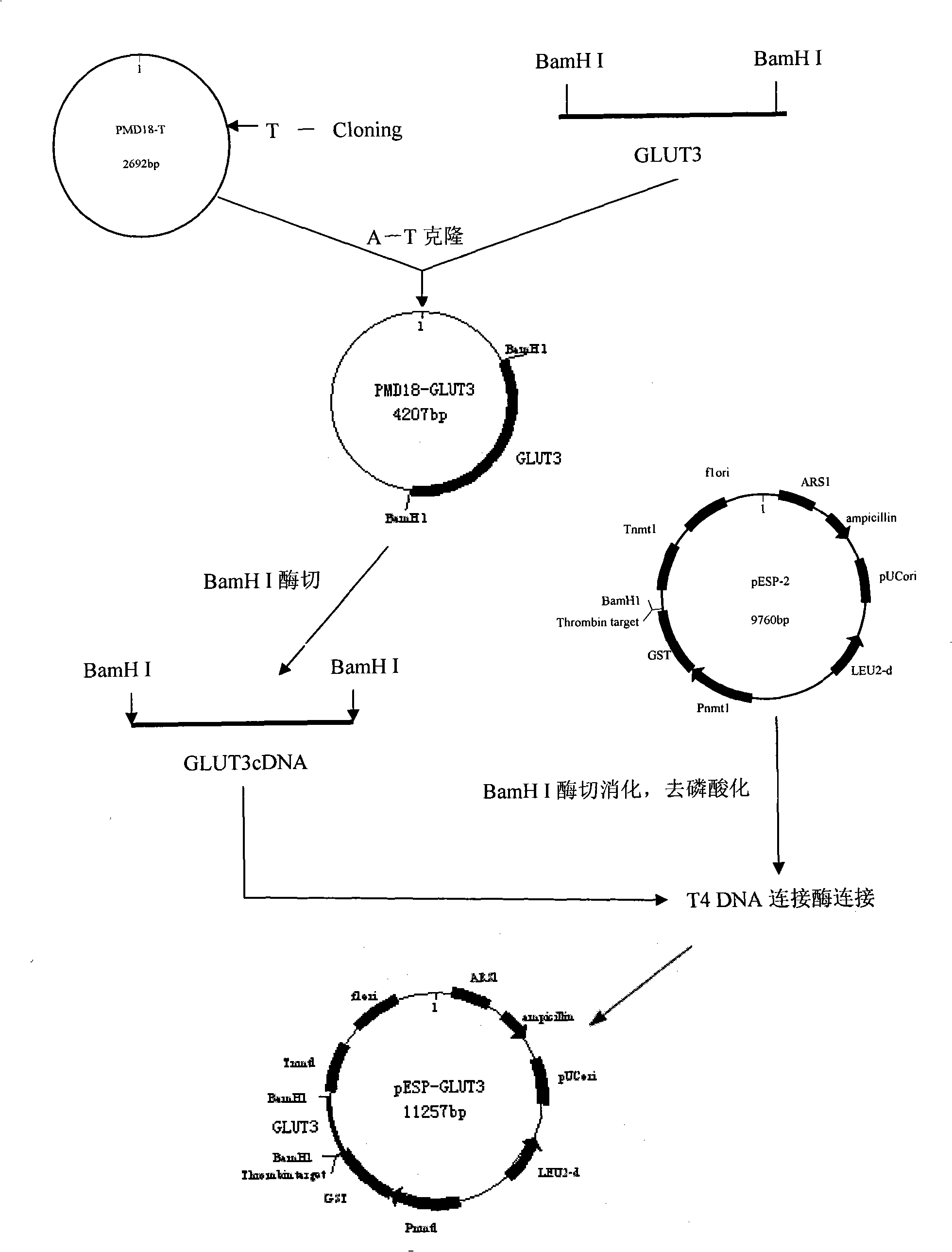
Process for heterologously expressing and purifying human glucose transporters GLUT1, GLUT2 and GLUT3
InactiveCN101285066AMaintain native conformationGuaranteed functionPeptide preparation methodsFermentationSAA proteinHeterologous
The invention discloses a method for heterologous expression and purification of members of glucose transporter family including CLUT1, CLUT2, and CLUT3 on human cell membrane, which comprises the following steps of: constructing an inducible fusion protein expression vectors of GST-CLUT1, GST-GLUT2, and GST-GLUT3 and transforming fission yeast; identifying transformants of the fission yeast; induced expression of the three fusion proteins of GST-CLUT1, GST-GLUT2, and GST-GLUT3 in the fission yeast; identifying the expression of the genes of GLUT1, GLUT2 and GLUT3 in the fission yeast; separating and purifying the three fusion protein; detecting and identifying the three separated and purified fusion proteins; separating and purifying human proteins of GLUT1, GLUT2 and GLUT3 from the three fusion proteins respectively.
Owner:CHONGQING UNIV
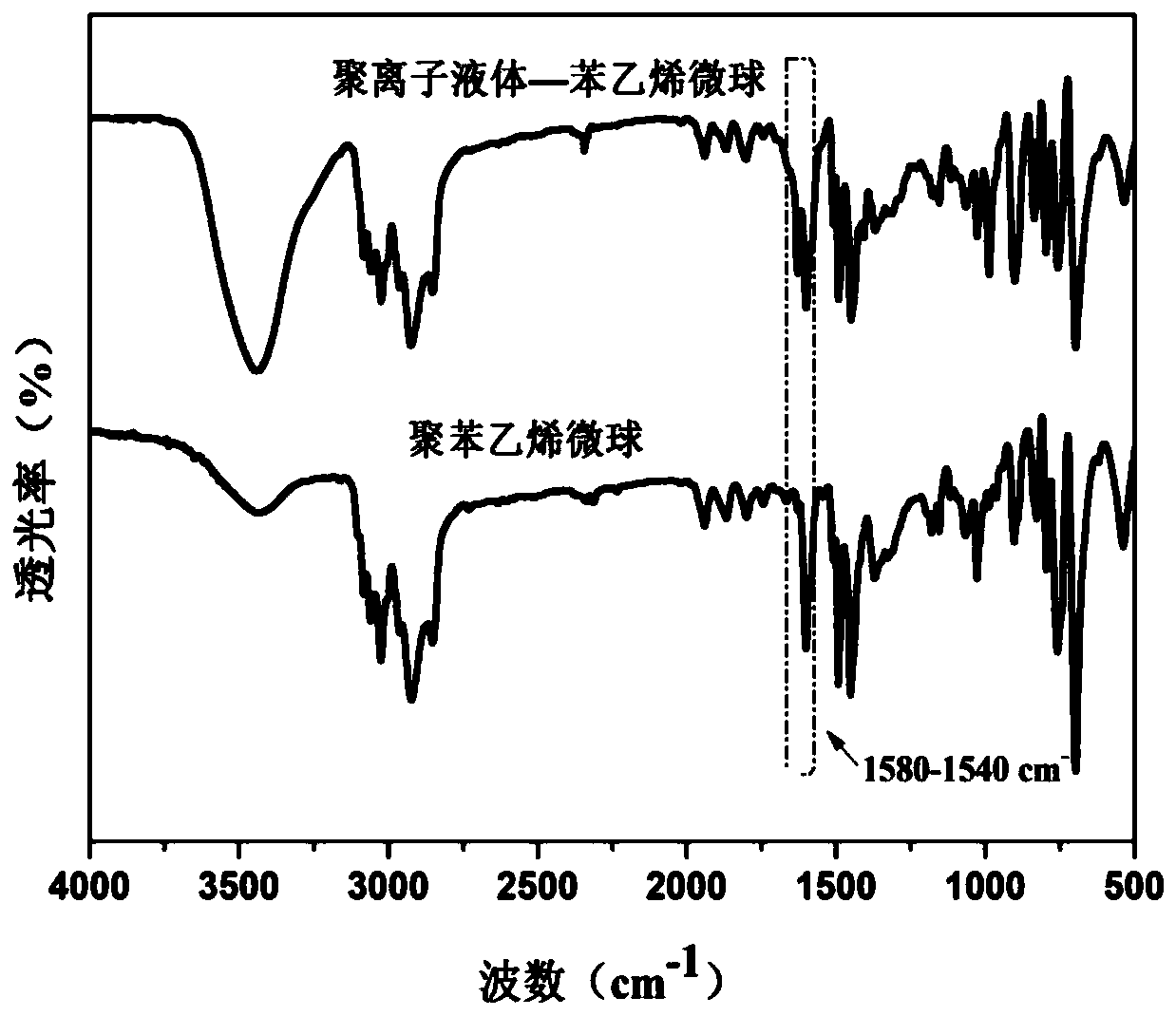
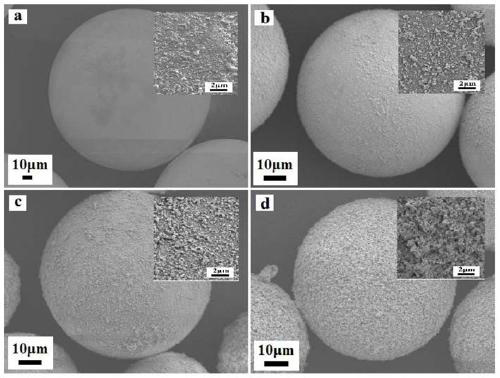
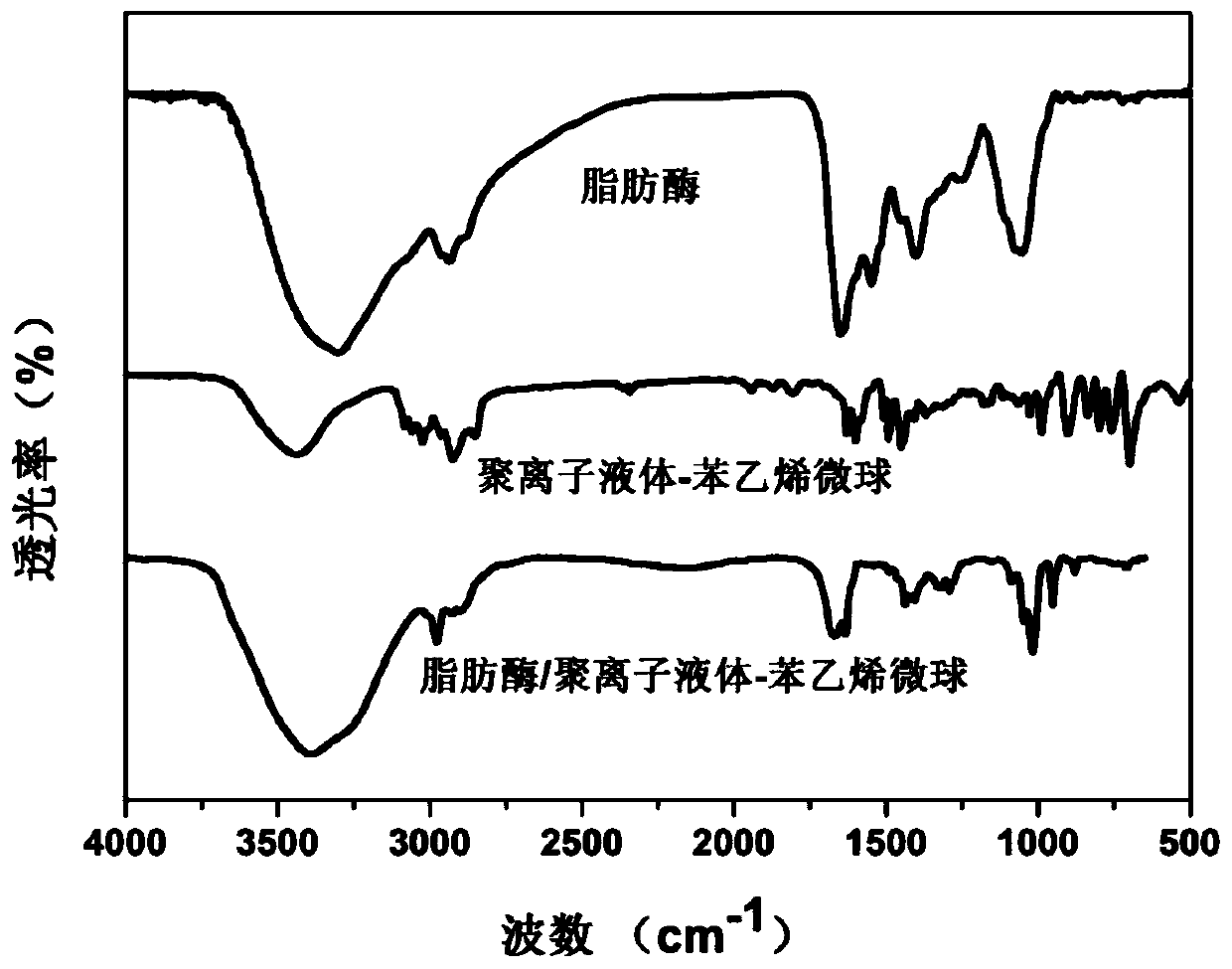
Lipase/polyion liquid-styrene microsphere/hydrogel catalytic material as well as preparation method and application thereof
ActiveCN111285951APrevent falling offEasy to separateHydrolasesOn/in organic carrierMicrosphereBiological organism
The invention relates to a lipase / polyion liquid-styrene microsphere / hydrogel catalytic material as well as a preparation method and application thereof, and belongs to the field of enzyme immobilization. The preparation method comprises the steps: performing polymerization reaction on styrene and imidazolium bromide to form polyion liquid-styrene microspheres; dispersing the polyion liquid-styrene microspheres into a lipase solution, immobilizing lipase onto the polyion liquid-styrene microspheres through physical adsorption to obtain lipase / polyion liquid-styrene microspheres, and carrying out secondary immobilization on the lipase / polyion liquid-styrene microspheres in hydrogel. The obtained catalytic material is environment-friendly and non-toxic, is easy to separate from a reaction system, and can be recycled, so that the catalytic material has a wide application value in various fields such as biocatalysis and food industry.
Owner:DALIAN POLYTECHNIC UNIVERSITY
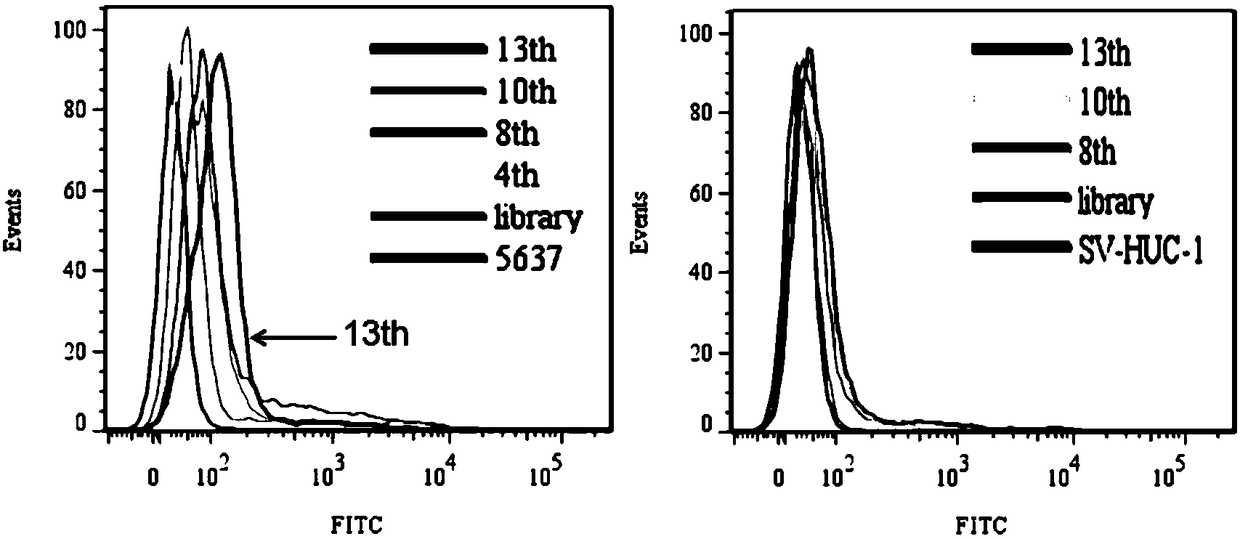
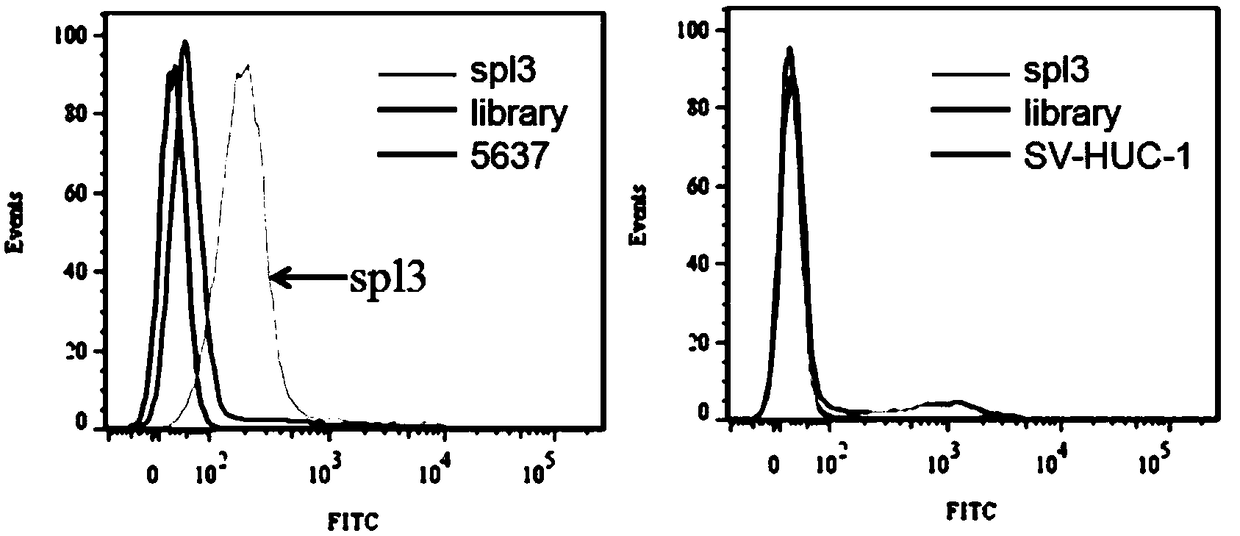
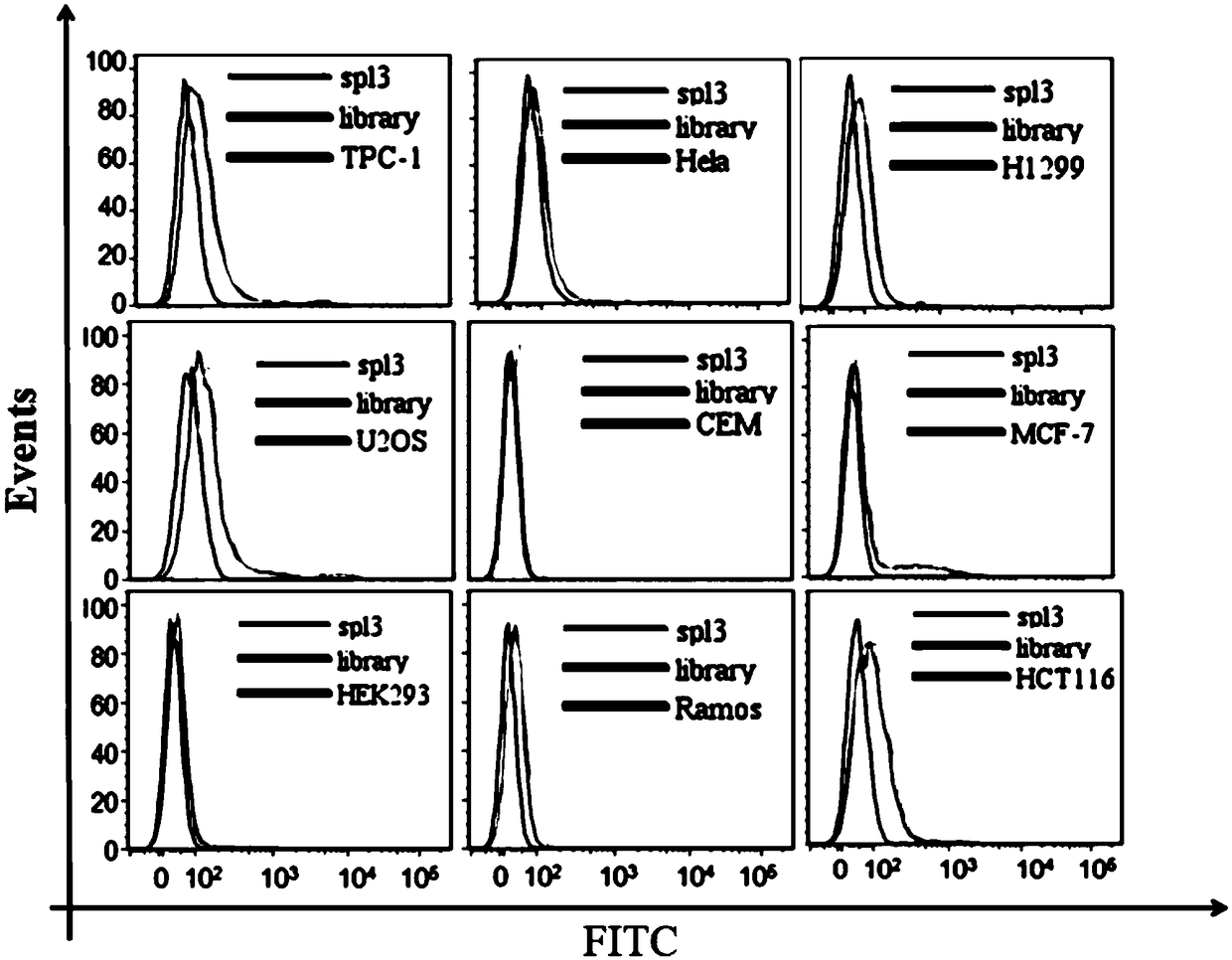
Nucleic acid aptamers for detecting bladder cancer and application thereof
ActiveCN109266654AEnsure specific identificationMaintain native conformationMaterial analysisDNA/RNA fragmentationBladder cancerAptamer
The invention discloses a nucleic acid aptamer for detecting bladder cancer and an application thereof. The nucleic acid aptamer for detecting bladder cancer has a unique stem ring structure under theconditions of 25 DEG C, 1.0mM Na+ and 0.5mM Mg2+, and has high recognition specificity and strong binding ability. Compared with the prior art, the present invention has the advantages that the aptamer screening does not need to define the conformation of the target molecule in advance, and molecular probes capable of specifically recognizing bladder cancer are directly screened from living cells. Nucleic acid aptamer has no immunogenicity. It can be chemically synthesized in vitro and can be modified and substituted in different parts. The sequence is stable and easy to be preserved.
Owner:HUNAN UNIV
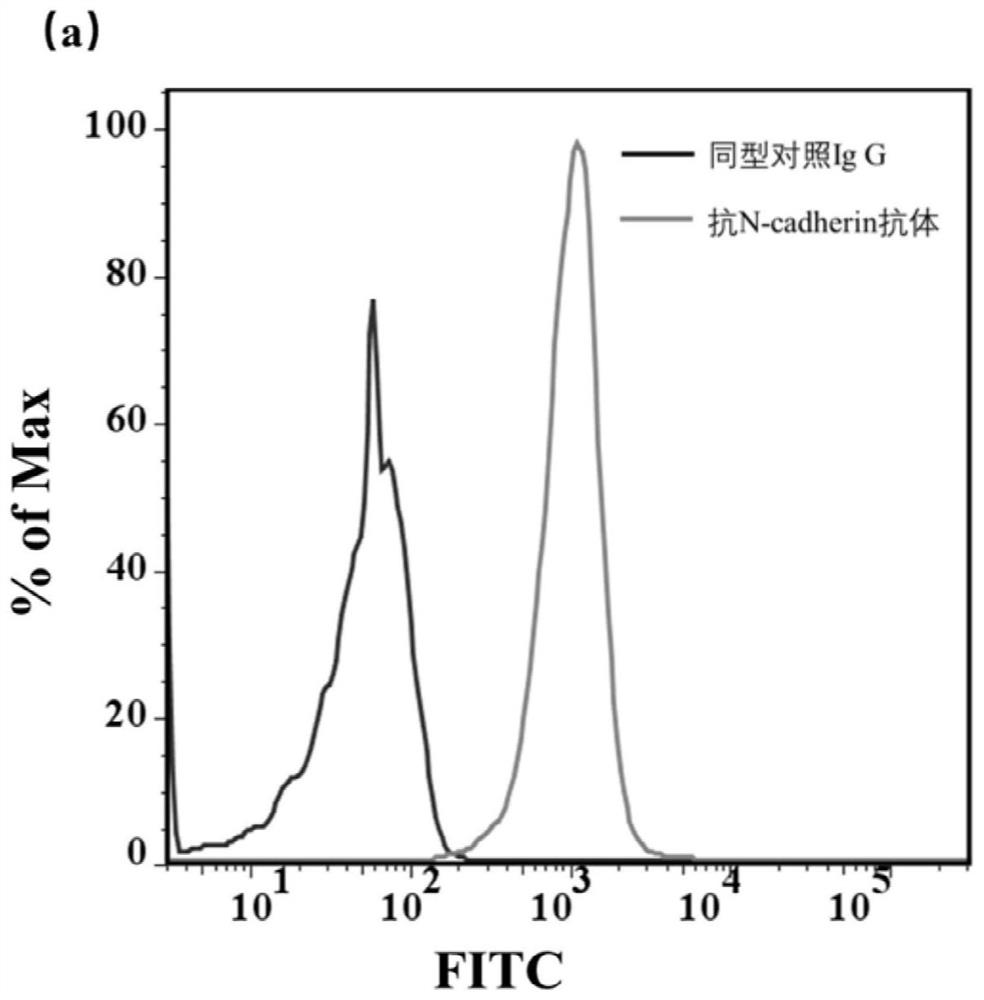
Screening and application of N-cadherin nucleic acid aptamer based on engineered cells
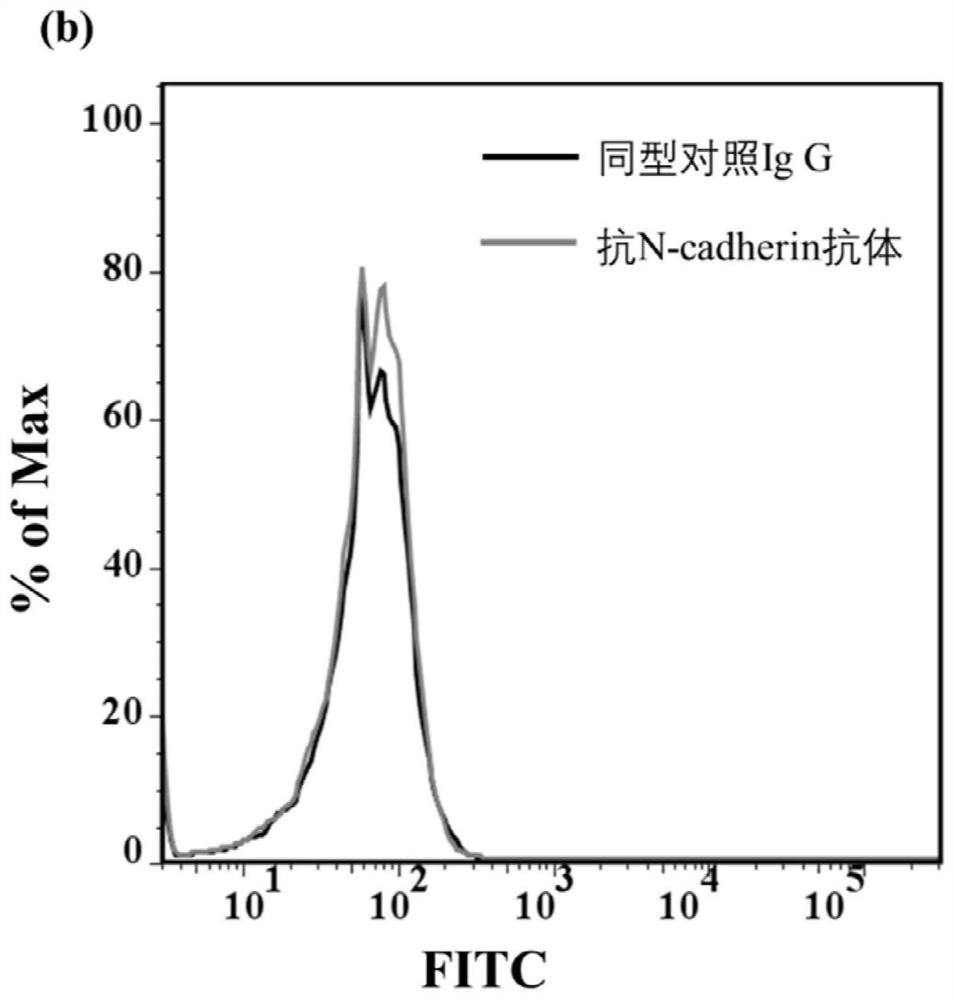
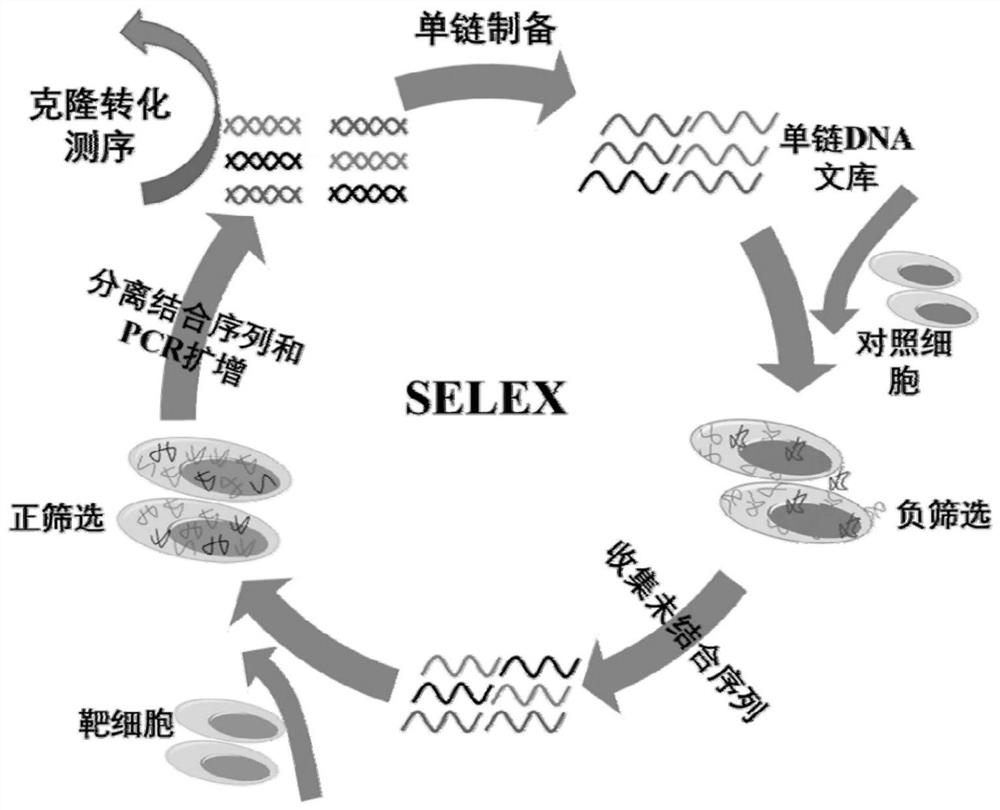
ActiveCN112126649AAchieve the purpose of captureMaintain native conformationCell dissociation methodsTumor/cancer cellsAptamerNucleotide
The invention discloses an N-cadherin nucleic acid aptamer. The nucleic acid aptamer has a nucleotide sequence as shown in SEQ ID No. 1 or SEQ ID No. 2. Preferably, the nucleotide sequence of the nucleic acid aptamer is as shown in SEQ ID No. 2. The invention also provides a screening method of the N-cadherin nucleic acid aptamer. The N-cadherin aptamer can specifically bind with circulating tumorcells with high expression of N-cadherin, so that the N-cadherin aptamer can be applied to capture of the circulating tumor cells.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI +1
ABC group meningococcal combined vaccine and preparation method thereof
ActiveCN104001166BBest combinationGood prevention effectAntibacterial agentsBacterial antigen ingredientsGenotypeInvasive disease
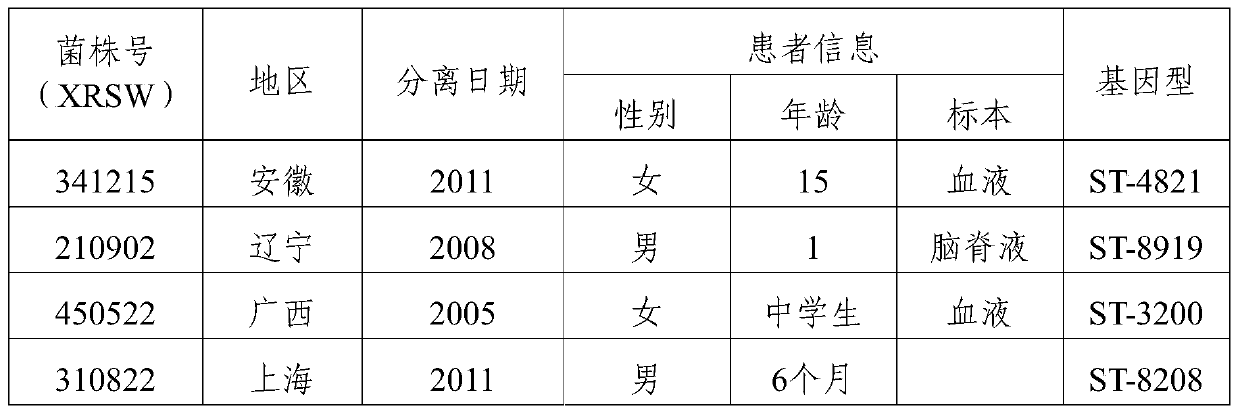
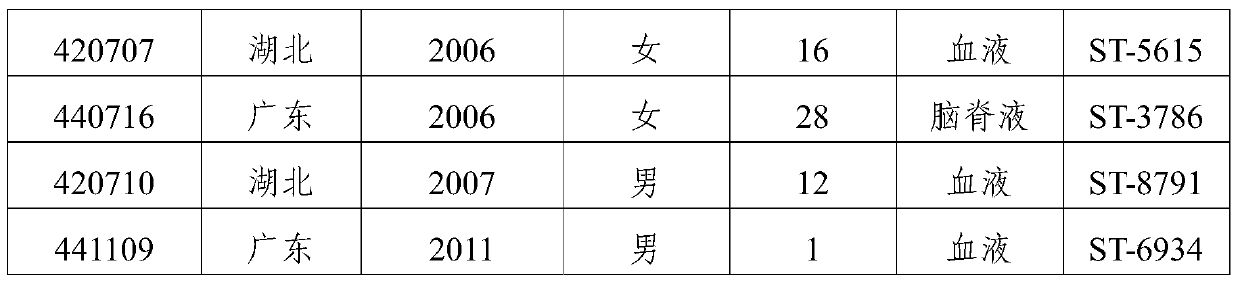
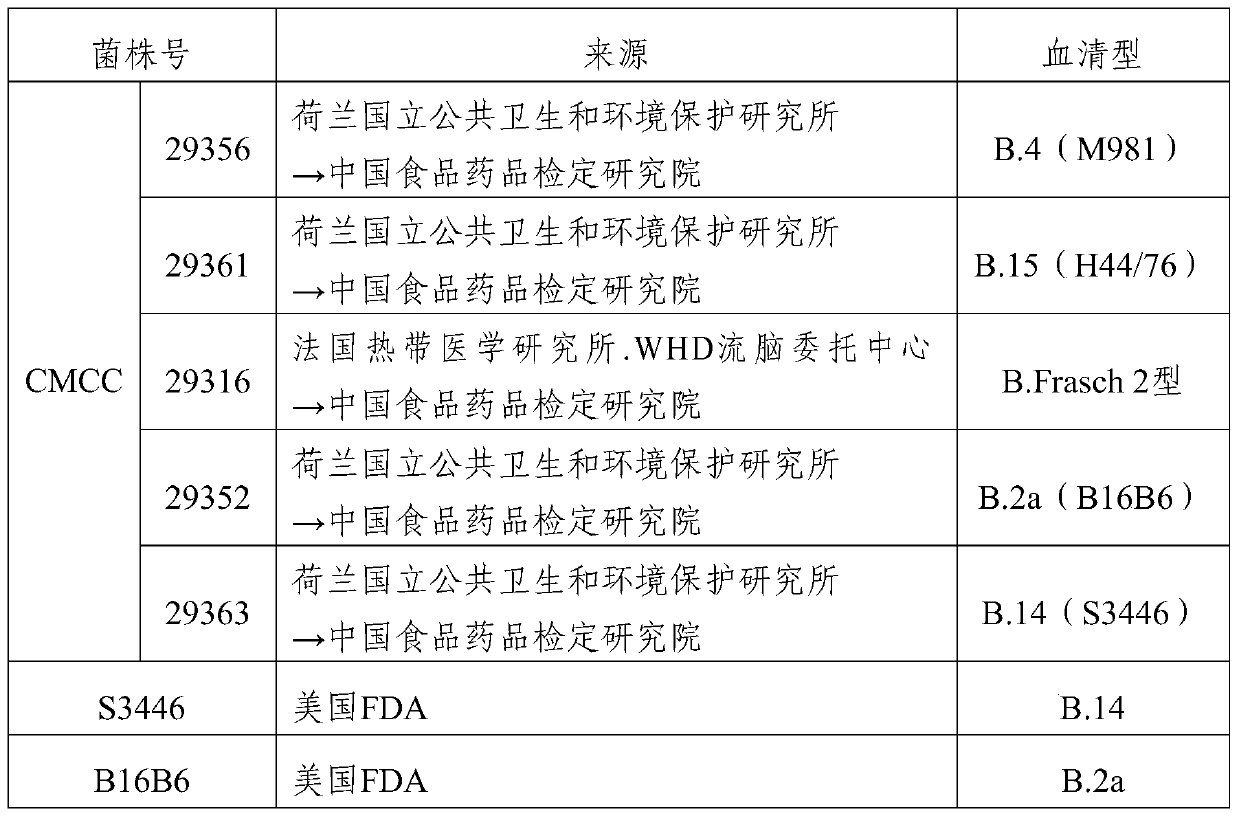
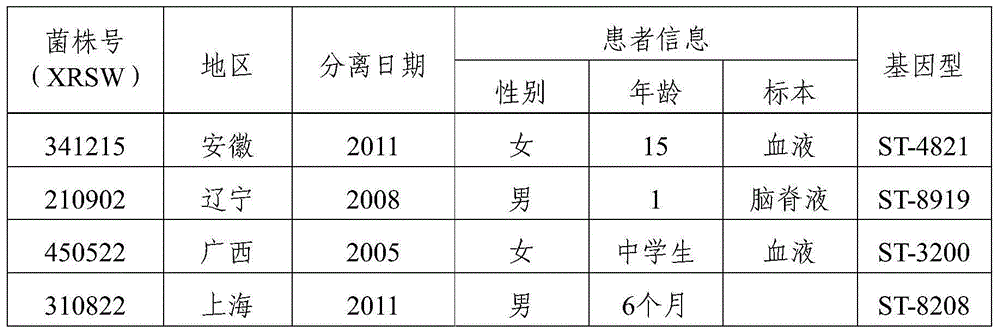
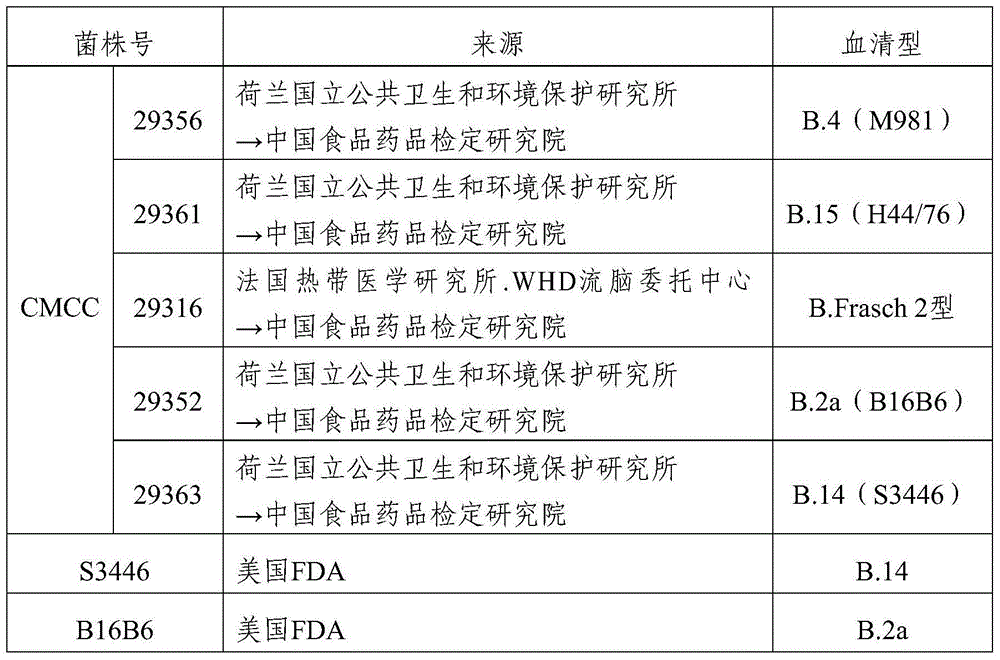
The invention provides a group A, group B and group C meningococcus combined vaccine which is composed of polysaccharide-protein conjugates and a group B ST-4821 genotype (xrsw341215) meningococcus outer membrane protein vesicle (OMV), wherein the polysaccharide-protein conjugates are obtained in a manner that a group A meningococcus capsular polysaccharide and a group C meningococcus capsular polysaccharide are each subjected to covalent binding with a group B serotype 4-type (CMCC29356) meningococcus outer membrane protein vesicle (OMV). The vaccine can simultaneously prevent group A, group B and group C meningococcus invasive diseases, has better protective effects especially on prevention aiming at epidemic cerebrospinal meningitis high-risk groups at the age of less than 2 years, and moreover, has better specificity and better protection effect aiming at nature group A, group B and group C meningococcus invasion.
Owner:CHENGDU OLYMVAX BIOPHARM
Glycoside hydrolase family 7 protein gene and its encoded protein and application
ActiveCN109280673BMaintain native conformationIncrease enzyme activityBacteriaEnzymesGlycoside hydrolase family 7Genetics
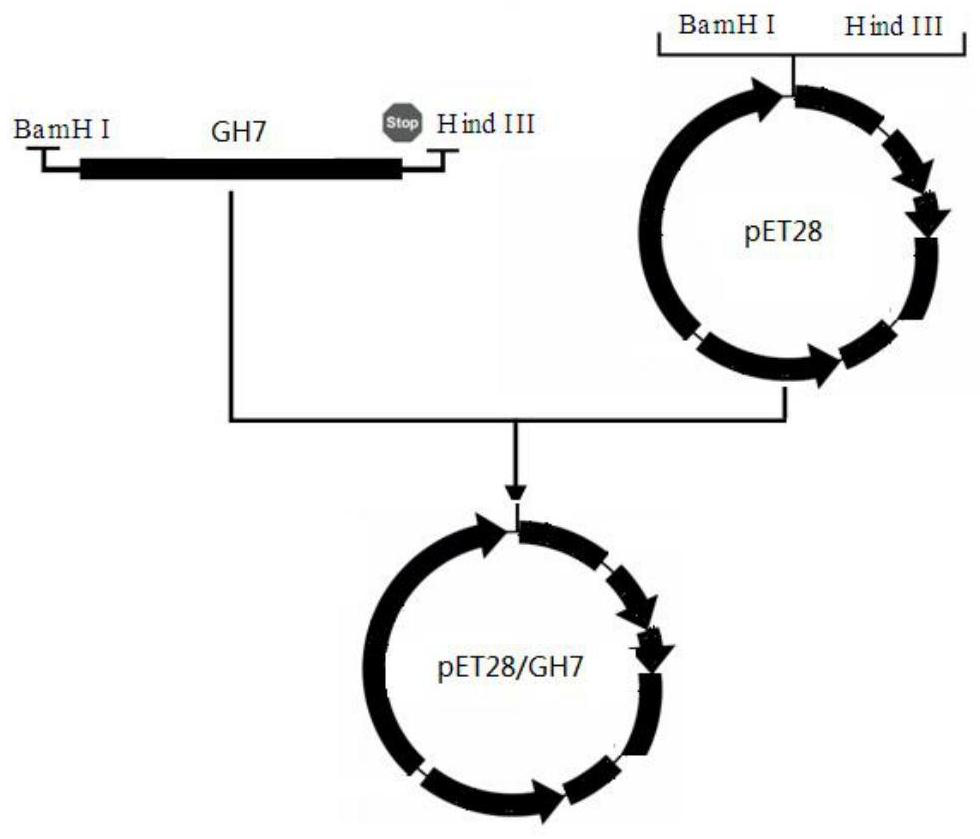
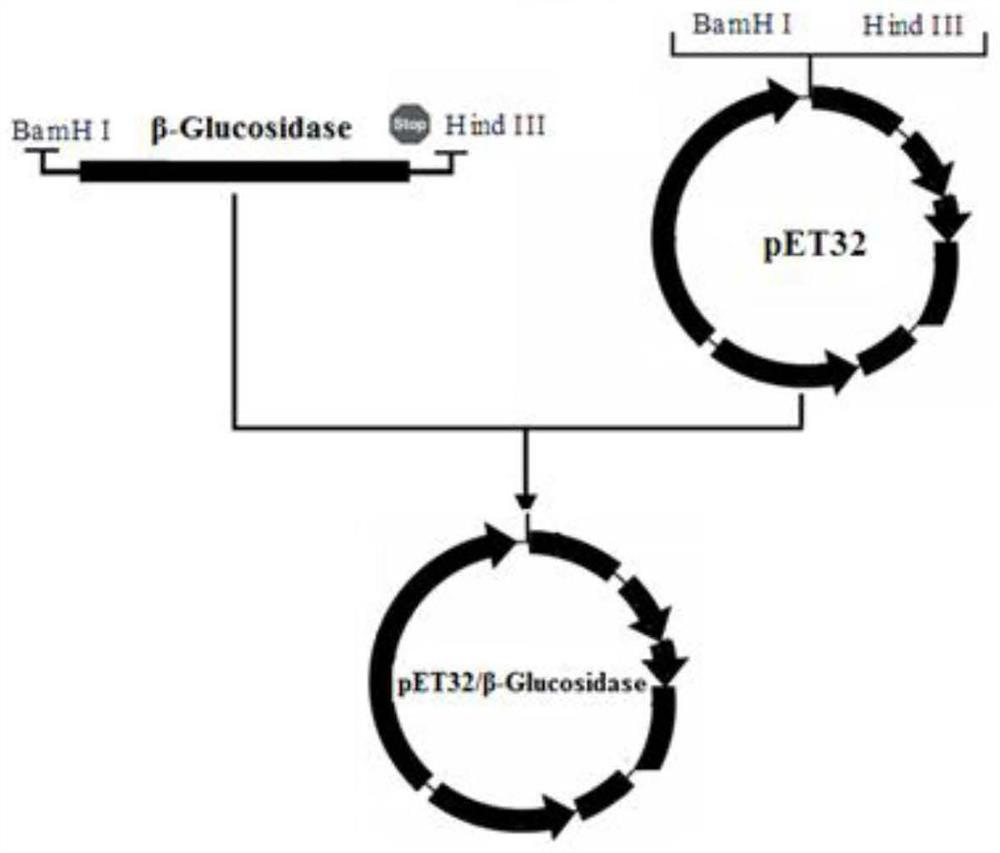
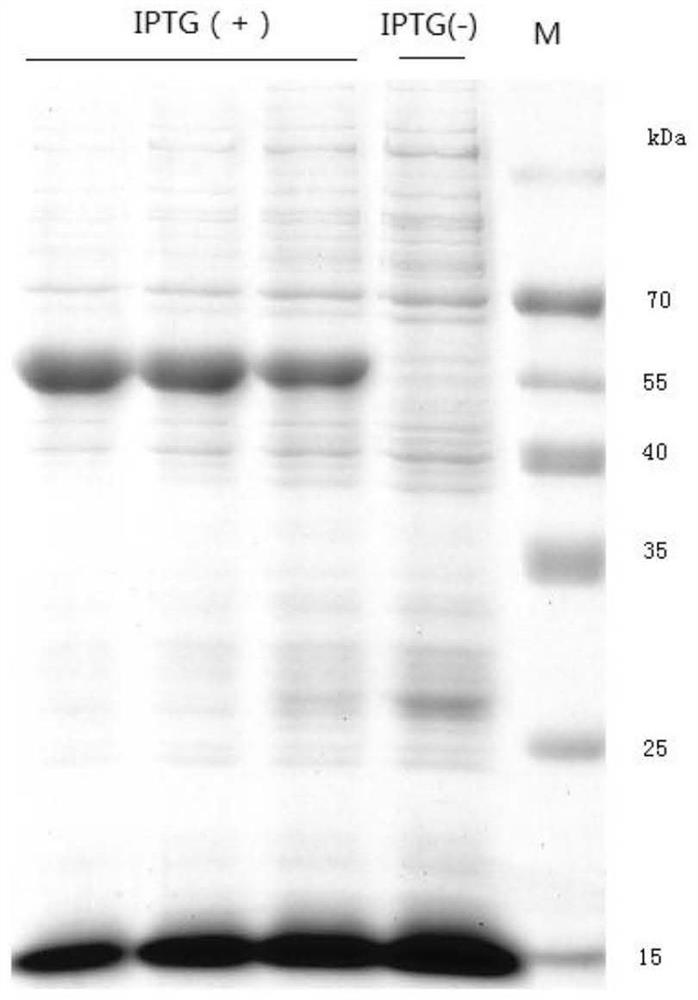
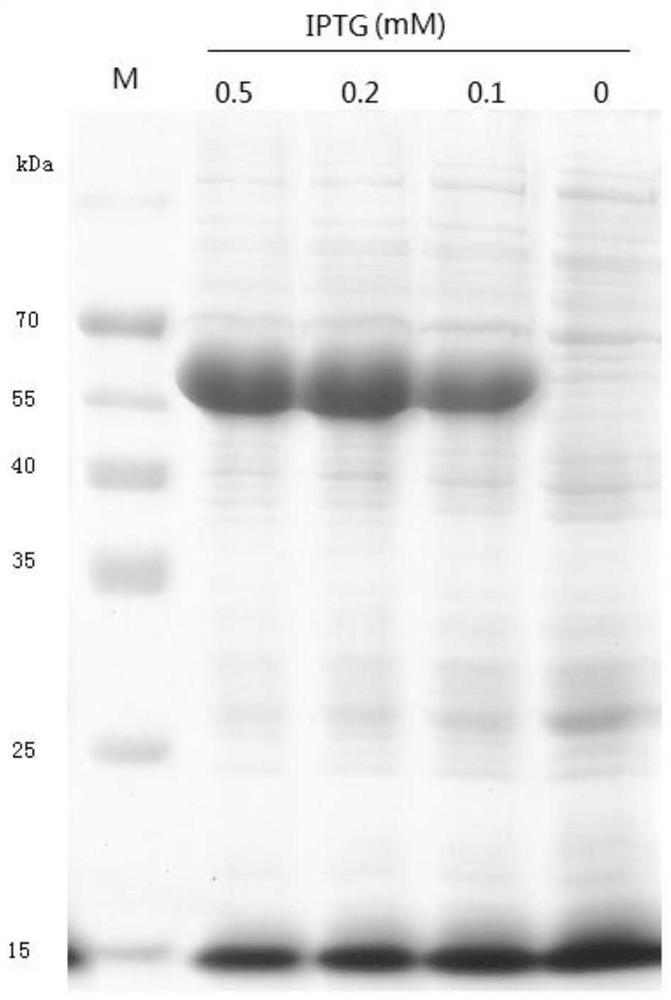
The present invention relates to a glycoside hydrolase family 7 protein gene, the nucleotide sequence of the glycoside hydrolase family 7 protein gene is shown in SEQ ID No.1. It also relates to the protein encoded by the gene, the amino acid sequence of which is shown in SEQ ID NO.2. The present invention designs a new gene sequence capable of expressing the glycoside hydrolase family 7 protein, and further utilizes the pET28 vector to construct a recombinant plasmid and transform it into an E. coli expression strain to realize the soluble expression of the expression product and obtain a large amount of soluble form of Recombinant glycoside hydrolase family 7 protein; in the expression system of the present invention, the recombinant glycoside hydrolase family 7 protein can be folded in an appropriate manner and maintain the natural conformation, and the prepared protein has the ability to hydrolyze sodium carboxymethyl cellulose to produce glucose It has good enzyme activity, and has good enzyme activity under the condition of pH3.5‑5.0, and has a certain activation effect in the presence of copper ions.
Owner:HUNAN BUTIAN PHARMA
Method for united typing detection of porcine contagious pleuropneumonia antibody and kit
InactiveCN101858912AMaintain native conformationHigh sensitivityFluorescence/phosphorescenceStatistical analysisBOAR
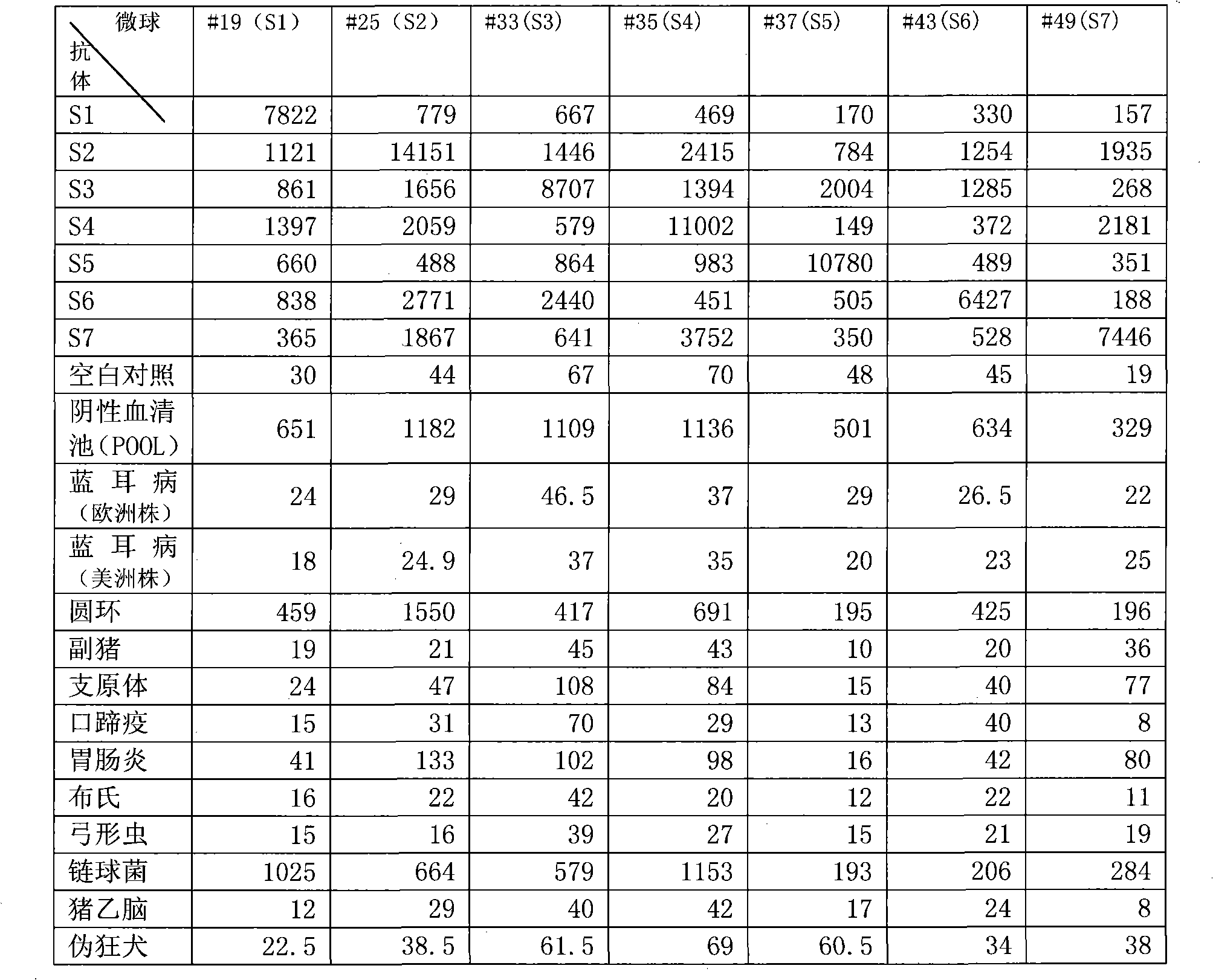
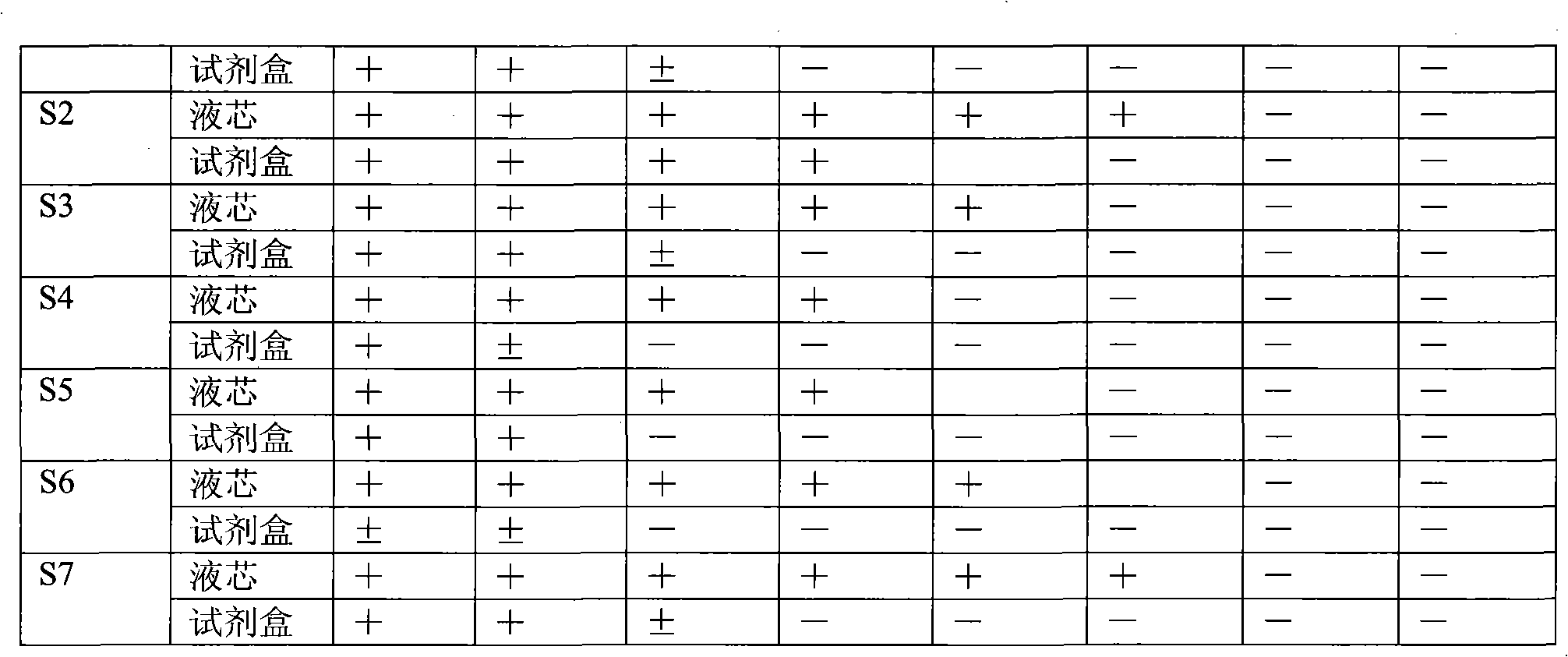
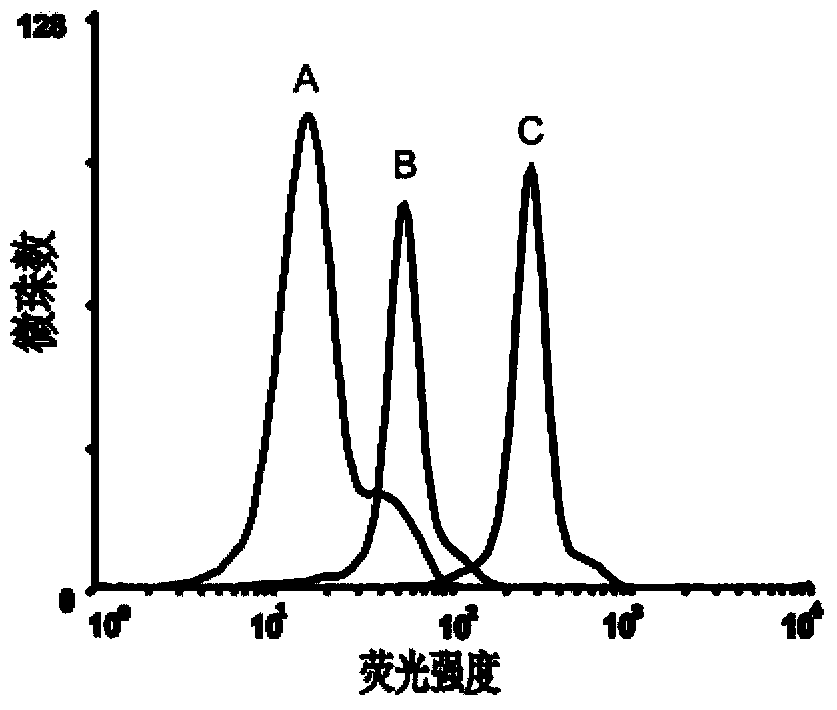
The invention discloses a method for united typing detection of a porcine contagious pleuropneumonia antibody. The method comprises the following steps: firstly preparing actinobacillus pleuropneumoniae polysaccharide antigen and rabbit anti-actinobacillus pleuropneumonia hyper-immune serum, and then purifying; coupling a liquid-phase chip microballoon by utilizing the purified rabbit anti-actinobacillus pleuropneumoniae hyper-immune serum, building a method for typing detection of the liquid-phase chip of the porcine pleuropneumonia antibody according to a double-sandwich ELISA principle, and determining the optimum experimental condition; and finally determining the threshold for positive and negative judgment of the liquid-phase chip through statistical analysis. In addition, the invention also discloses a kit for united typing detection of the liquid-phase chip of the porcine contagious pleuropneumoniae antibody. The invention can simultaneously carry out typing detection of the S1-S7-type serum antibody of porcine contagious pleuropneumonia, and the whole reaction can be completed within 3 hours; and the method has the characteristics of rapidly, sensitively, specifically andsimultaneously detecting a plurality of serum types, thus the method can be used for preliminarily screening entry and exit boars and diagnosing and monitoring porcine contagious pleuropneumoniae in hogpens of China.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Aptamer EpCAM (epithelial cell adhesion molecule) D of EpCAM and preparation method thereof
ActiveCN103387989AMaintain native conformationNo toxicityMicrobiological testing/measurementDNA preparationProtein targetNeural cell adhesion molecule
The invention provides an aptamer EpCAM (epithelial cell adhesion molecule) D of an EpCAM as well as a preparation method and application thereof, relating to nucleic acids. The aptamer has high specificity and affinity. The aptamer has a G tetramer structure or a stem-loop structure. The preparation method comprises the steps of designing and synthesizing a single-stranded DNA (deoxyribonucleic acid) random oligonucleotide bank, screening a target oligonucleotide sequence, and identifying the target protein binding specificity and affinity of the target oligonucleotide sequence by a flow analysis method. The screened aptamer does not have toxicity, has small molecular weight and good permeability, is easy to synthesize and label, only specifically identifies EpCAM proteins and cell lines expressing the EpCAM proteins, and does not have a function of identifying the cell lines which do not express the proteins.
Owner:XIAMEN UNIV
Antrodia camphorata immunomodulatory protein ACA1 and a coding gene thereof and application thereof
InactiveCN108753797ABiologically activeMaintain native conformationBacteriaDepsipeptidesA-DNAProtein C
The present invention relates to Antrodia camphorata immunomodulatory protein ACA1 and a coding gene thereof and application thereof, and the gene provided by the present invention is any one DNA molecule of the following (1) to (3): (1) a DNA molecule as shown in sequence 1 of a sequence table; (2) a DNA molecule that hybridizes with the DNA sequence defined in the (1) under stringent conditionsto code an immunomodulatory protein; (3) a DNA molecule that at least 90%- homologous, or at least 95%-homologous, at least 96%, at least 97%-homologous, at least 98%-homologous, or at least 99%-homologous to the DNA sequence defined in the (1) to code an immunomodulatory protein. The DNA sequence of the ACA1 is optimized, and a method for preparing a biologically active ACA1 recombinant protein is provided.
Owner:HUAIHUA UNIV
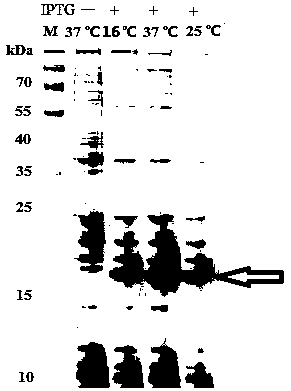
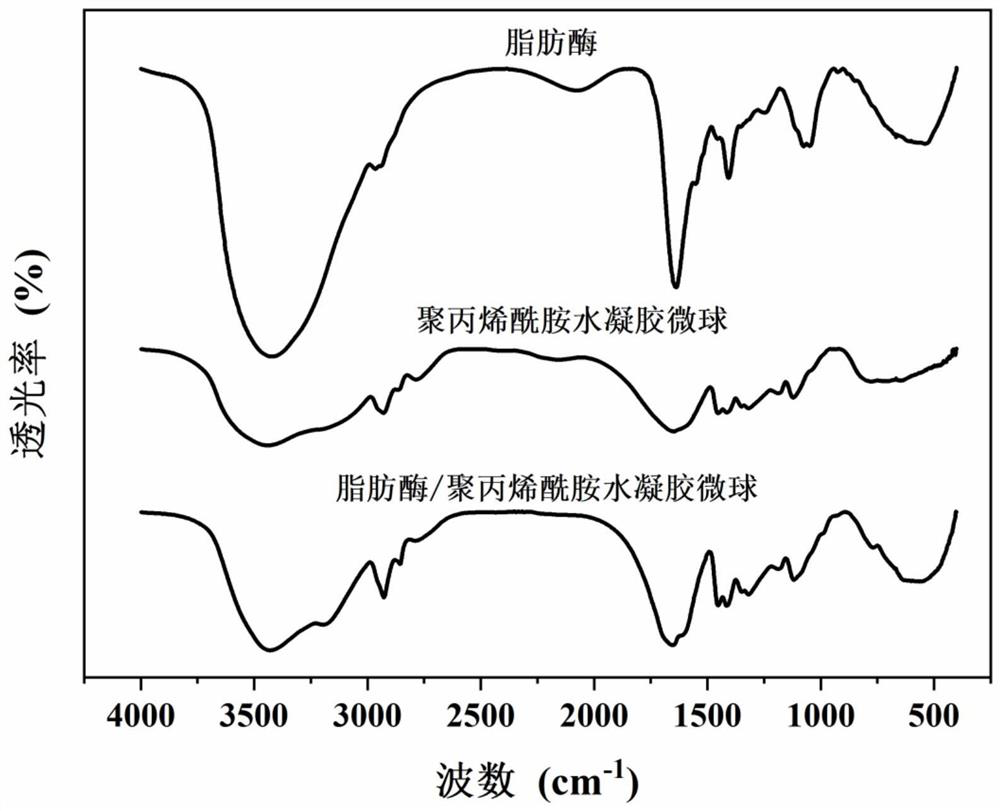
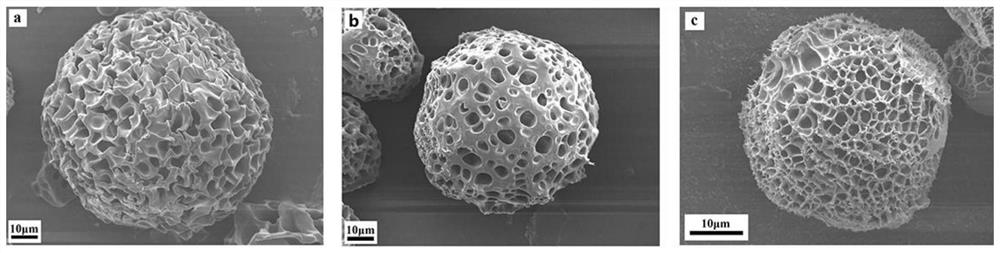
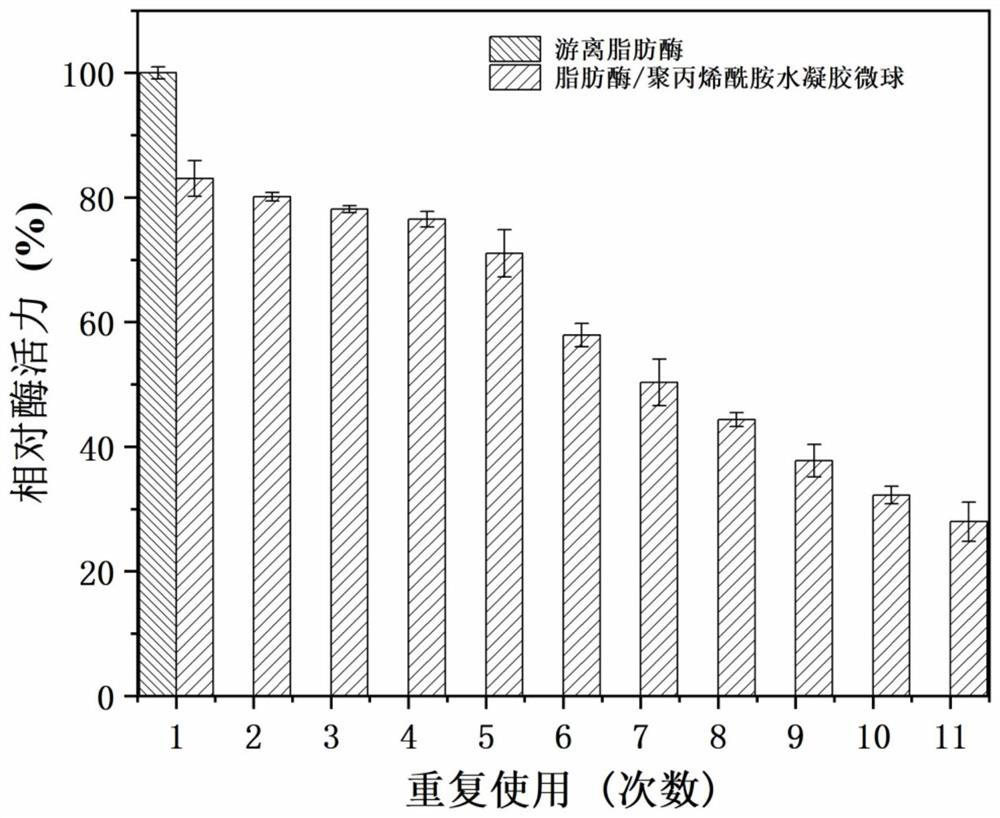
Lipase/polyacrylamide aquagel microsphere catalytic material, preparation method therefor and application of lipase/polyacrylamide aquagel microsphere catalytic material
ActiveCN112980826AMaintain native conformationDoes not reduce vitalityImmobilised enzymesHydrolasesFood industryPolymer science
The invention provides a lipase / polyacrylamide aquagel microsphere catalytic material, a preparation method therefor and application of the lipase / polyacrylamide aquagel microsphere catalytic material. The method comprises the steps: mixing an aqueous phase containing an acrylamide monomer and N,N-methylene-bisacrylamide with an organic phase containing a compounding emulsifier, and then, carrying out an out-phase emulsion polymerization reaction, so as to obtain polyacrylamide aquagel microspheres; and physically adsorbing lipase to the polyacrylamide aquagel microspheres, thereby obtaining the lipase / polyacrylamide aquagel microsphere catalytic material. The compounding emulsifier is prepared through mixing a Span emulsifier and a Tween emulsifier and has an HLB value of 3-7. The catalytic material prepared by the method is environmentally friendly and non-toxic, is easily separated from a reaction system, can be recycled and thus will have an extensive application value in various fields such as biocatalysis and food industry.
Owner:DALIAN POLYTECHNIC UNIVERSITY
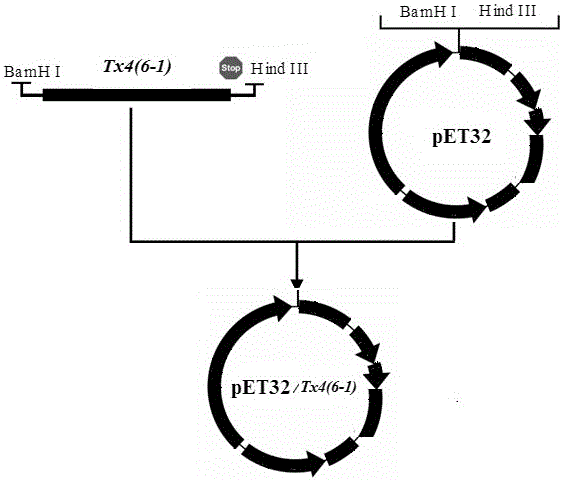
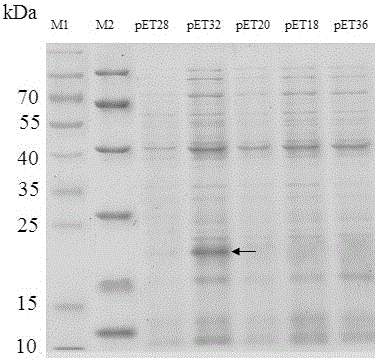
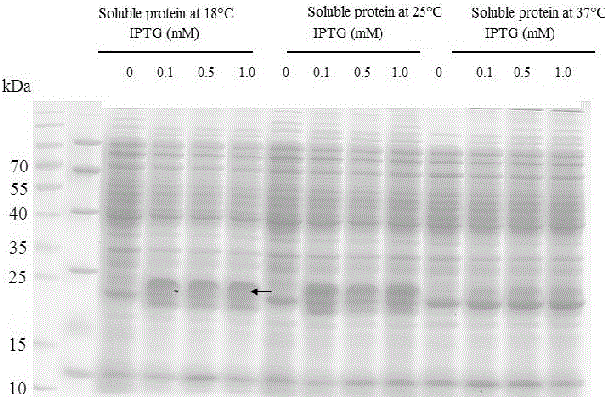
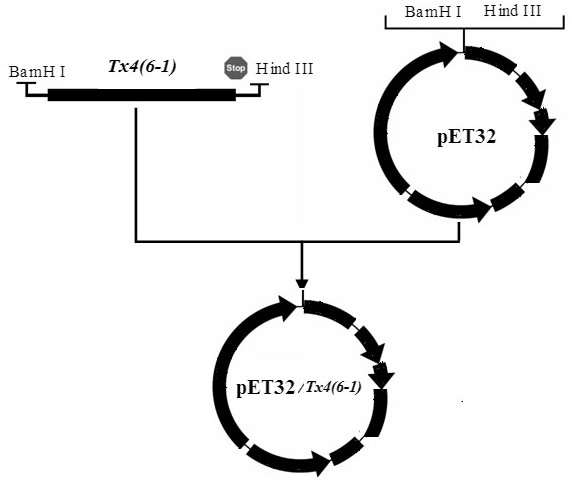
Production method of toxin Tx4 (6-1) label-free recombinant protein
ActiveCN106754945AQuick cutImprove biological activityBacteriaMicroorganism based processesEscherichia coliToxin protein
The invention relates to a production method of toxin Tx4 (6-1) label-free recombinant protein. Gene provided by the invention is DNA molecule shown by a sequence 1 in a sequence table. The production method includes that (1), soluble high-level fusion expression of the toxin gene in Escherichia coli can be realized by utilizing a gene sequence of the sequence table 1; (2), recombinant protein completely identical with naturally mature Tx4 (6-1) protein in amino acid sequence is obtained by cutting and removing non-target protein from soluble protein in fusion expression; (3), the obtained recombinant protein has high bioactivity. By the production method, DNA sequence of Tx4 (6-1) is optimized, and expression strain and expression vector are screened; a method for efficiently producing active Tx4 (6-1) recombinant protein is provided, thereby being conducive to conducting detained research on insect-resistant characteristic and bio-safety of the toxin protein and of important significance in screening corresponding insect-resistant plants and high-toxicity transgenic biological insecticides.
Owner:HUAIHUA UNIV
Preparation method of graphene immobilized laccase
PendingCN109971745AAvoid chemical damageRetain enzyme activityOxidoreductasesOn/in inorganic carrierCvd graphenePhenols
The invention provides a preparation method of graphene immobilized laccase. The graphene immobilized laccase is prepared by immobilizing laccase to a carrier of two-dimensional crystal material having excellent conductivity through nonspecific adsorbing action. The preparation method comprises the steps of preparing the carrier and immobilizing laccase; particularly, the carrier, graphene, is prepared by using a phenol as a raw material to carry out graphitization on sandwiched FePO4 / dodecylamine nanolayer composite, and stripping before synthesis; the immobilized laccase is immobilized by adsorbing laccase to the surface of the evenly dispersed graphene in nonspecific manner, centrifuging, washing, and drying to obtain the finished immobilized laccase. The carrier of graphene herein helps increase electron transfer rate of enzymatic catalytic reaction process and improve reaction efficiency; the immobilized laccase prepared via the preparation method has high fixing quantity, high activity and good stability, can be recycled from the reaction system, and is reusable; the preparation process is simple and feasible.
Owner:NANJING UNIV +1
Group A, group B and group C meningococcus combined vaccine and preparation method thereof
ActiveCN104001166ABest combinationGood prevention effectAntibacterial agentsBacterial antigen ingredientsGenotypeMeningococcal carriage
The invention provides a group A, group B and group C meningococcus combined vaccine which is composed of polysaccharide-protein conjugates and a group B ST-4821 genotype (xrsw341215) meningococcus outer membrane protein vesicle (OMV), wherein the polysaccharide-protein conjugates are obtained in a manner that a group A meningococcus capsular polysaccharide and a group C meningococcus capsular polysaccharide are each subjected to covalent binding with a group B serotype 4-type (CMCC29356) meningococcus outer membrane protein vesicle (OMV). The vaccine can simultaneously prevent group A, group B and group C meningococcus invasive diseases, has better protective effects especially on prevention aiming at epidemic cerebrospinal meningitis high-risk groups at the age of less than 2 years, and moreover, has better specificity and better protection effect aiming at nature group A, group B and group C meningococcus invasion.
Owner:CHENGDU OLYMVAX BIOPHARM
Method for preparing fructose lysine enzyme and application of preparing fructose lysine enzyme
ActiveCN102559643BHigh specific vitalityEasy extractionHydrolasesMicrobiological testing/measurementFructoseFractional Precipitation
The invention provides a method for preparing fructose lysine enzyme and a glycated albumin detection kit, wherein the glycated albumin detection kit is prepared by utilizing the fructose lysine enzyme that is prepared by adopting the method. The method comprises the operation steps as follows: strains are screened so as to obtain 11 to 82 aspergillus strains of the high-activity fructose lysine enzyme; the 11 to 82 aspergillus strains are cultivated, and mycelia are collected; the mycelia are suspended in buffer solution, and then are processed through cell disruption and centrifugation, and supernatant fluid is collected; the supernatant fluid is processed through fractional precipitation by adopting ammonium sulfate solution, and deposits are collected so as to obtain crude extracts; the crude extracts are processed through hydrophobic chromatography, and eluant is collected; in addition, the collected eluant is processed through affinity chromatography, and then eluant is collected, that is, fructose lysine enzyme solution is obtained. The method has the advantages as follows: the preparation steps are simple, and the obtained fructose lysine enzyme achieves high purity, high activity and high reaction specificity.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
A kind of production method of toxin tx4 (6-1) unlabeled recombinant protein
ActiveCN106754945BQuick cutImprove biological activityBacteriaMicroorganism based processesBiotechnologyEscherichia coli
The invention relates to a production method of toxin Tx4 (6-1) label-free recombinant protein. Gene provided by the invention is DNA molecule shown by a sequence 1 in a sequence table. The production method includes that (1), soluble high-level fusion expression of the toxin gene in Escherichia coli can be realized by utilizing a gene sequence of the sequence table 1; (2), recombinant protein completely identical with naturally mature Tx4 (6-1) protein in amino acid sequence is obtained by cutting and removing non-target protein from soluble protein in fusion expression; (3), the obtained recombinant protein has high bioactivity. By the production method, DNA sequence of Tx4 (6-1) is optimized, and expression strain and expression vector are screened; a method for efficiently producing active Tx4 (6-1) recombinant protein is provided, thereby being conducive to conducting detained research on insect-resistant characteristic and bio-safety of the toxin protein and of important significance in screening corresponding insect-resistant plants and high-toxicity transgenic biological insecticides.
Owner:HUAIHUA UNIV
A new pectinase gene and its protein expression, carrier and application
ActiveCN110747212BHigh purityIncrease concentrationBacteriaMicroorganism based processesEscherichia coliPectinase
The present invention relates to a new pectinase gene, which is shown in the nucleotide sequence of SEQ ID NO.1 in the sequence table; or has more than 90% of the nucleotide sequence shown in SEQ ID NO.1 A sequence that is homologous and encodes the same biological function protein; or a sequence that can hybridize with the nucleotide sequence shown in SEQ ID NO.1 and encodes the same biological function protein. The nucleotide sequence of SEQ ID NO.1 in the present invention can use the pET32 vector and Escherichia coli expression strain to realize the fusion of the expression product and the solubilizing factor Trx, and obtain a large amount of Trx pectinase fusion protein in soluble form , and purified by nickel affinity chromatography and DEAE anion exchange method to obtain high-purity and high-activity recombinant pectinase protein, which provides the basis for mass production of recombinant pectinase.
Owner:HUAIHUA UNIV
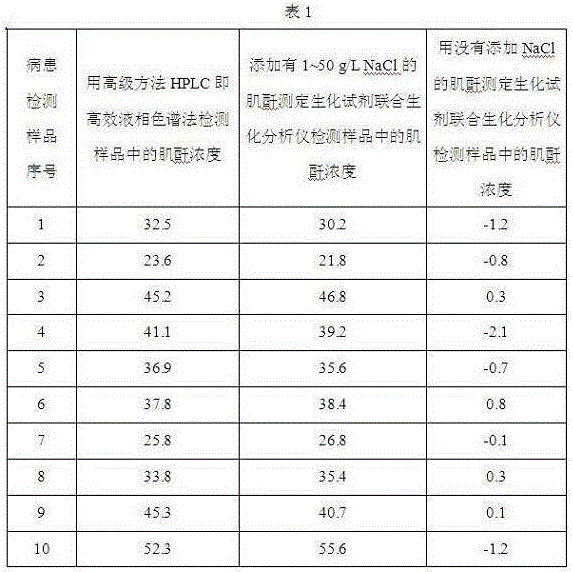
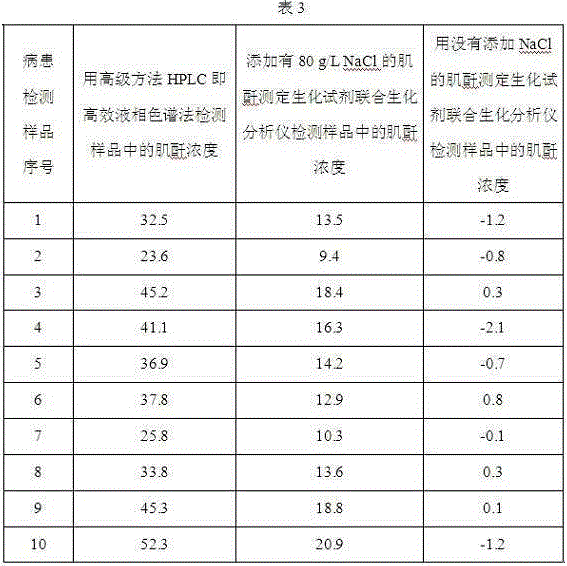
Application of chloride salt in the preparation of biochemical reagents that eliminate negative or zero values in assay results
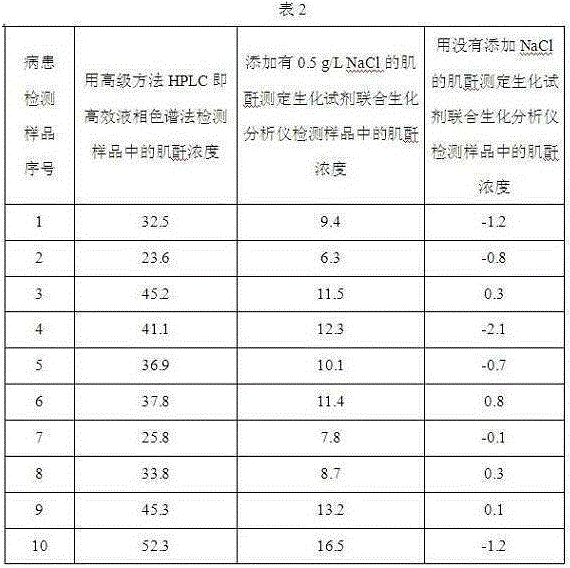
ActiveCN103397076BMaintain native conformationThe test result is accurateMicrobiological testing/measurementChloride saltDrug biological activity
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
Human platelet antigen genotyping liquid chip and human platelet antigen genotyping detection method thereof
InactiveCN102230017BImprove throughputIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHybridization reactionGene
The invention belongs to the technical field of molecular biology, and in particular relates to a human platelet antigen genotyping liquid phase chip and a detection method thereof. Human platelet antigen genotyping technology is an important technical means to provide patients with matching platelets and promote the safety and effectiveness of clinical platelet transfusion. However, the existing human platelet antigen genotyping technology has low throughput and high accuracy. Indexes such as sensitivity and sensitivity need to be improved. The liquid phase chip of the present invention includes primers 1-24, connection probes 25-46 and fluorescently encoded microspheres coated with detection probes 47-68, and the human platelet antigen gene is completed through amplification, connection and hybridization reactions. Typing detection. The liquid phase chip and detection method adopted in the present invention have the advantages of large flux, high sensitivity, strong specificity, good repeatability, wide linear range, simple operation, strong flexibility, and wide application range, and are clinically accurate, Efficient and practical human platelet antigen genotyping detection method.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
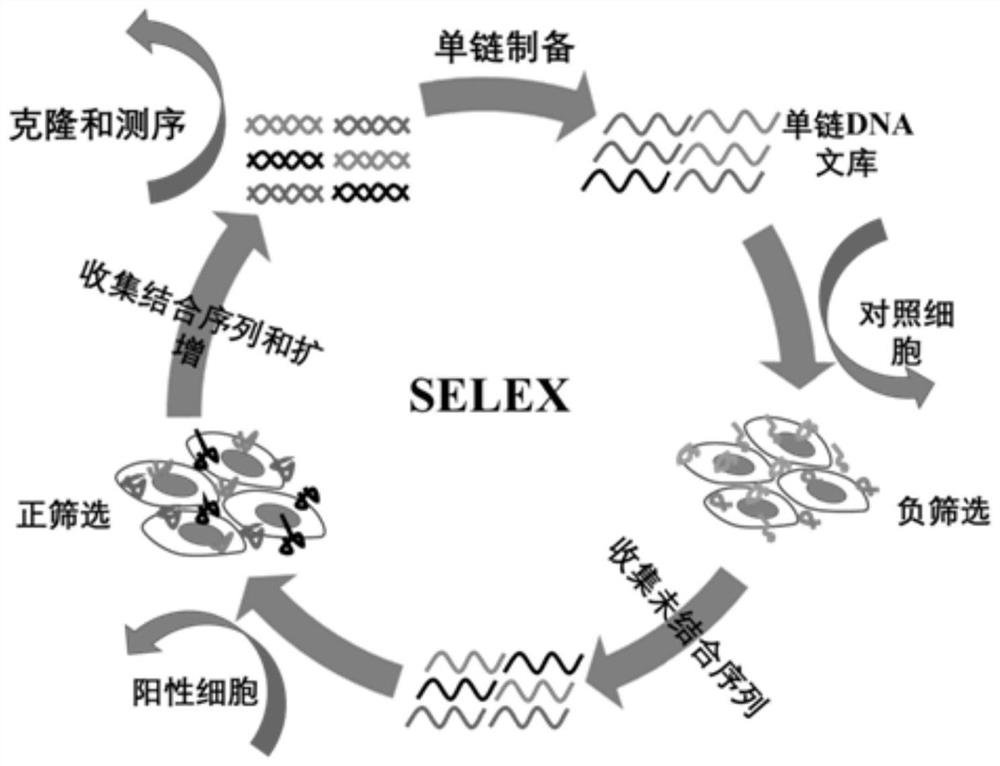
PD-L1 nucleic acid aptamer as well as screening method and application thereof
ActiveCN113699157ABinding blockAntagonize immunosuppressionOrganic active ingredientsMicrobiological testing/measurementAptamerNucleotide
The invention discloses a PD-L1 nucleic acid aptamer as well as a screening method and application thereof. The PD-L1 nucleic acid aptamer has a nucleotide sequence as shown in SEQ ID No. 1 (sequence identifier number 1). The screening method comprises the following steps: carrying out cell transfection on CHO-K1 cells by utilizing a lentivirus system to obtain a PD-L1 cell line with stable and high expression of PD-L1 for positive screening of target cells; selecting CHO / K1 cells which are not transfected as control cells for negative screening of target cells; and using a Cell-SELEX (systematic evolution of ligands by exponential enrichment) technology for screening the PD-L1 nucleic acid aptamer. According to the present invention, the PD-L1 nucleic acid aptamer can specifically bind to the PD-L1 high expression cell line, and can block the interaction between PD-1 and PD-L1 so as to antagonize the immunosuppression effect mediated by the PD-1 / PD-L1 signal pathway, such that the certain tumor inhibition effect is provided.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com