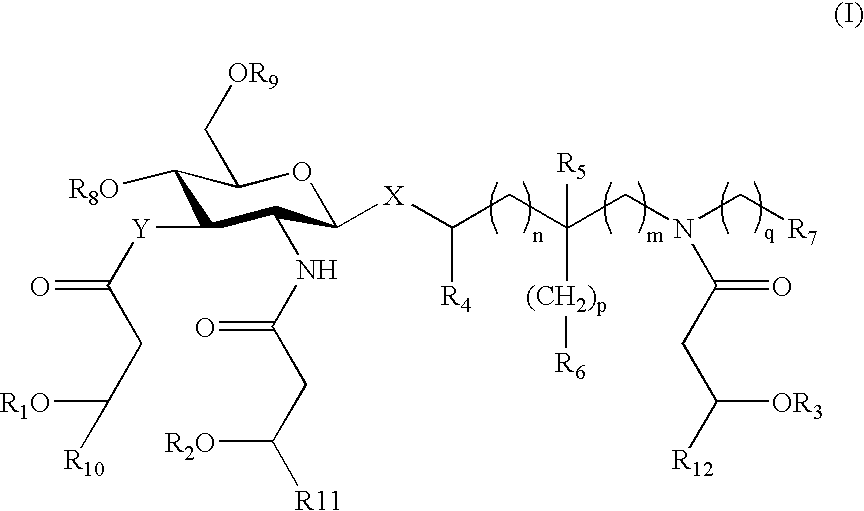
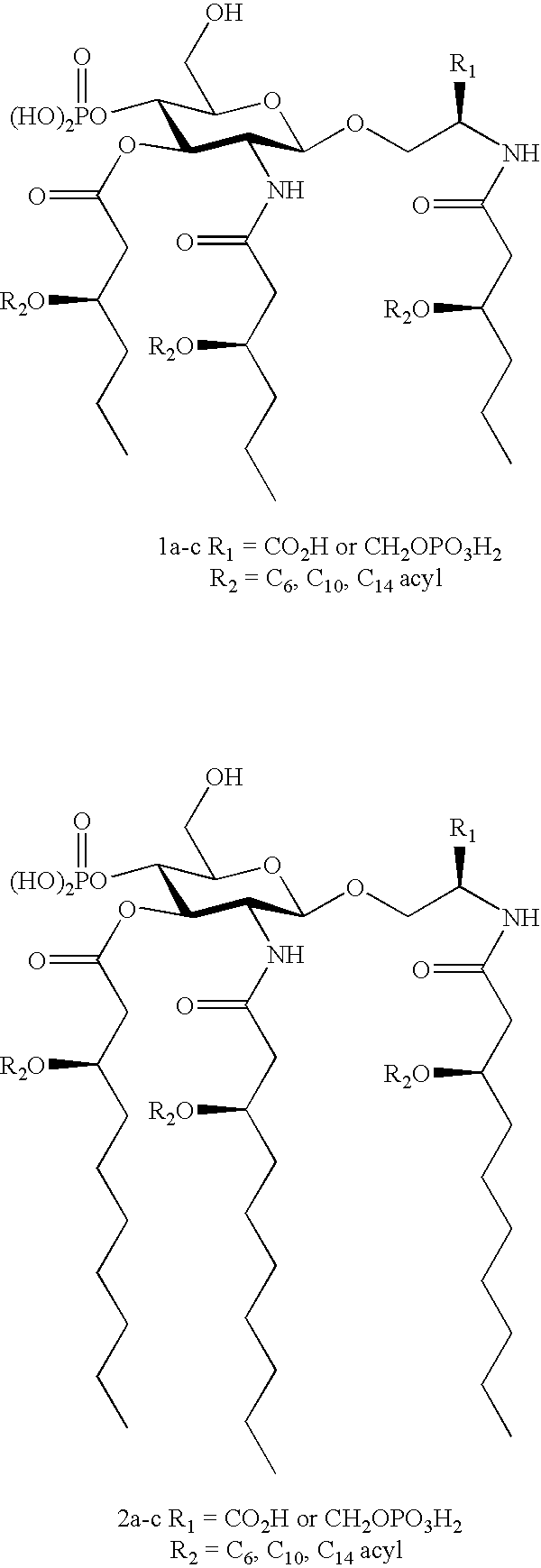
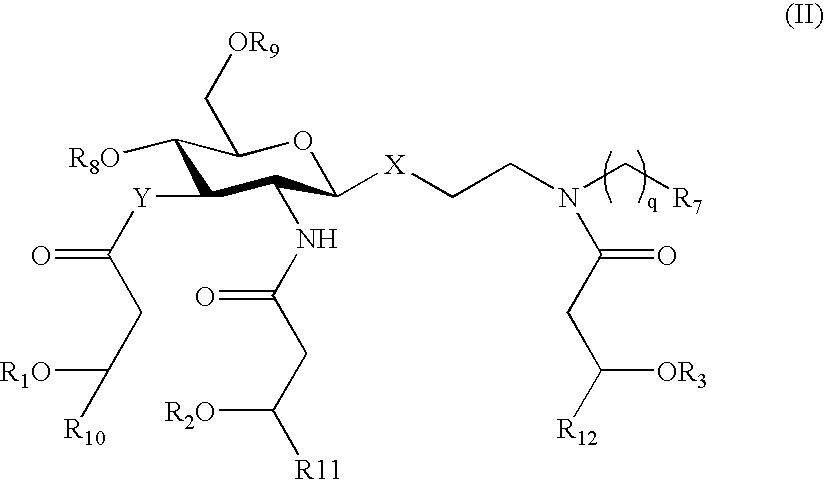
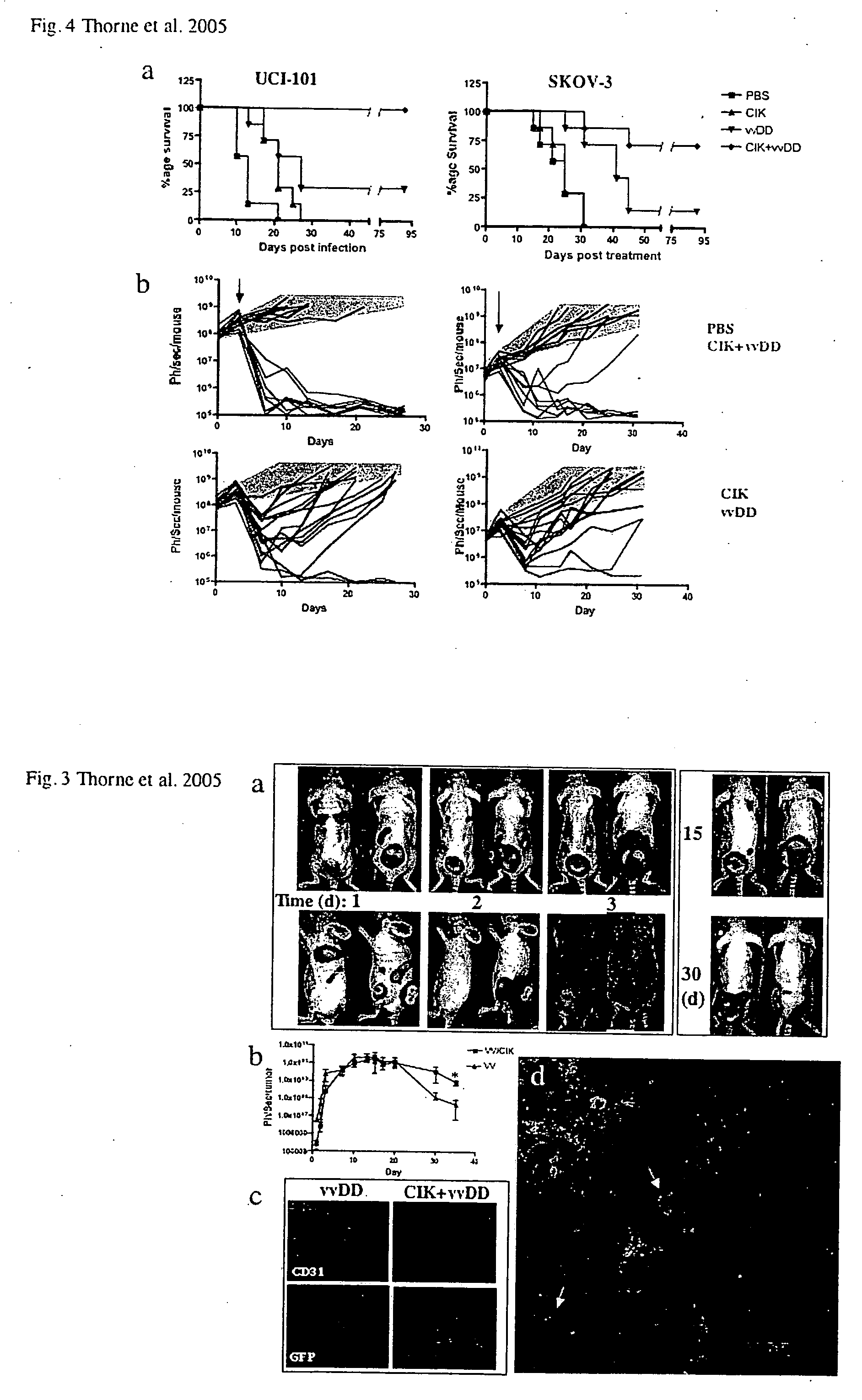
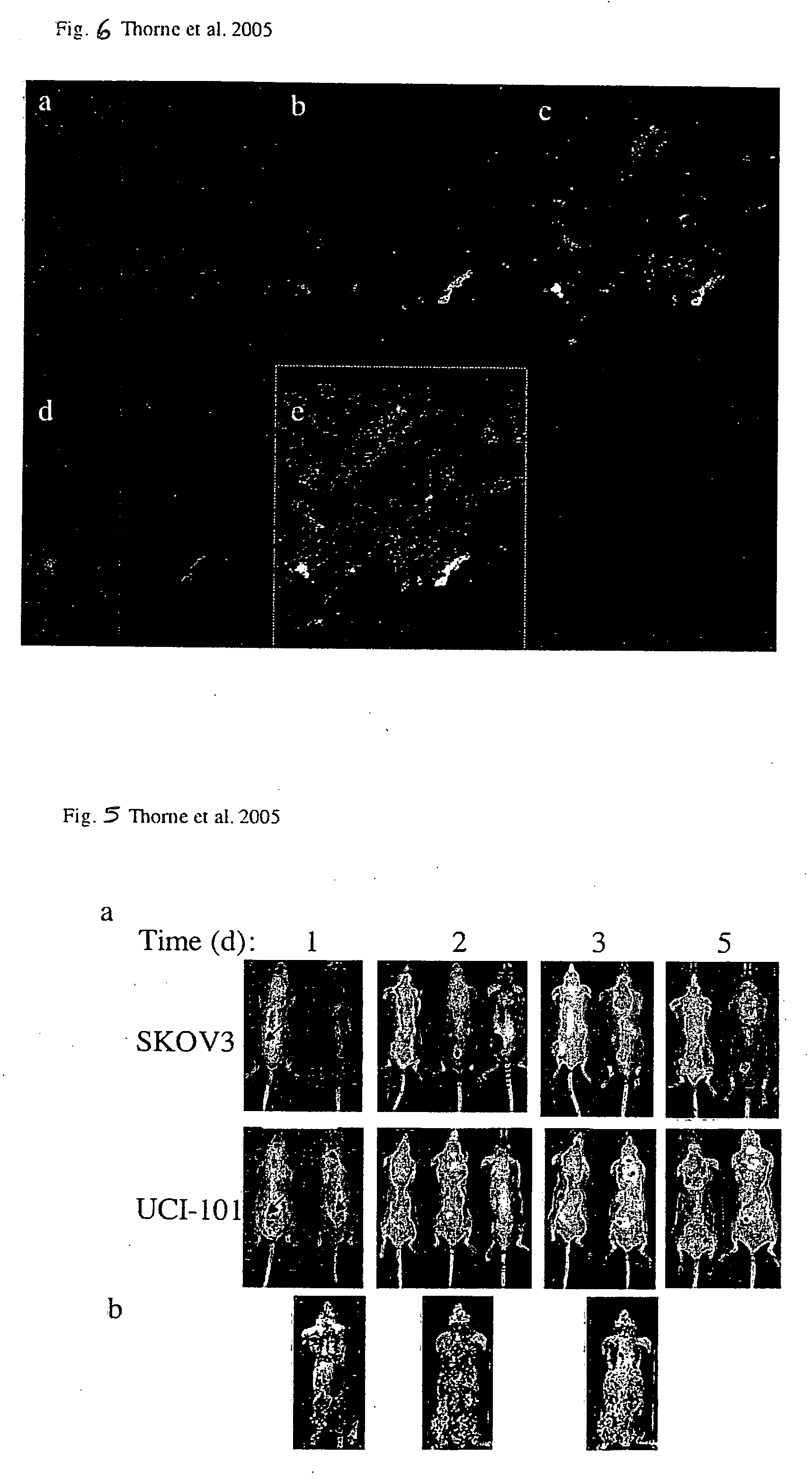
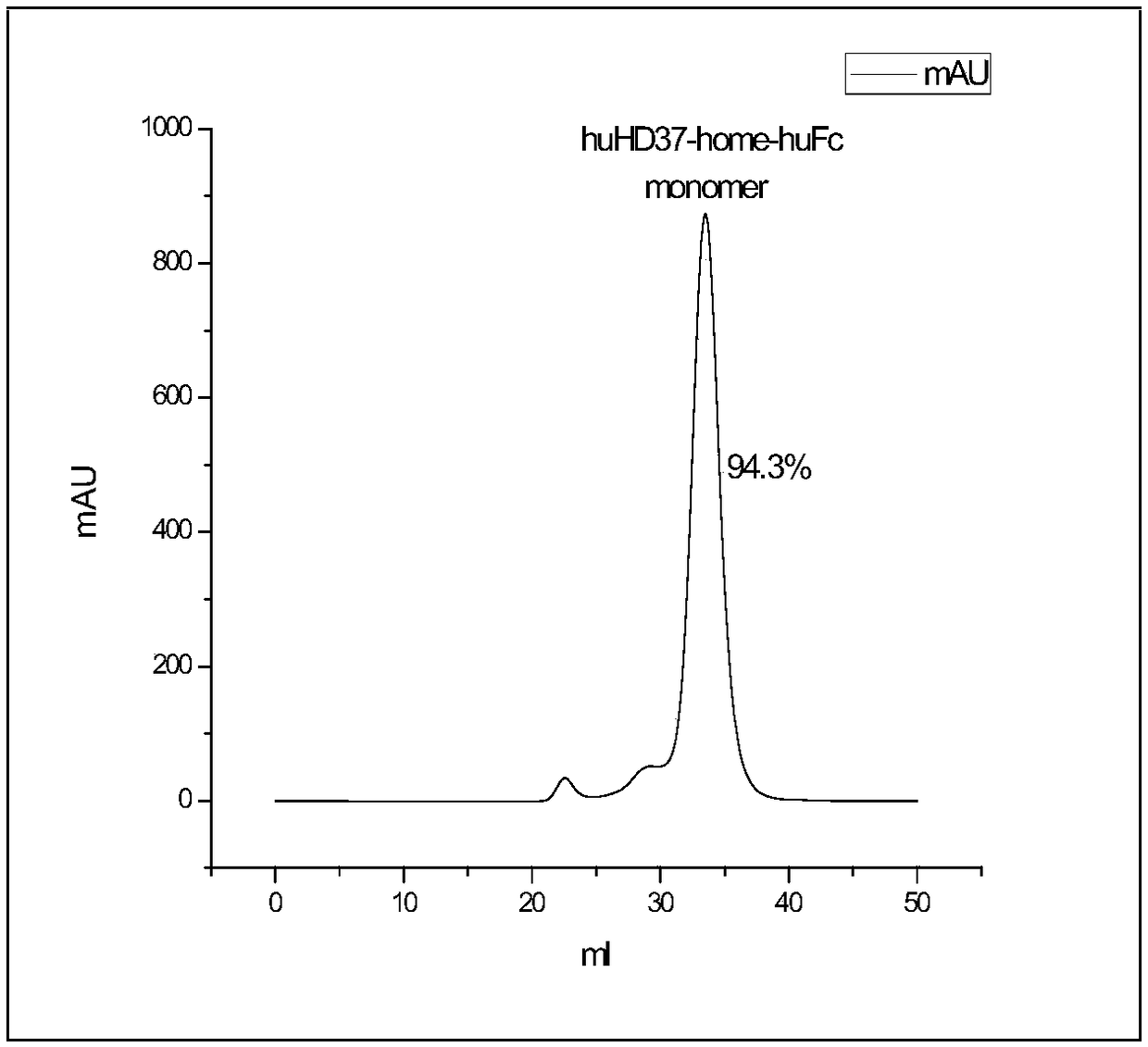
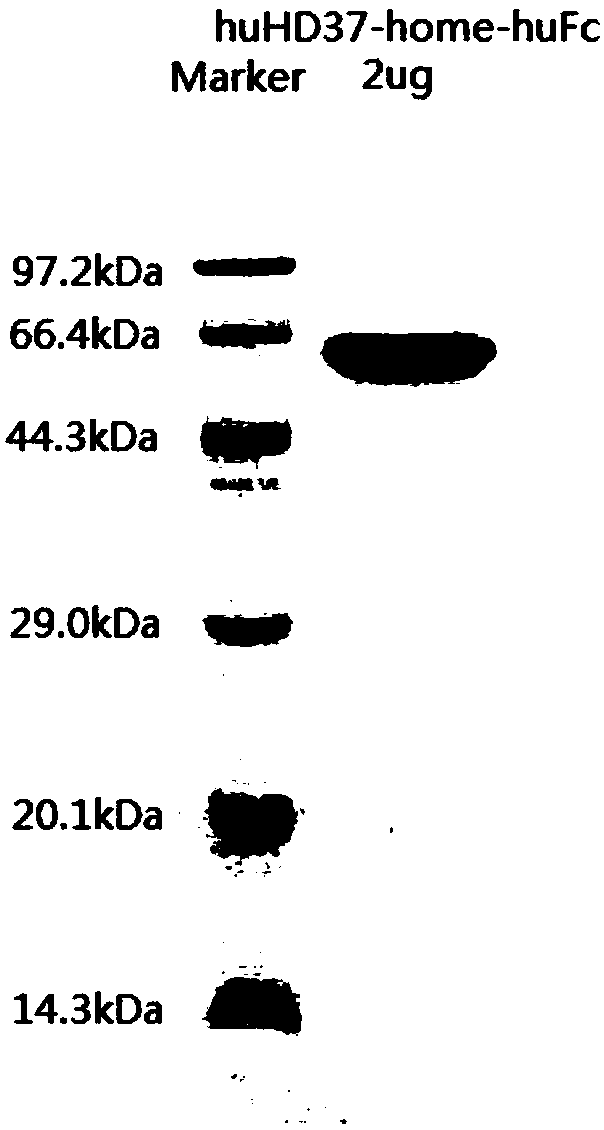
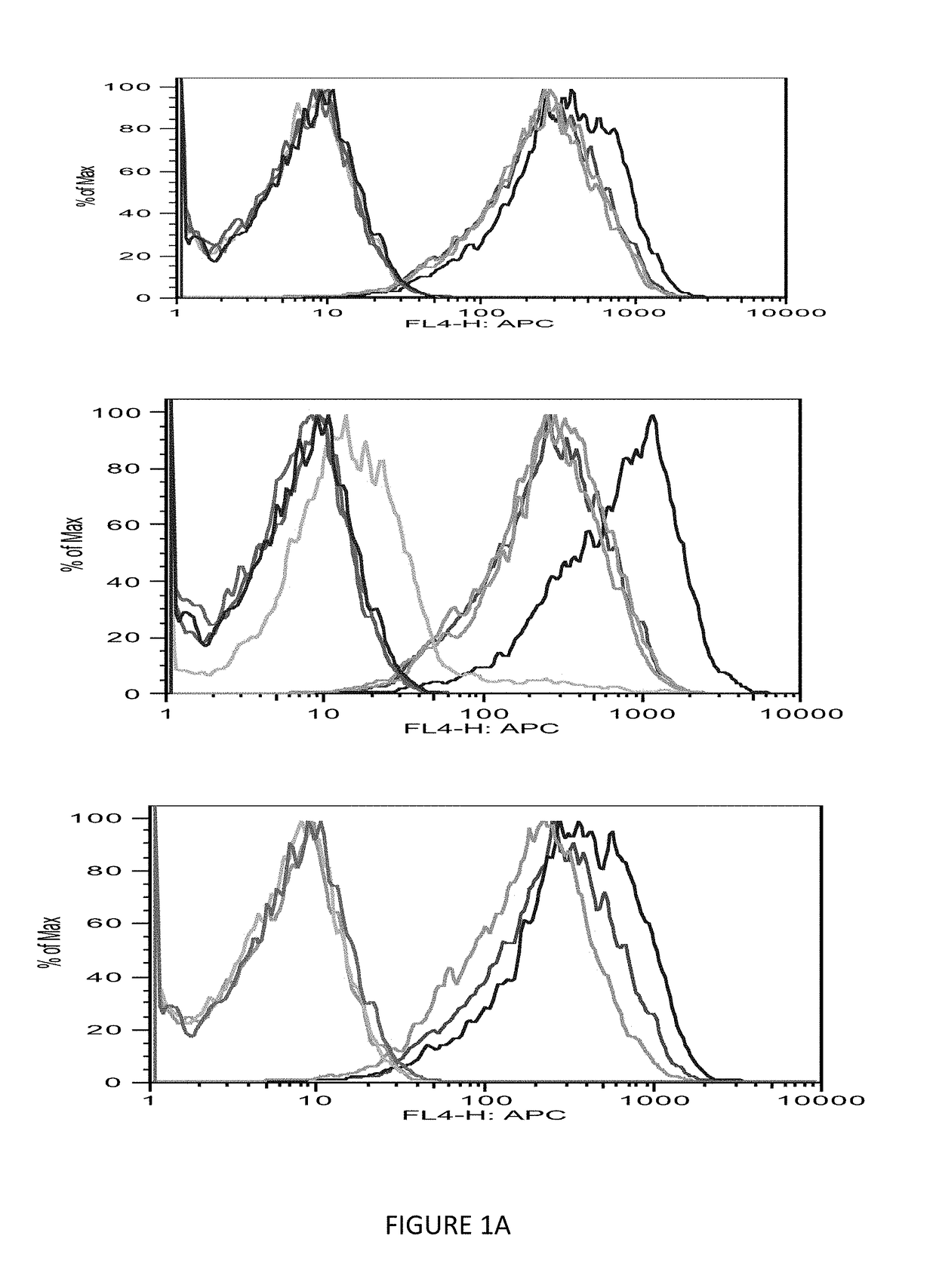
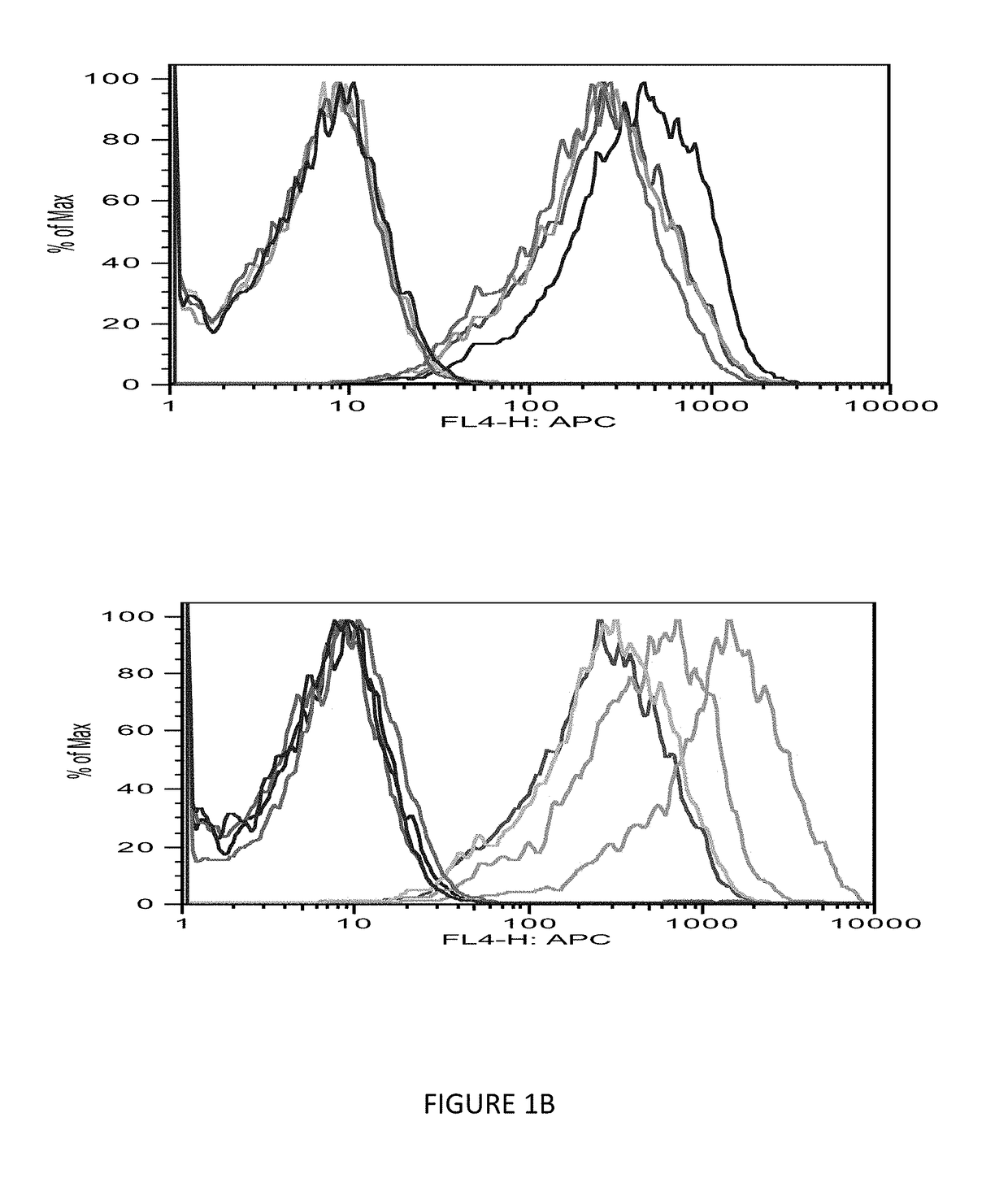
Patents
Literature
200 results about "Immune effector" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune effector cells are cells from the human body that have differentiated into a form capable of modulating or effecting an immune response. Cells naturally found in the body, such as B cells, dendritic cells, natural killer cells, and T cells are collected, transformed into a therapeutic product, and then administered to a patient.
Antibodies with immune effector activity and that internalize in folate receptor alpha-positive cells
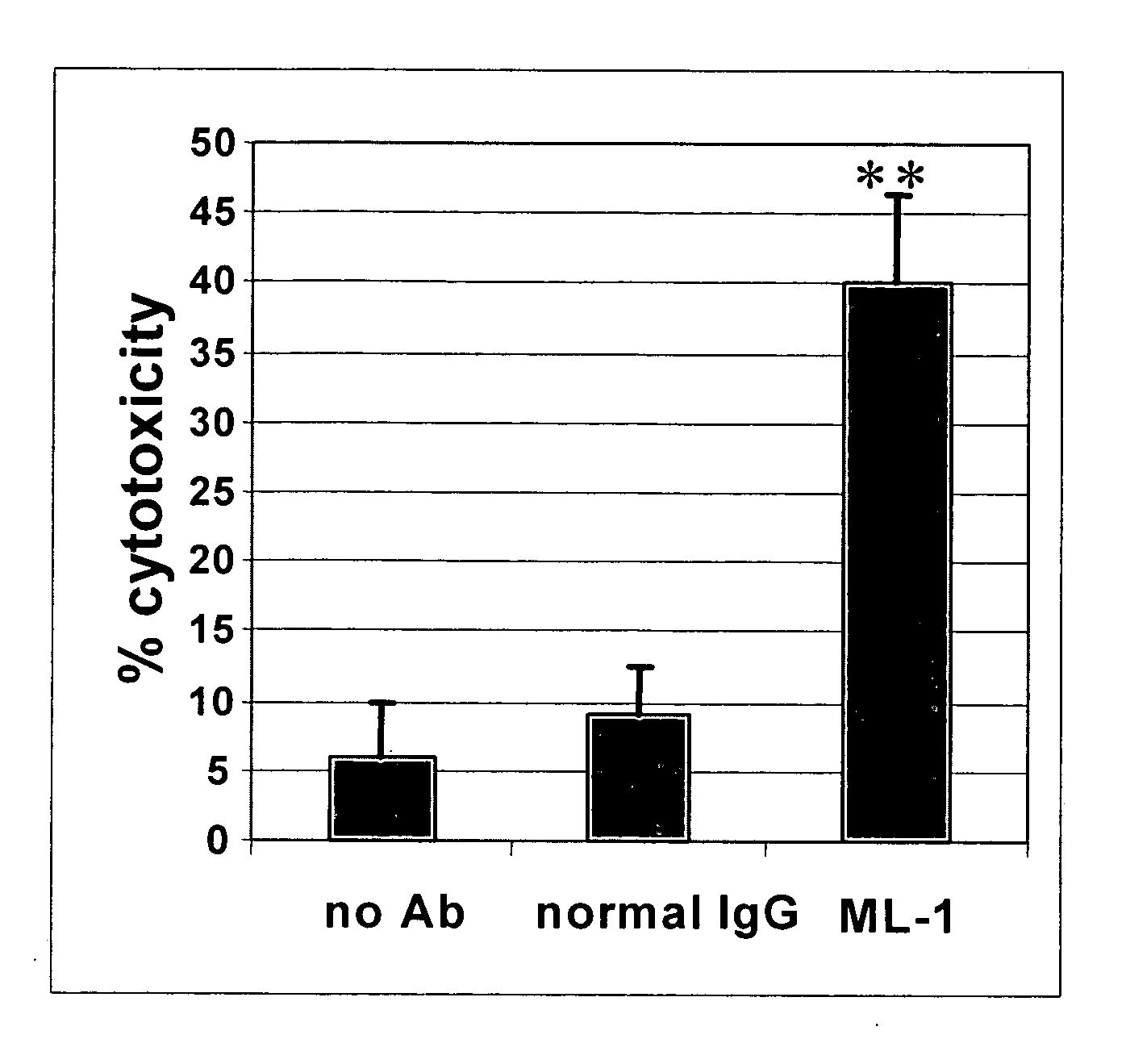
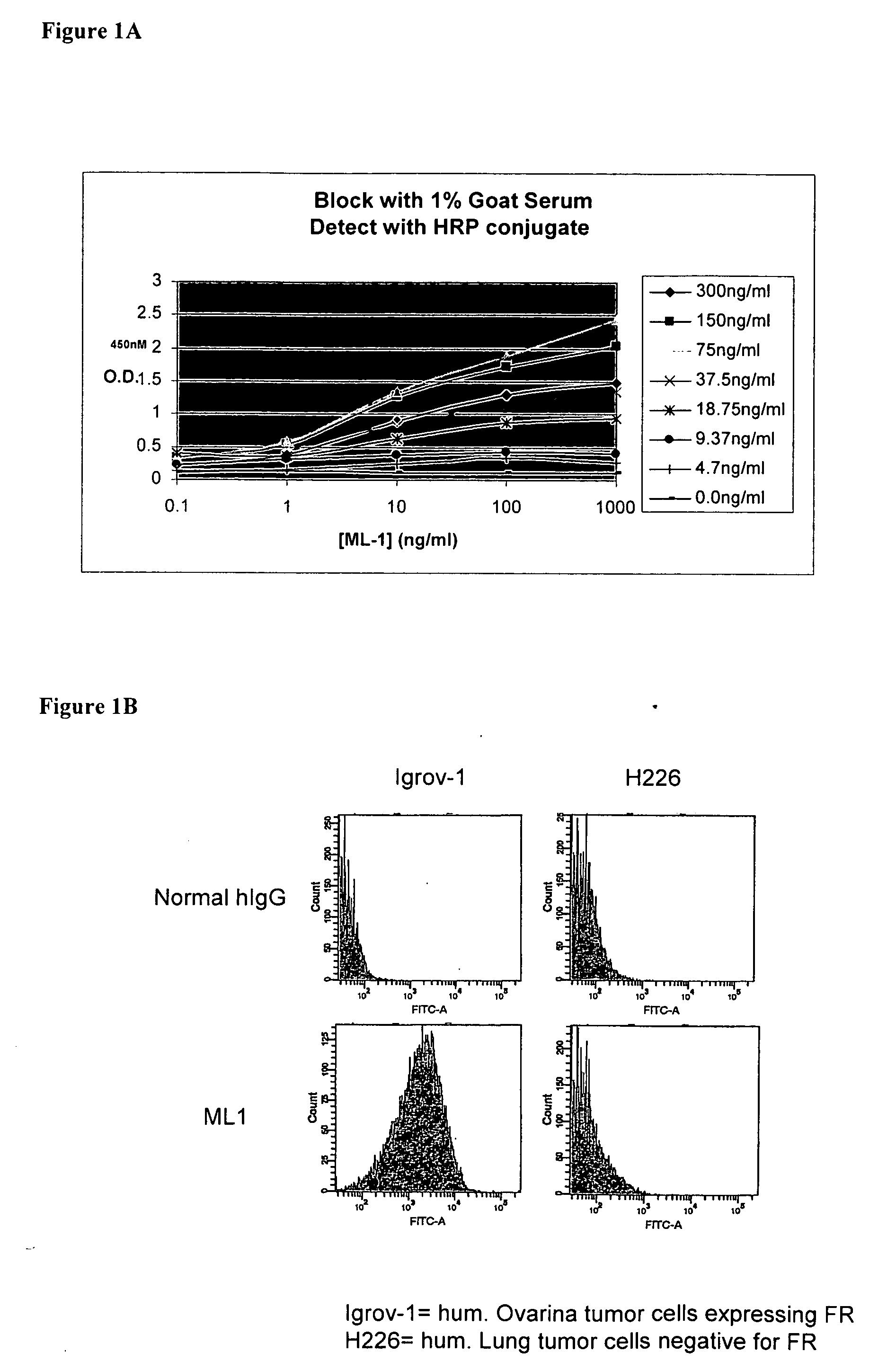
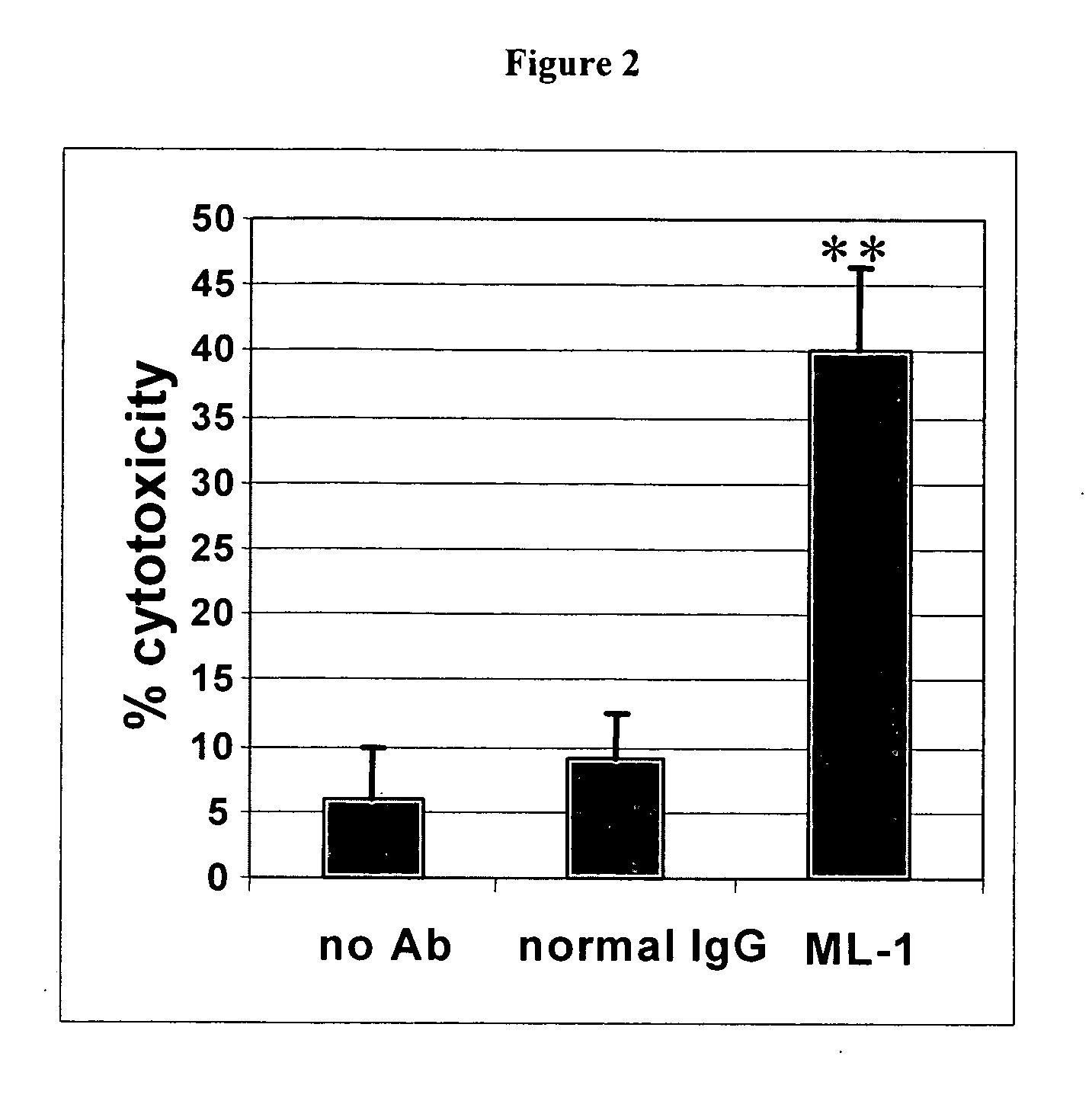
InactiveUS20060239910A1Improve anti-tumor activityEnhanced antibody-dependent cellular cytotoxicityOrganic active ingredientsNanomedicineDrug specific IgEFolate Receptor Alpha
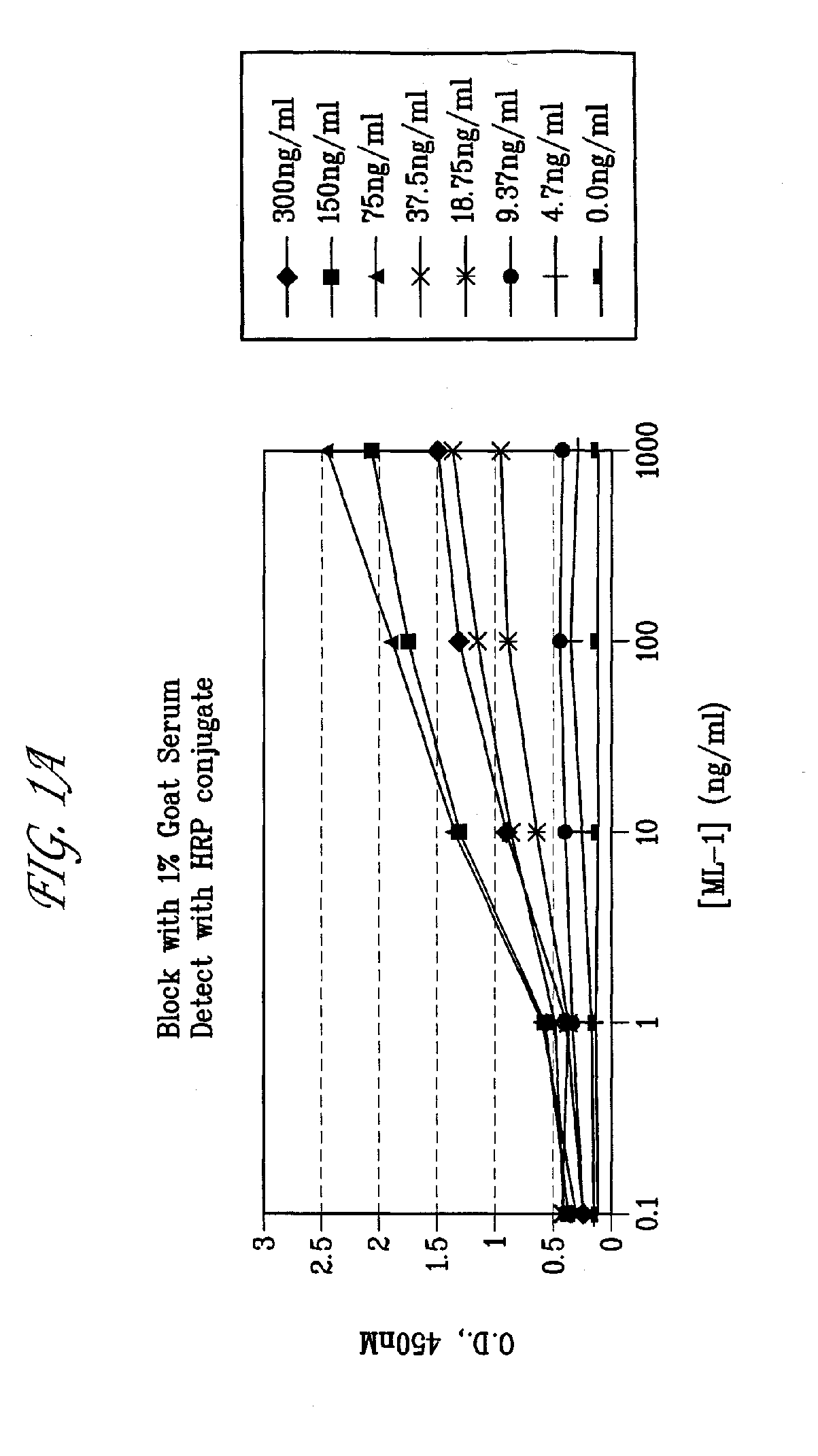
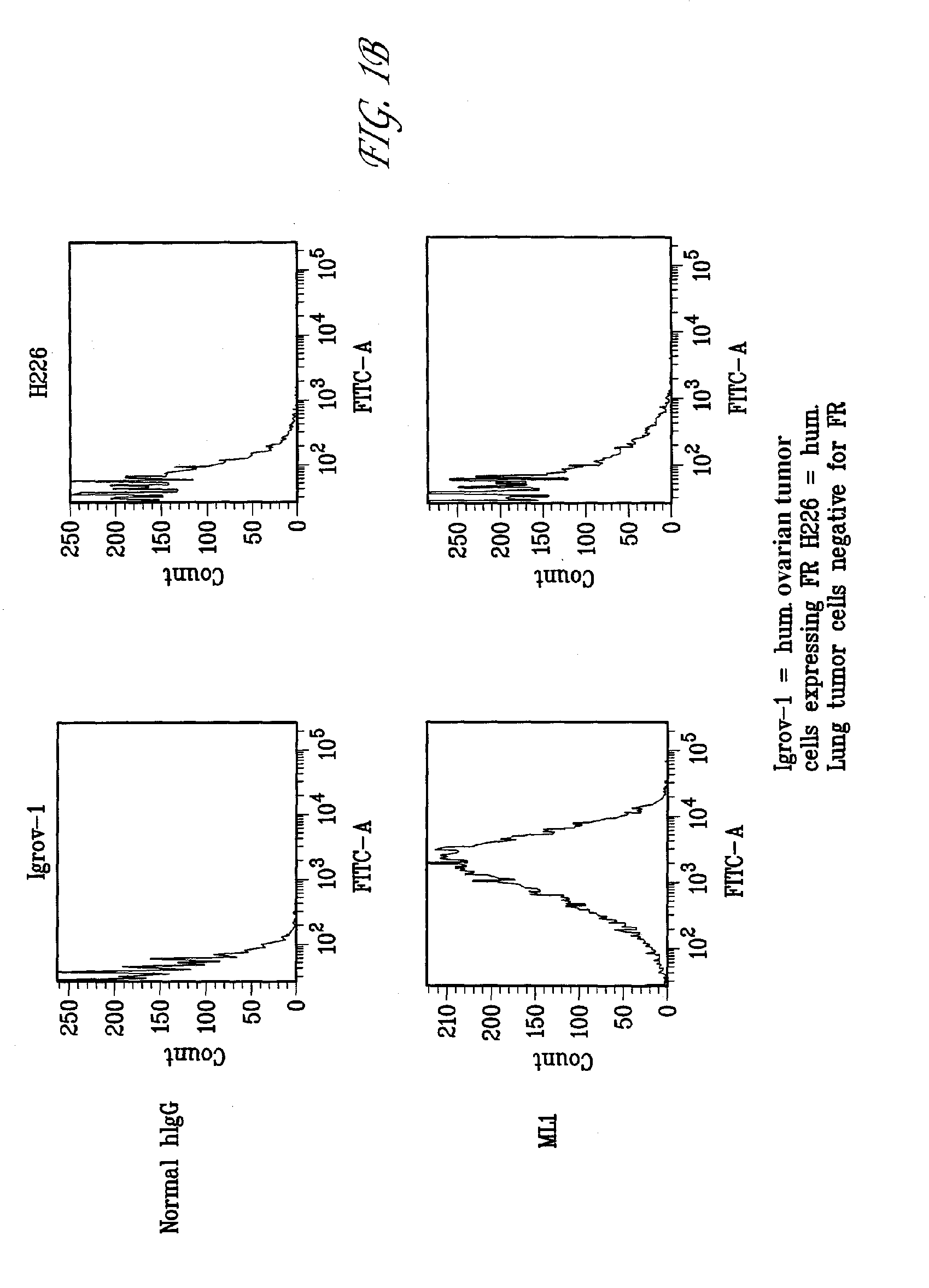
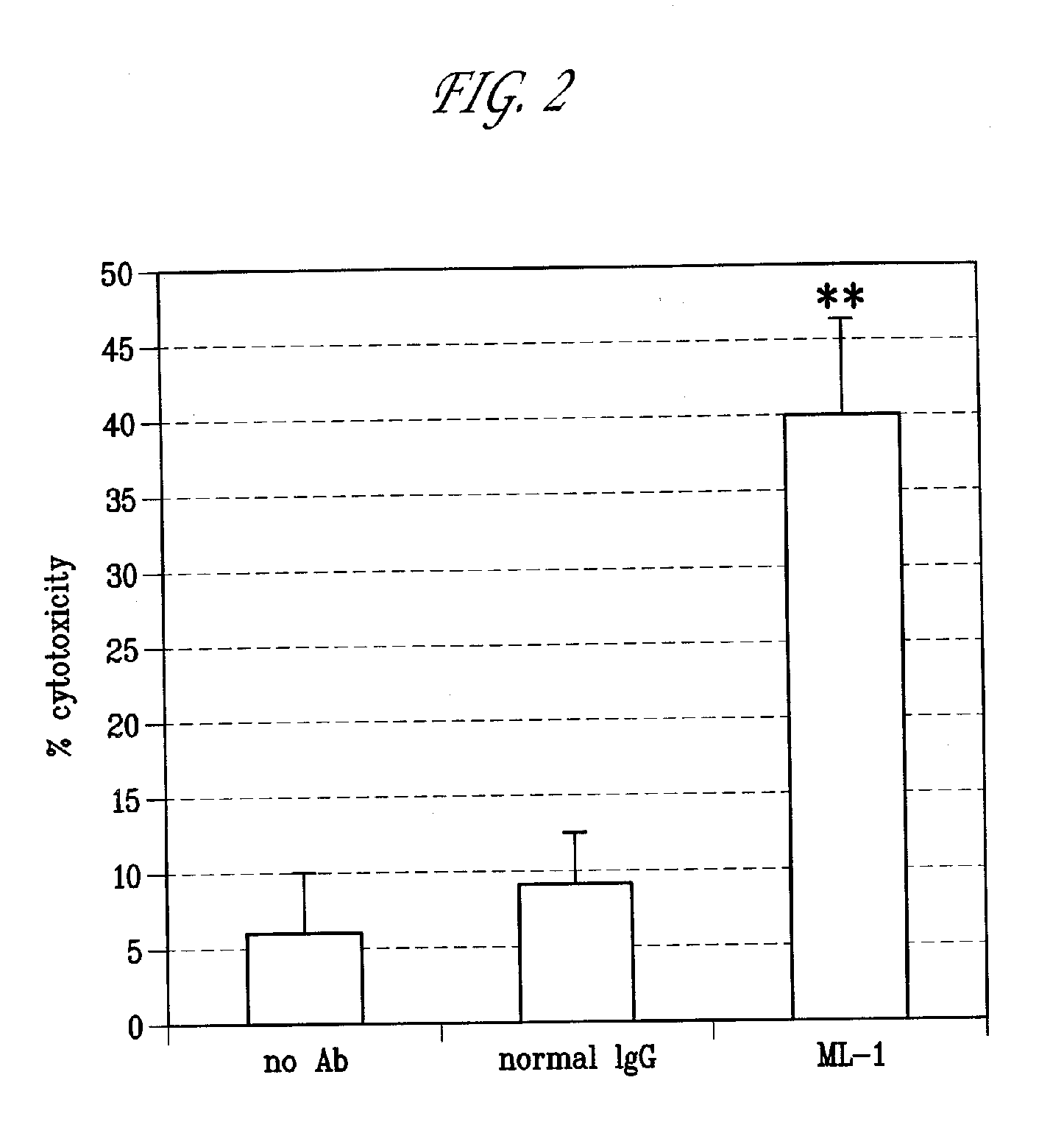
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing folate receptor alpha (FRA) and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to FRA-expressing cells as well as in eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer cells; and methods of treating cancer using the antibodies.
Owner:EISAI INC
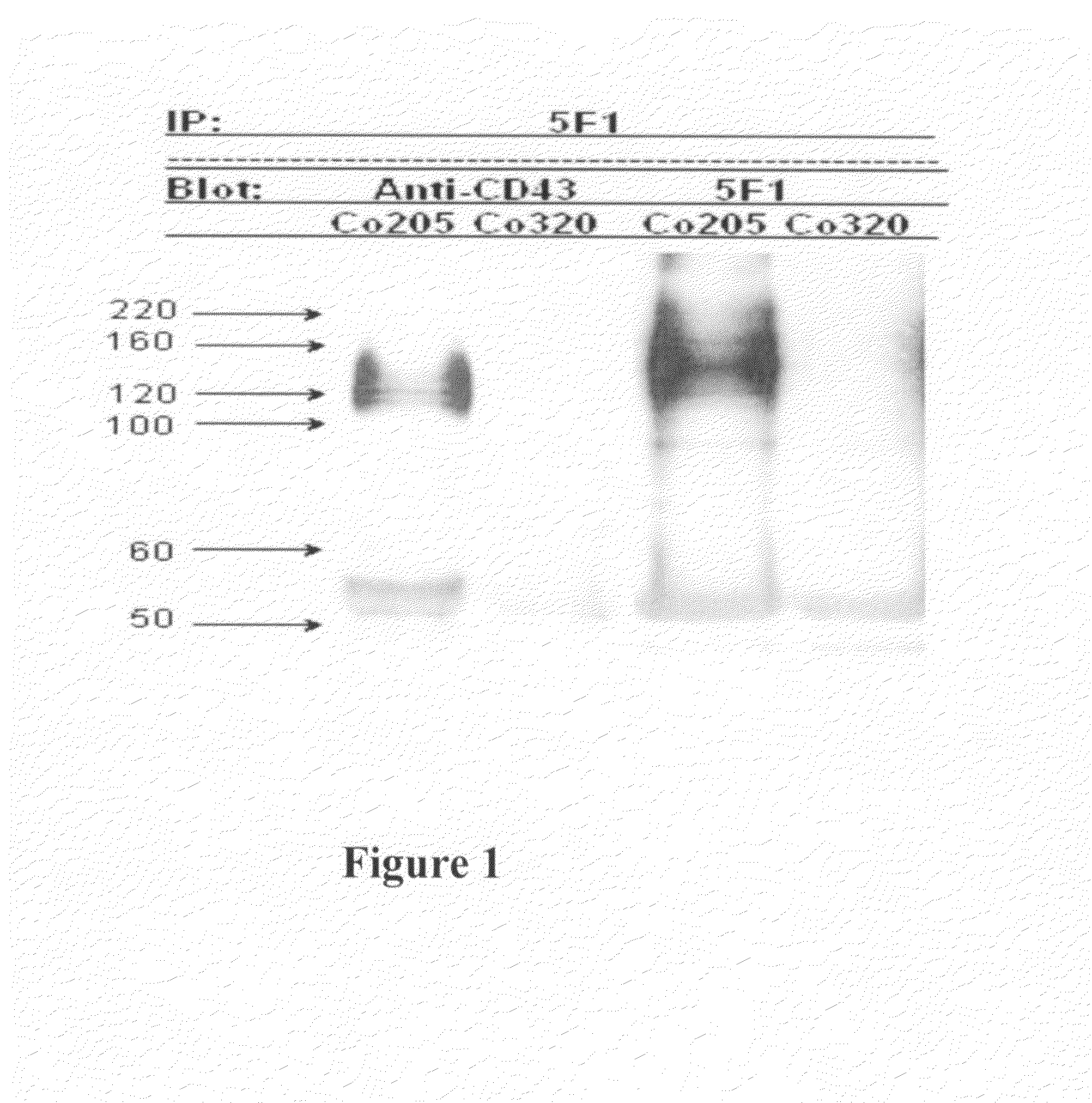
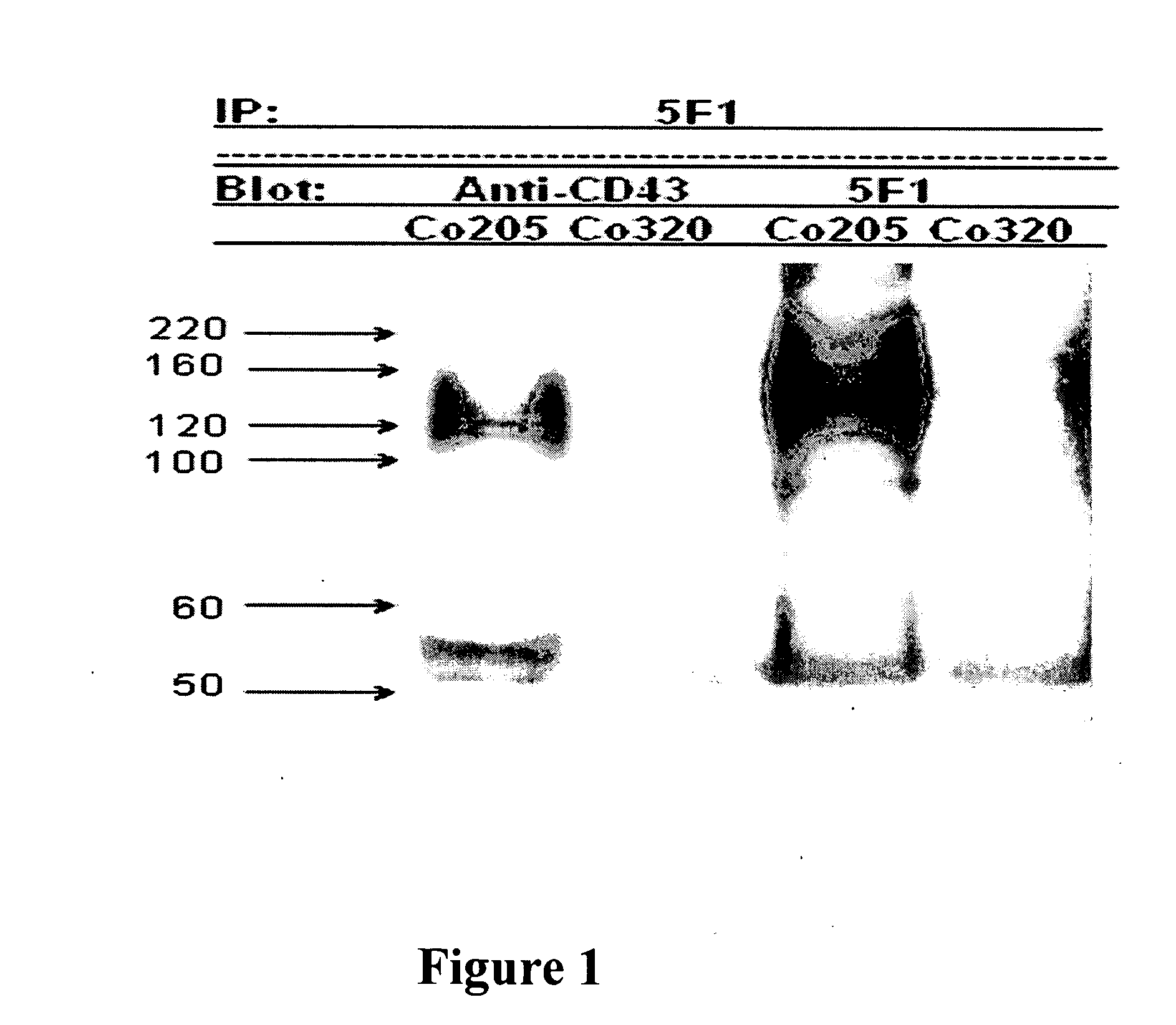
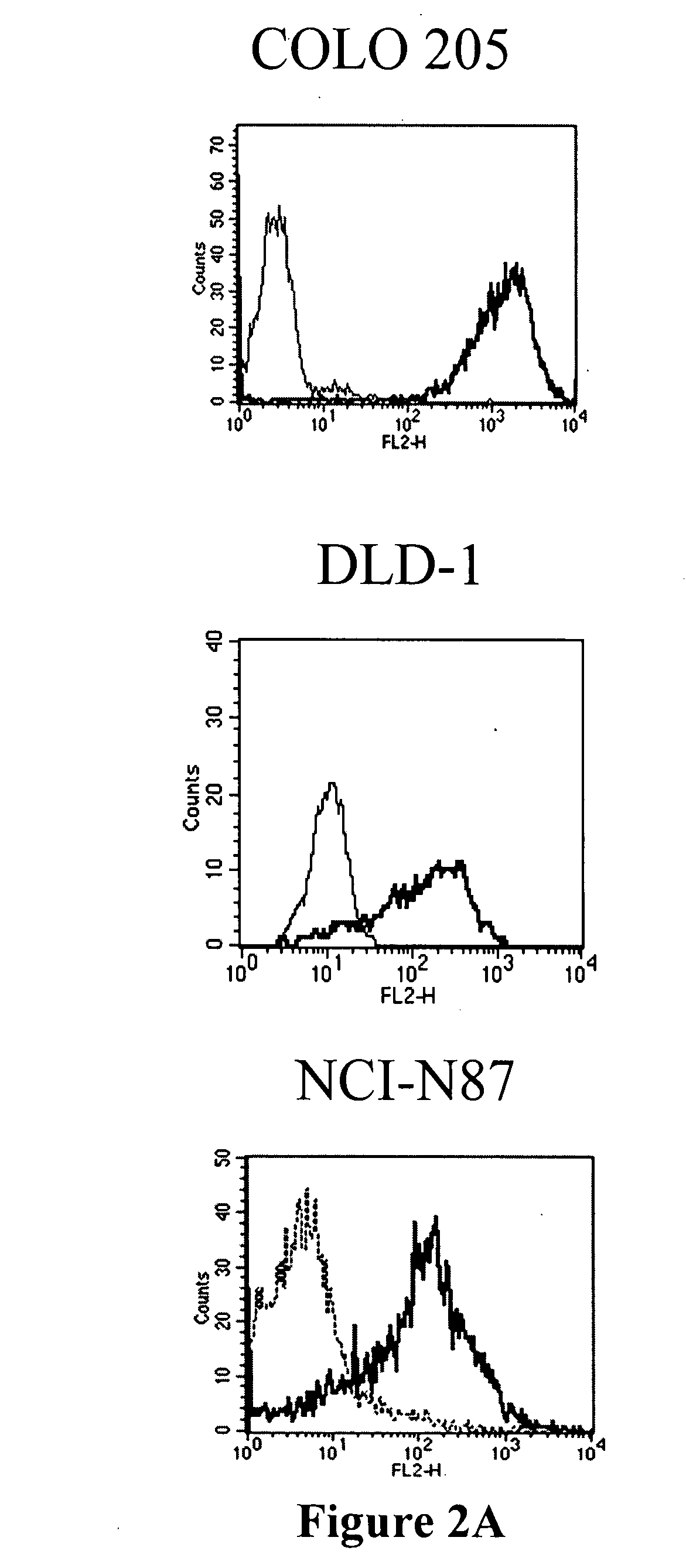
Antibodies recognizing a carbohydrate containing epitope on CD-43 and CEA expressed on cancer cells and methods using same
ActiveUS7674605B2Reduce in quantityInhibit growth and proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationHematopoietic cellCancer cell
The present invention provides novel antibodies specifically bind to an epitope on CD43 and CEA expressed on nonhematopoietic cancer cells, but do not specifically bind to a CD43 expressed by a leukocyte or by a Jurkat cell, and is capable of inducing apoptosis of the nonhematopoietic cancer cell after binding to the epitope on cell surface of the nonhematopoietic cancer cell in the absence of cytotoxin conjugation and immune effector function, wherein the epitope comprises a carbohydrate structure and the binding of the antibody to the epitope is inhibited by a carbohydrate comprising a Lea structure, a Lea-lactose structure, a LNDFH II structure, or a LNT structure. In addition, the present invention also provides use of the antibodies described herein for diagnostic and therapeutic purposes.
Owner:ALTRUBIO INC
Transcutaneous immunization without heterologous adjuvant
InactiveUS20060002949A1Increase skin hydrationIncrease local concentrationSsRNA viruses negative-senseAntibacterial agentsDendritic cellSpecific immunity
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response without the use of a heterologous adjuvant. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigens. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced. Immune responses that provide prophylactic and / or therapeutic treatments are preferred. Antigenic activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a dry or liquid formulation to the skin, patches and other medical devices may be used to deliver antigen for immunization.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE OFFICE OF THE COMMAND JUDGE ADVOCATE
Effectors of innate immunity
InactiveUS20070134261A1Reduce inflammationBlock/dampen inflammatory and/or septic responsesCompound screeningBacterial antigen ingredientsNucleotidePolynucleotide
The present invention provides a method of identifying agents that enhance innate immunity in a subject. The invention further provides a method of selectively supressing sepsis by suppressing expression of a proinflammatory gene while maintaining expression of an anti-inflammatory gene. Also provided are methods of identifying a polynucleotide or pattern of polynucleotides regulated by one or more sepsis or inflammatory inducing agents and inhibited by a peptide is described, methods of identifying a pattern of polynucleotide expression for inhibition of an inflammatory or septic response, and compounds and agents identified by the methods of the invention.
Owner:THE UNIV OF BRITISH COLUMBIA
Production of fc-fusion polypeptides in eukaryotic algae
InactiveUS20110151515A1Increase serum stabilityEfficient separation and purificationUnicellular algaeVaccinesHeterologousProtein regulation
Methods and compositions are disclosed to engineer plastids comprising heterologous genes encoding immuno-activating domains fused to an extracellular domain (ECD) of a receptor or surface glycoprotein, a growth factor or an enzyme and produced within a subcellular organelle, such as a chloroplast. The immuno-activating domains may include those regions of a protein capable of modulating the interaction between immune effector cells via proteins containing stereoselective binding domains and specific ligands, such as the Fc regions of antibodies. The present disclosure also demonstrates the utility of plants, including green algae, for the production of complex multi-domain fusion proteins as soluble bioactive therapeutic agents.
Owner:SAPPHIRE ENERGY
Compositions and methods to enhance the immune system
InactiveUS8728476B2Enhance in vivo efficacyImprove immunityOrganic active ingredientsPeptide/protein ingredientsTherapeutic antibodyCancer cell
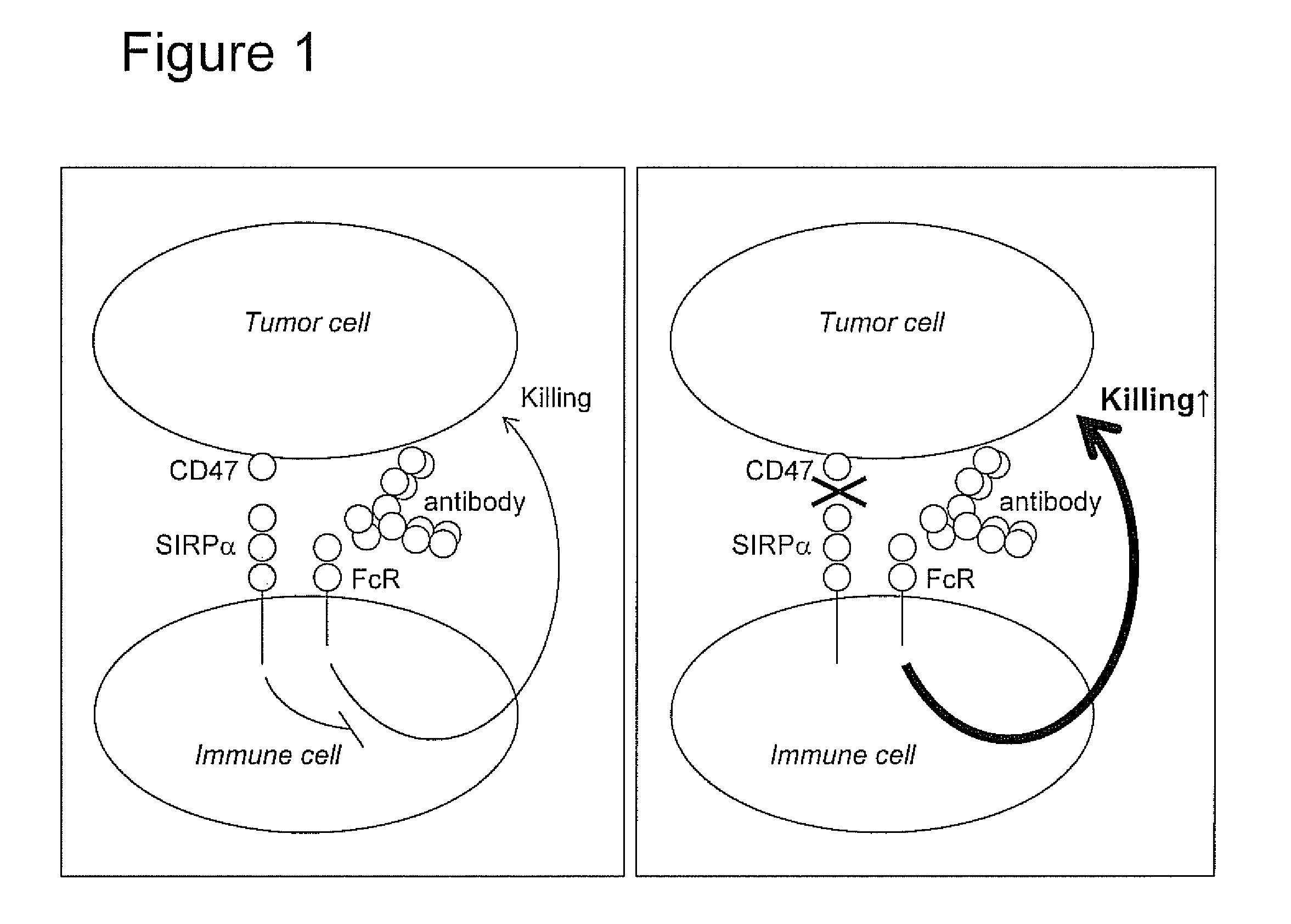
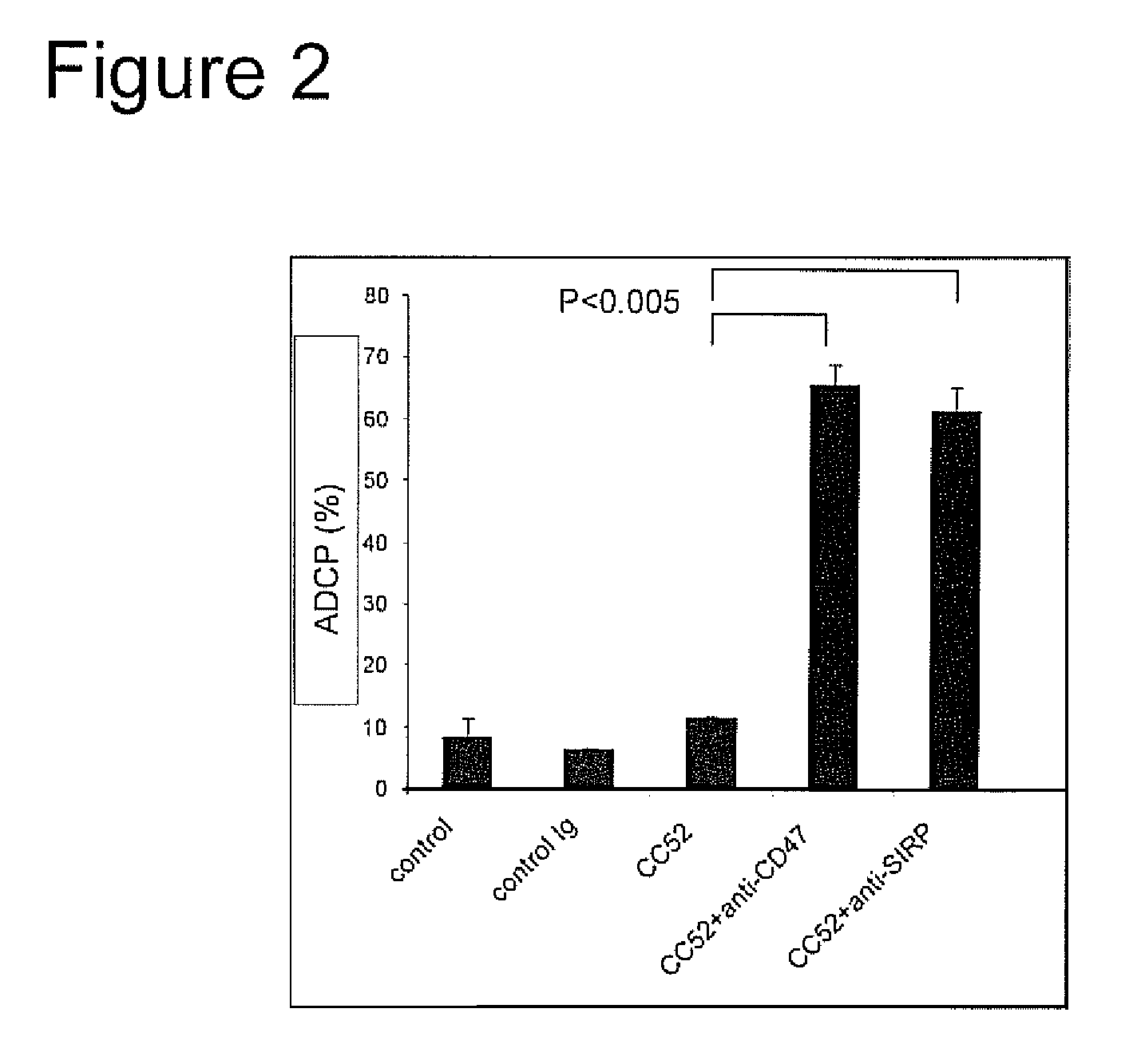
The invention relates to the field of molecular medicine. In particular, it relates to compositions and methods to enhance the clearance of aberrant cells, e.g. cancer cells or virus-infected cells, by the host's immune system. Provided is a composition comprising (i) a therapeutic compound that can trigger a host's immune effector cells against an aberrant cell, such as a therapeutic antibody, and (ii) at least one agent capable of reducing or preventing inhibitory signal transduction initiated via SIRPalpha.
Owner:STICHTING SANQUIN BLOEDVOORZIENING
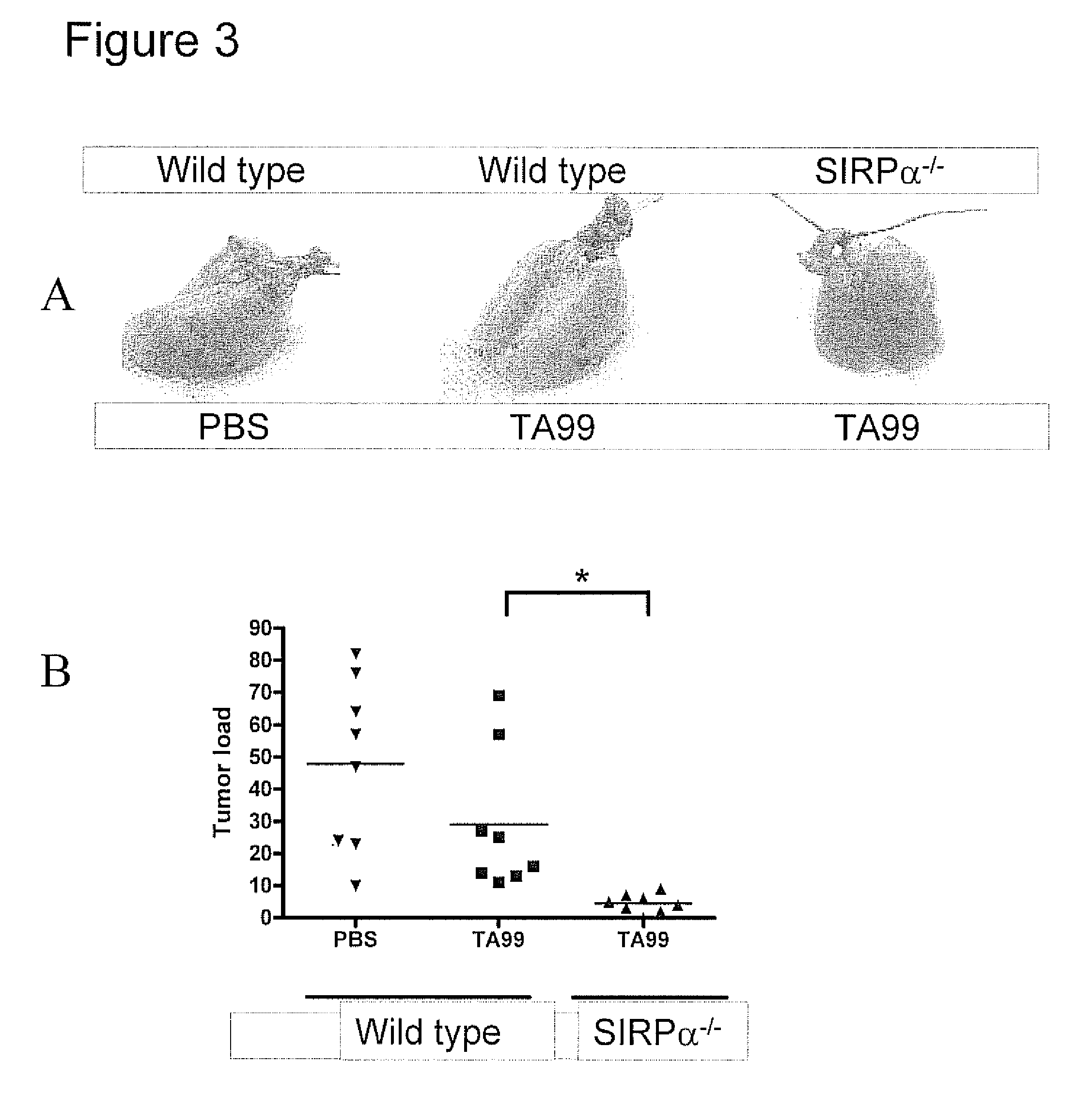
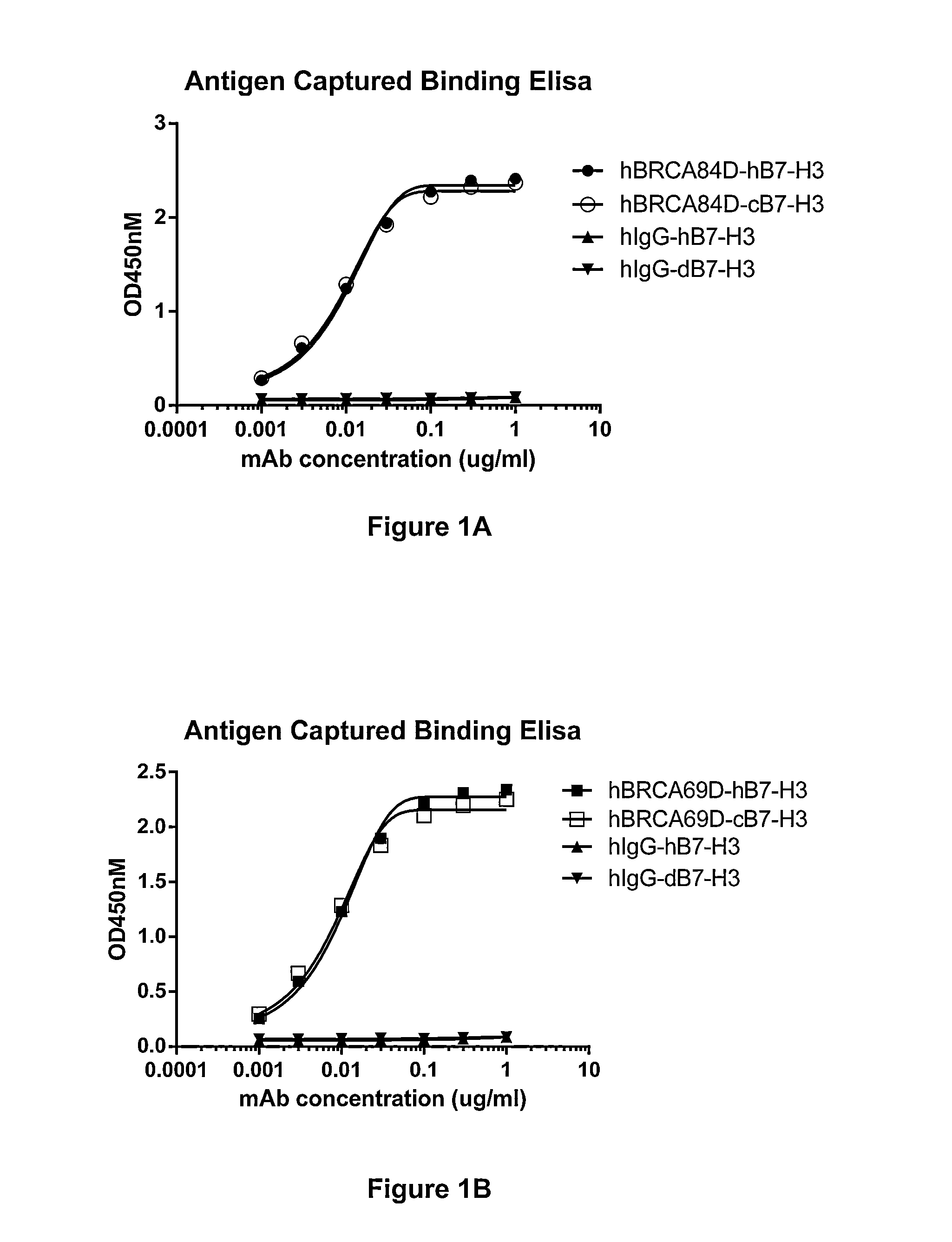
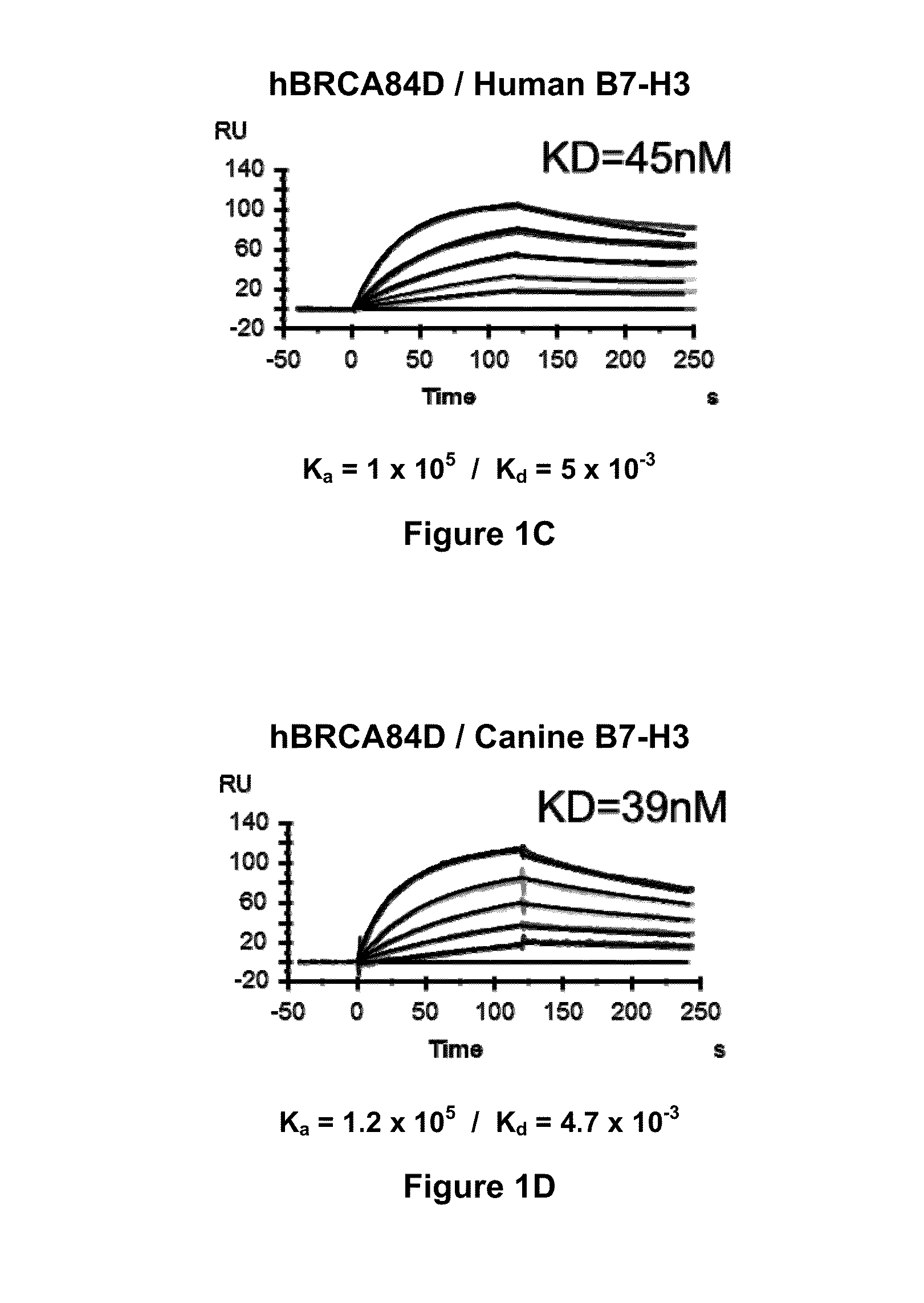
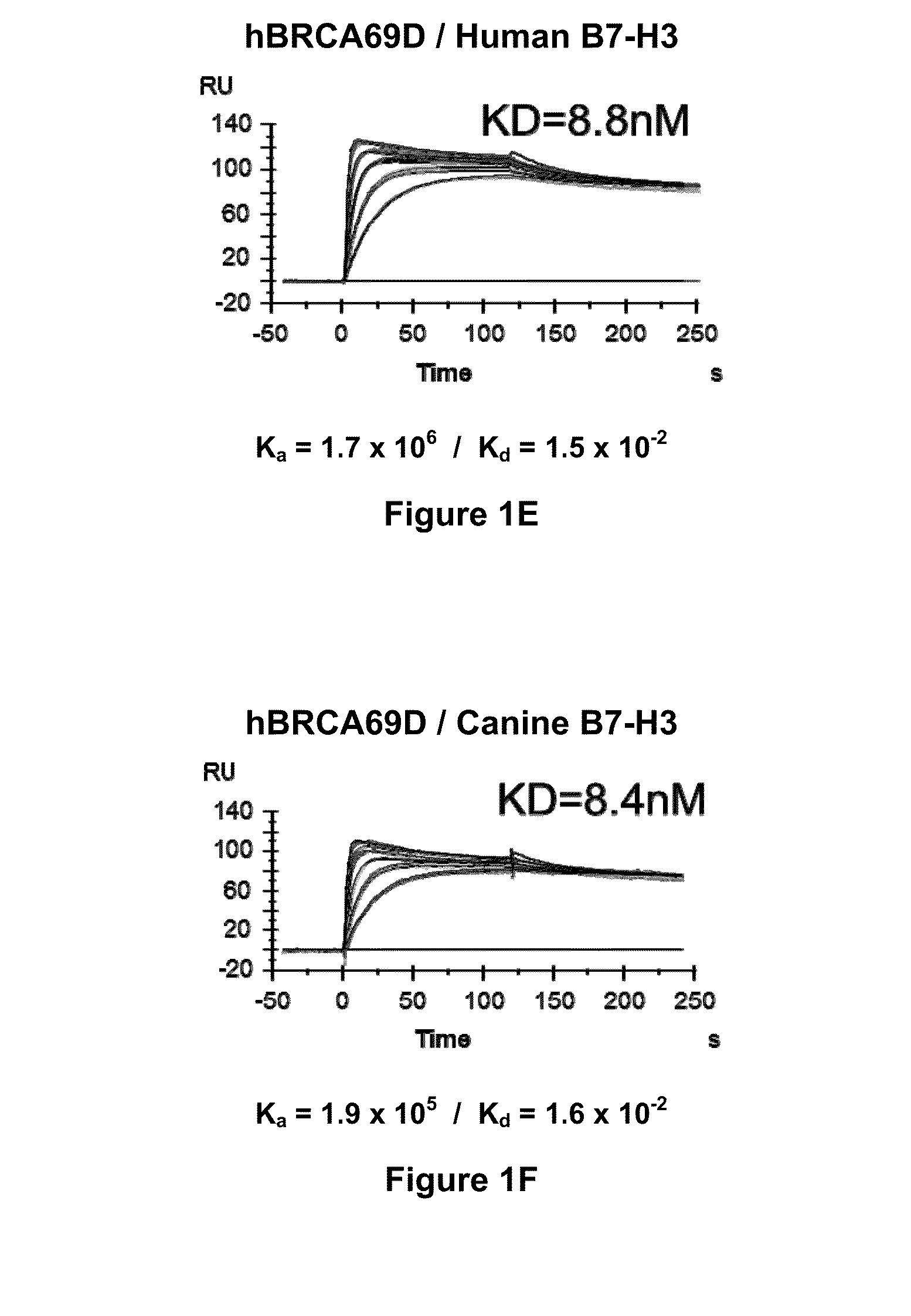
Bispecific Molecules That Are Immunoreactive with Immune Effector Cells of a Companion Animal That Express an Activating Receptor and Cells That Express B7-H3 and Uses Thereof
ActiveUS20140255407A1Facilitate killingIncreased activationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsImmune effector cellCancer therapy
The present invention relates to bispecific molecules that are immunoreactive to an activating receptor of a companion animal immune effector cell and to B7-H3, and to the use of such bispecific molecules in the treatment of cancer in companion animals.
Owner:MACROGENICS INC
Antibodies With Immune Effector Activity And That Internalize In Folate Receptor Alpha-Positive Cells
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing folate receptor alpha (FRA) and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to FRA-expressing cells as well as in eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer cells; and methods of treating cancer using the antibodies.
Owner:EISAI INC
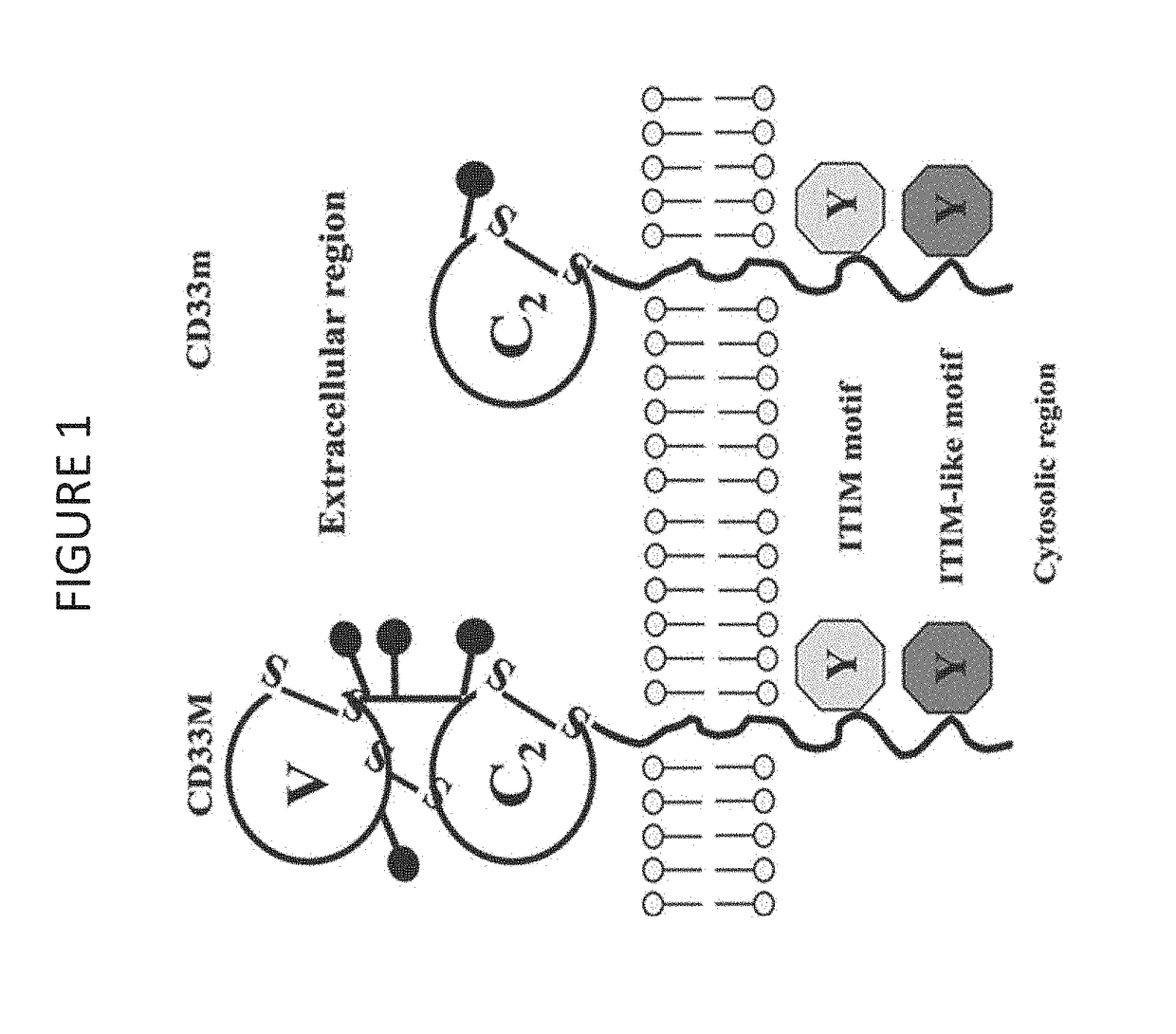
Cd33 specific chimeric antigen receptors
PendingUS20180002397A1Easy SurvivalEfficiently and specifically eliminatePolypeptide with localisation/targeting motifImmunoglobulin superfamilyMyeloid leukemiaCD33
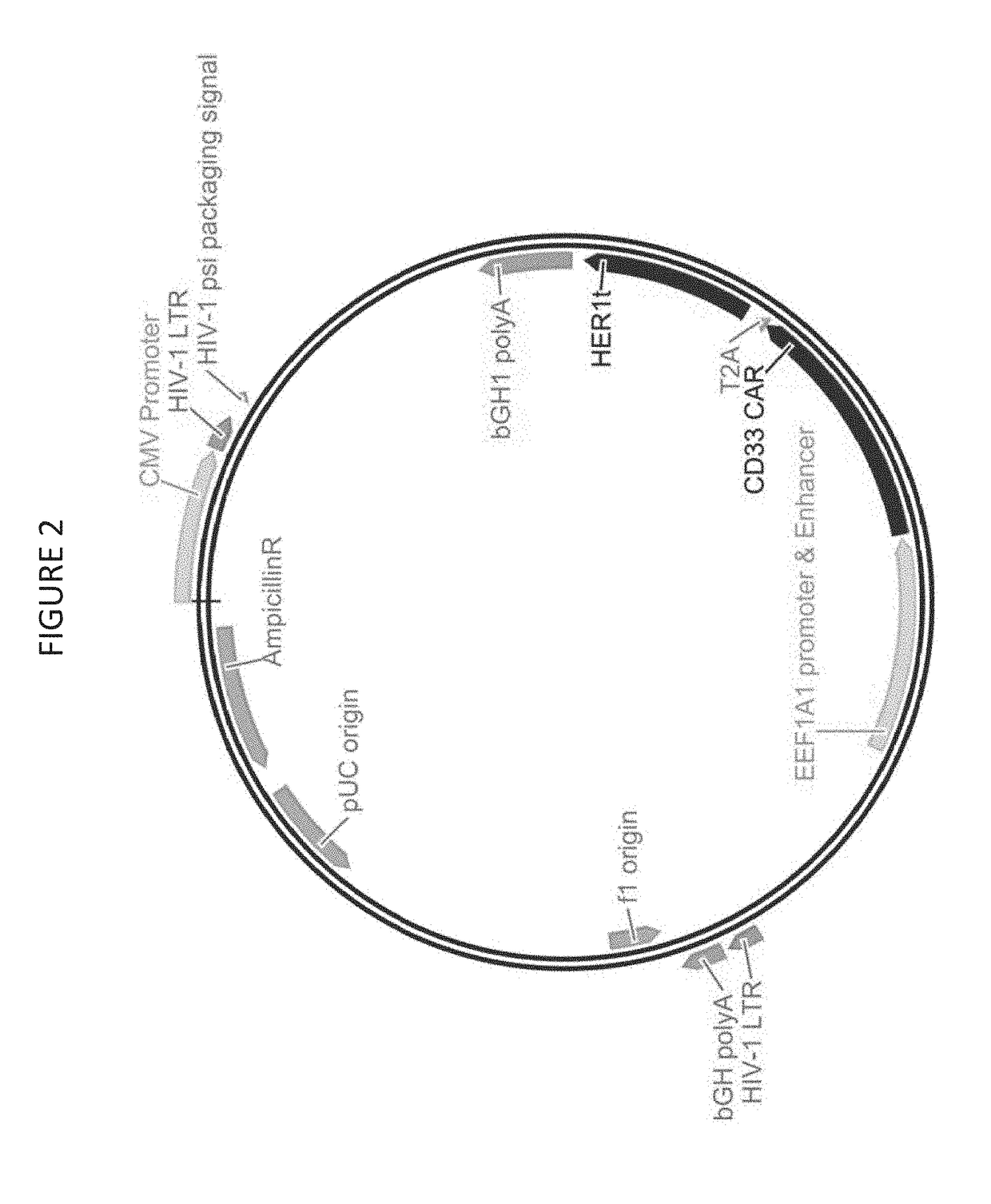
Provided herein are chimeric antigen receptors (CARs) for cancer therapy, and more particularly, CARs containing a scFv from a CD33 monoclonal antibody. Provided are immune effector cells containing such CARs, and methods of treating proliferative disorders such as acute myeloid leukemia (AML), and relapsed or refractory AML.
Owner:PRECIGEN INC
Methods and compositions for treating secondary tissue damage and other inflammatory conditions and disorders
InactiveUS7157418B1Enhance and aid in survivalPrevent proliferationAntibacterial agentsBiocidePhagocyteDendritic cell
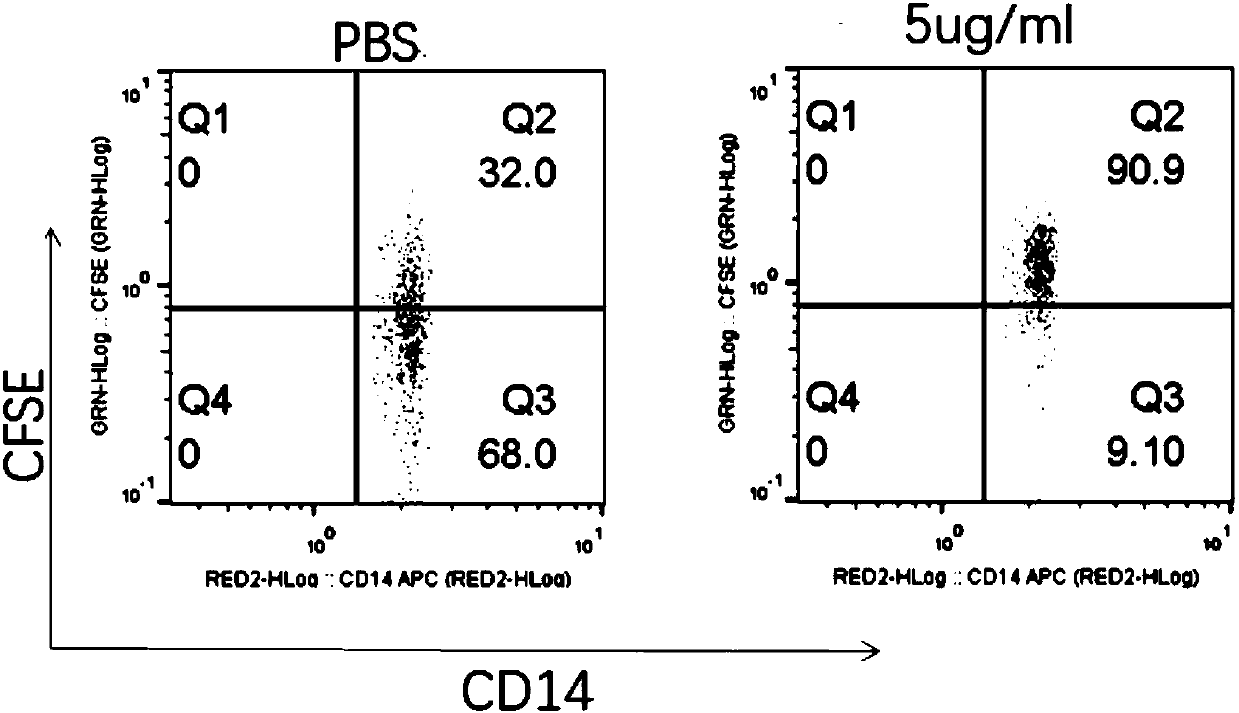
Conjugates containing as a ligand a chemokine receptor targeting agents, such as chemokines, and a targeted agent, such as a toxin are provided. These conjugates are used to treat inflammatory responses associated with activation, proliferation and migration of immune effector cells, including leukocyte cell types, neutrophiles, macrophages, and eosinophils. The conjugates provided herein are used to lessen or inhibit these processes to prevent or at least lessen the resulting secondary effects. In particular, the conjugates are used to target toxins to receptors on secondary tissue damage-promoting cells. The ligand moiety can be selected to deliver the cell toxin to such secondary tissue damage-promoting cells as mononuclear phagocytes, leukocytes, natural killer cells, dendritic cells, and T and B lymphocytes, thereby suppressing the proliferation, migration, or physiological activity of such cells. Among preferred conjugates are fusion proteins having a chemokine, or a biologically active fragment thereof, as the ligand moiety linked to a cell toxin via a peptide linker of from 2 to about 60 amino acid residues.
Owner:OSPREY PHARMA USA INC
Modified Monocytes/Macrophage Expressing Chimeric Antigen Receptors and Uses Thereof
ActiveUS20180244748A1Stimulate immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsDendritic cellGackstroemia
The present invention includes methods and compositions for treating cancer, whether a solid tumor or a hematologic malignancy. By expressing a chimeric antigen receptor in a monocyte, macrophage or dendritic cell, the modified cell is recruited to the tumor microenvironment where it acts as a potent immune effector by infiltrating the tumor and killing the target cells. One aspect includes a modified cell and pharmaceutical compositions comprising the modified cell for adoptive cell therapy and treating a disease or condition associated with immunosuppression.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Skin-sctive adjuvants for transcutaneous immuization
InactiveUS20060002959A1Increase skin hydrationEnhance immune responseAntibacterial agentsSsRNA viruses negative-senseDendritic cellSpecific immunity
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response. Use of skin-active adjuvants is preferred. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigen and adjuvant. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced; immune responses that result in prophylaxis and / or therapeutic treatments are preferred. Antigen and adjuvant activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens and adjuvants which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a liquid formulation, patches or other medical devices may be used to deliver antigen for immunization.
Owner:UNITED STATES OF AMERICA
Cytotoxic conjugates comprising a chemokine receptor targeting agent
InactiveUS7166702B1Enhance and aid in survivalPrevent proliferationPolypeptide with localisation/targeting motifChemokinesPhagocyteDendritic cell
Owner:OSPREY PHARMA USA INC
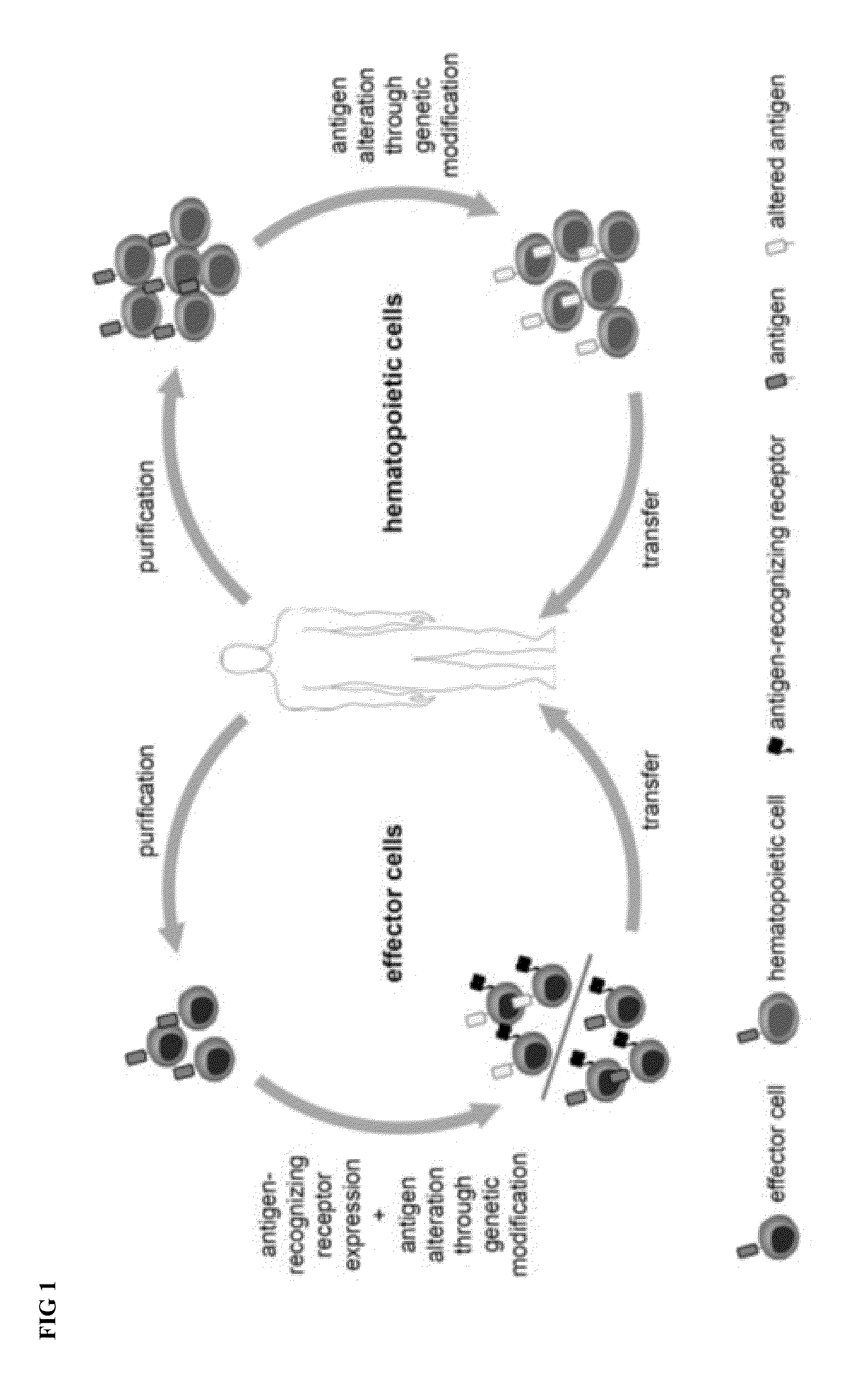
Combination immunotherapy of antigen-recognizing receptors and hematopoietic cells for the treatment of diseases
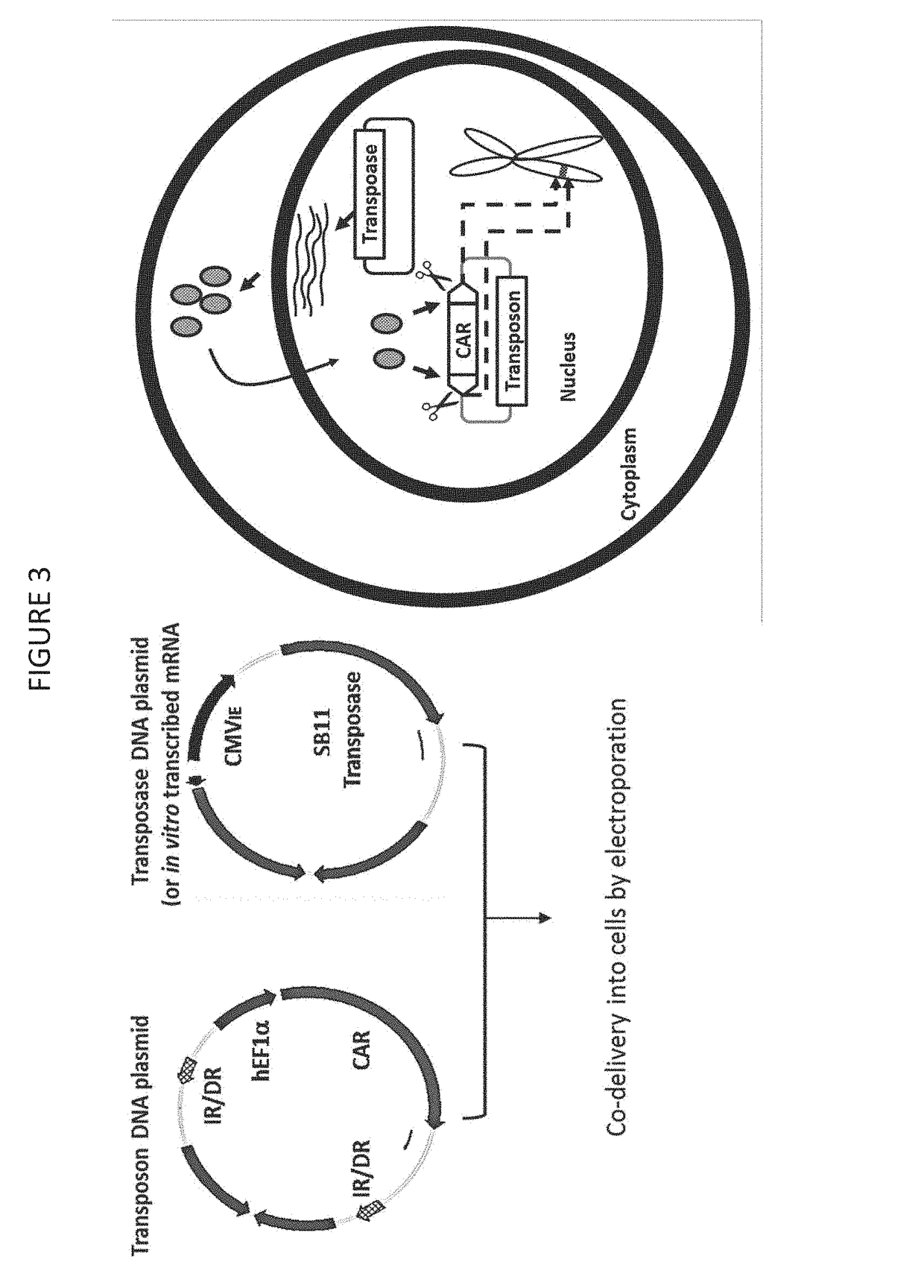
The invention provides a system that comprises pharmaceutical agents for use in immunotherapy for reducing the side-effects of an antigen-recognizing receptor against antigen-expressing non-target cells in an individual. The system includes an antigen-recognizing receptor that specifically recognizes an antigen on target cells and at least on one hematopoietic cell type in the individual. The antigen-recognizing receptor is exemplified by chimeric antigen receptors (CAR) be expressed on the surface of an immune effector cells. The system also includes hematopoietic cells resistant to recognition of the same antigen by the antigen-recognizing receptor.
Owner:MILTENYI BIOTEC B V & CO KG
Genetically modified mesenchymal stem cells expressing an immune response-stimulating cytokine to attract and/or activate immune cells
The invention relates to a genetically modified mesenchymal stem cell (MSC) and medical use thereof in the treatment of tumours, said MSC comprising one or more exogenous nucleic acid molecule(s), wherein said exogenous nucleic acid molecule(s) comprise a region encoding one or more immune response-stimulating or immune response-modulating cytokine(s) operably linked to a promoter or promoter / enhancer combination. The invention encompasses the use of said cells in modulating the tumour microenvironment in order to attract immune effector cells and facilitate their activation and / or adoption of a memory phenotype. One aspect of the invention relates to the use of said cells in anti-tumour treatment comprising the combined administration of said mesenchymal stem cells with anti-tumour immunotherapies, such as checkpoint inhibitors, immune cells, for example T cells, such as T cells with artificial T cell receptors, for example a chimeric antigen receptor (CAR-Ts) or exogenous T-Cell Receptor (TCR) transduced cells, NK cells or macrophages / monocytes, or a cancer vaccine.
Owner:APCETH GMBH & CO KG
Antibodies with immune effector activity and that internalize in endosialin-positive cells
ActiveUS20060239911A1Improve anti-tumor activityEnhanced antibody-dependent cellular cytotoxicityNanomedicineImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseNucleotide
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing endosialin and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to endosialin-expressing cells as well as in eliciting an immune-effector activity particularly on tumor and neovascular cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer and neovascular cells; and methods of treating cancer and neovascular disease using the antibodies, derivatives and fragments.
Owner:EISAI INC
Antibodies recognizing a carbohydrate containing epitope on CD-43 and CEA expressed on cancer cells and methods using same
ActiveUS20080171043A1Reduce in quantityInhibit growthImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationCancer cellHematopoietic cell
The present invention provides novel antibodies specifically bind to an epitope on CD43 and CEA expressed on nonhematopoietic cancer cells, but do not specifically bind to a CD43 expressed by a leukocyte or by a Jurkat cell, and is capable of inducing apoptosis of the nonhematopoietic cancer cell after binding to the epitope on cell surface of the nonhematopoietic cancer cell in the absence of cytotoxin conjugation and immune effector function, wherein the epitope comprises a carbohydrate structure and the binding of the antibody to the epitope is inhibited by a carbohydrate comprising a Lea structure, a Lea-lactose structure, a LNDFH II structure, or a LNT structure. In addition, the present invention also provides use of the antibodies described herein for diagnostic and therapeutic purposes.
Owner:ALTRUBIO INC
Diagnostic assay for measuring a cell mediated immune response
ActiveUS20050014205A1Reduce handling costsReduce exposureMicrobiological testing/measurementBiological testingWild lifeCell-mediated immune response
The present invention relates generally to a diagnostic assay and, more particularly, an assay for measuring cell-mediated immune reactivity. Even more particularly, the present invention provides an assay and a kit for measuring a cell-mediated response to an antigen using whole blood or other suitable biological sample. The assay may be conducted using ligands to immune effector molecules or at the nucleic acid level, screening for expression of genes encoding the immune effector molecules. The assay is useful in therapeutic and diagnostic protocols for human, livestock and veterinary and wild life applications.
Owner:CELLESTIS
Certain aminoalkyl glucosaminide phosphate compounds and their use
ActiveUS20050227943A1Little timeReduce the burden onAntibacterial agentsEsterified saccharide compoundsAdjuvantPhosphate
Compounds that are adjuvants and immunoeffectors are described and claimed. The compounds augment antibody production in immunized animals as well as stimulate cytokine production and activate macrophages. Compositions and methods for using the compounds as adjuvants and immunoeffectors are also disclosed.
Owner:CORIXA CORP
Immune effector cells pre-infected with oncolytic virus
InactiveUS20070077231A1Improved biodistributionMinimal viral infectionBiocideGenetic material ingredientsAbnormal tissue growthImmune effector cell
Compositions and methods are provided for the treatment of cancer. An immune effector cell population is pre-infected with an oncolytic virus. The combined therapeutic is safe and highly effective, producing an enhanced anti-tumor effect compared to either therapy alone. The methods of the invention thus provide for a synergistic effect based on the combined biotherapeutics.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
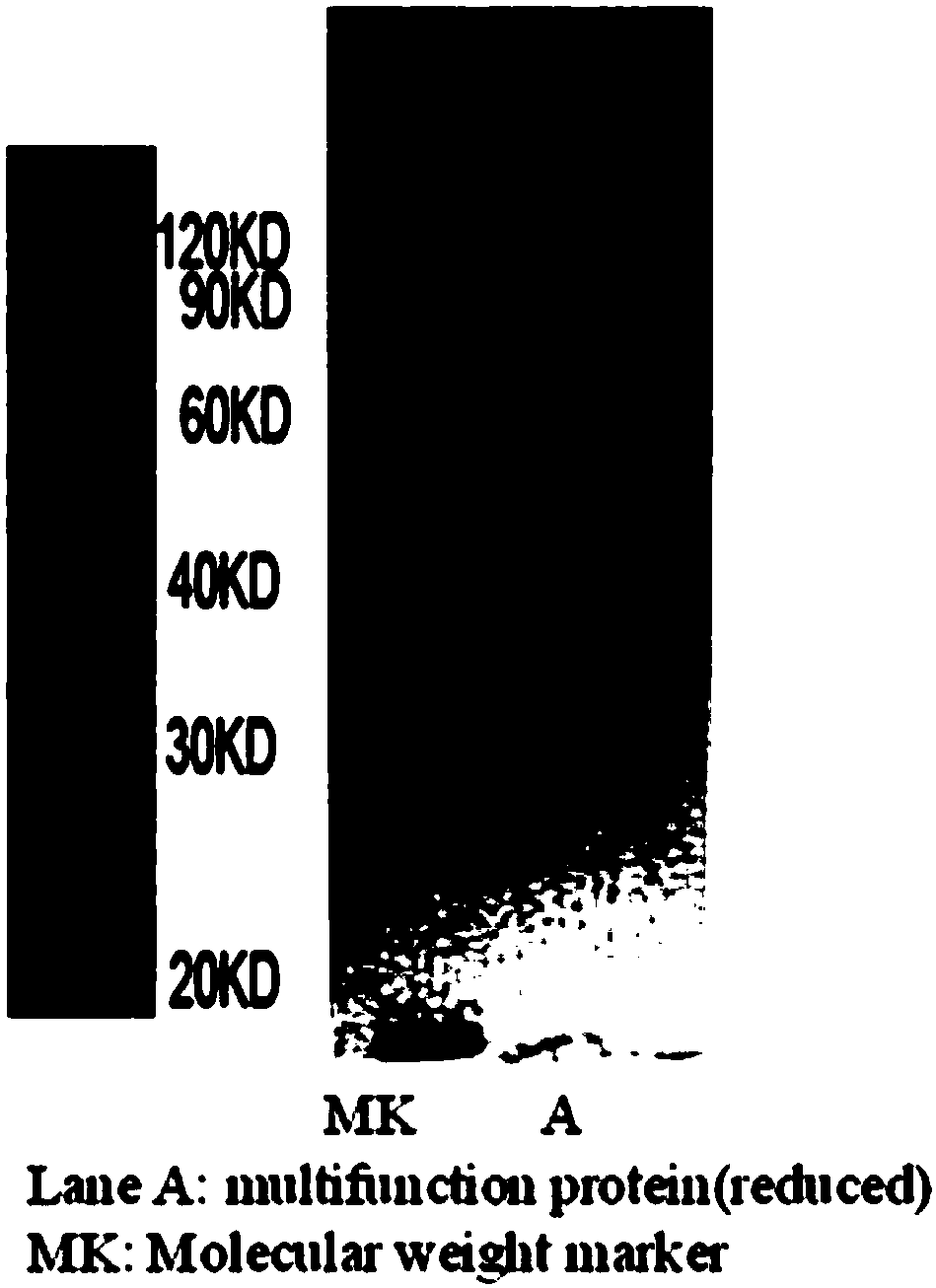
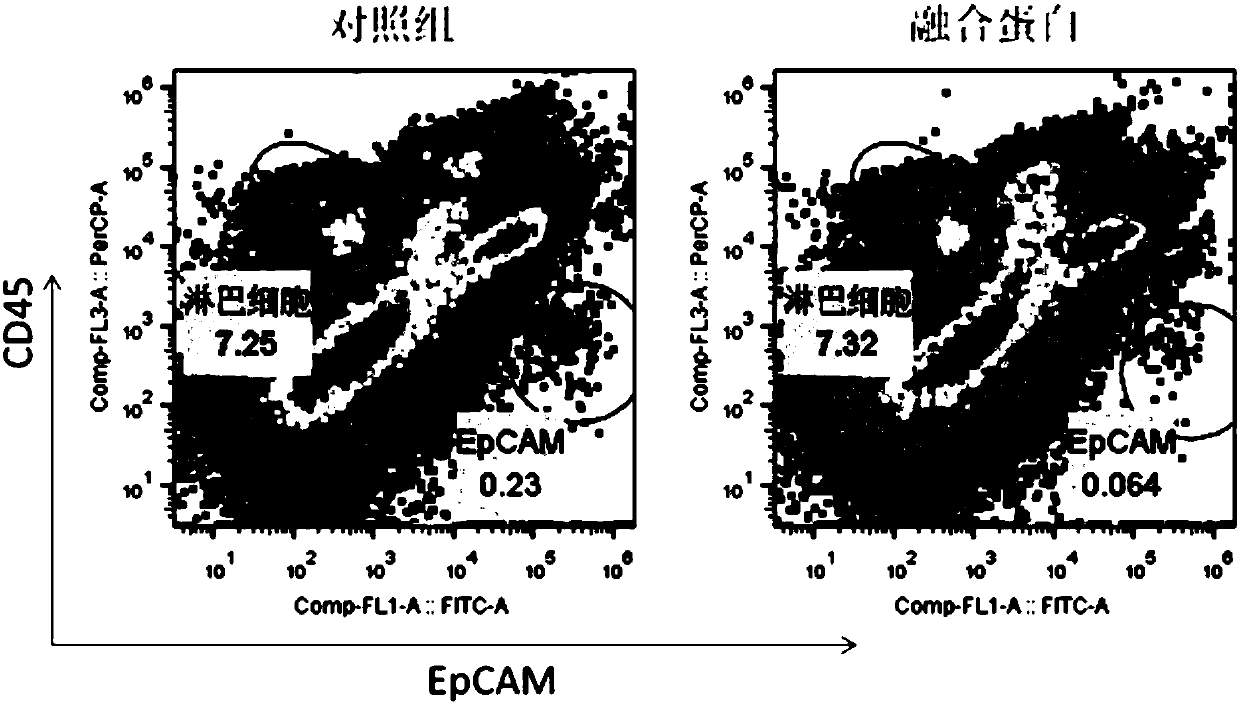
Multifunctional fusion protein and application thereof
InactiveCN107857819AMeet the needs of tumor immunotherapyInfection controlPeptide/protein ingredientsAntibody mimetics/scaffoldsFc(alpha) receptorAbnormal tissue growth
The invention relates to multifunctional fusion protein and an application thereof. The multifunctional fusion protein comprises: a. a functional area for identifying positive tumor cells CD47: an extracellular part of SIRP alpha, b. a functional area for identifying positive tumor cells PD-L1: an extracellular part of PD-1 and c. a functional area for being bonded to immune cells: a high-affinityhuman IgG1Fc part. The fusion protein disclosed by the invention can meet the needs of patients on immunotherapy of tumors; the recombinant fusion protein can identify positive tumor cells (CD47 andPD-L1) and can also be bonded to immune effector cells with Fc receptors; and due to clinical application of the fusion protein, the functions of inhibiting tumor growth and controlling viral infection can be enhanced, so that the fusion protein has very good clinical prospects and extensive range of application.
Owner:JIANGSU CTL BIOLOGICAL TECH CO LTD
Nucleic acid molecules encoding cytotoxic conjugates that contain a chemokine receptor targeting agent
InactiveUS7192736B2Enhance and aid in survivalPrevent proliferationBacteriaPeptide/protein ingredientsCell biologyWhite blood cell
Nucleic acid moleucles that encode conjugates containing as a ligand a chemokine receptor targeting agent, such as a chemokine, and a targeted agent, such as a toxin are provided. These conjugates are used to treat inflammatory responses associated with activation, proliferation and migration of immune effector cells, including leukocyte cell types, neutrophils, macrophages, and eosinophils.
Owner:SAMSUNG ENGINEERING CO LTD +1
Conjugates for immunotherapy
ActiveUS20170202902A1Enhance immune responseControlling signalPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseMelanoma
The current invention pertains to a molecular conjugate comprising an antagonist of a cell surface receptor specific to a target cell and an immune effector, such as a T cell modulator, conjugated to the antagonist. The target cell can be a cell responsible for development of a disease in a subject, for example, a cancer cell. In certain embodiments, the immune effector is an immune effector protein or an immune effector fragment thereof. The current invention also pertains to a method of treating a disease in a subject, the method comprising administering to the subject a pharmaceutically effective amount of the molecular conjugates of the current invention to the subject. The methods of the current invention can be used to treat cancer, such as breast cancer, ovarian cancer, prostate cancer, lung cancer, pancreatic cancer, or melanoma.
Owner:UNIV OF SOUTH FLORIDA +1
Humanized antibody against CD19 and immune effector cells targeting to CD19
The invention relates to an anti-CD19 humanized antibody prepared from a murine monoclonal antibody, a chimeric antigen receptor containing the humanized antibody, and immune cells expressing the humanized antibody. The humanized antibody of the invention does not produce an anti-antibody reaction (AAR) and a human anti-mouse antibody reaction (HAMA), has better affinity than a murine antibody, and presents excellent activity and safety, so a novel means is provided for treating tumors expressing CD19.
Owner:CARSGEN THERAPEUTICS
Monoclonal antigen-binding proteins to intracellular oncogene products
ActiveUS20180134804A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsRadio isotopesIntravenous gammaglobulin
Antigen binding proteins specific for an HLA-A2 restricted Ras peptide are disclosed. The antigen binding proteins encompass antibodies in a variety of forms, including full-length antibodies, substantially intact antibodies, Fab fragments, F(ab′)2 fragments, and single chain Fv fragments. Fusion proteins, such as scFv fusions with immunoglobulin or T-cell receptor domains, and bispecific antibodies incorporating the specificity of the antigen binding region for each peptide are also contemplated by the disclosure. Furthermore, immunoconjugates may include antibodies to which is linked a radioisotope, fluorescent or other detectable marker, cytotoxin, or other molecule are also encompassed by the disclosure. Among other things, immunoconjugates can be used for delivery of an agent to elicit a therapeutic effect or to facilitate an immune effector function.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT +1
Aminoalkyl glucosaminide phosphate compounds and their use as adjuvants and immunoeffectors
Aminoalkyl glucosaminide phosphate (AGP) compounds that are adjuvants and immunoeffectors are described and claimed. The compounds have a 2-deoxy-2-amino glucose in glycosidic linkage with an aminoalkyl (aglycon) group. Compounds are phosphorylated at the 4 or 6 carbon on the glucosaminide ring and comprise three 3-alkanoyloxyalkanoyl residues. The compounds augment antibody production in immunized animals as well as stimulate cytokine production and activate macrophages. Compositions and methods for using the compounds as adjuvants and immunoeffectors are also disclosed.
Owner:CORIXA CORP
Cytotoxic Antibody Directed Against Type B Lymphoid Hematopoietic Proliferations
ActiveUS20090053233A1Reduced dosLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsImmunological disordersCD20Cytotoxic antibody
The present invention relates to a monoclonal antibody directed against the CD20 antigen, wherein the variable region of each of the light chains thereof is encoded by a sequence which shares at least 70% identity with murine nucleic acid sequence SEQ ID No. 5, the variable region of each of the heavy chains thereof is encoded by a sequence which shares at least 70% identity with murine nucleic acid sequence SEQ ID No. 7, and the constant regions of light and heavy chains thereof are constant regions from a non-murine species, as well as for activation of FcγRIIIA receptors in immune effector cells, and for the manufacture of a drug especially for the treatment of leukaemia or lymphoma.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Diagnostic assay for measuring a cell mediated immune response
ActiveUS7608392B2Reduce exposureAmenable to analysisMicrobiological testing/measurementBiological testingImmune effectsWild life
The present invention relates generally to a diagnostic assay and, more particularly, an assay for measuring cell-mediated immune reactivity. Even more particularly, the present invention provides an assay and a kit for measuring a cell-mediated response to an antigen using whole blood or other suitable biological sample. The assay may be conducted using ligands to immune effector molecules or at the nucleic acid level, screening for expression of genes encoding the immune effector molecules. The assay is useful in therapeutic and diagnostic protocols for human, livestock and veterinary and wild life applications.
Owner:CELLESTIS
Dual specificity polypeptide molecule
PendingUS20190016801A1Hybrid immunoglobulinsImmunoglobulins against virusesEpitopeMajor histocompatibility
The present invention relates to a bispecific polypeptide molecule comprising a first polypeptide chain and a second polypeptide chain providing a binding region derived from a T cell receptor (TCR) being specific for a major histocompatibility complex (MHC)-associated peptide epitope, and a binding region derived from an antibody capable of recruiting human immune effector cells by specifically binding to a surface antigen of said cells, as well as methods of making the bispecific polypeptide molecule, and uses thereof.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Genetically modified mesenchymal stem cells expressing an immune response-stimulating cytokine to attract and/or activate immune cells
InactiveUS20170239297A1Lower Level RequirementsCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAbnormal tissue growthNatural Killer Cell Inhibitory Receptors
A genetically modified mesenchymal stem cell (MSC) and medical use thereof in the treatment of tumors, the MSC including one or more exogenous nucleic acid molecule(s), wherein the exogenous nucleic acid molecule(s) include a region encoding one or more immune response-stimulating or immune response-modulating cytokine(s) operably linked to a promoter or promoter / enhancer combination. The invention encompasses the use of the cells in modulating the tumor microenvironment in order to attract immune effector cells and facilitate their activation and / or adoption of a memory phenotype. One aspect of the invention relates to the use of the cells in anti-tumor treatment including combined administration of the mesenchymal stem cells with anti-tumor immunotherapies, such as checkpoint inhibitors, immune cells, for example T cells, such as T cells with artificial T cell receptors, for example a chimeric antigen receptor (CAR-Ts) or exogenous T-Cell Receptor (TCR) transduced cells, NK cells or macrophages / monocytes, or a cancer vaccine.
Owner:APCETH GMBH & CO KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com