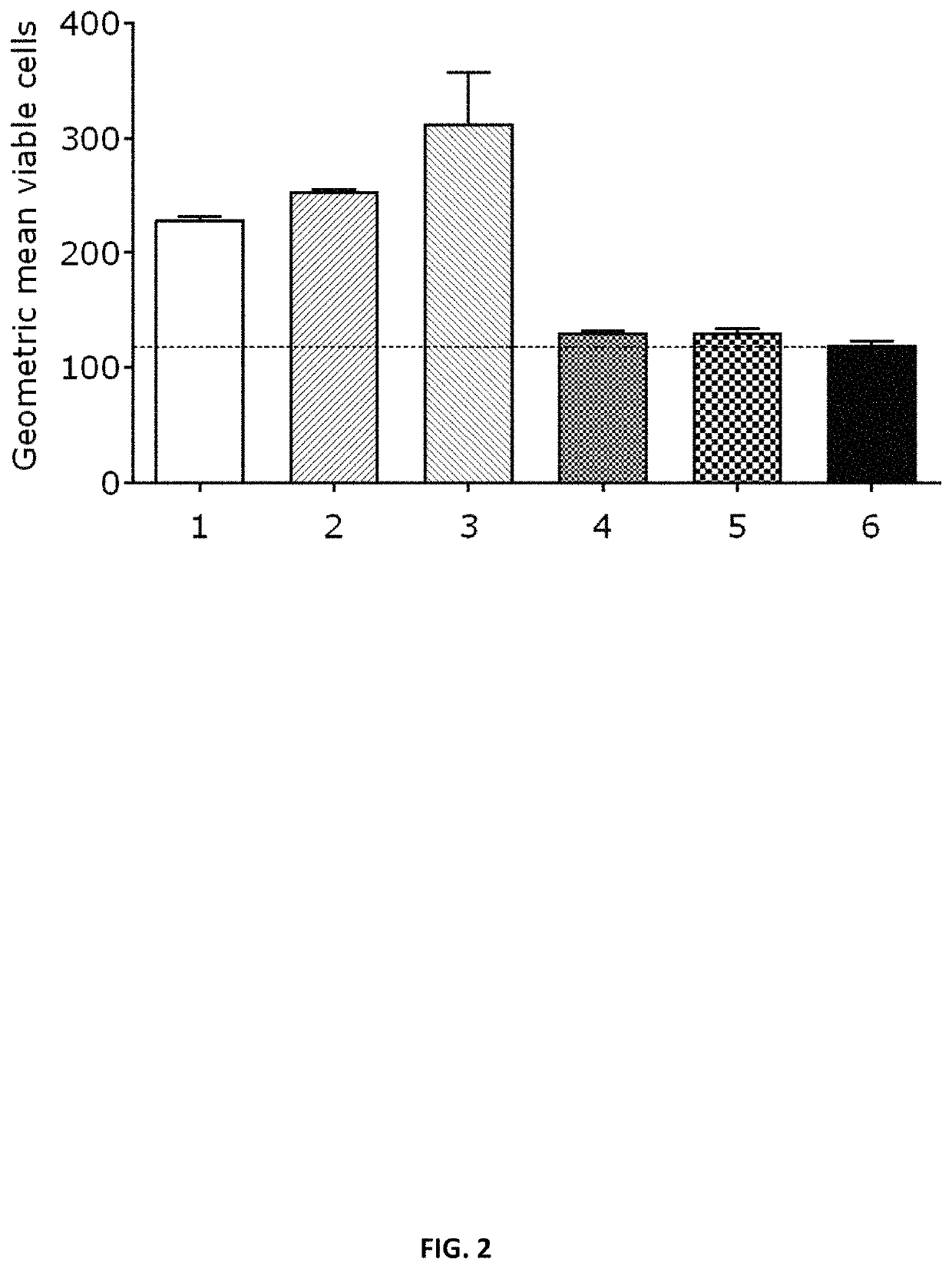
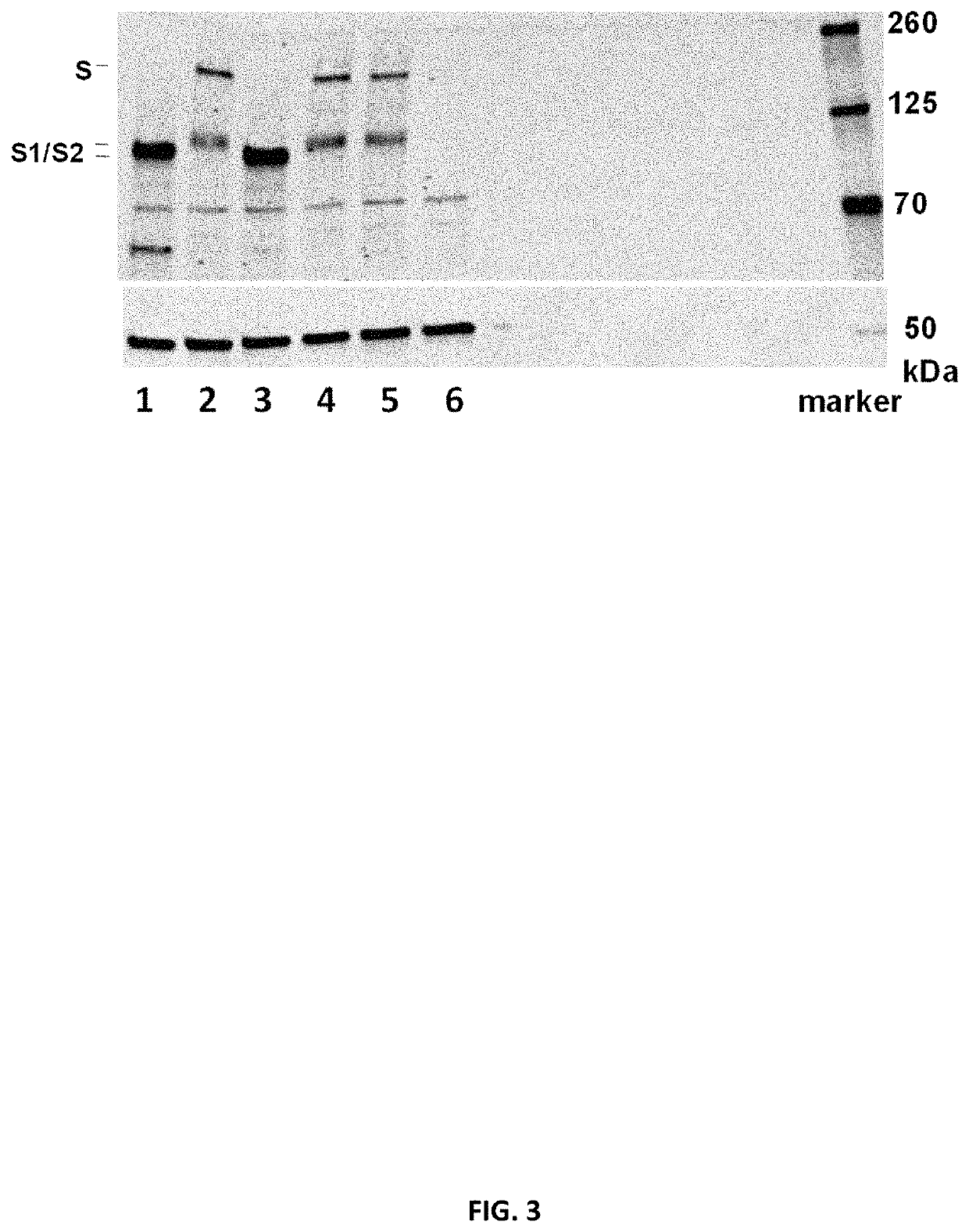
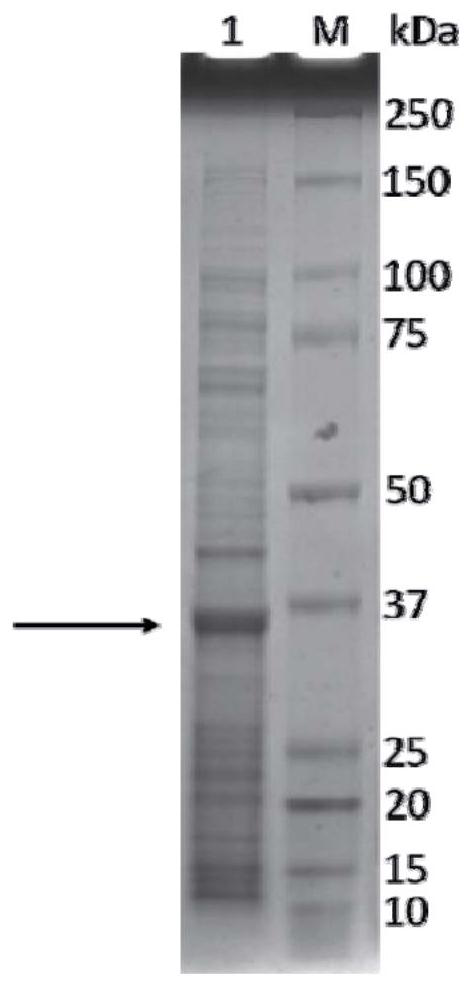
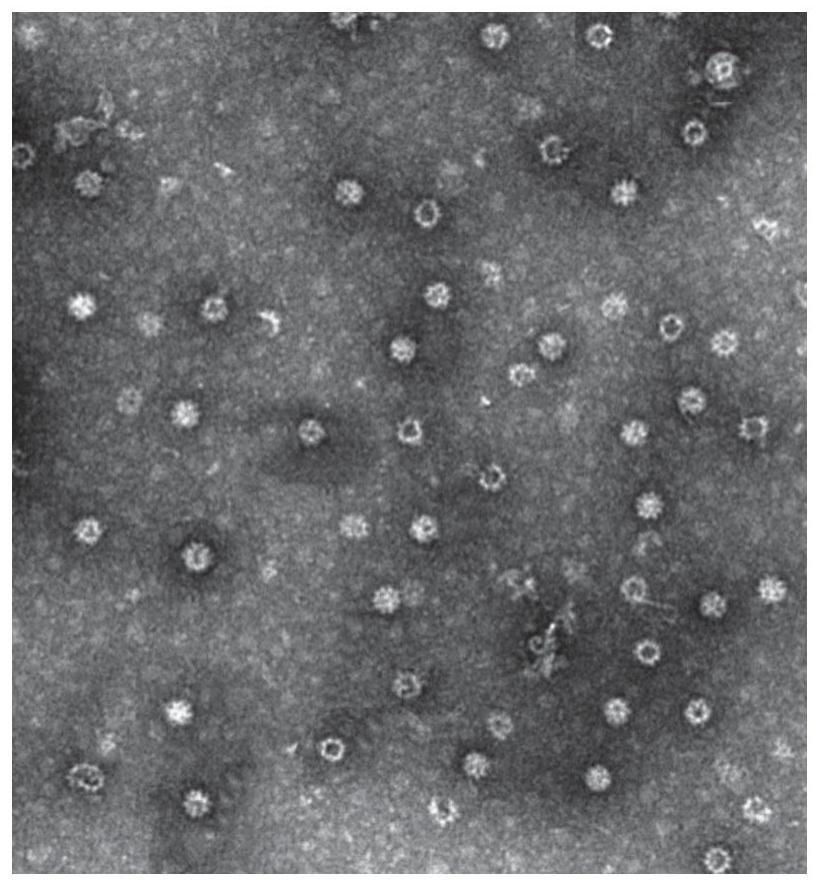
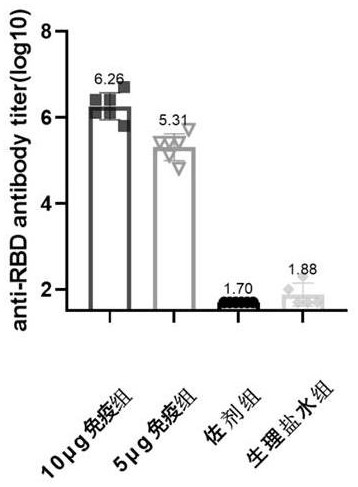
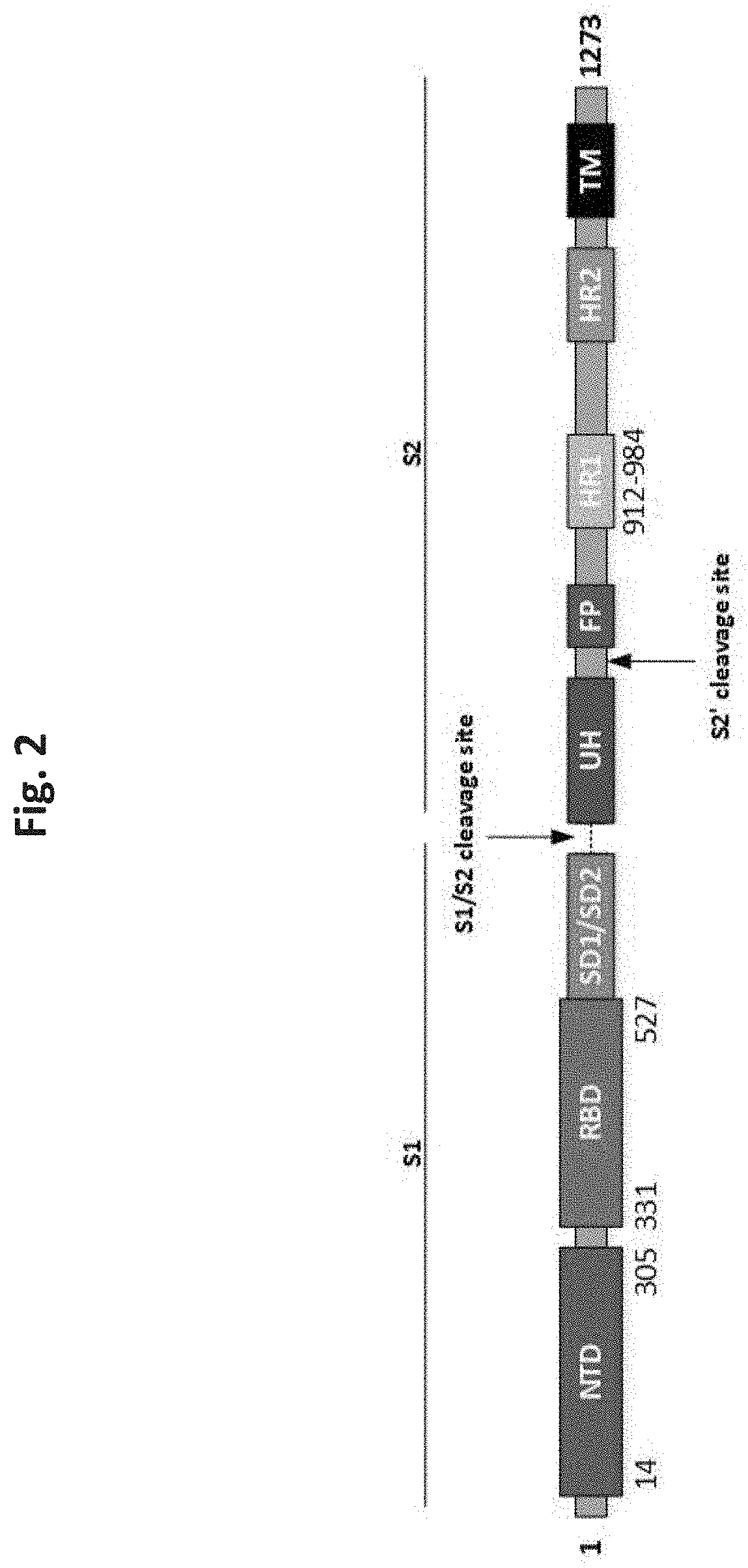
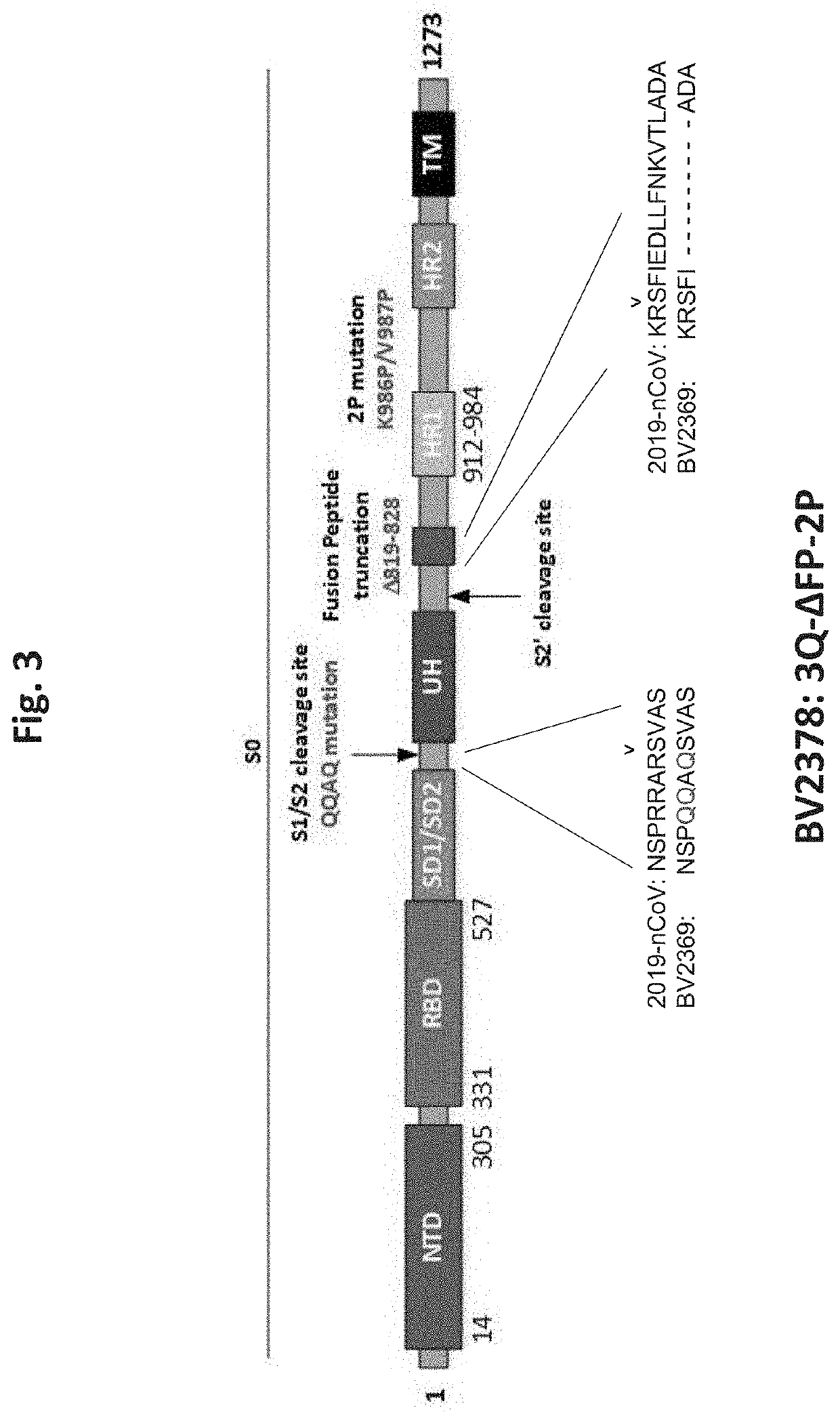
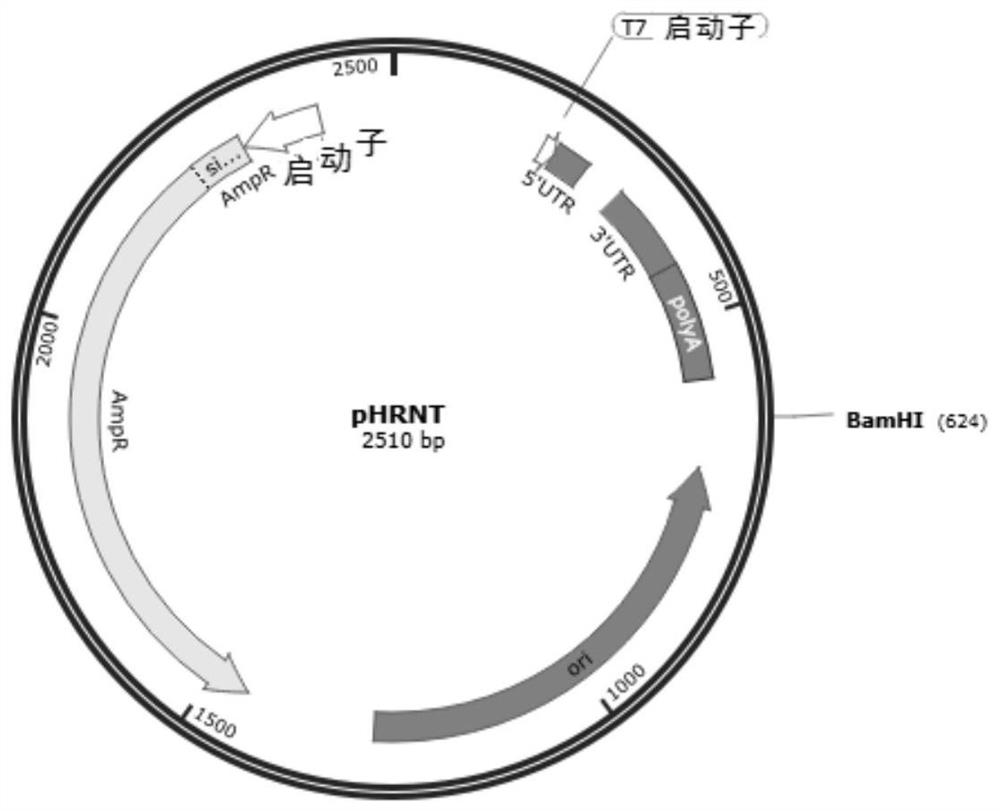
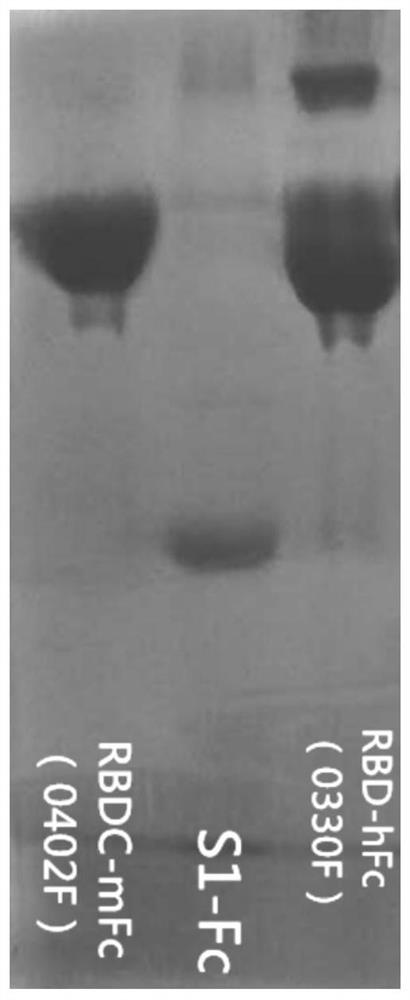
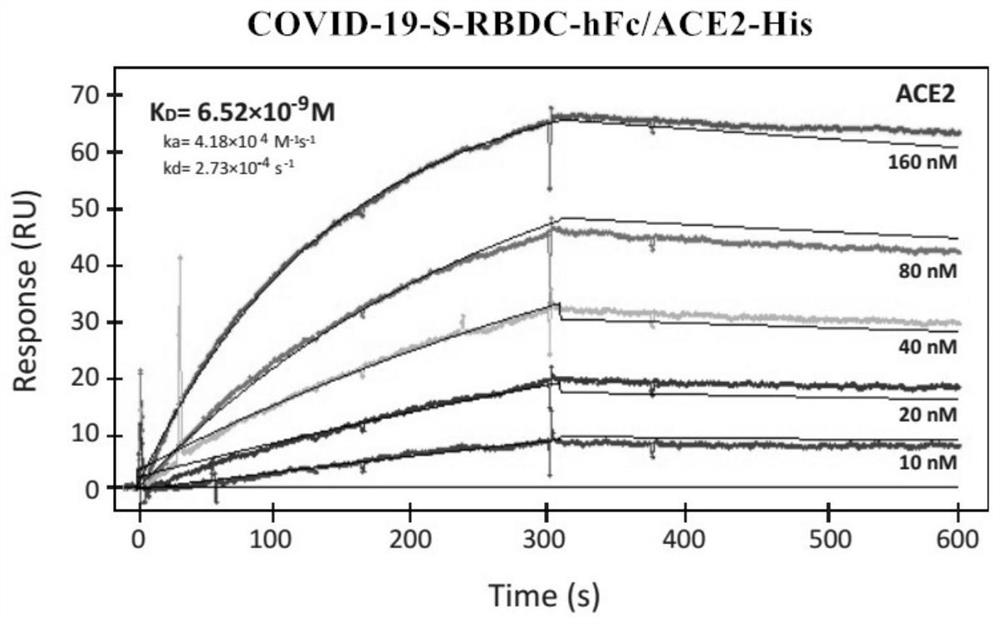
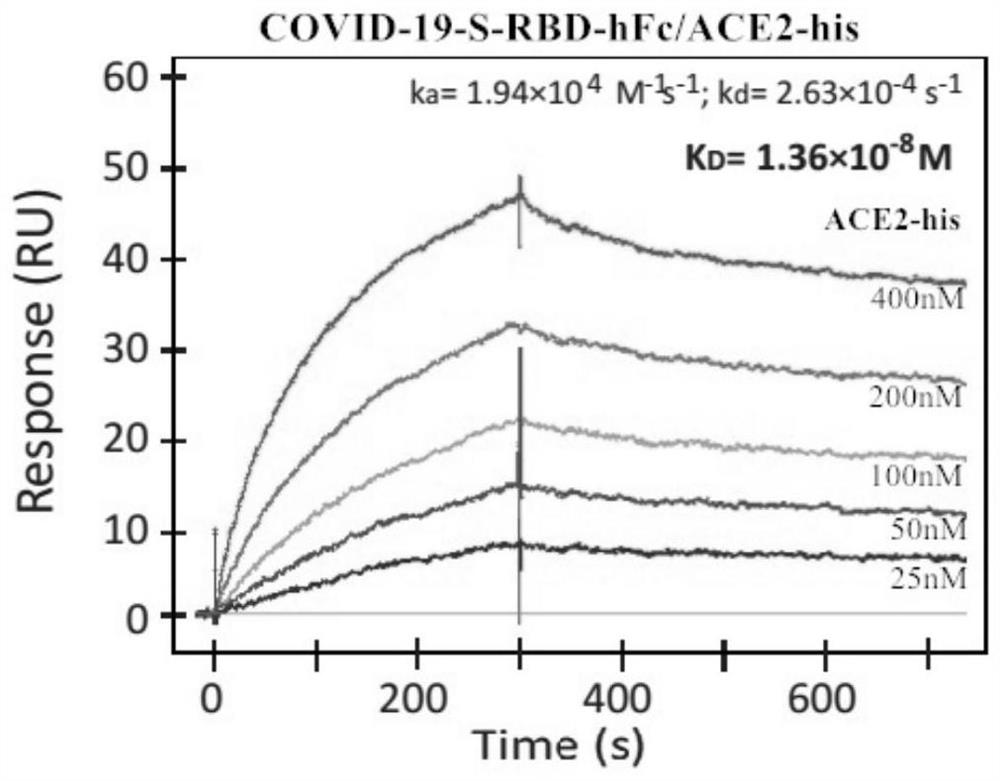
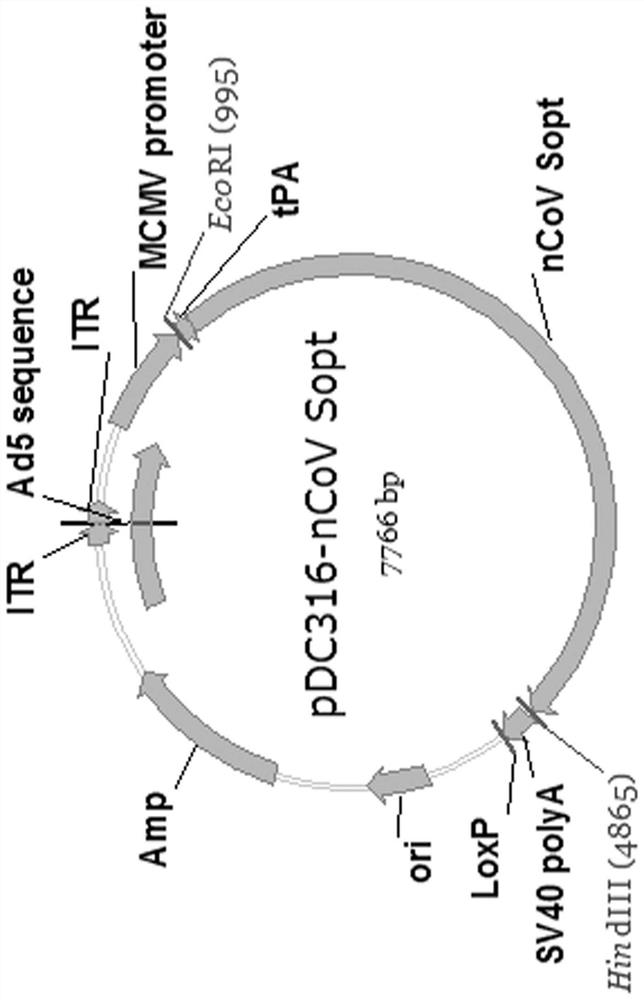
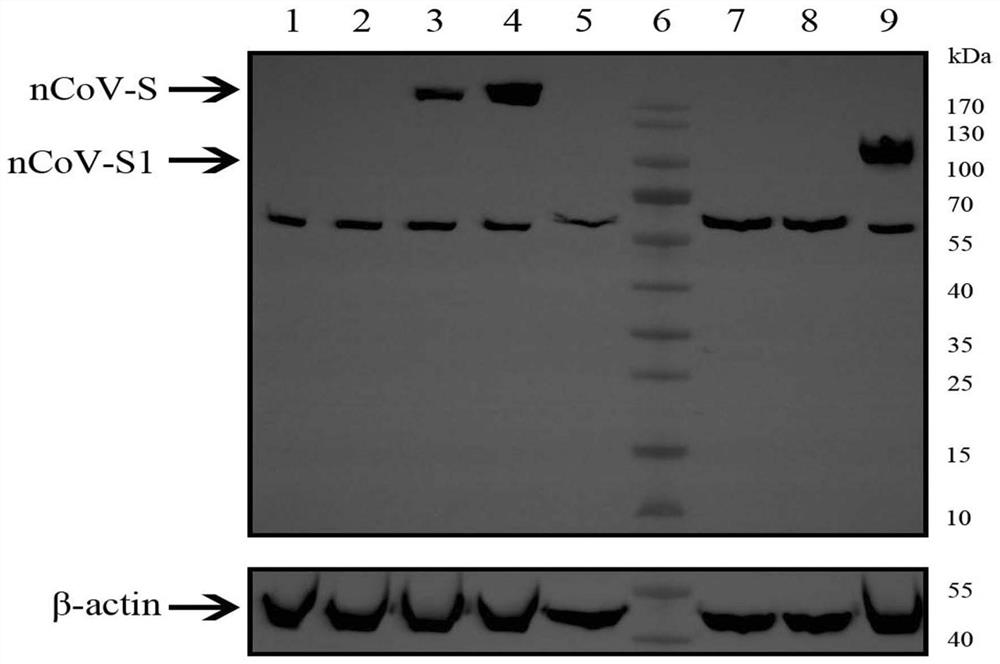
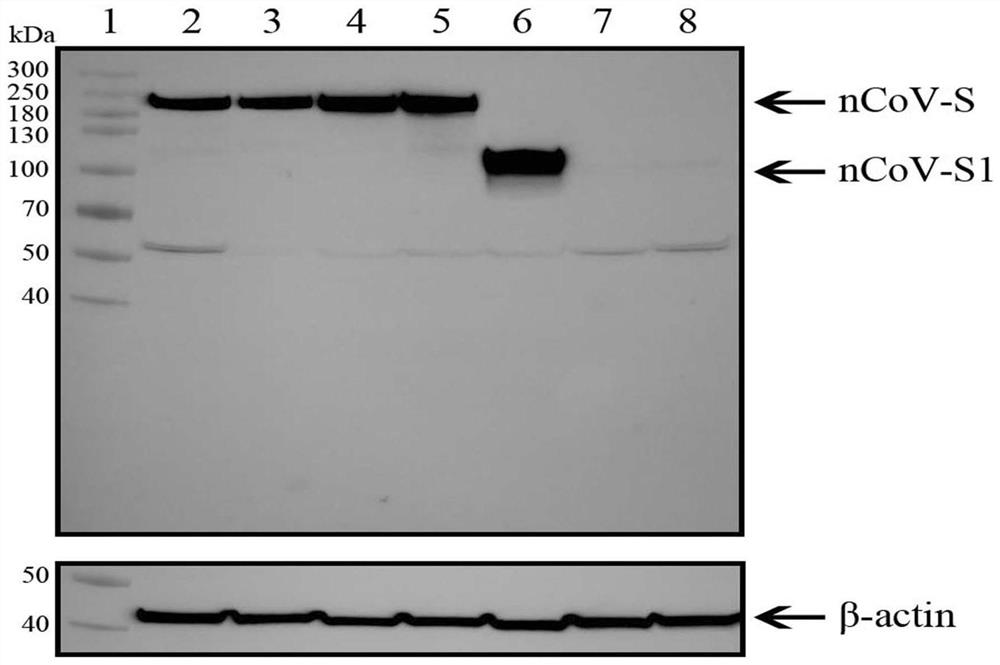
Patents
Literature
127 results about "Coronavirus vaccination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
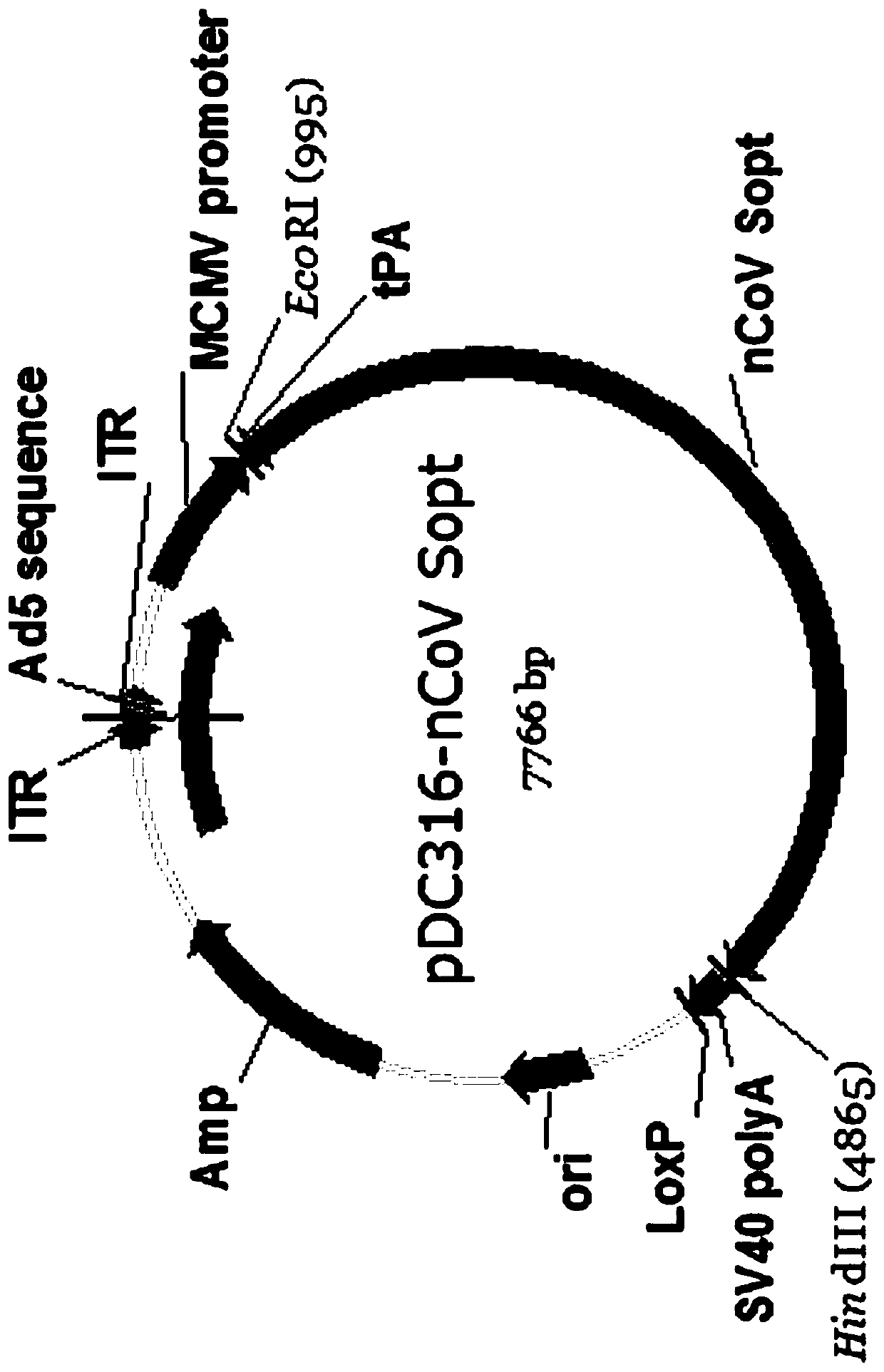
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Coronavirus vaccine formulations
ActiveUS10953089B1SsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationNanoparticle
Disclosed herein are coronavirus Spike (S) proteins and nanoparticles comprising the same, which are suitable for use in vaccines. The nanoparticles present antigens from pathogens surrounded to and associated with a detergent core resulting in enhanced stability and good immunogenicity. Dosages, formulations, and methods for preparing the vaccines and nanoparticles are also disclosed.
Owner:NOVAVAX
Subunit coronavirus vaccine for dimerization-based receptor binding domains
ActiveCN106928326AOvercoming the disadvantage of insufficient immunogenicityIncrease neutralizing antibody productionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationMiddle East respiratory syndrome coronavirus
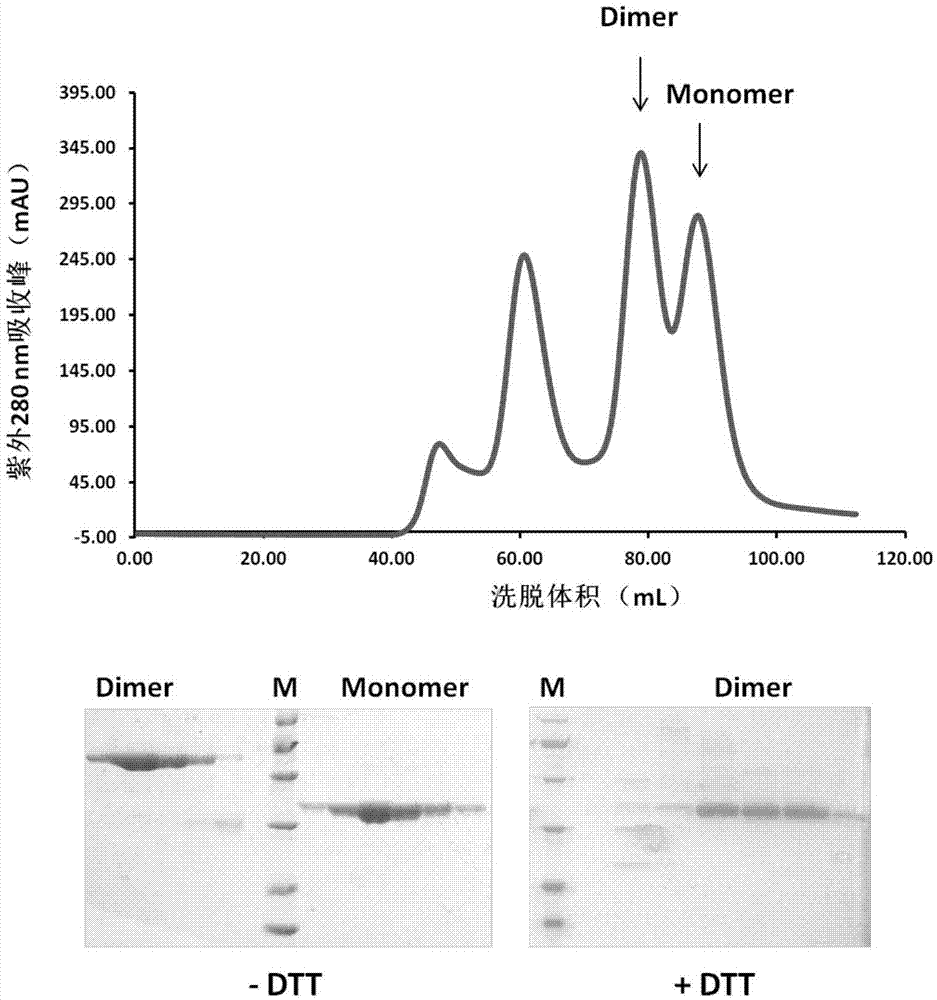
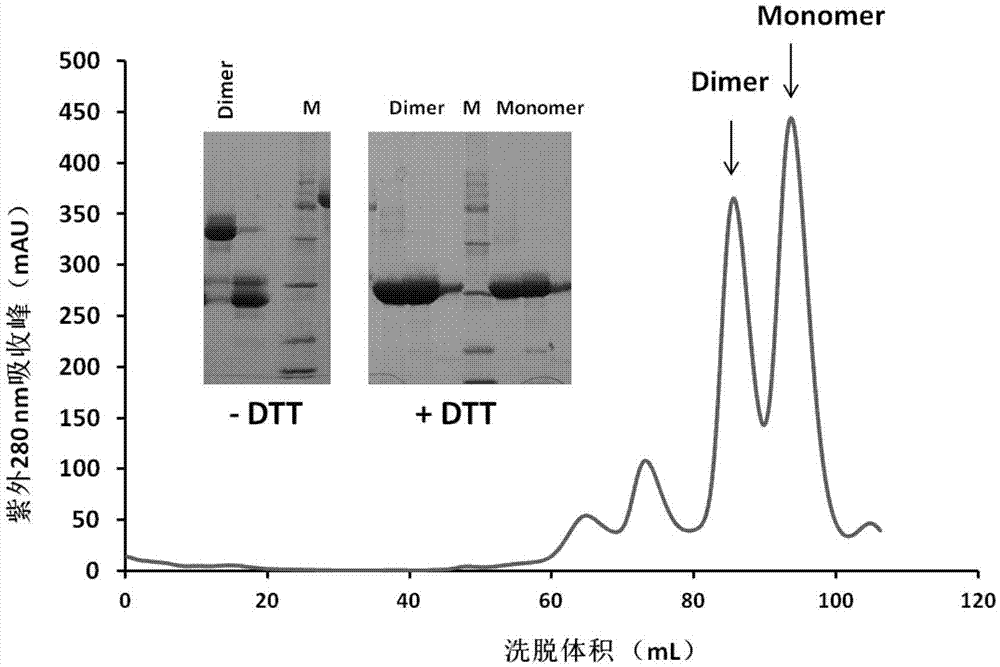
The invention discloses a subunit coronavirus vaccine for dimerization-based receptor binding domains and belongs to the technical field of medicine. A baculovirus expresses RBD (receptor binding domain (E367-Y606) of MERS-CoV (middle east respiratory syndrome coronavirus) protein and RBD (R294-F515) of SARS-CoV (severe acute respiratory syndrome coronavirus) in insect cells, the RBDs may form a dimer through cysteine residue at 603 of S (spike) protein or form a dimer through cysteine residue at 512 of the S protein, and purified RBD protein dimer and monomer are used respectively to immunize Bald / c mice. The dimerized RBDs have the advantages that the defect that RBD monomers have poor immunogenicity is overcome and the generation of neutralizing antibodies in against MERS-CoV is increased greatly.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD
Recombinant novel coronavirus as well as preparation method and application thereof
ActiveCN111560354ASolve the shortage problemSsRNA viruses negative-senseSsRNA viruses positive-senseAntigen epitopeAntigen
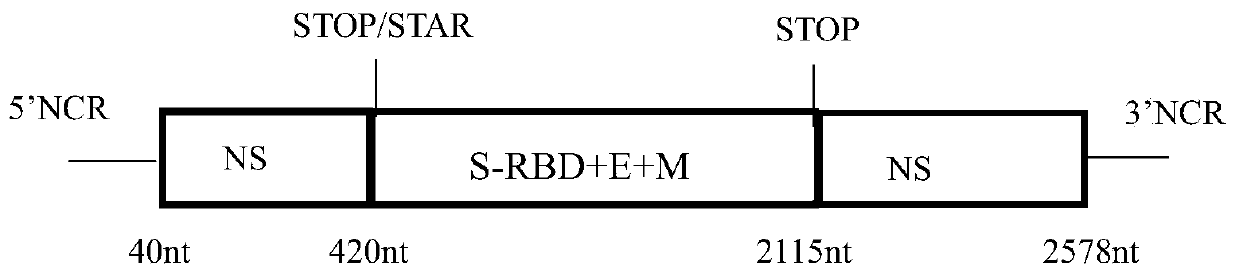
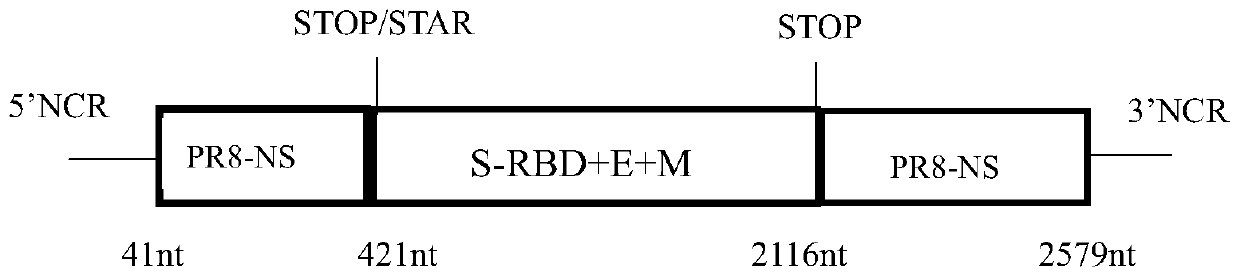
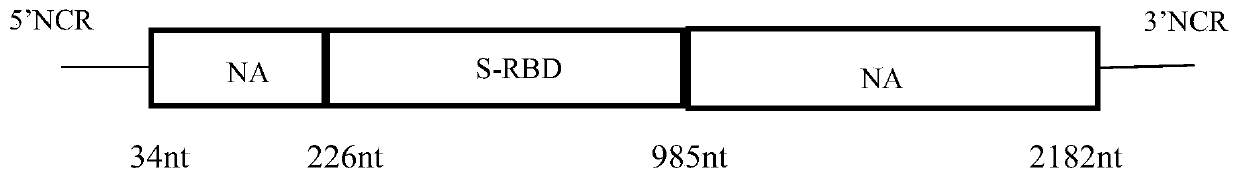
The invention discloses a recombinant novel coronavirus as well as a preparation method and application thereof. The invention firstly discloses a recombinant virus. Any one of an NS gene of an influenza virus, an HA gene of the influenza virus and an NA gene of the influenza virus of the recombinant virus is replaced. The invention further discloses application of the recombinant virus in preparation of a product for preventing and / or treating diseases caused by influenza virus and / or SARS-CoV-2. According to the invention, the SARS-CoV-2 antigen epitope and the influenza virus genome are operated from the gene level; the recombinant SARS-CoV-2 vaccine strain taking the influenza virus as the carrier is prepared on the basis of an RG technology, so that the problem of SARS-CoV-2 vaccine shortage is solved. The recombinant novel coronavirus will be a new milestone in the field of coronavirus vaccines, and the SARS-CoV-2 chimeric vaccine can protect more people from being harmed by theinfluenza virus and SARS-CoV-2.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL +1
Novel coronavirus antigen epitope and application thereof
ActiveCN111848753AImproving immunogenicityHigh titerSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigen epitopeCoronavirus vaccination
The invention provides a novel coronavirus antigen epitope and application thereof. A super computer is used for simulating S, E, M and N protein structures of the novel coronavirus, and novel coronavirus epitopes SEQ ID NO 1-38 are obtained through calculation. The epitopes have very good immunogenicity; an antibody generated by inducing a part of epitope polypeptide has the effect of neutralizing the novel coronavirus; the antigen epitope can be combined with antibodies in serum of novel coronavirus patients at home and abroad, has the potential of resisting various novel coronavirus strains, and also has the potential of identifying and applying different strains. The epitope provided by the invention can be used for (1) research and development of novel coronavirus vaccines and universal coronavirus vaccines such as MERS viruses and SARS viruses; and (2) preparing a novel coronavirus antibody, and further detecting and typing viruses and treating diseases caused by the viruses. Thenovel coronavirus antigen epitope has a wide application prospect in the aspects of prevention of coronavirus and propagation and outbreak thereof, and detection and diagnosis of virus strains.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Novel coronavirus vaccine and application thereof
ActiveCN112266411AHigh titerImprove expression levelSsRNA viruses positive-senseVirus peptidesCoronavirus vaccinationPharmaceutical drug
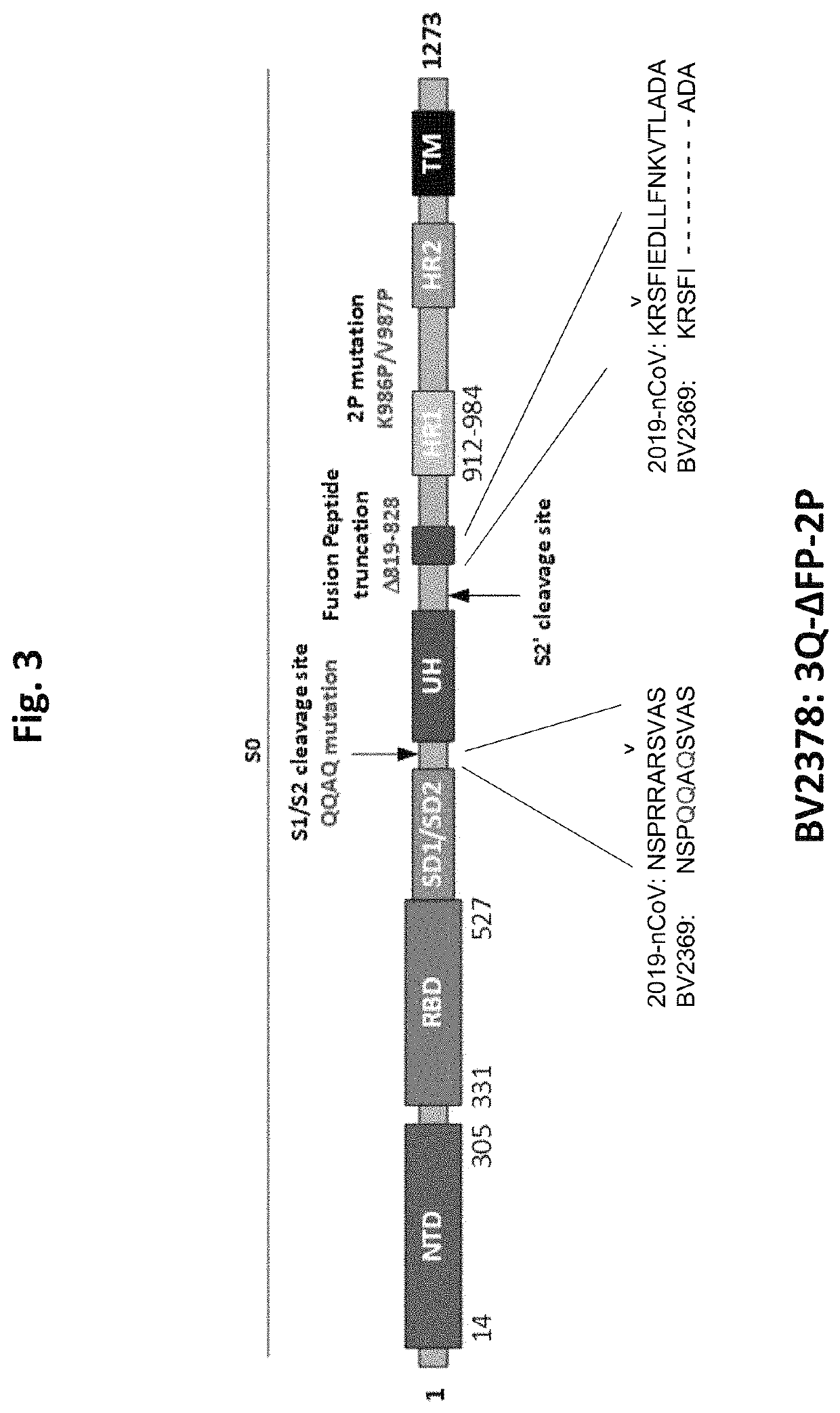
The invention relates to a novel coronavirus vaccine and application thereof, in particular to a truncated Spike protein, a fusion protein containing the truncated Spike protein, a nucleic acid molecule containing a nucleotide sequence encoding the truncated Spike protein or the fusion protein, and a vector and a host cell containing the nucleic acid molecule. The invention further relates to a pharmaceutical composition containing the truncated Spike protein, fusion protein, nucleic acid molecule or vector.
Owner:BEIJING NORTHLAND BIOTECH
New coronavirus vaccine based on chimpanzee adenovirus type 68 and MERS-CoV full length membrane protein
ActiveCN110616198AAvoiding deficiencies of pre-existing immunityRetain high titersSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationCell Membrane Proteins
The present invention discloses a new coronavirus vaccine based on chimpanzee adenovirus type 68 and MERS-CoV full length membrane protein. The recombinant adenovirus is obtained by transfecting an adenovirus packaging cell with a recombinant plasmid and then performing cell culture; the recombinant plasmid is obtained by inserting a specific DNA molecule into a delta-E1 region of a chimpanzee adenovirus vector AdC68; the specific DNA molecule has a full-length MERS-CoV Spike protein encoding gene; and the adenovirus packaging cell has an adenovirus E1 gene. The present invention also protectsthe recombinant adenovirus expressing the full-length MERS-CoV Spike protein; and a starting strain of the recombinant adenovirus is chimpanzee adenovirus type 68 or non-replicating chimpanzee adenovirus type 68. The developed vaccine against the new coronavirus MERS-CoV has important theoretical guidance value and broad application prospects, and provides a possibility for radical cure of MiddleEast respiratory syndrome.
Owner:TSINGHUA UNIV +1
SARS-CoV-2 coronavirus vaccine and preparation method thereof
ActiveCN112618707AMaintain native conformationImprove biological activitySsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationImmunogenicity
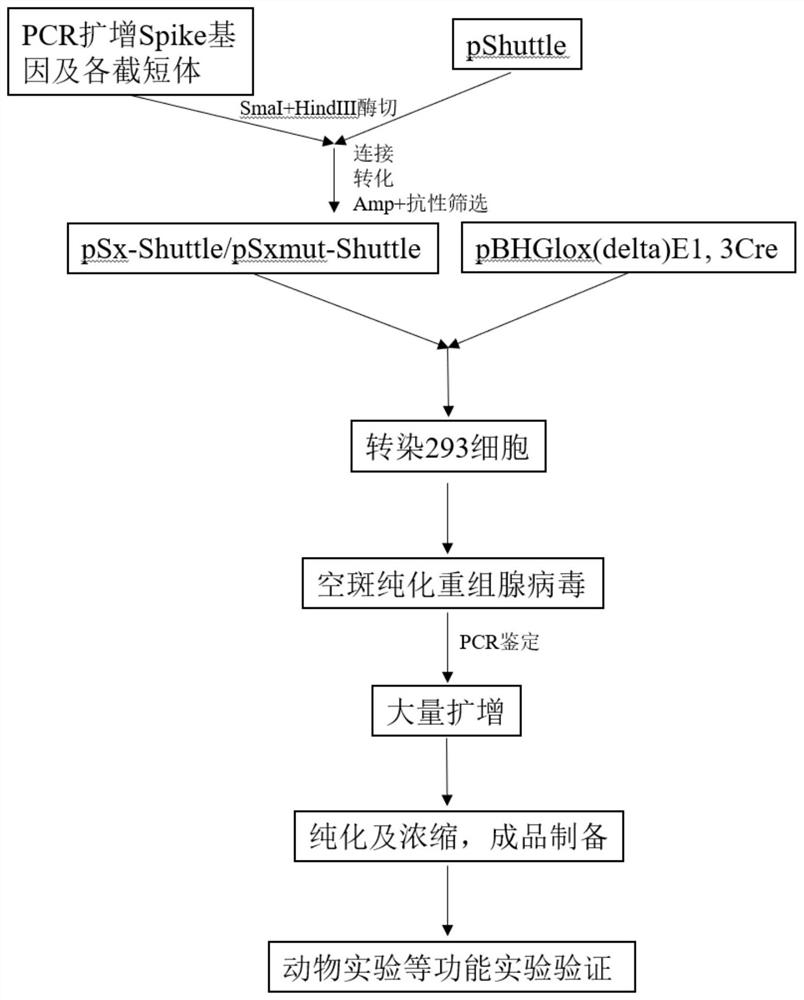
The invention discloses a SARS-CoV-2 coronavirus vaccine and a preparation method thereof. An S gene of a SARS-CoV-2 coronavirus is subjected to codon optimization, truncated body and mutant sequences of the S gene are introduced into a secretory defective adenovirus vector, and packaging is conducted to obtain a corresponding recombinant adenovirus; and the recombinant adenovirus can express SARS-CoV-2 virus related protein in vivo, processing, folding, glycosylation and other modifications are completed, the native conformation of S protein is basically maintained, and the characteristics of high biological activity, long half-life period, lasting immunogenicity and the like are achieved. According to the vaccine and the method, by adopting the defective adenovirus vector carrying secretory peptide, a recombinant adenovirus vaccine can be secreted out of cells after being expressed in vivo, so that the humoral immunity is activated.
Owner:GUANGZHOU DOUBLLE BIOPRODUCT CO LTD
Novel coronavirus vaccine and preparation method and application thereof
ActiveCN112500498AImproving immunogenicityEasy constructionSsRNA viruses positive-senseAntibody mimetics/scaffoldsAdjuvantCoronavirus vaccination
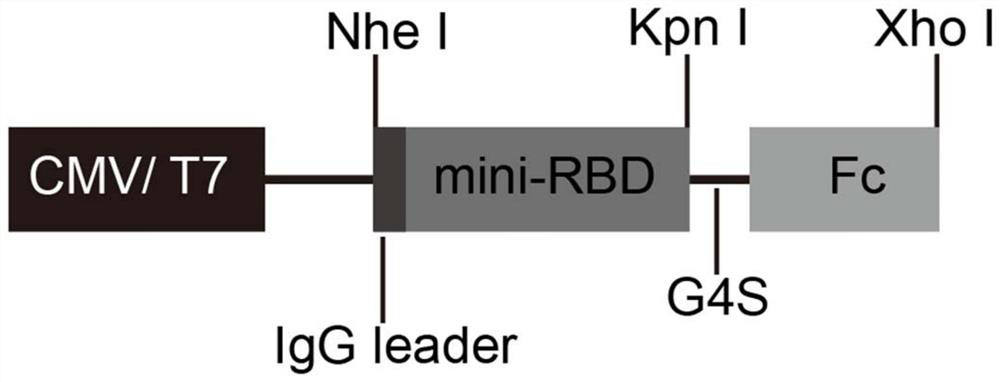
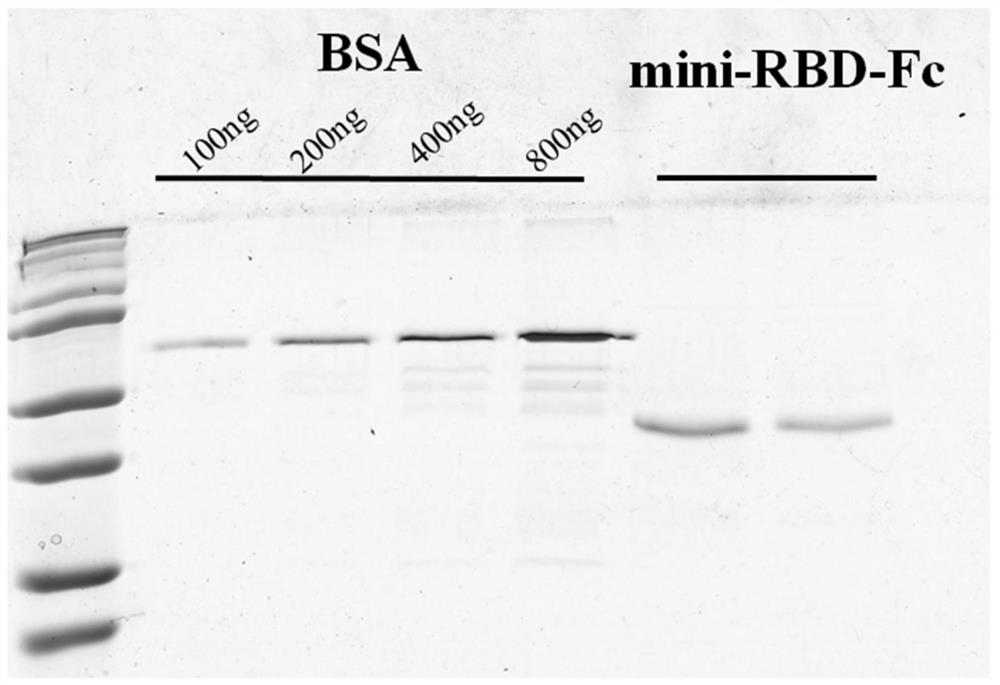
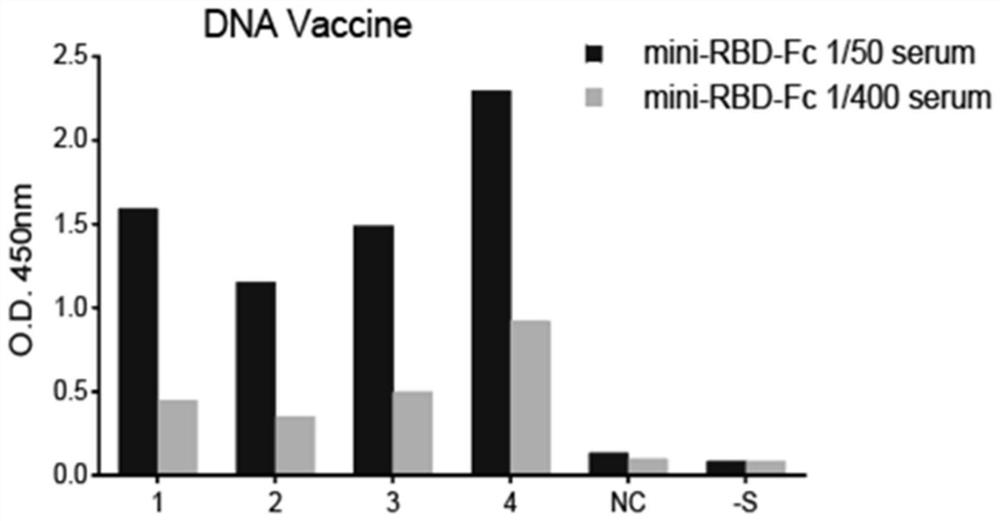
The invention provides a novel coronavirus vaccine and a preparation method and application thereof. The encoding gene of the vaccine comprises a peptide fragment sequence, a Kozak sequence, an IgG signal peptide sequence and a human IgG Fc structural domain sequence, wherein the peptide fragment sequence contains the novel coronavirus and is combined with ACE2 in an S protein receptor binding domain RBD; the Kozak sequence and the IgG signal peptide sequence are positioned at the 5 'end of the peptide fragment sequence combined with ACE2 in the RBD; and the human IgG Fc structural domain sequence is positioned at the 3' end of the peptide fragment sequence combined with ACE2 in the RBD. The novel coronavirus vaccine has the advantages that the 1168 to 1569 sites (containing the peptide fragment combined with ACE2 in the RBD) of the encoding sequence from S protein are used as the gene sequence of antigen protein, and are modified, so that the expression ability, secretion ability and immunogenicity of the antigen protein are improved; the prepared DNA vaccine, protein vaccine and mRNA vaccine are directly injected into a human body or are injected into the human body afterbeing packaged by an adjuvant to express the corresponding antigen, and the body is induced to generate immune response; and the broad application prospect is realized in the fields of COVID-19 prevention and treatment.
Owner:SICHUAN UNIV +1
Coronavirus vaccine
ActiveUS20210379181A1Improve temperature stabilityEasy to degradePowder deliverySsRNA viruses positive-senseDiseaseCoronavirus vaccination
The present invention is directed to a nucleic acid suitable for use in treatment or prophylaxis of an infection with a coronavirus, preferably with a Coronavirus SARS-CoV-2, or a disorder related to such an infection, preferably COVID-19. The present invention is also directed to compositions, polypeptides, and vaccines. The compositions and vaccines preferably comprise at least one of said nucleic acid sequences, preferably nucleic acid sequences in association a lipid nanoparticle (LNP). The invention is also directed to first and second medical uses of the nucleic acid, the composition, the polypeptide, the combination, the vaccine, and the kit, and to methods of treating or preventing a coronavirus infection, preferably a Coronavirus infection.
Owner:CUREVAC SE
Novel coronavirus vaccine based on chimeric virus-like particles
PendingCN111620952AEnhance immune responseGood immune protectionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationReceptor
Owner:SUZHOU MIDI BIOTECH CO LTD
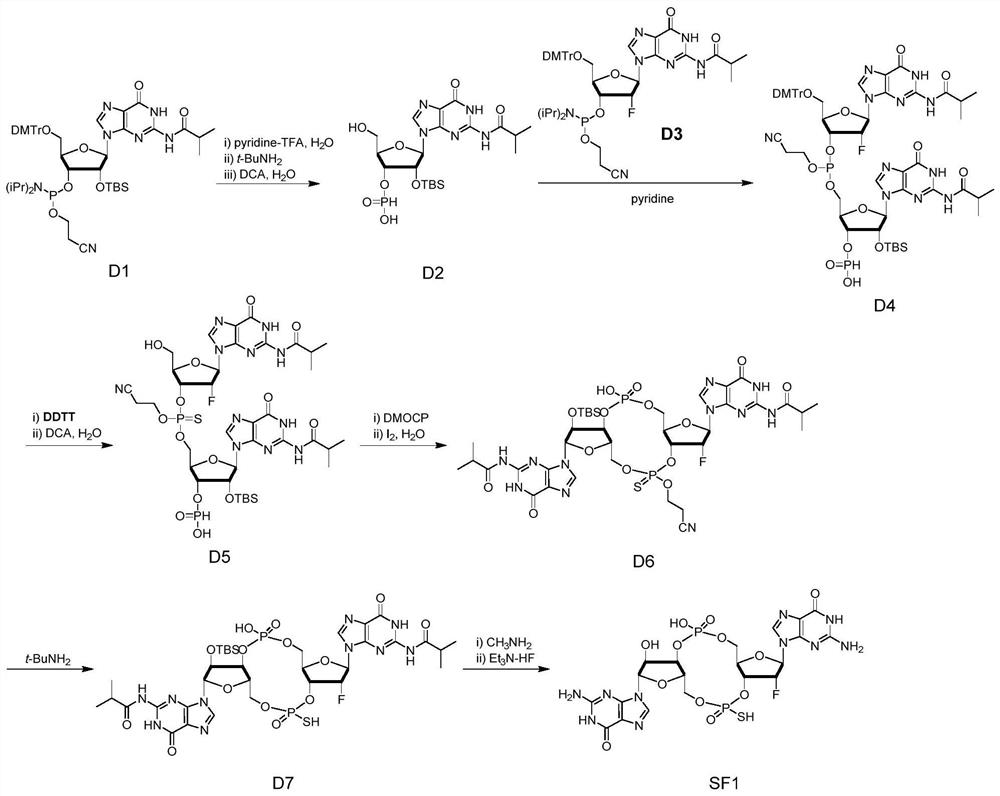
Novel vaccine adjuvant and application thereof in novel coronavirus pneumonia vaccine and other vaccines
ActiveCN111956797ASsRNA viruses positive-senseViral antigen ingredientsAntiendomysial antibodiesCoronavirus vaccination
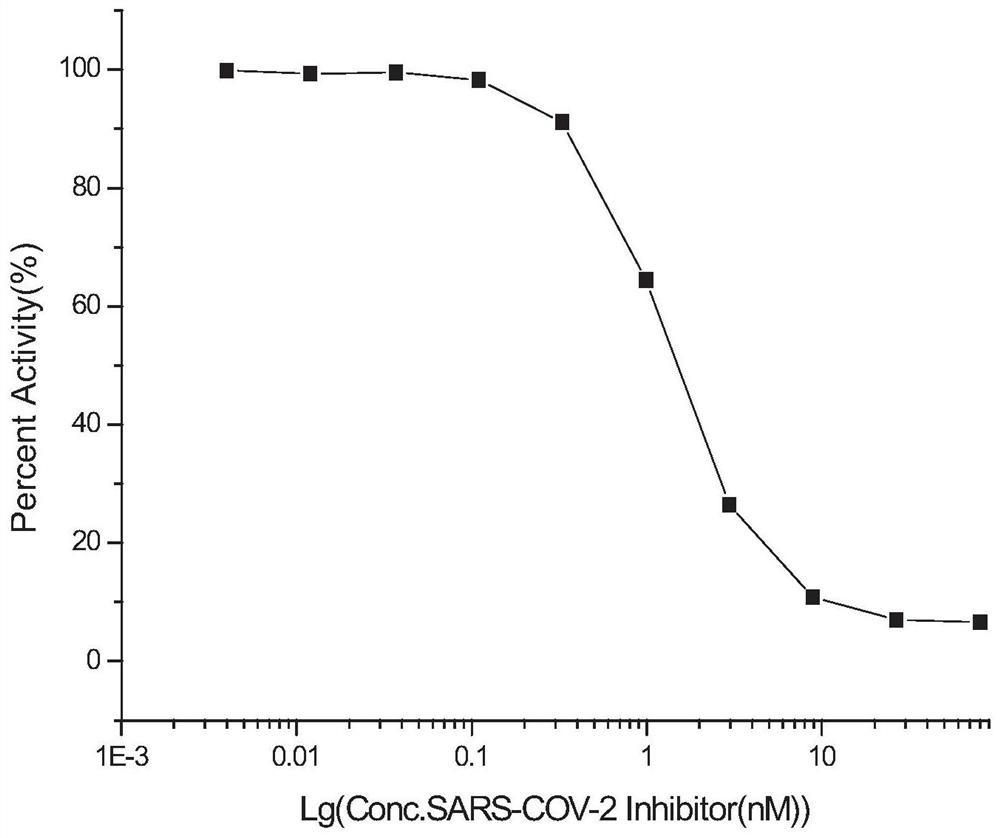
The invention relates to the field of biological medicines, in particular to a novel vaccine adjuvant and an application thereof in a novel coronavirus pneumonia vaccine and other vaccines. Chemicallymodified cyclic dinucleotide, namely an SF compound, is used as the vaccine adjuvant and is used in cooperation with the novel coronavirus vaccine, so that the SARS-CoV-2 virus antigen specific antibody titer and the generation of T cells can be remarkably improved; and the SF compound as the vaccine adjuvant is obviously superior to an aluminum adjuvant in immunopotentiation effect.
Owner:TSINGHUA UNIV
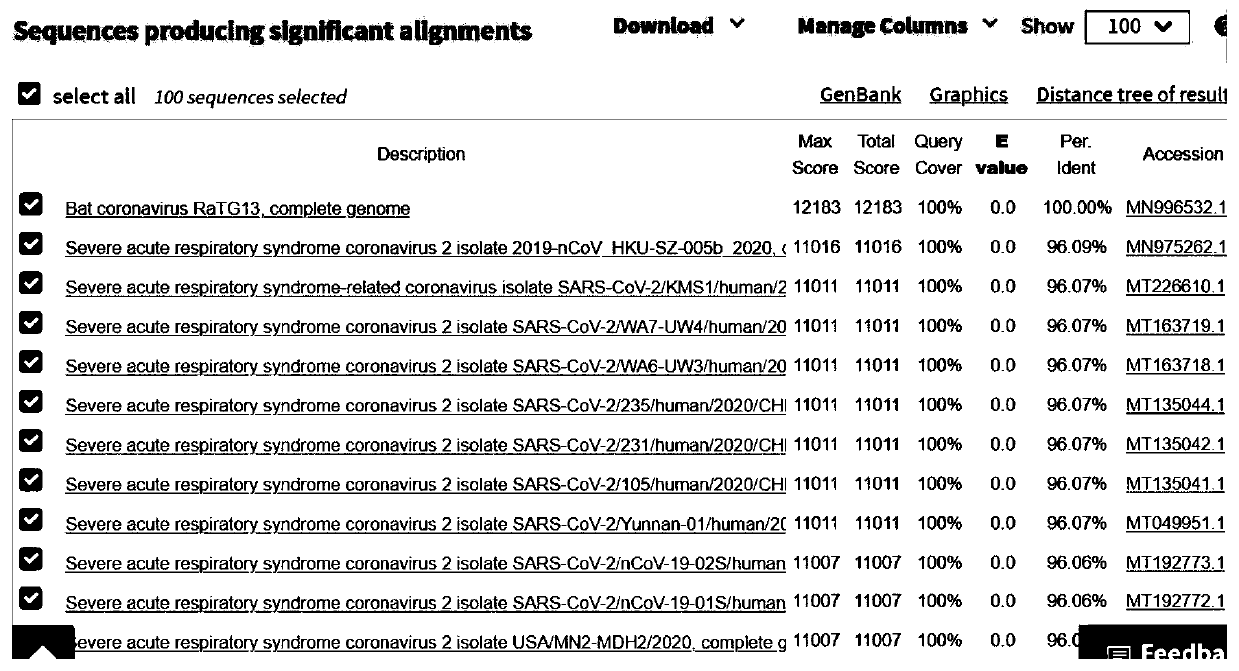
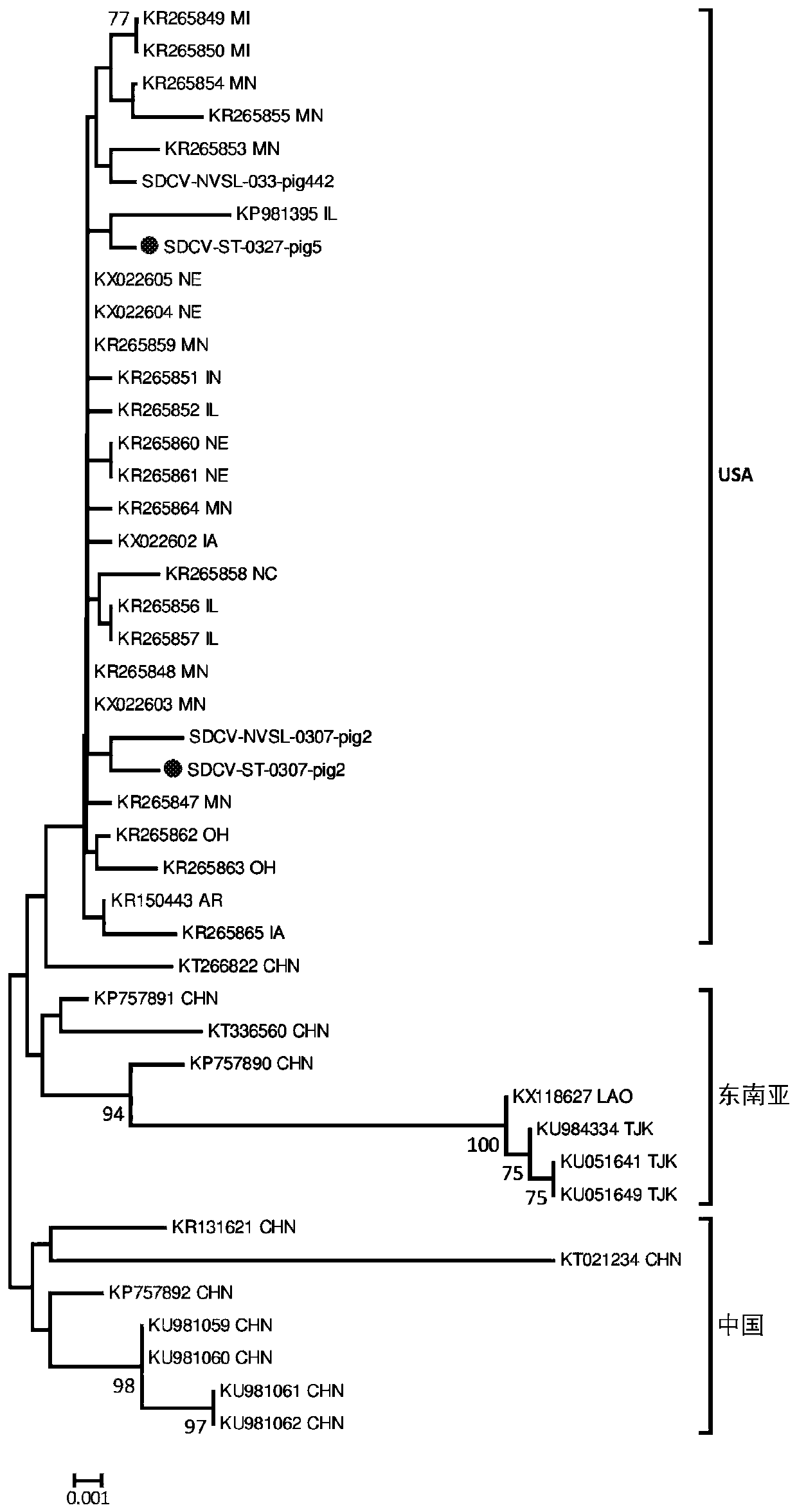
Bat-derived coronavirus vaccine for preventing COVID-19
PendingCN111437384ASsRNA viruses positive-senseViral antigen ingredientsDiseaseCoronavirus vaccination
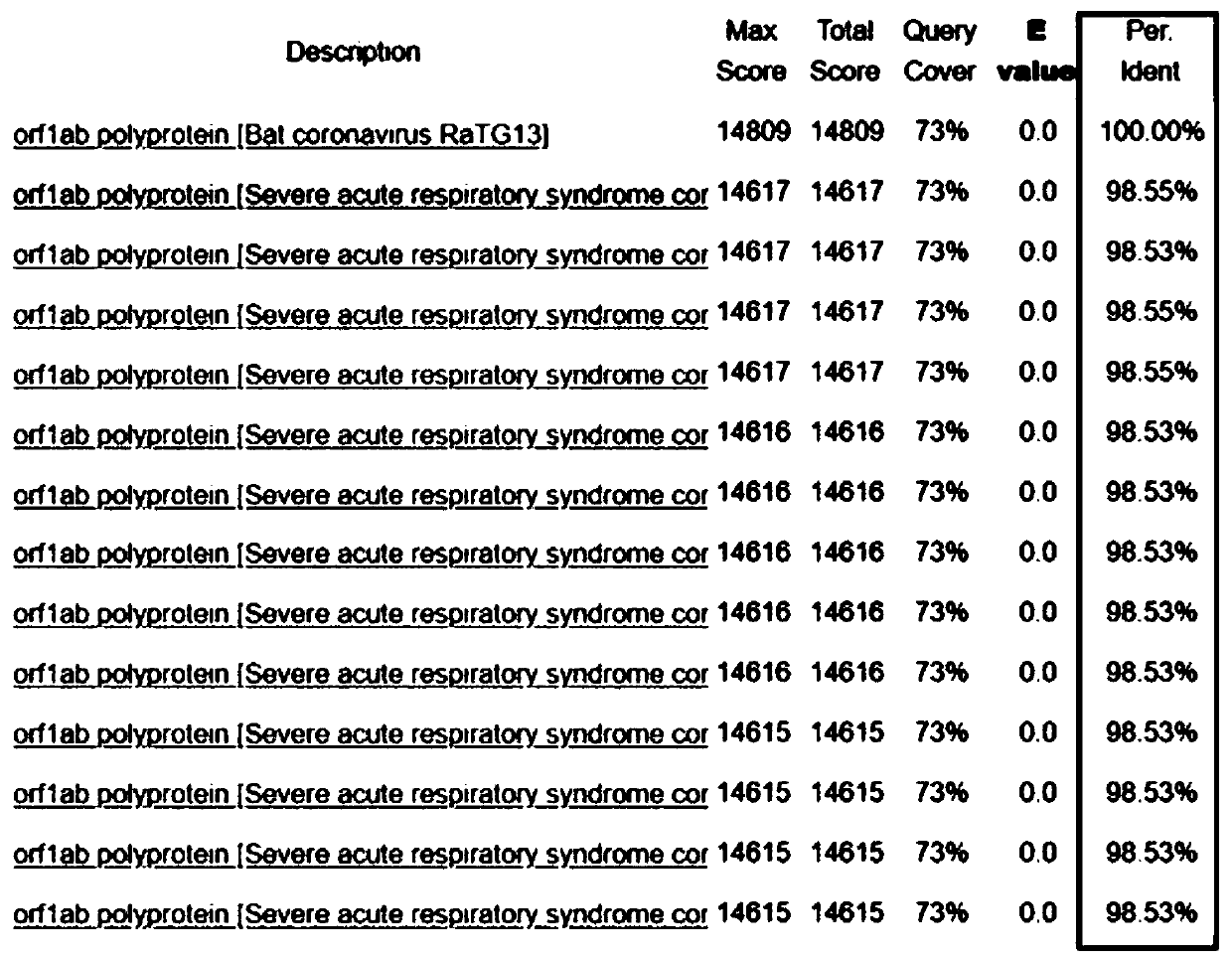
The invention discloses a bat-derived coronavirus vaccine for preventing COVID-19. The bat-derived coronavirus Bat / CovRaTG13 is adopted to produce the vaccine so as to control and prevent the ongoingCOVID-19 pandemic and the future epidemic of the disease. The genetic fingerprint of the Bat / CovRaTG13 is closest to the genetic fingerprint of the 2019 new coronavirus (SARS-CoV-2). Homology of all amino acid sequences not only determines the genetic relationship, but also determines the similarity of biological characteristics, and antigenicity is one of main factors for determining specificityand effectiveness of the vaccine. The vaccine strain comes from a Bat / CovRaTG13 family member, and has sequence homology. The bat coronavirus-derived vaccine can be prepared into three types of vaccines, namely a live vaccine, an inactivated vaccine and / or a recombinant vaccine, through corresponding construction and manufacturing method procedures so as to prevent COVID-19 pandemic.
Owner:SICHUAN CHENGYU BIOLOGICAL PROD INC
Gene of novel coronavirus B.1.351 South African mutant strain RBD and application of gene
ActiveCN112980852AIncreased clone expression efficiencySsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationNucleotide
The invention belongs to the technical field of biology, and particularly relates to a gene of a novel coronavirus B.1.351 South African mutant strain RBD and application of the gene. The nucleotide sequence of the gene of the novel coronavirus B.1.351 South African mutant strain RBD provided by the invention is shown as SEQ ID NO.1 or SEQ ID NO.6. The gene sequence of the wild type novel coronavirus South African B.1.351 South African mutant strain RBD is optimized, a relatively optimal sequence is determined by combining with screening, and the cloning expression efficiency generated by the optimized sequence is greatly improved as compared with the expression efficiency of the wild type novel coronavirus B.1.351 South African mutant strain RBD sequence, so that the gene of the novel coronavirus B.1.351 South African mutant strain RBD can be used for preparing a novel coronavirus vaccine.
Owner:BIO-BANK CORP
Coronavirus vaccine formulations
ActiveUS20210228709A1SsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationNanoparticle
Disclosed herein are coronavirus Spike (S) proteins and nanoparticles comprising the same, which are suitable for use in vaccines. The nanoparticles present antigens from pathogens surrounded to and associated with a detergent core resulting in enhanced stability and good immunogenicity. Dosages, formulations, and methods for preparing the vaccines and nanoparticles are also disclosed.
Owner:NOVAVAX
Novel coronavirus SARS-CoV-2 mRNA vaccines and preparation method and application thereof
ActiveCN113151312AProlong half-lifeEasy to getSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationSpecific igg
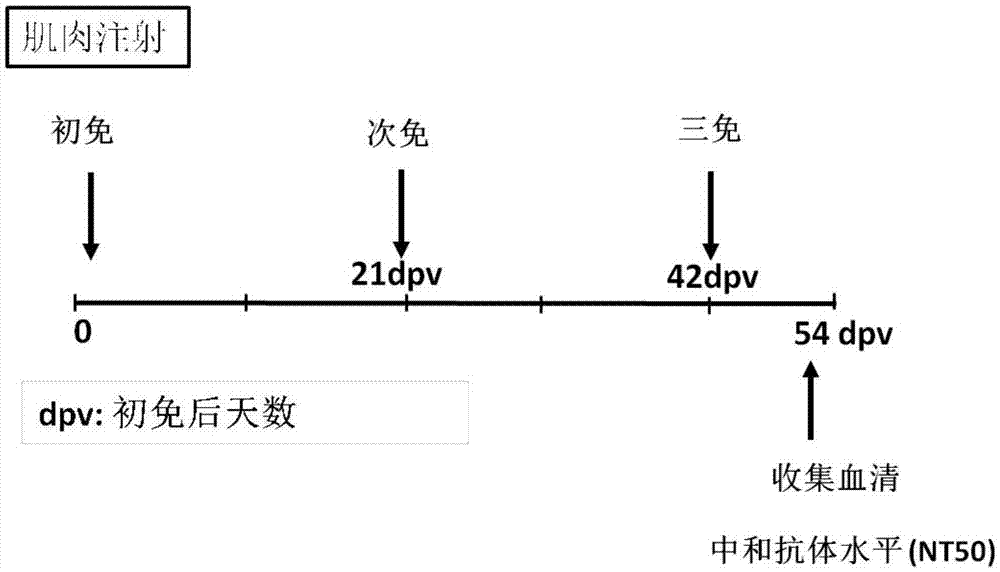
The invention provides novel coronavirus SARS-CoV-2mRNA vaccines and a preparation method and application thereof. The invention provides three mRNA vaccines, namely RBD, S1 and S vaccines. The RBD vaccine disclosed by the invention can induce a high-titer antigen-specific IgG antibody and a virus neutralization antibody after immunization with one dose, the high-titer neutralization antibody can be maintained for at least 26 weeks, and remarkable immune protection can be provided for human ACE2 transgenic mice in serum adoptive transfer protection experiments. The RBD and S vaccine disclosed by the invention can induce immune protection capable of completely resisting SARS-CoV-2 virus infection in the human ACE2 transgenic mice after immunization with two doses. A large number of experimental results show that the mRNA vaccine provided by the invention has good immunogenicity, forms powerful immune protection after immunizing an organism, and has a huge development potential.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Live attenuated Coronavirus vaccines
The present invention is directed live, attenuated coronavirus vaccines. The vaccine comprises a viral genome encoding a p59 protein having at mutation at a specific tyrosine residue, and may include other attenuating mutations. Such viruses show reduced growth and pathogenicity in vivo.
Owner:VANDERBILT UNIV
Coronavirus vaccine formulations
ActiveUS20210228708A1SsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationNanoparticle
Disclosed herein are coronavirus Spike (S) proteins and nanoparticles comprising the same, which are suitable for use in vaccines. The nanoparticles present antigens from pathogens surrounded to and associated with a detergent core resulting in enhanced stability and good immunogenicity. Dosages, formulations, and methods for preparing the vaccines and nanoparticles are also disclosed.
Owner:NOVAVAX
Live attenuated coronavirus vaccines
The present invention is directed live, attenuated coronavirus vaccines. The vaccine comprises a viral genome encoding a p59 protein having at mutation at a specific tyrosine residue, and may include other attenuating mutations. Such viruses show reduced growth and pathogenicity in vivo.
Owner:VANDERBILT UNIV
Recombinant virus and use thereof
InactiveUS20090214587A1Highly safe in preventing SARS infection and onsetSsRNA viruses positive-senseVectorsSARS coronavirusCoronavirus vaccination
The present invention provides a recombinant virus which is efficacious and highly safe in preventing the onset of SARS infection and a vaccine for SARS coronavirus containing the same. The recombinant virus of the invention can express a SARS coronavirus gene.
Owner:POST GENOME INST CO LTD
Porcine coronavirus vaccines
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Recombinant Newcastle disease virus vector novel coronavirus vaccine candidate strain as well as construction method and application thereof
PendingCN112011521AImprove growth characteristicsHas immune effectSsRNA viruses negative-senseSsRNA viruses positive-senseCoronavirus vaccinationNucleotide
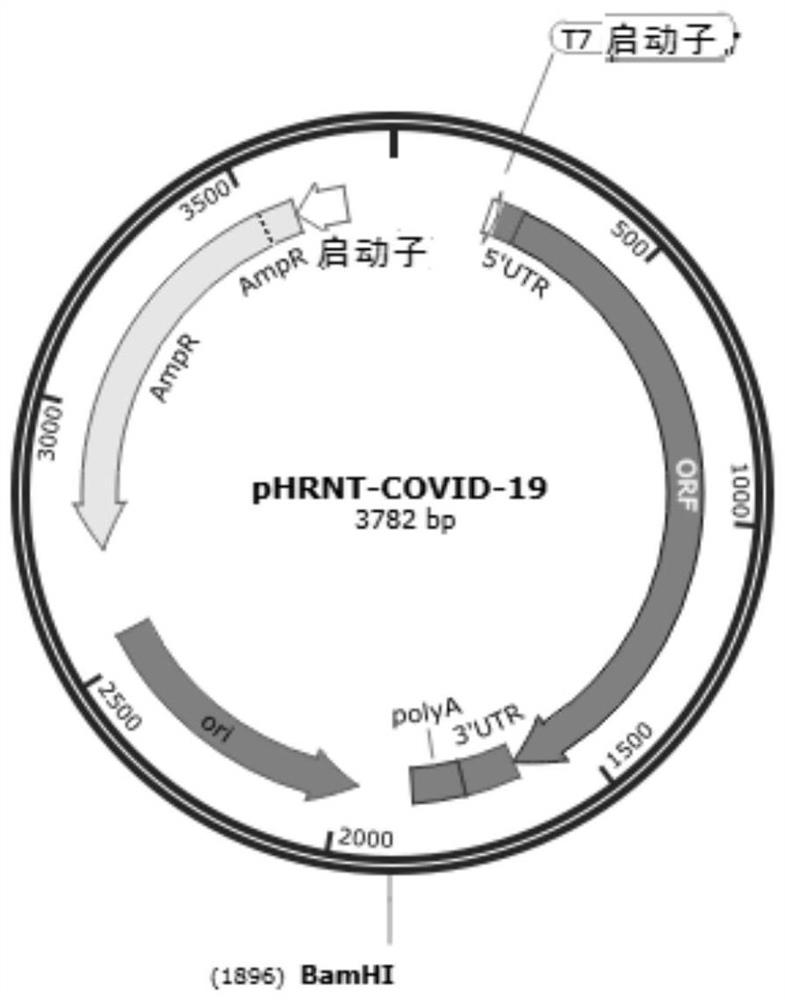
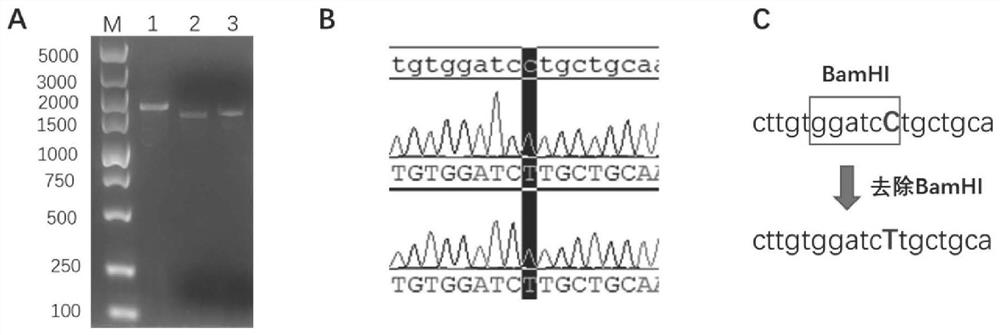
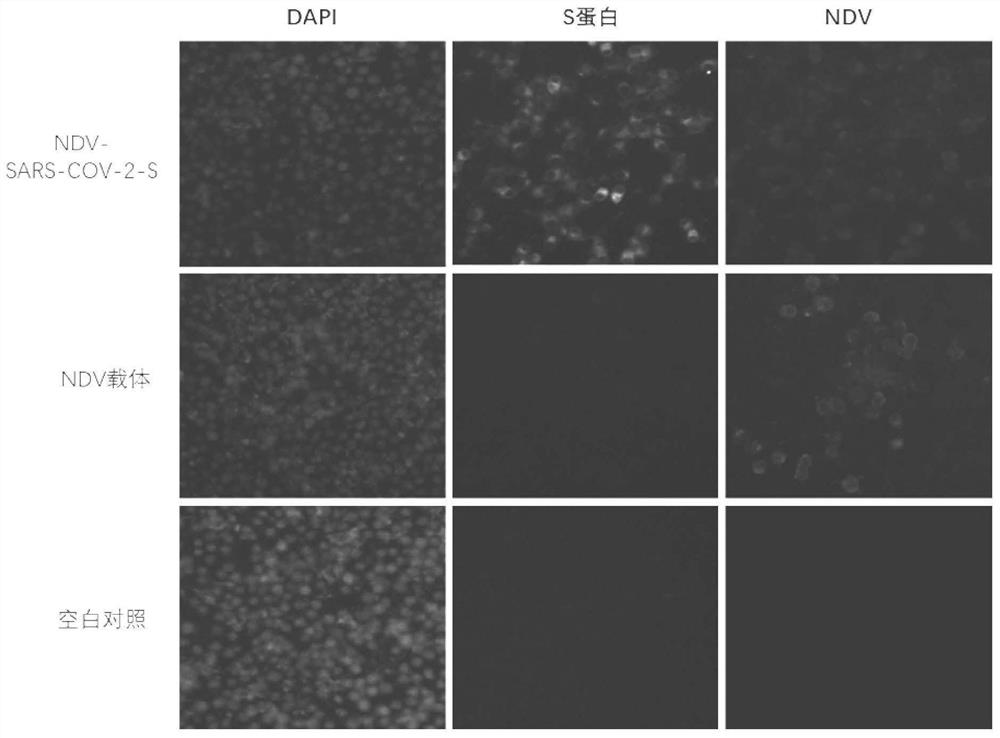
The invention relates to a recombinant Newcastle disease virus vector novel coronavirus vaccine candidate strain as well as a construction method and application thereof, and belongs to the technicalfield of genetic engineering vaccines. According to the vaccine candidate strain disclosed by the invention, a Newcastle disease virus LaSota strain is used as a carrier, and a mutated novel coronavirus S gene (C3756T, BamHI site is removed) is inserted between a P gene and an M gene of the Newcastle disease virus LaSota strain; and the nucleotide sequence of the mutated novel coronavirus S gene is shown as SEQ ID NO. 1. The recombinant Newcastle disease virus vector novel coronavirus vaccine candidate strain provided by the invention is safe, effective and low in production cost, can stimulate an organism to generate mucosal immunity through nasal cavity inoculation, and has important application value and outstanding public health safety significance.
Owner:ZHEJIAN DIFFERENCE BIOLOGICAL TECH CO LTD
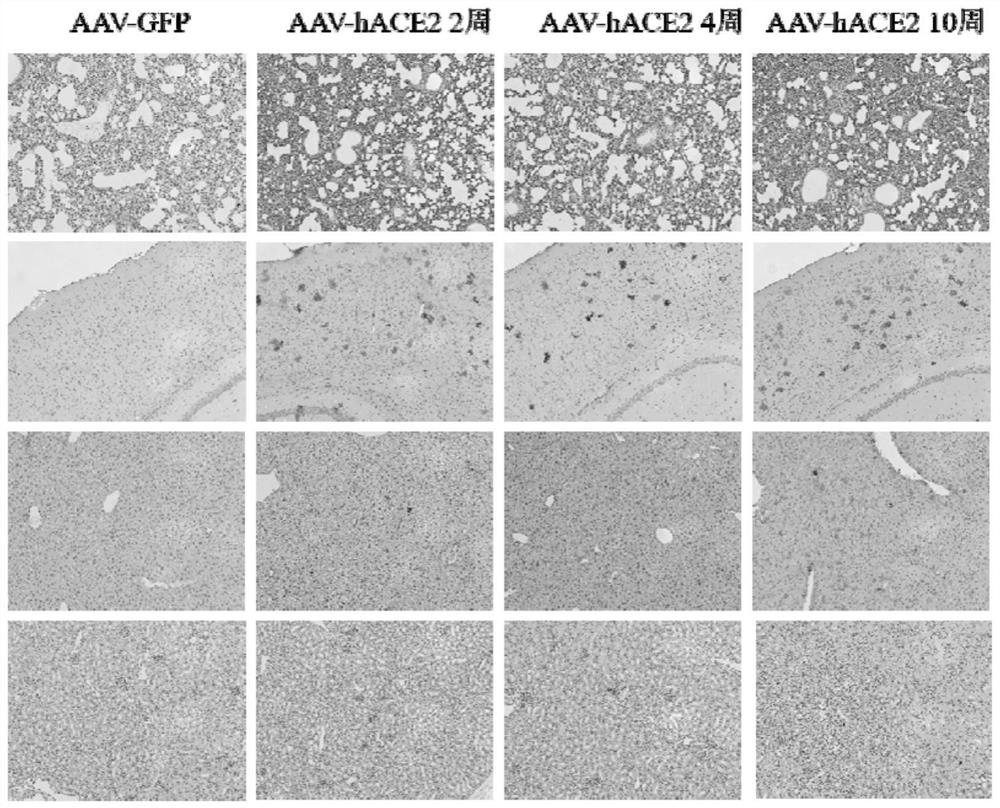
Animal model for expressing human ACE2 and application of animal model
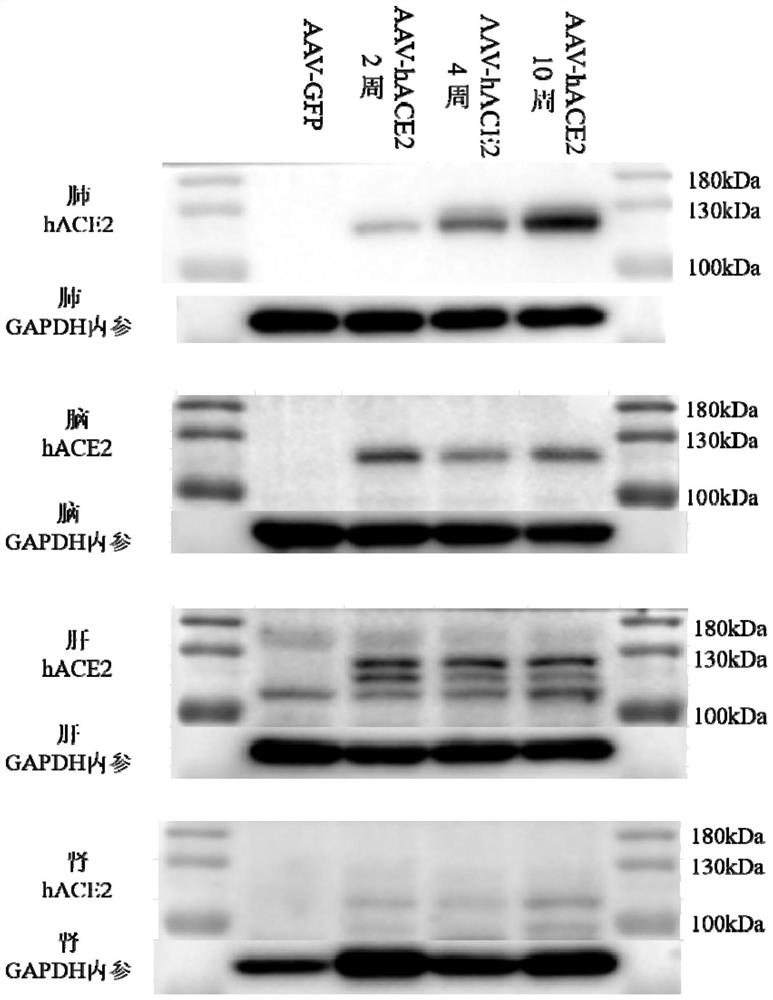
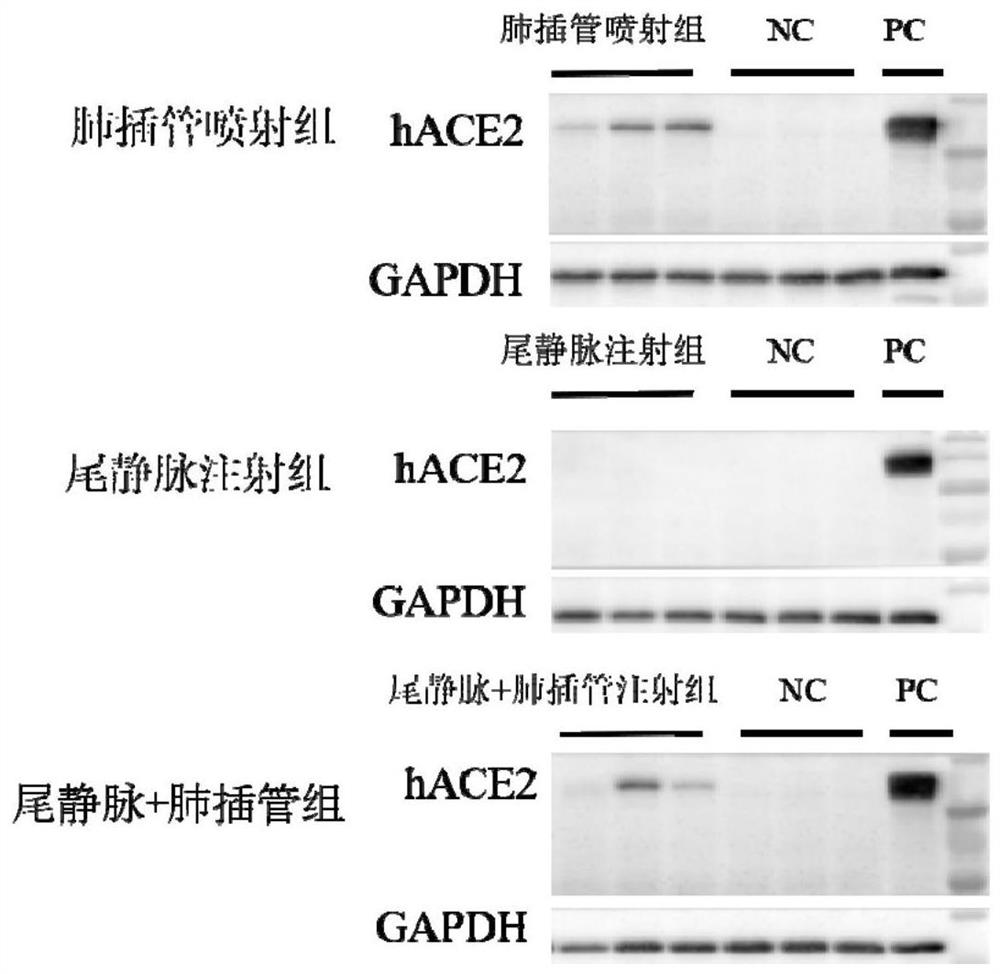
ActiveCN112680466AFast modelingModeling can be scaledViruses/bacteriophagesIn-vivo testing preparationsCoronavirus vaccinationTGE VACCINE
The invention discloses an animal model for expressing human ACE2 and application of the animal model. A mouse AC E2 gene signal peptide sequence and a human ACE2 gene mature peptide sequence are combined and subjected to codon optimization and amplification to obtain an hACE2 sequence, and a recombinant adeno-associated virus AAV-hACE2 is further prepared; and recombinant adeno-associated virus AAV-hACE2 is sprayed to the lung of the mouse by intubation for at least two weeks to obtain a mouse model with lung and brain tissue highly expressing human ACE2. According to the invention, the modeling speed for constructing the novel coronapneumonia virus susceptible mouse model is relatively high, and modeling can be succeeded only in two weeks. The animal model fully reveals pathological characteristics of AAV-hACE2 mice infected with novel coronavirus and application of the AAV-hACE2 mice in novel coronavirus vaccines, so that the model is clear to be suitable for pharmacodynamic evaluation of the novel coronavirus vaccines.
Owner:GUANGZHOU PACKGENE BIOTECH CO LTD +1
Coronavirus vaccine
ActiveUS20220218815A1Improve temperature stabilityPowder deliverySsRNA viruses positive-senseDiseaseCoronavirus vaccination
The present invention is directed to a nucleic acid suitable for use in treatment or prophylaxis of an infection with a coronavirus, preferably with a Coronavirus SARS-CoV-2, or a disorder related to such an infection, preferably COVID-19. The present invention is also directed to compositions, polypeptides, and vaccines. The compositions and vaccines preferably comprise at least one of said nucleic acid sequences, preferably nucleic acid sequences in association a lipid nanoparticle (LNP). The invention is also directed to first and second medical uses of the nucleic acid, the composition, the polypeptide, the combination, the vaccine, and the kit, and to methods of treating or preventing a coronavirus infection, preferably a Coronavirus infection.
Owner:CUREVAC SE
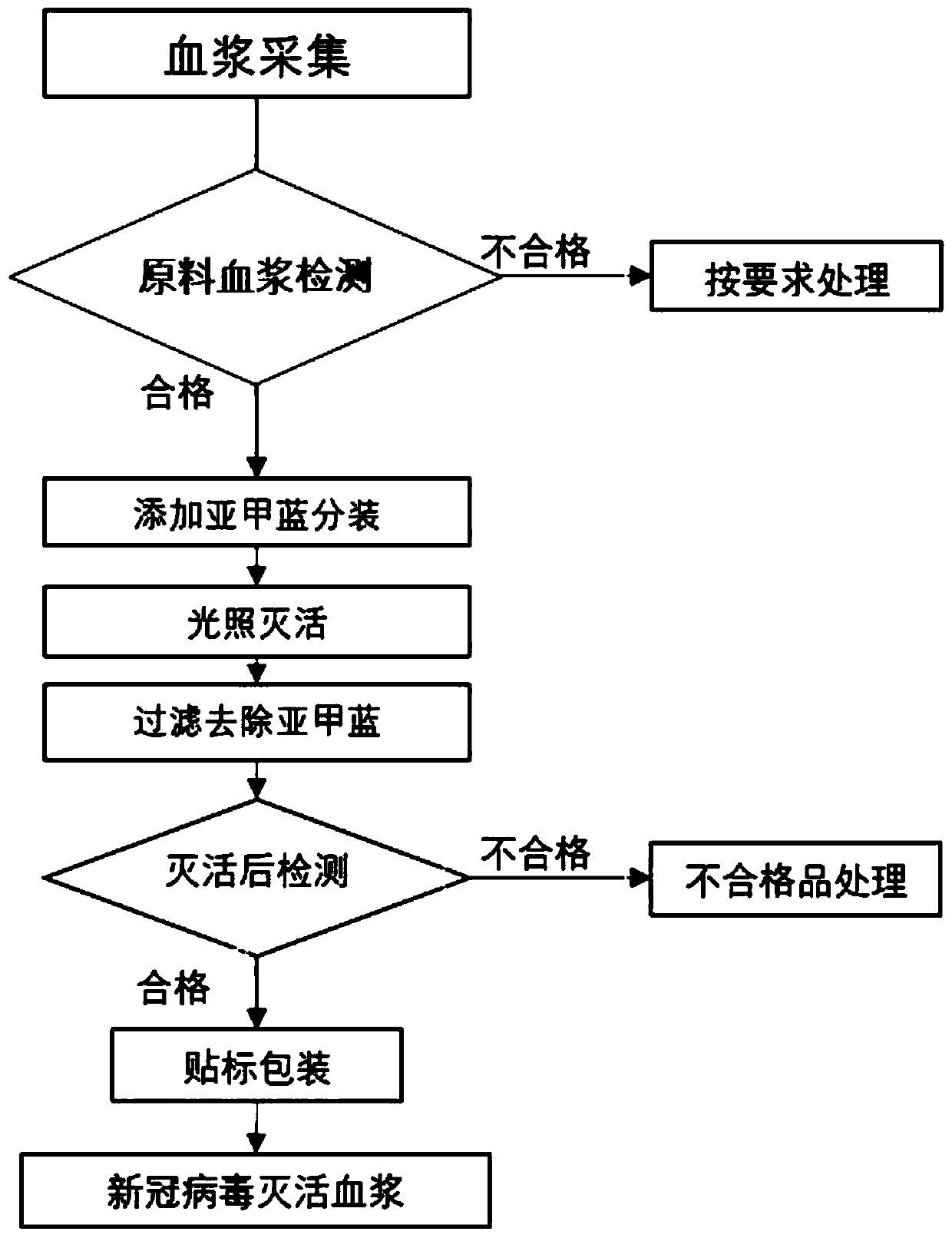
Production method of virus-inactivated plasma for treating COVID-19
InactiveCN111346108AQuick Preparation and AcquisitionHigh cure rateMammal material medical ingredientsAntiviralsVirus inactivationCoronavirus vaccination
The invention relates to a production method of virus-inactivated plasma for treating COVID-19. The method comprises the following steps: (1) collecting convalescent plasma of COVID-19 survivors or plasma immune to a Severe Acute Respiratory Syndrome Coronavirus 2 vaccine (SARS-CoV-2 vaccine), and conducting pretreatment; (2) adding methylene blue, and conducting photo-inactivation on the plasma;and (3) conducting labeling and packaging after the plasma passes tests.
Owner:国药集团武汉血液制品有限公司
Anti-novel coronavirus antibody and preparation method and application thereof
ActiveCN111548413ALarge range of protectionDoes not damage the ecological environmentEgg immunoglobulinsImmunoglobulins against virusesAntiendomysial antibodiesCoronavirus vaccination
The invention discloses an anti-novel coronavirus antibody. The antibody is a high-titer specific antibody extracted from chicken egg yolk, the chicken egg yolk is mainly obtained from eggs laid by immunized hens, and the immunized hens are healthy hens injected with novel coronavirus vaccines. The invention also discloses an adjuvant drug for preventing and controlling the novel coronavirus, which takes the antibody for resisting the novel coronavirus as a main component. The drug exerts the characteristics of the antibody for resisting the novel coronavirus, can be specifically combined withthe novel coronavirus of the oropharynx and the nasal cavity in a short time, is long in retention time, painless, non-irritant, good in safety performance and free of toxic and side effects, can beused for prevention and blocking of novel coronavirus infection and adjuvant therapy of early infection, and can be used for group virus prevention and control adjuvant medication.
Owner:CHENGDU YUKANG BIOTECHNOLOGY CO LTD
Truncated body based on novel coronavirus RBD-SD1 protein and application of truncated body
InactiveCN112094327AAvoid infectionStrong immune responseFungiSsRNA viruses positive-senseCoronavirus vaccinationNeutralising antibody
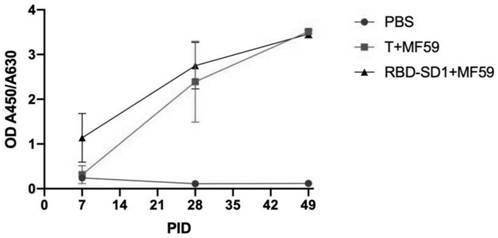
The invention relates to the field of biological medicine, in particular to a truncated body based on novel coronavirus RBD-SD1 protein and application of the truncated body. The protein truncated body disclosed by the invention has a sequence shown as follows: (I) an amino acid sequence shown as SEQ ID No. 1; and / or (II) an amino acid sequence which is obtained by substituting, deleting or addingone or more amino acids to the amino acid sequence shown in (I) and has the same function as the amino acid sequence shown in (I); and / or (III) an amino acid sequence having 80% or more identity withthe amino acid sequence shown in (I) or (II). The protein truncated body can induce relatively strong humoral immune response, has good immunogenicity, and can be applied to development of novel coronavirus vaccines and preparation of neutralizing antibodies.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE +1
Nucleic acid molecule encoding structural protein of novel coronavirus and novel coronavirus vaccine
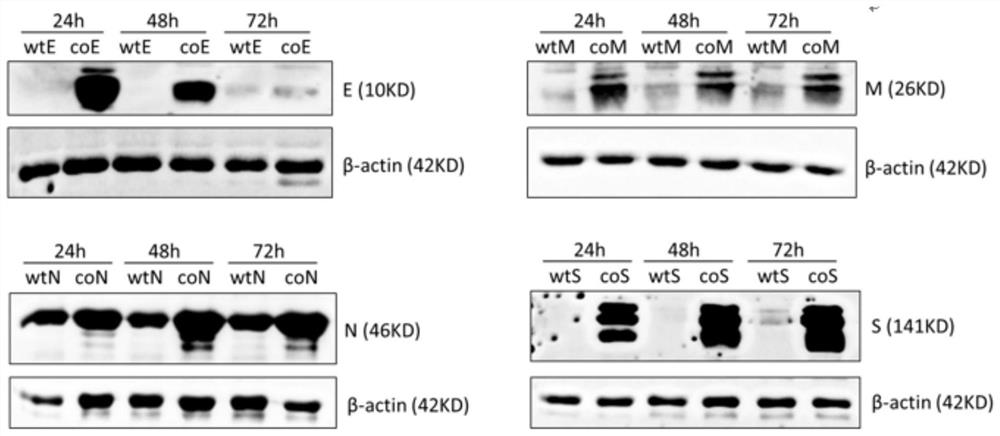
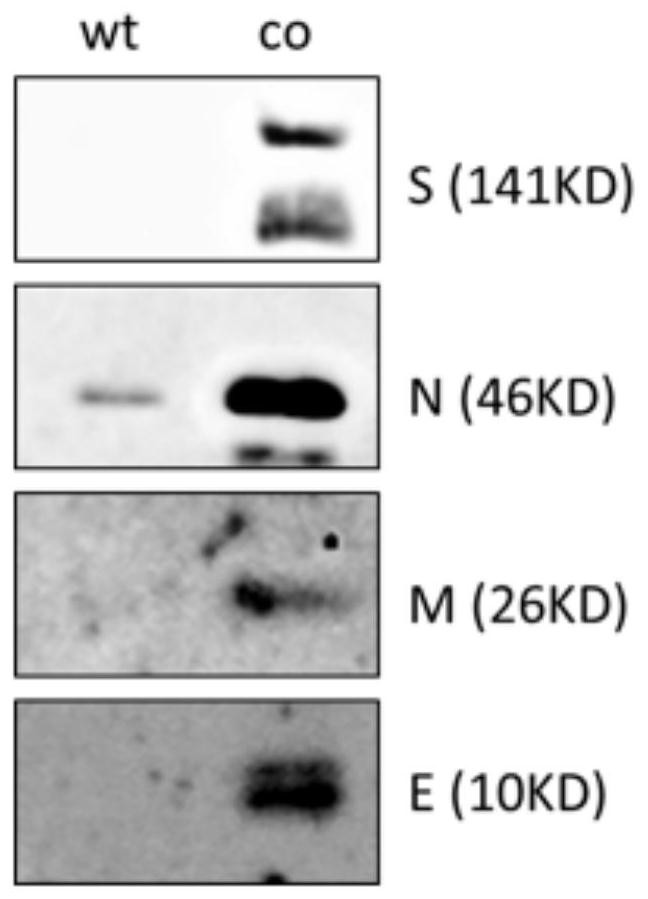
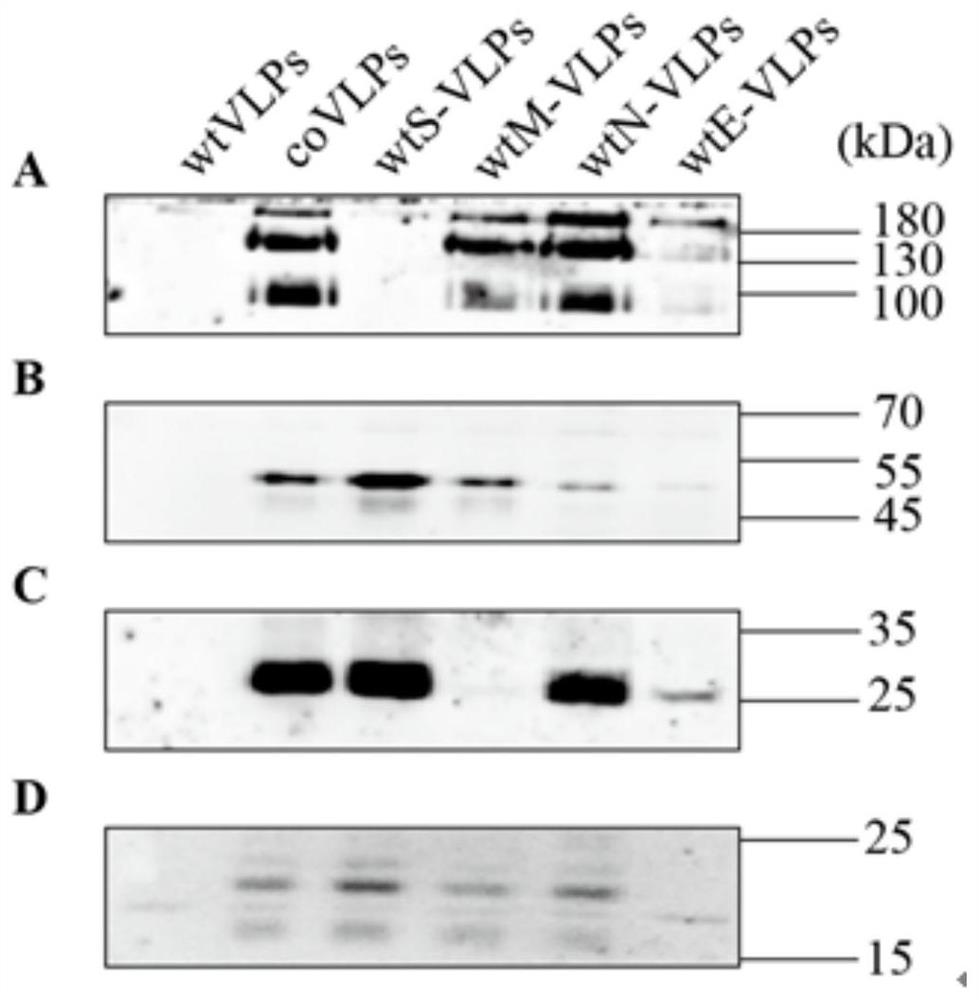
ActiveCN112575008AFast preparationMass preparationSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationStructural protein
The invention discloses a nucleic acid molecule for encoding a structural protein of a novel coronavirus and a novel coronavirus vaccine, and relates to the technical field of gene engineering. The base sequence of the nucleic acid molecule disclosed by the invention is as shown in any one of SEQ ID NO.1 to SEQ ID NO.4. The invention also discloses a preparation method of the nucleic acid molecule. The nucleic acid molecule provided by the invention can express the structural protein of the novel coronavirus more efficiently through codon optimization, and lays a foundation for development ofnovel coronapneumonia vaccines.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Kit for detecting new coronavirus neutralizing antibody and detection method
ActiveCN112710845AEasy to operateImprove accuracyBiological testingImmunoassaysDisulfide bondingCoronavirus vaccination
The invention relates to the field of antibody detection, in particular to a kit for detecting a new coronavirus neutralizing antibody and a detection method. The kit comprises a competition method kit and a double-sandwich method kit. The competition method kit comprises a detection card containing a recombinant S-RBD-C antigen marked by up-conversion luminescent particles, the double-sandwich method kit comprises a detection card containing a recombinant S-RBD-C antigen marked by up-conversion luminescent particles or an S1 antigen marked by up-conversion luminescent particles, and the recombinant S-RBD-C antigen contains at least one segment of additional amino acid sequence, and is used for forming disulfide bonds with free cysteine in the S-RBD antigen. The neutralizing antibody in a subject inoculated with the novel coronavirus vaccine or a human body infected with the novel coronavirus can be detected, quantitative evaluation can be carried out, the operation is simple, and the accuracy is high.
Owner:BEIJING HOTGEN BIOTECH CO LTD +1
A recombinant novel coronavirus vaccine based on human replication-defective adenovirus
ActiveCN111218459BReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
The invention provides a novel coronavirus vaccine using human type 5 replication-deficient adenovirus as a carrier. The vaccine uses a replication-defective human type 5 adenovirus with combined deletion of E1 and E3 as the carrier, HEK293 cells integrated with the adenovirus E1 gene as the packaging cell line, and the protective antigen gene carried is an optimized 2019 novel coronavirus (SARS‑CoV‑2) S protein gene (Ad5‑nCoV). After the S protein gene was optimized, the expression level in the transfected cells was significantly increased. The vaccine has good immunogenicity in both mouse and guinea pig models, and can induce strong cellular and humoral immune responses in the body in a short time. The protective effect study on hACE2 transgenic mice showed that a single immunization with Ad5-nCoV can significantly reduce the viral load in the lung tissue after 14 days, indicating that the vaccine has a good immune protective effect against 2019-nCoV. In addition, the preparation of the vaccine is quick and easy, and large-scale production can be realized in a short period of time for emergency outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com