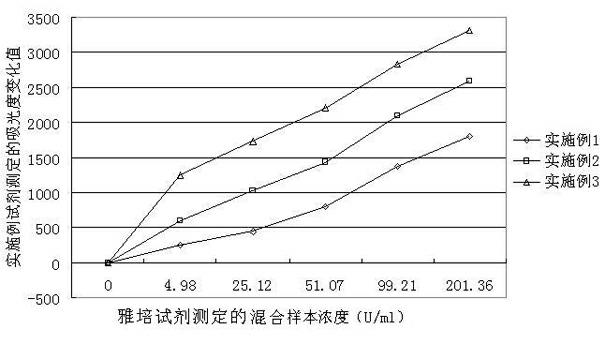
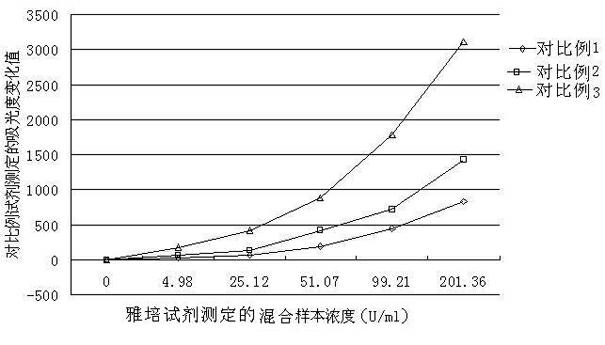
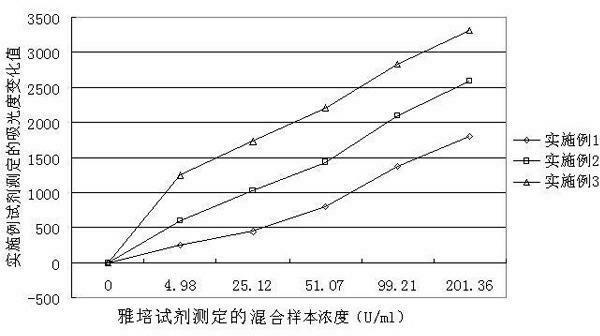
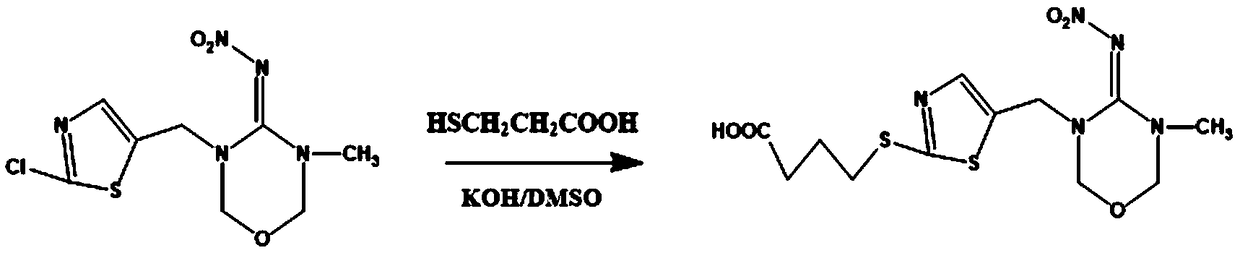
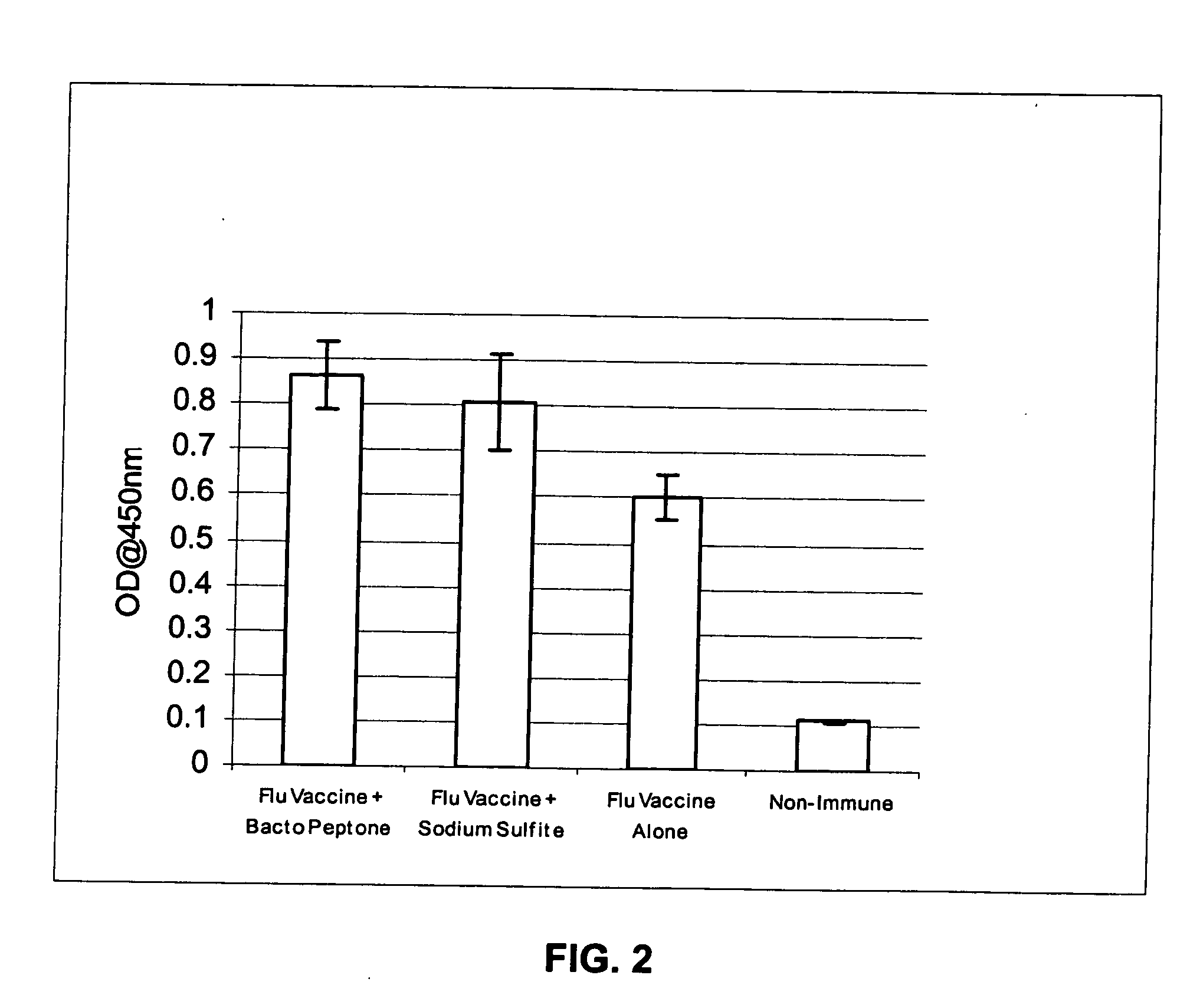
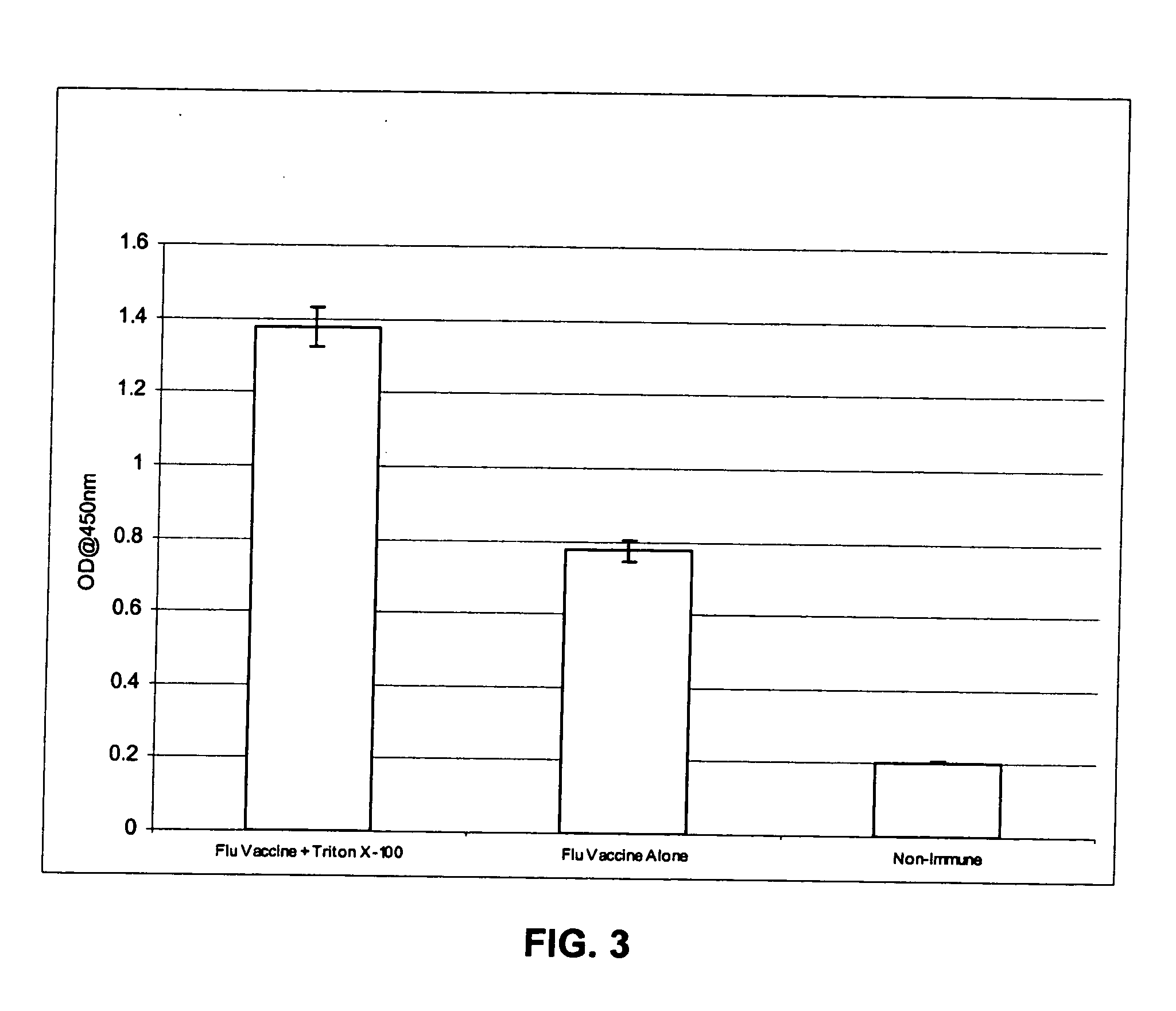
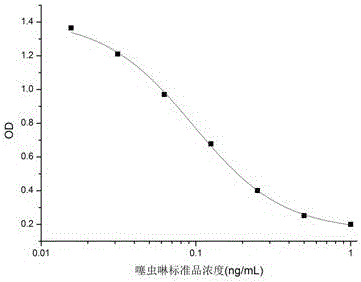
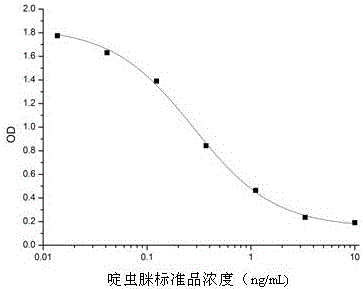
Patents
Literature
146 results about "Booster immunizations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050123550A1Enhances therapeutic efficacy and protective immune responseGood curative effectSsRNA viruses negative-senseOrganic active ingredientsInfluenza vaccineImmunogenicity
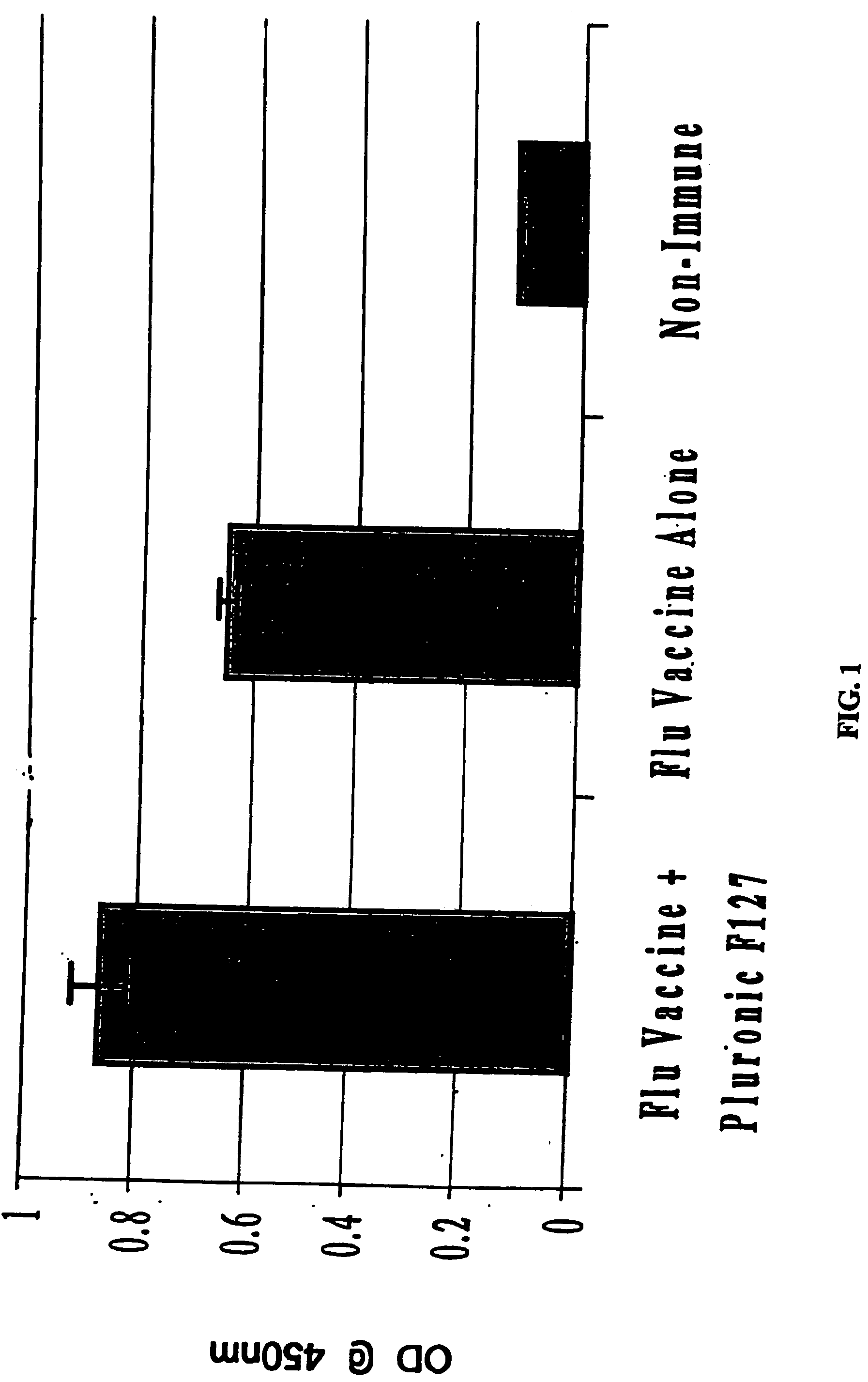
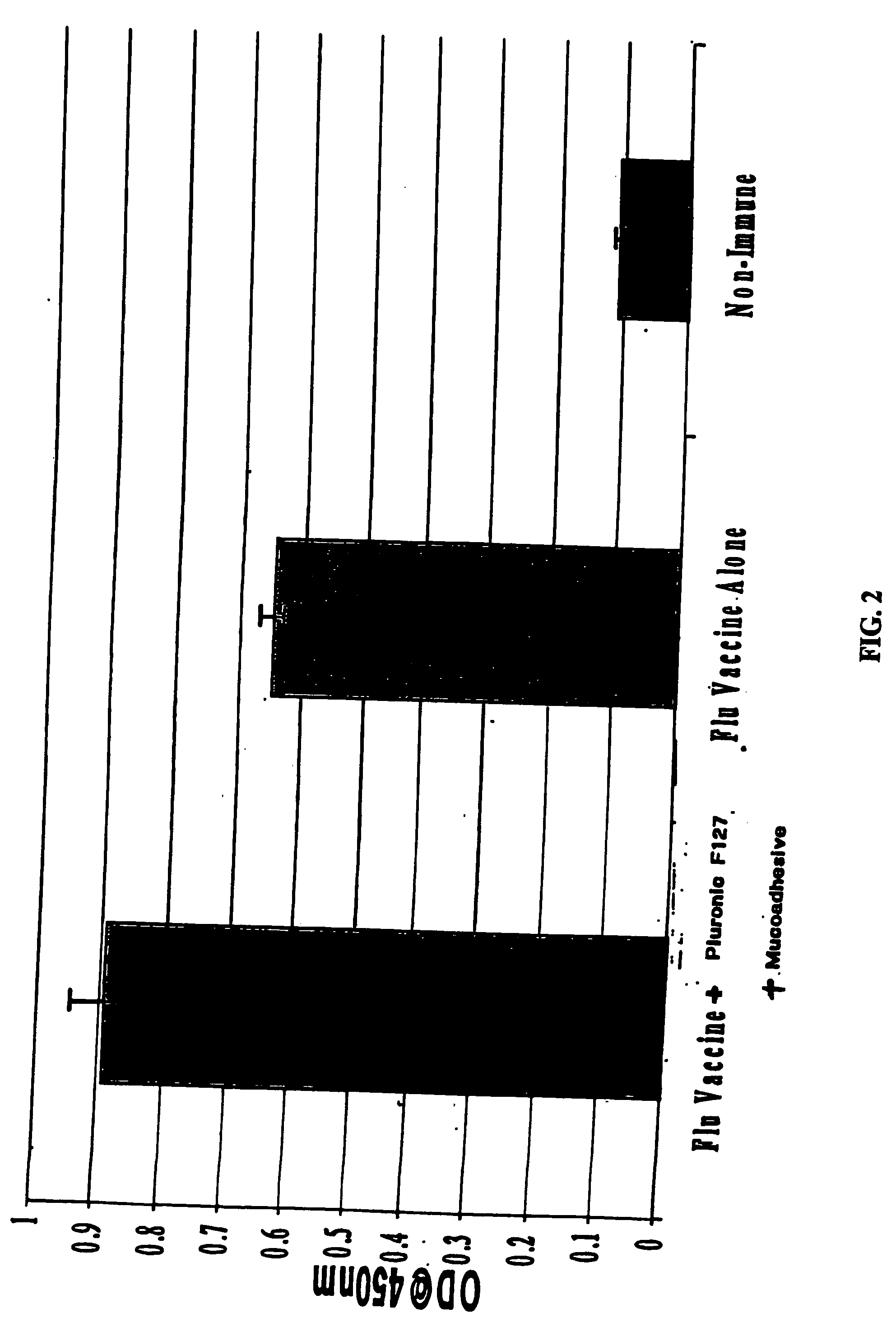
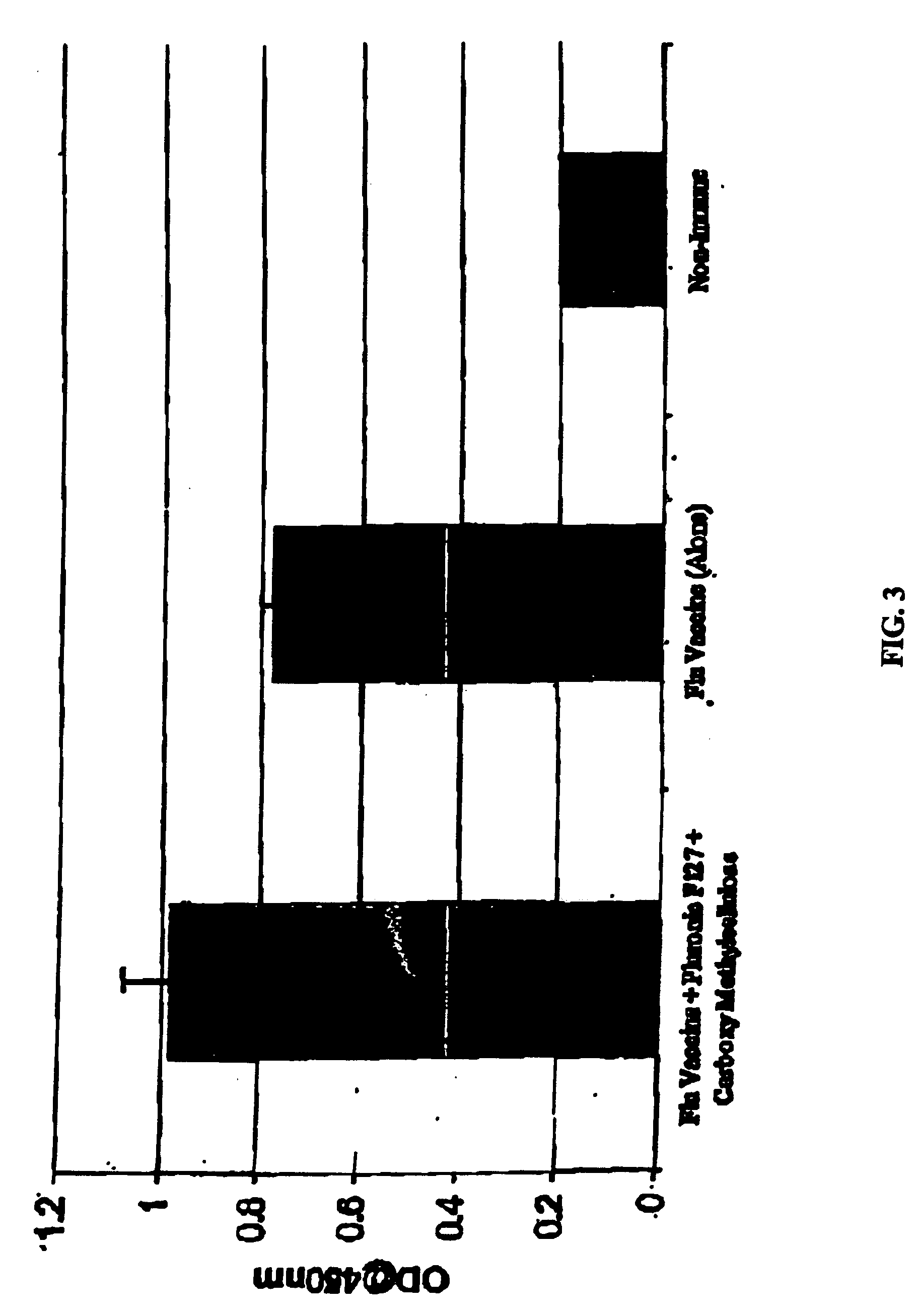
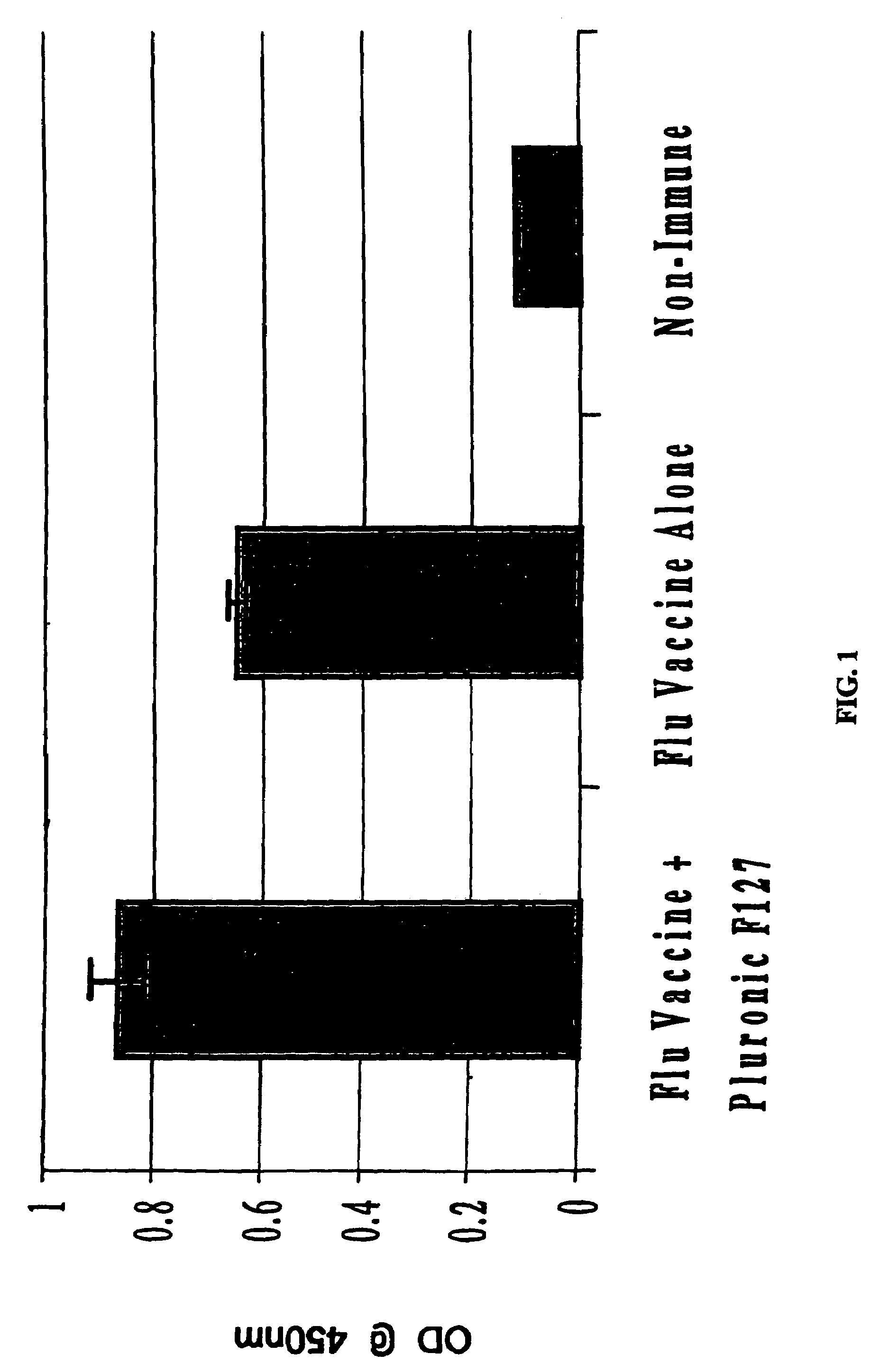
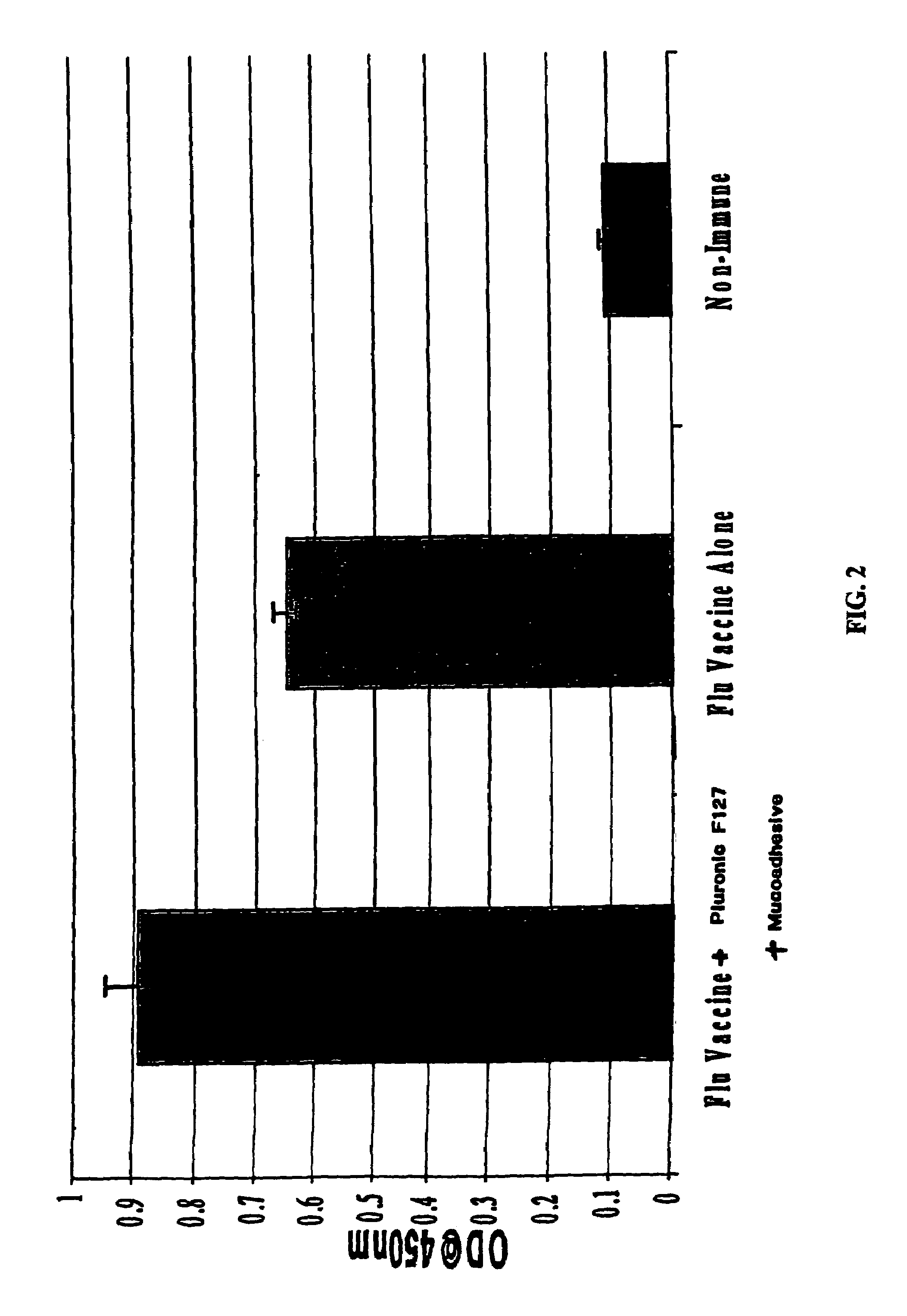
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
ALVAC/FIV constructs
InactiveUS7255862B1Improve security levelViral antigen ingredientsGenetic material ingredientsFeline immunodeficiency virusHeterologous
Recombinants containing and expressing lentivirus, retrovirus or immunodeficiency virus DNA and methods for making and using the same are disclosed and claimed. In an exemplified embodiment, attenuated recombinant viruses containing DNA encoding a feline immunodeficiency virus epitope such as an antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinants can be NYVAC or ALVAC recombinants. The DNA can encode at least one of: Env, Gag, Pol, or combinations thereof such as Gag and Pol or protease or Env, Gag and Pol or protease. The recombinants and gene products therefrom and antibodies generated by them have several preventive, therapeutic and diagnostic uses. DNA from the recombinants are useful as probes or, for generating PCR primers or for immunization. The immunogenicity and protective efficacy of immunization protocols involving ALVAC-FIV and priming with a recombinant canarypox virus ALVAC-FIV vaccine followed by a booster immunization with inactivated FIV-infected celled vaccine (ICV) was evaluated against FIV challenge in cats and the protocol was shown to effectively induce FIV-specific protective immune responses. Further, it was found that immunized cats were fully protected from an initial challenge with a slightly heterologous FIV strain (50CID50) and were partially protected from a second challenge with a distinctly heterologous FIV strain (75CID50) given eight months after the initial challenge without any intervening booster.
Owner:VIROGENETICS
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050255121A1Good antigenicityEnhance immune responseSsRNA viruses negative-senseBiocideInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
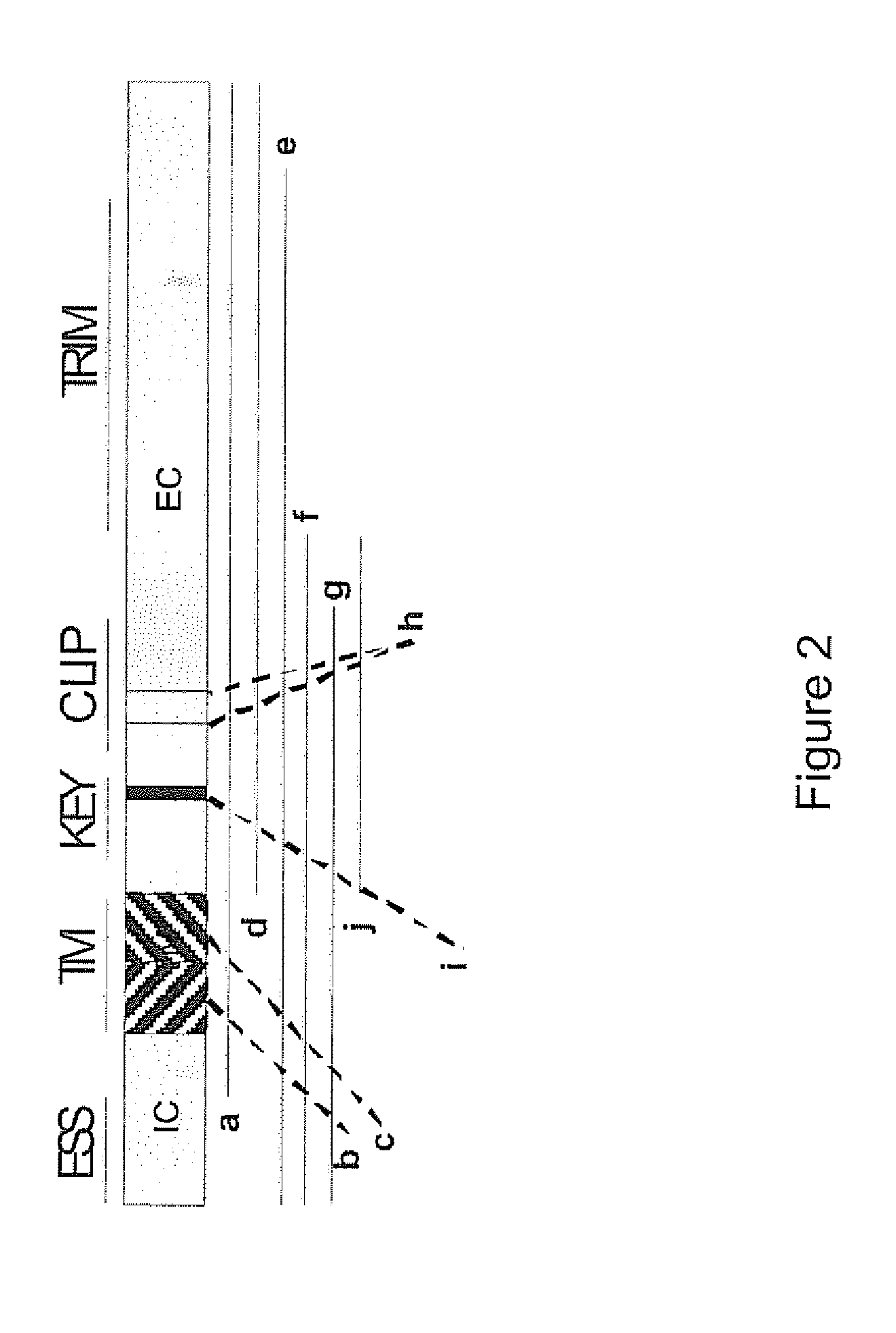
Priming of an immune response
InactiveUS20110293704A1Improve effectivenessReduce impactPowder deliveryVirusesBooster immunizationsInvariant chain
The present invention relates to a technology and method of priming of an immune response using invariant chain linked antigen, when these are used to prime a subsequent booster immunization using any suitable vacci.
Owner:UNIVERSITY OF COPENHAGEN
Encapsulated vaccines for the oral vaccination and boostering of fish and other animals
ActiveUS7998502B2Overcomes shortcomingAntibacterial agentsSsRNA viruses negative-senseVaccinationAquatic animal
The invention relates to a composition comprising a pharmaceutically active agent and a bioadhesive delivery system that provides for the oral delivery of a vaccine to animals, particularly aquatic animals.
Owner:INTERVET INC
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic polypeptide
InactiveCN103509107AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansPenicillinKeyhole-limpet haemocyanin
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic polypeptide. The method comprises the following steps: synthesizing a "CGAGAGSGAGAGS" polypeptide sequence by utilizing an Fmoc method, coupling the polypeptide with keyhole limpet hemocyanin (KLH) through the cysteine on the N terminus of the polypeptide so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain a primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antibody titer in the rabbit blood sample reaches 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate.
Owner:ZHEJIANG UNIV +1
Methods for vaccinating against malaria
ActiveUS20060188527A1Reduce chanceReduce severityBiocideGenetic material ingredientsVaccinationA-DNA
The invention pertains to methods for protecting against malaria infection by vaccination. The method of the invention involves priming an anti-malaria immune response with a DNA-based vaccine and boosting that response with a protein-based a vaccine. The method of the invention also relates to broadening the resulting immune response by boosting with a protein-based vaccine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Novel strategies for protein vaccines
InactiveUS20060275777A1Microbiological testing/measurementCancer antigen ingredientsTarget antigenPathogen
A prerequisite for clinical vaccines is the construction of safe and highly immunogenic reagents able to generate efficient immune responses against target antigens. Lipid based delivery vesicles, including virosomes, as clinically approved safe vaccines, can be used to elicit both humoral and cell-mediated responses against protein antigens and mediate effective immune responses against the target pathogen and / or induce tumor rejection. Thus the compositions of the present invention are useful either as a primary vaccination or as a boost in combination with other vaccines in a context of an adjuvant treatment plan.
Owner:PEVION BIOTECH
Porcine circovirus type II inactivated vaccine of and method for preparing same
ActiveCN101549155ABroad antigen spectrumGood immune effectViral antigen ingredientsAntiviralsOil adjuvantWindow period
The present invention belong to veterinary new biological medicine technology field, relates to porcine circovirus type II (PCV2) inactivated vaccine of and method for preparing same. The vaccine used seed virus is porcine circovirus type II DBN-SX07 strain, the preservation number is CGMCC No 3064, the virus strain is used as antigen preparation of inactivation by alkyl agents and emulsification by adding oil adjuvant. Using the invention provided PCV2 inactivated vaccine to immune pig can generate an uniform and effective protection force-shorting PCV2 infection windows period obviously, and prolong immune duration-reducing times of booster immunization.
Owner:兆丰华生物科技(南京)有限公司 +1
Microparticulated vaccines for the oral or nasal vaccination and boostering of animals including fish
ActiveUS20120040010A1Stabilizes sensitive bioactiveImprove thermal stabilitySsRNA viruses negative-sensePowder deliveryVaccinationAquatic animal
The invention relates to a composition and a method for manufacturing semi-dry or dry particles containing a mucoadhesive polymer and a bioactive agent such as, but not limited to, an Immunogenic Substance (e.g., a vaccine), that allows the oral or nasal administration and delivery of the bioactive agent essentially unaltered to mucosal surfaces in the animal, including an aquatic animal.
Owner:INTERVET INC
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic dodecapeptide
InactiveCN103509108AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansKeyhole-limpet haemocyaninPrimary immunization
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic dodecapeptide. The method comprises the following steps: synthesizing a polypeptide with a "CGYGAGAGAGYGA" sequence, coupling the polypeptide with keyhole limpet hemocyanin (KLH) so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, carrying out an emulsion treatment so as to obtain primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then carrying out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antiserum titer of rabbit arrives at 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate. The antibody prepared by the invention has a strong specificity, and can be used for detection and analysis of silk fibroin in textile, and the like.
Owner:ZHEJIANG UNIV +1
Yolk antibody of anti SARS coronavirus and its preparation method and liquid preparation
InactiveCN1556113AEasy to prepareFast preparation methodEgg immunoglobulinsImmunoglobulins against virusesYolkEpitope
A yolk antibody for SARS coronavirus is prepared through injecting the antigen, which may be recombinant genetic protein S, M, or E,or the antigen epitope for said protein able to represent SARS coronavirus, or the synthetic polypeptide of said protein, etc, in health hen for primary immunizing, booster immunizing, collecting its egg, extracting yolk, and extracting the yolk antibody from it. It can also be prepared to become liquid preparation. It can be used to prevent SARS.
Owner:BIOINFORBODY
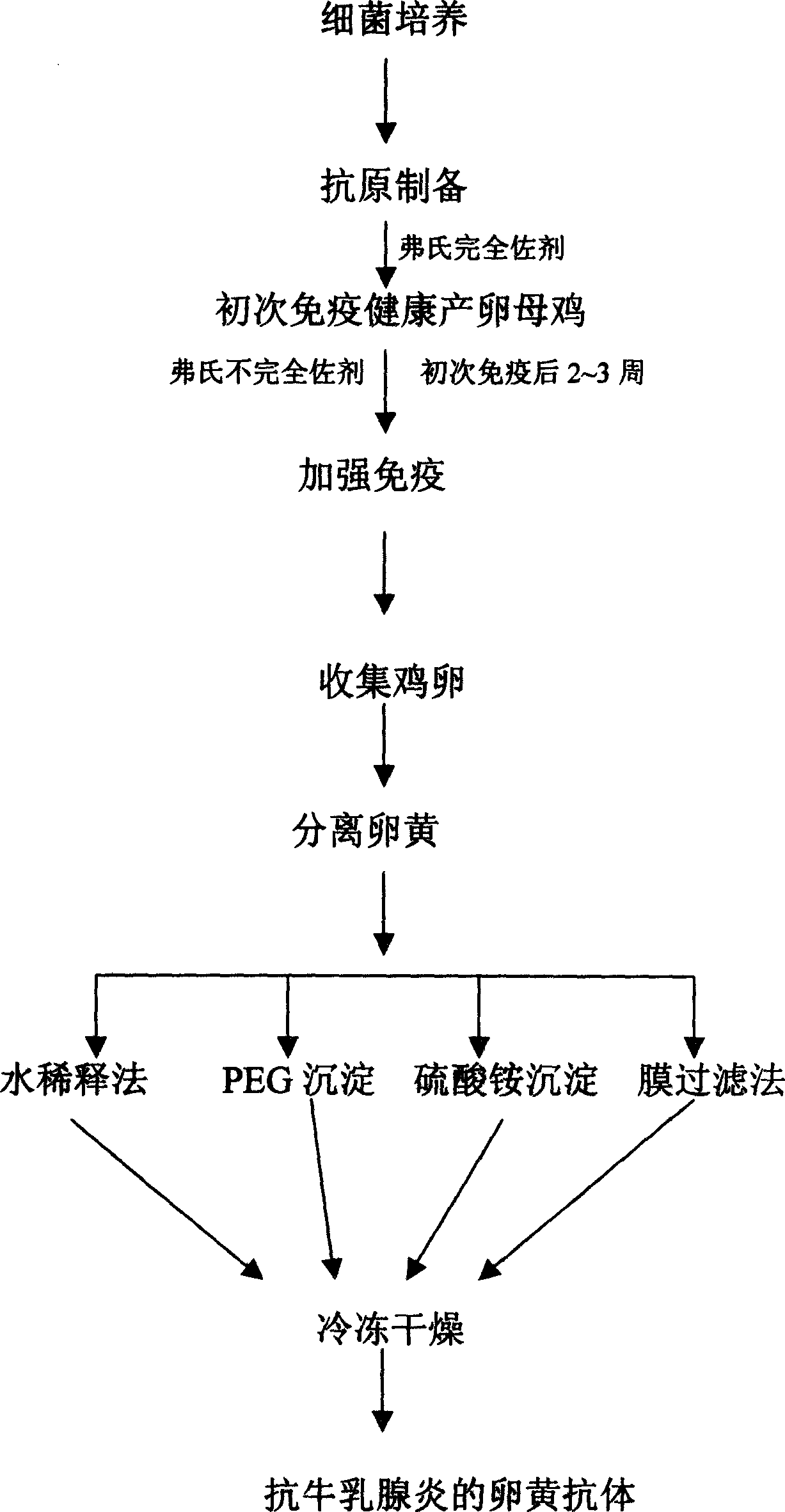
Bovine mastitis resistant yolk antibody and its preparation method and formulation
The invention discloses a vitelline antibody for resisting bovine mastitis, its preparing process and preparation thereof, wherein hens capable of healthy oviposition are used as immune animal, and pathogenic bacteria causing bovine mastitis diseases are used as antigens, the preparation process comprises the steps of first immunization, reinforced immunization, collecting eggs and extracting bovine mastitis resistant vitellus antibody from vitelline, the preparation includes liquid preparation, solid preparation and semi-solid ointment preparation. The vitelline antibody for resisting bovine mastitis and its preparation can be used in the prevention and cure of bovine mastitis caused by pathogenic bacteria.
Owner:BIOINFORBODY
Kit for determining anti-cyclic citrullinated peptide (Anti-CCP) antibody
ActiveCN102507918AEnhanced detection signalHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsAntiendomysial antibodiesCitrulline
The invention discloses a kit for determining an anti-cyclic citrullinated peptide (Anti-CCP) antibody. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a buffer solution, inorganic salt, surfactant, preservative, interference removing protein, modified CCP-sensitized nano poly styrene (PS) microspheres and ultrapure water; the reagent R2 comprises a buffer solution, inorganic salt, surfactant, preservative, an anti Anti-CCP second antibody and ultrapure water; and the weight ratio of the reagent R1 to the reagent R2 is (50-90):(10-50). According to the method, the Anti-CCP is determined by adding the anti Anti-CCP second antibody by a latex booster immunization transmission turbidimetry, a detection signal is subjected to two-stage amplification, the detection sensitivity is improved, and the determination range is enlarged; and the kit can be applied to an automatic biochemical analyzer to shorten detection time, and improve detection efficiency.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
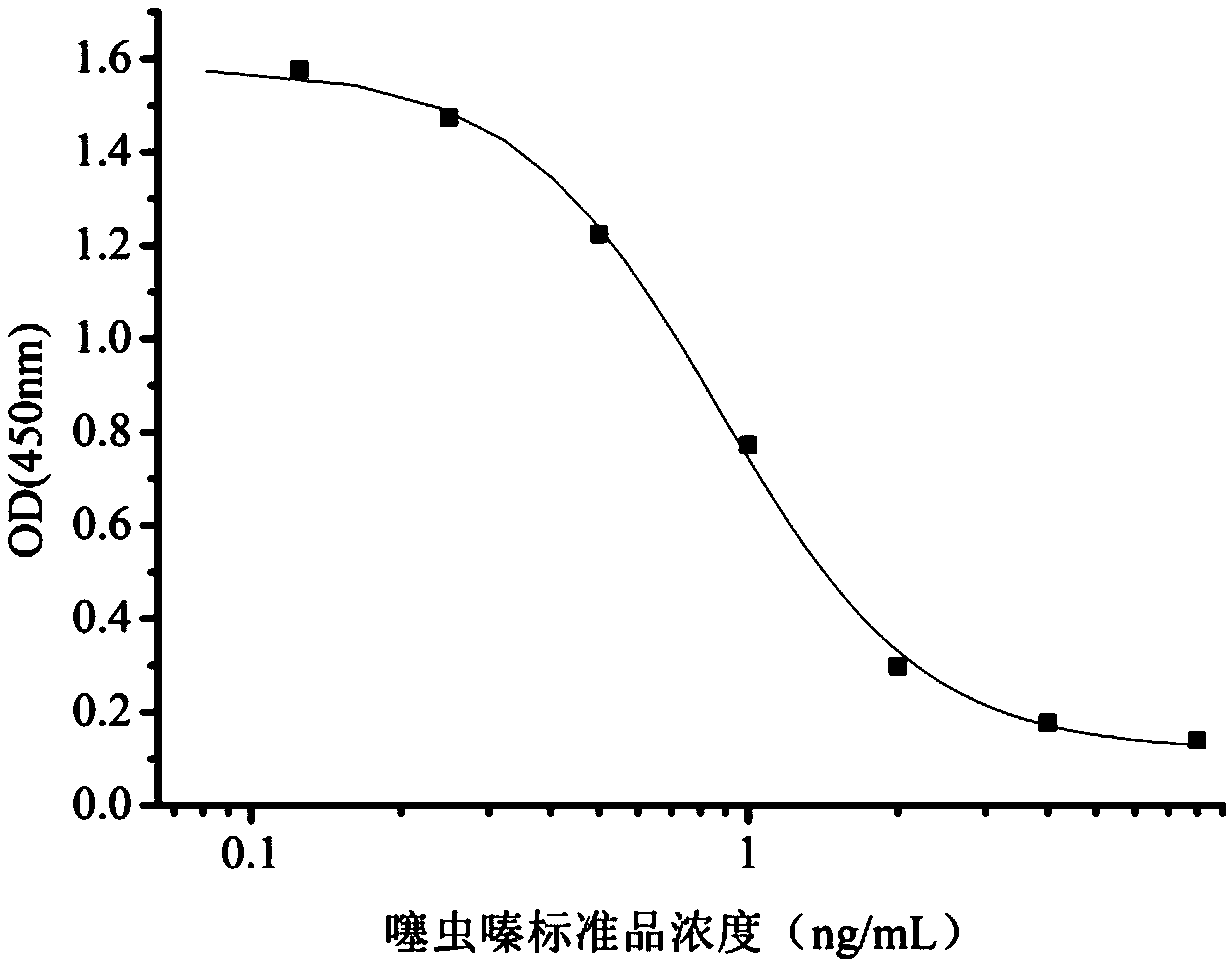
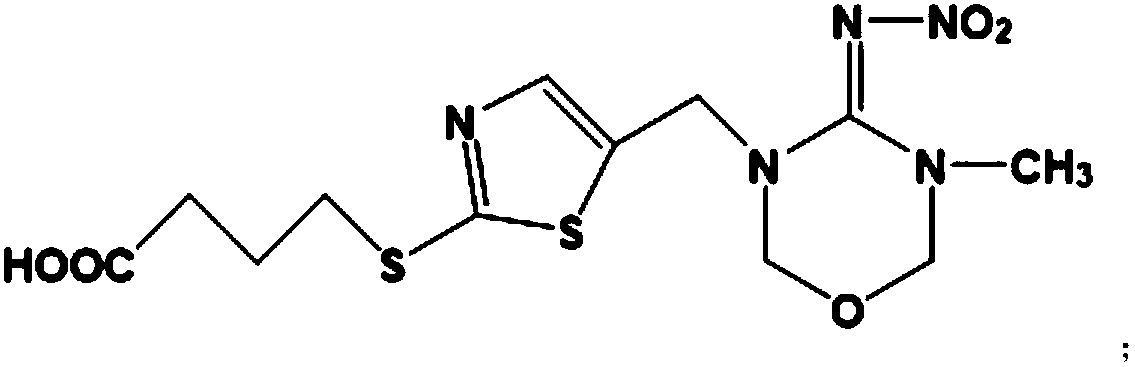
Hybridoma cell strain secreting thiamethoxam monoclonal antibody and application thereof
InactiveCN108998422AHigh detection sensitivityImprove featuresMicroorganism based processesDepsipeptidesBALB/cIc50 values
The invention relates to a hybridoma cell strain secreting thiamethoxacin monoclonal antibody and application thereof, belonging to the field of food safety immunodetection. The accession number of the hybridoma cell strain is CGMCC No. 14699. According to the invention, a complete Freund's adjuvant is used for primary immunization of a BALB / c mouse, then an incomplete Freund's adjuvant is used for booster immunization three times, and a thiamethoxam complete antigen containing no adjuvant is used for impact immunization once, so the BALB / c mouse is immunized; and then the high-titer low-IC50spleen cells of the immunized mouse are fused with mouse myeloma cells by using a PEG method, and then the cell strain is obtained through indirect competitive ELISA screening and subcloning three times. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity (with an IC50 value of 0.81 ng / mL) to thiamethoxam and can be used for detection of thiamethoxamresidues in food.
Owner:JIANGNAN UNIV +1
Methods and compositions for immunization against hiv
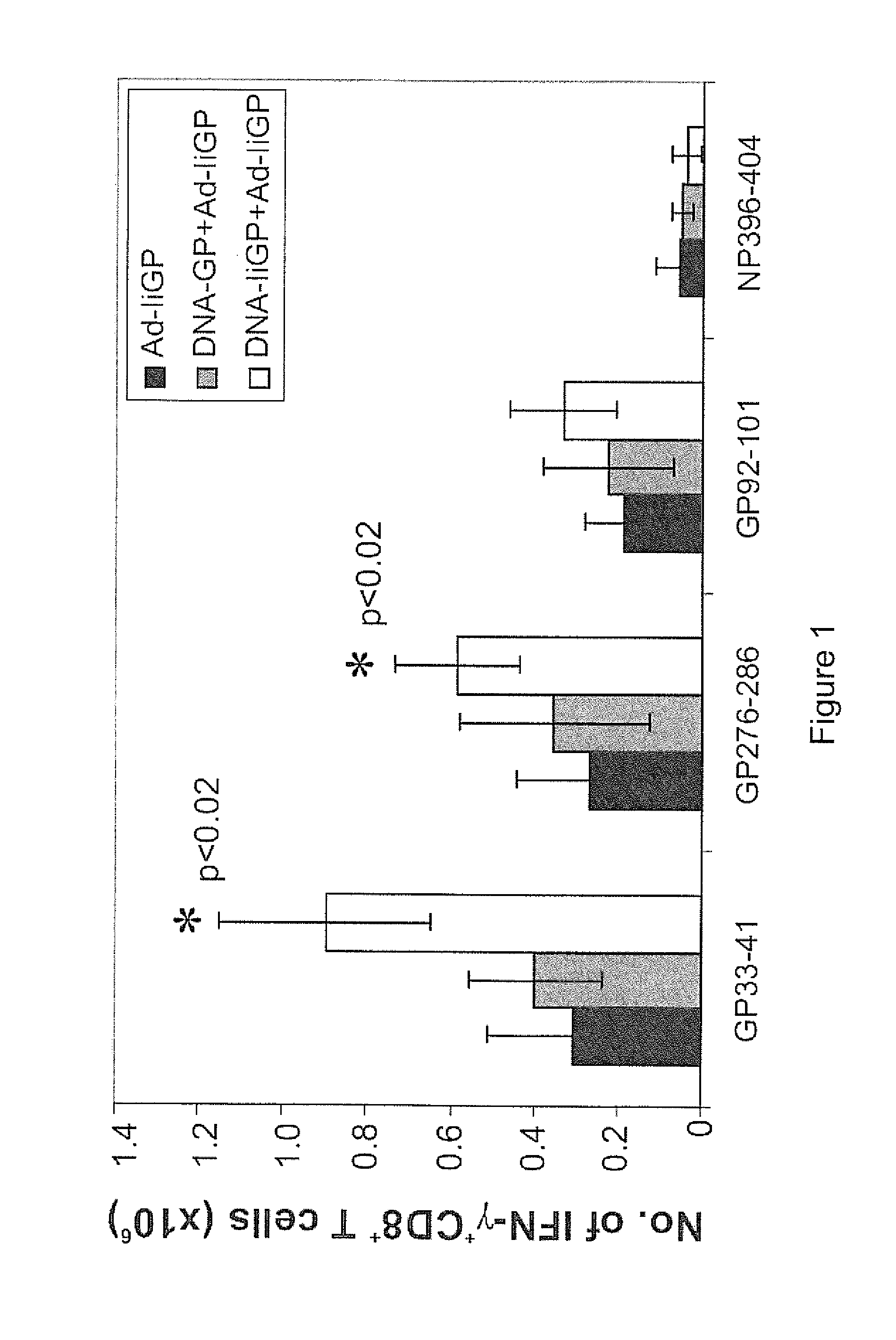
The present invention relates to nucleic acid and attenuated vaccinia vectors for prophylactic use against HIV infection, as well as methods of eliciting immune responses in subjects susceptible to HIV infection. The prophylactic vaccine regimen of the invention involves immunological priming with an inoculum comprising two novel DNA vectors, followed by boosting with a Modified Vaccinia Ankara (MVA) recombinant viral vector expressing the corresponding HIV proteins.
Owner:HUANG YAOXING +2
Recombinant HIV-1 gp120 immunogen with three different V3 loops from viruses of different clades
InactiveUS7847085B2Minimize potentialUtility in researchSugar derivativesViral antigen ingredientsDNA constructImmunogenicity
A novel immunogenic HIV-1 Env, particularly gp120, DNA construct is disclosed in which either the V1 / V2 loop and the V4 loop, or all three variable loops, including V3, are replaced with a V3 sequence each of which is from a different viral isolate. Preferably, each replacement V3 loop is a consensus sequence of V3 of a different clade. Such constructs are useful as immunogens as each presents three independent V3 epitopes, so that the immunized subject generates a more broadly reactive neutralizing antibody response than with conventional gp120 or V3 DNA or polypeptide immunogens. Also disclosed are methods of using the DNA construct to immunize a mammal, preferably a human, particularly in a priming regiment in which the DNA immunogen is followed by administration of a V3 fusion protein boosting immunogen.
Owner:NEW YORK UNIV +1
Methods of enhancing immune response in the intradermal compartment and compounds useful thereof
InactiveUS20050281832A1Good curative effectImprove presentationSsRNA viruses negative-senseBiocideAdjuvantImmunogenicity
The present invention relates to immunogenic compositions for intradermal delivery of an antigenic or immunogenic agent in combination with one or more excipients. The immunogenic compositions of the invention comprise an antigenic or immunogenic agent and at least one excipient which acts as an adjuvant, i.e., enhances the immune response to the antigenic or immunogenic agent, once delivered to the intradermal compartment of a subject's skin. The immunogenic compositions of the invention comprise an excipient which when administered to the intradermal compartment of skin in accordance with the invention demonstrate adjuvant activity. The immunogenic compositions of the invention have enhanced efficacy as the excipients of the composition cause an asymptomatic skin irritation and recruit antigen presenting cells to the intradermal compartment and thus enhance presentation and / or availability of the antigenic or immunogenic agent to the antigen presenting cells. The enhanced efficacy of the immunogenic compositions of the invention may result in a therapeutically effective immune response after a single intradermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations
Owner:BECTON DICKINSON & CO
Thiacloprid and acetamiprid monoclonal antibody hybridoma cell strain GW and application thereof
ActiveCN105754955AImprove featuresHigh detection sensitivityBiological material analysisMicroorganism based processesBALB/cAntigen
The invention discloses a thiacloprid and acetamiprid monoclonal antibody hybridoma cell strain GW and application thereof, and belongs to the field of food security immunodetection. Thiacloprid and acetamiprid complete antigen of the strain is uniformly mixed with an equal amount of QuickAntibody-Mouse 5W adjuvant, and is injected to BALB / c mice through leg muscle. The dosage is 100mu g / mouse for the first time of immunization, the dosage is 50[mu]g / mouse for multiple times of intensified immunization, the immunization is implemented at an interval of 21 days, and thiacloprid and acetamiprid complete antigen (25[mu]g / mouse, without adjuvant) is adopted for immunization impact for the last time. Splenocyte of high-potency low IC50 mice is taken and fused with mouse myeloma cells by using a PEG method, indirect competitive inhibition enzyme-linked immunosorbent assay is adopted for screening, and three times of subcloning is implemented, so as to obtain the hybridoma cell strain. A monoclonal antibody secreted from the cell strain has relatively good specificity and detection sensitivity (the IC50 values are 0.1ng / mL and 0.4ng / mL respectively) for thiacloprid and acetamiprid, detection on the residual amounts of thiacloprid and acetamiprid in water, fruits and vegetables and cereals can be achieved, conditions are provided for immunodetection on thiacloprid and acetamiprid residues in food can be provided, and practical use values can be made.
Owner:JIANGNAN UNIV
Adenovirus carrier vaccine carrying HIV gene
The invention describes an adenovirus vector which carries a HIV structure gene of codon optimization and / or a HIV regulatory and auxiliary gene as well as a heterogenous promoter and a transcription terminator. When a first-booster immunization proposal is adopted by a single or combined plasmid vector vaccine, the vector-viral vaccine can be used for preventing HIV infection.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Hybridoma cell strain CBC secreting monoclonal antibody of dicofol and application of hybridoma cell strain CBC
InactiveCN110607283AGood detection sensitivityHigh detection sensitivityDepsipeptidesTissue cultureLaboratory cultureTiter
The invention discloses a hybridoma cell strain CBC secreting a monoclonal antibody of dicofol and application of the hybridoma cell strain CBC, and belongs to the field of food safety immunoassay. The cell strain CBC is preserved in the China General Microbiological Culture Collection Center, the address is Institute of Microbiology, Chinese Academy of Sciences, No.3, Beichen West Road, ChaoyangDistrict, Beijing, the preservation date is March 7, 2019, and the preservation number is CGMCC No.17398. Complete antigens of the dicofol are mixed and emulsified with equivalent freund adjuvants, and BALB / c mice are immunized by subcutaneous multipoint injection of neck and back through first immunization, multiple strengthening immunization and sprint immunization. Spleen cells of the mice withhigh titer and low IC50 are fused with mouse myeloma cells by a PEC method, selective culture is conducted, then cells are screened and sub-cloned for three times through indirect competitive enzyme-linked immunosorbent assay, finally the cell strain CBC is obtained, the monoclonal antibody secreted by the cell strain CBC has good detection sensitivity to the dicofol (value of the IC50 is 19.09 ng / mL), and can be used for the detection of dicofol residues in food.
Owner:江苏权正检验检测有限公司 +1
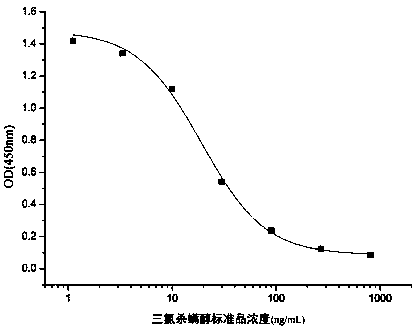
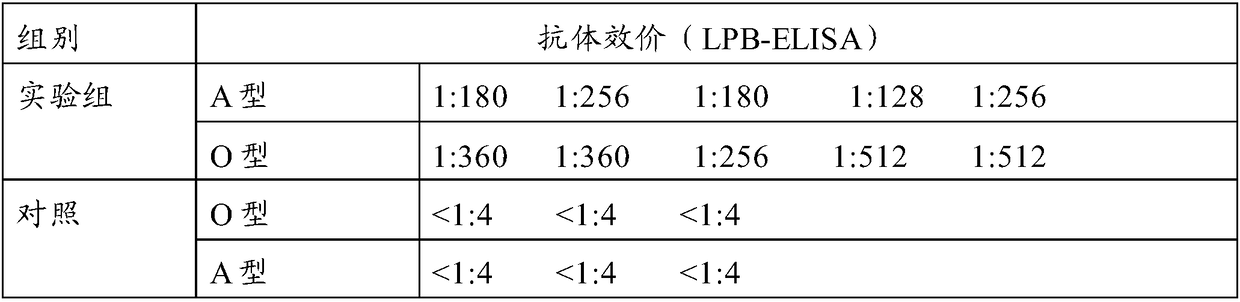
Pig foot-and-mouth disease virus O type and A type Fc polypeptide bivalent vaccine, as well as preparation method and application thereof
ActiveCN108273054AHigh-throughput soluble expressionHigh puritySsRNA viruses positive-senseViral antigen ingredientsNeutralizing antibodyAnimal husbandry
The invention discloses a pig foot-and-mouth disease virus O type and A type Fc polypeptide bivalent vaccine, as well as a preparation method and application thereof. The bivalent vaccine is preparedfrom effective components of pig foot-and-mouth disease virus A type Fc polypeptide and pig foot-and-mouth disease virus O type Fc polypeptide, wherein an amino acid sequence of the pig foot-and-mouthdisease virus A type Fc polypeptide is as shown in SEQ ID NO.3; an amino acid sequence of the pig foot-and-mouth disease virus O type Fc polypeptide is as shown in SEQ ID NO.5. Experimental results show that the bivalent vaccine can induce a body to produce a high-level neutralizing antibody against O type and A type foot-and-mouth disease viruses after being subjected to booster immunization, can protect immunized pigs against the attack of the O type and A type foot-and-mouth disease viruses, and realizes 5 / 5 protection (i.e. the protection rate is 100 percent). The pig foot-and-mouth disease virus O type and A type Fc polypeptide bivalent vaccine, as well as the preparation method and the application thereof provided by the invention provide a technical support and vaccine storage forguaranteeing healthy sustainable development of animal husbandry in China and realizing a mid-and-long-term prevention and control aim of foot-and-mouth disease countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS7588774B2Enhance immune responseGood curative effectSsRNA viruses negative-senseBiocideInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
Preparation method of spleen byproducts for producing homology anti-serum blood and transfer factor from fox, raccoon dog, mink
InactiveCN101422485AEasy to manufactureControl spreadAntibacterial agentsPeptide/protein ingredientsDiseaseBlood collection
The invention relates to a preparation method for obtaining blood used for preparing homologous antiserums and a spleen byproduct used for preparing transfer factors from the bodies of fur bearing animals such as foxes, raccoon dogs and minks; the furs of the foxes, the raccoon dogs and the minks are taken concentratedly in December and confirmed according to the fur-taking dates of different areas; healthy individuals are picked up 45 days before a predicated fur-taking date; the booster immunization of univalent vaccines or polyvalent vaccines of canine distemper, Canine parvovirus enteritis, adenovirus disease, corona virus laxness, parainfluenza, and pasteurellosis is carried out for 3 times by purified and condensed antigens on the foundation of carrying out immunization for once in summer; when the furs are taken, aseptic anticoagulation blood collection is carried out to a heart to improve the blood serum yield; and the spleens of the animals are collected for extracting specific or common transfer factors simultaneously. The blood can be conveniently prepared into the blood serums which resist communicable diseases, and the transfer factors can be extracted the spleen, thus providing guarantee for the healthy development of the breeding in the next year; the invention is used for curing the corresponding communicable diseases of the wild animals of the same species; and when a small amount of communicable disease cases appear in a breeding crowd, the corresponding pathogeny hyper-immune serums and transfer factors are mainly injected to a supposed healthy crowd after the pathogeny is confirmed so as to cure the affected animals in the early period of disease and control the spreading of the epidemic situations.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
GnRH antigen and application thereof in active immunization affecting castration effect and meat quality of oxen
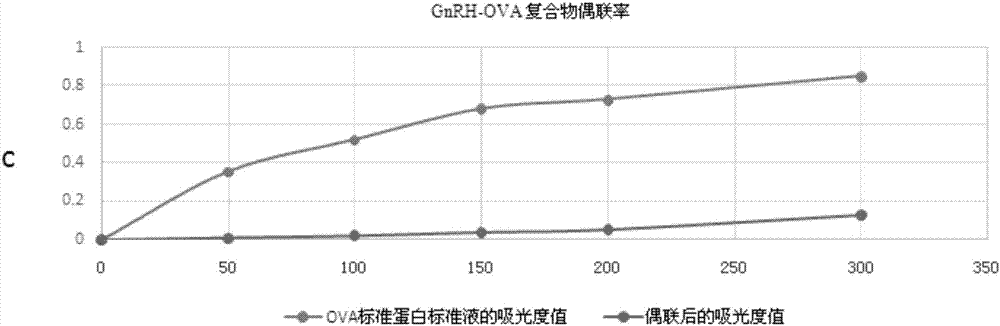
ActiveCN106986923AImproving immunogenicityEnhance antigen immunogenicityVertebrate antigen ingredientsLuteinising hormone-releasing hormoneActive immunizationMale rats
The invention discloses a GnRH antigen and application thereof in active immunization affecting the castration effect and meat quality of oxen. The invention provides a GnRH derivative which is obtained by inserting oligopeptides, which can form alpha helixes, among multiple serially-connected single GnRH antigens, and each single GnRH antigen is a polypeptide obtained by replacing the sixth-site glycine in the amino acid sequence of gonadotropin releasing hormone GnRH with D type lysine. When a GnRH two-string alpha-helix vaccine screened by experiments is used to actively immunize male rats and the oxen, the vaccine can allow the biological activity of testosterone to be partially or completely lost, an immunocastration effect is achieved, a good immunization enhancing effect is achieved, and the carcass quality of immunized animals can be increased to a certain degree.
Owner:XINJIANG ACADEMY OF AGRI & RECLAMATION SCI
Novel Neospora caninum Vaccine
InactiveUS20090208519A1Reduce severityPrevent and to decrease severityProtozoa antigen ingredientsViral antigen ingredientsBALB/cBuprestis novemmaculata
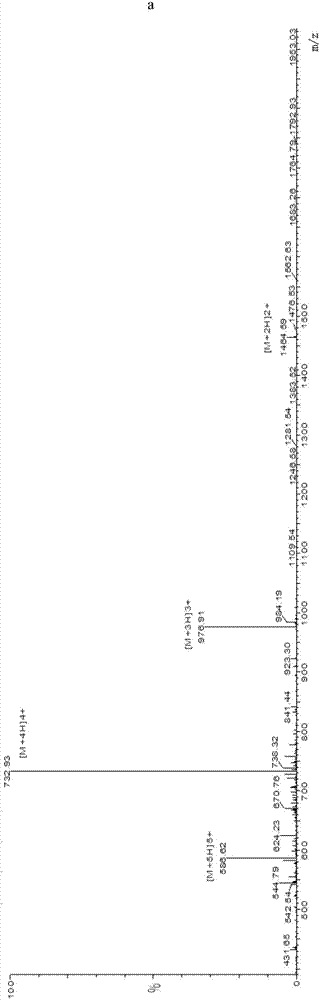
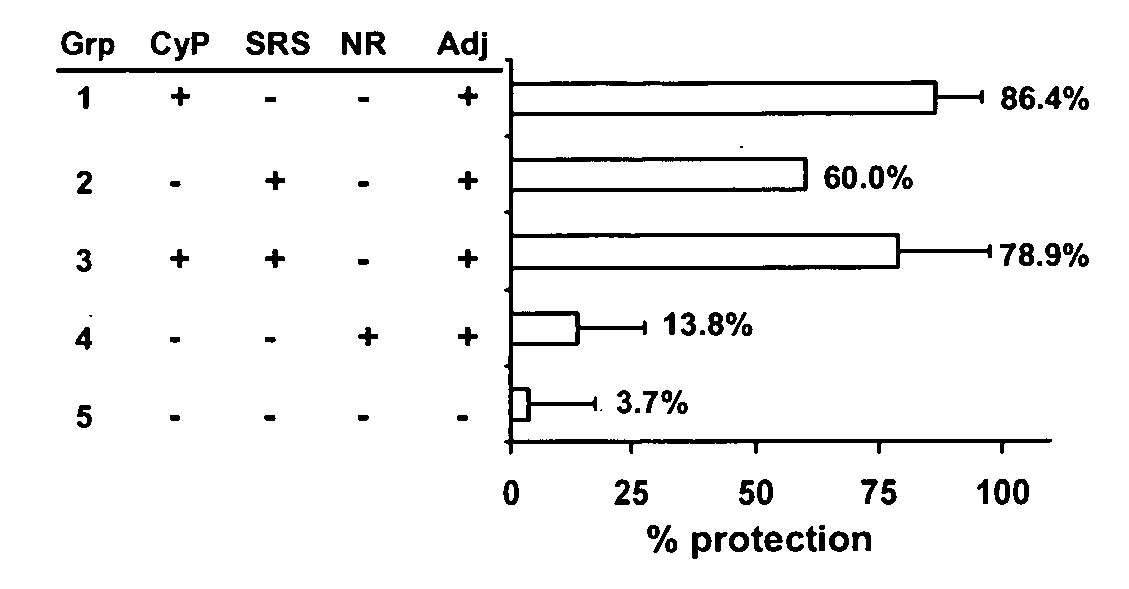
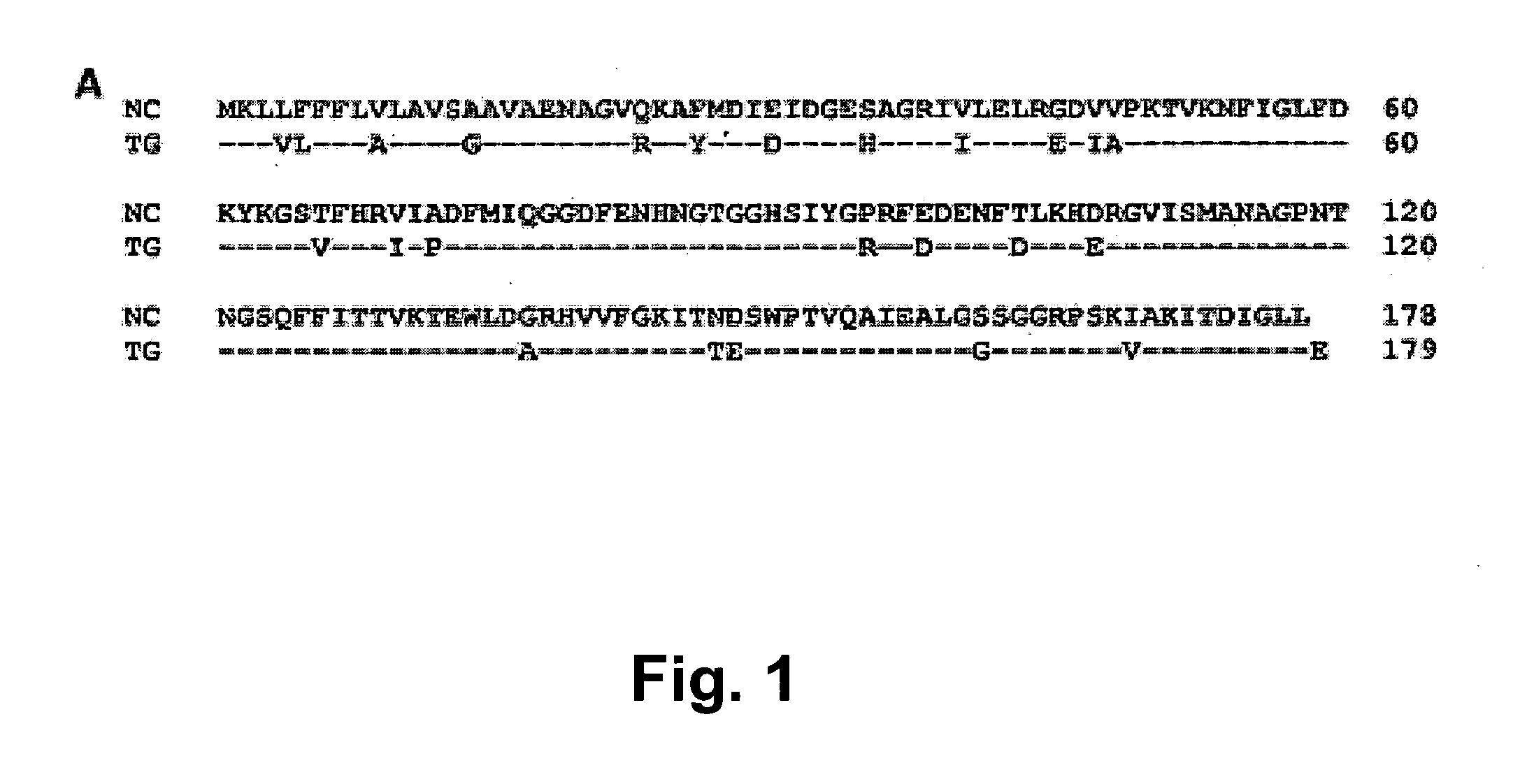
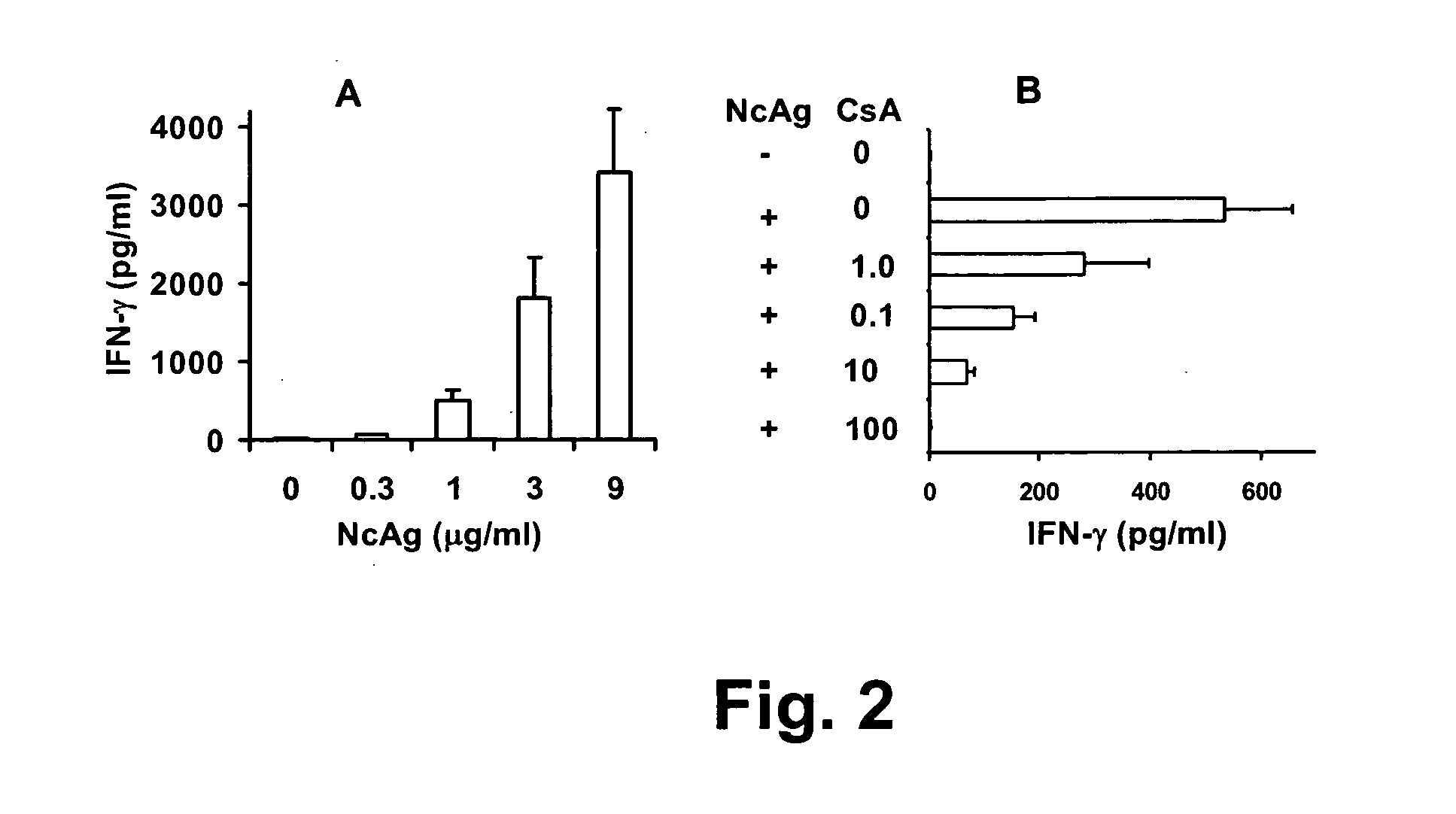
Neospora caninum is the causal agent of bovine neosporosis which results in high levels of abortion. The present study determined the protective efficacy of two Neospora antigens—Neospora cyclophilin (NcCyP) and NcSRS2. The ability of native NcCyP to upregulate mouse IFNγ was also confirmed in this study. Recombinant NcCyP or NcSRS2 were tested either alone or in combination and formulated with adjuvant ImmuMax-SR and CpG. Female BALB / c mice (n=15) of 10-12 weeks of age were immunized s.c. twice in a 2-week interval with vaccines containing either NcCyP alone, NcSRS2 alone, NcCyP plus NcSRS2, or non-recombinant bacterial antigen (NR) in 2 separate trials. All mice were challenge-infected 3 weeks following the booster immunization and necropsied 3 weeks after the challenge infection. Brain and serum were collected and Nc-specific DNA sequence in brain tissue and antibodies in serum were analyzed by PCR or ELISA / Western blotting. Results showed that mice vaccinated with rNcCyP, rNcSRS2, or both rNcCyP and rNcSRS2 responded with high levels of NcCyP or NcSRS2 specific antibodies. Overall, mice received vaccines formulated with either rNcCyP or rNcCyP and rNcSRS2 had a higher (p<0.01) percent protection when compared to the mock- or non-vaccinated mice. The groups immunized with rNcSRS2 alone exhibited slightly lower levels of protection, which was higher (p<0.05) than that of the non-vaccinated group but did not differ (p=0.06) from that of the mock-vaccinated group. The results of the present study indicate that NcCyP is a highly efficacious vaccine candidate which may be useful in protection against Neospora infection.
Owner:UNITED STATES OF AMERICA
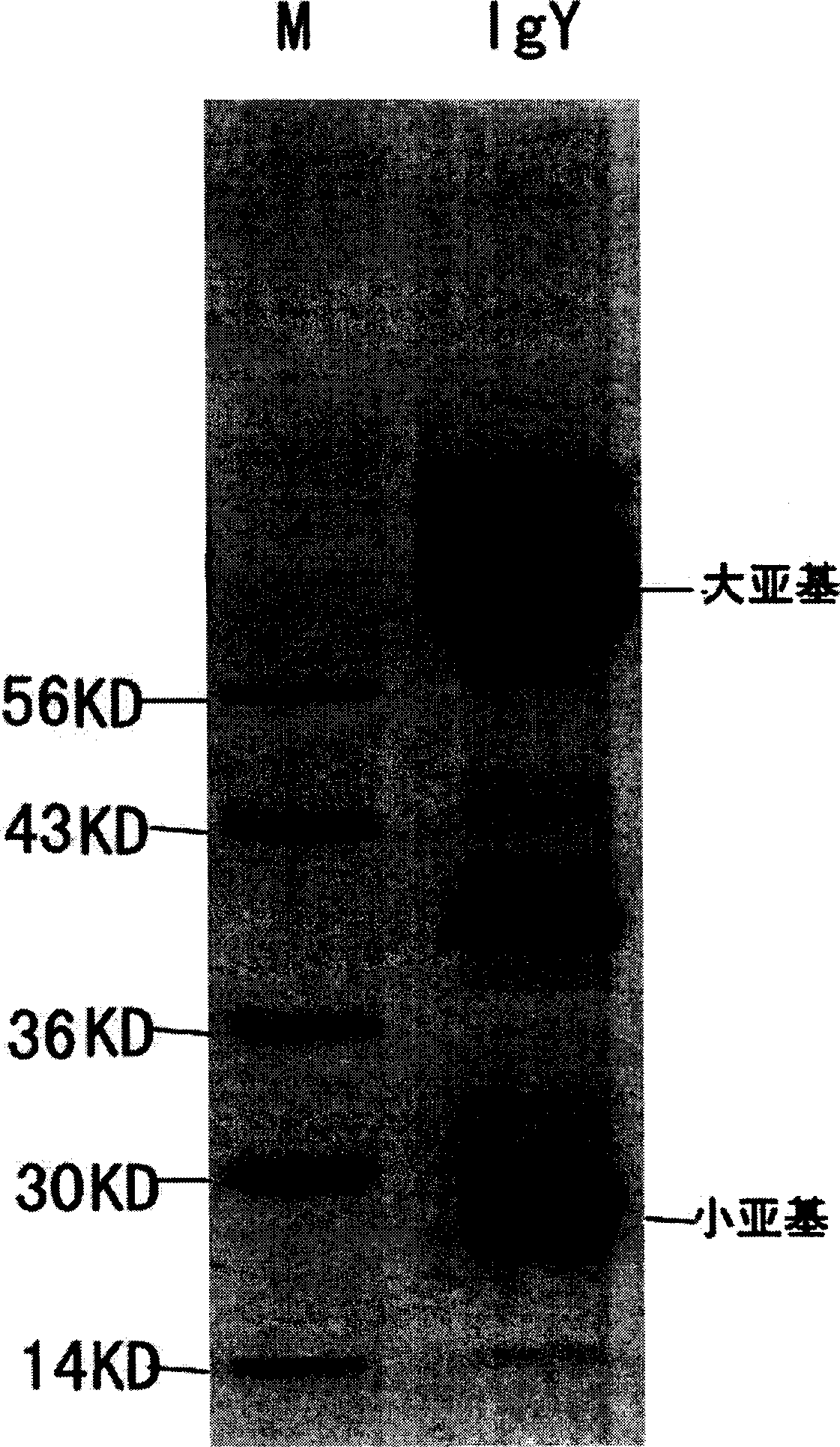
Method for preparing IgY for SARS
InactiveCN1546526ALong-term titer maintenanceEasy to feedEgg immunoglobulinsAntiviralsSerum igeNeutralizing antibody
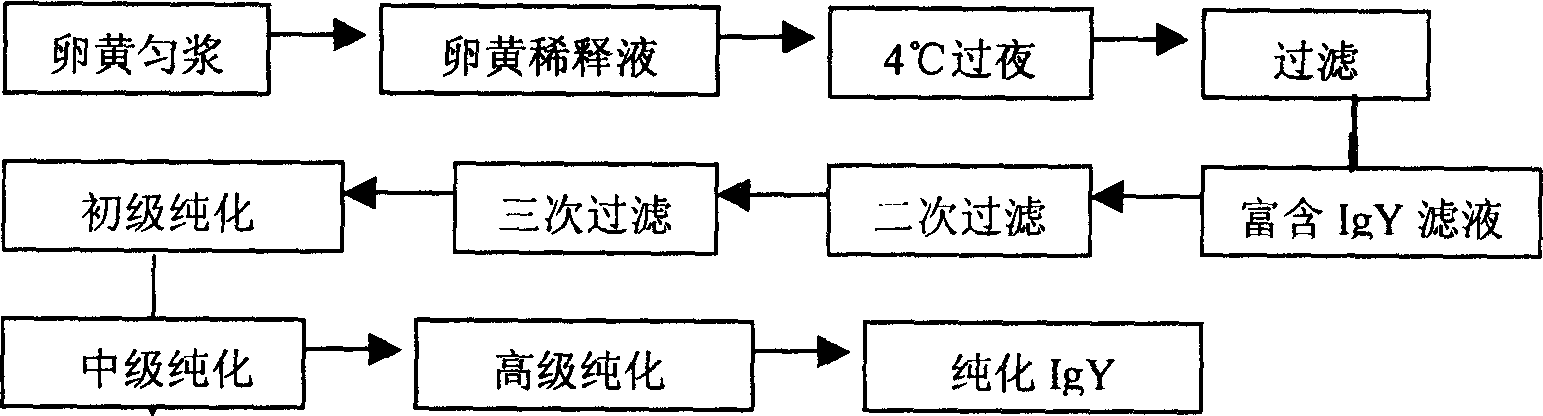
The invention discloses a method for preparing IgY for SARS, which belongs to a process for manufacturing anti-SARS biological pharmaceuticals. The process comprises the steps of making vaccinogen, preparing antibody, homogenating vitelline, diluting, low-temperature stewing, filtering, purifying, and pulverizing. The said antibody preparation process is disclosed in the invention. The process according to the invention can be applied in preparing anti-SARS medicament.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method of monoclonal antibody to chloramphenicol
InactiveCN102477097AStrong controllabilityGood repeatabilityMicroorganism based processesTissue cultureAscitesLymph
The invention discloses a preparation method of a monoclonal antibody to chloramphenicol (CAP). The method comprises the following steps of: (1) preparing an antigen of CAP-BSA by a diazotization method; (2) conducting lymph immunization to mice with the CAP-BSA antigen, leaving the antigen and a Freund's complete adjuvant to complete emulsification during the first time immunization, and during the second time and the third time immunization, leaving the antigen and a Freund's incomplete adjuvant to complete emulsification, and keeping an interval of 7 days between each time, and 3 days before fusion, carrying out direct antigen injection to the abdominal cavity to booster immunization; (3) measuring serum titers of the mice by an enzyme-linked immunosorbent assay (ELISA) method; (4) selecting a mouse with the highest serum titer, and taking a spleen cell and a myeloma cell for in vitro fusion; (5) culturing and screening a fusion cell in a selective medium, conducting positive cell detection and screening for cloning culture, further performing positive cell cloning and screening for positive cloning, expanding culture and cryopreservation; (6) injecting a cell strain undergoing expanded culture into the abdominal cavities of the mouse so as to produce a lot of ascites; (7) using a chromatographic column to purify the monoclonal antibody against chloramphenicol in the ascites. The prepared monoclonal antibody against CAP-BSA has the advantages of high sensitivity, strong specificity and good practicality.
Owner:ZHEJIANG UNIV OF TECH +1
Construction method of mycobacterium tuberculosis fusion protein Mtb 10.4-Hsp16.3, expression method thereof, purification method thereof and application thereof
ActiveCN102154324AEfficient purificationImproves and prolongs protective effectAntibacterial agentsBacterial antigen ingredientsCloning SiteTGE VACCINE
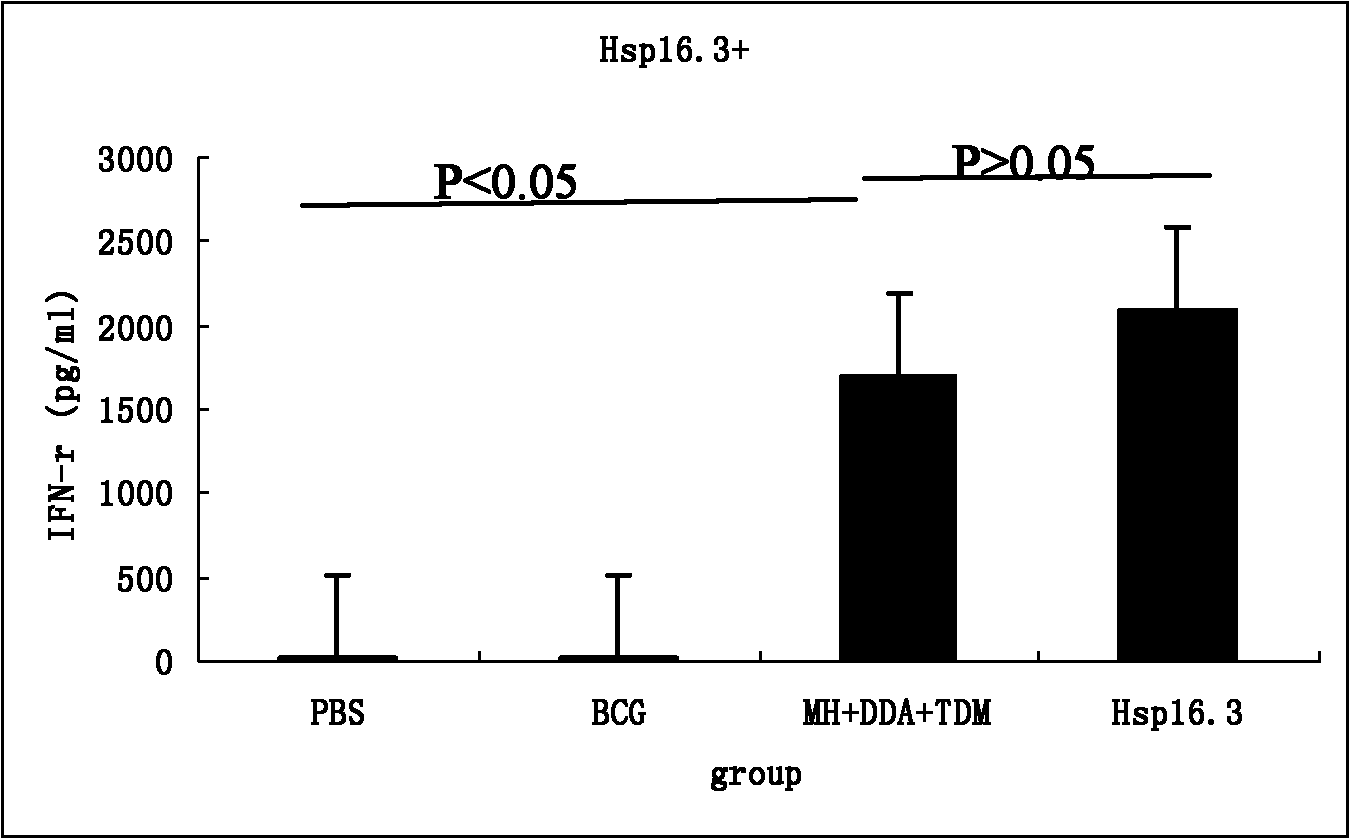
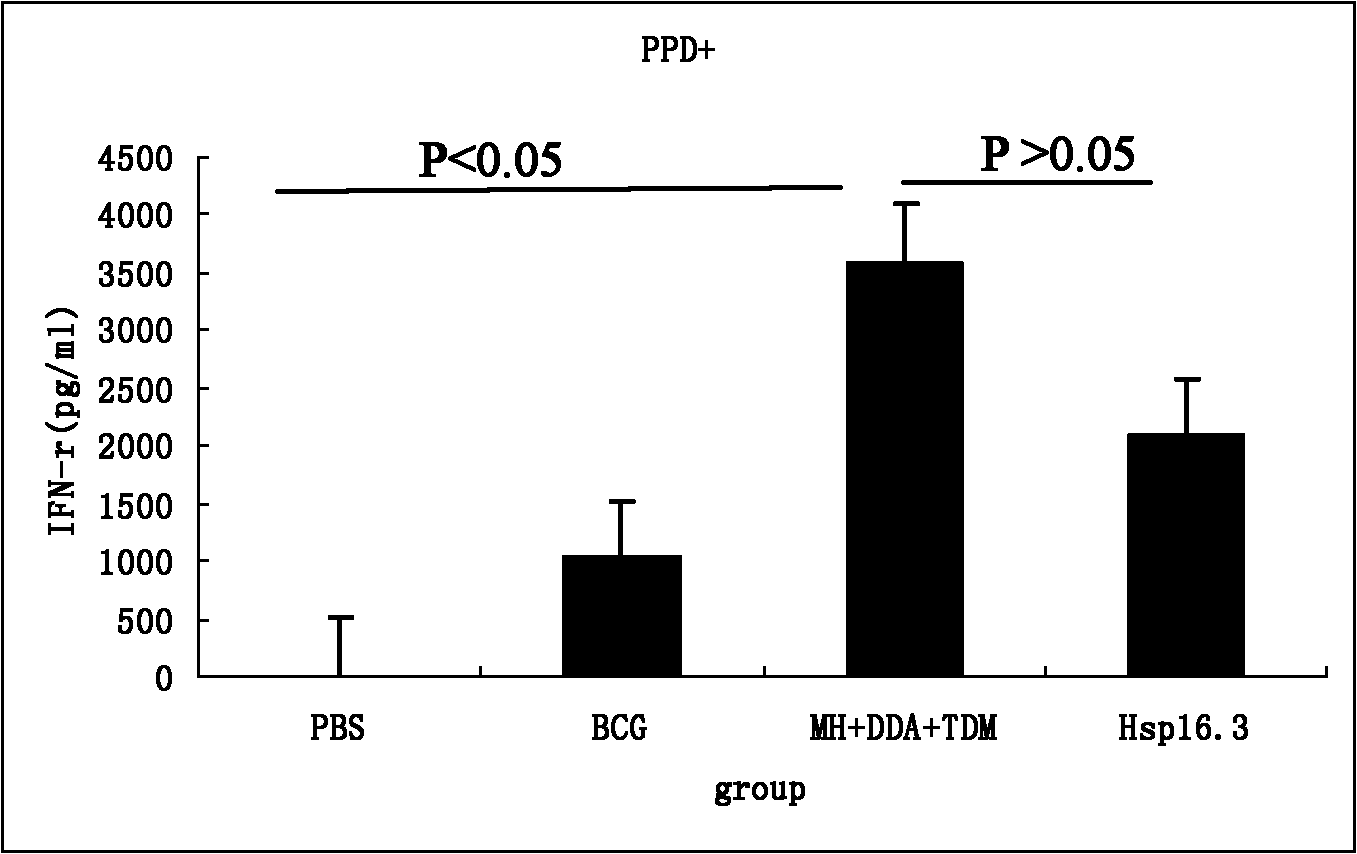
The invention discloses a construction method of mycobacterium tuberculosis fusion protein Mtb 10.4-Hsp16.3, an expression method thereof, a purification method thereof and application thereof. The construction method comprises the following steps: performing the PCR (polymerase chain reaction) amplification to Mtb 10.4 gene and Hsp16.3 gene, and sequentially inserting the amplified genes into a multi-clone site of a cloning vector to construct a recombinant vector; then expressing in the colibacillus; and finally purifying to obtain the fusion protein Mtb 10.4-Hsp16.3, wherein the fusion protein can be applied in a tuberculosis subunit vaccine. The invention has the advantages that the fusion protein vaccine can induce the higher cell of the animal to react with body fluid immunoreaction; the animal challenge test shows that the vaccine plays a part in immunity enhancing on the basis of the BCG (bacille calmette guerin) immunity, so that the protective effect of the BCG can be improved and prolonged; and the vaccine has no obvious side effects.
Owner:LANZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com