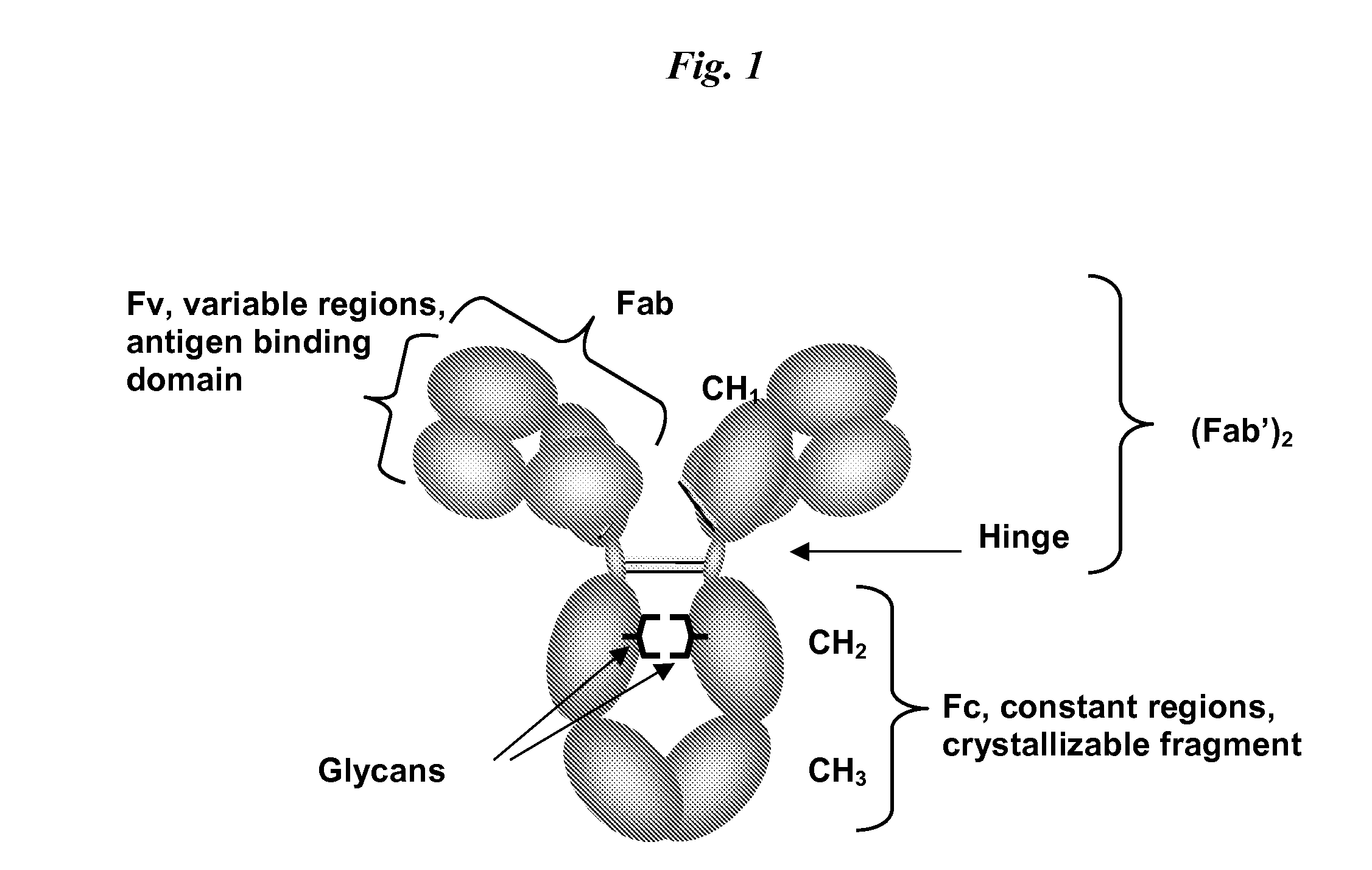
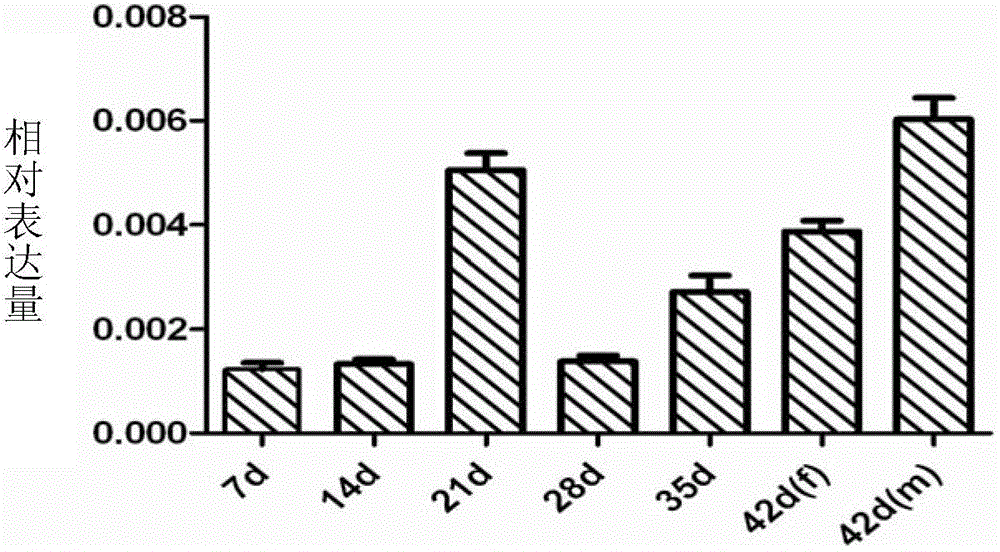
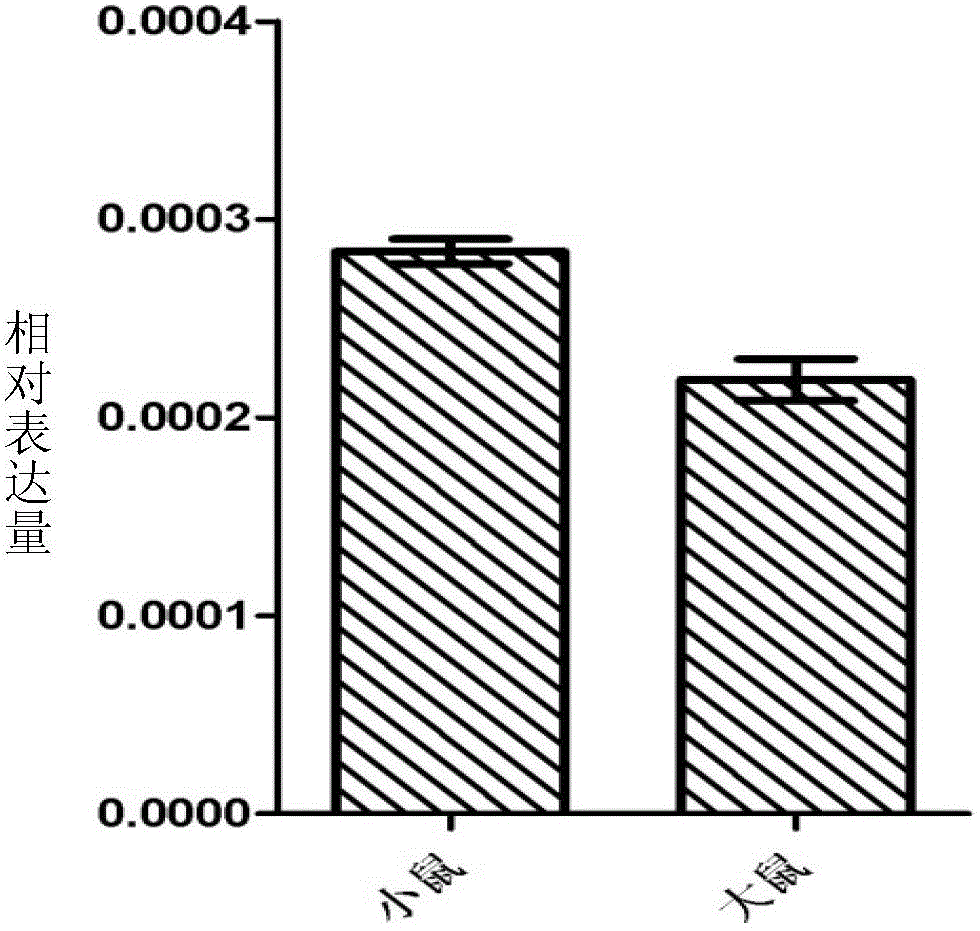
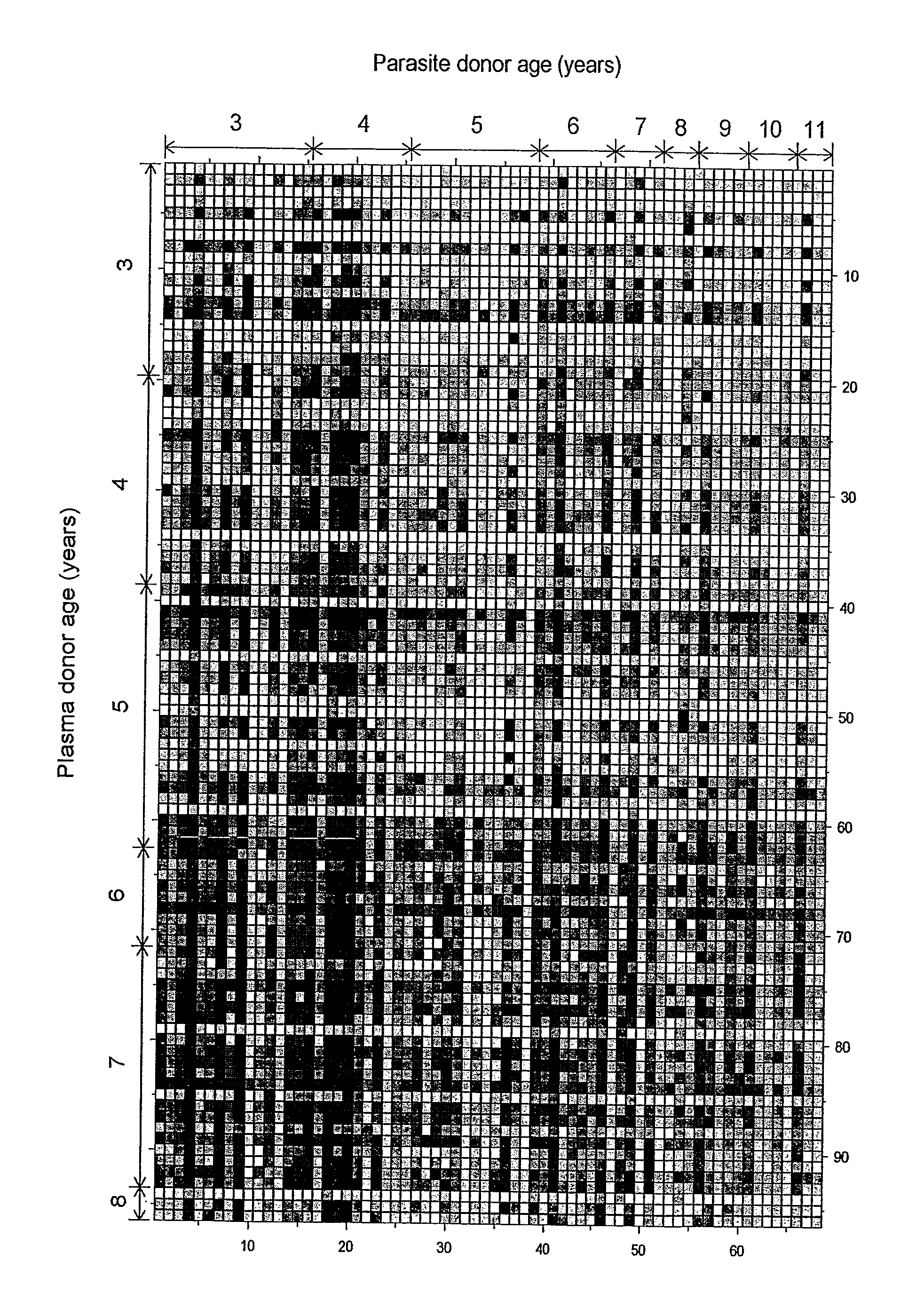
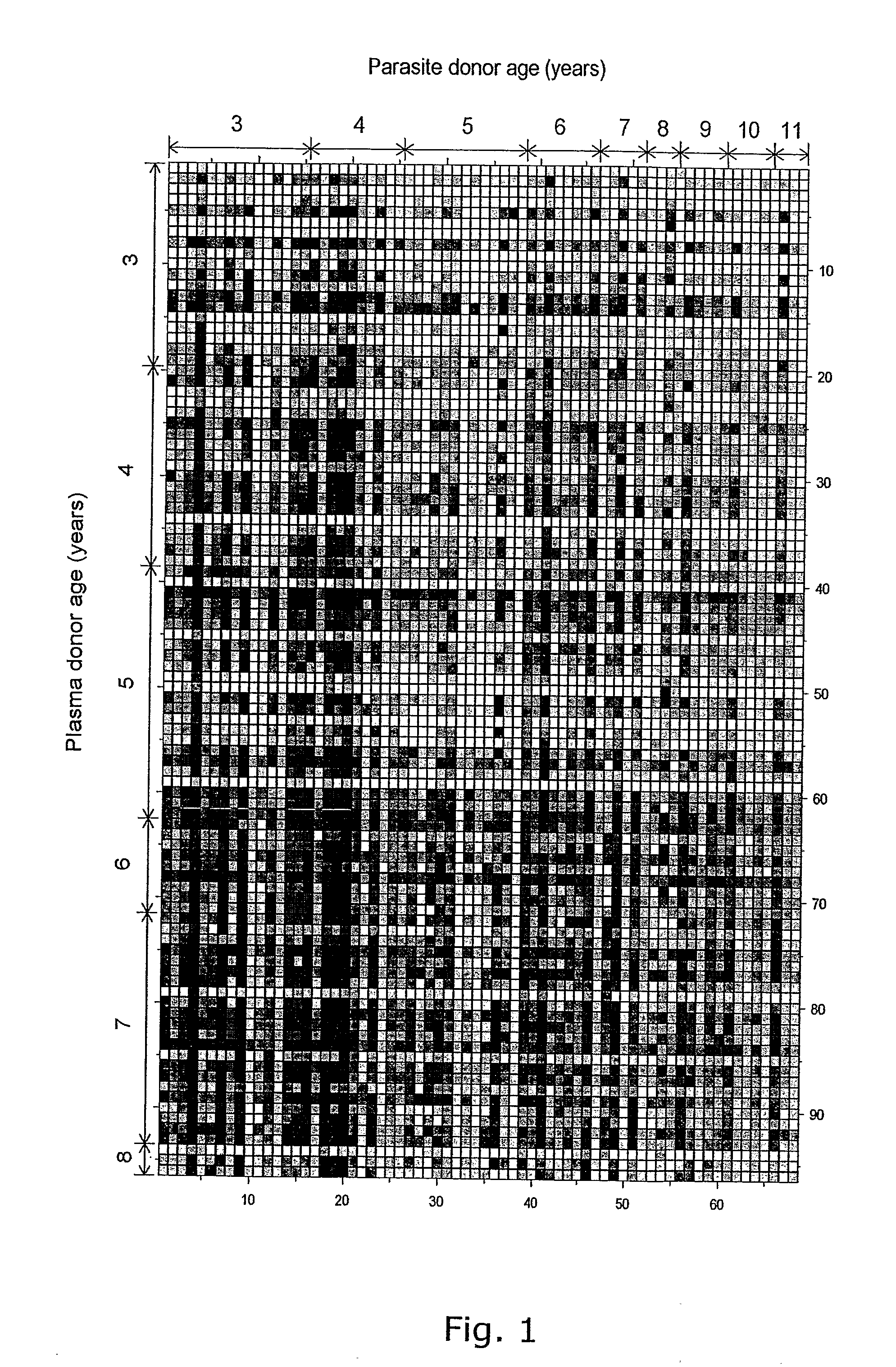
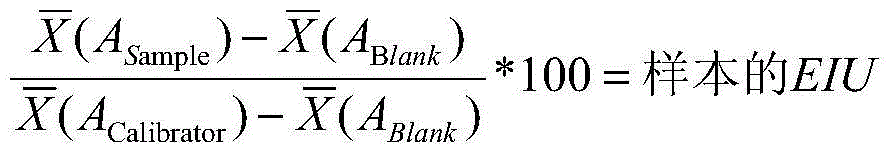Patents
Literature
95 results about "Specific igg" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
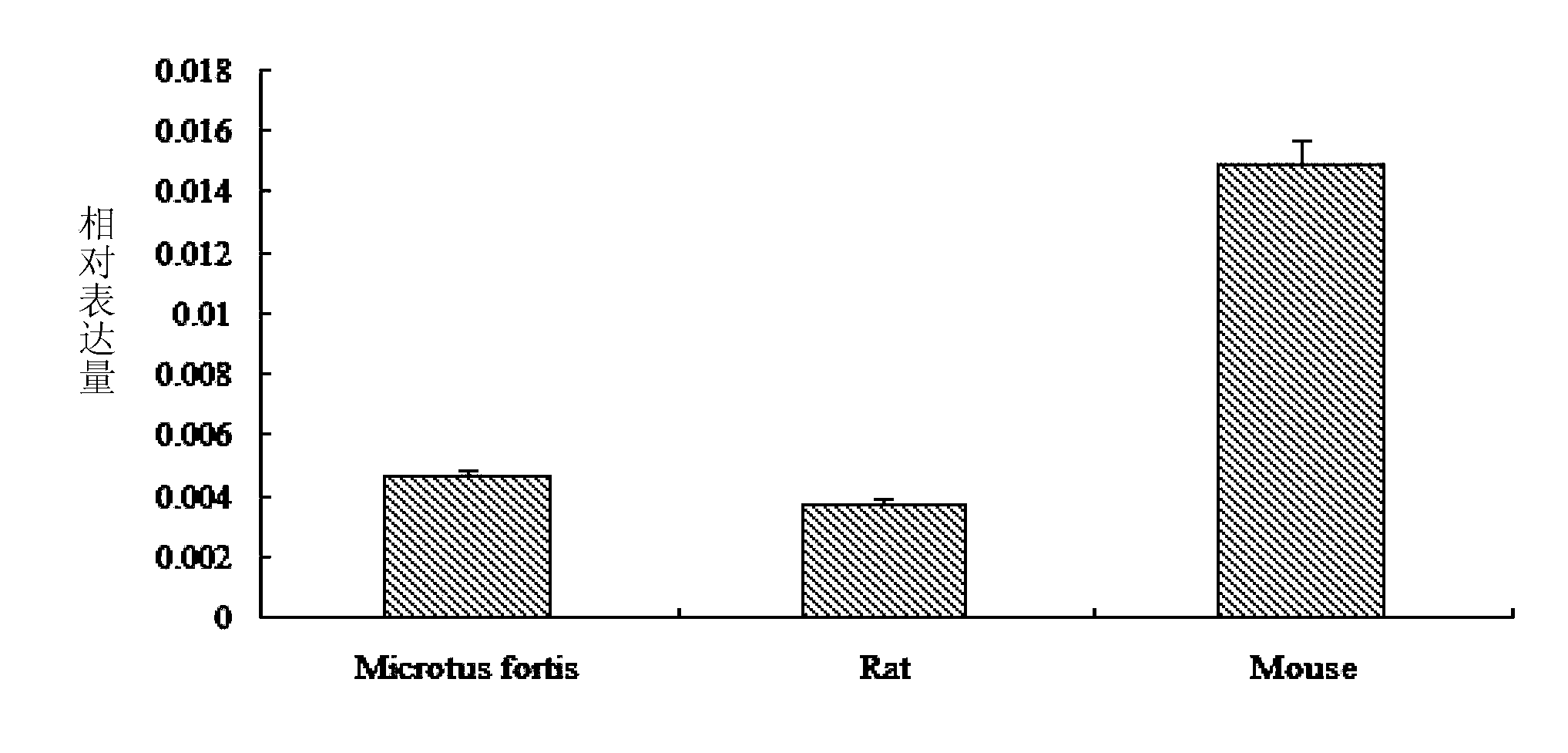
ImmunoCAP Specific IgG measures antigen-specific IgG antibodies in human serum and plasma. Specific IgG comprises antigen-specific antibodies of immunoglobulin class G. These antibodies are part of the natural defense system of the body and develop in response to contact with foreign substances.
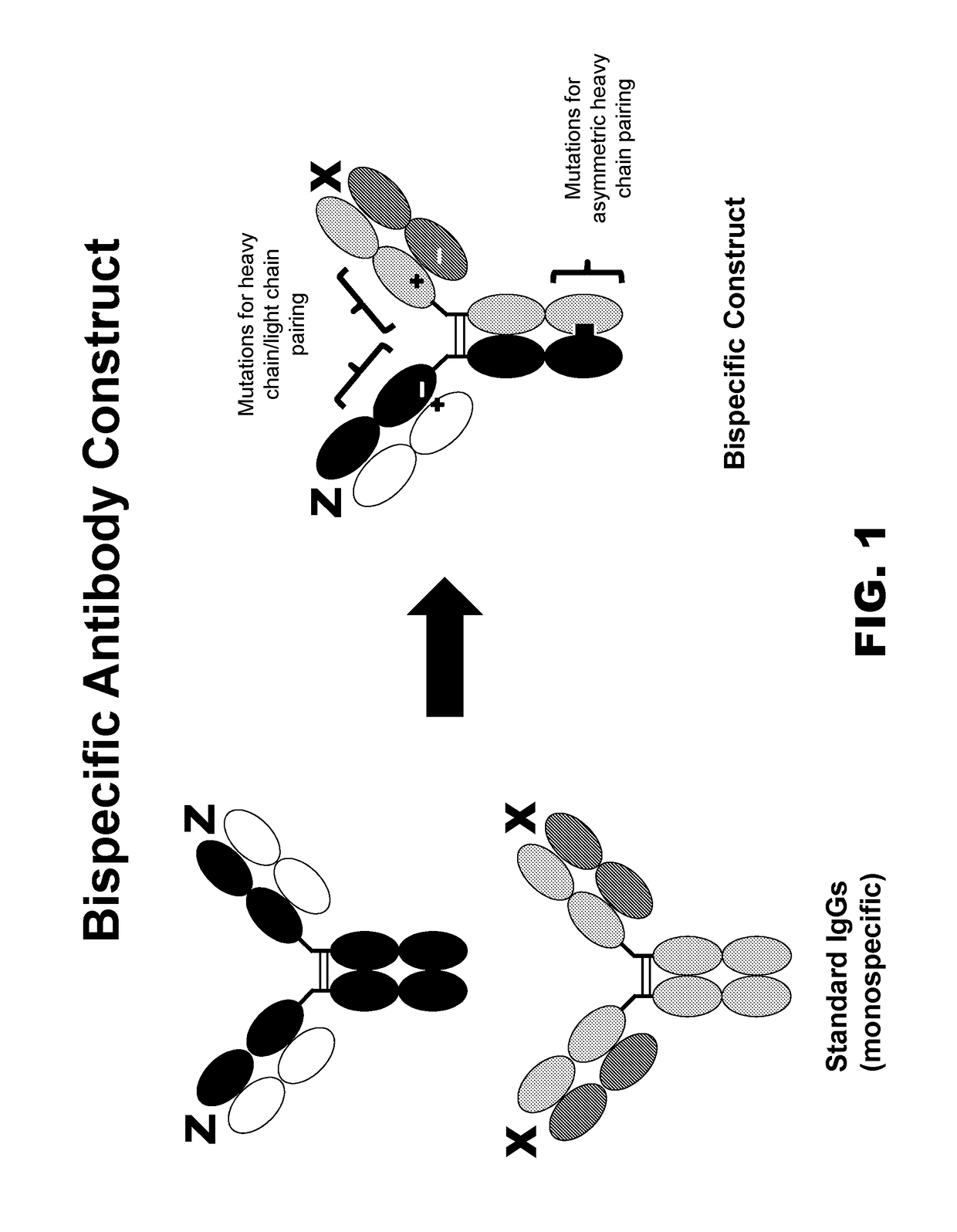
Bispecific igg antibodies as t cell engagers
ActiveUS20140120096A1Induce killingInduce target-specific activationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSpecific iggImmune effector cell
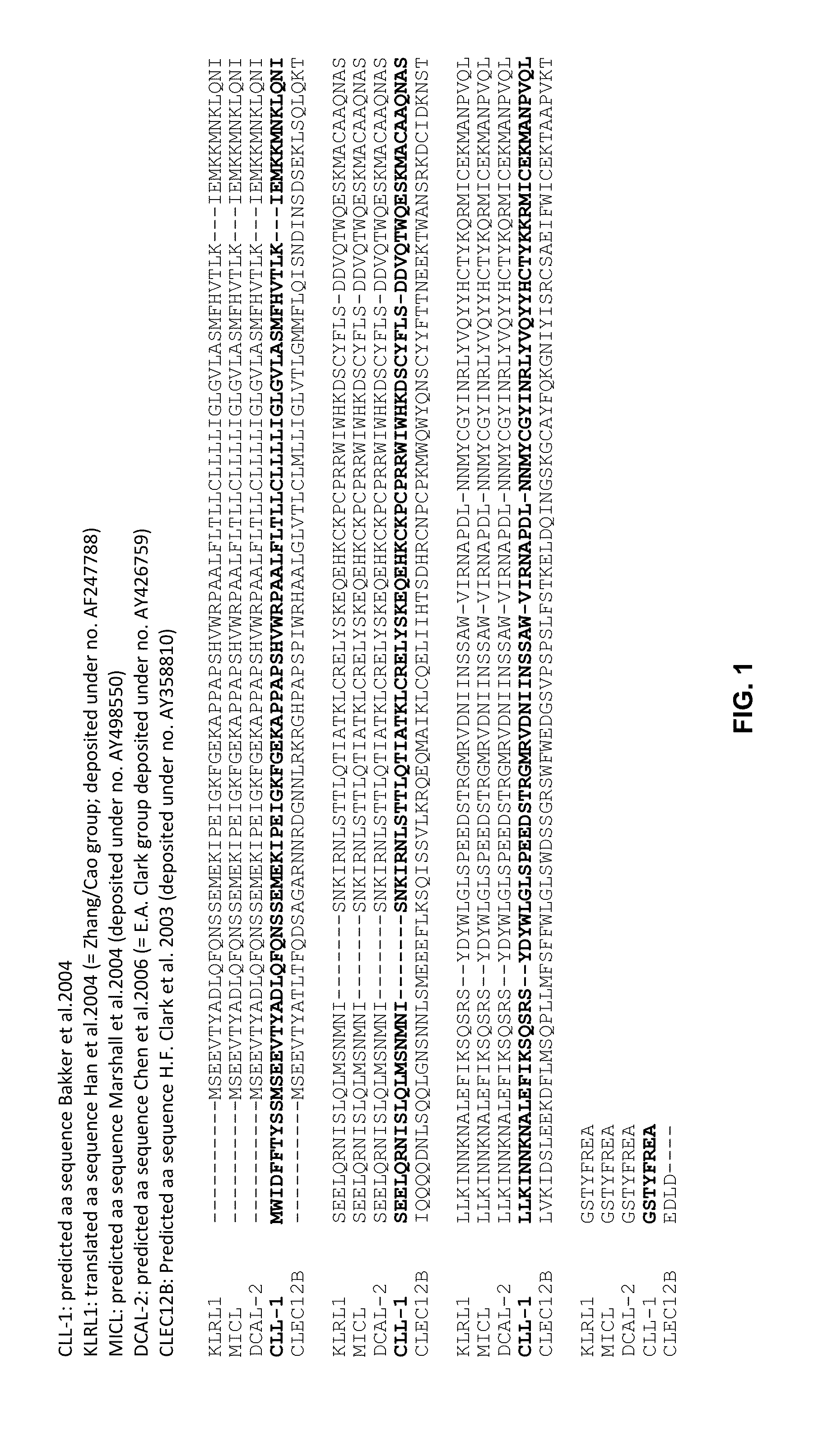
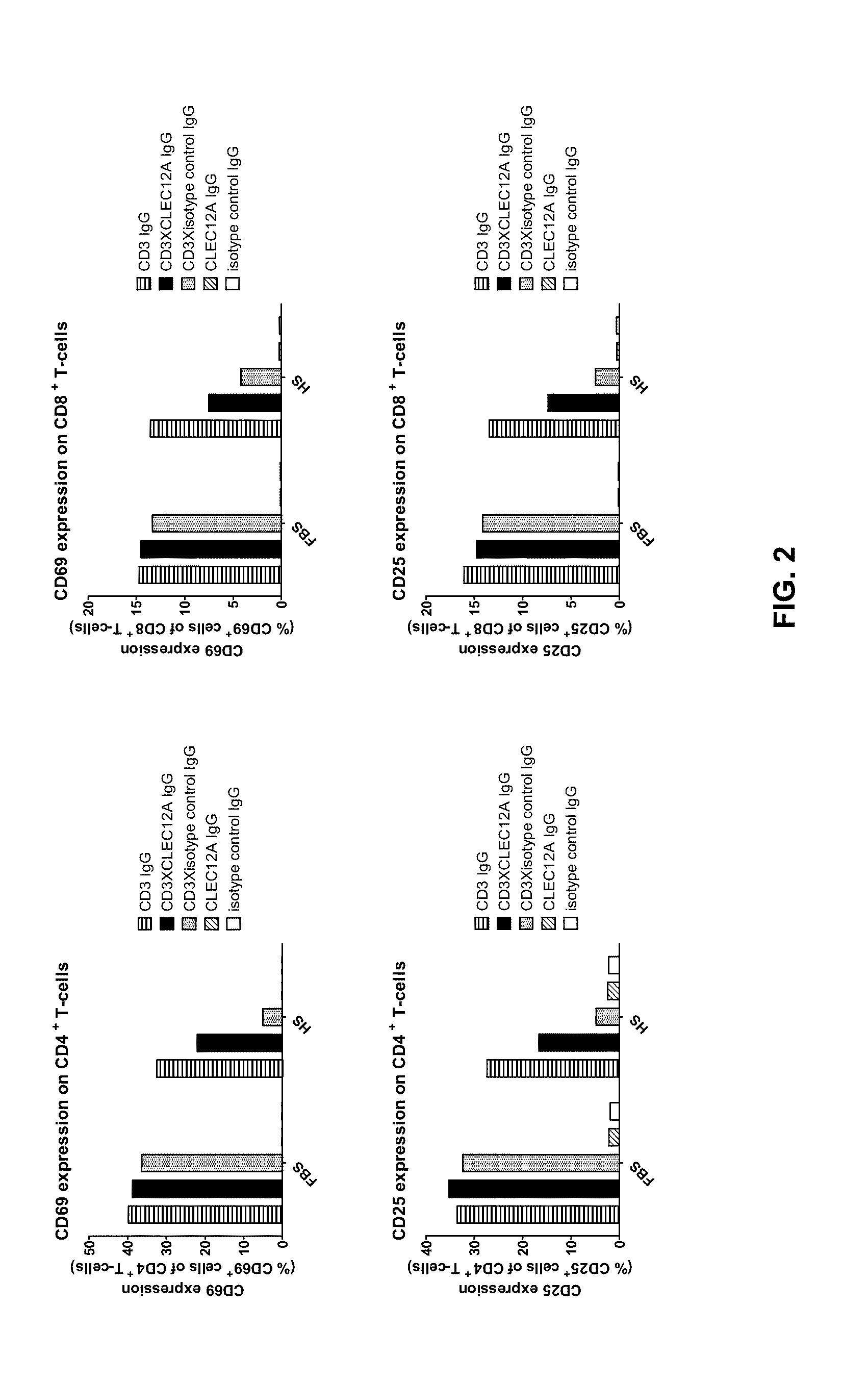
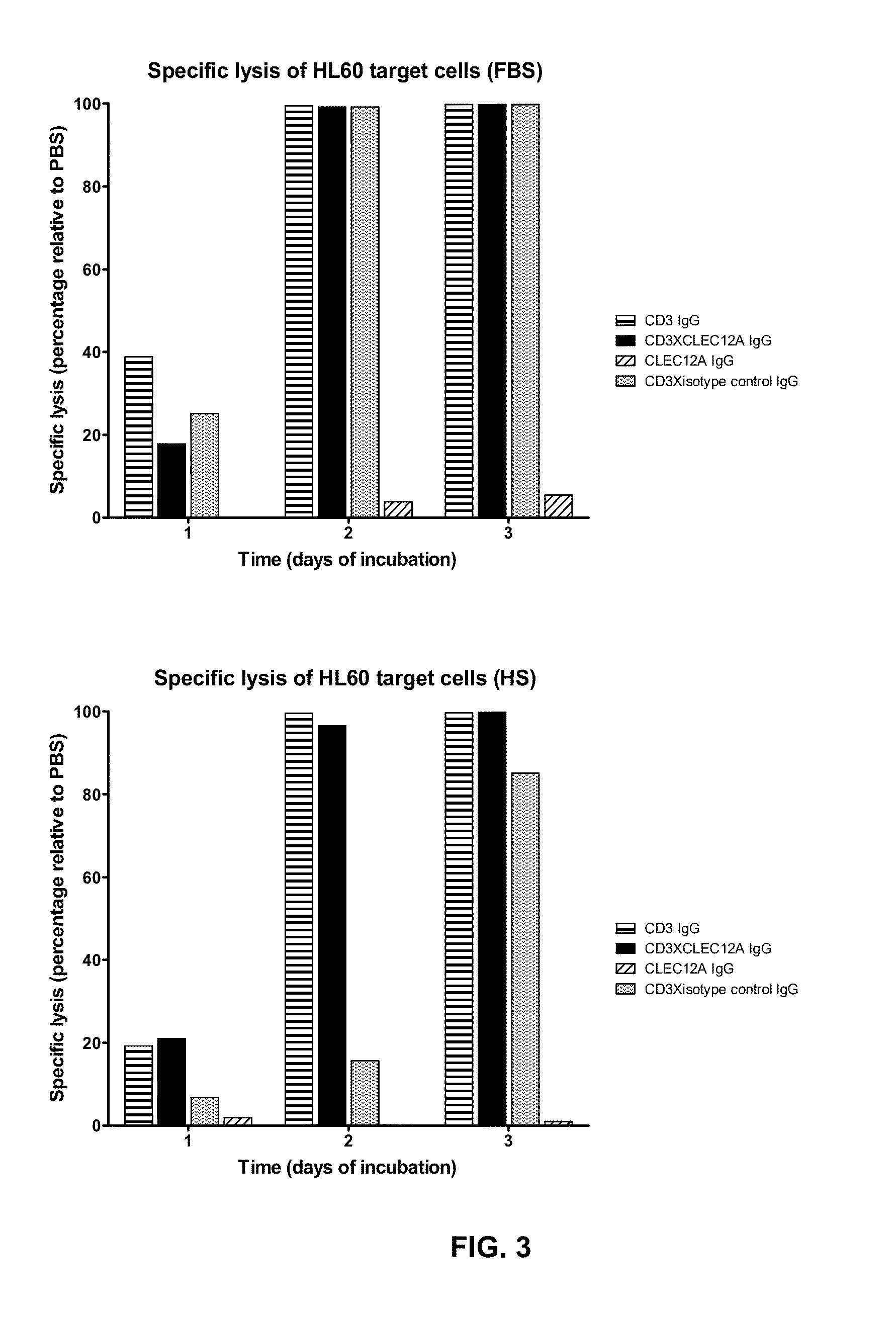
Bispecific IgG antibodies which bind to CLEC12A and an antigen on an immune effector cell are provided.
Owner:MERUS NV
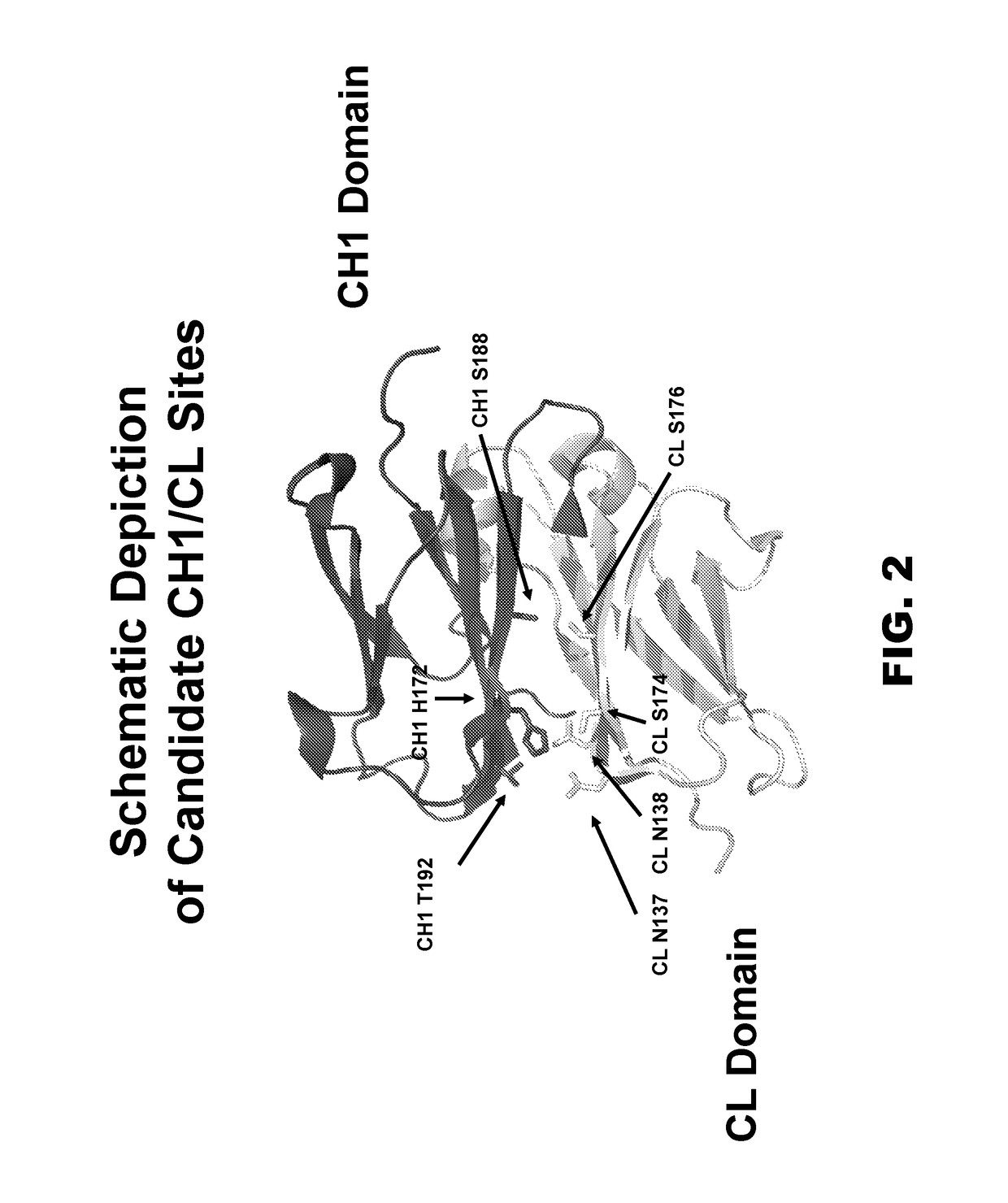
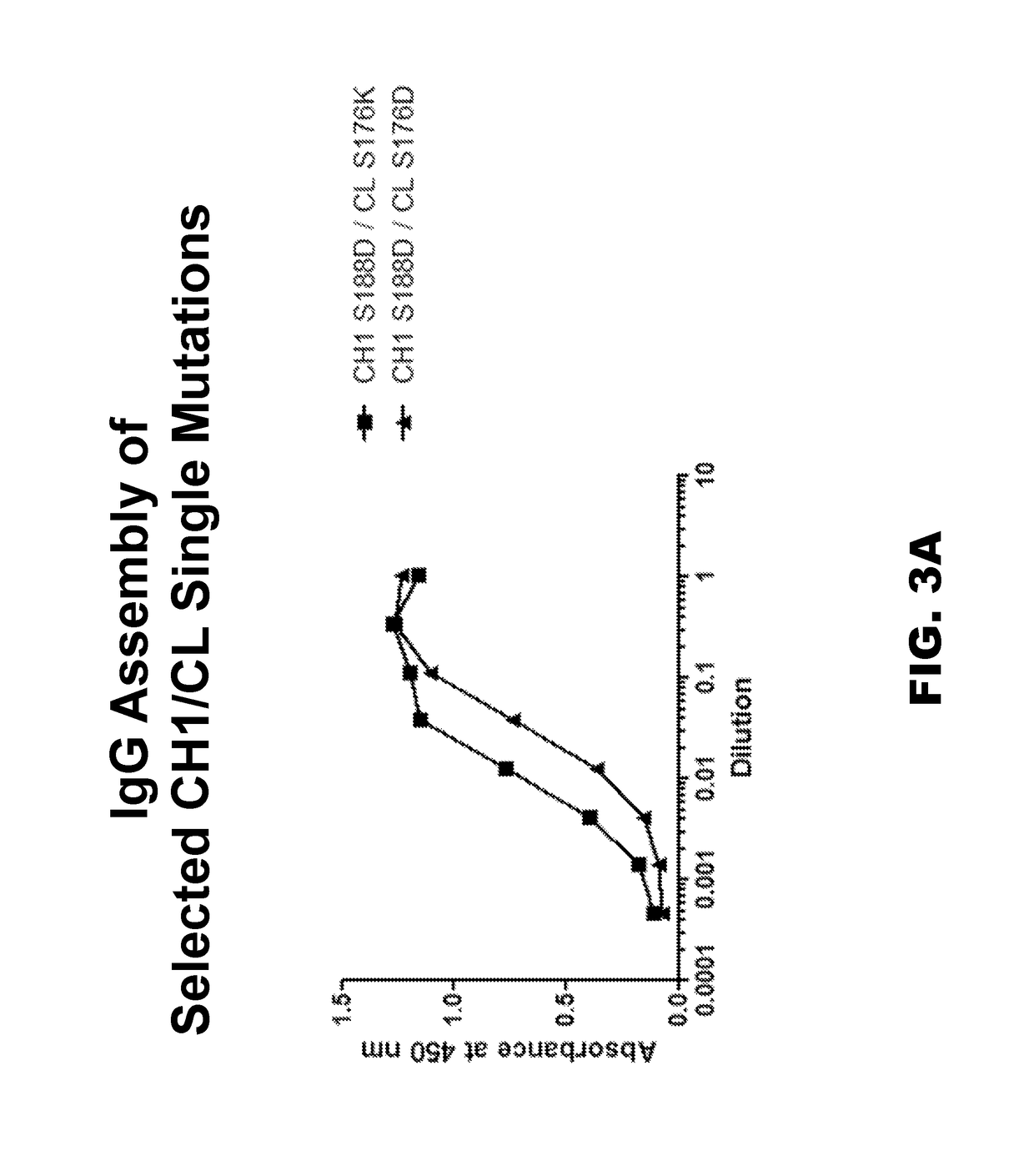
Novel multispecific constructs
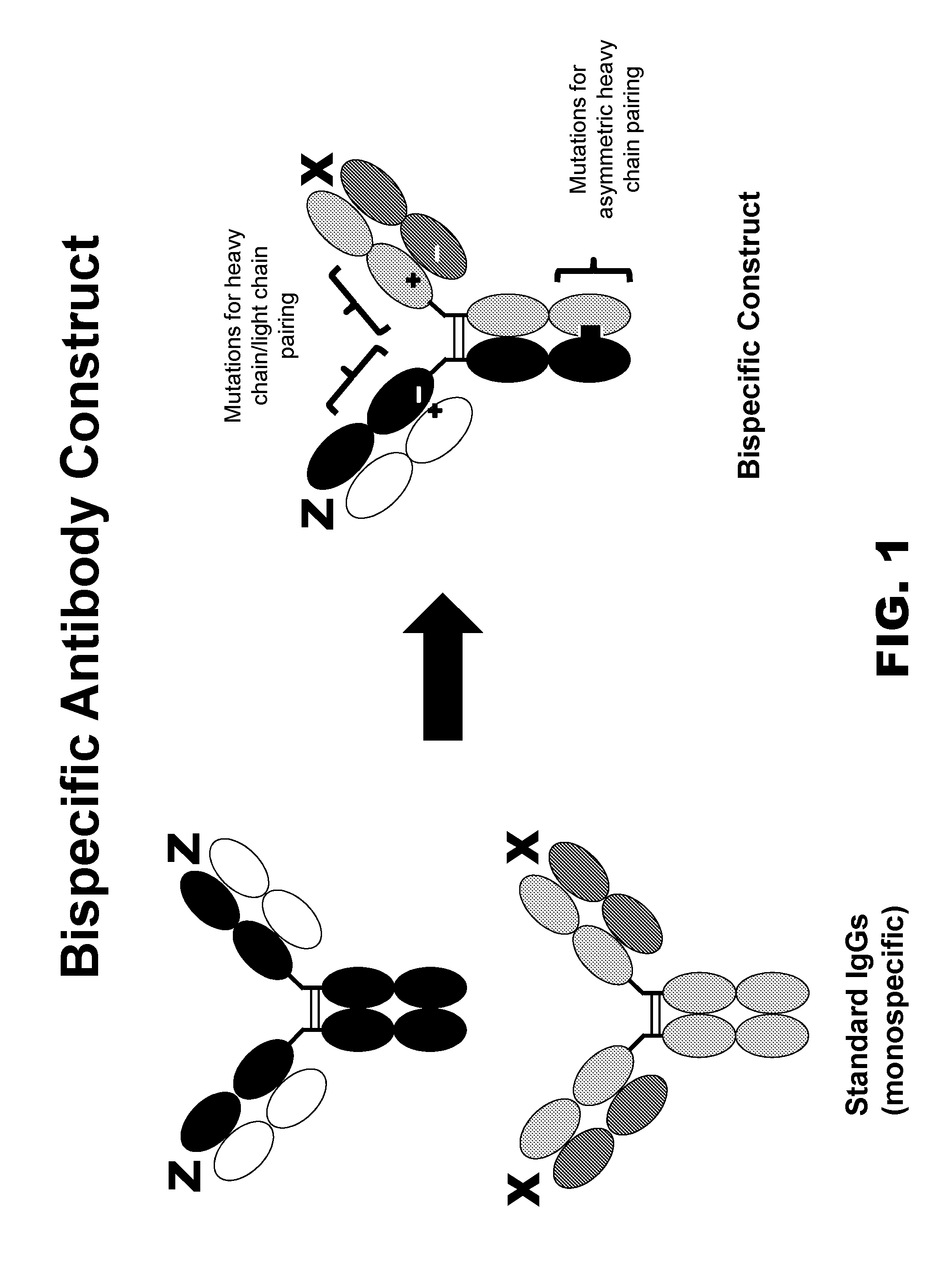
InactiveUS20150368352A1Easy pairingEasy to assembleImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationHeavy chainSpecific igg
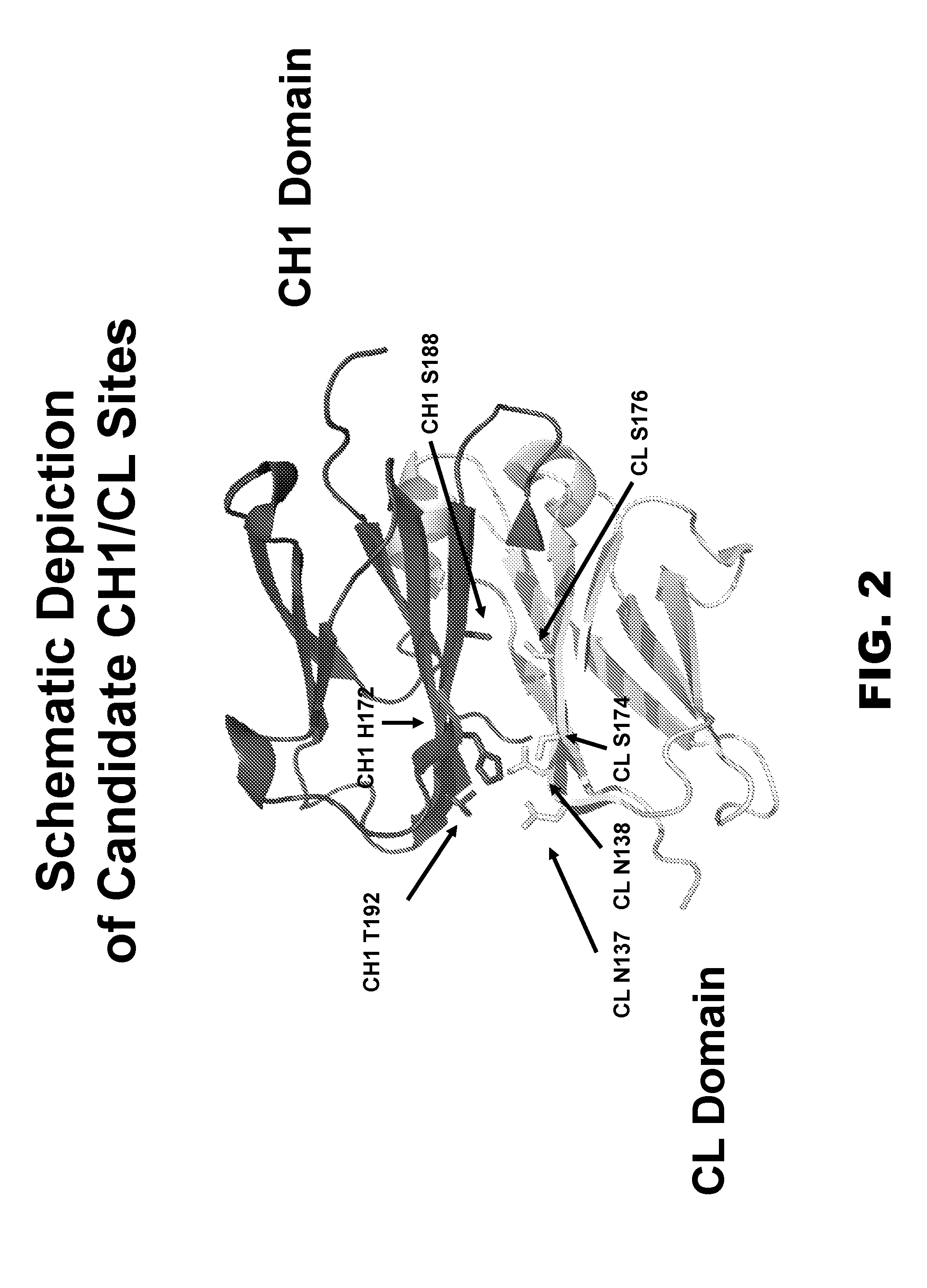
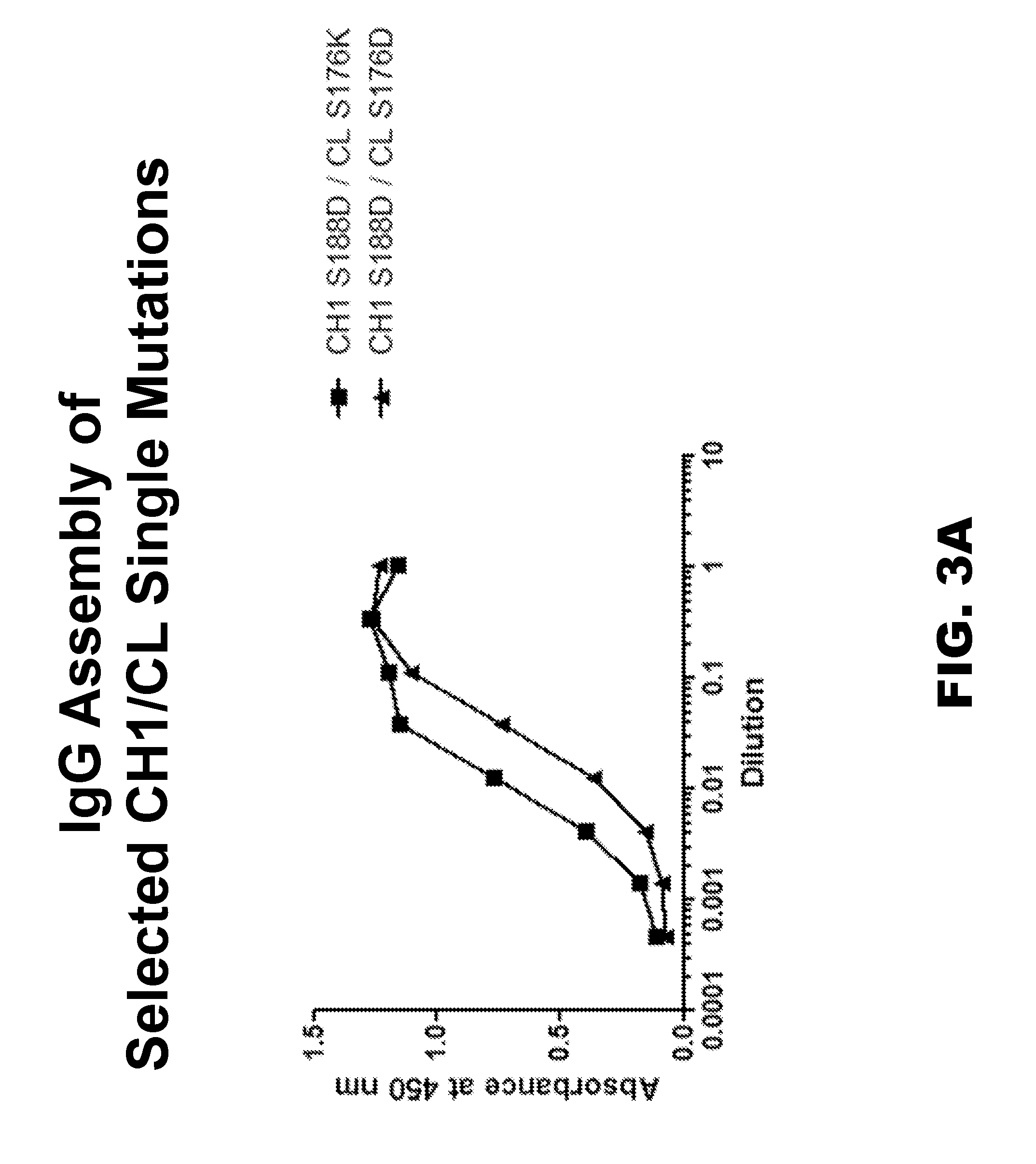
Provided are novel multispecific antibody constructs and multispecific antibody drug conjugates (ADCs), and methods of using such antibodies and ADCs to treat cancer. IgG-like bispecific antibodies have different binding specificities on each arm of the antibody. They are similar in structure to monospecific IgGs in that they contain two heavy chains with VH, CH1, CH2 and CH3 regions, and two light chains with VL and CL regions.
Owner:ABBVIE STEMCENTRX LLC
Microarray chip for detection of immunoglobulin
InactiveUS20060008895A1Determine hypersensitivity levelTime-effectiveBioreactor/fermenter combinationsSequential/parallel process reactionsTotal igeSpecific igm
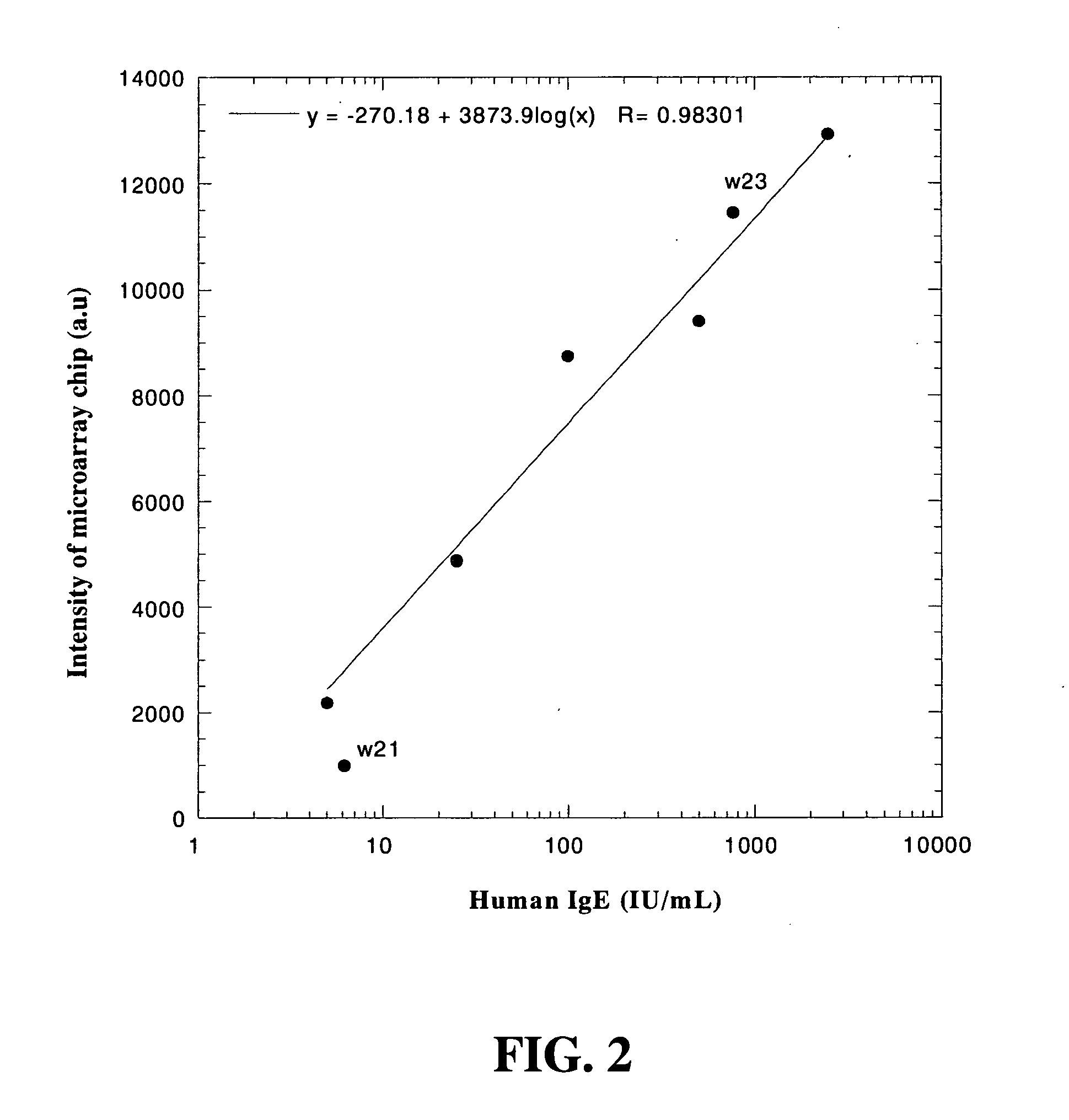
Disclosed is a microarray chip for allergy-related immunoglobulin detection, especially for quantitative detection of total IgE and allergen-specific immunoglobulins (such as specific IgE, specific IgG, and specific IgM), which comprises a solid substrate, a reactive layer fabricated on the solid substrate, and at least one allergen or substance capable of binding to immunoglobulin of interest. Whereby, use the result of quantitative detection for allergen-specific IgE to determine hypersensitivity level. In addition, a method for allergy-related immunoglobulin detection using the microarray chip is present, which uses a secondary monoclonal antibody to minimize non-specific binding and applies an enzymatic reaction to amplify reaction signal. An efficient way is thus obtained, which not only reduces time consumption but also provides quantitative measurement.
Owner:CHENG LOONG CORPORATION
Rapid and non-invasive method to evaluate immunization status of a patient
InactiveUS6927068B2Rapid and reliable and non-invasive and safe testingRapidly evaluate immunization status of a patientBioreactor/fermenter combinationsBiological substance pretreatmentsSpecific iggAnthrax protective antigen
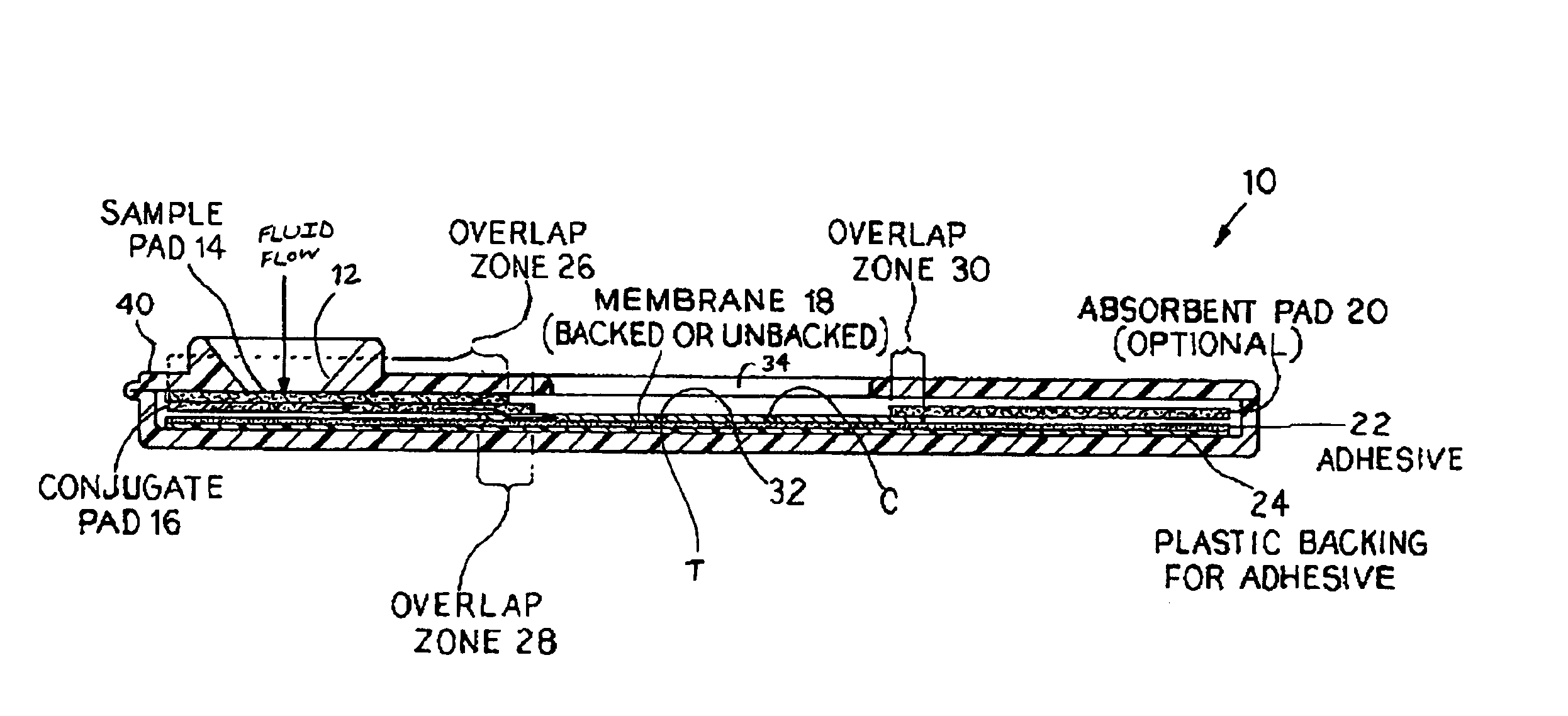
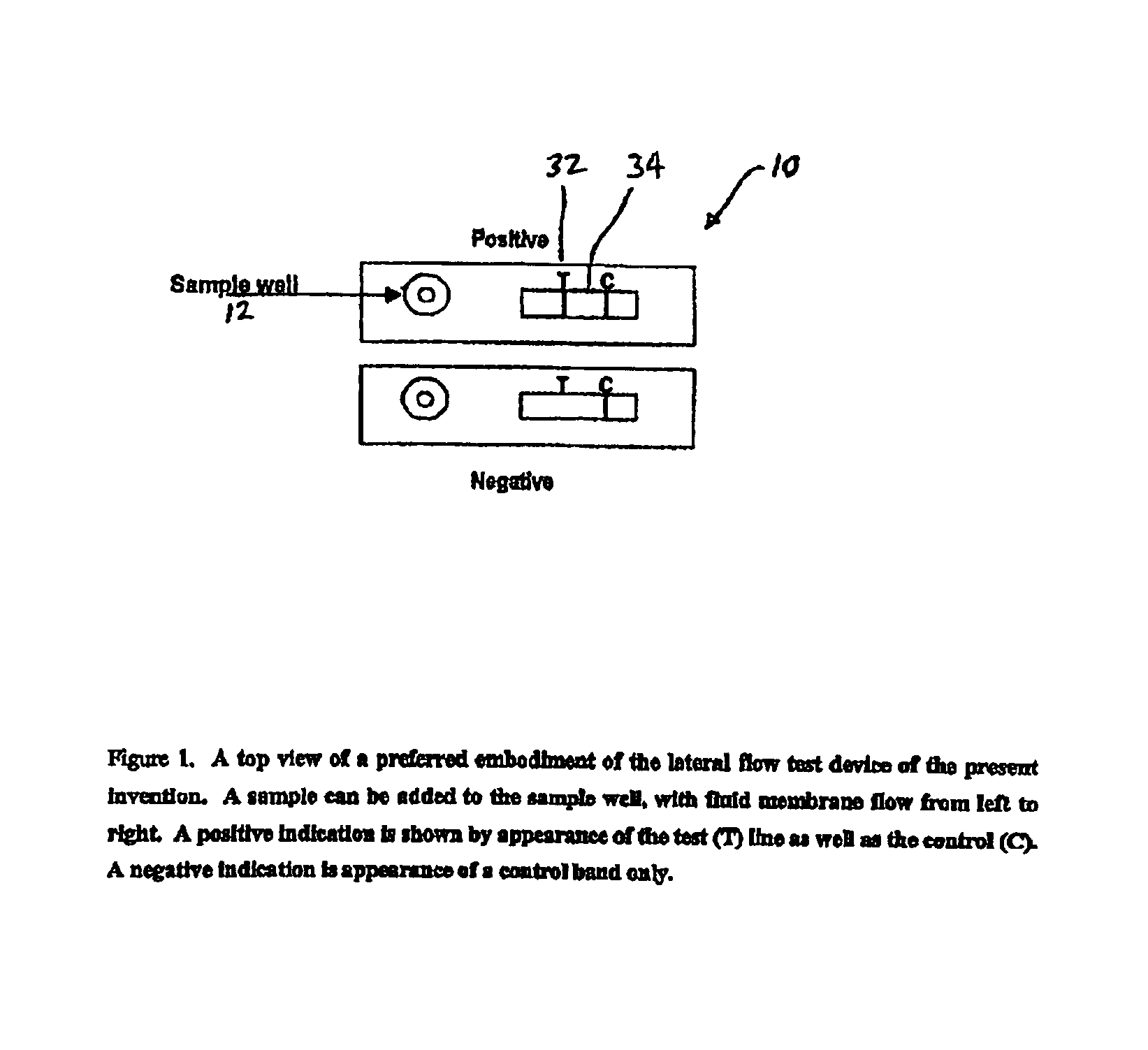
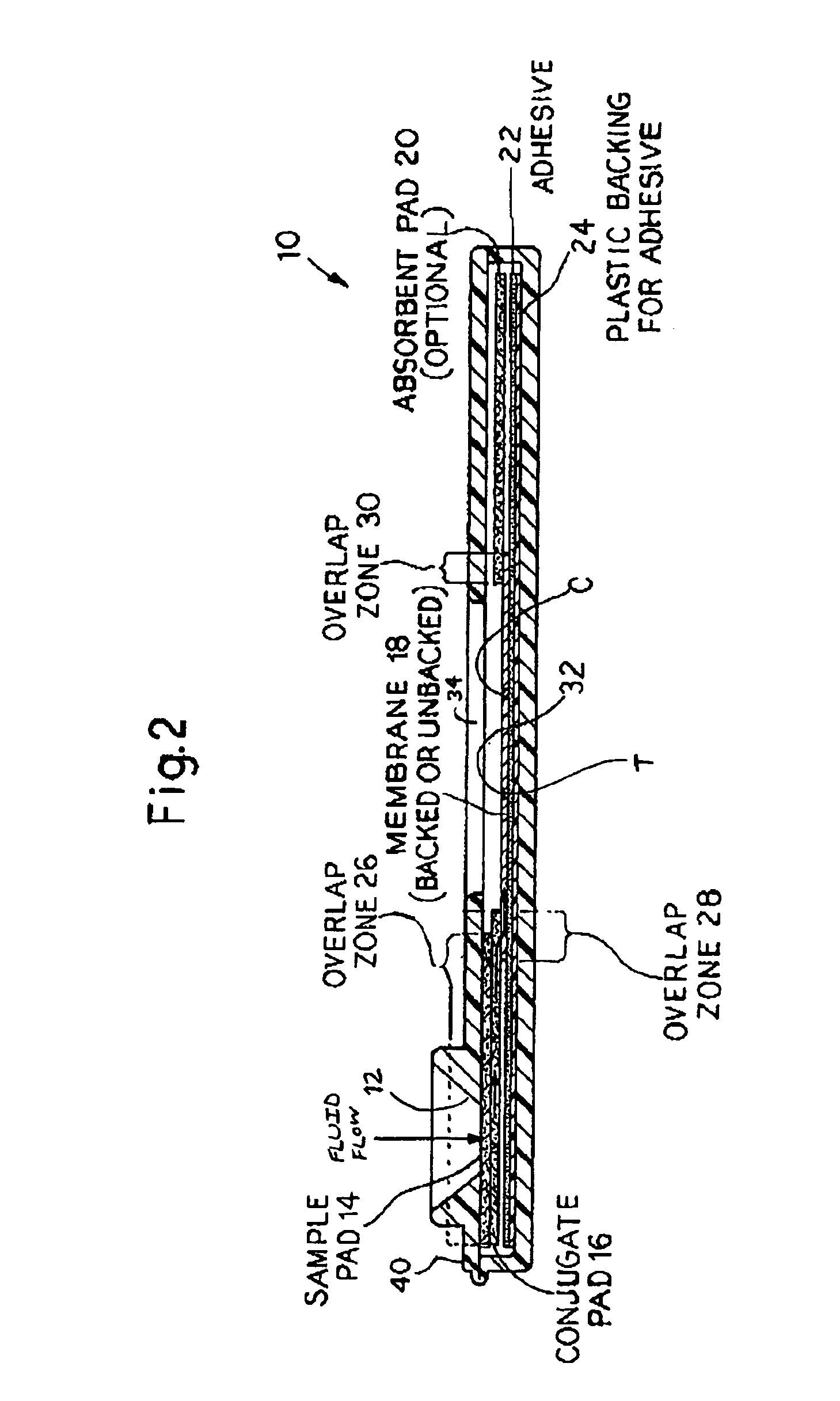
An assay method and kit for detecting the presence of a predesignated, target IgG antibody in a sample selected from one or more patient bodily fluids. The method comprises the following steps: (a) contacting the sample of one or more patient bodily fluids with a membrane-bound recombinant protective antigen to bind to the target IgG antibody in the sample; (b) previously, simultaneously or subsequently to step (a), binding the protective antigen (PA) with a conjugated label producing a detectable signal; and (c) detecting the signal whereby the presence of the target IgG antibody is determined in the sample by the intensity of the signal. The method can further comprise the step of evaluating immunization status of the patient from whom the sample came by comparing the signal or lack thereof with immunizations previously received by the patient. In a preferred embodiment, the recombinant protective antigen (PA) specifically binds to anthrax protective antigen-specific IgG antibodies. Preferably, the immunoassay of the present invention comprises a lateral-flow assay comprising a membrane, a conjugated label pad, and a recombinant protective antigen (PA) bound to the membrane.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Detection method and detection kit of antigen-specific IgG (immunoglobulin G) antibody of staphylococcus aureus SpA5 mutant
ActiveCN103645318AEliminate distractionsStrong specificityBiological material analysisSpecific iggStaphylococcus aureus
The invention belongs to the technical field of biology, and relates to a detection method and a detection kit of an antigen-specific IgG (immunoglobulin G) antibody of a staphylococcus aureus (SA) SpA5 mutant. By adopting the method disclosed by the invention, the specificity of the method is improved, and the method is simple and fast in operation, and good in repeatability.
Owner:CHENGDU OLYMVAX BIOPHARM +1
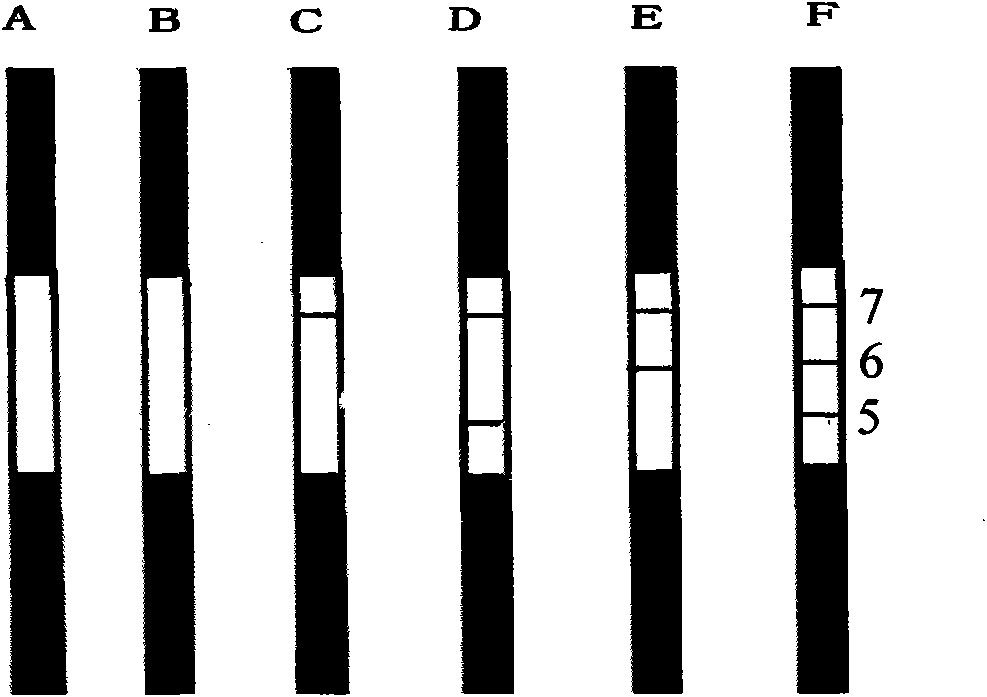
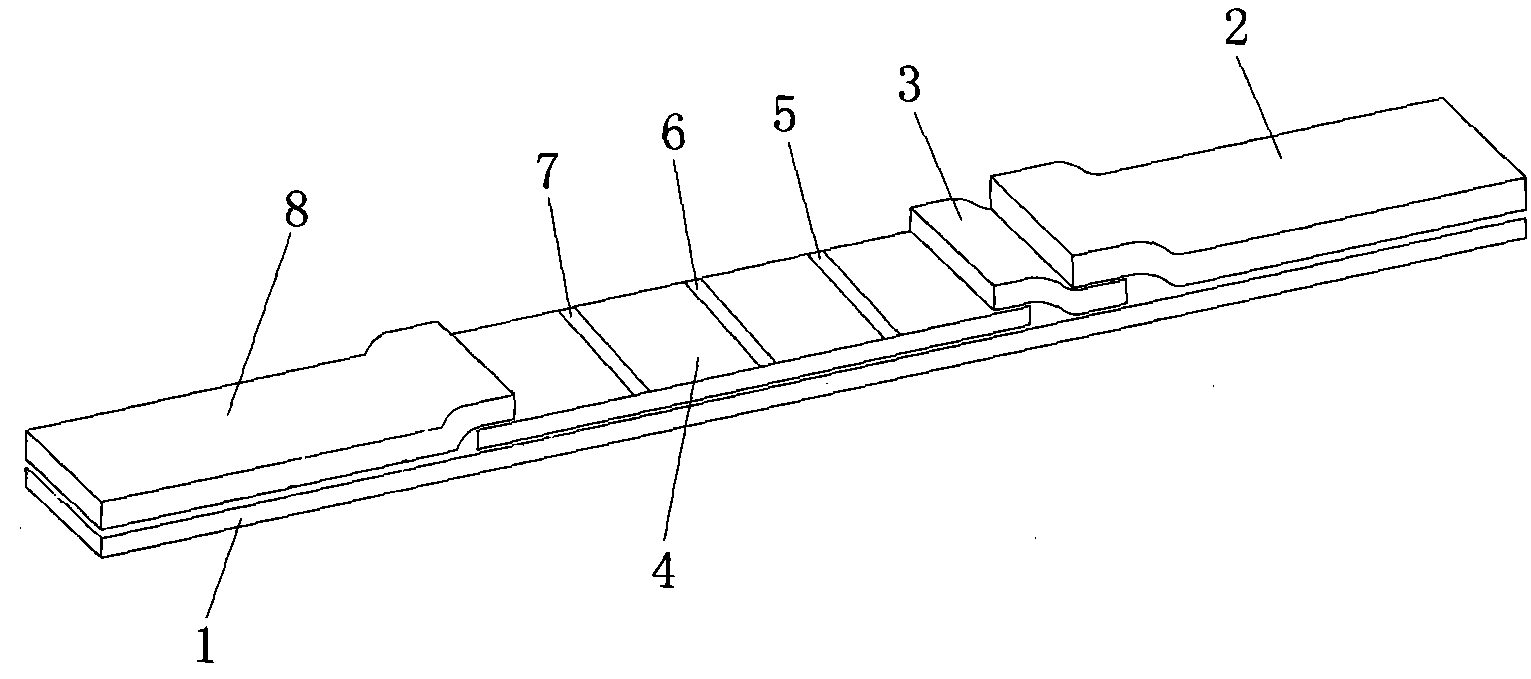
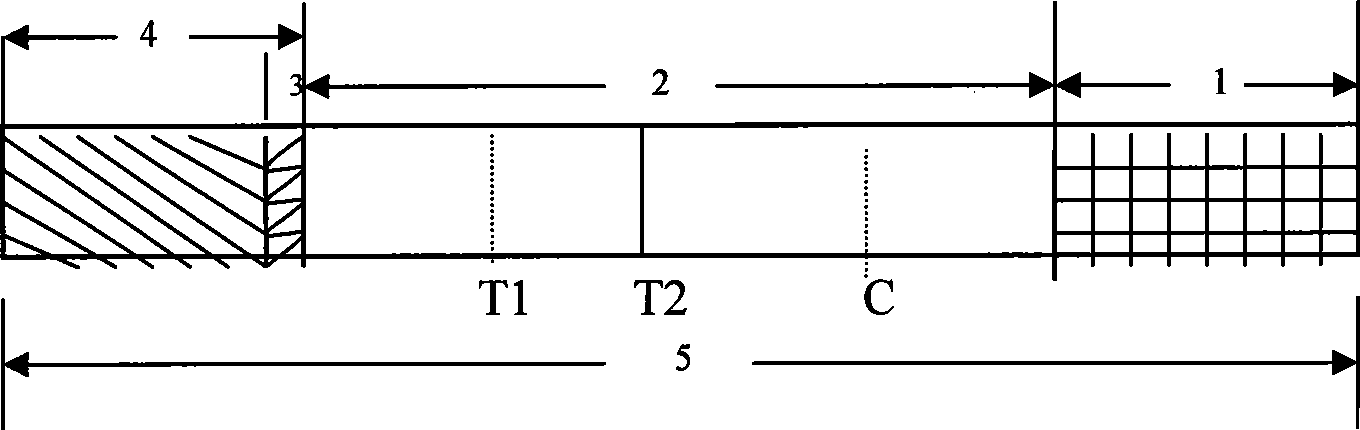
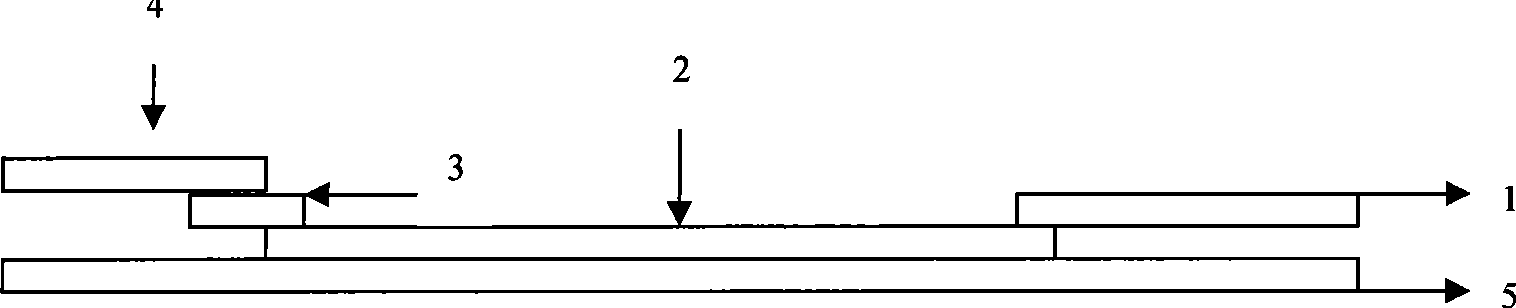
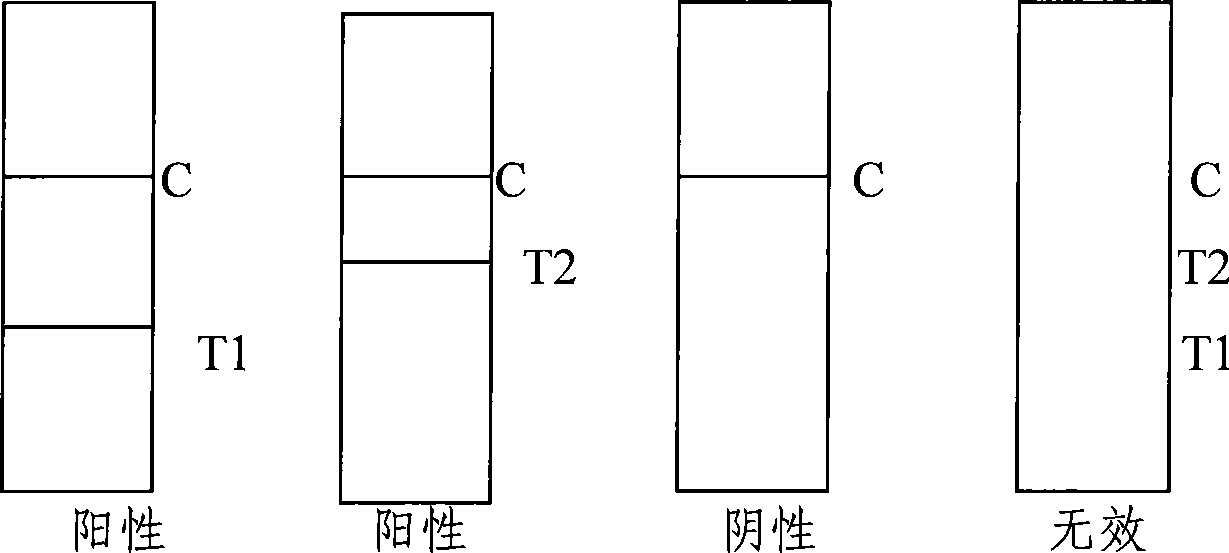
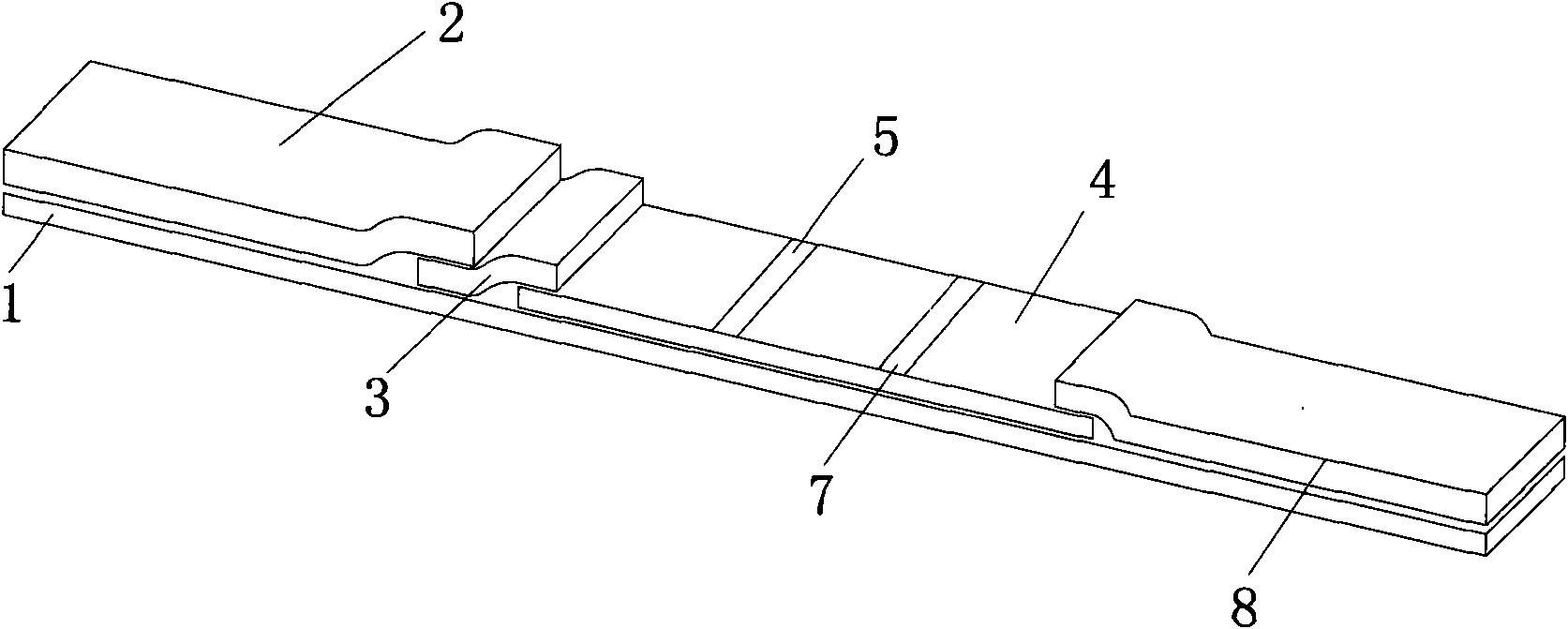
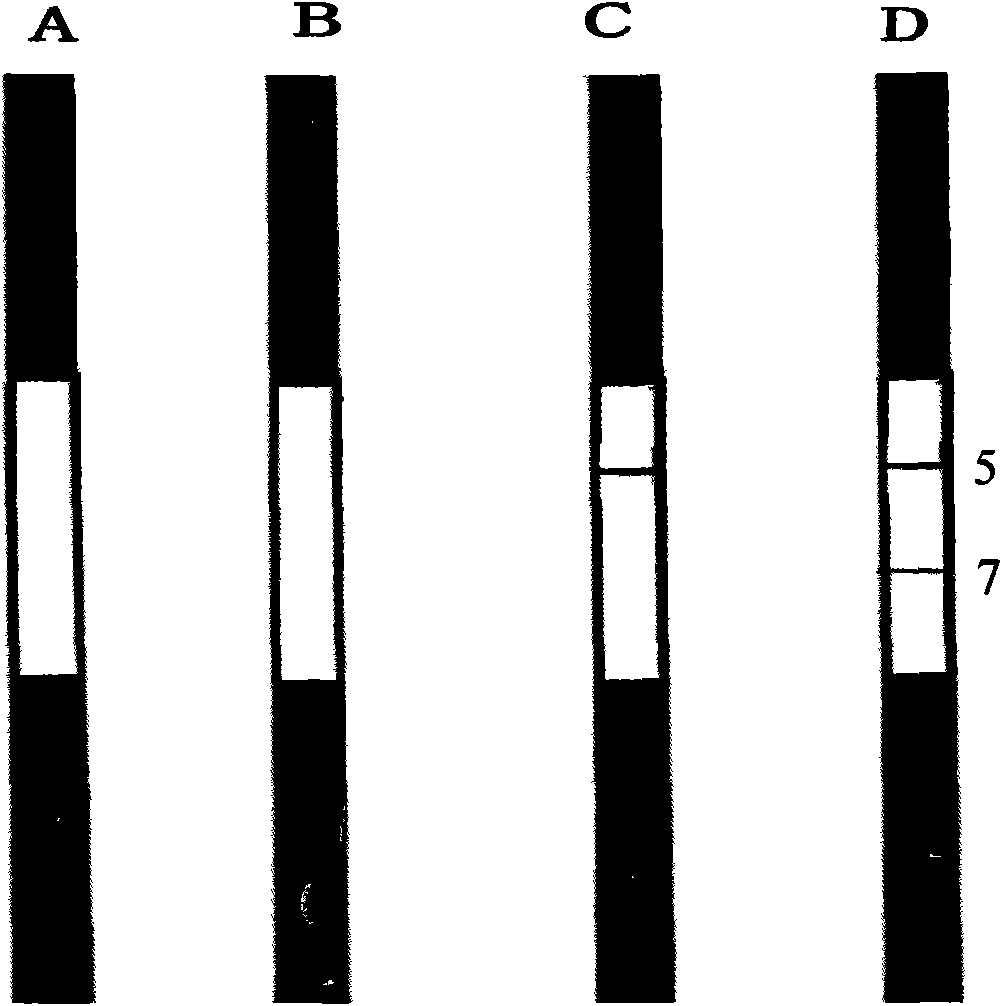
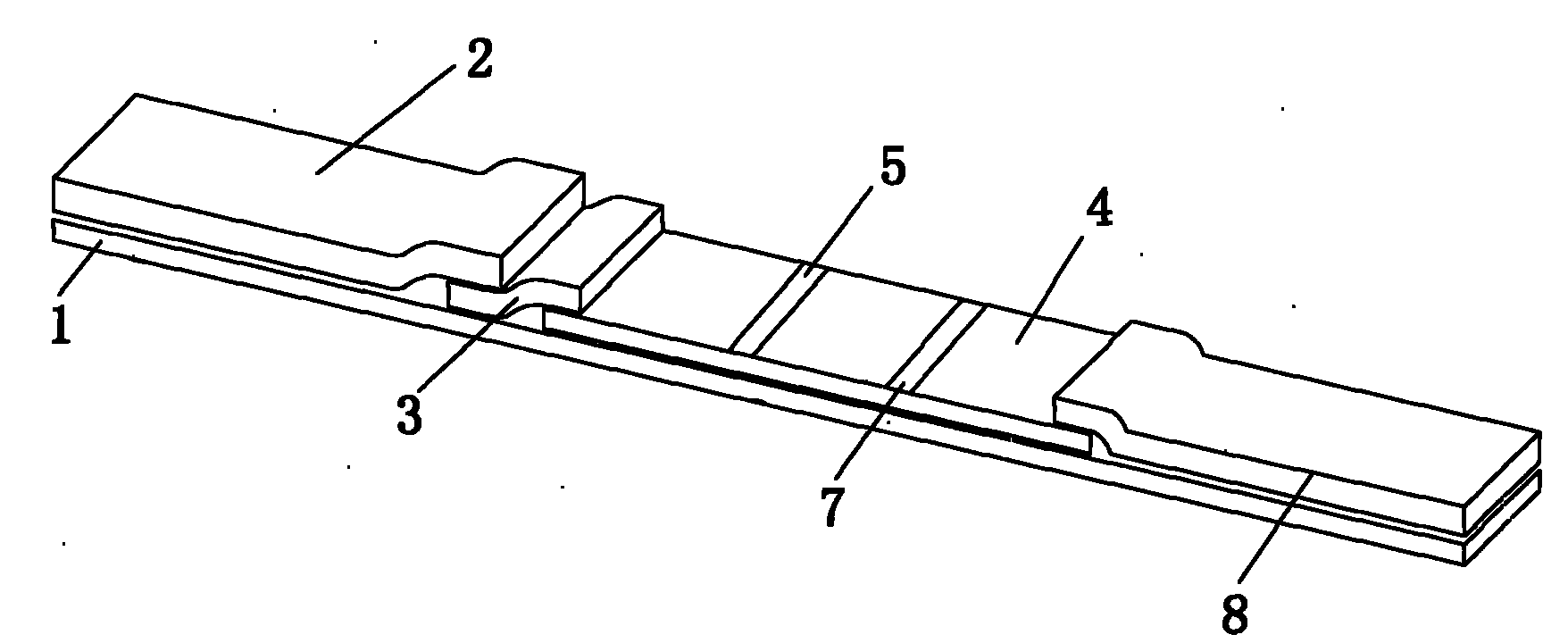
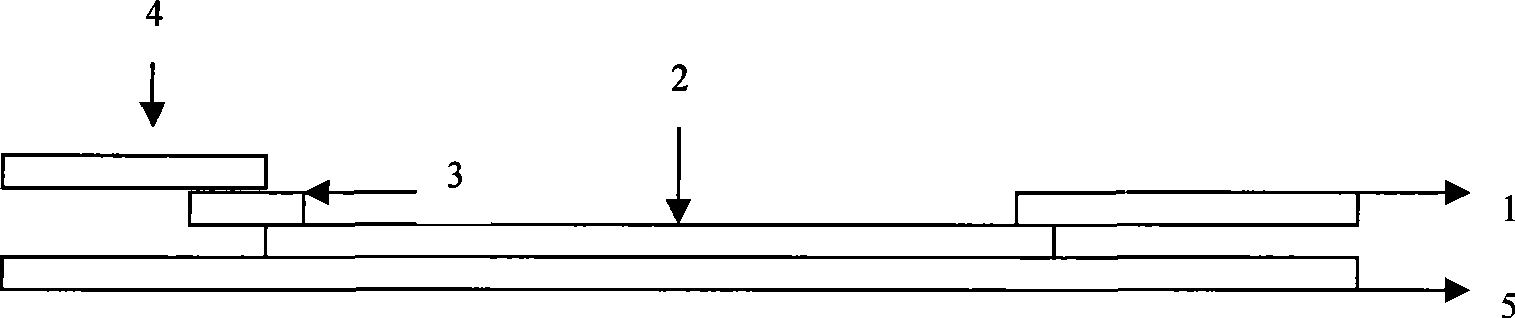
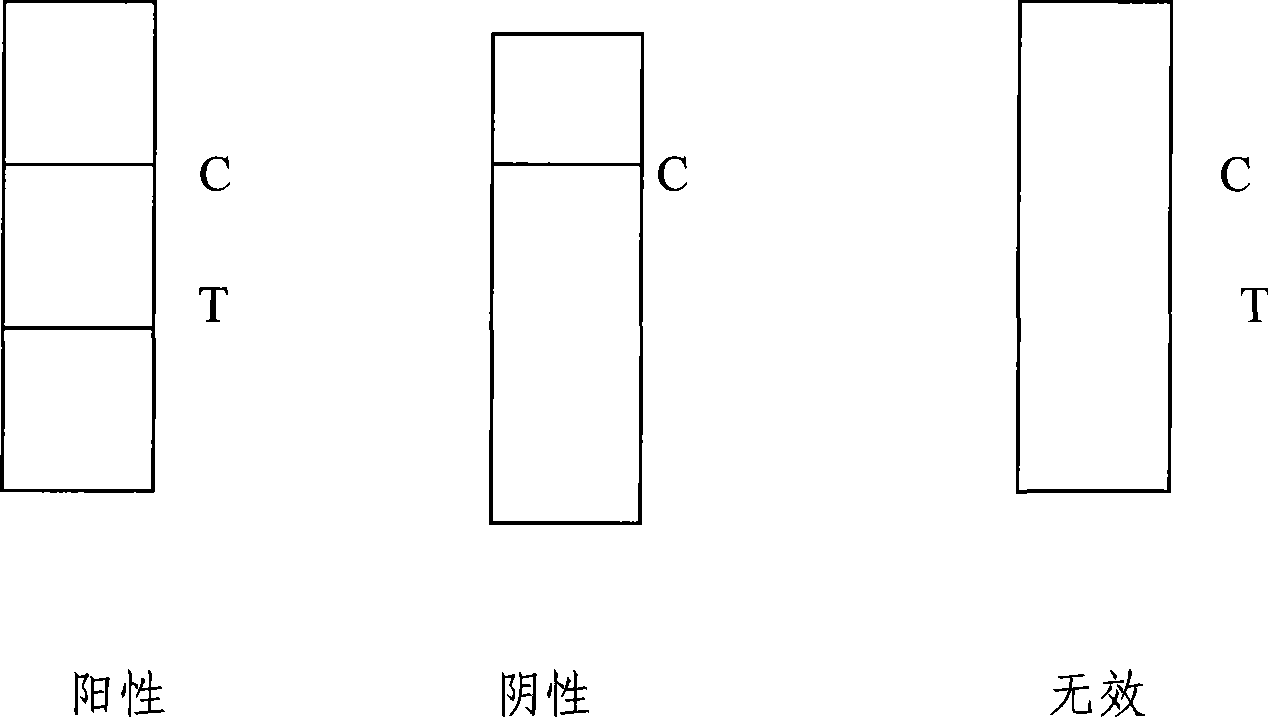
Reagent strip for joint detection of syphilis specific IgM and IgG antibodies and preparation method thereof
InactiveCN101825634AJoint detection implementationStrong specificityMaterial analysisReagent stripSyphilis
The invention provides a reagent strip for joint detection of a syphilis specific IgM and IgG antibodies and a preparation method thereof, relating to a reagent for detection of a syphilis specific antibody. The invention provides the reagent strip for joint detection of the syphilis specific IgM and IgG antibodies and the preparation method thereof. The reagent strip is provided with a vector plate, a loading pad, a colloidal gold pad, a nitrocellulose membrane, a syphilis specific IgG antibody detection line, a syphilis specific IgM antibody detection line, a contrast line and an absorption pad. The preparation method comprises the following steps: preparing sample application of nitrocellulose membranes for recombinant syphilis antigens TPN17 and TPN47, preparing colloidal gold, labeling syphilis specific antigen TPN17 and TPN47 with colloidal gold and preparing the reagent strip for joint detection of the syphilis specific IgM and IgG antibodies.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
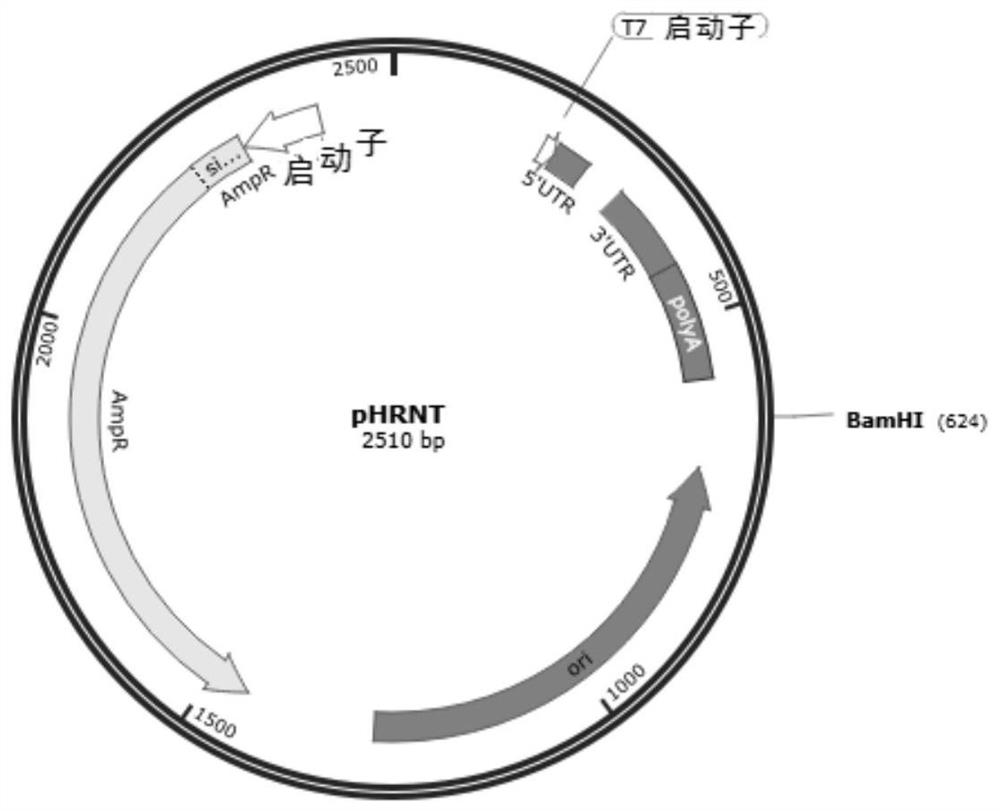
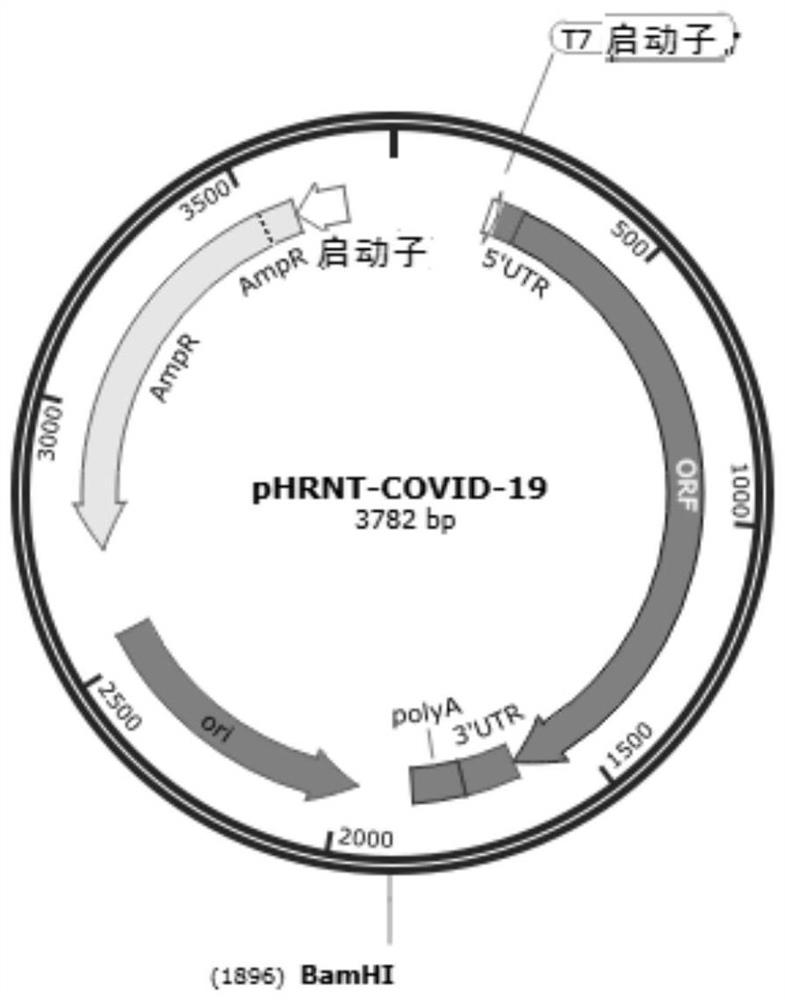
Novel coronavirus SARS-CoV-2 mRNA vaccines and preparation method and application thereof
ActiveCN113151312AProlong half-lifeEasy to getSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationSpecific igg
The invention provides novel coronavirus SARS-CoV-2mRNA vaccines and a preparation method and application thereof. The invention provides three mRNA vaccines, namely RBD, S1 and S vaccines. The RBD vaccine disclosed by the invention can induce a high-titer antigen-specific IgG antibody and a virus neutralization antibody after immunization with one dose, the high-titer neutralization antibody can be maintained for at least 26 weeks, and remarkable immune protection can be provided for human ACE2 transgenic mice in serum adoptive transfer protection experiments. The RBD and S vaccine disclosed by the invention can induce immune protection capable of completely resisting SARS-CoV-2 virus infection in the human ACE2 transgenic mice after immunization with two doses. A large number of experimental results show that the mRNA vaccine provided by the invention has good immunogenicity, forms powerful immune protection after immunizing an organism, and has a huge development potential.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Reagent strip for joint detection of syphilis specific IgG antibody and specific total antibody and preparation method thereof
The invention provides a reagent strip for joint detection of a syphilis specific IgG antibody and a specific total antibody and a preparation method thereof, relating to a reagent for joint detection of a syphilis specific IgG antibody and a specific total antibody. The reagent strip is provided with two reagent strips, wherein one is provided with a vector plate, a sample adding pad, a colloidal gold pad, a nitrocellulose membrane, a syphilis specific IgG antibody detection line, a control line and an absorbent pad; and the other one is provided with a vector plate, a sample adding pad, a colloidal gold pad, a nitrocellulose membrane, a specific total antibody detection line, a control line and an absorbent pad. The preparation method comprises the following steps: preparing recombinant syphilis antigens TPN17 and TPN47, applying samples of nitrocellulose membranes, preparing colloidal gold, labeling TPN17 and TPN47 with colloidal gold and preparing immunochromatography detection strips. The reagent strip can be used for detecting the syphilis specific IgG antibody and the specific total antibody in the specimens of whole blood, blood serum, blood plasma, cerebrospinal fluid and the like. During detection, the specimen amount is small, special instruments are not needed and the results are directly interpreted by the naked eyes. The reagent strip is simple, convenient, fast, accurate and reliable and has strong specificity and high sensitivity.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Test paper strip for rapidly detecting morbilli and rubella virus IgG antibody colloidal gold
ActiveCN101363856AHigh sensitivityImprove featuresMaterial analysisRubulavirus InfectionsSpecific igg
The invention provides a test strip for simultaneous detection of measles and rubella virus specific IgG antibodies, which comprises a reaction film and a conjugate release pad. The reaction film has a detection band simultaneously coated with measles virus H antigen and rubella virus E1 specific antigen, and a quality control band coated with double-antibody IgG. The conjugate release pad is coated with colloidal gold labeled anti-human IgG. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base and site detection and epidemiological investigation, has auxiliary and differential diagnosis effects on measles and rubella virus infection, and can be used for the immune effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
Reagent strip for testing syphilis specific IgG antibodies through gold immunochromatographic assay and preparation method thereof
InactiveCN101858915AThe amount of sample required for testing is smallSmall sample sizeMaterial analysisReagent stripSerum ige
The invention provides a reagent strip for testing the syphilis specific IgG antibodies in the specimens of whole blood, serum, blood plasma, cerebrospinal fluid and the like through gold immunochromatographic assay and a preparation method thereof and relates to a reagent for testing the syphilis specific IgG antibodies. The reagent strip is provided with a carrier plate, a sample-adding pad, a colloidal gold pad, a nitrocellulose membrane, a syphilis specific IgG antibody testing line, a control line and an absorption pad. The method comprises the following steps: preparing the recombinant syphilis antigens TPN17 and TPN47; applying samples of the nitrocellulose membrane; preparing the colloidal gold; labeling the colloidal gold and TPN17 and TPN47; and preparing the strip for testing through immunochromatographic assay. The invention has the following advantages: the specimen quantity needed for testing is small, special instruments are not needed, the results can be directly identified by naked eyes, testing is simple, convenient and rapid, the specificity is strong, the sensitivity is relatively high, testing is accurate and reliable, the cost is relatively low and the invention is widely applied.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Novel coronavirus SARS-CoV-2 detection method and novel coronavirus SARS-CoV-2 detection device
ActiveCN111650370ARealize qualitative detectionRealize detectionMaterial analysisCelluloseIgm antibody
The invention discloses a novel coronavirus SARS-CoV-2 detection method and a novel coronavirus SARS-CoV-2 detection device. A substrate is mainly prepared by oxidizing nano fibrillated cellulose paper by 2, 2, 6, 6-tetramethylpiperidine oxide. A substrate pattern is wax-printed on the surface of the substrate; qualitative detection of a novel coronavirus specific IgG antibody or IgM antibody in asample is realized on the substrate by utilizing an immunogold labeling technology; detection speed is fast, after calibration, the antibody concentration in the sample can be quantitatively detected; accuracy is high, the area of a surface-enhanced Raman scattering substrate for adding a reagent is not limited to the fixed substrate pattern; the size and style of the substrate pattern can be changed according to the use requirements and the detection environment, the detection of two novel coronavirus specific antibodies can be completed at the same time, and a plurality of samples to be detected can be detected at the same time, so that the sample detection is realized most efficiently.
Owner:苏州微湃医疗科技有限公司
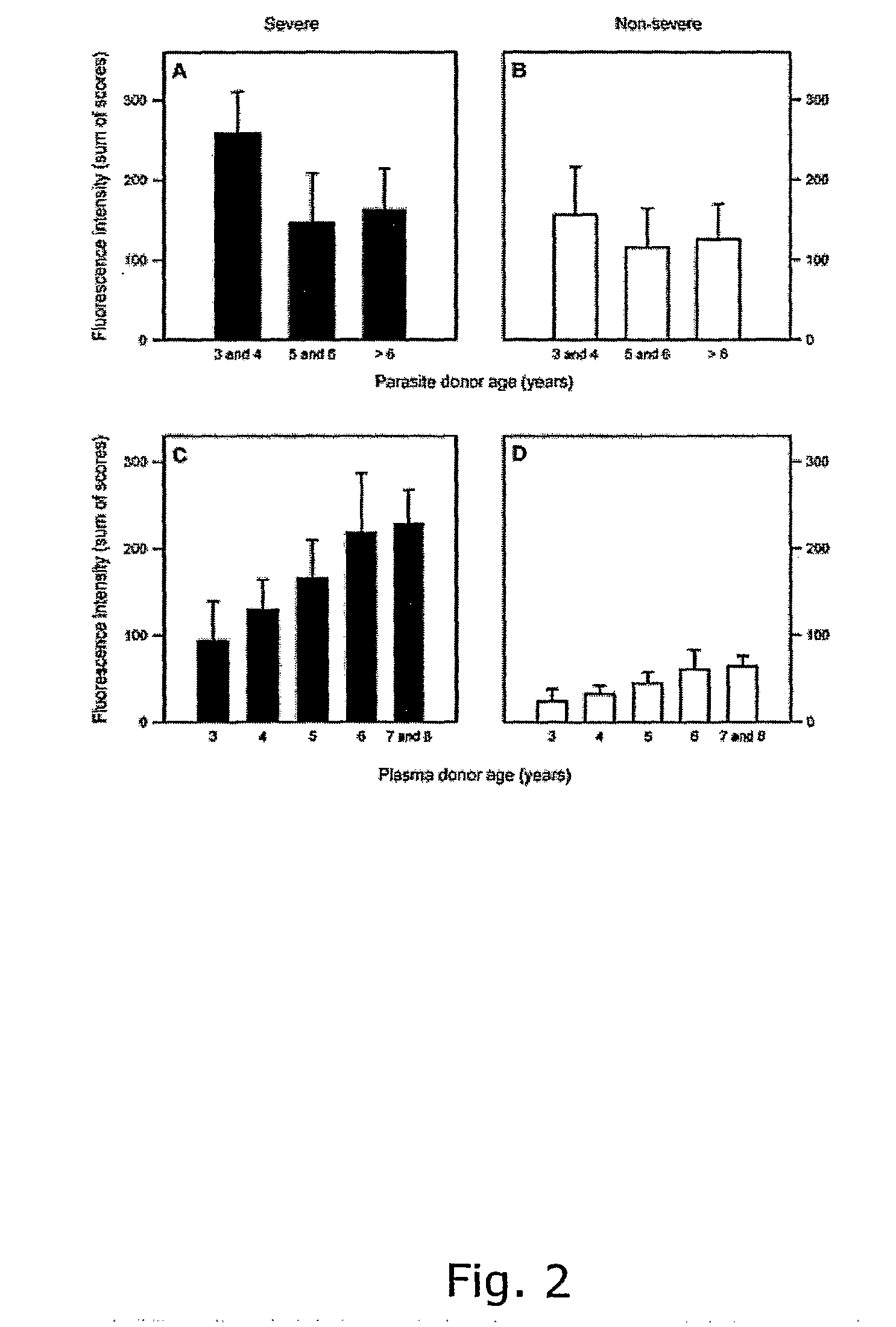
Compounds useful in the diagnosis and treatment of pregnancy-associated malaria
ActiveUS20070053928A1Avoid stickingRaise the possibilityPeptide/protein ingredientsProtozoaPregnancyRed blood cell
The present invention relates to nucleic acid molecules related to the var2csa gene family as well as amino acid sequences encoded by such nucleic acid molecules with respect to their role in mediating adhesion of infected red blood cells to chondroitin sulphate A (CSA) in the placenta which is characteristic for the pathogenesis of pregnancy associated malaria (PAM). Accordingly, The invention provides the use compounds that are related to VAR2CSA polypeptides var2csa nucleic acid molecules as medicaments, as well as it provides pharmaccutical compositions, in particular immunological compositions and vaccines, hereunder nucleotide-based vaccines comprising these compounds. In addition, the invention provides the use of the compounds mentioned for the manufacture of compositions, such as immunogenic compositions. Other aspects of the invention relates to methods of treatment and prevention of pregnancy associated malaria wherein these methods are based on the nucleic acid molecules and polypeptides the invention. As these compounds can also be used as biotechnological tools the invention provides in vitro diagnostic methods and kits comprising reagents and IgGs / antibodies designated to the use in such methods. The invention also relates to methods of identifying agents capable of modulating the VAR2CSA dependent adhesion to CSA and agent capable of interacting with VAR2CSA. Finally, a method for identifying polypeptides, which will induce a specific IgG / antibody response upon administration to a subject is provided by the invention.
Owner:UNIVERSITY OF COPENHAGEN
Diagnostic kit for mycobacterium tuberculosis dormant infection
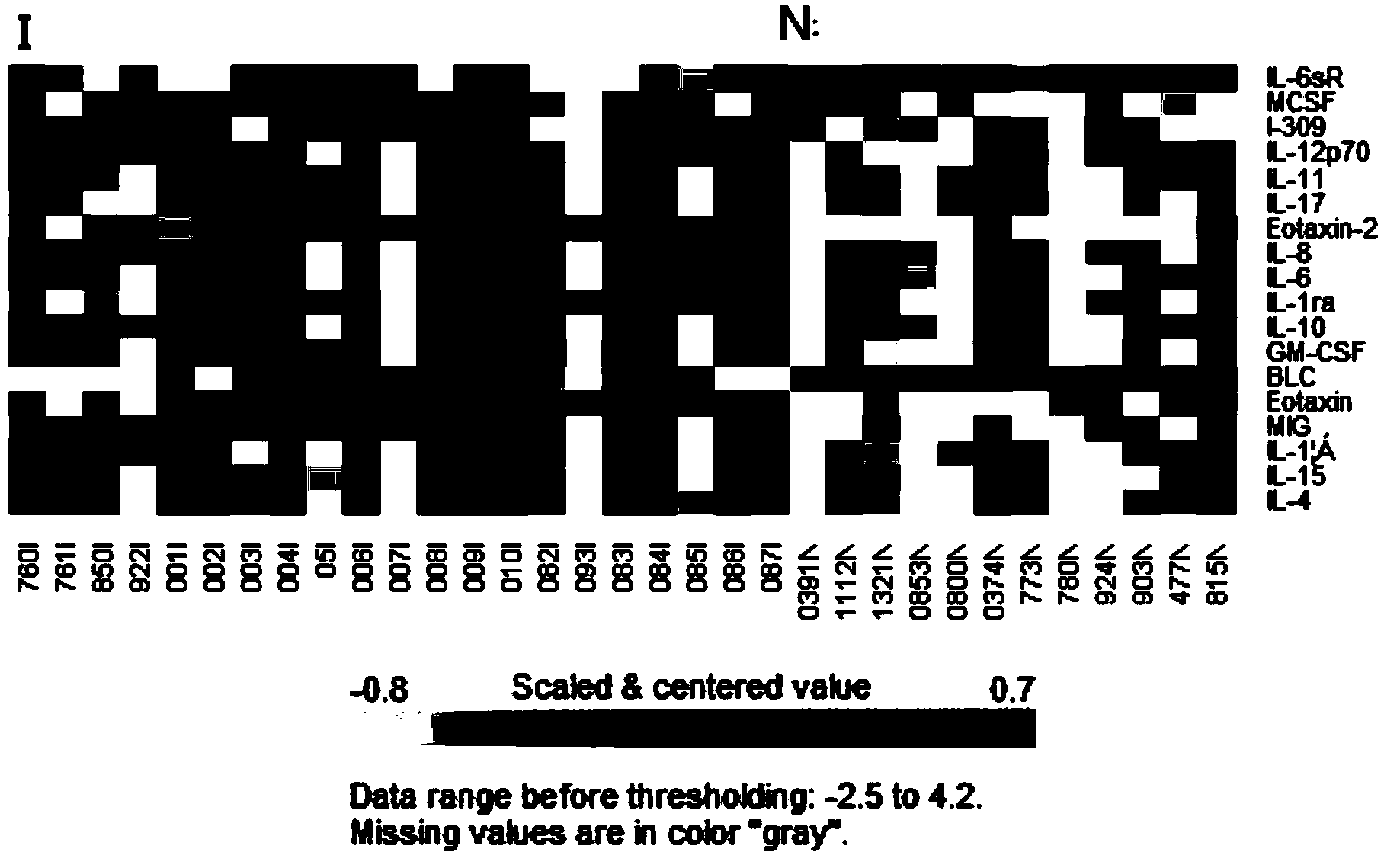
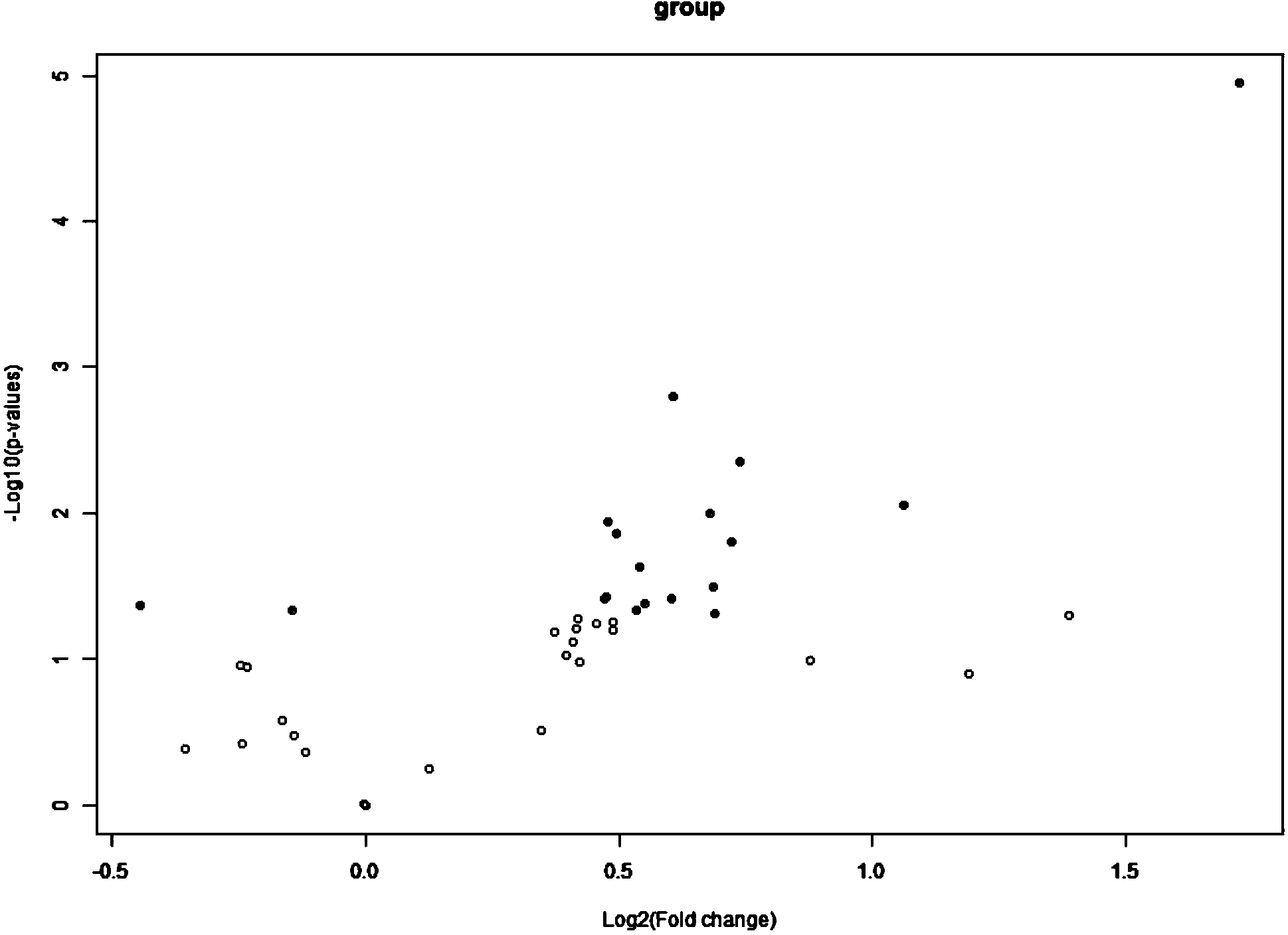
The invention discloses a diagnostic kit for mycobacterium tuberculosis dormant infection. The kit can specifically recognize at least one of cell factors Eotaxin-2, MIG, Eotaxin, MCSF or 1L-12p70, can quantitatively analyze the recognized cell factors and specifically recognize antigens 38-KDa, 32-KDa and 14-16-KDa and specific IgG antibody of Ag85B. The selected five cell factors have remarkable expression difference in uninfected population and mycobacteria dormant infection patients, are used as markers for detecting the mycobacteria dormant infection patients, can well differentiate dormant infection persons and uninfected persons, and has high expression quantity and high diagnostic sensitivity; besides, the kit is combined with the specific antibody for detecting four antigens, the diagnosis accuracy and the diagnosis sensitivity are further improved; the kit is directly used for detecting markers in serum, is convenient and rapid to operate and is suitable for popularization and application.
Owner:广东省结核病控制中心
Streptococcus pneumonia fusion protein and vaccine thereof
InactiveCN105968213AImprove protectionAntibacterial agentsBacterial antigen ingredientsSpecific iggStreptococcus mitis
The invention relates to the technical field of biology, in particular to streptococcus pneumonia fusion protein and a vaccine thereof. The fusion protein is an expression product formed by recombination of a streptococcus pneumonia virulence protein gene and a protein connector gene, and streptococcus pneumonia virulence protein is Ply or PspA or PsaA or PcpA or PhtD. After the vaccine of the streptococcus pneumonia fusion protein immunizes an animal through the nasal cavity, a high-potency serum specific IgG antibody can be induced, high-potency local mucosa specific IgA can be induced, and a good protective effect is achieved for nasal cavity toxin counteracting of different streptococcus pneumonia strains.
Owner:CHANGCHUN BCHT BIOTECH +1
Helicobacter pylori multiple-epitope fusion protein and multiple-epitope vaccine prepared by helicobacter pylori multiple-epitope fusion protein
InactiveCN102838680AAntibacterial agentsBacterial antigen ingredientsSpecific iggHelicobacter pylori
The invention relates to a helicobacter pylori multiple-epitope fusion protein and a multiple-epitope vaccine prepared by the helicobacter pylori multiple-epitope fusion protein. An amino acid sequence of the helicobacter pylori multiple-epitope fusion protein is shown as SEQ ID NO:1. Fusion proteins in the multiple-epitope vaccine can induce generation of specificity sIgA in stomach tissues and formation of phigh-potency specificity IgG in blood serum, can effectively reduce constant value quantity of helicobacter pylori in a stomach of a mouse and has obvious protection effects.
Owner:ARMY MEDICAL UNIV
Method for obtaining human antibody variable region by combination of SOLID sequencing and single-cell RT-PCR
InactiveCN102787118AGood technology platformFermentationPlant genotype modificationDiseaseSpecific igg
The present invention provides a method for obtaining a human antibody variable region by combination of SOLID sequencing and single-cell RT-PCR. According to the method, a cell surface molecular marker is adopted to screen a single cell expressing a specific antibody; mRNA of the single cell is subjected to a reverse transcription to obtain a cDNA sequence; then a designed specific IgG / M primer group is adopted to carry out a PCR amplification; the resulting amplification product is adopted as a template, and SOLID sequencing primers (Barcoded Primers) are adopted to carry out one PCR amplification; and finally the resulting amplification product by adopting the Barcoded Primers is adopted as a template, SOLID sequencing is performed, and the sequencing result is subjected to bioinformatics analysis to obtain a human antibody variable region gene sequence. The method of the present invention can be widely used for obtaining variable region sequences of heavy chains and light chains of specific antibodies in a variety of diseases, and advantages of rapidness and efficiency of the method provide an effective means for production of fully humanized antibody drugs.
Owner:孙毅 +3
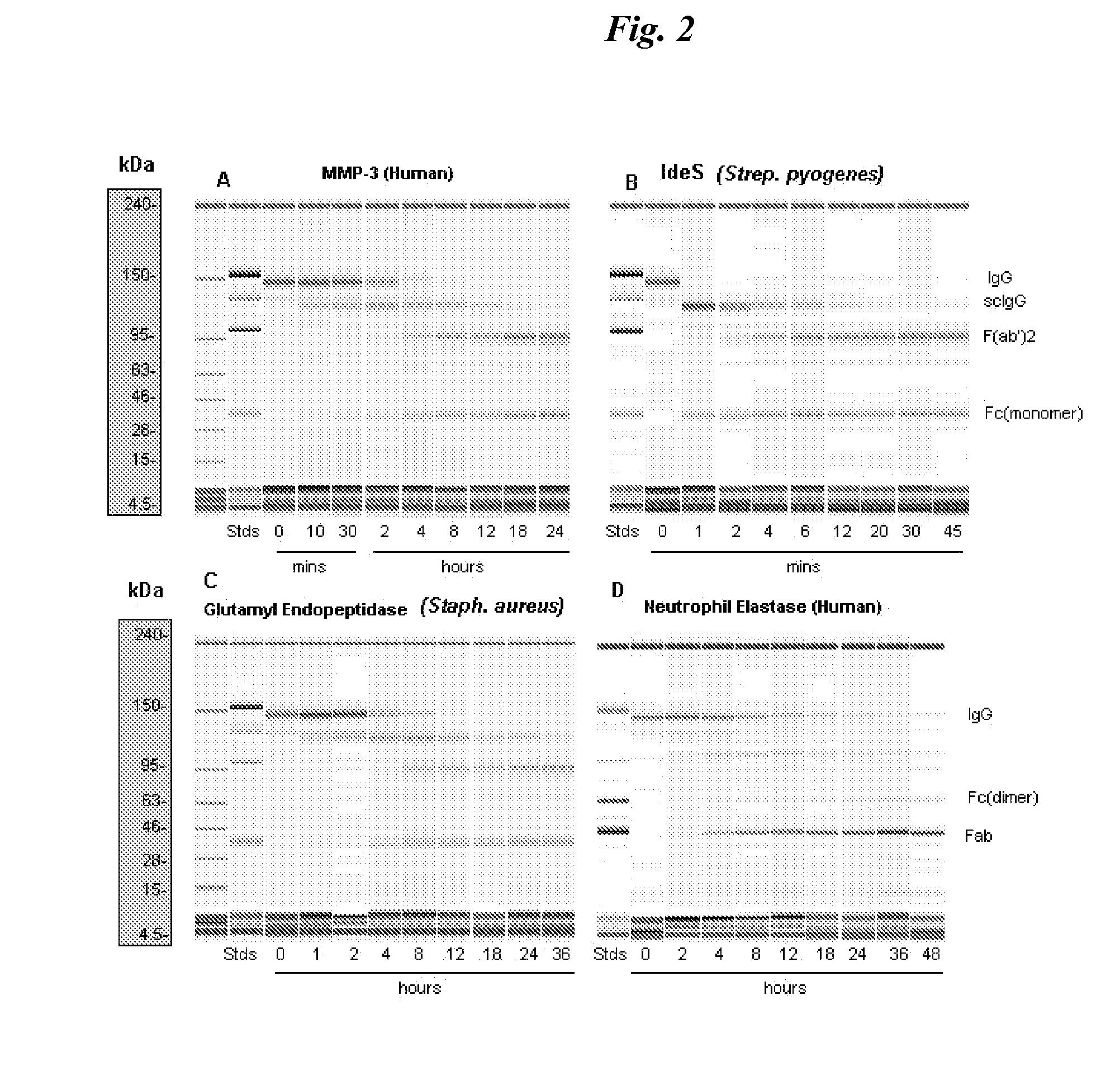
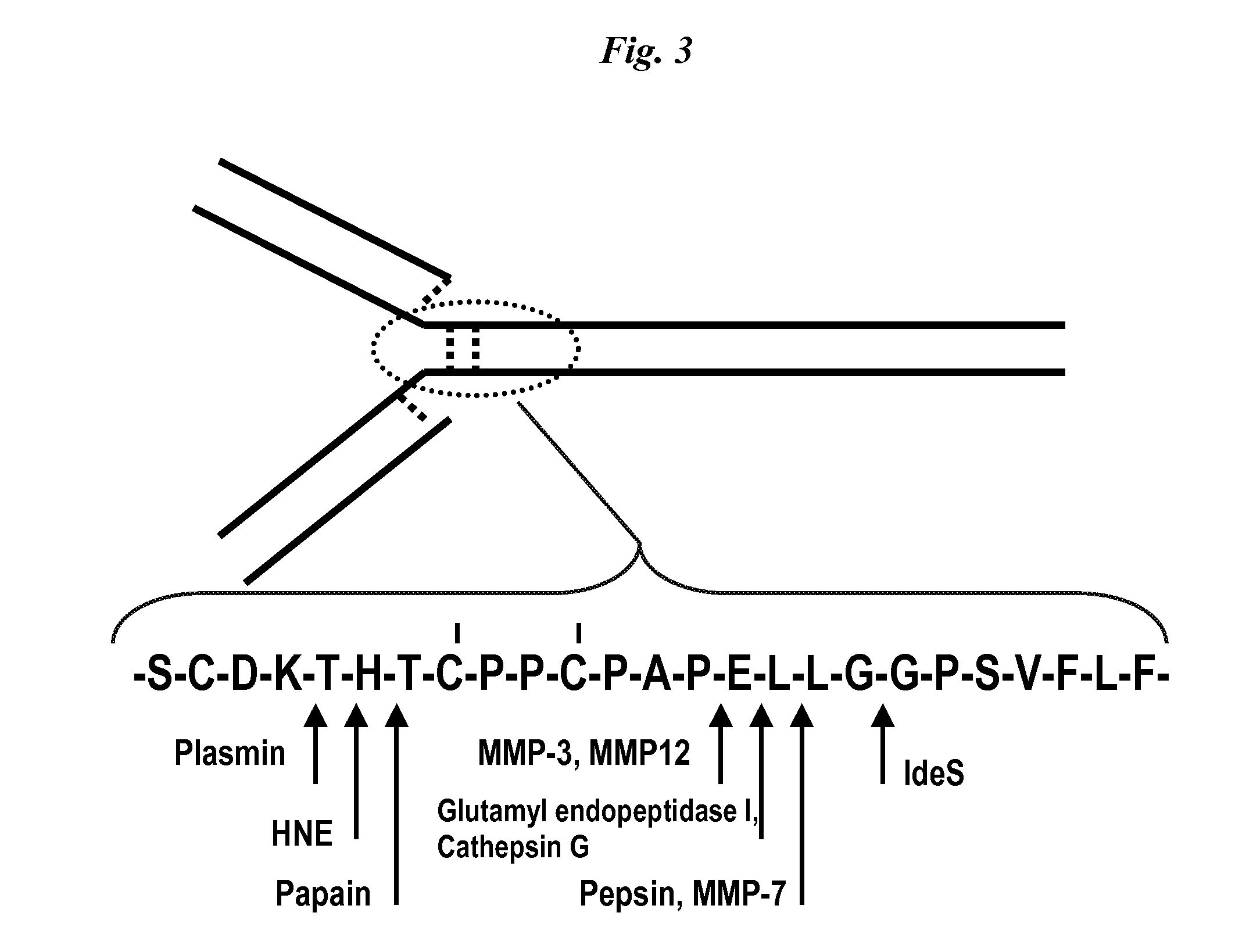
Immunoglobulin cleavage fragments as disease indicators and compositions for detecting and binding such
ActiveUS8501907B2Antibacterial agentsImmunoglobulins against blood coagulation factorsProteinase activitySpecific igg
The invention relates to antibody compositions and use of the composition to detect disease processes associated with elaboration of proteases. The reagents are directed to assessing an IgG breakdown product that is the result of such proteolytic cleavage. The invention further relates to the use of a therapeutic immunospecific for IgG protease cleavage products to restore effector function to antibody compositions that are subject to protease cleavage.
Owner:JANSSEN BIOTECH INC
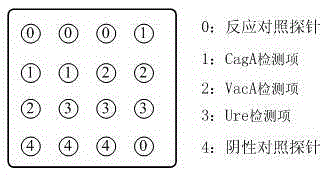
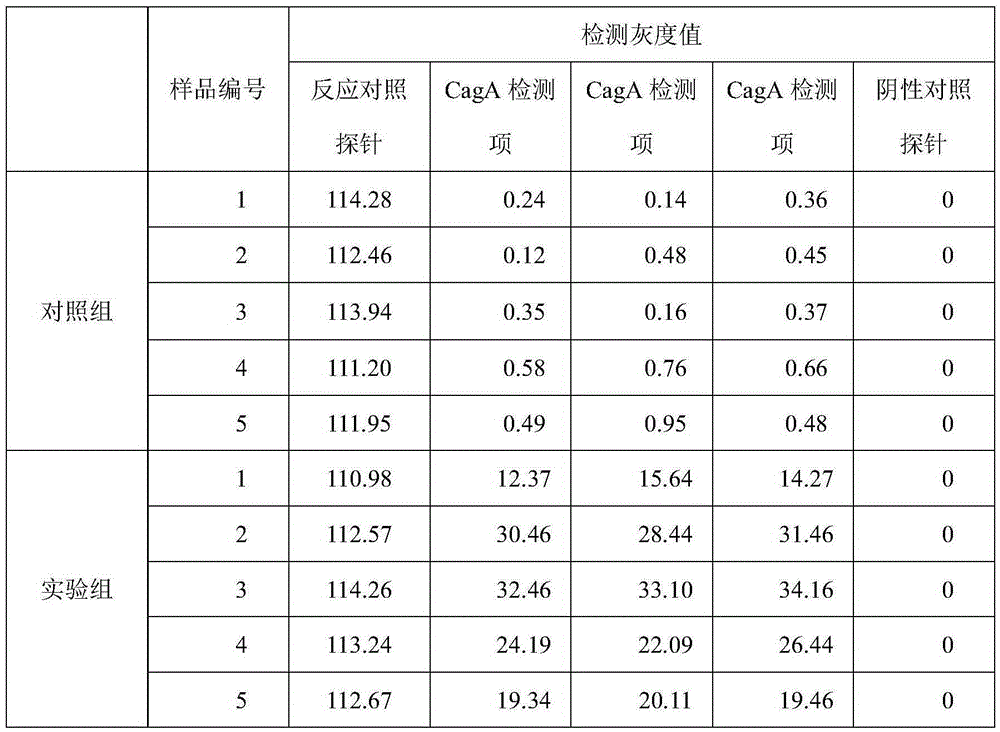
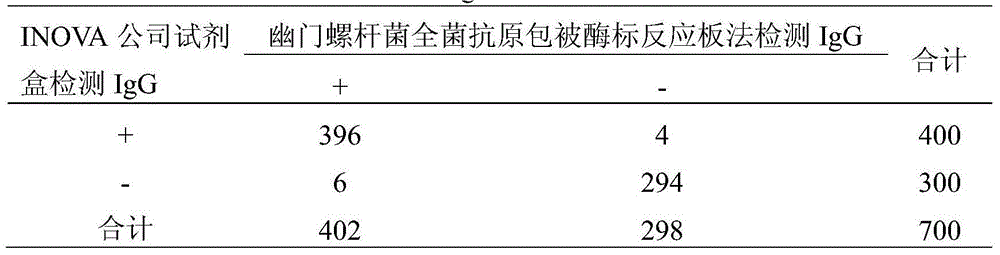
Protein chip for typing detection on helicobacter pylori infection
InactiveCN105277722AReduce dosageBeneficial to clinical treatmentBiological material analysisBiological testingSpecific iggProtein C
The invention provides a protein chip for typing detection on helicobacter pylori infection. The protein chip comprises a basement membrane and antigens which are respectively dotted on the basement membrane in the form of dot matrix, wherein the antigens include three types, i.e. CagA (cytotoxin-associated gene) antigens, VacA (Vacuolating cytotoxin A) antigens and Ure (ureaplasma urealyticum) antigens. Due to the fact that the detection antigens used by the protein chip are three single proteins, i.e. Ure, CagA and VacA, specific IgG antibodies for the three antigens in the blood serum of a patient can be detected at the same time, I-type infection or II-type infection can be judged, and clinic treatment is better facilitated; furthermore, the protein chip has the advantages of high detection sensitivity, quick, simple and convenient detection, instant readability, small using amount of antigen and the like.
Owner:TAIZHOU SYNO GENE DIGITAL TECH CO LTD
Helicobacter pylori IgG antibody ELISA semi-quantitative detection kit and application thereof
InactiveCN104833804AImprove consistencyImprove featuresBiological material analysisPositive controlSpecific igg
The invention provides a helicobacter pylori IgG antibody ELISA semi-quantitative detection kit. The kit is formed by a helicobacter pylori antigen coated enzyme labeled reaction plate, a sample diluting solution, a washing liquid, an enzyme labeled second antibody, a substrate solution, a stopping solution, a positive control solution, a negative control solution and a standard solution. A method for rapid and sensitive ELISA semi-quantitative detection of a helicobacter pylori specific IgG antibody in serum is established by using the kit. The helicobacter pylori IgG antibody standard solution is prepared, the helicobacter pylori antigen coated enzyme labeled reaction plate is prepared based on the standard solution, an indirect ELISA method is adopted to detect the specific helicobacter pylori IgG antibody in the serum, and the specific helicobacter pylori IgG antibody is quantified through calculation of an enzyme immunity unit (EIU). The kit has the advantages of high specificity and high sensitivity, and is mainly used for laboratory researches and clinic auxiliary diagnosis.
Owner:BIOHIT BIOTECH HEFEI
Schistosoma japonicum katsurada recombinant antigen as well as preparation method and application thereof
The invention discloses schistosoma japonicum katsurada recombinant antigen. The recombinant antigen comprises an amino acid sequence of schistosoma japonicum katsurada PGMRC2 protein indicated by SEQ ID No.1. The invention also discloses a preparation method of schistosoma japonicum katsurada antigen and application of the antigen in preparing a vaccine or a medicament for preventing or treating the schistosoma japonicum katsurada disease. By adopting the schistosoma japonicum katsurada recombinant antigen, the specificity IgG, IgG1 and IgG1a antibodies for resisting the recombinant antigen can be induced in a mice body, the worm reduction rates of 22.08 percent and 20.097 percent are respectively induced in two animal protection experiments, so that the recombinant antigen is suitable for being used as a candidate vaccine for resisting the schistosoma japonicum katsurada and has good application prospect.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
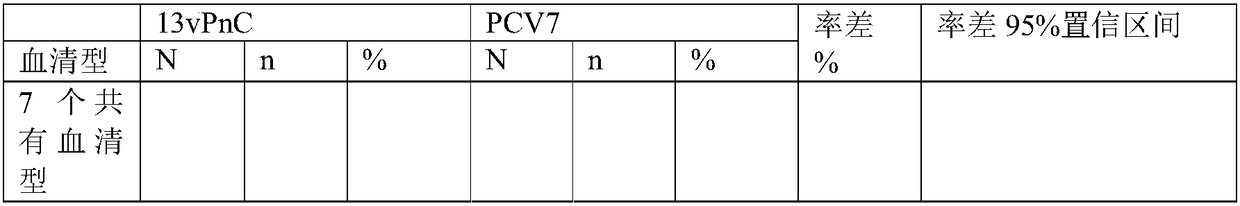
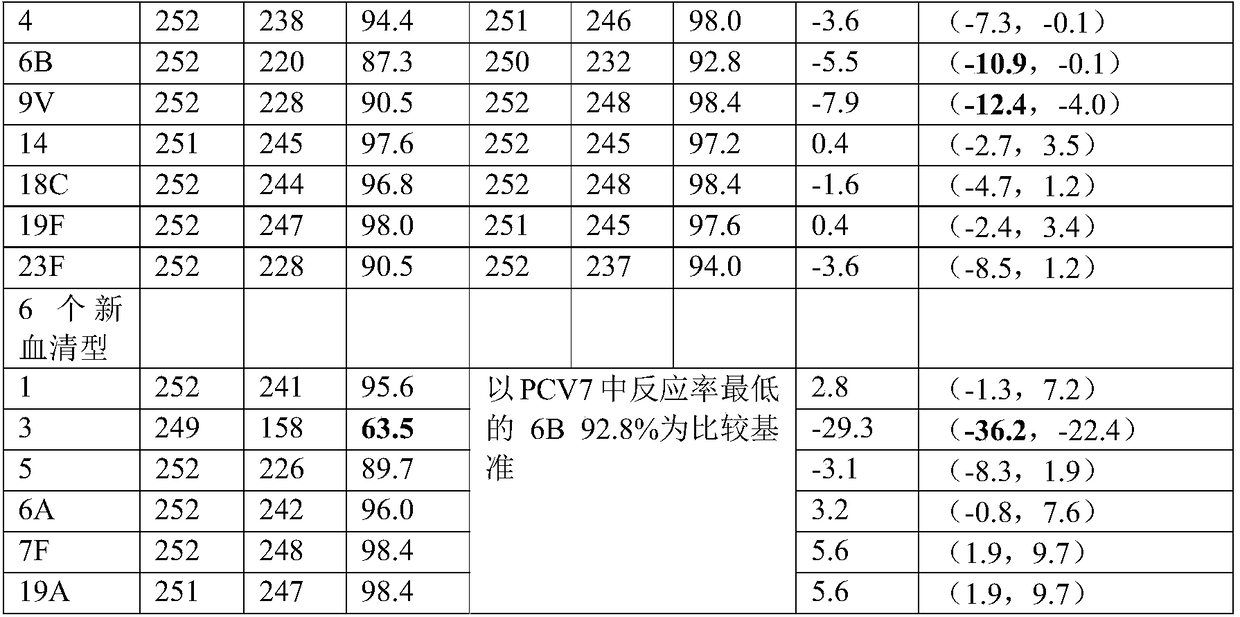
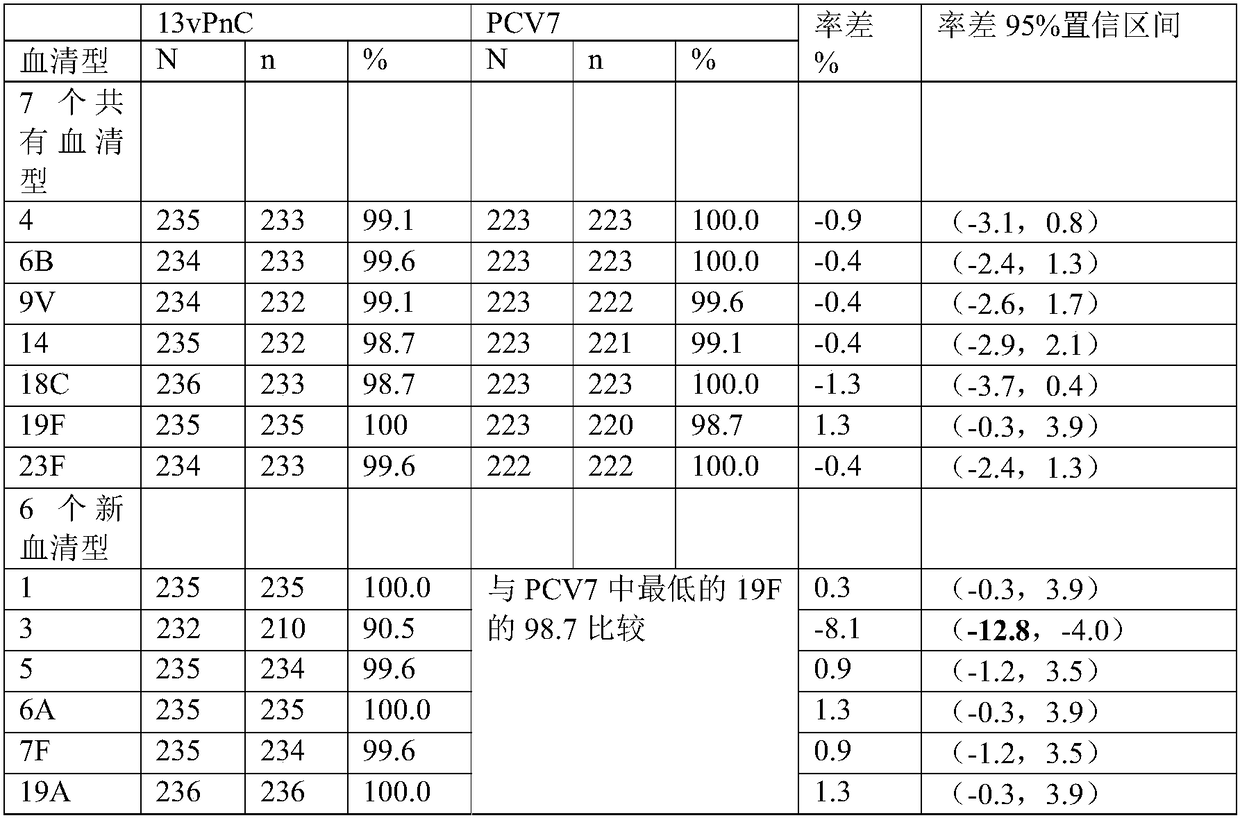
13-valent pneumococcal polysaccharide-protein conjugate composition and preparation method and application thereof
ActiveCN108079286AImproving immunogenicityReduce free proteinAntibacterial agentsBacterial antigen ingredientsPneumococcal serotypesThree stage
The invention discloses a 13-valent pneumococcal polysaccharide-protein conjugate composition and a preparation method and application thereof. The composition comprises 13 kinds of different pneumococcal polysaccharide-protein conjugates, wherein each pneumococcal polysaccharide-protein conjugate contains capsular polysaccharides and carrier protein from different pneumococcal serotypes, whereinthe capsular polysaccharides derive from type 1, type 3, type 4, type 5, type 6A, type 6B, type 7F, type 9V, type 14, type 18C, type 19A, type 19F and type 23F pneumococci, and the carrier protein isTT of which the monomer purity is greater than or equal to 90%. By virtue of three stages of clinical research and verification, the composition provided by the invention can realize induced production of serotype-specific IgG antibodies for people from 2 months old to 5 years old, wherein after main target crowds from 2 to 6 months old are subjected to three-needle fundamental immunization, the proportion that the concentration of all serotype-specific IgG is greater than or equal to 0.35mu g / ml is greater than or equal to 85%, and after reinforced immunization, the proportion is greater thanor equal to 95%.
Owner:云南沃森生物技术股份有限公司
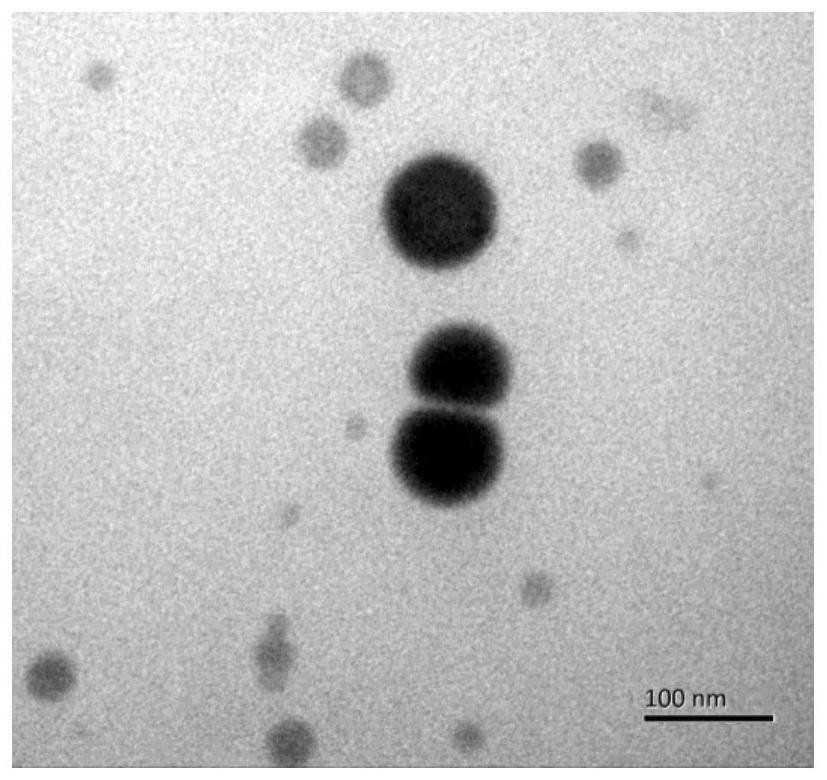
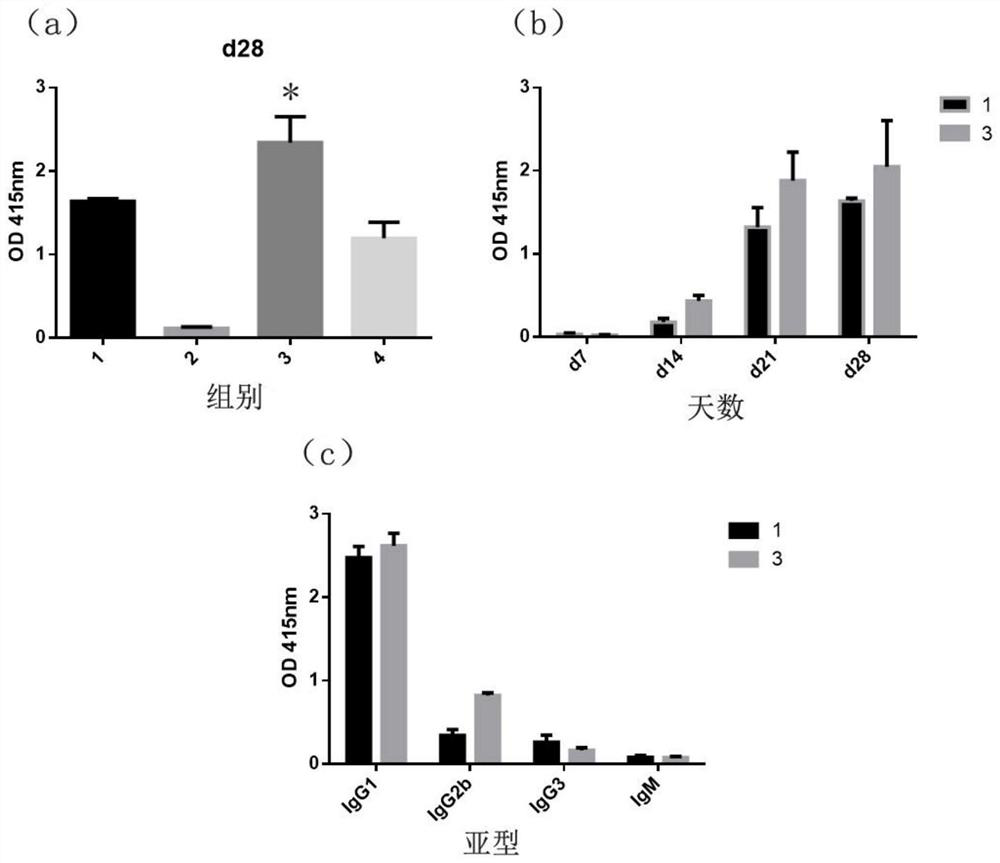
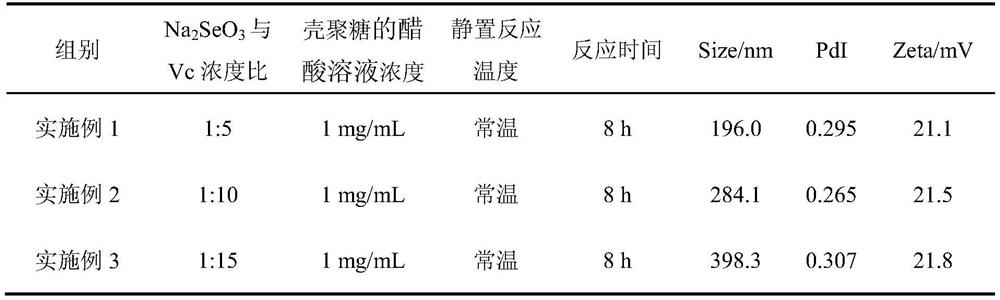
Chitosan nano-selenium particles, preparation method thereof and application of chitosan nano-selenium particles in vaccines
PendingCN112870348AHigh biosecurityImprove physical stabilityPowder deliveryCancer antigen ingredientsSpecific iggEngineering
The invention discloses chitosan nano-selenium particles, a preparation method thereof and application of the chitosan nano-selenium particles in vaccines, and the chitosan nano-selenium particles are prepared from chitosan, sodium selenite and other raw materials and can achieve efficient wrapping of antigens and vaccine adjuvants. The chitosan nano-selenium particles disclosed by the invention have extremely good biological safety. The chitosan nano-selenium particles have good physical stability and thermal stability, and are suitable for industrial preparation of vaccines. As an immune carrier or adjuvant, the chitosan nano-selenium particles can promote phagocytosis, treatment and presentation of antigen-presenting cells to antigens, induce generation of higher-level antigen-specific IgG antibody titer, and have important application value in the field of vaccine development.
Owner:JIANGNAN UNIV
Schistosoma japonicum recombinant antigen, and preparation method and application thereof
The invention discloses a schistosoma japonicum recombinant antigen, and the recombinant antigen comprises an amino acid sequence, shown as SEQ ID NO. 1, of schistosoma japonicum phosphoglyceric kinase. The invention also discloses a preparation method of the schistosoma japonicum recombinant antigen and application of the schistosoma japonicum recombinant antigen to prepare vaccines or medicines for preventing or controlling schistosomiasis. In mouse immunization experiments, the schistosoma japonicum recombinant antigen is capable of inducing a mouse to generate specific IgG, IgG1 and IgG2a antibodies capable of resisting the recombinant antigen in vivo at a relatively high level, and in animal protection experiments, through induction of the schistosoma japonicum recombinant antigen, the insect reducing rate is 34.5% and the ovum reducing rate is 32.2%. The experiment results prove that the recombinant antigen is applicable as a vaccine candidate for resisting schistosomiasis and has extremely good application prospect.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Compounds useful in the diagnosis and treatment of malaria
InactiveUS20090324628A1Reduce incidenceReduce the prevalenceDepsipeptidesImmunological disordersSpecific iggMalaria
The present invention relates to nucleic acid molecules related to the PFD1235w / MAL7P1.1, PF11_0008, and PF13_0003 gene families as well as amino acid sequences encoded by such nucleic acid molecules with respect to their role in mediating adhesion of infected red blood cells to endothelial cells, which is characteristic for the pathogenesis of severe malaria (SM). Accordingly, the invention provides pharmaceutical compositions and vaccines, hereunder nucleotide-based vaccines comprising compounds that are related to VAR4, VAR5, and / or VAR6 polypeptides and PFD1235w / MAL7P1.1 PF11_0008, and / or PF13_0003 nucleic acid molecules. The invention further relates to the use of these compounds as medicaments and for the manufacture of compositions, such as immunogenic compositions. In addition, the invention relates to methods of treatment and prevention of severe malaria wherein these methods are based on the nucleic acid molecules and polypeptides of the invention. As these compounds can also be used as biotechnological tools the invention provides in vitro diagnostic methods and kits comprising reagents and IgGs / antibodies designated to the use in such methods. The invention also relates to methods of identifying agents capable of modulating the VAR4, VAR5, and / or VAR6 dependent adhesion to endothelial cells and agent capable of interacting with VAR4, VAR5, and / or VAR6. Finally, a method for identifying polypeptides, which will induce a specific IgG / antibody response upon administration to a subject is provided by the invention.
Owner:KOBENHAVNS UNIVT PANIUM
Multispecific constructs
InactiveUS10047163B2Improve assembly and stabilityMediating cell killingHybrid immunoglobulinsImmunoglobulins against growth factorsHeavy chainSpecific igg
Provided are novel multispecific antibody constructs and multispecific antibody drug conjugates (ADCs), and methods of using such antibodies and ADCs to treat cancer. IgG-like bispecific antibodies have different binding specificities on each arm of the antibody. They are similar in structure to monospecific IgGs in that they contain two heavy chains with VH, CH1, CH2 and CH3 regions, and two light chains with VL and CL regions.
Owner:ABBVIE STEMCENTRX LLC
Compounds useful in the diagnosis and treatment of pregnancy-associated malaria
ActiveUS7745580B2Raise the possibilitySimple processPeptide/protein ingredientsProtozoaNucleotideSpecific igg
The present invention relates to nucleic acid molecules related to the var2csa gene family as well as amino acid sequences encoded by such nucleic acid molecules with respect to their role in mediating adhesion of infected red blood cells to chondroitin sulphate A (CSA) in the placenta which is characteristic for the pathogenesis of pregnancy associated malaria (PAM). Accordingly, The invention provides the use compounds that are related to VAR2CSA polypeptides var2csa nucleic acid molecules as medicaments, as well as it provides pharmaceutical compositions, in particular immunological compositions and vaccines, hereunder nucleotide-based vaccines comprising these compounds. In addition, the invention provides the use of the compounds mentioned for the manufacture of compositions, such as immunogenic compositions. Other aspects of the invention relates to methods of treatment and prevention of pregnancy associated malaria wherein these methods are based on the nucleic acid molecules and polypeptides the invention. As these compounds can also be used as biotechnological tools the invention provides in vitro diagnostic methods and kits comprising reagents and IgGs / antibodies designated to the use in such methods. The invention also relates to methods of identifying agents capable of modulating the VAR2CSA dependent adhesion to CSA and agent capable of interacting with VAR2CSA. Finally, a method for identifying polypeptides, which will induce a specific IgG / antibody response upon administration to a subject is provided by the invention.
Owner:UNIVERSITY OF COPENHAGEN
Test paper strip for detecting encephalitis virus specificity IgG antibody, method for making same and applications
ActiveCN101363864ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseSpecific igg
The invention provides a colloidal gold test strip for the detection of Japanese encephalitis virus specific IgG antibody. A Japanese encephalitis virus E gene antigen domain III and an anti III polyclonal antibody are coated on a nitrate cellulose film (NC film), and a membrane chromatography double antigen sandwich method is adopted to detect the Japanese encephalitis virus specific IgG antibody in an animal or human body serum specimen in combination with a colloidal gold labeled Japanese encephalitis virus E gene antigen domain III. Or the Japanese encephalitis virus E gene antigen domain III and an anti-mouse IgG are coated on the nitrate cellulose film (NC film), and a capture method is adopted to detect the Japanese encephalitis virus specific IgG antibody in the human body serum specimen in combination with a colloidal gold labeled antihuman monoclonal antibody. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, has auxiliary effect on the diagnosis of Japanese encephalitis virus infection, and can be used for the effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
Schistosoma japonicum katsurada recombinant protein and its preparation method and use
InactiveCN105566475AImmunoglobulins against animals/humansAntiparasitic agentsSpecific iggDrug target
The invention discloses a schistosoma japonicum katsurada recombinant protein. The schistosoma japonicum katsurada recombinant protein is prepared through expression of a schistosoma japonicum katsurada Vamp2 gene-containing recombinant vector. The invention also discloses a use of the schistosoma japonicum katsurada recombinant protein as a schistosoma japonicum katsurada diagnosis antigen and a use of the schistosoma japonicum katsurada recombinant protein in preparation of a vaccine or drug for preventing or treating bilharziasis. In a mouse immunization experiment, the schistosoma japonicum katsurada recombinant protein can induce generation of anti-recombinant protein specific IgG, IgG1 and IgG2a antibodies in a mouse and an antibody generation level is high. An animal protection experiment result shows that the recombinant protein has a potential as an anti-bilharziasis candidate vaccine and a novel drug target. A diagnosis antigen effect assessment experiment result shows that the recombinant protein has a potential as a diagnosis antigen and has a wide application prospect.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
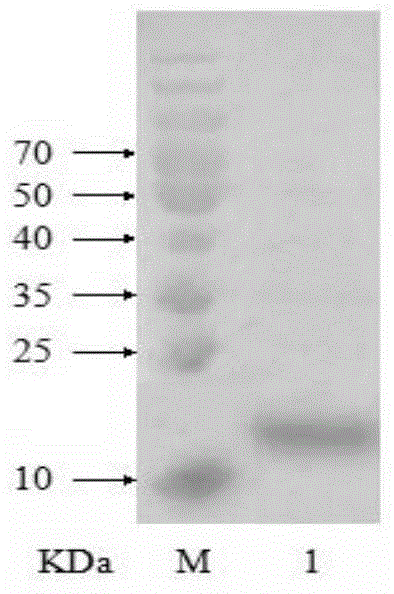
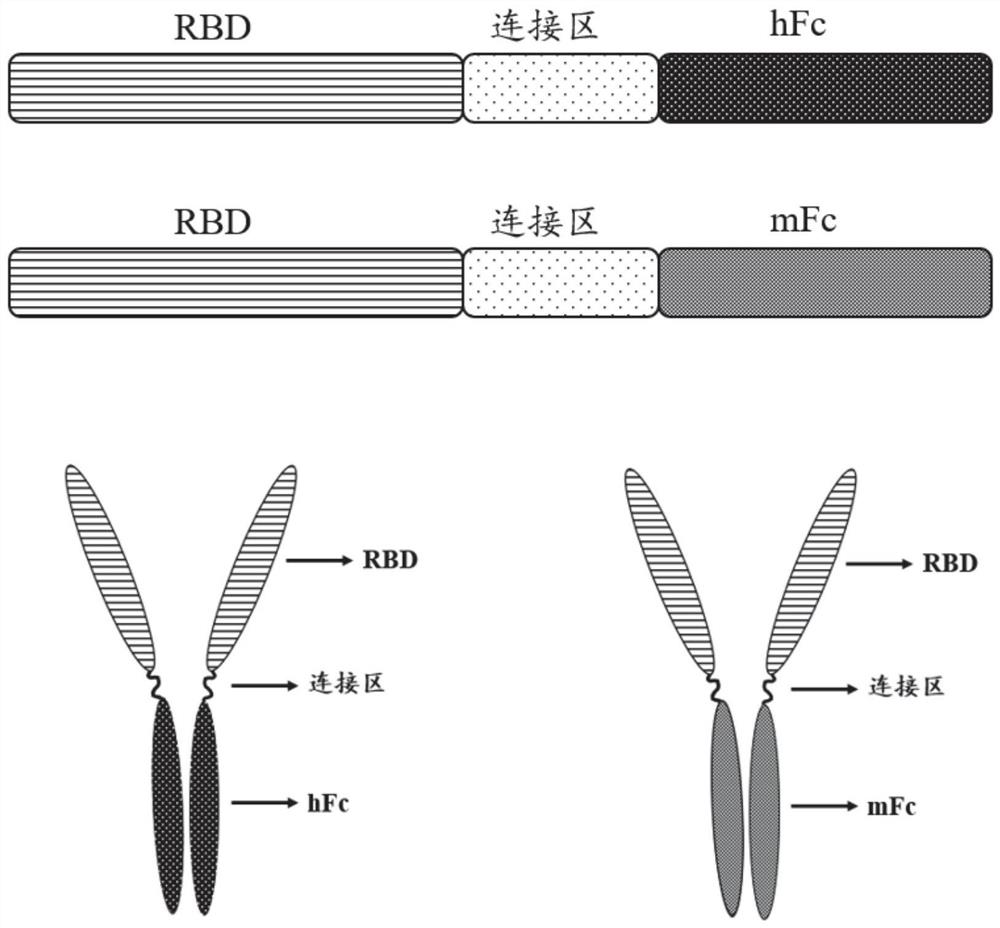
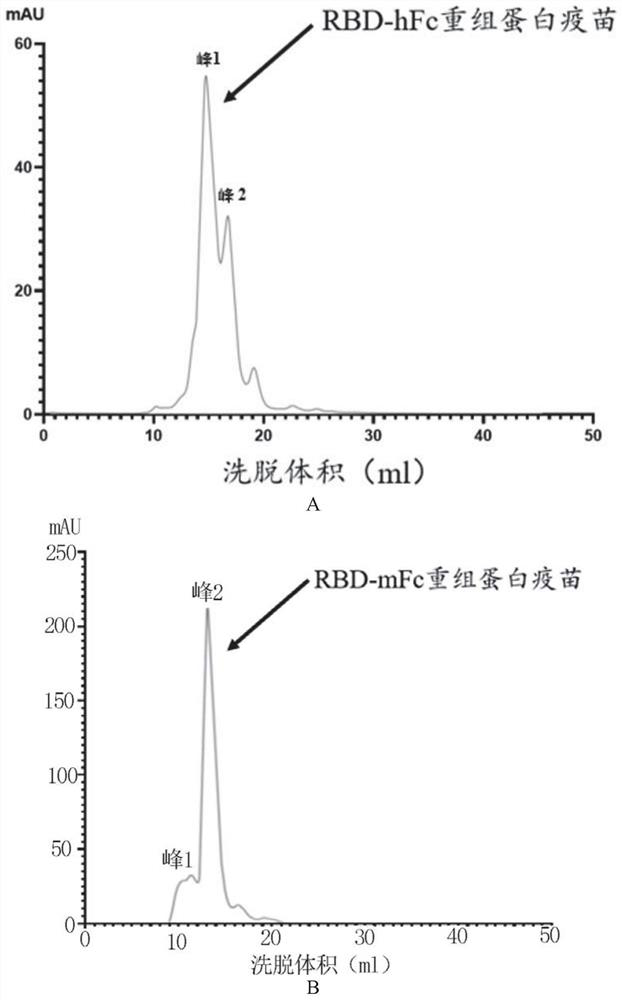
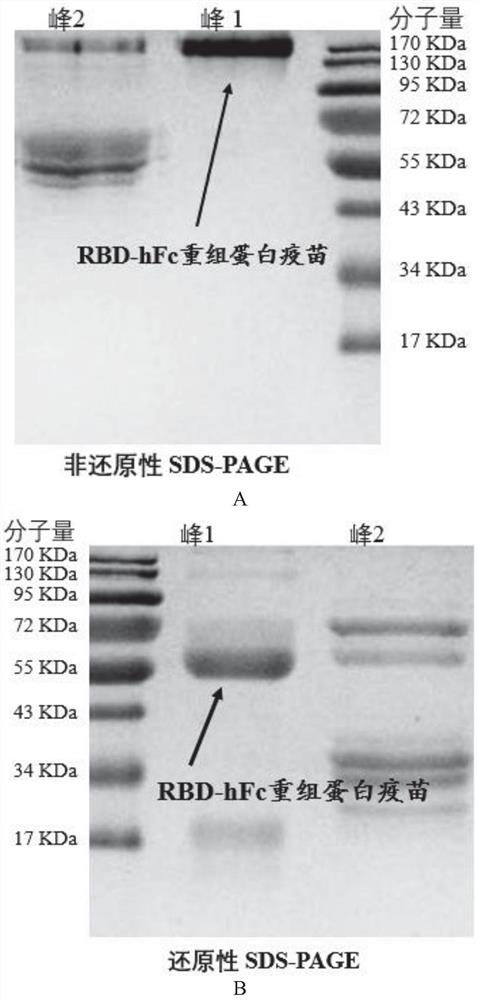
Fusion protein and application thereof in preparation of novel coronavirus subunit vaccine
PendingCN112851824AExtended half-lifeSsRNA viruses positive-senseAntibody mimetics/scaffoldsDisulfide bondingDimer
The invention discloses a fusion protein and application thereof in preparation of a novel coronavirus subunit vaccine. The fusion protein comprises an RBD region of SARS-CoV-2 virus S protein and an Fc region of an IgG antibody, wherein the RBD region and the Fc region are subjected to fusion expression. A recombinant fusion protein is obtained by performing fusion expression on an RBD structural domain of a spinous process protein of SARS-CoV-2 and an immunoglobulin Fc, and a hinge region and a constant region of the Fc can combine two heavy chains of an IgG antibody through a disulfide bond, so that an RBD dimer is formed; meanwhile, the prepared recombinant fusion protein can be used as a recombinant protein vaccine aiming at novel coronavirus pneumonia. The recombinant protein vaccine can induce generation of a high-titer specific IgG antibody and a neutralizing antibody in a mouse body, the antibody level can be maintained for more than 3 months, and the vaccine can induce the mouse to generate cellular immunity at the same time.
Owner:ZHEJIANG UNIV +1
High-throughput treponema pallidum specific antibody detection kit and preparation method thereof
ActiveCN104330562AAccurate detectionAccurate Epidemiological SurveyMaterial analysisSpiroplasmaSpecific igm
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com