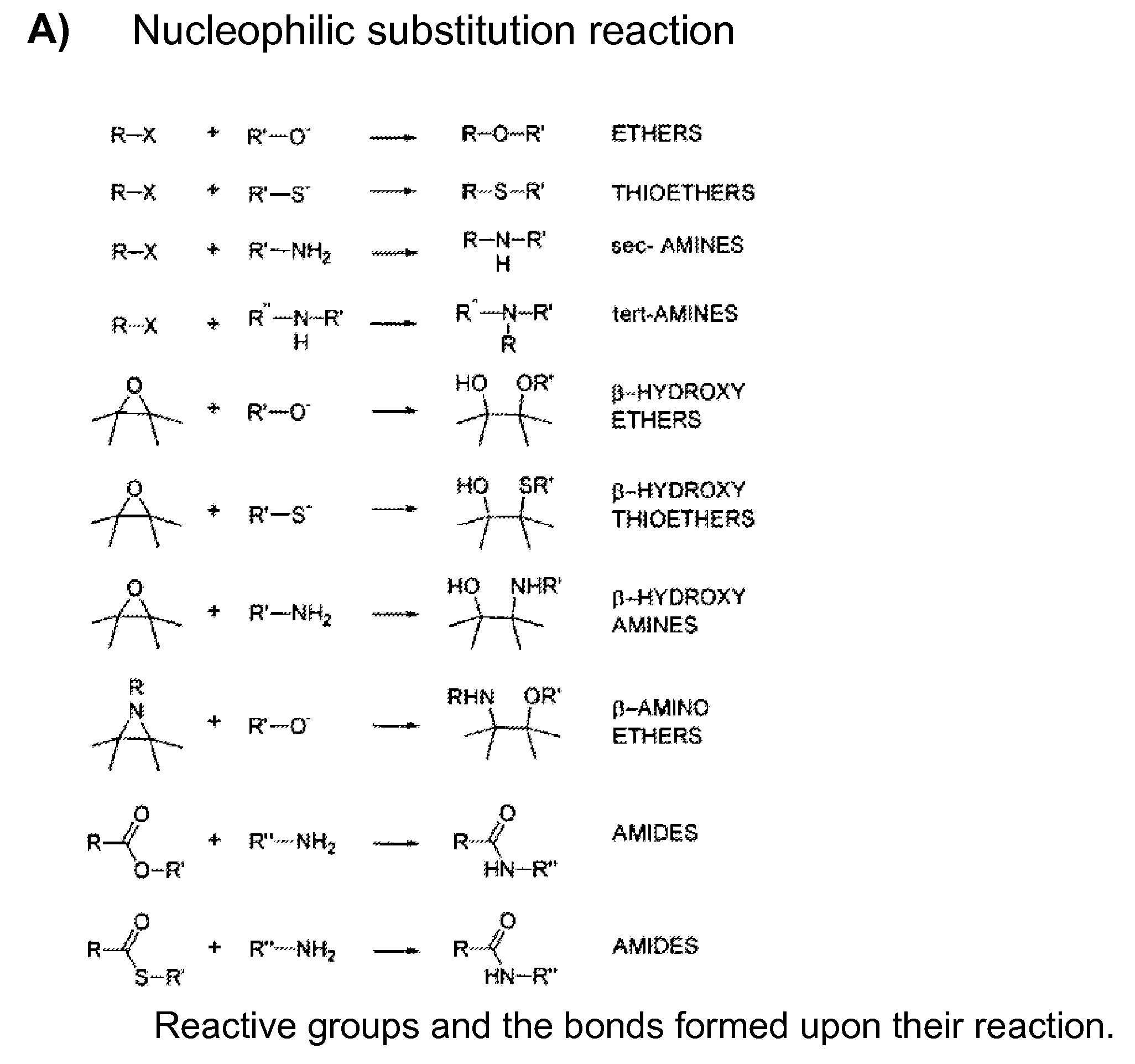
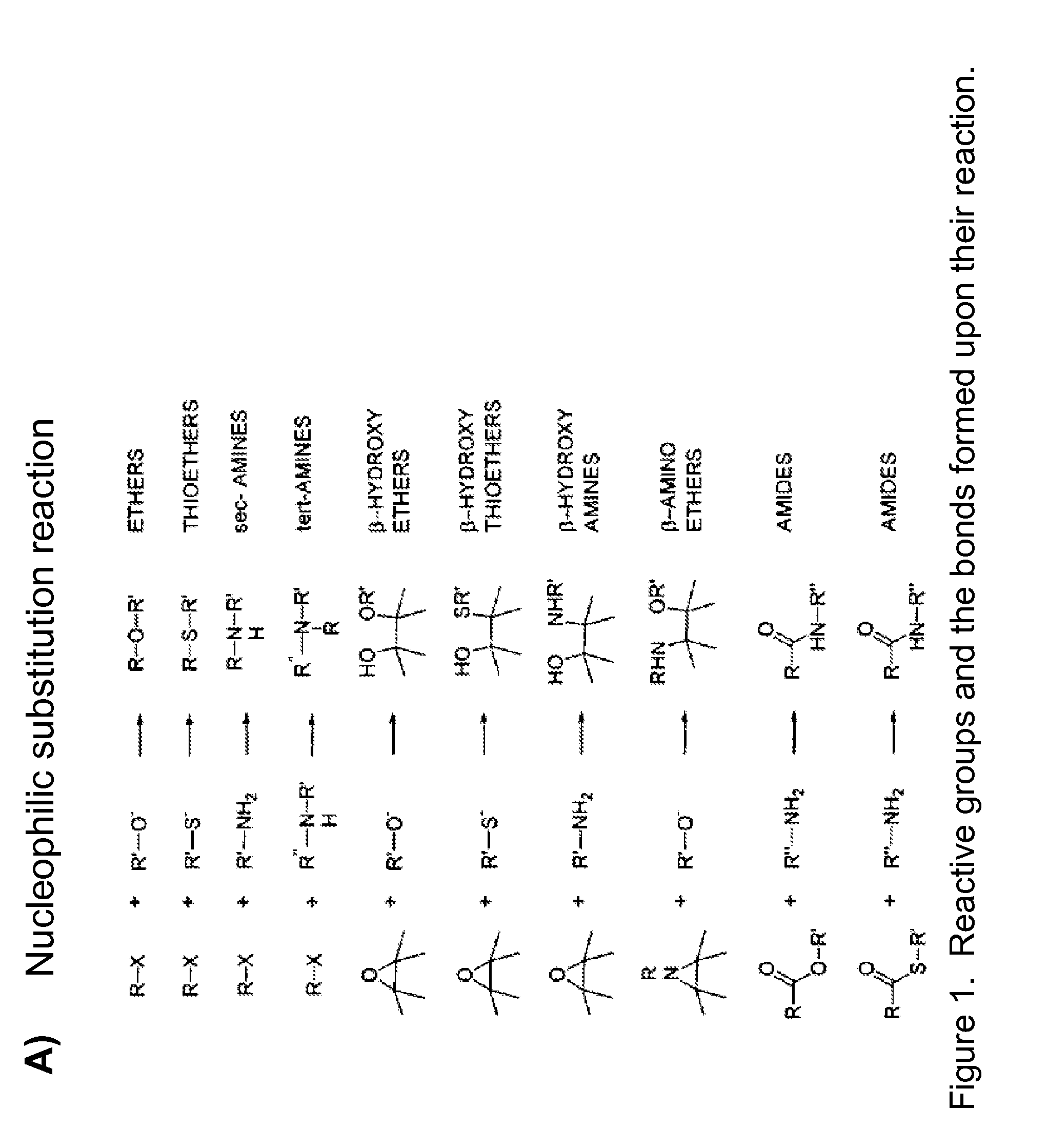
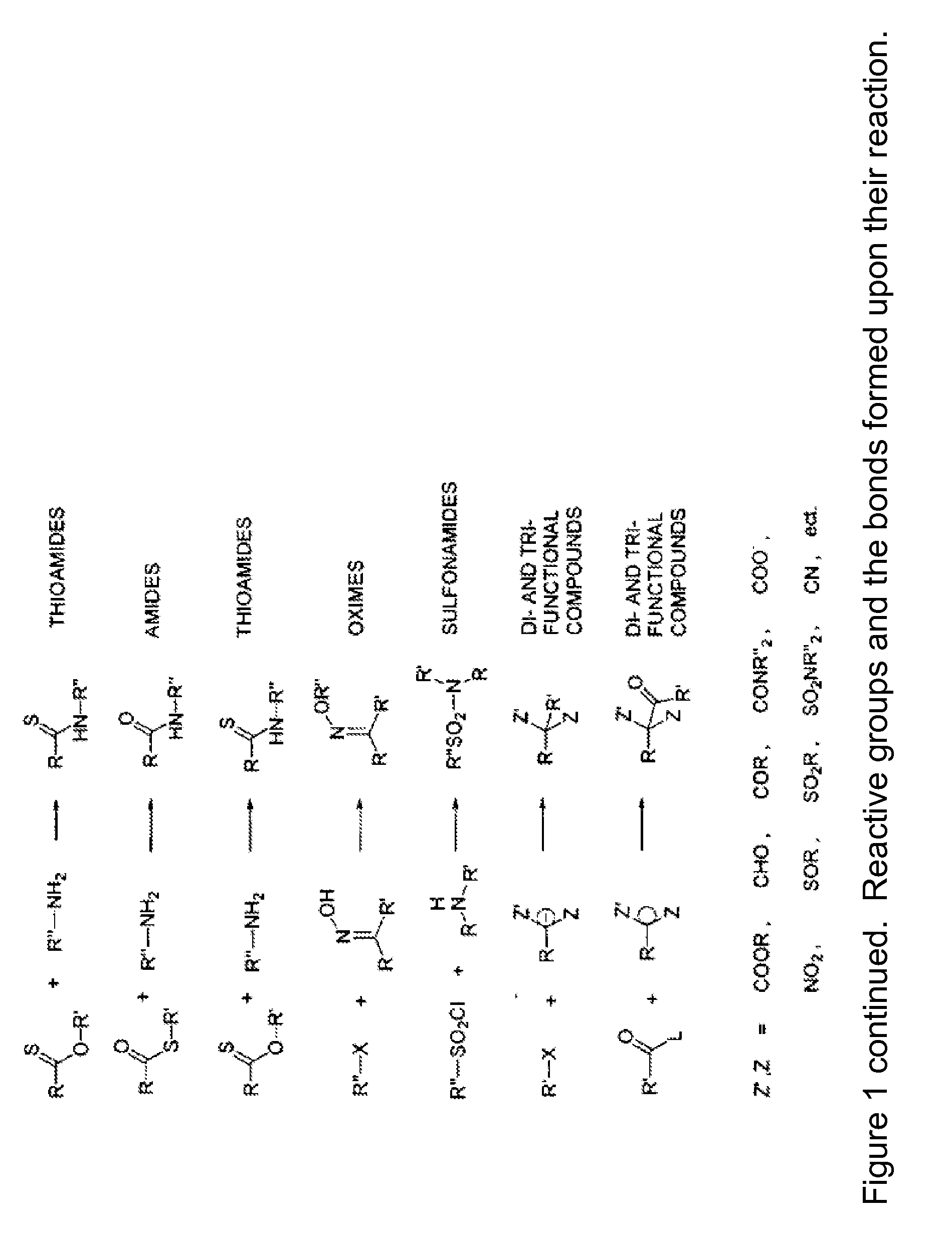
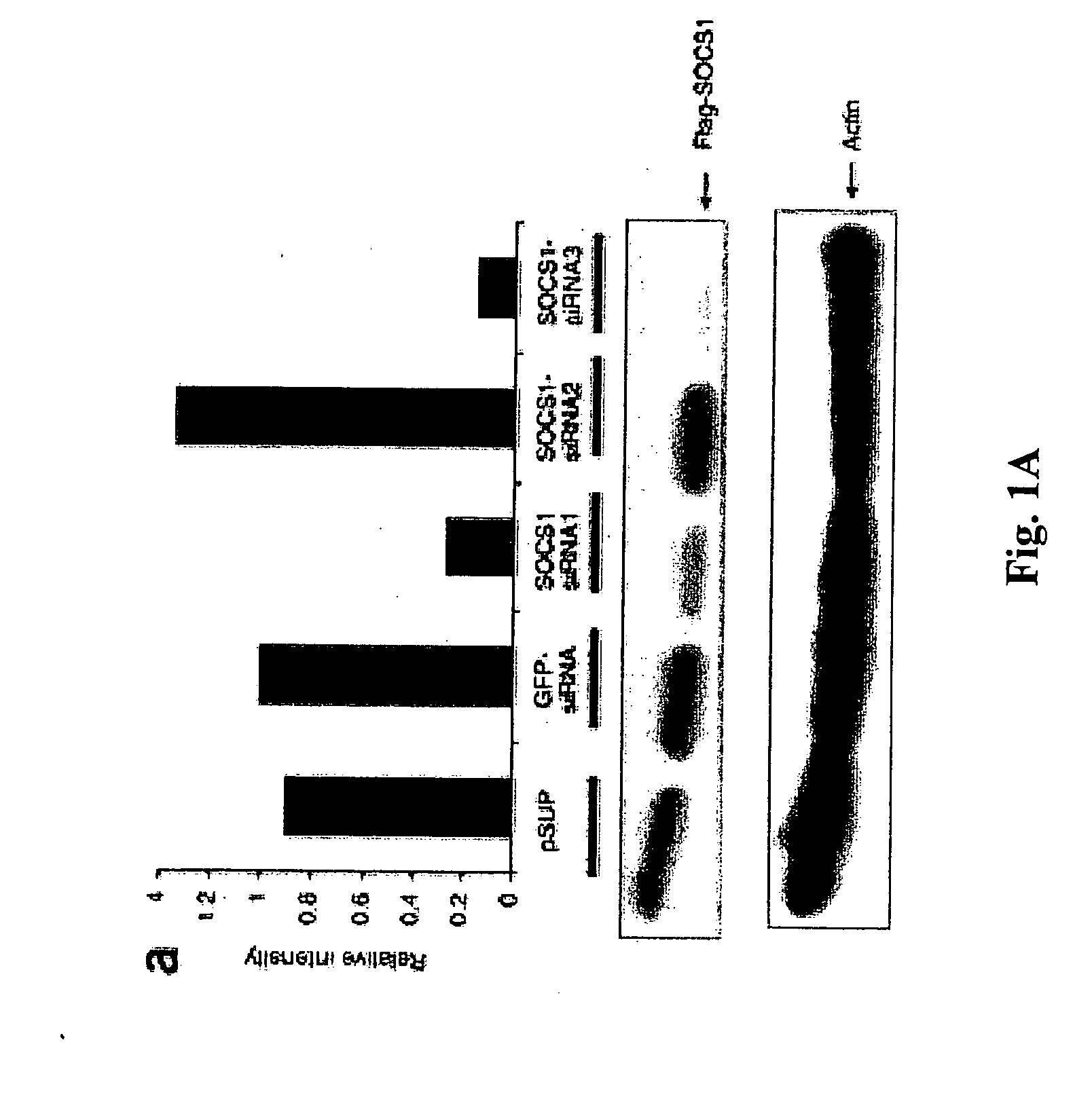
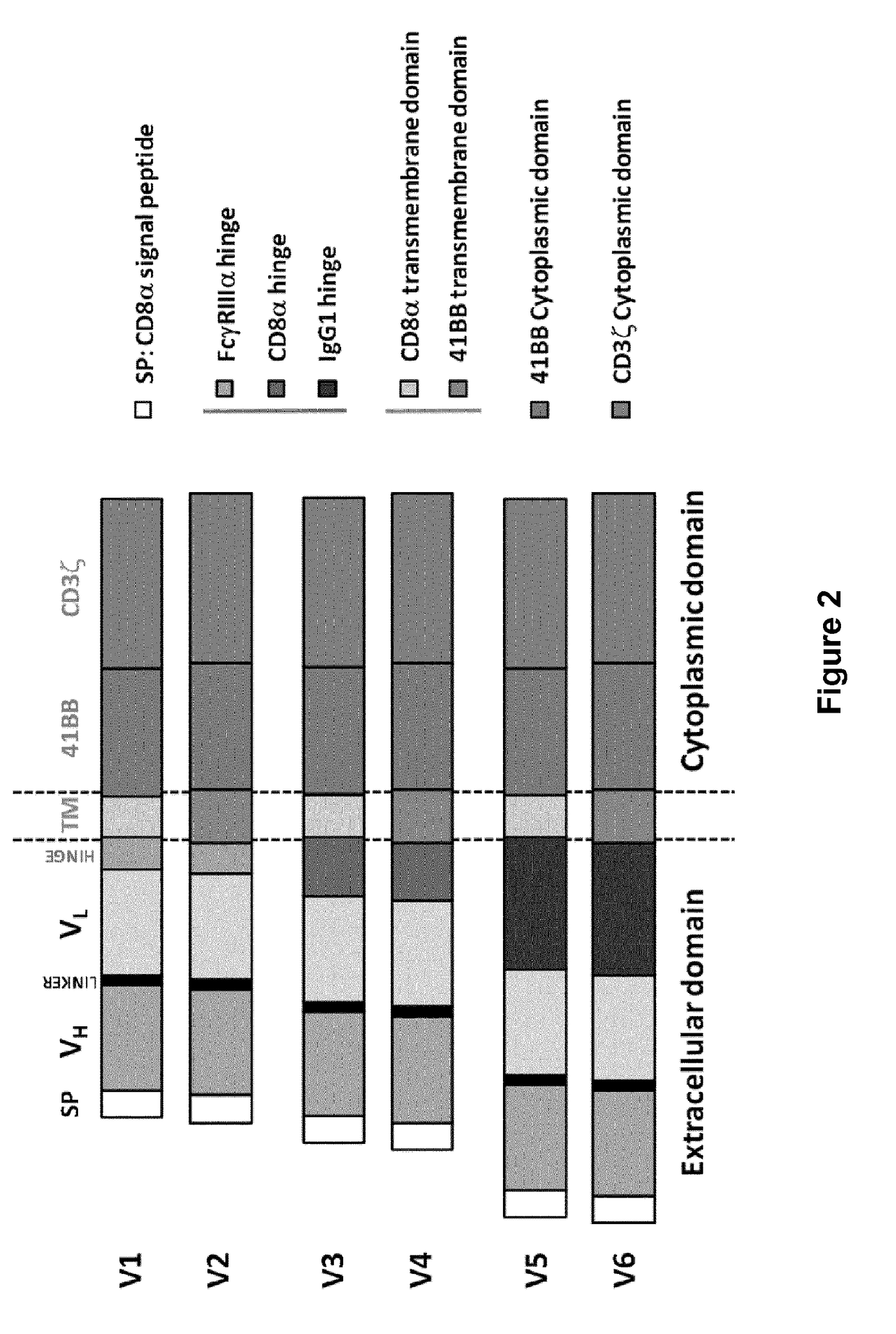
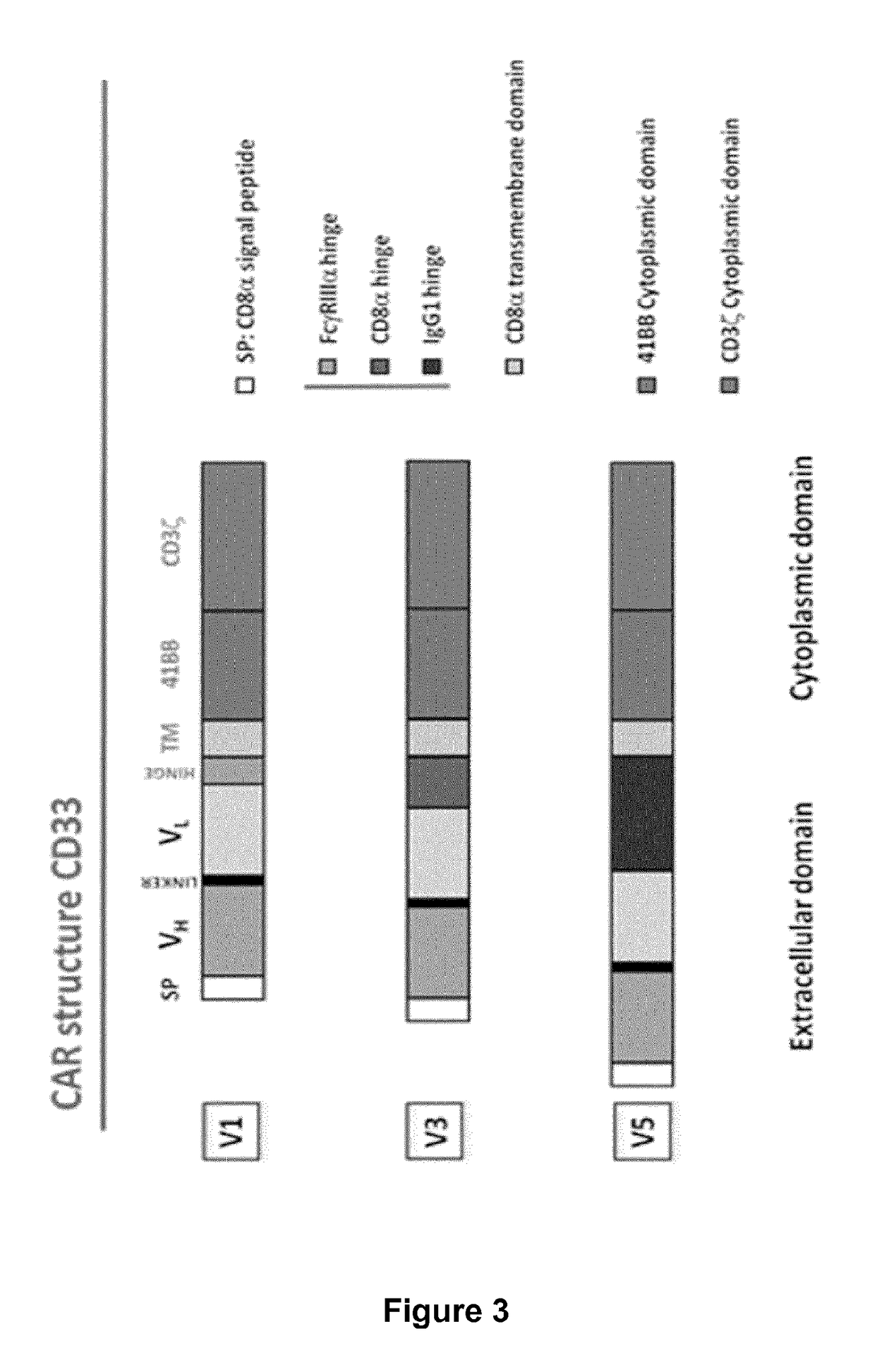
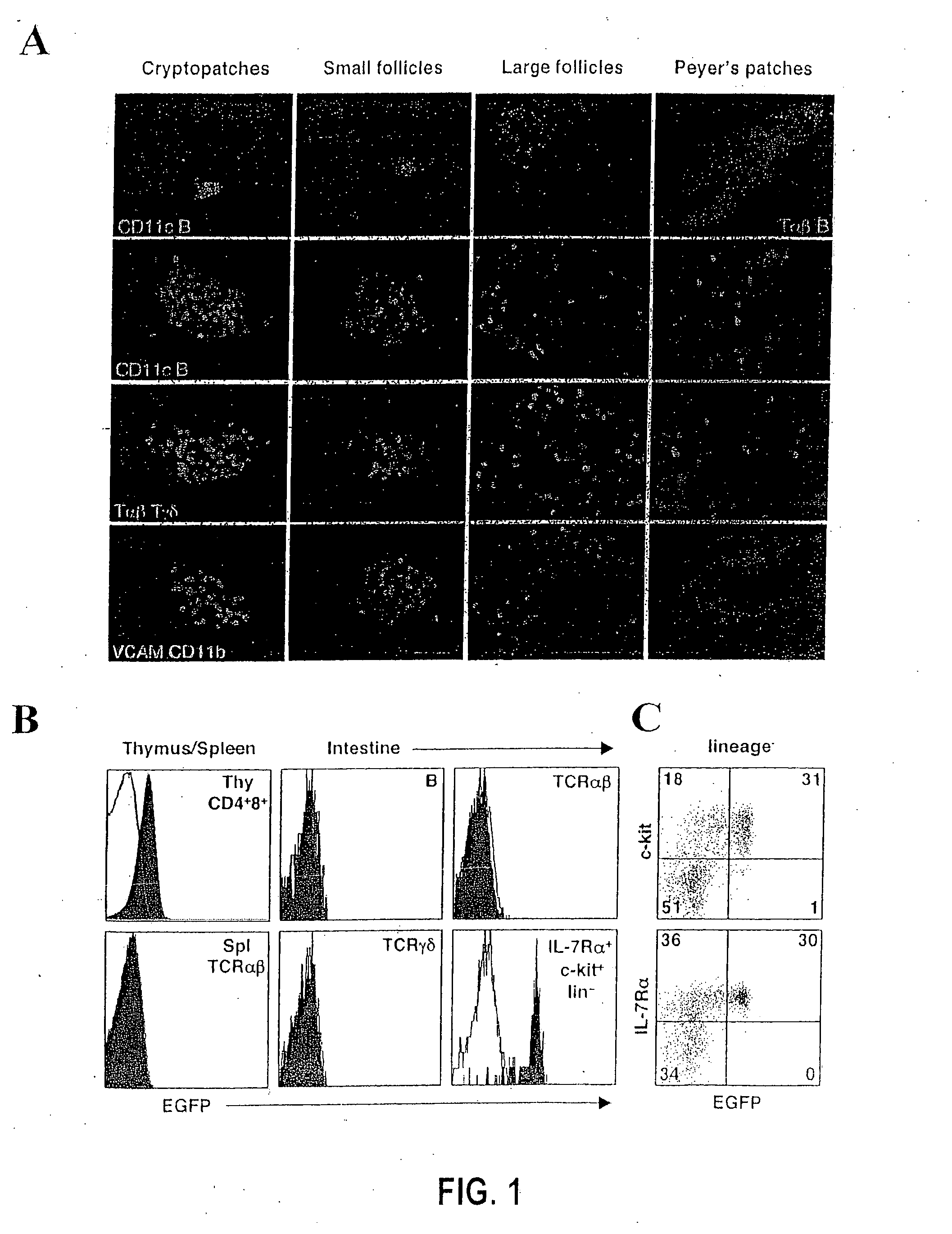
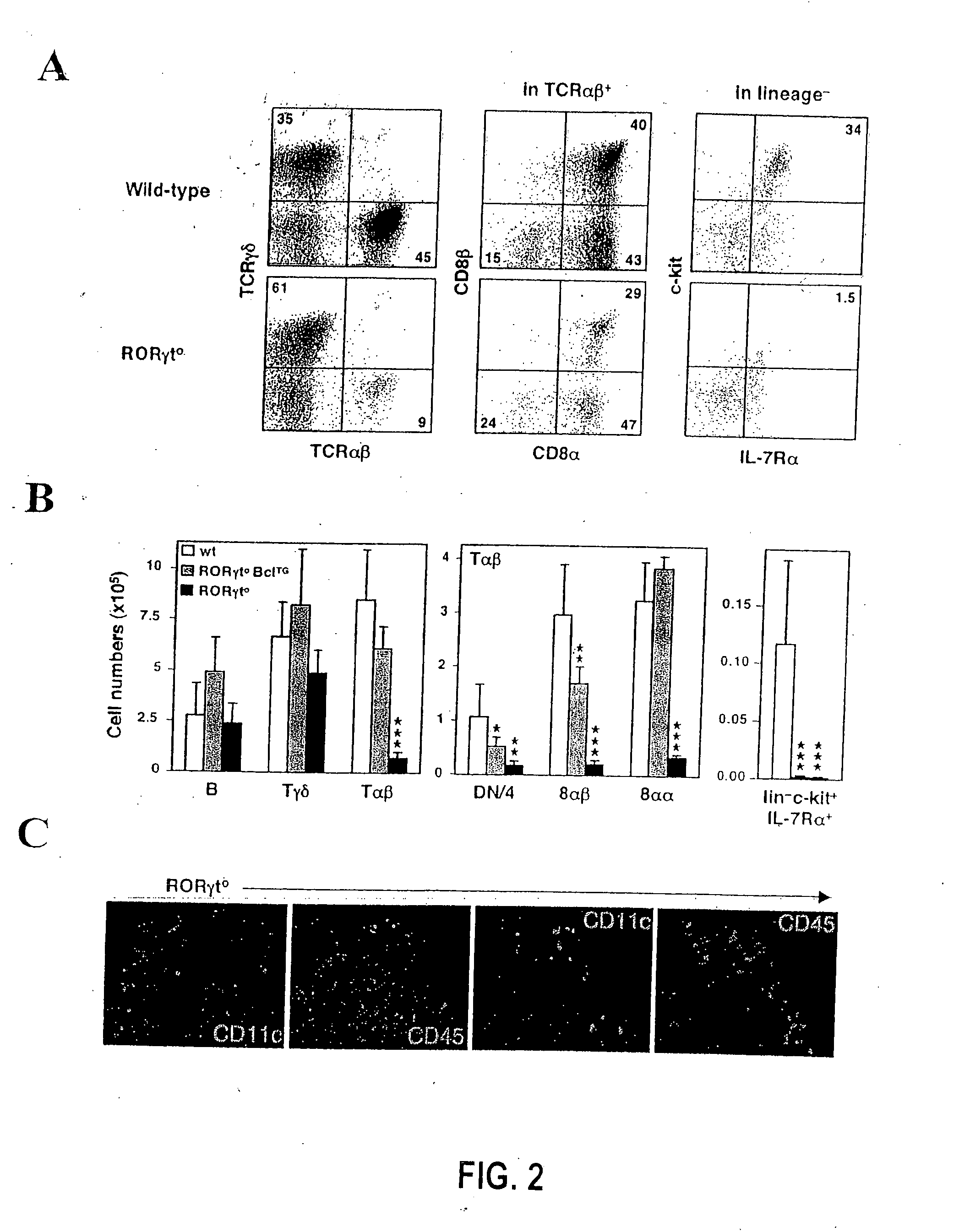
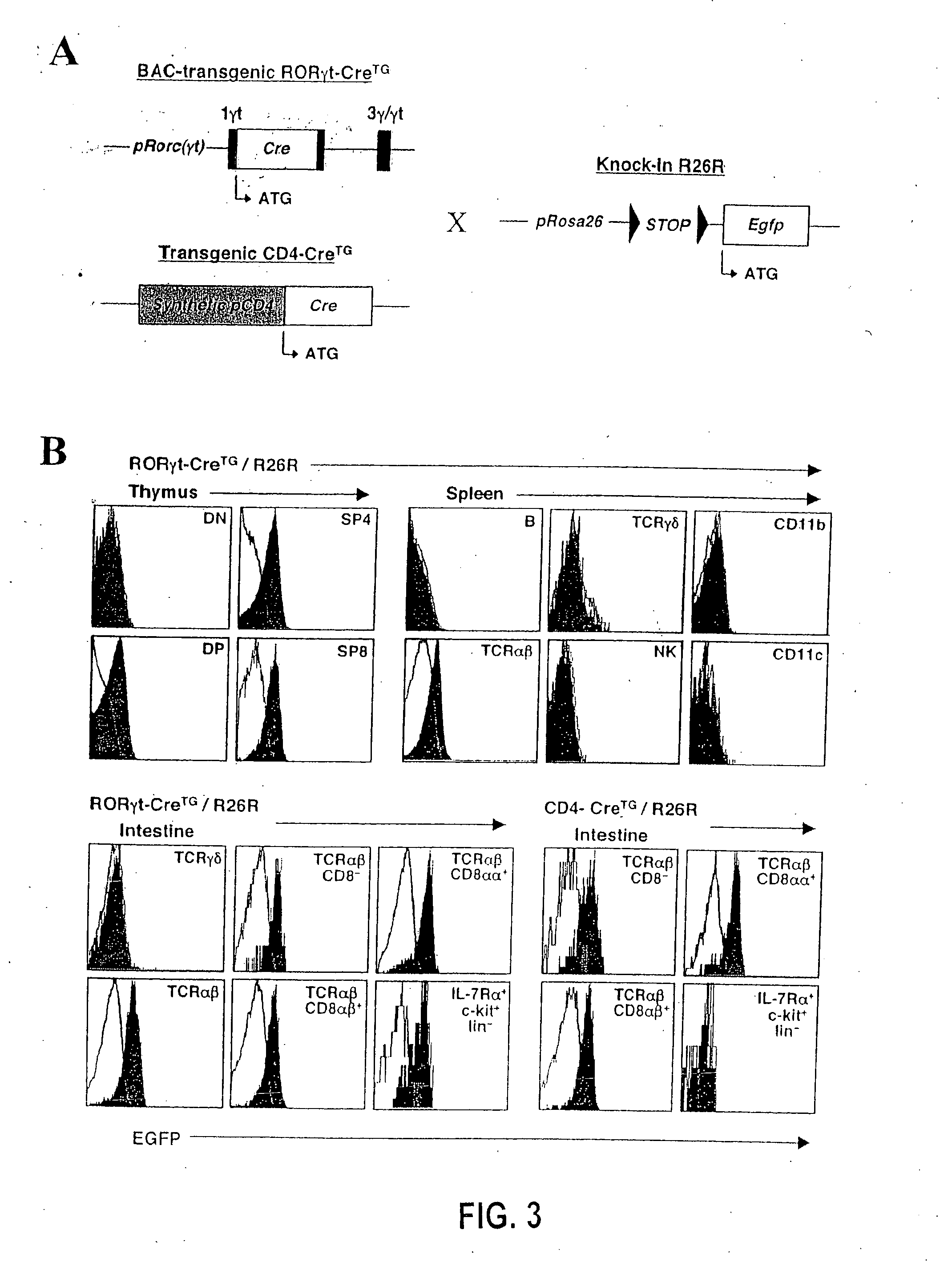
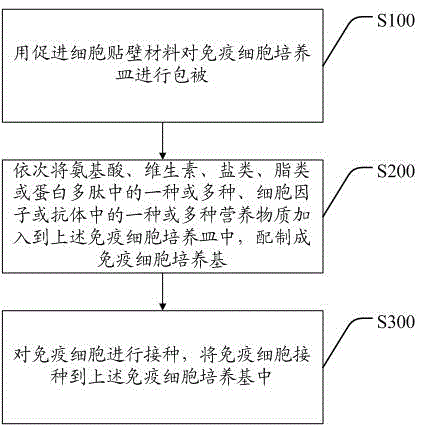
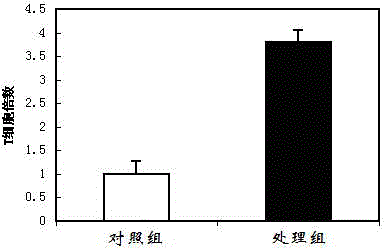
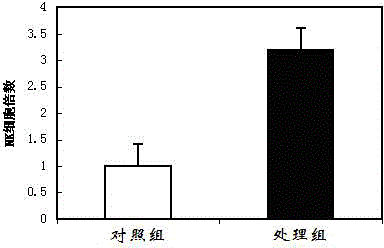
Patents
Literature
2027 results about "IMMUNOLOGIC CELLS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cells of the Immune System. The response to pathogens is orchestrated by the complex interactions and activities of the large number of diverse cell types involved in the immune response. The innate immune response is the first line of defense and occurs soon after pathogen exposure.
CE7-specific redirected immune cells
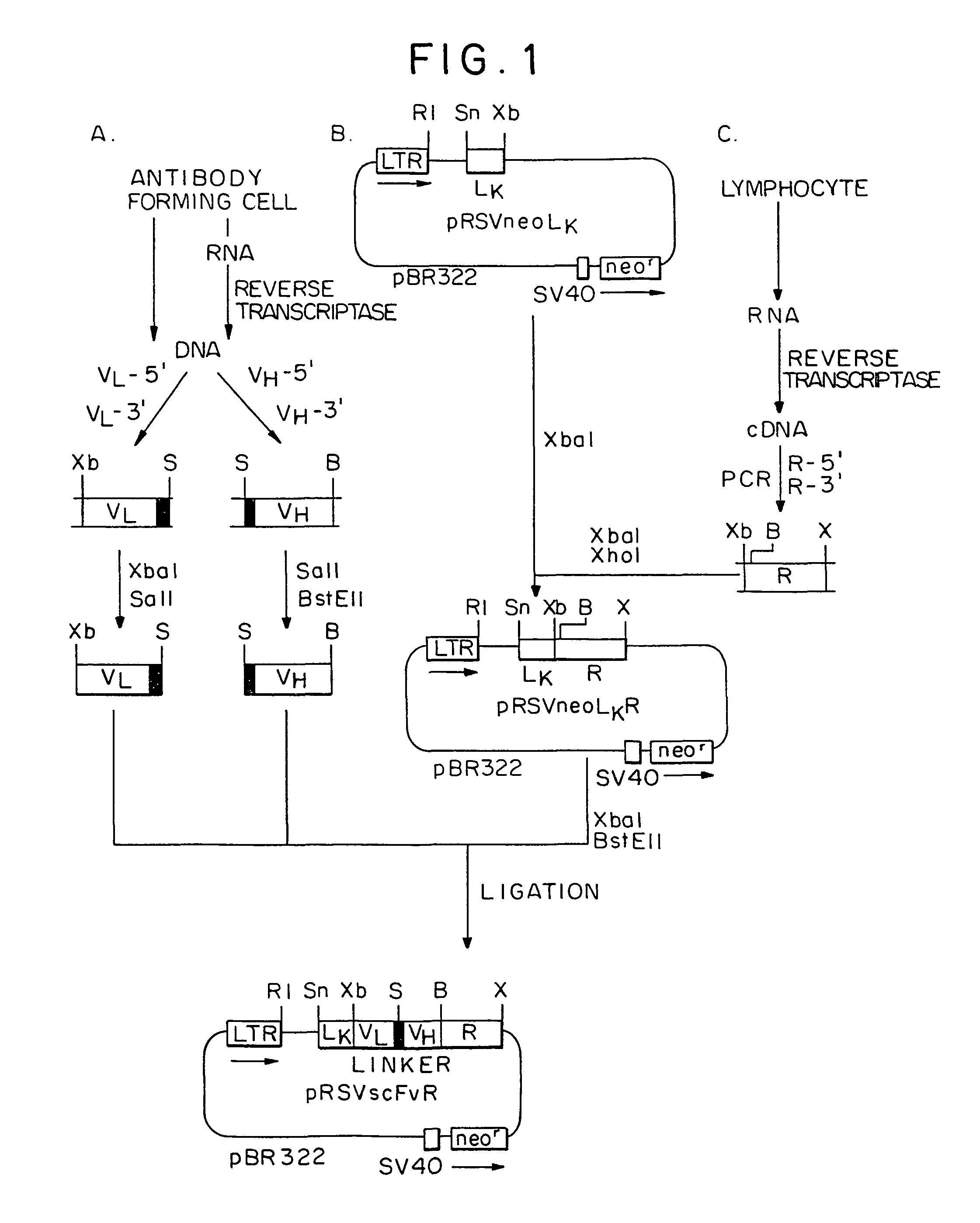
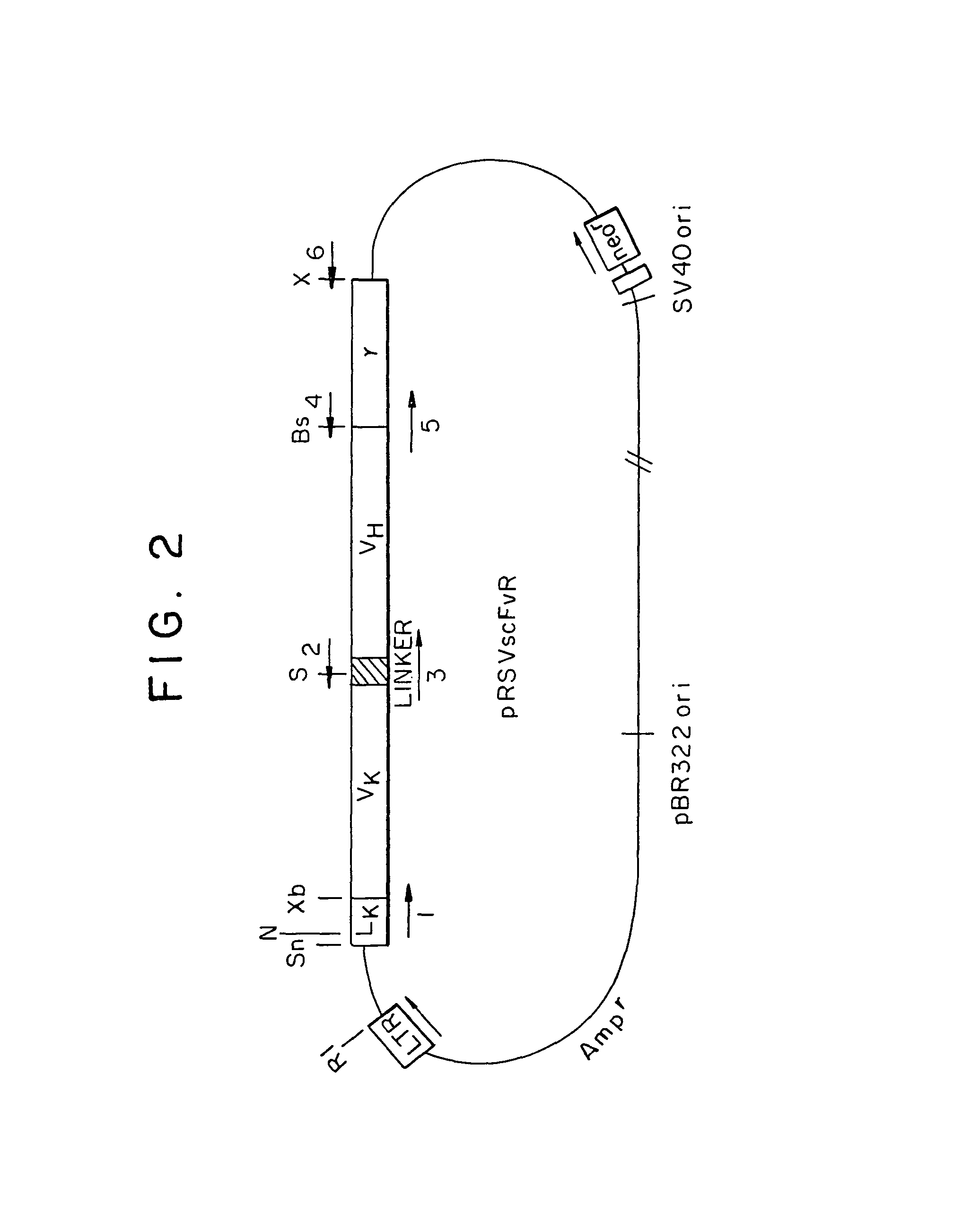
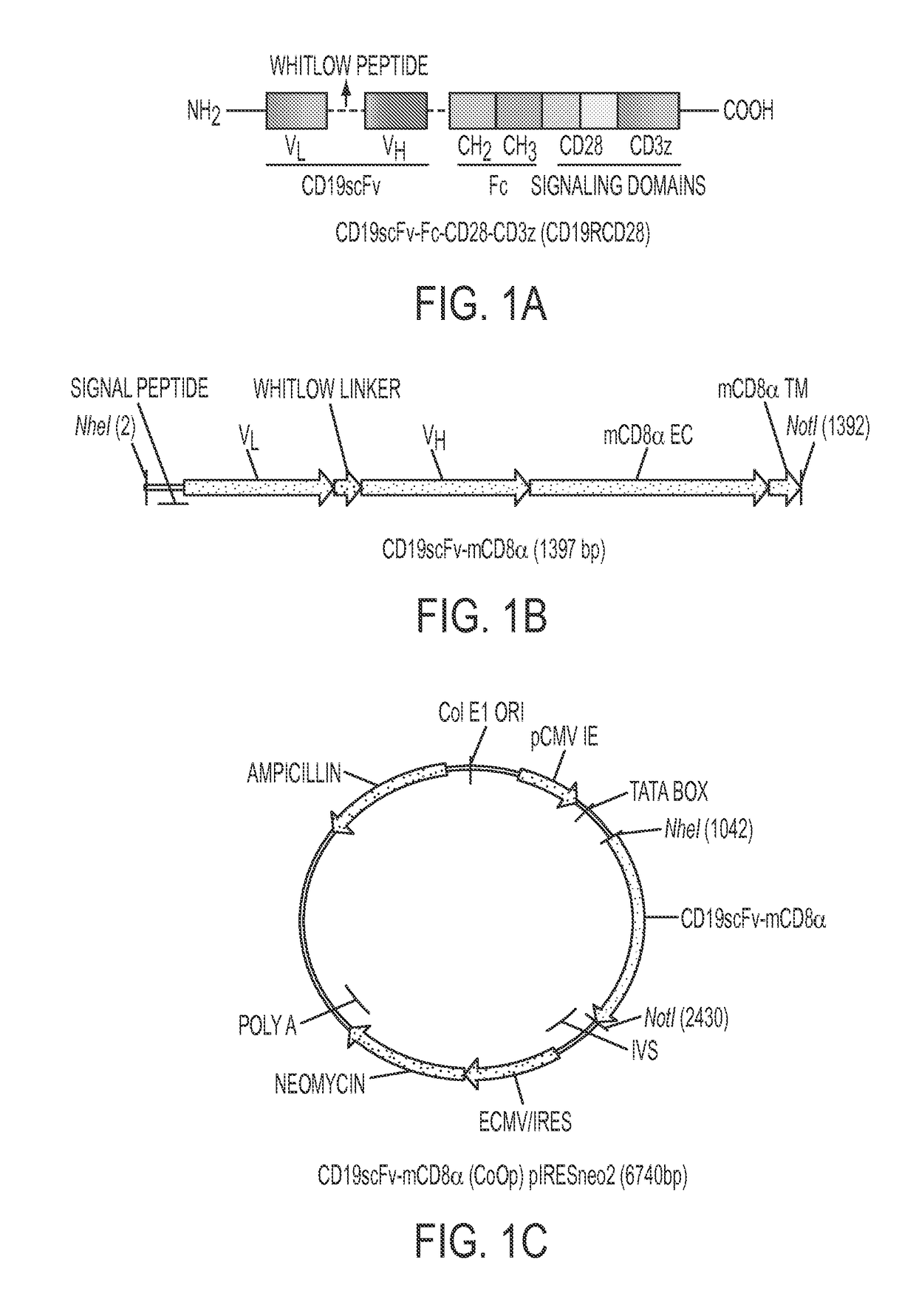
Genetically engineered, CE7-specific redirected immune cells expressing a cell surface protein having an extracellular domain comprising a receptor which is specific for CE7, an intracellular signaling domain, and a transmembrane domain, and methods of use for such cells for cellular immunotherapy of CE7+ neuroblastoma are disclosed. In one embodiment, the immune cell is a T cell and the cell surface protein is a single chain FvFc:ζ receptor where Fv designates the VH and VL chains of a single chain monoclonal antibody to CE7 linked by peptide, Fc represents a hinge —CH2—CH3 region of a human IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. DNA constructs encoding a chimeric T-cell receptor and a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor are also disclosed.
Owner:CITY OF HOPE
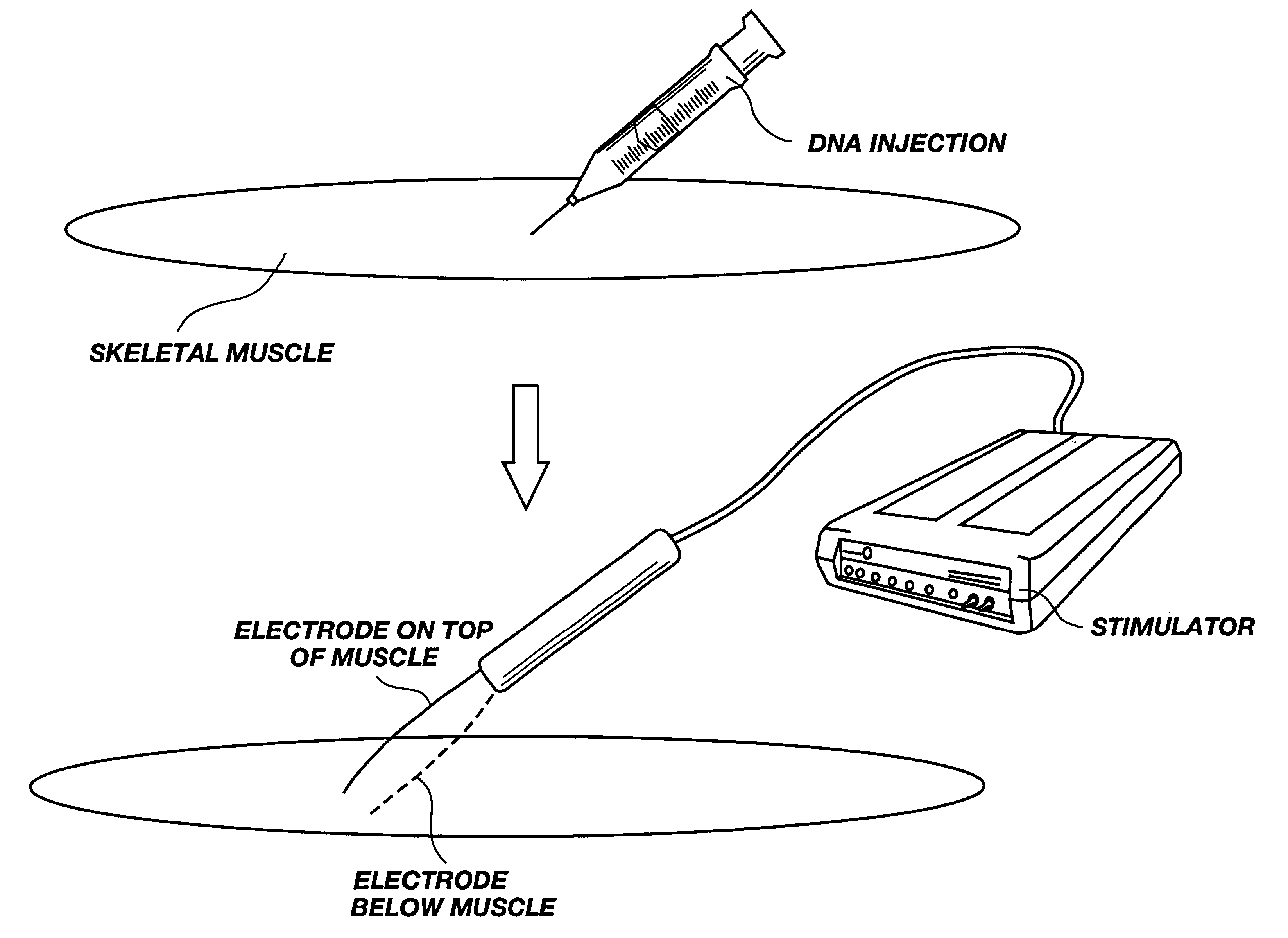
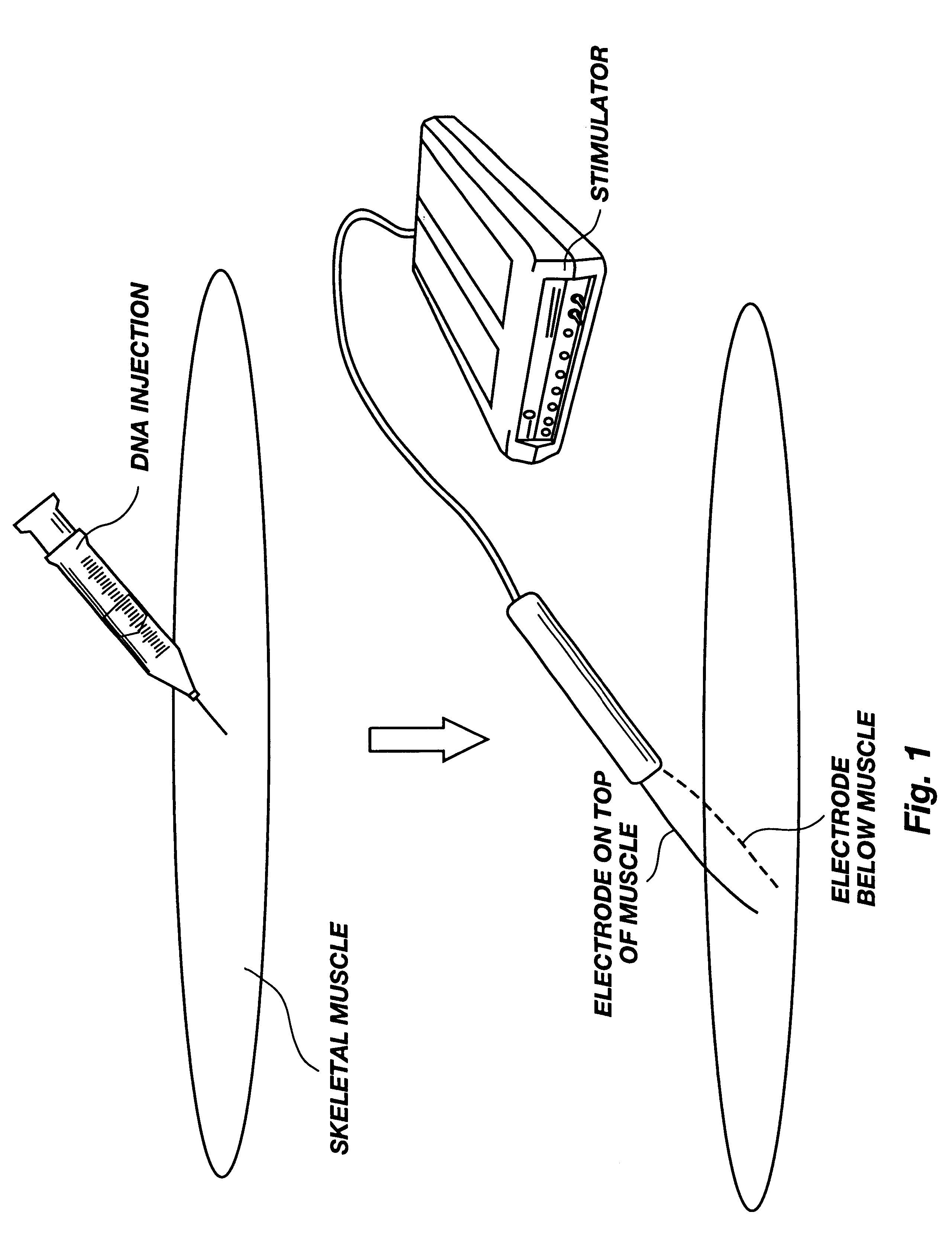
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
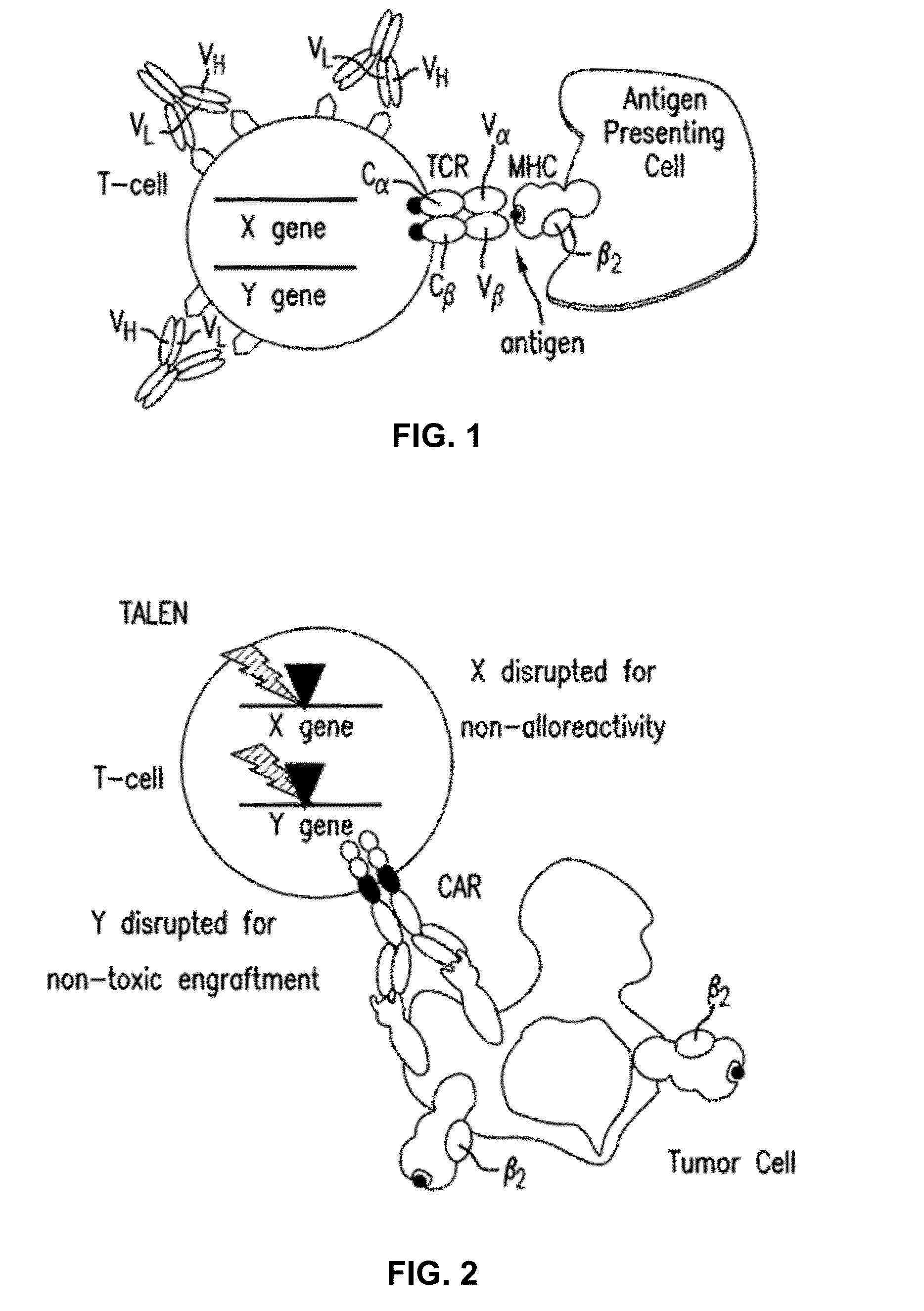
Chimeric receptor genes and cells transformed therewith
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:UNITED STATES OF AMERICA +1
Method and apparatus for heating inflammed tissue
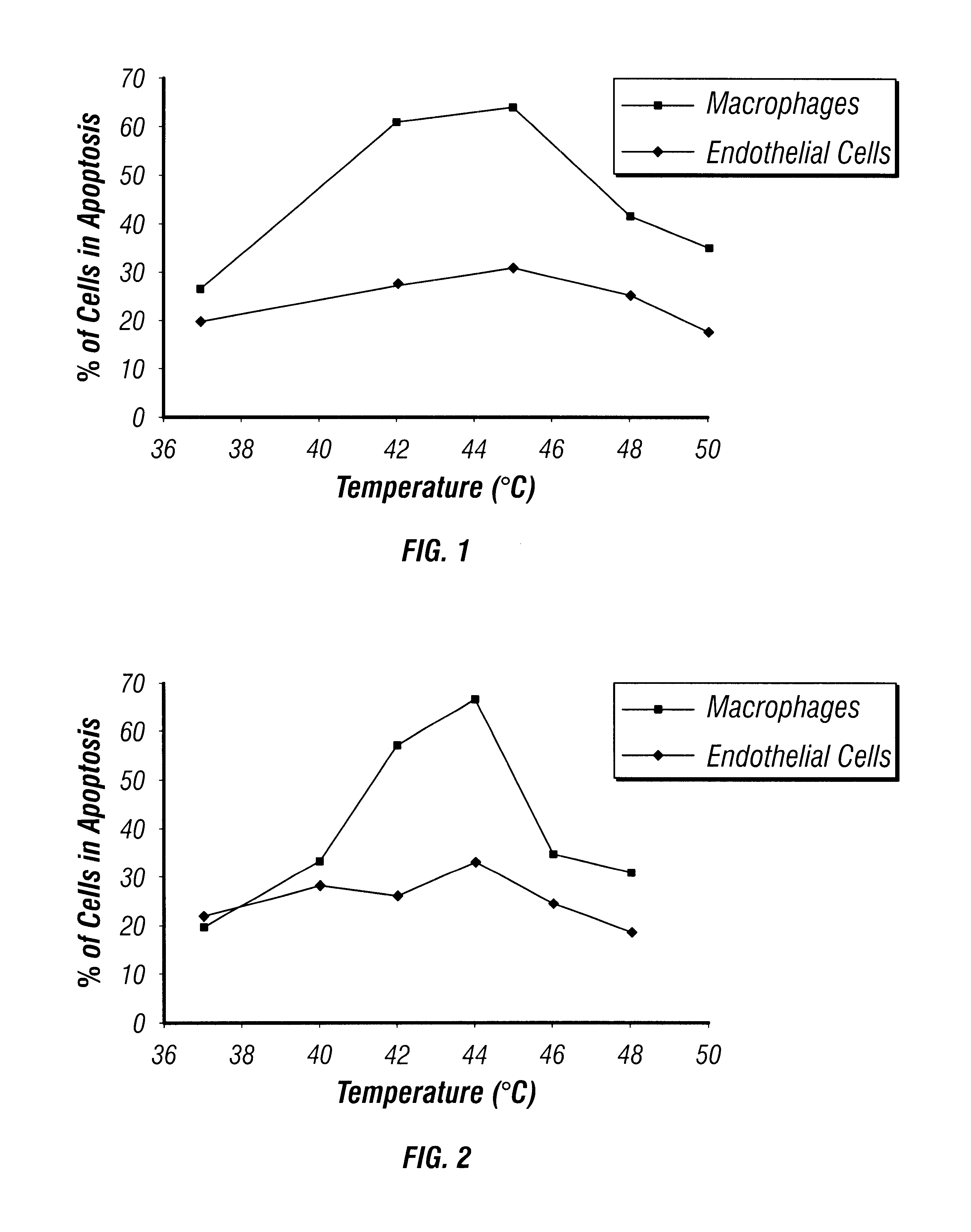
The present invention relates to methods for treating inflammation in body tissues. More specifically, certain disclosed methods relate to selectively inducing apoptosis in inflammatory immune cells by heating cells for a sufficient time and at a sufficient temperature to induce programmed cell death. The disclosed stents can be placed in contact with the inflammatory cells and heated under controlled conditions. The disclosed apparatus and methods are particularly suitable for treating athersclerotic plaques.
Owner:TEXAS HEART INST +1
Compositions and methods for modulating immune responses
ActiveUS20100151000A1Lower Level RequirementsDecreases functional activityPowder deliveryAntipyreticDiseaseAutoimmune responses
This invention discloses methods and compositions for modulating immune responses, which involve particulate delivery of agents to immune cells, wherein the agents comprise an inhibitor of the NF-κB signaling pathway and an antigen that corresponds to a target antigen. The methods and compositions of the present invention are particularly useful in the treatment or prophylaxis of an undesirable immune response associated with the target antigen, including autoimmune diseases, allergies and transplantation associated diseases.
Owner:THE UNIV OF QUEENSLAND
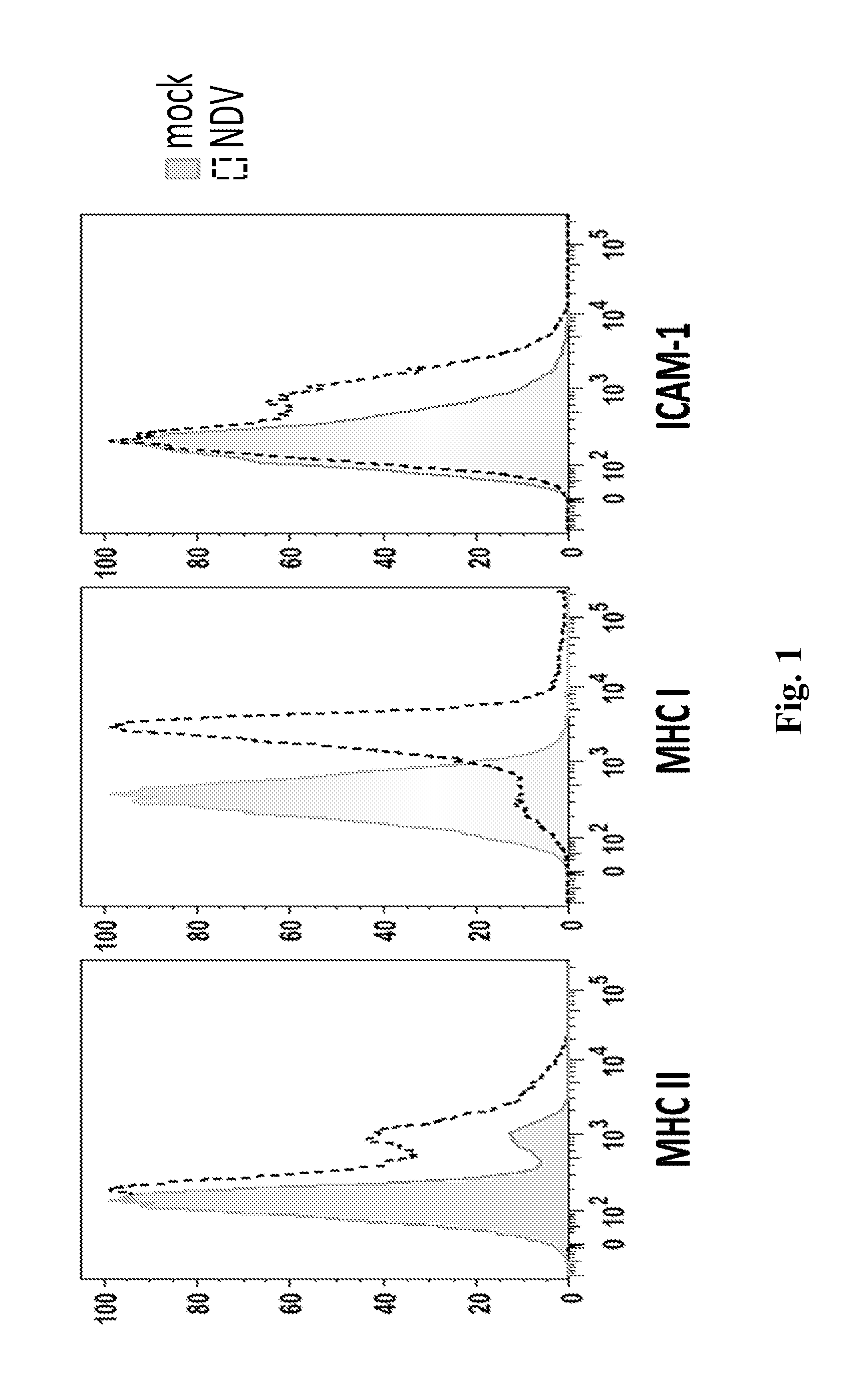
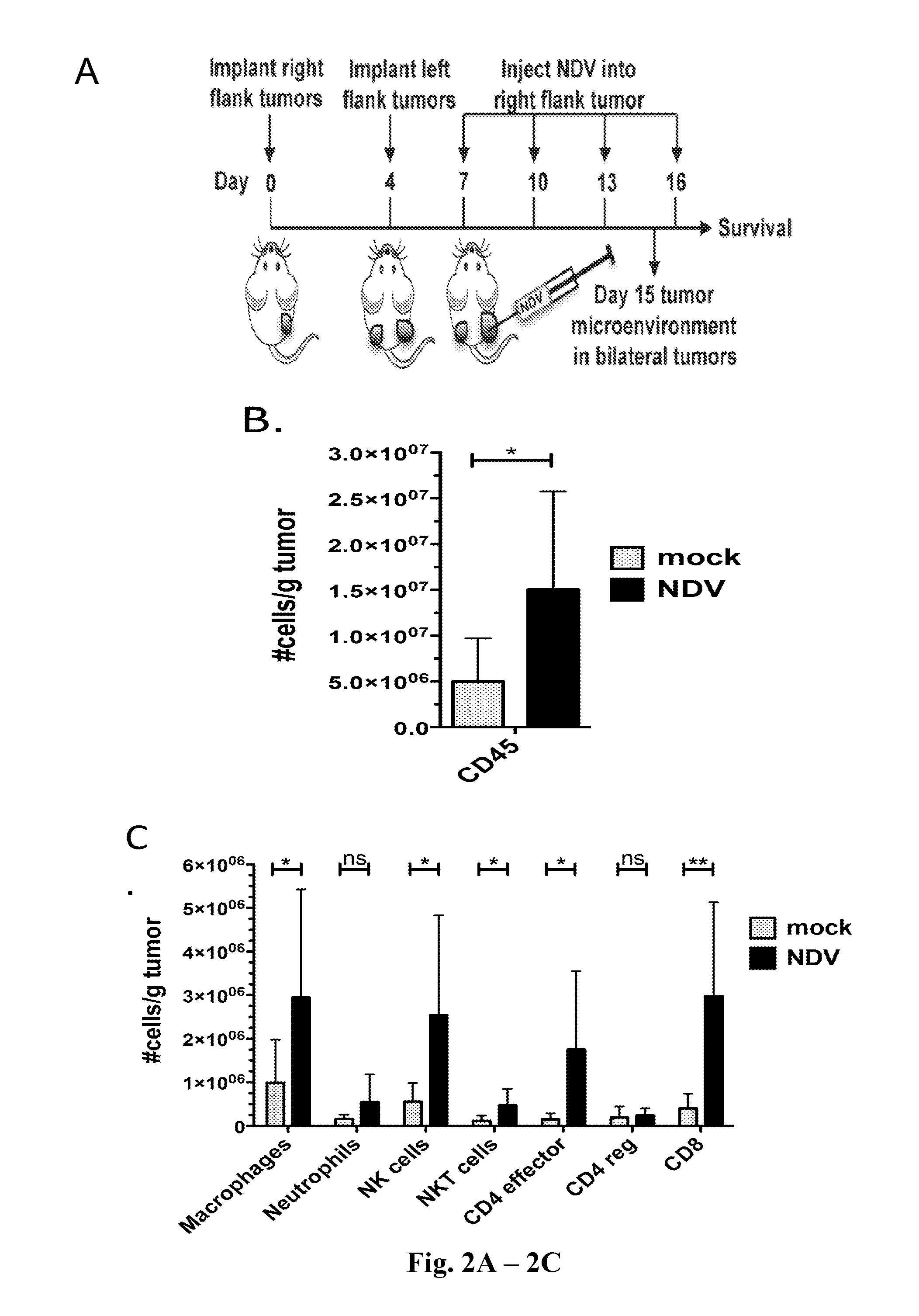
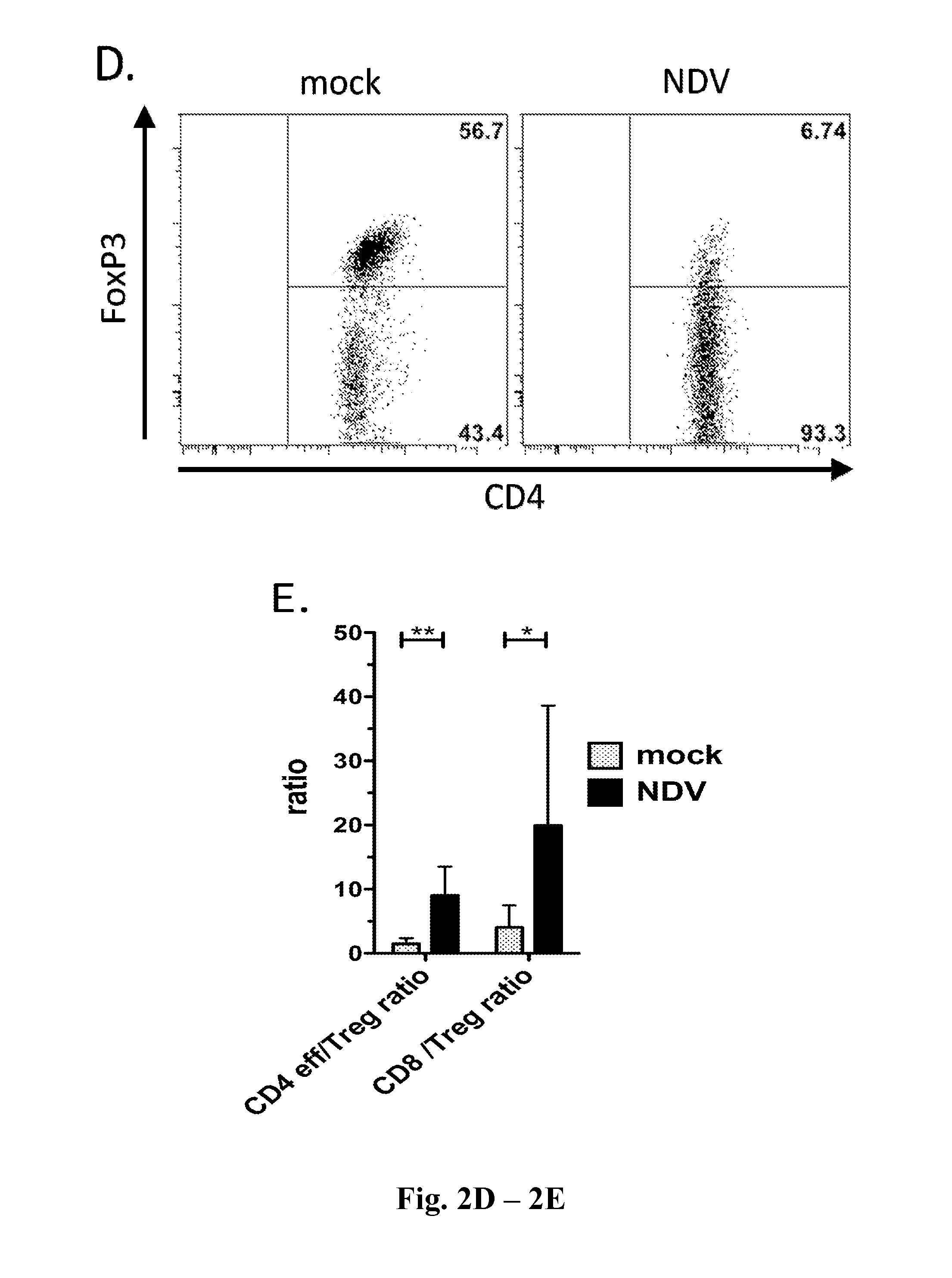
Newcastle Disease Viruses and Uses Thereof
InactiveUS20140271677A1Reduce severityPrevent relapseSsRNA viruses negative-senseViral antigen ingredientsNewcastle disease virus NDVAgonist
Described herein are chimeric Newcastle disease viruses engineered to express an agonist of a co-stimulatory signal of an immune cell and compositions comprising such viruses. Also described herein are chimeric Newcastle disease viruses engineered to express an antagonist of an inhibitory signal of an immune cell and compositions comprising such viruses. The chimeric Newcastle disease viruses and compositions are useful in the treatment of cancer. In addition, described herein are methods for treating cancer comprising administering Newcastle disease viruses in combination with an agonist of a co-stimulatory signal of an immune and / or an antagonist of an inhibitory signal of an immune cell.
Owner:MT SINAI SCHOOL OF MEDICINE +1
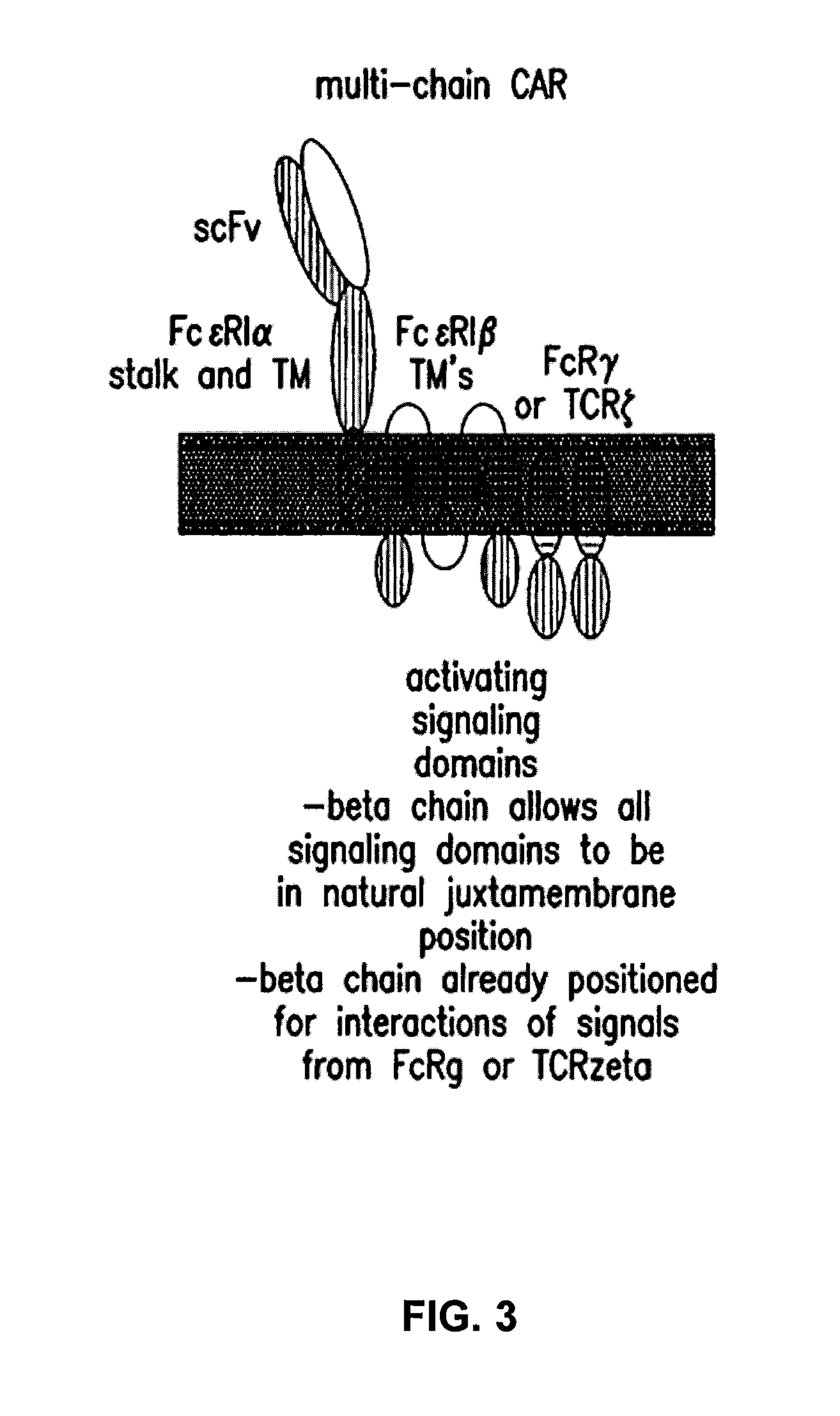
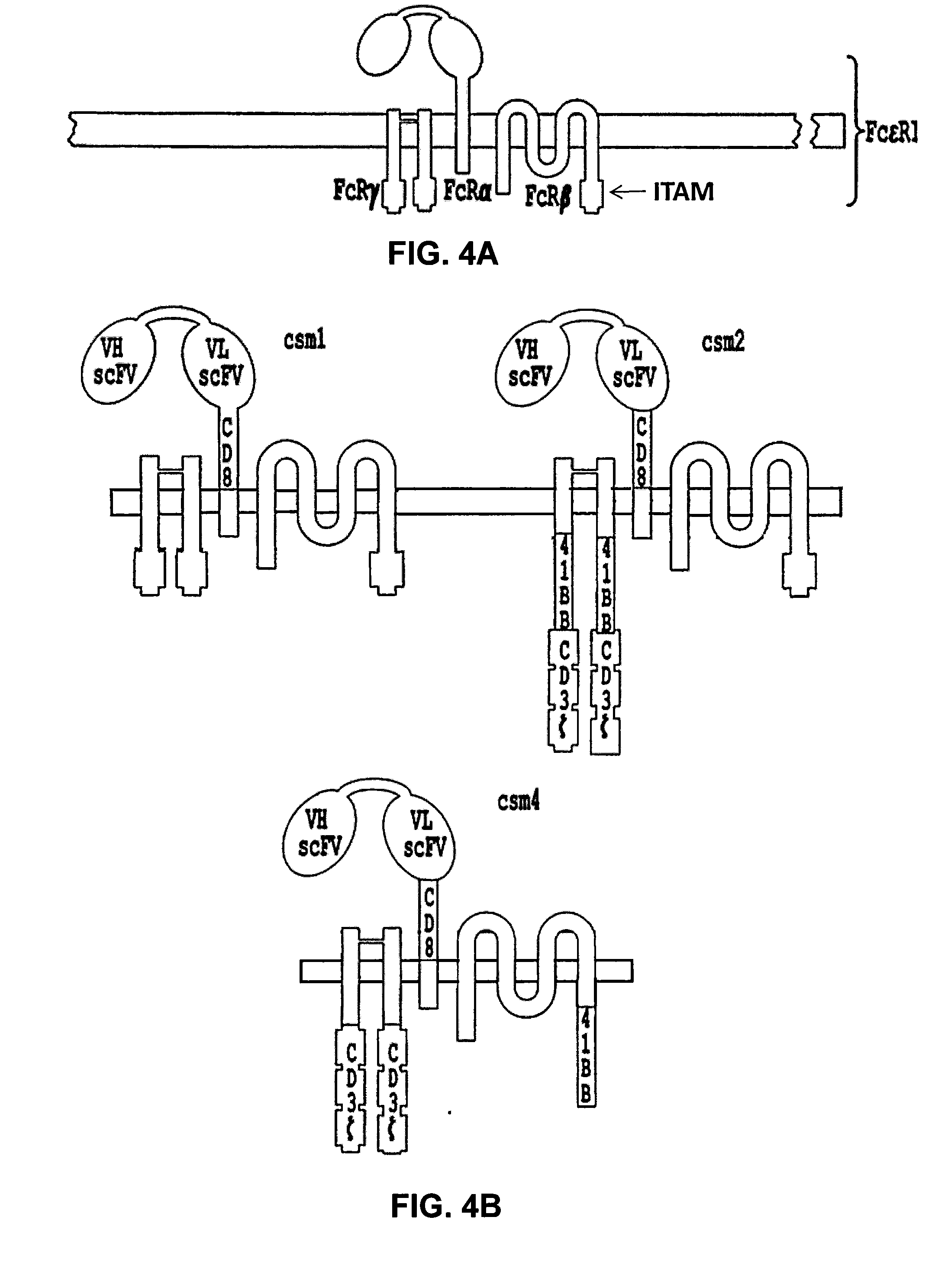
Multi-Chain Chimeric Antigen Receptor and Uses Thereof
ActiveUS20140134142A1Modulate activityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorReceptor
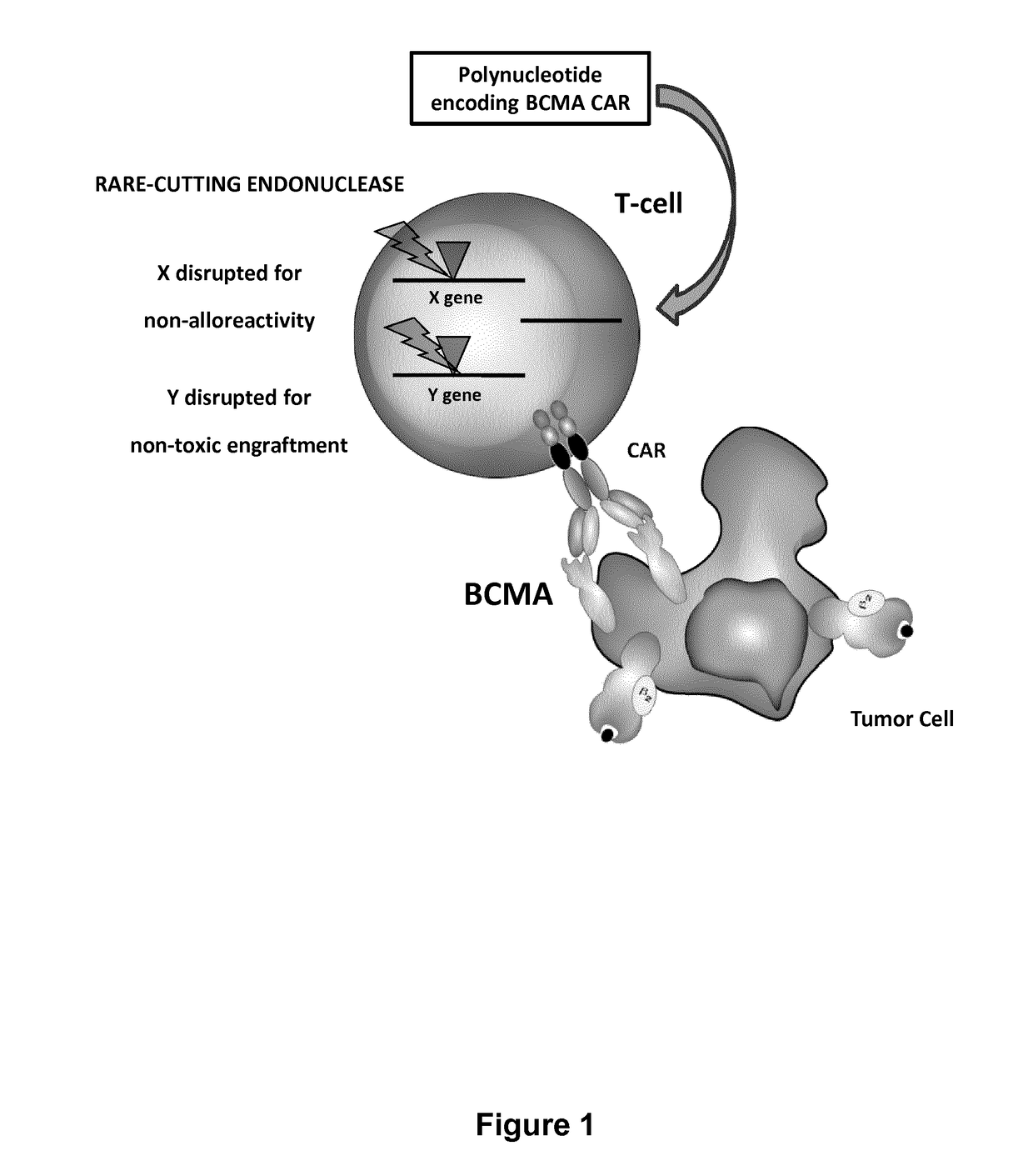
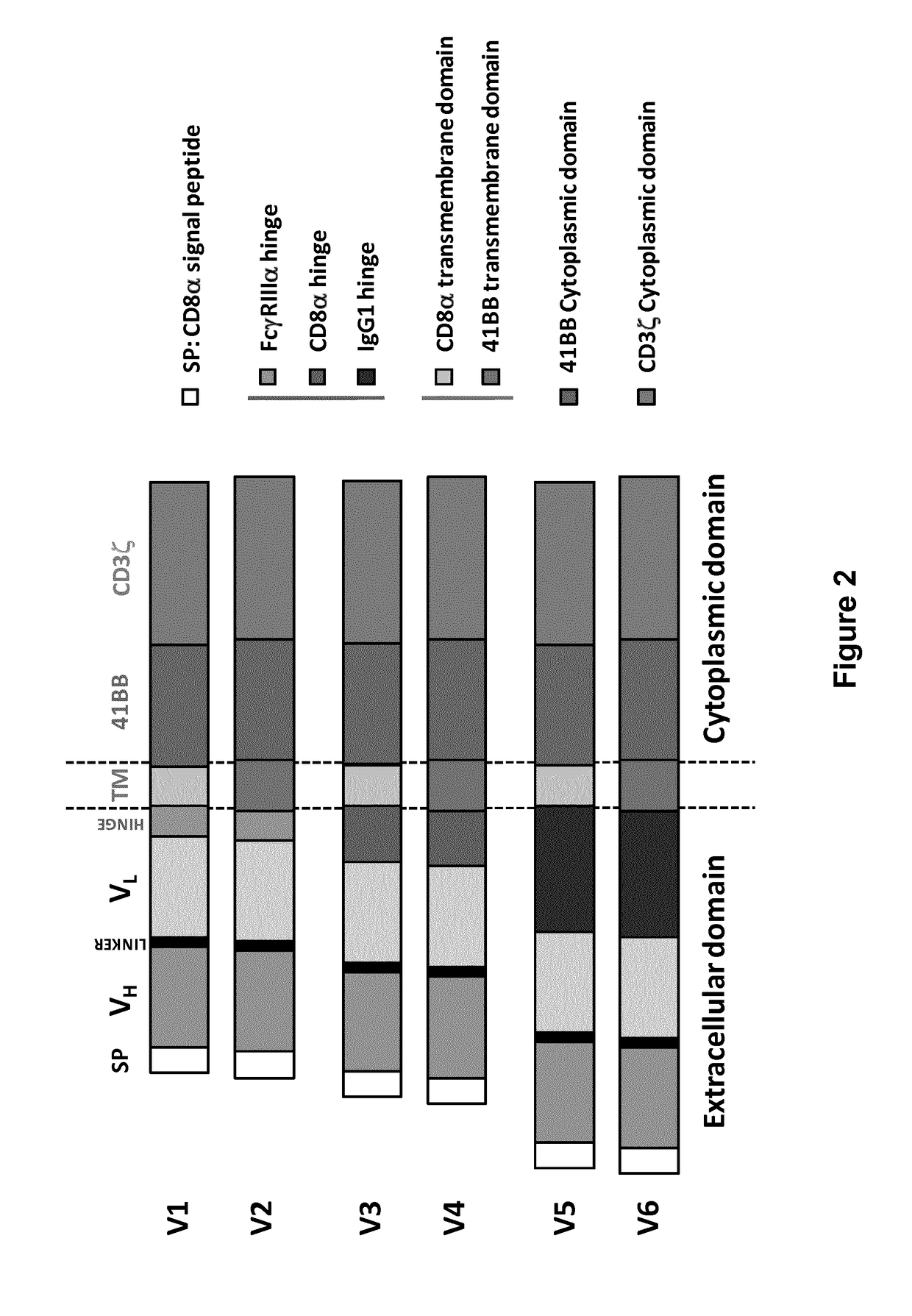
The present invention relates to a new generation of chimeric antigen receptors (CAR) referred to as multi-chain CARs. Such CARs, which aim to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties, comprise separate extracellular ligand binding and signaling domains in different transmembrane polypeptides. The signaling domains are designed to assemble in juxtamembrane position, which forms flexible architecture closer to natural receptors, that confers optimal signal transduction. The invention encompasses the polynucleotides, vectors encoding said multi-chain CAR and the isolated cells expressing them at their surface, in particularly for their use in immunotherapy. The invention opens the way to efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Bispecific antibody molecule
ActiveUS20150119555A1Easy to adaptHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsChemistryAntibody molecule
The present invention relates to a bispecific antibody molecule, as well as a method for producing the same, its use and a nucleic acid molecule encoding the bispecific antibody molecule. The invention in particular provides an antibody molecule that is capable of mediating target cell restricted activation of immune cells.
Owner:SYNIMMUNE
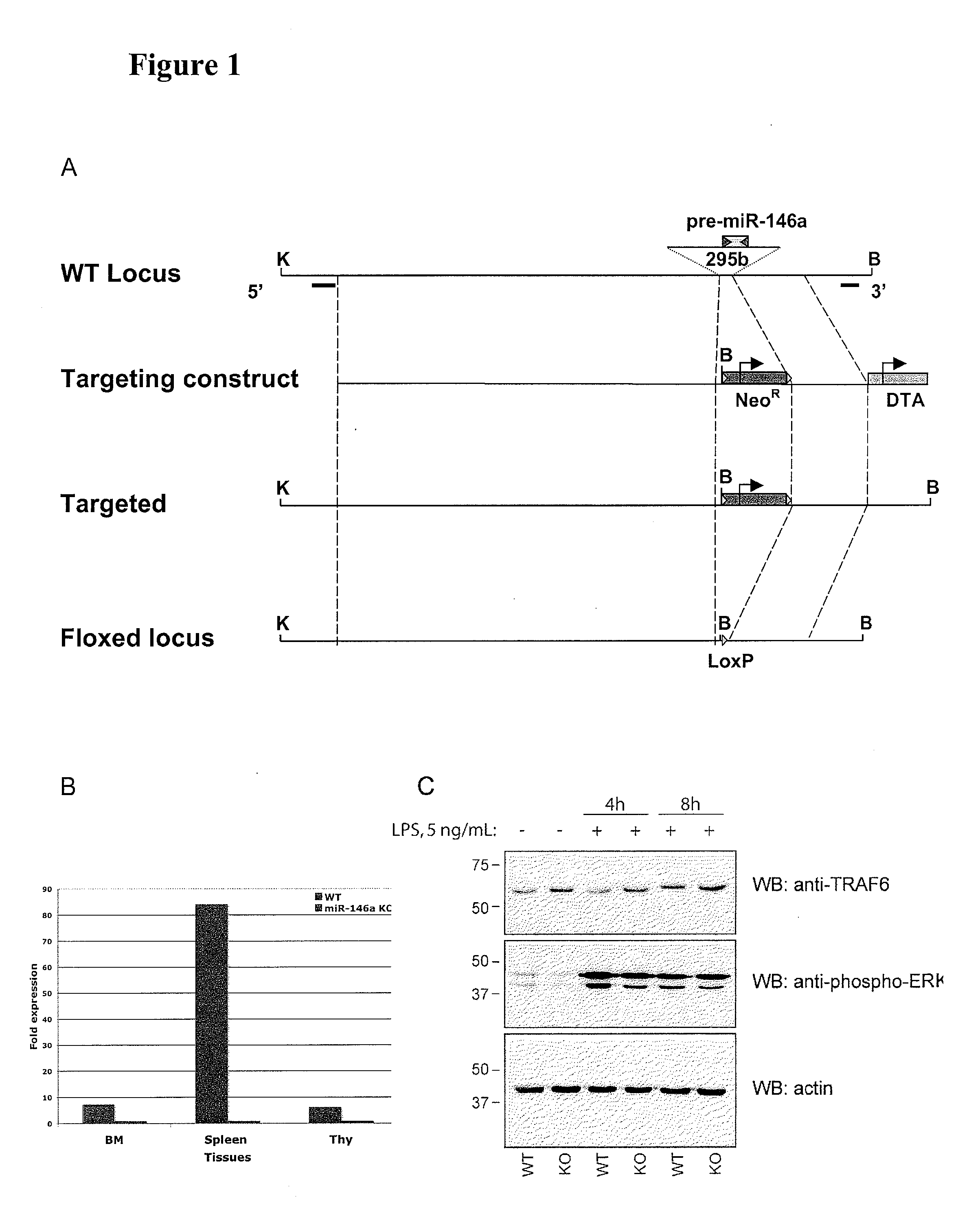
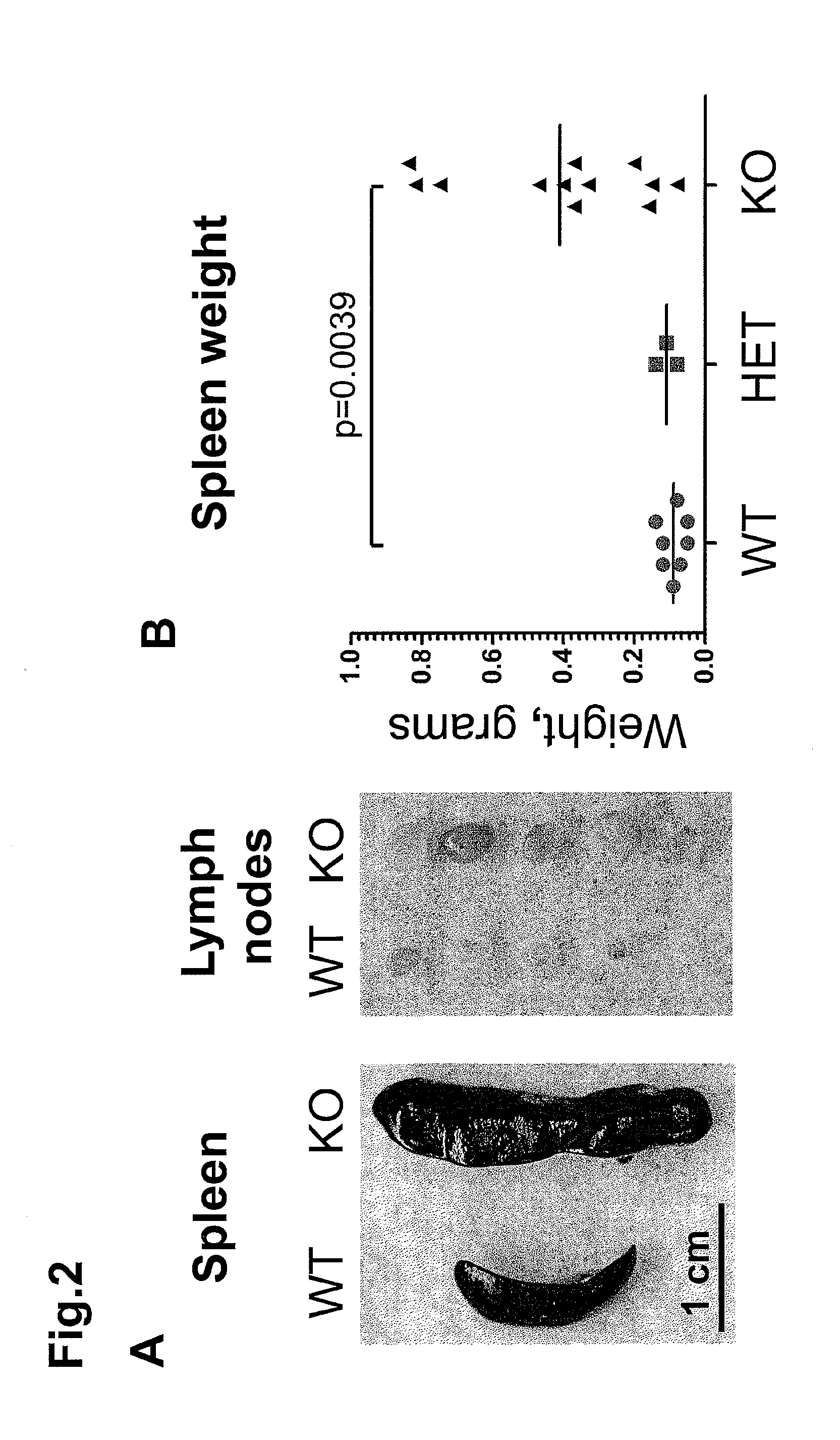
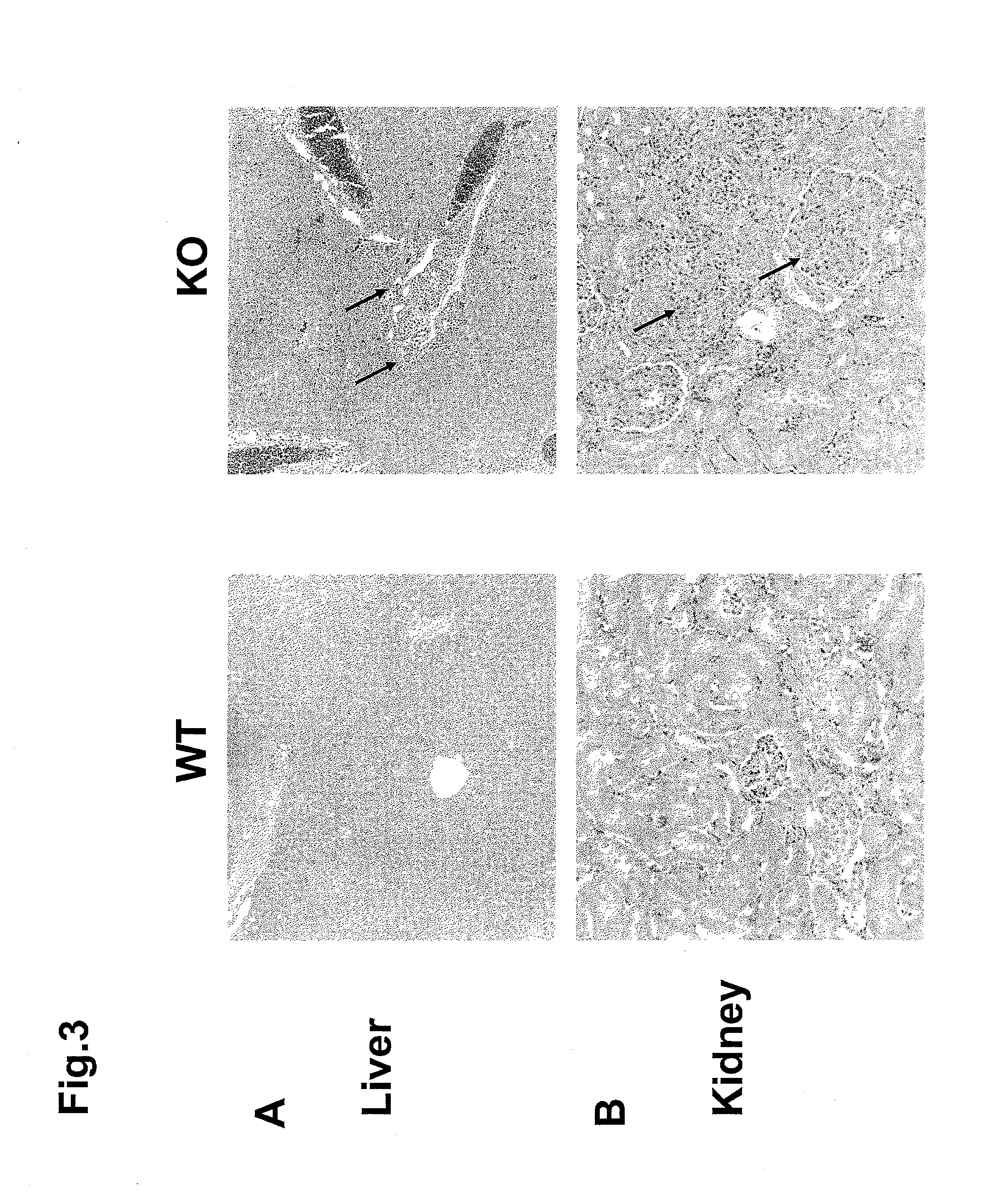
Modulating immune system development and function through microrna mir-146
InactiveUS20110258716A1Increasing proliferation and activityDecreasing proliferation and activityAnimal cellsAnimal husbandryPrecursor cellOligonucleotide
The present disclosure relates to the finding that microRNA-146 plays a role in modulating the development and function of the immune system. Immune cell development and function can be modulated by delivery of microRNA-146 (miR-146) or antisense miR-146 to target immune cells or precursor cells. For example, in some embodiments, activity and / or proliferation of certain immune cells is regulated by administering miR-146 oligonucleotides or anti-miR-146 oligonucleotides. In other embodiments, pro-inflammatory cytokine expression in immune cells is regulated by administering a miR-146 oligonucleotide or anti-miR-146. In further embodiments, methods of regulating macrophage activity using antisense miR-146 are provided. Additional methods and compositions for regulating immune system function and development using miR-146 are disclosed.
Owner:CALIFORNIA INST OF TECH
Mhc multimers, methods for their generation, labeling and use
The present invention describes novel methods to generate MHC multimers and methods to improve existing and new MHC multimers. The invention also describes improved methods for the use of MHC multimers in analysis of T-cells in samples including diagnostic and prognostic methods. Furthermore the use of MHC multimers in therapy are described, e.g. anti-tumour and anti-virus therapy, including isolation of antigen specific T-cells capable of inactivation or elimination of undesirable targeT-cells or isolation of specific T-cells capable of regulation of other immune cells.
Owner:AGILENT TECH INC
Modulation of negative immune regulators and applications for immunotherapy
ActiveUS20060292119A1Easy to integrateBiocideSsRNA viruses positive-senseVaccinationImmunocompetence
The invention includes compositions and methods for enhancing immunopotency of an immune cell by way of inhibiting a negative immune regulator in the cell. The present invention provides vaccines and therapies in which antigen presentation is enhanced through inhibition of negative immune regulators. The present invention also provides a mechanism to break self tolerance in tumor vaccination methods that rely on presentation of self tumor antigens.
Owner:BAYLOR COLLEGE OF MEDICINE
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
Chimeric receptor genes and cells transformed therewith
InactiveUS20120093842A1Limit acquisitionVirusesPeptide/protein ingredientsTherapeutic treatmentReceptor for activated C kinase 1
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:CABARET BIOTECH
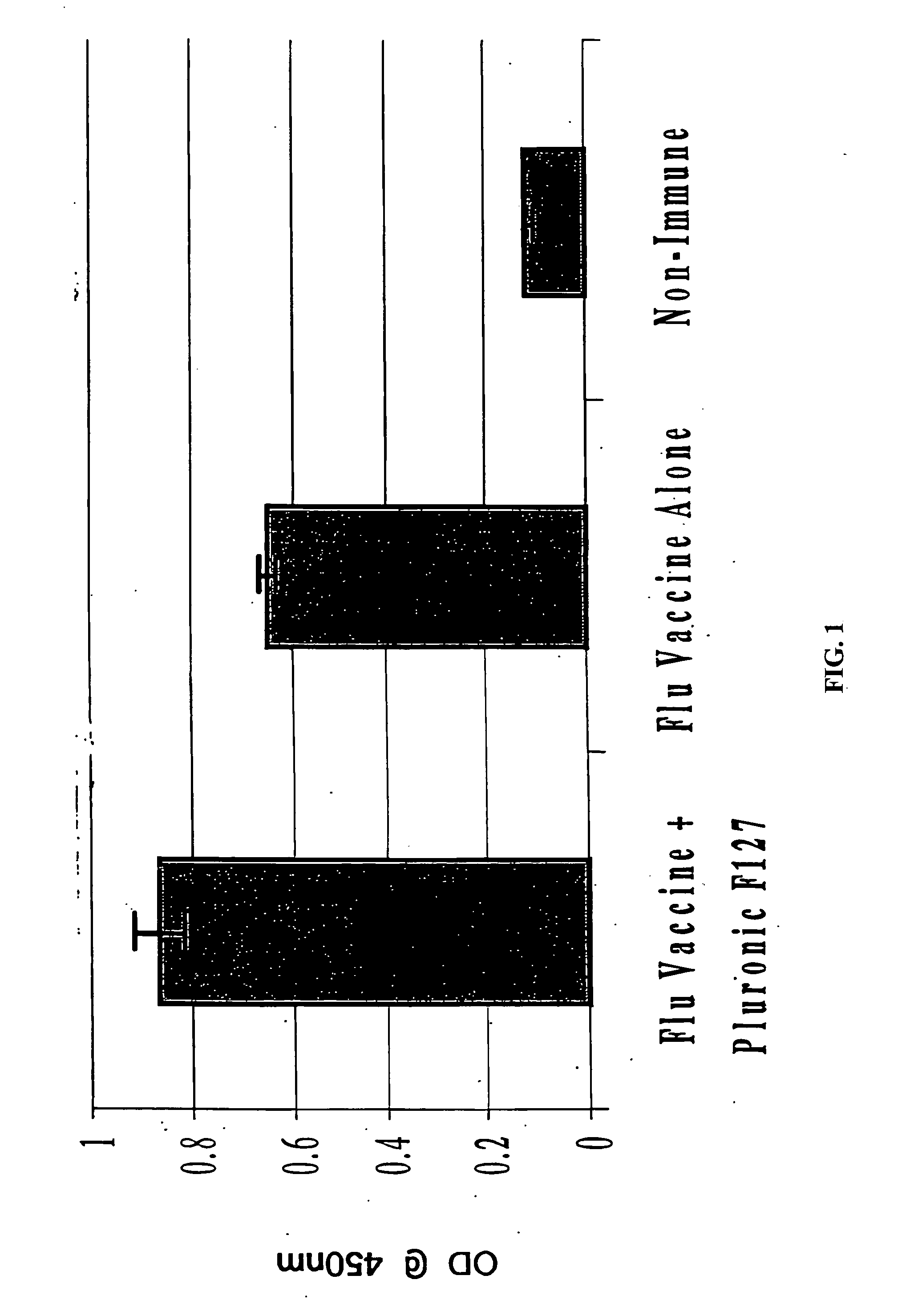
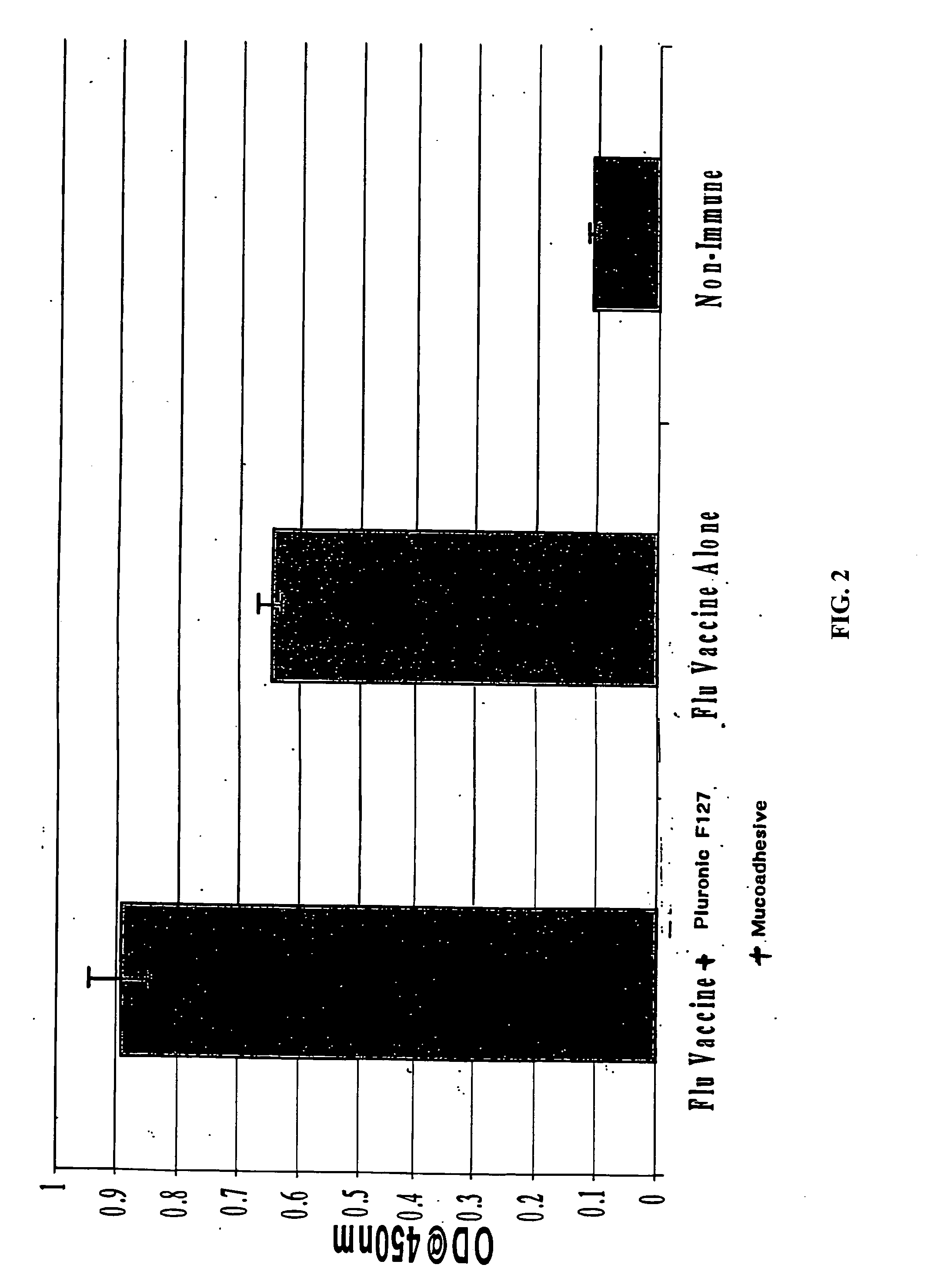
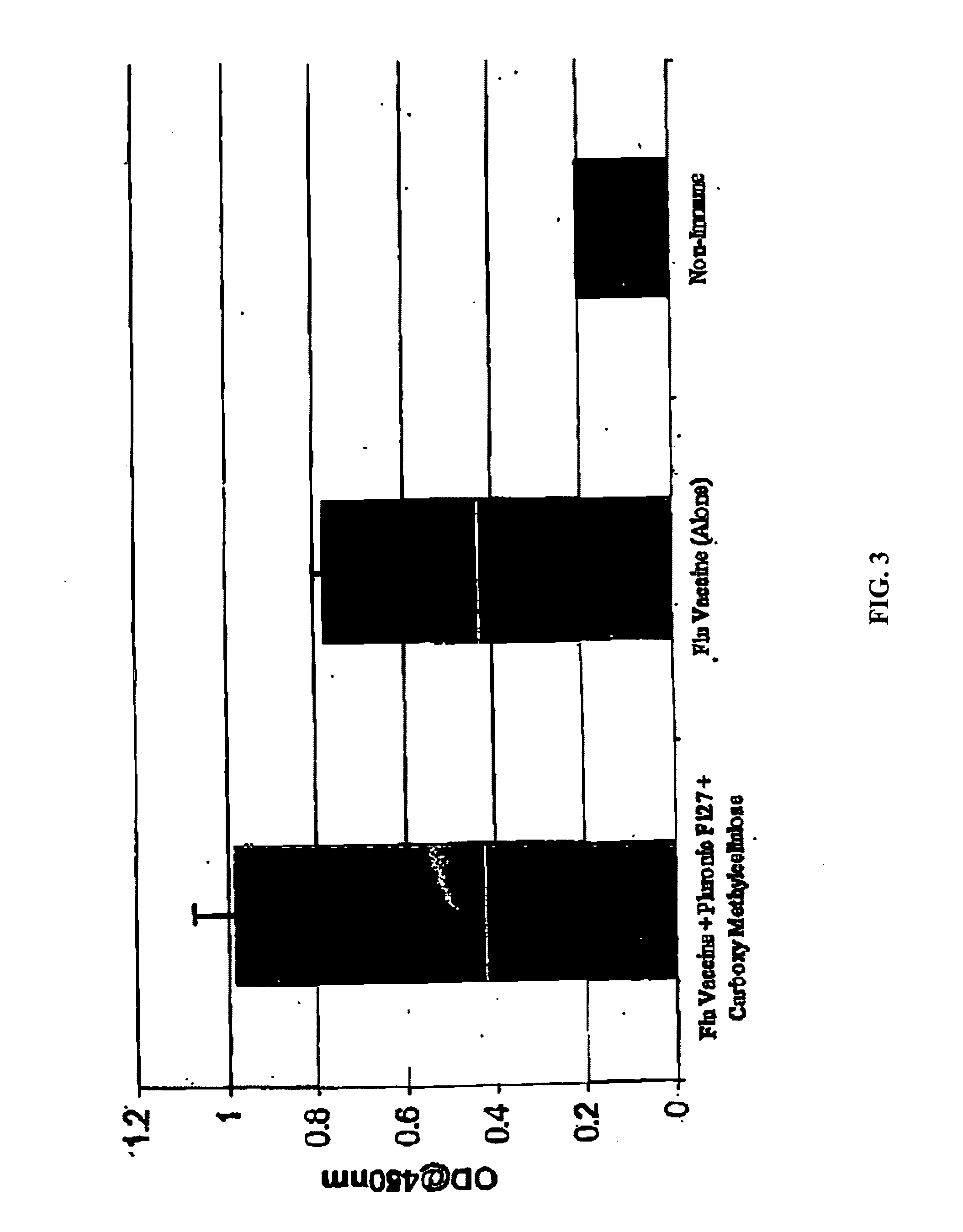
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050123550A1Enhances therapeutic efficacy and protective immune responseGood curative effectSsRNA viruses negative-senseOrganic active ingredientsInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
Tolerogenic synthetic nanocarriers for regulating innate immune responses
InactiveUS20120276160A1Suppressing antigen-specific activationReduce in quantityOrganic active ingredientsPowder deliveryB cellAntigen specific
Disclosed are synthetic nanocarrier methods, and related compositions, comprising administering B cell and / or MHC Class II-restricted epitopes of an antigen and immunosuppressants in order to reduce antigen-specific activation of innate immune cells.
Owner:SELECTA BIOSCI
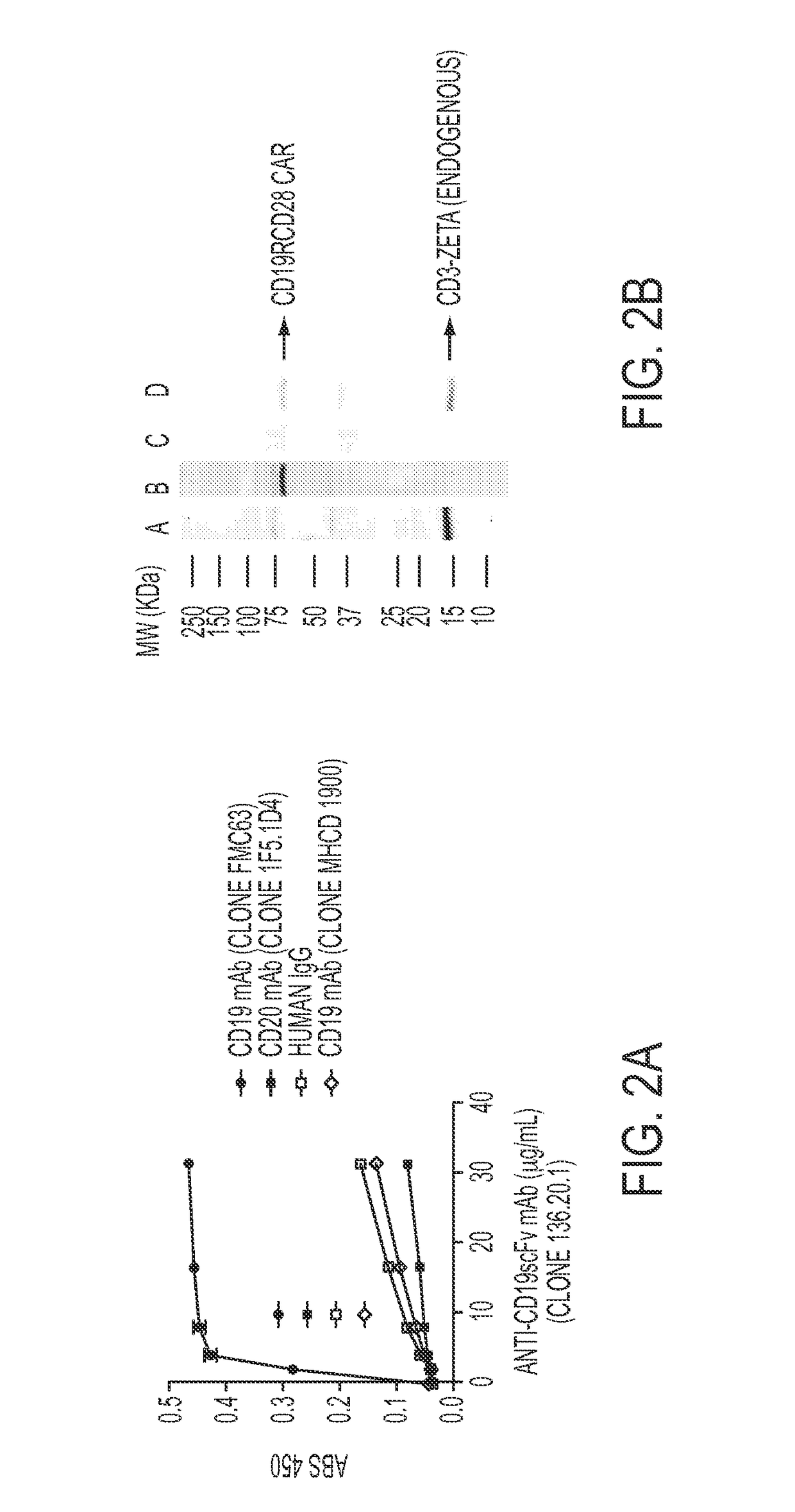
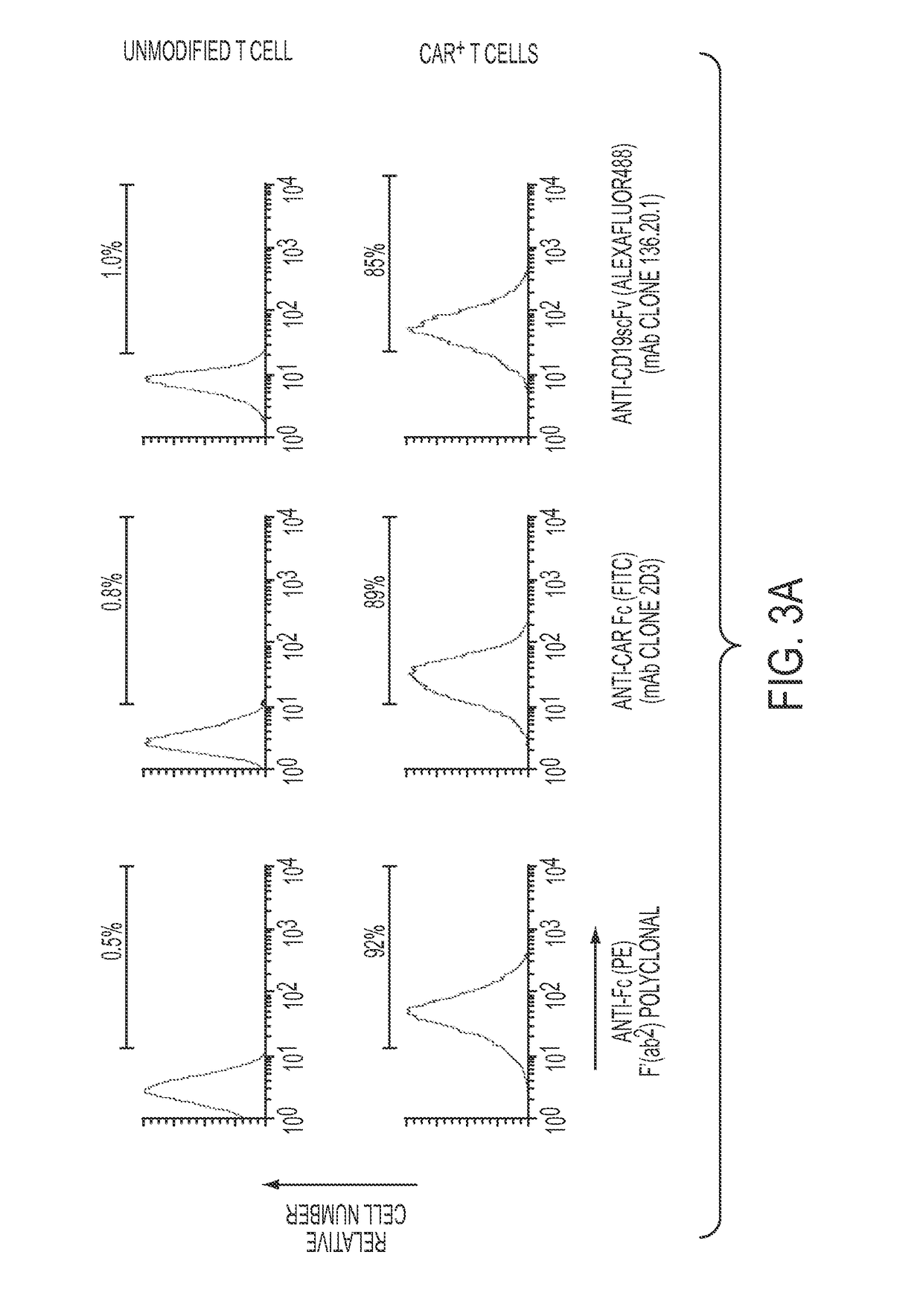
Anti-CD19 scFv (FMC63) polypeptide
ActiveUS9701758B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyMonoclonal antibodyBiological activation
Provided are monoclonal antibodies that detect CD 19 CAR-modified immune cells and CAR-modified immune cells irrespective of the tumor associated antigen they target. Methods of using these functional monoclonal antibodies include, but are not limited to, detection, quantification, activation, and selective propagation of CAR-modified immune cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Genetically modified tumor cells as cancer vaccines
InactiveUS20060165668A1Convenient treatmentEasy to detectBiocidePeptide/protein ingredientsAbnormal tissue growthCancer cell
The present invention provides methods and compositions for electroporation-mediated gene transfer to cancer cells. The transfected cancer cells are genetically modified to express one or more therapeutic proteins. In certain embodiments, the cancer cells are modified to express one or more cytokines capable of enhancing the immunogenicity of the transfected cancer cell. Administering the transfected cancer cell to a subject will lead to enhanced immune-cell mediated killing of tumors. Accordingly, the present invention provides methods and compositions for improved treatment and prevention of cancer and other hyperproliferative diseases.
Owner:MAXCYTE
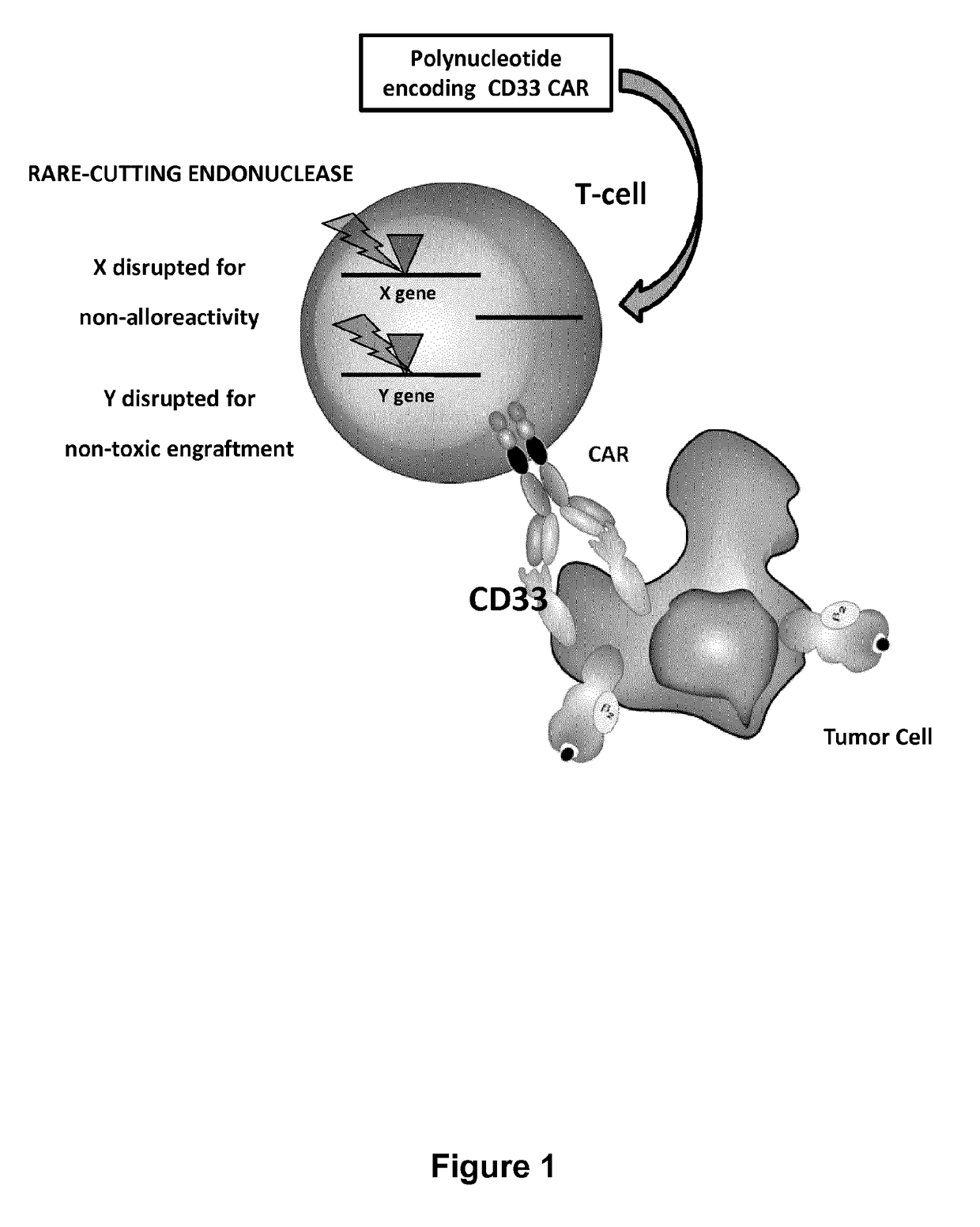
Cd33 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170145094A1Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD33
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD33 monoclonal antibody, conferring specific immunity against CD33 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
Compositions and methods for modulation of RORgammat functions
InactiveUS20070154487A1Reduce severityMany symptomSenses disorderNervous disorderAutoimmune conditionScreening method
The present invention relates to expression of RORγt in cells and tissues and the effect of expression of this gene on proliferation of specific immune cells and in promotion of immune cell aggregates and in induction of IL17 producing cells. Furthermore, the invention relates to methods and agents that may decrease function of the gene product (the protein) or expression of this gene in individuals experiencing an inflammatory condition, an autoimmune disease or a food allergy, or any other condition whereby it is desirable to inhibit an immune response. In addition, methods and agents useful for enhancing the function of RORγt with agonists or expression of this gene are also considered for use whereby it is desirable to increase immunity to a pathogen or tumor cell, for example, for use in conjunction with a vaccine. Screening methods for identifying novel modulators (antagonists and agonists) of RORγt are also disclosed.
Owner:NEW YORK UNIV
Immunocyte culture medium, and culture method and application of immunocytes
InactiveCN104818248AHigh speedExtended passage timesBlood/immune system cellsAntiendomysial antibodiesCell culture media
The invention discloses an immunocyte culture medium, and a culture method and application of immunocytes. The immunocyte culture medium is composed of one or more selected from the group consisting of amino acids, vitamins, salts, lipids or polypeptide, and one or more selected from the group consisting of cytokines or antibodies; the immunocyte culture medium can quickly amplify different types of immunocytes, enables the amplification speed of the immunocytes to be increased by 3 to 5 times than the amplification speed of the immunocytes cultured with a conventional culture medium, and prolongs passage times; meanwhile, the immunocytes cultured by the immunocyte culture medium provided by the invention can maintain good immunocyte functions and exert effects including, but not limited to, anti-cancer effect and anti-aging effect, so the immunocytes have good application prospects.
Owner:BIOTOWNTEK CO LTD
Method of treating autoimmune disease by inducing antigen presentation by tolerance inducing antigen presenting cells
InactiveUS20060257412A1Prevent proliferationAvoid enteringMetabolism disorderAntibody mimetics/scaffoldsAntigenAutoimmune condition
Antibodies to antigen presenting cells may be utilized to interfere with the interaction of the antigen presenting cell and immune cells, including T cells. Peptides may be linked to said antibodies thereby generating an immune response to such peptides. Preferably peptides linked to the antibodies are associated with autoimmunity.
Owner:ALEXION PHARMA INC
Genetically-engineered MHC molecules
The invention provides DNA molecules encoding a chimeric polypeptide comprising (a) a component of a MHC molecule capable of association on a cell surface with an endogenous MHC molecule component of the same class, and (b) an intracellular region of a signal transduction element capable of activating T cells. Component (a) may be a monomorphic component and is preferably beta 2-microglobulin, or a polymorphic class I or class II component. The signal transduction element (b) capable of activating T cells may be a component of T-cell receptor CD3, preferably the CD3 zeta (zeta) polypeptide, a B cell receptor polypeptide or an Fc receptor polypeptide. Immune cells such as a CTLs expressing said chimeric MHC molecules specifically eliminate or inactivate harmful T cells and are useful for treating graft rejection and autoimmune diseases.
Owner:GAVISH GALILEE BIO APPL
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050255121A1Good antigenicityEnhance immune responseSsRNA viruses negative-senseBiocideInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
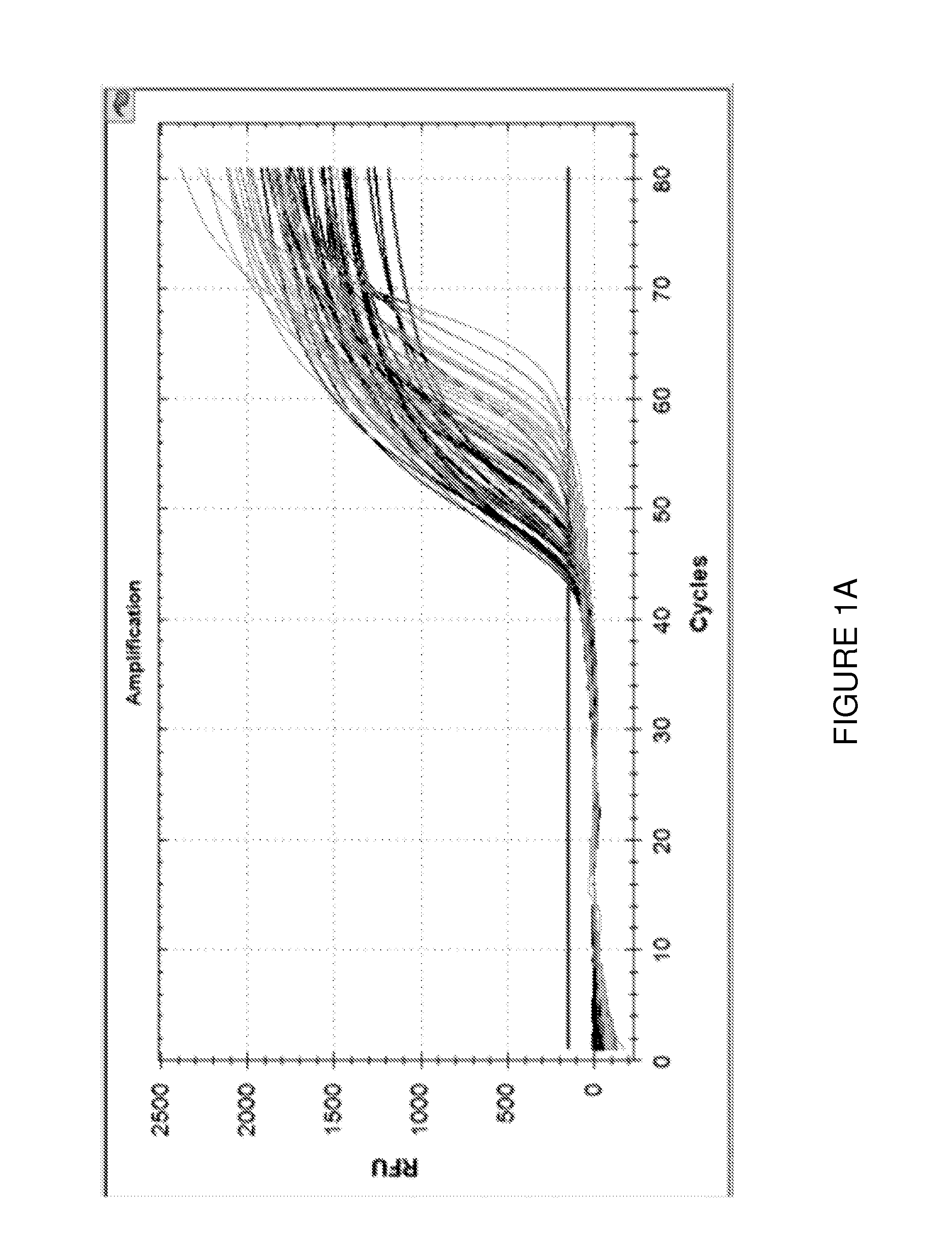
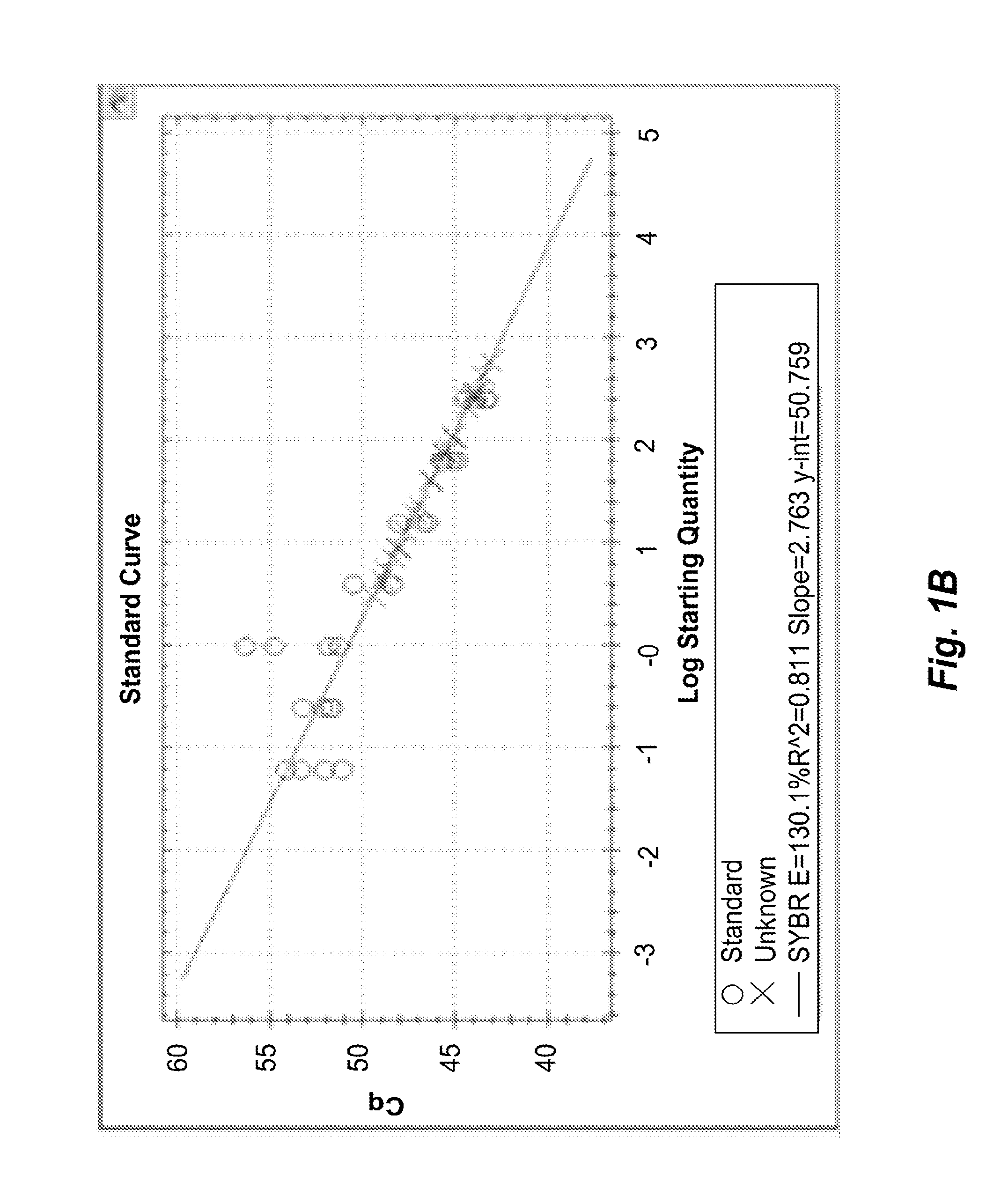
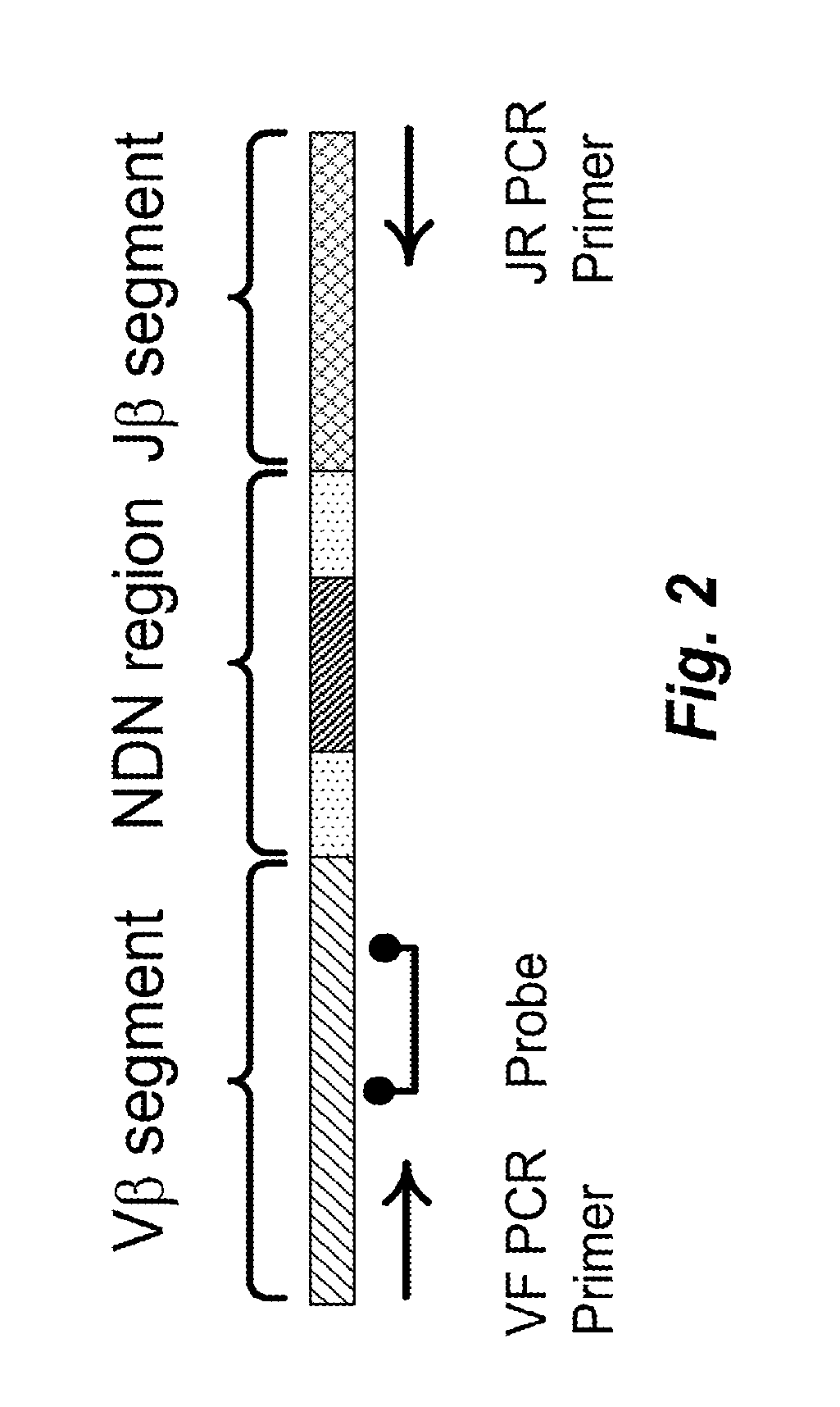
Quantification of Adaptive Immune Cell Genomes in a Complex Mixture of Cells
Compositions and methods are described for highly sensitive quantification of the relative representation of DNA from adaptive immune cells (e.g., T and / or B lymphocytes) in DNA extracted from complex mixtures of cells that include cells which are not adaptive immune cells. Included are methods for determining the relative presence in a tumor of tumor infiltrating lymphocytes (TIL), the relative presence of lymphocytes infiltrating a somatic tissue that is the target of an autoimmune disease, and the relative presence of lymphocytes infiltrating a transplanted organ.
Owner:ADAPTIVE BIOTECH +1
Immunomodulatory compositions and uses therefor
The poxvirus proteins designated A41L and 130L bind to three receptor-like protein tyrosine phosphatases (RPTP), leukocyte common antigen related protein (LAR), RPTP-δ, and RPTP-σ, that are present on the cell surface of immune cells. When a host is infected with the poxvirus, binding of A41L to cell surface proteins on the host cells results in suppression of the immune response. The present invention provides agents such as antibodies, and antigen-binding fragments thereof, small molecules, aptamers, small interfering RNAs, and peptide-IgFc fusion polypeptides that interact with one or more of LAR, RPTP-δ, and RPTP-σ expressed by immune cells or interact with a polynucleotide encoding the RPTP. Also provided are RPTP Ig domain oligomers and Fc fusion polypeptides. Such agents are useful for treating an immunological disorder in a subject according to the methods described herein.
Owner:VIRAL LOGIC SYST TECH CORP
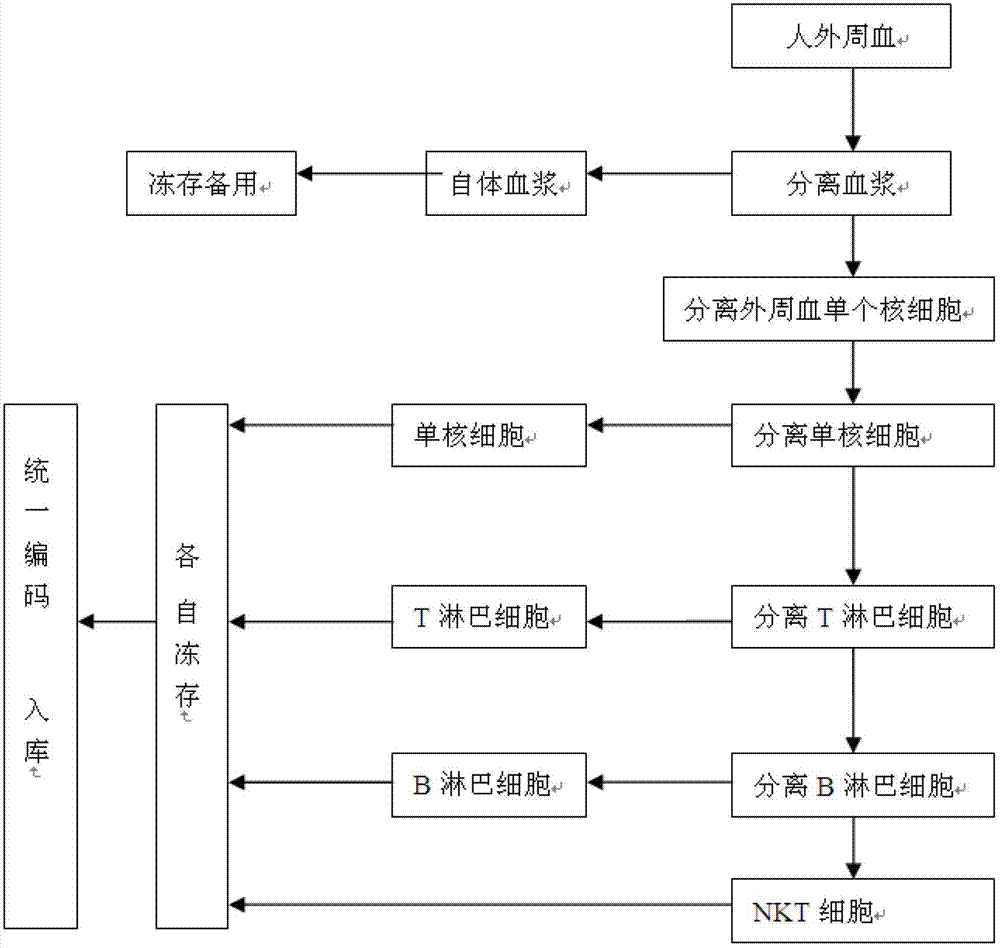
Method for constructing human peripheral blood immune cell bank
ActiveCN102758259AHigh activityHigh purityMicroorganism librariesBlood/immune system cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention discloses a method for constructing a human peripheral blood immune cell bank. The method comprises the following steps of: collecting human peripheral blood, separating autologous plasma, separating a peripheral blood mononuclear cell, separating a mononuclear cell by the peripheral blood mononuclear cell and freezing, separating a T lymphocyte by the peripheral blood mononuclear cell and freezing, separating a B lymphocyte by the peripheral blood mononuclear cell and freezing, separating an NK cell by the peripheral blood mononuclear cell and freezing, and encoding and puttingin storage. According to the invention, immune cells of health or young people are separated and are respectively independently frozen, and relative numbers are stored and put into storage, so that the stored human immune cells have the characteristics of high activity, high purity and convenience for use, and fetal calf serum can be replaced by the human autologous plasma, so that introduction of a foreign protein can be avoided. Meanwhile, the method, provided by the invention, has the advantages of low cost, low requirements on laboratory conditions, and wide application.
Owner:济南赛尔生物科技股份有限公司
Method for Evaluating and Comparing Immunorepertoires
Disclosed is a method for amplifying RNA and / or DNA from immune cell populations and using the amplified products to produce an immune response profile and evaluate the possible correlation between a normal or abnormal immune response and the development of a disease such as an autoimmune disease, cancer, diabetes, or heart disease.
Owner:CB BIOTECHNOLOGIES INC +1
In Vitro System for Assessing Immune Activity Using Pig Immune Cells
InactiveUS20090233301A1Strong cytotoxicityMicrobiological testing/measurementMaterial analysisImmunity activeIn vitro system
Owner:LEE SHING MOU
Dry Formulation for Transcutaneous Immunization
InactiveUS20090081244A1Easy to identifyEfficient deliverySsRNA viruses negative-senseAntibacterial agentsAdjuvantBacterial exotoxin
A transcutaneous immunization system delivers antigen to immune cells through the skin, and induces an immune response in an animal or human. For example, skin-active adjuvant (e.g., an ADP-ribosylating exotoxin) can be used to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a dry formulation containing antigen and adjuvant to skin of the animal or human. The dry formulation may be a powder or a unit-dose patch. Use of adjuvant is not required if the antigen is sufficiently antigenic. Transcutaneous immunization may be induced with or without penetration enhancement
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
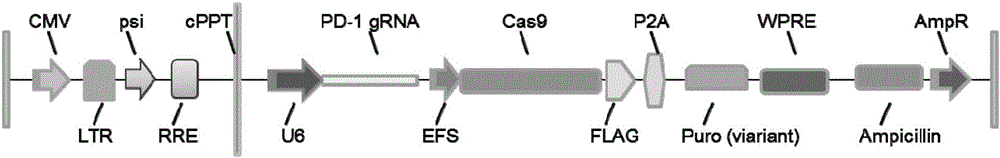
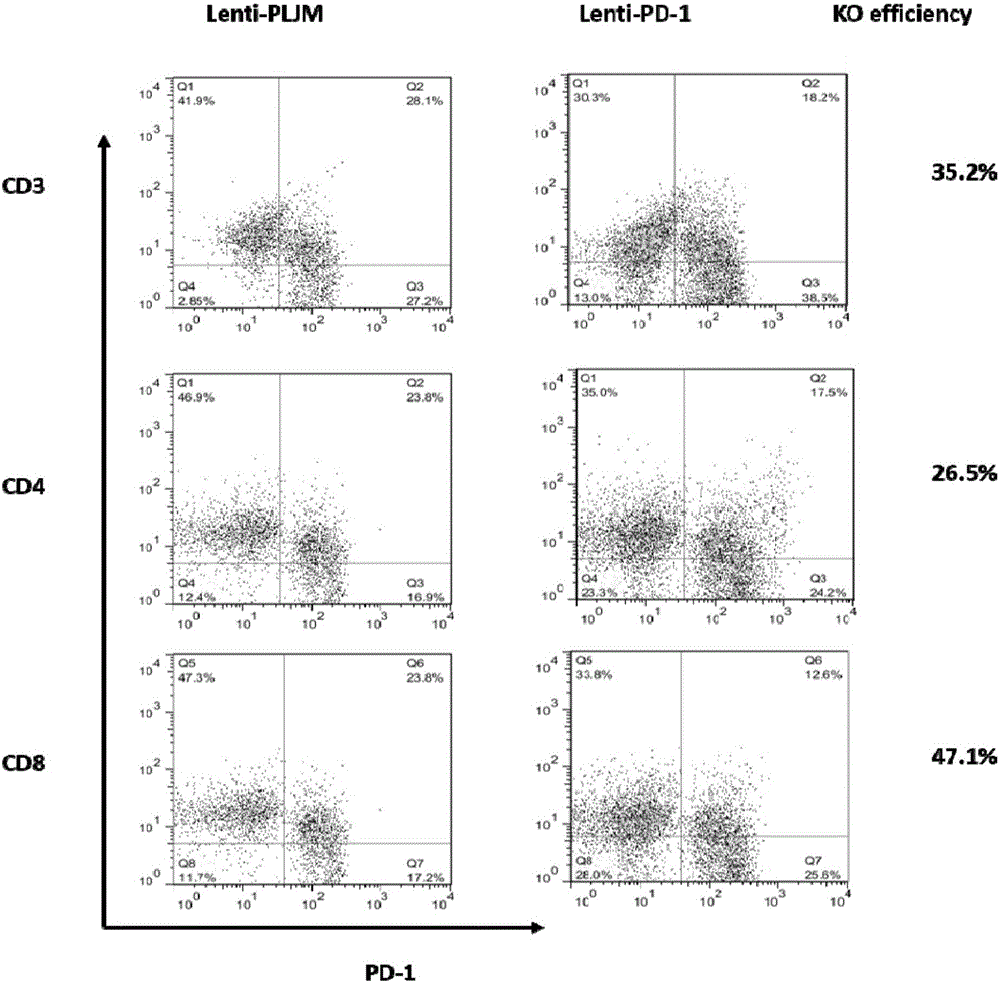
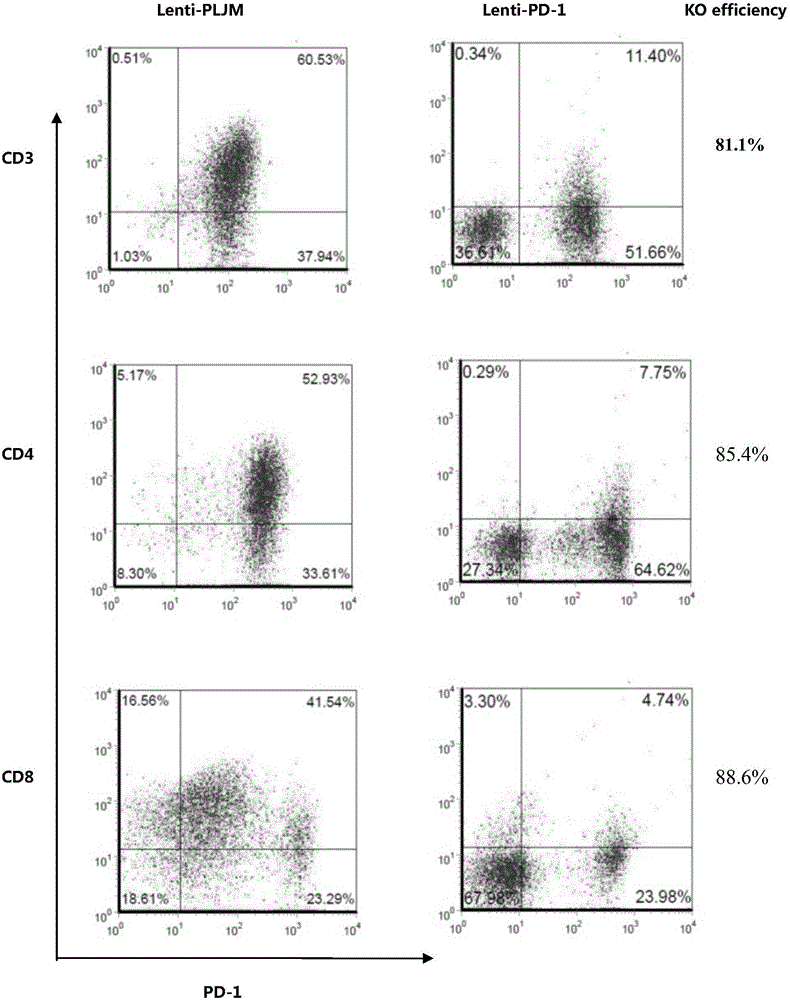
PD-1 gene recombinant virus plasmid, construction thereof, recombinant retrovirus Lenti-PD-1-Puro and packaging and application of recombinant retrovirus Lenti-PD-1-Puro
ActiveCN105671083AStrong specificityRelease the suppressed stateCell receptors/surface-antigens/surface-determinantsUnknown materialsT cellViral infection
The invention discloses a PD-1 gene recombinant virus. The collection number of the recombinant virus Lenti-PD-1-Puro is CCTCC No:V201601. According to the PD-1 gene recombinant virus, PD-1gRNA sequences are cloned into a retrovirus plasmid Lenti-CRISPR / Cas9-Puro, and a PD-1 recombinant virus plasmid Lenti-CRISPR / Cas9-PD-1-Puro is obtained; then the PD-1 recombinant virus plasmid Lenti-CRISPR / Cas9-PD-1-Puro, a plasmid pSPAX and pMD2.G are jointly transfected by 293T cells, and packaging of the recombinant virus Lenti-PD-1-Puro is completed. After T cells of peripheral blood of tumor patients are infected by the PD-1 recombinant virus, PD-1 on the T cells is successfully knocked out, the inhibitory state of the T cells of the tumor patients is relieved, the capacity of attacking tumor cells of the T cells embellished by the PD-1 recombinant virus is recovered accordingly, and the effect of immune cell treating is achieved.
Owner:安徽柯顿生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com