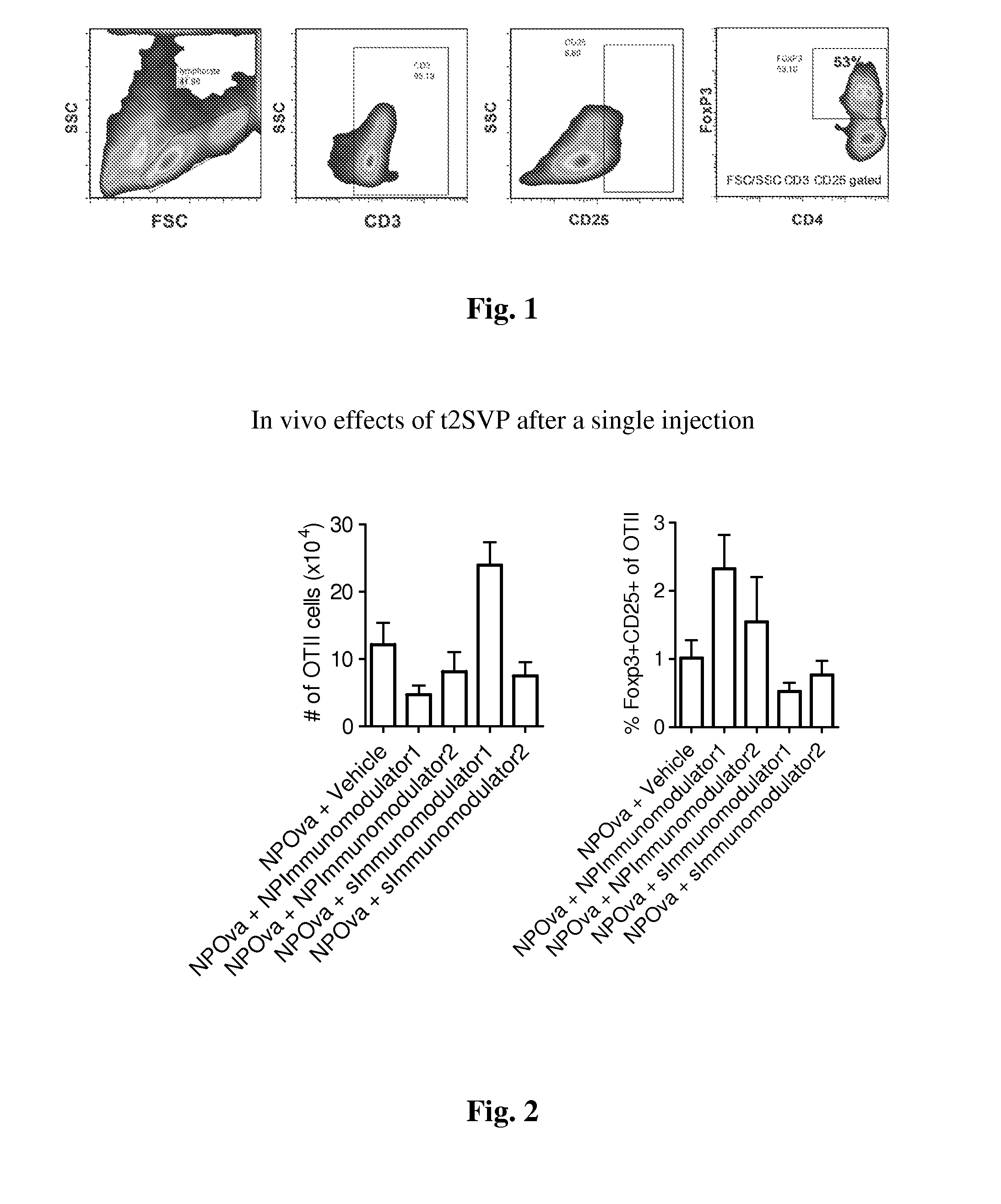
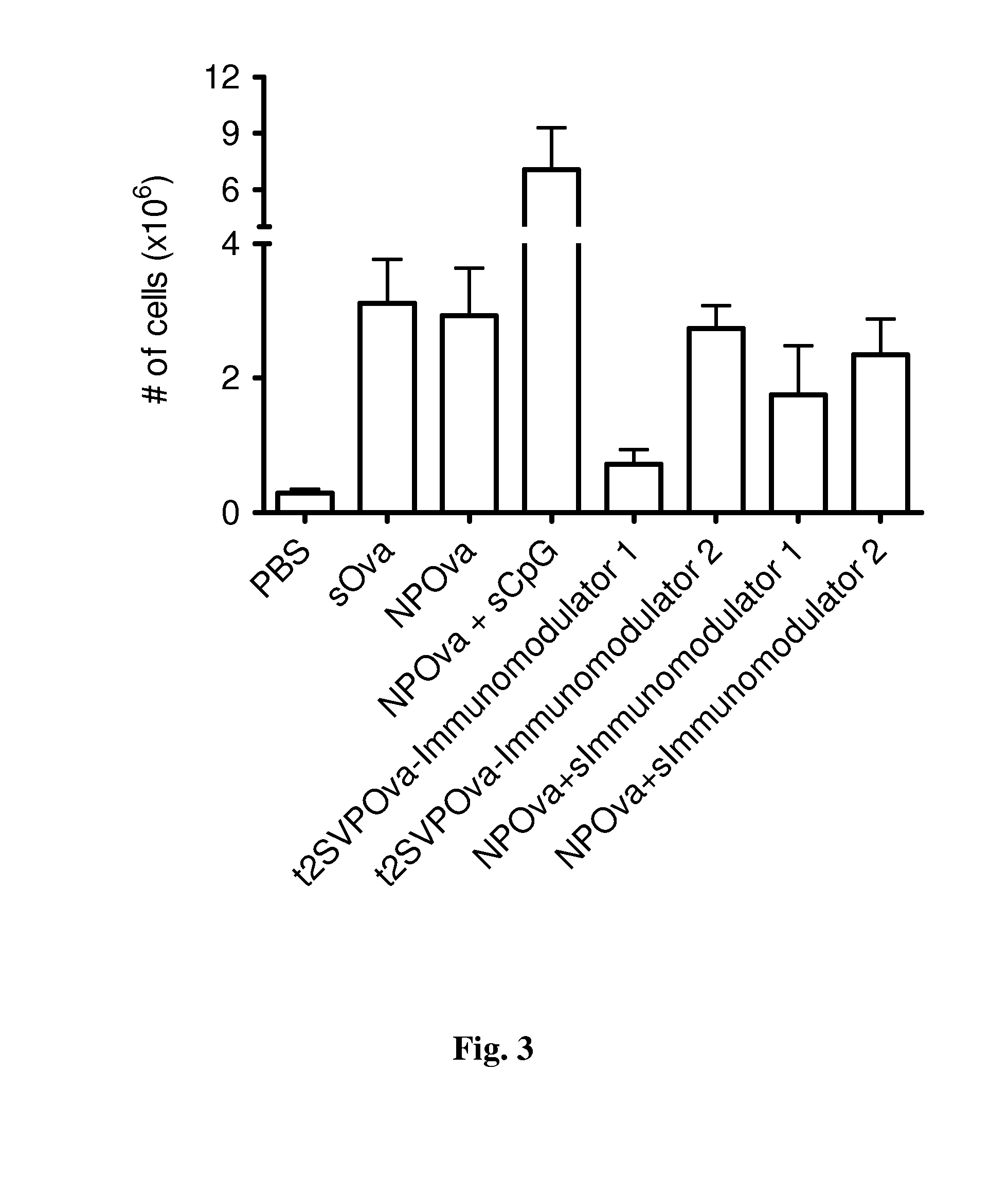
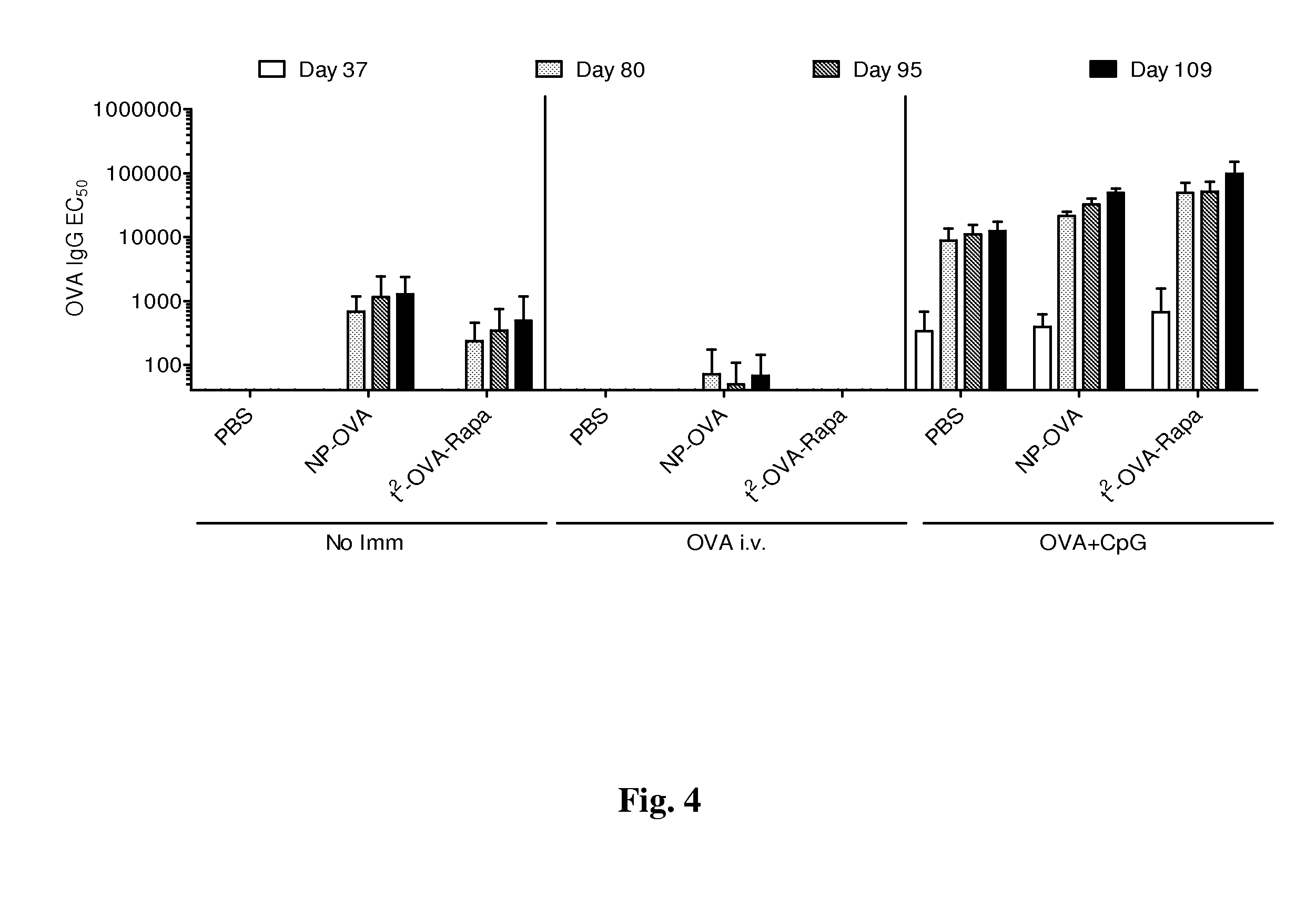
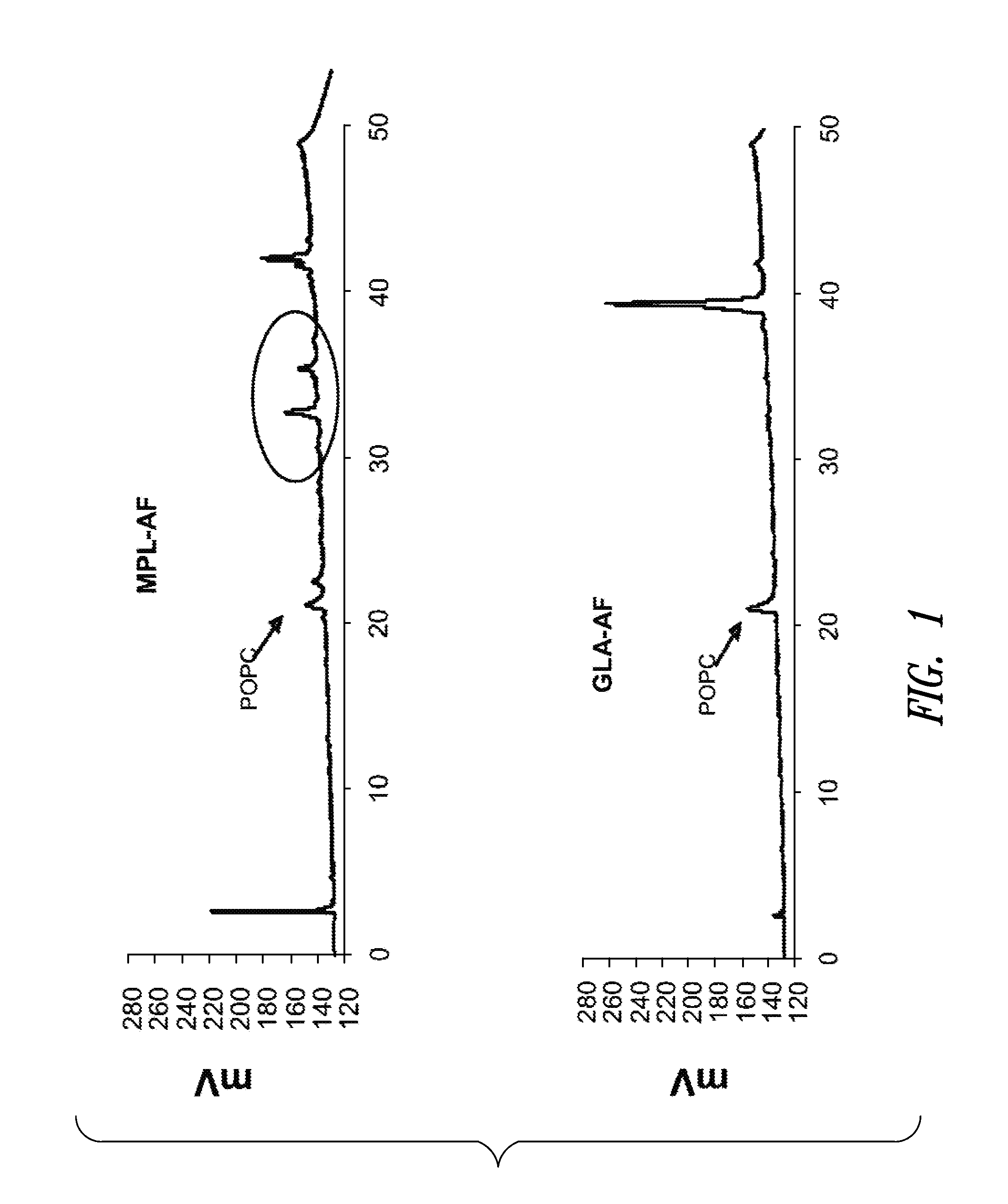
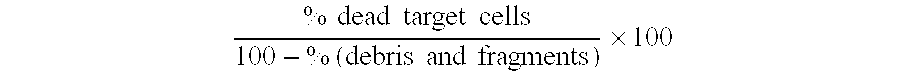Patents
Literature
526results about "Vertebrate antigen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
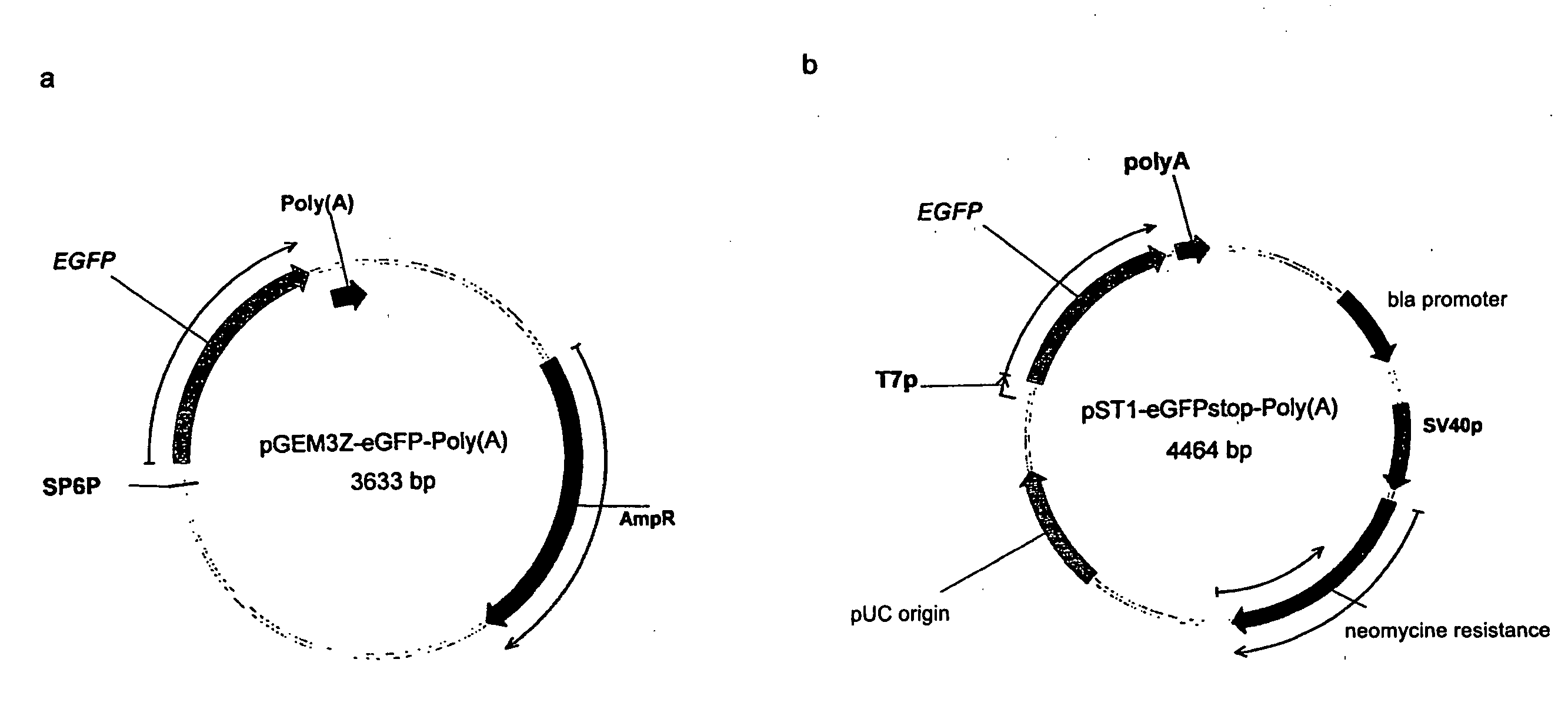
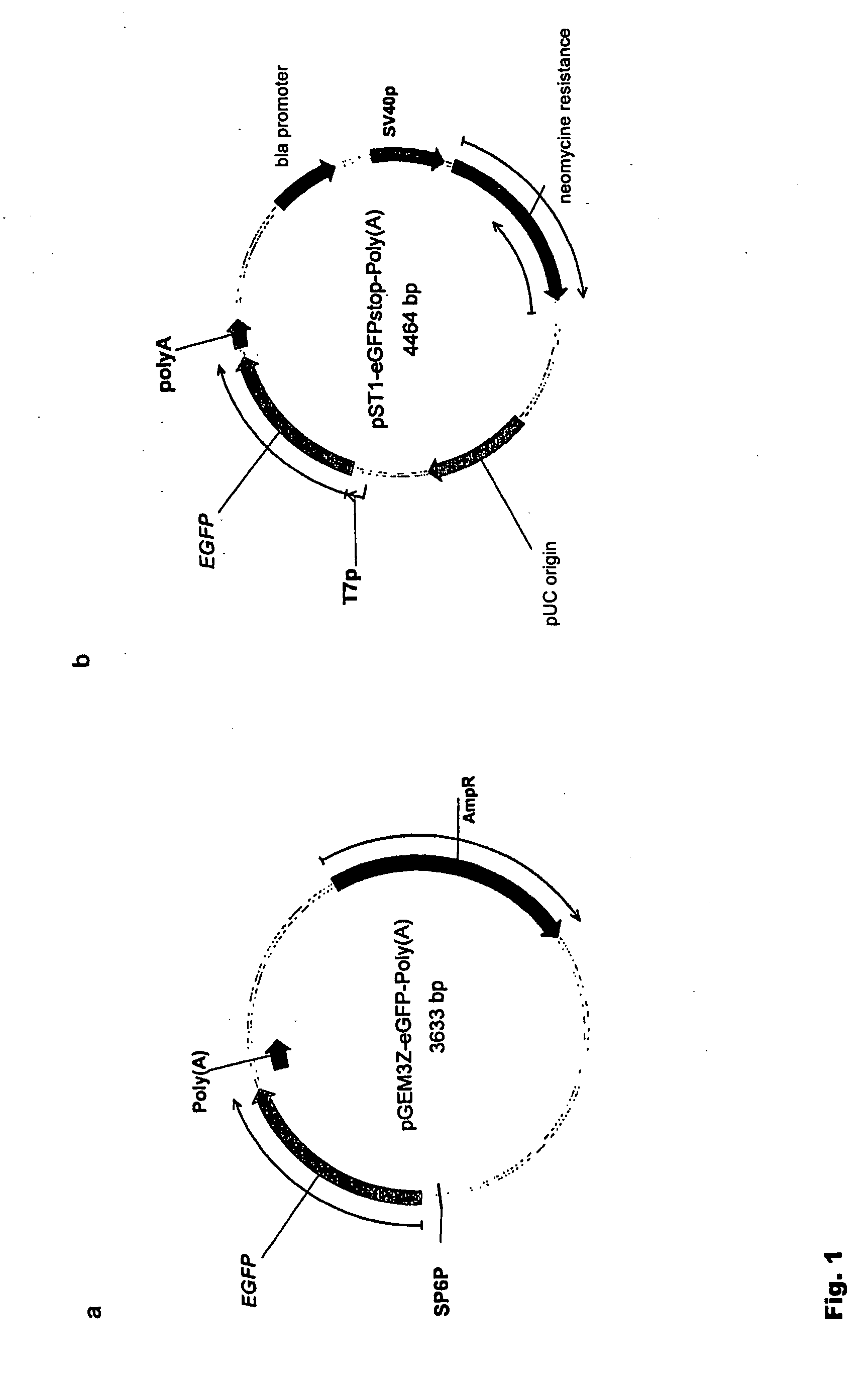
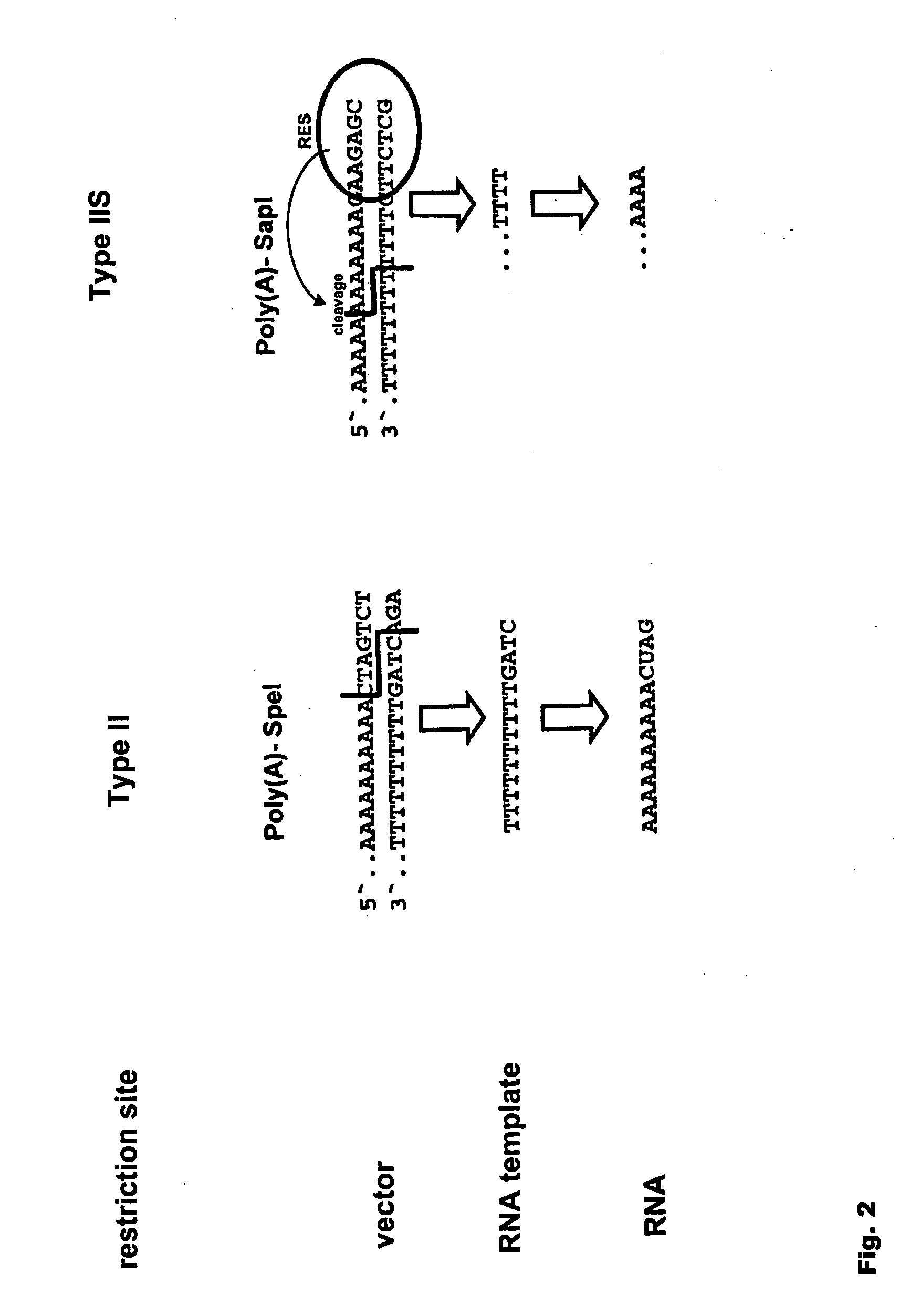
Modification of RNA, Producing an Increased Transcript Stability and Translation Efficiency
ActiveUS20100129877A1Improve stability efficiencyImprove translation efficiencySugar derivativesFermentationTranslational efficiencyCell biology
Owner:JOHANNES GUTENBERG UNIV MAINZ VERTRETEN DURCH DEN PRASIDENTEN
Nanocarriers possessing components with different rates of release
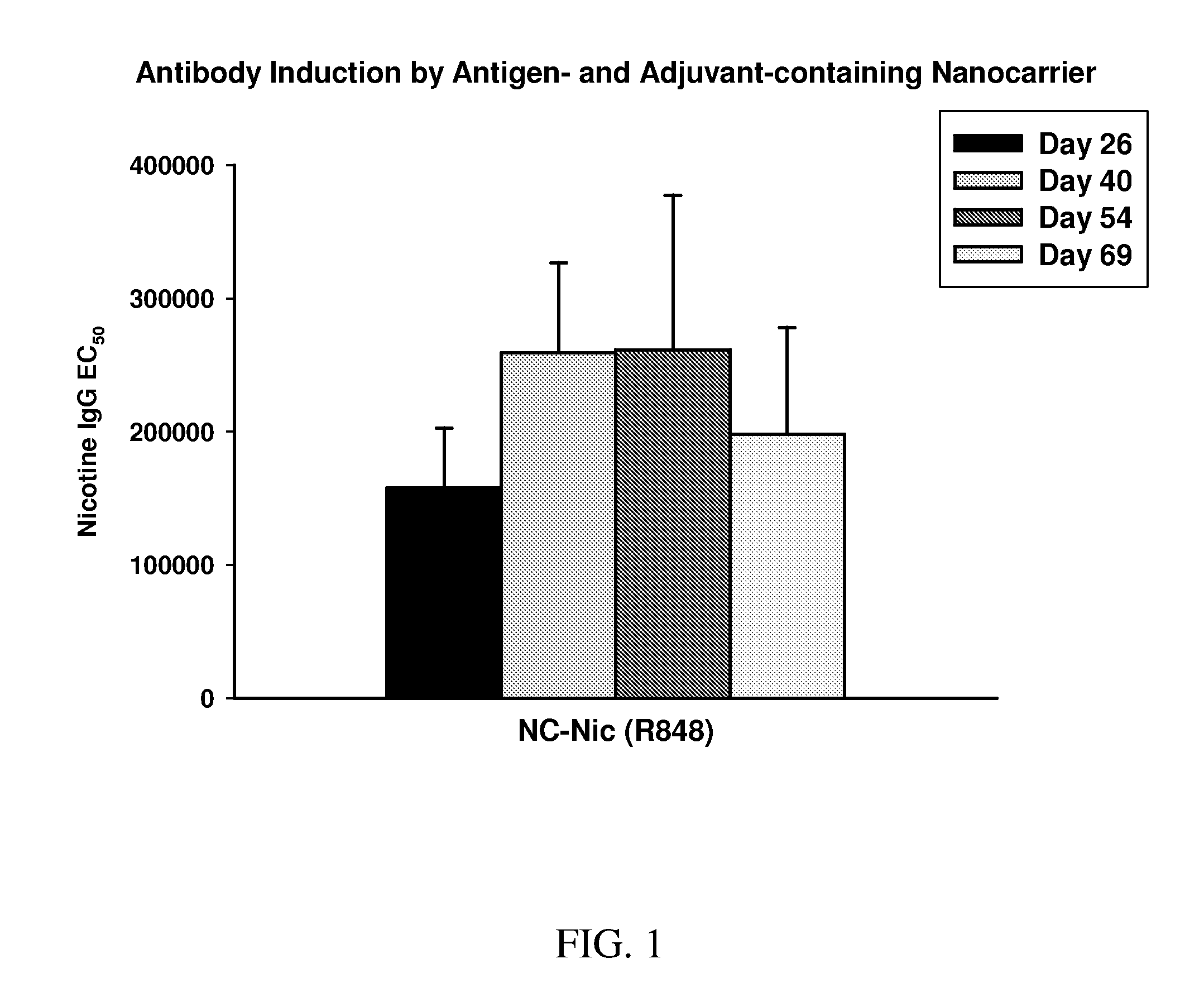
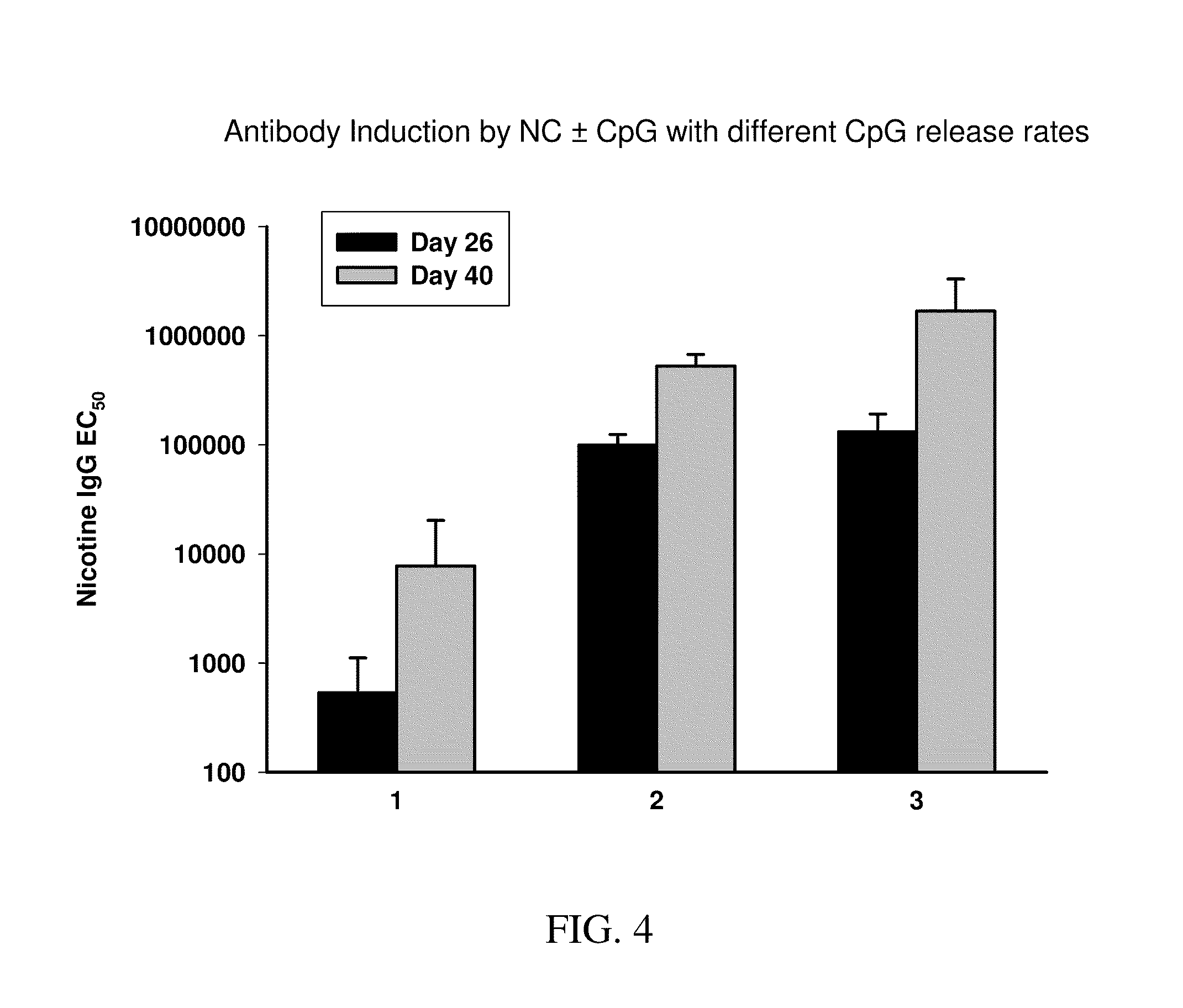
InactiveUS20100303850A1Induce and enhance immune responseStrong and long-term humoral immune responsePowder deliveryNervous disorderAntigenNanocarriers
This invention relates to compositions, and related methods, of synthetic nanocarriers that comprise immunomodulatory agents and antigens that are differentially released from the synthetic nanocarriers.
Owner:SELECTA BIOSCI
Targeted synthetic nanocarriers with ph sensitive release of immunomodulatory agents
This invention relates to compositions, and related methods, of synthetic nanocarriers that target sites of action in cells, such as antigen presenting cells (APCs), and comprise immunomodulatory agents that dissociate from the synthetic nanocarriers in a pH sensitive manner. Also disclosed are compositions and methods relating to synthetic nanocarriers that encapsulate labile immunomodulatory agents that dissociate from the synthetic nanocarriers in a pH sensitive manner.
Owner:SELECTA BIOSCI
Compositions capable of specifically binding particular human antigen presenting molecule/pathogen-derived antigen complexes and uses thereof
Owner:TECHNION RES & DEV FOUND LTD
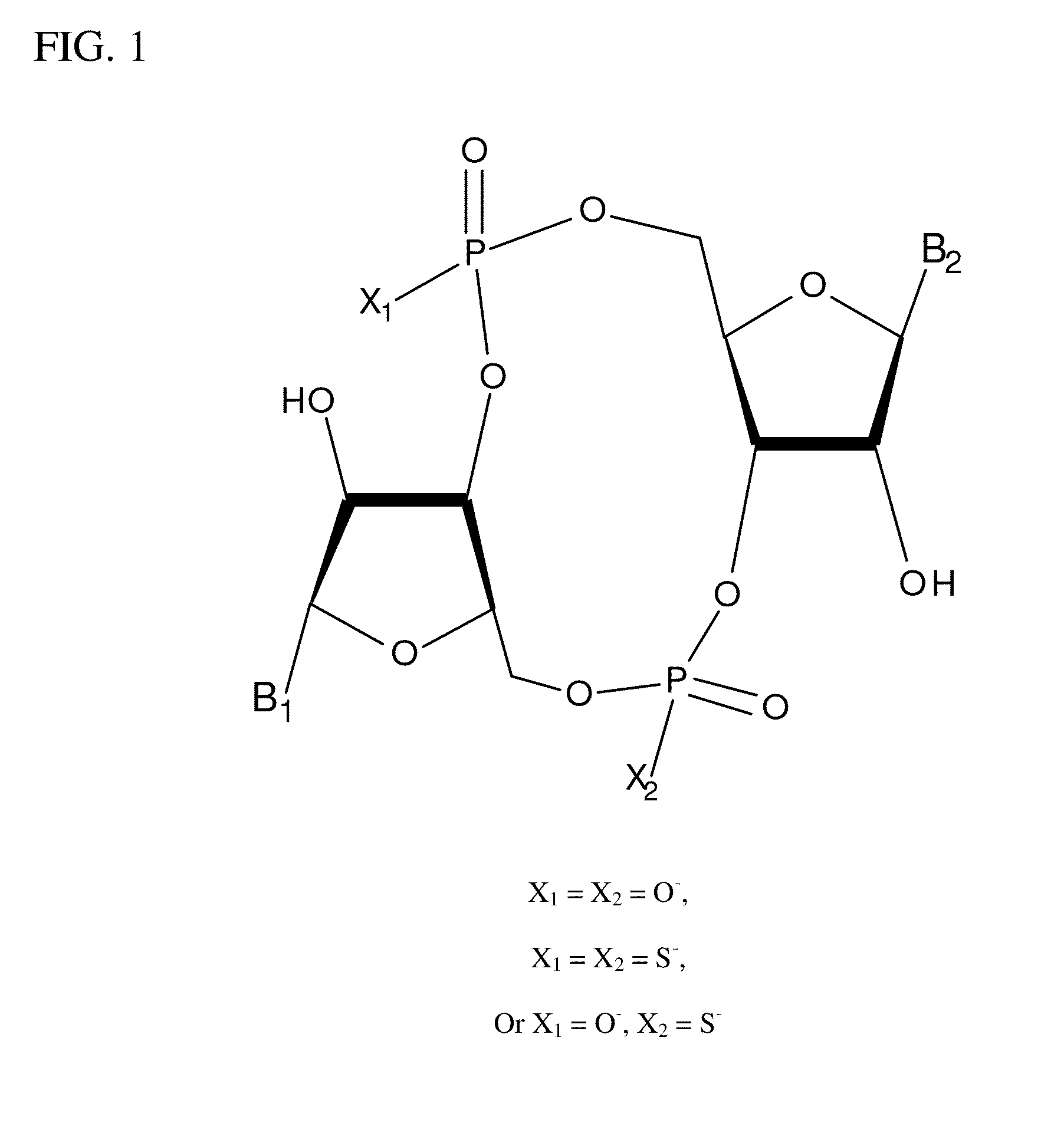
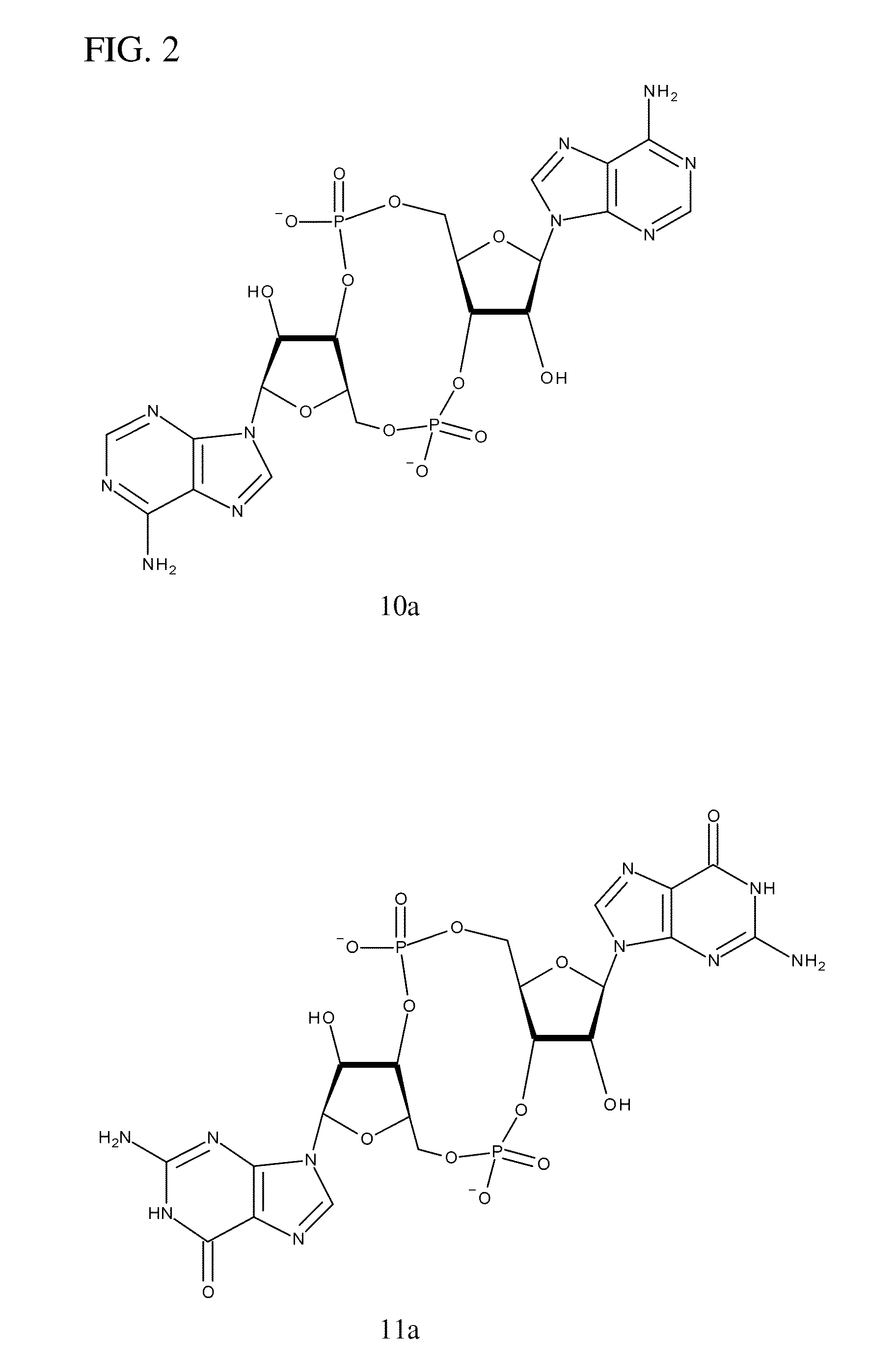
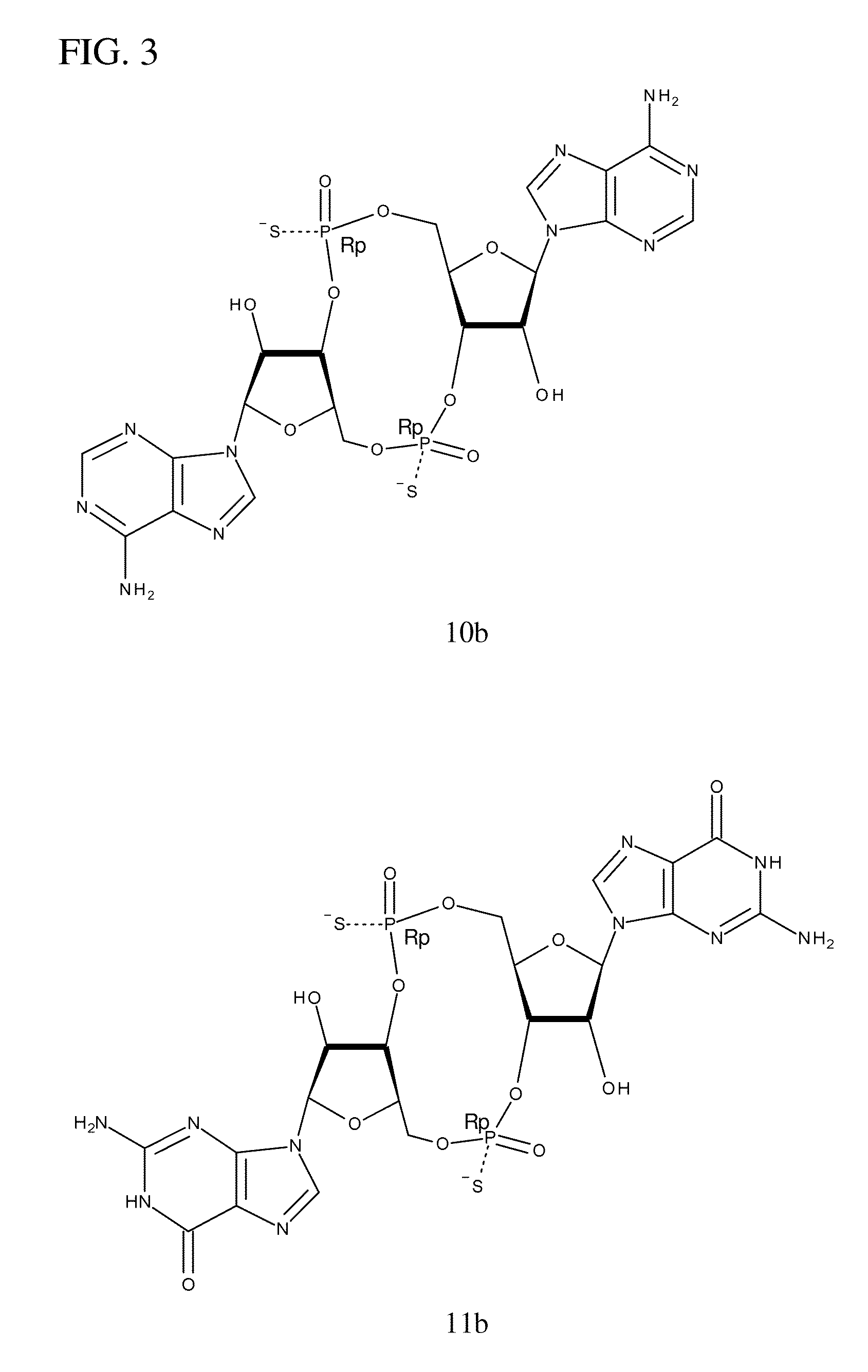
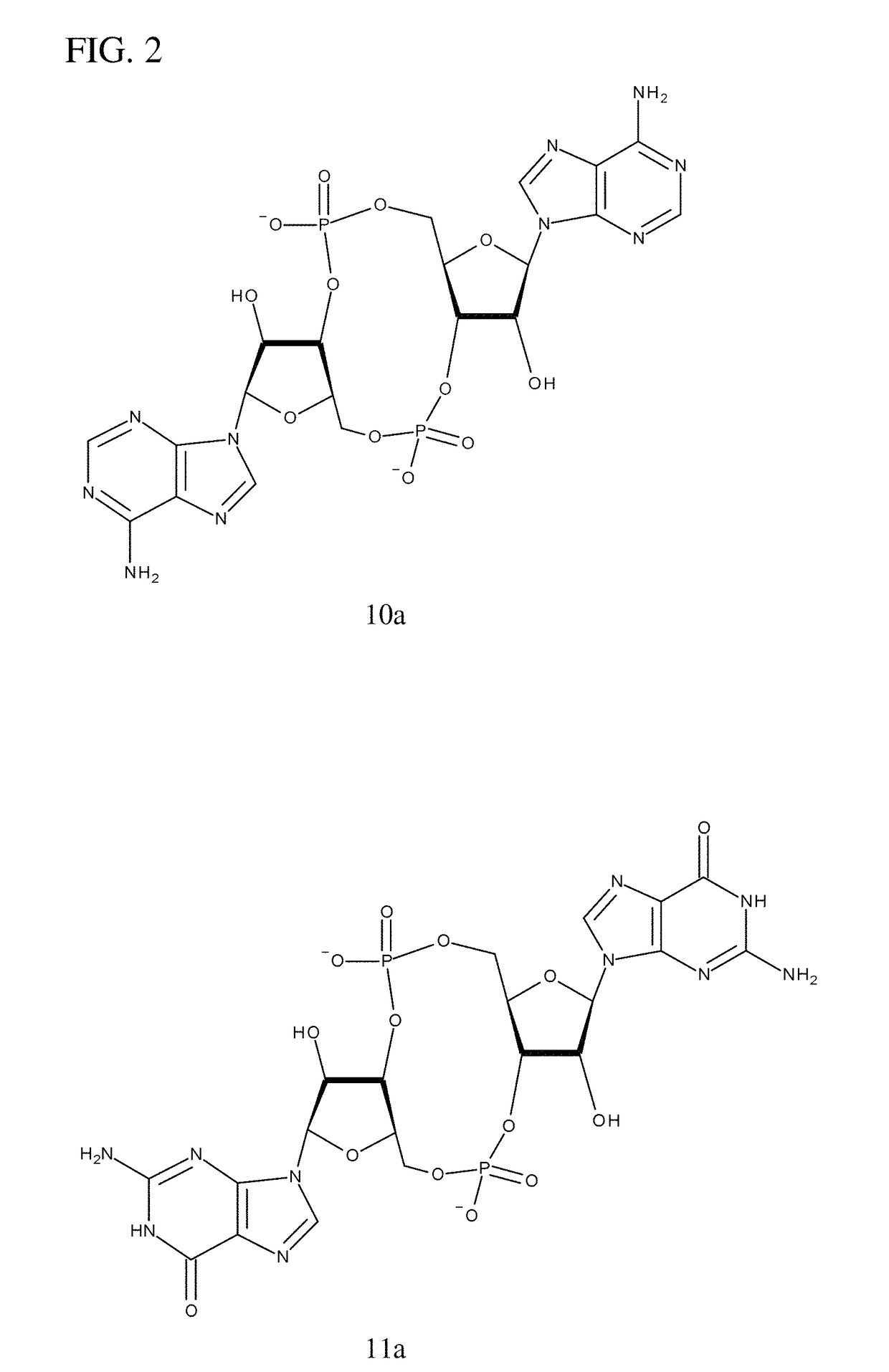
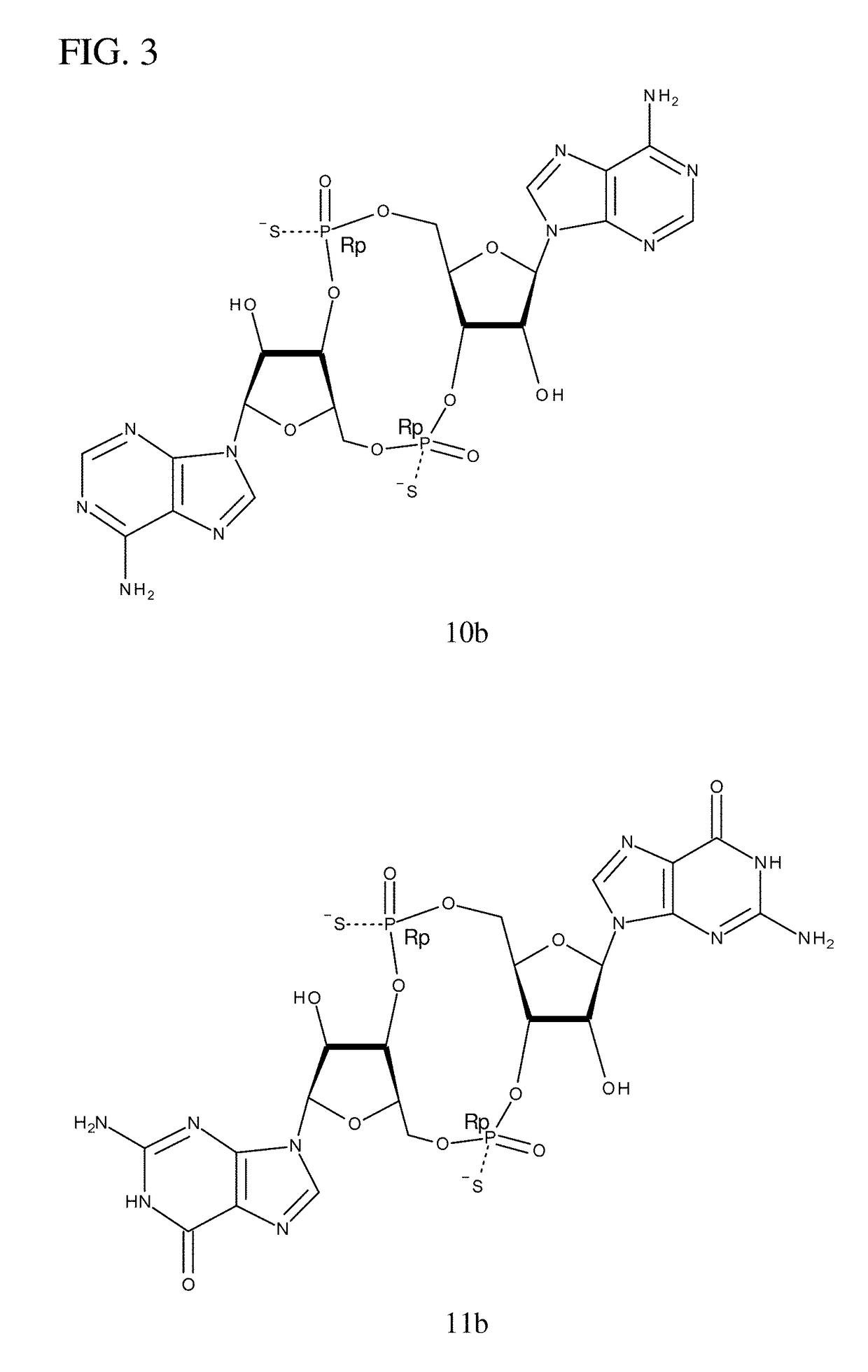
Compositions comprising cyclic purine dinucleotides having defined stereochemistries and methods for their preparation and use
ActiveUS20140205653A1Suppress overreactionOrganic active ingredientsBacterial antigen ingredientsEnantiomerPurine
It is an object of the present invention to provide novel and highly active cyclic-di-nucleotide (CDN) immune stimulators that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides that induce STING-dependent TBK1 activation, wherein the cyclic purine dinuclotides present in the composition are substantially pure Rp,Rp or Rp,Sp stereoisomers, and particularly substantially pure Rp,Rp, or RpSp CDN thiophosphate diastereomers.
Owner:CHINOOK THERAPEUTICS INC
Vaccine composition containing synthetic adjuvant
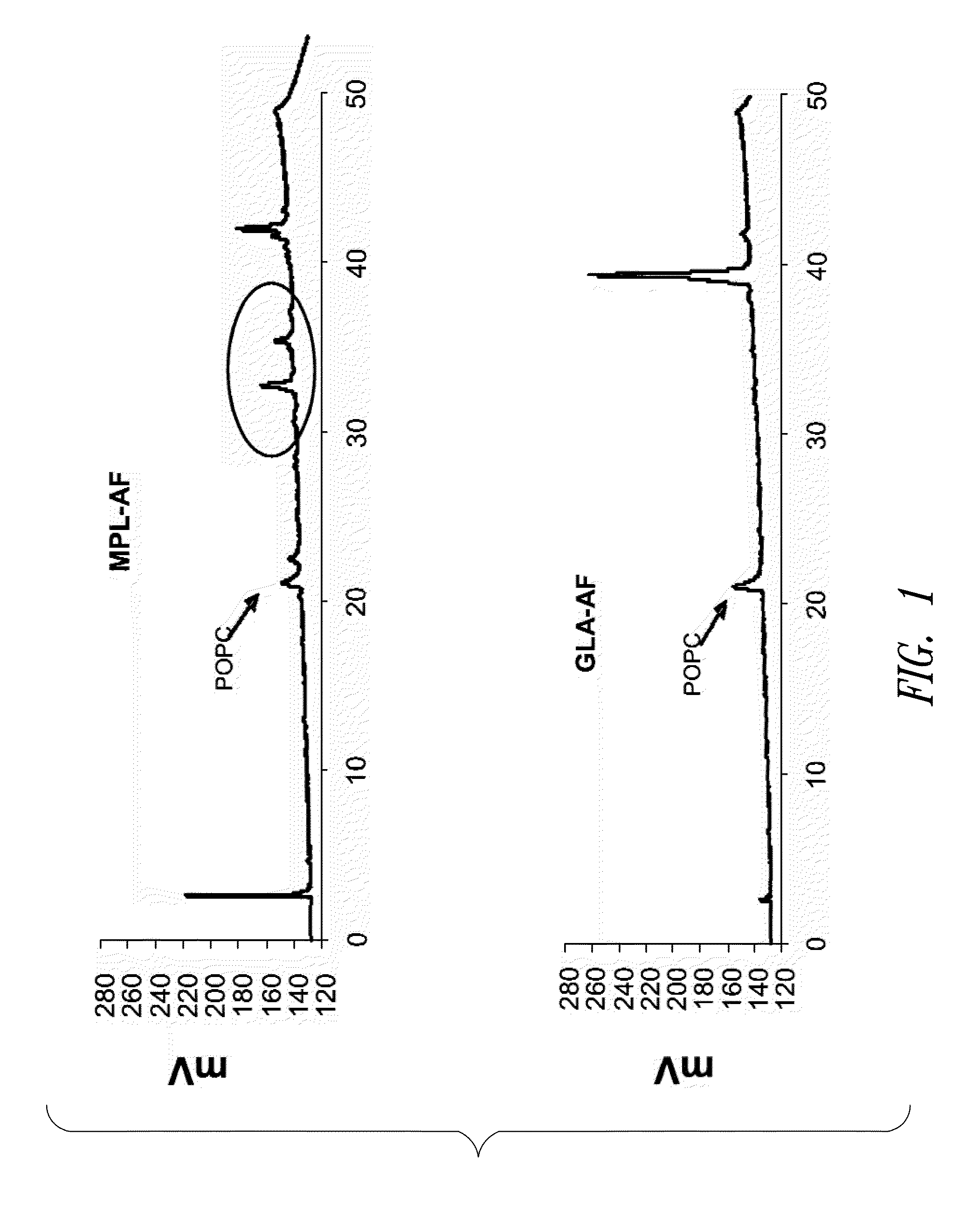
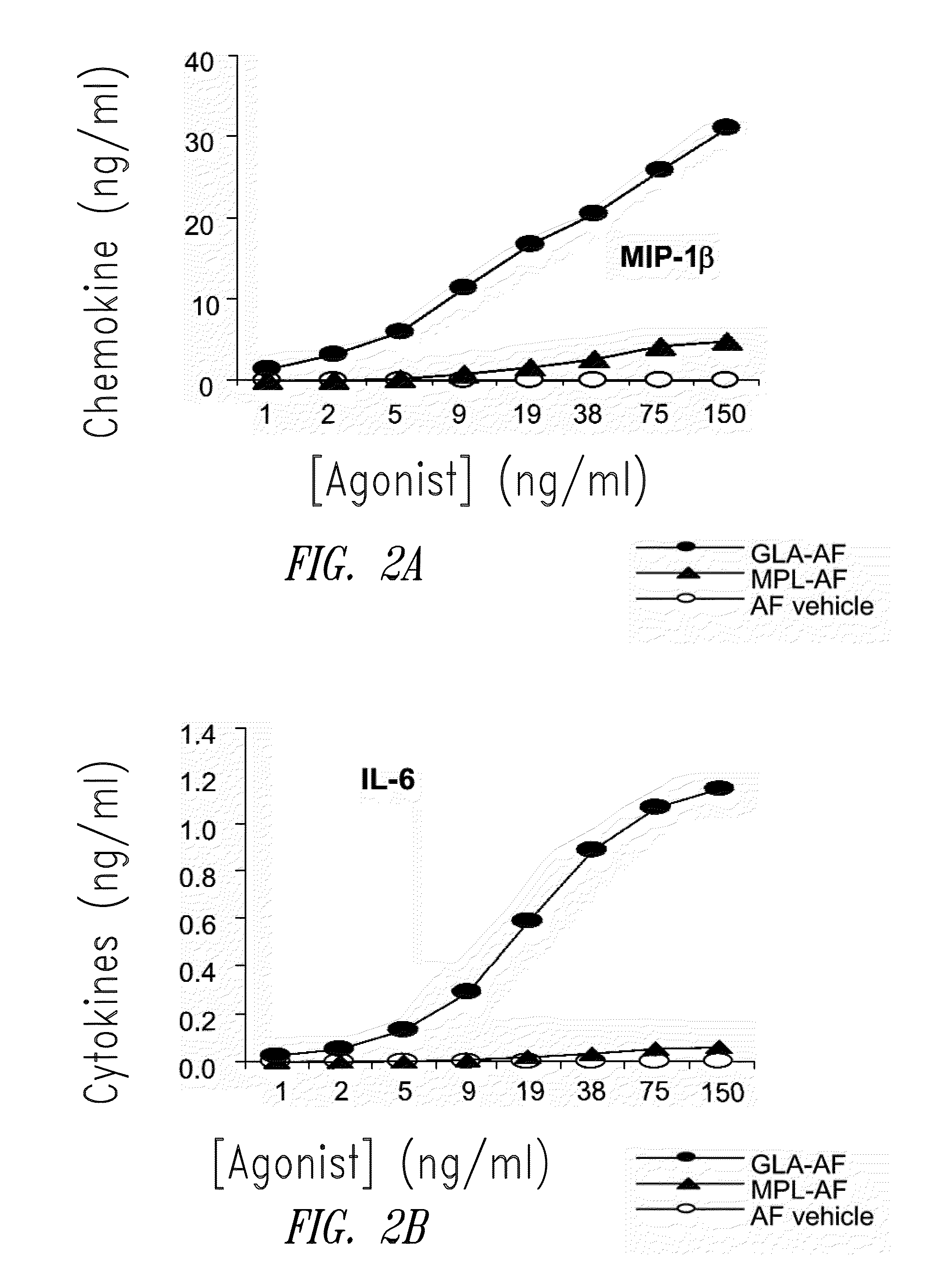
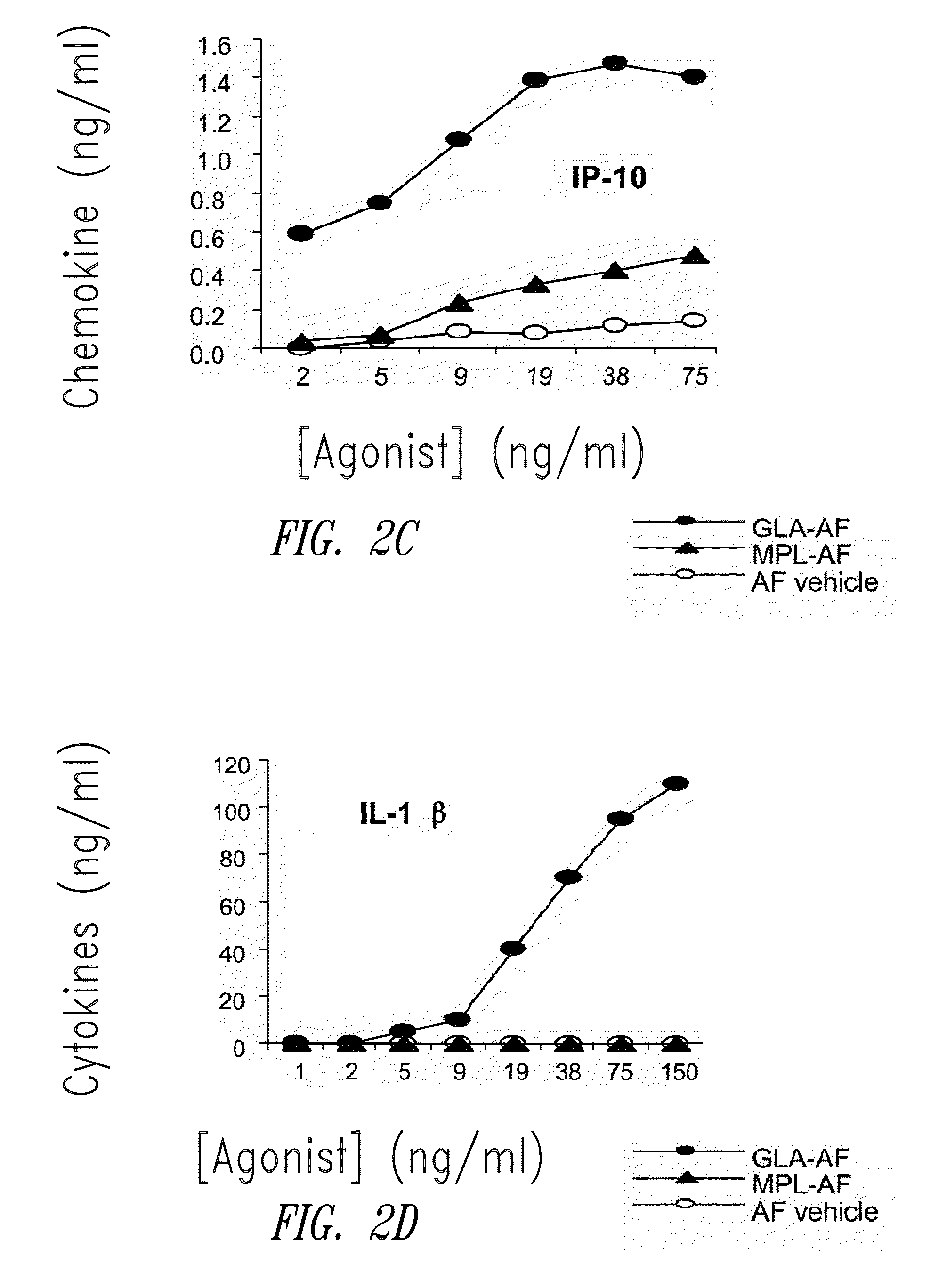
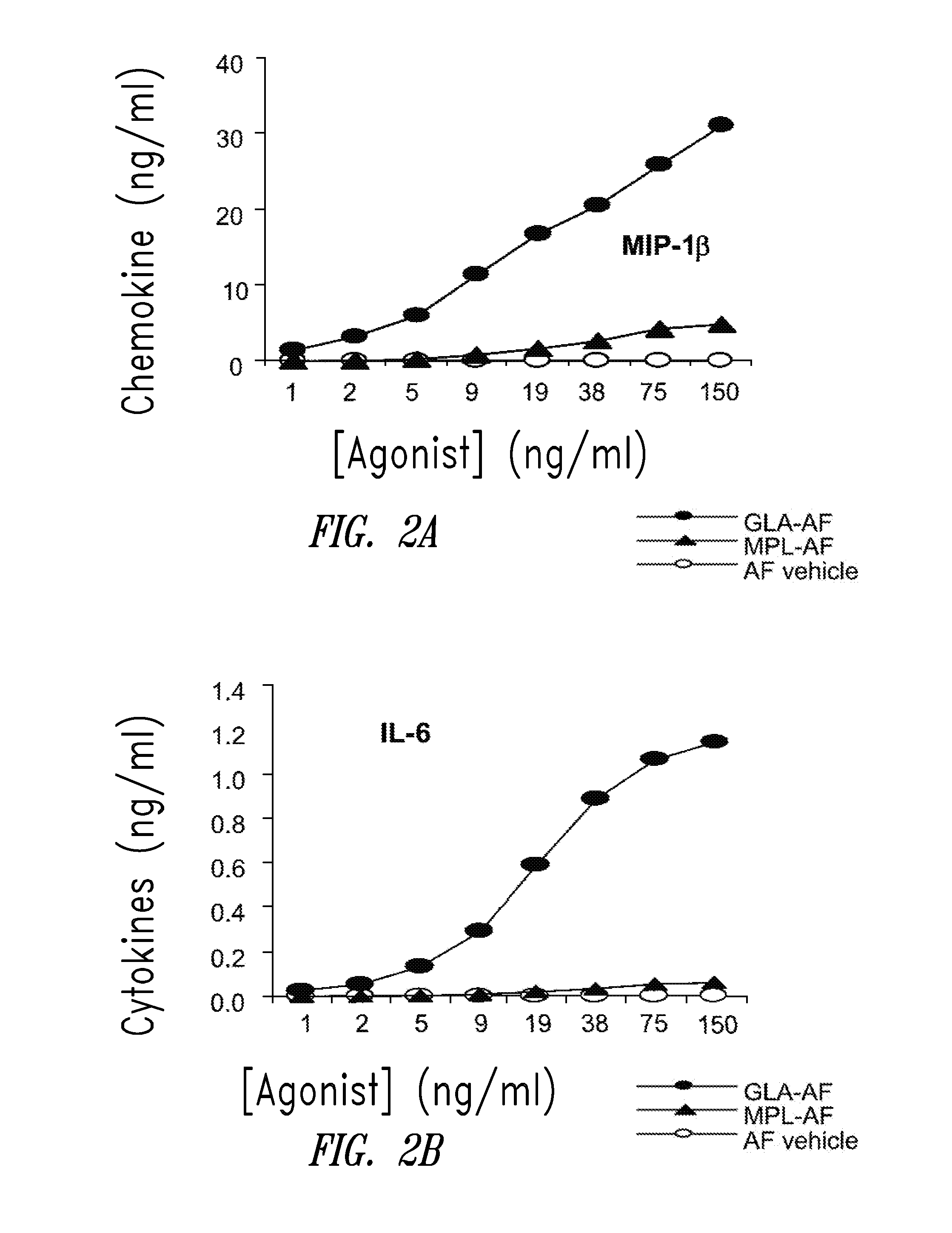
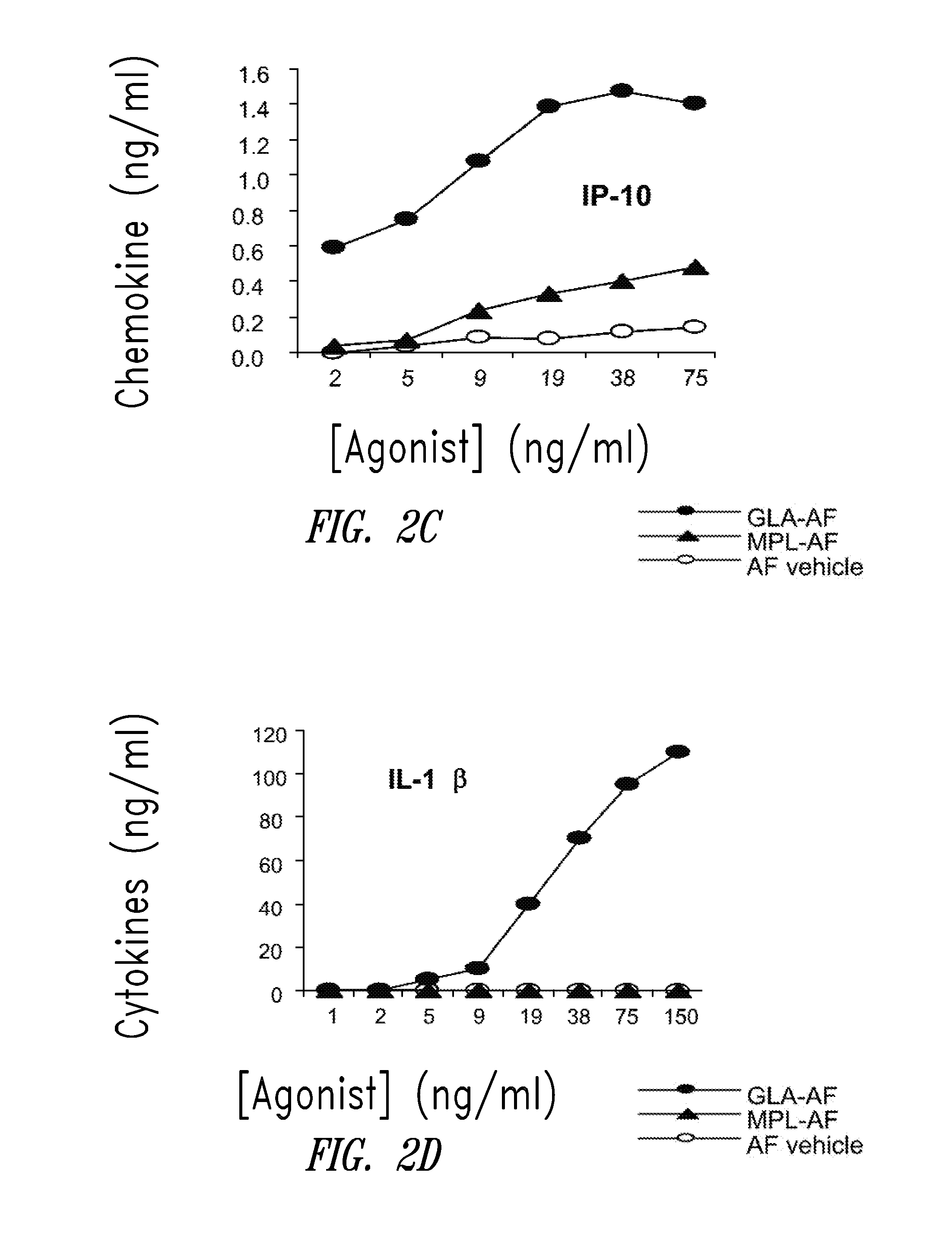
InactiveUS20090181078A1Promote maturitySsRNA viruses negative-senseVertebrate antigen ingredientsNatural productPharmaceutical drug
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:INFECTIOUS DISEASE RES INST
Compositions for allogeneic cell therapy
ActiveUS7435592B2Strong upregulationBiocideVertebrate antigen ingredientsAbnormal tissue growthAllogeneic cell
A method of manipulating allogeneic cells for use in allogeneic cell therapy protocols is described. The method provides a composition of highly activated allogeneic T-cells which are infused into immunocompetent cancer patients to elicit a novel anti-tumor immune mechanism called the “Mirror Effect”. In contrast to current allogeneic cell therapy protocols where T-cells in the graft mediate the beneficial graft vs. tumor (GVT) and detrimental graft vs. host (GVH) effects, the allogeneic cells of the present invention stimulate host T-cells to mediate the “mirror” of these effects. The mirror of the GVT effect is the host vs. tumor (HVT) effect. The “mirror” of the GVH effect is the host vs. graft (HVG) effect. The effectiveness and widespread application of the anti-tumor GVT effect is limited by the severe toxicity of the GVH effect. In the present invention, the anti-tumor HVT effect occurs in conjunction with a non-toxic HVG rejection effect. The highly activated allogeneic cells of the invention can be used to stimulate host immunity in a complete HLA mis-matched setting in patients that have not had a prior bone marrow transplant or received chemotherapy and / or radiation conditioning regimens.
Owner:MIRROR BIOLOGICS INC +1
Tolerogenic synthetic nanocarriers to reduce cytotoxic t lymphocyte responses
Disclosed are synthetic nanocarrier compositions, and related methods, comprising MHC Class I-restricted and / or MHC Class II-restricted epitopes associated with undesired CD8+ T cell responses and immunosuppressants that provide tolerogenic immune responses against antigens that comprise the epitopes.
Owner:SELECTA BIOSCI
Tolerogenic synthetic nanocarriers for antigen-specific deletion of t effector cells
Disclosed are synthetic nanocarrier methods, and related compositions, comprising administering immunosuppressants and MHC Class I-restricted and / or MHC Class II-restricted epitopes that can generate tolerogenic immune responses (e.g., antigen-specific T effector cell deletion).
Owner:SELECTA BIOSCI
Compositions that induce t cell help
The present invention relates, at least in part, to compositions, and related methods, comprising MHC II binding peptides. In one embodiment, the MHC II binding peptides comprise a peptide having at least 70% identity to a natural HLA-DP binding peptide, HLA-DQ binding peptide, or HLA-DR binding peptide.
Owner:SELECTA BIOSCI
Oral pharmaceutical composition
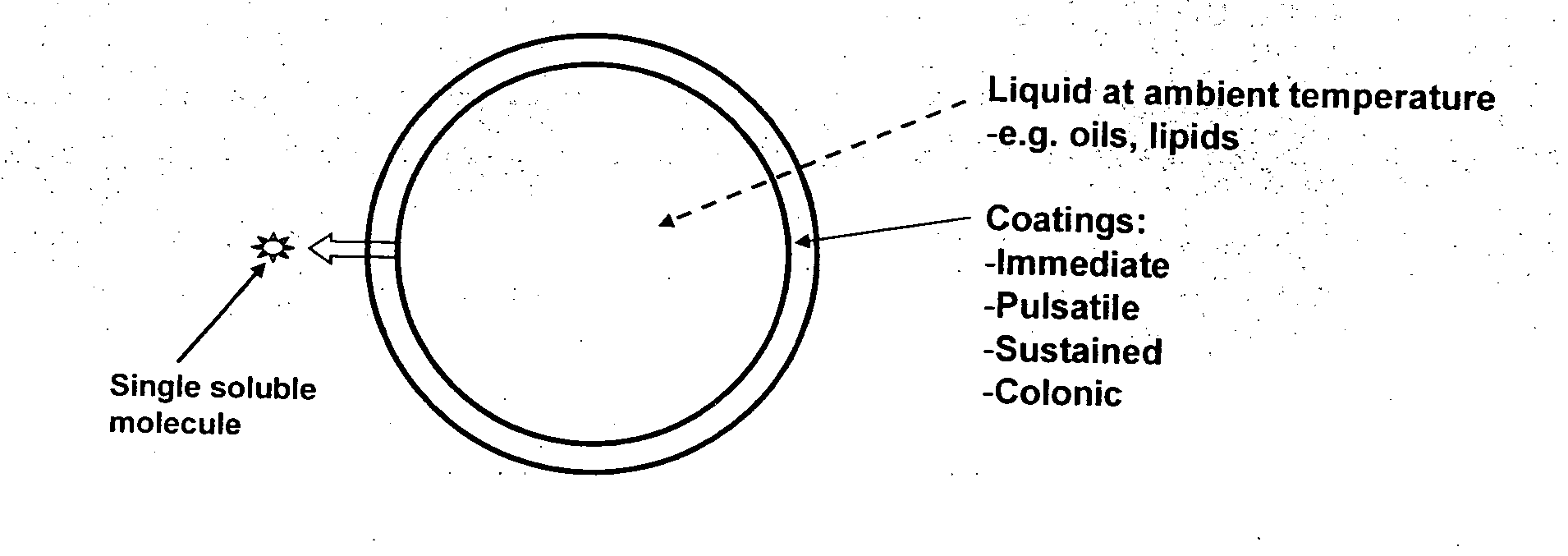
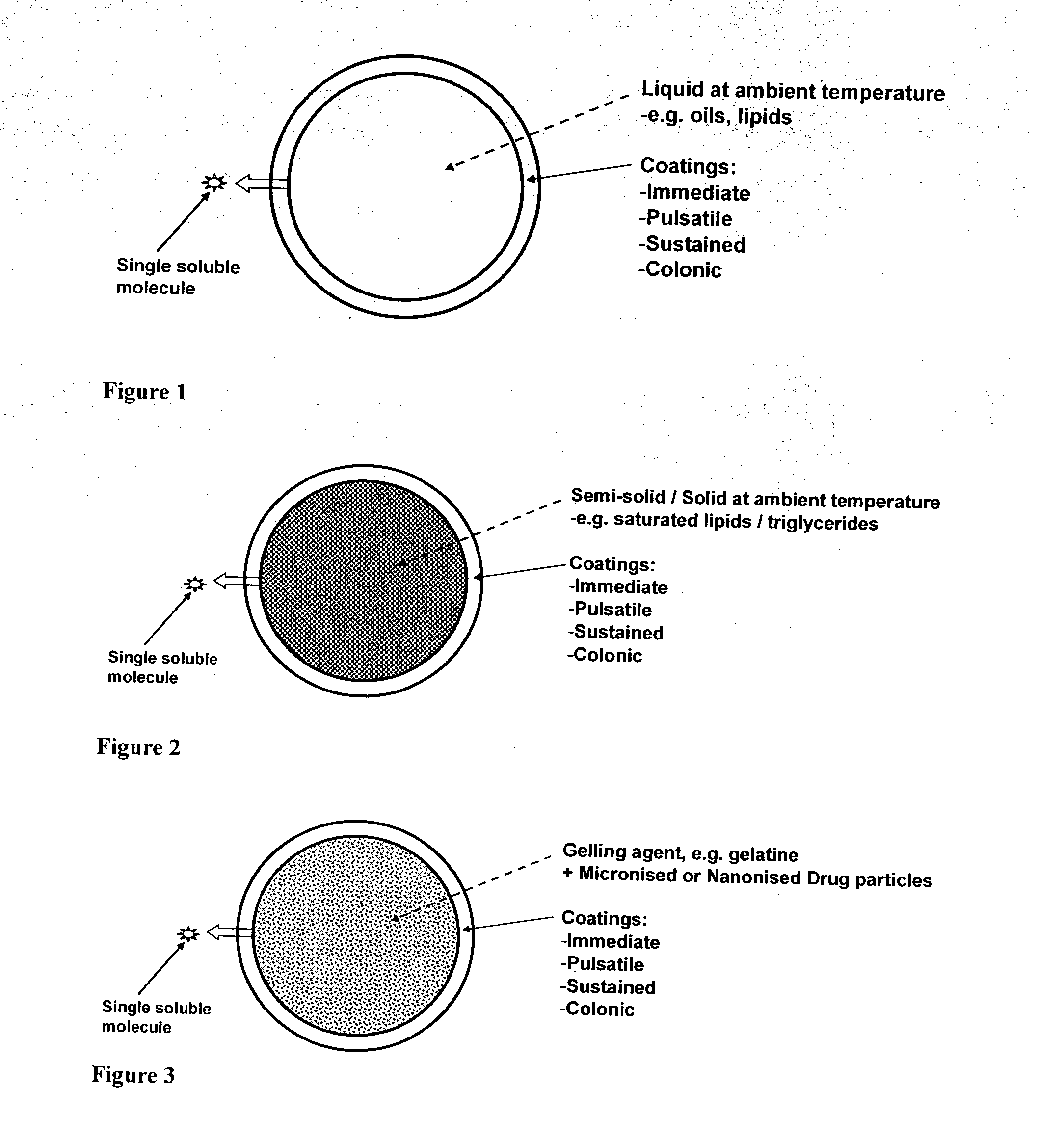
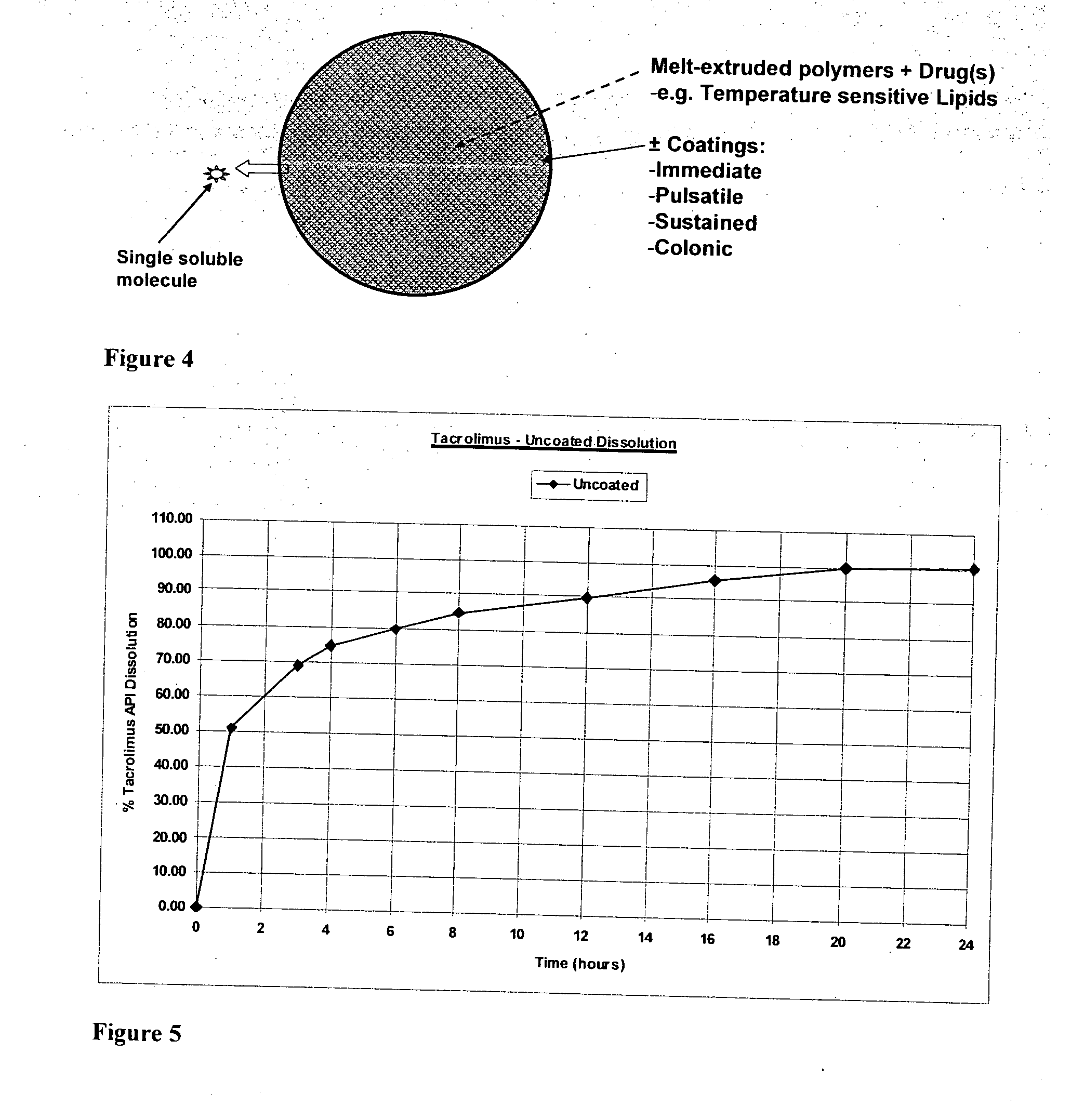
ActiveUS20100255087A1Improve solubilityImprove breathabilityOrganic active ingredientsNervous disorderSolid coreGastrointestinal tract
Owner:SUBLIMITY THERAPEUTICS LTD
Tolerogenic synthetic nanocarriers for regulating innate immune responses
InactiveUS20120276160A1Suppressing antigen-specific activationReduce in quantityOrganic active ingredientsPowder deliveryB cellAntigen specific
Disclosed are synthetic nanocarrier methods, and related compositions, comprising administering B cell and / or MHC Class II-restricted epitopes of an antigen and immunosuppressants in order to reduce antigen-specific activation of innate immune cells.
Owner:SELECTA BIOSCI
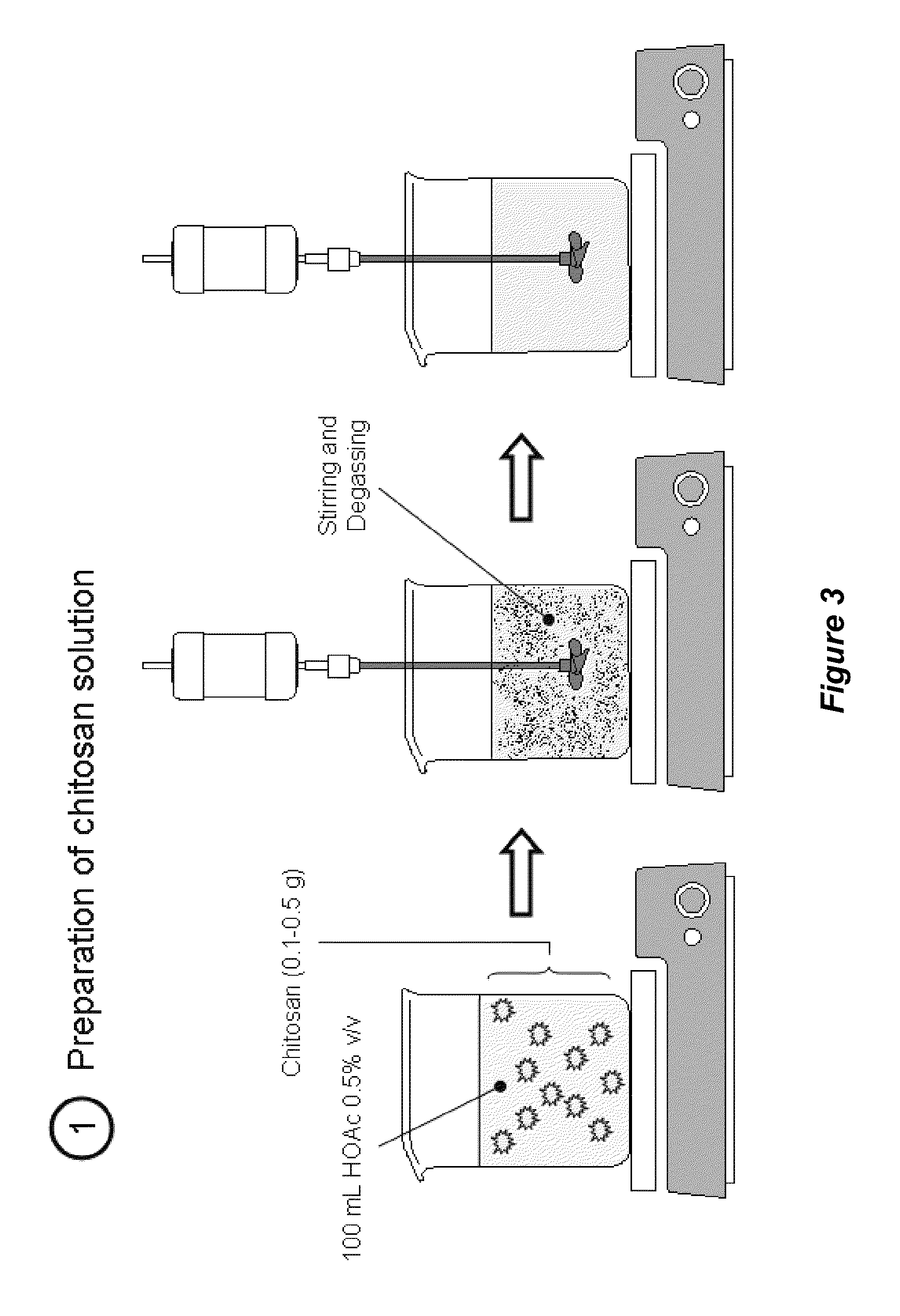
Tannin-chitosan composites
ActiveUS20110059162A1Improve mechanical propertiesImprove efficiencyAntibacterial agentsOrganic active ingredientsAdjuvantHydrogel film
Owner:WISCONSIN ALUMNI RES FOUND
Non-Natural Amino Acid Polypeptides Having Modified Immunogenicity
InactiveUS20090093405A1Low immunogenicityImproving immunogenicityPeptide/protein ingredientsTransferasesVaccine ImmunogenicityAmino acid
Non-naturally encoded amino acid polypeptides with modulated immunogenicity and uses thereof are provided.
Owner:AMBRX
Use of passive myostatin immunization
InactiveUS20020127234A1Increase in breast muscle and thigh muscle and testis and heart weightPromote growth ratePeptide/protein ingredientsVertebrate antigen ingredientsMyostatinBiomedical engineering
A method to alter the phenotype of animals, e.g., avians, which employs passive and active immunization is provided.
Owner:RGT UNIV OF MINNESOTA
Tolerogenic synthetic nanocarrier compositions with transplantable graft antigens and methods of use
InactiveUS20120276156A1Eliminate side effectsReduce generationAntipyreticAnalgesicsNanocarriersPlant Antigens
Disclosed are synthetic nanocarrier compositions, and related methods, comprising APC presentable transplant antigens and immunosuppressants that provide tolerogenic immune responses (e.g., a reduction in CD8+ T cell proliferation and / or activity) specific to the APC presentable transplant antigens.
Owner:SELECTA BIOSCI
Intradermal cellular delivery using narrow gauge micro-cannula
A method for delivering cells into a subject by administering cells into the intradermal space of the skin of the subject by a microneedle. The cells are associated with cellular based therapeutics and vaccines and delivered by perpendicular insertion of the microneedle. The microneedle is a hollow needle having an exposed height of between about 0 and 1 mm, a total length of between about 0.3 mm to 2.5 mm, and a size of equal to or less than 30 gauge. An array of microneedles can also be used.
Owner:BECTON DICKINSON & CO
Compositions comprising cyclic purine dinucleotides having defined stereochemistries and methods for their preparation and use
ActiveUS9695212B2Suppress overreactionOrganic active ingredientsBacterial antigen ingredientsEnantiomerPurine
It is an object of the present invention to provide novel and highly active cyclic-di-nucleotide (CDN) immune stimulators that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides that induce STING-dependent TBK1 activation, wherein the cyclic purine dinuclotides present in the composition are substantially pure Rp,Rp or Rp,Sp stereoisomers, and particularly substantially pure Rp,Rp, or RpSp CDN thiophosphate diastereomers.
Owner:CHINOOK THERAPEUTICS INC
Tannin-chitosan composites
ActiveUS8642088B2Improve mechanical propertiesImprove efficiencyAntibacterial agentsPowder deliveryAdjuvantHydrogel film
The invention provides a composition comprising a matrix of chitosan and a tannin wherein the chitosan is electrostatically bonded to the tannin to form a chitosan-tannin composite material. The chitosan can be partially or fully deacetylated, and the tannin can be a monomeric or an oligomeric proanthocyanidin or a hydrolysable tannin. The chitosan-tannin composite material can be a nanoparticle, a hydrogel film, a bio-foam, or a biogel, or the chitosan-tannin composite material can coat a liposome. The composite materials can be used for drug delivery, for antibacterial and / or antifungal applications, for tissue engineering applications, for wound healing applications, or they can be used as adjuvants for vaccination, including oral vaccinations. The invention also provides methods of preparing the composite materials and their various forms.
Owner:WISCONSIN ALUMNI RES FOUND
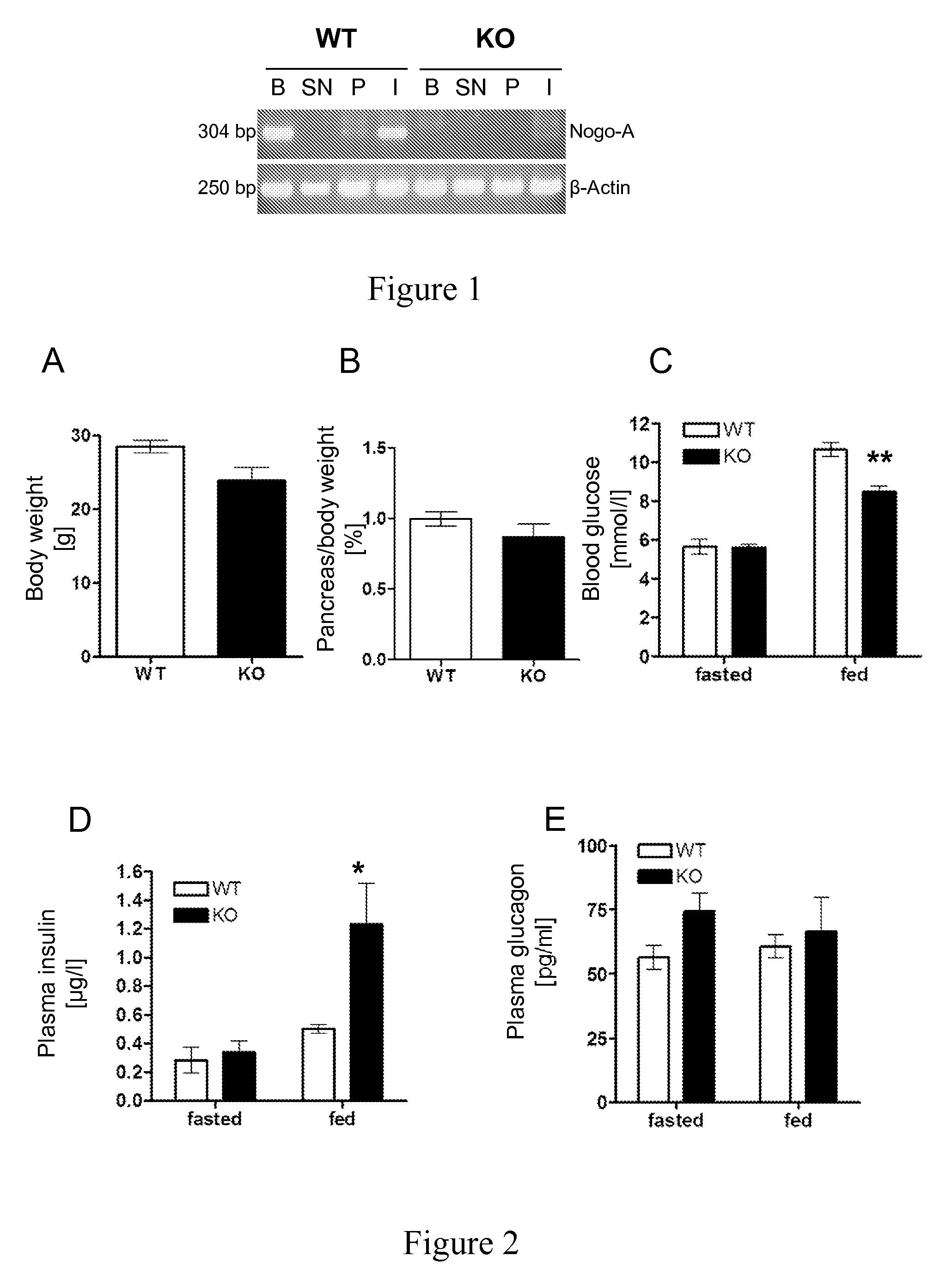
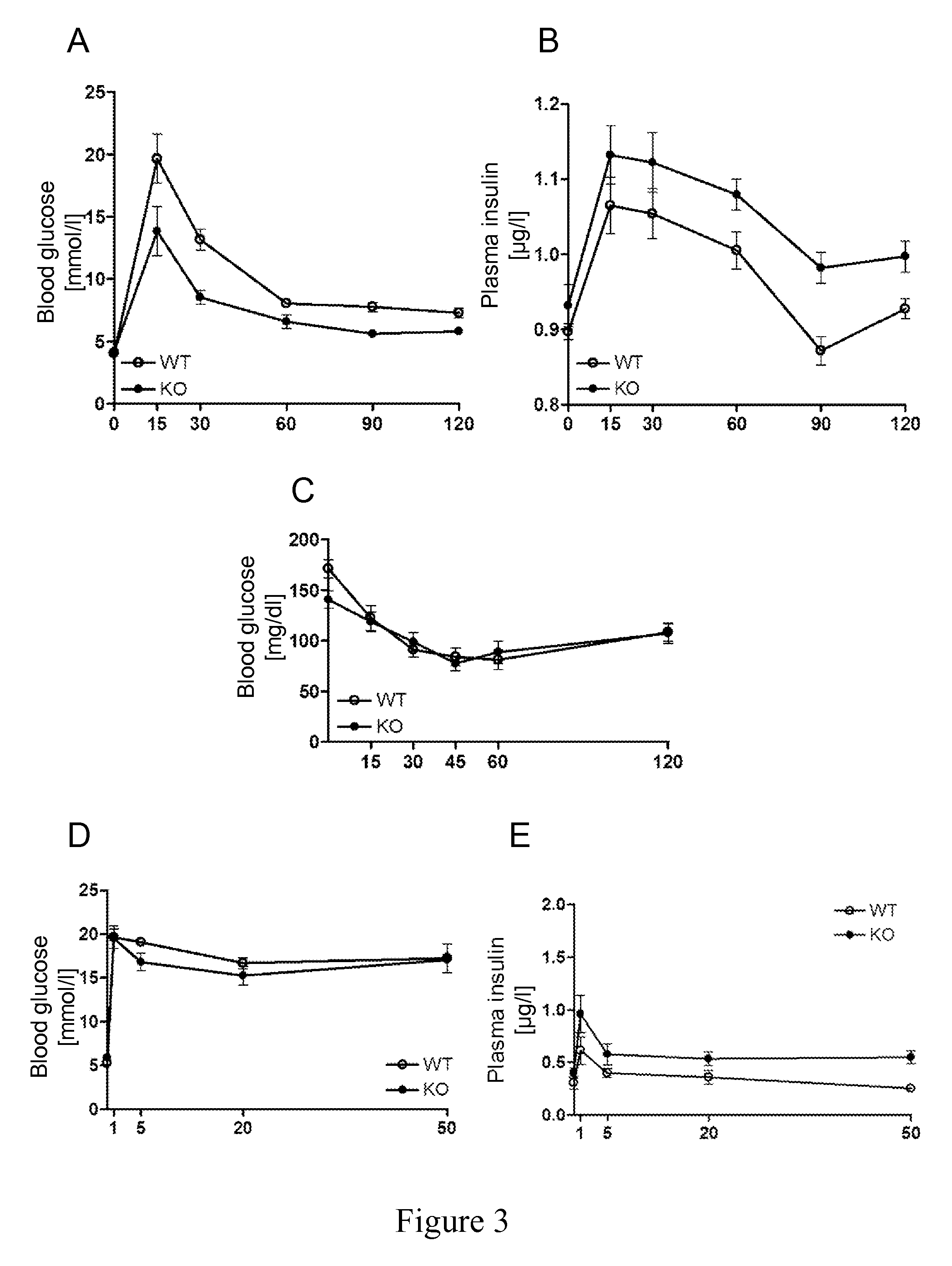
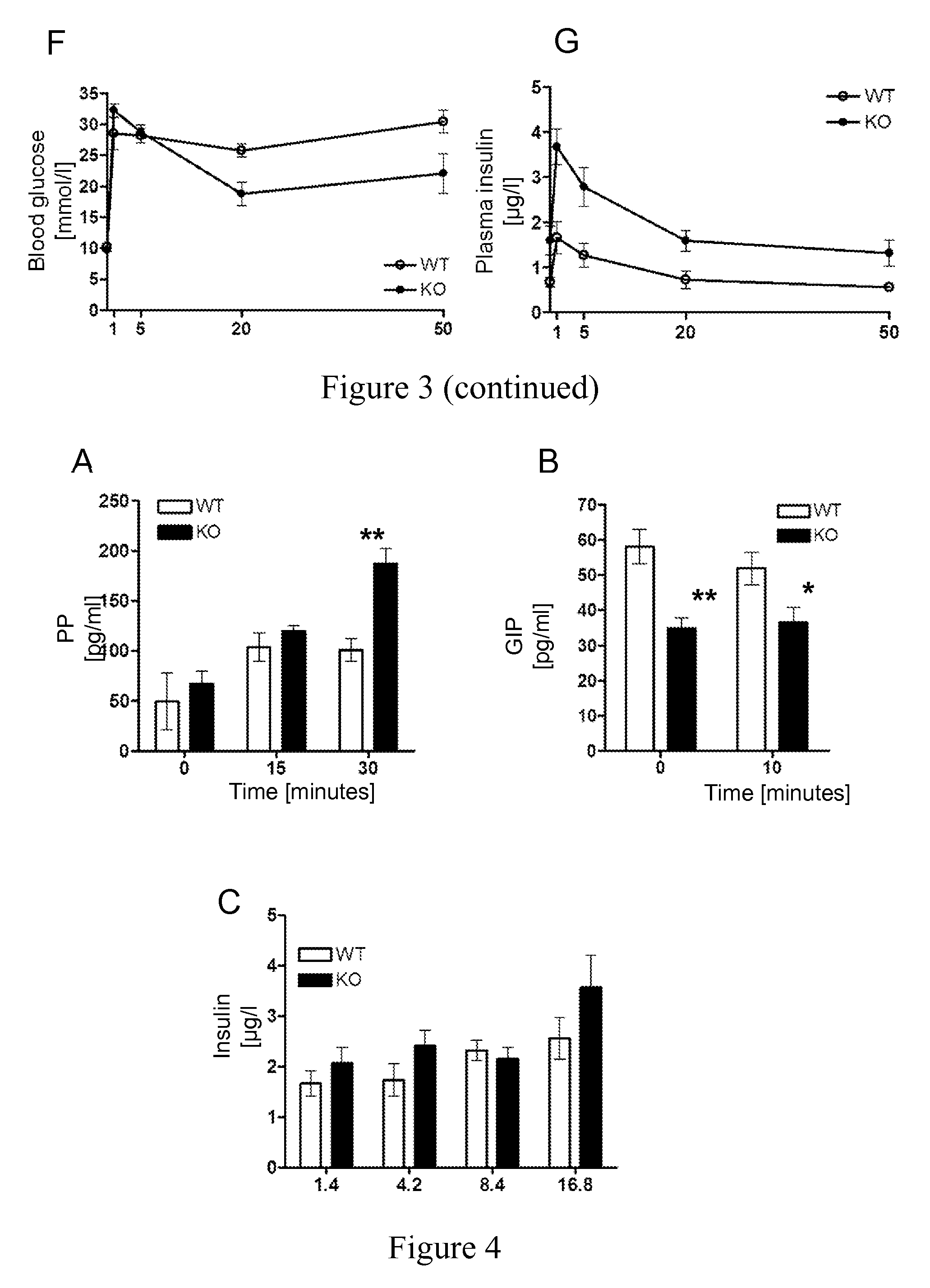
Uses of NOGO-A inhibitors and related methods
InactiveUS8828390B2High sensitivityIncrease secretionMetabolism disorderSnake antigen ingredientsBlood insulinD-Glucose
The present invention is directed to Nogo-A antagonists useful for the control of blood glucose or blood insulin levels in a subject and related use and formulation thereof. In particular, the invention is directed to Nogo-A antagonists useful for the prevention, repression or treatment insulin secretion deficiency and related methods and pharmaceutical formulations. In particular, the invention relates to Nogo-A antagonists useful in the treatment of diabetes mellitus.
Owner:UNIV ZURICH +1
Depletion of endogenous primordial germ cells in avian species
InactiveUS20060095980A1Decrease in primordial germ cell numberIncrease in primordial germ cell numberVertebrate antigen ingredientsFermentationAntigenHigh concentration
Methods for modulating primordial germ cell (PGC) numbers and / or development in avians are provided. In one embodiment, the presently disclosed subject matter provides a method for modulating primordial germ cells numbers in an avian embryo comprising immunizing a female bird with an antigen associated with primordial germ cells, whereby an egg produced by the female bird comprises a sufficiently high concentration of antibodies specific for the antigen to modulate numbers of endogenous PGCs in an avian embryo present within in the egg. Also provided are methods for producing chimeric avians, methods for increasing the proportion of male birds in a plurality of eggs, methods of producing avian gametes, and methods for enhancing germ line transmission of nucleic acids in birds.
Owner:NORTH CAROLINA STATE UNIV
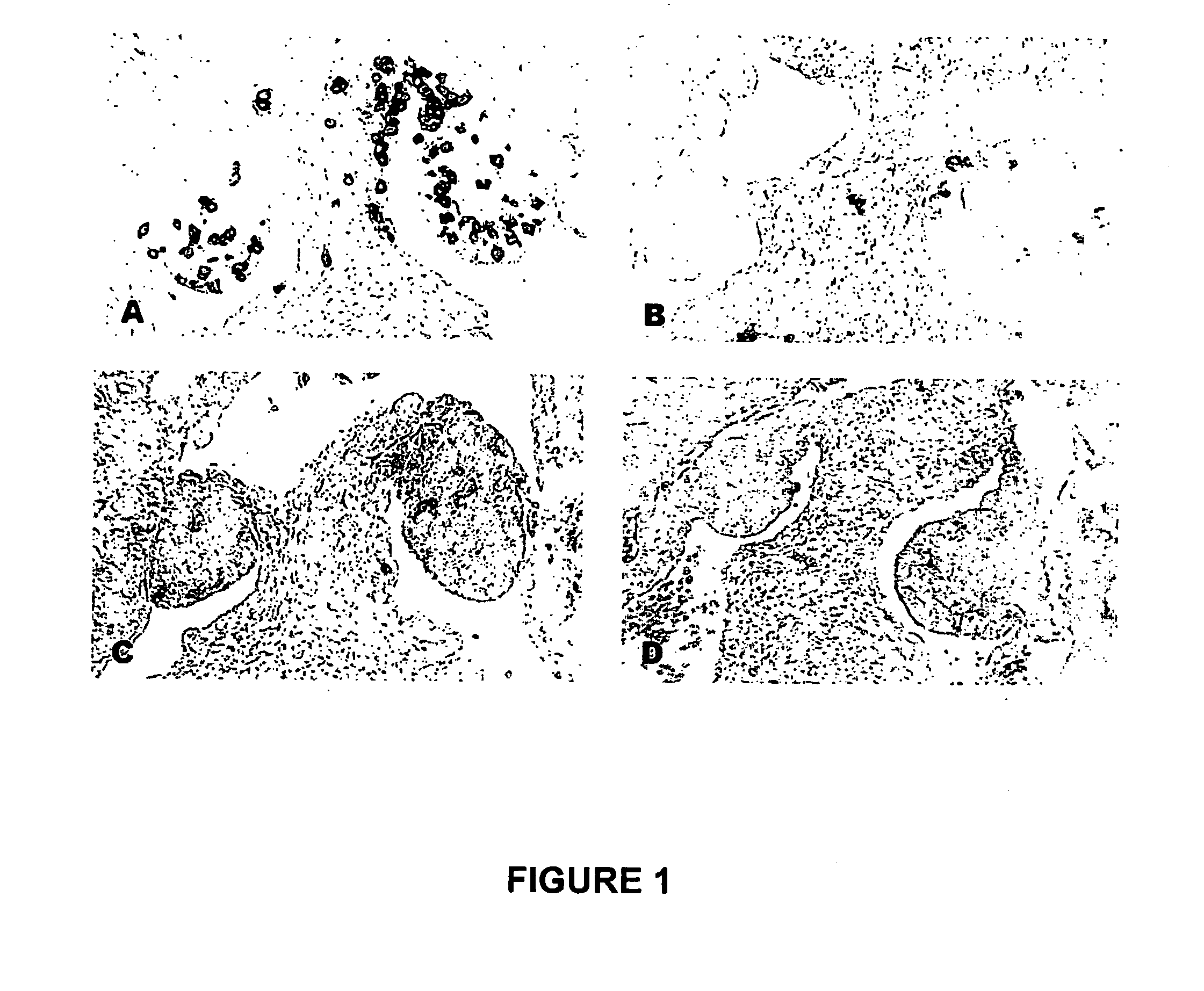
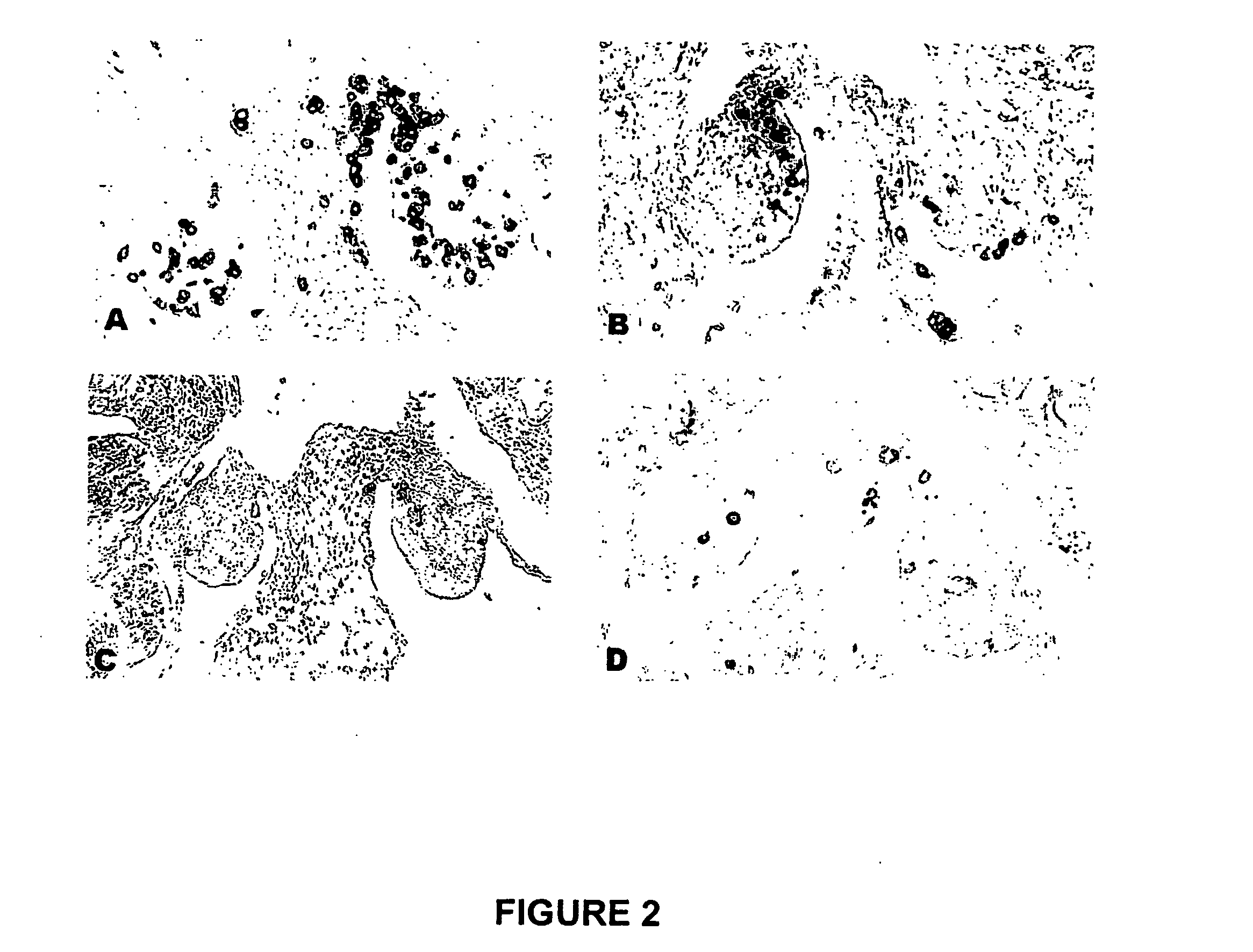
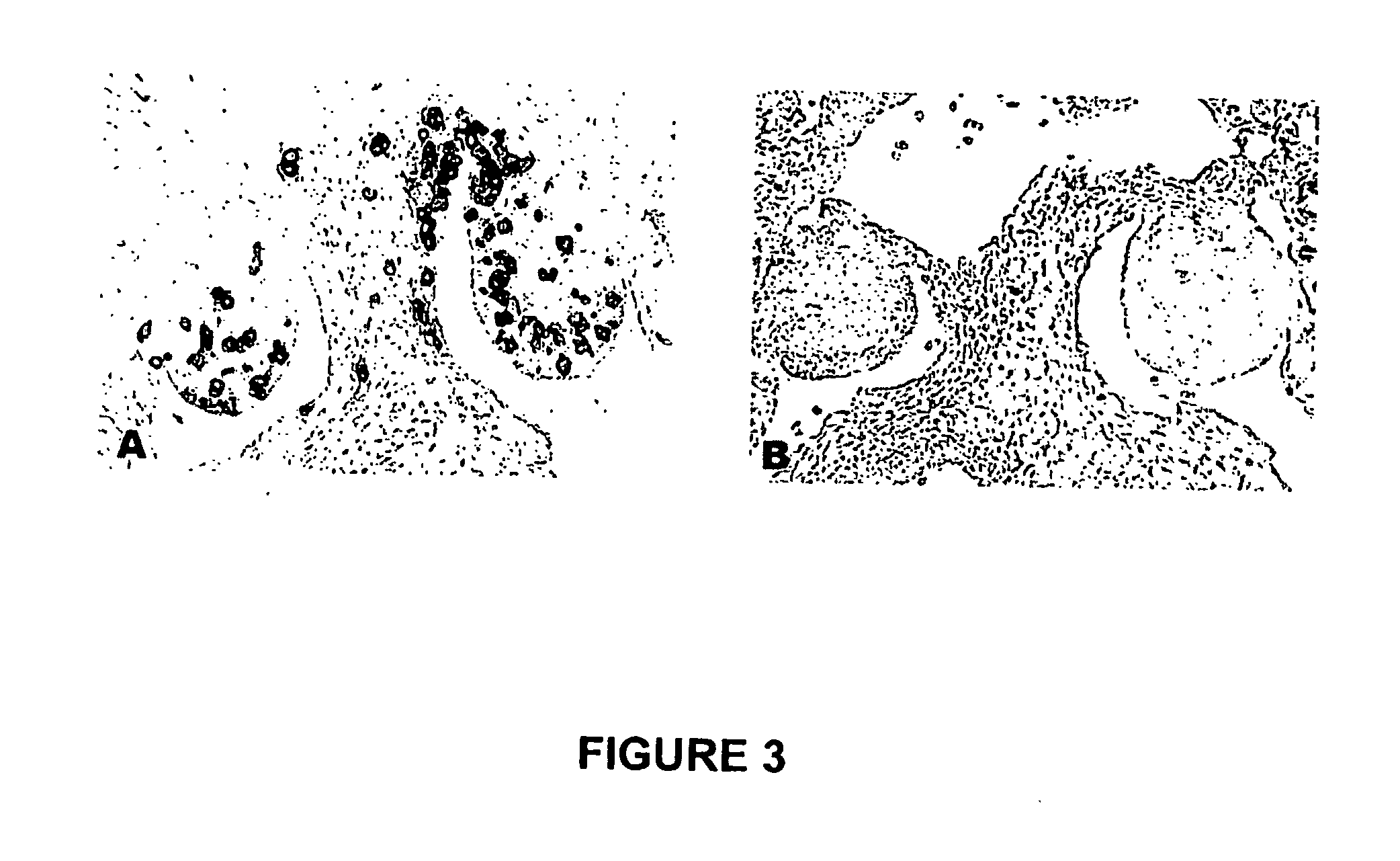
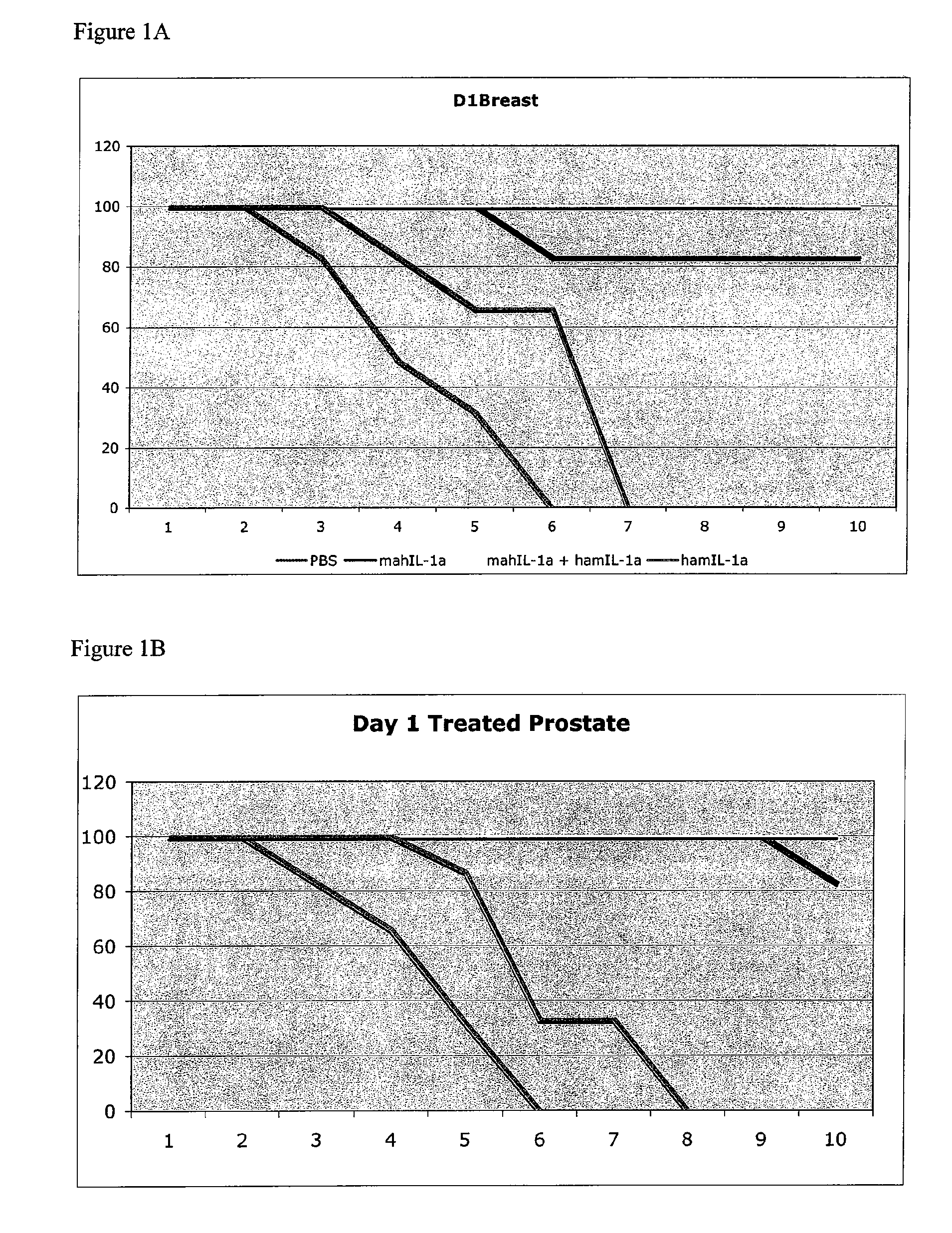
TREATMENT OF CANCER WITH ANTI-IL-1alpha ANTIBODIES
InactiveUS20100040574A1Peptide/protein ingredientsVertebrate antigen ingredientsCancer treatmentCancer research
Owner:XBIOTECH
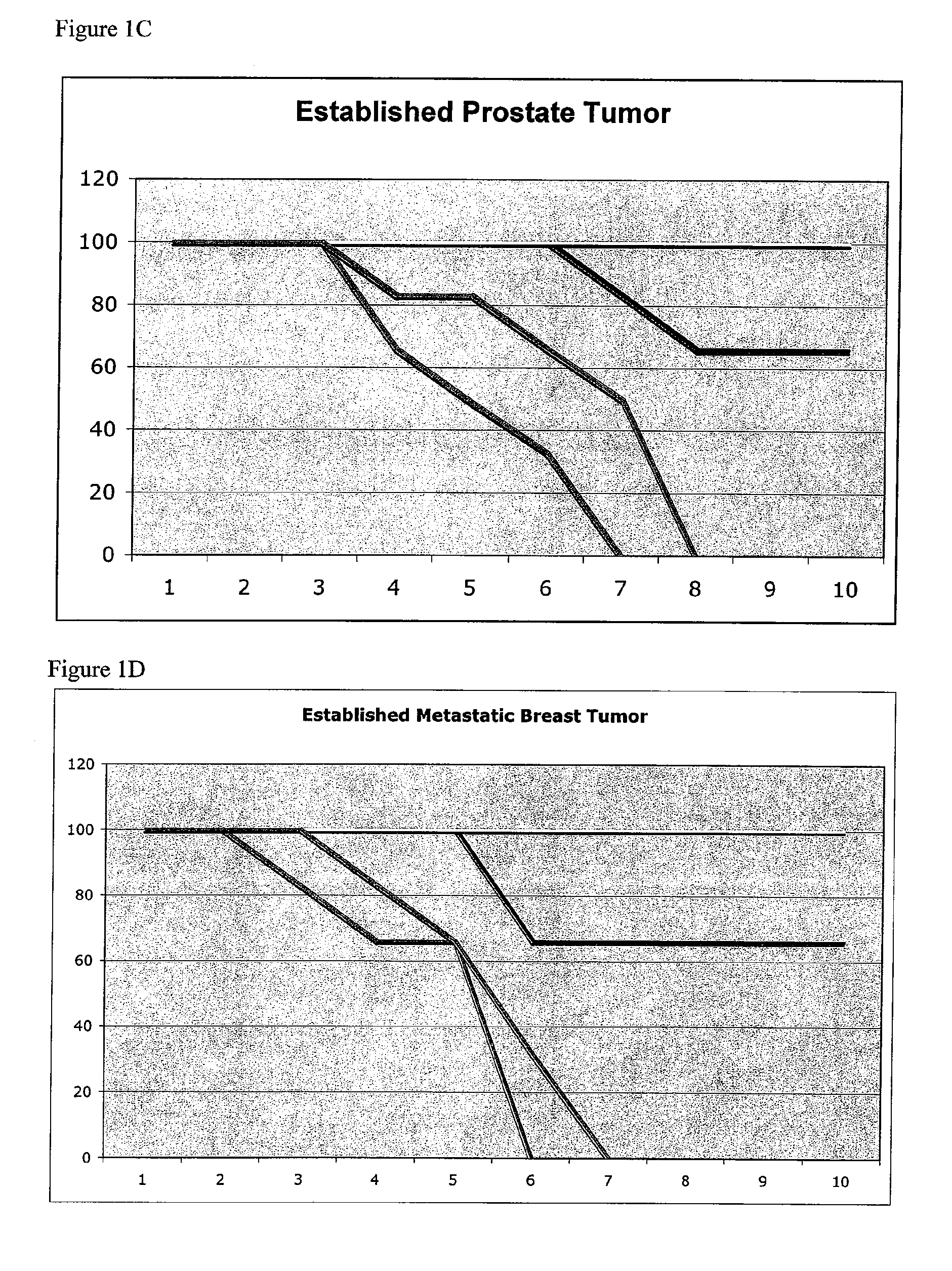
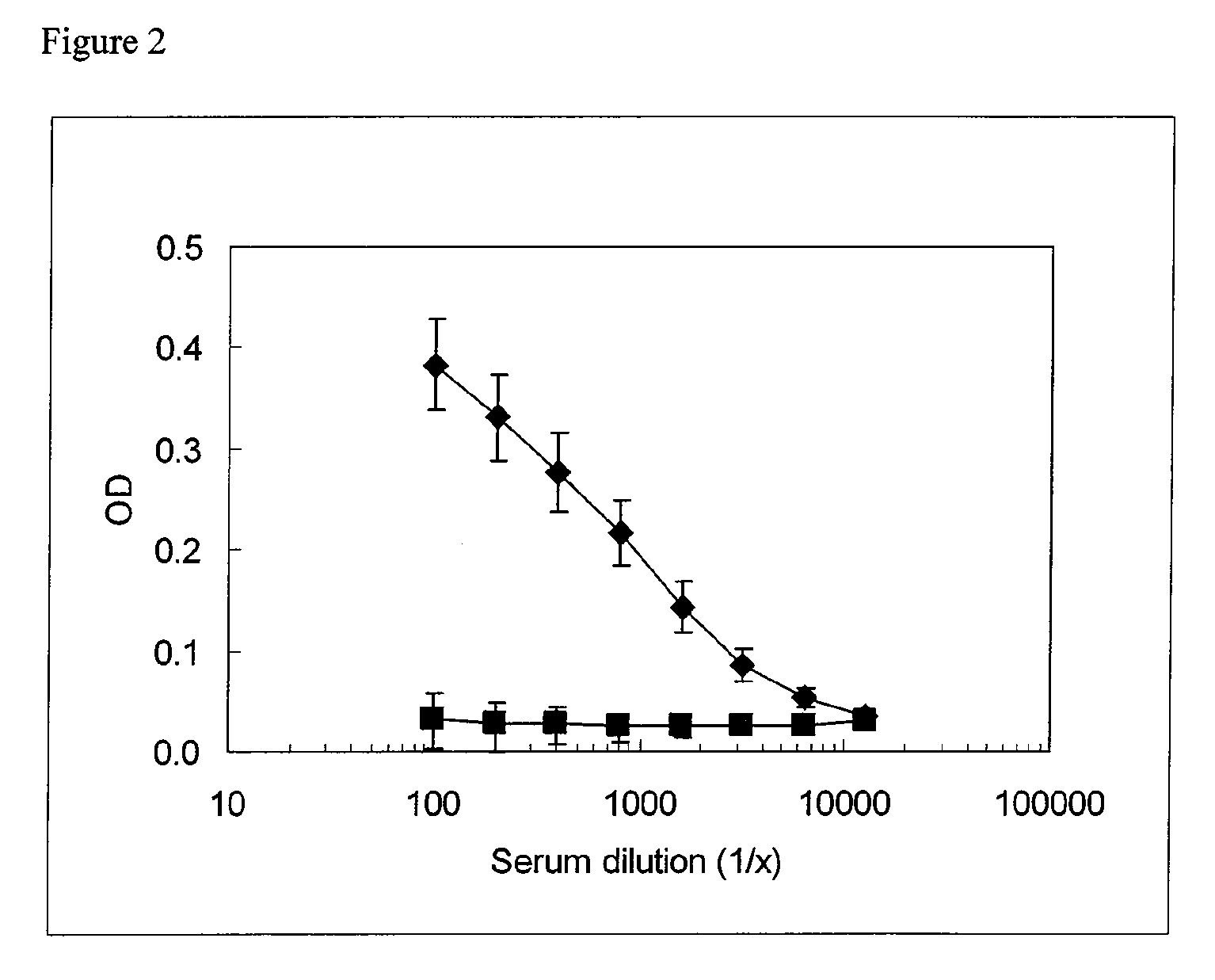
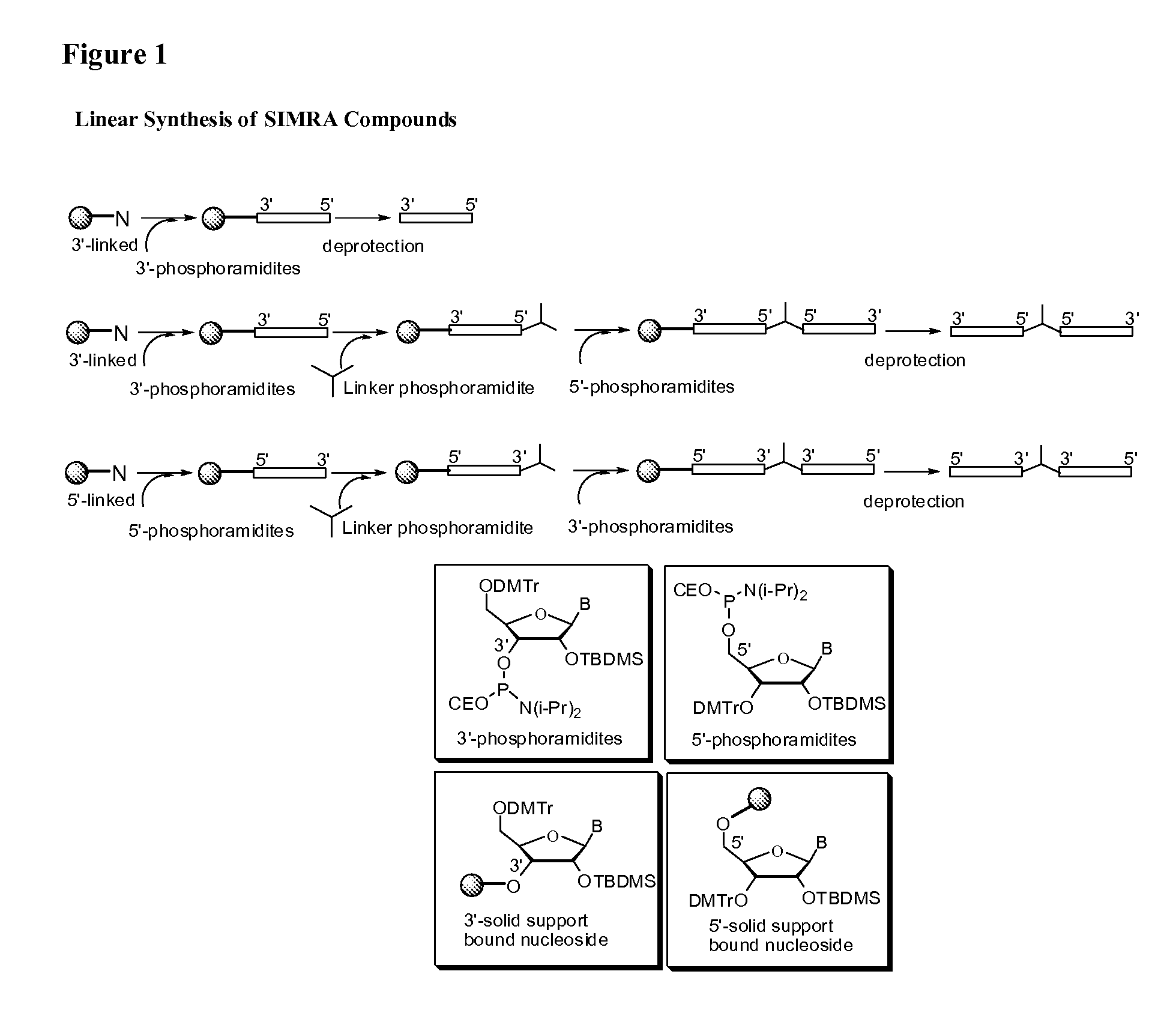
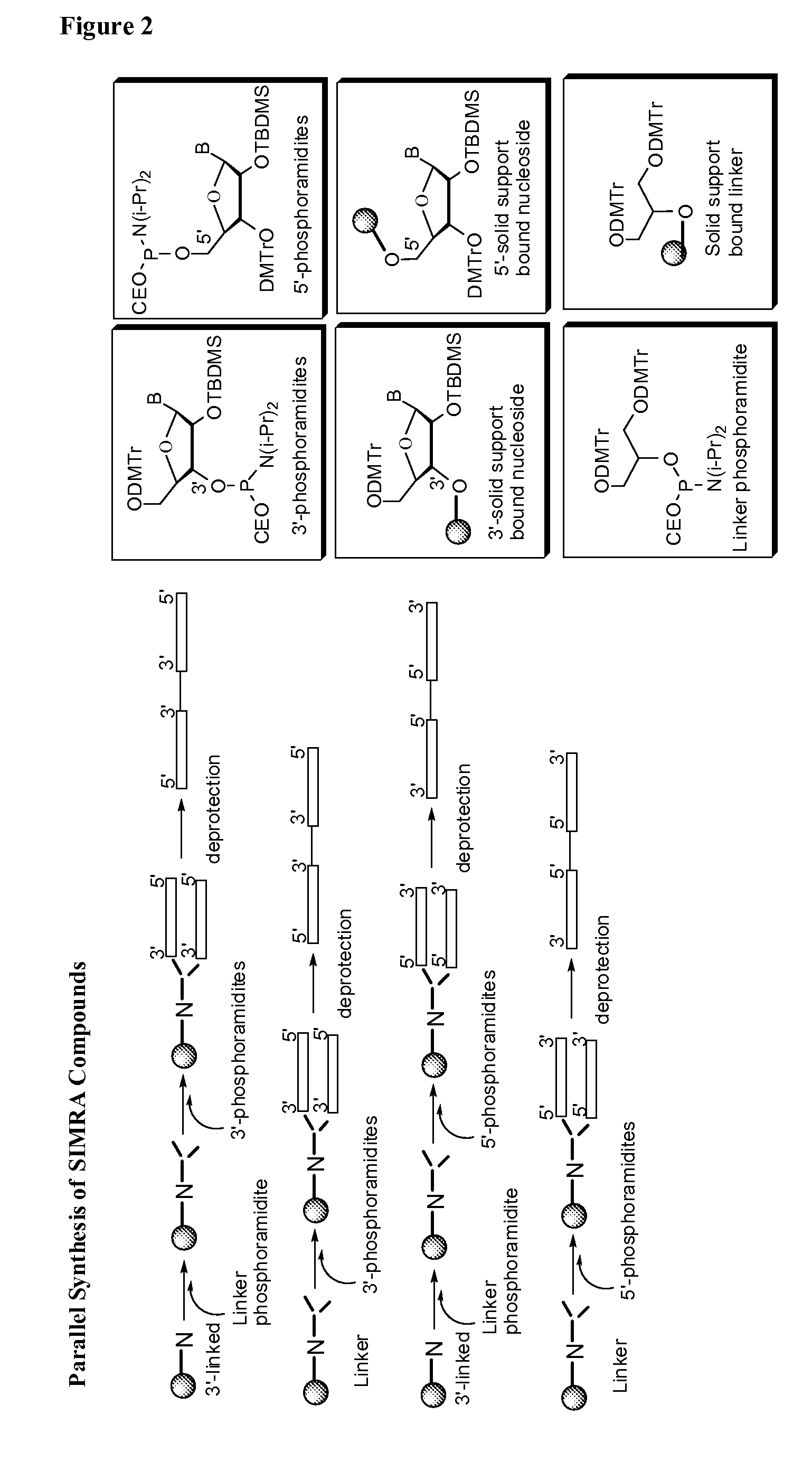
Stabilized immune modulatory RNA (SIMRA) compounds for tlr7 and tlr8
ActiveUS20080171712A1Improve stabilityImprove vaccination effectAntibacterial agentsOrganic active ingredientsOligoribonucleotidesTLR8
The invention relates to the therapeutic use of stabilized oligoribonucleotides as immune modulatory agents for immune therapy applications. Specifically, the invention provides RNA based oligoribonucleotides with improved nuclease and RNase stability and that have immune modulatory activity through TLR7 and / or TLR8.
Owner:IDERA PHARMA INC
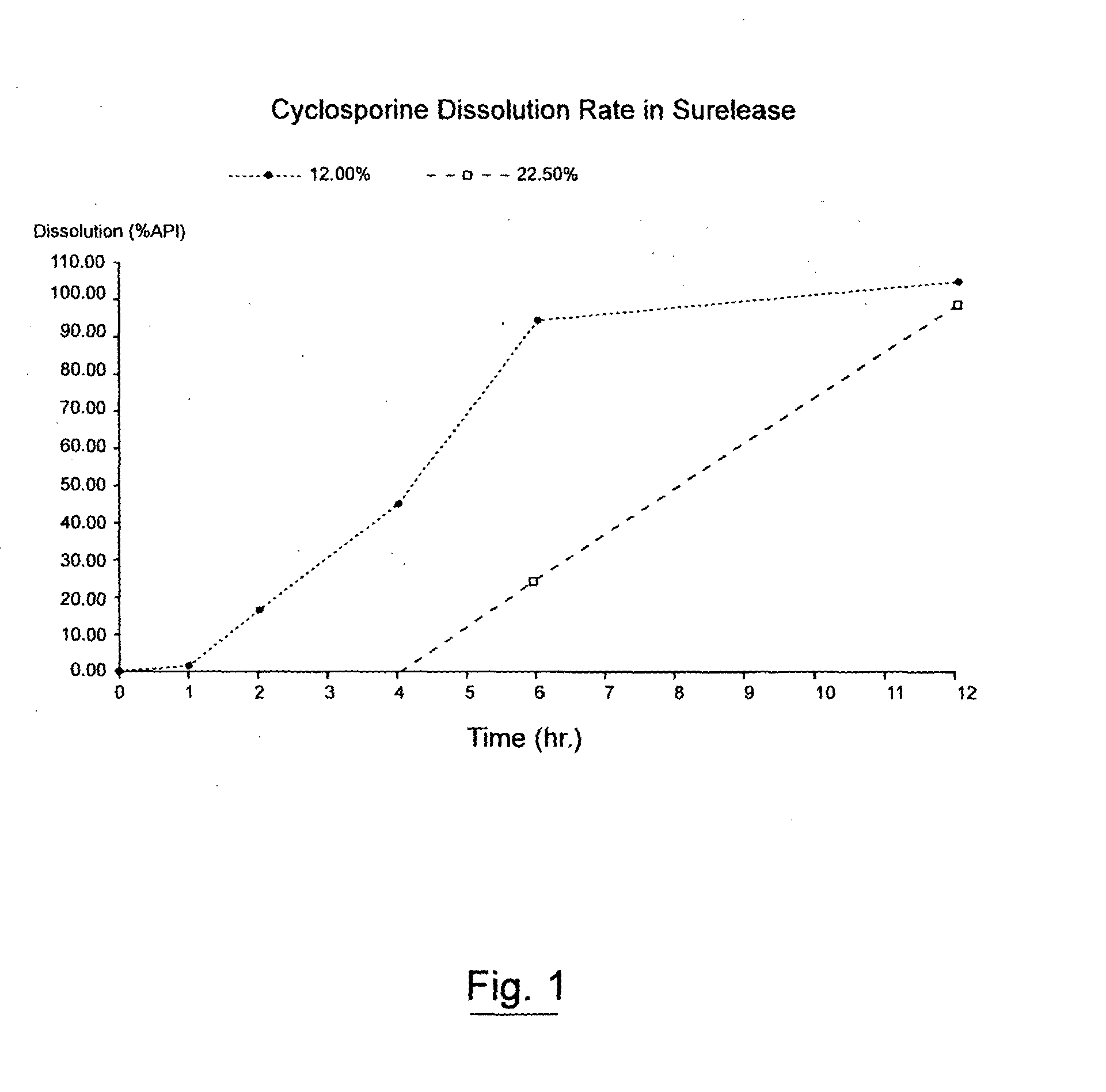
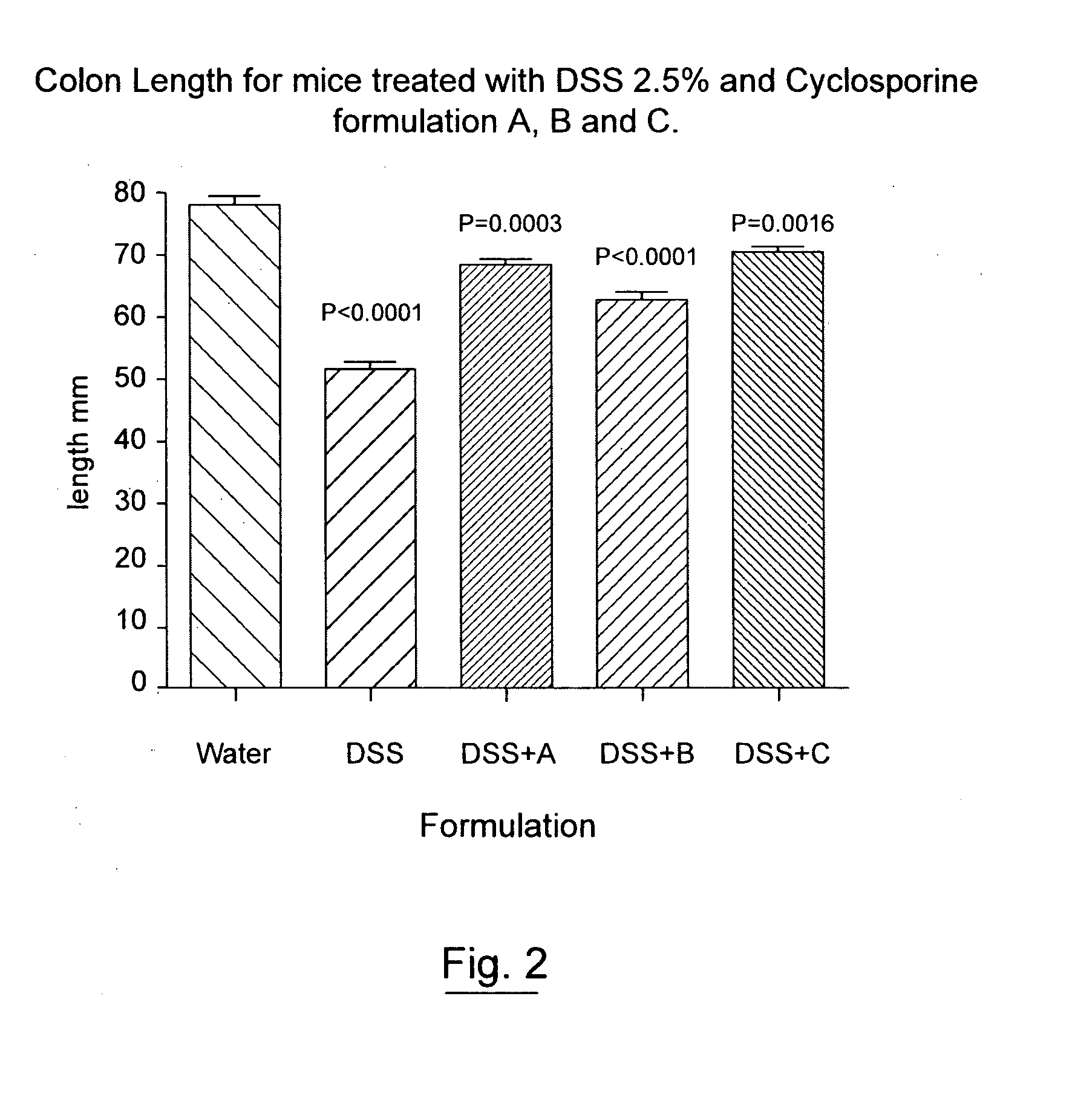
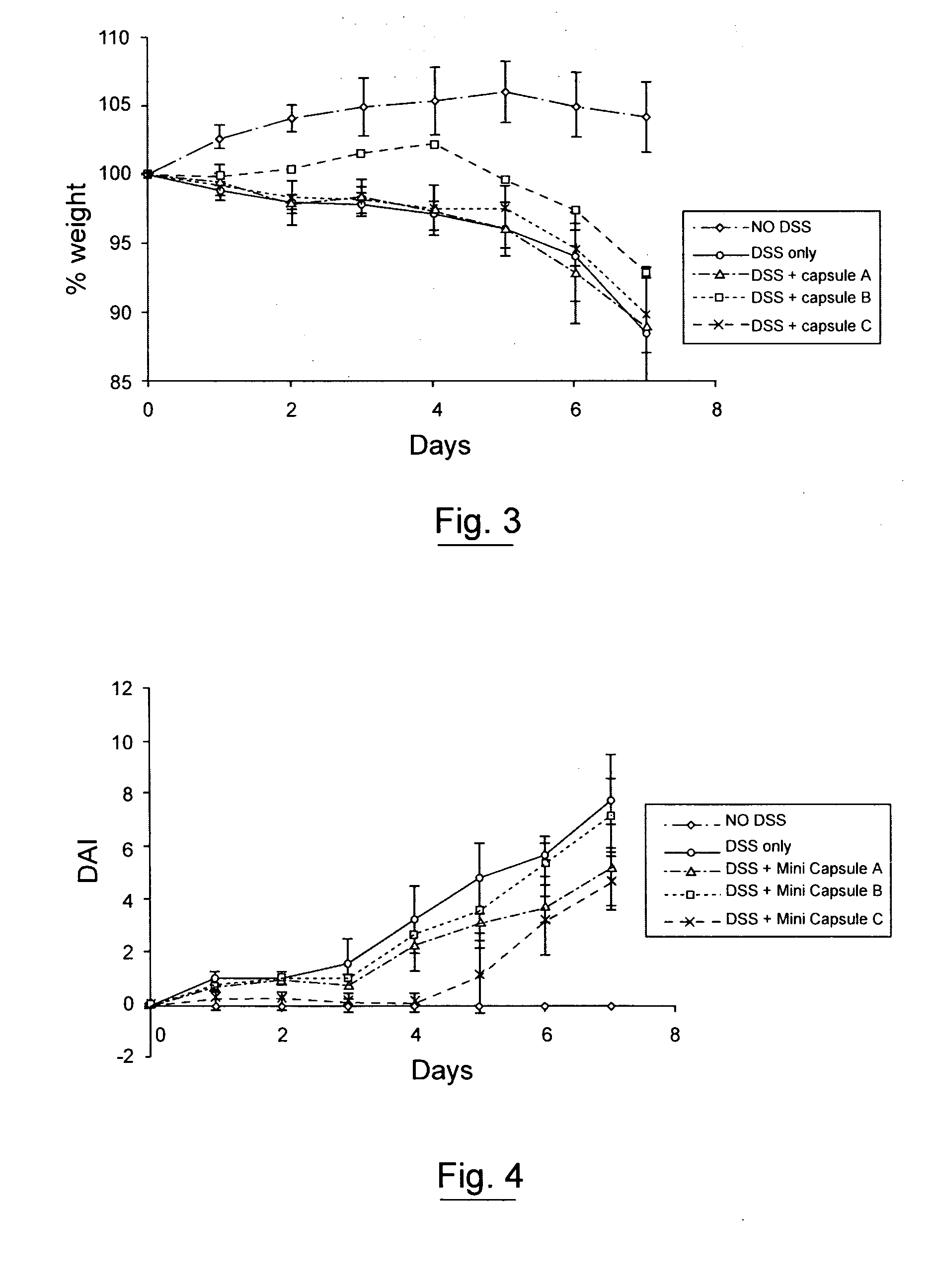
Pharmaceutical cyclosporin compositions
ActiveUS20100203120A1Maximize absorptionMaximize activityAntibacterial agentsOrganic active ingredientsDiseaseCyclosporins
An oral cyclosporin composition comprises minicapsules having a core containing a cyclosporin, especially cyclosporin A in a solubilised liquid form. The minicapsules have a release profile to release the pre-solubilised cyclosporin, at least in the colon. The composition may be used for treating a range of intestinal diseases [FIG. 10].
Owner:SUBLIMITY THERAPEUTICS LTD
Immunotherapy with binding agents
Binding agents that modulate the immune response are disclosed. The binding agents may include soluble receptors, polypeptides, and / or antibodies. Also disclosed are methods of using the binding agents for the treatment of diseases such as cancer.
Owner:ONCOMED PHARMA
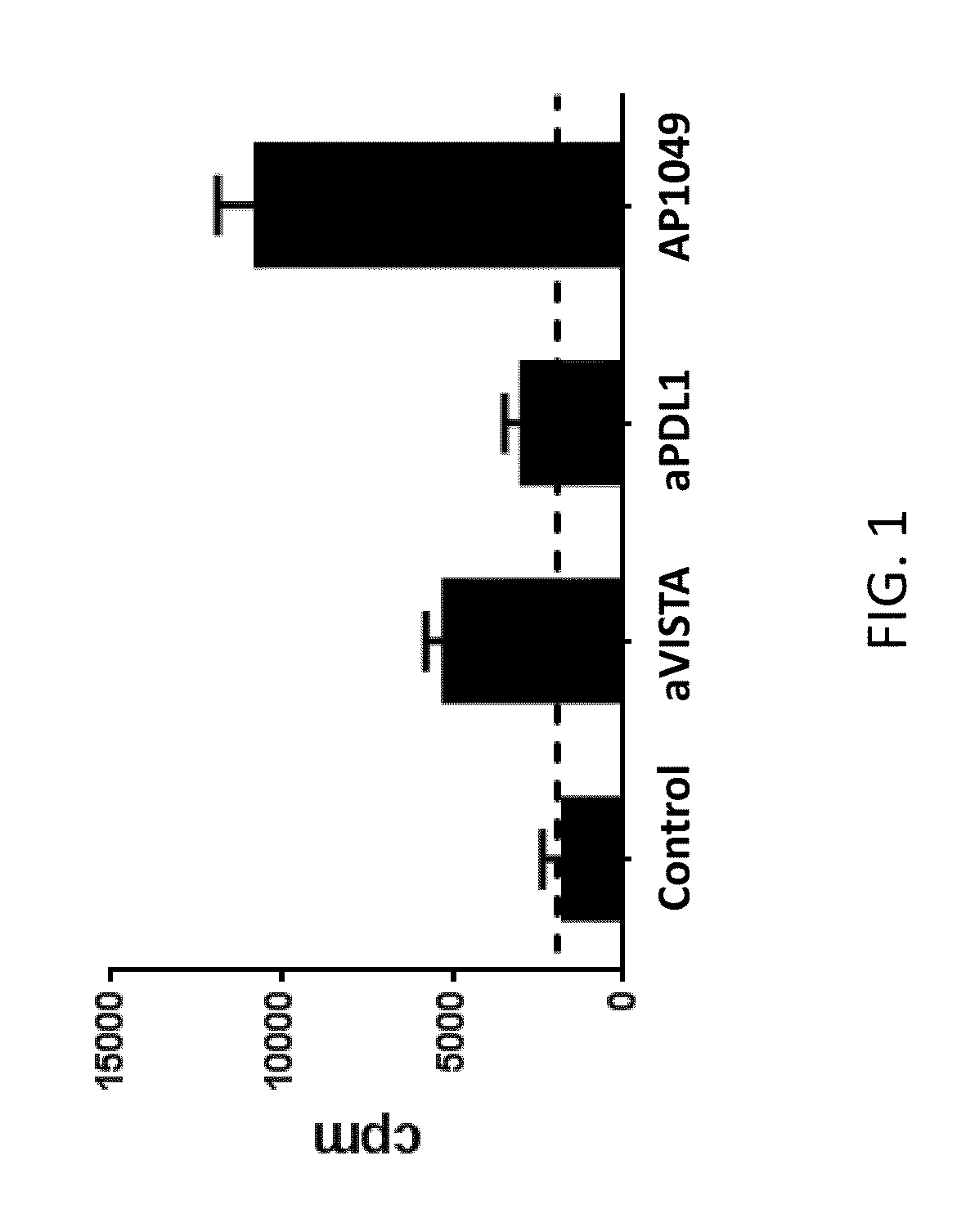
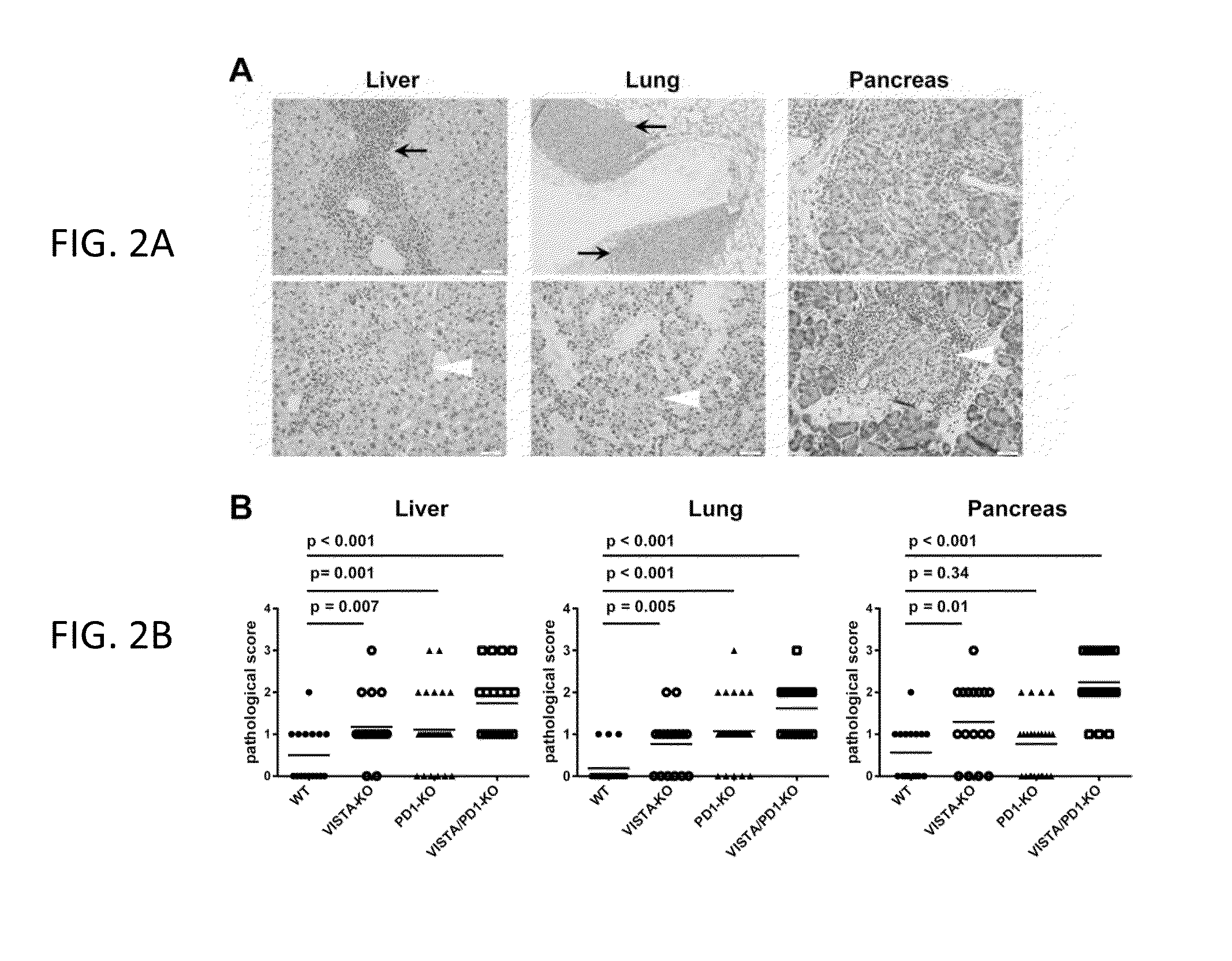
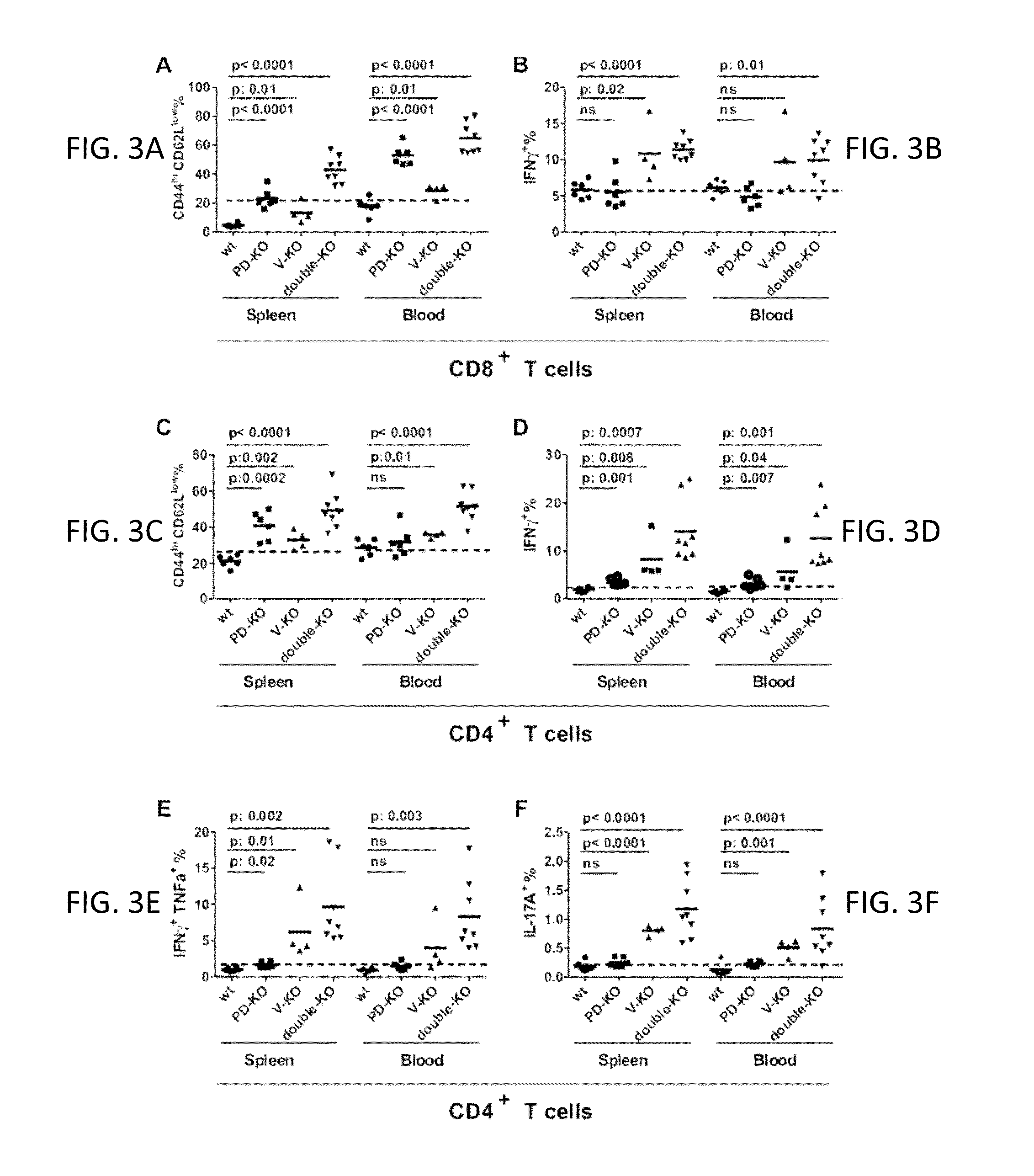
VISTA Antagonist and Methods of Use
The present invention is directed to synergic or additive therapies comprising the administration of a VISTA antagonist and a PD-1, PD-L1 or POD-L3 antagonist; or the combination of a VISTA agonist and a -1, PD-L1 or POD-L3 agonist which combinations respectively elicit an additive or synergistic effect at promoting T cell immunity or inhibiting T cell immunity, i.e., CD4, CD8 or Th1 immunity. The agonists and antagonists may be in the same or separate compositions and may be administered together or separately administered in either order.
Owner:NOELLE RANDOLPH J +4
Tolerogenic synthetic nanocarriers and therapeutic macromolecules for reduced or enhanced pharmacodynamic effects
ActiveUS20140328854A1Reduced pharmacodynamically effectiveLow effective dosePowder deliveryOrganic active ingredientsTolerabilityNanocarriers
Owner:SELECTA BIOSCI
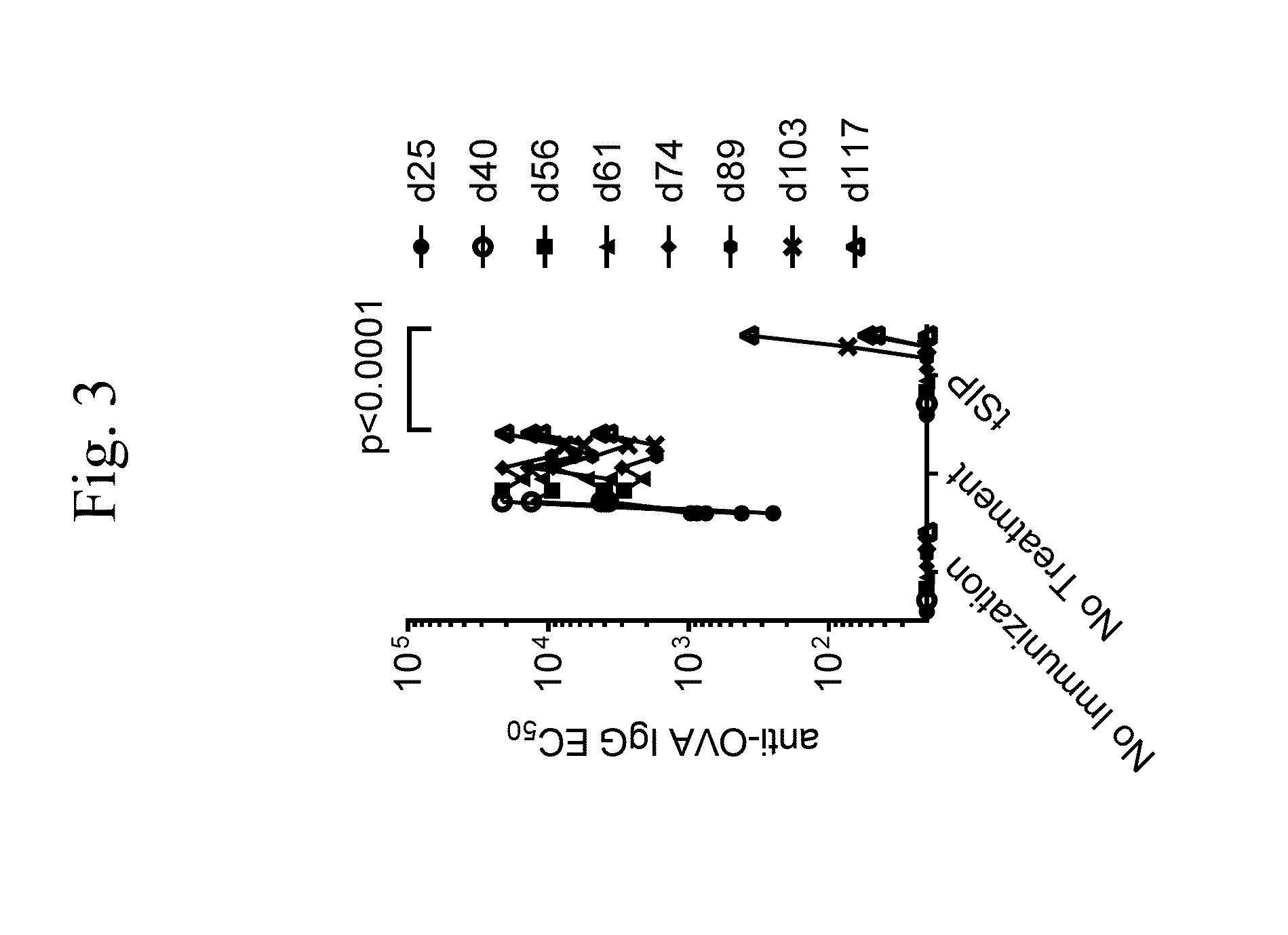
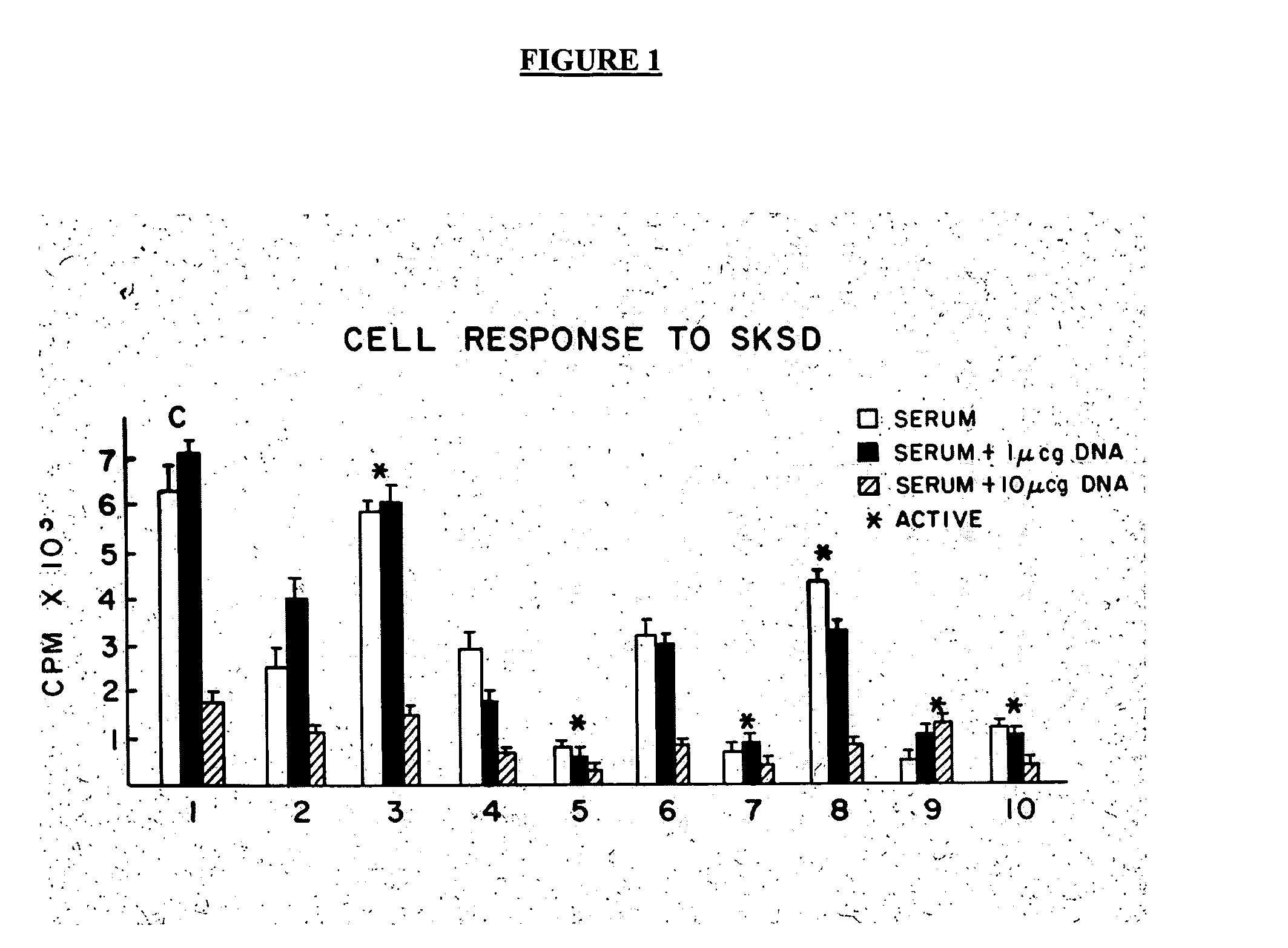
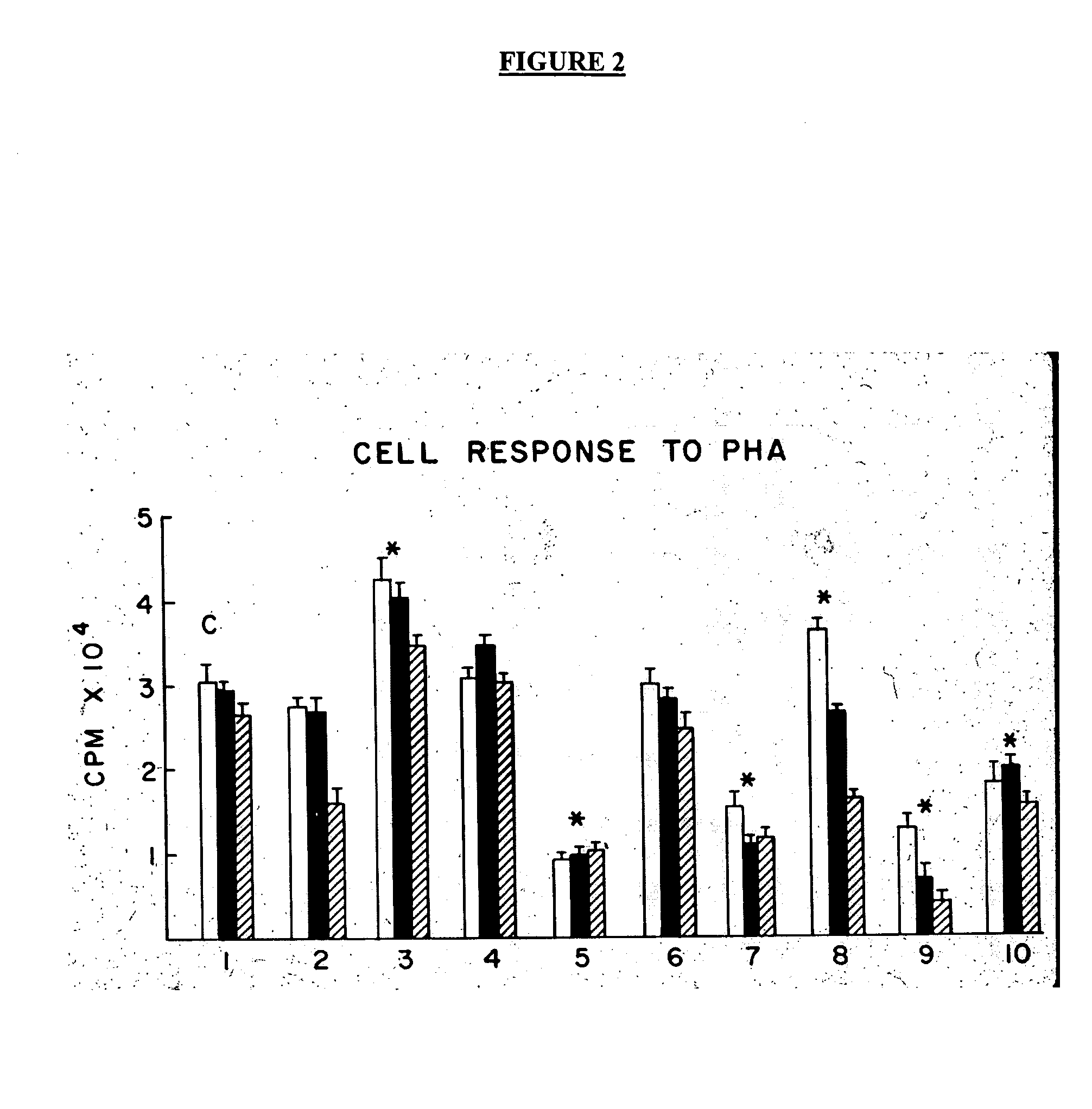
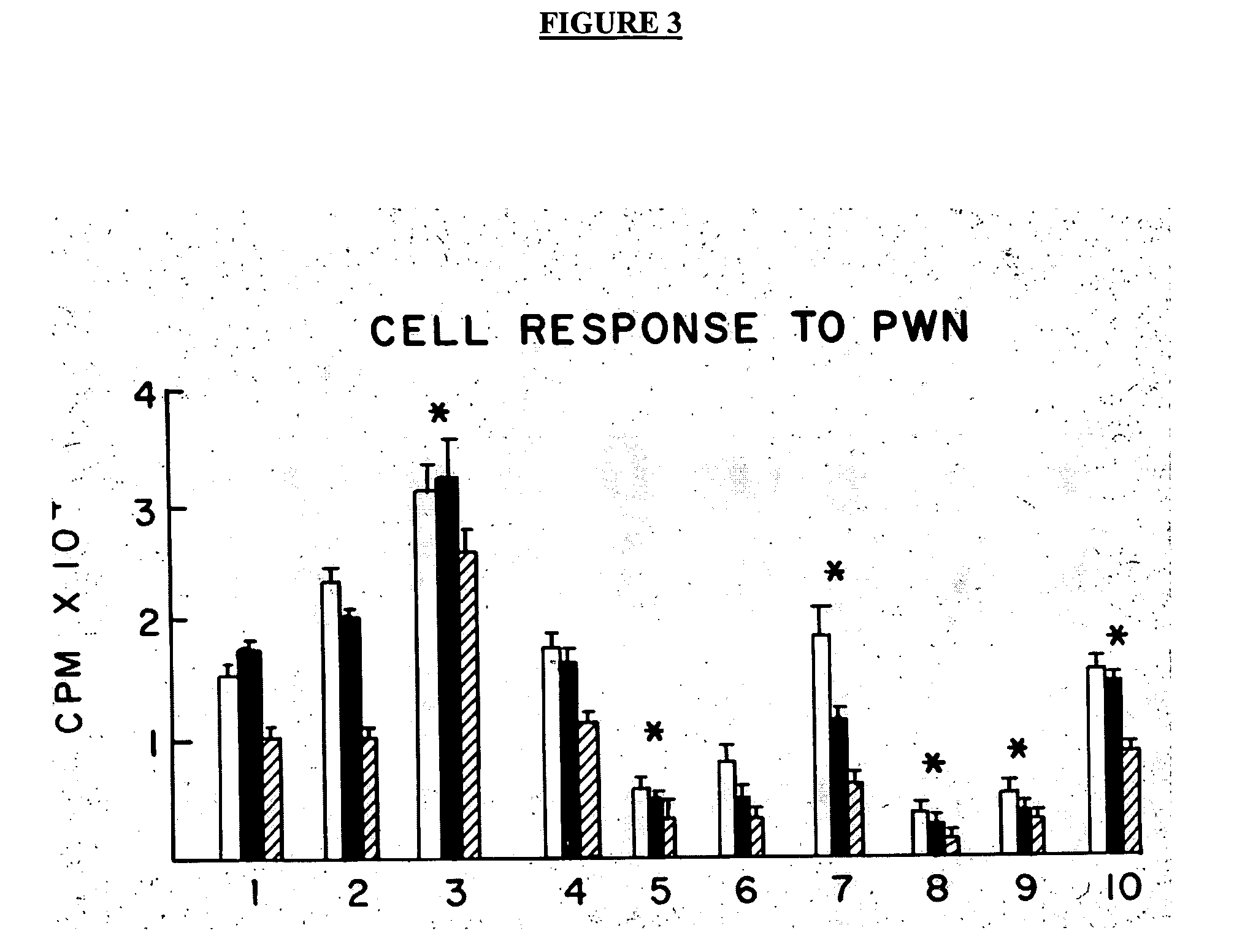
Treatment of inflammatory, non-infectious, autoimmune, vasculitic, degenerative vascular, host-v-graft diseases, Alzheimers disease, and amyloidosis using mammalian, dsDNA vaccination
The present invention relates generally to compositions and methods using mammalian, dsDNA (Double Stranded Deoxyribonucleic Acid) vaccination for the induction and maintenance of regulator suppressor T cells resulting in suppression of non infectious, and post infectious, inflammatory, allergic, auto-immune, vasculitic, certain degenerative vascular, and graft versus host diseases, with or without the use of IL-10, and with or without the use or TGFβ, with or without the use of anti-IL 6 receptor antibody, anti TNF antibody and or Plasmapheresis, IVIG, Corticosteroids, Methotrexate, Bromocriptine, and or vitamin D analogues.
Owner:LAWLESS OLIVER J
Tolerogenic synthetic nanocarriers to reduce immune responses to therapeutic proteins
InactiveUS20160220501A1Reduce in quantityReduce generationOrganic active ingredientsVertebrate antigen ingredientsAntigenNanocarriers
Disclosed are synthetic nanocarrier compositions, and related methods, comprising therapeutic protein APC presentable antigens and immunosuppressants that provide tolerogenic immune responses specific to therapeutic proteins.
Owner:SELECTA BIOSCI
Vaccine composition containing synthetic adjuvant
InactiveUS20110014274A1SsRNA viruses negative-senseBacterial antigen ingredientsNatural productDrug carrier
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:INFECTIOUS DISEASE RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com